Abstract
Background:
Nanocurcumin has antimicrobial properties and it is to be tested as a coating on gutta-percha against Enterococcusi faecalis.
Aim:
To evaluate the antimicrobial efficacy of nanocurcumin-coated gutta-percha against E. faecalis in comparison with conventional gutta-percha.
Materials and Methods:
The broth dilution method and colony-forming unit count assay were chosen for the evaluation of the minimum inhibitory concentration (MIC) and minimum bactericidal concentration of nanocurcumin against E. faecalis. ISO size 30, 4% taper gutta-percha cones were manually coated with nanocurcumin. All the coated and noncoated gutta-percha cones were examined under a scanning electron microscope to study the exterior surface. Antibacterial efficacy of nanocurcumin-coated gutta-percha and conventional gutta-percha was seen by agar diffusion method against E. faecalis.
Results:
MIC of nanocurcumin was observed at 50 mg/ml for E. faecalis. Nanocurcumin-coated gutta-percha showed a larger zone of inhibition when compared to conventional gutta-percha which showed a smaller zone of inhibition (P < 0.0001). Nanocurcumin-coated gutta-percha showed moderate antimicrobial activity, while conventional gutta-percha showed weak activity.
Conclusion:
The results of the study reveal that nanocurcumin has an antimicrobial activity against E. faecalis. The use of herbal alternatives in endodontics might prove to be advantageous.
Keywords: Enterococcus faecalis, gutta-percha, nanocurcumin
INTRODUCTION
The main objective of endodontic treatment is to completely remove microorganisms from the root canal system.[1] In the development of pulp and periapical pathosis, microorganisms play a critical role. The most prevalent bacteria isolated from nonhealing endodontic lesions is Enterococcus faecalis, a facultative anaerobic Gram-positive coccus.[2]
Research has looked into the antibacterial effects of antibacterial agents when used in combination with gutta-percha or sealer to prevent residual infection. Calcium hydroxide, zinc oxide, chlorhexidine, antibiotics, and iodoform are examples of antibacterial agents.
Curcumin is a chemical molecule extracted from the dried rhizome of turmeric that has antimicrobial, antidiabetic, and immunomodulatory properties. It can be used to relieve pain and it promotes wound healing, because of its anti-inflammatory effects.[3]
The aim of the present study was to evaluate the antimicrobial efficacy of nanocurcumin-coated gutta-percha against E. faecalis.
MATERIALS AND METHODS
Synthesis of nanocurcumin
Synthesis of nanocurcumin was done at the University Institute of Pharmaceutical Sciences, Panjab University, Chandigarh.
Curcumin nanoparticles were synthesized according to the procedure described by Nair et al.[4]
Coated gutta-percha preparation
ISO size 30, 4% taper gutta-percha cones were taken and cold sterilized by immersing in 5% sodium hypochlorite for 1 min followed by a final rinse with distilled water. After this, the sterilized gutta-percha cones were manually coated with nanocurcumin and then allowed to dry to form a film around the gutta-percha cones.
Scanning electron microscope
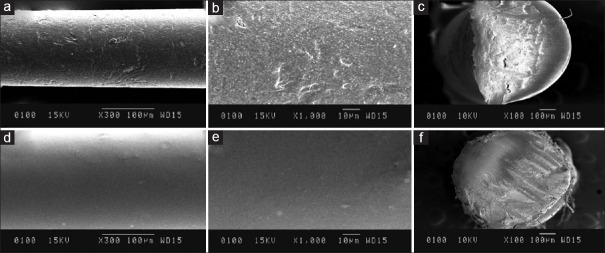
The external surface of the coated gutta-percha cones was scanned under the scanning electron microscope (JCM6100) with various magnifications. The gutta-percha cones were cut horizontally and the cross-sections of the cones were scanned to obtain cross-sectional scanning images [Figure 1a–f].
Figure 1.
