Abstract
Following a request from the European Commission, the EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA) was asked to deliver a scientific opinion on the tolerable upper intake level (UL) for vitamin B6. Systematic reviews of the literature were conducted by a contractor. The relationship between excess vitamin B6 intakes and the development of peripheral neuropathy is well established and is the critical effect on which the UL is based. A lowest‐observed‐effect‐level (LOAEL) could not be established based on human data. A reference point (RP) of 50 mg/day is identified by the Panel from a case–control study, supported by data from case reports and vigilance data. An uncertainty factor (UF) of 4 is applied to the RP to account for the inverse relationship between dose and time to onset of symptoms and the limited data available. The latter covers uncertainties as to the level of intake that would represent a LOAEL. This leads to a UL of 12.5 mg/day. From a subchronic study in Beagle dogs, a LOAEL of 50 mg/kg body weight (bw) per day can be identified. Using an UF of 300, and a default bw of 70 kg, a UL of 11.7 mg/day can be calculated. From the midpoint of the range of these two ULs and rounding down, a UL of 12 mg/day is established by the Panel for vitamin B6 for adults (including pregnant and lactating women). ULs for infants and children are derived from the UL for adults using allometric scaling: 2.2–2.5 mg/day (4–11 months), 3.2–4.5 mg/day (1–6 years), 6.1–10.7 mg/day (7–17 years). Based on available intake data, EU populations are unlikely to exceed ULs, except for regular users of food supplements containing high doses of vitamin B6.
Keywords: tolerable upper intake level, UL, vitamin B6, pyridoxine, pyridoxal, pyridoxamine, dietary reference value
1. Introduction
1.1. Background as provided by the European Commission
Article 6 of Regulation (EC) No 1925/2006 on the addition of vitamins and minerals and of certain other substances to foods and Article 5 of Directive 2002/46/EC on the approximation of the laws of the Member States relating to food supplements provide that maximum amounts of vitamins and minerals added to foods and to food supplements respectively, shall be set.
The above‐mentioned provisions lay down the criteria to be taken into account when establishing these maximum amounts that include the upper safe levels (ULs) of vitamins and minerals established by scientific risk assessment based on “generally accepted scientific data, taking into account, as appropriate, the varying degrees of sensitivity of different groups of consumers”.
To set maximum amounts of vitamins and minerals in fortified foods and food supplements, the Commission would like to ask the European Food Safety Authority (EFSA) to review the previous opinions of the Scientific Committee on Food (SCF) or the NDA Panel on the ULs for vitamin A, 1 folic acid/folate, 1 vitamin D, 1 vitamin E, 1 vitamin B6, 1 iron, 1 manganese 1 and β‐carotene 1 to take into account recent scientific developments and evidence.
In this context, EFSA should first review the guidelines of the SCF 1 for the development of Tolerable Upper Intake Levels for vitamins and minerals (adopted on 19 October 2000).
Tolerable Upper Intake Levels should be presented separately for the age group from 4/6 months onwards until 3 years of age and the general population group from 3 years onwards, taking into account, as appropriate, the varying degrees of sensitivity of different consumer groups. As foods intended for the general population are also consumed by young children, young children should be considered as a potentially sensitive consumer group.
1.2. Terms of Reference as provided by the European Commission
In accordance with Article 29(1)(a) of Regulation (EC) No 178/2002, the European Commission requests the European Food Safety Authority to:
Update the guidelines of the SCF for the development of Tolerable Upper Intake Levels for vitamins and minerals in the light of available recent scientific and methodological developments.
- Review existing scientific evidence and provide advice on Tolerable Upper Intake Levels for the following vitamins and minerals including their currently authorised forms for the addition to fortified foods and food supplements for the general population and, as appropriate, for vulnerable subgroups of the population:
- vitamin A
- folic acid/folate
- vitamin D
- vitamin E
- iron
- manganese
- β‐carotene
- vitamin B6
For nutrients for which there are no, or insufficient, data on which to base the establishment of an UL, an indication should be given on the highest level of intake where there is reasonable confidence in data on the absence of adverse effects.
1.3. Interpretation of the Terms of Reference
According to the mandate, EFSA has first reviewed the guidelines of the SCF for the development of tolerable upper intake levels (ULs) for vitamins and minerals (SCF, 2000). A draft guidance has been endorsed by the NDA Panel and published for a 1‐year pilot phase (EFSA NDA Panel, 2022), after which it will be revised and complemented as necessary, following a public consultation.
The Panel interprets that the UL for vitamin B6 should be revised according to the principles laid down in the above‐mentioned guidance, following a protocol developed for that purpose (Annex A) and covers all sources of vitamin B6 authorised for addition to foods and food supplements in the EU.
1.4. Context of the assessment
The SCF in (2000) set a UL of 25 mg/day for vitamin B6 in adults, including pregnant and lactating women, based on evidence of neurotoxicity from animal and human studies. Most of the available evidence in humans came from case‐reports, indicating the occurrence of clinical neuropathy at exposure levels between 100 and 6,000 mg/day vitamin B6 and at variable latency periods (1–72 months) (Schaumburg et al., 1983; Berger and Schaumburg, 1984; Parry and Bredesen, 1985; Friedman et al., 1986; Waterston and Gilligan, 1987). The first case series of vitamin B6‐related sensory neuropathy in humans was described by Schaumburg et al. (1983) in seven patients who had taken 2–6 g vitamin B6 daily mainly women who had taken vitamin B6 for the treatment of premenstrual symptoms (PMS) for 2–40 months. Four of these patients developed neuropathy and were unable to walk. Due to lack of sufficient data related to true incidence of neuropathy, the UL was calculated using data from the case–control study by Dalton and Dalton (1987). This study investigated women attending a clinic for the treatment of PMS and taking < 50–500 mg/day (exact supplemental intakes < 50 mg/day not reported) supplemental vitamin B6 for around 6 months and up to 5 years. The UL was calculated using the average daily vitamin B6 intakes, i.e. 100 mg, to which an uncertainty factor (UF) of 2 was applied to account for the fact that there was an inverse relationship between dose and time to development of peripheral neuropathy, and an additional UF of 2 to account for the limited available data. For children and adolescents, the SCF (2000)) extrapolated the UL from adults on a body weight basis, using reference weights derived by the SCF (1993). For infants, there was insufficient data (lack of case reports or adequate animal developmental neurotoxicity data) on neurological effects to establish an UL in these developmental stages. ULs set by the SCF (2000) are summarised in Table 1 and cover vitamin B6 intakes from all food sources, including food supplements.
Table 1.
Overview of existing Tolerable Upper Intake Levels (ULs) for vitamin B6, in mg/day
| Population group | SCF (2000) | IOM (1998) | EVM (2003) | WHO/FAO (2004) | NHMRC (2006) |
|---|---|---|---|---|---|
| Infants | |||||
| 0–6 months | nd | nd | nd | nd | nd |
| 7–12 months | nd | nd | nd | nd | nd |
| Children and adolescents | |||||
| 1–3 years | 5 (b) | 30 (a) | nd | nd | 15 (a) |
| 4–6 years | 7 (b) | nd | nd | ||
| 4–8 years | 40 (a) | nd | nd | 20 (a) | |
| 7–10 years | 10 (b) | nd | nd | ||
| 9–13 years | 60 (a) | nd | nd | 30 (a) | |
| 11–14 years | 15 (b) | nd | nd | ||
| 14–18 years | 80 (a) , (c) | nd | nd | 40 (a) , (c) | |
| 15–17 years | 20 (b) | nd | nd | ||
| Adults | |||||
| ≥ 18 years | 25 (c) | 10 (d) | 100 | ||
| ≥ 19 years | 100 (c) | 50 (c) | |||
nd: not defined; EVM: UK Expert Group on Vitamins and Minerals; IOM: Institute of Medicine; NHMRC: National Health and Medical Research Council; Australia and New Zealand; SCF: Scientific Committee on Food; WHO/FAO: World Health Organization/Food and Agriculture Organization.
Extrapolated from the UL for adults on a body size basis and growth considerations (for NHMRC only).
Extrapolated from the UL for adults on a body weight basis.
Including pregnant and lactating women.
0.17 mg/kg bw per day supplemental pyridoxine equivalent to 10 mg/day for a 60‐kg adult.
In 2016, the NDA Panel published a scientific opinion on dietary reference values (DRVs) for vitamin B6 (EFSA NDA Panel, 2016). As per the terms of reference for this task, a review of the UL for vitamin B6 was out of the scope of the assessment and the NDA Panel focused on providing advice on the requirement of the micronutrient. The Panel based the derivation of the average requirement (AR) and population reference intake (PRI) on the vitamin B6 intake required to maintain the mean concentration of plasma pyridoxal 5′‐phosphate (PLP) above 30 nmol/L. Six small intervention studies in young women, supported by larger cross‐sectional studies showing an age‐related decline in plasma PLP concentrations in older adults, informed the AR for all women, and this was set at 1.3 mg/day. For pregnant (AR 1.5 mg/day) and lactating (AR 1.4 mg/day) women, the AR was increased to account for the uptake of vitamin B6 by the fetal or maternal tissues during pregnancy and the losses of vitamin B6 in breast milk. Due to lack of reliable data to determine the dietary requirement of vitamin B6 in men, the Panel derived the AR for men (1.5 mg/day) by applying allometric scaling to the AR for all women, considering the differences in reference body weights. For children and adolescents, the ARs for vitamin B6 were extrapolated from the AR for adults by allometric scaling and application of a growth factor calculated as the proportional increase in protein requirement for growth relative to the maintenance requirement. The ARs for children ranged from 0.5 mg/day, for the youngest children, to 1.5 mg/day for adolescent boys. In the absence of information on the variability in the requirement, a coefficient of variation of 10% was used to calculate PRIs from the ARs for all age groups in children and in adults. The PRIs for children were 0.6 mg/day (1–3 years), 0.7 mg/day (4–6 years), 1 mg/day (7–10 years), 1.4 mg/day (11–14 years), 1.6 mg/day (15–17 years); and for adults the PRIs were 1.6 mg/day (≥ 18 years), 1.8 mg/day (pregnant women) and 1.7 mg/day (lactating women). For infants (7–11 months old), the Panel proposed an adequate intake (AI) of 0.3 mg/day, combining data from (i) upwards extrapolation from the estimated intake of vitamin B6 of exclusively breastfed infants up to 6 months of age and (ii) downwards extrapolation from the ARs for adults by using isometric scaling and application of a growth factor calculated as the proportional increase in protein requirement for growth relative to the maintenance requirement. The Panel stated that it was unnecessary to determine sex‐specific DRVs for infants and children up to 14 years of age.
1.5. Previous assessments by other bodies
The large heterogeneity in the ULs for vitamin B6 derived by different scientific bodies is mainly the result of having used different studies as the basis for deriving the UL (Table 1).
While the SCF (2000) had based the UL on the case–control study by Dalton and Dalton (1987), the US Institute of Medicine (IOM, 1998), followed by the WHO/FAO (2004), decided not to use this study because they considered it as not being sufficiently reliable in terms of administered vitamin B6 doses. They based the no‐observed‐adverse‐effect‐level (NOAEL) of 100 mg/day for adults on the publications by Bernstein and Lobitz (1988) and Del Tredici et al. (1985), which described studies on patients with diabetic neuropathy or carpal tunnel syndrome (CTS) in whom neuropathy did not get worse following vitamin B6 intakes of 100–300 mg/day. No UF was applied. The SCF (2000) had noted some discrepancies in reporting in relation to the study described by Bernstein and Lobitz (1988) in terms of number of patients involved and study duration. It also questioned the relevance of the patient population used and the appropriateness of the study duration. This is also addressed in Annex B in the report on the outcome of the public consultation.
Based on the studies described by Bernstein and Lobitz (1988) and Del Tredici et al. (1985), the Australian and New Zealand National Health and Medical Research Council (NHMRC, 2006) identified a NOAEL of 200 mg/day. Given that vitamin B6 exposure in these studies was 5–6 months or less and as it was assumed that symptoms may take longer to appear, an UF of 4 was used and a UL of 50 mg/day was derived.
The UK Expert Group on Vitamins and Minerals (EVM, 2003) derived the UL based on a study in Beagle dogs (Phillips et al., 1978) from which a lowest‐observed‐adverse‐effect‐level (LOAEL) of 50 mg/kg body weight (bw) per day was identified, to which a UF of 300 was applied.
2. Data and methodologies
For this scientific assessment, a protocol (Annex A) has been developed in line with EFSA's existing methodology (EFSA, 2020).
2.1. Problem formulation
In accordance with the draft NDA Panel guidance on establishing and applying ULs for vitamins and essential minerals (EFSA NDA Panel, 2022), the assessment questions underlying the UL evaluation are formulated as follows:
What is the maximum level of total chronic daily intake of vitamin B6 (from all sources) that is not expected to pose a risk of adverse health effects to humans? (Hazard identification and hazard characterisation)
What is the daily intake of vitamin B6 from all dietary sources in European Union (EU) populations? (Intake assessment)
What is the risk of adverse effects related to the intake of vitamin B6 in EU populations, including attendant uncertainties? (Risk characterisation)
The hazard identification and hazard characterisation relate to the identification of adverse health effects of a given nutrient and the qualitative and quantitative evaluation of the adverse health effects associated with the nutrient, including dose–response assessment and derivation of a UL, if possible.
Adverse (health) effects are defined as ‘a change in the morphology, physiology, growth, development, reproduction or life span of an organism, system or (sub)population that results in an impairment of functional capacity to compensate for additional stress or an increase in susceptibility to other influences (FAO/WHO, 2009; EFSA Scientific Committee, 2017a). The observable effects of high nutrient intake within the causal pathway of an adverse health effect can range from biochemical changes without functional significance (e.g. certain changes in enzyme activity) to irreversible clinical outcomes. Notably, some changes that occur before clinical manifestations could be used as surrogate or predictive markers of subsequent adverse health effects, i.e. biomarkers of effect’ (EFSA NDA Panel, 2022).
Priority adverse health effects were identified in consultation with a panel of qualified experts on vitamin B6 and after discussion by the Working Group on ULs and the NDA Panel, taking into account previous assessments from other scientific bodies (IOM, 1998; SCF, 2000; EVM, 2003).
Priority adverse health effects are those which are expected to play a critical role for establishing a UL. As a result of the problem formulation, the overarching risk assessment questions were further specified into assessment subquestions (sQs) and the methods to address each sQ were selected ( Table 2 ).
Table 2.
Assessment subquestions
| Subquestion | Method | |
|---|---|---|
| sQ1 |
|
Narrative review |
| sQ2 |
|
Narrative review |
| sQ3 |
|
Systematic review Narrative review |
| sQ4 | Is there a positive and causal relationship between vitamin B6 intake and developmental toxicity (including congenital defects) in humans and animals? | Systematic review |
| sQ5 | What other adverse effects have been reported to be associated with high intake of vitamin B6? | Narrative review |
| sQ6 |
|
Collection of data based on existing EFSA intake estimates and complementary searches in relevant databases and inquiries to competent authorities of European countries |
4‐PA, 4‐pyridoxic acid; ADME, absorption, distribution, metabolism and excretion; PL, pyridoxal; PLP, pyridoxal 5′‐phosphate; PN, pyridoxine; sQ, subquestion.
Intakes above the population reference intake.
2.2. Hazard identification and characterisation (sQ1 to sQ5)
2.2.1. Data
A description of the processes applied for evidence retrieval, study selection and data extraction is provided below. These steps were conducted by a contractor and were undertaken by the University of Copenhagen in collaboration with the University of Oslo and the Karolinska Institutet and are described in the final report of this outsourcing project (Tetens et al., 2023).
2.2.1.1. Priority adverse health effects (sQ3a, sQ3b and sQ4)
To address sQ3a, sQ3b and sQ4, relevant human studies (and animal studies for sQ4 only) on the selected adverse health effects were identified by the University of Copenhagen as contractor through systematic searches of the literature in MEDLINE (Ovid), Embase (Ovid) and Cochrane Central Register of Controlled Trials articles published in English. No date limit was applied. The search strategy was created by information specialists of the University of Oslo and peer reviewed by information specialists at the Karolinska Institutet and EFSA. It is further detailed in the final report of the outsourcing project (Tetens et al., 2023). Grey literature (i.e. literature not indexed in literature databases) was not searched.
Retrieved articles were screened in duplicate in Distiller SR® at title and abstract level, also with the use of the artificial intelligence tool of Distiller SR®, and at full text level for inclusion/exclusion according to the criteria defined in the protocol (Annex A). Conflicts were solved by a third reviewer, if necessary. Relevant systematic reviews, if available, were hand‐searched for additional pertinent studies. Reviews, expert opinions, editorials, letters to the editors, abstracts, posters and theses not reporting on original data were excluded.
Eligible designs: All experimental and observational study designs in humans (including case reports) were considered relevant.
Eligible study populations: Studies were eligible if they involved individuals of any age, either healthy individuals or diseased individuals if the disease was considered not to be related to the exposure‐outcome relationship. Studies in individuals with impaired vitamin B6 status were not eligible for sQ3a and sQ3b. For sQ4 (developmental toxicity), studies on the development of neural tube defects and oral clefts were excluded from further assessment, as insufficient rather than excessive vitamin B6 intakes are proposed to be related to these outcomes through involvement of vitamin B6 as a cofactor of enzymes involved in one‐carbon metabolism (Li et al., 2016), and no study had been retrieved in the search that had linked high vitamin B6 intakes to the development of neural tube defects and oral clefts.
Eligible exposure measurements: Studies were eligible if they measured vitamin B6 intake (either self‐reported or recorded) or used biomarkers of exposure, i.e. plasma concentrations of pyridoxine (PN), pyridoxal (PL) and PLP and urinary excretion of 4‐pyridoxic acid (4‐PA) (Section 3.3).
In relation to sQ3a and sQ3b, 3,793 unique references were identified after removing duplicates (see flow chart, Appendix A). The title and abstract screening left 69 relevant articles that underwent a full‐text review. Of those, 37 were excluded. The reasons for exclusion are reported in the final report of the outsourcing project (Tetens et al., 2023). A total of 32 publications reporting on 1 human controlled trial (HCT) and 30 observational studies were included.
In relation to sQ4, 4,941 unique references were identified after removing duplicates (see flow chart, Appendix A). The title and abstract screening left 82 relevant articles that underwent a full‐text review. Of those, 59 were excluded. The reasons for exclusion are reported in the final report of the outsourcing project (Tetens et al., 2023). An additional reference was excluded during the assessment step owing to implausible biomarker concentrations (see Section 3.5.2.1). A total of 22 publications reporting on 2 HCTs and 20 observational studies were included.
Data were extracted into Distiller SR® by two extractors of the University of Copenhagen. They were jointly discussed, compared and harmonised at several time points by the two extractors. Evidence tables were prepared in Microsoft Word® and are provided in Appendix D.
2.2.1.2. Other background information (sQ1, sQ2, sQ3c, sQ3d and sQ5)
The evidence used to inform sQ1, sQ2, sQ3c, sQ3d and sQ5 was retrieved through non‐systematic searches and was synthesised as narrative reviews.
2.2.2. Methodologies
The methodology for this assessment follows the methodology laid down by the SCF (2000) for deriving ULs for nutrients, the principles established by the EFSA NDA Panel (2022), EFSA's guidance on the application of the systematic review methodology in food and feed safety assessments (EFSA, 2010), its principles and processes for dealing with data and evidence in scientific assessments (EFSA, 2015), the guidance on statistical significance and biological relevance (EFSA Scientific Committee, 2011), the guidance on the assessment of the biological relevance of data in scientific assessments (EFSA Scientific Committee, 2017a), the guidance on the use of the weight of evidence approach in scientific assessments (EFSA Scientific Committee, 2017b) and the draft guidance on appraising and integrating evidence from epidemiological studies for use in EFSA's scientific assessments (EFSA Scientific Committee, 2020).
2.2.2.1. Evidence appraisal (sQ3a and sQ3b)
A risk of bias (RoB) appraisal, i.e. evaluation of the internal validity of studies, was applied to eligible studies that addressed sQ3a and sQ3b.
The appraisal was performed using the Office of Health Assessment and Translation (OHAT) RoB tool developed by the US National Toxicology Program (NTP) (OHAT‐NTP, 2015). The RoB criteria and rating instructions provided therein were tailored to the specific research questions, for the questions addressing: (1) consideration of potential confounders, (2) confidence in the exposure characterisation and (3) confidence in the outcome assessment (Appendix B).
The appraisal was performed in duplicate by independent experts of the University of Copenhagen. Discrepancies in the assessment in relation to the RoB judgement of each domain were discussed among the assessors. In case of disagreement, a third reviewer was involved.
The OHAT RoB tool proposes five response options for each RoB question: definitely low RoB (++), probably low RoB (+), not reported (NR), probably high RoB (−), definitely high RoB (−−).
Studies were categorised according to their overall RoB based on a three‐tier system (i.e. at low (tier 1), moderate (tier 2) or high (tier 3) RoB), according to the strategy proposed by OHAT (OHAT‐NTP, 2019) (Appendix B).
2.2.2.2. Evidence synthesis (sQ3a, sQ3b and sQ4)
Owing to the heterogeneity of studies retrieved, the evidence was synthesised narratively, and no data analyses were conducted.
2.2.2.3. Evidence integration and uncertainty analysis (sQ3a, sQ3b and sQ4)
Hazard identification
Regarding sQ3a and sQ3b, a causal relationship between ‘high’ vitamin B6 intake and peripheral neuropathy is well‐established. The assessment focused on the characterisation of the dose–response and no uncertainty analysis was carried out for these sQs.
Regarding sQ4, the hazard identification step consisted of assessing the evidence for a causal positive relationship between vitamin B6 intake and developmental toxicity. As the available body of evidence (BoE) did not suggest a positive relationship (i.e. the relationship appears to be negative or null), the evidence could not be used to inform the setting of the UL for vitamin B6. Therefore, no formal evidence integration and uncertainty analysis was carried out for this sQ.
Hazard characterisation
At this step, evidence is integrated to select the critical effect(s) and identify a reference point (RP) for establishing the UL. As proposed in the draft guidance for establishing and applying ULs for vitamins and essential minerals (EFSA NDA Panel, 2022), when available data are not suitable for dose–response modelling, a NOAEL or a LOAEL can be identified and used as the RP and this was applied in view of the available evidence. To derive the UL, a UF is applied to the RP to account for the uncertainties associated with extrapolating from the observed data to the general population. ULs should be protective for all members of the general population within a specific age and gender category, including sensitive individuals, throughout their lifetime. The rationale for the selection of the RP and UF are documented in the scientific opinion.
2.3. Dietary intake assessment (sQ6)
The assessment follows the approach outlined in the protocol for the intake assessments performed in the context of the revision of ULs for selected nutrients (EFSA, 2022). It is briefly summarised below.
2.3.1. Data
Vitamin B6 intakes for all population groups from foods, excluding food supplements, had previously been estimated in the context of the scientific opinion on DRVs for vitamin B6 (Roe et al., 2013; EFSA NDA Panel, 2016). Food intake data from the EFSA Comprehensive European Food Consumption Database (hereinafter referred as Comprehensive Database) 2 and data on vitamin B6 content in foods from the EFSA food composition database (FCDB) 3 were used. Given that the EFSA FCDB has not been updated since then and the number of national surveys that were newly integrated in the Comprehensive Database is limited, the intake estimates published in 2016 are still considered adequate for the purpose of the present assessment and were not updated, except for the addition of data for infants aged < 1 year (Section 2.3.2).
Regarding the use of vitamin B6 containing supplements, data in the Comprehensive Database suffer from important limitations, in particular due to partial reporting in the database of the nutrient(s) contained in food supplements. In view of the uncertainties associated with these data, the Panel relied on information available at national level.
To complement EFSA's intake assessment from 2016, vitamin B6 intake estimates from natural sources, from addition to foods and from food supplements based on nationally representative food consumption surveys and total diet studies (TDSs) published after 2016 were collected. Data on vitamin B6 intakes from fortified foods and/or food supplements published before 2016 were also considered as the contribution of those sources was not addressed in EFSA's previous assessment. Data were collected between September and November 2021 by contacting 64 competent authorities in 37 European countries through EFSA Focal Points 4 and the EFSA Food Consumption Network. 5 An additional search in sources of bibliographic information (Google Scholar, PubMed) was performed to collect reports of national surveys included in the Comprehensive Database that had not been obtained through the competent authorities. Between August and October 2022, EFSA contacted all EU Member States and Norway through the European Commission Working Group on Food supplements and Fortified foods 6 and collected data on the intake of vitamin B6 specifically from food supplements.
The Mintel Global New Products Database (GNPD) 7 was used as a data source to identify the type of vitamin B6 containing food supplements and fortified foods available on the EU market. The search was limited to the past 5 years, from September 2017 to September 2022.
2.3.2. Methodologies
EFSA's intake estimates were calculated by matching the food intake data from the Comprehensive Database and the data on vitamin B6 content in foods from the EFSA FCDB as available in 2016 (EFSA NDA Panel, 2016) (Section 3.4.2). Data on intake estimates for infants (≥ 4 to < 12 months), which were not in the remit of the DRV opinion from 2016, have been added to the present assessment. The methodology applied to estimate intakes in this population group is the same as for the other age groups.
Vitamin B6 intake data from recent national food consumption surveys, including specific estimates of vitamin B6 intake from food supplements and/or fortified foods, were extracted.
Information on food products fortified with vitamin B6 and vitamin B6‐containing supplements available on the EU market, and their vitamin B6 content as reported on the label, were extracted from the Mintel GNPD. These data were used qualitatively to describe the types of fortified foods and food supplements available and to gain insight into their potential contribution to total vitamin B6 intake.
2.4. Public consultation
In line with EFSA's policy on openness and transparency, and for EFSA to receive comments from the scientific community and stakeholders, the draft Scientific Opinion was released for public consultation from 13 January 2023 to 10 February 2023. The outcome of the public consultation is described in a technical report published as Annex D to this Scientific Opinion.
3. Assessment
3.1. Chemistry of vitamin B6
The term vitamin B6 is the generic descriptor for all 3‐hydroxy‐2‐methylpyridine derivatives exhibiting biological pyridoxine activity. Even though pyridoxine is sometimes used as synonym to vitamin B6, vitamin B6 is the recommended term to be used (IUPAC–IUB CBN, 1973). The term vitamin B6 covers three vitamers that differ by the one‐carbon substitution at the fourth position of the pyridine ring, i.e. the alcohol PN, the aldehyde PL and the amine pyridoxamine (PM) as well as their phosphate esters, i.e. pyridoxine 5′‐phosphate (PNP), pyridoxal 5′‐phosphate (PLP) and pyridoxamine 5′‐phosphate (PMP), and pyridoxine glucoside (PN‐5′‐β‐d‐glucoside (PNG)) (Figure 1) (EFSA NDA Panel, 2016).
Figure 1.
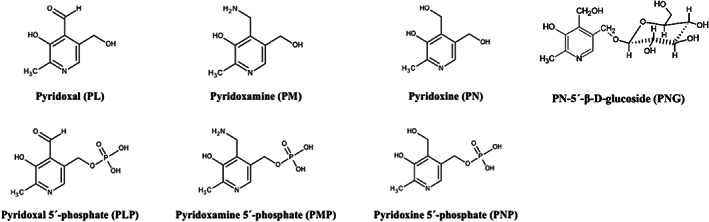
Structure of vitamin B6 vitamers (EFSA NDA Panel, 2016)
3.2. Absorption, distribution, metabolism and excretion (ADME)
Bioaccessibility of vitamin B6, i.e. the fraction that becomes available in the gastrointestinal tract for absorption, can in principle be almost complete from many animal‐based foods. However, thermal processing reduces the availability by 25–30% because of the reaction of PL and PLP with lysine to form PL‐ε‐lysine (Reynolds, 1988). Bioaccessibility of vitamin B6 vitamers present in plant‐based foods may differ depending on the vitamer. An in vitro study investigating the bioaccessibility of vitamin B6 vitamers from cereal‐based baby food found that the bioaccessibility in the intestinal phase of PN was 67–76%, depending on the gastric pH, while for PL and PM it was 38–53% and 36–50%, respectively (Yaman and Mizrak, 2019).
PL, PN and PM are absorbed without modification while the phosphorylated forms of the vitamers need to be dephosphorylated by alkaline phosphatase first (EFSA NDA Panel, 2016). Also, PNG, a vitamer found in fruits, vegetables, in particular crucifers and cereal‐grains and constituting 5–80% of their vitamin B6 content, requires hydrolysis before intestinal absorption, even though some intact PNG can also be absorbed and excreted unmetabolised in urine (Reynolds, 1988). The enzymatic hydrolysis of PNG at the intestinal epithelial brush‐border occurs with the involvement of pyridoxine‐5′‐β‐D‐glucoside hydrolase (Nakano et al., 1997) and lactase‐phlorizin hydrolase (Mackey et al., 2004). The hydrolysis is the rate limiting step in the absorption of this vitamer (Nakano et al., 1997; Mackey et al., 2004). Absorption takes place in the jejunum via diffusion (EFSA NDA Panel, 2016). In vitro studies have suggested that vitamin B6 absorption may also be carrier‐mediated by thiamine multi‐specific membrane transporters (Said et al., 2003; Said, 2011; EFSA NDA Panel, 2016; Yamashiro et al., 2020; Miyake et al., 2022). Evidence from in vitro studies suggests that vitamin B6 synthesised by the intestinal microbiota can be absorbed in the colon, but the amount absorbed in vivo in humans is unknown (Said, 2011; EFSA NDA Panel, 2016).
The absorption of PN from supplements in the form of PN hydrochloride (PN‐HCl), the most widespread form of vitamin B6 in food supplements, is almost complete with 95% absorption (EFSA NDA Panel, 2016). In a study comparing the bioavailability of PN‐HCl (4 mg/day) with equimolar doses of PM dihydrochloride monohydrate and PL‐HCl in humans (Wozenski et al., 1980), similar bioavailability of the three supplemental forms was reported, based on the assessment of urinary 4‐pyridoxic acid (PA) excretion and plasma total vitamin B6 and PLP concentrations. The bioavailability of PNG has been found to be around 50% lower than that of PN‐HCl (Gregory III et al., 1991; Nakano et al., 1997). However, as PNG contributes only about 15% to total vitamin B6 intakes, the reduced bioavailability of vitamin B6 from this vitamer is of little practical relevance (EFSA NDA Panel, 2016).
Overall, vitamin B6 absorption from mixed diets has been estimated to be around 75%. This estimation is based on a study using plasma PLP concentration and urinary vitamin B6 concentration as biomarkers and PN‐HCl as a reference (Tarr et al., 1981; EFSA NDA Panel, 2016), meaning that 1 mg vitamin B6 from food is equivalent to 0.8 mg PN‐HCl from food supplements when taking into account the lower absorption of vitamin B6 from mixed diets (EFSA NDA Panel, 2016).
Following uptake in the enterocytes, the dephosphorylated vitamers can become re‐phosphorylated by pyridoxal kinase (i.e. metabolic trapping) or be released into the portal vein by passive diffusion. In order for the metabolically trapped vitamers to be released into the portal vein, they need to be dephosphorylated again (EFSA NDA Panel, 2016).
In the liver, the vitamers are re‐phosphorylated in the hepatocytes by pyridoxal kinase. PNP and PMP are subsequently oxidised with the involvement of pyridoxine and pyridoxamine 5′‐phosphate oxidases to PLP, which is the principal active vitamer (Hadtstein and Vrolijk, 2021; Liu et al., 2022).
Before secretion into the circulatory system from hepatocytes, PLP is bound to lysine residues of proteins, mostly albumin, which is the main transport protein for PLP (Hadtstein and Vrolijk, 2021; Berger et al., 2022; Liu et al., 2022). PLP constitutes 70–90% of total vitamin B6 in plasma at normal intakes, with PL and 4‐PA being the other major vitamers (EFSA NDA Panel, 2016). At high vitamin B6 intakes, PL becomes the predominant vitamer in plasma, owing to the fact that only a limited amount of PLP can be bound to protein and unbound PLP will be dephosphorylated by alkaline phosphatase to PL (Vrolijk et al., 2020). At high PN intakes, both pyridoxal kinase and pyridoxine 5′‐phosphate oxidase become saturated which impedes the conversion of PN to PNP and to PLP. Therefore, PN that has been shown to be cytotoxic in experiments on cultured neuronal cells and that is usually not present in plasma, starts to appear in circulation, and long‐term supplementation may lead to the accumulation of this vitamer (Vrolijk et al., 2017; Vrolijk et al., 2020). Free PN may also be detected in some individuals after PLP supplementation, possibly owing to the presence of proteins with pyridoxal reductase activity in those individuals (Ramos et al., 2019; Vrolijk et al., 2020).
When reaching the target tissue, PLP disassociates from protein and is dephosphorylated by specific or non‐specific alkaline phosphatase. After entering cells, it is re‐phosphorylated by pyridoxal kinase (EFSA NDA Panel, 2016; Liu et al., 2022). The uptake of vitamin B6 after dephosphorylation is suggested to occur via a saturable process, exemplified by studies in liver and pancreatic cells (EFSA NDA Panel, 2016; Srinivasan et al., 2019). The intracellular concentrations of PLP are under tight regulation (Hadtstein and Vrolijk, 2021). Studies in humans have delineated the function of a newly discovered PLP‐binding protein, which binds cellular PLP, acting as a regulator of PLP homeostasis intracellularly (Darin et al., 2016; Plecko et al., 2017; Johnstone et al., 2019). Both pyridoxal reductase and PLP‐binding protein are suggested to prevent adverse reactions of the aldehyde moiety of PL/PLP with non‐specific cellular amino acids and amines (Johnstone et al., 2019; Ramos et al., 2019).
The main route for elimination of vitamin B6 is the urine, with 4‐PA being the main urinary metabolite, while some forms with vitamin B6 activity can also be found in urine (EFSA NDA Panel, 2016). 4‐PA is produced in the liver through the oxidation of PL by non‐specific aldehyde oxidases. It has been suggested that a non‐specific nicotinamide adenine dinucleotide (NAD)‐dependent aldehyde dehydrogenase is also involved (Liu et al., 2022).
The Panel notes that there are large inter‐individual differences in metabolism of vitamin B6 that have been described, especially in case of supplemental PN‐HCl intake (Vrolijk et al., 2020).
3.3. Biomarkers of intake
In its scientific opinion on DRVs for vitamin B6, the Panel had concluded that plasma PLP concentrations are a reliable marker of vitamin B6 intake and status as they correlate well with vitamin B6 intakes through habitual diets (EFSA NDA Panel, 2016). These may reach up to 5 mg/day in European populations based on data from the EFSA Comprehensive Database and the EFSA FCDB (Section 3.4.1). Plasma PLP concentrations of 30 nmol/L as a population mean are indicative of an adequate vitamin B6 status (EFSA NDA Panel, 2016).
Urinary 4‐PA excretion was considered a marker of short‐term intake (i.e. 5–7 days) but not of vitamin B6 status (EFSA NDA Panel, 2016). One study (Sharma et al., 2020) suggested that women excreted more 4‐PA/mmol creatinine than men.
Concentration of total vitamin B6 in plasma, the concentration of PL and PMP in plasma or red blood cells (RBCs), the concentration of PLP in RBCs, as well as ratios of concentrations of vitamin B6 vitamers in plasma, were deemed not to be suitable biomarkers of vitamin B6 intake and/or status at normal levels of intake (EFSA NDA Panel, 2016).
However, it has been suggested that plasma concentrations of PN, PL and 4‐PA might be useful markers of high vitamin B6 exposure (van Hunsel et al., 2018).
With respect to plasma PLP concentrations at high vitamin B6 intakes, it has been observed that they do not increase linearly anymore but start to level off, as reviewed by Hadtstein and Vrolijk (2021). The study by Edwards et al. (1990) tested doses of 10, 25, 100, 200, 400 and 800 mg/day vitamin B6 taken as PN‐HCl for 1 week (design not described) and found no substantial differences in the achieved plasma PLP concentrations (around 600 nmol/L (blood drawn 4 h after ingestion of the final dose) compared with around 70 nmol/L without supplementation). Other vitamers started to increase (PL and 4‐PA) or appear (PN and PNP) at a dose of 100 mg/day. Also, two unidentified metabolites appeared in plasma at intakes of 100 and 200 mg/day, respectively, with concentrations increasing at higher doses. Other reviewed studies (Speitling et al., 1990; Bor et al., 2003; Vrolijk et al., 2020) showed plasma PLP concentrations of 300–400 nmol/L at doses of 40, 50 and 300 mg/day taken for 12, 1 and 2 weeks, respectively. In a kinetic study in which 200 mg PM was administered once to five healthy male volunteers (van den Eynde et al., 2021), PLP concentrations increased up to a mean (± standard error; SE) of 2,787 ± 329 nmol/L within 10 h of supplementation and then remained stable (last measurement 25 h after administration). When the dose was divided into three doses consumed in 1 day, plasma PLP concentrations continued to rise to 3,282 ± 281 nmol/L, which was reached after 15 h (last intake at 12 h) and then levelled off. PN, PL, PM and PMP, peaked after around 5, 5, 3 and 3 h of the single dose, respectively. When using repeated doses, concentrations of the four vitamers declined after each peak associated with intake, but mostly not to baseline levels. In particular, PL and to a lesser extent PN seemed to accumulate, even though PN concentrations had returned to zero after 25 h (13 h after last intake).
Plasma PLP concentrations decline with age, possibly owing to changing metabolism with age (EFSA NDA Panel, 2016; Sharma et al., 2020), during pregnancy, to a greater extent than can be explained by the expanding blood volume, and in inflammatory conditions (EFSA NDA Panel, 2016). They are also influenced by albumin concentrations, alcohol consumption and alkaline phosphatase activity (Ueland et al., 2015). A reduction in albumin and an increase in alkaline phosphatase can explain the decreased plasma PLP concentrations observed in inflammatory states (Ueland et al., 2017). Alkaline phosphatase activity is also influenced by single nucleotide polymorphisms (SNPs) in the tissue non‐specific alkaline phosphatase gene, with lower alkaline phosphatase expression being associated with higher plasma PLP concentrations (Carter et al., 2015; Ueland et al., 2015; Loohuis et al., 2018). Reduced activities because of SNPs are also known for pyridoxine and pyridoxamine 5′‐phosphate oxidases, converting PNP or PMP to PLP (Alghamdi et al., 2021; Plecko and Mills, 2022). Similarly, variants have also been identified for pyridoxal kinase (Ghatge et al., 2021) and PLP‐binding protein (Plecko et al., 2017; Johnstone et al., 2019; Heath et al., 2021). The contribution of vitamin B6‐producing colonic bacteria to plasma PLP concentrations is unknown.
