Abstract
Alzheimer's disease (AD) is the most common cause of dementia worldwide. Pathological deposits of neurotoxic proteins within the brain, such as amyloid-ß and hyperphosphorylated tau tangles, are the prominent features in AD. According to recent studies, the newly discovered brain lymphatic system was demonstrated to be crucial in the clearance of metabolic macromolecules from the brain. Meningeal lymphatic vessels located in the dura mater drain the fluid, macromolecules, and immune cells from cerebrospinal fluid (CSF) and transport them, as lymph, to the deep cervical lymph nodes. The lymphatic system provides the perivascular exchange of CSF with interstitial fluid (ISF) and ensures the homeostasis of neuronal interstitial space. In this review, we aim to summarize recent findings on the role of the lymphatic system in AD pathophysiology and discuss possible therapeutic perspectives, targeting the lymphatic clearance mechanisms within the brain.
Keywords: Dementia, meningeal lymphatic vessels (MLVs), glymphatic system, cerebrospinal fluid (CSF), interstitial fluid (ISF), perivascular space, amyloid-β (Aβ)
1. INTRODUCTION
The main clinical manifestations of Alzheimer’s disease (AD) are gradual cognitive and physical impairments [1]. Accumulation of extracellular aggregates of amyloid - β (Aβ) in the form of plaques and intracellular deposits of neurofibrillary tangles of hyperphosphorylated forms of microtubule-associated protein tau has been recognized as a neuropathological hallmark in AD. The accumulation of these neurotoxic proteins within the brain results in growing damage and death of neurons and is thought to be a consequence of the discrepancy between their production and clearance [2, 3].
Throughout our body, the lymphatic system is the most important structure that facilitates the clearance of extracellular space. Initial, open-ended lymphatic capillaries drain the excess fluid and products of metabolism, cell breakdown products, including Danger/Damage Associated Molecular Patterns (DAMP), lipoproteins, immune cells, hormones, bacteria, and other infectious microorganisms from interstitial space. Larger collecting lymphatic vessels transport the lymph to the local lymph nodes and finally, through the thoracic duct, pass it on to the subclavian veins, at the base of the neck [4]. Lymphatic system prevents tissue oedema, provides an appropriate environment to the body cells, and is important for the initiation of immune response and maintenance of tolerance to self-antigens [5].
Lymphatic capillaries are present in the majority of organs, except bone marrow and avascular tissues such as cartilage, cornea, or epidermis [6]. Historically, it was thought that the central nervous system (CNS) lacks the lymphatic system [6]. Subarachnoid cerebrospinal fluid (CSF) and the interstitial fluid (ISF) have been thought to be absorbed by arachnoid granulations and released into the dural venous sinuses [7]. Recent studies, however, demonstrated that the cerebral lymphatic system exists and consists of two parts: meningeal lymphatic vessels (MLVs), located in the dura mater, and glymphatic system, situated in the brain interstitium [8-12]. MLVs transport the mixture of CSF and ISF from the brain to the deep cervical lymph nodes (dcLN) and can efficiently drain fluid with molecules and immune cells from the brain. The glymphatic system provides a perivascular passage way along which CSF and ISF are quickly exchanged. MLVs and the glymphatic system facilitates clearance of Aβ, tau, and other toxic proteins from the interstitial space in the brain. Dysfunction of this drainage system is crucial in the pathogenesis of AD [11, 13-15].
In this review, we summarize the most recent findings on the role of lymphatic system in the pathophysiology of AD. We also discuss possible new therapeutic strategies associated with lymphatic clearance mechanisms.
2. SEARCH STRATEGY AND SELECTION CRITERIA
References for this review were identified through searches of PubMed for articles published from January 2010, to July 2021, by the use of the following terms: “glymphatic”, “meningeal lymphatic vessels”, “dural lymphatics”, “brain clearance”, and the combination of the mentioned terms with: “Alzheimer”, “dementia”, neurological disorders” or “central nervous system” and “future treatments targeting the glymphatic system”. Articles resulting from these searches and relevant references cited in those articles were reviewed. Articles published in English were included.
3. MENINGEAL LYMPHATIC SYSTEM
3.1. Anatomy and Physiology of Meningeal Lymphatic Vessels
The first observation of the presence of lymphatic vessels on the surface of the human brain dated back to 1787 and was presented by Italian anatomist and physician Paolo Mascagni [16]. However, his studies have been largely ignored by the scientific community and it took more than 200 years to rediscover the MLVs networks and to undeniably confirm that MLVs are responsible for the drainage of CSF from the brain. Firstly, Louveau et al. and Aspelund et al. have independently described detailed anatomy and function of the intracranial lymphatic network in rodents using high-powered electron microscopes for scanning the brains [8, 9]. Then, analogous lymphatic structures were visualized in vivo by Absinta and colleagues in humans and nonhuman primates by using high-resolution magnetic resonance imaging (MRI) technique [10].
MLVs network is situated within the dura mater and MLVs are larger and more ramified in the transverse sinuses than in the superior sagittal sinus [9]. MLVs, although anatomically and molecularly resemble lymphatic vessels from other organs, also have some characteristic features [8, 9]. MLVs are narrower, frequently discontinuous, lack the intraluminal valves (except MLVs near the base of the skull), and form less complex network than lymphatics in other organisms [8, 9, 17]. The specificity of MVLs probably is a result of high CSF pressure within the CNS compared to the interstitial fluid pressure in peripheral tissues, which probably limit MVLs branching and spreading [9].
CSF is produced mostly by ependymal cells of the choroid plexus in the ventricular system of the brain. CSF flows through the ventricular system into the subarachnoid space surrounding the brain and the spinal cord [18]. MLVs drain the CSF from the adjacent subarachnoid space, and the ISF comes from the glymphatic system. This mixture fluid is further transported by MLVs into dcLNs, via foramina at the base of the skull [8]. Some lymph is also transported through the lymph vessels passing the cribriform plate with the olfactory nerves (Fig. 1).
Fig. (1).
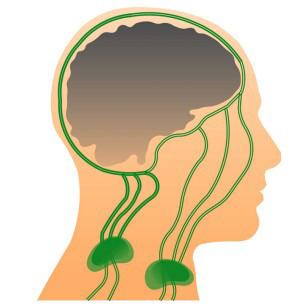
Scheme of the meningeal lymphatic system in humans. Meningeal lymphatic vessels (MLVs) are situated within the dura mater and drain the cerebrospinal fluid (CSF) from the adjacent subarachnoid space, and the interstitial fluid (ISF) coming from the glymphatic system. This mixture (CSF-ISF) fluid is further transported by MLVs into deep cervical lymph nodes (dcLNs) via foramina at the base of the skull. Some lymph is also transported through the lymph vessels passing the cribriform plate with the olfactory nerves into superficial cervical lymph nodes (dcLNs). Figure adapted and modified from Tamura, R. Neurosurg Rev 2020, 43, 1055-1064.
Masquita et al. demonstrated that MLVs provide proteostasis (proper concentration of proteins in brain fluids) and cognitive functions [13]. Interestingly, scavenging receptor class B type 1 (SR-B1), present in peripheral lymphatic endothelial cells [19], also binds fibrillar Aβ [20]. Although the presence of SR-B1 in MLVs was not confirmed yet, it would advocate active participation of MLVs in the clearance of Aβ from the subarachnoid space.
3.2. VEGF-C – VEGFR-3 Regulation of Meningeal Lymphatics
MLVs develop during the first postnatal month. It is much later in comparison to the time of fetal development of lymphatic vessels in other organs [6]. MLVs appear firstly around the foramina at the base of the skull. Then, they sprout alongside the cranial blood vessels and nerves within the meninges [21]. It was reported that vascular endothelial growth factor C (VEGF-C) and VEGF receptor 3 (VEGFR-3) are crucial for MLVs development, similarly as in other organs [8]. Interestingly, VEGF-C - VEGFR-3 signaling was demonstrated to be essential not only during developmental lymphangiogenesis of MLVs, but also for the maintenance of MLVs integrity during adulthood. Deletion or blocking of VEGF-C -VEGFR-3 signaling in adult mice resulted in MLVs regression and impairment of lymphatic drainage to dcLNs [21]. VEGF-D deletion was not significant [21]. Contrary, treatment of adult mice with VEGF-C resulted in meningeal lymphangiogenesis [21], an increase in MLVs diameter, increased brain perfusion, greater lymphatic drainage of macromolecules from CSF, and improved learning and memory performance [13].
4. GLYMPHATIC SYSTEM
4.1. Structure of Glymphatic System
The delicate structure of CNS requires strict regulation of the extracellular environment of neurons, including optimal drainage and intense removal of waste. Brain tissue lacks a typical lymphatic drainage system. However, recently, a specific cerebral lymphatic system named “glymphatic system” was described [11].
