Abstract
Anger has been associated with relative left frontal cortical activity, reflecting its approach (vs. withdrawal) motivational tendency. However, there may be contexts in which anger is associated with withdrawal motivation and, hence, relative right frontal cortical activity. Based on past research, we hypothesized that for some individuals, interracial interactions may be one such context, as societal pressure to be politically correct dictates that anger should not be expressed. Thus, in the context of an interracial interaction, the experience of anger may coincide with a desire to withdraw from the situation. Cortical activity was measured while White participants anticipated an interracial interaction. Consistent with expectations, self-reported anger was associated with relative right frontal cortical activity. In general, the motivational correlates of anger may be partially determined by the specific attributes of the person and the social context in which it occurs.
One of the primary functions of emotion is to motivate behavior, to draw us towards some things and push us away from others. The prominence of the motivational component of emotion has been underscored through work linking motivation to patterns of physiology. Specifically, cortical activity, as measured by surface electroencephalography (EEG), is one index of physiology that has been frequently used to explore issues in emotion. Early work measuring EEG during the experience of emotion linked frontal asymmetries in cortical activity to the valence of the emotion, with relative left frontal cortical activity linked to positive emotions and relative right frontal cortical activity linked to negative emotions (for review, see Coan & Allen, 2004).
However, more recent work has theorized that frontal asymmetries in cortical activity actually reflect the motivational direction of emotion (e.g., Harmon-Jones, 2003a). This relationship has been explored by exploiting the natural properties of anger, an emotion that is felt as negative but evokes approach motivational tendencies (Carver, 2004; Harmon-Jones, 2003b). Since work on the relationship between anger and asymmetrical frontal cortical activity began, evidence has suggested that anger is associated with relatively greater left-frontal cortical activity, consistent with a motivation-based model of frontal asymmetry rather than a valence-based model. Specifically, studies have found that trait anger is associated with greater relative left frontal activity at resting baseline (Harmon-Jones, 2004; Harmon-Jones & Allen, 1998; Hewig, Hagemann, Seifert, Naumann, & Bartussek, 2004). Other studies have found greater relative left frontal activation to anger-evoking situations for individuals high in trait anger (Harmon-Jones, in press) or who are prone toward mania (a state associated with increased behavioral approach; Harmon-Jones, Abramson, Sigelman, Bohlig, Hogan, & Harmon-Jones, 2002). Finally, manipulations of anger evoke greater relative left frontal activation (Harmon-Jones & Sigelman, 2001; Harmon-Jones, Vaughn-Scott, Mohr, Sigelman & Harmon-Jones, 2004), and manipulations of relative left frontal cortical activation affect attention to and memory for angry faces (e.g., van Honk & Schutter, 2006).
More recent experiments have revealed that it is the approach motivation aspect of anger that drives the increase in relative left frontal activation (Harmon-Jones, Lueck, Fearn, & Harmon-Jones, 2006; Harmon-Jones, Sigelman, Bohlig & Harmon-Jones, 2003). For example, in a study by Harmon-Jones and colleagues (2003), all participants were angered because of an impending tuition increase. However, half of the participants were first led to expect an opportunity to approach the source of the anger and possibly rectify the anger (by signing a petition against the possible tuition increase), whereas the other half of participants were first led to believe that there was nothing they could do to resolve the anger-evoking situation (because the tuition increase had already been approved). Both conditions evoked increases in self-reported anger as compared to baseline, but only participants in the approach action expectation condition showed an increase in relative left frontal activation. This effect suggests that the approach motivational character of anger drives the increase in relative left frontal activation.
It is important to note that in these experiments the removal of the approach motivational opportunity led to a symmetrical frontal activation rather than an increase in right frontal activation. Is it possible for anger to be associated with an increase in right frontal activation? Based on the motivational direction model of asymmetrical frontal cortical activity, we would expect that anger may be associated with right frontal activation if the anger evoked withdrawal motivational tendencies. We suspect, however, that anger may be evolutionarily prepared to evoke approach motivation, and it thus may be difficult for anger to activate withdrawal motivation. Indeed, research with infants (Lewis, Sullivan, Ramsay, & Alessandri, 1992) and non-human animals (Blanchard & Blanchard, 1984) suggests that anger is predominantly associated with approach motivational tendencies.
