Abstract
The gerP1 transposon insertion mutation of Bacillus cereus is responsible for a defect in the germination response of spores to both l-alanine and inosine. The mutant is blocked at an early stage, before loss of heat resistance or release of dipicolinate, and the efficiency of colony formation on nutrient agar from spores is reduced fivefold. The protein profiles of alkaline-extracted spore coats and the spore cortex composition are unchanged in the mutant. Permeabilization of gerP mutant spores by coat extraction procedures removes the block in early stages of germination, although a consequence of the permeabilization procedure in both wild type and mutant is that late germination events are not complete. The complete hexacistronic operon that includes the site of insertion has been cloned and sequenced. Four small proteins encoded by the operon (GerPA, GerPD, GerPB, and GerPF) are related in sequence. A homologous operon (yisH-yisC) can be found in the Bacillus subtilis genome sequence; null mutations in yisD and yisF, constructed by integrational inactivation, result in a mutant phenotype similar to that seen in B. cereus, though somewhat less extreme and equally repairable by spore permeabilization. Normal rates of germination, as estimated by loss of heat resistance, are also restored to a gerP mutant by the introduction of a cotE mutation, which renders the spore coats permeable to lysozyme. The B. subtilis operon is expressed solely during sporulation, and is sigma K-inducible. We hypothesize that the GerP proteins are important as morphogenetic or structural components of the Bacillus spore, with a role in the establishment of normal spore coat structure and/or permeability, and that failure to synthesize these proteins during spore formation limits the opportunity for small hydrophilic organic molecules, like alanine or inosine, to gain access to their normal target, the germination receptor, in the spore.
Spore germination is initiated by the interaction of the germinant molecule with a receptor in the spore. The nature of this receptor is not yet proven, but the available evidence suggests that the genes of the gerA family whose products are required for the response to specific germinants are likely to encode this receptor (16, 20). The trigger reaction commits spores to undergo a series of successive events which result in the loss of spore dormancy and resistance properties. Spores of Bacillus cereus initiate germination in response to l-alanine or ribosides, of which inosine is the most effective (8). Inhibition of the alanine racemase activity associated with spores by O-carbamyl d-serine is necessary to observe maximum rates of l-alanine-triggered germination, as d-alanine is a competitive inhibitor (8). The first measurable event after commitment is the loss of heat resistance (a rise in spore internal pH, a release of monovalent ions, and a release of dipicolinic acid (DPA) and calcium ions from spores are also early events), and later events include the activation of spore lytic enzymes (7, 17), selective cortex hydrolysis, and rehydration of the spore core. The genetic analysis of spore germination has concentrated on Bacillus subtilis; in addition to operons required for germinant-specific responses, such as gerA, gerB, and gerK, genes whose products are required for germination in several germinants have been identified, such as gerD. The products of genes required for the germination response to multiple types of germinant could represent proteins activated by the initial signal transduction mechanism (14). Analysis of B. cereus germination mutants has identified germinant-specific loci, such as gerI, a homologue of the gerA family of operons, required for inosine germination (5). In an attempt to isolate mutants with germination defects in both inosine and alanine, an operon has been identified which, rather than encoding a common element in the germination mechanism, appears to be required for the establishment of spore permeability properties.
MATERIALS AND METHODS
Strains and culture conditions.
Strains used in this study are listed in Table 1. Routine culture media were L broth for Escherichia coli and Oxoid nutrient broth for B. cereus and B. subtilis. Synchronous sporulation was by the resuspension method (25). Conditions for spore formation and washing and germination monitoring by loss of optical density (OD) and release of DPA were as previously described for B. cereus (5). Spores of B. subtilis were prepared and washed as previously described in reference 5, but germination conditions were as described in reference 15, except that the germination buffer was 10 mM Tris-HCl, pH 8.4, containing 2.24 mg of KCl ml−1.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant genotype and/or phenotype | Source or reference |
|---|---|---|
| Strains | ||
| B. cereus | ||
| 569 UM20.1 | trp-1 Strr (wild type for this study) | 1, 5 |
| AM1334 | Tn917-LTV1::gerPC1 | Transposon mutagenesis of UM20.1 |
| B. subtilis | ||
| 1604 | trpC2 (laboratory wild type) | 15 |
| BFS3015 | yisF::pMUTIN4 trpC2; lacZ fusion to yisF | This study |
| BFS3037 | yisD::pMUTIN4 trpC2; lacZ fusion to yisD | This study |
| AM1401 | pMUTIN4::yisF trpC2 | 1604 × DNA (BFS3015)a |
| AM1402 | pMUTIN4::yisD trpC2 | 1604 × DNA (BFS3037)a |
| AM1398 | trpC2 (parent of BFS strains) | |
| SH132 | trpC2 sigKΔ19::pVO12(Pspac-sigK) Cmr | 19 |
| AM1394 | trpC2 sigKΔ19::pVO12(Pspac-sigK) CmryisF::pMUTIN4 | AM1401 × DNA(SH132)a |
| AH64 | trpC2 metC3 ΔcotE::cat | A. Henriques |
| AM1423 | pMUTIN4::yisF trpC2 ΔcotE::cat | AM1401 × DNA (AH64)a |
| Plasmids | ||
| pRS11 | Pspac-sigF Cmr | R. Schmidt (22) |
| pDG180 | Pspac-sigE Kmr | 21 |
| pDG298 | Pspac-sigG Kmr | 26 |
Transformation cross showing the donor strain as DNA.
