Abstract
In the fight against malaria, transmission blocking interventions (TBIs) such as transmission blocking vaccines or drugs, are promising approaches to complement conventional tools. They aim to prevent the infection of vectors and thereby reduce the subsequent exposure of a human population to infectious mosquitoes. The effectiveness of these approaches has been shown to depend on the initial intensity of infection in mosquitoes, often measured as the mean number of oocysts resulting from an infectious blood meal in absence of intervention. In mosquitoes exposed to a high intensity of infection, current TBI candidates are expected to be ineffective at completely blocking infection but will decrease parasite load and therefore, potentially also affect key parameters of vector transmission. The present study investigated the consequences of changes in oocyst intensity on subsequent parasite development and mosquito survival. To address this, we experimentally produced different intensities of infection for Anopheles gambiae females from Burkina Faso by diluting gametocytes from three natural Plasmodium falciparum local isolates and used a newly developed non-destructive method based on the exploitation of mosquito sugar feeding to track parasite and mosquito life history traits throughout sporogonic development. Our results indicate the extrinsic incubation period (EIP) of P. falciparum and mosquito survival did not vary with parasite density but differed significantly between parasite isolates with estimated EIP50 of 16 (95% CI: 15–18), 14 (95% CI: 12–16) and 12 (95% CI: 12–13) days and median longevity of 25 (95% CI: 22–29), 15 (95% CI: 13–15) and 18 (95% CI: 17–19) days for the three isolates respectively. Our results here do not identify unintended consequences of the decrease of parasite loads in mosquitoes on the parasite incubation period or on mosquito survival, two key parameters of vectorial capacity, and hence support the use of transmission blocking strategies to control malaria.
Author summary
In the fight against malaria, it is recognized that the use of several complementary strategies is necessary to significantly reduce transmission and improve human health. Among these, transmission blocking strategies such as transmission blocking vaccines or drugs, aim to block the development of the parasites within mosquito vectors. This approach should prevent infection in most mosquitoes feeding on infectious hosts and thus block transmission. However, in some cases it may only reduce parasite load without fully clearing the infection. Here we identified potential risks: if reducing parasite load would reduce the incubation period of the parasite in mosquitoes or increase the longevity of the mosquitoes, undesirable consequences may occur with an increased efficiency of these vectors to transmit infection to humans. We tested these hypotheses and experimentally produced different infection loads in Anopheles gambiae by using dilutions of Plasmodium falciparum isolates from naturally infected human donors. We observed that the longevity of mosquitoes and the incubation period of the parasites were not affected by the parasite load. This is not consistent with the unintended risks that we identified and thus strengthens the potential of transmission blocking interventions in the toolbox to combat malaria.
Introduction
Despite significant progress in the fight against malaria in the last two decades, nearly half of the world’s population remains at risk of contracting the disease. The African region is the most affected, accounting for 94% of the global malaria burden [1]. Malaria control mainly relies on the use of antimalarial drugs, with an important contribution from artemisinin-based combination therapies, and vector control with the use of long-lasting insecticidal nets and indoor residual spraying. These tools enabled a significant reduction in the incidence and mortality due to malaria since the beginning of the century, but this decline has worryingly stalled in some countries and has even been reversed in some others in recent years with the spread of drug-resistance among parasites [2,3] and insecticide resistance in the main mosquito vectors [4]. As a complement to conventional tools targeting parasites in humans or seeking to kill mosquito vectors, new tools targeting parasites within mosquitoes appear promising [5–7]. These approaches, known as transmission blocking interventions (TBIs), target parasites within the mosquitoes where they are less numerous and express less variability than in human hosts. The principle of the current TBI candidates is to administrate drugs [8,9] or vaccines [10–13] to the human population so that the mosquitoes will not only ingest infectious gametocytes but also the drug or antibodies when taking a blood meal. These blocking agents will impede the development of infection at early stage within vectors and thereby reduce the subsequent exposure of human populations to infectious mosquitoes. The efficiency of TBIs is often measured by comparing oocyst intensities between groups of mosquitoes exposed to an infectious blood meal with versus without the blocking agent, and it has been shown to depend on the intensity of infection in the control group of mosquitoes [14–16]. In other words, when the intensity of infection is moderate or low (< 5 oocysts per mosquito in the absence of a TBI, as often found in naturally infected mosquitoes [17–19]), transmission-blocking strategies will be much more effective in reducing the prevalence of infection in mosquitoes compared to situations where mosquitoes carry higher intensities of infection. However, in nature, it has been shown that the distribution of oocysts is highly overdispersed, with a significant proportion of infected mosquitoes carrying dozens of oocysts and where a few mosquitoes harbor very high oocyst densities (> 50 oocysts per mosquito) [20]. Therefore, in mosquitoes exposed to high densities of parasites it is expected that imperfect TBIs will reduce the number of oocysts, but will be ineffective at completely blocking infection, which may lead to unexpected consequences.
Pathogen density is an important factor contributing to the virulence and transmission of disease [21,22]. Consequently, interventions altering pathogen density deserve attention. In general, theoretical assumptions predict that reduced densities in populations are associated with competitive release and increased fitness [23]. However the patchy resources, the diversity of hosts and environmental conditions complicate the predicted consequences of varying pathogen density for the transmission of disease [24]. In malaria vectors, host-parasite interactions shape important parameters of transmission [25,26]. Among them, the duration of the parasite’s development within the mosquito, from the ingestion of gametocytes to the invasion of salivary glands by sporozoites, also called the extrinsic incubation period (EIP), and mosquito longevity are the most influential [27]. These two parameters are critical as the EIP is often as long as a mosquito’s average lifespan thus limiting the time window for sporozoite transmission before mosquito death [28]. This intimate relationship between parasite EIP and mosquito longevity should theoretically favour the rapid development of parasites to their transmission stages (i.e. sporozoites in salivary glands), but trade-offs between multiple traits in response to the mosquito environment may constrain this evolution [26,29,30]. Evidence for genetic and environmental variability of EIP exists, although remains scarce. Still, it is well described that EIP is affected by temperature, with warming temperatures, until a threshold, that speed up the parasite development [31–35]. There are also suggestions of interspecific genetic influence as some Plasmodium species develop faster than others: P. mexicanum transmitted by short-lived sandflies has a short EIP compared to other Plasmodium species [36]. Whether variation in malaria parasite density may also influence its developmental schedule in mosquitoes remains to be explored. Other parasite species, or blood-stage malaria parasites [37,38] have been shown to speed up their investment into transmission stages in conditions of stress, when their transmission is compromised by the potential death of the host. This suggests that damage caused by malaria parasites to the vector, if related to parasite density, may induce density-dependant consequences on EIP. Moreover, EIP is now known to be affected by the nutritional status of the mosquito host, in larval or adult stages [39–42]. Parasite density in the mosquito may then interact with limited nutritional resources, which should become less restrictive if the intensity of infection decreases, allowing faster development [43].
Closely related to EIP, but easier to study and more documented, is mosquito survival in respect to Plasmodium infection. The effect of Plasmodium infection on the survival of its vectors has long been disputed with conflicting observations [44,45] but a general trend appears in natural and artificial combinations of vectors and parasites for negative effects of infection on mosquito longevity in combination with other stresses, such as; hydric stress [46], resistance to insecticides [47], exposure to insecticides [48] poor nutritional resources [49,50] exposure to predators [51] and was also found dependent on parasite genetics [44,49]. However, a density-dependent effect of Plasmodium infection on vector survival was observed only in experimental vector–parasite combinations or avian malaria systems [24,52–54] and to our knowledge never for the most deadly human parasite, Plasmodium falciparum. It is therefore important to investigate the effect of parasite density on this key transmission parameter and investigate whether interventions to control P. falciparum may unintentionally increase the life expectancy of infected vectors by reducing the intensity of infection and thus possibly facilitating the successful and sustained transmission of the pathogen [55].
As interventions to control malaria may affect parasite intensity in mosquitoes and possibly affect key parameters of vectorial transmission, the present study investigated the consequences of changes in the intensity of infection in vector mosquitoes on parasite development and mosquito survival. We experimentally produced different intensities of infection in Anopheles gambiae females by diluting gametocytes from natural isolates of P. falciparum and used a newly developed non-destructive method [56] based on the exploitation of mosquito sugar feeding to track parasite and mosquito life history traits throughout sporogonic development.
