Abstract
Electronic cigarettes are often used for smoking cessation as a harm reduction strategy, but studies comparing risks of electronic cigarettes (ECs) and tobacco cigarettes (TCs) are scarce. Ventricular repolarization in people who smoke TCs is abnormal. Baseline repolarization was compared among nonusers (people who do not use TCs or ECs) and people who use ECs or TCs. The acute effects of ECs and TCs on metrics of ventricular repolarization were then compared in people who chronically smoke. A total of 110 participants (59 female), including 35 people (21 females) in the TC cohort, 34 people (17 females) in the EC cohort, and 41 people (21 females) in the nonuser cohort, were included. None of the primary outcomes, Tpeak-end (Tp-e), Tp-e/QT, and Tp-e/QTc, were different among the three cohorts at supine baseline, even when adjusted for sex. When compared with the control exposure standing after acutely using the EC but not the TC, significantly prolonged all three primary indices of ventricular repolarization in people who smoke TCs. The major new finding in this study is that in people who smoke TCs, using an EC compared with a TC significantly prolongs ventricular repolarization. Furthermore, in our subgroup analysis by sex, this adverse effect on repolarization is found only in male, not female, smokers. In summary, chronic TC smoking is the most prevalent, modifiable risk factor for cardiovascular death, including sudden cardiac death. If used for smoking cessation, ECs should only be used in the short term since they too carry their own risks; this risk appears to be greatest in males compared with females who smoke.
NEW & NOTEWORTHY The major new finding in this study is that in people who smoke tobacco cigarettes, using an electronic cigarette but not a tobacco cigarette acutely and significantly prolongs several metrics of ventricular repolarization, including Tpeak-Tend, Tpeak-Tend/QT, and Tpeak-Tend/QTc. Furthermore, in our subgroup analysis by sex, this adverse effect on repolarization is found only in male, not female, smokers.
Keywords: electronic cigarettes, nicotine, tobacco cigarettes, Tpeak-Tend, ventricular repolarization
INTRODUCTION
Smoking commercial tobacco cigarettes remains the most prevalent modifiable risk factor for cardiovascular disease and sudden cardiac death in the United States today (1). Electronic cigarettes (ECs), which provide comparable nicotine but with lower levels of non-nicotine toxicants compared with tobacco cigarettes (2, 3), have been promoted as part of a harm reduction strategy aimed at smokers addicted to nicotine who are unwilling or unable to quit (4). However, their relative effects on sudden cardiac death risk remain unknown (5).
The observation that sudden death risk begins to decline toward that of a nonsmoker soon after smoking cessation has led to the hypothesis that increased smoking-related sudden death risk results from a direct toxic effect of constituent(s) in tobacco cigarette smoke (6). Nicotine, a sympathomimetic agent, may trigger sudden death by increasing ischemia risk, either by increasing myocardial oxygen demand or by eliciting coronary vasospasm, or importantly, nicotine may increase sudden death risk by prolonging ventricular repolarization, a known risk factor for sudden cardiac death (7, 8). Nicotine has been shown to prolong repolarization in isolated ventricular myocytes, devoid of sympathetic innervation, perhaps through direct effects on potassium channels (9–11).
Several studies have reported abnormal ventricular repolarization in people who smoke tobacco cigarettes (12–17). Ventricular repolarization can be estimated from the QT interval on the surface 12-lead electrocardiogram, but this interval includes both ventricular depolarization and repolarization and thus may lack sensitivity and precision in identifying increased sudden death risk (18, 19). Accordingly, the absolute Tpeak-to-Tend (Tp-e) interval, which only includes ventricular repolarization, and the ratio of the Tp-e-to-QT and Tp-e-to-QTc intervals have been promoted as superior predictors of sudden death risk compared with the QT or QTc intervals in many vulnerable populations (8, 18).
In a recent retrospective analysis, we reported that acutely smoking a tobacco cigarette markedly and significantly increased all three of these parameters of ventricular repolarization in people who chronically smoked tobacco cigarettes (20). In contrast, in people who exclusively used electronic cigarettes, only one of the three parameters, the Tp-e-to-QT ratio, was prolonged, and the increase was significantly less than that seen in smokers acutely using an equivalent “dose” of a tobacco cigarette. Although these findings support the use of electronic cigarettes as part of a harm reduction approach, this was a retrospective study in which only two of the 12 ECG leads were available for Tp-e analysis. In addition, although the acute effect of each tobacco product on repolarization was compared between cohorts who exclusively used electronic cigarettes versus those who exclusively smoked tobacco cigarettes, both products were not compared in the same individual—this was not a “switch study.” Accordingly, in this current prospective study in which all 12 ECG leads were recorded, we compare the acute effects of smoking a tobacco cigarette with the acute effects of using an electronic cigarette on ventricular repolarization in persons who chronically smoke tobacco cigarettes. In addition, we report the acute effects on ventricular repolarization of using an electronic cigarette in persons who exclusively use electronic cigarettes. To determine the effect of the nicotine versus non-nicotine constituents in mediating these changes, we compared tobacco cigarettes and electronic cigarettes with nicotine and without nicotine. Finally, to increase the sensitivity of our study for detecting abnormal repolarization, we performed a provocative, sympatho-excitatory maneuver during the ECG recording, abrupt standing, which has been advocated to unmask occult prolongation of ventricular repolarization in vulnerable populations (21–23).
MATERIALS AND METHODS
Study Population
Healthy male and female participants between the ages 21 and 45 yr meeting the following criteria were eligible for enrollment: 1) nonobese (≤30 kg/m2 body mass index), 2) no known health problems (including asthma, diabetes, heart disease, hypertension, or hyperlipidemia), 3) not pregnant (urine pregnancy test administered on the day of the study), 4) not competitive (nonintercollegiate) athletes, and 5) not taking prescription medications regularly (besides oral contraceptives). Finally, participants were screened through a questionnaire to ensure that they did not smoke marijuana, use illicit drugs regularly, or drink more than two alcoholic drinks per day. Plasma nicotine and urinary drug tests were administered at the start of each session to detect surreptitious use of nicotine products and drugs.
In conjunction with previously mentioned criteria, participants were eligible for specific groups based on their tobacco product use. Individuals were enrolled in the tobacco cigarette cohort if they had smoked tobacco cigarettes for >1 yr with or without concomitant electronic cigarette use before study enrollment. Individuals were enrolled in the electronic cigarette cohort if they had exclusively used electronic cigarettes for >1 yr before start of the study. People meeting enrollment criteria who currently use electronic cigarettes and who had previously smoked tobacco cigarettes were eligible for the study if they had quit smoking >1 yr before study enrollment. Individuals who had not smoked tobacco cigarettes or used electronic cigarettes for >1 yr before study enrollment were enrolled in the nonuser cohort. The experimental protocol was approved by the Institutional Review Board at the University of California, Los Angeles (UCLA), and written informed consent was obtained from each participant. This study is registered at ClinicalStudies.gov (NCT03916341).
Baseline Comparison Study
Baseline ECG parameters (Tp-e, Tp-e/QT, and Tp-e/QTc, QT, QTc) and heart rate (HR), recorded at the first experimental session supine and with abrupt standing, were compared among the three cohorts: 1) tobacco cigarette cohort, 2) electronic cigarette cohort, and 3) nonuser cohort.
Randomized Crossover Study
Participants in the tobacco cigarette cohort and electronic cigarette cohort then underwent a series of exposures (described in Exposures) on different session days in random order.
Experimental Session
All study participants were instructed to abstain from caffeine and exercise for 12 h before their study session. Participants were instructed to refrain from tobacco cigarette smoking and electronic cigarette use on the day of the study. Participants were situated in a quiet, temperature-controlled (21°C) room in the Human Physiology Laboratory located in the UCLA Clinical and Translational Research Center. Participants were placed in the supine position in a reclining chair with a footrest. Skin was cleaned with alcohol wipes and 10 electrodes (3 M Red Dot) were placed on the chest according to standard ECG protocol. Recording electrodes were foam silver-silver chloride conductors, 3.0 cm in diameter, with adhesive hydrogel. The ECG was recorded with digital recording software: LabChart Pro 8 with ECG module (AdInstruments, 1,000-Hz sampling frequency). Recordings were optimized to minimize noise and artifacts.
