Abstract
Peptidase B (PepB) of Salmonella enterica serovar Typhimurium is one of three broad-specificity aminopeptidases found in this organism. We have sequenced the pepB gene and found that it encodes a 427-amino-acid (46.36-kDa) protein, which can be unambiguously assigned to the leucyl aminopeptidase (LAP) structural family. PepB has been overexpressed and purified. The active enzyme shows many similarities to other members of the LAP family: it is a heat-stable (70°C; 20 min) hexameric (∼270-kDa) metallopeptidase with a pH optimum of 8.5 to 9.5. A detailed study of the substrate specificity of the purified protein shows that it differs from other members of the family in its ability to hydrolyze peptides with N-terminal acidic residues. The preferred substrates for PepB are peptides with N-terminal Asp or Glu residues. Comparison of the amino acid sequence of PepB with those of other LAPs leads to the conclusion that PepB is the prototype of a new LAP subfamily with representatives in several other eubacterial species and to the prediction that the members of this family share the ability to hydrolyze peptides with N-terminal acidic residues. Site-directed mutagenesis has been used to show that this specificity appears to be determined by a single Lys residue present in a sequence motif conserved in all members of the subfamily.
Salmonella enterica serovar Typhimurium and Escherichia coli require peptide hydrolases in order to utilize peptides supplied in the growth medium and to hydrolyze peptides generated inside the cell by proteolytic degradation and modification (15). Three “broad-specificity” aminopeptidases, peptidases N, A, and B (PepN, PepA, and PepB), have been shown to participate in these processes. PepN, PepA, and PepB can each remove the N-terminal amino acids from a broad range of peptides and, in conjunction with the dipeptidase PepD, they have been shown to be required for normal peptide degradation in vivo (28, 29). Although PepN, PepA, and PepB show broadly overlapping specificities, they are not equally effective at hydrolyzing all peptide bonds. None of them, for example, can hydrolyze a bond in which proline occupies the second position, and such peptides are specifically hydrolyzed by other peptidases (13, 16). Studies of the in vivo functions of these enzymes have suggested that, in spite of the apparent overlap in their specificities, PepA and PepN are more effective than PepB in restoring the capacity to carry out intracellular protein degradation in a pepN pepA pepB pepD mutant (28). In addition, mutant strains carrying only one of these peptidases differ slightly in the ability to use exogenously supplied peptides as amino acid sources.
PepA and PepN have been characterized in some detail both structurally and enzymologically. PepA is a member of the well-studied leucyl aminopeptidase family of metallopeptidases, while PepN belongs to the lysyl aminopeptidase family (3). Representatives of these families are widely distributed in both bacterial and eukaryotic species, and representatives of both families have recently been identified in the Archaea (9, 19, 24). At the beginning of these studies, little was known about the third broad-specificity aminopeptidase, PepB. PepB had not been placed in a structural family, nor had its specificity been thoroughly characterized. As part of a study aimed at determining the unique physiological roles of each of the three broad-specificity aminopeptidases, we have cloned and sequenced serovar Typhimurium pepB, overexpressed and purified its product, and carried out a detailed study of its substrate specificity.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
All Salmontella typhimuium strains listed in Table 1 are derivatives of strain LT2. E. coli strains are derivatives of CM89. Lennox L broth (LB) and Lennox L agar (GIBCO BRL) were used as rich media. E medium (26) supplemented with 0.4% glucose and with peptides (0.3 mM) and amino acids (0.3 mM) as required was used as minimal medium. Media for growth of plasmid-containing strains contained ampicillin (50 μg/ml) or chloramphenicol (25 μg/ml). Cultures were grown with aeration at 37°C.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Genotype or description |
|---|---|
| Serovar Typhimurium | |
| TN1246 | leuBCD485 pepB11 pepN90 pepA16 supQ302 Δ(proAB pepD) pepP1 pepQ1 |
| TN2617 | TN1246/pJG97 |
| TN2654 | leuBCD485 pepB11 pepN90 pepA16 supQ302 Δ(proAB pepD) pepP1 pepQ1 pepE1 pepT1 recA sor::Tn10 |
| TN4804 | TN2654/pCM265 |
| E. coli | |
| CM89 | leu-9 pepB1 pepN102 pepA11 pepQ10 Δpro-lac met |
| TN5179 | CM89/pCM370 |
| Plasmids | |
| pBluescript SK II(+) | Plasmid cloning vector (Stratagene) |
| pBR328 | Plasmid cloning vector (a derivative of pBR322; New England Biolabs) |
| pSE380 | Plasmid cloning vector containing Ptrc promoter and ribosome binding site |
| pJG97 | 10.7-kb fragment carrying the serovar Typhimurium pepB gene cloned into the BamHI site of pBR328 |
| pCM236 | 11.1-kb subclone of pJG97 obtained after SalI digestion of pJG97 |
| pCM265 | 2.1-kb SalI/PstI fragment of pJG236 carrying the pepB gene cloned into SalI-PstI-digested pBluescript SK II(+) |
| pCM370 | 1.329-kb fragment containing the ribosome binding site and the ORF of the serovar Typhimurium pepB gene amplified by PCR and cloned into EcoRI-KpnI-digested pSE380 vector |
DNA manipulations.
Restriction enzymes were obtained from GIBCO BRL or New England Biolabs and used according to the manufacturer's instructions. Fragments for subcloning were isolated from agarose gels and ligated with T4 DNA ligase (GIBCO BRL) according to the instructions of the manufacturer.
Cloning of S. typhimurium pepB.
S. typhimurium chromosomal DNA was partially digested with Sau3AI. The resulting fragments were ligated to BamHI-digested pBR328, and the resulting library was transformed into S. typhimurium LT2 strain TN1246 (pepN90 pepA16 pepB11 ΔsupQ302(proAB pepD) pepP1 pepQ1) and plated on LB-ampicillin. (TN1246 is unable to use Leu-Leu as a Leu source, but plasmids carrying either pepN, pepA, or pepB should restore the ability to use this peptide.) These transformants were screened for growth on minimal glucose medium containing Leu-Leu as the only Leu source (A. Kukral, unpublished data). Crude extracts were prepared from Leu-Leu utilizing transformants and were subjected to nondenaturing polyacrylamide gel electrophoresis, and the gel was stained for Leu-Leu hydrolysis activity (17). Strains carrying pepB plasmids were identified by the characteristic Rf (0.27) of this activity. One such strain, TN2617(pJG97), was retained.
Restriction mapping of pJG97 indicated that it contains a 10.7-kb chromosomal DNA insert. A SalI digest of pJG97 was electrophoresed on a 0.8% low-melting-point agarose (FMC Bioproducts) gel, and an 11.15-kb DNA fragment was isolated and religated in the gel. The religated plasmid was transformed into S. typhmurium TN2654, and ampicillin-resistant transformants were selected. The resulting strain contained a plasmid (pCM236) with a 6.5-kb insert and retained the ability to grow on Leu-Leu. A 2.1-kb fragment of pCM236 generated by digestion with SalI and PstI was ligated to SalI/PstI-digested pBluescript SKII(+) (Stratagene) to form pCM265. This plasmid was transformed into TN2654, and the resulting strain (TN4804) was able to utilize Leu-Leu, indicating that pCM265 carries a functional pepB gene.
Sequencing of the pepB gene.
