Highlights
-
•
The BA.1, BA.2 and BA.5 present mutations on antagonistic proteins to IFN-β.
-
•
Omicron BA.2 did not exert an in vitro downregulation of IFN-β secretion.
-
•
The in vitro reduced IFN-β secretion by BA.2 correlate with the ORF6 D61L mutation.
-
•
The recombinant mutated ORF6 protein fails to inhibit IFN-β production in vitro.
-
•
Post-transcriptional events can be involved in innate immunity escape by BA.1.
Keywords: SARS-CoV-2, Innate immunity, Omicron, Interferon antagonism, ORF6
Abstract
Although most of the attention was focused on the characterization of changes in the Spike protein among variants of SARS-CoV-2 virus, mutations outside the Spike region are likely to contribute to virus pathogenesis, virus adaptation and escape to the immune system. Phylogenetic analysis of SARS-CoV-2 Omicron strains reveals that several virus sub-lineages could be distinguished, from BA.1 up to BA.5. Regarding BA.1, BA.2 and BA.5, several mutations concern viral proteins with antagonistic activity to the innate immune system, such as NSP1 (S135R), which is involved in mRNAs translation, exhibiting a general shutdown in cellular protein synthesis. Additionally, mutations and/or deletions in the ORF6 protein (D61L) and in the nucleoprotein N (P13L, D31-33ERS, P151S, R203K, G204R and S413R) have been reported, although the impact of such mutations on protein function has not been further studied. The aim of this study was to better investigate the innate immunity modulation by different Omicron sub-lineages, in the attempt to identify viral proteins that may affect virus fitness and pathogenicity. Our data demonstrated that, in agreement with a reduced Omicron replication in Calu-3 human lung epithelial cells compared to the Wuhan-1 strain, a lower secretion of interferon beta (IFN-β) from cells was observed in all sub-lineages, except for BA.2. This evidence might be correlated with the presence of a mutation within the ORF6 protein (D61L), which is strikingly associated to the antagonistic function of the viral protein, since additional mutations in viral proteins acting as interferon antagonist were not detected or did not show significant influence. Indeed, the recombinant mutated ORF6 protein failed to inhibit IFN-β production in vitro. Furthermore, we found an induction of IFN-β transcription in BA.1 infected cells, that was not correlated with the cytokine release at 72 h post-infection, suggesting that post-transcriptional events can be involved in controlling the innate immunity.
1. Introduction
The Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) has been threatening humans since March 2019. The persistence of the pandemic is mostly driven by the emergence of new virus variants (Kockler and Gordenin, 2021). Taking advantage of the whole genome sequence campaigns adopted by many states, a global monitoring of SARS-CoV-2 distribution and evolution has been made and is still ongoing. Based on shared genome sequence databases, the World Health Organization (WHO) classified SARS-CoV-2 as variants of concern (VOCs), variants of interest (VOIs), and variants under monitoring (VUMs) (World Health Organization, 2022). Although most of the attention was focused on the characterization of changes in the Spike protein among VOCs/VOIs, mutations outside the Spike region are likely to contribute to virus adaptation and escape from the immune system. Progressive changes in VOCs transmissibility and clinical outcome have been shown since the Alpha (B.1.1.7) variant emerged in Great Britain in late 2020. Until now, several VOCs have followed one another, the most representative of whom were Beta (B.1.351), Gamma (P.1) and Delta (B.1.617.2). The Omicron variant (B.1.1.529) irrupted in the VOCs scenario on November 2021 (Kannan, 2021; WHO, 2021). Sequence analyses of the Omicron variant revealed more than 60 mutations in its genome (GISAID Tracking of variants, 2021). Considering that many of these mutations are localized in the Spike protein, which may negatively affect the protective efficacy of the current COVID-19 vaccines, and that the Omicron variant is 2.8 times more infectious than the previous Delta variant, huge concerns about its potential threat to public health and economy have been raised (Chen et al., 2022; Petersen et al., 2022; Ranjan, 2022; Callaway, 2021). Surprisingly, only few Omicron infected patients showed pneumonia, while the majority of people reported symptoms related to upper respiratory tract infections (Kim et al., 2022). In vitro studies demonstrated that the Omicron variant replicates faster than the Delta variant in human primary nasal epithelial cells, and it is associated to an increased transmission rate in vivo (Peacock et al., 2022). However, the Omicron replication proved to be lower in human alveolar cells. This could be related to reduced severe sequelae at pulmonary level (Hui et al., 2022). Moreover, the Omicron variant induces less inflammatory cytokines, which may contribute to the milder symptoms associated with COVID-19 (Du et al., 2022). Phylogenetic analysis of SARS-CoV-2 Omicron variant reveals that five sub-lineages could be distinguished: BA.1, BA.2, BA.3, BA.4 and BA.5 (Majumdar and Sarkar, 2021). The initial Omicron BA.1 and BA.2 variants have been progressively displaced by BA.5 in many countries. Compared to the BA.1 lineage, BA.2 further evolved by accumulating mutations, deletions and insertion which induced a peculiar behavior in the new variant (Dhawan et al., 2022; Rahimi et al., 2022). Since the sequences of BA.2 and BA.5 are substantially different from the one of BA.1, it is reasonable to assume that their immune escape strategies are also different from that of BA.1. Host innate immune defense is orchestrated by the production of type I interferons (IFN-α/β) which create the first line defense against viral infections. Similarly to many other viruses, the novel coronavirus encodes several proteins able to counteract the host immune system, which represents the most important factor in controlling viral pathogenesis (Shemesh et al., 2021; Yuen et al., 2020; Thorne et al., 2022; Chen et al., 2021; Gori Savellini et al., 2021). Indeed, a weak and delayed production of INF-β has been reported in individuals with acute SARS-CoV-2 viral infection (Hadjadj et al., 2020; Blanco-Melo et al., 2020; Acharya et al., 2020). Some viral proteins selectively suppress the host innate antiviral functions by blocking critical components of the pathway, such as the Pattern Recognition Receptors (PRRs) or transcription factors (Interferon Regulatory Factor 3; IRF-3), while some other proteins target the general host transcription/translation machinery, blocking the innate immunity as a collateral effect (Zheng et al., 2020; Wang et al., 2021; Xia et al., 2020; Finkel et al., 2021; Kumar et al., 2021). Several mutations in the genome of BA.1, BA.2 and BA.5 sub-lineages affect viral proteins with antagonistic activity to the innate immune system, such as NSP1 (S135R), which is involved in the inhibition of ribosome recognition and translation of mRNAs, as previously described for SARS-CoV and MERS-CoV viruses (Schubert et al., 2020). Additional mutations and/or deletions in NSP5 (P3395H), NSP6 (D3675/3677), ORF3a (T223I), ORF6 (D61L) proteins and in the nucleoprotein N (P13L, D31-33ERS, P151S, R203K, G204R and S413R) have been reported, although the impact of such mutations on protein function has not directly been investigated so far (Karthik et al., 2022). Thus, the aim of this study was to better investigate the innate immunity modulation by different Omicron lineages, in the attempt to identify the viral proteins that may affect virus fitness and pathogenicity. Most of the studies on interferon-antagonism of SARS-CoV-2 proteins have been performed on individually and ectopically expressed proteins. In the present study, we compared some Omicron sub-lineages with the Wuhan-1 strain in vitro, in order to assess whether the IFN-β expression could be correlated to a higher infectivity. Moreover, since mutations in the ORF6 protein were also described, the possible involvement of this protein in controlling the innate immune response to the viral infection was investigated in vitro by transient expression of a naturally-occurring deleted version of the protein lacking the D61 residue and the BA.2 native ORF6 bearing the D61L mutation.
2. Material and Methods
2.1. Cells and viruses
Human Calu-3 lung epithelial cells (ATCC HTB-55) were cultured in Dulbecco's Modified Eagle's medium (DMEM) (Lonza, Milan, Italy) supplemented with 100 U/mL penicillin/streptomycin (Hyclone Europe, Milan, Italy) and 10% heat-inactivated fetal bovine serum (FBS) (Lonza) at 37 °C. Vero E6 (ATCC CRL-1586) cells were maintained in DMEM supplemented with 5% FBS, while HEK-293T (Merk Life Science S.r.l., Milan, Italy) and A549 (ATCC CCL-185) cells were cultured in 10% FBS-containing DMEN. The SARS-CoV-2 lineages were isolated on Vero E6 cells from clinical specimen (according to the principles of the Declaration of Helsinki, with reference to BIOBANK-MIU-2010 document approved by the Ethics Committee with amendment No. 1, on February 17th, 2020. Prior to participating in this study, all subjects signed a written informed consent) at the Virology laboratory of ‘S. Maria alle Scotte’ Hospital in Siena, Italy. Virus stocks were sequenced by COVIDSeq test following manufacturers’ recommendations (Illumina, Milan, Italy) (GenBank accession numbers MT531537, OM956353, ON974845, ON974848 and ON974846, respectively).