3.4. Intake assessment
3.4.1. Sources of dietary vitamin B6
Dietary vitamin B6 intake from natural sources occurs primarily in the forms of PLP and PNP (Stover and Field, 2015). The main forms of vitamin B6 in animal tissues are PLP and PMP, whereas plant‐based foods mainly contain PN, PNP (IOM, 1998) and PNG (Stover and Field, 2015). Plant‐derived foods rich in vitamin B6 include grains (whole grain corn/maize, brown rice, sorghum, quinoa, wheat germ, buckwheat, barley, rye), pulses, nuts, seeds, white potatoes and other starchy vegetables (plantain, cassava, yam and taro), non‐citrus fruits (e.g. banana, avocado, apricot, peach, pear, berries, watermelon), vegetables (e.g. artichoke, asparagus, peas, green beans, beets, cabbage, cauliflower, broccoli, eggplant, mushrooms, onions, garlic) and herbs and spices such as basil, curry and ginger (IOM, 1998; EFSA NDA Panel, 2016). Animal‐derived foods rich in vitamin B6 include poultry (chicken and turkey), beef, organ meats (particularly beef liver), egg yolks and fish (particularly fresh tuna, salmon and trout) (IOM, 1998; Stover and Field, 2015; EFSA NDA Panel, 2016).
The content of total vitamin B6 in natural sources (unprocessed) from plants in mg per 100 g of grains is 0.13–0.77, of dried pulses is 0.06–0.58, of nuts is 0.10–0.54, of seeds is 0.09–0.96, of starchy vegetables is 0.08–0.45, of non‐citrus fruits is 0.12–0.42 and of vegetables is 0.05–0.48 (EFSA, online). Some plant sources with relatively higher contents included: garlic (0.85), fresh herbs (0.16–0.30), curry powder (0.86) and white ginger (0.84) (EFSA, online). The content of vitamin B6 from natural animal sources in mg per 100 g of egg yolks is~0.28, of fish and seafood is 0.08–0.88, of chicken and poultry is 0.51–0.53, of beef is 0.21–0.36, and of organ meat is 0.13–0.88 with liver having the highest content of 0.76–0.88 mg/100 g (EFSA, online).
Fortified foods
In the EU, authorised forms of vitamin B6 for addition to foods are PN‐HCl, PNP and pyridoxine dipalmitate. 8 The vitamin B6 content of infant and follow‐on formulae and of processed cereal‐based foods and baby foods for infants and children is regulated. 9
The main source of total vitamin B6 from fortified foods are fortified breakfast cereals (Anses, online; EFSA NDA Panel, 2016).
In the Mintel GNPD 10 a total of 7,333 packaged food products available in 24 EU Member States and Norway were identified as containing added vitamin B6 in the ingredients list. The majority of the products belong to the Mintel categories ‘sports and energy drinks’ (17%), ‘breakfast cereals’ (16%) ‘nutritional drinks and other beverages’ (16%) and ‘baby food’ (14%).
Data on vitamin B6 content per serving (as recommended by the manufacturer) based on labelled information were available for 31% of the products (n = 2,272). Among those, this information was available for 60% of ‘breakfast cereals’ (n = 687): range 0.06–3.2 mg vitamin B6 per serving (median = 0.36 mg vitamin B6), 41% of ‘nutritional drinks and other beverages’ (n = 490): range 0.1–17 mg vitamin B6 per serving (median = 0.7 mg vitamin B6) and 24% of ‘sports and energy drinks’ (n = 309): range 0.2–11.6 mg vitamin B6 per serving (median = 1.4 mg vitamin B6). The highest contents per serving were found in some ‘nutritional drinks’ (14–17 mg/serving) intended to be used as meal replacements and a hot beverage intended to relieve symptoms of PMS (65 mg/serving).
Food supplements
In the EU, authorised forms of vitamin B6 for use in food supplements are PN‐HCl, PNP and PLP. 11
In the Mintel GNPD, the category ‘vitamins and dietary supplements’, was searched. It yielded a total of 2,210 products available in 24 EU Member States and Norway. The labelled recommended dose per serving 12 ranged from 0.02 up to 90 mg of vitamin B6 (n = 2,145), with an average of 3 mg per dose (median 1.4 mg per dose) (Figure 2). Half of the food supplements contained between 1.01 and 2.0 mg per dose, within which lie the PRIs for adult women (1.6 mg/day) and men (1.7 mg/day). About 1.4% (n = 29) of the retrieved supplements contained a labelled dose per serving of more than 20 mg.
Figure 2.

Distribution of vitamin B6‐containing food supplements available in EU Member States and Norway as displayed on labels (mg/serving) Source: Mintel GNPD. Search for vitamin B6‐containing supplements available in the EU market in the last 5 years (from September 2017 to September 2022). A total of 2,210 products available in 25 EU Member States and Norway were identified, of which 2,145 contained complete data on mg/serving.
3.4.2. EFSA's intake assessment
Vitamin B6 intakes from food sources (excluding food supplements) in European populations were calculated in the context of the scientific opinion on DRVs for vitamin B6, based on the data from the EFSA Comprehensive Database and the EFSA FCDB (EFSA NDA Panel, 2016). Food consumption surveys of Finland, France, Germany, Ireland, Italy, Latvia, the Netherlands, Sweden were used for the assessment. The period of data collections covered by the surveys ranged between 2000 and 2012. Further information on the characteristics and methods used for the data collection in the respective surveys are provided in Annex B.
Food composition data from Finland, Germany, Italy, the Netherlands were used to calculate vitamin B6 intake in these countries. For nutrient intake estimates of Ireland and Latvia, food composition data from the UK and Germany, respectively, were used, because no specific composition data from these countries were available. The percentage of vitamin B6 values in the five composition databases that were borrowed from other composition databases varied as follows: Germany 100%, Italy 91%, the UK 68%, Finland 58% and the Netherlands 50%.
The intake assessment of 2016 did not distinguish between vitamin B6 ‘naturally present’ or ‘added’ to foods by manufacturers. However, data on the consumption of foods fortified with vitamin B6 available in the Comprehensive Database 13 and on the concentration of vitamin B6 in fortified foods available in the EFSA FCDB database are scarce. Survey participants are frequently not aware that they are consuming a fortified food, hence some of the eating occasions in the Comprehensive Database likely correspond to fortified foods. Similarly, 10‐fold differences among the vitamin B6 levels were found in the EFSA FCDB for similar foods, likely due to fortification (EFSA NDA Panel, 2016). Thus, EFSA's intake estimates reflect vitamin B6 intake from natural sources and fortified products although it is not possible to calculate their contribution separately.
The distributions of intake estimated by EFSA are presented below by age group, sex and country of origin (Figures 3 and 4). A summary overview, providing the ranges of means and 95th percentiles (P95) across EU surveys is given in Table 3 .
Figure 3.
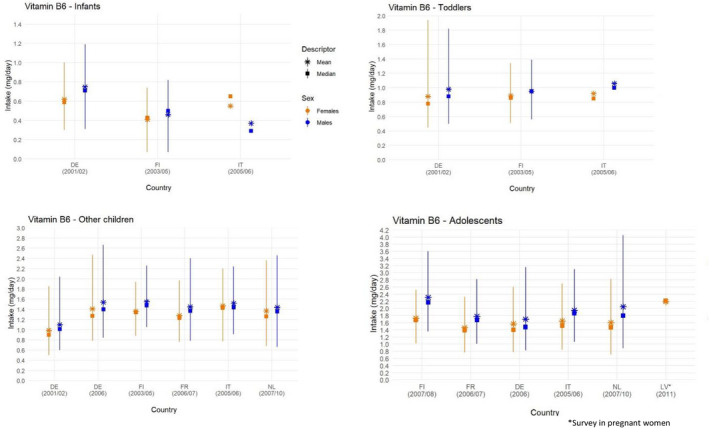
- Lines represent the range between the 5th and 95th percentiles. Estimated intakes from 5th and 95th percentiles are not presented when sample size is below 60 participants.. DE, Germany; FI, Finland; FR, France; IT, Italy; LV, Latvia; NL, The Netherlands.. Source: EFSA NDA Panel (2016), except for infants.
Figure 4.
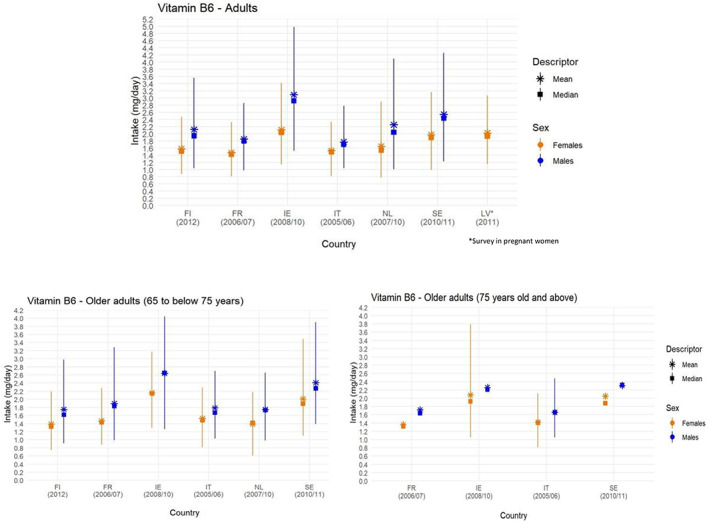
- Lines represent the range between the 5th and 95th percentiles. Estimated intakes from 5th and 95th percentiles are not presented when sample size is below 60 participants. FI, Finland; FR, France; IE, Ireland; IT, Italy; LV, Latvia; NL, The Netherlands; SE, Sweden. Source: EFSA NDA Panel (2016).
Table 3.
Minimum and maximum mean values and 95th percentiles of vitamin B6 daily intake from food sources (supplements excluded) across European dietary surveys by population group and sex
| Population group, age range | N of surveys | Vitamin B6 (mg/day) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Males | Females | ||||||||
| Mean | P95 (a) | Mean | P95 (a) | ||||||
| Min (b) | Max (b) | Min (b) | Max (b) | Min (b) | Max (b) | Min (b) | Max (b) | ||
| Infants, ≥ 4 to < 12 months | 3 | 0.4 | 0.7 | 0.8 | 1.2 | 0.4 | 0.6 | 0.7 | 1 |
| Toddlers, ≥ 1 to < 3 years | 3 | 0.9 | 1.1 | 1.4 | 1.8 | 0.9 | 0.9 | 1.3 | 1.9 |
| Other children, ≥ 3 to < 10 years | 6 | 1.1 | 1.5 | 2 | 2.7 | 1 | 1.5 | 1.9 | 2.5 |
| Adolescents, ≥ 10 to < 18 years | 5 | 1.7 | 2.3 | 2.8 | 4 | 1.5 | 1.7 | 2.3 | 2.8 |
| Adults, ≥ 18 to < 65 years | 6 | 1.8 | 3.1 | 2.8 | 5 | 1.5 | 2.1 | 2.3 | 3.4 |
| Older adults, ≥ 65 to < 75 years | 6 | 1.8 | 2.6 | 2.7 | 4 | 1.4 | 2.2 | 2.2 | 3.5 |
| Older adults, ≥ 75 years | 4 | 1.7 | 2.3 | 2.5 | 2.5 | 1.4 | 2.1 | 2.1 | 2.1 |
| Pregnant women | 1 | 2 | 2.2 | 3.1 | 3.1 | ||||
mo: months; n: number; P: percentile; y: years.
The 95th percentile estimates obtained from dietary surveys and population groups with fewer than 60 subjects may not be statistically robust (EFSA, 2011) and consequently are not considered in this table.
Minimum and maximum mean and 95th percentile estimates across European surveys, for each population group.
Source: EFSA NDA Panel (2016), except for infants.
Across population groups, the main food groups contributing to vitamin B6 intake were ‘food products for young population’ in infants and meat and meat products, milk and dairy products, grains and grain‐based products, fruit and fruit products and starchy roots and tuber and products thereof in all age groups. In addition, vegetables and vegetable products was one of the main contributors to vitamin B6 intake in the age groups of adolescents and adults. Differences in main contributors to vitamin B6 intake between genders were in most cases minor (EFSA NDA Panel, 2016) (Annex C).
3.4.3. Complementary information from national reports
3.4.3.1. Intake data of vitamin B6 from foods and fortified foods
There is no mandatory vitamin B6 fortification policy in EU countries.
Survey characteristics, mean and P95 intake estimates are presented in Annex C. Key information is summarised below.
Intake estimates from national consumption surveys
Reports from national consumption surveys providing estimates of vitamin B6 intake from foods, considering B6 fortification but excluding food supplements are available for 14 countries: Belgium (Enquête de Consommation Alimentaire 2014–2015), Bulgaria (National nutrition survey of infants and children 2007), Denmark (DANSDA 2011–2013), Estonia (Children's National Dietary Survey and National Dietary Survey among adults 2014), Finland (FINDIET 2017), France (INCA 3, 2014–2015), Greece (Hellenic National Nutrition and Health Survey (HNNHS) 2013–2015), Hungary (OTÁP, 2019), Ireland (National Teen's Food Consumption Survey (NTFS) II 2019–2020), the Netherlands (Dutch National Food Consumption Survey (DNFCS 2012–2016)), Norway (Småbarnskost 2007, Ungkost 3 2015 and 2016, Norkost 3 2015), Slovenia (National representative study on the dietary habits of Slovenian adolescents 2003–2005), Spain (Encuesta Nacional de Alimentación en la población Infantil y Adolescente (ENALIA 1) 2012–2014) and Sweden (Riksmaten adolescents 2016–2017, Riksmaten 2010–2011). The surveys in Austria (Österreichischer Ernährungsbericht 2012 and 2017), did not take fortified foods nor food supplements into consideration. Study characteristics, intake estimates and bibliographic references are provided in Annex C.
At the P95, estimated intakes of males reached 1.9 mg/day in infants (0–12 months; 2 countries), 2.1 mg/day in toddlers (1 to < 3 years; 4 countries), 2.5 mg/day in other children (3 to 10 years; 6 countries), 4.3 mg/day in adolescents (10 to 18 years; 7 countries) and 5.2 mg/day in adults (≥ 18 years; 6 countries). The highest P95 values for infants, toddlers and children were reported in the national survey in Spain, for adolescents highest P95 values were reported in the national surveys in Sweden, Ireland and Spain; and for adults for Austria in the 19‐ to 25‐year age range. Estimated intakes for females were generally lower than for males in all studies in all age groups, except for toddlers (1–3 years) and young adolescents (9–14 years) in France and Spain where they were higher; and in Hungary where the P95 intake of adult females (≥ 18 years) was higher than for adult males.
Contribution of fortified foods
The majority of the survey reports did not distinguish between vitamin B6 intake from natural sources and intake resulting from vitamin B6 addition to foods (fortified foods).
In an analysis of the DNFCS 2012–2016, among the consumers of fortified foods (75% of the Dutch population), the median contribution of voluntary fortification to the total vitamin B6 intake in the Netherlands was about 22%, while the P95 contribution reached up to about 67% (de Jong et al., 2022b). Foods fortified with vitamin B6 were among the most frequently consumed in the Netherlands among consumers of such foods, contributing up to 52% of the recall days, and the most frequently consumed was fortified syrup lemonade drink that accounted for 38% to the intake of vitamin B6 from fortified foods (de Jong et al., 2022b). Among children and adults of both genders, the median habitual intake of vitamin B6 of consumers of vitamin B6 fortified foods was significantly higher compared to non‐consumers of vitamin B6‐fortified foods (de Jong et al., 2022b). In a further analysis, the median contribution of vitamin B6‐fortified fats (margarines and other plant‐based fortified fats) to the vitamin B6 intake of the Dutch population from the DNFCS 2012–2016, was around 15% and the P95 contribution of fortified fats reached 40% (de Jong et al., 2022a).
3.4.3.2. Intake data of vitamin B6 from food supplements
Information on vitamin B6 intake from all sources, including supplements, are available for 17 dietary surveys conducted in 12 countries: Belgium (Enquête de Consommation Alimentaire 2014–2015), Denmark (DANSDA 2011–2013), Estonia (Children's National Dietary Survey and National Dietary Survey among adults 2014), Finland (FINDIET 2017), Germany (Nationale Verzehrsstudie (NVS) II, EsKiMo II), Ireland (National Teen Food Survey (NTFS II), National Children Food Survey (NCFS II)), Lithuania (Food consumption and nutrient intake study in Lithuania), the Netherlands (DNFCS 2012–2016), Norway (Småbarnskost 2007, Ungkost 3 2015 and 2016, Norkost 3 2015), Portugal (IAN‐AF 2015–2016), Spain (ANIBES 2013) and Sweden (Riksmaten 2010–2011). Study characteristics, and intake estimates are presented in Annex C.
Data collected on the use of vitamin B6 supplements in whole survey populations or in users only, including percent contribution to total vitamin B6 intake, absolute intakes and percent users in EU surveys, are briefly summarised in the following paragraphs.
Intake of vitamin B6 from foods supplements in the whole population
Six national dietary surveys from Belgium (n = 1), Ireland (n = 4) and the Netherlands (n = 1) provided information on the contribution of vitamin B6‐containing food supplements to total vitamin B6 intake in the whole study population, in different age groups. A summary of the data collected and the bibliographic references are provided in Table 4. The contribution of supplements to total vitamin B6 intake was estimated to be about 6% or less for toddlers, children and adolescents in all countries. Among adults aged 18–64 years (men and women combined), the contribution ranged between 4% in Belgium and 7% in Ireland.
Table 4.
Percent contribution of food supplements to total vitamin B6 intake in whole survey populations
|
Country Survey name (N subjects) Reference |
Dietary method (N of days) | Sex | Contribution of supplements to total mean vitamin B6 intake % (age) | ||||
|---|---|---|---|---|---|---|---|
| Toddlers | Other children | Adolescents | Adults | Elderly | |||
|
Belgium Enquête de Consommation Alimentaire 2014–2015 (n = 3,145) (Teppers, 2016) |
24‐h recall (2d) | m + f | NR |
Mean (95% CI) 3.5 (3–5 years) 2.2 (6–9 years) |
Mean (95% CI) 1.5 (10–13 years) 2.6 (14–17 years) |
Mean (95% CI) 4.3 (18–39 years) 4.4 (40–64 years) |
NR |
|
Ireland NPNS 2011–2012 (n = 500) NCFS II 2017–2018 (n = 600) NTFS II 2019–2020 (n = 428) NANS 2008–2010 (n = 1,500) (Kehoe and Walton, 2022) |
Weighted food diary (4 days) | m + f |
4.6 (1–4 years) |
6.1 (5–12 years) |
3.7 (13–18 years) |
7.4 (18–64 years) |
6.5 (65–90 years) |
|
Netherlands DNFCS 2012–2016 (n = 4,313) (van Rossum et al., 2020) |
Questionnaire (online/paper) | m + f | 9 (1–79 years) | ||||
|
Sweden Riksmaten 2003 (n = 2,495) (Barbieri et al., 2003) |
Dietary records | m + f | 4 (4 years) | 5 (9 years) | 4 (11 years) | NR | NR |
CI: confidence interval; d: day; DNFCS: Dutch National Food Consumption Survey; f: females; m: males; N: number; NANS: National Adult Nutrition Survey; NCFS: National Children's Food Survey; NPNS: National Pre‐School Nutrition Survey; NR: not reported in the publication; NTFS: National Teen's Food Consumption Survey; y: year.
Intake of vitamin B6 from food supplements in users
Toddlers, other children and adolescents
Data on the use of vitamin B6 supplements in toddlers, other children and adolescents were available from four national dietary surveys conducted in Denmark, Germany, Ireland and Norway. A summary of the data collected and bibliographic references are provided in Table 5 .
Table 5.
Percent vitamin B6 supplement users in EU surveys and vitamin B6 intake from food supplements among users
|
Country Survey name (N of subjects) Reference |
Dietary method (N of days) | Sex | Age range | % Vitamin B6 supplement users in total survey sample/ in supplements users | Vitamin B6 intake from supplements, P95 (mg/day) | Contribution of supplements to total vitamin B6 intake, mean (%) |
|---|---|---|---|---|---|---|
|
Denmark DANSDA 2011–2013 (n = 3,936) (Hindborg, 2015, unpublished) |
Face‐to‐face interview |
m f m f m f |
4–10 years 11–17 years 11–17 years 18–50 years 18–50 years 51–75 years 51–75 years |
61/NR 47/NR 43/NR 42/NR 52/NR 44/NR 58/NR |
NR |
37 52 57 51 59 60 67 |
|
Finland FINDIET 2017 (n = 1,655) (Valsta et al., 2018) |
FPQ |
m f |
18–74 years |
26/NR 36/NR |
(mean, mg/day) 8.7 7.6 |
79 80 |
|
Germany NVS II 2005–2007 (n = 13,753) (Heuer et al., 2012) |
24‐h recall (2 day) |
m f |
15–80 years |
7.3/NR 9.4/NR |
5.9 6 |
48 57 |
|
Germany EsKiMo II 2015–2017 (n = 2,644) (Mensink et al., 2021) (Perlitz et al, 2019) |
Questionnaire + weighing logs | m + f |
6–11 years 12–17 years |
2.2/NR 4.2/25.7 |
NR | NR |
|
Ireland NPNS 2011–2012 (n = 500) NCFS II 2017–2018 (n = 600) NTFS II 2019–2020 (n = 428) NANS 2008–2010 (n = 1,500) (Kehoe and Walton, 2022) |
Weighted food diary (4 days) | m + f |
1–4 years 5–12 years 13–18 years 18–64 years 65–90 years |
11/53 16/72 7/51 14/47 12/33 |
2.5 1.9 13.8 25 11.8 |
22 28 26 25 17.2 |
|
Norway Småbarnskost 2015 (n = 1,674) Ungkost 3 2016 (4 years, n = 399) Ungkost 3 2015 (9 years, n = 636) Ungkost 3 2015 (13 years, n = 687) Norkost 3 2015 (n = 1,787) (VKM, 2017) |
FFQ + food diary + 24‐h dietary interviews | m + f |
2 years 4 years 9 years 13 years 18–70 years |
33/NR 42/NR 33/NR 23/NR 23/NR |
1.2 1.4 1.2 1.7 5.3 |
40 56 41 43 56 |
|
Poland National Dietary Survey 2019–2020 (n = 1,831) (Stos et al., 2021) |
FPQ |
m f |
18–65+ years |
NR/40 NR/71 |
Mean ± SD (range) 3.4 ± 5.5 (0.7–25) 2.4 ± 4.1 (0.4–30) |
NA |
|
Sweden Riksmaten 2010–2011 (n = 1,797) (SFA, personal communication) |
Dietary records (x4) |
m + f |
18–80 years | NR/4 | NR | NA |
DANSDA: The Danish National Survey of Diet and Physical Activity; DNFCS: Dutch National Food Consumption Survey; EsKiMo: Eating study as a KiGGS Module; f: females; FINDIET: The Finnish National Dietary Survey in Adults and Elderly; FPQ: food propensity questionnaire; m: males; N: number; NA: cannot be calculated; NANS: National Adult Nutrition Survey; NCFS: National Children's Food Survey; NPNS: National Pre‐School Nutrition Survey; NR: not reported in the publication, NTFS: National Teen's Food Consumption Survey; NVS II: Nationale Verzehrsstudie II; SD: standard deviation; SFFQ: semiquantitative food frequency questionnaire; VKM: Vitenskapskomiteen for mat og miljø.
In children of different age groups who take supplements, contribution to total vitamin B6 intake from food supplements ranged from 30% in the Irish survey (5–12 years old) to 56% in the survey in Norway (Ungkost 3–4 years old). In adolescent users, the contribution of supplements to total vitamin B6 intake was 43% in Norway (Ungkost 3–13 years old) and 52% and 57% in male and female adolescents aged 11–17 years in Denmark (DANSDA 2011–2013). In Irish adolescents from the NTFS II, the contribution of supplements to total vitamin B6 intake was about 26%, leading to an absolute intake in high consumers (P95) of about 14 mg/day from vitamin B6 supplements only, possibly due to the availability in the market of supplements with daily doses up to 50 mg/day for adolescents.
Adults
Nine national dietary surveys conducted in Belgium, Denmark, Finland, Germany, Ireland, Norway, Poland and Sweden reported on the use of vitamin B6 supplements in adults ( Table 5 ).
Vitamin B6 supplements contributed more than 50% to total vitamin B6 intake in adults and older adults in all surveys and was up to 80% (in Finnish adults from the FINDIET 2017 survey). In Ireland, the contribution of supplements to total vitamin B6 intake in adults and older adults from the NANS survey, was lower than in all other surveys (25 and 17%, respectively). However, P95 intakes from food supplements only were about 25 and 12 mg/day, respectively in adults and older adults, possibly due to the availability in the market of supplements with daily doses up to 100 mg/day for the adult age groups. In a survey in adults in Poland, mean intake from vitamin B6 supplements was up to 30 mg/day.
Other information collected on food supplements among EU Member States
Nutritional guidelines or recommendations at national level in the EU do not advise on supplementation with vitamin B6. Among the 27 EU Member States contacted and Norway, four provided the maximum daily dose of vitamin B6 regulated for addition to food supplements: it was 4.2 mg/day in Luxembourg, 14 10 mg/day in Italy, 15 18 mg/day in Poland 16 and 21 mg/day in the Netherlands. 17 The maximum daily doses as recommended by the food business operators and notified to national authorities has been shared by a few countries: Norway (4.2 mg/day (VKM, 2017), Denmark 18 (50 mg/day) and Finland (400 mg/day) (unpublished data from the Finnish Food Safety Authority).
3.4.4. Overall conclusions on intake data
The Panel notes that the P95 estimated intake of vitamin B6 from food consumption only (i.e. without food supplements) is up to 1.2 mg/day in infants (4 to < 12 months), up to 1.9 mg/day in toddlers (1 to < 3 years), up to 2.7 mg/day in other children (3 to < 10 years), up to 4 mg/day in adolescents (10 to < 18 years) and up to 5 mg/day in adults (≥ 18 years), across surveys included in EFSA's intake assessment (EFSA NDA Panel, 2016) (Table 3 ). Intakes are slightly lower in females, except for female toddlers, mainly due to smaller quantities of food consumed per day. National reports of more recent data do not indicate that vitamin B6 intakes of high (P95) consumers in EU Member States may exceed EFSA estimates of intake from food sources only, but comparisons are difficult owing to the different methodologies and food composition tables used.
The Panel notes that fortified foods were found to contribute little to total vitamin B6 intake, at population levels, in the 15 countries for which information is available. The Panel notes the inherent uncertainties related to self‐reported intakes of fortified foods in food consumption surveys, as well as uncertainties in the composition data, which can hamper the accurate evaluation of the actual contribution of these foods. Additionally, in view of the high content of some fortified products in the EU market Section 3.4.1 the Panel notes that the contribution of fortified foods to total vitamin B6 intake could be significant among regular consumers of these foods but data are too limited for a reliable intake assessment of this group.
Data on the contribution of food supplements to total vitamin B6 intakes in supplement users are available for a limited number of European countries. Among supplement users, the Panel notes that, in children, food supplements were found to contribute more than 20% and up to about 60% to total vitamin B6 intake in children aged 9 years in Norway. In adolescents, percent contribution from vitamin B6 supplements was up to 60%, in Danish aged 11–17 years. In Irish adolescents and adults who were users of food supplements, vitamin B6 supplements contributed to about 17 to 26% of total vitamin B6 intake with P95 vitamin B6 intakes from supplements of about 14 mg/day in adolescents, 25 mg/day in adults and 12 mg/day in older adults. In adults in all other countries, vitamin B6 supplements contributed more than 50% to total vitamin B6 intake, and up to about 80% of total vitamin B6 intake in adults in Finland.
The Panel notes that data from the Mintel GNPD indicate that half of the food supplements on the market contain between 1.01 and 2.0 mg per serving dose and less than 1% of the supplements retrieved contain more than 25 mg per serving dose. However, the Panel notes the high variability in the maximum daily doses indicated by business operators in the labels of vitamin B6 containing food supplements notified to national authorities in the Member States (up to 4.2, 50 and 400 mg in Norway, Denmark and Finland, respectively). The Panel notes that in regular consumers of vitamin B6‐containing supplements, the contribution of supplements to total vitamin B6 intake can be substantial. The Panel also notes the high variability in the maximum amounts set by national authorities for addition of vitamin B6 to food supplements.
3.5. Hazard identification
3.5.1. Peripheral neuropathy
The evidence table is in Appendix D.1.
3.5.1.1. Evidence from human studies relating vitamin B6 intake to neuropathy
The relationship between high intakes of vitamin B6 and the development of peripheral neuropathy is well established both in humans and in animals (IOM, 1998; SCF, 2000).
Most of the studies retrieved as pertinent through the systematic search for the present assessment, had already been considered by the SCF (2000) in its previous evaluation. The pertinent studies are described in the following, including the considerations made by the SCF (2000) during their assessment, when relevant. The RoB of the overall BoE was moderate to high (Appendix B).
Some studies were not further considered in the assessment because of the reasons outlined in the following paragraphs.
Studies that used vitamin B6 vitamer concentrations in plasma/serum only as exposure markers and studies that provided too limited information on dietary vitamin B6 exposure were not used in the evaluation and are listed in Appendix C (Franzblau et al., 1996; Keniston et al., 1997; Scott et al., 2008; No authors listed, 2009; Chaudary and Cornblath, 2013; Falcone and Sowa, 2013; Kaur et al., 2014; Visser et al., 2014; Bacharach et al., 2017; Malek et al., 2020; Stewart et al., 2022).
Most studies that investigated supplemental vitamin B6 intakes that exceeded 500 mg/day were also not used in the evaluation and are listed in Appendix C (Schaumburg et al., 1982; Baer, 1984; Berger and Schaumburg, 1984; Vasile et al., 1984; Foca, 1985; Friedman et al., 1986; Waterston and Gilligan, 1987; Gdynia et al., 2008; No authors listed, 2009). This is the dose for which the SCF (2000) had already concluded that it represented a potentially toxic dose for humans associated with severe symptoms and which is also five times higher than the RP used by the SCF (2000) for setting the UL. Only the publications by Schaumburg et al. (1983) and Berger et al. (1992) are briefly described in the following because they established the occurrence of vitamin B6‐associated peripheral neuropathy in humans and the existence of an inverse relationship between the dose of vitamin B6 and the time to onset of symptoms.
The case series by Schaumburg et al. (1983) was the first to demonstrate that high vitamin B6 intakes at doses of ≥ 2,000 mg/day taken for 2 months to several years led to the development of peripheral neuropathy in humans, a relationship that had previously already been observed in rats and dogs (SCF, 2000). Symptoms in all individuals improved after withdrawal of vitamin B6 supplements.
The intervention study by Berger et al. (1992) established that an inverse relationship between dose and time to onset of symptoms of peripheral neuropathy existed in humans. In this study, five healthy volunteers received vitamin B6 in amounts of 12 mg/kg bw per day (two subjects), 19.5 mg/kg bw per day, 26.5 mg/kg bw per day or 56.9 mg/kg bw per day. In one individual who had consumed 12 mg/kg bw per day for 14 months, Quantitative sensory testing (QST) abnormalities developed without symptoms of neuropathy. However, serum PLP concentrations were only about 80 nmol/L compared with 650 nmol/L in the other individual who had also consumed vitamin B6 in an amount of 12 mg/kg bw per day for 7 months until symptoms of neuropathy and QST abnormalities occurred. In the other individuals, QST abnormalities alongside neuropathy symptoms showed after 4.5, 3.5 and 1.5 months of having consumed 19.5, 26.5 and 56.9 mg/kg bw per day, respectively. Serum PLP concentrations were around 300 nmol/L and 750 nmol/L in the individuals who had consumed 19.5 and 26.5 mg/kg bw per day, respectively, and were not reported for the fifth individual.
The following studies were used in the evaluation of the lower bound of daily vitamin B6 intake that is associated with the development of peripheral neuropathy.
One prospective cohort study (Shrim et al., 2006), two retrospective studies (Brush et al., 1988; Chaudary et al., 2003) and one case–control study (Dalton and Dalton, 1987) investigated the relationship between supplemental vitamin B6 intake and peripheral neuropathy at doses < 500 mg/day. Case reports (Dalton, 1985; Parry and Bredesen, 1985; Dalton and Dalton, 1987; Blackburn and Warren, 2017) and data from nutrivigilance systems of EU Member States (van Hunsel et al., 2018; No authors listed, 2020; Vrolijk et al., 2020) also support the occurrence of symptoms at supplemental doses < 500 mg/day.
Prospective cohort study
In the prospective cohort study, Shrim et al. (2006) followed 96 pregnant women up to birth who had taken vitamin B6 supplements during the first trimester of pregnancy in amounts of 50–150 mg/day for an average of 9 ± 4 (SD) weeks. Another 96 pregnant women not taking supplements were followed as well. The authors report that no adverse events related to vitamin B6 use were reported by participants. The Panel notes that it is unclear how adverse events were assessed in the study. The Panel also notes that the duration of consumption of vitamin B6 supplements may have been too short to induce symptoms of peripheral neuropathy. This limits the conclusions that can be drawn from this study.
Retrospective studies
Chaudary et al. (2003) conducted a retrospective cohort study in 555 individuals, aged 14–76 years, who had attended a nutritional therapy practice and who had been recommended to consume between 30 and 250 mg/day supplemental vitamin B6 for over 3 months. Before having started supplementation, the subjects had filled in a questionnaire that asked for the presence or absence of symptoms that could be associated with the deficiency of certain vitamins and minerals, among those tingling hands, insomnia, rashes and acne which could have been, as suggested by the authors, indicative of vitamin B6 deficiency. After 3–42 months of vitamin B6 supplementation, subjects were asked to fill in the questionnaire again. The authors of the study concluded that there was a reduction in frequency of symptoms, either individually or together rather than an increase. This reduction was largest in the individuals consuming 101–150 mg/day vitamin B6. The Panel notes that symptoms had already persisted before vitamin B6 supplementation and might have been due to vitamin B6 deficiency, as reported by the authors. The Panel notes that the improvement of symptoms potentially indicative of vitamin B6 deficiency cannot necessarily be interpreted as absence of adverse effects at the supplemented doses. This limits the conclusions that can be drawn from this study.
In the retrospective study by Brush et al. (1988), 630 women suffering from PMS had taken initially between 40 and 100 mg/day vitamin B6 and later on mostly between 120 and 200 mg/day. These women reported no symptoms of peripheral neuropathy. The total duration of vitamin B6 exposure was < 6 months in 46% of individuals and longer than 1 year in 19.5% (duration of exposure to different vitamin B6 dosages was not reported). In a follow‐up of the study which was described by the SCF (2000) 19 and which covered three additional years, five cases of dizziness and six cases of mild tingling were noted in 336 women taking 200 mg/day vitamin B6 (duration not reported). The Panel considers that the follow‐up of the study shows the occurrence of symptoms of peripheral neuropathy at supplemental vitamin B6 doses of 200 mg/day consumed for an unknown period of time.
In the case–control study described by Dalton and Dalton (1987), which was the study used by the SCF (2000) for the setting of the UL, 172 women with PMS, attending the same private practice, were recruited. The women were taking vitamin B6 supplements (range < 50 mg/day to 500 mg/day) and had serum PLP concentrations > 18 ng/mL (> 72.8 nmol/L). Women were asked to report symptoms indicative of peripheral neuropathy and in positive cases women were followed‐up by a neurological examination (not further described). A total of 103 women (60%) had neurological symptoms (paraesthesia, bone pains, hyperaesthesia, muscle weakness, fasciculation and numbness; cases), while 69 women did not have symptoms (controls). Serum PLP concentrations were above the upper limit of testing of 34 ng/mL (137.5 nmol/L) in 70% of cases and 55% of controls. The average vitamin B6 intake was 117 ± 92 mg/day (measure of spread not further specified) in cases and 116 ± 6 mg/day in controls. Cases had, however, taken supplements on average for longer than controls (mean 2.9 ± 1.9 vs. 1.6 ± 2.1 years, p < 0.01) without reporting on latency periods. Doses of < 50 mg/day (lower bound not reported) were consumed by 43 women (cases and controls combined) and 48% of these women showed symptoms of peripheral neuropathy. Exposure was > 6 months in all cases. With increasing doses, the percentage of women suffering from peripheral neuropathy increased, except in the highest dose group which, however, also included the smallest number of participants. The percentage of women with symptoms were 60%, 77% and 51% of 65, 42 and 22 women in the groups consuming 50–100 mg/day, 100–200 mg/day and 200–500 mg/day, respectively (data calculated by EFSA based on information provided in the publication). Following withdrawal of vitamin B6 supplements, 55% of women reported partial or complete recovery after 3 months and after 6 months all had recovered. The Panel notes that the SCF (2000) had previously used the average supplemental intake in this study of around 100 mg/day as a RP for the derivation of the UL. This was based on the observation that cases had taken vitamin B6 supplements on average for longer at the same mean intakes, indicating that an inverse relationship between dose and time to onset of symptoms existed at these mean intakes. The Panel, however, considers that this study not only shows an inverse relationship between dose and time to onset of symptoms, but also a dose‐dependent increase in the percentage of women with symptoms of peripheral neuropathy occurring at supplemental vitamin B6 intakes of < 50 mg/day (lower bound not reported) when consumed for > 6 months.
Case reports/series
In the context of a case series, Parry and Bredesen (1985) reported that neuropathy had manifested in an individual at supplemental vitamin B6 intakes of 200 mg/day consumed for 36 months.
In a case series, Dalton (1985) described 23 women who had consumed between 50 and 300 mg/day vitamin B6 for an unknown duration. However, it cannot be ruled out that this sample of women is also included in the study population described by Dalton and Dalton (1987).
The publication by Dalton and Dalton (1987), described above, also includes the description of a case of a woman with symptoms of neuropathy who had taken 75 mg/day vitamin B6 for 2 years and whose symptoms ceased within 3 months of having stopped supplementation. When she restarted taking vitamin B6 at a dose of 50 mg/day, the symptoms reappeared.
Blackburn and Warren (2017) described neuropathy in a male individual who had consumed six energy drinks per day, each containing 5.1 mg vitamin B6. In total, the individual had consumed 31 mg/day vitamin B6 from energy drinks and had reduced pinprick responses and absent vibration sensation in some parts of the extremities. Latency period, overall duration of consumption, the composition of the implicated energy drink other than vitamin B6 and other dietary habits were not reported.
Nutrivigilance data
Nutrivigilance data are described in three publications (van Hunsel et al., 2018; No authors listed, 2020; Vrolijk et al., 2020).
One publication (No authors listed, 2020) described 25 notifications of peripheral neuropathy attributed to vitamin B6‐containing products that the French Health Products Agency had received between 1986 and 2018. The products contained between 5 and 250 mg vitamin B6 and were consumed between 8 days and 4 years. There was no information on the actual doses taken. In ‘just under 10 individuals’ (as reported in the publication), vitamin B6 was consumed in the form of a combination of vitamins and minerals. Individuals did not consume other medicinal products that could have caused neuropathy.
