Abstract
Objective:
[1] review all studies utilizing SDM in the treatment of chronic rhinosinusitis (CRS) [2], increase awareness of otolaryngologists to shared decision-making, and [3] provide a framework for its incorporation into research and clinical practice.
Methods:
systematic search was performed in November 2019 using PubMed/MEDLINE 1947-, CINAHL Complete 1937-, the Cochrane Library, ClinicalTrials.gov, and Web of Science Core Collection (SCI-EXPANDED, SSCI, A&HCI, ESCI) 1900-. All databases were searched from their inception through the date of search. Studies were eligible if they involved a discussion of SDM in the management of CRS. Studies were excluded if they lacked original patient data or outcomes of interest. Identified studies were screened by title/abstract, followed by full-text review. PRISMA guidelines were strictly followed.
Results:
in total, 416 articles met screening criteria. Six were eligible for full text review. Only one study – an expert panel of the framework for the presurgical treatment of CRS - pertained to SDM. While this study mentions that SDM is a critically important piece to optimize care quality, it does not directly investigate the effects of SDM in CRS.
Conclusion:
this review represents a significant negative study that identifies a clear gap in the rhinology literature. Despite the recognized importance of SDM, there have been no interventional studies in the literature to investigate SDM in CRS. This review highlights the need for exploring the role of SDM in rhinological surgery, outlines an overview of SDM and its impact on patient outcomes, and provides a proposed framework for incorporating SDM in research and clinical practice.
Keywords: Shared decision-making, Chronic rhinosinusitis, Patient reported outcome measure, Evidence-based medicine, Rhinology
1. Introduction
Shared decision-making (SDM) is a well-established component of the patient-physician interaction that involves sharing information from physician and patient to inform a treatment plan agreed upon by both, consistent with best practices [1–6]. In doing so, the process of SDM is considered a combination of both evidence-based medicine and patient-centered care that has been shown to improve patient satisfaction and patient outcomes [6–9].
In other fields of medicine, the use of SDM in the treatment of chronic conditions found increase compliance to medical treatments by empowering patients in their own healthcare, and improved patient satisfaction measured by decreased of decisional conflict and decision regret [4,8–11]. Chronic rhinosinusitis (CRS) is a common, chronic condition affected 1 in 8 adults in the United States, with a large symptom burden that is high with direct and indirect costs. CRS is known to diminish sleep, productivity, mood, and even cognitive ability [12–14]. Optimal outcomes in CRS requires active patient participation in their care. For example, nasal saline irrigations, topical medications, and smoking cessation are known to be critically important components of CRS management and entirely dependent on patient engagement [15–17].
While SDM is considered the gold standard of patient care, its integration into the field of otolaryngology as a whole, and in particular rhinology, is still in its infancy [18]. The purpose of this study is to perform a scoping review to evaluate the current state of literature surrounding SDM in the treatment of CRS
2. Methods
A scoping review differs from a traditional systematic review in that the goal is to assess the current status of the medical literature surrounding a topic of interest, as opposed to the collection and consolidation of data regarding a clearly defined question [19]. This scoping review was conducted in accordance to the Preferred Reporting Items for systematic reviews and Meta-Analysis extension for Scoping Reviews (PRISMA-ScR). Given that this review is a scoping review, it was not eligible for registration on PROSPERO.
2.1. Search protocol and study selection
The published literature was searched using strategies created by a medical librarian (RV) to identify studies describing the use of shared decision-making in elective rhinological surgery. The search strategies were established using a combination of standardized terms and key words implemented in PubMed/MEDLINE 1947-, CINAHL Complete 1937-, the Cochrane Library, ClinicalTrials.gov, and Web of Science Core Collection (SCI-EXPANDED, SSCI, A&HCI, ESCI) 1900-. All databases were searched from their inception through November of 2019.
