To the Editor,
Volatile organic compounds (VOCs) such as benzene, toluene, ethylbenzene, xylenes, and styrene (BTEXS) and trihalomethanes are carbon-containing chemicals that readily evaporate at room temperature [1]. VOCs are ubiquitous mainly in indoor environments, but also outdoors, and are commonly found in cigarettes, degreasers, solvents, detergents, deodorizers, paints, furniture, pesticides, and personal care products [1]. Additionally, trihalomethanes travel into the air when showering with chlorinated water or washing dishes [1]. Due to inhalation being the main route of exposure to VOCs, these chemicals have raised concerns for respiratory health [2]. In in vitro and animal experimental studies, VOCs such as chlorobenzene, styrene, and xylene have been shown to trigger inflammation in lung epithelial cell lines [3]. Other VOCs, such as benzene have been reported to cause airway inflammation and irritation by reacting with mucous membrane epithelium to cause immunoglobulin (Ig) E responses [2]. Yet, epidemiological studies on the respiratory effects of VOCs in general populations have produced conflicting results [2]. Moreover, VOCs associated with either non-reversible or reversible airflow obstruction are unknown, and the association of trihalomethanes with impaired lung function has not been previously studied. Therefore, our objective was to assess the association of several blood VOCs with non-reversible and reversible airflow obstruction among U.S. adults.
Methods
We analyzed data on 5,988 adult participants in the 2007–2012 National Health and Nutrition Examination Surveys (NHANES) aged ≥20 years old who had blood VOC and spirometry measurements. NHANES methodology are detailed elsewhere [4] and a university-based IRB determined this study as not human subjects research.
Blood VOCs were analyzed using headspace solid-phase microextraction/gas chromatography/isotope dilution mass spectrometry and samples with levels below the limit of detection (LOD) were imputed and replaced with LOD/√2 [5]. Of the 41 blood VOCs included in NHANES, our analysis included the ones with a detection frequency ≥10%. Spirometry was performed on participants by trained technicians following a pretest screening questionnaire to determine medical safety. Each participant underwent five to eight maneuvers that were considered acceptable and reproducible based on American Thoracic Society (ATS) criteria [6]. Airflow obstruction was defined as pre-bronchodilator forced expiratory volume in 1 second (FEV1) to forced vital capacity (FVC) ratio <70% and was further classified as reversible (post-bronchodilator FEV1/FVC ≥70%) or non-reversible (post-bronchodilator FEV1/FVC <0.70) as per the Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria [7,8].
Logistic regression was used to estimate the odds ratio (OR) and 95% CI for the association of the chemicals with airflow obstruction. Using multinomial logistic regression, we further assessed the association of VOCs with non-reversible and reversible airflow obstruction compared to no airway obstruction. The models were adjusted for age, poverty income ratio (PIR), body mass index (BMI), and log-transformed pack-years of cigarette smoking as continuous variables, and sex, race/ethnicity, past and current cigarette smoking, exposure to environmental tobacco smoke (living with a household member who smoked combustible tobacco products inside the home), and work or leisure physical activity (none, moderate, and vigorous) as categorical variables. The PIR was used as a proxy for socioeconomic status; it is a ratio of family income to poverty estimated using guidelines and adjusted for family size, year and state [4]. To examine whether the association of VOCs with airflow obstruction differed with smoking status, we tested smoking for effect modification on a multiplicative scale by including an interaction term in the models. The analyses were performed in SAS (Version 9.4; SAS Institute, Cary, NC), accounting for the NHANES sampling weights and complex survey design to generate nationally representative estimates. P-values <0.05 were considered statistically significant in all analyses.
Results
Our analysis included 5,988 participants with a median age of 55.1 years old; 69.1% were non-Hispanic White, 11.0% were non-Hispanic Black, 7.8% were Mexican American, and 12.1% were of ‘other’ race/ethnicity. Half were female (51.0%); had a high PIR >3 (50.6%); 21.3% and 22.4% were current or former smokers, respectively; 31.3% had a normal BMI; and airflow obstruction prevalence was 14.0%. The detection frequencies of BTEXS chemicals, in decreasing order, were toluene (92.7%), m-/p-xylene (84.4%), 1,4-dichlorobenzene (52.1%), o-xylene (45.1%), styrene (42.2%), ethylbenzene (39.1%), and benzene (31.5%). The levels of trihalomethanes in decreasing order were chloroform (86.0%), bromodichloromethane (70.0%), dibromochloromethane (53.2%), methyl tert-butyl ether (MTBE) (26.0%), and bromoform (23.9%) (Figure 1A).
