Abstract
Background.
Mucopolysaccharidosis IVA (MPS IVA) is characterized by progressive skeletal dysplasia and respiratory issues with difficult anesthetic airway management.
Objective.
To characterize tracheal abnormalities in children and adults with MPS IVA including interplay of the trachea, vasculature, bones, and thyroid at the thoracic inlet.
Materials and Methods.
Computed tomography angiograms of the chest in patients with MPS IVA were analyzed for trachea shape, narrowing and deviation at the thoracic inlet, course of vasculature, bone alignment, and thyroid location. Tracheal cross-sectional area (TCSA) was measured at the cervical, thoracic inlet, and intrathoracic levels.
Results.
Thirty-seven patients (mean 18.1 years) were included. Mean TCSA narrowing at the thoracic inlet compared with intrathoracic trachea was 63.9% (range − 2.1 to 96%), with a trend for increased tracheal narrowing with older age in children. Trachea was commonly deviated rightward posterior (22/37). Thirty-one patients with T- or W-shaped tracheas had twice-greater TCsA narrowing compared with five patients with D- or U-shaped tracheas (p<0.05). The brachiocephalic artery was tortuous in 35/37 patients with direct impingement on the trachea in 24/37. Thyroid was located in the thoracic inlet in 28/37, significantly associated with TCSA narrowing (p=0.016).
Conclusion.
Narrowing, deviation, and abnormal shape of the trachea at the thoracic inlet are common in children and adults with MPS IVA, with a trend towards increased narrowing with advancing age in children. A W- or T-shaped trachea is associated with focal tracheal narrowing. Crowding of the thoracic inlet, due to vascular tortuosity, bony changes, and thyroid position, appears to play a major role.
Keywords: Trachea, Morquio syndrome, mucopolysaccharidosis, CT angiography, MPS IVA
Introduction
Mucopolysaccharidosis IVA (MPS IVA or Morquio A syndrome) is a rare autosomal recessive lysosomal storage disease caused by a deficiency in the N-acetylgalactosamine-6-sulfatase enzyme [1,2,3]. This enzymatic deficiency leads to accumulation of the glycosaminoglycans (GAGs) chondroitin-6-sulfate and keratan sulfate in a wide range of tissues throughout the body including bone, cartilage, aorta, heart muscle, heart valves, lung, and other visceral organs [4,5,6,7,8]. Clinically, MPS IVA is characterized by progressive skeletal dysplasia with short stature and neck, kyphoscoliosis, atlantoaxial instability, spinal cord compression, pectus carinatum, obstructive and restrictive lung, and joint laxity. Growth diminishes during early childhood and stops by age 7 or 8 years [9,10]. Patients with MPS IVA express the most severe phenotype when compared with those with other types of MPS and often require multiple orthopedic surgeries, wheelchair assistance by the teenage years, and shortened life expectancy [3,10].
In addition to orthopedic problems, respiratory issues and difficulty with anesthetic airway management are significant contributors to morbidity and mortality in MPS IVA, and perioperative deaths and paralysis have been reported due to the extreme challenges of airway management [11,12,13]. Patients with MPS IVA develop progressive respiratory difficulty due to a combination of factors, including restrictive chest deformity, obstructive airways (both upper and lower), and spinal cord compression. Upper airway obstruction is caused by a large tongue, short neck, and sub-mucosal and cartilaginous GAG deposits contributing to narrowing of the pharyngeal airway and abnormal vocal folds [2,11]. Lower airway obstruction is due to progressive buckling, twisting and narrowing of the trachea resulting in a tortuous and narrow trachea. As airway difficulties mount, patients often assume an extended head and neck position as though they were looking up at the sky to help maintain patency of their airways.
Although upper airway narrowing has been well recognized, tracheal abnormalities in MPS IVA have received scant attention in the medical literature with a few case reports describing tracheal stenosis and malacia resulting in respiratory failure and death [14,15]. Abnormal shape of the trachea has been described with computed tomography (CT) in the various forms of MPS, including MPS IVA. Shih et al. reported on airway changes in a mixed group of patients with MPS, including one two-year-old patient with MPS IVA [16]. In this study of 13 patients with a variety of MPS types, the group had significantly smaller mean tracheal cross-sectional area (TCSA) at the level of the T1 vertebra when compared with controls. In addition, eight patients had an abnormal “U” or “worm-shaped” trachea. Similarly, a study of 35 MPS patients (MPS I, II, III, IV, and VI) by Morimoto et al. [17] found abnormal tracheal shape on axial CT imaging in 60% of patients. In that study, the two patients with MPS IVA had abnormal shape at the T2 level [17].
At our institution, we became focused on the tracheal abnormalities after the experience with a teenaged boy with a severe phenotype of MPS IVA who presented with 2.5 years of progressive airway obstruction [18]. This child had known severe obstructive lung disease evident on pulmonary function testing, and CT angiography (CTA) of the chest showed severe focal narrowing of the trachea with direct impingement on the trachea by a tortuous brachiocephalic artery (BCA). Due to a short neck and thoracic inlet anatomy, tracheostomy was considered unsuitable and possibly dangerous with the risk of developing a tracheoinnominate fistula. Instead, he underwent successful surgical reconstruction of the trachea and translocation of the BCA with improvement in his symptoms.
Our group subsequently undertook a retrospective review of the trachea as seen on magnetic resonance imaging of the cervical spine, commonly performed for evaluation of spinal cord impingement in children and adults with MPS IVA [19]. Based on sagittal images, we noted at least 25% anteroposterior tracheal diameter narrowing at the thoracic inlet in 19 of 28 patients, and greater than 75% narrowing in eight patients. Furthermore, tracheal narrowing was age-dependent, with all patients older than 15 years (8/28) showing greater than 50% tracheal diameter narrowing. In addition, the crossing BCA appeared to directly impinge upon the trachea in 15 of 19 patients with tracheal narrowing. In this retrospective MRI study, however, we could not evaluate further the interplay of multidirectional tracheal deviation and narrowing, vascular impingement, and bony anatomy. We, therefore, undertook the evaluation of chest CTA to better understand the anatomic changes of the thoracic inlet in MPS IVA. Here, we report our experience with CTA of the chest performed for clinical concern of airway obstruction in children and adults with MPS IVA.
Materials and Methods
This retrospective review of clinically acquired imaging exams was performed after Institutional Review Board approval. The electronic medical record (EMR) and the picture archiving and communication system (PACS) of our tertiary-care children’s health system were queried for all patients with confirmed MPS IVA who underwent CTA of the chest from 2013 through 2018. If a patient had more than one CTA, the earliest scan was used. Patients with prior thoracic surgery were excluded. Images were analyzed using PACS as well as a three-dimensional (3D) workstation (Aquarius iNtuition, TeraRecon, Inc., Foster City, CA, USA) by a single pediatric radiologist with more than 13 years of experience in cross-sectional body imaging. The trachea, thoracic vasculature, bony anatomy of the thoracic inlet, and position of the thyroid gland were evaluated.
