Abstract
The application of modern ovarian reserve measures to women with sickle cell disease (SCD) may help answer longstanding questions about whether SCD or hydroxycarbamide affect women’s reproductive lifespan. Antimullerian hormone (AMH), an established marker of ovarian reserve, is used to asses the ovarian follicle pool. We used a standard clinical assay to measure AMH in 285 banked samples from 93 female subjects with haemoglobin SS from the historic Multicenter Study of Hydroxyurea (MSH) which led to the FDA approval of hydroxycarbamide for adults with SCD. No samples from the randomized portion of the MSH remain, so samples from the decade-long MSH follow-up studies were analyzed. Most subjects were exposed to hydroxycarbamide (86/93). Median AMH levels were lower in study subjects than in age- and sex-matched reference values. Median AMH levels consistent with diminished ovarian reserve, a risk factor for infertility, occurred in subjects starting at age 25–30 years; in healthy women, this occurs after age 40. In multivariate analysis, taking hydroxycarbamide was independently associated with a low AMH (Est Std .001, 95% CI −0.002 – 0.000, p=.006). These results suggest that ovarian reserve is prematurely reduced in women with haemoglobin SS and raise the possibilty that hydroxycarbamide contributes to this finding.
Keywords: Fertility, Women, Ovarian Reserve, Sickle Cell Disease, Hydroxyurea, Antimullerian Hormone, Infertility
Introduction
Sickle cell disease (SCD) is a congenital haemoglobinopathy characterized by anaemia, chronic end-organ damage and early death. Near universal survival through childhood has led to the identification of previously unappreciated and consequential end-organ damage(Niss et al, 2017; Lebensburger et al, 2019; Vichinsky et al, 2010). Whether the ovaries are injured by SCD or SCD therapies is not established(Chase et al, 2009). Oxidative and hypoxic-ischemic injury underlies SCD pathophysiology and causes ovarian injury in other settings(Soleimani et al, 2011; Agarwal et al, 2005; Chase et al, 2009). Sickling in the ovarian vasculature may cause primary ovarian insufficiency, a condition defined in women under 40 years by irregular menses and follicle-stimulating hormone levels in the post-menopausal range, indicating depletion of the egg supply and causing infertility(Chase et al, 2009). SCD treatments may indirectly or directly damage the ovaries and compromise existing oocyte quantity and/or quality. For example, iron overload secondary to chronic transfusions(Fung et al, 2006; Chang et al, 2011), hydroxycarbamide(Elchuri et al, 2015) and non-myeloablative haematopoietic stem cell transplant (HSCT)(Pecker et al, 2018; Carpinello et al, 2018) may compromise oocyte quantity or quality. Identifying whether SCD or its therapies reduce ovarian reserve matters to patients with SCD and parents of affected children, some of whom decline significant, life changing treatments because of concern that the therapy will compromise future fertility(Nahata et al, 2017; Oyeku et al, 2013; Thompson et al, 2013).
Females are born with a finite number of primordial ovarian follicles, representing their entire egg supply, that are continuously recruited over their lifespan. The recruitment of primordial follicles during ovulation causes a progressive decline in the follicle pool(Li et al, 2014). Contemporary measures of this follicle pool, called the ovarian reserve, include direct and indirect measure of the ovaries’ appearance and oocyte quantity. Primordial follicles produce antimullerian hormone (AMH), an established biomarker of the oocyte pool that is the earliest and most sensitive marker of ovarian reserve,(Li et al, 2014) and that is used to predict response to ovarian stimulation in women experiencing infertility(Visser et al, 2012). AMH levels beneath 1.1ng/mL contribute to the definition of diminished ovarian reserve (DOR), a finding that is normal in women over 40 years, but is a risk factor for infertility in younger women(Pastore et al, 2018). AMH is used to assess iatrogenic damage to ovarian reserve before and after exposure to gonadotoxic therapies(Peigné & Decanter, 2014; Perdrix et al, 2017; Elchuri et al, 2016; Helden & Weiskirchen, 2017). Pre-treatment AMH predicts post-therapy levels and helps predict if and when the ovarian follicle pool recovers from chemotherapy(Su et al, 2014; Anderson & Wallace, 2013).
In men, SCD and its therapies are associated with primary and secondary hypogonadism and compromised sperm quality and quantity(Berthaut et al, 2017), but little is established about ovarian reserve in women with SCD at baseline. A British study compared women with all genotypes of SCD to age-matched women being evaluated for infertility; AMH levels were lower in women with SCD at a younger age compared to controls with infertility(Kopeika et al, 2019). Although interpretation of this study is somewhat confounded by the inclusion of diverse SCD genotypes, these findings suggest that women with SCD have accelerated ovarian aging and a reduced reproductive lifespan.
The effects of hydroxycarbamide on ovarian reserve are not yet well defined. Hydroxycarbamide is considered “low risk” for infertility in women, but ovarian follicles are sensitive to DNA damage caused by this “milder” agent(Bedoschi et al, 2016). Limited data in mice and humans raises concern about the drug’s effect on oocytes: wild-type mice treated with hydroxycarbamide or placebo found that the hydroxycarbamide treated mice had lower ovarian weight, estradiol levels, and ovulation rates; while ex-vivo fertilization rates did not differ between groups, embryos with continuous Hydroxycarbamide exposure failed to develop to the blastocyst stage(Sampson et al, 2010). A single-center study comparing AMH and follicle stimulation hormone in adolescent girls with HbSS and haemoglobin Sβ0 (HbSβ0) found that teen subjects treated with hydroxycarbamide, but not with supportive care, had DOR (24%, n=8), a finding associated with older age and longer duration of hydroxycarbamide treatment(Elchuri et al, 2015). This study raised the possibility that hydroxycarbamide may reduce ovarian reserve.
The purpose of this study was to test the hypotheses that women who participated in the MSH would have lower AMH levels than age- and sex-matched reference values and (2) that hydroxycarbamide exposure is associated with lower AMH levels. These hypotheses were tested by measuring AMH levels in banked samples from the homogenous population of adult women with haemoglobin SS (HbSS) who participated in the Follow-up and Extension studies of the historic Multicenter Study of Hydroxyurea (MSH), the randomized controlled trial (RCT) that established hydroxycarbamide’s efficacy in adults with SCD, leading to hydroxycarbamide’s FDA approval(Charache et al, 1995a).
Methods
This study was approved by the Johns Hopkins University Institutional Review Board (IRB153296).
