Abstract
Currently, organic solvents are necessary for the preparation of anionic liposomes for siRNA delivery. The removal of organic solvent is time‐consuming and the residual organic solvent is not only a hidden danger, but also affects the stability of anionic liposomes. Glycerol, which is physiologically compatible and does not need to be removed, is used to promote the dispersion of lipids and the formation of anionic liposomes. Additionally, the preparation process is simple and not time‐consuming. The results showed that anionic liposomes, which were typically spherical with a particle size of 188.9 nm were successfully prepared with glycerol. And with the help of Ca2+, siRNA was encapsulated in anionic liposomes. The highest encapsulation efficiency at 2.4 mM Ca2+ reached 91%. And the formation of calcium phosphate could promote the endosomal escape of siRNA effectively. The results from cell viability showed that the anionic liposomes had no obvious cytotoxicity. It was also verified that anionic liposomes could improve the resistance of siRNA against degradation. Additionally, siRNA delivered by anionic liposomes could play an effective role in knockout. Therefore, anionic liposomes prepared with glycerol will be a safe and effective delivery platform for siRNA and even other nucleic acid drugs.
Keywords: anionic liposome, organic solvent, siRNA
Anionic liposomes can be prepared without organic solvents The anionic liposomes prepared with glycerol have high safety The anionic liposomes can be used for effective siRNA delivery.

1. INTRODUCTION
Due to the instability and negative charge of siRNA, the vehicles used to package siRNA for delivery are necessary [1, 2]. In addition, these vehicles must be able to overcome physiological barriers, in which endo/lysosomal degradation is most important [3, 4]. Cationic polymers and liposomes are the most used vehicles that utilise positive charges to compress siRNA with negative charges [5, 6, 7]. However, the use of excess positive charges often causes cytotoxicity [8, 9, 10]. These positively charged substances will bind to proteoglycans on the cell surface by electrostatic interactions, which will interfere with processes such as cell adhesion and cell division, thus resulting in cell death [11].
In order to overcome the defects of cationic liposomes, ionisable lipid nanoparticles were proposed to reduce the toxicity caused by positive charges. However, the acute immune responses and long‐term toxicity caused by ionisable lipids are still obstacles [12]. Anionic liposomes, which are composed of physiologically safe lipids and have a negative charge are a safer alternative to cationic liposomes [9, 13]. Anionic liposomes differ from cationic liposomes and ionisable lipid nanoparticles in that they do not cause inhibition of cell proliferation and cell death through charge interaction with cells due to their negative charge. In addition, they do not cause destabilisation of the cytoplasmic membrane. It has been reported in the literature that anionic liposomes do not cause cellular changes, including cell shrinkage, reduced mitochondrial numbers and cytoplasmic vacuolisation, which are usually caused by cationic lipids [8, 13]. However, the encapsulation efficiency, which is only 7%–9% of anionic liposomes for siRNA, is poor due to electrostatic repulsion between anionic liposomes and siRNA [14, 15]. Divalent cations (such as Mg2+, Mn2+, Ba2+ and Ca2+) are often used to help overcome the electrostatic repulsion between siRNA and anionic liposomes [16]. Among these divalent cations, Ca2+ is superior in gene transfection due to the promotion of endo/lysosomal escape. Many investigations reported that positively charged Ca2+ can bind to negatively charged phosphate groups in nucleic acids to form calcium phosphate (CaP) [17, 18, 19, 20]. CaP can dissolve rapidly in a weak acidic environment, which triggers the proton sponge effect and the rupture of endosomes, thereby achieving endo/lysosomal escape and releasing nucleic acids into the cytoplasm [20, 21, 22, 23, 24, 25, 26, 27].
Although anionic liposomes can be used for siRNA delivery with the help of Ca2+, there is still a great challenge. That is, the organic solvents such as chloroform and methanol need to be used during the preparation of anionic liposomes [28, 29, 30, 31]. These residual solvents have potential toxicity and affect the stability of anionic liposomes [28]. While there are ways to reduce residual organic solvents, such as dialysis, these processes are cumbersome and time‐consuming [30]. Glycerol is biocompatible, and is able to reduce the interaction between the hydrophobic section of lipids and water, to increase the dispersion of liposomes in water and to prevent the sedimentation of liposomes, thus increasing the stability of liposomes. In addition, there is no need to remove glycerol during preparation [32, 33, 34], because 3% v/v glycerol can also serve as an isotonic agent in the preparation of liposomes; this is beneficial to its application in vivo [35, 36]. It has been used in heating method to prepare and increase the stability of liposomes with no organic solvents [28, 29, 37]. Currently, anionic liposomes prepared with glycerol have been proved to have higher safety [28].
Here, to avoid the organic solvents, 3% v/v glycerol was used in the preparation of anionic liposomes for siRNA delivery. Additionally, Ca2+ was used to achieve the encapsulation of siRNA by anionic liposomes and the endosomal escape of siRNA by the formation of CaP. As shown in Scheme 1a, siRNA was encapsulated in anionic liposomes prepared without organic solvents by Ca2+. After being taken up into cells (Scheme 1b), anionic lipoplexes were captured by the endosomes. Next, the anionic liposomes fused with the endosomal membranes. Then the sponge effect induced by CaP caused the endosomes to rupture, thereby releasing the siRNA. The release of the siRNA into the cytoplasm inhibited protein expression. This siRNA delivery system prepared without organic solvents had higher safety and the process of preparation was convenient in which there was no need to remove organic solvents.
Scheme 1.

Schematic illustration of anionic lipoplexes (a) and the process of interfering with protein expression by anionic lipoplexes (b). After being taken up into cells, anionic lipoplexes are captured by endosomes. Next, the anionic liposomes fuse with the endosomal membranes, followed by the rupture of the endosomes by calcium phosphate (CaP), thereby releasing the siRNA.
