ABSTRACT
Fosmanogepix (FMGX), a novel antifungal available in intravenous (IV) and oral formulations, has broad-spectrum activity against pathogenic yeasts and molds, including fungi resistant to standard of care antifungals. This multicenter, open-label, single-arm study evaluated FMGX safety and efficacy for treatment of candidemia and/or invasive candidiasis caused by Candida auris. Eligible participants were ≥18 years, with established candidemia and/or invasive candidiasis caused by C. auris, (cultured within 120 h [for candidemia] or 168 h [for invasive candidiasis without candidemia] with accompanying clinical signs) and limited treatment options. Participants were treated with FMGX (≤42 days; loading dose: 1000 mg IV twice daily [Day 1], followed by 600 mg IV once daily [QD]). Switching to oral FMGX 800 mg QD was permitted from Day 4. Primary endpoint was treatment success (survival and clearance of C. auris from blood/tissue cultures without additional antifungals) at the end of the study treatment (EOST), assessed by an independent data review committee (DRC). Day 30 survival was a secondary endpoint. In vitro susceptibility of Candida isolates was assessed. Nine participants with candidemia (male:6, female:3; 21 to 76 years) in intensive care units in South Africa were enrolled; all received IV FMGX only. DRC-assessed treatment success at EOST and Day 30 survival were 89% (8/9). No treatment related adverse events or study drug discontinuations were reported. FMGX demonstrated potent in vitro activity against all C. auris isolates (MIC range: 0.008 to 0.015 μg/mL [CLSI]; 0.004–0.03 μg/mL [EUCAST]), with the lowest MICs compared to other antifungals tested. Thus, the results showed that FMGX was safe, well-tolerated, and efficacious in participants with candidemia caused by C. auris.
KEYWORDS: intensive care unit, APX001, Candida auris, Gwt1 inhibitor, candidemia, fosmanogepix
INTRODUCTION
Candida auris is a fungal pathogen that was first identified in 2009 and has since emerged as a global threat. C. auris isolates have been implicated in nosocomial outbreaks worldwide, with 5 distinct clades classified by region of independent emergence. Based on current profiling, these clades are referred to as Clade I (South Asian), Clade II (East Asian), Clade III (African), Clade IV (South American), and Clade V (Iranian) with different genetic determinants of resistance and resulting antifungal resistance profiles. Despite the classification of each clade based on the initial geographical region of detection, transmission in other areas has been reported, with multiple clades identified in Canada, Kenya, and the United States (1–7).
Invasive C. auris infections are associated with high mortality rates, with 60% of hospitalizations due to C. auris infections resulting in death (8). These rates are higher than the observed mortality rates for candidemia/invasive candidiasis, which are estimated to be 25% overall and 31%, respectively, for patients ≥65 years of age (9).
C. auris is well known for its multi-drug resistant characteristics, with most isolates resistant to fluconazole, an important first-line antifungal agent (3, 6, 10, 11). Furthermore, 41% of C. auris isolates from a global study were found to be resistant to 2 or more classes of antifungals (8). Indeed, echinocandins are now generally recognized as the drugs of choice for the treatment of C. auris infections (12). Increased rates of resistance, especially to the azoles, and high mortality rates demonstrate a significant unmet medical need for novel antifungal agents with activity against these Candida species.
Fosmanogepix (FMGX; PF-07842805, APX001, E1211) is the first member in the “gepix” class of antifungals, with a unique mechanism of action (MOA). FMGX is a prodrug that is rapidly converted in vivo by systemic phosphatases to the active moiety manogepix (MGX). MGX inhibits the conserved fungal glycosylphosphatidylinositol (GPI)-anchored wall transfer protein 1 (Gwt1) (13, 14). In yeasts, Gwt1 mediates cross-linking of cell wall mannoproteins to β-1,6-glucan. Inhibition of Gwt1 results in pleiotropic effects on the fungal cell including alterations in fungal adherence to surfaces, inhibition of biofilm and germ tube formation, and subsequently results in severe growth defects and yeast lethality (15). Broad-spectrum antifungal activity has been observed against pathogenic yeasts and molds, including activity against resistant strains (16–18).
Surveillance studies have demonstrated that MGX was the most mycologically active agent against over 400 diverse C. auris isolates, including strains which were multi-drug resistant (19, 20). Another study that focused on 200 C. auris strains collected between 2017 and 2020 in New York and New Jersey reported low MICs for MGX even against pan-resistant strains shown to be resistant to the 3 main classes of antifungal drugs (i.e., azoles, polyenes, and echinocandins) (21). When in vitro activity of MGX was evaluated against eight comparator agents against 122 wild type and non-wild type C. auris isolates, similar findings were reported, with MGX having the most potent antifungal activity against all C. auris strains (22). FMGX improved survival over anidulafungin, an echinocandin, in a disseminated C. auris infection model in immunocompromised mice (13). In a study assessing the efficacy of FMGX in reducing fungal burden and increasing survival of C. auris infected neutropenic mice, similar in vivo efficacy and improved survival was detected, even when treatment was delayed postinfection by 24 h (23). Additionally, with a wide tissue distribution, high oral bioavailability, and availability in both IV and oral formulations, FMGX provides drug characteristics that are favorable for treating invasive fungal infections, such as candidemia/invasive candidiasis caused by C. auris (24, 25).
