Abstract
Staphylococcus aureus is a common colonizer of the human gut and in doing so it must be able to resist the actions of the host’s innate defences. Bile salts are a class of molecules that possess potent antibacterial activity that control growth. Bacteria that colonize and survive in that niche must be able to resist the action of bile salts, but the mechanisms by which S. aureus does so are poorly understood. Here we show that FadB is a bile-induced oxidoreductase which mediates bile salt resistance and when heterologously expressed in Escherichia coli renders them resistant. Deletion of fadB attenuated survival of S. aureus in a model of the human distal colon.
Keywords: Staphylococcus aureus, FadB, dehydrogenase, cholate, bile acids, colon
Introduction
Infection by Staphylococcus aureus is a leading cause of community-acquired and nosocomial disease. Its ability to colonize the nares, which occurs in 20–25 % of the population at any one time [1, 2], is linked to infection which frequently occurs when the S. aureus spreads to normally sterile parts of the body such as the bloodstream [3]. While this has been well characterized, several recent studies have indicated that colonization of the intestine by S. aureus , which occurs in c. 20 % of individuals and has been much less well characterized, may have important clinical implications [4]. Carriage studies of methicillin-resistant S. aureus (MRSA) have reported gastrointestinal colonization in 11–89 % of those who were carrying the bacterium [5–9]. Such individuals display an increased frequency of skin colonization [10].
S. aureus intestinal colonization can serve as an important source of transmission when faecal contamination of the adjacent environment occurs [11–14], while screening for faecal carriage is proposed as a measure to reduce transmission [15]. A study of a long-term outbreak with S. aureus sequence type 228 (ST228) in a Swiss hospital reported persistence of a single clone which was adapted to colonize the rectum as the primary colonization niche, over the nares [16].
Although the extent and clinical implications of intestinal colonization by S. aureus are still relatively ill defined [17], it can be assumed that carriage is a risk for intestinal infection; S. aureus can cause pseudomembranous colitis that is histologically distinct from that caused by Clostridiodes difficile [18]. A study of intensive care and liver transplant units showed that patients with both rectal and nares colonization by MRSA was associated with a significantly higher risk of disease (40 %) than did patients with nasal colonization alone (18 %) [9]. Multiple studies have demonstrated frequent intestinal colonization in infants, particularly those who were breast fed, and that there is a positive correlation with the development of allergies [19–23]. While a role for S. aureus intestinal carriage in the development of systemic S. aureus disease has not been established, colonization of the intestinal lumen of mice can result in the pathogen crossing the intestinal epithelial barrier and spreading to mesenteric lymph nodes [24, 25]. Furthermore, a Trojan horse model has been proposed where intestinal colonization is a putative source of S. aureus -infected neutrophils which then disseminate the pathogen around the body [26].
The antibacterial activity of bile salts represents a survival challenge for bacteria in the gut [27] and helps to direct the structure of the microbiome, but some pathogens use them as an environmental signal to regulate colonization and virulence [28, 29]. Many members of the microbiota initiate bile acid metabolism via bile salt hydrolases, which hydrolyse and deconjugate the glycine or taurine from the sterol core of the primary bile acids. The deconjugated bile acids can subsequently undergo a variety of microbiota-induced transformations.
Bacteria employ a number of strategies in order to survive the antibacterial activity of bile salts. Gram-negative bacteria are generally more innately bile resistant than Gram-positive bacteria due to the presence of an outer membrane, which acts as a barrier [27] which with maintenance of membrane integrity by cell envelope lipopolysaccharide (LPS) imparts protection against the actions of bile salts [30, 31]. A number of pathogens possess bile efflux pumps, including S. aureus which uses MnhF to resist unconjugated bile acids and survive under conditions modelling the human colon [32]. The efflux pump AcrAB in Salmonella enterica serovar Typhi and S. enterica serovar Typhimurium allows these pathogens to grow at bile concentrations that are much higher than those encountered in vivo [33]. Similarly, HefC is an AcrB homologue that confers bile salt resistance to Helicobacter pylori [34]. The multidrug efflux pump CmeABC of Campylobacter jejuni mediates bile salt resistance and is required for colonization of chickens [35].
Thus bile salt resistance is important for intestinal survival of several enteric bacteria and while there is currently only limited understanding of how S. aureus resists bile, we have previously reported the role of MnhF in bile salt efflux [32]. Here, FadB, a putative oxidoreductase, was enriched in the cell envelope of bile-treated S. aureus , suggesting that it is involved in bile resistance and therefore survival of the pathogen under conditions that mimic the human colon.
Methods
Bacteria, plasmids and growth conditions
The bacterial strains and plasmids used in this study are listed in Tables 1 and 2, respectively. Escherichia coli strains were grown in lysogeny broth (LB) medium using selection with ampicillin at 100 µg ml−1 where appropriate. S. aureus was grown in Tryptic Soy Broth (TSB; Sigma), with inclusion of the following antibiotics, where appropriate: erythromycin at 5 µg ml−1 and lincomycin at 25 µg ml−1. Phage-mediated transductions were performed as described previously [36].
