Abstract
Francisella tularensis, the causative agent of tularemia, is a HHS Tier 1 select agent. Tularemia is the most commonly reported human and animal infection caused by a bacterial select agent in the United States. Because of the rarity of disease, low clinical suspicion, and the organism’s low infectious dose, F. tularensis poses a hazard for unsuspecting laboratorians, particularly those who handle cultures outside a biological safety cabinet or without use of appropriate personal protective equipment (PPE). We examined Form 4s and Form 3s submitted to the Federal Select Agent Program between 2011 and 2015 to assess laboratory methods used in the identification of F. tularensis and categorize reported occupational exposures. Culture, which is used in a confirmatory identification, was the primary method used in clinical laboratories. Reported occupational exposures in clinical, veterinary, and reference laboratories occurred at a rate of 33.8, 14.0, and 0.4/100 isolates, respectively. The number of exposed workers in clinical, reference, veterinary, and research laboratories was 3.2, 2.4, 5.1, and 0.9 workers per reported incident, respectively. Most reported occupational exposures occurred in clinical laboratorians working on the bench at BSL-2 conditions with isolated cultures with no suspicion that the organism was F. tularensis; the fewest occurred in research laboratories at BSL-3 where occupational exposures were prevented by prior knowledge that the organism was F. tularensis and the PPE that was used in these laboratories.
Keywords: Francisella tularensis, tularemia, occupational exposures, Form 3, Form 4, laboratory identification
Introduction
Over the past 20 years, events that have occurred in the United States, such as the fraudulent purchase of Yersinia pestis by a purported white supremacist1 and the dissemination of spores of Bacillus anthracis through the US mail,2 have resulted in the promulgation of regulations to enhance biosecurity by restricting access and possession of those pathogens and toxins (ie, biological select agents and toxins [BSAT]) that have potential to pose a severe threat to the health of humans, animals or plants, or animal or plant products to qualified institutions, laboratories, and scientists.3 The framework for the initial regulation was incorporated into the Antiterrorism and Effective Death Penalty Act of 1996 (Public Law 104–132).4 The resulting Department of Health and Human Services (HHS) regulation, which governed only the shipping and receipt of BSAT, included safety procedures, training, proper laboratory facilities for handling BSAT, as well as safeguards to prevent unlawful access to BSAT.5 The regulation was incorporated into Part 72 of Title 42 of the Code of Federal Regulations (CFR Part 72) and became effective April 15, 1997.
After the World Trade Center terrorist event on September 11, 2001, and the subsequent dissemination of B. anthracis spores through the US mail, controls to access BSAT were strengthened by passage of Public Law 107–56 titled Uniting and Strengthening America by Providing Appropriate Tools Required to Intercept and Obstruct Terrorism (USA PATRIOT) Act6 and the Public Health Security and Bioterrorism Preparedness and Response Act of 2002 (Public Law 107–188).7 The former defines who can handle or possess BSAT while the latter sets forth the requirements for the possession (including registration of the entity), use, and transfer of BSAT as well as establishing the US Department of Agriculture (USDA) Select Agent Program. The resulting regulation also addressed biosafety concerns by imposing specific requirements on laboratories working with BSAT.3 In 2010, in response to concerns raised in part by the scientific community, Executive Order 13546 was signed.8 This executive order directed the Secretaries of HHS and USDA to designate those BSAT that pose the greatest risk of deliberate misuse with the greatest potential for mass casualties or devastating effects to the economy, critical infrastructure, or public confidence as Tier 1 agents and toxins. A federal panel using several criteria, including input from the intelligence community, selected 13 BSAT deemed to be Tier 1 and subject to additional requirements.3
While the select agent regulations restricted access to BSAT, it also provided an exemption for clinical or diagnostic laboratories to possess, use, or transfer specimens presented for diagnosis or verification that may contain a BSAT. Most of these laboratories are nonregistered entities that are exempt from the regulatory requirements, provided that: (i) unless directed otherwise by the HHS/USDA Secretary within 7 calendar days after identification (note 1), the BSAT (ie, in this case viable Francisella tularensis) is either transferred to a registered entity or destroyed onsite by a recognized sterilization or inactivation process; (ii) the BSAT is secured against theft, loss, or release during the period between its identification and its transfer or destruction and any theft, loss, or release of such agent or toxin is reported; and (iii) the identification of the BSAT is reported to Animal and Plant Health Inspection Service (APHIS) or the Centers for Disease Control and Prevention (CDC) and other appropriate authorities when required by federal, state, or local law. However, the identification of a Tier 1 BSAT must be immediately reported to the Division of Select Agents and Toxins (DSAT) by telephone, facsimile, or email. This report must be followed by submission of APHIS/CDC Form 4 (Form 4) within 7 calendar days after identification with a copy of the record maintained for 3 years.3
The release of a BSAT causing a potential occupational exposure or the release of a BSAT outside of the primary barriers of the biocontainment area requires the immediate notification of CDC or APHIS. After this notification, the reporting entity must submit a completed APHIS/CDC Form 3 within 7 calendar days describing the circumstances surrounding the release event as well as specific details about the event. If the exposure occurred during the identification of a BSAT, it is first noted on the Form 4 submission and followed-up with a completed Form 3.