The initial search strategy was designed for PubMed/Medline using both natural language keywords and MEDLINE’s authority controlled Medical Subject Headings (MeSH). The databases were searched for numerous terms describing three main concepts: (a) rhinosinusitis, rhinitis, sinusitis, rhinologic, etc. (b) surgical procedures or surgery, etc. and (c) shared decision-making, patient decision-making, patient centric, etc. All three concepts were combined to identify relevant studies. The PubMed/MEDLINE search strategy was then translated and conducted in the other databases. No publication date restrictions were applied. Studies published in languages other than English were excluded. The full search strategy is provided in the Appendix. Eligible studies included any that discussed, implemented, or evaluated SDM in the management of CRS.
2.2. Data collection
Data extraction was performed by two authors (OAK, JAV). A standardized excel spreadsheet was created and used for this process. All disagreements were discussed between the two authors to arrive at consensus. Data collected included author, year, journal, title, abstract. From these data points, a screening for full-text articles was performed. After review of full-text articles, the eligibility for inclusion in the manuscript was determined.
3. Discussion/Observations
3.1. Study selection
All searches resulted in a total of 504 citations, including duplicates. After removing duplicates, 416 citations remained. All CINAHL results were duplicate results from the first two databases. No relevant articles were found in ClinicalTrails.gov or the Cochrane Library. Review of title and abstracts rendered 6 manuscripts eligible for full text review. Following this second review, only 1 article was determined to be eligible for inclusion into this scoping review. The remaining 5 articles discuss patient-centered decision making in the abstract, however on full text review this concept was defined differently than SDM. Fig. 1 is a visual depiction of the selection process.
Fig. 1.
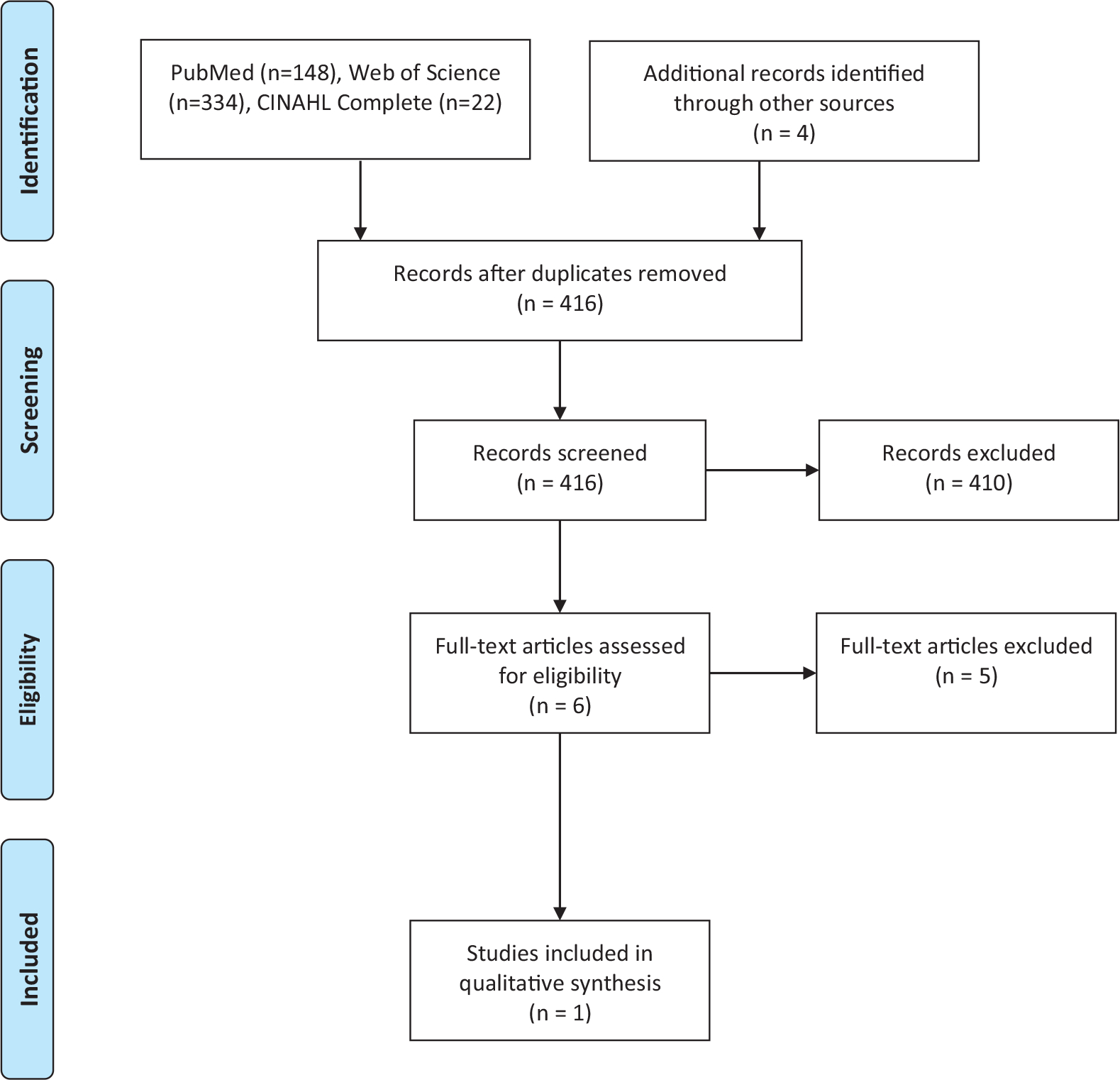
Flow Diagram of Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) for the Systematic Literature Search.
3.2. Selected study
The included manuscript by Mattos et al. is a review from the Quality Improvement Committee of the American Rhinologic Society (ARS) that discusses appropriate perioperative management of CRS. In this study, the authors provide a framework detailing the presurgical management of CRS patients targeting key quality metrics as demonstrated by the current literature to serve as a guide for otolaryngologists. The key metrics included in presurgical care include: diagnosis, medical management, surgical