Highlights
-
•
Low prevalence areas are not entirely safe from bacterial meningitis as time progresses.
-
•
Populations under identified hotspots are exceptionally at higher risk of outbreaks.
-
•
The spatial distribution of bacterial meningitis varied significantly in space and time and was not due to random chance.
Keywords: Spatial epidemiology, Bacterial meningitis, Geospatial analysis, Ghana, Upper West Region
Abstract
Background
The use of a Geographic Information System in identifying meningitis hotspots in the Upper West Region (UWR) remains underutilized, making spatial targeting of meningitis hotspots difficult. We therefore utilized surveillance data enabled with GIS technology to target meningitis outbreaks in the UWR.
Methods
Secondary data analysis was conducted in the study. The dynamics of bacterial meningitis in space and time were studied using epidemiological data from 2018 to 2020. Spot map and choropleths were used to depict the distribution of cases in the region. Moran's I statistics were used to assess spatial autocorrelation. Getis-Ord Gi*(d) and Anselin Local Moran’s statistics were used to identify hotspots and spatial outliers within the study area. A Geographic Weighted Regression model was also used to examine how socio bio-climatic conditions influence the spread of meningitis.
Results
There were 1176 cases of bacterial meningitis, 118 deaths, and 1058 survivors between 2018 and 2020. Nandom municipality had the highest Attack Rate (AR) at 492/100,000 persons, followed by Nadowli-Kaleo district at 314/100,000 persons. Jirapa had the highest case fatality rate (CFR) at 17%. The spatio-temporal analysis showed spatial diffusion of meningitis prevalence from the western half of the UWR to the east with a significant number of hotpots and cluster outliers.
Conclusion
Bacterial meningitis does not occur at random. Populations (10.9%) under sub-districts identified as hotspots are exceptionally at higher risk of outbreaks. Targeted interventions should be directed towards clustered hotspots, focusing on zones with low prevalence fenced off by high prevalence zones.
Introduction
Meningitis is an infection that occurs when the membranes (meninges) of the brain and spinal cord become inflamed (WHO, 2011). It is a nervous system disease caused by several agents, including viruses and bacteria of different forms (AM & MC Thomson, 2002, Putz et al., 2013). Nevertheless, most meningitis cases are caused by bacteria. A variety of bacteria can cause meningitis, the most prevalent of which are Streptococcus pneumoniae (S.P), Neisseria meningitidis (Serogroups W, A, C, and X), and Hemophilus influenzae Type B (Hib) (Mazamay et al., 2021, Tsang, 2021). Though bacterial meningitis is a worldwide disease, the epidemiology and pattern vary according to geographic region and season (Pace et al., 2020). However, the most serious ones occur primarily in sub-Saharan Africa's meningitis belt regions, extending from Senegal to Ethiopia, including Ghana (AM & MC Thomson, 2002, WHO, 2020). About four hundred million people across more than 26 countries live in this “African meningitis belt” Over 900,000 cases were reported in this region from 1995 to 2014. One in every ten people who contract meningitis dies, and one in every-five suffers from severe complications, including neurological sequelae (Paireau et al., 2012, WHO, 2021).
In Ghana, the northern territories (Upper West, Northern, North-East, Savanna, Oti, Bono East, and Upper East Regions) lie within the meningitis belt of Africa (WHO, 2020). More than 400 people died out of 4561 meningitis cases reported between 2010 and 2015 (GHS, 2017). In the 2015/16 meningitis season, the country experienced a mixed outbreak of meningitis caused by S. pneumoniae and Neisseria meningitidis Serotype W (NmW) that affected all regions in the country with severe infections in the Bono Region and Upper West Region (Aku et al., 2017, Domo et al., 2017, Kwarteng et al., 2017).
The Upper West Region (UWR) has continued to experience isolated outbreaks during the dry season. All communities in the UWR are contained by the African meningitis belt and have recorded a high incidence of meningitis cases every year throughout the dry season that usually stretches from November to April (WHO, 2020). Since 2013, the area has confirmed focal outbreaks of NmW and pneumococcal disease in Jirapa, Nadowli, and Nandom districts (Domo et al., 2017, GHS, 2012, GHS, 2020). Amid the novel COVID-19 pandemic in early 2020, the UWR suffered a double burden of a mixed meningitis outbreak (of NmX and S. pneumonia). COVID-19-induced anxiety and the global focus on the pandemic diverted the focus of crucial health system players from the seasonal outbreaks of meningitis, which led to almost 500 cases and 50 deaths, the majority of which were avoidable (GHS, 2020).
Human health and geographical location are inextricably correlated, positively or negatively affecting well-being. Hence, the spatial distribution of disease in a population is crucial evidence for researchers in public health, policymakers, and implementers to make evidence-based and informed decisions (Dummer, 2008, Weimann et al., 2020). Geographic Information System (GIS) technology has assisted in interpreting a wide range of disease outcomes (Mobasheri & Ahmadi, 2014). In Ghana, GIS has been used to map and create spatial clusters forecasting possible meningitis outbreaks in the Upper East Region (Akyereko et al., 2020). From what we know, a spatial epidemiological study of meningitis with spatio-temporal characteristics has not been conducted in the UWR. Therefore, this study relies on GIS-based geostatistical tools to assess spatio-temporal hotspots in the UWR region to predict possible local outbreak zones and enhance the region's meningitis surveillance and targeted interventions.
Materials and methods
Study setting
The Upper West Region is located in northern Ghana (between latitudes 9.8–11.O° North and longitudes 1.6°–3.0 West). It has a total land field of 18,476 km2 and shares boundaries with Burkina Faso, Savannah, and the Upper East Region. The region has 11 administrative districts and 1009 communities with a total estimated population of 868,479 in 2020, with a 1.9 % growth rate from the Population and Housing Census-2010 (GSS, 2013). For efficient health delivery, the districts in the region are further divided into seventy-five (75) sub-districts. These Sub-districts are governed by Sub-District Health Management Teams (SDHMTs).
Additionally, the region has eight district hospitals and one regional hospital. The vegetation is guinea savannah woodland, and the climate is tropical, with an annual average temperature of 22.6–40.0 °C. The rainy season peaks at 90 % of rainfall (from May to October) but decreases to 20 % in the dry season, and the average rainfall ranges from 100 to 120 cm, and humidity is between 110 % and 700 %. During harmattan, harsh, cold, dry, and dusty climatic conditions usually span from November to April. These climatic conditions make the region more vulnerable to cerebrospinal meningitis outbreaks annually (GHS, 2020).