Experimental efforts to investigate the anger-withdrawal association have underscored this difficulty. For example, Wacker, Heldmann, and Stemmler (2003) conducted an experiment in which soccer players were instructed to imagine that they were unfairly prevented from playing a soccer game by the coach. In the anger-approach condition, the participants imagined approaching the coach and protesting, whereas in the anger-withdrawal condition, they imagined backing out of the locker room and swearing silently at the coach. Results revealed that while both conditions evoked self-reported anger, they did not differ from one another in relative left frontal activation.
The elusiveness of the anger-withdrawal association may be because only some individuals have learned to associate feelings of anger with withdrawal motivation. This speculation has received some support; individuals higher in anger-control, the tendency to manage anger with active coping strategies, show relative right frontal cortical activity at rest compared to those high in anger-out, the tendency to openly express angry feelings (Hewig et al., 2004). However, these results do not directly support the existence of an anger-withdrawal relationship, because anger control can occur via strategies other than withdrawing from the angering situation.
Despite the lack of empirical evidence to date, the idea that anger can be associated with withdrawal motivational tendencies does hold some intuitive appeal (indeed, several reviewers of our past anger research have suggested this as a possibility). By examining responses to a situation that evokes anger and punishment concerns among some individuals (which may lead to a desire to withdraw), we may be better positioned to observe a relationship between feelings of anger and relative right frontal activation. For example, if the expression of anger is perceived to be socially inappropriate, some individuals may wish to withdraw from the social context rather than act on their anger.
To test these ideas, we needed a social context in which the experience of anger would be considered socially inappropriate. Given the strong norms encouraging political correctness and discouraging public expressions of racial prejudice (e.g., Plant & Devine, 1998), we believe a situation in which people must conform to politically correct (PC) pressure may provide a context for exploring the anger-withdrawal relationship. Specifically, research suggests that some people feel anger when required to comply with PC pressure to respond without racial prejudice (Plant & Devine, 2001). One instantiation of conforming to PC pressure would be an interracial interaction in which the PC standards are made relatively salient. In this situation, displaying anger would be perceived as socially inappropriate. To be clear, we do not expect that this type of situation will make all individuals angry; in fact, previous work has found that many people personally agree with egalitarian norms, and thus do not feel anger when conforming to PC pressure (Plant & Devine, 2001). However, to the extent that a given person feels angry in this type of situation, we believe it is likely that s/he will be motivated to withdraw from the interaction so as to avoid potential disapproval from others (Plant & Devine, 1998), and thus anger will be associated with relative right frontal cortical activity.
It is possible that anger felt in any social interaction would be perceived as socially inappropriate and thus, would be associated with the desire to withdrawal from the interaction. However, as a first test of the idea that anger may be associated with withdrawal motivation, we wanted to create the strongest possible situation in which this relationship could emerge. Research using self-reports has already provided evidence that interracial interactions would meet this criterion. Specifically, in an anticipated interaction study, Plant and Devine (2003) found that the degree of anger individuals felt about having an upcoming interaction with a Black person was strongly and positively correlated with a desire to avoid the interaction (r = .83). This finding provides evidence that, at least on self-report measures, feelings of anger and the desire to withdraw may be related when individuals are exposed to PC pressure associated with interracial interactions.
The goal of this study was to examine the relationship between self-reported anger and frontal cortical activity in a situation that had been previously found to evoke self-reported anger and withdrawal motivation for some individuals (i.e., conforming to PC pressure in an interracial interaction). Using a procedure similar to that used in Plant and Devine (2003), White participants were led to believe they were going to interact with a Black participant. To increase the likelihood that some participants would experience anger, the study rationale heightened PC pressure by emphasizing the importance of harmonious interracial interactions in today’s increasingly diverse society. After learning they were going to interact with a Black person, participants’ frontal cortical activity was assessed as they “mentally prepared” for the interaction. Immediately before the interaction was ostensibly about to take place, participants self-reported their affect (including anger) about the upcoming interaction. We hypothesized that, in this context, individuals’ anger would be related to the motivation to withdraw from the interaction altogether, as measured by relative right frontal cortical activity.
We also measured participants’ skin conductance level as an index of sympathetic nervous system (SNS) arousal. We predicted that individuals who were experiencing higher levels of negative affect (i.e., anger and anxiety) would have higher levels of SNS arousal. Finally, we measured the frequency of participants’ spontaneous blinks, as more frequent spontaneous blinking has been linked to attempts to suppress emotion (Gross & Levenson, 1993). Because anger is likely seen as unacceptable in this context, we expected that participants experiencing higher levels of anger would be more likely to suppress their negative emotion, and that this would lead to more frequent spontaneous blinking.
