Abstract
Prolonged exposure (PE) therapy is a first-line treatment for posttraumatic stress disorder (PTSD) and involves repeated presentation of trauma-related cues without aversive outcomes. A primary learning mechanism of PE is fear extinction (new learning that a dangerous cue is now safe) and its retention (maintaining this new learning over time). Extant research suggests extinction is impaired in PTSD patients. In this study, we employed an established fear-potentiated startle-based paradigm to examine fear acquisition, extinction learning and retention before and after completion of intensive outpatient treatment. First, PTSD patients undergoing PE (n = 55) were compared to trauma-exposed patients without PTSD (n = 57). We identified excessive fear in PTSD patients during acquisition and extinction before treatment compared to non-PTSD patients. At post-treatment, we examined the return of fear after extinction in PTSD patients showing high or low treatment response to PE (≥50% change in PTSD symptom severity vs. < 50%). High PE responders maintained fear extinction learning whereas low PE responders showed significant return of fear at post-treatment. These results replicate and extend previous findings of impaired extinction in PTSD and provide support for the proposed theoretical link between fear extinction and PE response.
Keywords: PTSD, Fear, Extinction, Exposure therapy, Psychophysiology, Startle
Posttraumatic stress disorder (PTSD) is a debilitating illness associated with significant distress and difficulties in functioning (Rodriguez, Holowka, & Marx, 2012). The development of PTSD fear-related symptoms has often been conceptualized as a result of fear conditioning (Bouton, Mineka, & Barlow, 2018; Foa, Steketee, & Rothbaum, 1989; Norrholm & Jovanovic, 2018; Rothbaum & Davis, 2003) in which a traumatic event (unconditioned stimulus; US) that evokes fear without any prior learning (unconditioned response; UR) becomes associated with previously neutral stimuli (e.g., sights, sounds, smells) that are present at the time of trauma. These cues (or triggers) can then become conditioned stimuli (CS) that elicit subsequent, robust fear reactions (conditioned responses; CRs). Within laboratory paradigms of fear conditioning and extinction, a neutral CS is repeatedly paired with a US (e.g., blast of air; Zuj, Palmer, Lommen, & Felmingham, 2016) such that an increased, or potentiated, conditioned fear response is elicited in the presence of the reinforced CS+. Fear extinction involves repeated presentation of the CS without the US, resulting in a decreased conditioned fear response (Myers & Davis, 2002). Fear extinction retention, or recall, is the retrieval and expression of the newly learned extinction memory following the passage of time (Bouton, Westbrook, Corcoran, & Maren, 2006; Jovanovic, Kazama, Bachevalier, & Davis, 2012; Milad et al., 2008; Norrholm et al., 2008). Individuals with PTSD, in addition to exhibiting altered fear conditioning, have been shown to have deficits in extinction learning and retention (Jovanovic et al., 2012; Milad & Quirk, 2012; Norrholm et al., 2011, 2015).
Fear extinction learning and extinction retention is one of the theoretical foundations of Prolonged Exposure (PE) therapy, a first line treatment for PTSD (APA, 2017). During PE, patients therapeutically confront trauma reminders and the trauma memories to facilitate fear extinction (Foa et al., 2018). Extinction retention, and the ability to appropriately maintain fear inhibition, is specifically relevant to PTSD treatment outcome in that this learning is believed to underlie the reduction of fear and its long-term suppression (Rauch et al., 2018). In clinical applications, extinction learning is achieved by the repeated recollection of trauma-related imagery and memories (Rothbaum & Davis, 2003). A large body of research indicates that PE is an effective treatment for PTSD and demonstrates large treatment effect sizes (Powers, Halpern, Ferenschak, Gillihan, & Foa, 2010; Watts et al., 2013). However, a subset of patients does not attain clinically meaningful symptom improvement in PE (Ready, Lamp, Rauch, Astin, & Norrholm, 2020). Thus, research is needed to understand mechanisms of change in order to improve outcomes. In other words, improved understanding of treatment-related changes in inhibitory and extinction learning is a key element to tailoring personalized approaches to medicine, including psychotherapy.
Previous research indicates psychophysiological data, either collected as part of traditional Pavlovian fear learning paradigms or in the presence of trauma-related cue presentation, can serve as a valuable source of empirical data regarding PTSD symptoms and treatment response (for review see Norrholm & Jovanovic, 2018). For example, a previous study found that high PTSD treatment responders demonstrated an increase followed by a decreased in trauma-potentiated startle over the course of treatment, whereas low responders demonstrated a flat response profile (Robison-Andrew et al., 2014). Our group has shown that high PTSD treatment responders are less reactive to trauma stimuli following PE treatment (Maples-Keller et al., 2019), elevated startle prior to virtual reality exposure therapy is associated with better treatment response (Norrholm et al., 2016), and fear extinction learning in Pavlovian fear conditioning tasks is altered in PTSD populations (e.g., Norrholm et al., 2011). Further, we recently employed pre- and post-treatment assessments of extinction learning before and after continuous positive airway pressure (CPAP) treatment in Veterans with obstructive sleep apnea (OSA) to show that improved sleep was associated with improved extinction learning (Reist et al., 2021). Yet, to our knowledge, no published studies have investigated fear extinction learning within the context of ongoing treatment specifically for PTSD.
While PTSD has been characterized by extinction deficits within this paradigm, most of the research has relied on cross-sectional methods (see Careaga, Girardi, & Suchecki, 2016). Cross sectional methods do not lend themselves well to considering the clinical implications of impaired extinction nor the potential capacity for observed deficits to be rescued by therapeutic intervention. For this reason, there is great value in studies that assess fear learning within an ongoing treatment environment. In the present study, we investigated fear extinction training and extinction retention at both pre- and post-treatment in Veterans and service members with and without PTSD who received PE (Foa et al., 2018; Rauch et al., 2020) or Unified Protocol for Transdiagnostic Treatment of Emotional Disorder (UP; Barlow et al., 2017), respectively in an intensive outpatient format. Intensive PE contains the same methods as traditional PE (i.e., imaginal exposure, in vivo exposure), is associated with large improvements in PTSD and depression symptoms (Rauch et al., 2020; Yasinski, Sherrill, Maples-Keller, Rauch, & Rothbaum, 2017) with notably high retention rates (96%; Rauch et al., 2020), and demonstrates non-inferiority to traditional spaced PE (Foa et al., 2018). The focus of investigation was 1) how within-session extinction (i.e., extinction training) and between-session extinction (i. e., retention/recall) differ in trauma-exposed Veterans with and without PTSD and 2) how extinction training and extinction retention was associated with PE treatment response for patients with PTSD. We hypothesized that trauma-exposed Veterans with PTSD would demonstrate impaired fear extinction at pre-treatment as compared to trauma-exposed Veterans without PTSD as seen in previous work (see Milad et al., 2008; Milad et al., 2009; Milad & Quirk, 2012; Norrholm & Jovanovic, 2018). In addition, we proposed that, among PTSD patients, PE treatment responders would show greater improvements in within-session fear extinction learning and between-session extinction retention compared to treatment non-responders.
1. Methods and materials
1.1. Participants
Participants were 123 post-9/11 Veterans or military service-members who participated in a two-week intensive outpatient program (IOP) and provided informed consent with study approved by the Emory University Institutional Review Board. The study sample consisted of 72 males and 51 females aged 20–58 years old with a mean age of 39.2 (±1.1) years. Participant self-reported race was 55.2% White/Caucasian (68/123), 28.4% Black/African American (35/1123), 4.9% Mixed Ancestry (6/123), and the remaining participants reporting to be Native American (2), Asian (3), or Unknown (9). Exclusionary criteria for entrance into the treatment program included conditions requiring a higher level of care such as unmanaged psychosis or bipolar disorder, severe alcohol or substance abuse or dependence, or acute suicidality. The use of psychotropic drugs was not exclusionary for the present study. The use of benzodiazepines was discouraged as these drugs have been shown to impede exposure therapy (Rothbaum et al., 2014).
1.2. Measures
All participants completed an intake assessment, which included: Clinician Administered PTSD Scale for DSM-5 (CAPS-5; Weathers et al., 2018), Mini International Neuropsychiatric Interview (Sheehan et al., 1998) or Diagnostic Interview for Anxiety, Mood, and Obsessive-Compulsive and Related Neuropsychiatric Disorders (DIAMOND; Tolin et al., 2018), and review of medical records in order to assess for primary and comorbid mental health diagnoses. Primary study outcome measure was the PTSD Checklist for DSM-5 (PCL-5; Blevins, Weathers, Davis, Witte, & Domino, 2015), a self-report measure of PTSD symptoms administered at pre- and post-treatment.
1.3. Treatment
PTSD Treatment.
Participants who had a primary diagnosis of PTSD attended a two-week PE IOP (Careaga et al., 2016; Rauch et al., 2020). The main goal of PE is to facilitate emotional processing of the trauma via systematic confrontation of trauma-related cues (Careaga et al., 2016) via imaginal and in-vivo exposures. Each day, participants engaged in an individual psychotherapy session which focused on imaginal exposure, in which they revisited the traumatic memory via their imagination and recounted it aloud to therapist and then engaged in processing. Next, participants attended a group-based psychotherapy session focused on in vivo exposure, in which they systematically and gradually confronted situations, people, places, or activities that they avoided due to trauma symptoms. Consistent with standard PE, participants listened to imaginal recording and completed in vivo exposures for homework. For a more detailed description of IOP PE see Rauch et al. (2020) or Yasinski et al. (2017).