Initially, animal studies have demonstrated that CSF flows from subarachnoid space to perivascular spaces within the brain [22, 23]. lliff end colleagues, based on in vivo two-photon imaging of small fluorescent tracers, have identified the entire pathway of subarachnoid CSF and its solute across brain parenchyma in mice [11]. In brief, they documented that CSF enters the brain via peri-arterial spaces surrounding penetrating cerebral arteries, then passes into the brain interstitium, taking away solutes from extracellular space and becoming ISF. After flowing through the brain via periarterial spaces, ISF drains into perivenous and perineuronal spaces around large-caliber cerebral veins and nerves. Finally, ISF from perivenous and perineuronal spaces drains from subarachnoid space into MLVs of the dura mater [11].
With regards to the dependence upon glial water transport and its similarity to the draining function of the lymphatic system, this fluid drainage pathway was termed the “glia-lymphatic (glymphatic) system” [11]. The interstitial flow of CSF-ISF between “influx” (para-arterial spaces) and “efflux” (perivenous or perineuronal spaces) sites was demonstrated to be mediated by aquaporin 4 water channels (AQP4), which are densely situated on perivascular astrocyte endfeet [11, 24].The continuous movement of fluid throughout the brain interstitium provides the clearance of waste from the CNS. Impaired glymphatic function is relevant in the pathogenesis of AD [11, 14, 15].
4.2. Factors Facilitating Flow in the Glymphatic System
CSF-ISF transport from subarachnoid space through para-arterial spaces to the brain interstitium is driven primarily by the cardiac cycle and mediated by arterial pulsation [25-27]. Interstitial brain fluid flows at the same frequency as the cardiac cycle, and in the same direction as the blood flow [11, 28]. Impairment of arterial pulsatility may contribute to the deposition of toxins, including Aβ accumulation [25]. Unilateral internal carotid artery ligation has resulted in reduced paravascular CSF-ISF exchange in the murine brain [25]. Arterial hypertension, which is associated with reduced pulsations of the arterial wall, was demonstrated to be a factor significantly decreasing CSF-ISF flow within the brain interstitium in humans [27]. Earlier, it was documented that arterial hypertension is associated with accumulation and aggregation of Aβ [29, 30]. Diabetes mellitus (DM) is also a known risk factor for AD [31]. Recently, quantitative MRI measurements analysis revealed that DM is associated with suppressed clearance of interstitial fluid in the hippocampus and hypothalamus. Therefore, it was suggested that the impairment of the glymphatic system in DM may contribute to cognitive deficits [32]. Similarly, congestive heart failure (CHF) is also associated with cognitive dysfunction, especially in the fields of executive functions, memory, language, and mental speed. The severity of CHF and the presence of the apolipoprotein E (apoE) epsilon 4 allele were the factors that significantly correlated with cognitive impairment in patients with chronic CHF [33].
A subsequent study using magnetic resonance encephalography performed in humans allowed to detect three types of physiological mechanisms affecting cerebral CSF-ISF pulsations: cardiac, respiratory, and slow vasomotor wave fluctuations. The cardiac pulsations are present in periarterial regions, extend centrifugally, and cover the brain in ~1 Hz cycles. The respiratory pulsations (0.3 Hz) occur mostly in perivenous areas. The third type of pulsation of very low (0.001–0.023 Hz) and low frequency (0.023–0.73 Hz) propagates with unique spatiotemporal patterns and probably is a result of fluctuation of the glymphatic fluid in the perivascular space surrounding the blood-brain barrier (BBB) [34]. These observations have been supported by the results of an MRI study showing that cardiac and respiratory impact on glymphatic flow is essential in humans [26].
Natural sleep is another key factor in brain clearance. During sleep, the volume of interstitial space within the brain increases, and this facilitates increased CSF-ISF exchange [35]. Glymphatic clearance of Aβ during sleep was more than doubled compared to the awake state in mice [35]. Conversely, sleep deprivation in mice causes a marked reduction in the clearance of various CSF metabolites [35-37]. Poor quality of night sleep is a known risk factor for cognitive decline and dementia in humans [38]. This connection might be well explained by reduced glymphatic function. In humans, a single night of sleep deprivation leads to an increase in Aβ concentration in the hippocampal, parahippocampal, and thalamic regions, which was quantified using positron emission tomography (PET) [39]. Interruption of slow-wave sleep also leads to elevated Aβ concentration in CSF [37]. Even body posture during sleep has an impact on glymphatic clearance. An animal study on mice has shown that glymphatic transport and Aβ clearance are greater with a lateral head position in comparison to the prone position [40]. Therefore, the body posture during sleep should be taken into account during the diagnostic imaging that assesses glymphatic transport [40]. It was further demonstrated that treatment of rats with acetazolamide, puncture of cisterna magna, AQP4 deletion, or sleep deprivation have resulted in significantly decreased CSF-ISF clearance and in an elevated concentration of brain lactate [37]. Opposite effect on brain lactate concentration had sleep or anesthesia. Brain lactate declines within minutes of sleep beginning. Manipulations that increase the concentration of lactate in the brain have also been shown to reduce lactate levels in the dcLNs, but not the inguinal lymph nodes. This suggests that the concentration of brain lactate inversely correlates with glymphatic clearance and has the potential to be a biomarker of the sleep-wake cycle [37].
It is still debatable whether the transport of CSF-ISF through the brain parenchyma is convective or via diffusion forces. Some researchers have raised the argument that the difference in pressure between para-arterial “entry” and paravenous “exit” is insufficient for convective transport [41-43] and they postulate that the transport through the brain is probably a simple diffusion [41-43]. Certainly, further studies are needed to clarify the exact mechanisms of cerebral fluid dynamics.
Table 1 presents the differences between the meningeal lymphatic system and glymphatic system.
Table 1.
Differences between meningeal lymphatic system and glymphatic system.
| Parameter | Meningeal Lymphatic System | Glymphatic System |
| localization | dura mater | brain interstitium |
| structure | resemble lymphatic vessels from other organs, however, have also some characteristic features | perivascular astrocyte endfeet wall and brain interstitium |
| forces of fluid movement | probably contractile activity of collecting mLVs wall (similarly as in lymphatic vessels in other organs) | dependent on arterial pressure, respiratory, sleep and position of head |
| function | drainage of CSF from subarachnoid space, including venous sinuses to the deep cervical lymph nodes | CSF-ISF exchange in the brain parenchyma |
Abbreviations: CSF - cerebrospinal fluid; ISF - interstitial fluid; mLVs - meningeal lymphatic vessels.
5. AGE-RELATED CHANGES IN MENINGEAL LYMPHATICS AND GLYMPHATIC SYSTEM
Advanced age is the greatest risk factor for developing AD [44]. Deterioration of the MLVs also occurs with age. MLVs in old mice display a decrease in their diameter and coverage [13, 45]. Basal MLVs in aged mice show hyperplastic phenotypes, which may be a sign of compensatory mechanisms in response to capillary lymphatic hypertension (similarly as in lymphoedema [46]), i.e. a dysmorphic distribution of type IV collagen, disrupted junctional structures between lymphatic endothelial cells (LECs), as well as impaired lymphatic valves in comparison to the MLVs samples from young mice [45]. These age-related structural alterations of MLVs are associated with reduced brain perfusion by CSF [13], a lower rate of lymphatic absorption of CSF [47] and decreased transport of macromolecules from CSF-ISF to dcLNs [13, 48]. The functional changes of MLVs with age were further confirmed by RNA-seq analysis, showing different gene expressions in MLVs endothelial cells in young-adult mice in comparison to old mice [13].
Impaired clearance of brain parenchyma in the aging brain may also be the result of glymphatic impairment. Old mice display impaired perivascular AQP4 polarization along the penetrating arteries and reduced pulsatility of intracortical arterioles. It was demonstrated that these deteriorations together might lead to a 40% reduction in intraparenchymally injected 125I-Aβ1-40 clearance rate [48]. Likewise, intraventricular and intracisternal tracers delivery to the CSF in mice show a significant delay in reaching dcLNs and the bloodstream in old, compared to young mice [17].
6. ACCUMULATION OF AΒ AND TAU PROTEIN AS A RESULT OF LYMPHATIC CLEARANCE IMPAIRMENT
Da Mesquita et al. investigated the effects of ablating the MLVs in two mouse models of AD. They found that ablation of MLVs leads to Aβ accumulation in the meninges and cognitive deficits [13]. These findings are consistent with Aβ accumulation observed in the meninges of AD patients [13]. Many studies have also established that MLVs play a key role in mediating extracellular tau clearance. A study on a mice model demonstrated that tau clearance was significantly impaired in the absence of mVLs [49].
