Abstract
Colorectal cancer (CRC) affects 1 in 23 males and 1 in 25 females, making it the third most common cancer. With roughly 608000 deaths worldwide, CRC accounts for 8% of all cancer-related deaths, making it the second most common cause of death due to cancer. Standard and conventional CRC treatments include surgical expurgation for resectable CRC and radiotherapy, chemotherapy, immunotherapy, and their combinational regimen for non-resectable CRC. Despite these tactics, nearly half of patients develop incurable recurring CRC. Cancer cells resist the effects of chemotherapeutic drugs in a variety of ways, including drug inactivation, drug influx and efflux modifications, and ATP-binding cassette transporter overexpression. These constraints necessitate the development of new target-specific therapeutic strategies. Emerging therapeutic approaches, such as targeted immune boosting therapies, non-coding RNA-based therapies, probiotics, natural products, oncolytic viral therapies, and biomarker-driven therapies, have shown promising results in preclinical and clinical studies. We tethered the entire evolutionary trends in the development of CRC treatments in this review and discussed the potential of new therapies and how they might be used in conjunction with conventional treatments as well as their advantages and drawbacks as future medicines.
Keywords: Colorectal cancer, Chemotherapy, Immunotherapy, RNA interference, Probiotics, Oncolytic viral therapy
Core Tip: This review highlights some of the latest colorectal cancer treatment approaches that have the potential to soon translate into standard care. A vast literature of patient data has revealed that conventional therapies are non-specific and have numerous secondary complications. Additionally, patients develop resistance to conventional chemotherapies. There is a need to develop new target-specific arrows in the cancer-fighting quiver.
INTRODUCTION
Starting from the industrial revolution, the scientific world has evolved progressively. After reaching the 21st century, certain diseases need novel therapeutic moieties for their cure, and colorectal cancer (CRC) is one of them. CRC is the third most prevalent cancer globally (6.1%) after lung cancer (11.6%) and breast cancer in females (11.6%) and prostate cancer in males (7.1%)[1]. It ranks second among all cancer in terms of mortality, accounting for 9.2% of all cases (9% male and 8% female)[1]. Estimates have shown that by the year 2035, there will be an increment in the cases of colon and rectal cancer by 71.5% and 60.0%, respectively[2]. This rapid disease progression has led to an economic burden on the countries and requires a major part of gross domestic product expenditure on public health.
The primary therapy for resectable CRC is surgical removal, and in non-resectable CRC, standard therapies include chemotherapy, radiotherapy, and immunotherapy. However, these therapies have certain drawbacks, such as being non-specific and cytotoxic to normal cells, which leads to secondary complications[3]. Depending upon the confinement and progression of the CRC, these therapies can be utilized in combinations. However, even with combinational therapies, more than half of patients relapse into acquired multidrug resistance CRC[4]. According to 2021 statistics, despite significant advancements in CRC screening, surgical resection, and adjuvant treatment, the death rate for CRC patients is still relatively high[1]. Therefore, there is a need to develop novel CRC therapies that render resistant tumors more sensitive to chemotherapeutic drugs.
Immune checkpoint inhibitors (ICIs), chimeric antigen receptor (CAR) T cell therapy, T cell receptor (TCR) alterations, and cytokine therapy have recently emerged as effective treatments for CRC. Also recent research on the use of probiotics[5], RNA-based therapies [small interfering RNA (siRNA), microRNA (miRNA), and RNA aptamer][6], oncolytic viral therapies[7], and natural products[8] in the treatment of CRC have yielded promising results. However, the survival rate of patients at an advanced stage remains a major problem. Scientists are working hard to understand the pathophysiology of CRC to have a better approach for treatment while enhancing current treatments like radiation therapy, targeted therapy, endoscopic resection, chemotherapy, and immunotherapy.
An individualized standard chemotherapeutic regimen is prescribed to a patient based on certain factors such as overall health profile, co-medication, comorbidities, patient compliance, psychosocial factors, results of prior resection, adjuvant therapy, route of administration, logistical support, consideration of side effect profiles, the biology of the tumor, the main location of the tumor, the presence of RAS and BRAF mutations, or microsatellite instability. Considering these factors, patients are divided into risk groups with varying treatment strategies. Since colon cancers and rectal cancers are different, multidisciplinary and distinct approaches must be taken depending on the staging information before treatment[9]. This review traced the evolutionary development of many standard CRC treatments and recent promising therapeutic approaches. We also reviewed the benefits and hurdles that still need to be overcome in treating CRC.
THERAPEUTIC APPROACHES
Local approaches
Radiation therapy: Neoadjuvant therapy, including radiotherapy and chemotherapy when used alone or in combination, has been recommended in rectal cancer and has effectively reduced tumor burden for intermediate and advanced stage cancer. The primary objective of radiotherapy is to decrease the risk of local recurrence and improve overall survival. However, evidence suggests that preoperative radiotherapy is more effective than postoperative therapy in reducing local recurrence, but it does not improve overall survival[10]. With two available adjuvant radiotherapies, short-course radiotherapy (RT) and long-course RT, there was always a question of which one was better. Higher rates of acute toxicity are observed with long-course RT than with short-course RT, but late side effect incidence rates do not show significant differences[11]. Radiation and adjuvant radiation therapy are better options for treating stage II and III CRC. However, radiation therapies have some plausible long-term toxicity impacts on vital organs.
New delivery methods, such as intensity-modulated RT (IMRT), have been widely adopted in clinical practice and have demonstrated potential benefits with decreased toxicity for patients with rectal cancer by lowering the radiation dose[12]. IMRT employs linear accelerators to safely deliver precise radiation to a tumor while reducing the exposure to nearby healthy tissue. With IMRT, radiation dosages to adjacent healthy organs can be limited while still being delivered at high doses to the tumor and nearby lymph nodes. By adjusting the dose in this way to avoid normal, unaffected tissues, it can lessen adverse effects and potentially improve the toxicity profile. Additionally, the use of IMRT for rectal cancer may help to speed up the time to surgery, promote better postoperative recovery, and enhance the tolerability of adjuvant chemotherapy[13]. IMRT may help reduce treatment interruptions, emergency department visits, and hospitalizations compared to 3-dimensional conformal radiation therapy[14]. In a United Kingdom study of radiotherapy facilities, 68% of respondents reported using IMRT for all rectal cancer patients[15]. In the future, more clinical data will be needed to support the use of IMRT for rectal cancer, which could encourage doctors to incorporate IMRT planning into preoperative chemoradiotherapy for rectal cancer.
Systemic approach
Chemotherapy: The ultimate treatment for locoregional CRC is surgical resection. Advances in primary and secondary treatments have improved the survival time in CRC. Notably, in some circumstances, chemotherapy or radiotherapy may be used as neoadjuvant or adjuvant treatments before or after surgery in order to significantly reduce and cure the tumor. Of note, chemotherapy is used to either eliminate cancer cells or stop them from proliferating. Cytotoxic drugs approved for CRC slow down disease progression and increase an individual’s lifespan. The medications approved are fluoropyrimidines, irinotecan, oxaliplatin, tri-fluridine-tipiracil, capecitabine, and 5-fluorouracil (5-FU), most commonly used as a chemotherapeutic agent for curing CRC.
Adjuvant fluoropyrimidine-based chemotherapy after surgery for CRC has been a standard treatment due to their ability to reduce the recurrence of the tumor and increase the survival as shown in trial by Moertel et al[16]. Leucovorin (LV, folinic acid), a chemoprotectant, potentiates the activity of 5-FU by forming a stable complex and preventing its adverse effects[17]. The fluorouracil + L-folinic acid (FU/LV) regimen given daily for 5 d in 6 cycles resulted in a 15% reduced risk of death at 5 years[18,19]. All 5-FU-based regimens must include LV as it has been demonstrated to improve patient survival and tumor response rate (RR) when combined with 5-FU[20]. Despite being one of the safest chemotherapeutic agents, 5-FU has side effects for some CRC patients, including fever, mucositis, stomatitis, leukopenia, and thrombocytopenia[21]. Cerebellar ataxia and other neurological diseases also affect 1% of patients[22].
Another imperative drug used is capecitabine, a prodrug of 5-FU. Its bioavailability is virtually 100%, and Cmax and area under the curve increase linearly with dosage. In patients with metastatic CRC, two phase III randomized studies compared capecitabine as a single drug to the typical 5-FU/LV therapy combination, and the RR in both studies were as efficient as 5-FU/LV[23].
Topoisomerase I inhibitor irinotecan and oxaliplatin were added to the 5-FU regimen as part of a cytotoxic combination therapy for metastatic colorectal cancer (mCRC) to enhance its efficacy. Oxaliplatin is a diaminocyclohexane platinum complex that has the potential to generate DNA adducts and interferes with the mechanism of DNA repair, thereby leading to a cytotoxic effect in CRC[22]. Patient survival improved due to the inclusion of oxaliplatin or irinotecan in the 5-FU regimen but at the same time has increased the toxicity levels. In the first-line treatment of mCRC, 5-FU/LV is coupled with oxaliplatin (FOLFOX), irinotecan (FOLFIRI), and both oxaliplatin and irinotecan (FOLFOXIRI). The combination therapies FOLFOX, FOLFIRI, and FOLFOXIRI have established themselves as effective cytotoxic regimens, with an average improvement in survival of about 2 years. According to the Gruppo Oncologico dell’Italia Meridionale trial, overall survival (OS) rates for FOLFIRI and FOLFOX were 14 mo and 15 mo, respectively. The GERCOR trial found that OS was 21.5 mo for patients who received FOLFOX first followed by FOLFIRI and 20.6 mo for those who received FOLFIRI first followed by FOLFOX. Trifluridine-tipiracil, a fluoropyrimidine, contains tipiracil hydrochloride, which prevents the degradation of trifluridine. The incorporation of trifluridine into DNA is the primary mechanism of action. Compared to the best supportive care alone, the prospective randomized clinical phase III trial RECOURSE found that it significantly increased median OS[24].
Tyrosine kinase signaling pathways typically prevent unchecked cell growth or boost sensitivity to trigger apoptosis. These signaling pathways are often genetically or epigenetically dysregulated in cancer cells, offering them a selective advantage. The human genome contains about 500 protein kinase genes, which constitute about 2% of all human genes. The structures of over 280 human protein kinases have been determined[25]. Regorafenib is a Food and Drug Administration (FDA)-approved tyrosine kinase inhibitor that targets numerous targets, including vascular endothelial growth factor (VEGF) receptor (VEGFR), platelet-derived growth factor, fibroblast growth factor, and BRAF in mCRC due to its better median OS and progression-free survival (PFS) in the phase III CORRECT trial[26] and the CONCUR trial[27]. Other promising tyrosine kinase inhibitors are listed in Table 1 with their specific target.
Table 1.
Tyrosine kinase inhibitors, plant based drugs, and selected target specific agents against colorectal cancer
|
Agent
|
Type of agent
|
Target/mechanism
|
FDA approval date/trial number/status
|
Sources/interventions
|
Results
|
| Sunitinib | TKI | VEGFR1-3 | NCT00457691. Completed | Phase II study: FOLFIRI and sunitinib for mCRC | Sunitinib did not add to the antitumor activity of FOLFIRI |
| Axitinib | TKI | VEGFR1-3 | NCT00460603. Completed | Phase II study: axitinib and/or bevacizumab with modified FOLFOX-6 as first-line therapy for mCRC | Neither the addition of continuous axitinib nor the axitinib/bevacizumab combination to FOLFOX-6 improved ORR, PFS, or OS compared with bevacizumab as first-line treatment of mCRC |
| Sorafenib | Kinase inhibitor | VEGFR | NCT00326495. Completed | Phase II study: cetuximab and sorafenib for the treatment of KRAS-mutated mCRC | No objective responses were observed |
| Regorafenib | Multikinase inhibitor | VEGFR1-3, TIE2, KIT, RET, RAF, PDGFR-B, FGFR | September 27, 2012 | Approved for ACRC, mCRC | - |
| Encorafenib | Kinase inhibitor | BRAF-V600E as well as wildtype BRAF and CRAF | April 8, 2020 | Approved for mCRC | - |
| Simtuzumab | Monoclonal antibody | LOXL2 | NCT01479465. Completed | Phase II study: efficacy of simtuzumab with FOLFIRI as second line treatment in CRC | The addition of simtuzumab to FOLFIRI did not improve clinical outcomes in patients with metastatic KRAS-mutant CRC |
| Lenvatinib | TKI of VEGFR | VEGFR1-3, KIT, RET, PDGFR-alpha, FGFR | NCT04776148. Ongoing | Phase III study ongoing: lenvatinib in combination with pembrolizumab for mCRC | Ongoing |
| Tivozanib | TKI of VEGFR | VEGFR1-3 | NCT01058655. Completed | Phase II study: everolimus (RAD001) and tivozanib (AV-951) in patients with refractory or mCRC | The oral combination of tivozanib and everolimus was well tolerated, with stable disease achieved in 50% of patients with refractory or mCRC |
| Tipifarnib | Farnesyltransferase inhibitor | Farnesyltransferase | NCT00005833. Completed | Phase II trial study: R-115777 given as a single agent | Ineffective in patients with mCRC |
| D-1553 | Small molecule KRasG12C inhibitor | KRAS G12C | NCT04585035. Ongoing | Phase I study using D-1553 in CRC with KRAS G12C mutation | Ongoing |
| Aflibercept | Recombinant fusion protein | VEGF-A and VEGF-B, PGF | NCT02181556. Completed | Phase II study: aflibercept in combination with FOLFIRI as first-line chemotherapy in patients with mCRC | Although the primary objective was not met, first-line FOLFIRI + aflibercept for mCRC resulted in median PFS and OS close to those reported with traditional doublet and targeted therapies |
| Berberine | Alkaloid | Anti-proliferation, cell cycle arrest | In vitro study | Plant/berberine | Berberine inhibited telomerase activity and induced cell cycle arrest and telomere erosion in colorectal cancer cell Line, HCT 116[149] |
| Piper nigrum ethanolic extract | Alkaloid | Antioxidative activity | In vitro study | Plant/EEPN | Time- and dose-dependent increase in the cytotoxic efficacy of 50% EEPN against colorectal carcinoma cell lines were noted[150] |
| Fucoidan | Polysaccharide | Inhibit growth and angiogenesis | In vitro study | Brown seaweed/combination of fucoidan with vitamin C | The combination of fucoidan with vitamin C showed significant inhibitory effects on HCT-116 colon cell viability[151] |
| Curcumin | Polyphenol | Apoptosis, antiangiogenesis, and cell cycle arrest | NCT02439385. Completed | Plant/phase II study: bevacizumab/FOLFIRI with ginsenoside-modifies nanostructured lipid carrier containing curcumin (G-NLC) in patients with mCRC | Bevacizumab/FOLFIRI with G-NLC increased long-term survival. Further randomized control studies are needed |
| NCT01490996. Completed | Phase I/II study: curcumin combined with FOLFOX | Curcumin with FOLFOX was safe and tolerable. The HR for PFS and OS was 0.57 and 0.34, respectively | |||
| Gingerol | Polyphenol | Antioxidative and anti-inflammatory | NCT01344538. Completed | Plants/phase II randomized control trial. Ginger for CRC prevention | Result suggested ginger may reduce proliferation and increase apoptosis |
| EPA | Polyunsaturated fatty acids | Inhibit angiogenic factors | NCT00398333. Terminated | Marine microalgae/phase IV | Due to small sample size further investigation needed |
| EGCG | Polyphenol | Apoptosis | NCT02891538. Ongoing | Plants/early phase 1 study: EGCG in CRC patients | Ongoing |
| PSK | Polysaccharide | Apoptosis and antiproliferative | NCT00497107. NA | Fungi/phase III study: oral tegafur/uracil plus PSK | Results suggested that there was reduction in recurrence and mortality by 43.6% and 40.2%, respectively in stage I and stage II |
| Resveratrol | Polyphenol | Apoptosis and antiproliferative | NCT00920803. Completed | Plants/phase I study: resveratrol for resectable CRC | Resveratrol was effective in treating CRC by modulating the Wnt pathway |
| Topotecan | Alkaloid | Antiproliferative | EORTC | Plants/phase II study: oral topotecan | Topotecan administered as a five times daily regimen has only minor activity as a single-agent therapy in colorectal cancer |
| Metformin | Alkaloid | Antiproliferative and antimetastatic | NCT03047837. NA | Plants/phase II study: using aspirin and metformin in stage I-II CRC | Result suggested that the given intervention delayed recurrence and improved prognosis |
| Everolimus | Macrolide | Antiproliferative and antimetastatic | NCT01387880. Completed | Bacterial/phase II study: irinotecan, cetuximab, and everolimus to patients with mCRC | Everolimus showed promising effects on CRC prognosis |
| NCT01058655. Completed | Phase II study: tivozanib and everolimus for patients with refractory mCRC | Oral combination of tivozanib and everolimus was well tolerated in 50% of the patient | |||
| Andrographolide | Diterpenoid | Apoptosis, antiproliferative, and cell cycle arrest | In vitro study | In vitro study using 5-FU with andrographolide | Andrographolide enhanced 5-FU induced antitumor effect in CRC via inhibition of the c-MET pathway[152] |
| Silymarin | Flavnoid | Apoptosis, antiproliferative | NCT03130634. Completed | Plants/phase IV study using silymarin in patients treated with first-line treatment FOLFIRI | Silymarin is a potential supplement for reducing toxicities in mCRC patients undergoing FOLFIRI plus bevacizumab first-line treatment |
| MMC | Hyleneimines | Antiproliferative | NCT00643877. NA | Streptomyces/phase III study using PHARC with oxaliplatin, MMC FUDR | Addition of PHRAC improved DFS in patients with stage II and stage III CRC |
| NCT03073694. Ongoing | Phase II study using MMC and melphalan | Ongoing |
5-FU: 5-fluorouracil; ACRC: Advanced colorectal cancer; BRAF-V600E: BRAF protein coding gene; CRC: Colorectal cancer; DFS: Disease-free survival; EEPN: Ethanolic extract of Piper nigrum; EGCG: Epigallocatechin gallate; EORTC: European Organization for Research and Treatment of Cancer; EPA: Eicosapentaenoic acid; FDA: Food and Drug Administration; FGFR: Fibroblast growth factor receptor; FOLFIRI: 5-flurouracil, leucovorin, and irinotecan; FOLFOX-6: 5-flurouracil, leucovorin, and oxaliplatin-6; FUDR: Fluorodeoxyuridine; G-NLC: Genistein-nanostructured lipid carriers; HR: Hazard ratio; KIT: Proto-oncogene receptor tyrosine kinase; LOXL2: Lysyl oxidase-like 2; mCRC: Metastatic colorectal cancer; MMC: Mitomycin C; NA: Not available; ORR: Objective response rate; OS: Overall survival; PDGFR-B: Platelet-derived growth factor receptor B; PFS: Progression-free survival; PGF: Placental growth factor; PHARC: Preoperative hepatic and regional atrial chemotherapy; PSK: Polysaccharide krestin; RAF: Rapidly activated fibrosarcoma; RET: Ret proto-oncogene; TIE2: Tyrosine kinase with immunoglobin and epidermal growth factor 2; TKI: Tyrosine kinase inhibitor; VEGFR1-3: Vascular endothelial growth factor 1-3.