1.4. Experimental startle paradigm
This well-validated fear-conditioning, extinction training, and extinction retention fear-potentiated startle (FPS) paradigm (for review see Norrholm & Jovanovic, 2018) involves conditioning with an aversive airblast US, of 250 ms duration and 140 psi pressure directed to the larynx, and conditioned stimuli (CSs) of different colored shapes displayed on a computer monitor. Airblasts were emitted by compressed airtank connected to polyethylene tubing and controlled by a solenoid switch. The airblast intensity was identical for each participant based on several of our previous studies in adult populations both traumatized and healthy controls (see Norrholm & Jovanovic, 2018). The startle probe was a 108 dB, 40 ms burst of white noise with near instantaneous rise time delivered binaurally through headphones (Norrholm et al., 2006). The eyeblink component of the acoustic startle response was assessed via electromyography (EMG) recordings of the right orbicularis oculi muscle with two 5-mm Ag/AgCl electrodes with a Biopac MP150 EMG module. All data was sampled at 1000 Hz and amplified with a gain of 2000 using EMG module of the Biopac system. To calculate the degree of potentiated startle (expressed in microVolts) to the conditioned stimuli during each phase of fear learning, a difference score was calculated by subtracting the mean startle magnitude to the noise probe alone (abbreviated NA) from the mean startle magnitude in the presence of each CS for each block. Blocks were defined as four presentations each of the NA, CS+, CS- stimuli. There were three Acquisition blocks for a total of 12 trials. Calculations which were performed using the same methods reported in several previous published works from our group (e.g., Norrholm et al., 2006).
This experimental paradigm was administered at pre- and post-treatment. Collection of acoustic startle data for each of three primary fear learning phases (Acquisition, Extinction Training, Recall) at both Pre- and Post-treatment was dependent on patient treatment schedule, successful completion of an audiological test to rule out patients with impaired hearing, and room/equipment availability. The three primary learning phases were preceded by a Pre-acquisition block that consisted of non-reinforced presentations of each CS to reduce the influence of novelty. The experimental session is illustrated in Fig. 1. Stimuli were presented using SuperLab 4.5 for Windows (Cedrus, Inc., San Pedro, CA) and synchronized with psychophysiological data acquisition using DIO card (Measurements Computing, Inc., Norton, MA) with the inter-trial interval ranging from 9 to 22 s. As in several previous studies using these methods (e.g., Norrholm et al., 2011), colored geometric shapes (square, triangle, star, circle assigned colors blue, orange, purple, or gray) were used as conditioned stimuli; reinforced conditioned stimulus abbreviated CS+; non-reinforced conditioned stimulus abbreviated CS-). To control for practice effects and the repeated presentation of an associative learning task, shapes and colors were counterbalanced for the Pre- and Post-treatment startle assessments such that individual participants were shown different shapes with different assigned colors at each time point. For the Acquisition phase, the shapes were presented for a total of 6 s. Startle probes were delivered on each trial for 40 ms, and occurred 5460 ms after shape presentation was initiated on CS + trials and 5960 ms after shape presentation started on CS- trials. On CS + trials, the CS + co-terminated with the presentation of the US 500 ms after the delivery of the auditory startle probe. For Extinction Training and Recall, the shapes were also presented for a total of 6 s. Startle probes were again delivered on each trial for 40 ms, and occurred 5960 ms after shape presentation on both (now non-reinforced) CS + trials and CS- trials.
Fig. 1.
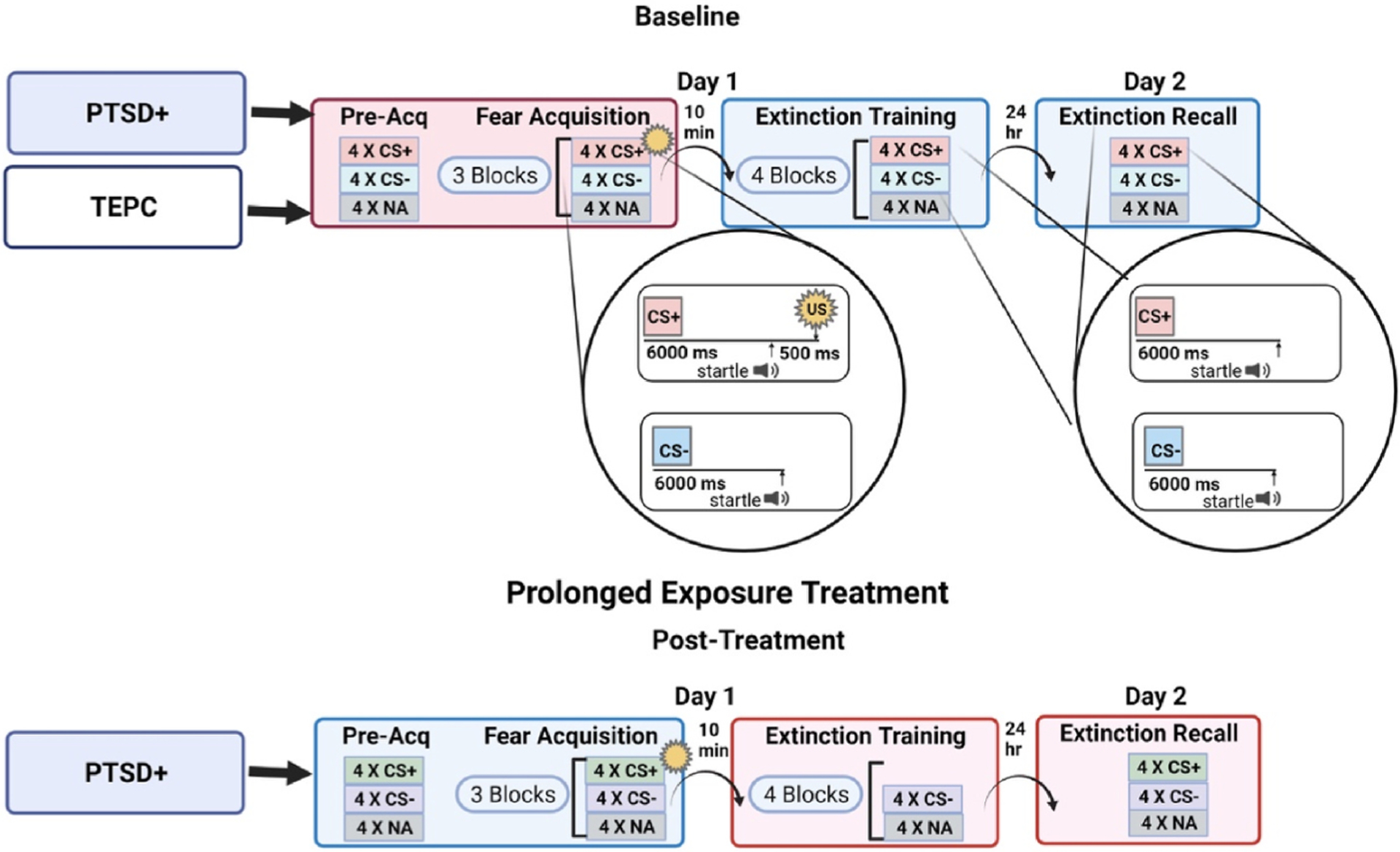
Schematic illustrating the session and trial definitions for the assessment of Fear Acquisition, Extinction Training, and test for extinction Recall in IOP participants at pre- (Trauma Exposed Psychiatric Controls, TEPC, and PTSD + groups) and post-treatment time points (PTSD + group only). The fear acquisition session contained a CS Habituation block and three Acquisition blocks. In the Acquisition blocks, there were 12 CSs (colored shapes) reinforced on 100% of the trials paired with the US (CS+), 12 colored shapes that were not reinforced (CS-), and 12 trials of the sound probe presented without a CS (noise alone, NA). CS+ = conditioned stimulus reinforced with airblast unconditioned stimulus (US); CS-= conditioned stimulus not paired with US; NA = noise alone (baseline). Created with Biorender.com.
The contexts in which the startle testing occurred included an ABB design such that the Acquisition session occurred in a dedicated acoustic startle laboratory (Context A) and Extinction Training and Recall occurred in a physically separate, and qualitatively distinct, dedicated research office complete with psychophysiological recording suite (Context B) with the contexts counterbalanced for the subsequent assessment (Context A for Pre-became Context B for Post and vice versa).
Fear extinction training and recall sessions (i.e., no US/airblast) occurred 10 min and 24 h later, respectively. During the extinction and extinction recall sessions, the same CSs from the fear acquisition session were presented on a computer monitor, but this time none were followed by an airblast. Extinction training involved 16 trials of each type (NA, CS+, CS-) and the extinction recall session included four trials of each type with the same varying ITIs as in the previous sessions (9–22s).
1.5. Data analysis
Baseline group differences.
A mixed three-way repeated-measures analysis of variance (RM-ANOVA) was used to test the effects of fear acquisition Block (4 levels: Pre-acquisition, Acquisition 1–3) by CS type (2 levels: CS+, CS-) and between-groups variable of primary diagnosis Group (2 levels: PTSD+, TEPC). Extinction was similarly tested in a 3-way RM-ANOVA of Extinction Block (1–4) by CS type by Group. FPS was the dependent variable, calculated as the difference score between startle during CS presentation and NA. To test for the return of fear 24 h after extinction training, a univariate ANOVA comparing FPS to the CS + between groups was performed, with FPS to CS + at the end of Extinction Training from the previous day included as a covariate, to control for individual differences in successful extinction training.
Treatment effect.
Three-way RM-ANOVAs were used to test the effects of Treatment separately for each phase, ie., Acquisition, Extinction Training, and Extinction Recall. The first analysis included Treatment (2 levels: Pre, Post) by Block (4 levels: Pre-acquisition, Acquisition Blocks 1–3) by CS type (2 levels: CS+, CS-). The second analysis examined Treatment (2 levels: Pre, Post) by Block (4 levels: Extinction Blocks 1–4) by CS type (2 levels: CS+, CS-). The analysis of Extinction Recall included Treatment (2 levels: Pre, Post) by CS type (2 levels: CS+, CS-).
Responder analysis.
