Objective
In SARS-CoV-2 (COVID-19) infection, it is unclear if continuation of preadmission antiplatelet regimens upon hospitalization will improve hypercoagulability outcomes. Methods: This retrospective cohort study analyzed adult patients hospitalized with confirmed COVID-19 infection for a 6-week period from March 13, 2020, to April 27, 2020. Preadmission antiplatelet regimen continuation for less than 75% of admission was compared to continuation for at least 75% of admission. Pregnancy, either death or withdrawal of care within 24 hours of admission, and admission beyond the studied timeframe were excluded. The primary endpoint was difference in World Health Organization COVID-19 Ordinal Scale for Clinical Improvement values (World Health Organization [WHO] scores) between maximum score during admission to that upon discharge. Secondary endpoints were mechanical ventilation requirement, mortality, radiologically confirmed venous thromboembolism, major bleeding, and length of stay. Results: This study included 171 patients. Patients failing to continue antiplatelet regimens for at least 75% of admission (n = 76) had significantly worse WHO score differences than those who did (n = 95) (median −1 vs 2; P < .05). Mechanical ventilation requirement (57% vs 27%; P < .05) and mortality (58% vs 29%; P < .05) also favored antiplatelet continuation. All other endpoints were not significantly different. Conclusion: Significantly improved WHO scores, mechanical ventilation requirement, and mortality occurred in patients continuing preadmission antiplatelet regimens in COVID-19 infection. Future prospective studies of COVID-19 patients with consistently collected baseline hypercoagulability markers (platelets, D-dimer, fibrinogen, and coagulation studies) and similar severe disease risk factors are required to confirm potential benefits of antiplatelet therapy during hospitalization.
Keywords: infectious disease, internal medicine, cardiology
Introduction
The SARS-CoV-2 (COVID-19) novel coronavirus pandemic originally emerged in Wuhan city, Hubei, China, in December 2019 as a severe, possibly fatal pneumonia.1,2 Since its early discovery, laboratory markers including thrombocytopenia, prolonged thrombin time, elevated fibrinogen, and elevated D-dimer levels now recognize the virus’s capacity to provoke hypercoagulability and possible disseminated intravascular coagulation (DIC).3-6 While hypercoagulability is the initial concern, this may progress to consumptive coagulopathy following the depletion of coagulation factors with concomitant fibrinolysis. Antiplatelet therapy given early in the course of COVID-19 infection may be beneficial due to inhibitory actions on platelet aggregation, thereby reducing thrombus formation, intravascular fibrin, and DIC development. However, antiplatelets may aggravate bleeding if administered during late infection in the presence of consumptive coagulopathy.
Previous studies of DIC in septic shock have demonstrated the potential utility of antiplatelet therapy for prevention. Cartin-Ceba and colleagues published a retrospective observational cohort study of 390 adult patients with septic shock which demonstrated that antiplatelet use was associated with decreased risk of DIC (odds ratio .23; 95% CI 0.10–.50) after adjusting for the probability of antiplatelet adherence, severity of illness, age, positive blood cultures, timely antibiotics, and adequate resuscitation. 7 Similarly, a second retrospective cohort study by Valerio-Rojas and colleagues of 651 patients with severe sepsis or septic shock demonstrated that chronic antiplatelet therapy was associated with both a decreased incidence of acute respiratory distress syndrome (ARDS) (odds ratio .50; 95% CI 0.35–.71; P < .001) and a decreased need for mechanical ventilation (odds ratio .62; 95% CI 0.45–.87; P = .005). 8 However, this study did not associate antiplatelet use with decreased mortality (odds ratio .73; 95% CI 0.46–1.16; P = .19).