Method
Participants
Sixty-one right-handed introductory psychology, White students (53% female) participated for extra course credit. Right-handed students were selected to avoid physiological differences due to brain laterality.
Procedure
Participants were run individually. They were told that the study involved “brain activity while people are in different kinds of situations”. After providing consent, participants were prepared for EEG and skin conductance recording. Following the attachment of electrodes, baseline EEG was recorded for eight 1-minute epochs, with alternating eyes-open and eyes-closed trials. Next, participants completed baseline self-report affect measures, including items indexing anger (bothered, annoyed, agitated, frustrated, mad, threatened, resentful, hostile, angry, irritated; alpha = .93), anxiety (nervous, uneasy, awkward, anxious, calm, worried, uncomfortable, tense, relaxed, apprehensive; alpha = .75), and positive affect (energetic, confident, active, interested, happy, optimistic, alert, excited, content, enthusiastic, inspired; alpha = .90). These items were compiled based on previous research assessing similar constructs (Harmon-Jones & Sigelman, 2001; Plant & Devine, 2001, 2003). Participants indicated the extent to which they were experiencing each feeling on a scale from 1 (does not apply at all) to 7 (applies very much).
Participants were then given information about the ostensible upcoming interaction. They were told:
Because diversity is such an important issue, both on campus and in the real world, our lab is interested in intergroup interactions between college students, and the physiological activity associated with these interactions. Given the increasingly diverse society that we live in, we’re interested in how people who are different from one another, like in race, gender, social class, get along. We think this may be a really important issue to help promote harmonious intergroup relations.
Participants were then informed that they would be talking with a Black student (matched for the participant’s gender), and were given their partner’s name (ostensibly to ensure the two were not previously acquainted). The experimenter explained that the interaction would take place in a nearby room that was equipped to collect physiological data from two participants simultaneously. Participants were told that during the interaction they should try to get to know their partner, and that following the interaction each participant would be taken back to his/her own room to fill out questionnaires regarding his/her impressions of the partner and the interaction generally. Finally, participants were told that before the interaction began, they would have two minutes to “mentally prepare”. During this time, participants were told that they should think about what they would like to discuss with their partner and how they thought the interaction would unfold.
The experimenter then cued participants to begin their mental preparation for the interaction. EEG and skin conductance were recorded during these two minutes. Immediately afterwards, participants completed a brief questionnaire packet, which included a questionnaire to assess anger (alpha = .92), anxiety (alpha = .93), and positive affect (alpha = .91) related to the upcoming interaction. Although this questionnaire included the same items as the baseline affect questionnaire, the order of items was altered. Upon completion of the questionnaire packet, participants were informed that the interaction would not take place and were orally debriefed. Participants also provided a rating of the degree to which they had believed they were going to have an interaction on a scale from 1 (not at all) to 7 (very much).
EEG Recording and Processing
EEG was recorded from 27 (22 homologous and 5 midline) tin electrodes mounted in a strech-lycra cap (ElectroCap, Eaton, OH), with a left-earlobe reference and a midline ground. All sites were abraded until the impedance was under 5,000 Ω (1,000 Ω for homologous sites). Electro-gel was used as the conductive medium. Frequencies from .05 to 100 Hz (60-Hz notch filter enabled) were digitized at 2500 Hz using Synamps acquisition hardware (Neuroscan Labs, Sterling, VA). Off-line, EEG was re-referenced to average earlobes, scored for movement artifact, and portions of data that included blinks were removed. All artifact-free epochs that were 2.048 s in duration were extracted through a Hamming window; contiguous epochs overlapped by 75%. An average of 630.59 artifact-free epochs (SD = 147.65) composed the resting baseline data, and an average of 118.72 artifact-free epochs (SD = 69.18) composed the experimental data.
A fast Fourier transform was used to calculate power in the alpha (8–13 Hz) frequency range across all epochs. Power values were log transformed to normalize the distributions. Asymmetry indices (log-right minus log-left alpha power) were computed. Asymmetry indices from three pairs of frontal sites (FP1/FP2; F3/F4; F7/F8) were averaged together. Because alpha power is inversely related to cortical activity, higher scores on the index indicate greater relative left-hemisphere cortical activity.
