Abstract
Small bowel adenocarcinoma (SBA) is a rare malignancy of the gastrointestinal tract that has increased in incidence across recent years. Often diagnosed at an advanced stage, outcomes for SBA are worse on average than for other related malignancies, including colorectal cancer. Due to the rarity of this disease, few studies have been done to direct optimal treatment, although recent data have shown that SBA responds to treatment differently than colorectal cancer, necessitating a separate approach to treatment. The NCCN Guidelines for Small Bowel Adenocarcinoma were created to establish an evidence-based standard of care for patients with SBA. These guidelines provide recommendations on the workup of suspected SBA, primary treatment options, adjuvant treatment, surveillance, and systemic therapy for metastatic disease. Additionally, principles of imaging and endoscopy, pathologic review, surgery, radiation therapy, and survivorship are described.
Overview
In 2019, an estimated 10,590 new cases of small bowel cancer will occur and 1,590 patients will die of this disease.1 Compared with cancers of other organs in the gastrointestinal tract, small bowel cancers are relatively rare, accounting for only about 3% of cancers occurring in this organ system.1 Small bowel cancers affect men and women relatively equally, with an incidence of 2.6 per 100,000 for men and 2.0 per 100,000 for women.2 The median age at diagnosis is 66 years. The incidence of small bowel cancers is increasing, with an annual percent increase of 1.8 between 2006 and 2015. This trend is in contrast to other gastrointestinal malignancies, including esophageal, gastric, colon, and rectum, which decreased in incidence across the same timeframe.2 The 4 most common histologies of cancers originating in the small bowel are adenocarcinomas, neuroendocrine tumors, gastrointestinal stromal tumors, and lymphomas.3,4 The treatment recommendations in this guideline only refer to small bowel adenocarcinoma (SBA), which comprise an estimated 30% to 40% incidence of small intestinal cancer diagnoses.4 Due to the rarity of this disease, very few established guidelines for management of SBA exist. In 2018, a French intergroup published the first clinical practice guidelines for SBA.5 These NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) for Small Bowel Adenocarcinoma are the second.
This discussion summarizes the NCCN Guidelines for SBA. These guidelines begin with the clinical presentation of the patient to the primary care physician or gastroenterologist and address diagnosis, pathologic staging, surgical management, perioperative treatment, patient surveillance, management of recurrent and metastatic disease, and survivorship. When reviewing these guidelines, clinicians should be aware of several things. First, these guidelines adhere to the TNM (tumor, node, metastases) staging system (see definitions of TNM in Table 1, available online, in these guidelines, at NCCN.org).6 Furthermore, all recommendations are classified as category 2A except where noted in the text or algorithm. Although the guidelines are believed to represent the optimal treatment strategy, participation in a clinical trial is especially encouraged for patients with SBA based on the dearth of clinical trial data on which to base treatment decisions for this disease.
Individual Disclosures for the NCCN Small Bowel Adenocarcinoma Panel
| Panel Member | Clinical Research Support/Data Safety Monitoring Board | Scientific Advisory Boards, Consultant, or Expert Witness | Promotional Advisory Boards, Consultant, or Speakers Bureau | Specialties |
|---|---|---|---|---|
| Mahmoud M. Al-Hawary, MD | None | None | None | Diagnostic/Interventional Radiology |
| Mustafa A. Arain, MD | None | None | Boston Scientific Corporation | Gastroenterology |
| Al B. Benson III, MD | Acerta Pharma, LLC; Amgen Inc.; Astellas Pharma US, Inc.; Bristol-Myers Squibb Company; Celgene Corporation; Infinity Pharmaceuticals, Inc.; MedImmune, Inc.; Merck & Co., Inc.; Novartis Pharmaceuticals Corporation; and Taiho Pharmaceuticals Co., Ltd. | Astellas Pharma US, Inc.; Bayer HealthCare; Bristol-Myers Squibb Company; Eli Lilly and Company; Exelixis Inc.; Genentech, Inc.; Merck & Co., Inc.; Purdue Pharma LP; and Taiho Pharmaceuticals Co., Ltd. | None | Medical Oncology |
| Yi-Jen Chen, MD, PhD | None | None | None | Radiotherapy/Radiation Oncology |
| Kristen K. Ciombor, MD | AbbVie, Inc.; Amgen Inc.; Array Biopharma Inc.; Bayer HealthCare; Bristol-Myers Squibb Company; Daiichi Sankyo Co.; Incyte Corporation; Merck & Co., Inc.; National Cancer Institute; NuCana Plc; Pfizer Inc.; and sanofi-aventis U.S. LLC | Bayer HealthCare, and Taiho Parmaceuticals Co., Ltd. | Foundation Medicine | Medical Oncology |
| Stacey A. Cohen, MD | Taiho Parmaceuticals Co., Ltd. | None | None | Medical Oncology |
| Harry S. Cooper, MD | None | None | None | Pathology |
| Dustin A. Deming, MD | Bristol-Myers Squibb Company; Genentech, Inc.; and Merck & Co., Inc. | Array Biopharma Inc.; Bayer HealthCare; Bristol-Myers Squibb Company; Genentech, Inc.; Ipsen; Novocure; and Taiho Pharmaceuticals Co., Ltd. | None | Medical Oncology |
| Ignacio Garrido-Laguna, MD, PhD | Agios, Inc.; Bayer HealthCare; Bristol-Myers Squibb Company; Eli Lilly and Company; Flatiron Health, Inc.; Halozyme, Inc.; Incyte Corporation; MedImmune, Inc.; Novartis Pharmaceuticals Corporation; OncoMed Pharmaceuticals; Inc.; Pfizer Inc.; and Taiho Pharmaceuticals Co., Ltd. |
Array Biopharma Inc. | None | Medical Oncology |
| Jean L. Grem, MD | Elion Oncology, and ICON plc | None | None | Medical Oncology |
| Sarah E. Hoffe, MD | Varian Medical Systems, Inc. | None | None | Radiotherapy/Radiation Oncology |
| Joleen Hubbard, MD | Bayer HealthCare; Boston Biomedical, Inc.; Incyte Corporation; Medpace; Merck & Co., Inc.; Senhwa Pharmaceuticals; Taiho Pharmaceuticals Co., Ltd.; and Treos Bio | Bayer HealthCare | None | Hematology/Hematology Oncology |
| Steven Hunt, MD | None | None | None | Surgery/Surgical Oncology |
| Ahmed Kamel, MD | Boston Scientific Corporation | Boston Scientific Corporation, and Sirtex Medical | Bard Peripheral Vascular, Inc., and Boston Scientific Corporation | Diagnostic/Interventional Radiology |
| Natalie Kirilcuk, MD | None | None | None | Surgery/Surgical Oncology |
| Smitha Krishnamurthi, MD | AbbVie, Inc. | None | None | Medical Oncology, and Internal Medicine |
| Wells A. Messersmith, MD | Aduro Biotech, Inc.; ALX Oncology; D3 Pharma; Genentech, Inc.; Immunomedics, Inc.; Incyte Corporation; Pfizer Inc.; Roche Laboratories, Inc.; and Tanabe Research Labs USA Inc. | Five Prime Therapeutics, Inc., and Gilead Sciences, Inc. | None | Medical Oncology |
| Jeffrey Meyerhardt, MD, MPH | Cota Healthcare | Taiho Pharmaceuticals Co., Ltd. | None | Medical Oncology |
| Eric D. Miller, MD, PhD | None | None | None | Radiotherapy/Radiation Oncology |
| Mary F. Mulcahy, MD | None | None | None | Hematology/Hematology Oncology, and Medical Oncology |
| Steven Nurkin, MD, MS | None | None | None | Surgery/Surgical Oncology |
| Michael J. Overman, MD | Bristol-Myers Squibb Company; MedImmune, Inc.; Merck & Co., Inc.; Nektar Therapeutics; and Roche Laboratories, Inc. | Bristol-Myers Squibb Company; MedImmune, Inc.; Novartis Pharmaceuticals Corporation; and Roche Laboratories, Inc. | None | Medical Oncology, and Hematology/ Hematology Oncology |
| Aparna Parikh, MD | Array Biopharma Inc.; Bristol-Myers Squibb Company; Guardant Health Inc.; Novartis Pharmaceuticals Corporation; Plexxikon Inc.; and TESARO, Inc. | Foundation Medicine, and Puretech Health | None | Medical Oncology |
| Hitendra Patel, MD | Bristol-Myers Squibb Company, and MedImmune, Inc. | None | None | Medical Oncology |
| Katrina S. Pedersen, MD, MS | Merck & Co., Inc. | None | None | Medical Oncology |
| Leonard B. Saltz, MD | Taiho Pharmaceuticals Co., Ltd. | None | None | Medical Oncology; Hematology/ Hematology Oncology; and Internal Medicine |
| Charles Schneider, MD | None | None | None | Medical Oncology |
| David Shibata, MD | None | None | None | Surgery/Surgical Oncology |
| John M. Skibber, MD | None | None | None | Surgery/Surgical Oncology |
| Constantinos T. Sofocleous, MD, PhD | Ethicon, Inc. | Ethicon, Inc., and Terumo Corporation | Ethicon, Inc. | Diagnostic/Interventional Radiology |
| Elena M. Stoffel MD, MPH | None | None | None | Gastroenterology |
| Eden Stotsky-Himelfarb, BSN, RN | None | None | None | Patient Advocate |
| Alan P. Venook, MD | Halozyme, Inc., and Pierre Fabre | Genentech, Inc., and Roche Laboratories, Inc. | None | Medical Oncology, and Hematology/ Hematology Oncology |
| Christopher G. Willett, MD | None | None | None | Radiotherapy/Radiation Oncology |
The NCCN Guidelines Staff have no conflicts to disclose.
Literature Search Criteria and Guidelines Update Methodology
Before the development of the NCCN Guidelines for SBA, an electronic search of the PubMed database was performed to obtain key literature in the field of small bowel cancer, using the following search terms: (small bowel cancer) OR (small intestine cancer) OR (jejunum cancer) OR (duodenum cancer) OR (ileum cancer). The PubMed database was chosen because it remains the most widely used resource for medical literature and indexes only peer-reviewed biomedical literature.7 The search results were narrowed by selecting studies in humans published in English. Results were confined to the following article types: Clinical Trial; Multicenter Study; Practice Guideline; Randomized Controlled Trial; Meta-Analysis; Systematic Reviews; and Validation Studies.
The data from key PubMed articles and articles from additional sources deemed as relevant to these guidelines and discussed by the panel have been included in this version of the discussion section (eg, e-publications ahead of print, meeting abstracts). Recommendations for which high-level evidence is lacking are based on the panel’s review of lower-level evidence and expert opinion.
The complete details of the “Development and Update of the NCCN Guidelines” are available at NCCN.org.
Risk Factors for SBA
Risk factors for SBA are similar to those for colorectal cancer (CRC), including lifestyle factors, inflammatory bowel disease (IBD), and certain familial syndromes such as Lynch syndrome (also known as hereditary nonpolyposis colorectal cancer), Peutz-Jeghers syndrome (PJS), and familial adenomatous polyposis (FAP). Therefore, it is recommended that all patients with small bowel cancer be queried regarding their family history and considered for risk assessment, as detailed in the NCCN Guidelines for Colorectal Cancer Screening (available at NCCN.org).