Blood was drawn for nicotine and cotinine levels, and after a short rest period, the 12-lead ECG was recorded continuously for 5 min. Participants were instructed to remain still and avoid speaking for the duration of the ECG recording. Use of digital devices during the recording was not allowed. Talking was minimized by research staff during data acquisition. At the end of the 5-min recording period, the footrest was abruptly lowered and the participant was instructed to stand up and remain still. The ECG recording was continued for 30 s with standing, capturing peak heart rate and its subsequent return toward baseline. The participant was then detached from the ECG electrodes and led to a smoking patio, where they underwent their assigned exposure (detailed below). Participants then returned to the study room and were placed once again in the supine position. Blood was drawn for nicotine levels. The ECG was again recorded for 5 min in the supine position and for 30 s following abrupt standing.
Exposures
Tobacco cigarette cohort.
On different days in random order, participants were asked to smoke 1) a commercial tobacco cigarette (participant’s own); 2) an electronic cigarette (JUUL) with 5% nicotine with mint flavoring, prompted by an audio recording to take a 3-s puff every 30 s for 15 min; 3) a research cigarette with negligible nicotine (Spectrum 20, class A cigarette) obtained from the Food and Drug Administration (FDA); and 4) an empty straw (control session) using the same predefined topography protocol as the electronic cigarette.
Electronic cigarette cohort.
On different days in random order, with the same predefined topography defined earlier, participants were asked to use 1) an electronic cigarette (JUUL) with 5% nicotine with mint flavoring, 2) an electronic cigarette (EZEE) with 0% nicotine with mint flavoring, and 3) an empty straw (control session).
ECG Recording Analysis
Twelve-lead ECG recordings were analyzed using commercially available software (Labchart Pro 8 with ECG module, AdInstruments) as previously described (24). For each of the 12 leads, all beats were averaged via block averaging resulting in one PQRST complex per lead for analysis. In the 5-min rest-supine recording, ∼300 beats were analyzed. In the brief standing recording, four to eight beats at peak heart rate were analyzed. The ECG Analysis Module software automatically identified the onset of the QRS complex, the peak of the T wave, and the end of the T wave. Cursors were placed on each autoidentified point, and placement was confirmed by at least one investigator (I.R.). The software designated the Tp-e interval as the difference between the peak of the T wave and the end of the T wave. The software automatically identified the end of the T wave as the intersection of the tangent to the T wave’s downslope and the isoelectric line (8). For inverted T waves, the Tp-e was measured as the interval from the nadir of the T wave to the end of the T wave (18). Leads in which T waves were low amplitude (<1.5 mm) or flattened were not included in the analysis (25). U waves were not included in the Tp-e interval (8). QTc was calculated using Bazett’s formula (25).
Nicotine and Cotinine Plasma Levels
Before and after each intervention, blood was drawn and sent to the UCLA Clinical Laboratory for measurement of plasma nicotine and cotinine levels.
Sample-Size Calculation
Based on preliminary data, in which the supine Tp-e change standard deviation was 29.4 ms, a sample size of n = 27/group was calculated to have 80% power to detect mean pre-post exposure differences of 18.6 ms using the usual two-sided P < 0.05 significance level. Other indices of ventricular repolarization were calculated to require even fewer subjects per group. Accordingly, we planned to enroll a minimum of 30 participants per group.
Statistical Methods
The P values for comparing continuous variables among groups at (visit 1) baseline were computed using the one-way analysis of variance (ANOVA) model if the data followed the normal distribution or with the nonparametric Kruskal–Wallis method otherwise.
Normal quantile plots (not shown) indicate that the post-pre change values for Tp-e, Tp-e/QT, Tp-e/QTc, QT, and QTc followed a normal distribution except for an occasional outlier. Therefore, mean comparisons across exposures were compared using a robust repeated-measure model for a 3 × 3 or 4 × 4 crossover design controlling for (adjusting for) possible visit effects. Robust methods were used to automatically down-weight outliers. Robust means are reported.
The overall Wald test across exposures was computed under the robust repeated-measure model for a given outcome (for example, for Tp-e). Pairwise robust mean comparisons between one exposure and another within a group were not considered statistically significant if the corresponding overall test was not significant (generalization of the Fisher least significant difference criterion). Using this criterion for significance reduces the probability of false positive (type I) errors.
The rank-based Spearman correlation was computed to assess the association between changes in nicotine versus changes in heart rate.
RESULTS
Human Subjects
A total of 110 participants participated in the study, including 35 people in the tobacco cigarette cohort who currently and chronically (>1 yr) smoked tobacco cigarettes (of whom 12 also used electronic cigarettes), 34 people in the electronic cigarette cohort, who exclusively and currently (>1 yr) used electronic cigarettes with nicotine (12 of whom were former smokers, quitting at least 1 yr before the study), and 41 people in the nonuser cohort, who did not currently smoke tobacco or electronic cigarettes (3 of whom were former smokers, quitting at least 1 yr before the study). Baseline characteristics are displayed in Table 1. Importantly, plasma cotinine levels tended to be low and were not different between the tobacco cigarette cohort compared with the electronic cigarette cohort, consistent with a similarly low level of daily tobacco product use in both groups.
Table 1.
Study population characteristics
| Nonusers | EC Users | TC Smokers | P Value | Total | |
|---|---|---|---|---|---|
| Sample size, n | 41 | 34 | 35 | 110 | |
| Age, yr | 24.4 ± 4.4 | 23.2 ± 2.6 | 24.2 ± 3.0 | 0.2992 | 23.9 ± 3.5 |
| Sex (female/male), n | 21/20 | 17/17 | 21/14 | 0.6547 | 59/51 |
| BMI, kg/m2 | 22.3 ± 3.1 | 22.9 ± 2.6 | 21.8 ± 2.4 | 0.1270 | 22.3 ± 2.7 |
| College education, n | |||||
| Yes | 41 | 34 | 35 | 110 | |
| No | 0 | 0 | 0 | 1.000 | 0 |
| Cotinine, ng/mL | <2 | 60.5 [12.3–170.0] | 56.0 [10.5–138.0] | 0.6084* | X |
Values are means ± SD or medians [interquartile ranges, Q1–Q3]; n, number of participants. BMI, body mass index; EC, electronic cigarette; TC, tobacco cigarette. *E-cigarette users vs. smokers.
Baseline Comparison Study
None of the primary outcomes, Tp-e, Tp-e/QT, and Tp-e/QTc, were different among the three cohorts while supine at baseline (Table 2), even when adjusted for sex (data not shown). Abrupt standing did not prolong any of the primary outcomes in the electronic cigarette or tobacco cigarette cohorts compared with the nonuser cohort; in fact, these variables were shorter in these groups compared with the nonuser cohort (Table 2). The secondary outcomes of resting HR, QT, and QTc were not different among the cohorts either supine or with abrupt standing.
Table 2.
Mean values preexposure
| Nonusers | EC Users | TC Smokers | P Value | |
|---|---|---|---|---|
| Supine | ||||
| Tp-e, ms | 106 ± 27 | 100 ± 42 | 105 ± 25 | 0.07 |
| Tp-e/QT | 0.28 ± 0.05 | 0.27 ± 0.11 | 0.27 ± 0.05 | 0.09 |
| Tp-e/QTc | 0.26 ± 0.05 | 0.26 ± 0.13 | 0.26 ± 0.05 | 0.14 |
| QT, ms | 413 ± 31 | 420 ± 42 | 428 ± 44 | 0.41 |
| QTc, ms | 431 ± 26 | 436 ± 30 | 437 ± 37 | 0.62 |
| HR, beats/min | 64.9 ± 9.1 | 65.3 ± 12.2 | 62.3 ± 10.5 | 0.61 |
| Standing | ||||
| Tp-e, ms | 122 ± 38 | 103 ± 37 | 105 ± 23 | 0.027 |
| Tp-e/QT | 0.32 ± 0.06 | 0.28 ± 0.09 | 0.27 ± 0.05 | 0.0029 |
| Tp-e/QTc | 0.25 ± 0.06 | 0.22 ± 0.08 | 0.22 ± 0.04 | 0.0046 |
| QT, ms | 408 ± 38 | 399 ± 47 | 417 ± 54 | 0.23 |
| QTc, ms | 512 ± 45 | 506 ± 57 | 516 ± 53 | 0.66 |
| HR, beats/min | 97.5 ± 9.3 | 98.9 ± 10.9 | 94.6 ± 12.2 | 0.27 |
Values are means ± SD. EC, electronic cigarette; HR, heart rate; TC, tobacco cigarette; Tp-e, Tpeak-Tend.