2. MATERIALS AND METHODS
2.1. Materials
siRNA (siGAPDH, sense 5′‐GUAUGACAACAGCCUCAAGdTdT‐3′ and antisense 5′‐CUUGAGGCUGUUGUCAUACdTdT‐3′) was synthesised by Sangon Biotech Co., Ltd. (Shanghai, China). Cy 5 was modified on the 5′ end of sense. (1,2‐Dioleoyl‐sn‐glycero‐3‐[phospho‐rac‐(1‐glycerol) (DOPG) and 1,2‐dioleoyl‐sn‐glycero‐3‐phosphoethanolamine (DOPE) were obtained from Shanghai Aladdin Biochemical Technology Co., Ltd. (Shanghai, China). Phosphate‐buffered saline and foetal bovine serum (FBS) were purchased from Gibco Life Technologies Co., Ltd. (Shanghai, China). DAPI, Lipo6000 and CCK8 were obtained from Beyotime Biotechnology (Shanghai, China). Dulbecco's modified Eagle's medium (DMEM) was purchased from KeyGEN Biotech (Nanjing, China). LysoTracker™ Green DND‐26 was obtained from Invitrogen Co., Ltd. (Shanghai, China). Glycerol was obtained from Sigma‒Aldrich (Shanghai, China). Then, 4% paraformaldehyde (Biosharp Life Sciences; Hefei, China) was used directly. RNase A was purchased from TransGen Biotech (Beijing, China). Anti‐GAPDH mouse monoclonal primary antibody was acquired from Abcam (Shanghai, China), anti‐bcl2 rabbit polyclonal antibody was obtained from Proteintech (Wuhan, China), and anti‐actin mouse monoclonal primary antibody was purchased from Cwbio Bio. (Beijing, China), both of which were used after dilution in Tris‐buffered saline with Tween‐20 (TBST). The secondary antibodies were horseradish peroxidase‐conjugated AffiniPure goat anti‐mouse and anti‐rabbit IgG (Elabscience Biotechnology Co., Ltd., Wuhan, China) and Alexa Fluor 488‐conjugated goat anti‐mouse and anti‐rabbit IgG (Sangon Biotech Co., Ltd., Shanghai, China).
2.2. Preparation of anionic liposomes and lipoplexes (anionic liposomes/Ca2+/siRNA)
Liposomes were prepared by the following method. DOPG and DOPE (molar ratio 4:1) were dispersed in phosphate‐buffered saline (pH 7.4) and stirred at 1000 rpm for 30 min at 70°C. Then, glycerol (final concentration 3% v/v) was added at 70°C and stirred for 15 min. Liposomes were collected and extruded into vesicles using a 100 nm polycarbonate membrane. The final liposomes were stored at 4°C for later use.
Anionic lipoplexes were prepared by incubating with different ratios of Ca2+/lipid/siRNA at 25°C for 20 min at 200 rpm. To study the preparation of lipoplexes, the concentration of siRNA was kept constant at 200 nM, the lipid concentration from 0.5 to 3 μg/ml and CaCl2 at a concentration of 0–9.6 mM. RNase A was used to verify whether siRNA was encapsulated inside the liposomes. siRNA (3 ng) was digested by RNase A (0.75 μg) at 37°C for 30 min.
2.3. Encapsulation efficiency
siRNA‐Cy 5 was used to determine the encapsulation efficiency. The intensity of Cy5 was detected by a Hitachi F‐4600 fluorescence spectrophotometer at λ ex = 649 nm, λ em = 664 nm before and after the 0.5% triton‐X 100 being added into the anionic lipoplexes. The encapsulation efficiency was calculated using the following formula given below:
2.4. Native polyacrylamide gel electrophoresis (PAGE)
Anionic lipoplexes were analysed using 6% native PAGE in 1× TBE buffer. The running condition was 100 V (constant voltage) at room temperature. Then, the gel was stained with gel red for 30 min at room temperature. Finally, the gel was imaged by a Tanon 5200 multi automatic chemiluminescence/fluorescence image analysis system (Tanon, Shanghai, China).
2.5. Dynamic light scattering
The diameter, polydispersity index (PDI) and zeta potentials of the samples were measured by a Zeta PALS (Brookhaven, USA).
2.6. Transmission electron microscopy
A drop of the sample was dropped onto the copper mesh, and then observed by Transmission electron microscopy (TEM). Samples were either observed directly or after being negatively stained with 2% phosphotungstic acid (FEI Tecnai G2, USA).
2.7. Stability of anionic lipoplexes
siRNA (2.6 ng) was incubated with RNase A (0.065 μg) at 37°C at different times. siRNA (3.25 ng) was incubated with FBS (1 μL) at 37°C at different times. The products of anionic lipoplexes were analysed by 6% native PAGE. The products of naked siRNA were analysed by 15% native PAGE.
2.8. Cell lines and cell culture
HeLa cells were cultured in DMEM containing 10% FBS, penicillin (50 UI/mL) and streptomycin (50 UI/mL). The culture environment was maintained at 37°C in suitable humidity with 5% CO2.
2.9. Cellular uptake
Cells were seeded in the dish at a density of 1 × 105 cells/dish and cultured for 24 h. Then, the cells were incubated with Lipo6000/siGA or anionic lipoplexes/siGA (final concentration of siRNA: 50 nM) at different times. Subsequently, the cells were fixed with 4% paraformaldehyde at room temperature for 10 min. Nuclei were stained with DAPI, and a confocal laser scanning microscope (CLSM, Leica TCS SP8) was used to acquire images.
