Abstract
Radiation for medical use is a well-established therapeutic method with an excellent prognosis rate for various cancer treatments. Unfortunately, a high dose of radiation therapy comes with its own share of side effects, causing radiation-induced non-specific cellular toxicity; consequently, a large percentage of treated patients suffer from chronic effects during the treatment and even after the post-treatment. Accumulating data evidenced that radiation exposure to the brain can alter the diverse cognitive-related signaling and cause progressive neurodegeneration in patients because of elevated oxidative stress, neuroinflammation, and loss of neurogenesis. Epidemiological studies suggested the beneficial effect of hormonal therapy using estrogen in slowing down the progression of various neuropathologies. Despite its primary function as a sex hormone, estrogen is also renowned for its neuroprotective activity and could manage radiation-induced side effects as it regulates many hallmarks of neurodegenerations. Thus, treatment with estrogen and estrogen-like molecules or modulators, including phytoestrogens, might be a potential approach capable of neuroprotection in radiation-induced brain degeneration. This review summarized the molecular mechanisms of radiation effects and estrogen signaling in the manifestation of neurodegeneration and highlighted the current evidence on the phytoestrogen mediated protective effect against radiation-induced brain injury. This existing knowledge points towards a new area to expand to identify the possible alternative therapy that can be taken with radiation therapy as adjuvants to improve patients' quality of life with compromised cognitive function.
Keywords: Radiation, brain, neurodegeneration, estrogen, phytoestrogen, radiation therapy
1. INTRODUCTION
During aging, a progressive decline in brain function is associated with many neurodegenerative disorders (NDDs) where both genetic and environmental stressors, such as radiation, play an essential role [1-4]. Radiation has a great impact on human life [5-8]. Humans are confronted with radiation daily, albeit in low doses, from natural or artificial sources [9]. Radiation from natural sources may include cosmic, terrestrial, radioactive molecules, and exposure from inhalation or intake of radiation-contaminated materials. In comparison, medical imaging is one of the essential and rapidly rising artificial sources of radiation [10, 11], such as X-ray for imaging [12], computed tomography [13], radiation therapy (RT) for cancer treatment [14-16], nuclear medicine [17, 18] etc [19]. Accumulating evidence showed that diagnostic medical and contributed to 20% of the global yearly per capita actual radiation acquaintance from 1997 to 2007 [20]. RT prolongs the lifespan of many patients diagnosed with brain tumors with primary and metastatic stages; however, it leaves many survivors with long-term degeneration in the brain and cognitive impairments. Every year, more than 100,000 patients with primary and metastatic brain tumors encounter radiation-induced brain injury and survive for more than six months. Radiation at 2 to 45 Gy doses can inhibit neurogenesis in the brain in a dose-dependent manner [21], where a single dose higher than 30 Gy can induce acute central nervous system (CNS) syndrome, and fractionated doses greater than 60 Gy produce necrosis in white matter [22]. Growing evidence suggests that radiation induces multifactorial pathogenicity in the brain, and one of these is neurodegeneration caused by long-term oxidative stress, rearrangement in cell-cell junctional complex, lipid bilayer oxidation, and alternation in microvascular permeability [23-25]. Furthermore, radiation either in low or high doses can alter the function of the protein clearance system, leading to senescence or apoptotic cell death and thus causing neurodegeneration [26]. Yet, the molecular events that changed during RT need further verification, as many studies have shown little success in clinical attempts to end the radiation-induced neurodegeneration [27, 28] to unveil a potential target for better therapy.
In line with the survivability rate in patients with brain tumors, several studies highlighted those female patients with gliomas have a high survival advantage over the females of reproductive age, where males confront glioma development more than females [29]. It is noteworthy to mention that many physiological and pathological states, such as brain development, neurodegeneration, and aging, are critically modulated by sex hormones, especially estrogen receptor (ER) signaling. Estrogen, also known as estradiol or 17β-estradiol (E2), confers a protective role in gliomas progression, reduces tumor progression, and increases chemotherapy response [30-34]. Moreover, E2 confers neuroprotective effects by increasing various growth factor expressions, reducing oxidative stress and inflammation, activating the protein clearance system, and promoting cell survival [35-38]. Furthermore, supplementation of ER agonists promotes neurogenesis, particularly axonal growth and dendritic spines, and improves cognitive and memory performance by increasing synaptic transmission and plasticity [39, 40].
In this context, the targeting of ERs can be an adjuvant in traditional RT to reduce radiation-induced brain injury. Therefore, in this appraisal, an effort was deployed to accumulate the current information regarding the therapeutic benefits of E2 or ER modulators on radiation-induced neurodegeneration and neuronal survival and propose an alternative strategy for better therapeutic outcomes in the management of radiation-induced cognitive impairment.
2. IMPACT OF RADIATION ON COGNITIVE FUNCTION AND AGING
The scenario of the brain’s response to radiation has changed substantially over the last few decades as accumulating evidence from various clinical and preclinical studies showed that radiation causes deflected cognitive function and promotes aging, where onset is more than 6 months after irradiation with or without noticeable functional abnormalities [41, 42]. Two major regions of the brain are mainly affected by radiation, namely the subgranular zone (SGZ) and the subventricular zone (SVZ) of the dentate gyrus (DG) of the hippocampus, where radiation alters neurogenesis particularly. RT can cause cognitive dysfunction and memory impairment in younger and elderly patients [43]. Different clinical studies have shown that RT for cancer treatment, especially for brain tumors and cancer, can induce brain injury leading to cognitive and motor dysfunction. Investigating 81 patients with brain metastasis who received RT [44], a 2018 study observed a significant increase in cognitive impairment following RT. Patients with low-grade glioma have also suffered from cognitive dysfunction after RT. The study comparatively assessed a case of glioblastoma patients receiving RT from 1 to 22 years prior with hematological patients (low-grade) and healthy subjects and identified a connection between RT and poor cognitive function. Precisely, patients who received RT at doses of more than 2 Gy encountered cognitive impairment predominantly in the memory domain [45]. In a case study of brain tumor patients, who received postoperative RT, Asai et al. found that patients receiving whole-brain RT encountered a higher rate of radiation-induced dementia and brain atrophy than those who received focal RT [46]. A study conducted in 2001 investigated the effect of whole-brain irradiation on cognitive function [47], where 25 Gy/single dose of RT to the adult rat induced cognitive dysfunction and memory loss following whole-brain irradiation for a year, along with a higher body weight gain. In 2011, an in vivo study reported fractionated radiation of 20 and 40 Gy (5 Gy/day, five days/week) caused cognitive impairment four weeks post-irradiation in the rat [48]. The study also reported that radiation treatment significantly increased the water content of the brain, which was probably due to an increase in the permeability of the blood-brain barrier by irradiation. It also concluded that fractionated irradiation could cause early memory and learning deficits. The effect of radiation on neurodegeneration is not limited to brain cancer patients. Patients with other cancers who received RT also had neuronal dysfunction and memory deficit. A 2008 human study [49] that included therapeutic cranial irradiation (TCI) group comprised 11 lung cancer patients, three breast cancer patients, and two gastrointestinal cancer patients with a control group of 15 breast cancer patients reported that whole-brain irradiation caused cognitive dysfunction, including verbal memory impairments in patients. Another study conducted in 2011 revealed a substantial level of cognitive impairment and neuropsychiatric symptoms in patients with cerebral radionecrosis when RT was delivered for nasopharyngeal carcinoma in a fractionated manner [50]. From these pieces of evidence, it can be concluded that RT plays a significant role in developing neurodegenerative symptoms in patients in later phases of life.
3. POSSIBLE ROLE OF RADIATION IN THE HALLMARKS OF NEURODEGENERATION
Multiple factors are associated with the etiology of different NDDs; nevertheless, one significant phenomenon that all NDDs share is the rise of brain oxidative stress [51]. Oxidative stress, characterized by an imbalance between reactive oxygen species (ROS) production and cellular antioxidant capacity, can be attained during aging [52, 53], dysfunctional mitochondria [54, 55], damage to mitochondrial DNA [56], or dysfunction of other ROS sources [3, 57]. Among other environmental factors, radiation, especially ionizing form, is also a principal source of ROS production [58], and this is accomplished by energy absorption. When radiation, such as infrared (IR) passes through water, water absorbs radiation energy, causing hydrolysis and resulting in a load of reactive species. Quantitatively, radiolysis of pure deaerated water is responsible for generating reactive species like e-aq, H•, H2, •OH, and H2O2 [59]. Besides, IR stimulates the overproduction of intracellular inducible nitric oxide synthase (iNOS) [60] and accordingly produces nitric oxide (•NO) in a higher amount in the cell. Excessive •NO produces peroxynitrite anion (ONOO−) following a direct reaction with O2•−, which is a higher rate constant than superoxide dismutase (SOD)-catalyzed dismutation of O2•− [61]. Therefore, under ambient oxygen, water radiolysis and early NOS activation act as significant ROS sources in irradiated cells. However, different types of radiation modulate ROS production, and the mode of cellular damage they cause also differs in terms of their concentration [62].
Numerous reports suggested that excessive oxidative stress can be induced either by environmental factors or other types of sources, leading to misfolded protein aggregation [63-65], which can also be a major pathogenic consequence of infrared irradiation [66]. IR decreases the protein stability by generating ROS, which ceases the protein’s static function by upregulating the breakage of the polypeptide chain [67-70]. Furthermore, IR-induced ROS can initiate protein misfolding by targeting the thiol moieties of protein that are primarily oxidized by the ROS pathway, which is a primary target of radiation-induced protein oxidation. Upregulated ROS promotes glycoxidation or lipid peroxidation reactions, and the end products of these reactions and ROS also cause protein oxidation by direct interaction with native protein. Protein carbonylation, where aldehyde and ketone groups are added to the protein side chains, is the most shared protein modification. All residues are subtle to ROS-derived oxidation; yet, cysteine and methionine are significantly more sensitive to oxidation [71].
The cellular proteostasis network repairs or removes modified or misfolded protein; nevertheless, the proteins with methionine oxidation cannot be repaired but are subject to degrade selectively by clearance systems [57, 72]. The clearance systems in the proteostasis network, including the ubiquitin-proteasome system (UPS) and autophagy [73-76], play an essential role in suppressing non-functional and damaged protein aggregates, but the excess production of ROS markedly impairs function. The misfolded and aggregated proteins are recognized by a specific pathway when accumulated in mitochondria and endoplasmic reticulum (EnR), which recruits proteostasis functions, such as protein synthesis and degradation, known as unfolded protein response (UPR) [77, 78]. The UPR is activated by the influx of misfolded polypeptides and other factors, such as ROS, which are responsible for producing EnR stress when it rises at a higher level in EnR. The activation of UPR is perceived by three major singling pathways, including inositol-requiring enzyme-1(IRE1), PKR-like ER kinase (PERK), and activating transcription factor 6 (ATF6), which downstream regulates expressions of foldases, chaperones expression, and various autophagy-related genes [79]. During EnR stress, UPR promotes EnR-associated degradation (ERAD) and autophagy, regulates redox balance, and controls protein synthesis to restore protein homeostasis. However, prolonged UPR activation due to extended EnR stress leads to cell death by initiating degenerative cascades [80-82].
Compelling evidence suggested that IR exposure enhances cellular stress response and causes defects in DNA repair, impacting cell cycle progression and survival [83-85]. IR can disrupt mitochondrial DNA (mtDNA) by directly acting on it or indirectly via the generation of ROS like reactive hydroxyl radicals, leading to mitochondrial dysfunction [86]. IR also reduced total mitochondrial synthesis by inducing damage to mtDNA [87]. Furthermore, IR exposure enhances the accumulation of Drp1 in mitochondria and accelerates mitochondrial fission to manage overproduced mitochondrial ROS [88]. In addition to cellular respiration, mitochondria plays an essential role in the regulation of calcium homeostasis [89]. The imbalance of calcium homeostasis has been reported to enhance protein aggregation, autophagy deficits, and apoptosis, leading to a drastic alteration in proteostasis networks [90-93].
Intracellular Ca2+ maintains a reciprocal relationship with ROS, where ROS generation is induced by Ca2+ signaling and vice-versa [94]. Increased intracellular Ca2+ exerts excitotoxic neuronal death, as reported in many NDDs [95], and demonstrated by overproduction and a delayed mitochondrial depolarization along with mitochondrial cytochrome c release [96]. The superoxide anion (O2-) is constantly being generated by the mitochondria. It has been shown that increased Ca2+ uptake by the mitochondria increases the production of reactive oxygen [97]. EnR stress is also a fundamental phenomenon related to the ROS production and Ca2+ excitotoxicity cycle [98].
Furthermore, soluble protein aggregates, following diffusion, colocalize and interact with the various membrane proteins and receptors, including Ca2+ regulating channels and receptors at the excitatory synapse, for example, like NMDARs [99] and mGluR5 [100]. These misfolded proteins assemblies with membrane receptors around the excitatory synapses initiate artificially activated signaling platforms that promote Ca2+ elevation and enhance ROS and other deleterious effects by causing excitotoxicity [101, 102]. Excessive excitotoxicity further impedes the proteostasis network and increases the total protein aggregates by establishing a “vicious cycle” [103, 104]. Oxidative stress derived from radiation and subsequent protein misfolding and protein aggregation, excitotoxicity, EnR stress together trigger neuroinflammation. Mounting evidence suggests that IR exposure induced a rapid rise in Ca2+ level, which persisted over time due to the higher increase of inositol triphosphate level [105-107]. Leach et al. proposed that IR causes mitochondrial depolarization by increasing Ca2+ permeability, where Ca2+ is involved in a chain reaction of ROS signaling propagation and amplification [108]. In immature dorsal root ganglion neurons, infrared irradiation with 24 Gy induced a high elevation of Ca2+ and promoted cell death in a dose-dependent manner [109]. Interestingly, nimodipine, a calcium antagonist, protected hippocampal neurons from the radiation-induced cognitive deficit and apoptosis in the rat model [110]. Thus, radiation-induced oxidative stress is closely related to the initiation of neurodegeneration, leading to NDDs.
4. ESTROGEN SIGNALING REGULATES THE HALL- MARKS OF NEURODEGENERATION
E2 is one of the primary sex hormones and has a key role in maintaining diverse signaling systems, leading to neuroprotection [111]. Although ovaries are the primary source of E2, this hormone is also available in adipose tissue and adrenal glands [112]. The typical functions of ER signaling are regulated by binding E2 as a ligand of mainly two classical nuclear receptors, estrogen receptor-alpha (ERα) and estrogen receptor-beta (ERβ). Besides, ER signaling is also modulated by GPR30, an additional category of membrane G protein-coupled receptor, called GPER [113]. However, both receptors, ERα and ERβ, primarily initiate cellular signaling mediated by E2, and both of these receptors show variations in the cellular distribution and regulations [114-116].
Structurally, both the ERs, ERα and ERβ, shared high homology and are composed of different functional and structural domains, including the N-terminal domain (NTD) bearing the first activation function domain (AF1), ligand-binding domain (LBD), including the second activation function domain (AF2), DNA binding domain (DBD) and hinge region. A study based on the sequence homology represented that the LBD of both isomers has a sequence identity of around ~55%, DBD shows more than 95% of sequence identity, while NTD bears a low sequence identity (around ~15%), as this portion in ERβ is comparatively not longer than that of ERα [116, 117]. Both AF-1 and AF-2 are involved in regulating the transcriptional activities of ERs, where hormones and steroids regulate AF2 actions, but the hormone-independent function is only represented by AF1 [118, 119]. Interestingly, ERs regulate gene transcriptions in nuclear signaling, known via classical and non-nuclear signaling approaches, dependent on ligand-induced activation [120, 121].
Accumulating evidence represented that non-nuclear ER signaling maintains a critical role in modulating the brain's proteostasis network and functional integrity [122]. In the human brain, both ERα and ERβ are present and modulate a broad range of brain functions, such as neuronal defense, neurogenesis, synaptic plasticity, reproductive processes, learning, memory, mood, and behavior [123]. E2 protects neurons by modulating the following pathways; axonal sprouting, synaptic transmission, regenerative responses, neurotransmitter receptor function, phosphorylation cascades, kinase signaling pathways, etc [124, 125]. E2 controls cell proliferation, differentiation, and apoptosis occurring in the normal mammary gland at lactation/involution cycles [126]. ER signaling protects mammary cells from EnR stress by modulating the UPR, where the modulators are exerted during milk protein synthesis and secretion [127]. The mechanistic premise of ER in modulating diverse signalings of degeneration cascades is discussed in the subsequent sections.
4.1. In the Attenuation of Ca2+ Mediated Excitotoxicity
Calcium appears to have a major role in brain aging that contributes to Alzheimer's disease (AD), and dementia has been established by several lines of evidence [128, 129]. Although physiological activities are required for intracellular Ca2+, neuronal dysfunction and cell death can be caused when the amount is excessive. There are a variety of enzymes, including proteases, endonucleases, phospholipases, and nitric oxide synthase (NOS), which can cause the elevation of Ca2+ level in the neuronal cell, thus there upregulated activity can be linked to neuronal cell death [130, 131]. In many neuronal signal transduction pathways, excessive intracellular calcium accumulation plays an essential role, where changes in the intracellular calcium homeostasis are crucial in brain aging, memory, and cell death. In such a case, one of the most common receptors, N-methyl-D-aspartic acid (NMDA), which regulates Ca2+ influx through glutamate-derived activation, triggers a broad range of cellular processes in the postsynaptic neuron, ranging from synaptic plasticity to cell death. Continuous activation of a high number of NMDA receptors causes a rise in intracellular calcium loading and catabolic enzyme activities, which can set off a chain of events that finally leads to apoptosis or necrosis. Excess glutamate levels in the CNS can produce an increase in intracellular Ca2+ levels, which causes an increase in Ca2+ concentration in sensitive organelles, such as mitochondria and the EnR [132]. Furthermore, increased Ca2+ entrance into the cytosol induced by NMDA activates calcineurin and causes death in rat hippocampus neurons and stable cell lines, such as HeLa cells [133, 134].
Accumulating studies evidenced that ER signaling regulates the expression of NMDA receptors, especially in the memory process. It was found that E2 enhances NR2B expression, a subunit of NMDAR, and accordingly enhances long-term potentiation (LTP) in the learning and memory process [135, 136]. Liu et al., 2008 found that LTP enhancement was directly mediated through ERβ activation [137], and ERβ lacking mice (ERβ-/-) failed to show E2 mediated neuroprotection against NMDA-induced excitotoxicity [138]. The evidence further suggests that E2-mediated neuroprotection reduces ischemia-induced excitotoxicity through interaction with the NMDA receptor [139, 140]. Weiss et al. showed that treatment of E2 in ovariectomized Wistar female rats decreased the NMDA mediated cerebral oxygen consumption, and the effect deviated from a reduced number of NMDA receptors [141]. As cortical neurons in the female brain have more immunoreactive ER than males, as shown by in vitro in Bryant et al., an ER-specific ligand propylpyrazoletriol (PPT) protected cortical neurons against glutamate-induced damage in females, while this effect was absent in males [142].
In ovariectomized rats, SERMs, such as tamoxifen, raloxifene, and bazedoxifene, protected neurons against kainic acid-induced hippocampus neuronal injury [143]. In ischemic rat brains, tamoxifen promoted neuroprotection against AMPA/NMDA receptor-mediated excitotoxicity caused by oxygen/glucose deprivation [144] and also against glutamate-induced excitotoxicity [145]. In the rat cerebral cortex, raloxifene, which acts as an ER agonist in the brain but an antagonist for the reproductive system [146], promoted neuroprotection against kainic acid-induced excitotoxicity as well as oxidative stress [147].
In addition to NMDA receptor modulation, ER can reduce calcium influx through L-type channels [148, 149]. As suggested by Zhao and Brinton [150], glutamate-mediated intracellular Ca2+ accumulation can be reduced by ER subtype-selective agonists PPT and diarylpropionitrile (DPN) through ERK/MAP kinase pathway, which was differentially regulated ERα and β in hippocampal neurons. Studies also suggested that ERα can interact with mGluR1 at the postsynaptic membrane of a hippocampal neuron when activated by E2 [151], and this interaction facilitates higher phosphorylation of the cAMP response element-binding protein (CREB) [152]. A similar phenomenon was also observed in the case of striatal neurons; however, the effect was mediated by the direct interaction of ERα to mGluR3 but not mGluR5 [153]. Both ERα and ERβ enhance mGluR2/3 activation and the Gi/0 pathway, which lowers L-Ca2+ channel function by inhibiting PKA phosphorylation of CREB.
4.2. In the Regulation of Oxidative Stress
ERs play essential roles in maintaining redox balance and protecting cells from oxidative damage [111]. Studies evidenced that ER modulators, such as E2, can initiate a chemical antioxidant system and regulate ROS signaling [154-158] by regulating expressions of antioxidant enzymes. Evidence derived from in vivo studies dealing with rat brains lacking estrogen revealed that estrogen deficiency downregulates the expression of SOD, CAT, NADH, and GSH/GSSG ratio [159], thus elevating oxidative stress along with anxiety, memory loss, and learning disability [160]. While in a similar experimental model, treatment of E2 showed a withdrawal effect of brain oxidative stress [161]. A detailed analysis by Rao et al. [162] represented that E2 treatment upregulates SOD1 in cortical neurons, which is negatively associated with the progression of oxidative stress induced brain damage. Yang et al. reported that knockdown of ERβ from HT-22 cells showed resting mitochondrial membrane potential becoming resistant to oxidative stress, leading to increased ROS production [163], suggesting that ERβ is associated with regulation of ROS in mitochondria. Siriphorn et al. stated that Schwann cells expressed ERα and ERβ and treatment with E2 had a protective effect and survival mechanism against H2O2 exposure, which was validated by ICI182780, an ER antagonist [164]. E2 consists of hydroxyl group which usually acts as a chemical shield during the protection from free radical [165]. In the case of mitochondrial ROS, Stirone et al. reported that E2 reduced the formation of hydrogen peroxide (H2O2] in female cerebral blood vessels [166]. E2 decreased bain mitochondrial ROS generation in Fischer 344 rats, by increasing aconitase and manganese superoxide dismutase (MnSOD), where enzymes break superoxide radicals in the male and female rat [167] to reduce mitochondrial ROS production.
In nongenomic pathways, overexpression of ERα and ERβ activates some cytosolic signaling molecules, including ERK and MAPK, leading to attenuating amyloid β-related ROS in the HT22 hippocampus-derived cell line [168]. Tsialtas et al. reported that mitochondrial ERβ was involved in reducing H2O2 mediated apoptosis via suppressing caspase-9 and -3 activation in the N2A cells line [169]. Razmara et al. reported that the E2-mediated protective effect decreases ROS-related mRNA in human cerebral endothelium cells through ERα [170]. Furthermore, investigations found that E2 offered neuroprotection in the brain tissue of male mice following permanent blockage of the middle cerebral artery, as well as in primary neurons exposed to ROS via an ER-independent anti-oxidant mechanism [171].