Transposon mutagenesis and mutant screening.
Transposon mutagenesis using pLTV1 was as described by Clements and Moir (5). The method of scoring potential germination mutants, modified from that of Irie et al. (10), involved transfer of spore-containing colonies to filter paper and thence onto agar containing specific germinants and 2,3,5-triphenyl tetrazolium chloride, described in detail in reference 5.
DNA sequencing.
The sequence of B. cereus clones was determined by cycle sequencing by using an ABI 373A DNA sequencer. Sequences were obtained on both strands and were fully overlapped. Staden programs (24) were used for sequence assembly and analysis.
Construction of null mutations in B. subtilis genes.
Integrational mutagenesis of B. subtilis genes with pMUTIN4 (27) used primers internal to the affected genes. For yisD, which has 399 bases in the reading frame, bases 34 to 51 and 228 to 245 were used for forward and reverse primers, respectively, with HindIII- and BamHI-bearing extensions, respectively, to allow cloning into pMUTIN4. For yisF (615 bases in coding sequence), primers extended from 123 to 141 (forward, with 5′ HindIII extension) and 386 to 404 (reverse, with 5′ BamHI extension). As discussed in reference 27, these constructions inactivate the gene and create a transcriptional fusion of the gene with a promoterless lacZ gene, allowing analysis of expression. A Pspac promoter is introduced downstream of the plasmid insertion.
Permeabilization procedures.
Permeabilization procedures for B. cereus were based on the UDS method of Brown et al. (3); spores (2 to 4 mg of dry weight ml−1) were incubated at 37°C for 90 min in 5 mM 2-(N-cyclohexylamino)ethanesulfonic acid (CHES) buffer, pH 8.6, containing 8 M urea, 70 mM dithiothreitol, and 1% (wt/vol) sodium dodecyl sulfate (SDS). The spores were then pelleted and washed five times with ice-cold distilled water. Spores were then examined by phase-contrast microscopy to confirm that they remained phase bright, and the permeability to lysozyme was checked by measuring the loss of OD at 580 nm (OD580) of an aliquot of the spore suspension incubated in NaCl (50 mM) with lysozyme (30 μg ml−1). This gave 30 to 40% OD loss in less than 30 min, demonstrating that the extraction had removed coat layers sufficiently to allow this enzyme to penetrate to the cortex and induce cortex lysis. The permeabilized spores were heat activated for 30 min at 70°C, then cooled and used within 2 h.
Permeabilization of B. subtilis spores (A. Atrih, personal communication) was in 10 mM Tris HCl, pH 8.5, 0.1 M NaCl, 0.1 M dithiothreitol, and 0.5% (wt/vol) SDS. Spore washing, confirmation of permeabilization by lysozyme, and heat activation were all as described for B. cereus.
Spore coat extraction procedures.
Spore coat extraction procedures with detergents or alkali were as described by Nicholson and Setlow (18). The assay of β-galactosidase during sporulation, using methylumbelliferyl-β-d-galactoside as substrate, was also as described by Nicholson and Setlow.
RESULTS
Isolation of the gerP1 mutant of B. cereus.
