Abstract
Many psychiatric patients do not respond to conventional therapy. There is a vast effort to investigate possible mechanisms involved in treatment resistance, trying to provide better treatment options, and several data points toward a possible involvement of inflammatory mechanisms. Microglia, glial, and resident immune cells are involved in complex responses in the brain, orchestrating homeostatic functions, such as synaptic pruning and maintaining neuronal activity. In contrast, microglia play a major role in neuroinflammation, neurodegeneration, and cell death. Increasing evidence implicate microglia dysfunction in neuropsychiatric disorders. The mechanisms are still unclear, but one pathway in microglia has received increased attention in the last 8 years, i.e., the NLRP3 inflammasome pathway. Stress response and inflammation, including microglia activation, can be attenuated by Cannabidiol (CBD). CBD has antidepressant, anti-stress, antipsychotic, anti-inflammatory, and other properties. CBD effects are mediated by direct or indirect modulation of many receptors, enzymes, and other targets. This review will highlight some findings for neuroinflammation and microglia involvement in stress-related psychiatric disorders, particularly addressing the NLRP3 inflammasome pathway. Moreover, we will discuss evidence and mechanisms for CBD effects in psychiatric disorders and animal models and address its potential effects on stress response via neuroinflammation and NLRP3 inflammasome modulation.
Keywords: Neuroinflammation, NLRP3 inflammasome, cannabidiol, stress, psychiatric disorders, animal models
1. NEUROINFLAMMATION, MICROGLIA, AND STRESS-RELATED DISORDERS
1.1. Immune System, Neuroinflammation, and Mood Disorders
There is increasing evidence for neuroinflammatory mechanisms in mood disorders, including major depressive disorder (MDD), posttraumatic stress disorder (PTSD), and bipolar disorder [1-4]. At first, inflammatory conditions, or treatment with immune system stimulants, such as interferons, induce sickness behavior, increase peripheral and SNC inflammation signs, and seem to contribute to the onset of psychiatric disorders [5-8]. Interestingly, an immune challenge with lipopolysaccharide (LPS) from Gram-negative bacteria in healthy humans and lab mice induces an immune response and sickness behavior in both species and emotional changes in humans [5]. In contrast, anti-cytokines treatment for inflammatory conditions attenuates depressive symptoms in these patients [9].
From another perspective, several studies report increased inflammation markers, for example, in many cases evaluated in untreated affective disorder patients [2, 10], or PTSD patients [11]. Moreover, increased levels of inflammatory mediators, such as IL-6 and CRP in MDD [12, 13] and CRP in PTSD [13], are associated with symptom severity. In one study, high basal levels of IL-6 and CRP were associated with persistent depressive symptoms over 5 years [14]. These changes are not reported in all studies, and other mediators are not found to be associated with symptoms severity [11, 12].
Several data indicate that higher levels of inflammatory markers, such as IL-1β, IL-6, and TNF-α, could predict resistance to conventional antidepressant treatment [15-18], whereas remission of symptoms could be associated with normalization of inflammatory markers [19]. However, several reports evidence the anti-inflammatory effects of antidepressant drugs, particularly SSRIs, in psychiatric patients who respond to the treatment [20-22]. Therefore, it seems that it is not the sole high levels of inflammatory mediators that predict antidepressant response or the ability of a drug to attenuate inflammatory response that counts, evidencing the complexity of psychiatric disorders neurobiology.
Nonetheless, a good amount of data shows that monotherapy or add-on treatment in MDD patients with anti-inflammatory or anti-cytokine drugs can improve depressive symptoms [23-25]. Anti-TNF-α therapy with Infliximab, for example, was only effective in treatment-resistant patients with high basal levels of inflammatory biomarkers [6]. Moreover, high levels of TNFR1/NF-κB contained in extracellular vesicles from a neuronal origin in Bipolar patients were attenuated by treatment with Infliximab along with improvement of depressive symptoms [26].
Despite evidence regarding an immune component, a key aspect to point out when discussing (neuro)inflammation and psychiatric disorders is that many psychiatric patients may not present increased levels of immune mediators [12, 15, 27]. Therefore, targeting immune mediators to improve behavioral outcomes or facilitate conventional treatment response seems to be relevant only for a subset of patients with signals of immune system activation [28, 29].
Most studies evaluate inflammatory markers in the blood, which in human studies are easy to detect. Some studies assess levels in the CSF, but this is invasive and, therefore, difficult to obtain. Generally, blood levels certainly do not reflect the entire picture of what is happening in the brain unless the evaluation of extracellular vesicles cargo of brain origin is investigated. Within the brain, glial cells produce, release, and respond to immune mediators. These cells exert a crucial role in neuronal functions, regulating neurotransmitters metabolism, and also complex functions, such as learning and memory [29]. In addition to mediators produced by these cells, now it is recognized that cytokines produced by circulating and resident immune cells in the meningeal lymphatic system compartment close to brain parenchyma can influence the brain function, including the production of cytokines by glial cells through a glymphatic route [30]. One of these cells is microglia; their role in neuroinflammation will discuss in this review.
1.2. Microglia in Neuroinflammation and Stress Response
Physical, cellular, or psychological stress exposure in lab rodents induces sterile inflammation in the brain by releasing danger/damage-associated molecular patterns (DAMPs), such as adenosine triphosphate (ATP), heat shock proteins (HSP), and high mobility group box 1 (HMGB-1). These molecules could be involved in an increased risk of developing psychiatric disorders or even worsening symptoms [1, 31, 32]. We will discuss the involvement of ATP later.
Simplistically, cytokines released in the periphery reach the brain through neural or humoral pathways [5, 33] or could influence brain activity after being sensed by endothelial cell receptors, such as IL-1R, in the blood-brain barrier [34], influencing brain function. The main target seems to be microglia, although a more recently direct effect on neurons has been identified [35]. In the brain, microglia, astrocytes, and oligodendrocytes, altogether called the glial cells, are an important source of cytokines and are essential regulators of brain homeostasis; therefore, these cells have crucial implications in different diseases [33].
Microglia are the only resident immune cells in the brain and several data suggested their involvement in neuropsychiatric disorders [4, 28, 36]. Microglia are originated from primitive myeloid cell precursors in the yolk sac and colonized the brain during the embryonic day E9.5 [37, 38]. These cells are essential for neuroplasticity and maintenance of brain homeostasis [38]. Still, their overactivation under persistent stress exposure, or aging processing, could initiate sterile inflammation in the brain, disrupt normal neuronal communication, and reduce brain homeostasis, leading to neuroinflammation and pathology [4, 36, 39]. In this context, microglia have been implicated in the neuroinflammation observed in several neuropsychiatric disorders.
Signs of microglia dysfunction are observed in mainly all kinds of psychiatric disorders [28, 40, 41]. For example, increased quinolinic acid (QUINA) levels were found in the subgenual and supracallosal parts of the anterior cingulate cortex of depressed patients who committed suicide [41, 42]. QUINA, a product from activation of the kynurenine pathway by pro-inflammatory cytokines in microglia, which could decrease available tryptophan for serotonin synthesis [43], is an NMDA agonist. Therefore, higher levels of QUINA could be involved in toxicity, oxidative stress, and neurodegeneration [17]. In the hippocampus, however, decreased levels of QUINA were observed, indicating potentially different mechanisms depending on the brain region [44], at least in depressed patients who committed suicide. However, other studies did not find indications of microglia activation in the brain [28], once again evidencing that not all psychiatric patients have microglia dysfunction.
Some studies showing microglia activation in the brain of depressive suicide patients [42] suggest that higher activation in some regions, including the anterior cingulate cortex and insula, could be related to symptoms severity, including suicidal thoughts [40, 45]. In line with the idea of microglia involvement in neuropsychiatric disorders, some human [46-49] and several animals studies [50-54] have evaluated the effectiveness of minocycline in attenuating stress effects. This tetracycline antibiotic inhibits microglia activation at low doses [49]. Overall, studies with minocycline have rendered some mixed but promising results [46].
A suggestion is that neuroinflammation and microglia could be involved in treatment resistance [55]. A recent small double-blind, placebo-controlled trial has evaluated the effect of adding minocycline to the usual treatment in treatment-resistant MDD patients for 12 weeks [47]. A significant decrease in scores on the Hamilton Rating Scale was reported, exerting an antidepressant activity in treatment-resistant patients [47].
Although microglia dysfunction is reported in several studies, there are still many open questions regarding their role in neuropsychiatric disorders. How can stress activate microglia? At which level can this be deleterious? Which microglia mechanisms after stress could result in behavioral changes? Are these mechanisms involved in resistance to conventional treatment? Can they contribute to the development of neuropsychiatric disorders? Many of these questions are only beginning to be addressed.
This review will focus on an innate immune mechanism essential for immune response to different stimuli in microglia, the NLRP3 inflammasome. Furthermore, we will present evidence pointing out the potential involvement of this pathway in neuropsychiatric disorders and mainly animal data showing that interference with this mechanism can effectively attenuate stress effects. Finally, we will address the beneficial effect of the Cannabis sativa plant compound Cannabidiol (CBD) in stress-related disorders and provide some evidence that led us to raise the hypothesis that microglial NLRP3 inflammasome is a potential target for cannabidiol effect in attenuating mood disorders.
2. NLRP3 INFLAMMASOME IN STRESS RESPONSE
The mammalian immune system comprehends several components, such as barriers (physical, chemical, and biological), cells, receptors, and soluble proteins that integrate innate or adaptive immunity. The innate immune mechanisms were formerly known as non-specific since they are constitutively expressed and always ready to be recruited. In contrast, the adaptive mechanisms were known as specific, depending on the antigen, allowing the formation of immunological memory (humoral and cellular) against that specific threat. Nowadays, innate immunity is considered and better referred to as the first line of protection and defense from external or internal danger signals. The pattern-recognition receptors (PRRs) are constitutively expressed and exert a vital role in sensing both damage- and pathogen-associated molecular patterns (DAMPs and PAMPS, respectively). PRRs are expressed not only by innate immune system cells, such as monocytes, macrophages, microglia, dendritic cells, and neutrophils but also by adaptive immune system cells.
Toll-like receptors (TLR) are the most studied PRRs, and they are present in cellular and endosomal membranes. In the last decades, a new family of PRRs was described, the cytosolic NOD-like receptors (NLRs), which contain 3 domains: a leucine-rich repeat (LRR) domain in its carboxyl(C)-terminal, a central NOD/NACHT domain (“Nucleotide-binding Oligomerization Domain”), and one amino(N)-terminal domain, which can vary between the different NLRs. The NLRP3 receptor type has a pyrin domain (PYD) in its N-terminal region, where its name partially derives from “pyrin domain-containing protein 3”. Of the known NLR receptors, some of them, including NLRP3, can form multiprotein inflammasome complexes under stimulation/activation [56, 57].
The NLRP3 inflammasome is a tripartite multiprotein structure that comprises the core sensor protein/receptor NLRP3, the adaptor protein ASC (apoptosis-associated speck-like protein containing a caspase-recruitment domain (CARD), and (pro-)caspase-1. In the absence of stimulus, the NLR is in its inactive form by autoinhibition, since the effector regions (NOD) are protected by the binding of the LRR region, blocking its activation. Once there is an activating stimulus, the LRR releases the NOD domain that undergoes oligomerization and homotypic interactions between NOD domains. The PYD domain from NLRP3 recruits and oligomerizes with ASC, which, in turn, recruits pro-caspase-1 through CARD-CARD interactions. Pro-caspase-1 is then able to self-cleavage into its active proteolytic form, caspase-1, which cleaves inactive pro-IL-1β and pro-IL-18 into their active forms, IL-1β and IL-18, that are secreted from the cell. In this way, the NLRP3 inflammasome plays a pivotal role in IL-1β activation and release [58] and its formation also triggers pyroptosis, a cell death related to inflammation [57, 59-61].
The canonical mechanism of activation of NLRP3 inflammasome is tightly regulated and generally occurs in two steps – priming (Fig. 1A) and activation (Fig. 1B). The priming process generally aims to upregulate the expression of NLRP3 inflammasome (Nlrp3, Pycard, Casp1) components as well as pro-IL-1β (Il1b) and pro-IL-18 (Il18) mRNAs. It is usually induced after a first signal, such as the recognition of DAMPS or PAMPS by other PRRs, such as TLR4, or direct action of some cytokines (TNF𝛼 and IL-1β), which, in turn, stimulates the transcription factor nuclear factor kβ (NF-kβ) [62-65]. Besides this, there is evidence that the priming process induces post-translational modifications that are important to the tight regulation of the NLRP3 inflammasome platform activation and to assure the rapid and precise activation of the system upon request [66-75]. The second signal, the recognition of an NLRP3 activator, precedes and induces receptor activation and complex formation. Several stimuli can activate the NLRP3 inflammasome platform, such as endogenous (self-derived) and external (foreign-derived) DAMPs, as well as PAMPs from bacterial, fungal, parasitical, and viral infections [57, 59, 76-78]. As examples of DAMPs that are already described to activate the platform, we can highlight ATP [79], oxidized mitochondrial DNA [80], amyloid-β [81], lysophosphatidylcholine [82], malarial hemozoin [83], crystals structures – cholesterol [84], uric acid [85] and calcium oxalate [86], among others. Bacterial toxins [87], β-Glucans [88], zymosan [89], microbial DNA, and RNA [90, 91], among others, are examples of PAMPS that, in turn, can activate the NLRP3. This wide range of activators with chemical and structural diversity suggests that NLRP3 senses a common cellular signal, presumably cellular stress or damage, and probably does not need physical interaction with its activators, unlike other PRRs that usually present a high specificity to be activated. Due to this, NLRP3 is considered a cytosolic damage sensor. The mechanisms by which NLRP3 senses signals, their identities, and pathways involved in NLRP3 inflammasome activation are not yet fully comprehended and are still a matter of debate. In this sense, several molecular and cellular upstream events are suggested to trigger the NLRP3 inflammasome activation, including potassium efflux (K+) [79, 87, 92-95], chloride efflux (Cl-) [96, 97], calcium flux / signaling (Ca2+) [98-101], lysosomal leakage, destabilization and rupture [81, 102-104], mitochondrial dysfunction, and reactive oxygen species (ROS) [80, 105-108], among others. For the purpose of this review, it is important to mention that for a long time, it was suggested that the K+ efflux occurred through P2X7 channels after its activation by ATP molecules [79, 109]. However, it was recently suggested that this event does not occur through P2X7 receptors in bone marrow derived macrophages. The proposed mechanism is that ATP activates P2X7, with subsequent Ca2+ and Na+ influx. The K+ efflux would be then mediated by TWIK2 channels in a coordinated manner [110]. If the K+ efflux really occurs through P2X7 is still a matter of discussion.
Fig. (1).
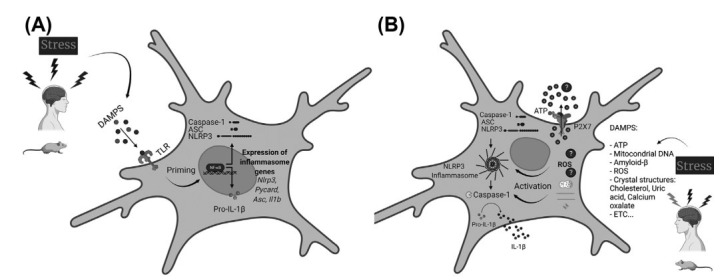
Schematic illustration of the microglial NLRP3 inflammasome priming (A) and activation (B). A. Stressful conditions promote the release of DAMPs, which will be sensed by membrane-associated PRRs (Pattern recognition receptors), here represented by TLRs. TLRs activation engages the NF-kB pathway. Active NF-kB translocates to the nucleus, culminating in the upregulation of the NLRP3 inflammasome-related genes (Nlrp3, Pycard, Casp1, Il1b, and Il18). After the translational process, the NLRP3-inflammasome-related proteins remain in their inactive forms inside the cellular cytoplasm. This phase is considered the priming process. B. A second signal or stimulus, also elicited by DAMPS, but now sensed by NLRP3, is necessary to assemble and activate the NLRP3 inflammasome. One proposed model to explain how this receptor senses the cytosolic damage involves the interaction between extracellular ATP and P2X7 receptors. ATP molecules would bind directly to the P2X7 receptor and promote K+ efflux, which will be sensed by the NLRP3 receptors and lead to the NLRP3 inflammasome oligomerization and activation. Lysosomal contents released after vesicles rupture are also suggested to induce the platform activation – this would be the mechanism caused by crystalline and particulate molecules (amyloid-β, for example). Another model proposes that DAMPs- and PAMPs-generated ROS will be sensed and responsible for the NLRP3 inflammasome activation. Besides these molecules and models, other mechanisms seem to be involved in the oligomerization and activation of the NLRP3 inflammasome platform. Independent of the exact mechanism by which this cytosolic sensor senses the stimulus, once the NLRP3 receptor detects an activating stimulus, this receptor recruits and oligomerizes with ASC, which, in turn, recruits pro-caspase-1. Pro-caspase-1 can self-cleavage into its active proteolytic form, caspase-1, which cleaves inactive pro-IL-1β into its active forms, IL-1β that will be finally secreted from the cell and induce its pro-inflammatory effects in other cells and tissues. DAMPs: Damage-associated molecular patterns (DAMPs); PAMPs: Pathogen-associated molecular patterns (DAMPs); PRRs: Pattern recognition receptors (PRRs); TLRs: Toll-like receptors; ROS: Reactive oxygen species. Created with BioRender.com.
Autophagic processes are essential negative regulators of NLRP3 inflammasome activation. Autophagy is a complex and tightly regulated intracellular process with notorious importance for cellular homeostasis that involves the capture and delivery of cytoplasmatic material to be digested by the lysosome [111-113]. Dysfunctions in autophagy are related to hyperinflammation as well as autoimmune, neurodegenerative, psychiatric, and inflammatory diseases [114-118]. Several different types of autophagic processes lead to different effects, but the discussion of these mechanisms is beyond the scope of this review. However, other authors have already reviewed these mechanisms elsewhere [111-114, 116, 117]. It is important to mention that NLRP3 also modulates autophagy in some circumstances. These inter-regulatory processes are crucial to keep a necessary balance between required and proper host immune defensive responses and the prevention of excessive, detrimental, or even harmful consequences [111].
In general, NLRP3 is still the most studied and consequently most comprehended inflammasome. Considering all the information above, the NLRP3 inflammasome platform is already associated with the onset and/or progression of several diseases and disorders, including autoimmune, metabolic, cardiovascular, oncologic, neurodegenerative, psychiatric conditions, among others [27, 77, 118-127].
2.1. Behavioral and Molecular Evidence of NLRP3 Activation in Preclinical and Clinical Studies of Mental Disorders and Consequences of its Modulation
In the last decade, increasing evidence suggested that the NLRP3 inflammasome is the primary source of IL-1β-induced inflammation in the central nervous system (CNS) [119, 128-130]. Therefore, its involvement and modulation in psychiatric preclinical and clinical studies are under extensive investigation.
It is already described that untreated major depressive patients have increased mRNA levels of NLRP3 and caspase-1 in peripheral blood mononuclear cells (PBMCs), as well as an increase in serum levels of IL-1β and IL-18 compared to amitriptyline treated-patients [131].
Preclinical studies with animal models of psychiatric disorders obtained similar results. Rats submitted to the chronic unpredictable stress model (CUS) for 12 weeks showed an increase in the protein levels of IL-1β, NLRP3, active caspase-1 fraction, P2X7, and TLR2 in the prefrontal cortex (PFC) region. In this same region, stressed rats showed an increase in the expression of CD11b integrin and Iba-1, markers of macrophage-like cells, including microglia, enhanced co-localization between NLRP3 and Iba-1, as well as decreased expression of GFAP, suggesting that microglial cells are important to activation of the inflammasome platform and IL-1β release in PFC. All these molecular alterations were concomitant with behavioral alterations predictive of depressive-like phenotype: sucrose preference levels and bodyweight reduction. Repeated treatment with Fluoxetine, a selective serotonin reuptake inhibitor (SSRI) widely used to treat stress-related conditions, prevented stress effects after 6 weeks [132]. Other in vitro and in vivo data suggested that Fluoxetine can suppress the NLRP3 inflammasome activation and improve the behavioral and inflammatory consequences of stress [133]. Moreover, several studies have demonstrated that in addition to Fluoxetine, different antidepressants and/or coadjuvant drugs used in the treatment of depressive disorders, such as ketamine, venlafaxine, mirtazapine, melatonin, and others, can inhibit the NLRP3 inflammasome in animal models [132, 134-136] or MDD patients [137].