Responder analyses were limited to the PTSD + group only, in order to limit the analyses to those individuals who received Prolonged Exposure treatment. High Responders were defined as demonstrating a 50% or more reduction in PTSD symptoms from pre- to post-treatment, as has been defined in our prior work (e.g., Maples-Keller et al., 2019; Norrholm et al., 2016; Robison-Andrew et al., 2014). RM-ANOVAs were used as described above with the addition of a between-groups variable of Responder (2 levels: Low Responder, High Responder), with FPS as the dependent variable. Again, a univariate ANOVA was used to test post-treatment extinction Recall by responder status, with and without FPS to CS + at the end of Extinction Training as a covariate. In order to test the return of fear, we also examined the change in FPS from end of Extinction Training on Day 1 to extinction Recall on Day 2, by subtracting the last block of extinction from extinction recall FPS for each CS type. We then conducted a RM-ANOVA of Treatment by CS type by Responder group using this difference score as the dependent variable. In addition, we examined PE response as percent change in PCL-5 scores from pre-to post-treatment as a continuous variable and tested Pearson correlations with extinction Recall. All analyses were performed in SPSS 27 for Windows (Armonk, NY), with alpha of 0.05. Within-subjects effects used the sphericity-assumed statistic. Group and treatment effect sizes are included with partial eta squared (η2). Significant within-subject interaction effects were compared with planned contrasts. Although all participants completed all fear conditioning phases and recall, sample sizes differ across conditions due to data loss related to noisiness, and computer and experimenter error. Sample sizes for each analysis are listed in the results section.
2. Results
2.1. Diagnostic groups
Participants (n = 123 with clinical data) were stratified by the presence or absence of PTSD as their primary clinical diagnosis at intake into Trauma-exposed psychiatric controls (TEPC, n = 58) and PTSD primary diagnosis (PTSD+, n = 65) groups. The mean PCL-5 scores at Pre-treatment were higher for the PTSD + group (49.95 ± 1.90) as compared to the TEPC group (39.95 ± 2.10; F(1,106) = 14.03, p <0.001). The TEPC group consisted of 41 males and 17 females while the PTSD + group included 31 males and 34 females, a significant difference in the proportion of biological sex in the groups (χ2 = 6.68, p = 0.01), with a higher percentage of women in the PTSD + group. The most common current comorbidities in the study sample were major depression, anxiety disorders (including panic and generalized anxiety disorder), insomnia, adjustment disorders, substance use disorder, and alcohol use disorder. There were no significant differences between the Trauma Exposed group and PTSD + group in terms of the rate of these comorbidities in each group (all χ2 analyses for the aforementioned psychiatric conditions had p > 0.1). Startle data for acquisition, extinction, and recall varied to some degree due to noisiness, experimenter error, or patients not willing to undergo startle testing. The sample size for each session is included in the respective results section.
2.2. Baseline group differences pre-treatment
2.2.1. Fear acquisition
Startle data were available for 112 patients for the pre-treatment Acquisition phase. A three-way RM-ANOVA of Block by CS type by Group (TEPC vs PTSD+) showed significant main effects of Block, F (3,330) = 8.91, p < 0.001, η2 = 0.08, CS type, F(1,110) = 11.56, p < 0.001, η2 = 0.095 and Block by CS type interaction, F(3, 330) = 9.15, p < 0.001, η2 = 0.08, see Fig. 2. The latter interaction indicated clear discrimination between the reinforced CS+ and non-reinforced CS-across Acquisition blocks. There was a significant Block × Group interaction F(3,330) = 4.44, p = 0.005, η2 = 0.04. Planned comparisons of Group within each block showed significantly greater FPS to both CS types in PTSD + compared to the TEPC group F(1,110) = 5.52, p = 0.02, η2 = 0.05. When including sex as a covariate, the group differences in FPS during Acquisition were no longer significant.
Fig. 2.
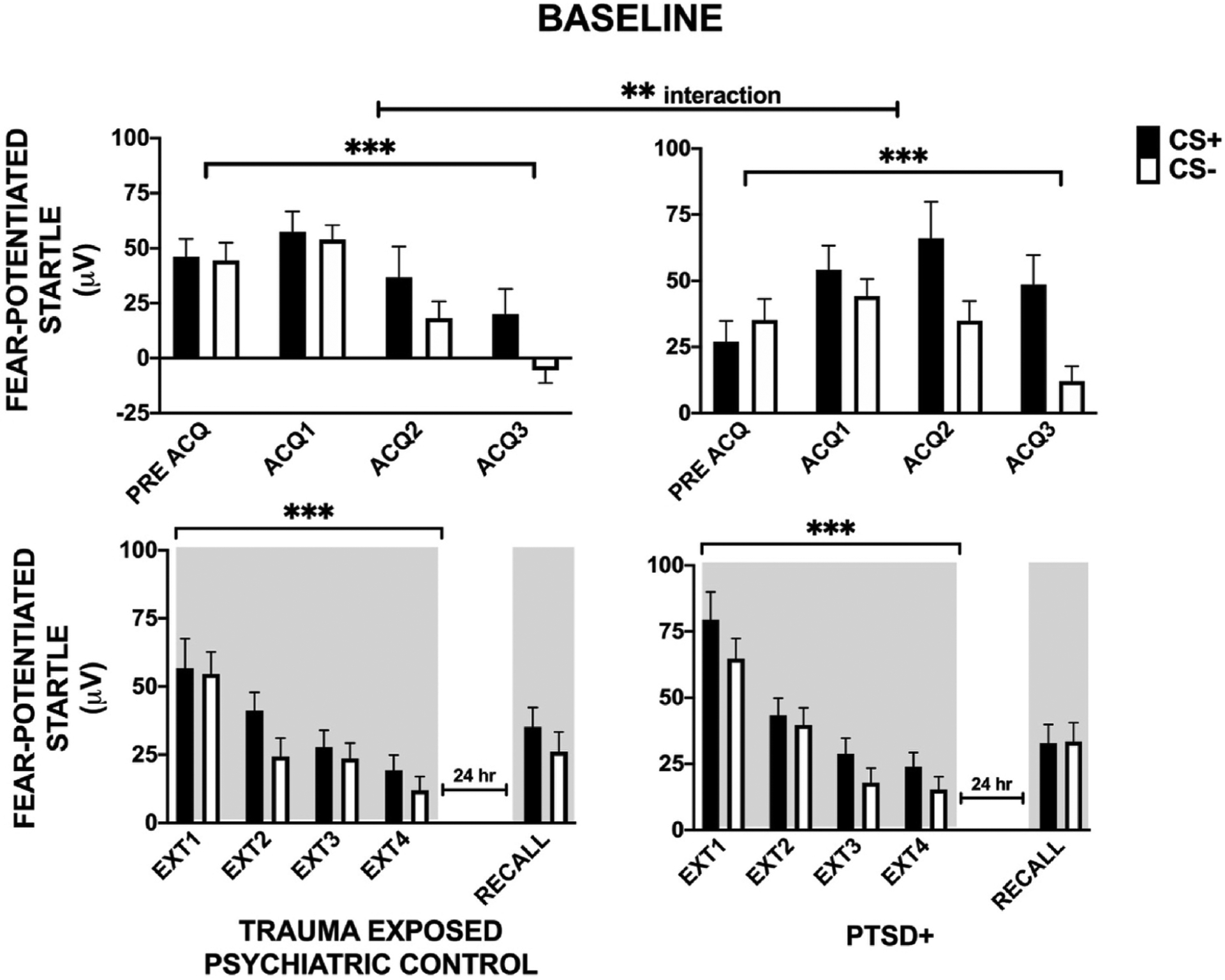
Baseline group differences Pre-treatment. Analysis of fear conditioning Phase (Habituation, Late Acquisition, Early and Late Extinction Training) and extinction Recall by CS type (CS+, CS-) by Group (Trauma Exposed and PTSD+). During Acquisition (ACQ), there were significant main effects of Block, CS type, and Block × CS type interaction supporting successful acquisition of fear-potentiated startle and CS discrimination. In addition, we observed a Group × Block interaction with the PTSD + group showing a greater increase in FPS during acquisition compared to the TEPC group. During extinction training (EXT), there were significant main effects of Block and CS type. ** - p < 0.01; ***p < 0.001; HAB = habituation; TEPC = trauma exposed; Shading indicates the context for extinction training session that occurred 10 min after acquisition, and extinction recall that occurred in the same context 24 h later.
2.2.2. Extinction training and recall
Startle data were available for 110 patients for the pre-treatment Extinction Training phase. A three-way RM-ANOVA showed significant main effects of Block F(3,324) = 59.42, p < 0.001, η2 = 0.36, and CS type F(1,108) = 15.35, p < 0.001, η2 = 0.12, with FPS decreasing over the extinction training blocks and, as such, demonstrating overall successful extinction training (see Fig. 2). There was not a significant Block by CS type interaction during Extinction Training.
We did not observe main or interaction effects of Group in Extinction Training or extinction Recall at baseline.
2.3. Treatment effects
2.3.1. Fear acquisition
Analyses of treatment effects were limited to the PTSD + group only because of the lack of an adequate intervention control group within this clinically derived sample. Namely, the TEPC patients received treatment as part of the Unified Protocol for Transdiagnostic Treatment of Emotional Disorder which contains some elements of extinction/exposure but spans several functional and clinical domains. Complete Preand Post-treatment FPS data for Acquisition were available for 41 PTSD + participants. A three-way RM-ANOVA of Treatment × Block × CS type showed a significant main effect of Block F(2,80) = 24.61, p < 0.001, η2 = 0.38, main effect of CS Type F(1,40) = 16.13, p < 0.001, η2 = 0.29, and two-way Block × CS Type interaction F(2,80) = 11.82, p < 0.001, η2 = 0.23 (see Fig. 3); these analyses showing that participants successfully re-acquired conditioned fear to the new threat cue. All main and interaction effects remained significant after covarying for biological sex.
Fig. 3.