Pools of B. cereus 569 UM20.1 cells carrying a chromosomal copy of Tn917-LTV1 were generated as described by Clements and Moir (5), and washed spore suspensions were prepared then enriched for mutants that remained chloroform resistant after incubation in a germinant mixture of alanine and inosine. To increase the proportion of potential germination mutants amongst the survivors, the enrichment procedure was repeated. A colony transfer method of scoring the reduction of tetrazolium chloride by germinating spores was used as a primary screen for germination mutants, by using separate plates with alanine and inosine as germinants. Germination mutants that were strongly germination defective in both alanine and inosine by this test were obtained from all 10 pools of mutagenized spores. However, there was a likelihood that some of the mutants could contain separate transposon insertions and independent point mutations in separate ger genes, selected during the cycling and enrichment procedures. Generalized transduction mediated by phage CP51ts, using a Trp+ B. cereus 569 strain as recipient, was used to test the linkage of the germination defect to the erythromycin and lincomycin resistance of the transposon. Out of 22 potential ger mutants tested, only for two, both derived from the same mutagenesis regime, was the complete germination defect 100% linked to the transposon resistance markers. Most strains contained combinations of a point mutation and a transposon mutation, separately affecting germination in alanine or inosine. Later work revealed that the two mutants were probably siblings, as they contained the identical transposon insertion, and therefore only one, strain AM1334, carrying the mutation Tn917 LTV1::gerP1, is described.
Germination behavior of the gerP1 mutant.
Suspensions of the parental strain germinate rapidly and synchronously in either l-alanine or inosine. In contrast, spores of AM1334 (gerP1) show no significant loss of OD in inosine, and the rate of OD loss in l-alanine is much reduced (Fig. 1A). Early events in germination do not proceed normally; spores lose heat resistance over a much longer timescale than normal (Fig. 1B), and little DPA is released (Fig. 1C). It was noted that the slower germination behavior was reflected in a reduced colony-forming ability of dormant mutant spores on L Agar or nutrient agar (20 to 25% that of the wild type [2]). Heat activation (70°C for 30 min) improved the colony-forming efficiency of both types of spores approximately threefold, so that 40% of wild-type spores (as counted by light microscopy) formed colonies, but the ratio of wild-type to mutant colony-forming ability remained approximately 5:1.
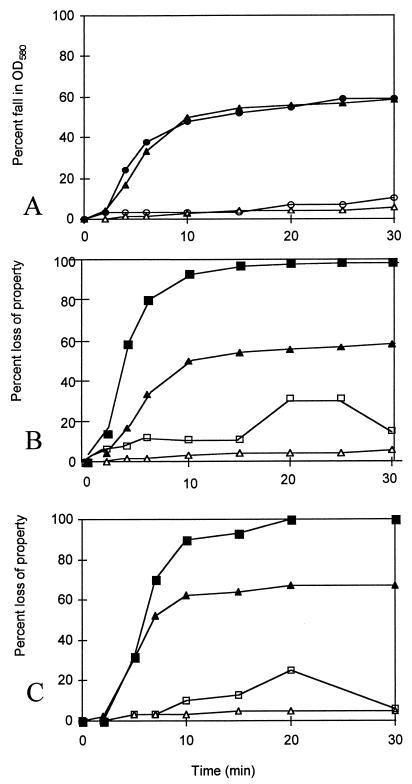
FIG. 1.
The germination properties of spore suspensions of B. cereus 569 (wild type) and AM1334 (gerPC1). (A) The fall in OD580 of B. cereus spore suspensions (wild type) in either l-alanine (●) or inosine (▴) and of gerPC1 in l-alanine (○) or inosine (▵). (B) The loss of heat resistance (■) and fall in OD580 (▴) of spore suspensions (wild type) and loss of heat resistance (□) and OD loss (▵) of gerPC1 in inosine. (C) The release of DPA (■) and fall in OD580 (▴) of the wild type and release of DPA (□) and fall in OD580 (▵) of gerPC1 spore suspensions, germinating in inosine. Panels A, B, and C represent separate experiments.
Germination properties of coat-extracted spores of the gerP1 mutant.
Washed permeabilized spores were heat activated, washed once with ice-cold water, and stored on ice. Their germination responses are presented in Fig. 2. Although loss of heat resistance in response to germinant is rapid, permeabilized spore suspensions of the wild type lose much less OD than normal, suggesting that some late germination event has been inhibited by the detergent extraction. However, the mutant now responds to germinant in precisely the same manner as the wild type. This demonstrates that the components required for specific germinant-induced early events are still intact in both wild-type and mutant spores. The colony-forming efficiencies of dormant wild-type and mutant spores, after permeabilization, were now identical, at 108 per OD unit.