Data collection
Data were extracted in January 2021 from the line-lists of meningitis cases from the routine disease surveillance data (2018–2020) using a data extraction sheet. References were made on the individual case-based forms and laboratory test results for missing data on the collated line list. The records were checked for completeness and accuracy for all the data elements to ensure quality. Again, the individual meningitis cases were geocoded by adding geographic identifiers to each case based on their town/village of residence. The cases were manually geocoded using Google Maps due to the non-availability of a reference database. Using ArcGIS, placemarks were used to extract locations and converted to geodatabase feature classes. The research area's shapefiles (Administrative levels 2 and 3) were acquired from the Ghana Health Service's database system, the DHIMS-2 (District Health Information Management System) platform.
Data analysis
The categorical variables were summarized into frequencies, percentages, and rates, while means were used to summarise continuous variables. An epidemic curve was generated from the case line list to illustrate the disease occurrence trend. Data were analyzed to identify patterns and spread of meningitis over time using choropleth as descriptive statistical techniques. The incidence of meningitis was calculated for the three preceding years (2018, 2019, and 2020). Further investigation of spatial and temporal consistency using a Spatio-temporal approach was used to examine the spatial trend of meningitis prevalence. The study findings were presented in maps, tables, and graphs. The data analysis was done using STATA version 14.0, Microsoft Excel (2019), ArcGIS version 10.5, and GeoDa version 1.18.0.
Assessing spatial autocorrelation of meningitis cases in UWR
We explored spatial autocorrelation using Moran's I statistics, both global and local. The spatial units were examined to evaluate whether or not meningitis cases cluster in the UWR. This is to generate a risk map for meningitis in a small area that is narrowed to the sub-district level to aid in developing a targeted response. Additionally, this analytical instrument is sensitive to eliciting critical details for decision-making.
The statistics of Anselin Local Moran I was utilized to identify potential outliers in the analysis. The tools were run in ArcGIS 10.5 and GeoDa version 1.18.0 using polygon contiguity. For the spatial autocorrelation, Moran's I statistic is given as:
For feature i, is the attribute's deviation from its mean . As shown in the equation, is the spatial weight between features i and j, n represents the number of features, and is the sum of all spatial weights:
The -score statistic is calculated as follows:
Were
The period prevalence of meningitis (cases per 10,000 population) was estimated for each unit polygon (Sub-District) during the analysis. Contiguity edges and corners (Queen's Case) were crucial in the study's conceptualization. The computations for the target polygon included polygons that shared an edge or a corner. While computing the target polygon feature, we considered sub-districts (polygon features) that shared boundaries or nodes. Again, portions of polygons that overlapped were considered neighbors. Using the Euclidean Distance Approach, a maximum peak of 78239.94 (derived from Incremental Spatial Autocorrelation computation) was deemed appropriate distant bandwidth for this analysis. As a weighting mechanism, row standardization was used to limit the aggregation scheme that could be imposed (Esri, 2020).
Analyzing meningitis prevalence hotspots
For each feature in the dataset, the hotspot analysis tool was used to calculate the Getis-Ord Gi*statistic. The generated z-scores and p-values show where high, and low-value features are spatially grouped. This tool analyses each feature in relation to its surroundings. A high-value feature is intriguing, but it may not be a statistically significant hot spot. In order to be a statistically significant hot spot, a feature must have a high value and be surrounded by other features with high values. The study used False Discovery Rate (FDR) correction to adjust for multiple testing and spatial dependence.
The formula is as follows:
is the feature j attribute value, and takes into account the spatial weight between feature and ; n is sum of features. As with the Global Moran I analysis, spatial relationships were conceptualized using polygon contiguousness with edges and corners. Incremental spatial autocorrelation analysis was conducted to choose a precise and realistic search radius that agrees with the neighborhood conceptualization. The analysis was performed using the Euclidean Distance method. Following the outcome of the computation, the highest peak distance value (78239.94 m) was chosen as the distance bandwidth or search radius.
Ethical consideration
Ethical approval was obtained from the Kwame Nkrumah University of Science and Technology’s (KNUST) Committee on Human Research, Publication, and Ethics (Ref Number: CHRPE/AP/270/21), and permission and approval were granted by the Ghana Health Service (GHS). We removed all traceable identifiers associated with the cases from the dataset. Global Positioning System (GPS) coordinates of case locations were randomly dotted within the community of origin, concealing the exact geographical household’s location of the cases.
Results
Descriptive epidemiology
The data analysis involved 1176 records of bacterial meningitis from the UWR from 2018 to 2020. It was observed that 118 deaths and 1058 survivors were recorded within the same period. Of all the bacterial meningitis cases recorded, 51 % (n = 605) have been males. Children under one year were the least (n = 34, 2.9 %) affected age group. Again, age-groups 5–14 years, as well as 15–29, were the most involved with 255 and 265 cases, respectively, as shown in Fig. 1.
Fig. 1.
Distribution of Meningitis Cases by Age group and Sex.
It was again found that the incidence of meningitis within the three years studied followed a cyclical trend, as shown in Fig. 2. The majority (n = 829, 70.5 %) of the cases occurred between epidemiological weeks 2 – 17 over the three years. These periods represent environmentally favorable conditions for meningitis transmission, given the predominance of dust, low humidity, and increased wind speed at the time. For the three years, the prevalence of meningitis cases was 52 per 100,000 population, 41 per 100,000 population, and 48 per 100,000 population for 2018, 2019, and 2020 respectively. Comparatively, the 2020 Case Fatality Rate (CFR) was 13.6 % which is almost two folds higher than 2019 (CFR 7.6 %) and 2018 (CFR 9.0 %).
Fig. 2.
Meningitis Weekly Incidence in Upper West Region, 2018 to 2020.
Results also indicate that Nandom municipality had the highest Attack Rate (AR) of 492 per 100,000 population, followed closely by the Nadowli-Kaleo district with an AR of 314 per 100,000 population. However, these reported cases of meningitis do not depict a population-cases ratio. In contrast, the disease burden was high at Nandom, although the municipality has a population relatively lower (55,925) than the Nadowli district (74,507). (Table 1).
Table 1.
