Abstract
Liver fibrosis plays a critical role in the evolution of most chronic liver diseases, and is characterized by a buildup of extracellular matrix, which can progress to cirrhosis, hepatocellular carcinoma, liver failure, or death. Currently there are no noninvasive methods available to accurately assess disease activity (fibrogenesis), to sensitively detect early onset of fibrosis, or to detect early response to treatment. Here, we hypothesized that extracellular allysine aldehyde (LysAld) pairs formed by collagen oxidation during active fibrosis could be a target for assessing fibrogenesis with a molecular probe. We showed that molecular magnetic resonance (MR) imaging using an extracellular probe targeting these LysAld pairs acts as a noninvasive biomarker of fibrogenesis and demonstrated its high sensitivity and specificity in detecting fibrogenesis in toxin- and dietary-induced mouse models, a cholestasis rat model of liver fibrogenesis, and in human fibrotic liver tissues. Quantitative molecular MRI was highly correlated with fibrogenesis markers and enabled non-invasive detection of early onset fibrosis and response to anti-fibrotic treatment, showing high potential for clinical translation.
One Sentence Summary:
Molecular MR imaging measures liver fibrogenesis in rodent models and human fibrotic liver tissues to enable disease detection and therapy monitoring.
INTRODUCTION
Chronic liver disease is caused by chronic injury from alcohol, drug abuse, viral injury, or metabolic derangements such as nonalcoholic steatohepatitis (NASH) (1). Chronic liver disease accounts for approximately 2 million deaths worldwide per year with more than 2 billion people at risk (2). If unchecked, it results in scarring of the liver (fibrosis) that can further lead to cirrhosis, primary liver cancer, liver failure, or death.
Biopsy is the gold standard to detect and stage fibrosis but is invasive, has sampling error, carries complication risk, and is not suited to serial monitoring (3). Ultrasound and magnetic resonance (MR) elastography methods and some blood biomarker panels are effective in detecting advanced fibrosis (4), but cannot detect early onset of liver fibrosis, nor measure disease activity / fibrogenesis (5). Sensing disease activity would provide the best guidance to reverse fibrosis and cure disease, enabling both disease detection and providing an early readout of treatment effectivity. Moreover, drug development efforts in NASH (6, 7) have been hampered by the inability to enroll patients with early-stage fibrosis, and instead recruit patients with advanced disease where drug therapies may be less effective. NASH trials also require a reduction in fibrosis stage as an endpoint, but fibrosis regression is a slow process; an early measure of treatment response would allow early termination of ineffective treatments and enable a better trial design to detect fibrosis reduction in promising therapies (8).
In liver fibrogenesis, regardless of cause, activated stellate cells secrete inflammatory mediators and synthesize extracellular matrix (ECM) components (9, 10). Excess accumulation of ECM and ECM crosslinking cause tissue stiffness and disrupt tissue architecture, resulting in liver dysfunction and ultimately liver failure (11). Collagens are the most abundant proteins in the fibrotic ECM (12). Noninvasive molecular imaging of collagen was explored in preclinical models and shown to be effective at staging fibrosis (13–15), but collagen imaging does not assess fibrogenesis and cannot distinguish ongoing disease from old injury.
Lysyl oxidase (LOX) and its paralogs are established markers of fibrogenesis (16). During liver fibrogenesis, the secretion and enzymatic activity of LOX and its paralogs are increased. Specifically, LOX catalyzes collagen crosslinking by preferentially oxidizing lysine amino pairs in close proximity, including two Lys residues in the C-telopeptide in collagen I (both α1–Lys16) and three in the N-telopeptide (two α1–Lys9 and one α2–Lys5) (17, 18). The oxidation products are allysine aldehyde (LysAld) pairs that subsequently react with one ε-amino group [2 + 1] in the receptor region of a neighboring collagen molecule to yield an intermolecular pyridinoline cross-link (Fig. 1A). Similar mechanisms were found for all collagen types and are conserved across species (18, 19).
Fig. 1. Probe design.
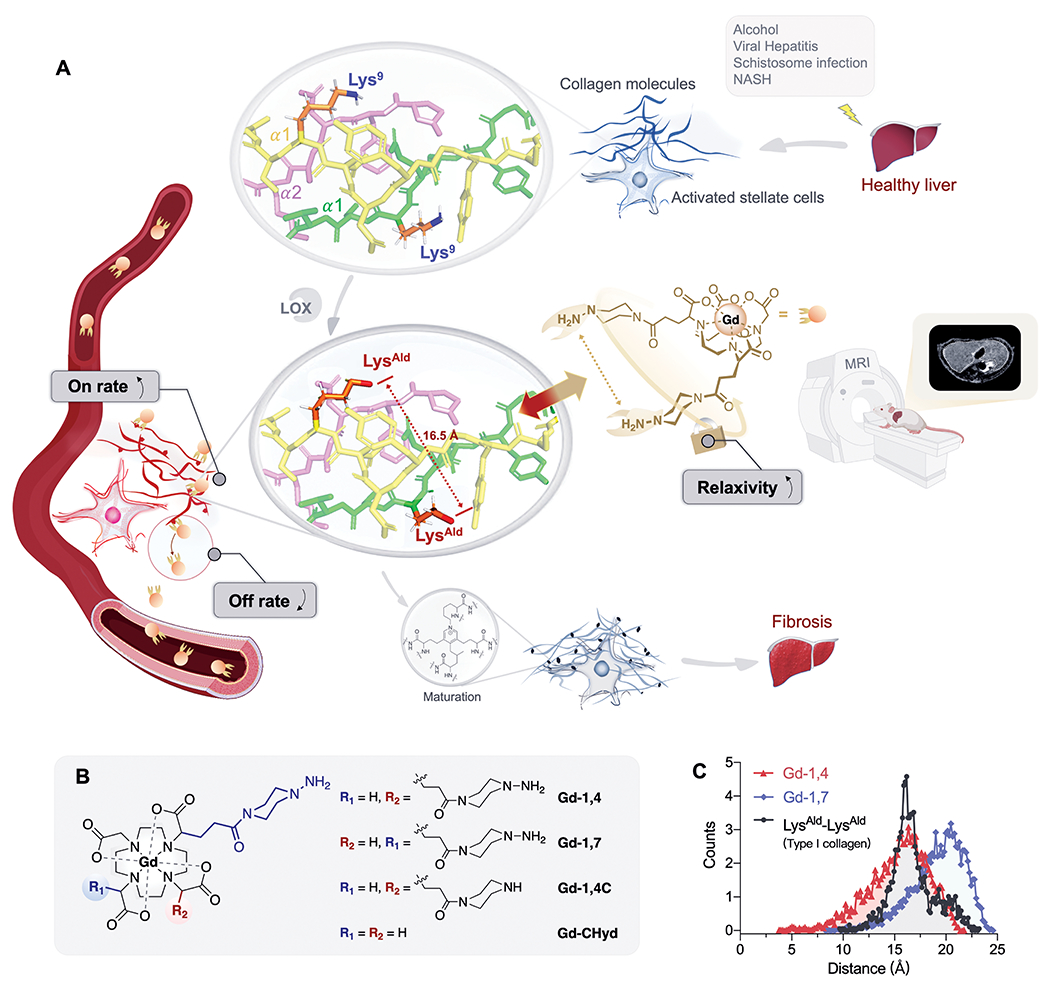
(A) Schematic illustration of the development of dual LysAld binding MRI probe for non-invasive detecting liver fibrogenesis. Chronic liver injury leads to the activation of stellate cells. In the remodeling of the extracellular matrix, closely separated Lys pairs on α1 chains of collagen telopeptide are oxidized by LOX to LysAld. The dual hydrazine Gd3+ probe precisely targets these LysAld pairs with high binding on-rate, high relaxivity upon binding and low off-rate, and results in increased dynamic range and noninvasive detection by MRI. (B) Chemical structures of Gd3+ complexes synthesized and studied in this work. (C) End-to-end distance distribution obtained from molecular dynamics simulation of the O-O distance between two α1–N9–LysAld residues in type-I collagen and the N-N distance in piperazino-hydrazine groups in Gd-1,4 and Gd-1,7.
We hypothesized that targeting these extracellular LysAld pairs, absent in normal liver tissues, could give a highly specific and sensitive biomarker of liver fibrogenesis. During active fibrosis, LysAld pair concentration is increased, but if fibrogenesis ceases, the aldehydes will be consumed by degradation. We reasoned that an extracellular MR probe with two hydrazine moieties in the molecule that can form reversible covalent bonds with the LysAld pairs could be an effective noninvasive reporter of fibrogenesis. Upon dual binding to LysAld pairs there will be a large turn-on in relaxivity as the MR probe goes from rapid tumbling in solution to forming a rather rigid macrocyclic structure with the protein (20), that is, the probe is turned-on by engaging its target. (Fig. 1A). Compared to a monobinder, the dual binding probe should have a higher reaction on-rate because of the higher local hydrazine concentration, and slower off-rate because two hydrazone bonds must be hydrolyzed. These probe design attributes should result in high MRI signal at the site of injury, and provide a non-invasive and sensitive readout for measuring the early onset of fibrogenesis.
RESULTS
Probe design
Precisely targeting LysAld pairs requires the targeting groups to closely match the distance between the two LysAld. Here, we used the diagnostic contrast agent Gd-DOTA as a core upon which to design our probes (Fig. 1B) because Gd-DOTA is known to be extracellularly distributed and is one of the most thermodynamically stable and kinetically inert Gd3+ complexes known (21, 22). We introduced two piperazino-hydrazine moieties on the α-carbons of two of the Gd-DOTA carboxylate arms to give either cis-1,4-Gd-(CHyd)2 (Gd-1,4) or trans-1,7-Gd-(CHyd)2 (Gd-1,7) that differed in the distance between the two hydrazine moieties (Fig. 1B). Two control compounds were prepared: Gd-1,4C, which possess one piperazino-hydrazine and one piperazine arm, and Gd-CHyd (23), which only has one piperazino-hydrazine moiety (Fig. 1B). Full synthetic details and characterizations are presented in the supplementary materials and fig. S1-S29.
To estimate how the regioisomers might react with oxidized collagen, we performed molecular dynamics simulations. The O-O distance between two α1–N9–LysAld residues on oxidized type I collagen was found to be centered at 16.5 Å (Fig. 1C). The N-N distance between the two piperazino-hydrazine groups in Gd-1,4 and Gd-1,7 was centered at 16.2 and 20.7 Å respectively. This suggested that Gd-1,4 may be preferred in targeting LysAld pairs.
Dual-binder probes have faster on-rates, slower off-rates, and higher relaxivity
Using butyraldehyde as a small-molecule model of LysAld, we measured hydrazone formation dynamically (Fig. 2A and fig. S30). Introducing a second hydrazine moiety increased the on-rate and conversion yield by a factor of two and improved affinity 2-fold for Gd-1,4 and Gd-1,7 over Gd-1,4C and Gd-CHyd (Fig. 2B and fig. S31).
Fig. 2. In vitro LysAld-conjugation characterization.