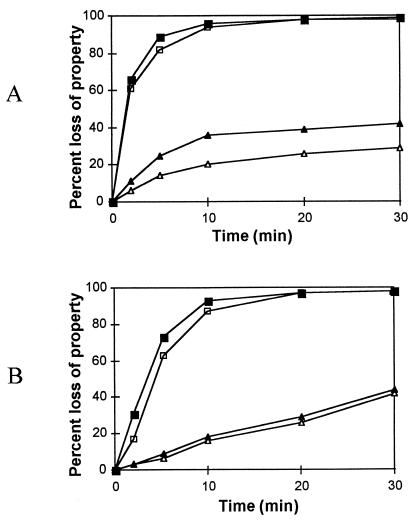
FIG. 2.
The germination properties of permeabilized spores of B. cereus 569 in inosine (A) or l-alanine (B). Loss of heat resistance (■) and fall in OD580 (▴) for spore suspensions of the wild type. The open symbols represent the percent loss of respective properties for the gerPC1 mutant spores.
Structure and resistance of gerP1 spores.
The retention of an active germination system in the gerP1 mutant seen in permeabilized spores suggests that the defect results from a failure of the germinant to gain access to its normal target, rather than from an absence of an essential germination component. The properties of mutant spores were therefore examined. Transmission electron microscopy of thin spore sections revealed no detectable difference between the wild type and the mutant (data not shown), and the profile of SDS-polyacrylamide gel electrophoresis-separated coat proteins extracted using detergent or NaOH was unchanged (2); spores of the mutant were lysozyme resistant (2). The colony-forming ability of spores of the wild type and AM1334 after heating in water at 80°C and at 95°C is shown in Fig. 3A and B, respectively. The wild type showed an almost constant logarithmic destruction during heating. The unusual plating behavior of the mutant results in a low recovery of unheated (zero time) spores. The heating of mutant spores resulted in biphasic destruction curves, including an initial increase of recovery, presumably due to activation of the spores, followed by a later logarithmic reduction, which closely matched the inactivation kinetics of wild-type spores. Therefore, an initial activation of the “super-dormant” mutant population appears to be superimposed on a thermal denaturation profile indistinguishable from that of the wild type. The spore cortices of AM1334 and the wild type appeared identical in high-pressure liquid chromatography analysis of digestion products of the cortices (A. Atrih, personal communication). Therefore, no gross defect in either cortex or coat could be detected.
FIG. 3.
Isothermal destruction curves for spore suspensions at 80°C (A) and at 95°C (B). Symbols: ⧫, wild-type spores; ■, gerPC1 spores. Suspensions of wild-type and mutant spores were adjusted to the same initial OD.
Characterization of the gerP locus.
Tn917-LTV1 has been designed to allow the rapid cloning in E. coli of DNA flanking the site of insertion, as it contains ColE1 replication functions, an antibiotic resistance gene selectable in E. coli, and a cluster of restriction sites (4). Chromosomal DNA was isolated from AM1334, digested with EcoRI, diluted, ligated, and then used to transform E. coli DH5α. The plasmid recovered (pJBD1) contained the expected vector fragment and a 1.8-kb insert. Only DNA from the lacZ-proximal side of the transposon is recovered by this means. A λZAP Express (Stratagene) library of B. cereus chromosomal DNA containing fragments of 4 to 9 kb from a partial Sau3A digest was constructed and probed with the insert fragment from pJBD1. Two hybridizing phages were purified, and phagemids pJB1 and pJB2 were excised. The larger, pJB1, contained a 5.5-kb insert, encompassing the complete gerP region (Fig. 4). The sequence of the cloned region on either side of the point of transposon insertion has been determined and deposited in GenBank (accession no. AF053927). This revealed a cluster of six genes (gerPA to gerPF) followed by a potential rho-independent terminator (Fig. 4). The putative operon is preceded by a small gene (named yisI, to correspond with its B. subtilis homologue, as discussed below). This gene would be transcribed in the same direction as gerP, but is separated from the gerP region by a potential rho-independent terminator. Potential ribosome binding sites (RBSs) are appropriately located for each open reading frame (ORF). Those for gerPB, gerPD, and gerPE all overlap with the end of the previous ORF; in contrast, there are two longer intergenic regions: a 52-base region between the stop codon of gerPB and the RBS of gerPC, which contains the site of transposon insertion in the gerP1 mutant, and a 26-base region between the stop codon of gerPE and the RBS of gerPF. The organization and relationships between gene products described below suggests that the six ORFs are likely to represent an operon. Another ORF on the gerP-distal side of yisI, and read in the opposite direction, was partially sequenced and was found to be a homologue of yisK of B. subtilis (2).
FIG. 4.