The data from the Dutch nutrivigilance system described by No authors listed (2020), van Hunsel et al. (2018) and Vrolijk et al. (2020) were also made available to EFSA as full data set. Between 2007 and 2022, 191 cases 20 of individuals who had experienced symptoms of neuropathy following supplemental vitamin B6 intake have been notified to the Dutch vigilance database. Of these, 10 were submitted by a physician, 7 by a pharmacist and the remaining 174 by consumers. In 76 cases, either the associated plasma PLP concentrations were reported, or a statement was available that the concentrations were elevated. There was no information on how neuropathy was diagnosed. For 47 cases the doses consumed, the latency times and the associated plasma PLP concentrations were available, which were considered the most reliable cases. Supplemental doses ranged from 1.4 mg/day to 209 mg/day. Forty‐one cases had taken supplemental vitamin B6 at doses ≤ 100 mg/day, three 125 mg/day for 5 days to 6 months and three between 200 and 209 mg/day for 2 weeks to 3 years. Table 6 provides an overview of those cases who had consumed vitamin B6 supplements in amounts ≤ 100 mg/day. The Panel notes the absence of information on the diagnosis of peripheral neuropathy, on the habitual diet of the individuals and on the actual (measured) concentrations of vitamin B6 in the consumed products (vs the labelled content). The Panel also notes that reported plasma PLP concentrations are likely not comparable owing to differences in the timing of blood sampling since the last supplemental intake. Finally, the Panel notes the uncertainties that are associated with self‐reported data.
Table 6.
Overview about individuals who had reported symptoms of neuropathy following supplemental vitamin B6 intakes ≤ 100 mg/day reported in the Dutch nutrivigilance system
| Dose | Cases | Latency | PLP concentrations | Symptoms, diagnosis (as reported) |
|---|---|---|---|---|
| 100 mg/day |
3 (1 M, 2 F) (2 reported by consumers, 1 by physician) |
7 d, 2 y, 18 y | Reported to be above normal (2 cases), 3,266 nmol/L (exposure: 7 d) | Tingling in feed and hands, pain, neuropathy |
| 74–75 mg/day |
5 (1 M, 4 F) (all reported by consumers) |
2 w, 2 m, 3 m, 16 m, 6 y | 190 nmol/L (exposure: 2 m) to 2,500 nmol/L (exposure: 6 y) | Neuropathy, neurologic symptoms, pain under the feet after short walking |
| 40–50 mg/day |
5 (2 M, 3 F) (4 reported by consumers, 1 by physician) |
6 m, 1 y, 2 y, 4 y, 10 y | Around 330 nmol/L (exposure; 6 m, 4 y, 10 y) to 4,338 nmol/L (exposure 2 y) | Neuropathy, numbness, paraesthesia of limbs |
| 25–28 mg/day |
5 (1 M, 4 F) (4 reported by consumers, 1 by pharmacist) |
10 d, 2 m, 3 y 4 y, 6 y | 88 nmol/L (exposure: 10 d) to 2,200 nmol/L (exposure: 6 y) | Neuropathy, tingling of extremities |
| 12–21 mg/day |
7 (1 M, 6 F) (6 reported by consumers, 1 by physician) |
3 w, 3 m, 11 m, 1 y, 5 y, 15 y, 20 y | Reported to be above normal (2 cases), 336 nmol/L (exposure: 5 y) to 3,600 nmol/L (exposure: 11 m) | Neuropathy, nerve damage, paraesthesia, muscular weakness |
| 5–8 mg/day |
5 (5 F) (all reported by consumers) |
3 m, 10 m, 1 y (2x), 15 m | Reported to be above normal (1 case), 158 nmol/L (exposure: 3 m) to 1,110 nmol/L (exposure: 15 m) | Neuropathy, paraesthesia, muscle weakness, nerve pain |
| 1.4–3.5 mg/day |
11 (3 M, 8 F) (all reported by consumers) |
12 h, 1 m, 5 m, 6 m (5x), 8 m, 3 y, 23 y | Reported to be above normal (2 cases), 158 nmol/L (exposure: 6 m) to > 1,200 nmol/L (exposure: 5 m), most were in the range of 200–400 nmol/L | Neuropathy, numbness of extremities, tingling in hands, pain in hands, paraesthesia |
D: days; F: female; h: hour; M: male; m: month(s); y: year(s).
Upon request from EFSA, other Member States' authorities also provided information on adverse effects reported to national vigilance systems or national authorities in relation to vitamin B6 supplementation:
Finland reported adverse effects at doses of 50 mg/day and beyond, but no cases of peripheral neuropathy at doses below the current UL of 25 mg/day.
Luxembourg and Denmark reported no notified cases of peripheral neuropathy.
France, through its nutrivigilance system, reported eight cases of neuropathy, all below the current UL of 25 mg/day, of which one at an intake of 21 mg/day with a latency period of 1 month with the causality judged to be likely. The other cases were related to a single intake of 13.3 mg and to intakes below 2.5 mg/day with latency periods between 1 day and 1 month, rated as likely or possible. The causality ratings do not refer to vitamin B6 only, but to the combination of ingredients present in the products that had been consumed by the individuals.
The Panel considers that there is evidence that symptoms of peripheral neuropathy may occur at supplemental vitamin B6 intakes that are below the RP of 100 mg/day used by the SCF (2000) to establish the UL.
The information provided in the individual publications (Dalton, 1985; Dalton and Dalton, 1987; Blackburn and Warren, 2017) and the data from Member States' vigilance systems either reported in publications (van Hunsel et al., 2018; No authors listed, 2020; Vrolijk et al., 2020) or made available to EFSA by Member States' authorities are limited and mostly insufficient to verify the causal link between vitamin B6 intake at the consumed doses and the development of neuropathy symptoms.
However, for drawing conclusions the following observations are to be considered:
In the case–control study by Dalton and Dalton (1987), 48% of women who had taken vitamin B6 supplements at doses < 50 mg/day for more than 6 months (the lowest dose group) showed symptoms of neuropathy and the percentage of women affected increased with increasing doses.
A woman developed symptoms of peripheral neuropathy for the second time after having taken 50 mg/day supplemental vitamin B6 for 4 months. This was following recovery from neuropathy that had originally developed after consumption of 75 mg/day vitamin B6 for 2 years (Dalton and Dalton, 1987). There is uncertainty whether vitamin B6 plasma concentrations had returned to normal between the two episodes. However, there was 1 year between the easing and reoccurrence of symptoms.
A man developed neuropathy (Blackburn and Warren, 2017) following energy drink consumption. These energy drinks had provided a total of 31 mg/day vitamin B6. However, information on the latency period, the overall duration of consumption, the composition of the implicated energy drink other than vitamin B6 and other dietary habits is lacking.
Dalton (1985) reports that 23 women with symptoms of neuropathy had consumed between 50 and 300 mg/day vitamin B6. It is unclear if these cases partly or fully overlap with the cases described by Dalton and Dalton (1987) and it is unknown how many women had consumed the dose of 50 mg/day.
The Dutch nutrivigilance system reports that 33 cases for which information on both the dose and the latency period were available had taken supplements that contained ≤ 50 mg/day vitamin B6.
The French nutrivigilance system indicates cases of peripheral neuropathy occurring at supplemental intakes < 25 mg/day.
The national competent Authority of Finland reports adverse effects at supplemental intakes of 50 mg/day and above.
The Panel notes that taken together the evidence indicates that symptoms may occur at daily supplemental vitamin B6 intakes of 50 mg. The Panel additionally notes the large interindividual differences in sensitivity to vitamin B6 toxicity described by Hadtstein and Vrolijk (2021).
The Panel also notes that plasma PLP concentrations show large interindividual variability in response to vitamin B6 supplementation as evidenced by the intervention study by Berger et al. (1992). In this study, plasma PLP concentrations were 80 nmol/L following consumption of supplemental vitamin B6 of 12 mg/kg bw per day for 24 months in one individual, 650 nmol/L when the same amount was consumed for 7 months in another individual, 300 nmol/L with a supplemental vitamin B6 intake of 19.5 mg/kg bw per day for 4.5 months and 750 nmol/L with a supplemental intake of 26.5 mg/kg bw per day for 3.5 months (one individual each).
The Panel concludes that the evidence allows establishing with sufficient certainty that peripheral neuropathy may occur at supplemental vitamin B6 intakes of 50 mg/day in some individuals.
3.5.1.2. Evidence from animal studies relating vitamin B6 intake to peripheral neuropathy
The description of the evidence in relation to animal studies that relate vitamin B6 intake to neuropathy is based on a narrative review of the evidence (Tetens et al., 2023). Only studies that investigated oral intake of vitamin B6 were considered.
Studies in rats
Only a few studies have been conducted in rats, in which vitamin B6 was provided orally. However, even at doses of 1,400–1,750 mg PN‐HCl/kg diet (recommended intake for rats 7 mg/kg diet) given for 6–10 weeks, no signs of peripheral neuropathy were reported (Schaeffer et al., 1989; Schaeffer et al., 1995). It should be noted that the studies were not designed to look at this endpoint. In a previous review, doses of 3,000–6,000 mg/kg bw per day were described to induce symptoms of peripheral neuropathy (e.g. ataxia) in rats (Cohen and Bendich, 1986).
Studies in dogs
Beagle dogs have a urinary excretion of vitamin B6 that is similar to humans in terms of metabolites and total vitamin B6 excreted in urine (Scudi et al., 1942). Beagle dogs have been widely used to investigate vitamin B6 toxicity (Montpetit et al., 1988) and seven studies were available for the present assessment. The Panel notes the absence of a control group in all studies, except in one (Phillips et al., 1978).
In one study in 10 Beagle dogs (five males, five females; age range: 13–15 months), PN‐HCl was administered at doses of 50 mg/kg bw per day for the first week, 100 mg/kg bw per day for the second week and 150 mg/kg bw per day from the 15th day to the end of the experimental period at 100–112 days. Neurological symptoms with signs of ataxia and gait abnormalities developed from day 18 of treatment onwards (Hoover and Carlton, 1981). Dogs had degenerative lesions in the dorsal funiculus, the trigeminal nerve fibres and the spinal tracts of the trigeminal nerves with a reduced number of axons and irregular and fragmented myelin (Hoover and Carlton, 1981).
In another study by the same authors testing the same PN doses administered for the same duration in three Beagle dogs, similar results were obtained (Hoover et al., 1981). Degeneration in the dorsal funiculus, the dorsal spinal roots, some fascicles of peripheral nerves, the spinal tracts of the trigeminal nerves and the trigeminal nerve fibres was observed. In the dorsal funiculus, degeneration of axons, loss of axons, collapse of myelin sheaths, degeneration and loss of myelin and astrocytic scarring occurred. The authors concluded that vitamin B6 produced axonal degeneration followed by nonspecific secondary degenerative changes in myelin.
Montpetit et al. (1988) administered 150 or 200 mg/kg bw per day PN‐HCl to 6 and 4 Beagle dogs (age range: 6–9 months), respectively. Signs of peripheral neuropathy started after 7–10 days of treatment and appeared at both dose levels. The findings suggested that the initial lesions occur in the soma of neurons of the dorsal root ganglia. Vacuoles appeared in the cytoplasm of large neurons, eventually substantially reducing the cytoplasm. This happened first in the lumbar dorsal root ganglia followed by the thoracic and cervical ones. In dorsal root, sciatic and sural nerves, an active breakdown of myelin, proportional in degree to the duration of vitamin B6 treatment, was observed. Evaluation of tissues evidenced that the unmyelinated processes of neurons in the dorsal root ganglia were swollen because of an accumulation of neurofilaments. Both in the soma and in the proximal myelinated processes, neurofilament microtubules were dissociated. The authors proposed that this cytoskeletal disruption was due to an increased rate of protein synthesis and that the vacuolar changes were probably a secondary reaction to the damaged cell processes.
Oral administration of vitamin B6 (300 mg/kg bw per day) to six Beagle dogs (7–11 months old) for 78 days induced gait abnormalities 4–9 days after beginning of treatment, and severe ataxia 8–30 days after start of treatment (Krinke et al., 1981). Morphological abnormalities were detected in neurons and processes of dorsal root and the trigeminal ganglia. Vacuoles appeared in the cytoplasm that over time reduced the cytoplasm to a small rim. Axon degeneration was present in dorsal roots, dorsal columns of the spinal cord, in the descending tract from the trigeminal nerve and in scattered fibres of trigeminal nerves with the most severe changes occurring in the sural nerve. Even in the most compromised nerves, normal fibres interspersed with damaged ones.
Schaeppi and Krinke (1982) provided 3,000 mg/day PN‐HCl (between 250 and 315 mg/kg bw per day, depending on the dog's body weight) to two female Beagle dogs for 8 and 26 days, respectively. Gait impairment started on day 6 in one dog and on day 20 in the other. There was selective damage of large, first‐order sensory neurons. Dorsal root ganglia showed a loss of dorsal root neurons as evidenced by an increased number of satellite cells. In the dorsal spinal roots and peripheral nerves, there was a decrease in the number of large, myelinated nerve fibres, but no degeneration was visible. A number of fibres were swollen and contained myelin fragments and shrunken fibre remnants.
Another study by the same authors tested a dose of PN‐HCl of 300 mg/kg bw per day consumed for 10–20 days by three dogs. The dogs had chronically implanted cortical electrodes (age range: 12–37 months) which showed impaired slow impulse transmission and a lesser effect on the faster one (Schaeppi and Krinke, 1985).
In a study in Beagle dogs with a control group (Phillips et al., 1978), the toxicity of different oral doses of PN‐HCl (0, 50 or 200 mg/kg bw per day) was tested (n = 14 in total; n = 4 for control group; n = 5 for each treatment group; age range: 7–8 months) for 100–114 days (average: 107 days). Animals were randomly assigned to the three groups and were fed a nutritionally balanced commercially available diet. Two dogs were housed in each pen. PN‐HCl was administered in gelatine capsules. The ‘high‐dose’ group initially received 250 mg/kg bw per day for a week, but as all dogs developed incoordination and ataxia, the dose was reduced to 200 mg/kg bw per day. Clinical examinations were carried out once per month and neurological assessments at biweekly intervals. Dogs in the ‘high‐dose’ group lost weight within the first week of treatment. The weight was not recovered during the duration of the study. There was also an increase in heart rate in this group from on average 118 beats per minute pre‐treatment to 160 beats per minute after 8 days of treatment which remained at this level until the end of the study. The signs that had been experienced by the dogs in the ‘high‐dose’ group within the first week disappeared within another week after the dose had been reduced to 200 mg/kg bw per day and reappeared between 40 to 75 days after the start of the study with 100% of animals affected. Ataxic gait, muscular weakness and loss of balance were the first signs to appear. The neurological impairment increased in severity with time until they were so marked that dogs had difficulties in standing. Histological findings showed a pronounced loss of myelin and axons in the dorsal fasciculi throughout the entire length of the spinal cord and loss of myelin and nerve fibre degeneration in the dorsal sensory nerve roots. In the ‘low‐dose’ group, signs of neurological disease did not develop, and they did not show lesions in the dorsal fasciculi. However, all five dogs in the group showed bilateral loss of myelin in the dorsal sensory nerve roots. No lesions were found in the control animals. The Panel considers that this study shows that supplemental vitamin B6 intake of 50 mg/kg bw per day in Beagle dogs for around 107 days leads to limited damage in the dorsal sensory nerve roots without the occurrence of symptoms of neurological disease.
The Panel concludes that the study by Phillips et al. (1978) provides evidence for a LOAEL of 50 mg/kg bw per day in Beagle dogs.
The Panel also concludes that the studies in Beagle dogs suggest that high vitamin B6 intake damages afferent nerves mostly through axon degeneration, myelin breakdown and vacuolisation of cytoplasm, with initial lesions mostly occurring in the dorsal root ganglia.
3.5.1.3. Mechanisms of toxicity
Hadtstein and Vrolijk (2021) recently reviewed four prevailing theories on the potential mechanisms by which high intakes of vitamin B6 could cause peripheral neuropathy. These have been summarised by the Tetens et al. (2023) based on Hadtstein and Vrolijk (2021) and are described in the following:
PL and PLP each contain an aldehyde group. Aldehydes are highly reactive and capable of altering structures and functions of macromolecules such as proteins and DNA (LoPachin and Gavin, 2014), not necessarily confined to neurons. In contrast to PLP, PL can exist in both aldehyde and hemiacetal forms. However, at neutral pH, such as in the human body, almost all PL is found in hemiacetal form which is not chemically reactive (Ink et al., 1982). PLP, on the other hand, could exert aldehyde toxicity. However, it is bound to protein in plasma and the body possesses mechanisms that counteract the accumulation of PLP (Hadtstein and Vrolijk, 2021). In a study in Lewis rats, it was observed that intraperitoneal injections of PL‐HCl, PLP and PM‐2HCl did not lead to clinical signs of neurotoxicity nor to histological lesions, while the injection of PN‐HCl did (Levine and Saltzman, 2004). The Panel considers that it is unlikely that the aldehyde groups of PL or PLP are responsible for neurotoxic effects of vitamin B6.
It has been suggested that quinone methide‐type intermediates could be involved in the generation of adverse effects of vitamin B6. Quinone‐methides possess electrophilic reactivity and can alkylate molecules (Hadtstein and Vrolijk, 2021). It has been shown in vitro that methide‐type intermediates that can be formed by irradiation of PN (Brousmiche and Wan, 2002). It has been proposed that they could potentially also be formed in vivo through enzymatic reaction (Frater‐Schröder and Mahrer‐Busato, 1975). Quinone methide‐type intermediates are expected to exert broad toxicological activity, not only confined to neurons. The Panel considers that is unlikely that quinone methide‐type intermediates are responsible for neurotoxic effects of vitamin B6.
As PN and/or PNP may form chemical complexes that hinder PLP from to binding to target enzymes, it has been proposed that high concentrations of PN in plasma may lead to vitamin B6 deficiency. In animal models, neuropathy is one of the manifested symptoms (Vrolijk et al., 2017). However, symptoms of neuropathy caused by vitamin B6 toxicity do not fully overlap with symptoms of vitamin B6 deficiency (Hadtstein and Vrolijk, 2021).
Mutations of the gene expressing pyridoxal kinase predominantly lead to injury of sensory neurons, causing axonal neuropathy and producing symptoms similar to vitamin B6‐induced neuropathy (Hadtstein and Vrolijk, 2021). High concentrations of PN may lead to inhibition of pyridoxal kinase in tissues with low PLP oxidase activity and thus to a depletion in PLP. Compounds that lead to inhibition of pyridoxal kinase have shown to inhibit γ‐aminobutyric acid (GABA) biosynthesis and GABAergic neurotransmission (Kasaragod et al., 2020). The presynaptic inhibition of GABA leads to an increased excitability of dorsal root ganglia neurons (Vargas‐Parada et al., 2021). It is known that overactivation of excitatory neurotransmitters result in degeneration of neurons (King et al., 2007) and it is plausible that the increased excitability of the dorsal root ganglia neurons could be the cause for the development of peripheral neuropathy. In addition, PLP is involved in the biosynthesis of most other neurotransmitters that may also contribute to the effect. It should, however, be noted that neurological symptoms caused by pyridoxal kinase deficiency or inhibition respond to vitamin B6 supplementation and that neuropathy caused by vitamin B6 intoxication does not affect all peripheral nerves to the same extent (Hadtstein and Vrolijk, 2021).
The Panel notes that some hypotheses have been put forward with respect to potential mechanisms by which high vitamin B6 intakes could lead to peripheral neuropathy, but the causal mechanisms are still unknown.
3.5.1.4. Conclusions on the evidence on the relationship between high vitamin B6 intakes and peripheral neuropathy
The Panel concludes that there is evidence from human studies that peripheral neuropathy may occur at supplemental vitamin B6 intakes of 50 mg/day in some individuals.
A subchronic study in Beagle dogs showed that supplemental vitamin B6 intakes of 50 mg/kg bw per day for around 107 days leads to limited damage in the dorsal sensory nerve roots without the occurrence of symptoms of neurological disease and provides evidence for a LOAEL of 50 mg/kg bw per day.
Studies in Beagle dogs have shown that high vitamin B6 intake damages afferent nerves mostly through axon degeneration, myelin breakdown and vacuolisation of cytoplasm, with initial lesions mostly occurring in the dorsal root ganglia.
Several proposed mechanisms have been put forward by which vitamin B6 may cause neurotoxicity, but the causal mechanisms are still unknown.
3.5.2. Developmental toxicity
In its evaluation of the UL for vitamin B6, the SCF (2000) indicated that there was a lack of data on the neuronal toxicity of excess vitamin B6 intakes during development of the nervous system.
The IOM (1998) noted isolated, uncontrolled case reports of congenital defects (Donaldson and Bury, 1982; Gardner et al., 1985; Philpot et al., 1995) and B6 dependency (Hunt et al., 1954) in newborn infants whose mothers were treated with vitamin B6 during the first half of pregnancy. In the study by Donaldson and Bury (1982), women used a supplement during pregnancy that contained 10 mg vitamin B6, but the daily exposure is unclear. In the study by Gardner et al. (1985), vitamin B6 was supplemented in amounts of 50 mg/day for the first 7 months of pregnancy. Hunt Jr. et al. (1954) administered 50 mg vitamin B6 intramuscularly 3–4 times per week during the second to fifth months of pregnancy. In the publication by Philpot et al. (1995), the exposure is not specified. Observational data in women taking up to 200 mg/day vitamin B6 orally (Weinstein et al., 1944; Ellis, 1987) during the first trimester of pregnancy suggested no adverse effects.
The SCF (2000) and IOM (1998) noted that studies in rats and monkeys (Schumacher et al., 1965; Khera, 1975; Hendrickx et al., 1985) had shown no evidence of teratogenicity.
In the following, the evidence retrieved through the systematic review is described. The evidence table is in Appendix D.2.
3.5.2.1. Congenital anomalies
Intervention study
One eligible intervention study was retrieved through the systematic search that assessed the effect of vitamin B6 supplementation on congenital anomalies (Shahraki et al., 2016).
Shahraki et al. (2016) conducted a randomised controlled trial (RCT) comparing the effect of ondansetron (n = 88), a serotonin 5‐HT3 receptor antagonist, with PN‐HCl supplementation (40 mg/day vitamin B6, n = 100) in pregnant women suffering from nausea and vomiting. Supplementation was initiated between 4 and 16 weeks of gestation and women were followed‐up until birth. No congenital anomalies were observed. The Panel notes that this study does not show an effect of vitamin B6 supplementation, initiated between 4 and 16 weeks of gestation, in amounts of 40 mg/day on congenital anomalies.
Observational studies on heart malformations
Prospective cohort study
Shrim et al. (2006) recruited 96 pregnant women with a vitamin B6 intake of > 50 mg/day during the first trimester of pregnancy and a control group (n = 96) with usual vitamin B6 intake. The mean (±SD) duration of supplementation was 9 ± 4.2 weeks with a mean dose of 132.3 ± 74 mg/day (range 50–510 mg/day). There was one major heart malformation (not further specified) in the supplement group and none in the control group. The Panel notes that the incidence of congenital heart defects in the general population lies between 0.4% and 5% of all live births (Hoffman and Kaplan, 2002; Marelli et al., 2007) and that the incidence in the study by Shrim et al. (2006) falls within this background risk, which does not allow the case observed in this study to be attributed to vitamin B6 supplementation.
Case–control studies
Zhang et al. (2021) included 396 newborn infants with non‐syndromic coronary heart defects and their mothers and 792 controls after propensity score matching to account for the imbalances in covariates between cases and controls. Dietary intake of vitamin B6, including supplements, during pregnancy was assessed by a semi‐quantitative food frequency questionnaire (FFQ). It was adjusted in the analysis for total energy intake using the residual method. There was a non‐significantly increased risk of neonatal coronary heart defects in the third tertile of total vitamin B6 intake as compared with the first tertile (median 3.1 vs 1.7 mg/day; adjusted odds ratio (aOR) 1.16 (95% confidence interval (CI) 0.83, 1.62), adjusted for total intake of other studied nutrients). Vitamin B6 intake from diet only was not associated with coronary heart defects. The Panel notes that total vitamin B6 intake in this study was within the intake range that can be expected from a habitual diet and that it is unlikely that the cases observed in the study can be attributed to the vitamin B6 intake of pregnant women, in particular as studies investigating higher vitamin B6 intakes have not observed an association.
Shaw et al. (2014) investigated the association between vitamin B6 status at mid‐pregnancy and increased risk for cono‐truncal heart defects ascertained through medical records and confirmed with instrumental checks in 420 mother‐infant pairs (140 cases, 280 controls). When using plasma PLP concentrations as marker of exposure, there was a non‐significant lower risk of developing cono‐truncal heart defects associated with the first (< 31.16 nmol/L) or the third (≥ 80.01 nmol/L) tertile compared with the second (31.16–80 nmol/L), i.e. OR (95% CI) 0.7 (0.4–1.2) and 0.7 (0.4–1.3), respectively (linear trend: p = 0.93). Using plasma PL concentrations, there was a non‐significant lower risk of developing cono‐truncal heart defects associated with the first tertile (< 16.86 nmol/L) compared with the second (16.86–49.56 nmol/L), i.e. OR (95% CI) 0.7 (0.4–1.3) and no association when comparing the third (≥ 49.57 nmol/L) with the second, i.e. OR (95% CI) 1.0 (0.6–1.7) (linear trend: p = 0.28). For plasma PA concentrations, there was a non‐significant lower risk of developing cono‐truncal heart defects associated with the first (< 14.37 nmol/L) or the third tertile (≥ 47.43 nmol/L) compared with the second (14.37–47.42 nmol/L), i.e. OR (95% CI) 0.6 (0.3–1.1) and 0.9 (0.5–1.5), respectively (linear trend: p = 0.29). These analyses were adjusted for maternal race and maternal age. The Panel notes that this study does not show a positive association between markers of vitamin B6 exposure and cono‐truncal heart defects.
Observational studies on other malformations
Case–control studies
Czeizel et al. (2004) used data from the Hungarian Case–Control Surveillance of Congenital Abnormalities study. Cases of congenital abnormalities were retrieved from the Hungarian Congenital Abnormality Register, while matched controls were drawn from the National Birth Registry. A total number of 22,843 cases and 38,151 controls were identified. Vitamin B6 supplements during pregnancy were consumed by 2,013 mothers of cases and 4,086 mothers of controls. Supplements were taken mostly during the second and third months of pregnancy at a dose of 60 mg/day because of nausea and vomiting during pregnancy. They were consumed for a mean duration of 2.5 months in the case group and 2.8 months in the control group. The likelihood of having taken vitamin B6 supplements during pregnancy was lower in cases than controls (aOR 0.8 (95% CI 0.7, 0.9). This also applied when supplementation during the first month and supplementation during the second and third month were considered separately. None of the 24 individual congenital abnormalities were significantly associated with a higher likelihood of taking vitamin B6 supplements. The Panel notes that this study does not show an association between the consumption of vitamin B6 supplements at a dose of 60 mg/day during the first 3 months of pregnancy and congenital abnormalities.
Goodman et al. (2019) matched cases of fetal gastroschisis at 24 gestational weeks (n = 30) to controls (n = 76) by race and maternal age. When adjusting for insurance, education, low body mass index (BMI) and nulliparity, this risk of gastroschisis in the fetus was non‐significantly lower in mothers with a serum vitamin B6 (compound unspecified) concentration ≥ 5 μg/L at the second trimester of pregnancy compared with < 5 μg/L (OR 0.55 (95% CI 0.04, 9.1)). In the unadjusted analysis, the risk estimates were inverted (OR 1.23 (0.18, 14.29)). The Panel notes that this study does not show a positive association between markers of vitamin B6 exposure and fetal gastroschisis.
The outcome of the studies reporting on congenital anomalies is summarised in Table 7 .
Table 7.
Summary of outcome of studies on congenital anomalies
| Reference | Study design | Exposure | Time of exposure | Compound | Unit | Exposure high | Exposure low | N exp. high | N exp. low | Outcome | OR (95% CI) | Cases exp. high | Cases exp. low | Adjusted |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Shahraki et al. (2016) | RCT | Supple‐mentation | From 4–16 gw onwards | Pyridoxine HCl | mg/day | 40 | Ondansetron | 100 | 88 | Congenital anomalies | NA | 0 | 0 | No |
| Shrim et al. (2006) | PC | Supple‐mentation | During 1st trimester | Pyridoxine HCl | mg/day | 50–150 | NR | 96 | 96 | Major heart malformation | NA | 1 | 0 | No |
| Zhang et al. (2021) | CC | Intake | During pregnancy | Dietary intake | mg/day | 3.1 | 1.7 | 396 | 396 | Non‐syndromic coronary heart defects | 1.16 (0.83, 1.62) | Yes | ||
| Shaw et al. (2014) | CC | Plasma | Drawn at mid pregnancy | PLP | nmol/L | ≥ 80.01 | 31.16–80 | 138 | 138 | Cono‐truncal heart defects | 0.7 (0.4, 1.3) | 33 | 78 | Yes |
| Shaw et al. (2014) | CC | Plasma | Drawn at mid pregnancy | Pyridoxal | nmol/L | ≥ 49.57 | 16.86–49.56 | 138 | 138 | Cono‐truncal heart defects | 1 (0.6, 1.7) | 40 | 71 | Yes |
| Shaw et al. (2014) | CC | Plasma | Drawn at mid pregnancy | 4‐PA | nmol/L | ≥ 47.43 | 14.37–47.42 | 138 | 138 | Cono‐truncal heart defects | 0.9 (0.5, 1.5) | 38 | 77 | Yes |
| Czeizel et al. (2004) | CC | Supple‐mentation | During 2nd and 3rd trimester | NR | mg/day | 60 | NR | 22843 (cases) | 38151 (controls) | Likelihood of consumption of vit B6 suppl. in cases of congenital anomalies compared with controls | 0.8 (0.7, 0.9) | 2013 (suppl. use in cases) | 4086 (suppl. use in controls) | Yes |
| Goodman et al. (2019) | CC | Serum | Drawn during the 2nd trimester | vitamin B6 (generic) | μg/L | ≥ 5 | < 5 | 84 | 8 | Fetal gastroschisis at 24 gw | 0.55 (0.04, 9.1) | 24 | 2 | Yes |
CC: case–control study; CI: confidence interval; exp.: exposure; gw: gestational weeks; HCl: hydrochloride; NA: not available; NR: not reported; OR: odds ratio; PA: pyridoxic acid; PC: prospective cohort study; PLP: pyridoxal phosphate; RCT: randomised controlled trial; suppl.: supplement.
The Panel notes that in one prospective cohort study (Shrim et al., 2006) in which 96 pregnant women consumed 50–150 mg/day vitamin B6 as PN‐HCl during the first trimester of pregnancy, one major heart malformation occurred in the cohort consuming vitamin B6 while none occurred in the cohort who followed the habitual diet. The Panel notes that the incidence of congenital heart defects in the general population lies between 0.4 and 5% of all live births (Hoffman and Kaplan, 2002; Marelli et al., 2007) and that the incidence in the study by Shrim et al. (2006) falls within this background risk, which does not allow the case observed in this study to be attributed to vitamin B6 supplementation. In a case–control study (Zhang et al., 2021), there was a non‐significant increase in the risk of non‐syndromic coronary heart defects in infants of women who had consumed 3.1 mg/day vitamin B6 during pregnancy compared with those who had consumed 1.7 mg/day (aOR 1.16 (95% CI 0.83, 1.62)). The Panel notes that the vitamin B6 intake in this study was within the range that is achieved by habitual diets, and it is unlikely that cases in this study can be attributed to the vitamin B6 intake of pregnant women, in particular as studies investigating higher vitamin B6 intakes have not observed an association. No congenital malformations occurred in an RCT in which pregnant women consumed 40 mg/day vitamin B6 as PN‐HCl or ondansetron from 4–16 gestational weeks onwards (Shahraki et al., 2016). Three case–control studies (Czeizel et al., 2004; Shaw et al., 2014; Goodman et al., 2019), among those one that included around 23,000 cases of congenital anomalies of all kind and 38,000 controls (Czeizel et al., 2004), did not show an increased risk of congenital malformations following vitamin B6 intakes of up to 60 mg/day.
The Panel considers that the available evidence from both intervention and observational studies does not suggest a positive relationship between vitamin B6 intake and congenital anomalies in humans over the range of exposure investigated.
3.5.2.2. Growth restriction in utero
With respect to growth restriction in utero, two prospective observational studies (Furness et al., 2013; Chen et al., 2015) as well as two case–control studies (Baker et al., 1977; Salcedo‐Bellido et al., 2017) were retrieved through the systematic search. The outcomes that were investigated in the studies were intrauterine growth restriction (IUGR) (Furness et al., 2013), the risk of an infant being born as small‐for‐gestational age (SGA) (Chen et al., 2015) and the risk of a term infant being born with a low birth weight between 1,500 and < 2,500 g (Baker et al., 1977).
IUGR was defined in the pertinent study as serial tapering of growth in abdominal circumference and of estimated fetal weight below the 10th percentile for their gestational age (Furness et al., 2013). SGA infants were defined as infants who were born with a weight that was below the 10th percentile for their gestational age (Chen et al., 2015) or were not further defined (Salcedo‐Bellido et al., 2017). However, as the definition used by Chen et al. (2015) is the most widely used definition (Schlaudecker et al., 2017), it is likely that it also applies to the study described by Salcedo‐Bellido et al. (2017). Even though IUGR and SGA are often considered as interchangeable, being born SGA is not necessarily a result of a pathological growth restriction but may reflect a genetic predisposition of the infant to be constitutionally light weight.
In the following, the studies are described.
Prospective observational studies
Furness et al. (2013) followed 137 pregnant women recruited from a high‐risk pregnancy clinic and from a routine antenatal clinic in whom aspartate aminotransferase activity coefficient, a measure of vitamin B6 status, was analysed in RBCs at gestational weeks 18–22. The aspartate aminotransferase activity coefficient was somewhat higher in the group in whom IUGR developed (n = 21) compared with normal pregnancy group (n = 63; mean (unadjusted), 95% CI; 50%, 45–55% vs 41.9%, 38–46%)), indicating a lower vitamin B6 status in the group with IUGR. The Panel notes that this study does not show a positive association between markers of vitamin B6 exposure and the risk of developing IUGR.
Using data from the Growing Up in Singapore Towards Healthy Outcomes (GUSTO) study, Chen et al. (2015) investigated the relationship between plasma vitamin B6 status at gestational weeks 26–28 (compound measured unspecified) and the risk of the infant being born SGA in 968 mother‐infant pairs. There was no association between log‐transformed maternal vitamin B6 status and the risk of being born SGA (aOR per one SD higher log vitamin B6 plasma concentration 0.98 (95% CI 0.79, 1.22)). The analyses were adjusted for infant sex, ethnicity, maternal age, gravidity, maternal height, pre‐pregnancy BMI, weight gain until 26 weeks, educational level and gestational diabetes mellitus. Median (interquartile range; IQR) plasma vitamin B6 concentrations at 26–28 gestational weeks were 61.8 (25.9–113) nmol/L. The Panel notes that this study does not show a positive association between markers of vitamin B6 exposure and the risk of being born SGA.
Case–control studies
A matched case–control study was conducted by Salcedo‐Bellido et al. (2017) in 518 mothers of infants born SGA and 518 controls. Vitamin B6 intake during pregnancy was estimated by a FFQ. Mothers in the highest quintile of vitamin B6 intake (> 2.9 mg/day) had a lower risk of giving birth to an infant who was SGA compared with the first quintile (< 1.9 mg/day) (aOR (95% CI 0.69 (0.43, 1.08), but this did not reach statistical significance. The analysis was adjusted for energy intake, preeclampsia, education level, pre‐pregnancy BMI, smoking, weight gain per week during pregnancy, and previous preterm/low‐birth‐weight newborn infant. The Panel notes that this study does not show a positive association between vitamin B6 intake and the risk if being born SGA.
Baker et al. (1977) reported that, in a matched case–control study, neither whole blood vitamin B6 concentrations (compound not reported) of the mother 30 min post‐partum nor cord blood concentrations were related to low birth weight for term deliveries. The study included 50 mother‐infant pairs with normal birth weight infants and the same number of mother‐infant pairs with low birth weight of the infant (1,500–2,500 g). Whole blood vitamin B6 concentrations were 0.5 ng/mL (CI not available) higher in mothers of infants with a low birthweight and in cord blood it was 0.4 ng/mL. The difference was not statistically significant. The Panel notes that even though a higher vitamin B6 exposure in this study was associated with low‐birth weight of infants, this association was not statistically significant.
The Panel notes that only one case–control study in 50 cases and 50 controls (Baker et al., 1977) reports a non‐statistically significant higher vitamin B6 exposure to be associated with low‐birth weight of infants, while the other three studies (Furness et al., 2013; Chen et al., 2015; Salcedo‐Bellido et al., 2017) with larger sample sizes, two of which were prospective cohort studies, did not show an association between vitamin B6 exposure and growth restriction in utero.
The Panel considers that the available evidence from observational studies does not suggest a positive relationship between vitamin B6 intake and growth restriction in utero in humans over the range of exposure investigated.
3.5.2.3. Birth weight and length
Birth weight and length could be used as surrogate markers of impaired fetal growth if biologically relevant lower birth weights are consistently associated with higher vitamin B6 intakes.
Two RCTs (Schuster et al., 1984; Shahraki et al., 2016), five prospective cohort studies (de Weerd et al., 2003; Lagiou et al., 2005; Shrim et al., 2006; Chen et al., 2015; McCullough et al., 2016) and one cross‐sectional study (Chang, 1999) investigated the relationship between vitamin B6 exposure and birth weight and birth length and were initially considered pertinent. During the assessment, the biologically implausible range of PLP concentrations reported in the publication by McCullough et al. (2016) was noted and this study was subsequently excluded from the evaluation.
The remaining seven studies are described in the following.
Intervention studies
Shahraki et al. (2016), described in Section 3.5.2.1, conducted a RCT comparing the effect of ondansetron (n = 88), a serotonin 5‐HT3 receptor antagonist, vs PN‐HCl supplementation (40 mg/day vitamin B6, n = 100) in pregnant women suffering from nausea and vomiting. Supplementation was initiated between 4 and 16 weeks of gestation and women were followed‐up until birth. There were no differences in birth weight (2,950 ± 457 g (SD, vitamin B6 group) vs 3,007 ± 442 g (ondansetron group)) and birth length (49.5 ± 1.5 cm vs 49.0 ± 1.5 cm) between groups. The Panel notes that this study does not show an effect of vitamin B6 supplementation, initiated between 4 and 16 weeks of gestation, in amounts of 40 mg/day on birth weight and birth length.
In the RCT by Schuster et al. (1984), 196 pregnant women were supplemented with 0, 2.1, 4.1, 6.2, 8.2, 12.3 or 16.5 mg pyridoxine equivalents (equivalent to 0, 2.6, 5, 7.5, 10, 15 or 20 mg PN‐HCl), starting between gestational weeks 6 and 21. Only 46 women at 30 weeks of gestation and 22 individuals at birth fully complied with the protocol and were included in the analysis. There were no differences in birth weight (3,287 g ± 429 (SD) vs 3,240 ± 505 g) and birth length (50.7 ± 2.1 cm vs 50.9 ± 2.7 cm) between the groups receiving 7.5–20 mg/day compared with the groups getting 0–5 mg/day. The Panel notes the high non‐compliance rate in this study and that this study did not show an effect of vitamin B6 supplementation, initiated between 6 and 21 weeks of gestation in amounts of up to 16.5 mg/day, on birth weight and birth length.
The Panel considers that the available evidence from RCTs does not suggest an inverse relationship between vitamin B6 intake and birth weight in humans over the range of exposure investigated.
Observational studies
Prospective observational studies.