CTA Technique
The origin of the studies (from our children’s health system or from outside facilities) was noted. When obtained at our institution, imaging was performed on a Somatom Definition Flash CT scanner (Siemens, Erlangen, Germany) typically using dual-energy CT angiographic technique with CARE (combined applications to reduce exposure) dose and quality reference of 141mAs. Imaging was acquired with inspiratory breath holding if the patient was able to cooperate or quiet breathing if the patient was sedated or unable to cooperate. Only images of acceptable quality for post-processing were included.
Trachea
The tracheal cross-sectional area (TCSA) was measured using a 3D post-processing centerline technique with automated edge detection to obtain a true cross-sectional measurement. The margins of the trachea were corrected manually if automated edge detection failed; any corrections were performed by the one radiologist who analyzed all scans. The TCSA measurements were obtained at three levels: the largest cervical tracheal lumen below the vocal folds and above the thoracic inlet, the narrowest tracheal lumen at the thoracic inlet, and the largest intrathoracic tracheal lumen. The length of tracheal luminal narrowing was measured using the centerline image, including areas of tapering both proximally and distally. The presence or absence of more than one level of tracheal narrowing was also assessed.
Additional descriptive features of the trachea were also recorded. The vertebral level adjacent to the level of greatest tracheal narrowing was selected using a sagittally reconstructed image. The shape of the tracheal lumen at the thoracic inlet on axial images was described using the following five categories [17,20]: C-shaped (circular except for flattening at the posterior membranous portion), D-shaped (transverse diameter greater than the anteroposterior diameter), U-shaped (anteroposterior diameter greater than the transverse diameter), W-shaped (“worm” or obliquely elliptical), and T-shaped (triangular with apex anterior). The C-shaped trachea was considered normal, D- and U-shaped tracheas were considered intermediate with variable severity, and the W- and T-shaped tracheas were considered abnormal (Fig. 1). The presence of tracheal deviation in the coronal and sagittal planes was also recorded.
Fig. 1.
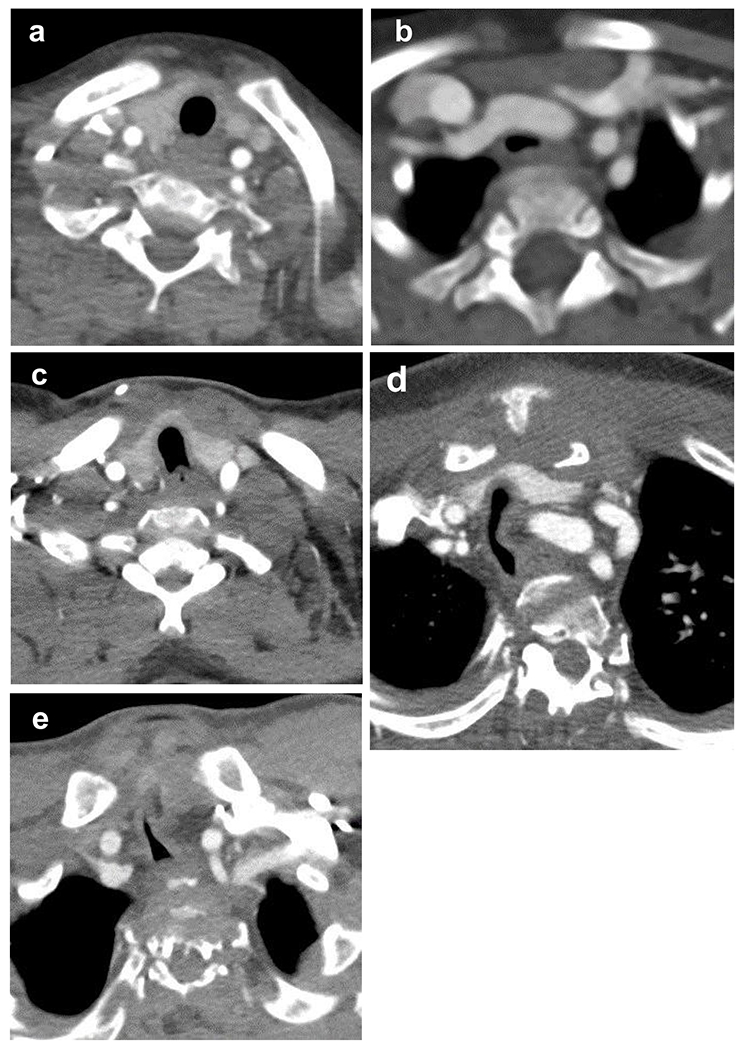
Spectrum of cross-sectional shape of the trachea on axial computed tomography angiogram images at the thoracic inlet in our cohort of children and adults with mucopolysaccharidosis type IVA (MPS IVA). Normal, C-shaped trachea in a 6-year old girl (a) without tracheal narrowing or deviation. A D-shaped trachea in a 1-year old girl (b) with 18.1% tracheal cross-sectional area narrowing at the thoracic inlet when compared with the intrathoracic trachea. A U-shaped trachea in a 17-year old girl (c) with 51.6% tracheal narrowing at the thoracic inlet. The U and D shapes span the border zone of normal and abnormal depending on the degree of tracheal deformation and can be seen in the general population as well as in MPS IVA. Worm or W-shaped trachea in a 16-year old boy (d) with 90.1% tracheal narrowing at the thoracic inlet. Triangular or T-shaped trachea in a 16-year old boy (e) with 91.9% tracheal narrowing at the thoracic inlet.
Vasculature
The presence of aortic tortuosity was qualitatively assessed at the level of the aortic arch and the descending thoracic aorta, including buckling of the descending aorta across midline. The course of the BCA was also categorized. In the coronal plane, the course of the BCA was described as transverse if the BCA made an approximately 90° turn and traveled left to right shortly after its vertical origin from the aortic arch; it was described as oblique if the BCA traveled obliquely from the aortic arch to its bifurcation into the subclavian and common carotid arteries without making an acute turn.
The relationship of the BCA and trachea narrowing was evaluated, noting whether the BCA crossed anterior to the trachea above, at, or below the level of tracheal narrowing. The appearance of direct impingement of the BCA upon the trachea was also assessed.
Bony Thoracic Inlet
The presence of pectus carinatum by qualitative assessment was recorded. The anteroposterior diameter of the thoracic inlet from manubrium to cervical spine and clavicle head to cervical spine was measured. The presence and levels of cervical spine fusion were recorded.