The APP/PS1 mouse model of AD expresses a chimeric mouse/human amyloid precursor protein (APP) and a mutant human presenilin 1 (PS1), and produces elevated levels of human Aβ. Ligation of dcLNs in APP/PS1 mice demonstrated that ablation of a major brain lymphatic drainage pathway from MLVs results in exacerbated AD-like phenotypes of APP/PS1 mice, manifested by even greater Aβ accumulation, neuroinflammation, synaptic protein loss, impaired polarization of AQP4, and deficits in cognitive and exploratory behaviors [50].
Ligation of dcLNs also causes an increase in total and phosphorylated tau protein levels in the hippocampus. Additionally, in simultaneously AQP4 knockout mice there was also an accumulation of hippocampal Aβ [51].
Accumulation of Aβ and tau may also be a result of changes in glymphatic flow. Influx and clearance rates of CSF tracers are reduced in the APP/PS1 mouse model of AD, because the accumulation of toxic Aβ results in reduced CSF transport into the brain [52]. However, interestingly, glymphatic dysfunction was found not only in aged transgenic animals with developed Aβ accumulation but also in young animals with no visible Aβ plaque formation [52]. After traumatic brain injury (TBI), glymphatic clearance is reduced by approximately 60% for at least 1 month after an injury. TBI is a known risk factor for early-onset dementia, including AD, and is associated with the accumulation of tau aggregates. These suggest that chronic deterioration of glymphatic clearance after TBI may be the factor leading to neurodegeneration [53].
CSF production rate is reduced in AD [54]. Lower clearance of CSF from the brain by MLVs and glymphatic flow in AD may result in its lower production [54]. PET performed in patients with AD showed that Aβ accumulation leads to reduced CSF turnover in humans. Patients with AD had a 23% reduction in ventricular CSF uptake of the tau tracer 18F-THK5117, along with 33% lower clearance of the tracer from the CSF [55]. This reduced CSF turnover was associated with elevated brain Aβ deposits visualized with dual tracer PET scanning [55]. However, it was impossible to state which pathology occurred as the first [55]. The answer was brought by the study in which Aβ was injected into CSF in normal mice. It has caused a reduction of glymphatic influx, suggesting that soluble Aβ in the CSF may, by itself, perturb glymphatic flow [52]. Hence, it seems that not only reduced glymphatic clearance leads to accumulation of Aβ, but also deposition of Aβ can directly or indirectly disrupt glymphatic flow. This suggests a vicious circle mechanism [50, 52].
7. IMMUNOLOGICAL IMPLICATIONS OF IMPAIRED BRAIN CLEARANCE IN ALZHEIMER’S DISEASE
Chronic inflammation within the CNS contributes to AD progression. T cells seem to be especially involved in AD pathogenesis [50, 56-58]. Patients with AD were demonstrated to have more T cells within brain parenchyma than control [9] and the predominance of CD8+ to CD4+ cells was marked [59].
MLVs provide a gateway for immune cells from the brain to dcLNs [9]. Impaired brain lymphatic clearance leads to the accumulation of immune cells within the brain, including T cells, dendritic cells (DCs), and macrophages [8, 9, 13]. Ablation of mVLs in the AD mouse model has resulted in the marked deposition of Aβ in the meninges and in significant increase in the number of macrophages around large Aβ aggregates [13]. Ligation of dcLNs in mice resulted in accumulation of T cells within the brain and dura mater, and reduced number of T cells in dcLNs [50]. Disruption of normal flow of meningeal T cells after the ligation of dcLNs in mice was associated with cognitive impairment [60].
The connection between the dcLNs ligation and reactive gliosis, which is also a characteristic feature for AD, was reported by Wang et colleagues. In both, wild-type and APP/PS1 mice, ligation of dcLNs resulted in a greater percentage of reactive astrocytes and microglia in the cerebral cortex and hippocampus. In APP/PS1 mice, glial activation was higher, together with increased concentrations of inflammatory factors, such as IL-1β and IL-6, and disturbed AQP4 polarity [50].
8. RELATIONSHIP BETWEEN APOLIPOPROTEIN E4 AND IMPAIRMENT OF MENINGEAL LYMPHATICS
Apolipoprotein E4 (APOE4) is the strongest genetic risk factor for sporadic AD, associated with greater production and reduced elimination of Aβ [61, 62]. Recent data show that APOE4 is associated with the impaired function of MLVs. Reanalysis of previously published RNA-Seq data showed that induced pluripotent stem cells (iPSCs) carrying the APOE4 allele (either as APOE4 knock-in or stemming from APOE4 patients) express lower levels of genes associated with lymphatic markers, and also genes for which well-characterized missense mutations have been linked to the peripheral lymphedema. These suggest that APOE4 may play a role in the premature shrinkage of MVLs (meningeal lymphosclerosis), leading to abnormal MVLs functions, and, in consequence, to reduced clearance of Aβ and other macromolecules, including inflammatory mediators, to exacerbation of AD manifestations and progression of the disease [63].
9. REGULATION OF GLYMPHATIC SYSTEM
9.1. Astroglial Water Channel Aquaporin 4 (AQP4) Polarization
Glymphatic clearance is dependent on the expression and perivascular localization of AQP4 on astrocytic endfeet. AQP4 facilitates brain waste clearance by providing a route for CSF transport from para-arterial spaces into the extracellular brain tissue and then an influx of ISF via bulk flow to the perivenous spaces (Fig. 2). Moreover, AQP4 provides water and potassium (K+) homeostasis in the brain [64]. Animals with AQP4 impairment exhibit decreased K+ clearance and increased predisposition to seizure [64].
Fig. (2).
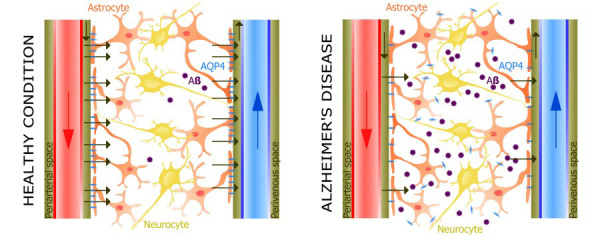
Schematic pictures of glymphatic system in healthy condition and in Alzheimer's disease. Interstitial flow of cerebrospinal fluid (CSF) - interstitial fluid (ISF) between “influx” (para-arterial spaces) and “efflux” (perivenous or perineuronal spaces) sites was demonstrated to be mediated by aquaporin 4 (AQP4) water channels situated on astrocytic endfeet. The continuous movement of fluid throughout the brain interstitium provides the clearance of waste from the brain. Impaired glymphatic function contribute to deposition of toxins, including Aβ accumulation. Figure adapted and modified from Mader, S. Cells, 2019, 8, 90.
Animals that lack AQP4 display a 70% decrease in CSF influx [24] and a 55% reduction in clearance of interstitial solutes compared to clearance in wild-type mice [11]. Deletion of AQP4 in APP/PS1 mice resulted in accumulation of Aβ and cognitive deficits [65]. Similarly, inhibition of AQP4 resulted also in decreased clearance of protein tau from the brain [65]. These imply that AQP4 impairment leads to induction or advancement of AD pathology [12]. The deletion of AQP4 did not alter the expression of proteins involved in the synthesis or degradation of Aβ, suggesting that deletion of AQP4 results in reduced glymphatic clearance of Aβ [12].
In the healthy brain, a membrane of astrocytic end foot displays even tenfold higher AQP4 concentration in comparison to the non-end foot membrane of astrocyte [66]. Appropriate polarization of AQP4 on astrocytic endfeet is crucial for efficient glymphatic CSF-ISF exchange [48, 53]. Impairment of AQP4 polarization increases with age [14, 48]. A loss of AQP4 polarization was observed in the tg-ArcSwe mouse model of AD, in which mice exhibited a redistribution of AQP4 from endfoot membranes to non-endfoot membranes of astrocytes [66]. Overexpression of AQP4 in astrocytes in tg-ArcSwe mouse was abnormally high at the sites of perivascular Aβ deposits, thus suggesting that the increase in Aβ accumulation is associated with loss of correctly polarized AQP4 expression. The possible consequences of impaired AQP4 polarization may be electrolyte imbalance within brain tissue, which in turn might explain the greater prevalence of epilepsy in patients with AD [66]. Moreover, deletion of AQP4 has been shown to result in greater brain water content [51]. Interestingly, it was revealed in mice that chronic unpredictable mild stress (CUMS) leads to decreased expression of AQP4 in the cortex and hippocampus and to Aβ accumulation within the brain. Treatment with antidepressant fluoxetine has reversed this negative consequence of CUMS on glymphatic clearance [15]. Earlier studies demonstrated that a history of depression is a risk factor of AD [67], and regarding the findings of the above study, this relationship can be explained by an impairment of glymphatic system [15].
