The poor efficacy of immunotherapy in clinical trials of solid tumours is mainly due to their highly immunosuppressive tumour microenvironment (TME).1 Regulatory T cells (Tregs), a subset of T cells that control the autoimmune response and are one of the main components of immunosuppressive TME, can regulate the immune response intensity and inhibit the function and activity of effector T cells, thus inducing immune tolerance and maintaining immune response homeostasis. In solid TME, Tregs mediate the immune escape of tumour cells by inhibiting the body’s immune response, affecting the efficacy of prognosis.2
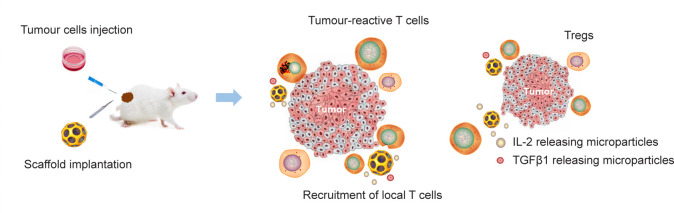
Based on the characteristics of Tregs in TME, reducing their infiltration and immunosuppressive effect helps explore new treatment strategies for solid tumours. The design, manufacture, and therapeutic properties of biologically degradable immunomodulatory macroporous scaffolds have been reported recently in Nature Biomedical Engineering.3 In this study, immunomodulatory macroporous scaffold implantation around the tumour released small-molecule inhibitors of transforming growth factor β, which selectively target Treg cells, while chemokines attracted effector T cells and antibodies to activate tumour cells. In animal models with malignant tumours, immunomodulatory macroporous scaffolds are beneficial to recruit and activate effector T cells into the tumours, released small-molecule inhibitors of transforming growth factor β, and suppressed Treg cells, which resulted in an “immunoectopic effect” against distant metastases and established long-term memory to prevent tumour recurrence. This study also revealed that immunomodulatory macroporous scaffolds can be regarded as a vehicle to deliver antigen-specific T cells into the tumours. Overall, implanting immunomodulatory macroporous scaffolds around tumours can enhance cellular immunity and avoid systemic toxicity (Figure 1).
Figure 1. The schematic representation showed that the anti-tumour immunity was enhanced by immunomodulatory macroporous scaffolds. Sustained release of scaffolds can recruit endogenous T cells, whereas the presentation of surface-conjugated activation cues and the sustained release of IL-2 can activate recruited T cells. Sustained release of TGF-β depletes Tregs in tumours. IL-2: interleukin-2; TGF-β: transforming growth factor β; TGFβ1: transforming growth factor β 1; Treg: regulatory T cell.
Mesoporous silica nanomaterials have the advantages of high porosity, high biocompatibility, and easy surface modification and are ideal materials for improving tumour immunotherapy efficacy.4 The two most common types of mesoporous-silica-based immunotherapies are internalization of mesoporous silica nanoparticles into antigen-presenting cells, and recruitment of antigen-presenting cells by micrometer-sized mesoporous silica rods that can form a three-dimensional (3D) space.4 mesoporous silica nanoparticle-based cancer vaccines can be taken up by peripheral or lymphoid cells to stimulate the immune system to fight cancer cells, and mesoporous silica rod cancer vaccines can aggregate immune cells into their scaffold to induce tumour-specific immunity. Therefore, mesoporous silica can successfully stimulate adaptive immune response and can treat cancer and other infectious diseases.5
There are many types of immunotherapy carriers of biological scaffolds, which are divided into different types according to their composition, preparation method, route of administration, or immune regulation principle.6 In addition to mesoporous silica, collagen, alginate, and hyaluronic acid are typically composed of natural ingredients or synthetic polymers.7 The preparation methods include simple crosslinking in vitro, rapid sol-gel phase transformation in vivo, in situ chemical polymerization assembly, or 3D printing and self-assembly technology. These scaffolds are also becoming more diverse with tumour antigens and adjuvants, immunostimulatory or immunosuppressive molecules, and biologically active immune cells.6 Moreover, because of the importance of 3D in vitro models in scientific research, 3D macroscale scaffolds have shown great potential for the development of biomimetic organoids.8
This work highlighted the unique advantage of using mesoporous materials for the modulation of cancer immunotherapy. However, such scaffold materials face challenges like limited penetration depth into solid tumours, the demand to deal with the high individual heterogeneity of tumours, the challenge to achieve precise regulation, and the hurdle in large-scale production and potential market transformation, etc. Further research efforts are necessary to further explore the molecular mechanism of tumour immune microenvironment regulation and the influence of biological scaffold materials on cell fate. Eventually, the development of more personalised biological scaffold materials in combination with advanced biomaterials manufacturing technology will provide more efficient immunotherapies for solid tumours and other cancers.
Footnotes
Author contributions: Conceptualization, investigation, and writing-original draft: FXC; software, supervision, and writing-review & editing: FFP. Both authors read and approved the final version of the manuscript.
Financial support: None.
Acknowledgement: None.
Conflicts of interest statement: None.
References
- 1.Ho W. J., Jaffee E. M., Zheng L. The tumour microenvironment in pancreatic cancer - clinical challenges and opportunities. Nat Rev Clin Oncol. 2020;17:527–540. doi: 10.1038/s41571-020-0363-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dees S., Ganesan R., Singh S., Grewal I. S. Regulatory T cell targeting in cancer: Emerging strategies in immunotherapy. Eur J Immunol. 2021;51:280–291. doi: 10.1002/eji.202048992. [DOI] [PubMed] [Google Scholar]
- 3.Majedi F. S., Hasani-Sadrabadi M. M., Thauland T. J., Keswani S. G., Li S., Bouchard L. S., Butte M. J. Systemic enhancement of antitumour immunity by peritumourally implanted immunomodulatory macroporous scaffolds. Nat Biomed Eng. 2023;7:56–71. doi: 10.1038/s41551-022-00977-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu A., Dai X., Wang Z., Chen H., Guo B., Huang L. Recent advances of mesoporous silica as a platform for cancer immunotherapy. Biosensors (Basel) 2022;12:109. doi: 10.3390/bios12020109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nguyen T. L., Choi Y., Kim J. Mesoporous silica as a versatile platform for cancer immunotherapy. Adv Mater. 2019;31:e1803953. doi: 10.1002/adma.201803953. [DOI] [PubMed] [Google Scholar]
- 6.Huang P., Wang X., Liang X., Yang J., Zhang C., Kong D., Wang W. Nano-, micro-, and macroscale drug delivery systems for cancer immunotherapy. Acta Biomater. 2019;85:1–26. doi: 10.1016/j.actbio.2018.12.028. [DOI] [PubMed] [Google Scholar]
- 7.Jung K., Corrigan N., Wong E. H. H., Boyer C. Bioactive synthetic polymers. Adv Mater. 2022;34:e2105063. doi: 10.1002/adma.202105063. [DOI] [PubMed] [Google Scholar]
- 8.Marchini A., Gelain F. Synthetic scaffolds for 3D cell cultures and organoids: applications in regenerative medicine. Crit Rev Biotechnol. 2022;42:468–486. doi: 10.1080/07388551.2021.1932716. [DOI] [PubMed] [Google Scholar]