Abstract
Septic shock impacts approximately 6% of hospitalized patients with cirrhosis and is associated with high rates of morbidity and mortality. Although a number of landmark clinical trials have paved the way for incremental improvements in the diagnosis and management of septic shock in the general population, patients with cirrhosis have largely been excluded from these studies and critical knowledge gaps continue to impact the care of these individuals. In this review, we discuss nuances in the care of patients with cirrhosis and septic shock using a pathophysiology-based approach. We illustrate that septic shock may be challenging to diagnose in this population in the context of factors such as chronic hypotension, impaired lactate metabolism, and concomitant hepatic encephalopathy. Furthermore, we demonstrate that the application of routine interventions such as intravenous fluids, vasopressors, antibiotics, and steroids should be carefully considered among those with decompensated cirrhosis in light of hemodynamic, metabolic, hormonal, and immunologic disturbances. We propose that future research should include and characterize patients with cirrhosis in a systematic manner, and clinical practice guidelines may need to be refined accordingly.
Keywords: Cirrhosis, Septic shock, Intravenous fluids, Vasopressors, Antibiotics, Steroids
Core Tip: Septic shock is an important cause of morbidity and mortality among hospitalized patients with cirrhosis. In turn, the pathophysiology of cirrhosis impacts both the diagnosis and management of septic shock in meaningful ways. However, patients with cirrhosis have been traditionally underrepresented in clinical trials for septic shock, leading to critical knowledge gaps. The optimal care of these patients depends on achieving an understanding of the current limitations and implementing strategies for future research to address these shortcomings.
INTRODUCTION
Among hospitalized patients with cirrhosis, approximately one-third develop sepsis and 6% develop septic shock[1]. Historically, due to unacceptably high mortality rates, individuals with cirrhosis and septic shock were generally considered poor candidates for admission to the intensive care unit (ICU). However, over the past three decades, the findings of randomized controlled trials (RCTs) have led to incremental progress in the management of septic shock, resulting in decreased mortality[2]. Although patients with cirrhosis were underrepresented in these trials, recent epidemiologic studies suggest parallel improvement in survival among this subset, indicating that management in the ICU is warranted[3-6]. While patients with compensated cirrhosis may respond to the same interventions and may have comparable outcomes to those without cirrhosis[7], patients with decompensated cirrhosis and clinically significant portal hypertension have marked local and systemic hemodynamic aberrations and hepatic functional impairment that may profoundly impact their management and prognosis. Consequently, the care of these patients should be appropriately tailored based on their unique pathophysiology. This review highlights the salient aspects of the management of septic shock among patients with cirrhosis and identifies critical knowledge gaps for future research.
PATHOPHYSIOLOGY OF PORTAL HYPERTENSION
Portal hypertension occurs as a result of increased resistance in the hepatic vasculature and nitric oxide (NO)-mediated splanchnic and peripheral arteriolar vasodilation. Together, decreased systemic vascular resistance and increased splanchnic pooling contribute to a state of decreased effective circulating volume. This results in the activation of neurohumoral mechanisms aimed at maintaining adequate tissue perfusion, including beta-adrenergic signaling and the renin-angiotensin-aldosterone system. In patients with portal hypertension, these mechanisms increase cardiac contractility and promote salt and water retention[7]. When a severe infection ensues, macrovascular[8,9] and microvascular[10] vasodilatory effects are exaggerated, further decreasing the effective circulating volume and potentiating the neurohumoral response (Figure 1A).
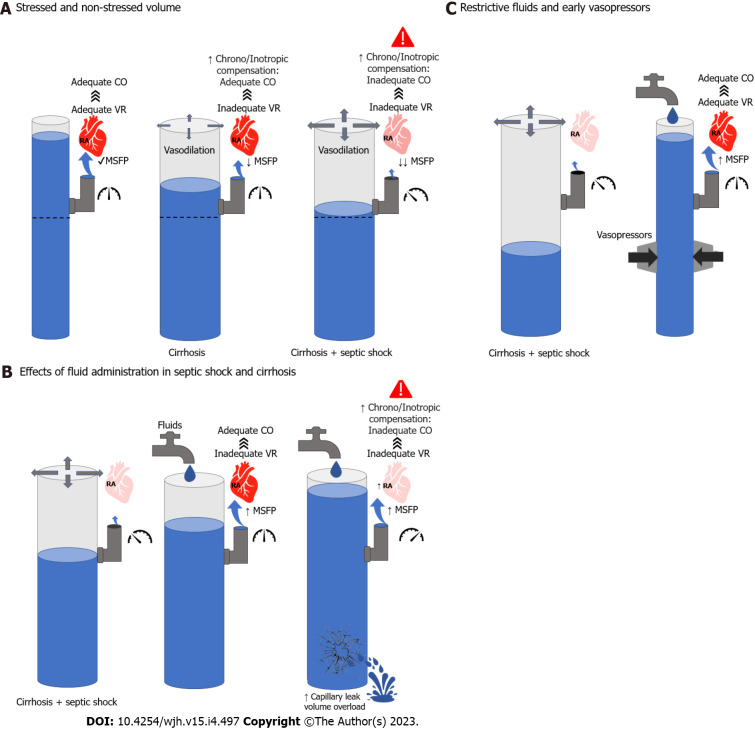
Figure 1.
Hemodynamic considerations in the management of cirrhosis and septic shock. A: Left; Normal mean systemic filling pressures (MSFP) leading to adequate venous return and cardiac output (CO). Middle; vasodilation in cirrhosis leading to lower MSFP and inadequate venous return (VR). However, compensatory mechanisms are able to maintain adequate CO. Right; further vasodilation leading to lower MSFP and inadequate VR. In this case, neurohumoral and cardiac compensation are not enough to maintain CO; B: Left; cirrhosis and septic shock pathophysiology. Middle; Effects of adequate volume resuscitation leading to increased MSFP. In the context of normal filling pressures, this will increase VR and CO. Right; Excessive fluid resuscitation will lead to high filling pressures which will decrease VR and CO. In addition, it may lead to volume overload and capillary leak; C: Left; cirrhosis and septic shock pathophysiology. Right; adjuvant effect of fluids and vasopressors on MSFP, VR and CO without leading to volume overload. CO: Cardiac output; MSFP: Mean systemic filling pressures; RA: Right atrium; VR: Venous return.
Irrespective of the etiology of liver disease, 50% of patients develop cirrhotic cardiomyopathy (CCM) as a byproduct of the neurohumoral mechanisms aimed at maintaining the effective circulating volume[11,12]. CCM can manifest with diastolic and/or systolic dysfunction, limiting further augmentation in cardiac contractility in response to hemodynamic stress. Likewise, CCM predisposes to anasarca if excess fluids are administered during resuscitation (Figure 2). Decreased oncotic pressures secondary to hypoalbuminemia and increased hydrostatic pressures secondary to portal hypertension enhance capillary leak.
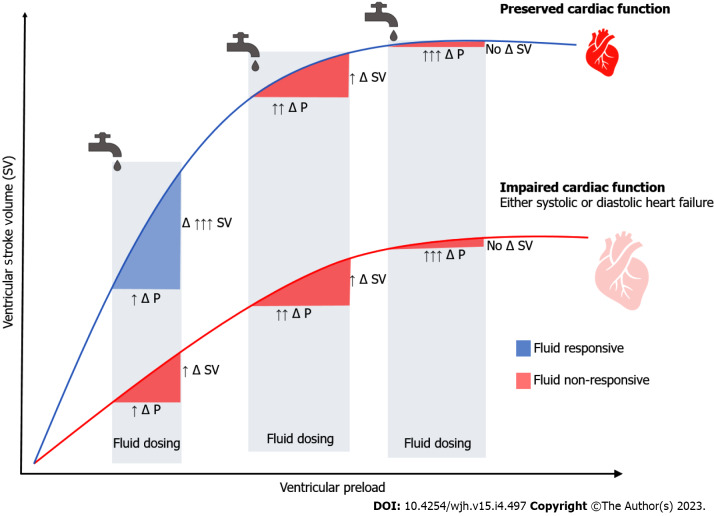
Figure 2.
Frank-Starling curves in septic shock. Every fluid bolus will lead in a change in pressure (Δ P) and a change in stroke volume (Δ SV). The effect of fluids on cardiac output among patients with normal (upper curve) and impaired (lower curve) myocardial function is depicted. Even among patients with normal myocardial reserve, excess fluid administration may significantly increase pressure without significantly increasing stroke volume, which may ultimately lead to anasarca. Δ P: Change in pressure; Δ SV: Change in stroke volume.
REPRESENTATION OF CIRRHOSIS IN SEPTIC SHOCK TRIALS
Although individual RCTs investigating diagnostic and therapeutic interventions for the management of septic shock have yielded controversial results, mortality has declined significantly over time. A possible explanation for the lack of benefit observed in trials is the frequent use of overall survival as the primary outcome. This endpoint may be suboptimal in the ICU setting, where the risk of death could be attributed to multiple competing causes[13,14]. In this context, isolated interventions are less likely to influence survival. Heterogeneity in patient selection and disease characteristics and the effects of confounding interventions are additional factors that may impact study results. A neutral association in a RCT may represent benefits for a particular subgroup of patients and harm to another[15]. To adequately interpret treatment effects among subpopulations such as cirrhosis, large pragmatic trials are required. Unfortunately, most contemporary septic shock trials have either underrepresented, excluded, or mischaracterized patients with cirrhosis (Table 1), limiting the potential applicability of common interventions in this patient population. In some cases, small RCTs of patients with cirrhosis have yielded conflicting results in comparison to those that excluded cirrhosis, leading to controversies in the care of these patients (Figure 3). Throughout the remainder of this review, we will highlight both evidence-based principles and areas of uncertainty.
Table 1.
Patients with liver disease in randomized controlled trials of sepsis and septic shock
|
Trial
|
Intervention
|
n
|
Liver disease present (%)
|
Cirrhosis excluded
|
Comments
|
| Rivers (2001) | EGDT vs Standard | 263 | 61 (23) | No | |
| ProMISe Trial (2014) | EGDT vs Standard | 1260 | 22 (1.8) | No | |
| ARISE Trial (2014) | EGDT vs Standard | 1600 | 83 (5) | No | |
| ProCESS (2014) | EGDT vs Standard | 1341 | 11 (0.8) | No | |
| ANDROMEDA-SHOCK (2019) | CRT vs Lactate clearance | 424 | 0 (0) | Yes | Excluded Child B and C |
| SMART Study (2018) | Balanced crystalloids vs 0.9% NS | 15802 | 180 (11) | No | |
| BaSICS Trial (2021) | Balanced crystalloids vs 0.9% NS and Slow vs Fast bolus | 11052 | 266 (2.4) | No | |
| PLUS Study (2022) | Balanced crystalloids vs 0.9% NS | 5037 | NR | No | |
| Classic Trial (2022) | Restrictive vs Liberal fluids | 1554 | NR | No | |
| SAFE Trial (2004) | 4% Albumin vs 0.9% NS | 6997 | NR | No | |
| ALBIOS Study (2014) | 20% Albumin + Crystalloids vs Crystalloids alone | 1818 | 27 (1.4) | No | Excluded cirrhotic patients with cirrhosis and ascites |
| VASST Trial (2008) | Vasopressin vs NE | 778 | 88 (11) | No | Excluded Na < 130 mEq/L and irreversible disease with less than six-month survival |
| VANISH Trial (2016) | Vasopressin vs NE - AKI | 409 | 14 (4) | No | Factorial design (vasopressin/hydrocortisone) |
| ATHOS-3 (2018) | Angiotensin-II vs Placebo | 344 | NR | Yes | Excluded MELD > 30 |
| CENSER (2019) | Early NE vs Placebo | 310 | 27 (9) | No | |
| CORTICUS Trial (2008) | Hydrocortisone vs Placebo | 499 | 40 (8) | No | |
| ADRENAL Trial (2018) | Hydrocortisone vs Placebo | 3800 | NR | No | |
| APROCCHSS Trial (2018) | Hydrocortisone + Fludrocortisone vs Placebo | 1241 | NR | Yes | Excluded Child C |
AKI: Acute kidney injury; CRT: Capillary refill time; EGDT: Early goal-directed therapy; NE: Norepinephrine; NR: Not reported; NS: Normal saline.
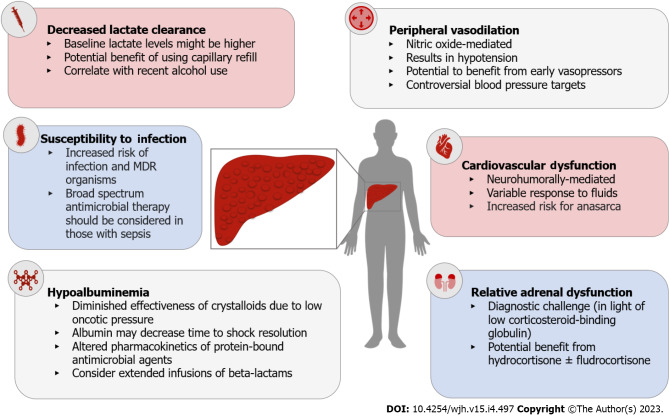
Figure 3.