4.3. In the Modulation of Neuroinflammation
Neuroinflammation is a response to specific CNS injuries [172], mediated by collaborative approaches of activated astrocytes and microglial cells that elevate pro-inflammatory mediators, such as ROS, chemokines, and pro-inflammatory cytokines, a vicious cycle that eventually causes neurodegeneration [173]. In such cases, ER signaling offers neuroprotection through the interactions with tissue and cell-specific co-regulators [2]. The neuromodulator causes the conformational change of ER and eventually causes receptor activations, and reduces neuroinflammation [2]. Interestingly, it is proved that all three ERs, ERα, ERβ, and GPR30, are involved in neuroprotection [174]. It is now evidenced that E2 inhibits the activation of NF-κB signaling [175]. NF-κB provokes the expression of several chemokines and cytokine-producing genes, including pro-inflammatory mediators [176]. The NF-κB family members (e.g. NF-κB1, NF-κB2, RelA, RelB, and c-Rel) facilitate around five hundred gene expressions, including iNOS, IL-1, IL-6, IL-8, TNF-α, which are responsible for inflammation [177]. The activated NF-κB signaling cascade is associated with many neurological disorders [176-178]. It is also found that ERK/MAP kinase activation is vital for E2-mediated neuroprotection [179]. Choi et al. showed that E2 activates PI3K/Akt signaling in cortical neurons [174, 180]. Xing et al. found that E2 activates ERβ, upregulates IκBα expression, and inhibits RelA (p65) binding to the inflammatory gene, which might reduce inflammation [181]. Tiwari-Woodruff showed that activation of ERα decreased the level of pro-inflammatory mediators TNF-α, IFN-γ, and IL-6 and upregulated the expression of anti-inflammatory cytokine IL-5 in EAE model animals [182]. Treatment with E2 led to reduced dendritic cell activation in the CNS of EAE mice and reduced the expression of IFN-γ, TNF-α, and IL-12 mRNA [183]. Remarkably, E2 also provides a neuroprotective effect through the activation of the GPR30 receptor [184]. The GPR30 agonist G-1 treatment also reduced CNS inflammation, axonal damage, and demyelination [185]. G-1 also reduced the number of macrophages in the CNS [186]. Blasko et al. showed that G-1 reduces the expression of chemokine and cytokine, including CCL2, CCL4, CCL5, TNF-α, IFN-γ, IL-17, and IL-23, [186]. Recently, Spence et al. have found that activation of ERα also declines the expression of chemokine CCL2 and CCL7 in EAE astrocytes [187].
4.4. In the Activation of Protein Clearance Systems
ER-mediated signaling is a complex process, and numerous anonymities need to be resolved. It is evidenced that ERα controls HSF1 signaling, the mediators which increase the expression of HSP70, HSP90, and HSP110 in stress conditions [188, 189]. Recently, Riar et al. have shown that activated ERα induces UPRmt activation, raises NRF1, and increases cellular proteasome systems in the ALS model [190]. The first outcome of UPR is the activation of BiP (GRP78) with a subsequent reduction of protein production, ultimately reducing EnR stress [191]. On the other hand, mitochondrial UPR upregulates CHOP [192], and CHOP influences the expression of HSP10, HSP60, and proteases [190].
Chung et al. found that E2 treatment activates autophagy, resulting in a higher level of Atg12, LC3B-II/LC3B-I, HSP70, and HO-1 expression in ovariectomized rats [193]. Activated ERα was also shown to increase p62 and reduce Bcl-2 expression, provoking autophagy [194, 195]. Besides, activated ERβ influences autophagy by controlling the expression of autophagy-associated markers, including p62 and LC3-I/II activating AMPK signaling, modulating P13K/ AKT/mTOR signaling [196], and detachment of BECN1-BCL2 complex [197, 198]. The mitochondrial ERα mediates autophagy by modulating p38 MAPK and ERK signaling [199]. It is now clear that ERs regulate several autophagy-related transcription factors. Li et al. showed that E2 stimulates Nrf2-ARE signaling and the expression of autophagy proteins in the brain [200].
5. ESTROGEN REPLACEMENT THERAPY IN NEURODEGENERATION
Many etiological studies established a relationship between sex preference of AD development. They found that women during the age of eighty are more likely to have a higher incidence of AD development, which is double that of men, though having a protective measures, including education. This finding also suggests that E2 deprivation is linked to AD. Many studies have been conducted so far to ensure the benefits or risks of hormone replacement therapy (HRT) in patients with various NDDs, where therapy includes either only several categories of E2 or in combination with exogenous or synthetic or progestogen. However, the implication of estrogen replacement therapy (ERT) for neurological disorders is not beyond question, as some studies also suggested that this treatment method may not be beneficial [201].
As E2 is predominately synthesized from the ovary and involved in many endocrine-related mechanisms of women, in the case of menopausal women, ERT has been applied and studied more extensively [202-204]. ERT could be beneficial in avoiding NDDs by boosting neuronal defense and memory systems based on E2-mediated neuroprotection [205]. Again, ERT can improve cognitive function performance in the aging brain [206]. A double-blind placebo-controlled clinical trial concluded that ERT would be an ineffective strategy for reversing the pathology of AD if it had already been initiated [207]. Another 12-week clinical trial investigation on 50 participants found that the E2 treatment group exhibited no significant improvement over the placebo group, indicating that ERT has limitations in treating AD [208]. In 2000, a clinical trial was conducted among mild to moderate AD patients. The study showed neither deterioration nor improvement in ERT patients [209]. It can, however, lessen the risk of AD or delay its development [210]. Numerous studies reported that ERT in older women showed improved cognitive function and memory. An early study conducted in 1952 showed ERT improved the verbal memory of old women patients compared to the placebo group [211]. In 1997, an investigation involving 472 peri-menopausal women showed that ERT provided a protective action against AD progression compared to non-user of ERT [212]. Another clinical trial study conducted among 278 post-menopausal women patients (aged 55 to 93) showed an improved memory function after ERT.
The study showed significant improvement in proper name recall in the treatment group patients [213]. A clinical investigation with 46 postmenopausal women was done to examine how E2 affected brain activity patterns during a working memory task. The study discovered that E2 increases activity in the inferior parietal lobule during the storage of verbal information and decreases activation during the storage of nonverbal information [214]. A recent meta-analysis study summarized the existing research and clinical data and illustrated a dramatically lower risk of AD and PD by ERT compared to the control group [215]. In 1994, one clinical trial study reported that dementia with AD is significantly reduced by ERT [216]. Also, ERT may help to improve the efficacy of other clinical therapy for AD. In 1996 a 30-week-long clinical trial study was conducted with 318 subjects, which concluded that ERT enhanced the efficacy of Tracine, an AD medicine [217].
PD is a progressive movement disorder developed by the degeneration of the dopaminergic neurons. The risk of PD begins to develop in pre-menopausal women, reducing the effectiveness of their medications. Furthermore, post-menopausal women have a higher risk of developing PD, suggesting ERT might play a crucial role against PD symptoms. Additionally, E2 has a modulatory role in lessening the degradation of dopaminergic neurons [218].
Several studies have reported the beneficial effect of ERT on PD during the menstrual cycle. Recently, song et al. showed that HRT reduced the risk of PD significantly in postmenopausal women compared to the control by using a random-effects meta-analysis where the odds ratio value was 0.470; 95% CI: 0.368-0.600 [215]. Along with this, some clinical studies have reported the more significant impact of ERT on PD. In a double-blind, parallel-group study, administration of conjugated E2 (Premarin; 0.625 mg/day) over eight weeks showed the reduction of motor fluctuation and improvement “on” and “off” times in postmenopausal women patients of PD (n=20) compared to placebo-treated patients. However, a double-blind, placebo-controlled study reported a short-term anti-Parkinsonian effect with mild-to-moderate PD in 8 postmenopausal women without altering dyskinesia scores [219]. In another placebo-controlled, randomized, double-blind trial with postmenopausal patients (12 females), the dopaminergic effect of E2 was not significant [220]. Thus, the null findings concluded the attenuation of PD symptoms in the menopause stage of women by ERT.
Multiple sclerosis (MS) is a persistent demyelinating disease of the central nervous system (CNS), affecting eyesight and causing a significant decrease in relapses [221]. Pregnant women with MS have the beneficial effect of the pregnancy hormone estriol in reducing lesions. Therefore, one clinical study was conducted to observe the role of estrogen treatment in MS. Non-pregnant patients with MS were (aged from 18 to 50 years) examined after the oral administration of estriol (8mg/day) for six months, where a significant decrease in lesion numbers was found compared to the control group [222]. Soldan and the team reported that the levels of CD4+, CD8+ T cells, and CD4+ CD45Ro+ (memory T cells) were decreased significantly while the levels of IL-5 and IL-10 increased during estriol (8 mg/day, for six months) treatment in ten female patients with MS, suggesting the correlation with depletion of lesions improvement [223].
Huntington's disease (HD) is a well-known inherited NDDs that causes the deterioration of nerve cells. Several studies revealed both enhancement and blocking of dopaminergic activity by E2 in animal models, but the study of ERT on HD patients was minimal. However, one clinical study reported the improvement in lower than one-third of patients with chorea in HD patients together with tardive dyskinesia [224].
Amyotrophic lateral sclerosis (ALS) is a fatal nervous system disease with various phenotypes. A case-control study in Ireland, Italy, and the Netherlands was performed to observe the effect of HRT in 653 patients and 1,217 controls over four years. The exogenous E2s combined with progestogens were found to reduce ALS risk in women [225]. Another study on HRT failed to demonstrate the beneficial effect of E2 in ALS patients. There was no significant difference in neuroprotection between postmenopausal women with ALS treated and those not taking ERT [226].
In brief, ERT is a newly established treatment that diminishes menopausal symptoms and the risk of postmenopausal diseases. The risk of NDDs, such as AD, PD, MS, HD, and ALS, might be lessened during the menopausal stages through ERT. Although several clinical studies were assessed, further studies are required with similar approaches to expand ERT-induced neuroprotection.
6. ROLE OF SELECTIVE ESTROGEN RECEPTOR MODULATORS IN RADIATION-INDUCED BRAIN INJURY
Several selective ER modulators (SERMs) are currently available and are being used to manage postmenopausal symptoms, osteoporosis, and cancers like breast cancer, which because of their tissue selective behavior, act as both agonists and antagonists of ERs. The binding and activation mode of these modulators are briefly described elsewhere [227-230]. Among these SERMs, tamoxifen has been widely used and prescribed for many diseases for several decades as adjuvant endocrine therapy. In the case of radiation-induced brain injury, Liu et al. summarized that tamoxifen at a concentration of 1 μM reduces the radiation-induced microglial inflammatory response through the negative effect of irradiation in a murine microglial cell line, BV-2 cells. When radiation was applied to 10 Gy, the expression of TNF-α and IL-1β increased and promoted inflammatory responses, which were significantly reduced by tamoxifen. Notably, tamoxifen administration at a dose of 5 mg/kg decreased the tissue edema formation and blood brain barrier (BBB) breakdown, glial activation, and neuronal death, caused by total brain irradiation in the adult male Sprague–Dawley rats [231]. Another ER signaling modulator [232], lithium, is also reported to reverse radiation-induced cognitive impairment, as Zanni et al. [223] reported. It has been found that lithium treatment promoted hippocampal neurogenesis in C57BL/6J mice, even four weeks after whole-brain radiation (a single dose of 4 Gy). Earlier mechanistic analysis showed that pretreatment lithium could increase the proliferation of hippocampal neural progenitor cells and rescues radiation-induced (3.5 Gy) G1 and G2/M phases of cell cycle arrest without causing apoptosis [233].
In addition to synthetic SERMs, many naturally occurring SERM, known as phytoestrogen, are also reported to ameliorate radiation-induced brain injury (Table 1). One of the popular phytoestrogens, quercetin, is a potential phytoestrogen that can protect against radiation-induced brain injury. Kale et al. found that quercetin treatment at 50 mg/kg can mitigate radiation-induced brain injury by altering antioxidant status, more explicitly regulating plasma total antioxidant status (TAS) and malondialdehyde (MDA) in male, Wistar-Albino rats [234]. Another experimental research, later on, revealed that quercetin (5-100 μM, 24 hrs) protected neurons against radiation (γ-ray, 2 Gy)-mediated EnR stress and inflammatory response through downregulating binding immunoglobulin protein (BiP) and C/EBP-homologous protein (CHOP), TNF-α, JNK, some EnR stress marker gene and upregulating Tuj1, a cytoskeletal protein with neurotrophin brain-derived neurotrophic factor, conducted by Chatterjee et al. [235]. Data obtained from a histopathological investigation of male Wister rats' brain tissues study, conducted by Mansour et al., stated that 5, 7-Dihydroxyflavone (DHF) or chrysin (50 mg/kg, 21 days) could reverse acrylamide (ACR) or γ-irradiation-induced neurotoxicity attributing increased catecholamine level and brain-derived neurotrophic factor (BDNF), leading to increased serum activity of creatinine kinase-BB but decreased acetylcholinesterase and caspase-3 activities and MDA and β-amyloid level [236]. Furthermore, chrysin upregulates glutathione (GSH) with downregulating MDA and TNF-α levels in ACR-treated Male albino rats (140-160g) [237]. Flaxseed oil (FSO), which contains a high level of phytoestrogenic lignans, was found to have neuroprotective effects against 7 Gy γ-irradiation and CCl4-induced brain injury in the irradiated Swiss albino (6-8 weeks, 25±2 gm) mice model through improving the antioxidant status, suppressing the inflammatory responses, and regulating the trace elements at a dose of 100 µl/mice/day for 21 days [238, 239]. Learning and memory improvement against 4Gy radiation-induced cerebral injury of curcumin (200 mg/kg) was mediated via improving antioxidant status through the underlying mechanism of increasing antioxidant transcription factor, i.e. nuclear erythroid 2 related factor 2 (Nrf2) and its downstream gene i.e. NADPH quinine oxidoreductase 1 (NQQ1], heme oxygenase-1 (HO-1), and γ-glutamyl cysteine synthetase (γ-GCS) with a concomitant significant increase in superoxide dismutase (SOD) and cerebral malonaldehyde (MDA) activity in 6–7 weeks Kunming mice [240]. In addition to the brain, curcumin (150 mg/kg, seven days) reduced radiation-induced heart injury by increasing IL-4 along with its receptor IL4Ra1, infiltrating some immune cells, i.e., lymphocytes and macrophages, and decreasing dual oxidase (Duox1 and Duox2) in γ-radiation (15 Gy) induced rat [241]. Resveratrol (5 and 10 mg/kg, 21 days) inhibits apoptosis induced by radiation and increases Sirt1 mRNA expression and activity with antagonizing oxidation and cellular ROS-scavenging effect in male Sprague-Dawley rats (200–220 g) [242]. Oral administration of epigallocatechin gallate (EGCG), with a dose of 2.5 and 5 mg/kg/d, significantly decreased amino acid homocysteine, immune molecules TNF-α and IL-6, and β amyloid with the simultaneous increase in the neurotransmitter dopamine, serotonin, and antioxidant status, including glutathione level, and the activities of glutathione peroxidase and glutathione reductase. They also found that EGCG prevents radiation-induced DNA damage to provide genomic stability and apoptosis via downregulating cyto-c, Bax, caspase-3, caspase-9, whereas upregulation of Bcl2 in the hippocampus [243]. Subsequently, silymarin (140 mg/kg/d) suppressed irradiation mediated damage that increased nucleic and histone protein stability and inhibited radiation-induced free radical production with lipid peroxidation against γ-ray (0.2 and 0.6 Gy/d) induced brain damage in the rat [244]. Baicalein protected neural progenitor cells in male C57BL/6 mice against γ-ray (5 Gy) -induced necrotic cell death. The author found that baicalein (10 mg/kg/d) alters neurogenesis by modulating oxidative stress and elevating BDNF-pCREB signaling, improving learning and memory capacity [245].
Table 1.
Phytoestrogens conferring neuroprotection against radiation-induced neuronal injury in vivo and in vitro.
| Compound | Structure | Treatment (Dose and Duration) | Experimental Model | Radiation (Type, Dose) |
Major
Outcomes |
Mechanism | References |
|---|---|---|---|---|---|---|---|
| Quercetin |
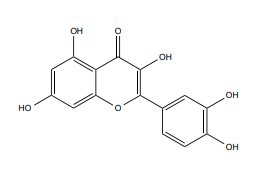
|
50 mg/kg | Male Wistar-Albino rats, 300–350 g | 20 Gy | ↑ Neuroprotection ↑ Antioxidant activity |
↑Regulate plasma MDA, TAS | [234] |
| 5-100 μM, 24 hrs | Dorsal root ganglion (DRG) neurons | γ-ray, 2 Gy | ↓ Inflammatory responses, ER stress, | ↓ BiP and C/EBP ↓ TNF-α, JNK ↑Tuj1, BDNF |
[235] | ||
| Chrysin |
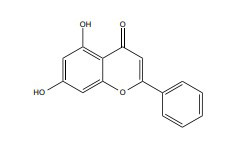
|
- | Male albino rats, 140-160 g | 5 Gy | ↓Oxidative damage | ↑ GSH,BDNF ↓MDA, TNF-Α, GABA |
[237] |
| 50 mg/kg 21days |
Male Wister rats, 120–150 g | γ-ray,5 Gy | ↓Oxidative damage | ↑Catecholamine content’ creatinine kinase-BB ↓ MDA, β-amyloid, acetylcholinesterase and caspase-3 |
[236] | ||
| Curcumin |
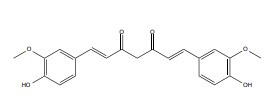
|
200 mg/kg | Kunming mice, 6–7 weeks | Heavy-ion radiation, 4 Gy. | ↑ Cognitive functions | ↑SOD, MDA ↓ NAD(P)H, NQO1, HO-1, γ-GCS |
[240] |
| 150 mg/kg, 7 days | - | γ-ray, 15 Gy |
↓ Heart injury | ↑IL-4 ↓ Duox1 and Duox2 |
[246] | ||
| Flaxseed oil | -- | 100 µl/mice/day (21days) |
Swiss albino 6-8 weeks, 25±2 gm | 7 Gy | ↓ Oxidative damage | ↓ Lipid peroxidation (LPO), ↑ Glutathione (GSH) |
[238] |
| Resveratrol |
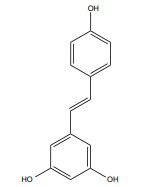
|
5 -10 mg/kg, 21 days |
Male Sprague Dawley rats, 200-220 g |
γ-ray, 4-Gy |
↑ Apoptosis ↑ Oxidative damage |
↑ Sirt1 mRNA ↑ ROS-scavenging |
[242] |
| Epigallocatechin gallate (EGCG) |
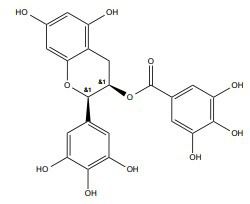
|
2.5-5 mg/kg/d | Male Wister rats | γ-ray, 4 Gy |
↓DNA damage and apoptosis | ↓ Homocysteine, ↓ Amyloid β, TNF-α, IL-6 levels ↑ Dopamine and serotonin ↓ Cytochrome-c, Bax, and caspase-3 and 9 ↑ Bcl-2 |
[243] |
| Silymarin |
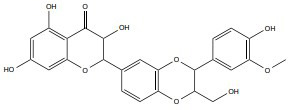
|
140 mg/kg/d | Rat model | γ-ray, 0.2-0.6 Gy |
↑ Repair DNA damage | ↑Nucleic acids, histone proteins stability ↓Free radical generation ↓Lipid peroxidation |
[244] |
| Baicalein |
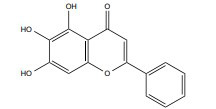
|
10 mg/kg/d | C57BL/6 mice | γ-ray, 5 Gy |
↑ Neurogenesis regulation | ↑BDNF-pCREB | [245] |
CONCLUDING REMARKS AND FUTURE PERSPECTIVES
Alongside regulating sexual behavior and reproductive functions, ER signaling has been renowned for its diverse functions in the CNS, including neurogenesis, synaptic plasticity, and neuroprotection, and eventually affecting motor activities, learning, cognition, and behavior [247-249]. Considering the substantial evidence highlighted in this review, it is inevitable that E2 or ER targeting modulators might hold the potential to delay the development and progression of various NDDs. Interestingly, it is evidenced that E2 or ER targeting modulators could successfully revert the expected pathological consequences in radiation-induced brain injury, including BBB breakdown, pro-inflammatory responses, and loss of hippocampal neurogenesis, where tamoxifen could be an example. Studies other than on radiation-induced injury revealed that the treatment of tamoxifen is effective in the brain and spinal cord injuries [250-252], promoting neuronal differentiation, preventing demyelination in the cerebral cortex [253], and providing neuroprotection against MS [254], stroke [255], PD [256], and dementia [257]. Nevertheless, other studies established that the use of tamoxifen failed to induce cognitive function when used as hormonal adjuvant therapy in breast cancer patients [258-260] and concluded that this drug could act like E2 only in the ovariectomized animals [261]. In the case of radiation-induced brain injury, Liu et al. observed a neuroprotective effect in the regular mice model [231], suggesting the importance of additional studies in different animal models to confirm the effectiveness and safety of radiation-induced neurodegeneration.
As both tumor radiosensitization and reduced toxicities in non-cancer cells are equally important goals in RT, ER signaling modulating agents from natural sources could be an attractive option to have the dual ability to enhance radiation damage in the malignancy, simultaneously protecting the normal cell from radiation injury [262, 263]. As highlighted in Table 1, several studies reported the potential of phytoestrogens in the neuroprotection against the radiation-induced brain or neuronal injury, where most of them are reported to increase radiosensitivity in conventional RT. For example, quercetin is reported to potentiate radiosensitivity against tumor cells in vivo and in vitro by regulating ATM-mediated pathways and acting as a radioprotective agent when used systemically [253]. A similar impression is also applied to curcumin, which also proved to increase radiosensitizing in various in vitro and in vivo cancer models [254, 255], while other studies suggested curcumin as a radioprotector [256-261].
Interestingly, all of the natural modulators listed in this study provide cumulative neuronal protection, acting on the multiple pathways of NDDs, including antioxidant defense systems, calcium excitotoxicity, proteostasis modulation, and neuroinflammation, as highlighted in Table 2. Since radiation exposure caused multi-path derived changes in brain signaling and function, the pharmacological modulation by these pleiotropic natural ER modulators compounds could open avenues to develop strategies for protecting brains against radiation-related incidents [264].
Table 2.