Results
Effect of infectious blood dilution on the prevalence and intensity of infection in mosquitoes
Infection prevalence and intensity in mosquito gut at 7 days post blood meal (dpbm)
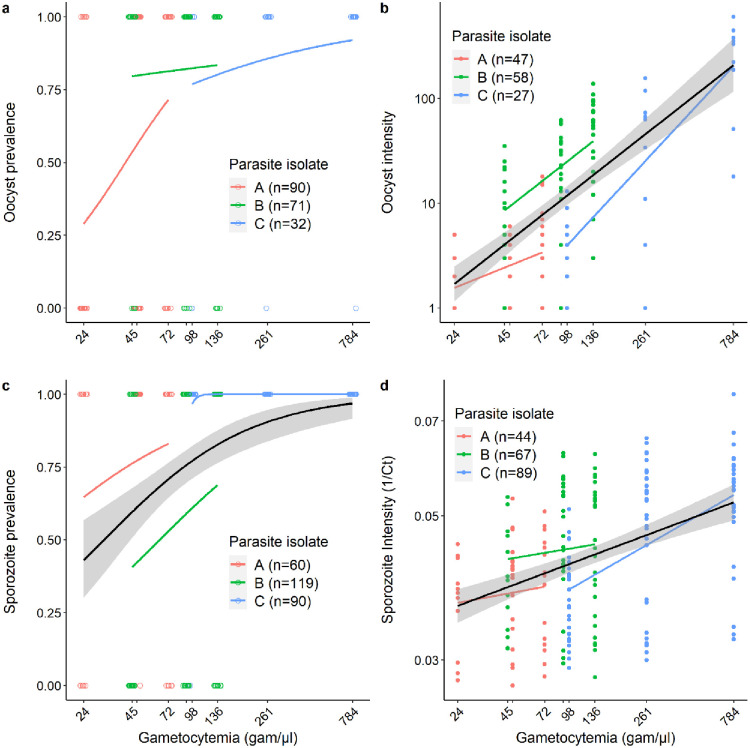
An. gambiae females were experimentally infected with the blood from one of three naturally infected gametocyte carriers (parasite isolates A, B and C) in Burkina Faso. The gametocyte-infected blood of each carrier was diluted to experimentally reduce the density of infectious gametocytes and create a range of parasite loads in mosquitoes. The midguts of 193 females were dissected at 7 dpbm for oocyst observation, among which 132 were positive for P. falciparum (68.4%). Dilution had no significant effect on the prevalence of infection (LRT X21 = 1.8, P = 0.177, Fig 1a), but had the intended effect of strongly reducing the intensity of infection among oocyst-infected mosquitoes throughout the dilution range (LRT X21 = 7.1, P = 0.008, Fig 1b).
Fig 1. Effect of infectious blood dilution on the prevalence and intensity of infection in mosquitoes.
(a) Oocyst prevalence 7 dpbm (number of mosquitoes with at least one oocyst in their midguts out of the total number of mosquitoes dissected) as a function of gametocytemia (the estimated number of infectious gametocytes per microliter of blood in each of the dilution groups: 24, 45, 48, 72, 91, 98, 136, 261 and 784 gametocytes/μl of blood, note that to avoid overlapping of the x-axis labels, the concentrations of 48 and 91 gam/μl are not indicated). Each colored circle represents a dissected mosquito (red: parasite isolate A with an initial gametocytemia of 72, green: parasite isolate B with initial gametocytemia of 136, and blue: parasite isolate C with an initial gametocytemia of 784). The colored lines represent the best-fit logistic growth curves for each parasite isolate. Note that the x-axis is on a log10 scale. (b) Oocyst intensity 7 dpbm (number of oocysts in infected mosquitoes) as a function of gametocytemia. Each colored circle represents a P. falciparum oocyst-positive midgut. The colored lines represent the linear relationship for each parasite isolate, while the black line (± se) for all data regardless of isolate origin. Note that the x- and y- axes are on a log10 scale. (c) Sporozoite prevalence (number of mosquitoes with heads/thoraxes detected positive to P. falciparum by qPCR at the time of death of the individual out of the total number of tested heads/thoraxes) as a function of gametocytemia. Each colored circle represents a tested head/thorax. The x-axis is on a log10 scale. (d) Amount of parasite DNA in mosquito heads/thoraxes expressed as the inverse of the qPCR cycle threshold (1/Ct, the higher the inverse of threshold cycle, the higher the intensity of infection). For each mosquito, 1/Ct value is the average over 4 to 6 technical replicates. The x- and y-axes are on a log10 scale. Each colored circle represents a P. falciparum positive head/thorax using qPCR.
Infection prevalence and intensity in mosquito head/thoraces upon death
At 7 dpbm, 269 An. gambiae females challenged with either parasite isolate A, B or C and that received the different dilution treatments (1/1, 1/3, 2/3 for parasite isolates A and B and 1/1, 1/3, 1/8 for parasite isolate C), were placed in individual tubes for saliva collection on cotton balls soaked with a 10% glucose solution. Upon mosquito death, the amount of parasite DNA in the heads/thoraxes of the females used to collect saliva was assessed using qPCR. Of a total of 269 An. gambiae females placed in individual tubes, 201 (75%) were found positive for P. falciparum by qPCR of the heads/thoraxes of mosquitoes at their time of death. The proportion of sporozoite-infected mosquitoes significantly increased with the density of infectious gametocytes (LRT X21 = 4.7, P = 0.030, Fig 1c). The global percentage of positive heads/thoraxes reached 88% (151 out of 172) when females that died before 14 dpbm (the time generally considered for sporozoites to have invaded mosquito salivary glands) were excluded. Consistent with observations made of mosquito midguts, the amount of parasite DNA in the heads and thoraxes of infected mosquitoes showed a positive relationship with gametocytemia (LRT X21 = 24.3, P< 0.001, Fig 1d).
Effect of parasite density on EIP
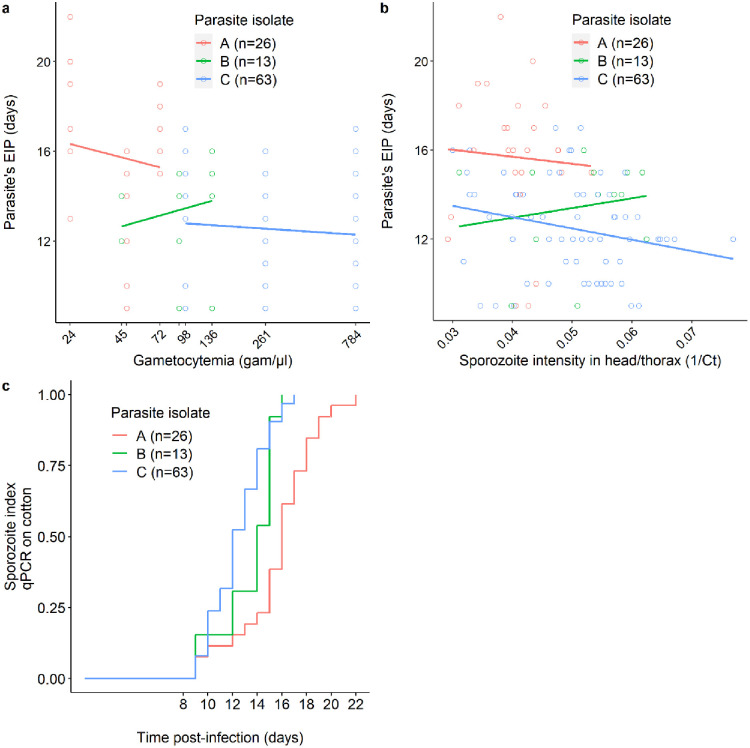
The presence of parasite DNA in the cotton balls used to collect saliva from infected mosquitoes (n = 201) was examined. A total of 1 997 cotton balls were analyzed and individual EIP was defined as the time between the infectious blood meal and the first day of positive qPCR detection of P. falciparum from a cotton wool substrate for a given infected female. Of the 201 females with an infected head/thorax, 102 (50.7%) generated at least one cotton ball containing detectable traces of parasite DNA. The infected females that did not produce any positive cottons over their lifespan were excluded from the analysis because no EIP values can be derived from these samples. The first positive cottons occurred at 9 dpbm in all dilution treatments. Among the mosquitoes that already produced a positive cotton ball, the proportion of positive cotton balls was 33, 81% (896 positive cottons out of 2650).
