Abstract
The tumor, nodes, metastasis (TNM) staging system has long been the gold standard for the classification and prognosis of solid tumors. However, the TNM staging system is not without limitations. Prognostic heterogeneity exists within patients at the same stage. Therefore, the pursuit of other biomarkers with the potential to classify patients with cancer has never stopped. One of them, tumor budding (TB), has gained much success in colorectal cancer. In recent years, TB in gastric cancer has attracted much attention from researchers, beginning to reveal the molecular and biological aspects of this phenomenon in gastric cancer, and has emerged as a promising prognostic biomarker in gastric cancer, predicting disease progression and unfavorable survival. Therefore, it is time and essential to provide a holistic overview of TB in gastric cancer, which has not been achieved and is the aim of this review.
Keywords: Tumor budding, Gastric cancer, Poorly differentiated cluster, Prognosis, Lymph node metastasis
Core Tip: Tumor budding has gained much success in colorectal cancer and has begun to attract much attention from researchers proficient in gastric cancer. Tumor budding showed promising prognostic potential in gastric cancer with the ability to predict disease progression and unfavorable survival. Therefore, it is time and essential to provide a holistic overview of tumor budding in gastric cancer, which has not been achieved and is the aim of this review, in which we summarize the current data on tumor budding in gastric cancer and discuss its clinical application prospects and challenges.
INTRODUCTION
Gastric cancer (GC) is one of the most common malignancies worldwide and is most commonly diagnosed in Eastern Asia, Central and Eastern Europe and South America[1]. Along with the eradication of Helicobacter pylori, the most associated risk factor for intestinal-type GC, the incidence of GC has dramatically decreased in countries and areas with high prevalence over the past 50 years[2]. However, despite tremendous triumphs, GC remains the fifth most common cancer and ranks fourth in the list of cancer-related deaths. According to GLOBCAN 2020, there have been more than 1 million new cases of GC, which caused 768793 deaths worldwide in 2020[1]. In recent years, the management of GC has advanced greatly, and multimodal treatment, including radical gastrectomy, perioperative chemotherapy, targeted therapy and immunotherapy, has been successfully applied to GC patients; however, the long-term survival is still dismal[3]. This is mainly attributed to delayed diagnosis, advanced disease, poor response to therapy and, an often overlooked reason, the lack of standardized and reproducible biomarkers for personalized health care.
In the clinical management of GC, several clinicopathological characteristics have long been used to classify patients with GC based on their prognostic and/or predictive significance[4]. The gold standard for classification of GC remains to be the tumor, nodes, metastasis (TNM) staging system, complemented by World Health Organization histologic categorization, cancer grade, Lauren subtypes, tumor size, neural and lymphovascular invasion. Some emerging new but less clinically translated prognostic biomarkers have also been explored, including the positive lymph node ratio (LNR), circulating tumor cells (CTCs) and neutrophil-lymphocyte ratio (NLR)[5-7]. However, the survival outcomes of GC patients remain heterogeneous, and some early-stage patients will experience recurrence or metastasis. Therefore, searching for new precise and reproducible biomarkers for personalized health care in GC has never been stopped. One of the promising factors is tumor budding (TB), also referred to as sprouting, which was first described by Imai more than 60 years ago to characterize isolated single or no more than four cancer cells at the invasive front[8]. Although first described in GC, TB is now most successfully applied in colorectal cancer and has become part of routine histopathological diagnostics to direct many clinical decisions[9]. The associations of TB with disease progression and survival have also been investigated in GC by various research groups in recent years. However, due to the lack of methodological standardization, findings reported across studies varied substantially, leaving the exact role of TB in GC unclear. Therefore, in this review, we aimed to summarize the evidence from the literature regarding TB assessment in GC to provide an overview of the clinical and prognostic role of TB in GC. We also discuss the methodological shortcomings, inconsistent scoring systems and future directions.
FINDINGS REGARDING TB IN GC
In GC, isolated single or small clusters of cancer cells have long been identified at the expansive border of the main tumor mass, but their significance has not been recognized[10]. Therefore, twenty years following the first attempt to explore the prognostic value of tumor cell dissociation (TCD), used synonymously to describe TB in GC patients, no studies on the same topic have been published[10]. Such a strange ignorance may be due to a lower prevalence of GC in Western countries compared to colorectal cancer, in which the assessing and reporting standardizations have been established[11]. In addition, the complex histological composition of GC, for example, almost all diffuse-type GC belongs to high-grade TB, may lead to conceptual difficulty. In addition, the lack of a consistent assessment and reporting criteria also limits the application of this biomarker in GC. However, during the International Tumor Budding Consensus Conference (ITBCC), a consensus was achieved for colorectal cancer on the definition of TB (single cancer cells or cell clusters of up to 4 cancer cells), assessment method (buds scored in the hotspot on a hematoxylin and eosin-stained slide using a 20× objective lens, followed by normalization to a field area of 0.785 mm2) and clinically relevant cutoff values (5 and 10 buds are used to categorize TB as low grade (Bd1), intermediate grade (Bd2) and high grade (Bd3), respectively)[11]. Therefore, the publication of ITBCC guidelines promotes research on TB in GC, and the corresponding publications have increased rapidly in recent years.
Despite the methodical variety, increased TB density has consistently been found to correlate with adverse features in GC, including tumor size, poor differentiation, Lauren class, advanced T stage, lymph node metastasis, distant metastasis, TNM stage, lymphovascular invasion (LVI), perineural invasion (PNI), and nonradical resection (Table 1). One meta-analysis pooled data from 7 of these studies and showed that TB, regardless of the methods used to determine it, was associated with overall cancer stage, lymph node metastasis, LVI and tumor differentiation in GC[12]. Among these clinicopathological characteristics, Lauren class and lymph node metastasis may have practical significance. In studies that included all Lauren subtypes, more than 90% of diffuse-type GC will be classified as high-grade TB, whose effects will be discussed in the sections below. Regarding lymph node metastasis, an important determinant of survival in GC patients, nearly all studies have explored its association with TB and demonstrated that high TB activity promotes the spreading of cancer cells to regional lymph nodes (Table 1). In reports by Ulase et al[13] and Szalai et al[14], not only lymph node metastasis itself but also the lymph node ratio (LNR) was significantly increased in patients with high-grade TB. In the context of early GC, this predictive value can be applied to identify patients who need additional curative gastrectomy, which will be discussed in the sections below.
Table 1.
Previous studies on tumor budding in gastric cancer
|
Ref.