Crossover Study: Supine
Tobacco cigarette cohort.
In people who chronically smoke tobacco cigarettes, we sought to determine if acutely switching from tobacco cigarettes to electronic cigarettes would result in less adverse changes in repolarization. To tease out the effect of nicotine versus non-nicotine constituents in smoke on ventricular repolarization, a research cigarette obtained from the FDA with negligible nicotine was also used during one session. Accordingly, the acute effect of four different exposures in random order on different days was measured: 1) commercial tobacco cigarette (participant’s own); 2) an electronic cigarette with nicotine, used according to our predefined topography protocol; 3) a research cigarette with negligible nicotine; and 4) an empty straw (control session), puffed according to the same predefined topography protocol as the electronic cigarette.
Increase in plasma nicotine following acute exposures.
The increase in plasma nicotine was similar after using the nicotine electronic cigarette compared with the commercial nicotine cigarette (7.63 ± 1.46 vs. 7.88 ± 1.21 ng/mL, respectively; P = 0.88). As expected, there was no change in plasma nicotine after using the research tobacco cigarette or the empty straw.
Changes in ECG indices of ventricular repolarization following acute exposures: supine.
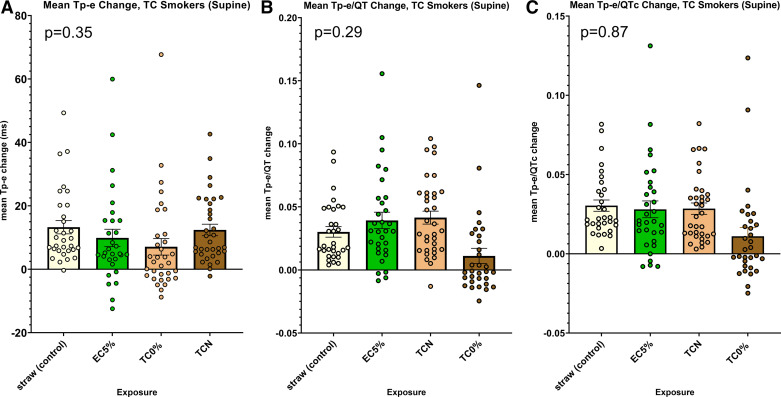
There were no significant differences in any of the primary indices of ventricular repolarization, Tp-e, Tp-e/QT, and Tp-e/QTc, among the four acute exposures when participants were supine (Fig. 1, A–C). Interestingly, the change in the secondary outcome, QT, was significantly less after either exposure with nicotine (electronic cigarette and commercial tobacco cigarette) compared with the no-nicotine research cigarette or the empty straw (Supplemental Fig. S1A; all Supplemental material is available at http://doi.org/10.6084/m9.figshare.22577797). This is likely explained by the increase in heart rate following each of these exposures compared with the no-nicotine research cigarette or the empty straw, since after correction for heart rate, there was no longer any difference in ventricular repolarization (QTc, Bazett’s) among the four exposures (Supplemental Fig. S1B).
Figure 1.
Changes in ECG indices of ventricular repolarization following acute exposures within the tobacco cigarette (TC) cohort: supine. A: no difference in the change in Tpeak-Tend (Tp-e) after any of the four exposures: straw control, electronic cigarette with 5% nicotine (EC5%), research tobacco cigarette with minimal nicotine (TC0%), or commercial tobacco cigarette with nicotine (TCN) (ANOVA, P = 0.35). B: no difference in the change in Tp-e/QT after any of the four exposures (as stated in A; ANOVA, P = 0.29). C: no difference in the change in Tp-e/QTc after any of the four exposures (as stated in A; ANOVA, P = 0.87).
Changes in ECG indices of ventricular repolarization following acute exposures: abrupt standing.
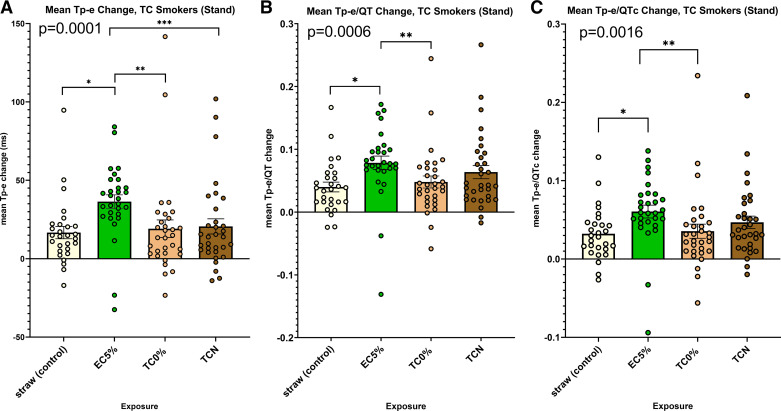
To unmask occult prolongation of the primary indices of ventricular repolarization induced by the various exposures, before and after each exposure, we had each subject stand up abruptly, a provocative maneuver that acutely increases cardiac sympathetic nerve activity (21, 22). When compared with the straw-control exposure, standing after acutely using the nicotine electronic cigarette, but not the commercial tobacco cigarette or the research tobacco cigarette, markedly and significantly prolonged all three primary indices of ventricular repolarization, including the Tp-e, Tp-e/QT, and Tp-e/QTc (Fig. 2, A–C). In fact, with the standing position after using the nicotine electronic cigarette, the prolongation of the Tp-e was significantly greater than after either of the other exposures: the commercial tobacco cigarette or the research cigarette.
Figure 2.
Changes in ECG indices of ventricular repolarization following acute exposures within the tobacco cigarette cohort: with the provocative maneuver, standing. A: change in Tpeak-Tend (Tp-e) was significantly longer after using the electronic cigarette with nicotine compared with each of the other four exposures (ANOVA, P = 0.0001). B: change in Tp-e/QT was significantly longer after using the electronic cigarette with nicotine compared with the straw control and the research tobacco cigarette with minimal nicotine (ANOVA, P = 0.0006). C: change in Tp-e/QTc was significantly longer after using the electronic cigarette with nicotine compared with the other straw control and the research tobacco cigarette with minimal nicotine (ANOVA, P = 0.0016); t test, *P < 0.01, **P < 0.006, ***P < 0.04. EC5%, electronic cigarette with 5% nicotine; TC, tobacco cigarette; TC0%, tobacco cigarette (research) with minimal nicotine; TCN, commercial tobacco cigarette with nicotine.
Subgroup analysis by sex.
The interaction term between exposure and sex for each of the primary outcomes of Tp-e, Tp-e/QT, and Tp-e/QTc was statistically significant or tended to be (shown in next section); therefore, the subgroup analysis was performed by sex.
Changes in ECG indices of ventricular repolarization following acute exposures: supine.