There was no effect of gametocytemia on EIP (LRT X21 = 0.9, P = 0.341, Fig 2a). Similar results were obtained when the explanatory variable, the gametocytemia, was substituted by the actual intensity of infection found in the head/thorax of each individual female upon their death (LRT X21 = 1.0, P = 0.317, Fig 2b). However, there was a significant effect of parasite isolate (LRT X22 = 32.8, P < 0.001) with an estimated EIP50 of 16 (95% CI: 15–18), 14 (95% CI: 12–16) and 12 (95% CI: 12–13) days for isolate A, B and C, respectively (Fig 2c).
Fig 2. Relationship between parasite density and EIP.
(a) EIP (the time between the infectious blood meal and the detection of P. falciparum in mosquito saliva collected from cotton balls) as a function of gametocytemia (the number of gametocytes per microliter of blood in each of the dilution groups: 24, 45, 48, 72, 91, 98, 136, 261 and 784 gametocytes/μl of blood, note that to avoid overlapping of the x-axis labels, the concentrations of 48 and 91 gam/μl are not indicated). Each colored circle represents an infected mosquito from which EIP was measured (red: parasite isolate A with an initial gametocytemia of 72, green: parasite isolate B with initial gametocytemia of 136, and blue: parasite isolate C with an initial gametocytemia of 784). The x-axis is on a log10 scale. (b) EIP as a function of 1/Ct in mosquito infected heads/thoraxes extracts (the higher the 1/Ct value, the higher the infection intensity). For each cotton ball, 1/Ct value is the average over 3 technical replicates. The colored lines in panels (a) and (b) represent the linear relationship for each parasite isolate. (c) Kaplan–Meier curves representing the temporal dynamics of sporozoite detection in cotton balls used to collect saliva from individual mosquitoes fed on each parasite isolate.
Effect of parasite density on mosquito survival
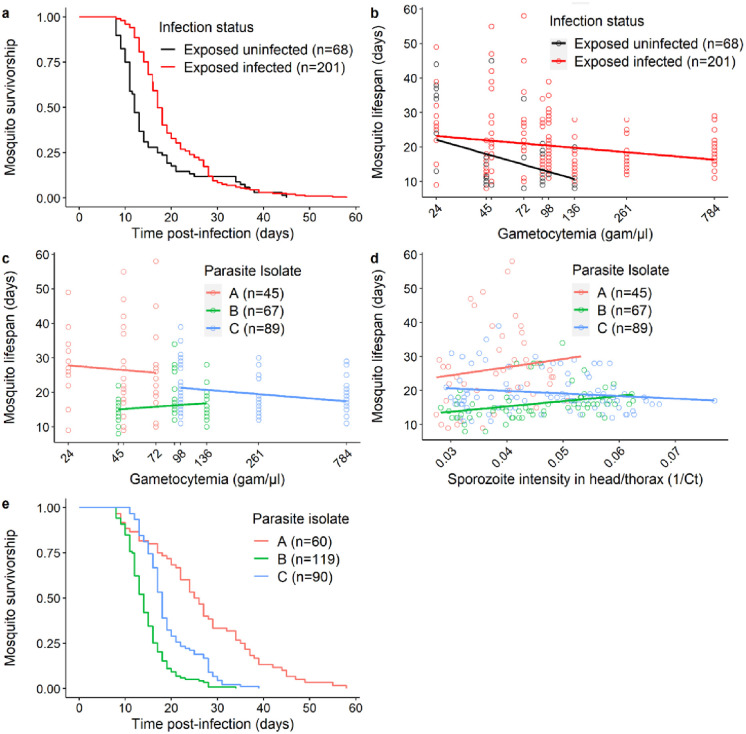
Mosquito survival was monitored daily for the 269 females placed in individual tubes, including 201 infected with P. falciparum and 68 fed on infectious blood but that did not develop an infection. Infected females survived better than those that did not become infected (LRT X21 = 14.2, P < 0.001, Fig 3a). No interaction between infection status and gametocytemia on mosquito survival was found (LRT X21 = 0, P = 0.968, Fig 3b). There was no effect of gametocytemia on the lifespan of infected mosquitoes (LRT X21 = 0.1, P = 0.783, Fig 3c) and no effect of infection intensity in the head/thorax of mosquitoes on the lifespan of infected mosquitoes (LRT X21 = 1.9, P = 0.164, Fig 3d). Finally, the lifespan of infected mosquitoes varied strongly depending on parasite isolate (LRT X21 = 46.6, P <0.001, Fig 3e), with a median longevity of 25 (95% CI: 22–29), 15 (95% CI: 13–15) and 18 (95% CI: 17–19) days for infected mosquitoes fed on isolate A, B and C, respectively.
Fig 3. Relationship between parasite density and mosquito longevity.
(a) Kaplan–Meier curves representing survival in days post blood meal for each infection status (red: mosquitoes exposed to infectious blood and having developed Plasmodium, black: mosquitoes exposed to infectious blood and which remained uninfected). (b) Mosquito longevity in days as a function of gametocytemia (the number of gametocytes per microliter of blood in each of the dilution groups: 24, 45, 48, 72, 91, 98, 136, 261 and 784 gametocytes/μl of blood, note that to avoid overlapping of the x-axis label, the concentrations of 48 and 91 gam/μl are not indicated). Each colored circle represents a mosquito exposed to the infectious blood (red: infected mosquitoes, black: mosquitoes that remained uninfected). The x-axis is on a log10 scale. (c) Mosquito longevity in days as a function of gametocytemia (the number of gametocytes per microliter of blood in each of the dilution groups: 24, 45, 48, 72, 91, 98, 136, 261 and 784 gametocytes/μl of blood, note that to avoid overlapping of the x-axis label, the concentrations of 48 and 91 gam/μl are not indicated). Each colored circle represents a mosquito exposed to one of the three parasite isolates (red: parasite isolate A with an initial gametocytemia of 72, green: parasite isolate B with initial gametocytemia of 136, and blue: parasite isolate C with an initial gametocytemia of 784). The x-axis is on a log10 scale. (d) mosquito longevity in days as a function of 1/Ct in mosquito infected heads/thoraxes extracts (the higher the 1/Ct value, the higher the infection intensity). For each cotton ball, 1/Ct value is the average over 3 technical replicates. Each colored circle represents an infected mosquito. The colored lines in panels (b) and (c) represent the linear relationship for each parasite isolate. (e) Kaplan–Meier curves representing survival in days post blood meal for each parasite isolate.
Discussion
In the present study, we questioned the importance of infection load in malaria-infected mosquitoes. We investigated the relationship between P. falciparum gametocyte densities in infectious blood and subsequent transmission parameters in mosquitoes, including infection prevalence, infection intensity at oocyst and sporozoite stages, and more originally the time taken for mosquitoes to become infectious (the parasite’s EIP) and their survival. EIP and mosquito survival are key parameters for transmission [26,29,57] and we explored the extent to which an intervention affecting the intensity of infection could affect them. Because the outcome of infection in mosquitoes depends on various parameters, such as, gametocyte maturity and sex ratio [58–61], genetics [44,62–64], parasite multiplicity of infection [61–65], as well as environmental conditions [51–66] we generated experimental ranges of parasite loads from infectious blood samples so that for a given parasite isolate, only the density of infectious gametocytes varied. To do this, we diluted field collected gametocyte-containing blood isolates by a volume of the same blood sample after it had been exposed to heat inactivation and we used a range of dilutions to expose mosquitoes in controlled conditions. This generated a wide range of infection loads, mimicking natural high infection loads in some mosquitoes [20] and a number of parasites reduced by an imperfect TBI in other individuals.