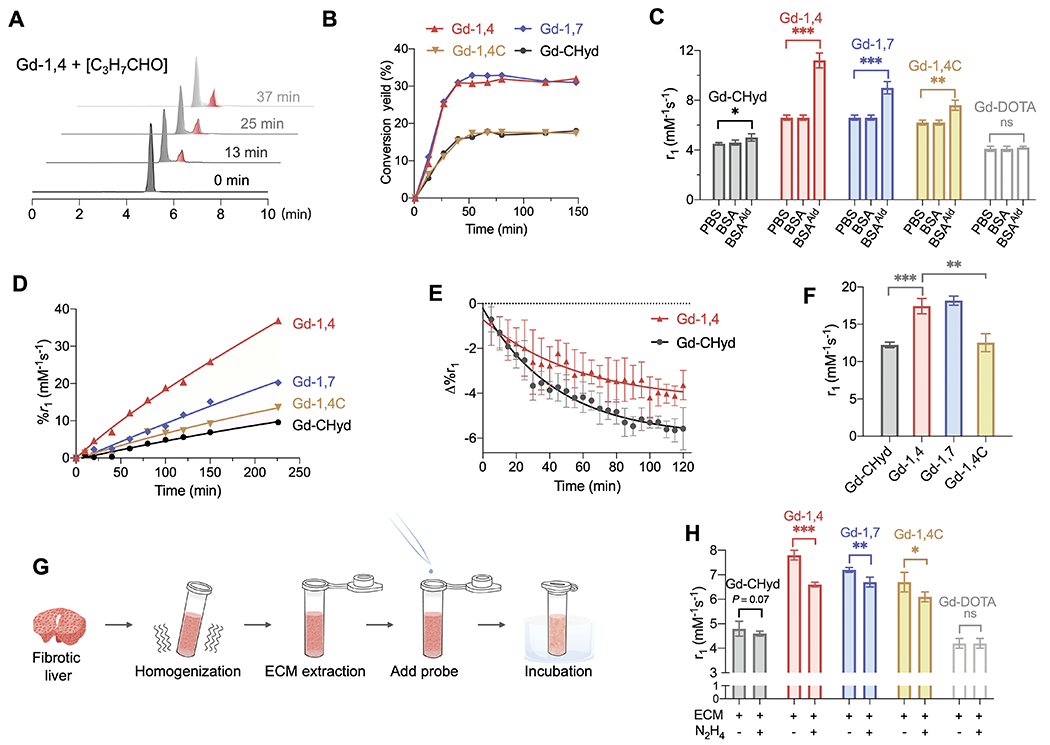
(A) Time course high-performance liquid chromatography in combination with inductively coupled plasma mass spectrometry (HPLC-ICP-MS) trace of Gd-1,4 (25 μM) in the presence of 100 μM butyraldehyde in PBS at room temperature (grey: Gd-1,4; red: product). (B) Conversion yield versus time of corresponding Gd3+ (25 μM) complexes in the presence of 100 μM butyraldehyde. (C) Relaxivity values in PBS, or in PBS with BSA or BSAAld (10 mg/mL, pH 7.4, 24 h, 37°C, 1.41T). Data are mean ± SD of three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001, ns: not significant, unpaired t-test, one-tailed. (D) Percentage r1 change of Gd complexes (0.1 mM) over time following incubation with 10 mg/mL BSAAld in PBS. (E) Hydrolysis of BSAAld bound Gd-CHyd and Gd-1,4 monitored by longitude relaxation in PBS (pH 7.4, 37°C, 1.41T). (F) Relaxivity of BSAAld-bound species in PBS (pH 7.4, 37°C, 1.41T). Data are mean ± SD of three independent experiments. **P < 0.01, ***P < 0.001, unpaired t-test, one-tailed. (G) Schematic illustration of the relaxivity measurement in ECM. (H) Relaxivity values in fibrotic rat liver ECM with or without addition of 100-fold excess of hydrazine (PBS, pH 7.4, 2 h, 37°C, 1.41T). Data are mean ± SD of three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001, ns: not significant, unpaired t-test, one-tailed.
We oxidized bovine serum albumin (BSA) to give a soluble LysAld bearing protein (BSAAld) to assess reactivity, affinity, and relaxivity of the probes. BSA contains 60 lysine residues, among which 19 pairs can be cross-linked by specific linker of 11.4 Å (24). We measured the longitudinal relaxivities (r1, 1.41 T, 37°C) of the probes in PBS alone, with excess unmodified BSA, or with excess of BSAAld. An increase in r1 compared to the PBS value is indicative of protein binding. In unmodified BSA, r1 values of Gd-CHyd, Gd-1,4C, Gd-1,4 and Gd-1,7 were 4.6 ± 0.1, 6.2 ± 0.2, 6.6 ± 0.2, 6.6 ± 0.2 mM−1s−1 respectively, and unchanged from those in PBS (4.5 ± 0.1, 6.2 ± 0.2, 6.6 ± 0.2, 6.6 ± 0.2 mM−1s−1), indicating no appreciable nonspecific protein binding of the complexes. However, relaxivities significantly increased in BSAAld compared to in PBS, in the order of Gd-CHyd (5.0 ± 0.3 mM−1s−1, 11% increase compared to PBS value, P = 0.0124) < Gd-1,4C (7.6 ± 0.4 mM−1s−1, 23% increase, P < 0.001) < Gd-1,7 (9.0 ± 0.5 mM−1s−1, 36% increase, P < 0.001) < Gd-1,4 (11.2 ± 0.6 mM−1s−1, 70% increase, P = 0.0019) (Fig. 2C). Gd-DOTA, which lacks a hydrazine moiety, showed no change in relaxivity (4.1 ± 0.2 mM−1s−1) between PBS, BSA, and BSAAld.
Gd-1,4 and Gd-1,7 reacted with BSAAld with 6- and 3-fold higher initial rates, respectively, compared to Gd-CHyd (Fig. 2D). Under these conditions, equilibrium binding to BSAAld was 14.6, 19.2, 39.2, and 28.1% for Gd-CHyd, Gd-1,4C, Gd-1,4 and Gd-1,7, respectively (fig. S32). The dual-binding Gd-1,4 probe had a slower off-rate from BSAAld compared to Gd-CHyd (Fig. 2E). We isolated the protein bound probes and measured their relaxivities directly; the dual-binding probes had higher protein-bound relaxivity than the mono-hydrazine probes, consistent with decreased internal rotation upon protein binding (25): Gd-CHyd (12.2 ± 0.4) < Gd-1,4C (12.5 ± 1.2) < Gd-1,4 (17.4 ± 1.0) ≈ Gd-1,7 (18.2 ± 0.6 mM−1s−1) (Fig. 2F). Sodium dodecyl sulfate polyacrylamide gel electrophoresis analysis of the protein-bound Gd-1,4 showed that Gd-1,4 was only bound to one BSAAld and did not crosslink two proteins (fig. S33).
We incubated ECM suspension extracted from fibrotic rat liver and measured relaxivity (Fig. 2G). Gd-CHyd (4.8 ± 0.3), Gd-1,4C (6.7 ± 0.4), Gd-1,4 (7.8 ± 0.2) and Gd-1,7 (7.2 ± 0.1 mM−1s−1) all showed enhanced relaxivity in ECM compared to PBS, and relaxivity was decreased by blocking ECM aldehydes with 100-fold excess of hydrazine (lowered to 4.6 ± 0.1, 6.1 ± 0.2, 6.6 ± 0.1, 6.7 ± 0.2 mM−1s−1 respectively) (Fig. 2H). Gd-DOTA, as negative control, showed similar relaxivity in ECM as in PBS.
Gd-1,4 specifically images liver fibrogenesis in the mouse CCl4 model
Following intravenous (i.v.) administration (100 μmol/kg) of Gd-CHyd, Gd-1,4 or Gd-1,7 to normal mice, dynamic whole-body MRI showed that all probes were eliminated exclusively via the kidneys into the bladder (fig. S34). The probes all showed an extracellular distribution with only transient liver enhancement consistent with blood pool. At 24 h post-injection >99% of the injected dose of gadolinium was eliminated from the body (table. S1).
Next, we compared Gd-DOTA, Gd-CHyd, Gd-1,4 and Gd-1,7 in mice gavaged with CCl4 for 12 weeks to induce liver fibrosis or with olive oil vehicle (26). All mice were imaged twice with different probes given randomly one day apart (Fig. 3A). Collagen staining of liver tissue with Sirius Red (SR, Fig. 3B) indicated consistent fibrosis in the CCl4 group, with collagen proportional area (CPA) elevated to 4.2 ± 1.2% vs. 0.7 ± 0.2% in vehicle group (Fig. 3C). Immunohistochemistry (IHC) of LOX on the liver sections revealed that the proportion of LOX positive tissue, hardly detectable in vehicle group (3.8 ± 1.2%), was strongly increased in the CCl4 group (28.2 ± 4.6%, Fig. 3D). The proportion of LysAld positive tissue evaluated by dinitrophenylhydrazine (DNPH) reactivity assay (27) also significantly increased in CCl4 injured mice (37.1 ± 13.0%) compared to vehicle controls (6.5 ± 4.4%, Fig. 3E, P < 0.0001). LOX immunoreactivity and LysAld were both primarily observed along the fibrotic septa (Fig. 3B) in a pattern similar to the distribution of collagen in SR and α-smooth muscle actin (α-SMA, fig. S35). Hydroxyproline, a marker of total tissue collagen (28), was elevated in mice that received CCl4 (426 ± 95 μg/g vs. vehicle 195 ± 30 μg/g liver, Fig. 3F).
Fig. 3. In vivo probe screening in a mouse model of CCl4-induced liver fibrosis.
