Abstract
Background:
Fear conditioning and extinction are well-characterized cross-species models of fear-related posttraumatic stress disorder (PTSD) symptoms, and recent animal data suggest that 3,4-methylenedioxymethamphetamine (MDMA) enhances fear extinction retention.
Aims:
This study investigated the effect of MDMA on fear learning, extinction training, and retention in healthy humans.
Methods:
The study involved a randomized placebo-controlled, two-group, parallel design trial in a sample of healthy adults, age 21–55 recruited from a major metropolitan area. The experimental paradigm included a fear acquisition session followed by an extinction training session 24 hours later, and 2 hours after study drug administration. Fear extinction retention was measured 48 hours after extinction training. Participants (N = 34; 70.6% male and 29.4% female) were randomly assigned in 1:1 ratio to 100 mg MDMA or placebo. All randomized participants completed the trial and were included in primary analyses. Safety was monitored via adverse events and vital signs. MDMA was well-tolerated with no serious adverse events.
Results:
Results indicated a significant main effect of session between extinction training and retention with no significant group differences. Significantly more participants in the MDMA group retained extinction learning compared to the placebo group (χ2 = 7.29, p = 0.007).
Conclusion:
Although we did not observe the hypothesized facilitation of extinction retention, the findings from this initial human trial provide compelling rationale to continue to explore the potential for MDMA to impact extinction retention.
Clinical Trials Registry Name and Identifier: Evaluation of MDMA on Startle Response (NCT0318176) https://clinicaltrials.gov/ct2/show/NCT03181763?term = MDMA&draw = 2&rank = 9
Keywords: Fear extinction, fear-potentiated startle, MDMA, psychophysiology, randomized trial
Posttraumatic stress disorder (PTSD) is a debilitating psychiatric illness associated with significant distress and difficulties in functioning (Kessler, 2000). Effective treatments for PTSD exist including trauma-focused psychotherapy and pharmacological treatments (American Psychological Association [APA], 2017; US Department of Veterans Affairs Department of Defense [VA/DoD], 2017). However, a subset of patients fails to benefit, and alternative treatment approaches are needed. Accumulating evidence provides support that 3,4-methylenedioxymethamphetamine (MDMA) in combination with psychotherapy is effective. MDMA is a phenethylamine derivative associated with subjective effects including experiences of euphoria, increased feelings of closeness and love for others, and better access to emotionally intense material (Bedi et al., 2010; Harris et al., 2002). MDMA enhances the release and inhibits the reuptake of serotonin, norepinephrine, and dopamine, resulting in increased neurotrans-mission of these monoamines; serotonergic effects are likely responsible for most of the subjective effects in humans (Farré et al., 2007; Young et al., 2015).
Across six randomized trials, after two blinded experimental sessions, the active MDMA dose groups (75–125 mg) demonstrated a greater reduction in PTSD symptoms compared to placebo or control dose groups (0–40 mg) (d = 0.8) (Mithoefer et al., 2019). In this research, participants engaged in three non-drug therapy preparatory sessions, two to three 8-hour MDMA or placebo sessions, and three to four integration therapy sessions following experimental sessions, using a non-directive therapeutic approach (Mithoefer et al., 2019). PTSD symptoms continued to decrease across follow-up assessments (Jerome et al., 2020), and pooled data indicate a favorable safety profile (Feduccia et al., 2019). Fear extinction learning and retention represent one of the theoretical foundations of prolonged exposure (PE) therapy, a first-line treatment for PTSD (APA, 2017; Institute of Medicine, 2007; VA/DoD, 2017), in which patients therapeutically confront trauma reminders and trauma memories (Rothbaum and Davis, 2003). PE demonstrates large treatment effect sizes (Cusack et al., 2016), but a subset of patients do not attain clinically meaningful symptom improvement in trauma-focused therapies for PTSD, and dropout rates are often significant (Bradley et al., 2005). Identifying potential mechanisms of MDMA’s impact on PTSD symptoms is vital to inform ongoing intervention research and enhancement of treatment efficacy, efficiency, and response rates. This study investigated MDMA’s impact on fear extinction learning and retention which could better determine MDMA’s potential to enhance outcomes of PE for PTSD through its action on fear circuitry and resulting behavior.
The administration of fear conditioning and subsequent extinction training is a well-characterized translational model for the study of fear-related PTSD symptoms (Briscione et al., 2014; Jovanovic et al., 2009; Norrholm et al., 2006), and improved extinction learning has been proposed as one of the mechanisms underlying the success of MDMA-assisted psychotherapy (Feduccia and Mithoefer, 2018). The fear-related symptoms of PTSD can occur following exposure to an extremely aversive event (unconditioned stimulus (US)) that can lead to an associative (conditioned) fear to cues associated with the US (conditioned stimuli (CS)) which can result in sustained behavioral expressions of fear (conditioned responses (CR)). According to the principles of associative fear learning (Rothbaum and Davis, 2003), fear-related PTSD symptoms (e.g. re-experiencing and hyperarousal) can involve conditioned fear responses such as physiological and psychological distress upon trauma reminders, avoidance of trauma-related cues, and exaggerated fear responses that do not diminish over time due in part to a failure of fear extinction (Rothbaum et al., 1992).
In the laboratory, fear extinction training (also termed within-session extinction) involves repeated presentation of the CS without the US, resulting in a decreased conditioned fear response (Myers and Davis, 2002). Fear extinction retention (also termed between-session extinction or extinction recall) is the retrieval and expression of the learned extinction memory following a delay. PTSD has been associated with extinction training and retention deficits (Jovanovic et al., 2012; Milad and Quirk, 2012; Norrholm et al., 2008, 2011). The fear memory is not erased during extinction, but new learning about the likelihood of further threat develops, inhibiting previously formed fear memories (Bouton et al., 2008; Briscione et al., 2014; Myers and Davis, 2002). Extinction retention, and the ability to appropriately maintain this inhibition of fear, is relevant to PTSD treatment outcome.
In rodent models, MDMA robustly enhanced long-term extinction when administered prior to extinction training (Young et al., 2015). MDMA’s extinction enhancement was associated with increased neuronal activity in the amygdala and medial prefrontal cortex and increased brain-derived neurotrophic factor (BDNF) expression in the amygdala (Young et al., 2015). A recent preclinical study replicated the finding that MDMA enhanced extinction retention as measured via fear-potentiated startle and found that acute and chronic treatment with a 5-HT transporter inhibitor blocked this effect (Young et al., 2017). Others have found that MDMA did not enhance extinction training but rather reduced conditioned fear when administered during the consolidation phase, suggesting MDMA may act to disrupt reconsolidation of fear memories (Hake et al., 2019). These rodent studies provide support for the promise of MDMA in enhancing fear extinction retention in PTSD patients.
This study investigated the impact of MDMA on fear extinction training and retention in a sample of healthy humans who completed a translational experimental fear acquisition and extinction startle paradigm (e.g. Norrholm et al., 2011) based largely on the rodent paradigms employed in the previously mentioned studies. In humans, the startle response is a phylogenetically well-preserved and sensitive indicator of conditioned fear that can be reliably measured and manipulated (Jovanovic et al., 2009) and has shown to be associated with effectiveness of PE therapy for PTSD (Glover et al., 2015; Maples-Keller et al., 2019; Norrholm et al., 2016; Robison-Andrew et al., 2014). We hypothesized that participants who received MDMA during extinction training would demonstrate a decreased startle response to aversive stimuli during extinction retention compared to participants who received placebo.
Materials and methods
Study design
The study was a parallel-group, randomized, placebo-controlled trial consisting of three visits conducted at Emory University in Atlanta, Georgia, from March 2018 to July 2020 when target enrollment was reached (N = 34). Adverse events were collected for duration of study participation. The protocol was approved by the Emory University IRB and conducted in accordance with the Declaration of Helsinki of 1975 and its amendments and was registered at ClinicalTrials.gov (NCT03181763) on 9 June 2017.