Table 1.
Bacterial strains
|
Strain |
Description/genotype |
Source or reference |
|---|---|---|
|
S. aureus SH1000 |
Wild-type |
[87] |
|
S. aureus RN4220 |
Accepts E. coli DNA |
[88] |
|
S. aureus ΔfadB |
ΔfadB mutation in SH1000 |
This study |
|
E. coli DH5α |
F– φ80lacZΔM15 Δ(lacZYA-argF)U169 recA1 endA1 hsdR17(rK –, mK +) phoA supE44 λ– thi-1 gyrA96 relA1 |
Invitrogen |
|
E. coli BL21 (DE3) |
F– ompT hsdSB (rB –, mB –) gal dcm (DE3) |
Invitrogen |
|
E. coli BW25113 |
Δ(araD-araB)567 ΔlacZ4787(::rrnB-3) λ- rph-1 Δ(rhaD-rhaB)568 hsdR514 |
[89] |
|
E. coli JW3822 |
E. coli BW25113 fadB |
[89] |
Table 2.
Plasmids
|
Plasmid name |
Description |
Antibiotic resistance |
Source or reference |
|---|---|---|---|
|
pMAD |
Temperature-sensitive (30 °C) E. coli – S. aureus shuttle vector. pE194ts:: pBR322 |
ApR ( E. coli ) EmR ( S. aureus ) |
[90] |
|
p∆fadB |
pMAD-based vector for ∆fadB mutation |
ApR ( E. coli ) EmR ( S. aureus ) |
This work |
|
pBAD His A |
Expression vector containing araBAD promoter |
ApR |
[91] |
|
pfadB |
pBAD His A containing fadB internal fragment |
ApR |
This work |
|
pET21a |
His6 tag overexpression vector |
ApR |
Novagen |
|
pAmjed1 |
pET21a containing internal fragment encoding rFadB |
ApR |
This work |
Preparation of cell envelope material
Cell envelope was extracted based on a previously described method [37]. Growing mid-log cultures of S. aureus in TSB (37 °C with orbital shaking at 250 r.p.m.) were harvested and diluted as appropriate to an optical density at 600 nm of ˜0.6 and resuspended to and OD600 of 0.6. Then, 50 ml was centrifuged at 16 100 g for 5 min at 4 °C, resuspended, and washed in 1 ml of TBS [50 mM Tris-HCl (pH 7.5), 0.1 M NaCl, 0.5 mM PMSF, 1 mg of iodoacetamide ml–1). Samples were centrifuged at 16 100 g for 5 min at 4 °C, and pellets were resuspended in 1 ml of TBS. Next, 0.5 ml of suspension was added to tube containing Lysing Matrix B (MP Biomedicals) containing glass beads, which was then shaken 10 times in a FastPrep-24 machine (MP Biomedicals) set at speed 60 for 40 s. The tubes were placed on ice and allowed to cool between each cycle. Glass beads were allowed to settle, and the supernatant containing insoluble cell wall material was removed. Insoluble material was recovered by centrifugation at 16 100 g for 10 min at 4 °C and washed in 1 ml cold 50 mM Tris-HCl (pH 7.5) followed by centrifugation at 16 100 g for 10 min at 4 °C before resuspension in SDS-PAGE buffer.
SDS-PAGE
Proteins were separated by SDS-PAGE with a 4 % (w/v) stacking gel and a 12 % (w/v) resolving gel in a Mini-Protean II gel apparatus (Bio-Rad).
Quantitative real-time PCR
mRNA from S. aureus was quantified using quantitative real-time PCR (qRT-PCR). Cells were grown as described above and then treated with RNAlater stabilization solution (Invitrogen), and RNA was isolated using RNeasy Mini Kits as per the manufacturer’s instructions. DNA was removed using Turbo DNase (Invitrogen). The quantity and quality of purified mRNA was determined using an Agilent RNA 6000 Nano Kit and Bioanalyzer. A total of 0.5 µg of RNA was reverse transcribed using the Tetro cDNA synthesis kit (Bioline) and reactions lacking RNA or reverse transcriptase were included as controls. qRT-PCR was performed using the Agilent qPCR system and iQ SYBR green supermix (Bio-Rad). Relative amounts of transcript were determined by relative quantification using gyr as the internal comparator gene, based on consistent levels observed in previous studies [38–41]. The oligonucleotides used for qRT-PCR are listed in Table 3.
Table 3.