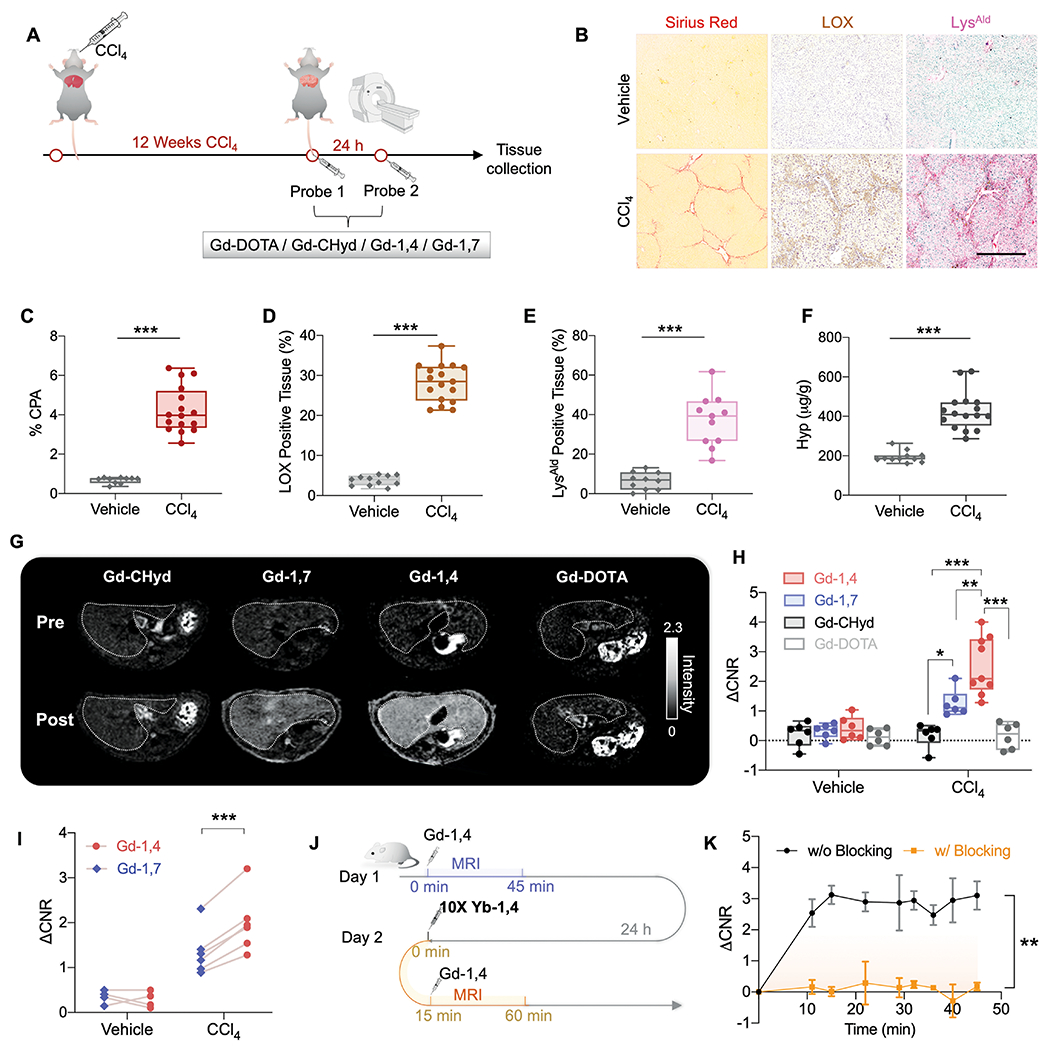
(A) Schematic illustration of the animal study design of CCl4-induced liver fibrosis. Mice were imaged after 12 weeks of oral gavage of CCl4 with probe 1 and then again the next day with probe 2 (100 μmol/kg i.v., probes chosen randomly from Gd-DOTA, Gd-CHyd, Gd-1,4 and Gd-1,7). (B) Representative images of Sirius red, LOX, and LysAld staining (scale bar: 500 μm). (C) Collagen proportional area (CPA) measured from Sirius red-stained tissue (n ≥ 10, ***P < 0.0001, unpaired t-test, two-tailed). (D) The percentage of LOX-positive tissue measured from IHC LOX-stained tissue (n ≥ 10, ***P < 0.0001, unpaired t-test, two-tailed). (E) The percentage of LysAld-positive tissue measured from DNPH reactivity assay (n ≥ 10, ***P < 0.0001, unpaired t-test, two-tailed). (F) Liver hydroxyproline (Hyp, μg/g) in vehicle- and CCl4-treated mice (n ≥ 10, ***P < 0.0001, unpaired t-test, two-tailed). (G) Axial liver (outlined in white) MR images of CCl4 mouse imaged before and 45 min post-injection of Gd-CHyd, Gd-1,7, Gd-1,4, and Gd-DOTA (100 μmol/kg i.v.). (H) Change in liver to muscle contrast to noise ratio (ΔCNR) relative to pre-injection image at 45 min post-injection (n ≥ 6 per group, *P = 0.0433, **P = 0.0084, ***P < 0.0001, one-way ANOVA, post hoc comparison, two-tailed). (I) Pairwise comparison of ΔCNR at 45 min for Gd-1,4 and Gd-1,7 imaged in the same mouse (100 μmol/kg i.v. injection order randomized) one day apart (***P = 0.0003, paired t-test, two-tailed). (J) Schematic illustration of the blocking study with Yb-1,4. (K) Time course of Gd-1,4 ΔCNR in CCl4 mice before and after pretreatment with 10-fold dose of MR-silent Yb-1,4 (n = 3, **P = 0.0045). All data shown as mean ± SD, *P < 0.05, **P < 0.01, ***P < 0.001.
Blood and kidney elimination of the probes in CCl4 injured mice was similar to that in the vehicle group (fig. S36-37), but marked differences were observed in the liver. At 10 mins post-injection when washout of the probes was negligible (fig. S37), there was a difference in ΔCNR among the probes in the order of Gd-1,4 > Gd-1,7 ≈ Gd-CHyd > Gd-DOTA, which may arise from their different on rates with LysAld. Over time, both Gd-1,4 and Gd-1,7 enhanced MRI show slower liver washout rates in CCl4 injured mice than Gd-CHyd and Gd-DOTA, and the enhancement in liver with Gd-1,4 and Gd-1,7 persisted until at least 45 mins post-injection. At 45 min post-injection, ΔCNR for Gd-1,4 and Gd-1,7 was 2.5 ± 1.0 and 1.3 ± 0.4 respectively in CCl4 injured mice, while there was no liver enhancement in the Gd-CHyd or Gd-DOTA groups, nor in any of the vehicle-treated mice (Fig. 3G-H). The half-life of Gd-1,4 and Gd-1,7 in CCl4 injured liver was estimated to be 49.6 ± 3.5 and 28.9 ± 2.7 min respectively. Representative images at pre- and 45 min post-injection of each Gd3+ probe are shown in Fig. 3G. Compared to Gd-CHyd, Gd-1,4 shows 10-fold higher ΔCNR. Importantly, Gd-1,4 showed significantly higher ΔCNR than Gd-1,7 (Fig. 3H, P < 0.01). To confirm this difference between isomers, we imaged mice with either Gd-1,4 or Gd-1,7 and then again the next day with the other probe. In CCl4 injured mice, but not vehicle-treated animals, ΔCNR was consistently and significantly higher in the mice imaged with Gd-1,4 compared to Gd-1,7 (P < 0.001, Fig. 3I).
Using Yb-1,4, we performed a blocking study in fibrotic mice. Yb-1,4 has the same structure as Gd-1,4 but Gd3+ is replaced by the MRI-inactive Yb3+ ion. We imaged CCl4 injured mice with 100 μmol/kg Gd-1,4 and then the next day gave a blocking dose of 1000 μmol/kg Yb-1,4, followed 15 minutes later by 100 μmol/kg of Gd-1,4 (Fig. 3J). The 10-fold dose of Yb-1,4 completely eliminated liver MRI enhancement with Gd-1,4 (P = 0.0045), demonstrating the specific binding of Gd-1,4 to the livers of fibrotic mice (Fig. 3K).
Gd-1,4 MRI can detect early onset of liver fibrogenesis and measure early response to treatment in a mouse model of NASH
We next examined how early in the fibrotic process could Gd-1,4 enhanced MRI detect disease and whether Gd-1,4 enhanced MRI would be sensitive to early reduction in fibrogenesis associated with therapeutic intervention. We imaged the natural history of the choline-deficient, L-amino acid-defined, high fat diet (CDAHFD) mouse model which carries many of the human NASH phenotypes including liver inflammation and steatosis followed by progressive fibrosis (29, 30). Disease can be reversed in this model by switching to normal chow (31).
Mice were fed with CDAHFD or standard chow for 2, 6, or 10 weeks; two additional groups of mice received CDAHFD for 10 weeks and then standard chow for 1 or 4 weeks (Fig. 4A). In vivo 20 min post-Gd-1,4 – pre-injection T1-weighted subtraction images in control and CDAHFD groups are presented in Fig. 4B. Gd-1,4 enhanced MRI showed progressively higher ΔCNR with longer duration on CDAHFD. Importantly, compared to controls (0.1 ± 0.4), significantly greater ΔCNR was seen in the livers of CDAHFD mice as early as 2 weeks on CDAHFD (1.3 ± 0.3, Fig. 4C, P < 0.001). Switching to standard chow for 1 week after 10 weeks of CDAHFD resulted in significantly lower ΔCNR (10 wk CDAHFD: 2.5 ± 0.5, switch 1wk: 0.9 ± 0.2, P < 0.001), which further reduced after 4 weeks of diet reversal (0.3 ± 0.2). The half-life of Gd-1,4 in the liver of CDAHFD mice increased from 17.6 ± 1.8 min in mice that received CDAHFD for 2 wk to 24.7 ± 3.6 min in 10 wk CDAHFD mice.
Fig. 4. Molecular MRI characterization of fibrotic disease activity and treatment response in a mouse model of NASH.