Additional data showed that mice exposed to 4 weeks of CUS presented depressive-like behaviors and had significantly higher levels of corticosterone and IL-1β. The hippocampi of stressed mice showed higher activity of caspase-1, followed by increased protein levels of active IL-1β, NRLP3, and ASC. Treatment with VX-765, a caspase-1 inhibitor, prevented the stress effects in both behavioral and molecular parameters [138]. Also, in a more causal study on NLRP3 knockout (KO) mice, or mice treated with minocycline, evaluated after a 7-days restraint-stress protocol; stress increased both transcripts and protein levels of NLRP3 and IL-1β in the hippocampus and adenosine, ATP, and the number of activated microglia in hippocampus and PFC. These molecular effects were accompanied also by depressive-like behavioral alterations in the forced swim, sucrose preference, and social interaction tests as well in the food consumption evaluation. The stress effects were not observed in NLRP3KO mice or after minocycline treatment [139], indicating that microglia and NLRP3 are crucial for stress consequences.
Recently, the involvement of NLRP3 in the fear conditioning process, a stress protocol used to evaluate fear memory and stress-related disorders, such as PTSD, was described. The contextual fear paradigm increased the hippocampal protein levels of cleaved caspase-1 and IL-1β three hours after electric foot shocks in wild-type (WT) but not NLRP3 KO mice. At the same time point, the microglial analysis showed that mRNA levels of IL-1β were also increased and the stressed group presented a higher number of activated cells (Iba1+) in comparison to the NLRP3 KO group. From the behavioral point of view, both deletion and pharmacological inhibition of NLRP3 (MCC950, a selective NLRP3 inhibitor), facilitate the fear extinction process and attenuate anxiety-related behaviors induced by stress [140].
Iwata and collaborators showed that acute restraint stress can rapidly increase the hippocampal extracellular levels of glutamate and ATP as well as IL-1β and TNF-α [141]. In astrocytes cell culture, it was verified that glutamate promotes ATP release through these cells. After this, using several approaches, like P2X7 pharmacological blockade, Nlrp3 null mutation, and IL-1β neutralization, as well as other molecular and behavioral investigations, the authors proposed a pathway by which the innate immune system senses the damage in the brain. Basically, psychological stress promotes glutamate release which then stimulates the astrocytes to release ATP, being able to activate microglial P2X7 receptors. Once activated, P2X7 would allow microglial NLRP3 assembling with consequent activation and release of IL-1β, which will mainly promote the behavioral alterations induced by stress [141], as exposed above. Clinical and preclinical evidence of the NLRP3 inflammasome involvement in mental disorders is summarized in Table 1.
Table 1.
Clinical and Preclinical evidence of the NLRP3 inflammasome involvement in mental disorders.
| Clinical Studies | |||
|---|---|---|---|
| Results | Patients’ Conditions | Comparison | References |
| ↑ mRNA expression of NRLP3 and caspase-1 in PBMCs ↑ Protein expression of NRLP3 in PBMCs ↑ IL-1β and IL-18 serum levels |
Major depressive patients with suicide attempts | No treated- compared to amitryptiline treated-patients (dose range of 62.5 ± 12 mg/day) |
[131] |
| ↑ mRNA expression of NLRP3 in PBMCs ↑ Serum levels of IL-1β and IL-18 |
Major depressive patients with suicide attempts | No treated patients compared to treated-patients with several different antidepressants, fluoxetine (40 mg/day), paroxetine (40 mg/day), mianserin (30 mg/day), mirtazapine (30 mg/day), venlafaxine (30mg/day), desvenlafaxine (50 mg/day), amitryptiline (25 mg/day), imipramine (50 mg/day), agomelatine (25 mg/day) | [137] |
| Preclinical Studies | |||
| Stress-Induced Effects | Species/Strain | Model | References |
| ↑ NLRP3, caspase-1 active fraction, IL-1β, P2X7, and TLR2 protein levels in PFC ↑ CD11b integrin and Iba-1 in PFC ↑ co-localization between NLRP3 and Iba-1 in PFC ↓ GFAP expression in PFC ↓ Sucrose preference and body weight |
Male Wistar Rats | CUS for 12 weeks | [132] |
| ↑ NLRP3 expression, caspase-1 cleavage, IL-1β production in HIP ↑ Depressive-like behavior (FST, TST, SPT) |
Male C57Bl/6 mice | CUS for 5 weeks | [133] |
| ↑ Depressive-like behavior (FST, TST) ↑ NLRP3, cleaved form of caspase-1 pro-IL-1β and IL-1β in HIP ↑ Iba-1 positive cells in HIP ↑ co-localization between NLRP3 and Iba-1 in HIP |
Female Balb/c mice | LPS (5 mg/kg, i.p.) 24 hrs. before behavioral tests | [134] |
| ↑ Depressive-like behavior (FST) ↑ IL-1β, IL-6 in PFC ↓ IL-10 in PFC |
Male Wistar Rats | LPS (1 mg/kg) 2 hrs. before behavioral tests | [136] |
| ↑ Depressive-like behavior (SPT, TST) ↑ Corticosterone and IL-1β serum levels ↑ Caspase-1 activity and proteins levels of IL-1β, NLRP3, and ASC in HIP - Concomitant treatment with a caspase-1 inhibitor prevented the stress-induced effects (VX-765, 50mg/kg, i.p. for 4 weeks) |
Male BALB/c mice | CUS for 4 weeks | [138] |
| ↑ mRNA and protein levels of NLRP3 and IL-1β in the HIP of WT submitted to stress ↑ Adenosine, ATP, and number of activated microglia in HIP and PFC of WT submitted to stress ↑ Depressive-like behavior (SPT, FST, SIT, Food intake) of WT mice submitted to stress |
Male WT and Nlrp3−/− transgenic mice on a CB57BL/6 background | Restraint stress for 7 days | [139] |
| ↑ Cleaved caspase-1 and IL-1β proteins levels in the HIP of WT stressed mice ↑ mRNA levels of L-1β in microglia of WT stressed mice. ↑ Iba+ positive cells - Nlrp3−/− and pharmacological inhibition of NLRP3 (MCC950, 1mg/kg, i.p.) enhance fear extinction and attenuate anxiety-like behavior (EPM and OFT) |
Male WT and Nlrp3−/− transgenic mice on a CB57BL/6 background. | Contextual fear conditioning (15 intermittent inescapable electric foot shocks, 0.8 mA, 10 s with 10 s interval) |
[140] |
| ↑ Extracellular ATP, glutamate, and IL-1β levels in the HIP of WT stressed mice | Male Sprague Dawley Rats | Acute restraint stress for 60 min | [141] |
| ↑ Depressive- and Anxiety-like behaviors (SPT, NSFT, EPM) - Treatment with P2X7 antagonist (A-804598, 10 mg/kg, i.p.) for the last 28 days of the protocol was able to attenuate the alterations induced by stress |
Male Sprague Dawley Rats | CUS for 56 days | [141] |
Abbreviations: PBMCs: Peripheral blood mononuclear cells; mRNA: Messenger ribonucleic acid; PFC: Prefrontal cortex; HIP: Hippocampus; GFAP: Glial fibrillary acidic protein; CUS: Chronic unpredictable stress; FST: Forced swim test; TST: Tail suspension test; SPT: Sucrose preference test; EPM: Elevated plus maze; NSFT: Novelty suppressed feeding test; LPS: Lipopolysaccharide; WT: Wild-type mice; KO: Knockout mice.
Aside from all the evidence about the NLRP3 direct involvement in psychiatric and mental conditions in clinical and preclinical studies, it is important to mention that data regarding IL-1β also supports the platform involvement in these situations. In this way, microglia, NLRP3, and IL-1β would be the essential bridge between psychological stress and mental disorders [119].
3. POTENTIAL USE OF CBD TO ATTENUATE THE STRESS RESPONSE
Cannabidiol (CBD) is one of the more than 140 phytocannabinoids present in the plant Cannabis sativa. It lacks the psychotomimetic and addictive effects of delta-9-tetrahydrocannabinol, the main cannabinoid present in the plant (for review, see [142]). Preclinical studies have indicated that CBD can be useful to treat several psychiatric disorders, such as schizophrenia, anxiety, depression, post-traumatic stress disorder (PTSD), drug addiction, and obsessive-compulsive disorder (OCD) [143-145]. The clinical efficacy of CBD in these disorders still needs more data. Anxiolytic effects of CBD have been demonstrated in healthy subjects exposed to anxiogenic situations [146, 147] and patients with social anxiety [148, 149]. Likewise, a few clinical trials did show antipsychotic effects of CBD in patients with schizophrenia [150, 151]. Also, it reduced cue-induced anxiety and craving in heroin-dependent patients [152].
Although these psychiatric disorders show different pathophysiology and symptomatology, they all suffer from a significant stress influence [153]. Preclinical studies show that CBD attenuates stress consequences after acute or repeated administration [143, 154-156]. Therefore, a general anti-stress effect of CBD could help to explain its broad therapeutic action in psychiatric disorders.
More than 60 molecular targets have been proposed to account for CBD effects [143, 157, 158]. Most of these targets have been described using in vitro experimental approaches [158]. The number of studies investigating these mechanisms in vivo is still limited, particularly in humans.
Several mechanisms are involved in the anti-stress action of CBD. Campos and Guimaraes (2008) firstly suggested that CBD acute anxiolytic effects in rats depend on the facilitation of 5-HT1A-mediated neurotransmission [159]. After that, several works have related this mechanism to the anxiolytic, antidepressant, and anti-stress effects of CBD [143, 145, 156]. However, this picture was complicated by the acute CBD effects in animal models associated with obsessive-compulsive disorder [160, 161] and aversive memory extinction and reconsolidation [162, 163]. These effects depend on CB1 rather than 5-HT1A receptors. Moreover, mice exposed to daily unpredictable stressors (CUS, chronic unpredictable stress) presented increased anxiety-like behaviors associated with decreased hippocampal neurogenesis and synaptic remodeling (reduced number of dendritic spines and branching). These changes were also prevented by CB1 or CB2, but not by 5-HT1A, receptor antagonists [154, 155].
The interaction of CBD with CB1 and CB2 receptors seems to be rather complex. In vitro studies, although showing a low affinity for these receptors, indicated that CBD could act as an antagonist or inverse agonist on CB1 and CB2 receptors, respectively [158]. In agreement with this possibility, even at high doses, CBD cannot induce the cannabinoid tetrad [164], a result compatible with its lack of psychotomimetic and addictive effects in humans.
CBD has also been reported to inhibit, in vitro, the metabolism/uptake of anandamide, one of the major endocannabinoids [165]. In the CUS model, two-week daily treatment with CBD increased AEA levels and decreased FAAH expression in the mice hippocampus [154, 155]. In humans, an increase in AEA plasmatic concentrations was reported in patients with schizophrenia repeatedly treated with CBD. The clinical improvement of these patients was associated with this increase [150]. There is still the intriguing possibility that CB1- and 5-HT1A-mediated neurotransmission interact to produce the anti-stress effects of CBD. For example, Sartim and collaborators (2016) showed that the antidepressant-like effects of CBD injected into the medial prefrontal cortex depend on both receptors [166]. Moreover, the anti-aggressive effect of acute systemic CBD injection in social-isolated mice was also mediated by both receptors [167].
An attractive possibility to explain a large number of CBD molecular targets, including the CB1, CB2, and 5-HT1A receptors, and the FAAH enzyme has been recently put forward by Martin and colleagues. It was suggested that CBD modifies these targets by interacting with a cholesterol site on transmembrane proteins [168].
As will be extensively discussed below, in addition to the interaction with these proteins, the anti-inflammatory properties of CBD have also been associated with its therapeutic actions in psychiatric conditions [169-172].
3.1. CBD Effects in Models of Depression and Investigated Mechanisms
Despite many behavioral effects having been previously described for CBD, there was a lack of evidence regarding the antidepressant properties of CBD until 2010. The evidence that CBD could bind and activate 5-HT1A receptors [173], a major target for antidepressant drugs [174], and alleviate stress-induced effects in rodents [156] motivated the research on the possible antidepressant properties of CBD. Using a simple experimental approach, Zanelati and colleagues first demonstrated that CBD reduces the immobility time in Swiss mice exposed to the forced swimming test [175], a prototype antidepressant-like effect in this test [176]. Interestingly, CBD effects were blocked by pre-treatment with the 5-HT1A receptor antagonist (WAY100635)[175], thus indicating the participation of serotonergic mechanisms in CBD-induced antidepressant effects. In line with that, it was recently demonstrated that the effects of CBD in the FST are not observed in animals that have been depleted of brain serotonin [177]. Furthermore, the combination of sub-effective doses of CBD and fluoxetine (SSRI), but not desipramine (noradrenaline reuptake inhibitor), produced synergistic effects in Swiss mice tested in the FST [177].
The antidepressant-like properties of CBD have also been observed after repeated treatment, both in mice and rats submitted to the FST [178, 179]. Interestingly, the effective doses of CBD were associated with increased levels of serotonin and noradrenaline in the hippocampus [178].
CBD has also shown promising effects in animal models with better face and construct validity, such as the rat learned helplessness (LH) [180], the chronic mild stress model (CMS) [181], the olfactory bulbectomized rodent model (OBX) [182], and in the rat lines that are genetically vulnerable for the depressive phenotype, the Flinders Sensitive Line (FSL) [180, 183], and the Wistar-Kyoto [183, 184]. In the OBX model, the antidepressant effect of CBD was again associated with increased release of serotonin and glutamate in the prefrontal cortex and sensitivity to the systemic blockade of 5-HT1A receptors [182].
CBD has shown promising effects in both male and female Wistar-Kyoto, but only in male FSL rats [184], thus indicating that CBD effects may be gender-dependent. More recently, it was demonstrated that CBD effects in females depend on the species and the strain used, as well as the time of treatment [185]. Although similar observations have been described for antidepressant drugs [186], it is not yet clear what drives such differences between males and females in CBD effects.
Interestingly, in contrast to conventional monoaminergic antidepressants, recent studies have suggested that CBD promotes rapid and sustained antidepressant effects in different animal models [180, 182]. Moreover, CBD counteracted the side effects induced by the rapid-acting antidepressant ketamine, while promoting its antidepressant properties [187]. Similar to ketamine, the rapid effects of CBD were associated with a rapid increase in BDNF and synaptic proteins in the prefrontal cortex [180], indicating that CBD effects involve the rapid modulation of synaptic plasticity. The observation that the blockade of TrkB receptors abrogated the behavioral effects of CBD further confirmed the involvement of BDNF [188]. In line with such observations, the intermittent administration of CBD (once weekly/4 weeks) reversed the behavioral deficits induced by chronic mild stress in mice, along with decreased microglial activation and increased BDNF and synaptogenesis in the prefrontal cortex [181].
Chronic treatment with CBD is also known to induce behavioral effects associated with changes in neuroplasticity, primarily neurogenesis, and synaptogenesis [155, 189]. Interestingly, the effects of CBD on neurogenesis are likely to involve the regulation of endocannabinoid levels, since they are attenuated by concomitant treatment with CB1 and CB2 antagonists, but not by 5-HT1A antagonists [155]. To what extent neurogenesis contributes to the effects of chronic CBD treatment is not yet known, since CBD effects can occur at doses that do not promote neurogenesis [189]. Contrasting results have also been shown for monoaminergic antidepressants, and it has been argued that not all antidepressant effects are dependent on hippocampal neurogenesis [190].
Altogether, the findings suggest that CBD produces antidepressant effects after acute, repeated, and intermittent administration in rodent models. Moreover, the antidepressant effects of CBD involve changes in synaptic plasticity characterized by increased BDNF and synaptogenesis in the PFC (acutely) and neurogenesis in the hippocampus (chronically). However, the molecular mechanism involved in these effects is not yet known.
Despite the promising evidence in animal studies, the evidence in humans is limited and inconclusive. In a cross-sectional study on CBD users, it was found that CBD was effective in alleviating depressive symptoms [191]. More recently, it was reported that CBD attenuated depressive symptoms in medical care professionals experiencing burnout syndrome [192]. Randomized and placebo-controlled clinical trials are still needed to clarify the therapeutic potential of CBD in depression.
3.2. A Therapeutic Approach with Cannabidiol in Posttraumatic Stress Disorder (PTSD)
Maladaptive responses to a single or repeated traumatic event may lead to the development of PTSD [193]. Many authors consider PTSD as a heterogeneous disorder, containing several subtypes explained in part by the diversity of symptoms [194]. The actual neural basis of PTSD is not fully understood. However, abnormalities across several neurotransmitter systems in concert with non-uniform responses of these dysfunctional circuits are known to be part of its pathophysiology [195].
PTSD treatment remains a tricky challenge for many other psychiatric conditions. A pharmacological intervention focused on a single system or neurotransmission may not be very efficient because of the miscellaneous pattern of the disorder. Therefore, CBD emerges as a potential approach to prevent and treat PTSD since different mechanisms appear to be involved in its pharmacological profile, as already discussed [196]. The efficacy of CBD in modulating the emotional memory process, stress-related responses, and anxiety-like behavior, together with actionability on mood and cognition properties, encompasses most of the symptom clusters of the diagnostic criteria for PTSD according to DSM-5 [197]. The discussion about the therapeutic potential of CBD in the aspects mentioned above is beyond the scope of this review and can be found in several excellent reviews [145, 193, 198-202]. This broad-spectrum action supports and stimulates the development of studies focused on evaluating and understanding the potential efficacy of CBD in animal models of PTSD.
3.3. CBD Reducing PTSD-like Symptoms: Learning, Emotional Memory, and Stress-related Responses
Trauma memory is usually generated and consolidated by associating a particular stimulus or environment with harmful physical and affective consequences of trauma exposure. Albeit such mechanisms endorse survival, dysregulation of these physiological processes is one of the key components of PTSD development [203]. The conditioned fear paradigm is considered a translational neuroscience approach to understanding PTSD, as it is based on associative learning, in which a specific environment or cue becomes predictive of a threat and induces emotional responses. Moreover, neural circuits that support fear conditioning are related to brain systems that play critical roles in PTSD (for review, see [199]). It is crucial to mention that a recent review proposed a contemporary rereading of the PTSD and fear condition association, drawing a critical analysis on conceptual parameters and suggesting actionable perspectives to improve translational research in PTSD [204].
Several studies indicated that CBD regulates fear learning and memory processing in translationally relevant paradigms to stress-related psychiatric conditions, such as PTSD (for review see, [193, 205]). Accumulating evidence indicates that CBD regulates different stages of the aversive conditioning process, decreases threat-related responses when administered before the acquisition of fear memories [206, 207], reduces either cardiovascular responses and anxiogenic effects caused by aversive conditioned context, both after systemic [208] and specific brain regions administration right before the retrieval session [209, 210], diminishes the expression of auditory fear memory [211, 212], under intense conditioning procedure, decreases contextual freezing following a single 20-min-extinction training [213], potentiates contextual fear extinction with an intra-cerebroventricular or infralimbic cortex (IL) infusions before three 9-min-retrieval sessions [162, 214], and disrupts the reconsolidation of contextual fear memory after a brief retrieving [215], reaching a long-lasting reduction in learned fear expression. These last studies demonstrating beneficial CBD effects on fear extinction and reconsolidation highlight its potential use as an alternative for mitigating the consequences of traumatic life experiences since those aspects are significantly impacted in PTSD.
The CBD findings on disrupting fear memory reconsolidation and enhancing fear extinction are, at least in part CB1-mediated, substantiating the endocannabinoid (ECB) system's role in emotional memory processing [162, 214, 215]. The action of CBD on aversive memories responses, as already mentioned, may be attributable to the indirect potentiation of the ECB system.
Preclinical evidence suggests that CBD impaired aversive memory formation through systemic [163] and brain-specific region infusions [207, 216, 217], involving a complex circuit interaction. A recent study showed that CBD effects on memory consolidation relied on distinct time-dependent mechanisms across the consolidation process in the dorsal hippocampus. This mechanism initially involves CB1 and CB2 receptors activation, followed by a later engagement of the peroxisome proliferator-activated receptor-gamma, PPAR-γ). These data suggest that the activation of CB1 or CB2 receptors represents an important step in the initial aversive memory consolidation process. In contrast, the inflammatory-related mechanisms (via PPAR-γ) appear to be significant in the delayed-memory formation (1 hour) in this brain area [216].
