Abstract
Objectives
C-reactive protein (CRP) and procalcitonin (PCT) are widely used biomarkers in high-income countries. However, evidence for their use in low- and middle-income countries (LMICs) is scant. Because many factors, including rates of endemic disease, comorbidities and genetics, may influence biomarkers’ behaviour, we aimed to review available evidence generated in LMICs.
Methods
We searched the PubMed database for relevant studies within the last 20 years that originated in regions of interest (Africa, Latin America, Middle East, South Asia or South East Asia), and full-text articles involving diagnosis, prognostication and evaluation of therapeutic response with CRP and/or PCT in adults (n = 88) were reviewed and categorized in 12 predefined focus areas.
Results
Overall, results were highly heterogeneous, at times conflicting, and often lacking clinically useful cut-off values. However, most studies demonstrated higher levels of CRP/PCT in patients with bacterial versus other infections. HIV and TB patients had consistently higher levels of CRP/PCT versus controls. In addition, higher CRP/PCT levels at baseline and follow-up in HIV, TB, sepsis and respiratory tract infections were associated with poorer prognosis.
Conclusions
Evidence generated from LMIC cohorts suggests that CRP and PCT may have potential to become effective clinical guiding tools particularly in respiratory tract infections, sepsis and HIV/TB. However, more studies are needed to define potential scenarios for use and cost-effectiveness. Consensus across stakeholders regarding target conditions, laboratory standards and cut-off values would support the quality and applicability of future evidence.
Introduction
The biomarkers C-reactive protein (CRP) and procalcitonin (PCT) have been used in clinical practice as tools to guide both diagnosis (negative predictive value of low CRP/PCT for bacterial infection), prognosis and antibiotic de-escalation (decreasing trend of CRP/PCT, mainly in acute respiratory infection). However, most of the evidence comes from high-income countries (HICs).1–3
Low- and middle-income countries (LMIC) settings are heterogeneous, with differing baseline rates of endemic disease and comorbidities such as malaria, which, combined with genetic factors, may mean that biomarkers behave differently than in HICs, where most evidence has been generated. For example, healthy adult males in Ghana were found to have a slightly lower baseline CRP compared with European counterparts (0.98 versus 1.52 mg/L).4 However, evidence from populations in LMICs suggests that use of CRP and PCT may avert unnecessary antibiotic use and improve patient outcomes in these settings as well.5–9 There are barriers to implementation, including cost. More recently, point-of-care testing (POCT), e.g. immunochromatographic methods, have been piloted also in primary care settings with evidence of potential feasibility also on a larger scale due to their reduced costs. More evidence to support the use of point-of-care CRP/PCT in acute febrile illnesses in sub-Saharan Africa and LMICs elsewhere is urgently needed.10
We performed a narrative review of existing evidence from LMICs on the utility of CRP and PCT in various clinical conditions.
Methods
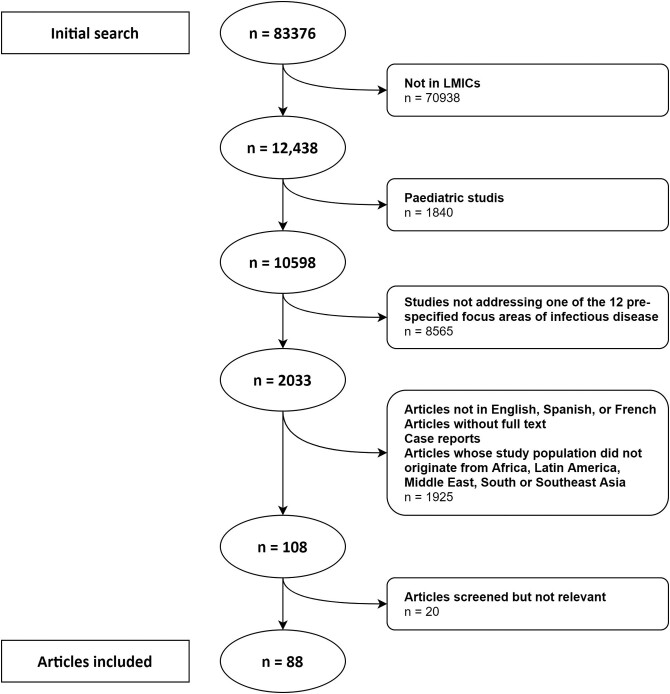
Using MeSH (Medical Subject Headings) terms and a Cochrane Database LMIC filter,11 we aimed at identifying studies from within the last 20 years that used biomarkers in adult populations (19 years and above) in LMIC settings for diagnosis, prognostication and evaluation of therapeutic response. The initial search yielded 83 376 articles using PCT or CRP in humans. After using the LMIC filter and excluding paediatric studies, the remaining 10 598 articles were considered for inclusion if they fell into 1 of 12 predefined focus areas (Table S1, available as Supplementary data at JAC-AMR Online) and selected 2033 articles. We subsequently excluded studies that were not from LMICs, publications not in English, French or Spanish, reviews, case reports, studies outside geographical regions of interest (Africa, Latin America, Middle East, South Asia or South East Asia), articles where full text was unavailable, and articles focusing on COVID-19. Abstracts of the remaining 108 relevant publications were screened by three independent reviewers, and 88 of these were included in the final review (Figure 1). The articles were categorized by use of biomarkers in diagnosis and prognostication, and further assigned to at least 1 of 12 predefined focus areas.
Figure 1.
Flowchart of inclusion/exclusion of publications for review.
Given the heterogeneity of the results, key findings were summarized in a narrative format. Laboratory values were converted to units of mg/L for CRP and ng/mL for PCT. When available, our review focused on objective and clinically applicable measures of diagnostic validity, such as biomarker cut-offs, sensitivity, specificity and predictive values (Table 1).
Table 1.
Summary of publications included for review
| Citation/ref. | Year | Study country | Study location | Study design | Disease tested | Study population | Biomarker(s) | Ranges (CRP/PCT) | Comparison | Outcomes tested |
|---|---|---|---|---|---|---|---|---|---|---|
| Sirijaichingkul et al.12 | 2005 | Thailand | Inpatient | Prospective cohort | CNS infections | 32 | CRP | Not specified | Non-comparative | Diagnostic value of CRP for bacterial meningitis |
| Abdelkader et al.13 | 2014 | Egypt | Emergency department | Prospective cohort | CNS infections | 40 | PCT | Not specified | Non-comparative | Diagnostic value of PCT at admission and 3 days after treatment for bacterial meningitis |
| Alavi et al.14 | 2012 | Iran | Inpatient | Prospective cohort | CNS infections | 36 | PCT | 0.5 ng/mL | Non-comparative | Prognostic value of early (24 h) decrease in PCT |
| Koegelenberg et al.15 | 2004. | S Africa | Inpatient | Prospective cohort | Endocarditis | 92 | CRP | Not specified | Non-comparative | Diagnostic value of CRP for infective endocarditis |
| Inan et al.16 | 2010 | Turkey | Inpatient (cardiac surgery) | Retrospective | Endocarditis (Brucella) | 31 | CRP | Not specified | Non-comparative | Diagnostic value of CRP for Brucella endocarditis |
| Mohanan et al.17 | 2018 | India | Inpatient | Prospective cohort | Endocarditis | 178 | CRP | <3 mg/L | Non-comparative | Prognostic value of CRP for infective endocarditis |
| Do et al.18 | 2016 | Vietnam | Outpatient | Randomized controlled trial | Acute respiratory tract infection | 2036 | CRP | 20 mg/L | No biomarker (routine care) | Diagnostic value of CRP in non-severe acute respiratory tract infection |
| Blake et al.19 | 2017 | Togo | Inpatient | Prospective cohort | Acute respiratory tract infection | 1684 | CRP | Not specified | Lyt A rtPCR, blood culture, CXR | Diagnostic value of CRP in community-acquired pneumonia |
| Borsi et al.20 | 2019 | Iran | Emergency department, and outpatient | Retrospective | Acute respiratory tract infection | 50 | PCT | Not specified | Non-comparative | Diagnostic value of PCT in acute exacerbation of COPD |
| Nyamande et al.21 | 2006 | S Africa | Inpatient | Prospective cohort | Acute respiratory tract infection | 266 | PCT | Not specified | Non-comparative | Diagnostic value of PCT for community-acquired pneumonia/and TB in HIV patients |
| Mendelson et al.22 | 2018 | S Africa | Inpatient | Prospective cohort | Acute respiratory tract infection | 210 | CRP, PCT | CRP 10 mg/L, PCT 0.02 μg/L | Composite reference standards | Diagnostic value of PCT and CRP for respiratory tract infections in HIV patients |
| Sharma et al.23 | 2016 | India | ICU | Prospective cohort | ARDS | 64 | CRP, PCT, IL-1β, IL-6, TNF-α | Not specified | Non-comparative | Prognostic value of PCT and CRP for ARDS patients |
| Wang et al.24 | 2019 | Uganda | Inpatient | Cross-sectional | Acute respiratory tract infection | 173 | CRP | Not specified | Non-comparative | Prognostic value of CRP for respiratory tract infections in HIV patients |
| Saldias-Penafiel et al.25 | 2019 | Chile | Inpatient | Prospective cohort | Acute respiratory tract infection | 823 | CRP | 0–0.5 mg/dL | Non-comparative | Diagnostic and prognostic value of CRP for community-acquired pneumonia |