Lifestyle Factors
Although data on the role of lifestyle factors in relation to the risk of developing SBA are very limited due to low incidence of disease, lifestyle factors that have been reported as raising the risk of SBA generally agree with known risk factors for CRC. A systematic review of the literature has reported that high levels of alcohol consumption, smoking, and dietary factors, including low intake of fiber and high intake of red/processed meat and sugary drinks, may increase the risk of SBA.8 Additionally, the results of a pooled analysis of more than 500,000 subjects in the Asia Cohort Consortium reported that elevated body mass index and high alcohol consumption were associated with a nonsignificant trend toward an increased risk of SBA, although this analysis did not identify smoking as a risk factor.9
IBD and Celiac Disease
It is well recognized that individuals with IBD (ulcerative colitis or Crohn’s disease) are at increased risk for CRC. Several studies have also reported an increased risk of distal SBA in patients with IBD.10–13 The results of a retrospective, multicenter observational cohort study of 9,100 patients with IBD found that the relative risk of small bowel cancer was 3.70 (95% CI, 1.23–11.13) for patients with IBD. The rate of death and cancer remission did not differ between patients who maintained IBD treatment compared with those who stopped IBD treatment.10 Additionally, although the data are mostly limited to case studies and literature reviews, cases of SBA have been reported in patients with celiac disease, suggesting a possible link between these conditions.14–16 The association with celiac disease is poorly understood and a distinct difference from CRC, for which celiac disease is not a risk factor.
Familial Syndromes
Due to the relative rarity of SBA and the disease’s association with several genetic syndromes, the NCCN panel recommends that all patients with a personal history of SBA should be counseled for familial malignancies and considered for risk assessment of various genetic syndromes, including Lynch syndrome, PJS, FAP, and other polypoid mutations. A brief description of some of these syndromes is included in subsequent sections. See the NCCN Guidelines for Genetic/Familial High-Risk Assessment: Colorectal (at NCCN.org) for more information.
Familial Adenomatous Polyposis
FAP is an autosomal dominant condition characterized by a germline mutation in the APC gene, located on chromosome 5q21.17,18 Patients with FAP develop large numbers of adenomatous polyps in the large bowel, beginning as early as adolescence, and most patients with classic FAP will develop polyps by the age of 25. Patients with attenuated FAP due to a germline MUTYH mutation develop fewer numbers of polyps and generally at a later age than those with classic FAP.17,18 Although the incidence of SBA in patients with FAP has not been well established, the lifetime risk has been estimated as 3% to 5%.19 The duodenum and periampullary region are the most common locations for SB Ain patients with FAP.19
Peutz-Jeghers Syndrome
PJS is an autosomal dominant condition mainly characterized by multiple hamartomatous and adenomatous gastrointestinal polyps, predominantly located in the jejunum and ileum.19,20 A majority of PJS cases occur due to mutations in the STK11 (LKB1) gene.21,22 However, other genetic mutations may be involved, because an estimated half of patients with PJS do not have detectable STK11/LKB1 mutations.23 SBA can arise from either hamartomatous or adenomatous polyps, predisposing patients with PJS to SBA. The relative risk of developing SBA has been estimated as 520 compared with the general population,24 and the lifetime risk of SBA has been estimated between 1.7% and 13% for individuals with PJS.19,24,25
Lynch Syndrome
Lynch syndrome is a hereditary syndrome resulting from germline mutations in DNA mismatch repair (MMR) genes (MLH1, MSH2, MSH6, and PMS2). Individuals with Lynch syndrome are estimated to have a lifetime risk of 4% of developing SBA, representing a relative risk of more than 100 compared with the general population.26,27 Although identifying a mutation in an MMR gene through germline sequencing is definitive for Lynch syndrome, patients usually undergo selection for screening by considering family history and performing an initial test on tumor tissue before sequencing. One of two (or both) different initial tests can be performed on SBA specimens to identify individuals who might have Lynch syndrome: (1) immunohistochemical analysis for MMR protein expression, which is often diminished because of mutation; or (2) polymerase chain reaction analysis for microsatellite instability (MSI), which results from MMR deficiency and is detected as changes in the length of repetitive DNA elements in tumor tissue caused by the insertion or deletion of repeated units.28
Many NCCN Member Institutions and other comprehensive cancer centers now perform immunohistochemistry (IHC) and sometimes MSI testing on all newly diagnosed CRC and endometrial cancers regardless of family history to determine which patients should have genetic testing for Lynch syndrome.29–32 This approach may also be applied to patients with a personal history of SBA, particularly since it has been reported that SBA has a higher percentage of MSI-high (MSI-H)/ MMR-deficient (dMMR) tumors compared with CRC.33,34 The NCCN Colon/Rectal/Anal Cancers Panel endorses universal MMR or MSI testing of all patients with a personal history of SBA to identify individuals with Lynch syndrome. This testing is also relevant for treatment selection in stage IV disease [see “Pembrolizumab or Nivolumab With or Without Ipilimumab (for dMMR/MSI-H tumors) as Subsequent-line Therapy,” page 1125). A more detailed discussion is available in the NCCN Guidelines for Colorectal Cancer Screening (available at NCCN.org).
Clinical Presentation and Workup
The treatment recommendations in this guideline only refer to SBA. For gastrointestinal stromal tumors, see the NCCN Guidelines for Soft Tissue Sarcoma; for neuroendocrine tumors, see the NCCN Guidelines for Neuroendocrine and Adrenal Tumors; and for small bowel lymphomas see the NCCN Guidelines for B-Cell Lymphomas (all available at NCCN.org).
Most cases of SBA arise in the duodenum, accounting for approximately 52% to 57% of cases. The remainder arise in the jejunum (18%–29%), ileum (10%–13%), or in an unspecified location of the small bowel (4%–14%).35–37 Patients with SBA tend to be younger at diagnosis and often present with a higher stage and grade compared with those with CRC.38 SBA often presents with a local complication of the tumor, most often gastric outlet obstruction in the case of a duodenal SBA or cramping abdominal pain in the case of a jejunal or ileal SBA.35,39,40 Occult gastrointestinal bleeding is another common presentation for SBA, occurring in approximately onequarter to one-third of cases.
Patients who present with small bowel cancer require a complete staging workup, including biopsy (if appropriate), pathologic tissue review, imaging studies (see “Imaging and Endoscopy,” below), complete blood count, chemistry profile, carbohydrate antigen 19–9, and carcinoembryonic antigen. Depending on the tumor’s location and the patient’s history, studies for celiac disease or IBD may be indicated. As discussed previously, MMR or MSI testing is recommended for all patients with SBA because MMR/MSI status can function as a prognostic and/or predictive marker and can help identify patients who should be tested for Lynch syndrome (see “Risk Factors for SBA,” page 1111).
Imaging and Endoscopy
Esophagogastroduodenoscopy with endoscopic ultrasound is recommended during initial workup and staging for detection and pathologic sampling when a duodenal malignancy is suspected. If obstruction is detected during imaging, palliative diversion or stenting may be considered.41 Endoscopic ultrasound is useful for pretherapeutic staging of proximal small bowel malignancies and may be used to discern duodenal lesions from ampullary, biliary, or pancreatic primaries.42
Other endoscopic techniques that are not required for routine staging, but may be useful in certain circumstances, include double balloon endoscopy and capsule endoscopy. A number of studies, both prospective and retrospective, have reported on the effectiveness and safety of double balloon endoscopy for workup of patients with small bowel cancer.43–45 Specifically, the use of this method may be of particular benefit for patients with small bowel strictures.46 Although capsule endoscopy allows for a more detailed examination of the entire small bowel mucosa, possibly resulting in the diagnosis of SBA when other imaging methods have failed to reveal a primary lesion, it is not the preferred method for initial workup due to its inability to biopsy tissue for diagnosis.47–49 In the case of a small bowel obstruction or stricture, the capsule may not be excreted naturally, requiring surgical removal. Therefore, capsule endoscopy is contraindicated for these conditions.41
CT or MRI may be used during initial workup of SBA to evaluate the extent of local tumor invasion and to assess for distant metastases. CT or MR enterography or enteroclysis, techniques involving administration of enteric contrast agent to the gastrointestinal system via oral intake or nasogastric tube, respectively, may improve imaging of the small bowel and, therefore, may be considered when conventional CT or MRI with contrast have failed to show a tumor.50–53 A prospective study comparing CT enterography to MR enterography in 150 patients with suspected small bowel disease, but negative findings on endoscopy, reported that MR enterography was more accurate than CT enterography, particularly for neoplastic diseases (P=.0412).54
Although PET/CT has not been formally evaluated for ability to detect metastatic SBA, or compared with MRI or CT, reports describing its usefulness for this disease have been published.55 Therefore, although PET/CT is not routinely indicated, it may be considered when CT or MR results are equivocal.
See “Posttreatment Surveillance” (page 1128) for the use of these imaging methods for posttreatment surveillance and for use in individuals with IBD, celiac disease, or familial syndromes.
Pathology and Staging
SBA staging is based on the TNM staging system.6 In the 8th edition of the AJCC Staging Manual, T1 tumors involve the lamina propria or submucosa; T2 tumors penetrate through the submucosa into the muscularis propria; T3 tumors penetrate through the muscularis propria into the subserosa or extend into non-peritonealized perimuscular tissue; and T4 tumors perforate the visceral peritoneum or directly invade other organs or structures. Regional lymph node classification includes N0 (no regional lymph node metastasis), N1 (1–2 positive lymph nodes), and N2 (3 or more positive nodes). SBA is classified as M1 when distant metastasis is present.6
SBA is staged as I or II when a tumor is present without regional lymph node or distant metastases (any T, N0, M0). Stage III disease includes disease with regional lymph node, but not distant metastasis (any T, N1–2, M0). Stage IV is distant metastatic disease (any T, any N, M1).6 A number of sources have reported stage III or IV SBA as having significantly worse outcomes compared with earlier stage disease.6,56,57
Other factors that may be useful for prognostication, but not used for staging, include the primary tumor site (ie, duodenum, jejunum, ileum); histologic grade; number of lymph nodes evaluated; margin status; lymphovascular invasion; MSI/MMR status; evidence/presence of celiac or IBD; and presence of polyps.6 The NCCN panel recommends reporting of these parameters during pathologic review.
Lymph Node Evaluation
Regional lymph nodes differ based on the site of the primary tumors: retropancreatic, hepatic artery, inferior pancreaticoduodenal, and superior mesenteric nodes are regional to the duodenum; cecal or ileocolic (terminal ileum only, superior mesenteric, or mesenteric [not otherwise specified]) nodes are regional to the jejunum and ileum.
Multiple analyses of patients with SBA in the SEER database have found that longer survival after resection is strongly associated with a lower ratio of positive-to-negative lymph nodes as well as with a higher number of regional lymph nodes assessed during surgery.38,58–60 Two of these analyses, which considered duodenal and jejunoileal adenocarcinomas separately, concluded that, for adequate staging, a minimum of 5 lymph nodes should be retrieved for duodenal tumors and a minimum of 9 lymph nodes for jejunal or ileal tumors.59,60 Analyses that pooled duodenal and jejunoileal tumors found that 8 regional lymph nodes should be assessed for adequate staging,38,58 although some data have suggested that harvesting even higher numbers of lymph nodes may better predict SBA survival outcomes.61 Based on these studies, NCCN recommends that a goal for all SBA resections should be the retrieval of at least 8 regional lymph nodes for evaluation.
