Keywords: myofibrillar protein synthesis, physiology, satellite cells, resistance training, ribosomes
Abstract
We sought to determine if the myofibrillar protein synthetic (MyoPS) response to a naïve resistance exercise (RE) bout, or chronic changes in satellite cell number and muscle ribosome content, were associated with hypertrophic outcomes in females or differed in those who classified as higher (HR) or lower (LR) responders to resistance training (RT). Thirty-four untrained college-aged females (23.4 ± 3.4 kg/m2) completed a 10-wk RT protocol (twice weekly). Body composition and leg imaging assessments, a right leg vastus lateralis biopsy, and strength testing occurred before and following the intervention. A composite score, which included changes in whole body lean/soft tissue mass (LSTM), vastus lateralis (VL) muscle cross-sectional area (mCSA), midthigh mCSA, and deadlift strength, was used to delineate upper and lower HR (n = 8) and LR (n = 8) quartiles. In all participants, training significantly (P < 0.05) increased LSTM, VL mCSA, midthigh mCSA, deadlift strength, mean muscle fiber cross-sectional area, satellite cell abundance, and myonuclear number. Increases in LSTM (P < 0.001), VL mCSA (P < 0.001), midthigh mCSA (P < 0.001), and deadlift strength (P = 0.001) were greater in HR vs. LR. The first-bout 24-hour MyoPS response was similar between HR and LR (P = 0.367). While no significant responder × time interaction existed for muscle total RNA concentrations (i.e., ribosome content) (P = 0.888), satellite cell abundance increased in HR (P = 0.026) but not LR (P = 0.628). Pretraining LSTM (P = 0.010), VL mCSA (P = 0.028), and midthigh mCSA (P < 0.001) were also greater in HR vs. LR. Female participants with an enhanced satellite cell response to RT, and more muscle mass before RT, exhibited favorable resistance training adaptations.
NEW & NOTEWORTHY This study continues to delineate muscle biology differences between lower and higher responders to resistance training and is unique in that a female population was interrogated. As has been reported in prior studies, increases in satellite cell numbers are related to positive responses to resistance training. Satellite cell responsivity, rather than changes in muscle ribosome content per milligrams of tissue, may be a more important factor in delineating resistance-training responses in women.
INTRODUCTION
Resistance training (RT) adaptations have been studied for decades, mainly in young adult male participants (1, 2). A 2014 study examining three major sport science journals from 2011 to 2013 found 39% of study participants were female and only 4–13% of studies contained participant groups that were females only (3). While advances have been made in the field, younger adult males do not appropriately represent how other populations will adapt to exercise (e.g., younger females, older individuals, older males). Therefore, there is a heightened need to address the literature gap of female participant inclusion in sport science.
Two RT adaptations that have been well documented include strength increases (4–6) and muscle growth (or hypertrophy) (7, 8). Moreover, certain lines of evidence suggest resistance training can improve muscle quality (i.e., force generation per muscle cross section), especially in older females (9, 10). While it is generally presumed that all participants will exhibit improvements in these variables, there are various lines of evidence demonstrating that “lower” and “higher” responders exist regarding strength (11) and hypertrophy outcomes (12–16), with most studies investigating hypertrophy responders. Importantly, these responder terms typically only apply to a few attributes and are not meant to imply a participant is a lower or nonresponder to exercise. A lower responder usually has a response to other attributes that might not be measured in that study. Specifically, being a lower hypertrophic responder could be a potential barrier to long-term training adherence, given that one of the chief motivations for a novice trainee is to gain skeletal muscle mass (17). Moreover, although lower hypertrophic responders can experience significant strength gains during training (13, 18, 19), there is some evidence suggesting lower hypertrophic responders to 12 wk of RT may also have impaired strength gains (11, 18). Thus deciphering variables that differentiate higher versus lower hypertrophic responders to RT is warranted, particularly in females.
Several studies from our laboratory (20–22) and others (16, 23, 24) have attempted to elucidate molecular variables that predict the hypertrophic response to RT. Collectively, these studies have demonstrated that three molecular variables, in particular, are associated with hypertrophy including 1) the ribosome biogenesis response to weeks of training, 2) the myofibrillar protein response days to weeks into training, and 3) the satellite cell response days to weeks into training. Nonetheless, there is debate pertaining to molecular variables being predictive of hypertrophic response to RT. Specifically, there is evidence in human, animal, and cell models to support (20, 25–32) and refute (7, 33) ribosome biogenesis and the myofibrillar protein synthetic responses being associated with muscle hypertrophy. Additionally, there are reports suggesting satellite cell proliferation and/or myonuclear accretion with training are (18, 34–37) and are not (38–40) associated with hypertrophic responses in animal models. Second, human studies have used both trained (5, 21, 41–45) and untrained participants (12, 16, 20, 31, 46–48). Training status influences adaptation and makes it difficult to compare findings between cohorts. Third, training interventions have ranged from 4 to 16 wk, raising the possibility of temporal variability among the reported molecular adaptations. Fourth, the criteria and statistics used to delineate responder clusters have vastly differed among studies, as well as what variable “responder” refers to (e.g., hypertrophy, strength, etc.). For example, some papers have used a single outcome variable to define the hypertrophic response, such as mean myofiber cross-sectional area (fCSA) (16), or ultrasound-determined muscle thickness (20, 49, 50). In contrast, others have adopted a composite score to delineate the hypertrophic response using multiple variables (16, 21, 22). Finally, most of these data are in males, and although women show a similar relative increase in muscle mass to RT (51), males and females adapt may show differential mechanistic responses to RT. For example, a prior study performed in younger and older males and females who underwent 16 wk of RT demonstrated that younger female participants generally did not exhibit hypertrophy assessed through mean fCSA (19). Likewise, it has been recently reported that men showed prolonged myofibrillar protein synthesis responses to a naïve resistance exercise bout compared to females (52).
To our knowledge, no one study has sought to determine if the acute myofibrillar protein synthetic response to a naïve resistance exercise bout, or chronic changes in muscle ribosome content or satellite abundance to RT, are predictive of muscle hypertrophy in younger adult females. Thus the aims of this study were to examine the following in female participants: 1) if the 24-h myofibrillar protein synthesis response to a naïve bout of RT was associated with hypertrophic outcomes, 2) if chronic changes in muscle ribosome content (RNA per mg tissue) and satellite cell abundance after 10 wk of RT were associated with hypertrophic outcomes, and 3) determine whether these three variables differed between higher versus lower responders (via a composite score of strength and hypertrophy metrics). Based on the current literature we hypothesized all three outcome variables would be associated with hypertrophy in all participants. We also hypothesized that higher responders would exhibit more favorable changes in these molecular markers compared to lower responders.
METHODS
Ethical Approval and Prescreening
This study was approved by the Auburn University Institutional Review Board (IRB) (Protocol No. 19-249 MR 1907), conformed to the standards set by the latest revisions of the Declaration of Helsinki, and was registered as a clinical trial (NCT04707963). Thirty-four young, college-aged, untrained female participants were recruited from Auburn University’s campus and the surrounding area via email, flyers, and word of mouth. The intent of the originally approved trial is to determine whether daily peanut protein (PP) supplementation, versus no supplementation, affects RT adaptations in college-aged males and females (53). In short, peanut protein supplementation did not affect hypertrophic or strength outcomes, so this study is a secondary analysis of the female participants from the trial.
The eligibility criteria for participation were defined as follows: between the ages of 18 and 30, body mass index <35 kg/m2; no recent participation in RT (less than one time per week for 6 months prior); no known peanut allergy; free of metal implants that may interfere with X-ray procedures; no medically necessary radiation exposure (excluding dental X-rays) for 6 months prior; free of obvious cardiovascular or metabolic disease; blood pressure below 140/90 mmHg (with or without medication); free of conditions contraindicating participation in exercise programs or donating muscle biopsies (i.e., taking blood thinners or blood clotting disorders); and not pregnant or trying to become pregnant. Those who were deemed eligible and agreed to participate received an informed consent packet and were verbally informed of all study procedures. Following verbal and written consent, participants completed a health history questionnaire and scheduled a time for pretesting (PRE).
Study Design
The timeline and testing battery for each visit can be visualized in Fig. 1A and is described in greater detail below. Participants reported to the laboratory a total of 5 times for testing and 20 times for RT.
Figure 1.
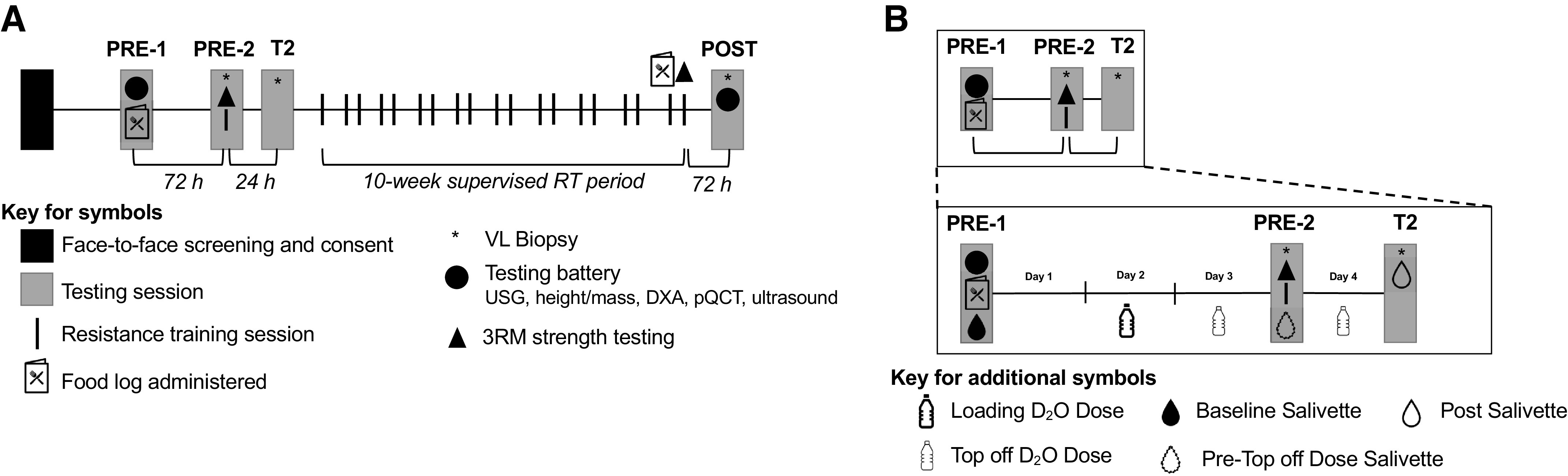
Study design. A: outline of the study design. More in-depth descriptions of procedures can be found in the text. B: outline of the protocol for D2O dosing and collection of salivette samples in relation to the points of data collection. DXA, dual energy X-ray absorptiometry; pQCT, peripheral quantitative computed tomography; 3RM, 3-repetition maximum; RT, resistance training; VL, vastus lateralis; PRE-1, first visit pretesting; PRE-2, second visit pretesting; T2, second skeletal muscle biopsy visit; USG, urine specific gravity.
The PRE time sample was completed in two visits. During the first visit (PRE-1), participants reported to the laboratory after fasting for at least 4 hours and underwent an array of testing, beginning with urine specific gravity (USG) to ensure hydration and a rapid pregnancy test. The following examinations were completed thereafter: 1) height and weight measurements, 2) whole body dual X-ray absorptiometry (DXA), 3) right leg midthigh peripheral quantitative computed tomography (pQCT) scan, 4) VL ultrasound at the midthigh, and 5) isokinetic dynamometry of the right knee extensors. The participants then chewed a salivette (SARSTEDT, Nümbrect, Germany) to provide saliva for the baseline assessment of whole body deuterium oxide (D2O). At the end of PRE-1, participants were given containers of D2O (70 atom percent; Sigma-Aldrich, St. Louis, MO) to take home and consume over the next 3 days and a 3-day food log. Participants consumed a total of 4.5 mL of D2O per kg of lean/soft tissue mass before the first muscle biopsy.
The second visit of PRE testing (PRE-2) occurred 3 days following PRE-1 and consisted of the baseline biopsy, strength assessment, and initial/naïve training bout. During PRE-2, participants chewed a salivette to monitor D2O enrichment, consumed a top-off dose of D2O (0.5 mL/kg), and donated skeletal muscle from the right VL via biopsy before performing baseline strength testing on the bilateral 45° leg press, barbell bench press, and hex-bar deadlift. This strength testing, with the addition of 2 sets of 10 repetitions on the leg press, bench press, and deadlifts at 50% of their estimated 1-repetition maximum strength (1-RM), was considered the first/naïve training session (training bout used for the 24-h myofibrillar protein synthesis response).
Twenty-four hours after PRE-2, participants returned to the laboratory at least 4 hours fasted for their second skeletal muscle biopsy visit (T2) and final salivette sampling for D2O enrichment. After the T2 visit, participants completed their 10-wk RT program, wherein the last session included maximum strength assessments and isokinetic dynamometry. The last testing session (POST) was conducted ∼72 h following the completion of the last RT session, and the following battery of tests was performed: 1) USG, 2) body mass, 3) DXA, 4) pQCT, 5) VL ultrasound, and 6) a muscle biopsy that was 1–2 cm proximal to the other two biopsy sites. The postintervention food log was given to participants in the week leading up to POST. Each of these tests is described in greater detail below.
Specific Testing Procedures
Urine specific gravity.
At the beginning of PRE-1 and POST, participants provided a urine sample (∼5 mL). The sample was immediately analyzed using a handheld refractometer (ATAGO; Bellevue, WA). All participants presented USG values 1.020 and were considered well hydrated.
Body composition.
Following USG assessments, body mass and height were assessed using a laboratory scale (Seca 769; Hanover, MD). Participants were then subjected to a whole body DXA scan (Lunar Prodigy; GE Corporation, Fairfield, CT) to assess bone-free lean/soft tissue mass (LSTM) and fat mass (FM). This instrument was calibrated using a phantom device, and quality assurance was tested on each day scans were performed before the test; participants were asked to remove any metal objects and to lie supine on the DXA scanner table underneath the scanner arm. Following a 5-min rest period, the scan was performed. Importantly, all scans were performed by the same investigator at both PRE and POST. Test-retest reliability from our laboratory using intraclass correlation coefficient 3,1 (ICC3,1), standard error of the measurement (SE), and minimal difference to be considered real (MD) was previously determined for whole body LSTM on 10 participants scanned ∼24 h apart. This resulted in an ICC3,1 of 0.99, SE of 0.36 kg, and MD of 0.99 kg (54).
Peripheral quantitative computed tomography.