| El Maghraby et al.26 | 2020 | Egypt | Inpatient | Cross-sectional | Acute respiratory tract infection | 240 | PCT | Not specified | Non-comparative | Diagnostic and prognostic value of PCT for community-acquired pneumonia |
| Tokman et al.27 | 2014 | Uganda | Inpatient | Prospective cohort | Acute respiratory tract infection | 635 | PCT | 0.02−0.5 ng/mL | Non-comparative | Prognostic value of PCT for lower respiratory tract infections in HIV patients |
| Seligman et al.28 | 2011 | Brazil | ICU | Observatonal cohort | Acute respiratory tract infection | 71 | PCT, CRP, MR-proANP, copeptin | CRP not specified, PCT 0.1 ng/mL, copeptin 2.25 pmol/L, MR-proANP 1 pmol/L | Non-comparative | Prognostic value of PCT and CRP for ventilator-associated pneumonia |
| Kiaei et al.29 | 2015 | Iran | ICU | Retrospective | Acute respiratory tract infection | 50 | PCT, CRP | Not specified | Non-comparative | Efficacy of PCT and CRP in determining antibiotic therapy duration in patients with ventilator-associated pneumonia |
| Wongsurakiat and Tulatamakit30 | 2018 | Thailand | ICU | Prospective non-randomized controlled study | Acute respiratory tract infection | 71 | PCT | 0.02–100 ng/mL | No biomarker (clinical score) | Efficacy of PCT in determining antibiotic therapy duration in patients with ventilator-associated pneumonia |
| Mohd et al.31 | 2021 | Malaysia | ICU | Prospective interventional single-blinded | Acute respiratory tract infection | 85 | PCT | < 0.25 ng/mL | No biomarker (routine care) | Efficacy of PCT in determining antibiotic therapy duration in patients with ventilator-associated pneumonia |
| El-Amin et al.32 | 2017 | Egypt | Inpatient | Cross-sectional descriptive study | Intra-abdominal infections | 100 | CRP | Not specified | Non-comparative | Diagnostic value of CRP for infection among patients with liver cirrhosis |
| Godinez-Vidal et al.33 | 2019 | México | Inpatient | Retrospective, descriptive, cross-sectional | Intra-abdominal infections | 99 | PCT | >10.1 ng/mL | Non-comparative | Prognostic value of PCT in abdominal sepsis |
| Orati et al.34 | 2013 | Brazil | ICU | Retrospective cohort | Intra-abdominal infections | 345 | CRP | Not specified | Non-comparative | Diagnostic value of CRP in abdominal sepsis |
| Trivedi et al.35 | 2016 | India | Inpatient | Retrospective | Urinary tract infections | 122 | CRP | Not specified | Non-comparative | Diagnostic value of CRP in urinary sepsis |
| Ndamason et al.36 | 2019 | Cameroon | Inpatient | Cross-sectional | Urinary tract infections | 128 | CRP | Not specified | Non-comparative | Diagnostic value of CRP in urinary sepsis |
| Arjunlal et al.37 | 2020 | India | Inpatient | Prospective | Urinary tract infections | 64 | CRP | Not specified | Non-comparative | Prognostic value of CRP in urinary sepsis |
| Agrawal et al.38 | 2015 | India | Inpatient | Retrospective | Bone and joint infection | 81 | CRP | Not specified | Non-comparative | Diagnostic value of CRP in osteoarticular infections |
| George et al.39 | 2019 | India | Inpatient | Retrospective | Bone and joint infection | 70 | CRP | Not specified | Non-comparative | Diagnostic value of CRP in septic arthritis |
| Waheed et al.40 | 2019 | Egypt | Inpatient | Prospective | Bone and joint infection | 44 | CRP | Not specified | Non-comparative | Prognostic value of CRP in patients with spondylodiscitis |
| Sharma et al.41 | 2012 | India | Inpatient | Prospective | Bone and joint infection | 20 | CRP | 11 mg/dL | Non-comparative | Prognostic value of CRP in patients with severe odontogenic infections |
| Umapathy et al.42 | 2018 | India | Inpatient | Cross-sectional case-control | Skin and soft tissue infections | 185 | PCT | 30 pg/mL | Biomarkers (CRP, ESR, WBC) | Prognostic value of PCT in diabetic patients with foot ulcers |
| Bammigatti et al.43 | 2019 | India | Inpatient | Prospective observational | Skin and soft tissue infections | 327 | PCT | 0.03 ng/mL | Non-comparative | Prognostic value of PCT in patients with snake bites |
| Yousef et al.44 | 2010 | Egypt | ICU | Prospective observational | Sepsis and bloodstream infections | 106 | CRP, leptin, IL-6 and TNF-α | Not specified | Non-comparative | Prognostic value of CRP in patients with sepsis |
| Mamani et al.45 | 2012 | Iran | Inpatient | Case-control | Sepsis and bloodstream infections | 90 | CRP, fibronectin | Not applicable | Non-comparative | Prognostic value of CRP in patients with sepsis |
| Ali et al.46 | 2017 | Pakistan | ICU | Case series | Sepsis and bloodstream infections | 32 | CRP, lactate | Not specified | Non-comparative | Prognostic value of CRP in patients with sepsis |
| Talebi-Taher et al.47 | 2014 | Iran | Emergency department | Prospective | Sepsis and bloodstream infections | 150 | CRP | PCT: 0.5 ng/mL; IL-6: 10 pg/µL, ESR: 17 mm/h for men and 25 mm/h for women; CRP ≥12 mg/L | ESR, PCT, IL-6 | Diagnostic value of CRP in elderly patients with sepsis |
| Premaratna et al.48 | 2015 | Sri Lanka | Inpatient | Prospective | Sepsis and bloodstream infections | 40 | CRP | Not specified | Non-comparative | Diagnostic value of CRP for sepsis in patients with dengue fever |
| Gupta et al.49 | 2019 | India | Inpatient | Prospective | Sepsis and bloodstream infections | 305 | PCT | >0.5 ng/mL | Non-comparative | Diagnostic value of PCT in patients with sepsis |
| Sinha et al.50 | 2011 | India | ICU | Prospective | Sepsis and bloodstream infections | 40 | PCT | >0.5 ng/mL | Non-comparative | Diagnostic value of PCT in patients with sepsis |
| Ghorbani51 | 2009 | Iran | Emergency department | Cross-sectional | Sepsis and bloodstream infections | 100 | PCT | 0.05 ng/mL | Non-comparative | Diagnostic value of PCT in patients with sepsis |
| Jain et al.52 | 2014 | India | ICU | Prospective | Sepsis and bloodstream infections | 54 | PCT | Not specifed | CRP | Prognostic value of PCT in patients with sepsis |
| Rebello et al.53 | 2017 | India | Inpatient, ICU | Prospective observational | Sepsis and bloodstream infections | 112 | PCT | Not applicable | Non-comparative | Prognostic value of PCT in patients with sepsis |
| Mehta et al.54 | 2016 | India | ICU | Retrospective observational study | Sepsis and bloodstream infections | 100 | PCT, BNP | Not specified | Non-comparative | Prognostic value of PCT and NTproBNP in ICU-admitted patients |
| Dolatabadi et al.55 | 2015 | Iran | Emergency department | Cross sectional | Sepsis and bloodstream infections | 170 | PCT | 0.5 ng/mL | PCT before, 6 h after and 24 h after ATBs | Prognostic value of PCT in patients with sepsis |
| Najafi et al.56 | 2015 | Iran | ICU | Prospective, single-blind randomized | Sepsis and bloodstream infections | 60 | PCT | Not specified | No biomarker | Prognostic value of PCT in patients with sepsis |
| Lubell et al.57 | 2015 | Thailand, Cambodia, Laos | Inpatient/outpatient | Retrospective | Undifferentiated fever | 1372 | CRP, PCT | Not specified | Non-comparative | Diagnostic value of CRP, PCT in patients with undifferentiated fever |
| Wangrangsimakul et al.58 | 2018 | Thailand | Inpatient | Prospective | Undifferentiated fever | 200 | CRP, PCT | Not specified | Non-comparative | Diagnostic value of CRP, PCT in patients with undifferentiated fever |
| Phatlhane et al.59 | 2016 | S Africa | Outpatient | Cross-sectional | HIV | 110 | PCT, IL-6, LBP, CRP, IgG, albumin | Not specified | Non-comparative | Prognostic value of CRP, PCT in HIV-infected patients with diarrhoea |
| Ramana et al.60 | 2013 | India | Outpatient | Prospective | HIV | 250 | CRP | Not specified | ESR, TLC, Hb, AEC | Prognostic value of CRP in HIV-infected patients with diarrhoea |
| Zulu et al.61 | 2008 | Zambia | Not specified | Retrospective | HIV | 80 | CRP, TNFR p55, MIF, IL-6, IL-12, IFN-γ | 0.2 mg/L | Non-comparative | Prognostic value of CRP and cytokines in HIV-infected patients with diarrhoea |
| Koethe et al.62 | 2011 | Zambia | Outpatient | Prospective observational cohort | HIV | 142 | CRP, albumin, ferritin | Not specified | Non-comparative | Prognostic value of CRP and other biomarkers in patients with malnutrition and advanced HIV |
| Bedell et al.63 | 2018 | Malawi | Outpatient | Retrospective | HIV | 469 | CRP | 3.0 - 480 mg/L | Non-comparative | Diagnostic value of CRP for tuberculosis and bloodstream infections in HIV-infected patients |
| Ledwaba et al.64 | 2012 | S Africa | Outpatient | Case-control | HIV | 187 | CRP, IL-6, D-dimer | CRP 1 mg/mL, IL-6 0.428-8.870 pg/L, D-Dimer 0.5 mg/L | Non-comparative | Prognostic value of CRP and other biomarkers in patients with advanced HIV |