candidacy, and presurgical counseling/discussion. The discussion of the last metric revolves around SDM and reinforces the importance of taking into account patient preferences and expectations. The review mentions that SDM is a critical part of the framework designed for presurgical management of patients with CRS. However, it does not directly investigate the effects of SDM in CRS, nor does it explore the components of SDM as it pertains to otolaryngology [20].
3.3. Introduction in shared-decision making
While the core elements of SDM has been described as early as 1997 [2], the process of implementing it in clinic practice has been lagging [21]. The lag of implementation is likely due to confusion surrounding SDM. At its conception, basic elements are described. However, the overall concept of SDM remained loosely defined, making measurement of SDM inconsistent and without clear outcomes [2,5]. Further work has provided a more tangible framework to SDM in the healthcare setting to provide a guide for clinicians [5]. The currently widely accepted framework includes a list of 9 actionable items including: (1) Define and explain the healthcare problem, (2) present options, (3) discuss pros and cons (benefits, risks, cost), (4) clarify patient values and preferences, (5) discuss patient ability and self-efficacy, (6) present what is known and make recommendations, (7) check and clarify the patients understand, (8) Make or explicitly defer a decision, (9) arrange follow-up.
The physician is tasked with sharing the best evidence regarding risks and benefits of the possible options; the patient shares personal values and situational context [1–5].
This was achieved by reviewing the current condition of their disease, providing evidence-based information regarding the expected trajectory of health status and establishing mutually decided goals. For any given clinical condition, various treatment options or combinations exist. This includes compiling the necessary information regarding different treatment options and presenting in a clear manner to the patient. Care teams can consider including information such as published ranges of improvement in quality-of-life scores with therapeutic options, survey results of most common pre-operative concerns and post-operative experiences, and impact of treatment timing on outcomes. The evidence in these areas in continually evolving and the responsibility of information management falls on the physician and health care team. Providing this information and engaging in informed discussions with patients leads to increased patient motivation resulting in behavioral changes, increased compliance, and overall improved disease control [4,10,11].