(a) SEM image showing the rough external surface of conventional gutta percha at ×300 (b) SEM image showing the rough external surface of conventional gutta percha at ×1000 (c) SEM image showing the cross-section of conventional gutta percha at ×100 (d) SEM image showing the smooth external surface of nanocurcumin coated gutta percha at ×300 (e) SEM image showing the smooth external surface of nanocurcumin coated gutta percha at ×1000 (f) SEM image showing cross sectional of gutta percha uniformly coated with nanocurcumin at ×100
Microorganism preparation and agar diffusion test
The tested microorganisms used in this study were E. faecalis ATCC29212.
Forty-four nutrient agar plates were heavily seeded uniformly with 0.1 ml of 105–106 cells/ml of the tested microorganism. The plates with microorganisms were then divided into two groups (conventional gutta-percha group n = 22 and coated gutta-percha group n = 22).
The plates were then incubated at 37°C for 24 h to allow maximum growth of the microorganisms.
The antibacterial activity was measured by the positive response shown as that is the zone of inhibition and its degree (distance) around gutta-percha.
The largest diameter of the inhibition zone around the gutta-percha was measured in millimeters using a digital vernier caliper.
Statistical analysis of data
Numerical results were statistically analyzed with IBM SPSS STATISTICS (version 23.0) for Windows using homogeneity of variances checked by Levene's test for equality of variances. Furthermore, group comparisons of values of data were made with independent t-tests for two groups. All statistical tests were two-sided and performed at a significance level of α = 0.05.
RESULTS
The minimum inhibitory concentration (MIC) of nanocurcumin against E. faecalis was 50 mg/ml.
A well-defined zone of inhibition was formed by nanocurcumin on a well diffusion assay [Figure 2a].
Figure 2.
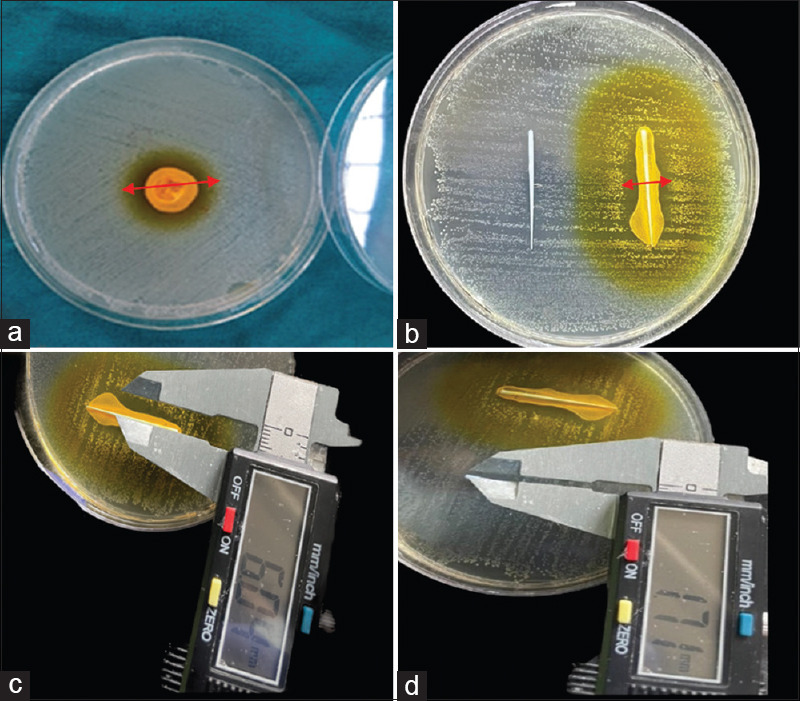
(a) Well diffusion assay showing ZOI for nanocurcumin against Enterococcus faecalis (red arrow),(b) ZOI (indicated by red arrows) of conventional gutta percha (left) and nanocurcumin coated gutta percha (right),(c) ZOI measured by vernier caliper of nanocurcumin coated gutta percha (d) ZOI measured by vernier caliper of conventional gutta percha. ZOI: Zone of inhibition
Antimicrobial activity of nanocurcumin-coated gutta-percha
Both the groups produced positive inhibitory action on tested microbial species. The inhibition zones around coated gutta-percha cones were more pronounced for nanocurcumin group by showing a larger zone of inhibition as indicated by the red arrow in the image (12.5 mm) [Figure 2b–c] than the conventional group (2.5 mm) of the tested microorganism [Figure 2d]. There was an extremely significant difference between the inhibitory effect of nanocurcumin-coated gutta-percha and conventional gutta-percha (P < 0.05).