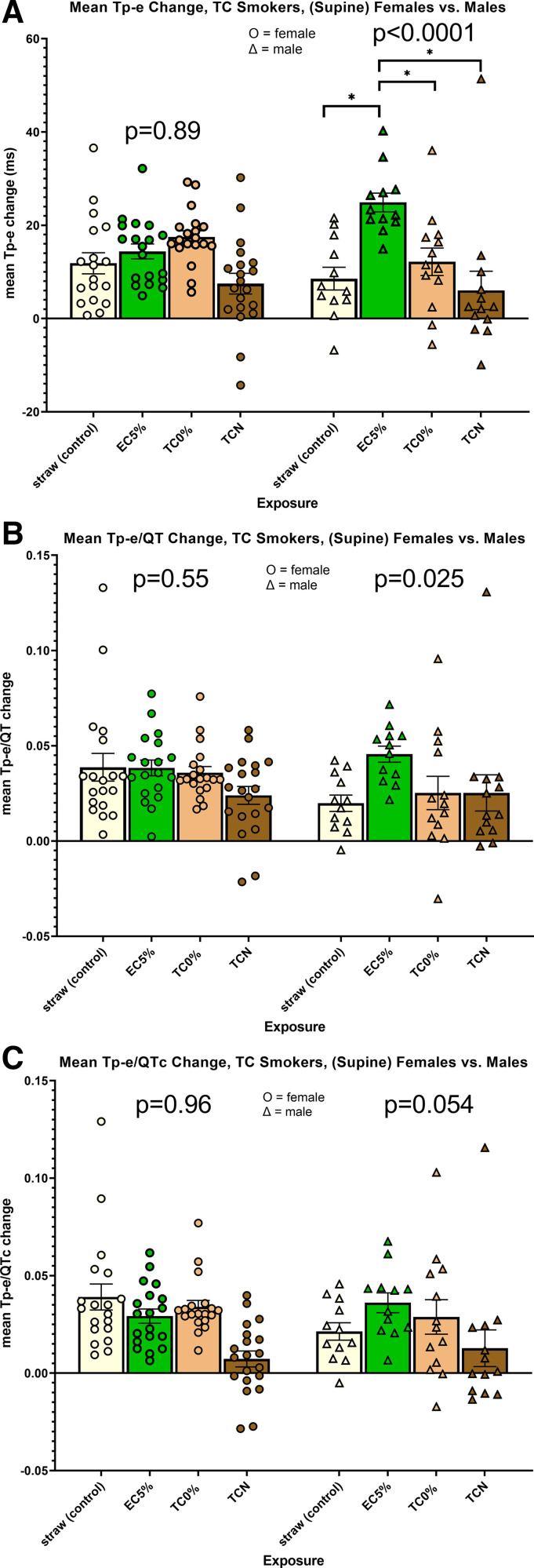
The interaction term between exposure and sex for the primary outcome of Tp-e was highly significant (P = 0.0008). When analyzed by sex, the overall P value among the four exposures in males (P < 0.00001) but not females (P = 0.89) was highly significant (Fig. 3A). In males only, prolongation of the Tp-e interval after using the electronic cigarette with nicotine was significantly greater compared with each of the other exposures. Similarly, the interaction term between exposure and sex for the primary outcome of Tp-e/QT was statistically significant (P = 0.039), and the overall P value among exposures in males was significant (P = 0.025), but not in females (P = 0.55; Fig. 3B). None of the pairwise individual comparisons among the four exposures reached statistical significance. Finally, the interaction term between exposure and sex for the primary outcome of Tp-e/QTc trended toward significance (P = 0.067), as did the overall P value among exposures in males (P = 0.054), but not females (P = 0.96; Fig. 3C). None of the pairwise individual comparisons among the four exposures reached statistical significance.
Figure 3.
Changes in ECG indices of ventricular repolarization following acute exposures within the tobacco cigarette cohort segregated by sex. A: interaction term between exposure and sex for the primary outcome of Tpeak-Tend (Tp-e) was highly significant (P = 0.0008). When analyzed by sex, the overall P value among the four exposures in males (P < 0.0001) but not females (P = 0.89) was highly significant. In males only, prolongation of the Tp-e interval after using the electronic cigarette with nicotine was significantly greater compared with all other exposures. B: interaction term between exposure and sex for the primary outcome of Tp-e/QT was statistically significant (P = 0.039), and the overall P value among exposures in males was significant (P = 0.025), but not in females (P = 0.55). None of the pairwise individual comparisons among exposures reached statistical significance. C: interaction term between exposure and sex for the primary outcome of Tp-e/QTc trended toward significance (P = 0.067), as did the overall P value among exposures in males (P = 0.054), but not females (P = 0.96); t test, *P < 0.0001. EC5%, electronic cigarette with 5% nicotine; TC, tobacco cigarette; TC0%, tobacco cigarette (research) with minimal nicotine; TCN, commercial tobacco cigarette with nicotine.
We considered that this sex difference reflected differences in nicotine exposure or metabolism between males and females during the electronic cigarette with nicotine exposure. However, nicotine levels, measured immediately after electronic cigarette exposure in all participants, did not differ between males and females (nicotine, 8.5 ± 1.5 vs. 8.4 ± 1.2 ng/mL, respectively; P = 0.75).
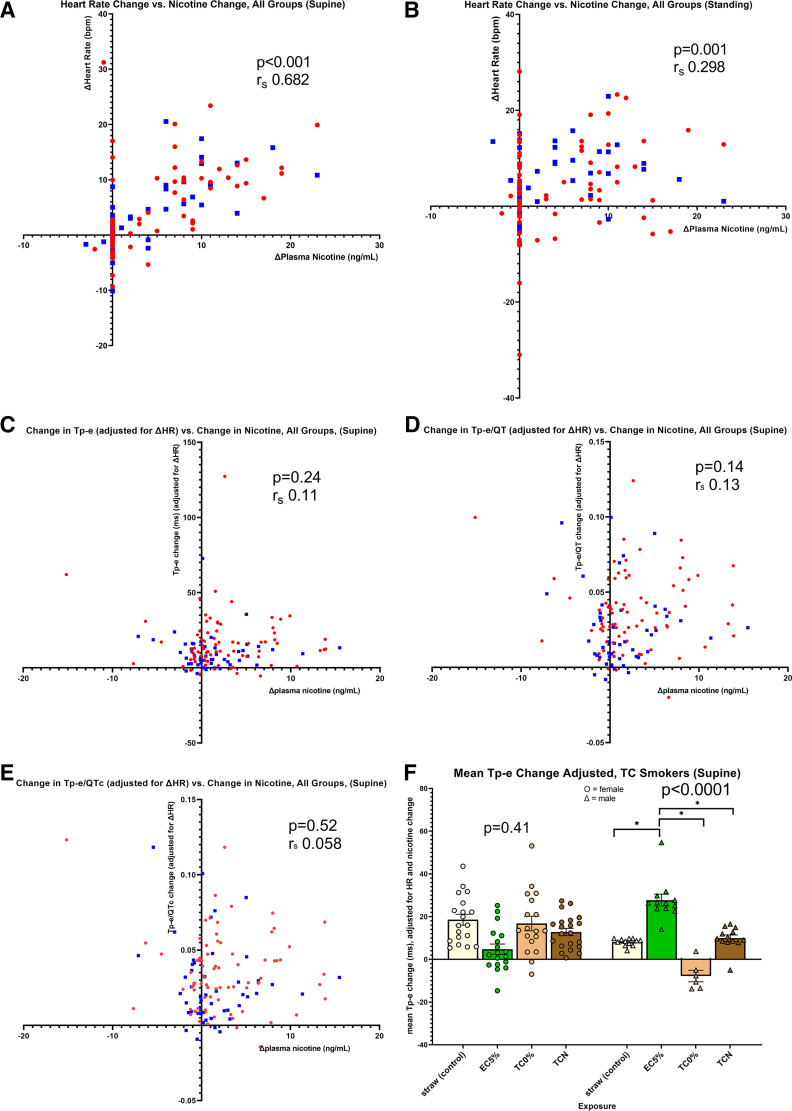
Relationship of each ECG indices of ventricular repolarization to changes in nicotine and heart rate.
There was an overall moderate, positive correlation between the change in nicotine and the change in heart rate that was also present in males and females when separately analyzed (Fig. 4A), consistent with the notion that nicotine drives the increase in heart rate observed during acute tobacco cigarette and electronic cigarette use. The correlation is weak but still detectable following standing, consistent with the notion that standing additionally increases cardiac sympathetic nerve activity and heart rate beyond the acute nicotine effect (Fig. 4B). Once again, this correlation was present in males and females when separately analyzed.
Figure 4.
Relationship between the change in nicotine and heart rate and between the change in each ECG index of ventricular repolarization to change in nicotine and heart rate. A: overall moderate, positive correlation between change in nicotine and change in heart rate (Spearman, rs, 0.682, P = 0.001), consistent with the notion that nicotine drives the increase in heart rate observed during acute tobacco cigarette and electronic cigarette use. This correlation was also present in males (blue squares; rs, 0.628, P < 0.001) and females (red circles; rs, 0.756, P < 0.001). B: correlation between the change in nicotine and change in heart rate is weak but still detectable following standing (Spearman, rs, 0.298, P < 0.001), consistent with the notion that standing additionally increases cardiac sympathetic nerve activity and heart rate beyond the acute nicotine effect. This correlation was present in males (rs, 0.260, P = 0.03) and females (rs, 0.378, P = 0.007) when separately analyzed. C: change in Tpeak-Tend (Tp-e) adjusted for heart rate was not correlated with the change in nicotine. This was true when the whole group was analyzed (rs, 0.11, P = 0.24) or separately analyzed by sex, (male: rs, 0.117, P = 0.31; female: rs, 0.058, P = 0.69). D: change in Tp-e/QT adjusted for heart rate was not correlated with the change in nicotine. This was true when the whole group was analyzed (rs, 0.130, P = 0.14) or separately analyzed by sex, (male: rs, 0.159, P = 0.17; female: rs, 0.044, P = 0.76). E: change in Tp-e/QTc adjusted for heart rate was not correlated with the change in nicotine. This was true when the whole group was analyzed (rs, 0.058, P = 0.52) or separately analyzed by sex, (male: rs, 0.0.085, P = 0.46; female: rs, 0.025, P = 0.856). F: mean Tp-e change adjusted for change in heart rate and nicotine. After the adjustment for nicotine and heart rate, Tp-e interval remained persistently prolonged in males (ANOVA, P < 0.0001) but not in females (P = 0.41), after using the electronic cigarette with nicotine. Change in Tp-e was significantly longer after using the electronic cigarette with nicotine compared with each of the other three exposures. Male, blue squares; female, red circles (t test, *P < 0.0001). EC5%, electronic cigarette with 5% nicotine; TC0%, tobacco cigarette (research) with minimal nicotine; TCN, commercial tobacco cigarette with nicotine.