2.10. Endosomal escape behaviour
Cells were seeded in the dish at 1 × 105 cells/dish. After 24 h, the cells were treated with Lipo6000/siGA or anionic lipoplexes/siGA (final concentration of siRNA: 50 nM) for 3 h. Subsequently, the cells were treated with LysoTracker™ Green DND‐26 (1:1000 dilution) for 2 h at 37°C. Then, the cells were fixed with 4% paraformaldehyde at room temperature for 10 min and dyed with DAPI at room temperature for 20 min. The fluorescence was detected by CLSM (Leica TCS SP8).
2.11. Immunofluorescence
Cells were seeded in the dish at a density of 5 × 104 cells/dish. After being cultured for 24 h, the cells were incubated with Lipo6000/siGA, Lipo6000/siNC, anionic lipoplexes/siGA, and anionic lipoplexes/siNC (final concentration of siRNA: 50 nM) for 5 h. After being cultured for 48 h, the cells were fixed with 4% paraformaldehyde for 40 min and treated with 0.1% Triton X‐100 for 5 min. Next, the cells were blocked with 5% bovine serum albumin at 37°C for 2 h. Then, the cells were incubated with primary GAPDH antibody (100 dilution) at 4°C for 16 h. Finally, after incubation with Alexa Fluor 488‐conjugated goat anti‐mouse IgG at 37°C (300 dilution) for 2 h, the results were analysed by a fluorescence microscope (Nikon ECLIPSE Ni).
2.12. Western blot
Cells were seeded into 6‐well plates at a density of 5 × 104 cells/dish. After 24 h, the cells were treated with Lipo6000/siGA, Lipo6000/siNC, anionic lipoplexes/siGA, or anionic lipoplexes/siNC (final concentration of siRNA: 50 nM) for 5 h. Then, the cells were cultured in a medium for 48 h. RIPA lysis buffer was used to collect the proteins. Twenty micrograms of protein were separated by 8% SDS‒PAGE and transferred to PVDF membranes (0.22 μm). Subsequently, PVDF membranes were blocked with 5% nonfat milk powder in TBST. After that, the primary GAPDH antibody at a dilution of 1:2000 and primary actin antibody at a dilution of 1:1000 were added to the PVDF membrane at 4°C for 16 h. Then, the membrane was incubated with horseradish peroxidase‐conjugated AffiniPure goat anti‐mouse IgG secondary antibody at 25°C (3000 dilution) for 1.5 h. ECL was used to visualise protein bands, and the bands were acquired by a Tanon 5200 multi automatic chemiluminescence/fluorescence image analysis system (Tanon, Shanghai, China).
2.13. Cell viability
A total of 3000 cells per plate were seeded onto 96‐well plates. When the cell confluence reached 50%, the cells were cultured in medium containing Lipo6000/siRNA and anionic lipoplexes at different concentrations and for varying amounts of time. After incubation for a predetermined time, DMEM containing CCK8 was added to each well. The absorbance at 450 nm was measured after incubation with CCK8 for 1 h.
2.14. Statistical analysis
All data that were repeated at least three times are expressed as mean ± standard deviation (SD). The significance of differences was analysed by GraphPad Prism version 8.0 (GraphPad Software Inc.) using one‐tailed Student's t‐test. p values < 0.05 were defined as significant.
3. RESULTS
3.1. Preparation and characterisation anionic liposomes
To verify whether anionic liposomes were obtained by glycerol successfully, Dynamic light scattering (DLS) and TEM were used to characterise the anionic liposomes. As shown in Figure 1a, the size of the anionic liposomes was 188.90 ± 13.03 nm (PDI: 0.196 ± 0.029) with a potential of −29.72 ± 7.25 mV. In addition, TEM images showed that the morphology of empty liposomes was obvious spherical structures with negative staining (Figure 1b) and the lipid bilayer was electron transparent without negative staining (Figure 1c).
FIGURE 1.

Characterisation of anionic liposomes. (a) The diameter of anionic liposomes prepared with glycerol. Transmission electron microscopy (TEM) images of anionic liposomes negatively stained with 2% phosphotungstic acid (b) and without negative staining (c).
3.2. Optimisation and characterisation of anionic lipoplexes
To investigate whether Ca2+ can realise the encapsulation of siRNA by anionic liposomes, PAGE was used to observe the changes before and after siRNA was encapsulated. We found (Figure 2a) that compared to the product without Ca2+ (Lane #1), an obvious band occurred at the sample holes, and the intensity of naked siRNA decreased significantly (Lanes #2–9). This indicated that in the absence of Ca2+, siRNA cannot be encapsulated by anionic liposomes due to electrostatic repulsion. The addition of Ca2+ successfully allowed encapsulation of the siRNA by anionic liposomes, so the migration rate of the band decreased. In addition, when the product was exposed to RNase A for 30 min at 37°C (Lanes #3, 5, 7, 9), the naked siRNA (the band at the bottom of the gel) disappeared, but the band of anionic lipoplexes (anionic liposomes/Ca2+/siRNA, the band at the sample hole) remained. This demonstrated that the siRNA was encapsulated inside the liposomes, thus resisting the degradation of RNase A.
FIGURE 2.

Optimisation and characterisation of anionic lipoplexes (anionic liposomes/Ca2+/siRNA). PAGE images of anionic lipoplexes prepared with different concentrations of Ca2+ (0, 1.2, 2.4, 4.8 and 9.6 mM) (a) and lipids (0.5, 1 and 3 μg/ml) (b). (c) The diameter of anionic lipoplexes prepared with 1 μg/ml lipids, 2.4 mM Ca2+ and 200 nM siRNA from Dynamic light scattering (DLS). Transmission electron microscopy (TEM) images of anionic lipoplexes negatively stained with 2% phosphotungstic acid (d) and without negative staining (e).