In a post-mortem immunohistochemical study of patients with AD, impaired AQP4 polarization was associated with older age and was a significant predictor of AD status and correlated with Aβ burden [14]. Particular genetic variants of AQP4 may modulate the degree of mental retardation after the onset of AD [68]. AQP4 genetic variants may also affect sleep quality and the impact of sleep quality on Aβ burden in cognitively normal older adults [69]. Therefore, genome-wide association studies in AD in combination with an evaluation of cerebral fluid dynamics and Aβ accumulation, are needed to identify other genes that would let to predict the course of the disease [69].
9.2. Effect of Melatonin on the Glymphatic System
In AD, decreased levels of melatonin in patients' cerebrospinal fluid correlated with the degree of disease development. With age, melatonin secretion by the pineal gland and the expression of the neuronal melatonin receptors (MT1 and MT2) decrease [70, 71]. Studies on murine and rat AD models have shown that melatonin enhances the functions of the glymphatic system in the clearance of the brain from Aβ and tau protein [72, 73]. In TG2567 mice, administration of melatonin for 15 months resulted in a reduction in the Aβ1-40 oligomers content in the brain by 55%, and Aβ1-42 oligomers by 30%. Notably, there was noted no appreciable increase in plasma Aβ concentrations, while a significant increase of Aβ was found in the dcLNs (by 150% and 60%, respectively). This suggests that melatonin increases brain clearance by enhancing the function of the glymphatic system rather than removing amyloids across the blood-brain barrier [74].
Numerous studies suggest that melatonin substitution in the elderly could prevent neurodegeneration, and in clinical cases of AD, it might slow the progression of the disease [72, 73, 74]. However, in the advanced stages of AD, the benefit of melatonin on cognition was small, and some studies showed no improvement after administration of the drug compared to the control group [74]. Based on experimental animal studies, it has been hypothesized that melatonin effectively prevents Aβ oligomerization in brain tissue by removing amyloid monomers via the glymphatic system, but with only relatively little effect on the degradation and removal of amyloid aggregates from the interstitial spaces of brain tissue [74].
9.3. Associations between Tissue Plasminogen Activator and Glymphatic System
Tissue plasminogen activator (tPA) is a serine protease primarily involved in dissolving fibrin clots into soluble degradation products [75]. Recent data have shown that tPA may have a role in the pathogenesis of cognitive dysfunction and dementia, because tPA initiates catabolism of Aβ [76]. Age-dependent decline of endogenous tPA [77] has an impact on glymphatic function and cognitive outcome in mice [76]. Increased fibrin deposition and clotting are common features in AD brain tissue and vessels [78]. Compared to age-matched wild-type mice, tPA(-/-) mice exhibit significantly decreased perivascular AQP-4 expression, and increased deposition of amyloid precursor protein (APP), Aβ, thrombin, D-dimer and fibrin. The tPA(-/-) mice exhibit axonal damage, decreased dendritic spine density, BBB leakage, neuroinflammation, impaired functioning of the glymphatic system, and significant cognitive impairment [76]. Increased thrombin inhibits AQP4 expression, thus may lead to further deterioration of glymphatic system [79].
10. DIAGNOSTIC PERSPECTIVES FOR ALZHEIMER'S DISEASE ASSOCIATED WITH IMPAIRED BRAIN LYMPHATIC CLEARANCE
Easily accessible method of cerebral lymphatic clearance assessment would facilitate earlier diagnosis and application of the appropriate treatment. It was demonstrated that CSF Aβ42/40 ratio better correlates with AD than the absolute value of CSF Aβ42. However, the reference value for the Aβ42/40 ratio is still not exactly defined [80]. MRI with intrathecal administration of gadobutrol as contrast agent (known as Gadovist) allows for evaluation of glymphatic transport in the human brain [32, 81], but, this assessment is also limited, as the size of perivasular spaces and MVLs is on the microscale. Ultra-fast magnetic resonance encephalography (MREG) in turn, enables the evaluation of brain fluid dynamics in humans, but it has been performed only in healthy subjects. Hence it is not known if it shows any difference between AD and non-AD patients [34]. Although there are also several invasive methods for the assessment of brain lymphatic clearance which have been used in animal studies [82], there is no direct method of brain's lymphatic system evaluation in humans. Therefore, more efforts are needed to find novel diagnostic tools in this regard.
11. NEW THERAPEUTIC PERSPECTIVES FOR ALZHEIMER'S DISEASE
Finding effective methods to increase brain lymphatic clearance represents an exciting new target for the prevention and treatment of AD [13]. Such strategy has the potential to open up a novel chapter in the management of AD.
Enhancing glymphatic flow by proper sleep hygiene and effective treatment of arterial hypertension, diabetes and CHD should be the basis of the prophylaxis of AD. Cardiovascular health is crucial in the maintenance of brain interstitium homeostasis [83].
Interestingly, it was demonstrated in AD mice models that focused ultrasound (FUS), which non-invasively provides ultrasonic energy to a small volume of the target tissues, in combination with microbubbles (MB) that are injected into the blood vessel, has resulted in a reduction of Aβ and tau deposition and improved memory [84, 85]. FUS-MB probably stimulates the vascular endothelium to mechanically open the BBB and thus increases the glymphatic flow [84, 86]. FUS-MB has the potential to be a preventive method in glymphatic system impairment in early AD [84]. Recently, several clinical human trials have begun to evaluate its clinical utility [84, 85]. FUS-MB might also enhance the effect of other therapies in AD, e.g., with the use of monoclonal antibodies [84].
Hypothetically, methods and possible drugs which would increase brain CSF and ISF secretion might be efficient in improving brain lymphatic flow and clearance of neurotoxic proteins. Based on experimental animal studies, melatonin seems to be effective in increasing glymphatic clearance [72, 73, 87]. Such influence of melatonin may explain findings of the studies with melatonin administration in humans, in which melatonin application in patients with mild to the moderately cognitive impairment has led not only to significant improvement in sleep, but also to higher mental performance [88, 89]. Increased CSF-ISF production, in theory, might be also a method to enhance the distribution of drugs to the brain tissue, including drugs applied in AD treatment and delivered to the brain, also via intrathecal dosing into the CSF [90, 91]. Similarly, greater CSF-ISF production is a possible target to improve potential genes [92, 93] and monoclonal antibody therapy in AD [94]. APQ4 mediates soluble Aβ clearance from the extracellular brain tissue, therefore, potential agonists of AQP4 might increase glymphatic flow [51]. Glymphatic system impairment results in altered brain immunity and greater accessibility of immune-derived cytokines to the brain tissue. Therefore, the possibility of modulation of cytokine exchange between CSF and ISF by increased activity of MLVs might be another novel target in AD therapy in the future [95]. The possibility of regeneration of MLVs, demonstrated in the recent studies with administration of VEGF-C, give promise for new therapeutic methods in AD, based on the process of lymphangiogenesis [13, 21].
Table 2 presents potential and hypothetical therapeutic methods targeting the impaired brain lymphatic system in AD.
Table 2.
New potential and hypothetical therapeutic methods targeting the impaired brain lymphatic system in Alzheimer's disease (AD).