In a Phase 2 study in 20 patients with invasive candidiasis, FMGX was safe, well-tolerated, and demonstrated a high treatment success rate of 80% at end of study treatment (EOST) and Day 30 survival of 85% (26). In the present Phase 2 study, we aimed to assess the efficacy and safety of FMGX in a similar population of patients with candidemia/invasive candidiasis caused by C. auris, with limited antifungal treatment options.
RESULTS
Disposition, demographics, and exposure.
The study enrolled 9 participants from 2 sites in South Africa (15 participants were planned for enrollment). However, the study was terminated early due to the impact of the COVID-19 pandemic on patient enrollment. The intent-to-treat and safety populations each included all 9 enrolled participants with candidemia (henceforth, study population). Eight participants completed a full course of treatment, and one did not complete treatment due to death. Seven participants completed the study through the 4-week follow-up, with an additional death reported during the follow-up period.
Demographics and baseline characteristics for enrolled participants were consistent with the epidemiology of C. auris infections (Table 1). The study was conducted in intensive care units (ICUs). Underlying diseases leading to C. auris infection included fractures, burns, trauma, and nervous system disorders. All participants had C. auris infections at baseline with no coinfections with other Candida spp. All participants had candidemia only, with no evidence of invasive candidiasis at other sites. All 9 patients received at least 1 dose of an echinocandin antifungal (for ≤4 days) prior to start of FMGX treatment. No other class of antifungal was administered prior to FMGX treatment.
TABLE 1.
Demographics and baseline characteristics
| Parameter | Fosmanogepix N = 9 |
|---|---|
| Age, mean (SDa) [range] yrsb | 49.8 (17.7) [21 to 76] |
| <65 yrs, n (%) | 7 (77.8) |
| ≥65 yrs, n (%) | 2 (22.2) |
| Gender, n (%) | |
| Male | 6 (66.7) |
| Female | 3 (33.3) |
| Race, n (%) | |
| Black or African American | 5 (55.6) |
| White | 3 (33.3) |
| Asian | 1 (11.1) |
| BMIc (kg/m2), mean (SD) | 28.11 (6.6) |
| APACHEd II score, mean (SD) | 12.7 (6.4) |
| <10 | 3 (33.3) |
| 10 to 19 | 4 (44.4) |
| 20 to 30 | 2 (22.2) |
| ICUe, n (%) | 9 (100.0) |
SD, standard deviation.
yrs, years.
BMI, body mass index.
APACHE, acute physiology and chronic health evaluation.
ICU, intensive care unit.
The mean (SD) duration of treatment with FMGX was 19 (5.83) days, ranging from 11 to 27 days; most participants (5/9 [55.6%]) received FMGX for >14 days but ≤28 days. All participants received FMGX via IV infusion; none were switched to the oral formulation. PK sampling was sparse and relatively few PK samples were collected, precluding a model-based compartmental analysis. However, a linear, two-compartment population PK model-based on previous phase I studies provided an adequate fit for the MGX concentrations observed. The PK data collected indicated that all participants had systemic exposures to MGX (median [range] total drug plasma AUC over duration of therapy: 93 [64.4 to 182] mg·h/L), consistent with prior exposure data from Phase 1 and Phase 1b studies in healthy volunteers and in patients with acute myeloid leukemia. MGX AUC:MIC ratios were also determined (median [range] total drug plasma AUC:MIC ratio over duration of therapy: 7147 [5194 to 12112] mg · h/L). Attainment of the total-drug plasma AUC:MIC ratio ED50 target was 100% for total-drug plasma AUC:MIC ratio evaluated on Day 7 and averaged over study treatment. FMGX exposures were minimal, as expected, given the rapid conversion by systemic phosphatases of parent (FMGX) to active moiety (MGX), as observed previously in Phase 1 and Phase 1b studies.
Efficacy.
(i) Primary efficacy endpoint: Treatment success at EOST. DRC-assessed treatment success at EOST was 89% (8/9) in the study population (Table 2). One (11%) participant was a treatment failure at EOST as death occurred during the FMGX treatment period. The participant initially presented with extensive burns (60% partial to full thickness) and acute renal failure 27 days prior to enrollment. The participant eventually developed Gram-negative sepsis and multi-organ failure on Day 10 of the study and died due to cardiac arrest on Day 11. This death was considered unrelated to FMGX. All 4 patients who did not have all preexisting intravascular catheters removed prior to receiving the first dose of FMGX were assessed as treatment successes by the DRC, as were the majority (4/5) of the patients who had their intravascular catheters removed.
TABLE 2.
Efficacy endpoints: treatment success and survival
| Endpoint | Fosmanogepix N = 9 |
|---|---|
| Response at EOSTa | |
| Treatment Successb, n (%) [95% CIc]d | 8 (88.9) [51.8, 99.7] |
| Treatment Failure, n (%) | 1 (11.1) |
| Reasons for failure at EOST | |
| Death, n (%) | 1 (11.1) |
| Response at 2 wks after EOST | |
| Treatment Success Sustained, n (%) [95% CI]d | 6 (66.7) [29.9, 92.5] |
| Clinical Relapse (Positive culture), n (%) | 1 (11.1) |
| Death, n (%) | 1 (11.1) |
| Response at 4 wks after EOST | |
| Treatment Success Sustained, n (%) | 6 (66.7) |
| Survival at Day 30, n (%) | 8 (88.9) |
EOST, end of study treatment.
Treatment Success was defined as meeting all of the following criteria: 2 consecutive blood cultures negative for Candida spp. and/or for participants with a deep-seated site of infection, at least 1 negative tissue culture or aspirate/fluid culture; alive at EOST; and no concomitant use of any other systemic antifungals through EOST.