Oligonucleotides; restriction endonuclease sites are underlined
|
Name* |
Sequence 5′−3′ |
|---|---|
|
FadBUpFor1 |
CTAAATGGATCCACAGTCACATGAACTGCG |
|
FadBUpRev2 |
TTACCCGGGTTGTCATAGTGATTCCTCCAATTTAGTTG |
|
FadBDownFor2 |
CATTACCCGGGCGTAATTAAAAGATAGTCATTAAGAGAGG |
|
FadBDownRev1 |
CGTTTGGGATCCAGAAGCAAATGCTTCGTTCAATTCG |
|
FadBOverFor3 |
GGAGATATACATATGATTGGAGGAATCACATATGAC |
|
FadBOverRev4 |
GTGGTGGTGCTCGAGATTACGTAATGGCTTA |
|
FadBCloneFor 5 |
CTAAGAGCTCATTGGAGGAATCACTATGACAATTAATAAAG |
|
FadBCloneRev1 |
GACTAGGTACCTCTTTTAATTACGTAATGGCTTACCAG |
|
fadBFor |
CACGGTCTATGTCTCGGAAATC |
|
fadBRev |
CAAGACGAAGCGGGACTATTT |
|
gyrBFor |
ATCGACTTCAGAGAGAGGTTTG |
|
gyrBRev |
CCGTTATCCGTTACTTTAATCCA |
|
Sau |
GAAGCAAGCTTCTCGTCCG |
*Restriction sites: 1 BamHI, 2 XmaI, 3 NdeI, 4 XhoI, 5 SacI.
Generation of an fadB mutant
To generate a ΔfadB mutant, DNA fragments corresponding to ~1 kb upstream and downstream of fadB were amplified using Pwo polymerase (Roche) with oligonucleotide pairs FadBUpFor/FadBUpRev and FadBDownFor/FadBDownRev (Table 3). PCR products were purified and then digested with BamHI/SmaI and cloned into pMAD. The resulting plasmid was used to transform electrocompetent S. aureus RN4220. Plasmid was transduced into S. aureus SH1000 using ϕ11 phage. The temperature-sensitive nature of plasmid replication was exploited to integrate the plasmid into the bacterial chromosome, by plating cells onto medium containing erythromycin and lincomycin at 42 °C. After further rounds of plating, erythromycin- and lincomycin-sensitive colonies were isolated and the loss of fadB was confirmed by PCR. Use of both erythromycin and lincomycin reduces the chance of unintentional selection of point mutantions.
Determination of MIC
The MICs of selected bile salts, sodium cholate (CA), sodium deoxycholate (DCA), sodium chenodeoxycholate (CDCA), sodium glycocholate (GCA) and sodium taurocholate (TCA) were determined by broth dilution. MICs were determined by stepwise dilutions and were reproduced in three independent experiments.
Time course measurement of bacterial viability upon exposure to bile salts
Overnight cultures of S. aureus were grown to mid-exponential phase in TSB at 37 °C with shaking. After harvesting, cells were washed twice with sterile 5 mM HEPES buffer (pH 7.2) containing 10 mM glucose and then resuspended in the same buffer to an OD600 of 0.5. Cells were incubated with various concentrations of bile salt which give reliable kill curves, for 30 min at 37 °C. At 10 min intervals, dilutions from each of the bile salt-treated groups were made with a sterile peptone saline diluent (Oxoid). Dilutions were plated onto tryptic soy agar plates and incubated overnight at 37 °C. Colonies were counted, and percentage viabilities were calculated based on the initial untreated cell suspension.
Cloning and overexpression of fadB
The fadB gene was amplified from S. aureus SH1000 DNA by PCR using Phusion DNA polymerase (Thermo Scientific). Oligonucleotides FadBOverFor and FadBOverRev were used to amplify the gene. PCR products were digested with NdeI and XhoI and ligated into similarly digested pET21a. The ligation mixture was transformed into E. coli DH5α, and transformants were selected for resistance to ampicillin (Apr) and checked by restriction digestion and sequenced to confirm the fidelity of the PCR. A representative plasmid, pAmjed1, was transformed into E. coli BL21(DE3).
For overexpression in E. coli BW25113, oligonucleotides FADBcloneFor and FADBcloneRev were used to generate a PCR product which was subsequently digested with SacI and KpnI and ligated into similarly digested pBAD/HisA to create plasmid pAmjed2, where fadB is fused to PBAD, which is under the tight control of the arabinose-inducible AraC-controlled promoters [42, 43].
Overexpression and purification of recombinant FadB
His6 tag recombinant rFadB was expressed by addition of 100 µM IPTG to growing cells. Purification was achieved using a pre-packed Ni-Sepharose column with the Biologic HR workstation (Bio-Rad). Eluted fractions were analysed by SDS-PAGE, and the protein concentration was determined using Bradford reagent. The validity of the rFadB protein overexpression was confirmed by submitting purified rFadB for MS analysis at the University of Birmingham. The protein sample was digested with trypsin and the masses of the recovered peptides were determined by LC-MS.
Measurement of FadB enzyme activity
A kinetic spectrophotometric assay was used to measure the enzymatic activity of recombinant FadB based on a previously described method [44]. The enzyme converts acetoacetyl-CoA to β-hydroxy butyryl-CoA in the presence of β-NADH. This reaction was measured by recording the decrease in NADPH absorption at 340 nm. One unit of enzyme activity was defined as conversion of 1 µmol acetoacetyl-CoA to β-hydroxy butyryl-CoA per minute at pH 7.3 at 37 °C in the presence of NADH.