When the concentration of Ca2+ was increased to 4.8 mM, a white precipitate appeared (Figure S1). When the concentration of Ca2+ was 9.6 mM, more white precipitate appeared (Figure S1). As expected, the diameter of anionic lipoplexes also increased significantly when the concentration of Ca2+ was above 4.8 mM (Table 1). This could be explained by the excessive Ca2+ resulting in uncontrolled growth of CaP. The formation of precipitates may be the reason that the total intensity of the bands in Lanes #6 and #8 was lower than those in Lanes #2 and #4. Furthermore, excess Ca2+ leads to an insufficient negative charge inside the anionic liposomes to bind with Ca2+/siRNAs, so some of the Ca2+/siRNAs were bound to the surface of the liposomes. That is why the band intensities at the sample holes of Lanes #7 and #9 decreased significantly after incubation with RNase A. Unlike from the lipoplexes prepared with 4.8‐ and 9.6‐mM Ca2+, the lipoplexes with siRNA prepared with 1.2‐ and 2.4‐mM Ca2+ were not significantly degraded by RNase A. The encapsulation efficiency of lipoplexes prepared with 2.4‐mM Ca2+ (91.0%) was higher than that of the lipoplexes with 1.2‐mM Ca2+ (82.4%). Since maximum encapsulation efficiency was obtained at 2.4 mM Ca2+, the optimal concentration of Ca2+ was 2.4 mM.
TABLE 1.
The diameter of anionic lipoplexes prepared with different concentrations of Ca2+.
| Ca2+(mM) | Diameter (nm) | PDI | Status |
|---|---|---|---|
| 0 | 188.90 ± 13.03 | 0.196 ± 0.029 | Transparent |
| 1.2 | 169.97 ± 5.35 | 0.189 ± 0.016 | Transparent |
| 2.4 | 268.77 ± 12.74 | 0.269 ± 0.013 | Transparent |
| 4.8 | 326.53 ± 36.90 | 0.303 ± 0.097 | White sediment |
| 9.6 | 1544.80 ± 738.73 | 2.671 ± 2.322 | White sediment |
Abbreviation: PDI, polydispersity index.
We also investigated the encapsulation efficiency of siRNA with different concentrations of lipids. The image (Figure 2b) showed that after anionic lipoplexes prepared with 0.5‐μg/ml lipid incubating with RNase, the band intensity of anionic lipoplexes at the loading hole decreased (Lanes #1 and #2). This may be explained using the insufficient amount of lipid, where induced siRNA could not be completely encapsulated by inner anionic liposomes. In addition, there was no significant difference in the encapsulation efficiency of siRNA by the lipoplexes (Figure 2b) when the concentration of lipid was ≥1 μg/ml (Lanes #3, #4, #5 and #6). The diameters of anionic lipoplexes prepared with different concentrations of lipids were shown in Table 2, which demonstrated that when the lipid concentration was between 0.5 and 3 μg/ml, the particle size of anionic lipoplexes was smaller. This may be because the higher the concentration of lipids, the lesser the distribution of siRNA in each liposome. The data also showed that the diameter of anionic lipoplexes prepared with 0.5‐μg/ml lipid was not uniform, which may be due to the uncontrollable growth of CaP (Ca2+/siRNA) on the surface of anionic lipoplexes.
TABLE 2.
The diameter of anionic lipoplexes prepared with different concentrations of lipids.
| Lipid (μg/ml) | Diameter (nm) | PDI |
|---|---|---|
| 0.5 | 308.10 ± 133.35 | 0.706 ± 0.762 |
| 1 | 263.57 ± 12.28 | 0.254 ± 0.024 |
| 3 | 214.10 ± 6.28 | 0.193 ± 0.041 |
Abbreviation: PDI, polydispersity index.
The PAGE images showed that anionic liposomes successfully encapsulated siRNA into anionic lipoplexes with the help of Ca2+. After encapsulating siRNA, the size of the lipoplexes became 268.77 ± 12.74 nm (PDI: 0.269 ± 0.013, Figure 2c). The potential of the lipoplexes increased to −15.12 ± 2.09 mV with the addition of Ca2+. In addition, TEM was performed to observe the morphology of lipoplexes and verify the formation of CaP inside the lipoplexes. The morphology of lipoplexes could be observed as obvious spherical structures with negative staining (Figure 2d). Moreover, the image (Figure 2e) also showed that the lipid bilayer was electron transparent without negative staining, and the dark particles without negative staining indicated the formation of inorganic CaP inside the lipoplexes. These results suggested that siRNA was successfully encapsulated inside the liposomes by Ca2+ with the formation of CaP.
3.3. Stability of anionic lipoplexes
Since siRNA is very unstable, the ideal vehicle will protect siRNA from degradation. Therefore, we tested the resistance to RNase A and FBS of siRNA after encapsulation by liposomes. The band of naked siRNA disappeared with the addition of RNase A (Figure 3a), which implied that naked siRNA had poor resistance to RNase A. In contrast to naked siRNA, siRNA encapsulated by liposomes maintained its integrity when exposed to RNase A for at least 2 h (Figure 3b).
FIGURE 3.

Stability of siRNA after encapsulation in anionic liposomes. (a) Stability of naked siRNA in RNase A as a control. Lane 1: naked siRNA. Lane 2: naked siRNA incubated with RNase A for 0 min before electrophoresis. Lanes 3–8: the products of naked siRNA incubated with RNase A for different times at 37°C. (b) Stability of anionic lipoplexes in RNase A. Lane 1: anionic lipoplexes. Lane 2: anionic lipoplexes incubated with RNase A for 0 min before electrophoresis. Lanes 3–8: the products of anionic lipoplexes incubated with RNase A at different times at 37°C. (c) Stability of anionic lipoplexes in foetal bovine serum (FBS). Lane 1: FBS. Lane 2: anionic lipoplexes. Lane 3: anionic lipoplexes incubated with FBS for 0 min before electrophoresis. Lanes 4–9: the products of anionic lipoplexes incubated with FBS at different times at 37°C. (d) Stability of naked siRNA in FBS. Lane 1: FBS. Lane 2: naked siRNA. Lane 3: naked siRNA incubated with FBS for 0 min before electrophoresis. Lanes 4–9: the products of naked siRNA incubated with FBS at different times at 37°C.
