Abstract
Background
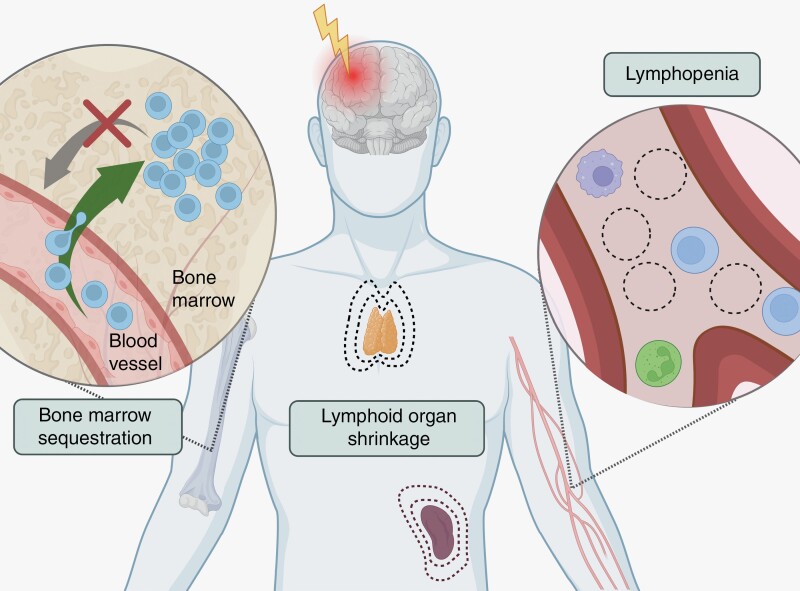
The nervous and immune systems interact in a reciprocal manner, both under physiologic and pathologic conditions. Literature spanning various CNS pathologies including brain tumors, stroke, traumatic brain injury and de-myelinating diseases describes a number of associated systemic immunologic changes, particularly in the T-cell compartment. These immunologic changes include severe T-cell lymphopenia, lymphoid organ contraction, and T-cell sequestration within the bone marrow.
Methods
We performed an in-depth systematic review of the literature and discussed pathologies that involve brain insults and systemic immune derangements.
Conclusions
In this review, we propose that the same immunologic changes hereafter termed ‘systemic immune derangements’, are present across CNS pathologies and may represent a novel, systemic mechanism of immune privilege for the CNS. We further demonstrate that systemic immune derangements are transient when associated with isolated insults such as stroke and TBI but persist in the setting of chronic CNS insults such as brain tumors. Systemic immune derangements have vast implications for informed treatment modalities and outcomes of various neurologic pathologies.
Keywords: brain injury, glioblastoma, mmunosuppression, lymphopenia, T-cells
Key Points.
Acute and chronic brain injuries induce systemic immune derangements including lymphopenia.
Understanding underpinnings of immune derangements secondary to brain injury will advance the clinical management of all patients with acute and chronic neurological diseases.
The field of neuroimmunology has continued to uncover reciprocal communication between the nervous and immune systems. While many studies have served to highlight the impact of inflammation on central nervous system (CNS) pathologies, there has been a dearth of studies that assess how CNS pathologies, in turn, are able to affect systemic immunity.
Over the last several decades, the immunologic consequences of neurological insults have been described in various case reports or can be found hidden among other pre-clinical or clinical datasets. However, to date, no dedicated study has truly examined the often-diminished state of peripheral immunity that can arise in the context of intracranial or other CNS pathologies.
While other groups have focused on local immunosuppression at the site of the tumor and how this impacts immunotherapeutic success and clinical trial design,1–4 our groups have authored recent mechanistic studies characterizing the state of peripheral immunity in both patients and various mouse models with intracranial tumors. Together, we have demonstrated numerous immune deficits ranging from downregulation of major histocompatibility complex II on blood-derived monocytes and B cells5 to memory T-cell dysfunction to potent immunosuppressive soluble factors that inhibit T-cell function and hinder cellular immunity.5 We have demonstrated anatomical changes in both primary and secondary lymphoid organs, including atrophy of the spleen and the thymus, as well as severe T-cell lymphopenia, resulting from a decreased absolute number, not percentage, of circulating T-cells.5,6 Our studies echo previous publications by others demonstrating that GBM patients have lower absolute counts of T-cells and are in a state of overall lowered T-cell immunity.7–11 We have additionally discovered that T-cells accumulate in the bone marrow of mice with intracranial tumors and that this T-cell sequestration is dependent upon the loss of sphingosine-1-phosphate receptor 1 (S1P1).6
In this review, we will highlight the presence of similar immunologic changes, particularly T-cell changes, across multiple CNS pathologies, and term these “systemic immune derangements.” Importantly, we highlight that these derangements are present not only in the setting of intracranial tumors but also in the setting of other insults (stroke, traumatic brain injury (TBI), multiple sclerosis (MS), and spinal cord injury (SCI)), provided these occur within the CNS (see Figure 1). The commonality we will reveal amidst varying CNS pathologies suggests a brain or CNS-intrinsic mechanism for limiting immune responses and secondary immune-mediated CNS damage, a mechanism that is perhaps activated by any of a number of inflammatory insults to the brain or spinal cord. Such a mechanism may be an evolutionarily adaptive response, consistent with the framework of classical definitions of CNS immune privilege. We propose here that the development of CNS pathology-driven systemic immune derangements represents a novel mechanism of immune privilege that serves to restrict the intensity of a peripheral immune response in the setting of CNS insults. We advance here a hypothesis that all brain injuries can induce some level of global immune derangement which impacts the peripheral immune organs and disrupts systemic immunity.
Figure 1.
Systemic immune derangements include T-cell lymphopenia, lymphoid organ contraction, and sequestration of T-cells within the bone marrow.
Intracranial Pathologies
Brain Tumors
Patients
Many studies have focused on T-cell exhaustion,4,12–14 including both progenitor and terminal exhaustion, within the tumor microenvironment and recent studies underscore the role of biological sex in the development of exhausted T-cells within the tumor.15 This review, however, will focus on peripheral immunosuppression. Both primary and metastatic brain tumors are difficult to treat with conventional therapies, as they display unique challenges including both intratumoral and peripheral immunosuppression. Peripheral immunosuppression includes various systemic immune derangements, which have been identified in patients,6,16 mice,5,6,17,18 and rats19 with brain tumors. In patients, these immune derangements have predominantly been studied in the setting of glioblastoma (GBM), the most common primary malignant brain tumor in adults.
While many cancers are associated with vague immunosuppressive features including T-cell anergy and exhaustion, GBM-induced immunosuppression is broader, more severe, and affects all stages of T-cell development, activation, and function. Studies dating back more than 50 years have described various phenotypic and functional evidence of immunosuppression in patients with primary intracranial tumors. These studies described unique features of immunosuppression in intracranial tumors which have not been observed in other cancers. For example, it was reported that T-cells isolated from GBM patients cannot be sensitized against primary tumor antigens, and normal hypersensitivity responses on skin tests are significantly delayed in GBM patients compared to controls.9,10 Further defects in T-cell activation, proliferation, and responses in patients with GBM were also reported.10 Similarly, it was described that T-cells isolated from GBM patients fail to be activated properly with mitogens or typical T-cell activating protocols.10,11,20,21 GBM patient T-cells make less IL-2, and their high affinity IL-2 receptor expression (CD25) during activation is diminished.11,21 Finally, early T-cell activation signaling cascades and calcium mobilization have been found to be defective in GBM patients.11,21,22 Together, these data indicated profound T-cell lymphopenia and additional functional immune defects in GBM patients and those patients with other primary brain tumors. Although lymphopenia has been observed in patients for decades, it has often been attributed to therapeutic interventions, including chemotherapy, radiation, and treatment with steroids such as dexamethasone. Importantly, however, lymphopenia and other systemic immune derangements arise in newly diagnosed, treatment-naïve patients, as demonstrated by Gustafson et al.7 and Chongsathidkiet et al.,.6 In the latter paper, lymphopenia was accompanied by both lymphoid organ contraction and sequestration of T-cells in the bone marrow. Additional prospective studies are needed to thoroughly characterize T-cell sequestration and lymphoid organ contraction in the setting of brain metastases.