Batch culture distal colon model system
An in vitro anaerobic batch culture system was used to simulate the main physiological and microbiological processes in the distal colon, including residence time, substrate availability and pH [45, 46]. The experiment was carried out in triplicate using faecal samples from three healthy volunteers. After obtaining verbal informed consent, a standard questionnaire to collect information regarding health status, drug use, clinical anamnesis and lifestyle was administered before the donor was asked to provide a faecal sample. No volunteers had received antibiotics, commercial probiotics or prebiotics, steroids or other drugs proven to have an impact on gut microbiota for at least 3 months before sampling. None of them had any history of gastrointestinal disorders. All healthy faecal donors had the experimental procedure explained to them and were provided with an opportunity to ask questions. The University of Reading research ethics committee exempted this study from review because no donors were involved in any intervention and waived the need for written consent because the samples were not collected by means of intervention. All faecal samples were collected on site, kept in an anaerobic cabinet (10 % H2, 10 % CO2, 80 % N2) and used within 15 min of collection. Samples were prepared on the day of the experiment and within 1 h of production, and were diluted to 1 : 10 (w/v) in anaerobic phosphate buffer (0.1 M; pH 7.4). Samples were homogenized in a stomacher for 2 min and the resulting slurry was inoculated into batch culture fermenters. The model was inoculated with S. aureus (˜2×1010 c.f.u. ml−1) as a single dose, suspended in colonic model media.
Survival of S. aureus was enumerated by fluorescence in situ hybridization (FISH), an efficient method for enumerating specific species in a mixed culture. Samples for FISH were fixed immediately in 4 % paraformaldehyde as described previously [47, 48], using Cy3-labelled Sau probe (Sigma-Aldrich) (Table 3), which is specific for this species [49].
Results
FadB is a bile salt-induced cell envelope protein
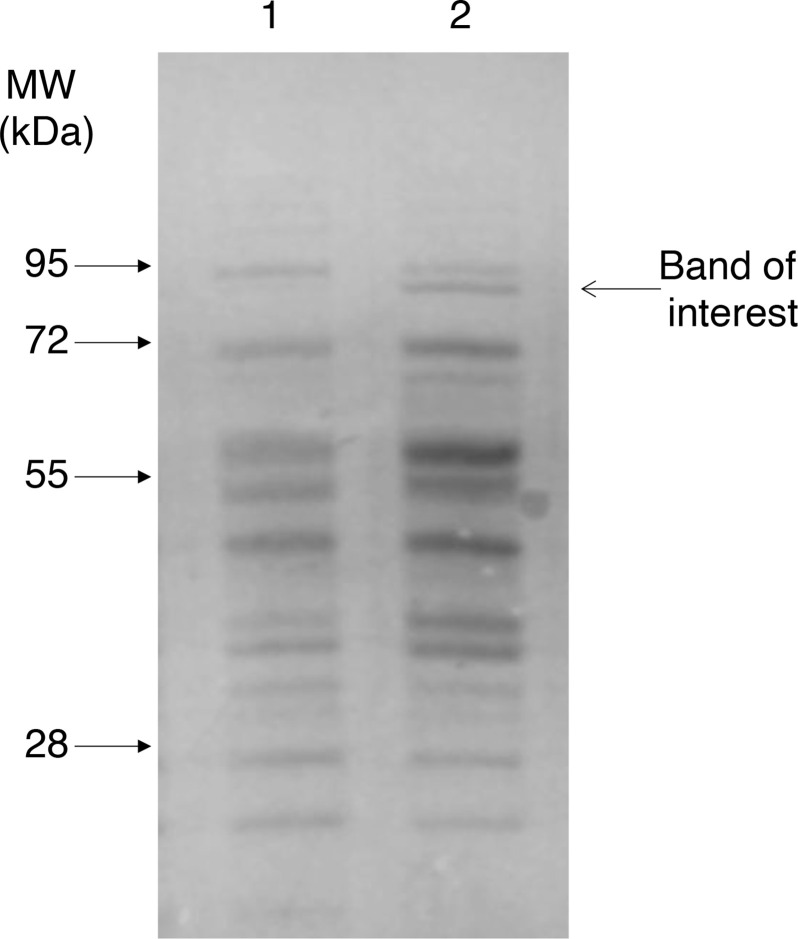
To determine whether exposure to bile salts caused differences in the cell envelope protein profile of S. aureus SH1000, cells were cultured in the presence of bile (8 % w/v bovine bile salts; Oxoid), which does not impede growth, until they reached OD600 ˜0.6 in TSB. SDS-PAGE showed the presence of a bile-induced protein of approximately 85 kDa (Fig. 1). The band was excised from the gel and submitted for analysis by MS (University of Birmingham, UK), revealing it to be a putative 3-hydroxyacyl-CoA dehydrogenase encoded by fadB (Table S1, available with the online version of this article).
Fig. 1.