| Woodd et al.65 | 2016 | Zambia, Tanzania | Outpatient | Prospective | HIV | 1815 | CRP | Not specified | Non-comparative | Prognostic value of CRP in patients with advanced HIV |
| Haddow et al.66 | 2012 | S Africa | Outpatient | Prospective | HIV | 498 | CRP | Not specified | Non-comparative | Prognostic value of CRP in patients with advanced HIV |
| Sereti et al.67 | 2020 | Kenya, Thailand, USA | Outpatient | Prospective observational | HIV | 506 | CRP | Not specified | Non-comparative | Prognostic value of CRP in patients with advanced HIV |
| Kroeze et al.68 | 2019 | Kenya, Nigeria, South Africa, Uganda, Zambia | Not specified | Retrospective | HIV | 398 | sCD14, sCD163, CRP, CXCL10, IL-6, CCL2, CXCL | Not specified | Non-comparative | Prognostic value of CRP in patients with advanced HIV |
| Kiefer et al.69 | 2018 | Rwanda | Outpatient | Prospective | HIV | 695 | CRP, D-dimer, transthyretin | Not specified | Non-comparative | Prognostic value of CRP in patients with advanced HIV |
| Chegou et al.70 | 2016 | S Africa, Uganda, Gambia, Malawi, Namibia | Outpatient | Prospective | 716 | TB | CRP, PCT | Not specified | IL-1ra, TGF-α, IFN-γ, IP-10, TNF-α, IFN-α2, VEGF, MMP-2, MMP-9, ApoA-1, Apo-CIII, transthyretin, CFH, SAA, SAP, fibrinogen, ferritin, TPA, haptoglobulin, α-2-macroglobulin | Diagnostic value of CRP, PCT and other biomarkers in patients with pulmonary TB |
| Jacobs et al.71 | 2016 | S Africa | Outpatient | Prospective | TB | 55 | CRP, PCT | Not specified | NCAM, SAP, IL-1β, sCD40L, IL-13 and Apo A-1 | Diagnostic value of CRP, PCT and other biomarkers in patients with pulmonary TB |
| Berrocal-Almanza et al.72 | 2016 | India | Outpatient | Prospective cohort | TB | 119 | CRP | Not specified | S100A12, sRAGE, esRAGE, HMGB-1, TNF-α, IFN-γ |
Prognostic value of CRP in TB |
| Worodria et al.73 | 2011 | Uganda | Outpatient | Prospective | TB | 247 | CRP | <5 mg/L | Non-comparative | Diagnostic value of CRP, in HIV patients with pulmonary TB |
| Yoon et al.74 | 2017 | Uganda | Outpatient | Prospective | TB | 1237 | CRP | >10 mg/L | Non-comparative | Diagnostic value of CRP for TB in people living with HIV/AIDS |
| Rajopadhye et al.75 | 2017 | India | Not specified | Prospective | TB | 50 | CRP, NO, TBARS, SOD | Not specified | Non-comparative | Diagnostic value of CRP for TB in people living with HIV/AIDS |
| Yoon et al.76 | 2019 | Uganda | Outpatient | Prospective | TB | 1245 | CRP | 8 mg/L | No biomarker (symptom-based screening) | Diagnostic value of CRP for TB in |
| Ciccacci et al.77 | 2019 | Mozambique | Outpatient | Retrospective | TB | 155 | CRP | 10 mg/L | Neopterin, IP-10 | Diagnostic value of CRP for TB in people living with HIV/AIDS |
| Olsson et al.78 | 2019 | Ethiopia | Outpatient | Prospective | TB | 260 | CRP, PCT | Not specified | CCL5, IP-10, IL-6, IL-12, IL-18, IL-27, IFN-γ, suPAR | Diagnostic value of CRP, PCT for TB in people living with HIV/AIDS |
| Wilson et al.79 | 2011 | S Africa | Outpatient | Prospective | TB | 364 | CRP | Method 1: 0–8 mg/L Method 2: 0–5 mg/L |
Non-comparative | Diagnostic value of CRP for TB in people living with HIV/AIDS |
| Lawn et al.80 | 2013 | S Africa | Outpatient | Prospective | TB | 496 | CRP | 50 mg/L | Non-comparative | Diagnostic and prognostic value of CRP for TB in people living with HIV/AIDS |
| Farr et al.81 | 2018 | Uganda | Not specified | Retrospective | TB | 865 | CRP, cytokines | Not specified | Non-comparative | Diagnostic value of CRP and cytokines for TB in people living with HIV/AIDS |
| de Oliveira et al.82 | 2019 | Brazil | Not specified | Retrospective | TB | 50 | CRP | <1 mg/dL | No biomarker (3D reconstructed lung imaging) | Prognostic value of CRP in TB |
| Wilson et al.83 | 2018 | S Africa | Outpatient | Prospective cohort | TB | 421 | CRP | Not specified | Non-comparative | Prognostic value of CRP in TB |
| Rasmussen et al.84 | 2011 | Guinea-Bissau | Outpatient | Prospective | TB | 218 | PCT CRP |
PCT 0.02–50 ng/mL CRP not specified |
CRP | Prognostic value of PCT in TB |
| Janssen et al.85 | 2017 | S Africa | Inpatient | Prospective cohort | TB | 60 | PCT | Not specified | Non-comparative | Prognostic value of PCT for TB among HIVpatients |
| Soedarsono et al.86 | 2019 | Indonesia | Outpatient | Prospective cohort | TB | 30 | CRP | 0.3−0.5 mg/dL | Non-comparative | Prognostic value of CRP in TB |
| Epelboin L et al.87 | 2013 | French Guiana | Emergency department | Retrospective | Undifferentiated febrile illness | 416 | CRP | >5 mg/L | No biomarker (clinical score) | Diagnostic value of CRP for malaria and dengue fever |
| Sanchez-Arcila et al.88 | 2014 | Brazil | Outpatient | Prospective | Malaria | 264 | CRP | 0.01–320 μg/mL | Non-comparative | Diagnostic value of CRP for malaria and intestinal parasites coinfection |
| Peto et al.89 | 2016 | Cambodia | Outpatient | Prospective | Malaria | 328 | CRP | Not applicable | Non-comparative | Diagnostic value of CRP for malaria |
| Gibson and Huddle90 | 1998 | Malawi | Outpatient | Prospective | Malaria | 152 | CRP | Not specified | Non-comparative | Diagnostic value of CRP for malaria in pregnancy |
| Mockenhaupt et al.91 | 2000 | Ghana | Outpatient | Cross-sectional | Malaria | 530 | CRP | >0.6 mg/dL | Non-comparative | Diagnostic value of CRP for malaria in pregnancy |
| Hinderaker et al.92 | 2002 | Tanzania | Outpatient | Prospective | Malaria | 2547 | CRP, ferritin, iron, TIBC, cobalamin, folate, vitamin A, LDH, TFsat | 10 mg/L | Non-comparative | Diagnostic value of CRP for malaria in pregnancy |
| Adegnika et al.93 | 2006 | Gabon | Inpatient | Prospective | Malaria | 145 | CRP | 0.5 −6 mg/L | Non-comparative | Prognostic value of CRP for malaria in pregnancy |
| Conroy et al.94 | 2011 | Malawi | Inpatient | Case-control | Malaria | 465 | CRP, C3a, C5a, angiopoietin-1, -2, sTie-2, sEndoglin, VEGF, sFlt-1, tissue factor, leptin | Not specified | Non-comparative | Prognostic value of CRP for malaria |
| Paul et al.95 | 2012 | India | Outpatient | Prospective | Malaria | 71 | CRP | ≤5 mg/L | Non-comparative | Prognostic value of CRP for malaria |
| Mendonca et al.96 | 2013 | Brazil | Outpatient | Retrospective | Malaria | 530 | CRP, liver transaminases, bilirubins, creatinine, fibrinogen, SOD-1, HO-1, cytokines, chemokines | Not specified | Non-comparative | Prognostic value of CRP for malaria |
| Bhardwaj, et al.97 | 2019 | India | Inpatient | Prospective | Malaria | 96 | CRP | Not applicable | Non-comparative | Prognostic value of CRP for malaria |
| Lima-Junior et al.98 | 2012 | Brazil | Outpatient | Prospective | Malaria | 71 | CRP, NO, platelets, neutrophils | 0.01–320 μg/mL | Non-comparative | Prognostic value of CRP for malaria |
AEC, absolute eosinophil count; ApoA-1, apolipoprotein A1; Apo-CIII, apolipoprotein CIII; ARDS, acute respiratory distress syndrome; ATB, antibiotic; BNP, brain natriuretic peptide; CCL, C motif chemokine ligand; CFH, complement factor H; COPD, chronic obstructive pulmonary disease; CRP, C-reactive protein; CXCL10, CXC motif chemokine 10; CXR, chest X-ray; ESR, erythrocyte sedimentation rate; esRAGE, endogenous secretory receptor for advanced glycation end products; Hb, haemoglobin; HMGB-1, high-mobility group protein 1; HO-1, haem oxygenase 1; IL-1ra, interleukin-1 receptor antagonist; IP-10, interferon gamma inducible protein 10; LBP, lipopolysaccharide-binding protein; LDH, lactate dehydrogenase; Lyt A, autolysin A; MIF, macrophage migration inhibitory factor; MMP, matrix metalloproteinase; MR-proANP, midregional pro-atrial natriuretic peptide; NCAM, neural cell adhesion molecule; NO, nitric oxide; PCT, procalcitonin; S100A12, S100 calcium binding protein A12; SAA, serum amyloid A; SAP, serum amyloid P-component; sCD, soluble cluster of differentiation; sCD40L, soluble CD40 ligand; sFlt-1, soluble Fms-like tyrosine kinase-1; SOD-1, superoxide dismutase 1; sRAGE, soluble receptor for advanced glycation end products; sTie-2, soluble tyrosine-kinase-receptor; suPAR, soluble urokinase-type plasminogen activator receptor; TBARS, thiobarbituric acid reactive substances; TFsat, transferrin saturation; TGF-α, transforming growth factor-α; TIBC, total iron-binding capacity; TLC, total leukocyte count; TNFR p55, tumour necrosis factor receptor p55; TPA, tissue polypeptide antigen; VEGF, vascular endothelial growth factor.