The lymphopenia noted in GBM patients for decades9 has recently been correlated with decreased overall survival.23 T-cell lymphopenia is more severe in the CD4+ compartment,5,6,17 and there is a concomitant increase in the percentage of regulatory T-cells (Tregs).24 Further, a retrospective study showed that nearly half of 200 newly diagnosed GBM patients exhibited decreased lymphocyte levels (<1500/µl) at baseline.16 Lymphopenia is also observed in patients with brain metastases and is negatively correlated with survival outcomes in breast cancer patients who develop brain metastases.25 On a related note, tumor resection surgery, which is typically performed in most GBM patients as a first line of therapy, can be considered an injury in itself further impacting peripheral lymphocyte counts. Accordingly, a recent study has shown that the number of circulating T-cells further decreases after surgical resection in a murine model of glioblastoma, though no clinical studies have examined this question to date.26
Animal Models
Immunocompetent mouse models of brain tumors including GBM have elegantly recapitulated major hallmarks of immune derangements observed in patients. Systemic immune derangements were initially described in patients and have been replicated in recent pre-clinical murine studies. A study by Chongsathidkiet et al. examined murine models of GBM in addition to patients and further demonstrated that T-cell sequestration in the bone marrow developed in mice when tumors were placed intracranially, but not subcutaneously, and that this trend was maintained across tumor types including GBM, melanoma, lung adenocarcinoma, and triple-negative breast cancer.6 A study by Ayasoufi et al. supported this finding by demonstrating an increase in CD4+ T-cells in the bone marrow.5
In addition to T-cell sequestration in the bone marrow, T-cell lymphopenia and lymphoid organ contraction have been observed in animal models of GBM and other brain tumors. Specifically, splenic atrophy has also been observed in mice with intracranial GBM.5,27 Thymic atrophy has been observed in mouse models of intracranial melanoma, diffuse intrinsic pontine glioma, GBM, and an RCAS-spontaneous glioma model,5,6,18,27 and rat models of intracranial gliomas.19 Finally, Ayasoufi et al. also demonstrated the presence of a potently immunosuppressive soluble factor in the serum of glioma-bearing mice capable of blocking T-cell function and proliferation.5
Stroke
Patients
Stroke patients are at risk for “stroke-induced immunodepression syndrome,” severe peripheral immunosuppression that arises after ischemic stroke. A thorough review of immunosuppression in stroke by Westendorp et al. demonstrates that infection complications arise in approximately one-third of stroke patients and, strikingly, nearly 50% of patients with post-stroke infections died, as compared to only 18% of patients without infection. It is important to note that the post-stroke immunosuppression cannot be attributed to simple aspiration pneumonia due to disability. Likewise, it was not avoided by prophylactic antibiotics in a large clinical trial, thus indicating a distinct mechanism for this immunosuppression beyond simple paralysis.28 These data suggest that brain injury-induced immunosuppression and, consequently, susceptibility to infection, plays a major role in post-stroke outcomes.29 In line with these findings, of all CNS pathologies, systemic immune derangements have been most thoroughly characterized in stroke patients30–38 and mouse models thereof.38–40
Given the feasibility of assessing lymphocyte counts in peripheral blood, lymphopenia is particularly well-described in stroke patients. Two independent retrospective studies showed that approximately one quarter of stroke patients presented with lymphopenia on admission.30,33 Lymphopenia on admission is independently associated with both an increased risk of infection30,33 and mortality at 90-days post-stroke.33 Interestingly, infarct volume is the factor that is most highly correlated with the development of lymphopenia in the first several days post-stroke.41 This demonstrates a direct correlation between the magnitude of the brain injury and the extent of the resulting peripheral immunosuppression. Given that infection is one of the most common complications from a stroke and is significantly associated with both death29 and larger infarct size,42 these data suggest that more severe strokes result in lymphopenia, which in turn results in susceptibility to infection and worse patient outcomes. These conclusions are further supported by an observational study of 855 patients demonstrating that lymphopenia on admission is associated with poorer neurological status and unfavorable outcomes in patients.35 Further, patients with sustained lymphopenia 5 days after their stroke did even worse than those who exhibited lymphopenia on admission, exhibiting increased rates of infection and poorer outcomes at 3 months of follow-up.35 A prospective study in stroke patients demonstrated that circulating T-cells, specifically, were decreased on admission, suggesting that a decrease in T-cells drives the lymphopenia observed across studies. The same prospective study revealed that patients exhibit T-cell lymphopenia for approximately 1 week before returning to baseline,31 while an additional study demonstrated that circulating T-cells reach their lowest levels around 12 hours post-stroke.43
Lymphoid organ contraction, particularly splenic atrophy, is also well-characterized in stroke patients. In a prospective study conducted by Vahidy et al. 158 healthy volunteers and an equal number of stroke patients had spleen measurements taken over the course of five consecutive days, or the first 24 hours post-stroke then daily, respectively. Splenic atrophy was seen in 40% of stroke patients.34 Further, patients who presented with severe strokes were more likely to present with splenic atrophy on admission, or at day 3 post-stroke.32 A prospective observational study showed that stroke patients maintained splenic atrophy until approximately four days post-stroke, then spleen size increased through day 8 post-stroke.37 Another study showed that the onset of both lymphopenia and splenic atrophy occurs less than 24 hours post-stroke but both systemic immune derangements disappear after 7–10 days post-stroke when T-cell numbers and spleen volumes returned to normal.38
Animal Models
Pre-clinical models of stroke, particularly transient middle cerebral artery occlusion (tMCAO), have been used to identify the timing of systemic immune derangements. Using pre-clinical models, various studies have shown splenic39,44,45 and thymic46 atrophy and circulating T-cell lymphopenia.46 In tMCAO models, thymic atrophy was observed and the number of thymocytes (developing T-cells in the thymus) was significantly lower than sham mice for the first two weeks after stroke, and only fully recovered after two months,46 while splenic atrophy and T-cell lymphopenia was only observed for one-week post-stroke.46
Multiple Sclerosis
Patients
MS is a neuroinflammatory disease that is associated with brain atrophy, demyelination, debilitating disability, loss of motor function, and cognitive decline. In the 1970s, broad immunosuppressants were identified as the most effective drugs for MS.47 MS is typically considered an inflammatory disease, hence the notion of peripheral immunosuppression in the context of MS might seem counterintuitive. However, upon close examination of the clinical and pre-clinical MS literature, we discovered evidence of peripheral immune derangements including T-cell lymphopenia.
Our systematic review of the literature indicated the presence of lymphopenia in MS patients. Early studies used rosetting techniques to demonstrate lymphopenia in MS patients when compared to healthy controls.48 Similarly, CD8 T-cell deficiencies in effector and memory responses of MS patients were observed when the functional and phenotypic analysis was used to compare MS patients to healthy controls.49 Interestingly, one study also correlated the extent of lymphopenia to active MS disease,50 but these studies are over 20 years old and do not use modern technologies to evaluate lymphopenia. Hence, direct studies to establish the extent of lymphopenia in MS are still needed. Nevertheless, it is crucial to discuss all studies that have reported data suggestive of immunosuppression as a direct result of MS alone and those effects that can be separated from treatment-induced lymphopenia.
While immune organ involution has not been directly measured in MS patients, thymus function has been evaluated in MS patients using human T-cell excision circles (TRECs). TRECs are an excellent proxy for T-cell output and, consequently, thymic size. These studies demonstrated that MS patients had reduced TRECs numbers in general among which regulatory T-cells were further reduced.51–54 In addition to defects seen in mature and newly generated T-cells, MS patients have reported defects in their T regs and B-cell.52,53,55
MS patients receive antiviral drugs, T-cell and B-cell depleting reagents, and fingolimod, which sequesters T-cells in secondary lymphoid organs.56–58 Each of these drugs is immunomodulatory in nature and can directly induce lymphopenia. However, several studies have measured lymphocyte counts prior to treatment and determined that patients with lower baseline counts were more prone to lymphopenia after treatment. The cause of lymphopenia at baseline, however, was not determined.59–63 Further, whether lymphopenia is due to homeostatic deficiencies downstream of these immunomodulatory drugs, or directly reflective of ongoing MS progression in the brain is unclear. Together, these studies support our hypothesis that MS activity in the brain is likely linked to lymphopenia in the blood.