Treatment of Stage I–III Small Bowel Adenocarcinoma
Surgical Management of Localized Resectable Disease
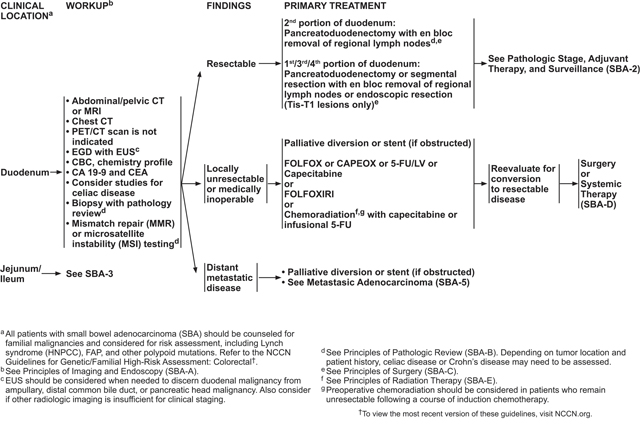
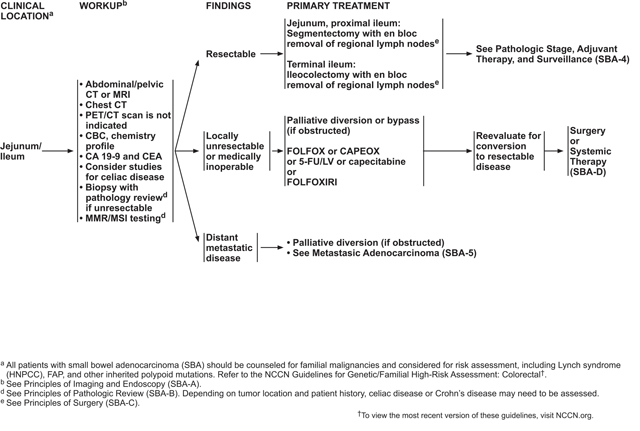
For local (stage I–III) SBA, primary treatment consists of surgical resection with en bloc removal of the regional lymph nodes. Although no prospective, randomized trials have been published to inform surgical technique, retrospective reviews on the subject have been published.62–64 Intraoperative staging of the abdomen—particularly including the mesentery, omentum, and peritoneum—should be completed in all cases.
The type of resection used to treat localized SBA depends on the location of the primary tumor. Segmental resection of the small bowel is often the mainstay of treatment, although duodenal tumors may require either pancreaticoduodenectomy or segmental duodenal resection. For tumors of the jejunum or ileum, segmentectomy is the preferred method of resection.
Pancreaticoduodenectomy, also known as the Whipple procedure, should be considered for all duodenal cancers and is particularly appropriate for those arising in the second portion of the duodenum or invading into any portion of the ampulla or pancreas. Minimally invasive procedures, such as laparoscopic surgery, may be considered for pancreaticoduodenectomy but should only be used by experienced surgeons. Limited segmentectomy may be considered in SBA involving the third and fourth segments of the duodenum and on the antimesenteric side of the intestine, although this approach is controversial based on reports of lower lymph node yields.64 However, a retrospective study of 1,611 patients with duodenal adenocarcinoma found that patients who were treated with radical resection did not show an improvement in overall survival (OS) or disease-specific survival compared with a simple removal of the primary site, after controlling for confounding factors.65 This finding was despite greater lymph node retrieval with radical resection. Case reports have suggested that segmentectomy and other limited resection methods may be considered for lesions in the first portion of the duodenum, particularly for those <2 cm in size and located on the mesenteric side of the intestine.63
NCCN recommends that a goal for all SBA resections should be the retrieval of at least 8 regional lymph nodes for evaluation based on the strong prognostic impact of lymph node metastases and studies showing improved outcomes with higher numbers of lymph nodes assessed during surgery.58–60 See “Lymph Node Evaluation” (page 1116) for more information on pathologic review of dissected lymph nodes.
Adjuvant Therapy
Localized SBAs are treated with surgical resection, but local and distant recurrences are common, and optimal perioperative therapy is unknown.66 Therefore, participation in a clinical trial is preferred for all patients with SBA who are considering adjuvant therapy. For discussion of neoadjuvant therapy, see “Primary Treatment of Unresectable Disease” (page 1120).
The ongoing, international phase III BALLAD trial is the first prospective trial investigating the role of adjuvant 5-FU/leucovorin (5-FU/LV) or 5-FU/LV plus oxaliplatin (FOLFOX) compared with observation alone for patients with stage I–III SBA.67,68 Until the results of BALLAD have been reported, the potential benefits of adjuvant therapy for SBA can be estimated only through retrospective reports. The data from retrospective studies or meta-analyses that have sought to assess the efficacy of adjuvant therapy (either chemotherapy or chemoradiotherapy) for SBA have been mixed, with some showing a benefit to adjuvant therapy,69–71 some showing no benefit,36,72,73 and some showing an equivocal or nonsignificant benefit.74,75 Data supporting the use of adjuvant chemoradiation are especially limited, with a recent retrospective review of patients with resected, nonmetastatic duodenal adenocarcinoma showing that patients who received adjuvant chemoradiotherapy (n=550) had no significant improvement in survival compared with those who received chemotherapy alone (n=694), even in high-risk cases.76 Therefore, chemoradiation should be considered only in highly selected patients.
MSI/MMR Status for Adjuvant Therapy
MSI/MMR is an important piece of information to consider when deciding whether to use adjuvant chemotherapy in patients with stage II SBA. Mutation of MMR genes or modifications of these genes (eg, methylation) can result in MMR protein deficiency and MSI.77 Tumors showing the presence of MSI are classified as either MSI-H or MSI-low, depending on the extent of instability in the markers tested, whereas tumors without this characteristic are classified as microsatellite-stable (MSS).78 Patients determined to have dMMR status are biologically the same population as those with MSI-H status.
Data from several large studies in colon cancer have shown that MSI-H (ie, dMMR) tumors have a decreased likelihood to metastasize and that MSI-H/dMMR may function as a prognostic marker for favorable outcomes in stage II disease.79–81 Some of these same studies also show that a dMMR/MSI-H tumor status may be a predictive marker of decreased benefit and possibly a detrimental impact from adjuvant therapy in patients with stage II colon cancer.80–82 However, a recent study of 1,913 patients with stage II CRC from the QUASAR study, half of whom received adjuvant chemotherapy, showed that although dMMR was prognostic, it did not predict benefit or detrimental impact of chemotherapy.83 A study of patients in the CALGB 9581 and 89803 trials came to a similar conclusion, though notably this used older, nonstandard chemotherapy regimens.84 Extrapolating from these colon cancer data, patients with stage II MSI-H/dMMR SBA may have a good prognosis and the benefit from adjuvant therapy is unclear.
NCCN Recommendations for Adjuvant Therapy
Based on the limited data available from retrospective studies of SBA, and extrapolation from studies of colon cancer, the NCCN Small Bowel Adenocarcinoma Panel recommends:
Six months of adjuvant treatment with FOLFOX, capecitabine plus oxaliplatin (CAPEOX), 5-FU/LV, or capecitabine for any locally advanced SBA with positive lymph nodes (stage III). Chemoradiation with capecitabine or infusional 5-FU is another option for stage III duodenal cancer that is margin-positive after resection.
Observation or 6 months of adjuvant treatment with FOLFOX, CAPEOX, 5-FU/LV, or capecitabine for stage II tumors that are MSS or MMR proficient (pMMR) and have high-risk features. High-risk features include T4 stage, close or positive surgical margins, few lymph nodes examined (<5 for duodenal or <8 for jejunal/ileal primary tumor location), or tumor perforation.38,58,72 Studies in CRC, and a retrospective report in SBA, have identified lymphovascular or perineural invasion and poorly differentiated histology as poor prognostic factors.72,85–87 Therefore, adjuvant therapy may be considered for patients with these factors as well. Chemoradiation with capecitabine or infusional 5-FU is another option for duodenal cancer that meets these criteria and is margin-positive after resection.
Observation or 6 months of adjuvant treatment with 5-FU/LV or capecitabine for T3, N0, M0 (stage IIA) tumors that are MSS or pMMR and have no high-risk features.
Observation after surgical treatment of all stage I tumors and for stage II tumors that are MSI-H or dMMR.
Due to poorer survival in stage III SBA compared with CRC and the fact that the trial enrolled no patients with SBA, extrapolation from the IDEA collaboration (wherein 3 months of fluoropyrimidine/oxaliplatin was shown to be noninferior to 6 months of therapy for CRC)88 is not currently recommended for SBA.
Primary Treatment of Unresectable Disease
For some patients with locally unresectable or medically inoperable SBA, conversion to resectable disease may be a goal. A limited amount of data has shown that neoadjuvant therapy may be beneficial in converting unresectable SBA to resectable disease. A retrospective study of patients with unresectable or recurrent duodenal adenocarcinoma who were treated with neoadjuvant chemotherapy or chemoradiation found that 9 of 10 patients showed conversion to resectable disease after neoadjuvant therapy. At data collection, 5 patients were still alive (ranging from 18–83 months postoperatively), suggesting prolonged survival after conversion to resectable disease.89 In addition, neoadjuvant chemoradiation was studied in 2 small prospective trials. A phase II trial including patients with duodenal or pancreatic adenocarcinomas reported that 4 of 5 patients with tumors in the duodenum were able to undergo resection after neoadjuvant chemoradiation.90 Another small prospective study of patients with duodenal or pancreatic adenocarcinomas reported that all 4 patients with duodenal cancer underwent curative resection after neoadjuvant chemoradiation and experienced a complete pathologic response.91
Because many small bowel cancers present at an advanced stage, malignant small bowel obstruction is a common complication. One retrospective Eastern European study reported that most patients with small bowel cancer presented due to an emergency situation.39 Obstruction was a common complication for SBA, accounting for 22% to 57.9% of these cases.39,92–94 Malignant small bowel obstruction may be treated palliatively with either surgical diversion or stenting. Although most of the literature on palliative treatment of malignant small bowel obstruction comes from pancreatic cancer, there are a few studies that include SBA cases.39,95–97 One retrospective study concluded that there was no difference in poststent survival between patients with pancreatic and nonpancreatic cancers, and that patients with nonpancreatic cancers (including SBA) showed a longer OS.95
Based on these data, the panel recommends that patients with locally unresectable or medically inoperable SBA may undergo neoadjuvant therapy, during which they should be routinely monitored for conversion to resectable disease. Neoadjuvant chemoradiation may be indicated for duodenal disease that remains unresectable after a course of induction chemotherapy, but this is controversial and should be considered on an individual case basis. Alternatively, in cases where conversion to resectable disease is not feasible, palliative chemotherapy may be considered. Palliative diversion or stenting is recommended if a small bowel obstruction is present.
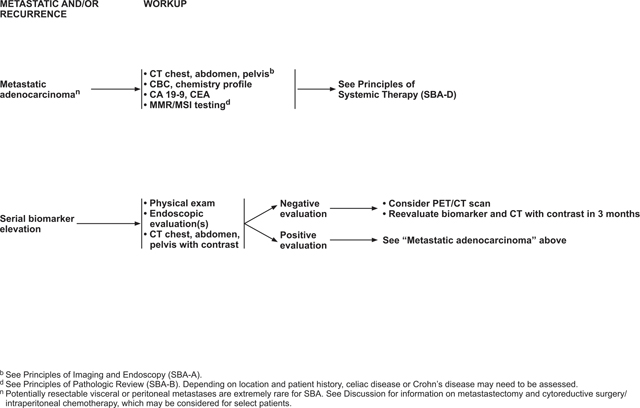
Treatment of Distant Metastatic (Stage IV) SBA
Approximately 32% of patients diagnosed with SBA have stage IV (distant metastatic) disease.38 The most common sites for metastatic spread include the peritoneal cavity and liver, consistent with other gastrointestinal malignancies.6 Although 5-year survival is relatively high (85%) for localized disease, patients with stage IV SBA have a 5-year relative survival of only 42%.98 In addition, recurrence rates of localized SBA treated with surgery are high, with many of these patients developing distant metastases.35 The NCCN recommendations for treatment of stage IV SBA are discussed subsequently.