CI, confidence interval.
95% CIs were two-sided exact binomial CIs.
Secondary efficacy endpoints. (i) Time to first negative blood culture. All participants had positive C. auris blood cultures during the enrollment period. A positive blood culture was reported for 3 participants on Day 1 before starting FMGX treatment (n = 3). For these participants, the mean (SD) time to first negative blood culture was 8.7 (5.51) days.
In the study population, 6 (66.7%) participants demonstrated eradication of C. auris bloodstream infection at EOST, 2 were indeterminate due to missing blood cultures, 1 participant had a recurrence 2 days after stopping FMGX (based on a single positive blood culture but was asymptomatic) (Table 3). All subsequent blood cultures were negative and therefore later assessed as eradication by the investigator. There were no further recurrences during the 4-week follow-up period.
TABLE 3.
Mycological outcomes
| Mycological outcome | Total (N = 9) | no. of Participants by Treatment Outcome | DRC assessed treatment outcome |
|---|---|---|---|
| EOST | |||
| Eradication | 6 | 6 | Treatment Success |
| Indeterminate | 2 | 1: no EOST culture taken | Treatment Success |
| 1: death on Day 11 | Treatment Failure | ||
| Recurrence | 1a | 1a | EOST Treatment Success, followed by early relapse |
| Follow-up period (2 and 4 wks after EOST combined) | |||
| Eradication | 7 | 1a | Recurrence at EOST, subsequently eradication (early relapse) |
| 6 | Treatment Success sustained | ||
| Indeterminate | 2 | 1 death on Day 11 | Treatment Failure at EOST |
| 1 death on Day 36 | Treatment Success at EOST, not sustained through follow-up | ||
| Recurrence | 0 | ||
Patient with investigator-assessed mycological recurrence at EOST visit was assessed as treatment success followed by early relapse by DRC, because the single C. auris in blood culture was sampled 2 days after stopping study drug, de facto in the early follow-up period. All subsequent blood cultures were negative and assessed as eradication by the investigator. EOST, end of study treatment.
(ii) Mycology. All screened C. auris isolates recovered from blood cultures had low MIC values to MGX using both CLSI and EUCAST assay methods (0.008 to 0.015 μg/mL [CLSI] and 0.004 to 0.03 μg/mL [EUCAST]; Table 4). Among all antifungals evaluated, MGX had the lowest MIC values across all C. auris isolates. In the single case of relapse, the C. auris isolate did not have a high MIC value at baseline, nor did the value increase during FMGX treatment. For the study population, no shift in MGX susceptibility was observed in C. auris isolates collected during FMGX treatment. Similar to a previous study on C. auris isolates (22), the agreement between CLSI and EUCAST MICs for MGX were high (equal to or within 1 dilution for 8/9 isolates) while anidulafungin MICs were different (2 to 16-fold) between CLSI and EUCAST methods for all 9 isolates.
TABLE 4.
Activity of manogepix and comparators against C. auris baseline isolates (CLSI and EUCAST methods)a
| MIC (CLSIb/EUCASTc; μg/mL) |
||||||
|---|---|---|---|---|---|---|
| Participant | Amphotericin | Anidulafungin | Micafungin | Fluconazole | Manogepix | Voriconazole |
| 1 | 1/0.5 | 0.5/1 | 0.25/0.25 | >128/>128 | 0.015/0.015 | 2/2 |
| 2 | 1/0.5 | 0.5/0.06 | 0.12/0.03 | >128/128 | 0.015/0.008 | 2/0.5 |
| 3 | 1/0.5 | 1/2 | 0.25/2 | 128/>128 | 0.015/0.03 | 2/8 |
| 4 | 1/0.5 | 1/0.25 | 0.25/0.25 | >128/128 | 0.015/0.004 | 2/0.5 |
| 5 | 1/0.5 | 1/0.06 | 0.25/0.12 | >128/>128 | 0.008/0.008 | 1/1 |
| 6 | 1/0.5 | 1/0.06 | 0.25/0.5 | >128/>128 | 0.015/0.008 | 2/1 |
| 7 | 1/0.5 | 0.5/0.12 | 0.25/0.25 | >128/>128 | 0.015/0.008 | 2/1 |
| 8 | 1/0.5 | 0.5/0.03 | 0.25/0.06 | >128/>128 | 0.008/0.004 | 2/4 |
| 9 | 1/0.5 | 1/0.06 | 0.25/0.12 | >128/>128 | 0.015/0.008 | 2/1 |
MICs of isolates collected at baseline shown for all participants.
CLSI, Clinical & Laboratory Standards Institute.
EUCAST, European Committee on Antimicrobial Susceptibility Testing.
(iii) Secondary safety endpoints. All participants experienced a treatment-emergent adverse event (TEAE); however, none were considered treatment related (Table 5). The most common TEAEs were pyrexia (3 [33.3%]), constipation, multiple organ dysfunction syndrome, pneumonia, pruritus, hypertension, and hypotension (2 [22.2%] each). Two participants experienced 5 serious adverse events (SAEs); all were considered unrelated to treatment. No clinically significant safety concerns related to routine laboratory investigations were identified.
TABLE 5.