2.2. Plasmids
The reporter plasmid encoding Firefly luciferase downstream of the complete interferon-beta promoter (pIFN-b) was a kind gift by Prof. Takashi Fujita (Tokyo Metropolitan Institute of Medical Science, Tokyo, Japan), while the pSV40 Renilla luciferase (pRL), constitutively expressing the reporter gene downstream the SV40 promoter, was purchased from Promega (Milan, Italy). The plasmids encoding the HA-tagged wild-type and M58R mutant ORF6 were kindly provided by Prof. Garcia-Sastre (Mount Sinai School of Medicine, NY, USA; CEIRS program, NIAD Centers of Excellence for Influenza Research and Surveillance). The naturally occurring D61 deleted ORF6 (ORF6Δ61) and the D61L (ORF6-D61L) mutant were isolated from viral RNA, purified from a clinical specimen (GenBank accession numbers OP002141 and ON974845, respectively) and cloned in the pCAGGS plasmid, in frame with a C-terminal HA-tag, by standard procedures.
2.3. Luciferase reporter assay
Calu-3 cells were seeded in 24-well plates (2x105/well) and transfected with 0.5 mg of pIFN-b and 0.05 mg of Renilla reporter plasmids the following day. Transfections were performed by using jetOPTIMUS Transfection Reagent (Polyplus, Illkirch, France), following the manufacturer's instructions. The day after, cells were counted and infected with a multiplicity of infection (MOI) of 0.01 of indicated SARS-CoV-2 lineages. Samples were collected at 48h post-infection (p.i.) and luciferase activities, expressed as Resonance Light Units (RLU), were measured in cell lysates by using the Dual-Luciferase reporter assay reagent (Promega), according to the manufacturer's instructions. After data normalization (Firefly/Renilla ratio), fold change in specific promoter activation was calculated. To study the ORF6 protein activity towards pIFN-b activity, HEK-293T (2x105/well) were seeded in 24-well plates and, after o/n incubation, cells were transfected with 0.2 mg and 0.02 mg of pIFN-β and pRL reporter plasmids, respectively alone or in combination with 0.01 mg of ORF6 or its mutant expressing plasmids, as previously described. Poly(I:C) stimulation was achieved at 36h post-transfection by transfecting 4 μg/mL of poly(I:C), using jetOPTIMUS transfection reagent. After additional 12h, cells were collected, luciferase activities were measured and pIFN-β fold change activation was determined. Results are given as mean fold change in IFN-b promoter activation ± standard deviations (SD) from at least three independent experiments. Protein expression was confirmed by immunoblotting on 50 mg of total cell lysates, quantified by Bradford's reagent (Bio-Rad, Milan, Italy), by using the anti-HA mouse monoclonal antibody (Merck Millipore, Milan, Italy) to detect the ORF6 and the anti-GAPDH (Life Technologies, Milan, Italy) as loading control. Horseradish peroxidase labeled anti-mouse IgG (Merck Millipore) was used as secondary antibody.
2.4. Kinetics of viral replication
Monolayers of Calu-3 cells were respectively seeded at 1x105 and 2x105 in 24-well plate in complete culture medium. The day after, cultures were infected with a MOI of 0.01 of selected SARS-CoV-2 lineages for 1h at 37 °C. After extensive washes to remove the inoculum, growth medium supplemented with 5% of FBS was added. Supernatants were collected at 24h, 48h and 72h post-infection (p.i.) and stored at -80 °C for further analysis. Release of infectious viral particles by infected Calu-3 cells was determined by virus microtitration assay on Vero E6 cells cultured in 96 multi-well plates, as described elsewhere. Ten-fold dilutions from each sample were used to infect cell cultures in four replicates. The cytopathic effect (CPE) was observed under a light microscope 4 days post-infection (p.i.). Reed-Muench formula was used to calculate viral titers as TCID50/ml. RNA samples from infected Calu-3 cells collected at 72h post-infection were analyzed by the TaqPath COVID-19 RT-qPCR Kit including primers/probe for S, N, and ORF1ab genes (Thermo Fisher Scientific, Milan, Italy). A 25 μL reaction was set up containing 5 μl of RNA, and reagents provided by the kit following manufacturer's instruction. Each assay was performed in triplicate on Applied Biosystems™ 7500 Real-Time PCR Systems (Thermo Fisher Scientific). Standard curve (1x104 – 1x10-2 copies/ml) was generated by using the control included in the kit and processed as the samples. Experimental viral gRNA copies (Cp)/ng of total RNA was calculated based on the standard curve and then normalized with respect to the imput RNA amount subjected to RT-qPCR determined by spectrophotometry. Results were reported as mean values ± standard deviation from at least three independent experiments.
2.5. Assessment of IFN-β expression
Medium from Calu-3 cells, infected as previously described, was collected at 24, 48, 72 h post-infection (p.i.). Likewise, the medium of A549 cells, transfected with empty plasmid or ORF6 variants expressing plasmids, was collected for further analysis. IFN-β quantification was assessed by VeriKine-HS Human IFN Beta TCM ELISA Kit (PBL assay science, NJ, USA), following manufacturer's instructions. Total RNA was purified from the respective cell pellets by the RNeasy mini kit (Qiagen, Milan, Italy) and RT-qPCR analyses for IFN-β mRNA was set up by using specific TaqMan assay (Life Technologies, Milan, Italy). IFN-β expression was expressed as fold relative to the mock-infected sample. Results were presented as mean values ± standard deviation from at least three independent experiments.
2.6. Statistics
The mean differences were statistically analyzed by using Fisher's test in GraphPad Prism 6 (GraphPad Software San Diego, CA), in order to compare prevalence rates among different study groups. Statistical significance was set at p<0.05.
3. Results
3.1. BA.2 omicron lineage leads to enhanced IFN-b secretion
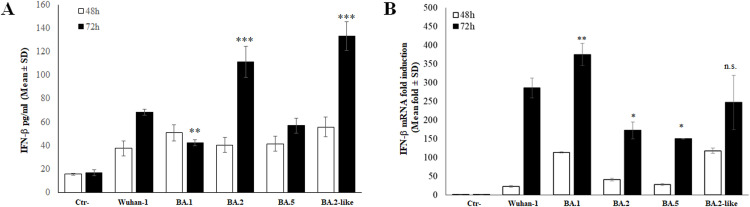
Several studies have provided contradictory results concerning the ability of Omicron BA.1 and BA.2 lineages to modulate the production of FN-b and, consequently, the innate immune response to viral infection. Since mutations in the well-known antagonistic proteins, such as ORF6, N and many NSP proteins, are present among Omicron sub-lineages, we investigated the role these changes could have controlling the host innate immunity. Additionally, a BA.2-like strain, carrying the D61I mutation in the ORF6, in place of D61L present in the parental BA.2 lineage, was included in the study. The Calu-3 human lung epithelial cell model was used for quantitative detection of secreted IFN-b in a time-course experiment upon SARS-CoV-2 infection. The selected viral VOCs did not lead to significant (p>0.05) changes in the basal IFN-β expression at early time as 24h post-infection (p.i.) (data not shown). Conversely, a prolonged infection (48h p.i.) induced IFN-β secretion in Calu-3 cells. Indeed, all tested strains had a significant stimulatory activity of IFN-β (∼2.5-fold increase; p<0.05); however, BA.1 and BA.2-like strain showed to be stronger inducers of IFN-b release (3.3-fold increase, p=0.0002; 3.6, p<0.0001, respectively) (Figure 1A). As the infection proceeded up to 72 h p.i., all but BA.1 and BA.5 showed a greater IFN-β stimulatory activity; in particular, BA.2 and the related BA.2-like lineage exerted massive stimulatory properties compared to the Wuhan-1 strain, leading to a 6.6- (p<0.0001) and 7.9-fold (p<0.0001), increase in IFN-β production, respectively (Figure 1A). These data suggest that both BA.1 and BA.5 sub-lineages are weaker inducers of IFN-b or a greater antagonistic function occurred.