The gene organization of the gerP locus in B. cereus and B. subtilis. The extent of B. cereus clones is indicated above the chromosomal region, which shows the point of transposon insertion. The B. cereus gerP operon and the homologous B. subtilis operon (yisH to yisC) are shown. Figures above the B. subtilis ORFs indicate percentage of amino acid identity to the equivalent B. cereus ORF.
Four of the GerP proteins are relatively small (64 to 73 residues), and all except GerPB have a predicted pI in the acidic range. The only region of hydrophobic amino acid sequence long enough to represent a membrane-spanning helix is found at the N terminus of GerPE. The GerPA, GerPB, GerPD, and GerPF proteins are related in sequence, with, for example, 42% identity between GerPA and GerPF (Fig. 5). GerPB and GerPD are related, and their N-terminal half shares homology with that of the GerPA-GerPF pair. The C-terminal half of GerPB-GerPD is less conserved, and it is rich in glycine, proline, and alanine residues, suggesting an extended structure. The GerPC protein, at 204 amino acids, and GerPE, at 128 residues, are encoded by the larger ORFs and have no homologues.
FIG. 5.
An alignment of the primary sequences of homologous protein products of the gerP locus of both B. cereus and B. subtilis. The equivalent genes in the two species are adjacent in the alignment.
An operon homologous to gerP is present in the B. subtilis genome sequence (13) (Fig. 4). The gene organization in the immediately surrounding region is identical in the two species, except that the flanking B. subtilis yisJ and yisB genes have no counterpart in this region in B. cereus. The degree of amino acid identity for the ORFs of the gerP operons of B. subtilis and B. cereus is indicated in Fig. 4 above the B. subtilis ORFs.
Mutation of the yisH-yisC (gerP) operon of B. subtilis.
The germination properties of spores of yisF and yisD mutants of B. subtilis, generated by integrational inactivation with pMUTIN4 (27), and transfer of the mutations into our laboratory strain, are compared with the wild-type parent in Fig. 6. Both mutants germinate slowly in alanine, and also slowly in the alternative combination of germinants for B. subtilis, asparagine, glucose, fructose, and KCl. The rate of OD loss is higher than that seen for the B. cereus mutant; the defect is less extreme in B. subtilis. This behavior is matched by the normal plating efficiency of these mutants in B. subtilis. Chemical permeabilization of the spores increased the germination rate in response to both germinants (Fig. 7 shows the data for germination in l-alanine). The permeabilization conditions used for B. subtilis were less harsh, and only 60% of the yisF mutant spores had been permeabilized to lysozyme (compared to >90% for the wild-type, yisD mutant, and B. cereus spores). This probably explains the lower response of the yisF mutant spores after chemical permeabilization compared to the other preparations.
FIG. 6.
Fall in OD580 of B. subtilis spore suspensions in 1 mM l-alanine and 10 mM KCl (A) or AGFK (20 mM asparagine, 8 mM glucose, 8 mM fructose, and 20 mM KCl) (B). Symbols: ■, 1604 wild type; ◊, AM1402 yisD; and ○, AM1401 yisF.
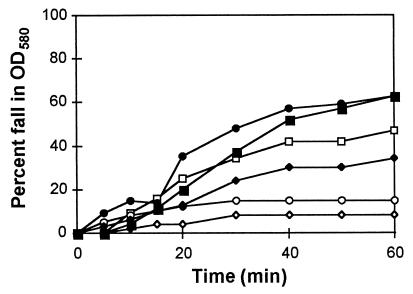
FIG. 7.
The fall in OD580 of B. subtilis spore suspensions after permeabilization. Symbols for different spore suspensions are □, wild-type spores; ○, yisD (AM1402); and ◊, yisF (AM1401) mutant. Open symbols represent intact, nonpermeabilized spores. Solid symbols represent the fall in OD580 of the respective suspensions of permeabilized spores.
In B. subtilis, the disruption of function of either the yisF or yisD gene causes a generally similar, though less extreme, defect in spore physiology to that observed in the gerPC transposon mutant of B. cereus, in which expression of the last four ORFs of the equivalent operon is disrupted by the transposon insertion. It is not known whether the less extreme defect introduced by the yisD and yisF mutations in B. subtilis reflects difference in the importance of these proteins in the B. subtilis spore coat or whether it results from residual function of the intact upstream genes (or downstream genes, as an uninduced Pspac promoter is present) in the gerP operon in these insertional mutants.
Consequences of introduction of a cotE mutation.
A cotE yisF mutant was constructed to test whether permeabilization of the coats by introduction of a cotE mutation (6) gave the same result as chemical permeabilization. The lysozyme sensitivity of spores of the double mutant was confirmed, and washed spores were germinated in l-alanine. Germination, as estimated by loss of heat resistance of spores, was increased twofold on introduction of the cotE mutation, restoring the germination response to that of the wild type (Fig. 8).
