Abstract
Introduction:
Chemotherapy induced thrombocytopenia (CIT) is a common complication of cancer treatment, frequently leads to reduced relative dose intensity, and is associated with reduced survival.
Given the lack of FDA-approved therapies for CIT, thrombopoietin receptor agonists (TPO-RAs) have received significant attention for treatment and prevention of CIT.
Areas covered:
This review will summarize the development of prior agents for treatment of CIT, discuss the existing literature investigating the use of TPO-RAs in CIT primarily in patients with solid tumor malignancies, and offer insights on the future direction of TPO-RAs and other therapeutics for CIT.
Expert opinion:
In alignment with NCCN guidelines, we recommend that patients with CIT participate in a clinical trial for consideration of TPO-RA treatment or consider off-label use of romiplostim when participation in clinical trials is not possible. The literature to date supports the use of TPO-RAs for treatment of persistent CIT. Further data is needed to describe the long-term efficacy, safety, and prescribing practices of TPO-RAs in a diverse patient population with a variety of tumor types and chemotherapy regimens in addition to exploring the underlying biology of CIT.
Keywords: Thrombocytopenia, chemotherapy induced thrombocytopenia, thrombopoietin, thrombopoietin receptor agonist, TPO-RA, romiplostim, eltrombopag, avatrombopag, platelet
1. Introduction to chemotherapy-induced thrombocytopenia (CIT)
Chemotherapy-induced thrombocytopenia (CIT) is a frequent challenge encountered in the care of cancer patients and represents a significant area of unmet need. Approximately 25% of all platelet transfusions in the United States are administered to patients receiving chemotherapy and nearly 10% of patients with solid tumors receiving chemotherapy require clinical intervention for thrombocytopenia. Despite the clinical challenges presented by CIT, there are currently no available agents approved by the U.S Food and Drug Agency (FDA) for treatment or prevention of CIT, and management had previously been limited to supportive platelet transfusions and reduction of chemotherapy relative dose intensity (RDI) [1].
Although there are no official guidelines defining platelet cutoffs for diagnosis of CIT, National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) stratify thrombocytopenia as Grade 1 if below the lower limit of normal to 75 × 109/L, Grade 2 if < 75 × 109/L, Grade 3 if < 50 × 109/L, and Grade 4 if < 25 × 109/L. While Grade 1 thrombocytopenia rarely is clinically meaningful, Grade 4 thrombocytopenia can significantly increase the risk of bleeding complications and spontaneous bleeding. However, the duration of thrombocytopenia is also important to consider. “Nadir CIT” describes significant thrombocytopenia during a chemotherapy cycle with at least partial recovery by the following cycle. “Persistent CIT,” on the other hand, describes more prolonged and often less severe thrombocytopenia that does not resolve despite chemotherapy delay [2]. Reflecting the importance of the time course of thrombocytopenia, many recent clinical studies investigating CIT use an inclusive platelet cutoff of 50–100 × 109/L if lasting ≥ 3–4 weeks recognizing that prolonged thrombocytopenia creates longer opportunity for adverse clinical impact [3–5].
On a more fundamental level, CIT becomes relevant when it precludes standard treatment. In response to CIT, clinicians are often pressed to modify the selection and/or number of agents in the treatment regimen, reduce doses, or delay chemotherapy cycles. Reduced RDI of any cause is associated with reduction in progression-free and overall survival in cancer patients [6,7]. CIT is also relevant when there is clinically significant bleeding attributed to the thrombocytopenia or when the intended use of anticoagulation therapy is precluded. CIT itself does not protect against cancer-associated thrombosis (CAT), the second leading cause of death in cancer patients only behind the malignancy itself. Challenging treatment decisions regarding dosing of anticoagulation may be required when CIT occurs in a patient population already prone to thromboembolic events, recurrence, and bleeding complications of anticoagulation therapy at a four-fold increased rate compared to the general population [8]. Concurrent CIT and CAT is clinically challenging due to the paucity of clear clinical guidelines in this scenario; thrombocytopenia is a common exclusion criterion in studies investigating anticoagulation in the cancer population.
The thrombopoietin receptor agonists (TPO-RAs) are a class of medications that act at the TPO receptor to promote megakaryocyte growth, differentiation, and platelet production. They include romiplostim, eltrombopag, lusutrombopag, avatrombopag, and, more recently, hetrombopag. Initially approved for the treatment of immune thrombocytopenia (ITP), the TPO-RAs have become widely adopted for treatment in other disorders of thrombocytopenia. This review will summarize the development of prior agents for treatment of CIT, discuss the existing literature investigating the use of TPO-RAs in CIT primarily in patients with solid tumor malignancies, and offer insights on the future direction of TPO-RAs and other therapeutics for CIT.
2. History of pharmaceutical development for treatment of chemotherapy-induced thrombocytopenia
Platelet transfusions are often employed as supportive therapy in CIT, but they alone fail to achieve sustainable and practical use as a mainstay of treatment for CIT. Platelet transfusions are transient in nature and significant resources are necessary to support a patient’s platelet count through ongoing chemotherapy cycles in a patient encountering persistent CIT. Longitudinally, recurrent transfusions not only increase the risk of alloimmunization within individual patients but they also exacerbate several healthcare systems issues of cost and availability [9]. In response to the challenges associated with platelet transfusion therapy, great efforts have been invested over the past several decades into development and identification of agents to provide sustainable increases in platelet counts in patients with CIT.
2.1. Oprelvekin
Recombinant human interleukin 11 (oprelvekin) is a thrombopoietic cytokine promoting megakaryocyte development that was previously approved by the US FDA for prevention and treatment of CIT. Although studies of oprelvekin produced data suggesting efficacy in raising platelet count and reducing about 30% of platelet transfusions [10], it was poorly tolerated in treated patients with notable toxicities of constitutional symptoms, fluid retention, dilutional anemia, and cardiac arrythmias. Pharmacoeconomic analysis showed that healthcare costs savings from reduction of platelet transfusions and associated transfusion reactions were greatly outweighed by the significant costs of oprelvekin (expected cost of $3,000–5,000 USD over a three week period in 2003) [11]. The financial impact and toxicities of oprelvekin outweighed the modest clinical efficacy, and oprelvekin was voluntarily withdrawn from the market by the manufacturer several years ago.
2.2. Physiology of thrombopoietin in platelet production and CIT
Thrombopoietin (TPO) was discovered in 1994 as the key hematopoietic growth factor regulating platelet production. TPO is constitutively produced by the liver and released into peripheral circulation where most TPO is bound and cleared by TPO receptors on platelets (acting as a “sink”), subsequently undergoing internalization and degradation. The residual TPO binds to bone marrow megakaryocytes, increases endomitosis and ploidy to expand the megakaryocyte pool, and stimulates maturation of megakaryocytes to increase platelet production; it also prevents apoptosis of early and late megakaryocytes [12]. This physiologic balance is demonstrated by the observation that the level of circulating TPO is inversely related to the rate of platelet production[13]. In patients receiving chemotherapy, platelet production is reduced due to cytotoxic and myelosuppressive effects of treatment (although there may be more diverse mechanisms of CIT as discussed later). The reduced clearance of TPO by platelets subsequently leads to an increase in circulating TPO and has been shown to demonstrate a log-linear relationship between the onset of thrombocytopenia and increase in TPO levels [14].
2.3. First generation recombinant thrombopoietins
Recombinant human thrombopoietins were subsequently developed to leverage physiologic thrombopoietic physiology. Recombinant human thrombopoietin (rhTPO) was a glycosylated TPO protein while pegylated recombinant human megakaryocyte growth and development factor (PEG-rHuMGDF) was a protein comprising the first 163 amino acids of TPO linked to polyethylene glycol. Several studies investigated the role of rhTPO [15,16] and PEG-rHuMGDF [17–23] in treatment or prevention of CIT (Table 1). Results were initially promising with greater efficacy in reducing both nadir and duration of thrombocytopenia as well as reducing platelet transfusion, with a tolerability profile much more favorable than oprelvekin.
Table 1.