Animal Models
Leading animal models of MS include the experimental autoimmune encephalitis (EAE) model, demyelination in certain strains of mice caused by Theiler’s murine encephalomyelitis virus (TMEV), and the cuprizone-mediated model of demyelination. Most MS lesions in patients are infiltrated by CD8+ T-cells64,65 while EAE pathology is CD4+ T-cell-mediated. Both CD4 and CD8 T-cells likely play crucial roles in MS pathogenesis yet the role of each T-cell type across mouse models of MS remains controversial. While addressing this controversy is beyond the scope of the review at hand, all models of MS are unanimously considered to induce severe neuroinflammation. The TMEV model is a direct model of virus-induced damage and brain injury that is due to both damage directly caused by the virus and damage caused by the immune system to the neurons.66–69 This model demonstrates the involvement of CD8 T-cells in the brain but ensues picornavirus viral infection which may not then directly model human MS. Finally, the cuprizone model is a chemically induced model of damage to the CNS that leads to eventual demyelination.70 While none of these models solely recapitulate the complexity and heterogeneity of all MS pathologies, they all contain significant levels of neuroinflammation. Interestingly, peripheral immune derangements, including T-cell lymphopenia, thymic, and splenic involution, have been documented in each of the pre-clinical models discussed above. Specifically, thymic and splenic involution was reported transiently in EAE, acutely post-intracranial TMEV infection, and during cuprizone-induced demyelination.5,71–74 While one paper reported reduced B and T-cell counts in the spleen within 6 days post EAE induction,75 another study reported that spleen involution in the EAE model is heterogenous and dependent on mouse strain, pathology, and time points measured.76
Additional Demyelinating Diseases
Current data establish Epstein-Barr Virus as a strong causative link to MS development.77–79 Therefore, we have also included studies that investigated systemic immune derangements in demyelinating viral infections below.
Progressive multifocal leukoencephalopathy
Progressive multifocal leukoencephalopathy (PML) is a rare, but severe complication of long-term immunosuppression.80 PML is caused by viral infections in the brain that induce encephalitis. Nearly all case reports on PML patients directly mention or show lymphopenia (lymphocyte counts < 1000), suggesting that active PML induces severe lymphopenia.
Krabbe Disease
Researchers have also described progressive peripheral lymphopenia in the context of other demyelinating diseases including Krabbe disease, a lysosomal beta-galactosylceramidase deficiency that results in severe demyelination. In a mouse model of this disease, the severity of neurological symptoms directly correlated with the extent of lymphopenia.81
Canine Distemper Virus
Lymphopenia following brain infection has also been reported after canine distemper virus (CDV) infection.82 Although CDV infection alone is known to directly lyse lymphocytes, strong evidence of severe lymphopenia and thymic involution only occurred with intracranial, not extracranial, CDV infections.83,84 Therefore, it is the intracranial locale of the infection that directly determined the extent of lymphopenia in these studies.
Traumatic Brain Injury
Patients
Each year, ~70 million people worldwide will suffer from a TBI. Similar to other brain pathologies, systemic immune derangements are present in patients,85 mice,86 and rats87,88 after TBI. Whereas approximately one quarter of stroke patients presented with lymphopenia, a 10-year retrospective study of over 2500 TBI patients determined that more than one-third of TBI patients presented with lymphopenia on admission.85 Further, lymphopenia on admission was associated with worse outcomes including longer length of hospital stay, a higher level of care required upon discharge, and an increased risk of mortality.85 Lymphopenia was also more prevalent in patients with more severe TBI85 and did not resolve until 1 week after the injury.89
Animal Models
There are a number of accepted animal models of TBI, including models of fluid percussion injury, controlled cortical impact injury, weight-drop impact acceleration injury, and blast injury (reviewed by Xiong et al.).90
In terms of lymphoid organ contraction, thymic atrophy is most well-described in the context of TBI. A study in mice demonstrated a 60% loss in thymus weight one-day post-injury when compared to sham controls86 and studies in rats have recapitulated thymic atrophy after TBI.87,88 Another study described a bimodal pattern of thymic T-cell loss and thymic atrophy, immune cell loss from the spleen, and depressed hematopoiesis in the bone marrow following the induction of TBI in mice.91
Other CNS Pathologies
Spinal Cord Injury
Although most frequently noted in the setting of intracranial pathologies, systemic immune derangements occur after spinal cord injury (SCI) as well, indicating the impact of a CNS-specific injury on systemic immune function. A cross-sectional study in individuals younger than 60 years demonstrated that patients with SCI had significantly lower lymphocyte concentrations in the blood compared with healthy individuals.92 The same study suggested that these decreased lymphocyte counts, contributing to general “immune frailty” were implicated in infection complications and decreased longevity.92 Further clinical studies have shown significant decreases in circulating lymphocytes in the first week after injury and have shown that lymphopenia at admission was correlated with higher level injuries.93,94 Murine studies of T3 SCI noted both lymphopenia95,96 and splenic atrophy.96–98
Epilepsy
Although there are relatively few papers detailing the extent of systemic immune derangements in seizure disorders, these have been noted in both epileptic patients and murine models of epilepsy. In particular, patients with convulsive status epilepticus, a seizure lasting longer than 5 minutes or more than one seizure in 5 minutes, exhibited lymphopenia in the first-hour post-seizure compared to a healthy control group.99,100 Further, patients exhibited decreased lymphocyte counts in the acute phase of status epilepticus (at the time of seizure) compared to the subacute phase (72 hours post-seizure).99 These same findings were noted in patients with generalized tonic-clonic (“Grand mal”) epileptic seizures.100 These data suggest a reversal of lymphopenia upon resolution of neurological insult. Notably, lymphopenia, splenic and thymic contraction, and decreased thymic cellularity have been described in murine models of epilepsy.5,101
Parkinson’s Disease
Attempts to understand the role of immunity in the pathogenesis of Parkinson’s disease (PD) have uncovered systemic immune derangements in patients. As early as 2001, studies demonstrated lymphopenia in the peripheral blood of PD patients.102,103 Notably, even studies that have not identified an absolute decrease in lymphocyte numbers have noted a lower CD4+:CD8+ T-cell ratio in PD patients compared to controls.104 One particularly compelling study from 2018 examined the peripheral blood of 60 PD patients compared to 30 healthy controls.105 In addition to observing a decreased mean T-cell count in the overall PD group, they found that advanced PD was associated with lower CD4+ and CD8+ T-cell counts than earlier-stage disease. This relationship between disease severity and the number of circulating T-cells was replicated by Bhatia et al. in 2021, showing that, for their cohort, reductions in CD8+ T-cells drove lymphopenia and were correlated with disease severity.106
Cardiac arrest and cardiopulmonary resuscitation
Just as the local ischemia and reperfusion within the CNS induced by stroke can cause systemic immune derangements, occlusion of CNS blood supply from other causes may have similar effects.107–109 As a pathology that indirectly impacts the CNS, decreased circulating lymphocytes have been identified in patients within the first days following CA/CPR.110,111 Consistent with findings in the single-insult pathologies described above, peripheral lymphocytes in CA/CPR patients hit their nadir approximately 90 minutes following the reperfusion event then begin to return to baseline.112
Many of the observations drawn from human patients following cardiac arrest are mirrored in mouse models. Both chemical and asphyxial murine models of CA/CPR have demonstrated peripheral blood lymphopenia, as well as splenic and thymic contraction within three days of mouse resuscitation.108,113 Together, these data indicate that immune derangements are associated with brain injuries even in instances where brain injury was an indirect result of cardiac insufficiency and resulting hypoxia.