Randomization
Randomization to MDMA or placebo was 1:1 from a list using permuted blocks generated by an independent biostatistician retained by MAPS Public Benefit Corporation (MAPS PBC), study sponsor designee, prior to the start of the study and sent directly to unblinded study physician, who maintained the list and the study drug supply. MDMA was manufactured by Zeeh Pharmaceutical Experimental Station within the University of Wisconsin in Madison, Wisconsin. No other study personnel were given access to the randomization list. At Visit 2, after all eligibility criteria had been confirmed, the study physician assigned the patient to the treatment arm indicated according to the randomization list and dispensed the study medication. Participant and all research staff other than study physician were blinded to group assignment. The unblinded study physician’s only other roles on the study included interpretation of laboratory and electrocardiogram data and availability for any medical issues arising after dosing.
Participants
Participants were recruited through community advertising and provided written informed consent. Inclusion criteria included (1) 21–55 years of age, (2) ability to read and understand the English language, (3) having previously used MDMA in recreational setting with no reported adverse experiences, (4) a negative pregnancy test in females of childbearing potential, and participants had to agree to use birth control through 10 days after study completion. Exclusion criteria included (1) lifetime diagnosis of bipolar disorder, primary psychotic disorder, dementia, or intellectual disorder, (2) lifetime diagnosis of moderate or severe substance use disorder, except caffeine or nicotine, (3) any psychiatric disorder in the 6 months prior to screening, (4) use of psychoactive medications during the 2 weeks prior to Visit 1, (5) use of MDMA more than 10 times in 10 years, (6) any use of MDMA in the past 6 months, (7) first-degree relative with diagnosis of schizophrenia or bipolar I disorder, (8) the presence of unstable or central nervous system-related medical illness that would interfere with study participation, (9) uncontrolled hyper-tension or clinically significant arrythmia as detected by electrocardiogram, (10) currently pregnant or breast feeding, (11) history of acute angle glaucoma, and (12) hearing impairment as detected by audiometer (i.e. unable to detect tones below 40 dB in right or left ear), and/or (13) positive urine drug screen at Visit 1 or 2. Participants were compensated $100 USD for Visits 1 and 2 and $150 for Visit 3.
Procedure
Prescreening, screening, and baseline evaluation (Visit 1).
Prospective participants were prescreened by telephone for basic eligibility criteria. Lifetime and current psychiatric diagnoses were assessed by a PhD-level assessor using the MINI-International Neuropsychiatric Interview, v.7.0.2 for DSM-5 (Sheehan et al., 1998). Medication and drug use history, a medical history and physical exam, electrocardiogram and screening laboratory test, including urine drug screen and pregnancy test (if applicable), were completed to confirm study eligibility. After completion of all eligibility procedures and confirmation that participant met all enrollment criteria (except for the pending laboratory results), the fear acquisition session was completed. At the end of the visit, participants were instructed to (1) ingest only alcohol-free liquids after midnight on the evening before experimental session, (2) refrain from use of psychoactive drugs with exception of caffeine or nicotine within 24 hours of the experimental session, (3) not use caffeine or nicotine 2 hours before or 6 hours after ingesting study drug, and (4) not use herbal supplementals after midnight.
Experimental session (Visit 2).
At the start of Visit 2, participants completed a urine drug screen and pregnancy test if applicable. The study physician administered the study medication which consisted of a white capsule containing either 100 mg MDMA or matching placebo. The visit occurred in a decorated office setting with patients sitting in a recumbent chair except when walked to and from the startle testing booth. Vital signs were assessed prior to study drug administration and 0.75, 1.25, 1.75, 3, 4, 5, and 6 hours following drug dosing. Vitals in the MDMA and placebo groups across Visit 2 timepoints are presented in the supplementary materials (Supplementary Figures S3–S6). The study physician was on site for the entirety of Visit 2 in case of medical complications. The extinction training session was implemented 2 hours after study drug administration (around Tmax for MDMA; de la Harris et al., 2002; De la Torre et al., 2000); other than this session, participants remained in the treatment room for 6 hours after dosing.
Extinction retention (Visit 3).
Visit 3 occurred 48 hours following Visit 2 and involved a urine drug screen and extinction retention phase of startle testing (described below).
Startle paradigm
The study used a well-validated fear conditioning, extinction training, and extinction retention fear-potentiated startle paradigm (Jovanovic et al., 2009; Norrholm et al., 2006, 2008, 2011). A diagram of this paradigm is presented in Figure 1. Acoustic startle response was measured using electromyography (EMG) of the right orbicularis oculi muscle. EMG activity was acquired at a sampling rate of 1 kHz and was amplified and digitized via the Biopac MP150 EMG module (Biopac Systems Inc, Aero Camino, California). Two 5-mm silver/silver chloride (Ag/AgCl) pre-gelled disposable electrodes were positioned approximately 1 cm under the pupil and 1 cm under the lateral canthus. The startle probe was a 108-dB sound pressure level, 40-ms burst of broadband noise with near instantaneous rise time per previously published methods (e.g. Norrholm et al., 2006).
Figure 1.
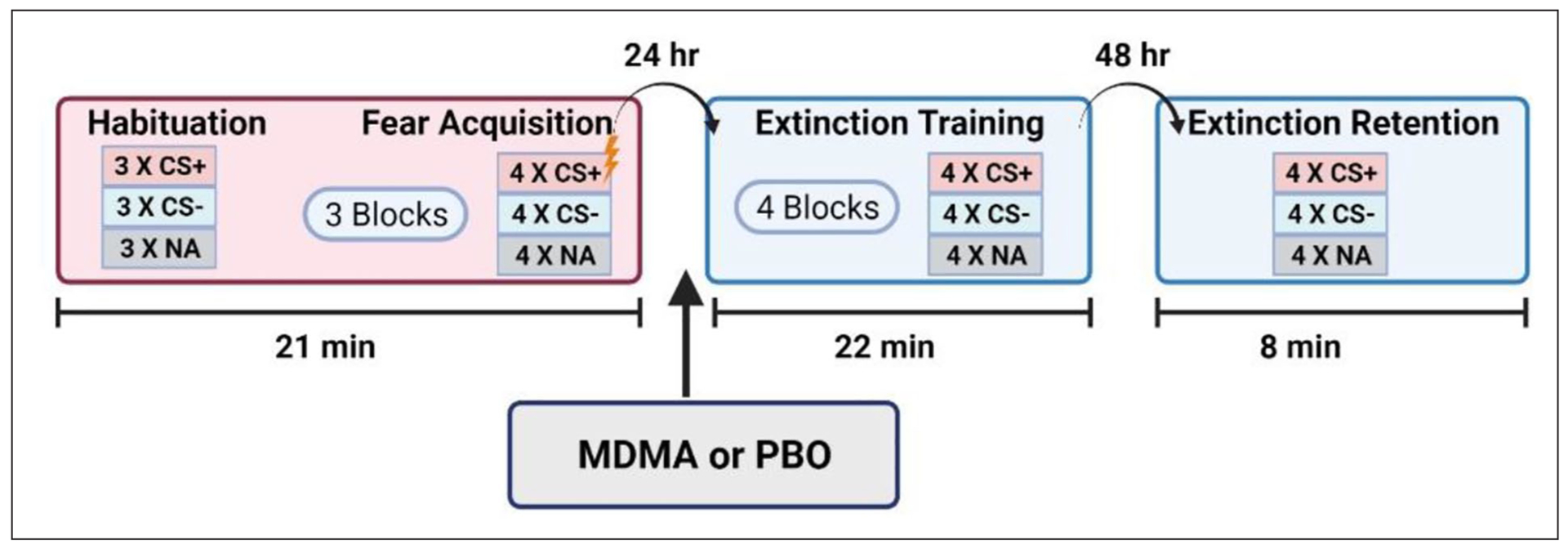
Schematic illustration of the experimental design for assessing CS habituation, fear acquisition, extinction training, and extinction retention (also termed recall).
NA = noise alone; CS = conditioned stimulus
Visit 1 (fear acquisition).