The range of P. falciparum infectious gametocyte densities generated by dilution resulted in proportional oocyst intensities of infection in An. gambiae mosquitoes. This relationship between gametocyte density and oocyst load in mosquitoes was previously demonstrated in a study using the same dilution protocol [67]. This dilution procedure also confirms the positive correlation between gametocyte density and oocyst load observed in the range of natural gametocyte densities, while reducing variance due to uncontrolled confounding factors occuring in natural conditions [58]. Here we observed similar relationships between gametocyte densities and sporozoite load in mosquito head/thorax extracts, consistent with the linear relation between oocyst number and sporozoite load in salivary glands [68,69]. Surprisingly, the sporozoite prevalence we observed by qPCR in head/thorax extracts of mosquitoes from 14 dpbm (88%) were higher than oocyst prevalence in midguts at 7 dpbm (68.4%). This suggests qPCR for sporozoite detection could, (i) be more sensitive than microscope detection of oocysts, (ii) may produce false positives, or (iii) that infected females could survive better than exposed but non-infected females. These three hypotheses are not mutually exclusive; the fact that we observed an important proportion of females positive for P. falciparum in their heads or thoraxes but that never produced a positive cotton (99 individuals out of 201) suggests that the inclusion of 4 to 6 technical replicates may have overestimated the proportion of positive cases by favoring false positives and support the second hypothesis. Moreover, females exposed to infectious blood but that did not develop an infection survived less well than their infected counterparts (Fig 3a), therefore providing support for the third hypothesis. As our study focused on life history traits of mosquitoes from which P. falciparum were detected in expelled saliva, the discrepancies between the ratio of mosquitoes positive for infection in the head/thorax compared to those positive for oocysts does not affect our conclusions.
Until recently, studying life history traits of infected mosquitoes and in particular following the dynamics of parasite development in mosquitoes required dissecting a large number of individuals and did not allow all variables to be recorded from a given female. Here, we took advantage of the recent development of a non-destructive technique to measure the presence of sporozoites in the saliva of mosquitoes as a proxy of their infectivity without sacrificing them [56]. This allows more parameters to be measured from the same individual, increasing the ability to detect trade-offs. Our results show that the manipulation of gametocyte density and subsequently of oocyst and sporozoite densities has no effect on the parasite’s extrinsic incubation period. Although not significant, mosquitoes exposed to the highest gametocyte densities and carrying the highest sporozoite loads had slightly shorter EIP. This could be due to a previously identified bias in our non-destructive method of sporozoite detection. Indeed, this technique, based on the detection of sporozoite DNA in absorbent cotton on which females have come to take a sugar meal and have left saliva infected with P. falciparum, is subject to limitations related to detection thresholds. Consistent with this is the fact that we observed a high proportion of mosquitoes positive for parasites in head-thorax by qPCR but never produced a positive cotton ball (99 out of 201) and the fact that only a minority of cottons are positive post EIP (896 out of 2650) This suggests that, in addition to some potential false-positives among head-thoraxes, qPCR is sensitive enough to detect thousands of sporozoites within a mosquito, but reaches the limit of its sensitivity in cotton balls where sporozoites are much less numerous [56]. A consequence is that higher sporozoite loads are more likely to be detected, so our technique may overestimate the extrinsic incubation time in mosquitoes carrying low sporozoite loads [56]. Thus, despite the observed trend, which remains non-significant and likely to be due to the expected technical bias, our results highlight that the EIP does not depend on the parasite density in the system tested here. This is not consistent with the prediction that lower intensities of infection in mosquitoes would limit competition for resources between parasites and therefore speed up their development (i.e. shorter EIP). Recent results obtained by using an inbred parasite strain supported this hypothesis [43], but our results suggest a more complex relationship where, for instance, different parasite genotypes or multiplicity of infection could induce variable effects on EIP.
Regarding the survival of infected mosquitoes, the expectation based on a density-dependent cost of infection observed in other Plasmodium-mosquito species combinations, was that higher parasite loads would reduce the longevity of females compared to females infected with lower loads [24,44,52,54,70]. If the question of the effect of Plasmodium infection on mosquito survival is under debate for decades because of variance due to a large diversity of biological systems and laboratory conditions between studies, a general trend shows that infection affects survival more clearly in artificial mosquito-parasite combinations and when additional stresses occur for natural combinations of species [44,46,48,51]. In contrast, the dose-dependent nature of these effects was poorly documented for human malaria parasites in their natural vectors. Our observations did not show a correlation between mosquito lifespan and parasite load. Besides, it appears females exposed to infection, but which remained uninfected, displayed reduced longevity compared to infected females. An explanation for this result could be a confounding effect of mosquito’s size which can be correlated to longevity [71,72]. Indeed, it can be expected that larger individuals will not only ingest bigger blood meals and more infectious parasites but will also be the ones with longer lifespans. However, the expected relationships between size, longevity and infectivity are not always observed [49,51,73]. Our observation may suggest either a mutualist interaction between P. falciparum and An. gambiae, with a benefit of being infected for the host, or a cost of resistance reducing the fitness of resistant hosts. Our present data do not allow us to discriminate between the two hypotheses as a non-exposed mosquito control group would have been needed to determine if infection increases/maintains the host’s lifespan or resistance a reduced one. However, to date several studies suggest that resisting infection does induce reduced survival for mosquitoes, consistent with a cost of resistance, although considerable variation was found between assays [40,49,74]. In addition, as Plasmodium transmits horizontally, its effect on mosquito fecundity has only an indirect impact on its transmission, meaning it could be an adaptive strategy of the parasite to increase mosquito survival through a trade-off in energy allocation between reproduction and survival [45,75,76]. Substantial effort has been invested to decipher the trade-offs between infection, survival and fecundity for malaria vectors, but results remain controversial [19,40,77], probably because of technicial difficulties to follow each of these traits for the same individual. Therefore, the non-destructive detection of parasite at the level of individual mosquitoes should allow us to better understand the possible associations between vector survival and fecundity and those between parasite load and EIP to better depict the interactions between malaria vectors and their parasites.
Regardless of the intensity of infection, our study reveals that EIP and survival varied greatly depending on parasite isolates and assay replicates. The fact that mosquitoes were exposed to the three isolates on three different days could induce confounding effects due to variation among mosquito batches. However standardized procedures in the insectary were used, including the use of a single serum sample for serum replacement during blood feeding for the entire experiment. This should have reduced batch effects, and the observed effect of parasite isolate is consistent with previous studies in which different parasites isolates were used simultanously [78,79]. In regions with high malaria transmission such as Burkina Faso, previous studies have found high genetic diversity in P. falciparum isolates [80,81]. Our results suggest there could be variation in the EIP and mosquito survival depending on the genetic makeup of the parasite isolates. It can be hypothesized that isolates with multiple genotypes would favor competition among genotypes and possibly for faster sporogony and more virulence [82–84]. Studies are ongoing to determine the effect of genetic diversity of parasite isolates on EIP and mosquito survival.
Our study provides evidence for the effects of Plasmodium parasite load in mosquito vectors on the life history traits of the mosquito and the parasite that could influence transmission. In this context, it sheds light on the potential consequences of transmission blocking interventions against malaria, which, if they do not always succeed in completely blocking the transmission of the parasite, could result in a decrease of the parasite load in mosquitoes. If a decrease in parasite load in the mosquito resulted in a strong decrease in EIP for the parasite or an increase in the longevity of vectors, the consequences in terms of transmission could be counterproductive, with an increase in the risk of human exposure to infectious bites. Our results do not show such consequences and therefore do not identify a risk associated with the decrease of parasite load in mosquitoes on the parasite’s extrinsic incubation time in the mosquito or on mosquito survival, thus supporting strategies for blocking the transmission of malaria.
Materials and methods
Mosquitoes
In this study, we used an outbred colony of An. gambiae that was established in 2008 and repeatedly replenished with F1 from wild-caught female mosquitoes collected in Soumousso, (11°23’14"N, 4°24’42"W), 40 km from Bobo Dioulasso, south-western Burkina Faso (West Africa). To do so, field collected fed or gravid Anopheles females, morphologically identified as belonging to the An. gambiae complex, were further identified by using a SINE-PCR [85] before pooling the eggs of An. gambiae s.s.. Mosquitoes were then held in 30 × 30 × 30 cm mesh-covered cages and maintained under standard insectary conditions (27 ± 2 °C, 70 ± 5% HR, 12:12 LD) in the IRSS (Institut de Recherche en Sciences de la Santé) laboratory in Bobo Dioulasso.