| Risk Factors of AD Associated with Impaired Brain Lymphatic System | New Potential and Hypothetical Therapeutic Methods in AD |
|---|---|
| age-related changes in mLVs [13] | - promoting the growth of meningeal lymphatic vessels (enhancing of VEGFC-VERFR-3 signaling) [13, 21] - genetic manipulations (e.g. mVEGF-C viral vectors injections) [13] - increase in CSF production - agonists for scavenging receptor class B type 1 (SR-B1), hypothetically present on MLVs endothelium [19, 20] |
| decreased glymphatic clearance | - potential drugs increasing brain ISF secretion, e.g. brain-derived arginine vasopressin (AVP) [74] |
| age-related changes in glymphatic system - impaired perivascular AQP4 polarization [48] - reduction of arterial pulsatility of intracortical arterioles [25, 48] - decreased melatonin secretion by the pineal gland and decreased expression of neuronal MT1 and MT2 receptors for melatonin [70, 71] |
- AQP4 receptor agonists [51] - hypothetical drugs which increase the expression of AQP4 in astrocytic end feet - hypothetical genetic manipulations that directly target AQP4 polarization [48, 92] - accurate treatment of arterial hypertension, diabetes and CHD [83] - focused ultrasound (FUS) in combination with microbubbles (MB) given into the blood vessel that mimic the arterial pulsality in the brain arterioles [84,86] - melatonin administration [88, 89] |
| impaired quality of night sleep [37, 39] | - proper sleep hygiene [37, 39] - accurate treatment of sleep apnea [37] |
| AQP4 mutations and polymorphisms [68, 69] | - hypothetical genetic manipulations targeting AQP4 |
| AQP4 impairment in the reaction to depression [15] | - fluoxetine and hypothetically other antidepressants [15] |
| traumatic brain injury (TBI) [53] | - hypothetical drugs enhancing the clearence of CSF-ISF through glymphatic system [53] |
| Aβ and tau deposition (after diagnosis of AD) | - focused ultrasound (FUS) with microbubbles (MB) [84,86] - monoclonal antibody therapy [94] - viral vectors for gene therapy [92, 93] |
| neuroinflammation within the brain tissue | - modulation of cytokine exchange between CSF and ISF by increased activity of mLVs [95] |
Abbreviations: AQP4 - aquaporin 4; Aβ - amyloid – β; CHD - congestive heart failure; CSF - cerebrospinal fluid; ISF - interstitial fluid; mLVs - meningeal lymphatic vessels; VEGFC - vascular endothelial growth factor C; VERFR-3 - vascular endothelial growth factor receptor 3.
CONCLUSION
Impairment of lymphatic brain clearance plays a direct role in Aβ and tau deposition and AD pathogenesis. Further studies are necessary to precisely understand the clearance mechanisms provided by MLVs and glymphatic system. New therapeutic strategies targeting the brain interstitium lymphatic clearance have the potential to become effective methods of prevention and treatment of AD, therefore requiring extensive investigation.
ACKNOWLEDGEMENTS
Declared none.
LIST OF ABBREVIATIONS
- Aβ
Amyloid – β
- AD
Alzheimer’s disease
- apoE
Apolipoprotein E
- APP
Amyloid precursor protein
- AQP4
Aquaporin 4
- BBB
Blood-brain barrier
- CHD
Congestive heart failure
- CNS
Central nervous system
- CSF
Cerebrospinal fluid
- CUMS
Chronic unpredictable mild stress
- DM
Diabetes mellitus
- dcLN
Deep cervical lymph nodes
- FUS
Focused ultrasound
- ISF
Interstitial fluid
- MB
Microbubbles
- mLVs
Meningeal lymphatic vessels
- MRI
Magnetic resonance imaging
- PET
Positron emission tomography
- PS1
Presenilin 1
- SR-B1
Scavenging receptor class B type 1
- TBI
Traumatic brain injury
- tPA
Tissue plasminogen activator
- VEGFC
Vascular endothelial growth factor C
- VERFR-3
Vascular endothelial growth factor receptor 3.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Patterson C. World Alzheimer Report 2018. The state of the art of dementia research: New frontiers. Alzheimer’s Disease International. London: ADI; 2018. [Google Scholar]
- 2.Blennow K., de Leon M.J., Zetterberg H. Alzheimer’s disease. Lancet. 2006;368(9533):387–403. doi: 10.1016/S0140-6736(06)69113-7. [DOI] [PubMed] [Google Scholar]
- 3.Wang M., Ding F., Deng S., Guo X., Wang W., Iliff J.J., Nedergaard M. Focal solute trapping and global glymphatic pathway impairment in a murine model of multiple microinfarcts. J. Neurosci. 2017;37(11):2870–2877. doi: 10.1523/JNEUROSCI.2112-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Földi M. The brain and the lymphatic system revisited. Lymphology. 1999;32(2):40–44. [PubMed] [Google Scholar]
- 5.Baluk P., Fuxe J., Hashizume H., Romano T., Lashnits E., Butz S., Vestweber D., Corada M., Molendini C., Dejana E., McDonald D.M. Functionally specialized junctions between endothelial cells of lymphatic vessels. J. Exp. Med. 2007;204(10):2349–2362. doi: 10.1084/jem.20062596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alitalo K., Tammela T., Petrova T.V. Lymphangiogenesis in development and human disease. Nature. 2005;438(7070):946–953. doi: 10.1038/nature04480. [DOI] [PubMed] [Google Scholar]
- 7.Szentistványi I., Patlak C.S., Ellis R.A., Cserr H.F. Drainage of interstitial fluid from different regions of rat brain. Am. J. Physiol. 1984;246(6 Pt 2):F835–F844. doi: 10.1152/ajprenal.1984.246.6.F835. [DOI] [PubMed] [Google Scholar]
- 8.Aspelund A., Antila S., Proulx S.T., Karlsen T.V., Karaman S., Detmar M., Wiig H., Alitalo K. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J. Exp. Med. 2015;212(7):991–999. doi: 10.1084/jem.20142290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Louveau A., Smirnov I., Keyes T.J., Eccles J.D., Rouhani S.J., Peske J.D., Derecki N.C., Castle D., Mandell J.W., Lee K.S., Harris T.H., Kipnis J. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015;523(7560):337–341. doi: 10.1038/nature14432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Absinta M., Ha S.K., Nair G., Sati P., Luciano N.J., Palisoc M., Louveau A., Zaghloul K.A., Pittaluga S., Kipnis J., Reich D.S. Human and nonhuman primate meninges harbor lymphatic vessels that can be visualized noninvasively by MRI. eLife. 2017;6:6. doi: 10.7554/eLife.29738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iliff J.J., Wang M., Liao Y., Plogg B.A., Peng W., Gundersen G.A., Benveniste H., Vates G.E., Deane R., Goldman S.A., Nagelhus E.A., Nedergaard M. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci. Transl. Med. 2012;4(147):147ra111. doi: 10.1126/scitranslmed.3003748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu Z., Xiao N., Chen Y., Huang H., Marshall C., Gao J., Cai Z., Wu T., Hu G., Xiao M. Deletion of aquaporin-4 in APP/PS1 mice exacerbates brain Aβ accumulation and memory deficits. Mol. Neurodegener. 2015;10(1):58. doi: 10.1186/s13024-015-0056-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Da Mesquita S., Louveau A., Vaccari A., Smirnov I., Cornelison R.C., Kingsmore K.M., Contarino C., Onengut-Gumuscu S., Farber E., Raper D., Viar K.E., Powell R.D., Baker W., Dabhi N., Bai R., Cao R., Hu S., Rich S.S., Munson J.M., Lopes M.B., Overall C.C., Acton S.T., Kipnis J. Functional aspects of meningeal lymphatics in ageing and Alzheimer’s disease. Nature. 2018;560(7717):185–191. doi: 10.1038/s41586-018-0368-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zeppenfeld D.M., Simon M., Haswell J.D., D’Abreo D., Murchison C., Quinn J.F., Grafe M.R., Woltjer R.L., Kaye J., Iliff J.J. Association of perivascular localization of aquaporin-4 with cognition and Alzheimer disease in aging brains. JAMA Neurol. 2017;74(1):91–99. doi: 10.1001/jamaneurol.2016.4370. [DOI] [PubMed] [Google Scholar]
- 15.Xia M., Yang L., Sun G., Qi S., Li B. Mechanism of depression as a risk factor in the development of Alzheimer’s disease: The function of AQP4 and the glymphatic system. Psychopharmacology (Berl.) 2017;234(3):365–379. doi: 10.1007/s00213-016-4473-9. [DOI] [PubMed] [Google Scholar]
- 16.Lukić I.K.; Gluncić V.; Ivkić G.; Hubenstorf, M.; Marusić A. Virtual dissection: A lesson from the 18th century. Lancet. 2003;362(9401):2110–2113. doi: 10.1016/S0140-6736(03)15114-8. [DOI] [PubMed] [Google Scholar]