Neoadjuvant chemotherapy (NACT) is an emerging field in cancer. It is mainly used to treat solid tumor malignancies such as gastric, esophageal, and rectal cancers[28], but its efficacy has not been fully explored in cases of CRC. Utilization of NACT before surgery can result in better outcomes in CRC patients with liver metastases. As a result, NACT is being investigated in primary rectal and colon cancers as a potential method to reduce the size of the tumor, enable a curative resection, and mitigate the chance of metastases. A potential benefit of NACT was reported in a trial on patients with stage III colon cancer, in which they received preoperative capecitabine + oxaliplatin (CAPOX) followed by adjuvant therapy (CAPOX) after resection. Tumor volume was reduced by 69.5%, without any progression of disease during therapy, and OS was 100%[29]. A similar protocol with an addition of 5-FU in some cases[30] resulted in the majority of patients experiencing a reduction in tumor volume of 62.5%. This finding was the basis of an ongoing randomized phase II study of neoadjuvant CAPOX in locally advanced colon cancer.
Based on the efficacy of FOLFOXIRI established in mCRC, the same triplet therapy was utilized as NACT in patients with stage IIIB colon cancer, followed by resection and then adjuvant therapy of either FOLFOXIRI or CAPOX. This trial documented a reduction in tumor in 91.3% of patients with toxicities of grade 3-4. At the end of trial, only 52.2% of patients were left with OS of 95.7% and 2-year recurrence rate of 26.1%[31]. There are several other proposed and ongoing randomized phase II and III clinical trials taking place utilizing NACT such as CAPOX, FOLFOX, ipilimumab + nivolumab ± celecoxib and many more[32]. Despite the controversial role of targeted agents in other cancers, these could be considered as a potential weapons in CRC, enhancing the efficacy of neoadjuvant therapy[33]. Treatment with bevacizumab and chemotherapy before surgery in CRC patients with liver metastases has been found to increase the survival in several phase II trials[34]. The efficacy of targeted neoadjuvant therapy requires studies involving larger population. Therefore, definite conclusions cannot be drawn from trials involving small population size, demanding further investigations.
Presently, progress in the adjuvant therapy following resection has taken a pause. A shift has been taken towards the utilization of NACT in both colon and rectal cancers instead. Large, randomized clinical trials are being conducted worldwide to investigate various strategies used for safe and accurate administration of treatment. However, certain parameters like standardization of regimen combination, standard intensity, and duration of therapy, postoperative settings, etc are still undefined because of lack of long-term oncological results of randomized phase II and phase III studies, warranting further investigation.
Target-specific approaches
Immunotherapy as a promising candidate for CRC treatment: Following early breakthroughs in the treatment of melanoma, immunotherapy has been quickly established as a prominent therapeutic strategy for a variety of solid tumors[35,36], including CRC. Cancer immunotherapy conquers the issue of specificity, which is a severe issue of chemotherapy, radiotherapy, and other approaches. Figure 1 illustrates all currently available and emerging immunotherapies for CRC. The cancer immunotherapy area has shown potential in treating a variety of solid tumors, with a growing number of FDA-approved monoclonal antibodies and single and combinational immunotherapeutic medicines throughout time[37].
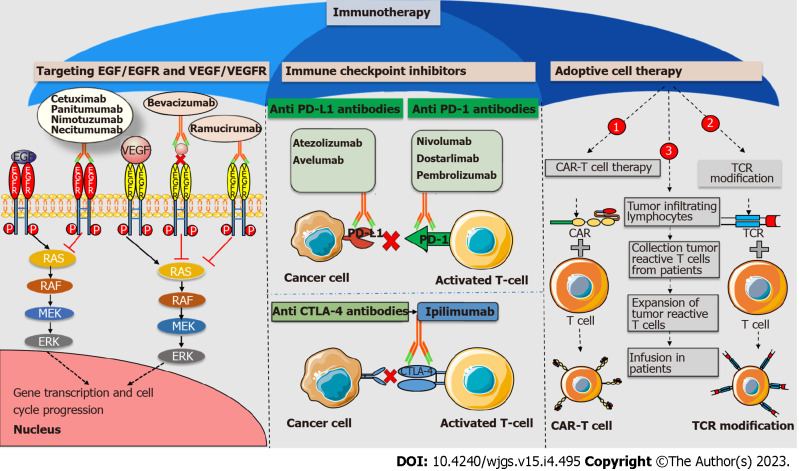
Figure 1.
Immunotherapeutic approaches against colorectal cancer. Antiangiogenic monoclonal antibodies like cetuximab, panitumumab, nimotuzumab, and necitumumab target epidermal growth factor (EGF) receptor (EGFR). Antiangiogenic monoclonal antibodies such as bevacizumab and ramucirumab target vascular EGF and its receptor, respectively. All antiangiogenic monoclonal antibodies downregulate the RAS-RAF-MEK-ERK pathway and prevent the transcription of genes involved in cell cycle progression. Immune checkpoint inhibitors (ICIs) like atezolizumab and avelumab target programmed cell death ligand 1 (PD-L1). ICIs like nivolumab, dostarlimab, and pembrolizumab target programmed cell death protein 1 (PD-1). The ICI ipilimumab targets cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4). ICIs act as immune brakes that prevent checkpoint proteins from interacting with their companion proteins, thus boosting T cell effector activity. T cell boosting therapy, like adoptive cell therapy, includes chimeric antigen receptor T cell therapy (CAR-T), T cell receptor modification (TCR), and tumor infiltrating lymphocyte boost T cell activity to combat cancer cell growth. VEGF: Vascular epidermal growth factor; VEGFR: Vascular epidermal growth factor receptor.
Anti-epidermal growth factor/receptor antibodies: The epidermal growth factor receptor (EGFR) signaling pathway is a complicated and closely controlled process that plays a role in proper cell development, proliferation, and survival. When this pathway malfunctions and continues unregulated, neoplastic cells can grow, proliferate, survive, and spread. With encouraging preclinical results, cetuximab, the first monoclonal antibody targeting EGFR, was introduced. Cetuximab is a chimeric immunoglobulin G (IgG) antibody that causes EGFR internalization and destruction[38]. According to the BOND trial, which supported the FDA’s 2004 approval of cetuximab for treating mCRC, cetuximab showed considerable promise in improving PFS and OS in patients with a low response to single-agent IRI therapy[39]. Cetuximab, in combination with other chemotherapies, showed encouraging outcomes as well. The phase III CRYSTAL trial found that the combination of cetuximab and the FOLFIRI regimen had better progression control than FOLFIRI alone [8.9 mo vs 8.0 mo, hazard ratio (HR): 0.85, P = 0.048][40]. However, being a chimeric antibody, cetuximab may ultimately result in immunogenic responses.
To minimize the immunogenic responses, the fully humanized antibody panitumumab was developed. Panitumumab, unlike cetuximab, does not cause antibody-dependent cell-mediated cytotoxicity[41]. In the PRIME trial, the combination regimen of FOLFOX plus panitumumab outperformed FOLFOX alone in terms of PFS (10.0 mo vs 8.6 mo, HR: 0.80, P = 0.01) and OS (23.9 mo vs 19.7 mo, HR: 0.88, P = 0.17), with further evidence of significance in the updated survival analysis (HR: 0.83, P = 0.003) in patients with mCRC[42,43].
Humanized antibodies are also a bit immunogenic compared to human antibodies. Necitumumab is a completely human monoclonal antibody approved by the FDA for advanced squamous non-small cell lung cancer. Necitumumab plus modified FOLFOX6 was studied in a phase II study for the first-line treatment of locally advanced or mCRC[44]. PFS and OS were 22.5 mo (11.0-30.0) and 10.0 mo (7.0-12.0), respectively. First-line necitumumab modified FOLFOX6 effectively treated locally advanced or mCRC with manageable side effects; further research on the effect of necitumumab on RAS-associated mutation is necessary. Humanized anti-EGFR antibodies, such as nimotuzumab, have a better safety record than most anti-EGFR antibodies.
In clinical practice, there were no discernible differences in the objective RR, disease control rate, PFS, or median survival between chemotherapy plus nimotuzumab and chemotherapy alone in patients with advanced CRC, indicating that these treatments were equally effective for treating the disease[45]. Both cetuximab and panitumumab are FDA-approved first-line treatments for CRC. Anti-EGFR medicines may be a low priority for second-line or beyond CRC treatment because cetuximab and panitumumab failed to achieve statistically superior PFS or OS for patients with CRC in multiple studies[46,47].
Anti-VEGF/VEGFR antibodies: Angiogenesis is required for tumor invasion and metastasis to progress beyond the size of a few centimeters. In CRC and other malignancies, VEGF is the most important angiogenic factor. As a result, multiple studies examining VEGF expression in CRC patients have been conducted, and several therapeutic drugs targeting the VEGF pathway have been tested. Bevacizumab, an antiangiogenic antibody, has proven to be more effective than chemotherapy when combined with IRI, 5-FU, and LV plus placebo in phase II and III AVF2107 studies based on antiangiogenic therapy for CRC. The AVF2107 study found that bevacizumab, a humanized IgG monoclonal antibody, targets VEGF-A and improves both PFS and OS in mCRC (RR: 44.0% vs 34.8%; OS: 20.3 mo vs 15.6 mo; HR: 0.66, P = 0.001; PFS: 10.6 mo vs 6.2 mo; HR: 0.54, P = 0.001).
In the E3200 trial, patients with CRC who progressed after FOLFOX therapy had a better PFS (7.3 mo vs 4.7 mo, HR: 0.61, P = 0.001) and OS (12.9 mo vs 10.8 mo, HR: 0.75, P = 0.0011) as well as a better RR (22.7% vs 8.6%, P = 0.0001) with a combination of FOLFOX and bevacizumab than FOLFOX alone. Bevacizumab is the only antibody approved by the FDA as a first- and second-line VEGF-targeted therapy for CRC. Another FDA-approved medication for second-line therapy of mCRC is ramucirumab, a completely humanized monoclonal VEGFR-2-targeted IgG antibody. Compared to FOLFIRI-placebo, a combination of ramucirumab and FOLFIRI significantly improved PFS (5.7 mo vs 4.5 mo; HR: 0.79, P = 0.0005) and OS (13.3 mo vs 11.7 mo, HR: 0.84, P = 0.022) based on the phase III RAISE trial. Table 2 enlists various anti-VEGF/VEGFR and anti-epidermal growth factor (EGF)/EGFR monoclonal antibodies that have been approved and are undergoing preclinical and clinical trials. All these medications increased OS from a few weeks to months. However, some tumor characteristics, such as genetic changes in endothelial cells, vasculogenic mimicry, and the unique therapeutic response of each tumor, interfere with these antiangiogenic therapeutic approaches. Furthermore, angiogenic treatments promote a hypoxic environment, which promotes tumor invasiveness[48].
Table 2.