F. tularensis, the causative agent of tularemia, is an HHS Tier 1 agent. It is highly infectious for humans with an aerosol ID50 of 10–50 organisms.9 Because of the rarity of human disease, low clinical suspicion, and the organism’s low infectious dose, F. tularensis poses a hazard for unsuspecting laboratorians handling diagnostic samples in BSL-2 laboratories. For research work involving F. tularensis, BSL-3 practices and biosafety equipment are utilized.10 Because tularemia is the most common infection caused by a bacterial select agent in the US and a common infection of domestic and wild animals, we examined the Form 4s submitted between 2011 and 2015 to assess laboratory methods used in the identification of F. tularensis and determine the extent to which molecular methods (eg, PCR) are used. In addition, because of the risk this agent poses to laboratory workers (including animal technicians and other staff) in general, we also reviewed Form 3 submissions to the Federal Select Agent Program (FSAP) for F. tularensis between 2011 and 2015 to categorize the reported occupational exposures.
Methods
Case Definitions
A confirmed human case of tularemia is defined as a patient with a clinically compatible case with an isolate of F. tularensis from a clinical specimen (blood, skin lesions, spinal fluid, lymph nodes, or respiratory secretions) or 4-fold or greater change in antibody titer to F. tularensis antigen. A presumptive case of tularemia is defined as a clinically compatible case with elevated serum antibody titer to F. tularensis antigen in a single serum specimen in a patient with no history of tularemia vaccination or detection of F. tularensis in a clinical specimen by direct fluorescent antibody (DFA) assay.11
Form 4
A total of 678 Form 4s that were submitted for the identification of F. tularensis between 2011 and 2015 (ie, primary Form 4) were examined. An additional 187 secondary Form 4s were also reviewed. A secondary Form 4 is related to the original Form 4 and was either requested by DSAT for additional information or submitted by the original submitter for other purposes. After listing by the submitting entity that was registered with FSAP, the following data were abstracted: date of submission to FSAP; name and location of primary clinical laboratory or agency requesting testing; type of specimen or culture provided; laboratory method(s) used for identification and/or confirmation; source of clinical specimen (ie, human or animal); zip code of patient; destruction, retention, or transfer of isolate or other material; and any potential exposures, which require the subsequent submission of a Form 3.
Form 3
A total of 272 Form 3s submitted to FSAP for the theft, loss, or release of F. tularensis between 2011 and 2015 were identified in the database. After elimination of duplicate forms (N = 2), reports of inventory discrepancies (N = 3), lost shipments (N = 2), misidentification (N = 2), and missing Form 3s (N = 11) (defined as an exposure noted on the Form 4 but a corresponding Form 3 could not be located, most likely because it was never submitted), the remaining forms (N = 252) were reviewed, and the following data were abstracted: submitting entity, corresponding Form 4 number, reasons for occupational exposure, laboratory methods used, number of individuals exposed, initial risk level as determined by DSAT review of incident, and the antibiotic used for prophylaxis.
Initial risk assessment of the release by DSAT used the following definitions: A low-risk incident has a predictable outcome and poses little or no public or occupational health threat, a moderate risk incident requires prompt attention and potentially threatens the health of a laboratory worker and no threat to the public, and a high-risk incident is an event that requires immediate attention by DSAT with potential threat to the public. High-risk incidents may not have a predictable outcome and may require an onsite investigation.
Results and Discussion
Form 4s
Overall, there were a total of 2404 Form 4s reporting the identification of a bacterial select agent submitted to FSAP between 2011 and 2015 (Table 1). During this time, the total number of submissions for each bacterial select agent ranged from a low of 0 (Rickettsia prowazekii) to a high of 678 (F. tularensis). F. tularensis accounted for 28.2% of the Form 4s and was the most common bacterial select agent detected in the US. However, when grouped together, the 3 species of Brucella (N = 794), namely, B. abortus, B. melitensis, and B. suis, were the most commonly reported genus, accounting for 33% of all Form 4s submitted with no single species accounting for more than 15.5% of Form 4s.
Table 1.
Form 4 Submissions for Bacterial Select Agents, 2011–2015.a
| Agent | Total Submissions | % of Total | Average Submissions/Year |
|---|---|---|---|
| Bacillus anthracis b,c | 69 | 2.9 | 13.8 |
| Brucella abortus c | 222 | 9.2 | 44.4 |
| Brucella melitensis c | 373 | 15.5 | 74.6 |
| Brucella suis c | 199 | 8.3 | 39.8 |
| Burkholderia mallei b,c | 1 | 0.04 | 0.2 |
| Burkholderia pseudomallei b,c | 93 | 3.9 | 18.6 |
| Clostridium botulinum b,d | 513 | 21.3 | 102.6 |
| Coxiella burnetii d | 118 | 4.9 | 23.6 |
| Francisella tularensis b,d | 678 | 28.2 | 135.6 |
| Rickettsia prowazekii d | 0 | 0.0 | 0 |
| Yersinia pestis b,d | 138 | 5.7 | 27.6 |
| Total | 2404 | 100 | 480.6 |
Form 4 reports represent the detection of a bacterial select agent in either human or animal specimens.
Tier 1 select agent.