The importance of SDM is reflected in its ability to improve outcomes. This is due to SDM’s inherently individualized plans that incorporate not only scientific evidence and medical best practices, but a number of other highly variable and critical factors. These factors include, but are not limited to, patient preferences, culture contexts, trust in medical decisions, perceived impact, and expectations. Engaging patients in treatment plans leads to increase adherence to treatment recommendations, and improved clinical or psychosocial outcomes [4,8,22]. In addition, SDM techniques have been demonstrated to correlate with improved scores on two metrics related to long-term patient satisfaction with surgery: decisional conflict and decisional regret [1,4,23–25].
Decisional conflict - uncertainty in deciding treatment pathway - and decision regret - remorse following treatment choice - are two measurable states that reflect the perception of the health status and best next steps in management of patients or patient families [24]. Decisional conflict occurs when patients have a lack of medical understanding, unclear values in regards to the medical ailment, inadequate support, and/or a sense of pessimism toward the outcome of any of the proposed treatment options [26]. Ultimately, this can lead to delay in health care decisions and emotional stress [4]. Several studies have demonstrated that SDM can significantly improve decision regret and decisional conflict while increasing the quality of the decision made [1,4,23–25]. This positive impact is independent of education level and invasiveness of procedure [27,28]. These concepts are important especially in fields like rhinology where there are multiple treatment options and surgeries are generally considered elective.
Shared decision-making tools (SDMT), like decision aids, are a common and effective way to incorporate SDM into clinical practice. They could easily be utilized in rhinological practice. Information about treatment options including benefits, risks, and the implications of each choice are provided. This can be via printed handout, multimedia module, or other mechanism. Ultimately, the goal is to help ensure that patient choice is aligned with their personal values [1,7,23]. SDMT has been validated in a number of medical conditions, both acute and chronic, and shown to increase participant knowledge, accuracy of risk perception, and decrease decisional conflict [7,23]. A 2017 Cochrane review conducted by Stacey et al. found that the use of SDMT positively affected the communication between health care provider and patients, while decreasing the time until a treatment decision is made [1,7,23]. SDMT are especially useful in situations where there is not a clear-cut evidence-based treatment option [4]. The key that separates SDMT from other forms of educational material is the inclusion of patient value clarification [1].
3.4. Shared decision-making in otolaryngology
In otolaryngology, where conditions often have more than one accepted treatment options, and many conditions are chronic, SDM provides an opportunity to increase the quality of care patients receive. Ikeda et al. identified several concrete examples within otolaryngology where an opportunity exists to make a large impact using SDM. These include, but are not limited to, chronic otitis media, mild obstructive sleep apnea in children, vestibular schwannoma, profound bilateral sensorineural hearing loss, chronic rhinosinusitis, laryngeal carcinoma, and thyroid nodule with indeterminate cytopathology [4]. To date, most studies of SDM in otolaryngology have occurred in the pediatric population [4,28,29]. Chorney et al. identify that pediatric otolaryngology is an ideal case-study of the impact of SDM due to the elective basis and equivocal evidence for surgical intervention versus watchful waiting treatment options in certain conditions such as chronic otitis media and mild obstructive sleep apnea [28]. In this population, parents have high levels of decisional conflict and decision regret, which negatively affects the perceived quality of care that parents report [24,25,27,28]. Conversely, and consistent with several other SDM studies, Hong et al. demonstrated that parents more engaged in the care of their child, as mediated through SDM, experienced significantly less decisional conflict and decision regret [24,25,27,28,30].