PPAR-γ receptors expression is described in glial cells and regulates genes attributable to central and peripheral inflammation, metabolic pathways, and immune responses [218-220]. The immune system and glial activation seem to be involved in the formation, maintenance [221, 222], reconsolidation, and extinction of traumatic memory [223] in rodents studies. Moreover, exacerbated pro-inflammatory cytokines response (mainly IL-6) disrupting memory extinction suggests the involvement of immune mediators in the persistence of fear memory in PTSD [223].
Numerous results evaluating CBD effects on mitigating neuroimmune and inflammation processes are frequently associated with PPAR-γ receptors [172, 224, 225]. Moreover, CB1 and CB2 receptors are protective in many inflammatory conditions [226] (for review see, [227]) and limit stress-induced neuroinflammatory responses [228, 229]. In this scenario, it is possible to speculate that PPAR-γ receptors and the ECB system are cross-talking to promote a global anti-neuroinflammatory action [230]. Accordingly, Hill and colleagues (2018) proposed that a flawed ECB system might represent a stress-susceptibility endophenotype predisposing to trauma-related psychopathology development [205]. In this way, CBD modulatory effects on the ECB system could be essential for its therapeutic potential to treat PTSD.
As mentioned before, CBD attenuates contextual conditioned emotional responses, which involve the 5-HT1A receptors [209, 231], similar to other responses discussed in this review. The molecular mechanism of 5-HT1A receptors activation by CBD still needs to be clarified; however, evidence suggests that it may be related to directly targeting 5-HT1A receptors, acting via allosteric sites modulation, and/or enhancing 5-HT1A receptor activation through interference with intracellular signaling [173, 232].
Despite the general effect of CBD in reducing fear memory, studies are exhibiting opposing results [210, 213, 233]. The apparent contradiction could be attributed to distinct neuroanatomical mechanisms, the nature of the threat, and the controllability and intensity of the stress [234]. CBD directly injected into the PL or IL PFC before fear retrieval, decreased or increased contextual fear expression, respectively; both effects are mediated by the 5-HT1A receptor [231, 233]. A plausible explanation is related to the particular role these medial PFC subregions exert, including opposing influences on learned fear, under some conditions [235-237]. Moreover, it is important to address that despite being based on learning and memory, contextual fear conditioning involves uncontrollable stressful experiences, which appears to be relevant to CBD effects. Experiments with relatively low threat levels evidenced opposite results to those detected in the fear conditioning model: CBD microinjected into the PL or IL PFC induced anxiogenic- or anxiolytic-like effects in the elevated plus-maze (EPM) test, respectively. When a prior stressful event (uncontrollable restraint stress) was applied before the EPM test, CBD induced a similar response profile to those observed in the contextual fear conditioning [213, 231, 233]. Likewise, although CBD enhanced fear extinction memory through local intracranial infusions [162, 214], acute systemic CBD administration induced bidirectional effects in the fear memory process, causing extinction impairment after weak conditioning while potentiated extinction after intense conditioning protocol [213]. In summary, findings suggest that stress levels might be a vital component of CBD impact and genuinely aversive events seem to be necessary for the efficient performance of this phytocannabinoid.
In a converging line of evidence, Szkudlarek and coworkers (2019) showed that intra- PL PFC injection of CBD impaired cognitive flexibility and working memory in naive rats while reversing the cognition impairments in rodents submitted to a state of prefrontal cortical pathology. The 5-HT1A receptor mediated CBD effects. The authors indicated that CBD's dissociable roles in modulating neuropsychiatric symptoms in cognitive domains require an established pathological state for its therapeutic effect [238].
3.4. CBD in Animal Models of PTSD
A “traumatic” foot shock paradigm, considered a PTSD model and relevant to evaluate fear overgeneralization, demonstrated that CBD reversed the behavioral consequences assessed in the light-dark emergence test, a novel context. The study suggests that CBD might be more effective under intense stress situations than under minimal stress conditions since no effect was observed in the light-dark test with no prior threat. Apparently, the behavior consequences were not worsened in this case [239]. Recently, Assareh and collaborators (2020), using a similar paradigm, have shown that CBD could not counteract anxiety-related behavior increased by shock exposure in the light-dark test [211]. The authors suggested that the discrepancy could be explained by the employment of different species, although the stress intensity could be another explanation. The former study applied a more robust foot shock protocol with 6 delivered shocks, while the second research used a single-foot shock protocol.
Using a broadly employed PTSD-animal model, the predator-exposure paradigm, repeated treatment with CBD prevented the anxiogenic effect induced by predator exposure 7 days later. The anti-stress effect of CBD appears to involve, at least partly, 5-HT1A-mediated neurotransmission. The stress exposure elicited upregulation of 5-HT1A gene expression in the PFC and hippocampus, without any changes induced by CBD. The authors speculated that 5-HT1A receptors changes could facilitate the anti-stress effect of CBD since the behavioral outcomes were not observed in naïve groups [240].
In a similar PTSD model, the predator-scent stress exposure, animals expressing a susceptible behavioral phenotype were evaluated in different paradigms. Acute systemic treatment with CBD induced an anxiolytic-like response in the light-dark box test but could not reduce freezing behavior in the predator context. CBD mechanisms were not evaluated. Concerning the lack of effect in contextual fear extinction, the authors suggested the possibility that the predator-scent exposure was not sufficient to evoke the appropriate neuroadaptations for CBD-mediated effects. In other words, the stress intensity, once again, appears to be relevant for CBD performance in emotional memory-related responses [241].
Recently, Gasparyan and collaborators (2021) proposed a chronic animal model of PTSD consisting of an alternating and unpredictable 5-week regimen with a combination of physical, psychogenic, and psychosocial stress [242]. Basal behavioral evaluations were conducted in the two weeks following the stress regimen, displaying an increased (and persistent) fear memory expression, hyperarousal, anxiogenic-like behaviors, in concert with gene expression changes related to the HPA-axis, cannabinoid receptor, and 5-HT transporter. CBD potentiated the fear extinction process by decreasing freezing behavior under acute and chronic administration and also induced anxiolytic-like responses evaluated in unlearned or innate fear paradigms. CBD anti-stress effect appears to involve a reduction in hippocampal glucocorticoid receptor expression, an up and down-regulation of CB1 and CB2 receptors expression in the amygdala, respectively, and an increase in expression of the 5-HT transporter in the dorsal raphe nucleus. The authors observed that a combination of CBD and sertraline induced more pronounced behavior effects for most of the evaluated parameters and suggested that this synergic effect appears to be promising [242]. The complex biomarkers gene regulation involved in stress response might indicate that the multi-target nature of CBD could be the key to its multipurpose therapeutic effect.
A collection of evidence suggests that a state of neuroinflammation might be a significant contributor to the pathophysiology of PTSD [243]. Despite the yielded mixed results, probably due to the heterogeneous profile of the disorder, a systemic review and meta-analysis evaluation showed that PTSD is associated with elevated pro-inflammatory cytokines and white blood cell (WBC), encompassing high levels of IL-1β, IL-2, IL-6, IFN-γ, TNF-α, and C-reactive protein (CRP) [244]. Recent preclinical reports have verified that animal models of PTSD induce a critical status of neuroinflammation [245, 246]. A single-cell mass cytometry verified that microglia comprise the majority of mouse brain immune cells and the numbers were increased in a PTSD model. Authors identified that microglial morphology and count changed in a time- and brain region-dependent manner after traumatic stress exposure (foot shocks) and that the stress also increased serum and hippocampal pro-inflammatory cytokines levels [245, 246]. Interestingly, inhibition of microglia or substances that ameliorated PTSD-related behavior, including an SSRI antidepressant and a ketone body (suggested as an NLRP3 inflammasome inhibitor), also attenuated the neuroinflammation state [245, 246]. Consistent with the potential involvement of the NLRP3 inflammasome in the consequences of traumatic exposure, Dong and coworkers demonstrated that the NLRP3 inflammasome was activated 3hrs. after the foot shock stress. This was not observed in the NLRP3 KO mice. Moreover, the NLRP3 inhibition attenuated later anxiety behavior and deficits in fear extinction [140].
In light of the anti-inflammatory properties of CBD, together with the modulation of inflammatory pathways by most of the CBD proposed molecular targets (direct or indirectly), and the observed activation of NLRP3 inflammasome in different stress conditions, it can be suggested that these mechanisms might be deeply related to the therapeutic CBD performance on stress-related disorders, such as PTSD and major depressive disorders.
4. CBD AND NEUROINFLAMMATION
The anti-inflammatory and immunosuppressive properties of CBD are widely described [247-249]. Discussions about the pharmacological effects of drugs are usually complex due to controversial findings in the literature since studies use different techniques, methodologies, distinct time points evaluation, and doses. Despite this, the acute and chronic administration of CBD decreased pro-inflammatory cytokines in several murine models of immunological challenge [250-255] and in vitro studies with microglia and peripheral immune cells [256].
Several mechanisms are proposed to explain the anti-inflammatory effects of CBD. Among them, there is the activation of PPAR-γ receptors [172, 257], control of iNOS expression [253], antioxidant activity, including suppression of ROS production [258-260], suppression of infiltration and activation of macrophages and T cells [261], and deregulation of the nuclear factor of activated T cells (NFAT) [262]. Especially in the last decade, an increasing amount of data indicated that microglia can be involved in the effects induced by repeated treatment with CBD [36, 171]. Some of these mechanisms will be explored in the next sessions.
4.1. Inflammatory-related Mechanisms of CBD in Animal and In Vitro Models of Mental Disorders and CNS Diseases
The first report on the potential effect of CBD in neuroinflammation, addressing its ability to modulate microglial cell migration in vitro, is dated 2003 [263]. Some years later, one of the first in vivo studies showed that systemic administration of CBD decreases neuroinflammation induced by intrahippocampal injection of amyloid-β (Aβ), an animal model of Alzheimer’s disease, through downregulating GFAP, IL-1β, and iNOS levels in mice [253]. Moreover, CBD was later shown to counteract the cognitive impairment induced by intracerebroventricular injection of Aβ [264], or in a model of hepatic encephalopathy associated with hippocampal inflammation [265].
Studies from our group demonstrated that CBD prevents the increase of microglial activation in the PFC and hippocampus of mice exposed to repeated treatment with NMDA receptors antagonist, a preclinical model of schizophrenia [170]. To our knowledge, this was one of the first studies linking the effects of CBD repeated administration to microglia activation using an animal model of mental disorder. CDB was also effective in mouse models of tardive dyskinesia, induced by repeated treatment with haloperidol or L-DOPA. Haloperidol increased striatal IL-1β levels, and haloperidol-induced vacuous chewing movements positively correlated with increased striatal microglial activation, and CBD treatment prevented these effects. The behavioral effect of CBD was mediated by PPAR-γ receptors [172], similarly to the CBD behavioral effects in the L-DOPA model, which was also mediated by TRPV1 receptors [257]. In the haloperidol model, although isolated treatments (CBD or haloperidol) did not change IL-10 levels, an anti-inflammatory cytokine that negatively regulates the NLRP3 pathway [266], the combination of both drugs increased its levels [172]. CBD treatment also alleviates the clinical symptoms and disease progression in mice submitted to an experimental model of autoimmune encephalomyelitis. These CBD-induced effects are related to the impairment of axonal damage, inflammation, and microglia activation (Iba-1 and Mac-2/Galectin-3) in the spinal cord [267].
In vitro experiments with microglia demonstrated a negative effect of CBD on ATP-induced intracellular Ca2+ increase, inhibition of LPS-induced NO/nitrite generation, and inhibitory effect in microglial cell migration via cannabinoid and adenosine A2 receptors [264]. Furthermore, in vitro, the exposure to CBD prevented LPS-induced NF-kβ nuclear translocation [169, 172], microglial ROS production, and activation (increased Iba-1 expression), involving PPAR-γ receptors [172]. LPS-stimulated BV-2 microglial cells upregulated the expression of pro-inflammatory miRNAs related to inflammatory, cell cycle, and cellular stress pathways; CBD treatment provides a better inhibitory effect on these miRNAs expression by targeting NF-kβ and other pathways than THC [268]. CBD also demonstrated antioxidant properties in an in vitro study with higher potency than THC [258]. Moreover, its antioxidant properties against glutamate neurotoxicity were demonstrated in neuronal cultures, with a better protective profile than the antioxidants ascorbate and tocopherol [269].
Recently, it has been demonstrated that CBD directly binds to caspase-1 protein in human keratinocyte HaCaT cells, and several NLRP3 inflammasome-related genes were proposed as potential molecular targets for its effects. This data suggests that CBD acts as a caspase-1 activity inhibitor [270], at least in this model. Even though this study has used a preclinical model not related to the purpose of this section, the unraveled mechanism is undoubtedly essential to the scope of this review, addressing a potential effect of CBD in stress response by modulating the NLRP3 inflammasome.
Repeated treatment with CBD favors the autophagic process in CUS-exposed mice by increasing essential autophagy-related and -inductor signaling proteins, such as mTOR, Beclin-1, and LC3 [143]. Similar positive effects were described in the Alzheimer’s mice model APPS/PS1 [271], or the alcohol-related steatosis model [272]. Evidence from in vitro studies reinforced CBD's pro-autophagic profile [273-276]. Recently, an in vitro study has shown that CBD-induced autophagy in neural cells (human neuroblastoma and murine astrocyte lines) was independent of mTOR but dependent on the autophagic factor ULK1 and mediated by ERK1/2 activation and suppression of AKT [276]. Although these data evidence some contradictions in the mechanism involving or not mTOR, it is essential to point out that they are very different experiments (in vitro and in vivo). Even though, these works evidence the pro-autophagy effect of CBD.
In general, CBD treatment: 1) negatively influences transcription, translation, and/or IL-1β release, the main product of NLRP3 inflammasome activation, 2) decreases the microglial activation, the main source of NLRP3 inflammasome activity in the central nervous system, and 3) enhances the autophagic process, a widely described negative regulator for NLRP3-inflammasome activation. Despite indirect, all the above results mentioned in this section support the idea that the NLRP3-inflammasome pathway can be involved in the mechanism of action by which CBD exerts its therapeutical effects in some diseases [277], especially in mental disorders.
4.2. Evidence of NLRP3 Inflammasome Involvement in the Effects of CBD Exposure
It is already suggested that CBD and other synthetic and phytocannabinoids can regulate NLRP3 activation [277]. Our focus will remain on the experimental studies that investigated the direct effects of CBD exposure on NLRP3 inflammasome activation. However, they do not necessarily involve mental disorders and central nervous system diseases. To our knowledge, there are no published studies investigating the causal or mechanistic relation between CBD and NLRP3 inflammasome in microglial cells. However, their involvement was already studied in monocytes/macrophages and stem cells, which will be discussed below.
Recently, Jiang and collaborators have described that CBD treatment mitigated liver injuries induced by ethanol-associated with a high-fat, high-cholesterol diet (EHFD) for 8 weeks in mice. Treatment with CBD during EHFD alleviated the hepatic oxidative stress, activation of the NF-kβ pathway, number of CD68+cells (a marker for monocyte lineage cells, such as macrophages), gene expression of inflammatory cytokines, and the levels of core proteins involved in the NLRP3 inflammasome pathway in the liver, NLRP3, ASC, Caspase-1 (p20), and IL-1β [278]. The same group previously described that CBD treatment counteracted the severity of liver injuries and inflammation related to the NLRP3 pathway in a mouse model of nonalcoholic steatosis submitted to the same diet protocol (high-fat diet, HFD), concomitant with CBD treatment [279]. These data suggested that both HFD, with or without ethanol, promotes hepatic damage due to inflammatory conditions. CBD ameliorates these changes, possibly due to the inhibition of NF-kβ and NLRP3 inflammasome pathways.
Similarly, CBD impaired NF-kβ and NLRP3 inflammasome activation induced by LPS and ATP in a macrophage cell lineage (RAW264.7). Experiments with pharmacological inhibition suggest that the blockade of NF-kβ translocation into the nucleus is at least one of the mechanisms for CBD inhibiting NLRP3 inflammasome activation since it is crucial for platform activation [279]. Despite methodological differences, the same inhibitory effect of CBD on IL-1β supernatant levels [169, 280] and NF-kβ nuclear translocation [169, 172] were already described in microglial cell cultures and could involve the NRLP3 inflammasome pathway. This possibility, however, was not investigated in the referred studies [169, 172]. Moreover, considering reactive oxygen species (ROS) can trigger NLRP3 inflammasome activation [80, 105-108] and CBD has antioxidant properties [258-260, 269]. Moreover, decreasing ROS production could be another mechanism by which CBD attenuates NLRP3 inflammasome activation.
Another piece of evidence for the CBD effect on the NLRP3 inflammasome pathway came from studies on human gingival mesenchymal stem cells (hGMSCs). Pretreatment with CBD for 24 hrs. decreased gene and protein expression levels of NLRP3, Caspase-1, and IL-18 [281]. Mechanistically, the antagonism of CB1 blocked the CBD-induced effects in both NLRP3 inflammasome and NF-kβ expression. Therefore, the pretreatment of stem cells with CBD favors cell survival rather than inflammatory processes. Considering that stem cells present a high therapeutical and medical potential due to their enhanced differentiation capacity, pretreatment with CBD could help against implant rejection and increase the long-term efficacy of transplanted patients [281].
Moreover, the NRLP3 pathway also seems to mediate CBD effects in the SARS-Cov-2 infection context. An in vitro study suggested that the inhibitory effect of CBD on cytotoxicity and inflammation induced by SARS-Cov-2 spike protein is PPAR-γ dependent and involves the suppression of protein levels of NLRP3 inflammasome components [282].
Finally, Liu and collaborators reported an inhibitory effect of CBD on the NLRP3 inflammasome pathway by another mechanism. In LPS-nigericin-stimulated human THP-1 monocytes, CBD reduced IL-1β media concentration similarly to pharmacological NLRP3 inhibitors and the nigericin-LPS-induced K+ efflux. The suggested mechanism for these effects was obtained by computational docking data analysis and proposed to involve the direct bind of CBD to P2X7 receptors, which, in turn, reduces K+ efflux [283]. Although the referred study did not investigate the causality between CBD effects and P2X7 receptors, for example, with a P2X7 antagonist, to verify a possible inhibition of CBD effects on K+ efflux, the computational analysis result reinforces the hypothesis that P2X7 is the channel by which the K+ efflux occurs [79, 109]. Evidence of the involvement of NLRP3 inflammasome in CBD effects is summarized in Table 2.
Table 2.