Meningitis Cases and Deaths by Districts, Upper West Region, 2018–2020.
| Districts | Cumulative Attack Rate (Per 100,000) |
Case Fatality Rate (CFR %) |
||
|---|---|---|---|---|
| No. | AR | No. | CFR% | |
| DBI | 60 | 151 | 6 | 10.0 % |
| Jirapa | 182 | 171 | 31 | 17.0 % |
| Lambussie | 81 | 129 | 12 | 14.8 % |
| Lawra | 162 | 245 | 12 | 7.4 % |
| Nadowli-Kaleo | 234 | 314 | 23 | 9.8 % |
| Nandom | 275 | 492 | 23 | 8.4 % |
| Sissala East | 12 | 17 | 0 | 0.0 % |
| Sissala West | 45 | 94 | 3 | 6.7 % |
| Wa East | 26 | 30 | 4 | 15.4 % |
| Wa Municipal | 50 | 39 | 2 | 4.0 % |
| Wa West | 49 | 50 | 3 | 6.1 % |
| Total | 1176 | 141 | 118 | 10.0 % |
Nandom recorded the highest case count, while Jirapa recorded the highest CFR (17 %), followed by Wa East (15.4 %) and Lambussie (14.8 %). Furthermore, a less severe burden of meningitis was observed at Sissala East as it recorded the lowest AR of 17 per 100,000 population and CFR of 0.0 %.
Assessing the spatial distribution of meningitis prevalence
Fig. 3 depicts the uneven regional distribution of meningitis cases in space and is not due to random chance, as homogeneity has not been observed. The western part of the region has more clusters than the eastern flank, as a sparse incidence of the disease was observed. This, therefore, warranted the investigation of spatial and temporal coherence in the disease incidence distribution observed.
Fig. 3.
Spot Map of Bacterial Meningitis Cases, Upper West Region, 2018–2020.
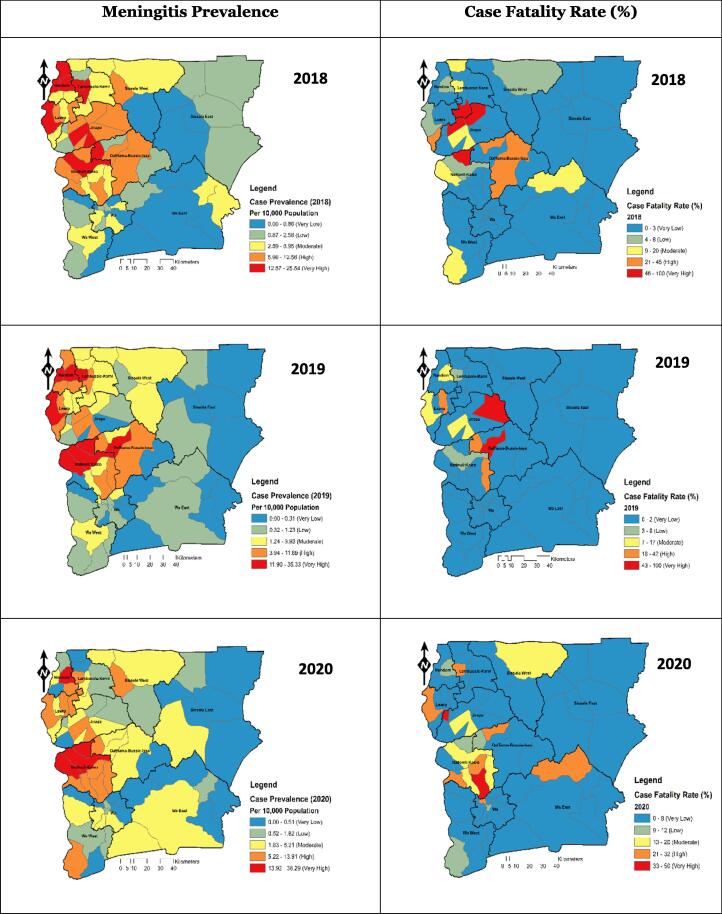
Fig. 4 shows that the incidence of meningitis was high around the north-western and the Middle Western part (Nandom, Jirapa, Lawra, Lambussie, and Nadowli-Kaleo) of the Upper West Region in 2018. However, there was a gradual spatial diffusion of the prevalence towards the middle belt of the study area in 2019. This spatial growth continued in 2020 as the prevalence of meningitis was widespread from the west to many parts of the region.
Fig. 4.
Spatio-Temporal Meningitis Case Prevalence and Case Fatality Rate, Upper West Region, 2018–2020.
Interestingly, CFRs are not equitably spread throughout the studied region. and do not precisely represent the spatial trend of the prevalence. They are strongly scattered in areas where a low prevalence of the disease was observed. It was also noted that CFR of meningitis was generally very low in most parts of the region. Nonetheless, Jirapa municipal's sub-municipalities reported a very high CFR of meningitis in 2018 and 2019. The northern part of Wa East also reported a high CFR of meningitis in 2020, as shown in Fig. 4.