Neuroprotective activities of phytoestrogens against neurodegenerative hallmarks.
| Compound | Estrogen Receptor Preference | Effect on Neurodegeneration | References | |||
| Dose | Experimental Model | Cellular Mechanism |
Molecular
Mechanism |
|||
| Quercetin | ERβ-selective agonist | 0-150 μM | P19 neurons | ↓Oxidative injury | ↓ROS production ↑Caspase-3/7 |
[265] |
| RSC96 and rat Schwann cells | ↓Autophagy | ↑Beclin-1 ↑LC3 |
[266] | |||
| 0-25 μM | HT22 cell | ↓Ca2+ excitotoxicity | ↓Glutamate-mediated Ca2+ influx ↓Bid and Bax, and Cyt-c release |
[267] | ||
| Chrysin | ERα, ERβ-selective agonist [268] | 100 mg/kg, p.o. | Male Wistar rats | ↓Neuroinflammation | ↓Pro-inflammatory markers, i.e, TNF-α, IL-1β, IL-6, NF-κB, iNOS and COX-2 |
[269] |
| 5 and 10 µg/ml | Wistar rats | ↓Oxidative stress | ↑NRF2/HO-1 pathway activation | [270] | ||
| Resveratrol | ERα, ERβ-selective agonist [271] | 10 and 50 μg/ml | NMDA induced neuronal injury | ↓Neuronal death | ↑Intracellular calcium ↑ROS generation |
[272] |
| 20 μM | SH-SY5Y cells | ↓Autophagic flux | ↑LC3-II ↑HO-1 expression. |
[273] | ||
| Epigallocatechin gallate (EGCG) |
ERα-selective agonist [274] | 1.5 or 3 mg/kg | PS2 transgenic mice model | ↓Protein aggregation | ↑Memory function ↑α-secretase activity ↓β- and γ-secretase activities |
[275] |
| 25-100 μM | GO induced neurotoxicity in H 19-7 cells |
↓Oxidative stress | ↑Nrf2 activation | [276] | ||
As their effects have only been recorded in pre-clinical investigations, the pharmacological potentials of the natural ER modulators remain somewhat imperceptible. In reality, no clinical trial has yet been carried out to assess their impact on radiation-derived brain injury; therefore, implementation of high-quality trials is essential to confirm these preventive effects in the various aspect of brain injury following RT. Unfortunately, the efficacy data highlighted in the present review only emphasized the single preclinical model focusing on specific signaling pathways. Thus, more mechanistic-based studies focusing on multiple pathways are needed to reveal the unknown mechanism of these modulators by emphasizing radiation-induced side effects.
In addition to regulating oxidative imbalance, ER signaling can also be targeted for mitochondrial stress adaption. Mitochondrial dysfunction has long been known to play a critical role in tumor growth and has been linked to the expression of hormone receptors in several kinds of malignancies [277]. Studies showed that higher intracellular ROS and Ca2+ caused by mitochondrial stress adaptation are linked to AREG expression and secretion, where upregulated AREG further activates ERα through PI3K/Akt/mTOR and ERK pathways [278]. As a result, mitochondrial stress adaption through ER signaling could be a potential therapeutic target for the treatment of drug resistance [279].
Given the therapeutic benefits of HRT in neuroprotection [180, 280-283], various animal studies and a randomized controlled trial by the Women's Health Initiative (WHI) evidenced increased ischemic lesions in HRT [284-287], albeit the E2 on stroke have been mainly remaining contradictory. Other studies have described this effect of E2 on stroke as hormesis [288], a biphasic dose-response relationship, in which cellular exposure to a particular toxin at low concentrations develops resistance to future interactions with more significant concentrations, which might be described as an adaptive response to ongoing cellular stressors [289-292]. It is evident that ER signaling has hormetic phenomena, as a single receptor regulates multiple complex pathways [293], and thus studies evidenced the detrimental effect of E2 because of prolonged and higher concentrations on stroke models, while physiological concentrations are protective [284-287]. While describing the hormesis regulation by E2, other studies implied that higher concentrations of the female hormone are anti-inflammatory; on the contrary, low concentrations showed pro-inflammatory action [283, 294-296].
Again, hormetic pathways activation is regulated by vitagene brain axis activation that encodes several protein systems, including molecular chaperons (HSPs), sirtuin, and thioredoxin, which are essential redox-dependent regulators of ROS that exert effects ranging from physiological signaling to physiopathological consequences [283, 297, 298]. As described earlier, both E2 and natural antioxidants, including phytoestrogens, have been neuroprotective by activating hormetic pathways regulated by the vitagene protein network [299]. Unless used as dietary supplements, phytoestrogens are generally present in the food in chemical combinations. While large amounts of phytoestrogens may have no deleterious impact on an adult, they may negatively affect infant growth [299]. As both E2 and phytoestrogens exhibit non-monotonic and hormetic dosage responses, the design of pre-clinical and clinical trials, as well as approaches for optimum patient dosing in the treatment of neurodegeneration, are more challenging [300]. These conflicting findings of whether concentration ranges of E2s are neurotoxic or neuroprotective may be due to organ variations or inconsistencies in the measured end-points. Thus bearing hormesis in mind, experiments aimed to reveal the appropriate biological and therapeutic effects of E2 should thus be designed and conducted with a broad range of dosages. The biological relevance of these doses should be evaluated by blood hormone readings and subsequent comparison with the desired biological/clinical scenario [301].
A subsequent question still remains regarding the bioavailability of natural products when the compounds are targeted for CNS diseases. Collecting evidence suggested that natural products generally fail to acquire adequate levels in the brain due to the poor bioavailability and BBB crossing ability. As poor bioavailabilities share the reason for clinical failure, more care should be taken when considering the natural products in clinical settings, emphasizing tolerances, safety, effectiveness, bioavailabilities, and biodistributions. Treatment with curcumin, for example, is challenging due to its instability, poor bioavailability, and instant body clearance [302]. In a clinical trial setting with Alzheimer's patients, curcumin was unsuccessful in reversing cognitive impairment and discontinued due to gastrointestinal issues [303]. Nevertheless, many efforts have improved curcumin bioavailability by applying different drug delivery approaches [304-307]. Hu et al. found that curcumin has minor impacts on cognition and mood in aged people and changes plasma biomarkers in a population of middle-aged people when administrated at 400 mg per day as a solid nanoparticle-based preparation, which ensured adequate plasma curcumin levels [308]. Chen et al. confirmed that mesoporous polydopamine nanoparticles loaded with curcumin enhance radioprotection in BEAS-2B cells and radiation pneumonitis rat model by increasing the anti-oxidant system, downregulating proinflammatory cytokines, and reducing apoptosis and tissue damages [309]. This novel observation calls for an extensive study to design a new delivery approach for natural ER modulators to prevent age-related diseases induced by radiation.
The therapeutic benefits of phytoestrogenic substances and the molecular processes underpinning their positive effects in reducing inflammation, oxidative stress, and neuronal loss after menopause are well documented [307]. These advantages might also explain why they influence cognitive deficiencies in animal models of different NDDs. Remarkably, a combination of herbal supplements (a mixture of estrogenic substances) in a recent clinical study is found to be beneficial and safe to reduce menopausal symptoms [310]. As phytoestrogens and their derivatives can replicate the therapeutic consequence of natural E2, targeting ER signaling with phytoestrogen is seen as a viable way to avoid the onset and progression of NDDs [307]. There has also been a growing interest in phytoestrogens as a radioprotective agent in other organ-specific damages. For instance, genistein is an isoflavone phytoestrogen [311], and is considered a potent SERM due to having a binding affinity with ERα [312], ERβ [313], and GPR30 [314]. This phytoestrogen was trialed clinically and could attenuate the adverse effects of chemotherapy and RT [315, 316]. Moreover, genistein provides protection against radiation-induced bone marrow [317, 318], lung damage [319], and DNA damage in several types of cells [320]. However, the therapeutic activities of genistein against radiation-induced brain damage are still unknown. Similarly, many potential phytoestrogens are already identified to modulate ER signaling (Table 3); some of them might provide cytoprotection against radiation-induced injury, while detailed information regarding the mechanism is scarce. As a result, this review recommends a high-throughput screening for the radiation-induced NDDs, along with pre-clinical and clinical studies to disclose the novel mechanisms of these phytoestrogens, which may yield promising pharmacological leads for the treatment of various NDDs.
Table 3.
List of phytoestrogens and their biological functions in numerous study models.
| Compound | Chemical Structure |
ER
Pharmacology |
Treatment (dose) | Experimental Model | Cellular Functions | References |
|---|---|---|---|---|---|---|
| Isoliquiritigenin |
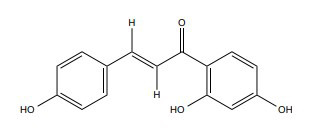
|
ERα | 25-50 mM |
In vitro (MDA-MB-231, MCF-7) |
↓MMP-2 and MMP-9, PI3K expression ↑p38 phosphorylation ↓NF-κB DNA binding and Akt kinase activity |
[321, 322] |
| Daidzein |
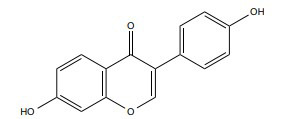
|
ERα | 0- 100 µM |
In vitro (MCF-7) |
↑ ROS generation, ↑ Bax, cyt-c. ↑ Caspase-9 and caspase-7, ↓ Bcl-2 protein level |
[323, 324] |
| Formononetin |
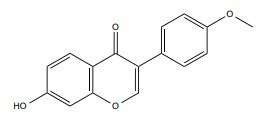
|
ERβ | 30–100 μM |
In vitro and in vivo (MCF-7) |
↓p-IGF-1 R, p-Akt, cyclin D1 protein and cyclin D1 mRNA expression ↑G0/G1 phase arrest ↓IGF1/IGF1R-PI3K/Akt pathways |
[325, 326] |
| Luteolin |
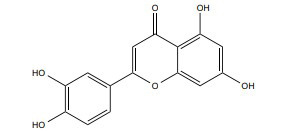
|
ERα | 40 µmol/L |
In vitro (MCF-7) |
↓pIGF-1 and PI3K-Akt pathway. | [327, 328] |
| Kaempferol |
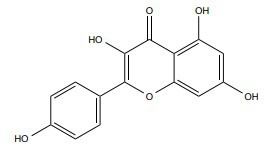
|
ERα |
In vitro (MDA-MB-231and BT474) |
↑ DNA damage ↑ Arrest at G1, G2 and G2/M phase ↑ Expression of γH2AX, ↓ Caspase 3 and 9 |
[329, 330] | |
| Apigenin |
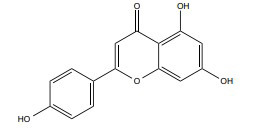
|
ERα | 5–20 μM |
In vitro and in vivo (MDA-MB231) |
↓Pro-intravasation trigger factors and MMP1 expression ↓ CYP1A1 activity |
[329, 331] |
| Glycitein |
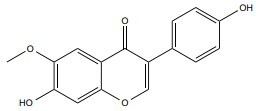
|
ERα | 10-100 mg/ml | In vitro SKBR-3 cells | ↑ Membrane permeability ↓ DNA synthesis |
[323, 332] |
| Gallocatechin-3-gallate |
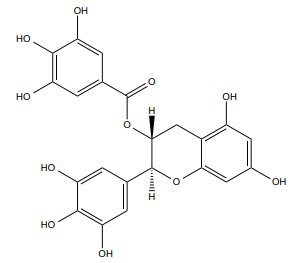
|
ERα | 20 μg/ml | in vitro and in vivo | ↓ miR-25 expression ↑Pro-caspase-3 and pro-caspase-9 and PARP. ↑ Arrest G2/M phase |
[274, 333] |
| Isorhapontigenin (ISO) |
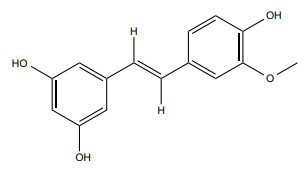
|
ERα |
In vitro (MCF7, T47D) |
↑ SPHK/tubulin destabilization ↑ ROS Production ↑ Cleaved PARP, cytoplasmic Cytochrome-C, cleaved caspase-3, and cleaved caspase-9 |
[334, 335] | |
| Pterostilbene |
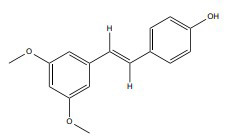
|
ERα | 50-75 μmol/L |
In vitro (MCF-7 and MDA-MB-231) |
↑Cytosolic Smac/DIABLO expression ↑MnSOD activity ↑Cytosolic calcium concentrations |
[336, 337] |
| Enterodiol |
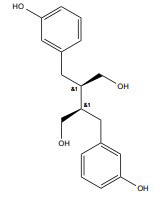
|
ERα, ERβ | 25- 75 μM |
In vitro (MDA-MB-231) |
↓ MAPK-p38 ↓ERK-1/2, NF-Κb ↓ Snail (mRNA & protein) |
[338, 339] |
| Sesamin |
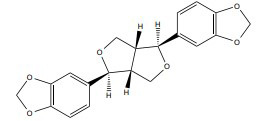
|
ERα | 12.5-100 μM |
In vitro (MCF-7) |
↑ Phosphorylation of RB and ↑Degradation of cyclin D1 | [340, 341] |
| Coumestrol |
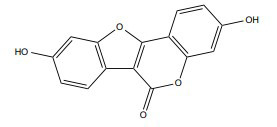
|
ERα, ERβ | 50 µM |
In vitro (MCF-7) |
↑ ROS production ↑ p53 and p21Cip1/WAF1 activity |
[342, 343] |
| Wedelolactone |
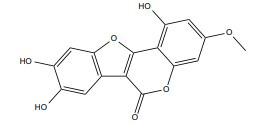
|
ERα, ERβ | 12.78- 27.8 μM |
In vitro (MDA-MB-231, MDA-MB-468 and T47D) |
↑ Chymotrypsin-like activity | [344, 345] |
| Psoralen |
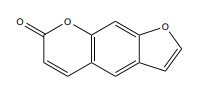
|
ERα | 0–65 μg/ml |
In vitro and in vivo (MCF-7 & MDA-MB-231) |
↑ G0/G1 & G2/M phase arrest ↓ Fra-1 & β-catenin expression ↑ Axin2 & phospho-(Y142) β-catenin expression |
[346, 347] |
| Anthocyanin |
|
ERα | In vitro and vivoN202/1A and N202/1E | ↑Oxidative stress ↑AMPK signalling pathway |
[348, 349] | |
| Delphinidin |
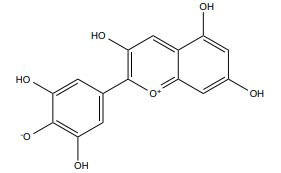
|
ERα | 25–1000 µg/ml |
In vitro (MDA-MB-453) |
↑ Cell cycle arrests the G1 phase. | [350, 351] |
| Ursolic acid |
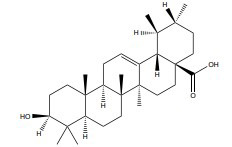
|
ERα | ≥10 μM |
In vitro (MDA-MB-231) |
↓ Nrf2 expression ↓ Keap1/Nrf2 pathway and EGFR/Nrf2 pathway |
[352, 353] |
| Sauchinone |
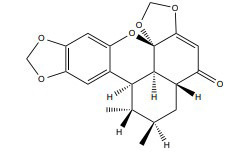
|
ERα | 50 g/ml |
In vitro (MCF-7) |
↓VEGF, cyclin D1, and Bcl-2 gene products ↑Activate caspase ↑ ERK signalling pathway |
[354, 355] |
| Lycopene |
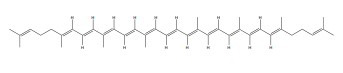
|
ERα, ERβ | 50 μM |
In vitro (MDA-MB-468 c) |
↑G0/G1 phase arrest ↑Activation of the ERK1/2 ↑p21 upregulation and Bax ↑Akt and mTOR activation |
[356, 357] |
| Phloridzin |
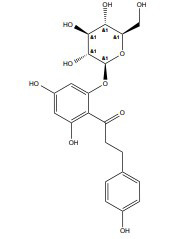
|
ERs | 10-150 μM |
In vitro (MDA-MB-231) |
↓Paxillin/FAK, Src, and α-Sma expression. ↑p53, p21, and E-cadherin ↓Type 2 glucose transporter. |
[358] |
| Oleuropein |
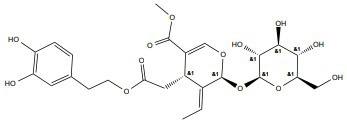
|
ERα | 100- 200 μg/ml |
In vitro (MCF-7) |
↑Blocks G1 to S ↑G0/G1 phase stability |
[359, 360] |
| Calycosin |
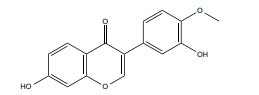
|
ERα, ERβ | 0-150 μM |
In vitro (MDA-MB-231) |
↓Rab27B-dependent signaling ↑β-catenin and VEGF modulation |
[361, 362] |
| α-Mangostin |
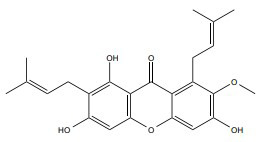
|
ERα | 10 µM |
In vitro (MCF-7) |
↑Activate-7, -8 and -9, Bax, p53, cytosolic cytochrome c and induced PARP cleavage ↓Bid and Bcl-2 |
[363] |
| Liquiritigenin |
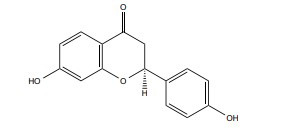
|
ERβ | 50 μM |
In vitro (HT22 cell) |
↓Ca2+ influx, ↓ROS production ↓Lipid peroxidation ↓Mitochondrial stress ↓MAPKs, ERK, c-JNK signaling pathways |
[364, 365] |
| Naringenin |
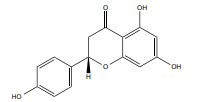
|
Partial ER agonist | 25-100 mg/kg/b.wt, |
In vivo C57BL/6J mice |
↓Lipid peroxidation ↑Glutathione reductase and catalase ↓iNOS expression |
[366, 367] |
| Genistein |
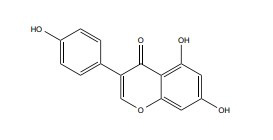
|
SERM | 0.01-1 uM |
In vivo Sprague–Dawley rat embryos |
↓Bcl-2/Bax ↓Caspase-9 and caspase-3 activities ↓NF-κB/p65, phosphorylation of p65 and IκB, ↓MAPK JNK and ERK signaling |
[368, 369] |
| Arctigenin |
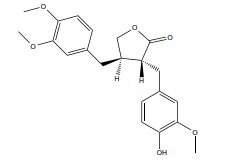
|
ERβ agonist |
In vitro (H89, SH-SY5Y cell) |
↓Aβdeposition, p-CREB. ↓Presenilin 1(PS1] |
[370], [312] | |
| Caffeic acid |
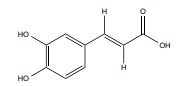
|
ERα | 10 µmol/kg (7 days) | Male Sprague-Dawley rats, 9–10 weeks, 110–120 |
↓ MDA, XO and ADA ↑ NO(x) and SOD |
[371, 372] |
| Cyanidin |
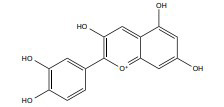
|
ERβ | 50-200, mg/kg/d | Kunming mice | Not mentioned | [348, 373] |
ACKNOWLEDGEMENTS
Declared none.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
This work was supported by the National Research Foundation of Korea (NRF) grant (No. NRF-2021R1A2C1008564) to ISM, funded by the Korean Ministry of Science and ICT.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Dash R., Ali M.C., Jahan I., Munni Y.A., Mitra S., Hannan M.A., Timalsina B., Oktaviani D.F., Choi H.J., Moon I.S. Emerging potential of cannabidiol in reversing proteinopathies. Ageing Res. Rev. 2021;65:101209. doi: 10.1016/j.arr.2020.101209. [DOI] [PubMed] [Google Scholar]
- 2.Dash R., Mitra S., Ali M.C., Oktaviani D.F., Hannan M.A., Choi S.M., Moon I.S. Phytosterols: Targeting neuroinflammation in neurodegeneration. Curr. Pharm. Des. 2021;27(3):383–401. doi: 10.2174/1381612826666200628022812. [DOI] [PubMed] [Google Scholar]
- 3.Hannan M.A., Dash R., Sohag A.A.M., Haque M.N., Moon I.S. Neuroprotection against oxidative stress: Phytochemicals targeting TrkB signaling and the Nrf2-ARE antioxidant system. Front. Mol. Neurosci. 2020;13:116. doi: 10.3389/fnmol.2020.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hannan M.A., Sohag A.A.M., Dash R., Haque M.N., Mohibbullah M., Oktaviani D.F., Hossain T., Choi H.J., Moon I.I.S. Phytosterols of marine algae: Insights into the potential health benefits and molecular pharmacology. Phytomedicine. 2020;69:153201. doi: 10.1016/j.phymed.2020.153201. [DOI] [PubMed] [Google Scholar]
- 5.Anand P., Kunnumakkara A.B., Sundaram C., Harikumar K.B., Tharakan S.T., Lai O.S., Sung B., Aggarwal B.B. Cancer is a preventable disease that requires major lifestyle changes. Pharm. Res. 2008;25(9):2097–2116. doi: 10.1007/s11095-008-9661-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gilbert E.S. Ionising radiation and cancer risks: What have we learned from epidemiology? Int. J. Radiat. Biol. 2009;85(6):467–482. doi: 10.1080/09553000902883836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Durazzo T.C., Mattsson N., Weiner M.W. Smoking and increased Alzheimer’s disease risk: A review of potential mechanisms. Alzheimers Dement. 2014;10(3) Suppl.:S122–S145. doi: 10.1016/j.jalz.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spagnuolo C., Napolitano M., Tedesco I., Moccia S., Milito A., Russo G.L. Neuroprotective role of natural polyphenols. Curr. Top. Med. Chem. 2016;16(17):1943–1950. doi: 10.2174/1568026616666160204122449. [DOI] [PubMed] [Google Scholar]
- 9.Kempf S.J., Azimzadeh O., Atkinson M.J., Tapio S. Long-term effects of ionising radiation on the brain: Cause for concern? Radiat. Environ. Biophys. 2013;52(1):5–16. doi: 10.1007/s00411-012-0436-7. [DOI] [PubMed] [Google Scholar]
- 10.Donya M., Radford M., ElGuindy A., Firmin D., Yacoub M.H. Radiation in medicine: Origins, risks and aspirations. Glob. Cardiol. Sci. Pract. 2014;2014(4):437–448. doi: 10.5339/gcsp.2014.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Timins J.K. Communication of benefits and risks of medical radiation: A historical perspective. Health Phys. 2011;101(5):562–565. doi: 10.1097/HP.0b013e3182259a71. [DOI] [PubMed] [Google Scholar]
- 12.Hoheisel M. Review of medical imaging with emphasis on X-ray detectors. Nucl. Instrum. Methods Phys. Res. A. 2006;563(1):215–224. doi: 10.1016/j.nima.2006.01.123. [DOI] [Google Scholar]
- 13.Villarraga-Gómez H., Herazo E.L., Smith S.T. X-ray computed tomography: From medical imaging to dimensional metrology. Precis. Eng. 2019;60:544–569. doi: 10.1016/j.precisioneng.2019.06.007. [DOI] [Google Scholar]
- 14.Fass L. Imaging and cancer: A review. Mol. Oncol. 2008;2(2):115–152. doi: 10.1016/j.molonc.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baskar R., Lee K.A., Yeo R., Yeoh K-W. Cancer and radiation therapy: Current advances and future directions. Int. J. Med. Sci. 2012;9(3):193–199. doi: 10.7150/ijms.3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mitra S., Dash R. Natural products for the management and prevention of breast cancer. Evid. Based Complement. Alternat. Med. 2018;2018:8324696. doi: 10.1155/2018/8324696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramamoorthy N. Impact of nuclear medicine and radiopharmaceuticals on health-care delivery: Advances, lessons, and need for an objective value-matrix. Indian J. Nucl. Med. 2018;33(4):273–276. doi: 10.4103/ijnm.IJNM_56_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hacker M., Beyer T., Baum R.P., Kalemis A., Lammertsma A.A., Lewington V., Talbot J.N., Verzijlbergen F. Nuclear medicine innovations help (drive) healthcare (benefits). Eur. J. Nucl. Med. Mol. Imaging. 2015;42(2):173–175. doi: 10.1007/s00259-014-2957-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holmberg O., Czarwinski R., Mettler F. The importance and unique aspects of radiation protection in medicine. Eur. J. Radiol. 2010;76(1):6–10. doi: 10.1016/j.ejrad.2010.06.031. [DOI] [PubMed] [Google Scholar]