Overlap (Department of Health and Human Services [HHS] and US Department of Agriculture) select agent.
HHS only select agent.
The F. tularensis Form 4s submitted between 2011 and 2015 were identified for further review. Data from 662 of 678 (97.6%) primary Form 4s in the database were abstracted and analyzed. The 16 Form 4s not included in the analyses were excluded based on: duplicate Form 4s (N = 10), proficiency testing specimens (N = 2), environmental sample (N = 1), misfiling as B. melitensis (N = 1), and further identification as the nonselect agent Francisella novicida (N = 2) by a CDC reference laboratory. It is important to note that during this period, there were at least 2 versions of Form 4. One version, used through 2011, requested the laboratory methods used to identify F. tularensis; Form 4s in use after 2011 did not specifically request laboratory methods, but some submitting entities included them in the comment box or notification email. Thus, incomplete data limit the strength of the conclusions that can be made regarding laboratory methods.
The 662 Form 4s encompassed 422 (63.7%) cultures and specimens from humans and 240 (36.2%) specimens and cultures from animals that were submitted to select agent registered laboratories for testing and/or confirmation and found to be positive for F. tularensis. The types of specimens received by these reference laboratories are shown in Table 2. From 2011 to 2015, Form 4s for identification of F. tularensis in specimens from humans were submitted from 43 of 50 states (86%); no Form 4s were received (and no cases reported) from Alabama, Connecticut, Georgia, Hawaii, Mississippi, New Hampshire, and South Carolina.12 Many human infections were initially identified and/or confirmed by multistate clinical laboratories, universities, or clinics that submitted Form 4s because they were FSAP-registered entities or sent cultures to State Public Health Laboratories for confirmation. A similar phenomenon was observed for animal specimens where laboratories processed specimens that were submitted to them from multiple states.
Table 2.
Distribution of Specimen Types Submitted to Select Agent Registered Laboratories for the Identification of Francisella tularensis, 2011–2015.
| Year | |||||||
|---|---|---|---|---|---|---|---|
| Source of Specimen | Type of Specimen | 2011 (%) | 2012 (%) | 2013 (%) | 2014 (%) | 2015 (%) | Average (%) |
| Human (N = 422) | Culture | 93 | 97 | 100 | 91 | 79 | 90 |
| Othera | 7 | 3 | 0 | 9 | 21 | 10 | |
| Animal (N = 240) | Culture | 29 | 38 | 29 | 18 | 19 | 25 |
| Othera | 67 | 51 | 54 | 70 | 53 | 58 | |
| Carcassb | 4 | 4 | 17 | 12 | 28 | 18 | |
Other specimen types include swab, tissue, and aspirates.
Carcasses or body parts.
The date (month) that the Form 4 was submitted was compared with historical data tracking the date reported (month of onset) for human cases.12 A peak was observed during the summer months (Figure 1). The curve reflecting Form 4 submissions was offset about a month, which was likely due to the time required to seek treatment and make a laboratory diagnosis. Form 4s submitted for infected animals also peaked in July but continued throughout the summer and fall. To compare Form 4 data with case reports of human infections, it was necessary to reassign 41 of 422 (9.7%) of the Form 4s to different states based on the zip code of residence for the patient. As expected, 34.0% to 50.3% (average 43.1%) of reported cases each year between 2011 and 2014 (the 2015 case report data were not available at the time this study was completed) are accounted for by a Form 4 indicating that F. tularensis was cultured from a clinical specimen (ie, confirmatory laboratory results). Three patterns were observed: (i) the number of Form 4s matched the number of cases reported in the National Notifiable Diseases Surveillance System (NNDSS), suggesting that all cases were confirmed culture positive cases; (ii) most commonly, the number of reported cases exceeded the number of Form 4s submitted, suggesting the difference was due to probable unconfirmed cases or the use of serology; and (iii) the number of Form 4s was greater than the number of reported cases, which may be due to missing patient zip codes preventing reassignment or the failure of some states to report cases.
Figure 1.