Thyroid
The location of the thyroid gland in relation to the thoracic inlet was described.
Data Analysis
Data were grouped by sex and age group (child or adult) and by anatomic feature (trachea, thoracic vasculature, bony anatomy of the thoracic inlet, and position of the thyroid gland). Ages birth through 18 years were considered children; adults included age 19 years and older.
Statistical analyses included descriptive statistics, normality testing, unpaired t-tests with Welsh’s correction, Fisher’s exact test, and two-way ANOVA with interactions to examine for sex and age effects. Data were analyzed using GraphPad Prism (version 8.4.2).
Results
Thirty-seven patients (19 females) with MPS IVA had a CTA examination available in the PACS system for review. Ages ranged from 1.1 years to 43.7 years (median 18.1 years, SD 9.5), with 20 pediatric patients 0-18 years (12.5 years, SD 4.8) and 17 adult patients 19 years and older (average 27 years, SD 7.3). Of the 20 children, nine were female, and of the 17 adults, 10 were female. The four youngest patients were female. Twenty-four exams were performed at our tertiary care pediatric hospital, and 14 scans were performed at other institutions. All image data sets were sufficient for 3-D post-processing.
Trachea
On axial imaging, the trachea was most commonly W-shaped with an oblique elliptical or “worm-like” configuration, seen in 21 patients (57%). A T-shaped or triangular trachea with apex anterior was the second-most common configuration, seen in 10 patients (27%). A U-shaped trachea was seen in four patients, and a C and D-shaped trachea were seen in one patient each (Fig. 1). The longitudinal course of the trachea was deviated at the thoracic inlet and upper chest in most but not all patients, being displaced to the right and posterior in an oblique fashion in 22 patients (59%), rightward only in seven patients (19%), and posteriorly only in one patient (3%) (Fig. 2). Tracheal narrowing was centered at the C7 or T1 levels in 76% of patients (13 and 15 patients, respectively), with the narrowed segment spanning an average of 2.4 cm craniocaudal (range 1.2 to 5.2 cm). More than one level of tracheal narrowing was observed in five patients, usually in the distal trachea in addition to the thoracic inlet (Fig. 2a).
Fig. 2.
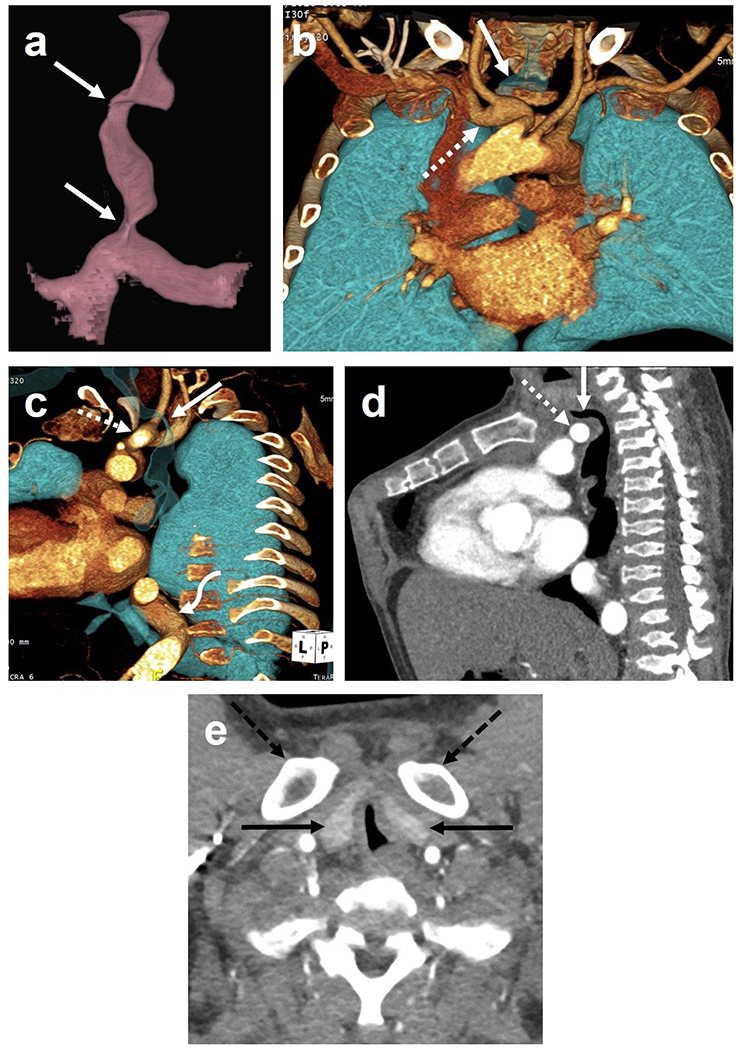
Computed tomography angiogram of a 28-year-old man with mucopolysaccharidosis type IVA (MPS IVA). Three-dimensional reconstruction of the trachea (a) shows two levels of tracheal narrowing: at the thoracic inlet and just above the carina (arrows). Coronal (b) and sagittal oblique (c) three-dimensional reconstructions of the vasculature and airways show deviation of the trachea (arrow) posteriorly and to the right at the thoracic inlet, draping around the tortuous and ectatic brachiocephalic artery (dashed arrow). Sagittal multiplanar reconstruction (d) similarly shows direct impingement of the brachiocephalic artery (dashed arrow) upon the trachea (arrow) at the thoracic inlet. Also note typical bony features of MPS IVA including pectus carinatum and platyspondyly. Axial image (e) shows T-shape trachea, both lobes of the thyroid (arrows) located in the thoracic inlet, and bony crowding from the clavicular heads (dashed arrows).
The TCSA of the cervical trachea was always equal to or larger than the TCSA of the intrathoracic trachea with an average difference of 29.7% (range, −0.6 to 53.1). Therefore, to avoid over-estimation of the tracheal caliber change, the smaller intrathoracic trachea was selected as the internal reference against which tracheal narrowing at the thoracic inlet was measured. On average, TSCA at the thoracic inlet was measured 63.9% smaller than the intrathoracic TSCA (range −2.1 to 96%), a finding that is statistically significant at p<0.001. The absolute TCSA measurement at the thoracic inlet averaged 32 mm2 (range 4.5 mm2 to 79.3 mm2).
Comparisons by Age
When the pediatric and adult MPS IVA groups were compared, children age 0-18 years had mildly smaller TCSA at both the cervical (128.8 mm2 child, 156.9 mm2 adult) and intrathoracic levels (85.6 mm2 child, 104.6 mm2 adult) and nearly the same TCSA at the thoracic inlet (32.2 mm2 child, 31.7 mm2 adult). There were no statistically significant differences in these measurements. There was a trend for increased tracheal narrowing at the thoracic inlet with older age in children (Fig. 3), but no age-related trend was observed in the adults (Fig. 4). The cohort was too small to further investigate differences in pre- and post-pubertal children.