CS was presented visually as colored shapes on a computer monitor. The aversive US was a 250-ms airblast with intensity of 140 pounds per square inch directed to the larynx. Airblasts were emitted by compressed airtank connected to polyethylene tubing and controlled by a solenoid switch. The conditioning session began with a CS habituation block, followed by three acquisition blocks. Across the acquisition blocks, there were a total of 12 CSs (colored shapes) reinforced with an airblast US (CS+) and 12 non-reinforced CSs (CS−), as well as 12 trials of the sound probe presented without a CS (noise alone (NA)). Trial order for each session was randomly determined during experimental design and all stimuli were presented in the same order for each participant. Stimuli were presented using SuperLab 4.5 for Windows (Cedrus, Inc., San Pedro, CA) and synchronized with psychophysiological data acquisition using DIO card (Measurements Computing, Inc). The inter-trial interval (ITI) ranged from 9 to 22 seconds.
Visit 2 (extinction training).
During the extinction session, the same CSs from the fear acquisition session were presented on a computer monitor, but this time, none were followed by an airblast US (Norrholm et al., 2011). Sixteen trials of each type (CS+, CS−, NA) were administered with same ITI range (9–22 seconds) as the prior session.
Visit 3 (extinction retention).
During extinction retention, the same CSs as previous sessions were repeatedly presented on the computer monitor without being followed by an airblast US (Norrholm et al., 2011). There were four trials of each type and same ITI range (9–22 seconds) as previously noted.
Statistical analysis
Consistent with previous studies that employed this paradigm (e.g. Norrholm et al., 2011), the primary outcome measure was fear-potentiated startle during extinction retention as defined as the increase in startle magnitude (in microVolts) when a conditioned stimulus was presented to the participant as compared to participant’s baseline acoustic startle response. In short, Repeated Measures Analysis of Variance (RM-ANOVA) was used to probe for fear acquisition, extinction training, and extinction retention effects. Acquisition of conditioned fear was tested with RM-ANOVA with Block (four levels—Habituation and three acquisition blocks) and Trial Type (two levels—NA and reinforced CS+) included as within-subjects variables and group (placebo vs MDMA) included as a between-subjects variable. The dependent variable in these analyses was startle magnitude on each trial type. Discrimination between reinforced CS+ and non-reinforced CS− was tested with RM-ANOVA with Block (four levels—habituation and three acquisition blocks) and trial type (2 levels—CS+ and CS−) included as within-subjects variables and group (placebo vs MDMA) included as a between-subjects variable. Therefore, the dependent variable in these analyses was fear-potentiated startle magnitude in the presence of each CS type expressed as a difference score from NA.
Extinction training was tested with RM-ANOVA with block (four extinction blocks) as within-subjects variables and group (placebo vs MDMA) as a between-subjects variable. Extinction retention was tested with RM-ANOVA with session (two levels—last block of extinction training and one block of extinction retention) as within-subjects variables and group (placebo vs MDMA) as a between-subjects variable. Extinction training and retention analyses were specific to the previously reinforced CS+; therefore, the dependent variable in those analyses was fear-potentiated startle to the CS+. Significant higher-order interaction effects were followed up by lower-order analyses and simple comparisons.
Given individual differences in fear extinction processes, best analytical practice for contemporary extinction models highlight the importance of investigating the proportion of individuals within a group that shows a specific response using clear bench-marks in addition to examining group-level mean differences (Shumake et al., 2018). As such, we conducted post hoc exploratory analyses to classify participants in each group according to the degree to which they showed a return of fear or retention of extinction learning and conducted chi-square analyses to compare the amount of extinction retainers in MDMA and placebo groups.
Results
Participants
Fifty-one people consented for the study. Thirty-four were randomized, all of whom completed Visits 2 and 3. Demographic information is presented in Supplementary Table S1, and CONSORT diagram is presented in Supplementary Figure S1. MDMA and placebo groups were compared across all demographic variables, and no significant differences were identified (Supplementary Table S1).
Fear acquisition
Acquisition of fear-potentiated startle was quantified and analyzed according to the methods reported in previous studies (Myers and Davis, 2002; Norrholm et al., 2006, 2011; Rothbaum et al., 2014). Across the four blocks of conditioning (one CS habituation + three acquisition), there was a significant three-way interaction of block × trial type × group (F(3, 93) = 3.34, p = 0.02) as well as a significant block × trial type interaction (F(3, 93) = 3.82, p = 0.012). In addition, there were main effects of block and trial type (See Figure 2; see Supplemental Table S2). The block × trial type interaction indicated greater acoustic startle responses in the presence of the reinforced CS+ as compared to NA with increasing trial type differences from CS habituation to the end of acquisition block 3, thus showing successful acquisition of fear-potentiated startle. We followed up the three-way interaction by analyzing a two-way RM-ANOVA with block × trial type in each group separately to ensure that both groups acquired fear to the CS+. Both groups showed higher startle magnitude to CS+ versus NA (Placebo: F(1, 45) = 25.56, p < 0.001; MDMA: F(1, 48) = 23.24, p < 0.001). We also conducted a univariate ANOVA comparing the groups on both trial types and found no significant differences between MDMA and placebo groups on any of the blocks of the acquisition phase. The fear acquisition data are shown in Figure 2 with the groups collapsed due to the lack of significant differences.
Figure 2.
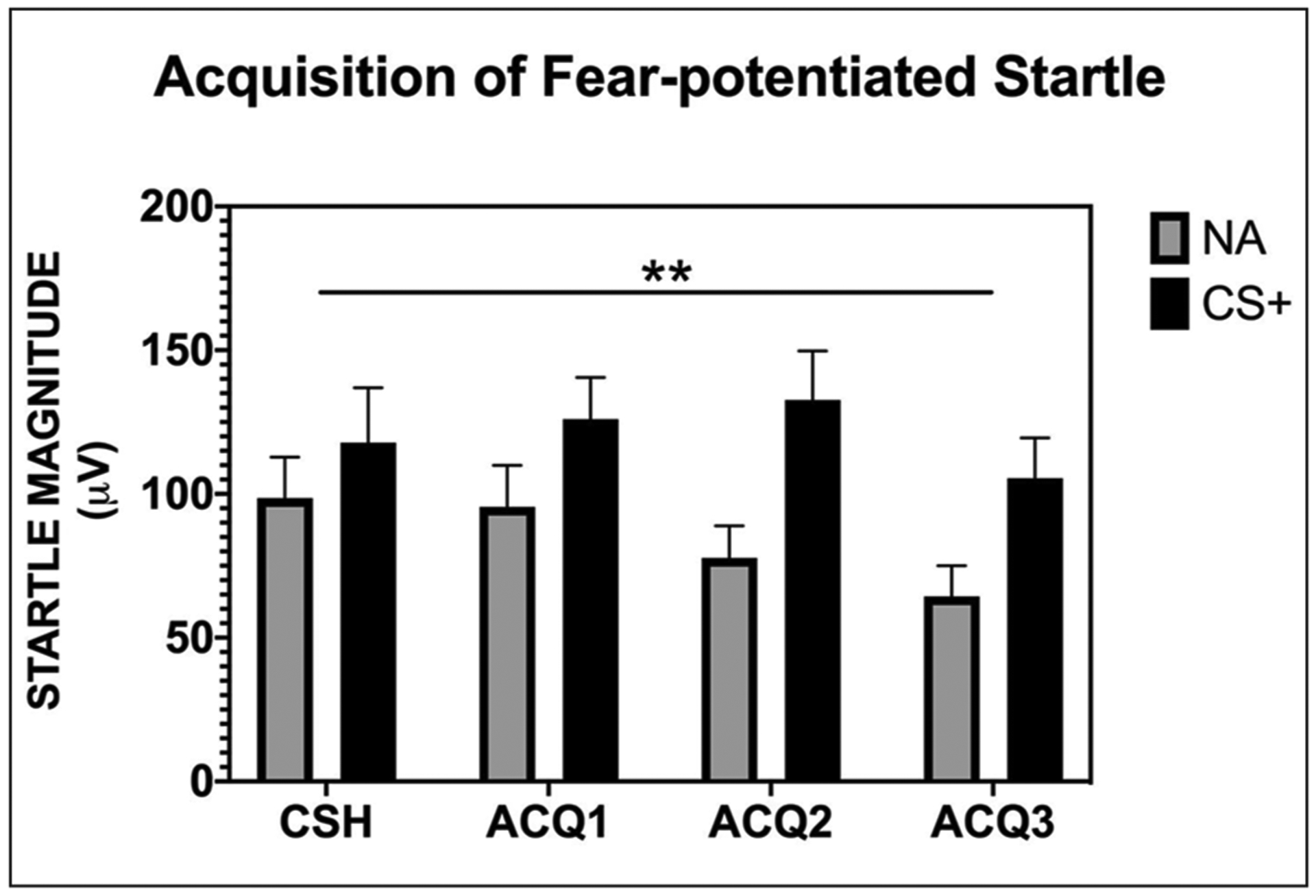
All participants, prior to any drug administration, displayed significant acquisition of fear-potentiated startle to the reinforced CS+ as compared to the noise probe alone.