After the DXA scan, a pQCT scan (Stratec XCT 3000, Stratec Medical, Pforzheim, Germany) of the right thigh was performed to measure midthigh muscle CSA (mCSA; cm2) and skeletal muscle density (mg/cm3) at 50% of the distance between the midinguinal crease and proximal patella. A permanent marker was used to indicate the precise transverse location of the scan, and a mark was made midbelly of the VL so that subsequent ultrasound images and muscle biopsy samples could be acquired from the exact location being imaged. Moreover, the biopsy scar was used as a reference point to ensure that POST images were captured at the same location as PRE. Each pQCT scan was captured using a scan speed of 20 mm/s, a 2.4-mm slice thickness, and a voxel size of 0.4 mm. Images were analyzed for mCSA and muscle density using the pQCT BoneJ plugin (55) freely available through ImageJ analysis software (NIH, Bethesda, MD). Importantly, all images were captured and analyzed by the same investigator who was blinded to group allocations. Test-retest reliability using ICC3,1, SE, and MD was previously determined for mCSA on 10 participants scanned ∼24 h apart resulting in an ICC3,1 of 0.99, SE of 0.84 cm2, and MD of 2.32 cm2.
Ultrasound.
Real-time B-mode ultrasonography (NextGen LOGIQe R8, GE Healthcare) utilizing a multifrequency linear-array transducer (L4-12T, 4–12 MHz, GE Healthcare) was used to capture images of the VL in the transverse plane for measurement of VL CSA. Before image acquisition, subjects rested supine on an examination table for a minimum of 5 minutes with the hip and knee fully extended. Images were captured at the same anatomical location as the pQCT scan as previously described above. For VL thickness, images were collected at a depth at which the edge of the femur was visible, and this depth was held constant for POST image collection. For VL CSA measurements, a high-density cork pad was placed around the circumference of the thigh and secured using an adjustable strap. The pad was used as a guide for the consistent placement and movement of the probe in the transverse plane. All VL CSA images were captured using a panoramic function (LogicView, GE Healthcare) with probe placement starting at the lateral aspect of the thigh and moving medially until the rectus femoris muscle was visible within the image. For all ultrasound images, a generous amount of water-soluble transmission gel was applied to both the skin and probe, and care was taken to apply a consistent probe pressure to maximize image quality without compressing the underlying tissue. All ultrasound settings (frequency: 10 MHz; gain: 50 dB; and dynamic range: 75), except for depth, were held constant across participants and sample times. One image per participant was obtained at each sample time. Images were analyzed using the freely available ImageJ software (National Institutes of Health, Bethesda, MD). VL CSA was calculated by manually tracing the border of the VL using the polygon function, with care taken to exclude any connective tissue within the region of interest. Again, these measurements were performed one time for each participant at each sample time. All ultrasound images were captured and analyzed by the same investigator with a previously determined test-retest reliability in 10 participants resulting in an ICC3,1, of 0.99, SE of 0.60 cm2, and MD of 1.65 cm2.
Strength assessments.
All participants began the testing session with a general warm-up consisting of 25 jumping jacks and 10 bodyweight squats, after which they completed a battery of strength testing in the order of 45° bilateral leg press, barbell bench press (flat bench), and hex-bar deadlifts. Each of the exercises began with three to five warm-up sets with the initial load being an unloaded barbell/machine; the load was then incrementally increased based on the participants’ perceived difficulty. As each set was completed, the participant was asked to rate the difficulty on a rating of perceived exertion (RPE) scale of 1–10 (“really easy” to “really hard”). An RPE of 1–4 resulted in a weight increase of 25%, an RPE of 5–7 with a 10% weight increase, and an RPE of 8–9 with a 2–3% weight increase. These guidelines were followed until the participant reached a load that could be completed for only five repetitions. Testing on each exercise finished with 1–3 sets of three repetitions with each of those sets increasing in intensity if the participant was able to complete all three repetitions. When a three-repetition maximum (3-RM) was achieved, that number was used to calculate an estimated 1-RM by dividing the 3-RM by 0.93 (56).
Skeletal Muscle Biopsies
Skeletal muscle biopsies were collected from the right leg VL at PRE-2, T2, and POST visits. At PRE-2, the biopsy was collected from the VL at the same location as the ultrasound and pQCT scans, and at T2 and POST the sample was taken 1–2 cm proximal of the initial biopsy site, using the previous scar as a reference. For biopsies, participants lay down on an athletic table and the upper thigh was shaved and cleansed with 70% isopropanol. A subcutaneous injection of 1% lidocaine (0.8 mL) was then administered. After 5 min, the area was cleansed with chlorhexidine solution (Hibiclens, Mölnlycke Health Care, Norcross, GA). Next, a pilot incision was made through the dermis with a single-use sterile No. 11 surgical blade (AD Surgical, Sunnyvale, CA). The 5-gauge biopsy needle was inserted into the pilot incision, through the muscle fascia, and ∼2 cm into the muscle to collect a 50- to 100-mg sample while applying suction (57). Tissue was immediately removed from the needle, cleared of blood and connective tissue, and separated for RNA, histological, and tracer analysis. Tissue allocated for RNA and tracer analysis was placed in prelabeled foils and immediately frozen in liquid nitrogen and stored at −80°C. Tissue allocated to histological analysis was embedded in optimum cutting temperature (OCT) gel to prevent freeze damage, slow-frozen in liquid nitrogen-cooled isopentane, and stored at −80°C until sectioned and stained.
Resistance Training Protocol
The RT protocol was modeled after a previous study from our laboratory (58), as well as American College of Sports Medicine Guidelines for Exercise Testing and Prescription (59). The protocol consisted of 10 wk in duration and consisted of 20 separate training sessions (2 days/wk; Table 1). The training session included the 45° bilateral leg press, barbell bench press (flat bench), seated leg extension, hex-bar deadlifts, and lat pull down. Each training session involved the following:
-
1)
A general warm-up of 25 jumping jacks and 10 body weight squats,
-
2)
A specific warm-up of 1 set of 10 reps at 50% of predetermined work-out weight, 1 set of 5 repetitions at 75% of work-out weight, and 1 set of 3 repetitions of 90% of work-out weight; and
-
3)
Either i) 4 sets of 10 repetitions (higher volume day) or ii) 5 sets of 6 reps per exercise (higher load day), each done once per week at the prescribed weight.
Table 1.
Resistance exercise training program
| Week/Day | Sets × Repetitions | %1-RM |
|---|---|---|
| Week 1 | ||
| Day 1 | 3-RM testing (+ 2 × 10) | 50% |
| Day 2 | 5 × 6 | 56% |
| Week 2 | ||
| Day 3 | 4 × 10 | 55% |
| Day 4 | 5 × 6 | 65% |
| Week 3 | ||
| Day 5 | 4 × 10 | 60% |
| Day 6 | 5 × 6 | 74% |
| Week 4 | ||
| Day 7 | 4 × 10 | 65% |
| Day 8 | 5 × 6 | 84% |
| Week 5 | ||
| Day 9 | 4 × 10 | 50% |
| Day 10 | 5 × 6 | 50% |
| Week 6 | ||
| Day 11 | 4 × 10 | 65% |
| Day 12 | 5 × 6 | 84% |
| Week 7 | ||
| Day 13 | 4 × 10 | 70% |
| Day 14 | 5 × 6 | 90% |
| Week 8 | ||
| Day 15 | 4 × 10 | 75% |
| Day 16 | 5 × 6 | 96% |
| Week 9 | ||
| Day 17 | 4 × 10 | 80% |
| Day 18 | 5 × 6 | 98% |
| Week 10 | ||
| Day 19 | 5 × 6 | 102% |
| Day 20 | 3-RM testing |
The training paradigm for the 10-wk progressive resistance training program is shown. Each training session included the 45° bilateral leg press, barbell bench press (flat bench), seated leg extension, hex-bar deadlifts, and lat pull down. 1-RM, 1-repetition maximum test; 3-RM, 3-repetition maximum test.
To provide progressive overload, weekly loads increased by ∼5% for the higher volume day and ∼9% for the higher load day for the first 4 wk. Participants were then given a week of reduced load training at week 5 (deload), consisting of 50% intensity for both training sessions. During week 6, progressive loading ensued from week 4 values. Although loads were preprogrammed for all participants based on percentages of their 1-RM, participant RPE was used during each training session to ensure the appropriate load was implemented and the individual programs were adapted accordingly.
Responder Composite Score
A composite pre- to postchange score consisting of the following variables was used to define “lower” versus “higher” responders: 1) whole body lean/soft tissue mass (LSTM) determined by dual X-ray absorptiometry (DXA), 2) vastus lateralis (VL) cross-sectional area (CSA) determined by ultrasound, 3) midthigh muscle CSA determined by peripheral quantitative computed tomography (pQCT), and 4) maximum deadlift strength determined by a three-repetition maximum test (3-RM). Crucially, both strength and hypertrophy measures were included in the multidimensional composite score to be more inclusive in capturing various levels of response and not limiting the assessment to strength or hypertrophy exclusively. All metrics carried the same weight within the composite score. Therefore, “responder” refers specifically to the sum of the composite score variables used in the present study. Based on the resultant composite scores, we identified upper and lower quartiles of n = 8 higher and n = 8 lower responders, respectively.
Wet Laboratory Analyses
Immunohistochemistry.
PRE and POST biopsies preserved in OCT were batch processed for 1) cryostat sectioning, 2) antibody-based immunohistochemistry, and 3) imaging and analysis. Initially, all samples were sliced into 10 µm thick sections and removed electrostatically from the cooled cryostat stage by a positively charged histology slide (Leica Biosystems; Buffalo Grove, IL). The slides were then stored at −80°C until antibody staining.
Staining for muscle fCSA and myonuclear number per fiber was performed (60). Briefly, slides with sections were removed from −80°C storage and dried for ∼10 min at room temperature. Triton-X (0.5%) in phosphate buffer solution (PBS) was then used to permeabilize the sections for 5 min. This was followed by a 5-min wash in PBS, and slides were subsequently incubated in a 100% concentration of blocking solution for 15 min (Pierce Super Blocker, Thermo Fisher Scientific). Slides were then incubated in primary antibody solution for 60 min. This solution contained PBS, 5% of Pierce Super Blocker Solution, and equal parts at 2% (1:50 dilution) of rabbit anti-dystrophin IgG1 (cat. no. GTX15277, Genetex Inc., Irvine, CA) and mouse anti-myosin I IgG1 (cat. no. A4.951 supernatant; Developmental Studies Hybridoma Bank, Iowa City, IA). Slides were then washed for 5 min in PBS and then incubated in a secondary antibody solution containing a 1× base of PBS and equal parts at 1% or (1:100 dilution) Texas Red-conjugated anti-rabbit IgG (cat. no. TI-1000; Vector Laboratories, Burlingame, CA) and Alexa Fluor 488-conjugated anti-mouse IgG1 (cat. no. A-11001; Thermo Fisher Scientific). This incubation occurred in the dark for 60 min. The slides were then washed for 5 min in PBS, dried, and mounted using a fluorescent media containing 4,6-diamidino-2-phenylindole (DAPI; cat. no. GTX16206; Genetex Inc.). Immediately following mounting, slides were imaged using a fluorescent microscope (Nikon Instruments, Melville, NY) with a ×10 objective lens. Exposure times were 200 ms for FITC, 600 ms for TRITC, and 100 ms for DAPI. Open-source software (MyoVision) was used to analyze all images for average fCSA, muscle fiber type, and myonuclear number per fiber (60). A conversion of 0.964 μm/pixel was used to adjust the image for size and bit-depth, and a fiber size threshold was set at a minimum of 500 μm2 and a maximum of 15,000 μm2 to ensure the exclusion of spaces between fibers or fibers in an oblong orientation. Resultant images were visually inspected for erroneous detection of fibers.
Determination of MyoPS.
D2O was administered as a stable isotope tracer to determine MyoPS [as pioneered by Previs et al. (61) and the Fluckey laboratory (62)], and the timeline for D2O loading and collection of salivette samples for the study is shown in Fig. 1B. Saliva samples obtained in the laboratory (or returned by participants) were stored at −20°C. After study completion, salivette tubes were centrifuged for 2 min at 1,000 g (2°C). Saliva obtained thereafter was frozen at −20°C. Frozen samples were then shipped to Metabolic Solutions (Nashua, NH) on dry ice for analysis. Saliva analysis for deuterium enrichment occurred using cavity ring-down spectroscopy. Instrumentation included Liquid Water Isotope Analyzer with an automated injection system and a version 2 upgrade (Los Gatos Research, Mountain View, CA). Samples were vortexed and spun at 8,000 rpm to remove any particulates. The aqueous phase of saliva was injected six times, and the last three measurements were averaged for data analysis. Standard curves were generated before and after sample runs for the determination of deuterium enrichment. Intrarun precision using this method is generally <2 delta per mil (parts per thousand), and interrun precision is generally <3.5 delta per mil.
Muscle biopsy samples from PRE-2 and 24 h were batch processed using the myofibril isolation and solubilization technique as previously described by our laboratory (63). Isolated myofibrils were frozen at −80°C, and frozen samples were shipped on dry ice to Metabolic Solutions for tracer analyses. This process first involved hydrolyzing myofibril pellets for 18 h with 3 mL of 6 N HCl (100°C). Dowex H+ resin (1 mL, 50WX8-100; Sigma-Aldrich, St. Louis, MO) was added to trap released alanine. Amino acid elution from the resin was performed using 2 mL of 3 N NH4OH, and eluates were evaporated to dryness. Thereafter, the N-acetyl-n-propyl (NAP) derivative of alanine was prepared, and the propyl ester was formed by adding 200 uL propyl acetate and 100 uL BF3:propanol (14%). Samples were heated at 110°C for 30 min, and solutions evaporated to dryness under N2 gas at 60°C. The N-acetyl group was formed by adding 100 uL of 0.1 M diethylamine (DEA) in hexane and 100 uL of acetic anhydride. This reaction was incubated for 20 min at 60°C and was subsequently dried down with N2 gas and low heat. Samples were reconstituted in 100 uL ethyl acetate and pipetted into autosampler vials. Deuterated alanine from myofibrillar preparations was detected using a Thermo Finnigan Delta V IRMS coupled to a Thermo Trace GC Ultra with a GC combustion interface III and Conflow IV. The NAP ester of alanine was analyzed using a splitless injection with CTC Pal autosampler (1 µL). Injections used a Zebron ZB-5 column of 30 m × 0.25 mm × 0.50 µm film thickness (Phenomenex, Torrance, CA), and the injection temperature was 250°C. The GC oven had an initial column temperature of 80°C with a 2-min hold and was followed by a ramp of 30°C per minute to 330°C. Compounds eluting off the column were directed into the pyrolysis reactor and heated to 1,450°C hydrogen gas conversion. Deuterium enrichment was first expressed in delta values compared to a calibrated hydrogen gas. These values were then converted to atom %D by standard equations. Methylpalmitate was used as the calibration standard for the reference hydrogen gas. Intrarun precision for alanine measurements is generally <2 delta per mil, and interrun precision is generally <3 delta per mil.