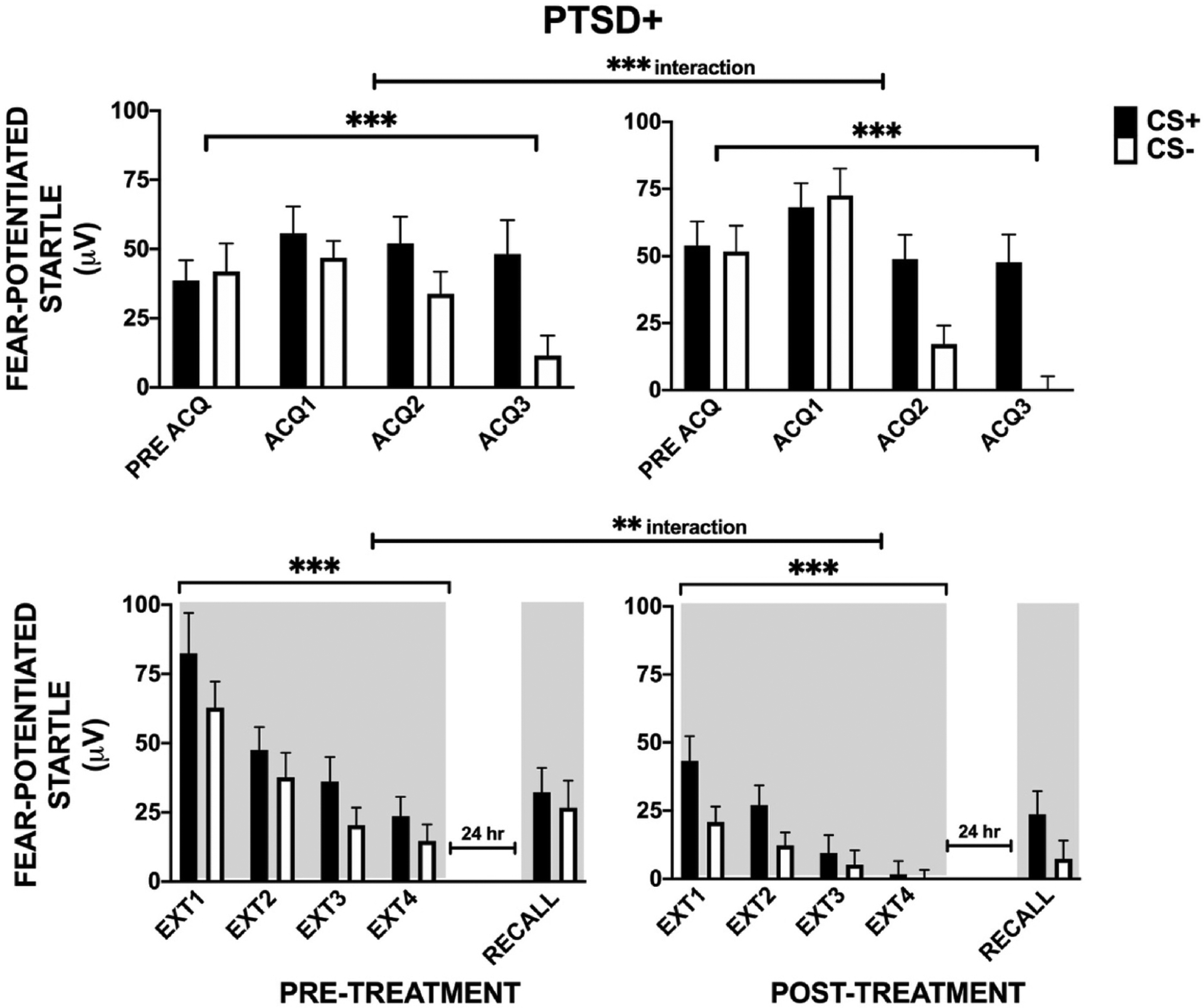
Pre- and Post-treatment effects in the PTSD + group by experimental phase. For Acquisition (ACQ), there were significant main effects of Block, CS-Type, and a 2-way interaction of Block × CS type. For extinction training (EXT), there were main effects of Treatment, Block, CS type, and a Treatment × Block interaction. There were no observed effects of treatment on Habituation (HAB) and Late Acquisition (ACQ) phases but there was a significant treatment-related decrease in FPS during Early and Late Extinction. There was a Treatment effect on extinction Recall, with significant discrimination between CS+ and CS- observed only at Post-treatment in both groups. ** - p < 0.01; ***p < 0.001; TEPC = trauma exposed; Shading indicates the context for extinction training session that occurred 10 min after acquisition, and extinction recall that occurred in the same context 24 h later.
There was also a two-way interaction of Treatment by Block, F(2,80) = 8.00, p < 0.001, η2 = 0.17 (see Fig. 3). Following up on this interaction by comparing Treatment within each Block showed that treatment did not affect FPS prior to Acquisition, p = 0.26, or at the end of Acquisition, p = 0.42, indicating a lack of carry-over effects from Pre-treatment fear learning. The lack of significant differences between the to-be-reinforced CS+ and non-reinforced CS- during the Pre-acquisition block of each Treatment time point alone shows a lack of baseline discrimination between the cues prior to conditioning as expected. The lack of this difference between the cues during Pre-acquisition at the post-treatment time point also supports a lack of carry-over learning/practice effects.
2.3.2. extinction training
Complete Pre- and Post-treatment FPS data for Extinction Training were available for 40 PTSD + participants. A RM-ANOVA of Treatment × Block × CS type revealed a main effects of Treatment F(1,39) = 15.10, p < 0.001, η2 = 0.28, Block F(3,117) = 30.98, p < 0.001, η2 = 0.44, and CS Type F(1,39) = 18.52, p < 0.001, η2 = 0.32 with a significant Treatment × Block interaction F(3,117) = 4.40, p = 0.006, η2 = 0.10 (see Fig. 3).
We then examined treatment effects within each extinction block separately. There were significant treatment effects in the first block F (1,39) = 17.87, p < 0.001, η2 = 0.31 and last block of Extinction Training F(1,39) = 6.38, p = 0.02, η2 = 0.14. We did not observe treatment by CS type interactions in either early or late extinction, suggesting that FPS decreased for both CS+ and CS-. These results demonstrate a reduced fear load in the PTSD + participants after treatment.
2.3.3. Extinction recall
Pre- and post-treatment extinction Recall data were available for 40 PTSD + participants. A RM-ANOVA of Treatment by CS type with extinction Recall as the dependent variable, showed no main effects of Treatment or CS Type nor any or interaction effects (see Fig. 3). However, while there was no discrimination between CS+ and CS- prior to treatment, FPS during extinction Recall was significantly higher for the CS + compared to CS- post treatment, F(1,42) = 8.93, p = 0.005, η2 = 0.18. Based on prior research in the literature regarding responses to a CS-, or safety signal, in anxiety disorders (e.g., Lissek et al., 2009), we also examined responses to CS- only. We found only a trend for a Treatment effect on CS- from Pre-to Post-treatment, F(1,39) = 2.99, p = 0.092.
2.4. Responder analyses
In order to examine whether treatment response was associated with fear Acquisition, Extinction Training, and Extinction Recall, we characterized participants as either Low Responders or High Responders (>50% reduction in PCL-5 score from pre-to post-treatment; Maples-Keller et al., 2019; Norrholm et al., 2016; Robison-Andrew et al., 2014). Low Responders (n = 19) displayed a small change in Pre-to Post--treatment PCL-5 from 51.7 (±2.9) to 40.3 (±3.0), while High Responders (n = 16) exhibited a robust Pre-to Post-treatment change from 48.0 (±2.6) to 13.2 (±1.9) in PCL-5 score. Given that the PTSD+ and TEPC Groups received different treatment, we limited the responder analysis to PTSD + participants in order to avoid confounding treatment type with patient and responder group. The biological sex distribution across responder groups was not significantly different, therefore, biological sex was not used as a covariate in the analyses.
We compared High and Low Responders at baseline and found no group difference in fear Acquisition, Extinction Training, or Recall. For Acquisition, a RM-ANOVA of Treatment × Block × CS type × Responder group showed a significant main effect of Block, F(3,105) = 10.26, p < 0.001, η2 = 0.23, main effect of CS type, F(1,35) = 12.51, p = 0.001, η2 = 0.26, as well as 2-way interaction effects of Block × CS type, F(3,105) = 10.78, p = 0.001, η2 = 0.24, and Treatment by Block, F(3,105) = 3.64, p = 0.02, η2 = 0.09, but no interaction effects with Responder status (see Fig. 4, panels A and B). The effects of Block and CS type were the same as above by demonstrating successful acquisition of fear.
Fig. 4.