Pathophysiologic changes in cirrhosis that impact the management of septic shock. MDR: Multi-drug resistant.
MANIFESTATIONS OF SHOCK IN CIRRHOSIS
Shock is a state of tissue hypoxia. It occurs when tissue oxygen demands cannot be met by the circulatory system or when tissue oxygen extraction is impaired, leading to cellular dysfunction[16]. It should be considered in patients who develop hypotension with additional clinical or biochemical findings of hypoperfusion, including altered mental status, acute kidney injury (AKI), or lactic acidosis[17]. Of the various subtypes, septic shock is most common among patients with cirrhosis[18]. It represents a dysregulated immune response to an infection, leading to systemic inflammation, vasodilation, and organ impairment[19].
In patients with cirrhosis and clinically significant portal hypertension, a low mean arterial pressure (MAP) is often present without overt signs and symptoms of hypoperfusion[20]. The ATTIRE trial[21] which included hospitalized patients with decompensated cirrhosis, defined hemodynamic dysfunction as a MAP < 60 mmHg rather than 65 mmHg, illustrating the point that a fixed MAP may not strictly reflect adequate tissue perfusion. Patients with advanced cirrhosis and a chronic state of systemic vasodilation have adaptive autoregulatory mechanisms to maintain perfusion to vital organs despite MAPs < 65 mmHg[22] whereas patients with early cirrhosis, metabolic syndrome, and chronic hypertension may develop tissue hypoperfusion despite MAPs 65 mmHg[23].
Therefore, in addition to assessing blood pressure, a determination of shock relies on assessing perfusion markers. In this respect, it is important to note that the clinical manifestations of hypoperfusion may be less reliable in cirrhosis. For instance, the neurological window for hypoperfusion might represent a diagnostic dilemma in cirrhosis, especially in patients with a history of hepatic encephalopathy (HE). In patients with new or unexplained HE, there should be a high index of suspicion for sepsis or septic shock. Similarly, skin mottling and other skin perfusion signs have lower sensitivity in patients with cirrhosis due to sustained peripheral vasodilation[24].
Another marker of hypoperfusion is type A hyperlactatemia. It occurs when lactate is produced under anaerobic conditions by lactate dehydrogenase[25] and is also confounded in cirrhosis in the context of altered lactate production and clearance. Septic shock is associated with normal to high tissue oxygen delivery but impaired oxygen extraction. Although tissue hypoxia may be present, direct clinical correlation with serum lactate levels may be unreliable in some instances[26-29]. However, peak lactic acid values and trends have prognostic significance[30]. The contemporary view of hyperlactatemia in septic shock relies on the observation that increased lactate production is driven by beta-adrenergic stimulation, otherwise referred to as stress hyperlactatemia[31]. Stress hyperlactatemia is believed to be a compensatory response to sepsis-induced vasodilation. In a stable hemodynamic state, patients with cirrhosis and more severe liver disease [i.e., those with decompensated disease and/or higher Child-Turcotte-Pugh (CTP) scores] have increased adrenergic tone and higher serum lactic acid values[32]. Because the liver provides up to 70% of the lactate clearance from the body[22], its disproportionate accumulation in patients with cirrhosis and critical illness is not surprising[33,34]. In a propensity score matched analysis accounting for potential confounding factors, Cheng et al[35] demonstrated that patients with cirrhosis had higher lactate levels. The difference was particularly robust in those with decompensated cirrhosis (4.08 mmol/L in patients with decompensated cirrhosis who survived vs 2.48 mmol/L in patients without cirrhosis who survived and 7.16 mmol/L in patients with decompensated cirrhosis who died vs 5.93 mmol/L in patients without cirrhosis who died). Similarly, Drolz et al[36] analyzed the predictive value of arterial lactate levels and clearance in critically ill patients with cirrhosis, demonstrating that values greater than 5 mmol/L were independently associated with 28-d mortality, and models such as the model for end-stage liver disease-lactate (MELD-LA) score have incorporated lactate values for prognostication[37]. Higher cutoffs for lactate levels have also been described in critically ill patients with cirrhosis and AKI[38] and in acute liver injury[39]. In the recent Baveno consensus conference, the criteria for futility in patients with variceal hemorrhage included lactate > 12 mmol/L[40]. Finally, it is important to note that, in patients with alcohol use disorder, ethanol oxidation decreases nicotinamide adenine dinucleotide (NAD+) thereby altering the NAD+/NADH ratio and shifting pyruvate metabolism toward lactate production. Although its impact on lactate levels appears to be modest[41], clinicians should consider the effects of alcohol use on lactate metabolism[42].
These cumulative data suggest that, although lactate remains useful as a predictor of mortality, the cutoff for normality may be higher in cirrhosis. Venous lactate levels > 2 mmol/L should raise suspicion for shock, but a multimodal approach that accounts for other signs and symptoms of organ hypoperfusion is warranted. In decompensated cirrhosis, a higher threshold (> 4 mmol/L) may be considered[35]. In patients without other signs of hypoperfusion, lactate elevations may indicate progressive physiologic stress and may correlate with poor prognoses but are not necessarily indicative of shock. This concept has important therapeutic implications.
FLUID RESUSCITATION
The initial management of sepsis is based on a practical evidence-based approach endorsed by the Society of Critical Care Medicine and the European Society of Intensive Care Medicine. The Surviving Sepsis Campaign (SSC) guidelines clarify best practices relating to critical aspects of care, including fluid resuscitation, vasopressor, antibiotic use, and steroid use, and hemodynamic monitoring, among other things. In the general population, the timely implementation of some components of this bundle are associated with improved outcomes[43].
Since inception, the SSC continues to suggest the use of at least 30 mL/kg of crystalloids within the first three hours as the initial management of patients with suspected septic shock[44], regardless of initial volume status or degree of volume responsiveness. However, the strength of the recommendation was downgraded from strong to weak in 2021 given the lack of robust data to support aggressive fluid resuscitation[45].
Septic shock is characterized by arteriolar vasodilation and venous pooling, further complicated by hypovolemia due to poor oral intake, insensible losses, and capillary leak in the context of endothelial dysfunction. The rationale for fluid administration is to increase the venous volume and augment the effective circulating volume. If right- and left-sided cardiac filling pressures are not elevated, the use of intravenous fluids may improve cardiac preload. On the dependent (steep) portion of the Frank-Starling curve, increased preload will ultimately augment cardiac output. However, if intravenous fluids are given in the setting of elevated cardiac filling pressures, or if the myocardium lacks inotropic reserve, fluids may not increase cardiac output (Figure 2). On the contrary, venous congestion, interstitial edema, and ineffective gas exchange will ensue (Figure 1B). Critically ill patients who develop anasarca have increased mortality for every liter of positive fluid balance[46,47]. Those with cirrhosis have an even greater risk for complications in light of decreased oncotic pressures and impaired cardiac reserve. Therefore, intravenous fluid therapy should be carefully administered, and as for any other medication, the type, dose, and duration need to be considered.
The type of intravenous fluids used for shock resuscitation are typically classified as crystalloids or colloids. Crystalloids include normal (0.9%) saline or balanced solutions such as Lactated Ringer’s, Plasma-Lyte, and Hartmann’s solution. Normal saline is the most ubiquitous worldwide, but its use associated with renal dysfunction[48], hyperchloremic metabolic acidosis, and decreased survival[49]. RCTs have demonstrated a potential benefit in favor of balanced crystalloid solutions in comparison to normal saline in critically ill patients, particularly when large volumes are necessary. Although patients with cirrhosis were underrepresented in these trials[50,51], there is no physiologic rationale against the use of balanced crystalloid in this population. Rather, in light of the risk for kidney injury, the use of hyperchloremic solutions should be limited in patients with cirrhosis.
As a result of the endothelial damage that occurs in sepsis, crystalloids remain in the intravascular compartment for minutes, whereas colloids, such as albumin, remain for up to three hours. In addition, the pleiotropic properties of albumin led to its use in critically ill patients, though RCTs have demonstrated mixed results[52-54]. The ALBIOS trial studied the addition of 20% albumin to crystalloids in hypoalbuminemic patients with severe sepsis or septic shock[54]. Although survival, length of stay, and organ failure scores did not improve, albumin use was associated with higher MAPs, lower net fluid balance, and decreased time to vasopressor or inotrope discontinuation. In a post-hoc analysis of only patients with septic shock, those randomized to the albumin arm had a 6.3% absolute reduction in 90-d mortality (RR 0.87, 95%CI 0.77-0.99; P = 0.03). However, less than 2% of the subjects had liver disease and patients with advanced cirrhosis were excluded from the trial. In the recent FRISC trial[55], investigators compared the use of 5% albumin with 0.9% saline in patients with advanced cirrhosis (mean CTP score of 12 and MELD-sodium score of 33) and sepsis-induced hypotension. The authors found improved hypotension reversal (primary outcome, defined as achieving a MAP ³ 65 mmHg at three hours), lower lactate levels, and resolution of tachycardia in the albumin arm. At one week, 43.5% of the patients in the albumin arm were alive in comparison to 38.3% in the normal saline arm (P = 0.03). Similarly, in the recent ALPS study[56] higher rates of short-term septic shock reversal were found using 20% albumin in comparison to Plasma-Lyte. Although albumin use was also associated with more rapid lactate clearance and lower rates of renal replacement therapy, there was no difference in mortality. One in every five patients in the albumin arm required discontinuation of the colloid due to pulmonary edema, most commonly among those with pneumonia. The safety concern of pulmonary edema with the rapid infusion of 20% albumin was also observed in the ATTIRE[21] and CONFIRM[57] studies. Thus, albumin may be effective for shock reversal in patients with cirrhosis, but due to the increased risk for pulmonary complications, close monitoring for volume overload is warranted, specifically in patients with AKI, lung disease, and higher MELD scores.
Finally, the volume of fluid administered also matters. Although no study has directly compared the initial 30 mL/kg of crystalloids to smaller volumes, recent studies have attempted to address the impact of volume. The CLASSIC trial compared restrictive (median 1798 mL) to liberal (median 3811 mL) fluid strategies for resuscitation after an initial administration of one liter of crystalloids[58]. The authors found no differences in 90-d mortality. However, the study provides valuable data regarding the safety of restrictive fluid resuscitation, which could be particularly useful in patients prone to develop volume overload, such as those with cirrhosis. The results of the CLOVERS trial, which tested a similar hypothesis[59] are pending, though the trial was terminated for futility after an interim analysis demonstrated no differences in 90-d survival between both groups. Regardless, it should be noted that weight-based fluid strategies should be reconsidered in some patients, especially those with underlying obesity or marked anasarca. In principle, individualizing fluid resuscitation is an essential principle as requirements and tolerance to fluids vary substantially among individuals[60].
VASOPRESSORS
The application of a restrictive fluid strategy hinges on the early use of vasopressors. Vasopressors target the vasodilatory physiology of septic shock by restoring vascular tone and mobilizing the pooled volume of blood to the heart (Figure 1C). Vasopressors consist of catecholamines such as norepinephrine, epinephrine, dopamine, or phenylephrine and non-catecholamines like vasopressin analogs and angiotensin II. In part, they increase the tone of the vascular bed in patients with septic shock via effects on alpha-1 (catecholamines), V1 (vasopressin analogs), or angiotensin II receptors (angiotensin II). The early use of vasopressors leads to a faster resolution of shock[61], whereas delay is associated with increased mortality[62]. In fact, there is an approximately 5.3% increased risk of death for every hour of delay[63]. However, common adverse effects of vasopressors include digital and splanchnic ischemia in a dose-dependent manner[61,62]. Catecholamine-based vasopressors can also lead to cardiac arrhythmias and ischemia due to their effect on beta-1 receptors[64-66].