To locate the pepB gene in pCM236, Tn1000 insertions (8) into the plasmid were isolated and the resulting strains were screened for growth on Leu-Leu as a Leu source. The strains unable to grow on this peptide were restriction mapped to determine the positions and orientations of the Tn1000 insertions. Primers carrying sequences from one or the other end of Tn1000 (12) were used to obtain the DNA sequence of the pepB insert in pCM236 using the Sequenase version 2.0 protocol (United States Biochemicals). Both strands were completely sequenced.
Construction of PepB expression plasmid.
The S. typhimurium pepB gene was amplified by PCR and cloned downstream of the Ptrc promoter of pSE380 to obtain high expression of the pepB gene for protein purification. The amplified product (1,338 bp) containing the entire pepB coding region and the proposed Shine-Dalgarno sequence was digested with EcoRI and KpnI and ligated to similarly digested pSE380, and the resulting ligation mixture was electroporated into TN2654 and plated on a medium containing LB and ampicillin (50 μg/ml). Transformants selected for ampicillin resistance were purified and shown to retain the ability to grow on Leu-Leu, indicating the presence of a functional pepB gene. One of these was electroporated into CM89 and saved as TN5179(pCM370).
Purification of PepB.
Strain TN5179 was grown in 4 liters of LB supplemented with 0.3 mM thymine, 0.05 mM thiamin, and 50 μg of ampicillin/ml to an optical density at 600 nm of 1.0; isopropyl β-d-thiogalactopyranoside was added to a final concentration of 1 mM; and the cells were grown for 16 h. The cells were washed and resuspended in 60 ml of 50 mM Tris-Cl, pH 8.5. The cells were lysed by sonication, and the resulting lysate was centrifuged in the cold (4°C) for 30 min at 29,000 × g. Crude cell extract (9.1 ml; 21.4 mg/ml of protein) derived from this step was applied to a HiLoad 26/10 Q Sepharose high-performance column (Pharmacia) in 20 mM Tris-Cl, pH 7.5. A 400-ml linear salt gradient from 300 to 500 mM KCl in 50 mM Tris-Cl (pH 7.5) was applied at a rate of 4 ml/min. Fractions with PepB activity were identified by using a plate assay similar to that described previously (5) but with Leu-Leu as a substrate. Active fractions were pooled and concentrated in 50 mM Tris-Cl (pH 8.5)–200 mM KCl–1 mM MgCl2. Twelve milliliters of this concentrated protein solution (7 mg/ml) was heated to 70°C for 15 min, and the precipitate was removed by centrifugation. Three milliliters of this solution (4 mg of protein/ml) was applied to a HiPrep Sephacryl S-300 16/60 column (Pharmacia). The column was eluted at 0.3 ml/min with 160 ml of 50 mM sodium phosphate (pH 7.5) containing 0.15 M NaCl. PepB activity eluted at a position corresponding to a molecular mass of approximately 270 kDa. The final product had a specific activity of 190 μmol min−1 mg−1. Two contaminating proteins copurified with PepB (see below). N-terminal sequence analysis showed that these proteins were fragments of PepB apparently generated by proteolytic cleavage.
N-terminal amino acid sequence determination and molecular mass determination by mass spectrometry.
Mass spectrometric analysis was carried out with a Micromass Quattro I (a quadrupole-hexapole-quadrupole mass spectrometer equipped with an electrospray source) in the Mass Spectrometry Laboratory, University of Illinois, Urbana-Champaign. N-terminal amino acid sequencing was carried with an Applied Biosystems 477A sequencer in the Protein Sciences Laboratory at the University of Illinois.
Assay of PepB.
The rate of hydrolysis of peptides by PepB was determined by reactions performed at 37°C in a solution of 50 mM Tris-Cl (pH 8.5), 50 mM KCl, 0.1 mM MgCl2, 5 mg of bovine serum albumin fraction V (Sigma)/ml, and 1 mM peptide in a final volume of 600 μl. Eighty-microliter aliquots of the reaction mixture were transferred at various times to a microcentrifuge tube containing 8 μl of 50% trichloroacetic acid (TCA). Proteins precipitated after treatment with TCA were removed by centrifugation in an Eppendorf 5415C microcentrifuge at 14,000 rpm for 15 min. Twenty microliters of the TCA supernatant was transferred to an amber microcentrifuge tube containing 80 μl of a 0.25 mM solution of an internal standard (usually isoleucine or asparagine) dissolved in 5% borax (1 g of sodium borate in 20 ml of 0.1 N KOH) and 200 μl of 2,4,6-trinitrobenzenesulfonic acid dihydrate (Pierce) solution (500 mg/ml). The derivatization reaction was stopped after 5 min with 5 μl of 6 N HCl. The products (trinitrophenyl derivatives of peptides or amino acids) were diluted with 891 μl of a mixture of 95% buffer A (0.1% trifluoroacetic acid in H2O) and 5% buffer B (0.1% trifluoroacetic acid in acetonitrile) and analyzed by high-pressure liquid chromatography using an Ultrasphere C-18 analytical reversed-phase column (Beckman) and the System Gold high-pressure liquid chromatography apparatus (Beckman). Diluted samples (100 μl) were injected into the column and eluted with a gradient of 95% buffer A plus 5% buffer B and 95% buffer B plus 5% buffer A in an interval of 45 min at a flow rate of 1 ml/min. The elution positions of the peptides and amino acids present in the reaction mixture were monitored by the absorbance of the trinitrophenyl group at 345 nm. The concentration of an amino acid in the reaction mixture was determined by using a Leu standard curve and the amount of internal standard present in the sample. Plots of the product produced or substrate hydrolyzed versus time were used to calculate the rate of hydrolysis. In all cases, the amount of peptide hydrolyzed at the last time point was <15% of the total amount of peptide present in the reaction mixture. One unit of PepB is defined as the amount of enzyme required to hydrolyze 1 nmol of Leu-Leu in 28 min at 37°C. The data reported in Tables 3 to 7 are derived from at least duplicate assays and in most cases from triplicate assays. Replicate observations differed from the mean by no more than 10% and in most cases by no more than 5%.
TABLE 3.
Hydrolysis of X-Leu peptides by PepB
| X-Leu | In vivoa | Sp act (μmol min−1 mg−1) | Relative activityb |
|---|---|---|---|
| DL | + | 550 | 100 |
| EL | + | 408 | 74 |
| LL | + | 223 | 40 |
| ML | + | 164 | 30 |
| HL | + | 156 | 28 |
| CL | + | 128 | 23 |
| NL | + | 126 | 23 |
| QL | − | 49 | 9 |
| FL | − | 39 | 7 |
| YL | − | 32 | 6 |
| WL | − | 25 | 4 |
| TL | + | 18 | 3 |
| AL | + | 18 | 3 |
| SL | + | 13 | 2 |
| IL | − | 11 | 2 |
| VL | − | 11 | 2 |
| KL | − | 1 | 0.2 |
| GL | − | 1 | 0.2 |
| RL | − | <0.5 | <0.09 |
| PL | − | <0.5 | <0.09 |
+, growth; −, no growth.
Relative activity with respect to Asp-Leu activity (550 μmol min−1 mg−1).
TABLE 7.
Kinetics of PepB-catalyzed hydrolysis
| Peptide | Km (mM) | kcat (s−1) | kcat/Km (s−1 mM−1) |
|---|---|---|---|
| DF | 0.3 | 500 | 1,670 |
| DH | 0.6 | 418 | 696 |
| DL | 0.4 | 666 | 1,670 |
| DQ | 1.8 | 761 | 422 |
| DY | 0.3 | 500 | 1,670 |
| DLG | 0.5 | 950 | 1,900 |
| DLK | 6 | 13 | 2 |
| DIGG | 3 | 26 | 9 |
| EL | 0.9 | 866 | 962 |
| LL | 4 | 918 | 229 |
| LY | 1 | 351 | 351 |
Properties of PepB.