Metastasectomy
Although resectable metastases are rare for SBA and the data supporting metastasectomy for SBA are limited, a retrospective analysis of patients with non-CRC, nonendocrine liver metastases (including 28 patients with small bowel cancers and 12 patients with duodenal cancers) showed promising survival rates after resection of liver metastases.99 The 5-year survival rate for small bowel cancers was 49% with a median survival of 58 months. For duodenal cancers, the 5-year survival rate was 21% with a median survival of 34 months. Recently, another retrospective study of 34 patients undergoing resection of SBA metastases reported a median OS of 28.2 months and a relapse-free survival of 18.7 months.100 In this study, 41.2% of patients survived more than 3 years; 88.2% of patients in this study received perioperative chemotherapy. Poor differentiation, invaded margins, and lymphatic invasion of the primary tumor were identified as poor prognostic factors. Therefore, certain patients with SBA and limited metastasis to visceral organs may be candidates for metastasectomy. If metastasectomy is being considered, a multidisciplinary team, including a surgeon experienced in the resection of metastases, should be consulted.
Peritoneal Carcinomatosis
Peritoneal carcinomatosis (peritoneal metastases) has been shown to affect 25% to 50% of patients with stage IV SBA. Peritoneal carcinomatosis occurs more frequently in tumors arising from the jejunum or ileum and less commonly in duodenal tumors.101 Peritoneal carcinomatosis generally carries a poor prognosis with a reported median OS of 5.9 months.102 The goal of treatment of unresectable peritoneal metastases is palliative and primarily consists of systemic therapy (see “Systemic Therapy for Metastatic Disease,” next section).
For resectable peritoneal carcinomatosis, surgical cytoreduction may be considered. For peritoneal metastases that present synchronously with the primary tumor, resection of the primary and cytoreduction of peritoneal metastases may be performed concurrently. A multidisciplinary team evaluation at an experienced center is important if considering this treatment approach. Data supporting the use of hyperthermic intraperitoneal chemotherapy (HIPEC) for SBA with peritoneal carcinomatosis are extremely limited, consisting entirely of small, retrospective studies.102–107 In addition, the recent phase III PRODIGE 7 study showed no benefit of oxaliplatin-based HIPEC in patients with CRC compared with cytoreduction alone.108 Significant morbidity and mortality are associated with this procedure. Various studies have reported morbidity rates ranging from 19% to 31% for serious adverse events and mortality rates ranging from 0% to 4%.103–107 Furthermore, recurrences after the procedure are common.106,107 Based on this lack of evidence, HIPEC cannot be recommended for this population unless more robust data become available.
Systemic Therapy for Metastatic Disease
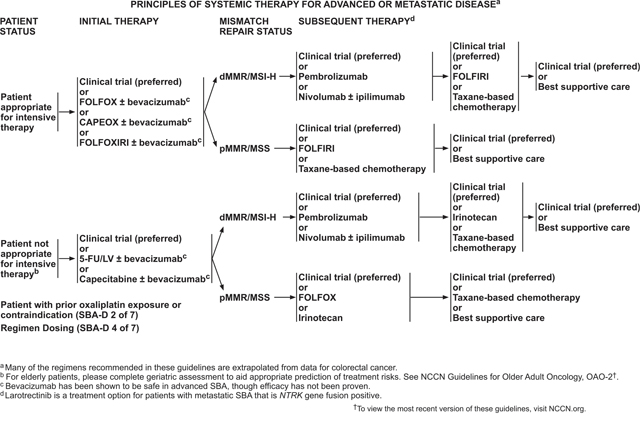
Data supporting systemic therapy for advanced adenocarcinoma of the small bowel were also almost entirely limited to retrospective reports,109–112 although recently several small phase II trials for SBA have been reported. Based on the results from these studies, several systemic therapy regimens are recommended for treatment of metastatic SBA. However, participation in clinical trials is especially encouraged for patients with SBA, based on the lack of data.
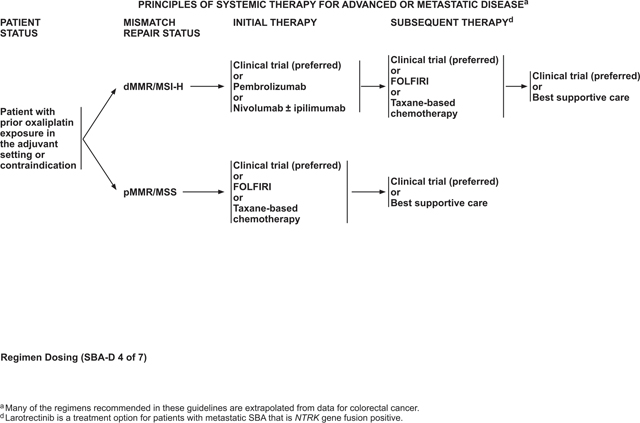
The choice of therapy is based on consideration of the goals of therapy, the type and timing of prior therapy, and the differing toxicity profiles of the constituent drugs. Furthermore, an evaluation of the efficacy and safety of these regimens for an individual patient must take into account the performance status of the patient. As initial therapy for advanced disease in a patient appropriate for intensive therapy (ie, one with a good tolerance for this therapy for whom a high tumor response rate would be potentially beneficial) without prior platinum resistance, the panel recommends a choice of 3 chemotherapy regimens: FOLFOX, CAPEOX, or FOLFOXIRI (infusional 5-FU, LV, oxaliplatin, irinotecan); any of which may be combined with bevacizumab. For patients who are not appropriate for intensive therapy, treatment options would exclude the more toxic components of these regimens, with 5-FU/LV or capecitabine with or without bevacizumab recommended as first-line therapy for these patients.
The choice of second-line therapy depends on the MMR/MSI status of the tumor. For tumors that are dMMR or MSI-H, checkpoint inhibitor therapy with anti-PD-1 inhibitors, alone or in combination with an anti-CTLA4 inhibitor, is recommended in the second-line setting. FOLFIRI or taxane-based chemotherapies are options in the second line for pMMR/MSS tumors, or those that are refractory to checkpoint inhibitor therapies. Larotrectinib is an option in subsequent lines of therapy for metastatic SBA with neurotrophic tyrosine receptor kinase (NTRK) gene fusion and no satisfactory alternative treatments.
Genetic Alterations in SBA
Emerging research has shown that SBA has a distinct genetic profile, which sets it apart from CRC or gastroesophageal cancers, the 2 cancer types SBA is most often likened to. Although KRAS and TP53 alterations are frequently identified in both SBA and CRC, APC mutations are significantly less common in SBA (27% in SBA vs 76% in CRC; P<.001).34 Considering the near ubiquity of APC mutation and its well-established role in CRC carcinogenesis, this suggests that neoplastic transformation in SBA is unique compared with CRC.33,34
SMAD4 and CDKN2A mutations are more commonly seen compared with gastroesophageal cancers and CRC. Though BRAF mutations occur at a similar rate as seen in CRC, only 10% of BRAF-mutant SBAs have a V600E alteration, compared with >70% in BRAF-mutant CRC.34 Importantly, human epidermal growth factor receptor 2 (HER2) alterations, MSI-H/dMMR, programmed death-ligand 1 (PD-L1) expression, and high tumor mutational burden are enhanced in SBA compared with CRC,34,113–115 and may reveal greater importance of targeted or immunotherapeutic treatments compared with current CRC treatment algorithms.
Regimens Not Recommended for SBA
Although many of the systemic therapy regimens recommended for treatment of metastatic SBA are extrapolated from data for CRC, several regimens are commonly used for metastatic CRC that are not recommended for SBA based either on a lack of data supporting their use or data suggesting that these regimens do not work for metastatic SBA.
A 2017 retrospective analysis reported that the efficacy of cetuximab-containing chemotherapy for RAS wild-type SBA was inconclusive.116 Subsequently, a phase II trial published in 2018 showed that panitumumab has no clinically meaningful activity in RAS wild-type SBA117; therefore, cetuximab or panitumumab should not be used for treatment of SBA.
While trifluridine-tipiracil or regorafenib are recommended as subsequent therapy options for metastatic CRC, no data are available to support their use for SBA. They are, therefore, not recommended.
FOLFOX or CAPEOX as First-line Therapy
Both FOLFOX and CAPEOX have been evaluated prospectively for first-line treatment of advanced SBA in phase II clinical trials. One of these trials evaluated CAPEOX in 30 patients with advanced adenocarcinomas of the small bowel and ampulla of Vater. The overall response rate (ORR) (the primary endpoint) was 50%, with 10% achieving complete response.118 A similar response rate of 48.5% (95% CI, 31%–67%) was seen in another small phase II study of 33 patients that assessed the efficacy of FOLFOX in first-line treatment of advanced SBA.119 Likewise, another phase II study reported an ORR of 45% for 24 patients with metastatic or unresectable SBA who were treated with FOLFOX, with a median progression-free survival (PFS) and OS of 5.9 and 17.3 months, respectively.120 These response rates to CAPEOX and FOLFOX were much higher than the 18% response rate seen in another small phase II study that evaluated 5-FU/doxorubicin/mitomycin C in patients with metastatic SBA.121 Adverse events reported across these 3 trials were similar, with neutropenia, thrombocytopenia, nausea, vomiting, diarrhea, peripheral neuropathy, and fatigue reported most frequently.118–120 Retrospective studies have supported the results of these trials, reporting that the combination of a fluoropyrimidine with oxaliplatin was the most effective first-line therapy for advanced SBA.111,122,123 Based on these data, FOLFOX or CAPEOX are recommended as first-line therapy options for treatment of patients with advanced SBA who are appropriate for intensive therapy.
FOLFOXIRI as First-line Therapy
Although the role of FOLFOXIRI for treatment of SBA has not been formally evaluated, CAPIRINOX (capecitabine, irinotecan, oxaliplatin) has been tested as first-line treatment in a phase II trial of 33 patients with advanced SBA.124 In this trial, CAPIRINOX—dose-adjusted according to UGT1A1 genotype—showed a response rate of 37.5% (95% CI, 21%–56%), with a median PFS and OS of 8.9 and 13.4 months, respectively. Neither hematologic toxicity nor tumor response rate differed significantly by UGT1A1 genotype, supporting the feasibility of genotype-directed dosing for CAPIRINOX. The NCCN panel does not recommend use of CAPIRINOX for SBA due to concerns about toxicity, but the recommendation for FOLFOXIRI is extrapolated from the results of this study.
FOLFOX, CAPEOX, or FOLFOXIRI Plus Bevacizumab as First-line Therapy
Although data supporting the addition of biologics to FOLFOX, CAPEOX, or FOLFOXIRI are currently extremely limited, a single-phase II trial has reported that CAPEOX in combination with bevacizumab is safe and efficacious in patients with SBA.125 Retrospective analyses have supported these results, reporting favorable outcomes in patients treated with bevacizumab-containing chemotherapy regimens without adding significant toxicity.116,126 Based on these data, FOLFOX, CAPEOX, or FOLFOXIRI may be given with or without bevacizumab as first-line therapy for advanced SBA.