Based on head-to-head RCTs comparing different adrenergic vasopressors, the SSC recommends norepinephrine as the first line vasopressor for the management of septic shock[45]. Nonetheless, most of these RCTs included less than 10% of patients with liver disease (Table 1). Multiple trials have demonstrated the benefit of vasopressin analogs in hepatorenal syndrome[67-70], a functional manifestation of end-stage portal hypertension characterized by systemic vasodilation and renal vasoconstriction, often precipitated by infections. In this setting, a recent network meta-analysis suggested that terlipressin may be more beneficial than norepinephrine[70]. Terlipressin, is a vasopressin analogue with greater affinity for V1 receptors. It has been proposed as an alternative vasopressor in septic shock. In a small RCT, Choudhury et al[71] compared the use of norepinephrine and terlipressin in patients with cirrhosis and septic shock. The authors observed higher rates of shock resolution, lower incidence of variceal bleeding, and improved time to vasopressor discontinuation with the use of terlipressin. However, a subsequent RCT in patients without cirrhosis showed a higher incidence of adverse events such as digital ischemia when compared to norepinephrine with no improvement in mortality or organ failure resolution[72]. To date, norepinephrine remains the first line vasopressor in patients with septic shock who do not respond to fluids. Although the results of the VASST[73] and VANISH[74] trials did not demonstrate that the early use of vasopressin improved mortality or renal outcomes, respectively, low-dose vasopressin remains the agent of choice after norepinephrine in septic shock because of its relatively favorable side-effect profile and possible pleiotropic effects[75]. Though it may be reasonable to consider vasopressin analogues such as terlipressin in some patients with cirrhosis, there is currently insufficient data to support their use over vasopressin[76-78]. The efficacy of new non-catecholamine based vasopressors such as angiotensin II might be limited in the setting of cirrhosis as hypoalbuminemia was a negative predictor for response in the ATHOS-3 trial[79].
GOALS OF RESUSCITATION
Although the trigger for initiating resuscitation in septic shock is well defined, the endpoint is less clear. The goal of resuscitation is to augment tissue perfusion. However, the resolution of organ dysfunction lags behind the sufficiency of resuscitation, which often leads to excess fluid administration[80]. The ideal targets for adequate resuscitation and the type of monitoring necessary continue to be heavily debated topics.
With the publication of the Rivers study[81], early goal-directed therapy (EGDT) became the cornerstone for the management of septic shock. In this single-center trial, the use of EGDT decreased mortality compared to standard therapy. EGDT consisted of a protocol for the administration of crystalloids, vasopressors, inotropes, and blood products to achieve specific hemodynamic goals (blood pressure, central venous pressure, central venous oxygen saturation, and hemoglobin levels). However, two decades later, three multicenter RCT demonstrated that EGDT does not improve outcomes[82-84] but leads to higher hospitalizations costs[85]. Of note, these trials included a small number of patients with chronic liver disease and cirrhosis (Table 1). Currently, the goals of septic shock resuscitation are to reverse derangements in the very same components that define it. This includes achieving an adequate MAP, improving the signs/symptoms of skin, renal and brain hypoperfusion, and decreasing lactate levels.
First, the SSC guidelines recommend targeting a goal MAP of 65 mmHg over higher values. This recommendation is based on the results of the SEPSISPAM trial which compared high (80-85 mmHg) vs low (65-70 mmHg) MAPs. The investigators found no difference in mortality. However, they observed a higher incidence of new-onset atrial fibrillation and lower rates of renal replacement therapy in the high MAP group[23]. The latter was observed only in those with chronic hypertension, suggesting that a personalized target for MAP must be considered, particularly in patients with chronic adaptive mechanisms of autoregulation to higher MAPs.
Of interest, among patients with chronic hypotension, Gershengorn and colleagues demonstrated a robust association between baseline low systolic blood pressure, prolonged use of vasopressors, and increased length of stay[86]. Whether these observations are a consequence of clinicians aiming for unrealistic MAP goals in chronically hypotensive patients or a reflection of more severe disease is unclear. Recently, the results of the 65 trial demonstrated the safety of more liberal MAP goals (60-65 mmHg) in patients with distributive shock[87]. Although these trials did not include patients with cirrhosis, they provide reassurance that lower conventional goals can achieve adequate oxygen delivery and may be adequate targets. Notably, however, as the etiology of cirrhosis has shifted to patients with metabolic syndrome, more patients with chronic arterial hypertension will present with septic shock.
Second, the SSC recommends guiding resuscitation to decrease serum lactate[45]. As mentioned in the previous sections, its use has limitations, particularly in the setting of cirrhosis. However, a decrease in lactate levels after initial resuscitation is associated with improved outcomes, even among patients with cirrhosis[88-91]. In a RCT, Jansen and colleagues tested a lactate-guided strategy for resuscitation based on lactate clearance which led to a significant decrease in mortality. The authors pursued a 20% decrease every two hours for the initial eight hours of management. Interestingly, the levels of lactate within groups were not significantly different, suggesting that perhaps closer monitoring with timely interventions for persistent hypoperfusion rather than lactate clearance is more consequential[91]. Given the caveats of lactate kinetics[92], which are impacted by hepatic clearance[93] and stress-induced production, new alternatives have been proposed.
The ANDROMEDA-SHOCK trial compared the use of capillary refill time (CRT) normalization to lactate clearance in patients with septic shock[94]. The authors demonstrated a non-significant trend towards improved 28-d mortality among the CRT group (HR 0.75, 95%CI 0.55 to 1.02; P = 0.06). Individuals randomized to the CRT arm did receive less fluids and had improvement in organ-dysfunction at 72 h. Zampieri et al[95] performed a Bayesian re-analysis of the data, finding a possible mortality benefit with the use of CRT. CRT is now recommended in the SSC guidelines as it offers an alternative for resuscitation targets, especially in patients in whom lactate clearance is impaired, such as in patients with cirrhosis.
HEMODYNAMIC MONITORING
Whether lactate clearance, CRT, or alternative markers are used as targets for adequate resuscitation, clinicians should assess whether their interventions are achieving the desired effect. Only half of patients with septic shock are volume-responsive during initial resuscitation, around 30% after two hours, and less than 20% after four hours[96]. The hemodynamic response to fluid administration can be assessed by dynamic tests that evaluate whether an increase in preload increases cardiac output[97,98].
Multiple RCTs have demonstrated the feasibility of using fluid responsiveness markers to monitor and guide fluid resuscitation. Their use led to a reduction in the amount of volume administered[99] and need for renal replacement therapy[100], albeit with no effect on survival[101]. Unfortunately, they have not been validated in patients with cirrhosis. Moving forward, the application of tools like point-of-care ultrasonography may help optimize fluid resuscitation in patients with cirrhosis, but studies are necessary to determine the parameters that are most applicable.
ANTIBIOTICS
Early antibiotic administration provides the greatest survival benefit in septic shock. Every hour of delay in their administration conveys an increased risk for mortality[102], even within the first six hours[103]. The SSC guidelines recommend the initiation of antibiotics within one hour of the diagnosis of sepsis with a particular emphasis on patients with shock, for which every hour of delay conveys a 7% increase in mortality[45]. Although the rapid initiation of antimicrobial therapy is essential, the adequacy of coverage and pharmacokinetics are also important in patients with cirrhosis.
Appropriate antibiotic initiation involves administering the drug most likely to eradicate the suspected organism while avoiding unnecessary antibiotic-associated toxicities and exposures that predispose to the development of multi-drug resistant (MDR) organisms[104,105]. In patients with cirrhosis, up to a third of bacterial infections are now due to resistant organisms[106], and these infections are associated with dismal prognoses[107]. Therefore, the choice of empiric treatment, specifically in septic shock, should account for local epidemiology and individual risk factors for MDR infections. Recent hospitalization, nosocomial infection, prior health-care exposure, ICU admission, and recent antibiotics use (within 90 d) predispose to MDR infections in patients with cirrhosis[108,109]. In individuals with these risk factors, broad spectrum antibiotics tailored to local antibiograms and site of infection are warranted, followed by de-escalation within 48-72 h, based on laboratory data and clinical status. Unfortunately, up to 50% of cases of sepsis are associated with insufficient or negative culture data, which complicates both antimicrobial de-escalation and the detection of resistant strains[110]. Rapid diagnostic techniques, which rely on molecular methods such as polymerase chain reaction, are now available for the identification of pathogens and resistance genes. They have been shown to be efficient and effective in isolating the cause of sepsis[111]. Their use is associated with improved antibiotic selection, decreased antimicrobial use[112], shortened hospital stays, and in the case of bloodstream infections, improved mortality[113]. When available, these techniques should be used to optimize the treatment of sepsis. Finally, although the prevalence of fungal infections is variable, patients with cirrhosis have functional defects in neutrophils function that increase the likelihood of infections due to Candida and Aspergillus species. In general, fungal infections should be strongly considered in patients with abdominal sepsis, exposure to broad spectrum antibiotics or steroids, parenteral nutrition, prolonged ICU stay[114], and ACLF[115].
In cirrhosis, altered pharmacokinetics and pharmacodynamics modify the efficacy of antimicrobial agents. For highly protein-bound antibiotics such as ceftriaxone, aztreonam, or carbapenems, hypoalbuminemia increases the unbound fraction and increases its clearance[116], resulting in lower drug levels over the minimal inhibitory concentration (MIC). For antibiotics such as beta-lactams, for which efficacy depends on the time over the MIC, this may lead to treatment failure[117]. In patients with hypoalbuminemia the use of ertapenem is associated with a fivefold increase in mortality, which is not observed with lower protein-bound carbapenems such as meropenem or imipenem[118]. Furthermore, patients with ascites have an increased volume of distribution, which may result in decreased peak concentrations of antibiotics, especially those which distribute extracellularly[119]. In the case of spontaneous bacterial peritonitis, a common source of sepsis among hospitalized patients with cirrhosis, peritoneal antibiotic penetration is an essential concept. While some agents like cephalosporins, fluoroquinolones, and meropenem[120-122] achieve high concentrations in ascitic fluid, others such as aminoglycosides and tigecycline have reduced penetration[123,124]. The use of continuous or extended infusions of beta-lactams increases the duration of antibiotic levels over the MIC and lead to higher cure rates and decreased mortality in RCTs among patients without cirrhosis[125-127]. In a secondary analysis of the BICHROME study, Bartoletti et al[128] compared extended infusions vs bolus dosing of carbapenems or piperacillin/tazobactam in patients with cirrhosis who had bloodstream infections. The authors found that extended infusions were associated with improved mortality and higher rates of hospital discharge. Currently, the use of prolonged infusions of beta-lactams is recommended in patients with sepsis and septic shock[45].
ADRENAL DYSFUNCTION
Corticosteroids provide anti-inflammatory counterbalance to the dysregulated inflammatory response. They counteract vasodilatation by acting on endothelial glucocorticoid receptors[129], potentiate catecholamine effects, and contribute to volume retention. Therefore, hydrocortisone is recommended for the treatment of septic shock refractory to norepinephrine (at doses > 0.25 mg/kg/min)[45], although multiple RCTs have yielded conflicting data about their efficacy[130-134]. In 2018 the results of the most recent trials ADRENAL[135] and APROCCHSS[136] were published. In the former, the investigators tested the administration of continuous intravenous hydrocortisone against placebo for seven days in patients with septic shock. Although the authors did not observe a mortality benefit, time to shock reversal, length of ICU stay, and mechanical ventilation duration were all reduced in the hydrocortisone group[137]. In the APROCCHSS trial, investigators compared bolus intravenous hydrocortisone plus oral fludrocortisone to placebo, demonstrating improved survival and faster shock resolution[136]. Based on these mixed results, Pirracchio et al[138] used data from these RCTs in a machine learning model to explore the individual treatment effect of corticosteroids based on individual estimates of benefit. The authors found that corticosteroid administration based on risk modeling yielded benefit compared to a treat-all-or-none approach. However, the impact of the presence or absence of cirrhosis was not assessed.
The number of patients with cirrhosis in studies evaluating the role of steroids in septic shock is low (Table 1). Nonetheless, 50%-80%[139-141] of patients with advanced cirrhosis have normal baseline cortisol secretion but impaired response to stress; a state called relative adrenal insufficiency (RAI). In stable patients with cirrhosis, RAI is diagnosed and managed according to the adrenal response to ACTH stimulation[142], but in critically ill patients, its use to characterize and manage RAI is discouraged[143].
The high prevalence of RAI would suggest a clear benefit in favor of corticosteroids among patients with cirrhosis, but the evidence for their efficacy is mixed. In a prospective observational study, Fernández et al[144] demonstrated that corticosteroids conferred improved survival and faster shock resolution. In a small RCT, Arabi et al[145] noted improvements in shock resolution but no survival benefit. Patients treated in the corticosteroid arm had a higher incidence of shock relapse, which supports the notion of unmasked RAI. A higher incidence of gastrointestinal bleeding was observed in the Arabi trial, but this was not replicated in larger observational studies[146]. Despite the mixed evidence, SSC guidelines currently recommend the use of corticosteroids in patients with refractory shock[45].