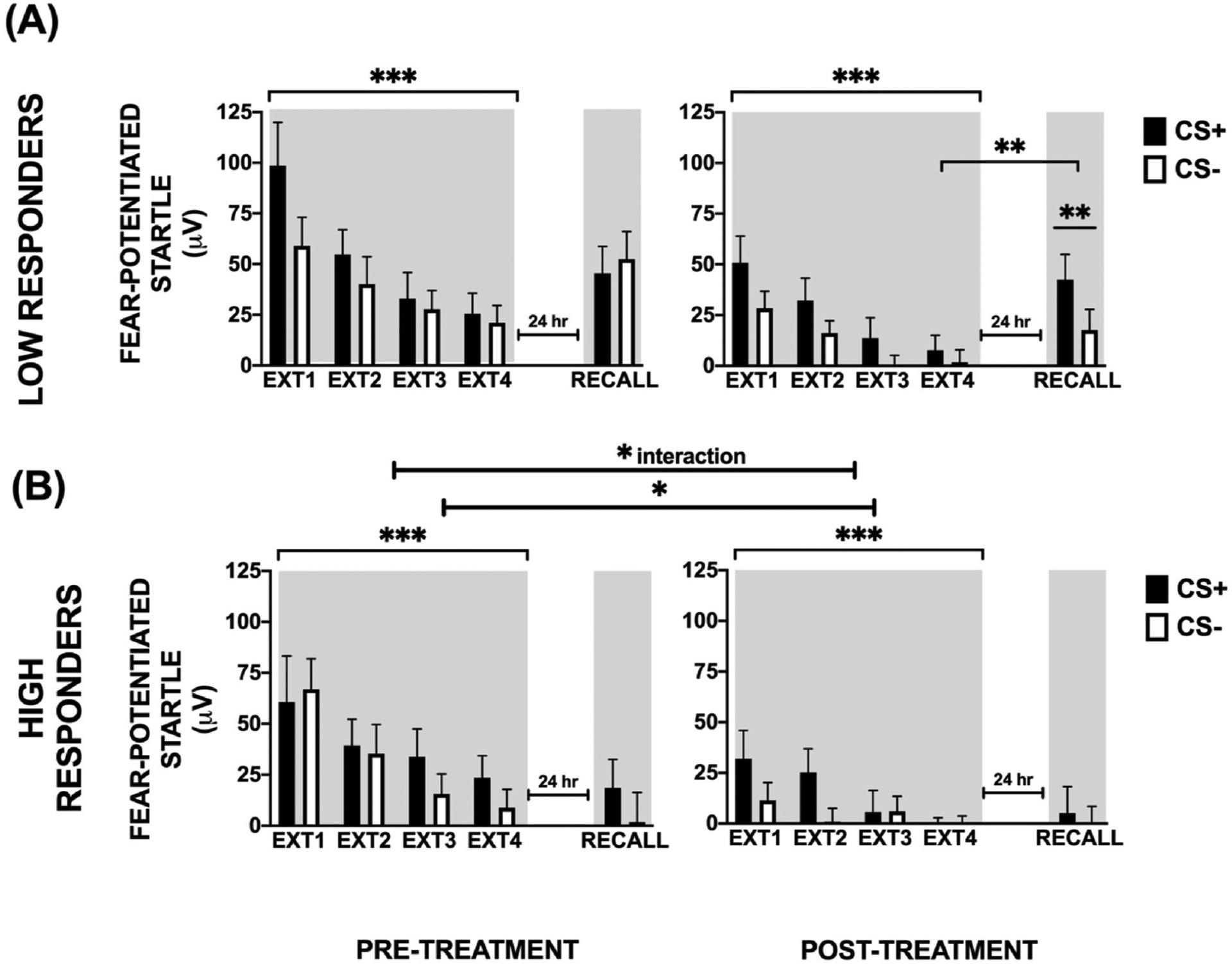
Pre- and Post-treatment effects in Low and High Responder groups among PTSD + participants. There was a 4-way interaction between Treatment × Block × CS type × Responder status. Panels (A) and (B) show separate analyses in each Responder group (Low and High Responders). (A) In the Low Responders, there was a significant main effect of Block but not CS type or Treatment. (B) In the High Responders, we observed main effects of Treatment and Block. The Habituation and Late Acquisition phases were not affected by treatment, however, FPS to the previously reinforced CS+ was lower at Early and Late Extinction Training (EXT). There was also a 3-way interaction of Treatment × CS type × Responder status on extinction Recall. High Responders had lower FPS to both CS’s compared to Low Responders at Post-treatment. In the Low Responders there was a significant CS type effect at Post-treatment, while both CS’s were equally low in the High Responders. * - p < 0.05; ** - p < 0.01; ***p < 0.001; Shading indicates the context for extinction training session that occurred 10 min after acquisition, and extinction recall that occurred in the same context 24 h later.
We next examined the effect of Responder status on Extinction Training using RM-ANOVA of Treatment × Block × CS type × Responder group. There was a significant main effect of Treatment, F(1,34) = 13.21, p = 0.001, η2 = 0.28, main effect of Block, F(3,102) = 25.02, p < 0.001, η2 = 0.42, main effect of CS type, F(1,34) = 15.48, p < 0.001, η2 = 0.31, a 2-way interaction of Treatment × Block, F(3,102) = 3.56, p = 0.02, η2 = 0.09, and a 4-way interaction with Responder status, F (3,102) = 3.53, p = 0.02, η2 = 0.09. We then followed up this interaction in order to answer the question of whether extinction to the threat cue (CS+) improved in the High Responders but not the Low Responders, by testing treatment effects on extinction within each Responder group. In the High Responders, there was a main effect of Treatment, F(1,16) = 7.00, p = 0.02, η2 = 0.30, indicating that FPS to CS + decreased from Pre-to Post-treatment in this group (see Fig. 4B). In the Low Responders, there was a much smaller, trend level effect of Treatment, F(1,18) = 4.17, p = 0.06, η2 = 0.19 (see Fig. 4A). We observed a main effect of Block in both groups: High Responders, F(3,48) = 7.30, p < 0.001, η2 = 0.31 and Low Responders, F(3,54) = 12.57, p < 0.001, η2 = 0.41. Since both groups showed an effect of Block, we examined whether there were effects specifically to the terminal level of extinction. Therefore, we compared FPS to CS+ in the last Block of Extinction Training, pre- and post-treatment within each Responder group. Here also we found a significant effect of Treatment in the High Responder group, F(1,16) = 4.50, p = 0.05, η2 = 0.22 but no effect in the Low Responder group, F (1,18) = 1.79, p = 0.20, η2 = 0.09 (see Fig. 4B). These data suggest that both groups show a within-session reduction in FPS during Extinction Training at the Pre- and Post-time points (a reduction in FPS from Extinction Block 1 to Extinction Block 4). However, only the High Responders show decreased between-session fear with Treatment across the Pre- and Post-time points (i.e., lower magnitude of FPS across all trial types) indicating that treatment response is associated with reductions in overall fear expression.
We recognize the limitations of the latter analysis approach which is likely related to low sample size. A larger sample in follow-up studies may allow for a more direct, between-group comparison of High and Low Responders.
Extinction Recall and PCL-5 data were available for 35 PTSD + individuals. A RM-ANOVA of Treatment by CS type by Responder group showed a significant 3-way interaction, F(1,34) = 6.15, p = 0.02, η2 = 0.15. While there were no differences between High and Low Responders Pre-treatment, High Responders had lower FPS to both CS’s compared to Low Responders at Post-treatment, F(1,34) = 4.80, p = 0.035, η2 = 0.12. We then compared FPS to CS+ and CS- at Recall before and after treatment in both groups. There was no effect of CS type pre-treatment, however, the Low Responders showed significantly greater FPS to CS + than CS- at post-treatment, F(1,20) = 9.37, p = 0.006, η2 =0.32. In the High Responders, FPS to both CS’s was low at post-treatment (Fig. 4 A and B). In order to specifically test the return of fear, we compared extinction Recall to CS + between High and Low Responder and found that High Responders had significantly less fear during Recall compared to Low Responders, F(1,34) = 5.36, p = 0.03, η2 = 0.13 (see Fig. 5). This difference remained significant after controlling for the terminal levels of fear at the end of Extinction Training on the previous day. In addition, the Low Responder group showed a distinct return of fear through spontaneous recovery (e.g., increase in FPS from end of Extinction Training to Recall test, F(1,18) = 8.25, p = 0.01, whereas the High Responder group showed successful extinction recall (lack of increase from end of Extinction Training to Recall test, F(1,15) = 1.46, p = 0.25).
Fig. 5.
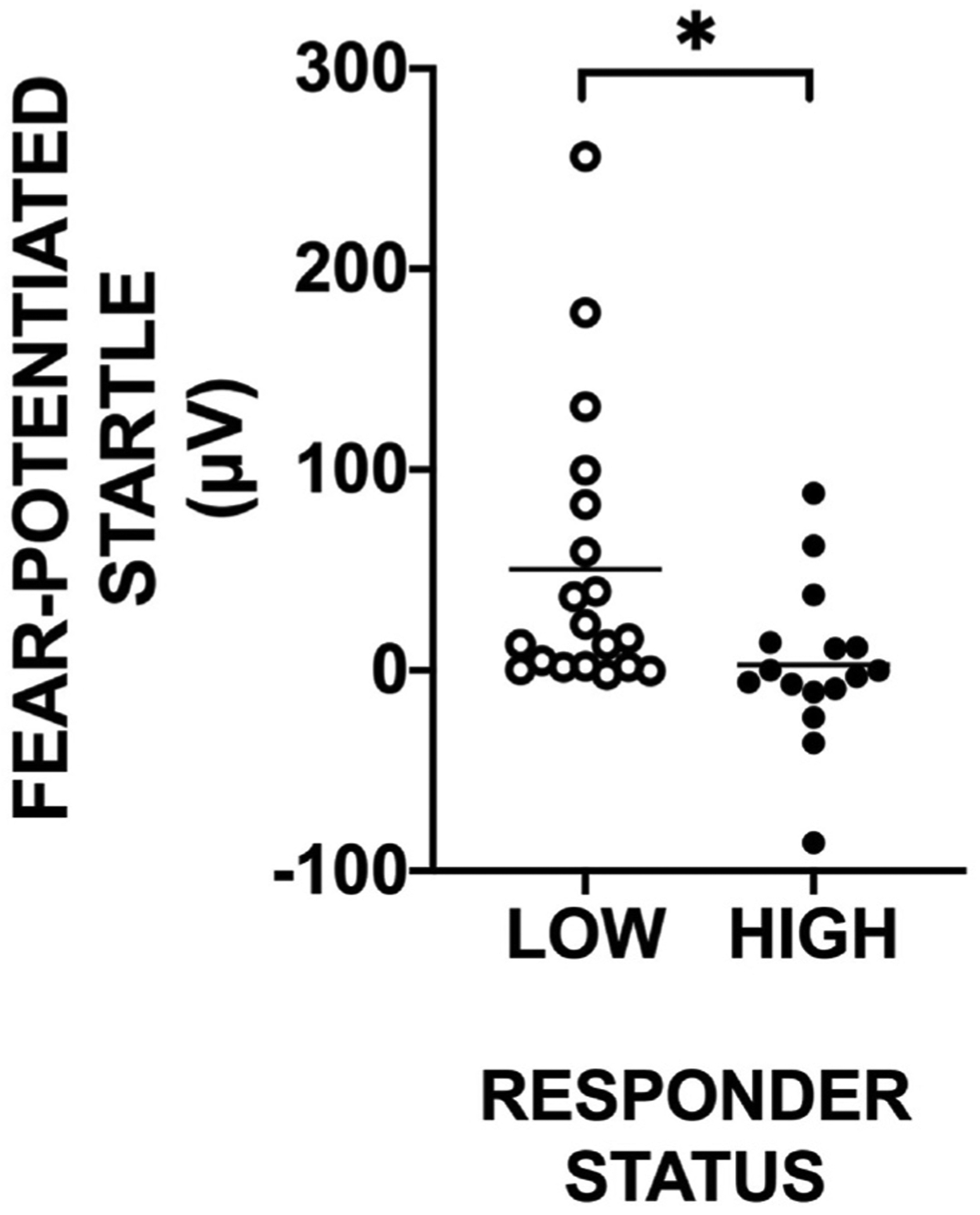
Scatterplot illustrating extinction Recall in Low and High PTSD treatment responders at the post-treatment time point. Low Responders exhibited a significantly greater return of fear in the presence of the previously extinguished CS + as compared to High Responders. * - p < 0.05.