Data to justify antiplatelet therapy for COVID-19 infection is currently limited. 3 An observational multicenter study of 192 patients at 5 Italian hospitals found no association between prehospitalization antithrombotics (antiplatelets or anticoagulants) and risk of ARDS at admission or death during hospitalization. 9 A second case-control study from 2 UK hospitals analyzed patients with COVID-19 on antithrombotic medications prior to hospitalization in comparison to those who were not. 10 There was no significant difference in ICU admissions (P = .83) or mortality (P = .516) between patients on antiplatelets (n = 29) and controls (n = 58); however, this study acknowledged that underlying conditions requiring such antithrombotic use may have confounded results. With limited data to determine potential benefits and risks of antiplatelet therapy during COVID-19, this study sought to determine the potential utility of these agents in patients taking regimens prior to hospitalization with this viral infection.
Methods
Study Design
This was an Institutional Review Board-approved retrospective chart review of admitted patients with confirmed COVID-19 infection and a prehospitalization antiplatelet regimen. The institutional setting was a large community teaching hospital in New Jersey located approximately 20 miles outside of New York City, a secondary epicenter of the initial US COVID-19 outbreak. Data from March 13, 2020, to April 27, 2020, determined eligible patients. All data was extracted from the institution’s electronic health record.
Inclusion and Exclusion Criteria
Adult patients taking antiplatelet agents prior to hospitalizations between March 13, 2020, to April 27, 2020, with a confirmed COVID-19 infection per nucleic acid test, rapid antigen test, or outpatient-reported positive result were included. Antiplatelet medications prior to the index hospitalization were recorded by the first documented medication reconciliation upon admission, and COVID-19 status was confirmed upon prehospitalization or admission laboratory findings. Antiplatelet agents included aspirin, clopidogrel, ticagrelor, prasugrel, aspirin with dipyridamole, or any combination of these. Pregnancy, either death or withdrawal of care within 24 hours of admission, and admission beyond April 27, 2020, were exclusion criteria. This site does not provide extracorporeal membrane oxygenation services.
Exposures
The control group consisted of patients taking antiplatelet medications for less than 75% of the index hospital admission. Patients taking any antiplatelet medication for 75% or more of hospital admission were included in the study group. The 75% duration of antiplatelet administration cutoff was chosen so that patients would receive medication for the majority of the stay with an allowance for an occasional missed dose. Occasional missed doses may have occurred due to short periods of nothing by mouth, procedures, or delayed admission medication orders. Administration of antiplatelets was confirmed by nursing documentation in the electronic medical record. The total percentage of medication administration was calculated by dividing the total number of days the patient received an antiplatelet agent by the total number of days in the hospital length of stay. Indications for antiplatelet medications and daily doses were not collected.
Endpoints
The primary endpoint of the study was the difference in World Health Organization COVID-19 Ordinal Scale for Clinical Improvement values (World Health Organization [WHO] scores) between the maximum score during admission to that upon discharge. WHO scores positively correlate with disease severity. A value of 0 indicates an uninfected patient, whereas a value of 8 indicates death. 11 Differences were calculated by subtracting the score upon discharge from the maximum score achieved during admission. Secondary endpoints included differences in requirement of mechanical ventilation, mortality, radiologically confirmed venous thromboembolism (VTE), major bleeding as defined by the International Society on Thrombosis and Haemostasis (ISTH), and length of stay. 12 All analyzed event occurrences resulted during the index hospitalization. Requirement of mechanical ventilation was defined as patients utilizing invasive mechanical ventilation or patients who died with do not intubate (DNI) and/or do not resuscitate (DNR) status.
Statistical Analyses
Investigators conducted statistical analyses for each endpoint with RStudio®. Primary endpoint statistical analysis utilized Mann–Whitney U testing for ordinal data. Secondary endpoints underwent chi-square testing for nominal data, Fisher’s exact testing for nominal data with small observed event frequencies, and Mann–Whitney U testing for continuous data. A P-value of less than .05 indicated statistical significance.