Fig. 3.
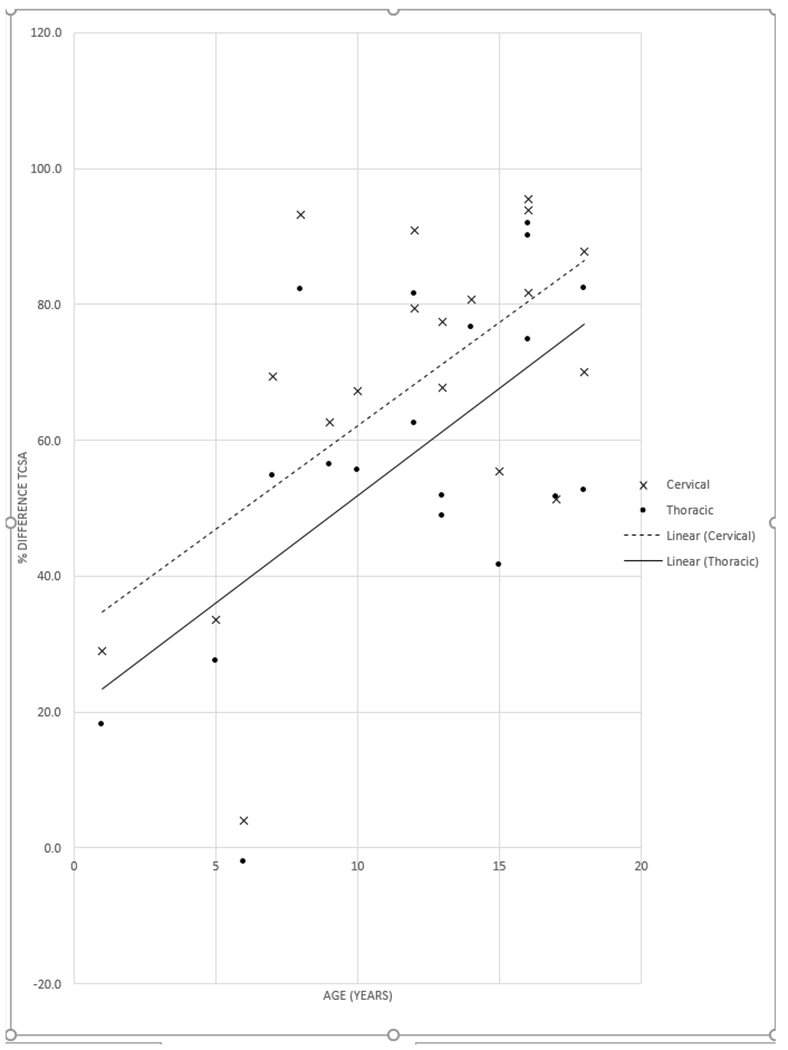
Percent narrowing of the tracheal cross-sectional area (TCSA) at the thoracic inlet versus age in children with mucopolysaccharidosis type IVA. When compared with either the cervical trachea (x) or the intrathoracic trachea (dot), there was a trend toward progressively increasing the percent narrowing of the TCSA at the thoracic inlet with age.
Fig. 4.
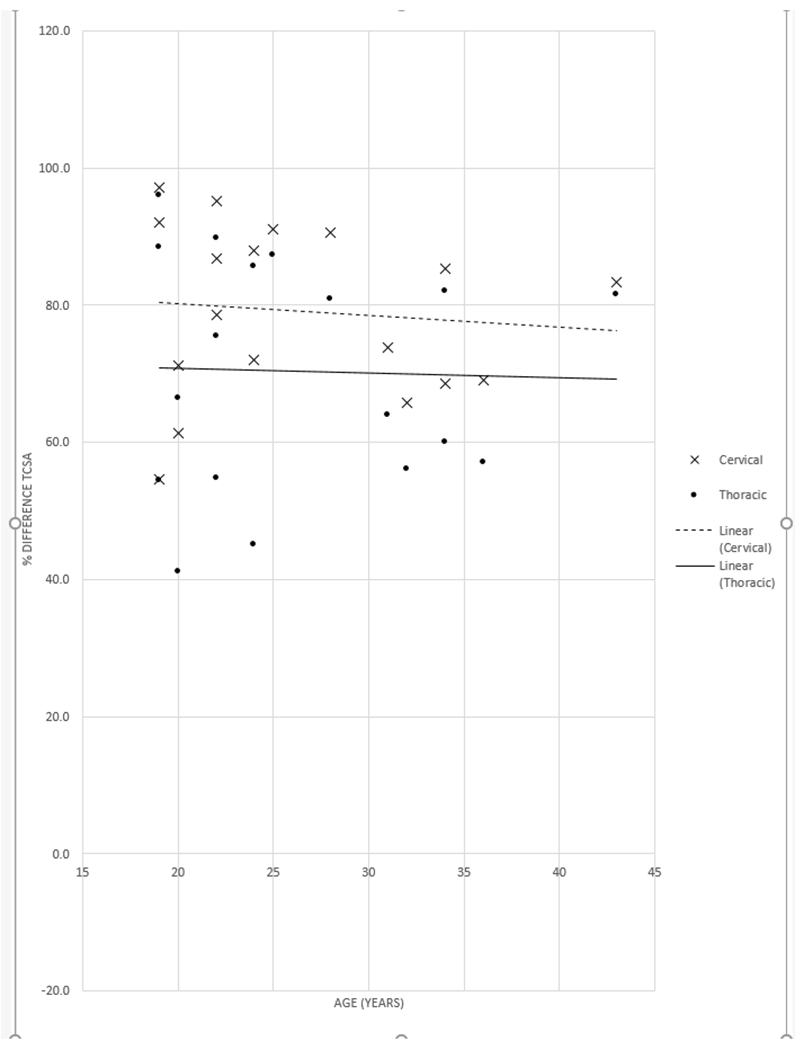
Percent narrowing of the tracheal cross-sectional area (TCSA) at the thoracic inlet versus age in adults with mucopolysaccharidosis type IVA. When compared with either the cervical trachea (x) or the intrathoracic trachea (dot), the percent narrowing of the TCSA at the thoracic inlet was similar across the age range of adults.
Comparisons by Sex
Females tended to have mildly smaller TCSA in both the cervical (115.7 mm2 female, 166.9 mm2 male) and intrathoracic levels (87 mm2 female, 102.1 mm2 male), but they had slightly larger TCSA at the thoracic inlet (35 mm2 female, 28.7 mm2 male). When all ages were considered together, there was no significant difference between the sexes in these measurements.