Treatment-emergent adverse eventsa
| Category | Fosmanogepix (N = 9) |
|
|---|---|---|
| Participants n (%) | Events n | |
| All TEAEs | 9 (100.0) | 48 |
| Study drug-related TEAEs | 0 | 0 |
| TEAEsb by Maximum Severity | ||
| Mild (CTCAE Grade 1) | 1 (11.1) | 2 |
| Moderate (CTCAE Grade 2) | 2 (22.2) | 5 |
| Severe (CTCAE Grade 3) | 4 (44.4) | 5 |
| Life-threatening (CTCAE Grade 4) | 0 (0.0) | 0 |
| Death (CTCAE Grade 5) | 2 (22.2) | 2 |
| Study drug related TEAEs | 0 (0.0) | 0 |
| SAEs | ||
| All SAEs | 2 (22.2) | 5 |
| Study drug related TESAEs | 0 (0.0) | 0 |
| TEAEs leading to discontinuation of study drug | 1 (11.1) | 1 |
| Study drug related TEAEs | 0 (0.0) | 0 |
AE = adverse event; CTCAE: Common Terminology Criteria for Adverse Events; SAE = serious adverse event; TEAE = treatment-emergent adverse event; TESAE = treatment-emergent serious adverse event.
TEAEs were defined as AEs occurring on or after the first dose of the study drug. Both participants and events are by maximum severity per patient.
Two deaths, both unrelated to FMGX treatment, were reported during the study. One occurred on treatment (Day 11) due to a cardiac arrest in a patient with Gram-negative sepsis, and the second occurred after treatment completion (Day 36) in a patient who developed ventilator-associated pneumonia and multi-organ failure.
DISCUSSION
The goal of this Phase 2 study was to assess the safety and efficacy of FMGX in participants with C. auris candidemia and/or invasive candidiasis. The enrolled population was consistent with the disease epidemiology. All participants were in the ICU and were generally younger (median age of 45 years) and with fewer comorbidities than participants evaluated in prior candidemia and invasive candidiasis studies (27). A high rate of treatment success at EOST and survival at Day 30 (both 88.9%) was reported, along with a durable mycological response. No discontinuations or treatment related AEs were reported. Furthermore, both deaths were considered unrelated to study treatment. MGX was the most active compound tested against all C. auris study isolates with an MIC range of 0.008 to 0.015 μg/mL (CLSI) and 0.004 to 0.03 μg/mL (EUCAST). This is consistent with previous in vitro studies showing MGX to be the most potent antifungal tested against C. auris isolates (21, 22).
C. auris, a well-documented global threat, has caused several independent outbreaks in different geographical areas. Currently, 5 clades have been identified, based on genetic sequence and geographical origin (2, 7). Outbreaks with high fatality have occurred in health care settings in immunosuppressed patients. More importantly, rapid nosocomial spread from person-to-person has also been documented (28). Almost all identified strains are resistant to at least 2 classes of antifungals (azoles and polyenes), with some strains demonstrating multi-drug resistance (azole-polyene-candin) (29). Given these characteristics, C. auris is classified as an urgent global threat by the Centers for Disease Control and Prevention (CDC), with endemic hospital-associated infections being reported worldwide (30). There is a high unmet need for novel antifungals to treat candidemia/invasive candidiasis caused by drug resistant C. auris.
In the United States, the occurrence of infection caused by pan-resistant C. auris isolates was recently reported in several regions. These isolates were resistant to echinocandins, the antifungal class of choice for the treatment of most systemic candida infections (31). It is important to note that these patients all had prior epidemiological links to health care facilities but no prior echinocandin exposure (32).
In addition, outbreaks of C. auris infection are associated with increased length of hospital stay and health care costs, treatment failures, and death. In an analysis of an outbreak involving 34 patients at a large teaching hospital in the United Kingdom, prior antifungal prescription was strongly associated with increased risk of infection. In this case, the cost of infection control was estimated to be more than £1 million, notwithstanding costs related to lost opportunity, consumables, and screening costs. At the same health care facility, outbreak management costs continued to remain high the subsequent year due to an increased persistence of C. auris in the environment (33).
The incidence of C. auris infections have also increased during the COVID-19 pandemic, straining health care capacity, and increasing the risk of multidrug resistant nosocomial infections (34). Thus, there is an urgent need to improve treatment options for affected patients and control the spread of infection in health care facilities worldwide. Although no patients in this study received oral treatment, FMGX has high oral bioavailability and maintenance of plasma exposures when switching from IV or oral dosing. In addition, FMGX has a wide tissue distribution and provides exposures to the eye, gut, and brain, common sites of Candida dissemination (24).
This pilot study had some limitations. The sample size was small since the study was terminated early due to the ICU burden of the COVID-19 pandemic. However, no trial related visits or procedures were negatively impacted by the pandemic, and all protocol mandated study data were collected. All active sites from which participants were enrolled were in South Africa, and the C. auris isolates were likely from Clade III so these results may not be generalizable to all clades. However, Clade III isolates (found in South Africa) have also been detected in other countries, including Canada and the United States (2, 35). In addition, study participants were at high risk of infection with C. auris. A high rate of treatment success at EOST was achieved despite an increased risk of adverse outcomes in participants admitted to intensive care units. However, although none of the study participants had deep-seated C. auris infections, in a delayed therapy animal infection model of C. auris invasive candidiasis, significant reductions in fungal burden were observed in both kidney and brain (consistent with a deep-seated infection) in all dosing cohorts of FMGX in the survival arm of the study (23).