The pH dependence of PepB activity was determined by using Leu-Leu (1 mM) as a substrate and 0.05 M bis-Tris HCl (pH 6 to 6.5), 0.05 M Tris HCl (pH 7 to 9.5), and 0.05 M 2-(cyclohexylamino)ethanesulfonic acid (pH 8.5 to 10). All buffers contained 1 mM MgCl2. The effect of salt (KCl) on PepB activity was determined in 0.05 M Tris-HCl (pH 8.5) with Leu-Leu (1 mM) as a substrate in the presence of MgCl2 (1 mM). To test the effect of divalent cations on PepB activity, purified PepB (0.5 μg/ml) was incubated in 500 μl of 50 mM Tris-Cl (pH 8.5) and 50 mM KCl at 37°C with or without EDTA (10 mM) for 4 h. The EDTA-treated PepB was then incubated separately with 1 mM CoCl2, MgCl2, MnCl2, or ZnCl2 at 37°C for 4 h, and activity was determined in the standard assay. To test the heat stability of PepB, a solution containing 0.2 μg of a crude extract of TN5179(pCM370)/μl in 50 mM Tris (pH 8.5), 200 mM KCl, and 10 mM MgCl2 was heated to 70°C. At various times, 60-μl samples were withdrawn and chilled on ice for 10 min before the activity was determined by the standard assay. The stability of PepB was also investigated after the first step of purification to determine if there was any difference between the activities of the crude extract and partially purified PepB. The partially purified PepB (after the Q Sepharose step) was resuspended in a solution of 50 mM Tris (pH 8.5), 200 mM KCl, and 10 mM MgCl2 at a concentration of 0.1 μg/μl. The suspension was heated at 70°C, and 60-μl samples were withdrawn and chilled on ice for 10 min before the activity was determined.
Substrate specificity studies.
Peptides were obtained from commercial sources (Bachem or Sigma) or, if not available commercially, were synthesized in the Protein Sciences Laboratory at the University of Illinois. All reactions were carried out in 50 mM Tris-Cl (pH 8.5), 50 mM KCl, 1 mM MgCl2, and 5 mg of bovine serum albumin/ml at 37°C. The substrate was added at a final concentration of 1 mM. At time points of 0, 7, 14, and 28 min, samples were withdrawn to analyze the products of the reaction using the standard assay described above. The range of substrate concentrations used for the kinetics studies was 0.05 to 10 mM. Kinetic constants were calculated with the program Hyper (version 1; 1992) to carry out a hyperbolic regression analysis.
Peptide utilization.
To test the abilities of various strains to use Leu peptides as Leu sources, cells grown overnight in a minimal glucose medium containing 0.1% Casamino Acids were washed and resuspended in E minimal glucose medium. One hundred microliters of the resuspended culture was plated in soft agar on an appropriately supplemented E minimal glucose plate. Sterile filter paper disks containing 1 μmol of the peptide were placed on the plate, and the presence or absence of growth around the disks was scored after overnight incubation.
Sequence analysis and phylogenetic inference.
Sequence data was obtained by using the BLAST program (2) to search the nonredundant database and the unfinished microbial genomes at the National Center for Biotechnology Information (NUMG) for proteins similar to PepB. Some partial genome sequences were obtained from the Sanger Sequencing Centre (SSC) and WIT (Interactive Metabolic Reconstruction on the Web; designed by the computational biology group at Argonne National Laboratory). Amino acid sequences were aligned using the CLUSTALW (version 1.7.4) program (25). From the alignment, 302 positions deemed to be confidently aligned were analyzed by protein maximum-parsimony methods using a heuristic search algorithm (PAUP* version 4 beta 2; D. Swofford, Sinauer Associates, Inc.). The 1,000 shortest trees were evaluated by maximum-likelihood criteria using the PROTML program (version 2.2) in the MOLPHY package (1) with the JTT model for amino acid substitutions. Bootstrap proportions for the 1,000 candidate trees were estimated using the RELL method (11). The CONSENSE program (J. Felsenstein, PHYLIP [phylogeny inference package] version 3.5c, Department of Genetics, University of Washington, Seattle, 1993) was used to construct a consensus tree from the bootstrap proportions.
RESULTS
PepB is a LAP.
The PepB open reading frame (ORF) (Fig. 1) is predicted to encode a protein of 46.36 kDa. A BLAST search of the protein sequence database revealed that this ORF is a member of the leucyl aminopeptidase (LAP) family of metallopeptidases. PepB is the second member of the LAP family shown to be present in S. typhimurium and related organisms. PepA had been shown earlier to belong to this family (20). The prototype for this family (family M17 of Rawlings and Barrett [18]) is bovine lens leucyl aminopeptidase (BLLAP), for which a crystal structure has been determined (4). Very recently, the structure of E. coli PepA has also been reported (22). Both BLLAP and PepA are homohexamers of ∼300 kDa. Each subunit has a C-terminal domain containing the peptidase active site and the two Zn2+ ions involved in catalysis and an N-terminal domain that is involved in multimerization of the protein. The family contains representatives from a variety of eukaryotes and bacteria (21, 27) and one recently identified sequence from the Archaea (9). The C-terminal domain of PepB (amino acids 111 to 403) is approximately equally similar to the corresponding region of BLLAP (38% identity; 47% similarity) and E. coli PepA (49% identity; 39% similarity). The N-terminal domain of PepB (amino acids 1 to 110) shows little similarity to the corresponding regions of either BLLAP (0% identity; 12% similarity) or PepA (14% identity; 21% similarity).
FIG. 1.
Nucleotide sequence of the pepB region and predicted amino acid sequence of PepB. The sequence for a potential ς70 promoter is indicated by a double underline, and the putative ribosome binding site is in boldface type. The N-terminal sequence of the purified PepB protein and the 22-kDa peptide (the N-terminal fragment of cleaved PepB) is underlined. The N-terminal sequence of the 24-kDa peptide (the C-terminal fragment of cleaved PepB) is indicated by a dashed underline.
A subgroup of the LAP family shows significantly greater similarity to PepB than to any other LAP. The C-terminal domain of S. typhimurium PepB is more closely related to the corresponding regions of ORFs found in Salmonella enterica serovar Typhi (99% identical), E. coli (93% identical), Yersinia pestis (82% identical), Actinobacillus actinomycetemcomitans (64% identical), Pasteurella multocida (62% identical), and Haemophilus influenzae (61% identical) than it is to E. coli PepA or BLLAP, supporting the notion that they belong to the same subfamily. In addition, the N-terminal domains of all of these putative PepBs show significant similarities with many positions conserved in the entire group. The N-terminal domain of the P. multocida enzyme, the least closely related of the group (for which the N-terminal sequence is available) to S. typihimurium PepB, is 44% identical to S. typhimurium PepB. In addition, the N-terminal domains of these putative PepBs are substantially shorter (106 to 111 amino acids) than those of most other LAPs (e.g., E. coli PepA [187 amino acids] or BLLAP [167 amino acids]). Based on these similarities, we propose that this group of enzymes constitutes a distinct subfamily of the LAPs, the PepB subfamily. As discussed below, the PepB subfamily forms a separate group in the phylogenetic tree of the LAP family. Additional evidence for the distinctness of this subfamily and the likely physiological role that the family members share is presented below.