When sex comparison was made by age group, however, there was a significant difference between adult females and adult males in cervical TCSA (131 mm2 female, 193.9 mm2male), p<0.05. There was no sex-related difference at other tracheal levels in adults and no sex-related difference in children at any tracheal level. The cohort was too small to further investigate differences in pre and post-pubertal children.
Shape Assessment
Tracheal shape correlated with severity of tracheal narrowing at the thoracic inlet. Patients with abnormal T- and W-shaped tracheas demonstrated twice-greater percent TCSA narrowing when compared with patients with intermediate U and D-shaped tracheas (p<0.01) (Table 1). One six-year-old girl had a normal C-shaped trachea and showed no tracheal narrowing, tracheal deviation, vascular tortuosity, or thyroid in the thoracic inlet. In fact, this girl had a slightly larger TCSA at the thoracic inlet compared with the intrathoracic trachea, thus resulting in −2.1% change.
Table 1.
Relationship of cross-sectional tracheal shape and percent tracheal cross-sectional area (TCSA) narrowing at the thoracic inlet as compared to the intrathoracic trachea
| Tracheal shape | Number of patients (gender) | Mean patient age in years (range) | Percent TCSA narrowing (standard deviation) | Range of percent TCSA narrowing |
|---|---|---|---|---|
| T- or W-shape | 31 (13 female) | 20.6 (7–43) | 70.1 (±16.0) | 41.7 to 96 |
| U- or D-shape | 5 (5 female) | 13(1–20) | 38.6 (±15.6) | 18.1 to 54.4 |
| C-shape | 1 (female) | 6.4 | −2.1 (±0) | – |
Patients with T- or W-shaped tracheas had significantly greater percent TCSA narrowing compared with those with intermediate U- and D-shaped tracheas (P<0.01)
Vasculature
The brachiocephalic artery was tortuous in 35 patients (95%), exhibiting a sharp rightward turn after its origin from the aortic arch and a transverse course at or just below the thoracic inlet. The BCA appeared to directly impinge upon the trachea in 24 (65%) patients. In 14 cases of vascular impingement, the BCA passed directly anterior to the level of greatest tracheal narrowing (Fig. 2). In 10 cases, however, the BCA impinged upon the trachea from below, with the narrowest portion of the trachea draped superiorly over the BCA. Direct impingement of the BCA on the trachea was not correlated with severity of tracheal narrowing at the thoracic inlet. Those patients without direct vascular impingement showed a mean percent TCSA narrowing of 63.2% (range −2.1 to 96%), similar to the study group as a whole (Fig. 5). The thoracic aorta was tortuous in most patients as well, including the aortic arch in 29 patients (78%) and the descending aorta in 31 patients (84%). In fact, the descending aorta took a zig-zag course in the lower chest crossing midline in 16 patients (43%) (Fig. 5c).
Fig. 5.
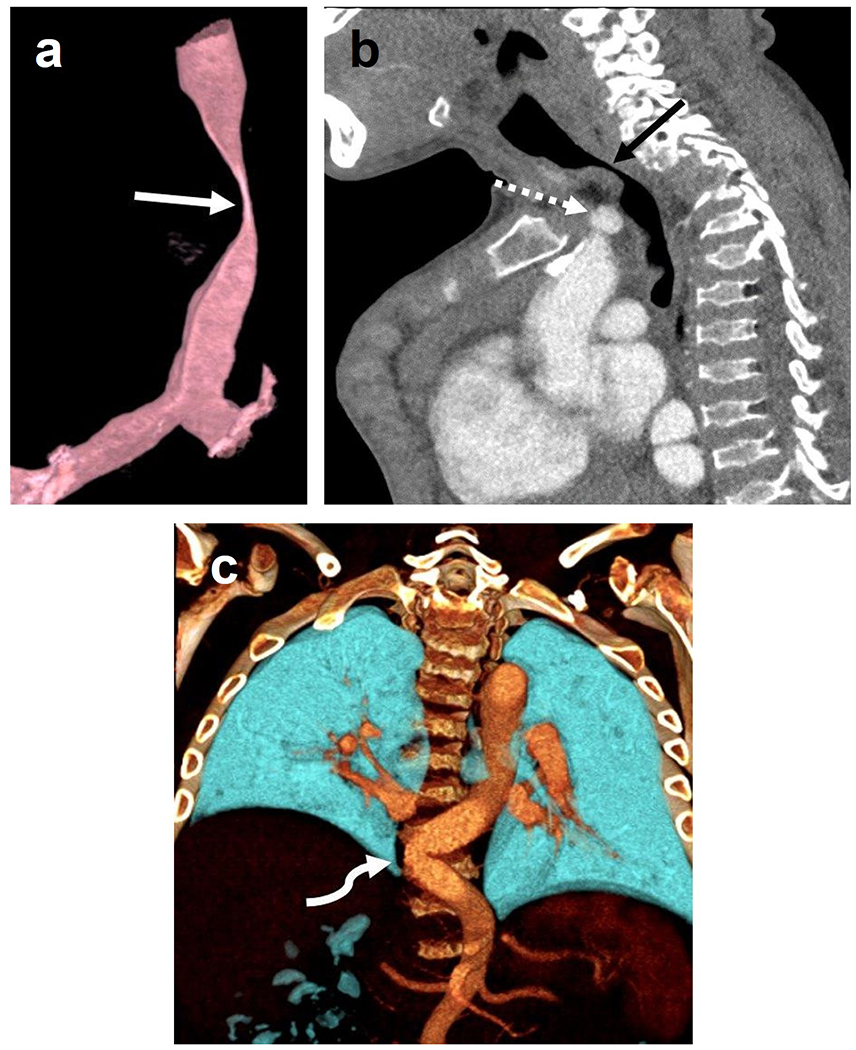
Computed tomography angiogram of a 16-year old boy with mucopolysaccharidosis type IVA. Three-dimensional reconstruction of the trachea (a) shows a severe narrowing of the trachea at the thoracic inlet (arrow). Sagittal image (b) shows that the brachiocephalic artery (dashed arrow) contributes to crowding at the thoracic inlet but does not directly indent the trachea (arrow). Three-dimensional coronal reconstruction (c) posteriorly in the chest shows tortuosity of the descending thoracic aorta, crossing midline (curved arrow).
Comparisons by Age
Direct impingement of the BCA upon the trachea at the thoracic inlet was seen more frequently in children than in adults with MPS IVA, 16/20 (80%) and 8/17 (47%), respectively, p<0.05. Tortuosity of the aorta, however, was more common in adults. The aortic arch appeared tortuous in 13/20 (49%) in children but was almost universal in adults, seen in 16/17 (94%), p<0.05. The descending thoracic aorta zig-zagged across the midline in 10/20 children (50%) and 12/17 adults (71%), without significant difference between the two age groups, p=0.31.