Stability of siRNA in the presence of FBS is also very important to achieve due to its tendency to degrade when exposed to various enzymes in the blood after systemic administration. Two different bands appeared after the lipoplexes were incubated with FBS at different times, including in the control (0 h). One band was at the sample holes, and the other was at the bottom of the gels (Lanes #4–9 in Figure 3c). To determine what these bands were, FBS, lipoplexes and the products that the lipoplexes incubated with FBS for 0 h before electrophoresis were used as the controls. The result illustrated that a band appeared at the bottom of the gel after adding FBS (Lane #3), whose position was the same as that of FBS (Lane #1). The band at the sample holes was consistent with the lipoplexes (Lane #2). These results demonstrated that the band at the bottom of the gel was FBS and the band at the sample hole consisted of lipoplexes. The intensity of bands at the sample holes showed that siRNA encapsulated in liposomes remained intact for at least 6 h.
A similar phenomenon in which bands changed after naked siRNA incubation with FBS was also observed (Lanes #4–9). Three control tests similar to those in Figure 3c were also carried out. The images from Lanes #2 and #3 in Figure 3d indicated that naked siRNA was degraded completely after mixing with FBS. Therefore, anionic liposomes could significantly improve the resistance of siRNA against degradation, which is beneficial for maintaining the activity of siRNA in vivo.
3.4. Cellular uptake of anionic lipoplexes
Next, we investigated whether anionic liposomes could deliver siRNA into cells, and Cy 5 was used to track the location of siRNA. Naked siRNA and siRNA delivered by cationic liposomes (Lipo6000) were used as controls. There was no fluorescence signal observed in cells treated with naked siRNA (Figure 4a). In contrast to naked siRNA, the red fluorescence (Figure 4b and c) and quantitative data (Figure 4d) showed significant difference in cellular uptake of siRNA delivered by anionic liposomes and Lipo6000. Although the transfection efficiency of anionic liposomes was less than Lipo6000, a large amount of siRNA was delivered into cells by anionic liposomes, which was favourable for realising the suppression of mRNA translation.
FIGURE 4.

Cellular uptake of anionic lipoplexes. Confocal laser scanning microscope (CLSM) images of naked siGA (a), Lipo6000/siGA (b) and anionic lipoplexes/siGA (c) taken into cells over time. Cy 5 and DAPI represent siGA and nucleus, respectively. (d) The quantitative data of the Cy 5 intensity by ImageJ. *** indicates p < 0.001, * indicates p < 0.05 versus the intensity of anionic lipoplexes/siGA at 4 h. ### indicates p < 0.001, # indicates p < 0.05 versus the intensity of Lipo6000/siGA at 4 h.
3.5. Endosomal escape behaviour of anionic lipoplexes
Due to the degradation of nucleases in endosomes/lysosomes, siRNA often fails to inhibit the expression of proteins. Here, the endosomal/lysosomal escape behaviour of siRNA after delivery by anionic liposomes and Lipo6000 was examined. The green signal represents the endosomes/lysosomes, and the red is the siRNA signal. The overlap of red and green signals was determined to investigate whether siRNA is trapped in endosomes/lysosomes. siRNA delivered into the cytoplasm by anionic liposomes and Lipo6000 exhibited a large amount of red fluorescence (Figure 5a). The Pearson coefficients (Figure 5b) of the overlap in anionic liposomes and Lipo6000 were 0.359 ± 0.114 and 0.287 ± 0.063, respectively, indicating that siRNA successfully escaped from endosomes.
FIGURE 5.

Endosomal escape behaviour of anionic lipoplexes/siGA. (a) Confocal images from Lyso‐Tracker Green (endosomes), Cy 5 (siRNA), DAPI (nucleus) and Merge (the overlap of red and green signals) after siGA delivered by Lipo6000 and anionic liposomes. (b) The correlation coefficient analysis from (a) by ImageJ.
3.6. In vitro gene silencing of GAPDH by anionic lipoplexes
To verify whether siRNA released in the cytoplasm could interrupt protein expression, immunofluorescence and western blot tests were developed to measure the protein expression level. GAPDH was used as a target protein. The green signal in the immunofluorescence images represents GAPDH. Our results showed that after treatment with anionic liposomes‐ and Lipo6000‐delivered siGA (the third and fifth columns of Figure 6a and b), the amount of GAPDH decreased significantly, indicating that both siRNAs could inhibit the expression of GAPDH. However, siNC, which was not specific to GAPDH, could not inhibit GAPDH expression (the second and fourth columns of Figure 6a and b), even when delivered by anionic liposomes and Lipo6000. This was also verified by western blot imaging (Figure 6c). These results demonstrated that siRNA delivered by anionic liposomes could inhibit protein expression effectively.
FIGURE 6.

In vitro gene silencing of GAPDH by anionic lipoplexes. (a) Immunofluorescence images of GAPDH silenced by siRNA. Green signals represent GAPDH. (b) The quantitative data of the GAPDH expression level according to the green signals. ** indicates p < 0.01, * indicates p < 0.05 versus control. # indicates p < 0.05 versus Lipo6000/siGA. Δ indicates p < 0.05 versus anionic lipoplexes/siGA. (c) Western blot images of GAPDH silenced by siRNA.