Fig. 1.
(A) Secreted IFN-β was assessed in Calu-3 cells infected (MOI=0.01) with Wuhan-1 SARS-CoV-2 or different Omicron sub-lineages by enzyme-linked immunoassay (ELISA) at 48h and 72h post-infection (p.i.). Negative control (Ctr-) was represented by uninfected Calu-3 cells. Quantitative evaluation was performed, based on relative standard curves. Results are reported as mean concentration (pg/ml) ± standard deviations (SD) from at least three independent experiments (n≥3). (B) The effects of SARS-CoV-2 lineages on IFN-β modulation were evaluated at transcriptional level in Calu-3. Total RNA was purified from mock-infected (Ctr-) or infected (MOI=0.01) cells collected at different times post infection. Specific IFN-β mRNA content was detected by quantitative reverse-transcription polymerase chain reaction (RT-qPCR). RNaseP gene expression was used for relative quantification based on 2-ΔΔCt method. At least three (n≥3) independent experiments were performed and representative data are presented as mean values ± standard deviations. With respect to Wuhan-1 reference strain, significance was determined using unpaired, two-tailed Student's t-test as p<0.0005, ⁎⁎⁎; p<0.005, ⁎⁎, p<0.05, *, p>0.05, n.s.
3.2. IFN-b gene expression is differently modulated in Omicron lineages
SARS-CoV-2 mediates a moderate and delayed activation of the IFN-β antiviral signaling, but few data (Miyamoto et al., 2022; Reuschl et al., 2022) are available regarding the impact of virus variants on the innate immune system. The regulation of IFN-β expression by BA.1 and BA.2 Omicron variants is poorly coherent so far: there are data stating that Omicron possesses reduced induction capacity. On the other hand, there are evidences in line with our own observations, showing a consistent activation of the early immune response during Omicron infection. The control of IFN-β in SARS-CoV-2 infection has been mostly assessed at transcriptional level rather than checking the cytokine release by infected cells, leading to possible misinterpretation because of post-transcriptional events. Thus, we also investigated this last aspect in order to assess discrepancies in methodological investigation. As shown in Fig. 1B, the RT-qPCR assay to quantitatively detect IFN-β messenger RNA (mRNA) evidenced that, among the SARS-CoV-2 lineages tested, only BA.1 and BA.2-like lineages were able to induce significant variations in IFN-β mRNA at 48h p.i., determining a 113- (p<0.0001) and 117-fold induction (p<0.0001), respectively. Conversely, we reported a strong stimulatory function on the cytokine gene transcription for all SARS-CoV-2 selected lineages at 72 h p.i. In particular, a 285-, 375- and 246-fold induction (p<0.0001) in IFN-β mRNA synthesis was observed in Calu-3 cells infected with the Wuhan-1 strain, BA.1 and BA.2-like sub-lineages, respectively (Fig. 1B). Nevertheless, BA.2 and BA.5 resulted in lower cytokine mRNA induction, leading to a 172- (p<0.0001) and 150-fold increase (p<0.0001) in specific transcripts (Fig. 1B). A correlation between IFN-β release by Calu-3 cells and its specific mRNA transcription was only observed in BA.5, BA.2 and BA.2-like sub-lineages. Conversely, BA.1 variant showed a different profile, with a high level of mRNA and a low amount of cytokine protein, indicating that post-transcriptional events might limit interferon release. As reported by Whole Genome Sequence (WGS), the R99C mutation in the Nsp1 gene of BA.1 lineage was reported (Table 1). However, since this mutation was previously demonstrated to marginally affected Nsp1 protein antagonistic function, we speculated that other post-transcriptional events might limit interferon release by BA.1 infected Calu-3 cells (Mendez et al., 2021). In contrast, the WGS approach identified point mutations targeting the Nsp3 and the Nsp6 of the BA.2 and the BA.2-like sub-lineages (Table 1). It was reported that Nsp3 and Nsp6 interacts with RIG-I and TBK-1, respectively, to inhibit activation of IRF3, in turn restricting type I IFN induction at transcriptional level rather that at subsequent steps (Shemesh et al., 2021). Although the mutations reported in our virus strains have not been further characterized, they may affect, along with other virus factors (e.g. dsRNA production), the reduced induction of IFN-β transcription (Figure 1B). However, both mutations in the Nsp3 and Nsp6 did not hinder IFN-β translation, thus the ORF6, with a well-established effect on mRNAs nuclear export, might be involved in BA.2 specific behavior (Figure 1A).
Table 1.
Mutations detected in virus strains used in the experimental procedures.
| Strain | Nextclade_pango | Clade | Clade_WHO | a.a. Substitutions | a.a. Deletions |
|---|---|---|---|---|---|
| BA.1 | BA.1 | 21K | Omicron | E:T9I,M:A63T,N:P13L,N:R203K,N:G204R,ORF1a:R99C,ORF1a:K856R,ORF1a:S2083I,ORF1a:A2710T,ORF1a:T3255I,ORF1a:P3395H,ORF1a:I3758V,ORF1b:P314L,ORF1b:I1566V, ORF9b:P10S | N:E31-,N:R32-,N:S33-,ORF1a:S2084-,ORF1a:L3674-,ORF1a:S3675-,ORF1a:G3676-,ORF9b:E27-,ORF9b:N28-,ORF9b:A29- |
| BA.2 | BA.2 | 21L | Omicron | E:T9I,M:Q19E,M:A63T,N:P13L,N:R203K,N:G204R,N:S413R,ORF1a:S135R,ORF1a:T842I,ORF1a:G1307S,ORF1a:T1760A,ORF1a:T1881I,ORF1a:L3027F,ORF1a:T3255I,ORF1a:P3395H,ORF1a:I3758V,ORF1b:P314L,ORF1b:R1315C,ORF1b:I1566V,ORF1b:T2163I,ORF3a:T223I,ORF6:D61L,ORF9b:P10S | N:E31-,N:R32-,N:S33-,ORF1a:S3675-,ORF1a:G3676-,ORF1a:F3677-,ORF9b:E27-,ORF9b:N28-,ORF9b:A29- |
| BA.2-like | BA.2 | 21L | Omicron | E:T9I,M:Q19E,M:A63T,N:P13L,N:R203K,N:G204R,N:S413R,ORF1a:S135R,ORF1a:T842I,ORF1a:G1307S,ORF1a:P2018S,ORF1a:P2046S,ORF1a:T2800I,ORF1a:L3027F,ORF1a:L3201F,ORF1a:T3255I,ORF1a:P3395H,ORF1a:L3606F,ORF1a:T4311I,ORF1b:P314L,ORF1b:R1315C,ORF1b:I1566V,ORF1b:T2163I,ORF3a:T223I,ORF6:D61I,ORF9b:P10S | N:E31-,N:R32-,N:S33-,ORF1a:S3675-,ORF1a:G3676-,ORF1a:F3677-,ORF9b:E27-,ORF9b:N28-,ORF9b:A29- |
| BA.5 | BA.5.1.8 | 22B | Omicron | E:T9I,M:D3N,M:Q19E,M:A63T,N:P13L,N:R203K,N:G204R,N:S413R,ORF1a:S135R,ORF1a:T842I,ORF1a:G1307S,ORF1a:L3027F,ORF1a:T3090I,ORF1a:T3255I,ORF1a:P3395H,ORF1a:I3758V,ORF1a:T4161I,ORF1b:P314L,ORF1b:R1315C,ORF1b:I1566V,ORF1b:T2163I,ORF3a:T223I,ORF9b:P10S | N:E31-,N:R32-,N:S33-,ORF1a:S3675-,ORF1a:G3676-,ORF1a:F3677-,ORF9b:E27-,ORF9b:N28-,ORF9b:A29- |
List of mutations targeting viral genes, except those in the Spike protein, detected by Whole Genome Sequence (WGS) analysis of selected virus stocks used in all the experimental procedures. In bold, are shown non-common mutations in proteins with well-established antagonistic properties on innate immunity.