Comparisons by Sex
Regarding direct impingement of the BCA upon the trachea, there was no significant difference according to sex in our cohort (12/19 or 63% female, 12/18 or 67% male). The aortic arch was tortuous in 14/19 (73%) of females and 15/18 (83%) of males. Likewise, there was no significant difference in the descending aorta crossing midline, seen in and 9/19 (47%) females and 13/18 (72%) males.
Bony Thoracic Inlet
As is typical for MPS IVA, pectus carinatum was seen in 34 patients (92%). One patient instead had marked pectus excavatum, while the sternum alignment was normal in two patients including a one-year-old girl with a completely normal exam and a 20-year-old woman with relatively mild tracheal narrowing. The mean manubrium-to-spine distance was 2.8 cm (range 1.5 to 4.1 cm), while the mean clavicle head-to-spine distance was 2.5 cm (range 1.4 to 4.1 cm). The clavicular heads specifically appeared to contribute to thoracic inlet crowding in 30 (81%) patients (Fig. 2e). There was no trend observed between the anteroposterior bony measurements of the thoracic inlet and the percent change in TCSA. Based on scout images, the cervical spine was surgically fused in 23 (62%) patients from the occiput to C2 in 14 patients and the occiput to other cervical levels in the remaining nine patients. The thoracic spine was fused in addition to occiput-C2 fusion in one patient.
Comparisons by Age
The mean manubrium-to-spine and clavicular head-to-spine distances were not significantly different between children (average 2.9 cm) and adults (average 2.7 cm). Likewise, the percentage of individuals with cervical spine fusion was similar between children (60%) and adults (65%).
Comparisons by Sex
There was no significant sex difference is the bony thorax measurements (2.6 cm female, 3.0 cm male) or rate of cervical spine fusion (60% female, 70% male).
Thyroid
The thyroid gland was located in the thoracic inlet in 28/37 (76%) patients; this appeared to contribute to crowding in addition to vascular and bony structures (Fig. 2e). There was 70.3% TCSA narrowing when the thyroid gland was located in the thoracic inlet compared with 44% TCSA narrowing when it was not (statistically significant at p=0.016).
Comparisons by Age
The thyroid gland was located in the thoracic inlet in 14/20 (70%) of children and in 14/17 (82%) of adults, not statistically significant.
Comparisons by Sex
The thyroid gland was located in the thoracic inlet in 12/19 (63%) of females and in 16/18 (89%) of males; findings were not statistically significant.
Discussion
Patients with MPS IVA frequently have progressive respiratory insufficiency and difficult anesthetic airway management, but few data have been reported about the anatomy of the thoracic inlet in this group. To date, this study represents the largest cohort of children and adults with MPS IVA evaluated for tracheal abnormalities and the only study to use CT angiography to determine the contribution of the surrounding vasculature, bones, and thyroid gland. As suggested from our prior investigation of the trachea using sagittal cervical spine MRI [19], there is progressively increasing tracheal narrowing at the thoracic inlet throughout childhood in patients with MPS IVA, even beyond the age when linear height growth ceases. Although the factors have not been fully elucidated, this phenomenon may be due to an imbalance of growth with the trachea continuing to lengthen in a disproportionate manner when compared with overall physical growth, constrained by the patient’s short spine and kyphoscolosis [18]. Furthermore, GAGs continue to deposit in tissues throughout the body, including the trachea, which alters their structural integrity [6]. In addition to focal narrowing, the trachea in MPS IVA typically deviates rightward and posterior at or just below the thoracic inlet, taking on a twisted configuration and an abnormal triangular or elliptical “worm” shape when seen in cross-section. Multiple sites of tracheal narrowing are possible.
As a whole, our cohort had smaller TCSA than pediatric and adult CT-derived reference values reported in the literature [21,22,23]. Compared with 96.2 mm2 and 116.8 mm2 in adult females and males with MPS IVA, respectively, the average intrathoracic TCSA in adults reported by Ulusoy et al. was 160.7 mm2 in females and 275.7 mm2 in males [21]. In another study of 294 children up to the age of 20 years with a similar age distribution to our cohort, the median TCSA 1 cm above the carina was 142 mm2 in females and 163 mm2 in males, noting differences according to sex beginning at age 14 years [22]. Our group of children aged 0-18 years with MPS IVA, in contrast, had mean intrathoracic TCSA of 76.7 mm2 in females and 90.3 mm2 in males. The only statistically significant difference according to sex in MPS IVA was seen at the cervical trachea level in adults, most likely related to the larger thyroid cartilage seen in men, the so-called Adam’s apple. Given the generally small cross-sectional dimensions of the trachea in MPS IVA as well as this difference in sex, the patient’s own intrathoracic trachea was used as an internal reference standard to determine the percent tracheal narrowing at the thoracic inlet.
There may be a slight impression on the trachea by the crossing BCA in both normal children and adults as well as slight change in caliber and morphology of the trachea as it passes through the thoracic inlet [24]. However, in normal subjects, this caliber alteration is slight, and the trachea typically maintains a rounded or slightly oval shape [21,24] and would be categorized as C-shaped or on the mild end of the D- and U-shaped spectrum. In contrast, most of our MPS IVA patients displayed markedly abnormal worm (W) or triangular (T) cross-sectional morphology of the trachea at the thoracic inlet. These abnormal shapes were significantly associated with greater tracheal narrowing at the thoracic inlet and can serve as a quick visual cue to the severity of tracheal abnormality.
In nearly all our patients, regardless of age, the BCA was tortuous and took a sharp rightward turn near the thoracic inlet. Furthermore, the thoracic aorta was also tortuous in most patients. Widespread vasculopathy has been previously reported in a 58-year-old woman with MPS IVA [25], and this appears to be a common finding. Aortic arch tortuosity was more common in adults than children in our study and may have a similar underlying cause to the tracheal narrowing due to imbalanced growth and GAG deposition in vascular walls. Tracheal narrowing was associated with direct impingement upon the trachea by the tortuous BCA in a majority of patients in our study, but the severity of tracheal narrowing was not dependent upon direct vascular impingement. Furthermore, there was greater direct vascular impingement in children than in adults. This seeming contradiction needs further investigation but suggests a complex interplay of structures leading to the potentially life-threatening tracheal narrowing seen in MPS IVA. When surgical reconstruction of the trachea is considered, the close relationship of the brachiocephalic artery and trachea is of paramount importance. The associated vasculopathy of MPS IVA needs to be considered in all cases, because surgical relocation of the tortuous BCA may provide greater space for the trachea regardless of direct impingement seen preoperatively [18].