Research assessing the use of SDMT within otolaryngology is lacking overall [1,23,28,29].; Bergeron et al. successfully performed a randomized controlled trial seeking to assess clinical outcomes after SDMT were used in the setting of OSA without tonsillar hypertrophy. They found families were in better agreement with treatment option, medical adherence increased, and calls for clarification of the treatment plan decreased. There was also a greater improvement in AHI and oxygen saturation nadir in the intervention group when compared to the control group [23]. An additional SDMT was developed by Petersen et al. for the treatment of laryngeal cancer. This online SDMT for advanced laryngeal cancer provided patients with a resource to educate patient on each treatment option along with the implication on quality of life. This tool also provides patients an opportunity to complete a knowledge and preference test that allow physicians to identify gaps in their knowledge and discuss their preferences. This feasibility trial found high levels of satisfaction in patients who utilized this tool; a multicenter clinical trial is underway [31]. Of note, the use of SDMT are designed as a supplement to conversation clinical encounters to increase patient knowledge and facilitate a more in-depth conversation, and cannot be used as a replacement to the physician-patient encounter [21].
3.5. Shared decision-making in rhinology
While investigations are advancing within the pediatric otolaryngology literature, the remainder of the otolaryngology literature still lags behind. As mentioned above, Ikeda et al. have identified several topics within otolaryngology that would be most amenable to SDM [4]. This study serves as the first step in investigating SDM in CRS. Chronic rhinosinusitis is an ideal target for SDM for several reasons: there is often not a clearly superior treatment choice between medical and surgical treatment of CRS [32), is largely influenced by patient preference, is a management of chronic disease [33], and patient compliance with rhinological care is highly necessary to achieve best outcomes [32,34]. Burden of disease on the individual and society is impressive and increasing. The direct cost of CRS is to be 4.5% of overall health care expenditure, with quality-of-life impact comparable to moderate COPD, CAD, and Parkinson’s disease [35,36].
Based on the current understanding of SDM, increasing its use in the treatment paradigm of CRS is therefore hypothesized to better the quality of care in this disease entity by increasing patient compliance and yield a lesser burden of disease. But as demonstrated in this scoping review, investigations on the impact of SDM on CRS is lacking, without a single interventional study having been conducted and only one review that advocates for the use of SDM in proper presurgical management of CRS.
SDMT are especially useful in situations where there is not a clear-cut evidence-based treatment option [4]. As mentioned earlier, the decision to continue medical management versus surgical management for the treatment of CRS is equivocal at times [32–34]. In this context, SDMT can help guide both clinicians and patients toward a catered treatment plan. Additionally, in rhinology, the importance of minimizing conflict and regret and optimizing patient engagement in the treatment course selected cannot be understated. This is because optimal outcomes require both active patient participation (e.g. adherence to topical routines, compliance with immunotherapy treatment course, etc.) and informed expectations about their clinical course.
3.6. Shared decision-making and patient-reported outcome measures
Notably, while conducting this scoping review within CRS the term “patient-centered” care was a recurring theme. Patient-centered care is an important construct in the management of CRS. However, as currently utilized, it does not indicate incorporating patient values and goals into care plans. Instead, it refers to tailoring patient care based on patient-reported outcome measures (PROM) [4,20,29]. A patient’s subjective improvement in burden of disease is, of course, an important consideration in presurgical management. These subjective improvements are often measured through the use of clinical surveys, most often with the Sinonasal Outcome Tool (SNOT). While this is a critically important factor in the decision-making process, it is important to clarify that this entity is distinct from SDM. In the context of CRS, PROM can be used as a part of the SDM process to discuss pros and cons of each medical option. In of themselves, PROM is not sufficiently comprehensive to be considered SDM [2,21,37].
As the field of otolaryngology works to implement SDM into clinical practice there are potential barriers that are important to address. One key perceived difficulty is the time-barrier of clinic visits. The process of engaging patients in SDM may cause increased difficulty within the time constraints of a clinical encounter [4,38,39]. This has been investigated in predominantly nonsurgical fields [38,39] and a Cochrane review determined that overall there is variable effect on length of consultation ranging from a reduction by 8 min to an additional 23 min. Overall, there was a median addition of 2.5 min. [7] However, previous work has implied a potential of long-term benefits in terms of time and resource savings when patients and their families are engaged in their health care, more adherent with treatment plans, experience less uncertainty, and utilize less health services [4].