CSH = conditioned stimuli habituation; ACQ = acquisition; NA = noise alone; CS+ = reinforced conditioned stimuli; μV = microvolts; ** = significant block × trial type interaction, p = 0.012
CS+/CS− discrimination
The discrimination between the CS+ and CS− cues was quantified and analyzed per previously reported methods (Norrholm et al., 2006, 2008, 2011; Rothbaum et al., 2014). Across the four blocks of Acquisition (one CS habituation + three acquisition), there was an interaction of block × CS type (F(3, 93) = 4.23, p = 0.008) as well as a main effect of CS type and block (See Figure 3 and Supplemental Table S2). We followed the two-way interaction by comparing CS type within each block and found that fear-potentiated startle responses in the presence of the CS+ were greater as compared to the CS− during the last two blocks of acquisition (both p values < 0.05). This demonstrates successful discrimination between the CS cues as is typically observed with this paradigm in healthy controls (Briscione et al., 2014). There were no significant main or interaction effects with Group, indicating no differences between MDMA and placebo groups during the acquisition session with regard to discriminative learning (F(1, 31) = 0.62, p = 0.44). The discrimination data are shown in Figure 3 with the groups collapsed due to the lack of significant differences.
Figure 3.
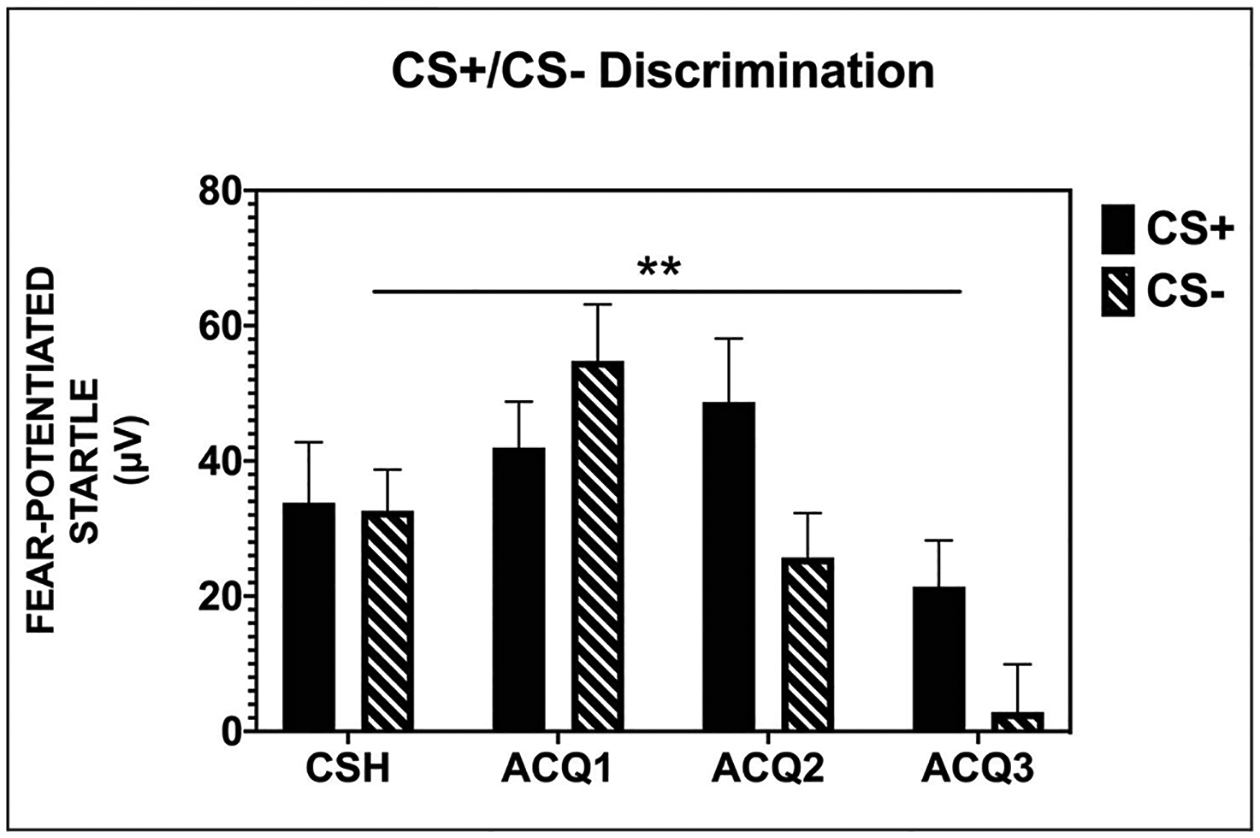
All participants, prior to any drug administration, showed successful discrimination between the reinforced CS+ and non-reinforced CS−.
CSH = conditioned stimuli habituation; ACQ = acquisition; CS+ = reinforced conditioned stimuli; CS− = non-reinforced conditioned stimuli; fear-potentiated startle = [mean startle magnitude to CS] – [mean startle magnitude to noise alone]; μV = microvolts; ** = significant block × CS type interaction, p = 0.008.
Extinction training
Given that study drug was administered prior to extinction training, we examined whether baseline startle was affected with drug on board. Importantly, we observed no effect of MDMA versus placebo on baseline startle (i.e. NA across four blocks) during the extinction session (F(1, 32) = 0.17, p = 0.68). The study sample demonstrated significant reduction of fear-potentiated startle responses to the CS+ across extinction blocks (expressed as a difference score from NA; F(3, 96) = 21.50, p < 0.001) with no significant difference between the MDMA and placebo groups (F(1,32) = 1.31, p = 0.26; Figure 4; see Supplemental Table S2).
Figure 4.
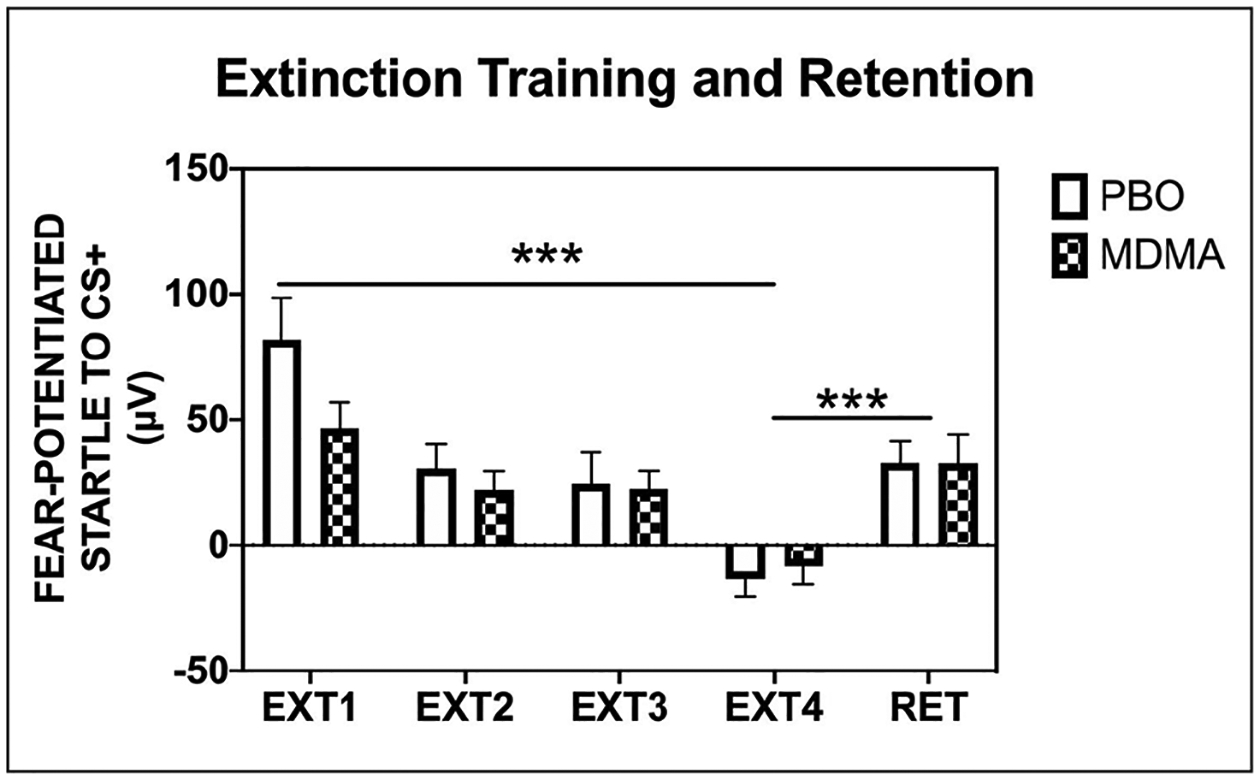
Participants assigned to either the placebo or drug administration exhibited a significant reduction in fear-potentiated startle to the CS+ across the blocks of extinction training to the previously reinforced CS+. In addition, both groups showed a significant return of fear at the extinction retention test.