FIG. 8.
Germination of B. subtilis spores in l-alanine, as measured by loss of heat resistance (70°C for 30 min). Squares represent 1604 (wild type), circles represent AM1401 (yisF), and triangles represent AM1423 (yisF cotE).
Regulation of expression of the yisH-yisC (gerP) genes of B. subtilis.
Although the transposon used for B. cereus mutagenesis carried a lacZ reporter, the insertion in B. cereus AM1334 (gerPC1) was in the wrong orientation to create a transcriptional fusion to the lacZ gene. The insertional mutagenesis in B. subtilis was designed specifically to create such fusions, and measurement of lacZ expression in AM1401 and AM1402 cultures induced to sporulate synchronously (25), by using a sensitive fluorescence assay, reveals that expression of yisF and yisD is switched on at the same time, after 3 h of sporulation (Fig. 9). This level of expression is just detectable using a classical o-nitrophenyl-β-d-galactopyranoside assay, but is easily measured with the fluorigenic substrate methylumbelliferyl-β-d-galactoside.
FIG. 9.
Expression of lacZ fusions to yisD (▴) and yisF (⧫) during synchronous sporulation. ■, the β-galactosidase activity of a control strain (1604). MUG, methylumbelliferyl-β-galactoside.
The spatial and temporal control of gene expression during sporulation is mediated by successive sporulation-specific sigma factors. The introduction of isopropyl-β-d-thiogalactopyranoside (IPTG)-inducible versions of these sporulation sigma factors, under Pspac control, resulted in expression of yisD on induction of sigma K, but not on induction of sigmas E, F, or G. (Fig. 10). Similar results were obtained for yisF (data not shown). Examination of the sequence upstream of the first gene of the cluster in both species reveals sequences that could represent potential sigma K-dependent promoters. Introduction of the yisD-lacZ or yisF-lacZ fusions into a gerE36 mutant background resulted in dramatic overexpression of these genes, as estimated by a plate assay, spraying the fluorigenic substrate on sporulating colonies. A more detailed analysis would be necessary to determine whether this reflects a direct role of GerE in negative regulation of these genes, or possibly an indirect effect, resulting from the increased levels of sigma K in a gerE mutant (9).
FIG. 10.
The expression of a yisD-lacZ fusion by induction of sporulation-specific sigma factors during vegetative growth. Symbols: ⧫, sigma F; ●, sigma E; ▴, sigma G; and ■, sigma K. With the exception of the sigma K-inducible strain (AM1394), the sigma factors were carried on the relevant plasmids described in Table 1, introduced into AM1402. Graphs for sigma F, sigma E, and sigma G induction are superimposed on the baseline—no lacZ induction was observed.
DISCUSSION
This work has identified a novel cluster of genes, organized in an operon-like arrangement, that is required for formation of functionally normal spores in both B. cereus and B. subtilis. These genes are only expressed during sporulation, in the mother cell compartment around the time of spore coat synthesis and assembly. Expression is sigma K-dependent and negatively regulated by GerE, a major regulator of coat protein gene expression (6). The proteins may be structural components of the spore or components required during the morphogenetic process but not represented in the mature spore. Immunochemical analysis would be required to distinguish these possibilities. The absence of some of these proteins does not appear to result in any major changes in spore structure, as revealed by transmission electron microscopy, or any major changes in coat protein composition, as demonstrated by gel electrophoresis of extracted proteins. The expression of the gene cluster in B. subtilis is easily detectable by lac fusion analysis, but the level of β-galactosidase synthesis is not as high as would be expected for highly expressed genes encoding major coat proteins.
The outer layers of spore coat and integument in B. subtilis and B. cereus are rather different in ultrastructure: B. cereus spores have a coat that appears thinner, in terms of the number of coat layers, and the spores are surrounded by an exosporium. The range and size of extractable spore coat proteins is also very different. Despite the extensive analysis of coat genes and proteins in B. subtilis, there has so far been little study of the molecular composition of integument layers in B. cereus. In both types of spores, however, the absence of at least some of the GerP proteins causes a defect in spore germination, more extreme in B. cereus, which can be relieved by extraction of coat layers sufficiently to permeabilize the spore to lysozyme. The residual defect in loss of heat resistance in response to germinant in a B. subtilis yisF mutant is overcome on introduction of a cotE mutation, which causes a defect in assembly of the spore outer coat and an increase in spore permeability (6).