P. falciparum natural isolates, infectious gametocytes dilution and mosquito infection
An. gambiae female mosquitoes were exposed to blood samples from donors naturally infected with P. falciparum gametocytes using a direct membrane feeding assay (DMFA) as described previously [86] and with a dilution procedure [67].
Briefly, thick blood smears were carried out from volunteers among 5–12 year-old schoolchildren in villages around Bobo-Dioulasso, air-dried, Giemsa-stained, and examined microscopically for the presence of P. falciparum. Asexual trophozoite parasite stages were counted against 200 leukocytes, while mature gametocyte stages were counted against 1,000 leukocytes and parasite densities were estimated on the basis of an average of 8,000 leukocytes/μl. Children with an asexual parasitaemia of > 1,000 parasites per microliter were treated according to national guidelines. Blood samples of three asymptomatic P. falciparum gametocyte carriers (called isolates A, B and C) were collected by venipuncture in heparinized tubes and their plasma was replaced by AB serum from a European donor. These blood samples underwent a series of dilutions. Dilutions involved heating part of each blood sample at 45°C for 20 minutes to inactivate the infectivity of gametocytes [73] and using this non-infectious blood to reduce the density of infectious parasites for each isolate. Isolate A, with 72 gametocytes/μl of blood, was treated to obtain three dilution factors, namely 1/1 (undiluted blood, 72 gametocytes/μl), 2/3 (48 gametocytes/μl) and 1/3 (24 gametocytes/μl). Isolate B with 136 gametocytes/μl of blood was diluted according to the same dilution factors as isolate A: 1/1 (undiluted blood, 136 gametocytes/μl), 2/3 (91 gametocytes/μl) and 1/3 (45 gametocytes/μl). The isolate C with 784 gametocytes/μl in blood was treated to obtain the dilution factor 1/1 (undiluted blood, 784 gametocytes/μl), 1/3 (261 gametocytes/μl) and 1/8 (98 gametocytes/μl).
The reconstituted blood samples were provided in feeders for one hour to female mosquitoes aged three to six days old, distributed in 500 ml paper cups at a density of 80 mosquitoes per cup, previously starved for 12 hours. Two paper cups of 80 female mosquitoes were fed using two different feeders for each blood dilution group. After exposure to a blood meal, the unfed or partially fed females were removed and discarded. The remaining fully engorged mosquitoes were placed in 30 × 30 × 30 cm mesh-covered cages by each experimental group and kept in a bio secure room, with restricted access and cold airlock, under standard conditions (27 ± 2°C, 70 ± 5% RH, 12:12 LD). The mosquitoes were given a 10% glucose solution on cotton wool after the blood meal. Mosquitoes were cold-anaesthetized for manipulation and counted at each step to verify that no accidental releases occurred. Mosquito feeding sessions were conducted three times, each time using a different parasite isolate.
Mosquito midgut dissection
On the seventh day post blood meal (dpbm), 30 females exposed to each dilution factor of isolate A, about 24 (+/- 1) females exposed to each dilution factor of isolate B and about 10 (+/- 2) females exposed to each dilution factor of isolate C were dissected. Midguts were stained in a 1% mercurochrome solution and observed by microscopy to estimate the prevalence and intensity of oocysts in each group of exposed mosquitoes.
Mosquito saliva collection and parasite DNA detection
A recently developed non-destructive sugar-feeding assay for parasite detection and estimating the extrinsic incubation period of P. falciparum in individual mosquito vectors was used [56]. Briefly, on the seventh dpbm, 20 to 40 females (median number = 30) exposed to each parasite isolate (A to C) and all experimental groups (dilution factors 1/1, 2/3, 1/3, 1/8) were individually placed in 28 ml plastic Drosophila tubes with a cotton ball (15 mg/piece) soaked with 10% glucose solution placed on each tube gauze. Cotton balls were placed at 17:00 hrs on the tubes and removed the day after at 7:00 hrs. New cotton balls were placed daily on the mosquito tubes from 8 to 22 dpbm, then at 24 dpbm and finally every four days until the mosquito died. When removed, cotton balls were stored in sterile 1.5 ml Eppendorf tubes at -20 °C for further processing.
Upon the death of all females used for saliva collection, DNA was extracted from the head and thorax of each female using the DNeasy Blood and Tissue Kit system (Qiagen, Manchester, UK) according to the manufacturer’s instructions and parasite detection was carried out by qPCR, using P. falciparum mitochondrial DNA specific primers: qPCR-PfF 5’-TTA CAT CAG GAA TGT TTT GC-3’ and qPCR-PfR 5’-ATA TTG GGA TCT CCT GCA AAT-3’ [87]. For all females found positive by qPCR for P. falciparum in head-thorax extracts, genomic DNA from saliva in the cotton samples was also extracted using the same Qiagen protocol and the presence of sporozoites tested by the same qPCR protocol.
The DNA extracts from the heads-thoraxes were tested 4 to 6 times each for the presence of parasite DNA by two different qPCR machines and the DNA extracts from cotton were run 3 times each by the same qPCR machine. Samples were considered positive for P. falciparum when at least one qPCR yielded a Ct < = 38 and 75 < = Tm< = 80.
Trait measurements
Oocyst prevalence and intensity at 7 dpbm
For all experimental groups and for the three parasites isolates, 10 to 30 females were dissected for microscopic estimation of oocyst prevalence and intensity. Oocyst prevalence is the ratio of the number of mosquitoes with at least one oocyst to the number of all individuals dissected for each experimental group and each isolate. Oocyst intensity is the average number of oocysts in infected females for each experimental group and each parasite isolate.
Sporozoite prevalence and intensity, extrinsic incubation period (EIP) and survival
The females placed in individual tubes to collect saliva from cotton balls were used to analyze the prevalence and intensity of sporozoites in carcasses (heads/thoraxes) and in saliva, to measure the EIP of parasites and mosquito survival.
Sporozoite prevalence was expressed as the number of mosquito head/thoraxes detected positive for P. falciparum by qPCR out of the total number of dissected head/thoraxes for each treatment group and for each parasite isolate. Sporozoite intensity was expressed as the inverse of the mean number of threshold cycle during qPCR (the higher the 1/Ct value, the higher the infection intensity) for each treatment group and for each parasite isolate.
EIP was defined as the time between the infectious blood meal and the first day of positive molecular detection by qPCR of P. falciparum from the cotton wool where the female deposited saliva during sugar feeding.
Dead mosquitoes in the individual tubes of each experimental group were recorded every morning at 8:00 hrs to record mosquito survival in each experimental group.
Statistical analyses
All statistical analyses were performed by R (version 4.0.2). The effect of gametocyte density on oocyst and sporozoite prevalence was tested using logistic regression for generalized linear mixed models (GLMM, binomial errors, logit link; “lme4 package”), and its effect on oocyst and sporozoite density was tested using a negative binomial GLMM and a linear mixed model (“lme4” package), respectively. In these models, gametocyte density was set as both a fixed and a random slope effect and parasite isolate as a random intercept. EIP was analysed using two LMMs, the first specifying gametocyte density as a fixed and a random slope factor and parasite isolate as a random intercept, the second specifying sporozoite load in mosquito head/thorax as a fixed and a random slope factor and parasite isolate as a random intercept. We also investigated the effect of parasite isolate (set as a fixed effect) on EIP using a Cox’s proportional hazards regression model. The effect of infection status (infected vs. uninfected) on mosquito survival was evaluated using a mixed Cox’s proportional hazards regression model (package “coxme”) with infection considered as a fixed effect and parasite isolate as a random intercept effect. Mosquito longevity was also analysed using two LMMs, the first specifying gametocyte density as a fixed and a random slope factor and parasite isolate as a random intercept, the second specifying sporozoite load in mosquito head/thorax as a fixed and a random slope factor and parasite isolate as a random intercept. Finally, we investigated the effect of parasite isolate (set as a fixed effect) on mosquito survival using a Cox’s proportional hazards regression model. For each model, the statistical significance of the fixed effects was evaluated using the “Anova” function of the “car” package.