Immunotherapeutic agents and vaccines against colorectal cancer
|
Antibodies/antigenic composition
|
Origin
|
Target/CRC stage
|
Approval date/trial number/yr
|
Description/interventions
|
Inference
|
| Monoclonal antibodies | |||||
| Cetuximab | Chimeric | EGFR | February 12, 2004 | Cetuximab alone for mCRC | Adding cetuximab to first-line chemotherapy in patients with WT KRAS mCRC was statistically beneficial for OS and PFS[153] |
| July 6, 2012 | For mCRC cetuximab + FOLFIRI | ||||
| Panitumumab | Humanized | EGFR | September 27, 2006 | For mCRC panitumumab + FOLFOX for WT KRAS mCRC. For WT RAS mutation mCRC | In WT KRAS mCRC, PFS was improved, objective response was higher, and there was a trend toward improved OS with panitumumab-FOLFOX4[42,43,154] |
| May 23, 2014 | |||||
| June 29, 2017 | |||||
| Nimotuzumab | Humanized | EGFR | NCT05278728. Completed | Phase II study nimotuzumab along with radiotherapy and concurrent capecitabine | No significant outcomes |
| NCT05278728. Completed | Phase IIa study of nimotuzumab to treat CRC | Ongoing | |||
| Necitumumab | Human | Cetuximab-resistant EGFR | NCT00835185. Completed | Phase II study necitumumab plus modified FOLFOX6 for locally advanced and mCRC | First-line necitumumab + mFOLFOX6 was active with manageable toxicity in locally advanced or mCRC |
| Bevacizumab | Humanized | VEGF | February 26, 2004 | For mCRC | The addition of bevacizumab to 5-fluorouracil-based combination significantly increased patient survival[155,156] |
| Ramucirumab | Human | VEGFR-2 | April 24, 2015 | Ramucirumab with FOLFIRI as second-line treatment for mCRC | The addition of ramucirumab to FOLFIRI improved patient outcomes in the RAISE trial[157] |
| Nivolumab | Human | PD-1 | August 1, 2017 | Nivolumab approved for MSI-H/dMMR mCRC | Nivolumab provided durable responses and disease control in pre-treated patients with dMMR/MSI-H mCRC[51] |
| Ipilimumab | Human | CTLA-4 | July 11, 2018 | Nivolumab plus low dose ipilimumab approved for previously treated MSI-H/dMMR mCRC | Clinical effect with nivolumab + low-dose ipilimumab was significant and long-lasting for MSI-H/dMMR mCRC[57] |
| Cemiplimab | Human | PD-1 | NCT04157985. Ongoing | Phase III study: evaluating length of treatment with cemiplimab and other inhibitors in solid tumor patients | Ongoing |
| Atezolizumab | Humanized | PD-L1 | NCT02788279. Completed | Phase III study: atezolizumab with or without cobimetinib vs regorafenib in previously treated mCRC | Did not meet its primary endpoint of improved OS with atezolizumab plus cobimetinib or atezolizumab vs regorafenib |
| NCT05118724. Ongoing | Phase II study: atezolizumab with/without IMM-101 in patients with MSI-H/dMMR stage III CRC ineligible for oxaliplatin | Ongoing | |||
| NCT05456165. Ongoing | Phase II study: atzolizumab in combination with neoantigen targeting vaccine | Ongoing | |||
| Avelumab | Human | PD-L1 | NCT03854799. Ongoing | Phase II study: avelumab + capecitabine combined with radiation | Ongoing |
| NCT03475953. Ongoing | Phase I/II Study: regorafenib plus avelumab in solid tumors | Ongoing | |||
| Dostarlimab | Humanized | PD-1 | NCT04165772. Ongoing | PD-1 blockade in dMMR, locally advanced rectal cancer | Ongoing: dMMR, locally advanced rectal cancer was highly sensitive to single-agent PD-1 blockade. Longer follow-up is needed to assess the duration of response |
| Pembrolizumab | Humanized | PD-1 | June 29, 2020 | Pembrolizumab for first-Line treatment of patients with unresectable or metastatic MSI-H or dMMR CRC | Approved based on Phase III Keynote-117 Trial in which pembrolizumab significantly reduced the risk of disease progression or death by 40%[158] |
| Relatimab | Human | LAG-3 | NCT05328908. Ongoing | Phase III study of nivolumab-relatlimab fixed-dose combination vs regorafenib or TAS-102 in participants with mCRC | Ongoing |
| NCT03642067. Ongoing | Study of nivolumab and relatlimab in patients with MSS advanced CRC | Ongoing | |||
| Peptide based vaccines[80] | |||||
| SART3 | - | Metastatic | 2001 | Used with adjuvant incomplete Freund’s adjuvant | Increased cellular immune responses to both CRC cells and the vaccinated peptide |
| Recombinant Ep-CAM (with liposome carrier) | - | I-III | 2001 | Used with adjuvant alum | The overall immune response was safe and effective for patients with CRC and advanced cancer against Ep-CAM |
| II-IV | 2004 | Used with adjuvant GM-CSF | |||
| Metastatic | 2004 | Used with adjuvant GM-CSF | |||
| CTP37-DT | - | III-IV | 2002 | Used with adjuvant Nor-MDP (Muramyl dipeptide) | Longer OS with an excellent safety profile in patients with CRC |
| Recombinant CEA expressed in baculovirus system | Expressed in baculovirus-insect cell system | Stage I-III | 2004 | Used with adjuvant alum and GM-CSF | Potent and long lasting antigen specific IgG and T cell response |
| Survivin-2B Human | - | Metastatic | 2004 | Used with adjuvant UFT (uracil-tegafur) | Excellent safety profile with potent immune response against HLA-A24-expression in patients with CRC |
| G17DT (N-terminus of gastrin 17) | - | Metastatic | 2014 | Used with adjuvant diphtheria toxoid | In combination with irinotecan this vaccine has an acceptable immune response with significantly longer survival |
| OCV-C02 | - | Metastatic | 2017 | Two peptide epitopes derived from RNF43 and TOMM34 and used with adjuvant montanide ISA 51 | Safe immune response in recurrent or advanced stage CRC patients resistant to standard chemotherapy |
| RNF43 and TOMM34-derived peptides | - | III | 2018 | Used with uracil-tegafur/leucovorin, montanide ISA 51 | Strong immune response with increased OS in patients with stage III CRC |
| PolyPEPI1018 | - | Metastatic | 2020 | Used with adjuvant montanide ISA 51 Human | Safe and well-tolerated and induced robust CRC-specific T cell responses, similar to personalized neoantigen vaccines |
| mRNA-based vaccines[80] | |||||
| NCI 4650 (mRNA 4650) | - | Metastatic | 2019 | - | Partly safe and neoantigen specific CD8 and CD4 T cells responses against CRC neoepitopes |
| mRNA 4157 | - | Metastatic | 2019 | In combination with pembrolizumab | Partly safe and strong neoantigen specific T cell responses against CRC neoepitopes |
| V 941 (mRNA 5671) | - | Metastatic | 2019, NCT03948763 | In combination with pembrolizumab | KRAS vaccine clinical trial is underway, and the results are eagerly awaited |
| RO 7198457 (RG 6180) | - | Metastatic | 2020 | In combination with atezolizumab | Partly safe and strong neoantigen specific immune responses |
| Cell based vaccines[80] | |||||
| Tumor cell | Tumor cell | II and III | 2000 | In combination with BCG | Less potency with 5-yr OS of 84.6% |
| Cancer Vax | Tumor cell | IV | 2001 | In combination with BCG | Significant increase in anti-TA90 IgG and IgM titers, and the OS was 21.9 mo |
| HSPPC-gp96 | Tumor cell | IV | 2003 | - | Two-year overall survival and disease-free survival improved |
| CEA mRNA | DCs | IV | 2003 | In combination with IL-2 | Well tolerated and safe immunization observed in patients with advanced malignancies |
| OPA-DC | DCs | Metastatic | 2011 | CEA peptide-loaded DCs matured with a combination of OK432, prostanoid, and interferon-α | Increased CEA-specific cytotoxic T cell response and NK cell levels in 8 patients with stable disease |
| Autologous tumor lysate DC (ADC) | DCs | Metastatic | 2016 | - | Not recommended: the use of ADC alone, in a phase III trial |
| Autologous tumor antigens-loaded DC | DCs | Metastatic | 2018 | In combination with 5-fluorouracil | Treatment was safe and had shown particularly prominent IL-12 production for immunization against neoantigens |
| Vector based vaccines | |||||
| ALVAC-CEA/B7 | Canary pox virus vector | Metastatic | 2008; 2013 | In combination with chemotherapy | Acceptable safety profile and induced CEA-specific T cell responses in patients with mCRC |
| AVX701 | Alphavirus vector | III | 2010 | VRP expressing CEA | Well tolerated and elicit robust CEA-specific T cell and antibody responses in patients with CRC |
| GI-6207 | Saccharomyces cerevisiae | Metastatic | 2014 | - | Strong antigen-specific CD8+ T cells and CD4+ T responses and extended stable disease |
| GI-6301 | Saccharomyces cerevisiae | Metastatic | 2015 | - | Decreased tumor density and serum CEA levels in CRC treated patients |
| pLADD | Listeria monocytogenes | Metastatic | 2017, NCT03189030 | Listeria bacterial vector in combination with neoantigens | Induced neoantigen-specific CD8+ T cells and gamma delta T cells |
| Cholera | Bacteria | I-IV | 2018 | - | Cholera vaccination largely decreased the mortality rate of CRC |
| GI-4000 | Saccharomyces cerevisiae | Metastatic | 2018 | - | Excellent safety profile and favorable immunogenicity in the majority of subjects |
| ADXS-NEO | - | Metastatic | 2019 | Bacteria expressing personalized tumor antigens | Increased CD4+/CD8+ T cell-mediated immune response |
ADC: Antibody drug conjugate; BCG: Bacillus Calmette–Guérin; CEA: Carcinoembryonic antigen; CRC: Colorectal cancer; CTLA-4: Cytotoxic T-Lymphocyte-associated antigen-4; DCs: Dendritic cells; dMMR/MSI-H: Microsatellite instability high and deficient mismatch repair; EGFR: Epidermal growth factor receptor; Ep-CAM: Epithelial cell adhesion molecule; FOLFIRI: 5-flurouracil, leucovorin, and irinotecan; FOLFOX4/6: 5-flurouracil, leucovorin, and oxaliplatin; GM-CSF: Granulocyte macrophage colony-stimulating factor; HLA: Human leukocyte antigen; HSPPC-gp96: Heat shock protein peptide glycoprotein Complex-96; Ig: Immunoglobin; IL: Interleukin; LAG-3: Lymphocyte activation gene 3; mCRC: Metastatic colorectal cancer; MSS: Microsatellite stable; NK: Natural killer cell; Nor-MDP: Nor-muramyl dipeptide; OS Overall survival; PFS: Progression-free survival; PD-1: Programmed cell death receptor 1; PD-L1: Programmed cell death ligand 1; RNF43: Ring finger protein 43; SART3: Squamous cell carcinoma antigen identified by T cells 3; TOMM34: Translocase of outer mitochondrial membrane 34; VEGF: Vascular endothelial growth factor; VEGFR: Vascular endothelial growth factor receptor; WT: Wild-type.
ICIs as immune boosters: The most common immunomodulatory antibodies are ICIs. ICIs are immune brakes that prevent checkpoint proteins from interacting with their companion proteins. The important immune checkpoints and their immunological inhibitors are listed in Table 2. One important target protein is cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4). CTLA-4 controls T cell activation and transmits inhibitory signals to T cells[49]. CTLA-4 inhibition by the anti-CTLA-4 antibody was shown to inhibit tumor progression by upregulating effector T cell activity and suppressing regulatory T cells[50]. The FDA has approved low-dose ipilimumab (CTLA-4 inhibitor) in combination with nivolumab for previously treated microsatellite instability-high and deficient mismatch repair (MSI-H/dMMR) mCRC[51].
Programmed cell death receptor programmed cell death protein 1 (PD-1) is another ICI target; it is a type 1 transmembrane protein that interacts with programmed cell death ligand 1 (PD-L1) (highly expressed in inflamed cells) and PD-L2 (expressed only on antigen-presenting cells). The interaction of PD-1 and PD-L1/PD-L2 is immunosuppressive, limiting the function of T effector cells to minimize the over storm of immune responses[52,53]. However, in CRC, overexpression of PD-1 and PD-L1/PD-L2 causes more than required immunosuppression[54]. Monoclonal antibodies were developed to bind and prevent PD-1/PD-L1 interactions and to assist T cells in killing cancerous cells by restoring their activation, proliferation, function, and downstream immune signaling[55]. There are five FDA-approved PD-1 inhibitors; three are approved for CRC (Table 2).
Pembrolizumab and nivolumab (PD-1 inhibitors) are used in CRC. Pembrolizumab improves CRC patients with dMMR/MSI-H based on the KEYNOTE-177 trial. Results showed a RR of up to 78% compared to 11% of patients with proficient mismatch-repair and microsatellite stability CRC[56]. Another successful PD-1 inhibitor is nivolumab, which shows durable responses with a 69% OS rate of 12 mo among patients with the dMMR mCRC[51]. Further, a higher RR of up to 94% in MSI-H/dMMR mCRC has been observed in the phase II CheckMate study (NCT02060188), which used the combination of nivolumab (a PD-1 targeting antibody) and ipilimumab (a CTLA-4-targeting antibody)[57]. This implies that the combination of immune checkpoint therapy can significantly increase the effectiveness of the treatment for MSI-H/dMMR mCRC patients.
Other potential immune checkpoints are lymphocyte-activation gene 3 (LAG-3), T cell immunoglobulin, and mucin domain-3-containing molecule 3 (TIM-3). They play an important role in T cell regulation and preventing autoimmune disorders. However, in CRC, LAG-3[58] and TIM-3[59] are overexpressed in the CRC microenvironment, which diminishes the effectiveness of T effector cells. Relatlimab is a monoclonal antibody that binds to LAG-3 and restores T cell effectiveness. Numerous clinical trials are currently being conducted on it (NCT03642067 and NCT05328908). IBI104 is a brand-new, highly-promising antibody that inhibits TIM-3-mediated suppressive signaling and as a result is crucial for the regulation of T cells. No research on the role of IBI104 in CRC has been conducted, and it can be added to the list of new explorable T cell boosters in CRC.
T-cell boosting therapies
Adoptive cell transfer therapy: Adoptive cell transfer therapy (ACT) is a cell-based therapy that uses cells from the patient (autologous transfer) or from other donors (allogeneic transfer) to improve immune function[60]. The ACT is performed in three ways: using tumor-infiltrating lymphocytes (TILs), inserting CAR, and modifying TCR. TILs are produced from the tumor-reactive T cells from the patients, expanded ex vivo, and infused in the patients. TILs are capable of identifying several targets in cancer cells. Numerous active clinical trials are investigating the potential of TILs to treat CRC (NCT01174121, NCT03935893, and NCT03610490). However, a significant shortcoming of ACT-TIL is the inability to produce tumor-specific T cells when employing patients’ own TIL.
Another ACT is CAR-T cells, in which T cells are derived from a patient or donor and fused with variable antibody fragments specific to the target antigen[61]. T cells are taken from a patient (autologous) or human leucocyte antigen-matched donor (allogeneic), cultivated ex vivo, and genetically transformed as CAR-T cells by inserting the CAR onto the T cells in the CAR approach. The main target of CAR-T cells are carcinoembryonic antigens (CEA), guanylyl cyclase C[62,63], tumor-associated glycoprotein 72[64], epithelial cell adhesion molecule[65], and major histocompatibility complex class I-related chain A and B. A clinical trial is ongoing targeting CEA to treat CRC liver metastases (NCT05240950).
Another newly developed ACT strategy includes altering TCR. Although it appears similar to CAR-T cells, its antigen-recognition processes are different. ACT, like many other approaches, has many drawbacks. Due to the necessity to create tumor-specific lymphocytes for each patient, this strategy is technically and economically demanding for both the business and patients. Second, the allogeneic transplant patient is frequently at risk of graft-versus-host disease. Although ACT offers considerable therapeutic promise, several issues still need to be carefully considered.
Vaccines: Cancer vaccines elicit an immune response by exposing tumor-associated antigens (whether in free form or expressed on the cell surface) to the immune system, leading to immune cell-mediated cancer cell death. Several clinical trials have been conducted over the past two decades introducing novel vaccines against CRC and other carcinomas[66]. Several possible tumor-associated antigens have been targeted over the past decades against CRC, such as squamous cell carcinoma antigen identified by T cells 3[67], survivin[68], CEA[69], melanoma-associated antigen[70], translocase of outer mitochondrial membrane 34[71], insulin-like growth factor–II mRNA binding protein 3[72], EGFR[73], transmembrane 4 superfamily member 5 protein[74], VEGFR1, Wilms tumor 1 protein[75], and ring finger protein 43[76]. These vaccines activate local immune cells by releasing tumor antigens and increase immune cell, like T cells and dendritic cells, infiltration to the site of action[77].
Several vaccination strategies have been developed against cancers based on antigenic composition. Depending upon the molecular composition of the antigen, vaccine methods can be classified as molecular-based, cell-based, and vector-based. Molecular-based vaccines consist of full-length peptides and DNA and mRNA vaccines. The CRC clinical trials showed that the mRNA and DNA vaccines have demonstrated impressive antitumor response with remarkable efficacy and safety profiles compared to the peptide vaccines. Cell-based vaccines consist of native and genetically modified tumor cells and activated dendritic cell vaccines. These vaccine strategies have reported limited efficacy in several trials but have shown potential efficacy in combination with chemotherapy and immunotherapy[78,79].
Live attenuated viruses, yeasts, and bacterial vectors are vector-based vaccines. The oncolytic viral vectors are used for the live attenuation virus vaccine strategy; however, due to increased immunogenicity, the clinical efficacy remains limited. The live attenuated bacterial and yeast vector-based vaccine are the emerging option for therapeutic vaccines against CRC[80]. These different type of vaccines discussed above have been evaluated in several clinical trials in the past decade, listed in Table 2.
Role of cytokines in CRC: Various cells, primarily immune cells (T cells, neutrophils, and macrophages), endothelial cells, fibroblasts, and other stromal cells, secrete cytokines[81]. Due to the complex network of cytokines in immune responses, cytokine-based medications are a complex task requiring a thorough understanding of cytokine biology and modern biotechnology to maximize antitumor activity while minimizing toxicity. Many cytokines therapy trials are currently ongoing for various cancer[82], but for CRC, there is a need for preclinical studies to assess their unexpected toxicity. From a future perspective, cytokines can be essential molecules because of their capacity to increase and reactivate natural killer (NK) cells and T lymphocytes, encourage lymphocyte infiltration of tumors, and persist in the tumor microenvironment (TME).
Despite the fact that immunotherapy is a cutting-edge and intriguing cancer treatment method, it comes with long-term effects and shortcomings. The main limitation of monoclonal antibodies is the decrease in efficacy because of the short half-life[83]. Immune checkpoints defend the body against infections and autoimmune diseases to maintain immune system equilibrium. ICIs induce the activation of T lymphocytes. However, sustained overactivation of these cells causes immune-related side effects including systemic toxicity[84]. ICIs do not elicit a response in all patients as their T cells are not potent enough to recognize malignant cells. Immunotherapy that boosts T cells does not work against solid tumor cells. Immunosuppressive cells that release chemokines like CXCL1, CXCL5, and others are prevalent in solid tumor cells, and they also have a lot of fibrous matrix[85,86].
CAR-T cells are not very effective in infiltration of solid tumor cells because of their architecture[87]. For each patient using the ACT approach, tumor-specific lymphocytes had to be produced. As a result, it is difficult financially and technically for both the industry and the patients. In the long run, the patients are at a significant risk of developing cytotoxicity, such as B cell hyperplasia, cytokine release syndrome, and graft-versus-host disease[83].