|
Country
|
Period
|
Cases
|
Lauren
|
Specimen
|
Staging
|
Assessment (%)
|
TB higher in
|
Outcomes (Multivariate analysis)
|
| Gulluoglu et al[33], 2015 | Turkey | 1993-2013 | 126 | I+D | Surgical resected | pT1a-1b | PTB, 400 ×; Positive/HPF (31) | LNM | LNM; pT1a: OR = 38.6 (1.91-781.70); pT1b: OR = 8.87 (2.79-22.16) |
| Che et al[68], 2017 | China | 2007-2010 | 296 | I+D | Surgical resected | I-IV | PTB, 400 ×; ≥ 5 buds, on average 10 HFP (49) | T stage, N stage, metastasis, TNM stage, differentiation | OS: 1.568 (1.044-2.354) |
| Olsen et al[16], 2017 | United States | 1999-2013 | 52 | I | Surgical resected | NA | Total-TB, 200 ×; ≥ 1 bud, median of 10 HFP (63) | Poor differentiation, LVI, PNI, T stage, N stage | Recurrence: P = 0.08; LNM: P = 0.51 |
| Kemi et al[61], 2019 | Finland | 1983-2016 | 583 | I+D | Surgical resected | I-IV | PTB, ITBCC; Bd1/2 (44.9), Bd3 (55.1) | Year of surgery, age, sex, T stage, Lauren class, grade; R | OS; Total: HR = 1.40 (1.12-1.76); I: HR = 1.39 (1.03-0.87); D: HR = 1.54 (1.01-1.2.34) |
| Du et al[34], 2019 | China | 2010-2016 | 621 | I+D | Surgical resected | pT1b | PTB, 200 ×; ≥ 1 bud/HPF (67) | NA | LNM; Total: OR = 3.3 (1.9-5.9); Well/moderately differentiated: OR = 3.3 (1.9-5.9) |
| Heckl et al[69], 2019 | Germany | 1997-2009 | 426 | I+D | Surgical resected | I-IV | PTB, ITBCC; Bd0 (24.9), Bd1/2 (22.8), Bd3 (52.3) | NA | NS |
| Dao et al[18], 2020 | Vietnam | 2012-2015 | 109 | I+D | Surgical resected | I-III | PTB, ITBCC; Bd1/2 (54.1), Bd3 (45.9) | Histopathological type, Differentiated grade, Lauren class, LVI, PNI, N stage | OS, HR = 20.899, P < 0.001; DFS, HR = 12.7, P < 0.001 |
| Ulase et al[13], 2020 | Germany | 1997-2009 | 456 | I+D | Surgical resected | I-IV | PTB, ITBCC; Bd0 (25.2), Bd1/2 (22.8), Bd3 (52.0) | Sex, Lauren class, differentiated grade, T stage, N stage, metastasis, TNM stage, LNR, LVI, PNI, R, HER-2, MSI, MET | OS, Total, I, D: NS. TSS, Total, I: NS; D: NA |
| Zhang et al[25], 2020 | China | 2001-2003 | 147 | I+D | Surgical resected | I-III | PTB, 200 ×; ≥ 6 buds, median of 5 HFP (46.3) | Tumor size, N stage, TNM stage, Lauren class, TILs | 10-yr OS, HR = 7.16 (3.35-15.29) |
| Qi et al[57], 2020 | China | 2000-2008 | 153 | I | Surgical resected | I-III | PTB and ITB, 400 ×; PTB: ≥ 8 buds, median of 5 HFP (48.4); ≥ 13 buds, median of 5 HFP (43.8) | PTB: Age, sex, location, N stage; ITB: Tumor size, N stage, TNM stage, grade | OS, PTB: HR = 1.92 (2.24-10.63); ITB: HR = 2.79 (2.24-10.63) |
| Cao et al[17], 2021 | China | 2009-2015 | 137 | I+D | ESD and Surgical resected | pT1 | PTB, NA; Positive (58.4) | Age, HP infection, N stage, recurrence, death | Recurrence, HR = 4.95, P = 0.01; Death, HR = 2.33, P = 0.043 |
| Sun et al[21], 2021 | China | 2004-2007 | 122 | I+D | Surgical resected | I-IV | PTB, 200 ×; > 5 buds, median of 10 HFP (57) | Lauren class, differentiation, LNM, metastasis, TNM stage | NA |
| Yim et al[32], 2021 | Korea | 2010-2021 | 289 | I+D | Surgical resected | pT1 | TB-YN: Present (57.1); TB-ITBCC: Bd2/3 (40.1); total-TB, ≥ 5 buds (419); SRCC excluded: mTB-YN: Present (48.8); mTB-ITBCC: Bd2/3 (29.1); total-mTB, ≥ 5 buds (29.8) | LNM | LNM, TB-YN: HR = 24.36 (3.15-188.17); TB-ITBCC: HR = 15.91 (5.41-46.80); Total-TB: HR = 25.50 (6.97-93.25); mTB-YN: HR = 21.07 (4.75-93.37); mTB-ITBCC: HR = 35.10 (12.11-101.76); Total-mTB: HR = 52.69 (15.69-179.97) |
| Kucuk[70], 2021 | Turkey | 2015-2020 | 43 | I+D | Surgical resected | pT2-4 | PTB, ITBCC; Bd1 (30.2), Bd2 (25.6), Bd3 (44.2) | Grade; T stage; N stage, differentiation | NA |
| Pun et al[15], 2022 | Canada | 2000-2018 | 76 | I | Surgical resected | IA-IV | PTB, ITBCC; Bd1 (21), Bd2 (21), Bd3 (58) | T stage, LNM, TNM stage, LVI, PNI, death, recurrence | OS: HR = 3.93 (0.34-45.44) |
| Jesinghaus et al[43], 2022 | Germany | 2001-2013 | 167 | I | Surgical resected | ypI-IV | PTB, ITBCC; Bd1 (29), Bd2 (25), Bd3 (46) | T stage, N stage, metastasis, TNM stage, R | OS: Bd2: HR = 2.60 (1.14-5.95); Bd3: HR = 4.74 (2.25-10.03) |
| Szalai et al[14], 2022 | Hungary | 2008-2018 | 290 | I+D | Surgical resected | IA-IV | PTB, ITBCC; Bd0 (1), Bd1 (6.6), Bd2 (13.8), Bd3 (78.6) | Lauren class, R; PNI; LVI; T stage, TNM stage, differentiation, LNM | OS, Total: HR = 1.65 (1.11-2.45); I: HR = 1.99 (1.23-3.22). DFS, Total, I: NS |
D: Diffuse; DFS: Disease-free survival; ESD: Endoscopic submucosal dissection; HPF: High power field; HR: Hazard ratio; I: Intestinal; ITB: Intratumoral budding; ITBCC: International Tumor Budding Consensus Conference; LNM: Lymph node metastasis; LNR: Lymph node ratio; LVI: Lymphovascular invasion; MSI: Microsatellite instability; NA: Not available; NS: No significance; OR: Odds ratio; OS: Overall survival; PNI: Perineural invasion; PTB: Peritumoral budding; R: Resection status; SRCC: Signet ring cancer cell; TB: Tumor budding; TILs: Tumor infiltrate lymphocytes; TSS: Tumor-specific survival.
As early as 1992, Gabbert et al[10] performed the first attempt to explore the prognostic value of TB in GC patients, although “TCD” was used to describe detached cancer cells in their report. Patients with GC were classified into four grades using a semiquantitative approach, and it was proven that patients with a higher density of detached cancer cells have poor survival independent of other prognostic factors[10]. Since then, the prognostic value of TB in GC has been overlooked. However, in the last decade, more evidence has emerged, except for a few studies, in which the majority of studies proved a poor prognosis in GC patients with high-grade TB (Table 1). One meta-analysis on the association between TB and GC has been published, in which 7 studies were included, and the prognostic value remained significant in the pooled results[12]. Nine of these studies reported the effects of TB on overall survival (OS), and only Pun et al[15] and Ulase et al[14] found that the prognostic significance of TB was lost in multivariable analyses for total cases or intestinal-type GC. Both studies assessed TB according to ITBCC guidelines; therefore, the conflicting findings may result from other confounding factors[13,15]. Two studies also set recurrence and death as the primary outcomes, and both reported a higher recurrence or death rate in patients with high-grade TB[16,17]. In addition to OS, only three studies reported the effects of TB on disease-free survival (DFS) or tumor-specific survival (TSS), and only one study was able to demonstrate the independent prognostic value for DFS in GC, while the other two studies reported that the association between TB and DFS or TSS was statistically significant only in univariate analysis[13,14,18]. Such findings are very intriguing, as high TB activity is intimately associated with adverse features in GC that may promote recurrence and metastasis, which will theoretically lead to poor cancer-specific survival. The conflicting findings may be due to the limited studies reporting the results of DFS and the retrospective design in all published studies. In retrospective studies, information on the exact disease recurrence status of some patients is difficult to collect. Regarding the value of TB in predicting DFS, a definite conclusion cannot be drawn at present, and more prospective studies with sufficient samples from various centers are needed.
MECHANISMS LINKING TB AND POOR PROGNOSIS
From the viewpoint of pathology, TB is a phenotype identified in various malignancies, in which the main cancer mass invades the adjacent stroma through finger-like projections, and a number of them eventually detach from the main cancer, leading to the formation of single cell or small cell clusters (Figure 1)[19]. Therefore, TB constitutes a distinctive part of the tumor microenvironment (TME), representing the first step of the cancer invasion-metastasis cascade. Currently, epithelial-mesenchymal transition (EMT), except for some debates, is widely accepted as an important biological program activated in the cancer invasion-metastasis cascade[20]. Therefore, TB has long been hypothesized as a specific histological manifestation of EMT, providing the significant prognostic potential of TB in various cancer types[8].
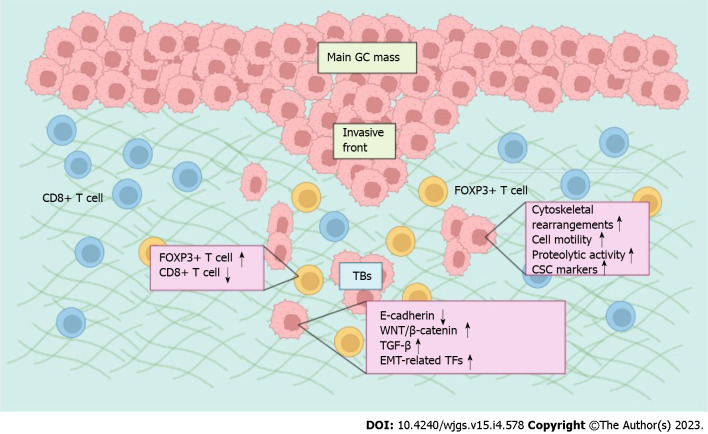
Figure 1.