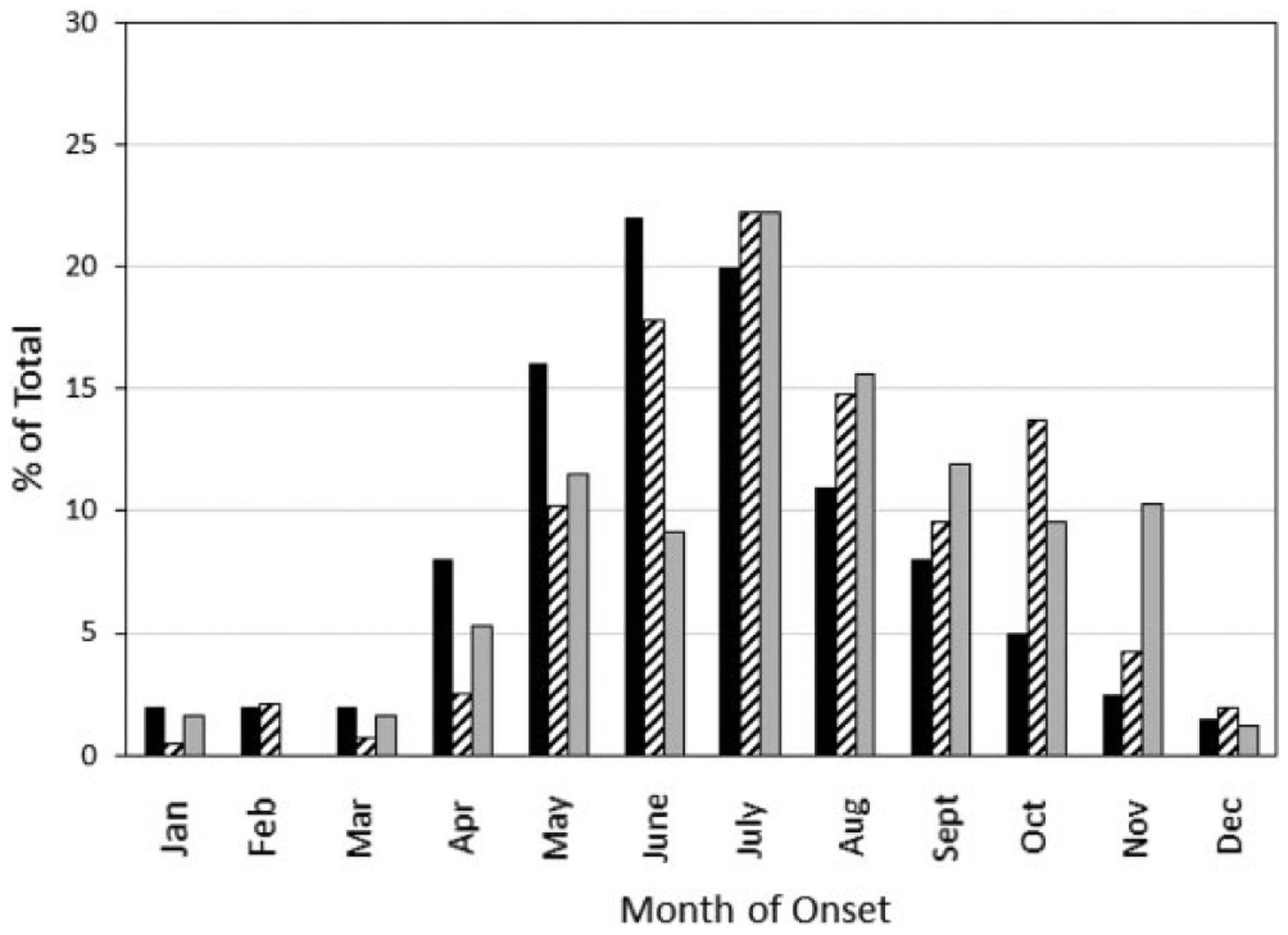
Human cases reported by month of onset (solid black bars) were obtained from CDC.12 Dates of Form 4 submission indicating the identification of Francisella tularensis in a specimen from a human (black and white bars bars) or in a specimen from an animal (gray bars) were obtained from the submitted form.
Animal infections are generally not tracked, and few data are available on prevalence of tularemia in domestic and wild animals. Animal specimens were more likely to be processed by out-of-state laboratories (52/240; 21.7%) than human specimens (41/422; 9.7%), which reflected the finding that there were fewer FSAP-registered veterinary reference laboratories than public health reference laboratories in the US that submitted a Form 4 for F. tularensis. Based on Form 4 data, animal specimens were tested for the presence of F. tularensis in both state and county public health laboratories, state veterinary diagnostic laboratories, universities, and federal reference laboratories. These laboratories functioned in both a reference capacity (eg, confirmation of an isolate as F. tularensis) and as a clinical laboratory (processing clinical specimens and conducting necropsies). Those submitting specimens to these laboratories were primarily veterinary clinics, national parks service, and state/county fish and game. In general, these submitters sent multiple specimens per animal. These specimens consisted of tissues, swabs, paraffin blocks, bones, blood, and aspirates. Animal carcasses were also submitted for necropsy, after which carcasses were incinerated. However, tissue samples were often retained by the FSAP-registered Form 4 submitter. The animal species was noted on 179 of 240 (74.5%) Form 4s. Among Form 4s that specified the animal species, cats (35.2%) and rabbits (31.8%) accounted for about two-thirds of all submitted specimens or necropsies. F. tularensis can infect a wide variety of domestic and wild animals as evidenced by the beavers (8.9%), primates (7.3%), prairie dogs (3.9%), muskrats (3.4%), voles (2.8%), squirrels (2.8%), dogs (2.2%), black bear (0.6%), Norwegian forest cat (0.6%), and Guam kingfisher (0.6%) found to be infected with this organism. Unlike reports for human infections, some Form 4s listed multiple F. tularensis positive animals.