In cases of vaccination, several trials have reported limited efficacy. This is due to the rejection upon identification as a foreign material[88]. In cytokine base therapy, increased levels of cytokines like interleukin-6 and interferon-γ in the bloodstream cause life-threatening poisoning. High toxicity and excessive immune response are caused by immunological signaling and immune cell stimulation brought on by an excess of cytokines[89]. When cytokines are used in excess for an extended period of time, neurological issues like hallucinations, comas, seizures, and verbal difficulties can also arise. Cytotoxicity and a cytokine storm are caused by them[83].
Other approaches
Non-coding RNA-mediated therapy: According to high-throughput genome-scale research, 5%-10% of the transcribed sequences are translated into mRNA or non-coding RNA (ncRNA), out of which only 1% is composed of protein-coding genes and the remaining 4%-9% is transcribed into ncRNAs. As a result, ncRNAs make up a sizable fraction of all RNA molecules[90]. Short regulatory ncRNAs, such as miRNAs and siRNAs, were first identified for their function and clinical significance. The regulatory functions of miRNAs in nearly all physiological and pathological processes in the body, including carcinogenesis, have been widely acknowledged. Several miRNAs are dysregulated in plasma samples of CRC patients; therefore, they have good diagnostic value for CRC screening[90].
Apart from tumor diagnosis and prognosis, ncRNAs can be interesting therapeutic targets for CRC given their tumor suppressive and oncogenic properties. Some ncRNAs play a crucial role in tumorigenesis by losing their tumor suppressive activity, and some are aberrantly overexpressed in CRC, contributing to the oncogenic activity. Therefore, these two mechanisms are used for the development of ncRNA-oriented therapies[91]. Over the past two decades, several synthetic miRNAs and siRNAs have been administered to animal models of various diseases. However, the proper delivery of synthetic miRNAs and siRNAs at the required site of action remains questionable. Several difficulties, including the inability of negatively charged genes to enter negatively charged cellular membranes, gene destruction by plasma nucleases, non-specificity towards targeted cells, and other issues, make in vivo direct administration of naked therapeutic genes impractical and challenging. Therefore several drug delivery strategies such as gene transfection with the help of viral or non-viral vectors, nanodelivery system, and others are safe and effective for delivery of siRNAs and miRNAs to the site of action[92].
Ever since the discovery of the potential role of miRNAs as therapeutic targets, several studies have been conducted aimed at various mechanisms such as miRNA-mediated chemosensitization and miRNA mimicking for tumor suppression and oncogenic miRNA targeting[93]. miRNA/siRNA combinatorial therapy with various chemotherapeutic and immunotherapeutic agents has increased the sensitivity of these drugs to cancers[94]. However, most of the studies performed on CRC are limited to animal models and CRC cell lines, and none were extrapolated to CRC patients[95]. Despite the advances in research, no miRNA or siRNA therapy are FDA approved for clinical usage against CRC or any other cancer, but several are in clinical trials (Table 3).
Table 3.
microRNAs and small interfering RNAs as therapeutics for colorectal cancer in clinical trials
|
Therapeutic name
|
Target gene/protein
|
Route of administration
|
Phase/status
|
Clinical trial identifier
|
Outcome
|
| siRNA targeted therapeutics | |||||
| ALN-VSP02 | VEGF, KSP | Systemic | Phase I (2011)/terminated | NCT00882180 | It was well-tolerated and had antitumor activity |
| IV infusion | Phase I (2012)/completed | NCT01158079 | |||
| Atu027 | PKN3 | Systemic | Phase I (2012) | NCT00938574 | It was safe in patients with advanced solid tumors |
| IV infusion | Phase I/II (2016)/completed | NCT01808638 | |||
| CALAA-01 | RRM 2 | Systemic; IV infusion | Phase I (2013)/terminated | NCT00689065 | It was well tolerated during the initial dose escalation portion of the phase Ia study |
| siRNA-EphA2-DOPC | EphA2 | Systemic; IV infusion | Phase I 2015/active | NCT01591356 | It was well tolerated at all doses tested in preclinical studies |
| TKM-PLK1 (TKM-080301) | PLK-1 | Systemic; IV infusion | Phase I/II (2016)/completed | NCT02191878 | It was tolerated and showed preliminary antitumor efficacy |
| DCR-MYC (DCRM1711) | MYC | Systemic | Phase I (2017) | NCT02110563 | It was well tolerated and showed promising initial clinical and metabolic responses across various dose levels |
| IV infusion | Phase Ib/2 (2016)/terminated | NCT02314052 | |||
| NBF-006 | GSTP | Systemic; IV infusion | Phase1 (2019)/active | NCT03819387 | Significant tumor growth inhibition and overall survival benefit was observed |
| miRNA targeted therapeutics | |||||
| MRX34 | miR-34a mimic | IV infusion | Phase I/terminated-2016. Phase I-II/withdrawn-2016 | NCT01829971 | Unexpected severe immune-mediated toxicities observed |
| NCT02862145 | |||||
DCR-MYC: Lipid nanoparticle-formulated small inhibitory RNA; EphA2: Ephrin type-A receptor 2; GSTP: Glutathione S-transferase pi gene; IV: Intravenous; KSP: Kinesin spindle protein; miRNA: MicroRNA; PKN3: Protein kinase N3; PLK-1: Polo-like kinase 1; RRM2: Ribonucleotide reductase regulatory subunit M2; siRNA: Small interfering RNA; VEGF: Vascular endothelial growth factor.
Oncolytic viral therapy: Oncolytic viral therapy or immuno-oncolytic virotherapy employs native or sometimes genetically modified viruses called oncolytic viruses to predominantly infect cancerous cells while avoiding healthy ones[96]. It has emerged as a promising treatment option for CRC. Oncolytic viruses are thought to exert antitumor activity via two distinct mechanisms: induction of systemic antitumor immunity and selective replication within neoplastic cells that have a direct lytic effect on the tumor cells. The specificity of oncolytic viruses for tumors can be increased by selective genetic modifications such as the expression of viral surface proteins that bind to cellular receptors found only on cancerous cells, deletion of virus fatal genes, and the addition of different immunostimulatory genes[97].
In past decades, several oncolytic viruses have been evaluated experimentally against CRC, including adenovirus, vaccinia virus, herpes simplex virus, vesicular stomatitis virus, measles virus, tanapox virus, echovirus, reovirus, and Newcastle disease virus. Several oncolytic viruses were also tested clinically in CRC patients as monotherapy, and combinatorial therapy of oncolytic viruses with chemotherapeutics[98], radiotherapy[99], and immunotherapeutics have also been examined against CRC[100]. In the first phase I/II trial, patients with refractory CRC treated with herpes simplex mutant NV120 had good safety profiles and increased chemosensitization[101]. A phase I clinical trial of an oncolytic Western Reserve strain genetically modified with deletion of vaccinia growth factor and thymidine kinase (vaccinia virus mutant) had shown an excellent safety profile in 17 patients with advanced and refractory CRC. However, only 1 patient experienced a true benefit from the therapy[102]. This therapy was intratumoral, and another trial with the same agent, when given intravenously in 11 patients, had established safety but limited antitumor activity[103].
A phase II clinical trial of FOLFOX/bevacizumab with or without an oncolytic reovirus pelareorep in 103 patients with mCRC showed a significantly improved response (combined therapy); however, the OS was poor[104]. In another case, patients with stage IV CRC treated with a combined regimen of oncolytic enterovirus, FOLFOX/bevacizumab, and surgical resection (RIGVIR) had impressive efficacy with complete remission for 7.7 years after diagnosis[105]. Despite the intensive research in this field, none of the oncolytic viral therapy is FDA approved for routine clinical use against CRC. Challenges like evading host antiviral immunity and the successful delivery of the virus to the target site cause a hindrance to achieve the optimal antitumor activity of oncolytic viruses. However, several promising strategies like using stem cells or immune cells as carriers are in the pipeline and may increase the antitumor activity of oncolytic viruses.
Cancer stage specific therapy: The cancer stage determines how it will be treated, as given in Figure 2, although other aspects may also be significant. Stage 0 CRC does not spread past the inner lining of the colon; therefore, a colonoscope can be used most of the time to remove the polyp. A partial colectomy can be performed if the cancer is too large to be eliminated by local excision[106]. Many cancers that were a remnant of a polyp are included in stage I and have penetrated further into the layers of the colon wall. They have not migrated to surrounding lymph nodes or outside of the colon wall itself. The complete removal of the cancerous polyp may not require further treatment; however, if the polyp is high-grade, more surgery may be recommended. If the cancer is not generated from the polyp, partial colectomy is the standard treatment[107].
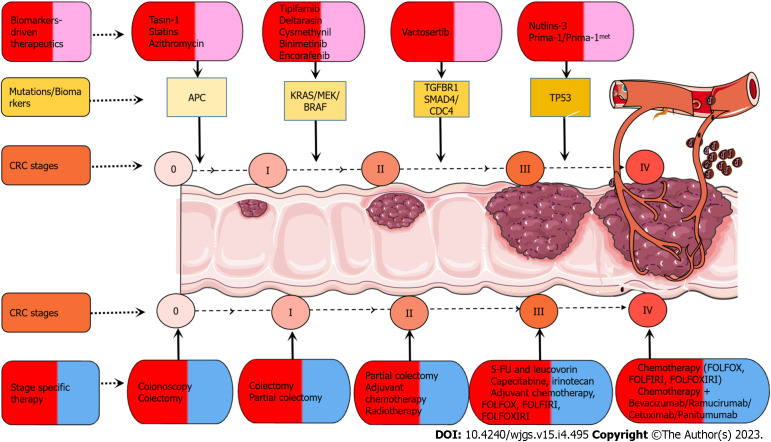
Figure 2.
Biomarker-driven therapeutics and cancer stage specific therapeutic strategies. Drugs like tasin-1, statins, and azithromycin specifically target adenomatous polyposis coli (APC). Drugs such as tipifarnib, deltarasin, and cysmethynil target KRAS mutations in colorectal cancer (CRC), and binimetinib and encorafenib target BRAF-V600 mutations in CRC. Vactosertib is a potent and selective transforming growth factor-beta receptor I kinase inhibitor. Small molecule nutlins-3 is an MDM2 antagonist blocking the interaction between MDM2 and p53. Small molecules prima-1/prima-1met convert mutant p53 to an active conformation. There are stage-specific therapies for different stages. For stage 0, colonoscopy and colectomy are recommended. For stage I, colectomy and partial colectomy are recommended. For stage II, partial colectomy, adjuvant chemotherapy, and radiotherapy are recommended. For stage III, chemotherapy includes 5-fluorouracil (5-FU), 5-FU/leucovorin, capecitabine, irinotecan, adjuvant chemotherapy, and combinational chemotherapy [5-FU + leucovorin + oxaliplatin (FOLFOX), 5-FU + leucovorin + irinotecan (FOLFIRI), 5-FU + leucovorin + oxaliplatin + irinotecan (FOLFOXIRI)]. For stage IV, chemotherapy, combinational chemotherapy, and immunotherapy include bevacizumab, ramucirumab, cetuximab, and panitumumab.
Stage II CRC has penetrated the wall of the colon and adjacent tissue but has not reached the lymph nodes. These require partial colectomy followed by adjuvant chemotherapy and/or radiotherapy to reduce the risk of relapse. However, adjuvant chemotherapy is not always used for stage II cancers due to severe adverse effects. Stage III CRC has penetrated the adjacent lymph nodes but has not migrated to the other body parts. The partial colectomy and adjoining lymph nodes followed by chemotherapy (FOLFOX or CAPOX) is the standard treatment regimen for this stage of CRC.
Cancers in stage IV have migrated to distant organs and tissues from the colon. The liver is where colon cancer spreads most frequently, although it can also affect the lungs, brain, peritoneum (the lining of the abdominal cavity), or distant lymph nodes. The treatment depends upon the severity of metastases. If the cancer is spread to a few small areas, it can be removed by surgery along with partial colectomy. If the metastases spread to many of the organs, chemotherapy is the primary treatment, and surgery may be an option if the tumor size shrinks[108,109].
Natural products for CRC treatment: Recent studies have uncovered the importance of numerous natural products as anticancerous agents because it enhances their quality of life due to their low toxicity and long-lasting nature. Numerous chemotherapeutic agents (almost 50%-60%) are well studied that are obtained from animals, plants, microorganisms, and marine microorganisms to exert anti-cancerous effects[110]. Some of the important natural anticancer agents against CRC are given in Table 1. These natural products are categorized as alkaloids, polysaccharides, polyphenols, terpenoids, and polyunsaturated fatty acids based on their chemical makeup.
Berberine is an isoquinoline natural alkaloid derived from the root, rhizomes, stem, and bark of various plants like Berberis vulgaris, European barberry, Oregon grape, and tree turmeric. Berberine inhibits nuclear factor-kappa B and Wnt/β-catenin signaling pathways and exerts an antiproliferative and antiapoptotic effect in cancer. It negatively regulates the action of arylamine N-acetyltransferase in a colon cancer cell line[111] and alters drug resistance by modulating pgp-10 expression in cancer cells[112].
Another alkaloid is irinotecan (CPT-11), a hydrophilic compound derived from camptothecin, which blocks RNA synthesis and prevents DNA synthesis by inhibiting DNA topoisomerase I[113]. CPT-11 effects have been enhanced in CRC when combined with 5-FU/formyltetrahydrofolate[114]. Piperine, an alkaloid of black pepper, enhances the bioavailability, increases absorption, and has favorable effects on the brush border epithelium ultrastructure[115]. Piperine arrests the cell cycle in the G1 phase by inhibiting the expression of cyclin D1 and D3 as well as their dependent kinases 4 and 6 in HT-29 colon carcinoma[116].
Some polysaccharides with anticancer properties, like fucoidan, obtained from seaweed, are a sulfated polysaccharides. Fucoidan plays a major role in suppressing the toxicity of anticancerous drugs in CRC[117]. Many polyphenols also play a significant role in preventing gastrointestinal malignancies. Curcumin is the most common polyphenol downregulating the gene products implicated in antiapoptosis, cell proliferation, invasion, and angiogenesis[118]. Curcumin shows anticancerous activities in in vitro (breast, cervical, colon, gastric, hepatic, leukemia, oral epithelial, ovarian, pancreatic, and prostate cancer cell lines) and preclinical animal models. Five studies of the anticancerous activities of curcumin in CRC were in phase I clinical trials. Each clinical trial found that curcumin is risk-free and has few side effects[119].
Gingerol is another potent polyphenol with various properties like antiemetic, antihyperlipidemic, anti-inflammatory, antioxidant, antiulcer, antihypertensive, immuno-stimulant, and cardiotonic. It regulates multiple cell signaling pathways and their constituents like AP-1, growth factors, p53, COX-2, mitogen-activated protein kinase (MAPK), VEGF, nuclear factor-kappaB, and cyclin D1, which further contribute to cancer initiation and progression[120,121]. In the human colon cancer cell line LoVo, 6-gingerol in a dose-dependent manner reduced the cell viability by arresting the cell cycle in the G2/M phase[122]. A phase II study revealed that andrographolide, a terpenoid, activates various proapoptotic signaling cascades and induces apoptosis (NCT01993472). Polyunsaturated fatty acids such as eicosapentaenoic acid and docosahexaenoic acid have shown the ability to treat malignancies including CRC, breast cancer, and pre-existing adenocarcinoma[123].
Role of probiotics in CRC: Probiotics are becoming progressively significant in basic and clinical examination. Studies have established a substantial relationship between probiotics and CRC and how some strains of good bacteria, i.e. probiotics, can have a therapeutic and preventive effect against CRC. Studies have shown that probiotics can also lower secondary complications arising from chemotherapies. In a CRC animal model, probiotic supplementation diminished the formation of aberrant crypts, improving the antitumor impact of 5-FU chemotherapy.
Probiotics can influence intestinal physiology either directly or indirectly by modulating endogenous microflora. Previously, researchers cocultured HT29 colorectal carcinoma cells with Propionibacterium acidipropionici strain CNRZ80, Propionibacterium freudenreichii subsp. Freudenreichii strain ITG18, and Propionibacterium freudenreichii subsp. Shermanii strain SI41. These strains were found to have a potent cytotoxic effect[124]. Another great bacterial variety, Lactobacillus, is a gut-resident probiotic that benefits host health. A study on Lactobacillus casei BL23 significantly protected mice against CRC development; specifically, Lactobacillus casei BL23 treatment reduced histological scores and proliferative index values[125]. Another in vivo study with Lactobacillus plantarum strains YYC-3 and YYCS prevented the occurrence of colon tumors and mucosal damage in APCMin/+ mice fed a high-fat diet. However, YYC-3 had a more robust anticancer effect[126].