Pembrolizumab or Nivolumab With or Without Ipilimumab (for dMMR/MSI-H tumors) as Subsequent-line Therapy
Pembrolizumab is a PD-1 inhibitor that was evaluated as a subsequent-line therapy for treatment-refractory metastatic cancers in a phase II study that included 3 cohorts: (1) dMMR colorectal adenocarcinomas, (2) MMR-proficient colorectal adenocarcinomas, and (3) dMMR cancers of types other than CRC.127 This third cohort included 2 patients with small bowel cancers. The immune-related objective response rate and immune-related PFS rate were 40% and 78%, respectively, for patients with dMMR CRC and 71% and 67% for patients with dMMR non-CRC. Common adverse events of clinical interest included rash or pruritus; thyroiditis, hypothyroidism, or hypophysitis; and asymptomatic pancreatitis.127 Based on the results of this study, the FDA granted accelerated approval to pembrolizumab in May 2017 for patients with unresectable or metastatic dMMR or MSI-H solid tumors that have progressed after prior treatment and have no satisfactory alternative treatment options.128 More recently, an abstract reported results of ZEBRA, a multicenter, phase II study of pembrolizumab in patients with previously treated, advanced SBA.129 The results of this study confirmed efficacy of pembrolizumab for dMMR/MSI-H SBA. Furthermore, although pembrolizumab did not achieve the goal ORR for this study, there was some evidence that this therapy may control disease in some patients with MSS SBA. In 18 patients with confirmed MSS SBA, a 50% disease control rate was shown, although further study is needed to confirm this result.129
Another PD-1 inhibitor, nivolumab—alone or in combination with the CTLA-4 inhibitor, ipilimumab—has been studied in patients with dMMR metastatic CRC in the phase II, multicohort CheckMate-142 trial.130,131 One cohort of this trial included 74 patients with dMMR CRC who were treated with nivolumab. ORR for these patients was 31.1% (95% CI, 20.8–42.9), with 69% of patients having disease control for at least 12 weeks. Median duration of response had not yet been reached at the time of data collection. PFS and OS were 50% and 73%, respectively, at 1 year. Grade 3 or 4 drug-related adverse events occurred in 20% of patients, with increased amylase and increased lipase being the most common.130 Another cohort of the CheckMate-142 trial included 119 patients with dMMR CRC who were treated with nivolumab in combination with ipilimumab. For this cohort, ORR was 55% (95% CI, 45.2–63.8) and the disease control rate for at least 12 weeks was 80%. PFS and OS were 71% and 85%, respectively, at 1 year. In addition, significant, clinically meaningful improvements were observed in patient-reported outcomes of functioning, symptoms, and quality of life. Grade 3 to 4 treatment-related adverse events occurred in 32% of patients, but were manageable.131
Based on these positive results for CRC and the data showing benefit of pembrolizumab in SBA, the NCCN panel recommends either pembrolizumab or nivolumab, with or without ipilimumab, as second-line treatment options for dMMR/MSI-H advanced SBA. SBA has been reported to have a higher incidence of dMMR/MSI-H and higher rates of PD-L1 IHC positivity compared with CRC,33,34,113 making checkpoint inhibition an important treatment option for some SBA patients.
Taxane-Based Chemotherapy as Subsequent-Line Therapy
Although almost all of the phase II trials of systemic therapy for SBA have focused on first-line therapy, a phase II trial including 13 patients with SBA studied the efficacy of nab-paclitaxel in the refractory disease setting.132 Patients with SBA in this trial had received a median of 2 prior lines of therapy including a fluoropyrimidine and oxaliplatin. Of the 10 patients with SBA who were evaluable for efficacy, 2 showed a partial response to nab-paclitaxel and an additional 3 had stable disease per RECIST criteria, yielding a disease control rate of 50%. Common grade 3 or 4 toxicities across the entire study population included fatigue (12%), neutropenia (9%), febrile neutropenia (9%), dehydration (6%), and thrombocytopenia (6%).132
A single-center, retrospective review reported on 20 patients with advanced SBA who were treated with taxane-based therapy (either as single therapy or in combination).133 Of these cases, 30% showed disease response, 35% showed stable disease, and 35% showed progression. Median time to progression was 3.8 months (95% CI, 2.9–4.6) and median OS was 10.7 months (95% CI, 3.1–18.3). Based on these data, taxane-based chemotherapy is a recommended option for second- or subsequent-line therapy, although only nab-paclitaxel has prospective, published data to support its use for treatment of SBA.
FOLFIRI as Subsequent-Line Therapy
A retrospective, multicenter study evaluated the efficacy of FOLFIRI as second-line therapy for patients with advanced SBA who had received platinum-based chemotherapy in the first-line setting.134 Of the 28 patients who fit this treatment paradigm, the ORR was 20% and disease control rate was 52%. The median PFS and OS were 3.2 and 10.5 months. Grade 3–4 toxicity was reported in 48% of patients. Based on these data, FOLFIRI is recommended as a treatment option for second- or subsequent-line treatment of advanced SBA.
Larotrectinib as Subsequent-Line Therapy
A pooled analysis of 3 studies (a phase I including adults, a phase I-II involving children, and a phase II involving adolescents and adults) studied the safety and efficacy of larotrectinib in patients with NTRK gene fusion-positive tumors, including 4 patients with colon cancer and 1 with cancer of the appendix.135 For the whole population, the ORR was 75% (95% CI, 61%–85%) by independent review and 80% (95% CI, 67%–90%) by investigator assessment. Larotrectinib was found to be well-tolerated as the majority (93%) of adverse events were grades 1 or 2 and no treatment-related adverse events of grades 3 or 4 occurred in more than 5% of patients.135 Based on these data, the FDA approved larotrectinib for metastatic solid tumors with NTRK gene fusion and no satisfactory alternative treatments on November 26, 2018.136
Posttreatment Surveillance
After curative-intent surgery and adjuvant chemotherapy, if administered, posttreatment surveillance of patients with SBA is performed to evaluate for possible therapeutic complications, identify disease recurrence, and discover new metachronous neoplasms at a preinvasive stage. A retrospective study of 146 patients with SBA who underwent cancer-directed surgery found that 39% subsequently developed disease recurrence, with a median time to recurrence of 25 months. Of the patients with disease recurrence, 57% developed distant metastases, 19% developed carcinomatosis, 7% recurred in the abdominal wall, and 17% developed local recurrences.35 Due to the lack of data regarding optimal surveillance following curative-intent treatment of SBA, a similar approach to CRC surveillance is recommended—including history and physical examination; carcinoembryonic antigen and/or carbohydrate antigen 19–9 measurement; and CT of the chest, abdomen, and pelvis. For data supporting the recommended surveillance approach for CRC, see the “Posttreatment Surveillance” section in the NCCN Guidelines for Colon Cancer, available at NCCN.org.
Patients with SBA who were determined to have Crohn’s disease or familial syndromes (ie, Lynch, FAP, PJS) may require more intensive surveillance due to their elevated risk of developing further SBAs.11,12 Endoscopy may be a feasible method for SBA surveillance in patients with Crohn’s disease,12,137 although one prospective study found a low (33%) sensitivity rate for SBA endoscopic screening.138 A number of studies have supported the use of endoscopy/enteroscopy for small bowel surveillance in patients with Lynch syndrome, FAP, or PJS.139–146 For further details on endoscopic small bowel evaluation, see “Imaging and Endoscopy” (page 1114).
Survivorship
Based on the rarity and poor prognosis of SBA, there is a dearth of data regarding survivorship for this disease. The panel recommendations for survivorship are largely extrapolated from the NCCN Guidelines for Colon Cancer, with some specific recommendations included for patients with celiac or Crohn’s disease who are at elevated risk of developing additional SBAs.11,12,14 This section provides an overview of the panel recommendations for survivorship; for more detailed information, see the “Survivorship” section in the NCCN Guidelines for Colon Cancer (available at NCCN.org).
The panel recommends that a prescription for survivorship and transfer of care to the primary care physician be written.147 The oncologist and primary care provider should have defined roles in the surveillance period, with roles communicated to the patient. Other recommendations include monitoring for late or long-term sequelae of treatment, such as oxaliplatin-induced peripheral neuropathy, fatigue, pain, sexual dysfunction, and emotional or social distress.148–152 Specific management interventions to address these and other side effects are described in a review.153 Disease preventive measures, such as immunizations; early disease detection through periodic screening for second primary cancers (eg, breast, cervical, or prostate cancers); and routine good medical care and monitoring are recommended. The NCCN Guidelines for Survivorship (at NCCN.org) provide screening, evaluation, and treatment recommendations for common consequences of cancer and cancer treatment to aid health care professionals who work with survivors of adult-onset cancer in the posttreatment period, including those in specialty cancer survivor clinics and primary care practices.
Summary
SBA is a rare malignancy, with a rising incidence in recent decades. Compared with CRC, SBA is more often diagnosed at advanced stages, suggesting the difficulty of detecting these cancers and highlighting the lack of screening programs, even for high-risk individuals. The majority of SBAs arise in the duodenum and are associated with poorer prognosis, with up to a third of resectable patients experiencing early relapse. To date, the only curative therapy for SBA is surgery.
For local disease, segmental resection of the small bowel is the mainstay of treatment, though duodenal tumors may require either pancreaticoduodenectomy or segmental duodenal resection. Database analyses have reported significantly improved outcomes when 8 or more lymph nodes are resected. In addition, the use of radiation therapy for retroperitoneal-based duodenal adenocarcinomas is a complex decision-making process. Fluoropyrimidine-based adjuvant therapy may be considered for some patients with SBA, though no studies have yet shown a definitive benefit of this approach. Results from the international, phase III adjuvant clinical study (BALLAD) investigating observation versus 5-FU versus FOLFOX for patients with resected stage I–III SBA should shed light on this approach in the coming years.
Metastatic SBA may rarely be treated with curative intent via primary tumor resection and metastasectomy; however, most patients with metastatic SBA are treated with systemic therapy. Systemic therapy options include fluoropyrimidine-based chemotherapy, taxane-based chemotherapy, or checkpoint inhibitors. Recently, SBA’s unique genetic profile has been a topic of research, which may lead to new targeted or immunotherapeutic treatment options for SBA.
NCCN CATEGORIES OF EVIDENCE AND CONSENSUS.
Category 1: Based upon high-level evidence, there is uniform NCCN consensus that the intervention is appropriate.
Category 2A: Based upon lower-level evidence, there is uniform NCCN consensus that the intervention is appropriate.
Category 2B: Based upon lower-level evidence, there is NCCN consensus that the intervention is appropriate.
Category 3: Based upon any level of evidence, there is major NCCN disagreement that the intervention is appropriate.
All recommendations are category 2A unless otherwise noted.
Clinical trials: NCCN believes that the best management of any patient with cancer is in a clinical trial. Participation in clinical trials is especially encouraged.
PLEASE NOTE
The NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) are a statement of evidence and consensus of the authors regarding their views of currently accepted approaches to treatment. Any clinician seeking to apply or consult the NCCN Guidelines is expected to use independent medical judgment in the context of individual clinical circumstances to determine any patient’s care or treatment. The National Comprehensive Cancer Network® (NCCN®) makes no representations or warranties of any kind regarding their content, use, or application and disclaims any responsibility for their application or use in any way. © National Comprehensive Cancer Network, Inc. 2019. All rights reserved. The NCCN Guidelines and the illustrations herein may not be reproduced in any form without the express written permission of NCCN.
Disclosures for the NCCN Small Bowel Adenocarcinoma Panel
At the beginning of each NCCN Guidelines Panel meeting, panel members review all potential conflicts of interest. NCCN, in keeping with its commitment to public transparency, publishes these disclosures for panel members, staff, and NCCN itself.
Individual disclosures for the NCCN Small Bowel Adenocarcinoma Panel members can be found on page 1133. (The most recent version of these guidelines and accompanying disclosures are available at NCCN.org.)
The complete and most recent version of these guidelines is available free of charge at NCCN.org.