3.7. Safety evaluation of anionic lipoplexes
Furthermore, to investigate the cytotoxicity of anionic lipoplexes, Lipo6000/siRNA was used as a control. The results (Figure 7a–c) showed that with the increasing concentration of anionic lipoplexes and incubation time, there was no obvious decrease in cell viability. The cytotoxicity of cationic lipoplexes (Lipo6000/siRNA) increased significantly with prolonged dosages and times. These results suggested that the anionic lipoplexes had better safety than cationic lipoplexes.
FIGURE 7.
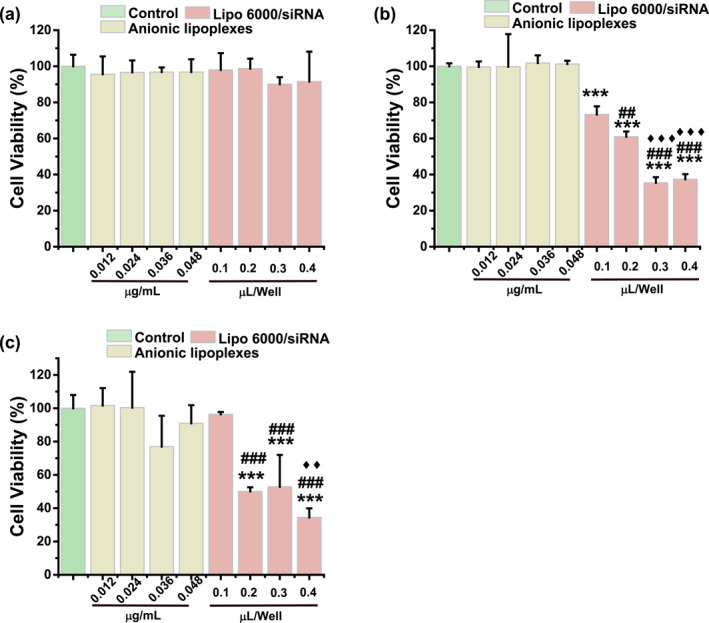
Cell viability treated with Lipo6000/siRNA and anionic lipoplexes with different concentration after (a) 24 h, (b) 48 h and (c) 72 h. The concentration of anionic liposomes corresponds to the concentration of Lipo6000 for transfecting the same molar amount of siRNA. *** indicates p < 0.001 versus Control. ### indicates p < 0.001, ## indicates p < 0.01 versus 0.1‐μL Lipo6000/well. ♦♦ indicates p < 0.01, ♦♦♦ indicates p < 0.001 versus 0.2‐μL Lipo6000/well.
4. DISCUSSION
We have identified that anionic liposomes can be prepared without using organic solvents, and the anionic liposomes can be used for siRNA delivery. We showed that glycerol can be used to prepare anionic liposomes and the process of preparation is simple with short time. Furthermore, high safety with no obvious cytotoxicity was observed. Moreover, the siRNA delivered by these anionic liposomes could effectively inhibit the protein expression.
As we know, most anionic liposomes used for siRNA delivery were prepared by the film hydration method [14, 16, 38]. Not only will the organic solvents, such as chloroform, be added in the preparation process using this method but the residual organic solvents will also need to be removed by overnight vacuum‐desiccation or dialysis [14, 39]. This process is complex and time‐consuming, with the risk of residual organic solvents [28]. In addition, the stability of liposomes will be affected by residual organic solvents [29, 40]. Here, anionic liposomes prepared from glycerol were used to deliver siRNA. Glycerol is biocompatible with no need to be removed, which makes the preparation process simple and less‐time consuming (within 2 h), and there is no potential risk of organic solvent residues [28, 29]. In addition, anionic liposomes prepared by glycerol are similar to those prepared by film hydration method, which can encapsulate siRNA with the help of Ca2+. In the presence of 2.4 mM Ca2+, the encapsulation efficiency of these two anionic liposomes can reach 90% [14]. Besides, the silencing efficiency of siRNA delivered by anionic liposomes prepared with glycerol is about 68.8%, which is similar to that prepared by film hydration method (∼70%) [14]. Therefore, anionic liposomes prepared from glycerol can play the same role as that prepared by film hydration method.
And we also proved that anionic liposomes could protect siRNA from degradation by RNase A and FBS, which is important for maintaining the activity of siRNA. Moreover, we compared the transfection efficiency, the ability to promote lysosomal escape and the silencing efficiency of the anionic lipoplexes, together with cationic lipoplexes (Lipo6000/siRNA). Although the transfection efficiency of anionic liposomes was lower than that of cationic liposomes (62.67%), there was no significant difference in the endosomal escape efficiency of siRNA between anionic liposomes and Lipo6000. Additionally, siRNA transferred by anionic liposomes into cells was sufficient to inhibit protein expression, with a similar inhibition efficiency to Lipo6000. However, anionic liposome had no obvious cytotoxicity which was different from cationic liposomes. In summary, anionic liposome prepared by glycerol is a safe and effective siRNA delivery platform, although its long‐term toxicity, safety and transfection efficiency in vivo must be further verified in the future.
5. CONCLUSION
In this paper, anionic lipoplexes, which are safer than cationic lipoplexes, were prepared without organic solvents. The formulation of these anionic lipoplexes was optimised for high encapsulation efficiency. The anionic liposomes not only can protect the integrity of siRNA in different physiological environments (at least 6 h in FBS), but also deliver siRNAs into cells effectively. Moreover, siRNA delivered by anionic liposomes could escape from endosomes into the cytoplasm to exert protein inhibition, and the protein inhibition efficiency of anionic lipoplexes was similar to that of cationic lipoplexes. We are confident that this liposome can be used in the delivery of other gene drugs, such as mRNA, in the future.