Patient cohort and samples
After the MSH RCT concluded, consented subjects were followed in the MSH Follow-up and Extension Studies, a non-randomized cohort study that monitored subjects for late toxicities of hydroxycarbamide exposure(Ballas et al, 2009). The Follow-up and Extension Studies consisted of 10 annual study visits. Subject age at enrollment in the MSH, but not during Follow-up visits, was available. Age at follow-up visits was therefore calculated by adding the following values in years: a) age at enrollment, b) number of years patient was enrolled in the RCT and c) number of years from the RCT’s closure to the time of follow-up visit.
AMH measurement
Through the NHLBI Biorepository, BioLINCC, we obtained clinical data from all MSH participants and banked serum samples from all female participants for whom a serum sample was available(Giffen et al, 2017). The serum samples were measured using a CLIA-certified, ultrasensitive electrochemiluminescent assay (Esoterix Laboratory Services, Calabasas, CA). This assay is used in clinical practice by the reproductive endocrinologists at the Johns Hopkins Fertility Center. The analytic range of the assay is 0.0015–15 ng/mL. The intra- and inter-assay sample precision for AMH determination were 9.3% and 12.9%, respectively. AMH levels that resulted beneath the assay’s limit of detection were run in duplicate when sufficient sample was available for analysis; this did not change results for any sample. Samples with AMH levels beneath the lower limit of detection were assigned a value of zero. DOR is a risk factor for developing primary ovarian insufficiency and associated infertility. The assay’s AMH cut-off for DOR is a level less than 1.1ng/mL.
Statistical analysis
Statistical analysis of the results was conducted in R(Team, 2019). Descriptive analyses are reported using ranges, means and medians as appropriate. Samples were analyzed according to subject age at the time of specimen collection. We described the AMH levels in the cohort, comparing median AMH values by age for the cohort to reported age- and sex- dependent reference values for the Esoterix Assay. To test the hypothesis that hydroxycarbamide treatment in the RCT of the MSH would be associated with lower AMH than placebo treatment at any follow-up visit, we compared age-matched AMH values by original MSH randomization.
We then analyzed outcomes among subjects for whom a study sample was available at the first follow-up visit. The rationale for this approach is that this was the visit at which there was a sample available for 56 of 93 subjects at the first follow-up visit; no other follow-up visit had more individual subjects available for analysis. The second rationale was that this is the closest visit to the RCT. We performed a dichotomous analysis of AMH in this sub-group, comparing subjects with DOR to those without. Univariate and multivariate linear regression analysis was used to estimate the strength of association between hydroxycarbamide exposure and AMH levels and MCV and AMH levels in patients while adjusting for possible confounders (age, body mass index (BMI), RCT randomization). Increased MCV is often, but not always, associated with hydroxycarbamide adherence. Established limitations in the MSH study related to tracking hydroxycarbamide use in the follow-up studies led to our use MCV as a proxy measure of hydroxycarbamide adherence and exposure.
Precise hydroxycarbamide exposure data in the MSH and follow-up studies is difficult to interpret(Ballas et al, 2009). For this reason, we considered hydroxycarbamide exposure in several ways (Figure 1). First, we evaluated AMH levels among all subjects based on whether they were randomized to hydroxycarbamide or placebo in the original MSH RCT. This analysis included 93 subjects and all 285 available samples. Second, we evaluated age adjusted AMH levels based on whether they were taking hydroxycarbamide at the time of the first study visit. This analysis included the 56 subjects with an existing sample from the first study visit. Third, we compared subjects by age with no hydroxycarbamide exposure throughout the study period to those with any exposure who were ≦ 40 years old at the time of their first available sample. Subjects were defined as having no hydroxycarbamide exposure if they were randomized to placebo in the original MSH and never took hydroxycarbamide thereafter. This analysis examined AMH level from the first available sample for each subject.
Figure 1. Explanation of study population and sample availability.
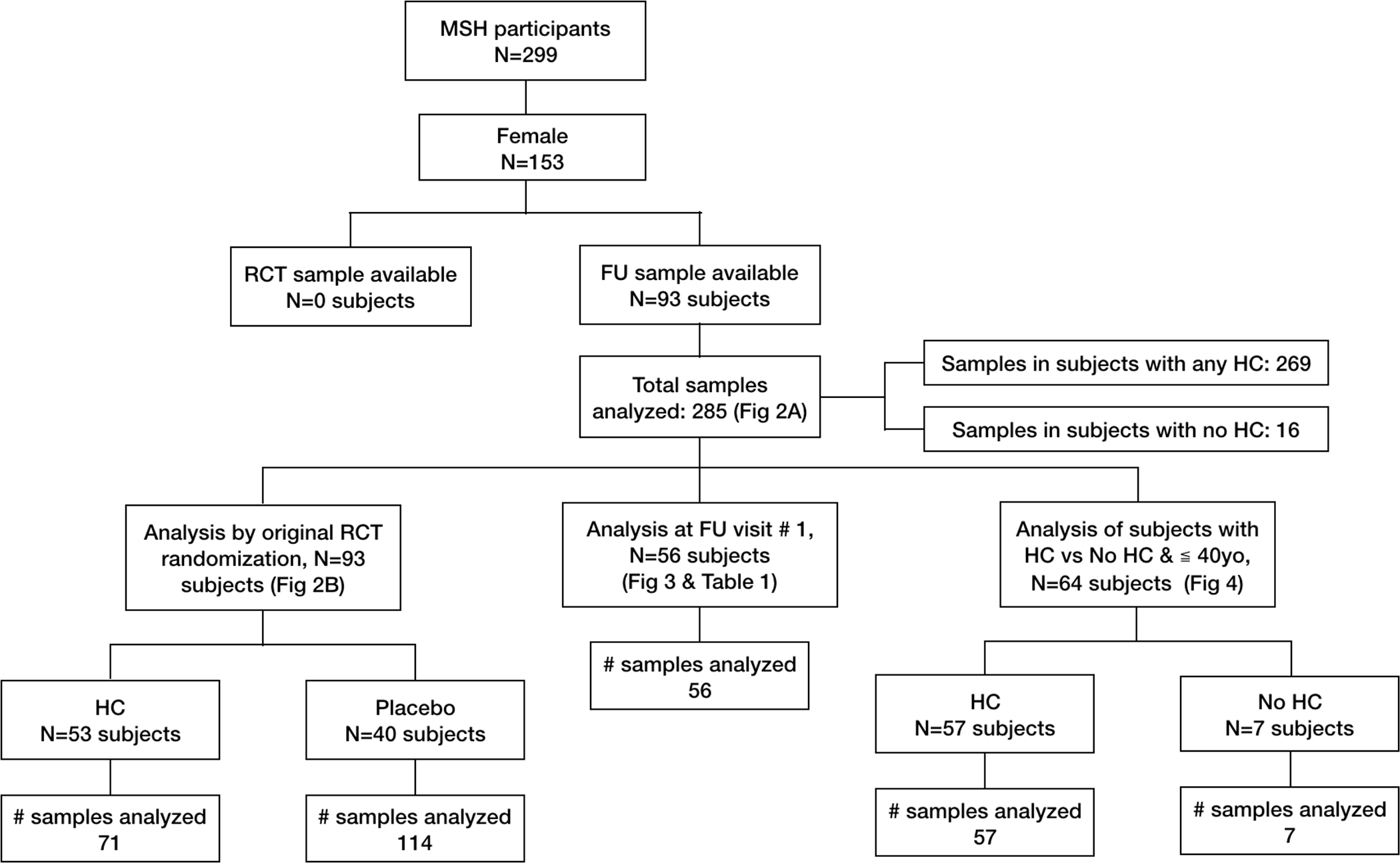
This figure outlines the study population, sample availability by hydroxycarbamide exposure, and populations and samples used in study analyses (Figures 2 – 4 and Table 1).