The bony measurements of the thoracic inlet were similar between children and adults with MPS IVA. Although our study was cross-sectional in design, the data support the lack of skeletal growth already well documented in MPS IVA. The absolute anteroposterior measurement of the bony thoracic inlet did not correlate with tracheal narrowing in our study, but the lack of growth may be the more important feature contributing to tracheal narrowing. For the first time, we have also documented abnormal location of the thyroid gland in children and adults with MPS IVA. The thyroid was located within the thoracic inlet in 70% of children and 82% of adults, and location was significantly correlated with tracheal narrowing. This fact may have implications for emergency airway management. If the cricoid cartilage is also located within the thoracic inlet, the patient may require sternotomy to access the crico-thyroid membrane during emergency tracheostomy.
Thus, the cause of tracheal narrowing, abnormal shape, and tortuosity at the thoracic inlet in MPS IVA is likely multifactorial and hypothesized to a combination of imbalanced growth of the trachea and vasculature, severe skeletal dysplasia with short spine and kyphoscoliosis, short neck with thyroid displaced into the thoracic inlet, clavicular heads encroaching upon the trachea and GAG deposition in tissues throughout the body. All these factors appear to contribute to crowding at the thoracic inlet leading to potentially life-threatening tracheal obstruction. In this progressive disorder, we have shown that focal tracheal narrowing correlates with advancing age in children as well. The youngest patients in our cohort had only mild tracheal narrowing. In fact, one six-year-old girl had no tracheal abnormality at all. Nearly all of our patients in adolescence and adulthood, however, had greater than 50% TCSA narrowing at the thoracic inlet and many greater than 75%. The most severely affected patient in our group was a 19-year-old female whose TCSA measured only 4.5 mm2 ( % of narrowing) at the thoracic inlet.
Characterizing the thoracic inlet anatomy in children and adults with MPS IVA may inform anesthetic care for these patients who undergo many orthopedic procedures during their lifetime. Based on our results, young children (approximately 7-8 years old without symptoms) probably do not need screening for tracheal abnormalities prior to surgery. Older children and adults with a severe phenotype or patients of any age with respiratory symptoms or “look up to the sky” head tilt in an effort to open the airway should be investigated with cross-sectional imaging such as CTA. Virtual bronchoscopy may help the anesthesiologist plan for successful intubation. Furthermore, novel tracheal reconstructive surgery with or without BCA relocation is now being offered to MPS IVA patients with critical airway stenosis in an effort to improve quality of life and life expectancy (Pizarro 2016). Detailed anatomic imaging is a key component of surgical planning.
This study has several limitations. Firstly, our study group included a convenience sample of MPS IVA patients who underwent CTA for a variety of indications ranging from prior difficult airway management during anesthesia to asymptomatic patients with parental concerns about the trachea. In spite of this, we believe our sample is representative of the MPS IVA population in general due to nearly all patients in United States with MPS IVA seek care at our Institution and the International Morquio registry is kept by our Biomedical department (provide a link or website here?) . In this study, we were unable to verify clinical data regarding the severity of the phenotype, respiratory symptoms, height, weight, and exposure to enzyme replacement therapy because scans were acquired for a variety of clinical indications and at multiple centers. Furthermore, the cross-sectional retrospective design of our study does not allow for longitudinal assessment of individual patients. Dynamic airway collapse throughout the respiratory cycle or with neck flexion was not included in the evaluation, which included a single CTA acquisition. Tracheomalacia has been described in MPS patients, and its role in critical airway obstruction in MPS IVA is not fully elucidated [14,26,27,28]. We assumed that airway caliber assessment in our patients reflects best-case conditions using inspiratory imaging in non-sedated patients and comfortable neck positioning on the CT table.
While we have made great progress in understanding critical airway tracheal abnormalities in MPS IVA patients, further work is needed to understand the natural history and clinical significance of such abnormalities in order to provide adequate information to the patients and potentially prevent sudden death among them, In addition, ongoing effort needs to be put in place to provide adequate information to the anesthetic community in order to safely anesthetize MPS IVA patients in order to provide loss of airway which is an ever present threat during their anesthetic care. Planned future investigations will correlate biometric data, respiratory status, and anesthetic history with airway imaging. Correlation of airway and thoracic inlet information gleaned from cervical spine MRI and radiographs with a more detailed CTA technique will be useful as well. Cervical spine MRI, a commonly ordered examination in patients with MPS IVA, may be able to serve as a screening tool for significant tracheal abnormalities, reserving CTA for those needing surgical planning.
Conclusion
Significant narrowing, deviation, and abnormal shape of the trachea at the thoracic inlet are common in children and adults with MPS IVA, with a trend towards increased narrowing with advancing age in children. A worm or triangular-shaped trachea on axial imaging is associated with focal tracheal narrowing, and multiple sites of tracheal narrowing are possible. Crowding of the thoracic inlet, due to vascular tortuosity, bony changes, and thyroid position appears to play a major role.
Footnotes
Conflicts of interest/Competing interests: none to report/not applicable
Ethics approval. This retrospective review of clinically acquired imaging exams was performed after Institutional Review Board approval.
Consent to participate: not applicable
Consent for publication: not applicable
Code availability: not applicable
Availability of data and material (data transparency) –
not applicable?