The mechanism of tumor budding in gastric cancer. Various signaling pathways are activated in budding cells, leading to increased expression of epithelial-mesenchymal transition (EMT)-related transcription factors and the loss of cell-cell adhesion. Tumor budding (TB) also shares many invasive abilities endowed by EMT, such as cytoskeletal rearrangements, cell motility and increased proteolytic activity. Furthermore, TB cells often display attributes of cancer stem cells (CSCs). The density of tumor-infiltrating lymphocytes changed from CD8+ > FOXP3+ T cells in the nonbudding area to FOXP3+ > CD8+ T cells in the budding area. This figure was created with BioRender.com. GC: Gastric cancer; TB: Tumor budding; EMT: Epithelial-mesenchymal transition; CSC: Cancer stem cells.
The association between TB and EMT has been extensively investigated in various solid tumors and has been reviewed in detail previously[8]. For example, E-cadherin, a critical protein responsible for cell-cell adhesion, is decreased when cancer cells lose their epithelial phenotype, especially within TBs at the invasive front of cancers[8]. In contrast, master regulators of EMT, including EMT-associated transcription factors (ZEB1, ZEB2, TWIST1, TWIST2, SNAI1 and SNAI2) and signaling pathways (WNT/β-catenin, TGF-β), are significantly overexpressed or activated in cancer cells obtained from TB[8]. TB also shares many invasive abilities endowed by EMT, such as cytoskeletal rearrangements, cell motility and increased proteolytic activity[8]. Furthermore, cancer stem cell markers, including CD44, LGR5 and ALDH1, often test positive on TB cells, indicating the anoikis resistance, self-renewal and metastasis-establishing capacity of these dissociated cells[8]. This evidence suggests that TB cells represent the morphological phenotype of EMT and are often identified adjacent to or within the endothelium of either lymphatic or blood vessels microscopically, indicating their possible intravasation and dissemination process. Studies on the association of TB and EMT or cancer stem cells (CSCs) are very limited in GC. In the invasive front of intestinal-type GC tissues, TB scores were positively correlated with the expression of ZBTB7A, which was shown to increase the expression of EGFR, leading to the activation of the PI3K-AKT-mTOR pathway and MAPK-ERK pathway, therefore greatly altering the expression of EMT markers[21]. In addition, although not examined specifically in TB cells, several studies have found elevated expression of EMT and CSC biomarkers at the invasive front of GC, supporting the activation of EMT and CSC programs in TB cells in GC tissues[22-24].
An attacker-defender model has been proposed to describe the interactions between TB cells and anticancer immunosurveillance. In this model, TB cells represent the attacker owing to their aggressive attributes, while innate and adaptive immune cells mediate the counterattack[8]. Therefore, the predictive and prognostic potential were improved significantly by the integration of TB and the immune microenvironment. In the invasive front of GC, TB counts were inversely associated with a high microsatellite instability (MSI) phenotype, owing to the local immune response capable of eradicating TB cells with increased generation of neoantigens[13]. The immune microenvironment analysis around TB in GC revealed that TB density is negatively correlated with the number of tumor-infiltrating lymphocytes (TILs), and the density of TILs changed from CD8+ > FOXP3+ > OX40+ > GrB+ T cells in the nonbudding area to FOXP3+ > CD8+ > OX40+ > GrB+ T cells in the budding area, indicating a privileged immune environment created by TB cells[25]. The recruitment of immunosuppressive cells enables evasion of the antitumor immune response by cancer cells and is proposed to be a critical step toward progression. Thus, the prognosis was significantly poor in patients who had a lower density of TILs and a higher density of TB[25].
Elucidating the acquisition of the EMT state and the immune escape mechanisms of TB as well as the interactions between TB cells and the surrounding stroma has the potential to reveal novel therapeutic targets. Thus, except for standardizing the assessment of TB as a promising predictive and prognostic biomarker in GC, the mechanisms underlying these clinical associations need to be further explored.
THE APPLICATION PROSPECT OF TB IN GC
Currently, several GC subtyping systems based on molecular markers have been proposed by various research organizations, such as The Cancer Genome Atlas and Asian Cancer Research Group[26,27]. However, these molecular tests are still costly, which hinders their wide application in clinical practice. A major advantage of GC patient stratification based on TB is that it can be counted along with conventional histopathological examination in H&E-stained tissue sections simultaneously without extra costs. Therefore, if more high-quality evidence is accumulated and consensus on definition and assessment methods is achieved in GC, TB could potentially be considered relevant in the following different clinical scenarios (Figure 2).
Figure 2.
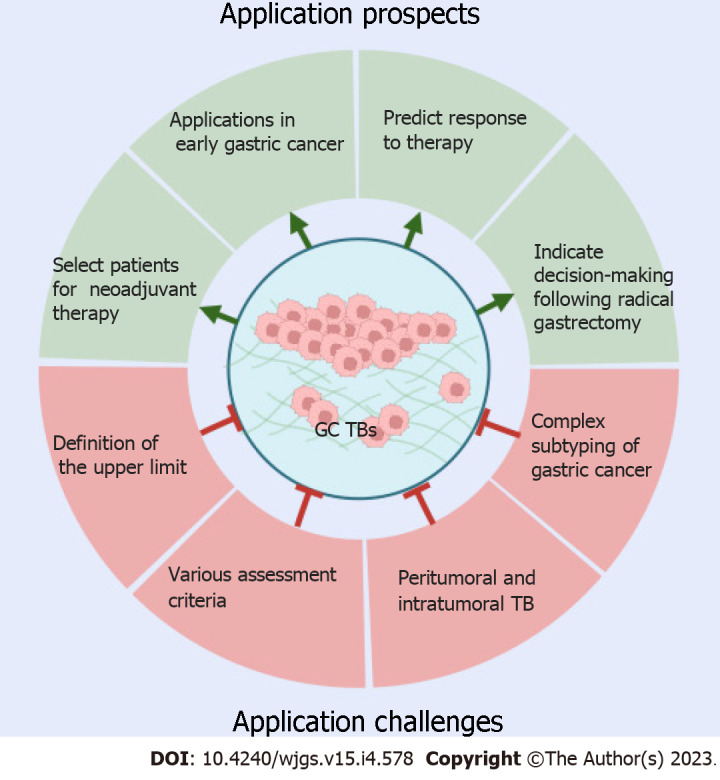
Application prospects and challenges of tumor budding in gastric cancer. Tumor budding (TB) could potentially be considered relevant in the selection of patients for neoadjuvant therapy, management of early gastric cancer (GC) following endoscopic resection, therapy response prediction, and decision-making after radical gastrectomy. However, before its routine clinical application, several challenges need to be overcome: Standardization of the definition and assessment of peritumoral and intratumoral TB in GC according to subtypes. This figure was created with BioRender.com. GC: Gastric cancer; TB: Tumor budding.
Selecting patients for neoadjuvant therapy
Due to unspecific symptoms and delayed presentation, GC is often diagnosed in an advanced stage, which requires preoperative chemotherapy to improve the curative resection rate and long-term survival. Currently, the preoperative treatment decision is determined mainly by TNM staging and patient performance status, and indications and modalities are recommended variously across different countries due to the lack of reliable prognostic and predictive biomarkers[28]. In addition to radically resected specimens, TB was also assessed in endoscopic biopsies with far less available cancer tissue and has been found to provide prognostic information[29]. In this regard, TB is a promising biomarker candidate that serves to select patients for neoadjuvant therapy. A study including both esophageal and gastroesophageal adenocarcinoma found that TB in preoperative endoscopic biopsies is strongly associated with poor survival[30]. Intriguingly, such prognostic significance was found only in stage Ⅱ but not stage Ⅲ cancers[30]. One possible explanation for such divergence may be that other strong negative prognostic factors in stage Ⅲ cancers dilute the patient stratification capability of TB, which can very well exhibit its influence on long-term outcomes in stage Ⅱ cancers[30]. However, in most countries, patients with stage II GC are often recommended to receive radical gastrectomy directly[28]. As the recurrence and metastasis of cancer following radical surgery is mainly due to distant disseminated cancer cells before surgery, whether neoadjuvant therapy is feasible and can improve long-term survival in patients with stage II GC warrants further prospective randomized controlled trials.