Skin Conductance and Spontaneous Blink Recording and Processing
Skin conductance level was continuously measured over the last two 1-minute epochs of the baseline period, and the entire two-minute anticipation period. Ag/AgCl electrodes were placed on the distal phalanges of the first and second fingers of the left hand, and a Contact Precision Instruments skin conductance module (SC5) was used to provide a constant voltage of .5V across the electrodes.
The number of spontaneous blinks was obtained by inspecting physiological data from the VEOG (vertical eye movement) channel off-line. Two independent judges counted the number of blinks across the four eyes-open minutes of the baseline period and the two-minute anticipation period. Inconsistencies were resolved through discussion.
Results
Overall, participants reported strong believability that the interaction would take place (M = 6.53, SD = 0.71, on a 7-point scale) and this believability did not vary as a function of gender, experimenter, or their interaction (all ps > .11). However, we thought it was critical that participants truly believe that the interaction was going to take place and thus, we eliminated three participants who scored below the midpoint of the scale. In addition, four participants had incomplete data sets, leaving a total of 54 - 58 participants (50% female)1. None of the self-reported affective or physiological measures varied as a function of gender.
Correlational analyses
To test our key hypotheses, we created indices that reflected the given construct during the pre-interaction phase, controlling for baseline levels of that construct, as recommended by Cohen, Cohen, West, and Aiken (2003)2. Thus, for each self-report measure, we created a new variable that reflected levels of the construct immediately prior to the interaction controlling for levels of that construct at baseline. For each physiological measure, we created a new variable that reflected levels of the construct during the two-minute mental preparation period, controlling for levels of that construct during the baseline recording. Then, we examined correlations between these residualized variables.
Correlations between self-report indices.
As seen in Table 1, residualized anger and residualized anxiety were positively correlated, r(55) = .53, p < .001, such that those participants who reported elevated levels of anger also reported higher levels of anxiety. Residualized positive affect was negatively correlated with residualized anger, r(55) = −.27, p = .04, and residualized anxiety, r(55) = −.28, p = .03, such that those participants who reported elevated levels of positive affect reported less anger and less anxiety (Table 1 about here).
Table 1.
Correlations Between Self-Report and Physiological Indices.
| Res. Anger |
Res. Anxiety |
Res. Positive Affect |
Res. Frontal Asymmetry |
Res. SCL |
Res. Blink Frequency |
|
|---|---|---|---|---|---|---|
| Residualized Anger | -- | |||||
| Residualized Anxiety | .534** | -- | ||||
| Residualized Positive Affect | −.273* | −.281* | -- | |||
| Residualized Frontal Asymmetry | −.373** | −.048 | −.045 | -- | ||
| Residualized SCL | .387** | .209 | −.153 | −.418** | -- | |
| Residualized Blink Frequency | .370** | .286* | −.009 | −.175 | .099 | -- |
| Residualized Parietal Asymmetry | −.154 | −.051 | −.073 | .441** | −.186 | −.095 |
Ns = 54 - 58
p < .05
p < .01
Correlations between physiological indices.
As seen in Table 1, residualized frontal asymmetry was negatively correlated with residualized skin conductance level, r(53) = −.42, p = .001, such that those participants who showed more right compared to left frontal activation were more aroused. Residualized spontaneous blink frequency was unrelated to residualized frontal asymmetry and residualized skin conductance level, ps > .18.
Correlations between self-report and physiological indices.
