Abstract
Background
There is a paucity of data on heart transplantation (HT) using COVID-19 donors.
Objectives
This study investigated COVID-19 donor use, donor and recipient characteristics, and early post-HT outcomes.
Methods
Between May 2020 and June 2022, study investigators identified 27,862 donors in the United Network for Organ Sharing, with 60,699 COVID-19 nucleic acid amplification testing (NAT) performed before procurement and with available organ disposition. Donors were considered “COVID-19 donors” if they were NAT positive at any time during terminal hospitalization. These donors were subclassified as “active COVID-19” (aCOV) donors if they were NAT positive within 2 days of organ procurement, or “recently resolved COVID-19” (rrCOV) donors if they were NAT positive initially but became NAT negative before procurement. Donors with NAT-positive status >2 days before procurement were considered aCOV unless there was evidence of a subsequent NAT-negative result ≥48 hours after the last NAT-positive result. HT outcomes were compared.
Results
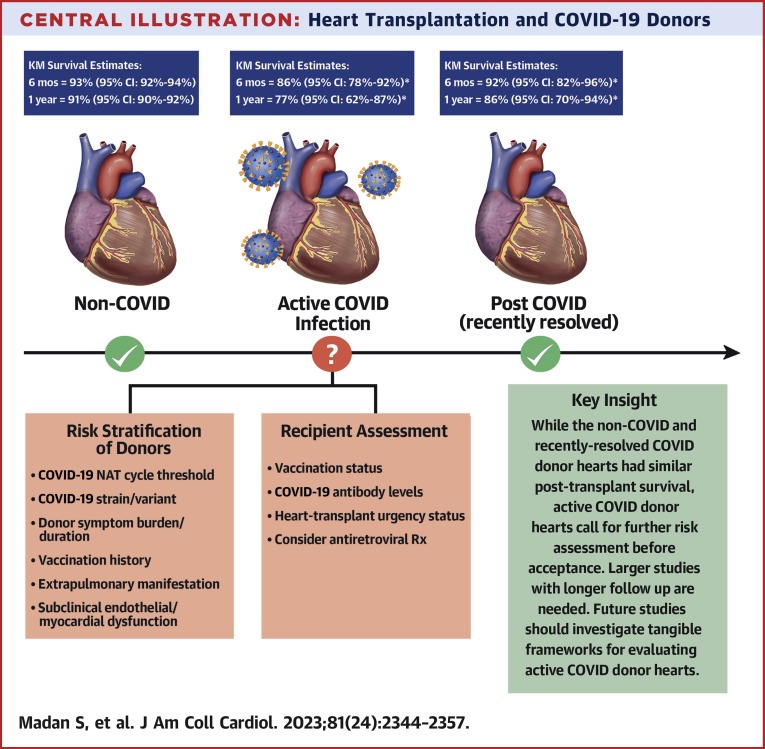
During the study period, 1,445 “COVID-19 donors” (COVID-19 NAT positive) were identified; 1,017 of these were aCOV, and 428 were rrCOV. Overall, 309 HTs used COVID-19 donors, and 239 adult HTs from COVID-19 donors (150 aCOV, 89 rrCOV) met study criteria. Compared with non-COV, COVID-19 donors used for adult HT were younger and mostly male (∼80%). Compared with HTs from non-COV donors, recipients of HTs from aCOV donors had increased mortality at 6 months (Cox HR: 1.74; 95% CI: 1.02-2.96; P = 0.043) and 1 year (Cox HR: 1.98; 95% CI: 1.22-3.22; P = 0.006). Recipients of HTs from rrCOV and non-COV donors had similar 6-month and 1-year mortality. Results were similar in propensity-matched cohorts.
Conclusions
In this early analysis, although HTs from aCOV donors had increased mortality at 6 months and 1 year, HTs from rrCOV donors had survival similar to that seen in recipients of HTs from non-COV donors. Continued evaluation and a more nuanced approach to this donor pool are needed.
Key Words: COVID-19 donors, donor characteristics, heart transplantation, transplant outcomes
Central Illustration
The COVID-19 pandemic has resulted in significant challenges for heart transplantation (HT), thereby adversely affecting both recipient management and organ procurement. Further, evaluation of potential donors with current or recent COVID-19 infection has presented a unique problem for HT centers,1 , 2 given that the outcomes of HTs from COVID-19 infected donors are not clearly established and newer variants of the virus continue to cause recurrent surges globally.3 Although the evidence thus far largely suggests absence of a viable transmittable virus outside of the respiratory (RS) tract, autopsy studies have detected COVID-19 viral proteins4 and genetic material in myocardial tissue even in patients with primarily COVID-19 pneumonia and low suspicion for myocarditis.5 Even though mortality related to COVID-19 in HT recipients has fallen,6 , 7 and recent single-center and smaller case series have suggested acceptable early short-term (weeks to months) outcomes of HT using donors with COVID-19 infection,8 , 9 data on long-term outcomes with a larger cohort are lacking. This is especially important because the COVID-19 virus can cause subclinical endothelial dysfunction and myocardial injury in potential donors, and studies involving non-HT groups have described an increased risk for adverse cardiovascular outcomes beyond the first 30 days of acute COVID-19 infection even among those persons who were never hospitalized.2 , 10 , 11 Hence, we evaluated the donor and recipient characteristics, use trends, and outcomes of HT using COVID-19 donors (both with active and recently resolved infection) in the United States.
Methods
Data source and study cohort
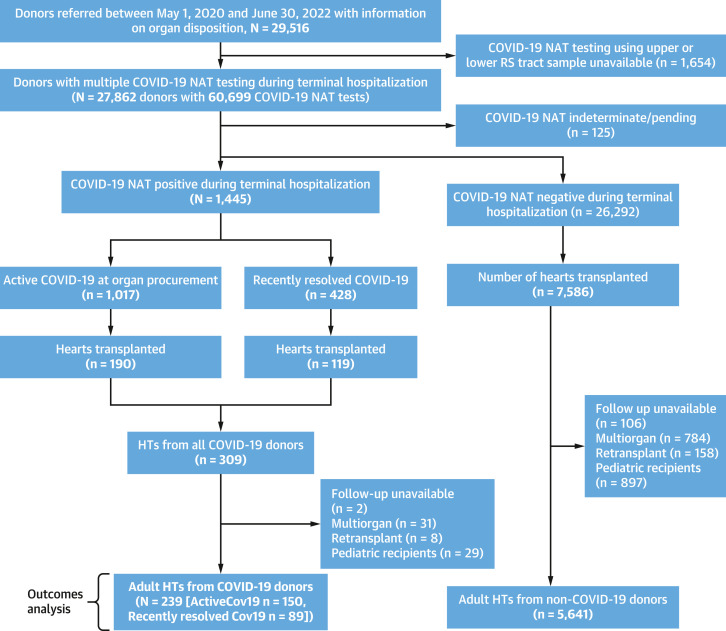
Patient-level nonidentifiable information was retrieved from the nationwide Organ Procurement and Transplant Network (OPTN) managed by the United Network for Organ Sharing (UNOS). The study was deemed exempt by the Institutional Review Board at Albert Einstein College of Medicine/Montefiore Medical Center, Bronx, New York, USA. UNOS started collecting data on donor COVID-19 status in DonorNet from April 21, 2020.12 We identified donors between May 2020 and June 2022 with available information on organ disposition and COVID-19 nucleic acid amplification testing (NAT) status in UNOS. Because potential organ donors may undergo COVID-19 testing multiple times during their terminal hospitalization and before organ procurement, additional data on multiple COVID-19 tests was requested from UNOS and matched with the Standard Transplant Analysis and Research UNOS files. COVID-19 NAT was done using upper RS (nasopharyngeal swabs) and/or lower RS samples (tracheal aspirate or bronchoalveolar lavage). Donors were considered “COVID-19 donors” if they were NAT positive at any time during terminal hospitalization. Donors were subclassified as “active COVID-19” (aCOV) if they were NAT positive within 2 days of organ procurement. Donors with a positive COVID-19 NAT result >2 days before organ procurement were also considered aCOV unless there was a subsequent negative NAT result ≥48 hours after the last positive NAT result to eliminate conflicting same-day testing and given the substantial heterogeneity in the frequency and timing of NAT across donors. Finally, donors were subclassified as “recently resolved COVID-19” (rrCOV) donors if they were NAT positive initially but became NAT negative before organ procurement (including a negative NAT result ≥48 hours after the last positive NAT result). Donors with other types of COVID-19 antigen or antibody tests where information on COVID-19 NAT was unavailable were excluded (Figure 1 ).