AUTHOR CONTRIBUTIONS
Xiu Han: Conceptualisation, Validation, Writing‐ Original draft preparation. Yan Lu and Zhaoluo Xu: Methodology, Investigation, Validation. Xueping Ma and Yanan Chu: Data Curation, Resources. Haiping Wu: Investigation, Formal analysis, Funding acquisition. Bingjie Zou and Guohua Zhou: Writing ‐ Review & Editing, Supervision, Project administration, Funding acquisition.
CONFLICT OF INTEREST STATEMENT
The authors declared that they have no conflicts of interest.
Supporting information
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by the National Natural Science Foundation of China (No.61871403) and the Fundamental Research Funds for the Central Universities (No.2022300326).
Han, X. , et al.: Anionic liposomes prepared without organic solvents for effective siRNA delivery. IET Nanobiotechnol. 17(3), 269–280 (2023). 10.1049/nbt2.12117
DATA AVAILABILITY STATEMENT
Data will be made available on reasonable request.
REFERENCES
- 1. Hickerson, R.P. , et al.: Stability study of unmodified siRNA and relevance to clinical use. Oligonucleotides 18(4), 345–354 (2008). 10.1089/oli.2008.0149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gavrilov, K. , Saltzman, W.M. : Therapeutic siRNA: principles, challenges, and strategies. Yale J. Biol. Med. 85(2), 187–200 (2012). PMC3375670.) [PMC free article] [PubMed] [Google Scholar]
- 3. Huang, H. , et al.: Ca 2+ participating self‐assembly of an apoferritin nanostructure for nucleic acid drug delivery. Nanoscale 12(13), 7347–7357 (2020). 10.1039/D0NR00547A [DOI] [PubMed] [Google Scholar]
- 4. Hu, B. , et al.: Clinical advances of siRNA therapeutics. J. Gene Med. 21(7), e3097 (2019). 10.1002/jgm.3097 [DOI] [PubMed] [Google Scholar]
- 5. Kang, L. , et al.: Nanocarrier‐mediated co‐delivery of chemotherapeutic drugs and gene agents for cancer treatment. Acta Pharm. Sin. B 5(3), 169–175 (2015). 10.1016/j.apsb.2015.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tsouris, V. , et al.: Nano carriers that enable co‐delivery of chemotherapy and RNAi agents for treatment of drug‐resistant cancers. Biotechnol. Adv. 32(5), 1037–1050 (2014). 10.1016/j.biotechadv.2014.05.006 [DOI] [PubMed] [Google Scholar]
- 7. Gary, D.J. , Puri, N. , Won, Y.Y. : Polymer‐based siRNA delivery: perspectives on the fundamental and phenomenological distinctions from polymer‐based DNA delivery. J. Contr. Release 121(1‐2), 64–73 (2007). 10.1016/j.jconrel.2007.05.021 [DOI] [PubMed] [Google Scholar]
- 8. Lv, H. , et al.: Toxicity of cationic lipids and cationic polymers in gene delivery. J. Contr. Release 114(1), 100–109 (2006). 10.1016/j.jconrel.2006.04.014 [DOI] [PubMed] [Google Scholar]
- 9. Dokka, S. , et al.: Oxygen radical‐mediated pulmonary toxicity induced by some cationic liposomes. Pharm. Res. (N. Y.) 17(5), 521–525 (2000). 10.1023/a:1007504613351 [DOI] [PubMed] [Google Scholar]
- 10. Nagahiro, I. , et al.: Toxicity of cationic liposome‐DNA complex in lung isografts. Transplantation 69(9), 1802–1805 (2000). 10.1097/00007890-200005150-00012 [DOI] [PubMed] [Google Scholar]
- 11. Payne, C.K. , et al.: Internalization and trafficking of cell surface proteoglycans and proteoglycan‐binding ligands. Traffic 8(4), 389–401 (2007). 10.1111/j.1600-0854.2007.00540.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Han, X. , et al.: An ionizable lipid toolbox for RNA delivery. Nat. Commun. 12(1), 7233 (2021). 10.1038/s41467-021-27493-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Patil, S.D. , Rhodes, D.G. , Burgess, D.J. : Anionic liposomal delivery system for DNA transfection. AAPS J. 6(4), 13–22 (2004). 10.1208/aapsj060429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kapoor, M. , Burgess, D.J. : Efficient and safe delivery of siRNA using anionic lipids: formulation optimization studies. Int. J. Pharm. 432(1‐2), 80–90 (2012). 10.1016/j.ijpharm.2012.04.058 [DOI] [PubMed] [Google Scholar]
- 15. Foged, C. , Nielsen, H. , Frokjaer, S. : Liposomes for phospholipase A2 triggered siRNA release: preparation and in vitro test. Int. J. Pharm. 331(2), 160–166 (2007). 10.1016/j.ijpharm.2006.11.010 [DOI] [PubMed] [Google Scholar]
- 16. Srinivasan, C. , Burgess, D.J. : Optimization and characterization of anionic lipoplexes for gene delivery. J. Contr. Release 136(1), 62–70 (2009). 10.1016/j.jconrel.2009.01.022 [DOI] [PubMed] [Google Scholar]
- 17. Kovtun, A. , Heumann, R. , Epple, M. : Calcium phosphate nanoparticles for the transfection of cells. Bio Med. Mater. Eng. 19(2‐3), 241–247 (2009). 10.3233/BME-2009-0586 [DOI] [PubMed] [Google Scholar]
- 18. Liu, T. , et al.: Calcium phosphate nanoparticles as a novel nonviral vector for efficient transfection of DNA in cancer gene therapy. Cancer Biother. Radiopharm. 20(2), 141–149 (2005). 10.1089/cbr.2005.20.141 [DOI] [PubMed] [Google Scholar]