Results
CNS infections
Diagnosis
CRP was higher in patients with bacterial meningitis (mean 209.25 mg/L) compared with viral meningitis (mean 67.05 mg/L) (Thailand).12 PCT was higher in bacterial compared with aseptic meningitis (Egypt).13
Treatment response
Patients with bacterial meningitis who responded to treatment had a decline in PCT after 24 h whereas those failing treatment had an increase (Iran).14
Cardiovascular infections
Diagnosis
Among patients with suspected endocarditis, elevated CRP was 97.9% sensitive for bacterial endocarditis (negative predictive value 87.5%) (South Africa).15 In Brucella endocarditis, the average CRP was 22.1 mg/L and, together with ESR (erythrocyte sedimentation rate), it was the only significant altered blood test in the study cohort (Turkey).16
Prognosis
Infective endocarditis with a CRP greater than 40 mg/L had a sensitivity of 76% and a specificity of 99% for complications (including need for urgent surgery due to embolic phenomena), and was associated with increased in-hospital mortality and 6 month mortality (India).17
Respiratory tract infections
Diagnosis, outpatients
A CRP below 20 mg/L was associated with a low likelihood of acute bacterial aetiology requiring antibiotics, and using this cut-off decreased antibiotic prescribing by 14% (Vietnam).18 Using a threshold of 40 mg/L led to a reduction in antibiotic use with no difference in clinical outcomes (Myanmar, Thailand).9
Diagnosis, inpatients
A CRP above 71 mg/L was associated with pneumococcal infection in patients admitted with community-acquired pneumonia (CAP) (Togo).19 PCT was mildly elevated among patients with acute chronic obstructive pulmonary disease (COPD) exacerbations compared with COPD patients without exacerbations (mean 0.27 versus 0.07 ng/mL) (Iran).20 Among people living with HIV (PLWH), PCT was higher in bacterial pneumonia compared with TB and Pneumocystis jirovecii pneumonia (PJP) (South Africa).21 A more recent study found significant differences in both biomarkers among bacterial pneumonia, TB and PJP in PLWH, but with too much overlap between groups to reliably differentiate cause of infection (South Africa).22
Prognosis
CRP and PCT were significantly higher in non-survivors compared with survivors (mean 76 versus 36 mg/L, and 2 versus 1.08 ng/mL) (India).23 CRP was higher in CAP among PLWH compared with HIV-negative patients (59.5 versus 20.1 mg/L), and higher CRP was associated with short-term mortality (Uganda).24 High mean admission CRP indicated risk of bacterial pneumonia (above 180 mg/L), septic shock (above 210 mg/L) and requirement of mechanical ventilation (above 280 mg/L), and lack of CRP decline within 3 days of hospitalization was associated with high risk of complications (Chile).25 Higher PCT was associated with risk of death in bacterial CAP (13.2 versus 3.4 ng/mL in survivors) (Egypt).26 PCT elevation greater than 0.5 ng/mL was an independent predictor of mortality for PLWH with pneumonia (Uganda).27 PCT was a significant predictor of in-hospital mortality in ventilator-acquired pneumonia (VAP) (Brazil).28
Treatment response
In patients with VAP responding to treatment, PCT and CRP began declining at 48 and 72 h, respectively (Iran).29 The combination of a low clinical pulmonary infection score (a validated score to predict the likelihood of VAP) plus a PCT below 0.5 ng/mL on Day 8 was a safe indication for discontinuation of antibiotics in VAP (Thailand).30 Patients with VAP and serial PCT measurements had on average 1.25 fewer days on antibiotics without increased mortality when compared with controls when discontinuation was based on 80% decrease to below 0.5 ng/mL, or any decrease below 0.25 ng/mL (Malaysia).31
Intra-abdominal infections including gastrointestinal and hepatobiliary
Diagnosis
Elevated CRP was an independent predictor of bacterial infection among admitted patients with liver cirrhosis (Egypt).32 Elevated PCT was associated with abdominal sepsis (most commonly appendicitis) (Mexico).33 Patients with abdominal sepsis had higher CRP than those with pulmonary sepsis (mean 178 versus 149 mg/L) (Brazil).34
Genitourinary
Diagnosis
CRP levels in pyelonephritis were often greater than 200 mg/L and were not affected by the presence of diabetes (India).35 CRP levels in lower urinary tract infection (UTI) were not altered by pregnancy (Cameroon).36 In males admitted with UTI the median CRP level was 22.3 mg/L (India).37
Musculoskeletal infections
Diagnosis
Patients with acute osteomyelitis and joint infections caused by MRSA had CRP levels greater than 13.9 mg/L (India).38 Patients with septic arthritis all had CRP levels greater than 75 mg/L (mean 132.5 mg/L) (India).39
Treatment response
CRP was elevated (mean 257 mg/L, range 60–980 mg/L) in patients with spondylodiscitis (bacterial, brucellar, mycobacterial, culture-negative) of more than 4 months duration, and decreased after 2 weeks of treatment to indicate response (Egypt).40 CRP decreased with treatment in severe odontogenic infections (India).41
Skin and soft tissue infections
Diagnosis
A PCT above 0.5 ng/mL had moderate sensitivity (54%) and excellent specificity (100%) for the diagnosis of infected diabetic foot ulcers, and outperformed CRP, WBC count and erythrocyte sedimentation rate in this setting (India).42 In patients with severe snake bites, PCT did not increase over time (0.29 ng/mL), supporting clinical suspicion of toxin-mediated inflammation and withholding antibiotics in patients with local manifestation mimicking those due to bacterial infection (India).43
Sepsis and bloodstream infections
Diagnosis, CRP
CRP was elevated on average to 67 mg/L in septic patients in their first day of admission to the ICU (Egypt).44 Patients with sepsis in the setting of UTI, pneumonia or soft tissue infection had a mean CRP of 89 mg/L (Iran).45 Patients with septic shock from pneumonia or UTI had a mean CRP of 29 mg/L (Pakistan).46 Patients older than 65 years with sepsis had a mean CRP of 58 mg/L (Iran).47 Prolonged fever in dengue (>5 days) with subsequent bacteraemia was associated with higher peak CRP (mean 600 compared with 160 mg/L in prolonged fever without bacteraemia) (Sri Lanka).48
Diagnosis, PCT
PCT was higher in sepsis with confirmed positive culture in blood, urine, sputum or other body fluid (2.2 versus 1.3 ng/mL), and a cut-off of 2.2 ng/mL was 98% sensitive and 89% specific for culture positivity (India).49 A PCT cut-off of 2 ng/mL was moderately sensitive (86%) and specific (95%) for sepsis (India).50 A PCT greater than 10 ng/mL (mean 30 ng/mL) was associated with septic shock, and higher PCT was associated with positive blood cultures (Iran).51
Prognosis
A PCT greater than 7 ng/mL on ICU admission was associated with mortality (India).52 Non-survivors in sepsis had rising PCT on Days 1–5 compared with those surviving (India).53 Among patients with acute respiratory distress syndrome from pneumonia and malaria, PCT was lower in survivors versus non-survivors (1.1 versus 2 ng/mL), as was CRP (36 versus 76 mg/L) (India).23 PCT did not predict all-cause mortality in an ICU population admitted for infectious and non-infectious conditions (India).54
Treatment response
Patients responding to treatment had a decrease in mean PCT from 9 to 5 ng/mL at 24 h (Iran).55 No difference in mortality was noted compared with standard care by withholding antibiotics in patients with suspected sepsis admitted to ICU with a low value of PCT at admission (less than 2 ng/mL) and serial follow-up measurements (Iran).56
Undifferentiated febrile illness
Diagnosis
A CRP cut-off of 10 mg/L was 95% sensitive and 49% specific for bacterial versus viral illness; increasing the cut-off to 20 mg/L lowered the sensitivity (86%) and increased the specificity (67%), and a PCT cut-off of 0.1 ng/mL was 90% sensitive and 39% specific (Laos, Thailand).57 In patients with undifferentiated fever on presentation, a low CRP (median 12.5 mg/L) was associated with viral compared with bacterial aetiology (median 139.5 mg/L). Median PCT in viral and bacterial undifferentiated febrile illness was 0.3 and 2.6 ng/mL, respectively (Thailand).58
HIV
This section includes publications addressing CRP and PCT related to HIV itself. Data on specific other conditions in PLWH have been described in other sections.