Purification of PepB.
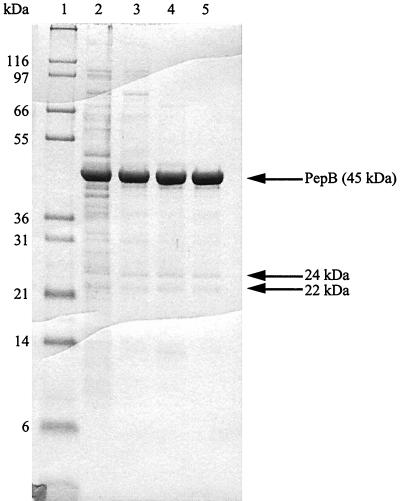
PepB was purified to greater-than-90% purity from extracts of an overproducing strain, TN5179 (Table 2 and Fig. 2). Mass spectrometric analysis indicated a monomer molecular mass of 46.36 kDa, in good agreement with that predicted from the sequence (46.362 kDa). The native molecular mass of the enzyme was determined by gel filtration to be 270 kDa, consistent with the expected homohexameric structure. Two polypeptides (24 and 26 kDa) consistently copurified with PepB (Fig. 2). N-terminal sequence analysis of these peptides showed that the 22-kDa protein is an N-terminal fragment of PepB (observed sequence, MTEAMKITLS) and the 24-kDa species is a C-terminal fragment starting at residue 216 (observed sequence, KSDMGGAATV). These species are not generated by autolysis because their levels do not increase on incubation of the purified enzyme (data not shown). We believe that these species are generated during purification, but we were unable to inhibit their formation with protease inhibitors.
TABLE 2.
Purification of serovar Typhimurium PepB
| Step | Total protein (mg) | Total activity (μmol min−1) | Sp act (μmol min−1 mg−1) | % Recovery | Fold purification |
|---|---|---|---|---|---|
| Crude extract | 195 | 12,900 | 66 | 100 | |
| Q Sepharose | 85 | 8,500 | 100 | 66 | 1.5 |
| Heat step | 12 | 2,280 | 190 | 18 | 2.8 |
| Superdex | 8 | 1,520 | 190 | 12 | 2.8 |
FIG. 2.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis of PepB purification. Four micrograms from each step of the purification of serovar Typhimurium PepB was loaded on a 10% Tris-tricine gel. Lanes: 1, molecular mass standards; 2, crude extract of TN5179; 3, Q Sepharose; 4, 70°C heat step; 5, Superdex. The arrows indicate purified PepB and the N-terminal and C-terminal cleavage products.
Enzymatic properties of PepB.
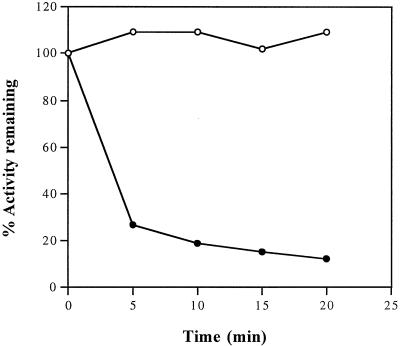
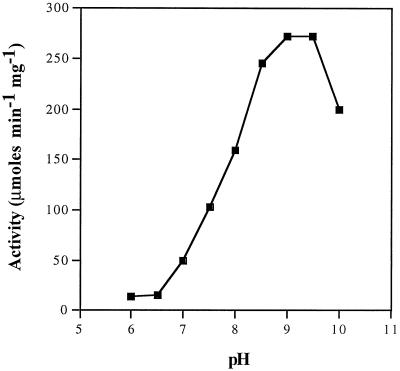
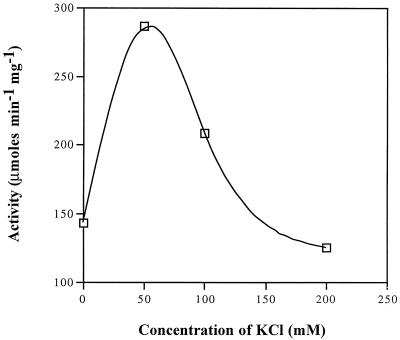
The enzymatic properties of PepB are similar to those of other members of the LAP family. The enzyme is heat stable (Fig. 3). Although PepB activity is quickly lost when a crude extract is heated to 70°C, the purified enzyme is stable under these conditions, as are most other LAPs. The pH optimum is 8.5 to 9.5 (Fig. 4), also typical for LAPs. The enzyme is stimulated by salt, with an optimum KCl concentration of 50 mM (Fig. 5). The enzyme is sensitive to EDTA. Activity can be restored to EDTA-dialyzed enzyme by divalent cations. The activities of the reactivated enzymes relative to undialyzed enzyme prepared in buffer containing Mg2+ are Mn2+ (4.3), Mg2+ (1.1), Co2+ (0.5), and Zn2+ (0.2). Bestatin (1 mM; 20 min; 37°C) completely inhibits PepB activity.
FIG. 3.
Heat stability of PepB. Crude extracts of strain TN5179 (●) and partially purified PepB (after the Q Sepharose column purification step) (○) were heated at 70°C. Samples were withdrawn at the indicated times and assayed for peptidase activity.
FIG. 4.
Effect of pH on PepB activity. The activity of PepB was determined in 50 mM bis-Tris at pH 6.0 and 6.5, in 50 mM Tris at pH 7.0 to 9.5, and in 50 mM 2-(cyclohexylamine) ethanesulfonic acid at pH 8.5 to 10.0 in the standard assay using 1 mM Leu-Leu as a substrate.
FIG. 5.
Effect of salt on PepB activity. The activity of PepB was determined by using the standard assay in 0.05 M Tris-HCl (pH 8.5) and 1 mM MgCl2 with 1 mM Leu-Leu as a substrate.
PepB hydrolyzes peptides with acidic N termini.
In an attempt to understand the physiological rationale for the presence in S. typhimurium and related organisms of three broad-specificity aminopeptidases, two of which are LAPs, we carried out a detailed study of the specificity of PepB. Since LAPs typically hydrolyze dipeptides as well as longer substrates, we began with a series of X-Leu and Leu-X dipeptides, where X is any of the 20 standard amino acids. The results of these studies are shown in Tables 3 and 4. Because most LAPs prefer hydrophobic residues at the N termini of their substrates, we were surprised to find that Asp-Leu was the most rapidly hydrolyzed of all the X-Leu dipeptides and Glu-Leu was hydrolyzed nearly as rapidly as Asp-Leu. Peptides with N-terminal Leu, Met, His, Cys, and Gln were all hydrolyzed well, and all supported the growth of a leucine auxotroph containing PepB as the only functional peptidase able to hydrolyze these peptides. Peptides with Gln or any of the aromatics at their N termini were hydrolyzed at 3 to 9% of the rate of Asp-Leu, but none of these peptides supported growth. Since other peptides with even lower hydrolysis rates (Thr-Leu, Ala-Leu, and Ser-Leu) support growth, it is unclear why the Gln and aromatic peptides do not. Not only do these peptides not support growth, they also inhibit the utilization of permissive peptides. We do not understand the basis of this observation, but it apparently does not involve inhibition of peptide uptake, since these peptides do not inhibit the utilization of other peptides in an isogenic strain dependent on PepA rather than PepB for peptide utilization. Peptides with N-terminal Ile and Val are slowly but detectably hydrolyzed but cannot support growth. N-terminal Gly and Lys peptides are very slowly hydrolyzed, and those with N-terminal Arg and Pro are not detectably hydrolyzed. None of these peptides supports growth.