PBO = placebo; MDMA = 3,4-methylenedioxymethamphetamine; EXT = extinction; RET = extinction retention; fear-potentiated startle = [mean startle magnitude to CS] – [mean startle magnitude to noise alone]; μV = microvolts; *** = significant effect of block, p < 0.001.
Extinction retention
As a whole, the participants showed a significant return of fear through spontaneous recovery when comparing fear-potentiated startle to the previously reinforced CS+ at the end of extinction training versus the beginning of the extinction retention session (F(1, 32) = 26.87, p < 0.001), no significant session × group interaction or main effect of group (Figure 4; see Supplemental Table S2). A return of fear-potentiated startle suggests a lack of extinction retention.
In our previous work, we have classified individuals based on the degree to which they learn to extinguish fear during extinction learning sessions (i.e. within-session extinction; Norrholm et al., 2006, 2008) In this study, there were no differences between the groups based on degree of within-session extinction learning. However, in the current analyses, we further explored this examination by classifying participants in each group according to the degree to which they showed a return of fear or retention of extinction learning. A participant that displayed an increase in fear-potentiated startle of <0 (i.e. no increase) when comparing fear-potentiated startle at the end of extinction training versus the beginning of extinction retention was defined as a “retainer.” Conversely, a participant that showed increased fear-potentiated startle from the end of extinction training to the beginning of extinction retention was defined as a “non-retainer.” A chi-square analysis revealed that there were significantly more retainers in the MDMA group (6/17) as compared to the placebo group (0/17; χ2 = 7.29, p = 0.007; see Figure 5 and Supplementary Figure S2). Within the MDMA group, the retainers demonstrated a full retention of extinction training across the retention session with no return of fear/spontaneous recovery, whereas the non-retainers demonstrated spontaneous recovery (repeated measures ANOVA, significant block × group interaction, F(1,15) = 9.34, p = 0.008; Figure 5(a)). A scatterplot of the distribution of retention scores is provided in Figure 5(b).
Figure 5.
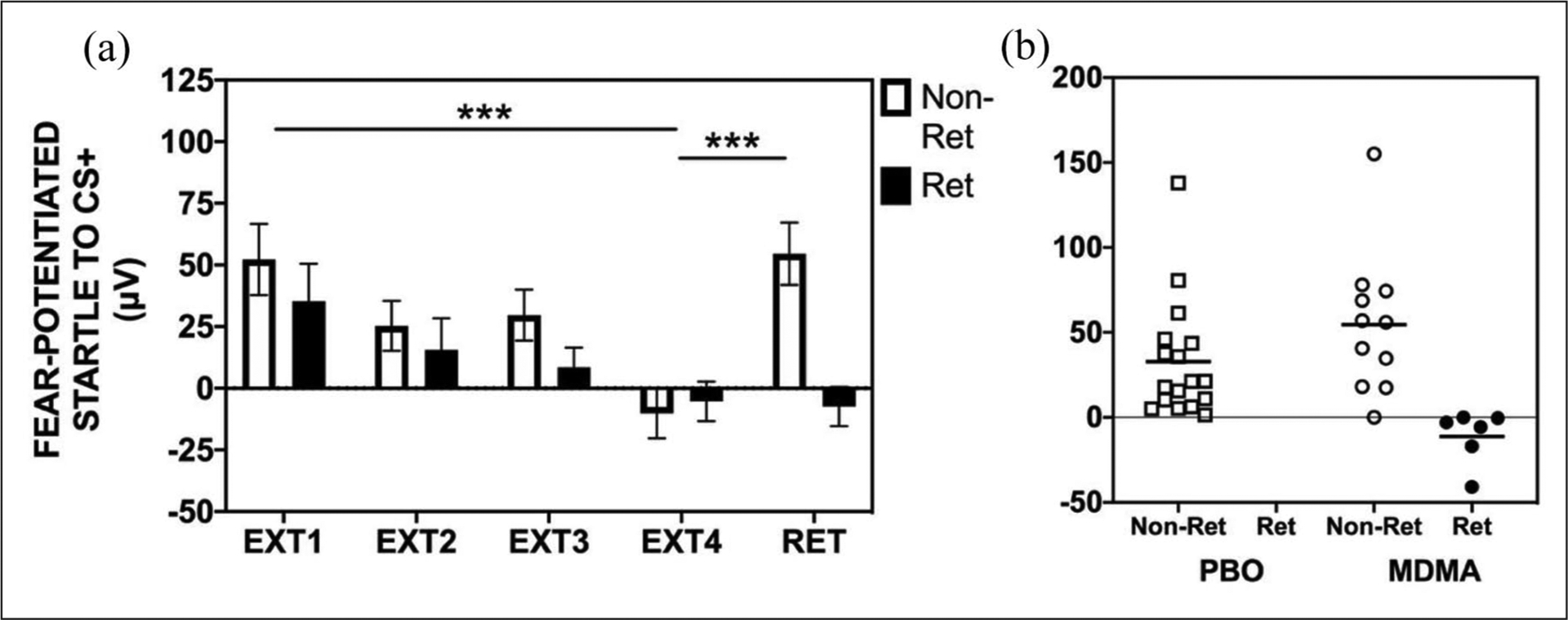
Extinction training and retention in MDMA group by retainers and non-retainers: (a) within the MDMA group, retainers (Ret) demonstrated full retention of extinction training from the end of extinction training (EXT4) to the extinction retention test (RET) with no return of fear via spontaneous recovery. The non-retainers (Non-Ret) demonstrated spontaneous recovery from EXT4 to RET (significant block × group interaction, F(1,15) = 9.34, p = 0.008). (b) Scatterplot demonstrating that there were no retainers (Ret) in the placebo (PBO) group but there were significantly more retainers (n = 6) in the MDMA group (χ2 = 7.29, p = 0.007).
PBO = Placebo; MDMA = 3,4-methylenedioxymethamphetamine; EXT = extinction; RET = extinction retention; fear-potentiated startle = [mean startle magnitude to CS] – [mean startle magnitude to noise alone]; μV = microvolts.
Tolerability and safety of MDMA
Adverse events were collected for the duration of study protocol including severity and relationship to study treatment. Study medication was well tolerated. There were no cardiovascular adverse events and no participants withdrew due to harms. The most common adverse events were elevated blood pressure (i.e. SBP ⩾ 160 or DBP ⩾ 110; 3/17 in MDMA group, 0/17 in placebo group), headache (2/17 in MDMA group, 1/17 in placebo group), anxiety (3/17 in MDMA group, 0/17 in placebo group), and tachycardia (i.e. HR ⩾ 110; 2/17 in MDMA group, 0/17 in placebo group). All adverse events ranged from mild to moderate severity and were transient in nature and did not require medication or intervention. There were no serious adverse events.