Representative studies of rhTPO and PEG-rHuMGDF for treatment/prevention of CIT
| Investigators | Study design | Major outcomes |
|---|---|---|
| Vadhan-Raj et al16 | 29 patients with gynecologic cancer receiving carboplatin and rhTPO before chemotherapy and after a second cycle of chemotherapy | Patient receiving rhTPO experienced higher mean platelet count nadir, greater number of days with platelet count < 50 x 109/L, shorter duration of thrombocytopenia, and fewer patients required platelet transfusions. |
| Vadhan-Raj et al15 | 66 patients with sarcoma receiving doxorubicin and ifosfamide and rhTPO before and/or after cycle 2 and subsequent cycles. | Compared to cycle 1 without rhTPO, patients who received rhTPO starting from 5 days before chemotherapy experienced higher mean platelet nadir count and fewer platelet transfusions. |
| Fanucchi et al17 | 53 patients with lung cancer receiving carboplatin and paclitaxel randomized to receive various doses of PEG-rHuMGDF or placebo | Patients receiving PEG-rHuMGDF experienced higher mean platelet count nadir and faster recovery to baseline platelet counts. |
| Basser et al18 | 41 patients with advanced cancers receiving carboplatin and cyclophosphamide randomized to receive various doses of PEG-rHuMGDF or placebo | Patients receiving PEG-rHuMGDF experienced faster recovery to baseline platelet counts. Platelet nadir occurred earlier but there was no difference in the depth of nadir. |
| Basser et al19 | 68 patients with advanced cancers receiving carboplatin and cyclophosphamide randomized to receive various doses of PEG-rHuMGDF | Compared with an initial cycle without post-chemotherapy PEG-rHuMGDF, subsequent cycles followed by PEG-rHuMGDF had higher mead platelet count nadirs and shorter duration of grade 3 or 4 CIT. |
| Moskowitz et al22 | 41 patients with diffuse large B-cell lymphoma receiving ICE (ifosfamide, carboplatin, etoposide) randomized to receive PEG-rHuMGDF or placebo | Patients receiving PEG-rHuMGDF experienced higher platelet count nadirs and fewer platelet transfusions, higher chemotherapy dose intensity, and improved survival. |
| Archimbaud et al20 | 108 patient with acute myeloid leukemia receiving daunorubicin, cytarabine, and etoposide followed by subsequent chemotherapy if not in remission randomized to receive PEG-rHuMGDF or placebo | Patient receiving PEG-rHuMGDF experienced greater peak platelet counts but no different in time to platelet recovery or platelet transfusion requirements. |
| Schiffer et al23 | 57 patients with acute myeloid leukemia receiving daunorubicin and cytarabine followed by high-dose cytarabine randomized to receive PEG-rHuMGDF or placebo | Patient receiving PEG-rHuMGDF experienced greater peak platelet counts but no different in time to platelet recovery or platelet transfusion requirements. |
| Geissler et al21 | 88 patients with acute myeloid leukemia receiving daunorubicin, cytarabine, and etoposide or high-dose cytarabine plus mitoxantrone randomized to receive PEG-rHuMGDF or placebo | Patients receiving PEG-rHuMGDF experienced no difference in time to platelet recovery or platelet transfusion requirements. |
CIT: chemotherapy induced thrombocytopenia, PEG-rHuMGDF: recombinant human pegylated megakaryocyte growth and development factor, rhTPO: recombinant human thrombopoietin
However, enthusiasm for these agents stalled after it was discovered that a minority of patients receiving PEG-rHuMGDF developed neutralizing antibodies capable of cross-reacting with native TPO [24,25]. This resulted in severe thrombocytopenia in these patients, and although they ultimately recovered to normal platelet counts, immunosuppression was often required. Such antibodies have not been demonstrated in patients treated with rhTPO. Nevertheless, development of both recombinant human thrombopoietins halted in the West due to their sequence homology with endogenous TPO. Of note, rhTPO has continued to be developed in China where it is now approved for CIT and routinely used [26].
3. Thrombopoietin receptor agonists
TPO-RAs represent the most recent and successful effort to develop thrombopoietic agents to increase platelet counts while avoiding antibody formation. A major difference between the TPO-RAs and the first generation recombinant thrombopoietins is that the TPO-RAs do not share sequence homology with native TPO so there is no risk of development of cross-reactive antibodies [27]. TPO-RAs currently approved for use in various countries worldwide include romiplostim, eltrombopag, lusutrombopag, avatrombopag, and hetrombopag. They are currently approved for management of immune thrombocytopenia, aplastic anemia, hepatitis C-associated thrombocytopenia, and periprocedural thrombocytopenia in patients with chronic liver disease, depending on the agent in question [28–32]. Successful use of TPO-RAs in other rare thrombocytopenic disorders and settings has also been described [33,34]. None is yet approved for management of CIT. Table 2 summarizes the properties of the TPO-RAs and their approved clinical uses. Table 3 summarizes representative studies of TPO-RAs for treatment and prevention of CIT.
Table 2.
Comparison of TPO-RAs.
| Romiplostim | Eltrombopag | Lusutrombopag | Avatrombopag | Hetrombopag | |
|---|---|---|---|---|---|
| Molecular structures | Peptide | Small molecule | Small molecule | Small molecule | Small molecule |
| TPO receptor site of action | Extracellular domain | Transmembrane domain | Transmembrane domain | Transmembrane domain | Transmembrane domain |
| Route of administration | Subcutaneous | Oral | Oral | Oral | Oral |
| Food interactions | N/A | Yes | No | No | Yes |
| Dose adjustments for renal or hepatic impairment | No adjustments | Dose reduction for hepatic impairment in ITP and severe aplastic anemia; no adjustment for renal impairment | No adjustments | No adjustments | No adjustments |
| FDA-approved indications and starting dosesa | Immune thrombocytopenia (1 mcg/kg weekly) | Immune thrombocytopenia (50 mg daily) Hepatitis C-associated thrombocytopenia (25 mg daily) Severe aplastic anemia (150 mg daily for first-line treatment, 50 mg daily for refractory disease) |
Periprocedural thrombocytopenia in CLD (3 mg daily) | Periprocedural thrombocytopenia in CLD (40 mg daily if platelets 40–50 x 109/L, 60 mg daily if platelets < 40 x 109/L) Immune thrombocytopenia (20 mg daily) |
No current FDA approvals (approved in China for ITP and aplastic anemia) |
Per drug label.
TPO, thrombopoietin. CLD, chronic liver disease. N/A, not applicable.
Table 3.