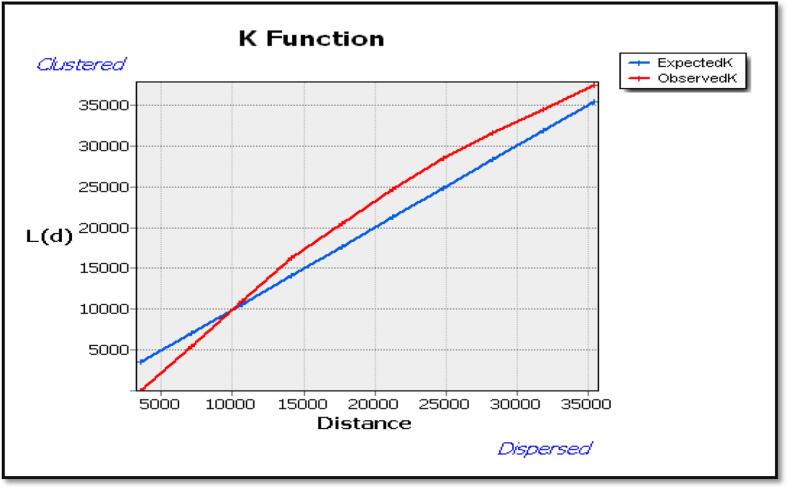
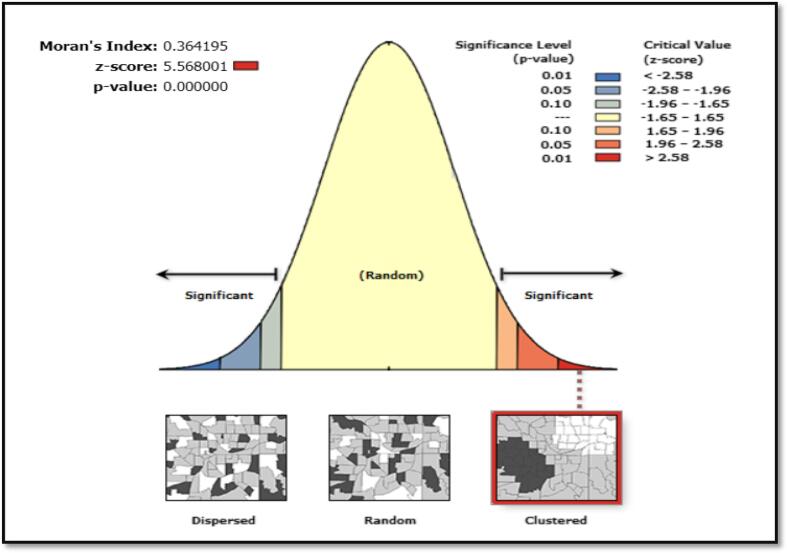
Nevertheless, Ripley’s K Function displayed how clustered or dispersed meningitis cases change at different distances in space. Fig. 5 depicts the average number of neighbors (L (d)) at an evaluation distance of 10,000 m is more than the anticipated average intensity of features. Hence, the distribution is more clustered at a distance of 10,000 m than dispersed. Fig. 6 also shows a high level of clustering in the area (0.364195, Z-Score = 5.568001 and a P < 0.001). Furthermore, using spatial autocorrelation analysis and a z-score of 5.568001, this clustering pattern of meningitis cases is unlikely to be a coincidence.
Fig. 5.
Multi-Distance Spatial Cluster Analysis using Ripley's K Function by ArcGIS, Upper West Region, and 2018–2020.
Fig. 6.
Spatial Autocorrelation Reports Produced by ArcGIS with additional Global Moran’s Index Statistics, Upper West Region, and 2018–2020.
The hotspot analysis predicts where meningitis cases are more concentrated. Sub-districts with statistically significant clusters are displayed in Fig. 7. The stronger the grouping of low values, the lower the Z-score (cold spot). however The more concentrated the grouping of high values, the higher the Z-score (hot spot). Statistical significance was determined based on 95 % and 99 % confidence.
Fig. 7.
Getis-Ord Gi* Cluster Map Showing Hot Spot for Meningitis in Sub-Districts of Upper West Region 2018–2020.
Eight significant clusters (P < 0.01) and five clusters (p < 0.05) were identified as displayed in Fig. 7. representing red and pink clusters in the study area. The white portion in Fig. 7. shows areas that are not statistically significant. The map clearly shows two distinct clusters of hotspots within the study area. The first hotspot zone lies in the UWR's northwestern corner covering Nandom and Lambussie districts. At the same time, the second zone can also be seen with the middle-west affecting Nadowli, DBI, and Jirapa districts. Details of the statistical output of the hotspots are presented in Table 2.
Table 2.
Hot Spot Analysis by Sub-Districts.
| District | Sub-District | Prevalence Per 10,000 Population | Z Score | P-Value | No. of Neighbors | Confidence Level |
|---|---|---|---|---|---|---|
| Lambussie | Lambussie | 45.6 | 3.337 | 0.001* | 5 | 99 % |
| Nadowli | Nadowli | 58.0 | 2.923 | 0.003* | 10 | 99 % |
| DBI | Daffiama | 22.5 | 2.994 | 0.003* | 7 | 99 % |
| Nandom | Nandom | 97.4 | 3.356 | 0.001* | 7 | 99 % |
| Nandom | Gengenkpe | 40.9 | 3.518 | 0.000* | 4 | 99 % |
| Nandom | Baseble | 8.9 | 3.444 | 0.001* | 7 | 99 % |
| Nandom | Ko | 16.6 | 3.070 | 0.002* | 7 | 99 % |
| Nandom | Puffien | 20.6 | 2.841 | 0.004* | 5 | 99 % |
| Lambussie | Billaw | 4.5 | 2.388 | 0.017* | 8 | 95 % |
| Nadowli | Charikpong | 45.9 | 2.510 | 0.012* | 4 | 95 % |
| Nadowli | Dapuori | 48.5 | 2.513 | 0.012* | 6 | 95 % |
| DBI | Fian | 35.8 | 2.083 | 0.037* | 5 | 95 % |
| Jirapa | Yagha | 2.8 | 2.025 | 0.043* | 7 | 95 % |
Significant Cluster.
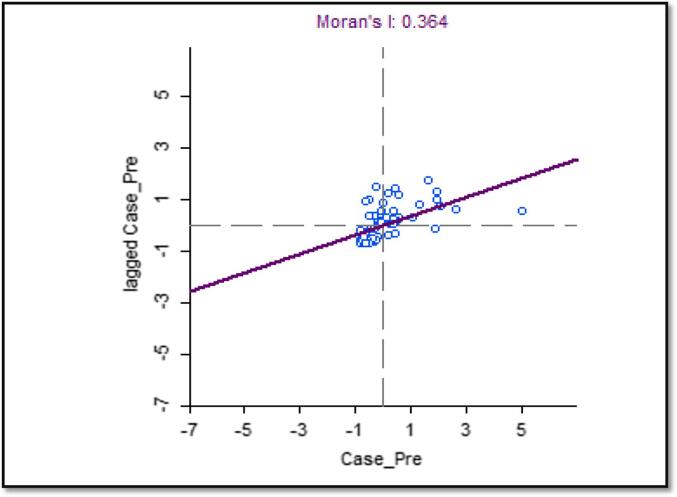
The scatterplot depicts the horizontal axis as the prevalence of meningitis cases, and the vertical axis is the spatial lag of meningitis prevalence. (see Fig. 8). The graph is split into four quadrants, and both variables are standardized: the upper-right quadrant shows sub-districts with high prevalence surrounded by sub-districts with a high prevalence of meningitis (High-High), and the Lower-Left quadrant also shows sub-districts with low meningitis prevalence surrounded by other sub-districts with lower prevalence rate (Low-Low). This is complemented with a positive slope (regression line), and Moran’s I index of 0.364 suggests a positive spatial autocorrelation (Because numerous similar numbers are close together, and Moran's I is close to + 1); Outliers in the bottom right (high-low) and upper left quadrants are also plausible (low–high).