Blood–brain barrier disruption
BBB disruption is a severe neuroinflammatory event that can be considered a form of brain injury. We demonstrated in a model of blood–brain barrier disruption where the damage is mediated by virus-specific resident memory CD8 T-cells, that BBB disruption is directly associated with peripheral lymphopenia in the blood.114 Importantly, BBB disruption and brain atrophy are two major brain pathologies confirmed in numerous COVID patients.115–119 In fact, the reported lymphopenia in COVID patients with severe brain involvement directly supports our interpretation that brain insults induced by BBB disruption or endothelial cell damage was linked to peripheral lymphopenia.108
Concluding Remarks
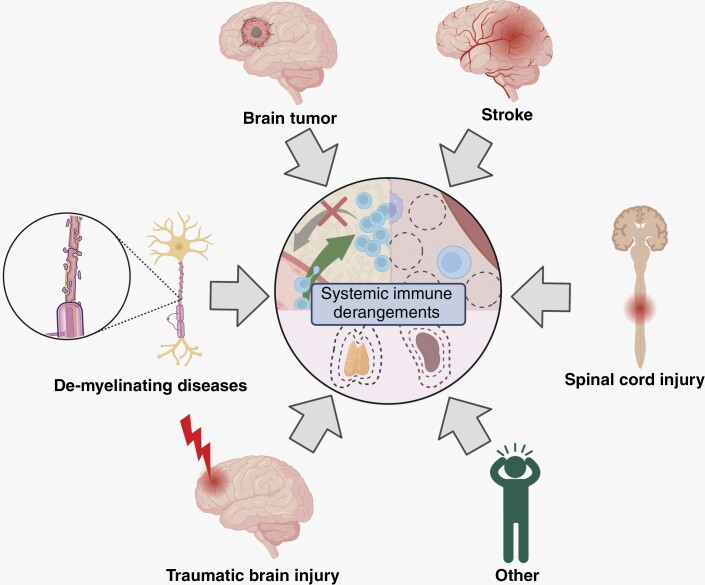
As described in the preceding sections, systemic immune derangements have been described across various CNS pathologies (Figure 2). Most of these systemic immune derangements have been noted in clinical, rather than pre-clinical, settings, indicating a lack of recognition of the connections between these phenomena and the need for further mechanistic studies to uncover their etiologies. However, several seminal mouse studies have paved the way for mechanistic studies into the origins of immunosuppression following brain injuries by recapitulating hallmark features of immune derangements in patients with acute and chronic neurological diseases (Table 1).
Figure 2.
Various CNS pathologies including brain tumors, stroke, SCI, traumatic brain injury, and de-myelinating diseases result in systemic immune derangements.
Table 1.
Summary of data on systemic immune derangements in human or animal models among the pathologies discussed in this review
| Lymphopenia | T cell sequestration to the bone marrow | Splenic contraction | Thymic contraction | T cell dysfunction/ other (see notes) | |
|---|---|---|---|---|---|
| Brain tumors | Patients Mouse models |
Patients, Mouse models |
Patients, Mouse Models |
Mouse models | Delayed hypersensitivity, defects in activation, proliferation |
| Stroke | Patients Mouse models |
Not reported | Patients Mouse models |
Mouse models | Not reported |
| Multiple Sclerosis/EAE | Patients Mouse models (strain and time point dependant) |
Not reported | Not reported in patients/Splenic T cell contraction reported in EAE, overall spleen atrophy depends on pathology, strain, and model | Patients (reduced TRECs =proxy) Mouse models (transient thymic involution in EAE) |
Defects in CD8+ effector and memory responses Defects in Trges |
| TMEV/cuprizone-induced demyelination | Mouse models (time point dependent) | Not reported | Not reported in TMEV/spleen atrophy in cuprizone models | Mouse models—TMEV and cuprizone | Not reported |
| Additional demyelinating diseases | Patients: PML, Krabbe disease, Dogs: canine distemper virus | Not reported | Not reported | Dogs: canine distemper virus | Not reported |
| Traumatic Brain Injury | Patients | Not reported | Mouse models have immune cell loss from spleen | Mouse and rat models | Depressed homeostasis in the bone marrow |
| Spinal Cord Injury | Patients Mouse models |
Not reported | Mouse models | Not reported | Not reported |
| Epilepsy | Patients Mouse models |
Not reported | Mouse models | Mouse models | Not reported |
| Parkinson’s disease | Patients | Not reported | Not reported | Not reported | Not reported |
| CA/CPR | Patients Mouse models |
Not reported | Mouse models | Mouse models | Not reported |
| BBB disruption | Patients Mouse models |
Not reported | Not reported | Not reported | Not reported |
Existing clinical studies have highlighted that an insult to the CNS, regardless of the nature of the insult, leads to several distinct and predictable phenotypes, namely, systemic immune derangements. Importantly, depending on the nature of the insult, systemic immune derangements are either chronic, as in the setting of brain tumors, or transient, in the setting of TBI, infections, and stroke. Although we propose that systemic immune derangements develop in response to acute or chronic injury, the transient nature of systemic immune derangements after acute injury supports the idea that this is an evolutionarily adaptive mechanism to protect the CNS from inflammation, and as such, represents a novel mechanism of immune privilege. The potential benefit vs. harm of mitigating immune derangement must be evaluated per intracranial pathology. In brain tumors, we argue, this mitigation results in benefits while in MS it might not. In parallel, profound immunosuppression in MS can cause further harm due to a lack of protective immunity. Hence, extensive discussions and specific targets will be required to specifically mitigate immune derangements while protecting the CNS. It is important for future studies, in brain tumors, to design ways to overcome these systemic immune derangements, as they likely limit immunotherapeutic efficacy, regardless of the protection against damaging inflammation this mechanism may provide the CNS. Should novel therapeutics overcome these systemic immune derangements, patients could be medically managed with steroids for life-threatening CNS inflammation but initiating sufficient anti-tumor responses in the CNS and initiating such inflammation remain the current challenge. Further mechanistic insights are needed in order to mitigate immune derangements in a pathology and cell type-specific manner in order to maximize patient outcomes.
Given that the preponderance of evidence for systemic immune derangements is clinical, it is important to note that sample availability impacts the frequency at which certain systemic immune derangements are observed. Importantly, this does not necessarily reflect isolated etiologies for specific immune derangements, but rather, limitations of retrospective human studies. For example, there are several retrospective studies that highlight lymphopenia, but none that assess T-cell sequestration in the bone marrow. This reflects the fact that patients regularly have blood drawn but do not routinely undergo invasive procedures, such as bone marrow aspiration, which is necessary to assess T-cell numbers in the bone marrow. Furthermore, retrospective studies often highlight lymphopenia but are unable to assess T-cell lymphopenia specifically, as many of the studies described in this review include patient data from the United States in a healthcare system that does not routinely collect complete blood counts with differentials. Therefore, pre-clinical data is needed to fill in the gaps.
Although there is a dearth of mechanistic studies to assess the etiology of systemic immune derangements following brain insults, there are three main mechanisms through which the CNS “communicates” with the rest of the body. The first is the Hypothalamic–Pituitary–Adrenal (HPA) axis, with hormonal outputs such as cortisol released from the adrenal cortex. The second is through the autonomic nervous system via the release of signaling molecules, catecholamines, and acetylcholine, as well as direct end-organ innervation. The third is through the release of non-steroidal soluble factors by the injured brain into the blood circulation that can affect functions of immune cells and immune organs at distant target organs. Both the HPA axis and autonomic nervous system mediate stress responses in the body and should be studied prospectively in the setting of various CNS pathologies to evaluate their contributions and potentially novel areas of intervention.