References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7–34. [DOI] [PubMed] [Google Scholar]
- 2.Noone AM, Howlader N, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2015, based on November 2017 SEER data submission, posted to the SEER web site, April 2018. National Cancer Institute, Bethesda, MD. 2018. Available at https://seer.cancer.gov/csr/1975_2015/. Accessed August 2, 2019. [Google Scholar]
- 3.Saif MW, editor. Small Bowel Adenocarcinoma. In: Gastrointestinal Malignancies. New York, NY: Demos Medical Publishing, LLC; 2010:171. [Google Scholar]
- 4.Bilimoria KY, Bentrem DJ, Wayne JD, et al. Small bowel cancer in the United States: changes in epidemiology, treatment, and survival over the last 20 years. Ann Surg 2009;249:63–71. [DOI] [PubMed] [Google Scholar]
- 5.Locher C, Batumona B, Afchain P, et al. Thésaurus National de Cancérologie Digestive (TNCD). Small bowel adenocarcinoma: French intergroup clinical practice guidelines for diagnosis, treatments and follow-up (SNFGE, FFCD, GERCOR, UNICANCER, SFCD, SFED, SFRO). Dig Liver Dis 2018;50:15–19. [DOI] [PubMed] [Google Scholar]
- 6.Amin MB, Edge SB, Greene F, et al. , editors. AJCC Cancer Staging Manual, 8th Ed. New York: Springer International Publishing; 2017. 10.1007/978-3-319-40618-3 [DOI] [Google Scholar]
- 7.U.S. National Library of Medicine-Key MEDLINE® Indicators. Available at: http://www.nlm.nih.gov/bsd/bsd_key.html. Accessed Octboer 8, 2018. [Google Scholar]
- 8.Bennett CM, Coleman HG, Veal PG, et al. Lifestyle factors and small intestine adenocarcinoma risk: a systematic review and meta-analysis. Cancer Epidemiol 2015;39:265–273. [DOI] [PubMed] [Google Scholar]
- 9.Boffetta P, Hazelton WD, Chen Y, et al. Body mass, tobacco smoking, alcohol drinking and risk of cancer of the small intestine--a pooled analysis of over 500,000 subjects in the Asia Cohort Consortium. Ann Oncol 2012;23:1894–1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Algaba A, Guerra I, Marín-Jiménez I, et al. Incidence, management, and course of cancer in patients with inflammatory bowel disease. J Crohn’s Colitis 2015;9:326–333. [DOI] [PubMed] [Google Scholar]
- 11.Cahill C, Gordon PH, Petrucci A, et al. Small bowel adenocarcinoma and Crohn’s disease: any further ahead than 50 years ago? World J Gastroenterol 2014;20:11486–11495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grolleau C, Pote NM, Guedj NS, et al. Small bowel adenocarcinoma complicating Crohn’s disease: a single-centre experience emphasizing the importance of screening for dysplasia. Virchows Arch 2017;471:611–617. [DOI] [PubMed] [Google Scholar]
- 13.Laukoetter MG, Mennigen R, Hannig CM, et al. Intestinal cancer risk in Crohn’s disease: a meta-analysis. J Gastrointest Surg 2011;15:576–583. [DOI] [PubMed] [Google Scholar]
- 14.Rampertab SD, Forde KA, Green PH. Small bowel neoplasia in coeliac disease. Gut 2003;52:1211–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benhammane H, El M’rabet FZ, Idrissi Serhouchni K, et al. Small bowel adenocarcinoma complicating coeliac disease: a report of three cases and the literature review. Case Rep Oncol Med 2012;2012:935183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zullo A, De Francesco V, Manta R, et al. A challenging diagnosis of jejunal adenocarcinoma in a celiac patient: case report and systematic review of the literature. J Gastrointestin Liver Dis 2017;26:411–415. [DOI] [PubMed] [Google Scholar]
- 17.Galiatsatos P, Foulkes WD. Familial adenomatous polyposis. Am J Gastroenterol 2006;101:385–398. [DOI] [PubMed] [Google Scholar]
- 18.Half E, Bercovich D, Rozen P. Familial adenomatous polyposis. Orphanet J Rare Dis 2009;4:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shenoy S Genetic risks and familial associations of small bowel carcinoma. World J Gastrointest Oncol 2016;8:509–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tomlinson IP, Houlston RS. Peutz-Jeghers syndrome. J Med Genet 1997;34:1007–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hemminki A, Markie D, Tomlinson I, et al. A serine/threonine kinase gene defective in Peutz-Jeghers syndrome. Nature 1998;391:184–187. [DOI] [PubMed] [Google Scholar]
- 22.Jenne DE, Reimann H, Nezu J, et al. Peutz-Jeghers syndrome is caused by mutations in a novel serine threonine kinase. Nat Genet 1998;18:38–43. [DOI] [PubMed] [Google Scholar]
- 23.Lim W, Hearle N, Shah B, et al. Further observations on LKB1/STK11 status and cancer risk in Peutz-Jeghers syndrome. Br J Cancer 2003;89:308–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giardiello FM, Brensinger JD, Tersmette AC, et al. Very high risk of cancer in familial Peutz-Jeghers syndrome. Gastroenterology 2000;119:1447–1453. [DOI] [PubMed] [Google Scholar]
- 25.Hearle N, Schumacher V, Menko FH, et al. Frequency and spectrum of cancers in the Peutz-Jeghers syndrome. Clin Cancer Res 2006;12:3209–3215. [DOI] [PubMed] [Google Scholar]
- 26.Koornstra JJ, Kleibeuker JH, Vasen HF. Small-bowel cancer in Lynch syndrome: is it time for surveillance? Lancet Oncol 2008;9:901–905. [DOI] [PubMed] [Google Scholar]
- 27.Vasen HF, Wijnen JT, Menko FH, et al. Cancer risk in families with hereditary nonpolyposis colorectal cancer diagnosed by mutation analysis. Gastroenterology 1996;110:1020–1027. [DOI] [PubMed] [Google Scholar]
- 28.Hendriks YM, de Jong AE, Morreau H, et al. Diagnostic approach and management of Lynch syndrome (hereditary nonpolyposis colorectal carcinoma): a guide for clinicians. CA Cancer J Clin 2006;56:213–225. [DOI] [PubMed] [Google Scholar]
- 29.Beamer LC, Grant ML, Espenschied CR, et al. Reflex immunohistochemistry and microsatellite instability testing of colorectal tumors for Lynch syndrome among US cancer programs and follow-up of abnormal results. J Clin Oncol 2012;30:1058–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burt RW. Who should have genetic testing for the Lynch syndrome? Ann Intern Med 2011;155:127–128. [DOI] [PubMed] [Google Scholar]
- 31.Matloff J, Lucas A, Polydorides AD, et al. Molecular tumor testing for Lynch syndrome in patients with colorectal cancer. J Natl Compr Canc Netw 2013;11:1380–1385. [DOI] [PubMed] [Google Scholar]
- 32.Ward RL, Hicks S, Hawkins NJ. Population-based molecular screening for Lynch syndrome: implications for personalized medicine. J Clin Oncol 2013;31:2554–2562. [DOI] [PubMed] [Google Scholar]
- 33.Aparicio T, Svrcek M, Zaanan A, et al. Small bowel adenocarcinoma phenotyping, a clinicobiological prognostic study. Br J Cancer 2013;109:3057–3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schrock AB, Devoe CE, McWilliams R, et al. Genomic profiling of small-bowel adenocarcinoma. JAMA Oncol 2017;3:1546–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dabaja BS, Suki D, Pro B, et al. Adenocarcinoma of the small bowel: presentation, prognostic factors, and outcome of 217 patients. Cancer 2004;101:518–526. [DOI] [PubMed] [Google Scholar]
- 36.Halfdanarson TR, McWilliams RR, Donohue JH, et al. A single-institution experience with 491 cases of small bowel adenocarcinoma. Am J Surg 2010;199:797–803. [DOI] [PubMed] [Google Scholar]
- 37.Howe JR, Karnell LH, Menck HR, et al. The American College of Surgeons Commission on Cancer and the American Cancer Society. Adenocarcinoma of the small bowel: review of the National Cancer Data Base, 1985–1995. Cancer 1999;86:2693–2706. [DOI] [PubMed] [Google Scholar]
- 38.Overman MJ, Hu CY, Kopetz S, et al. A population-based comparison of adenocarcinoma of the large and small intestine: insights into a rare disease. Ann Surg Oncol 2012;19:1439–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Negoi I, Paun S, Hostiuc S, et al. Most small bowel cancers are revealed by a complication. Einstein (Sao Paulo) 2015;13:500–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gustafsson BI, Siddique L, Chan A, et al. Uncommon cancers of the small intestine, appendix and colon: an analysis of SEER 1973–2004, and current diagnosis and therapy. Int J Oncol 2008;33:1121–1131. [PubMed] [Google Scholar]
- 41.Hara AK, Leighton JA, Sharma VK, et al. Imaging of small bowel disease: comparison of capsule endoscopy, standard endoscopy, barium examination, and CT. Radiographics 2005;25:697–711., discussion 711–718. [DOI] [PubMed] [Google Scholar]
- 42.Nylund K, Ødegaard S, Hausken T, et al. Sonography of the small intestine. World J Gastroenterol 2009;15:1319–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen WG, Shan GD, Zhang H, et al. Double-balloon enteroscopy in small bowel diseases: eight years single-center experience in China. Medicine (Baltimore) 2016;95:e5104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cazzato IA, Cammarota G, Nista EC, et al. Diagnostic and therapeutic impact of double-balloon enteroscopy (DBE) in a series of 100 patients with suspected small bowel diseases. Dig Liver Dis 2007;39:483–487. [DOI] [PubMed] [Google Scholar]
- 45.Mitsui K, Tanaka S, Yamamoto H, et al. Role of double-balloon endoscopy in the diagnosis of small-bowel tumors: the first Japanese multicenter study. Gastrointest Endosc 2009;70:498–504. [DOI] [PubMed] [Google Scholar]
- 46.Sunada K, Yamamoto H, Kita H, et al. Clinical outcomes of enteroscopy using the double-balloon method for strictures of the small intestine. World J Gastroenterol 2005;11:1087–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cobrin GM, Pittman RH, Lewis BS. Increased diagnostic yield of small bowel tumors with capsule endoscopy. Cancer 2006;107:22–27. [DOI] [PubMed] [Google Scholar]
- 48.Bailey AA, Debinski HS, Appleyard MN, et al. Diagnosis and outcome of small bowel tumors found by capsule endoscopy: a three-center Australian experience. Am J Gastroenterol 2006;101:2237–2243. [DOI] [PubMed] [Google Scholar]
- 49.Cheung DY, Lee IS, Chang DK, et al. Capsule endoscopy in small bowel tumors: a multicenter Korean study. J Gastroenterol Hepatol 2010;25:1079–1086. [DOI] [PubMed] [Google Scholar]
- 50.Boudiaf M, Jaff A, Soyer P, et al. Small-bowel diseases: prospective evaluation of multi-detector row helical CT enteroclysis in 107 consecutive patients. Radiology 2004;233:338–344. [DOI] [PubMed] [Google Scholar]
- 51.Soyer P, Aout M, Hoeffel C, et al. Helical CT-enteroclysis in the detection of small-bowel tumours: a meta-analysis. Eur Radiol 2013;23:388–399. [DOI] [PubMed] [Google Scholar]
- 52.Cronin CG, Lohan DG, Browne AM, et al. Magnetic resonance enterography in the evaluation of the small bowel. Semin Roentgenol 2009;44:237–243. [DOI] [PubMed] [Google Scholar]
- 53.Masselli G, Casciani E, Polettini E, et al. Magnetic resonance imaging of small bowel neoplasms. Cancer Imaging 2013;13:92–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Masselli G, Di Tola M, Casciani E, et al. Diagnosis of small-bowel diseases: prospective comparison of multi-detector row CT enterography with MR enterography. Radiology 2016;279:420–431. [DOI] [PubMed] [Google Scholar]
- 55.Cronin CG, Scott J, Kambadakone A, et al. Utility of positron emission tomography/CT in the evaluation of small bowel pathology. Br J Radiol 2012;85:1211–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sakae H, Kanzaki H, Nasu J, et al. The characteristics and outcomes of small bowel adenocarcinoma: a multicentre retrospective observational study. Br J Cancer 2017;117:1607–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Young JI, Mongoue-Tchokote S, Wieghard N, et al. Treatment and survival of small-bowel adenocarcinoma in the United States: a comparison with colon cancer. Dis Colon Rectum 2016;59:306–315. [DOI] [PubMed] [Google Scholar]
- 58.Overman MJ, Hu CY, Wolff RA, et al. Prognostic value of lymph node evaluation in small bowel adenocarcinoma: analysis of the surveillance, epidemiology, and end results database. Cancer 2010;116:5374–5382. [DOI] [PubMed] [Google Scholar]
- 59.Tran TB, Qadan M, Dua MM, et al. Prognostic relevance of lymph node ratio and total lymph node count for small bowel adenocarcinoma. Surgery 2015;158:486–493. [DOI] [PubMed] [Google Scholar]
- 60.Wilhelm A, Müller SA, Steffen T, et al. Patients with adenocarcinoma of the small intestine with 9 or more regional lymph nodes retrieved have a higher rate of positive lymph nodes and improved survival. J Gastrointest Surg 2016;20:401–410. [DOI] [PubMed] [Google Scholar]
- 61.Wu S, Chen JN, Zhang QW, et al. A new metastatic lymph node classification-based survival predicting model in patients with small bowel adenocarcinoma: A derivation and validation study. EBioMedicine 2018;32:134–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang S, Yuan W, Zhang J, et al. Clinicopathological features, surgical treatments, and survival outcomes of patients with small bowel adenocarcinoma. Medicine (Baltimore) 2017;96:e7713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hashimoto D, Arima K, Chikamoto A, et al. Limited resection of the duodenum for nonampullary duodenal tumors, with review of the literature. Am Surg 2016;82:1126–1132. [PubMed] [Google Scholar]
- 64.Onkendi EO, Boostrom SY, Sarr MG, et al. 15-year experience with surgical treatment of duodenal carcinoma: a comparison of periampullary and extra-ampullary duodenal carcinomas. J Gastrointest Surg 2012;16:682–691. [DOI] [PubMed] [Google Scholar]
- 65.Cloyd JM, Norton JA, Visser BC, et al. Does the extent of resection impact survival for duodenal adenocarcinoma? Analysis of 1,611 cases. Ann Surg Oncol 2015;22:573–580. [DOI] [PubMed] [Google Scholar]
- 66.Raghav K, Overman MJ. Small bowel adenocarcinomas--existing evidence and evolving paradigms. Nat Rev Clin Oncol 2013;10:534–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.U.S. National Library of Medicine. Phase III Trial Investigating the Potential Benefit of Adjvant Chemotherapy for Small Bowel Adenocarcinoma (BALLAD). ClinicalTrials.gov: 2015. Available at: https://clinicaltrials.gov/ct2/show/NCT02502370. Accessed January 9, 2019.