Representative studies of TPO-RAs for treatment/prevention of CIT
| Investigators | Study design | Patient population | Efficacy outcomes | Safety outcomes |
|---|---|---|---|---|
| Soff et al3,36 | Phase 2 trial: patients randomized to romiplostim for treatment of CIT or untreated observation Long-term extension study for patients who continued romiplostim treatment for at least one year |
60 patients with solid tumor cancers receiving various chemotherapy regimens; 52 patients receiving romiplostim after conversion to single-arm study 20 patients included in long-term extension study |
85% of patients receiving romiplostim in phase 2 study achieved platelet count > 100 x 109/L compared with 12% of patients in observation group. Only 7% of these patients required chemotherapy dose reduction/delay. 70% of patients in the extension study experienced no further CIT |
10% of patients in phase 2 study experienced VTE. Two patients in the extension study experienced VTE. Overall, rates were similar to historical controls of cancer associated VTE |
| Al-Samkari et al4 | Observational cohort of patients receiving romiplostim for treatment of CIT | 153 solid tumor and 20 lymphoma/myeloma patients receiving various chemotherapy regimens | 71% achieved median platelet count > 75 x 109/L and > 30 x 109/L. 79% avoided chemotherapy dose reduction/delay, and 89% avoided platelet transfusions. Predictors of nonresponse included: invasion of bone marrow by tumor, prior pelvic irradiation, and temozolomide 10% response rate in patients with lymphoma/myeloma with bone marrow involvement |
VTE rate of 14 per 100 patient years similar to historical controls |
| Kellum et al43 | Phase 2 trial Patients randomized to placebo or eltrombopag 50, 75, or 100 mg for prevention of CIT |
183 chemotherapy-naïve patients with advanced solid tumor cancers receiving carboplatin and paclitaxel 134 patients completed 2 chemotherapy cycles and were evaluated |
Although the primary end point (the difference in platelet counts between cycle 2 day 1 and cycle 2’s platelet nadir) was not met, nadir platelet counts as well as platelet counts at start of subsequent chemotherapy cycles were higher in eltrombopag group. | Similar rates of adverse events in both groups with safety profile similar to placebo with most being unrelated to study drug. Rates of VTE were similar between placebo (7%), 50 and 75 mg groups (5%), and 100 mg group (13%). |
| Winer et al44 | Phase 1 trial Patients randomized to placebo or eltrombopag 100 mg for prevention of CIT |
26 patients with pancreatic cancer receiving gemcitabine or gemcitabine plus cisplatin or carboplatin | Fewer patients required chemotherapy dose reductions or delays in the eltrombopag group (14%) versus placebo (50%). Mean platelet nadirs were higher in the eltrombopag group. |
3 patients receiving eltrombopag experienced VTE, but none were considered related to study drug and treatment was continued. |
| Winer et al45 | Phase 2 trial Patients randomized to placebo or eltrombopag 100 mg for prevention of CIT |
75 patients with solid tumor cancers receiving gemcitabine or gemcitabine plus cisplatin or carboplatin 26 patients completed planned number of chemotherapy cycles |
Fewer patients required dose reductions or delays in both eltrombopag arms versus placebo (monotherapy: 77 vs 91%, combination therapy: 62 vs 83%). Fewer patients had platelet counts < 100 x 109/L at nadir. |
Adverse events were less frequent in both eltrombopag arms versus placebo. Rates of thromboembolic events were higher in monotherapy group (13 vs 8%) and lower in combination therapy group (5 vs 9%). |
| Al-Samkari et al49 | Phase 3 trial Patients randomized to avatrombopag 60 mg daily or placebo for treatment of nadir CIT |
122 patients with ovarian, lung, or bladder cancer receiving various chemotherapy regimens | Similar proportions of patients reached the primary endpoint (reducing platelet transfusions, chemotherapy dose reductions, and chemotherapy dose delays) in the avatrombopag (70%) and placebo groups (73%) attributed to frequent spontaneous platelet recovery in the placebo group. | Similar rates of adverse events in both groups with safety profile similar to placebo Rates of VTE were similar between avatrombopag (2.4%) and placebo groups (2.5%) |
CIT: chemotherapy induced thrombocytopenia, TPO-RA: thrombopoietin receptor agonist, VTE: Venous thromboembolism
3.1. Romiplostim
Romiplostim was the first medication developed in the current class of TPO-RAs. In 1996, a 14-amino acid peptide was identified that bound to the extracellular domain of the TPO receptor and had no sequence homology to native TPO. Romiplostim in its current form was subsequently created by inserting this peptide into an IgG4 heavy chain to improve the half-life to 120 hours [35]. Romiplostim is administered subcutaneously on a weekly basis and is currently FDA-approved for treatment of ITP in adults and children. A representative treatment course for a patient receiving romiplostim for treatment of CIT is illustrated in Figure 1.
Figure 1.
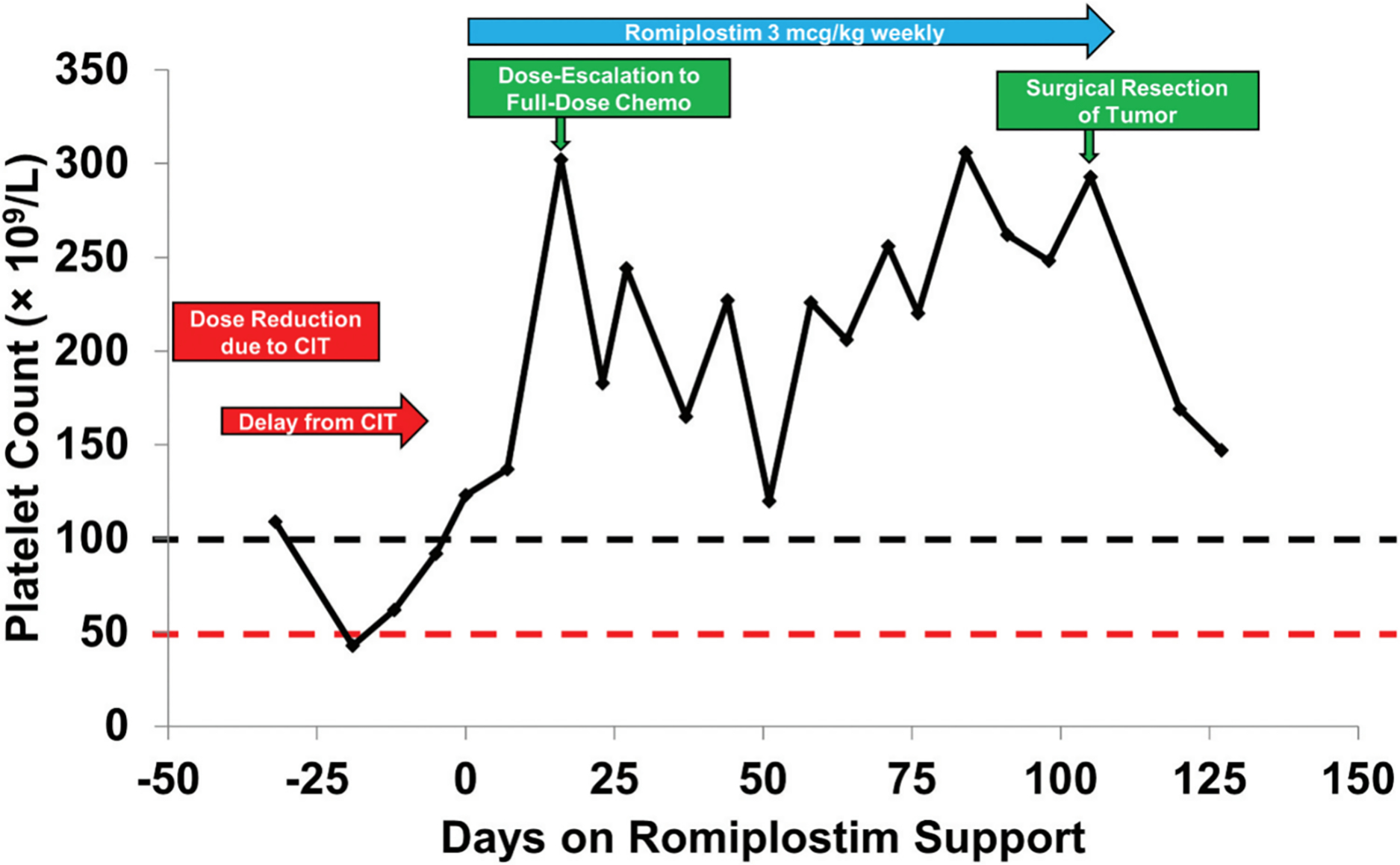
Platelet counts in a representative patient prior to and during romiplostim treatmentPatient was a 39-year-old man with oligometastatic colorectal cancer involving the liver treated for potential curative intent. Prior to initiation of romiplostim, he developed CIT prompting both dose reduction and treatment delay while receiving treatment with fluorouracil, oxaliplatin and leucovorin (FOLFOX). His platelet count improved dramatically with romiplostim treatment allowing resumption of full-dose FOLFOX and without further treatment delays. He completed his remaining prescribed cycles of chemotherapy, which sufficiently treated his cancer to allow for surgical excision of his liver metastasis, achieving cure. Reproduced with permission from Al-Samkari et al<sup>1</sup>.
Soff and colleagues successfully performed a phase II clinical trial investigating the use of romiplostim in CIT [3]. The study was designed as an open-label trial of romiplostim versus untreated observation. Patients were eligible if they had a nonhematologic malignancy with a platelet count of < 100 × 109/L for at least 4 weeks despite dose reduction or delay of prior marrow-suppressive chemotherapy. After 23 patients were enrolled, preliminary analysis demonstrated that 14 of the 15 patients treated with romiplostim had met the primary end point of platelet recovery (defined as platelet count of > 100 × 109/L) within three weeks. The mean platelet count of the patients receiving romiplostim increased from 63 × 109/L to 141 × 109/L within two weeks. Only 1 of the 8 observed patients achieved spontaneous platelet recovery and there was no significant increase in mean platelet counts. The remaining 7 patients crossed over to receive romiplostim and 6 of these patients achieved correction of platelet counts within three weeks; the one additional patient who crossed over to receive romiplostim passed away prior to receiving three weeks of romiplostim. After 23 patients were enrolled, the study converted from a randomized design to a single-arm study in which all patients received romiplostim, given the dramatic difference between the two arms observed at interim analysis. Ultimately, 44 of 52 (85%) patients assigned to romiplostim achieved platelet recovery. These patients all resumed chemotherapy with ongoing romiplostim support and only 3 patients (6.8%) required subsequent chemotherapy dose reduction or treatment delay.