- 20.Schulz R.J., Albert R.E. Follow-up study of patients treated by x-ray epilation for tinea capitis. 3. Dose to organs of the head from the x-ray treatment of tinea capitis. Arch. Environ. Health. 1968;17(6):935–950. doi: 10.1080/00039896.1968.10665350. [DOI] [PubMed] [Google Scholar]
- 21.Acharya M.M., Patel N.H., Craver B.M., Tran K.K., Giedzinski E., Tseng B.P., Parihar V.K., Limoli C.L. Consequences of low dose ionizing radiation exposure on the hippocampal microenvironment. PLoS One. 2015;10(6):e0128316. doi: 10.1371/journal.pone.0128316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greene-Schloesser D., Robbins M.E., Peiffer A.M., Shaw E.G., Wheeler K.T., Chan M.D. Radiation-induced brain injury: A review. Front. Oncol. 2012;2:73. doi: 10.3389/fonc.2012.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giusti A.M., Raimondi M., Ravagnan G., Sapora O., Parasassi T. Human cell membrane oxidative damage induced by single and fractionated doses of ionizing radiation: A fluorescence spectroscopy study. Int. J. Radiat. Biol. 1998;74(5):595–605. doi: 10.1080/095530098141177. [DOI] [PubMed] [Google Scholar]
- 24.Azzam E.I., de Toledo S.M., Little J.B. Expression of CONNEXIN43 is highly sensitive to ionizing radiation and other environmental stresses. Cancer Res. 2003;63(21):7128–7135. [PubMed] [Google Scholar]
- 25.Dayal D., Martin S.M., Owens K.M., Aykin-Burns N., Zhu Y., Boominathan A., Pain D., Limoli C.L., Goswami P.C., Domann F.E., Spitz D.R. Mitochondrial complex II dysfunction can contribute significantly to genomic instability after exposure to ionizing radiation. Radiat. Res. 2009;172(6):737–745. doi: 10.1667/RR1617.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sharma N.K., Sharma R., Mathur D., Sharad S., Minhas G., Bhatia K., Anand A., Ghosh S.P. Role of ionizing radiation in neurodegenerative diseases. Front. Aging Neurosci. 2018;10:134. doi: 10.3389/fnagi.2018.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shaw E.G., Rosdhal R., D’Agostino R.B., Jr, Lovato J., Naughton M.J., Robbins M.E., Rapp S.R. Phase II study of donepezil in irradiated brain tumor patients: Effect on cognitive function, mood, and quality of life. J. Clin. Oncol. 2006;24(9):1415–1420. doi: 10.1200/JCO.2005.03.3001. [DOI] [PubMed] [Google Scholar]
- 28.Rapp S.R., Case L.D., Peiffer A., Naughton M.M., Chan M.D., Stieber V.W., Moore D.F., Jr, Falchuk S.C., Piephoff J.V., Edenfield W.J., Giguere J.K., Loghin M.E., Shaw E.G. Donepezil for irradiated brain tumor survivors: A phase III randomized placebo-controlled clinical trial. J. Clin. Oncol. 2015;33(15):1653–1659. doi: 10.1200/JCO.2014.58.4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kabat G.C., Etgen A.M., Rohan T.E. Do steroid hormones play a role in the etiology of glioma? Cancer Epidemiol. Biomarkers Prev. 2010;19(10):2421–2427. doi: 10.1158/1055-9965.EPI-10-0658. [DOI] [PubMed] [Google Scholar]
- 30.Ström A., Hartman J., Foster J.S., Kietz S., Wimalasena J., Gustafsson J.A. Estrogen receptor beta inhibits 17beta-estradiol-stimulated proliferation of the breast cancer cell line T47D. Proc. Natl. Acad. Sci. USA. 2004;101(6):1566–1571. doi: 10.1073/pnas.0308319100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hartman J., Edvardsson K., Lindberg K., Zhao C., Williams C., Ström A., Gustafsson J.A. Tumor repressive functions of estrogen receptor beta in SW480 colon cancer cells. Cancer Res. 2009;69(15):6100–6106. doi: 10.1158/0008-5472.CAN-09-0506. [DOI] [PubMed] [Google Scholar]
- 32.Liu J., Sareddy G.R., Zhou M., Viswanadhapalli S., Li X., Lai Z., Tekmal R.R., Brenner A., Vadlamudi R.K. Differential effects of estrogen receptor β isoforms on glioblastoma progression. Cancer Res. 2018;78(12):3176–3189. doi: 10.1158/0008-5472.CAN-17-3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sareddy G.R., Nair B.C., Gonugunta V.K., Zhang Q.G., Brenner A., Brann D.W., Tekmal R.R., Vadlamudi R.K. Therapeutic significance of estrogen receptor β agonists in gliomas. Mol. Cancer Ther. 2012;11(5):1174–1182. doi: 10.1158/1535-7163.MCT-11-0960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou M., Sareddy G.R., Li M., Liu J., Luo Y., Venkata P.P., Viswanadhapalli S., Tekmal R.R., Brenner A., Vadlamudi R.K. Estrogen receptor beta enhances chemotherapy response of GBM cells by down regulating DNA damage response pathways. Sci. Rep. 2019;9(1):6124. doi: 10.1038/s41598-019-42313-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bellanti F., Matteo M., Rollo T., De Rosario F., Greco P., Vendemiale G., Serviddio G. Sex hormones modulate circulating antioxidant enzymes: Impact of estrogen therapy. Redox Biol. 2013;1(1):340–346. doi: 10.1016/j.redox.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rekkas P.V., Wilson A.A., Lee V.W., Yogalingam P., Sacher J., Rusjan P., Houle S., Stewart D.E., Kolla N.J., Kish S., Chiuccariello L., Meyer J.H. Greater monoamine oxidase a binding in perimenopausal age as measured with carbon 11-labeled harmine positron emission tomography. JAMA Psychiatry. 2014;71(8):873–879. doi: 10.1001/jamapsychiatry.2014.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Singh M., Meyer E.M., Simpkins J.W. The effect of ovariectomy and estradiol replacement on brain-derived neurotrophic factor messenger ribonucleic acid expression in cortical and hippocampal brain regions of female Sprague-Dawley rats. Endocrinology. 1995;136(5):2320–2324. doi: 10.1210/endo.136.5.7720680. [DOI] [PubMed] [Google Scholar]
- 38.Solum D.T., Handa R.J. Estrogen regulates the development of brain-derived neurotrophic factor mRNA and protein in the rat hippocampus. J. Neurosci. 2002;22(7):2650–2659. doi: 10.1523/JNEUROSCI.22-07-02650.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wallace M., Luine V., Arellanos A., Frankfurt M. Ovariectomized rats show decreased recognition memory and spine density in the hippocampus and prefrontal cortex. Brain Res. 2006;1126(1):176–182. doi: 10.1016/j.brainres.2006.07.064. [DOI] [PubMed] [Google Scholar]
- 40.Luine V., Frankfurt M. Interactions between estradiol, BDNF and dendritic spines in promoting memory. Neuroscience. 2013;239:34–45. doi: 10.1016/j.neuroscience.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bálentová S., Hnilicová P., Kalenská D., Murín P., Hajtmanová E., Lehotský J., Adamkov M. Effect of whole-brain irradiation on the specific brain regions in a rat model: Metabolic and histopathological changes. Neurotoxicol. 2017;60:70–81. doi: 10.1016/j.neuro.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 42.Sundgren P.C., Cao Y. Brain irradiation: Effects on normal brain parenchyma and radiation injury. Neuroimaging Clin. N. Am. 2009;19(4):657–668. doi: 10.1016/j.nic.2009.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Armstrong C.L., Gyato K., Awadalla A.W., Lustig R., Tochner Z.A. A critical review of the clinical effects of therapeutic irradiation damage to the brain: The roots of controversy. Neuropsychol. Rev. 2004;14(1):65–86. doi: 10.1023/B:NERV.0000026649.68781.8e. [DOI] [PubMed] [Google Scholar]
- 44.Cheng H., Chen H., Lv Y., Chen Z., Li C.R. Prospective memory impairment following whole brain radiotherapy in patients with metastatic brain cancer. Cancer Med. 2018;7(10):5315–5321. doi: 10.1002/cam4.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Klein M., Heimans J.J., Aaronson N.K., van der Ploeg H.M., Grit J., Muller M., Postma T.J., Mooij J.J., Boerman R.H., Beute G.N., Ossenkoppele G.J., van Imhoff G.W., Dekker A.W., Jolles J., Slotman B.J., Struikmans H., Taphoorn M.J. Effect of radiotherapy and other treatment-related factors on mid-term to long-term cognitive sequelae in low-grade gliomas: A comparative study. Lancet. 2002;360(9343):1361–1368. doi: 10.1016/S0140-6736(02)11398-5. [DOI] [PubMed] [Google Scholar]
- 46.Asai A., Matsutani M., Kohno T., Nakamura O., Tanaka H., Fujimaki T., Funada N., Matsuda T., Nagata K., Takakura K. Subacute brain atrophy after radiation therapy for malignant brain tumor. Cancer. 1989;63(10):1962–1974. doi: 10.1002/1097-0142(19890515)63:10<1962:AID-CNCR2820631016>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 47.Akiyama K., Tanaka R., Sato M., Takeda N. Cognitive dysfunction and histological findings in adult rats one year after whole brain irradiation. Neurol. Med. Chir. (Tokyo) 2001;41(12):590–598. doi: 10.2176/nmc.41.590. [DOI] [PubMed] [Google Scholar]
- 48.Zhou H., Liu Z., Liu J., Wang J., Zhou D., Zhao Z., Xiao S., Tao E., Suo W.Z. Fractionated radiation-induced acute encephalopathy in a young rat model: Cognitive dysfunction and histologic findings. AJNR Am. J. Neuroradiol. 2011;32(10):1795–1800. doi: 10.3174/ajnr.A2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Welzel G., Fleckenstein K., Schaefer J., Hermann B., Kraus-Tiefenbacher U., Mai S.K., Wenz F. Memory function before and after whole brain radiotherapy in patients with and without brain metastases. Int. J. Radiat. Oncol. Biol. Phys. 2008;72(5):1311–1318. doi: 10.1016/j.ijrobp.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 50.Wu X., Gu M., Zhou G., Xu X., Wu M., Huang H. Cognitive and neuropsychiatric impairment in cerebral radionecrosis patients after radiotherapy of nasopharyngeal carcinoma. BMC Neurol. 2014;14(1):10. doi: 10.1186/1471-2377-14-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Patten D.A., Germain M., Kelly M.A., Slack R.S. Reactive oxygen species: Stuck in the middle of neurodegeneration. J. Alzheimers Dis. 2010;20(s2) Suppl. 2:S357–S367. doi: 10.3233/JAD-2010-100498. [DOI] [PubMed] [Google Scholar]
- 52.Wyss-Coray T. Ageing, neurodegeneration and brain rejuvenation. Nature. 2016;539(7628):180–186. doi: 10.1038/nature20411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Castelli V., Benedetti E., Antonosante A., Catanesi M., Pitari G., Ippoliti R., Cimini A., d’Angelo M. Neuronal cells rearrangement during aging and neurodegenerative disease: Metabolism, oxidative stress and organelles dynamic. Front. Mol. Neurosci. 2019;12:132. doi: 10.3389/fnmol.2019.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schapira A.H.V. Mitochondria in the aetiology and pathogenesis of Parkinson’s disease. Lancet Neurol. 2008;7(1):97–109. doi: 10.1016/S1474-4422(07)70327-7. [DOI] [PubMed] [Google Scholar]
- 55.Anandatheerthavarada H.K., Biswas G., Robin M-A., Avadhani N.G. Mitochondrial targeting and a novel transmembrane arrest of Alzheimer’s amyloid precursor protein impairs mitochondrial function in neuronal cells. J. Cell Biol. 2003;161(1):41–54. doi: 10.1083/jcb.200207030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nissanka N., Moraes C.T. Mitochondrial DNA damage and reactive oxygen species in neurodegenerative disease. FEBS Lett. 2018;592(5):728–742. doi: 10.1002/1873-3468.12956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hannan M.A., Dash R., Haque M.N., Mohibbullah M., Sohag A.A.M., Rahman M.A., Uddin M.J., Alam M., Moon I.S. Neuroprotective potentials of marine algae and their bioactive metabolites: Pharmacological insights and therapeutic advances. Mar. Drugs. 2020;18(7):E347. doi: 10.3390/md18070347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Burgio E., Piscitelli P., Migliore L. Ionizing radiation and human health: Reviewing models of exposure and mechanisms of cellular damage. An epigenetic perspective. Int. J. Environ. Res. Public Health. 2018;15(9):1971. doi: 10.3390/ijerph15091971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Spinks J.W.T., Woods R.J. An introduction to radiation chemistry. United States: John Wiley and Sons Inc; 1990. [Google Scholar]
- 60.Mikkelsen R.B., Wardman P. Biological chemistry of reactive oxygen and nitrogen and radiation-induced signal transduction mechanisms. Oncogene. 2003;22(37):5734–5754. doi: 10.1038/sj.onc.1206663. [DOI] [PubMed] [Google Scholar]
- 61.Jay-Gerin J-P., Ferradini C. Are there protective enzymatic pathways to regulate high local Nitric Oxide (NO) concentrations in cells under stress conditions? Biochimie. 2000;82(2):161–166. doi: 10.1016/S0300-9084(00)00062-6. [DOI] [PubMed] [Google Scholar]
- 62.Goodhead D.T. The initial physical damage produced by ionizing radiations. Int. J. Radiat. Biol. 1989;56(5):623–634. doi: 10.1080/09553008914551841. [DOI] [PubMed] [Google Scholar]
- 63.Mehta N.J., Marwah P.K., Njus D. Are proteinopathy and oxidative stress two sides of the same coin? Cells. 2019;8(1):E59. doi: 10.3390/cells8010059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lévy E., El Banna N., Baïlle D., Heneman-Masurel A., Truchet S., Rezaei H., Huang M.E., Béringue V., Martin D., Vernis L. Causative links between protein aggregation and oxidative stress: A review. Int. J. Mol. Sci. 2019;20(16):3896. doi: 10.3390/ijms20163896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Squier T.C. Oxidative stress and protein aggregation during biological aging. Exp. Gerontol. 2001;36(9):1539–1550. doi: 10.1016/S0531-5565(01)00139-5. [DOI] [PubMed] [Google Scholar]
- 66.Ganguly G., Chakrabarti S., Chatterjee U., Saso L. Proteinopathy, oxidative stress and mitochondrial dysfunction: cross talk in Alzheimer’s disease and Parkinson’s disease. Drug Des. Devel. Ther. 2017;11:797–810. doi: 10.2147/DDDT.S130514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Aivazidis S., Anderson C.C., Roede J.R. Toxicant-mediated redox control of proteostasis in neurodegeneration. Curr. Opin. Toxicol. 2019;13:22–34. doi: 10.1016/j.cotox.2018.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Grimm S., Höhn A., Grune T. Oxidative protein damage and the proteasome. Amino Acids. 2012;42(1):23–38. doi: 10.1007/s00726-010-0646-8. [DOI] [PubMed] [Google Scholar]
- 69.Hawkins C.L., Davies M.J. Generation and propagation of radical reactions on proteins. Biochim. Biophys. Acta. 2001;1504(2-3):196–219. doi: 10.1016/S0005-2728(00)00252-8. [DOI] [PubMed] [Google Scholar]
- 70.Baraibar M.A., Liu L., Ahmed E.K., Friguet B. Protein oxidative damage at the crossroads of cellular senescence, aging, and age-related diseases. Oxid. Med. Cell. Longev. 2012;2012:919832. doi: 10.1155/2012/919832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Berlett B.S., Stadtman E.R. Protein oxidation in aging, disease, and oxidative stress. J. Biol. Chem. 1997;272(33):20313–20316. doi: 10.1074/jbc.272.33.20313. [DOI] [PubMed] [Google Scholar]
- 72.Dash R., Jahan I., Ali M.C., Mitra S., Munni Y.A., Timalsina B., Hannan M.A., Moon I.S. Potential roles of natural products in the targeting of proteinopathic neurodegenerative diseases. Neurochem. Int. 2021;145:105011. doi: 10.1016/j.neuint.2021.105011. [DOI] [PubMed] [Google Scholar]
- 73.Vendruscolo M., Knowles T.P.J., Dobson C.M. Protein solubility and protein homeostasis: A generic view of protein misfolding disorders. Cold Spring Harb. Perspect. Biol. 2011;3(12):a010454. doi: 10.1101/cshperspect.a010454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ciechanover A. Intracellular protein degradation: From a vague idea thru the lysosome and the ubiquitin-proteasome system and onto human diseases and drug targeting. Best Pract. Res. Clin. Haematol. 2017;30(4):341–355. doi: 10.1016/j.beha.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 75.Dikic I. Proteasomal and autophagic degradation systems. Annu. Rev. Biochem. 2017;86:193–224. doi: 10.1146/annurev-biochem-061516-044908. [DOI] [PubMed] [Google Scholar]
- 76.Boland B., Yu W.H., Corti O., Mollereau B., Henriques A., Bezard E., Pastores G.M., Rubinsztein D.C., Nixon R.A., Duchen M.R., Mallucci G.R., Kroemer G., Levine B., Eskelinen E.L., Mochel F., Spedding M., Louis C., Martin O.R., Millan M.J. Promoting the clearance of neurotoxic proteins in neurodegenerative disorders of ageing. Nat. Rev. Drug Discov. 2018;17(9):660–688. doi: 10.1038/nrd.2018.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Haeri M., Knox B.E. Endoplasmic reticulum stress and unfolded protein response pathways: Potential for treating age-related retinal degeneration. J. Ophthalmic Vis. Res. 2012;7(1):45–59. [PMC free article] [PubMed] [Google Scholar]
- 78.Snapp E.L. Unfolded protein responses with or without unfolded proteins? Cells. 2012;1(4):926–950. doi: 10.3390/cells1040926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Labbadia J., Morimoto R.I. The biology of proteostasis in aging and disease. Annu. Rev. Biochem. 2015;84(1):435–464. doi: 10.1146/annurev-biochem-060614-033955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Scheper W., Hoozemans J.J. The unfolded protein response in neurodegenerative diseases: A neuropathological perspective. Acta Neuropathol. 2015;130(3):315–331. doi: 10.1007/s00401-015-1462-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Feng J., Yang Y., Zhou Y., Wang B., Xiong H., Fan C., Jiang S., Liu J., Ma Z., Hu W., Li T., Feng X., Xu J., Jin Z. Bakuchiol attenuates myocardial ischemia reperfusion injury by maintaining mitochondrial function: The role of silent information regulator 1. Apoptosis. 2016;21(5):532–545. doi: 10.1007/s10495-016-1225-6. [DOI] [PubMed] [Google Scholar]
- 82.Hetz C., Saxena S. ER stress and the unfolded protein response in neurodegeneration. Nat. Rev. Neurol. 2017;13(8):477–491. doi: 10.1038/nrneurol.2017.99. [DOI] [PubMed] [Google Scholar]
- 83.Fike J.R., Rola R., Limoli C.L. Radiation response of neural precursor cells. Neurosurg. Clin. N. Am. 2007;18(1):115–127. doi: 10.1016/j.nec.2006.10.010. x. [DOI] [PubMed] [Google Scholar]
- 84.Fike J.R., Rosi S., Limoli C.L. Neural precursor cells and central nervous system radiation sensitivity. Semin. Radiat. Oncol. 2009;19(2):122–132. doi: 10.1016/j.semradonc.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tseng B.P., Giedzinski E., Izadi A., Suarez T., Lan M.L., Tran K.K., Acharya M.M., Nelson G.A., Raber J., Parihar V.K., Limoli C.L. Functional consequences of radiation-induced oxidative stress in cultured neural stem cells and the brain exposed to charged particle irradiation. Antioxid. Redox Signal. 2014;20(9):1410–1422. doi: 10.1089/ars.2012.5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Prithivirajsingh S., Story M.D., Bergh S.A., Geara F.B., Ang K.K., Ismail S.M., Stevens C.W., Buchholz T.A., Brock W.A. Accumulation of the common mitochondrial DNA deletion induced by ionizing radiation. FEBS Lett. 2004;571(1-3):227–232. doi: 10.1016/j.febslet.2004.06.078. [DOI] [PubMed] [Google Scholar]
- 87.Malakhova L., Bezlepkin V.G., Antipova V., Ushakova T., Fomenko L., Sirota N., Gaziev A.I. The increase in mitochondrial DNA copy number in the tissues of gamma-irradiated mice. Cell. Mol. Biol. Lett. 2005;10(4):721–732. [PubMed] [Google Scholar]
- 88.Kobashigawa S., Suzuki K., Yamashita S. Ionizing radiation accelerates Drp1-dependent mitochondrial fission, which involves delayed mitochondrial reactive oxygen species production in normal human fibroblast-like cells. Biochem. Biophys. Res. Commun. 2011;414(4):795–800. doi: 10.1016/j.bbrc.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 89.Kwon M.J., Kim J-H., Kim T., Lee S.B. Pharmacological intervention of early neuropathy in neurodegenerative diseases. Pharmacol. Res. 2017;119:169–177. doi: 10.1016/j.phrs.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 90.Wileman T., Kane L.P., Carson G.R., Terhorst C. Depletion of cellular calcium accelerates protein degradation in the endoplasmic reticulum. J. Biol. Chem. 1991;266(7):4500–4507. doi: 10.1016/S0021-9258(20)64351-4. [DOI] [PubMed] [Google Scholar]