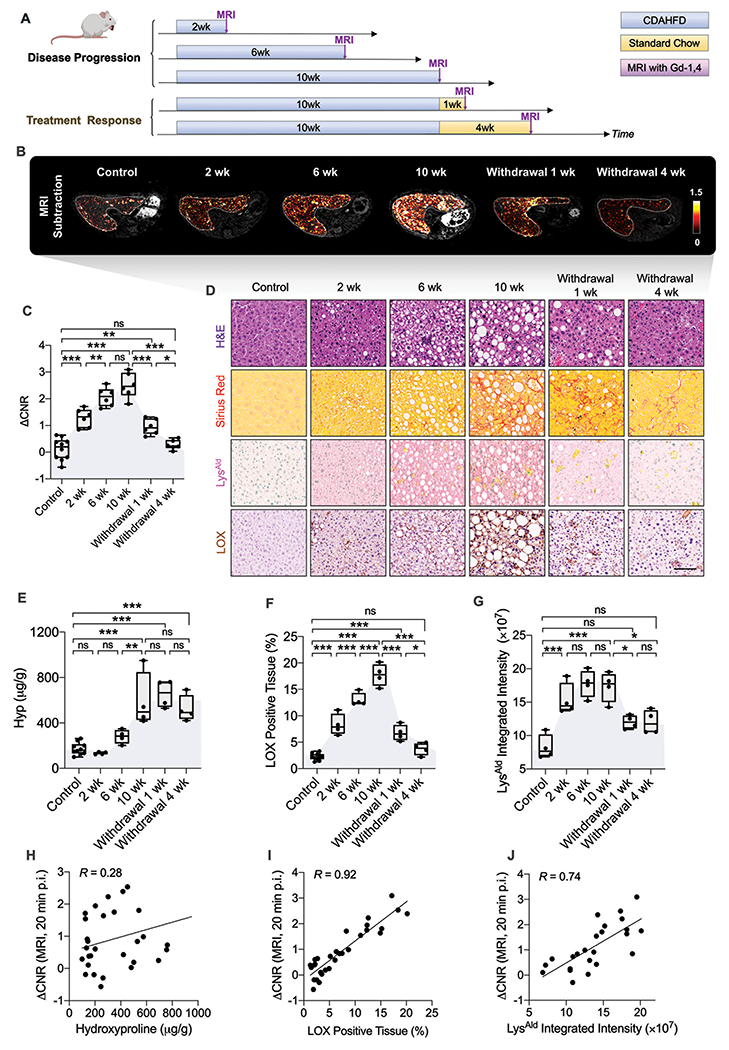
(A) Diagram shows experimental design, animal group classification, and in vivo MRI imaging. Adult C57BL/6 mice receiving standard chow served as age-matched (n = 10) controls. Mice were fed with a choline-deficient, l-amino acid–defined, high-fat diet (CDAHFD) for 2, 6, or 10 weeks (n = 6 per group) to study disease progression of nonalcoholic steatohepatitis (NASH). Mice that received 10 weeks of CDAHFD were subsequently either switched back to standard chow for 1 or 4 weeks (n = 6 per group) to study treatment efficacy. At each time point, mice underwent Gd-1,4-enhanced MRI followed by sacrifice and ex vivo characterization of the liver. (B) Subtraction (20 min post-injection 100 μmol Gd-1,4/kg – pre-injection) T1-weighted images in control and CDAHFD groups. (C) Quantitative analyses of liver to muscle ΔCNR at 20 min in each group (all data shown as mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001, ns: not significant, one-way ANOVA, post hoc comparison, two-tailed). (D) Representative H&E, Sirius red, LysAld, and LOX staining of livers from control mice and CDAHFD fed mice (scale bar: 100 μm). (E) Total collagen quantification assessed by liver hydroxyproline (Hyp) content as a fibrosis measure (n ≥ 4 per group, all data shown as mean ± SD, **P < 0.01, ***P < 0.001, ns: not significant, one-way ANOVA, post hoc comparison, two-tailed). (F) The percentage of LOX-positive tissue measured from IHC LOX-stained tissue (n ≥ 4 per group, all data shown as mean ± SD. *P < 0.05, ***P < 0.001, ns: not significant, one-way ANOVA, post hoc comparison, two-tailed). (G) Quantitative analyses of the integrated intensity of LysAld from DNPH reactivity assay as measures of fibrogenesis (n ≥ 4 per group, all data shown as mean ± SD, *P < 0.05, ***P < 0.001, ns: not significant, one-way ANOVA, post hoc comparison, two-tailed). (H) Correlation analysis between ΔCNR at 20 min and hydroxyproline content (R = 0.28, P = 0.13). Each data point represents one mouse. (I) Pearson’s correlation analysis between ΔCNR at 20 min and the percentage of LOX-positive tissue (R = 0.92, P < 0.0001). Each data point represents one mouse. (J) Pearson’s, correlation analysis between ΔCNR at 20 min and the integrated intensity of LysAld (R = 0.74, P < 0.0001). Each data point represents one mouse.
Histological analyses (Fig. 4D and fig. S38A) showed that steatosis, apparent as lipid droplets, is present after 2 weeks on CDAHFD and increases with time on diet. Fibrosis quantified by hydroxyproline and SR showed that compared to controls, neither was significantly increased after 2 weeks on CDAHFD (P > 0.05) but were significantly elevated after 6 or 10 weeks (P < 0.001) on CDAHFD (Fig. 4E and fig. S38B). However, IHC assessment of fibrogenesis markers LOX, LysAld and α-SMA were significantly elevated compared to control livers even at 2 weeks of CDAHFD (P < 0.001 for LysAld, P = 0.0399 for α-SMA). The percentage of LOX positive tissue increased from 2.3 ± 0.6% in the control group to 8.3 ± 2.0%, 13.0 ± 1.3%, and 17.7 ± 2.1% after 2, 6, and 10 weeks of CDAHFD, respectively (Fig. 4F). The average integrated intensity of LysAld increased 86%, 114%, 108% at 2, 6, 10 weeks of CDAHFD compared to control group (Fig. 4G).
Animals fed CDAHFD for 10 weeks and then switched to standard chow for one week still showed prominent fibrosis (Hyp, Fig. 4E) but marked reductions in LOX (Fig. 4F), LysAld, and α-SMA (fig. S38C-D) compared to 10 wk. These biomarkers of fibrogenesis (LOX, LysAld, α-SMA) change quickly with the treatment of diet reversal, while Hyp as a biomarker of fibrosis does not show a significant decrease even 4 weeks after CDAHFD withdrawal when disease activity was further resolved (Fig. 4D, P > 0.05).
Gd-1,4 ΔCNR did not correlate with total liver collagen content as assessed biochemically by Hyp (Fig. 4H, R = 0.28, P = 0.13), but tracked well with measures of fibrogenesis like expression of LOX (Fig. 4I, R = 0.92, P < 0.0001), LysAld (Fig. 4J, R = 0.74, P < 0.0001) and α-SMA (R = 0.85, P < 0.0001, fig. S38E).
Gd-1,4 MRI detects liver fibrogenesis in a rat model of obstructive cholestatic disease
Bile duct ligation (BDL) induced cholestatic liver disease is a model that progresses into liver injury following liver inflammation and fibrosis (9). Ten days after BDL surgery, rats were imaged before and after 100 μmol/kg i.v. Gd-1,4 to detect hepatic fibrogenesis in a second species (Fig. 5A). Gd-1,4 enhanced MRI showed higher liver signal in BDL rats than in sham-operated rats at 30 min post-injection (Fig. 5B). Both the change of liver longitudinal relaxation rate ΔR1 (0.6 ± 0.2, P = 0.0003) and ΔCNR (3.4 ± 1.6, P = 0.0081) were significantly enhanced in the BDL animals (Fig. 5C-D), compared to sham rats (ΔR1 = 0.1 ± 0.03, ΔCNR = 0.6 ± 0.2). Liver fibrosis/fibrogenesis in the BDL rats was confirmed by the presence of elevated CPA, LOX, LysAld and hydroxyproline content (Fig. 5E and fig. S39A-C).
Fig. 5. Molecular MR imaging in a rat model of bile duct ligation-induced liver fibrogenesis.

(A) Diagram shows experimental design. Bile duct ligation surgery was performed on 7-9-week-old male CD rats (n = 7) and the rats were imaged 10 days later both before and after 100 μmol/kg i.v. Gd-1,4 (n = 4 for sham group) for 30 min. After imaging, liver was harvested and sectioned. (B) Coronal MRI (greyscale) of BDL rat with pre-injection and 30 min post-injection longitudinal relaxation rate (R1) maps (color scale). (C) The change in liver longitudinal relaxation rate (ΔR1) at 30 min post-injection induced by Gd-1,4 was 5-fold higher in BDL rats compared to sham rats (***P = 0.0003, unpaired t-test, two-tailed). (D) Liver to muscle ΔCNR at 30 min was 4-fold higher in BDL rats compared to sham rats (**P = 0.0081, unpaired t-test, two-tailed). (E) The percentage of LysAld positive liver tissue measured from DNPH reactivity assay and LOX-positive liver tissue measured from LOX IHC was both significantly higher in BDL rats compared to sham rats (n = 4, ***P = 0.0014, ***P = 0.0006, respectively, unpaired t-test, two-tailed). (F) Consecutive liver slices from a BDL rat after Gd-1,4 enhanced MRI. Left to right: LA-ICP-MS images of gadolinium distribution in fresh harvested BDL liver, BDL liver incubated with Gd-1,4, BDL liver incubated with Gd-1,4+N2H4, and LysAld staining (scale bar: 500 μm). (G) Colocalization of Gd distribution and LysAld along the line indicated by the arrow in (F). All data shown as mean ± SD. **P < 0.01, ***P < 0.001.
Ex vivo elemental Gd imaging of fibrotic rodent livers corresponds with in vivo binding
We imaged rat livers ex vivo using laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS). Since Gd is not an endogenous element, imaging Gd represents a direct measure of Gd-1,4 in tissue. Liver tissue from BDL and sham rats imaged with Gd-1,4 was harvested 30 min post-injection. LA-ICP-MS showed higher liver Gd concentration in BDL rats (Fig. 5F) compared to sham rats (fig. S39D), with specific accumulation of Gd (~50 ppm) in fibrotic septa colocalizing with the presence of LysAld (Fig. 5G). On an adjacent liver slice we incubated with additional Gd-1,4 and NaBH3CN to make an irreversible linkage which resulted in further increased Gd concentration in fibrotic septa; on another adjacent slice we co-incubated Gd-1,4 with a 100-fold excess of hydrazine which blocked further binding of Gd-1,4 and demonstrated the specificity of the probe for tissue aldehyde (Fig. 5F).
Gd-1,4 binds to human fibrotic liver tissues
We next investigated the applicability of Gd-1,4 to assess fibrogenesis in human liver specimens. Resected human fibrotic/cirrhotic liver associated with NASH (n = 5) and normal liver tissue (n = 4) (Table S2) were sectioned and stained for LysAld. Increased LysAld staining was observed in fibrotic regions of liver, whereas there was negligible staining in normal liver (Fig. 6A, B). Positive staining of collagen and LOX were also observed in the fibrotic septa (Fig. 6C). We incubated adjacent slices from the fibrotic liver with Gd-1,4 or Gd-1,4 with excess N2H4. LA-ICP-MS imaging showed that the average Gd concentration in LysAld positive areas (30-200 ppm Gd) was much higher than in regions with low LysAld (< 30 ppm Gd, Fig. 6C-D), but excess N2H4 blocks Gd-1,4 binding, demonstrating the specificity of the probe to fibrotic human liver. Across all samples (Fig. S40-41), the average Gd concentration in fibrotic human liver was 80.4 ± 22.1 ppm, compared to 23.7 ± 10.8 ppm in normal liver (Fig. 6E-F).
Fig. 6. Translation potential in detecting human liver fibrogenesis.