However, the changes in the primary indices of ventricular repolarization while supine or standing was not correlated, or only weakly correlated, with change in heart rate or nicotine (Fig. 4, C–E). This was true when the group was analyzed as one, or separately analyzed according to sex.
Importantly, even after adjusting for changes in nicotine and heart rate, the Tp-e interval remained persistently prolonged in males, but not in females, after using the nicotine electronic cigarette (Fig. 4F). This result is consistent with the notion that this acute prolongation of the Tp-e interval is attributable to neither the nicotine delivered by the electronic cigarette nor the change in HR rate which accompanied acute electronic cigarette use and implicates the non-nicotine constituents in the emissions from electronic cigarettes.
Electronic cigarette cohort.
In persons who chronically use electronic cigarettes, which are believed to be harmless by many, we sought to determine if acutely using an electronic cigarette was associated with detectable adverse changes in ventricular repolarization and, if so, whether this effect was attributable to the nicotine versus non-nicotine constituents in electronic cigarette emissions. Participants underwent three exposures using the same puffing topography in random order on different days: 1) an electronic cigarette with nicotine, 2) an electronic cigarette without nicotine, and 3) an empty straw (control).
Increase in plasma nicotine following acute exposure.
Using the electronic cigarette with nicotine increased the plasma nicotine by 10.0 ± 1.4 ng/mL. As expected, there was no increase in plasma nicotine following the other two exposures.
Changes in ECG indices of ventricular repolarization following acute exposures: supine.
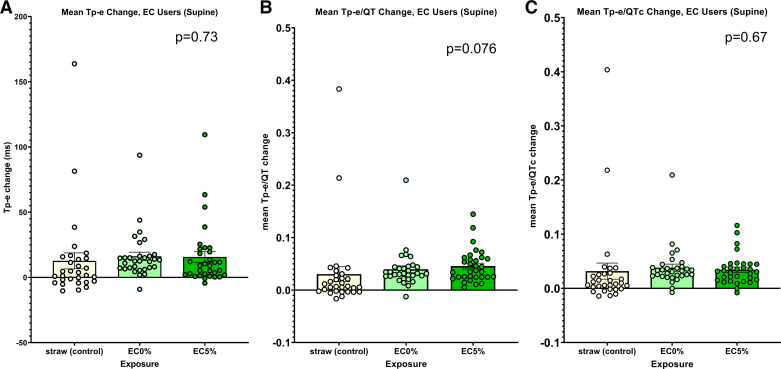
There were no significant differences in any of the primary indices of ventricular repolarization, Tp-e, Tp-e/QT, and Tp-e/QTc, among the three acute exposures when participants were supine (Fig. 5, A–C). Interestingly, the change in the secondary outcome, QT, was less after the nicotine electronic cigarette compared with the no-nicotine electronic cigarette or the empty straw (Supplemental Fig. S2A). As was the case in tobacco cigarette cohort, this is likely explained by the increase in heart rate following the nicotine exposure, since after correction for heart rate (QTc, Bazett), there was no longer any difference in ventricular repolarization (Supplemental Fig. S2B) among the three exposures.
Figure 5.
Changes in ECG indices of ventricular repolarization following acute exposures within the electronic cigarette cohort: supine. A. no difference in the change in Tpeak-Tend (Tp-e) after any of the three exposures: straw control, electronic cigarette with 5% nicotine, or electronic cigarette with 0% nicotine. B: no difference in the change in Tp-e/QT after any of the three exposures (as stated in A). C: no difference in the change in Tp-e/QTc after any of the three exposures (as stated in A). EC, electronic cigarette; EC5%, electronic cigarette with 5% nicotine.
Changes in ECG indices of ventricular repolarization following acute exposures: abrupt standing.
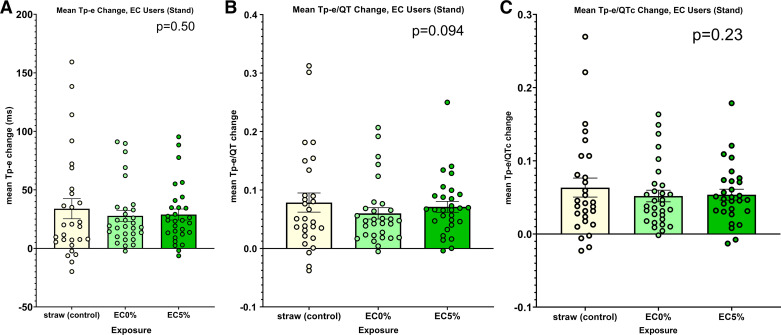
The effect of abrupt standing, a provocative maneuver that acutely increases cardiac sympathetic nerve activity, on the primary indices of ventricular before and after each exposure, was next measured. Once again, there were no significant differences in any of the primary indices of ventricular repolarization, Tp-e, Tp-e/QT, and Tp-e/QTc, among the three acute exposures induced by this provocative maneuver (Fig. 6, A–C).
Figure 6.
Changes in ECG indices of ventricular repolarization following acute exposures within the electronic cigarette cohort: standing. A: there was no difference in the change in Tpeak-Tend (Tp-e) after any of the three exposures: straw control, electronic cigarette with 5% nicotine, or electronic cigarette with 0% nicotine. B: no difference in the change in Tp-e/QT after any of the three exposures (as stated in A). C: no difference in the change in Tp-e/QTc after any of the three exposures (as stated in A). EC, electronic cigarette; EC5%, electronic cigarette with 5% nicotine.
Subgroup analysis by sex.
The interaction term between exposure and sex for each of the primary outcomes of Tp-e (P = 0.074), Tp-e/QT (P = 0.13), and Tp-e/QTc (P = 0.28) was not statistically significant therefore the subgroup analysis was not performed by sex.
DISCUSSION
In 2020, ∼34.1 million American adults were current combusted tobacco cigarette smokers (26), and it is predicted that half, which is 17 million Americans who smoke, will die from tobacco-related diseases, most frequently cardiovascular disease (1). It is widely recognized that combusted tobacco cigarettes are the most prevalent, reversible risk factor for cardiovascular death, including sudden cardiovascular death, in the United States. Also, in 2020, ∼5.66 million adults reported current use of electronic cigarettes, the vast majority of whom were either current or former tobacco cigarette smokers (26). Importantly, almost 70% of current smokers and 80% of former smokers reported using electronic cigarettes for smoking cessation, as part of a harm reduction approach (26). Nonetheless, the cardiovascular risks of electronic cigarette use relative to combusted tobacco cigarette smoking remain uncertain. In our prior study, we found that acutely using an electronic cigarette increased the Tp-e-to-QT ratio in people who chronically used electronic cigarettes, but the effects of acute electronic cigarette use were not tested in people who smoke tobacco cigarettes, nor were the effects on ventricular repolarization directly compared after acute tobacco and electronic cigarette use (20). In summary, the prevalence of current tobacco and/or electronic cigarette use in the United States today reveals the salience of this research question.
The major new finding in this study is that in people who chronically smoke tobacco cigarettes, using an electronic cigarette with nicotine compared with smoking a commercial tobacco cigarette significantly and acutely prolongs ventricular repolarization, an adverse effect potentially associated with increased sudden death risk. Furthermore, in our subgroup analysis by sex, this adverse effect on repolarization is found only in male smokers, not female smokers. These findings have implications for the use of electronic cigarettes as part of a harm reduction strategy.