Acknowledgments
We thank all volunteers for participating in this study as well as the local authorities for their support. We are very grateful to all the students and technicians at the IRSS/IRD who provided valuable assistance for the experiments in this study and to Philip Agnew for correcting our English.
Data Availability
The data and scripts are available on DataSuds repository: https://dataverse.ird.fr/dataset.xhtml?persistentId=doi:10.23708/OJCZQD.
Funding Statement
The work described in our manuscript was funded by support from the following sources: AC received support from the Malaria Vaccine Initiative, a program of the global non-profit PATH organization (Seattle, USA) and the European Union’s Horizon 2020 research and innovation program under grant agreement No 733273. TL received support from the ANR Grant “STORM” No. 16-CE35-0007. DFDSH received support from the JEAI IRD program Grant No. AAP2018_JEAI_PALUNEC. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.OMS. World Malaria Report 2020. 2020.
- 2.Dondorp AM, Nosten F, Yi P, Das D, Phyo AP, Tarning J, et al. Artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med. 2009;361. doi: 10.1056/NEJMoa0808859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Uwimana A, Legrand E, Stokes BH, Ndikumana JLM, Warsame M, Umulisa N, et al. Emergence and clonal expansion of in vitro artemisinin-resistant Plasmodium falciparum kelch13 R561H mutant parasites in Rwanda. Nat Med. 2020; doi: 10.1038/s41591-020-1005-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ranson H, Lissenden N. Insecticide resistance in African Anopheles mosquitoes: a worsening situation that needs urgent action to maintain malaria control. Trends Parasitol. 2016. doi: 10.1016/j.pt.2015.11.010 [DOI] [PubMed] [Google Scholar]
- 5.Delves MJ, Angrisano F, Blagborough AM. Antimalarial transmission-blocking interventions: past, present, and future. Trends Parasitol. 2018. doi: 10.1016/j.pt.2018.07.001 [DOI] [PubMed] [Google Scholar]
- 6.Sinden RE. Developing transmission-blocking strategies for malaria control. PLoS Pathog. 2017. doi: 10.1371/journal.ppat.1006336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blagborough AM, Churcher TS, Upton LM, Ghani AC, Gething PW, Sinden RE. Transmission-blocking interventions eliminate malaria from laboratory populations. Nat Commun. 2013;4. doi: 10.1038/ncomms2840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burrows JN, Duparc S, Gutteridge WE, Hooft Van Huijsduijnen R, Kaszubska W, Macintyre F, et al. New developments in anti-malarial target candidate and product profiles. Malar J. 2017. doi: 10.1186/s12936-016-1675-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Birkholtz LM, Alano P, Leroy D. Transmission-blocking drugs for malaria elimination. Trends Parasitol. 2022;38: 390–403. doi: 10.1016/j.pt.2022.01.011 [DOI] [PubMed] [Google Scholar]
- 10.Wu Y, Sinden RE, Churcher TS, Tsuboi T, Yusibov V. Development of malaria transmission-blocking vaccines: From concept to product. Adv Parasitol. 2015;89. doi: 10.1016/bs.apar.2015.04.001 [DOI] [PubMed] [Google Scholar]
- 11.Acquah FK, Adjah J, Williamson KC, Amoah LE. Transmission-blocking vaccines: Old friends and new prospects. Infect Immunity. 2019. doi: 10.1128/IAI.00775-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duffy PE. Transmission-blocking vaccines: harnessing herd immunity for malaria elimination. Expert RevVaccines. 2021. doi: 10.1080/14760584.2021.1878028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Challenger JD, Olivera Mesa D, Da DF, Yerbanga RS, Lefèvre T, Cohuet A, et al. Predicting the public health impact of a malaria transmission-blocking vaccine. Nat Commun. 2021;12. doi: 10.1038/s41467-021-21775-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Churcher TS, Blagborough AM, Delves M, Ramakrishnan C, Kapulu MC, Williams AR, et al. Measuring the blockade of malaria transmission—An analysis of the Standard Membrane Feeding Assay. Int J Parasitol. 2012;42. doi: 10.1016/j.ijpara.2012.09.002 [DOI] [PubMed] [Google Scholar]
- 15.Miura K, Swihart BJ, Deng B, Zhou L, Pham TP, Diouf A, et al. Transmission-blocking activity is determined by transmission-reducing activity and number of control oocysts in Plasmodium falciparum standard membrane-feeding assay. Vaccine. 2016;34. doi: 10.1016/j.vaccine.2016.06.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bompard A, Da DF, Yerbanga RS, Biswas S, Kapulu M, Bousema T, et al. Evaluation of two lead malaria transmission blocking vaccine candidate antibodies in natural parasite-vector combinations. Sci Rep. 2017;7. doi: 10.1038/s41598-017-06130-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pringle G. A quantitative study of naturally-acquired malaria infections in Anopheles gambiae and Anopheles funestus in a highly malarious area of East Africa. Trans R Soc Trop Med Hyg. 1966;60. doi: 10.1016/0035-9203(66)90009-5 [DOI] [PubMed] [Google Scholar]
- 18.Billingsley PF, Medley GF, Charlwood D, Sinden RE. Relationship between prevalence and intensity of Plasmodium falciparum infection in natural populations of Anopheles mosquitoes. Am J Trop Med Hyg. 1994;51. doi: 10.4269/ajtmh.1994.51.260 [DOI] [PubMed] [Google Scholar]
- 19.Hogg JC, Hurd H. The effects of natural Plasmodium falciparum infection on the fecundity and mortality of Anopheles gambiae s.l. in north east Tanzania. Parasitology. 1997;114. doi: 10.1017/S0031182096008542 [DOI] [PubMed] [Google Scholar]
- 20.Bompard A, Da DF, Yerbanga SR, Morlais I, Awono-Ambéné PH, Dabiré RK, et al. High Plasmodium infection intensity in naturally infected malaria vectors in Africa. Int J Parasitol. 2020;50. doi: 10.1016/j.ijpara.2020.05.012 [DOI] [PubMed] [Google Scholar]
- 21.Bush AO, Lotz JM. The ecology of « Crowding ». J Parasitol. 2000. doi: 10.1645/0022-3395(2000)086[0212:TEOC]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- 22.Dietz K. Density-dependence in parasite transmission dynamics. Parasitology Today. 1988. doi: 10.1016/0169-4758(88)90034-8 [DOI] [PubMed] [Google Scholar]
- 23.Begon M, Townsend CR, Harper JL. Ecology: from individuals to ecosystems, 4th Edition. Blackwell Publishing. 2005.