Applications in early GC
An increasing number of patients with early GC have been treated by endoscopic mucosectomy and endoscopic submucosal dissection because of their similar survival outcomes but lower incidence of complications[31]. Initially, endoscopic resection was only applied to patients with an absolute indication that the risk of lymph node metastasis is less than 1%; then, indications were expanded and relative indications were established to identify additional patients suitable for endoscopic treatment, which raises the requirement for improving the accuracy of histopathological examination on endoscopically resected specimens to predict lymph node metastasis, in which case additional radical gastrectomy is needed[31]. TB is a well-known predictor for lymph node metastasis in early-stage colorectal cancer and has already been considered a standard to determine the curability following endoscopic resection[9]. Recently, it has also emerged as a potential risk factor for lymph node metastasis in GC. However, the majority of these studies included patients with all stages, and only a few studies specifically focused on early GC. In a study reported by Yim et al[32], the risk for lymph node metastasis in early GC with high-grade TB (Bd2 and Bd3) increased by nearly 16 times, and the predictive efficacy was obviously improved when TB was added to conventional clinicopathological factors[32]. In this study, the risks of TB for lymph node metastasis in patients with pT1a and pT1b were not determined separately[32]. As only 4.3% of patients with pT1a disease developed lymph node metastasis, the predictive value may mainly work in pT1b patients[32]. In another study, although TB was reported to be the only variable that remained significant as an independent predictor of lymph node positivity in both pT1a and pT1b diseases, there were only 5 patients with pT1a found to be lymph node positive, and only 2 of them had high-grade TB[33]. Therefore, as the number of pT1a patients with TB is small, the application potential of TB in this subgroup of patients is still unknown at present. Therefore, a multicenter clinicopathologic study only included patients with pT1b disease and concluded that TB is a significant high-risk factor for lymph node metastasis in submucosal early GC[34]. Supporting these findings indirectly, additional gastrectomy with lymph node dissection needs to be considered when high-grade TB is observed in endoscopically resected specimens, especially in cancers invading the submucosa. Except for these studies assessing TB activity in surgically resected specimens, one study also assessed TB activity in endoscopically resected specimens and found that patients with high-grade TB activity who only underwent endoscopic therapy had a lower DFS and OS rate than patients who received an additional radical gastrectomy[21]. Collectively, TB is a powerful independent predictor of lymph node metastasis and poor survival in early GC; thus, it is recommended that radical gastrectomy should be applied to patients with high-grade TB scores in their endoscopic resected specimens.
On the other hand, currently, the majority of guidelines on GC recommend that D1 lymph node dissection is sufficient in patients with early GC who are treated with curative surgery[35-37]. However, the evidence supporting such a recommendation was based on studies including patients without further stratification. Because high TB scores indicate a higher probability of lymph node involvement, whether more extensive lymph node dissection is required in early GC with high TB activity warrants validation in comparative trials.
Predicting response to therapy
Although GC is one of the most common malignancies worldwide, a relatively small number of options for effective therapy are available to clinicians compared with other solid cancers. Currently, chemotherapy based on fluoropyrimidine, platinums, taxanes, and irinotecan is still the foundation of treatment for advanced GC[3]. However, no robust biomarkers for predicting the response to these drugs have been developed. Therefore, the administration of these drugs is only based on the clinical stage and the performance of patients. As a cost-effective histopathological biomarker, TB has been explored to predict the response to chemotherapy in some cancers, and a poor response was reported in patients with high TB activity[38]. However, data in GC are limited, possibly owing to the difficulty of obtaining tissues for TB assessment in advanced GC, in which setting it is feasible to assess therapy response.
Nevertheless, TB assessment in surgically resected specimens may provide indirect information for therapy response for other strategies, such as targeted therapy and immunotherapy, which were introduced into the multidisciplinary treatment of GC in recent years. For example, TB activity is inversely correlated with the expression of HER-2 but positively correlated with the expression of MET[13]. Both HER-2 and MET are well-defined therapeutic targets in cancer, and several agents targeting them have already advanced to clinical practice[39]. However, as robust predictive biomarkers for these targeted therapies have been developed, the clinical application significance of TB in these scenarios is limited.
In contrast, there is an unmet need to identify better predictive biomarkers of response to immunotherapy at present. TB is regarded as a special morphology of EMT, which has been shown to correlate with the activation of various immune checkpoint molecules, including programmed cell death protein 1 ligand[40]. Thus, whether high TB activity may be a potential predictor of immune checkpoint inhibitor therapy deserves further research. However, in GC, increasing peritumoral inflammation leads to decreased TB activity due to inflammation-related destruction of cancer cells at the invasive front, although the survival influence was not evaluated[13]. In addition, MSI-high tumors are often characterized by a pushing border type invasion front, no or low TB and a strong peritumoral inflammatory infiltrate[13]. The immune microenvironment analysis around TB in GC has revealed that TB density is negatively correlated with the number of TILs, indicating a privileged immune environment created by TB cells[25]. Therefore, these findings indicate that high-grade TB in GC may represent a promising negative biomarker for immunotherapy.
Collectively, the predictive value of TB in response to therapy is only speculated from indirect evidence, while direct supporting data are lacking in the present literature. In consideration of its assessment convenience and lack of additional cost, the predictive values can be easily verified when the efficacy of various therapy strategies is explored in clinical trials.
Applications in the management following radical gastrectomy
Currently, early-stage GC is believed to have a very high 5-year survival rate after endoscopic resection or curative gastrectomy; therefore, adjuvant therapy is not recommended in such patients. However, recurrence and metastasis are still inevitable in a small number of these patients[41]. Therefore, in addition to helping identify patients with high lymph node metastasis probability following endoscopic resection for whom an additional gastrectomy should be considered, TB also provides critical information regarding the decision of adjuvant chemotherapy after curative surgery. On the other hand, all guidelines on the management of GC recommended that all patients with locally advanced GC should receive adjuvant chemotherapy after curative resection. However, more than half of the patients with stage Ⅱ who receive only curative gastrectomy will not experience recurrence; therefore, whether assessment of TB will help decision-making in these scenarios needs to be investigated in future studies.
In the postneoadjuvant therapy setting, although ypTNM and tumor regression grade (TRG) have been routinely examined in resected specimens, other prominent prognostic histopathological parameters are usually omitted, and clinical guidelines directing further management of these patients are still lacking[42]. Therefore, additional biomarkers with potential prognostic or predictive value in this specific condition are urgently needed to stratify patients into groups with different prognoses that may require different management. In contrast to TB assessment in primary gastrectomy specimens, there has only been one study investigating the prognostic relevance of TB in resected GC specimens following preoperative therapy[43]. In 167 intestinal-type GC patients in the post neoadjuvant setting, TB was reported according to the ITBCC recommendation and was identified as an independent prognostic factor, even after adjusting for post neoadjuvant stage and TRG[43]. The authors concluded that post neoadjuvant assessment of TB categories according to the ITBCC criteria is feasible and that the prognostic power of TB is not disrupted by chemotherapy effects[43]. Although data are limited and more evidence is needed in GC, parallel with the consistent findings in other cancer types, such as esophageal, colorectal, and breast cancer, the routine implementation of TB assessment in the post neoadjuvant setting may be feasible and essential in patients with GC[44-46].
Therefore, assessment of TB would aid clinicians in decision-making. By this, they would make adjuvant therapy in patients more likely to experience recurrence or, on the other hand, avoid unnecessary side effects in those least likely.
CHALLENGES LIMITING THE APPLICATION OF TB IN GC
Despite the abovementioned promising clinical application potential in GC, TB is still not routinely assessed in daily practice, largely owing to the lack of GC-specific standardized assessment methods, including definition, cutoff determination, and location to count. In addition, the complex histopathological heterogeneity of GC further impedes the consistent validation of this biomarker in various studies (Figure 2).
Four cancer cells to define the upper limit of TB
Throughout the literature, a single tumor cell or a cluster of no more than four cohesive tumor cells separated from the main cancer mass is consistently used to define TB, and an agreement has been achieved in colorectal cancer at the ITBCC[11]. However, although clinical applications have already gained great success in colorectal cancer, this cutoff is an arbitrary determination, and whether it is the best definition feasible for GC has not been extensively validated. Although the prognostic power of TB is not affected by the number of cells that define a cluster in other cancer types and studies have recurrently demonstrated its prognostic and predictive values in GC, no prospective large cohort studies have compared the differences among TBs defined by various upper limits, except for another tumor cluster presentation with more than four cancer cells, poorly differentiated clusters (PDCs), which have been proposed.
PDCs have been investigated in several cancer types and have shown prognostic potential[47]. Compared with TB, whose accurate determination may be challenged when TBs are mixed with degenerating normal glands or inflammatory stoma without cytokeratin immunohistochemical staining, PDCs are more easily and accurately recognized[48]. Therefore, in colorectal cancer, PDCs were found to have stronger prognostic potential than TBs[49-51]. Theoretically, TB and PDCs indeed represent the same biological phenomenon, EMT, which underlies their prognostic roles. Therefore, some authors have integrated them into a single scoring system and proved the value of the combined effects in prognosis for squamous cell carcinomas[52,53]. Only a limited number of studies have been conducted to explore the prognostic value of PDCs in GC, and findings from these studies are somewhat different from those in other cancer types. In a study with a small sample size (n = 50), a positive association between PDC categories and poor prognosis was observed[54]. In contrast, in another study with a relatively large sample size (n = 290), the presence of PDCs was marginally associated with the features of local tumor spread, such as PNI, LVI and advanced T stage, but not with lymph node metastasis and poorer survival[14]. Therefore, the application of PDCs in GC prognosis is inconclusive at present.