Results
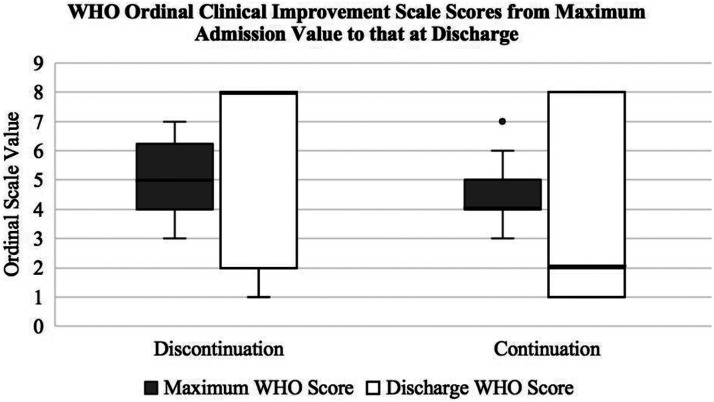
A total of 171 admitted patients with prehospitalization antiplatelet regimens were included in the analysis from March 13, 2020, to April 27, 2020 (Table 1). The most common antiplatelet regimen in either group was aspirin monotherapy. Dose intensity of aspirin therapy was not evaluated. Upon admission, 95 patients continued prehospitalization antiplatelet regimens for at least 75% of the index hospitalization and 76 did not. The majority of patients in the control group did not have a reported reason for antiplatelet discontinuation (n = 39). Listed reasons for discontinuation included bleeding (n = 9), patient inability or unwillingness to perform (n = 8), periods of nothing by mouth (n = 6), comfort care (n = 5), or other (n = 9). In comparison to the antiplatelet discontinuation group, the antiplatelet continuation group had a significantly improved difference in WHO scores between the maximum score during admission to that at discharge (−1 vs 2; P < .05) (Figure 1). A negative difference indicates a less favorable condition upon discharge.
Table 1.
Baseline Characteristics.
| Discontinuation n = 76 | Continuation n = 95 | P Value | |
|---|---|---|---|
| Mean age (SD) | 71 (±11.7) | 71 (±12.1) | .92 |
| Mean symptom onset history in days (SD) | 6 (±4.4) | 6 (±4.7) | .94 |
| Mean antiplatelet days (SD) | 1.4 (±2.9) | 8.1 (±5.1) | <.05 |
| Male sex (%) | 40 (53) | 57 (60) | .42 |
| Race (%) | <.05 | ||
| Black | 42 (55) | 35 (37) | <.05 |
| White | 20 (26) | 39 (41) | .06 |
| Other | 14 (18) | 21 (22) | .69 |
| Preadmission antiplatelet regimen (%) | .68 | ||
| Aspirin | 59 (78) | 69 (73) | .57 |
| Clopidogrel | 6 (8) | 13 (14) | .34 |
| Aspirin + clopidogrel | 9 (12) | 10 (11) | .98 |
| Other | 2 (3) | 3 (3) | 1 |
| Death while DNR and/or DNI (%) | 23 (30) | 10 (11) | <.05 |
| Admission from long-term care facility (%) | 23 (30) | 22 (23) | .38 |
| Oxygen supplementation prior to admission (%) | 2 (3) | 4 (4) | .69 |
| Any hydroxychloroquine during admission (%) | 59 (78) | 85 (89) | .06 |
| Any Tocilizumab during admission (%) | 8 (11) | 6 (6) | .48 |
| Any anticoagulation ≥50% of admission (%) | 59 (78) | 81 (85) | .28 |
| Any therapeutic anticoagulation | 13 (17) | 13 (14) | .68 |
| Any prophylactic anticoagulation | 54 (71) | 74 (78) | .40 |
| Any corticosteroid during admission (%) | 23 (30) | 22 (23) | .38 |
| Initial white blood cell value (IQR) | 6.9 (5.4–9.8) n = 75 | 6.2 (4.8–8.1) n = 95 | .08 |
| Initial hemoglobin value (IQR) | 12.6 (10.3–14) n = 75 | 12.6 (11.4–14.1) n = 95 | .36 |
| Initial hematocrit value (IQR) | 39.4 (32–43.1) n = 75 | 38.4 (35–43.1) n = 95 | .55 |
| Initial platelet value (IQR) | 181 (148–251) n = 75 | 198 (148–275) n = 95 | .28 |
| PT in first 48 hours of admission (IQR) | 14.3 (13.4–15.4) n = 24 | 12.8 (12.4–13.9) n = 11 | <.05 |
| PTT in first 48 hours of admission (IQR) | 29.7 (29.1–32.4) n = 20 | 30.1 (27.3–31.6) n = 8 | .78 |
| INR in first 48 hours of admission (IQR) | 1.3 (1.2–1.4) n = 25 | 1.1 (1.1–1.2) n = 13 | <.05 |
| D-dimer in first 48 hours of admission (IQR) | 731 (17–714) n = 24 | 439 (72–538) n = 28 | .13 |
| Fibrinogen in first 48 hours of admission (IQR) | 700 (643–700) n = 21 | 662 (552–700) n = 6 | .25 |
SD, standard deviation; DNR, do not resuscitate; DNI, do not intubate; IQR, interquartile range.