3.3. Transcriptional control of IFN-b during Omicron sub-lineages infection
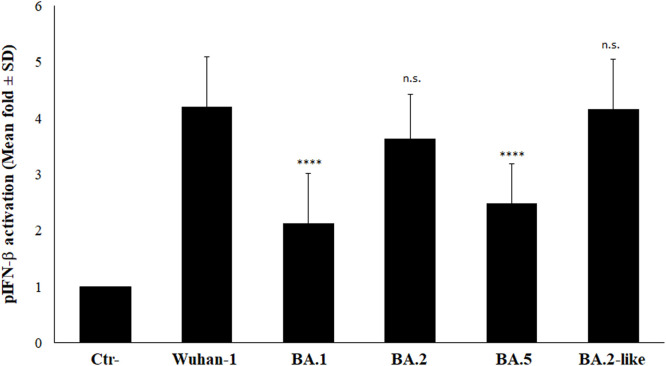
Interferon-b production is mainly controlled at transcriptional level by a tight regulation of the components involved in the signaling cascade, leading to specific promoter activation. Among them, the cytoplasmic Pattern Recognition Receptors (PRRs) and the transcription factor Interferon Regulatory Factor (IRF)-3 play a predominant role in controlling the cytokine expression. Many viruses, including SARS-CoV-2, have developed escape strategies by blocking the signaling pathway at different steps. It is well established that SARS-CoV-2 ORF6 protein is a potent interferon antagonist, while NSP1 protein, blocking the mRNA recognition by ribosomes, exerts a more generic shut-down of protein synthesis (Simeoni et al., 2021; Thoms et al., 2020; Schubert et al., 2020). In the present work, we describe that, while the BA.1 virus substantially induced IFN-β at transcriptional level, a reduced amount of the cytokine was secreted by infected Calu-3 cells. On the contrary, a higher level of IFN-β mRNA corresponded to a higher amount of IFN-β for both BA.2 and BA.2-like lineages. To further investigate whether these apparent discrepancies were related to a reduced activation of PRRs and/or IRF-3, rather than to downstream translation mechanisms, the IFN-β promoter (pIFN-b) activation was assessed by reporter gene assay. In Calu-3 cells, the pIFN-b was significantly activated (p<0.0001) by all the virus lineages included in the study. Among them, a similar activation rate (p=n.s.) was described for the Wuhan-1, BA.2 and BA.2-like sub-lineages (fold induction 4.3±0.26; 3.7±0.22 and 4.28±0.25, respectively), whereas the BA.1 and BA.5 sub-lineages exerted a reduced (2.19±0.12 and 2.56±0.15, respectively) stimulation of IFN-β at transcriptional level (Figure 2). Since the stimulatory function exerted by the BA.2 sub-lineages was similar (p=n.s.) to that of the reference strain, we concluded that the signaling cascade leading to IFN-β production was not hampered by viral strains of BA.2 sub-lineage. This evidence was further supported in IFN-β-deficient Vero E6 cells which, despite the lack in IFN-β production due to a gene deletion, possesses the up-stream and down-stream intact signaling pathways and represent a valid system to investigate transcription factors activation during viral infection (supplementary Figure 1). While the pIFN-β activation rate reported for the Wuhan-1, BA.1 and BA.5 lineages was similar (fold induction 9.6, 8.6 and 6.3, respectively), a 25- and 19-fold promoter activation (p<0.0001 and p=0.0002, respectively) was observed in the BA.2 and BA.2-like infected cultures (supplementary Figure 1), suggesting that the transcriptional machinery controlling IFN-β was not impaired by BA.2 viral strains. Thus, the previously described reduction of IFN-β mRNA in BA.2- and B.2-like-infected Calu-3 cells (Figure 1B) was not a consequence of specific transcription factors’ activity modulation, but resides elsewhere.
Fig. 2.
Stimulatory activity of different SARS-COV-2 lineages towards IFN-β promoter was investigated in Calu-3 cells transfected with the IFN-β promoter-mediated firefly luciferase (pIFN-β) and the SV40 promoter-mediated Renilla luciferase reporter plasmids. At 12h post-transfection, cells were mock-infected or infected with a MOI=0.01 of selected virus variants. Firefly and Renilla luciferase activities were estimated with respect to the mock sample at 48h post-infection. Three (n=3) independent experiments were performed. Representative data are presented as mean logarithm values of relative luminescence unit (RLU) ± standard deviations (SD). With respect to Wuhan-1 reference strain, significance was determined using unpaired, two-tailed Student's t-test as p<0.0001, ⁎⁎⁎⁎; p>0.05, n.s.
3.4. Omicron replication in human epithelial lung cells
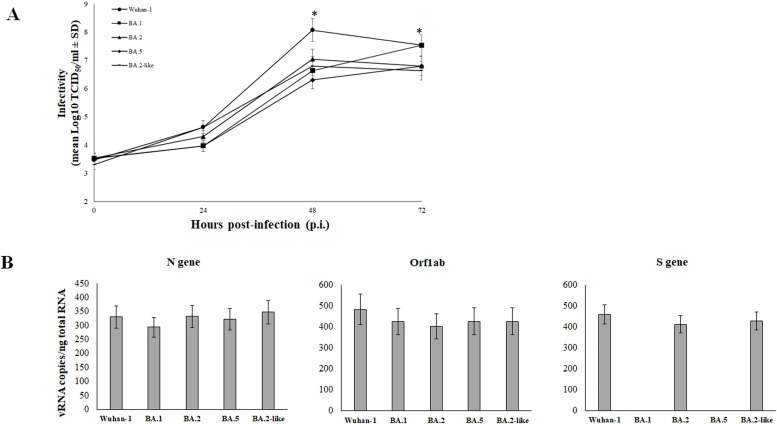
We examined the replication kinetics of the SARS-CoV-2 Omicron variants and the Wuhan-1 strain in Calu-3 cells, in order to establish whether replicative properties of selected lineages could explain discrepancies observed in IFN-β secretion. The virus growth was monitored in culture supernatants of Calu-3 cells infected at a MOI of 0.01 of each virus variant and collected at 24, 48 and 72h p.i. by standard microtitration method (TCID50/ml). Replication kinetics showed a replicative disadvantage of Omicron lineages in comparison to the Wuhan-1 reference strain lower (p<0.03) at 48h p.i. (Fig. 3A). Among the Omicron sub-lineages, only the BA.1 showed a better growth capacity over time, reaching a peak at 72 h p.i. similar to that of the reference strain (Fig. 3A). To address if the reduced Omicron lineages replication in Calu-3 cells was a consequence of new mutations in the virus stocks used, the furin cleavage site (FCS) of the Spike protein was sequenced. Indeed, it is well established that SARS-CoV-2 virus propagation in Vero E6 cells might induce mutation at the FCS (R-R-A-R) and at the upstream QTQTN motif of the Spike protein to alter virus infectivity. The presence of conserved FCS was confirmed in all the virus strains used (Supplementary Fig. 2). The upstream QTQTN site, which as associated to efficient virus infectivity in airway epithelial cells, was deleted in the Wuhan-1 strain, leading to the conclusion that this virus variants might have an altered infectivity and replication in the selected cellular model. However, as reported in Fig. 3A, the Wuhan-1 efficiently infected and replicated in Calu-3 cells. Thus, we speculated that the different IFN-b modulation by viruses within the Omicron group was not due to altered entry pathways and infectivity but resides elsewhere. The infectivity and replication of the selected virus strains in Calu-3 cells was further confirmed by intracellular genome (gRNA) copies quantification. As shown in Fig. 3B, at 72h p.i. when the IFN-b modulation was more prominent, none of the Omicron sub-lineages showed significant variation (p=n.s.) in viral gene synthesis and replication (Fig. 3B). To support the evidence that specific antagonistic proteins (e.g. ORF6) present in the BA.2 and BA.2-like SARS-CoV-2 Omicron sub-lineages were responsible for differences in the modulation of the IFN response, the cytokine expression at both transcriptional and secretion levels by infected Calu-3 cells was normalized to virus replication rate (supplementary Figure 3). As shown, the increased secretion of mature IFN-β reported for the BA.2 sub-lineages was not a consequence of altered virus replication, but was mostly caused by different levels of viral proteins that induce IFN-β release.
Fig. 3.