In conclusion, FMGX was safe and well-tolerated and demonstrated activity in participants with candidemia caused by C. auris. Based on the results from this pilot study, as well as a previously completed Phase 2 study in patients with candidemia, FMGX has the potential to be a safe and effective treatment option for patients with candidemia/invasive candidiasis. Additional studies are planned to further assess the safety and efficacy of FMGX in patients with invasive fungal infections.
MATERIALS AND METHODS
This multicenter, open-label, non-comparative, single-arm study (NCT04148287), evaluated the safety and efficacy of FMGX for the treatment of candidemia and/or invasive candidiasis caused by C. auris. The study was planned at 4 sites in South Africa and one site in Panama. Participants were enrolled at 2 sites in South Africa, between 10 Dec 2019 and 23 Oct 2020, in accordance with International Conference on Harmonization Guidelines for Good Clinical Practice, applicable regulatory requirements, and the Declaration of Helsinki.
Eligible participants (males and females, ≥ 18 years of age) had an established diagnosis of candidemia and/or invasive candidiasis caused by C. auris from blood collected <120 h (for participants with candidemia) or from a normally sterile site <168 h (for participants with invasive candidiasis without candidemia) from the time of enrollment, and had limited or no treatment options due to either antifungal resistance, contraindication(s), intolerance, or lack of clinical response to standard of care antifungal therapy, as advocated by the relevant regional/country treatment guidelines (36, 37).
Mycological and clinical diagnoses criteria were: ≥ 1 positive culture from blood or other normally sterile site for C. auris (including possible co-infection with other Candida spp., except C. krusei), attributable clinical signs (fever [>38°C], or hypothermia [<36°C], or hypotension [SBP <90 mmHg or decrease of >30 mmHg]) within the permitted 120 h or 168 h enrollment period for candidemia or invasive candidiasis, respectively.
Exclusion criteria included severe or moderate hepatic impairment (total bilirubin > 3x upper limit of normal and alanine aminotransferase or aspartate aminotransferase > 5x upper limit of normal); concomitant use of strong CYP inhibitors; investigator-assessed life expectancy <7 days; diagnosis of C. krusei infection, deep-seated Candida-related infections requiring >42 days of treatment; and pregnancy or lactation. Participants with severe or moderate renal impairment were initially excluded, but became eligible later on, based on a protocol amendment.
Treatment.
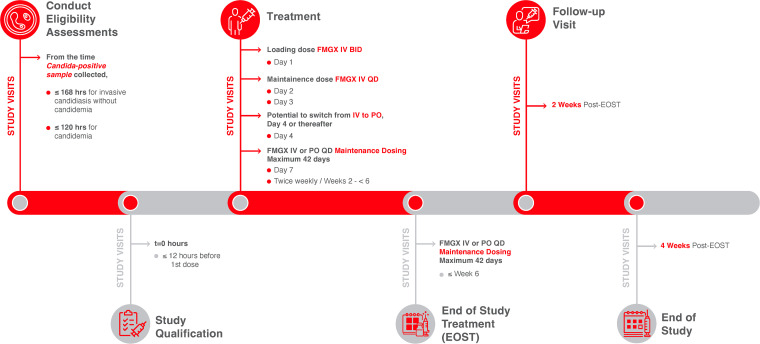
Participants were treated with FMGX within 12 h of study enrollment (Fig. 1). Participants received a 1000 mg FMGX loading dose (3-h IV infusion, twice daily [BID], 12 h apart). The maintenance dose was 600 mg IV, administered as a 3-h infusion once daily (QD). A switch to oral FMGX on Day 4 onwards (800 mg QD maintenance dose) was permitted at the discretion of the investigator if the participant was clinically stable and able to swallow tablets. FMGX was administered for 14 days after clearance of Candida from the bloodstream (2 consecutive negative blood cultures), and in accordance with clinical judgment as applicable for other infected sites, up to a maximum of 42 days. Participants were monitored for continued safety and efficacy at 2 weeks and 4 weeks after EOST.
FIG 1.
Study Design. For participants with invasive candidiasis, maintenance dosing was to continue until there was at least 1 negative tissue culture or aspirate/fluid. For participants with a deep-seated site of infection from which a tissue culture was not obtainable, maintenance dosing was to continue until after the resolution of the attributable clinical signs of infection recorded at baseline, and as applicable, radiological improvement associated with the site of infection. BID, twice daily; EOST, end of study treatment; FMGX, fosmanogepix; IV, intravenous; PO, orally; QD, once daily.
Plasma samples for pharmacokinetic (PK) analysis of FMGX and MGX levels were collected at baseline (pre-dose), twice weekly during treatment, at EOST/early termination (ET), and 2 weeks after EOST. Adverse events (AEs) were recorded from the date of informed consent through study completion, and coded (using Medical Dictionary for Regulatory Activities [MedDRA] version 22.0).
The primary efficacy endpoint was the percentage of participants with DRC-assessed treatment success at EOST, defined as clearance of C. auris from the blood/infection site with no additional antifungal treatment and survival. Clearance was defined as 2 consecutive blood cultures negative for Candida spp., and/or ≥ 1 negative tissue culture or aspirate/fluid culture for deep-seated infections. For participants with a deep-seated infection involving visceral organs from which a tissue culture was not obtainable, resolution of the attributable clinical signs of infection that were recorded at baseline, and as applicable, radiological improvement associated with the site of infection, were required.
Secondary efficacy parameters included all-cause mortality through Day 30, and time to first negative blood culture. The DRC also assessed treatment success at 2 and 4 weeks after EOST. Investigator-assessed secondary parameters included percentage of participants with successful mycological outcomes, treatment success at EOST and 2 and 4 weeks after EOST, and the number of participants with TEAEs.