In addition, PDCs represent another group of cancer cell clusters with more than 4 cells but are not a new definition of TB. Therefore, whether the upper limit of four cancer cells is truly the optimal choice in GC warrants further validation. Nevertheless, both TB and PDCs are defined by the number of tumor cells residing in the invasive front or the center of the cancer mass, and they are just quantitative but not qualitative characteristics. In other words, are TB and PDCs observed on tissue slides truly TB and PDCs? In fact, some cancer cells, which were determined to be detached cells/cell clusters from the main cancer mass in the two-dimensional plane of histological sections, are actually found to be part of the main tumor in 3D reconstruction[55]. In addition, is EMT or CSC programs activated in all TBs and PDCs counted by pathologists according to the present recommended criteria? In fact, the invasive and metastatic potential of TB cells varies substantially, suggesting overt differences in the prognosis of patients with colorectal cancer depending on the profile of CSC markers in TB cells[56]. Therefore, more studies are needed to investigate whether assessment of TB with activation of EMT or CSC programs will provide stronger prognostic and predictive values.
Various criteria to assess TB and to define high-grade TB
Before and even after the publication of the ITBCC guidelines, the methods used to assess TB in GC varied across studies. For example, Gabbert et al[10] examined the TCD, used synonymously to describe TB, at the invasive front of GC by using semiquantitative scoring (TCD0-3). Then, some studies reported the mean or median TB counts of several hotspots at the cancer invasive front, and cutoffs ranging from 1 (presence or absence) to 10 have been used to categorize GC into low- and high-grade TB (Table 1). After the publication of the ITBCC guidelines, most studies reported and classified TB in GC according to the ITBCC guidelines, and in some studies, small modifications have been made. For example, some authors classified bud 0 as Bd0, and some authors defined high-grade TB activity as Bd3 or the integration of Bd2 and Bd3 (Table 1). However, few cutoffs have been de novo established based on a GC cohort and validated in a separate validation cohort. Therefore, similar to the upper limit of TB definition, the optimal cutoffs to define different TB activities are also not clear, especially in diffuse-type GC, the majority of which may fall within high-grade TB according to ITBCC criteria. To date, only one study has determined the cutoff value of the TB score by receiver operating characteristic curves, and the optimal cutoff value of the TB score was set to 5, which had the best predictive significance for lymph node metastasis[21]. However, only 122 total and 71 intestinal-type patients were included, which may prevent generalization. In another study, the authors compared the differences in prognostic values among different categorizations of TB[32]. This study showed that TB activity classified by ITBCC criteria predicts lymph node metastasis better than TB presence, at least in early GC[32]. Therefore, although the ITBCC criteria are now adopted as the standard method to assess TB for reproducibility, the optimal definition of high-grade TB in GC is still unclear.
Peritumoral and intratumoral TB
Traditionally, TB was assessed mainly at the invasive front of cancer, termed peritumoral budding (PTB). However, as the pathologists who prepare and read tissue slides may not be the same, it is difficult to ensure that the invasive front used to determine PTB truly represents the patients’ cancer infiltration state, leading to inaccurate predictive and prognostic values[57]. In addition, in some conditions, it is impossible to determine the invasive front of GC. For example, the majority of GC patients were initially diagnosed by biopsies, which were often obtained from the cancer center rather than from its invasive front, and the biopsy specimens are the only tissues available for TB assessment because some patients may not undergo gastrectomy for unresectability or poor performance status. In the conditions of some early GC, it is also difficult to specify an invasive front in the intrusive cancer. Moreover, after neoadjuvant therapy, the architecture of cancer is often disrupted to some extent, especially in cancers with a strong response. The invasive front is hard to determine, and assessing peritumoral TB is very difficult or infeasible. Thus, intratumoral TB from the cancer center was suggested in those cases and was demonstrated to have a very high correlation with peritumoral TB activity[58,59]. A significant positive correlation between ITB and PTB was also found in GC, and on the same slide, the number of ITB was higher than that of PTB[57]. Both ITB and PTB predicted a shorter OS, while the simultaneous presence of ITB and PTB had a stronger prognostic value[57]. Some authors have tried to assess both peritumoral TB and intratumoral TB, also referred to as total TB, which seems to be superior to peritumoral TB in several cancer types. In GC, although statistical significance has not been achieved, total TB showed a tendency to predict lymph node metastasis better[32]. Therefore, assessment of ITB activity may not only provide similar predictive and prognostic value as PTB but can also replace PTB when the assessment of the latter is infeasible.
Complex subtyping of GC
In contrast to other cancer types, such as colorectal cancer and breast cancer, many biomarkers have not yet achieved full application in the routine clinical prognostic and predictive categorization of GC patients due to the complex histopathological characteristics of GC and the lack of consistent explicitness in all subtypes. The widely used simple histopathological classification system, proposed by Lauren in 1965, divides GC into intestinal, diffuse and mixed types[60]. In studies on the association between GC and TB, some studies included all subtypes, while others only included intestinal-type GC (Table 1). As indicated by its definition, diffuse GC grows with a highly dissociative pattern that would assign the majority of them into the high-grade TB category, which may confound the prognostic power of TB in patients with intestinal-type GC. Therefore, whether a GC with a diffuse or mixed component should be included to assess TB is still under debate. Some authors stated that TB and tumor grade are not the same, as high TB was not consistently found in all poorly differentiated cancers; thus, excluding this subgroup of patients is not necessary[13]. In contrast, Kemi et al[61] concluded that assessing TB in diffuse-type GC provides no prognostic information based on findings from 583 patients; therefore, routinely examining TB in diffuse-type GC is not recommended.
Signet ring cell (SRC) cancer, a distinctive subtype classified in the WHO classification system, is another poorly cohesive GC, which may also increase the difficulty of TB counting. Theoretically, SRC cancer belongs to diffuse-type GC and is more likely to display TB activity. However, compared with other poorly cohesive cancers, SRC-type GC showed a favorable prognosis in patients with early disease[62,63]. Therefore, Yim et al[32] developed a modified TB determining method, which excluded SRCs to overcome this contradiction. In early GC, modified TB was more predictive of lymph node involvement than conventional TB. In contrast, SRC-matched TB had no significant association with lymph node metastasis; rather, it showed a greater tendency toward patients without lymph node metastasis[32]. This finding was also supported by targeted genomic sequencing data, which found that many EMT-related molecules are not involved in the early onset of SRC cancer[63-65]. Therefore, as an EMT marker, SRCs are inappropriate to be identified as TBs for GC. Currently, such a conclusion may only be applied to early GC, as no such findings have been reported in advanced GC, in which a higher percentage of SRC is also inversely related to tumor aggressiveness and predicts better survival[66,67].
CONCLUSION
Although TB is promising in the management of GC in several clinical scenarios, further research is needed before its implementation in routine practice. There are many unknowns about the roles of TB in the management of GC. Considering the assessment method, although consensus has already been achieved in colorectal cancer and the ITBCC criteria are now widely accepted as the standardized method in studies on other cancer types, inconsistent criteria are still used to assess TB in GC. Therefore, multicenter studies are needed to yield a standardized methodology in GC similar to that in colorectal cancer for clinical decision-making. As in many settings in GC, it is difficult to determine the invasive front of cancer in available tissues, and studies on the use of ITB in these settings need further exploration. As discussed above, the complex histopathological subtypes of GC cause inconsistent conclusions, indicating the requirement for exploring the applications in each subgroup separately. In addition, TB is intimately associated with activation of EMT and CSC programs, leading to an increased capability for cancer cell dissociation, migration and metastasis. The interactions between TB and the immune microenvironment also facilitate the spread of GC. Therefore, as an easily available and cost-effective biomarker, TB assessment holds important prognostic, predictive and potentially therapeutic implications. A deeper understanding of the molecular and pathogenetic mechanisms underpinning TB may lead to an area of ‘anti-TB therapies’ in GC.
ACKNOWLEDGEMENTS
The author thanks the Health Commission of Mianyang City and the Science and Education Department of the Third Hospital of Mianyang for their support. The space limitations of this review have unfortunately meant that we have not been able to separately cite many of the original publications that have contributed substantially to the literature. We sincerely apologize to the authors of these publications. All figures in this review were created with BioRender.com.