Fig. 8.
Global Moran’s Index Scatter Plot Graph in GeoDa, Upper West Region, and 2018–2020.
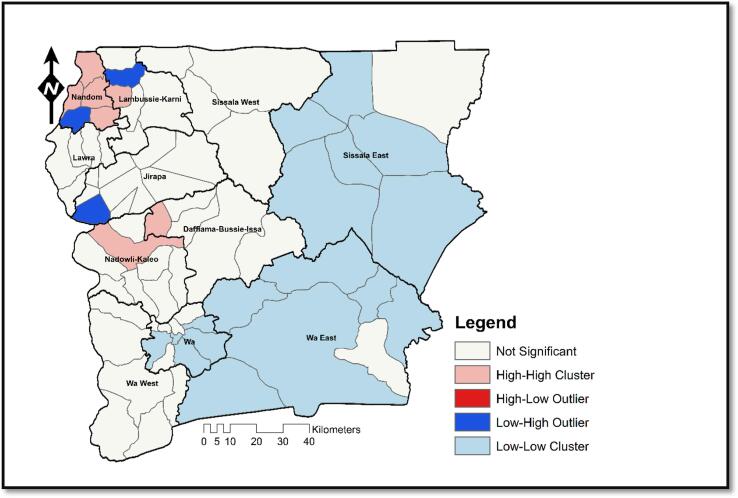
The existence of Low-Low, High-High, and Low-High clusters is seen on the local Moran's I map. There are no High-Low clusters, though. Fig. 9 illustrates the occurrence of High-High clusters in the Nandom, Nadowli, Lambussie, and Daffiama Bussie Issa districts (DBI).
Fig. 9.
Anselin Local Moran’s I Map Showing Spatial Outliers for Meningitis in Sub-Districts of Upper West Region 2018–2020.
The western part of the study area also depicts a cluster of sub-districts with Low values surrounded by sub-district with a Low prevalence of meningitis. Such districts do not deserve emergency response as immediate neighborhood in-diffusion is considerably less likely. Three sub-districts (Billaw, Yagha and Baseble) show noticeable Low-High outliers in the region, indicating low prevalence sub-districts that are surrounded by high prevalence areas (Fig. 9).
Discussion
To the best of our knowledge, this is the first study to explore the geographic and temporal pattern of bacterial meningitis in the Upper West Region. The findings revealed that the disease's incidence remained high (n = 1176) throughout time, with a cumulative attack rate of 141 per 100,000 population, which is greater than the 100/100,000 population seasonal incidence generally recorded in the meningitis belt. (Trotter et al., 2015). However, this is comparable to previous outbreaks reported in Burkina Faso during the 2010 season, with a cumulative incidence of 120 per 100,000 (Delrieu et al., 2011). There were 1176 cases and 118 deaths recorded for the period under study. The average CFR reported in this study (10.8 %) was within the WHO range of 10–30 % (WHO, 2017). More than half of the sub-districts (58 %) with the highest CFR are among the most deprived in the region without hospitals or polyclinics and with few critical categories of health staff. Case management in these districts would likely be inadequate, leading to higher mortalities. Again, there was a substantial spatial variation in CFR among districts ranging from 0.0 % in Sissala East to 17.0 % in Jirapa. The implication is that Jirapa has the highest level of disease burden as compared to Sissala East hence constant monitoring and adequate logistics are needed in the affected sub-districts to mitigate this burden.
The prevalence of meningitis varied significantly among districts, with cumulative incidence ranging from 12 cases in Sissala East to 275 cases in the Nandom district with an AR of 17/100,000 and 492/100,000, respectively. Nevertheless, the disease burden was higher among males than females, representing 605 (51 %) and 571 (49 %) of total cases. These findings agreed with similar studies conducted in the United States and Iraq (Dharmarajan et al., 2015, Neamah and Abdullah, 2020). This sex variation may be attributed to the health-seeking attitude and behavioral differences among males and females (Akyereko et al., 2020, Dharmarajan et al., 2015). Consequently, public education should be intensive among men about the etiological dynamics of the disease. Based on the age factor, children under 1 year were the least affected; however, those aged between 5 and 14 (21.7 %) and 15–29 (22.5 %) years were the most affected age group, which is consistent with what (Domo et al., 2017) found. This can be attributable to immune system strength differences between children and adults. Children and young adults have weak immune systems and are more susceptible to developing meningitis than matured adults (Kwarteng et al., 2017, Opare et al., 2015). This consequently presents a significant socio-economic burden since most youths are likely to contract the illness, increasing ailment and reducing productivity, thus, aggravating poverty in the region. Targeting older children and young adults in the study area with conjugate vaccination may effectively reduce bacterial meningitis spread.
Following the temporal prevalence, it was found that within the three years studied (2018, 2019, and 2020), more cases are usually recorded within epidemiological weeks 2–17, where climatological and environmental conditions such as low humidity, less rainfall, and increased dust are favorable (Dukić et al., 2012). From a macro-temporal perspective, it was discovered that 2018 had the highest prevalence (52/100,000) and reduced in 2019 (41/100,000), and increased in 2020 (51/100,000). The higher prevalence in 2018 can be attributed to the weakened vaccine protective effect over time. There was a mass vaccination campaign around 2014 in the UWR and since the reactive vaccine protective effect of the vaccine weakens within two years (Clark and Borrow, 2020, Ramsay et al., 2003), it is therefore arguable that more significant portion of the people would have entered into the susceptible etiological class since no mass vaccination after 2014 (Akyereko et al., 2020, Clark and Borrow, 2020, Opare et al., 2015). The study suggests that the districts along the Region's north-western border had the highest incidence of the disease compared to the rest of the region (Fig. 4). This observation has been recurring annually during the dry season (GHS, 2020, Nuoh et al., 2016). The significant mobility of people (both native and international) between the major towns of Wa, Lawra, and Nandom and the Hamile border may also have played a pivotal role in the disease's westward spread. It is worth noting that these major towns are all along the Ghana National Highway 12 (N12) road that routes through seven districts (7/11) to other neighboring Sahel countries, including Burkina Faso, Mali, Niger, Chad, and Senegal, all within the meningitis belt. More investigations are needed to understand how the presence of the N12 road affects the dynamics of meningitis within UWR (MoF, 2010).