TABLE 4.
Hydrolysis of Leu-X peptides by PepB
| Leu-X | In vivoa | Sp act (μmol min−1 mg−1) | Relative activityb |
|---|---|---|---|
| LI | + | 326 | 59 |
| LL | + | 223 | 40 |
| LY | + | 204 | 37 |
| LM | + | 167 | 30 |
| LC | + | 135 | 24 |
| LF | + | 65 | 12 |
| LH | + | 51 | 9 |
| LW | − | 34 | 6 |
| LQ | + | 33 | 6 |
| LE | + | 32 | 6 |
| LS | + | 30 | 5 |
| LA | + | 28 | 5 |
| LV | + | 23 | 4 |
| LN | + | 17 | 3 |
| LG | − | 17 | 3 |
| LT | − | 8 | 1 |
| LD | − | 4 | 0.7 |
| LR | − | 3 | 0.5 |
| LK | − | 2 | 0.4 |
| LP | − | <0.5 | <0.09 |
+, growth; −, no growth.
Relative activity with respect to Asp-Leu activity (550 μmol min−1 mg−1).
To probe the specificity of PepB for the amino acid occupying the position next to the N terminus, a series of Leu-X peptides was tested for hydrolysis using the purified enzyme (Table 4). All peptides tested showed a detectable rate of hydrolysis except Leu-Pro. Most broad-specificity peptidases cannot hydrolyze peptides with Pro next to the N terminus, and two specialized X-Pro hydrolyases are present in S. typhmurium and related organisms to deal with these peptides (16). Most neutral amino acids are tolerated at the C terminus, and hydrophobic amino acids (Ile, Leu, and Tyr) are preferred. Although Glu is tolerated at this position, peptides containing the other charged amino acids (Asp, Arg, and Lys) are very poorly hydrolyzed.
Because Asp-Leu is the most rapidly hydrolyzed of the X-Leu peptides, we wished to see if other N-terminal Asp peptides are hydrolyzed and, if so, to determine if the preferences for second amino acids found with the Leu-X series would also apply in a series of Asp-X peptides. As shown in Table 5, nearly all Asp-X peptides are hydrolyzed more rapidly than the corresponding Leu-X peptides. In addition, the second amino acid specificity in the Asp-X series is very similar to that observed for Leu-X peptides. Except for Glu, substrates with charged amino acids or Pro in the second position are very poorly hydrolyzed. Most other amino acids are tolerated, and substrates with hydrophobic amino acids are the most rapidly hydrolyzed. These rules are essentially the same as those deduced from the Leu-X peptides. Note that because of the presence in these strains of other enzymes that hydrolyze N-terminal Asp dipeptides, it is not possible to test the PepB-dependent utilization of Asp-X peptides (5). The data in Table 5 also confirm that other N-terminal Glu peptides (besides Glu-Leu) are good substrates, although most of them are hydrolyzed at a slightly lower rate than the corresponding Asp peptides.
TABLE 5.
Hydrolysis of Asp-X and Glu-X peptides by PepB
| Peptide | Sp act (μmol min−1) | Relative activitya |
|---|---|---|
| DI | 659 | 120 |
| DF | 579 | 105 |
| DL | 550 | 100 |
| DY | 476 | 86 |
| DC | 348 | 63 |
| DH | 301 | 55 |
| DQ | 257 | 47 |
| DE | 200 | 36 |
| DW | 164 | 30 |
| DA | 177 | 32 |
| DS | 126 | 23 |
| DV | 119 | 22 |
| DN | 71 | 13 |
| DM | 69 | 12 |
| DG | 59 | 11 |
| DT | 25 | 4 |
| DD | 21 | 4 |
| DR | 2 | 0.4 |
| DP | 0.7 | 0.1 |
| DK | <0.5 | <0.09 |
| EW | 124 | 22 |
| EY | 114 | 21 |
| EV | 43 | 8 |
| EA | 41 | 7 |
| EG | 26 | 5 |
| EK | 16 | 3 |
Relative activity with respect to Asp-Leu activity (550 μmol min−1 mg−1).
To determine if the specificity rules deduced for dipeptides also apply to tripeptides, we tested a series of X-Gly-Gly peptides (Table 6). These peptides were chosen because they are available at reasonable cost. Presumably because Gly in position 2 leads to relatively poor hydrolysis (Tables 4 and 5), all of these peptides are hydrolyzed more slowly than the corresponding X-Leu dipeptides. The results of these experiments show that the specificity rules for the N-termini of tripeptides are essentially identical to those deduced using dipeptides.
TABLE 6.
Hydrolysis of X-Gly-Gly peptides by PepB
| Peptide | Hydrolysis rate (μmol min−1) | Relative activitya |
|---|---|---|
| DGG | 32 | 6 |
| EGG | 12 | 2 |
| LGG | 12 | 2 |
| HGG | 9 | 2 |
| MGG | 4 | 0.7 |
| CGG | 3 | 0.5 |
| NGG | 3 | 0.5 |
| AGG | 2 | 0.4 |
| FGG | 2 | 0.4 |
| WGG | 2 | 0.4 |
| YGG | 2 | 0.4 |
| QGG | 2 | 0.4 |
| IGG | 1 | 0.2 |
| TGG | 1 | 0.2 |
| SGG | 1 | 0.2 |
| VGG | 0.6 | 0.1 |
| GGG | <0.5 | <0.09 |
| KGG | <0.5 | <0.09 |
| PGG | <0.5 | <0.09 |
| RGG | <0.5 | <0.09 |
Relative activity with respect to the hydrolysis rate of Asp-Leu (550 μmol min−1 mg−1).
Kinetics of PepB-catalyzed peptide hydrolysis.
Table 7 shows kinetic data for the PepB-catalyzed hydrolysis of several peptides. These data indicate that the observed specificity for Asp is mainly a Km effect. The Kms of all of the Asp-X dipeptides where X is uncharged are between 0.3 and 0.6 mM. The Km for Glu-Leu is also in this range (0.9 mM). Kms for the N-terminal Leu peptides tested are 1 mM or greater.
Structural basis for PepB specificity.
In an attempt to define the structural basis for the ability of PepB to hydrolyze peptides with N-terminal acidic residues, we compared the amino acid sequences of the regions of BLLAP believed to contact substrates with the corresponding regions of PepB. An examination of the structure of BLLAP with the peptide analog inhibitor amastatin bound in the active site (10, 23) and comparison with the corresponding residues in PepB led us to speculate that Lys310 and/or Arg402 might interact with the negative charge of acidic N-terminal PepB substrates. Both of these residues are conserved in all members of the proposed PepB subfamily. Lys310 is present in a region with significant conservation among all LAPs, but only the proposed PepB subfamily contains a Lys residue at this position (Fig. 6). Arg402 is part of a larger motif, CSATYRK. This motif is present in all members of the PepB subfamily, and the underlined amino acids are not present in a similar motif at the corresponding positions in any other LAP. Except for three enzymes that are related to PepB and that may have similar specificities (described below), no other members of the LAP family have positively charged residues at the positions corresponding to either Lys310 or Arg402. To test the hypothesis that these residues contribute to the specificity of PepB for peptides with N-terminal acidic residues, we used site-directed mutagenesis to change the Lys310 to Val. Val is the amino acid found at the corresponding position in E. coli PepA, which does not hydrolyze peptides with N-terminal acidic residues. We also constructed an Arg402-to-Trp mutant, again substituting the corresponding residue from E. coli PepA. Plasmids carrying these strains were introduced into a peptidase-deficient S. typhimurium strain, and crude extracts were prepared and used as a source of PepB in assays with Leu-Leu and Asp-Leu as substrates. The results of these assays (Table 8) indicate that either of these changes reduces the ability of PepB to hydrolyze Asp-Leu without decreasing its ability to attack Leu-Leu. The effect of the K310V mutant is particularly striking. This mutation leads to a more-than-200-fold reduction in the rate of Asp-Leu hydrolysis with no decrease in the rate of Leu-Leu hydrolysis. Thus, a single amino acid residue, K310, appears to play a dominant role in PepB substrate recognition.