Some anaerobic microscopic organisms process prebiotics like oligosaccharides into short-chain fatty acids. Short-chain fatty acids, such as acetate, propionate, and butyrate, have many beneficial effects like improving tight junctions, antiproliferative function[127], and anti-inflammatory function by stimulating immunosuppressive cytokines such as interleukin-10[128]. According to the findings, administering specific fecal bacteria, also known as fecal microbiota transplantation (FMT), into a patient’s colon may improve a the response to immunosuppressive medications that increase the capacity of the immune system to identify and eradicate tumor cells. Numerous clinical trials have shown cure rates of about 90% when repeated FMTs are included for the treatment of recurrent Clostridioides difficile infection[129,130]. By replacing CRC-associated dysbiosis and restoring eubiosis in chronic illnesses, the adoption of FMT protocols could lessen the activation of inflammatory, proliferative, and procarcinogenic pathways as well as microbiota-induced genotoxicity. Even though fecal transplantation has not been well investigated in CRC, future studies will significantly advance this field of study[131].
Biomarker-driven therapy: Conventional drugs are less effective, non-specific, and have more secondary consequences than benefits. Therefore, it is necessary to focus research on a different direction. The following signaling pathways in CRC are dysregulated: APC (Wnt pathway), RAS, RAF, KRAS, RET, MAPK, P53, and SMAD. Figure 2 lists therapeutic agents that target these mutations at particular stages of CRC. CRC shows a sequence of mutations. The very first step in the chromosomal instability pathway begins with the APC mutations. APC is a tumor suppressor protein that leads to familial and sporadic types of CRC. It coordinates with axin, β-catenin, and glycogen synthase kinase-3 β and regulates β-catenin in the Wnt signaling pathway. Tasin-1 and statins are drugs that target APC. Tasin-1 induces endoplasmic reticulum stress-dependent jun N-terminal kinase activation and apoptosis in mutated APC human colon cancer cells. It also suppresses AKT activity in a cholesterol-dependent manner[132]. APC mutations are followed by the mutational activation of oncogene KRAS and SMAD-4 and inactivation of tumor suppressor gene p53. About 40%-50% of CRC patients have KRAS mutations.
Mutated RAS enhances the activation of downstream signaling pathway molecules like RAF and MAP kinase. It leads to a malfunctioning GTPase activity and more frequently affects exons 2, 33, and 34. Drugs like tipifarnib, deltarasin, and cysmethynil target RAS and KRAS mutations in CRC. Cysmethynil inhibits cell growth in the colon cancer cell line in an isoprenylcysteine carboxyl methyltransferase-dependent fashion[133]. SMAD4 is a tumor suppressor protein and an important molecule of transforming growth factor-beta. Truncated and mutated SMAD-4 is involved in tumor progression and metastasis. It promotes distant metastasis as compared to lymphatic metastasis of CRC[134]. SMAD-4 is targeted via the drug vactosertib. In phase I/II (NCT03724851) trials, vactosertib in combination with pembrolizumab significantly decreased the transforming growth factor-beta-related vactosertib responsive gene signature, and the extent of decrease was more significant in responders compared to non-responders[135].
Mutation in p53 contributes to 35%-40% of sporadic CRC and almost half in all other cancers[136]. p53 is a tumor suppressor protein involved in various processes like cell cycle, apoptosis, and angiogenesis regulation. A preclinical study on nutlins-3, an Mdm2 inhibitor in the mouse azoxymethane colon cancer model, reduced both cell proliferation and apoptosis[137]. A study on a small molecule PRIMA-1MET, a mutant p53 reactivator, has shown promising results in restoring the wild-type structure of p53 and inducing massive p53-dependent apoptosis[138].
The CpG island methylator phenotype route of CRC pathogenesis is linked to BRAF hypermethylation, which results in truncated BRAF. BRAF is stimulated by RAS and involved in various processes like apoptosis, cell growth, cell proliferation, cell differentiation, cell survival, and cell migration. This mutation is 8%-10% in CRC patients. This is important for the EGFR-mediated cell signaling pathway, MAP kinase pathway, and Bcl-2 expression. The truncated BRAF is targeted by vemurafenib and encorafenib drugs, which reduce signaling through the aberrant MAPK pathway. A phase II study (NCT02164916) has shown that addition of vemurafenib improved PFS and the primary endpoint (HR: 0.50, P = 0.001)[139]. In the phase III CRC study (NCT02928224), encorafenib plus cetuximab improved OS, objective RR, and PFS in previously treated patients in the metastatic setting compared with standard chemotherapy[140].
Most promising therapy among new therapeutic approaches
The aforementioned strategies are innovative methods for creating contemporary cancer treatments, and they are characterized by benefits, including specificity, effectiveness, minimal to no side effects, a higher survival rate, and a lower risk of CRC recurrence. Based on their similarities and differences, these strategies might be grouped. Natural products, probiotics, and oncolytic viral treatments have various anticancer qualities and can be given at any stage of CRC, which is one similarity. However, biomarker-driven therapy and RNA-based therapy target specific molecular entities depending on their expression at a particular stage, making them extremely stage-specific.
The function of therapy, whether diagnostic, prognostic, preventative, or therapeutic, is another commonality in the similarity index. Probiotics and naturopathic remedies serve largely as preventative[141]. However, RNA-based therapy, oncolytic viral therapy, and biomarker-driven therapy have therapeutic potential[93,98-100,142,143]. In addition, RNA-based methods can be used for prognostic and diagnostic purposes[144]. These methods also differ in how they work; for example, certain natural products and RNA-based therapies work at the mRNA level to halt translation by degrading mRNA. Natural products also have several anticancer properties, such as antiproliferative, antiapoptotic, and RNA/DNA synthesis prevention. On the other hand, oncolytic viral medicines cause cellular lysis by inducing systemic antitumor immunity and selective replication within neoplastic cells, which have a lytic effect on the tumor cells. Additionally, probiotics play a preventative role by enhancing tight junction and antiproliferative activity.
Overall, each strategy has its benefits and drawbacks. But RNA-based treatments have drawn more attention, primarily because of their versatility in modifying a wide range of targets and their capacity to target disease genes that were previously inaccessible for manipulation. The inability of the negatively charged RNA to enter negatively charged cellular membranes; poor cellular uptake, non-specificity, and RNA destruction by nucleases in the plasma are a few difficulties that must be addressed[145]. Advances in medicinal chemistry and nanotechnology will help to solve those issues, and RNA-based therapies will become more widely adopted. RNA-based therapies and oncolytic viral therapies hold the most promising potential. One of the main advantages of oncolytic viruses are they can be engineered to target specific tumor cells without damaging healthy cells. The specificity of oncolytic viruses for tumors can be increased by selective genetic modifications such as the expression of viral surface proteins that bind to cellular receptors found only on cancerous cells, deletion of virus fatal genes, and addition of different immunostimulatory genes[97].
CONCLUSION
Conclusion and future directions
Human genomic, transcriptional, proteomic, and epigenetic information has never been more accessible than now thanks to advances in medical sciences and electronics. Since every patient’s TME is unique, a single CRC treatment strategy was never an option. Moreover, individualized therapeutic approaches are required due to tissue heterogeneity. Although conventional cytotoxic drugs are always the first line of treatment for solid tumors, drug resistance causes patients to develop incurable recurring CRC. As a result of these shortcomings, the development of novel approaches with significant benefits and minimal drawbacks as future perspectives is required. Radiotherapy is also a promising option for rectal cancer patients. But, it has some plausible and long-term toxicity effects on vital organs that must be overcome by modifying radiation intensities[12].
Chemotherapy has become the mainstay of CRC treatment due to studies conducted in recent decades showing that utilizing it has increased the OS time of CRC patients, particularly those with metastases, to approximately 20 mo[20]. Chemotherapy has several drawbacks, including systemic toxicity, an unsatisfactory RR, fever, mucositis, stomatitis, leukopenia and thrombocytopenia[21], and a lack of tumor-specific selectivity. Recently, this has led to the idea of molecular targeted therapy for CRC.
Various monoclonal antibodies against angiogenic factors/receptors have been developed, including anti-EGFR/EGF, anti-VEGFR/VEGF, and ICIs (CTLA-4, PD-1/PD-L1, TIM-3, anti-LAG-3), which act directly on cancer cells by boosting immune cells such as T cells and NK cells. The impediment of antiangiogenic treatments is that they make cancers impervious to them. Tumor-associated fibroblasts continuously secrete proangiogenic growth factors like EGF, insulin-like growth factor, and particularly platelets-derived growth factor-C, which is a key factor for maintaining angiogenesis even in the presence of antiangiogenic therapies[146]. To address these issues and treat this fatal cancer, more target-specific treatment methods, such as immunotherapeutic and target-specific approaches, including ACT therapies[60], vaccines, and cytokine-based therapies[66], are required. ACT, vaccines, and cytokine-based therapies can defeat the inadequacies raised because of chemotherapeutic drugs as they have the ability to increase and reactivate effector NK and T lymphocytes, promote lymphocyte infiltration of tumors, and persist in the TME. Therapeutic cancer vaccines have the potential to be as effective as monotherapies when used in premalignant cancer and carcinoma in situ. Many cytokine therapy trials are currently ongoing for various cancers[82]. But for CRC, there is a need for preclinical studies to assess their unexpected toxicity.
Along with the aforementioned approaches, new emerging approaches for treating CRC, including RNA interference, oncolytic viral therapy, use of natural products, probiotics, and biomarker-driven therapy, have shown promising results. RNA interference has been proposed as another remedial approach that offers significant benefits over customary medicines, with high explicitness and intensity and low toxicity[147]. Utilizing genetically altered oncolytic viruses is another remedial strategy. Currently, only one oncolytic virus therapy has been FDA approved to treat cancer. T-VEC (Imlygic®) is a modified herpes simplex virus that targets tumor cells and aids in their demise. It has been approved for specific melanoma patient subsets. Numerous studies have shown that many natural products have potent anti-CRC effects and could replace chemotherapy agents in treating CRC.
Furthermore, the composition of microbiota appears to influence the development of CRC. As per scientific literature, utilizing probiotics can assist with forestalling CRC growth[148] by utilizing anticarcinogenic activity via potential biologically host-dependent and strain-specific physiological mechanisms[141]. The time has come to modify the available therapeutic approaches and foster novel methodologies with negligible drawbacks and higher advantages for CRC treatments.
Footnotes
Conflict-of-interest statement: All the authors report no relevant conflicts of interest for this article.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: October 21, 2022
First decision: January 3, 2023
Article in press: March 3, 2023
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Luo W, China; Wang LH, China S-Editor: Fan JR L-Editor: Filipodia P-Editor: Fan JR
Contributor Information
Anil Kumar, Department of Pharmacology, Post Graduate Institute of Medical Education and Research, Chandigarh 160012, India.
Vipasha Gautam, Department of Pharmacology, Post Graduate Institute of Medical Education and Research, Chandigarh 160012, India.
Arushi Sandhu, Department of Pharmacology, Post Graduate Institute of Medical Education and Research, Chandigarh 160012, India.
Kajal Rawat, Department of Pharmacology, Post Graduate Institute of Medical Education and Research, Chandigarh 160012, India.
Antika Sharma, Department of Pharmacology, Post Graduate Institute of Medical Education and Research, Chandigarh 160012, India.
Lekha Saha, Department of Pharmacology, Post Graduate Institute of Medical Education and Research, Chandigarh 160012, India. lekhasaha@rediffmail.com.
References
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin. 2021;71:7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 2.Sawicki T, Ruszkowska M, Danielewicz A, Niedźwiedzka E, Arłukowicz T, Przybyłowicz KE. A Review of Colorectal Cancer in Terms of Epidemiology, Risk Factors, Development, Symptoms and Diagnosis. Cancers (Basel) 2021;13 doi: 10.3390/cancers13092025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schuell B, Gruenberger T, Kornek GV, Dworan N, Depisch D, Lang F, Schneeweiss B, Scheithauer W. Side effects during chemotherapy predict tumour response in advanced colorectal cancer. Br J Cancer. 2005;93:744–748. doi: 10.1038/sj.bjc.6602783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou J, Ji Q, Li Q. Resistance to anti-EGFR therapies in metastatic colorectal cancer: underlying mechanisms and reversal strategies. J Exp Clin Cancer Res. 2021;40:328. doi: 10.1186/s13046-021-02130-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ding S, Hu C, Fang J, Liu G. The Protective Role of Probiotics against Colorectal Cancer. Oxid Med Cell Longev. 2020;2020:8884583. doi: 10.1155/2020/8884583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu J, Guo B. RNA-based therapeutics for colorectal cancer: Updates and future directions. Pharmacol Res. 2020;152:104550. doi: 10.1016/j.phrs.2019.104550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fukuhara H, Ino Y, Todo T. Oncolytic virus therapy: A new era of cancer treatment at dawn. Cancer Sci. 2016;107:1373–1379. doi: 10.1111/cas.13027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rajamanickam S, Agarwal R. Natural products and colon cancer: current status and future prospects. Drug Dev Res. 2008;69:460–471. doi: 10.1002/ddr.20276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van de Velde CJ, Boelens PG, Borras JM, Coebergh JW, Cervantes A, Blomqvist L, Beets-Tan RG, van den Broek CB, Brown G, Van Cutsem E, Espin E, Haustermans K, Glimelius B, Iversen LH, van Krieken JH, Marijnen CA, Henning G, Gore-Booth J, Meldolesi E, Mroczkowski P, Nagtegaal I, Naredi P, Ortiz H, Påhlman L, Quirke P, Rödel C, Roth A, Rutten H, Schmoll HJ, Smith JJ, Tanis PJ, Taylor C, Wibe A, Wiggers T, Gambacorta MA, Aristei C, Valentini V. EURECCA colorectal: multidisciplinary management: European consensus conference colon & rectum. Eur J Cancer. 2014;50:1.e1–1.e34. doi: 10.1016/j.ejca.2013.06.048. [DOI] [PubMed] [Google Scholar]