Figure 1.
Study Flowchart
Flowchart showing the study cohort derivation and design. Cov19 = COVID-19; HTs = heart transplants; NAT = nucleic acid amplification testing; RS = respiratory.
Post-HT outcomes were compared between adult (≥18 years) recipients of HTs from non–COVID-19 (referred to as non-COV) and COVID-19 donors (aCOV and rrCOV donors), with follow-up available through September 30, 2022. Multiorgan transplantations, retransplantations, pediatric recipients, or HTs with missing follow-up were excluded from the outcomes analyses (Figure 1). We also compared and analyzed characteristics of adult COVID-19 organ donors (aCOV and rrCOV) during the study period on the basis of their use for HT. We did not have cycle threshold (Ct) values for COVID-19 NATs. After meeting the study’s inclusion and exclusion criteria, data were available for all except when indicated in study tables.
Other definitions and study outcomes
Size mismatch was defined using the predicted heart mass as the donor to recipient predicted heart mass ratio of <0.86.13 Sex mismatch was defined as transplantation of a female donor heart to a male recipient.14 The primary outcome of analysis for the study was all-cause mortality up to 6 months and 1 year of follow-up. Secondary outcomes included in-hospital stroke, hemodialysis, pacemaker insertion, and post-HT hospital length of stay. To put these data into context of the overall COVID-19 infections in the United States, we also compared the number of HTs from COVID-19 donors to the 7-day average of COVID-19 cases in the United States during the study period (Supplemental Methods).
Statistical analysis
Baseline recipient and donor characteristics were expressed as percentages for categorical variables and median (IQR) for continuous variables. The Fisher exact test was used to compare categorical variables, and the Kruskal-Wallis test was used to compare continuous variables. Recipient mortality was compared using unadjusted and adjusted Cox proportional hazards regression (Cox HR) models and Kaplan-Meier (KM) analysis. All recipients were censored at 1 year of post-HT follow-up. Cox models were adjusted for donor and recipient characteristics known to be associated with post-HT outcomes in previous studies.
Because of differences in sample size and the possibility of confounding variables, we also used the propensity score (PS) matching method. Two unique PSs were generated for 2 pairwise comparisons (ie, aCOV vs non-COV donor HTs and rrCOV vs non-COV donor HTs). PSs were estimated using a multivariable logistic regression model on the basis of recipient factors including recipient age at transplantation, sex, race (Black vs others), sex mismatch, size mismatch, ischemic origin, UNOS urgency status at transplantation, blood type (O vs others), body mass index ≥35 kg/m2, life support with inotropic agents, intra-aortic balloon pump use, recipient durable left ventricular assist device use, extracorporeal membrane oxygenation support, and year of transplant, as well as donor factors including donor age, sex, donation after circulatory death (DCD) status, donor left ventricular ejection fraction (LVEF) <50%, and donor cause of death. Patients with missing information on these variables were excluded from PS matching. Matching was performed using 1:2 (greedy algorithm) without replacement using a caliper width of 0.2 of the SD of the logit of PSs. After PS matching, all standardized differences were <0.10 (or <10%). A standardized difference of <0.10 (or <10%) indicates adequate balance among the PS-matched cohorts.15 , 16 Postmatching analysis was performed using the Fisher exact test for categorical variables and Student’s t-test for continuous variables (expressed as mean ± SD). Post-HT survival in the PS-matched cohorts was compared using Cox HR and KM analysis. All statistical analyses were done using Stata software version 16 (StataCorp LLC). Two-sided P values <0.05 were considered significant.
Results
Study group
We identified 27,862 donors with available information on multiple COVID-19 NATs and organ disposition during the study period. Of these donors, 1,445 had at least 1 positive COVID-19 NAT result during terminal hospitalization and were classified as COVID-19 donors. The remaining 26,292 donors were COVID-19 NAT negative at all times during testing before procurement and were classified as non-COV. Donors with indeterminate or pending testing were excluded (n = 125) (Figure 1). Of the 1,445 COVID-19 donors, 1,017 were aCOV donors, and 428 were rrCOV donors. A total of 309 HTs were performed during the study period from COVID-19 donors (190 from aCOV and 119 from rrCOV donors) (Figure 1). After excluding multiorgan transplantations, retransplantations, pediatric recipients, and those with missing follow-up, 239 adult HTs from COVID-19 donors (150 aCOV and 89 rrCOV donors) were included in the outcomes analyses cohort and were compared with 5,641 adult HTs from non-COV donors during the same period (Figure 1).
Baseline adult HT characteristics on the basis of donor COVID-19 status
On comparing HT recipients from non-COV donors, HT recipients from COVID-19 donors were similar in age (55 years [IQR: 42-63 years] vs 57 years [IQR: 46-64 years]), female sex (23.85% vs 27.03%), Black race (23.43% vs 24.22%), size mismatch (12.13% vs 11.59%), ischemic origin (29.29% vs 28.01%), UNOS urgency status, intra-aortic balloon pump use (27.20% vs 26.98%), left ventricular assist device use (29.71% vs 30.99%) or extracorporeal membrane oxygenation support (5.02% vs 5.78%) (all P > 0.05). HT recipients from COVID-19 donors trended toward more blood group O (47.28% vs 40.77%; P = 0.051) (Table 1 ).
Table 1.
Baseline Adult (≥18 y) Heart Transplant Characteristics by Donor COVID-19 NAT Status During Terminal Hospitalization
| COVID-19 Donor (n = 239) | Non–COVID-19 DONOR (n = 5,641) | P Value | |
|---|---|---|---|
| Recipient | |||
| Age at transplant, y | 55 (42-63) | 57 (46-64) | 0.113 |
| Female | 57 (23.85) | 1,525 (27.03) | 0.298 |
| Recipient race: Black vs others | 56 (23.43) | 1,366 (24.22) | 0.817 |
| Sex mismatch (female donor to male recipient) | 17 (7.11) | 598 (10.60) | 0.104 |
| Size mismatch (donor recipient PHM ratio <0.86) | 29 (12.13) | 654 (11.59) | 0.758 |
| Origin of heart failure (ischemic) | 70 (29.29) | 1,580 (28.01) | 0.660 |
| Total wait-list time, mo | 0.97 (0.30-5.20) | 0.97 (0.30-4.60) | 0.700 |
| UNOS status at time of transplant | |||
| 1 | 24 (10.04) | 554 (9.82) | 0.739 |
| 2 | 109 (45.61) | 2,753 (48.8) | — |
| 3 | 36 (15.06) | 843 (14.94) | — |
| 4, 5, 6 | 70 (29.29) | 1,491 (26.43) | — |
| Recipient creatinine at transplant, mg/dL | 1.13 (0.90-1.46) [212] | 1.12 (0.90-1.40) [5,411] | 0.543 |
| Recipient blood type O | 113 (47.28) | 2,300 (40.77) | 0.051 |
| Recipient BMI at transplant, kg/m2 | 28.2 (24.2-32.0) | 27.6 (24.1-31.4) | 0.258 |
| Life support with inotropic agents | 83 (34.73) | 2,079 (36.86) | 0.538 |
| Recipient on LVAD at transplant | 71 (29.71) | 1,748 (30.99) | 0.721 |
| Recipient on IABP | 65 (27.20) | 1,522 (26.98) | 0.941 |
| Recipient on ECMO | 12 (5.02) | 326 (5.78) | 0.776 |
| Donor | |||
| Age, y | 30 (23-37) | 32 (25-40) | 0.002 |
| Female | 47 (19.67) | 1,573 (27.89) | 0.005 |
| DCD donors | 16 (6.69) | 344 (6.10) | 0.679 |
| HCV NAT positive | 16 (6.69) | 383 (6.79) | 0.999 |
| Any blood infection | 26 (10.88) | 649 (11.51) | 0.836 |
| LVEF, % | 61 (58-65) [239] | 60 (57-65) [5,639] | 0.058 |
| Donor LVEF <50% | 3 (1.26) [239] | 78 (1.38) [5,639] | 0.999 |
| Ischemic time, h | 3.5 (2.9-4.0) [211] | 3.5 (2.9-4.0) [5,390] | 0.999 |
| Donor cause of death | |||
| Brain anoxia | 105 (43.93) | 2,602 (46.13) | 0.023 |
| CVA/stroke | 18 (7.53) | 709 (12.57) | — |
| Head trauma | 107 (44.77) | 2,200 (39.00) | — |
| CNS tumor/others | 9 (3.77) | 130 (2.30) | — |
Values are median (IQR), n (%), median (IQR) [N], or n (%) [N]. Data were available for the complete cohort except when indicated by [N].