- 24.Pollitt LC, Churcher TS, Dawes EJ, Khan SM, Sajid M, Basáñez MG, et al. Costs of crowding for the transmission of malaria parasites. Evol Appl. 2013;6: 617–629. doi: 10.1111/eva.12048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cohuet A, Harris C, Robert V, Fontenille D. Evolutionary forces on Anopheles: what makes a malaria vector? Trends Parasitol. 2010. doi: 10.1016/j.pt.2009.12.001 [DOI] [PubMed] [Google Scholar]
- 26.Lefevre T, Ohm J, Dabiré KR, Cohuet A, Choisy M, Thomas MB, et al. Transmission traits of malaria parasites within the mosquito: Genetic variation, phenotypic plasticity, and consequences for control. Evol Appl. 2018; doi: 10.1111/eva.12571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith DL, McKenzie FE. Statics and dynamics of malaria infection in Anopheles mosquitoes. Malar J. 2004;3: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Killeen GF, McKenzie FE, Foy BD, Schieffelin C, Billingsley PF, Beier JC. A simplified model for predicting malaria entomologic inoculation rates based on entomologic and parasitologic parameters relevant to control. Am J Trop Med Hyg. 2000;62. doi: 10.4269/ajtmh.2000.62.535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ohm JR, Baldini F, Barreaux P, Lefevre T, Lynch PA, Suh E, et al. Rethinking the extrinsic incubation period of malaria parasites. Parasit and Vectors. 2018. doi: 10.1186/s13071-018-2761-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koella JC. An evolutionary view of the interactions between anopheline mosquitoes and malaria parasites. Microbes Infect. 1999;1: 303–308. doi: 10.1016/s1286-4579(99)80026-4 [DOI] [PubMed] [Google Scholar]
- 31.Shapiro LLM, Whitehead SA, Thomas MB. Quantifying the effects of temperature on mosquito and parasite traits that determine the transmission potential of human malaria. PLoS Biol. 2017;15: 1–21. doi: 10.1371/journal.pbio.2003489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Noden BH, Kent MD, Beier JC. The impact of variations in temperature on early Plasmodium falciparum development in Anopheles stephensi. Parasitology. 1995;111. doi: 10.1017/S0031182000077003 [DOI] [PubMed] [Google Scholar]
- 33.Okech BA, Gouagna LC, Kabiru EW, Walczak E, Beier JC, Yan G, Githure JI. Resistance of early midgut stages of natural Plasmodium falciparum parasites to high temperatures in experimentally infected Anopheles gambiae (Diptera: Culicidae). J Parasitol. 2004. Aug; 90:764–768. doi: 10.1645/GE-135R1 [DOI] [PubMed] [Google Scholar]
- 34.Murdock CC, Paaijmans KP, Cox-Foster D, Read AF, Thomas MB. Rethinking vector immunology: The role of environmental temperature in shaping resistance. Nat Rev Microbiol. 2012. p. 869–876. doi: 10.1038/nrmicro2900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Detinova TS. Age-grouping methods in Diptera of medical importance with special reference to some vectors of malaria. Monogr Ser World Health Organ. 1962;47. doi: 10.2307/3275215 [DOI] [PubMed] [Google Scholar]
- 36.Fialho RF, Schall JJ. Thermal ecology of a malarial parasite and its insect vector: consequences for the parasite’s transmission success. J Anim Ecol. 1995;64: 553. doi: 10.2307/5799 [DOI] [Google Scholar]
- 37.Donnell DM, Hunter MS. Developmental rates of two congeneric parasitoids, Encarsia formosa and E. pergandiella (Hymenoptera: Aphelinidae), utilizing different egg provisioning strategies. J Insect Physiol. 2002;48. doi: 10.1016/S0022-1910(02)00070-7 [DOI] [PubMed] [Google Scholar]
- 38.Mideo N, Reece SE. Plasticity in parasite phenotypes: Evolutionary and ecological implications for disease. Future Microbiol. 2012;7. doi: 10.2217/fmb.11.134 [DOI] [PubMed] [Google Scholar]
- 39.Shapiro LLM, Murdock CC, Jacobs GR, Thomas RJ, Thomas MB. Larval food quantity affects the capacity of adult mosquitoes to transmit human malaria. Proc R Soc B Biol Sci. 2016;283. doi: 10.1098/rspb.2016.0298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hien DF, Dabiré KR, Roche B, Diabaté A, Yerbanga SR, Cohuet A, et al. Plant-mediated effects on mosquito capacity to transmit human malaria. PLoS Pathog. 2016;12: e1005773. doi: 10.1371/journal.ppat.1005773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shaw WR, Holmdahl IE, Itoe MA, Werling K, Marquette M, Paton DG, et al. Multiple blood feeding in mosquitoes shortens the Plasmodium falciparum incubation period and increases malaria transmission potential. PLoS Pathog. 2020;16. doi: 10.1371/journal.ppat.1009131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shaw WR, Marcenac P, Catteruccia F. Plasmodium development in Anopheles: a tale of shared resources. Trends Parasitol. 2022. doi: 10.1016/j.pt.2021.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Habtewold T, Sharma AA, Wyer CAS, Masters EKG, Windbichler N, Christophides GK. Plasmodium oocysts respond with dormancy to crowding and nutritional stress. Sci Rep. 2021;11: 1–10. doi: 10.1038/s41598-021-81574-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ferguson HM, Read AF. Why is the effect of malaria parasites on mosquito survival still unresolved? Trends Parasitol. 2002;18: 256–261. doi: 10.1016/s1471-4922(02)02281-x [DOI] [PubMed] [Google Scholar]
- 45.Vézilier J, Nicot A, Gandon S, Rivero A. Plasmodium infection decreases fecundity and increases survival of mosquitoes. Proc R Soc B Biol Sci. 2012;279. doi: 10.1098/rspb.2012.1394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aboagye-Antwi F, Guindo A, Traore AS, Hurd H, Coulibaly M, Traore S, et al. Hydric stress-dependent effects of Plasmodium falciparum infection on the survival of wild-caught Anopheles gambiae female mosquitoes. Malar J. 2010;9: 243–254. doi: 10.1186/1475-2875-9-243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alout H, Dabiré RK, Djogbénou LS, Abate L, Corbel V, Chandre F, Cohuet A. Interactive cost of Plasmodium infection and insecticide resistance in the malaria vector Anopheles gambiae. Sci Rep. 2016. Jul 19;6:29755. doi: 10.1038/srep29755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alout H, Yameogo BK, Djogbénou LS, Chandre F, Dabiré KR, Corbel V, et al. Interplay between Plasmodium infection and resistance to insecticides in vector mosquitoes. J Infect Dis. 2014;210: 1464–1470. doi: 10.1093/infdis/jiu276 [DOI] [PubMed] [Google Scholar]
- 49.Sangare I, Dabiré KR, Yameogo BK, Da DF, Michalakis Y, Cohuet A. Stress dependent infection cost of the human malaria agent Plasmodium falciparum on its natural vector Anopheles coluzzii. Infect Genet Evol. 2014;25: 57–65. doi: 10.1016/j.meegid.2014.04.006 [DOI] [PubMed] [Google Scholar]
- 50.Lalubin F, Delédevant A, Glaizot O, Christe P. Natural malaria infection reduces starvation resistance of nutritionally stressed mosquitoes. J Anim Ecol. 2014;83. doi: 10.1111/1365-2656.12190 [DOI] [PubMed] [Google Scholar]
- 51.Roux O, Vantaux A, Roche B, Yameogo BK, Dabiré KR, Diabaté A, et al. Evidence for carry-over effects of predator exposure on pathogen transmission potential. Proc R Soc B Biol Sci. 2015;282: 1–9. doi: 10.1098/rspb.2015.2430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dawes EJ, Churcher TS, Zhuang S, Sinden RE, Basáñez MG. Anopheles mortality is both age- and Plasmodium-density dependent: Implications for malaria transmission. Malar J. 2009;8. doi: 10.1186/1475-2875-8-228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Klein TA, Harrison BA, Grove JS, Dixon S V., Andre RG. Correlation of survival rates of Anopheles dirus A (Diptera: culicidae) with different infection densities of Plasmodium cynomolgi. Bull World Health Organ. 1986;64: 901–907. [PMC free article] [PubMed] [Google Scholar]