Physiological indices including pulse rate, blood pressure, and body temperature were assessed during the drug/placebo session (Visit 2) at eight timepoints (0 (pre-drug), 0.75, 1.25, 1.75, 3, 4, 5, and 6 hours post-drug administration). We observed a significant time × group interaction across the eight pulse assessment timepoints during Visit 2 (Repeated Measures ANOVA, F(1,31) = 51.37, p < 0.001). The MDMA group displayed significantly higher pulse rate over the course of the session (Supplementary Figure S3). We found a significant time × group interaction on both blood pressure indices (SBP: F(1,31) = 78.73, p < 0.001; DBP: F(1,31) = 18.69, p < 0.001; Supplementary Figures S4 and S5). The MDMA group higher SBP and DBP compared to the placebo group. On measures of body temperature, there was a significant time × group interaction across the eight timepoints during Visit 2 (repeated measures ANOVA, F(1,30) = 14.62, p = 0.001) with body temperature steadily increasing in the MDMA group over the course of the test session; however, temperature in both groups remained within normal range throughout the Visit 2 session (Supplementary Figure S6).
Discussion
MDMA-assisted psychotherapy has shown potential as a PTSD treatment (Feduccia et al., 2019; Jerome et al., 2020; Mitchell et al., 2021; Mithoefer et al., 2018; Oehen et al., 2013) and mechanistic research is needed to inform understanding of treatment efficacy, efficiency, and response rates. Clinical investigators have suggested enhanced extinction retention as a primary mechanism by which MDMA may produce positive treatment outcomes in PTSD (Feduccia and Mithoefer, 2018). Early evidence from rodent models supported this proposed mechanism of action by showing that MDMA enhanced retention of extinction when administered prior to extinction training (Young et al., 2015, 2017) This study sought to translate these pre-clinical rodent trials to human fear learning paradigms and hypothesized that MDMA would be associated with enhanced extinction retention in healthy humans.
The results of this study are summarized as follows: (1) all participants successfully acquired and then extinguished conditioned fear in this established paradigm, (2) pre-extinction training administration of MDMA, and its associated experiential and physiological effects, did not interfere with participants’ ability to learn to extinguish recently acquired conditioned fear such that both groups demonstrated extinction learning, (3) acute MDMA administration did not overall enhance within-session extinction learning nor enhance extinction retention and (4) there was a significantly greater number of participants who retained extinction learning in the MDMA versus placebo group.
Recently MDMA research has significantly increased with growing evidence for the safety and efficacy of MDMA-assisted therapy (Feduccia et al., 2019; Jerome et al., 2020; Mitchell et al., 2021; Mithoefer et al., 2018; Oehen et al., 2013; Reiff et al., 2020; Warren et al., 2014). As previously shown in a large analysis (Vizeli and Liechti, 2017), in this study, MDMA was safe and well tolerated; pulse rate, systolic and diastolic blood pressure, and body temperature demonstrated transient increases that normalized over the 6-hour session. This study provides support for the feasibility of implementing experimental procedures in MDMA clinical research. No previously published study has investigated the effects of acute MDMA administration on extinction learning in healthy human adults, thus it was previously unknown whether conditioned fear could be extinguished while participants were under acute effects of MDMA. During extinction training, the entire group demonstrated a decrease in fear-potentiated startle responses, providing evidence that under MDMA administration participants were able to successfully attend to and engage in this experimental paradigm. Notably, there was not a significant group difference across the blocks of extinction training. This provides support for the use of extinction experimental research under MDMA administration, as the MDMA group demonstrated comparable acoustic startle responses (i.e. baseline startle to NA) and extinction learning to placebo group despite acute subjective effects and increase in physiological arousal. This may represent a potential floor effect in startle responding, as the total sample demonstrated virtually complete extinction of fear-potentiated startle by the end of the four extinction blocks. Extinction retention was the primary outcome for this study based on previous research in rodent models indicate that MDMA enhances retention of extinction learning when administered prior to extinction training (Young et al., 2015, 2017). We did not replicate this effect in this study as a significant difference in extinction retention was not identified when directly comparing groups. Nearly all participants showed a return of fear through spontaneous recovery when comparing fear-potentiated startle at the end of extinction training versus extinction retention. Return of fear is the normative response in this paradigm (Jovanovic and Norrholm, 2011; Norrholm et al., 2006, 2008) for a number of reasons, including context, occasion setting, configural learning, and demand characteristics. For a clinical treatment, a higher dose of extinction training (e.g. more trials (exposures) and/or more sessions) may be required to see higher rates of extinction retention.
While the primary hypothesis was not supported, the results of this study provide valuable new insight into the mechanisms by which MDMA may or may not elicit clinical gains in PTSD treatment. Our extinction retainer analyses, based on our group’s previous work with extinction learning in humans, provides a compelling rationale to continue this avenue of translational research. This rationale coupled with increasing calls to investigate the proportion of individuals who demonstrate a specific response in fear extinction studies in addition to examining group-level mean differences (Shumake et al., 2018) led us to consider the proportion of extinction retainers across the treatment groups via exploratory analyses. The MDMA group demonstrated significantly more participants who retained extinction learning (χ2 = 7.29, p = 0.007) with 6/17 participants classified as retainers compared to 0/17 in the placebo group. It should also be noted that our definition of retention of extinction was strictly defined as any return of fear as compared to terminal levels of extinction during extinction training (i.e. any response greater than 0). This restricted definition does not rely on nuanced data transformations and removes the potential for setting a relatively arbitrary degree of fear return (e.g. % of fear observed at acquisition).
This indicates that it is possible that MDMA facilitates extinction retention in a subset of individuals, and future research should investigate potential individual differences that may interact with MDMA in facilitating fear extinction processes. These differences may be hormonal, genomic, experiential, or a combination of these factors (Briscione et al., 2014). While promising, given that this was not a primary objective of this study, it will be beneficial for future research to investigate return of fear mechanisms specific to MDMA in larger sample sizes or in samples elected specifically for deficits in fear extinction learning. The potential relevance of MDMA to fear extinction learning is supported by research indicating that the effects of MDMA and the mediation of fear extinction processes are associated with overlapping neural circuity. Neuroimaging research in healthy adults indicates that MDMA results in decreased cerebral blood flow to the right amygdala and hippocampus, which correlated with self-reported intensity of subjective effects of MDMA, and increased resting state functional connectivity between the amygdala and hippocampus (Carhart-Harris et al., 2015). Neuroimaging research using fear extinction training and retention experimental paradigms suggest involvement of the amygdala, hippocampus, and ventromedial prefrontal cortex (Milad et al., 2007). Future research may benefit from using fewer extinction trials or from investigating extinction learning in PTSD samples in which there is a pre-existing impairment (Sessa, 2017) that may be “rescued.”
In a recent rodent study, MDMA administration prior to fear extinction training did not enhance the fear extinction memory, but MDMA administered during reconsolidation phase resulted in a reduction in conditioned fear (Hake et al., 2019); as such, future research may also benefit from investigating MDMA’s potential role in disrupting reconsolidation of fear memories in human samples. Imaging studies testing MDMA’s impact on fear extinction and reconsolidation neurocircuitry may be beneficial in further elucidating how MDMA might impact these distinct processes (Feduccia and Mithoefer, 2018). In addition to the hypothesized effects of MDMA on fear extinction, MDMA has been shown to have emotional effects that could be useful in MDMA-assisted therapy including reduced recognition of negative emotions including fear, enhanced emotional empathy, and increased feelings of well-being, trust, and openness (Bedi et al., 2010; Bershad et al., 2016; Holze et al., 2020; Hysek et al., 2014a, 2014b; Schmid et al., 2014); future research can investigate how these factors and other factors that they may impact emotional processing of a traumatic memory (e.g. autobiographical memory recall and therapeutic alliance) may be associated with its therapeutic benefit within PTSD therapy.