BMI = body mass index; CNS = central nervous system; CVA = cerebrovascular accident; DCD = donation after circulatory death; ECMO = extracorporeal membrane oxygenation; HCV = hepatitis C virus; IABP = intra-aortic balloon pump; LVAD = left ventricular assist device; LVEF = left ventricular ejection fraction; NAT = nucleic acid amplification testing; PHM = predicted heart mass; UNOS = United Network for Organ Sharing.
Compared with non-COV donors, COVID-19 donors were younger (30 years [IQR: 23-37 years] vs 32 years [IQR: 25-40 years]; P = 0.002), less likely to be female (19.67% vs 27.89%; P = 0.005), and more likely to have head trauma as a cause of death (44.77% vs 39.00%; P = 0.023); otherwise, the 2 cohorts were similar in DCD status and donor LVEF (Table 1).
When comparing aCOV and rrCOV donor HTs only, the baseline recipient and donor characteristics were similar (Supplemental Table 1).
All-cause mortality and secondary outcomes
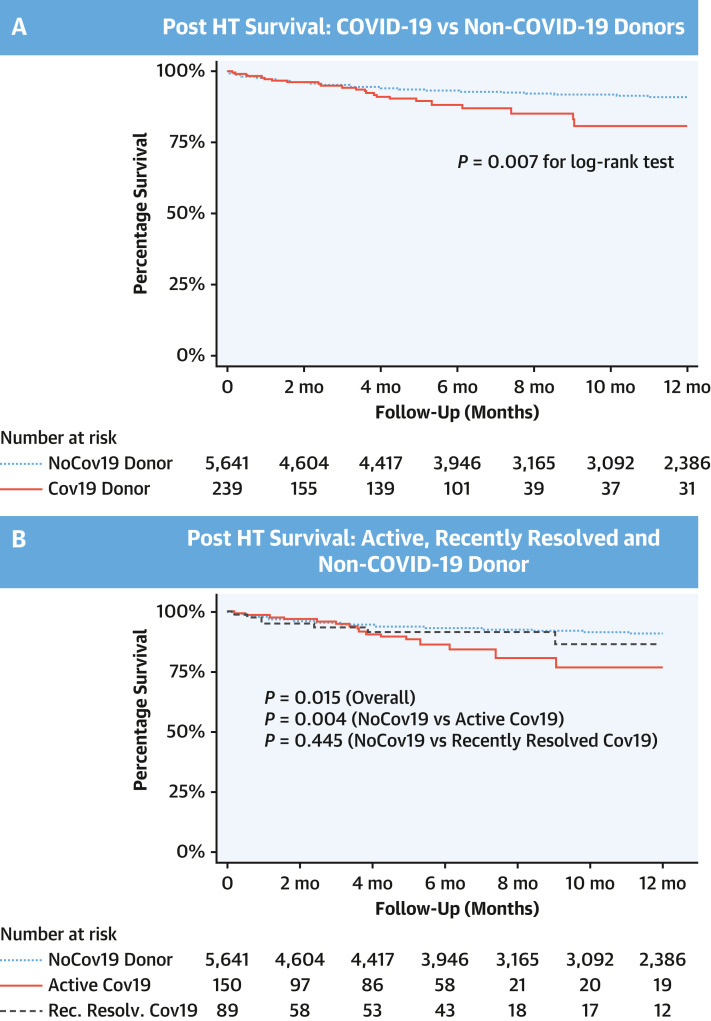
The overall median follow-up for the outcomes analysis cohort was 11.2 months (IQR: 5.4-12.3 months), and for adult HTs from COVID-19 donors only was 5.7 months (IQR: 1.5-6.5 months). KM estimates of survival for the overall cohort at 6 months and 1 year were 92.8% (95% CI: 92.1%-93.5%) and 90.5% (95% CI: 89.6%-91.4%), respectively. Compared with HT recipients from non-COV donors, HT recipients from COVID-19 donors had a trend toward increased mortality at 6 months in unadjusted Cox models (HR: 1.55; 95% CI: 0.99-2.44; P = 0.054) that was statistically significant after adjustment for baseline HT characteristics (HR: 1.61; 95% CI: 1.03-2.53; P = 0.038) (Table 2 ). Further, HT recipients from COVID-19 donors had increased mortality at 1 year in unadjusted Cox models (HR: 1.74; 95% CI: 1.15-2.63; P = 0.008) and adjusted Cox models (HR: 1.81; 95% CI: 1.20-2.73; P = 0.005) (Table 2, Figure 2A ).
Table 2.
Post–Heart Transplantation Survival in Heart Transplantations From All COVID-19, Active COVID-19, and Recently Resolved COVID-19 Donors
| 6-Month Cumulative KM Survival Estimates, % (95% CI) |
1-Year Cumulative KM Survival Estimates, % (95% CI) |
Unadjusted and Adjusted 6-Month Mortality, Cox HR (95% CI) | Unadjusted and Adjusted 1-Year Mortality, Cox HR (95% CI) | |
|---|---|---|---|---|
| Non–COVID-19 donors (n = 5,641) | 93.0 (92.2-93.7) | 90.8 (89.9-91.6) | ||
| All COVID-19 donors (n = 239) | 88.2 (82.2-92.3) | 80.7 (70.5-87.7) | 1.55 (0.99-2.44); P = 0.054a | 1.74 (1.15-2.63); P = 0.008a |
| 1.61 (1.03-2.53); P = 0.038b | 1.81 (1.20-2.73); P = 0.005b | |||
| 1.62 (1.03-2.55); P = 0.036c | 1.83 (1.21-2.77); P = 0.004c | |||
| Active COVID-19 donors (n = 150) | 86.2 (77.6-91.6) | 76.8 (61.9-86.5) | 1.74 (1.02-2.96); P = 0.043a | 1.98 (1.22-3.22); P = 0.006a |
| 1.79 (1.05-3.07); P = 0.033b | 2.05 (1.26-3.34); P = 0.004b | |||
| 1.81 (1.07-3.11); P = 0.029c | 2.10 (1.29-3.42); P = 0.003c | |||
| Recently resolved COVID-19 donors (n = 89) | 91.5 (81.8-96.1) | 86.4 (69.5-94.3) | 1.26 (0.56-2.82); P = 0.579a | 1.43 (0.64-2.84); P = 0.439a |
| 1.31 (0.58-2.94); P = 0.511b | 1.41 (0.67-2.97); P = 0.372b | |||
| 1.30 (0.58-2.91); P = 0.525c | 1.40 (0.66-2.94); P = 0.371c |
KM = Kaplan-Meier; other abbreviations as in Table 1.
Unadjusted model.