- 54.Gutiérrez-López R, Martínez-de la Puente J, Gangoso L, Yan J, Soriguer R, Figuerola J. Experimental reduction of host Plasmodium infection load affects mosquito survival. Sci Rep. 2019;9. doi: 10.1038/s41598-019-45143-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Churcher TS, Dawes EJ, Sinden RE, Christophides GK, Koella JC, Basáñez MG. Population biology of malaria within the mosquito: Density-dependent processes and potential implications for transmission-blocking interventions. Malar J. 2010;9. doi: 10.1186/1475-2875-9-311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guissou E, Waite JL, Jones M, Bell AS, Suh E, Yameogo KB, et al. A non-destructive sugar-feeding assay for parasite detection and estimating the extrinsic incubation period of Plasmodium falciparum in individual mosquito vectors. Sci Rep. 2021;11. doi: 10.1038/s41598-021-88659-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Matthews J, Bethel A, Osei G. An overview of malarial Anopheles mosquito survival estimates in relation to methodology. Parasit & Vectors. 2020. doi: 10.1186/s13071-020-04092-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bradley J, Stone W, Da DF, Morlais I, Dicko A, Cohuet A, et al. Predicting the likelihood and intensity of mosquito infection from sex specific Plasmodium falciparum gametocyte density. Elife. 2018;7: 1–13. doi: 10.7554/eLife.34463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mitri C, Thiery I, Bourgouin C, Paul REL. Density-dependent impact of the human malaria parasite Plasmodium falciparum gametocyte sex ratio on mosquito infection rates. Proc R Soc B Biol Sci. 2009;276. doi: 10.1098/rspb.2009.0962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Paul REL, Brey PT, Robert V. Plasmodium sex determination and transmission to mosquitoes. Trends in Parasitology. 2002. doi: 10.1016/S1471-4922(01)02122-5 [DOI] [PubMed] [Google Scholar]
- 61.Reece SE, Drew DR, Gardner A. Sex ratio adjustment and kin discrimination in malaria parasites. Nature. 2008;453. doi: 10.1038/nature06954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Harris C, Lambrechts L, Rousset F, Abate L, Nsango SE, Fontenille D, et al. Polymorphisms in Anopheles gambiae immune genes associated with natural resistance to Plasmodium falciparum. PLoS Pathog. 2010;6. doi: 10.1371/journal.ppat.1001112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lambrechts L, Halbert J, Durand P, Gouagna LC, Koella JC. Host genotype by parasite genotype interactions underlying the resistance of anopheline mosquitoes to Plasmodium falciparum. Malar J. 2005;4. doi: 10.1186/1475-2875-4-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Grignard L, Gonçalves BP, Early AM, Daniels RF, Tiono AB, Guelbéogo WM, et al. Transmission of molecularly undetectable circulating parasite clones leads to high infection complexity in mosquitoes post feeding. Int J Parasitol. 2018;48: 671–677. doi: 10.1016/j.ijpara.2018.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nsango SE, Abate L, Thoma M, Pompon J, Fraiture M, Rademacher A, et al. Genetic clonality of Plasmodium falciparum affects the outcome of infection in Anopheles gambiae. Int J Parasitol. 2012;42: 589–595. doi: 10.1016/j.ijpara.2012.03.008 [DOI] [PubMed] [Google Scholar]
- 66.Lefèvre T, Vantaux A, Dabiré KR, Mouline K, Cohuet A. Non-genetic determinants of mosquito competence for malaria parasites. PLoS Pathog. 2013;9. doi: 10.1371/journal.ppat.1003365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Da DF, Churcher TS, Yerbanga RS, Yameogo BK, Sangaré I, Bosco J, et al. Experimental study of the relationship between Plasmodium gametocyte density and infection success in mosquitoes: implications for the evaluation of malaria transmission-reducing interventions. Exp Parasitol. 2015;149: 74–83. doi: 10.1016/j.exppara.2014.12.010 [DOI] [PubMed] [Google Scholar]
- 68.Stone WJR, Eldering M, Van Gemert GJ, Lanke KHW, Grignard L, Van De Vegte-Bolmer MG, et al. The relevance and applicability of oocyst prevalence as a read-out for mosquito feeding assays. Sci Rep. 2013;3. doi: 10.1038/srep03418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Miura K, Swihart BJ, Deng B, Zhou L, Pham TP, Diouf A, et al. Strong concordance between percent inhibition in oocyst and sporozoite intensities in a Plasmodium falciparum standard membrane-feeding assay. Parasit and Vectors. 2019;12. doi: 10.1186/s13071-019-3470-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ferguson HM, Mackinnon MJ, Chan BH, Read AF. Mosquito mortality and the evolution of malaria virulence. Evolution (N Y). 2003;57: 2792–2804. doi: 10.1111/j.0014-3820.2003.tb01521.x [DOI] [PubMed] [Google Scholar]
- 71.Barreaux AMG, Stone CM, Barreaux P, Koella JC. The relationship between size and longevity of the malaria vector Anopheles gambiae (s.s.) depends on the larval environment. Parasit and Vectors. 2018;11. doi: 10.1186/s13071-018-3058-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lehmann T, Dalton R, Kim EH, Dahl E, Diabate A, Dabire R, et al. Genetic contribution to variation in larval development time, adult size, and longevity of starved adults of Anopheles gambiae. Infect Genet Evol. 2006;6. doi: 10.1016/j.meegid.2006.01.007 [DOI] [PubMed] [Google Scholar]
- 73.Sangare I, Michalakis Y, Yameogo B, Dabire R, Morlais I, Cohuet A. Studying fitness cost of Plasmodium falciparum infection in malaria vectors: validation of an appropriate negative control. Malar J. 2013;12: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hien DFDS, Paré PSL, Cooper A, Koama BK, Guissou E, Yaméogo KB, et al. Contrasting effects of the alkaloid ricinine on the capacity of Anopheles gambiae and Anopheles coluzzii to transmit Plasmodium falciparum. Parasit Vectors. 2021;14: 1–11. doi: 10.1186/s13071-021-04992-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schwartz A, Koella JC. Trade-offs, conflicts of interest and manipulation in Plasmodium–mosquito interactions. Trends Parasitol. 2001;17: 189–194. [DOI] [PubMed] [Google Scholar]
- 76.Ferguson HM, Rivero A, Read AF. The influence of malaria parasite genetic diversity and anaemia on mosquito feeding and fecundity. Parasitology. 2003;127. doi: 10.1017/s0031182003003287 [DOI] [PubMed] [Google Scholar]
- 77.Alout H, Dabiré RK, Djogbénou LS, Abate L, Corbel V, Chandre F, et al. Interactive cost of Plasmodium infection and insecticide resistance in the malaria vector Anopheles gambiae. Sci Rep. 2016;6. doi: 10.1038/srep29755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ferguson HM, Mackinnon MJ, Chan BH, Read a F. Mosquito mortality and the evolution of malaria virulence. Evolution (N Y). 2003;57: 2792–2804. doi: 10.1111/j.0014-3820.2003.tb01521.x [DOI] [PubMed] [Google Scholar]
- 79.Ferguson HM, Read AF. Genetic and environmental determinants of malaria parasite virulence in mosquitoes. Proc R Soc B Biol Sci. 2002;269. doi: 10.1098/rspb.2002.2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Somé AF, Bazié T, Zongo I, Yerbanga RS, Nikiéma F, Neya C, et al. Plasmodium falciparum msp1 and msp2 genetic diversity and allele frequencies in parasites isolated from symptomatic malaria patients in Bobo-Dioulasso, Burkina Faso. Parasit Vectors. 2018; doi: 10.1186/s13071-018-2895-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sondo P, Derra K, Rouamba T, Nakanabo Diallo S, Taconet P, Kazienga A, et al. Determinants of Plasmodium falciparum multiplicity of infection and genetic diversity in Burkina Faso. Parasit Vectors. 2020;13. doi: 10.1186/s13071-020-04302-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.De Roode JC, Pansini R, Cheesman SJ, Helinski MEH, Huijben S, Wargo AR, et al. Virulence and competitive ability in genetically diverse malaria infections. Proc Natl Acad Sci U S A. 2005;102. doi: 10.1073/pnas.0500078102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Alizon S, de Roode JC, Michalakis Y. Multiple infections and the evolution of virulence. Ecol Lett. 2013;16. doi: 10.1111/ele.12076 [DOI] [PubMed] [Google Scholar]
- 84.Alizon S, Lion S. Within-host parasite cooperation and the evolution of virulence. Proc R Soc B Biol Sci. 2011;278. doi: 10.1098/rspb.2011.0471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Santolamazza F, Mancini E, Simard F, Qi Y, Tu Z, Della Torre A. Insertion polymorphisms of SINE200 retrotransposons within speciation islands of Anopheles gambiae molecular forms. Malar J. 2008; doi: 10.1186/1475-2875-7-163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ouédraogo AL, Guelbéogo WM, Cohuet A, Morlais I, King JG, Gonçalves BP, et al. A protocol for membrane feeding assays to determine the infectiousness of P. falciparum naturally infected individuals to Anopheles gambiae. Malar World J. 2013;4: 17–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Boissière A, Gimonneau G, Tchioffo MT, Abate L, Bayibeki A, Awono-Ambéné PH, et al. Application of a qPCR assay in the investigation of susceptibility to malaria infection of the M and S molecular forms of An. gambiae s.s. in Cameroon. PLoS One. 2013;8. doi: 10.1371/journal.pone.0054820 [DOI] [PMC free article] [PubMed] [Google Scholar]