Saliva and myofibril enrichments were used to calculate MyoPS rates over the 24-h period following the first naïve training bout. The equation follows the one published by Bell et al. (64) (see below; FSR, fractional synthesis rate).
Briefly, the difference in deuterium (2H) enrichment from the first two biopsies (EAla2 = 24-h muscle sample enrichment; EAla2 = PRE-2 muscle sample enrichment) was divided by the product total body enrichment of 2H (in atom % excess) (EBW = 2H from PRE-2 and 24-h saliva – baseline 2H from PRE-2 saliva) and the number of days that D2O was consumed at the loading dose. This quotient was then multiplied by 3.7 to adjust for the number of 2H atoms typically bound to alanine. Finally, the resultant value was multiplied by 100 to achieve myofibrillar synthesis rate in percent per day.
Determination of satellite cells.
Satellite cells were quantified using Pax7 immunohistochemistry. For this analysis, separate slides with adjacent sections were removed from −80°C storage and dried for ∼10 min at room temperature. Triton-X (0.5%) in PBS was used to permeabilize the sections for 5 min. This was followed by a 5-min wash in PBS, and slides were subsequently incubated in a 100% concentration of blocking solution for 15 min (Pierce Super Blocker). Slides were then incubated in primary antibody solution for 60 min. This solution contained PBS, 5% of Pierce Super Blocker Solution, and equal parts at 2% (1:50 dilution) of rabbit anti-dystrophin IgG1 (cat. no. GTX15277; Genetex Inc.; Irvine, CA) and mouse anti-Pax7 IgG (cat. no. PAX7 supernatant; Hybridoma Bank). Sections were then washed for 5 min in 1× PBS and incubated in the dark for 1 h with a secondary antibody solution containing Alexa Fluor 488-conjugated anti-rabbit IgG (Vector Laboratories),and Texas Red-conjugated anti-mouse IgG (Thermo Fisher Scientific) (10 µL of all secondary antibodies per 1 mL of blocking solution). Sections were then washed for 5 min in PBS thereafter, air-dried, and mounted with fluorescent media containing DAPI (Genetex). Following mounting, slides were imaged using a fluorescent microscope (Nikon Instruments) with a ×20 objective lens. Exposure times were 200 ms for FITC, 600 ms for TRITC, and 100 ms for DAPI. Satellite cells were manually counted by an investigator blinded to the PRE and POST time points using a grid function in ImageJ and a handheld tally counter. Due to imaging constraints (i.e., three fluorescent detection filters), satellite cells were not specified as type I or type II fiber specific.
Determination of muscle ribosome content.
For total RNA analysis, ∼15–30 mg of powdered tissue was weighed using an analytical scale with a sensitivity of 0.001 g (Mettler-Toledo, Columbus, OH). Tissue was then homogenized in 1.7-mL microcentrifuge tubes containing 500 μL of Ribozol (Ameresco, Solon, OH) via micropestle manipulation and RNA isolation was performed per manufacturer recommendations. Total RNA concentrations were determined in duplicate using a NanoDrop Lite spectrophotometer (Thermo Fisher Scientific, Waltham, MA), and total RNA per wet muscle weight was used as a surrogate for muscle ribosome content as in past publications (65, 66).
Food Log Analysis
The 3-day food log packets were used to obtain nutritional intakes for 1 weekend day and 2 weekdays in the week leading up to PRE-2 and POST visits and ensure the lack of change in participant diet. Therefore, it could be assumed the change in the outcome metrics was due to the exercise intervention and not to other changes in participant habits. Each food log contained an example of 1 day, with three additional blank sheets. Participants were instructed to write down as many details of their food as possible. If a food log was returned incomplete, we met with the participant to gather more information. Participants were asked to maintain their normal dietary habits throughout the duration of the study, except for daily consumption of the protein supplement in the PP group. Data from the food logs was entered into the Nutrition Data System for Research (NDSR) (NDSR 2014: University of Minnesota). Calories and macronutrients were averaged from the three days of food logs for a mean intake (kcal/day, g/day, or g/kg/day) at each sample time.
Additional Questionnaires
Self-reported sex, gender, race, and ethnicity were collected via questionnaires during the Informed Consent visit. Additionally, prior exercise history, menstrual cycle information, and contraceptive use were recorded during this visit. Day of the menstrual cycle was self-reported, with the first day of bleeding considered day 1. Finally, at POST, menstrual cycle information was collected again, as well as subjective sleep quality using the Pittsburgh Sleep Quality Index (67).
Statistical Analysis
Statistical analysis was performed in SPSS v26.0 (IBM Corp., Armonk, NY). Before statistical analysis, normality testing was performed on all dependent variables using Shapiro-Wilk tests at PRE and POST sample times.
Dependent samples t tests were performed to examine changes in outcome variables for all participants. For menstrual cycle analysis, independent samples t tests were used to compare the average day of cycle during PRE and POST testing. Additionally, dependent samples t tests were used to examine differences in menstrual cycle day within each cohort. Pearson correlations were also performed between each individual variable in the composite score and VL, CSA, and mean fCSA, and these tests included all participants.
For the higher responder (HR) versus lower responder (LR) analysis, independent samples t-tests were used to compare the 24-h MyoPS rates, baseline differences in select phenotypes, as well as self-reported sleep duration between cohorts. Two-way (group × time) repeated measure ANOVAs were used to determine changes in dependent variables before and following the training intervention in the HR versus LR cohorts. When a significant group × time interaction was observed, least significant differences post hocs were performed to determine differences within each group from pre- to postintervention and between groups at each sample time. Statistical significance was established a priori at P < 0.05.
RESULTS
Participant Characteristics
Baseline participant characteristics can be found in Table 2 We assessed 81 females for eligibility (43 were excluded, with the majority waitlisted based on our maximum limit of participants), 38 female participants began the study, and 34 female participants (21 ± 2.1 yr, >90% Caucasian, body mass index of 23.4 ± 3.4 kg/m2) completed the study. All participants completed above 90% of the workouts (i.e., at least 18 training sessions).
Table 2.
Participant characteristics and general training adaptations
| Variable | All (n = 34) | HR (n = 8) | LR (n = 8) | Interaction P value | P Value HR vs. LR |
|---|---|---|---|---|---|
| Age, years | 21 ± 2 | 21 ± 1 | 21 ± 1 | 0.693 | |
| Height, m | 1.7 ± 0.1 | 1.7 ± 0.1 | 1.7 ± 0.1 | 0.909 | |
| PRE body mass, kg | 68.2 ± 10.3 | 69.7 ± 11.1 | 62.5 ± 10.8 | 0.477 | 0.214 |
| POST body mass, kg | 68.2 ± 10.3 | 71.7 ± 10.9 | 63.5 ± 9.9 | 0.138 | |
| PRE DXA LSTM, kg | 42.1 ± 4.1 | 44.2 ± 3.3 | 39.2 ± 3.5 | <0.001 | 0.010 |
| POST DXA LSTM, kg | 43.2 ± 4.3* | 46.5 ± 3.2† | 39.5 ± 3.7 | 0.001 | |
| PRE DXA fat mass, kg | 23.0 ± 8.0 | 22.4 ± 8.6 | 21.8 ± 8.2 | 0.490 | 0.876 |
| POST DXA fat mass, kg | 22.5 ± 7.5 | 22.6 ± 9.0 | 21.4 ± 7.1 | 0.763 | |
| PRE 1-RM leg press, kg | 83 ± 39 | 111.8 ± 29 | 50.6 ± 39.4 | 0.580 | 0.003 |
| POST 1-RM leg press, kg | 168 ± 48+ | 184.9 ± 28.8 | 118.3 ± 33.3 | 0.001 | |
| PRE 1-RM bench press, kg | 32 ± 6 | 34.2 ± 5.4 | 27.2 ± 6.0 | 0.033 | 0.026 |
| POST 1-RM bench press, kg | 39 ± 7+ | 44.0 ± 5.1 | 33.3 ± 4.6 | 0.001 | |
| PRE 1-RM deadlift, kg | 60 ± 14 | 64.4 ± 11.6 | 51.7 ± 12.6 | <0.001 | 0.055 |
| POST 1-RM deadlift, kg | 82 ± 23* | 101.0 ± 11.1† | 64.7 ± 14.4† | <0.001 | |
| PRE relative 1-RM deadlift, kg/kg body mass | 0.91 ± 0.24 | 0.95 ± 0.25 | 0.84 ± 0.21 | 0.005 | 0.365 |
| POST relative 1-RM deadlift, kg/kg body mass | 1.22 ± 0.34* | 1.43 ± 0.23† | 1.03 ± 0.19† | 0.002 |
DXA LSTM, lean/soft tissue mass according to the dual energy X-ray absorptiometry scans; 1-RM, 1-repetition maximum; HR, higher responder; LR, lower responder *Significance between pretesting (PRE) and posttesting (POST) for all participants. †Increase within HR or LR from PRE to POST.
At PRE, participants weighed 68.2 ± 10.3 kg, with 42.1 ± 4.1 kg being LSTM and 23.0 ± 8.0 kg being FM. Body weight and FM did not change significantly over time. However, LSTM significantly increased with training (43.16 ± 4.27 kg, P < 0.001). There were no baseline differences between the HR and LR cohorts regarding age, height, or FM. Interestingly, at both PRE and POST, the HR cohort had significantly higher LSTM (PRE: P = 0.010; POST: P = 0.001; see Table 2 for values).
Training Volume and Strength Metrics
The HR cohort engaged in a higher training volume compared to LR throughout the duration of the study (HR = 151,101 ± 18,478 kg; LR = 101,145 ± 26,204 kg; P < 0.001). However, this may have been due to HR participants weighing more. When considering relative training volume throughout the study (i.e., total training volume divided by PRE body mass), no difference was evident between the HR and LR cohorts (HR = 2203 ± 348; LR = 1931 ± 1076; P = 0.506).
All strength metrics are presented in Table 2 For all participants, 1-RM values increased by 102.4% for leg press, 24.6% for bench press, and 36.9% for deadlift from PRE to POST (P < 0.001 for all). At PRE, the HR cohort had significantly greater 1-RM leg press and bench press (75.4% greater, P = 0.003 and 22.9% greater, P = 0.026, respectively). All POST strength metrics were significantly higher in the HR than the LR cohort (leg press, 44% greater, P < 0.001; bench press, 27.7% greater, P < 0.001; and deadlift, 43.8% greater, P < 0.001). Additionally, the change in strength between cohorts significantly differed for bench press (HR = 9.8 ± 1.7 kg; LR = 6.1 ± 3.8 kg; P = 0.025) and deadlift (HR = 36.5 ± 14.7 kg; LR = 13.0 ± 7.1 kg; P = 0.001) but not leg press (HR = 73.0 ± 14.5 kg; LR = 67.7 ± 23.3 kg; P = 0.588).
Responder Cohorts and Clustering Variables
Figure 2 displays the training adaptations from PRE to POST for the four variables used in the composite score for the responder analysis including DXA LSTM, VL CSA, midthigh mCSA, and estimated 1-RM deadlift. For all participants, the change values were as follows: 1.07 ± 1.11 kg, 2.67 ± 2.70 cm2, 8.9 ± cm2, and 24 ± 13 kg, respectively (Fig. 2A, P < 0.001, for all variables from pre- to posttraining). The HR cohort showed increases in these variables from PRE to POST (P < 0.001 for all variables). Midthigh mCSA (PRE = 98.8 ± 6.3 cm2; POST = 102.1 ± 5.9 cm2; P = 0.011) and estimated 1-RM deadlift (PRE = 51.7 ± 12.6 kg; POST = 64.7 ± 14.4 kg; P = 0.001) increased in the LR cohort. However, DXA LSTM (PRE = 39.2 ± 3.5 kg; POST = 39.5 ± 3.7 kg; P = 0.177) and VL CSA (PRE = 17.6 ± 1.9 cm2; POST = 18.1 ± 1.9 cm2; P = 0.067) did not. Finally, the HR cohort showed significantly greater increases than the LR cohort for all four variables (Fig. 2B, P < 0.001, for all variables).
Figure 2.
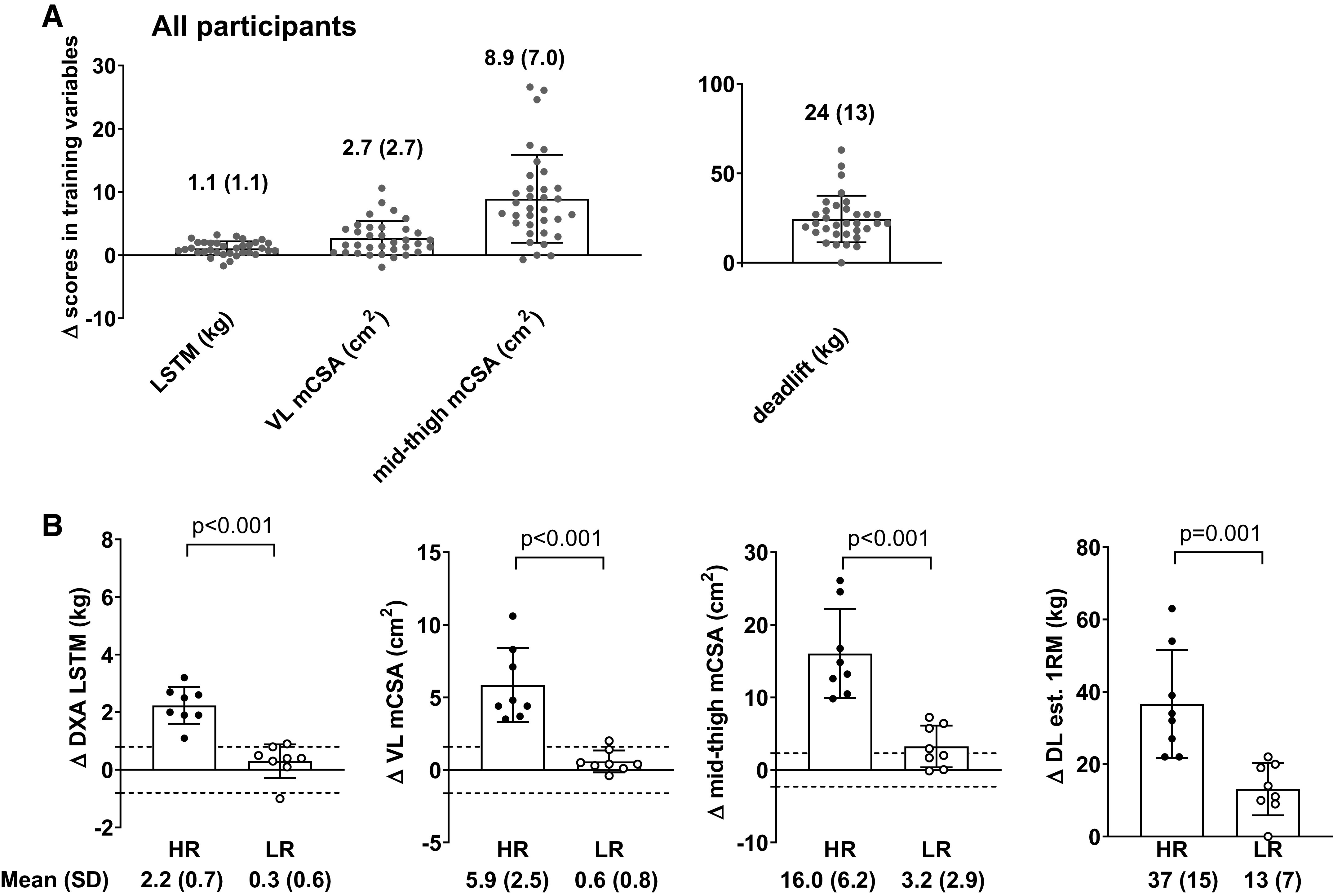
Adaptations in variables used for classifying response groups. A: pre- to postintervention change scores for outcome variables used to generate response groups in all 34 female participants. B: pre- to postintervention change score differences between the higher (n = 8) and lower (n = 8) responders. For DXA LSTM, VL mCSA, and midthigh mCSA, dashed lines in B represent minimal difference scores based on test-retest statistics discussed in methods. All bar graphs are expressed as means ± SD values and contain individual respondent values as well. DXA, dual energy X-ray absorptiometry; HR, higher responder; LR, lower responder; LSTM, lean/soft tissue mass; mCSA, muscle cross-sectional area; VL, vastus lateralis; 1RM, 1-repetition maximum; DL est., deadlift estimated.