The Wuhan-1 SARS-CoV-2 variant outcompeted the replication of Omicron variants. (A) Calu-3 cells were infected by Wuhan-1 and Omicron BA.1, BA.2, BA.5 and BA.2-like variants at a MOI of 0.01. Cell culture supernatants were collected at 24h, 48h and 72h post-infection (p.i.) and viable released virus content was assessed by microtitration assay. Results were presented as mean viral titer expressed as tissue culture dose 50 (TCID50)/ml ± standard deviations (SD) from three separate experiments. Significance was determined using unpaired, two-tailed Student's t-test as p<0.05, *. (B) Intracellular viral copy numbers (Cp) of N, S and Orf1ab genes was assessed by RT-qPCR on Calu-3 cells infected at MOI=0.01 with Wuhan-1 and Omicron BA.1, BA.2, BA.5 and BA.2-like variants, collected at 72h p.i. The S gene target detection was impacted by the presence of a six-nucleotide deletion corresponding to the amino-acid 69-70 (D69-70) present in the BA.1 and BA.5 Omicron sub-lineages, rather than to the lack of gene expression. Results were presented as mean Cp/ng of input purified total RNA ± standard deviations (SD) from three separate experiments. Significance was determined using unpaired, two-tailed Student's t-test as p>0.05, n.s.
3.5. Mutations within the ORF6 protein favor efficient IFN-b expression
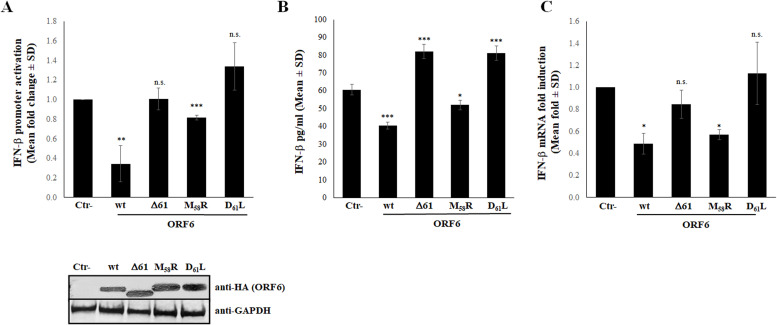
Many studies have been conducted in order to identify the antagonistic activity of SARS-CoV-2 viral proteins to the innate immunity. We and others previously reported that SARS-CoV-2 evolved enhanced innate immune evasion, associated with ORF6 and N antagonists (Shemesh et al., 2021; Yuen et al., 2020; Thorne et al., 2022; Chen et al., 2021; Gori Savellini et al., 2021; Miorin et al., 2020; Addetia et al., 2021; Gori Savellini et al., 2022). Recently, Reuschl et al. (2022) reported that BA.5 expressed higher levels of ORF6 and N proteins. Since virus genetic evolution determined accumulation of several mutations in the Spike, but also in immunity antagonistic proteins, we investigated whether these changes altered protein function. Our results suggest that the ORF6 protein could be involved in controlling host innate immunity, since no significant differences in replication kinetics were reported among Omicron sub-lineages, respect to the Wuhan-1 strain in Calu-3 cells. BA.2 and BA.2-like Omicron sub-lineages induced the highest level of IFN-β in vitro, although the cytokine-specific mRNA was less abundant than the ones detected during BA.1 and Wuhan-1 infection. The C-terminus of the ORF6 protein is critical for its function. Indeed, it has been previously demonstrated that the M58R ORF6 mutant is deficient in binding Rae1/Nup98 nucleopore components due to alteration of a diacidic motif located at the end of the viral protein (aa 53-56) (Miorin et al., 2020; Gori Savellini et al., 2022; Addetia et al., 2021). Thus, we can speculate that the D61 substitution, present in both the BA.2 and BA.2-like Omicron sub-lineages (D61L and D61I, respectively), could explain the reduced antagonistic activity noted in these virus variants. Several sequences are available to demonstrate that, within the BA.2 sub-lineage, the ORF6 was mutated at the 61 position, although independently from the nature of substitution. Thus, exploiting a naturally ORF6 mutated variant carrying the D61 deletion (ORF6Δ61) detected in a clinical sample (GeneBank accession number OP002141) and the BA.2 ORF6 variant bearing the D61L mutation, we demonstrated that the aspartic acid at the last position of the ORF6 protein was fundamental to protein antagonistic function. Thus, a reporter gene assay, based on the firefly luciferase expression mediated by the IFN-β promoter (pIFN-β), was applied in order to assess the antagonistic nature of ORF6Δ61 and ORF6-D61L with respect to the Wuhan-1 protein (wild-type, wt). The transient expression of both ORF6Δ61 and ORF6-D61L was unable to antagonize pIFN-β activation upon poly(I:C) stimulation (fold change 1 and 1.3, respectively; p=n.s.) when over-expressed in HEK-293T cells (Fig. 4A), accordingly to a high expression of IFN-β in A549 cultured cells (fold increase 1.3; p=0.0001 and p=0.0006, respectively) (Fig. 4B). Conversely, the wt-ORF6 massively antagonized IFN-β expression (pIFN-β activation fold change 0.3; p=0.002), while the inactive ORF6 M58R mutant with a substitution next to the carboxy-terminal part of the protein correlated to a moderate, although significant (p=0.0002) inhibition of pIFN-β (Figure 4A). Of notes, a not significant variation in ORF6 variants expression was evidenced in transfected HEK-293T cells, as reported in Fig. 4A, lower panel. Thus, the described proteins activity was not a consequence of differences in their expression rate and cellular accumulation. As shown in Fig. 4B, the wt protein efficiently (p=0.0002) hampered the cytokine expression and release, while the M58R mutant exerted a reduced antagonistic function (0.66-fold decrease, p=0.03) to IFN-β secretion. Moreover, these data were confirmed by investigating the expression rate of IFN-β by RT-qPCR in A549 cells expressing ORF6 variants, in comparison to the negative control (Ctr-), represented by cells transfected with empty plasmid, and to the Wuhan-1 ORF6 protein. When cytokines expression was examined, the wt-ORF6 significantly downregulated endogenous IFN-β expression in transfected cells. As reported in Fig. 4C, a 2-fold decrease was observed in the IFN-β expression (p=0.012). Although less pronounced, a similar activity was also noted in the M58R mutant (1.7-fold decrease, p=0.02) (Fig. 4C). On the contrary, neither the ORF6Δ61 nor the ORF6-D61L mutants showed a significant inhibition of IFN-β mRNA (p=0.3 and p>0.9999, respectively) (Fig. 4C).
Fig. 4.
Inhibitory activity of SARS-COV-2 ORF6 protein variants. (A) HEK-293T cells were co-transfected with the IFN-β promoter-mediated firefly luciferase (pIFN-β) reporter plasmid in combination with wild-type, Δ61 and the D61L or M58R ORF6 inactive mutant expressing plasmids or with empty vector (Ctr-). At 36h post-transfection, cells were poly(I:C)-stimulated by transfection. Firefly and Renilla luciferase activities were evaluated at 48h post-transfection. Three (n=3) independent experiments were performed. Representative data are presented as mean fold change of relative luminescence unit (RLU) ± standard deviations (SD). (B) The production of IFN-β was tested by enzyme-linked immunoassay (ELISA) in supernatant of wild-type, M58R, D61L or Δ61 ORF6 expressing A549 cells. Negative control (Ctr-) was represented by A549 cells transfected with empty plasmid alone. Quantitative evaluation, based on relative standard curves, was performed. Results are reported as mean concentration (pg/ml) ± standard deviations (SD) from at least three separate experiments (n>3). (C) IFN-β expression was evaluated in A549 cells expressing different ORF6 mutants by specific IFN-β mRNA quantification using quantitative reverse-transcription polymerase chain reaction (RT-qPCR). Ribonuclease P (RNaseP) gene expression was used for relative quantification based on 2-ΔΔCt method. With respect to Wuhan-1 reference strain, significance was determined using unpaired, two-tailed Student's t-test as p<0.0005, ⁎⁎⁎; p<0.005, ⁎⁎; p<0.05, *; p>0.05, n.s.