Patients remained on romiplostim if both the patient and treating clinician felt it was beneficial. A long-term efficacy and safety analysis was recently published describing the 21 patients who continued romiplostim treatment for at least one year [36]. The majority of patients who were not included in the analysis discontinued romiplostim due to death, change in goals of care, discontinuation of chemotherapy or enrollment on a new chemotherapy clinical trial unrelated to CIT, and continuity of care. 14 of 20 analyzed patients experienced no further episodes of CIT, 4 patients required a single delay in chemotherapy dose due to CIT without dose reduction, and 2 patients required a chemotherapy dose reduction. 1 patient was not included in the analysis due to receiving chemotherapy at an outside facility although platelet counts always remained > 100 × 109/L.
Safety results were encouraging particularly with regards to the theoretical concern of thromboembolic events. In the initial phase II study, 6 of the 59 treated patients (10%) developed VTE while on romiplostim treatment, with two patients developing pulmonary emboli, two patients experiencing proximal lower extremity DVT, and two patients experiencing distal lower extremity DVT. In the extension study, one patient developed a deep vein thrombosis and remained in the study while being anticoagulated with enoxaparin. Another patient from the extension study developed multiple ischemic thrombotic events but was previously known to be heterozygous for prothrombin G20210A mutation and have prior history of DVT. Overall, the rate of thromboembolic events was felt to be within expectation for this heavily pretreated population with advanced malignancy and metastatic burden; epidemiologic studies of similar populations have described VTE rates of 10–14 events per 100 patient-years [37,38]. VTE onset did not prompt romiplostim discontinuation. While a theoretical thrombotic risk remains, these data, as well as the fact that romiplostim has been demonstrated to have no significant impact on platelet aggregation, or result in spontaneous platelet hyperreactivity, in subjects with ITP [39], provide reassurance.
A recent observational cohort study of 173 patients also further supports the clinical efficacy and safety of romiplostim for treatment of CIT [4]. The study retrospectively evaluated 173 patients with CIT treated with off-label romiplostim by longstanding institutional dosing protocols at four United States institutions. Of the 153 solid tumor patients, 71% of patients achieved the primary endpoint of a romiplostim response (defined as a median on-treatment platelet count of ≥ 75 × 109/L and ≥ 30 × 109/L above pretreatment baseline), and 79% avoided chemotherapy dose reductions or treatment delays. When excluding patients with risk factors for nonresponse derived from multivariable regression (which were identified as bone marrow tumor invasion, pelvic irradiation, and prior receipt of temozolomide), these numbers improved to 95% and 82%, respectively. Figure 2 illustrates the difference in median weekly platelet counts when accounting for these predictors of nonresponse. 89% of patients avoided platelet transfusions. Major bleeding was only observed in 1% of chemotherapy cycles, less than the rate observed in historical CIT cohorts, and thrombosis rates were similar to prior cohorts [6,37,38]. Weekly dosing of romiplostim was superior to intracycle dosing (administering romiplostim during weeks without chemotherapy; twice per month on average) with increased rates of platelet recovery and less chemotherapy dose reduction or delays. Median weekly romiplostim dose was 3 mg/kg.
Figure 2.
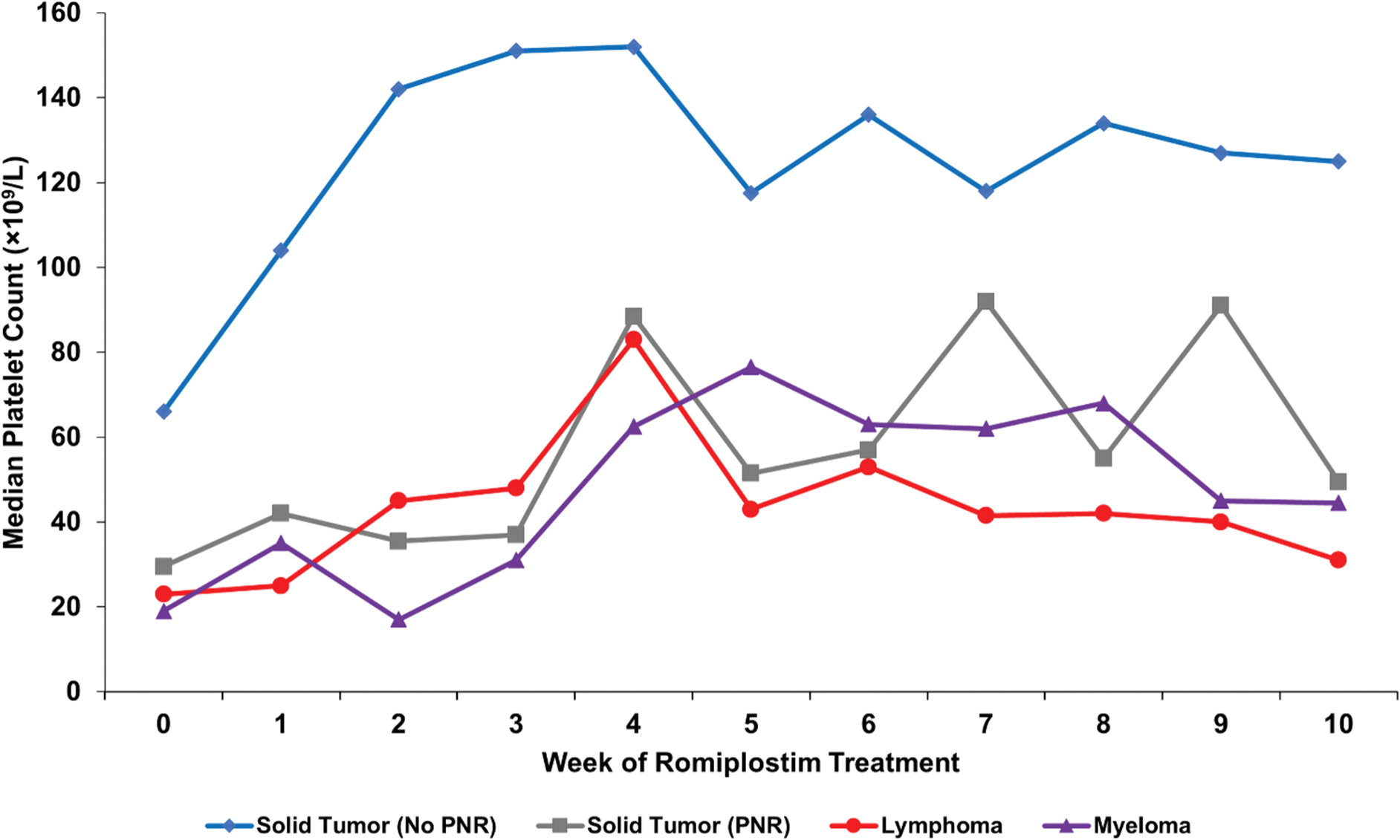
Median weekly platelet counts for various patient populations treated for CIT with romiplostim from a study of 173 patients with CITSolid tumor patients with no predictors of romiplostim non-response (N = 122, blue); solid tumor patients with predictors of romiplostim non-response (N = 31, gray) including bone marrow invasion by tumor, prior pelvic irradiation, or prior temozolomide treatment; aggressive lymphoma patients (N = 13, red); and myeloma patients (N = 7, purple). All lymphoma and myeloma patients had known marrow involvement by tumor. PNR, predictors of non-response. Reproduced with permission from Al-Samkari et al<sup>4</sup>.
Overall, these results provide support for the long-term efficacy and safety of romiplostim for treatment of CIT. For patients with no other known reason for thrombocytopenia and diagnosis of CIT, NCCN guidelines now recommend the following in addition to consideration of platelet transfusions and chemotherapy dose adjustment/delay: 1) enrollment in a clinical trial of TPO-RAs or 2) off-label treatment with romiplostim following a full discussion of potential benefits and harms. Romiplostim is currently the only TPO-RA recommended by the NCCN for potential off-label treatment of CIT [40].
Romiplostim may also have a role in the maintenance of platelet counts in patients receiving targeted cancer therapies [41], though data at present is very limited. Two phase III clinical trials of romiplostim for treatment of CIT are currently enrolling: one (NCT03937154) for adult patients with non-small cell lung cancer, ovarian cancer, or breast cancer receiving carboplatin-based chemotherapy and a second (NCT03362177) for adult patients with gastrointestinal, pancreatic, or colorectal cancer receiving oxaliplatin-based chemotherapy. A phase II clinical trial (NCT04673266) is also enrolling patients with lymphoma and CIT.