General issues and prognosis
PCT levels were not elevated in asymptomatic untreated HIV infection compared with controls (South Africa).59 A CRP above 12 mg/L was associated with a CD4 count of <350 cells/mm3 (India).60 Elevated CRP was linked with disease severity in AIDS-related diarrhoea, and associated with short-term mortality in PLWH (Zambia).61 A CRP above 15 mg/L was associated with increased 90 day mortality among malnourished adults initiating ART (Zambia).62 A CRP above 10 mg/L at the time of ART initiation was associated with TB, bloodstream infection and early mortality (Malawi).63 A pre-ART elevated CRP was a strong predictor of death in advanced HIV (South Africa).64 The mortality rate in malnourished PLWH increased with baseline CRP and was five times higher with a measurement above 160 mg/L compared with below 10 mg/L (Tanzania, Zambia).65 Elevated baseline CRP was associated with immune reconstitution inflammatory syndrome and increased mortality after ART initiation (Thailand, Kenya, South Africa).66,67
Treatment response
PLWH had elevated CRP prior to initiating ART compared with HIV-negative controls; this varied by country of origin, and post-ART CRP declined but did not completely normalize (Kenya, South Africa, Nigeria, Uganda, Zambia).68 Higher CRP was associated with HIV infection with more advanced immune suppression, but ART was not associated with a decrease in CRP (Rwanda).69
TB
Diagnosis
Patients with TB had elevated CRP compared with controls (South Africa, Malawi, The Gambia, Namibia, Uganda),70 with a sensitivity of 82% and specificity of 90% when using a cut-off of 9 mg/L (South Africa).71 Mean baseline CRP in smear-positive TB was elevated compared with controls (6.74 compared with 3.18 mg/L) (India).72
Diagnosis, PLWH
A CRP above 5 mg/L was associated with TB unmasking at ART initiation (Uganda).73 CRP above 10 mg/L was 89% sensitive and 72% specific for TB in PLWH with CD4 <350 cells/mm3 (Uganda) and was associated with TB (Malawi).63,74 CRP was elevated in TB, and more so in HIV-positive (mean 44.7 mg/L) than HIV-negative (mean 3.67 mg/L) patients, compared with controls (1.4 mg/L) (India).75 Using a CRP above 8 mg/L as a criterion for TB testing with GeneXpert in PLWH decreased cost and number of tests by around 50% without lowering overall sensitivity (Uganda).76 Average CRP among GeneXpert-positive PLWH was 15.7 mg/L compared with 1.1 mg/L in GeneXpert-negative controls (Mozambique).77 PLWH with TB had higher CRP levels if smear- or GeneXpert-positive (50 mg/L and 49.5 mg/L, respectively) compared with patients who were only positive by culture (9.1 mg/L), and PCT was low amongst all patients (Ethiopia).78 A CRP above 5 mg/L had sensitivity of 98% and specificity of 59% for smear-negative TB in a population with high HIV prevalence (South Africa).79 A CRP below 1.5 mg/L excluded TB among a cohort of PLWH (South Africa).80 Average CRP among PLWH with TB admitted to the hospital was 140 mg/L compared with 69 mg/L in PLWH without TB (Uganda).81 CRP elevation correlated with volume of TB-affected lung on 3D CT reconstructions (Brazil).82
Prognosis
In PLWH with TB, a CRP above 50 mg/L was associated with higher mortality, disseminated disease and increased mycobacterial load (South Africa).80 Failure of CRP to decrease to below 55% of baseline value at Week 2 predicted hospitalization or death among symptomatic TB patients initiating treatment (South Africa).83 CRP correlated with disease severity and mortality (Guinea Bissau).84 Among PLWH with TB, non-survivors had higher PCT than survivors (mean 8.28 versus 1.31 ng/mL) (South Africa).85
Treatment response
Mean baseline CRP in smear-positive TB decreased to 4.4 mg/L at 2 months of treatment (India).72 After 2 months of treatment for smear-positive TB, the mean CRP decreased from 64 to 12 mg/L (Indonesia).86 CRP declined with treatment (South Africa).71
Malaria
Diagnosis
CRP was higher in malaria than dengue fever, and a cut-off of 5 mg/L had a sensitivity of 99.5% and specificity of 35% (French Guiana).87 Patients with intestinal protozoan and malarial coinfection did not have increased CRP compared with those infected with malaria alone (Brazil).88 Median CRP was similar in patients with subclinical parasitaemia (0.66 mg/L) and healthy matched controls (0.52 mg/L) (Thailand).89
Diagnosis, pregnancy
Of asymptomatic pregnant women, 31.3% tested positive for malaria, yet only 6% of these had a CRP above 15 mg/L (Malawi).90 Of 528 pregnant women, 51% had a CRP above 6 mg/L, and 82% of these had a positive test for malaria compared with 31% in the total cohort (Ghana).91 A higher CRP level was associated with a degree of anaemia but not with a microscopic diagnosis of malaria in pregnant women (Tanzania).92 CRP was elevated in microscopic but not submicroscopic Plasmodium falciparum malaria (average 34 versus 7 mg/L) (Gabon).93 In patients with asymptomatic placental P. falciparum malaria, average CRP was 60.2 compared with 18.5 mg/L in those without infection, and a cut-off of 30.5 mg/L had a sensitivity of 73.9% and a specificity 68.3% (Malawi).94
Prognosis
In patients with P. falciparum malaria, CRP increase was associated with severity of organ dysfunction and mortality (mean 47.1 mg/L compared with 16.4 mg/L in survivors), and a CRP greater than 35 mg/L had a sensitivity above 95% for mortality (India).95 Mean CRP varied with severity of Plasmodium vivax malaria: deaths 34.4 mg/L, severe malaria survivors 13.2 mg/L, uncomplicated cases 15.5 mg/L, asymptomatic cases 7.9 mg/L, and endemic controls 5.2 mg/L (Brazil).96 The CRP level was slightly higher in severe P. falciparum malaria (20 mg/L) compared with uncomplicated malaria (14 mg/L) and healthy controls (1 mg/L) (India).97
Treatment response
Of symptomatic patients with P. vivax or P. falciparum malaria, 87% had elevated CRP (mean 27.8 mg/L), which decreased with successful therapy (mean 3 mg/L on Day 15) (Brazil).98
Discussion
CRP and PCT both have the potential to improve clinical outcomes and antimicrobial stewardship in LMICs. Notable areas where available data are particularly promising are the diagnosis of bacterial respiratory infections, sepsis and TB, and in monitoring response to treatment in these infections.
In the outpatient setting, using low CRP measurements to support withholding antibiotics for respiratory symptoms is perhaps the most straightforward specific area of implementation, although it would require POCT in order to be practically useful and have added value compared with clinical indicators alone. High concentrations of both CRP and PCT could also help identify patients at risk of adverse outcome of respiratory tract infection who are in need of admission or closer outpatient follow-up.
In the inpatient setting, CRP and PCT should be interpreted in the light of clinical findings and ideally together with a range of additional tests such as culture of blood and respiratory specimens and biochemical markers of organ dysfunction. The absence of these additional paraclinical safety nets in many LMICs is an important limitation in the implementation of CRP/PCT for both diagnosis and monitoring purposes. This is particularly important in the diagnostic workup and treatment monitoring in sepsis, where mortality is high and the consequence of withholding effective treatment can be fatal.
In TB, CRP has the specific potential to rationalize the use of more expensive diagnostic tests, and WHO recently recommended CRP as one of the screening tools for TB.99 The cost-effectiveness of this strategy should be evaluated.
There are important limitations to this review. The general lack of well-defined cut-off values validated in more than one setting will be an important limiting factor in the practical implementation of both CRP and PCT. Half of the included publications were carried out in only three countries—South Africa, India and Uganda (Table 1). In addition, there was a high degree of methodological heterogeneity between studies. Some studies used different definitions of normal range measurements, most studies did not include laboratory cut-offs for normal, different assays were used, some studies used non-standard units when reporting CRP and PCT, and some did not include any units and authors had to be contacted for this information. Finally, studies with a focus on COVID-19 were excluded from the review. The impact of the pandemic may change the future generalizability of studies of respiratory tract infections and may also mean less available funding for implementation of new laboratory tests in LMICs.
Despite numerous limitations, there were consistent results across many areas, and almost half of identified publications were from the last 5 years, perhaps indicating increasing interest and availability of CRP and PCT in LMICs. Consensus across stakeholders regarding target-conditions and laboratory standards, in particular cut-off values, would support the quality and applicability of future evidence.
Supplementary Material
Contributor Information
Amin Lamrous, Médecins Sans Frontières, Operational Center Barcelona, Barcelona, Spain.
Ernestina Repetto, Médecins Sans Frontières, Operational Center Geneva, Geneva, Switzerland; Infectious Diseases Department, Université Libre de Bruxelles (ULB), CHU Saint-Pierre, Brussels, Belgium.
Tim Depp, Emergency Medicine, University of South Carolina School of Medicine, Greenville, SC, USA.
Carolina Jimenez, Médecins Sans Frontières, Operational Center Paris, Paris, France.
Arlene C Chua, Medical Department, Médecins Sans Frontières—International, Geneva, Switzerland.
Rupa Kanapathipillai, Médecins Sans Frontières, Operational Center Paris, Paris, France.
Tomas O Jensen, Médecins Sans Frontières, Operational Center Paris, Paris, France; CHIP Center of Excellence for Health, Immunity, and Infections, Rigshospitalet, Copenhagen, Denmark.