Evidence of the NLRP3 inflammasome pathway involvement in CBD treatment effects.
| Model | Species/Strain | CBD Treatment | CBD Effects | Possible Mechanism of Action | References |
|---|---|---|---|---|---|
| Ethanol-associated with a high-fat, high-cholesterol diet for 8 weeks | Male C57B/6J mice | 5 mg/kg, daily gavage for 8 weeks | ↓ Hepatocellular lipid accumulation and inflammation ↓ Hepatic TC and TG ↓ Serum ALT ↓ Hepatic oxidative stress ↓ Hepatic MDA ↑ Hepatic GSH/GSSG ratio ↓ Number of CD68+ cells ↓ mRNA levels of inflammatory mediators (F4/80, IL-1β, MCP-1, and TNF-α) in the liver ↓ NF-kβ pathway activation (pIκBα, pNF-κB and the ratios pIκBα/IκBα, pNF-κB/NF-κB) ↓ NLRP3 Inflammasome activation in the liver (NLRP3, ASC, caspase-1 (p20), Gasdermin D, and IL-1β protein levels) |
Not determined | [274] |
| High-fat high-cholesterol diet for 8 weeks | Male C57B/6J mice | 5 mg/kg, daily gavage for 8 weeks | ↓ Hepatocellular lipid accumulation and inflammation ↓ Serum TC, TG, ALT ↓ Number of CD68+ cells ↓ mRNA levels of inflammatory mediators (F4/80, IL-1β, MCP-1, and TNF-α) in the liver ↓ NF-kβ pathway activation (pIκBα, pNF-κB and the ratios pIκBα/IκBα, pNF-κB/NF-κB) and its nuclear translocation ↓ NLRP3 Inflammasome activation in the liver (NLRP3, ASC, caspase-1 (p20), pro- IL-1β and IL-1β protein levels) |
Not determined | [275] |
| LPS and ATP stimulation | RAW264.7 cells | Pretreatment with CBD 5 μM (2 h) | ↓ NF-kβ pathway activation (pIκBα, pNF-κB and the ratios pIκBα/IκBα, pNF-κB/NF-κB) ↓ mRNA levels of inflammatory mediators (IL-1β, MCP-1, and TNF-α) ↓ NLRP3 Inflammasome activation in the liver (NLRP3, ASC, caspase-1 (p20), pro- IL-1β and IL-1β protein levels) |
Suppression of NF-kβ activation | [275] |
| Cellular pretreatment with CBD | Human gingival mesenchymal stem cells | CBD 5 μM (24 h) | ↓ Expression of pro-inflammatory genes ↓ Expression of genes and proteins related to the NLRP3 Inflammasome (NLRP3, caspase-1, IL-18) |
CB1 receptors | [277] |
| SARS-CoV-2 recombinant human novel coronavirus spike glycoprotein (S) | Caco-2 cell | CBD 10-9, 10-8, 10-7 M (24 h) | ↓ Cytotoxicity and Pyroptosis ↓ TLR4 and ACE-2 protein expression ↑ RhoA GTP ↓ NRLP3 and caspase-1 cytoplasmic protein ↓ TNF-α, IL-6, IL-1β and IL-18 secretion in supernatant ↓ Spike protein-induced epithelial damage |
PPAR-γ receptors | [278] |
| Nigericin and LPS-nigericin stimulation | Human THP-1 monocytes | CBD 1 and 10 μM (1 h) | ↓ IL-1β in supernatant ↓ K+ efflux (CBD 10 μM) |
A specific inhibitor of NLRP3 activation Direct bind to P2X7 |
[279] |
Abbreviations: CBD: Cannabidiol; TC: Hepatic total cholesterol; TG: Hepatic triglyceride; ALT: Serum alanine aminotransferase; MDA: Malondialdehyde; GSH: Reduced glutathione; GSSG: oxidized glutathione; MCP-1: Monocyte chemoattractant protein-1; LPS: Lipopolysaccharide; ATP: Adenosine triphosphate; ACE-2: Angiotensin-converting enzyme 2; RhoA GTP: Ras homolog A-GTPase, K+: Potassium.
CONCLUSION AND FUTURE PERSPECTIVES
The use of CBD to treat various medical problems has become increasingly popular. For example, in a recent survey, it has been estimated that 26% and 16% of USA and Canada respondents, respectively, have used CBD products in the last year [284]. The primary reason for this employment was the relief of anxiety, stress, insomnia, and pain symptoms [285]. However, this wild use, together with the legal regulatory confusion and the lack of pharmaceutical standardization between the hundreds of CBD products present in the market, has been worrying the scientific community [286]. Even so, as discussed above, several pre-clinical and clinical studies suggested that CBD can attenuate stress consequences by several mechanisms, including interference with neuroinflammation.
Neuroinflammation has been considered an important factor in stress reactivity, neuropsychiatric disorders, and treatment resistance. Therefore, in conditions where immune activation signals are observed, treatments targeting immune mechanisms, particularly neuroimmune ones, could be a better approach and improve treatment response.
Considering all the data presented and discussed in this review, it seems clear that the NLRP3 inflammasome inhibition is partially involved in the anti-inflammatory and potential therapeutical effects of CBD. The evidence implicating CBD as an anxiolytic, antidepressant, antipsychotic, and anti-inflammatory agent, among others, and that some effects are mediated by NLRP3 inflammasome, allow us to propose that this mechanism is also implicated in the anti-stress effects of CBD (Fig. 2). However, their relation and interaction in mental disorders and neurological diseases still need to be investigated appropriately. Most of the recent studies are pioneers on the topic and are usually conducted indirectly or are correlational. It is also essential to identify if the mechanisms in microglia are the same as in macrophages, considering that these last cells are the most studied cells in this topic.
Fig. (2).
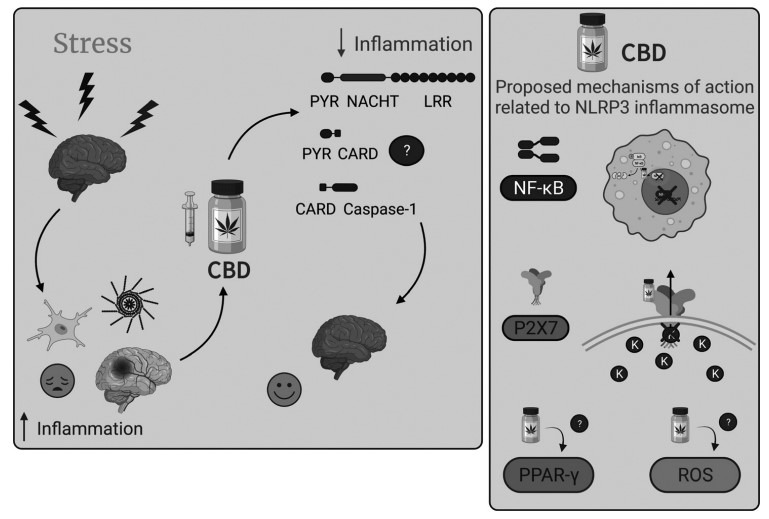
Stress-induced inflammation and the proposed mechanisms of action of CBD related to NLRP3 inflammasome. Left Panel. Stressful events are important risk factors for the development of mental disorders. One of the proposed theories to explain these effects relies on the increase of inflammatory processes in the brain. Microglia, the brain-resident immune cells, play a significant role in neuroinflammatory and neurodegenerative processes. The NLRP3 inflammasome pathway is the primary source of IL-1β, responsible for pro-IL-1β cleavage and activation in the CNS. The anti-stress and anti-inflammatory effects of CBD, the main non-psychotomimetic compound of the Cannabis sativa plant, have also been extensively described. Despite the absence of studies investigating the involvement of the microglial NLRP3 inflammasome pathway in the mechanisms by which CBD improves stress-related conditions, there is increasing evidence suggesting that the NLRP3 inflammasome is involved in the mechanism by which CBD reduces inflammatory processes. In this way, more studies are necessary to verify if NLRP3 is involved in the mechanism of action of CBD and the improvement of behavioral alterations induced by stress – Does it happen due to the disassembling of the NLRP3 inflammasome components? Is it through a direct or indirect interaction? Right Panel. Proposed mechanisms of action of CBD that involve the NLRP3 inflammasome pathway. In macrophages, CBD appears to inhibit the nuclear translocation of NF-kB and, consequently, reduces the NLRP3 inflammation assembling and activation. A molecular docking study suggests that CBD binds to P2X7 receptors and blocks K+ efflux, a critical step in promoting platform oligomerization and activation. There are also reports on PPAR-γ and ROS involvement in the mechanism by which CBD decreases NLRP3 inflammasome activation, although the exact mechanism involved in these effects is less precise than the other two described. CNS: Central nervous system; CBD: Cannabidiol. Images created with BioRender.com.
Due to the wide range of targets by which the phytocannabinoid CBD exerts its effects, causal studies are needed to understand their interactions better. It is relevant, curious, and outstanding prospect that CBD can also influence innate immune responses to treat diseases/disorders and improve clinical conditions, especially those related to inflammatory states. Therefore, we hope these remaining questions can be well addressed in the following years and help improve the treatment and quality of life of unresponsive-treatment patients, especially in the neuropsychiatric field.
ACKNOWLEDGEMENTS
Declared none.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
S.F. Lisboa received a fellowship from FAPESP (2017/19731-6), CNPq (420818-2018-9), and L’Oreal/ABC For Women in Science. F.S. Guimarães and S.R.L. Joca received a fellowship from FAPESP (2017/24304-0). S.R.L. Joca received a fellowship from Aarhus University Research Foundation (AUFF-E-2020-7-19). A.Hartmann received a fellowship from FAPESP (2016/14282-6).
CONFLICT OF INTEREST
Francisco S. Guimarães is a co-inventor (Mechoulam R, JC, Guimaraes FS, AZ, JH, Breuer A) of the patent “Fluorinated CBD compounds, compositions and uses thereof. Pub. No.: WO/2014/108899. International Application No.: PCT/IL2014/050023” Def. US no. Reg. 62193296; 29/07/2015; INPI on 19/08/2015 (BR1120150164927). The University of São Paulo has licensed the patent to Phytecs Pharm (USP Resolution No. 15.1.130002.1.1). The University of São Paulo has an agreement with Prati-Donaduzzi (Toledo, Brazil) to “develop a pharmaceutical product containing synthetic cannabidiol and prove its safety and therapeutic efficacy in the treatment of epilepsy, schizophrenia, Parkinson's disease, and anxiety disorders.”
REFERENCES
- 1.Franklin T.C., Xu C., Duman R.S. Depression and sterile inflammation: Essential role of danger associated molecular patterns. Brain Behav. Immun. 2018;72:2–13. doi: 10.1016/j.bbi.2017.10.025. [DOI] [PubMed] [Google Scholar]
- 2.Jones K.A., Thomsen C. The role of the innate immune system in psychiatric disorders. Mol. Cell. Neurosci. 2013;53:52–62. doi: 10.1016/j.mcn.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 3.Pape K., Tamouza R., Leboyer M., Zipp F. Immunoneuropsychiatry - novel perspectives on brain disorders. Nat. Rev. Neurol. 2019;15(6):317–328. doi: 10.1038/s41582-019-0174-4. [DOI] [PubMed] [Google Scholar]
- 4.Troubat R., Barone P., Leman S., Desmidt T., Cressant A., Atanasova B., Brizard B., El Hage W., Surget A., Belzung C., Camus V. Neuroinflammation and depression: A review. Eur. J. Neurosci. 2021;53(1):151–171. doi: 10.1111/ejn.14720. [DOI] [PubMed] [Google Scholar]
- 5.Lasselin J., Schedlowski M., Karshikoff B., Engler H., Lekander M., Konsman J.P. Comparison of bacterial lipopolysaccharide-induced sickness behavior in rodents and humans: Relevance for symptoms of anxiety and depression. Neurosci. Biobehav. Rev. 2020;115:15–24. doi: 10.1016/j.neubiorev.2020.05.001. [DOI] [PubMed] [Google Scholar]
- 6.Raison C.L., Capuron L., Miller A.H. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27(1):24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raison C.L., Dantzer R., Kelley K.W., Lawson M.A., Woolwine B.J., Vogt G., Spivey J.R., Saito K., Miller A.H. CSF concentrations of brain tryptophan and kynurenines during immune stimulation with IFN-alpha: relationship to CNS immune responses and depression. Mol. Psychiatry. 2010;15(4):393–403. doi: 10.1038/mp.2009.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wichers M.C., Koek G.H., Robaeys G., Verkerk R., Scharpé S., Maes M. IDO and interferon-alpha-induced depressive symptoms: a shift in hypothesis from tryptophan depletion to neurotoxicity. Mol. Psychiatry. 2005;10(6):538–544. doi: 10.1038/sj.mp.4001600. [DOI] [PubMed] [Google Scholar]
- 9.Kappelmann N., Lewis G., Dantzer R., Jones P.B., Khandaker G.M. Antidepressant activity of anti-cytokine treatment: a systematic review and meta-analysis of clinical trials of chronic inflammatory conditions. Mol. Psychiatry. 2018;23(2):335–343. doi: 10.1038/mp.2016.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dahl J., Ormstad H., Aass H.C., Malt U.F., Bendz L.T., Sandvik L., Brundin L., Andreassen O.A. The plasma levels of various cytokines are increased during ongoing depression and are reduced to normal levels after recovery. Psychoneuroendocrinology. 2014;45:77–86. doi: 10.1016/j.psyneuen.2014.03.019. [DOI] [PubMed] [Google Scholar]
- 11.Lindqvist D., Wolkowitz O.M., Mellon S., Yehuda R., Flory J.D., Henn-Haase C., Bierer L.M., Abu-Amara D., Coy M., Neylan T.C., Makotkine I., Reus V.I., Yan X., Taylor N.M., Marmar C.R., Dhabhar F.S. Proinflammatory milieu in combat-related PTSD is independent of depression and early life stress. Brain Behav. Immun. 2014;42:81–88. doi: 10.1016/j.bbi.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 12.Haapakoski R., Mathieu J., Ebmeier K.P., Alenius H., Kivimäki M. Cumulative meta-analysis of interleukins 6 and 1β tumour necrosis factor α and C-reactive protein in patients with major depressive disorder. Brain Behav. Immun. 2015;49:206–215. doi: 10.1016/j.bbi.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lamers F., Milaneschi Y., Smit J.H., Schoevers R.A., Wittenberg G., Penninx B.W.J.H. Longitudinal association between depression and inflammatory markers: results from the netherlands study of depression and anxiety. Biol. Psychiatry. 2019;85(10):829–837. doi: 10.1016/j.biopsych.2018.12.020. [DOI] [PubMed] [Google Scholar]
- 14.Zalli A., Jovanova O., Hoogendijk W.J., Tiemeier H., Carvalho L.A. Low-grade inflammation predicts persistence of depressive symptoms. Psychopharmacology (Berl.) 2016;233(9):1669–1678. doi: 10.1007/s00213-015-3919-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beurel E., Toups M., Nemeroff C.B. The bidirectional relationship of depression and inflammation: double trouble. Neuron. 2020;107(2):234–256. doi: 10.1016/j.neuron.2020.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cattaneo A., Gennarelli M., Uher R., Breen G., Farmer A., Aitchison K.J., Craig I.W., Anacker C., Zunsztain P.A., McGuffin P., Pariante C.M. Candidate genes expression profile associated with antidepressants response in the GENDEP study: differentiating between baseline ‘predictors’ and longitudinal ‘targets’. Neuropsychopharmacology. 2013;38(3):377–385. doi: 10.1038/npp.2012.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haroon E., Raison C.L., Miller A.H. Psychoneuroimmunology meets neuropsychopharmacology: translational implications of the impact of inflammation on behavior. Neuropsychopharmacology. 2012;37(1):137–162. doi: 10.1038/npp.2011.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hodes G.E., Pfau M.L., Leboeuf M., Golden S.A., Christoffel D.J., Bregman D., Rebusi N., Heshmati M., Aleyasin H., Warren B.L., Lebonté B., Horn S., Lapidus K.A., Stelzhammer V., Wong E.H., Bahn S., Krishnan V., Bolaños-Guzman C.A., Murrough J.W., Merad M., Russo S.J. Individual differences in the peripheral immune system promote resilience versus susceptibility to social stress. Proc. Natl. Acad. Sci. USA. 2014;111(45):16136–16141. doi: 10.1073/pnas.1415191111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lanquillon S., Krieg J.C., Bening-Abu-Shach U., Vedder H. Cytokine production and treatment response in major depressive disorder. Neuropsychopharmacology. 2000;22(4):370–379. doi: 10.1016/S0893-133X(99)00134-7. [DOI] [PubMed] [Google Scholar]
- 20.Sutcigil L., Oktenli C., Musabak U., Bozkurt A., Cansever A., Uzun O., Sanisoglu S.Y., Yesilova Z., Ozmenler N., Ozsahin A., Sengul A. Pro- and anti-inflammatory cytokine balance in major depression: effect of sertraline therapy. Clin. Dev. Immunol. 2007;2007:76396. doi: 10.1155/2007/76396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsao C.W., Lin Y.S., Chen C.C., Bai C.H., Wu S.R. Cytokines and serotonin transporter in patients with major depression. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2006;30(5):899–905. doi: 10.1016/j.pnpbp.2006.01.029. [DOI] [PubMed] [Google Scholar]
- 22.Tuglu C., Kara S.H., Caliyurt O., Vardar E., Abay E. Increased serum tumor necrosis factor-alpha levels and treatment response in major depressive disorder. Psychopharmacology (Berl.) 2003;170(4):429–433. doi: 10.1007/s00213-003-1566-z. [DOI] [PubMed] [Google Scholar]
- 23.Köhler O., Benros M.E., Nordentoft M., Farkouh M.E., Iyengar R.L., Mors O., Krogh J. Effect of anti-inflammatory treatment on depression, depressive symptoms, and adverse effects: a systematic review and meta-analysis of randomized clinical trials. JAMA Psychiatry. 2014;71(12):1381–1391. doi: 10.1001/jamapsychiatry.2014.1611. [DOI] [PubMed] [Google Scholar]
- 24.Więdłocha, M.; Marcinowicz, P.; Krupa, R.; Janoska-Jaździk, M.; Janus, M.; Dębowska, W.; Mosiołek, A.; Waszkiewicz, N.; Szulc, A. Effect of antidepressant treatment on peripheral inflammation markers - A meta-analysis. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2018;80(Pt C):217–226. doi: 10.1016/j.pnpbp.2017.04.026. [DOI] [PubMed] [Google Scholar]
- 25.Bai S., Guo W., Feng Y., Deng H., Li G., Nie H., Guo G., Yu H., Ma Y., Wang J., Chen S., Jing J., Yang J., Tang Y., Tang Z. Efficacy and safety of anti-inflammatory agents for the treatment of major depressive disorder: a systematic review and meta-analysis of randomised controlled trials. J. Neurol. Neurosurg. Psychiatry. 2020;91(1):21–32. doi: 10.1136/jnnp-2019-320912. [DOI] [PubMed] [Google Scholar]
- 26.Mansur R.B., Delgado-Peraza F., Subramaniapillai M., Lee Y., Iacobucci M., Rodrigues N., Rosenblat J.D., Brietzke E., Cosgrove V.E., Kramer N.E., Suppes T., Raison C.L., Chawla S., Nogueras-Ortiz C., McIntyre R.S., Kapogiannis D. Extracellular vesicle biomarkers reveal inhibition of neuroinflammation by infliximab in association with antidepressant response in adults with bipolar depression. Cells. 2020;9(4):E895. doi: 10.3390/cells9040895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaufmann F.N., Costa A.P., Ghisleni G., Diaz A.P., Rodrigues A.L.S., Peluffo H., Kaster M.P. NLRP3 inflammasome-driven pathways in depression: Clinical and preclinical findings. Brain Behav. Immun. 2017;64:367–383. doi: 10.1016/j.bbi.2017.03.002. [DOI] [PubMed] [Google Scholar]
- 28.Mondelli V., Vernon A.C., Turkheimer F., Dazzan P., Pariante C.M. Brain microglia in psychiatric disorders. Lancet Psychiatry. 2017;4(7):563–572. doi: 10.1016/S2215-0366(17)30101-3. [DOI] [PubMed] [Google Scholar]
- 29.Stevenson R., Samokhina E., Rossetti I., Morley J.W., Buskila Y. Neuromodulation of glial function during neurodegeneration. Front. Cell. Neurosci. 2020;14:278. doi: 10.3389/fncel.2020.00278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Da Mesquita S., Fu Z., Kipnis J. The meningeal lymphatic system: A new player in neurophysiology. Neuron. 2018;100(2):375–388. doi: 10.1016/j.neuron.2018.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen G.Y., Nuñez G. Sterile inflammation: sensing and reacting to damage. Nat. Rev. Immunol. 2010;10(12):826–837. doi: 10.1038/nri2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fleshner M., Frank M., Maier S.F. Danger signals and inflammasomes: stress-evoked sterile inflammation in mood disorders. Neuropsychopharmacology. 2017;42(1):36–45. doi: 10.1038/npp.2016.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salvador A.F., de Lima K.A., Kipnis J. Neuromodulation by the immune system: a focus on cytokines. Nat. Rev. Immunol. 2021;21(8):526–541. doi: 10.1038/s41577-021-00508-z. [DOI] [PubMed] [Google Scholar]