Spatio-temporal analysis from 2018 to 2020 revealed that the concentration of the meningitis cases had shown locational expansion over the three years. For example, places with very low to low prevalence classes in 2018 began to exhibit moderate levels of prevalence in 2020 (1.24–3.83/100,000). While population growth may account for this spatial growth, there is also the possibility that changing phases of economic activities, increased mobility, and climate change dynamics may be strong predictors of the expanding nature of the epidemics (Amegah et al., 2016, Dovie et al., 2017, Kwarteng et al., 2017). Therefore, it is essential that policies that incorporate the dynamics of socio-environmental conditions be formulated. In exploring statistically significant hotspots, eight hotspots at 99 % confidence level were discovered based on the aggregated temporal prevalence (2018–2020), whereas five at 95 % confidence level were observed. This phenomenon illustrates that these districts though having a high prevalence of meningitis, are also surrounded by districts with similar prevalence.
Consequently, they may expect to exhibit this trend of high concentration of cases over time. Hence, these hotspot zones are more likely to record local meningitis outbreaks if specific interventions are not employed to prevent future epidemics. About eight cold spots were found at a 90 % confidence level (Fig. 7). However, a greater portion of the region did not have significant hotspots. This trajectory is expected since the cases were not evenly distributed in space. This finding should be a pointer for the health system to focus interventions and resources on the identified hot spots while maintaining surveillance across all sub-districts.
Interestingly, Wa, the regional capital with the highest population, recorded no significant hotspot of meningitis. Improved housing structures can explain this phenomenon with good ventilation, access to health promotion messages, higher literacy, and smaller household size in urban settlements than in areas such as Nandom, Jirapa, Nadowli, etc., where a majority of the population is rural. Secondly, most of the sub-districts that recorded local-level clusters (p < 0.01 and 0.05) were found in the northern part of the region and thus proximate to drier environmental conditions (Akyereko et al., 2020). These areas are more likely to experience drier conditions and pervasive northeast trade winds originating from the Sahara desert (Opare et al., 2015). For example, Abdussalam et al., 2014, Dukić et al., 2012 discovered that climatic conditions and differentiation among different sub-districts were responsible for differing levels of meningitis prevalence. Other studies conducted in Nigeria (Abdussalam et al., 2014) and Congo (Mazamay et al., 2020) affirm the impact of different climatic conditions on the prevalence of meningitis. While an attempt to establish natural greenery may be difficult, the study findings suggest an equitable distribution of resources, public education, mass vaccination, and improved healthcare facilities as the most feasible approach in thinning out the effect of the epidemics in the region as suggested by other researchers (Akyereko et al., 2020, Nuoh et al., 2016, Opare et al., 2015).
The sub-districts with low prevalence are not entirely safe as time progresses, especially if higher prevalence sub-districts surround them. This trend is of significant worry since they are likely to be impacted by the surrounding district. Regular monitoring is therefore needed to prevent mobility-based transmission. For full details on the cluster-outlier outcome, refer to Table 3 (Akyereko et al., 2020). Spatial outliers were examined using local-based clustering analysis (Anselin Local Moran’s I). Results (Fig. 9) show that seven sub-districts in Lambussie, Nandom, and Kaleo had a high prevalence and were equally surrounded by high prevalence sub-districts. This implies that these areas will continue to experience higher levels of outbreaks if interventions are not implemented. More vigorous attention must therefore be accorded to these areas. It was also discovered that three sub-districts had less prevalence and were bordered by high prevalence sub-districts. Thus, emergency response or urgent logistics allocation is needed to safeguard these low prevalent sub-districts. The study did not observe any high-low spatial outliers. In most parts of the region, low-low clusters were observed predominantly in Wa and Wa East districts. Unfortunately, these are areas where resources and improved healthcare facilities are concentrated because of the proximity to the regional capital. From the mid-north to the southwest, no significant outlier was observed regarding meningitis prevalence. Any policy hoping to achieve maximum output should target districts with spatial hotspots and outliers.
Table 3.
Local Moran’s I Index Analysis by Sub-Districts.
| District | Sub-District | Prevalence Per 10,000 Population | Local Moran I Index | Z Score | P-Value | No. of Neighbours | Risk Level |
|---|---|---|---|---|---|---|---|
| DBI | Daffiama | 22.5 | 0.636 | 3.000 | 0.010* | 6 | High-High |
| Jirapa | Yagha | 2.8 | −0.595 | −2.593 | 0.019** | 6 | Low-High |
| Lambussie | Lambussie | 45.6 | 2.535 | 2.609 | 0.029* | 4 | High-High |
| Lambussie | Billaw | 4.5 | −0.514 | −2.524 | 0.022** | 7 | Low-High |
| Nadowli | Nadowli | 58.0 | 1.748 | 2.407 | 0.023* | 9 | High-High |
| Nandom | Nandom | 97.4 | 2.854 | 2.093 | 0.027* | 6 | High-High |
| Nandom | Gengenkpe | 40.9 | 2.845 | 2.902 | 0.019* | 3 | High-High |
| Nandom | Baseble | 8.9 | −0.386 | −3.832 | 0.002** | 6 | Low-High |
| Nandom | Ko | 16.6 | 0.244 | 3.261 | 0.005* | 6 | High-High |
| Nandom | Puffien | 20.6 | 0.616 | 2.969 | 0.014* | 4 | High-High |
| Sissala East | Nabulo | 1.1 | 0.468 | 1.678 | 0.006* | 6 | Low-Low |
| Sissala East | Wallembelle | 4.0 | 0.324 | 1.617 | 0.009* | 7 | Low-Low |
| Sissala East | Kulfuo | 0.0 | 0.539 | 1.600 | 0.003* | 5 | Low-Low |
| Sissala East | Nabugubelle | 2.0 | 0.478 | 1.462 | 0.005* | 4 | Low-Low |
| Sissala East | Sakai | 0.0 | 0.445 | 1.353 | 0.037* | 5 | Low-Low |
| Sissala East | Tumu | 2.5 | 0.400 | 1.530 | 0.014* | 5 | Low-Low |
| Wa East | Loggu | 3.0 | 0.362 | 1.261 | 0.040* | 4 | Low-Low |
| Wa East | Baayiri | 1.1 | 0.396 | 1.460 | 0.029* | 7 | Low-Low |
| Wa East | Funsi | 1.2 | 0.458 | 1.633 | 0.010* | 6 | Low-Low |
| Wa East | Yaala | 5.0 | 0.342 | 1.599 | 0.001* | 5 | Low-Low |
| Wa East | Bulenga | 3.5 | 0.405 | 1.772 | 0.001* | 6 | Low-Low |
| Wa East | Kundungu | 2.8 | 0.421 | 1.202 | 0.039* | 3 | Low-Low |
| Wa Municipal | Kambali | 6.5 | 0.218 | 1.326 | 0.040* | 6 | Low-Low |
| Wa Municipal | Bamahu | 8.1 | 0.198 | 1.489 | 0.011* | 5 | Low-Low |
| Wa Municipal | Charingu | 3.7 | 0.402 | 1.475 | 0.008* | 4 | Low-Low |
| Wa Municipal | Busa | 0.0 | 0.458 | 1.689 | 0.014* | 7 | Low-Low |
| Wa Municipal | Wa South | 2.6 | 0.342 | 1.449 | 0.030* | 6 | Low-Low |
Significant Cluster.