In conclusion, we present data characterizing a group of immune deficits common to various CNS pathologies that we have collectively termed ‘systemic immune derangements. These derangements represent a novel mechanism of immune privilege that includes T-cell lymphopenia, lymphoid organ contraction, and T-cell sequestration in the bone marrow. To the best of our knowledge, this is the first body of work to highlight these derangements as a collective and demonstrate their conserved presence across pathologies. We further demonstrate that the persistence of these pathologies is dependent upon whether the CNS insult is acute or chronic. These systemic immune derangements have important implications for disease pathology and outcomes, particularly in chronic pathologies such as brain tumors. As a mechanism of persistent immunosuppression, systemic immune derangements in brain tumor patients present a unique challenge to immunotherapeutic success. Therefore, mechanistic insights are critical for licensing immunotherapies in this patient population. Understanding the etiology and mechanistic underpinnings of immune derangements secondary to brain injury will advance the clinical management of all patients with acute and chronic neurological diseases.
Acknowledgement
All figures were prepared on biorender.com.
Contributor Information
Selena J Lorrey, Department of Immunology, Duke University, Durham, NC, USA; Brain Tumor Immunotherapy Program, Duke University, Durham, NC, USA.
Jessica Waibl Polania, Brain Tumor Immunotherapy Program, Duke University, Durham, NC, USA; Department of Pathology, Duke University, Durham, NC, USA.
Lucas P Wachsmuth, Brain Tumor Immunotherapy Program, Duke University, Durham, NC, USA; Department of Pathology, Duke University, Durham, NC, USA; Medical Scientist Training Program, Duke University, Durham, NC, USA.
Alexandra Hoyt-Miggelbrink, Brain Tumor Immunotherapy Program, Duke University, Durham, NC, USA; Department of Pathology, Duke University, Durham, NC, USA.
Zachariah P Tritz, Department of Immunology, Mayo Clinic, Rochester, MN, USA.
Ryan Edwards, Brain Tumor Immunotherapy Program, Duke University, Durham, NC, USA.
Delaney M Wolf, Department of Immunology, Mayo Clinic, Rochester, MN, USA.
Aaron J Johnson, Department of Immunology, Mayo Clinic, Rochester, MN, USA.
Peter E Fecci, Department of Immunology, Duke University, Durham, NC, USA; Brain Tumor Immunotherapy Program, Duke University, Durham, NC, USA; Department of Pathology, Duke University, Durham, NC, USA; Department of Neurosurgery, Duke University, Durham, NC, USA.
Katayoun Ayasoufi, Department of Immunology, Mayo Clinic, Rochester, MN, USA.
Funding
Funding sources below have generously provided salaries for all authors who contributed to this manuscript. K99NS117799-01A (KA); Brain Together for a Cure Foundation (KA); R01 NS 103212 (AJJ), RF1 NS122174 (AJJ), and P50CA190991 (PEF). K99, R01, and RF1 are all from NINDS, National Institute of Neurological disorders and stroke. P50CA190991 is from NCI, National Cancer Institute.
Conflicts of Interests
None.
All authors have read this manuscript and agree with the statements written.
References
- 1. Singh K, Batich KA, Wen PY, et al. Designing clinical trials for combination immunotherapy: a framework for glioblastoma. Clin Cancer Res. 2022;28(4):585–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ott M, Prins RM, Heimberger AB.. The immune landscape of common CNS malignancies: implications for immunotherapy. Nat Rev Clin Oncol. 2021;18(11):729–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Platten M, Ochs K, Lemke D, Opitz C, Wick W.. Microenvironmental clues for glioma immunotherapy. Curr Neurol Neurosci Rep. 2014;14(4):440. [DOI] [PubMed] [Google Scholar]
- 4. Lakshmanachetty S, Mitra SS.. Mapping the tumor-infiltrating immune cells during glioblastoma progression. Nat Immunol. 2022;23(6):826–828. [DOI] [PubMed] [Google Scholar]
- 5. Ayasoufi K, Pfaller CK, Evgin L, et al. Brain cancer induces systemic immunosuppression through release of non-steroid soluble mediators. Brain. 2020;143(12):3629–3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chongsathidkiet P, Jackson C, Koyama S, et al. Sequestration of T cells in bone marrow in the setting of glioblastoma and other intracranial tumors. Nat Med. 2018;24(9):1459–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gustafson MP, Lin Y, New KC, et al. Systemic immune suppression in glioblastoma: the interplay between CD14+HLA-DRlo/neg monocytes, tumor factors, and dexamethasone. Neuro Oncol. 2010;12(7):631–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brooks WH, Netsky MG, Normansell DE, Horwitz DA.. Depressed cell-mediated immunity in patients with primary intracranial tumors: characterization of a humoral immunosuppressive factor. J Exp Med. 1972;136(6):1631–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brooks WH, Roszman TL, Mahaley MS, Woosley RE.. Immunobiology of primary intracranial tumours. II. Analysis of lymphocyte subpopulations in patients with primary brain tumours. Clin Exp Immunol. 1977;29(1):61–66. [PMC free article] [PubMed] [Google Scholar]
- 10. Dix A, Brooks W, Roszman T, Morford L.. Immune defects observed in patients with primary malignant brain tumors. J Neuroimmunol. 1999;100(1–2):17. [DOI] [PubMed] [Google Scholar]
- 11. Elliott LH, Brooks WH, Roszman TL.. Suppression of high affinity IL-2 receptors on mitogen activated lymphocytes by glioma-derived suppressor factor. J Neurooncol. 1992;14(1):1–7. [DOI] [PubMed] [Google Scholar]
- 12. Woroniecka K, Chongsathidkiet P, Rhodin K, et al. T-cell exhaustion signatures vary with tumor type and are severe in glioblastoma. Clin Cancer Res. 2018;24(17):4175–4186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Choi BD, Maus MV, June CH, Sampson JH.. Immunotherapy for glioblastoma: adoptive T-cell strategies. Clin Cancer Res. 2019;25(7):2042–2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Davidson TB, Lee A, Hsu M, et al. Expression of PD-1 by T cells in malignant glioma patients reflects exhaustion and activation. Clin Cancer Res. 2019;25(6):1913–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lee J, Nicosia M, Silver DJ, et al. Sex-specific T cell exhaustion drives differential immune responses in glioblastoma. bioRxiv. 2022. doi: 10.1101/2022.08.17.503211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kim WJ, Dho YS, Ock CY, et al. Clinical observation of lymphopenia in patients with newly diagnosed glioblastoma. J Neurooncol. 2019;143(2):321–328. [DOI] [PubMed] [Google Scholar]
- 17. Fecci PE, Sweeney AE, Grossi PM, et al. Systemic anti-CD25 monoclonal antibody administration safely enhances immunity in murine glioma without eliminating regulatory T cells. Clin Cancer Res. 2006;12(14 Pt 1):4294–4305. [DOI] [PubMed] [Google Scholar]
- 18. Andaloussi AE, Han Y, Lesniak MS.. Progression of intracranial glioma disrupts thymic homeostasis and induces T-cell apoptosis in vivo. Cancer Immunol Immunother. 2008;57(12):1807–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Prins RM, Graf MR, Merchant RE, Black KL, Wheeler CJ.. Thymic function and output of recent thymic emigrant T cells during intracranial glioma progression. J Neurooncol. 2003;64(1-2):45–54. [DOI] [PubMed] [Google Scholar]
- 20. Elliott LH, Brooks WH, Roszman TL.. Inability of mitogen-activated lymphocytes obtained from patients with malignant primary intracranial tumors to express high affinity interleukin 2 receptors. J Clin Invest. 1990;86(1):80–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Morford LA, Elliott LH, Carlson SL, Brooks WH, Roszman TL.. T cell receptor-mediated signaling is defective in T cells obtained from patients with primary intracranial tumors. J Immunol. 1997;159(9):4415–4425. [PubMed] [Google Scholar]