4. Discussion
Many studies suggest that SARS-CoV-2 is able to antagonize innate immunity by suppressing type I interferon response (Lei et al., 2020; Blanco-Melo et al., 2020; Acharya et al., 2020; Channappanavar et al., 2016). Viral recognition and sensing by the innate immune system is primed by the activation of pattern recognition receptors (PRRs), including Toll-like receptors (TLRs) and cytosolic RIG-I-like receptors (RLRs). The recognition of the viral component, such as double-strand RNA (dsRNA) produced during viral replication, by PRRs mediates the activation of signaling pathways, leading to the expression of type I interferon (Sampaio et al., 2020; Mazaleuskaya et al., 2012; Reikine et al., 2014; Rehwinkel et al., 2020). There are growing evidences that like its ancestors SARS-CoV and MERS-CoV, SARS-CoV-2 has evolved multiple immune evasion strategies that interfere at multiple steps between viral sensing and the subsequent interferon-induced antiviral effector proteins (Lei et al., 2020; Noor et al., 2021). Several SARS-CoV-2 proteins were recognized to have IFN-antagonistic properties, including non-structural proteins (e.g. NSP1 and NSP13) and structural proteins (e.g. N), while accessory proteins (e.g. ORF6) can evade host immunity by inhibiting the nuclear translocation of the signaling STAT1 and nucleopore traffic of newly synthetized mRNAs (Fung et al., 2022; Min et al., 2021; Miorin et al., 2020; Addetia et al., 2021; Gori Savellini et al., 2021; Shemesh et al., 2021; Yuen et al., 2020; Gori Savellini et al., 2022; Chen et al., 2020). SARS-CoV-2 B.1.1.529 variant, which emerged in November 2021, was highly transmissible and has become dominant in all regions of the world, rapidly replacing the previously circulating Delta variant. Omicron variant has subsequently evolved in BA.1, BA.2, BA.3, BA.4 and BA.5 sub-lineages (World Health Organization. WHO announces simply, easy-to-say labels for SAR S-CoV-2 Variants of, 2021; World Health Organization, 2022). Among them, BA.2 has rapidly displaced the BA.1 variant, circulating worldwide since the beginning of 2022 (Rahimi et al., 2022). Several epidemiological data suggested that BA.2 was more transmissible than BA.1. However, the difference in transmissibility between BA.1 and BA.2 was much smaller than the difference between BA.1 and its ancestor, Delta. The BA.5 sub-lineage is spreading all around the globe and it is currently being monitored together with other sub-variants, such as BQ.1.1 and BF.7 (Mohapatra et al., 2022; European Centre for Disease Prevention and Control ECDC, 2022). All these sub-lineages differ from the Wuhan-1 strain by genetic mutations, mostly located in the Spike protein, while other viral proteins are less affected by virus genetic evolution. We focused on the viral accessory protein ORF6 which represents the major IFN-β viral antagonist, in addition to the structural protein N (Gori Savellini et al., 2021; Chen et al., 2020; Yuen et al., 2020; Miorin et al., 2020; Addetia et al., 2021; Gori Savellini et al., 2022). BA.2, BA.3, BA.4 and BA.5 share mutation in the NSP1 portion of ORF1a (S135R) and several substitutions in the N structural protein (Gangavarapu et al., 2022). Whereas, the ORF6 D61L substitution has been described for BA.2 and BA.4 sub-lineages only and, since this viral protein is strikingly associated to the innate immunity antagonism, its impact on IFN-β modulation deserves to be considered. In the present study, we further investigated the effects of selected SARS-CoV-2 lineages on IFN-b cytokine expression in pulmonary epithelial Calu-3 cells (Miyamoto et al., 2022; Reuschl et al., 2022; Shalamova et al., 2022). While precocious times of infection, such as 24h and 48h, were negligible for IFN-β secretion by infected Calu-3 cells, mature cytokine release was markedly modulated at 72h after infection. A peculiar profile for BA.1 sub-lineage was reported. Indeed, transcription of IFN-β did not correlate with the release of the cytokine, leading to hypothesize the occurrence of a post transcriptional hindrance. However, we have not found an explanation for this evidence so far, although differences in the generation of dsRNA molecules to induce IFN-β might be considered (Thorne et al., 2022). Notwithstanding, it has been recently shown that among the Omicron sub-lineages several viral proteins were expressed at different levels during the infection (Reuschl et al., 2022). This characteristic was mostly described for the BA.5 sub-lineage, which showed an increased expression of the ORF6 and N antagonist to evade host defence (Reuschl et al., 2022). However, none of the remaining viral protein, such as the NSP1, was investigated. Therefore, we cannot rule out but neither can we confirm that an increased expression in the viral antagonistic gene NSP1 may occur by BA.1 infection in order to prevent IFN-β translation. A different behavior was observed in BA.2 sub-lineages. To better understand this phenomenon, we focused on ORF6 by analyzing transient expression of the protein in vitro. Although this approach does not take into account neither the natural expression level and timing of the viral proteins’ expression driven by virus infection and replication, nor their interaction/cooperation with other viral proteins or cellular component, it represents a common method to study protein function. The ORF6 D61L substitution, close to the key amino acid M58 necessary for protein function, was described in Omicron BA.2 sub-lineage, but not in BA.1 and BA.5 sub-lineages (Miorin et al., 2020; Addetia et al., 2021; Gori Savellini et al., 2022). Hence, taking advantage of a BA.2 Omicron sub-lineage (BA.2-like) carrying the D61 mutated to isoleucine (D61I) in the ORF6 gene, we further investigated the impact of the viral protein on IFN-β during viral replication. We observed that BA.2 and BA.2-like exhibited a reduced replication in Calu-3 cells, as compared to wild-type SARS-CoV-2. This is in line with a reduced transcriptional stimulation of interferon beta. Notwithstanding, the cytokine secretion was not inhibited at all. Thus, we speculated by an in vitro infection model that the D61 amino-acid is part of the ORF6 functional domain which, when mutated, does not affect the cytokine mRNA movement from the nucleus to the cytoplasm to be translated. Further experiments on ectopically expressed ORF6 protein variants confirmed the incisive role of the C-terminus domain of the protein, linked to the D61 mutation more than to the upstream M58 amino-acid. Indeed, the over-expression of the D61 deleted or mutated ORF6 variants completely lost antagonistic activity toward IFN-β, in comparison to the wild-type protein and, to a lesser extent, to the M58R mutant. Afterall, the amino-acid location (D61) affects protein function more prevalent than the nature of substitution. Indeed, the last position of the protein was frequently mutated to amino-acid with chemical-physical properties divergent from the aspartic acid present in the ancestral virus strain. Notwithstanding, the two BA.2 sub-lineages included in the study shares the ORF6 D61 mutation with an amino-acid (leucine and isoleucine) with similar characteristics from the point of view of chemical properties (isoelectric point, hydrophobicity, aliphatic side-chain). Taken together, our data showed that a naturally occurring mutant of ORF6, lacking or mutated at the last amino-acid, completely lost antagonistic function on IFN-β expression. This also corroborated the hypothesis that the variability in cytokine induction, observed in different virus lineages, might not only be related to a different replication and stimulatory profile of SARS-CoV-2 virus variants, but also to different properties of antagonistic viral proteins, such as the ORF6.
5. Conclusions
In the present study, we demonstrated that a reduction in interferon beta (IFN-β) production from Calu-3 cells occurred in all Omicron sub-lineages, except for BA.2. This evidence might be correlated with the presence of a mutation within the ORF6 protein (D61L), which is strikingly associated to the antagonistic function of the viral protein. Indeed, a naturally-occurring ORF6 mutated protein failed to inhibit IFN-β production in vitro similarly to the D61L mutant described for the BA.2 sub-lineage. Furthermore, the induction of IFN-β transcription in BA.1 infected Calu-3 cells, that was not correlated with the cytokine release, suggested that post-transcriptional events can be involved in controlling the innate immunity. In conclusion, our data supported the hypothesis that the variability in cytokine induction, observed in different SARS-CoV-2 lineages, might not only be a consequence of different replication and stimulatory profile of the virus variants, but also to different properties of antagonistic viral proteins, such as ORF6.
Funding
This work was supported by Piano di Sostegno alla Ricerca (PSR) 2022, University of Siena, Italy; and by Piano Nazionale di Ripresa e Resilienza (PNRR), Italian Ministry of University and Research (grant number CN00000041).