We calculated a difference score from the end of Extinction Training on day 1 to the end of Extinction Recall on day 2. A RM-ANOVA of Treatment × CS type × Responder group with this Difference Score as the dependent variable showed a significant main effect of Responder, F (1,28) = 5.13, p = 0.03, η2 = 0.155. This analysis shows less retention of extinction (thus greater return of fear on day 2) in the Low Responder group.
Additionally, we treated percent change on PCL-5 as a continuous variable and correlated it with FPS to CS+ during extinction recall at post-treatment. A negative but non-significant association was revealed (r = 0.21, p = 0.20) between these factors suggesting that the relationship may not be a linear one.
Based on criteria from our previous work (Norrholm et al., 2006, 2008; Maples-Keller et al., 2022), we then classified participants in each responder group according to the degree to which they showed a return of fear or extinction retention. No increase in FPS from the end of Extinction Training to extinction Recall identified a “retainer.” Conversely, a participant that showed increased FPS from the end of Extinction Training to extinction recall identified a “non-retainer.” Chi-square analysis revealed significantly more retainers in Responders (10/16) as compared to Non-responders (3/19; χ2 = 6.35, p = 0.01). Low and High Responders did not significantly differ on number of PE sessions and thus received a comparable dose of PE (Low Responders: mean number of sessions = 8.5 ± 0.75; High Responders: mean number of sessions = 7.4 ± 0.7, F(1,34) = 1.20, p = 0.29).
3. Discussion
Impaired extinction learning has been identified as a central underlying mechanism for the expression of PTSD symptoms (Foa et al., 1989; Zuj et al., 2016) and enhancing extinction retention is a target for improving the efficacy of PE (Rothbaum & Davis, 2003; Zuj & Norrholm, 2019). To our knowledge, this is the first evaluation of the three primary phases of translational, Pavlovian fear learning (acquisition, extinction training, and extinction recall/return of fear) within a PTSD treatment environment. Several previous studies have reported PTSD-specific differences in the degree of conditioned fear acquired, discrimination between danger (CS+) and safety (CS-), the ability to learn to extinguish within-session, and the degree to which extinguished fear is maintained between-sessions. In general, the fear- and anxiety-related symptoms of PTSD have been characterized as an over-expression of fear that is resistant to extinction. The results of the present work are consistent with this conceptualization. It is important to note that most of the empirical study in this area has been cross-sectional with PTSD symptom severity and fear acquisition and extinction assessed at the same or a time proximal to the clinical assessment; our-pre-treatment results in the present study mirror these prior, cross sectional studies indicating excessive fear in PTSD + patients (Norrholm et al., 2015) as compared to trauma-exposed, non- PTSD patients during both the acquisition and extinction training phases. However, it is possible that these group differences were associated with sex differences in fear expression, since controlling for biological sex accounted for the baseline group differences. Given that the PTSD + group had proportionally more females, and that female sex is both a risk factor for PTSD, and associated with higher physiological fear responses (Ressler et al., 2011), the over-expression of conditioned fear may be part of the mechanisms underlying risk for PTSD.
PTSD+ and TEPC groups showed significant, yet incomplete, extinction training that remained evident a day later at the pre-treatment extinction recall test. This pattern of extinction learning is markedly different from what we have observed in psychiatrically healthy controls using the same procedures (Norrholm et al., 2008; Warren et al., 2014). Control participants routinely show a total absence of conditioned fear by the end of extinction training. Previous research has revealed three primary extinction training trajectories that are preserved across species: (1) “normative” complete within-session extinction, (2) incomplete within-session extinction in the absence of excessive fear as compared to control conditions, and (3) excessive fear at the outset of extinction coupled with incomplete within-session extinction (Galatzer-Levy & Bryant, 2013; Galatzer-Levy, Ma, Statnikov, Yehuda, & Shalev, 2017). In the present work, at pre-treatment, the PTSD + group showed both increased fear and incomplete extinction. These group differences were medium in effect size (η2 = 0.06–0.08)
The results of the present study represent an extension of findings that have been reported as part of cross-sectional, single time point studies of extinction learning. Namely, we found altered fear extinction in the PTSD + group in the form of an over-expression of conditioned fear to the CS+ (termed fear load; Norrholm et al., 2011; Fani et al., 2012; Norrholm et al., 2015; Orcutt et al., 2017). We now have empirical evidence to suggest that this fear load phenomenon may be susceptible to interruption by extinction-based, clinical exposure therapy, although in the absence of a non-treatment waitlist control group it remains unclear whether fear load could be reduced by PE, passage of time, or other unanticipated causes. However, comparing low and high treatment responders addresses some of these concerns, and supports the finding that PE is impacting extinction learning. This could represent a significant step forward for the field as this paradigm appears to: (1) possess the capacity to assess reliably fear learning repeatedly (i.e., test-retest); (2) have the sensitivity to detect pre-treatment differences in fear extinction; and (3) detect treatment specific changes in extinction learning that can be used as a potential objective biomarker when applied in concert with clinician and self-report ratings.
Based on our a priori hypothesis that poor extinction learning would be related to greater symptom severity and worse treatment outcome (Shumake, Jones, Auchter, & Monfils, 2018), we next investigated the aforementioned fear learning phases in PTSD + participants classified as low and high treatment responders (e.g. Norrholm et al., 2016). There were no significant differences between the low and high treatment responders in terms of the successful acquisition of conditioned fear and its within-session extinction before and after treatment. However, high responders showed an intact retention of extinction training, and low responders did not show a retention of extinction training, with fear returning to a level equivalent to the start of post-treatment extinction training. The effect size difference between the two groups was large (η2 = 0.15). This failure in extinction recall is consistent with several reports from the literature (e.g., Helpman et al., 2016; Milad et al., 2008; Milad et al., 2009). The results of the present study now indicate a direct link between improved outcomes of exposure treatment for PTSD and successful extinction recall at the time of intervention, consistent with clinical models indicating the importance of extinction retention within PE.
There has been some ambiguity in the field regarding whether or not extinction learning predicts cognitive-behavioral treatment outcomes (for discussions see Stojek, McSweeney, & Rauch, 2018). In the present study, successful extinction training at pre-treatment (>50% reduction in FPS across the session; as in Norrholm et al., 2008; Norrholm et al., 2006), was not predictive of high treatment responder status as assessed via repeated measures analysis of variance with within-session extinction status (≥ or < 50% reduction in conditioned fear) as a between groups variable (F(1,68) = 0.25, p = 0.62) nor as a function of class membership (≥ or < reduction in conditioned fear and responder versus low-responder status (χ2 = 0.7, p = 0.4). As such, fear extinction retention at post-treatment may represent an indicator of treatment outcome, whereas we did not identify fear extinction as a pre-treatment predictor of response.
3.1. Limitations and future directions
At present, there is evidence from prior psychophysiological studies to suggest PTSD-associated extinction impairment is a robust and potentially central feature to fear-related PTSD psychopathology (Zuj & Norrholm, 2018). However, the expanding body of evidence is comprised of data acquired via different indices, namely FPS and skin conductance responding. Fear-potentiated startle paradigms, on the one hand, have revealed notable differences in within-session extinction training whereas the majority of investigations utilizing skin conductance have shown PTSD-associated impairments in between-session learning, or extinction recall. The methodological divergence in much of this work is related to whether or not neuroimaging was included as part of the experimental design as skin conductance can be practical in this context while it proves more difficult for acoustic startle. The present study did not include self-reported learning metrics, such as ratings of US expectancy. Future analyses and endeavors should integrate multiple indices concurrently, including multiple psychophysiological indicators as well as self-reported learning metrics, to allow for better synthesis of these findings and enhanced clinical application. Additionally, the present study uses an intensive outpatient PE format. While this treatment protocol is largely similar to standard PE, involving the same main intervention methods (i.e., imaginal and in vivo exposure) with the primary difference being the timeline of sessions, future research can investigate if the findings replicate in PTSD patients receiving standard spaced PE. Future research can also investigate if the same results are observed in different exposure-based treatments for other anxiety disorders, such as exposure and response prevention for obsessive-compulsive disorder or exposure therapy for specific phobia.
3.2. Clinical implications
Clinical investigators focused on PTSD, its neurobiological correlates, and the relationship of these correlates to treatment selection and outcome have sought to identify objective measures of fear and anxiety to complement clinician- and self-report assessments. The results of the present study, taken together with the growing body of literature coupling therapeutic approaches with technology, suggest that determining one’s capacity to acquire and extinguish conditioned fear pre-treatment may provide an additional diagnostic tool at treatment intake. Further, the employment of an objective, psychophysiologically-based index of treatment outcome to supplement gold-standard PTSD symptom rating measures may allow both clinician and client to observe directly the connection between brain, mind, and body and how this relationship changes with treatment be it cognitive behavior therapy, pharmacotherapy, or an adjuvant combination of these approaches. We believe the results presented here illustrate an exciting new avenue to pursue when addressing the fear-related features of PTSD and its comorbidities and could inform strategies to augment traditional exposure approaches for those at risk of sub-optimal treatment response.