- 10.van Gijn W, Marijnen CA, Nagtegaal ID, Kranenbarg EM, Putter H, Wiggers T, Rutten HJ, Påhlman L, Glimelius B, van de Velde CJ Dutch Colorectal Cancer Group. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol. 2011;12:575–582. doi: 10.1016/S1470-2045(11)70097-3. [DOI] [PubMed] [Google Scholar]
- 11.Ngan SY, Burmeister B, Fisher RJ, Solomon M, Goldstein D, Joseph D, Ackland SP, Schache D, McClure B, McLachlan SA, McKendrick J, Leong T, Hartopeanu C, Zalcberg J, Mackay J. Randomized trial of short-course radiotherapy versus long-course chemoradiation comparing rates of local recurrence in patients with T3 rectal cancer: Trans-Tasman Radiation Oncology Group trial 01.04. J Clin Oncol. 2012;30:3827–3833. doi: 10.1200/JCO.2012.42.9597. [DOI] [PubMed] [Google Scholar]
- 12.Dröge LH, Weber HE, Guhlich M, Leu M, Conradi LC, Gaedcke J, Hennies S, Herrmann MK, Rave-Fränk M, Wolff HA. Reduced toxicity in the treatment of locally advanced rectal cancer: a comparison of volumetric modulated arc therapy and 3D conformal radiotherapy. BMC Cancer. 2015;15:750. doi: 10.1186/s12885-015-1812-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Samuelian JM, Callister MD, Ashman JB, Young-Fadok TM, Borad MJ, Gunderson LL. Reduced acute bowel toxicity in patients treated with intensity-modulated radiotherapy for rectal cancer. Int J Radiat Oncol Biol Phys. 2012;82:1981–1987. doi: 10.1016/j.ijrobp.2011.01.051. [DOI] [PubMed] [Google Scholar]
- 14.Jabbour SK, Patel S, Herman JM, Wild A, Nagda SN, Altoos T, Tunceroglu A, Azad N, Gearheart S, Moss RA, Poplin E, Levinson LL, Chandra RA, Moore DF, Chen C, Haffty BG, Tuli R. Intensity-modulated radiation therapy for rectal carcinoma can reduce treatment breaks and emergency department visits. Int J Surg Oncol. 2012;2012:891067. doi: 10.1155/2012/891067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hanna CR, Slevin F, Appelt A, Beavon M, Adams R, Arthur C, Beasley M, Duffton A, Gilbert A, Gollins S, Harrison M, Hawkins MA, Laws K, O'Cathail S, Porcu P, Robinson M, Sebag-Montefiore D, Teo M, Teoh S, Muirhead R. Intensity-modulated Radiotherapy for Rectal Cancer in the UK in 2020. Clin Oncol (R Coll Radiol) 2021;33:214–223. doi: 10.1016/j.clon.2020.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moertel CG, Fleming TR, Macdonald JS, Haller DG, Laurie JA, Goodman PJ, Ungerleider JS, Emerson WA, Tormey DC, Glick JH. Levamisole and fluorouracil for adjuvant therapy of resected colon carcinoma. N Engl J Med. 1990;322:352–358. doi: 10.1056/NEJM199002083220602. [DOI] [PubMed] [Google Scholar]
- 17.Santi DV, McHenry CS, Sommer H. Mechanism of interaction of thymidylate synthetase with 5-fluorodeoxyuridylate. Biochemistry. 1974;13:471–481. doi: 10.1021/bi00700a012. [DOI] [PubMed] [Google Scholar]
- 18.Efficacy of adjuvant fluorouracil and folinic acid in colon cancer. International Multicentre Pooled Analysis of Colon Cancer Trials (IMPACT) investigators. Lancet. 1995;345:939–944. [PubMed] [Google Scholar]
- 19.Francini G, Petrioli R, Lorenzini L, Mancini S, Armenio S, Tanzini G, Marsili S, Aquino A, Marzocca G, Civitelli S. Folinic acid and 5-fluorouracil as adjuvant chemotherapy in colon cancer. Gastroenterology. 1994;106:899–906. doi: 10.1016/0016-5085(94)90748-x. [DOI] [PubMed] [Google Scholar]
- 20.Gustavsson B, Carlsson G, Machover D, Petrelli N, Roth A, Schmoll HJ, Tveit KM, Gibson F. A review of the evolution of systemic chemotherapy in the management of colorectal cancer. Clin Colorectal Cancer. 2015;14:1–10. doi: 10.1016/j.clcc.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 21.Evans RM, Laskin JD, Hakala MT. Effect of excess folates and deoxyinosine on the activity and site of action of 5-fluorouracil. Cancer Res. 1981;41:3288–3295. [PubMed] [Google Scholar]
- 22.Latchman J, Guastella A, Tofthagen C. 5-Fluorouracil toxicity and dihydropyrimidine dehydrogenase enzyme: implications for practice. Clin J Oncol Nurs. 2014;18:581–585. doi: 10.1188/14.CJON.581-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Comella P. A review of the role of capecitabine in the treatment of colorectal cancer. Ther Clin Risk Manag. 2007;3:421–431. [PMC free article] [PubMed] [Google Scholar]
- 24.Mayer RJ, Van Cutsem E, Falcone A, Yoshino T, Garcia-Carbonero R, Mizunuma N, Yamazaki K, Shimada Y, Tabernero J, Komatsu Y, Sobrero A, Boucher E, Peeters M, Tran B, Lenz HJ, Zaniboni A, Hochster H, Cleary JM, Prenen H, Benedetti F, Mizuguchi H, Makris L, Ito M, Ohtsu A RECOURSE Study Group. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med. 2015;372:1909–1919. doi: 10.1056/NEJMoa1414325. [DOI] [PubMed] [Google Scholar]
- 25.Modi V, Dunbrack RL Jr. A Structurally-Validated Multiple Sequence Alignment of 497 Human Protein Kinase Domains. Sci Rep. 2019;9:19790. doi: 10.1038/s41598-019-56499-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grothey A, Van Cutsem E, Sobrero A, Siena S, Falcone A, Ychou M, Humblet Y, Bouché O, Mineur L, Barone C, Adenis A, Tabernero J, Yoshino T, Lenz HJ, Goldberg RM, Sargent DJ, Cihon F, Cupit L, Wagner A, Laurent D CORRECT Study Group. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:303–312. doi: 10.1016/S0140-6736(12)61900-X. [DOI] [PubMed] [Google Scholar]
- 27.Li J, Qin S, Xu R, Yau TC, Ma B, Pan H, Xu J, Bai Y, Chi Y, Wang L, Yeh KH, Bi F, Cheng Y, Le AT, Lin JK, Liu T, Ma D, Kappeler C, Kalmus J, Kim TW CONCUR Investigators. Regorafenib plus best supportive care versus placebo plus best supportive care in Asian patients with previously treated metastatic colorectal cancer (CONCUR): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2015;16:619–629. doi: 10.1016/S1470-2045(15)70156-7. [DOI] [PubMed] [Google Scholar]
- 28.Van Cutsem E, Van de Velde C, Roth A, Lordick F, Köhne CH, Cascinu S, Aapro M European Organisation for Research and Treatment of Cancer (EORTC)-gastrointestinal cancer group. Expert opinion on management of gastric and gastro-oesophageal junction adenocarcinoma on behalf of the European Organisation for Research and Treatment of Cancer (EORTC)-gastrointestinal cancer group. Eur J Cancer. 2008;44:182–194. doi: 10.1016/j.ejca.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 29.Arredondo J, Pastor C, Baixauli J, Rodríguez J, González I, Vigil C, Chopitea A, Hernández-Lizoáin JL. Preliminary outcome of a treatment strategy based on perioperative chemotherapy and surgery in patients with locally advanced colon cancer. Colorectal Dis. 2013;15:552–557. doi: 10.1111/codi.12119. [DOI] [PubMed] [Google Scholar]
- 30.Arredondo J, Baixauli J, Pastor C, Chopitea A, Sola JJ, González I, A-Cienfuegos J, Martínez P, Rodriguez J, Hernández-Lizoain JL. Mid-term oncologic outcome of a novel approach for locally advanced colon cancer with neoadjuvant chemotherapy and surgery. Clin Transl Oncol. 2017;19:379–385. doi: 10.1007/s12094-016-1539-4. [DOI] [PubMed] [Google Scholar]
- 31.Zhou H, Song Y, Jiang J, Niu H, Zhao H, Liang J, Su H, Wang Z, Zhou Z, Huang J. A pilot phase II study of neoadjuvant triplet chemotherapy regimen in patients with locally advanced resectable colon cancer. Chin J Cancer Res. 2016;28:598–605. doi: 10.21147/j.issn.1000-9604.2016.06.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roth M, Eng C. Neoadjuvant Chemotherapy for Colon Cancer. Cancers. 2020;12:2368. doi: 10.3390/cancers12092368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xie YH, Chen YX, Fang JY. Comprehensive review of targeted therapy for colorectal cancer. Signal Transduct Target Ther. 2020;5:22. doi: 10.1038/s41392-020-0116-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bisschop C, van Dijk TH, Beukema JC, Jansen RLH, Gelderblom H, de Jong KP, Rutten HJT, van de Velde CJH, Wiggers T, Havenga K, Hospers GAP. Short-Course Radiotherapy Followed by Neoadjuvant Bevacizumab, Capecitabine, and Oxaliplatin and Subsequent Radical Treatment in Primary Stage IV Rectal Cancer: Long-Term Results of a Phase II Study. Ann Surg Oncol. 2017;24:2632–2638. doi: 10.1245/s10434-017-5897-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Atkins MB, Lotze MT, Dutcher JP, Fisher RI, Weiss G, Margolin K, Abrams J, Sznol M, Parkinson D, Hawkins M, Paradise C, Kunkel L, Rosenberg SA. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol. 1999;17:2105–2116. doi: 10.1200/JCO.1999.17.7.2105. [DOI] [PubMed] [Google Scholar]
- 36.Goldstein D, Laszlo J. Interferon therapy in cancer: from imaginon to interferon. Cancer Res. 1986;46:4315–4329. [PubMed] [Google Scholar]
- 37.Hemmatzadeh M, Mohammadi H, Babaie F, Yousefi M, Ebrazeh M, Mansoori B, Shanehbandi D, Baradaran B. Snail-1 Silencing by siRNA Inhibits Migration of TE-8 Esophageal Cancer Cells Through Downregulation of Metastasis-Related Genes. Adv Pharm Bull. 2018;8:437–445. doi: 10.15171/apb.2018.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mendelsohn J, Prewett M, Rockwell P, Goldstein NI. CCR 20th anniversary commentary: a chimeric antibody, C225, inhibits EGFR activation and tumor growth. Clin Cancer Res. 2015;21:227–229. doi: 10.1158/1078-0432.CCR-14-2491. [DOI] [PubMed] [Google Scholar]
- 39.Jonker DJ, O'Callaghan CJ, Karapetis CS, Zalcberg JR, Tu D, Au HJ, Berry SR, Krahn M, Price T, Simes RJ, Tebbutt NC, van Hazel G, Wierzbicki R, Langer C, Moore MJ. Cetuximab for the treatment of colorectal cancer. N Engl J Med. 2007;357:2040–2048. doi: 10.1056/NEJMoa071834. [DOI] [PubMed] [Google Scholar]
- 40.Van Cutsem E, Köhne CH, Hitre E, Zaluski J, Chang Chien CR, Makhson A, D'Haens G, Pintér T, Lim R, Bodoky G, Roh JK, Folprecht G, Ruff P, Stroh C, Tejpar S, Schlichting M, Nippgen J, Rougier P. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360:1408–1417. doi: 10.1056/NEJMoa0805019. [DOI] [PubMed] [Google Scholar]
- 41.Yarom N, Jonker DJ. The role of the epidermal growth factor receptor in the mechanism and treatment of colorectal cancer. Discov Med. 2011;11:95–105. [PubMed] [Google Scholar]
- 42.Douillard JY, Siena S, Cassidy J, Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham D, Jassem J, Rivera F, Kocákova I, Ruff P, Błasińska-Morawiec M, Šmakal M, Canon JL, Rother M, Oliner KS, Tian Y, Xu F, Sidhu R. Final results from PRIME: randomized phase III study of panitumumab with FOLFOX4 for first-line treatment of metastatic colorectal cancer. Ann Oncol. 2014;25:1346–1355. doi: 10.1093/annonc/mdu141. [DOI] [PubMed] [Google Scholar]
- 43.Douillard JY, Siena S, Cassidy J, Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham D, Jassem J, Rivera F, Kocákova I, Ruff P, Błasińska-Morawiec M, Šmakal M, Canon JL, Rother M, Oliner KS, Wolf M, Gansert J. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol. 2010;28:4697–4705. doi: 10.1200/JCO.2009.27.4860. [DOI] [PubMed] [Google Scholar]
- 44.Elez E, Hendlisz A, Delaunoit T, Sastre J, Cervantes A, Varea R, Chao G, Wallin J, Tabernero J. Phase II study of necitumumab plus modified FOLFOX6 as first-line treatment in patients with locally advanced or metastatic colorectal cancer. Br J Cancer. 2016;114:372–380. doi: 10.1038/bjc.2015.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bennouna J, Deslandres M, Senellart H, de Labareyre C, Ruiz-Soto R, Wixon C, Botbyl J, Suttle AB, Delord JP. A phase I open-label study of the safety, tolerability, and pharmacokinetics of pazopanib in combination with irinotecan and cetuximab for relapsed or refractory metastatic colorectal cancer. Invest New Drugs. 2015;33:138–147. doi: 10.1007/s10637-014-0142-1. [DOI] [PubMed] [Google Scholar]
- 46.Sobrero AF, Maurel J, Fehrenbacher L, Scheithauer W, Abubakr YA, Lutz MP, Vega-Villegas ME, Eng C, Steinhauer EU, Prausova J, Lenz HJ, Borg C, Middleton G, Kröning H, Luppi G, Kisker O, Zubel A, Langer C, Kopit J, Burris HA 3rd. EPIC: phase III trial of cetuximab plus irinotecan after fluoropyrimidine and oxaliplatin failure in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:2311–2319. doi: 10.1200/JCO.2007.13.1193. [DOI] [PubMed] [Google Scholar]
- 47.Cohn AL, Shumaker GC, Khandelwal P, Smith DA, Neubauer MA, Mehta N, Richards D, Watkins DL, Zhang K, Yassine MR. An open-label, single-arm, phase 2 trial of panitumumab plus FOLFIRI as second-line therapy in patients with metastatic colorectal cancer. Clin Colorectal Cancer. 2011;10:171–177. doi: 10.1016/j.clcc.2011.03.022. [DOI] [PubMed] [Google Scholar]
- 48.Chanmee T, Ontong P, Konno K, Itano N. Tumor-associated macrophages as major players in the tumor microenvironment. Cancers (Basel) 2014;6:1670–1690. doi: 10.3390/cancers6031670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015;348:56–61. doi: 10.1126/science.aaa8172. [DOI] [PubMed] [Google Scholar]
- 50.Nishikawa H, Sakaguchi S. Regulatory T cells in tumor immunity. Int J Cancer. 2010;127:759–767. doi: 10.1002/ijc.25429. [DOI] [PubMed] [Google Scholar]
- 51.Overman MJ, McDermott R, Leach JL, Lonardi S, Lenz HJ, Morse MA, Desai J, Hill A, Axelson M, Moss RA, Goldberg MV, Cao ZA, Ledeine JM, Maglinte GA, Kopetz S, André T. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol. 2017;18:1182–1191. doi: 10.1016/S1470-2045(17)30422-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Latchman Y, Wood CR, Chernova T, Chaudhary D, Borde M, Chernova I, Iwai Y, Long AJ, Brown JA, Nunes R, Greenfield EA, Bourque K, Boussiotis VA, Carter LL, Carreno BM, Malenkovich N, Nishimura H, Okazaki T, Honjo T, Sharpe AH, Freeman GJ. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol. 2001;2:261–268. doi: 10.1038/85330. [DOI] [PubMed] [Google Scholar]
- 53.Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC, Horton HF, Fouser L, Carter L, Ling V, Bowman MR, Carreno BM, Collins M, Wood CR, Honjo T. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kitsou M, Ayiomamitis GD, Zaravinos A. High expression of immune checkpoints is associated with the TIL load, mutation rate and patient survival in colorectal cancer. Int J Oncol. 2020;57:237–248. doi: 10.3892/ijo.2020.5062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pernot S, Terme M, Voron T, Colussi O, Marcheteau E, Tartour E, Taieb J. Colorectal cancer and immunity: what we know and perspectives. World J Gastroenterol. 2014;20:3738–3750. doi: 10.3748/wjg.v20.i14.3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, Biedrzycki B, Donehower RC, Zaheer A, Fisher GA, Crocenzi TS, Lee JJ, Duffy SM, Goldberg RM, de la Chapelle A, Koshiji M, Bhaijee F, Huebner T, Hruban RH, Wood LD, Cuka N, Pardoll DM, Papadopoulos N, Kinzler KW, Zhou S, Cornish TC, Taube JM, Anders RA, Eshleman JR, Vogelstein B, Diaz LA Jr. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med. 2015;372:2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lenz HJ, Van Cutsem E, Luisa Limon M, Wong KYM, Hendlisz A, Aglietta M, García-Alfonso P, Neyns B, Luppi G, Cardin DB, Dragovich T, Shah U, Abdullaev S, Gricar J, Ledeine JM, Overman MJ, Lonardi S. First-Line Nivolumab Plus Low-Dose Ipilimumab for Microsatellite Instability-High/Mismatch Repair-Deficient Metastatic Colorectal Cancer: The Phase II CheckMate 142 Study. J Clin Oncol. 2022;40:161–170. doi: 10.1200/JCO.21.01015. [DOI] [PubMed] [Google Scholar]