- 91.Torres M., Encina G., Soto C., Hetz C. Abnormal calcium homeostasis and protein folding stress at the ER: A common factor in familial and infectious prion disorders. Commun. Integr. Biol. 2011;4(3):258–261. doi: 10.4161/cib.4.3.15019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Grzybowska E.A. Calcium-binding proteins with disordered structure and their role in secretion, storage, and cellular signaling. Biomolecules. 2018;8(2):E42. doi: 10.3390/biom8020042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bezprozvanny I. Calcium signaling and neurodegenerative diseases. Trends Mol. Med. 2009;15(3):89–100. doi: 10.1016/j.molmed.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Görlach A., Bertram K., Hudecova S., Krizanova O. Calcium and ROS: A mutual interplay. Redox Biol. 2015;6:260–271. doi: 10.1016/j.redox.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ruiz A., Matute C., Alberdi E. Endoplasmic reticulum Ca(2+) release through ryanodine and IP(3) receptors contributes to neuronal excitotoxicity. Cell Calcium. 2009;46(4):273–281. doi: 10.1016/j.ceca.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 96.Atlante A., Calissano P., Bobba A., Azzariti A., Marra E., Passarella S. Cytochrome c is released from mitochondria in a reactive oxygen species (ROS)-dependent fashion and can operate as a ROS scavenger and as a respiratory substrate in cerebellar neurons undergoing excitotoxic death. J. Biol. Chem. 2000;275(47):37159–37166. doi: 10.1074/jbc.M002361200. [DOI] [PubMed] [Google Scholar]
- 97.Luetjens C.M., Bui N.T., Sengpiel B., Münstermann G., Poppe M., Krohn A.J., Bauerbach E., Krieglstein J., Prehn J.H. Delayed mitochondrial dysfunction in excitotoxic neuron death: cytochrome c release and a secondary increase in superoxide production. J. Neurosci. 2000;20(15):5715–5723. doi: 10.1523/JNEUROSCI.20-15-05715.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tadic V., Prell T., Lautenschlaeger J., Grosskreutz J. The ER mitochondria calcium cycle and ER stress response as therapeutic targets in amyotrophic lateral sclerosis. Front. Cell. Neurosci. 2014;8:147. doi: 10.3389/fncel.2014.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Guivernau B., Bonet J., Valls-Comamala V., Bosch-Morató M., Godoy J.A., Inestrosa N.C., Perálvarez-Marín A., Fernández-Busquets X., Andreu D., Oliva B., Muñoz F.J. Amyloid-β peptide nitrotyrosination stabilizes oligomers and enhances NMDAR-mediated toxicity. J. Neurosci. 2016;36(46):11693–11703. doi: 10.1523/JNEUROSCI.1081-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Renner M., Lacor P.N., Velasco P.T., Xu J., Contractor A., Klein W.L., Triller A. Deleterious effects of amyloid beta oligomers acting as an extracellular scaffold for mGluR5. Neuron. 2010;66(5):739–754. doi: 10.1016/j.neuron.2010.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hipp M.S., Kasturi P., Hartl F.U. The proteostasis network and its decline in ageing. Nat. Rev. Mol. Cell Biol. 2019;20(7):421–435. doi: 10.1038/s41580-019-0101-y. [DOI] [PubMed] [Google Scholar]
- 102.Shrivastava A.N., Aperia A., Melki R., Triller A. Physico-pathologic mechanisms involved in neurodegeneration: Misfolded protein-plasma membrane interactions. Neuron. 2017;95(1):33–50. doi: 10.1016/j.neuron.2017.05.026. [DOI] [PubMed] [Google Scholar]
- 103.Seneci P. Chapter 6 - Targeting assembly and disassembly of protein aggregates: A raggle-taggle bunch with high hopes. In: Seneci P., editor. Chemical Modulators of Protein Misfolding and Neurodegenerative Disease. San Diego: Academic Press; 2015. pp. 173–228. [DOI] [Google Scholar]
- 104.Hipp M.S., Park S.H., Hartl F.U. Proteostasis impairment in protein-misfolding and -aggregation diseases. Trends Cell Biol. 2014;24(9):506–514. doi: 10.1016/j.tcb.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 105.Szumiel I., Sochanowicz B., Buraczewska I. Ca2+ mobilization is related to the lethal effect of X-irradiation in L5178Y cells. Int. J. Radiat. Biol. 1990;58(1):125–131. doi: 10.1080/09553009014551481. [DOI] [PubMed] [Google Scholar]
- 106.Uckun F.M., Tuel-Ahlgren L., Song C.W., Waddick K., Myers D.E., Kirihara J., Ledbetter J.A., Schieven G.L. Ionizing radiation stimulates unidentified tyrosine-specific protein kinases in human B-lymphocyte precursors, triggering apoptosis and clonogenic cell death. Proc. Natl. Acad. Sci. USA. 1992;89(19):9005–9009. doi: 10.1073/pnas.89.19.9005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hallahan D.E., Bleakman D., Virudachalam S., Lee D., Grdina D., Kufe D.W., Weichselbaum R.R. The role of intracellular calcium in the cellular response to ionizing radiation. Radiat. Res. 1994;138(3):392–400. doi: 10.2307/3578688. [DOI] [PubMed] [Google Scholar]
- 108.Leach J.K., Van Tuyle G., Lin P.S., Schmidt-Ullrich R., Mikkelsen R.B. Ionizing radiation-induced, mitochondria-dependent generation of reactive oxygen/nitrogen. Cancer Res. 2001;61(10):3894–3901. [PubMed] [Google Scholar]
- 109.Tong J.X., Vogelbaum M.A., Drzymala R.E., Rich K.M. Radiation-induced apoptosis in dorsal root ganglion neurons. J. Neurocytol. 1997;26(11):771–777. doi: 10.1023/A:1018566431912. [DOI] [PubMed] [Google Scholar]
- 110.Tong J., Li J., Zhang Q-S., Yang J-K., Zhang L., Liu H-Y., Liu Y.Z., Yuan J.W., Su X.M., Zhang X.X., Jiao B.H. Delayed cognitive deficits can be alleviated by calcium antagonist nimodipine by downregulation of apoptosis following whole brain radiotherapy. Oncol. Lett. 2018;16(2):2525–2532. doi: 10.3892/ol.2018.8968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhao L., Chen Q., Diaz Brinton R. Neuroprotective and neurotrophic efficacy of phytoestrogens in cultured hippocampal neurons. Exp. Biol. Med. (Maywood) 2002;227(7):509–519. doi: 10.1177/153537020222700716. [DOI] [PubMed] [Google Scholar]
- 112.Fuentes N., Silveyra P. Estrogen receptor signaling mechanisms. Adv. Protein Chem. Struct. Biol. 2019;116:135–170. doi: 10.1016/bs.apcsb.2019.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Haas E., Bhattacharya I., Brailoiu E. Damjanović M.; Brailoiu, G.C.; Gao, X.; Mueller-Guerre, L.; Marjon, N.A.; Gut, A.; Minotti, R.; Meyer, M.R.; Amann, K.; Ammann, E.; Perez-Dominguez, A.; Genoni, M.; Clegg, D.J.; Dun, N.J.; Resta, T.C.; Prossnitz, E.R.; Barton, M. Regulatory role of G protein-coupled estrogen receptor for vascular function and obesity. Circ. Res. 2009;104(3):288–291. doi: 10.1161/CIRCRESAHA.108.190892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mosselman S., Polman J., Dijkema R. ER beta: identification and characterization of a novel human estrogen receptor. FEBS Lett. 1996;392(1):49–53. doi: 10.1016/0014-5793(96)00782-X. [DOI] [PubMed] [Google Scholar]
- 115.Tremblay G.B., Tremblay A., Copeland N.G., Gilbert D.J., Jenkins N.A., Labrie F., Giguère V. Cloning, chromosomal localization, and functional analysis of the murine estrogen receptor beta. Mol. Endocrinol. 1997;11(3):353–365. doi: 10.1210/mend.11.3.9902. [DOI] [PubMed] [Google Scholar]
- 116.Kuiper G.G., Enmark E., Pelto-Huikko M., Nilsson S., Gustafsson J.A. Cloning of a novel receptor expressed in rat prostate and ovary. Proc. Natl. Acad. Sci. USA. 1996;93(12):5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cowley S.M., Hoare S., Mosselman S., Parker M.G. Estrogen receptors alpha and beta form heterodimers on DNA. J. Biol. Chem. 1997;272(32):19858–19862. doi: 10.1074/jbc.272.32.19858. [DOI] [PubMed] [Google Scholar]
- 118.Beato M., Arnemann J., Chalepakis G., Slater E., Willmann T. Gene regulation by steroid hormones. J. Steroid Biochem. 1987;27(1-3):9–14. doi: 10.1016/0022-4731(87)90288-3. [DOI] [PubMed] [Google Scholar]
- 119.Berry M., Metzger D., Chambon P. Role of the two activating domains of the oestrogen receptor in the cell-type and promoter-context dependent agonistic activity of the anti-oestrogen 4-hydroxytamoxifen. EMBO J. 1990;9(9):2811–2818. doi: 10.1002/j.1460-2075.1990.tb07469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mendelsohn M.E., Karas R.H. Molecular and cellular basis of cardiovascular gender differences. Science. 2005;308(5728):1583–1587. doi: 10.1126/science.1112062. [DOI] [PubMed] [Google Scholar]
- 121.Sader M.A., Celermajer D.S. Endothelial function, vascular reactivity and gender differences in the cardiovascular system. Cardiovasc. Res. 2002;53(3):597–604. doi: 10.1016/S0008-6363(01)00473-4. [DOI] [PubMed] [Google Scholar]
- 122.Ueda K., Karas R.H. Emerging evidence of the importance of rapid, non-nuclear estrogen receptor signaling in the cardiovascular system. Steroids. 2013;78(6):589–596. doi: 10.1016/j.steroids.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 123.Gillies G.E., McArthur S. Estrogen actions in the brain and the basis for differential action in men and women: a case for sex-specific medicines. Pharmacol. Rev. 2010;62(2):155–198. doi: 10.1124/pr.109.002071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Garcia-Segura L.M., Azcoitia I., DonCarlos L.L. Neuroprotection by estradiol. Prog. Neurobiol. 2001;63(1):29–60. doi: 10.1016/S0301-0082(00)00025-3. [DOI] [PubMed] [Google Scholar]
- 125.Raz L., Khan M.M., Mahesh V.B., Vadlamudi R.K., Brann D.W. Rapid estrogen signaling in the brain. Neurosignals. 2008;16(2-3):140–153. doi: 10.1159/000111559. [DOI] [PubMed] [Google Scholar]
- 126.Gjorevski N., Nelson C.M. Integrated morphodynamic signalling of the mammary gland. Nat. Rev. Mol. Cell Biol. 2011;12(9):581–593. doi: 10.1038/nrm3168. [DOI] [PubMed] [Google Scholar]
- 127.Clarke R., Cook K.L., Hu R., Facey C.O., Tavassoly I., Schwartz J.L., Baumann W.T., Tyson J.J., Xuan J., Wang Y., Wärri A., Shajahan A.N. Endoplasmic reticulum stress, the unfolded protein response, autophagy, and the integrated regulation of breast cancer cell fate. Cancer Res. 2012;72(6):1321–1331. doi: 10.1158/0008-5472.CAN-11-3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tong B.C., Wu A.J., Li M., Cheung K.H. Calcium signaling in Alzheimer’s disease & therapies. Biochim. Biophys. Acta Mol. Cell Res. 2018;1865(11) 11 Pt B:1745–1760. doi: 10.1016/j.bbamcr.2018.07.018. [DOI] [PubMed] [Google Scholar]
- 129.Chandran R., Kumar M., Kesavan L., Jacob R.S., Gunasekaran S., Lakshmi S., Sadasivan C., Omkumar R.V. Cellular calcium signaling in the aging brain. J. Chem. Neuroanat. 2019;95:95–114. doi: 10.1016/j.jchemneu.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 130.Agranoff B.W., Siegel G.J. Basic neurochemistry: Molecular, cellular, and medical aspects. Raven Press; 1994. [Google Scholar]
- 131.Sattler R., Tymianski M. Molecular mechanisms of calcium-dependent excitotoxicity. J. Mol. Med. (Berl.) 2000;78(1):3–13. doi: 10.1007/s001090000077. [DOI] [PubMed] [Google Scholar]
- 132.Friedman L.K. Calcium: a role for neuroprotection and sustained adaptation. Mol. Interv. 2006;6(6):315–329. doi: 10.1124/mi.6.6.5. [DOI] [PubMed] [Google Scholar]
- 133.Youn H.D., Sun L., Prywes R., Liu J.O. Apoptosis of T cells mediated by Ca2+-induced release of the transcription factor MEF2. Science. 1999;286(5440):790–793. doi: 10.1126/science.286.5440.790. [DOI] [PubMed] [Google Scholar]
- 134.Szado T., Vanderheyden V., Parys J.B., De Smedt H., Rietdorf K., Kotelevets L., Chastre E., Khan F., Landegren U., Söderberg O., Bootman M.D., Roderick H.L. Phosphorylation of inositol 1,4,5-trisphosphate receptors by protein kinase B/Akt inhibits Ca2+ release and apoptosis. Proc. Natl. Acad. Sci. USA. 2008;105(7):2427–2432. doi: 10.1073/pnas.0711324105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Morissette M., Le Saux M., D’Astous M., Jourdain S., Al Sweidi S., Morin N., Estrada-Camarena E., Mendez P., Garcia-Segura L.M., Di Paolo T. Contribution of estrogen receptors alpha and beta to the effects of estradiol in the brain. J. Steroid Biochem. Mol. Biol. 2008;108(3-5):327–338. doi: 10.1016/j.jsbmb.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 136.Smith C.C., Vedder L.C., McMahon L.L. Estradiol and the relationship between dendritic spines, NR2B containing NMDA receptors, and the magnitude of long-term potentiation at hippocampal CA3-CA1 synapses. Psychoneuroendocrinology. 2009;34(Suppl. 1):S130–S142. doi: 10.1016/j.psyneuen.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Liu F., Day M., Muñiz L.C., Bitran D., Arias R., Revilla-Sanchez R., Grauer S., Zhang G., Kelley C., Pulito V., Sung A., Mervis R.F., Navarra R., Hirst W.D., Reinhart P.H., Marquis K.L., Moss S.J., Pangalos M.N., Brandon N.J. Activation of estrogen receptor-beta regulates hippocampal synaptic plasticity and improves memory. Nat. Neurosci. 2008;11(3):334–343. doi: 10.1038/nn2057. [DOI] [PubMed] [Google Scholar]
- 138.Aguirre C., Jayaraman A., Pike C., Baudry M. Progesterone inhibits estrogen-mediated neuroprotection against excitotoxicity by down-regulating estrogen receptor-β. J. Neurochem. 2010;115(5):1277–1287. doi: 10.1111/j.1471-4159.2010.07038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wong M., Moss R.L. Long-term and short-term electrophysiological effects of estrogen on the synaptic properties of hippocampal CA1 neurons. J. Neurosci. 1992;12(8):3217–3225. doi: 10.1523/JNEUROSCI.12-08-03217.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Weaver C.E., Jr, Park-Chung M., Gibbs T.T., Farb D.H. 17beta-Estradiol protects against NMDA-induced excitotoxicity by direct inhibition of NMDA receptors. Brain Res. 1997;761(2):338–341. doi: 10.1016/S0006-8993(97)00449-6. [DOI] [PubMed] [Google Scholar]
- 141.Weiss H.R., Doshi D., Sinha A.K., Liu X., Chi O.Z. 17Beta-estradiol blocks NMDA-induced increases in regional cerebral O(2) consumption. Brain Res. 2002;951(2):177–182. doi: 10.1016/S0006-8993(02)03158-X. [DOI] [PubMed] [Google Scholar]
- 142.Bryant D.N., Dorsa D.M. Roles of estrogen receptors alpha and beta in sexually dimorphic neuroprotection against glutamate toxicity. Neuroscience. 2010;170(4):1261–1269. doi: 10.1016/j.neuroscience.2010.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ciriza I., Carrero P., Azcoitia I., Lundeen S.G., Garcia-Segura L.M. Selective estrogen receptor modulators protect hippocampal neurons from kainic acid excitotoxicity: differences with the effect of estradiol. J. Neurobiol. 2004;61(2):209–221. doi: 10.1002/neu.20043. [DOI] [PubMed] [Google Scholar]
- 144.Zhang H., Xie M., Schools G.P., Feustel P.F., Wang W., Lei T., Kimelberg H.K., Zhou M. Tamoxifen mediated estrogen receptor activation protects against early impairment of hippocampal neuron excitability in an oxygen/glucose deprivation brain slice ischemia model. Brain Res. 2009;1247:196–211. doi: 10.1016/j.brainres.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.O’Neill K., Chen S., Diaz Brinton R. Impact of the selective estrogen receptor modulator, tamoxifen, on neuronal outgrowth and survival following toxic insults associated with aging and Alzheimer’s disease. Exp. Neurol. 2004;188(2):268–278. doi: 10.1016/j.expneurol.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 146.Huang Y., Huang Y.L., Lai B., Zheng P., Zhu Y.C., Yao T. Raloxifene acutely reduces glutamate-induced intracellular calcium increase in cultured rat cortical neurons via inhibition of high-voltage-activated calcium current. Neuroscience. 2007;147(2):334–341. doi: 10.1016/j.neuroscience.2007.04.037. [DOI] [PubMed] [Google Scholar]
- 147.Armagan G., Kanit L., Terek C.M., Sozmen E.Y., Yalcin A. The levels of glutathione and nitrite-nitrate and the expression of Bcl-2 mRNA in ovariectomized rats treated by raloxifene against kainic acid. Int. J. Neurosci. 2009;119(2):227–239. doi: 10.1080/00207450802330959. [DOI] [PubMed] [Google Scholar]
- 148.Kurata K., Takebayashi M., Kagaya A., Morinobu S., Yamawaki S. Effect of beta-estradiol on voltage-gated Ca(2+) channels in rat hippocampal neurons: a comparison with dehydroepiandrosterone. Eur. J. Pharmacol. 2001;416(3):203–212. doi: 10.1016/S0014-2999(01)00880-9. [DOI] [PubMed] [Google Scholar]
- 149.Mermelstein P.G., Becker J.B., Surmeier D.J. Estradiol reduces calcium currents in rat neostriatal neurons via a membrane receptor. J. Neurosci. 1996;16(2):595–604. doi: 10.1523/JNEUROSCI.16-02-00595.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zhao L., Brinton R.D. Estrogen receptor alpha and beta differentially regulate intracellular Ca(2+) dynamics leading to ERK phosphorylation and estrogen neuroprotection in hippocampal neurons. Brain Res. 2007;1172:48–59. doi: 10.1016/j.brainres.2007.06.092. [DOI] [PubMed] [Google Scholar]
- 151.Huang G.Z., Woolley C.S. Estradiol acutely suppresses inhibition in the hippocampus through a sex-specific endocannabinoid and mGluR-dependent mechanism. Neuron. 2012;74(5):801–808. doi: 10.1016/j.neuron.2012.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Mermelstein P.G. Membrane-localised oestrogen receptor alpha and beta influence neuronal activity through activation of metabotropic glutamate receptors. J. Neuroendocrinol. 2009;21(4):257–262. doi: 10.1111/j.1365-2826.2009.01838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Grove-Strawser D., Boulware M.I., Mermelstein P.G. Membrane estrogen receptors activate the metabotropic glutamate receptors mGluR5 and mGluR3 to bidirectionally regulate CREB phosphorylation in female rat striatal neurons. Neuroscience. 2010;170(4):1045–1055. doi: 10.1016/j.neuroscience.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Sonoda J., Laganière J., Mehl I.R., Barish G.D., Chong L.W., Li X., Scheffler I.E., Mock D.C., Bataille A.R., Robert F., Lee C.H., Giguère V., Evans R.M. Nuclear receptor ERR alpha and coactivator PGC-1 beta are effectors of IFN-gamma-induced host defense. Genes Dev. 2007;21(15):1909–1920. doi: 10.1101/gad.1553007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hong E.J., Levasseur M.P., Dufour C.R., Perry M.C., Giguère V. Loss of estrogen-related receptor α promotes hepatocarcinogenesis development via metabolic and inflammatory disturbances. Proc. Natl. Acad. Sci. USA. 2013;110(44):17975–17980. doi: 10.1073/pnas.1315319110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Murray J., Auwerx J., Huss J.M. Impaired myogenesis in estrogen-related receptor γ (ERRγ)-deficient skeletal myocytes due to oxidative stress. FASEB J. 2013;27(1):135–150. doi: 10.1096/fj.12-212290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kim J.H., Choi Y.K., Byun J.K., Kim M.K., Kang Y.N., Kim S.H., Lee S., Jang B.K., Park K.G. Estrogen-related receptor γ is upregulated in liver cancer and its inhibition suppresses liver cancer cell proliferation via induction of p21 and p27. Exp. Mol. Med. 2016;48(3):e213. doi: 10.1038/emm.2015.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wu Y.M., Chen Z.J., Jiang G.M., Zhang K.S., Liu Q., Liang S.W., Zhou Y., Huang H.B., Du J., Wang H.S. Inverse agonist of estrogen-related receptor α suppresses the growth of triple negative breast cancer cells through ROS generation and interaction with multiple cell signaling pathways. Oncotarget. 2016;7(11):12568–12581. doi: 10.18632/oncotarget.7276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Sandhir R., Sethi N., Aggarwal A., Khera A. Coenzyme Q10 treatment ameliorates cognitive deficits by modulating mitochondrial functions in surgically induced menopause. Neurochem. Int. 2014;74:16–23. doi: 10.1016/j.neuint.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 160.Patki G., Allam F.H., Atrooz F., Dao A.T., Solanki N., Chugh G., Asghar M., Jafri F., Bohat R., Alkadhi K.A., Salim S. Grape powder intake prevents ovariectomy-induced anxiety-like behavior, memory impairment and high blood pressure in female Wistar rats. PLoS One. 2013;8(9):e74522. doi: 10.1371/journal.pone.0074522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Pourganji M., Hosseini M., Soukhtanloo M., Zabihi H., Hadjzadeh M.A. Protective role of endogenous ovarian hormones against learning and memory impairments and brain tissues oxidative damage induced by lipopolysaccharide. Iran. Red Crescent Med. J. 2014;16(3):e13954. doi: 10.5812/ircmj.13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Rao A.K., Dietrich A.K., Ziegler Y.S., Nardulli A.M. 17β-Estradiol-mediated increase in Cu/Zn superoxide dismutase expression in the brain: a mechanism to protect neurons from ischemia. J. Steroid Biochem. Mol. Biol. 2011;127(3-5):382–389. doi: 10.1016/j.jsbmb.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Yang S.H., Sarkar S.N., Liu R., Perez E.J., Wang X., Wen Y., Yan L.J., Simpkins J.W. Estrogen receptor beta as a mitochondrial vulnerability factor. J. Biol. Chem. 2009;284(14):9540–9548. doi: 10.1074/jbc.M808246200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Siriphorn A., Chompoopong S., Floyd C.L. 17β-estradiol protects Schwann cells against H2O2-induced cytotoxicity and increases transplanted Schwann cell survival in a cervical hemicontusion spinal cord injury model. J. Neurochem. 2010;115(4):864–872. doi: 10.1111/j.1471-4159.2010.06770.x. [DOI] [PubMed] [Google Scholar]