FIG. 6.
Sequence alignment of the C-terminal domains of a subset of the LAP family. All members of the proposed PepB subfamily and its close relatives are presented, and a diverse group of other LAPs chosen to represent some of the major subgroupings predicted by the phylogenetic tree (Fig. 7) is included for comparison. Positions that are identical for all seven PepBs are indicated by black shading. The conserved residues in the PepB subfamily are indicated in the consensus sequence above the alignment, and the residues that are identical in all members of the LAP family are underlined. The asterisks indicate the positions of K310 and the CSATYRK motif. The arrows indicate the metal binding sites. The reported sequence for A. actinomycetemcomitans PepB shows a G rather than an E in the universally conserved NTDAEGR metal binding site (line 3 of the alignment). We believe that this is likely to be a sequencing error in this incomplete genome sequence and have replaced the G with an E. A.aPepB, A. actinomycetemcomitans PepB (NUMG); P.mPepB, P. multocida PepB (NUMG); H.iPepB, H. influenzae PepB (P45334); S.tiPepB, serovar Typhi PepB (SSC); S.tmPepB, serovar Typhimurium PepB (AF201078 [this work]); E.cPepB, E. coli PepB (P37095); Y.pPepB, Y. pestis PepB (SSC); S.pLAP3, S. putrefaciens LAP3 (NUMG); C.cLAP3, C. crescentus LAP3 (NUMG); L.pLAP, L. pneumophila LAP (NUMG); R.cLAP3, R. capsulatus LAP3 (blast search of WIT at http://www.wit.mcs.anl.gov); E.cPepA, E. coli PepA (P11648); P.aPepA, Pseudomonas aeruginosa PepA (AAD04821); B.tLAP, Bos taurus kidney (P00727); A.pLAP, Aeropyrum pernix LAP (NUMG); SynecLAP, Synechocystis sp. LAP (P73971); B.sLAP, Bacillus subtilis LAP (Z99120); S.aLAP, Staphylococcus aureus LAP (NUMG); R.pPepA, Rickettsia prowazekii PepA (P27888); R.cLAP2, R. capsulatus LAP2 (WIT).
TABLE 8.
Peptide hydrolysis by wild-type and mutant PepBs
| PepB | Sp act (μmol min−1 mg−1)
|
|
|---|---|---|
| Leu-Leu | Asp-Leu | |
| Wild-type | 129 | 239 |
| K310V | 310 | 1 |
| R402W | 137 | 63 |
Phylogenetic relationships among members of the LAP family.
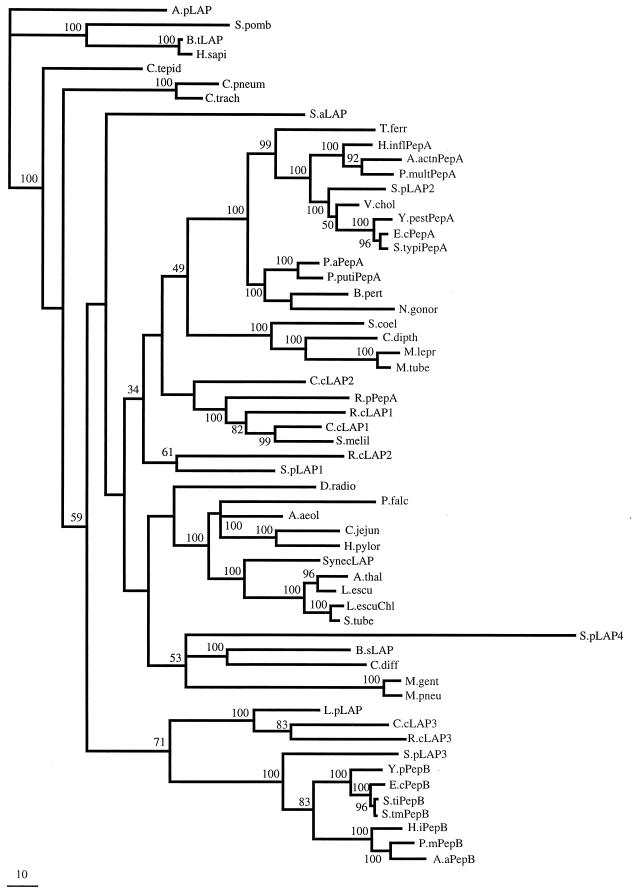
Figure 7 shows a phylogenetic tree of the C-terminal domains of a diverse group of LAPs. This tree includes sequences from incomplete genomes as well as from GenBank. All of these sequences show complete conservation of the metal binding sites, and it is likely that all of them are indeed peptidases. Several conclusions can be drawn from this tree. (i) The group of enzymes proposed above as the PepB subfamily clearly forms a distinct and deeply branching subfamily. There is an additional group of proteins that are more closely related to the PepBs than to other LAPs. These enzymes (Shewanella putrefaciens LAP3, Legionella pneumoniae LAP, Caulobacter crescentus LAP3, and Rhodobacter capsulatus LAP3) share substantial similarity in both size and amino acid sequence in their N-terminal domains, further establishing their relationship to the PepBs. None of these four proteins retains the CSATYRK motif, although three of them (L. pneumoniae LAP, C. crescentus LAP3, and R. capsulatus LAP3) retain a basic residue at the position corresponding to K310 in the S. typhimurium and E. coli enzymes, suggesting that they may also be able to hydrolyze substrates with an N-terminal acidic residue. In the fourth enzyme (S. putrefaciens LAP3), a valine occupies this position, and it seems unlikely, therefore, that this enzyme shares the PepB specificity pattern. (ii) This tree is consistent with the idea that the PepB family evolved in an ancestor of the Proteobacteria, since all PepBs are present in either alpha- or gamma-proteobacteria. (iii) The presence of multiple LAPs in a single organism is relatively common. All of the proteobacteria appear to have at least two, and some have three (C. crescentus and R. capsulatus) or four (S. putrefaciens).
FIG. 7.