F. tularensis is highly infectious. The Biosafety in Microbiological and Biomedical Laboratories (BMBL) 5th edition10 estimates that the infectious dose for F. tularensis subsp. tularensis (Type A) and F. tularensis subsp. holartica (Type B) is 10 to 50 organisms. Tularemia is also a commonly reported laboratory-acquired infection,13 which can occur following direct contact of the organism with skin or mucous membranes, accidental parenteral inoculation, ingestion, or exposure to aerosol and infectious droplets. Laboratory-acquired infections are more commonly associated with handling cultures outside of a biological safety cabinet than with handling clinical materials or infected animals.13 For these reasons, BSL-2 is recommended when working with clinical materials of human or animal origin. BSL-3 and ABSL-3 are recommended for all manipulations of cultures and necropsies, respectively. Furthermore, to reduce occupational exposures, clinicians should alert the laboratory when tularemia is suspected. Form 4s describing an exposure incident comprised 21.9% (145/662) of all Form 4s submitted for F. tularensis. Importantly, more than three-fourths of Form 4s for F. tularensis did not report an occupational exposure. Nevertheless, FSAP-registered reference laboratories that received cultures for confirmation and/or specimens from unregistered clinical laboratories often submitted a Form 4 that indicated there was a potential exposure in the unregistered clinical laboratory that had originally isolated F. tularensis by culture (Table 3). Over this 5-year period, clinical laboratories reported an average of 33.8 exposure incidents/100 isolates of F. tularensis. During this same period, the rate of reported exposures per 100 isolates decreased from a high of 49.3 in 2012 to a low of 21.1 in 2015, a 57% decrease. This decrease in reported exposures occurred despite an 81% increase in the number of positive cultures that were handled in these laboratories. The reasons for this decrease are unclear, but it may be due to increased clinical recognition of tularemia, educational outreach on proper ways to handle suspected cultures of F. tularensis, or a general emphasis on biosafety in clinical laboratories.
Table 3.
Estimated Reported Occupational Exposure Incidents While Handling Cultures or Processing Specimens for the Detection of Francisella tularensis: 2011–2015.
| Year | ||||||
|---|---|---|---|---|---|---|
| 2011 | 2012 | 2013 | 2014 | 2015 | Total | |
| Human specimens | ||||||
| Positive cultures in clinical laboratoriesa | 67 | 75 | 70 | 78 | 95 | 385 |
| Occupational exposure incidentsb | 29 | 37 | 24 | 20 | 20 | 130 |
| Incidents/100 isolates handled | 43.3 | 49.3 | 34.3 | 25.6 | 21.1 | 33.8 |
| Cultures handled in reference laboratoriesc | 139 | 152 | 140 | 164 | 216 | 81 |
| Occupational exposure incidentsb | 0 | 1 | 1 | 1 | 0 | 3 |
| Incidents/100 isolates handled | 0 | 0.7 | 0.7 | 0.6 | 0 | 0.4 |
| Animal specimens | ||||||
| Positive cultures in veterinary laboratoriesa | 7 | 17 | 7 | 9 | 18 | 58 |
| Occupational exposure incidentsb | 0 | 1 | 1 | 1 | 3 | 6 |
| Incidents/100 isolates handled | 0 | 5.9 | 14.3 | 11.1 | 16.7 | 10.3 |
| Necropsies performed | 2 | 5 | 4 | 6 | 26 | 43 |
| Occupational exposure incidentsb | 2 | 1 | 1 | 1 | 1 | 6 |
| Incidents/100 necropsies | 100 | 20 | 25 | 16.7 | 3.8 | 14.0 |
Data abstracted from submitted Form 4s.
Incident is defined as a Form 4 with occupational exposure noted.
Estimated based on observation that reference laboratories handled the cultures submitted by the clinical laboratory (N = 385) and made a subculture (N = 385) prior to performing confirmatory tests. They also cultured clinical specimens (N = 41) that were received from hospitals and clinics.
FSAP-registered reference laboratories handled more than twice as many cultures of F. tularensis as clinical laboratories yet had an occupational exposure incident rate/100 isolates that was 84-fold less than that of clinical laboratories (Table 3). The numbers of cultures handled in reference laboratories was estimated based on the observation that these laboratories handled isolates submitted by the clinical laboratory (N = 385) and made at least 1 subculture (N = 385) prior to performing confirmatory tests. They also cultured clinical specimens (N = 41) that were received directly from hospitals and clinics. Based on Form 4 data, there were only 3 reports of occupational exposures in registered reference laboratories between 2011 and 2015. Two of these occurred because the submitting nonregistered clinical laboratory misidentified F. tularensis as Haemophilus influenzae, a BSL-2 agent, using automated methods and had sent the cultures to a reference laboratory along with a request for typing.
Reported exposures were less common in laboratories processing specimens from animals. Between 2011 and 2015, there were only 58 positive cultures submitted to veterinary reference laboratories by laboratories or clinics processing specimens from animals with an occupational exposure incident rate of 10.3/100 isolates. This rate was 70% lower than that reported for laboratories processing human specimens. However, performing a necropsy on an infected animal had an occupational exposure incident rate of 14.0/100 necropsies, which was 39% greater than that of handling cultures. Thus, the overall reported exposure incident rate for laboratories processing animal specimens (necropsies and cultures) was 11.9/100.