Nanocurcumin coating was more uniform and closely adherent to the gutta-percha cone under the scanning electron microscope [Figure 1d–f].
DISCUSSION
Failure of the endodontic therapy and the re-emergence of periapical lesions are two possible outcomes of incomplete disinfection of infected root canals. The intracanal medicament is strongly advised to eradicate as many leftover microorganisms as possible during disinfection and to inhibit their recolonization.[5]
This study was performed to evaluate the antimicrobial efficacy of nanocurcumin-coated gutta-percha against E. faecalis. E. faecalis was chosen as the test organism because it is a facultative organism that can survive high temperatures and high pH environment. E. faecalis is frequently recovered from canals when therapy has failed, implying that it is an opportunistic pathogen whose presence in the root canal poses a serious therapeutic challenge.[6]
Nowadays, many plant extracts have been introduced as phytomedicines in dentistry to overcome the possible side effects caused by chemical agents.[7] One of them is curcumin.
Curcumin (C21H20O6), a yellow-colored phenolic pigment, is the most important fraction which is responsible for the biological activities of turmeric.[8]
Extensive research on curcumin has demonstrated a wide spectrum of therapeutic effects such as anti-inflammatory, antibacterial, antiviral, and antifungal.[8,9] Curcumin possesses antibacterial properties against a number of bacteria.[10]
Enhancement of the bioavailability of curcumin
Although curcumin has been described as a promising antibacterial drug with therapeutic potential, one of the major drawbacks of using curcumin alone is its low bioavailability, which appears to be mostly owing to poor absorption, quick metabolism, and rapid elimination.[11] Curcumin microemulsions made from food-grade components such as Tween 20, lecithin, Vitamin E, and ethanol increase curcumin water solubility in water by 1000–10,000 times.[12] Nanocurcumin is also proposed as a means of increasing curcumin bioavailability.[13] Curcumin nanoparticles in size of 2–40 nm have more significant antibacterial action against Staphylococcus aureus, Escherichia coli, and Pseudomonas aeruginosa. Curcumin encapsulation in liposomes improves its water dispersibility and chemical stability, as well as its antioxidant and anti-inflammatory activities.[12]
Antimicrobial effect of curcumin on pathogenic bacteria
Curcumin inhibits bacteria by disrupting their membranes, according to a research. Curcumin causes membrane leakage in Gram-negative and Gram-positive bacteria such as S. aureus, E. faecalis, E. coli, and P. aeruginosa, according to a membrane permeabilization analysis.[14,15] Therefore, its incorporation into gutta-percha could be effective in eliminating E. faecalis from canals.
Conventional gutta-percha has been shown to have an intrinsic antibacterial effect on very few bacteria species.[16,17] However, Melker et al. in their investigation clearly demonstrated the failure of standard gutta-percha to kill essential endodontic microorganisms.[18]
A research has also been conducted on the antibacterial activity of gutta-percha points containing established root canal medications, including calcium hydroxide, zinc oxide, chlorhexidine, and iodine–polyvinylpyrrolidone alone or used in combination.[19]
In a study involving coated gutta-percha cones with various medications, Jain et al. discovered that gutta-percha coated with antibiotics (amoxicillin–clavulanic acid combination) was efficient against E. faecalis. Similarly, in another investigation, Bodrumlu et al. compared tetracycline-integrated gutta-percha (TGP) to conventional gutta-percha and found TGP to be a more efficient antibacterial than conventional gutta-percha cones.[19,20] The present study also showed a similar observation that coated gutta-percha had greater antibacterial activity.