Results
Descriptive
Ninety-three of 153 original female MSH participants had at least one banked sample available for AMH analysis and there were 285 samples available for analysis of AMH at one or more of nine annual follow-up study visits. Figure 1 provides an overview of the study population, samples and those used in the analyses performed. According to MSH inclusion criteria, all subjects had HbSS disease and averaged 3-painful crises per year(Charache et al, 1995a). Among the 93-subjects for whom a serum sample was available for analysis: median age at enrollment in the RCT was 30 years (Inter-Quartile Range (IQR) 37) and BMI was 21.5kg/m2 (IQR 4.6). Fifty-three (57%) were randomized to receive hydroxycarbamide during the MSH RCT, and they had a median of 25.3 (IQR 27) months of hydroxycarbamide treatment. During the RCT or in the Follow-up and Extension studies, most subjects (n=86) took hydroxycarbamide at some point. Seven subjects had no hydroxycarbamide exposure up to the time of the first AMH level.
Comparison of female MSH participants with (n=93) and without (n=60) an available sample showed no difference in the number of subjects randomized to hydroxycarbamide (57% vs 40%, p=0.0592), median age at enrollment (30 vs 31.5 years, p=0.131) or BMI (21.5 vs 21.2kg/m2, p=0.153), indicating no selection bias based on clinically relevant factors. As might be expected, subjects for whom a banked serum sample was available were universally enrolled in the MSH Follow-up and Extension Studies whereas fewer than half of subjects without a banked sample available had enrolled in the MSH Follow-up studies (100% vs 43%, p<0.01).
AMH levels are low in adult women with SCD
The median AMH level by subject age at the time of the sample is shown in Figure 2A. At every age, AMH levels were lower in women with SCD than reference values for age-matched women. Median AMH levels consistent with DOR occurred in subjects starting at age 25–30 years of age; in reference norms, this occurs after 40 years of age. Figure 2B shows a trend of higher AMH levels in subjects randomized to placebo compared to hydroxycarbamide; this difference was significant at ages 30 – 35 (median AMH 1.23ng/mL vs 0.87ng/mL, p<0.001) and 40 – 46 years old (median AMH 0.045ng/mL vs 0.05ng/mL, p=.005).
Figure 2. AMH is lower than reference values for age in women with HbSS.
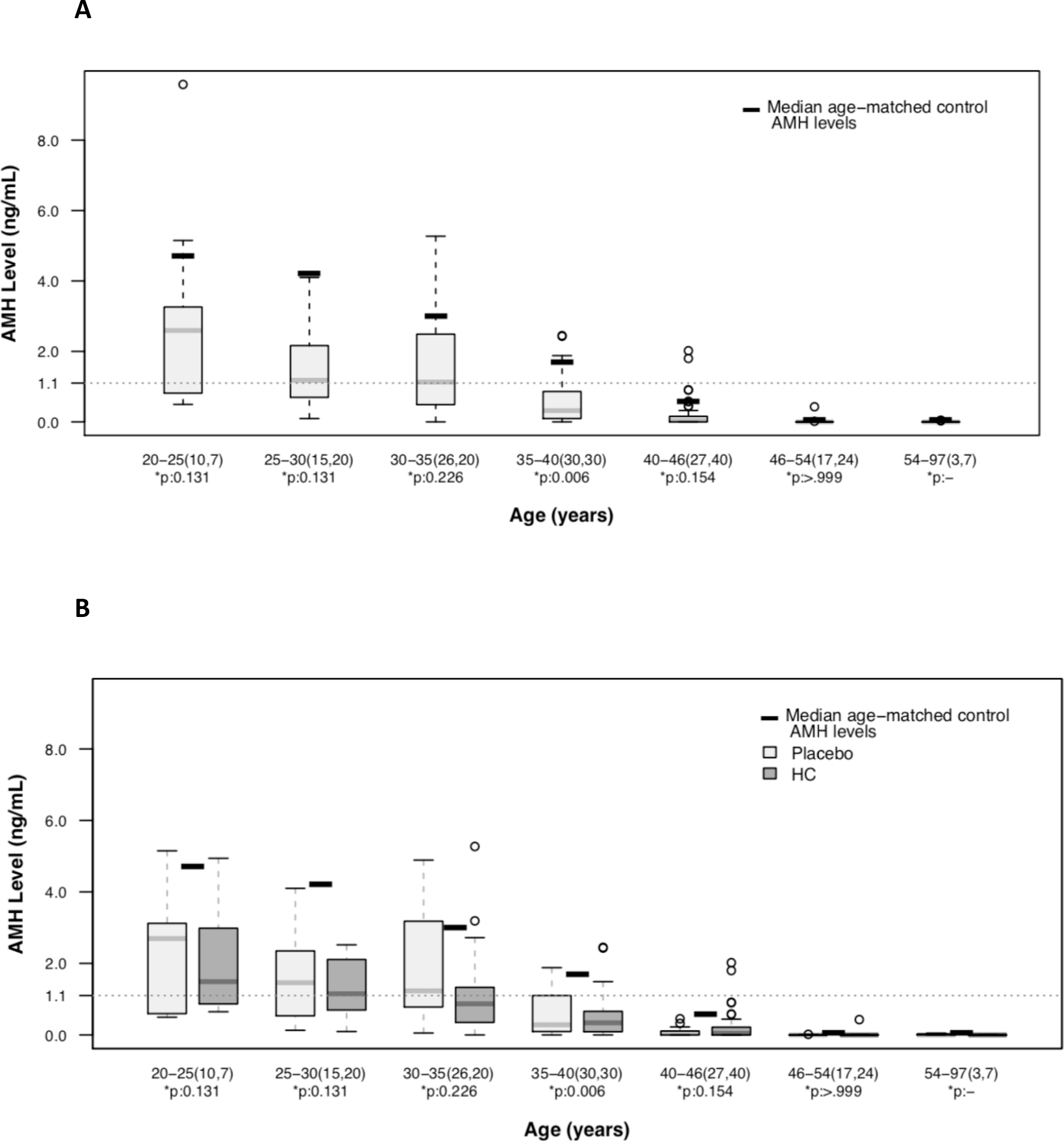
Box plots of 285 AMH levels in 93 subjects by subject age at time of specimen collection. As expected, AMH decreased in an age-dependent manner. Median AMH by age as reported for this assay is indicated by a thick black line. Median AMH for age is lower than median age-dependent values in the general population. The dotted line indicates AMH less than 1.1ng/mL, a level consistent with diminished ovarian reserve, and a risk factor for infertility. (A) Most study subjects had an AMH less than 1.1ng/mL by age 30 years. (B) Box plots of AMH level by subject age and original randomization in the MSH.