Strengths of the current study include 1) analysis from all 12 leads of the electrocardiogram (24); 2) the implementation of a provocative test to acutely increase sympathetic nerve activity to unmask abnormal ventricular repolarization (21, 22); 3) the inclusion of exposures to tobacco products with and without nicotine, most notably the research tobacco cigarette, which emits negligible nicotine; and 4) the study design in which the effects of acute tobacco and electronic cigarette use on ventricular repolarization are directly compared in individuals who smoke tobacco cigarettes (a “switch study”). This approach extends and expands our previous retrospective report that included analysis of only 2 ECG leads, no provocative test, no non-nicotine combusted tobacco cigarette, and no direct comparison within the same individual of the effects of tobacco and electronic cigarettes (20). Similar to our prior study (20), in the current study, there were no differences in the three cohorts at baseline, in the absence of acute exposure. However, the results following acute exposure to tobacco or electronic cigarettes in the current study differed in several ways from our prior, retrospective report.
In our retrospective study of tobacco cigarette smokers, we reported that smoking a tobacco cigarette acutely increased all three indices of ventricular repolarization primary outcomes (Tp-e, Tp-e/QT, and Tp-e/QTc; 20), but in the current study, acute tobacco smoking did not alter ventricular repolarization. We considered several possible explanations for this difference, first considering differences in study populations in the two studies. The number of participants who smoked tobacco cigarettes was similar in both the retrospective and prospective studies, but in the retrospective study, the level of smoking in the tobacco cigarette group was heavier, as estimated by the mean plasma cotinine level, which was over twice that of the prospective study (20). It has been proposed that the frequent repetition of tobacco product use with its electrophysiological effects, may lead to long-term remodeling of cardiac myocytes, characterized by increased vulnerability to abnormal repolarization (27–29). It follows that participants with higher levels of smoking would be more likely to undergo electrical remodeling and thereby be more likely to experience adverse electrophysiological sequelae.
We considered additional explanations for differing results in our retrospective and prospective studies, such as the tobacco cigarette smoking protocol and the approach to ventricular repolarization measurement and analysis. The acute tobacco cigarette exposure protocol and the acute increase in plasma nicotine with smoking were not different between the retrospective and prospective studies (20). The technique for recording and analyzing the ECG recordings was the same in both studies, except that in the current, prospective study all 12 ECG leads were recorded simultaneously, compared with only 2 ECG leads previously (20). This difference certainly raises the possibility that important data points were missed with the prior approach, and when all leads were recorded, no true differences were present. This seems unlikely, however, since in prior studies of tobacco cigarette smokers (12, 13, 15–17, 30), many, but not all, have reported abnormal ventricular repolarization at baseline and/or following acute tobacco cigarette smoking, often recording all 12 ECG leads (12, 15, 17, 31). Importantly, most studies purposefully enrolled people who were heavy tobacco cigarette smokers (variously defined; 12, 13, 15–17) and when reported, the degree of prolongation in ventricular repolarization was directly related to smoking burden (17). Although the explanation for the differing responses to acute tobacco cigarette smoking in our retrospective and prospective studies remains uncertain, we speculate it may be related to differing levels of baseline smoking burden between the study populations.
In our retrospective study, we found a modest effect of acute electronic cigarette use on only one of the three indices of ventricular repolarization (Tp-e/QT) in the chronic electronic cigarette users (20), and this effect was not reproduced in the current study, in which acute electronic cigarette use did not alter repolarization. Unlike the retrospective study, in the prospective study, tobacco cigarette smokers also used a nicotine electronic cigarette to compare the effects of switching from a tobacco to an electronic cigarette as part of a harm reduction strategy. In tobacco cigarette smokers, using an electronic cigarette acutely produced a marked and significant prolongation in ventricular repolarization that was unmasked with our provocative maneuver. Furthermore, the prolongation in ventricular repolarization seemed to be independent of the nicotine, implicating the non-nicotine constituents in electronic cigarette emissions.
The exact mechanism underlying this robust and adverse effect of electronic cigarettes on repolarization in people who smoke tobacco cigarettes, which was not seen after acute tobacco cigarette smoking, is unknown. Furthermore, it appears to be confined to the male tobacco cigarette smokers. The increase in plasma nicotine was similar after using the tobacco cigarette and the electronic cigarette, and similar in male and female tobacco cigarette smokers, supporting the notion that the increase in nicotine cannot be the sole explanation. It is tempting to assume that a toxicant, or specific combination of toxicants present in electronic cigarette emissions is responsible for this effect. In addition to nicotine, e-liquid contains solvents, typically vegetable glycerin (VG) and/or propylene glycol (PG), and flavorings. When heated, electronic cigarette liquids generate aerosols that contain aldehydes and particulate matter at levels similar to, or that exceed, tobacco cigarette smoke, and these toxicants have been found to be increased in the presence of menthol flavoring (27, 32). In fact, 92% of the cardiovascular toxicity from smoking has been attributed to aldehyde, acrolein, formaldehyde, and acetaldehydes, all of which are degradation products produced by VG and PG heating (33). Carll et al. (27) have recently reported that exposure to aerosols generated from heating VG and/or PG prolonged ventricular repolarization in mice, and these effects were greater in male compared with female mice. Furthermore, aerosols generated from menthol flavoring or PG also increased ventricular ectopy frequency. Again, this proarrhythmic effect was significantly more pronounced in male compared with female mice (27). PG and VG, when heated, rapidly degrade into aldehydes, especially acrolein. Menthol-flavored e-liquid generates formaldehyde and acrolein at higher levels than tobacco-flavored electronic cigarettes and comparable levels to combusted tobacco smoke (32, 34). These investigators propose, and their experiments support, the notion that solvents have their own arrhythmogenic effect that is potentiated by menthol and other flavorings (27). Conversely, these investigators found that some electrophysiologic effects of flavorings and solvents were opposed by nicotine. They conclude that the individual effects of nicotine, solvents, flavorings, and their degradation products in electronic cigarette aerosols “are particularly challenging to disentangle” mandating further studies (27, 35). At this point, it seems safe only to conclude that the culprit underlying the electronic cigarette effects on ventricular repolarization in our study is not nicotine. Nonetheless, the adverse effect of electronic cigarette use on ventricular repolarization in smokers is concerning, with implications for public health perceptions and messaging regarding the role of electronic cigarettes as part of a harm reduction strategy.
The observation that the adverse effect on repolarization of acute electronic cigarette use was significant only in males, not female smokers is intriguing. However, this was a subgroup analysis and will have to be prospectively confirmed. Nonetheless, it is widely recognized that there is a sex difference in ventricular repolarization; repolarization as measured by the QT interval is longer in females compared with males (36), and preclinical studies have also revealed sex differences (27). Importantly, smoking confers a sevenfold greater risk of suffering an acute cardiac event, including sudden cardiac death in premenopausal females compared with premenopausal females who do not smoke, a much greater adverse effect than that found in similarly aged males who smoke (37). Our findings suggest that females who switch from lethal tobacco cigarettes to electronic cigarettes will likely benefit, since the acute adverse effect of electronic cigarettes on repolarization were apparent only in males in this subgroup analysis.
Limitations
We recognize several limitations to our study. First, we examined the acute effects of using an electronic cigarette compared with a comparable “dose” of a tobacco cigarette (as measured by acute increase in plasma nicotine levels) on ventricular repolarization in people who smoke and found a marked and significant acute increase in ventricular repolarization as measured by the Tp-e interval. However, we did not determine if this acute, adverse effect would recur indefinitely after each electronic cigarette use or after smoking cessation. This is an important question, since the majority of adults who use electronic cigarettes in the United States are former smokers (26). Only 12 people who chronically use electronic cigarettes in our study were former smokers, an insufficient number to address this question. Future studies in a larger group of electronic cigarette users who are former smokers would be of interest to determine if, and for how long, the adverse effects of electronic cigarette use on ventricular repolarization persist after complete smoking cessation. Until this important question is answered, it is logical to conclude that the shortest interval that an individual uses electronic cigarettes to ensure complete smoking cessation would be the safest approach. In addition, we only used one type of electronic cigarette and one flavor in these studies, and yet there are many different generations of devices and literally thousands of different flavors, each derived from different chemical(s). We chose the JUUL brand filled with mint-flavored liquid, because at the time of study development, it was the most popular brand with the largest market share (38). Although we acknowledge that it would be of interest to know if this adverse effect of one brand and flavor of electronic cigarette is generalizable to most electronic cigarette devices and flavors, testing many devices and flavors was beyond the scope of the current study. Finally, although the major focus of the current study was on the relative effects of switching from tobacco to electronic cigarettes on ventricular repolarization as a form of harm reduction, we recognize that there are many other risk markers and pathologies in which electronic cigarettes have been shown to be less harmful than tobacco cigarettes, including flow-mediated dilatation (39), cellular inflammation (40, 41), and oxidative stress (42), all risk factors for atherosclerotic coronary disease. Despite our findings, we believe electronic cigarettes still have an important role in harm reduction. Nonetheless, this role is diminished by our results, and results from the current study will help inform people who smoke in their decision-making in choosing whether, and how long, to use an electronic cigarette for smoking cessation.