- 19. Sokolova, V.V. , et al.: Effective transfection of cells with multi‐shell calcium phosphate‐DNA nanoparticles. Biomaterials 27(16), 3147–3153 (2006). 10.1016/j.biomaterials.2005.12.030 [DOI] [PubMed] [Google Scholar]
- 20. Khalifehzadeh, R. , Arami, H. : Biodegradable calcium phosphate nanoparticles for cancer therapy. Adv. Colloid Interface Sci. 279, 102157 (2020). 10.1016/j.cis.2020.102157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li, J. , et al.: Biodegradable calcium phosphate nanoparticle with lipid coating for systemic siRNA delivery. J. Contr. Release 142(3), 416–421 (2010). 10.1016/j.jconrel.2009.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lee, M.S. , et al.: Target‐specific delivery of siRNA by stabilized calcium phosphate nanoparticles using dopa–hyaluronic acid conjugate. J. Contr. Release 192, 122–130 (2014). 10.1016/j.jconrel.2014.06.049 [DOI] [PubMed] [Google Scholar]
- 23. Choi, B. , et al.: Glutamine‐chitosan modified calcium phosphate nanoparticles for efficient siRNA delivery and osteogenic differentiation. J. Mater. Chem. B 3(31), 6448–6455 (2015). 10.1039/C5TB00843C [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kapoor, M. , Burgess, D.J. : Physicochemical characterization of anionic lipid‐based ternary siRNA complexes. Biochim. Biophys. Acta Biomembr. 1818(7), 1603–1612 (2012). 10.1016/j.bbamem.2012.03.013 [DOI] [PubMed] [Google Scholar]
- 25. Straubinger, R.M. , et al.: Endocytosis of liposomes and intracellular fate of encapsulated molecules: encounter with a low pH compartment after internalization in coated vesicles. Cell 32(4), 1069–1079 (1983). 10.1016/0092-8674(83)90291-x [DOI] [PubMed] [Google Scholar]
- 26. Xu, E. , Saltzman, W.M. , Piotrowski‐Daspit, A.S. : Escaping the endosome: assessing cellular trafficking mechanisms of non‐viral vehicles. J. Contr. Release 335, 465–480 (2021). 10.1016/j.jconrel.2021.05.038 [DOI] [PubMed] [Google Scholar]
- 27. Xia, Y. , Tian, J. , Chen, X. : Effect of surface properties on liposomal siRNA delivery. Biomaterials 79, 56–68 (2016). 10.1016/j.biomaterials.2015.11.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mozafari, M.R. , et al.: Cytotoxicity evaluation of anionic nanoliposomes and nanolipoplexes prepared by the heating method without employing volatile solvents and detergents. Pharmazie 62(3), 205–209 (2007). 10.1691/ph.2007.3.6045 [DOI] [PubMed] [Google Scholar]
- 29. Mortazavi, S.M. , et al.: Preparation of liposomal gene therapy vectors by a scalable method without using volatile solvents or detergents. J. Biotechnol. 129(4), 604–613 (2007). 10.1016/j.jbiotec.2007.02.005 [DOI] [PubMed] [Google Scholar]
- 30. Lin, M. , Qi, X.‐R. : Purification Method of Drug‐Loaded Liposome (2021)
- 31. Large, D.E. , et al.: Liposome composition in drug delivery design, synthesis, characterization, and clinical application. Adv. Drug Deliv. Rev. 176, 113851 (2021). 10.1016/j.addr.2021.113851 [DOI] [PubMed] [Google Scholar]
- 32. Kabarkouhi, Z. , et al.: Liposome, nanoliposome and allied Technologies in Covid‐19 vaccines: Key roles and functionalities. Curr. Drug Deliv. 19(10), 1001–1011 (2022). 10.2174/1567201819666220427125342 [DOI] [PubMed] [Google Scholar]
- 33. Rani, V. , Venkatesan, J. , Prabhu, A. : Liposomes‐A promising strategy for drug delivery in anticancer applications. J. Drug Deliv. Sci. Technol. 76, 103739 (2022). 10.1016/j.jddst.2022.103739 [DOI] [Google Scholar]
- 34. Ismail, M.F. , et al.: Potential therapeutic effect of nanobased formulation of rivastigmine on rat model of Alzheimer's disease. Int. J. Nanomed. 8, 393–406 (2013). 10.2147/ijn.s39232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mozafari, M.R. : Liposomes: an overview of manufacturing techniques. Cell. Mol. Biol. Lett. 10(4), 711–719 (2005) [PubMed] [Google Scholar]
- 36. Khorasani, S. , Danaei, M. , Mozafari, M. : Nanoliposome technology for the food and nutraceutical industries. Trends Food Sci. Technol. 79, 106–115 (2018). 10.1016/j.tifs.2018.07.009 [DOI] [Google Scholar]
- 37. Gbian, D.L. , Omri, A. : Lipid‐Based drug delivery systems for diseases managements. Biomedicines 10(9), 2137 (2022). 10.3390/biomedicines10092137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mozafari, M.R. , et al.: Formation and characterisation of non‐toxic anionic liposomes for delivery of therapeutic agents to the pulmonary airways. Cell. Mol. Biol. Lett. 7(2), 243–244 (2002) [PubMed] [Google Scholar]
- 39. Dua, J. , et al.: Liposome: methods of preparation and applications. Int J Pharm Stud Res 3(2), 14–20 (2012) [Google Scholar]
- 40. Meure, L.A. , Foster, N.R. , Dehghani, F. : Conventional and dense gas techniques for the production of liposomes: a review. AAPS PharmSciTech 9(3), 798–809 (2008). 10.1208/s12249-008-9097-x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
Data will be made available on reasonable request.