CONCLUSION
The management of patients with cirrhosis and septic shock is largely based on data extrapolated from RCTs of patients without cirrhosis. However, in light of key differences in pathophysiology, basic interventions may be associated with different outcomes in this subset. Although the SSC guidelines have streamlined and improved the management of septic shock in the general population, these recommendations must ultimately be individualized for patients with cirrhosis using evidence-based strategies. In light of the growing impact of cirrhosis on the care of critically ill patients, future research in septic shock should focus on including and accurately characterizing this population in an effort to overcome critical knowledge gaps.
Footnotes
Conflict-of-interest statement: All the authors have no conflicts of interest to report.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: December 28, 2022
First decision: January 5, 2023
Article in press: March 23, 2023
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ferrarese A, Italy; Moldovan CA, Romania S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
Contributor Information
Jose Victor Jimenez, Section of Digestive Diseases, Yale School of Medicine, New Haven, CT 06520, United States.
Guadalupe Garcia-Tsao, Section of Digestive Diseases, Yale School of Medicine, New Haven, CT 06520, United States.
Saad Saffo, Section of Digestive Diseases, Yale School of Medicine, New Haven, CT 06520, United States. saad.saffo@yale.edu.
References
- 1.Safi W, Elnegouly M, Schellnegger R, Umgelter K, Geisler F, Reindl W, Saugel B, Hapfelmeier A, Umgelter A. Infection and Predictors of Outcome of Cirrhotic Patients after Emergency Care Hospital Admission. Ann Hepatol. 2018;17:948–958. doi: 10.5604/01.3001.0012.7195. [DOI] [PubMed] [Google Scholar]
- 2.Bauer M, Gerlach H, Vogelmann T, Preissing F, Stiefel J, Adam D. Mortality in sepsis and septic shock in Europe, North America and Australia between 2009 and 2019- results from a systematic review and meta-analysis. Crit Care. 2020;24:239. doi: 10.1186/s13054-020-02950-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sauneuf B, Champigneulle B, Soummer A, Mongardon N, Charpentier J, Cariou A, Chiche JD, Mallet V, Mira JP, Pène F. Increased survival of cirrhotic patients with septic shock. Crit Care. 2013;17:R78. doi: 10.1186/cc12687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levesque E, Hoti E, Azoulay D, Ichaï P, Habouchi H, Castaing D, Samuel D, Saliba F. Prospective evaluation of the prognostic scores for cirrhotic patients admitted to an intensive care unit. J Hepatol. 2012;56:95–102. doi: 10.1016/j.jhep.2011.06.024. [DOI] [PubMed] [Google Scholar]
- 5.Galbois A, Aegerter P, Martel-Samb P, Housset C, Thabut D, Offenstadt G, Ait-Oufella H, Maury E, Guidet B Collège des Utilisateurs des Bases des données en Réanimation (CUB-Réa) Group. Improved prognosis of septic shock in patients with cirrhosis: a multicenter study*. Crit Care Med. 2014;42:1666–1675. doi: 10.1097/CCM.0000000000000321. [DOI] [PubMed] [Google Scholar]
- 6.Baudry T, Hernu R, Valleix B, Jahandiez V, Faucher E, Simon M, Cour M, Argaud L. Cirrhotic Patients Admitted to the ICU With Septic Shock: Factors Predicting Short and Long-Term Outcome. Shock. 2019;52:408–413. doi: 10.1097/SHK.0000000000001282. [DOI] [PubMed] [Google Scholar]
- 7.Vora RS, Subramanian RM. Hypotension in Cirrhosis. Clin Liver Dis (Hoboken) 2019;13:149–153. doi: 10.1002/cld.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Armentano RL, Arbeitman CR, Cymberknop LJ, Farro I, Viotti R, Cardelino J. Flow Mediated Dilation in Cirrhosis: A Pilot Study in Different Stages of the Disease. Annu Int Conf IEEE Eng Med Biol Soc. 2018;2018:4564–4566. doi: 10.1109/EMBC.2018.8513192. [DOI] [PubMed] [Google Scholar]
- 9.Cazzaniga M, Salerno F, Visentin S, Cirello I, Donarini C, Cugno M. Increased flow-mediated vasodilation in cirrhotic patients with ascites: relationship with renal resistive index. Liver Int. 2008;28:1396–1401. doi: 10.1111/j.1478-3231.2008.01847.x. [DOI] [PubMed] [Google Scholar]
- 10.Hariri G, Urbina T, Lavillegrand JR, Gasperment M, Mazerand S, Abdelmalek A, Bigé N, Baudel JL, Guidet B, Maury E, Ait-Oufella H. Exaggerated Microvascular Vasodilating Responses in Cirrhotic Patients With Septic Shock. Crit Care Med. 2021;49:e404–e411. doi: 10.1097/CCM.0000000000004846. [DOI] [PubMed] [Google Scholar]
- 11.Kaur H, Premkumar M. Diagnosis and Management of Cirrhotic Cardiomyopathy. J Clin Exp Hepatol. 2022;12:186–199. doi: 10.1016/j.jceh.2021.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wehmeyer MH, Heuer AJ, Benten D, Püschel K, Sydow K, Lohse AW, Lüth S. High Rate of Cardiac Abnormalities in a Postmortem Analysis of Patients Suffering From Liver Cirrhosis. J Clin Gastroenterol. 2015;49:866–872. doi: 10.1097/MCG.0000000000000323. [DOI] [PubMed] [Google Scholar]
- 13.Veldhoen RA, Howes D, Maslove DM. Is Mortality a Useful Primary End Point for Critical Care Trials? Chest. 2020;158:206–211. doi: 10.1016/j.chest.2019.11.019. [DOI] [PubMed] [Google Scholar]
- 14.Granholm A, Alhazzani W, Derde LPG, Angus DC, Zampieri FG, Hammond NE, Sweeney RM, Myatra SN, Azoulay E, Rowan K, Young PJ, Perner A, Møller MH. Randomised clinical trials in critical care: past, present and future. Intensive Care Med. 2022;48:164–178. doi: 10.1007/s00134-021-06587-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iwashyna TJ, Burke JF, Sussman JB, Prescott HC, Hayward RA, Angus DC. Implications of Heterogeneity of Treatment Effect for Reporting and Analysis of Randomized Trials in Critical Care. Am J Respir Crit Care Med. 2015;192:1045–1051. doi: 10.1164/rccm.201411-2125CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vincent JL, De Backer D. Circulatory shock. N Engl J Med. 2013;369:1726–1734. doi: 10.1056/NEJMra1208943. [DOI] [PubMed] [Google Scholar]
- 17.Cecconi M, De Backer D, Antonelli M, Beale R, Bakker J, Hofer C, Jaeschke R, Mebazaa A, Pinsky MR, Teboul JL, Vincent JL, Rhodes A. Consensus on circulatory shock and hemodynamic monitoring. Task force of the European Society of Intensive Care Medicine. Intensive Care Med. 2014;40:1795–1815. doi: 10.1007/s00134-014-3525-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weiss E, Paugam-Burtz C, Jaber S. Shock Etiologies and Fluid Management in Liver Failure. Semin Respir Crit Care Med. 2018;39:538–545. doi: 10.1055/s-0038-1672139. [DOI] [PubMed] [Google Scholar]
- 19.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ge PS, Runyon BA. Treatment of Patients with Cirrhosis. N Engl J Med. 2016;375:2104–2105. doi: 10.1056/NEJMc1612334. [DOI] [PubMed] [Google Scholar]
- 21.China L, Freemantle N, Forrest E, Kallis Y, Ryder SD, Wright G, Portal AJ, Becares Salles N, Gilroy DW, O'Brien A ATTIRE Trial Investigators. A Randomized Trial of Albumin Infusions in Hospitalized Patients with Cirrhosis. N Engl J Med. 2021;384:808–817. doi: 10.1056/NEJMoa2022166. [DOI] [PubMed] [Google Scholar]
- 22.Licata A, Mazzola A, Ingrassia D, Calvaruso V, Cammà C, Craxì A. Clinical implications of the hyperdynamic syndrome in cirrhosis. Eur J Intern Med. 2014;25:795–802. doi: 10.1016/j.ejim.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 23.Asfar P, Meziani F, Hamel JF, Grelon F, Megarbane B, Anguel N, Mira JP, Dequin PF, Gergaud S, Weiss N, Legay F, Le Tulzo Y, Conrad M, Robert R, Gonzalez F, Guitton C, Tamion F, Tonnelier JM, Guezennec P, Van Der Linden T, Vieillard-Baron A, Mariotte E, Pradel G, Lesieur O, Ricard JD, Hervé F, du Cheyron D, Guerin C, Mercat A, Teboul JL, Radermacher P SEPSISPAM Investigators. High versus low blood-pressure target in patients with septic shock. N Engl J Med. 2014;370:1583–1593. doi: 10.1056/NEJMoa1312173. [DOI] [PubMed] [Google Scholar]
- 24.Galbois A, Bigé N, Pichereau C, Boëlle PY, Baudel JL, Bourcier S, Maury E, Guidet B, Ait-Oufella H. Exploration of skin perfusion in cirrhotic patients with septic shock. J Hepatol. 2015;62:549–555. doi: 10.1016/j.jhep.2014.10.012. [DOI] [PubMed] [Google Scholar]
- 25.Weinberger J, Klompas M, Rhee C. What Is the Utility of Measuring Lactate Levels in Patients with Sepsis and Septic Shock? Semin Respir Crit Care Med. 2021;42:650–661. doi: 10.1055/s-0041-1733915. [DOI] [PubMed] [Google Scholar]
- 26.James JH, Luchette FA, McCarter FD, Fischer JE. Lactate is an unreliable indicator of tissue hypoxia in injury or sepsis. Lancet. 1999;354:505–508. doi: 10.1016/S0140-6736(98)91132-1. [DOI] [PubMed] [Google Scholar]
- 27.Boekstegers P, Weidenhöfer S, Kapsner T, Werdan K. Skeletal muscle partial pressure of oxygen in patients with sepsis. Crit Care Med. 1994;22:640–650. doi: 10.1097/00003246-199404000-00021. [DOI] [PubMed] [Google Scholar]
- 28.Ronco JJ, Fenwick JC, Wiggs BR, Phang PT, Russell JA, Tweeddale MG. Oxygen consumption is independent of increases in oxygen delivery by dobutamine in septic patients who have normal or increased plasma lactate. Am Rev Respir Dis. 1993;147:25–31. doi: 10.1164/ajrccm/147.1.25. [DOI] [PubMed] [Google Scholar]
- 29.Levy B, Gibot S, Franck P, Cravoisy A, Bollaert PE. Relation between muscle Na+K+ ATPase activity and raised lactate concentrations in septic shock: a prospective study. Lancet. 2005;365:871–875. doi: 10.1016/S0140-6736(05)71045-X. [DOI] [PubMed] [Google Scholar]
- 30.Borthwick HA, Brunt LK, Mitchem KL, Chaloner C. Does lactate measurement performed on admission predict clinical outcome on the intensive care unit? Ann Clin Biochem. 2012;49:391–394. doi: 10.1258/acb.2011.011227. [DOI] [PubMed] [Google Scholar]
- 31.Garcia-Alvarez M, Marik P, Bellomo R. Sepsis-associated hyperlactatemia. Crit Care. 2014;18:503. doi: 10.1186/s13054-014-0503-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jeppesen JB, Mortensen C, Bendtsen F, Møller S. Lactate metabolism in chronic liver disease. Scand J Clin Lab Invest. 2013;73:293–299. doi: 10.3109/00365513.2013.773591. [DOI] [PubMed] [Google Scholar]
- 33.Sauer CM, Gómez J, Botella MR, Ziehr DR, Oldham WM, Gavidia G, Rodríguez A, Elbers P, Girbes A, Bodi M, Celi LA. Understanding critically ill sepsis patients with normal serum lactate levels: results from U.S. and European ICU cohorts. Sci Rep. 2021;11:20076. doi: 10.1038/s41598-021-99581-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Jonghe B, Cheval C, Misset B, Timsit JF, Garrouste M, Montuclard L, Carlet J. Relationship between blood lactate and early hepatic dysfunction in acute circulatory failure. J Crit Care. 1999;14:7–11. doi: 10.1016/s0883-9441(99)90002-3. [DOI] [PubMed] [Google Scholar]