- 68.Evans TRJ, Aparicio T, Malicot KL, et al. GLOBAL BALLAD: an International Rare Cancers Initiative trial to evaluate the potential benefit of adjuvant chemotherapy for small bowel adenocarcinoma (IRCI 002). [abstract] J Clin Oncol 2016;34(15_suppl):TPS4154–TPS4154. [Google Scholar]
- 69.Ecker BL, McMillan MT, Datta J, et al. Efficacy of adjuvant chemotherapy for small bowel adenocarcinoma: a propensity score-matched analysis. Cancer 2016;122:693–701. [DOI] [PubMed] [Google Scholar]
- 70.Swartz MJ, Hughes MA, Frassica DA, et al. Adjuvant concurrent chemoradiation for node-positive adenocarcinoma of the duodenum. Arch Surg 2007;142:285–288. [DOI] [PubMed] [Google Scholar]
- 71.Kelsey CR, Nelson JW, Willett CG, et al. Duodenal adenocarcinoma: patterns of failure after resection and the role of chemoradiotherapy. Int J Radiat Oncol Biol Phys 2007;69:1436–1441. [DOI] [PubMed] [Google Scholar]
- 72.Aydin D, Sendur MA, Kefeli U, et al. Evaluation of prognostic factors and adjuvant chemotherapy in patients with small bowel adenocarcinoma who underwent curative resection. Clin Colorectal Cancer 2017;16:220–227. [DOI] [PubMed] [Google Scholar]
- 73.Ye X, Zhang G, Chen H, et al. Meta-analysis of postoperative adjuvant therapy for small bowel adenocarcinoma. PLoS One 2018;13:e0200204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kim K, Chie EK, Jang JY, et al. Role of adjuvant chemoradiotherapy for duodenal cancer: a single center experience. Am J Clin Oncol 2012;35:533–536. [DOI] [PubMed] [Google Scholar]
- 75.Overman MJ, Kopetz S, Lin E, et al. Is there a role for adjuvant therapy in resected adenocarcinoma of the small intestine? Acta Oncol 2010;49:474–479. [DOI] [PubMed] [Google Scholar]
- 76.Ecker BL, McMillan MT, Datta J, et al. Adjuvant chemotherapy versus chemoradiotherapy in the management of patients with surgically resected duodenal adenocarcinoma: a propensity score-matched analysis of a nationwide clinical oncology database. Cancer 2017;123:967–976. [DOI] [PubMed] [Google Scholar]
- 77.Markowitz SD, Bertagnolli MM. Molecular origins of cancer: molecular basis of colorectal cancer. N Engl J Med 2009;361:2449–2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kim GP, Colangelo LH, Wieand HS, et al. Prognostic and predictive roles of high-degree microsatellite instability in colon cancer: a National Cancer Institute-National Surgical Adjuvant Breast and Bowel Project Collaborative Study. J Clin Oncol 2007;25:767–772. [DOI] [PubMed] [Google Scholar]
- 79.Klingbiel D, Saridaki Z, Roth AD, et al. Prognosis of stage II and III colon cancer treated with adjuvant 5-fluorouracil or FOLFIRI in relation to microsatellite status: results of the PETACC-3 trial. Ann Oncol 2015;26:126–132. [DOI] [PubMed] [Google Scholar]
- 80.Ribic CM, Sargent DJ, Moore MJ, et al. Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N Engl J Med 2003;349:247–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sargent DJ, Marsoni S, Monges G, et al. Defective mismatch repair as a predictive marker for lack of efficacy of fluorouracil-based adjuvant therapy in colon cancer. J Clin Oncol 2010;28:3219–3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kim JE, Hong YS, Kim HJ, et al. Defective mismatch repair status was not associated with DFS and OS in stage II colon cancer treated with adjuvant chemotherapy. Ann Surg Oncol 2015; 22(S3, Suppl 3)S630–S637. [DOI] [PubMed] [Google Scholar]
- 83.Hutchins G, Southward K, Handley K, et al. Value of mismatch repair, KRAS, and BRAF mutations in predicting recurrence and benefits from chemotherapy in colorectal cancer. J Clin Oncol 2011;29:1261–1270. [DOI] [PubMed] [Google Scholar]
- 84.Bertagnolli MM, Redston M, Compton CC, et al. Microsatellite instability and loss of heterozygosity at chromosomal location 18q: prospective evaluation of biomarkers for stages II and III colon cancer--a study of CALGB 9581 and 89803. J Clin Oncol 2011;29:3153–3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Compton CC, Fielding LP, Burgart LJ, et al. Prognostic factors in colorectal cancer. College of American Pathologists Consensus Statement 1999. Arch Pathol Lab Med 2000;124:979–994. [DOI] [PubMed] [Google Scholar]
- 86.Fujita S, Shimoda T, Yoshimura K, et al. Prospective evaluation of prognostic factors in patients with colorectal cancer undergoing curative resection. J Surg Oncol 2003;84:127–131. [DOI] [PubMed] [Google Scholar]
- 87.Liebig C, Ayala G, Wilks J, et al. Perineural invasion is an independent predictor of outcome in colorectal cancer. J Clin Oncol 2009;27:5131–5137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Grothey A, Sobrero AF, Shields AF, et al. Duration of adjuvant chemotherapy for stage III colon cancer. N Engl J Med 2018;378:1177–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Onkendi EO, Boostrom SY, Sarr MG, et al. Neoadjuvant treatment of duodenal adenocarcinoma: a rescue strategy. J Gastrointest Surg 2012;16:320–324. [DOI] [PubMed] [Google Scholar]
- 90.Yeung RS, Weese JL, Hoffman JP, et al. Neoadjuvant chemoradiation in pancreatic and duodenal carcinoma. A Phase II Study. Cancer 1993;72:2124–2133. [DOI] [PubMed] [Google Scholar]
- 91.Coia L, Hoffman J, Scher R, et al. Preoperative chemoradiation for adenocarcinoma of the pancreas and duodenum. Int J Radiat Oncol Biol Phys 1994;30:161–167. [DOI] [PubMed] [Google Scholar]
- 92.Minardi AJ Jr., Zibari GB, Aultman DF, et al. Small-bowel tumors. J Am Coll Surg 1998;186:664–668. [DOI] [PubMed] [Google Scholar]
- 93.Ciresi DL, Scholten DJ. The continuing clinical dilemma of primary tumors of the small intestine. Am Surg 1995;61:698–702., discussion 702–703. [PubMed] [Google Scholar]
- 94.Ojha A, Zacherl J, Scheuba C, et al. Primary small bowel malignancies: single-center results of three decades. J Clin Gastroenterol 2000;30:289–293. [DOI] [PubMed] [Google Scholar]
- 95.Oh SY, Edwards A, Mandelson M, et al. Survival and clinical outcome after endoscopic duodenal stent placement for malignant gastric outlet obstruction: comparison of pancreatic cancer and nonpancreatic cancer. Gastrointest Endosc 2015;82:460–8.e2. [DOI] [PubMed] [Google Scholar]
- 96.van den Berg MW, Haijtink S, Fockens P, et al. First data on the Evolution duodenal stent for palliation of malignant gastric outlet obstruction (DUOLUTION study): a prospective multicenter study. Endoscopy 2013;45:174–181. [DOI] [PubMed] [Google Scholar]
- 97.Upchurch E, Ragusa M, Cirocchi R. Stent placement versus surgical palliation for adults with malignant gastric outlet obstruction. Cochrane Database Syst Rev 2018;5:CD012506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Facts CS. Small Intestine Cancer. 2019. Available at: https://seer.cancer.gov/statfacts/html/smint.html. Accessed August 7, 2019.