Figure 1.
The above box plot depicts studied patients’ WHO 8-point ordinal clinical improvement scale scores from the maximum score during admission to that upon discharge. WHO scores positively correlate with disease severity. Clinical improvement occurs as scores decrease. WHO: World Health Organization.
Secondary Outcomes
There were significant differences favoring inpatient antiplatelet continuation for requirement of mechanical ventilation (57% vs 27%; P < .05) and mortality (58% vs 29%; P < .05). There was no significant difference determined for radiologically confirmed VTE, major bleeding events as defined by the ISTH, or length of hospital stay (Table 2). 12
Table 2.
Research Outcomes.
| Discontinuation (n = 76) | Continuation (n = 95) | P Value | |
|---|---|---|---|
| Primary outcome | |||
| Median WHO score differences (IQR) | −1 (−3−3) | 2 (−1−3) | <.05 |
| Secondary outcomes | |||
| Requirement of mechanical ventilation (%) | 43 (57) | 26 (27) | <.05 |
| Mortality (%) | 44 (58) | 28 (29) | <.05 |
| Radiologically confirmed VTE (%) | 1 (1) | 3 (3) | .63 |
| Major bleeding per ISTH (%) | 1 (1) | 1 (1) | 1 |
| Length of hospital stay in days (SD) | 8.6 (±5.6) | 8.3 (±5.2) | .84 |
WHO, World Health Organization; IQR, interquartile range; ISTH, International Society on Thrombosis and Haemostasis.
Discussion
In this retrospective cohort study, inpatients who continued a prehospitalization antiplatelet regimen during confirmed COVID-19 infection had significantly improved WHO clinical improvement score differences at discharge from the maximum value attained during admission. Patients who continued antiplatelet regimens for 75% or more of admission also had significantly decreased mortality and requirement of mechanical ventilation. Despite these positive results, it was difficult to determine whether antiplatelet agents were discontinued due to severity of disease or other reasons. Of note, similar numbers of patients were on oxygen at baseline, so this characteristic is unlikely to have affected WHO score differences. However, there were significantly more patients of black race and those who died while DNR or DNI status. Since these patient populations are expected to have more severe disease and worse outcomes, this does represent a significant confounder in our analysis.13,14
Variations in prescriber documentation made it difficult to determine a patient’s baseline severity of disease. As data began to emerge during the timeframe of this research regarding the utility of DIC laboratory markers in assessing disease severity upon presentation, many patients did not have these values collected until the later portion of admission. 4 Decisions to discontinue antiplatelets were rarely documented in the electronic medical record, and it was mostly unclear if the admission orders for these medications were forgotten or intentionally discontinued. A large limitation in this research came from the inability to determine if the patient’s disease was so severe that antiplatelets became futile or if the discontinuation of antiplatelet agents was associated with a progression of disease severity from the sequelae of hypercoagulability.