Model adjusted for recipient age, sex, race (Black vs others), sex mismatch, size mismatch, ischemic origin, UNOS status, inotropic agent use, IABP, LVAD, ECMO status, and donor age, sex, DCD status, and total ischemic time >4 hours.
Model adjusted for recipient age, sex, race (Black vs others), sex mismatch, size mismatch, ischemic origin, blood group O, UNOS status, inotropic agent use, IABP, LVAD, ECMO status, and donor age, sex, DCD status, donor LVEF <50%, and cause of death.
Figure 2.
Kaplan-Meier Curves for Different COVID-19 Donor Heart Transplantation Cohorts
Kaplan-Meier curves for freedom from all-cause mortality in (A) non–COVID-19 (No Cov19) and COVID-19 donor heart transplantation cohorts and (B) active COVID-19 (Active Cov19), recently resolved COVID-19 (Rec. resolv. Cov19), and non–COV donor heart transplantation cohorts.
When aCOV and rrCOV donor HTs were compared with non-COV donor HTs, aCOV donor HTs had increased mortality at 6 months in both unadjusted Cox models (Cox HR: 1.74; 95% CI: 1.02-2.96; P = 0.043) and adjusted Cox models (Cox HR: 1.81; 95% CI: 1.07-3.11; P = 0.029). The increased mortality in aCOV donor HTs persisted at 1 year in both unadjusted models (Cox HR: 1.98; 95% CI: 1.22-3.22; P = 0.006) and adjusted models (Cox HR: 2.10; 95% CI: 1.29-3.42; P = 0.003) (Table 2, Figure 2B). However, compared with non-COV donor HTs, rrCOV donor HTs cohort had similar mortality at 6 months and 1 year of follow-up in unadjusted and adjusted Cox models (Table 2, Figure 2B). Causes of post-HT recipient mortality in different COVID-19 donor HT cohorts are listed in Supplemental Table 2.
COVID-19 donor HTs and non-COV donor HTs were similar in terms of secondary outcomes, including in-hospital stroke (1.70% vs 3.64%), hemodialysis (17.30% vs 14.11%), pacemaker insertion (1.28% vs 1.46%), and post-HT length of stay (16 days [IQR: 11-27 days] vs 17 days [12-25 days]) (all P > 0.05). These secondary in-hospital outcomes were also similar between aCOV and rrCOV donor HTs (Supplemental Table 3).
Propensity-matched cohorts
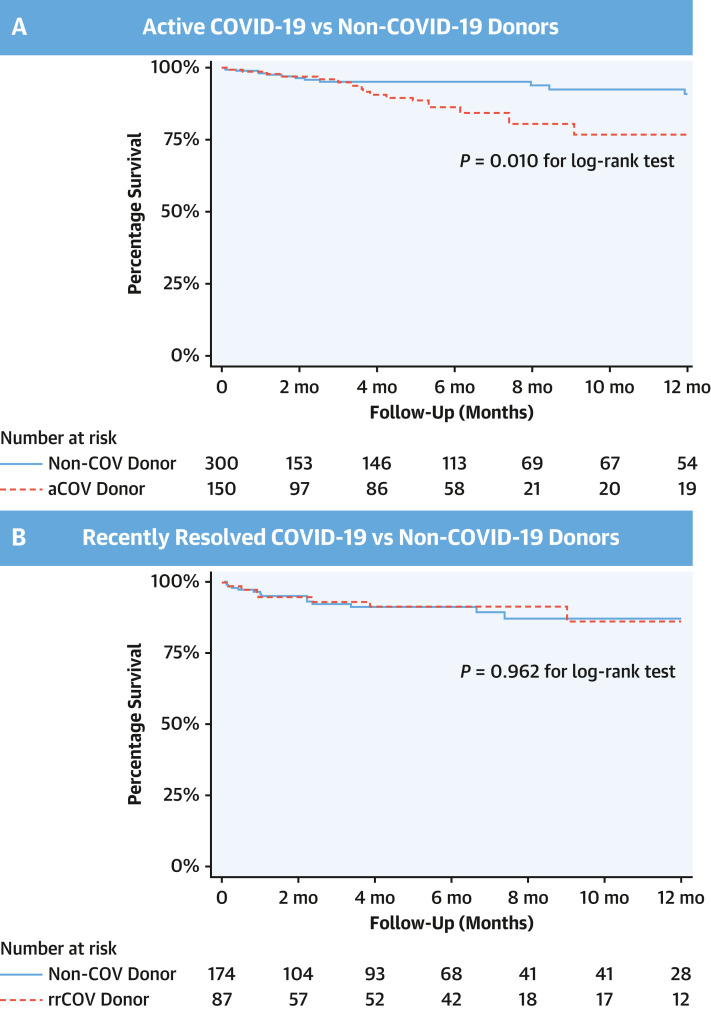
With the use of PSs on the basis of donor and recipient factors listed earlier and in Table 3 , 150 aCOV donor HTs were matched to 300 non-COV donor HTs (1:2 matching) (Table 3), and 87 rrCOV donor HTs were matched to 174 non-COV donor HTs (1:2 matching) (Supplemental Table 4). After PS matching, there were no significant differences in the baseline HT characteristics between cohorts, and all standardized differences were <0.1 (or <10%), indicating adequate balance (Table 3, Supplemental Table 4). Histograms of the 2 unique estimated PSs before and after matching for the 2 separate pairwise comparison cohorts (aCOV vs non-COV and rrCOV vs non-COV donor HTs) are shown in Supplemental Figures 1A, 1B, 2A, and 2B. Although total ischemic time was not used in the generation of PSs, mean total ischemic time was similar in the propensity-matched cohorts (P = 0.80 for non-COVID vs aCOV donor HTs and P = 0.37 for non-COVID vs rrCOV donor HTs). Post–PS matching survival analysis also showed similar results (ie, compared with non-COV donor HTs, the aCOV donor HT cohort had increased mortality up to 6-month and 1 year of follow-up by using both Cox models and KM analysis) (Figure 3A , Table 4 ). However, compared with non-COVID donor HTs, recipients of rrCOV donor HTs had similar mortality up to 6 months and 1 year of follow-up (Figure 3B, Supplemental Table 5).
Table 3.
Baseline Adult Heart Transplantation Characteristics in Propensity-Matched Cohorts of Active COVID-19 vs Non–COVID-19 Donor Heart Transplantations
| Active COVID-19 Donors (n = 150) | Non–COVID-19 Donors (n = 300) | P Value | Absolute SD | |
|---|---|---|---|---|
| Recipient | ||||
| Age at transplant, y | 52.47 ± 13.71 | 53.68 ± 13.59 | 0.375 | 0.089 |
| Female | 36 (24.00) | 74 (24.67) | 0.908 | 0.015 |
| Recipient race: Black vs others | 39 (26.00) | 85 (28.33) | 0.655 | 0.052 |
| Sex mismatch (female donor to male recipient) | 11 (7.33) | 21 (7.00) | 0.839 | 0.013 |
| Size mismatch (donor recipient PHM ratio <0.86) | 16 (10.67) | 30 (10.00) | 0.869 | 0.022 |
| Origin of heart failure (Ischemic) | 43 (28.67) | 82 (27.33) | 0.823 | 0.030 |
| UNOS status | ||||
| 1 | 15 (10.00) | 27 (9.00) | 0.588 | 0.064 |
| 2 | 67 (44.67) | 153 (51.00) | ||
| 3 | 25 (16.67) | 40 (13.33) | ||
| 4, 5, 6 | 43 (28.67) | 80 (26.67) | ||
| Recipient blood type O | 73 (48.67) | 143 (47.67) | 0.842 | 0.020 |
| Recipient BMI ≥35 kg/m2 | 9 (6.00) | 17 (5.67) | 0.999 | 0.014 |
| Life support with inotropic agents | 54 (36.00) | 113 (37.67) | 0.757 | 0.034 |
| Recipient on LVAD at transplant | 47 (31.33) | 93 (31.00) | 0.999 | 0.007 |
| Recipient on IABP | 40 (26.67) | 83 (27.67) | 0.911 | 0.022 |
| Recipient on ECMO | 6 (4.00) | 18 (6.00) | 0.505 | 0.092 |
| Year of transplant | ||||
| 2020 (May to December) | 2 (1.33) | 4 (1.33) | 0.944 | 0.020 |
| 2021 | 50 (33.33) | 97 (32.33) | ||
| 2022 (January to June) | 98 (65.33) | 199 (66.33) | ||
| Donor | ||||
| Age, y | 30.17 ± 9.70 | 30.58 ± 9.69 | 0.670 | 0.043 |
| Female | 28 (18.67) | 60 (20.00) | 0.801 | 0.034 |
| DCD donors | 10 (6.67) | 21 (7.00) | 0.999 | 0.013 |
| Donor LVEF <50% | 1 (0.67) | 6 (2.00) | 0.433 | 0.096 |
| Donor cause of death | ||||
| Brain anoxia | 64 (42.67) | 113 (37.67) | 0.140 | 0.027 |
| CVA/stroke | 10 (6.67) | 35 (11.67) | ||
| Head trauma | 70 (46.67) | 147 (49.00) | ||
| CNS tumor/others | 6 (4.00) | 5 (1.67) |
Values are mean ± SD or n (%), unless otherwise indicated.