- 17.Ma Q., Ineichen B.V., Detmar M., Proulx S.T. Outflow of cerebrospinal fluid is predominantly through lymphatic vessels and is reduced in aged mice. Nat. Commun. 2017;8(1):1434. doi: 10.1038/s41467-017-01484-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johanson C.E., Duncan J.A., III, Klinge P.M., Brinker T., Stopa E.G., Silverberg G.D. Multiplicity of cerebrospinal fluid functions: New challenges in health and disease. Cerebrospinal Fluid Res. 2008;5(1):10. doi: 10.1186/1743-8454-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lim H.Y., Thiam C.H., Yeo K.P., Bisoendial R., Hii C.S., McGrath K.C., Tan K.W., Heather A., Alexander J.S., Angeli V. Lymphatic vessels are essential for the removal of cholesterol from peripheral tissues by SR-BI-mediated transport of HDL. Cell Metab. 2013;17(5):671–684. doi: 10.1016/j.cmet.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 20.Thanopoulou K., Fragkouli A., Stylianopoulou F., Georgopoulos S. Scavenger receptor class B type I (SR-BI) regulates perivascular macrophages and modifies amyloid pathology in an Alzheimer mouse model. Proc. Natl. Acad. Sci. USA. 2010;107(48):20816–20821. doi: 10.1073/pnas.1005888107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Antila S., Karaman S., Nurmi H., Airavaara M., Voutilainen M.H., Mathivet T., Chilov D., Li Z., Koppinen T., Park J.H., Fang S., Aspelund A., Saarma M., Eichmann A., Thomas J.L., Alitalo K. Development and plasticity of meningeal lymphatic vessels. J. Exp. Med. 2017;214(12):3645–3667. doi: 10.1084/jem.20170391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rennels M.L., Gregory T.F., Blaumanis O.R., Fujimoto K., Grady P.A. Evidence for a ‘paravascular’ fluid circulation in the mammalian central nervous system, provided by the rapid distribution of tracer protein throughout the brain from the subarachnoid space. Brain Res. 1985;326(1):47–63. doi: 10.1016/0006-8993(85)91383-6. [DOI] [PubMed] [Google Scholar]
- 23.Dobson H., Sharp M.M., Cumpsty R., Criswell T.P., Wellman T., Finucane C., Sullivan J.M., Weller R.O., Verma A., Carare R.O. The perivascular pathways for influx of cerebrospinal fluid are most efficient in the midbrain. Clin. Sci. (Lond.) 2017;131(22):2745–2752. doi: 10.1042/CS20171265. [DOI] [PubMed] [Google Scholar]
- 24.Mestre H., Hablitz L.M., Xavier A.L., Feng W., Zou W., Pu T., Monai H., Murlidharan G., Castellanos Rivera R.M., Simon M.J., Pike M.M., Pla V., Du T., Kress B.T., Wang X., Plog B.A., Thrane A.S., Lundgaard I., Abe Y., Yasui M., Thomas J.H., Xiao M., Hirase H., Asokan A., Iliff J.J., Nedergaard M. Aquaporin-4-dependent glymphatic solute transport in the rodent brain. eLife. 2018:7. doi: 10.7554/eLife.40070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iliff J.J., Wang M., Zeppenfeld D.M., Venkataraman A., Plog B.A., Liao Y., Deane R., Nedergaard M. Cerebral arterial pulsation drives paravascular CSF-interstitial fluid exchange in the murine brain. J. Neurosci. 2013;33(46):18190–18199. doi: 10.1523/JNEUROSCI.1592-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ringstad G., Valnes L.M., Dale A.M., Pripp A.H., Vatnehol S.S., Emblem K.E., Mardal K.A., Eide P.K. Brain-wide glymphatic enhancement and clearance in humans assessed with MRI. J.C.I. 2018;3(13):e121537. doi: 10.1172/jci.insight.121537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mestre H., Tithof J., Du T., Song W., Peng W., Sweeney A.M., Olveda G., Thomas J.H., Nedergaard M., Kelley D.H. Flow of cerebrospinal fluid is driven by arterial pulsations and is reduced in hypertension. Nat. Commun. 2018;9(1):4878. doi: 10.1038/s41467-018-07318-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bedussi B., Almasian M., de Vos J., VanBavel E., Bakker E.N. Paravascular spaces at the brain surface: Low resistance pathways for cerebrospinal fluid flow. J. Cereb. Blood Flow Metab. 2018;38(4):719–726. doi: 10.1177/0271678X17737984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carnevale D., Mascio G., D’Andrea I., Fardella V., Bell R.D., Branchi I., Pallante F., Zlokovic B., Yan S.S., Lembo G. Hypertension induces brain β-amyloid accumulation, cognitive impairment, and memory deterioration through activation of receptor for advanced glycation end products in brain vasculature. Hypertension. 2012;60(1):188–197. doi: 10.1161/HYPERTENSIONAHA.112.195511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gentile M.T., Poulet R., Di Pardo A., Cifelli G., Maffei A., Vecchione C., Passarelli F., Landolfi A., Carullo P., Lembo G. Beta-amyloid deposition in brain is enhanced in mouse models of arterial hypertension. Neurobiol. Aging. 2009;30(2):222–228. doi: 10.1016/j.neurobiolaging.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 31.Baglietto-Vargas D., Shi J., Yaeger D.M., Ager R., LaFerla F.M. Diabetes and Alzheimer’s disease crosstalk. Neurosci. Biobehav. Rev. 2016;64:272–287. doi: 10.1016/j.neubiorev.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 32.Jiang Q., Zhang L., Ding G., Davoodi-Bojd E., Li Q., Li L., Sadry N., Nedergaard M., Chopp M., Zhang Z. Impairment of the glymphatic system after diabetes. J. Cereb. Blood Flow Metab. 2017;37(4):1326–1337. doi: 10.1177/0271678X16654702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vogels R.L., Oosterman J.M., van Harten B., Scheltens P., van der Flier W.M., Schroeder-Tanka J.M., Weinstein H.C. Profile of cognitive impairment in chronic heart failure. J. Am. Geriatr. Soc. 2007;55(11):1764–1770. doi: 10.1111/j.1532-5415.2007.01395.x. [DOI] [PubMed] [Google Scholar]
- 34.Kiviniemi V., Wang X., Korhonen V., Keinänen T., Tuovinen T., Autio J., LeVan P., Keilholz S., Zang Y.F., Hennig J., Nedergaard M. Ultra-fast magnetic resonance encephalography of physiological brain activity - Glymphatic pulsation mechanisms? J. Cereb. Blood Flow Metab. 2016;36(6):1033–1045. doi: 10.1177/0271678X15622047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xie L., Kang H., Xu Q., Chen M.J., Liao Y., Thiyagarajan M., O’Donnell J., Christensen D.J., Nicholson C., Iliff J.J., Takano T., Deane R., Nedergaard M. Sleep drives metabolite clearance from the adult brain. Science. 2013;342(6156):373–377. doi: 10.1126/science.1241224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Plog B.A., Dashnaw M.L., Hitomi E., Peng W., Liao Y., Lou N., Deane R., Nedergaard M. Biomarkers of traumatic injury are transported from brain to blood via the glymphatic system. J. Neurosci. 2015;35(2):518–526. doi: 10.1523/JNEUROSCI.3742-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lundgaard I., Lu M.L., Yang E., Peng W., Mestre H., Hitomi E., Deane R., Nedergaard M. Glymphatic clearance controls state-dependent changes in brain lactate concentration. J. Cereb. Blood Flow Metab. 2017;37(6):2112–2124. doi: 10.1177/0271678X16661202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ju Y.E., Lucey B.P., Holtzman D.M. Sleep and Alzheimer disease pathology--a bidirectional relationship. Nat. Rev. Neurol. 2014;10(2):115–119. doi: 10.1038/nrneurol.2013.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ju Y.S., Ooms S.J., Sutphen C., Macauley S.L., Zangrilli M.A., Jerome G., Fagan A.M., Mignot E., Zempel J.M., Claassen J.A.H.R., Holtzman D.M. Slow wave sleep disruption increases cerebrospinal fluid amyloid-β levels. Brain. 2017;140(8):2104–2111. doi: 10.1093/brain/awx148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee H., Xie L., Yu M., Kang H., Feng T., Deane R., Logan J., Nedergaard M., Benveniste H. The effect of body posture on brain glymphatic transport. J. Neurosci. 2015;35(31):11034–11044. doi: 10.1523/JNEUROSCI.1625-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jin B.J., Smith A.J., Verkman A.S. Spatial model of convective solute transport in brain extracellular space does not support a “glymphatic” mechanism. J. Gen. Physiol. 2016;148(6):489–501. doi: 10.1085/jgp.201611684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Holter K.E., Kehlet B., Devor A., Sejnowski T.J., Dale A.M., Omholt S.W., Ottersen O.P., Nagelhus E.A., Mardal K.A., Pettersen K.H. Interstitial solute transport in 3D reconstructed neuropil occurs by diffusion rather than bulk flow. Proc. Natl. Acad. Sci. USA. 2017;114(37):9894–9899. doi: 10.1073/pnas.1706942114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith A.J., Yao X., Dix J.A., Jin B.J., Verkman A.S. Test of the ‘glymphatic’ hypothesis demonstrates diffusive and aquaporin-4-independent solute transport in rodent brain parenchyma. eLife. 2017:6. doi: 10.7554/eLife.27679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guerreiro R., Bras J. The age factor in Alzheimer’s disease. Genome Med. 2015;7(1):106. doi: 10.1186/s13073-015-0232-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ahn J.H., Cho H., Kim J.H., Kim S.H., Ham J.S., Park I., Suh S.H., Hong S.P., Song J.H., Hong Y.K., Jeong Y., Park S.H., Koh G.Y. Meningeal lymphatic vessels at the skull base drain cerebrospinal fluid. Nature. 2019;572(7767):62–66. doi: 10.1038/s41586-019-1419-5. [DOI] [PubMed] [Google Scholar]