Phylogenetic tree of the C-terminal domain of the LAP family. The tree is rooted with the Aeropyrum pernix sequence as an outgroup. The scale bar represents 10 amino acid substitutions per 100 positions. The numbers near each node are the bootstrap proportions estimated by the RELL method from candidate trees. The nodes without bootstrap values cannot be assigned with confidence. A.actn PepA, A. actinomycetemcomitans PepA; A.aPepB, A. actinomycetemcomitans PepB; A.aeol, Aquifex aeolicus (AAC07829); A.pLAP, A. pernix LAP (NUMG); A.thal, Arabidopsis thaliana (P30184); B.sLAP, Bacillus subtilis LAP; B.pert, Bordetella pertussis (NUMG); B.tLAP, Bos taurus kidney (P00727); C.cLAP2, C. crescentus LAP2; C.jejun, Campylobacter jejuni (NUMG); C.pneum, Chlamydia pneumoniae (AE001623); C.trach, Chlamydia trachomatis (AE001279); C.tepid, Chlorobium tepidum (NUMG); C.diff, Clostridium difficile (NUMG); C.dipth, Corynebacterium diptheriae (NUMG); D.radio, Deinococcus radiodurans (AE001928); E.cPepA, E. coli; PepA; H.inflPepA, Haemophilus influenzae PepA (AAC23347); H.iPepB, H. influenzae PepB; H.pylor, Helicobacter pylori (AE001485); H.sapi, Homo sapiens (AAD17527); L.escu, Lycopersicon esculentum (Q42876); L.escuChl, L. esculentum Chloroplast (Q10712); L.pLAP, L. pneumophila LAP; M.lepr, Mycobacterium leprae (CAB11379); M.tube, Mycobacterium tuberculosis (Q10401); M.gent, Mycoplasma genitalium (P47631); M.pneu, Mycoplasma pneumoniae (P75206); N.gonor, Neisseria gonorrhoeae (NUMG); P.aPepA, Pseudomonas aeruginosa PepA; P.mPepB, P. multocida PepB; P.multPepA, P. multocida PepA (NUMG); P.falc, Plasmodium falciparum (NUMG); P.putiPepA, Pseudomonas putida PepA (CAA09054); R.cLAP1, R. capsulatus LAP1; R.pPepA, Rickettsia prowazekii PepA (P2788); S.typiPepA, serovar Typhi PepA (SSC); S.pomb, Schizosaccharomyces pombe (Q09735); S.melil, Sinorhizobium meliloti (NUMG); S.tiPepB, serovar Typhi PepB; S.tmPepB, serovar Typhimurium PepB; S.tube, Solanum tuberosum (P31427); S.aLAP, Staphylococcus aureus LAP (NUMG); S.coel, Streptomyces coelicolor (CAB51263); S.pLAP2, S. putrefaciens LAP2; SynecLAP, Synechocystis sp. LAP; T.ferr, Thiobacillus ferrooxidans (NUMG); V.chol, Vibrio cholerae (NUMG); Y.pestPepA, Y. pestis PepA (SSC); Y.PepB, Y. pestis PepB. In organisms where there is more than one LAP, a numerical designation has been arbitrarily assigned. In several cases, we have assigned names that have not been previously assigned in the literature based on location in the tree: A.actnPepA and -PepB and P.multPepA and -PepB.
DISCUSSION
The results presented in this paper show that in many respects PepB is a typical member of the LAP family of enzymes. It is a heat-stable, homohexameric, bestatin-inhibited metalloaminopeptidase able to hydrolyze a variety of different N-terminal amino acids from its substrates. It differs strikingly from the other well-characterized members of the family, however, in its preference for N-terminal acidic residues. Other LAPs prefer hydrophobic N-terminal amino acids and are unable to cleave N-terminal acidic residues.
It seems likely that PepB's ability to hydrolyze peptides with N-terminal acidic residues plays a physiologically important role in peptide degradation. The ATP-dependent enzymes that are involved in the initial steps of many intracellular protein degradation pathways all produce peptides as products, and these peptides are rapidly degraded to produce free amino acids. Apparently this peptide degradation process requires the cooperation of several different peptidases, each with its special role in the degradation process. The other well-characterized N-terminal Asp-hydrolyzing enzyme, PepE, is strictly a dipeptidase (5, 7) and is unable to attack larger peptides. PepE is also unable to hydrolyze N-terminal Glu peptides. It seems likely, therefore, that PepB is primarily responsible for the degradation of peptides with N-terminal acidic residues.
PepA, the other LAP present in E. coli and S. typhimurium, has been shown to carry out two additional functions unrelated to its ability to hydrolyze peptides. PepA is required for the resolution of ColE1 plasmid dimers (20), and it is a transcriptional regulator of the carAB operon (6). Both of these activities are believed to involve the noncatalytic N-terminal domain of PepA acting as a DNA binding protein (22). Because PepB shows essentially no sequence similarity to PepA in this domain, it seems unlikely that it possesses any DNA binding capability.
We believe that S. typhimurium PepB represents the prototype of a new subfamily of LAPs characterized by sequence similarities in the N-terminal domain and by the existence of conserved sequence motifs in the C-terminal domain among PepB family members that are not present in any other LAPs. The findings that the specificity for N-terminal acidic residues is abolished by mutations at Lys310 in S. typhimurium pepB and that this residue is one of those conserved in all members of the proposed PepB subfamily (and not found in any other LAP) leads us to propose that all of these enzymes share PepB's preference for peptides with N-terminal acidic amino acids.
Representatives of the LAP family are found in all of the domains of life. Representatives of the PepB subfamily have been identified only in proteobacteria, however, and this subfamily appears to represent a distinct and ancient division of the LAP family. We predict that all close relatives of S. typhimurium and E. coli PepB (those enzymes with both 310K and the CSATYRK motif) will be found to hydrolyze N-terminal acidic peptides. Some of the enzymes that show greater overall similarity to PepB than to other LAPs lack the CSATYRK motif but have a basic residue (R rather than K) in the position corresponding to K310. These enzymes may also be able to hydrolyze acidic substrates. One of the PepB relatives (S. putrefaciens LAP3) has a valine at the position corresponding to K310, and it is predicted, based on the properties of our K310V mutant and the specificity of PepA, which also has a Val at this position, to be unable to hydrolyze peptides with N-terminal acidic residues. It is tempting to speculate that this substitution represents the conversion of an ancestral acid-specific enzyme to a more PepA-like specificity. Many proteobacteria contain more than one LAP, and one (S. putrefaciens) contains four. It seems likely that many of these groups of enzymes differ from each other in their specificities for peptide substrates, and a closer study based on the phylogenetic analysis of Fig. 7 may reveal other subfamilies with unique enzymatic properties that distinguish them from other LAPs.
One of the goals of this work was to learn whether the broad-specificity aminopeptidases could be differentiated based on special functions carried out by one but not by the others. The complete degradation of cellular proteins requires that the cell be able to hydrolyze essentially all possible small peptides. Degradation of these peptides is highly efficient, since peptide intermediates do not accumulate under conditions of extensive intracellular protein breakdown (14). It seems unlikely a priori that an enzyme can be designed that is an efficient catalyst of peptide bond hydrolysis while lacking any specificity for the amino acids that form the peptide bond. The results reported here clearly reveal one of the special functions of PepB, as well as the structural basis for this function.
ACKNOWLEDGMENTS
This work was supported by a grant (AI10333) from the National Institute for Allergy and Infectious Diseases.
We thank Kjell Håkansson for help in modeling the structure of PepB and David Graham and Gary Olsen for help in constructing the phylogenetic tree. We also thank Chris Conlin for isolating the Tn1000 insertions and Jane Glazebrook for isolating pJ697.