- 58.Rhyner Agocs G, Assarzadegan N, Kirsch R, Dawson H, Galván JA, Lugli A, Zlobec I, Berger MD. LAG-3 Expression Predicts Outcome in Stage II Colon Cancer. J Pers Med. 2021;11 doi: 10.3390/jpm11080749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xu B, Yuan L, Gao Q, Yuan P, Zhao P, Yuan H, Fan H, Li T, Qin P, Han L, Fang W, Suo Z. Circulating and tumor-infiltrating Tim-3 in patients with colorectal cancer. Oncotarget. 2015;6:20592–20603. doi: 10.18632/oncotarget.4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tran KQ, Zhou J, Durflinger KH, Langhan MM, Shelton TE, Wunderlich JR, Robbins PF, Rosenberg SA, Dudley ME. Minimally cultured tumor-infiltrating lymphocytes display optimal characteristics for adoptive cell therapy. J Immunother. 2008;31:742–751. doi: 10.1097/CJI.0b013e31818403d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ramos CA, Dotti G. Chimeric antigen receptor (CAR)-engineered lymphocytes for cancer therapy. Expert Opin Biol Ther. 2011;11:855–873. doi: 10.1517/14712598.2011.573476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Magee MS, Abraham TS, Baybutt TR, Flickinger JC Jr, Ridge NA, Marszalowicz GP, Prajapati P, Hersperger AR, Waldman SA, Snook AE. Human GUCY2C-Targeted Chimeric Antigen Receptor (CAR)-Expressing T Cells Eliminate Colorectal Cancer Metastases. Cancer Immunol Res. 2018;6:509–516. doi: 10.1158/2326-6066.CIR-16-0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Parkhurst MR, Yang JC, Langan RC, Dudley ME, Nathan DA, Feldman SA, Davis JL, Morgan RA, Merino MJ, Sherry RM, Hughes MS, Kammula US, Phan GQ, Lim RM, Wank SA, Restifo NP, Robbins PF, Laurencot CM, Rosenberg SA. T cells targeting carcinoembryonic antigen can mediate regression of metastatic colorectal cancer but induce severe transient colitis. Mol Ther. 2011;19:620–626. doi: 10.1038/mt.2010.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hege KM, Bergsland EK, Fisher GA, Nemunaitis JJ, Warren RS, McArthur JG, Lin AA, Schlom J, June CH, Sherwin SA. Safety, tumor trafficking and immunogenicity of chimeric antigen receptor (CAR)-T cells specific for TAG-72 in colorectal cancer. J Immunother Cancer. 2017;5:22. doi: 10.1186/s40425-017-0222-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang BL, Li D, Gong YL, Huang Y, Qin D-Y, Jiang L, Liang X, Yang X, Gou H-F, Wang YS, Wei YQ, Wang W. Preclinical Evaluation of Chimeric Antigen Receptor–Modified T Cells Specific to Epithelial Cell Adhesion Molecule for Treating Colorectal Cancer. Hum Gene Ther. 2019;30:402–412. doi: 10.1089/hum.2018.229. [DOI] [PubMed] [Google Scholar]
- 66.Wagner S, Mullins CS, Linnebacher M. Colorectal cancer vaccines: Tumor-associated antigens vs neoantigens. World J Gastroenterol. 2018;24:5418–5432. doi: 10.3748/wjg.v24.i48.5418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Miyagi Y, Imai N, Sasatomi T, Yamada A, Mine T, Katagiri K, Nakagawa M, Muto A, Okouchi S, Isomoto H, Shirouzu K, Yamana H, Itoh K. Induction of cellular immune responses to tumor cells and peptides in colorectal cancer patients by vaccination with SART3 peptides. Clin Cancer Res. 2001;7:3950–3962. [PubMed] [Google Scholar]
- 68.Tsuruma T, Hata F, Torigoe T, Furuhata T, Idenoue S, Kurotaki T, Yamamoto M, Yagihashi A, Ohmura T, Yamaguchi K, Katsuramaki T, Yasoshima T, Sasaki K, Mizushima Y, Minamida H, Kimura H, Akiyama M, Hirohashi Y, Asanuma H, Tamura Y, Shimozawa K, Sato N, Hirata K. Phase I clinical study of anti-apoptosis protein, survivin-derived peptide vaccine therapy for patients with advanced or recurrent colorectal cancer. J Transl Med. 2004;2:19. doi: 10.1186/1479-5876-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu KJ, Wang CC, Chen LT, Cheng AL, Lin DT, Wu YC, Yu WL, Hung YM, Yang HY, Juang SH, Whang-Peng J. Generation of carcinoembryonic antigen (CEA)-specific T-cell responses in HLA-A*0201 and HLA-A*2402 late-stage colorectal cancer patients after vaccination with dendritic cells loaded with CEA peptides. Clin Cancer Res. 2004;10:2645–2651. doi: 10.1158/1078-0432.ccr-03-0430. [DOI] [PubMed] [Google Scholar]
- 70.Kavanagh B, Ko A, Venook A, Margolin K, Zeh H, Lotze M, Schillinger B, Liu W, Lu Y, Mitsky P, Schilling M, Bercovici N, Loudovaris M, Guillermo R, Lee SM, Bender J, Mills B, Fong L. Vaccination of metastatic colorectal cancer patients with matured dendritic cells loaded with multiple major histocompatibility complex class I peptides. J Immunother. 2007;30:762–772. doi: 10.1097/CJI.0b013e318133451c. [DOI] [PubMed] [Google Scholar]
- 71.Okuno K, Sugiura F, Hida JI, Tokoro T, Ishimaru E, Sukegawa Y, Ueda K. Phase I clinical trial of a novel peptide vaccine in combination with UFT/LV for metastatic colorectal cancer. Exp Ther Med. 2011;2:73–79. doi: 10.3892/etm.2010.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hazama S, Nakamura Y, Takenouchi H, Suzuki N, Tsunedomi R, Inoue Y, Tokuhisa Y, Iizuka N, Yoshino S, Takeda K, Shinozaki H, Kamiya A, Furukawa H, Oka M. A phase I study of combination vaccine treatment of five therapeutic epitope-peptides for metastatic colorectal cancer; safety, immunological response, and clinical outcome. J Transl Med. 2014;12:63. doi: 10.1186/1479-5876-12-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ahsan A, Ramanand SG, Bergin IL, Zhao L, Whitehead CE, Rehemtulla A, Ray D, Pratt WB, Lawrence TS, Nyati MK. Efficacy of an EGFR-specific peptide against EGFR-dependent cancer cell lines and tumor xenografts. Neoplasia. 2014;16:105–114. doi: 10.1593/neo.14182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kwon S, Kim YE, Park JA, Kim DS, Kwon HJ, Lee Y. Therapeutic effect of a TM4SF5-specific peptide vaccine against colon cancer in a mouse model. BMB Rep. 2014;47:215–220. doi: 10.5483/BMBRep.2014.47.4.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shimodaira S, Sano K, Hirabayashi K, Koya T, Higuchi Y, Mizuno Y, Yamaoka N, Yuzawa M, Kobayashi T, Ito K, Koizumi T. Dendritic Cell-Based Adjuvant Vaccination Targeting Wilms' Tumor 1 in Patients with Advanced Colorectal Cancer. Vaccines (Basel) 2015;3:1004–1018. doi: 10.3390/vaccines3041004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Taniguchi H, Iwasa S, Yamazaki K, Yoshino T, Kiryu C, Naka Y, Liew EL, Sakata Y. Phase 1 study of OCV-C02, a peptide vaccine consisting of two peptide epitopes for refractory metastatic colorectal cancer. Cancer Sci. 2017;108:1013–1021. doi: 10.1111/cas.13227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sagiv-Barfi I, Czerwinski DK, Levy S, Alam IS, Mayer AT, Gambhir SS, Levy R. Eradication of spontaneous malignancy by local immunotherapy. Sci Transl Med. 2018;10 doi: 10.1126/scitranslmed.aan4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Azadi A, Golchini A, Delazar S, Abarghooi Kahaki F, Dehnavi SM, Payandeh Z, Eyvazi S. Recent Advances on Immune Targeted Therapy of Colorectal Cancer Using bi-Specific Antibodies and Therapeutic Vaccines. Biol Proced Online. 2021;23:13. doi: 10.1186/s12575-021-00147-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Grenier JM, Yeung ST, Khanna KM. Combination Immunotherapy: Taking Cancer Vaccines to the Next Level. Front Immunol. 2018;9:610. doi: 10.3389/fimmu.2018.00610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shahnazari M, Samadi P, Pourjafar M, Jalali A. Therapeutic vaccines for colorectal cancer: The progress and future prospect. Int Immunopharmacol. 2020;88:106944. doi: 10.1016/j.intimp.2020.106944. [DOI] [PubMed] [Google Scholar]
- 81.Rusch T, Bayry J, Werner J, Shevchenko I, Bazhin AV. Immunotherapy as an Option for Cancer Treatment. Arch Immunol Ther Exp (Warsz) 2018;66:89–96. doi: 10.1007/s00005-017-0491-5. [DOI] [PubMed] [Google Scholar]
- 82.Conlon KC, Miljkovic MD, Waldmann TA. Cytokines in the Treatment of Cancer. J Interferon Cytokine Res. 2019;39:6–21. doi: 10.1089/jir.2018.0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Johdi NA, Sukor NF. Colorectal Cancer Immunotherapy: Options and Strategies. Front Immunol. 2020;11:1624. doi: 10.3389/fimmu.2020.01624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kennedy LB, Salama AKS. A review of cancer immunotherapy toxicity. CA Cancer J Clin. 2020;70:86–104. doi: 10.3322/caac.21596. [DOI] [PubMed] [Google Scholar]
- 85.Wang G, Lu X, Dey P, Deng P, Wu CC, Jiang S, Fang Z, Zhao K, Konaparthi R, Hua S, Zhang J, Li-Ning-Tapia EM, Kapoor A, Wu CJ, Patel NB, Guo Z, Ramamoorthy V, Tieu TN, Heffernan T, Zhao D, Shang X, Khadka S, Hou P, Hu B, Jin EJ, Yao W, Pan X, Ding Z, Shi Y, Li L, Chang Q, Troncoso P, Logothetis CJ, McArthur MJ, Chin L, Wang YA, DePinho RA. Targeting YAP-Dependent MDSC Infiltration Impairs Tumor Progression. Cancer Discov. 2016;6:80–95. doi: 10.1158/2159-8290.CD-15-0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Feig C, Jones JO, Kraman M, Wells RJ, Deonarine A, Chan DS, Connell CM, Roberts EW, Zhao Q, Caballero OL, Teichmann SA, Janowitz T, Jodrell DI, Tuveson DA, Fearon DT. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proc Natl Acad Sci U S A. 2013;110:20212–20217. doi: 10.1073/pnas.1320318110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kershaw MH, Wang G, Westwood JA, Pachynski RK, Tiffany HL, Marincola FM, Wang E, Young HA, Murphy PM, Hwu P. Redirecting migration of T cells to chemokine secreted from tumors by genetic modification with CXCR2. Hum Gene Ther. 2002;13:1971–1980. doi: 10.1089/10430340260355374. [DOI] [PubMed] [Google Scholar]
- 88.Yanagi T, Nagai K, Shimizu H, Matsuzawa SI. Melanoma antigen A12 regulates cell cycle via tumor suppressor p21 expression. Oncotarget. 2017;8:68448–68459. doi: 10.18632/oncotarget.19497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lee DW, Gardner R, Porter DL, Louis CU, Ahmed N, Jensen M, Grupp SA, Mackall CL. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124:188–195. doi: 10.1182/blood-2014-05-552729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Xu MD, Qi P, Du X. Long non-coding RNAs in colorectal cancer: implications for pathogenesis and clinical application. Mod Pathol. 2014;27:1310–1320. doi: 10.1038/modpathol.2014.33. [DOI] [PubMed] [Google Scholar]
- 91.Ling H. Non-coding RNAs: Therapeutic Strategies and Delivery Systems. Adv Exp Med Biol. 2016;937:229–237. doi: 10.1007/978-3-319-42059-2_12. [DOI] [PubMed] [Google Scholar]
- 92.Kara G, Calin GA, Ozpolat B. RNAi-based therapeutics and tumor targeted delivery in cancer. Adv Drug Deliv Rev. 2022;182:114113. doi: 10.1016/j.addr.2022.114113. [DOI] [PubMed] [Google Scholar]
- 93.Biswas S. MicroRNAs as Therapeutic Agents: The Future of the Battle Against Cancer. Curr Top Med Chem. 2018;18:2544–2554. doi: 10.2174/1568026619666181120121830. [DOI] [PubMed] [Google Scholar]
- 94.Chakraborty C, Sharma AR, Sharma G, Sarkar BK, Lee SS. The novel strategies for next-generation cancer treatment: miRNA combined with chemotherapeutic agents for the treatment of cancer. Oncotarget. 2018;9:10164–10174. doi: 10.18632/oncotarget.24309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jung E, Choi J, Kim JS, Han TS. MicroRNA-Based Therapeutics for Drug-Resistant Colorectal Cancer. Pharmaceuticals (Basel) 2021;14 doi: 10.3390/ph14020136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kana SI, Essani K. Immuno-Oncolytic Viruses: Emerging Options in the Treatment of Colorectal Cancer. Mol Diagn Ther. 2021;25:301–313. doi: 10.1007/s40291-021-00517-7. [DOI] [PubMed] [Google Scholar]
- 97.Conrad SJ, El-Aswad M, Kurban E, Jeng D, Tripp BC, Nutting C, Eversole R, Mackenzie C, Essani K. Oncolytic tanapoxvirus expressing FliC causes regression of human colorectal cancer xenografts in nude mice. J Exp Clin Cancer Res. 2015;34:19. doi: 10.1186/s13046-015-0131-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang B, Ogata H, Takishima Y, Miyamoto S, Inoue H, Kuroda M, Yamada K, Hijikata Y, Murahashi M, Shimizu H, Okazaki T, Nakanishi Y, Tani K. A Novel Combination Therapy for Human Oxaliplatin-resistant Colorectal Cancer Using Oxaliplatin and Coxsackievirus A11. Anticancer Res. 2018;38:6121–6126. doi: 10.21873/anticanres.12963. [DOI] [PubMed] [Google Scholar]
- 99.Pokrovska TD, Jacobus EJ, Puliyadi R, Prevo R, Frost S, Dyer A, Baugh R, Rodriguez-Berriguete G, Fisher K, Granata G, Herbert K, Taverner WK, Champion BR, Higgins GS, Seymour LW, Lei-Rossmann J. External Beam Radiation Therapy and Enadenotucirev: Inhibition of the DDR and Mechanisms of Radiation-Mediated Virus Increase. Cancers (Basel) 2020;12 doi: 10.3390/cancers12040798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rojas JJ, Sampath P, Hou W, Thorne SH. Defining Effective Combinations of Immune Checkpoint Blockade and Oncolytic Virotherapy. Clin Cancer Res. 2015;21:5543–5551. doi: 10.1158/1078-0432.CCR-14-2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Geevarghese SK, Geller DA, de Haan HA, Hörer M, Knoll AE, Mescheder A, Nemunaitis J, Reid TR, Sze DY, Tanabe KK, Tawfik H. Phase I/II study of oncolytic herpes simplex virus NV1020 in patients with extensively pretreated refractory colorectal cancer metastatic to the liver. Hum Gene Ther. 2010;21:1119–1128. doi: 10.1089/hum.2010.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zeh HJ, Downs-Canner S, McCart JA, Guo ZS, Rao UN, Ramalingam L, Thorne SH, Jones HL, Kalinski P, Wieckowski E, O'Malley ME, Daneshmand M, Hu K, Bell JC, Hwang TH, Moon A, Breitbach CJ, Kirn DH, Bartlett DL. First-in-man study of western reserve strain oncolytic vaccinia virus: safety, systemic spread, and antitumor activity. Mol Ther. 2015;23:202–214. doi: 10.1038/mt.2014.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Downs-Canner S, Guo ZS, Ravindranathan R, Breitbach CJ, O'Malley ME, Jones HL, Moon A, McCart JA, Shuai Y, Zeh HJ, Bartlett DL. Phase 1 Study of Intravenous Oncolytic Poxvirus (vvDD) in Patients With Advanced Solid Cancers. Mol Ther. 2016;24:1492–1501. doi: 10.1038/mt.2016.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jonker DJ, Tang PA, Kennecke H, Welch SA, Cripps MC, Asmis T, Chalchal H, Tomiak A, Lim H, Ko YJ, Chen EX, Alcindor T, Goffin JR, Korpanty GJ, Feilotter H, Tsao MS, Theis A, Tu D, Seymour L. A Randomized Phase II Study of FOLFOX6/Bevacizumab With or Without Pelareorep in Patients With Metastatic Colorectal Cancer: IND.210, a Canadian Cancer Trials Group Trial. Clin Colorectal Cancer. 2018;17:231–239.e7. doi: 10.1016/j.clcc.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 105.Tilgase A, Olmane E, Nazarovs J, Brokāne L, Erdmanis R, Rasa A, Alberts P. Multimodality Treatment of a Colorectal Cancer Stage IV Patient with FOLFOX-4, Bevacizumab, Rigvir Oncolytic Virus, and Surgery. Case Rep Gastroenterol. 2018;12:457–465. doi: 10.1159/000492210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lawler M, Johnston B, Van Schaeybroeck S, Salto-Tellez M, Wilson R, Dunlop M, and Johnston PG. Chapter 74–Colorectal Cancer. In: Niederhuber JE, Armitage JO, Dorshow JH, Kastan MB, Tepper JE, eds. Abeloff’s Clinical Oncology. 6th ed. Philadelphia: Elsevier, 2020. [Google Scholar]
- 107.Libutti SK, Saltz LB, Willett CG, and Levine RA. Ch 62 - Cancer of the Colon. In: DeVita VT, Hellman S, Rosenberg SA, eds. DeVita, Hellman, and Rosenberg’s Cancer: Principles and Practice of Oncology. 11th ed. Philadelphia: Lippincott-Williams & Wilkins, 2019. [Google Scholar]
- 108.National Cancer Institute. Physician Data Query (PDQ). [cited 10 October 2023]. Available from: https://www.cancer.gov/types/colorectal/patient/colorectal-treatment-pdq .
- 109.National Comprehensive Cancer Network (NCCN) Guidelines. [cited 10 October 2023]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf .
- 110.Angulo P, Kaushik G, Subramaniam D, Dandawate P, Neville K, Chastain K, Anant S. Natural compounds targeting major cell signaling pathways: a novel paradigm for osteosarcoma therapy. J Hematol Oncol. 2017;10:10. doi: 10.1186/s13045-016-0373-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lin JG, Chung JG, Wu LT, Chen GW, Chang HL, Wang TF. Effects of berberine on arylamine N-acetyltransferase activity in human colon tumor cells. Am J Chin Med. 1999;27:265–275. doi: 10.1142/S0192415X99000306. [DOI] [PubMed] [Google Scholar]
- 112.Lin HL, Liu TY, Wu CW, Chi CW. Berberine modulates expression of mdr1 gene product and the responses of digestive track cancer cells to Paclitaxel. Br J Cancer. 1999;81:416–422. doi: 10.1038/sj.bjc.6690710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Font A, Sanchez JM, Rosell R, Taron M, Martinez E, Guillot M, Manzano JL, Margeli M, Barnadas A, Abad A. Phase I study of weekly CPT-11 (irinotecan)/docetaxel in patients with advanced solid tumors. Lung Cancer. 2002;37:213–218. doi: 10.1016/s0169-5002(02)00081-8. [DOI] [PubMed] [Google Scholar]
- 114.Rougier P, Bugat R, Douillard JY, Culine S, Suc E, Brunet P, Becouarn Y, Ychou M, Marty M, Extra JM, Bonneterre J, Adenis A, Seitz JF, Ganem G, Namer M, Conroy T, Negrier S, Merrouche Y, Burki F, Mousseau M, Herait P, Mahjoubi M. Phase II study of irinotecan in the treatment of advanced colorectal cancer in chemotherapy-naive patients and patients pretreated with fluorouracil-based chemotherapy. J Clin Oncol. 1997;15:251–260. doi: 10.1200/JCO.1997.15.1.251. [DOI] [PubMed] [Google Scholar]
- 115.Khajuria A, Thusu N, Zutshi U. Piperine modulates permeability characteristics of intestine by inducing alterations in membrane dynamics: influence on brush border membrane fluidity, ultrastructure and enzyme kinetics. Phytomedicine. 2002;9:224–231. doi: 10.1078/0944-7113-00114. [DOI] [PubMed] [Google Scholar]
- 116.Yaffe PB, Power Coombs MR, Doucette CD, Walsh M, Hoskin DW. Piperine, an alkaloid from black pepper, inhibits growth of human colon cancer cells via G1 arrest and apoptosis triggered by endoplasmic reticulum stress. Mol Carcinog. 2015;54:1070–1085. doi: 10.1002/mc.22176. [DOI] [PubMed] [Google Scholar]
- 117.Ikeguchi M, Yamamoto M, Arai Y, Maeta Y, Ashida K, Katano K, Miki Y, Kimura T. Fucoidan reduces the toxicities of chemotherapy for patients with unresectable advanced or recurrent colorectal cancer. Oncol Lett. 2011;2:319–322. doi: 10.3892/ol.2011.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bharti AC, Donato N, Aggarwal BB. Curcumin (diferuloylmethane) inhibits constitutive and IL-6-inducible STAT3 phosphorylation in human multiple myeloma cells. J Immunol. 2003;171:3863–3871. doi: 10.4049/jimmunol.171.7.3863. [DOI] [PubMed] [Google Scholar]
- 119.Johnson JJ, Mukhtar H. Curcumin for chemoprevention of colon cancer. Cancer Lett. 2007;255:170–181. doi: 10.1016/j.canlet.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 120.Lee TY, Lee KC, Chen SY, Chang HH. 6-Gingerol inhibits ROS and iNOS through the suppression of PKC-alpha and NF-kappaB pathways in lipopolysaccharide-stimulated mouse macrophages. Biochem Biophys Res Commun. 2009;382:134–139. doi: 10.1016/j.bbrc.2009.02.160. [DOI] [PubMed] [Google Scholar]
- 121.Ray A. Cancer preventive role of selected dietary factors. Indian J Cancer. 2005;42:15–24. doi: 10.4103/0019-509x.15095. [DOI] [PubMed] [Google Scholar]
- 122.Lin CB, Lin CC, Tsay GJ. 6-Gingerol Inhibits Growth of Colon Cancer Cell LoVo via Induction of G2/M Arrest. Evid Based Complement Alternat Med. 2012;2012:326096. doi: 10.1155/2012/326096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Nauroth JM, Liu YC, Van Elswyk M, Bell R, Hall EB, Chung G, Arterburn LM. Docosahexaenoic acid (DHA) and docosapentaenoic acid (DPAn-6) algal oils reduce inflammatory mediators in human peripheral mononuclear cells in vitro and paw edema in vivo. Lipids. 2010;45:375–384. doi: 10.1007/s11745-010-3406-3. [DOI] [PubMed] [Google Scholar]
- 124.Jan G, Belzacq AS, Haouzi D, Rouault A, Métivier D, Kroemer G, Brenner C. Propionibacteria induce apoptosis of colorectal carcinoma cells via short-chain fatty acids acting on mitochondria. Cell Death Differ. 2002;9:179–188. doi: 10.1038/sj.cdd.4400935. [DOI] [PubMed] [Google Scholar]
- 125.Jacouton E, Chain F, Sokol H, Langella P, Bermúdez-Humarán LG. Probiotic Strain Lactobacillus casei BL23 Prevents Colitis-Associated Colorectal Cancer. Front Immunol. 2017;8:1553. doi: 10.3389/fimmu.2017.01553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yue Y, Ye K, Lu J, Wang X, Zhang S, Liu L, Yang B, Nassar K, Xu X, Pang X, Lv J. Probiotic strain Lactobacillus plantarum YYC-3 prevents colon cancer in mice by regulating the tumour microenvironment. Biomed Pharmacother. 2020;127:110159. doi: 10.1016/j.biopha.2020.110159. [DOI] [PubMed] [Google Scholar]
- 127.Thirabunyanon M, Boonprasom P, Niamsup P. Probiotic potential of lactic acid bacteria isolated from fermented dairy milks on antiproliferation of colon cancer cells. Biotechnol Lett. 2009;31:571–576. doi: 10.1007/s10529-008-9902-3. [DOI] [PubMed] [Google Scholar]
- 128.Cox MA, Jackson J, Stanton M, Rojas-Triana A, Bober L, Laverty M, Yang X, Zhu F, Liu J, Wang S, Monsma F, Vassileva G, Maguire M, Gustafson E, Bayne M, Chou CC, Lundell D, Jenh CH. Short-chain fatty acids act as antiinflammatory mediators by regulating prostaglandin E(2) and cytokines. World J Gastroenterol. 2009;15:5549–5557. doi: 10.3748/wjg.15.5549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cammarota G, Masucci L, Ianiro G, Bibbò S, Dinoi G, Costamagna G, Sanguinetti M, Gasbarrini A. Randomised clinical trial: faecal microbiota transplantation by colonoscopy vs. vancomycin for the treatment of recurrent Clostridium difficile infection. Aliment Pharmacol Ther. 2015;41:835–843. doi: 10.1111/apt.13144. [DOI] [PubMed] [Google Scholar]
- 130.EISEMAN B, SILEN W, BASCOM GS, KAUVAR AJ. Fecal enema as an adjunct in the treatment of pseudomembranous enterocolitis. Surgery. 1958;44:854–859. [PubMed] [Google Scholar]
- 131.Filip M, Tzaneva V, Dumitrascu DL. Fecal transplantation: digestive and extradigestive clinical applications. Clujul Med. 2018;91:259–265. doi: 10.15386/cjmed-946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhang L, Kim SB, Luitel K, Shay JW. Cholesterol Depletion by TASIN-1 Induces Apoptotic Cell Death through the ER Stress/ROS/JNK Signaling in Colon Cancer Cells. Mol Cancer Ther. 2018;17:943–951. doi: 10.1158/1535-7163.MCT-17-0887. [DOI] [PubMed] [Google Scholar]
- 133.Winter-Vann AM, Baron RA, Wong W, dela Cruz J, York JD, Gooden DM, Bergo MO, Young SG, Toone EJ, Casey PJ. A small-molecule inhibitor of isoprenylcysteine carboxyl methyltransferase with antitumor activity in cancer cells. Proc Natl Acad Sci U S A. 2005;102:4336–4341. doi: 10.1073/pnas.0408107102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhang B, Halder SK, Kashikar ND, Cho YJ, Datta A, Gorden DL, Datta PK. Antimetastatic role of Smad4 signaling in colorectal cancer. Gastroenterology. 2010;138:969–80.e1. doi: 10.1053/j.gastro.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lee KW, Park YS, Ahn JB, Lee JK, Ryu J, Oh B, Ock CY, Hwang S, Hahm KB, Kim SJ, Kim TW. 332 Novel TGF-β signatures in metastatic colorectal cancer patients treated with vactosertib in combination with pembrolizumab. In: Regular and young investigator award abstracts. BMJ Publishing Group Ltd. 2020:A203.2–A204. [Google Scholar]
- 136.Li XL, Zhou J, Chen ZR, Chng WJ. P53 mutations in colorectal cancer - molecular pathogenesis and pharmacological reactivation. World J Gastroenterol. 2015;21:84–93. doi: 10.3748/wjg.v21.i1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Rigatti MJ, Verma R, Belinsky GS, Rosenberg DW, Giardina C. Pharmacological inhibition of Mdm2 triggers growth arrest and promotes DNA breakage in mouse colon tumors and human colon cancer cells. Mol Carcinog. 2012;51:363–378. doi: 10.1002/mc.20795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Li XL, Zhou J, Chan ZL, Chooi JY, Chen ZR, Chng WJ. PRIMA-1met (APR-246) inhibits growth of colorectal cancer cells with different p53 status through distinct mechanisms. Oncotarget. 2015;6:36689–36699. doi: 10.18632/oncotarget.5385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kopetz S, Guthrie KA, Morris VK, Lenz HJ, Magliocco AM, Maru D, Yan Y, Lanman R, Manyam G, Hong DS, Sorokin A, Atreya CE, Diaz LA, Allegra C, Raghav KP, Wang SE, Lieu CH, McDonough SL, Philip PA, Hochster HS. Randomized Trial of Irinotecan and Cetuximab With or Without Vemurafenib in BRAF-Mutant Metastatic Colorectal Cancer (SWOG S1406) J Clin Oncol. 2021;39:285–294. doi: 10.1200/JCO.20.01994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Tabernero J, Grothey A, Van Cutsem E, Yaeger R, Wasan H, Yoshino T, Desai J, Ciardiello F, Loupakis F, Hong YS, Steeghs N, Guren TK, Arkenau HT, Garcia-Alfonso P, Elez E, Gollerkeri A, Maharry K, Christy-Bittel J, Kopetz S. Encorafenib Plus Cetuximab as a New Standard of Care for Previously Treated BRAF V600E-Mutant Metastatic Colorectal Cancer: Updated Survival Results and Subgroup Analyses from the BEACON Study. J Clin Oncol. 2021;39:273–284. doi: 10.1200/JCO.20.02088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Drago L. Probiotics and Colon Cancer. Microorganisms. 2019;7 doi: 10.3390/microorganisms7030066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Schirripa M, Cohen SA, Battaglin F, Lenz HJ. Biomarker-driven and molecular targeted therapies for colorectal cancers. Semin Oncol. 2018;45:124–132. doi: 10.1053/j.seminoncol.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Jebelli A, Baradaran B, Mosafer J, Baghbanzadeh A, Mokhtarzadeh A, Tayebi L. Recent developments in targeting genes and pathways by RNAi-based approaches in colorectal cancer. Med Res Rev. 2021;41:395–434. doi: 10.1002/med.21735. [DOI] [PubMed] [Google Scholar]
- 144.He J, Wu F, Han Z, Hu M, Lin W, Li Y, Cao M. Biomarkers (mRNAs and Non-Coding RNAs) for the Diagnosis and Prognosis of Colorectal Cancer - From the Body Fluid to Tissue Level. Front Oncol. 2021;11:632834. doi: 10.3389/fonc.2021.632834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Alshaer W, Zureigat H, Al Karaki A, Al-Kadash A, Gharaibeh L, Hatmal MM, Aljabali AAA, Awidi A. siRNA: Mechanism of action, challenges, and therapeutic approaches. Eur J Pharmacol. 2021;905:174178. doi: 10.1016/j.ejphar.2021.174178. [DOI] [PubMed] [Google Scholar]
- 146.Ribatti D, Annese T, Ruggieri S, Tamma R, Crivellato E. Limitations of Anti-Angiogenic Treatment of Tumors. Transl Oncol. 2019;12:981–986. doi: 10.1016/j.tranon.2019.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Prados J, Melguizo C, Roldan H, Alvarez PJ, Ortiz R, Arias JL, Aranega A. RNA interference in the treatment of colon cancer. BioDrugs. 2013;27:317–327. doi: 10.1007/s40259-013-0019-4. [DOI] [PubMed] [Google Scholar]
- 148.Brasiel PGA, Dutra Luquetti SCP, Peluzio MDCG, Novaes RD, Gonçalves RV. Preclinical Evidence of Probiotics in Colorectal Carcinogenesis: A Systematic Review. Dig Dis Sci. 2020;65:3197–3210. doi: 10.1007/s10620-020-06062-3. [DOI] [PubMed] [Google Scholar]
- 149.Samad MA, Saiman MZ, Abdul Majid N, Karsani SA, Yaacob JS. Berberine Inhibits Telomerase Activity and Induces Cell Cycle Arrest and Telomere Erosion in Colorectal Cancer Cell Line, HCT 116. Molecules. 2021;26 doi: 10.3390/molecules26020376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Prashant A, Rangaswamy C, Yadav AK, Reddy V, Sowmya MN, Madhunapantula S. In vitro anticancer activity of ethanolic extracts of Piper nigrum against colorectal carcinoma cell lines. Int J Appl Basic Med Res. 2017;7:67–72. doi: 10.4103/2229-516X.198531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Al Monla R, Dassouki Z, Sari-Chmayssem N, Mawlawi H, Gali-Muhtasib H. Fucoidan and Alginate from the Brown Algae Colpomenia sinuosa and Their Combination with Vitamin C Trigger Apoptosis in Colon Cancer. Molecules. 2022;27 doi: 10.3390/molecules27020358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Su M, Qin B, Liu F, Chen Y, Zhang R. Andrographolide enhanced 5-fluorouracil-induced antitumor effect in colorectal cancer via inhibition of c-MET pathway. Drug Des Devel Ther. 2017;11:3333–3341. doi: 10.2147/DDDT.S140354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bokemeyer C, Van Cutsem E, Rougier P, Ciardiello F, Heeger S, Schlichting M, Celik I, Köhne CH. Addition of cetuximab to chemotherapy as first-line treatment for KRAS wild-type metastatic colorectal cancer: pooled analysis of the CRYSTAL and OPUS randomised clinical trials. Eur J Cancer. 2012;48:1466–1475. doi: 10.1016/j.ejca.2012.02.057. [DOI] [PubMed] [Google Scholar]
- 154.Giusti RM, Shastri KA, Cohen MH, Keegan P, Pazdur R. FDA drug approval summary: panitumumab (Vectibix) Oncologist. 2007;12:577–583. doi: 10.1634/theoncologist.12-5-577. [DOI] [PubMed] [Google Scholar]
- 155.Avastin Approved for Metastatic Colorectal Cancer. Onco Times. 2004;26:20. [PubMed] [Google Scholar]
- 156.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, Ferrara N, Fyfe G, Rogers B, Ross R, Kabbinavar F. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 157.Yoshino T, Portnoy DC, Obermannová R, Bodoky G, Prausová J, Garcia-Carbonero R, Ciuleanu T, García-Alfonso P, Cohn AL, Van Cutsem E, Yamazaki K, Lonardi S, Muro K, Kim TW, Yamaguchi K, Grothey A, O'Connor J, Taieb J, Wijayawardana SR, Hozak RR, Nasroulah F, Tabernero J. Biomarker analysis beyond angiogenesis: RAS/RAF mutation status, tumour sidedness, and second-line ramucirumab efficacy in patients with metastatic colorectal carcinoma from RAISE-a global phase III study. Ann Oncol. 2019;30:124–131. doi: 10.1093/annonc/mdy461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Andre T, Amonkar M, Norquist JM, Shiu KK, Kim TW, Jensen BV, Jensen LH, Punt CJA, Smith D, Garcia-Carbonero R, Sevilla I, De La Fouchardiere C, Rivera F, Elez E, Diaz LA Jr, Yoshino T, Van Cutsem E, Yang P, Farooqui M, Le DT. Health-related quality of life in patients with microsatellite instability-high or mismatch repair deficient metastatic colorectal cancer treated with first-line pembrolizumab versus chemotherapy (KEYNOTE-177): an open-label, randomised, phase 3 trial. Lancet Oncol. 2021;22:665–677. doi: 10.1016/S1470-2045(21)00064-4. [DOI] [PubMed] [Google Scholar]