MDMA-assisted psychotherapy shows promise as a novel intervention for PTSD (Feduccia et al., 2019; Jerome et al., 2020; Mithoefer et al., 2019). PE is an established first-line treatment for PTSD (APA, 2017; VA/DoD, 2017) and is based on extinction principles. Recent work suggests that one of the most consistent predictors of long-term fear was fear at the outset of extinction training (Brown et al., 2017); while not significant in this study, the placebo group showed elevated fear responding during extinction Block 1 compared to the MDMA group. The present results suggest that MDMA does not impair the extinction of learned fear nor does it directly improve extinction learning in human subjects in this paradigm. This report is the first investigation of acute administration of MDMA on fear learning in humans. Further research may reveal that, if present, the most beneficial use of MDMA may be as a “rescue” drug for impaired extinction learning and retention in PTSD samples receiving PE; however, this is speculative and requires empirical investigation.
Limitations and future directions
This study used stringent inclusion and exclusion criteria as such may not be generalizable to the general public. MDMA was safe and well tolerated consistent with previous research (Vizeli and Liechti, 2017) providing support for future research investigating MDMA in more representative samples. Another limitation was the difficulty in maintaining blinding with MDMA compared to an inert placebo; however, the study outcomes were objective psychophysiological measures. The small sample size was a limitation. In this study, MDMA was administered 2 hours prior to extinction training to align around Tmax for MDMA (de la Harris et al., 2002; De la Torre et al., 2000); animal studies of MDMA and extinction administered MDMA 30 minutes prior to extinction training (Young et al., 2017); as such, future research may also investigate how different timing of drug administration may impact extinction processes. Future research would benefit from investigating the pharmacological specificity of MDMA’s impact on fear extinction; for instance, given shared features with stimulant drugs such as amphetamines, future research could compare MDMA and amphetamines and their impact on facilitation of fear extinction learning versus general effects on memory. Given that PTSD is associated with fear extinction deficits (Young et al., 2017), it will be beneficial to investigate fear extinction retention in more deeply phenotyped and genotyped PTSD patients to investigate to whom MDMA may hold the greatest potential to rescue deficits.
Supplementary Material
Acknowledgements
The authors extend our gratitude to the Abraham J. and Phyllis Katz Foundation and Multidisciplinary Association for Psychedelic Studies (MAPS) for their support in making this study possible. This study was supported with funding from the Abraham J. and Phyllis Katz Foundation and was supported in part by NIH grant P50MH100023. This study was supported by the Building Interdisciplinary Research Careers in Women’s Health of the National Institutes of Health under Award Number K12HD085850 and UL1TR002378 (Georgia CTSA). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The Multidisciplinary Association for Psychedelic Studies (MAPS) was the study sponsor, and its wholly owned subsidiary MAPS Public Benefit Corporation (MAPS PBC) was the sponsor designee and trial organizer.
Declaration of conflicting interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: This study was supported with funding from the Abraham J. and Phyllis Katz Foundation and was supported in part by NIH grant P50MH100023. J.L.M-.K. is supported by Building Interdisciplinary Research Careers in Women’s Health of the National Institutes of Health under Award Number K12HD085850 and UL1TR002378 and has received research funding from COMPASS Pathways. S.N. has received research funding from Department of Veterans Affairs (VA) and the Congressionally Directed Medical Research Program (CDMRP). B.W.D. has received research support from Acadia, Compass, Aptinyx, NIMH, Sage, and Takeda, and has served as a consultant to Greenwich Biosciences, Myriad Neuroscience, Otsuka, Sage, and Sophren Therapeutics. S.R. receives support from Wounded Warrior Project (WWP), Department of Veterans Affairs (VA), National Institute of Health (NIH), McCormick Foundation, Tonix Pharmaceuticals, Woodruff Foundation, and Department of Defense (DOD) and receives royalties from Oxford University Press and American Psychological Association Press. T.J. has received research funding from NIH and Brain and Behavior Research Foundation. B.O.R. has funding from Wounded Warrior Project, Department of Defense Clinical Trial Grant No.W81XWH-10-1-1045, and McCormick Foundation. B.O.R. receives royalties from Oxford University Press, Guilford, APPI, and Emory University and received advisory board payments from Genentech, Jazz Pharmaceuticals, Nobilis Therapeutics, Sophren, Neuronetics, and Aptinyx. B.O.R. is a consultant to and owns equity in Virtually Better, Inc. that creates virtual environments. The terms of these arrangements have been reviewed and approved by Emory University in accordance with its conflict of interest policies. J.R. has received honoraria from SMI Clinical Advisor and FOCUS and research support from American Board or Psychiatry and Neurology, Otsuka, Takeda, and Compass. The sponsor had no involvement in the data collection, data analysis, or manuscript preparation. The remaining authors have no conflicts to declare.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Abraham J. and Phyllis Katz Foundation, NIH grant P50MH100023.
Footnotes
Supplemental material
Supplemental material for this article is available online.
References
- American Psychological Association (2017) Clinical Practice Guideline for the Treatment of Posttraumatic Stress Disorder (PTSD) in Adults. Washington, DC: American Psychological Association. [Google Scholar]
- Bedi G, Hyman D and de Wit H (2010) Is ecstasy an “empathogen”? Effects of ±3,4-methylenedioxymethamphetamine on prosocial feelings and identification of emotional states in others. Biological Psychiatry 68: 1134–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bershad AK, Miller MA, Baggott MJ, et al. (2016) The effects of MDMA on socio-emotional processing: Does MDMA differ from other stimulants? Journal of Psychopharmacology 30: 1248–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton ME, Vurbic D and Woods AM (2008) D-cycloserine facilitates context-specific fear extinction learning. Neurobiology of Learning and Memory 90(3): 504–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley R, Greene J, Russ E, et al. (2005) A multidimensional meta-analysis of psychotherapy for PTSD. American Journal of Psychiatry 162(2): 214–227. [DOI] [PubMed] [Google Scholar]
- Briscione MA, Jovanovic T and Norrholm SD (2014) Conditioned fear associated phenotypes as robust, translational indices of trauma-, stressor-, and anxiety-related behaviors. Frontiers in Psychiatry 5: 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown LA, LeBeau RT, Chat K-Y, et al. (2017) Associative learning versus fear habituation as predictors of long-term extinction retention. Cognition and Emotion 31(4): 687–698. [DOI] [PubMed] [Google Scholar]
- Carhart-Harris RL, Murphy K, Leech R, et al. (2015) The effects of acutely administered 3, 4-methylenedioxymethamphetamine on spontaneous brain function in healthy volunteers measured with arterial spin labeling and blood oxygen level–dependent resting state functional connectivity. Biological Psychiatry 78(8): 554–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusack K, Jonas DE, Forneris CA, et al. (2016) Psychological treatments for adults with posttraumatic stress disorder: A systematic review and meta-analysis. Clinical Psychology Review 43: 128–141. [DOI] [PubMed] [Google Scholar]
- De la Torre R, Farre M, Roset PN, et al. (2000) Pharmacology of MDMA in humans. Annals of the New York Academy of Sciences 914(1): 225–237. [DOI] [PubMed] [Google Scholar]
- Farré M, Abanades S, Roset PN, et al. (2007) Pharmacological interaction between 3,4-methylenedioxymethamphetamine (ecstasy) and paroxetine: Pharmacological effects and pharmacokinetics. Journal of Pharmacology and Experimental Therapeutics 323(3): 954–962. [DOI] [PubMed] [Google Scholar]
- Feduccia AA and Mithoefer MC(2018) MDMA-assisted psychotherapy for PTSD: Are memory reconsolidation and fear extinction underlying mechanisms? Progress in Neuro-Psychopharmacology and Biological Psychiatry 84: 221–228. [DOI] [PubMed] [Google Scholar]