- 34.Liu X., Nemeth D.P., McKim D.B., Zhu L., DiSabato D.J., Berdysz O., Gorantla G., Oliver B., Witcher K.G., Wang Y., Negray C.E., Vegesna R.S., Sheridan J.F., Godbout J.P., Robson M.J., Blakely R.D., Popovich P.G., Bilbo S.D., Quan N. Cell-type-specific interleukin 1 receptor 1 signaling in the brain regulates distinct neuroimmune activities. Immunity. 2019;50(2):317–333.e6. doi: 10.1016/j.immuni.2018.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kipnis J., Filiano A.J. Neuroimmunology in 2017: The central nervous system: privileged by immune connections. Nat. Rev. Immunol. 2018;18(2):83–84. doi: 10.1038/nri.2017.152. [DOI] [PubMed] [Google Scholar]
- 36.Lisboa S.F., Gomes F.V., Guimaraes F.S., Campos A.C. Microglial cells as a link between cannabinoids and the immune hypothesis of psychiatric disorders. Front. Neurol. 2016;7:5. doi: 10.3389/fneur.2016.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ginhoux F., Prinz M. Origin of microglia: current concepts and past controversies. Cold Spring Harb. Perspect. Biol. 2015;7(8):a020537. doi: 10.1101/cshperspect.a020537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Prinz M., Jung S., Priller J. Microglia biology: One century of evolving concepts. Cell. 2019;179(2):292–311. doi: 10.1016/j.cell.2019.08.053. [DOI] [PubMed] [Google Scholar]
- 39.Jurgens H.A., Johnson R.W. Dysregulated neuronal-microglial cross-talk during aging, stress and inflammation. Exp. Neurol. 2012;233(1):40–48. doi: 10.1016/j.expneurol.2010.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Holmes S.E., Hinz R., Conen S., Gregory C.J., Matthews J.C., Anton-Rodriguez J.M., Gerhard A., Talbot P.S. Elevated translocator protein in anterior cingulate in major depression and a role for inflammation in suicidal thinking: A positron emission tomography study. Biol. Psychiatry. 2018;83(1):61–69. doi: 10.1016/j.biopsych.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 41.Steiner J., Walter M., Gos T., Guillemin G.J., Bernstein H.G., Sarnyai Z., Mawrin C., Brisch R., Bielau H., Meyer zu Schwabedissen L., Bogerts B., Myint A.M. Severe depression is associated with increased microglial quinolinic acid in subregions of the anterior cingulate gyrus: evidence for an immune-modulated glutamatergic neurotransmission? J. Neuroinflammation. 2011;8(1):94. doi: 10.1186/1742-2094-8-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Torres-Platas S.G., Cruceanu C., Chen G.G., Turecki G., Mechawar N. Evidence for increased microglial priming and macrophage recruitment in the dorsal anterior cingulate white matter of depressed suicides. Brain Behav. Immun. 2014;42:50–59. doi: 10.1016/j.bbi.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 43.Hori H., Kim Y. Inflammation and post-traumatic stress disorder. Psychiatry Clin. Neurosci. 2019;73(4):143–153. doi: 10.1111/pcn.12820. [DOI] [PubMed] [Google Scholar]
- 44.Busse M., Busse S., Myint A.M., Gos T., Dobrowolny H., Müller U.J., Bogerts B., Bernstein H.G., Steiner J. Decreased quinolinic acid in the hippocampus of depressive patients: evidence for local anti-inflammatory and neuroprotective responses? Eur. Arch. Psychiatry Clin. Neurosci. 2015;265(4):321–329. doi: 10.1007/s00406-014-0562-0. [DOI] [PubMed] [Google Scholar]
- 45.Setiawan E., Wilson A.A., Mizrahi R., Rusjan P.M., Miler L., Rajkowska G., Suridjan I., Kennedy J.L., Rekkas P.V., Houle S., Meyer J.H. Role of translocator protein density, a marker of neuroinflammation, in the brain during major depressive episodes. JAMA Psychiatry. 2015;72(3):268–275. doi: 10.1001/jamapsychiatry.2014.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dean O.M., Data-Franco J., Giorlando F., Berk M. Minocycline: therapeutic potential in psychiatry. CNS Drugs. 2012;26(5):391–401. doi: 10.2165/11632000-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 47.Husain M.I., Chaudhry I.B., Husain N., Khoso A.B., Rahman R.R., Hamirani M.M., Hodsoll J., Qurashi I., Deakin J.F., Young A.H. Minocycline as an adjunct for treatment-resistant depressive symptoms: A pilot randomised placebo-controlled trial. J. Psychopharmacol. 2017;31(9):1166–1175. doi: 10.1177/0269881117724352. [DOI] [PubMed] [Google Scholar]
- 48.Kato T.A., Watabe M., Tsuboi S., Ishikawa K., Hashiya K., Monji A., Utsumi H., Kanba S. Minocycline modulates human social decision-making: possible impact of microglia on personality-oriented social behaviors. PLoS One. 2012;7(7):e40461. doi: 10.1371/journal.pone.0040461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Soczynska J.K., Mansur R.B., Brietzke E., Swardfager W., Kennedy S.H., Woldeyohannes H.O., Powell A.M., Manierka M.S., McIntyre R.S. Novel therapeutic targets in depression: minocycline as a candidate treatment. Behav. Brain Res. 2012;235(2):302–317. doi: 10.1016/j.bbr.2012.07.026. [DOI] [PubMed] [Google Scholar]
- 50.Bassett B., Subramaniyam S., Fan Y., Varney S., Pan H., Carneiro A.M.D., Chung C.Y. Minocycline alleviates depression-like symptoms by rescuing decrease in neurogenesis in dorsal hippocampus via blocking microglia activation/phagocytosis. Brain Behav. Immun. 2021;91:519–530. doi: 10.1016/j.bbi.2020.11.009. [DOI] [PubMed] [Google Scholar]
- 51.Liu H.Y., Yue J., Hu L.N., Cheng L.F., Wang X.S., Wang X.J., Feng B. Chronic minocycline treatment reduces the anxiety-like behaviors induced by repeated restraint stress through modulating neuroinflammation. Brain Res. Bull. 2018;143:19–26. doi: 10.1016/j.brainresbull.2018.08.015. [DOI] [PubMed] [Google Scholar]
- 52.Henry C.J., Huang Y., Wynne A., Hanke M., Himler J., Bailey M.T., Sheridan J.F., Godbout J.P. Minocycline attenuates lipopolysaccharide (LPS)-induced neuroinflammation, sickness behavior, and anhedonia. J. Neuroinflammation. 2008;5(1):15. doi: 10.1186/1742-2094-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Miura H., Ando Y., Noda Y., Ozaki N., Isobe K. Effects of minocycline on changes in brain tryptophan metabolism and the behavior of juvenile mice elicited by inescapable-predator stress. J. Trauma. Stress Disord. Treat. 2013;2(3):7. doi: 10.4172/2324-8947.1000107. [DOI] [Google Scholar]
- 54.Wang B., Huang X., Pan X., Zhang T., Hou C., Su W.J., Liu L.L., Li J.M., Wang Y.X. Minocycline prevents the depressive-like behavior through inhibiting the release of HMGB1 from microglia and neurons. Brain Behav. Immun. 2020;88:132–143. doi: 10.1016/j.bbi.2020.06.019. [DOI] [PubMed] [Google Scholar]
- 55.Bhattacharya A., Drevets W.C. Role of neuro-immunological factors in the pathophysiology of mood disorders: Implications for novel therapeutics for treatment resistant depression. Curr. Top. Behav. Neurosci. 2017;31:339–356. doi: 10.1007/7854_2016_43. [DOI] [PubMed] [Google Scholar]
- 56.Schroder K., Tschopp J. The inflammasomes. Cell. 2010;140(6):821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 57.Swanson K.V., Deng M., Ting J.P. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 2019;19(8):477–489. doi: 10.1038/s41577-019-0165-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Martinon F., Burns K., Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol. Cell. 2002;10(2):417–426. doi: 10.1016/S1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 59.He Y., Hara H., Núñez G. Mechanism and regulation of NLRP3 inflammasome activation. Trends Biochem. Sci. 2016;41(12):1012–1021. doi: 10.1016/j.tibs.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee S., Suh G.Y., Ryter S.W., Choi A.M. Regulation and function of the nucleotide binding domain leucine-rich repeat-containing receptor, pyrin domain-containing-3 inflammasome in lung disease. Am. J. Respir. Cell Mol. Biol. 2016;54(2):151–160. doi: 10.1165/rcmb.2015-0231TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Martinon F., Mayor A., Tschopp J. The inflammasomes: guardians of the body. Annu. Rev. Immunol. 2009;27(1):229–265. doi: 10.1146/annurev.immunol.021908.132715. [DOI] [PubMed] [Google Scholar]
- 62.Bauernfeind F.G., Horvath G., Stutz A., Alnemri E.S., MacDonald K., Speert D., Fernandes-Alnemri T., Wu J., Monks B.G., Fitzgerald K.A., Hornung V., Latz E. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J. Immunol. 2009;183(2):787–791. doi: 10.4049/jimmunol.0901363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Franchi L., Eigenbrod T., Núñez G. Cutting edge: TNF-alpha mediates sensitization to ATP and silica via the NLRP3 inflammasome in the absence of microbial stimulation. J. Immunol. 2009;183(2):792–796. doi: 10.4049/jimmunol.0900173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Harder J., Franchi L., Muñoz-Planillo R., Park J.H., Reimer T., Núñez G. Activation of the Nlrp3 inflammasome by Streptococcus pyogenes requires streptolysin O and NF-kappa B activation but proceeds independently of TLR signaling and P2X7 receptor. J. Immunol. 2009;183(9):5823–5829. doi: 10.4049/jimmunol.0900444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xing Y., Yao X., Li H., Xue G., Guo Q., Yang G., An L., Zhang Y., Meng G. Cutting Edge: TRAF6 Mediates TLR/IL-1R Signaling-Induced Nontranscriptional Priming of the NLRP3 Inflammasome. J. Immunol. 2017;199(5):1561–1566. doi: 10.4049/jimmunol.1700175. [DOI] [PubMed] [Google Scholar]
- 66.Barry R., John S.W., Liccardi G., Tenev T., Jaco I., Chen C.H., Choi J., Kasperkiewicz P., Fernandes-Alnemri T., Alnemri E., Drag M., Chen Y., Meier P. SUMO-mediated regulation of NLRP3 modulates inflammasome activity. Nat. Commun. 2018;9(1):3001. doi: 10.1038/s41467-018-05321-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Guo C., Xie S., Chi Z., Zhang J., Liu Y., Zhang L., Zheng M., Zhang X., Xia D., Ke Y., Lu L., Wang D. Bile acids control inflammation and metabolic disorder through inhibition of NLRP3 inflammasome. Immunity. 2016;45(4):802–816. doi: 10.1016/j.immuni.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 68.Han S., Lear T.B., Jerome J.A., Rajbhandari S., Snavely C.A., Gulick D.L., Gibson K.F., Zou C., Chen B.B., Mallampalli R.K. Lipopolysaccharide primes the NALP3 inflammasome by inhibiting its ubiquitination and degradation mediated by the SCFFBXL2 E3 ligase. J. Biol. Chem. 2015;290(29):18124–18133. doi: 10.1074/jbc.M115.645549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Juliana C., Fernandes-Alnemri T., Kang S., Farias A., Qin F., Alnemri E.S. Non-transcriptional priming and deubiquitination regulate NLRP3 inflammasome activation. J. Biol. Chem. 2012;287(43):36617–36622. doi: 10.1074/jbc.M112.407130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Py B.F., Kim M.S., Vakifahmetoglu-Norberg H., Yuan J. Deubiquitination of NLRP3 by BRCC3 critically regulates inflammasome activity. Mol. Cell. 2013;49(2):331–338. doi: 10.1016/j.molcel.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 71.Rodgers M.A., Bowman J.W., Fujita H., Orazio N., Shi M., Liang Q., Amatya R., Kelly T.J., Iwai K., Ting J., Jung J.U. The linear ubiquitin assembly complex (LUBAC) is essential for NLRP3 inflammasome activation. J. Exp. Med. 2014;211(7):1333–1347. doi: 10.1084/jem.20132486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Song H., Liu B., Huai W., Yu Z., Wang W., Zhao J., Han L., Jiang G., Zhang L., Gao C., Zhao W. The E3 ubiquitin ligase TRIM31 attenuates NLRP3 inflammasome activation by promoting proteasomal degradation of NLRP3. Nat. Commun. 2016;7(1):13727. doi: 10.1038/ncomms13727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Song N., Liu Z.S., Xue W., Bai Z.F., Wang Q.Y., Dai J., Liu X., Huang Y.J., Cai H., Zhan X.Y., Han Q.Y., Wang H., Chen Y., Li H.Y., Li A.L., Zhang X.M., Zhou T., Li T. NLRP3 Phosphorylation Is an Essential Priming Event for Inflammasome Activation. Mol. Cell. 2017;68(1):185–197.e6. doi: 10.1016/j.molcel.2017.08.017. [DOI] [PubMed] [Google Scholar]
- 74.Stutz A., Kolbe C.C., Stahl R., Horvath G.L., Franklin B.S., van Ray O., Brinkschulte R., Geyer M., Meissner F., Latz E. NLRP3 inflammasome assembly is regulated by phosphorylation of the pyrin domain. J. Exp. Med. 2017;214(6):1725–1736. doi: 10.1084/jem.20160933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang Z., Meszaros G., He W.T., Xu Y., de Fatima Magliarelli H., Mailly L., Mihlan M., Liu Y., Puig Gámez M., Goginashvili A., Pasquier A., Bielska O., Neven B., Quartier P., Aebersold R., Baumert T.F., Georgel P., Han J., Ricci R. Protein kinase D at the Golgi controls NLRP3 inflammasome activation. J. Exp. Med. 2017;214(9):2671–2693. doi: 10.1084/jem.20162040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jo E.K., Kim J.K., Shin D.M., Sasakawa C. Molecular mechanisms regulating NLRP3 inflammasome activation. Cell. Mol. Immunol. 2016;13(2):148–159. doi: 10.1038/cmi.2015.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kelley N., Jeltema D., Duan Y., He Y. The NLRP3 inflammasome: An overview of mechanisms of activation and regulation. Int. J. Mol. Sci. 2019;20(13):E3328. doi: 10.3390/ijms20133328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sutterwala F.S., Haasken S., Cassel S.L. Mechanism of NLRP3 inflammasome activation. Ann. N. Y. Acad. Sci. 2014;1319(1):82–95. doi: 10.1111/nyas.12458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Perregaux D., Gabel C.A. Interleukin-1 beta maturation and release in response to ATP and nigericin. Evidence that potassium depletion mediated by these agents is a necessary and common feature of their activity. J. Biol. Chem. 1994;269(21):15195–15203. doi: 10.1016/S0021-9258(17)36591-2. [DOI] [PubMed] [Google Scholar]
- 80.Shimada K., Crother T.R., Karlin J., Dagvadorj J., Chiba N., Chen S., Ramanujan V.K., Wolf A.J., Vergnes L., Ojcius D.M., Rentsendorj A., Vargas M., Guerrero C., Wang Y., Fitzgerald K.A., Underhill D.M., Town T., Arditi M. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity. 2012;36(3):401–414. doi: 10.1016/j.immuni.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Halle A., Hornung V., Petzold G.C., Stewart C.R., Monks B.G., Reinheckel T., Fitzgerald K.A., Latz E., Moore K.J., Golenbock D.T. The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat. Immunol. 2008;9(8):857–865. doi: 10.1038/ni.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Freeman L., Guo H., David C.N., Brickey W.J., Jha S., Ting J.P. NLR members NLRC4 and NLRP3 mediate sterile inflammasome activation in microglia and astrocytes. J. Exp. Med. 2017;214(5):1351–1370. doi: 10.1084/jem.20150237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dostert C., Guarda G., Romero J.F., Menu P., Gross O., Tardivel A., Suva M.L., Stehle J.C., Kopf M., Stamenkovic I., Corradin G., Tschopp J. Malarial hemozoin is a Nalp3 inflammasome activating danger signal. PLoS One. 2009;4(8):e6510. doi: 10.1371/journal.pone.0006510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Duewell P., Kono H., Rayner K.J., Sirois C.M., Vladimer G., Bauernfeind F.G., Abela G.S., Franchi L., Nuñez G., Schnurr M., Espevik T., Lien E., Fitzgerald K.A., Rock K.L., Moore K.J., Wright S.D., Hornung V., Latz E. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464(7293):1357–1361. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Martinon F., Pétrilli V., Mayor A., Tardivel A., Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440(7081):237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 86.Mulay S.R., Kulkarni O.P., Rupanagudi K.V., Migliorini A., Darisipudi M.N., Vilaysane A., Muruve D., Shi Y., Munro F., Liapis H., Anders H.J. Calcium oxalate crystals induce renal inflammation by NLRP3-mediated IL-1β secretion. J. Clin. Invest. 2013;123(1):236–246. doi: 10.1172/JCI63679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Muñoz-Planillo R. Kuffa, P.; Martínez-Colón, G.; Smith, B.L.; Rajendiran, T.M.; Núñez, G. K⁺ efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity. 2013;38(6):1142–1153. doi: 10.1016/j.immuni.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kankkunen P., Teirilä L., Rintahaka J., Alenius H., Wolff H., Matikainen S. (1,3)-beta-glucans activate both dectin-1 and NLRP3 inflammasome in human macrophages. J. Immunol. 2010;184(11):6335–6342. doi: 10.4049/jimmunol.0903019. [DOI] [PubMed] [Google Scholar]
- 89.Lamkanfi M., Malireddi R.K., Kanneganti T.D. Fungal zymosan and mannan activate the cryopyrin inflammasome. J. Biol. Chem. 2009;284(31):20574–20581. doi: 10.1074/jbc.M109.023689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Franchi L., Eigenbrod T., Muñoz-Planillo R., Ozkurede U., Kim Y.G., Arindam C., Gale M., Jr, Silverman R.H., Colonna M., Akira S., Núñez G. Cytosolic double-stranded RNA activates the NLRP3 inflammasome via MAVS-induced membrane permeabilization and K+ efflux. J. Immunol. 2014;193(8):4214–4222. doi: 10.4049/jimmunol.1400582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sha W., Mitoma H., Hanabuchi S., Bao M., Weng L., Sugimoto N., Liu Y., Zhang Z., Zhong J., Sun B., Liu Y.J. Human NLRP3 inflammasome senses multiple types of bacterial RNAs. Proc. Natl. Acad. Sci. USA. 2014;111(45):16059–16064. doi: 10.1073/pnas.1412487111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Katsnelson M.A., Rucker L.G., Russo H.M., Dubyak G.R.K. K+ efflux agonists induce NLRP3 inflammasome activation independently of Ca2+ signaling. J. Immunol. 2015;194(8):3937–3952. doi: 10.4049/jimmunol.1402658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pétrilli V., Papin S., Dostert C., Mayor A., Martinon F., Tschopp J. Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death Differ. 2007;14(9):1583–1589. doi: 10.1038/sj.cdd.4402195. [DOI] [PubMed] [Google Scholar]
- 94.Rühl S., Broz P. Caspase-11 activates a canonical NLRP3 inflammasome by promoting K(+) efflux. Eur. J. Immunol. 2015;45(10):2927–2936. doi: 10.1002/eji.201545772. [DOI] [PubMed] [Google Scholar]
- 95.Walev I., Reske K., Palmer M., Valeva A., Bhakdi S. Potassium-inhibited processing of IL-1 beta in human monocytes. EMBO J. 1995;14(8):1607–1614. doi: 10.1002/j.1460-2075.1995.tb07149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Domingo-Fernández R., Coll R.C., Kearney J., Breit S., O’Neill L.A.J. The intracellular chloride channel proteins CLIC1 and CLIC4 induce IL-1β transcription and activate the NLRP3 inflammasome. J. Biol. Chem. 2017;292(29):12077–12087. doi: 10.1074/jbc.M117.797126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tang T., Lang X., Xu C., Wang X., Gong T., Yang Y., Cui J., Bai L., Wang J., Jiang W., Zhou R. CLICs-dependent chloride efflux is an essential and proximal upstream event for NLRP3 inflammasome activation. Nat. Commun. 2017;8(1):202. doi: 10.1038/s41467-017-00227-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Brough D., Le Feuvre R.A., Wheeler R.D., Solovyova N., Hilfiker S., Rothwell N.J., Verkhratsky A. Ca2+ stores and Ca2+ entry differentially contribute to the release of IL-1 beta and IL-1 alpha from murine macrophages. J. Immunol. 2003;170(6):3029–3036. doi: 10.4049/jimmunol.170.6.3029. [DOI] [PubMed] [Google Scholar]