In summary, chronic tobacco cigarette smoking is the most prevalent, modifiable risk factor for cardiovascular death, including sudden cardiac death. If used for smoking cessation, electronic cigarettes, often perceived to be harmless, should only be used in the short term since they too carry their own risks. This risk appears to be greatest in males compared with females who smoke.
DATA AVAILABILITY
Data will be made available upon reasonable request.
SUPPLEMENTAL DATA
Supplemental Figs. S1 and S2: http://doi.org/10.6084/m9.figshare.22577797.
GRANTS
This work was supported by Tobacco Related Diseases Research Program Grant T29IP0319 and by the National Institutes of Health National Center for Advancing Translational Science, Univ. of California, Los Angeles, Clinical and Translational Science Institute Grant L1TR001881.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.G. and H.R.M. conceived and designed research; I.R., K.L., and R.N. performed experiments; I.R. and J.G. analyzed data; H.R.M. interpreted results of experiments; I.R. and H.R.M. drafted manuscript; K.L., R.N., J.G., and H.R.M. edited and revised manuscript; I.R., K.L., R.N., J.G., and H.R.M. approved final version of manuscript.
ACKNOWLEDGMENTS
The investigators are grateful to the staff of the Univ. of California, Los Angeles, Clinical and Translational Research Center for expertise and professionalism in helping conduct these studies, even during the pandemic, and to the participants who volunteered for our study.
REFERENCES
- 1. Jha P, Ramasundarahettige C, Landsman V, Rostron B, Thun M, Anderson RN, McAfee T, Peto R. 21st-century hazards of smoking and benefits of cessation in the United States. N Engl J Med 368: 341–350, 2013. doi: 10.1056/NEJMsa1211128. [DOI] [PubMed] [Google Scholar]
- 2. Shahab L, Goniewicz ML, Blount BC, Brown J, McNeill A, Alwis KU, Feng J, Wang L, West R. Nicotine, carcinogen, and toxin exposure in long-term E-cigarette and nicotine replacement therapy users: a cross-sectional study. Ann Intern Med 166: 390–400, 2017. doi: 10.7326/M16-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Goniewicz ML, Gawron M, Smith DM, Peng M, Jacob P 3rd, Benowitz NL. Exposure to nicotine and selected toxicants in cigarette smokers who switched to electronic cigarettes: a longitudinal within-subjects observational study. Nicotine Tob Res 19: 160–167, 2017. doi: 10.1093/ntr/ntw160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Green SH, Bayer R, Fairchild AL. Evidence, policy, and E-cigarettes–will England reframe the debate? N Engl J Med 374: 1301–1303, 2016. doi: 10.1056/NEJMp1601154. [DOI] [PubMed] [Google Scholar]
- 5. Buchanan ND, Grimmer JA, Tanwar V, Schwieterman N, Mohler PJ, Wold LE. Cardiovascular risk of electronic cigarettes: a review of preclinical and clinical studies. Cardiovasc Res 116: 40–50, 2020. doi: 10.1093/cvr/cvz256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sandhu RK, Jimenez MC, Chiuve SE, Fitzgerald KC, Kenfield SA, Tedrow UB, Albert CM. Smoking, smoking cessation, and risk of sudden cardiac death in women. Circ Arrhythm Electrophysiol 5: 1091–1097, 2012. doi: 10.1161/CIRCEP.112.975219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Middlekauff HR, Park J, Moheimani RS. Adverse effects of cigarette and noncigarette smoke exposure on the autonomic nervous system: mechanisms and implications for cardiovascular risk. J Am Coll Cardiol 64: 1740–1750, 2014. doi: 10.1016/j.jacc.2014.06.1201. [DOI] [PubMed] [Google Scholar]
- 8. Panikkath R, Reinier K, Uy-Evanado A, Teodorescu C, Hattenhauer J, Mariani R, Gunson K, Jui J, Chugh SS. Prolonged Tpeak-to-tend interval on the resting ECG is associated with increased risk of sudden cardiac death. Circ Arrhythm Electrophysiol 4: 441–447, 2011. doi: 10.1161/CIRCEP.110.960658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bébarová M, Horáková Z, Kula R. Addictive drugs, arrhythmias, and cardiac inward rectifiers. Europace 19: 346–355, 2017. doi: 10.1093/europace/euw071. [DOI] [PubMed] [Google Scholar]
- 10. Wang H, Shi H, Wang Z. Nicotine depresses the functions of multiple cardiac potassium channels. Life Sci 65: PL143–PL149, 1999. doi: 10.1016/s0024-3205(99)00370-7. [DOI] [PubMed] [Google Scholar]
- 11. Wang H, Yang B, Zhang L, Xu D, Wang Z. Direct block of inward rectifier potassium channels by nicotine. Toxicol Appl Pharmacol 164: 97–101, 2000. doi: 10.1006/taap.2000.8896. [DOI] [PubMed] [Google Scholar]
- 12. Akbarzadeh MA, Yazdani S, Ghaidari ME, Asadpour-Piranfar M, Bahrololoumi-Bafruee N, Golabchi A, Azhari A. Acute effects of smoking on QT dispersion in healthy males. ARYA Atheroscler 10: 89–93, 2014. [PMC free article] [PubMed] [Google Scholar]
- 13. Andrássy G, Szabo A, Dunai A, Simon E, Nagy T, Trummer Z, Tahy A, Varro A. Acute effects of cigarette smoking on the QT interval in healthy smokers. Am J Cardiol 92: 489–492, 2003. doi: 10.1016/s0002-9149(03)00678-7. [DOI] [PubMed] [Google Scholar]
- 14. D'Alessandro A, Boeckelmann I, Hammwhöner M, Goette A. Nicotine, cigarette smoking and cardiac arrhythmia: an overview. Eur J Prev Cardiol 19: 297–305, 2012. doi: 10.1177/1741826711411738. [DOI] [PubMed] [Google Scholar]
- 15. Ileri M, Yetkin E, Tandoğan I, Hisar I, Atak R, Senen K, Cehreli S, Demirkan D. Effect of habitual smoking on QT interval duration and dispersion. Am J Cardiol 88: 322–325, 2001. doi: 10.1016/s0002-9149(01)01653-8. [DOI] [PubMed] [Google Scholar]
- 16. İlgenli TF, Tokatlı A, Akpınar O, Kılıçaslan F. The effects of cigarette smoking on the Tp-e interval, Tp-e/QT ratio and Tp-e/QTc ratio. Adv Clin Exp Med 24: 973–978, 2015. doi: 10.17219/acem/28114. [DOI] [PubMed] [Google Scholar]
- 17. Taşolar H, Ballı M, Bayramoğlu A, Otlu YÖ, Cetin M, Altun B, Cakıcı M. Effect of smoking on Tp-e interval, Tp-e/QT and Tp-e/QTc ratios as indices of ventricular arrhythmogenesis. Heart Lung Circ 23: 827–832, 2014. doi: 10.1016/j.hlc.2014.03.016. [DOI] [PubMed] [Google Scholar]
- 18. Antzelevitch C. Heterogeneity and cardiac arrhythmias: an overview. Heart Rhythm 4: 964–972, 2007. doi: 10.1016/j.hrthm.2007.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Antzelevitch C. T peak-Tend interval as an index of transmural dispersion of repolarization. Eur J Clin Invest 31: 555–557, 2001. doi: 10.1046/j.1365-2362.2001.00849.x. [DOI] [PubMed] [Google Scholar]