3.2. Eltrombopag
Eltrombopag and the other TPO-RAs differ from romiplostim in that they are orally administered small molecules that bind to the transmembrane domain of the TPO receptor. Eltrombopag is administered daily (although its half-life allows for off-label dosing less frequently than once daily [42]). Eltrombopag is currently FDA-approved for treatment of ITP in adults and children, hepatitis C-associated thrombocytopenia, and severe aplastic anemia. Unlike studies of romiplostim, most studies evaluating eltrombopag in CIT evaluate its use for the prevention of CIT in unselected patients with cancer receiving chemotherapy, rather than its use as a treatment for patients who have already developed CIT.
The first study to investigate the potential role for eltrombopag for prevention of CIT was a phase 2 study of 183 patients receiving carboplatin and paclitaxel randomized to receive placebo or eltrombopag (50 mg, 75 mg, 100 mg daily on days 2–11 of a chemotherapy cycle). Although the primary end point (the difference in platelet counts between cycle 2 day 1 and cycle 2’s platelet nadir) was not met, nadir platelet counts in cycles 1 and 2 were higher in the eltrombopag group [43].
A phase I study of patients with solid tumors receiving gemcitabine alone (9 patients) or gemcitabine plus either cisplatin or carboplatin (10 patients) with platelet counts of < 300 × 109/L randomized (3:1) treatment with eltrombopag 100 mg daily versus placebo on days −5 to −1 and days 2–6 starting from cycle 2 of chemotherapy [44]. Mean platelet count in cycles 2–6 was higher in patients receiving eltrombopag versus placebo in both chemotherapy cohorts: 143 × 109/L versus 103 × 109/L in gemcitabine only and 115 × 109/L versus 53 × 109/L in gemcitabine plus cisplatin or carboplatin. 50% of patients receiving placebo required dose reductions or delays compared with only 14% of patients receiving eltrombopag. Three thromboembolic events occurred but were attributed to factors not including eltrombopag treatment.
A phase II study similarly investigated patients with gemcitabine alone (42 patients) or gemcitabine plus carboplatin or cisplatin (32 patients) randomized (2:1) to treatment with eltrombopag 100 mg daily versus placebo with the same dosing schedule as the prior study [45]. The geometric mean platelet count was higher in patients receiving eltrombopag (246 × 109/L) versus patients receiving placebo (193 × 109/L); this difference did not meet statistical significance. There were fewer dose reductions and treatment delays attributed to thrombocytopenia in the eltrombopag cohorts.
A real-world retrospective observational study was recently published evaluating patients with lymphoma and CIT whose platelet counts dropped < 30 × 109/L who were treated with eltrombopag (51 patients) or rhTPO (50 patients); a group of 52 patients who did not receive either agent served as a control group [46]. Nadir and mean platelet count at days 5, 7, and 10 as well as time to platelet recovery were significantly higher in both the eltrombopag and rhTPO groups compared with the control group but without significant difference between the two treatment groups.
There is one single-arm study evaluating eltrombopag for treatment of CIT in patients who have not responded to rhTPO or rhIL-11 treatment (NCT04600960).
3.3. Avatrombopag
Avatrombopag is a more recently approved small molecule that also binds to the transmembrane domain of the TPO receptor. Avatrombopag is FDA approved for adults with chronic ITP and patients with periprocedural thrombocytopenia in the setting of chronic liver disease [32,47,48].
A recent global randomized, double-blind, placebo-controlled phase III study was recently published investigated the use of avatrombopag in chemotherapy-naïve patients with CIT receiving chemotherapy for ovarian, small cell lung, non-small cell lung, or bladder cancer [49]. Patients were included if they had CIT as defined by at least two platelet counts < 50 × 109/L during a chemotherapy cycle (functionally, nadir CIT). Exclusion criteria included receipt of multiple prior chemotherapy regimens and any prior CIT. 122 patients were randomized (2:1) to avatrombopag 60 mg daily versus placebo given 5 days before and after chemotherapy. Although avatrombopag did increase platelet counts, there was no statistically significant different in the primary endpoint of reducing platelet transfusions, chemotherapy dose reductions, and chemotherapy dose delays (69.5% in the avatrombopag group versus 72.5% in the placebo group). Although there was no significant difference in the primary endpoint, this was attributed to frequent spontaneous platelet recovery in the placebo group without use of TPO-RA therapy in this relatively chemotherapy-naïve population. These results suggest that relatively chemotherapy-naïve patients experiencing nadir CIT that does not persist to the start of the following chemotherapy cycle may not benefit from TPO-RA treatment due to a low likelihood of future recurrence. Avatrombopag was safe and well-tolerated without an increased incidence of thromboembolic events compared with placebo.
There is one single-arm study evaluating avatrombopag for treatment of CIT in patients who have not responded to rhTPO or IL-11 treatment (NCT05218226).
3.4. Hetrombopag
Hetrombopag is the newest TPO-RA and is also a small molecule that binds to the transmembrane domain of the TPO receptor. Hetrombopag is currently only approved in China with treatment of ITP and severe aplastic anemia [50–52]. A clinical trial evaluating the role of hetrombopag in solid tumor patients with CIT is currently underway (NCT03976882).
4. Principles of TPO-RA treatment for CIT
Although none of the TPO-RAs are currently approved by the FDA for treatment of CIT, romiplostim is currently the only TPO-RA recommended by NCCN guidelines for consideration of off-label use. Romiplostim currently has the most robust literature supporting this off-label use [40]. For patients with persistent CIT, we recommend that patients should be enrolled in a clinical trial investigating the use of TPO-RA for which several studies are currently enrolling if such a trial is available. If patients are not eligible for clinical trial participation or a trial is unavailable, we recommend consideration of off-label use of romiplostim in those patients with persistent CIT or severe nadir CIT (platelet count < 20 × 109/L) or those with nadir CIT who have already experienced clinically relevant bleeding events.
Prior studies have demonstrated that weekly dosing of romiplostim is superior to intracycle dosing on chemotherapy-off weeks. Romiplostim can be initiated at 2–4 ug/kg weekly and titrated by increments of 1–2 ug/kg to achieve a modest platelet count of 100 × 109/L at the beginning of chemotherapy cycles, a threshold which would be unlikely to require any chemotherapy dose reduction or delay. If platelet counts do not meaningfully recover after several weeks of maximal romiplostim dosing (10 ug/kg), romiplostim should not be considered for further use.
Not all patients respond well to romiplostim whether they require significant dose titration or whether there is lack of response at all. Prior retrospective analysis of 173 patients receiving romiplostim identified three key predictors of non-response via multivariable regression: bone marrow tumor invasion, pelvic irradiation, and prior receipt of temozolomide [4]. Baseline endogenous TPO levels may also be a predictive biomarker for response to TPO therapy with a recent analysis demonstrating that lower baseline TPO levels predicted for likelihood and depth of response to romiplostim as well as a lower effective dose of romiplostim [53,54]. Regardless of these predictors of response or non-response, it is reasonable that any patient with persistent CIT be considered for a trial of romiplostim treatment as some patients with unfavorable predictive factors did ultimately respond in these studies.
History of thromboembolic events is not an absolute contraindication for TPO-RA treatment. Prior clinical trials permitted inclusion of patients with recent thromboembolic events if they were being anticoagulated without complications. As discussed previously, the clinical trials to date and retrospective analyses have not demonstrated an increased signal for thromboembolic events compared with historical cancer patient cohorts although none of the trials were powered to evaluate for differences in rates of thromboembolic events. However, significant attention is warranted for patients with strong thrombophilia such as history of recurrent VTE despite therapeutic anticoagulation or triple-positive antiphospholipid syndrome. In these patients, use of TPO-RA should be decided on a case by case basis given the lack of data evaluating TPO-RA use in these high-risk patient populations [2].
5. Expert Opinion
Data supporting the use of TPO-RAs for treatment of CIT have been strongest for romiplostim, and definitive phase 3 trials are ongoing. Further studies are needed exploring the use of the oral TPO-RAs for treatment of CIT. Prior studies of eltrombopag have mostly focused on prevention of CIT and the recent phase III study of avatrombopag for treatment of CIT did not meet its primary endpoint due to high rates of spontaneous platelet recovery in the placebo group. Therefore, the oral TPO-RAs are only recommended for use in CIT in the setting of a clinical trial.