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
CRediT authorship contribution statement
Gianni Gori Savellini: Conceptualization, Investigation, Funding acquisition, Methodology, Formal analysis, Data curation, Project administration, Writing – review & editing. Gabriele Anichini: Investigation, Formal analysis, Funding acquisition, Methodology. Maria Grazia Cusi: Writing – review & editing, Funding acquisition, Formal analysis.
Declaration of Competing Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.virusres.2023.199134.
Appendix. Supplementary materials
Data availability
Data will be made available on request.
References
- Acharya D., Liu G., Gack M.U. Dysregulation of type I interferon responses in COVID-19. Nat. Rev. Immunol. 2020;20:397–398. doi: 10.1038/s41577-020-0346-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addetia A., Lieberman N.A.P., Phung Q., Hsiang T.Y., Xie H., Roychoudhury P., Shrestha L., Loprieno M.A., Huang M.L., Gale M.Jr, Jerome K.R., Greninger A.L. SARS-CoV-2 ORF6 disrupts bidirectional nucleocytoplasmic transport through interactions with Rae1 and Nup98. mBio. 2021;12:e00065–e00069. doi: 10.1128/mBio.00065-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco-Melo D., Nilsson-Payant B.E., Liu W.C., Uhl S., Hoagland D., Møller R., Jordan T.X., Oishi K., Panis M., Sachs D., Wang T.T., Schwartz R.E., Lim J.K., Albrecht R.A., tenOever B.R. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell. 2020;181:1036–1045. doi: 10.1016/j.cell.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaway E. Heavily mutated Omicron variant puts scientists on alert. Nature. 2021;600:21. doi: 10.1038/d41586-021-03552-w. [DOI] [PubMed] [Google Scholar]
- Channappanavar R., Fehr A.R., Vijay R., Mack M., Zhao J., Meyerholz D.K., Perlman S. Dysregulated type I Interferon and inflammatory monocyte-macrophage responses cause lethal pneumonia in SARS-CoV-infected mice. Cell host microbe. 2016;19:181–193. doi: 10.1016/j.chom.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Wang R., Gilby N.B., Wei G.W. Omicron variant (B.1.1.529): infectivity, vaccine breakthrough, and antibody resistance. J. Chem. Inf. Model. 2022;62:412–422. doi: 10.1021/acs.jcim.1c01451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K., Xiao F., Hu D., Ge W., Tian M., Wang W., Pan P., Wu K., Wu J. SARS-CoV-2 nucleocapsid protein interacts with RIG-I and represses RIG-mediated IFN-β production. Viruses. 2020;13:47. doi: 10.3390/v13010047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhawan M., Priyanka, Choudhary O.P. Emergence of omicron sub-variant BA.2: Is it a matter of concern amid the COVID-19 pandemic? Int. J. Surg. 2022;99 doi: 10.1016/j.ijsu.2022.106581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du X., Tang H., Gao L., Wu Z., Meng F., Yan R., Qiao S., An J., Wang C., Qin F.X. Omicron adopts a different strategy from Delta and other variants to adapt to host. Signal Transduct. Target. Ther. 2022;7:45. doi: 10.1038/s41392-022-00903-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel Y., Gluck A., Nachshon A., Winkler R., Fisher T., Rozman B., Mizrahi O., Lubelsky Y., Zuckerman B., Slobodin B., Yahalom-Ronen Y., Tamir H., Ulitsky I., Israely T., Paran N., Schwartz M., Stern-Ginossar N. SARS-CoV-2 uses a multipronged strategy to impede host protein synthesis. Nature. 2021;594:240–245. doi: 10.1038/s41586-021-03610-3. [DOI] [PubMed] [Google Scholar]
- Fung S.Y., Siu K.L., Lin H., Chan C.P., Yeung M.L., Jin D.Y. SARS-CoV-2 NSP13 helicase suppresses interferon signaling by perturbing JAK1 phosphorylation of STAT1. Cell Biosci. 2022;12:36. doi: 10.1186/s13578-022-00770-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gori Savellini G., Anichini G., Gandolfo C., Cusi M.G. Nucleopore traffic is hindered by SARS-CoV-2 ORF6 protein to efficiently suppress IFN-β and IL-6 secretion. Viruses. 2022;14:1273. doi: 10.3390/v14061273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gori Savellini G., Anichini G., Gandolfo C., Cusi M.G. SARS-CoV-2 N protein targets TRIM25-mediated RIG-I activation to suppress innate immunity. Viruses. 2021;13:1439. doi: 10.3390/v13081439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjadj J., Yatim N., Barnabei L., Corneau A., Boussier J., Smith N., Péré H., Charbit B., Bondet V., Chenevier-Gobeaux C., Breillat P., Carlier N., Gauzit R., Morbieu C., Pène F., Marin N., Roche N., Szwebel T.A., Merkling S.H., Treluyer J.M., …, Terrier B. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science. 2020;369:718–724. doi: 10.1126/science.abc6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui K., Ho J., Cheung M.C., Ng K.C., Ching R., Lai K.L., Kam T.T., Gu H., Sit K.Y., Hsin M., Au T., Poon L., Peiris M., Nicholls J.M., Chan M. SARS-CoV-2 Omicron variant replication in human bronchus and lung ex vivo. Nature. 2022;603:715–720. doi: 10.1038/s41586-022-04479-6. [DOI] [PubMed] [Google Scholar]
- Kannan S., Shaik Syed Ali P., Sheeza A. Omicron (B.1.1.529) - variant of concern - molecular profile and epidemiology: a mini review. Eur. Rev. Med. Pharmacol. Sci. 2021;25:8019–8022. doi: 10.26355/eurrev_202112_27653. [DOI] [PubMed] [Google Scholar]
- Kim M.K., Lee B., Choi Y.Y., Um J., Lee K.S., Sung H.K., Kim Y., Park J.S., Lee M., Jang H.C., Bang J.H., Chung K.H., Jeon J. Clinical characteristics of 40 patients infected with the SARS-CoV-2 omicron variant in Korea. J. Korean Med. Sci. 2022;37:e31. doi: 10.3346/jkms.2022.37.e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kockler Z.W., Gordenin D.A. From RNA world to SARS-CoV-2: the edited story of RNA viral evolution. Cells. 2021;10:1557. doi: 10.3390/cells10061557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A., Ishida R., Strilets T., Cole J., Lopez-Orozco J., Fayad N., Felix-Lopez A., Elaish M., Evseev D., Magor K.E., Mahal L.K., Nagata L.P., Evans D.H., Hobman T.C. SARS-CoV-2 nonstructural protein 1 inhibits the interferon response by causing depletion of key host signaling factors. J. Virol. 2021;95 doi: 10.1128/JVI.00266-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei X., Dong X., Ma R., Wang W., Xiao X., Tian Z., Wang C., Wang Y., Li L., Ren L., Guo F., Zhao Z., Zhou Z., Xiang Z., Wang J. Activation and evasion of type I interferon responses by SARS-CoV-2. Nature Commun. 2020;11:3810. doi: 10.1038/s41467-020-17665-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumdar S., Sarkar R. Mutational and phylogenetic analyses of the two lineages of the Omicron variant. J. Med. Virol. 2022;94:1777–1779. doi: 10.1002/jmv.27558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazaleuskaya L., Veltrop R., Ikpeze N., Martin-Garcia J., Navas-Martin S. Protective role of Toll-like Receptor 3-induced type I interferon in murine coronavirus infection of macrophages. Viruses. 2012;4:901–923. doi: 10.3390/v4050901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez A.S., Ly M., González-Sánchez A.M., Hartenian E., Ingolia N.T., Cate J.H., Glaunsinger B.A. The N-terminal domain of SARS-CoV-2 nsp1 plays key roles in suppression of cellular gene expression and preservation of viral gene expression. Cell Rep. 2021;37 doi: 10.1016/j.celrep.2021.109841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min Y.Q., Huang M., Sun X., Deng F., Wang H., Ning Y.J. Immune evasion of SARS-CoV-2 from interferon antiviral system. Comput.Struct. Biotechnol. J. 2021;19:4217–4225. doi: 10.1016/j.csbj.2021.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miorin L., Kehrer T., Sanchez-Aparicio M.T., Zhang K., Cohen P., Patel R.S., Cupic A., Makio T., Mei M., Moreno E., Danziger O., White K.M., Rathnasinghe R., Uccellini M., Gao S., Aydillo T., Mena I., Yin X., Martin-Sancho L., Krogan N.J., García-Sastre A. SARS-CoV-2 Orf6 hijacks Nup98 to block STAT nuclear import and antagonize interferon signaling. Proc. Natl Acad. Sci. 2020;117:28344–28354. doi: 10.1073/pnas.2016650117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto Y., Itoh Y., Suzuki T., Tanaka T., Sakai Y., Koido M., Hata C., Wang C.X., Otani M., Moriishi K., Tachibana T., Kamatani Y., Yoneda Y., Okamoto T., Oka M. SARS-CoV-2 ORF6 disrupts nucleocytoplasmic trafficking to advance viral replication. Commun Biol. 2022;5:483. doi: 10.1038/s42003-022-03427-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohapatra R.K., Kandi V., Sarangi A.K., Verma S., Tuli H.S., Chakraborty S., Chakraborty C., Dhama K. The recently emerged BA.4 and BA.5 lineages of Omicron and their global health concerns amid the ongoing wave of COVID-19 pandemic - Correspondence. Int. J. Surg. 2022;103 doi: 10.1016/j.ijsu.2022.106698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noor R. A comparative review of pathogenesis and host innate immunity evasion strategies among the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), severe acute respiratory syndrome coronavirus (SARS-CoV) and the Middle East respiratory syndrome coronavirus (MERS-CoV) Arch. Microbiol. 2021;203:1943–1951. doi: 10.1007/s00203-021-02265-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Centre for Disease Prevention and Control (ECDC). 2022. Implications of the emergence and spread of the SARS-CoV-2 variants of concern BA.4 and BA.5 for the EU/EEA. ECDC: Stockholm. Available at: https://www.ecdc.europa.eu/en/news-events/implications-emergence-spread-sars-cov-2-variants-concern-ba4-and-ba5.