Form 3s
Reported occupational exposures were further investigated by reviewing 252 Form 3s submitted for F. tularensis between 2011 and 2015 that were listed in the database (Table 4). There were 191 reported incidents that had both a Form 4 and a Form 3 (ie, an occupational exposure noted on the Form 4 during isolation of F. tularensis from a clinical specimen that is described in detail on a Form 3). However, there was a discrepancy between the number of incidents reported on Form 4s (N = 191) and the number reported on Form 3s (N = 245). The latter number includes 43 Form 3s submitted by research institutions where there was no identification of F. tularensis in a clinical specimen and therefore no requirement for a Form 4. In addition, there were Form 4s with multiple Form 3s (N = 7). After adjustment, there were 200 Form 3s, which indicated that 95.5% of Form 4s had a corresponding Form 3. However, there were 20 Form 3s with no corresponding Form 4. These 20 Form 3s consisted of reports from clinical laboratories (N = 14), multistate clinical laboratories (N = 3), and veterinary laboratories (N = 3); only 1 of the laboratories (a veterinary laboratory) was registered with the FSAP. It is possible that the absence of a Form 4 indicates that the presence of F. tularensis was not confirmed by the reference laboratory.
Table 4.
Analysis of Occupational Exposures to Francisella tularensis From Form 3 Data: 2011–2015.
| Form 3s | Exposed Individuals | |||||
|---|---|---|---|---|---|---|
| Submitting Entity | No. | % | No. | Prophylaxis | % | Mean/Incident |
| Clinical laboratorya | 180 | 71.4 | 577 | 227 | 39.3 | 3.2 |
| Reference laboratoryb | 5 | 2.0 | 12 | 2 | 16.7 | 2.4 |
| Veterinary laboratoryc | 24 | 9.5 | 122 | 11 | 9.0 | 5.1 |
| Research Laboratoryb,d | 43 | 17.1 | 37 | 10 | 27.0 | 0.9 |
| Total | 252 | 748 | 250 | 33.4 | 3.0 | |
Most clinical laboratories are not registered with Federal Select Agent Program (FSAP).
All entities registered with FSAP.
Most entities registered with FSAP.
Research laboratories are defined as government, academic, and private laboratories that are conducting basic and/or applied research with F. tularensis.
The number of occupationally exposed individuals was determined for each type of submitting entity (Table 4). The total number of occupationally exposed individuals reported from all submitting entities during the study period was 748. Clinical laboratories processing human specimens accounted for 577 of 748 (77%) of the reported exposures, veterinary laboratories and clinics 122 of 748 (16.3%), research institutions 37 of 748 (4.9%), and public health reference laboratories 12 of 748 (1.6%). Thus, clinical laboratories and veterinary laboratories and clinics, which are often working with unknown samples and are probably unaware that the specimen contains F. tularensis or are handling infected animals, account for more than 93% of all reported exposures. The mean number of exposed individuals per reported incident was estimated by type of submitting entity (Table 4). Veterinary laboratories were the highest with an average of 5 individuals per incident. Necropsies were an important reason for the elevated level of occupational exposures reported by veterinary laboratories. Most necropsies were performed at teaching institutions during which students were observing the necropsy of an animal with an unknown infection status. Research institutions had the lowest number of individuals exposed per incident with less than 1 per incident. The observation that 11 of 45 incidents (24.4%) in research institutions versus 4 of 209 (1.9%) in all other submitting entities reported that there were no occupational exposures provides an explanation for the small number of exposed individuals per incident. The use of personal protective equipment (PPE) worn by individuals working in BSL-3 laboratories as noted on the Form 3 likely reduced the risk of potential exposures. Despite the number of reported occupational exposures, there were no confirmed cases of laboratory acquired tularemia during the study period. There was 1 occupationally acquired case involving a veterinary technician in an unregistered facility who was bitten on the finger by a cat determined later to be infected with F. tularensis.
After medical assessment of the reported occupational exposures, antimicrobial prophylaxis was provided to 33% of the exposed individuals (Table 4). Individuals working in clinical laboratories had the highest rate of antimicrobial prophylaxis (39%) while those working in veterinary laboratories had the lowest (9%). The antibiotic provided was mentioned for 100 of 250 (40%) of those receiving prophylaxis. Doxycycline was prescribed for 91 individuals while ciprofloxacin was prescribed for 9 individuals.
Occupational exposures were categorized as low, moderate, or high-risk by DSAT personnel during review of the Form 3 submission. Low-risk exposures accounted for 221 of 252 (87.7%), moderate risk 23 of 252 (9.1%), and high-risk 8 of 252 (3.2%). Submissions from research institutions (N = 45) accounted for 5 of the moderate- and 3 of the high-risk exposures. The moderate- and high-risk exposures at research institutions resulted from cuts (N = 3), needle sticks (N = 2), or animal bites (N = 3) (Table 5). Most incidents reported by research laboratories (37/45; 82.2%) occurred within a BSL-3 laboratory where exposure of individuals was limited by use of PPE. Laboratory workers in clinical laboratories who sniffed plates (n = 4) were deemed to be at moderate risk. Most of the low-risk exposures that occurred in clinical laboratories were the result of subculturing positive blood culture bottles, performing gram stains, and other clinical tests conducted at BSL-2. The mixing of bacterial suspensions using a vortex on an open bench top at BSL-2 for use in automated identification systems was considered to be of moderate risk. Once F. tularensis was recognized as a possibility, plates and other activities were often moved to a biological safety cabinet. One observation that clinical laboratories could use to suggest the presence of F. tularensis was slow growth of a culture on chocolate agar (ca. 48 hours). The microorganism most often confused with F. tularensis by clinical laboratories based on Gram staining, other biochemical tests, and the use of automated identification systems was Haemophilus spp. (N = 13). Other bacteria mistaken for F. tularensis were Campylobacter spp. (N = 2), Pasteurella spp. (N = 1), and Legionella spp. (N = 1). Among the 5 occupational exposures in reference laboratories based on Form 3 submissions, 3 were due to misidentification of F. tularensis as Haemophilus spp. by the clinical laboratory with subsequent requested work (eg, typing) by reference laboratories performed at BSL-2. The other 2 potential exposures in reference laboratories occurred while identifying an unknown bacterium, which was later confirmed as F. tularensis.