The results of this study did not demonstrate significant differences in radiologically confirmed VTE, major bleeding, or hospital length of stay. During the pandemic, strict isolation measures made it logistically more difficult for providers to perform diagnostic imaging in this patient population. 15 Therefore, there was a higher threshold to perform this type of diagnostic imaging leading to a potential underdiagnosis of VTE events. Notably, similar numbers of patients in either group received prophylactic or therapeutic anticoagulation at any point during admission. However, exact anticoagulation regimens administered in relation to mechanical ventilation initiation or death were not collected. Resuming antiplatelet regimens in the setting of COVID-19 infection did not increase occurrences of major bleeding as defined by the ISTH, but acquisition of defined criteria through the electronic medical record was also limited. 12 Length of stay was not significantly different, but an increased number of deaths in the discontinuation of antiplatelets group may have reduced these durations.
It is still unclear if antiplatelet agents can lead to better patient outcomes with COVID-19 infection. While 2 small prior studies in the United Kingdom and Italy did not find significant benefit or harm with prehospitalization antiplatelet use, it was difficult to account for underlying comorbidities in patients requiring such antithrombotics in comparison to patients who did not.9,10 Indications for antiplatelet therapy were not collected in this study, but all patients were on antiplatelet medications at baseline. Additionally, as aspirin is an over-the-counter medication, doses were not collected. We refrained from collecting this information as it would be difficult to confirm the precise daily dose since accuracy would rely entirely on patient report. This may be a significant confounder in our results, especially since patients with a history of coronary artery disease would be expected to have higher risk of severe disease in comparison to those taking antiplatelets solely for primary prevention. However, we chose to evaluate the number of patients that received other proposed COVID-19 treatments including hydroxychloroquine, tocilizumab, anticoagulation, and corticosteroids which were similar in each group.16-21 Other information regarding potential underlying predictors of disease severity such as blood type as well as emerging therapies including convalescent plasma and remdesivir were not collected.22-25
Although our research accounted for antiplatelet therapy prior to admission, baseline DIC laboratory markers indicative of severe infection were rarely collected in either group for the studied timeframe as the utility of this data was simultaneously emerging. 4 Therefore, it was difficult to determine if either group was truly similar at baseline, especially when many patients elected for DNR or DNI status in the setting of poor outcomes in severe infection for this novel virus. While there are limitations to the conclusions that may be drawn from this data, it is hypothesis generating and potentially encouraging. Future studies surrounding antiplatelet use in patients with COVID-19 not previously prescribed antiplatelet therapy could be considered.
Conclusion
Continuation of a prehospitalization antiplatelet regimen while admitted for COVID-19 infection may be associated with significantly increased clinical improvement, decreased requirement of mechanical ventilation, and decreased mortality in this retrospective cohort study. However, the utility of identifying severe disease through DIC laboratory markers was just emerging during the researched timeframe, and these values were rarely collected upon admission. 4 Therefore, future prospective studies of patients with COVID-19, consistently collected baseline DIC laboratory markers, and similar baseline risk for severe disease are required to determine similarities of disease severity and the potential benefits of antiplatelet use.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs
Angela Antoniello https://orcid.org/0000-0002-5565-6707
Alison Brophy https://orcid.org/0000-0002-8478-8493
References
- 1.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet. 2020;395(10229):1054-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou X, Li Y, Yang Q. Antiplatelet Therapy after percutaneous coronary intervention in patients with COVID-19: Implications from clinical features to pathologic findings. Circulation. 2020;141(22):1736-1738. [DOI] [PubMed] [Google Scholar]