Cell envelope proteins in bile-treated S. aureus . Coomassie-stained SDS-PAGE (12 %, w/v) gel of cell envelope extracts grown in the absence (lane 1) or presence (lane 2) of bovine bile (8 %, w/v).
Transcription of fadB was measured using qRT-PCR to determine whether transcription of the gene is induced by bile salts. S. aureus was grown in the presence of bile as described above and the levels of transcripts were quantified. The level of fadB mRNA in bile-treated cells was approximately four times higher than in untreated cells (Fig. 2).
Fig. 2.
Transcription of fadB is upregulated in the presence of bile acids. qRT-PCR was performed to quantify amounts of transcript in S. aureus SH1000. Data represent means±sd from three independent experiments. White circles indicate data points. P<0.01, Student’s t-test.
FadB mediates resistance to bile salts
We hypothesized that as FadB is found in the cell envelope of bile-treated cells, it may influence S. aureus bile acid sensitivity. To test this, an unmarked, in-frame ΔfadB strain was created in S. aureus SH1000. A mutant lacking an antibiotic resistance phenotype was necessary for subsequent use of our colonic model, where adding such genes to complex mixtures of gut microbes should be avoided. The mutant strain had no growth defect when grown on/in TSB solid or liquid medium in the absence of bile salts (results not shown). A selection of bile acids with differeing pKa values were used to test susceptibility of the mutant. S. aureus ΔfadB had a 2–3-fold reduced MIC for cholic acid and deoxycholic acid, but not chenodeoxycholic acid or conjugated bile acids (Table 4). In killing assays, the ΔfadB strain was significantly more sensitive than the parent (Fig. 3). Increased sensitivity of the mutant strain was only observed with certain unconjugated bile salts but it should be noted that as in previous studies, we were unable to determine the S. aureus MIC of conjugated bile salts as they were insoluble at >200 mM [32].
Table 4.
MICs of bile salts for S. aureus SH1000 and ΔfadB
|
Bile salt |
Wild-type (mM) |
ΔfadB (mM) |
|---|---|---|
|
CA |
22 |
7 |
|
DCA |
1.2 |
0.6 |
|
CDCA |
1.2 |
1.2 |
|
GCA |
>200 |
>200 |
|
TCA |
>200 |
>200 |
CA, sodium cholate; CDCA, sodium deoxycholate; DCA, sodium deoxycholate; GCA, sodium glycocholate; TCA, sodium taurocholate.
Fig. 3.

FadB protects S. aureus against the bactericidal activity of bile salts. Viability of S. aureus SH1000 (■) and ΔfadB (•) treated with (a) 2 mM cholic acid, (b) 0.25 mM deoxycholic acid and (c) 25 mM glycocholic acid. Data represent means±sd from three independent experiments. *P<0.05; all other time points P>0.05, Student’s t-test.
To confirm a role for fadB in resistance to bile salts, the gene was cloned under the control of the arabinose-inducible inducible PBAD promoter of plasmid pBAD/HisA, which allowed arabinose dose-dependent expression of FadB in E. coli JW3822, an isogenic fadB mutant of E. coli BW25113. E. coli JW3822 had a lower MIC for cholic acid, glycocholic acid and taurocholic acid than its parent (Table 5). Expression of FadB increased the MIC of cholic acid and conjugated bile salts in an arabinose-dependent manner (Table 5) and exclusion of arabinose reduced the MIC to the same level as the background strain lacking the plasmid. Similarly, expression of FadB in E. coli also decreased the bacteriostatic effects of bile salts on that bacterium in an arabinose dose-dependent fashion (Fig. 4).
Table 5.
MICs of bile salts for wild-type (BW25113), fadB mutant (JW3822) and recombinant E. coli expressing FadB at different levels of arabinose induction
|
Bile salt |
E. coli BW25113 |
JW3822 |
JW3822 pBAD |
E. coli JW3822 pfadB |
||
|---|---|---|---|---|---|---|
|
0 % Arabinose |
0.02 % Arabinose |
2 % Arabinose |
||||
|
CA |
60 |
30 |
30 |
30 |
50 |
50 |
|
DCA |
4 |
4 |
4 |
4 |
4 |
4 |
|
CDCA |
4 |
4 |
4 |
4 |
4 |
4 |
|
GCA |
120 |
80 |
80 |
80 |
100 |
100 |
|
TCA |
120 |
80 |
80 |
80 |
100 |
100 |
Inclusion of 2 % arabinose did not affect the MIC of the control strains.
CA, sodium cholate; CDCA, sodium deoxycholate; DCA, sodium deoxycholate; GCA, sodium glycocholate; TCA, sodium taurocholate.
Fig. 4.
Heterologous expression of FadB in E. coli protects against the bacteriostatic effects of bile salts. Data show the viability of E. coli JW3822 (fadB) and E. coli JW3822 (pfadB) cells in LB medium containing cholic acid (CA, 10 mM), deoxycholic acid (DCA, 2 mM), glycocholic acid (GCA, 50 mM) and taurocholic acid (TCA, 50 mM) and then grown for 16 h at 37 °C. Cell counts were determined by viable plate counting. Data represent means±sd from three independent experiments. *P<0.005, Student’s t-test of arabinose treated versus no arabinose.