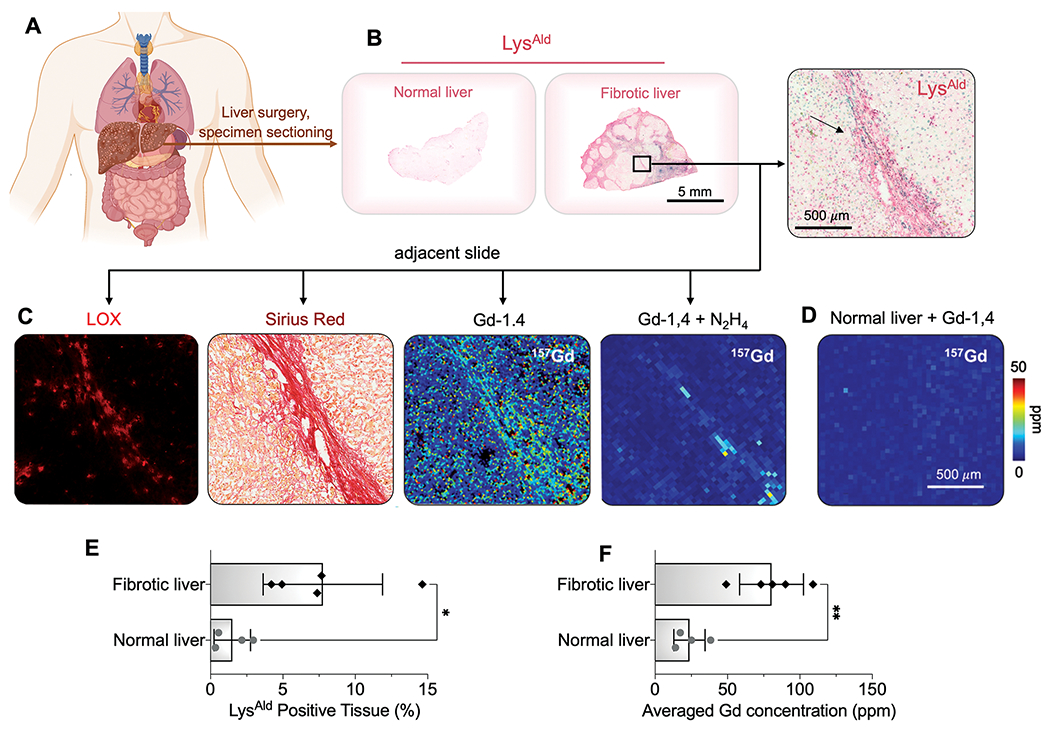
(A) Human fibrotic/cirrhotic liver associated with NASH and normal liver specimens were obtained from surgery and sectioned. (B) Representative liver sections stained for LysAld by DNPH reactivity assay to show the distribution of extracellular aldehydes (arrow marks fibrotic band). (C) Adjacent fibrotic liver sections are stained for LOX (immunofluorescence), Sirius red, or incubated with Gd-1,4 without or with 100-fold N2H4 and assessed by LA-ICP-MS. (D) Normal human liver was incubated with Gd-1,4 and assessed by LA-ICP-MS. (E) The percentage of LysAld positive tissue for normal and fibrotic human liver measured from DNPH reactivity assay (*P = 0.0232, unpaired t-test, two-tailed). (F) The average Gd concentration in human fibrotic and normal tissues incubated with Gd-1,4 and assessed by LA-ICP-MS (**P = 0.0023, unpaired t-test, two-tailed). All data shown as mean ± SD. *P < 0.05, **P < 0.01.
DISCUSSION
Liver fibrosis is a progressive form of most chronic liver disease that can progress to cirrhosis, hepatocellular carcinoma, liver failure, and death (1, 9). No noninvasive methods exist to assess disease activity, to sensitively detect early onset of disease, or to detect early response to treatment (3). Here, we proposed that LysAld pairs transiently produced in the ECM can act as a general fibrogenesis marker. Rational design and systematic probe screening gave extracellular MR probe Gd-1,4 with two hydrazine moieties that can precisely form reversible covalent hydrazone bonds with LysAld pairs on collagen telopeptides. The dual binding approach resulted in a faster on-rate (600%), slower off-rate (50%), and higher protein-bound relaxivity (50%) compared to a monobinder, and led to markedly superior performance (10-fold higher ΔCNR) in vivo for measuring liver fibrogenesis. Gd-1,4 specifically detected liver fibrogenesis in toxin- and dietary-induced mouse models and a cholestasis rat model of liver fibrogenesis. The Gd-1,4 enhanced MRI signal was reflective of LOX mediated LysAld cross-linking related disease activity.
In a mouse model of NASH, Gd-1,4 molecular MRI detected the early onset of liver fibrosis (prior to increases in liver hydroxyproline) and was sensitive to a reduction in fibrogenesis following a therapeutic intervention. The change in molecular MRI signal preceded the presence or resolution of fibrosis assessed biochemically and histologically which depended on the change of collagen concentration, highlighting the potential of this method for early disease detection and as an early readout of response to effective therapy.
The potential of Gd-1,4 for clinical translation is high. Gd-1,4 is prepared in 5 synthetic steps with high overall yield (> 50%) and is based on the inert Gd-DOTA chelate, which itself has been administered millions of times to humans with no unconfounded gadolinium-associated toxicity (21, 22). Gd-1,4 showed no nonspecific protein binding, nor did it accumulate in normal tissue. Gd-1,4 exhibited similar pharmacokinetics to Gd-DOTA with fast renal elimination (terminal blood half-life estimated from imaging in healthy mice was 5.8 ± 0.8 min for Gd-1,4 and 6.0 ± 0.3 min for Gd-DOTA) and that at 24 h post-injection, >99% of the injected gadolinium was eliminated from the body. The blocking experiment with a 10-fold higher dose of Yb3+-labeled compound not only demonstrated the specificity of Gd-1,4 in vivo, but also revealed that a dose of 1 mmol/kg was well-tolerated in mice. The MR signal change observed with Gd-1,4 was robust across different models and species. Of note, the Gd-1,4 MR signal enhancement in fibrotic liver depended on the extent of fibrogenesis; animals with increased fibrogenesis had both larger enhancement and slower liver half-lives across different disease models. This suggests that fibrogenesis can be staged by quantitative imaging. Ex vivo analysis of human liver specimens shows absence of extracellular aldehyde in normal liver, but high concentrations in fibrotic regions. Incubating Gd-1,4 with human fibrotic liver revealed Gd concentrations similar to those observed in the rat model, where we observed ΔR1 = 0.6 s−1 with in vivo imaging, strongly suggesting that robust MR signal changes will be seen in patients with chronic liver disease. In addition, in experiments performed on liver fibrosis mice on consecutive days, we observed no retention of probe in liver as the liver MR signal had returned to its baseline value on the second day. Therefore, we do not anticipate any safety concerns with respect to retention of the probe in fibrotic liver. The imaging protocols performed here utilized standard T1-weighted and T1-mapping sequences that are available on commercial clinical scanners. A specific Gd-1,4-enhanced liver signal was observed within minutes of administration, indicating that Gd-1,4 MRI could readily be adapted to existing clinical protocols.
However, there are some limitations of the study. Due to the long IND application process, currently we were unable to test the probe in patients in vivo. There remains concern around the use of Gd-based contrast agents in patients with poor renal function or in vulnerable patients who may require multiple Gd contrast enhanced MRIs because of the potential for nephrogenic systemic fibrosis (32, 33) or gadolinium tissue retention (34). We sought to minimize these risks by using the very stable Gd-DOTA chelate and to design a molecule that has little nonspecific protein-binding and rapid plasma elimination in rodents. Further nonclinical testing will be required to establish safety and pharmacokinetics/elimination in different species before initiating human studies. Although Gd-1,4 enhanced MRI can noninvasively measure hepatic fibrogenesis, it does not report on fibrosis stage. However, Gd-1,4 MRI is readily combined with existing techniques like elastography or serum tests. For instance, a positive Gd-1,4 MRI combined with a negative elastography exam could be indicative of disease with F1/F2 fibrosis, whereas negative Gd-1,4 MRI and negative elastography could indicate true absence of fibrosis.
In conclusion, we determined that extracellular LysAld pairs formed during active fibrosis serve as a specific marker of fibrogenesis that is quantifiable by a dual hydrazine equipped MR probe. The dual binding approach boosted on-rate, lowered off-rate, and increased MR signal upon binding. Gd-1,4 MRI was highly sensitive to early onset of liver fibrogenesis and robustly detected treatment response prior to changes in liver collagen concentration.
MATERIALS AND METHODS
Study design
The objective of this study was to design a molecular MRI probe to target lysine aldehyde pairs formed during fibrogenesis to enable early diagnosis of liver fibrosis and to monitor treatment response. These goals were addressed by designing a series of mono and dual hydrazine equipped probes and investigation of their in vitro reactivity with lysine aldehyde; screening the probes in vivo using the CCl4 induced liver fibrosis mouse model; selecting the best probe and testing its ability to detect the onset of liver fibrosis and to monitor treatment response in a diet induced nonalcoholic steatohepatitis mouse model; testing the probe in a second species in a rat model of obstructive cholestatic disease; and demonstrating the potential translation of the probe using human liver specimens. All animal experiments were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and in compliance with the ARRIVE guidelines and approved by the MGH Institutional Animal Care and Use Committee (Protocol 2009N000207 for the mouse studies and 2008N000204 for the rat study). Human liver tissues were obtained from a total of 9 anesthetized patients who underwent surgical resection at Massachusetts General Hospital, and analyzed in accordance with a protocol approved by the Massachusetts General Hospital’s Institutional Review Board (Protocol 1999P004983). All animals were housed in an animal facility under controlled temperature and 12-hour light/12-hour dark cycle conditions. Animals were randomized to different groups and control groups were matched for age and gender. Evaluation of the probes in animals was performed in a nonblinded fashion. Investigators were blinded for histology analysis. No animal was excluded from the study and no sample or data points were omitted from analysis. Numbers of biological replicates and statistical results are indicated in figure legends.
Synthetic Protocols
(S)-5-Benzyl-1-tert-butyl 2-(methylsulfonyloxy)pentanedioate (35), 1,4-DO2A-t-Bu (36), 1,7-DO2A-t-Bu (37) and Gd-CHyd (23) were obtained as described previously. All other reactants and reagents were of commercial grade and used without further purification. NMR spectra were recorded on a JEOL ECZ 500R 11.7 T NMR system equipped with a 5 mm broadband probe (1H: 499.81 MHz, 13C: 125.68 MHz). Quantification of gadolinium was carried out using an Agilent 8800-QQQ ICP-MS system. Longitudinal (T1) relaxation measurements were recorded using a Bruker mq60 Minispec at 1.41 T and 37 °C. High resolution electrospray ionization mass spectra (HR-ESI-MS) were acquired with Bruker Maxis Impact LC-q-TOF Mass Spectrometer. Detailed syntheses and characterizations can be found in the supplementary information.
Molecular dynamic simulation
The systems with Gd3+ complexes were constructed on the basis of the crystal structure of Gd-DOTA (CCDC: 1882455) (38) and modeling structure of type-I collagen (39). All the three chains of type-I collagen were considered in our study. The missing hydrogen atoms were added by the LEAP module in Amber 18 (40). The Amber ff14SB (41) force field was employed for the protein residues. The general AMBER force field (GAFF (42)) was used for the ligand of Gd3+ complexes, while the partial atomic charges were quantified by the RESP method (43), using HF/6-31G*. The force field for the Gd3+ complex was parametrized using MCPB (44). Then, Na+ ions were added to the surface of the protein to neutralize the total charge of the protein. Last, the neutral system was solvated in a cube of TIP3P (45) waters with a 15 Å water layer.
After setup, the system was minimized by a combined steepest descent and conjugate gradient method. Then the system was heated from 0 to 300 K under a canonical ensemble for 0.2 ns with a weak restraint of 15 kcal/(mol Å). To achieve a uniform density after heating dynamics, 1 ns of density equilibration was performed under the NPT ensemble at the target temperature of 300 K and target pressure of 1.0 atm. Afterward, we further equilibrated the system for 4 ns under the NPT ensemble to get an equilibrated pressure and temperature using Langevin thermostat and Berendsen barostat. Last, a MD run was conducted for 50 ns. The covalent bonds containing hydrogen were constrained using SHAKE (46, 47). 5000 snapshots were sampled from the MD trajectories every 10 ps to calculate the distance distribution between the interested groups. During all the minimization, equilibrium and NPT MD processes, both the N- and C- terminals of α1, α1 and α2 chain of type-I collagen were fixed with a strong restraint of 500 kcal/(mol Å).