Chen et al. (2015) (GUSTO study), described in Section 3.5.2.2, reported no significant association between log‐transformed maternal vitamin B6 status at gestational weeks 26–28 and birth weight (adjusted mean per one SD higher log vitamin B6 plasma concentration (95% CI) ‐3.9 (−30.7, 22.9) g) and birth length (−0.08 (−0.21, 0.05) cm). The analyses were adjusted for infant sex, ethnicity, maternal age, gravidity, maternal height, pre‐pregnancy BMI, weight gain until 26 weeks, educational level and gestational diabetes mellitus. The Panel notes that this study does not show an inverse association between markers of vitamin B6 exposure and birth weight and birth length.
Shrim et al. (2006), described in Section 3.5.1.1, recruited 96 pregnant women with a vitamin B6 intake of > 50 mg/day during the first trimester of pregnancy and a control group (n = 96) with usual vitamin B6 intake. The mean (SD) duration of supplementation was 9 ± 4 weeks with a mean dose of 132.3 ± 74 mg/day (range 50–510 mg/day). Birth weight was higher in the group consuming vitamin B6 compared with the group not consuming it (3,542 ± 512 g vs 3,321 ± 562 g, p = 0.01, unadjusted). The Panel notes that this study shows a positive, rather than a negative association, between vitamin B6 intake and birth weight in an unadjusted analysis.
Lagiou et al. (2005) investigated the relationship between dietary vitamin B6 intake during the first 27 gestational weeks and birth weight and birth length in 222 mother‐infant pairs. There were no significant differences in birth weight and birth length (adjusted mean differences per SD higher vitamin B6 intake 2.7 g (95% CI –54.4, 59.7) and −0.04 cm (−0.32, 0.25), respectively). The analyses were adjusted for age, education, parity, height, pre‐pregnancy BMI, oral contraceptives prior to index pregnancy, smoking, gender of offspring, exact gestational age at delivery and total energy intake. Median (IQR) vitamin B6 intake from diet and supplements, assessed with a semi‐quantitative FFQ, was 4.1 (95% CI 2.6–5.9) mg/day. The Panel notes that this study does not show an inverse association between vitamin B6 intake and birth weight.
de Weerd et al. (2003) found no association between whole blood PLP concentrations in 240 women measured at 6 and 10 gestational weeks and birth weight (results not reported). Median whole blood PLP concentrations at 6 gestational weeks (n = 188) were 47 (IQR 40–56) nmol/L. The Panel notes that this study does not show an inverse association between markers of vitamin B6 exposure and birth weight and birth length.
Cross sectional studies
Chang (1999) investigated 209 newborn infants whose mothers had been supplemented with 0, 1, 2 or 3 mg/day PN‐HCl during pregnancy. The habitual diet, as estimated from a FFQ, provided around 1 mg/day vitamin B6. Infants in the highest group of cord blood PLP (≥ 50 nmol/L) were heavier than infants in the lowest group (< 40 nmol/L) (mean ± SD 3,400 ± 300 g vs. 3,000 ± 300 g, p < 0.001) and also longer (50.7 ± 2.4 vs. 48.7 ± 1.6), even though the latter difference was not statistically significant. All results were unadjusted. Supplementation was not associated with birth weight and birth length (numerical results not reported in the publication). The Panel notes that this study shows a positive, rather than a negative association, between markers of vitamin B6 exposure and birth weight in an unadjusted analysis.
A summary of the outcome of the studies on birth weight in which exposure was used as categorical variable are given in Table 8 and those in which exposure was used as a continuous variable in Table 9. Those on birth length are given in Tables 10 and 11, respectively. 95% CI were calculated by EFSA when not given in the publication to ease comparability of study results.
Table 8.
Summary of the outcome of studies on birth weight in which exposure was used as categorical variable
| Reference | Study design | Exposure | Timing of exposure/assessment | Com‐pound | Unit | Exposure high | Exposure low | n expos. high | n expos. low | n total | Mean difference (g) (95% CI) | Adjusted |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Shahraki et al. (2016) | RCT | Supple‐mentation | From 4 to 16 gw onwards | Pyridoxine HCl | mg/day | 40 | Ondan‐setron | 100 | 88 | 188 | −57 (−187, 73) | No |
| Schuster et al. (1984) | RCT | Supple‐mentation | From 6 to 21 gw onwards | Pyridoxine HCl | mg/day | 7.5–20 | 0–5 | 112 | 84 | 196 | 47 (−85, 179) | No |
| Shrim et al. (2006) | PC | Supple‐mentation | During 1st trimester | Pyridoxine HCl | mg/day | 50–150 | NR | 96 | 96 | 192 | 221 (68, 374) | No |
| de Weerd et al. (2003) | PC | Whole blood | Drawn at 6 gw | PLP | nmol/L | NR | NR | NR | NR | 240 | NS | NR |
| Chang (1999)(a) | CS | Cord blood | Birth | PLP | nmol/L | ≥ 50 | < 40 | NR | NR | 209 | 400 (299, 502) | No |
CI: confidence interval; CS: cross‐sectional study; expos.: exposure; gw: gestational weeks; HCl: hydrochloride; NR: not reported; NS: not significant; PC: prospective cohort study; PLP: pyridoxal phosphate; RCT: randomised controlled trial.
Table 9.
Summary of the outcome of studies on birth weight in which exposure was used as continuous variable
| Reference | Study design | Exposure | Timing of exposure/assessment | Compound | Unit | Exposure ‐ study median (IQR) | n Total | Unit | Mean difference (95% CI) | Adjusted |
|---|---|---|---|---|---|---|---|---|---|---|
| Chen et al. (2015) | PC | Plasma | Drawn at 26–28 gw | Vitamin B6 (generic) | nmol/L | 61.8 (25.9–113) | 968 | g per one SD higher log vitamin B6 plasma concentration | −3.9 (−30.7, 22.9) | Yes |
| Lagiou et al. (2005) | PC | Intake | During first 27 gw | Dietary intake | mg/day | 4.1 (2.6–5.9) | 222 | g per one SD higher vitamin B6 intake | 2.7 (−54.4, 59.7) | Yes |
CI: confidence interval; gw: gestational weeks; IQR: interquartile range; PC: prospective cohort study; SD: standard deviation.
Table 10.
Summary of the outcome of studies on birth length in which exposure was used as categorical variable
| Reference | Study design | Exposure | Timing of exposure/assessment | Com‐pound | Unit | Exposure high | Exposure low | n expos. high | n expos. low | n total | Mean difference (cm) (95% CI) | Ad‐justed |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Shahraki et al. (2016) | RCT | Supple‐mentation | From 4–16 gw onwards | Pyridoxine HCl | mg/day | 40 | Ondan‐setron | 100 | 88 | 188 | 0.5 (0.07, 0.93) | No |
| Schuster et al. (1984) | RCT | Supple‐mentation | From 6–21 gw onwards | Pyridoxine HCl | mg/day | 7.5–20 | 0–5 | 112 | 84 | 196 | −0.2 (−0.88, 0.48) | No |
| Chang (1999) | CS | Cord blood | Birth | PLP | nmol/L | ≥ 50 | < 40 | NR | NR | 209 | 2 (1.3, 2.7) | No |
CI: confidence interval; CS: cross‐sectional study; expos.: exposure; gw: gestational weeks; HCl: hydrochloride; NR: not reported; PLP: pyridoxal phosphate; RCT: randomised controlled trial.
Table 11.
Summary of the outcome of studies on birth length in which exposure was used as continuous variable
| Reference | Study design | Exposure | Timing of exposure/ assessment | Compound | Unit | Exposure study median (IQR) | Total n | Unit | Mean difference (95% CI) | Ad‐justed |
|---|---|---|---|---|---|---|---|---|---|---|
| Chen et al. (2015) | PC | Plasma | Drawn at 26–28 gw | Vitamin B6 (generic) | nmol/L | 61.8 (25.9–113) | 968 | cm per one SD higher log vitamin B6 plasma concentration | −0.08 (−0.21, 0.05) | Yes |
| Lagiou et al. (2005) | PC | Intake | During first 27 gw | Dietary intake | mg/day | 4.1 (2.6–5.9) | 222 | cm per one SD higher vitamin B6 intake | −0.04 (−0.32, 0.25) | Yes |
CI: confidence interval; gw: gestational weeks; IQR: interquartile range; PC: prospective cohort study; SD: standard deviation.
The Panel notes that one prospective cohort study (Shrim et al., 2006) and one cross‐sectional study (Chang, 1999) found, in unadjusted analyses, that higher vitamin B6 exposure was associated with a higher birth weight. The cross‐sectional study by Chang (1999) also found that infants with higher vitamin B6 exposure were not only heavier but also longer, while Shrim et al. (2006) did not report on birth length. Two RCTs (Schuster et al., 1984; Shahraki et al., 2016) and three prospective cohort studies (de Weerd et al., 2003; Lagiou et al., 2005; Chen et al., 2015) did not observe vitamin B6 intake or markers of vitamin B6 exposure to be associated with birth weight or birth length, with exposures up to 40 mg/day consumed from 4–16 gestational weeks onwards in one of the RCTs (Shahraki et al., 2016).
The Panel considers that the available evidence from observational studies does not suggest an inverse relationship between vitamin B6 intake and birth weight and birth length in humans over the range of exposures investigated.
3.5.2.4. Developmental toxicity in animals
Four studies in rats and two studies in pigs were retrieved through the systematic search and are described in the following.
Marathe and Thomas (1986) studied four groups of 12 female Wistar rats each. They were given normal feed or 100, 400 or 800 mg/kg bw per day vitamin B6 in distilled water from days 6–15 of gestation (period of organogenesis) via water. On day 20 of gestation, the rats were sacrificed. The total number of live pups and their weights were comparable between groups. There were no visceral or skeletal malformations.
In another study on female Wister rats (Khera, 1975), rats were fed 0, 20, 40, 60 or 80 mg/kg bw per day vitamin B6 as PN‐HCl in water from days 6–15 of gestation until day 22 when they were sacrificed. The number of pregnant rats per dose group ranged between 16 and 19. fetal weight, the number of live and dead foetuses and resorption sites were similar between groups.
The studies by Alton‐Mackey and Walker (1973) and Alton‐Mackey and Walker (1978) were designed to study the effect of vitamin B6 deficiency on developmental outcomes, but also included groups of female Wistar rats (n not reported), which were fed 100 or 400% of their vitamin B6 requirement from mating until delivery. There was no difference in litter weight (Alton‐Mackey and Walker, 1973, 1978) and litter size (Alton‐Mackey and Walker, 1973) between groups.
Two studies in pigs (Ritchie et al., 1960; Dalto et al., 2016) also did not show an effect of vitamin B6 supplementation on litter weight (Ritchie et al., 1960; Dalto et al., 2016) and size (Ritchie et al., 1960). In the study by Ritchie et al. (1960), pregnant sows (n = 18) and gilts (n = 24) had received either a control diet with a vitamin B6 content that covered requirements (~ 0.9 mg/kg feed) or a diet that contained around 10 mg/kg feed. Sows received 5 kg and gilts 2.5 kg of the feed. The study by Dalto et al. (2016) in gilts had a factorial design in which both the effect of selenium and vitamin B6 on litter development were tested. The control diet had contained 0.6 mg/kg feed selenium and 2.4 mg/kg vitamin B6, while the diet high in vitamin B6 had provided 0.6 mg/kg feed selenium and 12.4 mg/kg vitamin B6. The gilts had been fed with 2.8 kg/day of feed from before pregnancy to day 30 after insemination.
The Panel notes that the available evidence from animal studies does not suggest a relationship between high vitamin B6 intakes and developmental toxicity.
3.5.2.5. Conclusions on the evidence on the relationship between high vitamin B6 intakes and developmental toxicity
Overall, the Panel concludes that the available evidence does not suggest a relationship between high vitamin B6 intakes and outcomes indicative of developmental toxicity.
3.5.3. Other adverse health effects
Other adverse health effects that have been reported to be associated with high vitamin B6 intakes have been reviewed and summarised by the Tetens et al. (2023) as described in the following.
3.5.3.1. Photosensitisation and dermatological lesions
Case reports on subepidermal vesicular dermatosis and photosensitive dermatitis, caused by high vitamin B6 intake (2 g and 75 mg PN daily, respectively) have been published (Friedman et al., 1986; Murata et al., 1998). The conditions presented may be induced by the ability of vitamin B6 vitamers to act as photosensitisers, which can cause photooxidative damage upon irradiation as shown in in vitro studies (Sato et al., 1993; Maeda et al., 2000; Wondrak et al., 2004; Justiniano et al., 2017). The photosensitisation ability of vitamin B6 vitamers is assumed to be due to the 3‐hydroxypyridine structure shared among all the vitamers (Wondrak and Jacobson, 2012) and may depend on vitamer and type of irradiation (Wang et al., 2015).
3.5.3.2. Risk of hip fracture
An association between high vitamin B6 intake and hip fracture was found in a secondary pooled analysis from two Norwegian RCTs, originally investigating the effect of B‐vitamins on cardiovascular disease. For the secondary analysis, trial participant data were linked to a national database for hip fractures. For individuals consuming vitamin B6 (40 mg PN daily) with or without other B vitamins (folic acid and vitamin B12), compared to groups not consuming vitamin B6, the risk of hip fractures was non‐statistically significantly increased (during the trial period (median follow‐up 3.3 years, hazard ratio (HR) 1.42 (95% CI 0.78, 2.61)) and significantly increased when considering also the extended follow‐up period after the end of the trial period (median follow up 11.1 years; HR 1.42 (95% CI 1.09, 1.83)) (Lopez et al., 2017). Similar findings were obtained in an analysis of data from the Nurses' Health Study (US), a prospective cohort study in 61,445 women. A significantly increased risk of hip fracture was observed among women with vitamin B6 intakes ≥ 35 mg/day compared with women with vitamin B6 intake < 2 mg/day (adjusted risk ratio (aRR) 1.29 (95% CI 1.03, 1.59), however there was a lack of dose–response relationship when only B6 supplement usage was tested (Meyer et al., 2019). An analysis on data from the prospective Singapore Chinese Health Study in 63,257 participants (Dai et al., 2013) showed a non‐statistically significant increased risk of hip fractures between the fourth quartile of vitamin B6 intakes (0.78–1.7 mg/1,000 kcal/day) and the first quartile (0.36–0.61 mg/1,000 kcal/day), i.e. adjusted hazard ratio (aHR) 1.29 (95% CI 0.99, 1.68), although the vitamin B6 intakes in quartile four were still within the range of intake that can be achieved through the habitual diet. An analysis on 1,002 women of the Framingham Osteoporosis Study (McLean et al., 2008) found that the risk of hip fractures was non‐significantly increased when individuals were vitamin B6 deficient (based on plasma PLP concentrations) compared with those who had an adequate vitamin B6 status. The relationship with high PLP concentrations was not investigated. Finally, in a case–control study (Lumbers et al., 2001), the 75 women with hip fractures had significantly lower vitamin B6 intakes than the 50 controls (details on intakes not reported).
3.5.3.3. Impaired memorisation
Molimard et al. (1980) described two intervention studies. One was in 69 medical students who received 0, 100 or 500 mg/day vitamin B6 for 10 days. The improvement in performance in a digital coding test between the test done at the beginning of the study and 15 days after the end was better in the placebo group than in the 500 mg/day vitamin B6 group. There were no differences in improvement of performance between the vitamin B6 groups and placebo when comparing the results at the end of the study with those at the beginning. There was no effect on tests that related to the syllabus taught during the intervention and on two numerical application problems. The other study was a study in 30 obese patients on a low‐calorie diet who received placebo, 20 or 1,000 mg/day vitamin B6 for 15 days. Even though the improvement in performance in the digital coding test was greater in the placebo group than in the vitamin B6 groups, differences were not statistically significant. The results in a word memorisation test, a visual memorisation test and a visual retention test were not different between groups.
3.5.3.4. Anti‐lactogenic effect
An anti‐lactogenic effect or inhibition of lactation by high intakes of vitamin B6 has been systematically reviewed (Al Saad et al., 2017). The total population in the seven human studies included in the review amounted to 1,155 post‐partum women, of which 471 received vitamin B6. Three studies were RCTs, and four studies were non‐randomised controlled trials. Daily doses of 450–600 mg for 5–7 days were consumed by the participants. In two (out of the seven) included studies, lactation was suppressed in approximately 95% of the included participants following consumption of 600 mg/day for 6 days. In the remaining studies, no such effect was observed.
A study in dogs also found that PN at different intake levels (10 mg/kg bw per day and 50 mg/kg bw per day for 20 days) could suppress lactation at the highest dose (da Silva et al., 2021).
3.5.3.5. Testicular toxicity
In a systematic review, 12 studies were identified, which related to the effect of vitamin B6 on semen quality (Banihani, 2017). Eight studies were conducted in animals, and four studies were performed in humans.
Of the included human studies, one investigated monoamine oxidase in fertile and infertile men (Roberge et al., 1984) and one primarily considered other nutrients than vitamin B6 (folate and derivatives) (Forges et al., 2007). One observational human study found seminal plasma PLP, folate and cobalamin concentration to be inversely correlated with ejaculation volume (Boxmeer et al., 2009), and another observational human study showed that a specific dietary pattern consisting of high intakes of fruit, vegetables, fish and whole grains significantly correlated positively with blood PLP concentration, and inversely with sperm DNA damage (Vujkovic et al., 2009).
The animal studies showed that high doses of vitamin B6 (125–1,000 mg/kg bw per day) administered daily for 4–9 weeks, led to lower weights of epididymis, testis, prostate gland and seminal vesicle, but also resulted in changes in testicular enzymatic activity. Further, adverse effects on spermatids, spermiation, Sertoli cells and sperm motility and count were also observed.
3.5.3.6. Conclusions on the evidence on high vitamin B6 intakes and on other adverse health effects
The Panel notes that there is some evidence that vitamin B6 supplementation at a dose of 35–40 mg/day is associated with an increased risk of hip fracture. However, the evidence to date is insufficient to conclude on a causal relationship and a RP based on this observation cannot be used on its own. There is also an indication that vitamin B6 consumed at doses of 600 mg/day may suppress lactation, a level of intake that is beyond the RP originally used by the SCF (2000) to derive the UL. Evidence for a relationship of high vitamin B6 intakes with other outcomes discussed above is insufficient.
3.6. Hazard characterisation
3.6.1. Selection of the critical effect and the reference point
Experimental animal data show that vitamin B6 causes peripheral neuropathy in a dose related manner. A similar relationship has been shown in humans, and peripheral neuropathy is the critical effect for setting the UL for vitamin B6.
The SCF (2000) had identified a RP of 100 mg/day based on peripheral neuropathy and mean vitamin B6 intakes in the human case–control study by Dalton and Dalton (1987). Using the same study but a different observation, the Panel identified a lower RP than the SCF (2000), which is also supported by other available data.
In the study by Dalton and Dalton (1987) neuropathy was reported in 48% of all women having taken vitamin B6 supplements < 50 mg/day for at least 6 months (i.e. the lowest dose group). This percentage increased in a dose‐dependent manner with higher vitamin B6 intakes. Based on the study by Dalton and Dalton (1987) and supported by other case reports (Dalton, 1985; Dalton and Dalton, 1987; Blackburn and Warren, 2017) and Member States' vigilance data, a RP of 50 mg/day can be identified. Taking all evidence together, the available data indicate that symptoms may occur at daily vitamin B6 intakes of 50 mg and below with a large interindividual variability. The value of 50 mg/day represents the lowest level of vitamin B6 intake that is associated with certainty with the development of neuropathy when consumed for more than 6 months, however smaller but unconfirmed B6 doses were also linked to this adverse effect.
A LOAEL and a NOAEL cannot be identified from human studies based on the available data.
A subchronic study in Beagle dogs (Phillips et al., 1978) allows the establishment of a LOAEL of 50 mg/kg bw per day. All Beagle dogs (n = 5) consuming this dose for around 100 days showed bilateral loss of myelin in the dorsal sensory nerve roots, but no symptoms of neurotoxicity. The group of five dogs consuming 200 mg/kg bw per day, on the other hand, showed a pronounced damage to dorsal fasciculi and severe neurological impairments. No lesions were found in the control animals.
3.6.2. Derivation of the UL
Default UFs in this Section are based on the default values recommended by the EFSA Scientific Committee (2012). Default body weight are those recommended by the EFSA NDA Panel (2022).
3.6.2.1. Adults
The Panel notes that since the assessment of the SCF (2000) no additional data became available to revise the UF of 4 originally proposed by the SCF (2000) in the derivation of the UL for vitamin B6. Therefore, the Panel proposes to apply a factor of 2 to account for the fact that there is an inverse relationship between dose and time to onset of symptoms of peripheral neuropathy. This factor covers the uncertainties as to whether the duration of exposure in the study by Dalton and Dalton (1987) was sufficiently long to cover this aspect. An additional UF of 2, as already used by the SCF, is proposed to be applied to account for the limited available data. This covers the uncertainties as to the level of intake that would represent a LOAEL and the uncertainties as to whether the study by Dalton and Dalton (1987) was sufficiently representative and large to account for interindividual variability and adequately covered sensitive groups of the population. It should also be noted that the RP to which the UFs are applied is based on supplemental vitamin B6 intake (not considering the background intake from the diet). Therefore, the Panel considers that the UF of 4 that is proposed to be applied is sufficiently conservative if the UL covers both dietary and supplemental vitamin B6 intake.
Applying a UF of 4 to the RP of 50 mg/day identified from the study by Dalton and Dalton (1987), and supported by other available data, results in a UL of 12.5 mg/day for adults. There is no indication from the literature that men would be more susceptible than women. Therefore, the UL is considered to be also protective for men.
When using the LOAEL of 50 mg/kg bw per day derived from the subchronic study in Beagle dogs (Phillips et al., 1978) as a basis and applying an UF of 300, a UL of 0.17 mg/kg bw per day is derived, corresponding to 11.7 mg/day using a default body weight of 70 kg for adults. The UF of 300 is composed of the following: (a) a factor of 2 to account for interspecies variability in toxicokinetics; the default value of 4 that is suggested to be used for this purpose by the EFSA Scientific Committee (2012) has been reduced by the Panel to 2, because of the similarities in vitamin B6 excretion in terms of metabolites and total vitamin B6 between dogs and humans (Scudi et al., 1942), (b) a default factor of 2.5 for interspecies variability in toxicodynamics, (c) a default factor of 10 to account for intra‐human variability in toxicokinetics and toxicodynamics (default factors of 3.16 each), (d) a default factor of 2 to extrapolate from subchronic to chronic exposure, even though this default factor had been established for studies in rodents and (e) a factor of 3 which was considered sufficient by the Panel to account for the absence of a NOAEL.
The Panel notes that the derivation of the UL from the study in humans leads to a similar UL as when the study in dogs is used as a basis. This increases the confidence in the resulting UL. The Panel proposes to establish the UL at the midpoint of these two ULs and round down.
Therefore, based on the evidence in both humans and dogs, the Panel derives an UL of 12 mg/day for adults. The UL includes dietary and supplemental intake and covers all vitamin B6 vitamers.
3.6.2.2. Pregnant and lactating women
There is no indication for a specific risk or increased susceptibility to adverse effects of excessive vitamin B6 intake during pregnancy or lactation. A potential anti‐lactogenic effect has been observed at intakes much higher than the UL (Section 3.5.3.4).
In line with what was previously proposed by the SCF (2000), the Panel considers that the UL for adults also applies during pregnancy and lactation.
3.6.2.3. Children and adolescents
There are no data to support a derivation of a UL for children. On the other hand, there is no indication from the literature that children may be more susceptible than adults to vitamin B6 toxicity. The Panel considers that it is appropriate to extrapolate the UL from adults to children based on allometric scaling (bw0.75), as it is the preferred method to adjust for metabolic differences between age groups, using reference body weights (EFSA NDA Panel, 2022).
ULs are established for the age categories proposed by the Panel in its guidance on establishing ULs (EFSA NDA Panel, 2022). Values are rounded to the closest 0.1 mg (Table 12).
Table 12.
UL for children and adolescents aged 1–17 years
| Age range | Reference bw males and females (kg) (a) | UL males and females (mg/day) |
|---|---|---|
| 1–3 years | 11.9 | 3.2 |
| 4–6 years | 19.0 | 4.5 |
| 7–10 years | 28.7 | 6.1 |
| 11–14 years | 44.6 | 8.6 |
| 15–17 years | 60.3 | 10.7 |
Bw: body weight; UL: tolerable upper intake level.
3.6.2.4. Infants
There are no data to support a derivation of an UL for infants. On the other hand, there is no indication from the literature that infants may be more susceptible than adults to vitamin B6 toxicity. The Panel considers that it is appropriate to extrapolate the UL from adults to infants aged ≥ 4 months based on allometric scaling (bw0.75), as it is the preferred method to adjust for metabolic differences between age groups, using reference body weights (EFSA NDA Panel, 2022).
ULs are established for infants aged 4–6 months and 7–11 months, respectively. Values are rounded to the closest 0.1 mg (Table 13).
Table 13.
UL for infants aged 4 months to 11 months
| Age range | Reference bw males and females (kg) (a) | UL males and females (mg/day) |
|---|---|---|
| 4–6 months | 7.2 | 2.2 |
| 7–11 months | 8.6 | 2.5 |
Bw: body weight; UL: tolerable upper intake level.
The averages of the median weights‐for‐age for boys and girls at 5 and 9 months, respectively, were used as reference weights (WHO Multicentre Growth Reference Study Group, 2006).
3.7. Risk characterisation
The ULs apply to the general European population and relate to vitamin B6 intake from all dietary sources and all vitamin B6 vitamers.
Data on the intake of vitamin B6 from fortified foods and food supplements in European population are scarce (Section 3.4). The Panel considers that it is unlikely that the UL for vitamin B6 is exceeded in European populations, except for regular users of food supplements containing high doses of vitamin B6 (Section 3.4.3.2).
Conclusions
The following ULs are established:
| Age group | UL males and females (mg/day) |
|---|---|
| 4–6 months | 2.2 |
| 7–11 months | 2.5 |
| 1–3 years | 3.2 |
| 4–6 years | 4.5 |
| 7–10 years | 6.1 |
| 11–14 years | 8.6 |
| 15–17 years | 10.7 |
| Adults | 12 |
| Pregnant women | 12 |
| Lactating women | 12 |
UL: tolerable upper intake level.
Recommendations for research
Additional research is needed regarding potential differences in the toxicity profile of the different vitamers of vitamin B6.
Additional research on toxicokinetics and toxicodynamics could help to refine the derivation of a UF.
Further investigation of the mechanisms of vitamin B6 toxicity is needed and the identification of genetic traits that may influence individual susceptibility.
Additional research is required to investigate the impact of other factors, such as age and sex and epigenetics, on vitamin B6 neurotoxicity.
Further investigations or analysis of the existing datasets would be needed to clarify the role of high vitamin B6 intake on bone health.
Abbreviations
- 4‐PA
4‐pyridoxic acid
- ADME
absorption, distribution, metabolism and excretion
- AI
adequate intake
- ANIBES
anthropometric data, macronutrients and micronutrients intake, practice of physical activity, socioeconomic data and lifestyles in Spain
- AR
average requirement
- BMI
body mass index
- BoE
body of evidence
- bw
body weight
- CC
case–control
- CHD
congenital heart defects
- CI
confidence interval
- CIAP
chronic idiopathic axonal polyneuropathy
- CS
cross sectional
- CT
controlled trial
- CTS
carpal tunnel syndrome
- DANSDA
The Danish National Survey of Diet and Physical Activity
- DB
double blind
- DNFCS
Dutch National Food Consumption Survey
- DRV
dietary reference value
- ENALIA
Encuesta Nacional de Alimentación en la población Infantil y Adolescente
- EsKiMo
Eating study as a KiGGS Module
- EVM
Expert Group on Vitamins and Minerals
- FAO
Food and Agricultural Organization of the United Nations
- FCDB
EFSA food composition database
- FFQ
food frequency questionnaire
- FINDIET
Finnish National Dietary Survey in Adults and Elderly
- FPQ
food propensity questionnaire
- GABA
γ‐aminobutyric acid
- GNPD
Mintel Global New Products Database
- GUSTO
Growing Up in Singapore Towards Healthy Outcomes
- HCT
human controlled trial
- HNNHS
Hellenic National Nutrition and Health Survey
- HR
hazard ratio
- IAN‐AF
Inquérito Alimentar Nacional e de Atividade Física
- INCA
étude individuelle nationale des consommations alimentaires
- IOM
US Institute of Medicine
- IQR
interquartile range
- IUGR
intrauterine growth restriction
- IUPAC‐IUB CBN
International Union of Pure and Applied Chemistry–International Union of Biochemistry, Commission on biochemical nomenclature.
- KiGGS
Studie zur Gesundheit von Kindern und Jugendlichen in Deutschland
- LLD
longitudinal limb deficiency
- LOAEL
lowest‐observed‐adverse‐effect‐level
- MM
median motoneuropathy
- MRC
medical research council
- NAD
nicotinamide adenine dinucleotide
- NANS
National Adult Nutrition Survey
- NCFS
National Children's Food Survey
- NDA
Panel on Nutrition, Novel Foods and Food Allergens.
- NHMRC
Australian and New Zealand National Health and Medical Research Council
- NOAEL
no‐observed‐adverse‐effect‐level
- NPNS
National Pre‐School Nutrition Survey
- NTFS
National Teen's Food Consumption Survey
- NTP
US National Toxicology Program
- NVS
Nationale Verzehrsstudie
- OHAT
Office of Health Assessment and Translation
- OR
odds ratio
- PC
prospective cohort
- PL
pyridoxal
- PLP
pyridoxal 5′‐phosphate
- PM
pyridoxamine
- PMP
pyridoxamine 5′‐phosphate
- PMS
premenstrual symptoms
- PN
pyridoxine
- PNG
pyridoxine glucoside
- PNP
pyridoxine 5′‐phosphate
- PRI
population reference intake
- QST
Quantitative Sensory Testing
- RCT
randomised controlled trial
- RDA
recommended daily allowance
- RDI
recommended daily intake
- RoB
risk of bias
- RP
reference point
- RR
risk ratio
- SCF
Scientific Committee on Food
- SEM
standard error or the mean
- SFFQ
semiquantitative food frequency questionnaire
- SGA
small‐for‐gestational age
- SNP
single nucleotide polymorphisms
- sQ
subquestion
- TDL
trans limb deficiency
- TDS
total diet studies
- UF
uncertainty factor
- UL
tolerable upper intake level
- VKM
Vitenskapskomiteen for mat og miljø
- WHO
World Health Organization
Appendix A – Literature screening and selection – Flow charts (Moher et al., 2009)
1.
sQ3a and 3b
What is the dose–response relationship between vitamin B6 intake and the development of peripheral neuropathy in humans?
What is the relationship between biomarkers of B6 exposure and the development of peripheral neuropathy in humans? Could a dose‐response be characterised?

sQ4
Is there a positive and causal relationship between vitamin B6 intake and developmental toxicity (including congenital defects) in humans and animals?
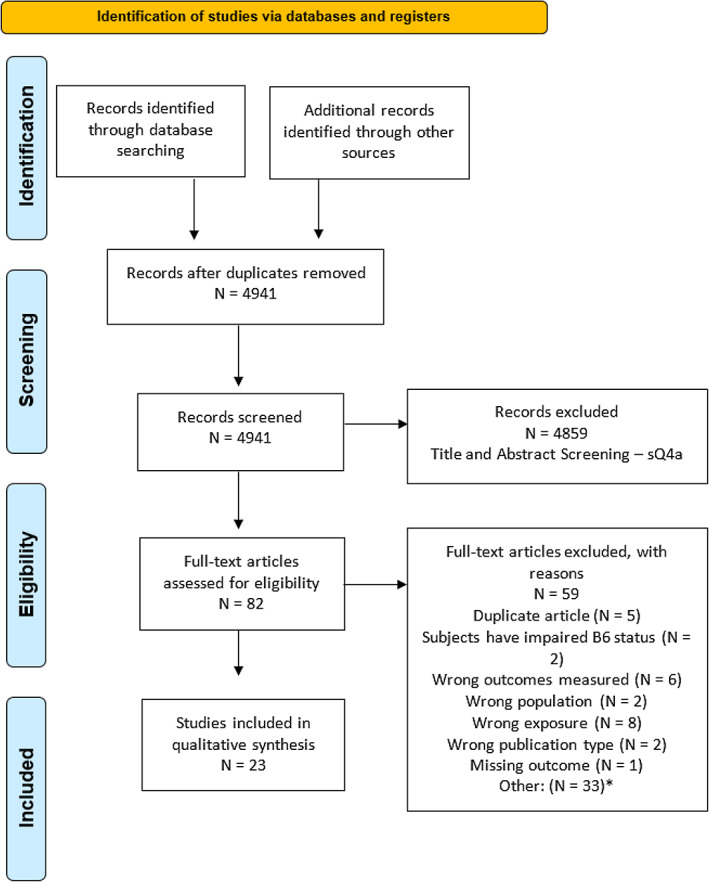
Appendix B – Risk of bias appraisal of studies investigating the relationship between vitamin B6 exposure and the development of peripheral neuropathy
Intervention study
| References | Risk of bias domains (a) | Tier (b) | ||||||
|---|---|---|---|---|---|---|---|---|
| Exposure KEY | Outcome KEY | Randomisation KEY | Allocation concealment | Blinding | Attrition | Other threats to internal validity | ||
| Berger et al. ( 1992 ) | NR | + | −− | −− | −− | − | − | 2 |
Expert judgement was translated into a rating scale for each question to be answered as follows: (++): definitely low RoB; (+): probably low RoB; (NR): not reported; (−): probably high RoB; (− −): definitively high RoB.
The individual rating for each question was combined by an algorithm and translated to an overall tier of reliability for each individual study (RoB tier 1: low RoB; RoB tier 2: moderate RoB; RoB tier 3: high RoB).
Observational studies
| References | Risk of bias domains (a) | Tier (b) | |||||
|---|---|---|---|---|---|---|---|
| Exposure KEY | Outcome KEY | Confounding KEY | Inappropriate selection | Attrition | Other sources of bias/statistics | ||
| Bacharach et al. ( 2017 ) | + | + | −− | NA | NA | − | 2 |
| Baer (1984) | NR | NR | −− | NA | NA | − | 3 |
| Berger and Schaumburg (1984) | NR | + | −− | NA | NA | − | 2 |
| Blackburn and Warren (2017) | NR | NR | −− | NA | NA | − | 3 |
| Brush et al. ( 1988 ) | − | −− | −− | −− | NR | −− | 3 |
| Chaudary et al. ( 2003 ) | − | −− | NR | NR | + | − | 3 |
| Chaudary and Cornblath (2013) | + | + | −− | NA | NA | − | 2 |
| Dalton and Dalton (1987) | + | −− | −− | NR | ++ | − | 2 |
| Dalton ( 1985 ) | + | NR | −− | NA | NA | −− | 2 |
| Falcone and Sowa (2013) | + | NR | −− | NA | NA | −− | 2 |
| Foca ( 1985 ) | + | + | −− | NA | NA | −− | 2 |
| Franzblau et al. ( 1996 ) | + | + | NR | NR | + | − | 2 |
| Friedman et al. ( 1986 ) | NR | + | −− | NA | NA | − | 2 |
| Gdynia et al. ( 2008 ) | + | + | −− | NA | NA | − | 2 |
| Kaur et al. ( 2014 ) | + | + | −− | NA | NA | − | 2 |
| Keniston et al. ( 1997 ) | + | + | + | − | + | − | 2 |
| Malek et al. ( 2020 ) | + | + | −− | NA | NA | − | 2 |
| No authors listed ( 2009 ) | NR | NR | −− | NA | NA | −− | 3 |
| No authors listed ( 2020 ) | NR | NR | −− | NA | NA | −− | 3 |
| Parry and Bredesen (1985) | NR | − | −− | NA | NA | −− | 3 |
| Schaumburg et al. ( 1982 ) | NR | + | −− | NA | NA | − | 2 |
| Schaumburg et al. ( 1983 ) and No authors listed (1984) | NR | + | −− | NA | NA | − | 2 |
| Scott et al. ( 2008 ) | + | + | −− | NA | NA | − | 2 |
| Shrim et al. ( 2006 ) | −− | −− | −− | NR | ++ | − | 3 |
| Stewart et al. ( 2022 ) | + | + | + | + | − | + | 1 |
| van Hunsel et al. ( 2018 ) | NR | −− | −− | NA | NA | − | 3 |
| Vasile et al. ( 1984 ) | −− | + | −− | NA | NA | − | 2 |
| Visser et al. ( 2014 ) | + | + | + | − | ++ | + | 1 |
| Vrolijk et al. ( 2020 ) | − | −− | −− | NA | NA | −− | 3 |
| Waterston and Gilligan (1987) | −− | + | −− | NA | NA | − | 2 |
Expert judgement was translated into a rating scale for each question to be answered as follows: (++): definitely low RoB; (+): probably low RoB; (NR): not reported; (−): probably high RoB; (− −): definitively high RoB.
The individual rating for each question was combined by an algorithm and translated to an overall tier of reliability for each individual study (RoB tier 1: low RoB; RoB tier 2: moderate RoB; RoB tier 3: high RoB).
Appendix C – Studies investigating the association between vitamin B6 exposure and peripheral neuropathy which could not be used to inform the setting of a RP for the derivation an UL
1.
Studies not used because of insufficient information on dietary exposure to vitamin B6:
Case 1 described in No authors listed (2009) had consumed 50 mg/day vitamin B6 for 3 months but had taken in addition several not further specified multivitamin preparations that probably also contained vitamin B6;
The case reported in Bacharach et al. (2017) had consumed a large amount of energy drinks with > 2,000% the DRV for vitamin B6 and had PA concentrations of 463 μg/L (normal range 3–30 μg/L, serum, plasma or whole blood not specified);
Falcone and Sowa (2013) did not specify the vitamin B6 intake of the five cases on which they reported; serum pyridoxine concentrations were 53.4–148.5 ng/mL (216–601 nmol/L) (normal range 2.1–21.7 ng/mL);
The case described by Malek et al. (2020) had consumed up to 30 mg/day vitamin B6 plus a number of energy drinks containing high amounts of vitamin B6; pyridoxine concentrations were 60.2 ng/mL (244 nmol/L) (normal range 3.6–18 ng/mL, serum, plasma or whole blood not specified);
Kaur et al. (2014) reported on a case who had PLP concentrations of 116 μg/L (469 nmol/L; normal range 5–50 μg/L) and PA concentrations of 37 μg/L (normal range 3–30 μg/L, serum, plasma or whole blood not specified);
Scott et al. (2008) described 26 cases with neuropathy who had serum pyridoxine levels of 23.3–177.3 ng/mL (94–717 nmol/L; normal range 3–30 ng/mL). Two patients have reported vitamin B6 intakes of around 50 mg/day for 34 years;
Franzblau et al. (1996) reported on 125 randomly selected workers who self‐reported symptoms of carpal tunnel syndrome (CTS). Vitamin B6 status as measured by plasma PLP concentrations and glutamic pyruvic transaminase assay was unrelated to CTS symptoms and electrophysiologically determined median or ulnar nerve function;
Keniston et al. (1997) in a cross‐sectional study on 441 individuals reported that in multivariable analysis taking vitamin B6 containing supplements (amount not reported) was positively associated with the prevalence of definite CTS, but not with the prevalence of probable or confirmed CTS;
Visser et al. (2014) studied 381 patients with chronic idiopathic axonal polyneuropathy and 140 healthy controls. Even though patients used more vitamin B6 containing supplements, plasma vitamin B6 concentrations (vitamer not specified) were not significantly different between patients and controls. There was also no correlation between plasma vitamin B6 concentrations and severity of polyneuropathy;
Chaudary and Cornblath (2013) reported on 51 patients with neuropathy of unknown origin who all had elevated vitamin B6 concentrations in blood (> 26 ng/mL (range 31–348 ng/mL, vitamer not reported). Vitamin B6 concentrations were uncorrelated with severity of neuropathy. There was no improvement of symptoms following cessation of supplementation;
Stewart et al. (2022) patients with chronic idiopathic axonal polyneuropathy enrolled in the Peripheral Neuropathy Research Registry. There was no association between elevated plasma PLP concentration (> 50 ng/mL) and self‐reported severity of neuropathy or the results of nerve conduction studies.