Mean fCSA Changes
Changes in mean fCSA and the responder analysis for this variable can be seen in Fig. 3, A and B. For these and other histology analyses, 31 participants were included due to 3 participant samples yielding low-quality sections at one of the sampling time points. Mean fCSA significantly increased from PRE to POST on average by 17.7% for all participants (PRE = 4,290 ± 989 µm2; POST = 5,049 ± 1429 µm2; P = 0.001). However, there was no cluster × time interaction observed for the responder analysis (P = 0.606; Fig. 3B).
Figure 3.
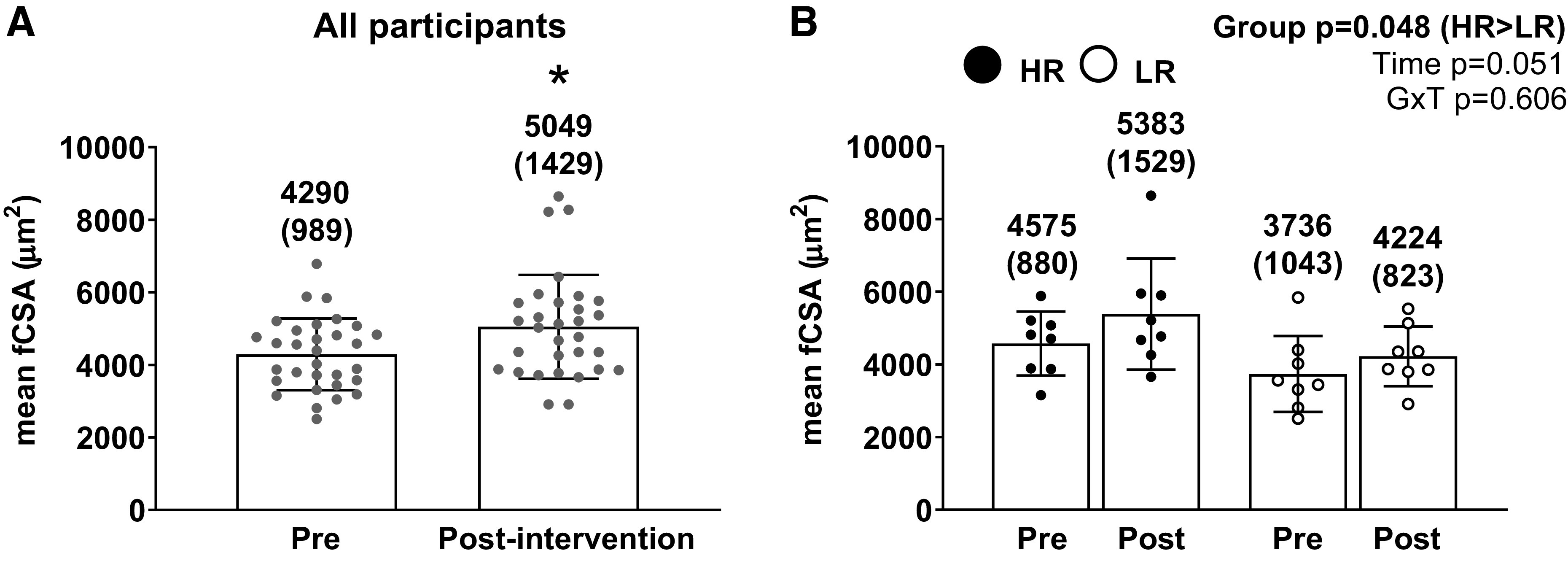
Mean fCSA changes in all participants and higher vs. lower responders. A: pre- and postintervention mean fCSA values in the 31 of 34 female participants that yielded enough tissue for analyses (*P < 0.05 comparing pre to post). B: pre- and postintervention mean fCSA values between the higher (n = 8) and lower (n = 8) responders. All bar graphs are expressed as means ± SD values and contain individual respondent values as well. fCSA, fiber cross-sectional area; GxT, group × time interaction; HR, higher responder; LR, lower responder.
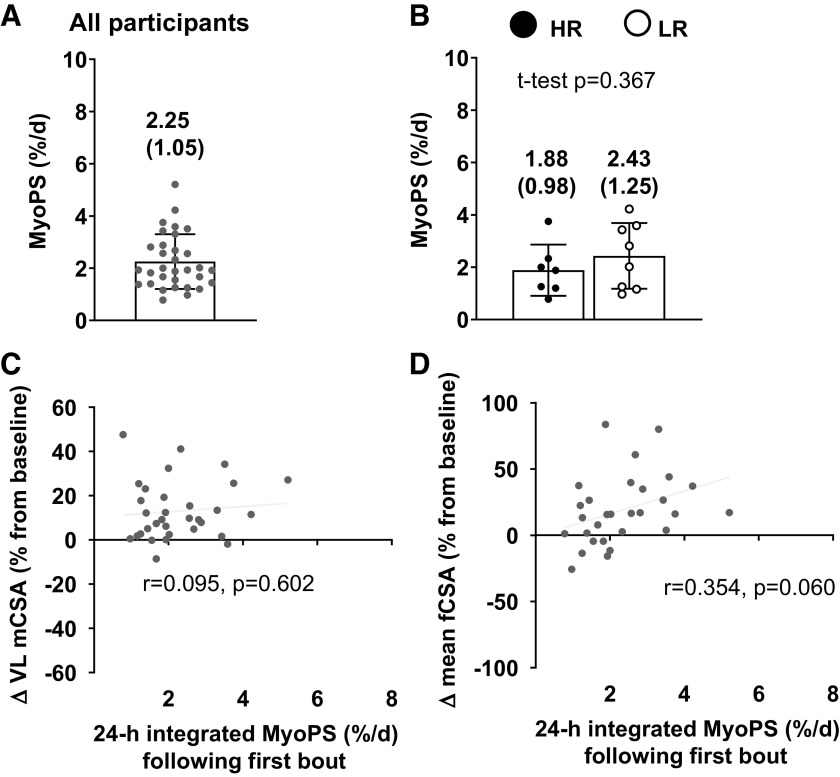
Twenty-four-hour MyoPS Response to First Bout of Training
The 24-h integrated MyoPS response to the first naïve bout of resistance exercise and additional analyses related to this variable are presented in Fig. 4. For the initial analysis, 31 participants were included due to three participant samples lacking appropriate yield amounts from isolated myofibrillar proteins. MyoPS rates were 2.25 ± 1.05% per day during this 24-h period (Fig. 4A). There was no significant difference between HR and LR cohorts (HR = 1.88 ± 0.98% per day; LR = 2.43 ± 1.25% per day; P = 0.370; Fig. 4B). There was no significant association between pre- to posttraining percentage change in VL CSA and the 24-h integrated MyoPS (Fig. 4C; r = 0.095; P = 0.602). Likewise, there was no significant association between the pre- to posttraining percentage change in mean fCSA and 24-h MyoPS response (Fig. 4D; r = 0.354; P = 0.060).
Figure 4.
Integrated MyoPS response to the first bout of training in all participants and higher vs. lower responders. A: 24-h integrated MyoPS response to the first bout of training in the 32 of 34 female participants that yielded enough tissue for analyses. B: same values as in A between the higher (n = 7) and lower (n = 8) responders. All bar graphs are expressed as means ± SD values and contain individual respondent values as well. C: scatterplot showing the association between the MyoPS responses presented in A and the pre- to postchange in VL mCSA in all participants. D: scatterplot showing the association between the MyoPS responses presented in panel a and the pre- to postchange in mean fCSA in all participants. mCSA, muscle cross-sectional area; fCSA, fiber cross-sectional area; HR, higher responder; LR, lower responder; MyoPS, myofibrillar protein synthetic; VL, vastus lateralis.
Satellite Cell and Myonuclear Number
Satellite cell number changes from PRE to POST and correlations to hypertrophy can be seen in Fig. 5. Satellite cell number per muscle fiber increased in all participants by ∼25% (P = 0.025; Fig. 5A). A significant cluster × time interaction was observed for the number of satellite cells per muscle fiber (P = 0.025), but there was no main effect of time (P = 0.103) or cluster (P = 0.563; Fig. 5B). Post hoc testing indicated a significant increase occurred from PRE to POST in higher responders (PRE = 0.064 ± 0.020; POST = 0.113 ± 0.046; P = 0.026), whereas no significant difference was evident from PRE to POST in lower responders (PRE = 0.083 ± 0.048; POST = 0.075 ± 0.043; P = 0.118). Importantly, there was no significant difference between higher responders and lower responders at PRE (P = 0.305). When considering all participants for correlations, there was no significant association between the pre- to posttraining percentage changes in satellite cells per fiber and VL CSA (r = 0.095 P = 0.609; Fig. 5C). However, there was a positive correlation between the pre- to posttraining percentage changes in satellite cells per fiber and mean fCSA (r = 0.471; P = 0.007; Fig. 5D). Figure 5E provides a representative histology image for PRE. Figure 5F provides a representative histology image for POST of the same participant.
Figure 5.
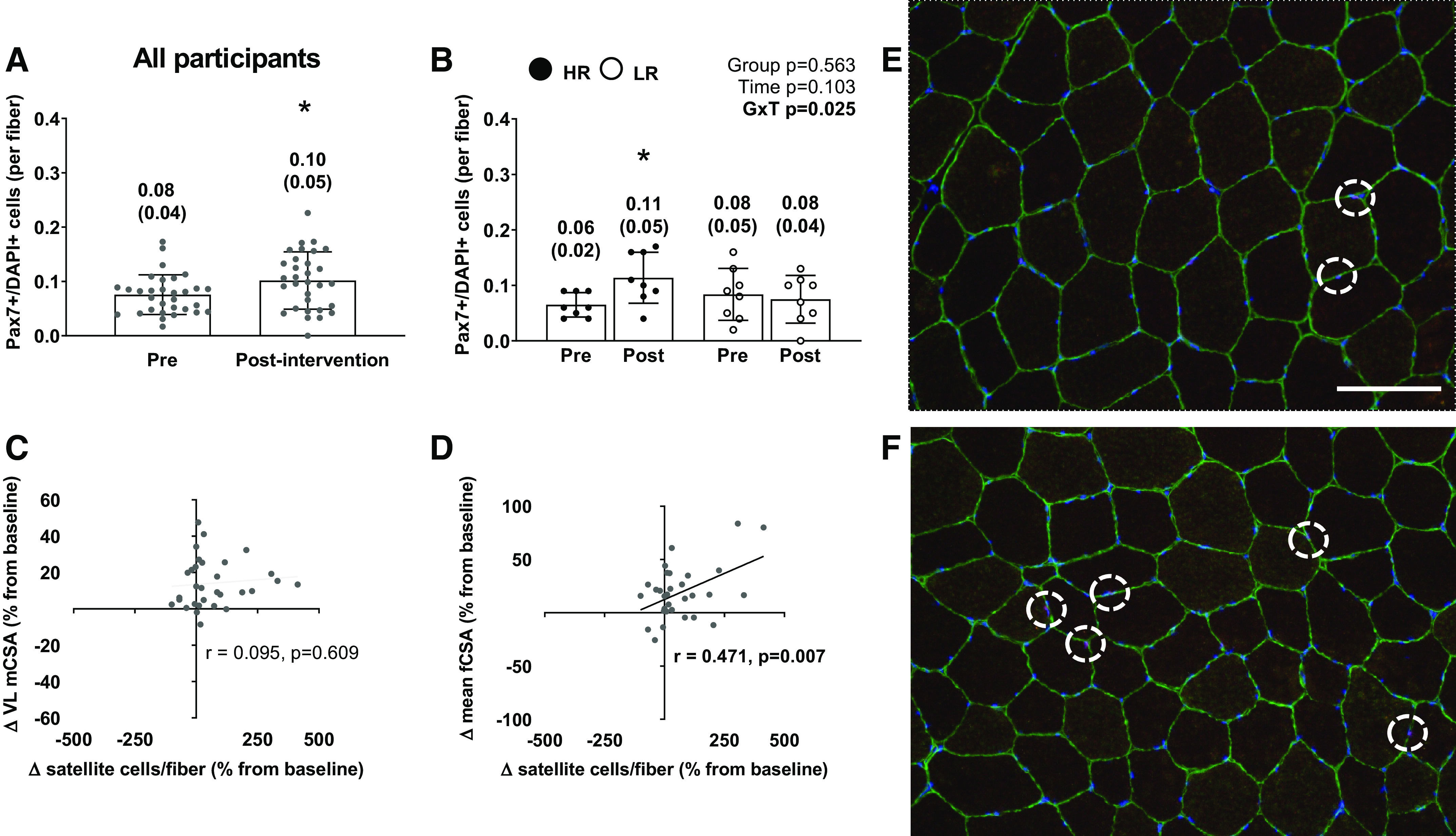
The satellite cell response to training in all participants and higher vs. lower responders. A: PAX7+/DAPI+ (or satellite cell) response to 10 wk of training in the 31 of 34 female participants that yielded enough tissue for analyses. B: same values as in A between the higher (n = 8) and lower (n = 8) responders. *Increase in HR from pre to post. All bar graphs are expressed as means ± SD values and contain individual respondent values as well. C: scatterplot showing the association between the pre- to postchange in satellite cells and the pre- to postchange in VL mCSA in all participants. D: scatterplot showing the association between pre- to postchange in satellite cells and the pre- to postintervention change in mean fCSA in all participants. E: ×20 representative image of PRE (scale bar = 100 µm). F: representative image of POST of the same participant (white circles identify satellite cells; colors: green, laminin; blue, DAPI; red, Pax-7; purple, satellite cell). mCSA, muscle cross-sectional area; fCSA, fiber cross-sectional area; GxT, group × time interaction; HR, higher responder; LR, lower responder; VL, vastus lateralis.