This study has a stated overall goal to describe the state of the literature overall, including the impact of implementation in other surgical domains, and the present gap in rhinology. In addition, information regarding the core tenants of SDM upon which future interventions can be designed has been provided. From these tenants, a measurable framework can be created within which to implement and observe SDM. This step is critically important for the future of this field within rhinology, and is paramount for any outcomes research. Future investigations should work to develop rhinology-specific SDM metrics and assess impact of their implementation in CRS management.
It is unlikely that decision-making will truly be equally shared, even in the best shared decision-making model. Rather, decision-making should be viewed as a continuum [5]. On one end, the physician leads the discussion and unilaterally makes decisions for the patient. On the other end, the patient leads the discussion and autonomously makes her or his own decisions. Truly shared decision-making occurs somewhere in the middle of these two extremes.
4. Conclusion
This scoping review identifies the gap in the rhinology literature of the use of SDM in the management of CRS. Despite the unanimous recognition that SDM is important to improve quality of care within otolaryngology, and the surplus of literature on the benefits in fields outside of otolaryngology, there have been no interventional studies in rhinology to investigate SDM. This review highlights the need for studies that investigate outcomes of CRS with the use of SDM to fully understand its potential impact within the CRS patient population. Furthermore, this study provides a framework for the otolaryngologist to increase understanding of SDM and incorporation into the clinical setting.
Supplementary Material
Acknowledgments
The first author, Omar A. Karadaghy, and principal investigator, Jennifer Villwock, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Declaration of Competing Interest
Research reported in this publication was not supported by any grants or source of funding. There are no potential conflicts of interest or disclosures to report.
This study was accepted as a poster presentation for the American Rhinologic Society (ARS) at COSM 2020, but was unable to be presented due to COVID-19 pandemic.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.anl.2021.03.008.
References
- [1].Boss EF, Mehta N, Nagarajan N, Links A, Benke JR, Berger Z, et al. Shared decision making and choice for elective surgical care: a systematic review. Otolaryngol Head Neck Surg 2016;154(3):405–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Charles C, Gafni A, Whelan T. Shared decision-making in the medical encounter: what does it mean? (or it takes at least two to tango). Soc Sci Med 1997;44(5):681–92. [DOI] [PubMed] [Google Scholar]
- [3].Charles C, Gafni A, Whelan T. Decision-making in the physician-patient encounter: revisiting the shared treatment decision-making model. Soc Sci Med 1999;49(5):651–61. [DOI] [PubMed] [Google Scholar]
- [4].Ikeda AK, Hong P, Ishman SL, Joe SA, Randolph GW, Shin JJ. Evidence-based medicine in otolaryngology part 7: introduction to shared decision making. Otolaryngol Head Neck Surg 2018;158(4):586–93. [DOI] [PubMed] [Google Scholar]
- [5].Makoul G, Clayman ML. An integrative model of shared decision making in medical encounters. Patient Educ Couns 2006;60(3):301–12. [DOI] [PubMed] [Google Scholar]
- [6].Hoffmann TC, Montori VM, Del Mar C. The connection be- tween evidence-based medicine and shared decision making. JAMA 2014;312(13):1295–6. [DOI] [PubMed] [Google Scholar]