Several factors may impact patient preference and adherence to different TPO-RAs [55,56]. Romiplostim is administered as a subcutaneous injection which may be less favorable for patients compared to the straight administration of an oral medication but may be favorable for patients experiencing ongoing nausea, vomiting, aspiration risk, or malabsorption. Eltrombopag requires a 4 to 6-hour period of fasting around administration as it is poorly absorbed when taken with high-fat and high-calcium meals due to drug chelation of polyvalent cations. Eltrombopag and hetrombopag both require liver chemistry monitoring due to risk of hepatotoxicity. The logistical simplicity of dose adjustments is also different between TPO-RAs, and the relative potency is not defined in CIT; as in other diseases, patients not responding to one agent may respond to another [57,58]. Once sufficient efficacy and safety data are reported for the oral TPO-RAs to consider routine use for treatment of CIT, real world observational data will be needed to assess patient preference and adherence.
Several large retrospective analyses describe the heterogeneous rates of CIT associated with different chemotherapy regimens. In addition to impact from variable dosing, different chemotherapy regimens may have variable propensity for inducing CIT based on each chemotherapy’s impact on physiologic megakaryocyte and platelet development. The pathophysiology of CIT is diverse with different chemotherapy agents having variable pathologic impact on different stages of megakaryocyte and platelet development. In addition to variable dosing of chemotherapy, this may explain the vastly heterogeneous rates of CIT associated with different chemotherapy regimens. More studies are needed to explore the efficacy of TPO-RAs for treatment of CIT in the setting of different chemotherapy regimens.
Although a distinct disease entity from ITP, the potential role of the immune system in CIT may be an area for further exploration. As one example, single-agent fludarabine for treatment of lymphomas has been associated with an antiplatelet antibody-mediated ITP that is responsive to rituximab [59,60]. Many myelosuppressive and cytotoxic agents have more subtle but nevertheless deleterious impacts on the immune system, as does active cancer itself. Conversely, TPO-RAs have also been shown to have potential immunomodulatory effect in patients with ITP. Recent studies have associated TPO-RAs with: correction of increased M1-characteristics, correction of IFN-γ/IL-4 ratio, and reduced peripheral monocyte expansion [61,62]; reduction in platelet autoantibody levels [63]; promotion of regulatory B-cell activity [64]; and reversal of reduced regulatory T cell number and activity in patients with ITP [65–68]. There are essentially no studies evaluating any significant pathophysiologic role of immune dysregulation, which is common in patients with cancer, in CIT. Given the potential positive impact of TPO-RA treatment on these immune parameters, this is an intriguing potential topic of future study.
Lastly, this review has thus far discussed the use of TPO-RA for management of CIT in patient with solid tumor malignancies. In the previously discussed retrospective analysis of 173 patients with CIT receiving romiplostim, 20 patients had a non-myeloid hematologic malignancy and only 10% of them achieved response to romiplostim, but all of the patients with hematologic malignancy had bone marrow involvement by the tumor [4]. Until further study is done, the existing literature primarily supports the use of TPO-RAs for treatment of CIT in patients with solid tumor malignancies and is insufficient to support the use of TPO-RAs in patients with non-myeloid hematologic malignancies. Although TPO-RAs are sometimes used off-label in patients with MDS with severe thrombocytopenia [70], this is for a different use than CIT. Discussion of TPO-RA use in patients with myeloid malignancies or post-stem cell transplant is beyond the scope of this review and we refer the reader to a recent review by Desborough and colleagues [69].
In conclusion, CIT is a common complication of cancer treatment which may lead to reduced chemotherapy relative dose intensity and treatment delay. Although there are no FDA-approved agents for treatment of CIT, NCCN guidelines recommend participation in a clinical trial for consideration of TPO-RA treatment or considering off-label use of romiplostim when participation in clinical trials is not possible. The literature to date supports the use of TPO-RAs for treatment of persistent CIT. Further data is needed to describe the long-term efficacy, safety, and prescribing practices of TPO-RAs in a diverse patient population with a variety of tumor types and chemotherapy regimens in addition to exploring the underlying biology of CIT.
Article Highlights:
Despite the clinical challenges presented by CIT, there are currently no available agents approved by the U.S Food and Drug Agency (FDA) for treatment or prevention of CIT. Management had previously been limited to supportive platelet transfusions and reduction of chemotherapy relative dose intensity.
NCCN guidelines recommend that patients with CIT should participate in a clinical trial for consideration of TPO-RA treatment or consider off-label use of romiplostim. Romiplostim currently has the most robust literature supporting this off-label use.
TPO-RAs have been safe and well-tolerated. Despite a theoretical risk of thrombosis, rates of thromboembolic events in studies of TPO-RAs for CIT are comparable with historical rates of cancer-associated thrombosis.
The existing literature primarily supports the use of TPO-RAs for treatment of CIT in patients with solid tumor malignancies and is insufficient to support the use of TPO-RAs in patients with non-myeloid hematologic malignancies.
Footnotes
Declaration of interest:
H. Al-Samkari is the recipient of the American Society of Hematology Scholar Award. H Al-Samkari has also received research funding from Agios, Dova/Sobi and Amgen; and consultancy from Agios, Dova/Sobi, Forma, Argenx, Rigel, Moderna, and Novartis. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer Disclosures:
A peer review on this manuscript is an advisory board member for Amgen and Sobieer. Peer reviewers on this manuscript have no other relevant financial or other relationships to disclose.
References
Papers of special note have been highlighted as either of interest (*) or of considerable interest (**) to readers.
- 1.Al-Samkari H, Soff GA. Clinical challenges and promising therapies for chemotherapy-induced thrombocytopenia. Expert Rev Hematol. 2021. May;14(5):437–448. [DOI] [PubMed] [Google Scholar]
- 2.Al-Samkari H Thrombopoietin receptor agonists for chemotherapy-induced thrombocytopenia: a new solution for an old problem. Hematology Am Soc Hematol Educ Program. 2022. Dec 9;2022(1):286–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Soff GA, Miao Y, Bendheim G, et al. Romiplostim Treatment of Chemotherapy-Induced Thrombocytopenia. J Clin Oncol. 2019. Nov 1;37(31):2892–2898. ** This definitive phase II randomized trial of demonstrated romiplostim’s safety and efficacy for treatment of persistent CIT.
- 4. Al-Samkari H, Parnes AD, Goodarzi K, et al. A multicenter study of romiplostim for chemotherapy-induced thrombocytopenia in solid tumors and hematologic malignancies. Haematologica. 2021. Apr 1;106(4):1148–1157. ** This observational cohort study of 173 patients describes the safety, efficacy, and predictors of nonresponse to romiplostim.
- 5.Al-Samkari H, Marshall AL, Goodarzi K, et al. The use of romiplostim in treating chemotherapy-induced thrombocytopenia in patients with solid tumors. Haematologica. 2018. Apr;103(4):e169–e172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Elting LS, Rubenstein EB, Martin CG, et al. Incidence, cost, and outcomes of bleeding and chemotherapy dose modification among solid tumor patients with chemotherapy-induced thrombocytopenia. J Clin Oncol. 2001. Feb 15;19(4):1137–46. * This large cohort study emphasizes the bleeding rates in CIT and rates of dose modification over 1000 chemotherapy cycles.