Change in myonuclear number per fiber from PRE to POST in all participants is presented in Fig. 6A. There was a significant increase in myonuclei per fiber from PRE to POST in all participants (P = 0.013). With the responder cluster analysis, there was a significant main effect of time from PRE to POST (PRE = 1.16 ± 0.43 myonuclei/fiber; POST = 1.45 ± 0.64 myonuclei/fiber; P = 0.049; Fig. 6B). However, the cluster effect and cluster × time interaction was not significant (P = 0.163 and P = 0.233 respectively; Fig. 6B). There was a significant positive correlation between percent changes in myonuclei per fiber and mean fCSA from PRE to POST (r = 0.523; P = 0.003; Fig. 6D) but no significant correlation between percent change in VL CSA (P = 0.159; Fig. 6C). Figure 6E provides a representative histology image for PRE. Figure 6F provides a representative histology image for POST of the same participant.
Figure 6.
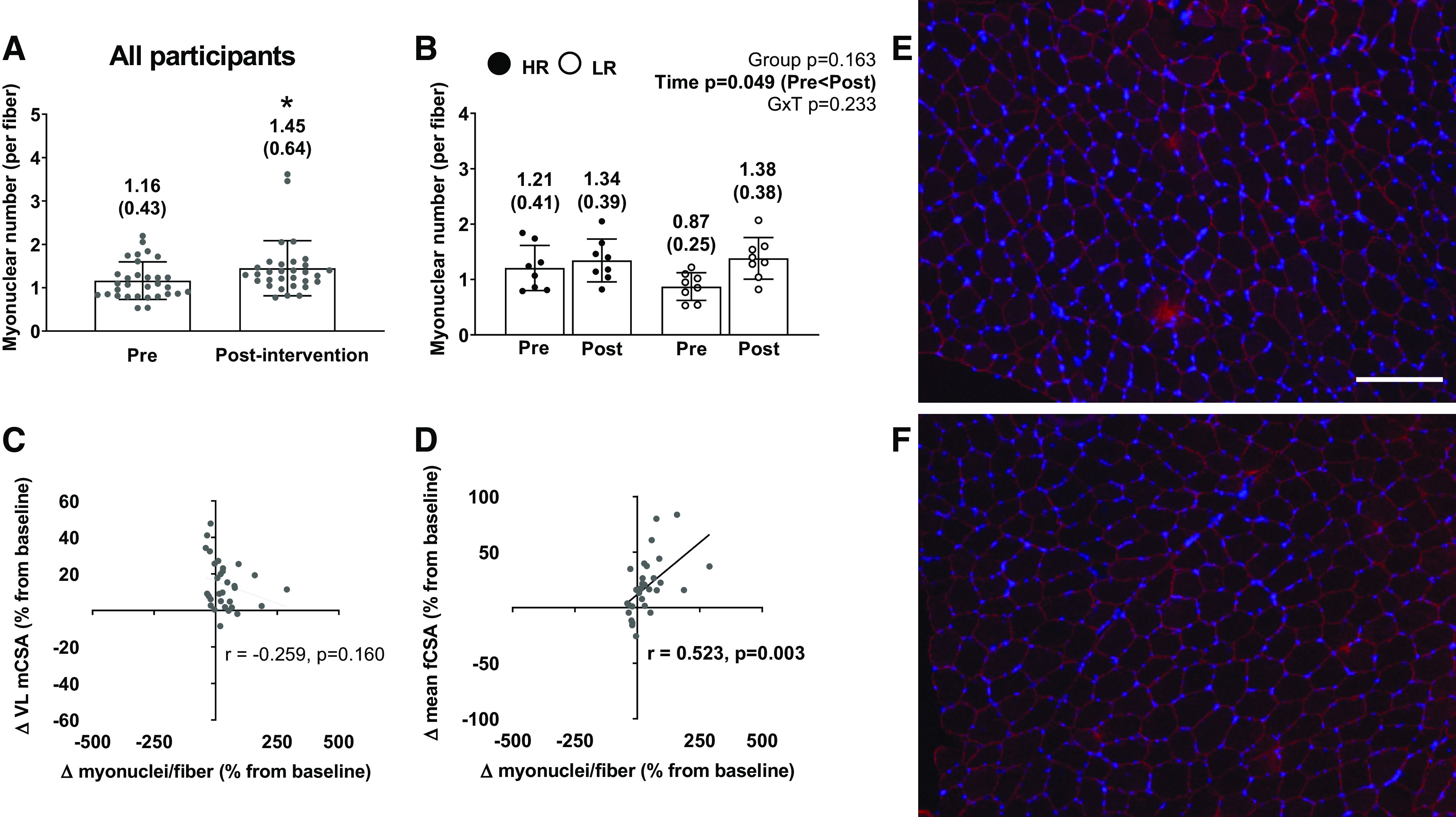
The myonuclear response to training in all participants and higher vs. lower responders. A: myonuclear number response to 10 wk of training in the 31 of 34 female participants that yielded enough tissue for analyses. B: same values as in A between the higher (n = 8) and lower (n = 8) responders. All bar graphs are expressed as means ± SD values and contain individual respondent values as well. C: scatterplot showing the association between the pre- to postchange in myonuclear number and the pre- to postchange in VL mCSA in all participants. D: scatterplot showing the association between pre- to postchange in myonuclear number and the pre- to postchange in mean fCSA in all participants. E and F: ×10 representative image of PRE (E) and representative image of POST (F) of the same participant (colors: red, dystrophin; blue, DAPI; white scale bar = 200 µm). mCSA, muscle cross-sectional area; fCSA, fiber cross-sectional area; GxT, group × time interaction; HR, higher responder; LR, lower responder.
Muscle Ribosome Content
Changes in a marker of muscle ribosome content (RNA per mg of wet muscle) and associations with hypertrophic outcomes can be found in Fig. 7. For this analysis, 33 participants were included due to 1 participant sample lacking adequate tissue amount to isolate RNA. There was no difference in RNA content per mg of wet muscle between PRE and POST for all participants (PRE = 1,221 ± 667 ng/mg; POST = 1,261 ± 729 ng/mg; P = 0.684). However, because hypertrophy occurred in all participants (i.e., both fCSA and VL CSA), and the RNA content per mg per wet muscle did not change, this indicates the absolute amount of total RNA content increased. For responder analysis, the cluster × time interaction and main effects of time and cluster were all not significant (P = 0.157, P = 0.778, and P = 0.888 respectively; Fig. 7B). The correlations between percentage changes in muscle ribosome content and both hypertrophy measures were not significant (VL CSA: P = 0.060; mean fCSA: P = 0.574; Fig. 7, C and D). Furthermore, when removing on participant outlier from Fig. 7C, this yielded a lower association between changes in muscle ribosome content and VL mCSA (r = 0.014; P = 0.937).
Figure 7.
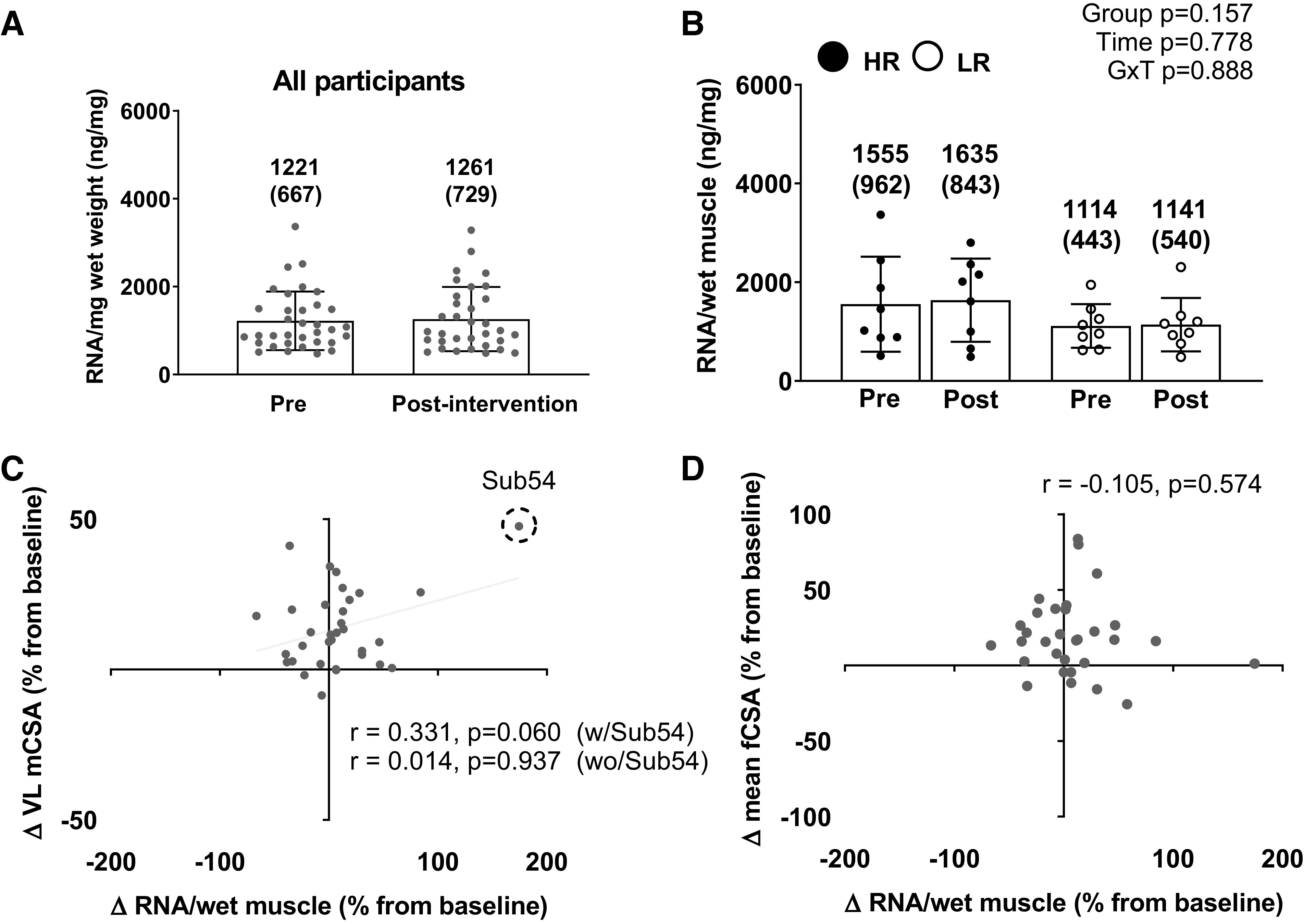
The muscle ribosome content response to training in all participants and higher vs. lower responders. A: muscle total RNA concentration (per wet tissue mass, or ribosome density) response to 10 wk of training in the 33 of 34 female participants that yielded enough tissue for analyses. B: same values as in A between the higher (n = 8) and lower (n = 8) responders. All bar graphs are expressed as means ± SD values and contain individual respondent values as well. C: scatterplot showing the association between the pre- to postchange in ribosome density and the pre- to postchange in VL mCSA in all participants. Notably, the removal of one subject (subject 54) changes the P value from 0.060 to 0.937. D: scatterplot showing the association between the pre- to postchange in ribosome density and the pre- to postchange score in mean fCSA in all participants. mCSA, muscle cross-sectional area; fCSA, fiber cross-sectional area; GxT, group × time interaction; HR, higher responder; LR, lower responder; VL, vastus lateralis.
Other Differences between Higher and Lower Responders
Table 3 compares other variables of interest between higher and lower responders. Along with Table 2 data indicating PRE DXA LSTM was greater in HR versus LR (HR PRE = 44.2 ± 3.3 kg; LR PRE = 39.2 ± 3.5 kg; P = 0.010), PRE midthigh mCSA (HR = 116.6 ± 8.0 cm2; LR PRE = 98.8 ± 6.3 cm2), and PRE VL mCSA (HR = 20.1 ± 2.2 cm2; LR = 17.6 ± 1.9 cm2) were significantly greater in HR versus LR (P < 0.001 and P = 0.028, respectively). PRE self-reported energy intake, POST self-reported energy intake, self-reported sleep, and self-reported day of menstrual cycle at PRE were not significantly different between response groups.
Table 3.
Other differences between higher and lower responders
| Variable | HR (n = 8) | LR (n = 8) | P Value |
|---|---|---|---|
| PRE midthigh mCSA, cm2 | 117 ± 8 | 99 ± 6 | <0.001 |
| PRE VL mCSA, cm2 | 20.1 ± 2.2 | 17.6 ± 1.9 | 0.028 |
| PRE energy intake, kcal/day | 1,268 ± 388 | 1,512 ± 474 | 0.279 |
| POST energy intake, kcal/day | 1,312 ± 448 | 1,627 ± 618 | 0.263 |
| PRE protein intake, g/day | 61 ± 32 | 65 ± 30 | 0.807 |
| POST protein intake, g/day | 68 ± 22 | 77 ± 31 | 0.521 |
| PRE HEI-2015 | 48.1 ± 7.3 | 46.4 ± 12.7 | 0.691 |
| POST HEI-2015 | 46.8 ± 8.1 | 44.9 ± 10.3 | 0.492 |
| Self-reported sleep, h/night | 9.3 ± 1.1 | 9.4 ± 2.1 | 0.870 |
| Day of menstrual cycle (at PRE) | 15 ± 8 | 18 ± 7 | 0.480 |
HR, higher responder; LR, lower responder; HEI-2015, healthy eating index-2015; mCSA, muscle cross-sectional area; VL, vastus lateralis; PRE, pretesting; POST, posttesting.