- 35.Cheng CY, Kung CT, Wu KH, Chen FC, Cheng HH, Cheng FJ, Huang JB, Su CM. Liver cirrhosis affects serum lactate level measurement while assessing disease severity in patients with sepsis. Eur J Gastroenterol Hepatol. 2021;33:1201–1208. doi: 10.1097/MEG.0000000000001826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Drolz A, Horvatits T, Rutter K, Landahl F, Roedl K, Meersseman P, Wilmer A, Kluwe J, Lohse AW, Kluge S, Trauner M, Fuhrmann V. Lactate Improves Prediction of Short-Term Mortality in Critically Ill Patients With Cirrhosis: A Multinational Study. Hepatology. 2019;69:258–269. doi: 10.1002/hep.30151. [DOI] [PubMed] [Google Scholar]
- 37.Chen XF. Prognostic Role of MELD-Lactate in Cirrhotic Patients' Short- and Long-Term Prognosis, Stratified by Causes of Cirrhosis. Can J Gastroenterol Hepatol. 2022;2022:8449579. doi: 10.1155/2022/8449579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun DQ, Zheng CF, Lu FB, Van Poucke S, Chen XM, Chen YP, Zhang L, Zheng MH. Serum lactate level accurately predicts mortality in critically ill patients with cirrhosis with acute kidney injury. Eur J Gastroenterol Hepatol. 2018;30:1361–1367. doi: 10.1097/MEG.0000000000001189. [DOI] [PubMed] [Google Scholar]
- 39.Dugas AF, Mackenhauer J, Salciccioli JD, Cocchi MN, Gautam S, Donnino MW. Prevalence and characteristics of nonlactate and lactate expressors in septic shock. J Crit Care. 2012;27:344–350. doi: 10.1016/j.jcrc.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de Franchis R, Bosch J, Garcia-Tsao G, Reiberger T, Ripoll C Baveno VII Faculty. Corrigendum to 'Baveno VII - Renewing consensus in portal hypertension' [J Hepatol (2022) 959-974] J Hepatol. 2022;77:271. doi: 10.1016/j.jhep.2021.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.MacDonald L, Kruse JA, Levy DB, Marulendra S, Sweeny PJ. Lactic acidosis and acute ethanol intoxication. Am J Emerg Med. 1994;12:32–35. doi: 10.1016/0735-6757(94)90193-7. [DOI] [PubMed] [Google Scholar]
- 42.Dezman ZD, Comer AC, Narayan M, Scalea TM, Hirshon JM, Smith GS. Alcohol consumption decreases lactate clearance in acutely injured patients. Injury. 2016;47:1908–1912. doi: 10.1016/j.injury.2016.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seymour CW, Gesten F, Prescott HC, Friedrich ME, Iwashyna TJ, Phillips GS, Lemeshow S, Osborn T, Terry KM, Levy MM. Time to Treatment and Mortality during Mandated Emergency Care for Sepsis. N Engl J Med. 2017;376:2235–2244. doi: 10.1056/NEJMoa1703058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dellinger RP, Carlet JM, Masur H, Gerlach H, Calandra T, Cohen J, Gea-Banacloche J, Keh D, Marshall JC, Parker MM, Ramsay G, Zimmerman JL, Vincent JL, Levy MM. Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Intensive Care Med. 2004;30:536–555. doi: 10.1007/s00134-004-2210-z. [DOI] [PubMed] [Google Scholar]
- 45.Evans L, Rhodes A, Alhazzani W, Antonelli M, Coopersmith CM, French C, Machado FR, Mcintyre L, Ostermann M, Prescott HC, Schorr C, Simpson S, Joost Wiersinga W, Alshamsi F, Angus DC, Arabi Y, Azevedo L, Beale R, Beilman G, Belley-Cote E, Burry L, Cecconi M, Centofanti J, Yataco AC, De Waele J, Dellinger RP, Doi K, Du B, Estenssoro E, Ferrer R, Gomersall C, Hodgson C, Møller MH, Iwashyna T, Jacob S, Kleinpell R, Klompas M, Koh Y, Kumar A, Kwizera A, Lobo S, Masur H, McGloughlin S, Mehta S, Mehta Y, Mer M, Nunnally M, Oczkowski S, Osborn T, Papathanassoglou E, Perner A, Puskarich M, Roberts J, Schweickert W, Seckel M, Sevransky J, Sprung CL, Welte T, Zimmerman J, Levy M. Executive Summary: Surviving Sepsis Campaign: International Guidelines for the Management of Sepsis and Septic Shock 2021. Crit Care Med. 2021;49:1974–1982. doi: 10.1097/CCM.0000000000005357. [DOI] [PubMed] [Google Scholar]
- 46.Messmer AS, Zingg C, Müller M, Gerber JL, Schefold JC, Pfortmueller CA. Fluid Overload and Mortality in Adult Critical Care Patients-A Systematic Review and Meta-Analysis of Observational Studies. Crit Care Med. 2020;48:1862–1870. doi: 10.1097/CCM.0000000000004617. [DOI] [PubMed] [Google Scholar]
- 47.Boyd JH, Forbes J, Nakada TA, Walley KR, Russell JA. Fluid resuscitation in septic shock: a positive fluid balance and elevated central venous pressure are associated with increased mortality. Crit Care Med. 2011;39:259–265. doi: 10.1097/CCM.0b013e3181feeb15. [DOI] [PubMed] [Google Scholar]
- 48.Semler MW, Self WH, Wanderer JP, Ehrenfeld JM, Wang L, Byrne DW, Stollings JL, Kumar AB, Hughes CG, Hernandez A, Guillamondegui OD, May AK, Weavind L, Casey JD, Siew ED, Shaw AD, Bernard GR, Rice TW SMART Investigators and the Pragmatic Critical Care Research Group. Balanced Crystalloids versus Saline in Critically Ill Adults. N Engl J Med. 2018;378:829–839. doi: 10.1056/NEJMoa1711584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hammond DA, Lam SW, Rech MA, Smith MN, Westrick J, Trivedi AP, Balk RA. Balanced Crystalloids Versus Saline in Critically Ill Adults: A Systematic Review and Meta-analysis. Ann Pharmacother. 2020;54:5–13. doi: 10.1177/1060028019866420. [DOI] [PubMed] [Google Scholar]
- 50.Finfer S, Micallef S, Hammond N, Navarra L, Bellomo R, Billot L, Delaney A, Gallagher M, Gattas D, Li Q, Mackle D, Mysore J, Saxena M, Taylor C, Young P, Myburgh J PLUS Study Investigators and the Australian New Zealand Intensive Care Society Clinical Trials Group. Balanced Multielectrolyte Solution versus Saline in Critically Ill Adults. N Engl J Med. 2022;386:815–826. doi: 10.1056/NEJMoa2114464. [DOI] [PubMed] [Google Scholar]
- 51.Zampieri FG, Machado FR, Biondi RS, Freitas FGR, Veiga VC, Figueiredo RC, Lovato WJ, Amêndola CP, Serpa-Neto A, Paranhos JLR, Guedes MAV, Lúcio EA, Oliveira-Júnior LC, Lisboa TC, Lacerda FH, Maia IS, Grion CMC, Assunção MSC, Manoel ALO, Silva-Junior JM, Duarte P, Soares RM, Miranda TA, de Lima LM, Gurgel RM, Paisani DM, Corrêa TD, Azevedo LCP, Kellum JA, Damiani LP, Brandão da Silva N, Cavalcanti AB BaSICS investigators and the BRICNet members. Effect of Intravenous Fluid Treatment With a Balanced Solution vs 0.9% Saline Solution on Mortality in Critically Ill Patients: The BaSICS Randomized Clinical Trial. JAMA. 2021;326:1–12. doi: 10.1001/jama.2021.11684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Finfer S, Bellomo R, Boyce N, French J, Myburgh J, Norton R SAFE Study Investigators. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med. 2004;350:2247–2256. doi: 10.1056/NEJMoa040232. [DOI] [PubMed] [Google Scholar]
- 53.Annane D, Siami S, Jaber S, Martin C, Elatrous S, Declère AD, Preiser JC, Outin H, Troché G, Charpentier C, Trouillet JL, Kimmoun A, Forceville X, Darmon M, Lesur O, Reignier J, Abroug F, Berger P, Clec'h C, Cousson J, Thibault L, Chevret S CRISTAL Investigators. Effects of fluid resuscitation with colloids vs crystalloids on mortality in critically ill patients presenting with hypovolemic shock: the CRISTAL randomized trial. JAMA. 2013;310:1809–1817. doi: 10.1001/jama.2013.280502. [DOI] [PubMed] [Google Scholar]
- 54.Caironi P, Tognoni G, Masson S, Fumagalli R, Pesenti A, Romero M, Fanizza C, Caspani L, Faenza S, Grasselli G, Iapichino G, Antonelli M, Parrini V, Fiore G, Latini R, Gattinoni L ALBIOS Study Investigators. Albumin replacement in patients with severe sepsis or septic shock. N Engl J Med. 2014;370:1412–1421. doi: 10.1056/NEJMoa1305727. [DOI] [PubMed] [Google Scholar]
- 55.Philips CA, Maiwall R, Sharma MK, Jindal A, Choudhury AK, Kumar G, Bhardwaj A, Mitra LG, Agarwal PM, Sarin SK. Comparison of 5% human albumin and normal saline for fluid resuscitation in sepsis induced hypotension among patients with cirrhosis (FRISC study): a randomized controlled trial. Hepatol Int. 2021;15:983–994. doi: 10.1007/s12072-021-10164-z. [DOI] [PubMed] [Google Scholar]
- 56.Maiwall R, Kumar A, Pasupuleti SSR, Hidam AK, Tevethia H, Kumar G, Sahney A, Mitra LG, Sarin SK. A randomized-controlled trial comparing 20% albumin to plasmalyte in patients with cirrhosis and sepsis-induced hypotension [ALPS trial] J Hepatol. 2022;77:670–682. doi: 10.1016/j.jhep.2022.03.043. [DOI] [PubMed] [Google Scholar]
- 57.Wong F, Pappas SC, Curry MP, Reddy KR, Rubin RA, Porayko MK, Gonzalez SA, Mumtaz K, Lim N, Simonetto DA, Sharma P, Sanyal AJ, Mayo MJ, Frederick RT, Escalante S, Jamil K CONFIRM Study Investigators. Terlipressin plus Albumin for the Treatment of Type 1 Hepatorenal Syndrome. N Engl J Med. 2021;384:818–828. doi: 10.1056/NEJMoa2008290. [DOI] [PubMed] [Google Scholar]
- 58.Meyhoff TS, Hjortrup PB, Wetterslev J, Sivapalan P, Laake JH, Cronhjort M, Jakob SM, Cecconi M, Nalos M, Ostermann M, Malbrain M, Pettilä V, Møller MH, Kjær MN, Lange T, Overgaard-Steensen C, Brand BA, Winther-Olesen M, White JO, Quist L, Westergaard B, Jonsson AB, Hjortsø CJS, Meier N, Jensen TS, Engstrøm J, Nebrich L, Andersen-Ranberg NC, Jensen JV, Joseph NA, Poulsen LM, Herløv LS, Sølling CG, Pedersen SK, Knudsen KK, Straarup TS, Vang ML, Bundgaard H, Rasmussen BS, Aagaard SR, Hildebrandt T, Russell L, Bestle MH, Schønemann-Lund M, Brøchner AC, Elvander CF, Hoffmann SKL, Rasmussen ML, Martin YK, Friberg FF, Seter H, Aslam TN, Ådnøy S, Seidel P, Strand K, Johnstad B, Joelsson-Alm E, Christensen J, Ahlstedt C, Pfortmueller CA, Siegemund M, Greco M, Raděj J, Kříž M, Gould DW, Rowan KM, Mouncey PR, Perner A CLASSIC Trial Group. Restriction of Intravenous Fluid in ICU Patients with Septic Shock. N Engl J Med. 2022;386:2459–2470. doi: 10.1056/NEJMoa2202707. [DOI] [PubMed] [Google Scholar]
- 59.Self WH, Semler MW, Bellomo R, Brown SM, deBoisblanc BP, Exline MC, Ginde AA, Grissom CK, Janz DR, Jones AE, Liu KD, Macdonald SPJ, Miller CD, Park PK, Reineck LA, Rice TW, Steingrub JS, Talmor D, Yealy DM, Douglas IS, Shapiro NI CLOVERS Protocol Committee and NHLBI Prevention and Early Treatment of Acute Lung Injury (PETAL) Network Investigators. Liberal Versus Restrictive Intravenous Fluid Therapy for Early Septic Shock: Rationale for a Randomized Trial. Ann Emerg Med. 2018;72:457–466. doi: 10.1016/j.annemergmed.2018.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vincent JL, Singer M, Einav S, Moreno R, Wendon J, Teboul JL, Bakker J, Hernandez G, Annane D, de Man AME, Monnet X, Ranieri VM, Hamzaoui O, Takala J, Juffermans N, Chiche JD, Myatra SN, De Backer D. Equilibrating SSC guidelines with individualized care. Crit Care. 2021;25:397. doi: 10.1186/s13054-021-03813-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Permpikul C, Tongyoo S, Viarasilpa T, Trainarongsakul T, Chakorn T, Udompanturak S. Early Use of Norepinephrine in Septic Shock Resuscitation (CENSER). A Randomized Trial. Am J Respir Crit Care Med. 2019;199:1097–1105. doi: 10.1164/rccm.201806-1034OC. [DOI] [PubMed] [Google Scholar]