- 46.Gousopoulos E., Proulx S.T., Scholl J., Uecker M., Detmar M. Prominent lymphatic vessel hyperplasia with progressive dysfunction and distinct immune cell infiltration in lymphedema. Am. J. Pathol. 2016;186(8):2193–2203. doi: 10.1016/j.ajpath.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 47.Pollay M. The function and structure of the cerebrospinal fluid outflow system. Cerebrospinal Fluid Res. 2010;7(1):9. doi: 10.1186/1743-8454-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kress B.T., Iliff J.J., Xia M., Wang M., Wei H.S., Zeppenfeld D., Xie L., Kang H., Xu Q., Liew J.A., Plog B.A., Ding F., Deane R., Nedergaard M. Impairment of paravascular clearance pathways in the aging brain. Ann. Neurol. 2014;76(6):845–861. doi: 10.1002/ana.24271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Patel T.K., Habimana-Griffin L., Gao X., Xu B., Achilefu S., Alitalo K., McKee C.A., Sheehan P.W., Musiek E.S., Xiong C., Coble D., Holtzman D.M. Dural lymphatics regulate clearance of extracellular tau from the CNS. Mol. Neurodegener. 2019;14(1):11. doi: 10.1186/s13024-019-0312-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang L., Zhang Y., Zhao Y., Marshall C., Wu T., Xiao M. Deep cervical lymph node ligation aggravates AD-like pathology of APP/PS1 mice. Brain Pathol. 2019;29(2):176–192. doi: 10.1111/bpa.12656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cao X., Xu H., Feng W., Su D., Xiao M. Deletion of aquaporin-4 aggravates brain pathology after blocking of the meningeal lymphatic drainage. Brain Res. Bull. 2018;143:83–96. doi: 10.1016/j.brainresbull.2018.10.007. [DOI] [PubMed] [Google Scholar]
- 52.Peng W., Achariyar T.M., Li B., Liao Y., Mestre H., Hitomi E., Regan S., Kasper T., Peng S., Ding F., Benveniste H., Nedergaard M., Deane R. Suppression of glymphatic fluid transport in a mouse model of Alzheimer’s disease. Neurobiol. Dis. 2016;93:215–225. doi: 10.1016/j.nbd.2016.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Iliff J.J., Chen M.J., Plog B.A., Zeppenfeld D.M., Soltero M., Yang L., Singh I., Deane R., Nedergaard M. Impairment of glymphatic pathway function promotes tau pathology after traumatic brain injury. J. Neurosci. 2014;34(49):16180–16193. doi: 10.1523/JNEUROSCI.3020-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Silverberg G.D., Heit G., Huhn S., Jaffe R.A., Chang S.D., Bronte-Stewart H., Rubenstein E., Possin K., Saul T.A. The cerebrospinal fluid production rate is reduced in dementia of the Alzheimer’s type. Neurology. 2001;57(10):1763–1766. doi: 10.1212/WNL.57.10.1763. [DOI] [PubMed] [Google Scholar]
- 55.de Leon M.J., Li Y., Okamura N., Tsui W.H., Saint-Louis L.A., Glodzik L., Osorio R.S., Fortea J., Butler T., Pirraglia E., Fossati S., Kim H.J., Carare R.O., Nedergaard M., Benveniste H., Rusinek H. Cerebrospinal fluid clearance in Alzheimer disease measured with dynamic PET. J. Nucl. Med. 2017;58(9):1471–1476. doi: 10.2967/jnumed.116.187211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Weiner H.L., Frenkel D. Immunology and immunotherapy of Alzheimer’s disease. Nat. Rev. Immunol. 2006;6(5):404–416. doi: 10.1038/nri1843. [DOI] [PubMed] [Google Scholar]
- 57.Baruch K., Kertser A., Porat Z., Schwartz M. Cerebral nitric oxide represses choroid plexus NFκB-dependent gateway activity for leukocyte trafficking. EMBO J. 2015;34(13):1816–1828. doi: 10.15252/embj.201591468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dansokho C., Ait Ahmed D., Aid S., Toly-Ndour C., Chaigneau T., Calle V., Cagnard N., Holzenberger M., Piaggio E., Aucouturier P., Dorothée G. Regulatory T cells delay disease progression in Alzheimer-like pathology. Brain. 2016;139(Pt 4):1237–1251. doi: 10.1093/brain/awv408. [DOI] [PubMed] [Google Scholar]
- 59.Togo T., Akiyama H., Iseki E., Kondo H., Ikeda K., Kato M., Oda T., Tsuchiya K., Kosaka K. Occurrence of T cells in the brain of Alzheimer’s disease and other neurological diseases. J. Neuroimmunol. 2002;124(1-2):83–92. doi: 10.1016/S0165-5728(01)00496-9. [DOI] [PubMed] [Google Scholar]
- 60.Kipnis J., Gadani S., Derecki N.C. Pro-cognitive properties of T cells. Nat. Rev. Immunol. 2012;12(9):663–669. doi: 10.1038/nri3280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Giri M., Zhang M., Lü Y. Genes associated with Alzheimer’s disease: An overview and current status. Clin. Interv. Aging. 2016;11:665–681. doi: 10.2147/CIA.S105769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ye S., Huang Y., Müllendorff K., Dong L., Giedt G., Meng E.C., Cohen F.E., Kuntz I.D., Weisgraber K.H., Mahley R.W. Apolipoprotein (apo) E4 enhances amyloid beta peptide production in cultured neuronal cells: ApoE structure as a potential therapeutic target. Proc. Natl. Acad. Sci. USA. 2005;102(51):18700–18705. doi: 10.1073/pnas.0508693102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mentis A.A., Dardiotis E., Chrousos G.P. Apolipoprotein E4 and meningeal lymphatics in Alzheimer disease: A conceptual framework. Mol. Psychiatry. 2021;26(4):1075–1097. doi: 10.1038/s41380-020-0731-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Amiry-Moghaddam M., Williamson A., Palomba M., Eid T., de Lanerolle N.C., Nagelhus E.A., Adams M.E., Froehner S.C., Agre P., Ottersen O.P. Delayed K+ clearance associated with aquaporin-4 mislocalization: Phenotypic defects in brains of alpha-syntrophin-null mice. Proc. Natl. Acad. Sci. USA. 2003;100(23):13615–13620. doi: 10.1073/pnas.2336064100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Harrison I.F., Ismail O., Machhada A., Colgan N., Ohene Y., Nahavandi P., Ahmed Z., Fisher A., Meftah S., Murray T.K., Ottersen O.P., Nagelhus E.A., O’Neill M.J., Wells J.A., Lythgoe M.F. Impaired glymphatic function and clearance of tau in an Alzheimer’s disease model. Brain. 2020;143(8):2576–2593. doi: 10.1093/brain/awaa179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang J., Lunde L.K., Nuntagij P., Oguchi T., Camassa L.M., Nilsson L.N., Lannfelt L., Xu Y., Amiry-Moghaddam M., Ottersen O.P., Torp R. Loss of astrocyte polarization in the tg-ArcSwe mouse model of Alzheimer’s disease. J. Alzheimers Dis. 2011;27(4):711–722. doi: 10.3233/JAD-2011-110725. [DOI] [PubMed] [Google Scholar]
- 67.Kessing L.V., Nilsson F.M. Increased risk of developing dementia in patients with major affective disorders compared to patients with other medical illnesses. J. Affect. Disord. 2003;73(3):261–269. doi: 10.1016/S0165-0327(02)00004-6. [DOI] [PubMed] [Google Scholar]