Data availability.
Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions and exceptions, Pfizer may also provide access to the related individual de-identified participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.
ACKNOWLEDGMENTS
We thank all investigators, staff, and patients for participating in study-related activities and procedures. Milli Kapoor (PhD, employee of Pfizer) assisted with in vitro data collection and analysis; Abhijeet Jakate (PhD, employee of Pfizer) assisted with confirmation of the PK data included. The authors acknowledge Michelle Merrigan (PhD) and Kripa Madnani (PhD, CMPP; employee of Pfizer Inc.) for providing medical writing assistance under the guidance of the authors. Sonia Philipose (PhD, CMPP) and Varkha Agrawal (PhD, CMPP), employees of Pfizer Inc., provided editorial assistance.
The study was funded by Amplyx, now a subsidiary of Pfizer Inc.
Jose Vazquez received grant support from Cidara, Scynexis and was a consultant for Cidara, Scynexis, F2G, Amplyx. Michel R Hodges, Eric Ople, Pamela Wedel were employees of Amplyx (now a Pfizer Inc subsidiary). Michel R Hodges was previously an employee of Pfizer and holds Pfizer stock. Paul A. Bien and Margaret Tawadrous are employees of Pfizer and hold Pfizer stock. Iwona Oborska was a consultant for Amplyx, previously a Pfizer employee, and Pfizer shareholder. Fathima Paruk has served on the speaker bureaus for Thermofischer Scientific, Pfizer, bioMérieux, MSD and Dr Reddy's. Peter Pappas received grant support from Astellas, Gilead, Mayne, Cidara, Scynexis and provided advisory and consultancy services to Amplyx, Mayne, Scynexis. Kenneth Boffard has no conflicts of interest to declare.
REFERENCES
- 1.Ben-Ami R, Berman J, Novikov A, Bash E, Shachor-Meyouhas Y, Zakin S, Maor Y, Tarabia J, Schechner V, Adler A, Finn T. 2017. Multidrug-resistant Candida haemulonii and C. auris, Tel Aviv, Israel. Emerg Infect Dis 23:195–203. doi: 10.3201/eid2302.161486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chow NA, Muñoz JF, Gade L, Berkow EL, Li X, Welsh RM, Forsberg K, Lockhart SR, Adam R, Alanio A, Alastruey-Izquierdo A, Althawadi S, Araúz AB, Ben-Ami R, Bharat A, Calvo B, Desnos-Ollivier M, Escandón P, Gardam D, Gunturu R, Heath CH, Kurzai O, Martin R, Litvintseva AP, Cuomo CA. 2020. Tracing the evolutionary history and global expansion of Candida auris using population genomic analyses. mBio 11:e03364-19. doi: 10.1128/mBio.03364-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Magobo RE, Corcoran C, Seetharam S, Govender NP. 2014. Candida auris-associated candidemia, South Africa. Emerg Infect Dis 20:1250–1251. doi: 10.3201/eid2007.131765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Satoh K, Makimura K, Hasumi Y, Nishiyama Y, Uchida K, Yamaguchi H. 2009. Candida auris sp. nov., a novel ascomycetous yeast isolated from the external ear canal of an inpatient in a Japanese hospital. Microbiol Immunol 53:41–44. doi: 10.1111/j.1348-0421.2008.00083.x. [DOI] [PubMed] [Google Scholar]
- 5.Spivak ES, Hanson KE. 2018. Candida auris: an emerging fungal pathogen. J Clin Microbiol 56:e01588-17. doi: 10.1128/JCM.01588-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vallabhaneni S, Kallen A, Tsay S, Chow N, Welsh R, Kerins J, Kemble SK, Pacilli M, Black SR, Landon E, Ridgway J, Palmore TN, Zelzany A, Adams EH, Quinn M, Chaturvedi S, Greenko J, Fernandez R, Southwick K, Furuya EY, Calfee DP, Hamula C, Patel G, Barrett P, Lafaro P, Berkow EL, Moulton-Meissner H, Noble-Wang J, Fagan RP, Jackson BR, Lockhart SR, Litvintseva AP, Chiller TM. 2017. Investigation of the first seven reported cases of Candida auris, a globally emerging invasive, multidrug-resistant fungus-United States, May 2013-August 2016. Am J Transplant 17:296–299. doi: 10.1111/ajt.14121. [DOI] [PubMed] [Google Scholar]
- 7.Spruijtenburg B, Badali H, Abastabar M, Mirhendi H, Khodavaisy S, Sharifisooraki J, Armaki MT, de Groot T, Meis JF. 2022. Confirmation of fifth Candida auris clade by whole genome sequencing. Emerg Microbes Infect 11:2405–2411. doi: 10.1080/22221751.2022.2125349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lockhart SR, Etienne KA, Vallabhaneni S, Farooqi J, Chowdhary A, Govender NP, Colombo AL, Calvo B, Cuomo CA, Desjardins CA, Berkow EL, Castanheira M, Magobo RE, Jabeen K, Asghar RJ, Meis JF, Jackson B, Chiller T, Litvintseva AP. 2017. Simultaneous emergence of multidrug-resistant Candida auris on 3 continents confirmed by whole-genome sequencing and epidemiological analyses. Clin Infect Dis 64:134–140. doi: 10.1093/cid/ciw691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsay SV, Mu Y, Williams S, Epson E, Nadle J, Bamberg WM, Barter DM, Johnston HL, Farley MM, Harb S, Thomas S, Bonner LA, Harrison LH, Hollick R, Marceaux K, Mody RK, Pattee B, Davis SS, Phipps EC, Tesini BL, Gellert AB, Zhang AY, Schaffner W, Hillis S, Ndi D, Graber CR, Jackson BR, Chiller T, Magill S, Vallabhaneni S. 2020. Burden of Candidemia in the United States, 2017. Clin Infect Dis 71:e449–e453. doi: 10.1093/cid/ciaa193. [DOI] [PubMed] [Google Scholar]