AMH levels at first follow-up visit
Fifty-six subjects (60%) had samples available for the first follow-up visit after the MSH RCT closed. The median age of this group was 35.9 (IQR 11.5) years. Among these subjects, 33 (59%) were randomized to hydroxycarbamide and 23 (41%) to placebo in the RCT, and 44 (79%) were taking hydroxycarbamide at the time of the visit. In univariate analysis (Figure 3), AMH was significantly higher in women under age 35 years, not taking hydroxycarbamide and with an MCV less than 100fL. Variables associated with DOR were analyzed with AMH as a dichotomous variable (Table 1). Variables associated with low AMH were older age, taking hydroxycarbamide at the time of the visit, and MCV.
Figure 3. Low AMH is associated with age, taking hydroxycarbamide and MCV.
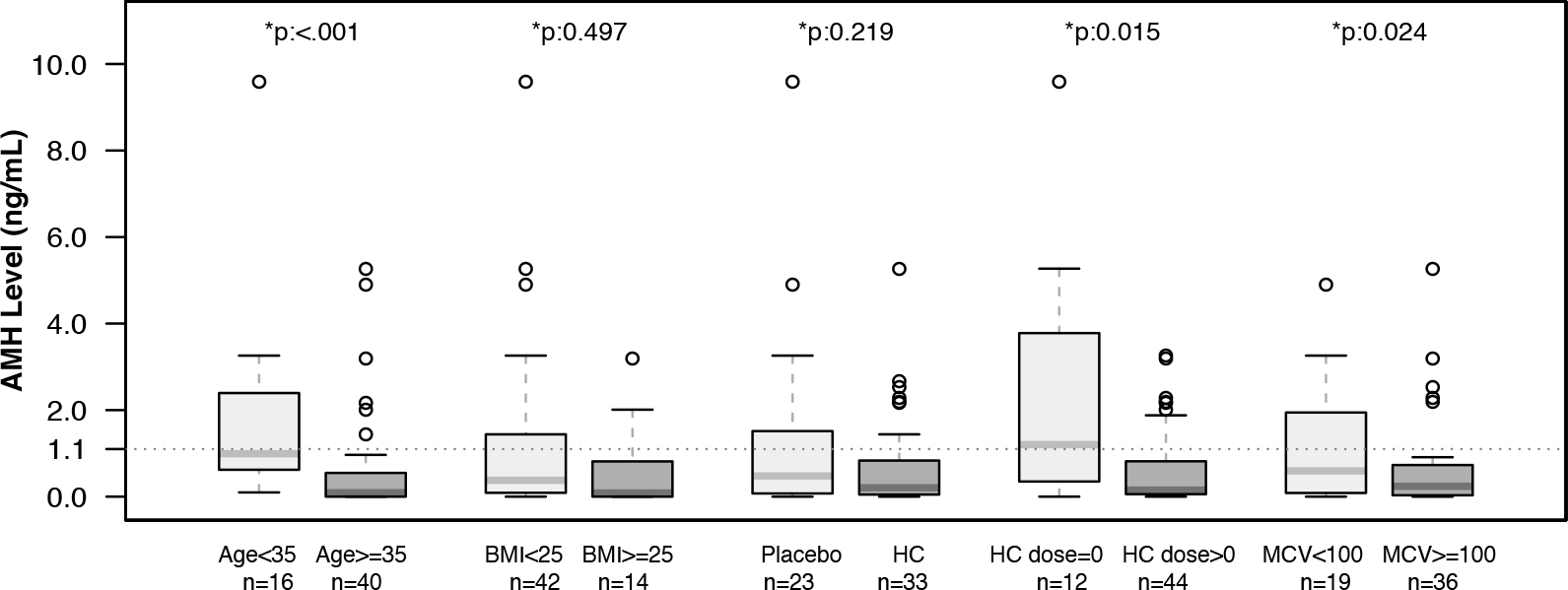
Box plots of univariate analysis of AMH in the first MSH follow-up visit. Fifty six subjects had a sample available at the first visit. Box plots compare subjects ≥/< 35 years of age, with BMI ≥/< 25, by randomization to placebo or hydroxycarbamide in the MSH RCT, hydroxycarbamide use at the time of sample, and MCV ≥/< 100fL. AMH is lower in older subjects, those taking hydroxycarbamide and those with an MCV greater than 100fL.
Table 1.