- 62.Beck V, Chateau D, Bryson GL, Pisipati A, Zanotti S, Parrillo JE, Kumar A Cooperative Antimicrobial Therapy of Septic Shock (CATSS) Database Research Group. Timing of vasopressor initiation and mortality in septic shock: a cohort study. Crit Care. 2014;18:R97. doi: 10.1186/cc13868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bai X, Yu W, Ji W, Lin Z, Tan S, Duan K, Dong Y, Xu L, Li N. Early versus delayed administration of norepinephrine in patients with septic shock. Crit Care. 2014;18:532. doi: 10.1186/s13054-014-0532-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Landry GJ, Mostul CJ, Ahn DS, McLafferty BJ, Liem TK, Mitchell EL, Jung E, Abraham CZ, Azarbal AF, McLafferty RB, Moneta GL. Causes and outcomes of finger ischemia in hospitalized patients in the intensive care unit. J Vasc Surg. 2018;68:1499–1504. doi: 10.1016/j.jvs.2018.01.050. [DOI] [PubMed] [Google Scholar]
- 65.Martin C, Medam S, Antonini F, Alingrin J, Haddam M, Hammad E, Meyssignac B, Vigne C, Zieleskiewicz L, Leone M. NOREPINEPHRINE: NOT TOO MUCH, TOO LONG. Shock. 2015;44:305–309. doi: 10.1097/SHK.0000000000000426. [DOI] [PubMed] [Google Scholar]
- 66.McIntyre WF, Um KJ, Alhazzani W, Lengyel AP, Hajjar L, Gordon AC, Lamontagne F, Healey JS, Whitlock RP, Belley-Côté EP. Association of Vasopressin Plus Catecholamine Vasopressors vs Catecholamines Alone With Atrial Fibrillation in Patients With Distributive Shock: A Systematic Review and Meta-analysis. JAMA. 2018;319:1889–1900. doi: 10.1001/jama.2018.4528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sanyal AJ, Boyer T, Garcia-Tsao G, Regenstein F, Rossaro L, Appenrodt B, Blei A, Gülberg V, Sigal S, Teuber P Terlipressin Study Group. A randomized, prospective, double-blind, placebo-controlled trial of terlipressin for type 1 hepatorenal syndrome. Gastroenterology. 2008;134:1360–1368. doi: 10.1053/j.gastro.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sanyal AJ, Boyer TD, Frederick RT, Wong F, Rossaro L, Araya V, Vargas HE, Reddy KR, Pappas SC, Teuber P, Escalante S, Jamil K. Reversal of hepatorenal syndrome type 1 with terlipressin plus albumin vs. placebo plus albumin in a pooled analysis of the OT-0401 and REVERSE randomised clinical studies. Aliment Pharmacol Ther. 2017;45:1390–1402. doi: 10.1111/apt.14052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Best LM, Freeman SC, Sutton AJ, Cooper NJ, Tng EL, Csenar M, Hawkins N, Pavlov CS, Davidson BR, Thorburn D, Cowlin M, Milne EJ, Tsochatzis E, Gurusamy KS. Treatment for hepatorenal syndrome in people with decompensated liver cirrhosis: a network meta-analysis. Cochrane Database Syst Rev. 2019;9:CD013103. doi: 10.1002/14651858.CD013103.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pitre T, Kiflen M, Helmeczi W, Dionne JC, Rewa O, Bagshaw SM, Needham-Nethercott N, Alhazzani W, Zeraatkar D, Rochwerg B. The Comparative Effectiveness of Vasoactive Treatments for Hepatorenal Syndrome: A Systematic Review and Network Meta-Analysis. Crit Care Med. 2022;50:1419–1429. doi: 10.1097/CCM.0000000000005595. [DOI] [PubMed] [Google Scholar]
- 71.Choudhury A, Kedarisetty CK, Vashishtha C, Saini D, Kumar S, Maiwall R, Sharma MK, Bhadoria AS, Kumar G, Joshi YK, Sarin SK. A randomized trial comparing terlipressin and noradrenaline in patients with cirrhosis and septic shock. Liver Int. 2017;37:552–561. doi: 10.1111/liv.13252. [DOI] [PubMed] [Google Scholar]
- 72.Liu ZM, Chen J, Kou Q, Lin Q, Huang X, Tang Z, Kang Y, Li K, Zhou L, Song Q, Sun T, Zhao L, Wang X, He X, Wang C, Wu B, Lin J, Yuan S, Gu Q, Qian K, Shi X, Feng Y, Lin A Study Group of investigators, Guan XD. Terlipressin versus norepinephrine as infusion in patients with septic shock: a multicentre, randomised, double-blinded trial. Intensive Care Med. 2018;44:1816–1825. doi: 10.1007/s00134-018-5267-9. [DOI] [PubMed] [Google Scholar]
- 73.Russell JA, Walley KR, Singer J, Gordon AC, Hébert PC, Cooper DJ, Holmes CL, Mehta S, Granton JT, Storms MM, Cook DJ, Presneill JJ, Ayers D VASST Investigators. Vasopressin versus norepinephrine infusion in patients with septic shock. N Engl J Med. 2008;358:877–887. doi: 10.1056/NEJMoa067373. [DOI] [PubMed] [Google Scholar]
- 74.Gordon AC, Mason AJ, Thirunavukkarasu N, Perkins GD, Cecconi M, Cepkova M, Pogson DG, Aya HD, Anjum A, Frazier GJ, Santhakumaran S, Ashby D, Brett SJ VANISH Investigators. Effect of Early Vasopressin vs Norepinephrine on Kidney Failure in Patients With Septic Shock: The VANISH Randomized Clinical Trial. JAMA. 2016;316:509–518. doi: 10.1001/jama.2016.10485. [DOI] [PubMed] [Google Scholar]
- 75.Wieruszewski PM, Khanna AK. Vasopressor Choice and Timing in Vasodilatory Shock. Crit Care. 2022;26:76. doi: 10.1186/s13054-022-03911-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Svoboda P, Scheer P, Kantorová I, Doubek J, Dudra J, Radvan M, Radvanova J. Terlipressin in the treatment of late phase catecholamine-resistant septic shock. Hepatogastroenterology. 2012;59:1043–1047. doi: 10.5754/hge10550. [DOI] [PubMed] [Google Scholar]
- 77.Wang J, Shi M, Huang L, Li Q, Meng S, Xu J, Xue M, Xie J, Liu S, Huang Y. Addition of terlipressin to norepinephrine in septic shock and effect of renal perfusion: a pilot study. Ren Fail. 2022;44:1207–1215. doi: 10.1080/0886022X.2022.2095286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sahoo P, Kothari N, Goyal S, Sharma A, Bhatia PK. Comparison of Norepinephrine and Terlipressin vs Norepinephrine Alone for Management of Septic Shock: A Randomized Control Study. Indian J Crit Care Med. 2022;26:669–675. doi: 10.5005/jp-journals-10071-24231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Khanna A, English SW, Wang XS, Ham K, Tumlin J, Szerlip H, Busse LW, Altaweel L, Albertson TE, Mackey C, McCurdy MT, Boldt DW, Chock S, Young PJ, Krell K, Wunderink RG, Ostermann M, Murugan R, Gong MN, Panwar R, Hästbacka J, Favory R, Venkatesh B, Thompson BT, Bellomo R, Jensen J, Kroll S, Chawla LS, Tidmarsh GF, Deane AM ATHOS-3 Investigators. Angiotensin II for the Treatment of Vasodilatory Shock. N Engl J Med. 2017;377:419–430. doi: 10.1056/NEJMoa1704154. [DOI] [PubMed] [Google Scholar]
- 80.Pinsky MR, Cecconi M, Chew MS, De Backer D, Douglas I, Edwards M, Hamzaoui O, Hernandez G, Martin G, Monnet X, Saugel B, Scheeren TWL, Teboul JL, Vincent JL. Effective hemodynamic monitoring. Crit Care. 2022;26:294. doi: 10.1186/s13054-022-04173-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, Peterson E, Tomlanovich M Early Goal-Directed Therapy Collaborative Group. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 82.Mouncey PR, Osborn TM, Power GS, Harrison DA, Sadique MZ, Grieve RD, Jahan R, Harvey SE, Bell D, Bion JF, Coats TJ, Singer M, Young JD, Rowan KM ProMISe Trial Investigators. Trial of early, goal-directed resuscitation for septic shock. N Engl J Med. 2015;372:1301–1311. doi: 10.1056/NEJMoa1500896. [DOI] [PubMed] [Google Scholar]
- 83.ARISE Investigators ANZICS Clinical Trials Group, Peake SL, Delaney A, Bailey M, Bellomo R, Cameron PA, Cooper DJ, Higgins AM, Holdgate A, Howe BD, Webb SA, Williams P. Goal-directed resuscitation for patients with early septic shock. N Engl J Med. 2014;371:1496–1506. doi: 10.1056/NEJMoa1404380. [DOI] [PubMed] [Google Scholar]
- 84.ProCESS Investigators, Yealy DM, Kellum JA, Huang DT, Barnato AE, Weissfeld LA, Pike F, Terndrup T, Wang HE, Hou PC, LoVecchio F, Filbin MR, Shapiro NI, Angus DC. A randomized trial of protocol-based care for early septic shock. N Engl J Med. 2014;370:1683–1693. doi: 10.1056/NEJMoa1401602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.PRISM Investigators, Rowan KM, Angus DC, Bailey M, Barnato AE, Bellomo R, Canter RR, Coats TJ, Delaney A, Gimbel E, Grieve RD, Harrison DA, Higgins AM, Howe B, Huang DT, Kellum JA, Mouncey PR, Music E, Peake SL, Pike F, Reade MC, Sadique MZ, Singer M, Yealy DM. Early, Goal-Directed Therapy for Septic Shock - A Patient-Level Meta-Analysis. N Engl J Med. 2017;376:2223–2234. doi: 10.1056/NEJMoa1701380. [DOI] [PubMed] [Google Scholar]
- 86.Gershengorn HB, Stelfox HT, Niven DJ, Wunsch H. Association of Premorbid Blood Pressure with Vasopressor Infusion Duration in Patients with Shock. Am J Respir Crit Care Med. 2020;202:91–99. doi: 10.1164/rccm.201908-1681OC. [DOI] [PubMed] [Google Scholar]
- 87.Lamontagne F, Richards-Belle A, Thomas K, Harrison DA, Sadique MZ, Grieve RD, Camsooksai J, Darnell R, Gordon AC, Henry D, Hudson N, Mason AJ, Saull M, Whitman C, Young JD, Rowan KM, Mouncey PR 65 trial investigators. Effect of Reduced Exposure to Vasopressors on 90-Day Mortality in Older Critically Ill Patients With Vasodilatory Hypotension: A Randomized Clinical Trial. JAMA. 2020;323:938–949. doi: 10.1001/jama.2020.0930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nguyen HB, Rivers EP, Knoblich BP, Jacobsen G, Muzzin A, Ressler JA, Tomlanovich MC. Early lactate clearance is associated with improved outcome in severe sepsis and septic shock. Crit Care Med. 2004;32:1637–1642. doi: 10.1097/01.ccm.0000132904.35713.a7. [DOI] [PubMed] [Google Scholar]
- 89.Jones AE, Shapiro NI, Trzeciak S, Arnold RC, Claremont HA, Kline JA Emergency Medicine Shock Research Network (EMShockNet) Investigators. Lactate clearance vs central venous oxygen saturation as goals of early sepsis therapy: a randomized clinical trial. JAMA. 2010;303:739–746. doi: 10.1001/jama.2010.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lee SG, Song J, Park DW, Moon S, Cho HJ, Kim JY, Park J, Cha JH. Prognostic value of lactate levels and lactate clearance in sepsis and septic shock with initial hyperlactatemia: A retrospective cohort study according to the Sepsis-3 definitions. Medicine (Baltimore) 2021;100:e24835. doi: 10.1097/MD.0000000000024835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jansen TC, van Bommel J, Schoonderbeek FJ, Sleeswijk Visser SJ, van der Klooster JM, Lima AP, Willemsen SP, Bakker J LACTATE study group. Early lactate-guided therapy in intensive care unit patients: a multicenter, open-label, randomized controlled trial. Am J Respir Crit Care Med. 2010;182:752–761. doi: 10.1164/rccm.200912-1918OC. [DOI] [PubMed] [Google Scholar]