Reaction with butyraldehyde monitored by HPLC-ICP-MS
Reactions were conducted in pH 7.4 phosphate-buffered saline with butyraldehyde concentration of 100 μM and hydrazine probes at 25 μM. Concentrations of unreacted starting material and condensation products were determined based on their relative integrations at 12 min time intervals over 2 hours.
Preparation of BSAAld
Preparation of BSAAld and binding of Gd3+ probe to protein was carried out according to modified procedures (48). To a solution of bovine serum albumin (BSA) (100 mg) dissolved in phosphate buffered saline (4 mL, pH 7.4, 0.25 mM) was added sodium ascorbate (20 mg), ferric chloride (120 μL, 10 mM) and 20 μL H2O2 (30% w). The reaction was stirred at 37 °C for 24 h, and sodium ascorbate (20 mg) was added repeatedly every 8 h. After 24 h, the protein was purified using PD-10 Sephadex G25 desalting columns (GE Healthcare), eluted with PBS. Protein concentration was assessed using the ‘Micro BCA Protein Assay Kit’ (Thermo Scientific, 23235). Protein carbonyl concentration was determined by ‘Protein Carbonyl Content Assay Kit’ (Sigma-Aldrich, MAK094-1KT). BSAAld had an aldehyde concentration of 4 aldehyde/protein. The protein solutions were kept at a concentration of 20 mg/mL for further use.
Relaxivity measurements in BSAAld
BSAAld (10 mg/mL) or BSA (10 mg/mL) were treated for 24 h at 37 °C with the corresponding molecular probe at a range of concentrations (0.01-0.2 mM), with a total volume of 300 μL maintained for all samples. After 24 h, an aliquot of the solution (50 μL) was used for longitudinal (T1) relaxation measurements, recorded using a Bruker mq60 Minispec at 1.41 T and 37 °C. Longitudinal (T1) relaxation times were measured via an inversion recovery experiment using 10 inversions of duration ranging between 0.05 x T1 and 10 x T1. Solutions (concentration range: 0.1 mM-1.0 mM) in PBS only were run in parallel. To measure the relaxivity of the protein-bound species, sodium cyanoborohydride (10 mg) was added to the solution to reduce the hydrazone bond and irreversibly bind the probe to the protein. After a further 2 h incubation at 37 °C, protein-bound and protein-free solutions were separated by ultrafiltration. Then 200 μL PBS was added to the residue to dissolve the protein. Longitudinal (T1) relaxation times for the bounded species were measured using a Bruker mq60 Minispec at 1.41 T and 37 °C. After the measurement, concentration of corresponding Gd3+ in the residue and filter were both determined using an Agilent 8800 ICP-MS system. Relaxivity (r1) was determined from the slope of a plot of 1/T1 vs metal concentration for 5 concentrations.
On/off rate with BSAAld
BSAAld (10 mg/mL) was incubated with 200 μM corresponding molecular probe at 37 °C in pH 7.4 PBS, with a total volume of 300 μL. The dynamic longitudinal (T1) relaxation time were measured using a Bruker mq60 Minispec at 1.41 T and 37 °C. After 24 h, protein-bound and protein-free solutions were separated by ultrafiltration. Then 200 μL PBS was added to the residue to dissolve the protein-bound species. The change of longitudinal (T1) relaxation time was then measured with a Bruker mq60 Minispec at 1.41 T and 37 °C.
Sodium dodecyl sulfate polyacrylamide gel electrophoresis analysis
BSAAld or BSA (10 mg/mL, 300 μL) was incubated with 200 μM Gd-1,4 at 37 °C in pH 7.4 PBS for 24 h. Then sodium cyanoborohydride (10 mg) was added to the solution and reacted for an additional further 2 h to irreversibly bind the probe to the protein. After the protein species were collected by ultrafiltration, 200 μL PBS was added to redissolve the protein. An aliquot of the solution was diluted in Tris-Glycine SDS sample buffer (1X) to give a final protein concentration of ~4 μg/μL, with a total volume of 40 μL. Then 5 μL of the samples and a molecular weight standard (PageRuler Prestained Protein Ladder No. 26619) were resolved electrophoretically using a 4-20% Tris-Glycine Gel (Thermo Fisher Scientific, XP04200BOX). Subsequently the proteins were then identified in the gel using silver staining (Thermo Fisher Scientific, 24600).
ECM extraction
Liver fibrosis was induced in male Sprague Dawley rats by ligation of the common bile duct (CD, Charles River Labs). Ten days after common bile duct ligation, the fibrotic liver was harvested and sequential extractions were performed using the CNMCS (Cytosol/Nucleus/Membrane/Cytoskeleton) Compartmental Protein Extraction kit (Cytomol) according to manufacturer’s instructions. In brief, frozen tissues (150–200 mg) were homogenized and with solvents provided in the kit to sequentially to remove: cytosolic proteins, nuclear proteins, membrane proteins, and cytoskeletal proteins, leaving a final insoluble fraction enriched for ECM proteins (49).
Relaxivity measurements in ECM
The enriched ECM proteins from 800 mg of fibrotic rat liver were suspended in 1 ml PBS. The ECM protein concentration was about 10% (5 mg in a 50 μL aliquot determined by weight after lyophilization). An aliquot of the solution (50 μL) was treated for 2 h at 37 °C with the corresponding molecular probe at a range of concentrations (0.01-0.1 mM) and then longitudinal (T1) relaxation measurements was recorded using a Bruker mq60 Minispec at 1.41 T and 37 °C. For the blocking experiment with N2H4, a 100-fold-excess of N2H4 referred to the concentration of molecular probe was added to the solution before the incubation. Longitudinal (T1) relaxation times were measured via an inversion recovery experiment using 10 inversions of duration ranging between 0.05 x T1 and 10 x T1. Relaxivity (r1) was determined from the slope of a plot of 1/T1 vs metal concentration for 4 concentrations.
Stability of the compounds in human plasma
A 16 μL aliquot of the molecular probe (1.0 mM) was added to 500 μL of human plasma (Lampire Biological laboratories). The solution was then incubated for 3 h at 37 °C. Aliquots (50 μL) were removed at 3 hours. Proteins were precipitated by the addition of 150 μL of acetonitrile and removed following centrifugation. The supernatant was then analyzed using HPLC-ICP-MS. No unchelated gadolinium was observed nor the appearance of any new Gd-containing species.
Animal Studies
A total of 100 mice and 12 rats were used in this study. Animals were imaged on a 4.7 Tesla MRI scanner (Bruker) using a custom-built volume coil.
CCl4 liver fibrosis mouse model
Male C57BL/6 mice (6-week old, Charles River Laboratories) were treated with carbon tetrachloride for 12 weeks (0.1 ml of 20% CCl4 in olive oil the first week, 30% the second week, and 40% from weeks 3-12) by oral gavage, 2-3 times per week. Control mice were fed vehicle (olive oil) only.
A total of 40 C57BL/6 mice were included to study CCl4 induced hepatic fibrosis:
Group A, CCl4 for 12 weeks (n = 16). Each mouse was imaged with probe 1 and then imaged again 24 hours later with probe 2. Probe 1 and Probe 2 were randomly choosing from Gd-DOTA, Gd-CHyd, Gd-1,4 and Gd-1,7.
Group B, vehicle treated control (n = 16). Each mouse was imaged with probe 1 and then imaged again 24 hours later with probe 2. Probe 1 and Probe 2 were randomly choosing from Gd-DOTA, Gd-CHyd, Gd-1,4, Gd-1,7.
Group C, CCl4 for 12 weeks (n = 3, another 3 pair-wise comparison have been done in group A). Each mouse was imaged with probe 1 and then imaged again 24 hours later with probe 2. Probe 1 and Probe 2 were randomly choosing from Gd-1,4 and Gd-1,7.
Group D, vehicle treated control (n = 2, another 2 pair-wise comparison have been done in group B). Each mouse was imaged with probe 1 and then imaged again 24 hours later with probe 2. Probe 1 and Probe 2 were randomly choosing from Gd-1,4 and Gd-1,7.
Group E, CCl4 for 12 weeks (n = 3). Each mouse was imaged with Gd-1,4. After 24 h, the same mouse was first injected with 1000 μmol/kg of Yb-1,4 and then injected with 100 μmol/kg of Gd-1,4 15 min after the Yb-1,4 injection.
CDAHFD mouse model
A total of 40 C57BL/6 mice were used in this study and randomized to each study group. To induce NASH, 6-week old, male C57BL/6 mice (Charles River Labs) were fed a CDAHFD consisting of 60 kcal% fat and 0.1% methionine (A06071302; Research Diets) by weight. No animals were excluded from the study.
Three groups of mice were studied:
Group A, mice were fed CDAHFD for 2 (n = 6), 6 (n = 6), 10 (n = 6) weeks.
Group B, mice were fed CDAHFD for 10 weeks followed by normal chow for 1 (n = 6) or 4 weeks (n = 6).
Group C, mice were fed normal chow for 2, 6, 10, 11 or 14 weeks (n = 10).
MR imaging and analysis for mouse studies
Animals were anesthetized with isoflurane (1–2%) and placed in a specially designed cradle with body temperature maintained at 37°C. The inhaled isoflurane concentration was adjusted to maintain a respiration rate of 60 ± 5 breaths per minute. The tail vein was cannulated for i.v. delivery of the molecular probe while the animal was positioned in the scanner. Imaging was performed at 4.7 T using a small bore animal scanner with a custom-built volume coil. Mice were imaged with a dose of 100 μmol/kg of molecular probe from 30 mM stock solution, as determined by ICP-MS.
MR imaging.
A series of baseline images (3D T1 weighted fast low angle shot magnetic resonance imaging (FLASH)) were first acquired (repetition time/echo time = 15/2 ms; flip angle, 30°; field of view, 48 × 30 mm2; matrix size, 136 × 136 mm2; slice thickness, 0.25 mm; acquisition time, 3 minutes 20 seconds), then a bolus of molecular probe was administered i.v. and imaging performed for a period of 45 min post-injection with the repetition acquisition of 3D T1 weighted FLASH sequences. Following the imaging session, animals were sacrificed (75 min post-injection) and liver tissue was subjected to histopathologic analysis.
Data analysis.
A region of interest (ROI) was manually traced encompassing the liver parenchyma while avoiding major blood vessels. A second ROI was placed on the dorsal muscle visible in the same image slice to quantify the signal intensity in the muscle for comparison. Seven ROIs were placed in the field of view without any tissue (air) to measure the variation in background signal. We analyzed more than 20 axial slices per mouse across the entire liver in this fashion. The same analysis was performed on the pre- and 45-min post injection images acquired with the FLASH sequence. Image visualization and quantification was performed in Horos. CNR was calculated by subtracting the signal intensity in the muscle from that in the liver and normalizing to the standard deviation of the signal in the air outside the animal, eq S1. ΔCNR was calculated by subtracting the CNRPre from CNRPost, eq S2.