- 22. Dix AR, Brooks WH, Roszman TL, Morford LA.. Immune defects observed in patients with primary malignant brain tumors. J Neuroimmunol. 1999;100(1-2):216–232. [DOI] [PubMed] [Google Scholar]
- 23. Marini A, Dobran M, Aiudi D, et al. Pre-operative hematological markers as predictive factors for overall survival and progression free survival in glioblastomas. Clin Neurol Neurosurg. 2020;197:106162. [DOI] [PubMed] [Google Scholar]
- 24. Fecci PE, Mitchell DA, Whitesides JF, et al. Increased regulatory T-cell fraction amidst a diminished CD4 compartment explains cellular immune defects in patients with malignant glioma. Cancer Res. 2006;66(6):3294–3302. [DOI] [PubMed] [Google Scholar]
- 25. Le Scodan R, Massard C, Jouanneau L, et al. Brain metastases from breast cancer: proposition of new prognostic score including molecular subtypes and treatment. J Neurooncol. 2012;106(1):169–176. [DOI] [PubMed] [Google Scholar]
- 26. Otvos B, Alban TJ, Grabowski MM, et al. Preclinical modeling of surgery and steroid therapy for glioblastoma reveals changes in immunophenotype that are associated with tumor growth and outcome. Clin Cancer Res. 2021;27(7):2038–2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chongsathidkiet P, Jackson C, Koyama S, et al. Author correction: sequestration of T cells in bone marrow in the setting of glioblastoma and other intracranial tumors. Nat Med. 2019;25(3):529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stanley D, Mason LJ, Mackin KE, et al. Translocation and dissemination of commensal bacteria in post-stroke infection. Nat Med. 2016;22(11):1277–1284. [DOI] [PubMed] [Google Scholar]
- 29. Westendorp WF, Nederkoorn PJ, Vermeij JD, Dijkgraaf MG, van de Beek D.. Post-stroke infection: a systematic review and meta-analysis. BMC Neurol. 2011;11:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Carneiro T, Spears W, LeClair J, et al. Admission lymphocytopenia is associated with urinary tract infection and nosocomial infections in hemorrhagic stroke. J Stroke Cerebrovasc Dis. 2021;30(11):106079. [DOI] [PubMed] [Google Scholar]
- 31. Wong CH, Jenne CN, Tam PP, et al. Prolonged activation of invariant natural killer T cells and TH2-skewed immunity in stroke patients. Front Neurol. 2017;8:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nous A, Peeters I, Nieboer K, et al. Post-stroke infections associated with spleen volume reduction: a pilot study. PLoS One. 2020;15(5):e0232497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Morotti A, Marini S, Jessel MJ, et al. Lymphopenia, infectious complications, and outcome in spontaneous intracerebral hemorrhage. Neurocrit Care. 2017;26(2):160–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vahidy FS, Parsha KN, Rahbar MH, et al. Acute splenic responses in patients with ischemic stroke and intracerebral hemorrhage. J Cereb Blood Flow Metab. 2016;36(6):1012–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Giede-Jeppe A, Bobinger T, Gerner ST, et al. Lymphocytopenia is an independent predictor of unfavorable functional outcome in spontaneous intracerebral hemorrhage. Stroke. 2016;47(5):1239–1246. [DOI] [PubMed] [Google Scholar]
- 36. Chiu NL, Kaiser B, Nguyen YV, et al. The volume of the spleen and its correlates after acute stroke. J Stroke Cerebrovasc Dis. 2016;25(12):2958–2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sahota P, Vahidy F, Nguyen C, et al. Changes in spleen size in patients with acute ischemic stroke: a pilot observational study. Int J Stroke. 2013;8(2):60–67. [DOI] [PubMed] [Google Scholar]
- 38. Liu Q, Jin WN, Liu Y, et al. Brain ischemia suppresses immunity in the periphery and brain via different neurogenic innervations. Immunity. 2017;46(3):474–487. [DOI] [PubMed] [Google Scholar]
- 39. Offner H, Subramanian S, Parker SM, et al. Splenic atrophy in experimental stroke is accompanied by increased regulatory T cells and circulating macrophages. J Immunol. 2006;176(11):6523–6531. [DOI] [PubMed] [Google Scholar]
- 40. Amantea D, La Russa D, Frisina M, et al. Ischemic preconditioning modulates the peripheral innate immune system to promote anti-inflammatory and protective responses in mice subjected to focal cerebral ischemia. Front Immunol. 2022;13:825834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hug A, Dalpke A, Wieczorek N, et al. Infarct volume is a major determiner of post-stroke immune cell function and susceptibility to infection. Stroke. 2009;40(10):3226–3232. [DOI] [PubMed] [Google Scholar]
- 42. Urra X, Laredo C, Zhao Y, et al. Neuroanatomical correlates of stroke-associated infection and stroke-induced immunodepression. Brain Behav Immun. 2017;60:142–150. [DOI] [PubMed] [Google Scholar]
- 43. Vogelgesang A, Grunwald U, Langner S, et al. Analysis of lymphocyte subsets in patients with stroke and their influence on infection after stroke. Stroke. 2008;39(1):237–241. [DOI] [PubMed] [Google Scholar]
- 44. Jin R, Zhu X, Liu L, et al. Simvastatin attenuates stroke-induced splenic atrophy and lung susceptibility to spontaneous bacterial infection in mice. Stroke. 2013;44(4):1135–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ajmo CT, Jr, Collier LA, Leonardo CC, et al. Blockade of adrenoreceptors inhibits the splenic response to stroke. Exp Neurol. 2009;218(1):47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kim M, Kim SD, Kim KI, et al. Dynamics of T lymphocyte between the periphery and the brain from the acute to the chronic phase following ischemic stroke in mice. Exp Neurobiol. 2021;30(2):155–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ring J, Seifert J, Lob G, et al. Intensive immunosuppression in the treatment of multiple sclerosis. Lancet. 1974;2(7889):1093–1096. [DOI] [PubMed] [Google Scholar]
- 48. Naess A, Nyland H.. Lymphocyte subpopulations in multiple sclerosis: variations in rosette tests using erythrocytes from different sheep. Acta Pathol Microbiol Scand C. 1980;88(6):293–297. [DOI] [PubMed] [Google Scholar]
- 49. Pender MP, Csurhes PA, Pfluger CM, Burrows SR.. Deficiency of CD8+ effector memory T cells is an early and persistent feature of multiple sclerosis. Mult Scler. 2014;20(14):1825–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Dziuba AN, Frolov VM, Peresadin NA. Znachenie estestvennogo ingibiruiushchego faktora v patogeneze rasseiannogo skleroza [The significance of a natural inhibitory factor in the pathogenesis of disseminated sclerosis]. Lik Sprava. Feb–Mar 1993;(2-3):93–95. [PubMed] [Google Scholar]
- 51. Jones JL, Thompson SA, Loh P, et al. Human autoimmunity after lymphocyte depletion is caused by homeostatic T-cell proliferation. Proc Natl Acad Sci U S A. 2013;110(50):20200–20205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Haas J, Fritzsching B, Trübswetter P, et al. Prevalence of newly generated naive regulatory T cells (Treg) is critical for Treg suppressive function and determines Treg dysfunction in multiple sclerosis. J Immunol. 2007;179(2):1322–1330. [DOI] [PubMed] [Google Scholar]
- 53. Haas J, Korporal M, Schwarz A, Balint B, Wildemann B.. The interleukin-7 receptor α chain contributes to altered homeostasis of regulatory T cells in multiple sclerosis. Eur J Immunol. 2011;41(3):845–853. [DOI] [PubMed] [Google Scholar]
- 54. Haegert DG, Hackenbroch JD, Duszczyszyn D, et al. Reduced thymic output and peripheral naïve CD4 T-cell alterations in primary progressive multiple sclerosis (PPMS). J Neuroimmunol. 2011;233(1-2):233–239. [DOI] [PubMed] [Google Scholar]