ISO size 30 gutta-percha cones were selected in this study since it has been shown that the narrow surface of smaller size gutta-percha points could only sustain a small amount of nanocurcumin on its surface; thus, it was hypothesized that expanding the overall gutta-percha surface would promote a larger and faster antibacterial effect. Moreover, studies have found that root canals prepared to an apical size of 30, 4% taper could effectively clean the canals.[21,22]
Well diffusion assay
Agar well diffusion method is widely used to evaluate the efficacy of antimicrobial agents. This assay is simple, inexpensive, and has the ability to test the enormous number of microorganisms and antimicrobial agents with ease to interpret the results provided. It also allows direct comparison of the antimicrobial agents against the microorganisms, indicating their potential to eliminate bacteria in the local microenvironment of the root canal system.[23]
Hence, in this study, well diffusion assay was used to compare and evaluate nanocurcumin-coated gutta-percha and conventional gutta-percha against E. faecalis. A well-defined zone of inhibition was seen against E. faecalis [Figure 2a].
The mean zone of inhibition was recorded with an unaided eye using a digital vernier caliper as this is an upgraded version of the vernier caliper with a least count of 0.01 mm which is more accurate as per the protocol followed by Cui et al. and Routh et al.,[24,25] and the results were tabulated and statistically analyzed using independent t-test. Nanocurcumin-coated gutta-percha has shown moderate antimicrobial activity (12.5 mm), while conventional gutta-percha showed weak activity (2.5 mm) against E. faecalis as per the classification given by Rota et al.[26] In the present study, conventional gutta-percha was weakly effective with a comparatively very smaller zone of inhibition (2.5 mm), against E. faecalis. This is in accordance with Moorer and Genet who investigated that the major component of gutta-percha cones that is zinc oxide was shown to be responsible for some antibacterial properties of the cones.[27,28]
Scanning electron microscopy
Further, a scanning electron microscope examination was also carried out for visual inspection of the exterior surface of traditional gutta-percha and nanocurcumin surface coating. Nanocurcumin coating was uniform and closely adherent to the gutta-percha cones [Figure 1a–f]. SEM allows the visualization of images at high magnification (×50–×10.000 and above). In addition, since SEM figures are in grayscale, it does not influence obtaining a correct focus, a limitation which is found in optical stereomicroscopes.[29]
In the present study, nanocurcumin-coated gutta-percha had greater antibacterial activity than conventional gutta-percha against E. faecalis. Its use in the form of coating over gutta-percha as an interappointment medicament can be explored further. Incorporating an antibacterial agent into obturating materials may be beneficial in limiting bacterial growth once root canal therapy is completed. However, taking into account every aspect, such as the impact of heat and the effects of sealers on the gutta-percha and canal walls' interface (as applied in continuous wave compaction) Further research is needed on how will be the medication interacts with the gutta percha and how long the antibacterial component of the medication will be active there.[30] Nanocurcumin incorporation into gutta-percha could be effective as an obturating material also. Further studies can be undertaken to check for the time period for which nanocurcumin remains effective as an antimicrobial in the canals. In the future, ex vivo studies could be carried out for understanding the diffusion through dentinal tubules of this endodontic medicament.
CONCLUSION
From the present in vitro study, it can be concluded as follows:
Nanocurcumin-coated gutta-percha shows antimicrobial activity against E. faecalis.
The MIC of nanocurcumin against E. faecalis is 50 mg/ml.
Colony-forming unit counts revealed that the higher the concentration of nanocurcumin, the lesser the microbial colony formed, and hence, the antibacterial efficacy is directly proportional to the concentration of nanocurcumin.
Scanning electron microscopy revealed a uniform layer of nanocurcumin coating over the gutta-percha points, which points to a uniform distribution of intracanal medicament in the root canal.