Rat liver fibrosis model
Liver fibrosis was induced in male Sprague Dawley rats (n = 7) by ligation of the common bile duct (CD, Charles River Labs). Control animals (n = 4) underwent a sham procedure. The BDL and sham rats were imaged 10 days following ligation with a dose of 100 μmol/kg of Gd-1,4 based on body surface area.
MR imaging and analysis for rat study
Animals were anesthetized with isoflurane (1–2%) and placed in a specially designed cradle with body temperature maintained at 37°C. The inspired isoflurane concentration was adjusted to maintain a respiration rate of 60 ± 5 breaths per minute. The tail vein was cannulated for i.v. delivery of the molecular probe while the animal was positioned in the scanner. Imaging was performed at 4.7 T using a large bore animal scanner with a custom-built volume coil.
MR imaging.
A series of baseline images (T1-map and 3D T1 weighted FLASH) were first acquired, then a bolus of molecular probe was administered i.v. and imaging performed for a period of 30 min post-injection with the repetition acquisition of T1-mapping and 3D T1 weighted FLASH sequences. T1-mapping was performed with a flow sensitive alternating inversion recovery (FAIR) sequence: repetition time/echo time = 11000/33.8 ms; flip angle, 90°; inversion times: 100, 200, 400, 500, 600, 1000, 1500, 2000 ms; field of view, 60 × 60 mm2; matrix size, 140 × 140; slice thickness, 2 mm; acquisition time, 3 minutes 34 seconds. T1 weighted imaging with 3D FLASH: repetition time/echo time = 20/2.3 ms; flip angle, 30°; field of view, 60 × 60 mm2; matrix size: 127 × 127; slice thickness, 0.5 mm; acquisition time, 3 minutes 10 seconds. Following the imaging session, animals were sacrificed (30 min post-injection) and liver tissue was subjected to histopathologic analysis.
Data analysis.
Longitudinal relaxation rate (R1) maps were generated from the T1 mapping images using a custom written MATLAB (Mathworks) program for voxel wise fitting of the inversion recovery signal intensities as a function of the inversion time. A region of interest (ROI) was manually traced encompassing the liver parenchyma while avoiding major blood vessels. ΔR1 was calculated by subtracting the R1Pre from R1Post, eq S3. ΔCNR were analyzed from 3D T1 weighted FLASH images, the same as that in mouse.
Tissue analysis for mice and rats
General method.
After imaging, the animals were sacrificed under anesthesia, and the liver tissues were harvested. A piece of left lobe of the liver was fixed in 4% paraformaldehyde in PBS, dehydrated, embedded in paraffin, and then sectioned into 5-μm-thick slices for later staining with Sirius red and hematoxylin and eosin (H&E). Another piece of left lobe was fixed in methacarn (methanol-Carnoy) (50), dehydrated, embedded in paraffin, and sectioned into 7-μm-thick slices for detection of aldehyde. A remaining piece of left lobe was quickly frozen in liquid nitrogen for later hydroxyproline analysis.
The collagen proportional area (CPA), defined as the percentage of the area stained positive by Sirius red, was measured with ImageJ (Fiji, version 1.0) as previously described (48). For NASH components, H&E sections were evaluated and morphometric quantitation of hepatic steatosis, expressed as percentage of lipid vacuolization, was performed using ImageJ (Fiji, version 1.0) (51).
LOX protein detection.
LOX protein expression in the liver tissue was detected using immunohistochemistry assays with antibody against LOX (NB100-2527, 1:100, Novus Biologicals). After deparaffinization and hydration of the paraffin-embedded liver sections, the endogenous peroxidase activity was inhibited using 0.3% hydrogen peroxide and antigens were retrieved using 10 mM sodium citrate buffer (pH 6.0) at 110oC for 30 min. After the tissue was blocked using 2% triton, they were reacted with the anti-LOX antibodies at room temperature overnight. Subsequently, the slices were incubated with anti-goat IgG conjugated with peroxidase for 1 h at room temperature and then treated with DAB and counterstained with hematoxylin before dehydration and mounting.
Hydroxyproline assay.
Hydroxyproline in liver was quantified by HPLC analysis using a reported method (52), and was expressed as amounts per wet weight of tissue.
DNPH reactivity assay for determining LysAld.
The staining was carried out as previously described (27). After deparaffinization and hydration were performed on the 7 μm-thick methacarn-fixed liver sections, the tissue was reacted with 1 mg/mL dinitrophenylhydrazine (DNPH; TCI D0846, VWR) in 2 M HCl for 30 min. After the sections were neutralized with PBS, they were blocked with horse serum and then sequentially exposed to anti-DNP antibody (D9656, 1:2,000, Sigma-Aldrich), biotinylated secondary antibody, enzyme linked avidin-biotin complex, and Vector Red substrate (SK-5100, Vector Laboratories) diluted in levamisole (SP-5000, Vector Laboratories) and 100 mM Tris-HCl, pH 8.8 for 4 min before being counterstained with methyl green, dehydrated, and having a coverslip applied. The slides were scanned using NanoZoomer Slide Scanner (Hamamatsu) at 40X original magnification. Subsequently, four nonoverlapping, randomly oriented, 1000 x 600, 250 x 150 ,1000 x 600 μm image fields were obtained for the CCl4, NASH and BDL study, respectively, were picked for each tissue, avoiding large blood vessels and the tissue edge. For CCl4 and BDL study, the percentage of DNPH-reactive tissue in the image fields was then measured with ImageJ (Fiji, version 1.0), similar to the calculation of CPA (48). For the NASH study, the images were segmented using a uniform method to exclude cell nucleus and lipids, and the mean integrated intensities were determined using ImageJ (Fiji, version 1.0). During the image sampling, segmentation, and staining intensity determination, the involved investigator that was masked with respect to the animal treatment group.
Human sample characteristics
The clinical characteristics of the subjects are summarized in table. S2.
Tissue analysis for human tissues
The dissected tissues were immediately snap frozen in OCT using liquid nitrogen, sectioned into 10-μm-thick slices and stored at −80°C. Subsequently, the tissue sections were first allowed to warm to room temperature for 10 min and then fixed in 60% ethanol for 30 min, and washed with PBS. The following steps were the same as described above for the Sirius red staining and DNPH-reactivity assay.
Protein expression of LOX in the human tissue sections was detected using indirect immunofluorescence assays using with antibodies against LOX (ab174316, 1:50, Abcam), fluoroprobe-conjugated secondary antibody (A32733, 1:300, Invitrogen). The nuclei were identified using DNA-staining with DAPI. Last, LOX-positive cells were detected using a fluorescence microscope (EVOS FL).
LA-ICP-MS gadolinium imaging analysis
Sample preparation.
Harvested rat livers after MR imaging were immediately snapfrozen in OCT using liquid nitrogen, sectioned into 10-μm-thick slices, stored in −80°C. One slice was assessed by LA-ICP-MS directly. One adjacent slide was incubated with 0.1 mM Gd-1,4 at 37°C for 2 h, followed with 0.2 mM NaBH3CN for 2 h and washed with PBS (3×10 min). Another adjacent slide was incubated with 0.1 mM Gd-1,4 and 10 mM hydrazine at 37°C for 2 h, followed with 0.2 mM NaBH3CN for 2 h and washed with PBS (3×10 min). Similar procedures were carried out for human tissues. Slides were incubated with 0.1 mM Gd-1,4 at 37°C for 2h, followed with 0.2 mM NaBH3CN for 2 h and washed with PBS (3×10 min).
Gadolinium mapping.
Elemental images were collected from sections of liver tissue via laser ablation inductively coupled plasma mass spectrometry at the Biomedical National Elemental Imaging Resource (BNEIR). The instrumentation used was a New Wave Research 213 nm laser ablation system with a 10 cm2 sample chamber, interfaced with an Agilent 7900 ICP-MS system. Samples were ablated in no-gas mode, with helium as a carrier gas at a flow rate of 600 L min−1. The laser power was 65% and the frequency was 20 Hz. Ablation patterns consisted of continuous lines, with either a 12 μm or 20 μm square beam and a scan speed of 120 μm sec−1 or 200 μm sec−1 respectively, as noted. The acquisitions time for gadolinium (mass 157) was 0.015 s. Elemental imaging data was quantified using metal-doped gelatin standards prepared at BNEIR (53) and National Institute of Standards and Technology (NIST) certified references material 1515 (apple leaves). Data reduction was performed in the Iolite software application (54, 55), using carbon (mass 13) as an internal standard to normalize the data.
Statistics
Data are displayed as box plots with the dark band inside the box representing the mean, the bottom and top of the box representing the first and third quartiles, and the whiskers the minimum and maximum values. Data are reported as the mean ± standard deviation. For relaxivity measurements, the data distribution was assumed normal based on normality tests of historical laboratory relaxivity studies (data file S1). Differences between two groups were tested with one-tailed unpaired t-test. For biological and imaging data, data were tested for normality prior to use of parametric tests. Differences between two groups for un-paired studies were tested with two-tailed unpaired t-test. Differences between two groups for paired studies were tested with two-tailed paired t-test. Differences among more than two groups were tested with one-way analysis of variance (ANOVA) followed by Tukey’s post-hoc test with P < 0.05 considered significant. All statistical analyses were performed using GraphPad Prism 9.0 (GraphPad software).
Supplementary Material
Acknowledgments:
We thank Dr. Yuhang Yao of Peking University for helpful discussions on the molecular dynamics simulation. We thank Jonah Weigand-Whittier of Massachusetts General Hospital for assistance with MRI scanning.
Funding:
P.C. acknowledges grant support from the National Institute of Diabetes and Digestive and Kidney Diseases (DK121789) and the National Institutes of Health Office of the Director (OD025234 and OD010650). J.D.R. acknowledges support from the National Heart, Lung, and Blood Institute (HL125715 and HL147863). H.K.D. acknowledges support from the German Research Foundation (DFG, DR 1161/1-1). Laser ablation elemental imaging was performed at the Dartmouth Biomedical National Elemental Imaging Resource (BNEIR) supported by the National Institute for General Medical Sciences (GM141194, B.P.J.).
Competing interests:
YN, EA and PC are inventors of a filed patent based on the work here (Molecular probes for in vivo detection of aldehydes. PCT/US2022/072310). P.C. has equity in and is a consultant to Collagen Medical LLC, has equity in Reveal Pharmaceuticals Inc, and has research support from Pliant Therapeutics, Takeda, and Janssen. The other authors declare that they have no competing interests.
Data and materials availability:
All data are available in the main text or the supplementary materials. Detailed synthesis and characterization data, experimental details, materials, and methods. Primary data used to generate the graphs was in data file S1.