- 165.Prokai L., Prokai-Tatrai K., Perjesi P., Zharikova A.D., Perez E.J., Liu R., Simpkins J.W. Quinol-based cyclic antioxidant mechanism in estrogen neuroprotection. Proc. Natl. Acad. Sci. USA. 2003;100(20):11741–11746. doi: 10.1073/pnas.2032621100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Stirone C., Duckles S.P., Krause D.N., Procaccio V. Estrogen increases mitochondrial efficiency and reduces oxidative stress in cerebral blood vessels. Mol. Pharmacol. 2005;68(4):959–965. doi: 10.1124/mol.105.014662. [DOI] [PubMed] [Google Scholar]
- 167.Razmara A., Duckles S.P., Krause D.N., Procaccio V. Estrogen suppresses brain mitochondrial oxidative stress in female and male rats. Brain Res. 2007;1176:71–81. doi: 10.1016/j.brainres.2007.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Fitzpatrick J.L., Mize A.L., Wade C.B., Harris J.A., Shapiro R.A., Dorsa D.M. Estrogen-mediated neuroprotection against beta-amyloid toxicity requires expression of estrogen receptor alpha or beta and activation of the MAPK pathway. J. Neurochem. 2002;82(3):674–682. doi: 10.1046/j.1471-4159.2002.01000.x. [DOI] [PubMed] [Google Scholar]
- 169.Tsialtas I., Georgantopoulos A., Karipidou M.E., Kalousi F.D., Karra A.G., Leonidas D.D., Psarra A.G. Anti-apoptotic and antioxidant activities of the mitochondrial estrogen receptor beta in N2A neuroblastoma cells. Int. J. Mol. Sci. 2021;22(14):7620. doi: 10.3390/ijms22147620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Razmara A., Sunday L., Stirone C., Wang X.B., Krause D.N., Duckles S.P., Procaccio V. Mitochondrial effects of estrogen are mediated by estrogen receptor alpha in brain endothelial cells. J. Pharmacol. Exp. Ther. 2008;325(3):782–790. doi: 10.1124/jpet.107.134072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Culmsee C., Vedder H., Ravati A., Junker V., Otto D., Ahlemeyer B., Krieg J.C., Krieglstein J. Neuroprotection by estrogens in a mouse model of focal cerebral ischemia and in cultured neurons: evidence for a receptor-independent antioxidative mechanism. J. Cereb. Blood Flow Metab. 1999;19(11):1263–1269. doi: 10.1097/00004647-199911000-00011. [DOI] [PubMed] [Google Scholar]
- 172.Brambilla R. Neuroinflammation, the thread connecting neurological disease: Cluster: “Neuroinflammatory mechanisms in neurodegenerative disorders”. Acta Neuropathol. 2019;137(5):689–691. doi: 10.1007/s00401-019-02009-9. [DOI] [PubMed] [Google Scholar]
- 173.Qiu A.W., Liu Z., Guo J., Peng Y.P. Relationship between neuroinflammation and neurodegenerative diseases. Sheng Li Ke Xue Jin Zhan. 2011;42(5):353–358. [PubMed] [Google Scholar]
- 174.Simpkins J.W., Singh M., Brock C., Etgen A.M. Neuroprotection and estrogen receptors. Neuroendocrinology. 2012;96(2):119–130. doi: 10.1159/000338409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Murphy A.J., Guyre P.M., Pioli P.A. Estradiol suppresses NF-kappa B activation through coordinated regulation of let-7a and miR-125b in primary human macrophages. J. Immunol. 2010;184(9):5029–5037. doi: 10.4049/jimmunol.0903463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Liu T. Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017;2(1):17023. doi: 10.1038/sigtrans.2017.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Shih R.H., Wang C.Y., Yang C.M. NF-kappaB signaling pathways in neurological inflammation: A mini review. Front. Mol. Neurosci. 2015;8:77. doi: 10.3389/fnmol.2015.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Dresselhaus E.C., Meffert M.K. Cellular specificity of NF-κB function in the nervous system. Front. Immunol. 2019;10:1043. doi: 10.3389/fimmu.2019.01043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Jover-Mengual T., Zukin R.S., Etgen A.M. MAPK signaling is critical to estradiol protection of CA1 neurons in global ischemia. Endocrinology. 2007;148(3):1131–1143. doi: 10.1210/en.2006-1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Choi Y.C., Lee J.H., Hong K.W., Lee K.S. 17 Beta-estradiol prevents focal cerebral ischemic damages via activation of Akt and CREB in association with reduced PTEN phosphorylation in rats. Fundam. Clin. Pharmacol. 2004;18(5):547–557. doi: 10.1111/j.1472-8206.2004.00284.x. [DOI] [PubMed] [Google Scholar]
- 181.Xing D., Oparil S., Yu H., Gong K., Feng W., Black J., Chen Y.F., Nozell S. Estrogen modulates NFκB signaling by enhancing IκBα levels and blocking p65 binding at the promoters of inflammatory genes via estrogen receptor-β. PLoS One. 2012;7(6):e36890. doi: 10.1371/journal.pone.0036890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Tiwari-Woodruff S., Morales L.B., Lee R., Voskuhl R.R. Differential neuroprotective and antiinflammatory effects of estrogen receptor (ER)alpha and ERbeta ligand treatment. Proc. Natl. Acad. Sci. USA. 2007;104(37):14813–14818. doi: 10.1073/pnas.0703783104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Spence R.D., Voskuhl R.R. Neuroprotective effects of estrogens and androgens in CNS inflammation and neurodegeneration. Front. Neuroendocrinol. 2012;33(1):105–115. doi: 10.1016/j.yfrne.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Yates M.A., Li Y., Chlebeck P.J., Offner H. GPR30, but not estrogen receptor-alpha, is crucial in the treatment of experimental autoimmune encephalomyelitis by oral ethinyl estradiol. BMC Immunol. 2010;11(1):20. doi: 10.1186/1471-2172-11-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Wang C., Dehghani B., Li Y., Kaler L.J., Proctor T., Vandenbark A.A., Offner H. Membrane estrogen receptor regulates experimental autoimmune encephalomyelitis through up-regulation of programmed death 1. J. Immunol. 2009;182(5):3294–3303. doi: 10.4049/jimmunol.0803205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Blasko E., Haskell C.A., Leung S., Gualtieri G., Halks-Miller M., Mahmoudi M., Dennis M.K., Prossnitz E.R., Karpus W.J., Horuk R. Beneficial role of the GPR30 agonist G-1 in an animal model of multiple sclerosis. J. Neuroimmunol. 2009;214(1-2):67–77. doi: 10.1016/j.jneuroim.2009.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Spence R.D., Wisdom A.J., Cao Y., Hill H.M., Mongerson C.R., Stapornkul B., Itoh N., Sofroniew M.V., Voskuhl R.R. Estrogen mediates neuroprotection and anti-inflammatory effects during EAE through ERα signaling on astrocytes but not through ERβ signaling on astrocytes or neurons. J. Neurosci. 2013;33(26):10924–10933. doi: 10.1523/JNEUROSCI.0886-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Joutsen J., Sistonen L. Tailoring of proteostasis networks with heat shock factors. Cold Spring Harb. Perspect. Biol. 2019;11(4):a034066. doi: 10.1101/cshperspect.a034066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Whitesell L., Lindquist S. Inhibiting the transcription factor HSF1 as an anticancer strategy. Expert Opin. Ther. Targets. 2009;13(4):469–478. doi: 10.1517/14728220902832697. [DOI] [PubMed] [Google Scholar]
- 190.Riar A.K., Burstein S.R., Palomo G.M., Arreguin A., Manfredi G., Germain D. Sex specific activation of the ERα axis of the mitochondrial UPR (UPRmt) in the G93A-SOD1 mouse model of familial ALS. Hum. Mol. Genet. 2017;26(7):1318–1327. doi: 10.1093/hmg/ddx049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Mori K. Signalling pathways in the unfolded protein response: development from yeast to mammals. J. Biochem. 2009;146(6):743–750. doi: 10.1093/jb/mvp166. [DOI] [PubMed] [Google Scholar]
- 192.Aldridge J.E., Horibe T., Hoogenraad N.J. Discovery of genes activated by the mitochondrial unfolded protein response (mtUPR) and cognate promoter elements. PLoS One. 2007;2(9):e874. doi: 10.1371/journal.pone.0000874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Chung M.T., Lee Y.M., Shen H.H., Cheng P.Y., Huang Y.C., Lin Y.J., Huang Y.Y., Lam K.K. Activation of autophagy is involved in the protective effect of 17β-oestradiol on endotoxaemia-induced multiple organ dysfunction in ovariectomized rats. J. Cell. Mol. Med. 2017;21(12):3705–3717. doi: 10.1111/jcmm.13280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Felzen V., Hiebel C., Koziollek-Drechsler I., Reißig S., Wolfrum U., Kögel D., Brandts C., Behl C., Morawe T. Estrogen receptor α regulates non-canonical autophagy that provides stress resistance to neuroblastoma and breast cancer cells and involves BAG3 function. Cell Death Dis. 2015;6(7):e1812. doi: 10.1038/cddis.2015.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Ruddy S.C., Lau R., Cabrita M.A., McGregor C., McKay B.C., Murphy L.C., Wright J.S., Durst T., Pratt M.A. Preferential estrogen receptor β ligands reduce Bcl-2 expression in hormone-resistant breast cancer cells to increase autophagy. Mol. Cancer Ther. 2014;13(7):1882–1893. doi: 10.1158/1535-7163.MCT-13-1066. [DOI] [PubMed] [Google Scholar]
- 196.Yang Z.M., Yang M.F., Yu W., Tao H.M. Molecular mechanisms of estrogen receptor β-induced apoptosis and autophagy in tumors: implication for treating osteosarcoma. J. Int. Med. Res. 2019;47(10):4644–4655. doi: 10.1177/0300060519871373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Zeng M., Chen B., Qing Y., Xie W., Dang W., Zhao M., Zhou J. Estrogen receptor β signaling induces autophagy and downregulates Glut9 expression. Nucleosides Nucleotides Nucleic Acids. 2014;33(7):455–465. doi: 10.1080/15257770.2014.885045. [DOI] [PubMed] [Google Scholar]
- 198.Wei Y., Huang J. Role of estrogen and its receptors mediated-autophagy in cell fate and human diseases. J. Steroid Biochem. Mol. Biol. 2019;191:105380. doi: 10.1016/j.jsbmb.2019.105380. [DOI] [PubMed] [Google Scholar]
- 199.Barbati C., Pierdominici M., Gambardella L., Malchiodi Albedi F., Karas R.H., Rosano G., Malorni W., Ortona E. Cell surface estrogen receptor alpha is upregulated during subchronic metabolic stress and inhibits neuronal cell degeneration. PLoS One. 2012;7(7):e42339. doi: 10.1371/journal.pone.0042339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Li L., Chen J., Sun S., Zhao J., Dong X., Wang J. Effects of Estradiol on Autophagy and Nrf-2/ARE Signals after Cerebral Ischemia. Cell. Physiol. Biochem. 2017;41(5):2027–2036. doi: 10.1159/000475433. [DOI] [PubMed] [Google Scholar]
- 201.LeBlanc E.S., Neiss M.B., Carello P.E., Samuels M.H., Janowsky J.S. Hot flashes and estrogen therapy do not influence cognition in early menopausal women. Menopause. 2007;14(2):191–202. doi: 10.1097/01.gme.0000230347.28616.1c. [DOI] [PubMed] [Google Scholar]
- 202.Schmidt R., Fazekas F., Reinhart B., Kapeller P., Fazekas G., Offenbacher H., Eber B., Schumacher M., Freidl W. Estrogen replacement therapy in older women: a neuropsychological and brain MRI study. J. Am. Geriatr. Soc. 1996;44(11):1307–1313. doi: 10.1111/j.1532-5415.1996.tb01400.x. [DOI] [PubMed] [Google Scholar]
- 203.Compton J., van Amelsvoort T., Murphy D. HRT and its effect on normal ageing of the brain and dementia. Br. J. Clin. Pharmacol. 2001;52(6):647–653. doi: 10.1046/j.0306-5251.2001.01492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Kampen D.L., Sherwin B.B. Estrogen use and verbal memory in healthy postmenopausal women. Obstet. Gynecol. 1994;83(6):979–983. doi: 10.1097/00006250-199406000-00017. [DOI] [PubMed] [Google Scholar]
- 205.Dubal D.B., Wise P.M. Estrogen and neuroprotection: from clinical observations to molecular mechanisms. Dialogues Clin. Neurosci. 2002;4(2):149–161. doi: 10.31887/DCNS.2002.4.2/ddubal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Resnick S.M., Maki P.M. Effects of hormone replacement therapy on cognitive and brain aging. Ann. N. Y. Acad. Sci. 2001;949(1):203–214. doi: 10.1111/j.1749-6632.2001.tb04023.x. [DOI] [PubMed] [Google Scholar]
- 207.Henderson V.W., Paganini-Hill A., Miller B.L., Elble R.J., Reyes P.F., Shoupe D., McCleary C.A., Klein R.A., Hake A.M., Farlow M.R. Estrogen for Alzheimer’s disease in women: randomized, double-blind, placebo-controlled trial. Neurology. 2000;54(2):295–301. doi: 10.1212/WNL.54.2.295. [DOI] [PubMed] [Google Scholar]
- 208.Wang P.N., Liao S.Q., Liu R.S., Liu C.Y., Chao H.T., Lu S.R., Yu H.Y., Wang S.J., Liu H.C. Effects of estrogen on cognition, mood, and cerebral blood flow in AD: a controlled study. Neurology. 2000;54(11):2061–2066. doi: 10.1212/WNL.54.11.2061. [DOI] [PubMed] [Google Scholar]
- 209.Mulnard R.A., Cotman C.W., Kawas C., van Dyck C.H., Sano M., Doody R., Koss E., Pfeiffer E., Jin S., Gamst A., Grundman M., Thomas R., Thal L.J. Estrogen replacement therapy for treatment of mild to moderate Alzheimer disease a randomized controlled trial. JAMA. 2000;283(8):1007–1015. doi: 10.1001/jama.283.8.1007. [DOI] [PubMed] [Google Scholar]
- 210.Simpkins J.W., Perez E., Wang X., Yang S., Wen Y., Singh M. The potential for estrogens in preventing Alzheimer’s disease and vascular dementia. Ther. Adv. Neurol. Disord. 2009;2(1):31–49. doi: 10.1177/1756285608100427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Caldwell B.M., Watson R.I. An evaluation of psychologic effects of sex hormone administration in aged women. I. Results of therapy after six months. J. Gerontol. 1952;7(2):228–244. doi: 10.1093/geronj/7.2.228. [DOI] [PubMed] [Google Scholar]
- 212.Kawas C., Resnick S., Morrison A., Brookmeyer R., Corrada M., Zonderman A., Bacal C., Lingle D.D., Metter E. A prospective study of estrogen replacement therapy and the risk of developing Alzheimer’s disease: the Baltimore Longitudinal Study of Aging. Neurology. 1997;48(6):1517–1521. doi: 10.1212/WNL.48.6.1517. [DOI] [PubMed] [Google Scholar]
- 213.Robinson D., Friedman L., Marcus R., Tinklenberg J., Yesavage J. Estrogen replacement therapy and memory in older women. J. Am. Geriatr. Soc. 1994;42(9):919–922. doi: 10.1111/j.1532-5415.1994.tb06580.x. [DOI] [PubMed] [Google Scholar]
- 214.Shaywitz S.E., Shaywitz B.A., Pugh K.R., Fulbright R.K., Skudlarski P., Mencl W.E., Constable R.T., Naftolin F., Palter S.F., Marchione K.E., Katz L., Shankweiler D.P., Fletcher J.M., Lacadie C., Keltz M., Gore J.C. Effect of estrogen on brain activation patterns in postmenopausal women during working memory tasks. JAMA. 1999;281(13):1197–1202. doi: 10.1001/jama.281.13.1197. [DOI] [PubMed] [Google Scholar]
- 215.Song Y-J., Li S-R., Li X-W., Chen X., Wei Z-X., Liu Q-S., Cheng Y. The effect of estrogen replacement therapy on Alzheimer’s disease and Parkinson’s disease in postmenopausal women: A meta-analysis. Front. Neurosci. 2020;14:157. doi: 10.3389/fnins.2020.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Ohkura T., Isse K., Akazawa K., Hamamoto M., Yaoi Y., Hagino N. Evaluation of estrogen treatment in female patients with dementia of the Alzheimer type. Endocr. J. 1994;41(4):361–371. doi: 10.1507/endocrj.41.361. [DOI] [PubMed] [Google Scholar]
- 217.Schneider L.S., Farlow M.R., Henderson V.W., Pogoda J.M. Effects of estrogen replacement therapy on response to tacrine in patients with Alzheimer’s disease. Neurol. 1996;46(6):1580–1584. doi: 10.1212/WNL.46.6.1580. [DOI] [PubMed] [Google Scholar]
- 218.Di Paolo T. Modulation of brain dopamine transmission by sex steroids. Rev. Neurosci. 1994;5(1):27–41. doi: 10.1515/REVNEURO.1994.5.1.27. [DOI] [PubMed] [Google Scholar]
- 219.Blanchet P.J., Fang J., Hyland K., Arnold L.A., Mouradian M.M., Chase T.N. Short-term effects of high-dose 17beta-estradiol in postmenopausal PD patients: a crossover study. Neurol. 1999;53(1):91–95. doi: 10.1212/WNL.53.1.91. [DOI] [PubMed] [Google Scholar]
- 220.Strijks E., Kremer J.A., Horstink M.W. Effects of female sex steroids on Parkinson’s disease in postmenopausal women. Clin. Neuropharmacol. 1999;22(2):93–97. doi: 10.1097/00002826-199903000-00005. [DOI] [PubMed] [Google Scholar]
- 221.Trapp B.D., Nave K.A. Multiple sclerosis: an immune or neurodegenerative disorder? Annu. Rev. Neurosci. 2008;31(1):247–269. doi: 10.1146/annurev.neuro.30.051606.094313. [DOI] [PubMed] [Google Scholar]
- 222.Sicotte N.L., Liva S.M., Klutch R., Pfeiffer P., Bouvier S., Odesa S., Wu T.C., Voskuhl R.R. Treatment of multiple sclerosis with the pregnancy hormone estriol. Ann. Neurol. 2002;52(4):421–428. doi: 10.1002/ana.10301. [DOI] [PubMed] [Google Scholar]
- 223.Soldan S.S., Retuerto A.I.A., Sicotte N.L., Voskuhl R.R. Immune Modulation in Multiple Sclerosis Patients Treated with the Pregnancy Hormone Estriol. 2003;171(11):6267–6274. doi: 10.4049/jimmunol.171.11.6267. [DOI] [PubMed] [Google Scholar]
- 224.Koller W.C., Barr A., Biary N. Estrogen treatment of dyskinetic disorders. Neurology. 1982;32(5):547–549. doi: 10.1212/WNL.32.5.547. [DOI] [PubMed] [Google Scholar]
- 225.Rooney J.P.K., Visser A.E., D’Ovidio F., Vermeulen R., Beghi E., Chio A., Veldink J.H., Logroscino G., van den Berg L.H., Hardiman O. A case-control study of hormonal exposures as etiologic factors for ALS in women: Euro-MOTOR. Neurology. 2017;89(12):1283–1290. doi: 10.1212/WNL.0000000000004390. [DOI] [PubMed] [Google Scholar]
- 226.Rudnicki S.A. Estrogen replacement therapy in women with amyotrophic lateral sclerosis. J. Neurol. Sci. 1999;169(1-2):126–127. doi: 10.1016/S0022-510X(99)00234-8. [DOI] [PubMed] [Google Scholar]
- 227.Brufsky A.M., Dickler M.N. Estrogen receptor-positive breast cancer: Exploiting signaling pathways implicated in endocrine resistance. Oncologist. 2018;23(5):528–539. doi: 10.1634/theoncologist.2017-0423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Dhingra K. Selective estrogen receptor modulation: the search for an ideal hormonal therapy for breast cancer. Cancer Invest. 2001;19(6):649–659. doi: 10.1081/CNV-100104293. [DOI] [PubMed] [Google Scholar]
- 229.Martini P.G., Katzenellenbogen B.S. Modulation of estrogen receptor activity by selective coregulators. J. Steroid Biochem. Mol. Biol. 2003;85(2-5):117–122. doi: 10.1016/S0960-0760(03)00207-3. [DOI] [PubMed] [Google Scholar]
- 230.Patel H.K., Bihani T. Selective estrogen receptor modulators (SERMs) and selective estrogen receptor degraders (SERDs) in cancer treatment. Pharmacol. Ther. 2018;186:1–24. doi: 10.1016/j.pharmthera.2017.12.012. [DOI] [PubMed] [Google Scholar]
- 231.Liu J.L., Tian D.S., Li Z.W., Qu W.S., Zhan Y., Xie M.J., Yu Z.Y., Wang W., Wu G. Tamoxifen alleviates irradiation-induced brain injury by attenuating microglial inflammatory response in vitro and in vivo. Brain Res. 2010;1316:101–111. doi: 10.1016/j.brainres.2009.12.055. [DOI] [PubMed] [Google Scholar]
- 232.Valdes J.J., Weeks O.I. Lithium: a potential estrogen signaling modulator. J. Appl. Biomed. 2009;7(4):175–188. doi: 10.32725/jab.2009.020. [DOI] [Google Scholar]
- 233.Zanni G., Di Martino E., Omelyanenko A., Andäng M., Delle U., Elmroth K., Blomgren K. Lithium increases proliferation of hippocampal neural stem/progenitor cells and rescues irradiation-induced cell cycle arrest in vitro. Oncotarget. 2015;6(35):37083–37097. doi: 10.18632/oncotarget.5191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Kale A., Piskin Ö., Bas Y., Aydin B.G., Can M., Elmas Ö., Büyükuysal Ç. Neuroprotective effects of Quercetin on radiation-induced brain injury in rats. J. Radiat. Res. (Tokyo) 2018;59(4):404–410. doi: 10.1093/jrr/rry032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Chatterjee J., Langhnoja J., Pillai P.P., Mustak M.S. Neuroprotective effect of quercetin against radiation-induced endoplasmic reticulum stress in neurons. J. Biochem. Mol. Toxicol. 2019;33(2):e22242. doi: 10.1002/jbt.22242. [DOI] [PubMed] [Google Scholar]