S. aureus FadB is a dehydrogenase
FadB is proposed to convert acetoacetyl-CoA to hyrdoxybutyryl-CoA in the presence of β-NADH (Fig. 5a). Using purified rFadB (Fig. S1) this activity was demonstrated by measuring the decrease of NADPH absorption at 340 nm as described previously [44]. The enzyme showed catalytic activity at 0.53.5 mM of substrate acetoacetyl-CoA in the presence of 0.1 mM NADH (Fig. 5b), but no activity was observed in the absence of rFadB. Thus, S. aureus FadB was demonstrated to exhibit dehydrogenase activity in the presence of acetoacetyl-CoA.
Fig. 5.
Hydroxyacyl-CoA dehydrogenase enzyme assay. (a) Conversion of acetoacetyl-CoA to hyrdoxybutyryl-CoA in the presence of β-NADH. (b) The catalytic activity of the enzyme by converting acetoacetyl-CoA to hydroxybutyryl-CoA in the presence of NADH as a cofactor was determined spectrometrically (A 340). The serial dilution of the substrate (acetoacetyl-CoA) was used to measure the activity rate of the enzyme. Data represent means±sd from three independent experiments. No activity was observed in the absence of enzyme.
FadB is required for survival of S. aureus in a human gut model
To examine the role of FadB in survival of S. aureus under conditions found in the human distal colon, we used a temperature- and pH-controlled faecal batch culture model system (37 °C, pH 6.8) containing bile. In vivo studies of colonic bacteria are hampered by a lack of suitable animal models as they do not correctly simulate the physicochemical conditions or gut microbiota found in the human colon. We have previously used similar in vitro models to study the survival of S. aureus and the impact of infection on the host’s colonic microflora [32, 48].
We ran parallel models, each containing either S. aureus ΔfadB or the parental wild-type. The culture vessel was inoculated with S. aureus to a final concentration of 1010 c.f.u. ml−1 in a single dose. Survival of S. aureus ΔfadB was significantly attenuated compared to that of its parental strain (Fig. 6). Thus, FadB mediates S. aureus survival under human colonic conditions.
Fig. 6.
FadB is required for S. aureus survival in a human distal colon model. Survival of S. aureus SH1000 (■) and ΔfadB (×) cells in a human colonic model. Samples were taken at 4, 8, 24 and 48 h post-infection. Data represent means±sd from three independent experiments. *P<0.01, Student’s t-test.
Discussion
The interaction between S. aureus and its human host is complex and is built upon a range of interactions and adaptations. As a pathogen of great medical significance and as a common commensal, S. aureus must be able to resist host innate antimicrobials such as peptides, fatty acids and bile, a complex cocktail composed principally of bile salts, phospholipids, cholesterol, proteins and bilirubin [50]. The human liver secretes up to 1 litre of bile per day into the gut [27] and molecules secreted by bacteria during infection, including S. aureus , are an important cause of metabolic cholestasis, an inability of hepatocytes to produce bile [51]. Additionally, bile salts are present in human serum at micromolar concentrations [52–54].
In addition to anti-bacterial effects, bile salts serve endocrine functions [55–57], consequently regulating their own synthesis, conjugation, transport and detoxification, as well as lipid, glucose and energy homeostasis [58]. Moreover, bile salts have an important role in maintaining intestinal barrier function and induce genes encoding antimicrobial peptides and lectins [59]. Thus, by modulating the composition of the bile acid pool in the gut, bacteria can exert multiple effects on host physiology.
Many antibacterial agents act by disrupting the cytoplasmic membrane resulting in loss of proton gradients and electrical potential across the membrane, leading eventually to cell death [60]. Due to their structural and chemical properties, bile salts are generally considered to be weak acids which decrease intracellular pH and dissipate transmembrane potential. In S. aureus and other bacteria, bile salts act by disrupting the cytoplasmic membrane, which results in dissipation of the membrane’s proton gradient and electrical potential, resulting in cell death [61, 62].
In the human colon, bile salts are modified by the normal microbiota [63]. The ‘gateway’ modification is usually regarded as hydrolysis of an amino acid conjugate by bile salt hydrolase [64]. However, unconjugated bile salts are more active against S. aureus than either glycocholic or taurocholic acids [32, 62]. Major modifications include deconjugation, oxidation of hydroxyl groups at C-3, C-7 and C-12, and 7α/β-dehydroxylation [65, 66]. The ability of bacteria to remove the 3-, 7- and 12-hydroxyl groups is dependent in part on 3α-, 7α- and 12α-hydroxysteroid dehydrogenase (HSDH) activity [67–71]. Members of the gut microbiome are capable of removing the 7α-hydroxyl group from cholic acid and chenodeoxycholic acid, forming deoxycholic acid and lithocholic acid, respectively [66, 72]. Deoxycholic acid and chenodeoxycholic acid are the principal bile acids found in the stool of healthy humans [73].