- 99.Lee G.S., Subramanian N., Kim A.I., Aksentijevich I., Goldbach-Mansky R., Sacks D.B., Germain R.N., Kastner D.L., Chae J.J. The calcium-sensing receptor regulates the NLRP3 inflammasome through Ca2+ and cAMP. Nature. 2012;492(7427):123–127. doi: 10.1038/nature11588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Murakami T., Ockinger J., Yu J., Byles V., McColl A., Hofer A.M., Horng T. Critical role for calcium mobilization in activation of the NLRP3 inflammasome. Proc. Natl. Acad. Sci. USA. 2012;109(28):11282–11287. doi: 10.1073/pnas.1117765109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rossol M., Pierer M., Raulien N., Quandt D., Meusch U., Rothe K., Schubert K., Schöneberg T., Schaefer M., Krügel U., Smajilovic S., Bräuner-Osborne H., Baerwald C., Wagner U. Extracellular Ca2+ is a danger signal activating the NLRP3 inflammasome through G protein-coupled calcium sensing receptors. Nat. Commun. 2012;3(1):1329. doi: 10.1038/ncomms2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chu J., Thomas L.M., Watkins S.C., Franchi L., Núñez G., Salter R.D. Cholesterol-dependent cytolysins induce rapid release of mature IL-1beta from murine macrophages in a NLRP3 inflammasome and cathepsin B-dependent manner. J. Leukoc. Biol. 2009;86(5):1227–1238. doi: 10.1189/jlb.0309164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hornung V., Bauernfeind F., Halle A., Samstad E.O., Kono H., Rock K.L., Fitzgerald K.A., Latz E. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat. Immunol. 2008;9(8):847–856. doi: 10.1038/ni.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Orlowski G.M., Colbert J.D., Sharma S., Bogyo M., Robertson S.A., Rock K.L. Multiple cathepsins promote Pro-IL-1β synthesis and NLRP3-mediated IL-1β activation. J. Immunol. 2015;195(4):1685–1697. doi: 10.4049/jimmunol.1500509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bauernfeind F., Bartok E., Rieger A., Franchi L., Núñez G., Hornung V. Cutting edge: reactive oxygen species inhibitors block priming, but not activation, of the NLRP3 inflammasome. J. Immunol. 2011;187(2):613–617. doi: 10.4049/jimmunol.1100613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dostert C., Pétrilli V., Van Bruggen R., Steele C., Mossman B.T., Tschopp J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science. 2008;320(5876):674–677. doi: 10.1126/science.1156995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lawlor K.E., Vince J.E. Ambiguities in NLRP3 inflammasome regulation: is there a role for mitochondria? Biochim. Biophys. Acta. 2014;1840(4):1433–1440. doi: 10.1016/j.bbagen.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 108.Zhou R., Yazdi A.S., Menu P., Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469(7329):221–225. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
- 109.Surprenant A., Rassendren F., Kawashima E., North R.A., Buell G. The cytolytic P2Z receptor for extracellular ATP identified as a P2X receptor (P2X7). Science. 1996;272(5262):735–738. doi: 10.1126/science.272.5262.735. [DOI] [PubMed] [Google Scholar]
- 110.Di A., Xiong S., Ye Z., Malireddi R.K.S., Kometani S., Zhong M., Mittal M., Hong Z., Kanneganti T.D., Rehman J., Malik A.B. The TWIK2 potassium efflux channel in macrophages mediates NLRP3 inflammasome-induced inflammation. Immunity. 2018;49(1):56–65.e4. doi: 10.1016/j.immuni.2018.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Biasizzo M., Kopitar-Jerala N. Interplay between NLRP3 inflammasome and autophagy. Front. Immunol. 2020;11:591803. doi: 10.3389/fimmu.2020.591803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Saitoh T., Akira S. Regulation of inflammasomes by autophagy. J. Allergy Clin. Immunol. 2016;138(1):28–36. doi: 10.1016/j.jaci.2016.05.009. [DOI] [PubMed] [Google Scholar]
- 113.Zheng D., Liwinski T., Elinav E. Inflammasome activation and regulation: toward a better understanding of complex mechanisms. Cell Discov. 2020;6(1):36. doi: 10.1038/s41421-020-0167-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Alirezaei M., Kemball C.C., Whitton J.L. Autophagy, inflammation and neurodegenerative disease. Eur. J. Neurosci. 2011;33(2):197–204. doi: 10.1111/j.1460-9568.2010.07500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Houtman J., Freitag K., Gimber N., Schmoranzer J., Heppner F.L., Jendrach M. Beclin1-driven autophagy modulates the inflammatory response of microglia via NLRP3. EMBO J. 2019;38(4):e99430. doi: 10.15252/embj.201899430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jiang P., Mizushima N. Autophagy and human diseases. Cell Res. 2014;24(1):69–79. doi: 10.1038/cr.2013.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mizushima N., Levine B. Autophagy in human diseases. N. Engl. J. Med. 2020;383(16):1564–1576. doi: 10.1056/NEJMra2022774. [DOI] [PubMed] [Google Scholar]
- 118.Pierone B.C., Pereira C.A., Garcez M.L., Kaster M.P. Stress and signaling pathways regulating autophagy: From behavioral models to psychiatric disorders. Exp. Neurol. 2020;334:113485. doi: 10.1016/j.expneurol.2020.113485. [DOI] [PubMed] [Google Scholar]
- 119.Iwata M., Ota K.T., Duman R.S. The inflammasome: pathways linking psychological stress, depression, and systemic illnesses. Brain Behav. Immun. 2013;31:105–114. doi: 10.1016/j.bbi.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mangan M.S.J., Olhava E.J., Roush W.R., Seidel H.M., Glick G.D., Latz E. Targeting the NLRP3 inflammasome in inflammatory diseases. Nat. Rev. Drug Discov. 2018;17(8):588–606. doi: 10.1038/nrd.2018.97. [DOI] [PubMed] [Google Scholar]
- 121.Meyers A.K., Zhu X. The NLRP3 inflammasome: Metabolic regulation and contribution to inflammaging. Cells. 2020;9(8):E1808. doi: 10.3390/cells9081808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Pereira C.F., Santos A.E., Moreira P.I., Pereira A.C., Sousa F.J., Cardoso S.M., Cruz M.T. Is Alzheimer’s disease an inflammasomopathy? Ageing Res. Rev. 2019;56:100966. doi: 10.1016/j.arr.2019.100966. [DOI] [PubMed] [Google Scholar]
- 123.Pirzada R.H., Javaid N., Choi S. The roles of the NLRP3 inflammasome in neurodegenerative and metabolic diseases and in relevant advanced therapeutic interventions. Genes (Basel) 2020;11(2):E131. doi: 10.3390/genes11020131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Shao B.Z., Xu Z.Q., Han B.Z., Su D.F., Liu C. NLRP3 inflammasome and its inhibitors: a review. Front. Pharmacol. 2015;6:262. doi: 10.3389/fphar.2015.00262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tong Y., Wang Z., Cai L., Lin L., Liu J., Cheng J. NLRP3 inflammasome and its central role in the cardiovascular diseases. Oxid. Med. Cell. Longev. 2020;2020:4293206. doi: 10.1155/2020/4293206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zahid A., Li B., Kombe A.J.K., Jin T., Tao J. Pharmacological inhibitors of the NLRP3 inflammasome. Front. Immunol. 2019;10:2538. doi: 10.3389/fimmu.2019.02538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhou W., Chen C., Chen Z., Liu L., Jiang J., Wu Z., Zhao M., Chen Y. NLRP3: A novel mediator in cardiovascular disease. J. Immunol. Res. 2018;2018:5702103. doi: 10.1155/2018/5702103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gordon R., Albornoz E.A., Christie D.C., Langley M.R., Kumar V., Mantovani S., Robertson A.A.B., Butler M.S., Rowe D.B., O’Neill L.A., Kanthasamy A.G., Schroder K., Cooper M.A., Woodruff T.M. Inflammasome inhibition prevents α-synuclein pathology and dopaminergic neurodegeneration in mice. Sci. Transl. Med. 2018;10(465):eaah4066. doi: 10.1126/scitranslmed.aah4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Walsh J.G., Muruve D.A., Power C. Inflammasomes in the CNS. Nat. Rev. Neurosci. 2014;15(2):84–97. doi: 10.1038/nrn3638. [DOI] [PubMed] [Google Scholar]
- 130.Wong M.L., Inserra A., Lewis M.D., Mastronardi C.A., Leong L., Choo J., Kentish S., Xie P., Morrison M., Wesselingh S.L., Rogers G.B., Licinio J. Inflammasome signaling affects anxiety- and depressive-like behavior and gut microbiome composition. Mol. Psychiatry. 2016;21(6):797–805. doi: 10.1038/mp.2016.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Alcocer-Gómez E., de Miguel M., Casas-Barquero N., Núñez-Vasco J., Sánchez-Alcazar J.A., Fernández-Rodríguez A., Cordero M.D. NLRP3 inflammasome is activated in mononuclear blood cells from patients with major depressive disorder. Brain Behav. Immun. 2014;36:111–117. doi: 10.1016/j.bbi.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 132.Pan Y., Chen X.Y., Zhang Q.Y., Kong L.D. Microglial NLRP3 inflammasome activation mediates IL-1β-related inflammation in prefrontal cortex of depressive rats. Brain Behav. Immun. 2014;41:90–100. doi: 10.1016/j.bbi.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 133.Du R.H., Tan J., Sun X.Y., Lu M., Ding J.H., Hu G. Fluoxetine inhibits NLRP3 inflammasome activation: Implication in depression. Int. J. Neuropsychopharmacol. 2016;19(9):pyw037. doi: 10.1093/ijnp/pyw037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Arioz B.I., Tastan B., Tarakcioglu E., Tufekci K.U., Olcum M., Ersoy N., Bagriyanik A., Genc K., Genc S. Melatonin attenuates LPS-induced acute depressive-like behaviors and microglial NLRP3 inflammasome activation through the SIRT1/Nrf2 pathway. Front. Immunol. 2019;10:1511. doi: 10.3389/fimmu.2019.01511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Li J.M., Liu L.L., Su W.J., Wang B., Zhang T., Zhang Y., Jiang C.L. Ketamine may exert antidepressant effects via suppressing NLRP3 inflammasome to upregulate AMPA receptors. Neuropharmacology. 2019;146:149–153. doi: 10.1016/j.neuropharm.2018.11.022. [DOI] [PubMed] [Google Scholar]
- 136.Yang C., Shen J., Hong T., Hu T.T., Li Z.J., Zhang H.T., Zhang Y.J., Zhou Z.Q., Yang J.J. Effects of ketamine on lipopolysaccharide-induced depressive-like behavior and the expression of inflammatory cytokines in the rat prefrontal cortex. Mol. Med. Rep. 2013;8(3):887–890. doi: 10.3892/mmr.2013.1600. [DOI] [PubMed] [Google Scholar]
- 137.Alcocer-Gómez E., Casas-Barquero N., Williams M.R., Romero-Guillena S.L., Cañadas-Lozano D., Bullón P., Sánchez-Alcazar J.A., Navarro-Pando J.M., Cordero M.D. Antidepressants induce autophagy dependent-NLRP3-inflammasome inhibition in Major depressive disorder. Pharmacol. Res. 2017;121:114–121. doi: 10.1016/j.phrs.2017.04.028. [DOI] [PubMed] [Google Scholar]
- 138.Zhang Y., Liu L., Liu Y.Z., Shen X.L., Wu T.Y., Zhang T., Wang W., Wang Y.X., Jiang C.L. NLRP3 inflammasome mediates chronic mild stress-induced depression in mice via neuroinflammation. Int. J. Neuropsychopharmacol. 2015;18(8):pyv006. doi: 10.1093/ijnp/pyv006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Alcocer-Gómez E., Ulecia-Morón C., Marín-Aguilar F., Rybkina T., Casas-Barquero N., Ruiz-Cabello J., Ryffel B., Apetoh L., Ghiringhelli F., Bullón P., Sánchez-Alcazar J.A., Carrión A.M., Cordero M.D. Stress-induced depressive behaviors require a functional NLRP3 inflammasome. Mol. Neurobiol. 2016;53(7):4874–4882. doi: 10.1007/s12035-015-9408-7. [DOI] [PubMed] [Google Scholar]
- 140.Dong Y., Li S., Lu Y., Li X., Liao Y., Peng Z., Li Y., Hou L., Yuan Z., Cheng J. Stress-induced NLRP3 inflammasome activation negatively regulates fear memory in mice. J. Neuroinflammation. 2020;17(1):205. doi: 10.1186/s12974-020-01842-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Iwata M., Ota K.T., Li X.Y., Sakaue F., Li N., Dutheil S., Banasr M., Duric V., Yamanashi T., Kaneko K., Rasmussen K., Glasebrook A., Koester A., Song D., Jones K.A., Zorn S., Smagin G., Duman R.S. Psychological stress activates the inflammasome via release of adenosine triphosphate and stimulation of the purinergic type 2X7 receptor. Biol. Psychiatry. 2016;80(1):12–22. doi: 10.1016/j.biopsych.2015.11.026. [DOI] [PubMed] [Google Scholar]
- 142.Bitencourt R.M., Takahashi R.N., Carlini E.A. From an alternative medicine to a new treatment for refractory epilepsies: Can cannabidiol follow the same path to treat neuropsychiatric disorders? Front. Psychiatry. 2021;12:638032. doi: 10.3389/fpsyt.2021.638032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Campos A.C., Fogaça M.V., Scarante F.F., Joca S.R.L., Sales A.J., Gomes F.V., Sonego A.B., Rodrigues N.S., Galve-Roperh I., Guimarães F.S. Plastic and neuroprotective mechanisms involved in the therapeutic effects of cannabidiol in psychiatric disorders. Front. Pharmacol. 2017;8:269. doi: 10.3389/fphar.2017.00269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.García-Gutiérrez M.S., Navarrete F., Gasparyan A., Austrich-Olivares A., Sala F., Manzanares J. Cannabidiol: A potential new alternative for the treatment of anxiety, depression, and psychotic disorders. Biomolecules. 2020;10(11):E1575. doi: 10.3390/biom10111575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Silote G.P., Sartim A., Sales A., Eskelund A., Guimarães F.S., Wegener G., Joca S. Emerging evidence for the antidepressant effect of cannabidiol and the underlying molecular mechanisms. J. Chem. Neuroanat. 2019;98:104–116. doi: 10.1016/j.jchemneu.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 146.Zuardi A.W., Cosme R.A., Graeff F.G., Guimarães F.S. Effects of ipsapirone and cannabidiol on human experimental anxiety. J. Psychopharmacol. 1993;7(1) Suppl.:82–88. doi: 10.1177/026988119300700112. [DOI] [PubMed] [Google Scholar]
- 147.Zuardi A.W., Rodrigues N.P., Silva A.L., Bernardo S.A., Hallak J.E.C., Guimarães F.S., Crippa J.A.S. Inverted U-Shaped dose-response curve of the anxiolytic effect of cannabidiol during public speaking in real life. Front. Pharmacol. 2017;8:259. doi: 10.3389/fphar.2017.00259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bergamaschi M.M., Queiroz R.H., Chagas M.H., de Oliveira D.C., De Martinis B.S., Kapczinski F., Quevedo J., Roesler R., Schröder N., Nardi A.E., Martín-Santos R., Hallak J.E., Zuardi A.W., Crippa J.A. Cannabidiol reduces the anxiety induced by simulated public speaking in treatment-naïve social phobia patients. Neuropsychopharmacology. 2011;36(6):1219–1226. doi: 10.1038/npp.2011.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Masataka N. Anxiolytic effects of repeated cannabidiol treatment in teenagers with social anxiety disorders. Front. Psychol. 2019;10:2466. doi: 10.3389/fpsyg.2019.02466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Leweke F.M., Piomelli D., Pahlisch F., Muhl D., Gerth C.W., Hoyer C., Klosterkötter J., Hellmich M., Koethe D. Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Transl. Psychiatry. 2012;2(3):e94. doi: 10.1038/tp.2012.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.McGuire P., Robson P., Cubala W.J., Vasile D., Morrison P.D., Barron R., Taylor A., Wright S. Cannabidiol (CBD) as an adjunctive therapy in schizophrenia: A multicenter randomized controlled trial. Am. J. Psychiatry. 2018;175(3):225–231. doi: 10.1176/appi.ajp.2017.17030325. [DOI] [PubMed] [Google Scholar]
- 152.Hurd Y.L., Spriggs S., Alishayev J., Winkel G., Gurgov K., Kudrich C., Oprescu A.M., Salsitz E. Cannabidiol for the reduction of cue-induced craving and anxiety in drug-abstinent individuals with heroin use disorder: A double-blind randomized placebo-controlled trial. Am. J. Psychiatry. 2019;176(11):911–922. doi: 10.1176/appi.ajp.2019.18101191. [DOI] [PubMed] [Google Scholar]
- 153.Frank M.G., Weber M.D., Watkins L.R., Maier S.F. Stress-induced neuroinflammatory priming: A liability factor in the etiology of psychiatric disorders. Neurobiol. Stress. 2015;4:62–70. doi: 10.1016/j.ynstr.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Campos A.C., Ortega Z., Palazuelos J., Fogaça M.V., Aguiar D.C., Díaz-Alonso J., Ortega-Gutiérrez S., Vázquez-Villa H., Moreira F.A., Guzmán M., Galve-Roperh I., Guimarães F.S. The anxiolytic effect of cannabidiol on chronically stressed mice depends on hippocampal neurogenesis: involvement of the endocannabinoid system. Int. J. Neuropsychopharmacol. 2013;16(6):1407–1419. doi: 10.1017/S1461145712001502. [DOI] [PubMed] [Google Scholar]
- 155.Fogaça M.V., Campos A.C., Coelho L.D., Duman R.S., Guimarães F.S. The anxiolytic effects of cannabidiol in chronically stressed mice are mediated by the endocannabinoid system: Role of neurogenesis and dendritic remodeling. Neuropharmacology. 2018;135:22–33. doi: 10.1016/j.neuropharm.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 156.Resstel L.B., Tavares R.F., Lisboa S.F., Joca S.R., Corrêa F.M., Guimarães F.S. 5-HT1A receptors are involved in the cannabidiol-induced attenuation of behavioural and cardiovascular responses to acute restraint stress in rats. Br. J. Pharmacol. 2009;156(1):181–188. doi: 10.1111/j.1476-5381.2008.00046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Campos A.C., Ferreira F.R., Guimarães F.S. Cannabidiol blocks long-lasting behavioral consequences of predator threat stress: possible involvement of 5HT1A receptors. J. Psychiatr. Res. 2012;46(11):1501–1510. doi: 10.1016/j.jpsychires.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 158.Ibeas Bih C., Chen T., Nunn A.V., Bazelot M., Dallas M., Whalley B.J. Molecular targets of cannabidiol in neurological disorders. Neurotherapeutics. 2015;12(4):699–730. doi: 10.1007/s13311-015-0377-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Campos A.C., Guimarães F.S. Involvement of 5HT1A receptors in the anxiolytic-like effects of cannabidiol injected into the dorsolateral periaqueductal gray of rats. Psychopharmacology (Berl.) 2008;199(2):223–230. doi: 10.1007/s00213-008-1168-x. [DOI] [PubMed] [Google Scholar]
- 160.Casarotto P.C., Gomes F.V., Resstel L.B., Guimarães F.S. Cannabidiol inhibitory effect on marble-burying behaviour: involvement of CB1 receptors. Behav. Pharmacol. 2010;21(4):353–358. doi: 10.1097/FBP.0b013e32833b33c5. [DOI] [PubMed] [Google Scholar]
- 161.Nardo M., Casarotto P.C., Gomes F.V., Guimarães F.S. Cannabidiol reverses the mCPP-induced increase in marble-burying behavior. Fundam. Clin. Pharmacol. 2014;28(5):544–550. doi: 10.1111/fcp.12051. [DOI] [PubMed] [Google Scholar]