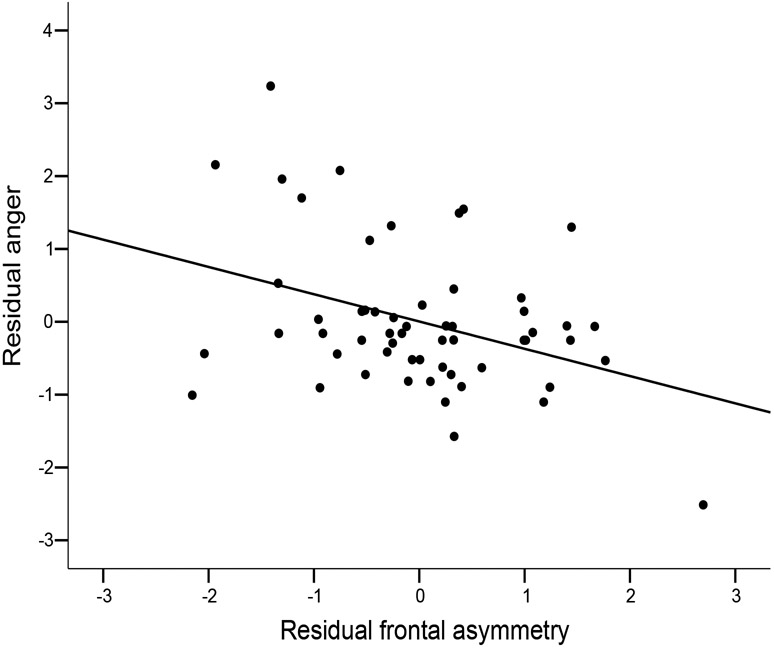
Consistent with our key prediction, residualized anger was negatively correlated with residualized frontal asymmetry, r(55) = −.37, p = .004, such that those participants who experienced greater anger showed more right relative to left frontal cortical activity (see Figure 1). Independent levels of residualized left frontal cortical activity and residualized right frontal cortical activity were not correlated with residualized anger (ps >.68), indicating that frontal asymmetry is the critical index (Harmon-Jones et al., 2002). Also, residualized parietal asymmetrical activity (P3/P4) was not correlated with residualized anger, anxiety, or positive affect (ps > .25) (Figure 1 about here).
Figure 1. Relationship between residualized self-reported anger and residualized frontal asymmetry.
Note. Greater positive values indicate greater relative left frontal cortical activity.
Residualized anger was also positively correlated with residualized skin conductance level, r(52) = .39, p = .004, suggesting that participants who reported heightened anger were more aroused. In addition, residualized anger, r(55) = .37, p = .005, and residualized anxiety, r(55) = .29, p = .03, were positively correlated with residualized spontaneous blink frequency, suggesting that those participants who reported elevated levels of negative affect evidenced more spontaneous blinks and perhaps a greater desire to suppress their negative emotions. No other correlations were significant, ps > .13.
Although the zero-order correlations supported our hypothesis, we wanted to investigate the independent effect of anger on frontal asymmetry. This is important because residualized anger and anxiety were strongly correlated, and anxiety has been linked to relative right frontal cortical activity during anticipation of an interpersonal situation (Davidson, Marshall, Tomarken, & Henriques, 2000). In addition, residualized skin conductance level was strongly correlated with anger and frontal asymmetry. Thus, we conducted a regression analysis simultaneously predicting residualized frontal asymmetry from residualized skin conductance level, residualized anxiety, and residualized anger. Consistent with predictions, residualized anger was an independent predictor of residualized frontal asymmetry (ß = −.37, p = .02). Residualized skin conductance was also an independent predictor of residualized frontal asymmetry (ß = −.30, p = .03), whereas residualized anxiety was not (ß = .13, p = .38). Including parietal asymmetry in the analysis did not change this pattern of significance.3
Discussion
Although anger is primarily associated with an approach motivational state, the current study explored the possibility that certain individuals in particular contexts may experience anger accompanied by withdrawal motivation. We hypothesized that because of societal pressure to behave in a PC manner, feelings of anger regarding an interracial interaction would be associated with a desire to withdraw from the situation (Plant & Devine, 2003). Consistent with this hypothesis, the current study found that in anticipation of an interracial interaction, self-reported feelings of anger correlated with relative right frontal cortical activity, a physiological indicator of withdrawal motivation. Self-reported anger also correlated with skin conductance level, suggesting that participants who experienced more anger also were more aroused. Finally, self-reported anger and anxiety related to spontaneous blink frequency, which has been linked to emotion suppression efforts (Gross & Levenson, 1993). It is likely that in this situation, participants attempted to suppress their negative feelings because they knew these emotions would be seen by others as inappropriate.
As this was a first step in establishing a relationship between anger and withdrawal motivation (assessed via frontal asymmetries in cortical activity), we wanted to create the strongest possible situation in which this relationship could emerge. Thus, we created a situation in which the expression of anger would be considered socially inappropriate: an interracial interaction. While we speculate that the interracial nature of the interaction led to the anger-withdrawal relationship, it is possible that any constrained social interaction may produce a similar relationship. Indeed, future research could experimentally manipulate the situational context in order to clearly delineate which aspects of the situation lead to anger withdrawal.