4. Conclusions
Taken together, the results of the current study and findings available in the literature support the existing view that extinction learning is impaired in PTSD as evident in numerous psychophysiological studies. Additionally, while fear extinction is a proposed mechanism underlying PE, this is the first published study to investigate an experimental fear extinction paradigm in a PE sample at pre- and post-treatment, with results providing support that PTSD-related altered fear extinction (fear load) may be responsive to extinction-based exposure therapy and that high PE responders demonstrate extinction recall following PE treatment. This finding can inform future research into PE augmentation and personalized medicine approaches.
Funding
This work is supported by Building Interdisciplinary Research Careers in Women’s Health of the National Institutes of Health (JMK; Award Nos. K12HD085850, UL1TR002378), The authors acknowledge, with gratitude, critical support from Wounded Warrior Project (B.O.R.), who supports the Emory Healthcare Veterans Program and serves as a partner in the Warrior Care Network, dedicated to filling gaps in mental health care for the invisible wounds of war in service members, veterans, and military families, and to the Robert McCormick Foundation. We thank Manessa Riser for her assistance in the preparation of this work.
Disclosure
Dr. Maples-Keller has funding from the Building Interdisciplinary Research Careers in Women’s Health of the National Institutes of Health under Award Number K12HD085850, UL1TR002378 (Georgia CTSA) and has funding from COMPASS Pathways and has received a speaking fee from COMPASS Pathways. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Drs. Watkins, Nylocks, and Yasinski report no financial relationships with commercial interests.
Ms. Coghlan reports no financial relationships with commercial interests.
Mrs. Black reports no financial relationships with commercial interests.
Dr. Jovanovic has funding from the National Institutes of Health (NIH: R01MH111682; R01MH108641; R01HD099178; R01MH122867; UM1NS118922). Dr. Jovanovic reports no financial relationships with commercial interests.
Dr. Rauch receives support from Wounded Warrior Project (WWP), Department of Veterans Affairs (VA), National Institute of Health (NIH#UL1TR000433; R33MH111935; PI: Rabinak), McCormick Foundation, Tonix Pharmaceuticals, Woodruff Foundation, and Department of Defense (DOD #W81XWH-11-1-0073; PI: Rauch). Dr. Rauch receives royalties from Oxford University Press and American Psychological Association Press.
Dr. Rothbaum has or recently had funding from Wounded Warrior Project, National Science Foundation, Cohen Veteran Bioscience, Bob Woodruff Foundation, The Hidden Heroes Fund (an initiative of the Elizabeth Dole Foundation), Department of Defense Clinical Trial Grant No.W81XWH-10-1-1045, and McCormick Foundation. Dr. Rothbaum receives royalties from Oxford University Press, Guilford, APPI, and Emory University and received advisory board payments from Genentech, Jazz Pharmaceuticals, Nobilis Therapeutics, Sophren, Neuronetics, and Aptinyx. Dr. Rothbaum is a consultant to and owns equity in Virtually Better, Inc. that creates virtual environments. The terms of these arrangements have been reviewed and approved by Emory University in accordance with its conflict of interest policies.
Dr. Norrholm has funding from the Department of Defense/PASA Consortium (AS140026-A5). Dr. Jovanovic reports no financial relationships with commercial interests.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Jessica Maples-Keller reports financial support was provided by National Institutes of Health. Jessica Maples-Keller reports financial support was provided by COMPASS Pathways Plc. Jessica Maples-Keller reports financial support was provided by Emory University School of Medicine (Georgia CTSA NIH award; UL1-TR002378). Tanja Jovanovic reports financial support was provided by National Institutes of Health. Sheila Rauch reports financial support was provided by Wounded Warrior Project. Sheila Rauch reports financial support was provided by US Department of Veterans Affairs. Sheila Rauch reports financial support was provided by National Institutes of Health. Sheila Rauch reports financial support was provided by Tonix Pharmaceuticals Holding Corp. Sheila Rauch reports financial support was provided by Robert W Woodruff Foundation. Sheila Rauch reports financial support was provided by US Department of Defense. Sheila Rauch reports financial support was provided by Robert R McCormick Foundation. Barbara Rothbaum reports financial support was provided by Wounded Warrior Project. Barbara Rothbaum reports financial support was provided by National Science Foundation. Barbara Rothbaum reports financial support was provided by Cohen Veterans Bioscience. Barbara Rothbaum reports financial support was provided by Robert W Woodruff Foundation. Barbara Rothbaum reports financial support was provided by The Elizabeth Dole Foundation. Barbara Rothbaum reports financial support was provided by US Department of Defense. Barbara Rothbaum reports financial support was provided by Robert R McCormick Foundation. Seth D. Norrholm reports financial support was provided by US Department of Defense. Barbara Rothbaum reports a relationship with Genentech Inc that includes: consulting or advisory. Barbara Rothbaum reports a relationship with Jazz Pharmaceuticals Inc that includes: consulting or advisory. Barbara Rothbaum reports a relationship with Nobilis Health that includes: consulting or advisory. Barbara Rothbaum reports a relationship with Sophren that includes: consulting or advisory. Barbara Rothbaum reports a relationship with Neuronetics that includes: consulting or advisory. Barbara Rothbaum reports a relationship with Aptinyx Inc that includes: consulting or advisory. Barbara Rothbaum reports a relationship with Virtually Better, Inc that includes: equity or stocks. Sheila Rauch reports a relationship with Oxford University Press that includes: consulting or advisory. Sheila Rauch reports a relationship with American Psychological Association that includes: consulting or advisory. Royalties from Oxford University Press, Guilford, APPI, Emory University - B.O.R.
Footnotes
CRediT authorship contribution statement
Jessica Maples-Keller: Conceptualization, Methodology, Formal analysis, Data curation, Writing – original draft, Writing – review & editing. Laura E. Watkins: Conceptualization, Methodology, Writing – review & editing. K. Maria Nylocks: Conceptualization, Methodology, Writing – review & editing. Carly Yasinski: Conceptualization, Methodology, Writing – review & editing. Callan Coghlan: Data curation, Writing – review & editing. Kathryn Black: Data curation, Project administration, Writing – review & editing. Tanja Jovanovic: Conceptualization, Methodology, Formal analysis, Writing – review & editing, Supervision. Sheila A.M. Rauch: Conceptualization, Methodology, Investigation, Writing – review & editing, Supervision. Barbara O. Rothbaum: Conceptualization, Methodology, Investigation, Writing – review & editing, Supervision, Resources, Funding acquisition. Seth Davin Norrholm: Conceptualization, Methodology, Investigation, Writing – review & editing, Supervision, Visualization.
References
- American Psychological Association. (2017). Clinical practice guideline for the treatment of posttraumatic stress disorder (PTSD) in adults. Washington, DC. [Google Scholar]
- Barlow DH, Farchione TJ, Bullis JR, Gallagher MW, Murray-Latin H, Sauer-Zavala S, et al. (2017). The unified protocol for transdiagnostic treatment of emotional disorders compared with diagnosis-specific protocols for anxiety disorders: A randomized clinical trial. JAMA Psychiatry, 74(9), 875–884. 10.1001/jamapsychiatry.2017.2164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blevins CA, Weathers FW, Davis MT, Witte TK, & Domino JL (2015). The posttraumatic stress disorder checklist for DSM-5 (PCL-5): Development and initial psychometric evaluation. Journal of Traumatic Stress, 28(6), 489–498. 10.1002/jts.22059 [DOI] [PubMed] [Google Scholar]
- Bouton ME, Mineka S, & Barlow DH (2018). A modern learning theory perspective on the etiology of panic disorder. The Neurotic Paradox, 265–324. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Westbrook RF, Corcoran KA, & Maren S (2006). Contextual and temporal modulation of extinction: Behavioral and biological mechanisms. Biological Psychiatry, 60(4), 352–360. 10.1016/j.biopsych.2005.12.015 [DOI] [PubMed] [Google Scholar]
- Careaga MBL, Girardi CEN, & Suchecki D (2016). Understanding posttraumatic stress disorder through fear conditioning, extinction and reconsolidation. Neuroscience & Biobehavioral Reviews, 71, 48–57. 10.1016/j.neubiorev.2016.08.023 [DOI] [PubMed] [Google Scholar]
- Fani N, Tone EB, Phifer J, Norrholm SD, Bradley B, Ressler KJ, … Jovanovic T (2012). Attention bias toward threat is associated with exaggerated fear expression and impaired extinction in PTSD. Psychological Medicine, 42(3), 533–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foa EB, McLean CP, Zang Y, Rosenfield D, Yadin E, Yarvis JS, et al. (2018). Effect of prolonged exposure therapy delivered over 2 weeks vs 8 weeks vs present-centered therapy on PTSD symptom severity in military personnel: A randomized clinical trial. JAMA, 319(4), 354–364. 10.1001/jama.2017.21242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foa EB, Steketee G, & Rothbaum BO (1989). Behavioral/cognitive conceptualizations of post-traumatic stress disorder. Behavior Therapy, 20(2), 155–176. 10.1016/S0005-7894(89)80067-X [DOI] [Google Scholar]
- Galatzer-Levy IR, & Bryant RA (2013). 636,120 ways to have posttraumatic stress disorder. Perspectives on Psychological Science, 8(6), 651–662. 10.1016/j.nlm.2013.10.014 [DOI] [PubMed] [Google Scholar]