Funding
No funding was provided for this work.
Transparency declarations
We have no conflicts of interest.
Supplementary data
Table S1 is available as Supplementary data at JAC-AMR Online.
References
- 1. Sridharan P, Chamberlain RS. The efficacy of procalcitonin as a biomarker in the management of sepsis: slaying dragons or tilting at windmills? Surg Infect (Larchmt) 2013; 14: 489–511. 10.1089/sur.2012.028 [DOI] [PubMed] [Google Scholar]
- 2. Lopez AF, Cubells CL, García JGet al. . Procalcitonin in pediatric emergency departments for the early diagnosis of invasive bacterial infections in febrile infants: results of a multicenter study and utility of a rapid qualitative test for this marker. Pediatr Infect Dis J 2003; 22: 895–904. 10.1097/01.inf.0000091360.11784.21 [DOI] [PubMed] [Google Scholar]
- 3. Zhang Y, La M, Sun Jet al. . Diagnostic value and prognostic significance of procalcitonin combined with C-reactive protein in patients with bacterial bloodstream infection. Comput Math Methods Med 2022; 2022: 6989229. 10.1155/2022/6989229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Eriksson UK, van Bodegom D, May Let al. . Low C-reactive protein levels in a traditional West-African population living in a malaria endemic area. PLoS One 2013; 8: e70076. 10.1371/journal.pone.0070076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lee CC, Kwa ALH, Apisarnthanarak Aet al. . Procalcitonin (PCT)-guided antibiotic stewardship in Asia-Pacific countries: adaptation based on an expert consensus meeting. Clin Chem Lab Med 2020; 58: 1983–91. 10.1515/cclm-2019-1122 [DOI] [PubMed] [Google Scholar]
- 6. Lubell Y, Althaus T, Blacksell SDet al. . Modelling the impact and cost-effectiveness of biomarker tests as compared with pathogen-specific diagnostics in the management of undifferentiated fever in remote tropical settings. PLoS One 2016; 11: e0152420. 10.1371/journal.pone.0152420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shafiq N, Gautam V, Pandey AKet al. . A meta-analysis to assess usefulness of procalcitonin-guided antibiotic usage for decision making. Indian J Med Res 2017; 146: 576–84. https://doi.org/10.4103%2Fijmr.IJMR_613_15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yebyo H, Medhanyie AA, Spigt Met al. . C-reactive protein point-of-care testing and antibiotic prescribing for acute respiratory tract infections in rural primary health centres of North Ethiopia: a cross-sectional study. NPJ Prim Care Respir Med 2016; 26: 15076. 10.1038/npjpcrm.2015.76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Althaus T, Greer RC, Swe MMMet al. . Effect of point-of-care C-reactive protein testing on antibiotic prescription in febrile patients attending primary care in Thailand and Myanmar: an open-label, randomised, controlled trial. Lancet Glob Health 2019; 7: e119–31. 10.1016/S2214-109X(18)30444-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. van Griensven J, Cnops L, De Weggheleire Aet al. . Point-of-care biomarkers to guide antibiotic prescription for acute febrile illness in Sub-Saharan Africa: promises and caveats. Review Open Forum Infect Dis 2020; 7: ofaa260. 10.1093/ofid/ofaa260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cochrane LMIC filters. Cochrane Collaboration. https://epoc.cochrane.org/lmic-filters
- 12. Sirijaichingkul S, Tiamkao S, Sawanyawisuth Ket al. . C reactive protein for differentiating bacterial from aseptic meningitis in Thai patients. J Med Assoc Thai 2005; 88: 1251–6. [PubMed] [Google Scholar]
- 13. Abdelkader NA, Mahmoud WA, Saber SM. Serum procalcitonin in Egyptian patients with acute meningitis and a negative direct cerebrospinal fluid examination. J Infect Public Health 2014; 7: 106–13. 10.1016/j.jiph.2013.07.005 [DOI] [PubMed] [Google Scholar]
- 14. Alavi SM, Shokri S. Can serum procalcitonin measurement help monitor the treatment of acute bacterial meningitis? A prospective study. Caspian J Intern Med 2012; 3: 382–5. [PMC free article] [PubMed] [Google Scholar]
- 15. Koegelenberg CF, Doubell AF, Orth Het al. . Infective endocarditis: improving the diagnostic yield. Cardiovasc J S Afr 2004; 15: 14–20. 10.1093/qjmed/hcg028 [DOI] [PubMed] [Google Scholar]
- 16. Inan MB, Eyileten ZB, Ozcinar Eet al. . Native valve Brucella endocarditis. Clin Cardiol 2010; 33: E20–6. 10.1002/clc.20606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mohanan S, Gopalan Nair R, Vellani Het al. . Baseline C-reactive protein levels and prognosis in patients with infective endocarditis: a prospective cohort study. Indian Heart J 2018; 70Suppl 3: S43–S9. 10.1016/j.ihj.2018.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Do NT, Ta NT, Tran NTet al. . Point-of-care C-reactive protein testing to reduce inappropriate use of antibiotics for non-severe acute respiratory infections in Vietnamese primary health care: a randomised controlled trial. Lancet Glob Health 2016; 4: e633–41. 10.1016/S2214-109X(16)30142-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Blake A, Njanpop-Lafourcade BM, Telles JNet al. . Evaluation of chest radiography, lytA real-time PCR, and other routine tests for diagnosis of community-acquired pneumonia and estimation of possible attributable fraction of pneumococcus in northern Togo. Epidemiol Infect 2017; 145: 583–94. 10.1017/S0950268816002211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Borsi H, Nia EP, Mal-Amir MDet al. . Relationship between serum procalcitonin level and chronic obstructive pulmonary disease. J Family Med Prim Care 2019; 8: 738–40. https://doi.org/10.4103%2Fjfmpc.jfmpc_468_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nyamande K, Lalloo UG. Serum procalcitonin distinguishes CAP due to bacteria, Mycobacterium tuberculosis and PJP. Int J Tuberc Lung Dis 2006; 10: 510–15. [PubMed] [Google Scholar]
- 22. Mendelson F, Griesel R, Tiffin Net al. . C-reactive protein and procalcitonin to discriminate between tuberculosis, Pneumocystis jirovecii pneumonia, and bacterial pneumonia in HIV-infected inpatients meeting WHO criteria for seriously ill: a prospective cohort study. BMC Infect Dis 2018; 18: 399. 10.1186/s12879-018-3303-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sharma SK, Gupta A, Biswas Aet al. . Aetiology, outcomes and predictors of mortality in acute respiratory distress syndrome from a tertiary care centre in north India. Indian J Med Res 2016; 143: 782–92. https://doi.org/10.4103%2F0971-5916.192063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang RJ, Moore J, Moisi Det al. . HIV Infection is associated with elevated biomarkers of immune activation in Ugandan adults with pneumonia. PLoS One 2019; 14: e0216680. 10.1371/journal.pone.0216680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Saldias-Penafiel F, Salinas-Rossel G, Farcas-Oksenberg Ket al. . Immunocompetent adults hospitalized for a community-acquired pneumonia: serum C-reactive protein as a prognostic marker. Rev Med Chil 2019; 147: 983–92. 10.4067/s0034-98872019000800983 [DOI] [PubMed] [Google Scholar]
- 26. El Maghraby HM, Ismail NA, Mohammed HA. Serum procalcitonin as a diagnostic and prognostic marker for bacterial community—acquired pneumonia. Egypt J Immunol 2020; 27: 37–44. [PubMed] [Google Scholar]
- 27. Tokman S, Barnett CF, Jarlsberg LGet al. . Procalcitonin predicts mortality in HIV-infected Ugandan adults with lower respiratory tract infections. Respirology 2014; 19: 382–8. 10.1111/resp.12237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Seligman R, Seligman BG, Teixeira PJ. Comparing the accuracy of predictors of mortality in ventilator-associated pneumonia. J Bras Pneumol 2011; 37: 495–503. 10.1590/S1806-37132011000400012 [DOI] [PubMed] [Google Scholar]
- 29. Kiaei BA, Ghiasi F, Moradi D. Precalcitonin and C-reactive protein as markers in response to antibiotic treatment in ventilator-associated pneumonia in intensive care unit-hospitalized patients. Adv Biomed Res 2015; 4: 240. https://doi.org/10.4103%2F2277-9175.168607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wongsurakiat P, Tulatamakit S. Clinical pulmonary infection score and a spot serum procalcitonin level to guide discontinuation of antibiotics in ventilator-associated pneumonia: a study in a single institution with high prevalence of nonfermentative gram-negative bacilli infection. Ther Adv Respir Dis 2018; 12: 1753466618760134. https://doi.org/10.1177%2F1753466618760134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mohd ZM, Mohd AHI, Saedah Aet al. . Efficacy and safety of the point-of-care procalcitonin test for determining the antibiotic treatment duration in patients with ventilator-associated pneumonia in the intensive care unit: a randomised controlled trial. Anaesthesiol Intensive Ther 2021; 53: 207–14. 10.5114/ait.2021.104300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. El-Amin H, Sabry AMM, Ahmed REet al. . Types and microbiological spectrum of infections in patients with cirrhosis: a single-centre experience in Upper Egypt. Arab J Gastroenterol 2017; 18: 159–64. 10.1016/j.ajg.2017.09.005 [DOI] [PubMed] [Google Scholar]