REFERENCES
- 1.Adachi J, Hasegawa M. MOLPHY version 2.3: programs for molecular phylogenetics based on maximum likelihood. Comput Sci Monogr. 1996;28:1–150. [Google Scholar]
- 2.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 3.Barrett A J, Rawlings N D, Woessner J F. Handbook of proteolytic enzymes. San Diego, Calif: Academic Press; 1998. [Google Scholar]
- 4.Burley S K, David P R, Sweet R M, Taylor A, Lipscomb W N. Structure determination and refinement of bovine lens leucine aminopeptidase and its complex with bestatin. J Mol Biol. 1992;224:113–140. doi: 10.1016/0022-2836(92)90580-d. [DOI] [PubMed] [Google Scholar]
- 5.Carter T H, Miller C G. Aspartate-specific peptidases in Salmonella typhimurium: mutants deficient in peptidase E. J Bacteriol. 1984;159:453–459. doi: 10.1128/jb.159.2.453-459.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Charlier D, Hassanzadeh G, Kholti A, Gigot D, Pierard A, Glansdorff N. carP, involved in pyrimidine regulation of the Escherichia coli carbamoylphosphate synthetase operon encodes a sequence-specific DNA-binding protein identical to XerB and PepA, also required for resolution of ColE1 multimers. J Mol Biol. 1995;250:392–406. doi: 10.1006/jmbi.1995.0385. [DOI] [PubMed] [Google Scholar]
- 7.Conlin C A, Håkansson K, Liljas A, Miller C G. Cloning and nucleotide sequence of the cyclic AMP receptor protein-regulated Salmonella typhimurium pepE gene and crystallization of its product, an α-aspartyl dipeptidase. J Bacteriol. 1994;176:166–172. doi: 10.1128/jb.176.1.166-172.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guyer M S. Uses of the transposon gamma delta in the analysis of cloned genes. Methods Enzymol. 1983;101:362–369. doi: 10.1016/0076-6879(83)01027-7. [DOI] [PubMed] [Google Scholar]
- 9.Kawarabayasi Y, Hino Y, Horikawa H, Yamazaki S, Haikawa Y, Jin-no K, Takahashi M, Sekine M, Baba S, Ankai A, Kosugi H, Hosoyama A, Fukui S, Nagai Y, Nishijima K, Nakazawa H, Takamiya M, Masuda S, Funahashi T, Tanaka T, Kudoh Y, Yamazaki J, Kushida N, Oguchi A, Kikuchi H, et al. Complete genome sequence of an aerobic hyper-thermophilic crenarchaeon, Aeropyrum pernix K1. DNA Res. 1999;6:83–101. doi: 10.1093/dnares/6.2.83. , 145–152. [DOI] [PubMed] [Google Scholar]
- 10.Kim H, Lipscomb W N. X-ray crystallographic determination of the structure of bovine lens leucine aminopeptidase complexed with amastatin: formulation of a catalytic mechanism featuring a gem-diolate transition state. Biochemistry. 1993;32:8465–8478. doi: 10.1021/bi00084a011. [DOI] [PubMed] [Google Scholar]
- 11.Kishino H, Miyata T, Hasegawa M. Maximum likelihood inference of protein phylogeny and the origin of chloroplasts. J Mol Evol. 1990;31:151–160. [Google Scholar]
- 12.Liu L, Whalen W, Das A, Berg C M. Rapid sequencing of cloned DNA using a transposon for bidirectional priming: sequence of the Escherichia coli K-12 avtA gene. Nucleic Acids Res. 1987;15:9461–9469. doi: 10.1093/nar/15.22.9461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McHugh G L, Miller C G. Isolation and characterization of proline peptidase mutants of Salmonella typhimurium. J Bacteriol. 1974;120:364–371. doi: 10.1128/jb.120.1.364-371.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller C G. Protein degradation and proteolytic modification. In: Neidhardt F C, Ingraham J L, Low K B, Magasanik B, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella typhimurium: cellular and molecular biology. Washington, D.C.: American Society for Microbiology; 1987. pp. 680–691. [Google Scholar]
- 15.Miller C G. Protein degradation and proteolytic modification. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. Washington, D.C.: American Society for Microbiology; 1996. pp. 938–954. [Google Scholar]
- 16.Miller C G, Green L. Degradation of proline peptides in peptidase-deficient strains of Salmonella typhimurium. J Bacteriol. 1983;153:350–356. doi: 10.1128/jb.153.1.350-356.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller C G, Mackinnon K. Peptidase mutants of Salmonella typhimurium. J Bacteriol. 1974;120:355–363. doi: 10.1128/jb.120.1.355-363.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rawlings N, Barrett A J. Evolutionary families of metallopeptidases. Methods Enzymol. 1995;248:183–228. doi: 10.1016/0076-6879(95)48015-3. [DOI] [PubMed] [Google Scholar]
- 19.Sensen C W, Klenk H P, Singh R K, Allard G, Chan C C, Liu Q Y, Penny S L, Young F, Schenk M E, Gaasterland T, Doolittle W F, Ragan M A, Charlebois R L. Organizational characteristics and information content of an archaeal genome: 156 kb of sequence from Sulfolobus solfataricus P2. Mol Microbiol. 1996;22:175–191. doi: 10.1111/j.1365-2958.1996.tb02666.x. [DOI] [PubMed] [Google Scholar]
- 20.Stirling C J, Colloms S D, Collins J F, Szatmari G, Sherratt D J. xerB, an Escherichia coli gene required for plasmid ColE1 site-specific recombination, is identical to pepA, encoding aminopeptidase A, a protein with substantial similarity to bovine lens leucine aminopeptidase. EMBO J. 1989;8:1623–1627. doi: 10.1002/j.1460-2075.1989.tb03547.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sträter N, Lipscomb W N. Leucyl aminopeptidase (animal and plant) In: Barrett A J, Rawlings N D, Woessner J F, editors. Handbook of proteolytic enzymes. San Diego, Calif: Academic Press; 1998. [Google Scholar]
- 22.Sträter N, Sherratt D J, Colloms S D. X-ray structure of aminopeptidase A from Escherichia coli and a model for the nucleoprotein complex in Xer site-specific recombination. EMBO J. 1999;18:4513–4522. doi: 10.1093/emboj/18.16.4513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sträter N, Sun L, Kantrowitz E R, Lipscomb W N. A bicarbonate ion as a general base in the mechanism of peptide hydrolysis by dizinc leucine aminopeptidase. Proc Natl Acad Sci USA. 1999;96:11151–11155. doi: 10.1073/pnas.96.20.11151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tamura N, Lottspeich F, Baumeister W, Tamura T. The role of tricorn protease and its aminopeptidase-interacting factors in cellular protein degradation. Cell. 1998;95:637–648. doi: 10.1016/s0092-8674(00)81634-7. [DOI] [PubMed] [Google Scholar]
- 25.Thompson J D, Higgins D G, Gibson T J. CLUSTAL W: improving the sensitivity of progressive multiple alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1984;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vogel H J, Bonner D M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956;218:97–106. [PubMed] [Google Scholar]
- 27.Wood D O. Leucyl aminopeptidase (bacteria) In: Barrett A J, Rawlings N D, Woessner J F, editors. Handbook of proteolytic enzymes. San Diego, Calif: Academic Press; 1998. pp. 1389–1391. [Google Scholar]
- 28.Yen C, Green L, Miller C G. Degradation of intracellular protein in Salmonella typhimurium peptidase mutants. J Mol Biol. 1980;143:21–33. doi: 10.1016/0022-2836(80)90122-9. [DOI] [PubMed] [Google Scholar]
- 29.Yen C, Green L, Miller C G. Peptide accumulation during growth of peptidase deficient mutants. J Mol Biol. 1980;143:35–48. doi: 10.1016/0022-2836(80)90123-0. [DOI] [PubMed] [Google Scholar]