References and Notes
- 1.Pellicoro A, Ramachandran P, Iredale JP, Fallowfield JA, Liver fibrosis and repair: immune regulation of wound healing in a solid organ. Nat Rev Immunol 14, 181–194 (2014). [DOI] [PubMed] [Google Scholar]
- 2.Asrani SK, Devarbhavi H, Eaton J, Kamath PS, Burden of liver diseases in the world. J Hepatol 70, 151–171 (2019). [DOI] [PubMed] [Google Scholar]
- 3.Tapper EB, Lok AS, Use of Liver Imaging and Biopsy in Clinical Practice. N Engl J Med 377, 756–768 (2017). [DOI] [PubMed] [Google Scholar]
- 4.Tapper EB, Loomba R, Noninvasive imaging biomarker assessment of liver fibrosis by elastography in NAFLD. Nat Rev Gastroenterol Hepatol 15, 274–282 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sanyal AJ et al. , Non-Invasive Biomarkers of Nonalcoholic Steatohepatitis: the FNIH NIMBLE project. Nat Med, (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sheka AC et al. , Nonalcoholic steatohepatitis: a review. Jama 323, 1175–1183 (2020). [DOI] [PubMed] [Google Scholar]
- 7.Ratziu V, Goodman Z, Sanyal A, Current efforts and trends in the treatment of NASH. J Hepatol 62, S65–75 (2015). [DOI] [PubMed] [Google Scholar]
- 8.Davison BA et al. , Suboptimal reliability of liver biopsy evaluation has implications for randomized clinical trials. J Hepatol 73, 1322–1332 (2020). [DOI] [PubMed] [Google Scholar]
- 9.Kisseleva T, Brenner D, Molecular and cellular mechanisms of liver fibrosis and its regression. Nat Rev Gastroenterol Hepatol 18, 151–166 (2021). [DOI] [PubMed] [Google Scholar]
- 10.Roehlen N, Crouchet E, Baumert TF, Liver Fibrosis: Mechanistic Concepts and Therapeutic Perspectives. Cells 9, 875 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bonnans C, Chou J, Werb Z, Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol 15, 786–801 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nimni ME, et al. , Collagen. M. Nimni, Ed., (CRC press, FL, USA, 2018). [Google Scholar]
- 13.Farrar CT et al. , 3D molecular MR imaging of liver fibrosis and response to rapamycin therapy in a bile duct ligation rat model. J Hepatol 63, 689–696 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Désogère P et al. , Type I collagen–targeted PET probe for pulmonaryfibrosis detection and staging in preclinical models. Sci. Transl. Med. 9, 384 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salarian M et al. , Early detection and staging of chronic liver diseases with a protein MRI contrast agent. Nat Commun 10, 4777 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen W et al. , Lysyl Oxidase (LOX) Family Members: Rationale and Their Potential as Therapeutic Targets for Liver Fibrosis. Hepatology 72, 729–741 (2020). [DOI] [PubMed] [Google Scholar]
- 17.Kawahara K et al. , Spatiotemporal regulation of PEDF signaling by type I collagen remodeling. Proc Natl Acad Sci U S A 117, 11450–11458 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamauchi M, Sricholpech M, Lysine post-translational modifications of collagen. Essays Biochem 52, 113–133 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mouw JK, Ou G, Weaver VM, Extracellular matrix assembly: a multiscale deconstruction. Nat Rev Mol Cell Biol 15, 771–785 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caravan P, Strategies for increasing the sensitivity of gadolinium based MRI contrast agents. Chem Soc Rev 35, 512–523 (2006). [DOI] [PubMed] [Google Scholar]
- 21.Wahsner J, Gale EM, Rodriguez-Rodriguez A, Caravan P, Chemistry of MRI Contrast Agents: Current Challenges and New Frontiers. Chem Rev 119, 957–1057 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramalho J et al. , Gadolinium-Based Contrast Agent Accumulation and Toxicity: An Update. AJNR Am J Neuroradiol 37, 1192–1198 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Akam EA et al. , Improving the reactivity of hydrazine-bearing MRI probes for in vivo imaging of lung fibrogenesis. Chem Sci 11, 224–231 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang BX, Kim HY, Dass C, Probing three-dimensional structure of bovine serum albumin by chemical cross-linking and mass spectrometry. J Am Soc Mass Spectrom 15, 1237–1247 (2004). [DOI] [PubMed] [Google Scholar]
- 25.Zhang Z et al. , Multilocus binding increases the relaxivity of protein-bound MRI contrast agents. Angew Chem Int Ed 44, 6766–6769 (2005). [DOI] [PubMed] [Google Scholar]
- 26.Iredale JP, Models of liver fibrosis: exploring the dynamic nature of inflammation and repair in a solid organ. J Clin Invest 117, 539–548 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhong Y et al. , Lysyl oxidase regulation and protein aldehydes in the injured newborn lung. Am J Physiol Lung Cell Mol Physiol 322, L204–L223 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rantakari P et al. , Stabilin-1 expression defines a subset of macrophages that mediate tissue homeostasis and prevent fibrosis in chronic liver injury. Proc Natl Acad Sci U S A 113, 9298–9303 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matsumoto M et al. , An improved mouse model that rapidly develops fibrosis in non-alcoholic steatohepatitis. Int J Exp Pathol 94, 93–103 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brown ZJ, Heinrich B, Greten TF, Mouse models of hepatocellular carcinoma: an overview and highlights for immunotherapy research. Nat Rev Gastroenterol Hepatol 15, 536–554 (2018). [DOI] [PubMed] [Google Scholar]
- 31.Zhou IY et al. , Advanced MRI of Liver Fibrosis and Treatment Response in a Rat Model of Nonalcoholic Steatohepatitis. Radiology 296, 67–75 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weinreb JC et al. , Use of Intravenous Gadolinium-Based Contrast Media in Patients With Kidney Disease: Consensus Statements from the American College of Radiology and the National Kidney Foundation. Kidney Med 3, 142–150 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goldstein KM et al. , Risk of Nephrogenic Systemic Fibrosis after Exposure to Newer Gadolinium Agents. Ann Intern Med 173, 110–119 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Noda SM et al. , Gadolinium retention: should pediatric radiologists be concerned, and how to frame conversations with families. Pediatr Radiol 52, 345–353 (2022). [DOI] [PubMed] [Google Scholar]
- 35.Levy SG et al. , Development of a Multigram Asymmetric Synthesis of 2-(R)-2-(4,7,10-Tris tert-Butylcarboxymethyl-1,4,7,10-tetraazacyclododec-1-yl)-pentanedioic Acid, 1-tert-Butyl Ester, (R)-tert-Bu4-DOTAGA. Org. Process Res. Dev. 13, 535–542 (2009). [Google Scholar]
- 36.Li C, Wong WT, A simple, regioselective synthesis of 1,4-bis(tert-butoxycarbonylmethyl)- tetraazacyclododecane. J Org Chem 68, 2956–2959 (2003). [DOI] [PubMed] [Google Scholar]
- 37.Hopper LE, Allen MJ, Rapid synthesis of 1,7-bis(t-butoxycarbonylmethyl)-1,4,7,10-tetraazacyclododecane (DO2A-t-Bu ester). Tetrahedron Lett 55, 5560–5561 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Janicki R, Mondry A, Structural and thermodynamic aspects of hydration of Gd(III) systems. Dalton Trans 48, 3380–3391 (2019). [DOI] [PubMed] [Google Scholar]
- 39.Malone JP, George A, Veis A, Type I collagen N-telopeptides adopt an ordered structure when docked to their helix receptor during fibrillogenesis. Proteins 54, 206–215 (2004). [DOI] [PubMed] [Google Scholar]
- 40.Brozell SR et al. , AMBER 2018. (University of California, San Francisco, 2018). [Google Scholar]
- 41.Maier JA et al. , ff14SB: Improving the Accuracy of Protein Side Chain and Backbone Parameters from ff99SB. J. Chem. Theory Comput. 11, 3696–3713 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang J, Wolf RM, Caldwell JW, Kollman PA, Case DA, Development and testing of a general amber force field. J. Comput. Chem. 25, 1157–1174 (2004). [DOI] [PubMed] [Google Scholar]
- 43.Bayly CI, Cieplak P, Cornell WD, Kollman PA, A Well-Behaved Electrostatic Potential Based Method Using Charge Restraints for Deriving Atomic Charges - the Resp Model. J. Phys. Chem 97, 10269–10280 (1993). [Google Scholar]
- 44.Li P, Merz KM Jr., MCPB.py: A Python Based Metal Center Parameter Builder. J. Chem. Inf. Model 56, 599–604 (2016). [DOI] [PubMed] [Google Scholar]
- 45.Jorgensen WL, Chandrasekhar J, Madura JD, Impey RW, Klein ML, Comparison of Simple Potential Functions for Simulating Liquid Water. J. Chem. Phys 79, 926–935 (1983). [Google Scholar]
- 46.Ryckaert JP, Ciccotti G, Berendsen HJC, Numerical integration of the cartesian equations of motion of a system with constraints: molecular dynamics of n-alkanes. J. Comput. Phys 23, 327–341 (1977). [Google Scholar]
- 47.Zhang X et al. , H-Bonding Networks Dictate the Molecular Mechanism of H2O2 Activation by P450. ACS Catalysis 11, 8774–8785 (2021). [Google Scholar]
- 48.Chen HH et al. , Molecular imaging of oxidized collagen quantifies pulmonary and hepatic fibrogenesis. JCI Insight 2, e91506 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Naba A et al. , The matrisome: in silico definition and in vivo characterization by proteomics of normal and tumor extracellular matrices. Mol Cell Proteomics 11, M111.014647 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Puchtler H, Waldrop FS, Meloan SN, Terry MS, Conner HM, Methacarn (methanol-Carnoy) fixation. Practical and theoretical considerations. Histochemie 21, 97–116 (1970). [DOI] [PubMed] [Google Scholar]
- 51.Erstad DJ et al. , Molecular magnetic resonance imaging accurately measures the antifibrotic effect of EDP-305, a novel farnesoid X receptor agonist. Hepatol Commun 2, 821–835 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hutson PR, Crawford ME, Sorkness RL, Liquid chromatographic determination of hydroxyproline in tissue samples. J. Chromatogr. B 791, 427–430 (2003). [DOI] [PubMed] [Google Scholar]
- 53.Sala M, Selih VS, van Elteren JT, Gelatin gels as multi-element calibration standards in LA-ICP-MS bioimaging: fabrication of homogeneous standards and microhomogeneity testing. Analyst 142, 3356–3359 (2017). [DOI] [PubMed] [Google Scholar]
- 54.Paul B et al. , CellSpace: A module for creating spatially registered laser ablation images within the Iolite freeware environment. J. Anal. At. Spectrom 27, 700–706 (2012). [Google Scholar]
- 55.Paton C, Hellstrom J, Paul B, Woodhead J, Hergt J, Iolite: Freeware for the visualisation and processing of mass spectrometric data. J. Anal. At. Spectrom 26, 2508–2518 (2011). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available in the main text or the supplementary materials. Detailed synthesis and characterization data, experimental details, materials, and methods. Primary data used to generate the graphs was in data file S1.