- 55. Knippenberg S, Peelen E, Smolders J, et al. Reduction in IL-10 producing B cells (Breg) in multiple sclerosis is accompanied by a reduced naïve/memory Breg ratio during a relapse but not in remission. J Neuroimmunol. 2011;239(1-2):80–86. [DOI] [PubMed] [Google Scholar]
- 56. Brinkmann V, Davis MD, Heise CE, et al. The immune modulator FTY720 targets sphingosine 1-phosphate receptors. J Biol Chem. 2002;277(24):21453–21457. [DOI] [PubMed] [Google Scholar]
- 57. Huwiler A, Zangemeister-Wittke U.. The sphingosine 1-phosphate receptor modulator fingolimod as a therapeutic agent: recent findings and new perspectives. Pharmacol Ther. 2018;185:34–49. [DOI] [PubMed] [Google Scholar]
- 58. Mandala S, Hajdu R, Bergstrom J, et al. Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science. 2002;296(5566):346–349. [DOI] [PubMed] [Google Scholar]
- 59. Abbadessa G, Maida E, Miele G, et al. Lymphopenia in multiple sclerosis patients treated with ocrelizumab is associated with an effect on CD8 T cells. Mult Scler Relat Disord. 2022;60:103740. [DOI] [PubMed] [Google Scholar]
- 60. Landi D, Grimaldi A, Bovis F, et al. Influence of previous disease-modifying drug exposure on T-lymphocyte dynamic in patients with multiple sclerosis treated with ocrelizumab. Neurol Neuroimmunol Neuroinflamm. 2022;9(3). doi: 10.1212/nxi.0000000000001157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Dinoto A, Sartori A, Cheli M, et al. Lymphopenia during treatment with dimethyl fumarate in patients with multiple sclerosis: prevalence, predicting factors and clinical outcomes. Mult Scler Relat Disord. 2022;57:103357. [DOI] [PubMed] [Google Scholar]
- 62. Sainz de la Maza S, Sabin Muñoz J, Pilo de la Fuente B, et al. Early predictive risk factors for dimethyl fumarate-associated lymphopenia in patients with multiple sclerosis. Mult Scler Relat Disord. 2022;59:103669. doi: 10.1016/j.msard.2022.103669. [DOI] [PubMed] [Google Scholar]
- 63. Boffa G, Bruschi N, Cellerino M, et al. Fingolimod and dimethyl-fumarate-derived lymphopenia is not associated with short-term treatment response and risk of infections in a real-life MS population. CNS Drugs. 2020;34(4):425–432. [DOI] [PubMed] [Google Scholar]
- 64. Beltrán E, Gerdes LA, Hansen J, et al. Early adaptive immune activation detected in monozygotic twins with prodromal multiple sclerosis. J Clin Invest. 2019;129(11):4758–4768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Friese MA, Fugger L.. Pathogenic CD8(+) T cells in multiple sclerosis. Ann Neurol. 2009;66(2):132–141. [DOI] [PubMed] [Google Scholar]
- 66. Huseby Kelcher AM, Atanga PA, Gamez JD, et al. Brain atrophy in picornavirus-infected FVB mice is dependent on the H-2Db class I molecule. FASEB J. 2017;31(6):2267–2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Johnson HL, Chen Y, Suidan GL, et al. A hematopoietic contribution to microhemorrhage formation during antiviral CD8 T cell-initiated blood-brain barrier disruption. J Neuroinflammation. 2012;9(1):60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Malo CS, Huggins MA, Goddery EN, et al. Non-equivalent antigen presenting capabilities of dendritic cells and macrophages in generating brain-infiltrating CD8 (+) T cell responses. Nat Commun. 2018;9(1):633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. McDole J, Johnson AJ, Pirko I.. The role of CD8+ T-cells in lesion formation and axonal dysfunction in multiple sclerosis. Neurol Res. 2006;28(3):256–261. [DOI] [PubMed] [Google Scholar]
- 70. Behrangi N, Heinig L, Frintrop L, et al. Siponimod ameliorates metabolic oligodendrocyte injury via the sphingosine-1 phosphate receptor 5. Proc Natl Acad Sci U S A. 2022;119(40):e2204509119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. das Neves SP, Serre-Miranda C, Nobrega C, et al. Immune thymic profile of the MOG-induced experimental autoimmune encephalomyelitis mouse model. Front Immunol. 2018;9:2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Solti I, Kvell K, Talaber G, et al. Thymic atrophy and apoptosis of CD4+CD8+ thymocytes in the cuprizone model of multiple sclerosis. PLoS One. 2015;10(6):e0129217e0129217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Barnard AL, Chidgey AP, Bernard CC, Boyd RL.. Androgen depletion increases the efficacy of bone marrow transplantation in ameliorating experimental autoimmune encephalomyelitis. Blood. 2009;113(1):204–213. [DOI] [PubMed] [Google Scholar]
- 74. Sui RX, Miao Q, Wang J, et al. Protective and therapeutic role of Bilobalide in cuprizone-induced demyelination. Int Immunopharmacol. 2019;66:69–81. [DOI] [PubMed] [Google Scholar]
- 75. Barthelmes J, Tafferner N, Kurz J, et al. Induction of experimental autoimmune encephalomyelitis in mice and evaluation of the disease-dependent distribution of immune cells in various tissues. J Vis Exp. 2016;(111). doi: 10.3791/53933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Tsunoda I, Libbey JE, Kuang LQ, Terry EJ, Fujinami RS.. Massive apoptosis in lymphoid organs in animal models for primary and secondary progressive multiple sclerosis. Am J Pathol. 2005;167(6):1631–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Angelini DF, Serafini B, Piras E, et al. Increased CD8+ T cell response to Epstein-Barr virus lytic antigens in the active phase of multiple sclerosis. PLoS Pathog. 2013;9(4):e1003220e1003220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Bjornevik K, Cortese M, Healy BC, et al. Longitudinal analysis reveals high prevalence of Epstein-Barr virus associated with multiple sclerosis. Science. 2022;375(6578):296–301. [DOI] [PubMed] [Google Scholar]
- 79. Schneider-Hohendorf T, Gerdes LA, Pignolet B, et al. Broader Epstein-Barr virus-specific T cell receptor repertoire in patients with multiple sclerosis. J Exp Med. 2022;219(11). doi: 10.1084/jem.20220650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Boumaza X, Bonneau B, Roos-Weil D, et al. Progressive multifocal leukoencephalopathy treated by immune checkpoint inhibitors. Ann Neurol. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Galbiati F, Basso V, Cantuti L, et al. Autonomic denervation of lymphoid organs leads to epigenetic immune atrophy in a mouse model of Krabbe disease. J Neurosci. 2007;27(50):13730–13738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Ayasoufi K, Pfaller CK.. Seek and hide: the manipulating interplay of measles virus with the innate immune system. Curr Opin Virol. 2020;41:18–30. [DOI] [PubMed] [Google Scholar]
- 83. Tipold A, Vandevelde M, Wittek R, et al. Partial protection and intrathecal invasion of CD8(+) T cells in acute canine distemper virus infection. Vet Microbiol. 2001;83(3):189–203. [DOI] [PubMed] [Google Scholar]
- 84. McCullough B, Krakowka S, Koestner A.. Experimental canine distemper virus-induced lymphoid depletion. Am J Pathol. 1974;74(1):155–170. [PMC free article] [PubMed] [Google Scholar]
- 85. Campbell B, Budreau D, Williams-Perez S, et al. Admission lymphopenia predicts infectious complications and mortality in traumatic brain injury victims. Shock. 2022;57(2):189–198. [DOI] [PubMed] [Google Scholar]
- 86. Ritzel RM, Doran SJ, Barrett JP, et al. Chronic alterations in systemic immune function after traumatic brain injury. J Neurotrauma. 2018;35(13):1419–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Belabed L, Charrueau C, Besson V, et al. Impairment of lymphocyte function in head-injured rats: effects of standard and immune-enhancing diets for enteral nutrition. Clin Nutr. 2006;25(5):832–841. [DOI] [PubMed] [Google Scholar]