Figure 1:
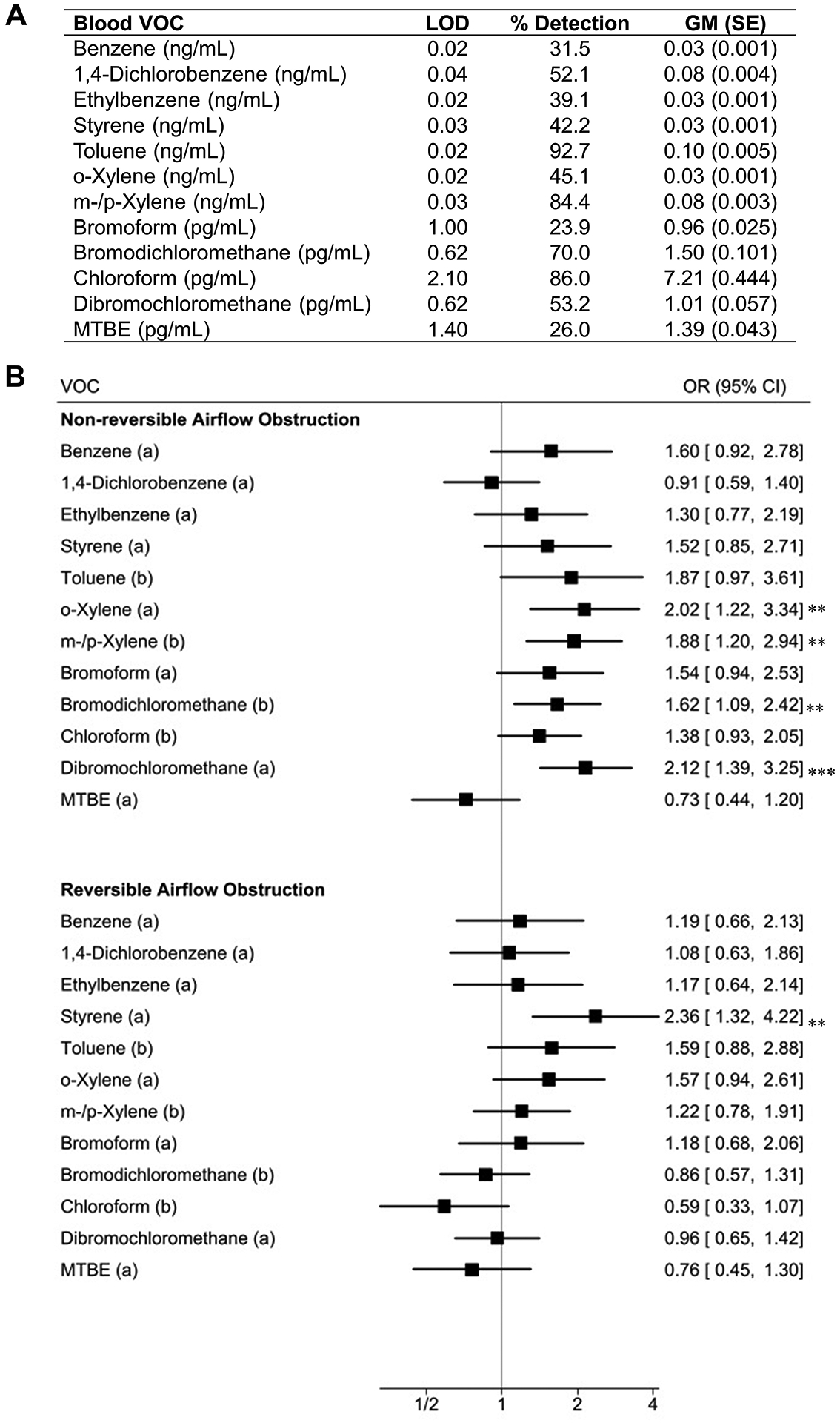
Detection frequency and levels of blood VOCs (A) and forest plot for the association of VOCs with reversible and non-reversible airflow obstruction (B).
** P < 0.01 *** P < 0.001
(a) Detection of chemical (b) Log10-transformed concentrations of chemical
Abbreviations: VOC, volatile organic compound; MTBE, methyl-tert-butyl ether.
Models adjusted for age, poverty income ratio, body mass index, and log-transformed pack-years of cigarette smoking used as continuous variables, and sex, race/ethnicity, past and current smoking status, and exposure to environmental tobacco smoke used as categorical variables.