- [7].Stacey D, Legare F, Col NF, Bennett CL, Barry MJ, Eden KB, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev 2014(1):CD001431. [DOI] [PubMed] [Google Scholar]
- [8].Stewart MA. Effective physician-patient communication and health outcomes: a review. CMAJ 1995;152(9):1423–33. [PMC free article] [PubMed] [Google Scholar]
- [9].Montori VM, Breslin M, Maleska M, Weymiller AJ. Creating a conversation: insights from the development of a decision aid. PLoS Med 2007;4(8):e233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hussain T, Allen A, Halbert J, Anderson CA, Boonyasai RT, Cooper LA. Provider perspectives on essential functions for care management in the collaborative treatment of hypertension: the P.A.R.T.N.E.R. framework. J Gen Intern Med 2015;30(4):454–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Varming AR, Hansen UM, Andresdottir G, Husted GR, Willaing I. Empowerment, motivation, and medical adherence (EMMA): the feasibility of a program for patient-centered consultations to support medication adherence and blood glucose control in adults with type 2 diabetes. Patient Prefer Adheren 2015;9:1243–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Jang DW, Lachanas VA, Segel J, Kountakis SE. Budesonide nasal irrigations in the postoperative management of chronic rhinosinusitis. Int Forum Allergy Rhinol 2013;3(9):708–11. [DOI] [PubMed] [Google Scholar]
- [13].DeConde AS, Soler ZM. Chronic rhinosinusitis: epidemiology and burden of disease. Am J Rhinol Allergy 2016;30(2):134–9. [DOI] [PubMed] [Google Scholar]
- [14].Cox DR, Ashby S, DeConde AS, Mace JC, Orlandi RR, Smith TL, et al. Dyad of pain and depression in chronic rhinosinusitis. Int Forum Allergy Rhinol 2016;6(3):308–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Christensen DN, Franks ZG, McCrary HC, Saleh AA, Chang EH. A systematic review of the association between cigarette smoke exposure and chronic rhinosinusitis. Otolaryngol Head Neck Surg 2018;158(5):801–16. [DOI] [PubMed] [Google Scholar]
- [16].Chong LY, Head K, Hopkins C, Philpott C, Glew S, Scadding G, et al. Saline irrigation for chronic rhinosinusitis. Cochrane Database Syst Rev 2016;4:CD011995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Orlandi RR, Kingdom TT, Hwang PH. International Consensus statement on allergy and rhinology: rhinosinusitis executive summary. Int Forum Allergy Rhinol. 2016;6(Suppl 1):S3–21. [DOI] [PubMed] [Google Scholar]
- [18].Legare F, Stacey D, Turcotte S, Cossi MJ, Kryworuchko J, Graham ID, et al. Interventions for improving the adoption of shared decision making by healthcare professionals. Cochrane Database Syst Rev 2014(9):CD006732. [DOI] [PubMed] [Google Scholar]
- [19].Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med 2018;169(7):467–73. [DOI] [PubMed] [Google Scholar]
- [20].Mattos JL, Soler ZM, Rudmik L, Manes PR, Higgins TS, Lee J, et al. A framework for quality measurement in the presurgical care of chronic rhinosinusitis: a review from the Quality Improvement Committee of the American Rhinologic Society. Int Forum Allergy Rhinol 2018;8(12):1380–8. [DOI] [PubMed] [Google Scholar]
- [21].Stiggelbout AM, Pieterse AH, De Haes JC. Shared decision making: concepts, evidence, and practice. Patient Educ Couns 2015;98(10):1172–9. [DOI] [PubMed] [Google Scholar]
- [22].Hauser K, Koerfer A, Kuhr K, Albus C, Herzig S, Matthes J. Out-come-relevant effects of shared decision making. Dtsch Arztebl Int 2015;112(40):665–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Bergeron M, Duggins A, Chini B, Ishman SL. Clinical outcomes after shared decision-making tools with families of children with obstructive sleep apnea without tonsillar hypertrophy. Laryngoscope 2019;129(11):2646–51. [DOI] [PubMed] [Google Scholar]