In E. coli , fadB encodes a 3-hyrdroxyacyl-CoA dehydrogenase [74, 75] involved in fatty acid degradation [76]. Bile stress commonly induces proteins involved in fatty acid metabolism [77] and, in Salmonella enterica , fad genes are upregulated in response to bile exposure [78].
In response to the detergent action of bile acids, bacteria change the lipid metabolism and therefore the lipid and protein profiles of their cell membrane, which can lead to alterations in the physical properties of the membrane [79, 80]. Modification of fatty acid composition can maintain membrane fluidity, a phenomenon known as homeoviscous adaptation [81]. In Lactobacillus reuteri , these changes include decreased amounts of phospholipids and a lower ratio of saturated to unsaturated fatty acids, influencing the physical properties of the cell membrane, potentially adapting the bacterium to the conditions found in the human gut [82].
In this study, we show that FadB, a putative 3-hydroxyacyl-CoA dehydrogenase, protects S. aureus against the bactericidal activity of bile acids, including cholic acid. Bacteria commonly modify bile acids in vivo including dehydrogenation, and thus the activity of FadB may result in a derivative of cholic acid that is less toxic to S. aureus .
Alternatively, bile salts have well-characterized effects on bacterial membranes, showing greater lytic activity towards membranes with increasing fluidity [83]. FadB shortens fatty acids [84–86] and may thus increase membrane fluidity, rendering the cell more susceptible to the surfactant nature of bile acids. It remains to be determined whether either of these two phenomena accounts for the resistance phenotype observed in this study, but it seems entirely plausible that either or both could, at least in part, account for our observations.
Supplementary Data
Funding information
This work was funded by an Iraqi Government PhD studentship to A.A.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Footnotes
Abbreviations: FISH, Fluorescence in situ hybridisation; MIC, Minimum inhibitory concentration; MRSA, methicillin-resistant S. aureus; NADH, Nicotinamide adenine dinucleotide; qRT-PCR, quantitative real-time PCR; TSB, Tryptic soy broth.
One supplementary figure and one supplementary table are available with the online version of this article.
References
- 1.Peacock SJ, de Silva I, Lowy FD. What determines nasal carriage of Staphylococcus aureus? Trends Microbiol. 2001;9:605–610. doi: 10.1016/s0966-842x(01)02254-5. [DOI] [PubMed] [Google Scholar]
- 2.Wertheim HF, Vos MC, Ott A, van Belkum A, Voss A, et al. Risk and outcome of nosocomial Staphylococcus aureus bacteraemia in nasal carriers versus non-carriers. Lancet. 2004;364:703–705. doi: 10.1016/S0140-6736(04)16897-9. [DOI] [PubMed] [Google Scholar]
- 3.Smyth DS, Kafer JM, Wasserman GA, Velickovic L, Mathema B, et al. Nasal carriage as a source of agr-defective Staphylococcus aureus bacteremia. J Infect Dis. 2012;206:1168–1177. doi: 10.1093/infdis/jis483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Acton DS, Tempelmans Plat-Sinnige M, van Wamel W, de Groot N, van Belkum A. Intestinal carriage of Staphylococcus aureus: how does its frequency compare with that of nasal carriage and what is its clinical impact? Eur J Clin Microbiol Infect Dis. 2009;28:115–127. doi: 10.1007/s10096-008-0602-7. [DOI] [PubMed] [Google Scholar]
- 5.Batra R, Eziefula AC, Wyncoll D, Edgeworth J. Throat and rectal swabs may have an important role in MRSA screening of critically ill patients. Intensive Care Med. 2008;34:1703–1706. doi: 10.1007/s00134-008-1153-1. [DOI] [PubMed] [Google Scholar]
- 6.Buehlmann M, Frei R, Fenner L, Dangel M, Fluckiger U, et al. Highly effective regimen for decolonization of methicillin-resistant Staphylococcus aureus carriers. Infect Control Hosp Epidemiol. 2008;29:510–516. doi: 10.1086/588201. [DOI] [PubMed] [Google Scholar]
- 7.Dupeyron C, Campillo B, Bordes M, Faubert E, Richardet J-P, et al. A clinical trial of mupirocin in the eradication of methicillin-resistant Staphylococcus aureus nasal carriage in a digestive disease unit. J Hosp Infect. 2002;52:281–287. doi: 10.1053/jhin.2002.1287. [DOI] [PubMed] [Google Scholar]
- 8.Silvestri L, Milanese M, Oblach L, Fontana F, Gregori D, et al. Enteral vancomycin to control methicillin-resistant Staphylococcus aureus outbreak in mechanically ventilated patients. Am J Infect Control. 2002;30:391–399. doi: 10.1067/mic.2002.122255. [DOI] [PubMed] [Google Scholar]