- 7.Denduluri N, Patt DA, Wang Y, et al. Dose Delays, Dose Reductions, and Relative Dose Intensity in Patients With Cancer Who Received Adjuvant or Neoadjuvant Chemotherapy in Community Oncology Practices. J Natl Compr Canc Netw. 2015. Nov;13(11):1383–93. [DOI] [PubMed] [Google Scholar]
- 8.Patell R, Zwicker JI. Evidence-Based Minireview: Full dose, modified dose, or no anticoagulation for patients with cancer and acute VTE and thrombocytopenia. Hematology Am Soc Hematol Educ Program. 2022. Dec 9;2022(1):312–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goodnough LT, DiPersio JF. Issues in the management of cancer-related thrombocytopenia. Oncology (Williston Park). 2002. Nov;16(11):1558–67; discussion 1570, 1572–4. [PubMed] [Google Scholar]
- 10.Wilde MI, Faulds D. Oprelvekin: a review of its pharmacology and therapeutic potential in chemotherapy-induced thrombocytopenia. BioDrugs. 1998. Aug;10(2):159–71. [DOI] [PubMed] [Google Scholar]
- 11.Cantor SB, Elting LS, Hudson DV Jr., et al. Pharmacoeconomic analysis of oprelvekin (recombinant human interleukin-11) for secondary prophylaxis of thrombocytopenia in solid tumor patients receiving chemotherapy. Cancer. 2003. Jun 15;97(12):3099–106. [DOI] [PubMed] [Google Scholar]
- 12.Kuter DJ. Managing thrombocytopenia associated with cancer chemotherapy. Oncology (Williston Park). 2015. Apr;29(4):282–94. [PubMed] [Google Scholar]
- 13.Emmons RV, Reid DM, Cohen RL, et al. Human thrombopoietin levels are high when thrombocytopenia is due to megakaryocyte deficiency and low when due to increased platelet destruction. Blood. 1996. May 15;87(10):4068–71. [PubMed] [Google Scholar]
- 14.Kuter DJ. The physiology of platelet production. Stem Cells. 1996;14 Suppl 1:88–101. [DOI] [PubMed] [Google Scholar]
- 15.Vadhan-Raj S, Patel S, Bueso-Ramos C, et al. Importance of predosing of recombinant human thrombopoietin to reduce chemotherapy-induced early thrombocytopenia. J Clin Oncol. 2003. Aug 15;21(16):3158–67. [DOI] [PubMed] [Google Scholar]
- 16.Vadhan-Raj S, Verschraegen CF, Bueso-Ramos C, et al. Recombinant human thrombopoietin attenuates carboplatin-induced severe thrombocytopenia and the need for platelet transfusions in patients with gynecologic cancer. Ann Intern Med. 2000. Mar 7;132(5):364–8. [DOI] [PubMed] [Google Scholar]
- 17.Fanucchi M, Glaspy J, Crawford J, et al. Effects of polyethylene glycol-conjugated recombinant human megakaryocyte growth and development factor on platelet counts after chemotherapy for lung cancer. N Engl J Med. 1997. Feb 6;336(6):404–9. [DOI] [PubMed] [Google Scholar]
- 18.Basser RL, Rasko JE, Clarke K, et al. Randomized, blinded, placebo-controlled phase I trial of pegylated recombinant human megakaryocyte growth and development factor with filgrastim after dose-intensive chemotherapy in patients with advanced cancer. Blood. 1997. May 1;89(9):3118–28. [PubMed] [Google Scholar]
- 19.Basser RL, Underhill C, Davis I, et al. Enhancement of platelet recovery after myelosuppressive chemotherapy by recombinant human megakaryocyte growth and development factor in patients with advanced cancer. J Clin Oncol. 2000. Aug;18(15):2852–61. [DOI] [PubMed] [Google Scholar]
- 20.Archimbaud E, Ottmann OG, Yin JA, et al. A randomized, double-blind, placebo-controlled study with pegylated recombinant human megakaryocyte growth and development factor (PEG-rHuMGDF) as an adjunct to chemotherapy for adults with de novo acute myeloid leukemia. Blood. 1999. Dec 1;94(11):3694–701. [PubMed] [Google Scholar]
- 21.Geissler K, Yin JA, Ganser A, et al. Prior and concurrent administration of recombinant human megakaryocyte growth and development factor in patients receiving consolidation chemotherapy for de novo acute myeloid leukemia--a randomized, placebo-controlled, double-blind safety and efficacy study. Ann Hematol. 2003. Nov;82(11):677–83. [DOI] [PubMed] [Google Scholar]
- 22.Moskowitz CH, Hamlin PA, Gabrilove J, et al. Maintaining the dose intensity of ICE chemotherapy with a thrombopoietic agent, PEG-rHuMGDF, may confer a survival advantage in relapsed and refractory aggressive non-Hodgkin lymphoma. Ann Oncol. 2007. Nov;18(11):1842–50. [DOI] [PubMed] [Google Scholar]
- 23.Schiffer CA, Miller K, Larson RA, et al. A double-blind, placebo-controlled trial of pegylated recombinant human megakaryocyte growth and development factor as an adjunct to induction and consolidation therapy for patients with acute myeloid leukemia. Blood. 2000. Apr 15;95(8):2530–5. [PubMed] [Google Scholar]
- 24.Basser RL, O’Flaherty E, Green M, et al. Development of pancytopenia with neutralizing antibodies to thrombopoietin after multicycle chemotherapy supported by megakaryocyte growth and development factor. Blood. 2002. Apr 1;99(7):2599–602. [DOI] [PubMed] [Google Scholar]
- 25.Li J, Yang C, Xia Y, et al. Thrombocytopenia caused by the development of antibodies to thrombopoietin. Blood. 2001. Dec 1;98(12):3241–8. [DOI] [PubMed] [Google Scholar]
- 26.Consensus Committee of Chemotherapy Induced Thrombocytopenia CSoCO. [Consensus on clinical diagnosis, treatment and prevention management of chemotherapy induced thrombocytopenia in China(2018)]. Zhonghua Zhong Liu Za Zhi. 2018. Sep 23;40(9):714–720. [DOI] [PubMed] [Google Scholar]
- 27.Mytych DT, Park JK, Kim J, et al. Assessment of romiplostim immunogenicity in adult patients in clinical trials and in a global postmarketing registry. Br J Haematol. 2020. Sep;190(6):923–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Al-Samkari H, Grace RF, Kuter DJ. The role of romiplostim for pediatric patients with immune thrombocytopenia. Therapeutic advances in hematology. 2020;11:2040620720912992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ayad N, Grace RF, Al-Samkari H. Thrombopoietin receptor agonists and rituximab for treatment of pediatric immune thrombocytopenia: A systematic review and meta-analysis of prospective clinical trials. Pediatr Blood Cancer. 2022. Mar;69(3):e29447. [DOI] [PubMed] [Google Scholar]
- 30.Cheloff AZ, Al-Samkari H. Avatrombopag for the treatment of immune thrombocytopenia and thrombocytopenia of chronic liver disease. J Blood Med. 2019;10:313–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nagrebetsky A, Al-Samkari H, Davis NM, et al. Perioperative thrombocytopenia: evidence, evaluation, and emerging therapies. Br J Anaesth. 2019. Jan;122(1):19–31. [DOI] [PubMed] [Google Scholar]
- 32.Song AB, Al-Samkari H. An updated evaluation of avatrombopag for the treatment of chronic immune thrombocytopenia. Expert Rev Clin Immunol. 2022. Aug;18(8):783–791. [DOI] [PubMed] [Google Scholar]
- 33.Park AK, Park JC, Al-Samkari H. Pembrolizumab-Induced Acquired Amegakaryocytic Thrombocytopenia and Successful Combination Treatment With Eltrombopag, Romiplostim and Cyclosporine: A Brief Communication. J Immunother. 2022. Sep 1;45(7):321–323. [DOI] [PubMed] [Google Scholar]
- 34.Al-Samkari H, Marshall AL, Goodarzi K, et al. Romiplostim for the management of perioperative thrombocytopenia. Br J Haematol. 2018. Jul;182(1):106–113. [DOI] [PubMed] [Google Scholar]
- 35.Cwirla SE, Balasubramanian P, Duffin DJ, et al. Peptide agonist of the thrombopoietin receptor as potent as the natural cytokine. Science. 1997. Jun 13;276(5319):1696–9. [DOI] [PubMed] [Google Scholar]
- 36. Wilkins CR, Ortiz J, Gilbert LJ, et al. Romiplostim for chemotherapy-induced thrombocytopenia: Efficacy and safety of extended use. Res Pract Thromb Haemost. 2022. Mar;6(3):e12701. * This extension study of the phase II trial by Soff et al. describes the long-term safety and efficacy of romiplostim in patients who were treated with romiplostim for at least one year.