The incorporation of the antibacterial component of nanocurcumin at a concentration of 50 mg/ml and above into the obturating material, i.e., gutta-percha may be useful in preventing bacterial regrowth after completion of root canal therapy.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Fabricius L, Dahlén G, Holm SE, Möller AJ. Influence of combinations of oral bacteria on periapical tissues of monkeys. Eur J Oral Sci. 1982;90:200–6. doi: 10.1111/j.1600-0722.1982.tb00728.x. [DOI] [PubMed] [Google Scholar]
- 2.Donlan RM, Costerton JW. Biofilms: Survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev. 2002;15:167–93. doi: 10.1128/CMR.15.2.167-193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tyagi P, Singh M, Kumari H, Kumari A, Mukhopadhyay K. Bactericidal activity of curcumin I is associated with damaging of bacterial membrane. PLoS One. 2015;10:e0121313. doi: 10.1371/journal.pone.0121313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nair RS, Morris A, Billa N, Leong CO. An evaluation of curcumin-encapsulated chitosan nanoparticles for transdermal delivery. AAPS PharmSciTech. 2019;20:69. doi: 10.1208/s12249-018-1279-6. [DOI] [PubMed] [Google Scholar]
- 5.Estrela C, Pimenta FC, Ito IY, Bammann LL. Antimicrobial evaluation of calcium hydroxide in infected dentinal tubules. J Endod. 1999;25:416–8. doi: 10.1016/S0099-2399(99)80269-6. [DOI] [PubMed] [Google Scholar]
- 6.Balakrishnan R, Dhole TK, Dubey S, Boruah LC, Langde SP. Enterococcus faecalis and Candida albicans. ENDO (Lond Engl) 2012;6:1–5. [Google Scholar]
- 7.Jyoti BB. Phytotherapeutics in conservative dentistry & endodontics – A review. J Conserv Dent. 2005;8:31. [Google Scholar]
- 8.Chattopadhyay I, Biswas K, Bandyopadhyay U, Banerjee RK. Curr Sci. 2004. Turmeric and curcumin: Biological actions and medicinal applications; pp. 44–53. [Google Scholar]
- 9.Kohli K, Ali J, Ansari MJ, Raheman Z. Indian J Pharmacol. 2005. Curcumin: A natural anti. inflammatory agent; pp. 141–7. [Google Scholar]
- 10.Alpers DH. The potential use of curcumin in management of chronic disease: Too good to be true? Curr Opin Gastroenterol. 2008;24:173–5. doi: 10.1097/MOG.0b013e3282f44a19. [DOI] [PubMed] [Google Scholar]
- 11.Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Mol Pharm. 2007. Bioavailability of curcumin: Problems and promises; pp. 07–18. [DOI] [PubMed] [Google Scholar]
- 12.Patil VM, Das S, Balasubramanian K. Quantum chemical and docking insights into bioavailability enhancement of curcumin by piperine in pepper. J Phys Chem A. 2016;120:3643–53. doi: 10.1021/acs.jpca.6b01434. [DOI] [PubMed] [Google Scholar]
- 13.Sanidad KZ, Sukamtoh E, Xiao H, McClements DJ, Zhang G. Annu Rev Food Sci Technol. 2019. Curcumin: Recent advances in the development of strategies to improve oral bioavailability; pp. 597–617. [DOI] [PubMed] [Google Scholar]
- 14.Mandroli PS, Bhat K. An in vitro evaluation of antibacterial activity of curcumin against common endodontic bacteria. J Appl Pharm Sci. 2013;3:106–8. [Google Scholar]
- 15.Negahdari R, Ghavimi MA, Barzegar A, Memar MY, Balazadeh L, Bohlouli S, et al. Antibacterial effect of nanocurcumin inside the implant fixture: An in vitro study. Clin Exp Dent Res. 2021;7:163–9. doi: 10.1002/cre2.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Podbielski A, Boeckh C, Haller B. Growth inhibitory activity of gutta-percha points containing root canal medications on common endodontic bacterial pathogens as determined by an optimized quantitative in vitro assay. J Endod. 2000;26:398–403. doi: 10.1097/00004770-200007000-00005. [DOI] [PubMed] [Google Scholar]