The effect of coat protein extraction on germination of B. cereus T spores in a mixture of alanine and inosine has already been described (12, 23). Extraction does not inhibit the response of the spore to germinants as determined by loss of heat resistance, although it does reduce the amount of OD loss observed. Germination by inosine and alanine is dependent on more than one class of GerA homologues (5), but the response to each of these individually in the gerP mutant is similarly affected. As the germinant-specific response is still observed, the primary initiating target for germinants in the spore is unaffected by this extraction of outer layer proteins, although the completion of later events is severely disturbed. The intact spores of the B. cereus gerP mutant represent a type of super-dormant spore, whose latency can be overcome to some extent by extreme heating or by extraction of the spore, permeabilizing it to molecules of the size of lysozyme. It appears that the integument in the gerP mutant may be abnormally impermeable to germinants, as on its removal, they can once more access their primary target(s) with at least the normal kinetics. The inner coat layers may represent a general barrier to the passage of small organic molecules, as reported for glucose at 4°C in Bacillus megaterium QMB1551 (11).
The GerP proteins could contribute directly to a structural element normally present in the spore that facilitates transfer of such molecules across the integument under physiological conditions; an alternative interpretation is that they are required for proper assembly of other coat proteins into a structure that allows passage of germinants. Whichever is the correct interpretation, the phenotype of the gerP mutants focuses attention on our lack of understanding of the permeability properties of the outer layers of the bacterial spore and on the need of germinant to traverse these layers to initiate the early stages of the germination response.
To give these genes a designation based on the germination defect of mutants is not ideal, but this at least indicates an associated phenotype. In the absence of evidence of a direct coat defect, we have adopted the gerP terminology for B. cereus and suggest that the same gene designations be adopted in B. subtilis.
ACKNOWLEDGMENTS
This work was supported by a postgraduate studentship award to J.B. from the Ministry of Health and Medical Education, Iran; by the European Union Bacillus subtilis functional analysis programme (contract BIO4-CT95-0278) to the laboratories of A. Moir and Simone Seror; and by a BBSRC project grant to A. Moir.
Emma Ratcliffe and Mark Gidley are thanked for their contribution to the analysis of cotE mutant spores.
REFERENCES
- 1.Battisti L, Green B D, Thorne C B. Mating system for transfer of plasmids among Bacillus anthracis, Bacillus cereus, and Bacillus thuringiensis. J Bacteriol. 1985;162:543–550. doi: 10.1128/jb.162.2.543-550.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Behravan J. Characterisation of the gerP spore germination operon of Bacillus cereus. Ph.D. thesis. Sheffield, England: University of Sheffield; 1998. [Google Scholar]
- 3.Brown W C, Vellom D, Ho I, Mitchell N, McVay P. Interaction between a Bacillus cereus spore hexosaminidase and specific germinants. J Bacteriol. 1982;149:969–976. doi: 10.1128/jb.149.3.969-976.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Camilli A, Portnoy D A, Youngman P. Insertional mutagenesis of Listeria monocytogenes with a novel Tn917 derivative that allows direct cloning of DNA flanking transposon insertions. J Bacteriol. 1990;172:3738–3744. doi: 10.1128/jb.172.7.3738-3744.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clements M O, Moir A. Role of the gerI operon of Bacillus cereus 569 in the response of spores to germinants. J Bacteriol. 1998;180:6729–6735. doi: 10.1128/jb.180.24.6729-6735.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Driks A. Bacillus subtilis spore coat. Microbiol Mol Biol Rev. 1999;63:1–21. doi: 10.1128/mmbr.63.1.1-20.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Foster S J, Johnstone K. Pulling the trigger: the mechanism of bacterial spore germination. Mol Microbiol. 1990;4:137–141. doi: 10.1111/j.1365-2958.1990.tb02023.x. [DOI] [PubMed] [Google Scholar]