References
- 1.Hendriksz CJ, Berger KI, Giugliani R, Harmatz P, Kampmann C, Mackenzie WG, Raiman J, Villarreal MS, Savarirayan R (2015) International guidelines for the management and treatment of Morquio A syndrome. Am J Med Genet A 167A(1):11–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khan S, Alméciga-Díaz CJ, Sawamoto K, Mackenzie WG, Theroux MC, Pizarro C, Mason RW, Orii T, Tomatsu S (2017) Mucopolysaccharidosis IVA and glycosaminoglycans. Mol Genet Metab 120(1-2):78–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peracha H, Sawamoto K, Averill L, Kecskemethy H, Theroux M, Thacker M, Nagao K, Pizarro C, Mackenzie W, Kobayashi H, Yamaguchi S, Suzuki Y, Orii K, Orii T, Fukao T, Tomatsu S (2018) Diagnosis and prognosis of mucopolysaccharidosis IVA. Mol Genet Metab 125(1-2):18–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yasuda E, Fushimi K, Suzuki Y, Shimizu K, Takami T, Zustin J, Patel P, Ruhnke K, Shimada T, Boyce B, Kokas T, Barone C, Theroux M, Mackenzie W, Nagel B, Ryerse JS, Orii KE, Iida H, Orii T, Tomatsu S (2013) Pathogenesis of Morquio A syndrome: an autopsied case reveals systemic storage disorder. Mol Genet Metab 109(3):301–311. [DOI] [PubMed] [Google Scholar]
- 5.Tomatsu S, Montano AM, Oikawa H, Smith M, Barrera L, Chinen Y, Thacker MM, Mackenzie WG, Suzuki Y, Orii T (2011) Mucopolysaccharidosis type IVA (Morquio disease): clinical review and current treatment: a special review. Curr Pharm Biotechnol 12:931–945 [DOI] [PubMed] [Google Scholar]
- 6.Doherty C, Averill LW, Theroux M, Mackenzie WG, Pizarro C, Mason RW, Tomatsu S (2017) Natural history of Morquio A patient with tracheal obstruction from birth to death. Mol Genet Metab Rep 14:59–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shimada T, Tomatsu S, Mason RW, Yasuda E, Mackenzie WG, Hossain J, Shibata Y, Montaño AM, Kubaski F, Giugliani R, Yamaguchi S, Suzuki Y, Orii KE, Fukao T, Orii T. Di-sulfated keratan sulfate as a novel biomarker for mucopolysaccharidosis II, IVA, and IVB. JIMD Rep. 2015; 21:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shimada T, Tomatsu S, Yasuda E, Mason RW, Mackenzie WG, Shibata Y, Kubaski F, Giugliani R, Yamaguchi S, Suzuki Y, Orii K, Orii T (2014) Chondroitin 6-sulfate as a novel biomarker for mucopolysaccharidosis IVA and VII. JIMD Rep 16:15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Montano AM, Tomatsu S, Gottesman GS, Smith M, Orii T (2007) International Morquio A registry: Clinical manifestation and natural course of Morquio A disease. J Inherit Metab Dis 30:165–174 [DOI] [PubMed] [Google Scholar]
- 10.Melbouci M, Mason RW, Suzuki Y, Fukao T, Orii T, Tomatsu S. Growth impairment in mucopolysaccharidoses. Mol Genet Metab 2018;124(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Theroux MC, Nerker T, Ditro C, Mackenzie WG (2012) Anesthetic care and perioperative complications of children with Morquio syndrome. Pediatr Anesth 22:901–907 [DOI] [PubMed] [Google Scholar]
- 12.Ransford AO, Crockard HA, Stevens JM, Modaghegh S (1996) Occipito-atlanto-axial fusion in Morquio-Brailsford syndrome. A ten-year experience. J Bone Joint Surg Br 78:307–13. [PubMed] [Google Scholar]
- 13.Drummond JC, Krane EJ, Tomatsu S, Theroux MC, Lee RR. Paraplegia after epidural-general anesthesia in a Morquio patient with moderate thoracic spinal stenosis. Can J Anaesth. 2015; 62(1):45–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pelley CJ, Kwo J, Hess DR (2007) Tracheomalacia in an adult with respiratory failure and Morquio syndrome. Respir Care 52(3):278–282 [PubMed] [Google Scholar]
- 15.Walker PP, Rose E, Williams JG (2003) Upper airways abnormalities and tracheal problems in Morquio’s disease. Thorax 58:458–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shih SL, Lee YJ, Lin SP, Sheu CY, Blickman JG (2002) Airway changes in children with mucopolysaccharidoses: CT evaluation. Acta Radiol 43:40–43 [DOI] [PubMed] [Google Scholar]
- 17.Morimoto N, Kitamura M, Kosuga M, Okuyama T (2014) CT and endoscopic evaluation of larynx and trachea in mucopolysaccharidoses. Mol Genet Metab 112:154–159 [DOI] [PubMed] [Google Scholar]
- 18.Pizarro C, Davies RR, Theroux M, Spurrier EA, Averill LW, Tomatsu S (2016) Surgical reconstruction for severe tracheal obstruction in Morquio A syndrome. Ann Thorac Surg 102(4):e329–e331 [DOI] [PubMed] [Google Scholar]
- 19.Tomatsu S, Averill LW, Sawamoto K, Mackenzie WG, Bober MB, Pizarro C, Goff CJ, Orii T, Theroux M (2016) Obstructive airway in Morquio A syndrome, the past, the present and the future. Mol Genet Metab 117(2):150–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mackenzie CF, McAslan C, Shin B, Schellinger D, Helrich M (1978) The shape of the human adult trachea. Anesthesiology 49(1):48–50 [DOI] [PubMed] [Google Scholar]
- 21.Ulusoy M, Uysal II, Kivrak AS, Ozbek S, Karabulut AK, Paksoy Y, Dogan NU (2016) Age and gender-related changes in bronchial tree: a morphometric study with multidetector CT. Eur Rev Med Pharmacol Sci 20:3351–3357. [PubMed] [Google Scholar]
- 22.Kuo W, Ciet P, Andrinopoulou ER, Chen Y, Pullens B, Garcia-Pena P, Fleck RJ, Paoletti M, McCartin M, Vermeulen F, Morana G, Lee EY, Tiddens HAWM, Normal Chest CT Study Group (2018) Reference values for central airway dimensions on CT images of children and adolescents. AJR Am J Roentgenol 210:423–430. [DOI] [PubMed] [Google Scholar]
- 23.Szelloe P, Weiss M, Schraner T, Dave MH (2017) Lower airway dimensions in pediatric patients: a computed tomography study. Pediatr Anesth 27:1043–1049. [DOI] [PubMed] [Google Scholar]
- 24.Fawcett SL, Gomez AC, Hughes JA, Set P (2010) Anatomical variation in the position of the brachiocephalic trunk (innominate artery) with respect to the trachea: a computed tomography-based study and literature review of innominate artery compression syndrome. Clin Anat 23:61–69. [DOI] [PubMed] [Google Scholar]
- 25.Powell AW, Taylor MD, Burrow TA, Hopkin RL, Prada CE, Jeffries JL (2017) Widespread vasculopathy in a patient with Morquio A syndrome. Tex Heart Inst J 44:420–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kampmann C, Wiethoff CM, Huth RG, Staaz G, Mengel E, Beck M, Gehring S, Mewes T, Abu-Tair T (2017) Management of life-threatening tracheal stenosis and tracheomalacia in patients with mucopolysaccharidoses. JIDM Rep 33:33–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nagano R, Takizawa S, Hayama N, Umemura S, Uesugi T, Nakagawa S, Okamoto S, Yanagimachi N, Takagi S (2007) Three-dimensional CT and histopathological findings of airway malacia in Hunter syndrome. Tokai J Exp Clin Med 32:59–61. [PubMed] [Google Scholar]
- 28.Rutten M, Ciet P, van den Biggelaar R, Oussoren E, Langendonk JG, van der Ploeg AT, Lagneveld M (2016) Severe tracheal and bronchial collapse in adults with type II mucopolysaccharidosis. Orphanet J Rare Dis 11:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
not applicable?