It is important to note that the motivational model is not the only model of frontal cortical asymmetry. Recent work based on Gray’s (1994) model of behavioral approach and inhibition has linked the Behavioral Activation System (BAS; mediates approach behavior) to relative left frontal cortical activity, and the Behavioral Inhibition System (BIS; mediates goal conflict) to relative right frontal cortical activity (Wacker et al., 2003). Our data are somewhat consistent with this model, as participants who experienced high levels of anger may also be those participants who experienced goal conflict (because they did not want to comply with PC pressure, but thought they would receive punishment from others if they responded with prejudice) and thus relative right frontal activity. However, according to this model, we would also expect anxiety (stemming from the goal conflict) to be related to relative right frontal cortical activity, but this relationship is not present in our data. More work is needed to fully understand and separate these two models.
Researchers are beginning to investigate how affective dimensions as well as specific emotions are associated with various motivational orientations (Harmon-Jones et al., 2003; Hewig et al., 2004; Wacker et al., 2003). Although specific emotions are generally tied to particular motivational tendencies, the current study suggests that these motivational correlates of an emotion may be partially determined by the specific attributes of the person and social context in which the emotion occurs. Person and contextual factors have not received their due attention in emotion research; indeed, this may be part of the reason that identifying unique physiological markers for the basic emotions has been elusive (Lang, Bradley & Cuthbert, 1990). By considering emotions as a function of the person and context, rather than in a vacuum, researchers will be better positioned to understand emotion and motivation, as well as their neural and physiological underpinnings.
Acknowledgements
This research was supported in part by a grant from the National Science Foundation (BCS-0350435) and by National Institute of Mental Health Emotion Training Grant T32-MH18931-14.
We gratefully acknowledge the assistance of Jed Rosenkrantz, Naomi Cigan, and Vivenne Yeh in collecting the data reported in this article.
Footnotes
Analyses including all 61 participants revealed an identical pattern of results.
Because change scores can produce misleading results, Cohen et al. (2003) recommend using residualized scores. However, the pattern of results was identical using change scores (pre-interaction - baseline).
From baseline (M = 1.98, SD = 0.96) to pre-interaction (M = 1.52, SD = 0.70), participants showed a significant decrease in anger, t(56) = −4.95, p < .001, d = .66, but a significant increase in anxiety (baseline: M = 2.92, SD = 0.87; pre-interaction: M = 3.23, SD = 1.20), t(56) = 2.24, p = .03, d = .30, and positive affect (baseline: M = 3.89, SD = 1.00; pre-interaction: M = 4.12, SD = 0.94), t(56) = 1.98, p = .05, d = .26. Participants showed a marginally significant decrease in relative left frontal cortical activity (baseline: M = .0152, SD = .0425; pre-interaction: M = .0069, SD = .0433), t(57) = −1.88, p = .065, d = .25, and a significant increase in skin conductance level (Baseline: M = 3.29, SD = 2.02; pre-interaction: M = 4.65, SD = 1.85), t(54) = 9.22, p < .001, d = 1.24 and spontaneous blinks per minute (baseline: M = 7.81, SD = 5.92; pre-interaction: M = 13.80, SD = 10.23), t(57) = 6.71, p < .001, d = .89. It is important to note that although mean levels of anger decreased over time, some individuals showed more anger in the pre-interaction compared to baseline, and it is these individuals who also experienced more relative right-frontal cortical activity. This was consistent with our expectation that this situation would not elicit anger in all individuals, as complying with PC pressure is compatible with many people’s egalitarian values.
Contributor Information
Leah R. Zinner, University of Wisconsin, Madison
Amanda B. Brodish, University of Wisconsin, Madison
Patricia G. Devine, University of Wisconsin, Madison
Eddie Harmon-Jones, Texas A & M University.