Footnotes
Conflict-of-interest statement: There are no conflicts of interest to report.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: December 21, 2022
First decision: February 1, 2023
Article in press: March 21, 2023
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Chen X, China; Lotz G, Hungary; Mokni M, Tunisia S-Editor: Yan JP L-Editor: A P-Editor: Yan JP
Contributor Information
Shuo-Meng Xiao, Department of Gastric Surgery, Sichuan Cancer Hospital, Chengdu 610041, Sichuan Province, China.
Jian Li, Department of General Surgery, The Third Hospital of Mianyang, Sichuan Mental Health Center, Mianyang 621000, Sichuan Province, China. 654747973@qq.com.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Ajani JA, Lee J, Sano T, Janjigian YY, Fan D, Song S. Gastric adenocarcinoma. Nat Rev Dis Primers. 2017;3:17036. doi: 10.1038/nrdp.2017.36. [DOI] [PubMed] [Google Scholar]
- 3.Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet. 2020;396:635–648. doi: 10.1016/S0140-6736(20)31288-5. [DOI] [PubMed] [Google Scholar]
- 4.Díaz Del Arco C, Ortega Medina L, Estrada Muñoz L, García Gómez de Las Heras S, Fernández Aceñero MJ. Is there still a place for conventional histopathology in the age of molecular medicine? Histol Histopathol. 2021;36:587–613. doi: 10.14670/HH-18-309. [DOI] [PubMed] [Google Scholar]
- 5.Zhu J, Xue Z, Zhang S, Guo X, Zhai L, Shang S, Zhang Y, Lu H. Integrated analysis of the prognostic role of the lymph node ratio in node-positive gastric cancer: A meta-analysis. Int J Surg. 2018;57:76–83. doi: 10.1016/j.ijsu.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 6.Li TT, Liu H, Yu J, Shi GY, Zhao LY, Li GX. Prognostic and predictive blood biomarkers in gastric cancer and the potential application of circulating tumor cells. World J Gastroenterol. 2018;24:2236–2246. doi: 10.3748/wjg.v24.i21.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Szor DJ, Dias AR, Pereira MA, Ramos MFKP, Zilberstein B, Cecconello I, Ribeiro-Júnior U. Prognostic Role of Neutrophil/Lymphocyte Ratio in Resected Gastric Cancer: A Systematic Review and Meta-analysis. Clinics (Sao Paulo) 2018;73:e360. doi: 10.6061/clinics/2018/e360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lugli A, Zlobec I, Berger MD, Kirsch R, Nagtegaal ID. Tumour budding in solid cancers. Nat Rev Clin Oncol. 2021;18:101–115. doi: 10.1038/s41571-020-0422-y. [DOI] [PubMed] [Google Scholar]
- 9.Haddad TS, Lugli A, Aherne S, Barresi V, Terris B, Bokhorst JM, Brockmoeller SF, Cuatrecasas M, Simmer F, El-Zimaity H, Fléjou JF, Gibbons D, Cathomas G, Kirsch R, Kuhlmann TP, Langner C, Loughrey MB, Riddell R, Ristimäki A, Kakar S, Sheahan K, Treanor D, van der Laak J, Vieth M, Zlobec I, Nagtegaal ID. Improving tumor budding reporting in colorectal cancer: a Delphi consensus study. Virchows Arch. 2021;479:459–469. doi: 10.1007/s00428-021-03059-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gabbert HE, Meier S, Gerharz CD, Hommel G. Tumor-cell dissociation at the invasion front: a new prognostic parameter in gastric cancer patients. Int J Cancer. 1992;50:202–207. doi: 10.1002/ijc.2910500208. [DOI] [PubMed] [Google Scholar]
- 11.Lugli A, Kirsch R, Ajioka Y, Bosman F, Cathomas G, Dawson H, El Zimaity H, Fléjou JF, Hansen TP, Hartmann A, Kakar S, Langner C, Nagtegaal I, Puppa G, Riddell R, Ristimäki A, Sheahan K, Smyrk T, Sugihara K, Terris B, Ueno H, Vieth M, Zlobec I, Quirke P. Recommendations for reporting tumor budding in colorectal cancer based on the International Tumor Budding Consensus Conference (ITBCC) 2016. Mod Pathol. 2017;30:1299–1311. doi: 10.1038/modpathol.2017.46. [DOI] [PubMed] [Google Scholar]
- 12.Guo YX, Zhang ZZ, Zhao G, Zhao EH. Prognostic and pathological impact of tumor budding in gastric cancer: A systematic review and meta-analysis. World J Gastrointest Oncol. 2019;11:898–908. doi: 10.4251/wjgo.v11.i10.898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ulase D, Heckl S, Behrens HM, Krüger S, Röcken C. Prognostic significance of tumour budding assessed in gastric carcinoma according to the criteria of the International Tumour Budding Consensus Conference. Histopathology. 2020;76:433–446. doi: 10.1111/his.13997. [DOI] [PubMed] [Google Scholar]
- 14.Szalai L, Jakab Á, Kocsmár I, Szirtes I, Kenessey I, Szijártó A, Schaff Z, Kiss A, Lotz G, Kocsmár É. Prognostic Ability of Tumor Budding Outperforms Poorly Differentiated Clusters in Gastric Cancer. Cancers (Basel) 2022;14 doi: 10.3390/cancers14194731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pun C, Luu S, Swallow C, Kirsch R, Conner JR. Prognostic Significance of Tumour Budding and Desmoplastic Reaction in Intestinal-Type Gastric Adenocarcinoma. Int J Surg Pathol. 2022:10668969221105617. doi: 10.1177/10668969221105617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olsen S, Jin L, Fields RC, Yan Y, Nalbantoglu I. Tumor budding in intestinal-type gastric adenocarcinoma is associated with nodal metastasis and recurrence. Hum Pathol. 2017;68:26–33. doi: 10.1016/j.humpath.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 17.Cao L, Wang Z, Duan L, Wei L. Analysis of Endoscopy Findings to Identify Early Gastric Cancers with Tumor Budding: A Retrospective Study. J Gastrointest Surg. 2021;25:1706–1715. doi: 10.1007/s11605-020-04862-6. [DOI] [PubMed] [Google Scholar]
- 18.Dao TV, Nguyen CV, Nguyen QT, Vu HTN, Phung HT, Bui OT, Nguyen DK, Luong BV, Tran TV. Evaluation of Tumor Budding in Predicting Survival for Gastric Carcinoma Patients in Vietnam. Cancer Control. 2020;27:1073274820968883. doi: 10.1177/1073274820968883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grigore AD, Jolly MK, Jia D, Farach-Carson MC, Levine H. Tumor Budding: The Name is EMT. Partial EMT. J Clin Med. 2016;5 doi: 10.3390/jcm5050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dongre A, Weinberg RA. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat Rev Mol Cell Biol. 2019;20:69–84. doi: 10.1038/s41580-018-0080-4. [DOI] [PubMed] [Google Scholar]
- 21.Sun Y, He J, Shi DB, Zhang H, Chen X, Xing AY, Gao P. Elevated ZBTB7A expression in the tumor invasive front correlates with more tumor budding formation in gastric adenocarcinoma. J Cancer Res Clin Oncol. 2021;147:105–115. doi: 10.1007/s00432-020-03388-3. [DOI] [PubMed] [Google Scholar]
- 22.Kodama H, Murata S, Ishida M, Yamamoto H, Yamaguchi T, Kaida S, Miyake T, Takebayashi K, Kushima R, Tani M. Prognostic impact of CD44-positive cancer stem-like cells at the invasive front of gastric cancer. Br J Cancer. 2017;116:186–194. doi: 10.1038/bjc.2016.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bornschein J, Seidel T, Langner C, Link A, Wex T, Selgrad M, Jechorek D, Meyer F, Bird-Lieberman E, Vieth M, Malfertheiner P. MMP2 and MMP7 at the invasive front of gastric cancer are not associated with mTOR expression. Diagn Pathol. 2015;10:212. doi: 10.1186/s13000-015-0449-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tanaka K, Shimura T, Kitajima T, Kondo S, Ide S, Okugawa Y, Saigusa S, Toiyama Y, Inoue Y, Araki T, Uchida K, Mohri Y, Kusunoki M. Tropomyosin-related receptor kinase B at the invasive front and tumour cell dedifferentiation in gastric cancer. Br J Cancer. 2014;110:2923–2934. doi: 10.1038/bjc.2014.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang N, Wang D, Duan Y, Ayarick VA, Cao M, Wang Y, Zhang G. The special immune microenvironment of tumor budding and its impact on prognosis in gastric adenocarcinoma. Pathol Res Pract. 2020;216:152926. doi: 10.1016/j.prp.2020.152926. [DOI] [PubMed] [Google Scholar]