- 92.Bakker J, de Backer D, Hernandez G. Lactate-guided resuscitation saves lives: we are not sure. Intensive Care Med. 2016;42:472–474. doi: 10.1007/s00134-016-4220-z. [DOI] [PubMed] [Google Scholar]
- 93.Hernandez G, Bellomo R, Bakker J. The ten pitfalls of lactate clearance in sepsis. Intensive Care Med. 2019;45:82–85. doi: 10.1007/s00134-018-5213-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hernández G, Ospina-Tascón GA, Damiani LP, Estenssoro E, Dubin A, Hurtado J, Friedman G, Castro R, Alegría L, Teboul JL, Cecconi M, Ferri G, Jibaja M, Pairumani R, Fernández P, Barahona D, Granda-Luna V, Cavalcanti AB, Bakker J The ANDROMEDA SHOCK Investigators and the Latin America Intensive Care Network (LIVEN), Hernández G, Ospina-Tascón G, Petri Damiani L, Estenssoro E, Dubin A, Hurtado J, Friedman G, Castro R, Alegría L, Teboul JL, Cecconi M, Cecconi M, Ferri G, Jibaja M, Pairumani R, Fernández P, Barahona D, Cavalcanti AB, Bakker J, Hernández G, Alegría L, Ferri G, Rodriguez N, Holger P, Soto N, Pozo M, Bakker J, Cook D, Vincent JL, Rhodes A, Kavanagh BP, Dellinger P, Rietdijk W, Carpio D, Pavéz N, Henriquez E, Bravo S, Valenzuela ED, Vera M, Dreyse J, Oviedo V, Cid MA, Larroulet M, Petruska E, Sarabia C, Gallardo D, Sanchez JE, González H, Arancibia JM, Muñoz A, Ramirez G, Aravena F, Aquevedo A, Zambrano F, Bozinovic M, Valle F, Ramirez M, Rossel V, Muñoz P, Ceballos C, Esveile C, Carmona C, Candia E, Mendoza D, Sanchez A, Ponce D, Ponce D, Lastra J, Nahuelpán B, Fasce F, Luengo C, Medel N, Cortés C, Campassi L, Rubatto P, Horna N, Furche M, Pendino JC, Bettini L, Lovesio C, González MC, Rodruguez J, Canales H, Caminos F, Galletti C, Minoldo E, Aramburu MJ, Olmos D, Nin N, Tenzi J, Quiroga C, Lacuesta P, Gaudín A, Pais R, Silvestre A, Olivera G, Rieppi G, Berrutti D, Ochoa M, Cobos P, Vintimilla F, Ramirez V, Tobar M, García F, Picoita F, Remache N, Granda V, Paredes F, Barzallo E, Garcés P, Guerrero F, Salazar S, Torres G, Tana C, Calahorrano J, Solis F, Torres P, Herrera L, Ornes A, Peréz V, Delgado G, López A, Espinosa E, Moreira J, Salcedo B, Villacres I, Suing J, Lopez M, Gomez L, Toctaquiza G, Cadena Zapata M, Orazabal MA, Pardo Espejo R, Jimenez J, Calderón A, Paredes G, Barberán JL, Moya T, Atehortua H, Sabogal R, Ortiz G, Lara A, Sanchez F, Hernán Portilla A, Dávila H, Mora JA, Calderón LE, Alvarez I, Escobar E, Bejarano A, Bustamante LA, Aldana JL. Effect of a Resuscitation Strategy Targeting Peripheral Perfusion Status vs Serum Lactate Levels on 28-Day Mortality Among Patients With Septic Shock: The ANDROMEDA-SHOCK Randomized Clinical Trial. JAMA. 2019;321:654–664. doi: 10.1001/jama.2019.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zampieri FG, Damiani LP, Bakker J, Ospina-Tascón GA, Castro R, Cavalcanti AB, Hernandez G. Effects of a Resuscitation Strategy Targeting Peripheral Perfusion Status versus Serum Lactate Levels among Patients with Septic Shock. A Bayesian Reanalysis of the ANDROMEDA-SHOCK Trial. Am J Respir Crit Care Med. 2020;201:423–429. doi: 10.1164/rccm.201905-0968OC. [DOI] [PubMed] [Google Scholar]
- 96.Kattan E, Ospina-Tascón GA, Teboul JL, Castro R, Cecconi M, Ferri G, Bakker J, Hernández G ANDROMEDA-SHOCK Investigators. Systematic assessment of fluid responsiveness during early septic shock resuscitation: secondary analysis of the ANDROMEDA-SHOCK trial. Crit Care. 2020;24:23. doi: 10.1186/s13054-020-2732-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.De Backer D, Aissaoui N, Cecconi M, Chew MS, Denault A, Hajjar L, Hernandez G, Messina A, Myatra SN, Ostermann M, Pinsky MR, Teboul JL, Vignon P, Vincent JL, Monnet X. How can assessing hemodynamics help to assess volume status? Intensive Care Med. 2022;48:1482–1494. doi: 10.1007/s00134-022-06808-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Perez Nieto OR, Wong A, Lopez Fermin J, Zamarron Lopez EI, Meade Aguilar JA, Deloya Tomas E, Carrion Moya JD, Castillo Gutierrez G, G Olvera Ramos M, García Montes X, Alberto Guerrero Gutiérrez M, George Aguilar F, Salvador Sánchez Díaz J, Soriano Orozco R, Ríos Argaiz E, Hernandez-Gilsoul T, Secchi Del Rio R, Ñamendys-Silva SA, L N G Malbrain M. Aiming for zero fluid accumulation: First, do no harm. Anaesthesiol Intensive Ther. 2021;53:162–178. doi: 10.5114/ait.2021.105252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chen C, Kollef MH. Targeted Fluid Minimization Following Initial Resuscitation in Septic Shock: A Pilot Study. Chest. 2015;148:1462–1469. doi: 10.1378/chest.15-1525. [DOI] [PubMed] [Google Scholar]
- 100.Douglas IS, Alapat PM, Corl KA, Exline MC, Forni LG, Holder AL, Kaufman DA, Khan A, Levy MM, Martin GS, Sahatjian JA, Seeley E, Self WH, Weingarten JA, Williams M, Hansell DM. Fluid Response Evaluation in Sepsis Hypotension and Shock: A Randomized Clinical Trial. Chest. 2020;158:1431–1445. doi: 10.1016/j.chest.2020.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Azadian M, Win S, Abdipour A, Kim CK, Nguyen HB. Mortality Benefit From the Passive Leg Raise Maneuver in Guiding Resuscitation of Septic Shock Patients: A Systematic Review and Meta-Analysis of Randomized Trials. J Intensive Care Med. 2022;37:611–617. doi: 10.1177/08850666211019713. [DOI] [PubMed] [Google Scholar]
- 102.Im Y, Kang D, Ko RE, Lee YJ, Lim SY, Park S, Na SJ, Chung CR, Park MH, Oh DK, Lim CM, Suh GY Korean Sepsis Alliance (KSA) investigators. Time-to-antibiotics and clinical outcomes in patients with sepsis and septic shock: a prospective nationwide multicenter cohort study. Crit Care. 2022;26:19. doi: 10.1186/s13054-021-03883-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Liu VX, Fielding-Singh V, Greene JD, Baker JM, Iwashyna TJ, Bhattacharya J, Escobar GJ. The Timing of Early Antibiotics and Hospital Mortality in Sepsis. Am J Respir Crit Care Med. 2017;196:856–863. doi: 10.1164/rccm.201609-1848OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Garnacho-Montero J, Gutiérrez-Pizarraya A, Escoresca-Ortega A, Fernández-Delgado E, López-Sánchez JM. Adequate antibiotic therapy prior to ICU admission in patients with severe sepsis and septic shock reduces hospital mortality. Crit Care. 2015;19:302. doi: 10.1186/s13054-015-1000-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Suberviola Cañas B, Jáuregui R, Ballesteros MÁ, Leizaola O, González-Castro A, Castellanos-Ortega Á. Effects of antibiotic administration delay and inadequacy upon the survival of septic shock patients. Med Intensiva. 2015;39:459–466. doi: 10.1016/j.medin.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 106.The Lancet Gastroenterology Hepatology. The problem of antimicrobial resistance in chronic liver disease. Lancet Gastroenterol Hepatol. 2022;7:495. doi: 10.1016/S2468-1253(22)00130-3. [DOI] [PubMed] [Google Scholar]
- 107.Salerno F, Borzio M, Pedicino C, Simonetti R, Rossini A, Boccia S, Cacciola I, Burroughs AK, Manini MA, La Mura V, Angeli P, Bernardi M, Dalla Gasperina D, Dionigi E, Dibenedetto C, Arghittu M AISF Investigators. The impact of infection by multidrug-resistant agents in patients with cirrhosis. A multicenter prospective study. Liver Int. 2017;37:71–79. doi: 10.1111/liv.13195. [DOI] [PubMed] [Google Scholar]
- 108.Fernández J, Prado V, Trebicka J, Amoros A, Gustot T, Wiest R, Deulofeu C, Garcia E, Acevedo J, Fuhrmann V, Durand F, Sánchez C, Papp M, Caraceni P, Vargas V, Bañares R, Piano S, Janicko M, Albillos A, Alessandria C, Soriano G, Welzel TM, Laleman W, Gerbes A, De Gottardi A, Merli M, Coenraad M, Saliba F, Pavesi M, Jalan R, Ginès P, Angeli P, Arroyo V European Foundation for the Study of Chronic Liver Failure (EF-Clif) Multidrug-resistant bacterial infections in patients with decompensated cirrhosis and with acute-on-chronic liver failure in Europe. J Hepatol. 2019;70:398–411. doi: 10.1016/j.jhep.2018.10.027. [DOI] [PubMed] [Google Scholar]
- 109.Piano S, Singh V, Caraceni P, Maiwall R, Alessandria C, Fernandez J, Soares EC, Kim DJ, Kim SE, Marino M, Vorobioff J, Barea RCR, Merli M, Elkrief L, Vargas V, Krag A, Singh SP, Lesmana LA, Toledo C, Marciano S, Verhelst X, Wong F, Intagliata N, Rabinowich L, Colombato L, Kim SG, Gerbes A, Durand F, Roblero JP, Bhamidimarri KR, Boyer TD, Maevskaya M, Fassio E, Kim HS, Hwang JS, Gines P, Gadano A, Sarin SK, Angeli P International Club of Ascites Global Study Group. Epidemiology and Effects of Bacterial Infections in Patients With Cirrhosis Worldwide. Gastroenterology. 2019;156:1368–1380.e10. doi: 10.1053/j.gastro.2018.12.005. [DOI] [PubMed] [Google Scholar]
- 110.Gupta S, Sakhuja A, Kumar G, McGrath E, Nanchal RS, Kashani KB. Culture-Negative Severe Sepsis: Nationwide Trends and Outcomes. Chest. 2016;150:1251–1259. doi: 10.1016/j.chest.2016.08.1460. [DOI] [PubMed] [Google Scholar]
- 111.Vincent JL, Brealey D, Libert N, Abidi NE, O'Dwyer M, Zacharowski K, Mikaszewska-Sokolewicz M, Schrenzel J, Simon F, Wilks M, Picard-Maureau M, Chalfin DB, Ecker DJ, Sampath R, Singer M Rapid Diagnosis of Infections in the Critically Ill Team. Rapid Diagnosis of Infection in the Critically Ill, a Multicenter Study of Molecular Detection in Bloodstream Infections, Pneumonia, and Sterile Site Infections. Crit Care Med. 2015;43:2283–2291. doi: 10.1097/CCM.0000000000001249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.D'Onofrio V, Salimans L, Bedenić B, Cartuyvels R, Barišić I, Gyssens IC. The Clinical Impact of Rapid Molecular Microbiological Diagnostics for Pathogen and Resistance Gene Identification in Patients With Sepsis: A Systematic Review. Open Forum Infect Dis. 2020;7:ofaa352. doi: 10.1093/ofid/ofaa352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Timbrook TT, Morton JB, McConeghy KW, Caffrey AR, Mylonakis E, LaPlante KL. The Effect of Molecular Rapid Diagnostic Testing on Clinical Outcomes in Bloodstream Infections: A Systematic Review and Meta-analysis. Clin Infect Dis. 2017;64:15–23. doi: 10.1093/cid/ciw649. [DOI] [PubMed] [Google Scholar]
- 114.Fernández J, Piano S, Bartoletti M, Wey EQ. Management of bacterial and fungal infections in cirrhosis: The MDRO challenge. J Hepatol. 2021;75 Suppl 1:S101–S117. doi: 10.1016/j.jhep.2020.11.010. [DOI] [PubMed] [Google Scholar]
- 115.Bartoletti M, Rinaldi M, Pasquini Z, Scudeller L, Piano S, Giacobbe DR, Maraolo AE, Bussini L, Del Puente F, Incicco S, Angeli P, Giannella M, Baldassarre M, Caraceni P, Campoli C, Morelli MC, Cricca M, Ambretti S, Gentile I, Bassetti M, Viale P. Risk factors for candidaemia in hospitalized patients with liver cirrhosis: a multicentre case-control-control study. Clin Microbiol Infect. 2021;27:276–282. doi: 10.1016/j.cmi.2020.04.030. [DOI] [PubMed] [Google Scholar]