- 236.Mansour S.Z., Moawed F.S.M., Elmarkaby S.M. Protective effect of 5, 7-dihydroxyflavone on brain of rats exposed to acrylamide or γ-radiation. J. Photochem. Photobiol. B. 2017;175:149–155. doi: 10.1016/j.jphotobiol.2017.08.034. [DOI] [PubMed] [Google Scholar]
- 237.A Rashed L, F Al-Saeed H, M El-Shawwa M. Neuroprotective effects of chrysin on adult male albino rats exposed to acrylamide and radiation. Azhar Med. J. 2016;45(3):493–504. doi: 10.12816/0033118. [DOI] [Google Scholar]
- 238.Patni S., Sisodia R., Shrivastava P. Modulation of radiation induced oxidative damage in brain of Swiss albino mice by flaxseed oil. Asian J. Exp. Sci. 2012;26(2):61–70. [Google Scholar]
- 239.Ismail A.F., Salem A.A., Eassawy M.M. Modulation of gamma-irradiation and carbon tetrachloride induced oxidative stress in the brain of female rats by flaxseed oil. J. Photochem. Photobiol. B. 2016;161:91–99. doi: 10.1016/j.jphotobiol.2016.04.031. [DOI] [PubMed] [Google Scholar]
- 240.Xie Y., Zhao Q.Y., Li H.Y., Zhou X., Liu Y., Zhang H. Curcumin ameliorates cognitive deficits heavy ion irradiation-induced learning and memory deficits through enhancing of Nrf2 antioxidant signaling pathways. Pharmacol. Biochem. Behav. 2014;126:181–186. doi: 10.1016/j.pbb.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 241.Kolivand S., Amini P., Saffar H., Rezapoor S., Motevaseli E., Najafi M., Nouruzi F., Shabeeb D., Musa A.E. Evaluating the radioprotective effect of curcumin on rat’s heart tissues. Curr. Radiopharm. 2019;12(1):23–28. doi: 10.2174/1874471011666180831101459. [DOI] [PubMed] [Google Scholar]
- 242.Li J., Feng L., Xing Y., Wang Y., Du L., Xu C., Cao J., Wang Q., Fan S., Liu Q., Fan F. Radioprotective and antioxidant effect of resveratrol in hippocampus by activating Sirt1. Int. J. Mol. Sci. 2014;15(4):5928–5939. doi: 10.3390/ijms15045928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.El-Missiry M.A., Othman A.I., El-Sawy M.R., Lebede M.F. Neuroprotective effect of epigallocatechin-3-gallate (EGCG) on radiation-induced damage and apoptosis in the rat hippocampus. Int. J. Radiat. Biol. 2018;94(9):798–808. doi: 10.1080/09553002.2018.1492755. [DOI] [PubMed] [Google Scholar]
- 244.Gakova N., Mishurova E., Kropachova K. [Effects of flavobion on nucleic acids in tissues of rats irradiated with gamma rays]. Biull. Eksp. Biol. Med. 1992;113(3):275–277. [Effects of flavobion on nucleic acids in tissues of rats irradiated with gamma rays] [PubMed] [Google Scholar]
- 245.Ogle W.O., Speisman R.B., Ormerod B.K. Potential of treating age-related depression and cognitive decline with nutraceutical approaches: a mini-review. Gerontology. 2013;59(1):23–31. doi: 10.1159/000342208. [DOI] [PubMed] [Google Scholar]
- 246.Kolivand S., Amini P., Saffar H., Rezapoor S., Motevaseli E., Najafi M., Nouruzi F., Shabeeb D., Musa A.E. Evaluating the radioprotective effect of curcumin on rat’s heart tissues. Curr. Radiopharm. 2019;12(1):23–28. doi: 10.2174/1874471011666180831101459. [DOI] [PubMed] [Google Scholar]
- 247.Shi C., Xu J., Yew D.T.W. Effects of phytoestrogen in brain and neurodegenerative diseases. Neuroembryology Aging. 2006;4(3):162–164. doi: 10.1159/000109348. [DOI] [Google Scholar]
- 248.Bustamante-Barrientos F.A., Méndez-Ruette M., Ortloff A., Luz-Crawford P., Rivera F.J., Figueroa C.D., Molina L., Bátiz L.F. The impact of estrogen and estrogen-like molecules in neurogenesis and neurodegeneration: Beneficial or harmful? Front. Cell. Neurosci. 2021;15:636176. doi: 10.3389/fncel.2021.636176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Engler-Chiurazzi E.B., Brown C.M., Povroznik J.M., Simpkins J.W. Estrogens as neuroprotectants: Estrogenic actions in the context of cognitive aging and brain injury. Prog. Neurobiol. 2017;157:188–211. doi: 10.1016/j.pneurobio.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Guptarak J., Wiktorowicz J.E., Sadygov R.G., Zivadinovic D., Paulucci-Holthauzen A.A., Vergara L., Nesic O. The cancer drug tamoxifen: a potential therapeutic treatment for spinal cord injury. J. Neurotrauma. 2014;31(3):268–283. doi: 10.1089/neu.2013.3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Tian D.S., Liu J.L., Xie M.J., Zhan Y., Qu W.S., Yu Z.Y., Tang Z.P., Pan D.J., Wang W. Tamoxifen attenuates inflammatory-mediated damage and improves functional outcome after spinal cord injury in rats. J. Neurochem. 2009;109(6):1658–1667. doi: 10.1111/j.1471-4159.2009.06077.x. [DOI] [PubMed] [Google Scholar]
- 252.Ismailoğlu, O.; Oral, B.; Görgülü, A.; Sütçü, R.; Demir, N. Neuroprotective effects of tamoxifen on experimental spinal cord injury in rats. J. Clin. Neurosci. 2010;17(10):1306–1310. doi: 10.1016/j.jocn.2010.01.049. [DOI] [PubMed] [Google Scholar]
- 253.Franco Rodríguez N.E., Dueñas Jiménez J.M., De la Torre Valdovinos B., López Ruiz J.R., Hernández Hernández L., Dueñas Jiménez S.H. Tamoxifen favoured the rat sensorial cortex regeneration after a penetrating brain injury. Brain Res. Bull. 2013;98:64–75. doi: 10.1016/j.brainresbull.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 254.Gonzalez G.A., Hofer M.P., Syed Y.A., Amaral A.I., Rundle J., Rahman S., Zhao C., Kotter M.R.N. Tamoxifen accelerates the repair of demyelinated lesions in the central nervous system. Sci. Rep. 2016;6(1):31599. doi: 10.1038/srep31599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Kimelberg H.K., Feustel P.J., Jin Y., Paquette J., Boulos A., Keller R.W., Jr, Tranmer B.I. Acute treatment with tamoxifen reduces ischemic damage following middle cerebral artery occlusion. Neuroreport. 2000;11(12):2675–2679. doi: 10.1097/00001756-200008210-00014. [DOI] [PubMed] [Google Scholar]
- 256.Latourelle J.C., Dybdahl M., Destefano A.L., Myers R.H., Lash T.L. Risk of Parkinson’s disease after tamoxifen treatment. BMC Neurol. 2010;10(1):23. doi: 10.1186/1471-2377-10-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Sun L.M., Chen H.J., Liang J.A., Kao C.H. Long-term use of tamoxifen reduces the risk of dementia: a nationwide population-based cohort study. QJM. 2016;109(2):103–109. doi: 10.1093/qjmed/hcv072. [DOI] [PubMed] [Google Scholar]
- 258.Breckenridge L.M., Bruns G.L., Todd B.L., Feuerstein M. Cognitive limitations associated with tamoxifen and aromatase inhibitors in employed breast cancer survivors. Psychooncology. 2012;21(1):43–53. doi: 10.1002/pon.1860. [DOI] [PubMed] [Google Scholar]
- 259.Van Dyk K., Crespi C.M., Bower J.E., Castellon S.A., Petersen L., Ganz P.A. The cognitive effects of endocrine therapy in survivors of breast cancer: A prospective longitudinal study up to 6 years after treatment. Cancer. 2019;125(5):681–689. doi: 10.1002/cncr.31858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Hermelink K., Henschel V., Untch M., Bauerfeind I., Lux M.P., Munzel K. Short-term effects of treatment-induced hormonal changes on cognitive function in breast cancer patients: results of a multicenter, prospective, longitudinal study. Cancer. 2008;113(9):2431–2439. doi: 10.1002/cncr.23853. [DOI] [PubMed] [Google Scholar]
- 261.Novick A.M., Scott A.T., Neill Epperson C., Schneck C.D. Neuropsychiatric effects of tamoxifen: Challenges and opportunities. Front. Neuroendocrinol. 2020;59:100869. doi: 10.1016/j.yfrne.2020.100869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Hazra B., Ghosh S., Kumar A., Pandey B.N. The prospective role of plant products in radiotherapy of cancer: a current overview. Front. Pharmacol. 2012;2:94. doi: 10.3389/fphar.2011.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Rezghi M., Farahani A.M., Asadi F., Mitra S., Dash R., Mozaffarpour S.A., Memariani Z. Application of natural products in radiotherapy-induced dermatitis: A comprehensive review. . Trad. Integrat. Med. 2021;6(3) doi: 10.18502/tim.v6i3.7314. [DOI] [Google Scholar]
- 264.Sato T., Kinoshita M., Yamamoto T., Ito M., Nishida T., Takeuchi M., Saitoh D., Seki S., Mukai Y. Treatment of irradiated mice with high-dose ascorbic acid reduced lethality. PLoS One. 2015;10(2):e0117020. doi: 10.1371/journal.pone.0117020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Jazvinšćak Jembrek, M.; Vuković L.; Puhović J.; Erhardt, J.; Oršolić N. Neuroprotective effect of quercetin against hydrogen peroxide-induced oxidative injury in P19 neurons. J. Mol. Neurosci. 2012;47(2):286–299. doi: 10.1007/s12031-012-9737-1. [DOI] [PubMed] [Google Scholar]
- 266.Qu L., Liang X., Gu B., Liu W. Quercetin alleviates high glucose-induced Schwann cell damage by autophagy. Neural Regen. Res. 2014;9(12):1195–1203. doi: 10.4103/1673-5374.135328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Yang E.J., Kim G.S., Kim J.A., Song K.S. Protective effects of onion-derived quercetin on glutamate-mediated hippocampal neuronal cell death. Pharmacogn. Mag. 2013;9(36):302–308. doi: 10.4103/0973-1296.117824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Kuiper G.G., Lemmen J.G., Carlsson B., Corton J.C., Safe S.H., van der Saag P.T., van der Burg B., Gustafsson J.A. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 1998;139(10):4252–4263. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- 269.Mani R., Natesan V., Arumugam R. Neuroprotective effect of chrysin on hyperammonemia mediated neuroinflammatory responses and altered expression of astrocytic protein in the hippocampus. Biomed. Pharmacother. 2017;88:762–769. doi: 10.1016/j.biopha.2017.01.081. [DOI] [PubMed] [Google Scholar]
- 270.Zhang Y., Zhao J., Afzal O., Kazmi I., Al-Abbasi F.A., Altamimi A.S.A., Yang Z. Neuroprotective role of chrysin-loaded poly(lactic-co-glycolic acid) nanoparticle against kindling-induced epilepsy through Nrf2/ARE/HO-1 pathway. J. Biochem. Mol. Toxicol. 2021;35(2):e22634. doi: 10.1002/jbt.22634. [DOI] [PubMed] [Google Scholar]
- 271.Bowers J.L., Tyulmenkov V.V., Jernigan S.C., Klinge C.M. Resveratrol acts as a mixed agonist/antagonist for estrogen receptors alpha and beta. Endocrinology. 2000;141(10):3657–3667. doi: 10.1210/endo.141.10.7721. [DOI] [PubMed] [Google Scholar]
- 272.Ban J.Y., Cho S.O., Choi S.H., Ju H.S., Kim J.Y., Bae K., Song K.S., Seong Y.H. Neuroprotective effect of Smilacis chinae rhizome on NMDA-induced neurotoxicity in vitro and focal cerebral ischemia in vivo. J. Pharmacol. Sci. 2008;106(1):68–77. doi: 10.1254/jphs.FP0071206. [DOI] [PubMed] [Google Scholar]
- 273.Lin T.K., Chen S.D., Chuang Y.C., Lin H.Y., Huang C.R., Chuang J.H., Wang P.W., Huang S.T., Tiao M.M., Chen J.B., Liou C.W. Resveratrol partially prevents rotenone-induced neurotoxicity in dopaminergic SH-SY5Y cells through induction of heme oxygenase-1 dependent autophagy. Int. J. Mol. Sci. 2014;15(1):1625–1646. doi: 10.3390/ijms15011625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Farabegoli F., Barbi C., Lambertini E., Piva R. (-)-Epigallocatechin-3-gallate downregulates estrogen receptor alpha function in MCF-7 breast carcinoma cells. Cancer Detect. Prev. 2007;31(6):499–504. doi: 10.1016/j.cdp.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 275.Lee J.W., Lee Y.K., Ban J.O., Ha T.Y., Yun Y.P., Han S.B., Oh K.W., Hong J.T. Green tea (-)-epigallocatechin-3-gallate inhibits beta-amyloid-induced cognitive dysfunction through modification of secretase activity via inhibition of ERK and NF-kappaB pathways in mice. J. Nutr. 2009;139(10):1987–1993. doi: 10.3945/jn.109.109785. [DOI] [PubMed] [Google Scholar]
- 276.Romeo L., Intrieri M., D’Agata V., Mangano N.G., Oriani G., Ontario M.L., Scapagnini G. The major green tea polyphenol, (-)- epigallocatechin-3-gallate, induces heme oxygenase in rat neurons and acts as an effective neuroprotective agent against oxidative stress. J. Am. Coll. Nutr. 2009;28(sup4)(Suppl.):492S–499S. doi: 10.1080/07315724.2009.10718116. [DOI] [PubMed] [Google Scholar]
- 277.Lappano R., Todd L.A., Stanic M., Cai Q., Maggiolini M., Marincola F., Pietrobon V. Multifaceted interplay between hormones, growth factors and hypoxia in the tumor microenvironment. Cancers (Basel) 2022;14(3):539. doi: 10.3390/cancers14030539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Wang S.F., Chang Y.L., Tzeng Y.D., Wu C.L., Wang Y.Z., Tseng L.M., Chen S., Lee H.C. Mitochondrial stress adaptation promotes resistance to aromatase inhibitor in human breast cancer cells via ROS/calcium up-regulated amphiregulin-estrogen receptor loop signaling. Cancer Lett. 2021;523:82–99. doi: 10.1016/j.canlet.2021.09.043. [DOI] [PubMed] [Google Scholar]
- 279.Velloso F.J., Bianco A.F., Farias J.O., Torres N.E., Ferruzo P.Y., Anschau V., Jesus-Ferreira H.C., Chang T.H., Sogayar M.C., Zerbini L.F., Correa R.G. The crossroads of breast cancer progression: insights into the modulation of major signaling pathways. OncoTargets Ther. 2017;10:5491–5524. doi: 10.2147/OTT.S142154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Rau S.W., Dubal D.B., Böttner M., Gerhold L.M., Wise P.M. Estradiol attenuates programmed cell death after stroke-like injury. J. Neurosci. 2003;23(36):11420–11426. doi: 10.1523/JNEUROSCI.23-36-11420.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Simpkins J.W., Rajakumar G., Zhang Y.Q., Simpkins C.E., Greenwald D., Yu C.J., Bodor N., Day A.L. Estrogens may reduce mortality and ischemic damage caused by middle cerebral artery occlusion in the female rat. J. Neurosurg. 1997;87(5):724–730. doi: 10.3171/jns.1997.87.5.0724. [DOI] [PubMed] [Google Scholar]
- 282.Sandstrom N.J., Rowan M.H. Acute pretreatment with estradiol protects against CA1 cell loss and spatial learning impairments resulting from transient global ischemia. Horm. Behav. 2007;51(3):335–345. doi: 10.1016/j.yhbeh.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.O’Donnell M.E., Lam T.I., Tran L.Q., Foroutan S., Anderson S.E. Estradiol reduces activity of the blood-brain barrier Na-K-Cl cotransporter and decreases edema formation in permanent middle cerebral artery occlusion. J. Cereb. Blood Flow Metab. 2006;26(10):1234–1249. doi: 10.1038/sj.jcbfm.9600278. [DOI] [PubMed] [Google Scholar]
- 284.Paganini-Hill A., Ross R.K., Henderson B.E. Postmenopausal oestrogen treatment and stroke: a prospective study. BMJ. 1988;297(6647):519–522. doi: 10.1136/bmj.297.6647.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Falkeborn M., Persson I., Terént A., Adami H.O., Lithell H., Bergström R. Hormone replacement therapy and the risk of stroke. Follow-up of a population-based cohort in Sweden. Arch. Intern. Med. 1993;153(10):1201–1209. doi: 10.1001/archinte.1993.00410100035005. [DOI] [PubMed] [Google Scholar]
- 286.Finucane F.F., Madans J.H., Bush T.L., Wolf P.H., Kleinman J.C. Decreased risk of stroke among postmenopausal hormone users. Results from a national cohort. Arch. Intern. Med. 1993;153(1):73–79. doi: 10.1001/archinte.1993.00410010097008. [DOI] [PubMed] [Google Scholar]
- 287.Rossouw J.E., Anderson G.L., Prentice R.L., LaCroix A.Z., Kooperberg C., Stefanick M.L., Jackson R.D., Beresford S.A., Howard B.V., Johnson K.C., Kotchen J.M., Ockene J. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288(3):321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 288.Strom J.O., Theodorsson A., Theodorsson E. Dose-related neuroprotective versus neurodamaging effects of estrogens in rat cerebral ischemia: a systematic analysis. J. Cereb. Blood Flow Metab. 2009;29(8):1359–1372. doi: 10.1038/jcbfm.2009.66. [DOI] [PubMed] [Google Scholar]
- 289.Calabrese V., Cornelius C., Dinkova-Kostova A.T., Calabrese E.J., Mattson M.P. Cellular stress responses, the hormesis paradigm, and vitagenes: novel targets for therapeutic intervention in neurodegenerative disorders. Antioxid. Redox Signal. 2010;13(11):1763–1811. doi: 10.1089/ars.2009.3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Calabrese V., Mancuso C., Calvani M., Rizzarelli E., Butterfield D.A., Stella A.M. Nitric oxide in the central nervous system: neuroprotection versus neurotoxicity. Nat. Rev. Neurosci. 2007;8(10):766–775. doi: 10.1038/nrn2214. [DOI] [PubMed] [Google Scholar]
- 291.Calabrese V., Copani A., Testa D., Ravagna A., Spadaro F., Tendi E., Nicoletti V.G., Giuffrida S.A.M. Nitric oxide synthase induction in astroglial cell cultures: effect on heat shock protein 70 synthesis and oxidant/antioxidant balance. J. Neurosci. Res. 2000;60(5):613–622. doi: 10.1002/(SICI)1097-4547(20000601)60:5<613::AID-JNR6>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 292.Dattilo S., Mancuso C., Koverech G., Di Mauro P., Ontario M.L., Petralia C.C., Petralia A., Maiolino L., Serra A., Calabrese E.J., Calabrese V. Heat shock proteins and hormesis in the diagnosis and treatment of neurodegenerative diseases. Immun. Ageing. 2015;12(1):20. doi: 10.1186/s12979-015-0046-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Conolly R.B., Lutz W.K. Nonmonotonic dose-response relationships: mechanistic basis, kinetic modeling, and implications for risk assessment. Toxicol. Sci. 2004;77(1):151–157. doi: 10.1093/toxsci/kfh007. [DOI] [PubMed] [Google Scholar]
- 294.Holladay S.D., Ehrich M., Gogal R.M., Jr Commentary on hormetic dose-response relationships in immunology: occurrence, quantitative features of the dose response, mechanistic foundations, and clinical implications. Crit. Rev. Toxicol. 2005;35(2-3):299–302. doi: 10.1080/10408440590917062. [DOI] [PubMed] [Google Scholar]
- 295.Straub R.H. The complex role of estrogens in inflammation. Endocr. Rev. 2007;28(5):521–574. doi: 10.1210/er.2007-0001. [DOI] [PubMed] [Google Scholar]
- 296.Polan M.L., Daniele A., Kuo A. Gonadal steroids modulate human monocyte interleukin-1 (IL-1) activity. Fertil. Steril. 1988;49(6):964–968. doi: 10.1016/S0015-0282(16)59945-2. [DOI] [PubMed] [Google Scholar]
- 297.Cornelius C., Trovato Salinaro A., Scuto M., Fronte V., Cambria M.T., Pennisi M., Bella R., Milone P., Graziano A., Crupi R., Cuzzocrea S., Pennisi G., Calabrese V. Cellular stress response, sirtuins and UCP proteins in Alzheimer disease: role of vitagenes. Immun. Ageing. 2013;10(1):41. doi: 10.1186/1742-4933-10-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Pennisi M., Crupi R., Di Paola R., Ontario M.L., Bella R., Calabrese E.J., Crea R., Cuzzocrea S., Calabrese V. Inflammasomes, hormesis, and antioxidants in neuroinflammation: Role of NRLP3 in Alzheimer disease. J. Neurosci. Res. 2017;95(7):1360–1372. doi: 10.1002/jnr.23986. [DOI] [PubMed] [Google Scholar]
- 299.Calabrese V., Scapagnini G., Davinelli S., Koverech G., Koverech A., De Pasquale C., Salinaro A.T., Scuto M., Calabrese E.J., Genazzani A.R. Sex hormonal regulation and hormesis in aging and longevity: role of vitagenes. J. Cell Commun. Signal. 2014;8(4):369–384. doi: 10.1007/s12079-014-0253-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Calabrese V., Cornelius C., Cuzzocrea S., Iavicoli I., Rizzarelli E., Calabrese E.J. Hormesis, cellular stress response and vitagenes as critical determinants in aging and longevity. Mol. Aspects Med. 2011;32(4-6):279–304. doi: 10.1016/j.mam.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 301.Strom J.O., Theodorsson A., Theodorsson E. Hormesis and female sex hormones. Pharmaceuticals (Basel) 2011;4(5):726–740. doi: 10.3390/ph4050726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Amini P., Saffar H., Nourani M.R., Motevaseli E., Najafi M., Ali Taheri R., Qazvini A. Curcumin mitigates radiation-induced lung pneumonitis and fibrosis in rats. Int. J. Mol. Cell. Med. 2018;7(4):212–219. doi: 10.22088/IJMCM.BUMS.7.4.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Ringman J.M., Frautschy S.A., Teng E., Begum A.N., Bardens J., Beigi M., Gylys K.H., Badmaev V., Heath D.D., Apostolova L.G., Porter V., Vanek Z., Marshall G.A., Hellemann G., Sugar C., Masterman D.L., Montine T.J., Cummings J.L., Cole G.M. Oral curcumin for Alzheimer’s disease: tolerability and efficacy in a 24-week randomized, double blind, placebo-controlled study. Alzheimers Res. Ther. 2012;4(5):43. doi: 10.1186/alzrt146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Du L., Feng X., Xiang X., Jin Y. Wound healing effect of an in situ forming hydrogel loading curcumin-phospholipid complex. Curr. Drug Deliv. 2016;13(1):76–82. doi: 10.2174/1567201813666151202195437. [DOI] [PubMed] [Google Scholar]