- 26.Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202–209. doi: 10.1038/nature13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cristescu R, Lee J, Nebozhyn M, Kim KM, Ting JC, Wong SS, Liu J, Yue YG, Wang J, Yu K, Ye XS, Do IG, Liu S, Gong L, Fu J, Jin JG, Choi MG, Sohn TS, Lee JH, Bae JM, Kim ST, Park SH, Sohn I, Jung SH, Tan P, Chen R, Hardwick J, Kang WK, Ayers M, Hongyue D, Reinhard C, Loboda A, Kim S, Aggarwal A. Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. Nat Med. 2015;21:449–456. doi: 10.1038/nm.3850. [DOI] [PubMed] [Google Scholar]
- 28.Joshi SS, Badgwell BD. Current treatment and recent progress in gastric cancer. CA Cancer J Clin. 2021;71:264–279. doi: 10.3322/caac.21657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamadera M, Shinto E, Nagata K, Shiraishi T, Kajiwara Y, Mochizuki S, Okamoto K, Kishi Y, Ueno H. Proposal for a tumor budding predictive score derived from endoscopic biopsy samples in colorectal cancer. Int J Clin Oncol. 2022;27:756–764. doi: 10.1007/s10147-021-02104-6. [DOI] [PubMed] [Google Scholar]
- 30.Beer A, Reber A, Paireder M, Schoppmann SF, Heber S, Schiefer AI. Tumor cell budding in preoperative biopsies of esophageal and gastroesophageal junction carcinoma independently predicts survival in a grade-dependent manner. Surgery. 2022;172:567–574. doi: 10.1016/j.surg.2022.02.020. [DOI] [PubMed] [Google Scholar]
- 31.Ono H, Yao K, Fujishiro M, Oda I, Nimura S, Yahagi N, Iishi H, Oka M, Ajioka Y, Ichinose M, Matsui T. Guidelines for endoscopic submucosal dissection and endoscopic mucosal resection for early gastric cancer. Dig Endosc. 2016;28:3–15. doi: 10.1111/den.12518. [DOI] [PubMed] [Google Scholar]
- 32.Yim K, Jang WM, Lee SH. Modified Tumor Budding as a Better Predictor of Lymph Node Metastasis in Early Gastric Cancer: Possible Real-World Applications. Cancers (Basel) 2021;13 doi: 10.3390/cancers13143405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gulluoglu M, Yegen G, Ozluk Y, Keskin M, Dogan S, Gundogdu G, Onder S, Balik E. Tumor Budding Is Independently Predictive for Lymph Node Involvement in Early Gastric Cancer. Int J Surg Pathol. 2015;23:349–358. doi: 10.1177/1066896915581200. [DOI] [PubMed] [Google Scholar]
- 34.Du M, Chen L, Cheng Y, Wang Y, Fan X, Zhang Y, Zhou X, Guo L, Xu G, Zou X, Huang Q. Tumor Budding and Other Risk Factors of Lymph Node Metastasis in Submucosal Early Gastric Carcinoma: A Multicenter Clinicopathologic Study in 621 Radical Gastrectomies of Chinese Patients. Am J Surg Pathol. 2019;43:1074–1082. doi: 10.1097/PAS.0000000000001276. [DOI] [PubMed] [Google Scholar]
- 35.Ajani JA, D'Amico TA, Bentrem DJ, Chao J, Cooke D, Corvera C, Das P, Enzinger PC, Enzler T, Fanta P, Farjah F, Gerdes H, Gibson MK, Hochwald S, Hofstetter WL, Ilson DH, Keswani RN, Kim S, Kleinberg LR, Klempner SJ, Lacy J, Ly QP, Matkowskyj KA, McNamara M, Mulcahy MF, Outlaw D, Park H, Perry KA, Pimiento J, Poultsides GA, Reznik S, Roses RE, Strong VE, Su S, Wang HL, Wiesner G, Willett CG, Yakoub D, Yoon H, McMillian N, Pluchino LA. Gastric Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2022;20:167–192. doi: 10.6004/jnccn.2022.0008. [DOI] [PubMed] [Google Scholar]
- 36.Wang FH, Shen L, Li J, Zhou ZW, Liang H, Zhang XT, Tang L, Xin Y, Jin J, Zhang YJ, Yuan XL, Liu TS, Li GX, Wu Q, Xu HM, Ji JF, Li YF, Wang X, Yu S, Liu H, Guan WL, Xu RH. The Chinese Society of Clinical Oncology (CSCO): clinical guidelines for the diagnosis and treatment of gastric cancer. Cancer Commun (Lond) 2019;39:10. doi: 10.1186/s40880-019-0349-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Japanese Gastric Cancer Association. Japanese Gastric Cancer Treatment Guidelines 2021 (6th edition) Gastric Cancer. 2023;26:1–25. doi: 10.1007/s10120-022-01331-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jäger T, Neureiter D, Fallaha M, Schredl P, Kiesslich T, Urbas R, Klieser E, Holzinger J, Sedlmayer F, Emmanuel K, Dinnewitzer A. The potential predictive value of tumor budding for neoadjuvant chemoradiotherapy response in locally advanced rectal cancer. Strahlenther Onkol. 2018;194:991–1006. doi: 10.1007/s00066-018-1340-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nakamura Y, Kawazoe A, Lordick F, Janjigian YY, Shitara K. Biomarker-targeted therapies for advanced-stage gastric and gastro-oesophageal junction cancers: an emerging paradigm. Nat Rev Clin Oncol. 2021;18:473–487. doi: 10.1038/s41571-021-00492-2. [DOI] [PubMed] [Google Scholar]
- 40.Jiang Y, Zhan H. Communication between EMT and PD-L1 signaling: New insights into tumor immune evasion. Cancer Lett. 2020;468:72–81. doi: 10.1016/j.canlet.2019.10.013. [DOI] [PubMed] [Google Scholar]
- 41.Ito H, Inoue H, Ikeda H, Onimaru M, Yoshida A, Hosoya T, Sudo K, Eleftheriadis N, Maselli R, Maeda C, Wada Y, Sando N, Hamatani S, Kudo SE. Clinicopathological characteristics and treatment strategies in early gastric cancer: a retrospective cohort study. J Exp Clin Cancer Res. 2011;30:117. doi: 10.1186/1756-9966-30-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gervaso L, Pellicori S, Cella CA, Bagnardi V, Lordick F, Fazio N. Biomarker evaluation in radically resectable locally advanced gastric cancer treated with neoadjuvant chemotherapy: an evidence reappraisal. Ther Adv Med Oncol. 2021;13:17588359211029559. doi: 10.1177/17588359211029559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jesinghaus M, Herz AL, Kohlruss M, Silva M, Grass A, Lange S, Novotny A, Ott K, Schmidt T, Gaida M, Hapfelmeier A, Denkert C, Weichert W, Keller G. Post-neoadjuvant assessment of tumour budding according to ITBCC subgroups delivers stage- and regression-grade independent prognostic information in intestinal-type gastric adenocarcinoma. J Pathol Clin Res. 2022;8:448–457. doi: 10.1002/cjp2.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lohneis P, Rohmann J, Gebauer F, Hieggelke L, Bruns C, Schröder W, Büttner R, Löser H, Quaas A. International Tumor Budding Consensus Conference criteria determine the prognosis of oesophageal adenocarcinoma with poor response to neoadjuvant treatment. Pathol Res Pract. 2022;232:153844. doi: 10.1016/j.prp.2022.153844. [DOI] [PubMed] [Google Scholar]
- 45.Kim S, Huh JW, Lee WY, Yun SH, Kim HC, Cho YB, Park YA, Shin JK. Prognostic Impact of Lymphatic Invasion, Venous Invasion, Perineural Invasion and Tumor Budding In Rectal Cancer Treated With Neoadjuvant Chemoradiotherapy Followed By Total Mesorectal Excision. Dis Colon Rectum. 2022 doi: 10.1097/DCR.0000000000002266. [DOI] [PubMed] [Google Scholar]
- 46.Mozarowski P, Rasaiah B, Reed M, Lewis A, Walde N, Voutsadakis IA. Prognostic Role of Tumor Budding in Breast Cancer Patients Receiving Neo-Adjuvant Therapy. J Clin Med. 2021;10 doi: 10.3390/jcm10040827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shivji S, Conner JR, Barresi V, Kirsch R. Poorly differentiated clusters in colorectal cancer: a current review and implications for future practice. Histopathology. 2020;77:351–368. doi: 10.1111/his.14128. [DOI] [PubMed] [Google Scholar]