References
- Blanchard DC, & Blanchard RJ (1984). Affect and aggression: An animal model applied to human behavior. Advances in the study of aggression, 1, 1–62. [Google Scholar]
- Carver CS (2004). Negative affects deriving from the behavioral approach system. Emotion, 4, 3–22. [DOI] [PubMed] [Google Scholar]
- Coan JA, & Allen JJB (2004). Frontal EEG asymmetry as a moderator and mediator of emotion. Biological Psychology, 67, 7–49. [DOI] [PubMed] [Google Scholar]
- Cohen J, Cohen P, West SG, & Aiken LS (2003). Applied multiple regression/correlation analysis for the behavioral sciences, 3rd edition. [Google Scholar]
- Davidson RJ, Marshall JR, Tomarken AJ & Henriques JB (2000). While a phobic waits: Regional brain electrical and autonomic activity in social phobics during anticipation of public speaking. Biological Psychiatry, 47, 85–95. [DOI] [PubMed] [Google Scholar]
- Gray JA (1994). Three fundamental emotion systems. In Ekman P & Davidison RJ (Eds.), The nature of emotion: Fundamental questions (pp. 243–247). New York: Oxford University Press. [Google Scholar]
- Gross JJ & Levenson RW (1993). Emotional suppression: Physiology, self-report, and expressive behavior. Journal of Personality and Social Psychology, 64, 970–986. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E (2003a). Anger and the behavioral approach system. Personality and Individual Differences, 35, 995–1005. [Google Scholar]
- Harmon-Jones E (2003b). Clarifying the emotive functions of asymmetrical frontal cortical activity. Psychophysiology, 40, 838–848. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E (in press). Trait anger predicts relative left frontal cortical activation to anger-inducing stimuli. International Journal of Psychophysiology. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E (2004). On the relationship of frontal brain activity and anger: Examining the role of attitude toward anger. Cognition & Emotion, 18, 337–361. [Google Scholar]
- Harmon-Jones E, Abramson LY, Sigelman J, Bohlig A, Hogan ME, & Harmon-Jones C (2002). Proneness to hypomania/mania symptoms or depression symptoms and asymmetrical frontal cortical responses to an anger-evoking event. Journal of Personality and Social Psychology, 82, 610–618. [PubMed] [Google Scholar]
- Harmon-Jones E, & Allen JJB (1998). Anger and frontal brain activity: EEG asymmetry consistent with approach motivation despite negative affective valence. Journal of Personality and Social Psychology, 74, 1310–1316. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E, Lueck L, Fearn M, & Harmon-Jones C (2006). The effect of personal relevance and approach-related action expectation on relative left frontal cortical activity. Psychological Science, 17, 434–440. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E, & Sigelman J (2001). State anger and prefrontal brain activity: Evidence that insult-related relative left-prefrontal activation is associated with experienced anger and aggression. Journal of Personality and Social Psychology, 80, 797–803. [PubMed] [Google Scholar]
- Harmon-Jones E, Sigelman JD, Bohlig A, & Harmon-Jones C (2003). Anger, coping, and frontal cortical activity: The effect of coping potential on anger-induced left frontal activity. Cognition and Emotion, 17, 1–24. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E, Vaughn-Scott K, Mohr S, Sigelman J, & Harmon-Jones C (2004). The effect of manipulated sympathy and anger on left and right frontal cortical activity. Emotion, 4, 95–101. [DOI] [PubMed] [Google Scholar]
- Hewig J, Hagemann D, Seifert J, Naumann E, & Bartussek D (2004). On the selective relation of frontal cortical asymmetry and anger-out versus anger-control. Journal of Personality and Social Psychology, 87, 926–939. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, & Cuthbert BN (1990). Emotion, attention, and the startle reflex. Psychological Review, 97, 377–395. [PubMed] [Google Scholar]
- Lewis M, Sullivan MW, Ramsay DS, & Alessandri SM (1992). Individual differences in anger and sad expressions during extinction: Antecedents and consequences. Infant Behavior & Development, 15, 443–452. [Google Scholar]
- Plant EA, & Devine PG (1998). Internal and external motivation to respond without prejudice. Journal of Personality and Social Psychology, 75, 811–832. [DOI] [PubMed] [Google Scholar]
- Plant EA, & Devine PG (2001). Responses to other-imposed pro-Black pressure: Acceptance or backlash. Journal of Experimental Social Psychology, 37, 486–501. [Google Scholar]
- Plant EA, & Devine PG (2003). The antecedents and implications of interracial anxiety. Personality and Social Psychology Bulletin, 29, 790–801. [DOI] [PubMed] [Google Scholar]
- Wacker J, Heldmann M, Stemmler G (2003). Separating emotion and motivational direction in fear and anger: Effects on frontal asymmetry. Emotion, 3, 167–193. [DOI] [PubMed] [Google Scholar]
- van Honk J & Schutter DJLG (2006). From affective valence to motivational direction. Psychological Science, 17, 963–965. [DOI] [PubMed] [Google Scholar]