Absolute SD = absolute standardized difference; other abbreviations as in Table 1.
Figure 3.
Kaplan-Meier Curves for Propensity-Matched Heart Transplantation Cohorts
Kaplan-Meier curves for freedom from all-cause mortality in propensity-matched (A) active-COVID-19 (aCOV) vs non–COVID-19 (non-COV) and (B) recently resolved COVID-19 (rrCOV) vs non–COVID-19 donor heart transplantation cohorts.
Table 4.
Post–Heart Transplantation Mortality in Propensity-Matched Cohort of Heart Transplantations From Active COVID-19 and Non–COVID-19 Donors
| 6-Month Mortality Cumulative KM Estimates, % (95% CI) |
1-Year Mortality Cumulative KM Estimates, % (95% CI) |
6-Month Mortality, Cox HR (95% CI) | 1-Year Mortality, Cox HR (95% CI) | |
|---|---|---|---|---|
| Non–COVID-19 donors (n = 300) | 4.9 (2.6-8.9) | 9.2 (5.0-16.8) | — | — |
| Active COVID-19 donors (n = 150) | 13.8 (8.4-22.4) | 23.2 (13.5-38.1) | 2.41 (1.07-5.43); P = 0.033 | 2.51 (1.21-5.20); P = 0.013 |
KM = Kaplan-Meier.
Trend for HTs from COVID-19 donors
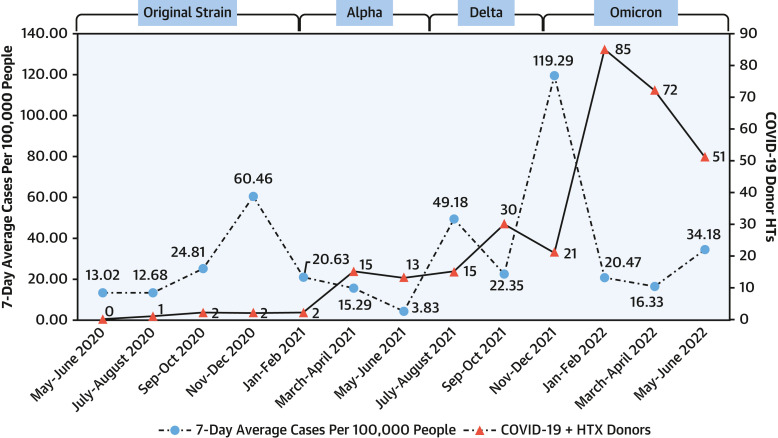
The number of overall COVID-19 donor HTs increased during the study period (P < 0.001 for trend) (Supplemental Table 6). Interestingly, the peaks for HTs with COVID-19 donors followed the peaks of the community COVID-19 surges in the United States (Figure 4 ). Although we have approximate estimates of the dominant COVID-19 virus strain that was prevalent in the community at large, we did not have the exact donor COVID-19 virus strain.
Figure 4.
Heart Transplantations From COVID-19 Donors and Community COVID-19 Cases in the United States
Heart transplantations (HTs) (adult and pediatric) from COVID-19 heart transplant (HTX) donors and 7-day average of COVID-19 cases per 100,000 U.S. population.
COVID-19 NAT testing of potential donors
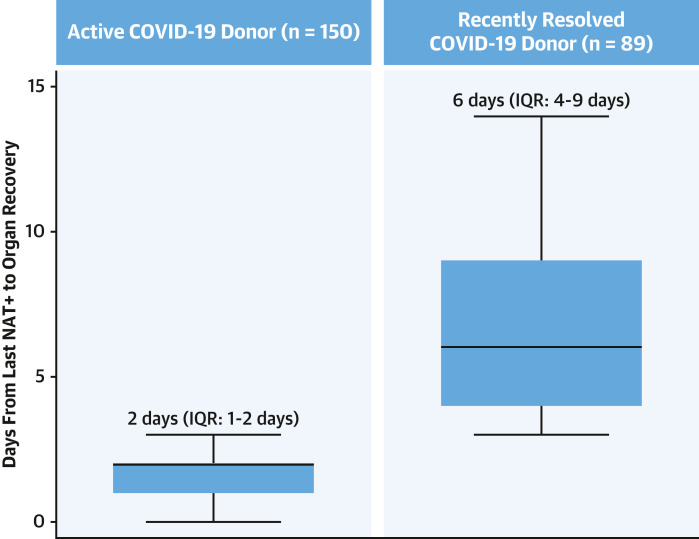
For the 27,862 donors with available information on organ disposition (Figure 1), there were 60,699 COVID-19 NATs performed during terminal hospitalization and before organ procurement (median 2 tests per donor [IQR: 2-3 tests per donor]). Of these, 44,961 (74.07%) tests were done using upper RS and 15,738 (25.93%) using lower RS samples. For the 1,445 COVID-19 donors (with ≥1 COVID-19 NAT positive test), there were 4,467 COVID-19 NATs done (3 tests per donor [IQR: 3-5 tests per donor]). Of these, 3,389 (75.87%) tests were done using upper RS and 1,078 (24.13%) using lower RS samples. As expected, the number of COVID-19 tests done per donor before organ procurement was higher for COVID-19 donors than for non-COV donors (3 [IQR: 3-5] vs 2 [IQR: 2-3]; P < 0.001). For the 239 COVID-19 donors used for adult HT and included in the final outcomes cohort, there were 893 COVID-19 NATs done (4 tests per donor [IQR: 3-5 tests per donor]). Of these tests, 622 (69.65%) used upper RS and 271 (30.35%) used lower RS samples. Finally, the median number of days between the last positive COVID-19 NAT result and organ procurement was 2 days (IQR: 1-2 days) for donors with aCOV and 6 days (IQR: 4-9 days) for donors with rrCOV used for adult HT (Figure 5 ). For non-COV donor HTs, the median number of days from the last negative COVID-19 NAT result to organ procurement was 2 days (IQR: 1-3 days).
Figure 5.
Days From Last COVID-19 NAT+ Result to Organ Procurement
Number of days from last COVID-19–positive nucleic acid amplification testing (NAT+) result for active COVID-19 and recently resolved COVID-19 donors used for adult heart transplantation.
Characteristics of adult donors on the basis of use for HT and COVID-19 status
Because the allocation system for pediatric and adult heart donors is different in the United States, we restricted the utilization analysis to adult (≥18 years) donors only and excluded pediatric donors (Supplemental Figure 3).