DISCUSSION
The main findings from the present study include 1) 24-h integrated MyoPS following the first training bout did not predict hypertrophy outcomes, 2) muscle ribosome content (per mg tissue) did change with training, and 3) satellite cell proliferation only occurred in higher responders. Furthermore, there was a moderately strong positive correlation between the percent change in satellite cell number and mean fCSA in all participants (r = 0.471; P = 0.007). Myonuclear number per fiber increased significantly following training, regardless of response cluster, and a moderate positive correlation was found between percentage changes in this variable and mean fCSA in all participants (r = 0.523; P = 0.003). Finally, females that began training with more lean tissue mass and larger leg muscles (as assessed using DXA and ultrasound, respectively) were typically higher responders.
Satellite cells are distinctively defined by their proximity to skeletal muscle fibers and are positioned between the sarcolemma and basement membrane. Mechanical overload stimulates satellite cell proliferation, differentiation, and fusion to preexisting myofibers (68), and an increase in satellite cell-mediated myonuclear addition in growing myofibers likely provides cellular support for the increased number of cellular macromolecules (e.g., ribosomes and muscle proteins) (69). Importantly, several groups have reported that satellite cell proliferation increases in response to one bout of resistance exercise (70–75). Moreover, our findings align with those of several studies that have demonstrated a similar response to multiple weeks of RT in males (76–78) and females (78–80) and continue to support the body of literature suggesting an increase in satellite cell number to one or multiple bouts of resistance training differentiates higher and lower responders (71, 80).
Several studies have shown that increased muscle RNA content per mg tissue through ribosome biogenesis coincides with muscle hypertrophy following weeks to months of mechanical overload in both animal models (30, 81–83) and humans (25, 84, 85). Contrary to these data, the female participants herein did not demonstrate increases in muscle RNA content per milligram of tissue, and changes in this variable were not predictive of hypertrophy (i.e., ΔVL CSA or ΔVL mean fCSA). This lack of predictability does not discount the role that ribosome biogenesis plays in muscle hypertrophy, and it is possible that more dynamic responses could have occurred earlier in training. However, this hypothesis is speculative given that we did not obtain time course biopsies throughout training. Moreover, and as stated in the previous section, while ribosome content (RNA per mg tissue) remained unaltered with chronic training, we contend that ribosome biogenesis did occur given that muscle hypertrophy occurred in lieu of ribosome content values remained stable. However, unlike past studies in previously untrained men indicating that an increased ribosome content per milligram of muscle tissue delineates higher and lower hypertrophic responders after shorter term (i.e., 4 wk) (31) and longer term (i.e., 12 wk) resistance training (20), we did not observe that phenomenon in previously untrained women following a 10-wk resistance training protocol.
As well, the 24-h integrated MyoPS response to the first bout of resistance exercise was not predictive of hypertrophy (i.e., ΔVL CSA or ΔVL mean fCSA), agreeing with prior studies and a review reporting low associations exist between the muscle protein synthetic response to a naïve bout of resistance exercise and muscle mass changes weeks into training (7, 86). The measured level of MyoPS (2.29 ± 1.05%) aligns with previous studies showing heightened MyoPS rates 24 h following RE in males (7, 87). We hypothesize that this could be due to other factors affecting net muscle protein balance early into training. Specifically, muscle protein breakdown in an untrained individual during the initial sessions of RT is likely increased due to the novelty of the stimulus and the initial muscle damage that ensues. Therefore, this level of breakdown activity will affect the net protein balance and therefore hypertrophy. Additionally, the MyoPS response over the entirety of the training intervention was not measured, and the magnitude and duration of MyoPS increases become more refined as individuals become more trained (88, 89). Thus it is likely that we assessed the “damage-synthesis” MyoPS response, whereas ascertaining this variable later in training may have yielded more insightful findings. Notably, this phenomenon has been observed in previously untrained men by Damas et al. (86) who reported that the elevations in MyoPS to a naïve training bout, when muscle damage metrics were concomitantly elevated, were not significantly associated with muscle hypertrophy after 10 wk of resistance training. However, the MyoPS response to a week 10 training bout was strongly correlated (r ∼0.90) with the muscle hypertrophy induced by 10 wk of resistance training. Whether this finding holds true in females, however, remains to be determined. What finally should be noted is that our approach may not have been sensitive enough to detect between-cluster differences in MyoPS given that a large majority of D2O-labeled alanine was likely integrated into myofibrils by the first biopsy based on our dosing schematic shown in Fig. 1.
It has been implied that satellite cell-mediated myonuclear accretion coincides with hypertrophy (15, 77, 90). However, hypertrophy without myonuclear accretion has also been observed in humans and, more specifically, in previously untrained female participants. For instance, Herman-Montemayor et al. (91) demonstrated a 30% increase in type II fiber hypertrophy, a 29% increase in the size of myonuclear domains, and no myonuclear accretion. Petrella et al. (71) reported similar findings after 16 wk of knee extensor training in untrained female participants. Specifically, mean fCSA and myonuclear domain size increased while myonuclear number remained unchanged. Interestingly, we observed a significant increase in satellite cell number in the HR cluster, whereas the LR cluster did not exhibit this phenotype. However, greater increases in satellite cell number did not translate to enhancements in myonuclear number in HR, as the latter metric demonstrated a main effect of training but not group × time interaction. There are speculative explanations for this observation. First, an enhancement in myonuclear accretion may have eventually become evident in HR if the training program was longer in duration. Second, beyond contributing to the myonuclear pool, rodent data indicate that satellite cells have nonfusion roles. For example, satellite cells secrete exosomes that communicate with fibroblasts to downregulate collagen production (92, 93). Given the pervasiveness of collagen in the extracellular matrix, an increase in satellite cell number in HR may have acted to reduce collagen synthesis to better facilitate myofiber and tissue growth. However, changes in muscle tissue collagen characteristics, satellite cell exosome cargo, and other molecular variables to test this hypothesis were not performed. Notwithstanding, these data support the notion that training-induced increases in satellite cell numbers coincide with skeletal muscle hypertrophy and may play a role in the responsiveness to RT.
Other findings herein are also notable. First, the nutritional intake, average hours of sleep, and day of menstrual cycle at PRE and POST were not statistically different between responder clusters. However, there is a growing interest in the field investigating the impact of the amount of sleep (94) and timing within the menstrual cycle (95–97) on training adaptations. While the present study collected these data, it did not thoroughly investigate these variables. An interesting finding in the present study is the HR cluster had higher lean mass values at PRE. This agrees in principle with a study by Van Etten et al. (13) who reported that individuals with a “solid body build” presented gains in lean body mass, whereas a separate cohort of those who possessed a “slender” build did not. In contrast to these reports, however, our laboratory has observed different findings in males, seeing that there was no difference in lean tissue mass, and, tangentially, LR had higher type II fCSA values at PRE than HR (36). While these data allude to the role that pretraining body composition plays in the response to RT, more research is needed to fully address this relationship.
Limitations
As with any study, there are limitations that must be considered, and the first is the length of training. Previous literature demonstrates that as little as 3–6 wk of RT increases hypertrophy measures in untrained male participants (45, 98, 99); however, less investigation has been done in female participants. While the present study was 10 wk in duration, a longer intervention would have broadened the opportunity to observe training adaptations. Second, the analysis of “higher responder” versus “non/lower responder” is highly debated. Some researchers attribute this differentiation to a technical error in the measurements being used to assess skeletal muscle hypertrophy or plainly interindividual variation. Conversely, others (including our laboratory) believe there are characteristics that lead an individual to respond to RT in varying magnitudes. A key limitation to the responder analysis itself is contingent on the training regimen used; therefore, a higher or lower responder is referring to the magnitude of response for the specific training intervention used. For example, the training stimulus may have been too low for a lower responder to see similar increases in performance outcomes as a higher responder (i.e., if participants trained 4 or 5 times a week and/or completed more volume, we could see different results). Additionally, there is a lack of standardization in how response clusters are defined in the field, making this analysis seemingly subjective. However, to address this concern, data are presented separately for both responder clusters as well as all participants with associations. It also should be noted that histology was performed on a small section of skeletal muscle, and analyses were performed in cross-section. As our laboratory has contended in the past, limitations in what information can be gleaned with skeletal muscle biopsy analyses whereby two-dimensional analysis is used to extrapolate three-dimensional attributes of multinucleated cells should be more recognized by readership (100). Finally, the methods to collect information on sleep were self-reported, and therefore, those data are limited to the discretion of the participant. Using wearables to collect this data would increase the accuracy of this data.
Conclusions
These data support prior research suggesting satellite cells are involved with resistance training-induced skeletal muscle hypertrophy. As discussed throughout, more research is needed in determining other molecular factors that delineate lower versus higher responders to RT in females. Moreover, designing lifestyle and training interventions to optimize exercise adaptations in lower responders is warranted.
DATA AVAILABILITY
All raw data can be obtained upon reasonable request and establishment of a material transfer agreement by emailing the corresponding author (mdr0024@auburn.edu).
GRANTS
Participant compensation was provided by the Peanut Institute Foundation (Albany, GA) and discretionary laboratory funds of M.D.R. Reagent costs were provided by indirect cost recovery to M.D.R. from the Auburn School of Kinesiology. The Auburn School of Kinesiology provided publishing fees for this article.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.A.S., A.D.F., L.B.G., and M.D.R. conceived and designed research; M.A.S., C.L.S., K.A.S., S.C.O., J.S.G., J.P.B., and B.A.R., K.C.Y., and M.D.R. performed experiments; M.A.S., C.L.S., J.S.G., and J.P.B. analyzed data; M.A.S. and J.S.G. interpreted results of experiments; M.A.S. prepared figures; M.A.S. drafted manuscript; M.A.S., C.L.S., K.A.S., S.C.O., J.S.G., J.P.B., B.A.R., M.D.G., J.L.E, A.D.F., A.T.R., L.B.G., K.C.Y., and M.D.R. edited and revised manuscript; M.A.S., C.L.S., K.A.S., S.C.O., J.S.G., J.P.B., B.A.R., M.D.G., J.L.E., A.D.F., A.T.R., L.B.G., K.C.Y., and M.D.R. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the participants for partaking in the study. We also thank Dr. David Wagner (Metabolic Solutions) for his assistance regarding the mass spectrometry work. Finally, we thank Dr. Karyn Esser (University of Florida) for her insight. This experiment was performed at Auburn University’s School of Kinesiology in the Molecular and Applied Sciences Laboratory.
REFERENCES
- 1. Sheel AW. Sex differences in the physiology of exercise: an integrative perspective. Exp Physiol 101: 211–212, 2016. doi: 10.1113/EP085371. [DOI] [PubMed] [Google Scholar]
- 2. Miller VM. Sex-based physiology prior to political correctness. Am J Physiol Endocrinol Metab 289: E359–E360, 2005. doi: 10.1152/classicessays.00035.2005. [DOI] [PubMed] [Google Scholar]
- 3. Costello JT, Bieuzen F, Bleakley CM. Where are all the female participants in Sports and Exercise Medicine research? Eur J Sport Sci 14: 847–851, 2014. doi: 10.1080/17461391.2014.911354. [DOI] [PubMed] [Google Scholar]
- 4. Kraemer WJ, Ratamess NA, French DN. Resistance training for health and performance. Curr Sports Med Rep 1: 165–171, 2002. doi: 10.1249/00149619-200206000-00007. [DOI] [PubMed] [Google Scholar]
- 5. Stone M, Potteiger J, Pierce K, Proulx C, O'Bryant H, Johnson R, Stone M. Comparison of the effects of three different weight-training programs on the one repetition maximum squat. J Strength Cond Res 14: 332–337, 2000. [Google Scholar]
- 6. Staron RS, Karapondo DL, Kraemer WJ, Fry AC, Gordon SE, Falkel JE, Hagerman FC, Hikida RS. Skeletal muscle adaptations during early phase of heavy-resistance training in men and women. J Appl Physiol (1985) 76: 1247–1255, 1994. doi: 10.1152/jappl.1994.76.3.1247. [DOI] [PubMed] [Google Scholar]