- Feduccia AA, Jerome L, Klosinski B, et al. (2019) Breakthrough for trauma treatment: Safety and efficacy of MDMA-assisted psychotherapy compared to paroxetine and sertraline. Frontiers in Psychiatry 10: 650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover EM, Jovanovic T and Norrholm SD (2015) Estrogen and extinction of fear memories: Implications for posttraumatic stress disorder treatment. Biological Psychiatry 78(3): 178–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hake HS, Davis JK, Wood RR, et al. (2019) 3, 4-methylenedioxymeth-amphetamine (MDMA) impairs the extinction and reconsolidation of fear memory in rats. Physiology & Behavior 199: 343–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris DS, Baggott M, Mendelson JH, et al. (2002) Subjective and hormonal effects of 3, 4-methylenedioxymethamphetamine (MDMA) in humans. Psychopharmacology 162(4): 396–405. [DOI] [PubMed] [Google Scholar]
- Holze F, Vizeli P, Muller F, et al. (2020) Distinct acute effects of LSD, MDMA, and D-amphetamine in healthy subjects. Neuropsychopharmacology 45: 462–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hysek CM, Schmid Y, Simmler LD, et al. (2014a) MDMA enhances emotional empathy and prosocial behavior. Social Cognitive and Affective Neuroscience 9: 1645–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hysek CM, Simmler LD, Schillinger N, et al. (2014b) Pharmacokinetic and pharmacodynamic effects of methylphenidate and MDMA administered alone and in combination. International Journal of Neuropsychopharmacology 17: 371–381. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine (US). Committee on Treatment of Posttraumatic Stress Disorder (2007) Treatment of posttraumatic stress disorder: An assessment of the evidence. In Institute of Medicine (Eds.), Appendix D, Analysis and Interpretation of Studies with Missing Data, Washington, DC: Institute of Medicine, pp. 185–194. [Google Scholar]
- Jerome L, Feduccia AA, Wang JB, et al. (2020) Long-term follow-up outcomes of MDMA-assisted psychotherapy for treatment of PTSD: A longitudinal pooled analysis of six phase 2 trials. Psychopharmacology 237: 2485–2497. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Jovanovic T and Norrholm SD (2011) Neural mechanisms of impaired fear inhibition in posttraumatic stress disorder. Frontiers in Behavioral Neuroscience 5: 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Kazama A, Bachevalier J, et al. (2012) Impaired safety signal learning may be a biomarker of PTSD. Neuropharmacology 62(2): 695–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Norrholm SD, Fennell JE, et al. (2009) Posttraumatic stress disorder may be associated with impaired fear inhibition: Relation to symptom severity. Psychiatry Research 167(1–2): 151–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC (2000) Posttraumatic stress disorder: The burden to the individual and to society. The Journal of Clinical Psychiatry 61: 4–14. [PubMed] [Google Scholar]
- Maples-Keller JL, Rauch SA, Jovanovic T, et al. (2019) Changes in trauma-potentiated startle, skin conductance, and heart rate within prolonged exposure therapy for PTSD in high and low treatment responders. Journal of Anxiety Disorders 68: 102147. [DOI] [PubMed] [Google Scholar]
- Milad MR and Quirk GJ (2012) Fear extinction as a model for translational neuroscience: Ten years of progress. Annual Review of Psychology 63: 129–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Wright CI, Orr SP, et al. (2007) Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biological Psychiatry 62(5): 446–454. [DOI] [PubMed] [Google Scholar]
- Mitchell JM, Bogenschutz M, Lilienstein A, et al. (2021) MDMA-assisted therapy for severe PTSD: A randomized, double-blind, placebo-controlled phase 3 study. Nature Medicine 27(6): 1025–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mithoefer MC, Feduccia AA, Jerome L, et al. (2019) MDMA-assisted psychotherapy for treatment of PTSD: Study design and rationale for phase 3 trials based on pooled analysis of six phase 2 randomized controlled trials. Psychopharmacology 236(9): 2735–2745. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Mithoefer MC, Mithoefer AT, Feduccia AA, et al. (2018) 3,4-methylenedioxymethamphetamine (MDMA)-assisted psychotherapy for post-traumatic stress disorder in military veterans, firefighters, and police officers: A randomised, double-blind, dose-response, phase 2 clinical trial. Lancet Psychiatry 5(6): 486–497. [DOI] [PubMed] [Google Scholar]
- Myers KM and Davis M (2002) Behavioral and neural analysis of extinction. Neuron 36(4): 567–584. [DOI] [PubMed] [Google Scholar]
- Norrholm SD, Jovanovic T, Gerardi M, et al. (2016) Baseline psychophysiological and cortisol reactivity as a predictor of PTSD treatment outcome in virtual reality exposure therapy. Behaviour Research and Therapy 82: 28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrholm SD, Jovanovic T, Olin IW, et al. (2011) Fear extinction in traumatized civilians with posttraumatic stress disorder: Relation to symptom severity. Biological Psychiatry 69(6): 556–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrholm SD, Jovanovic T, Vervliet B, et al. (2006) Conditioned fear extinction and reinstatement in a human fear-potentiated startle paradigm. Learning & Memory 13(6): 681–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrholm SD, Vervliet B, Jovanovic T, et al. (2008) Timing of extinction relative to acquisition: A parametric analysis of fear extinction in humans. Behavioral Neuroscience 122(5): 1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oehen P, Traber R, Widmer V, et al. (2013) A randomized, controlled pilot study of MDMA (±3, 4-Methylenedioxymethamphetamine)-assisted psychotherapy for treatment of resistant, chronic Post-Traumatic Stress Disorder (PTSD). Journal of Psychopharmacology 27(1): 40–52. [DOI] [PubMed] [Google Scholar]
- Reiff CM, Richman EE, Nemeroff CB, et al. (2020) Psychedelics and psychedelic-assisted psychotherapy. AMerican Journal of Psychiatry 177(5): 391–410. [DOI] [PubMed] [Google Scholar]
- Robison-Andrew EJ, Duval ER, Nelson CB, et al. (2014) Changes in trauma-potentiated startle with treatment of posttraumatic stress disorder in combat Veterans. Journal of Anxiety Disorders 28(4): 358–362. [DOI] [PubMed] [Google Scholar]
- Rothbaum BO and Davis M (2003) Applying learning principles to the treatment of post-trauma reactions. Annals of the New York Academy of Sciences 1008(1): 112–121. [DOI] [PubMed] [Google Scholar]
- Rothbaum BO, Foa EB, Riggs DS, et al. (1992) A prospective examination of post-traumatic stress disorder in rape victims. Journal of Traumatic Stress 5(3): 455–475. [Google Scholar]
- Rothbaum BO, Price M, Jovanovic T, et al. (2014) A randomized, double-blind evaluation of D-cycloserine or alprazolam combined with virtual reality exposure therapy for posttraumatic stress disorder in Iraq and Afghanistan War veterans. American Journal of Psychiatry 171(6): 640–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid Y, Hysek CM, Simmler LD, et al. (2014) Differential effects of MDMA and methylphenidate on social cognition. Journal of Psychopharmacology 28: 847–856. [DOI] [PubMed] [Google Scholar]
- Sessa B (2017) MDMA and PTSD treatment: “PTSD: From novel patho-physiology to innovative therapeutics.” Neuroscience Letters 649: 176–180. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, et al. (1998) The Mini-International Neuropsychiatric Interview (MINI): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. Journal of Clinical Psychiatry 59(20): 22–33. [PubMed] [Google Scholar]
- Shumake J, Jones C, Auchter A, et al. (2018) Data-driven criteria to assess fear remission and phenotypic variability of extinction in rats. Philosophical Transactions of the Royal Society B: Biological Sciences 373(1742): 20170035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Department of Veterans Affairs Department of Defense (2017) Va/dod Clinical Practice Guideline for the Management of Posttraumatic Stress Disorder and Acute Stress Disorder. Washington, DC: US Department of Veterans Affairs Department of Defense. Available at: https://healthquality.va.gov/HEALTHQUALITY/guidelines/MH/ptsd/VADoDPTSDCPGFinal012418.pdf [Google Scholar]
- Vizeli P and Liechti ME (2017) Safety pharmacology of acute MDMA administration in healthy subjects. Journal of Psychopharmacology 31(5): 576–588. [DOI] [PubMed] [Google Scholar]
- Warren VT, Anderson KM, Kwon C, et al. (2014) Human fear extinction and return of fear using reconsolidation update mechanisms: The contribution of on-line expectancy ratings. Neurobiology of Learning and Memory 113: 165–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young MB, Andero R, Ressler KJ, et al. (2015) 3, 4-Methylenedioxymethamphetamine facilitates fear extinction learning. Translational Psychiatry 5: e634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young MB, Norrholm SD, Khoury LM, et al. (2017) Inhibition of serotonin transporters disrupts the enhancement of fear memory extinction by 3, 4-methylenedioxymethamphetamine (MDMA). Psychopharmacology 234(19): 2883–2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