- Galatzer-Levy IR, Ma S, Statnikov A, Yehuda R, & Shalev AY (2017). Utilization of machine learning for prediction of post-traumatic stress: A re-examination of cortisol in the prediction and pathways to non-remitting PTSD. Translational Psychiatry, 7(3), Article e1070–e1070. 10.1038/2ftp.2017.38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helpman L, Marin MF, Papini S, Zhu X, Sullivan GM, Schneier F, et al. (2016). Neural changes in extinction recall following prolonged exposure treatment for PTSD: A longitudinal fMRI study. NeuroImage: Clinica, 12, 715–723. 10.1016/j.nicl.2016.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Kazama A, Bachevalier J, & Davis M (2012). Impaired safety signal learning may be a biomarker of PTSD. Neuropharmacology, 62(2), 695–704. 10.1016/j.neuropharm.2011.02.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissek S, Rabin SJ, McDowell DJ, Dvir S, Bradford DE, Geraci M, et al. (2009). Impaired discriminative fear-conditioning resulting from elevated fear responding to learned safety cues among individuals with panic disorder. Behaviour Research and Therapy, 47(2), 111–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maples-Keller JL, Norrholm SD, Burton M, Reiff C, Coghlan C, Jovanovic T, et al. (2022). A randomized controlled trial of 3,4-methylenedioxymethamphetamine (MDMA) and fear extinction retention in healthy adults. Journal of Psychopharmacology, 36(3), 368–377. 10.1177/02698811211069124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maples-Keller JL, Rauch SA, Jovanovic T, Yasinski CW, Goodnight JM, Sherrill A, et al. (2019). Changes in trauma-potentiated startle, skin conductance, and heart rate within prolonged exposure therapy for PTSD in high and low treatment responders. Journal of Anxiety Disorders, 68, Article 102147. 10.1016/j.janxdis.2019.102147 [DOI] [PubMed] [Google Scholar]
- Milad MR, Orr SP, Lasko NB, Chang Y, Rauch SL, & Pitman RK (2008). Presence and acquired origin of reduced recall for fear extinction in PTSD: Results of a twin study. Journal of Psychiatric Research, 42(7), 515–520. 10.1016/j.jpsychires.2008.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB, et al. (2009). Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biological Psychiatry, 66(12), 1075–1082. 10.1016/j.biopsych.2009.06.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, & Quirk GJ (2012). Fear extinction as a model for translational neuroscience: Ten years of progress. Annual Review of Psychology, 63, 129–151. 10.1146/annurev.psych.121208.131631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers KM, & Davis M (2002). Behavioral and neural analysis of extinction. Neuron, 36(4), 567–584. 10.1016/S0896-6273(02)01064-4 [DOI] [PubMed] [Google Scholar]
- Norrholm SD, Glover EM, Stevens JS, Fani N, Galatzer-Levy IR, Bradley B, et al. (2015). Fear load: The psychophysiological over-expression of fear as an intermediate phenotype associated with trauma reactions. International Journal of Psychophysiology, 98(2), 270–275. 10.1016/j.ijpsycho.2014.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrholm SD, & Jovanovic T (2018). Fear processing, psychophysiology, and PTSD. [DOI] [PubMed]
- Harvard Review of Psychiatry, 26(3), 129–141. 10.1097/HRP.0000000000000189 [DOI] [PubMed] [Google Scholar]
- Norrholm SD, Jovanovic T, Gerardi M, Breazeale KG, Price M, Davis M, et al. (2016). Baseline psychophysiological and cortisol reactivity as a predictor of PTSD treatment outcome in virtual reality exposure therapy. Behaviour Research and Therapy, 82, 28–37. 10.1016/j.brat.2016.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrholm SD, Jovanovic T, Olin IW, Sands LA, Bradley B, & Ressler KJ (2011). Fear extinction in traumatized civilians with posttraumatic stress disorder: Relation to symptom severity. Biological Psychiatry, 69(6), 556–563. 10.1016/j.biopsych.2010.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrholm SD, Jovanovic T, Vervliet B, Myers KM, Davis M, Rothbaum BO, et al. (2006). Conditioned fear extinction and reinstatement in a human fear-potentiated startle paradigm. Learning & Memory, 13(6), 681–685. 10.1101/lm.393906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrholm SD, Vervliet B, Jovanovic T, Boshoven W, Myers KM, Davis M, et al. (2008). Timing of extinction relative to acquisition: A parametric analysis of fear extinction in humans. Behavioral Neuroscience, 122(5), 1016. https://psycnet.apa.org/doi/10.1037/a0012604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orcutt HK, Hannan SM, Seligowski AV, Jovanovic T, Norrholm SD, Ressler KJ, et al. (2017). Fear-potentiated startle and fear extinction in a sample of undergraduate women exposed to a campus mass shooting. Frontiers in Psychology, 7, 2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers MB, Halpern JM, Ferenschak MP, Gillihan SJ, & Foa EB (2010). A meta-analytic review of prolonged exposure for posttraumatic stress disorder. Clinical Psychology Review, 30(6), 635–641. 10.1016/j.cpr.2010.04.007 [DOI] [PubMed] [Google Scholar]
- Rauch SA, King A, Kim HM, Powell C, Rajaram N, Venners M, et al. (2020). Cortisol awakening response in PTSD treatment: Predictor or mechanism of change. Psychoneuroendocrinology, 118, Article 104714. 10.1016/j.psyneuen.2020.104714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch SA, Koola C, Post L, Yasinski C, Norrholm SD, Black K, et al. (2018). In session extinction and outcome in virtual reality exposure therapy for PTSD. Behaviour Research and Therapy, 109, 1–9. 10.1016/j.brat.2018.07.003 [DOI] [PubMed] [Google Scholar]
- Ready DJ, Lamp K, Rauch SA, Astin MC, & Norrholm SD (2020). Extending prolonged exposure for veterans with posttraumatic stress disorder: When is enough really enough? Psychological Services, 17(2), 199. https://psycnet.apa.org/doi/10.1037/ser0000309. [DOI] [PubMed] [Google Scholar]
- Reist C, Jovanovic T, Kantarovich D, Weingast L, Hollifield M, Novin M, et al. (2021). An analysis of fear inhibition and fear extinction in a sample of veterans with obstructive sleep apnea (OSA): Implications for co-morbidity with post-traumatic stress disorder (PTSD). Behavioural Brain Research, 404, Article 113172. [DOI] [PubMed] [Google Scholar]
- Ressler KJ, Mercer KB, Bradley B, Jovanovic T, Mahan A, Kerley K, et al. (2011). Post-traumatic stress disorder is associated with PACAP and the PAC1 receptor. Nature, 470(7335), 492–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robison-Andrew EJ, Duval ER, Nelson CB, Echiverri-Cohen A, Giardino N, Defever A, … Rauch SA (2014). Changes in trauma-potentiated startle with treatment of posttraumatic stress disorder in combat Veterans. Journal of Anxiety Disorders, 28(4), 358–362. [DOI] [PubMed] [Google Scholar]
- Rodriguez P, Holowka DW, & Marx BP (2012). Assessment of posttraumatic stress disorder-related functional impairment: A review. Journal of Rehabilitation Research and Development, 49(5), 649–666. 10.1682/jrrd.2011.09.0162 [DOI] [PubMed] [Google Scholar]
- Rothbaum BO, & Davis M (2003). Applying learning principles to the treatment of post-trauma reactions. Annals of the New York Academy of Sciences, 1008(1), 112–121. 10.1196/annals.1301.012 [DOI] [PubMed] [Google Scholar]
- Rothbaum BO, Price M, Jovanovic T, Norrholm SD, Gerardi M, Dunlop B, et al. (2014). A randomized, double-blind evaluation of D-cycloserine or alprazolam combined with virtual reality exposure therapy for posttraumatic stress disorder in Iraq and Afghanistan War veterans. American Journal of Psychiatry, 171(6), 640–648. 10.1176/appi.ajp.2014.13121625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. (1998). The mini-international neuropsychiatric interview (MINI): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. Journal of Clinical Psychiatry, 59(20), 22–33. [PubMed] [Google Scholar]
- Shumake J, Jones C, Auchter A, & Monfils MH (2018). Data-driven criteria to assess fear remission and phenotypic variability of extinction in rats. Philosophical Transactions of the Royal Society B: Biological Sciences, 373(1742), Article 20170035. 10.1038/tp.2017.38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stojek MM, McSweeney LB, & Rauch SA (2018). Neuroscience informed prolonged exposure practice: Increasing efficiency and efficacy through mechanisms. Frontiers in Behavioral Neuroscience, 12, 281. 10.3389/fnbeh.2018.00281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolin DF, Gilliam C, Wootton BM, Bowe W, Bragdon LB, Davis E, et al. (2018). Psychometric properties of a structured diagnostic interview for DSM-5 anxiety, mood, and obsessive-compulsive and related disorders. Assessment, 25(1), 3–13. 10.1177/2f1073191116638410 [DOI] [PubMed] [Google Scholar]
- Warren VT, Anderson KM, Kwon C, Bosshardt L, Jovanovic T, Bradley B, et al. (2014). Human fear extinction and return of fear using reconsolidation update mechanisms: The contribution of on-line expectancy ratings. Neurobiology of Learning and Memory, 113, 165–173. 10.1016/j.nlm.2013.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts BV, Schnurr PP, Mayo L, Young-Xu Y, Weeks WB, & Friedman MJ (2013). Meta-analysis of the efficacy of treatments for posttraumatic stress disorder. Journal of Clinical Psychiatry, 74(6), 541–550. 10.4088/JCP.12r08225 [DOI] [PubMed] [Google Scholar]
- Weathers FW, Bovin MJ, Lee DJ, Sloan DM, Schnurr PP, Kaloupek DG, et al. (2018). The Clinician-Administered PTSD Scale for DSM–5 (CAPS-5): Development and initial psychometric evaluation in military veterans. Psychological Assessment, 30(3), 383. https://psycnet.apa.org/doi/10.1037/pas0000486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasinski C, Sherrill AM, Maples-Keller JL, Rauch SAM, & Rothbaum BO (2017). Intensive outpatient prolonged exposure for PTSD in post-9/11 veterans and service-members: Program structure and preliminary outcomes of the emory Healthcare veterans program. Trauma Psychology News, 12(3), 14–17. [Google Scholar]
- Zuj DV, & Norrholm SD (2019). The clinical applications and practical relevance of human conditioning paradigms for posttraumatic stress disorder. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 88, 339–351. 10.1016/j.pnpbp.2018.08.014 [DOI] [PubMed] [Google Scholar]
- Zuj DV, Palmer MA, Lommen MJ, & Felmingham KL (2016). The centrality of fear extinction in linking risk factors to PTSD: A narrative review. Neuroscience & Biobehavioral Reviews, 69, 15–35. 10.1016/j.neubiorev.2016.07.014 [DOI] [PubMed] [Google Scholar]