- 7. Mitchell CJ, Churchward-Venne TA, Parise G, Bellamy L, Baker SK, Smith K, Atherton PJ, Phillips SM. Acute post-exercise myofibrillar protein synthesis is not correlated with resistance training-induced muscle hypertrophy in young men. PLoS One 9: e89431, 2014. doi: 10.1371/journal.pone.0089431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nordez A, Jolivet E, Südhoff I, Bonneau D, de Guise JA, Skalli W. Comparison of methods to assess quadriceps muscle volume using magnetic resonance imaging. J Magn Reson Imaging 30: 1116–1123, 2009. doi: 10.1002/jmri.21867. [DOI] [PubMed] [Google Scholar]
- 9. Da Boit M, Sibson R, Meakin JR, Aspden RM, Thies F, Mangoni AA, Gray SR. Sex differences in the response to resistance exercise training in older people. Physiol Rep 4: e12834, 2016. doi: 10.14814/phy2.12834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cunha PM, Nunes JP, Tomeleri CM, Nascimento MA, Schoenfeld BJ, Antunes M, Gobbo LA, Teixeira D, Cyrino ES. Resistance training performed with single and multiple sets induces similar improvements in muscular strength, muscle mass, muscle quality, and IGF-1 in older women: a randomized controlled trial. J Strength Cond Res 34: 1008–1016, 2020. doi: 10.1519/JSC.0000000000002847. [DOI] [PubMed] [Google Scholar]
- 11. Ahtiainen JP, Walker S, Peltonen H, Holviala J, Sillanpää E, Karavirta L, Sallinen J, Mikkola J, Valkeinen H, Mero A, Hulmi JJ, Häkkinen K. Heterogeneity in resistance training-induced muscle strength and mass responses in men and women of different ages. Age (Dordr) 38: 10, 2016. doi: 10.1007/s11357-015-9870-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bamman MM, Petrella JK, Kim JS, Mayhew DL, Cross JM. Cluster analysis tests the importance of myogenic gene expression during myofiber hypertrophy in humans. J Appl Physiol (1985) 102: 2232–2239, 2007. doi: 10.1152/japplphysiol.00024.2007. [DOI] [PubMed] [Google Scholar]
- 13. Van Etten LM, Verstappen FT, Westerterp KR. Effect of body build on weight-training-induced adaptations in body composition and muscular strength. Med Sci Sports Exerc 26: 515–521, 1994. doi: 10.1249/00005768-199404000-00018. [DOI] [PubMed] [Google Scholar]
- 14. Yannoulopoulos BD, Meeus JB. Arterial hypertension and pregnancy: detection and monitoring. Soins Gynecol Obstet Pueric Pediatr 127–128: 22–26, 1991. [PubMed] [Google Scholar]
- 15. Petrella JK, Kim JS, Mayhew DL, Cross JM, Bamman MM. Potent myofiber hypertrophy during resistance training in humans is associated with satellite cell-mediated myonuclear addition: a cluster analysis. J Appl Physiol (1985) 104: 1736–1742, 2008. doi: 10.1152/japplphysiol.01215.2007. [DOI] [PubMed] [Google Scholar]
- 16. Davidsen PK, Gallagher IJ, Hartman JW, Tarnopolsky MA, Dela F, Helge JW, Timmons JA, Phillips SM. High responders to resistance exercise training demonstrate differential regulation of skeletal muscle microRNA expression. J Appl Physiol (1985) 110: 309–317, 2011. doi: 10.1152/japplphysiol.00901.2010. [DOI] [PubMed] [Google Scholar]
- 17. Gjestvang C, Abrahamsen F, Stensrud T, Haakstad LA. Motives and barriers to initiation and sustained exercise adherence in a fitness club setting–a one-year follow-up study. Scand J Med Sci Sports 30: 1796–1805, 2020. doi: 10.1111/sms.13736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Roberts MD, Haun CT, Mobley CB, Mumford PW, Romero MA, Roberson PA, Vann CG, McCarthy JJ. Physiological differences between low versus high skeletal muscle hypertrophic responders to resistance exercise training: current perspectives and future research directions. Front Physiol 9: 834, 2018. doi: 10.3389/fphys.2018.00834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kosek DJ, Kim JS, Petrella JK, Cross JM, Bamman MM. Efficacy of 3 days/wk resistance training on myofiber hypertrophy and myogenic mechanisms in young vs. older adults. J Appl Physiol (1985) 101: 531–544, 2006. doi: 10.1152/japplphysiol.01474.2005. [DOI] [PubMed] [Google Scholar]
- 20. Mobley CB, Haun CT, Roberson PA, Mumford PW, Kephart WC, Romero MA, Osburn SC, Vann CG, Young KC, Beck DT, Martin JS, Lockwood CM, Roberts MD. Biomarkers associated with low, moderate, and high vastus lateralis muscle hypertrophy following 12 weeks of resistance training. PLoS One 13: e0195203, 2018. doi: 10.1371/journal.pone.0195203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Haun CT, Vann CG, Mobley CB, Osburn SC, Mumford PW, Roberson PA, Romero MA, Fox CD, Parry HA, Kavazis AN, Moon JR, Young KC, Roberts MD. Pre-training skeletal muscle fiber size and predominant fiber type best predict hypertrophic responses to 6 weeks of resistance training in previously trained young men. Front Physiol 10: 297, 2019. doi: 10.3389/fphys.2019.00297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Roberts MD, Romero MA, Mobley CB, Mumford PW, Roberson PA, Haun CT, Vann CG, Osburn SC, Holmes HH, Greer RA, Lockwood CM, Parry HA, Kavazis AN. Skeletal muscle mitochondrial volume and myozenin-1 protein differences exist between high versus low anabolic responders to resistance training. PeerJ 6: e5338, 2018. doi: 10.7717/peerj.5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Thalacker-Mercer A, Stec M, Cui X, Cross J, Windham S, Bamman M. Cluster analysis reveals differential transcript profiles associated with resistance training-induced human skeletal muscle hypertrophy. Physiol Genomics 45: 499–507, 2013. doi: 10.1152/physiolgenomics.00167.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Timmons JA. Variability in training-induced skeletal muscle adaptation. J Appl Physiol (1985) 110: 846–853, 2011. doi: 10.1152/japplphysiol.00934.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Figueiredo VC, Caldow MK, Massie V, Markworth JF, Cameron-Smith D, Blazevich AJ. Ribosome biogenesis adaptation in resistance training-induced human skeletal muscle hypertrophy. Am J Physiol Endocrinol Metab 309: E72–E83, 2015. doi: 10.1152/ajpendo.00050.2015. [DOI] [PubMed] [Google Scholar]
- 26. Terzis G, Georgiadis G, Stratakos G, Vogiatzis I, Kavouras S, Manta P, Mascher H, Blomstrand E. Resistance exercise-induced increase in muscle mass correlates with p70S6 kinase phosphorylation in human subjects. Eur J Appl Physiol 102: 145–152, 2008. doi: 10.1007/s00421-007-0564-y. [DOI] [PubMed] [Google Scholar]
- 27. Phillips SM. A brief review of critical processes in exercise-induced muscular hypertrophy. Sports Med 44: S71–S77, 2014. doi: 10.1007/s40279-014-0152-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hartman JW, Tang JE, Wilkinson SB, Tarnopolsky MA, Lawrence RL, Fullerton AV, Phillips SM. Consumption of fat-free fluid milk after resistance exercise promotes greater lean mass accretion than does consumption of soy or carbohydrate in young, novice, male weightlifters. Am J Clin Nutr 86: 373–381, 2007. doi: 10.1093/ajcn/86.2.373. [DOI] [PubMed] [Google Scholar]
- 29. Burd NA, West DW, Staples AW, Atherton PJ, Baker JM, Moore DR, Holwerda AM, Parise G, Rennie MJ, Baker SK, Phillips SM. Low-load high volume resistance exercise stimulates muscle protein synthesis more than high-load low volume resistance exercise in young men. PLoS One 5: e12033, 2010. doi: 10.1371/journal.pone.0012033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nakada S, Ogasawara R, Kawada S, Maekawa T, Ishii N. Correlation between ribosome biogenesis and the magnitude of hypertrophy in overloaded skeletal muscle. PLoS One 11: e0147284, 2016. doi: 10.1371/journal.pone.0147284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Stec MJ, Kelly NA, Many GM, Windham ST, Tuggle SC, Bamman MM. Ribosome biogenesis may augment resistance training-induced myofiber hypertrophy and is required for myotube growth in vitro. Am J Physiol Endocrinol Metab 310: E652–E661, 2016. doi: 10.1152/ajpendo.00486.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lambert BS, Shimkus KL, Fluckey JD, Riechman SE, Greene NP, Cardin JM, Crouse SF. Anabolic responses to acute and chronic resistance exercise are enhanced when combined with aquatic treadmill exercise. Am J Physiol Endocrinol Metab 308: E192–E200, 2015. doi: 10.1152/ajpendo.00689.2013. [DOI] [PubMed] [Google Scholar]
- 33. Mitchell CJ, Churchward-Venne TA, West DW, Burd NA, Breen L, Baker SK, Phillips SM. Resistance exercise load does not determine training-mediated hypertrophic gains in young men. J Appl Physiol (1985) 113: 71–77, 2012. doi: 10.1152/japplphysiol.00307.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schiaffino S, Bormioli SP, Aloisi M. The fate of newly formed satellite cells during compensatory muscle hypertrophy. Virchows Arch B Cell Pathol 21: 113–118, 1976. doi: 10.1007/BF02899148. [DOI] [PubMed] [Google Scholar]
- 35. Murach KA, Vechetti IJ, Van Pelt DW, Crow SE, Dungan CM, Figueiredo VC, Kosmac K, Fu X, Richards CI, Fry CS, McCarthy JJ, Peterson CA. Fusion-independent satellite cell communication to muscle fibers during load-induced hypertrophy. Function (Oxf) 1: zqaa009, 2020. doi: 10.1093/function/zqaa009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rosenblatt JD, Parry DJ. Gamma irradiation prevents compensatory hypertrophy of overloaded mouse extensor digitorum longus muscle. J Appl Physiol (1985) 73: 2538–2543, 1992. doi: 10.1152/jappl.1992.73.6.2538. [DOI] [PubMed] [Google Scholar]
- 37. O'Connor RS, Pavlath GK. Point:Counterpoint: Satellite cell addition is/is not obligatory for skeletal muscle hypertrophy. J Appl Physiol (1985) 103: 1099–1100, 2007. doi: 10.1152/japplphysiol.00101.2007. [DOI] [PubMed] [Google Scholar]
- 38. McCarthy JJ, Mula J, Miyazaki M, Erfani R, Garrison K, Farooqui AB, Srikuea R, Lawson BA, Grimes B, Keller C, Van Zant G, Campbell KS, Esser KA, Dupont-Versteegden EE, Peterson CA. Effective fiber hypertrophy in satellite cell-depleted skeletal muscle. Development 138: 3657–3666, 2011. doi: 10.1242/dev.068858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. McCarthy JJ, Esser KA. Counterpoint: Satellite cell addition is not obligatory for skeletal muscle hypertrophy. J Appl Physiol (1985) 103: 1100–1102, 2007. doi: 10.1152/japplphysiol.00101.2007a. [DOI] [PubMed] [Google Scholar]
- 40. Jackson JR, Mula J, Kirby TJ, Fry CS, Lee JD, Ubele MF, Campbell KS, McCarthy JJ, Peterson CA, Dupont-Versteegden EE. Satellite cell depletion does not inhibit adult skeletal muscle regrowth following unloading-induced atrophy. Am J Physiol Cell Physiol 303: C854–C861, 2012. doi: 10.1152/ajpcell.00207.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Vann CG, Sexton CL, Osburn SC, Smith MA, Haun CT, Rumbley MN, Mumford PW, Ferguson BK, Montgomery NT, Fox CD, Ruple BA, McKendry J, McLeod J, Bashir A, Beyers RJ, Brook MS, Smith K, Atherton PJ, Beck DT, McDonald JR, Young KC, Phillips SM, Roberts MD. Effects of high-volume versus high-load resistance training on skeletal muscle growth and molecular adaptations. Front Physiol 13: 857555, 2022. doi: 10.3389/fphys.2022.857555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Morton RW, Sato K, Gallaugher MP, Oikawa SY, McNicholas PD, Fujita S, Phillips SM. Muscle androgen receptor content but not systemic hormones is associated with resistance training-induced skeletal muscle hypertrophy in healthy, young men. Front Physiol 9: 1373, 2018. doi: 10.3389/fphys.2018.01373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Phillips SM, Tipton KD, Ferrando AA, Wolfe RR. Resistance training reduces the acute exercise-induced increase in muscle protein turnover. Am J Physiol Endocrinol Metab 276: E118–E124, 1999. doi: 10.1152/ajpendo.1999.276.1.E118. [DOI] [PubMed] [Google Scholar]
- 44. Damas F, Phillips S, Vechin FC, Ugrinowitsch C. A review of resistance training-induced changes in skeletal muscle protein synthesis and their contribution to hypertrophy. Sports Med 45: 801–807, 2015. doi: 10.1007/s40279-015-0320-0. [DOI] [PubMed] [Google Scholar]
- 45. Haun CT, Vann CG, Osburn SC, Mumford PW, Roberson PA, Romero MA, Fox CD, Johnson CA, Parry HA, Kavazis AN, Moon JR, Badisa VL, Mwashote BM, Ibeanusi V, Young KC, Roberts MD. Muscle fiber hypertrophy in response to 6 weeks of high-volume resistance training in trained young men is largely attributed to sarcoplasmic hypertrophy. PLoS One 14: e0215267, 2019. doi: 10.1371/journal.pone.0215267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Walker DK, Dickinson JM, Timmerman KL, Drummond MJ, Reidy PT, Fry CS, Gundermann DM, Rasmussen BB. Exercise, amino acids, and aging in the control of human muscle protein synthesis. Med Sci Sports Exerc 43: 2249–2258, 2011. doi: 10.1249/MSS.0b013e318223b037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Haun CT, Vann CG, Roberts BM, Vigotsky AD, Schoenfeld BJ, Roberts MD. A critical evaluation of the biological construct skeletal muscle hypertrophy: size matters but so does the measurement. Front Physiol 10: 247, 2019. doi: 10.3389/fphys.2019.00247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Staron RS, Leonardi MJ, Karapondo DL, Malicky ES, Falkel JE, Hagerman FC, Hikida RS. Strength and skeletal muscle adaptations in heavy-resistance-trained women after detraining and retraining. J Appl Physiol (1985) 70: 631–640, 1991. doi: 10.1152/jappl.1991.70.2.631. [DOI] [PubMed] [Google Scholar]
- 49. Abe T, DeHoyos DV, Pollock ML, Garzarella L. Time course for strength and muscle thickness changes following upper and lower body resistance training in men and women. Eur J Appl Physiol 81: 174–180, 2000. doi: 10.1007/s004210050027. [DOI] [PubMed] [Google Scholar]
- 50. Räntilä A, Ahtiainen JP, Avela J, Restuccia J, Dawson K, Häkkinen K. High responders to hypertrophic strength training also tend to lose more muscle mass and strength during detraining than low responders. J Strength Cond Res 35: 1500–1511, 2021. doi: 10.1519/JSC.0000000000004044. [DOI] [PubMed] [Google Scholar]
- 51. Roberts BM, Nuckols G, Krieger JW. Sex differences in resistance training: a systematic review and meta-analysis. J Strength Cond Res 34: 1448–1460, 2020. doi: 10.1519/JSC.0000000000003521. [DOI] [PubMed] [Google Scholar]
- 52. Abou Sawan S, Hodson N, Malowany JM, West DW, Tinline-Goodfellow C, Brook MS, Smith K, Atherton PJ, Kumbhare D, Moore DR. Trained integrated postexercise myofibrillar protein synthesis rates correlate with hypertrophy in young males and females. Med Sci Sports Exerc 54: 953–964, 2022. doi: 10.1249/MSS.0000000000002878. [DOI] [PubMed] [Google Scholar]