Comparison of subjects at first follow-up visit by AMH shows AMH levels consistent with diminished ovarian reserve are an associated with age, taking HC and higher MCV.
|
AMH ≥ 1.1
(n=14) |
AMH < 1.1
(n=42) |
p-value** | |
|---|---|---|---|
| Age at enrollment, years | 24.5 (8.0) | 34.5 (9.0) | <0.0001 |
| Age at first follow-up visit, years | 29.1 (7.5) | 39.3 (8.1) | <0.0001* |
| Months after MSH enrollment to follow-up visit, months median(IQR) | 29.5 (8.75) | 31(6) | 0.35 |
| BMI, kg/m2, median (IQR) | 20.1 (2.1) | 21.3 (7.3) | 0.54 |
| Received HC in RCT, n(%) | 7 (50%) | 26 (62%) | 0.43 |
| Taking HC at time of sample, n(%) | 8 (57%) | 36 (86%) | 0.024* |
| Months HC exposure during RCT, median (IQR) | 11.0 (28.0) | 23.5(33.0) | 0.44 |
| ANC, K/mcL, median (IQR) | 4.7 (2.2) | 5.6 (3.8) | 0.75 |
| MCV, fL, median (IQR) | 95.8 (11.9) | 105.3 (14.3) | 0.03* |
p-value was calculated using Wilcoxon rank-sum test for continuous variables and Pearson Chi-Square test for categorical variables
In a multi-variable linear regression analysis controlling for age, BMI and original randomization, MCV was not associated with low AMH (Est Std −.019, 95% CI −0.045 – 0.008, p=.181), but taking hydroxycarbamide at the time of the study visit was associated with a lower AMH(Est Std .001, 95% CI −0.002 – 0.000, p=.006).
AMH levels are normal in reproductive age women with HbSS and no hydroxycarbamide exposure
Very few subjects (n=7) in this study had no hydroxycarbamide exposure documented at any point before a sample was available. Figure 4 compares median AMH levels between subjects with any hydroxycarbamide exposure, no hydroxycarbamide exposure and to age- and sex-matched reference values. The small number of subjects makes statistical comparison difficult, however in the four subjects under age 36 years without hydroxycarbamide exposure, AMH was comparable to reference values.
Figure 4. AMH is normal in young women without hydroxycarbamide exposure.
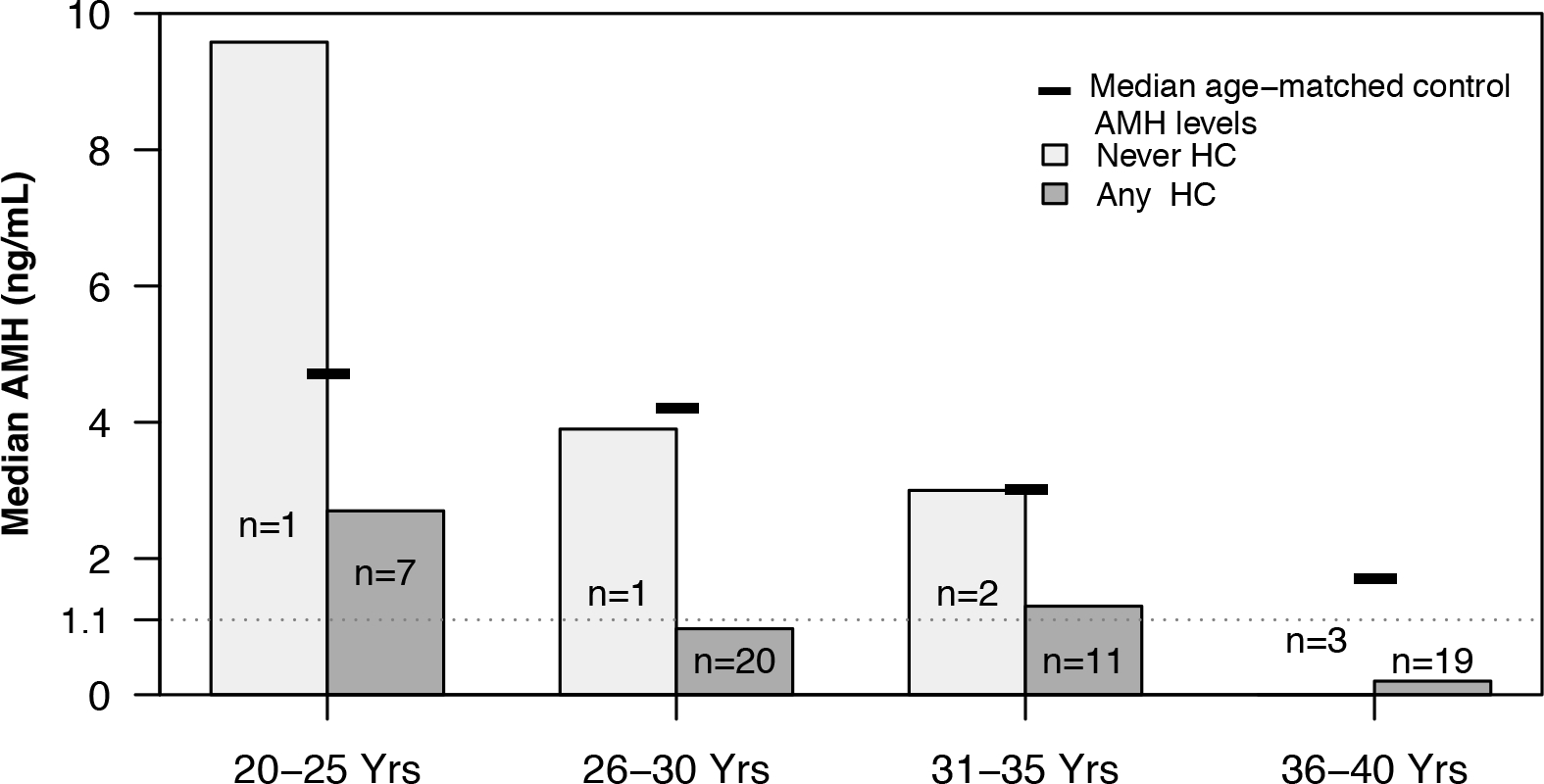
Bar graphs show an age-stratified comparison of subjects never exposed to hydroxycarbamide and those exposed to hydroxycarbamide when ≦ 40 years of age. Only one sample per subject is included and the included sample for each subject was their earliest AMH level. AMH levels in subjects 35 years of age or less with no hydroxycarbamide exposure is normal for age while those with any hydroxycarbamide exposure are low. The few hydroxycarbamide unexposed subjects preclude statistical comparison.