Significant Outlier.
Limitation of the study
The study acknowledged some limitations. One significant limitation of the current study is the scale of data used (spatial resolution). Since we considered Sub-district-level centroids, there is a higher propensity to mask local-level, meaning no matter how hard we tried to optimize the modeling process. Also, ecological fallacy could not be prevented entirely. However, considering the scale –Sub-district, the extent of such ecological fallacy is minimal. Again, the study primarily relied on self-reported meningitis cases at the various health facilities in the Upper West Region. These cases may not accurately represent the disease burden at the community level. The population distribution was based on a projection from the 2010 population and housing census, which may not also represent the actual population of the various units of analysis. Finally, the data collected was restricted to completed line lists from 2018 to 2020. Even better, the three-year trend of 1176 cases of meningitis was fit to conduct the analyses and generate the disease risk maps.
Conclusion
GIS has always been a forerunner in evaluating and monitoring public health interventions to control infectious diseases. As a result, identifying the geographical distribution, clusters, and socio-bioclimatic risk factors responsible for spatial patterns of meningitis prevalence based on GIS is vital in eradicating meningitis. Disease surveillance data and regional geodata were combined with GIS to detect meningitis clusters and forecast hotspots responsible for local meningitis outbreaks in the Upper West Region. Meningitis incidence and mortality rates in the region have significantly increased (2018–2020), though there are sub-district variations. The epidemic does not occur at random, according to the evidence. Populations (10.9 %) within hotspot sub-districts are excessively at higher risk of the disease. Resources and targeted interventions should be directed toward clustered hotspots, emphasizing low prevalence zones fenced by high prevalence zones.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Abdussalam A.F., Monaghan A.J., Steinhoff D.F., Dukic V.M., Hayden M.H., Hopson T.M., Thornes J.E., Leckebusch G.C. The impact of climate change on meningitis in northwest Nigeria: An assessment using CMIP5 climate model simulations. Weather Clim. Soc. 2014;6(3):371–379. doi: 10.1175/WCAS-D-13-00068.1. [DOI] [Google Scholar]
- Aku F.Y., Lessa F.C., Asiedu-Bekoe F., Balagumyetime P., Ofosu W., Farrar J., Ouattara M., Vuong J.T., Issah K., Opare J., Ohene S.-A., Okot C., Kenu E., Ameme D.K., Opare D., Abdul-Karim A. Meningitis outbreak caused by vaccine-preventable bacterial pathogens—northern Ghana, 2016. MMWR Morb. Mortal. Wkly Rep. 2017;66(30):806–810. doi: 10.15585/mmwr.mm6630a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akyereko E., Ameme D., Nyarko K., Asiedu-Bekoe F., Sackey S., Issah K., Wuni B., Kenu E. Geospatial clustering of meningitis: an early warning system (hotspot) for potential meningitis outbreak in upper east region of Ghana. Original Article Www.Ghanamedj.Org. 2020;54(2):32–39. doi: 10.4314/gmj.v54i2s.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AM, M., & MC Thomson, 2002. Where is the meningitis belt? Defining an area at risk of epidemic meningitis in Africa. Academic.Oup.Com. https://academic.oup.com/trstmh/article-abstract/96/3/242/1921676. [DOI] [PubMed]
- Amegah A.K., Rezza G., Jaakkola J.J.K. vol. 91. Elsevier Ltd.; 2016. Temperature-related morbidity and mortality in Sub-Saharan Africa: A systematic review of the empirical evidence; pp. 133–149. (Environment International). [DOI] [PubMed] [Google Scholar]
- Clark S.A., Borrow R. Herd Protection against Meningococcal Disease through Vaccination. Microorganisms. 2020;8(11):1–16. doi: 10.3390/MICROORGANISMS8111675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delrieu, I., Yaro, S., One, T. T.-P., & 2011, U., n.d. Emergence of Epidemic Neisseria meningitidis Serogroup X Meningitis in Togo and Burkina Faso. J. Plos. Org., 2011. Retrieved September 9, 2021, from https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0019513. [DOI] [PMC free article] [PubMed]
- Dharmarajan L., Salazar L., Hasbun R. Gender Differences in Community-acquired Meningitis in Adults: Clinical Presentations and Prognostic Factors. J. Meningitis. 2015;01(01) doi: 10.4172/2572-2050.1000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domo N.R., Nuolabong C., Nyarko K.M., Kenu E., Balagumyetime P., Konnyebal G., Noora C.L., Ameme K.D., Wurapa F., Afari E. Uncommon mixed outbreak of pneumococcal and meningococcal meningitis in Jirapa District, Upper West Region, Ghana, 2016. Ghana Med. J. 2017;51(4):149–155. doi: 10.4314/gmj.v51i4.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dovie D.B.K., Dzodzomenyo M., Ogunseitan O.A. Sensitivity of health sector indicators’ response to climate change in Ghana. Sci. Total Environ. 2017;574:837–846. doi: 10.1016/j.scitotenv.2016.09.066. [DOI] [PubMed] [Google Scholar]
- Dukić V., Hayden M., Forgor A.A., Hopson T., Akweongo P., Hodgson A., Monaghan A., Wiedinmyer C., Yoksas T., Thomson M.C., Trzaska S., Pandya R. The Role of Weather in Meningitis Outbreaks in Navrongo, Ghana: A Generalized Additive Modeling Approach. J. Agric. Biol. Environ. Stat. 2012;17(3):442–460. doi: 10.1007/s13253-012-0095-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dummer T.J.B. Health geography: supporting public health policy and planning. Can. Med. Assoc. J. 2008;178(9):1177–1180. doi: 10.1503/cmaj.071783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esri, 2020. Spatial Autocorrelation (Global Moran’s I) (Spatial Statistics)—ArcGIS Pro | Documentation. https://pro.arcgis.com/en/pro-app/2.7/tool-reference/spatial-statistics/spatial-autocorrelation.htm.