- 99.Adam R, Chiche L, Aloia T, et al. ; Association Française de Chirurgie. Hepatic resection for noncolorectal nonendocrine liver metastases: analysis of 1,452 patients and development of a prognostic model. Ann Surg 2006;244:524–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rompteaux P, Gagniére J, Gornet JM, et al. Resection of small bowel adenocarcinoma metastases: Results of the ARCAD-NADEGE cohort study. Eur J Surg Oncol 2019;45:331–335. [DOI] [PubMed] [Google Scholar]
- 101.Rovers KP, de Bree E, Yonemura Y, et al. Treatment of peritoneal metastases from small bowel adenocarcinoma. Int J Hyperthermia 2017;33:571–578. [DOI] [PubMed] [Google Scholar]
- 102.Legué LM, Simkens GA, Creemers GM, et al. Synchronous peritoneal metastases of small bowel adenocarcinoma: Insights into an underexposed clinical phenomenon. Eur J Cancer 2017;87:84–91. [DOI] [PubMed] [Google Scholar]
- 103.Elias D, Glehen O, Pocard M, et al. A comparative study of complete cytoreductive surgery plus intraperitoneal chemotherapy to treat peritoneal dissemination from colon, rectum, small bowel, and nonpseudomyxoma appendix. Ann Surg 2010;251:896–901. [DOI] [PubMed] [Google Scholar]
- 104.Liu Y, Ishibashi H, Takeshita K, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal dissemination from small bowel malignancy: results from a single specialized center. Ann Surg Oncol 2016;23:1625–1631. [DOI] [PubMed] [Google Scholar]
- 105.Liu Y, Yonemura Y, Levine EA, et al. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy for peritoneal metastases from a small bowel adenocarcinoma: multi-institutional experience. Ann Surg Oncol 2018;25:1184–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Saxena A, Valle SJ, Liauw W, et al. Recurrence and survival outcomes after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for small bowel adenocarcinoma. Anticancer Res 2017;37:5737–5742. [DOI] [PubMed] [Google Scholar]
- 107.van Oudheusden TR, Lemmens VE, Braam HJ, et al. Peritoneal metastases from small bowel cancer: results of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in The Netherlands. Surgery 2015;157:1023–1027. [DOI] [PubMed] [Google Scholar]
- 108.Quenet F, Elias D, Roca L, et al. A UNICANCER phase III trial of hyperthermic intra-peritoneal chemotherapy (HIPEC) for colorectal peritoneal carcinomatosis (PC): PRODIGE 7 [abstract]. J Clin Oncol 2018;36(suppl):Abstract LBA3503. [Google Scholar]
- 109.Czaykowski P, Hui D. Chemotherapy in small bowel adenocarcinoma: 10-year experience of the British Columbia Cancer Agency. Clin Oncol (R Coll Radiol) 2007;19:143–149. [DOI] [PubMed] [Google Scholar]
- 110.Jigyasu D, Bedikian AY, Stroehlein JR. Chemotherapy for primary adenocarcinoma of the small bowel. Cancer 1984;53:23–25. [DOI] [PubMed] [Google Scholar]
- 111.Zhang L, Wang LY, Deng YM, et al. Efficacy of the FOLFOX/CAPOX regimen for advanced small bowel adenocarcinoma: a three-center study from China. J BUON 2011;16:689–696. [PubMed] [Google Scholar]
- 112.Aydin D, Sendur MA, Kefeli U, et al. Evaluation of prognostic factors and treatment in advanced small bowel adenocarcinoma: report of a multi-institutional experience of Anatolian Society of Medical Oncology (ASMO). J BUON 2016;21:1242–1249. [PubMed] [Google Scholar]
- 113.Pedersen K, Smyrk TC, Harrington S, et al. Programmed death-ligand 1 (PD-L1) expression in small bowel adenocarcinomas (SBA) [abstract]. J Clin Oncol 2015;33(15_suppl):3619. [Google Scholar]
- 114.Thota R, Gonzalez RS, Berlin J, et al. Could the PD-1 pathway be a potential target for treating small intestinal adenocarcinoma? Am J Clin Pathol 2017;148:208–214. [DOI] [PubMed] [Google Scholar]
- 115.Laforest A, Aparicio T, Zaanan A, et al. ERBB2 gene as a potential therapeutic target in small bowel adenocarcinoma. Eur J Cancer 2014;50:1740–1746. [DOI] [PubMed] [Google Scholar]
- 116.Takayoshi K, Kusaba H, Uenomachi M, et al. Suggestion of added value by bevacizumab to chemotherapy in patients with unresectable or recurrent small bowel cancer. Cancer Chemother Pharmacol 2017;80:333–342. [DOI] [PubMed] [Google Scholar]
- 117.Gulhati P, Raghav K, Shroff R, et al. Phase II study of panitumumab in RAS wild-type metastatic adenocarcinoma of small bowel or ampulla of Vater. Oncologist 2018;23:277–e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Overman MJ, Varadhachary GR, Kopetz S, et al. Phase II study of capecitabine and oxaliplatin for advanced adenocarcinoma of the small bowel and ampulla of Vater. J Clin Oncol 2009;27:2598–2603. [DOI] [PubMed] [Google Scholar]
- 119.Xiang XJ, Liu YW, Zhang L, et al. A phase II study of modified FOLFOX as first-line chemotherapy in advanced small bowel adenocarcinoma. Anticancer Drugs 2012;23:561–566. [DOI] [PubMed] [Google Scholar]
- 120.Horimatsu T, Nakayama N, Moriwaki T, et al. A phase II study of 5-fluorouracil/L-leucovorin/oxaliplatin (mFOLFOX6) in Japanese patients with metastatic or unresectable small bowel adenocarcinoma. Int J Clin Oncol 2017;22:905–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gibson MK, Holcroft CA, Kvols LK, et al. Phase II study of 5-fluorouracil, doxorubicin, and mitomycin C for metastatic small bowel adenocarcinoma. Oncologist 2005;10:132–137. [DOI] [PubMed] [Google Scholar]
- 122.Tsushima T, Taguri M, Honma Y, et al. Multicenter retrospective study of 132 patients with unresectable small bowel adenocarcinoma treated with chemotherapy. Oncologist 2012;17:1163–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zaanan A, Costes L, Gauthier M, et al. Chemotherapy of advanced small-bowel adenocarcinoma: a multicenter AGEO study. Ann Oncol 2010;21:1786–1793. [DOI] [PubMed] [Google Scholar]
- 124.McWilliams RR, Foster NR, Mahoney MR, et al. North Central Cancer Treatment Group N0543 (Alliance): A phase 2 trial of pharmacogenetics-based dosing of irinotecan, oxaliplatin, and capecitabine as first-line therapy for patients with advanced small bowel adenocarcinoma. Cancer 2017;123:3494–3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gulhati P, Raghav K, Shroff RT, et al. Bevacizumab combined with capecitabine and oxaliplatin in patients with advanced adenocarcinoma of the small bowel or ampulla of Vater: a single-center, open-label, phase 2 study. Cancer 2017;123:1011–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Aydin D, Sendur MA, Kefeli U, et al. Evaluation of bevacizumab in advanced small bowel adenocarcinoma. Clin Colorectal Cancer 2017;16:78–83. [DOI] [PubMed] [Google Scholar]
- 127.Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatchrepair deficiency. N Engl J Med 2015;372:2509–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.U.S. Food and Drug Administration. FDA grants accelerated approval to pembrolizumab for first tissue/site agnostic indication. 2017. Available at: https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm560040.htm. Accessed January 8, 2019.
- 129.Pedersen K, Foster N, Overman M, et al. ZEBRA: an ACCRU/IRCI multicenter phase 2 study of pembrolizumab in patients with advanced small bowel adenocarcinoma (SBA) [abstract]. Ann Oncol 2019;30(Suppl):Abstract # O-007. [Google Scholar]
- 130.Overman MJ, McDermott R, Leach JL, et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol 2017;18:1182–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Overman MJ, Lonardi S, Wong KYM, et al. Durable clinical benefit with nivolumab plus ipilimumab in DNA mismatch repair-deficient/microsatellite instability-high metastatic colorectal cancer. J Clin Oncol 2018;36:773–779. [DOI] [PubMed] [Google Scholar]
- 132.Overman MJ, Adam L, Raghav K, et al. Phase II study of nab-paclitaxel in refractory small bowel adenocarcinoma and CpG island methylator phenotype (CIMP)-high colorectal cancer. Ann Oncol 2018;29:139–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Aldrich JD, Raghav KPS, Varadhachary GR, et al. Retrospective analysis of taxane-based therapy in small bowel adenocarcinoma. Oncologist 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zaanan A, Gauthier M, Malka D, et al. Second-line chemotherapy with fluorouracil, leucovorin, and irinotecan (FOLFIRI regimen) in patients with advanced small bowel adenocarcinoma after failure of first-line platinum-based chemotherapy: a multicenter AGEO study. Cancer 2011;117:1422–1428. [DOI] [PubMed] [Google Scholar]
- 135.Drilon A, Laetsch TW, Kummar S, et al. Efficacy of larotrectinib in TRK fusion-positive cancers in adults and children. N Engl J Med 2018;378:731–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.U.S. Food and Drug Administration. FDA approves larotrectinib for solid tumors with NTRK gene fusions. 2018. Available at: https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm626720.htm. Accessed November 30, 2018.
- 137.Kim M, Jang HJ. The role of small bowel endoscopy in small bowel Crohn’s disease: when and how? Intest Res 2016;14:211–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Simon M, Cosnes J, Gornet JM, et al.GETAID group. Endoscopic detection of small bowel dysplasia and adenocarcinoma in Crohn’s disease: A prospective cohort-study in high-risk patients. J Crohn’s Colitis 2017;11:47–52. [DOI] [PubMed] [Google Scholar]
- 139.Goverde A, Korsse SE, Wagner A, et al. Small-bowel surveillance in patients with Peutz-Jeghers syndrome: comparing magnetic resonance enteroclysis and double balloon enteroscopy. J Clin Gastroenterol 2017;51:e27–e33. [DOI] [PubMed] [Google Scholar]
- 140.Haanstra JF, Al-Toma A, Dekker E, et al. Prevalence of small-bowel neoplasia in Lynch syndrome assessed by video capsule endoscopy. Gut 2015;64:1578–1583. [DOI] [PubMed] [Google Scholar]
- 141.Katsinelos P, Kountouras J, Chatzimavroudis G, et al. Wireless capsule endoscopy in detecting small-intestinal polyps in familial adenomatous polyposis. World J Gastroenterol 2009;15:6075–6079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Koornstra JJ. Small bowel endoscopy in familial adenomatous polyposis and Lynch syndrome. Best Pract Res Clin Gastroenterol 2012;26:359–368. [DOI] [PubMed] [Google Scholar]
- 143.Postgate A, Hyer W, Phillips R, et al. Feasibility of video capsule endoscopy in the management of children with Peutz-Jeghers syndrome: a blinded comparison with barium enterography for the detection of small bowel polyps. J Pediatr Gastroenterol Nutr 2009;49:417–423. [DOI] [PubMed] [Google Scholar]
- 144.Serrano M, Mão-de-Ferro S, Pinho R, et al. Double-balloon enteroscopy in the management of patients with Peutz-Jeghers syndrome: a retrospective cohort multicenter study. Rev Esp Enferm Dig 2013;105:594–599. [DOI] [PubMed] [Google Scholar]
- 145.van Lier MG, Wagner A, Mathus-Vliegen EM, et al. High cancer risk in Peutz-Jeghers syndrome: a systematic review and surveillance recommendations. Am J Gastroenterol 2010;105:1258–1264., author reply 1265. [DOI] [PubMed] [Google Scholar]
- 146.Drini M, Speer A, Dow C, et al. Management of duodenal adenomatosis in FAP: single centre experience. Fam Cancer 2012;11:167–173. [DOI] [PubMed] [Google Scholar]
- 147.Hewitt M, Greenfield S, and Stovall E, (eds). From Cancer Patient to Cancer Survivor: Lost in Transition. Committee on Cancer Survivorship: Improving Care and Quality of Life, Institute of Medicine and National Research Council. Washington, DC: National Academy of Sciences; 2006. Available at http://www.nap.edu/catalog/11468.html [Google Scholar]
- 148.Jansen L, Herrmann A, Stegmaier C, et al. Health-related quality of life during the 10 years after diagnosis of colorectal cancer: a population-based study. J Clin Oncol 2011;29:3263–3269. [DOI] [PubMed] [Google Scholar]
- 149.Lynch BM, Steginga SK, Hawkes AL, et al. Describing and predicting psychological distress after colorectal cancer. Cancer 2008;112:1363–1370. [DOI] [PubMed] [Google Scholar]
- 150.Mols F, Beijers T, Lemmens V, et al. Chemotherapy-induced neuropathy and its association with quality of life among 2- to 11-year colorectal cancer survivors: results from the population-based PROFILES registry. J Clin Oncol 2013;31:2699–2707. [DOI] [PubMed] [Google Scholar]
- 151.Thong MS, Mols F, Wang XS, et al. Quantifying fatigue in (long-term) colorectal cancer survivors: a study from the population-based patient reported outcomes following initial treatment and long term evaluation of survivorship registry. Eur J Cancer 2013;49:1957–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wright P, Downing A, Morris EJ, et al. Identifying social distress: a cross-sectional survey of social outcomes 12 to 36 months after colorectal cancer diagnosis. J Clin Oncol 2015;33:3423–3430. [DOI] [PubMed] [Google Scholar]
- 153.Denlinger CS, Barsevick AM. The challenges of colorectal cancer survivorship. J Natl Compr Canc Netw 2009;7:883–894. [DOI] [PMC free article] [PubMed] [Google Scholar]