-
b
Studies not used because doses of vitamin B6 exceeded 500 mg/day, i.e. the dose considered by the SCF (2000) to represent a potentially toxic dose to humans and five times above the RP used by the SCF (2000) to derive the UL
Case 2 described in No authors listed (2009) had consumed 600 mg/day vitamin B6 for 3–4 years;
The case described by Baer (1984) reported an intake of 4,000 mg/day for a total of 4 years, however with shorter latency time which is not reported;
Foca (1985) described a case who had taken 4,300 mg/day vitamin B6 for 3 weeks;
The case reported by Gdynia et al. (2008) had taken 9,600 mg/day for 12 months;
The two cases described by Schaumburg et al. (1982) had taken 2,000 and 3,000 mg/day for 11 and 3 months, respectively;
Vasile et al. (1984) published on a case who had consumed 3,000 mg/day for 6 months;
The female case of neuropathy reported by Berger and Schaumburg (1984) had taken 200 mg/day vitamin B6 for 2 years and thereafter 500 mg/day for 1 year to which 300 mg once per week was added;
The female case of neuropathy reported by Waterston and Gilligan (1987) had consumed 1,000 mg/day vitamin B6 for 1 year; and
The female case who had developed neuropathy described by Friedman et al. (1986) had taken 2,000 mg/day vitamin B6 for 2 years.
Appendix D – Evidence tables
D.1. Peripheral neuropathy
D.1.1. Intervention study on peripheral neuropathy
|
Reference Country Duration Funding |
Design | Subject characteristics at baseline | Intervention | Outcome ascertainment | Results |
|---|---|---|---|---|---|
|
Berger et al. (1992) USA Subject 1: 7 months Subject 2: 4.5 months Subject 3: 14 months (QST results became abnormal) Subject 4: 3.5 months Subject 5: 1.5 months Note: Pyridoxine discontinued at first evidence of sensory disturbance NR |
CT Inclusion/exclusion criteria: NR (cases neurologically normal, except one with poliomyelitis as a child, but not described as in‐ or exclusion criteria) N = 5 |
Sex (% women): 20 Age (range): 29–55 Serum pyridoxal phosphate (PLP), nmol/L (after 1 month of intervention): Subject 1: 920 Subject 2: 280 Subject 3: NR Subject 4: 690 Subject 5: NR |
Vitamin B6 (pyridoxine) dose (mg/day) Subject 1: 1,000 Subject 2: 1,000 Subject 3: 1,000 Subject 4: 3,000 Subject 5: 3,000 Vitamin B6 (mg/kg bw): Subject 1: 12 Subject 2: 19.6 Subject 3: 12 Subject 4: 42.8 Subject 5: 56.9 Background nutrient intake: NR Compliance: NR |
Time to onset of clinical symptoms (reflected in duration of exposure) Quantitative sensory threshold (QST) index Method: Clinical neurologic examinations including testing of deep tendon reflexes appreciation to pin, cold, and vibration, motor strength, and gait Physiologic evaluations including 1) Quantitative measurement of the sural sensory potential amplitude and conduction velocity 2) Quantitative vibration thresholds 3) Quantitative thermal thresholds |
Symptoms progressed with increasing PLP concentration and after PLP returned to normal Serum PLP (nM) *Subjects 3 + 5: NR Subject 1 Month 1: 920 Month 4: 690 Month 7: 680 Month 8: 10 Subject 2 Month 1: 280 Month 5: 280 Month 5.5: 50 Month 6: 20 Subject 3 did not experience symptoms Subject 4: Month 1: 690 Month 3: 750 Month 5: 0 Thermal and vibrational QST post PLP Subject: 1: returned to normal after 1 month Subject 2: returned to normal over 1 month+ vibrational QST remained elevated Subject 4: continued to worsen over 2.5 months Subject 5: continued to worsen over 1 month Time until serum PLP levels returned to normal after discontinuation (week): Subject 1: 4 Subject 2: 2 Subject 4: 6 |
bw: body weight; CT: clinical trial; d: day; NR: not reported; PLP: pyroxidal phosphate; QST: quantitative sensory threshold; USA: United States of America; wk: week.
D.1.2. Observational studies on peripheral neuropathy
|
Reference Country Study design Follow‐up Funding |
Original cohort (N total) Population sampled Exclusion criteria Study population |
Ascertainment of outcome |
Exposure groups Duration Exposure assessment method |
Incident cases | Results |
|---|---|---|---|---|---|
|
Anonymous (2009) Australia Case series NA NR |
N = 2 Population: one woman with symptoms of neuropathy and one woman diagnosed with neuropathy Exclusion criteria: NA Lost to follow‐up (%): NA Sex (% of women): 100 Age (y): Case 1: 39 Case 2: 69 |
Reported to the Australian pharmacovigilance system |
Vitamin B6 (mg/day) Case 1: 50 + multivitamins (may contain B6) Case 2: 600 Duration Case 1: daily intake for 3 month Case 2: daily intake for 3–4 years Type of vitamer: NR Method: Self‐report |
NA |
Symptoms experienced Case 1: Burning and electric shock sensations Case 2: Gait and clinical diagnosis of sensory neuropathy |
|
Franzblau et al. (1996) USA CS NR NR |
N = 125 Company 1: (n = 50), company 2: (n = 75) Population sampled: Workers from two companies (automotive parts manufacturer) Inclusion criteria: Company 1: all workers Company 2: only workers in selected jobs Lost to follow‐up (%): 0 n = 125 Sex (% women): 67 Age (y): 36.6 ± 11.3 |
1. Self‐reported (questionnaire) of 9 symptoms at 15 body locations. CTS symptoms defined as: numbness, tingling, burning or pain in wrists, hands or fingers 2. Electrophysiologic testing: – Sensory nerve conduction studies (median and ulnar sensory nerve) – Mid‐palm temperature tests CTS definition: Reported symptoms potentially consistent with CTS and evidence of median mononeuropathy (MM) in the same hand. |
PLP (n = 112), nmol/L: 128.0 ± 81.1 (23.6–379.6) Deficiency: < 30 nmol/L Method: radioenzymatic assay |
Cases, n Dominant hand CTS symptoms: 34 No CTS symptoms: 78 MM: 20, 17.6% No MM: 92 CTS: 10 No CTS: 102 Non‐dominant hand CTS symptoms: 27 No CTS symptoms: 85 MM: 15, 13.6% No MM: 97 CTS: 5 No CTS: 107 Either hand CTS symptoms: 39 No CTS symptoms: 73 MM: 29, 24.8% No MM: 83 CTS: 14 No CTS: 98 |
PLP concentration (nmol/L) Dominant hand CTS symptoms: 130.7 ± 93.3 No CTS symptoms: 126.8 ± 75.8 p > 0.25 MM: 145.4 ± 104.3 No MM: 124.2 ± 75.3 p > 0.25 CTS: 145.0 ± 122.8 No CTS: 126.3 ± 76.5 p > 0.25 Non‐dominant hand CTS symptoms: 133.9 ± 95.6 No CTS symptoms: 126.1 ± 76.5 p > 0.25 MM: 111.0 ± 75.8 No MM: 130.6 ± 82.0 p > 0.25 CTS: 117.6 ± 75.5 No CTS: 128.5 ± 81.7 p > 0.25 Either hand CTS symptoms: 133.3 ± 90.9 No CTS symptoms: 125.1 ± 75.9 p > 0.25 MM: 1.06 ± 0.17 No MM: 127.1 ± 76.6 p > 0.25 CTS: 138.4 ± 110.8 No CTS: 126.5 ± 76.6 p > 0.25 |
|
Keniston et al. (1997) USA CS NR NR |
N = 441 Population sampled: Volunteer male and female workers from six industries, workers at university, homemakers employed in various occupations and from an exercise study at university Exclusion criteria: None Lost to follow up (%): 0 n = 441 Sex (% women): 39 Age (y): 44.5 ± 9.3 (19–71) |
Self‐reported presence, frequency, and nature of hand and wrists symptoms (via interview), including: – Numbness, tingling or nocturnal awakening (defined as specific CTS symptoms) – Pain, tightness and clumsiness (defined as non‐specific CTS symptoms, not relevant if isolated presence) Nerve conduction studies of the median nerve CTS definition: Minimum 1 specific symptom and confirmed by a positive median nerve slowing. Cut‐off at 100 nmol/L plasma PLP, at which level vitamin B6 intake is associated with 3.5 mg/d (supplementation ~ > 2 mg/d) |
Self‐reported vitamin B6 supplementation (mg/day): All: 0–200 Women, median: 1 Men, median: 0 G1 (n = 87): no symptoms or slowing (control) G2 (n = 56): symptoms only G3 (n = 115): slowing only G4 (n = 183): CTS (slowing and symptoms) Plasma PLP, nmol/L (n = 441): mean ± SD, median G1: 63.2 ± 72.4, 45.9 G2: 64.8 ± 70.7, 39.6 G3: 62.3 ± 64.5, 44.6 G4: 67.4 ± 73.5, 43.5 Plasma PLP, nmol/L (n = 218), mean ± SD, median no vitamin supplements and plasma PLP ≤ 100 nmol/L) G1: 38.7 ± 16.5, 35.6 G2: 30.9 ± 15.6, 29.0 G3: 38.0 ± 18.2, 36.3 G4: 38.8 ± 19.1, 35.6 |
Plasma PLP > 100 nmol/L (n = 50): 20% (n = 10) were diagnosed with CTS, of which 80% (n = 8) took B6 |
Chi2 + F‐test: G1 (control) + G2 + G3 + G4: X 2 = 2.5, p = 0.476 + F = 0.144, p = 0.933 Stepwise multiple regression analysis*: Vitamin B6 supplementation independent predictor of: – Prevalence of definite CTS, p = 0.020 (supplemented and unsupplemented males and females) – Carpal tunnel release surgery, p = 0.040 (supplemented and unsupplemented males and females), p = 0.101 (all females) Plasma PLP independent predictor (in unsupplemented female) of: – Prevalence confirmed CTS, p = 0.020 (Significant correlation between PLP and frequency of tingling, [R = −0.18], p = 0.031) and frequency of awakening, [R = ‐0.23], p = 0.005) *Adjusted for age; gender; BMI; alkaline phosphatase; cigarette smoking |
|
Shrim et al. (2006) Canada PC B6 intake: 9 ± 4.2 wks Total: ≈ 32 wks Unclear |
N = 192 Population sampled: General population + health professionals Exclusion criteria: NR % lost to follow up: 0 n = 192 Sex (% women): 100 Age (y): G1: 32.5 ± 4.4 G2: 33.1 ± 4.2 |
Self‐report of adverse events, specifically during the period of B6 intake |
Vitamin B6 intake via supplements (mg/d): G1 (n = 96): 132.3 ± 74 (> 5 0), duration: ≈ week 7–16 G2 (n = 96): 0 Type of vitamer: NR Method: NR |
NR |
No adverse effects Heterogeneity: Parity, p = 0.03, smoking status, p = 0.002 |
|
Blackburn and Warren (2017) NR Case report NA NR |
N = 1 Population: A male with symptoms of peripheral neuropathy Exclusion criteria: NA Lost to follow up (%): NA Sex (% women): 0 Age (y): 65 |
– Self‐report of physical limitations – Testing of tendon reflexes and sensations to modalities – Pinprick and vibratory sensational testing Clinical examination (mentation, orientation, cranial nerves, motor strength and tone and balance (Romberg test)) |
Vitamin B6 intake: 6 energy drinks/d (content: 300% DRV/drink) Vitamin B6 intake, mg/d: 30.6 Vitamin B6 status (blood), ng/mL: 62.3 Duration: NR Type of vitamer: NR Method: Self‐report + blood sample |
NA |
– Decreased sensation to multiple modalities – Decreased pinprick in bilateral lower extremities – Absence of vibratory sensation in great toes, medial malleoli and hands bilaterally – Normal mentation, orientation, cranial nerves, motor strength and ton Romberg test (balance test): positive Vitamin B6 cessation improved neuropathy symptoms |
|
Falcone and Sowa (2013) USA Case series NA NR |
N = 5 Population: Patients with neuropathy symptoms reporting to clinic asked to replace B6 supplementation with P5P Exclusion criteria: NA Lost to follow up (%): NA Sex (% women): 80 Age (y): 39–81 |
Self‐reported neuropathic pain in distal limbs Electrodiagnostic measurements: Large fibre polyneuropathy n = 2 Chronic right L4‐5 radiculopathy: n = 1 |
Serum pyridoxine, ng/mL: 53.4–148.5 Normative range, ng/mL: 2.1–21.7 Method: Blood sample |
NA | Improved neuropathic pain |
|
Dalton and Dalton (1987) UK Study 1: CC Study 2: case‐report Follow‐up (Study 1): 6 months after B6‐discontinuation NR |
Study 1: N = 172 Study 2: N = 1 Population sampled: Study 1: women with premenstrual syndrome Study 2: woman with neurological symptoms Exclusion criteria: Study 1: NR Study 2: NA % lost to follow‐up: 0 Sex (% women): 100 Age (y): Study 1 Cases: 41.5 ± 8.8 Controls: 41.9 ± 9.8 Study 2: 49 |
Study 1: Self‐report of altered sensations in limbs or skin and notice of muscle weakness or pains Study 2: Self‐reported measures: – Complains of paraesthesia of hands at night, – Electric shock pains – Numbness of fingertips – Itching between shoulder blades – Hypersensitivity to stoking with cotton wool on her back and lower limbs Reflexes and muscle power normal Lhermitte's test negative |
Vitamin B6 intake, mg/d Study 1: Cases: 117 ± 92 Controls: 116 ± 66 Study 2: 75 Duration (y): Study 1 Cases (neurotoxic): 2.9 ± 1.9 Controls: 1.6 ± 2.1 p < 0.01 Study 2: 2 Serum vit B6 Study 1, % > 34 ng/mL Cases: 70 Controls: 55 *34 ng/mL = upper limit of testing Study 2, ng/mL: > 34 Normative range: 3.6–18 ng/mL Method: Self‐report and blood sample |
Study 1: % with neurological symptoms (serum B6 > 18 ng/mL) Cases < 50: 20 < 100: 38 < 200: 31 < 500: 11 Controls < 50: 32 < 100: 38 < 200: 14 < 500: 16 Cases (neurological symptoms in serum B6 > 18 ng/mL, n = 103) Paraesthesia: 59 Bone pains: 45 Hyperesthesia: 33 Muscle weakness: 33 Numbness: 21 Fasciculation: 18 Study 2: NA |
Study 1: After 2 months of B6‐discontinuation: B6 levels within the normal range except for 11 women (controls: 4), whose levels were below normal (3.6 ng/mL) After 3 months: 55% reported partial or complete recovery of neurological symptoms After 6 months of B6‐discontinuation: all reported complete recovery of neurological symptoms Cases (n = 7) who did not stop B6 intake: Continuation of neurological symptoms + B6 level remained elevated until discontinuation of B6 Study 2: B6 discontinuation eased symptoms within 3 months Return with neurological symptoms: B6 levels were > 34 ng/mL after restarting with B6 supplements |
|
Visser et al. (2014) Netherlands CC NR Supported by grant (WAR 07–24) from the Prinses Beatrix Fonds |
N = 577 Population sampled: G1: patients with chronic idiopathic axonal polyneuropathy (CIAP*) G2: healthy controls *CIAP defined as predominantly sensory polyneuropathy without ataxia, predominantly sensory polyneuropathy with ataxia or sensorimotor polyneuropathy Exclusion criteria: Vit B6 supplementation unknown; vit B6 supplementation after debut of polyneuropathy symptoms; pure small fibre neuropathy or typical sensory ataxic polyneuropathy. % Lost to follow up/excluded: 13 n = 521 G1: 381 G2: 140 Sex (% women): G1: 30 G2: 38 Age (y, mean): G1: 64.7 G2: 64.0 |
– All patients clinically evaluated for polyneuropathy in a standardised fashion – Severity of the polyneuropathy graded by calculation and summation of sensory and motor sum scores of the lower limbs – Manual muscle strength testing performed of tibialis anterior, gastrocnemius, peroneal, and toe extensor muscles, and graded according to the Medical Research Council (MRC) scale resulting in a motor sum score from 0 to 40 for both lower limbs. |
Whole blood PLP (nmol/L, median (range)): Non‐users of B6 supplements G1: 90 (38–255) G2: 94 (41–826) Users of B6 supplements G1: 135 (43–2,967) G2: 165 (94–2,373) Vit B6 supplements, mg/d (median, range): G1 (n = 112): 2.1 (0.5–200) G2 (n = 29): 2.8 (0.4–71) Duration (median (range) y): 7.4 (0.5–81) Type of vitamer: Blood: PLP (normative range: 26–102 nmol/L) Supplements: NR Method: Blood: Reversed‐phase fluorescence detection Use of supplements: Questionnaire |
CIAP, n G1: 381 G2: 0 Predominantly sensory polyneuropathy without ataxia, n G1: 245 G2: 0 Predominantly sensory polyneuropathy with ataxia, n G1: 33 G2: 0 Sensorimotor polyneuropathy, n G1: 103 G2: 0 |
CIAP not significantly (NS) associated with elevated vitamin B6 levels Note : More patients (31%) than controls (22%) used B6 supplements, (OR 1.7 (95% CI 1.0–2.7) p = 0.032) Vitamin B6 levels NS different between patients and controls, p = 0.58* Follow‐up of patients confirming cessation of vitamin B6 supplement‐use showed slow progression of symptoms in 64%, stabilisation in 26%, and regression in 10%. ‘An association between CIAP and vitamin B6 exposure or elevated vitamin B6 levels appears unlikely’ *Initially adjusted for gender and age Further adjusted for smoking and alcohol use (did not change overall conclusions) |
|
Baer (1984) USA Case report NA NR |
N = 1 Population: A woman with cutaneous skin changes and neuropathy Lost to follow‐up: NA Sex (% women): 100 Age (y): 36 |
History and clinical manifestations, neurologic and general physical examination and routine laboratory tests |
Pyridoxine: 4 g/day Duration: 4 years (discontinuation of intake after she had informed the dermatologist about her intake) Method: Self‐reported |
NA |
Symptoms: Developing numbness of the feet and tips of the fingers Discontinuation of pyridoxine for 3 months did not improve the neurologic manifestations |
|
Scott et al. (2008) USA Case series Patients (n = 14): Follow‐up phone calls after 6 months of B6 discontinuation NR |
N = 26 Population: Patients with elevated vit B6 levels and neuropathic symptoms Exclusion criteria: Patients with hereditary neuropathy and laboratory abnormalities of any kind (besides elevated B6 levels) that could explain their symptoms Lost to follow‐up (%): NA Sex (% women): 35 Age (y, mean): At presentation: 57.6 (28–84) At onset of symptoms: 53.8 (27–76) |
Quantitative sensory testing (QST) Electromyography (EMG) Nerve conduction studies (NCS) |
Patients with daily use of specific vit B6 supplements or B‐complex vitamins + daily multivitamins, n (%): 14 (53.8) Reported B6 dose (mg/d) Patient 1 + 2: 53 Other patients: NR Duration (y): Patients 1 + 2: 34 Other patients (23/26): > 1 Blood pyridoxine level (ng/mL, mean (range)): 68.8 (23.3–177.3) Normal level 3–20 Method: Self‐reports + blood sample |
NA |
QST (n = 10): Abnormal, n (%): 8 (80) EMG/NCS findings (n = 26), n (%): Normal: 17 (65) Mild sensorimotor axonal neuropathy: 7 (27) Mild sensory axonal neuropathy: 1 (4) Moderate sensorimotor axonal neuropathy: 1 (4) Self‐reported symptoms, %: Numbness: 96 Burning pain: 50 Tingling: 58 Balance difficulties: 31 Weakness: 8 Progressive involvement of distal extremities (legs worse than arms): 100 Lower extremities involvement: 35 Upper and lower extremities involvement: 62 Only upper limb involvement: 1 Deficits in facial sensation and taste: 1 No patient had strictly motor symptoms Neurological examinations, %: Decreased pinprick: 39 Decreased vibration: 42 Deficits in proprioception and/or light touch: 19 Gait dysfunction: 12 Decreased deep‐tendon reflex abnormalities: 12 Weakness and muscle atrophy: 8 |
|
Chaudary et al. (2003) UK RC 3–42 mo (this is performed before inclusion into the study, c.f. the retrospective design) NR |
N = 584 Population sampled: Men and women attending a nutritional therapy practice for help with various complaints and prescribed >30 mg/day vitamin B6 supplements for more than 3 months. Lost to follow‐up (%): 0 n = 555 Sex (% women): NR Age (y): 14–76 |
Self‐reported on the Nutrition Programme Questionnaire that reported predefined symptoms: Tingling hands, insomnia (night restlessness), acne, and skin rashes The questionnaire by the nutritional therapist was done before and after vitamin B6 supplementation (≥ 30 mg/day) |
Vitamin B6 from supplements (mg/day): 30–230 (Individual mean dose calculated from start dose to end dose) Duration: At least 3 months supplementation before entry into the study Background intake: NA Type of vitamer (%): Pyridoxine: 93.3 PLP: 6.3 PN‐HCl: 0.4 |
NA |
Symptoms (tingling hands, insomnia, rash, acne) reduced in frequency after 3–42 months of vitamin B6 supplementation over the full dose range (p < 0.001) ‘On analysis the majority of the participants in both the ‘no change in tingling hands’ group and ‘improvements in tingling hands’ group had complained about fatigue… [and other symptoms of vitamin B6 deficiency]’. 114 participants attended the nutrition therapy clinic with no reported symptoms, 91 did not develop symptoms associated with toxicity and 23 experienced symptoms after vitamin B6 supplementation (majority with 101–150 mg/day). |
|
Vrolijk et al. (2020) Netherlands Case series NA The Netherlands Pharmacovigilance Centre Lareb and Natuuren Gezondheidsproducten Nederland (NPN) |
N = 173 Population sampled: Cases of vitamin B6‐induced neuropathies from 1991 to 2020 (see under ‘comments’ to this paper) reported to the Netherland Pharmacovigilance Centre Lareb Lost to follow‐up (%): NA Sex (% women): NR Age (y): 54 ± 15 |
Self‐reports by the cases, other methods NR (no quality check of supplements as reported by patients, and no check of adherence to the advised daily doses) |
Vitamin B6, mg/d (intake from supplements (PLP, PN)): 0.5–250 36.7 ± 24.4 96 out of 173 used vitamin B6 supplementation without co‐medication Plasma PLP, nmol/L (n = 70): 1078 ± 1124 (88–5656) Duration: NR Type of vitamer: Supplements: Pyridoxal 5′ phosphate (PLP) and pyridoxine (PN) Blood: Plasma PLP Method: Self‐reported |
Complaints reported: Cases using vitamin B6 supplements < 21 mg/day: 39% (67/173 cases) neurological complaints Cases using vitamin B6 supplements > 21 mg/day: 45% (78/173 cases) complaints (no information about dose in 16% [28 cases]) |
PLP: 3.5% of reported cases PN: 96.5% of reported cases Pearson correlation analysis: NS correlation between the dose of PN and plasma level of PLP, r = 0.15 |
|
Chaudary and Cornblath (2013) USA Case series NA NR |
N total = 78 Population sampled: Patients with neuropathy and B6 levels < 26 ng/mL Exclusion criteria: Patients with well‐recognised causes of neuropathy (diabetes, chemotherapy, CIPD, alcohol, CMT, and multiple myeloma) Lost to follow‐up (%): 35 n = 51 Sex (% of women): 47 Age (y, median): 58 |
Sensory symptoms: Small fibre sensory symptoms Large fibre sensory symptoms Loss of modality Reflexes Weakness Normal sensory nerve action potential (SNAP) amplitudes CAMP Skin biopsy, intraepidermal nerve fibre density (IENFD) |
Blood pyridoxine levels (ng/mL mean (range)): 114 (31–348) Elevated levels: >26 ng/mL Duration: NR Method: Blood samples |
N, with symptoms Small fibre sensory symptoms: 46 Large fibre sensory symptoms: 15 Small fibre modality loss: 26 Large fibre modality loss: 17 Reduced reflexes:15 Mild distal weakness: 6 Reduced SNAP amplitudes: 9 Reduced CAMP: 4 Reduced skin biopsy, IEFND: 18 Mean pyridoxine level did not correlate with neuropathy severity Very high dose pyridoxine (NR) associated with a predominantly sensory, small fibre > large fibre, length‐dependent or independent neuropathy |
|
|
Berger et al. (1984) USA Case report NA NR |
N = 1 Population: one woman (general) Lost to follow‐up (%): NA Sex (% women): 100 Age (y): 34 |
Self‐reported symptoms Clinical examination at the hospital |
Vit B6 intake (mg/d): First two years: 200 Third year: 500 Once per week in the last year: 800 Duration: 200 mg/day for two years, increased to 500 mg/day for one year Type of vitamer: NR Method: Self‐reported |
NA |
Signs of peripheral neuropathy Signs decreased 3 wks after vit B6 supplementation cessation and continued to decrease in following months Self‐reported: – Electric shocks down the spine – Progressive gait unsteadiness – worse when walking and in darkness – Numbness and paresthesias of both feet – Numbness of both hands, lips and left cheek Clinical examination: – Marked sensory ataxia – Absent tendon reflexes and flexor plantar responses – Needed assistance to walk – Somatosensory evoked potential showed prolonged latency between lumbar and cervical sites |
|
Foca (1985) USA Case report NA NR |
N = 1 Population: Woman with difficulty walking and frequent falling. History of bilateral carpal tunnel syndrome (CTS) Lost to follow‐up (%): 0 Sex (% women): 100 Age (y): 81 |
Clinical examination*: Test of voluntary movement Reflexes Pinprick Position and vibratory sense * at 3 wks post cessation of vit B6 intake |
Pyridoxine intake (g/d): 4.5 Blood vit B6 (ng/mL): 9 At time of admission medications: Dyazide (triamterene hydrochlorothiazide), vit A, C, D, and B complex vitamins Duration: Gradually increasing vit B6 for several months, 4.5 g/d for the last three wks Normative range: Blood: 3.6–18 ng/mL Intake: 2–4 mg/d Method: Self‐reported + blood samples |
NA |
Clinical examination – Sensation to pin in lower extremities (more distally and more in the right lower extremity) – Position sense was absent in both lower extremities – Vibratory sense was absent in toes and reduced up to the knees bilaterally – Ambulation reduced with ataxia and poor foot placement – Depressed reflexes bilaterally Electrodiagnostic testing: Slowing motor conduction in both peroneal and tibial nerves; sensory latency was unobtainable in right slow in left sural nerve |
|
Brush et al. (1988) UK RC NA NR |
N = 630 Population sampled: General population, women attending the PMS Clinic Inclusion criteria: PMS Exclusion criteria: Menstrual distress. Intermittent conditions liable to occur at any stages of the cycle Lost to follow‐up (%): 0 n = 630 Sex (% women): 100 Age (y % patients at 1st visit): < 14: 0.2 15–25: 10 26–35: 46.2 36–45: 40.2 > 45: 3.3 |
Clinical diagnosis of peripheral neuropathy Definition of PMS: Occurring in the second half of the menstrual cycle and finishing within 24–48 h of the onset of menstruation. Most common symptoms: mood changes, fluid retention and/or redistribution, breast discomfort and tension headache |
Pyridoxine hydrochloride supplement (mg/d) initially at 40–100, followed by 120–200 Duration and % patients < 6 mo: 45.9 6–12 mo: 34.6 1–2 y: 12.6 3–4 y: 4.6 4–5 y: 2.3 Normative range: NR Method: NR |
% response to treatment recorded as good (no significant residual complains) of patients taking 100–150 mg/d pyridoxine: 40 % response to treatment recorded as good of patients taking 160–200 mg/d pyridoxine: 60 |
No symptoms consistent with a diagnosis of peripheral neuropathy reported |
|
Schaumburg et al. (1982) USA Case report NA NR |
N = 2 Population: A man and woman with sensory neuropathy symptoms Exclusion criteria: NA Lost follow‐up (%): NA Sex (% women): 50 Age (y): Man: 25 Woman: 37 |
Neurological examinations (pinprick test, sensory test of temperature, touch and vibration, test of distal tendon reflexes) |
Pyridoxine supplement (mg/d): Man: 3000 Woman: 2000 RDI: 2.5 Duration (mo): Man: 3 Woman: 11 Method: Self‐report |
NA |
Sensory neuropathy measures (n = 2): – Numbness in feet and hands – No dysesthesias, facial involvement or weakness – Moderately diminished sensation to pinprick, temperature, and touch sensation – Severe loss of vibration sense – Distal tendon reflexes absent – Normal strength – Electromyograms normal – Motor nerve conduction normal Discontinuation of pyridoxine after 1 y: No improvements in sensory neuropathy |
|
Waterston and Gilligan (1987) Australia Case report NA NR |
N = 1 Population: one woman Lost to follow‐up (%): NA Sex (% women): 100 Age (y): 20 |
Self‐reported (3‐mo recall) symptoms and examined in the hospital Physical examination power and coordination, Knee‐ and ankle‐jerks. Gait. Romberg's test Sensory examination (proprioception, vibration and temperature sensation, light touch sensation) |
Pyridoxine supplementation (mg/d): 1000 (Average daily requirement ≈ 1.5 mg/d with 100 g of protein) Duration: 12 months Method: Self‐reported |
NA |
Self‐reported symptoms: – Increased numbness and paraesthesia in both feet – Aching legs – Balance deteriorated, especially poor in darkness Physical examination: – Impaired proprioception, vibration, and temperature sensation – Positive for Romberg's sign – Slowing in sensory fibres in lower and upper limbs – Prolonged distal motor latency Symptoms worsened 3 wks later – moved to upper limbs Symptoms slowly decreased after 4 months of vitamin B6 supplementation termination |
|
Dalton et al. (1985) UK Case series 2 months after vit B6 discontinuation NR |
N = 58 Population: Women taking pyridoxine for premenstrual syndrome Exclusion criteria: NA Lost to follow‐up (%): 53 n = 58 Sex (% women) 100 Age (y): 42 ± 9 |
Sensory neuropathy Burning, shooting, tingling pains Paraesthesia of limbs Clumsiness Ataxia Perioral numbness |
Vitamin B6 supplementation (mg/d): 50–300 Normative range Intake : 2–4 mg/d Blood: 3–18 ng/mL Method: Self‐reported |
Symptoms of sensory neuropathy, n per patient (B6 serum levels > 18 ng/mlL: At first visit (n = 58): 3.4 2 months after termination of B6: (n = 27): 1.04 | Improvement in neuropathy symptoms 2 months after vitamin B6 discontinuation: p < 0.001 |
|
Bacharach et al. (2017) USA Case report 1 y after initial presentation NR |
N = 1 Population: One woman with progressive burning, numbness, tingling, and weakness in all 4 extremities for 2 years Lost to follow‐up (%): NA Sex (% women): 100 Age (y): 41 |
1. Physical examinations (touch, vibration, proprioception temperature, pinprick, gait test, Romberg test) 2. Electromyography/ nerve conduction study 3. Quantitative sudomotor axon reflex test (QSART) |
Intake: NR Duration: NR (symptoms >2 y) Initial pyridoxic acid (PA) concentration, μg/L: 463 (B‐vitamin complex, dose NA) *Normative range: 3–30 μg/L PA 4 mo after restriction of vit B6 (food and supplements)*, μg/L: 2440 PA 4 mo after restriction of energy drinks high in vit B6, μg/L: 760 *accidentally consumed energy drinks high in vit B6 Method: Blood sample |
NA |
1. Symptoms consistent with small fibre neuropathy 2. Nerve conduction study (left arm and leg): Moderate chronic sensory polyneuropathy + left‐sided, mild median mononeuropathy across the wrist 3. QSART: Abnormal postganglionic sympathetic sudomotor responses at the proximal/distal leg and foot sites Follow‐up 1 y after initial presentation: no significant improvement in symptoms |
|
Vasile et al. (1984) USA Case report 3 mo after cessation of vit B6 NR |
N = 1 Population: A woman experiencing difficulty with ambulation over a 4‐month period. Known with mild osteoarthritis (non‐medicated), no history of syphilis, alcohol abuse, or degenerative disorder. Took vitamin B6 supplementation for carpal tunnel syndrome, as ordered by her physician. Lost to follow‐up (%): 0 Sex (% women): 100 Age (y): 58 |
Physical examination and electrodiagnostic tests (sensory conduction studies, electromyography, nerve biopsy) |
Pyridoxine intake (mg/d): 3000 Duration (mo): 6 Method: NR |
NA |
Physical examination: – Abnormal gait compatible with sensory neuropathy – Absent vibration and proprioceptive modalities in the distal lower extremities, and reduced awareness in the upper extremities – Lhermitte's sign – Muscle testing normal in all extremities – Achilles jerk reflexes absent bilaterally – Biceps and patellar reflexes diminished bilaterally Electrodiagnostic tests: – Normal motor conduction velocities and terminal latencies in all extremities (but CTS present) – Evoked response to sural nerve testing – Electromyography negative for acute or chronic denervation – Chronic active axonal neuropathy found in sural nerve biopsy Progression of symptoms while on vitamin B6 treatment: awareness to pinprick stimulation in a glove and stocking distribution, and gait abnormality 3 months after cessation of vit B6: Improved gait pattern and sensory awareness. Lhermitte's sign absent, normal sensory conduction velocity in upper extremities. Sural nerve response still present. |
|
Malek et al. (2020) Lebanon Case report 1st: 1 mo 2nd: 18 mo NR |
N = 1 Population: A non‐smoking man working as an energy consultant with no history of exposure to hazardous substances, no history of alcohol use or abuse. Treated for depression with escitalopram. Progressive numbness and imbalance for 12 years. Lost to follow‐up (%): NA Sex (% women): 0 Age (y): 54 |
Self‐reported (10 y post onset of symptoms) and clinical examination Physical examination Gait, Romberg's test, reflexes Sensory examination (vibration sense) Electrophysiological examination Electromyography (EMG) Somatosensory‐evoked potentials (SSEP) |
Vitamin B6 intake from self‐prescribed medication containing pyridoxine (mg/d): 30* *also consumed energy drinks with high amounts of vitamin B6 (exact amount NR) Vit B6 level at 1st visit (ng/mL): 60.2 (reference 3.6–18) Vit B6 level at follow‐up after cessation of medication and energy drinks (ng/mL): 20.9 (reference 5–30) Duration (y): ~ > 9 Type of vitamer (blood): NR Normative range: 3.6–18 ng/mL Methods: Self‐reported and blood sample |
NA |
Symptoms at first visit: Self‐reported: Gait instability Near falls Medical examination: Positive Romberg's test Decreased vibratory sense distally Absent deep tendon reflexes Electrophysiological examination: Severe, generalised, and non‐length dependent sensory neuronopathy/ganglionopathy EMG and SSEP absent sensory responses in bilateral sural, median and ulnar nerves Abnormal tibial SSEP responses 1st Follow‐up: Pyridoxine‐induced diffuse sensory ganglionopathy 2nd Follow‐up: Pyridoxine‐induced diffuse sensory ganglionopathy remained stable |
|
Schaumburg et al. (1983) and Anonymous (1984) (S6) USA Case series & Case report 7 mo after pyridoxine withdrawal NR |
N = 7 Population: General population with symptoms of peripheral neuropathy Subject 1 (S1): Case with ‘typical clinical presentation’ exemplifying other cases Subject 2–7 (S2‐S7): Other cases Lost to follow‐up (%): NA Sex (% women): 71 Age (y): 20–43 |
Self‐reported and clinical examined S1 reported in more detail S2‐6 reported together S6 reported in separate publication Examination: Sensory profile Sural‐nerve biopsy Sensory‐, motor, tibial‐ and median‐nerve conduction Somatosensory response S1 and S6: Physical examination: Walking gait Reflexes Babinski test Sensory examination: Touch, temperature, pinprick, vibration, joint position Electrophysiologic examination: Motor nerve, sensory‐nerve, needle, electromyography, somatosensory response, tibial‐nerve, median‐nerve |
Pyridoxine intake (g/d) S6: – 2 y before seeking medical attention: 0.5 – 1 y before seeking medical attention: 5 S1‐7: 2–6 (max daily dose; Only S2 and S5 started out with high doses, the other cases started with 0.5–1 g/day and increased the intake steadily. None experienced any symptoms at doses < 2 g/day Duration: 2–40 months Pyridoxine in blood (ng/mL): S7: >30 Normative range: 3.6–18 Method: Self‐reported and blood samples |