Adult donors not used for HT
Compared with non-COV donors, aCOV and rrCOV donors that were not used for HT were more likely to be younger (51 years [IQR: 39-59 years] vs 47 years [IQR: 37-56 years] vs 49 years [IQR: 39-56 years]) and to have less donor LVEF <50% (23.77% vs 18.39% vs 18.29%) and were less likely to have a history of hypertension (49.44% vs 39.02% vs 45.61%), smoking (30.05% vs 19.84% vs 19.93%), heavy alcohol intake (26.58% vs 19.31% vs 16.91%), cocaine use (23.93% vs 18.53% vs 17.52%), intravenous drug use (12.08% vs 10.84% vs 7.47%), or Centers for Disease Control and Prevention high risk (19.94% vs 15.03% vs 13.79%); and they were less likely to have brain anoxia as a cause of death (47.89% vs 40.15% vs 39.31%) (all P < 0.05) (Table 5 ). However, aCOV and rrCOV donors that were not used for HT were more likely to be DCD (38.00% vs 47.22% vs 49.31%; P < 0.001).
Table 5.
Baseline Characteristics of Adult (≥18 Years) Donors on the Basis of Use for Heart Transplantation and COVID-19 Status
| Not Transplanted |
P Value | Transplanted |
P Value | |||||
|---|---|---|---|---|---|---|---|---|
| Non–COVID-19 (n = 17,988) | Active COVID-19 (n = 792) | Recently Resolved COVID-19 (n = 290) | Non–COVID-19 (n = 6,585) | Active COVID-19 (n = 167) | Recently Resolved COVID-19 (n = 96) | |||
| Age, y | 51 (39-59) | 47 (37-56) | 49 (39-56) | <0.001 | 32 (25-40) | 30 (24-37) | 30 (25-39) | 0.058 |
| Female | 7,375 (41.00) | 314 (39.65) | 127 (43.79) | 0.463 | 1,867 (28.35) | 35 (20.96) | 19 (19.79) | 0.021 |
| Black race | 2,640 (14.68) | 104 (13.13) | 39 (13.45) | 0.437 | 1,070 (16.25) | 26 (15.57) | 20 (20.83) | 0.463 |
| Donor LVEF, % | 60 (50-65) [10,084] | 60 (55-65) [397] | 60 (55-65) [164] | <0.001 | 60 (57-65) [6,582] | 61 (58-65) [167] | 61 (57-65) [96] | 0.455 |
| Donor LVEF <50% | 2,397 (23.77) [10,084] | 73 (18.39) [397] | 30 (18.29) [164] | 0.013 | 86 (1.31) [6,582] | 1 (0.60) [167] | 3 (3.12) [96] | 0.166 |
| Blood type O | 8,265 (45.95) | 360 (45.45) | 135 (46.55) | 0.943 | 3,537 (53.71) | 92 (55.09) | 59 (61.46) | 0.306 |
| Donor BMI, kg/m2 | 28.5 (24.4-33.8) | 29.7 (25.2-34.7) | 30.2 (26.1-35.5) | <0.001 | 26.9 (23.7-31.2) | 25.9 (23.1-31.3) | 27.4 (24.7-32.3) | 0.277 |
| Donor medical history | ||||||||
| Diabetes | 2,871 (16.64) [17,253] | 110 (14.53) [757] | 42 (15.27) [275] | 0.273 | 221 (3.42) [6,463] | 6 (3.68) [163] | 6 (6.59) [91] | 0.218 |
| Hypertension | 8,748 (49.44) [17,694] | 304 (39.02) [779] | 130 (45.61) [285] | <0.001 | 999 (15.40) [6,486] | 24 (14.72) [163] | 10 (10.87) [92] | 0.526 |
| Coronary artery disease | 1,920 (10.91) [17,593] | 46 (5.93) [776] | 23 (8.13) [283] | <0.001 | 14 (0.22) [6,484] | 0 (0) [163] | 0 (0) [91] | 0.999 |
| CDC high risk | 3,586 (19.94) | 119 (15.03) | 40 (13.79) | <0.001 | 1,905 (28.93) | 41 (24.55) | 32 (33.33) | 0.289 |
| Blood infection in donor | 2,643 (14.69) | 160 (20.20) | 78 (26.90) | <0.001 | 754 (11.45) | 14 (8.38) | 14 (14.58) | 0.284 |
| Donor serology | ||||||||
| HCV NAT positive | 1,117 (6.21) | 39 (4.92) | 8 (2.76) | 0.014 | 455 (6.91) | 9 (5.39) | 11 (11.46) | 0.169 |
| CMV antibody positive | 11,324 (62.95) | 487 (61.49) | 177 (61.03) | 0.561 | 4,119 (62.55) | 95 (56.89) | 45 (46.88) | 0.003 |
| Donor lifestyle factors | ||||||||
| History of any smoking | 5,226(30.05) [17,389] | 151 (19.84) [761] | 56(19.93) [281] | <0.001 | 785(12.28) [6,391] | 25(15.43) [162] | 8 (8.89) [90] | 0.309 |
| History of heavy alcohol use (2+ drinks/d) | 4,605 (26.58) [17,327] | 146 (19.31) [756] | 47 (16.91) [278] | <0.001 | 1,291 (20.39) [6,332] | 30 (18.99) [158] | 20 (22.99) [87] | 0.749 |
| History of any cocaine use | 4,105 (23.93) [17,156] | 139 (18.53) [750] | 48 (17.52) [274] | <0.001 | 1,752 (27.81) [6,301] | 44 (28.39) [155] | 27 (30.34) [89] | 0.837 |
| History of any IV drug use | 2,125 (12.08) [17,591] | 84 (10.84) [775] | 21 (7.47) [281] | 0.034 | 1,191 (18.52) [6,430] | 26 (16.05) [162] | 23 (25.84) [89] | 0.152 |
| DCD | 6,835(38.00) | 374 (47.22) | 143(49.31) | <0.001 | 375 (5.69) | 9 (5.39) | 6 (6.25) | 0.931 |
| Brain anoxia | 8,615 (47.89) | 318 (40.15) | 114 (39.31) | <0.001 | 2,993 (45.45) | 74 (44.31) | 44 (45.83) | 0.965 |
Values are median (IQR), n (%), median (IQR) [N], or n (%) [N]. Data were available for the complete cohort except when indicated by [N].
CDC = Centers for Disease Control and Prevention; CMV = cytomegalovirus; HBV = hepatitis B virus; IV = intravenous; other abbreviations as in Table 1.
Adult donors used for HT
Compared with non-COV donors, aCOV and rrCOV donors that were used for HT were less likely to be female (28.35% vs 20.96% vs 19.79%; P = 0.021), were less CMV antibody positive (62.55% vs 56.89% vs 46.88%; P = 0.003), and had a trend toward being younger (32 years [IQR: 25-40 years] vs 30 years [IQR: 24-37 years] vs 30 years [IQR: 25-39 years]; P = 0.058). Otherwise, the donor cohorts were similar in terms of donor LVEF, history of diabetes, hypertension, smoking, cocaine use, and other characteristics (Table 5).
Discussion
We present our analysis using the UNOS database and evaluating donor and recipient characteristics and outcomes of HTs from COVID-19 donors (aCOV and rrCOV). Our principal findings are as follows. First, there was a significant trend toward increased use of COVID-19 donors (both aCOV and rrCOV) during the study period. Second, as expected, HT centers have been selective in their use of COVID-19 donors for HT, primarily using younger donors who were mostly male (∼80%). Third, potential donors underwent multiple COVID-19 NATs before organ procurement, numbers that were higher for donors who had ≥1 positive COVID-19 NAT result (ie, COVID-19 donors) compared with donors who tested NAT negative (3 tests per donor [IQR: 3-5 tests per donor] vs 2 tests per donor [IQR: 2-3 tests per donor]). Fourth, we found a concerning increase in 6-month and 1-year mortality among adult HT recipients from aCOV donors in both unadjusted and adjusted survival models (Table 2, Figure 2B). HT recipients from rrCOV donors had mortality similar to that of HT recipients from non-COV donors at 6 months and 1 year of follow-up (Table 2, Figure 2B). These results held true in propensity-matched cohorts analysis as well (Table 4, Figures 3A and 3B). Because most of the COVID-19 donor hearts were transplanted more recently and given that we ended the overall study cohort on June 30, 2022, 1-year outcomes of this analysis should be interpreted with caution. However, the difference in HT recipient survival in different cohorts was also becoming apparent at 6 months.