- 33. Godinez-Vidal AR, Veronica RH, Montero-Garcia PJet al. . Evaluation of the serum procalcitonin level as an indicator of severity and mortality in abdominal sepsis due to secondary peritonitis. Cir Cir 2019; 87: 255–9. 10.24875/ciru.18000301 [DOI] [PubMed] [Google Scholar]
- 34. Orati JA, Almeida P, Santos Vet al. . Serum C-reactive protein concentrations in early abdominal and pulmonary sepsis. Rev Bras Ter Intensiva 2013; 25: 6–11. 10.1590/S0103-507X2013000100003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Trivedi SC, Phatak SR, Trivedi RS. Retrospective comparison of clinical characteristics and in-hospital outcomes among diabetic and non-diabetic adults with acute pyelonephritis. J Clin Diagn Res 2016; 10: OC26–OC9. 10.7860/jcdr/2016/22830.8720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ndamason LM, Marbou WJ, Kuete V. Urinary tract infections, bacterial resistance and immunological status: a cross sectional study in pregnant and non-pregnant women at Mbouda Ad-Lucem hospital. Afr Health Sci 2019; 19: 1525–35. 10.4314/ahs.v19i1.26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Arjunlal TS, Deepanjali S, Manikandan Ret al. . Frequency and clinical significance of prostatic involvement in men with febrile urinary tract infection: a prospective observational study. F1000Res 2020; 9: 617. https://doi.org/10.12688%2Ff1000research.24094.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Agrawal R, Sharma D, Dhiman Pet al. . Clinical and haematological predictors of acute hematogenous methicillin resistant Staphylococcus aureus (MRSA) osteomyelitis and septic arthritis. J Orthop 2015; 12: 137–41. 10.1016/j.jor.2015.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. George J, Chandy VJ, Premnath Jet al. . Microbiological profile of septic arthritis in adults: lessons learnt and treatment strategies. Indian J Med Microbiol 2019; 37: 29–33. 10.4103/ijmm.IJMM_19_134 [DOI] [PubMed] [Google Scholar]
- 40. Waheed G, Soliman MAR, Ali AMet al. . Spontaneous spondylodiscitis: review, incidence, management, and clinical outcome in 44 patients. Neurosurg Focus 2019; 46: E10. 10.3171/2018.10.FOCUS18463 [DOI] [PubMed] [Google Scholar]
- 41. Sharma A, Gokkulakrishnan S, Shahi AKet al. . Efficacy of serum CRP levels as monitoring tools for patients with fascial space infections of odontogenic origin: a clinicobiochemical study. Natl J Maxillofac Surg 2012; 3: 148–51. https://doi.org/10.4103%2F0975-5950.111369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Umapathy D, Dornadula S, Rajagopalan Aet al. . Potential of circulatory procalcitonin as a biomarker reflecting inflammation among South Indian diabetic foot ulcers. J Vasc Surg 2018; 67: 1283–91.e2. 10.1016/j.jvs.2017.02.060 [DOI] [PubMed] [Google Scholar]
- 43. Bammigatti C, Reddy PA, Hanumanthappa Net al. . Serum procalcitonin concentration and its relationship with local manifestations after snakebites. Am J Trop Med Hyg 2019; 100: 146–9. https://doi.org/10.4269%2Fajtmh.17-0892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yousef AA, Amr YM, Suliman GA. The diagnostic value of serum leptin monitoring and its correlation with tumor necrosis factor-alpha in critically ill patients: a prospective observational study. Crit Care 2010; 14: R33. 10.1186/cc8911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mamani M, Hashemi SH, Hajilooi Met al. . Evaluation of fibronectin and C-reactive protein levels in patients with sepsis: a case-control study. Acta Med Iran 2012; 50: 404–10. [PubMed] [Google Scholar]
- 46.. Ali A, Abbasi AS, Sheikh M. Outcomes of intensive care patients having septic shock at a tertiary care hospital of Islamabad. J Ayub Med Coll Abbottabad 2017; 29: 455–61. https://inis.iaea.org/search/searchsinglerecord.aspx? recordsFor=SingleRecord&RN=48102457 [PubMed] [Google Scholar]
- 47. Talebi-Taher M, Babazadeh S, Barati M, et al. . Serum inflammatory markers in the elderly: are they useful in differentiating sepsis from SIRS? Acta Med Iran 2014; 52: 438–42. 10.4038/cmj.v60i1.7165 [DOI] [PubMed] [Google Scholar]
- 48. Premaratna R, Dissanayake D, Silva FH, et al. . Secondary bacteraemia in adult patients with prolonged dengue fever. Ceylon Med J 2015; 60: 10–12. 10.4038/cmj.v60i1.7165 [DOI] [PubMed] [Google Scholar]
- 49. Gupta S, Jaswani P, Sharma RKet al. . Procalcitonin as a diagnostic biomarker of sepsis: a tertiary care centre experience. J Infect Public Health 2019; 12: 323–9. 10.1016/j.jiph.2018.11.004 [DOI] [PubMed] [Google Scholar]
- 50. Sinha M, Desai S, Mantri Set al. . Procalcitonin as an adjunctive biomarker in sepsis. Indian J Anaesth 2011; 55: 266–70. https://doi.org/10.4103%2F0019-5049.82676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ghorbani G. Procalcitonin role in differential diagnosis of infection stages and non infection inflammation. Pak J Biol Sci 2009; 12: 393–6. 10.3923/pjbs.2009.393.396 [DOI] [PubMed] [Google Scholar]
- 52. Jain S, Sinha S, Sharma SKet al. . Procalcitonin as a prognostic marker for sepsis: a prospective observational study. BMC Res Notes 2014; 7: 458. 10.1186/1756-0500-7-458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Rebello A, Thabah MM, Dutta TKet al. . Procalcitonin levels in sepsis and its association with clinical outcome in southern India. Trop Doct 2017; 47: 331–6. 10.1177/0049475517702314 [DOI] [PubMed] [Google Scholar]
- 54. Mehta C, Dara B, Mehta Yet al. . Retrospective study on prognostic importance of serum procalcitonin and amino-terminal pro-brain natriuretic peptide levels as compared to acute physiology and chronic health evaluation IV score on intensive care unit admission, in a mixed intensive care unit population. Ann Card Anaesth 2016; 19: 256–62. https://doi.org/10.4103%2F0971-9784.179616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Dolatabadi AA, Memary E, Amini Aet al. . Efficacy of measuring procalcitonin levels in determination of prognosis and early diagnosis of bacterial resistance in sepsis. Niger Med J 2015; 56: 17–22. https://doi.org/10.4103%2F0300-1652.149165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Najafi A, Khodadadian A, Sanatkar M, et al. . The comparison of procalcitonin guidance administer antibiotics with empiric antibiotic therapy in critically ill patients admitted in intensive care unit. Acta Med Iran 2015; 53: 562–7. http://acta.tums.ac.ir/index.php/acta/article/view/4294/4249 [PubMed] [Google Scholar]
- 57. Lubell Y, Blacksell SD, Dunachie Set al. . Performance of C-reactive protein and procalcitonin to distinguish viral from bacterial and malarial causes of fever in Southeast Asia. BMC Infect Dis 2015; 15: 511. 10.1186/s12879-015-1272-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wangrangsimakul T, Althaus T, Mukaka Met al. . Causes of acute undifferentiated fever and the utility of biomarkers in Chiangrai, northern Thailand. PLoS Negl Trop Dis 2018; 12: e0006477. 10.1371/journal.pntd.0006477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Phatlhane DV, Ipp H, Erasmus RTet al. . Evaluating the use of procalcitonin in an asymptomatic, HIV-infected antiretroviral therapy-naive, South African cohort. Clin Chem Lab Med 2016; 54: 501–8. 10.1515/cclm-2015-0549 [DOI] [PubMed] [Google Scholar]
- 60. Ramana KV, Sabitha V, Rao R. A study of alternate biomarkers in HIV disease and evaluating their efficacy in predicting T CD4+ cell counts and disease progression in resource poor settings in highly active antiretroviral therapy (HAART) era. J Clin Diagn Res 2013; 7: 1332–5. https://doi.org/10.7860%2FJCDR%2F2013%2F5306.3138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zulu I, Hassan G, Njobvu RNLet al. . Cytokine activation is predictive of mortality in Zambian patients with AIDS-related diarrhoea. BMC Infect Dis 2008; 8: 156. 10.1186/1471-2334-8-156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Koethe JR, Blevins M, Nyirenda Cet al. . Nutrition and inflammation serum biomarkers are associated with 12-week mortality among malnourished adults initiating antiretroviral therapy in Zambia. J Int AIDS Soc 2011; 14: 19. 10.1186/1758-2652-14-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Bedell RA, van Lettow M, Meaney Cet al. . Predictive value of C-reactive protein for tuberculosis, bloodstream infection or death among HIV-infected individuals with chronic, non-specific symptoms and negative sputum smear microscopy. Trop Med Int Health 2018; 23: 254–62. 10.1111/tmi.13025 [DOI] [PubMed] [Google Scholar]