- 88. Hamani D, Charrueau C, Butel MJ, et al. Effect of an immune-enhancing diet on lymphocyte in head-injured rats: what is the role of arginine? Intensive Care Med. 2007;33(6):1076–1084. [DOI] [PubMed] [Google Scholar]
- 89. Mrakovcic-Sutic I, Tokmadzic VS, Laskarin G, et al. Early changes in frequency of peripheral blood lymphocyte subpopulations in severe traumatic brain-injured patients. Scand J Immunol. 2010;72(1):57–65. [DOI] [PubMed] [Google Scholar]
- 90. Xiong Y, Mahmood A, Chopp M.. Animal models of traumatic brain injury. Nat Rev Neurosci. 2013;14(2):128–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Schwulst SJ, Trahanas DM, Saber R, Perlman H.. Traumatic brain injury-induced alterations in peripheral immunity. J Trauma Acute Care Surg. 2013;75(5):780–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Pavlicek D, Krebs J, Capossela S, et al. Immunosenescence in persons with spinal cord injury in relation to urinary tract infections—a cross-sectional study. Immun Ageing. 2017;14:22. ecollection doi: 10.1186/s12979-017-0103-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Jogia T, Lubstorf T, Jacobson E, et al. Prognostic value of early leukocyte fluctuations for recovery from traumatic spinal cord injury. Clin Transl Med. 2021;11(1):e272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Furlan JC, Krassioukov AV, Fehlings MG.. Hematologic abnormalities within the first week after acute isolated traumatic cervical spinal cord injury: a case-control cohort study. Spine. 2006;31(23):2674–2683. [DOI] [PubMed] [Google Scholar]
- 95. Stirling DP, Yong VW.. Dynamics of the inflammatory response after murine spinal cord injury revealed by flow cytometry. J Neurosci Res. 2008;86(9):1944–1958. [DOI] [PubMed] [Google Scholar]
- 96. Zhang Y, Guan Z, Reader B, et al. Autonomic dysreflexia causes chronic immune suppression after spinal cord injury. J Neurosci. 2013;33(32):12970–12981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Lucin KM, Sanders VM, Jones TB, Malarkey WB, Popovich PG.. Impaired antibody synthesis after spinal cord injury is level dependent and is due to sympathetic nervous system dysregulation. Exp Neurol. 2007;207(1):75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Mironets E, Fischer R, Bracchi-Ricard V, et al. Attenuating neurogenic sympathetic hyperreflexia robustly improves antibacterial immunity after chronic spinal cord injury. J Neurosci. 2020;40(2):478–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Ozdemir HH, Akil E, Acar A, et al. Changes in serum albumin levels and neutrophil-lymphocyte ratio in patients with convulsive status epilepticus. Int J Neurosci. 2017;127(5):417–420. [DOI] [PubMed] [Google Scholar]
- 100. Gunes M, Buyukgol H.. Relationship between generalized epileptic seizure and neutrophil/lymphocyte ratio, platelet/lymphocyte ratio, and neutrophil mediated inflammation. Int J Neurosci. 2020;130(11):1095–1100. [DOI] [PubMed] [Google Scholar]
- 101. Shevtsova Z, Garrido M, Weishaupt J, et al. CNS-expressed cathepsin D prevents lymphopenia in a murine model of congenital neuronal ceroid lipofuscinosis. Am J Pathol. 2010;177(1):271–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Hisanaga K, Asagi M, Itoyama Y, Iwasaki Y.. Increase in peripheral CD4 bright+ CD8 dull+ T cells in Parkinson disease. Arch Neurol. 2001;58(10):1580–1583. [DOI] [PubMed] [Google Scholar]
- 103. Bas J, Calopa M, Mestre M, et al. Lymphocyte populations in Parkinson’s disease and in rat models of Parkinsonism. J Neuroimmunol. 2001;113(1):146–152. [DOI] [PubMed] [Google Scholar]
- 104. Baba Y, Kuroiwa A, Uitti RJ, Wszolek ZK, Yamada T.. Alterations of T-lymphocyte populations in Parkinson disease. Parkinsonism Relat Disord. 2005;11(8):493–498. [DOI] [PubMed] [Google Scholar]
- 105. Hu ZX, Song WN, Lu XD, Zhou ML, Shao JH.. Peripheral T lymphocyte immunity and l-dopamine in patients with Parkinson’s disease. J Biol Regul Homeost Agents. 2018;32(3):687–691. [PubMed] [Google Scholar]
- 106. Bhatia D, Grozdanov V, Ruf WP, et al. T-cell dysregulation is associated with disease severity in Parkinson’s disease. J Neuroinflammation. 2021;18(1):250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Miyatake H, Fujino K, Tanaka S, et al. Association between lymphocyte count and neurological outcomes in post-cardiac arrest patients treated with mild therapeutic hypothermia. Acute Med Surg. 2019;6(1):30–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Zhao Q, Shen Y, Li R, et al. Cardiac arrest and resuscitation activates the hypothalamic-pituitary-adrenal axis and results in severe immunosuppression. J Cereb Blood Flow Metab. 2021;41(5):1091–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Haeusler KG, Schmidt WU, Foehring F, et al. Immune responses after acute ischemic stroke or myocardial infarction. Int J Cardiol. 2012;155(3):372–377. [DOI] [PubMed] [Google Scholar]
- 110. Cour M, Jahandiez V, Bochaton T, et al. Cyclosporine A prevents ischemia-reperfusion-induced lymphopenia after out-of-hospital cardiac arrest: a predefined sub-study of the CYRUS trial. Resuscitation. 2019;138:129–131. [DOI] [PubMed] [Google Scholar]
- 111. Venet F, Cour M, Demaret J, Monneret G, Argaud L.. Decreased monocyte HLA-DR expression in patients after non-shockable out-of-hospital cardiac arrest. Shock. 2016;46(1):33–36. [DOI] [PubMed] [Google Scholar]
- 112. Boag SE, Das R, Shmeleva EV, et al. T lymphocytes and fractalkine contribute to myocardial ischemia/reperfusion injury in patients. J Clin Invest. 2015;125(8):3063–3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Wang W, Li R, Miao W, et al. Development and evaluation of a novel mouse model of asphyxial cardiac arrest revealed severely impaired lymphopoiesis after resuscitation. J Am Heart Assoc. 2021;10(11):e019142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Ayasoufi K, Wolf D, Namen S, et al. Brain resident memory T cells rapidly expand and initiate neuroinflammatory responses following CNS injury and viral infection. bioRxiv. 2022. doi: 10.1101/2022.04.08.487707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Alonazi B, Farghaly AM, Mostafa MA, et al. Brain MRI in SARS-CoV-2 pneumonia patients with newly developed neurological manifestations suggestive of brain involvement. Sci Rep. 2021;11(1):20476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Collantes MEV, Espiritu AI, Sy MCC, Anlacan VMM, Jamora RDG.. Neurological manifestations in COVID-19 infection: a systematic review and meta-analysis. Can J Neurol Sci. 2021;48(1):66–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Fernández-Castañeda A, Lu P, Geraghty AC, Song E, Lee MH, Wood J, Yalçın B, Taylor KR, Dutton S, Acosta-Alvarez L, Ni L, Contreras-Esquivel D, Gehlhausen JR, Klein J, Lucas C, Mao T, Silva J, Peña-Hernández MA, Tabachnikova A, Takahashi T, Tabacof L, Tosto-Mancuso J, Breyman E, Kontorovich A, McCarthy D, Quezado M, Hefti M, Perl D, Folkerth R, Putrino D, Nath A, Iwasaki A, Monje M.. Mild respiratory SARS-CoV-2 infection can cause multi-lineage cellular dysregulation and myelin loss in the brain. bioRxiv [Preprint]. 2022. Jan 10:2022.01.07.475453. doi: 10.1101/2022.01.07.475453. PMID: 35043113; PMCID: PMC8764721. [DOI] [Google Scholar]
- 118. Pellegrini L, Albecka A, Mallery DL, et al. SARS-CoV-2 infects the brain choroid plexus and disrupts the blood-CSF barrier in human brain organoids. Cell Stem Cell. 2020;27(6):951–961.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Yachou Y, El Idrissi A, Belapasov V, Ait Benali S.. Neuroinvasion, neurotropic, and neuroinflammatory events of SARS-CoV-2: understanding the neurological manifestations in COVID-19 patients. Neurol Sci. 2020;41(10):2657–2669. [DOI] [PMC free article] [PubMed] [Google Scholar]