S1: – Lhermitte's sign (tingling sensation down the neck into feet and toes when flexing neck) – Walk with cane only – Gait broad‐based and stamping – Unable to walk with eyes closed – Marked pseudoarthrosis of outstretched arms – Limb reflexes and Babinski signs absent – No sensory‐ nerve action potentials – Motor‐nerve conduction and electromyogram normal – Somatosensory evoked response studies: Unilateral tibial nerve stimulation, no response – Bilateral tibial nerve stimulation: No response over the lumbar or cervical spine but a cerebral response of very low amplitude was noted S2‐7: – Sensory loss in extremities – Sensations of touch, temperature, pinprick, vibration and joint position severely impaired in the upper and lower limbs – Sural nerve biopsy (non‐specific axonal degeneration), n = 2 – Somatosensory evoked responses, n = 2 – Distal sensory‐nerve conduction: Absent in all nerves, n = 4 – Mild subjective alteration of touch‐pressure and pinprick sensation over the cheeks and lips, n = 6 – Sensory‐nerve action potentials not be elicited unilateral tibial nerve and bilateral tibial nerve stimulation produced no response, n = 6 S6: Self–reported symptoms: – Difficulty walking especially in darkness – Difficulty handling small objects – Changes in feeling in lips and tongue – Lhermitte's sign Clinical examination findings: – Walk with cane only and unable to walk with closed eyes – Gait broad–based and stamping – Marked pseudoathetosis of the outstretched arms – Limb reflexes and Babinski signs absent – Sensations of touch, temperature, pinprick, vibration and joint position were severely impaired in upper and lower limbs After stopping pyridoxine supplementation symptoms decreased in all cases 7 months after pyridoxine withdrawal: – Sensory nerve responses were absent – Motor nerve conduction normal – Somatosensory evoked responses definite improvement in central conduction and mild improvement in peripheral conduction |
|
|
Parry and Bredesen (1985) USA Case series 3–18 months after vit B6 supplementation stopped NR |
N = 16 Population: Women with symptoms of peripheral neuropathy, one patient with inherited neuropathy Lost to follow up (%): 31 Sex (% women): 100 Age (y): 25–53 |
Clinical examination 8 patients at clinic and 8 by telephone interview Electrophysiologic studies (n = 7) Sural nerve biopsy (n = 2) Follow‐up evaluations, n: By telephone: 8 At examination: 5 |
Pyridoxine supplementation (mg/d): 200–5000 Duration (mo): 1–72 Normative range: NR Method: Self‐reported |
NA |
Symptoms (n = 16), % of patients Numbness: 100 Paresthesia: 100 Ataxia: 81 Lhermitte's sign: 50 Pain: 31 Weakness: 6 Signs (n = 8), % of patients Sensory deficit: 100 Sensory ataxia: 88 Romberg's sign: 88 Loss of Achilles reflexes: 100 Loss of other deep tendon reflexes: 0 Weakness: 13 Electrophysiologic studies, n: Needle EMG normal: 7 Sensory nerve conduction velocity slowed mild: 7 Sensory nerve action potential absent or severely reduced amplitude: 7 Compound muscle action potential amplitudes and motor nerve conduction velocity normal: 7 Sural nerve biopsy (n = 2): myelinated fibre density moderately reduced, myelin debris indicated axonal degeneration, no segmental demyelination or myelinated fibre regeneration |
|
Gdynia et al. (2008) Germany Case report 1 y post‐supplementation NR |
N = 1 Population: Man, wheel‐chair‐bound; subject was self‐medicated; self‐diagnosed with a pendulum to estimate his deficiencies Exclusion criteria: NA Lost to follow‐up (%): NA Sex (% women): 0 Age (y): 75 |
Blood concentration of pyridoxine Neurological examination: – Symmetric tetraparesis – Romberg's test – Deep‐tendon reflexes – Touch, temperature, pinprick, vibration, and joint‐position – Skin colour Electrophysiological examination: – Sensorimotor mixed axonal‐demyelination – Distal motor latency (DML) – Motor nerve action potential (MSAP) – Nerve conduction velocity (NCV) – Fibrillation potentials – Signs of reinnervation |
Pyridoxine supplementation (+range of other vits): 9.6 g/d Duration (y): 3 Blood pyridoxine (μg/L*): 1850 *Normative range: 40–120 μg/L Method: Self‐reported + blood samples |
NA |
Neurological examination: Symmetric tetraparesis: distally pronounced muscle weakness Romberg's test: positive Deep‐tendon reflexes: reduced or absent. Touch, temperature, pinprick, vibration and joint‐position: loss at all distal limbs Skin colour: yellowish‐brown Electrophysiological examination: Sensorimotor mixed axonal‐demyelinating polyneuropathy DML (ms): Above normal in both tibial nerves MSAP (mV): Below normal in both tibial nerves, left median nerve, and both radial nerves NCV (ms): Below normal in both tibial and radial nerves Fibrillation potentials: Positive in right tibialis anterior and left vastus medialis Signs of reinnervation: Positive in right tibialis anterior, right flexor pollicis brevis and left vastus medialis Follow‐up (1 y post supplementation): Electrophysiological examinations: DML: Above normal in both tibial nerves, but decreased MSAP: Below normal in both tibial nerves, left median nerve, and both radial nerves, but increased NCV: Below normal in tibial and radial nerves Increased in tibial nerves Fibrillation potentials: All negative Signs of reinnervation: Positive in right tibialis anterior, right flexor pollicis brevis and left vastus medialis Ataxic signs more pronounced than motor signs |
|
Friedman et al. (1986) USA Case report NA NR |
N = 1 Population: Woman with good health (general) Lost to follow‐up (%): NA Sex (% women): 100 Age (y): 49 |
Self‐reported: Numbness bilaterally in great toes Physical examination at hospital: Walking, gait, Romberg's test, Achilles' tendon reflexes, muscle strength Sensory examination: Position sense Temperature Pinprick sensation |
Pyridoxine supplementation (g/d): 2 Duration (y): 1 Method: Self‐reported |
NA |
Self‐reported: Numbness in toes Physical examination at hospital: Diagnosed with sensory peripheral neuropathy Walking: unable to tandem walk Gait: unsteady Romberg's sign test: swaying when eyes closed Achilles' tendon reflexes: absent Muscle strength: normal Sensory examination: Position sense: loss Vibration sense in toes: impaired bilateral sensory deficits Temperature: loss of temperature over feet and toes Pinprick sensation: loss over feet and toes |
|
Stewart et al. (2022) USA RC NA Supported by the Foundation for Peripheral Neuropathy |
N = 261 Population sampled: Patients with CIAP enrolled at the Peripheral Neuropathy Research Registry (a multicentre database and biorepository) prior to December 2020 with a complete dataset including plasma vitamin B6 available (within 3 years from ‘study enrolment date’). Cases of vitamin B6 deficiency (0–4.9 μg/L) were excluded. Participants had negative tests for other common peripheral neuropathies aetiologies Lost to follow‐up (%): 0 n = 261 Sex (% women): 44 Age (y): 61.2 ± 13.6 |
Painful CIAP determined by physician + self‐reported by the participant (questionnaire) Neurological exam: Muscular strength, deep tendon, reflexes, sensory examination findings, gait evaluations, and Romberg test Nerve conduction studies (NCS): Major motor and sensory nerve Intra‐epidermal nerve fibre density measured by skin biopsy (IENFD) History questionnaire: Nature and severity of peripheral neuropathy symptoms, including pain, numbness, weakness, balance, and autonomic symptoms. Also included medication intake Overall neuropathy severity (n = 236): Total Neuropathy Score‐reduced (TNSr), including pinprick sensibility, vibration sensibility, muscular strength, and absence of deep tendon reflexes + degree of paresthesia extension measured by pain and numbness (TNSr 0–5 = mild impairment, 6–9 = moderate, 10–16 = severe) |
Vitamin B6, B‐complex or multivitamin (dose NR; n (%)): Normal plasma B6: 28 (15.8) Elevated plasma B6: 38 (33.3) Plasma PLP (μg/L): Overall (mean): 57.7 Normal range (5–50 μg/L), %: 67.8 Elevated (50.1–99.9 μg/L), %: 15.9 Highly elevated (100 μg/L), %: 15.9 With vit B6, B‐complex or multivitamin supplements Normal and elevated plasma B6: 78.8 ± 82.7 Method: Intake: Self‐reported Blood: Mayo Clinic catalogue, Test ID: B6PA |
NA |
Those taking vit B6 supplement, B‐complex, or a multivitamin had higher plasma vitamin B6 level than those not taking pyridoxine supplements, p = 0.0272 (t‐test) Elevated plasma vitamin B6 levels NS related to any patient‐reported neuropathy sign or symptom, p > 0.05 NCS and IENFD findings: No higher odds for nearly all NCS results, including peroneal or sural velocities or amplitudes, nor for IENFD, in the elevated plasma B6 group vs. normal level group, p > 0.09 The groups with elevated plasma B6 levels were more likely to have an abnormal left peroneal CMAP Amplitude, p = 0.046, but no other relation seen for other peroneal or sural results, p > 0.09 TNSr findings: Physical exam: No higher odds for exam features including ankle and toe strength, vibration sense, deep tendon reflexes, pinprick border, or gait abnormality in the elevated vitamin B6 groups vs. normal levels, p > 0.1 TNSr score: No higher odds for mild vs. moderate or severe TNSr, p = 0.561 Self‐report: There were no differences in frequency or severity of patient‐reported pain, pain types (i.e. sharp, hot, or deep pain), or numbness, p > 0.1 All tests adjusted for age and time elapsed since the onset of symptoms (in years) |
|
Anonymous (2020) Netherlands and France Case series NA NR |
N = 115 Population: Netherlands: 90 cases with peripheral sensory, motor, and autonomic nervous system neuropathy from the Netherland Pharmacovigilance Centre (1991–2017) France: 25 reports on neuropathy from the French Health Products Agency (1986–2018) % lost to follow up: NA n, Netherlands: 90 France: 25 Sex (% women): Netherlands: 80 France: 56 Age (y): Netherlands (mean (range)): 53 (3–85) France (range): 25–92 |
Netherlands: Adverse drug reaction reports linked to vit B6 intake France: Reports of neuropathy linked attributed to vitamin B6‐containing drugs |
B6 supplementation: Netherlands (mg/tablet; dose in tablet known for 63 cases, usually at least 25 mg and > 50 mg in 1/3 of cases): 1.4–100 (number of daily tablet intake NR) France (mg): – 5–250 (amount in culprit drugs, daily dose NR) – Vitamin B6 doses taken as combinations of vitamins and minerals: < 10 cases Duration: B6 supplementation: Netherlands: average 2 y France: 8 d – 4 y Type of vitamer: NR European normative range: 0.3–2 mg/day |
Neuropathy, n Netherlands : Peripheral sensory, motor and autonomic nervous system neuropathy: 90 France: Neuropathy: 25 |
Netherlands: Cases had either peripheral neuropathy, paraesthesia, hypoesthesia, neuropathic pain, chronic polyneuropathy and burning sensations or muscle weakness Symptoms regressed in 30 patients after ending B6 supplementation France: Cases had either neuritis, polyneuritis, neuropathy, heaviness of the limbs, distal paraesthesia, motor, sensory or sensorimotor peripheral neuropathy, inability to walk, muscle pain or cutaneous burning sensation Heterogeneity: In many cases participants were taking other substances known to increase risk of neuropathy |
|
Van Hunsel et al. (2018) Netherlands Case series NA NR |
N = 139 Population: All patients with adverse drug reactions (ADRs) related to neuropathy and health supplements that contain vitamin B6 reported to the Netherlands Pharmacovigilance Centre Lareb from 1991 (establishment of the center) until July 2017 Lost to follow‐up (%): NA n = 90 Sex (% women): 80 Age (y): 53.2 ± 15.7 (3–85) |
Data extracted from the database as an adverse drug reaction coded with a MedDRA preferred term (PT) within the MedDRA version 20.1 standardised MedDRA query (SMQ) ‘peripheral neuropathy’. Symptoms were self‐reported, and some symptoms could be neuropathy in general and not specifically peripheral neuropathy. Many patients were seen by a neurologist, but few diagnostic tests were reported. Some patients reported symptoms, but electromyogram was normal Causality assessed using the Bradford Hill criteria (strength of association, consistency of the cases, specificity, temporality, biological gradient, coherence, experiment, analogy) |
Vitamin B6 intake from supplements (mg/tablet or %DRV): 1.4–100 or 200–400 *number of tablets taken daily NR. Some cases took > 1 tablet/d with a dose > 100 mg/d *In 38 cases, vitamin B6 dose reached ≥ maximum acceptable daily intake of 25 mg for adults, and 22 of these cases had a dosage ≥50 mg. The dose was unknown in 27 cases Latency period (n = 57; mean d [y] ± SD): 807 [2.2] ± 1461 [4.0] (‘minutes after start’ ‐ [23]) ≤ 2 mo: n = 2 Serum vit B6 (n = 36; nmol/L, mean, median (range)): 907, 945 (88–4,338) (reference 51–183 nmol/L) Type of vitamer: NR Method: NR |
NA |
NS correlation (Pearson correlation r = − 0.876, p = 0.65) between vit B6 dose and serum levels (n = 29), no dose–response analysis was possible – The highest observed serum vit B6 level was caused by 50 mg/d of pyridoxine supplementation for 2 years – Another case used 75 mg/day for 2.5 months and reached a serum level > 500 nmol/L. After 1 month of withdrawal, the serum level decreased to 261 nmol/L and symptoms improved Recovery seen 2.5 y after cessation of vitamin supplements after vit B6 intake for 3.5 y (dose NR) Self‐reported symptoms: Case 1: Tingling of legs aggravating to muscle weakness of lower limb, numbness of arm and thumb and neuropathic pain (‘numbness in leg and balance difficulty’) Case 2: Strange feeling in right thigh, sensory disturbance, muscle twitching, involuntary muscle movements at rest, burning pain in both knees and fibula cups, burning pain, mushy feeling buttocks, and painful hips after sleep Case 3: Coldness of limbs and tingling feet, aching feet, very difficult to walk (= symptoms of small fibre peripheral neuropathy) Other cases: increased and burning sensation in the foot and lower leg + numbness of the big toe’ + tingling of lower legs Causality conclusion: ‘It is plausible for the B6 supplements to have caused complaints such as neuropathies. This is especially the case with higher dosages and prolonged use, but dosages < 50 mg/day also cannot be excluded’. ‘The dosage and timing at which patients are prone to develop adverse reactions is subject to a patient's individual susceptibility’. |
|
Kaur et al. (2014) NR Case report 3 mo post‐supplementation NR |
N = 1 Population: One case with progressive distal lower extremities weakness and paraesthesia 2 weeks following an episode of diarrhoea Exclusion criteria: NA Lost to follow‐up (%): NA Sex (% of women): 100 Age (y): 44 |
1. Physical examination 2. Electrodiagnostic studies |
Blood PLP, μg/L: 116 Blood PA, μg/L: 37 Vit B6 intake, mg: Reported as megadose, but mg NR Duration: 1 year Normative range: PLP: 5–30 μg/L, PA: 3–30 μg/L Method: Blood sample and self‐reported B6 intake |
NA |
1. Weakness of bilateral extensor hallucis longus and foot plantar flexion, hypoflexia at the ankles bilaterally and sensory loss to all primary modalities up to bilateral mid calves 2. Subacute sensorimotor axonal polyneuropathy Dorsal root ganglion cells and subsequent degeneration of both sensory peripheral nerve fibres and dorsal column axons = sensory neuropathy/neuronopathy After stopping vit B6 supplementation for 3 mo: Mild improvement in symptoms but motor weakness persisted |
Mean ± SD (standard deviation), unless specified otherwise.
BMI: body mass index; CC: case–control; CIAP: chronic idiopathic axonal polyneuropathy; CS: cross‐sectional; CTS: carpal tunnel syndrome; d: day; DRV: dietary reference value; hr: hour; MM: median motoneuropathy; mo: month; MRC: Medical research Council; NA: not applicable; NR: not reported; NS: non‐significant; PA: pyridoxic acid; PC: prospective cohort; PLP: pyridoxal‐5′‐phosphate; RC: retrospective cohort; RDI: recommended daily intake; UK: United Kingdom; USA: United Sates of America; vit: vitamin; y: year; wk/wks: week/s
D.2. Developmental toxicity
D.2.1. Intervention studies on developmental toxicity
|
Reference Study Country Duration Funding |
Design | Subject characteristics at baseline | Intervention | Results |
|---|---|---|---|---|
|
Shahraki et al. (2016) NR Iran Start: wk 4–16 of gestation End: delivery NR |
RCT, DB Inclusion: Primipara pregnant females; pregnancy‐related nausea and vomiting Exclusion: Multi‐gestational; refusal of antiemetic medication; drug allergy; underlying disorders; history of other types of antiemetic drugs in recent weeks; addiction; reproductive assisted technologies n = 200/188/188 G1: n = 88 (ondansetron) G2: n = 100 (B6) |
Sex (% women): G1: 100 G2: 100 Age (y, [mean ± SD]): G1: 24.8 ± 3.3 G2: 24.9 ± 4.0 |
Nutrient form: G1: 2 tablets/d ondansetron G2: 2 tablets/d vitamin B6 Doses: G1: 4 mg × 2/d G2: 40 mg × 2/d Background nutrient intake: NR Compliance: NR |
Birth weight (g): G1: 3,007 ± 442 G2: 2,950 ± 457 p = 0.67 Type of analysis: Chi‐square/Fisher's exact test T‐test/Mann–Whitney U‐Test No congenital anomalies in both groups |
|
Schuster et al. (1984) NR USA Start: wk 15 ± 4 (range 6–21) End: wk 30 or delivery Public |
RCT, DB Inclusion criteria: Good health at first visit; < 22 weeks pregnant; > 17 years; no B6 supplementation, no long‐term history (> 1 year) of oral contraceptives N = NR G1: (pregnant): n = 196 G2: (non‐pregnant control): n = 26 |
Sex (% women): 100 Age (y): G1: 17–38 G2: 19–34 PLP (pmol/mL): G1 (pregnant): 37.1 ± 25.3 – SubG1: 38.8 ± 17.3 – SubG2: 50.0 ± 32.5 – SubG3: 31.4 ± 10.0 – SubG4: 37.3 ± 6.2 – SubG5: 25.8 ± 13.5 – SubG6: 32.5 ± 31.3 – SubG7: 28.2 ± 12.8 G2 (non‐pregnant controls): 59.0 ± 19.0 |
Nutrient form: Pyridoxine*HCL (PN*HCL) (mg/d) in tablets: Pregnant women received in random order: 0, 2.6, 5, 7.5, 10, 15, 20 SubG1‐3 (pregnant): 0–5 SubG4‐7 (pregnant): 7.5–20 Background dietary B6 intake (mg/d): 1.43 ± 1.28 (83% consumed less than RDA [2.6 mg/d]) B6 intake/energy intake (mg/1,000 kcal): 0.67 ± 0.63 Compliance: NR |
Birth weight (g, mean ± SD) SubG1‐3 (pregnant): 3,240 ± 505 SubG4‐7 (pregnant): 3,287 ± 429 p > 0.05 |
Mean ± SD, unless specified otherwise.
d: day; DB: double blind; Gx: group x; NA: not applicable; NR: not reported; PLP: pyridoxal 5‐phosphate; PN*HCL: pyridoxine hydrochloride; RCT: randomised controlled trial; RDA: recommended dietary allowance; SD: standard deviation; SubGx: sub‐group x; USA: United States of America; wk: week; y: year.
D.2.2. Observational studies on developmental toxicity
|
Reference Study name Country Study design Follow‐up Funding |
Original cohort (N total) Exclusion criteria Population sampled Study population |
Ascertainment of outcome |
Exposure groups n/ person‐years Exposure assessment method |
Incident cases | Model covariates | Results |
|---|---|---|---|---|---|---|
|
Shrim et al. (2006) NR Canada PC ~ 32 weeks NR |
N = 192 Population sampled: General population B6 intake G1: Pregnant women: > 50 mg/day) G2: Pregnant women: 0 mg Exclusion criteria: NA % lost to follow up: 0 n = 192 G1: 96 G2: 96 Sex (% women): 100 Age (y, mean ± SD): G1: 32.5 ± 4.4 G2: 33.1 ± 4.2 Heterogeneity: Smoking status, n: G1: 1 G2: 12 p = 0.002 Parity (mean) : G1: 1.04 G2: 0.73 p = 0.03 |
Self‐reported birth weight Gestational age Malformation Live birth Twins |
Vit B6 intake (mg, mean ± SD): G1: 132.3 ± 74 G2: 0 Duration: Vit B6 intake: 9 ± 4.2 weeks Method: Self‐report via telephone interview |
Birth weight (g, mean ± SD): G1: 3,542 ± 512 G2: 3,321 ± 562 Malformation, n: G1: 1 G2: 0 Live birth, n: G1: 91 G2: 92 Twins, n: G1: 1 G2: 2 |
NA |
Birth weight p = 0.01 Malformation p = 0.3 Live birth p = 0.7 Twins NA |
|
Chang (1999) NR Taiwan CS During pregnancy Public |
N = 209 Population sampled: General population, healthy pregnant women Exclusion criteria: Diseases such as, diabetes, chronic hypertension, or kidney disease. Use of oral contraceptives or drugs known to interfere with B6 metabolism within 1 year of pregnancy n = 209 G1: 83 G2: 63 G3: 43 G4: 20 Sex (% women): 100 Age (y, mean ± SD): G1: 28.3 ± 4.1 G2: 30.2 ± 3.4 G3: 30.3 ± 1.8 G4: 29.4 ± 3.1 Heterogeneity: BMI (kg/m 2 , mean ± SD): G1: 19.3 ± 3.6 G2: 20.3 ± 2.0 G3: 20.3 ± 1.0 G4: 19.8 ± 1.1 Parity, n (mean ± SD): G1: 1.9 ± 0.8 G2: 1.7 ± 0.8 G3: 1.5 ± 0.5 G4: 1.3 ± 0.4 |
Measurement of birth weight |
Dose of pyridoxine*HCL supplement (mg/day): G1: 0 G2: 1 G3: 2 G4: 3 Intake of B6: ~ 1.0 mg/day for all groups Method: Blood samples (10 mL cord blood) at time of delivery used for determining PLP, PL, B6 aldehyde in cord plasma B6 intake estimated with Food frequency table |
NA | NA |
Maternal measurements Cord plasma concentrations of PLP (nM/L, mean ± SD): G1: 29 ± 6 G2: 40 ± 8 G3: 78 ± 2 G4: 90 ± 7 Cord plasma concentrations of PL (nM/L, mean ± SD): G1: 15 ± 8 G2: 14 ± 4 G3: 14 ± 2 G4: 16 ± 6 Cord plasma concentrations of B6 aldehyde (nM/L, mean ± SD): G1: 44 ± 11 G2: 55 ± 9 G3: 91 ± 2 G4: 106 ± 6 Correlations between supplementation and – Cord plasma PLP: 0.78 p < 0.0001 – Cord plasma PL: −0.14 p = NS – Cord total B6 aldehyde: 0.61 p < 0.0002 Neonate measurements: Birth weight with various conc. of PLP (nM/L) in cord blood (kg, mean ± SD): < 40: 3.0 ± 0.3 40–50: 3.3 ± 0.4 > 50: 3.4 ± 0.3 significantly different between < 40 and > 50 |
|
Goodman et al. (2019) NR USA CC 22 months Public |
N = 106 Population samples: G1 (case): Pregnant women diagnosed with fetal gastroschisis G2 (control): Maternal age and race/ethnicity matched pregnant women Exclusion criteria: Multiple pregnancies. Fetus known to have lethal anomalies, and/or chromosome abnormalities % lost to follow up: 0 n = 106 G1: 30 G2: 76 Sex (% women): 100 Age (y, mean ± SD): G1: 20.9 ± 4.5 G2: 22.7 ± 4.5 Heterogeneity: Parity, n (%): G1: Nulliparous: 18 (60.0) Parous: 12 (40.0) G2: Nulliparous: 28 (36.8) Parous: 48 (63.2) Conception BMI (kg/m 2 ), n (%): G1: < 19: 3 (10) 19–24: 12 (40) 25–29: 11 (37) > 30: 4 (130) G2: < 19: 3 (4)) 19–24: 32 (42) 25–29: 17 (22) ≥ 30: 24 (32) |
Mid‐pregnancy ultrasound was performed at < 24 wk gestation. The diagnosis of gastroschisis was confirmed by a Maternal‐Fetal Medicine physician |
Vit B6 supplementation n, (%): Prior to conception: G1: 2 (6.7) G2: 9 (11.8) After positive pregnancy test: G1: 12 (40.0) G2: 29 (38.2) After 6 weeks of gestation: G1: 15 (50.0) G2: 26 (34.2) None: G1: 1 (3.3) G2: 11 (14.5) Missing: G1: 0 (0.0) G2: 1 (1.3) Vit B6 concentration (ug/L), n (%): G1: Q1 (< 5): 2 (6.7) Q2 (≥ 5): 24 (80.0) Missing: 4 (11.3) G2: Q1 (< 5): 6 (7.9) Q2 (≥ 5): 60 (79.0) Missing: 10 (13.2) Method: 25 cc maternal blood sample (complete blood count of Vit B6) |
Fetal gastroschisis, n: G1: 30 G2: 0 |
Model 1: Unadjusted Model 2: Adjusted for insurance, education, BMI, and nulliparity |
Model 1: OR (95% CI) for gastroschisis: Q1: 0.81 (0.07–5.53) Q2: ref Model 2: OR (95% CI) for gastroschisis: Q1: 1.81 (0.11–22.32) Q2: ref |
|
Salcedo‐Bellido et al. (2017) NR Spain CC NR |
N = 1,066 Population sampled: General population, women who gave birth to a singleton baby Inclusion criteria: G1 (cases): Small for gestational age (SGA) infants without congenital malformations G2 (controls): non‐SGA infants without congenital malformations. Age matched to cases Excluded: G1: 15 G2: 15 Sex (% women): 100 n = 1,036 G1: 518 G2: 518 Sex (% women): 100 Smoking during pregnancy, n: G1: 149 G2: 80 p < 0,001 Previous preterm/LBW infant, n: G1: 64 G2: 26 p < 0,001 Preeclampsia, n: G1: 46 G2: 11 p < 0,001 Intrauterine growth retardation, n: G1: 141 G2: 8 p < 0.001 Pre‐pregnancy BMI (kg/m2, mean ± SD): G1: 23.1 ± 4.5 G2: 23.9 ± 4.1 p < 0.001 |
Birth weight was measured in the delivery room (data obtained from the clinical charts) |
G1: Vit B6 intake level (mg/day), n per quintile: Q1 (< 1.949): 134 Q2 (1.950–2.257): 116 Q3 (2.258–2.508): 80 Q4 (2.509–2.858): 105 Q5 (> 2.858): 83 G2: Vit B6 intake level (mg/day), n per quintile: Q1 (< 1.949): 104 Q2 (1.950–2.257): 104 Q3 (2.258–2.508): 103 Q4 (2.509–2.858): 104 Q5 (> 2.858): 103 Method: Semi‐quantitative FFQ. Nutrient scores were computed using computer software |
SGA, n: G1: 518 G2: 0 |
Model 1: No adjustments Model 2: Adjusted for energy intake, preeclampsia, education level, pre‐pregnancy body mass index, smoking, weight gain per week during pregnancy, and previous preterm/LBW newborn |
Model 1: OR (95% CI) for SGA: Q1: 1 (ref) Q2: 0.85 (0.58–1.23) Q3: 0.62* (0.42–0.91) Q4: 0.76 (0.52–1.12) Q5: 0.62* (0.42–0.93) * = significant association Model 2: OR (95% CI) for SGA: Q1: 1 (ref) Q2: 0.80 (0.52–1.22) Q3: 0.62* (0.40–0.96) Q4: 0.70 (0.45–1.08) Q5: 0.69 (0.43–1.08) * = significant association |
|
Hobbs et al. (2005) NR USA CC NR |
Sex (% women): 100 Population sampled: General population G1 (cases): Women with pregnancies affected by congenital heart defects (CHD) G2 (controls): Women with pregnancies not affected by CHDs Inclusion criteria: G1: Live‐born infant, stillborn infant or elective termination. Diagnosis of a nonsyndromic septal, cono‐truncal, or right‐ or left‐sided obstructive heart defect, confirmed by prenatal or postnatal echocardiogram, surgery, or autopsy report or all 3. Only non‐syndromic CHDs. G2: No birth defects G1 + G2: participation in the National Birth Defects Prevention Study Exclusion criteria: G1 + G2: Single gene disorder, chromosomal abnormality or syndrome. Pregnancy at the time of blood samples. Folate antagonist medication n = 456 G1: 331 G2: 125 Sex (% women): 100 Age, n: G1: < 30: 211 > 30: 120 G2: < 30: 74 > 30: 51 Heterogeneity: Smoking status, n: G1: 93 G2: 23 p = 0.0401 |
CHD knowledge was ascertained through the Arkansas Reproductive Health Monitoring System |
G1: B6 concentrations (nmol/L), n: Q1 (< 31.86 (30th)): 161 Q2 (< 27.90 (20th)): 111 Q3 (< 24.52 (10th)): 67 G2: B6 concentrations (nmol/L), n: Q1 (< 31.86 (30th)): 37 Q2 (< 27.90 (20th)): 24 Q3 (< 24.52 (10th)): 12 Method: Plasma concentration of pyridoxal‐5′‐phosphate measured in blood samples using an HPLC method |
CHD, n: G1: 331 G2: 0 |
Model 1: No adjustments Model 2: Adjusted for age, race, education, number of cigarettes smoked per day, alcohol consumption, vitamin intake, caffeine intake, breastfeeding status, and the interval between the end of pregnancy and study participation (multivariate linear regression) |
Model 1: B6 plasma concentration (nmol/L, mean ± SD): G1: 34.22 ± 11.40 r2: 37.23 ± 12.00 p = 0.0066 OR (95% CI) for CHD: Q1: 2.25 (1.45–3.50) Q2: 2.12 (1.29–3.50) Q3: 2.39 (1.24–4.59) Model 2: B6 plasma concentration (nmol/L, mean ± SD): G1: 34.22 ± 11.40 G2: 37.23 ± 12.00 p = 0.0023 OR (95% CI) for CHD: Q1: 2.52 (1.54–4.11) Q2: 2.63 (1.49–4.64) Q3: 2.86 (1.38–5.93) |
|
Zang et al. (2019) NR China CC NR |
N = 2000 Population sampled: General population, G1 (Cases): Women who gave birth to a child with CHD G2 (controls): Women who gave birth to a child without CHD Exclusion criteria: NR n = 2000 G1: 500 G2: 1500 Sex (% women): 100 Age: NR |
NR | Vitamin B6 intake: G1: NR G2: NR (G1 had higher intake of B6 than G2) Method: Semi‐quantitative FFQ |
CHD, n: G1: 500 G2: 0 |
NA | OR (95% CI) for CHD: Highest tertile vs lowest tertile: 0.44 (0.28–0.70) |
|
Furness et al. (2013) NR Australia PC Start: 18–20 weeks gestation End: after delivery Public |
N = 143 Population sampled: General population, pregnant women G1: Low risk for adverse pregnancy outcome G2: High risk for adverse pregnancy outcome Inclusion criteria: 6–20 weeks gestation Exclusion criteria: Condition requiring termination of pregnancy. Major fetal anomaly or fetal demise. Twins. Disorders requiring systemic steroids. Pre‐existing maternal renal disease % loss to follow up/excluded: 4.2% (n = 6) n = 137 G1: 46 G2: 91 Sex (%women): 100 Age (y, mean (95% CI)): G1: 31.1 (29.5–32.4) G2: 34.0 (32.4–35.6) p = 0.020 BMI (kg/m2, mean (95% CI)): G1: 26.5 (25.4–27.8) G2: 29.5 (28.1–31.1) p = 0.010 Smokers, n (%): G1: 3 (6.5%) G2: 18 (19.8%) p = 0.042 |
Risks of adverse pregnancy outcome were obtained from clinical records by senior obstetricians High risk: Obstetric risk factors, including a history of one or more of pre‐eclampsia/ eclampsia, early‐onset IUGR (< 34 weeks gestation and birthweight < 10th centile), placental abruption, preterm birth < 34 weeks gestation, recurrent pregnancy loss (three or more miscarriages) and previous fetal demise Low risk: No known pre‐existing medical (including chronic hypertension and diabetes mellitus) or obstetric disorders, and had had a previous normal pregnancy (birth > 37 weeks gestation, customised birthweight > 10th centile, with no gestational hypertension) |
Red blood cell (RBC) B6 concentration (nmol/L, mean (95% CI)): Total cohort: 41.9 (39.1–44.7) B6 supplementation, n: G1: 21 (45.7%) G2: 44 (48.4%) B6 supplementation dosage (ug, mean (95% CI)): G1: 1031 (653–1426) G2: 1301 (898–1703) Method: RCB B6 concentration: Blood samples Supplements: Dietary questionnaire data (type, dosage) |
Adverse pregnancy outcomes, n: G1: 7 G2: 67 |
NA |
RBC B6 concentration in different pregnancy outcomes (nmol/L, mean (95% CI)): Normal pregnancy: 41.9 (38–46) Pre‐eclampsia: 41.5 (29–54) IUGR: 50.0 (45–55) Other: 38.0 (33–44) p = 0.062 |
|
Carmichael et al. (2010) National Birth Defects Prevention Study USA CC 8–13 months from delivery until study interview Public |
N = 3181 Population sampled: General population G1 (cases): Women who gave birth to an infant with hypospadias G2 (controls): Women who gave birth to a male nonmalformed infant Inclusion criteria: Only second and third‐degree hypospadias Exclusion criteria: Infants with recognised single gene disorders or chromosomal abnormalities. Each case received a final review by 1 clinical geneticist to ensure that cases from each study centre met standard eligibility criteria n = 3111 G1: 893 G2: 2218 (those with data on dietary intake) Sex (% women): 100 Age, n (%) G1: < 25: 217 (22.7) 25–29: 209 (21.8) 30–34: 324 (33.8) > 35: 208 (21.7) G2: < 25: 852 (33.6) 25–29: 617 (24.4) 30–34: 664 (26.2) > 35: 345 (13.6) |
Medical record information (including operative reports when available) was reviewed by a clinical geneticist, who decided whether to include the case in the National Birth Defects Prevention Study database |
Dietary intake of B6 (mg) for those that did not take folate supplements, n: G1: Q1 (< 1.36): 20 Q2 (1.36–1.80): 15 Q3 (1.81–2.54): 19 Q4 (>2.55): 22 G2: Q1 (< 1.36): 75 Q2 (1.36–1.80): 58 Q3 (1.81–2.54): 84 Q4 (>2.55): 87 Dietary intake of B6 (mg) for those that did take folate supplements, n: G1: Q1 (< 1.36): 226 Q2 (1.36–1.80): 231 Q3 (1.81–2.54): 212 Q4 (>2.55): 148 G2: Q1 (< 1.36): 483 Q2 (1.36–1.80): 509 Q3 (1.81–2.54): 470 Q4 (>2.55): 452 Method: FFQ ‐ intake of food the last year FFQ ‐ intake the last 3 months before pregnancy |
Hypospadias, n: G1: 893 G2: 0 |
Model 1: Adjusted for energy intake, maternal race/ethnicity, education, age, number of previous live births, body mass index, plurality, fertility treatments or procedures and study site |
Model 1: No folate supplement: OR (95% CI) for hypospadias Q1: ref Q2: 1.0 (0.4–2.4) Q3: 1.0 (0.4–2.4) Q4: 1.5 (0.5–4.8) Folate supplement: OR (95% CI) for hypospadias Q1: ref Q2: 1.0 (0.8–1.3) Q3: 1.2 (0.9–1.6) Q4: 0.9 (0.6–1.3) |
|
McCullough et al. (2016) NR USA PC 3 years NR |
N = 496 Population sampled: An ethnically diverse population of women Exclusion criteria: No intend of using one of the participating obstetric facilities for delivery. Planned to relinquish custody of the child. Move from the area in the subsequent 3 years. HIV infection. Infant death before, during or soon after delivery % loss to follow up: 45 n = 496 (at delivery) n = 273 (after 3 years) Sex (% women): 100 Age (at delivery), n (%): 18‐ < 20: 22 (4) 20–29: 278 (56) 30–35: 147 (30) > 36: 50 (10) | Extraction of parturition data from medical records by trained personnel | Vit B6 concentration (nM/L, range): PLP: Q1: (ref) ≤ 3.76 Q2: 3.77–7.47 Q3: 7.48–12.05 Q4: > 12.05 PA: Q1: (ref) ≤ 2.06 Q2: 2.07–3.21 Q3: 3.22–5.93 Q4: > 5.93 Method: Maternal serum concentrations of pyridoxal phosphate (PLP) and 4‐pyridoxic acid (PA) | Birth weight (g, mean ± SD): 3294 ± 541 g | Model 1: Adjusted for gestational age at delivery, gestational age at blood draw, maternal pre‐pregnancy BMI, maternal race/ethnicity, parity, household income, maternal smoking | Model 1: Regression coefficients: Values: beta coefficient, standard error, p‐value Birth weight: PLP Q1: (ref) Q2: −72.75, 71.50, 0.31 Q3: −45.61, 73.22, 0.53 Q4: 39.81, 75.75, 0.60 PA: Q1: (ref) Q2: −13.80, 70.48, 0.84 Q3: −35.02, 70.51, 0.62 Q4: 9.95, 72.43, 0.89 |
|
Chen et al. (2015) Growing Up in Singapore Towards Healthy Outcomes (GUSTO) Singapore PC Study: ≈ week 26–39 Public |
N = 1247 Population sampled: General population, pregnant women Exclusion criteria: Mothers or fathers whose parents were of the different ethnicity. Serious health conditions such as psychosis and type 1 diabetes. In vitro fertilisation % lost to follow up: 1 (n = 15) n = 986 Sex (% women): 100 Age (y): 30.6 ± 5.2 | Information on birth weight, gestational age, infant sex, and birth order was retrieved from birth delivery reports | Vit B6 concentration (nmol/L, median (IQRs)): Q1: 15.4 (12.4–18.8) Q5: 152 (135–177) Method: Maternal fasting blood sample at 26th‐28th week of pregnancy |
Birth weight (g, mean ± SD): 3101 ± 449 |