- 53. Sexton CL, Smith MA, Smith KS, Osburn SC, Godwin JS, Ruple BA, Hendricks AM, Mobley CB, Goodlett MD, Fruge AD, Young KC, Roberts MD. Effects of peanut protein supplementation on resistance training adaptations in younger adults. Nutrients 13: 3981, 2021. doi: 10.3390/nu13113981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kephart WC, Wachs TD, Thompson RM, Brooks Mobley C, Fox CD, McDonald JR, Ferguson BS, Young KC, Nie B, Martin JS, Company JM, Pascoe DD, Arnold RD, Moon JR, Roberts MD. Ten weeks of branched-chain amino acid supplementation improves select performance and immunological variables in trained cyclists. Amino Acids 48: 779–789, 2016. [Erratum in Amino Acids 50(10): 1495, 2018]. doi: 10.1007/s00726-015-2125-8. [DOI] [PubMed] [Google Scholar]
- 55. Rantalainen T, Nikander R, Heinonen A, Daly RM, Sievanen H. An open source approach for regional cortical bone mineral density analysis. J Musculoskelet Neuronal Interact 11: 243–248, 2011. [PubMed] [Google Scholar]
- 56. Haff G, Triplett T. Essentials of Strength Training and Conditioning. Colorado Springs, CO: National Strength and Conditioning Association, 2016. [Google Scholar]
- 57. Evans WJ, Phinney SD, Young VR. Suction applied to a muscle biopsy maximizes sample size. Med Sci Sports Exerc 14: 101–102, 1982. [PubMed] [Google Scholar]
- 58. Ruple BA, Godwin JS, Mesquita PH, Osburn SC, Sexton CL, Smith MA, Ogletree JC, Goodlett MD, Edison JL, Ferrando AA, Fruge AD, Kavazis AN, Young KC, Roberts MD. Myofibril and mitochondrial area changes in type I and II fibers following 10 weeks of resistance training in previously untrained men. Front Physiol 12: 728683, 2021. doi: 10.3389/fphys.2021.728683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.American College of Sports Medicine. American College of Sports Medicine position stand. Progression models in resistance training for healthy adults. Med Sci Sports Exerc 41: 687–708, 2009. doi: 10.1249/MSS.0b013e3181915670. [DOI] [PubMed] [Google Scholar]
- 60. Wen Y, Murach KA, Vechetti IJ Jr, Fry CS, Vickery C, Peterson CA, McCarthy JJ, Campbell KS. MyoVision: software for automated high-content analysis of skeletal muscle immunohistochemistry. J Appl Physiol (1985) 124: 40–51, 2018. doi: 10.1152/japplphysiol.00762.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Previs SF, Fatica R, Chandramouli V, Alexander JC, Brunengraber H, Landau BR. Quantifying rates of protein synthesis in humans by use of 2H2O: application to patients with end-stage renal disease. Am J Physiol Endocrinol Metab 286: E665–E672, 2004. doi: 10.1152/ajpendo.00271.2003. [DOI] [PubMed] [Google Scholar]
- 62. Gasier HG, Riechman SE, Wiggs MP, Previs SF, Fluckey JD. A comparison of 2H2O and phenylalanine flooding dose to investigate muscle protein synthesis with acute exercise in rats. Am J Physiol Endocrinol Metab 297: E252–E259, 2009. doi: 10.1152/ajpendo.90872.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Roberts MD, Young KC, Fox CD, Vann CG, Roberson PA, Osburn SC, Moore JH, Mumford PW, Romero MA, Beck DT, Haun CT, Badisa VL, Mwashote BM, Ibeanusi V, Kavazis AN. An optimized procedure for isolation of rodent and human skeletal muscle sarcoplasmic and myofibrillar proteins. J Biol Methods 7: e127, 2020. doi: 10.14440/jbm.2020.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Bell KE, Brook MS, Snijders T, Kumbhare D, Parise G, Smith K, Atherton PJ, Phillips SM. Integrated myofibrillar protein synthesis in recovery from unaccustomed and accustomed resistance exercise with and without multi-ingredient supplementation in overweight older men. Front Nutr 6: 40, 2019. [Erratum in Front Nutr 7: 611389]. doi: 10.3389/fnut.2019.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Nader GA, McLoughlin TJ, Esser KA. mTOR function in skeletal muscle hypertrophy: increased ribosomal RNA via cell cycle regulators. Am J Physiol Cell Physiol 289: C1457–C1465, 2005. doi: 10.1152/ajpcell.00165.2005. [DOI] [PubMed] [Google Scholar]
- 66. Mobley CB, Fox CD, Thompson RM, Healy JC, Santucci V, Kephart WC, McCloskey AE, Kim M, Pascoe DD, Martin JS, Moon JR, Young KC, Roberts MD. Comparative effects of whey protein versus L-leucine on skeletal muscle protein synthesis and markers of ribosome biogenesis following resistance exercise. Amino Acids 48: 733–750, 2016. doi: 10.1007/s00726-015-2121-z. [DOI] [PubMed] [Google Scholar]
- 67. Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 28: 193–213, 1989. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 68. Fry CS, Lee JD, Jackson JR, Kirby TJ, Stasko SA, Liu H, Dupont-Versteegden EE, McCarthy JJ, Peterson CA. Regulation of the muscle fiber microenvironment by activated satellite cells during hypertrophy. FASEB J 28: 1654–1665, 2014. doi: 10.1096/fj.13-239426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kirby TJ, Patel RM, McClintock TS, Dupont-Versteegden EE, Peterson CA, McCarthy JJ. Myonuclear transcription is responsive to mechanical load and DNA content but uncoupled from cell size during hypertrophy. Mol Biol Cell 27: 788–798, 2016. doi: 10.1091/mbc.E15-08-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Crameri RM, Langberg H, Magnusson P, Jensen CH, Schroder HD, Olesen JL, Suetta C, Teisner B, Kjaer M. Changes in satellite cells in human skeletal muscle after a single bout of high intensity exercise. J Physiol 558: 333–340, 2004. doi: 10.1113/jphysiol.2004.061846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Petrella JK, Kim JS, Cross JM, Kosek DJ, Bamman MM. Efficacy of myonuclear addition may explain differential myofiber growth among resistance-trained young and older men and women. Am J Physiol Endocrinol Metab 291: E937–E946, 2006. doi: 10.1152/ajpendo.00190.2006. [DOI] [PubMed] [Google Scholar]
- 72. Kim JS, Kosek DJ, Petrella JK, Cross JM, Bamman MM. Resting and load-induced levels of myogenic gene transcripts differ between older adults with demonstrable sarcopenia and young men and women. J Appl Physiol (1985) 99: 2149–2158, 2005. doi: 10.1152/japplphysiol.00513.2005. [DOI] [PubMed] [Google Scholar]
- 73. Kim JS, Cross JM, Bamman MM. Impact of resistance loading on myostatin expression and cell cycle regulation in young and older men and women. Am J Physiol Endocrinol Metab 288: E1110–E1119, 2005. doi: 10.1152/ajpendo.00464.2004. [DOI] [PubMed] [Google Scholar]
- 74. Walker DK, Fry CS, Drummond MJ, Dickinson JM, Timmerman KL, Gundermann DM, Jennings K, Volpi E, Rasmussen BB. PAX7+ satellite cells in young and older adults following resistance exercise. Muscle Nerve 46: 51–59, 2012. doi: 10.1002/mus.23266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. O'Reilly C, McKay B, Phillips S, Tarnopolsky M, Parise G. Hepatocyte growth factor (HGF) and the satellite cell response following muscle lengthening contractions in humans. Muscle Nerve 38: 1434–1442, 2008. doi: 10.1002/mus.21146. [DOI] [PubMed] [Google Scholar]
- 76. Conceicao MS, Vechin FC, Lixandrao M, Damas F, Libardi CA, Tricoli V, Roschel H, Camera D, Ugrinowitsch C. Muscle fiber hypertrophy and myonuclei addition: a systematic review and meta-analysis. Med Sci Sports Exerc 50: 1385–1393, 2018. doi: 10.1249/MSS.0000000000001593. [DOI] [PubMed] [Google Scholar]
- 77. Kadi F, Schjerling P, Andersen LL, Charifi N, Madsen JL, Christensen LR, Andersen JL. The effects of heavy resistance training and detraining on satellite cells in human skeletal muscles. J Physiol 558: 1005–1012, 2004. doi: 10.1113/jphysiol.2004.065904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Roth SM, Martel GF, Ivey FM, Lemmer JT, Tracy BL, Metter EJ, Hurley BF, Rogers MA. Skeletal muscle satellite cell characteristics in young and older men and women after heavy resistance strength training. J Gerontol A Biol Sci Med Sci 56: B240–B247, 2001. doi: 10.1093/gerona/56.6.B240. [DOI] [PubMed] [Google Scholar]
- 79. Kadi F, Thornell LE. Concomitant increases in myonuclear and satellite cell content in female trapezius muscle following strength training. Histochem Cell Biol 113: 99–103, 2000. doi: 10.1007/s004180050012. [DOI] [PubMed] [Google Scholar]
- 80. Abou Sawan S, Hodson N, Babits P, Malowany JM, Kumbhare D, Moore DR. Satellite cell and myonuclear accretion is related to training-induced skeletal muscle fiber hypertrophy in young males and females. J Appl Physiol (1985) 131: 871–880, 2021. doi: 10.1152/japplphysiol.00424.2021. [DOI] [PubMed] [Google Scholar]
- 81. Kirby TJ, Lee JD, England JH, Chaillou T, Esser KA, McCarthy JJ. Blunted hypertrophic response in aged skeletal muscle is associated with decreased ribosome biogenesis. J Appl Physiol (1985) 119: 321–327, 2015. doi: 10.1152/japplphysiol.00296.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Chaillou T, Lee JD, England JH, Esser KA, McCarthy JJ. Time course of gene expression during mouse skeletal muscle hypertrophy. J Appl Physiol (1985) 115: 1065–1074, 2013. doi: 10.1152/japplphysiol.00611.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Laurent GJ, Sparrow MP. Changes in RNA, DNA and protein content and the rates of protein synthesis and degradation during hypertrophy of the anterior latissimus dorsi muscle of the adult fowl (Gallus domesticus). Growth 41: 249–262, 1977. [PubMed] [Google Scholar]
- 84. Figueiredo VC, Wen Y, Alkner B, Fernandez-Gonzalo R, Norrbom J, Vechetti IJ, Valentino T, Mobley CB, Zentner GE, Peterson CA, McCarthy JJ, Murach KA, von Walden F. Genetic and epigenetic regulation of skeletal muscle ribosome biogenesis with exercise. J Physiol 599: 3363–3384, 2021. doi: 10.1113/JP281244. [DOI] [PubMed] [Google Scholar]
- 85. Hammarstrom D, Ofsteng S, Koll L, Hanestadhaugen M, Hollan I, Apro W, Whist JE, Blomstrand E, Ronnestad BR, Ellefsen S. Benefits of higher resistance-training volume are related to ribosome biogenesis. J Physiol 598: 543–565, 2020. doi: 10.1113/JP278455. [DOI] [PubMed] [Google Scholar]
- 86. Damas F, Phillips SM, Libardi CA, Vechin FC, Lixandrão ME, Jannig PR, Costa LA, Bacurau AV, Snijders T, Parise G, Tricoli V, Roschel H, Ugrinowitsch C. Resistance training-induced changes in integrated myofibrillar protein synthesis are related to hypertrophy only after attenuation of muscle damage. J Physiol 594: 5209–5222, 2016. doi: 10.1113/JP272472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Brook MS, Wilkinson DJ, Mitchell WK, Lund JN, Szewczyk NJ, Greenhaff PL, Smith K, Atherton PJ. Skeletal muscle hypertrophy adaptations predominate in the early stages of resistance exercise training, matching deuterium oxide-derived measures of muscle protein synthesis and mechanistic target of rapamycin complex 1 signaling. FASEB J 29: 4485–4496, 2015. doi: 10.1096/fj.15-273755. [DOI] [PubMed] [Google Scholar]
- 88. Tang JE, Perco JG, Moore DR, Wilkinson SB, Phillips SM. Resistance training alters the response of fed state mixed muscle protein synthesis in young men. Am J Physiol Regul Integr Comp Physiol 294: R172–R178, 2008. doi: 10.1152/ajpregu.00636.2007. [DOI] [PubMed] [Google Scholar]
- 89. Burd NA, Holwerda AM, Selby KC, West DW, Staples AW, Cain NE, Cashaback JG, Potvin JR, Baker SK, Phillips SM. Resistance exercise volume affects myofibrillar protein synthesis and anabolic signalling molecule phosphorylation in young men. J Physiol 588: 3119–3130, 2010. doi: 10.1113/jphysiol.2010.192856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Bruusgaard JC, Johansen IB, Egner IM, Rana ZA, Gundersen K. Myonuclei acquired by overload exercise precede hypertrophy and are not lost on detraining. Proc Natl Acad Sci U S A 107: 15111–15116, 2010. doi: 10.1073/pnas.0913935107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Herman-Montemayor JR, Hikida RS, Staron RS. Early-phase satellite cell and myonuclear domain adaptations to slow-speed vs. traditional resistance training programs. J Strength Cond Res 29: 3105–3114, 2015. doi: 10.1519/JSC.0000000000000925. [DOI] [PubMed] [Google Scholar]
- 92. Fry CS, Kirby TJ, Kosmac K, McCarthy JJ, Peterson CA. myogenic progenitor cells control extracellular matrix production by fibroblasts during skeletal muscle hypertrophy. Cell Stem Cell 20: 56–69, 2017. doi: 10.1016/j.stem.2016.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Murach KA, Fry CS, Kirby TJ, Jackson JR, Lee JD, White SH, Dupont-Versteegden EE, McCarthy JJ, Peterson CA. Starring or supporting role? Satellite cells and skeletal muscle fiber size regulation. Physiology (Bethesda) 33: 26–38, 2018. doi: 10.1152/physiol.00019.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Knowles OE, Drinkwater EJ, Urwin CS, Lamon S, Aisbett B. Inadequate sleep and muscle strength: Implications for resistance training. J Sci Med Sport 21: 959–968, 2018. doi: 10.1016/j.jsams.2018.01.012. [DOI] [PubMed] [Google Scholar]
- 95. Bruinvels G, Burden RJ, McGregor AJ, Ackerman KE, Dooley M, Richards T, Pedlar C. Sport, exercise and the menstrual cycle: where is the research? Br J Sports Med 51: 487–488, 2017. doi: 10.1136/bjsports-2016-096279. [DOI] [PubMed] [Google Scholar]
- 96. Carmichael MA, Thomson RL, Moran LJ, Wycherley TP. The impact of menstrual cycle phase on athletes' performance: a narrative review. Int J Environ Res Public Health 18: 1667, 2021. doi: 10.3390/ijerph18041667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Blagrove RC, Bruinvels G, Pedlar CR. Variations in strength-related measures during the menstrual cycle in eumenorrheic women: A systematic review and meta-analysis. J Sci Med Sport 23: 1220–1227, 2020. doi: 10.1016/j.jsams.2020.04.022. [DOI] [PubMed] [Google Scholar]
- 98. Seynnes OR, de Boer M, Narici MV. Early skeletal muscle hypertrophy and architectural changes in response to high-intensity resistance training. J Appl Physiol (1985) 102: 368–373, 2007. doi: 10.1152/japplphysiol.00789.2006. [DOI] [PubMed] [Google Scholar]
- 99. DeFreitas JM, Beck TW, Stock MS, Dillon MA, Kasishke PR. An examination of the time course of training-induced skeletal muscle hypertrophy. Eur J Appl Physiol 111: 2785–2790, 2011. doi: 10.1007/s00421-011-1905-4. [DOI] [PubMed] [Google Scholar]
- 100. Roberts MD, Haun CT, Vann CG, Osburn SC, Young KC. Sarcoplasmic hypertrophy in skeletal muscle: a scientific “unicorn” or resistance training adaptation? Front Physiol 11: 816, 2020. doi: 10.3389/fphys.2020.00816. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All raw data can be obtained upon reasonable request and establishment of a material transfer agreement by emailing the corresponding author (mdr0024@auburn.edu).