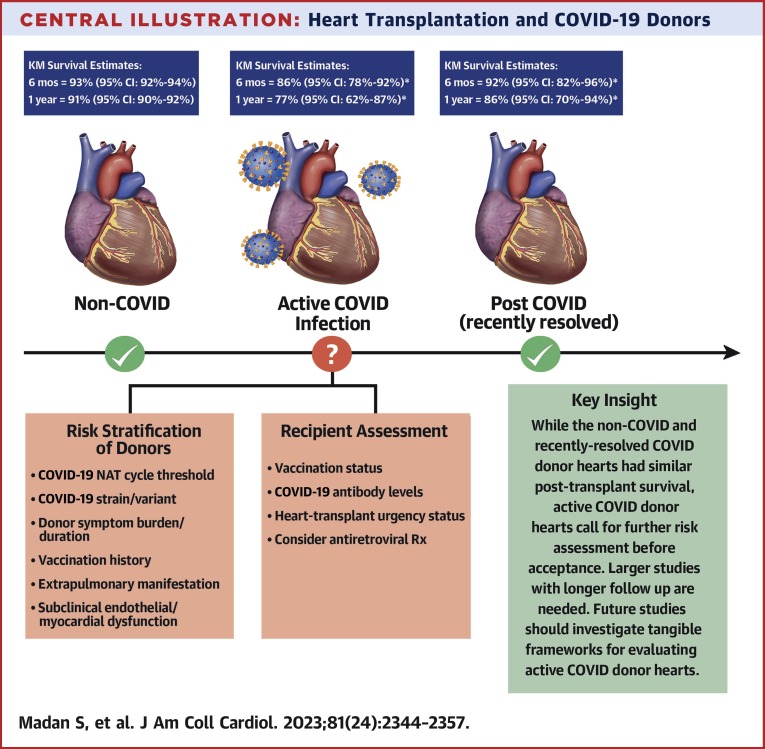
Although expansion of the donor pool with use of hepatis C virus (HCV)–infected donors17 and those with DCD status18 has benefited patients on the HT wait list in recent years, the COVID-19 pandemic has created multiple challenges for HT centers. COVID-19 has adversely affected all facets of HT, including wait-list mortality, recipient survival, and donor heart procurement.1 HT centers have had to modify recipient and donor management practices continuously during the pandemic as our understanding of the COVID-19 virus has evolved. Our early analysis suggests that whereas HTs from rrCOV donors appear to be safe, HTs from aCOV donors may be associated with increased mortality (Central Illustration ). Of note, >50% of HT recipients in the study cohort were UNOS status 1 or 2 at the time of HT. The proportion of HTs from UNOS status 2 has been on the rise in the new allocation system, as illustrated in the 2021 OPTN report.19 Despite the limitations of our study, these early trends should be concerning enough that HT centers need thoroughly to evaluate and continue to weigh the risks and benefits of using hearts from aCOV donors. Although the original strain of the virus was reported to be much more lethal in HT recipients (∼25% short-term mortality),20 more recent data suggested that COVID-19–associated mortality in HT recipients is likely to be substantially lower with subsequent strains of the virus and with the availability of newer vaccinations and treatments.7 Nonetheless, the impact of immunosuppression on COVID-19 disease severity in HT recipients remains unclear because the pathogenesis of organ involvement beyond the RS system in COVID-19 depends on both direct virally mediated injury and the associated immune response by the host.21 , 22 Further, COVID-19 infection-mediated myocardial injury in potential organ donors may manifest only subclinically before organ procurement, and the effect of this injury on long-term post-HT outcomes remains unclear.2
Central Illustration.
Heart Transplantation and COVID-19 Donors
Donor COVID-19 status, risk stratification, and early post–heart transplantation survival. ∗Wide CI due to limited study cohort size. KM = Kaplan-Meier.
Although a recent single-center institutional experience of 12 HTs (in 11 recipients) using donors with any positive COVID-19 testing results showed good post-HT outcomes with no development of COVID-19 infection in recipients, the majority of the organ donors either tested negative for COVID-19 infection closest to organ procurement (after having tested positive initially) or had a high polymerase chain reaction Ct of ≥34 (suggesting a lower viral load). Further, none of the donors had clinically significant COVID-19 symptoms.8 Another recent analysis using the UNOS database showed an equivalent survival in HTs from COVID-19 and non-COV donors, but the median follow-up time for the COVID-19 cohort was only 35 days, which may be too short to detect any significant difference.23
We found an increase in 6-month and 1-year mortality in this early analysis of HT recipients from aCOV donors. However, HTs from rrCOV donors had survival similar to HTs from non-COV donors. COVID-19 can have a wide spectrum of disease severity and extrapulmonary manifestations. Thus, the aCOV donors (as defined by our study criteria) likely included donors with varying degrees of disease severity. For example, of the 150 aCOV donors included in the study outcomes cohort, 7 (4.67%) were used for lung transplantations as well. However, notably, all the 7 aCOV donors used for lung transplantation had positive NAT results from the upper RS sample but had negative NAT results from the lower RS samples. Conversely, the percentage of donors used for lung transplantation in the rrCOV cohort was much higher, at 20 (22.47%) (of 89 rrCOV donors). All 20 of these but 1 were listed as being positive only from the upper RS sample. Although the association between lower Ct values (indicating higher virus burden) for positive COVID-19 NAT results and adverse clinical outcomes is yet to be clearly elucidated, some studies have found an association between lower Ct values and higher rates of multiorgan failure and increased mortality in those persons infected.24 , 25 We did not find a clear signal to explain the foregoing differences in HT recipient mortality in different COVID-19 donor cohorts when evaluating causes of recipient death post-HT (Supplemental Table 2). It is possible that hearts from aCOV donors may have been used for HT recipients who were sicker, but our registry-based analysis may have been unable to capture that in its entirety.
Study limitations
First, the current study had the inherent limitations of a retrospective, registry-based analysis and should be interpreted as such. Second, although OPTN recommends testing potential donors for COVID-19 as close as possible to (or at least within 72 hours of) organ procurement to reduce the risk of virus transmission, there was significant heterogeneity in the timing and frequency of COVID-19 testing during terminal hospitalization for potential donors.26 Hence, to identify rrCOV donors, we applied the 48-hour threshold for evidence of a subsequent NAT negative result after a positive NAT result to eliminate conflicting same-day testing. Third, we did not have detailed information on donor COVID-19 disease activity, including Ct values (which may be indicative of viral loads), donor infection history (including date of disease onset and symptom burden), vaccination status for both donor and recipients, or any specific treatments given to HT recipients from COVID-19 donors. Hence, we are unable to determine whether any of these factors or constraints in access and delivery of care contributed to the observed differences in HT recipient survival in different COVID-19 donor cohorts (Central Illustration). Fourth, we did not have specific information on the COVID-19 virus strain in potential donors. Hence we are unable to determine the outcomes by different COVID-19 “pandemic waves.” However, most HTs from COVID-19 donors were performed when the Delta and Omicron strains of the virus were prevalent in the United States (Figure 4). Fifth, our study was not sufficiently powered to do a direct subgroup comparison between aCOV and rrCOV donor HTs. Further, most of the aCOV and rrCOV donor HTs were performed more recently, and to account for this, we also added “year of transplant” in generation of propensity-matched cohorts. Although the current analysis has a longer follow-up than previous reports, it must be emphasized that these data are still early, and continued evaluation of COVID-19 donors with more granular data, larger sample size, longer follow-up, and newer variants of the COVID-19 virus is needed. Finally, we did not have information on antiretroviral therapies administered around the time of HT and are unable to determine whether that factor may have influenced outcomes for aCOV and rrCOV donor HTs.