Discussion
In adult women with HbSS disease in the MSH, almost all of whom were exposed to hydroxycarbamide, expected age-associated decline in AMH occurred, but levels were lower than median levels in age- and sex-matched reference values. AMH is an established measure of the ovarian oocyte pool. Normal values cover a wide range, and extremely low levels are a risk factor for infertility. In this cohort, median AMH levels consistent with DOR occurred between 25 and 30 years of age, well before the expected age of 40 more more years. The American College of Obstetrics and Gynecology recently called for further study of AMH in women with risks of premature ovarian aging or infertility(ACOG Committee Opinion No. 773: The Use of Antimüllerian Hormone in Women Not Seeking Fertility Care., 2019). The preponderance of AMH levels consistent with DOR in women with HbSS of reproductive age in this study suggests that women with HbSS and hydroxycarbamide exposure need further study.
Our findings add to two previous studies of ovarian reserve in SCD. Kopeika et al also found that AMH is lower in women with SCD and that low AMH occurred at an earlier age in women with SCD compared to controls (Kopeika et al, 2019). However Kopeika et al included women with compound heterozygous SCD, few subjects (n=8) took hydroxycarbamide., and found that most subjects ages 25 – 30 years had normal AMH. In contrast, we find AMH levels consistent with DOR starting at 25 – 30 years. This difference might be explained by the exclsuive analysis of women with HbSS in our cohort and/or that most subjects in our study had hydroxycarbamide exposure. While it is plausible that both severe forms of SCD and hydroxycarbamide treatment may accelerate reductions in AMH, we found normal AMH in the few subjects aged 20 – 35 years without hydroxycarbamide exposure. A previous study of AMH in adolecent girls with HbSS and HbSβ0 similarly found normal AMH in subjects without hydroxycarbamide exposure and DOR in some hydroxycarbamide exposed subjects(Elchuri et al, 2015). The presence of normal AMH in two, small hydroxycarbamide naiive populations with sickle cell anaemia suggests that SCD itself may not affect ovarian reserve in young women of reproductive age. Further clarification of the effects of hydroxycarbamide on ovarian reserve is especially important for the emerging generation of young adults who started hydroxycarbamide in infancy or early childhood(Thornburg et al, 2012; Hankins et al, 2014), for girls and women treated in sub-Saharan Africa where both fixed dose and maximally tolerated dose treatment strategies are under investigation(Akingbola et al, 2019), and in women pursuing fertility preservation interventions before HSCT or gene therapy(Pecker et al, 2018; Carpinello et al, 2018).
Many patients, caregivers and prescribers are concerned about whether hydroxycarbamide affects female fertility. Our results, especially given their harmony with previous publications, may conservatively inform the care of women with HbSS. Existing recommendations for initiating infertility evaluations depends on age: if over age 35-years, work-up is indicated after failure to conceive for 6 or more months of unprotected intercourse, if under age 35-years of age evaluation is indicated after at least 12 of unprotected intercourse. These cut-offs reflect the more rapid age-associated decline in ovarian reserve in healthy women after age 35 years(Practice Committee of the American Society for Reproductive Medicine. Electronic address: ASRM@asrm.org, 2020). Since DOR is seen in our study population at age 25 – 30 years, standard indications for infertility evaluations may not apply to women with HbSS. Given the intense interest in biological reproduction captured in studies of the parents of children with SCD and young adults with SCD(Oyeku et al, 2013; Chakrabarti & Bareford, 2007; Nahata et al, 2017), counseling about the potential for a reduced reproductive lifespan, at least in women with HbSS treated with hydroxycarbamide, may be indicated. Further studies will inform approaches to treatment, fertility preservation or how hydroxycarbamide treatment compares to gonadotoxic HSCT regimens.
This study has several strengths. Subjects enrolled in the MSH represent a homogenous population of adult women only with HbSS disease. This is the largest study of AMH levels in girls or women with SCD to date and included mostly hydroxycarbamide-exposed subjects. This study addresses a significant challenge in studying ovarian reserve in women with SCD: differentiating the possible effect of hydroxyucarbamide from those of SCD complications on ovarian reserve. This is further confounded by the higher likelihood that adults with severe SCD at baseline will take hydroxycarbamide. In our study, the study population met disease severity inclusion criteria to participate in the MSH(Charache et al, 1995b). If disease complications rather than hydroxycarbamide treatment accounted for diminishment in ovarian reserve, we would expect lower AMH levels in placebo-treated subjects who had more disease complications in the MSH(Charache et al, 1995a). This study shows the opposite: placebo-treated subjects had higher AMH levels thus implicating hydroxycarbamide rather than disease complications in the finding of low AMH levels.
This study is limited because the primary outcome of the MSH was unrelated to AMH and the study was not designed to determine whether SCD or hydroxycarbamide were associated with AMH levels. Hydroxycarbamide exposure data is, as noted, difficult to parse in the MSH Extension and Follow-Up Studies(Ballas et al, 2009), furthermore nearly the whole population was hydroxycarbamide-exposed. There are limited definitive conclusions that can be drawn about hydroxycarbamide’s effect on ovarian reserve. This study offers no information about the effect of hydroxycarbamide on AMH when started in infancy or before puberty, about ovarian reserve in women with compound heterozygous forms of SCD, and there are no historic race and age matched controls available for comparison. Finally, while AMH is an established marker of ovarian reserve, it does not predict spontaneous pregnancy in women without a diagnosis of infertility(Steiner et al, 2017).
The findings from this historic cohort suggest that HbSS and hydroxycarbamide therapy are risk factors for early ovarian aging and a reduced reproductive lifespan. Hydroxycarbamide remains a fundamental and life changing SCD therapy. Prospective studies are needed to determine whether hydroxyurea use is associated with poor oocyte quantity or quality during fertility preservation procedures, to definitively determine the effect of hydroxycarbamide on ovarian reserve and to establish whether this leads to infertility. Such studies will enhance the informed consent process for hydroxycarbamide initiation and may lead to new care standards to shape family planning guidance, specialist referrals to reproductive endocrinology and indications for fertility preservation for affected girls and women.
Acknowledgements:
This work was supported by National Institutes of Health National Heart, Lung and Blood Institute grant R21HL144973-02 (L.H.P., N.Z., S.H., M.S.C., S.L.). The authors acknowledge Ni Zhao, Hyunwok Koh at the Johns Hopkins School of Public Health, Baltimore, MD and Morgan Herle and Dave Golec at Esoterix Labs, Calabassas, CA.