- [24].Hong P, Gorodzinsky AY, Taylor BA, Chorney JM. Parental decision making in pediatric otoplasty: the role of shared decision making in parental decisional conflict and decisional regret. Laryngoscope 2016;126(Suppl 5):S5–S13. [DOI] [PubMed] [Google Scholar]
- [25].Hong P, Maguire E, Purcell M, Ritchie KC, Chorney J. Decision-making quality in parents considering adenotonsillectomy or tympanostomy tube insertion for their children. JAMA Otolaryngol Head Neck Surg 2017;143(3):260–6. [DOI] [PubMed] [Google Scholar]
- [26].LeBlanc A, Kenny DA, O’Connor AM, Legare F. Decisional conflict in patients and their physicians: a dyadic approach to shared decision making. Med Decis Making 2009;29(1):61–8. [DOI] [PubMed] [Google Scholar]
- [27].Aarthun A, Akerjordet K. Parent participation in decision-making in health-care services for children: an integrative review. J Nurs Manag 2014;22(2):177–91. [DOI] [PubMed] [Google Scholar]
- [28].Chorney J, Haworth R, Graham ME, Ritchie K, Curran JA, Hong P. Understanding shared decision making in pediatric otolaryngology. Otolaryngol Head Neck Surg 2015;152(5):941–7. [DOI] [PubMed] [Google Scholar]
- [29].Ikeda AK, Hong P, Ishman SL, Joe SA, Randolph GW, Shin JJ. Evidence-based medicine in otolaryngology, part 8: shared decision making-impact, incentives, and instruments. Otolaryngol Head Neck Surg 2018;159(1):11–16. [DOI] [PubMed] [Google Scholar]
- [30].Hess EP, Knoedler MA, Shah ND, Kline JA, Breslin M, Branda ME, et al. The chest pain choice decision aid: a randomized trial. Circ Cardiovasc Qual Outcomes 2012;5(3):251–9. [DOI] [PubMed] [Google Scholar]
- [31].Petersen JF, Berlanga A, Stuiver MM, Hamming-Vrieze O, Hoebers F, Lambin P, et al. Improving decision making in larynx cancer by developing a decision aid: a mixed methods approach. Laryngoscope 2019;129(12):2733–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Harvey R, Hannan SA, Badia L, Scadding G. Nasal saline irrigations for the symptoms of chronic rhinosinusitis. Cochrane Database Syst Rev 2007(3):CD006394. [DOI] [PubMed] [Google Scholar]
- [33].Ah-See KL, MacKenzie J, Ah-See KW. Management of chronic rhinosinusitis. BMJ 2012;345:e7054. [DOI] [PubMed] [Google Scholar]
- [34].Kennedy DW. Defining quality in chronic rhinosinusitis management. Int Forum Allergy Rhinol 2018;8(12):1367–8. [DOI] [PubMed] [Google Scholar]
- [35].DeConde AS, Smith TL. Classification of chronic rhinosinusitis–working toward personalized diagnosis. Otolaryngol Clin North Am 2017;50(1):1–12. [DOI] [PubMed] [Google Scholar]
- [36].Remenschneider AK, D’Amico L, Gray ST, Holbrook EH, Gliklich RE, Metson R. The EQ-5D: a new tool for studying clinical outcomes in chronic rhinosinusitis. Laryngoscope 2015;125(1):7–15. [DOI] [PubMed] [Google Scholar]
- [37].Hoffmann TC, Legare F, Simmons MB, McNamara K, McCaffery K, Trevena LJ, et al. Shared decision making: what do clinicians need to know and why should they bother? Med J Aust 2014;201(1):35–9. [DOI] [PubMed] [Google Scholar]
- [38].Legare F, Thompson-Leduc P. Twelve myths about shared decision making. Patient Educ Couns 2014;96(3):281–6. [DOI] [PubMed] [Google Scholar]
- [39].Whelan T, Sawka C, Levine M, Gafni A, Reyno L, Willan A, et al. Helping patients make informed choices: a randomized trial of a decision aid for adjuvant chemotherapy in lymph node-negative breast cancer. J Natl Cancer Inst 2003;95(8):581–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