- 4.Tang N, Li D, Wang X, et al. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844-847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thachil J, Tang N, Gando S, et al. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost. 2020;18(5):1023-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iba T, Levy JH, Warkentin TE, et al. ; Scientific and standardization committee on DIC, and the scientific and standardization committee on perioperative and critical care of the international society on thrombosis and haemostasis. Diagnosis and management of sepsis-induced coagulopathy and disseminated intravascular coagulation. J Thromb Haemost. 2019;17(11):1989-1994. [DOI] [PubMed] [Google Scholar]
- 7.Cartin-Ceba R, Valerio-Rojas J, Cabello-Garza J, et al. Antiplatelet therapy and disseminated intravascular coagulation in septic shock patients: Propensity score analysis. Am J Respir Crit Care Med .2011;183:A4698. [Google Scholar]
- 8.Valerio-Rojas JC, Jaffer IJ, Kor DJ, et al. Outcomes of severe sepsis and septic shock patients on chronic antiplatelet treatment: A historical cohort study. Crit Care Res Pract. 2013;2013:782573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Russo V, Di Maio M, Attena E, et al. Clinical impact of pre-admission antithrombotic therapy in hospitalized patients with COVID-19: A multicenter observational study. Pharmacol Res. 2020;159:104965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sivaloganathan H Ladikou EEand Chevassut T. COVID-19 mortality in patients on anticoagulants and antiplatelet agents. Br J Haematol 2020;190(4):e192-e195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization . WHO R&D Blueprint novel Coronavirus COVID-19 Therapeutic Trial Synopsis [Internet]. Geneva, Switzerland: WHO Press; 2020[cited 2020 Apr 27]. 12 p. https://www.who.int/blueprint/priority-diseases/key-action/COVID-19_Treatment_Trial_Design_Master_Protocol_synopsis_Final_18022020.pdf [Google Scholar]
- 12.Schulman S, Angerås U, Bergqvist D, et al. Subcommittee on control of anticoagulation of the scientific and standardization committee of the international society on thrombosis and haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in surgical patients. J Thromb Haemost. 2010;8(1):202-204. [DOI] [PubMed] [Google Scholar]
- 13.Centers for Disease Control . COVID-19 Hospitalization and Death by Race/Ethnicity [Internet]. Atlanta, Georgia: CDC; 2021[cited 2021 Apr 1]. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/investigations-discovery/hospitalization-death-by-race-ethnicity.html [Google Scholar]
- 14.Alhatem A, Spruijt O, Heller DS, Chokshi RJ, Schwartz RA, Lambert WC. “Do-not-resuscitate (DNR)” status determines mortality in patients with COVID-19. Clin Dermatol. 2020;39(3):510-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aryal MR, Gosain R, Donato A, et al. Venous thromboembolism in COVID-19: Towards an ideal approach to thromboprophylaxis, screening, and treatment. Curr Cardiol Rep. 2020;22(7):52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berlin DA, Gulick RM, Martinez FJ. Severe Covid-19. N Engl J Med. 2020;383(25):2451-2460. [DOI] [PubMed] [Google Scholar]
- 17.Gao J, Tian Z, Yang X. Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends. 2020;14(1):72-73. [DOI] [PubMed] [Google Scholar]
- 18.Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30(3):269-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guaraldi G, Meschiari M, Cozzi-Lepri A, et al. Tocilizumab in patients with severe COVID-19: A retrospective cohort study. Lancet Rheumatol. 2020;2(8):e474-e484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klok FA, Kruip MJHA, van der Meer NJM, et al. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: An updated analysis. Thromb Res. 2020;191:148-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Horby P, Lim WS, Emberson JR, RECOVERY Collaborative Group , Dexamethasone in hospitalized patients with Covid-19 - preliminary report. N Engl J Med. 2020;384(8):693-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Latz CA, DeCarlo C, Boitano L, Blood type and outcomes in patients with COVID-19. Ann Hematol. 2020;99(9):2113-2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li L, Zhang W, Hu Y, et al. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: A randomized clinical trial. JAMA. 2020;324(5):460-470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Y, Zhang D, Du G, et al. Remdesivir in adults with severe COVID-19: A randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395(10236):1569-1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beigel JH, Tomashek KM, Dodd LE, ACTT-1 Study Group Members , Remdesivir for the treatment of Covid-19 - final report. N Engl J Med. 2020;383(19):1813-1826. [DOI] [PMC free article] [PubMed] [Google Scholar]