- Petersen E., Ntoumi F., Hui D.S., Abubakar A., Kramer L.D., Obiero C., Tambyah P.A., Blumberg L., Yapi R., Al-Abri S., Pinto T., Yeboah-Manu D., Haider N., Asogun D., Velavan T.P., Kapata N., Bates M., Ansumana R., Montaldo C., Mucheleng'anga L., Zumla A. Emergence of new SARS-CoV-2 Variant of Concern Omicron (B.1.1.529) - highlights Africa's research capabilities, but exposes major knowledge gaps, inequities of vaccine distribution, inadequacies in global COVID-19 response and control efforts. Int. J. Infect. Dis. 2022;114:268–272. doi: 10.1016/j.ijid.2021.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahimi F., Talebi Bezmin Abadi A. The Omicron subvariant BA.2: Birth of a new challenge during the COVID-19 pandemic. Int. J. Surg. 2022;99 doi: 10.1016/j.ijsu.2022.106261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peacock T.P., Brown J.C., Zhou J., Thakur N., Newman J., Kugathasan R., Sukhova K., Kaforou M., Bailey D., Barclay W.S. The SARS-CoV-2 variant, Omicron, shows rapid replication in human primary nasal epithelial cultures and efficiently uses the endosomal route of entry. bioRxiv. 2022;474653 doi: 10.1101/2021.12.31.474653. [DOI] [Google Scholar]
- Ranjan R. Omicron impact in India: analysis of the ongoing COVID-19 third wave based on global data. medRxiv.22268969. 2022 doi: 10.1101/2022.01.09.22268969. [DOI] [Google Scholar]
- Reikine S., Nguyen J.B., Modis Y. Pattern Recognition and Signaling Mechanisms of RIG-I and MDA5. Front. Immunol. 2014;5:342. doi: 10.3389/fimmu.2014.00342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuschl A.K., Thorne L.G., Whelan M.V.X., Mesner D., Ragazzini R., Dowgier G., Bogoda N., Turner J.L.E., Furnon W., Cowton V.M., de Lorenzo G., Bonfanti P., Palmarini M., Patel A.H., Jolly C., Towers G.J. Enhanced innate immune suppression by SARS-CoV-2 Omicron subvariants BA.4 and BA.5. Biorxiv.499603. 2022 doi: 10.1101/2022.07.12.499603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert K., Karousis E.D., Jomaa A., Scaiola A., Echeverria B., Gurzeler L.A., Leibundgut M., Thiel V., Mühlemann O., Ban N. SARS-CoV-2 Nsp1 binds the ribosomal mRNA channel to inhibit translation. Nat. Struct. Mol. Biol. 2020;27:959–966. doi: 10.1038/s41594-020-0511-8. [DOI] [PubMed] [Google Scholar]
- Shemesh M., Aktepe T.E., Deerain J.M., McAuley J.L., Audsley M.D., David C.T., Purcell D., Urin V., Hartmann R., Moseley G.W., Mackenzie J.M., Schreiber G., Harari D. SARS-CoV-2 suppresses IFNβ production mediated by NSP1, 5, 6, 15, ORF6 and ORF7b but does not suppress the effects of added interferon. PLOS Pathog. 2021;17(8) doi: 10.1371/journal.ppat.1009800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simeoni M., Cavinato T., Rodriguez D., Gatfield D. I(nsp1)ecting SARS-CoV-2-ribosome interactions. Commun. Biol. 2021;4:715. doi: 10.1038/s42003-021-02265-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoms M., Buschauer R., Ameismeier M., Koepke L., Denk T., Hirschenberger M., Kratzat H., Hayn M., Mackens-Kiani T., Cheng J., Straub J.H., Stürzel C.M., Fröhlich T., Berninghausen O., Becker T., Kirchhoff F., Sparrer K., Beckmann R. Structural basis for translational shutdown and immune evasion by the Nsp1 protein of SARS-CoV-2. Science. 2020;369:1249–1255. doi: 10.1126/science.abc8665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne L.G., Bouhaddou M., Reuschl A.K., Zuliani-Alvarez L., Polacco B., Pelin A., Batra J., Whelan M., Hosmillo M., Fossati A., Ragazzini R., Jungreis I., Ummadi M., Rojc A., Turner J., Bischof M.L., Obernier K., Braberg H., Soucheray M., Richards A., Krogan N.J. Evolution of enhanced innate immune evasion by SARS-CoV-2. Nature. 2022;602:487–495. doi: 10.1038/s41586-021-04352-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Zhou Z., Xiao X., Tian Z., Dong X., Wang C., Li L., Ren L., Lei X., Xiang Z., Wang J. SARS-CoV-2 nsp12 attenuates type I interferon production by inhibiting IRF3 nuclear translocation. Cell Mol. Immunol. 2021;18:945–953. doi: 10.1038/s41423-020-00619-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization Classification of omicron (B.1.1.529): SARS-CoV-2 variant of concern. https://www.who.int/news/item/26-11-2021-classification-of-omicron-(b.1.1.529)-sars-cov-2-variant-of-concern
- World Health Organization. 2022. Tracking SARS-CoV-2 variants. https://www.who.int/activities/tracking-SARS-CoV-2-variants.
- World Health Organization. WHO announces simply, easy-to-say labels for SAR S-CoV-2 Variants of. Interest and Concern. 2021 https://www.who.int/news/item/31-05-2021-who-announces-simple-easy-to-say-labels-for-sars-cov-2-variants-of-interest-and-concern [Google Scholar]
- Xia H., Cao Z., Xie X., Zhang X., Chen J.Y., Wang H., Menachery V.D., Rajsbaum R., Shi P.Y. Evasion of type I interferon by SARS-CoV-2. Cell Rep. 2020;33 doi: 10.1016/j.celrep.2020.108234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen C.K., Lam J.Y., Wong W.M., Mak L.F., Wang X., Chu H., Cai J.P., Jin D.Y., To K.K., Chan J.F., Yuen K.Y., Kok K.H. SARS-CoV-2 nsp13, nsp14, nsp15 and orf6 function as potent interferon antagonists. Emerg. Microbes Infect. 2020;9:1418–1428. doi: 10.1080/22221751.2020.1780953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y., Zhuang M.W., Han L., Zhang J., Nan M.L., Zhan P., Kang D., Liu X., Gao C., Wang P.H. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) membrane (M) protein inhibits type I and III interferon production by targeting RIG-I/MDA-5 signaling. Signal Transduct. Target. Ther. 2020;5:299. doi: 10.1038/s41392-020-00438-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.