Abbreviations
- AMH
Antimullerian Hormone
- BMI
Body Mass Index
- DOR
Diminished Ovarian Reserve
- HbSβ0
Haemoglobin Sβ0
- HbSS
Haemoglobin SS
- HC
Hydroxycarbamide
- HSCT
Haematopoietic Stem Cell Transplant
- IQR
Inter-Quartile Range
- MSH
Multicenter Study of Hydroxyurea
- NHLBI
National Heart, Lung, Blood Institute
- POI
Primary ovarian insufficiency
- RCT
Randomized Controlled Trial
- SCD
Sickle Cell Disease
Footnotes
Conflict of Interest: The authors declare no competing financial interests
References
- ACOG Committee Opinion No. 773: The Use of Antimüllerian Hormone in Women Not Seeking Fertility Care. (2019) ACOG Committee Opinion No. 773: The Use of Antimüllerian Hormone in Women Not Seeking Fertility Care. Obstetrics and Gynecology, 133, e274–e278. [DOI] [PubMed] [Google Scholar]
- Agarwal A, Gupta S & Sharma RK (2005) Role of oxidative stress in female reproduction. Reproductive biology and endocrinology : RB&E, 3, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akingbola TS, Tayo BO, Ezekekwu CA, Sonubi O, Zhang X, Saraf SL, Molokie R, Hsu LL, Han J, Cooper RS & Gordeuk VR (2019) “Maximum tolerated dose” vs ‘fixed low-dose’ hydroxyurea for treatment of adults with sickle cell anemia. American journal of hematology, 94, E112–E115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RA & Wallace WHB (2013) Antimüllerian hormone, the assessment of the ovarian reserve, and the reproductive outcome of the young patient with cancer. Fertility and sterility, 99, 1469–1475. [DOI] [PubMed] [Google Scholar]
- Ballas SK, McCarthy WF, Guo N, DeCastro L, Bellevue R, Barton BA, Waclawiw MA Multicenter Study of Hydroxyurea in Sickle Cell Anemia (2009) Exposure to hydroxyurea and pregnancy outcomes in patients with sickle cell anemia. Journal of the National Medical Association, 101, 1046–1051. [DOI] [PubMed] [Google Scholar]
- Bedoschi G, Navarro PA & Oktay K (2016) Chemotherapy-induced damage to ovary: mechanisms and clinical impact. Future Oncology, 12, 2333–2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthaut I, Bachir D, Kotti S, Chalas C, Stankovic K, Eustache F, Ravel C, Habibi A, Brailly-Tabard S, Lévy-Dutel L, Bleibtreu A, Simon T, Galacteros F, Lionnet F & Mandelbaum J (2017) Adverse effect of hydroxyurea on spermatogenesis in patients with sickle cell anemia after six months of treatment. Blood, blood–2017–03–771857. [DOI] [PubMed] [Google Scholar]
- Carpinello OJ, Kosturakis AK, Hsieh MM, Fitzhugh CD, Tisdale JF, Wolff E, Plowden TC, Hill MJ & DeCherney AH (2018) Post- transplant ovarian function in women with sickle cell disease. Fertility and sterility, 110, e315–e316. [Google Scholar]
- Chakrabarti S & Bareford D (2007) A survey on patient perception of reduced-intensity transplantation in adults with sickle cell disease. Bone marrow transplantation, 39, 447–451. [DOI] [PubMed] [Google Scholar]
- Chang H-H, Chen M-J, Lu M-Y, Chern JPS, Lu C-Y, Yang Y-L, Jou S-T, Lin D-T, Yang Y-S & Lin K-H (2011) Iron overload is associated with low anti-müllerian hormone in women with transfusion-dependent β-thalassaemia. BJOG : an international journal of obstetrics and gynaecology, 118, 825–831. [DOI] [PubMed] [Google Scholar]
- Charache S, Terrin ML, Moore RD, Dover GJ, Barton FB, Eckert SV, McMahon RP & Bonds DR (1995a) Effect of hydroxyurea on the frequency of painful crises in sickle cell anemia. Investigators of the Multicenter Study of Hydroxyurea in Sickle Cell Anemia. New England Journal of Medicine, 332, 1317–1322. [DOI] [PubMed] [Google Scholar]
- Charache S, Terrin ML, Moore RD, Dover GJ, McMahon RP, Barton FB, Waclawiw M & Eckert SV (1995b) Design of the multicenter study of hydroxyurea in sickle cell anemia. Investigators of the Multicenter Study of Hydroxyurea. Controlled clinical trials, 16, 432–446. [DOI] [PubMed] [Google Scholar]
- Chase AR, Howard J & Oteng-Ntim E (2009) Ovarian sickling as a proposed mechanism for premature ovarian failure necessitating ovum donation. Menopause International, 15, 70–71. [DOI] [PubMed] [Google Scholar]
- Elchuri SV, Patterson BC, Brown M, Bedient C, Record E, Wasilewski-Masker K, Mertens AC & Meacham LR (2016) Low Anti-Müllerian Hormone in Pediatric Cancer Survivors in the Early Years after Gonadotoxic Therapy. Journal of pediatric and adolescent gynecology, 29, 393–399. [DOI] [PubMed] [Google Scholar]
- Elchuri SV, Williamson RS, Clark Brown R, Haight AE, Spencer JB, Buchanan I, Hassen-Schilling L, Brown MR, Mertens AC & Meacham LR (2015) The effects of hydroxyurea and bone marrow transplant on Anti-Müllerian hormone (AMH) levels in females with sickle cell anemia. Blood Cells, Molecules, and Diseases, 55, 56–61. [DOI] [PubMed] [Google Scholar]
- Fung EB, Harmatz PR, Lee PDK, Milet M, Bellevue R, Jeng MR, Kalinyak KA, Hudes M, Bhatia S, Vichinsky EPMulti-Centre Study of Iron Overload Research Group (2006) Increased prevalence of iron-overload associated endocrinopathy in thalassaemia versus sickle-cell disease. British Journal of Haematology, 135, 574–582. [DOI] [PubMed] [Google Scholar]
- Giffen CA, Wagner EL, Adams JT, Hitchcock DM, Welniak LA, Brennan SP & Carroll LE (2017) Providing researchers with online access to NHLBI biospecimen collections: The results of the first six years of the NHLBI BioLINCC program. PLOS ONE, 12, e0178141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankins JS, Aygun B, Nottage K, Thornburg C, Smeltzer MP, Ware RE & Wang WC (2014) From infancy to adolescence: fifteen years of continuous treatment with hydroxyurea in sickle cell anemia. Medicine, 93, e215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helden JV & Weiskirchen R (2017) Age-independent anti-Müllerian hormone (AMH) standard deviation scores to estimate ovarian function. European journal of obstetrics, gynecology, and reproductive biology, 213, 64–70. [DOI] [PubMed] [Google Scholar]