- 305.Sun Y., Du L., Liu Y., Li X., Li M., Jin Y., Qian X. Transdermal delivery of the in situ hydrogels of curcumin and its inclusion complexes of hydroxypropyl-β-cyclodextrin for melanoma treatment. Int. J. Pharm. 2014;469(1):31–39. doi: 10.1016/j.ijpharm.2014.04.039. [DOI] [PubMed] [Google Scholar]
- 306.Zhang T., Chen Y., Ge Y., Hu Y., Li M., Jin Y. Inhalation treatment of primary lung cancer using liposomal curcumin dry powder inhalers. Acta Pharm. Sin. B. 2018;8(3):440–448. doi: 10.1016/j.apsb.2018.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Hu Y., Li M., Zhang M., Jin Y. Inhalation treatment of idiopathic pulmonary fibrosis with curcumin large porous microparticles. Int. J. Pharm. 2018;551(1-2):212–222. doi: 10.1016/j.ijpharm.2018.09.031. [DOI] [PubMed] [Google Scholar]
- 308.Hu S., Maiti P., Ma Q., Zuo X., Jones M.R., Cole G.M., Frautschy S.A. Clinical development of curcumin in neurodegenerative disease. Expert Rev. Neurother. 2015;15(6):629–637. doi: 10.1586/14737175.2015.1044981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Chen T., Zhuang B., Huang Y., Liu Y., Yuan B., Wang W., Yuan T., Du L., Jin Y. Inhaled curcumin mesoporous polydopamine nanoparticles against radiation pneumonitis. Acta Pharm. Sin. B. 2021 doi: 10.1016/j.apsb.2021.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Rattanatantikul T., Maiprasert M., Sugkraroek P., Bumrungpert A. Efficacy and safety of nutraceutical on menopausal symptoms in post-menopausal women: A randomized, double-blind, placebo-controlled clinical trial. J. Diet. Suppl. 2020:1–15. doi: 10.1080/19390211.2020.1853648. [DOI] [PubMed] [Google Scholar]
- 311.Lai C.C., Shih T.P., Ko W.C., Tang H.J., Hsueh P.R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges. Int. J. Antimicrob. Agents. 2020;55(3):105924. doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Riplinger L., Piera-Jiménez J., Dooling J.P. Patient identification techniques - approaches, implications, and findings. Yearb. Med. Inform. 2020;29(1):81–86. doi: 10.1055/s-0040-1701984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Popp F.A. Properties of biophotons and their theoretical implications. Indian J. Exp. Biol. 2003;41(5):391–402. [PubMed] [Google Scholar]
- 314.Thomas P., Dong J. Binding and activation of the seven-transmembrane estrogen receptor GPR30 by environmental estrogens: a potential novel mechanism of endocrine disruption. J. Steroid Biochem. Mol. Biol. 2006;102(1-5):175–179. doi: 10.1016/j.jsbmb.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 315.Tacyildiz N., Ozyoruk D., Yavuz G., Unal E., Dincaslan H., Dogu F., Sahin K., Kucuk O. Soy isoflavones ameliorate the adverse effects of chemotherapy in children. Nutr. Cancer. 2010;62(7):1001–1005. doi: 10.1080/01635581.2010.509841. [DOI] [PubMed] [Google Scholar]
- 316.Ahmad I.U., Forman J.D., Sarkar F.H., Hillman G.G., Heath E., Vaishampayan U., Cher M.L., Andic F., Rossi P.J., Kucuk O. Soy isoflavones in conjunction with radiation therapy in patients with prostate cancer. Nutr. Cancer. 2010;62(7):996–1000. doi: 10.1080/01635581.2010.509839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Landauer M.R., Srinivasan V., Seed T.M. Genistein treatment protects mice from ionizing radiation injury. J. Appl. Toxicol. 2003;23(6):379–385. doi: 10.1002/jat.904. [DOI] [PubMed] [Google Scholar]
- 318.Davis T.A., Clarke T.K., Mog S.R., Landauer M.R. Subcutaneous administration of genistein prior to lethal irradiation supports multilineage, hematopoietic progenitor cell recovery and survival. Int. J. Radiat. Biol. 2007;83(3):141–151. doi: 10.1080/09553000601132642. [DOI] [PubMed] [Google Scholar]
- 319.Para A.E., Bezjak A., Yeung I.W., Van Dyk J., Hill R.P. Effects of genistein following fractionated lung irradiation in mice. Radiother. Oncol. 2009;92(3):500–510. doi: 10.1016/j.radonc.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 320.Rosen E.M., Day R., Singh V.K. New approaches to radiation protection. Front. Oncol. 2015;4:381. doi: 10.3389/fonc.2014.00381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Maggiolini M., Statti G., Vivacqua A., Gabriele S., Rago V., Loizzo M., Menichini F., Amdò S. Estrogenic and antiproliferative activities of isoliquiritigenin in MCF7 breast cancer cells. J. Steroid Biochem. Mol. Biol. 2002;82(4-5):315–322. doi: 10.1016/S0960-0760(02)00230-3. [DOI] [PubMed] [Google Scholar]
- 322.Wang K.L., Hsia S.M., Chan C.J., Chang F.Y., Huang C.Y., Bau D.T., Wang P.S. Inhibitory effects of isoliquiritigenin on the migration and invasion of human breast cancer cells. Expert Opin. Ther. Targets. 2013;17(4):337–349. doi: 10.1517/14728222.2013.756869. [DOI] [PubMed] [Google Scholar]
- 323.Poschner S., Maier-Salamon A., Zehl M., Wackerlig J., Dobusch D., Pachmann B., Sterlini K.L., Jäger W. The impacts of genistein and daidzein on estrogen conjugations in human breast cancer cells: A targeted metabolomics approach. Front. Pharmacol. 2017;8:699. doi: 10.3389/fphar.2017.00699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Jin S., Zhang Q.Y., Kang X.M., Wang J.X., Zhao W.H. Daidzein induces MCF-7 breast cancer cell apoptosis via the mitochondrial pathway. Ann. Oncol. 2010;21(2):263–268. doi: 10.1093/annonc/mdp499. [DOI] [PubMed] [Google Scholar]
- 325.El-Bakoush A., Olajide O.A. Formononetin inhibits neuroinflammation and increases estrogen receptor beta (ERβ) protein expression in BV2 microglia. Int. Immunopharmacol. 2018;61:325–337. doi: 10.1016/j.intimp.2018.06.016. [DOI] [PubMed] [Google Scholar]
- 326.Chen J., Zeng J., Xin M., Huang W., Chen X. Formononetin induces cell cycle arrest of human breast cancer cells via IGF1/PI3K/Akt pathways in vitro and in vivo. Horm. Metab. Res. 2011;43(10):681–686. doi: 10.1055/s-0031-1286306. [DOI] [PubMed] [Google Scholar]
- 327.Puranik N.V., Srivastava P., Bhatt G., John Mary D.J.S., Limaye A.M., Sivaraman J. Determination and analysis of agonist and antagonist potential of naturally occurring flavonoids for estrogen receptor (ERα) by various parameters and molecular modelling approach. Sci. Rep. 2019;9(1):7450. doi: 10.1038/s41598-019-43768-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Wang L.M., Xie K.P., Huo H.N., Shang F., Zou W., Xie M.J. Luteolin inhibits proliferation induced by IGF-1 pathway dependent ERα in human breast cancer MCF-7 cells. APJCP. 2012;13(4):1431–1437. doi: 10.7314/apjcp.2012.13.4.1431. [DOI] [PubMed] [Google Scholar]
- 329.Long X., Fan M., Bigsby R.M., Nephew K.P. Apigenin inhibits antiestrogen-resistant breast cancer cell growth through estrogen receptor-alpha-dependent and estrogen receptor-alpha-independent mechanisms. Mol. Cancer Ther. 2008;7(7):2096–2108. doi: 10.1158/1535-7163.MCT-07-2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Zhu L., Xue L. Kaempferol Suppresses Proliferation and Induces Cell Cycle Arrest, Apoptosis, and DNA Damage in Breast Cancer Cells. Oncol. Res. 2019;27(6):629–634. doi: 10.3727/096504018X15228018559434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Hong J., Fristiohady A., Nguyen C.H., Milovanovic D., Huttary N., Krieger S., Hong J., Geleff S., Birner P., Jäger W., Özmen A., Krenn L., Krupitza G. Apigenin and luteolin attenuate the breaching of MDA-MB231 breast cancer spheroids through the Lymph endothelial barrier in vitro. Front. Pharmacol. 2018;9:220. doi: 10.3389/fphar.2018.00220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Zhang B., Su J.P., Bai Y., Li J., Liu Y.H. Inhibitory effects of O-methylated isoflavone glycitein on human breast cancer SKBR-3 cells. Int. J. Clin. Exp. Pathol. 2015;8(7):7809–7817. [PMC free article] [PubMed] [Google Scholar]
- 333.Zan L., Chen Q., Zhang L., Li X. Epigallocatechin gallate (EGCG) suppresses growth and tumorigenicity in breast cancer cells by downregulation of miR-25. Bioengineered. 2019;10(1):374–382. doi: 10.1080/21655979.2019.1657327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Zeng X., Xu Z., Gu J., Huang H., Gao G., Zhang X., Li J., Jin H., Jiang G., Sun H., Huang C. Induction of miR-137 by Isorhapontigenin (ISO) directly targets Sp1 protein translation and mediates its anticancer activity both in vitro and in vivo. Mol. Cancer Ther. 2016;15(3):512–522. doi: 10.1158/1535-7163.MCT-15-0606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Subedi L., Teli M.K., Lee J.H., Gaire B.P., Kim M.H., Kim S.Y. A stilbenoid isorhapontigenin as a potential anti-cancer agent against breast cancer through inhibiting sphingosine kinases/tubulin stabilization. Cancers (Basel) 2019;11(12):E1947. doi: 10.3390/cancers11121947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Pan C., Hu Y., Li J., Wang Z., Huang J., Zhang S., Ding L. Estrogen receptor-α36 is involved in pterostilbene-induced apoptosis and anti-proliferation in in vitro and in vivo breast cancer. PLoS One. 2014;9(8):e104459. doi: 10.1371/journal.pone.0104459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Moon D., McCormack D., McDonald D., McFadden D. Pterostilbene induces mitochondrially derived apoptosis in breast cancer cells in vitro. J. Surg. Res. 2013;180(2):208–215. doi: 10.1016/j.jss.2012.04.027. [DOI] [PubMed] [Google Scholar]
- 338.Carreau C., Flouriot G., Bennetau-Pelissero C., Potier M. Enterodiol and enterolactone, two major diet-derived polyphenol metabolites have different impact on ERalpha transcriptional activation in human breast cancer cells. J. Steroid Biochem. Mol. Biol. 2008;110(1-2):176–185. doi: 10.1016/j.jsbmb.2008.03.032. [DOI] [PubMed] [Google Scholar]
- 339.Mali A.V., Joshi A.A., Hegde M.V., Kadam S.S. Enterolactone modulates the ERK/NF-κB/Snail signaling pathway in triple-negative breast cancer cell line MDA-MB-231 to revert the TGF-β-induced epithelial-mesenchymal transition. Cancer Biol. Med. 2018;15(2):137–156. doi: 10.20892/j.issn.2095-3941.2018.0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Jin J.S., Lee J.H., Hattori M. Ligand binding affinities of arctigenin and its demethylated metabolites to estrogen receptor alpha. Molecules. 2013;18(1):1122–1127. doi: 10.3390/molecules18011122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Bergman Jungeström M., Thompson L.U., Dabrosin C. Flaxseed and its lignans inhibit estradiol-induced growth, angiogenesis, and secretion of vascular endothelial growth factor in human breast cancer xenografts in vivo. Clin. Cancer Res. 2007;13(3):1061–1067. doi: 10.1158/1078-0432.CCR-06-1651. [DOI] [PubMed] [Google Scholar]
- 342.Wang C., Li C., Zhou H., Huang J. High-throughput screening assays for estrogen receptor by using coumestrol, a natural fluorescence compound. J. Biomol. Screen. 2014;19(2):253–258. doi: 10.1177/1087057113502673. [DOI] [PubMed] [Google Scholar]
- 343.Lee Y.H., Yuk H.J., Park K.H., Bae Y.S. Coumestrol induces senescence through protein kinase CKII inhibition-mediated reactive oxygen species production in human breast cancer and colon cancer cells. Food Chem. 2013;141(1):381–388. doi: 10.1016/j.foodchem.2013.03.053. [DOI] [PubMed] [Google Scholar]
- 344.Nehybova T., Smarda J., Daniel L., Brezovsky J., Benes P. Wedelolactone induces growth of breast cancer cells by stimulation of estrogen receptor signalling. J. Steroid Biochem. Mol. Biol. 2015;152:76–83. doi: 10.1016/j.jsbmb.2015.04.019. [DOI] [PubMed] [Google Scholar]
- 345.Nehybová T., Šmarda J., Daniel L., Stiborek M., Kanický V. Spasojevič I.; Preisler, J.; Damborský, J.; Beneš, P. Wedelolactone acts as proteasome inhibitor in breast cancer cells. Int. J. Mol. Sci. 2017;18(4):E729. doi: 10.3390/ijms18040729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Xin D., Wang H., Yang J., Su Y.F., Fan G.W., Wang Y.F., Zhu Y., Gao X. -.M. Phytoestrogens from psoralea corylifolia reveal estrogen receptor-subtype selectivity. Phytomedicine. 2010;17(2):126–131. doi: 10.1016/j.phymed.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 347.Wang X., Xu C., Hua Y., Cheng K., Zhang Y., Liu J., Han Y., Liu S., Zhang G., Xu S., Yang Z. Psoralen induced cell cycle arrest by modulating Wnt/β-catenin pathway in breast cancer cells. Sci. Rep. 2018;8(1):14001. doi: 10.1038/s41598-018-32438-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Nanashima N., Horie K., Maeda H. Phytoestrogenic activity of blackcurrant anthocyanins is partially mediated through estrogen receptor beta. Molecules. 2017;23(1):E74. doi: 10.3390/molecules23010074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Mazzoni L., Giampieri F., Alvarez Suarez J.M., Gasparrini M., Mezzetti B., Forbes Hernandez T.Y., Battino M.A. Isolation of strawberry anthocyanin-rich fractions and their mechanisms of action against murine breast cancer cell lines. Food Funct. 2019;10(11):7103–7120. doi: 10.1039/C9FO01721F. [DOI] [PubMed] [Google Scholar]
- 350.Dayoub O., Le Lay S., Soleti R., Clere N., Hilairet G., Dubois S., Gagnadoux F., Boursier J., Martínez M.C., Andriantsitohaina R. Estrogen receptor α/HDAC/NFAT axis for delphinidin effects on proliferation and differentiation of T lymphocytes from patients with cardiovascular risks. Sci. Rep. 2017;7(1):9378. doi: 10.1038/s41598-017-09933-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Herrera-Sotero M.Y., Cruz-Hernández C.D., Oliart-Ros R.M., Chávez-Servia J.L., Guzmán-Gerónimo R.I., González-Covarrubias V., Cruz-Burgos M., Rodríguez-Dorantes M. Anthocyanins of blue corn and tortilla arrest cell cycle and induce apoptosis on breast and prostate cancer cells. Nutr. Cancer. 2020;72(5):768–777. doi: 10.1080/01635581.2019.1654529. [DOI] [PubMed] [Google Scholar]
- 352.Kim H.I., Quan F.S., Kim J.E., Lee N.R., Kim H.J., Jo S.J., Lee C.M., Jang D.S., Inn K.S. Inhibition of estrogen signaling through depletion of estrogen receptor alpha by ursolic acid and betulinic acid from Prunella vulgaris var. lilacina. Biochem. Biophys. Res. Commun. 2014;451(2):282–287. doi: 10.1016/j.bbrc.2014.07.115. [DOI] [PubMed] [Google Scholar]
- 353.Zhang X., Li T., Gong E.S., Liu R.H. Antiproliferative Activity of Ursolic Acid in MDA-MB-231 Human Breast Cancer Cells through Nrf2 Pathway Regulation. J. Agric. Food Chem. 2020;68(28):7404–7415. doi: 10.1021/acs.jafc.0c03202. [DOI] [PubMed] [Google Scholar]
- 354.Billam M., Witt A.E., Davidson N.E. The silent estrogen receptor--can we make it speak? Cancer Biol. Ther. 2009;8(6):485–496. doi: 10.4161/cbt.8.6.7582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Kim H.Y., Choi T.W., Kim H.J., Kim S.M., Park K.R., Jang H.J., Lee E.H., Kim C.Y., Jung S.H., Shim B.S., Ahn K.S. A methylene chloride fraction of Saururus chinensis induces apoptosis through the activation of caspase-3 in prostate and breast cancer cells. Phytomedicine. 2011;18(7):567–574. doi: 10.1016/j.phymed.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 356.Raj M.V., Selvakumar K., Krishnamoorthy G., Revathy S., Elumalai P., Arunakaran J. Impact of lycopene on epididymal androgen and estrogen receptors’ expression in polychlorinated biphenyls-exposed rat. Reprod. Sci. 2014;21(1):89–101. doi: 10.1177/1933719113492213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Takeshima M., Ono M., Higuchi T., Chen C., Hara T., Nakano S. Anti-proliferative and apoptosis-inducing activity of lycopene against three subtypes of human breast cancer cell lines. Cancer Sci. 2014;105(3):252–257. doi: 10.1111/cas.12349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Wu K.H., Ho C.T., Chen Z.F., Chen L.C., Whang-Peng J., Lin T.N., Ho Y.S. The apple polyphenol phloretin inhibits breast cancer cell migration and proliferation via inhibition of signals by type 2 glucose transporter. J. Food Drug Anal. 2018;26(1):221–231. doi: 10.1016/j.jfda.2017.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Sirianni R., Chimento A., De Luca A., Casaburi I., Rizza P., Onofrio A., Iacopetta D., Puoci F., Andò S., Maggiolini M., Pezzi V. Oleuropein and hydroxytyrosol inhibit MCF-7 breast cancer cell proliferation interfering with ERK1/2 activation. Mol. Nutr. Food Res. 2010;54(6):833–840. doi: 10.1002/mnfr.200900111. [DOI] [PubMed] [Google Scholar]
- 360.Han J., Talorete T.P., Yamada P., Isoda H. Anti-proliferative and apoptotic effects of oleuropein and hydroxytyrosol on human breast cancer MCF-7 cells. Cytotechnology. 2009;59(1):45–53. doi: 10.1007/s10616-009-9191-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Chen J., Zhao X., Ye Y., Wang Y., Tian J. Estrogen receptor beta-mediated proliferative inhibition and apoptosis in human breast cancer by calycosin and formononetin. Cell. Physiol. Biochem. 2013;32(6):1790–1797. doi: 10.1159/000356612. [DOI] [PubMed] [Google Scholar]
- 362.Wu G., Niu M., Qin J., Wang Y., Tian J. Inactivation of Rab27B-dependent signaling pathway by calycosin inhibits migration and invasion of ER-negative breast cancer cells. Gene. 2019;709:48–55. doi: 10.1016/j.gene.2019.04.005. [DOI] [PubMed] [Google Scholar]
- 363.Won Y. -.S.; Lee, J-.H.; Kwon, S-.J.; Kim, J-.Y.; Park, K-.H.; Lee, M-.K.; Seo, K-.II. α-Mangostin-induced apoptosis is mediated by estrogen receptor α in human breast cancer cells. Food Chem. Toxicol. 2014;66:158–165. doi: 10.1016/j.fct.2014.01.040. [DOI] [PubMed] [Google Scholar]
- 364.Mersereau J.E., Levy N., Staub R.E., Baggett S., Zogovic T., Chow S., Ricke W.A., Tagliaferri M., Cohen I., Bjeldanes L.F., Leitman D.C. Liquiritigenin is a plant-derived highly selective estrogen receptor beta agonist. Mol. Cell. Endocrinol. 2008;283(1-2):49–57. doi: 10.1016/j.mce.2007.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Yang E.J., Park G.H., Song K.S. Neuroprotective effects of liquiritigenin isolated from licorice roots on glutamate-induced apoptosis in hippocampal neuronal cells. Neurotoxicology. 2013;39:114–123. doi: 10.1016/j.neuro.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 366.Kim S., Park T.I. Naringenin: a partial agonist on estrogen receptor in T47D-KBluc breast cancer cells. Int. J. Clin. Exp. Med. 2013;6(10):890–899. [PMC free article] [PubMed] [Google Scholar]
- 367.Sugumar M., Sevanan M., Sekar S. Neuroprotective effect of naringenin against MPTP-induced oxidative stress. Int. J. Neurosci. 2019;129(6):534–539. doi: 10.1080/00207454.2018.1545772. [DOI] [PubMed] [Google Scholar]
- 368.Duan X., Li Y., Xu F., Ding H. Study on the neuroprotective effects of Genistein on Alzheimer’s disease. Brain Behav. 2021;11(5):e02100. doi: 10.1002/brb3.2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Qian Y., Cao L., Guan T., Chen L., Xin H., Li Y., Zheng R., Yu D. Protection by genistein on cortical neurons against oxidative stress injury via inhibition of NF-kappaB, JNK and ERK signaling pathway. Pharm. Biol. 2015;53(8):1124–1132. doi: 10.3109/13880209.2014.962057. [DOI] [PubMed] [Google Scholar]
- 370.Wu X., Tong B., Yang Y., Luo J., Yuan X., Wei Z., Yue M., Xia Y., Dai Y. Arctigenin functions as a selective agonist of estrogen receptor β to restrict mTORC1 activation and consequent Th17 differentiation. Oncotarget. 2016;7(51):83893–83906. doi: 10.18632/oncotarget.13338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Rezaei-Seresht H., Cheshomi H., Falanji F., Movahedi-Motlagh F., Hashemian M., Mireskandari E. Cytotoxic activity of caffeic acid and gallic acid against MCF-7 human breast cancer cells: An in silico and in vitro study. Avicenna J. Phytomed. 2019;9(6):574–586. doi: 10.22038/AJP.2019.13475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Mansour H.H., Tawfik S.S. Early treatment of radiation-induced heart damage in rats by caffeic acid phenethyl ester. Eur. J. Pharmacol. 2012;692(1-3):46–51. doi: 10.1016/j.ejphar.2012.06.037. [DOI] [PubMed] [Google Scholar]
- 373.Fan Z.L., Wang Z.Y., Zuo L.L., Tian S.Q. Protective effect of anthocyanins from lingonberry on radiation-induced damages. Int. J. Environ. Res. Public Health. 2012;9(12):4732–4743. doi: 10.3390/ijerph9124732. [DOI] [PMC free article] [PubMed] [Google Scholar]