- 162.Bitencourt R.M., Pamplona F.A., Takahashi R.N. Facilitation of contextual fear memory extinction and anti-anxiogenic effects of AM404 and cannabidiol in conditioned rats. Eur. Neuropsychopharmacol. 2008;18(12):849–859. doi: 10.1016/j.euroneuro.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 163.Stern C.A.J., da Silva T.R., Raymundi A.M., de Souza C.P., Hiroaki-Sato V.A., Kato L., Guimarães F.S., Andreatini R., Takahashi R.N., Bertoglio L.J. Cannabidiol disrupts the consolidation of specific and generalized fear memories via dorsal hippocampus CB1 and CB2 receptors. Neuropharmacology. 2017;125:220–230. doi: 10.1016/j.neuropharm.2017.07.024. [DOI] [PubMed] [Google Scholar]
- 164.Silva N.R., Gomes F.V., Fonseca M.D., Mechoulam R., Breuer A., Cunha T.M., Guimaraes F.S. Antinociceptive effects of HUF101, a fluorinated cannabidiol derivative. Prog Neuropsychopharmacol Biol Psychiatry. 2017;79(Pt B):369–377. doi: 10.1016/j.pnpbp.2017.07.012. [DOI] [PubMed] [Google Scholar]
- 165.Bisogno T., Hanus L., De Petrocellis L., Tchilibon S., Ponde D.E., Brandi I., Moriello A.S., Davis J.B., Mechoulam R., Di Marzo V. Molecular targets for cannabidiol and its synthetic analogues: effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br. J. Pharmacol. 2001;134(4):845–852. doi: 10.1038/sj.bjp.0704327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Sartim A.G., Guimarães F.S., Joca S.R. Antidepressant-like effect of cannabidiol injection into the ventral medial prefrontal cortex-Possible involvement of 5-HT1A and CB1 receptors. Behav. Brain Res. 2016;303:218–227. doi: 10.1016/j.bbr.2016.01.033. [DOI] [PubMed] [Google Scholar]
- 167.Hartmann A., Lisboa S.F., Sonego A.B., Coutinho D., Gomes F.V., Guimarães F.S. Cannabidiol attenuates aggressive behavior induced by social isolation in mice: Involvement of 5-HT1A and CB1 receptors. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2019;94:109637. doi: 10.1016/j.pnpbp.2019.109637. [DOI] [PubMed] [Google Scholar]
- 168.Martin L.J., Banister S.D., Bowen M.T. Understanding the complex pharmacology of cannabidiol: Mounting evidence suggests a common binding site with cholesterol. Pharmacol. Res. 2021;166:105508. doi: 10.1016/j.phrs.2021.105508. [DOI] [PubMed] [Google Scholar]
- 169.Dos-Santos-Pereira M., Guimarães F.S., Del-Bel E., Raisman-Vozari R., Michel P.P. Cannabidiol prevents LPS-induced microglial inflammation by inhibiting ROS/NF-κB-dependent signaling and glucose consumption. Glia. 2020;68(3):561–573. doi: 10.1002/glia.23738. [DOI] [PubMed] [Google Scholar]
- 170.Gomes F.V., Llorente R., Del Bel E.A., Viveros M.P., López-Gallardo M., Guimarães F.S. Decreased glial reactivity could be involved in the antipsychotic-like effect of cannabidiol. Schizophr. Res. 2015;164(1-3):155–163. doi: 10.1016/j.schres.2015.01.015. [DOI] [PubMed] [Google Scholar]
- 171.Scarante F.F., Ribeiro M.A., Almeida-Santos A.F., Guimarães F.S., Campos A.C. Glial cells and their contribution to the mechanisms of action of cannabidiol in neuropsychiatric disorders. Front. Pharmacol. 2021;11:618065. doi: 10.3389/fphar.2020.618065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Sonego A.B., Prado D.S., Vale G.T., Sepulveda-Diaz J.E., Cunha T.M., Tirapelli C.R., Del Bel E.A., Raisman-Vozari R., Guimarães F.S. Cannabidiol prevents haloperidol-induced vacuos chewing movements and inflammatory changes in mice via PPARγ receptors. Brain Behav. Immun. 2018;74:241–251. doi: 10.1016/j.bbi.2018.09.014. [DOI] [PubMed] [Google Scholar]
- 173.Russo E.B., Burnett A., Hall B., Parker K.K. Agonistic properties of cannabidiol at 5-HT1a receptors. Neurochem. Res. 2005;30(8):1037–1043. doi: 10.1007/s11064-005-6978-1. [DOI] [PubMed] [Google Scholar]
- 174.Haddjeri N., Blier P., de Montigny C. Long-term antidepressant treatments result in a tonic activation of forebrain 5-HT1A receptors. J. Neurosci. 1998;18(23):10150–10156. doi: 10.1523/JNEUROSCI.18-23-10150.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Zanelati T.V., Biojone C., Moreira F.A., Guimarães F.S., Joca S.R. Antidepressant-like effects of cannabidiol in mice: possible involvement of 5-HT1A receptors. Br. J. Pharmacol. 2010;159(1):122–128. doi: 10.1111/j.1476-5381.2009.00521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Cryan J.F., Valentino R.J., Lucki I. Assessing substrates underlying the behavioral effects of antidepressants using the modified rat forced swimming test. Neurosci. Biobehav. Rev. 2005;29(4-5):547–569. doi: 10.1016/j.neubiorev.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 177.Sales A.J., Crestani C.C., Guimarães F.S., Joca S.R.L. Antidepressant-like effect induced by Cannabidiol is dependent on brain serotonin levels. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2018;86:255–261. doi: 10.1016/j.pnpbp.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 178.Abame M.A., He Y., Wu S., Xie Z., Zhang J., Gong X., Wu C., Shen J. Chronic administration of synthetic cannabidiol induces antidepressant effects involving modulation of serotonin and noradrenaline levels in the hippocampus. Neurosci. Lett. 2021;744:135594. doi: 10.1016/j.neulet.2020.135594. [DOI] [PubMed] [Google Scholar]
- 179.Réus G.Z., Stringari R.B., Ribeiro K.F., Luft T., Abelaira H.M., Fries G.R., Aguiar B.W., Kapczinski F., Hallak J.E., Zuardi A.W., Crippa J.A., Quevedo J. Administration of cannabidiol and imipramine induces antidepressant-like effects in the forced swimming test and increases brain-derived neurotrophic factor levels in the rat amygdala. Acta Neuropsychiatr. 2011;23(5):241–248. doi: 10.1111/j.1601-5215.2011.00579.x. [DOI] [PubMed] [Google Scholar]
- 180.Sales A.J., Fogaça M.V., Sartim A.G., Pereira V.S., Wegener G., Guimarães F.S., Joca S.R.L. Cannabidiol induces rapid and sustained antidepressant-like effects through increased BDNF signaling and synaptogenesis in the prefrontal cortex. Mol. Neurobiol. 2019;56(2):1070–1081. doi: 10.1007/s12035-018-1143-4. [DOI] [PubMed] [Google Scholar]
- 181.Xu C., Chang T., Du Y., Yu C., Tan X., Li X. Pharmacokinetics of oral and intravenous cannabidiol and its antidepressant-like effects in chronic mild stress mouse model. Environ. Toxicol. Pharmacol. 2019;70:103202. doi: 10.1016/j.etap.2019.103202. [DOI] [PubMed] [Google Scholar]
- 182.Linge R., Jiménez-Sánchez L., Campa L., Pilar-Cuéllar F., Vidal R., Pazos A., Adell A., Díaz Á. Cannabidiol induces rapid-acting antidepressant-like effects and enhances cortical 5-HT/glutamate neurotransmission: role of 5-HT1A receptors. Neuropharmacology. 2016;103:16–26. doi: 10.1016/j.neuropharm.2015.12.017. [DOI] [PubMed] [Google Scholar]
- 183.Shoval G., Shbiro L., Hershkovitz L., Hazut N., Zalsman G., Mechoulam R., Weller A. Prohedonic effect of cannabidiol in a rat model of depression. Neuropsychobiology. 2016;73(2):123–129. doi: 10.1159/000443890. [DOI] [PubMed] [Google Scholar]
- 184.Shbiro L., Hen-Shoval D., Hazut N., Rapps K., Dar S., Zalsman G., Mechoulam R., Weller A., Shoval G. Effects of cannabidiol in males and females in two different rat models of depression. Physiol. Behav. 2019;201:59–63. doi: 10.1016/j.physbeh.2018.12.019. [DOI] [PubMed] [Google Scholar]
- 185.Silote G.P., Gatto M.C., Eskelund A., Guimarães F.S., Wegener G., Joca S.R.L. Strain-, sex-, and time-dependent antidepressant-like effects of cannabidiol. Pharmaceuticals (Basel) 2021;14(12):1269. doi: 10.3390/ph14121269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Adu-Nti F., Ghartey-Kwansah G., Aboagye B. Sex differences in the antidepressant effects of ketamine in animal models of depression. Int J Depress Anxiety. 2019;2(2):13. [Google Scholar]
- 187.Sartim A.G., Marques J., Silveira K.M., Gobira P.H., Guimarães F.S., Wegener G., Joca S.R. Co-administration of cannabidiol and ketamine induces antidepressant-like effects devoid of hyperlocomotor side-effects. Neuropharmacology. 2021;195:108679. doi: 10.1016/j.neuropharm.2021.108679. [DOI] [PubMed] [Google Scholar]
- 188.Sartim A.G., Sales A.J., Guimarães F.S., Joca S.R. Hippocampal mammalian target of rapamycin is implicated in stress-coping behavior induced by cannabidiol in the forced swim test. J. Psychopharmacol. 2018;32(8):922–931. doi: 10.1177/0269881118784877. [DOI] [PubMed] [Google Scholar]
- 189.Schiavon A.P., Bonato J.M., Milani H., Guimarães F.S., Weffort de Oliveira R.M. Influence of single and repeated cannabidiol administration on emotional behavior and markers of cell proliferation and neurogenesis in non-stressed mice. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2016;64:27–34. doi: 10.1016/j.pnpbp.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 190.Kraus C., Castrén E., Kasper S., Lanzenberger R. Serotonin and neuroplasticity - Links between molecular, functional and structural pathophysiology in depression. Neurosci. Biobehav. Rev. 2017;77:317–326. doi: 10.1016/j.neubiorev.2017.03.007. [DOI] [PubMed] [Google Scholar]
- 191.Corroon J., Phillips J.A. A cross-sectional study of cannabidiol users. Cannabis Cannabinoid Res. 2018;3(1):152–161. doi: 10.1089/can.2018.0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Pacheco J.C., Souza J.D.S., Hallak J.E.C., Osório F.L., Campos A.C., Guimarães F.S., Zuardi A.W., Crippa J.A.S. Cannabidiol as a treatment for mental health outcomes among health care workers during the coronavirus disease pandemic. J. Clin. Psychopharmacol. 2021;41(3):327–329. doi: 10.1097/JCP.0000000000001405. [DOI] [PubMed] [Google Scholar]
- 193.Bitencourt R.M., Takahashi R.N. Cannabidiol as a therapeutic alternative for post-traumatic stress disorder: from bench research to confirmation in human trials. Front. Neurosci. 2018;12:502. doi: 10.3389/fnins.2018.00502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Stein J.Y., Wilmot D.V., Solomon Z. Does one size fit all? Nosological, clinical, and scientific implications of variations in PTSD Criterion A. J. Anxiety Disord. 2016;43:106–117. doi: 10.1016/j.janxdis.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 195.Shalev A., Liberzon I., Marmar C. Post-traumatic stress disorder. N. Engl. J. Med. 2017;376(25):2459–2469. doi: 10.1056/NEJMra1612499. [DOI] [PubMed] [Google Scholar]
- 196.Campos A.C., Fogaça M.V., Sonego A.B., Guimarães F.S. Cannabidiol, neuroprotection and neuropsychiatric disorders. Pharmacol. Res. 2016;112:119–127. doi: 10.1016/j.phrs.2016.01.033. [DOI] [PubMed] [Google Scholar]
- 197.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: 2013. [Google Scholar]
- 198.Blessing E.M., Steenkamp M.M., Manzanares J., Marmar C.R. Cannabidiol as a potential treatment for anxiety disorders. Neurotherapeutics. 2015;12(4):825–836. doi: 10.1007/s13311-015-0387-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Careaga M.B.L., Girardi C.E.N., Suchecki D. Understanding posttraumatic stress disorder through fear conditioning, extinction and reconsolidation. Neurosci. Biobehav. Rev. 2016;71:48–57. doi: 10.1016/j.neubiorev.2016.08.023. [DOI] [PubMed] [Google Scholar]
- 200.Crippa J.A., Guimarães F.S., Campos A.C., Zuardi A.W. Translational investigation of the therapeutic potential of cannabidiol (CBD): Toward a new age. Front. Immunol. 2018;9:2009. doi: 10.3389/fimmu.2018.02009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Lee J.L.C., Bertoglio L.J., Guimarães F.S., Stevenson C.W. Cannabidiol regulation of emotion and emotional memory processing: relevance for treating anxiety-related and substance abuse disorders. Br. J. Pharmacol. 2017;174(19):3242–3256. doi: 10.1111/bph.13724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Lisboa S.F., Vila-Verde C., Rosa J., Uliana D.L., Stern C.A.J., Bertoglio L.J., Resstel L.B., Guimaraes F.S. Tempering aversive/traumatic memories with cannabinoids: a review of evidence from animal and human studies. Psychopharmacology (Berl.) 2019;236(1):201–226. doi: 10.1007/s00213-018-5127-x. [DOI] [PubMed] [Google Scholar]
- 203.Ross D.A., Arbuckle M.R., Travis M.J., Dwyer J.B., van Schalkwyk G.I., Ressler K.J. An integrated neuroscience perspective on formulation and treatment planning for posttraumatic stress disorder: An educational review. JAMA Psychiatry. 2017;74(4):407–415. doi: 10.1001/jamapsychiatry.2016.3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Bienvenu T.C.M., Dejean C., Jercog D., Aouizerate B., Lemoine M., Herry C. The advent of fear conditioning as an animal model of post-traumatic stress disorder: Learning from the past to shape the future of PTSD research. Neuron. 2021;109(15):2380–2397. doi: 10.1016/j.neuron.2021.05.017. [DOI] [PubMed] [Google Scholar]
- 205.Hill M.N., Campolongo P., Yehuda R., Patel S. Integrating endocannabinoid signaling and cannabinoids into the biology and treatment of posttraumatic stress disorder. Neuropsychopharmacology. 2018;43(1):80–102. doi: 10.1038/npp.2017.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Levin R., Almeida V., Peres F.F., Calzavara M.B., da Silva N.D., Suiama M.A., Niigaki S.T., Zuardi A.W., Hallak J.E., Crippa J.A., Abílio V.C. Antipsychotic profile of cannabidiol and rimonabant in an animal model of emotional context processing in schizophrenia. Curr. Pharm. Des. 2012;18(32):4960–4965. doi: 10.2174/138161212802884735. [DOI] [PubMed] [Google Scholar]
- 207.Norris C., Loureiro M., Kramar C., Zunder J., Renard J., Rushlow W., Laviolette S.R. Cannabidiol modulates fear memory formation through interactions with serotonergic transmission in the mesolimbic system. Neuropsychopharmacology. 2016;41(12):2839–2850. doi: 10.1038/npp.2016.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Resstel L.B., Joca S.R., Moreira F.A., Corrêa F.M., Guimarães F.S. Effects of cannabidiol and diazepam on behavioral and cardiovascular responses induced by contextual conditioned fear in rats. Behav. Brain Res. 2006;172(2):294–298. doi: 10.1016/j.bbr.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 209.Gomes F.V., Reis D.G., Alves F.H., Corrêa F.M., Guimarães F.S., Resstel L.B. Cannabidiol injected into the bed nucleus of the stria terminalis reduces the expression of contextual fear conditioning via 5-HT1A receptors. J. Psychopharmacol. 2012;26(1):104–113. doi: 10.1177/0269881110389095. [DOI] [PubMed] [Google Scholar]
- 210.Lemos J.I., Resstel L.B., Guimarães F.S. Involvement of the prelimbic prefrontal cortex on cannabidiol-induced attenuation of contextual conditioned fear in rats. Behav. Brain Res. 2010;207(1):105–111. doi: 10.1016/j.bbr.2009.09.045. [DOI] [PubMed] [Google Scholar]
- 211.Assareh N., Gururajan A., Zhou C., Luo J.L., Kevin R.C., Arnold J.C. Cannabidiol disrupts conditioned fear expression and cannabidiolic acid reduces trauma-induced anxiety-related behaviour in mice. Behav. Pharmacol. 2020;31(6):591–596. doi: 10.1097/FBP.0000000000000565. [DOI] [PubMed] [Google Scholar]
- 212.Jurkus R., Day H.L., Guimarães F.S., Lee J.L., Bertoglio L.J., Stevenson C.W. Cannabidiol regulation of learned fear: Implications for treating anxiety-related disorders. Front. Pharmacol. 2016;7:454. doi: 10.3389/fphar.2016.00454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Song C., Stevenson C.W., Guimaraes F.S., Lee J.L. Bidirectional effects of cannabidiol on contextual fear memory extinction. Front. Pharmacol. 2016;7:493. doi: 10.3389/fphar.2016.00493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Do Monte F.H., Souza R.R., Bitencourt R.M., Kroon J.A., Takahashi R.N. Infusion of cannabidiol into infralimbic cortex facilitates fear extinction via CB1 receptors. Behav. Brain Res. 2013;250:23–27. doi: 10.1016/j.bbr.2013.04.045. [DOI] [PubMed] [Google Scholar]
- 215.Stern C.A., Gazarini L., Takahashi R.N., Guimarães F.S., Bertoglio L.J. On disruption of fear memory by reconsolidation blockade: evidence from cannabidiol treatment. Neuropsychopharmacology. 2012;37(9):2132–2142. doi: 10.1038/npp.2012.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Raymundi A.M., da Silva T.R., Zampronio A.R., Guimarães F.S., Bertoglio L.J., Stern C.A.J. A time-dependent contribution of hippocampal CB1, CB2 and PPARγ receptors to cannabidiol-induced disruption of fear memory consolidation. Br. J. Pharmacol. 2020;177(4):945–957. doi: 10.1111/bph.14895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Rossignoli M.T., Lopes-Aguiar C., Ruggiero R.N., Do Val da Silva R.A., Bueno-Junior L.S., Kandratavicius L., Peixoto-Santos J.E., Crippa J.A., Cecilio Hallak J.E., Zuardi A.W., Szawka R.E., Anselmo-Franci J., Leite J.P., Romcy-Pereira R.N. Selective post-training time window for memory consolidation interference of cannabidiol into the prefrontal cortex: Reduced dopaminergic modulation and immediate gene expression in limbic circuits. Neuroscience. 2017;350:85–93. doi: 10.1016/j.neuroscience.2017.03.019. [DOI] [PubMed] [Google Scholar]
- 218.Bensinger S.J., Tontonoz P. Integration of metabolism and inflammation by lipid-activated nuclear receptors. Nature. 2008;454(7203):470–477. doi: 10.1038/nature07202. [DOI] [PubMed] [Google Scholar]
- 219.Bernardo A., Minghetti L. Regulation of glial cell functions by PPAR-gamma natural and synthetic agonists. PPAR Res. 2008;2008:864140. doi: 10.1155/2008/864140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Moraes L.A., Piqueras L., Bishop-Bailey D. Peroxisome proliferator-activated receptors and inflammation. Pharmacol. Ther. 2006;110(3):371–385. doi: 10.1016/j.pharmthera.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 221.Clark S.M., Soroka J.A., Song C., Li X., Tonelli L.H. CD4(+) T cells confer anxiolytic and antidepressant-like effects, but enhance fear memory processes in Rag2(-/-) mice. Stress. 2016;19(3):303–311. doi: 10.1080/10253890.2016.1191466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Yu Z., Fukushima H., Ono C., Sakai M., Kasahara Y., Kikuchi Y., Gunawansa N., Takahashi Y., Matsuoka H., Kida S., Tomita H. Microglial production of TNF-alpha is a key element of sustained fear memory. Brain Behav. Immun. 2017;59:313–321. doi: 10.1016/j.bbi.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 223.Young M.B., Howell L.L., Hopkins L., Moshfegh C., Yu Z., Clubb L., Seidenberg J., Park J., Swiercz A.P., Marvar P.J. A peripheral immune response to remembering trauma contributes to the maintenance of fear memory in mice. Psychoneuroendocrinology. 2018;94:143–151. doi: 10.1016/j.psyneuen.2018.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Hughes B., Herron C.E. Cannabidiol reverses deficits in hippocampal LTP in a model of Alzheimer’s disease. Neurochem. Res. 2019;44(3):703–713. doi: 10.1007/s11064-018-2513-z. [DOI] [PubMed] [Google Scholar]