- 10.Chowdhary A, Kumar VA, Sharma C, Prakash A, Agarwal K, Babu R, Dinesh KR, Karim S, Singh SK, Hagen F, Meis JF. 2014. Multidrug-resistant endemic clonal strain of Candida auris in India. Eur J Clin Microbiol Infect Dis 33:919–926. doi: 10.1007/s10096-013-2027-1. [DOI] [PubMed] [Google Scholar]
- 11.Schelenz S, Hagen F, Rhodes JL, Abdolrasouli A, Chowdhary A, Hall A, Ryan L, Shackleton J, Trimlett R, Meis JF, Armstrong-James D, Fisher MC. 2016. First hospital outbreak of the globally emerging Candida auris in a European hospital. Antimicrob Resist Infect Control 5:35. doi: 10.1186/s13756-016-0132-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kordalewska M, Lee A, Park S, Berrio I, Chowdhary A, Zhao Y, Perlin DS. 2018. Understanding echinocandin resistance in the emerging pathogen Candida auris. Antimicrob Agents Chemother 62:e00238-18. doi: 10.1128/AAC.00238-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miyazaki M, Horii T, Hata K, Watanabe NA, Nakamoto K, Tanaka K, Shirotori S, Murai N, Inoue S, Matsukura M, Abe S, Yoshimatsu K, Asada M. 2011. In vitro activity of E1210, a novel antifungal, against clinically important yeasts and molds. Antimicrob Agents Chemother 55:4652–4658. doi: 10.1128/AAC.00291-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Watanabe NA, Miyazaki M, Horii T, Sagane K, Tsukahara K, Hata K. 2012. E1210, a new broad-spectrum antifungal, suppresses Candida albicans hyphal growth through inhibition of glycosylphosphatidylinositol biosynthesis. Antimicrob Agents Chemother 56:960–971. doi: 10.1128/AAC.00731-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McLellan CA, Whitesell L, King OD, Lancaster AK, Mazitschek R, Lindquist S. 2012. Inhibiting GPI anchor biosynthesis in fungi stresses the endoplasmic reticulum and enhances immunogenicity. ACS Chem Biol 7:1520–1528. doi: 10.1021/cb300235m. [DOI] [PubMed] [Google Scholar]
- 16.Hager CL, Larkin EL, Long L, Abidi FZ, Shaw KJ, Ghannoum MA. 2018. In vitro and in vivo evaluation of the antifungal activity of APX001A/APX001 against Candida auris. Antimicrob Agents Chemother 62:e02319-17. doi: 10.1128/AAC.02319-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shaw KJ, Schell WA, Covel J, Duboc G, Giamberardino C, Kapoor M, Moloney M, Soltow QA, Tenor JL, Toffaletti DL, Trzoss M, Webb P, Perfect JR. 2018. In vitro and in vivo evaluation of APX001A/APX001 and other Gwt1 inhibitors against cryptococcus. Antimicrob Agents Chemother 62:e00523-18. doi: 10.1128/AAC.00523-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao M, Lepak AJ, VanScoy B, Bader JC, Marchillo K, Vanhecker J, Ambrose PG, Andes DR. 2018. In vivo pharmacokinetics and pharmacodynamics of APX001 against Candida spp. in a neutropenic disseminated candidiasis mouse model. Antimicrob Agents Chemother 62:e02542-17. doi: 10.1128/AAC.02542-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berkow EL, Lockhart SR. 2018. Activity of novel antifungal compound APX001A against a large collection of Candida auris. J Antimicrob Chemother 73:3060–3062. doi: 10.1093/jac/dky302. [DOI] [PubMed] [Google Scholar]
- 20.Maphanga TG, Naicker SD, Kwenda S, Muñoz JF, Schalkwyk EV, Wadula J, Nana T, Ismail A, Coetzee J, Govind C, Mtshali PS, Mpembe RS, Govender NP, for GERMS-S . 2021. In vitro antifungal resistance of Candida auris isolates from bloodstream infections, South Africa. Antimicrob Agents Chemother 65:e0051721. doi: 10.1128/AAC.00517-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu Y, Kilburn S, Kapoor M, Chaturvedi S, Shaw KJ, Chaturvedi V. 2020. In vitro activity of manogepix against multidrug-resistant and panresistant Candida auris from the New York outbreak. Antimicrob Agents Chemother 64:e01124-20. doi: 10.1128/AAC.01124-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arendrup MC, Chowdhary A, Jørgensen KM, Meletiadis J. 2020. Manogepix (APX001A) in vitro activity against Candida auris: head-to-head comparison of EUCAST and CLSI MICs. Antimicrob Agents Chemother 64:e00656-20. doi: 10.1128/AAC.00656-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wiederhold NP, Najvar LK, Shaw KJ, Jaramillo R, Patterson H, Olivo M, Catano G, Patterson TF. 2019. Efficacy of delayed therapy with fosmanogepix (APX001) in a murine model of Candida auris invasive candidiasis. Antimicrob Agents Chemother 63:e01120-19. doi: 10.1128/AAC.01120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mansbach R, Shaw KJ, Hodges MR, Coleman S, Fitzsimmons ME. 2017. Absorption, distribution, and excretion of (14)C-APX001 after single-dose administration to rats and monkeys. Open Forum Infect Dis 4:S472. doi: 10.1093/ofid/ofx163.1209. [DOI] [Google Scholar]