- 64. Ledwaba L, Tavel JA, Khabo Pet al. . Pre-ART levels of inflammation and coagulation markers are strong predictors of death in a South African cohort with advanced HIV disease. PLoS One 2012; 7: e24243. 10.1371/journal.pone.0024243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Woodd SL, Kelly P, Koethe JRet al. . Risk factors for mortality among malnourished HIV-infected adults eligible for antiretroviral therapy. BMC Infect Dis 2016; 16: 562. 10.1186/s12879-016-1894-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Haddow LJ, Moosa MY, Mosam Aet al. . Incidence, clinical spectrum, risk factors and impact of HIV-associated immune reconstitution inflammatory syndrome in South Africa. PLoS One 2012; 7: e40623. 10.1371/journal.pone.0040623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Sereti I, Sheikh V, Shaffer Det al. . Prospective international study of incidence and predictors of immune reconstitution inflammatory syndrome and death in people living with human immunodeficiency virus and severe lymphopenia. Clin Infect Dis 2020; 71: 652–60. 10.1093/cid/ciz877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kroeze S, Wit FW, Rossouw TMet al. . Plasma biomarkers of human immunodeficiency virus-related systemic inflammation and immune activation in Sub-Saharan Africa before and during suppressive antiretroviral therapy. J Infect Dis 2019; 220: 1029–33. 10.1093/infdis/jiz252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kiefer EM, Hoover DR, Shi Qet al. . Longitudinal evaluation of markers of inflammation in HIV-positive and HIV-negative Rwandan women. HIV Med 2018; 19: 734–44. 10.1111/hiv.12665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Chegou NN, Sutherland JS, Malherbe Set al. . Diagnostic performance of a seven-marker serum protein biosignature for the diagnosis of active TB disease in African primary healthcare clinic attendees with signs and symptoms suggestive of TB. Thorax 2016; 7: 785–94. 10.1136/thoraxjnl-2015-207999 [DOI] [PubMed] [Google Scholar]
- 71. Jacobs R, Malherbe S, Loxton AGet al. . Identification of novel host biomarkers in plasma as candidates for the immunodiagnosis of tuberculosis disease and monitoring of tuberculosis treatment response. Oncotarget 2016; 7: 57581–92. https://doi.org/10.18632%2Foncotarget.11420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Berrocal-Almanza LC, Goyal S, Hussain Aet al. . S100a12 is up-regulated in pulmonary tuberculosis and predicts the extent of alveolar infiltration on chest radiography: an observational study. Sci Rep 2016; 6: 31798. 10.1038/srep31798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Worodria W, Massinga-Loembe M, Mayanja-Kizza Het al. . Antiretroviral treatment-associated tuberculosis in a prospective cohort of HIV-infected patients starting ART. Clin Dev Immunol 2011; 2011: 758350. 10.1155/2011/758350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Yoon C, Semitala FC, Atuhumuza Eet al. . Point-of-care C-reactive protein-based tuberculosis screening for people living with HIV: a diagnostic accuracy study. Lancet Infect Dis 2017; 17: 1285–92. 10.1016/S1473-3099(17)30488-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Rajopadhye SH, Mukherjee SR, Chowdhary ASet al. . Oxidative stress markers in tuberculosis and HIV/TB co-infection. J Clin Diagn Res 2017; 11: BC24–BC8. 10.7860/jcdr/2017/28478.10473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Yoon C, Semitala FC, Asege Let al. . Yield and efficiency of novel intensified tuberculosis case-finding algorithms for people living with HIV. Am J Respir Crit Care Med 2019; 199: 643–50. 10.1164/rccm.201803-0490OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Ciccacci F, Floridia M, Bernardini Ret al. . Plasma levels of CRP, neopterin and IP-10 in HIV-infected individuals with and without pulmonary tuberculosis. J Clin Tuberc Other Mycobact Dis 2019; 16: 100107. 10.1016/j.jctube.2019.100107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Olsson O, Bjorkman P, Jansson Met al. . Plasma profiles of inflammatory markers associated with active tuberculosis in antiretroviral therapy-naive human immunodeficiency virus-positive individuals. Open Forum Infect Dis 2019; 6: ofz015. 10.1093/ofid/ofz015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Wilson D, Badri M, Maartens G. Performance of serum C-reactive protein as a screening test for smear-negative tuberculosis in an ambulatory high HIV prevalence population. PLoS One 2011; 6: e15248. 10.1371/journal.pone.0015248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Lawn SD, Kerkhoff AD, Vogt Met al. . Diagnostic and prognostic value of serum C-reactive protein for screening for HIV-associated tuberculosis. Int J Tuberc Lung Dis 2013; 17: 636–43. 10.5588/ijtld.12.0811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Farr K, Ravindran R, Strnad Let al. . Diagnostic performance of blood inflammatory markers for tuberculosis screening in people living with HIV. PLoS One 2018; 13: e0206119. 10.1371/journal.pone.0206119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. de Oliveira M, Duarte SB, Giacomini Get al. . A lung image reconstruction from computed radiography images as a tool to tuberculosis treatment control. J Venom Anim Toxins Incl Trop Dis 2019; 25: e144918. 10.1590/1678-9199-JVATITD-1449-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Wilson D, Moosa MS, Cohen Tet al. . Evaluation of tuberculosis treatment response with serial C-reactive protein measurements. Open Forum Infect Dis 2018; 5: ofy253. 10.1093/ofid/ofy253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Rasmussen TA, Sogaard OS, Camara Cet al. . Serum procalcitonin in pulmonary tuberculosis. Int J Tuberc Lung Dis 2011; 15: 251–6. [PubMed] [Google Scholar]
- 85. Janssen S, Schutz C, Ward Aet al. . Mortality in severe human immunodeficiency virus-tuberculosis associates with innate immune activation and dysfunction of monocytes. Clin Infect Dis 2017; 65: 73–82. 10.1093/cid/cix254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Soedarsono S, Subiantoro MC. Changes of CRP serum levels in pulmonary TB patients with AFB smear-positive sputum before and two months after receiving anti-tuberculosis drug treatment. Indian J Tuberc 2019; 66: 134–8. 10.1016/j.ijtb.2018.07.007 [DOI] [PubMed] [Google Scholar]
- 87. Epelboin L, Boulle C, Ouar-Epelboin Set al. . Discriminating malaria from dengue fever in endemic areas: clinical and biological criteria, prognostic score and utility of the C-reactive protein: a retrospective matched-pair study in French Guiana. PLoS Negl Trop Dis 2013; 7: e2420. 10.1371/journal.pntd.0002420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Sanchez-Arcila JC, Perce-da-Silva DS, Vasconcelos MPet al. . Intestinal parasites coinfection does not alter plasma cytokines profile elicited in acute malaria in subjects from endemic area of Brazil. Mediators Inflamm 2014; 2014: 857245. 10.1155/2014/857245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Peto TJ, Tripura R, Lee SJet al. . Association between subclinical malaria infection and inflammatory host response in a pre-elimination setting. PLoS One 2016; 11: e0158656. 10.1371/journal.pone.0158656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Gibson RS, Huddle JM. Suboptimal zinc status in pregnant Malawian women: its association with low intakes of poorly available zinc, frequent reproductive cycling, and malaria. Am J Clin Nutr 1998; 67: 702–9. 10.1093/ajcn/67.4.702 [DOI] [PubMed] [Google Scholar]
- 91. Mockenhaupt FP, Rong B, Gunther Met al. . Anaemia in pregnant Ghanaian women: importance of malaria, iron deficiency, and haemoglobinopathies. Trans R Soc Trop Med Hyg 2000; 94: 477–83. 10.1016/S0035-9203(00)90057-9 [DOI] [PubMed] [Google Scholar]
- 92. Hinderaker SG, Olsen BE, Lie RTet al. . Anemia in pregnancy in rural Tanzania: associations with micronutrients status and infections. Eur J Clin Nutr 2002; 56: 192–9. 10.1038/sj.ejcn.1601300 [DOI] [PubMed] [Google Scholar]
- 93. Adegnika AA, Verweij JJ, Agnandji STet al. . Microscopic and sub-microscopic Plasmodium falciparum infection, but not inflammation caused by infection, is associated with low birth weight. Am J Trop Med Hyg 2006; 75: 798–803. 10.4269/ajtmh.2006.75.798 [DOI] [PubMed] [Google Scholar]
- 94. Conroy AL, Liles WC, Molyneux MEet al. . Performance characteristics of combinations of host biomarkers to identify women with occult placental malaria: a case-control study from Malawi. PLoS One 2011; 6: e28540. 10.1371/journal.pone.0028540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Paul R, Sinha PK, Bhattacharya Ret al. . Study of C reactive protein as a prognostic marker in malaria from Eastern India. Adv Biomed Res 2012; 1: 41. 10.4103/2277-9175.100140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Mendonca VR, Queiroz AT, Lopes FMet al. . Networking the host immune response in Plasmodium vivax malaria. Malar J 2013; 12: 69. 10.1186/1475-2875-12-69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Bhardwaj N, Ahmed MZ, Sharma Set al. . C-reactive protein as a prognostic marker of Plasmodium falciparum malaria severity. J Vector Borne Dis 2019; 56: 122–6. 10.4103/0972-9062.263727 [DOI] [PubMed] [Google Scholar]
- 98. Lima-Junior JC, Rodrigues-da-Silva RN, Pereira VAet al. . Cells and mediators of inflammation (C-reactive protein, nitric oxide, platelets and neutrophils) in the acute and convalescent phases of uncomplicated Plasmodium vivax and Plasmodium falciparum infection. Mem Inst Oswaldo Cruz 2012; 107: 1035–41. 10.1590/S0074-02762012000800012 [DOI] [PubMed] [Google Scholar]
- 99.World Health Organization. Rapid communication on the systematic screening for tuberculosis. 2020. https://www.who.int/publications/i/item/rapid-communication-on-the-systematic-screening-for-tuberculosis
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.