Model 1: Unadjusted Model 2: Adjusted for infant sex, ethnicity, maternal age, gravidity, maternal height, prepregnancy BMI, weight gain up until 26 wk, educational level, and gestational diabetes mellitus |
Model 1: Birth weight: β per 1 SD of log vitamin B6 concentration (95% CI): 5.8 (−22.1–33.7) p = 0.69 Small for gestational age: OR (95% CI): 0.95 (0.77–1.17) p = 0.62 Model 2: Birth weight: β per 1 SD of log vitamin B6 concentration (95% CI): −3.9 (−30.7–22.9) p = 0.78 Small for gestational age: OR (95% CI): 0.98 (0.79–1.22) p = 0.87 |
| Robitaille et al. (2009) National Birth Defects Prevention Study (NBDPS) 1997–2003 USA CC NA Public | N = 5,464 Population sampled: General population G1a + b (cases): Women who gave birth to an infant with trans limb deficiency (TLD) or longitudinal limb deficiency (LLD) G2 (controls): Women who gave birth to nonmalformed infants Exclusion criteria: G1a + b: Identified single‐gene mutations or clinical histories suggestive of a mendelian disorder. Evidence of amnion rupture sequence or central axis deficiencies (split hand/split foot). Maternal diabetes pre pregnancy % loss to follow up: (did not answer maternal interview) G1a: 16 G1b: 21 G2: 20 n = 4366 G1a (TLD): 272 G1b (LLD): 125 G2: 3969 Age, n (%): G1a: < 24: 119 (36.7) 25–34: 168 (51.9) > 35: 37 (11.4) G1b: < 24: 84 (40.4) 25–34: 100 (48.1) > 35: 24 (11.5) G2: < 24: 1665 (33.4) 25–34: 26336 (52.9) > 35: 684 (13.7) Race/ethnicity, n (%): Non‐Hispanic white: G1a: 180 (55.7) G1b: 116 (55.8) G2: 2993 (60.2) Non‐Hispanic black or African American black: G1a: 27(8.4) G1b: 27 (13.0) G2: 583 (11.72) Hispanic: G1a: 100 (31.0) G1b: 54 (26.0) G2: 1119 (22.5) > 8% higher in Gr1a compared to Gr2: Other: G1a: 16 (5.0) G1b: 11 (5.3) G2: 275 (5.5) | NBDPS records of limb deficiencies were reviewed independently by a clinical geneticist (R.S.O.) Clinical geneticists reviewed abstracted records of physical examinations, radiographs, laboratory investigations, autopsies, surgical reports, and other relevant medical information | Dietary vitamin B6 intake (mg) for those that did not take folate supplements, n, per quartile: G1a: Q1 (< 1.35): 16 Q2 (1.35–1.84): 16 Q3 (1.84–2.60): 12 Q4 (> 2.60): 19 G1b: Q1 (< 1.35): 14 Q2 (1.35–1.84): 3 Q3 (1.84–2.60): 11 Q4 (> 2.60): 11 G2: Q1 (< 1.35): 215 Q2 (1.35–1.84): 212 Q3 (1.84–2.60): 226 Q4 (> 2.60): 311 Dietary intake of B6 (mg) for those that did take folate supplements, n: G1a: Q1 (< 1.35): 58 Q2 (1.35–1.84): 57 Q3 (1.84–2.60): 52 Q4 (>2.60): 42 G1b: Q1 (< 1.35): 24 Q2 (1.35–1.84): 27 Q3 (1.84–2.60): 26 Q4 (> 2.60): 16 G2: Q1 (< 1.35): 788 Q2 (1.35–1.84): 790 Q3 (1.84–2.60): 758 Q4 (> 2.60): 669 | TLD, n: G1a: 272 G1b: 0 G2: 0 LLD, n: G1a: 0 G1b: 125 G2: 0 | Model 1: Adjustment for maternal age, maternal race or ethnicity, maternal education, smoking tobacco during the periconceptional period and drinking alcohol during the periconceptional period, energy intake (kcal), and body mass index | Model 1: B6 with No folate supplement: OR (95% CI) for TLD: Q1: 1.53 (0.52–4.50) Q2: 1.44 (0.54–3.85) Q3: 0.95 (0.36–2.47) Q4: 1.0 OR (95% CI) for LLD: Q1: 4.36 (0.93–20.48) Q2: 1.47 (0.28–7.57) Q3: 4.39 (1.19–16.12) Q4: 1.0 B6 with Folate supplement: OR (95% CI) for TLD: Q1: 1.30 (0.73–2.31) Q2: 1.19 (0.71–2.00) Q3: 1.11 (0.69–1.80) Q4: 1.0 OR (95% CI) for LLD: Q1: 1.15 (0.49–2.73) Q2: 1.32 (0.61–2.83) Q3: 1.39 (0.69–2.78) Q4: 1.0 |
| de Weerd et al. (2003) NR Netherlands PC From preconce‐ption to 6 wk and 10‐wk amenorrhea Public |
N = 253 Population sampled: General population, pregnant women Exclusion criteria: Treatment for infectious, endocrine, metabolic or malignant diseases. Twin‐pregnancy. Low birth weight (< 2.3 percentile). Preterm delivery (< 37 wk). Post term birth (> 42 wk). Intra‐uterine fetal death Controls: No first degree relative with known genetic disorder known to cause major congenital malformations n = 240 G1 (Normal birth): 194 G2 (miscarriages): 46 (119 women were diagnosed with epilepsy (99 were treated for it). 15 women with previous birth of a child with NTD. 106 healthy women) Age (y, mean ± SD) G1: 30 ± 3.5 G2: 30 ± 4.2 |
Birth weight was ascertained from the medical records Early pregnancy loss was defined as spontaneous abortion ending before or at 16 weeks amenorrhea | Vit B6 concentration (nmol/L, median (25–75 quartiles)): Preconceptional G1 (n = 96): 49 (43–59) G2 (n = 20): 47 (41–54) 6 weeks: G1 (n = 188): 47 (40–56) G2 (n = 41): 43 (37–51) 10 weeks: G1 (n = 174): 48 (41–57) G2 (n = 13): 44 (34–56) | Miscarriages, n: G1: 0 G2: 46 | NA | Birth weight and Early pregnancy loss: Periconceptional and first trimester B6 levels were unrelated to low birth weight and early loss of pregnancy (no significant differences in B6 levels at the different time points) |
|
Lagiou et al., 2005 NR USA PC 27th gestational wk until birth (~ 11–13 wks) Public |
N = 325 Population sampled: General population, pregnant women. Inclusion criteria: Caucasian < 40 years old Exclusion criteria: Parity > 2. Hormonal medication during index pregnancy. Diabetes or thyroid diseases. Known major fetus abnormalities. Spontaneous or planned abortion. Twins. Pregnancy lasted less than 37 or more than 42 weeks. Preeclampsia % loss to follow up: 32 (n = 103) n = 222 Sex (% women): 100 Age, n: 18–24: 5 25–29: 60 30–34: 138 ≥ 35: 19 Heterogeneity: Pre‐pregnancy BMI (kg/m 2 ), n: < 18.9: 26 19–21.9: 107 22–24.9: 59 ≥ 25: 30 Smoking in pregnancy, n: Yes: 11 No: 211 Parity, n: 1: 136 2: 86 | Birth weight, birth length and head circumference measured at delivery by study collaborators | B6 dietary intake (mg, mean ± SD and median (1th‐3th quartile)): 5.6 ± 7.7 4.1 (2.6–5.9) Method: Semi quantitative FFQ including vitamin supplements | NA | Model 1: No adjustments Model 2: Adjusted for age, education, parity, height, pre‐pregnancy BMI, oral contraceptives prior to index pregnancy, smoking, gender of offspring, exact gestational age at delivery and total energy intake |
Model 1: Birth weight (g, mean change (95% CI)): + 6.8 (−57.5 to +71.2) p = 0.84 Birth length (cm, mean change (95% CI)): − 0.02 (−0.33 to +0.30) p = 0.92 Head circumference (cm, mean change (95% CI)): + 0.01 (−0.22 to +0.24) p = 0.94 Model 2: Birth weight (g, mean change (95% CI)): + 2.7 (−54.4 to +59.7) p = 0.93 Birth length (cm, mean change (95% CI)): – 0.04 (−0.32 to +0.25) p = 0.79 Head circumference (cm, mean change (95% CI)): − 0.02 (−0.23 to +0.19) p = 0.87 |
|
Shaw et al., 2014 NR USA CC B6 measured: 15th‐18th wk of pregnancy End: after delivery Public |
N = 420 Population sampled: General population G1 (cases): Pregnant women with infants with cono‐truncal heart defects (d‐transposition of the great arteries (dTGA) and tetralogy of Fallot (TOF)) G2 (controls): Pregnant women with nonmalformed infants Exclusion criteria: Single gene disorders or chromosomal aneusomies % loss to follow up: 1.4% (n = 6) n = 414 G1: 137 G2: 177 Sex (as % women): 100 Age, n (%): G1: < 25: 41 (29.3) 25–29: 37 (26.4) 30–34: 40 (28.6) > 34: 22 (15.7) G2: < 25: 105 (37.5) 25–29: 72 (25.7) 30–34: 73 (26.1) > 34: 30 (10.7) Heterogeneity: Race/ethnicity, n (%): G1: Hispanic: 67 (48.9) White non‐Hispanic: 45 (33.0) Asian: 12 (8.8) Black: 8 (5.8) Other: 5 (3.7) G2: Hispanic: 160 (57.8) White non‐Hispanic: 63 (22.7) Asian: 26 (9.4) Black: 13 (4.7) Other: 15 (5.4) |
Case information was abstracted from multiple hospital reports and medical record |
Pyridoxal‐5‐phosphate concentration (nmol/L, mean ± SD): G1: 65.40 ± 52.36 G2: 69.38 ± 72.38 G1, n per quartile (nmol/L) Q1 (< 31.16): 26 Q2 (31.16–80.00): 78 Q3 (≥ 80.01): 33 G2, n per quartile (nmol/L) Q1 (< 31.16): 69 Q2 (31.16–80.00): 139 Q3 (≥ 80.01): 69 Pyridoxal (nmol/L, mean ± SD): G1: 51.45 ± 79.23 G2: 75.07 ± 208.62 G1, n per quartile (nmol/L) Q1 (< 16.86): 26 Q2 (16.86–49.56): 71 Q3 (≥49.57): 40 G2, n per quartile (nmol/L) Q1 (< 16.86): 70 Q2 (16.86–49.56): 137 Q3 (≥ 49.57): 70 Pyridoxic acid (nmol/L, mean ± SD): G1: 50.57 ± 87.25 G2: 67.08 ± 167.10 G1, n per quartile (nmol/L) Q1 (< 14.37): 22 Q2 (14.37–47.42): 77 Q3 (≥ 47.43): 38 G2, n per quartile (nmol/L): Q1 (< 14.37): 68 Q2 (14.37–47.42): 139 Q3 (≥ 47.43): 70 Pyridoxine (nmol/L, mean ± SD): G1: 0.03 ± 0.19 G2: 3.63 ± 30.76 Method: Serum samples were taken using BDTM Vacutainer 3.5 mL serum separator tubes with no anticoagulants or preservatives and centrifuged within 30 min |
Cono‐truncal heart defects, n: G1: 140 G2: 0 | Model 1: Adjusted for maternal race and age | Model 1: Pyridoxal‐5‐phosphate: OR (95% CI): Q1: 0.7 (0.4–1.2) Q2: Ref Q3: 0.7 (0.4–1.3) Linear trend: p = 0.93 Pyridoxal: OR (95% CI): Q1: 0.7 (0.4–1.3) Q2: Ref Q3: 1.0 (0.6–1.7) Linear trend: p = 0.28 Pyridoxic acid: OR (95% CI): Q1: 0.6 (0.3–1.1) Q2: Ref Q3: 0.9 (0.5–1.5) Linear trend: p = 0.29 |
| Czeizel et al., 2004 Hungarian Case–Control Surveillance of Congenital Abnormalities Hungary CC 17 years Public | N = 70,074 Population sampled: General population G1 (cases): Women with newborns or foetuses with congenital abnormalities (CA) G2 (controls): Women with infants without CA Exclusion criteria: NR % loss to follow‐up: 13 (n = 9,080) n = 60,994 Gr1: 22,843 Gr2: 38,151 Sex (% women): 100 Age (y, means ± SD): Gr1: 25.5 ± 5.3 Gr2: 25.5 ± 4.9 | Cases are selected from the Hungarian Congenital Abnormality Registry. It is mandatory for physicians to register CA in the registry | B6 supplement, n (%): G1: 2013 (8.8) G2: 4086 (10.7) General dose of B6 was 60 mg/day as treatment for nausea and vomiting during pregnancy Mean duration of treatment: G1: 2.5 months G2: 2.8 months Method: B6 data from antenatal care logbook and/or medical records. Or from questionnaire about supplementation use during pregnancy | CA, n: G1: 22,843 G2: 0 | Model 1: Adjusted for birth order, maternal age and employment status, nausea/vomiting during the second and third months of pregnancy |
Model 1: B6 supplementation the entire pregnancy G1: POR (95% CI) for total CA: 0.8 (0.7–0.9) G2: POR (95% CI) for total CA: Ref B6 supplementation the first month of pregnancy G1: POR (95% CI) for total CA: 0.7 (0.6–0.8) G2: POR (95% CI) for total CA: Ref B6 supplementation the second to third month of pregnancy G1: POR (95% CI) for total CA: 0.8 (0.7–0.9) G2: POR (95% CI) for total CA: Ref Medically B6 supplementation without folic acid G1 (n = 765): POR (95% CI) for total CA: 0.89 (0.80–0.98) G2 (n = 1645): POR (95% CI) for total CA: Ref |
|
Baker et al. ( 1977 ) NR USA Matched CC 1 year Private |
N = 100 Population sampled: General population, mothers of a child birthed at term Exclusion criteria: Supplemental vitamins, hard drugs, signs of malnutrition, C‐section n = 100 G1 (low birth weight (< 2500 g): 50 G2 (normal birth weight (> 2,500 g): 50 Sex (% women): 100 Age: (y, median): G1: 28 G2: 27 |
Birth weight measured at birth | Plasma B6 concentration (ng/mlL mean ± SD): Gr1: 22.3 ± 1.51 Gr2: 21.8 ± 1.50 Method: Blood samples from antecubital vein 30 min. post‐partum | Low birth weight, n: G1: 50 G2: 0 | NA | No significant differences in Vit B6 concentration between G1 and G2 |
Mean ± SD, unless specified otherwise.
BMI: body mass index; CC: case–control; CI: confidence interval; CHD: congenital heart defects; CrSe: cross sectional; FFQ: food frequency questionnaire; Gx: group; LLD: longitudinal limb deficiency; TDL: trans limb deficiency; IUGR: intrauterine growth retardation; NA: not applicable; NR: not reported; OR: odds ratio; PA: pyridoxic acid PC: prospective cohort; POR: prevalence odds ratio; PLP: pyridoxal phosphate; PL: pyridoxal; Qx: quartile or quintile; SGA: small for gestational age; SD: standard deviation; y: year.
D.2.3. Animal observational studies on developmental toxicity
|
Reference Study name Country Duration Funding |
Design | Subject characteristics at baseline | Intervention | Incident cases | Results |
|---|---|---|---|---|---|
|
Marathe et al. (1986) NR India B6: Day 6–15 of gestation Study: 0–20 weeks of gestation NR |
Animal (rat) study Parallel N = 48 G1: 12 (control) G2: 12 G3: 12 G4: 12 |
Sex (% female): 100 Wistar rats, weight range 200–220 g |
Pyridoxine hydrochloride: G1: 0 mg/kg per day G2: 200 mg/kg per day G3: 400 mg/kg per day G4: 800 mg/kg per day Background nutrient intake: All fed Hindustan Lever feed and water ad libitum B6 intake: NR Compliance: 100% |
Pup weight |
Pup weight (g, mean ± SD) G1: 6.57 ± 0.16 G2: 6.37 ± 0.16 G3: 6.26 ± 0.15 G4: 6.21 ± 0.12 Difference NS |
|
Schumacher et al. (1965) NR USA 2 weeks pre‐pregnancy + during pregnancy NR |
CT, parallel (rats) Inclusion criteria: Long‐Evans rat strain N = 55 G1 (control): n = 40 G2 (+pyridoxine): n = 15 |
Sex (% female): 100 Age: NR Weight (g, mean): G1 (control): 300 G2 (+pyridoxine): 300 |
Pyridoxine Doses (g/100 g diet) G1: 0.25 G2: 6.25 Background nutrient intake: NR Compliance: NR |
Birth weight |
Birth weight (g/litter, mean) (average weight provided only) G1: 6.2 G2: 6.1 Difference NS |
|
Alton‐Mackey and Walker (1973) NR Canada 14 days pre‐pregnancy + during pregnancy NR |
CT, parallel (rats) N = 48 |
Sex (% female): 100 Wistar rats, virgin Weight (g): 200 |
Basal diet supplemented with 400% National Research Council (NRC) recommendations for pyridoxine for 2 weeks prior to mating (control) During pregnancy, rats divided into six dietary groups. Basal diet supplemented with pyridoxine, % of NRC: G1: 400 G2: 100 G3: 75 G4: 50 G5: 25 G6: 0 Group size: NR Compliance: NR |
Birth weight |
Birth weight (g/four litters, mean ± SEM) G1: 78.2 ± 3.8 G2: 74.3 ± 3.6 G3: 66.7 ± 3.1 G4: 60.9 ± 3.7 G5: 47.8 ± 3.6 G6: 44.8 ± 3.1 G4, G5, G6 lower than G1, G2, G3, p < 0.05 |
|
Alton‐Mackey and Walker (1978) NR Canada 14 days prepregnancy + pregnancy NR |
CT, parallel (rats) N = 186 |
Sex (% female): 100 Wistar rats, virgin Weight (g): 200 |
Basal diet supplemented with 400% National Research Council (NRC) recommendations for pyridoxine for 2 weeks prior to mating (control) During pregnancy, basal diet supplemented with pyridoxine, % of NRC: G1 (n = 31): 400 G2 (n = 31): 100 G3 (n = 31): 75 G4 (n = 31): 50 G5 (n = 31): 25 G6 (n = 31): 0 Group size: NR Compliance: NR |
Birth weight |
Birth weight (g/litter, mean) (read from Figure 1) G1: 50 G2: 41 G3: 39 G4: 38 G5: 30 G6: 7 G5 and G6 (0–25%) very low birth weight Birth weight of G1, G2, G3, and G4 normal Tests + p‐values NR |
|
Ritchie et al. (1960) NR USA ~ 2nd month of pregnancy – delivery Public |
RCT, parallel (swine) Inclusion criteria: Basis for equal allotment to two treatments: Size, sex, breed, litter N (randomised/completed) = 44/42 G1 (randomised/completed): n = 22/21 G2 (randomised/completed): n = 22/21 |
Sex (% female): 100 G1: Control G2: Intervention Pregnant |
Pyridoxine‐HCl G1: Total pyridoxine (pyridoxine + pyridoxal + pyridoxamine) G2: Pyridoxine‐HCl Doses (mg/pound of diet) G1: 0.45 G2: 5 Background nutrient intake: NR but controlled and similar in both groups |
Birth weight |
Birth weight (pounds/litter mean ± SEM) G1: 29.1 ± 2.0 G2: 28.7 ± 2.0 Difference NS |
|
Khera (1975) NR Canada Gestation day 6–15 Public |
RCT, parallel (rats) Female Wistar rats N = NR n = 89 |
Sex (% women): 100 Weight range (g): 175–200 |
Pyridoxine hydrochloride Doses (mg/kg day): G1: 0 G2: 20 G3: 40 G4: 60 G5: 80 |
Birth weight + anomalies Types of anomalies investigated: Wavy ribs; lumbar ribs; sternal defects; and runts. Less commonly: pericardial haemorrhage and subcutaneous oedema |
Birth weight (g/animal, mean ± SD) G1: 4.8 ± 0.4 G2: 4.8 ± 0.3 G3: 4.8 ± 0.3 G4: 4.9 ± 0.3 G5: 4.8 ± 0.4 Anomalies (no/total examine, frequency) G1: 10/201 G2: 12/193 G3: 10/206 G4: 6/230 G5: 9/243 |
|
Dalto et al. (2016) NR Canada 30 days Private |
RCT, parallel (gilts) Inclusion criteria: NR N (randomised/completed/analysed) = 84/77/32 |
Sex (% female): 100 Age (d [range]): 135–170 Body weight (kg ± SD): 91.4 ± 1.0 |
Pyridoxine hydrochloride Doses (mg/kg feed) G1: 2.4 G2: 12.4 Compliance: NR |
Embryo weight at 30 days of gestation |
Embryo weight at 30 d of gestation (g, mean) G1: 22.75 G2: 20.12 Tests and p‐values NR |
Mean ± SD, unless specified otherwise.
CT: controlled trial; d: day; Gx: group; HCL: hydrochloride; NR: not reported; NS: not significant; RCT: randomised controlled trial; SEM: standard error of the mean; SD: standard deviation; USA: United States of America.
List of Annexes
1.
Annex A – Protocol for the Scientific Opinion on the revision of the EFSA's Tolerable Upper Intake Level of vitamin B6
Annex B – EFSA's intake assessment of vitamin B6
Annex C – Intake data from Competent Authorities in European Member States
Annex D – Outcome of the public consultation on the draft Scientific opinion on the Tolerable Upper Intake Level for vitamin B6
Supporting information
Protocol for the Scientific Opinion on the revision of the EFSA’s Tolerable Upper Intake Level of vitamin B6
EFSA’s intake assessment of vitamin B6
Intake data from Competent Authorities in European Member States
Outcome of the public consultation on the draft Scientific opinion on the Tolerable Upper Intake Level for vitamin B6
Suggested citation: EFSA NDA Panel (EFSA Panel on Nutrition, Novel Foods and Food Allergens) , Turck D, Bohn T, Castenmiller J, de Henauw S, Hirsch‐Ernst K‐I, Knutsen HK, Maciuk A, Mangelsdorf I, McArdle HJ, Pelaez C, Pentieva K, Siani A, Thies F, Tsabouri S, Vinceti M, Fairweather‐Tait S, Vrolijk M, Fabiani L, Titz A and Naska A, 2023. Scientific opinion on the tolerable upper intake level for vitamin B6. EFSA Journal 2023;21(5):8006, 110 pp. 10.2903/j.efsa.2023.8006
Requestor European Commission
Question number EFSA‐Q‐2021‐00369
Panel members Dominique Turck, Torsten Bohn, Jacqueline Castenmiller, Stefaan de Henauw, Karen‐Ildico Hirsch‐Ernst, Helle Katrine Knutsen, Alexandre Maciuk, Inge Mangelsdorf, Harry J McArdle, Carmen Pelaez, Kristina Pentieva, Alfonso Siani, Frank Thies, Sophia Tsabouri, Marco Vinceti and Androniki Naska.
Declarations of interest If you wish to access the declaration of interests of any expert contributing to an EFSA scientific assessment, please contact interestmanagement@efsa.europa.eu.
Acknowledgements The Panel wishes to thank for their contribution to this output: the WG on Upper Levels: Peter Aggett, Brandy Beverly, Torsten Bohn, Julia Bornhorst, Marta Crous Bou, Francesco Cubadda, Aymeric Dopter, Susan Fairweather‐Tait, Susan Lanham New, Georg Lietz, Harry J McArdle, Anne Molloy, Androniki Naska, Giovanni Passeri, Kristina Pentieva, Marco Vinceti and Misha Vrolijk; and EFSA staff members: Pedro Daniel Das Neves Ferreira, Rita Sofia Ferreira De Sousa, Irene Muñoz Guajardo, Zsuzsanna Horvath, Roanne Marie Saad and Angeliki Sofroniou. The Panel acknowledges Inge Tetens, Caroline Filskov Petersen, Sine Højlund Christensen, Trine Wilkens and Lasse Sommer Mikkelsen for the preparatory work as part of a procurement procedure. The Panel also wishes to acknowledge the contribution of all national institutions in European countries that provided consumption data for this scientific output and the authors of published papers on vitamin B6 who provided additional information upon request.
EFSA may include images or other content for which it does not hold copyright. In such cases, EFSA indicates the copyright holder and users should seek permission to reproduce the content from the original source.
Adopted: 29 March 2023
Annex A,B,C,D are available under the Supporting Information section
Notes
SCF, 2000. Scientific Committee on Food. Guidelines of the Scientific Committee on Food for the Development of Tolerable Upper Intake Levels for Vitamins and Minerals. in: Scientific Committee on Food, Scientific Panel on Dietetic Products, Nutrition and Allergies (2006). Tolerable Upper Intake Levels for Vitamins and Minerals. European Food Safety Authority. SCF, 2001. Scientific Committee on Food. Opinion of the Scientific Committee on Food on the Tolerable Upper Intake Level of Magnesium. in: Scientific Committee on Food, Scientific Panel on Dietetic Products, Nutrition and Allergies (2006). Tolerable Upper Intake Levels for Vitamins and Minerals. European Food Safety Authority.
Available online: https://www.efsa.europa.eu/it/data-report/food-consumption-data
Available online: https://www.efsa.europa.eu/it/data-report/food-composition-data
Working Group consisting of representatives of 27 EU Member States and Norway.
The Mintel GNPD contains information on over 3 million food and beverage products, of which more than 1 million are or have been available on the European food market. A total of, twenty five out of the 27 EU Member States and Norway are present in the database. The database provides the compulsory ingredient information reported on product labels and the nutrition declaration when available. https://www.mintel.com/globalnew-products-database
Regulation (EC) No 1925/2006 of the European Parliament and of the Council of 20 December 2006 on the addition of vitamins and minerals and of certain other substances to foods, OJ L 404, 30.12.2006, p. 26.
Commission Directive 2006/141/EC of 22 December 2006 on infant formulae and follow‐on formulae and amending Directive1999/21/EC, OJ L 401, 30.12.2006, p.1 and Commission Directive 2006/125/EC of 5 December 2006 on processed cereal‐based foods and baby foods for infants and young children, OJ L 339, 6.12.2006, p. 16.
Directive 2002/46/EC of the European Parliament and of the Council of 10 June 2002 on the approximation of the laws of the Member States relating to food supplements, OJ L 183, 12.7.2002, p. 51.
The Mintel GNPD provides data on the content of supplements per serving which may not always reflect the daily dose recommended by the manufacturer. This dose could be higher than the reported dose per serving and may also vary depending on the consumer's use.
Indicatively, 2.2% of the overall eating occasions reported a fortification descriptor (e.g. ‘F09.Fortification agent’) in the latest version of the Comprehensive Database (updated in July 2021, of which 0.01% report a vitamin B6‐related fortification descriptor, for foods such as sport drinks, biscuits and multivitamin juices. These figures are likely to underestimate the actual consumption of fortified foods, as some surveys did not address the consumption fortified foods or because the survey participants did not know if the food consumed was fortified.
Annex I of Règlement grand‐ducal du 11 décembre 2003 concernant les compléments alimentaires, which has last been modified by Règlement grand‐ducal du 26 novembre 2013 modifiant le règlement grand‐ducal modifié du 11 décembre 2003 concernant les compléments alimentaires. Available at: https://legilux.public.lu/eli/etat/leg/rgd/2013/11/26/n1/jo
Decreto Legislativo 21 maggio 2004, n. 169. Available at: https://www.salute.gov.it/imgs/C_17_pagineAree_1268_5_file.pdf
Available online https://www.gov.pl/web/gis/zespol-do-spraw-suplementow-diety
Warenwetregeling vrijstelling voedingssupplementen, Artikel 4. Available at: https://wetten.overheid.nl/BWBR0041264/2018-10-01
Registered with the Danish Veterinary and Food Administration. Available at: https://www.foedevarestyrelsen.dk/Foedevarer/Kosttilskud/Sider/S%C3%B8gIKosttilskud.aspx
The data on the follow‐up of the study were reported in a book chapter and were thus not retrieved in the present systematic review. The description of the results, therefore, relies on the reporting by the SCF (2000b).
The full dataset submitted to EFSA contained 234 entries. Cases with symptoms not necessarily indicative of neuropathy were not included in the description of the data set.
References
- Al Saad D, Awaisu A, El salem S, Abdulrouf PV, Thomas B and AlHail M, 2017. Is pyridoxine effective and safe for post‐partum lactation inhibition? A systematic review. Journal of Clinical Pharmacy and Therapeutics, 42, 373–382. 10.1111/jcpt.12526 [DOI] [PubMed] [Google Scholar]
- Alghamdi M, Bashiri FA, Abdelhakim M, Adly N, Jamjoom DZ, Sumaily KM, Alghanem B and Arold ST, 2021. Phenotypic and molecular spectrum of pyridoxamine‐5′‐phosphate oxidase deficiency: a scoping review of 87 cases of pyridoxamine‐5′‐phosphate oxidase deficiency. Clinical Genetics, 99, 99–110. 10.1111/cge.13843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alton‐Mackey MG and Walker BL, 1973. Graded levels of pyridoxine in the rat diet during gestation and the physical and neuromotor development of offspring. The American Journal of Clinical Nutrition, 26, 420–428. 10.1093/ajcn/26.4.420 [DOI] [PubMed] [Google Scholar]
- Alton‐Mackey MG and Walker BL, 1978. Physical and neuromotor development of progeny of pyridoxine‐restricted rats cross‐fostered with control or isonutritional dams. American Journal of Clinical Nutrition, 31, 241–246. 10.1093/ajcn/31.2.241 [DOI] [PubMed] [Google Scholar]
- Anses , online. Table de composition nutritionnelle des aliments Ciqual. Available online: https://ciqual.anses.fr/ [Accessed: 3 August 2022].
- Bacharach R, Lowden M and Ahmed A, 2017. Pyridoxine toxicity small fiber neuropathy with dysautonomia: a case report. Journal of Clinical Neuromuscular Disease, 19, 43–46. 10.1097/cnd.0000000000000172 [DOI] [PubMed] [Google Scholar]
- Baer RL, 1984. Cutaneous skin changes probably due to pyridoxine abuse. Journal of the American Academy of Dermatology, 10, 527–528. 10.1016/s0190-9622(84)80115-2 [DOI] [PubMed] [Google Scholar]
- Baker H, Thind IS, Frank O, DeAngelis B, Caterini H and Louria DB, 1977. Vitamin levels in low‐birth‐weight newborn infants and their mothers. American Journal of Obstetrics and Gynecology, 129, 521–524. 10.1016/0002-9378(77)90090-4 [DOI] [PubMed] [Google Scholar]
- Banihani SA, 2017. A systematic review evaluating the effect of vitamin B6 on semen quality. Urology Journal, 15, 1–5. 10.22037/uj.v0i0.3808 [DOI] [PubMed] [Google Scholar]
- Barbieri HE, Pearson M and Becker W (Livsmedelsverket), 2003. Riksmaten – barn 2003. Livsmedels‐ och näringsintag bland barn i Sverige. [Google Scholar]
- Berger A and Schaumburg HH, 1984. More on neuropathy from pyridoxine abuse. New England Journal of Medicine, 311, 986–987. [PubMed] [Google Scholar]
- Berger AR, Schaumburg HH, Schroeder C, Apfel S and Reynolds R, 1992. Dose response, coasting, and differential fiber vulnerability in human toxic neuropathy: a prospective study of pyridoxine neurotoxicity. Neurology, 42, 1367–1370. 10.1212/wnl.42.7.1367 [DOI] [PubMed] [Google Scholar]
- Berger MM, Shenkin A, Schweinlin A, Amrein K, Augsburger M, Biesalski HK, Bischoff SC, Casaer MP, Gundogan K, Lepp HL, de Man AME, Muscogiuri G, Pietka M, Pironi L, Rezzi S and Cuerda C, 2022. ESPEN micronutrient guideline. Clinical Nutrition, 41, 1357–1424. 10.1016/j.clnu.2022.02.015 [DOI] [PubMed] [Google Scholar]
- Bernstein A and Lobitz C, 1988. A clinical and electrophysiologic study of the treatment of painful diabetic neuropathies with pyridoxine. In: Leklem JE and Reynolds RD (eds). Clinical and physiological applications of vitamin B‐6. Liss, New York, Alan R. pp. 415–423. [Google Scholar]
- Blackburn K and Warren K, 2017. A case of peripheral neuropathy due to pyridoxine toxicity in association with NOS energy drink consumption (P4.043). Neurology, 88:P4.043. [Google Scholar]
- Bor MV, Refsum H, Bisp MR, Bleie Ø, Schneede J, Nordrehaug JE, Ueland PM, Nygard OK and Nexø E, 2003. Plasma vitamin B6 vitamers before and after oral vitamin B6 treatment: a randomized placebo‐controlled study. Clinical Chemistry, 49, 155–161. 10.1373/49.1.155 [DOI] [PubMed] [Google Scholar]
- Boxmeer JC, Smit M, Utomo E, Romijn JC, Eijkemans MJ, Lindemans J, Laven JS, Macklon NS, Steegers EA and Steegers‐Theunissen RP, 2009. Low folate in seminal plasma is associated with increased sperm DNA damage. Fertility and Sterility, 92, 548–556. 10.1016/j.fertnstert.2008.06.010 [DOI] [PubMed] [Google Scholar]
- Brousmiche DW and Wan P, 2002. Photogeneration of quinone methide‐type intermediates from pyridoxine and derivatives. Journal of Photochemistry and Photobiology A: Chemistry, 149, 71–81. 10.1016/S1010-6030(01)00654-2 [DOI] [Google Scholar]
- Brush MG, Bennett T and Hansen K, 1988. Pyridoxine in the treatment of premenstrual syndrome: a retrospective survey in 630 patients. British Journal of Clinical Practice, 42, 448–452. [PubMed] [Google Scholar]
- Carter TC, Pangilinan F, Molloy AM, Fan R, Wang Y, Shane B, Gibney ER, Midttun Ø, Ueland PM and Cropp CD, 2015. Common variants at putative regulatory sites of the tissue nonspecific alkaline phosphatase gene influence circulating pyridoxal 5′‐phosphate concentration in healthy adults. The Journal of Nutrition, 145, 1386–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SJ, 1999. Adequacy of maternal pyridoxine supplementation during pregnancy in relation to the vitamin B6 status and growth of neonates at birth. Journal of Nutritional Science and Vitaminology, 45, 449–458. 10.3177/jnsv.45.449 [DOI] [PubMed] [Google Scholar]
- Chaudary AN, Porter‐Blake A and Holford P, 2003. Indices of pyridoxine levels on symptoms associated with toxicity: a retrospective study. Journal of Orthomolecular Medicine, 18, 65–76. [Google Scholar]
- Chaudary V and Cornblath D, 2013. Is pyridoxine associated with peripheral neuropathy. Journal of the Peripheral Nervous System, 18, S20–S21. [Google Scholar]
- Chen LW, Lim AL, Colega M, Tint MT, Aris IM, Tan CS, Chong YS, Gluckman PD, Godfrey KM, Kwek K, Saw SM, Yap F, Lee YS, Chong MF and van Dam RM, 2015. Maternal folate status, but not that of vitamins B‐12 or B‐6, is associated with gestational age and preterm birth risk in a multiethnic Asian population. Journal of Nutrition, 145, 113–120. 10.3945/jn.114.196352 [DOI] [PubMed] [Google Scholar]
- Cohen M and Bendich A, 1986. Safety of pyridoxine — a review of human and animal studies. Toxicology Letters, 34, 129–139. 10.1016/0378-4274(86)90202-x [DOI] [PubMed] [Google Scholar]
- Czeizel AE, Puhó E, Bánhidy F and Acs N, 2004. Oral pyridoxine during pregnancy: potential protective effect for cardiovascular malformations. Drugs in R and D, 5, 259–269. 10.2165/00126839-200405050-00002 [DOI] [PubMed] [Google Scholar]
- da Silva MC, Guedes PEB, Silva FL and Snoeck P, 2021. Use of pyridoxine hydrochloride in the interruption of lactation in female dogs with pseudopregnancy. Animal Reproduction, 18, e20200062. 10.1590/1984-3143-AR2020-0062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Z, Wang R, Ang LW, Yuan JM and Koh WP, 2013. Dietary B vitamin intake and risk of hip fracture: the Singapore Chinese Health Study. Osteoporosis International, 24, 2049–2059. 10.1007/s00198-012-2233-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalto DB, Audet I, Lapointe J and Matte JJ, 2016. The importance of pyridoxine for the impact of the dietary selenium sources on redox balance, embryo development, and reproductive performance in gilts. Journal of Trace Elements in Medicine and Biology, 34, 79–89. 10.1016/j.jtemb.2016.01.001 [DOI] [PubMed] [Google Scholar]
- Dalton K, 1985. Pyridoxine overdose in premenstrual syndrome. Lancet, 1, 1168–1169. 10.1016/s0140-6736(85)92480-8 [DOI] [PubMed] [Google Scholar]
- Dalton K and Dalton MJ, 1987. Characteristics of pyridoxine overdose neuropathy syndrome. Acta Neurologica Scandinavica, 76, 8–11. 10.1111/j.1600-0404.1987.tb03536.x [DOI] [PubMed] [Google Scholar]
- Darin N, Reid E, Prunetti L, Samuelsson L, Husain RA, Wilson M, El Yacoubi B, Footitt E, Chong WK, Wilson LC, Prunty H, Pope S, Heales S, Lascelles K, Champion M, Wassmer E, Veggiotti P, de Crecy‐Lagard V, Mills PB and Clayton PT, 2016. Mutations in PROSC disrupt cellular pyridoxal phosphate homeostasis and cause vitamin‐B6‐dependent epilepsy. American Journal of Human Genetics, 99, 1325–1337. 10.1016/j.ajhg.2016.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong MH, Nawijn EL and Verkaik‐Kloosterman J, 2022a. Contribution of fortified margarines and other plant‐based fats to micronutrient intake in The Netherlands. European Journal of Nutrition, 61, 1893–1904. 10.1007/s00394-021-02757-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong MH, Nawijn EL and Verkaik‐Kloosterman J, 2022b. Contribution of voluntary fortified foods to micronutrient intake in The Netherlands. European Journal of Nutrition, 61, 1649–1663. 10.1007/s00394-021-02728-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Weerd S, Steegers‐Theunissen RP, de Boo TM, Thomas CM and Steegers EA, 2003. Maternal periconceptional biochemical and hematological parameters, vitamin profiles and pregnancy outcome. European Journal of Clinical Nutrition, 57, 1128–1134. 10.1038/sj.ejcn.1601654 [DOI] [PubMed] [Google Scholar]
- Del Tredici A, Bernstein A and Chinn K, 1985. Carpal tunnel syndrome and vitamin B‐6 therapy. In: Reynolds R and Leklem J (eds). Vitamin B‐6: Its role in health and disease. Liss, New York, Alan R. pp. 459–462. [Google Scholar]
- Donaldson GL and Bury RG, 1982. Multiple congenital‐abnormalities in a newborn boy associated with maternal use of fluphenazine enanthate and other drugs during pregnancy. Acta Paediatrica Scandinavica, 71, 335–338. 10.1111/j.1651-2227.1982.tb09428.x [DOI] [PubMed] [Google Scholar]
- Edwards P, Liu PK and Rose GA, 1990. Liquid chromatographic studies of vitamin B6 metabolism in man. Clinica Chimica Acta, 190, 67–80. 10.1016/0009-8981(90)90281-v [DOI] [PubMed] [Google Scholar]
- EFSA , online. Food composition data. Available online: https://www.efsa.europa.eu/en/microstrategy/food-composition-data [Accessed: 22 November 2022].