Conclusions
In this early cohort of HTs from COVID-19 donors, we found an increased risk of mortality in HT recipients from aCOV donors. However, HT recipients from rrCOV donors had survival similar to that of HT recipients from non-COV donors. The current study highlights the importance of continued evaluation and probably the need for a more nuanced approach toward using this new donor pool.
Perspectives.
COMPETENCY IN PATIENT CARE AND PROCEDURAL SKILLS: The outcomes of HT from donors who recently recovered from COVID-19 are similar to those from other donors, but recipients of hearts from donors with aCOV may have shorter post-transplant survival.
TRANSLATIONAL OUTLOOK: Further studies of larger numbers of patients, longer follow-up, and more detailed clinical data are needed to assess the outcomes of HT from COVID-19–infected donors.
Funding Support and Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
Listen to this manuscript's audio summary by Editor-in-Chief Dr Valentin Fuster onwww.jacc.org/journal/jacc.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For an expanded Methods section as well as supplemental figures and tables, please see the online version of this paper.
Appendix
References
- 1.DeFilippis E.M., Farr M.A., Givertz M.M. Challenges in heart transplantation in the era of COVID-19. Circulation. 2020;141:2048–2051. doi: 10.1161/CIRCULATIONAHA.120.047096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sadeh B., Ugolini S., Pinzon O.W., et al. Medical decisions in organ donors and heart transplant candidates with history of COVID-19 infection: an international practice survey. Clin Transplant. 2022;36(7) doi: 10.1111/ctr.14733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alexandridi M., Mazej J., Palermo E., Hiscott J. The coronavirus pandemic–2022: viruses, variants & vaccines. Cytokine Growth Factor Rev. 2022;63:1–9. doi: 10.1016/j.cytogfr.2022.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gaussen A., Hornby L., Rockl G., et al. Evidence of SARS-CoV-2 infection in cells, tissues, and organs and the risk of transmission through transplantation. Transplantation. 2021;105:1405–1422. doi: 10.1097/TP.0000000000003744. [DOI] [PubMed] [Google Scholar]
- 5.Kogan E., Berezovskiy Y., Blagova O., et al. Morphologically, immunohistochemically and PCR proven lymphocytic viral peri-, endo-, myocarditis in patients with fatal COVID-19. Diagn Pathol. 2022;17:1–7. doi: 10.1186/s13000-022-01207-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nimmo A., Gardiner D., Ushiro-Lumb I., Ravanan R., Forsythe J.L. The global impact of COVID-19 on solid organ transplantation: two years into a pandemic. Transplantation. 2022 doi: 10.1097/TP.0000000000004151. 10.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kanaan C.N., Iskandar J.P., Gad M.M., et al. Outcomes associated with COVID-19 hospitalization in heart transplantation patients. Transplant Proc. 2022;54(10):2688–2691. doi: 10.1016/j.transproceed.2022.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eichenberger E.M., Coniglio A.C., Milano C., et al. Transplanting thoracic COVID-19 positive donors: an institutional protocol and report of the first 14 cases. J Heart Lung Transplant. 2022;41:1376–1381. doi: 10.1016/j.healun.2022.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de la Villa S., Valerio M., Salcedo M., et al. Heart and liver transplant recipients from donor with positive SARS-CoV-2 RT-PCR at time of transplantation. Transpl Infect Dis. 2021;23(5) doi: 10.1111/tid.13664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xie Y., Xu E., Bowe B., Al-Aly Z. Long-term cardiovascular outcomes of COVID-19. Nat Med. 2022;28:583–590. doi: 10.1038/s41591-022-01689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tobler D.L., Pruzansky A.J., Naderi S., Ambrosy A.P., Slade J.J. Long-term cardiovascular effects of COVID-19: emerging data relevant to the cardiovascular clinician. Curr Atheroscler Rep. 2022;24(7):563–570. doi: 10.1007/s11883-022-01032-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.New donor data collection for COVID-19 testing and results added to DonorNet. United Network for Organ Sharing; 2020. p. 2022. Accessed August 15, 2022. https://unos.org/news/new-donor-data-collection-for-covid-19-testing-and-results-added-to-donornet/#:∼:text=New%20data%20collection%20elements%20have,this%20information%20is%20not%20mandatory. [Google Scholar]
- 13.Kransdorf E.P., Kittleson M.M., Benck L.R., et al. Predicted heart mass is the optimal metric for size match in heart transplantation. J Heart Lung Transplant. 2019;38:156–165. doi: 10.1016/j.healun.2018.09.017. [DOI] [PubMed] [Google Scholar]
- 14.Madan S., Saeed O., Shin J., et al. Donor troponin and survival after cardiac transplantation: an analysis of the United Network of Organ Sharing Registry. Circ Heart Fail. 2016;9 doi: 10.1161/CIRCHEARTFAILURE.115.002909. [DOI] [PubMed] [Google Scholar]
- 15.Austin P.C. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat Simul Comput. 2009;38:1228–1234. [Google Scholar]
- 16.Bangalore S., Guo Y., Samadashvili Z., Blecker S., Xu J., Hannan E.L. Everolimus-eluting stents or bypass surgery for multivessel coronary disease. N Engl J Med. 2015;372:1213–1222. doi: 10.1056/NEJMoa1412168. [DOI] [PubMed] [Google Scholar]
- 17.Madan S., Patel S.R., Rahgozar K., et al. Utilization rates and clinical outcomes of hepatitis C positive donor hearts in the contemporary era. J Heart Lung Transplant. 2019;38:907–917. doi: 10.1016/j.healun.2019.06.023. [DOI] [PubMed] [Google Scholar]
- 18.Madan S., Saeed O., Forest S.J., Goldstein D.J., Jorde U.P., Patel S.R. Feasibility and potential impact of heart transplantation from adult donors after circulatory death. J Am Coll Cardiol. 2022;79:148–162. doi: 10.1016/j.jacc.2021.10.042. [DOI] [PubMed] [Google Scholar]
- 19.Colvin M.M., Smith J.M., Ahn Y.S., et al. OPTN/SRTR 2021 annual data report: heart. Am J Transplant. 2023;23(suppl):S300–S378. doi: 10.1016/j.ajt.2023.02.008. [DOI] [PubMed] [Google Scholar]
- 20.Latif F., Farr M.A., Clerkin K.J., et al. Characteristics and outcomes of recipients of heart transplant with coronavirus disease 2019. JAMA Cardiol. 2020;5:1165–1169. doi: 10.1001/jamacardio.2020.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen G., Wu D., Guo W., et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130:2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ram-Mohan N., Kim D., Zudock E.J., et al. SARS-CoV-2 RNAemia predicts clinical deterioration and extrapulmonary complications from COVID-19. Clin Infect Dis. 2022;74:218–226. doi: 10.1093/cid/ciab394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DeFilippis E.M., Wayda B., Lala A., Givertz M.M., Khush K.K. Utilization of COVID-19 positive donors for heart transplantation and associated short-term outcomes. J Heart Lung Transplant. 2023;42(5):651–659. doi: 10.1016/j.healun.2022.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hagman K., Hedenstierna M., Widaeus J., et al. Correlation of SARS-CoV-2 nasopharyngeal CT values with viremia and mortality in adults hospitalized with COVID-19. Open Forum Infect Dis. 2022;9:ofac463. doi: 10.1093/ofid/ofac463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shah V.P., Farah W.H., Hill J.C., et al. Association between SARS-CoV-2 cycle threshold values and clinical outcomes in patients with COVID-19: a systematic review and meta-analysis. Open Forum Infect Dis. 2021;8:ofab453. doi: 10.1093/ofid/ofab453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.OPTN Ad Hoc Disease Transmission Advisory Committee (DTAC) 2022. Summary of current evidence and information– donor SARS-CoV-2 testing & organ recovery from donors with a history of COVID-19. Accessed December 12, 2022. https://optn.transplant.hrsa.gov/media/kkhnlwah/sars-cov-2-summary-of-evidence.pdf. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.