Table 5.
Characterization of Form 3 Reports from Research Institutions.a
| Type of Laboratory | No. | Low | Medium | High | A | B | C | D | E | F | G |
|---|---|---|---|---|---|---|---|---|---|---|---|
| University | 29 | 24 | 3 | 2 | 7 | 9 | 5 | 0 | 6 | 1 | 1 |
| Government | 5 | 4 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 1 |
| Private/contractor | 11 | 9 | 1 | 1 | 5 | 1 | 1 | 1 | 2 | 0 | 1 |
| Totals | 45 | 37 | 5 | 3 | 13 | 11 | 6 | 2 | 9 | 1 | 3 |
Type of incident: A, needle stick, cut, or animal bite; B, dropped culture or animal cage; C, leak; D, contamination; E, equipment malfunction; F, torn glove; G, miscellaneous.
As might be expected, the highest risk of potential exposure occurred under situations where the laboratorian or pathologist was unaware of the presence of F. tularensis. Education and better communication with clinicians could reduce this risk. Registered research laboratories use PPE and work with known F. tularensis under BSL-3 conditions where exposures are fewer and are due primarily to breaks in containment. Risk reduction efforts to reduce needle stick injuries, cuts, and animal bites will reduce incidents in research institutions. Prompt evaluation of potential exposures by health professionals coupled with appropriate use of antimicrobial prophylaxis limited the number of occupationally acquired infections to 1 individual during this 5-year period.
Laboratory Methods
Despite the development of molecular diagnostic assays such as polymerase chain reaction (PCR), serology remains the most commonly used method for the diagnosis of tularemia (Paul Mead, Division of Vector Borne Diseases, CDC Ft. Collins, personal communication). Nevertheless, PCR assays for F. tularensis are available in many public health laboratories14 for use as a rapid test for presumptive identification of F. tularensis in cultures (as stated on the Form 4) and for animal specimens.
APHIS/CDC Form 4s can provide information concerning the use of PCR for the identification of F. tularensis. An examination of Form 4s indicated that real-time PCR was used in these laboratories together with standard methods (eg, DFA, slide agglutination) for culture confirmation and the presumptive identification of F. tularensis DNA in clinical specimens. Large academic reference laboratories reported that they used 16 S PCR with amplicon sequencing on human specimens. This method was used to identify a subculture of F. tularensis as well as detect F. tularensis DNA in a lymph node biopsy specimen sent from a hospital in Colorado. The Form 4 reporting the latter result made no mention of a confirmed laboratory diagnosis. PCR of a biopsy specimen was used to diagnose a laboratory-acquired case of tularemia15; however, this case occurred in 2009 (Roseanne Ressner, personal communication) and therefore was not included in our analysis.
PCR was also used for the diagnosis of tularemia in animals, although the extent to which it was used as a replacement or an adjunct to culture was difficult to determine. Four types of PCR assays were mentioned on the Form 4 as being used in registered entities. A real-time PCR assay was used by state and federal public health laboratories that processed animal specimens.14 Other laboratories that processed animal specimens reported using published PCR assays14,16 for the identification of F. tularensis. Since FDA approval is not required for veterinary diagnostic assays, the sensitivity and specificity of these assays for the various specimen types are questionable given that internal validation data and quality control by laboratories using these assays is unknown. Furthermore, Form 4s only capture positive PCR results. Thus, the number of false negative PCR results are unknown. Nevertheless, when used properly, PCR remains an ideal culture-independent method to identify F. tularensis given that the preponderance (76%) of animal specimens were clinical specimens (Table 2) that were either submitted by veterinary clinics, where shipping conditions and viability of F. tularensis are unknown, and specimens taken during necropsy of a carcass (also of unknown quality). PCR assays were used with DNA from extracted tissues or on extracted cells after culture of the clinical specimen in the reference laboratory. PCR was also used to detect F. tularensis in formalin-fixed tissue or in slides of paraffin-embedded fixed tissue where there were no viable cells to culture.
Many specimens containing F. tularensis DNA as determined by PCR were reported to FSAP by means of a Form 4 indicating the identification of a BSAT. A confirmed positive culture of F. tularensis is required for reporting the identification of this agent on a Form 4, whereas a positive PCR result on a primary specimen without isolation of a viable organisms is not. However, it remains unknown to what extent specimens shown to contain F. tularensis DNA solely by PCR go unreported. Based on our data, it is more likely to occur with specimens from animals than with those from humans.