- 68.Burfeind K.G., Murchison C.F., Westaway S.K., Simon M.J., Erten-Lyons D., Kaye J.A., Quinn J.F., Iliff J.J. The effects of noncoding aquaporin-4 single-nucleotide polymorphisms on cognition and functional progression of Alzheimer’s disease. Alzheimers Dement. (N. Y.) 2017;3(3):348–359. doi: 10.1016/j.trci.2017.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rainey-Smith S.R., Mazzucchelli G.N., Villemagne V.L., Brown B.M., Porter T., Weinborn M., Bucks R.S., Milicic L., Sohrabi H.R., Taddei K., Ames D., Maruff P., Masters C.L., Rowe C.C., Salvado O., Martins R.N., Laws S.M. Genetic variation in Aquaporin-4 moderates the relationship between sleep and brain Aβ-amyloid burden. Transl. Psychiatry. 2018;8(1):47. doi: 10.1038/s41398-018-0094-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Brunner P., Sözer-Topcular N., Jockers R., Ravid R., Angeloni D., Fraschini F., Eckert A., Müller-Spahn F., Savaskan E. Pineal and cortical melatonin receptors MT1 and MT2 are decreased in Alzheimer’s disease. Eur. J. Histochem. 2006;50(4):311–316. [PubMed] [Google Scholar]
- 71.Wu Y.H., Feenstra M.G., Zhou J.N., Liu R.Y., Toranõ J.S., Van Kan H.J., Fischer D.F., Ravid R., Swaab D.F. Molecular changes underlying reduced pineal melatonin levels in Alzheimer disease: Alterations in preclinical and clinical stages. J. Clin. Endocrinol. Metab. 2003;88(12):5898–5906. doi: 10.1210/jc.2003-030833. [DOI] [PubMed] [Google Scholar]
- 72.Cardinali D.P. Melatonin: Clinical perspectives in neurodegeneration. Front. Endocrinol. (Lausanne) 2019;10:480. doi: 10.3389/fendo.2019.00480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li Y., Zhang J., Wan J., Liu A., Sun J. Melatonin regulates Aβ production/clearance balance and Aβ neurotoxicity: A potential therapeutic molecule for Alzheimer’s disease. Biomed. Pharmacother. 2020;132:110887. doi: 10.1016/j.biopha.2020.110887. [DOI] [PubMed] [Google Scholar]
- 74.Plog B.A., Nedergaard M. The glymphatic system in central nervous system health and disease: Past, present, and future. Annu. Rev. Pathol. 2018;13(1):379–394. doi: 10.1146/annurev-pathol-051217-111018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cheng J.W., Zhang X.J., Cheng L.S., Li G.Y., Zhang L.J., Ji K.X., Zhao Q., Bai Y. Low-dose tissue plasminogen activator in acute ischemic stroke: A systematic review and meta-analysis. J. Stroke Cerebrovasc. Dis. 2018;27(2):381–390. doi: 10.1016/j.jstrokecerebrovasdis.2017.09.014. [DOI] [PubMed] [Google Scholar]
- 76.Yu P., Venkat P., Chopp M., Zacharek A., Shen Y., Liang L., Landschoot-Ward J., Liu Z., Jiang R., Chen J. Deficiency of tPA exacerbates white matter damage, neuroinflammation, glymphatic dysfunction and cognitive dysfunction in aging mice. Aging Dis. 2019;10(4):770–783. doi: 10.14336/AD.2018.0816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bi Oh S., Suh N., Kim I., Lee J.Y. Impacts of aging and amyloid-β deposition on plasminogen activators and plasminogen activator inhibitor-1 in the Tg2576 mouse model of Alzheimer’s disease. Brain Res. 2015;1597:159–167. doi: 10.1016/j.brainres.2014.11.042. [DOI] [PubMed] [Google Scholar]
- 78.Cortes-Canteli M., Paul J., Norris E.H., Bronstein R., Ahn H.J., Zamolodchikov D., Bhuvanendran S., Fenz K.M., Strickland S. Fibrinogen and beta-amyloid association alters thrombosis and fibrinolysis: A possible contributing factor to Alzheimer’s disease. Neuron. 2010;66(5):695–709. doi: 10.1016/j.neuron.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tang Y., Cai D., Chen Y. Thrombin inhibits aquaporin 4 expression through protein kinase C-dependent pathway in cultured astrocytes. J. Mol. Neurosci. 2007;31(1):83–93. doi: 10.1007/BF02686120. [DOI] [PubMed] [Google Scholar]
- 80.Hansson O., Lehmann S., Otto M., Zetterberg H., Lewczuk P. Advantages and disadvantages of the use of the CSF Amyloid β (Aβ) 42/40 ratio in the diagnosis of Alzheimer’s Disease. Alzheimers Res. Ther. 2019;11(1):34. doi: 10.1186/s13195-019-0485-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Eide P.K., Ringstad G. MRI with intrathecal MRI gadolinium contrast medium administration: A possible method to assess glymphatic function in human brain. Acta Radiol. Open. 2015;4(11):2058460115609635. doi: 10.1177/2058460115609635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sun B.L., Wang L.H., Yang T., Sun J.Y., Mao L.L., Yang M.F., Yuan H., Colvin R.A., Yang X.Y. Lymphatic drainage system of the brain: A novel target for intervention of neurological diseases. Prog. Neurobiol. 2018;163-164:118–143. doi: 10.1016/j.pneurobio.2017.08.007. [DOI] [PubMed] [Google Scholar]
- 83.Nedergaard M., Goldman S.A. Glymphatic failure as a final common pathway to dementia. Science. 2020;370(6512):50–56. doi: 10.1126/science.abb8739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lee Y., Choi Y., Park E.J., Kwon S., Kim H., Lee J.Y., Lee D.S. Improvement of glymphatic-lymphatic drainage of beta-amyloid by focused ultrasound in Alzheimer’s disease model. Sci. Rep. 2020;10(1):16144. doi: 10.1038/s41598-020-73151-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Poon C.T., Shah K., Lin C., Tse R., Kim K.K., Mooney S., Aubert I., Stefanovic B., Hynynen K. Time course of focused ultrasound effects on β-amyloid plaque pathology in the TgCRND8 mouse model of Alzheimer’s disease. Sci. Rep. 2018;8(1):14061. doi: 10.1038/s41598-018-32250-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chen K.T., Wei K.C., Liu H.L. Theranostic Strategy of Focused Ultrasound Induced Blood-Brain Barrier Opening for CNS Disease Treatment. Front. Pharmacol. 2019;10:86. doi: 10.3389/fphar.2019.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pappolla M.A., Matsubara E., Vidal R., Pacheco-Quinto J., Poeggeler B., Zagorski M., Sambamurti K. Melatonin treatment enhances Aβ lymphatic clearance in a transgenic mouse model of amyloidosis. Curr. Alzheimer Res. 2018;15(7):637–642. doi: 10.2174/1567205015666180411092551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wade A.G., Farmer M., Harari G., Fund N., Laudon M., Nir T., Frydman-Marom A., Zisapel N. Add-on prolonged-release melatonin for cognitive function and sleep in mild to moderate Alzheimer’s disease: A 6-month, randomized, placebo-controlled, multicenter trial. Clin. Interv. Aging. 2014;9:947–961. doi: 10.2147/CIA.S65625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cardinali D.P., Vigo D.E., Olivar N., Vidal M.F., Furio A.M., Brusco L.I. Therapeutic application of melatonin in mild cognitive impairment. Am. J. Neurodegener. Dis. 2012;1(3):280–291. [PMC free article] [PubMed] [Google Scholar]
- 90.Wolak D.J., Thorne R.G. Diffusion of macromolecules in the brain: Implications for drug delivery. Mol. Pharm. 2013;10(5):1492–1504. doi: 10.1021/mp300495e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pizzo M.E., Wolak D.J., Kumar N.N., Brunette E., Brunnquell C.L., Hannocks M.J., Abbott N.J., Meyerand M.E., Sorokin L., Stanimirovic D.B., Thorne R.G. Intrathecal antibody distribution in the rat brain: Surface diffusion, perivascular transport and osmotic enhancement of delivery. J. Physiol. 2018;596(3):445–475. doi: 10.1113/JP275105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rasmussen M.K., Mestre H., Nedergaard M. The glymphatic pathway in neurological disorders. Lancet Neurol. 2018;17(11):1016–1024. doi: 10.1016/S1474-4422(18)30318-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Murlidharan G., Crowther A., Reardon R.A., Song J., Asokan A. Glymphatic fluid transport controls paravascular clearance of AAV vectors from the brain. JCI Insight. 2016;1(14):e88034. doi: 10.1172/jci.insight.88034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Vulchanova L., Schuster D.J., Belur L.R., Riedl M.S., Podetz-Pedersen K.M., Kitto K.F., Wilcox G.L., McIvor R.S., Fairbanks C.A. Differential adeno-associated virus mediated gene transfer to sensory neurons following intrathecal delivery by direct lumbar puncture. Mol. Pain. 2010;6:31. doi: 10.1186/1744-8069-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Da Mesquita S., Fu Z., Kipnis J. The meningeal lymphatic system: a new player in neurophysiology. Neuron. 2018;100(2):375–388. doi: 10.1016/j.neuron.2018.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]