- 25.Shaw KJ, Ibrahim AS. 2020. Fosmanogepix: a review of the first-in-class broad spectrum agent for the treatment of invasive fungal infections. J Fungi (Basel) 6:239. doi: 10.3390/jof6040239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pappas P, Kullberg BJ, Vazquez JA, Oren I, Rahav G, Aoun M, Bulpa P, Ben-Ami R, Ferrer R, McCarty TP, Thompson GR, III, Barbat S, Wedel P, Oborska I, Schlamm HT, Hodges M. 2020. Clinical safety and efficacy of novel antifungal, fosmanogepix, in the treatment of candidemia: results from a phase 2 proof of concept trial. Open Forum Infect Dis 7:S203–S204. doi: 10.1093/ofid/ofaa439.457. [DOI] [Google Scholar]
- 27.U.S. National Library of Medicine. 2017. Open-label study to evaluate the efficacy and safety of oral ibrexafungerp (SCY-078) in patients with candidiasis caused by Candida auris (CARES) (CARES). https://clinicaltrials.gov/ct2/show/record/NCT03363841. Accessed 25 January 2023.
- 28.Friedman DZP, Schwartz IS. 2019. Emerging fungal infections: new patients, new patterns, and new pathogens. J Fungi 5:67. doi: 10.3390/jof5030067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Al-Obaid I, Asadzadeh M, Ahmad S, Alobaid K, Alfouzan W, Bafna R, Emara M, Joseph L. 2022. Fatal breakthrough candidemia in an immunocompromised patient in Kuwait due to Candida auris exhibiting reduced susceptibility to echinocandins and carrying a novel mutation in hotspot-1 of FKS1. J Fungi 8:267. doi: 10.3390/jof8030267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.U.S. Department of Health and Human Services. 2019. Antibiotic resistance threats in the United States. https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf. Accessed 14 March 2022. [Google Scholar]
- 31.Pappas PG, Kauffman CA, Andes DR, Clancy CJ, Marr KA, Ostrosky-Zeichner L, Reboli AC, Schuster MG, Vazquez JA, Walsh TJ, Zaoutis TE, Sobel JD. 2016. Clinical practice guideline for the management of candidiasis: 2016 update by the infectious diseases society of America. Clin Infect Dis 62:e1–e50. doi: 10.1093/cid/civ933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lyman M, Forsberg K, Reuben J, Dang T, Free R, Seagle EE, Sexton DJ, Soda E, Jones H, Hawkins D, Anderson A, Bassett J, Lockhart SR, Merengwa E, Iyengar P, Jackson BR, Chiller T. 2021. Notes from the field: transmission of pan-resistant and echinocandin-resistant Candida auris in health care facilities ― Texas and the District of Columbia, January–April 2021. MMWR Morb Mortal Wkly Rep 70:1022–1023. doi: 10.15585/mmwr.mm7029a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taori SK, Khonyongwa K, Hayden I, Ad Athukorala GD, Letters A, Fife A, Desai N, Borman AM. 2019. Candida auris outbreak: mortality, interventions and cost of sustaining control. J Infect 79:601–611. doi: 10.1016/j.jinf.2019.09.007. [DOI] [PubMed] [Google Scholar]
- 34.Hanson BM, Dinh AQ, Tran TT, Arenas S, Pronty D, Gershengorn HB, Ferreira T, Arias CA, Shukla BS. 2021. Candida auris invasive infections during a COVID-19 case surge. Antimicrob Agents Chemother 65:e0114621. doi: 10.1128/AAC.01146-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De Luca DG, Alexander DC, Dingle TC, Dufresne PJ, Hoang LM, Kus JV, Schwartz IS, Mulvey MR, Bharat A. 2021. Four genomic clades of Candida auris identified in Canada, 2012–2019. Medical Mycology 60:myab079. doi: 10.1093/mmy/myab079. [DOI] [PubMed] [Google Scholar]
- 36.Govender NP, Avenant T, Brink A, Chibabhai V, Cleghorn J, Du Toit B, Govind C, Lewis E, Lowman W, Mahlangu H, Maslo C, Messina A, Mer M, Pieton K, Seetharam S, Sriruttan C, Swart K, van Schalkwyk E. 2019. Federation of infectious diseases societies of Southern Africa guideline: recommendations for the detection, management and prevention of healthcare-associated Candida auris colonisation and disease in South Africa. S Afr J Infect Dis 34:163. doi: 10.4102/sajid.v34i1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hodges MR, Ople E, Shaw KJ, Mansbach R, van Marle S, van Hoogdalem E, Wedel P, Kramer W. 2017. First-in-human study to assess safety, tolerability and pharmacokinetics of APX001 administered by intravenous infusion to healthy subjects. Open Forum Infect Dis 4:S526–S526. doi: 10.1093/ofid/ofx163.1370. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions and exceptions, Pfizer may also provide access to the related individual de-identified participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.