- 225.Esposito G., Scuderi C., Valenza M., Togna G.I., Latina V., De Filippis D., Cipriano M., Carratù M.R., Iuvone T., Steardo L. Cannabidiol reduces Aβ-induced neuroinflammation and promotes hippocampal neurogenesis through PPARγ involvement. PLoS One. 2011;6(12):e28668. doi: 10.1371/journal.pone.0028668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Wen J., Ribeiro R., Tanaka M., Zhang Y. Activation of CB2 receptor is required for the therapeutic effect of ABHD6 inhibition in experimental autoimmune encephalomyelitis. Neuropharmacology. 2015;99:196–209. doi: 10.1016/j.neuropharm.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 227.Turcotte C., Blanchet M.R., Laviolette M., Flamand N. The CB2 receptor and its role as a regulator of inflammation. Cell. Mol. Life Sci. 2016;73(23):4449–4470. doi: 10.1007/s00018-016-2300-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Zoppi S., Madrigal J.L., Caso J.R., García-Gutiérrez M.S., Manzanares J., Leza J.C., García-Bueno B. Regulatory role of the cannabinoid CB2 receptor in stress-induced neuroinflammation in mice. Br. J. Pharmacol. 2014;171(11):2814–2826. doi: 10.1111/bph.12607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Zoppi S., Pérez Nievas B.G., Madrigal J.L., Manzanares J., Leza J.C., García-Bueno B. Regulatory role of cannabinoid receptor 1 in stress-induced excitotoxicity and neuroinflammation. Neuropsychopharmacology. 2011;36(4):805–818. doi: 10.1038/npp.2010.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Pistis M., O’Sullivan S.E. The role of nuclear hormone receptors in cannabinoid function. Adv. Pharmacol. 2017;80:291–328. doi: 10.1016/bs.apha.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 231.Fogaça M.V., Reis F.M., Campos A.C., Guimarães F.S. Effects of intra-prelimbic prefrontal cortex injection of cannabidiol on anxiety-like behavior: involvement of 5HT1A receptors and previous stressful experience. Eur. Neuropsychopharmacol. 2014;24(3):410–419. doi: 10.1016/j.euroneuro.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 232.Rock E.M., Bolognini D., Limebeer C.L., Cascio M.G., Anavi-Goffer S., Fletcher P.J., Mechoulam R., Pertwee R.G., Parker L.A. Cannabidiol, a non-psychotropic component of cannabis, attenuates vomiting and nausea-like behaviour via indirect agonism of 5-HT(1A) somatodendritic autoreceptors in the dorsal raphe nucleus. Br. J. Pharmacol. 2012;165(8):2620–2634. doi: 10.1111/j.1476-5381.2011.01621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Marinho A.L., Vila-Verde C., Fogaça M.V., Guimarães F.S. Effects of intra-infralimbic prefrontal cortex injections of cannabidiol in the modulation of emotional behaviors in rats: contribution of 5HT₁A receptors and stressful experiences. Behav. Brain Res. 2015;286:49–56. doi: 10.1016/j.bbr.2015.02.023. [DOI] [PubMed] [Google Scholar]
- 234.Lisboa S.F., Stecchini M.F., Corrêa F.M., Guimarães F.S., Resstel L.B. Different role of the ventral medial prefrontal cortex on modulation of innate and associative learned fear. Neuroscience. 2010;171(3):760–768. doi: 10.1016/j.neuroscience.2010.09.048. [DOI] [PubMed] [Google Scholar]
- 235.Giustino T.F., Maren S. The role of the medial prefrontal cortex in the conditioning and extinction of fear. Front. Behav. Neurosci. 2015;9:298. doi: 10.3389/fnbeh.2015.00298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Sotres-Bayon F., Quirk G.J. Prefrontal control of fear: more than just extinction. Curr. Opin. Neurobiol. 2010;20(2):231–235. doi: 10.1016/j.conb.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Vidal-Gonzalez I., Vidal-Gonzalez B., Rauch S.L., Quirk G.J. Microstimulation reveals opposing influences of prelimbic and infralimbic cortex on the expression of conditioned fear. Learn. Mem. 2006;13(6):728–733. doi: 10.1101/lm.306106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Szkudlarek H.J. Desai, S.J.; Renard, J.; Pereira, B.; Norris, C.; Jobson, C.E.L.; Rajakumar, N.; Allman, B.L.; Laviolette, S.R. Δ-9-Tetrahydrocannabinol and Cannabidiol produce dissociable effects on prefrontal cortical executive function and regulation of affective behaviors. Neuropsychopharmacology. 2019;44(4):817–825. doi: 10.1038/s41386-018-0282-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Rock E.M., Limebeer C.L., Petrie G.N., Williams L.A., Mechoulam R., Parker L.A. Effect of prior foot shock stress and Δ9-tetrahydrocannabinol, cannabidiolic acid, and cannabidiol on anxiety-like responding in the light-dark emergence test in rats. Psychopharmacology (Berl.) 2017;234(14):2207–2217. doi: 10.1007/s00213-017-4626-5. [DOI] [PubMed] [Google Scholar]
- 240.Campos A.C., Moreira F.A., Gomes F.V., Del Bel E.A., Guimarães F.S. Multiple mechanisms involved in the large-spectrum therapeutic potential of cannabidiol in psychiatric disorders. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2012;367(1607):3364–3378. doi: 10.1098/rstb.2011.0389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Shallcross J., Hámor P., Bechard A.R., Romano M., Knackstedt L., Schwendt M. The divergent effects of CDPPB and cannabidiol on fear extinction and anxiety in a predator scent stress model of PTSD in rats. Front. Behav. Neurosci. 2019;13:91. doi: 10.3389/fnbeh.2019.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Gasparyan A., Navarrete F., Manzanares J. Cannabidiol and sertraline regulate behavioral and brain gene expression alterations in an animal model of PTSD. Front. Pharmacol. 2021;12:694510. doi: 10.3389/fphar.2021.694510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Passos I.C., Vasconcelos-Moreno M.P., Costa L.G., Kunz M., Brietzke E., Quevedo J., Salum G., Magalhães P.V., Kapczinski F., Kauer-Sant’Anna M. Inflammatory markers in post-traumatic stress disorder: a systematic review, meta-analysis, and meta-regression. Lancet Psychiatry. 2015;2(11):1002–1012. doi: 10.1016/S2215-0366(15)00309-0. [DOI] [PubMed] [Google Scholar]
- 244.Yang J.J., Jiang W. Immune biomarkers alterations in post-traumatic stress disorder: A systematic review and meta-analysis. J. Affect. Disord. 2020;268:39–46. doi: 10.1016/j.jad.2020.02.044. [DOI] [PubMed] [Google Scholar]
- 245.Li S., Liao Y., Dong Y., Li X., Li J., Cheng Y., Cheng J., Yuan Z. Microglial deletion and inhibition alleviate behavior of post-traumatic stress disorder in mice. J. Neuroinflammation. 2021;18(1):7. doi: 10.1186/s12974-020-02069-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Yamanashi T., Iwata M., Shibushita M., Tsunetomi K., Nagata M., Kajitani N., Miura A., Matsuo R., Nishiguchi T., Kato T.A., Setoyama D., Shirayama Y., Watanabe K., Shinozaki G., Kaneko K. Beta-hydroxybutyrate, an endogenous NLRP3 inflammasome inhibitor, attenuates anxiety-related behavior in a rodent post-traumatic stress disorder model. Sci. Rep. 2020;10(1):21629. doi: 10.1038/s41598-020-78410-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Nagarkatti P., Pandey R., Rieder S.A., Hegde V.L., Nagarkatti M. Cannabinoids as novel anti-inflammatory drugs. Future Med. Chem. 2009;1(7):1333–1349. doi: 10.4155/fmc.09.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Nichols J.M., Kaplan B.L.F. Immune responses regulated by cannabidiol. Cannabis Cannabinoid Res. 2020;5(1):12–31. doi: 10.1089/can.2018.0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Zuardi A.W. Cannabidiol: from an inactive cannabinoid to a drug with wide spectrum of action. Rev. Bras. Psiquiatr. 2008;30(3):271–280. doi: 10.1590/S1516-44462008000300015. [DOI] [PubMed] [Google Scholar]
- 250.Barichello T., Ceretta R.A., Generoso J.S., Moreira A.P., Simões L.R., Comim C.M., Quevedo J., Vilela M.C., Zuardi A.W., Crippa J.A., Teixeira A.L. Cannabidiol reduces host immune response and prevents cognitive impairments in Wistar rats submitted to pneumococcal meningitis. Eur. J. Pharmacol. 2012;697(1-3):158–164. doi: 10.1016/j.ejphar.2012.09.053. [DOI] [PubMed] [Google Scholar]
- 251.Borrelli F., Aviello G., Romano B., Orlando P., Capasso R., Maiello F., Guadagno F., Petrosino S., Capasso F., Di Marzo V., Izzo A.A. Cannabidiol, a safe and non-psychotropic ingredient of the marijuana plant Cannabis sativa, is protective in a murine model of colitis. J. Mol. Med. (Berl.) 2009;87(11):1111–1121. doi: 10.1007/s00109-009-0512-x. [DOI] [PubMed] [Google Scholar]
- 252.Campos A.C., Brant F., Miranda A.S., Machado F.S., Teixeira A.L. Cannabidiol increases survival and promotes rescue of cognitive function in a murine model of cerebral malaria. Neuroscience. 2015;289:166–180. doi: 10.1016/j.neuroscience.2014.12.051. [DOI] [PubMed] [Google Scholar]
- 253.Esposito G., Scuderi C., Savani C., Steardo L., Jr, De Filippis D., Cottone P., Iuvone T., Cuomo V., Steardo L. Cannabidiol in vivo blunts beta-amyloid induced neuroinflammation by suppressing IL-1beta and iNOS expression. Br. J. Pharmacol. 2007;151(8):1272–1279. doi: 10.1038/sj.bjp.0707337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Mecha M., Feliú A., Iñigo P.M., Mestre L., Carrillo-Salinas F.J., Guaza C. Cannabidiol provides long-lasting protection against the deleterious effects of inflammation in a viral model of multiple sclerosis: a role for A2A receptors. Neurobiol. Dis. 2013;59:141–150. doi: 10.1016/j.nbd.2013.06.016. [DOI] [PubMed] [Google Scholar]
- 255.Vuolo F., Petronilho F., Sonai B., Ritter C., Hallak J.E., Zuardi A.W., Crippa J.A., Dal-Pizzol F. Evaluation of serum cytokines levels and the role of cannabidiol treatment in animal model of asthma. Mediators Inflamm. 2015;2015:538670. doi: 10.1155/2015/538670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Srivastava M.D., Srivastava B.I., Brouhard B. Delta9 tetrahydrocannabinol and cannabidiol alter cytokine production by human immune cells. Immunopharmacology. 1998;40(3):179–185. doi: 10.1016/S0162-3109(98)00041-1. [DOI] [PubMed] [Google Scholar]
- 257.Dos-Santos-Pereira M., da-Silva C.A., Guimarães F.S., Del-Bel E. Co-administration of cannabidiol and capsazepine reduces L-DOPA-induced dyskinesia in mice: Possible mechanism of action. Neurobiol. Dis. 2016;94:179–195. doi: 10.1016/j.nbd.2016.06.013. [DOI] [PubMed] [Google Scholar]
- 258.Hayakawa K., Mishima K., Nozako M., Ogata A., Hazekawa M., Liu A.X., Fujioka M., Abe K., Hasebe N., Egashira N., Iwasaki K., Fujiwara M. Repeated treatment with cannabidiol but not Delta9-tetrahydrocannabinol has a neuroprotective effect without the development of tolerance. Neuropharmacology. 2007;52(4):1079–1087. doi: 10.1016/j.neuropharm.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 259.Booz G.W. Cannabidiol as an emergent therapeutic strategy for lessening the impact of inflammation on oxidative stress. Free Radic. Biol. Med. 2011;51(5):1054–1061. doi: 10.1016/j.freeradbiomed.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Petrovici A.R., Simionescu N., Sandu A.I., Paraschiv V., Silion M., Pinteala M. New insights on hemp oil enriched in cannabidiol: Decarboxylation, antioxidant properties and in vitro anticancer effect. Antioxidants. 2021;10(5):738. doi: 10.3390/antiox10050738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Liu D.Z., Hu C.M., Huang C.H., Wey S.P., Jan T.R. Cannabidiol attenuates delayed-type hypersensitivity reactions via suppressing T-cell and macrophage reactivity. Acta Pharmacol. Sin. 2010;31(12):1611–1617. doi: 10.1038/aps.2010.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Kaplan B.L., Springs A.E., Kaminski N.E. The profile of immune modulation by cannabidiol (CBD) involves deregulation of nuclear factor of activated T cells (NFAT). Biochem. Pharmacol. 2008;76(6):726–737. doi: 10.1016/j.bcp.2008.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Walter L., Franklin A., Witting A., Wade C., Xie Y., Kunos G., Mackie K., Stella N. Nonpsychotropic cannabinoid receptors regulate microglial cell migration. J. Neurosci. 2003;23(4):1398–1405. doi: 10.1523/JNEUROSCI.23-04-01398.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Martín-Moreno A.M., Reigada D., Ramírez B.G., Mechoulam R., Innamorato N., Cuadrado A., de Ceballos M.L. Cannabidiol and other cannabinoids reduce microglial activation in vitro and in vivo: Relevance to Alzheimer’s disease. Mol. Pharmacol. 2011;79(6):964–973. doi: 10.1124/mol.111.071290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Magen I., Avraham Y., Ackerman Z., Vorobiev L., Mechoulam R., Berry E.M. Cannabidiol ameliorates cognitive and motor impairments in bile-duct ligated mice via 5-HT1A receptor activation. Br. J. Pharmacol. 2010;159(4):950–957. doi: 10.1111/j.1476-5381.2009.00589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Gurung P., Li B., Subbarao Malireddi R.K., Lamkanfi M., Geiger T.L., Kanneganti T.D. Chronic TLR Stimulation controls NLRP3 inflammasome activation through IL-10 mediated regulation of NLRP3 expression and caspase-8 activation. Sci. Rep. 2015;5(1):14488. doi: 10.1038/srep14488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Kozela E., Lev N., Kaushansky N., Eilam R., Rimmerman N., Levy R., Ben-Nun A., Juknat A., Vogel Z. Cannabidiol inhibits pathogenic T cells, decreases spinal microglial activation and ameliorates multiple sclerosis-like disease in C57BL/6 mice. Br. J. Pharmacol. 2011;163(7):1507–1519. doi: 10.1111/j.1476-5381.2011.01379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Juknat A., Gao F., Coppola G., Vogel Z., Kozela E. miRNA expression profiles and molecular networks in resting and LPS-activated BV-2 microglia-Effect of cannabinoids. PLoS One. 2019;14(2):e0212039. doi: 10.1371/journal.pone.0212039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Hampson A.J., Grimaldi M., Lolic M., Wink D., Rosenthal R., Axelrod J. Neuroprotective antioxidants from marijuana. Ann. N. Y. Acad. Sci. 2000;899(1):274–282. doi: 10.1111/j.1749-6632.2000.tb06193.x. [DOI] [PubMed] [Google Scholar]
- 270.Liu C., Li H., Xu F., Jiang X., Ma H., Seeram N.P. Cannabidiol protects human skin Keratinocytes from hydrogen-peroxide-induced oxidative stress via modulation of the Caspase-1-IL-1β axis. J. Nat. Prod. 2021;84(5):1563–1572. doi: 10.1021/acs.jnatprod.1c00083. [DOI] [PubMed] [Google Scholar]
- 271.Hao F., Feng Y. Cannabidiol (CBD) enhanced the hippocampal immune response and autophagy of APP/PS1 Alzheimer’s mice uncovered by RNA-seq. Life Sci. 2021;264:118624. doi: 10.1016/j.lfs.2020.118624. [DOI] [PubMed] [Google Scholar]
- 272.Yang L., Rozenfeld R., Wu D., Devi L.A., Zhang Z., Cederbaum A. Cannabidiol protects liver from binge alcohol-induced steatosis by mechanisms including inhibition of oxidative stress and increase in autophagy. Free Radic. Biol. Med. 2014;68:260–267. doi: 10.1016/j.freeradbiomed.2013.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Böckmann S., Hinz B. Cannabidiol promotes endothelial cell survival by heme oxygenase-1-mediated autophagy. Cells. 2020;9(7):E1703. doi: 10.3390/cells9071703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Gugliandolo A., Pollastro F., Bramanti P., Mazzon E. Cannabidiol exerts protective effects in an in vitro model of Parkinson’s disease activating AKT/mTOR pathway. Fitoterapia. 2020;143:104553. doi: 10.1016/j.fitote.2020.104553. [DOI] [PubMed] [Google Scholar]
- 275.Shrivastava A., Kuzontkoski P.M., Groopman J.E., Prasad A. Cannabidiol induces programmed cell death in breast cancer cells by coordinating the cross-talk between apoptosis and autophagy. Mol. Cancer Ther. 2011;10(7):1161–1172. doi: 10.1158/1535-7163.MCT-10-1100. [DOI] [PubMed] [Google Scholar]
- 276.Vrechi T.A.M., Leão A.H.F.F., Morais I.B.M., Abílio V.C., Zuardi A.W., Hallak J.E.C., Crippa J.A., Bincoletto C., Ureshino R.P., Smaili S.S., Pereira G.J.S. Cannabidiol induces autophagy via ERK1/2 activation in neural cells. Sci. Rep. 2021;11(1):5434. doi: 10.1038/s41598-021-84879-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Suryavanshi S.V., Kovalchuk I., Kovalchuk O. Cannabinoids as key regulators of inflammasome signaling: A current perspective. Front. Immunol. 2021;11:613613. doi: 10.3389/fimmu.2020.613613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Jiang X., Gu Y., Huang Y., Zhou Y., Pang N., Luo J., Tang Z., Zhang Z., Yang L. CBD alleviates liver injuries in alcoholics with high-fat high-cholesterol diet through regulating NLRP3 inflammasome-pyroptosis pathway. Front. Pharmacol. 2021;12:724747. doi: 10.3389/fphar.2021.724747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Huang Y., Wan T., Pang N., Zhou Y., Jiang X., Li B., Gu Y., Huang Y., Ye X., Lian H., Zhang Z., Yang L. Cannabidiol protects livers against nonalcoholic steatohepatitis induced by high-fat high cholesterol diet via regulating NF-κB and NLRP3 inflammasome pathway. J. Cell. Physiol. 2019;234(11):21224–21234. doi: 10.1002/jcp.28728. [DOI] [PubMed] [Google Scholar]
- 280.Rimmerman N., Juknat A., Kozela E., Levy R., Bradshaw H.B., Vogel Z. The non-psychoactive plant cannabinoid, cannabidiol affects cholesterol metabolism-related genes in microglial cells. Cell. Mol. Neurobiol. 2011;31(6):921–930. doi: 10.1007/s10571-011-9692-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Libro R., Scionti D., Diomede F., Marchisio M., Grassi G., Pollastro F., Piattelli A., Bramanti P., Mazzon E., Trubiani O. Cannabidiol modulates the immunophenotype and inhibits the activation of the inflammasome in human gingival mesenchymal stem cells. Front. Physiol. 2016;7:559. doi: 10.3389/fphys.2016.00559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Corpetti C., Del Re A., Seguella L., Palenca I., Rurgo S., De Conno B., Pesce M., Sarnelli G., Esposito G. Cannabidiol inhibits SARS-Cov-2 spike (S) protein-induced cytotoxicity and inflammation through a PPARγ-dependent TLR4/NLRP3/Caspase-1 signaling suppression in Caco-2 cell line. Phytother. Res. 2021;35(12):6893–6903. doi: 10.1002/ptr.7302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Liu C., Ma H., Slitt A.L., Seeram N.P. Inhibitory effect of cannabidiol on the activation of NLRP3 inflammasome is associated with its modulation of the P2X7 receptor in human monocytes. J. Nat. Prod. 2020;83(6):2025–2029. doi: 10.1021/acs.jnatprod.0c00138. [DOI] [PubMed] [Google Scholar]
- 284.Goodman S., Wadsworth E., Schauer G., Hammond D. Use and perceptions of cannabidiol products in Canada and in the United States. Cannabis Cannabinoid Res. 2020;7(3):355–364. doi: 10.1089/can.2020.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Moltke J., Hindocha C. Reasons for cannabidiol use: a cross-sectional study of CBD users, focusing on self-perceived stress, anxiety, and sleep problems. J Cannabis Res. 2021;3(1):5. doi: 10.1186/s42238-021-00061-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Pavlovic R., Nenna G., Calvi L., Panseri S., Borgonovo G., Giupponi L., Cannazza G., Giorgi A. Quality traits of “cannabidiol oils”: Cannabinoids content, terpene fingerprint and oxidation stability of european commercially available preparations. Molecules. 2018;23(5):E1230. doi: 10.3390/molecules23051230. [DOI] [PMC free article] [PubMed] [Google Scholar]