- GHS, 2012. Annual Health Performance Report, Upper West Region.
- GHS, 2017. Annual Performance Review Report, Upper West Region.
- GHS, 2020. Annual Health Performance Report, Upper West Region.
- GSS, 2013. 2010 Population & Housing Census National Analytical Report.
- Kwarteng A., Amuasi J., Annan A., Ahuno S., Opare D., Nagel M., Vinnemeier C., May J., Owusu-Dabo E. Current meningitis outbreak in Ghana: Historical perspectives and the importance of diagnostics. Acta Trop. 2017;169:51–56. doi: 10.1016/j.actatropica.2017.01.014. [DOI] [PubMed] [Google Scholar]
- Mazamay S., Broutin H., Bompangue D., Muyembe J.J., Guégan J.F. The environmental drivers of bacterial meningitis epidemics in the democratic republic of congo, central africa. PLoS Negl.Trop. Dis. 2020;14(10):1–16. doi: 10.1371/journal.pntd.0008634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazamay S., Guégan J.F., Diallo N., Bompangue D., Bokabo E., Muyembe J.J., Taty N., Vita T.P., Broutin H. An overview of bacterial meningitis epidemics in Africa from 1928 to 2018 with a focus on epidemics “outside-the-belt”. BMC Infect. Dis. 2021;21(1):1–13. doi: 10.1186/S12879-021-06724-1/FIGURES/5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobasheri M., Ahmadi A. Incidence Patterns and Spatial Analysis of the Most Common Cancers in Southeastern Iran Using Geographic Information System (GIS) Acad. J. Cancer Res. 2014;7(2):141–145. doi: 10.5829/idosi.ajcr.2014.7.2.83269. [DOI] [Google Scholar]
- MoF, G., 2010. Integrated Transport Plan for Ghana Volume 6 : Transport Model Calibration. In: Egis Bceom International, vol. 6, Issue June.
- Neamah S.R., Abdullah Y.J. Identification, Epidemiology and Seasonal Variation of Neisseria meningitidis in Al-Nasiriya City, South of Iraq. Curr. Appl. Sci. Technol. 2020;22(3):527–534. 4(4) [Google Scholar]
- Nuoh R.D., Nyarko K.M., Nortey P., Sackey S.O., Lwanga N.C., Ameme D.K., Nuolabong C., Abdulai M., Wurapa F., Afari E. Review of meningitis surveillance data, upper West Region, Ghana 2009–2013. Pan Afric. Med. J. 2016;25(Supp 1):9. doi: 10.11604/pamj.supp.2016.25.1.6180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opare, J.K.L., Awoonor-Williams, J.K., Odoom, J.K., 2015. Bacterial Meningitis: A Review in the Upper East Region of Ghana 2010-2014 Bacterial Meningitis : A Review in the Upper East Region of Ghana 2010–2014. January. 10.9734/IJTDH/2015/19398. [DOI]
- Pace D., Gauci C., Barbara C. The epidemiology of invasive meningococcal disease and the utility of vaccination in Malta. Eur. J. Clin. Microbiol. Infect. Dis. 2020;39(10):1885–1897. doi: 10.1007/s10096-020-03914-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paireau J., Girond F., Collard J.M., Maïnassara H.B., Jusot J.F. Analyzing spatio-temporal clustering of meningococcal meningitis outbreaks in Niger Reveals opportunities for improved disease control. PLoS Negl.Trop. Dis. 2012;6(3) doi: 10.1371/journal.pntd.0001577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putz, K., Hayani, K., Practice, F. Z.-C. C. in O.U., 2013. Meningitis. Primarycare.Theclinics.Com. https://www.primarycare.theclinics.com/article/S0095-4543(13)00063-8/abstract.
- Ramsay M.E., Andrews N.J., Trotter C.L., Kaczmarski E.B., Miller E. Herd immunity from meningococcal serogroup C conjugate vaccination in England: database analysis. BMJ: British Med. J. 2003;326(7385):365. doi: 10.1136/BMJ.326.7385.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotter, C., Cibrelus, L., Fernandez, K., Vaccine, C.L.-U., 2015. Response thresholds for epidemic meningitis in sub-Saharan Africa following the introduction of MenAfriVac®. Elsevier. https://www.sciencedirect.com/science/article/pii/S0264410X15014097. [DOI] [PubMed]
- Tsang R.S.W. A Narrative Review of the Molecular Epidemiology and Laboratory Surveillance of Vaccine Preventable Bacterial Meningitis Agents: Streptococcus pneumoniae, Neisseria meningitidis, Haemophilus influenzae and Streptococcus agalactiae. Microorganisms. 2021;9(2):449. doi: 10.3390/MICROORGANISMS9020449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weimann A., Kabane N., Jooste T., Hawkridge A., Smit W., Oni T. Health through human settlements: Investigating policymakers’ perceptions of human settlement action for population health improvement in urban South Africa. Habitat Int. 2020;103:102203. doi: 10.1016/J.HABITATINT.2020.102203. [DOI] [Google Scholar]
- WHO Meningococcal vaccines: WHO position paper, November 2011. Releve Epidemiologique Hebdomadaire. 2011;86(47):521–539. [PubMed] [Google Scholar]
- WHO, 2020. Meningococcal Meningitis Facts. WHO. https://www.who.int/news-room/fact-sheets/detail/meningococcal-meningitis.
- WHO, 2017. Standard Operating Procedures for case-based surveillance of meningitis, preparedness and response to meningitis epidemics in Table of Contents (Issue October).
- WHO, 2021. Meningitis Fact Sheet. https://www.who.int/news-room/fact-sheets/detail/meningitis.