The odds of airflow obstruction were 20% to 82% higher with blood detection of styrene (OR: 1.82, 95% CI: 1.23–2.68), o-xylene (OR: 1.43, 95% CI: 1.07–1.89), bromoform (OR: 1.41, 95% CI: 1.06–1.88), and dibromochloromethane (OR: 1.20, 95% CI: 1.01–1.41) after accounting for covariates. Airflow obstruction odds were also 20% to 50% higher with a 10-fold increase in blood m-/p-xylene (OR: 1.50, 95% CI: 1.17–1.93) and bromodichloromethane (OR: 1.20, 95% CI: 1.01–1.41). The associations of toluene and o-xylene with airflow obstruction differed with smoking status (Pinteraction=0.002 and Pinteraction=0.02 respectively). Both chemicals were associated with airflow obstruction only in current smokers (OR: 3.50, 95% CI: 1.56–7.85 for toluene and OR: 3.52, 95% CI: 1.46–8.49 for o-xylene), but not in never or former smokers.
Blood detections of o-xylene (OR: 2.02, 95% CI: 1.22–3.34) and dibromochloromethane (OR: 2.12, 95% CI: 1.39–3.25) as well as 10-fold increases in blood m-/p-xylene (OR: 1.88, 95% CI: 1.20–2.94) and bromodichloromethane (OR:1.62, 95% CI: 1.09–2.42) were associated with non-reversible airflow obstruction. Blood detection of styrene was associated with reversible airflow obstruction (OR: 2.36, 95% CI: 1.32–4.22) (Figure 1B).
Discussion
Using representative samples of the U.S. adult population, we found that blood xylenes, bromodichloromethane, and dibromochloromethane were associated with non-reversible airflow obstruction, while blood styrene was associated with reversible airflow obstruction. Blood toluene and o-xylene were associated with airflow obstruction only in current smokers.
Our study is the first to identify VOCs associated with non-reversible versus reversible airflow obstruction, and the first on trihalomethanes and lung function in a general population. It is also the first to report an association of styrene with adverse respiratory function in non-occupational settings. Prior evidence on xylene and impaired lung function in a general population was limited to one study showing that urinary xylene metabolites may be linked to reduced FEV1, FEV1/FVC, and mid-expiratory flow in older adults [9]. Consistent with the present study’s findings, exposure to styrene can cause irritation, trigger interferon γ production, and promote allergic sensitization by increasing antigen-presenting activity leading to IgE production, eosinophilic inflammation, and airway hyperreactivity [10]. Xylene has been shown to cause cellular apoptosis in bronchus-associated lymphoid and lung tissues in both young and adult Sprague-Dawley rats by activating caspase-3 [11]. This can cause epithelial apoptosis, elastolytic activity in bronchoalveolar lavage, and emphysema, a phenotype of chronic obstructive pulmonary disease [12]. The mechanisms by which the trihalomethanes bromodichloromethane and dibromochloromethane may be associated with non-reversible airflow obstruction is unclear. Blood trihalomethanes have been reported to be associated with oxidative stress, and this could be a possible pathway between exposure and impaired lung function [13]. In the U.S., a recent NHANES analysis identified dibromochloromethane as potentially associated with asthma in adolescents [14].
The main limitation of this analysis was the cross-sectional design which precludes assessing temporality between VOC exposure and airflow obstruction. Other limitations included the measurement of VOCs in a single blood sample and the analysis of certain VOCs as binary variables comparing chemical detection versus non-detection, due to their low detection frequency. The strengths of the study include the large sample size, blood VOCs measurements which capture both indoor and outdoor exposure, individual level blood VOCs assessment by the Centers for Disease Control and Prevention with rigorous quality control and quality assurance procedures, spirometry testing, and the analysis adjusted for several important covariates, which minimized residual confounding.
In conclusion, xylenes, bromodichloromethane, and dibromochloromethane exposure may be associated with non-reversible airflow obstruction, whereas blood styrene may be associated with reversible airflow obstruction. Additionally, toluene and o-xylene exposure may be associated with impaired lung function in smokers. However, the associations reported in this study are cross-sectional and should be confirmed by prospective investigations with repeated exposure measures. Future studies to understand their underlying mechanisms and studies to examine whether reducing VOC exposure can help prevent the VOC-associated airflow obstruction are warranted. If the observed associations are causal, measures to reduce exposure to xylenes, bromodichloromethane, dibromochloromethane, and styrene may improve or prevent airflow obstruction.