- 8.Gould G W. Stimulation of l-alanine-induced germination of Bacillus cereus spores by d-cycloserine and O-carbamyl-d-serine. J Bacteriol. 1966;92:1261–1262. doi: 10.1128/jb.92.4.1261-1262.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ichikawa H, Halberg R, Kroos L. Negative regulation by the Bacillus subtilis GerE protein. J Biol Chem. 1999;274:8322–8327. doi: 10.1074/jbc.274.12.8322. [DOI] [PubMed] [Google Scholar]
- 10.Irie R, Okamoto T, Fujita Y. A germination mutant of Bacillus subtilis deficient in response to glucose. J Gen Appl Microbiol. 1982;28:345–354. [Google Scholar]
- 11.Koshikawa T, Beaman T C, Pankratz H S, Nakashio S, Corner T R, Gerhardt P. Resistance and permeability correlates of Bacillus megaterium spores successively divested of integument layers. J Bacteriol. 1984;159:624–632. doi: 10.1128/jb.159.2.624-632.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kutima P M, Foegeding P M. Involvement of the spore coat in germination of Bacillus cereus T spores. Appl Environ Microbiol. 1987;53:47–52. doi: 10.1128/aem.53.1.47-52.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Medina N, Vannier F, Roche B, Autret S, Levine A, Seror S J. Sequencing of regions downstream of addA (98°) and citG (289°) in Bacillus subtilis. Microbiology. 1997;143:3305–3308. doi: 10.1099/00221287-143-10-3305. [DOI] [PubMed] [Google Scholar]
- 14.Moir A, Smith D A. The genetics of bacterial spore germination. Ann Rev Microbiol. 1990;44:531–553. doi: 10.1146/annurev.mi.44.100190.002531. [DOI] [PubMed] [Google Scholar]
- 15.Moir A, Lafferty E, Smith D A. Genetic analysis of spore germination mutants of Bacillus subtilis 168: the correlation of phenotype with map location. J Gen Microbiol. 1979;111:165–180. doi: 10.1099/00221287-111-1-165. [DOI] [PubMed] [Google Scholar]
- 16.Moir A, Kemp E H, Robinson C, Corfe B M. The genetic analysis of bacterial spore germination. J Appl Bacteriol. 1994;76:9S–16S. [PubMed] [Google Scholar]
- 17.Moriyama R, Kudoh S, Miyata S, Nonobe S, Hattori A, Makino S. A germination-specific spore cortex-lytic enzyme from Bacillus cereus spores: cloning and sequencing of the gene and molecular characterisation of the enzyme. J Bacteriol. 1996;178:5330–5332. doi: 10.1128/jb.178.17.5330-5332.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nicholson W L, Setlow P. Sporulation, germination and outgrowth. In: Harwood C R, Cutting S M, editors. Molecular biological methods for Bacillus. Chichester, England: John Wiley & Sons; 1990. pp. 391–450. [Google Scholar]
- 19.Oke V, Losick R. Multilevel regulation of the sporulation transcription factor ςK in Bacillus subtilis. J Bacteriol. 1993;175:7341–7347. doi: 10.1128/jb.175.22.7341-7347.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paidhungat M, Setlow P. Isolation and characterization of mutations in Bacillus subtilis that allow spore germination in the novel germinant d-alanine. J Bacteriol. 1999;181:3341–3350. doi: 10.1128/jb.181.11.3341-3350.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Popham D, Setlow P. Cloning, characterisation and expression of the spoVB gene of Bacillus subtilis. J Bacteriol. 1991;173:7942–7949. doi: 10.1128/jb.173.24.7942-7949.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmidt R, Margolis P, Duncan L, Coppolacchia R, Moran C P, Losick R. Control of development transcription factor ςF by sporulation regulatory proteins SpoIIAA and SpoIIAB in Bacillus subtilis. Proc Natl Acad Sci USA. 1990;87:9221–9225. doi: 10.1073/pnas.87.23.9221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Senesi S, Freer G, Batoni G, Barnini S, Capaccioli A, Cercignani G. Role of spore coats in the germinative response of Bacillus cereus to adenosine and its analogues. Can J Microbiol. 1992;38:38–44. doi: 10.1139/m92-006. [DOI] [PubMed] [Google Scholar]
- 24.Staden R. Finding protein coding regions in genomic sequences. Methods Enzymol. 1990;183:163–180. doi: 10.1016/0076-6879(90)83012-x. [DOI] [PubMed] [Google Scholar]
- 25.Sterlini J M, Mandelstam J. Commitment to sporulation in Bacillus subtilis and its relationship to development of actinomycin resistance. Biochem J. 1969;113:29–37. doi: 10.1042/bj1130029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun D, Stragier P, Setlow P. Identification of a new ς-factor involved in compartmentalised gene expression during sporulation of Bacillus subtilis. Genes Dev. 1989;3:141–149. doi: 10.1101/gad.3.2.141. [DOI] [PubMed] [Google Scholar]
- 27.Vagner V, Dervyn E, Ehrlich S D. A vector for systematic gene inactivation in Bacillus subtilis. Microbiology. 1998;144:3097–3104. doi: 10.1099/00221287-144-11-3097. [DOI] [PubMed] [Google Scholar]