- 116.Kowalska-Krochmal B, Dudek-Wicher R. The Minimum Inhibitory Concentration of Antibiotics: Methods, Interpretation, Clinical Relevance. Pathogens. 2021;10 doi: 10.3390/pathogens10020165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zoratti C, Moretti R, Rebuzzi L, Albergati IV, Di Somma A, Decorti G, Di Bella S, Crocè LS, Giuffrè M. Antibiotics and Liver Cirrhosis: What the Physicians Need to Know. Antibiotics (Basel) 2021;11 doi: 10.3390/antibiotics11010031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zusman O, Farbman L, Tredler Z, Daitch V, Lador A, Leibovici L, Paul M. Association between hypoalbuminemia and mortality among subjects treated with ertapenem versus other carbapenems: prospective cohort study. Clin Microbiol Infect. 2015;21:54–58. doi: 10.1016/j.cmi.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 119.Bergman SJ, Speil C, Short M, Koirala J. Pharmacokinetic and pharmacodynamic aspects of antibiotic use in high-risk populations. Infect Dis Clin North Am. 2007;21:821–846, x. doi: 10.1016/j.idc.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 120.Dalmau D, Layrargues GP, Fenyves D, Willems B, Turgeon F, Turgeon P. Cefotaxime, desacetyl-cefotaxime, and bactericidal activity in spontaneous bacterial peritonitis. J Infect Dis. 1999;180:1597–1602. doi: 10.1086/315053. [DOI] [PubMed] [Google Scholar]
- 121.Terg R, Cobas S, Fassio E, Landeira G, Ríos B, Vasen W, Abecasis R, Ríos H, Guevara M. Oral ciprofloxacin after a short course of intravenous ciprofloxacin in the treatment of spontaneous bacterial peritonitis: results of a multicenter, randomized study. J Hepatol. 2000;33:564–569. doi: 10.1034/j.1600-0641.2000.033004564.x. [DOI] [PubMed] [Google Scholar]
- 122.Griemsmann M, Grote-Koska D, Cornberg M, Schmidt JJ, Maasoumy B CirPK - Study Group. Plasma and ascites pharmacokinetics of meropenem in patients with decompensated cirrhosis and spontaneous bacterial peritonitis. J Hepatol. 2022;76:230–233. doi: 10.1016/j.jhep.2021.07.015. [DOI] [PubMed] [Google Scholar]
- 123.Westphal JF, Brogard JM. Clinical pharmacokinetics of newer antibacterial agents in liver disease. Clin Pharmacokinet. 1993;24:46–58. doi: 10.2165/00003088-199324010-00004. [DOI] [PubMed] [Google Scholar]
- 124.Scheetz MH, Reddy P, Nicolau DP, Noskin GA, Postelnick MJ, Stosor V, Zembower TR. Peritoneal fluid penetration of tigecycline. Ann Pharmacother. 2006;40:2064–2067. doi: 10.1345/aph.1H229. [DOI] [PubMed] [Google Scholar]
- 125.Abdul-Aziz MH, Sulaiman H, Mat-Nor MB, Rai V, Wong KK, Hasan MS, Abd Rahman AN, Jamal JA, Wallis SC, Lipman J, Staatz CE, Roberts JA. Beta-Lactam Infusion in Severe Sepsis (BLISS): a prospective, two-centre, open-labelled randomised controlled trial of continuous versus intermittent beta-lactam infusion in critically ill patients with severe sepsis. Intensive Care Med. 2016;42:1535–1545. doi: 10.1007/s00134-015-4188-0. [DOI] [PubMed] [Google Scholar]
- 126.Dulhunty JM, Roberts JA, Davis JS, Webb SA, Bellomo R, Gomersall C, Shirwadkar C, Eastwood GM, Myburgh J, Paterson DL, Lipman J. Continuous infusion of beta-lactam antibiotics in severe sepsis: a multicenter double-blind, randomized controlled trial. Clin Infect Dis. 2013;56:236–244. doi: 10.1093/cid/cis856. [DOI] [PubMed] [Google Scholar]
- 127.Roberts JA, Abdul-Aziz MH, Davis JS, Dulhunty JM, Cotta MO, Myburgh J, Bellomo R, Lipman J. Continuous versus Intermittent β-Lactam Infusion in Severe Sepsis. A Meta-analysis of Individual Patient Data from Randomized Trials. Am J Respir Crit Care Med. 2016;194:681–691. doi: 10.1164/rccm.201601-0024OC. [DOI] [PubMed] [Google Scholar]
- 128.Bartoletti M, Giannella M, Lewis RE, Caraceni P, Tedeschi S, Paul M, Schramm C, Bruns T, Merli M, Cobos-Trigueros N, Seminari E, Retamar P, Muñoz P, Tumbarello M, Burra P, Torrani Cerenzia M, Barsic B, Calbo E, Maraolo AE, Petrosillo N, Galan-Ladero MA, D'Offizi G, Zak-Doron Y, Rodriguez-Baño J, Baldassarre M, Verucchi G, Domenicali M, Bernardi M, Viale P ESGBIS/BICHROME study group. Extended Infusion of β-Lactams for Bloodstream Infection in Patients With Liver Cirrhosis: An Observational Multicenter Study. Clin Infect Dis. 2019;69:1731–1739. doi: 10.1093/cid/ciz032. [DOI] [PubMed] [Google Scholar]
- 129.Goodwin JE, Feng Y, Velazquez H, Sessa WC. Endothelial glucocorticoid receptor is required for protection against sepsis. Proc Natl Acad Sci U S A. 2013;110:306–311. doi: 10.1073/pnas.1210200110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Annane D, Bellissant E, Bollaert PE, Briegel J, Keh D, Kupfer Y, Pirracchio R, Rochwerg B. Corticosteroids for treating sepsis in children and adults. Cochrane Database Syst Rev. 2019;12:CD002243. doi: 10.1002/14651858.CD002243.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Annane D, Sébille V, Charpentier C, Bollaert PE, François B, Korach JM, Capellier G, Cohen Y, Azoulay E, Troché G, Chaumet-Riffaud P, Bellissant E. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA. 2002;288:862–871. doi: 10.1001/jama.288.7.862. [DOI] [PubMed] [Google Scholar]
- 132.Keh D, Trips E, Marx G, Wirtz SP, Abduljawwad E, Bercker S, Bogatsch H, Briegel J, Engel C, Gerlach H, Goldmann A, Kuhn SO, Hüter L, Meier-Hellmann A, Nierhaus A, Kluge S, Lehmke J, Loeffler M, Oppert M, Resener K, Schädler D, Schuerholz T, Simon P, Weiler N, Weyland A, Reinhart K, Brunkhorst FM SepNet–Critical Care Trials Group. Effect of Hydrocortisone on Development of Shock Among Patients With Severe Sepsis: The HYPRESS Randomized Clinical Trial. JAMA. 2016;316:1775–1785. doi: 10.1001/jama.2016.14799. [DOI] [PubMed] [Google Scholar]
- 133.Sprung CL, Annane D, Keh D, Moreno R, Singer M, Freivogel K, Weiss YG, Benbenishty J, Kalenka A, Forst H, Laterre PF, Reinhart K, Cuthbertson BH, Payen D, Briegel J CORTICUS Study Group. Hydrocortisone therapy for patients with septic shock. N Engl J Med. 2008;358:111–124. doi: 10.1056/NEJMoa071366. [DOI] [PubMed] [Google Scholar]
- 134.Volbeda M, Wetterslev J, Gluud C, Zijlstra JG, van der Horst IC, Keus F. Glucocorticosteroids for sepsis: systematic review with meta-analysis and trial sequential analysis. Intensive Care Med. 2015;41:1220–1234. doi: 10.1007/s00134-015-3899-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Venkatesh B, Finfer S, Cohen J, Rajbhandari D, Arabi Y, Bellomo R, Billot L, Correa M, Glass P, Harward M, Joyce C, Li Q, McArthur C, Perner A, Rhodes A, Thompson K, Webb S, Myburgh J ADRENAL Trial Investigators and the Australian–New Zealand Intensive Care Society Clinical Trials Group. Adjunctive Glucocorticoid Therapy in Patients with Septic Shock. N Engl J Med. 2018;378:797–808. doi: 10.1056/NEJMoa1705835. [DOI] [PubMed] [Google Scholar]
- 136.Annane D, Renault A, Brun-Buisson C, Megarbane B, Quenot JP, Siami S, Cariou A, Forceville X, Schwebel C, Martin C, Timsit JF, Misset B, Ali Benali M, Colin G, Souweine B, Asehnoune K, Mercier E, Chimot L, Charpentier C, François B, Boulain T, Petitpas F, Constantin JM, Dhonneur G, Baudin F, Combes A, Bohé J, Loriferne JF, Amathieu R, Cook F, Slama M, Leroy O, Capellier G, Dargent A, Hissem T, Maxime V, Bellissant E CRICS-TRIGGERSEP Network. Hydrocortisone plus Fludrocortisone for Adults with Septic Shock. N Engl J Med. 2018;378:809–818. doi: 10.1056/NEJMoa1705716. [DOI] [PubMed] [Google Scholar]
- 137.Annane D, Pirracchio R, Billot L, Waschka A, Chevret S, Cohen J, Finfer S, Gordon A, Hammond N, Myburgh J, Venkatesh B, Delaney A ULYSSES IPDMA Collaborators. Effects of low-dose hydrocortisone and hydrocortisone plus fludrocortisone in adults with septic shock: a protocol for a systematic review and meta-analysis of individual participant data. BMJ Open. 2020;10:e040931. doi: 10.1136/bmjopen-2020-040931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Pirracchio R, Hubbard A, Sprung CL, Chevret S, Annane D Rapid Recognition of Corticosteroid Resistant or Sensitive Sepsis (RECORDS) Collaborators. Assessment of Machine Learning to Estimate the Individual Treatment Effect of Corticosteroids in Septic Shock. JAMA Netw Open. 2020;3:e2029050. doi: 10.1001/jamanetworkopen.2020.29050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Marik PE, Gayowski T, Starzl TE Hepatic Cortisol Research and Adrenal Pathophysiology Study Group. The hepatoadrenal syndrome: a common yet unrecognized clinical condition. Crit Care Med. 2005;33:1254–1259. doi: 10.1097/01.ccm.0000164541.12106.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kim G, Huh JH, Lee KJ, Kim MY, Shim KY, Baik SK. Relative Adrenal Insufficiency in Patients with Cirrhosis: A Systematic Review and Meta-Analysis. Dig Dis Sci. 2017;62:1067–1079. doi: 10.1007/s10620-017-4471-8. [DOI] [PubMed] [Google Scholar]
- 141.Wentworth BJ, Schliep M, Novicoff W, Siragy HM, Geng CX, Henry ZH. Relative adrenal insufficiency in the non-critically ill patient with cirrhosis: A systematic review and meta-analysis. Liver Int. 2023;43:660–672. doi: 10.1111/liv.15473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wentworth BJ, Siragy HM. Adrenal Insufficiency in Cirrhosis. J Endocr Soc. 2022;6:bvac115. doi: 10.1210/jendso/bvac115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Téblick A, Gunst J, Van den Berghe G. Critical Illness-induced Corticosteroid Insufficiency: What It Is Not and What It Could Be. J Clin Endocrinol Metab. 2022;107:2057–2064. doi: 10.1210/clinem/dgac201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Fernández J, Escorsell A, Zabalza M, Felipe V, Navasa M, Mas A, Lacy AM, Ginès P, Arroyo V. Adrenal insufficiency in patients with cirrhosis and septic shock: Effect of treatment with hydrocortisone on survival. Hepatology. 2006;44:1288–1295. doi: 10.1002/hep.21352. [DOI] [PubMed] [Google Scholar]
- 145.Arabi YM, Aljumah A, Dabbagh O, Tamim HM, Rishu AH, Al-Abdulkareem A, Knawy BA, Hajeer AH, Tamimi W, Cherfan A. Low-dose hydrocortisone in patients with cirrhosis and septic shock: a randomized controlled trial. CMAJ. 2010;182:1971–1977. doi: 10.1503/cmaj.090707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Piccolo Serafim L, Simonetto DA, Anderson AL, Choi DH, Weister TJ, Hanson AC, Kamath PS, Gajic O, Gallo de Moraes A. Clinical Effect of Systemic Steroids in Patients with Cirrhosis and Septic Shock. Shock. 2021;56:916–920. doi: 10.1097/SHK.0000000000001822. [DOI] [PubMed] [Google Scholar]