Funding:
This work was supported in part by the National Institute on Drug Abuse (Grant Number K01DA044313), the National Institute of Environmental Health Sciences (Grant Numbers R21ES032161, R01ES030743, R01ES027815, and P30ES006096), R01ES030743, R01ES027815, and P30ES006096), National Heart, Lung, and Blood Institute (Grant Number R01HL132344), National Human Genome Research Institute (Grant Number R01HG011411) of the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Disclosures: The authors have no disclosure related to the submitted manuscript.
Data Sharing:
AM takes full responsibility for the integrity of the dataset and the analysis results. The SAS codes and datasets will be made available for the sole purpose of reproducing the findings upon request.
REFERENCES
- 1.Cakmak S, Cole C, Hebbern C, Andrade J, Dales R. Associations between blood volatile organic compounds, and changes in hematologic and biochemical profiles, in a population-based study. Environ Int 2020;145:106121. [DOI] [PubMed] [Google Scholar]
- 2.Alford KL, Kumar N. Pulmonary Health Effects of Indoor Volatile Organic Compounds—A Meta-Analysis. Int J Environ Res Public Health. 2021;18(4):1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuang H, Li Z, Lv X, Wu P, Tan J, Wu Q, et al. Exposure to volatile organic compounds may be associated with oxidative DNA damage-mediated childhood asthma. Ecotoxicol Environ Saf 2021;210:111864. [DOI] [PubMed] [Google Scholar]
- 4.U.S. Department of Health and Human Services (DHHS), Centers for Disease Control and Prevention (CDC), National Center for Health Statistics (NCHS). The National Health and Nutrition Examination Survey (NHANES). https://www.cdc.gov/nchs/nhanes/index.htm. Last Accessed April 24, 2022.
- 5.Kirman CR, Aylward LL, Blount BC, Pyatt DW, Hays SM. Evaluation of NHANES biomonitoring data for volatile organic chemicals in blood: application of chemical-specific screening criteria. J Expo Sci Environ Epidemiol 2012;22(1):24–34. [DOI] [PubMed] [Google Scholar]
- 6.Hankinson JL, Bang KM. Acceptability and reproducibility criteria of the American Thoracic Society as observed in a sample of the general population. Am Rev Respir Dis 1991;143(3):516–521. [DOI] [PubMed] [Google Scholar]
- 7.Mendy A, Forno E, Niyonsenga T, Gasana J. Blood biomarkers as predictors of long‐term mortality in COPD. Clin Respir J 2018;12(5):1891–1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mendy A, Forno E, Niyonsenga T, Carnahan R, Gasana J. Prevalence and features of asthma‐COPD overlap in the United States 2007–2012. Clin Respir J 2018;12(8):2369–2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoon H, Hong Y, Cho S, Kim H, Kim Y, Sohn JR, et al. Exposure to volatile organic compounds and loss of pulmonary function in the elderly. Eur Respir J 2010;36(6):1270–1276. [DOI] [PubMed] [Google Scholar]
- 10.Diez U, Kroeßner T, Rehwagen M, Richter M, Wetzig H, Schulz R, et al. Effects of indoor painting and smoking on airway symptoms in atopy risk children in the first year of life results of the LARS-study. Int J Hyg Environ Health 2000;203(1):23–28. [DOI] [PubMed] [Google Scholar]
- 11.Sandikci M, Seyrek K, Aksit H, Kose H. Inhalation of formaldehyde and xylene induces apoptotic cell death in the lung tissue. Toxicol Ind Health 2009;25(7):455–461. [DOI] [PubMed] [Google Scholar]
- 12.Demedts IK, Demoor T, Bracke KR, Joos GF, Brusselle GG. Role of apoptosis in the pathogenesis of COPD and pulmonary emphysema. Respir Res 2006;7(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu C, Wang Y, Chen Y, Sun Y, Huang L, Cheng Y, et al. Blood and urinary biomarkers of prenatal exposure to disinfection byproducts and oxidative stress: a repeated measurement analysis. Environ Int 2020;137:105518. [DOI] [PubMed] [Google Scholar]
- 14.Sun Y, Xia P, Xie J, Mustieles V, Zhang Y, Wang Y, et al. Association of blood trihalomethane concentrations with asthma in US adolescents: nationally representative cross-sectional study. Eur Respir J 2022;59(5):210144. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
AM takes full responsibility for the integrity of the dataset and the analysis results. The SAS codes and datasets will be made available for the sole purpose of reproducing the findings upon request.