- 17.Vishwanath V, Rao HM. Gutta-percha in endodontics – A comprehensive review of material science. J Conserv Dent. 2019;22:216–22. doi: 10.4103/JCD.JCD_420_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Melker KB, Vertucci FJ, Rojas MF, Progulske-Fox A, Bélanger M. Antimicrobial efficacy of medicated root canal filling materials. J Endod. 2006;32:148–51. doi: 10.1016/j.joen.2005.10.032. [DOI] [PubMed] [Google Scholar]
- 19.Jain VM, Karibasappa GN, Dodamani AS, Vishwakarma PK, Mali GV. Comparative assessment of antimicrobial efficacy of different antibiotic coated gutta-percha cones on Enterococcus faecalis an in vitro study. J Clin Diagn Res. 2016;10:ZC65–8. doi: 10.7860/JCDR/2016/20699.8541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bodrumlu E, Alaçam T, Semiz M. The antimicrobial and antifungal efficacy of tetracycline-integrated gutta-percha. Indian J Dent Res. 2008;19:112–5. doi: 10.4103/0970-9290.40464. [DOI] [PubMed] [Google Scholar]
- 21.Khademi A, Yazdizadeh M, Feizianfard M. Determination of the minimum instrumentation size for penetration of irrigants to the apical third of root canal systems. J Endod. 2006;32:417–20. doi: 10.1016/j.joen.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 22.Mickel AK, Chogle S, Liddle J, Huffaker K, Jones JJ. The role of apical size determination and enlargement in the reduction of intracanal bacteria. J Endod. 2007;33:21–3. doi: 10.1016/j.joen.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 23.Severina E, Severin A, Tomasz A. Antibacterial efficacy of nisin against multidrug-resistant Gram-positive pathogens. J Antimicrob Chemother. 1998;41:341–7. doi: 10.1093/jac/41.3.341. [DOI] [PubMed] [Google Scholar]
- 24.Cui ZH, He HL, Wu SB, Dong CL, Lu SY, Shan TJ, et al. Rapid screening of essential oils as substances which enhance antibiotic activity using a modified well diffusion method. Antibiotics (Basel) 2021;10:463. doi: 10.3390/antibiotics10040463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Routh S, Pai MB, Rajesh G, Shenoy R. Effect of lactobacillus acidophilus and streptococcus salivarius on growth of periodontal pathogens – An in-vitro study. Int J Adv Res. 2018;6:607–12. [Google Scholar]
- 26.Rota C, Carramiñana JJ, Burillo J, Herrera A. In vitro antimicrobial activity of essential oils from aromatic plants against selected foodborne pathogens. J Food Prot. 2004;67:1252–6. doi: 10.4315/0362-028x-67.6.1252. [DOI] [PubMed] [Google Scholar]
- 27.Moorer WR, Genet JM. Evidence for antibacterial activity of endodontic gutta-percha cones. Oral Surg Oral Med Oral Pathol. 1982;53:503–7. doi: 10.1016/0030-4220(82)90467-4. [DOI] [PubMed] [Google Scholar]
- 28.Moorer WR, Genet JM. Antibacterial activity of gutta-percha cones attributed to the zinc oxide component. Oral Surg Oral Med Oral Pathol. 1982;53:508–17. doi: 10.1016/0030-4220(82)90468-6. [DOI] [PubMed] [Google Scholar]
- 29.Paradella TC, Bottino MA. Scanning Electron Microscopy in modern dentistry research. Braz Dent Sci. 2012;15:43–8. [Google Scholar]
- 30.Szep S, Grumann L, Ronge K, Schriever A, Schultze M, Heidemann D. In vitro cytotoxicity of medicated and nonmedicated gutta-percha points in cultures of gingival fibroblasts. J Endod. 2003;29:36–40. doi: 10.1097/00004770-200301000-00010. [DOI] [PubMed] [Google Scholar]