- 48.Lee VWK, Chan KF. Tumor budding and poorly-differentiated cluster in prognostication in Stage II colon cancer. Pathol Res Pract. 2018;214:402–407. doi: 10.1016/j.prp.2017.12.019. [DOI] [PubMed] [Google Scholar]
- 49.Barresi V, Reggiani Bonetti L, Ieni A, Branca G, Tuccari G. Histologic prognostic markers in stage IIA colorectal cancer: a comparative study. Scand J Gastroenterol. 2016;51:314–320. doi: 10.3109/00365521.2015.1084646. [DOI] [PubMed] [Google Scholar]
- 50.Ueno H, Kajiwara Y, Shimazaki H, Shinto E, Hashiguchi Y, Nakanishi K, Maekawa K, Katsurada Y, Nakamura T, Mochizuki H, Yamamoto J, Hase K. New criteria for histologic grading of colorectal cancer. Am J Surg Pathol. 2012;36:193–201. doi: 10.1097/PAS.0b013e318235edee. [DOI] [PubMed] [Google Scholar]
- 51.Barresi V, Reggiani Bonetti L, Branca G, Di Gregorio C, Ponz de Leon M, Tuccari G. Colorectal carcinoma grading by quantifying poorly differentiated cell clusters is more reproducible and provides more robust prognostic information than conventional grading. Virchows Arch. 2012;461:621–628. doi: 10.1007/s00428-012-1326-8. [DOI] [PubMed] [Google Scholar]
- 52.Jesinghaus M, Boxberg M, Konukiewitz B, Slotta-Huspenina J, Schlitter AM, Steiger K, Specht K, Wieczorek K, Warth A, Schmidt T, Hartmann A, Demir IE, Feith M, Ott K, Weichert W. A Novel Grading System Based on Tumor Budding and Cell Nest Size Is a Strong Predictor of Patient Outcome in Esophageal Squamous Cell Carcinoma. Am J Surg Pathol. 2017;41:1112–1120. doi: 10.1097/PAS.0000000000000865. [DOI] [PubMed] [Google Scholar]
- 53.Weichert W, Kossakowski C, Harms A, Schirmacher P, Muley T, Dienemann H, Warth A. Proposal of a prognostically relevant grading scheme for pulmonary squamous cell carcinoma. Eur Respir J. 2016;47:938–946. doi: 10.1183/13993003.00937-2015. [DOI] [PubMed] [Google Scholar]
- 54.Sorrentino L, De Ruvo N, Serra F, Salati M, Ricciardolo AA, Bonetti LR, Gelmini R. Role of poorly differentiated cluster in gastric cancer: is it a new prognosis factor? Scand J Gastroenterol. 2022;57:44–49. doi: 10.1080/00365521.2021.1974932. [DOI] [PubMed] [Google Scholar]
- 55.Bronsert P, Enderle-Ammour K, Bader M, Timme S, Kuehs M, Csanadi A, Kayser G, Kohler I, Bausch D, Hoeppner J, Hopt UT, Keck T, Stickeler E, Passlick B, Schilling O, Reiss CP, Vashist Y, Brabletz T, Berger J, Lotz J, Olesch J, Werner M, Wellner UF. Cancer cell invasion and EMT marker expression: a three-dimensional study of the human cancer-host interface. J Pathol. 2014;234:410–422. doi: 10.1002/path.4416. [DOI] [PubMed] [Google Scholar]
- 56.Hostettler L, Zlobec I, Terracciano L, Lugli A. ABCG5-positivity in tumor buds is an indicator of poor prognosis in node-negative colorectal cancer patients. World J Gastroenterol. 2010;16:732–739. doi: 10.3748/wjg.v16.i6.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Qi B, Liu L, Pan Y, Xu S, Li J. Prognostic significance of peritumoural and intratumoural budding in intestinal-type gastric adenocarcinoma. Arab J Gastroenterol. 2020;21:111–116. doi: 10.1016/j.ajg.2020.04.005. [DOI] [PubMed] [Google Scholar]
- 58.Thies S, Guldener L, Slotta-Huspenina J, Zlobec I, Koelzer VH, Lugli A, Kröll D, Seiler CA, Feith M, Langer R. Impact of peritumoral and intratumoral budding in esophageal adenocarcinomas. Hum Pathol. 2016;52:1–8. doi: 10.1016/j.humpath.2016.01.016. [DOI] [PubMed] [Google Scholar]
- 59.Lugli A, Vlajnic T, Giger O, Karamitopoulou E, Patsouris ES, Peros G, Terracciano LM, Zlobec I. Intratumoral budding as a potential parameter of tumor progression in mismatch repair-proficient and mismatch repair-deficient colorectal cancer patients. Hum Pathol. 2011;42:1833–1840. doi: 10.1016/j.humpath.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 60.Lauren P. The Two Histological Main Types Of Gastric Carcinoma: Diffuse And So-Called Intestinal-Type Carcinoma. An Attempt At A Histo-Clinical Classification. Acta Pathol Microbiol Scand. 1965;64:31–49. doi: 10.1111/apm.1965.64.1.31. [DOI] [PubMed] [Google Scholar]
- 61.Kemi N, Eskuri M, Ikäläinen J, Karttunen TJ, Kauppila JH. Tumor Budding and Prognosis in Gastric Adenocarcinoma. Am J Surg Pathol. 2019;43:229–234. doi: 10.1097/PAS.0000000000001181. [DOI] [PubMed] [Google Scholar]
- 62.Chon HJ, Hyung WJ, Kim C, Park S, Kim JH, Park CH, Ahn JB, Kim H, Chung HC, Rha SY, Noh SH, Jeung HC. Differential Prognostic Implications of Gastric Signet Ring Cell Carcinoma: Stage Adjusted Analysis From a Single High-volume Center in Asia. Ann Surg. 2017;265:946–953. doi: 10.1097/SLA.0000000000001793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kwon CH, Kim YK, Lee S, Kim A, Park HJ, Choi Y, Won YJ, Park DY, Lauwers GY. Gastric poorly cohesive carcinoma: a correlative study of mutational signatures and prognostic significance based on histopathological subtypes. Histopathology. 2018;72:556–568. doi: 10.1111/his.13383. [DOI] [PubMed] [Google Scholar]
- 64.Shu Y, Zhang W, Hou Q, Zhao L, Zhang S, Zhou J, Song X, Zhang Y, Jiang D, Chen X, Wang P, Xia X, Liao F, Yin D, Zhou X, Zhang D, Yin S, Yang K, Liu J, Fu L, Zhang L, Wang Y, Zhang J, An Y, Cheng H, Zheng B, Sun H, Zhao Y, Xie D, Ouyang L, Qiu M, Fu X, Dai L, He G, Yang H, Cheng W, Yang L, Liu B, Li W, Dong B, Zhou Z, Wei Y, Peng Y, Xu H, Hu J. Prognostic significance of frequent CLDN18-ARHGAP26/6 fusion in gastric signet-ring cell cancer. Nat Commun. 2018;9:2447. doi: 10.1038/s41467-018-04907-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Humar B, Blair V, Charlton A, More H, Martin I, Guilford P. E-cadherin deficiency initiates gastric signet-ring cell carcinoma in mice and man. Cancer Res. 2009;69:2050–2056. doi: 10.1158/0008-5472.CAN-08-2457. [DOI] [PubMed] [Google Scholar]
- 66.Roviello F, Marano L, Ambrosio MR, Resca L, D'Ignazio A, Petrelli F, Petrioli R, Costantini M, Polom K, Macchiarelli R, Biviano I, Marrelli D. Signet ring cell percentage in poorly cohesive gastric cancer patients: A potential novel predictor of survival. Eur J Surg Oncol. 2022;48:561–569. doi: 10.1016/j.ejso.2021.09.003. [DOI] [PubMed] [Google Scholar]
- 67.Li Y, Zhong Y, Xu Q, Zhu Z, Tian Y. Prognostic Significance of Signet Ring Cells in Gastric Cancer: The Higher Proportion, The Better Survival. Front Oncol. 2021;11:713587. doi: 10.3389/fonc.2021.713587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Che K, Zhao Y, Qu X, Pang Z, Ni Y, Zhang T, Du J, Shen H. Prognostic significance of tumor budding and single cell invasion in gastric adenocarcinoma. Onco Targets Ther. 2017;10:1039–1047. doi: 10.2147/OTT.S127762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Heckl SM, Wiesener V, Behrens HM, Ulase D, Krüger S, Röcken C. The expression of the insulin receptor in gastric cancer correlates with the HER2 status and may have putative therapeutic implications. Gastric Cancer. 2019;22:1130–1142. doi: 10.1007/s10120-019-00964-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kucuk S. Prognostic value of tumour budding in stomach cancers. Int J Clin Pract. 2021;75:e14922. doi: 10.1111/ijcp.14922. [DOI] [PubMed] [Google Scholar]