- EFSA (European Food Safety Authority) , 2010. Application of systematic review methodology to food and feed safety assessments to support decision making. EFSA Journal 2010;8(6):1637, 90 pp. 10.2903/j.efsa.2010.1637 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , 2011. Use of the EFSA Comprehensive European Food Consumption Database in exposure assessment. EFSA Journal 2011;9(3):2097, 34 pp. 10.2903/j.efsa.2011.2097 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , 2015. Scientific report on principles and process for dealing with data and evidence in scientific assessments. EFSA Journal 2015;13(5):4121, 35 pp. 10.2903/j.efsa.2015.4121 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , 2020. Draft framework for protocol development for EFSA's scientific assessments. EFSA supporting publication 2020;17(4):EN‐1843, 46 pp. 10.2903/sp.efsa.2020.EN-1843 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , 2022. Protocol for the intake assessments performed in the context of the revision of Tolerable Upper Intake Levels for selected nutrients. EFSA Supporting publication 2022:e200801, 15 pp. 10.2903/sp.efsa.2022.e200801 [DOI] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies) , 2016. Scientific opinion on Dietary Reference Values for vitamin B6. EFSA Journal 2016;14(6):4485, 79 pp. 10.2903/j.efsa.2016.4485 [DOI] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Nutrition, Novel Foods and Food Allergens) , 2022. Guidance for establishing and applying tolerable upper intake levels for vitamins and essential minerals. EFSA Journal 2022;20(1):e200102, 27 pp. 10.2903/j.efsa.2022.e200102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee , 2011. Statistical significance and biological relevance. EFSA Journal 2011;9(9):2372, 17 pp. 10.2903/j.efsa.2011.2372 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2012. Guidance on selected default values to be used by the EFSA Scientific Committee, Scientific Panels and Units in the absence of actual measured data. EFSA Journal 2012;10(3):2579, 32 pp. 10.2903/j.efsa.2012.2579 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2017a. Guidance on the assessment of the biological relevance of data in scientific assessments. EFSA Journal 2017;15(8):4970, 73 pp. 10.2903/j.efsa.2017.4970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee , 2017b. Guidance on the use of the weight of evidence approach in scientific assessments. EFSA Journal 2017;15(8):4971, 69 pp. 10.2903/j.efsa.2017.4971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee , 2020. Draft for internal testing Scientific Committee guidance on appraising and integrating evidence from epidemiological studies for use in EFSA's scientific assessments. EFSA Journal 2020;18(8):6221, 83 pp. 10.2903/j.efsa.2020.6221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis JM, 1987. Treatment of carpal‐tunnel syndrome with vitamin‐B6. Southern Medical Journal, 80, 882–884. 10.1097/00007611-198707000-00018 [DOI] [PubMed] [Google Scholar]
- EVM (Expert Group on Vitamins and Minerals) , 2003. Safe Upper Levels for vitamins and minerals, 360. [Google Scholar]
- Falcone TD and Sowa GA, 2013. Abnormal vitamin B6 and response to supplementation with pyridoxal 5‐phosphate (P5P) in patients with neuropathic pain: a case series. PM&R, 5, S216. 10.1016/j.pmrj.2013.08.323 [DOI] [Google Scholar]
- FAO/WHO (Food and Agriculture Organization of the United Nations/World Health Organization) , 2009. Annex 1 Glossary of Terms. In: Principles and methods for the risk assessment of chemicals in food. Environmental Health Criteria 240. WHO, Geneva. 34 pp. Available online: https://www.who.int/publications/i/item/9789241572408 [Google Scholar]
- Foca FJ, 1985. Motor and sensory neuropathy secondary to excessive pyridoxine ingestion. Archives of Physical Medicine and Rehabilitation, 66, 634–636. [PubMed] [Google Scholar]
- Forges T, Monnier‐Barbarino P, Alberto JM, Gueant‐Rodriguez RM, Daval JL and Gueant JL, 2007. Impact of folate and homocysteine metabolism on human reproductive health. Human Reproduction Update, 13, 225–238. 10.1093/humupd/dml063 [DOI] [PubMed] [Google Scholar]
- Franzblau A, Rock CL, Werner RA, Albers JW, Kelly MP and Johnston EC, 1996. The relationship of vitamin B6 status to median nerve function and carpal tunnel syndrome among active industrial workers. Journal of Occupational and Environmental Medicine, 38, 485–491. 10.1097/00043764-199605000-00009 [DOI] [PubMed] [Google Scholar]
- Frater‐Schröder M and Mahrer‐Busato M, 1975. A model reaction demonstrating alkylating properties of pyridoxol, involving an o‐quinone methide intermediate. Bioorganic Chemistry, 4, 332–341. 10.1016/0045-2068(75)90045-0 [DOI] [Google Scholar]
- Friedman MA, Resnick JS and Baer RL, 1986. Subepidermal vesicular dermatosis and sensory peripheral neuropathy caused by pyridoxine abuse. Journal of the American Academy of Dermatology, 14, 915–917. 10.1016/s0190-9622(86)70112-6 [DOI] [PubMed] [Google Scholar]
- Furness D, Fenech M, Dekker G, Khong TY, Roberts C and Hague W, 2013. Folate, vitamin B12, vitamin B6 and homocysteine: impact on pregnancy outcome. Maternal and Child Nutrition, 9, 155–166. 10.1111/j.1740-8709.2011.00364.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner LI, Welshsloan J and Cady RB, 1985. Phocomelia in infant whose mother took large doses of pyridoxine during pregnancy. Lancet, 1, 636. [DOI] [PubMed] [Google Scholar]
- Gdynia HJ, Müller T, Sperfeld AD, Kühnlein P, Otto M, Kassubek J and Ludolph AC, 2008. Severe sensorimotor neuropathy after intake of highest dosages of vitamin B6. Neuromuscular Disorders, 18, 156–158. 10.1016/j.nmd.2007.09.009 [DOI] [PubMed] [Google Scholar]
- Ghatge MS, Al Mughram M, Omar AM and Safo MK, 2021. Inborn errors in the vitamin B6 salvage enzymes associated with neonatal epileptic encephalopathy and other pathologies. Biochimie, 183, 18–29. 10.1016/j.biochi.2020.12.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman JR, Peck JD, Landmann A, Williams M and Elimian A, 2019. An evaluation of nutritional and vasoactive stimulants as risk factors for gastroschisis: a pilot study. Journal of Maternal‐Fetal and Neonatal Medicine, 32, 2346–2353. 10.1080/14767058.2018.1433657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory JF III, Trumbo PR, Bailey LB, Toth JP, Baumgartner TG and Cerda JJ, 1991. Bioavailability of pyridoxine‐5′‐β‐D‐glucoside determined in humans by stable‐isotopic methods. The Journal of Nutrition, 121, 177–186. 10.1093/jn/121.2.177 [DOI] [PubMed] [Google Scholar]
- Hadtstein F and Vrolijk M, 2021. Vitamin B‐6‐induced neuropathy: exploring the mechanisms of pyridoxine toxicity. Advances Nutrition, 12, 1911–1929. 10.1093/advances/nmab033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath O, Pitt J, Mandelstam S, Kuschel C, Vasudevan A and Donoghue S, 2021. Early‐onset vitamin B6‐dependent epilepsy due to pathogenic PLPBP variants in a premature infant: a case report and review of the literature. JIMD Reports, 58, 3–11. 10.1002/jmd2.12183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickx AG, Cukierski M, Prahalada S, Janos G, Booher S and Nyland T, 1985. Evaluation of Bendectin embryotoxicity in nonhuman primates: II. Double‐blind study in term cynomolgus monkeys. Teratology, 32, 191–194. 10.1002/tera.1420320206 [DOI] [PubMed] [Google Scholar]
- Heuer T, Walter C, Krems C and Hoffmann I, 2012. In: Deutsche Gesellschaft für Ernährung (ed). Nährstoffzufuhr über Supplemente ‐ Ergebnisse der Nationalen Verzehrsstudie II. 12. Ernährungsbericht edn, Bonn, DGE. pp. 86–97. [Google Scholar]
- Hindborg H (DTU Food National Institute) , 2015, unpublished. Dietary supplements may contribute to an adequate micronutrient intake in Danish adults but increase the risk of overdose in children.
- Hoffman JIE and Kaplan S, 2002. The incidence of congenital heart disease. Journal of the American College of Cardiology, 39, 1890–1900. 10.1016/S0735-1097(02)01886-7 [DOI] [PubMed] [Google Scholar]
- Hoover DM and Carlton WW, 1981. The subacute neurotoxicity of excess pyridoxine HCl and clioquinol (5‐chloro‐7‐iodo‐8‐hydroxyquinoline) in beagle dogs I. Clinical disease. Veterinary Pathology, 18, 745–756. 10.1177/030098588101800605 [DOI] [PubMed] [Google Scholar]
- Hoover DM, Carlton WW and Henrikson CK, 1981. Ultrastructural lesions of pyridoxine toxicity in beagle dogs. Veterinary Pathology, 18, 769–777. 10.1177/030098588101800607 [DOI] [PubMed] [Google Scholar]
- Hunt AD Jr, Stokes J Jr, McCrory WW and Stroud HH, 1954. Pyridoxine dependency: report of a case of intractable convulsions in an infant controlled by pyridoxine. Pediatrics, 13, 140–145. [PubMed] [Google Scholar]
- Ink SL, Mehansho H and Henderson LM, 1982. The binding of pyridoxal to hemoglobin. Journal of Biological Chemistry, 257, 4753–4757. [PubMed] [Google Scholar]
- IOM (Institute of Medicine) , 1998. Dietary Reference Intakes for thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B12, pantothenic acid, biotin, and choline. Food and Nutrition Board. National Academy Press, Washington, DC, USA. pp. 591. [PubMed] [Google Scholar]
- IUPAC–IUB CBN (International Union of Pure and Applied Chemistry–International Union of Biochemistry, Commission on biochemical nomenclature) , 1973. Nomenclature for vitamins B‐6 and related compounds. Recommendations 1973. European Journal of Biochemistry, 40, 325–327. [PubMed] [Google Scholar]
- Johnstone DL, Al‐Shekaili HH, Tarailo‐Graovac M, Wolf NI, Ivy AS, Demarest S, Roussel Y, Ciapaite J, van Roermund CWT, Kernohan KD, Kosuta C, Ban K, Ito Y, McBride S, Al‐Thihli K, Abdelrahim RA, Koul R, Al Futaisi A, Haaxma CA, Olson H, Sigurdardottir LY, Arnold GL, Gerkes EH, Boon M, Heiner‐Fokkema MR, Noble S, Bosma M, Jans J, Koolen DA, Kamsteeg EJ, Drogemoller B, Ross CJ, Majewski J, Cho MT, Begtrup A, Wasserman WW, Bui T, Brimble E, Violante S, Houten SM, Wevers RA, van Faassen M, Kema IP, Lepage N, Care4Rare Canada C, Lines MA, Dyment DA, RJA W, Verhoeven‐Duif N, Ekker M, Boycott KM, Friedman JM, Pena IA and van Karnebeek CDM, 2019. PLPHP deficiency: clinical, genetic, biochemical, and mechanistic insights. Brain, 142, 542–559. 10.1093/brain/awy346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justiniano R, Williams JD, Perer J, Hua A, Lesson J, Park SL and Wondrak GT, 2017. The B6 ‐vitamer Pyridoxal is a Sensitizer of UVA‐induced Genotoxic Stress in Human Primary Keratinocytes and Reconstructed Epidermis. Photochemistry and Photobiology, 93, 990–998. 10.1111/php.12720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasaragod VB, Pacios‐Michelena A, Schaefer N, Zheng F, Bader N, Alzheimer C, Villmann C and Schindelin H, 2020. Pyridoxal kinase inhibition by artemisinins down‐regulates inhibitory neurotransmission. Proceedings of the National Academy of Sciences of the United States of America, 117, 33235–33245. 10.1073/pnas.2008695117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur D, Velazquez‐Rodriguez Y and Ahmed A, 2014. When expected turns unexpected: a case of subacute progressive weakness and paresthesias of the distal lower extremities following a brief diarrheal episode. Neurology, 82, P6.111. [Google Scholar]
- Kehoe L and Walton J, 2022. Report on the intake of micronutrients (Vitamin A, β‐carotene, retinol, vitamin D, vitamin E, vitamin B6, folic acid, iron and manganese) from food supplements in the Irish population. Department of Biological Sciences, Munster Technological University; Report commissioned by the Food Safety Authority of Ireland, 7 pp. [Google Scholar]
- Keniston RC, Nathan PA, Leklem JE and Lockwood RS, 1997. Vitamin B6, vitamin C, and carpal tunnel syndrome. A cross‐sectional study of 441 adults. Journal of Occupational and Environmental Medicine, 39, 949–959. 10.1097/00043764-199710000-00007 [DOI] [PubMed] [Google Scholar]
- Khera KS, 1975. Teratogenicity study in rats given high doses of pyridoxine (vitamin‐B6) during organogenesis. Experientia, 31, 469–470. 10.1007/bf02026385 [DOI] [PubMed] [Google Scholar]
- King AE, Dickson TC, Blizzard CA, Foster SS, Chung RS, West AK, Chuah MI and Vickers JC, 2007. Excitotoxicity mediated by non‐NMDA receptors causes distal axonopathy in long‐term cultured spinal motor neurons. European Journal of Neuroscience, 26, 2151–2159. 10.1111/j.1460-9568.2007.05845.x [DOI] [PubMed] [Google Scholar]
- Krinke G, Schaumburg HH, Spencer PS, Suter J, Thomann P and Hess R, 1981. Pyridoxine megavitaminosis produces degeneration of peripheral sensory neurons (sensory neuronopathy) in the dog. Neurotoxicology, 2, 13–24. [PubMed] [Google Scholar]
- Lagiou P, Mucci L, Tamimi R, Kuper H, Lagiou A, Hsieh CC and Trichopoulos D, 2005. Micronutrient intake during pregnancy in relation to birth size. European Journal of Nutrition, 44, 52–59. 10.1007/s00394-004-0491-1 [DOI] [PubMed] [Google Scholar]
- Levine S and Saltzman A, 2004. Pyridoxine (vitamin B6) neurotoxicity: enhancement by protein‐deficient diet. Journal of Applied Toxicology, 24, 497–500. 10.1002/jat.1007 [DOI] [PubMed] [Google Scholar]
- Li K, Wahlqvist ML and Li D, 2016. Nutrition, one‐carbon metabolism and neural tube defects: a review. Nutrients, 8, 741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Farkas P, Wang K, Kohli MO and Fitzpatrick TB, 2022. B vitamin supply in plants and humans: the importance of vitamer homeostasis. Plant Journal, 111, 662–682. 10.1111/tpj.15859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loohuis LM, Albersen M, de Jong S, Wu T, Luykx JJ, Jans JJM, Verhoeven‐Duif NM and Ophoff RA, 2018. The alkaline phosphatase (ALPL) locus is associated with B6 vitamer levels in CSF and plasma. Genes (Basel), 10. 10.3390/genes10010008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoPachin RM and Gavin T, 2014. Molecular mechanisms of aldehyde toxicity: a chemical perspective. Chemical Research in Toxicology, 27, 1081–1091. 10.1021/tx5001046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez MG, Bonaa KH, Ebbing M, Eriksen EF, Gjesdal CG, Nygard O, Tell GS, Ueland PM and Meyer HE, 2017. B vitamins and hip fracture: secondary analyses and extended follow‐up of two large randomized controlled trials. Journal of Bone and Mineral Research, 32, 1981–1989. 10.1002/jbmr.3189 [DOI] [PubMed] [Google Scholar]
- Lumbers M, New SA, Gibson S and Murphy MC, 2001. Nutritional status in elderly female hip fracture patients: comparison with an age‐matched home living group attending day centres. British Journal of Nutrition, 85, 733–740. 10.1079/bjn2001350 [DOI] [PubMed] [Google Scholar]
- Mackey AD, McMahon RJ, Townsend JH and Gregory JF 3rd, 2004. Uptake, hydrolysis, and metabolism of pyridoxine‐5′‐beta‐D‐glucoside in Caco‐2 cells. Journal of Nutrition, 134, 842–846. 10.1093/jn/134.4.842 [DOI] [PubMed] [Google Scholar]
- Maeda T, Taguchi H, Minami H, Sato K, Shiga T, Kosaka H and Yoshikawa K, 2000. Vitamin B6 phototoxicity induced by UVA radiation. Archives Dermatology Research, 292, 562–567. 10.1007/s004030000174 [DOI] [PubMed] [Google Scholar]
- Malek E, Doumiati H and Salameh JS, 2020. Pyridoxine‐induced sensory ataxic ganglionopathy: a case report and literature review. Acta Neurologica Belgica, 120, 413–414. 10.1007/s13760-018-0919-7 [DOI] [PubMed] [Google Scholar]
- Marathe MR and Thomas GP, 1986. Absence of teratogenicity of pyridoxine in Wistar rats. Indian Journal of Physiology and Pharmacology, 30, 264–266. [PubMed] [Google Scholar]
- Marelli AJ, Mackie AS, Ionescu‐Ittu R, Rahme E and Pilote L, 2007. Congenital heart disease in the general population. Circulation, 115, 163–172. 10.1161/CIRCULATIONAHA.106.627224 [DOI] [PubMed] [Google Scholar]
- Mensink GB, Haftenberger M, Lage‐Barbosa C, Brettschneider AK, Lehmann F, Frank M, Heide K, Moosburger R, Patelakis E and Perlitz H 2021. EsKiMo II ‐ Die Ernährungsstudie als KiGGS‐Modul. doi: 10.25646/7028.2 [DOI]
- McCullough LE, Miller EE, Mendez MA, Murtha AP, Murphy SK and Hoyo C, 2016. Maternal B vitamins: effects on offspring weight and DNA methylation at genomically imprinted domains. Clinical Epigenetics, 8, 8. 10.1186/s13148-016-0174-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean RR, Jacques PF, Selhub J, Fredman L, Tucker KL, Samelson EJ, Kiel DP, Cupples LA and Hannan MT, 2008. Plasma B vitamins, homocysteine, and their relation with bone loss and hip fracture in elderly men and women. Journal of Clinical Endocrinology and Metabolism, 93, 2206–2212. 10.1210/jc.2007-2710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer HE, Willett WC, Fung TT, Holvik K and Feskanich D, 2019. Association of high intakes of vitamins B6 and B12 from food and supplements with risk of hip fracture among postmenopausal women in the Nurses' Health Study. JAMA Network Open, 2, e193591. 10.1001/jamanetworkopen.2019.3591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake K, Yasujima T, Takahashi S, Yamashiro T and Yuasa H, 2022. Identification of the amino acid residues involved in the species‐dependent differences in the pyridoxine transport function of SLC19A3. Journal of Biological Chemistry, 298, 102161. 10.1016/j.jbc.2022.102161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J and Altman DG, 2009. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. BMJ, 339, b2535. 10.1136/bmj.b2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molimard R, Marillaud A, Paille A, Le Devehat C, Lemoine A and Dougny M, 1980. Impairment of memorization by high doses of pyridoxine in man. Biomedicine, 32, 88–92. [PubMed] [Google Scholar]
- Montpetit VJ, Clapin DF, Tryphonas L and Dancea S, 1988. Alteration of neuronal cytoskeletal organization in dorsal root ganglia associated with pyridoxine neurotoxicity. Acta Neuropathology, 76, 71–81. 10.1007/BF00687682 [DOI] [PubMed] [Google Scholar]
- Murata Y, Kumano K, Ueda T, Araki N, Nakamura T and Tani M, 1998. Photosensitive dermatitis caused by pyridoxine hydrochloride. Journal of the American Academy of Dermatology, 39, 314–317. 10.1016/s0190-9622(98)70379-2 [DOI] [PubMed] [Google Scholar]
- Nakano H, McMahon LG and Gregory JF III, 1997. Pyridoxine‐5′‐β‐D‐glucoside exhibits incomplete bioavailability as a source of vitamin B‐6 and partially inhibits the utilization of co‐ingested pyridoxine in humans. The Journal of Nutrition, 127, 1508–1513. 10.1093/jn/127.8.1508 [DOI] [PubMed] [Google Scholar]
- NHMRC (National Health and Medical Research Council, Australian Government Department of Health and Ageing, New Zealand Ministry of Health) , 2006. Nutrient Reference Values for Australia and New Zealand. National Health and Medical Research Council, Canberra. 320 p.. [Google Scholar]
- No authors listed , 2009. Vitamin B6: peripheral neuropathy. Dependent on dose and duration of use. Prescrire International, 18, 257. [PubMed] [Google Scholar]
- No authors listed , 2020. Vitamin B6: More cases of neuropathy through chronic overdosing. Prescrire International, 29, 21–22. [Google Scholar]
- OHAT‐NTP (Office of Health Assessment and Translation‐Division of the National Toxicology Program) , 2015. OHAT risk of bias rating tool for human and animal studies, 37 pp. Available online. https://ntp.niehs.nih.gov/ntp/ohat/pubs/riskofbiastool_508.pdf [Google Scholar]
- OHAT‐NTP (Office of Health Assessment and Translation‐Division of the National Toxicology Program) , 2019. Handbook for conducting a literature‐based health assessment using OHAT approach for systematic review and evidence integration. Available online: https://ntp.niehs.nih.gov/ntp/ohat/pubs/handbookjan2015_508.pdf [Google Scholar]
- Perlitz H, Mensink GBM, Lage Barbosa C, Richter A, Brettschneider AK, Lehmann F, Patelakis E, Frank M, Heide K and Haftenberger M 2019. Use of vitamin and mineral supplements among adolescents living in Germany‐Results from EsKiMo II. Nutrients, 11. doi: 10.3390/nu11061208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry GJ and Bredesen DE, 1985. Sensory neuropathy with low‐dose pyridoxine. Neurology, 35, 1466–1468. 10.1212/wnl.35.10.1466 [DOI] [PubMed] [Google Scholar]
- Phillips WEJ, Mills JHL, Charbonneau SM, Tryphonas L, Hatina GV, Zawidzka Z, Bryce FR and Munro IC, 1978. Subacute toxicity of pyridoxine hydrochloride in the beagle dog. Toxicology and Applied Pharmacology, 44, 323–333. 10.1016/0041-008x(78)90194-1 [DOI] [PubMed] [Google Scholar]
- Philpot J, Muntoni F, Skellett S and Dubowitz V, 1995. Congenital symmetrical weakness of the upper limbs resembling brachial plexus palsy: a possible sequel of drug toxicity in first trimester of pregnancy? Neuromuscular Disorders, 5, 67–69. 10.1016/0960-8966(94)e0028-7 [DOI] [PubMed] [Google Scholar]
- Plecko B and Mills P, 2022. PNPO Deficiency. In: Adam MP, Mirzaa GM, Pagon RA, Wallace SE, Bean LJH, Gripp KW and Amemiya A (eds). GeneReviews® [Internet]. University of Washington, Seattle, Seattle (WA). [PubMed] [Google Scholar]
- Plecko B, Zweier M, Begemann A, Mathis D, Schmitt B, Striano P, Baethmann M, Vari MS, Beccaria F, Zara F, Crowther LM, Joset P, Sticht H, Papuc SM and Rauch A, 2017. Confirmation of mutations in PROSC as a novel cause of vitamin B 6 ‐dependent epilepsy. Journal of Medical Genetics, 54, 809–814. 10.1136/jmedgenet-2017-104521 [DOI] [PubMed] [Google Scholar]
- Ramos RJ, Albersen M, Vringer E, Bosma M, Zwakenberg S, Zwartkruis F, Jans JJM and Verhoeven‐Duif NM, 2019. Discovery of pyridoxal reductase activity as part of human vitamin B6 metabolism. Biochimica et Biophysica Acta: General Subjects, 1863, 1088–1097. 10.1016/j.bbagen.2019.03.019 [DOI] [PubMed] [Google Scholar]
- Reynolds RD, 1988. Bioavailability of vitamin B‐6 from plant foods. The American Journal of Clinical Nutrition, 48, 863–867. 10.1093/ajcn/48.3.863 [DOI] [PubMed] [Google Scholar]
- Ritchie HD, Miller ER, Ullrey DE, Hoefer JA and Luecke RW, 1960. Supplementation of the Swine gestation diet with pyridoxine. The Journal of Nutrition, 70, 491–496. 10.1093/jn/70.4.491 [DOI] [PubMed] [Google Scholar]
- Roberge AG, Moufarege A, Lavoie J, Roberge C and Tremblay RR, 1984. Biochemical properties and kinetic parameters of monoamine oxydase in human seminal plasma. International Journal of Fertility, 29, 180–185. [PubMed] [Google Scholar]
- Roe MA, Bell S, Oseredczuk M, Christensen T, Westenbrink S, Pakkala H, Presser K, Finglas PM and Consortium E, 2013. Updated food composition database for nutrient intake. EFSA supporting publication. 10.2903/sp.efsa.2013.EN-355 [DOI] [Google Scholar]
- Said HM, 2011. Intestinal absorption of water‐soluble vitamins in health and disease. Biochemical Journal, 437, 357–372. 10.1042/BJ20110326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Said HM, Ortiz A and Ma TY, 2003. A carrier‐mediated mechanism for pyridoxine uptake by human intestinal epithelial Caco‐2 cells: regulation by a PKA‐mediated pathway. American Journal of Physiology. Cell Physiology, 285, C1219–C1225. 10.1152/ajpcell.00204.2003 [DOI] [PubMed] [Google Scholar]
- Salcedo‐Bellido I, Martínez‐Galiano JM, Olmedo‐Requena R, Mozas‐Moreno J, Bueno‐Cavanillas A, Jimenez‐Moleon JJ and Delgado‐Rodríguez M, 2017. Association between vitamin intake during pregnancy and risk of small for gestational age. Nutrients, 9. 10.3390/nu9121277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K, Taguchi H, Maeda T and Yoshikawa K, 1993. Pyridoxine toxicity to cultured fibroblasts caused by near‐ultraviolet light. Journal of Investigative Dermatology, 100, 266–270. 10.1111/1523-1747.ep12469648 [DOI] [PubMed] [Google Scholar]
- SCF (Scientific Committee for Food) , 1993. Nutrient and energy intakes for the European Community. Reports of the Scientific Committee for Food, 31st Series. 248 pp. [DOI] [PubMed] [Google Scholar]
- SCF (Scientific Committee on Food) , 2000. Opinion of the Scientific Committee on Food on the Tolerable Upper Intake Level of vitamin B6, 15. [Google Scholar]
- Schaeffer MC, Sampson DA, Skala JH, Gietzen DW and Grier RE, 1989. Evaluation of vitamin B‐6 status and function of rats fed excess pyridoxine. Journal of Nutrition, 119, 1392–1398. 10.1093/jn/119.10.1392 [DOI] [PubMed] [Google Scholar]
- Schaeffer MC, Gretz D, Mahuren JD and Coburn SP, 1995. Tissue B‐6 vitamer concentrations in rats fed excess vitamin B‐6. Journal of Nutrition, 125, 2370–2378. 10.1093/jn/125.9.2370 [DOI] [PubMed] [Google Scholar]
- Schaeppi U and Krinke G, 1982. Pyridoxine neuropathy: correlation of functional tests and neuropathology in beagle dogs treated with large doses of vitamin B6. Agents and Actions, 12, 575–582. 10.1007/BF01965944 [DOI] [PubMed] [Google Scholar]
- Schaeppi U and Krinke G, 1985. Differential vulnerability of 3 rapidly conducting somatosensory pathways in the dog with vitamin B6 neuropathy. Agents and Actions, 16, 567–579. 10.1007/BF01983664 [DOI] [PubMed] [Google Scholar]
- Schaumburg H, Kaplan J, Rasmus S and Vick N, 1982. Pyridoxine megavitaminosis produces sensory neuropathy (neuronopathy?) in humans. Annals of Neurology, 12, P98. [Google Scholar]
- Schaumburg H, Kaplan J, Windebank A, Vick N, Rasmus S, Pleasure D and Brown MJ, 1983. Sensory neuropathy from pyridoxine abuse. A new megavitamin syndrome. New England Journal of Medicine, 309, 445–448. 10.1056/nejm198308253090801 [DOI] [PubMed] [Google Scholar]
- Schlaudecker EP, Munoz FM, Bardají A, Boghossian NS, Khalil A, Mousa H, Nesin M, Nisar MI, Pool V, Spiegel HML, Tapia MD, Kochhar S and Black S, 2017. Small for gestational age: Case definition & guidelines for data collection, analysis, and presentation of maternal immunisation safety data. Vaccine, 35, 6518–6528. 10.1016/j.vaccine.2017.01.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher MF, Williams MA and Lyman RL, 1965. Effect of high intakes of thiamine, riboflavin and pyridoxine on reproduction in rats and vitamin requirements of the offspring. Journal of Nutrition, 86, 343–349. 10.1093/jn/86.4.343 [DOI] [PubMed] [Google Scholar]
- Schuster K, Bailey LB and Mahan CS, 1984. Effect of maternal pyridoxine HCl supplementation on the vitamin B‐6 status of mother and infant and on pregnancy outcome. Journal of Nutrition, 114, 977–988. 10.1093/jn/114.5.977 [DOI] [PubMed] [Google Scholar]
- Scott K, Zeris S and Kothari MJ, 2008. Elevated B6 levels and peripheral neuropathies. Electromyography and Clinical Neurophysiology, 48, 219–223. [PubMed] [Google Scholar]
- Scudi JV, Buhs RP and Hood DB, 1942. The metabolism of vitamin B6. Journal of Biological Chemistry, 142, 323–328. 10.1016/S0021-9258(18)72727-0 [DOI] [Google Scholar]
- Shahraki Z, Hashemi Bonjar ZS, Forghani F and Nakhai R, 2016. Comparing neonatal outcome following the use of ondansetron versus vitamin B6 in pregnant females with morning sickness: A randomized clinical trial., 7, e37081. 10.17795/compreped-37081 [DOI] [Google Scholar]
- Sharma P, Han SM, Gillies N, Thorstensen EB, Goy M, Barnett MPG, Roy NC, Cameron‐Smith D and Milan AM, 2020. Circulatory and urinary B‐vitamin responses to multivitamin supplement ingestion differ between older and younger adults. Nutrients, 12. 10.3390/nu12113529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw GM, Yang W, Carmichael SL, Vollset SE, Hobbs CA, Lammer EJ and Ueland PM, 2014. One‐carbon metabolite levels in mid‐pregnancy and risks of conotruncal heart defects. Birth Defects Research Part A, Clinical and Molecular Teratology, 100, 107–115. 10.1002/bdra.23224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrim A, Boskovic R, Maltepe C, Navios Y, Garcia‐Bournissen F and Koren G, 2006. Pregnancy outcome following use of large doses of vitamin B6 in the first trimester. Journal of Obstetrics and Gynaecology, 26, 749–751. 10.1080/01443610600955826 [DOI] [PubMed] [Google Scholar]
- Speitling A, Heseker H and Kübler W, 1990. Pharmacokinetic properties of the plasma B6 vitamers after single and chronic oral pyridoxine mega doses. Annals of the New York Academy of Sciences, 585, 557–559. 10.1111/j.1749-6632.1990.tb28104.x [DOI] [Google Scholar]
- Srinivasan P, Ramesh V, Wu J, Heskett C, Chu BD and Said HM, 2019. Pyridoxine and pancreatic acinar cells: transport physiology and effect on gene expression profile. American Journal of Physiology. Cell Physiology, 317, C1107–C1114. 10.1152/ajpcell.00225.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart SL, Thomas S, Höke E, Simpson D, Singleton JR and Höke A, 2022. Vitamin B6 levels do not correlate with severity of neuropathy in chronic idiopathic axonal polyneuropathy. Journal of the Peripheral Nervous System, 27, 31–37. 10.1111/jns.12480 [DOI] [PubMed] [Google Scholar]
- Stos K, Wozniak A, Rychlik E, Ziolkowska I, Glowala A and Oltarzewski M, 2021. Assessment of food supplement consumption in Polish population of adults. Frontiers in Nutrition, 8. 10.3389/fnut.2021.733951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stover PJ and Field MS, 2015. Vitamin B‐6. Advances in Nutrition, 6, 132–133. 10.3945/an.113.005207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarr JB, Tamura T and Stokstad EL, 1981. Availability of vitamin B6 and pantothenate in an average American diet in man. American Journal of Clinical Nutrition, 34, 1328–1337. 10.1093/ajcn/34.7.1328 [DOI] [PubMed] [Google Scholar]
- Teppers E, 2016. Vitamine B6. In: Bel S and Tafforeau J (eds). Enquête de Consommation Alimentaire 2014–2015. Bruxelles, WIV‐ISP. [Google Scholar]
- Tetens I, Petersen CF, Christensen SH, Wilkens T and Mikkelsen LS, 2023. Preparatory work for the update of the tolerable upper intake levels for vitamin B6. EFSA supporting publication 2023;EN‐7814, 141 pp. 10.2903/sp.efsa.2023.EN-7814. Available online: https://www.efsa.europa.eu/en/supporting/pub/en-7814 [DOI] [Google Scholar]
- Ueland PM, Ulvik A, Rios‐Avila L, Midttun Ø and Gregory JF, 2015. Direct and functional biomarkers of vitamin B6 status. Annual Review of Nutrition, 35, 33–70. 10.1146/annurev-nutr-071714-034330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueland PM, McCann A, Midttun Ø and Ulvik A, 2017. Inflammation, vitamin B6 and related pathways. Molecular Aspects of Medicine, 53, 10–27. 10.1016/j.mam.2016.08.001 [DOI] [PubMed] [Google Scholar]
- Valsta L, Kaartinen N, Tapanainen H, Männistö S and Sääksjärvi K, 2018. Ravitsemus Suomessa. FinRavinto 2017 ‐tutkimus (Nutrition in Finland – The National FinDiet 2017 Survey). THL Raportti 12/2018, Helsinki, Finland. pp. 311. [Google Scholar]
- van Buuren S, Schönbeck Y and van Dommelen P, 2012. Collection, collation and analysis of data in relation toreference heights and reference weights for female and male children and adolescents (0–18 years) in the EU,as well as in relation to the age of onset of puberty and the age at which different stages of puberty arereached in adolescents in the EU. Project developed on the procurement project CT/EFSA/NDA/2010/01. EFSA Supporting Publication 2012;EN‐255, 59 pp. [Google Scholar]
- van den Eynde MDG, Scheijen JLJM, Stehouwer CDA, Miyata T and Schalkwijk CG, 2021. Quantification of the B6 vitamers in human plasma and urine in a study with pyridoxamine as an oral supplement; pyridoxamine as an alternative for pyridoxine. Clinical Nutrition, 40, 4624–4632. 10.1016/j.clnu.2021.05.028 [DOI] [PubMed] [Google Scholar]
- van Hunsel F, van de Koppel S, van Puijenbroek E and Kant A, 2018. Vitamin B(6) in health supplements and neuropathy: Case series assessment of spontaneously reported cases. Drug Safety, 41, 859–869. 10.1007/s40264-018-0664-0 [DOI] [PubMed] [Google Scholar]
- van Rossum CTM, Buurma‐Rethans EJM, Dinnissen CS, Beukers MH, Brants HAM, Dekkers ALM and Ocké MC, 2020. The diet of the Dutch. Results of the Dutch National Food Consumption Survey 2012–2016. National Institute for Public Health and the Environment, RIVM report 2020–0083. 388 pp.
- Vargas‐Parada A, Loeza‐Alcocer E, González‐Ramírez R, Rodríguez‐Sánchez M, Raya‐Tafolla G, Florán B, Felix R and Delgado‐Lezama R, 2021. γ‐Aminobutyric acid (GABA) from satellite glial cells tonically depresses the excitability of primary afferent fibers. Neuroscience Research, 170, 50–58. 10.1016/j.neures.2020.08.007 [DOI] [PubMed] [Google Scholar]
- Vasile A, Goldberg R and Kornberg B, 1984. Pyridoxine toxicity: report of a case. The Journal of the American Osteopathic Association, 84, 67–69. 10.1515/jom-1984-840711 [DOI] [PubMed] [Google Scholar]
- Visser NA, Notermans NC, Degen LA, de Kruijk JR, van den Berg LH and Vrancken AF, 2014. Chronic idiopathic axonal polyneuropathy and vitamin B6: a controlled population‐based study. Journal of the Peripheral Nervous System, 19, 136–144. 10.1111/jns5.12063 [DOI] [PubMed] [Google Scholar]
- VKM (Norwegian Scientific Committee for Food Safety) , 2017. Assessment of vitamin B6 intake in relation to tolerable upper intake levels. Oslo. Available online: www.vkm.no [Google Scholar]
- Vrolijk MF, Opperhuizen A, Jansen E, Hageman GJ, Bast A and Haenen G, 2017. The vitamin B6 paradox: supplementation with high concentrations of pyridoxine leads to decreased vitamin B6 function. Toxicology In Vitro, 44, 206–212. 10.1016/j.tiv.2017.07.009 [DOI] [PubMed] [Google Scholar]
- Vrolijk MF, Hageman GJ, van de Koppel S, van Hunsel F and Bast A, 2020. Inter‐individual differences in pharmacokinetics of vitamin B6: A possible explanation of different sensitivity to its neuropathic effects. PharmaNutrition, 12, 100188. 10.1016/j.phanu.2020.100188 [DOI] [Google Scholar]
- Vujkovic M, de Vries JH, Dohle GR, Bonsel GJ, Lindemans J, Macklon NS, van der Spek PJ, Steegers EA and Steegers‐Theunissen RP, 2009. Associations between dietary patterns and semen quality in men undergoing IVF/ICSI treatment. Human Reproduction, 24, 1304–1312. 10.1093/humrep/dep024 [DOI] [PubMed] [Google Scholar]
- Wang S‐C, Ji H‐X, Hsiao C‐L, Wang T‐C, Syu Y‐R, Miao C‐E, Hou L‐L, Lin S‐S, Chang W‐S and Tsai C‐W, 2015. Protective effects of pyridoxamine against UVC‐induced programmed cell death in HaCaT cells. In Vivo, 29, 379–383. [PubMed] [Google Scholar]
- Waterston JA and Gilligan BS, 1987. Pyridoxine neuropathy. Medical Journal of Australia, 146, 640–642. 10.5694/j.1326-5377.1987.tb120445.x [DOI] [PubMed] [Google Scholar]
- Weinstein BB, Wohl Z, Mitchell GJ and Sustendal GF, 1944. Oral administration of pyridoxme hydrochloride in the treatment of nausea and vomiting of pregnancy. American Journal of Obstetrics and Gynecology, 47, 389–394. 10.1016/s0002-9378(15)30753-5 [DOI] [Google Scholar]
- WHO Multicentre Growth Reference Study Group , 2006. WHO Child Growth Standards based on length/height, weight and age. Acta Paediatrica Supplement, 450, 76–85. 10.1111/j.1651-2227.2006.tb02378.x [DOI] [PubMed] [Google Scholar]
- WHO/FAO (World Health Organization and Food and Agriculture Organization of the United Nations) , 2004. Vitamin and mineral requirements in human nutrition. Report of a joint FAO/WHO expert consultation, Bangkok, Thailand, 21–30, (September 1998), 362. [Google Scholar]
- Wondrak GT and Jacobson EL, 2012. Vitamin B6: beyond coenzyme functions. Sub‐Cellular Biochemistry, 56, 291–300. 10.1007/978-94-007-2199-9_15 [DOI] [PubMed] [Google Scholar]
- Wondrak GT, Roberts MJ, Jacobson MK and Jacobson EL, 2004. 3‐hydroxypyridine chromophores are endogenous sensitizers of photooxidative stress in human skin cells. Journal of Biological Chemistry, 279, 30009–30020. 10.1074/jbc.M404379200 [DOI] [PubMed] [Google Scholar]
- Wozenski JR, Leklem JE and Miller LT, 1980. The metabolism of small doses of vitamin B‐6 in men. Journal of Nutrition, 110, 275–285. 10.1093/jn/110.2.275 [DOI] [PubMed] [Google Scholar]
- Yaman M and Mizrak OF , 2019. Determination and evaluation of in vitro bioaccessibility of the pyridoxal, pyridoxine, and pyridoxamine forms of vitamin B6 in cereal‐based baby foods. Food Chemistry, 298, 125042. 10.1016/j.foodchem.2019.125042 [DOI] [PubMed] [Google Scholar]
- Yamashiro T, Yasujima T, Said HM and Yuasa H, 2020. pH‐dependent pyridoxine transport by SLC19A2 and SLC19A3: implications for absorption in acidic microclimates. Journal of Biological Chemistry, 295, 16998–17008. 10.1074/jbc.RA120.013610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Guo L, Zhao D, Qu P, Dang S and Yan H, 2021. Maternal B‐vitamin intake and B‐vitamin supplementation during pregnancy in relation to neonatal congenital heart defects: a case‐control study with propensity score matching. European Journal of Clinical Nutrition, 75, 782–791. 10.1038/s41430-020-00804-2 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Protocol for the Scientific Opinion on the revision of the EFSA’s Tolerable Upper Intake Level of vitamin B6
EFSA’s intake assessment of vitamin B6
Intake data from Competent Authorities in European Member States
Outcome of the public consultation on the draft Scientific opinion on the Tolerable Upper Intake Level for vitamin B6