- Kopeika J, Oyewo A, Punnialingam S, Reddy N, Khalaf Y, Howard J, Mononen S & Oteng-Ntim E (2019) Ovarian reserve in women with sickle cell disease. PLOS ONE, 14, e0213024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebensburger JD, Aban I, Pernell B, Kasztan M, Feig DI, Hilliard LM & Askenazi DJ (2019) Hyperfiltration during early childhood precedes albuminuria in pediatric sickle cell nephropathy. American journal of hematology, 94, 417–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HWR, Lee VCY, Lau EYL, Yeung WSB, Ho PC & Ng EHY (2014) Ovarian response and cumulative live birth rate of women undergoing in-vitro fertilisation who had discordant anti-Mullerian hormone and antral follicle count measurements: a retrospective study. PLOS ONE, 9, e108493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahata L, Caltabellotta NM, Ball K, O’Brien SH & Creary SE (2017) Desire for parenthood and reproductive health knowledge in adolescents and young adults with sickle cell disease and their caregivers. Pediatric Blood \& Cancer, 179, e26829. [DOI] [PubMed] [Google Scholar]
- Niss O, Fleck R, Makue F, Alsaied T, Desai P, Towbin JA, Malik P, Taylor MD & Quinn CT (2017) Association between diffuse myocardial fibrosis and diastolic dysfunction in sickle cell anemia. Blood, blood–2017–02–767624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyeku SO, Driscoll MC, Cohen HW, Trachtman R, Pashankar F, Mullen C, Giardina PJ, Velazco N, Racine AD & Green NS (2013) Parental and other factors associated with hydroxyurea use for pediatric sickle cell disease. Pediatric Blood \& Cancer, 60, 653–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastore LM, Christianson MS, Stelling J, Kearns WG & Segars JH (2018) Reproductive ovarian testing and the alphabet soup of diagnoses: DOR, POI, POF, POR, and FOR. Journal of assisted reproduction and genetics, 35, 17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecker LH, Maher JY, Law JY, Beach MC, Lanzkron S & Christianson MS (2018) Risks associated with fertility preservation for women with sickle cell anemia. Fertility and sterility, 110, 720–731. [DOI] [PubMed] [Google Scholar]
- Peigné M & Decanter C (2014) Serum AMH level as a marker of acute and long-term effects of chemotherapy on the ovarian follicular content: a systematic review. Reproductive biology and endocrinology : RB&E, 12, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perdrix A, Saint-Ghislain M, Degremont M, David M, Khaznadar Z, Loeb A, Leheurteur M, Di Fiore F & Clatot F (2017) Influence of adjuvant chemotherapy on anti-Müllerian hormone in women below 35 years treated for early breast cancer. Reproductive biomedicine online. [DOI] [PubMed] [Google Scholar]
- Practice Committee of the American Society for Reproductive Medicine. Electronic address: ASRM@asrm.org (2020) Definitions of infertility and recurrent pregnancy loss: a committee opinion. Fertility and sterility, 113, 533–535. [DOI] [PubMed] [Google Scholar]
- Sampson M, Archibong AE, Powell A, Strange B, Roberson S, Hills ER & Bourne P (2010) Perturbation of the developmental potential of preimplantation mouse embryos by hydroxyurea. International journal of environmental research and public health, 7, 2033–2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soleimani R, Heytens E & Oktay K (2011) Enhancement of neoangiogenesis and follicle survival by sphingosine-1-phosphate in human ovarian tissue xenotransplants. PLOS ONE, 6, e19475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner AZ, Pritchard D, Stanczyk FZ, Kesner JS, Meadows JW, Herring AH & Baird DD (2017) Association Between Biomarkers of Ovarian Reserve and Infertility Among Older Women of Reproductive Age. JAMA : the journal of the American Medical Association, 318, 1367–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su H-CI, Haunschild C, Chung K, Komrokian S, Boles S, Sammel MD & DeMichele A (2014) Prechemotherapy antimullerian hormone, age, and body size predict timing of return of ovarian function in young breast cancer patients. Cancer, 120, 3691–3698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Team, R.S. (2019)RStudio: integrated development for R.[internet]. Boston, MA: RStudio, Inc.; 2015. [Google Scholar]
- Thompson AL, Bridley A, Twohy E, Dioguardi J, Sande J, Hsu LL, Kamani N & Meier ER (2013) An educational symposium for patients with sickle cell disease and their families: results from surveys of knowledge and factors influencing decisions about hematopoietic stem cell transplant. Pediatric Blood \& Cancer, 60, 1946–1951. [DOI] [PubMed] [Google Scholar]
- Thornburg CD, Files BA, Luo Z, Miller ST, Kalpatthi R, Iyer R, Seaman P, Lebensburger J, Alvarez O, Thompson B, Ware RE, Wang WCBABY HUG investigators (2012) Impact of hydroxyurea on clinical events in the BABY HUG trial. Blood, 120, 4304–10– quiz 4448 Available at: http://www.scopus.com/inward/record.url?eid=2-s2.0-84869803950&partnerID=40&md5=bb0201ae1e495d7756d67c13f678d7b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vichinsky EP, Neumayr LD, Gold JI, Weiner MW, Rule RR, Truran D, Kasten J, Eggleston B, Kesler K, McMahon L, Orringer EP, Harrington T, Kalinyak K, De Castro LM, Kutlar A, Rutherford CJ, Johnson C, Bessman JD, Jordan LB, Armstrong FD, et al. (2010) Neuropsychological dysfunction and neuroimaging abnormalities in neurologically intact adults with sickle cell anemia. JAMA : the journal of the American Medical Association, 303, 1823–1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser JA, Schipper I, Laven JSE & Themmen APN (2012) Anti-Müllerian hormone: an ovarian reserve marker in primary ovarian insufficiency. Nature reviews. Endocrinology, 8, 331–341. [DOI] [PubMed] [Google Scholar]