- 37.Wun T, White RH. Epidemiology of cancer-related venous thromboembolism. Best Pract Res Clin Haematol. 2009. Mar;22(1):9–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khorana AA, Dalal M, Lin J, et al. Incidence and predictors of venous thromboembolism (VTE) among ambulatory high-risk cancer patients undergoing chemotherapy in the United States. Cancer. 2013. Feb 1;119(3):648–55. [DOI] [PubMed] [Google Scholar]
- 39.Al-Samkari H, Van Cott EM, Kuter DJ. Platelet aggregation response in immune thrombocytopenia patients treated with romiplostim. Ann Hematol. 2019. Mar;98(3):581–588. [DOI] [PubMed] [Google Scholar]
- 40.Griffiths EA, Roy V, Alwan L, et al. NCCN Guidelines(R) Insights: Hematopoietic Growth Factors, Version 1.2022. J Natl Compr Canc Netw. 2022. May;20(5):436–442. [DOI] [PubMed] [Google Scholar]
- 41.Cheloff AZ, Al-Samkari H. Romiplostim for PARP inhibitor-induced thrombocytopenia in solid tumor malignancies. Platelets. 2022. Nov 17;33(8):1312–1313. [DOI] [PubMed] [Google Scholar]
- 42.Al-Samkari H, Kuter DJ. An alternative intermittent eltrombopag dosing protocol for the treatment of chronic immune thrombocytopenia. Br J Clin Pharmacol. 2018. Nov;84(11):2673–2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kellum A, Jagiello-Gruszfeld A, Bondarenko IN, et al. A randomized, double-blind, placebo-controlled, dose ranging study to assess the efficacy and safety of eltrombopag in patients receiving carboplatin/paclitaxel for advanced solid tumors. Curr Med Res Opin 2010. Oct;26(10):2339–46. [DOI] [PubMed] [Google Scholar]
- 44.Winer ES, Safran H, Karaszewska B, et al. Eltrombopag with gemcitabine-based chemotherapy in patients with advanced solid tumors: a randomized phase I study. Cancer Med. 2015. Jan;4(1):16–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Winer ES, Safran H, Karaszewska B, et al. Eltrombopag for thrombocytopenia in patients with advanced solid tumors receiving gemcitabine-based chemotherapy: a randomized, placebo-controlled phase 2 study. Int J Hematol. 2017. Dec;106(6):765–776. * This phase 2 randomized study evaluated use of eltrombopag to prevent CIT in patients with advanced solid tumors receiving gemcitabine-based chemotherapy. It is the most recent major study to evaluate eltrombopag in CIT.
- 46.Zhu Q, Yang S, Zeng W, et al. A Real-World Observation of Eltrombopag and Recombinant Human Thrombopoietin (rhTPO) in Lymphoma Patients With Chemotherapy Induced Thrombocytopenia. Front Oncol. 2021;11:701539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Virk ZM, Kuter DJ, Al-Samkari H. An evaluation of avatrombopag for the treatment of thrombocytopenia. Expert Opin Pharmacother. 2021. Feb;22(3):273–280. [DOI] [PubMed] [Google Scholar]
- 48.Al-Samkari H Avatrombopag maleate for the treatment of periprocedural thrombocytopenia in patients with chronic liver disease. Drugs of today. 2018. Nov;54(11):647–655. [DOI] [PubMed] [Google Scholar]
- 49. Al-Samkari H, Kolb-Sielecki J, Safina SZ, et al. Avatrombopag for chemotherapy-induced thrombocytopenia in patients with non-haematological malignancies: an international, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Haematol. 2022. Mar;9(3):e179–e189. ** This phase III randomized trial investigated avatrombopag for treatment of CIT. Both the avatrombopag and placebo groups achieved high rates of the primary endpoint suggesting that chemotherapy-naïve patients experiencing a transient nadir CIT may not require TPO-RA treatment before spontaneous recovery.
- 50.Mei H, Liu X, Li Y, et al. A multicenter, randomized phase III trial of hetrombopag: a novel thrombopoietin receptor agonist for the treatment of immune thrombocytopenia. J Hematol Oncol. 2021. Feb 25;14(1):37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Syed YY. Hetrombopag: First Approval. Drugs. 2021. Sep;81(13):1581–1585. [DOI] [PubMed] [Google Scholar]
- 52.Peng G, He G, Chang H, et al. A multicenter phase II study on the efficacy and safety of hetrombopag in patients with severe aplastic anemia refractory to immunosuppressive therapy. Ther Adv Hematol. 2022;13:20406207221085197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Song AB, Goodarzi K, Karp Leaf R, et al. Thrombopoietin level predicts response to treatment with romiplostim in chemotherapy-induced thrombocytopenia. Am J Hematol. 2021. Dec 1;96(12):1563–1568. [DOI] [PubMed] [Google Scholar]
- 54.Al-Samkari H, Kuter DJ. Thrombopoietin level predicts response to treatment with eltrombopag and romiplostim in immune thrombocytopenia. Am J Hematol. 2018. Dec;93(12):1501–1508. [DOI] [PubMed] [Google Scholar]
- 55.Al-Samkari H, Kuter DJ. Optimal use of thrombopoietin receptor agonists in immune thrombocytopenia. Therapeutic advances in hematology. 2019;10:2040620719841735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Al-Samkari H, Kuter DJ. Immune Thrombocytopenia in Adults: Modern Approaches to Diagnosis and Treatment. Semin Thromb Hemost. 2020. Apr;46(3):275–288. [DOI] [PubMed] [Google Scholar]
- 57.Al-Samkari H, Kuter DJ. Relative potency of the thrombopoietin receptor agonists eltrombopag, avatrombopag and romiplostim in a patient with chronic immune thrombocytopenia. Br J Haematol. 2018. Oct;183(2):168. [DOI] [PubMed] [Google Scholar]
- 58.Al-Samkari H, Jiang D, Gernsheimer T, et al. Adults with immune thrombocytopenia who switched to avatrombopag following prior treatment with eltrombopag or romiplostim: A multicentre US study. Br J Haematol. 2022. May;197(3):359–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Leach M, Parsons RM, Reilly JT, et al. Autoimmune thrombocytopenia: a complication of fludarabine therapy in lymphoproliferative disorders. Clin Lab Haematol. 2000. Jun;22(3):175–8. [DOI] [PubMed] [Google Scholar]
- 60.Hegde UP, Wilson WH, White T, et al. Rituximab treatment of refractory fludarabine-associated immune thrombocytopenia in chronic lymphocytic leukemia. Blood. 2002. Sep 15;100(6):2260–2. [PubMed] [Google Scholar]
- 61.Yang F, Zong H, Li F, et al. Eltrombopag modulates the phenotypic evolution and potential immunomodulatory roles of monocytes/macrophages in immune thrombocytopenia. Platelets. 2023. Dec;34(1):2135694. [DOI] [PubMed] [Google Scholar]
- 62.Di Paola A, Palumbo G, Merli P, et al. Effects of Eltrombopag on In Vitro Macrophage Polarization in Pediatric Immune Thrombocytopenia. Int J Mol Sci. 2020. Dec 24;22(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kapur R, Aslam R, Speck ER, et al. Thrombopoietin receptor agonist (TPO-RA) treatment raises platelet counts and reduces anti-platelet antibody levels in mice with immune thrombocytopenia (ITP). Platelets. 2020;31(3):399–402. [DOI] [PubMed] [Google Scholar]
- 64.Li X, Zhong H, Bao W, et al. Defective regulatory B-cell compartment in patients with immune thrombocytopenia. Blood. 2012. Oct 18;120(16):3318–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bao W, Bussel JB, Heck S, et al. Improved regulatory T-cell activity in patients with chronic immune thrombocytopenia treated with thrombopoietic agents. Blood. 2010. Nov 25;116(22):4639–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Monzon Manzano E, Alvarez Roman MT, Justo Sanz R, et al. Platelet and immune characteristics of immune thrombocytopaenia patients non-responsive to therapy reveal severe immune dysregulation. Br J Haematol. 2020. Jun;189(5):943–953. [DOI] [PubMed] [Google Scholar]
- 67.Kapur R Regulatory T cells are replenished in the splenic microenvironment of patients with immune thrombocytopenia by treatment with thrombopoietin receptor agonists. Br J Haematol. 2022. Sep;198(5):803–804. [DOI] [PubMed] [Google Scholar]
- 68.Pizzi M, Vianello F, Binotto G, et al. Thrombopoietin receptor agonists increase splenic regulatory T-cell numbers in immune thrombocytopenia. Br J Haematol. 2022. Sep;198(5):916–922. [DOI] [PubMed] [Google Scholar]
- 69.Desborough M, Estcourt LJ, Doree C, et al. Alternatives, and adjuncts, to prophylactic platelet transfusion for people with haematological malignancies undergoing intensive chemotherapy or stem cell transplantation. Cochrane Database Syst Rev. 2016. Aug 22;2016(8):CD010982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fenaux P, Muus P, Kantarjian H, et al. Romiplostim monotherapy in thrombocytopenic patients with myelodysplastic syndromes: long-term safety and efficacy. Br J Haematol. 2017. Sep;178(6):906–913. [DOI] [PMC free article] [PubMed] [Google Scholar]