Conclusions
Because of the rarity of tularemia, low clinical suspicion, and the causative agent’s low infectious dose, F. tularensis poses a hazard for unsuspecting laboratorians, particularly those who handle cultures outside a biological safety cabinet or without the use of appropriate PPE. Culture, which is currently used in confirmatory identification, was the primary method used in clinical and reference laboratories. Most reported occupational exposures occurred in clinical laboratorians working on the bench with cultures at BSL-2 conditions with no suspicion that the organism was F. tularensis; the fewest occurred in research laboratories at BSL-3 where prior knowledge of the organism required the use of PPE.
Confirmatory identification of an isolate as F. tularensis by the reference laboratory was primarily (>85%) accomplished by DFA, as stated on the Form 4. Most, if not all, cultures received by the reference laboratory were subcultured prior to confirmation. Thus, DFA and culture are currently the primary methods used by laboratories for the identification of F. tularensis. Molecular methods such as PCR are not FDA approved but are used for the identification of F. tularensis in specimens from animals and for presumptive identification in specimens from humans. It is likely that the use of molecular methods and whole genome sequencing will become more common in the future as these technologies are incorporated into clinical laboratories.17
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
The select agent regulations, which became effective on March 21, 2017, was modified as follows: Unless directed otherwise by the HHS Secretary, within 7 calendar days after identification of the select agent or toxin (except for Botulinum neurotoxin and/or Staphylococcal enterotoxin [Subtypes A–E]) or within 30 calendar days after identification of Botulinum neurotoxin and/or Staphylococcal enterotoxin (Subtypes A–E), the select agent or toxin is transferred in accordance with § 73.16 or destroyed onsite by a recognized sterilization or inactivation process.
References
- 1.Carus WS. Bioterrorism and Biocrimes: The Illicit Use of Biological Agents Since 1900. Amsterdam, Netherlands: Fredonia Books; 2002. [Google Scholar]
- 2.Willman D The Mirage Man. Bruce Ivins, The Anthrax Attacks, and America’s Rush to War. New York, NY: Bantam Books; 2011. [Google Scholar]
- 3.Morse SA. Pathogen security-help or hindrance? Frontiers Bioeng Biotechnol. 2015;2:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Public Law 104–132. Antiterrorism and Effective Death Penalty Act of 1996. 1996. www.gpo.gov/fdsys/pkg/PLAW-104publ132/pdf/PLAW-104publ132.pdf. Accessed April 25, 2016.
- 5.Department of Health and Human Services. Additional requirements for facilities transferring or receiving select infectious agents. Fed Reg. October 24 issue, 1996;61:55190–55520. [Google Scholar]
- 6.Public Law 107–56. Uniting and Strengthening America by Providing Appropriate Tools Required to Intercept and Obstruct Terrorism; 2001. www.gpo.gov/fdsys/pkg/PLAW-107publ56/pdf/PLAW-107publ56.pdf. Accessed April 25, 2016.
- 7.Public Law 107–188. Public Health Security and Bioterrorism Preparedness and Response Act of 2002; 2002. www.gpo.gov/fdsys/pkg/PLAW-107publ188/pdf/PLAW-107publ188.pdf. Accessed April 25, 2016.
- 8. The President. Executive order 13546: optimizing the security of biological select agents and toxins in the United States. Fed Reg. July 8 issue, 2010;75:39439–39442. [Google Scholar]
- 9.United States Army Medical Research Institute for Infectious Diseases. Medical Management of Biological Casualties Handbook. 7th ed. Fort Detrick, MD; 2011. [Google Scholar]
- 10.Centers for Disease Control and Prevention, National Institutes of Health. Biosafety in Microbiological and Biomedical Laboratories. 5th ed. U.S. Department of Health and Human Services, HHS Publication No. (CDC), U.S. Govt. Printing Office. 21–1112; 2009. [Google Scholar]
- 11.Centers for Disease Control and Prevention. Tularemia (Francisella tularensis) 1999 case definition; 1999. https://wwwn.cdc.gov/nndss/conditions/tularemia/case-definitions/1999/. Accessed April 25, 2016.
- 12.Centers for Disease Control and Prevention. Tularemia statistics. http://www.cdc.gov/tularemia/statistics/index.html. Accessed December 7, 2016.
- 13.Harding L, Byers KB. Epidemiology of laboratory-associated infections. In: Fleming DO, Hunt DL, eds. Biological Safety. Principles and Practices Washington, DC: ASM Press; 2000:35–54. [Google Scholar]
- 14.Versage JL, Severin DD, Chu MC, Petersen JM. Development of a multitarget real-time TaqMan PCR assay for enhanced detection of Francisella tularensis in complex assays. J Clin Microbiol. 2003;41(12):5492–5499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lam ST. Laboratory-acquired tularemia successfully treated with ciprofloxacin. Infect Dis Clin Pract. 2012;20(3):204–207. [Google Scholar]
- 16.Kugeler KJ, Gurfield N, Creek JG, Mahoney KS, Versage JL, Petersen JM. Discrimination between Francisella tularensis and Francisella-like endosymbionts when screening ticks by PCR. J Clin Microbiol. 2005;71(11):7594–7597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doggett NA, Mukundan H, Lefkowitz EJ, et al. Culture-independent diagnostics for health security. Health Secur. 2016; 14(3):121–142. [DOI] [PubMed] [Google Scholar]