- 20. Ip M, Diamantakos E, Haptonstall K, Choroomi Y, Moheimani RS, Nguyen KH, Tran E, Gornbein J, Middlekauff HR. Tobacco and electronic cigarettes adversely impact ECG indexes of ventricular repolarization: implication for sudden death risk. Am J Physiol Heart Circ Physiol 318: H1176–H1184, 2020. doi: 10.1152/ajpheart.00738.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Adler A, van der Werf C, Postema PG, Rosso R, Bhuiyan ZA, Kalman JM, Vohra JK, Guevara-Valdivia ME, Marquez MF, Halkin A, Benhorin J, Antzelevitch C, Wilde AA, Viskin S. The phenomenon of “QT stunning”: the abnormal QT prolongation provoked by standing persists even as the heart rate returns to normal in patients with long QT syndrome. Heart Rhythm 9: 901–908, 2012. doi: 10.1016/j.hrthm.2012.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Viskin S, Postema PG, Bhuiyan ZA, Rosso R, Kalman JM, Vohra JK, Guevara-Valdivia ME, Marquez MF, Kogan E, Belhassen B, Glikson M, Strasberg B, Antzelevitch C, Wilde AA. The response of the QT interval to the brief tachycardia provoked by standing: a bedside test for diagnosing long QT syndrome. J Am Coll Cardiol 55: 1955–1961, 2010. doi: 10.1016/j.jacc.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wong JA, Gula LJ, Klein GJ, Yee R, Skanes AC, Krahn AD. Utility of treadmill testing in identification and genotype prediction in long-QT syndrome. Circ Arrhythm Electrophysiol 3: 120–125, 2010. doi: 10.1161/CIRCEP.109.907865. [DOI] [PubMed] [Google Scholar]
- 24. Ruedisueli I, Ma J, Nguyen R, Lakhani K, Gornbein J, Middlekauff HR. Optimizing ECG lead selection for detection of prolongation of ventricular repolarization as measured by the Tpeak-end interval. Ann Noninvasive Electrocardiol 27: e12958, 2022. doi: 10.1111/anec.12958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rautaharju PM, Surawicz B, Gettes LS, Bailey JJ, Childers R, Deal BJ, Gorgels A, Hancock EW, Josephson M, Kligfield P, Kors JA, Macfarlane P, Mason JW, Mirvis DM, Okin P, Pahlm O, van Herpen G, Wagner GS, Wellens H; American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; American College of Cardiology Foundation; Heart Rhythm Society. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part IV: the ST segment, T and U waves, and the QT interval: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society. Endorsed by the International Society for Computerized Electrocardiology. J Am Coll Cardiol 53: 982–991, 2009. doi: 10.1016/j.jacc.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 26. Mayer M, Reyes-Guzman C, Grana R, Choi K, Freedman ND. Demographic characteristics, cigarette smoking, and e-cigarette use among US adults. JAMA Netw Open 3: e2020694, 2020. doi: 10.1001/jamanetworkopen.2020.20694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Carll AP, Arab C, Salatini R, Miles MD, Nystoriak MA, Fulghum KL, Riggs DW, Shirk GA, Theis WS, Talebi N, Bhatnagar A, Conklin DJ. E-cigarettes and their lone constituents induce cardiac arrhythmia and conduction defects in mice. Nat Commun 13: 6088, 2022. doi: 10.1038/s41467-022-33203-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cutler MJ, Jeyaraj D, Rosenbaum DS. Cardiac electrical remodeling in health and disease. Trends Pharmacol Sci 32: 174–180, 2011. doi: 10.1016/j.tips.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tsuji Y, Zicha S, Qi XY, Kodama I, Nattel S. Potassium channel subunit remodeling in rabbits exposed to long-term bradycardia or tachycardia: discrete arrhythmogenic consequences related to differential delayed-rectifier changes. Circulation 113: 345–355, 2006. doi: 10.1161/CIRCULATIONAHA.105.552968. [DOI] [PubMed] [Google Scholar]
- 30. Dilaveris P, Pantazis A, Gialafos E, Triposkiadis F, Gialafos J. The effects of cigarette smoking on the heterogeneity of ventricular repolarization. Am Heart J 142: 833–837, 2001. doi: 10.1067/mhj.2001.118737. [DOI] [PubMed] [Google Scholar]
- 31. Singh K. Effect of smoking on QT interval, QT dispersion and rate pressure product. Indian Heart J 56: 140–142, 2004. [PubMed] [Google Scholar]
- 32. Conklin DJ, Ogunwale MA, Chen Y, Theis WS, Nantz MH, Fu XA, Chen LC, Riggs DW, Lorkiewicz P, Bhatnagar A, Srivastava S. Electronic cigarette-generated aldehydes: the contribution of e-liquid components to their formation and the use of urinary aldehyde metabolites as biomarkers of exposure. Aerosol Sci Technol 52: 1219–1232, 2018. doi: 10.1080/02786826.2018.1500013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Haussmann HJ. Use of hazard indices for a theoretical evaluation of cigarette smoke composition. Chem Res Toxicol 25: 794–810, 2012. doi: 10.1021/tx200536w. [DOI] [PubMed] [Google Scholar]
- 34. Ogunwale MA, Li M, Ramakrishnam Raju MV, Chen Y, Nantz MH, Conklin DJ, Fu XA. Aldehyde detection in electronic cigarette aerosols. ACS Omega 2: 1207–1214, 2017. doi: 10.1021/acsomega.6b00489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Spahn JE, Stavchansky SA, Cui Z. Critical research gaps in electronic cigarette devices and nicotine aerosols. Int J Pharm 593: 120144, 2021. doi: 10.1016/j.ijpharm.2020.120144. [DOI] [PubMed] [Google Scholar]
- 36. Al-Khatib SM, LaPointe NM, Kramer JM, Califf RM. What clinicians should know about the QT interval. JAMA 289: 2120–2127, 2003. [Erratum in JAMA 290: 1318, 2003] doi: 10.1001/jama.289.16.2120. [DOI] [PubMed] [Google Scholar]
- 37. Njølstad I, Arnesen E, Lund-Larsen PG. Smoking, serum lipids, blood pressure, and sex differences in myocardial infarction. A 12-year follow-up of the Finnmark Study. Circulation 93: 450–456, 1996. doi: 10.1161/01.cir.93.3.450. [DOI] [PubMed] [Google Scholar]
- 38. Huang J, Duan Z, Kwok J, Binns S, Vera LE, Kim Y, Szczypka G, Emery SL. Vaping versus JUULing: how the extraordinary growth and marketing of JUUL transformed the US retail e-cigarette market. Tob Control 28: 146–151, 2019. doi: 10.1136/tobaccocontrol-2018-054382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Haptonstall KP, Choroomi Y, Moheimani R, Nguyen K, Tran E, Lakhani K, Ruedisueli I, Gornbein J, Middlekauff HR. Differential effects of tobacco cigarettes and electronic cigarettes on endothelial function in healthy young people. Am J Physiol Heart Circ Physiol 319: H547–H556, 2020. doi: 10.1152/ajpheart.00307.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kelesidis T, Zhang Y, Tran E, Sosa G, Middlekauff HR. Increased expression of proatherogenic proteins in immune cell subtypes in tobacco cigarette smokers but not in electronic cigarette vapers. Can J Cardiol 37: 1175–1180, 2021. doi: 10.1016/j.cjca.2021.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kelesidis T, Zhang Y, Tran E, Sosa G, Middlekauff HR. Expression of key inflammatory proteins is increased in immune cells from tobacco cigarette smokers but not electronic cigarette vapers: implications for atherosclerosis. J Am Heart Assoc 10: e019324, 2021. doi: 10.1161/JAHA.120.019324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gupta R, Lin Y, Luna K, Logue A, Yoon AJ, Haptonstall KP, Moheimani R, Choroomi Y, Nguyen K, Tran E, Zhu Y, Faull KF, Kelesidis T, Gornbein J, Middlekauff HR, Araujo JA. Electronic and tobacco cigarettes alter polyunsaturated fatty acids and oxidative biomarkers. Circ Res 129: 514–526, 2021. doi: 10.1161/CIRCRESAHA.120.317828. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figs. S1 and S2: http://doi.org/10.6084/m9.figshare.22577797.
Data Availability Statement
Data will be made available upon reasonable request.