- 9.Squier C, Rihs JD, Risa KJ, Sagnimeni A, Wagener MM, et al. Staphylococcus aureus rectal carriage and its association with infections in patients in a surgical intensive care unit and a liver transplant unit. Infect Control Hosp Epidemiol. 2002;23:495–501. doi: 10.1086/502095. [DOI] [PubMed] [Google Scholar]
- 10.Bhalla A, Aron DC, Donskey CJ. Staphylococcus aureus intestinal colonization is associated with increased frequency of S. aureus on skin of hospitalized patients. BMC Infect Dis. 2007;7:1–7. doi: 10.1186/1471-2334-7-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boyce JM. Environmental contamination makes an important contribution to hospital infection. J Hosp Infect. 2007;65 Suppl 2:50–54. doi: 10.1016/S0195-6701(07)60015-2. [DOI] [PubMed] [Google Scholar]
- 12.Boyce JM, Havill NL, Maria B. Frequency and possible infection control implications of gastrointestinal colonization with methicillin-resistant Staphylococcus aureus . J Clin Microbiol. 2005;43:5992–5995. doi: 10.1128/JCM.43.12.5992-5995.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boyce JM, Havill NL, Otter JA, Adams NM. Widespread environmental contamination associated with patients with diarrhea and methicillin-resistant Staphylococcus aureus colonization of the gastrointestinal tract. Infect Control Hosp Epidemiol. 2007;28:1142–1147. doi: 10.1086/520737. [DOI] [PubMed] [Google Scholar]
- 14.Masaki H, Asoh N, Watanabe H, Tao M, Watanabe K, et al. Possible relationship between Staphylococcus aureus colonizing the respiratory tract and rectum and S. aureus isolated in a geriatric hospital environment. Intern Med. 2003;42:281–282. doi: 10.2169/internalmedicine.42.281. [DOI] [PubMed] [Google Scholar]
- 15.Claassen-Weitz S, Shittu AO, Ngwarai MR, Thabane L, Nicol MP, et al. Fecal carriage of Staphylococcus aureus in the hospital and community setting: a systematic review. Front Microbiol. 2016;7:449. doi: 10.3389/fmicb.2016.00449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Senn L, Clerc O, Zanetti G, Basset P, Prod’hom G, et al. The stealthy superbug: the role of asymptomatic enteric carriage in maintaining a long-term hospital outbreak of ST228 methicillin-resistant Staphylococcus aureus . mBio. 2016;7:e02039–15. doi: 10.1128/mBio.02039-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Belkum A. Hidden Staphylococcus aureus carriage: overrated or underappreciated? mBio. 2016;7:e00079–16. doi: 10.1128/mBio.00079-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Froberg MK, Palavecino E, Dykoski R, Gerding DN, Peterson LR, et al. Staphylococcus aureus and Clostridium difficile cause distinct pseudomembranous intestinal diseases. Clin Infect Dis. 2004;39:747–750. doi: 10.1086/423273. [DOI] [PubMed] [Google Scholar]
- 19.Adlerberth I, Strachan DP, Matricardi PM, Ahrné S, Orfei L, et al. Gut microbiota and development of atopic eczema in 3 European birth cohorts. J Allergy Clin Immunol. 2007;120:343–350. doi: 10.1016/j.jaci.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 20.Björkstén B, Naaber P, Sepp E, Mikelsaar M. The intestinal microflora in allergic Estonian and Swedish 2-year-old children. Clin Exp Allergy. 1999;29:342–346. doi: 10.1046/j.1365-2222.1999.00560.x. [DOI] [PubMed] [Google Scholar]
- 21.Lindberg E, Adlerberth I, Hesselmar B, Saalman R, Strannegård I-L, et al. High rate of transfer of Staphylococcus aureus from parental skin to infant gut flora. J Clin Microbiol. 2004;42:530–534. doi: 10.1128/JCM.42.2.530-534.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lindberg E, Nowrouzian F, Adlerberth I, Wold AE. Long-time persistence of superantigen-producing Staphylococcus aureus strains in the intestinal microflora of healthy infants. Pediatr Res. 2000;48:741–747. doi: 10.1203/00006450-200012000-00007. [DOI] [PubMed] [Google Scholar]
- 23.Lundell A-C, Adlerberth I, Lindberg E, Karlsson H, Ekberg S, et al. Increased levels of circulating soluble CD14 but not CD83 in infants are associated with early intestinal colonization with Staphylococcus aureus . Clin Exp Allergy. 2007;37:62–71. doi: 10.1111/j.1365-2222.2006.02625.x. [DOI] [PubMed] [Google Scholar]
- 24.Hess DJ, Garni RM, Henry-Stanley MJ, Wells CL. Escherichia coli modulates extraintestinal spread of Staphylococcus aureus . Shock. 2005;24:376–381. doi: 10.1097/01.shk.0000180615.75822.fe. [DOI] [PubMed] [Google Scholar]
- 25.Nakamura Y, Aramaki Y, Kakiuchi T. A mouse model for postoperative fatal enteritis due to Staphylococcus infection. J Surg Res. 2001;96:35–43. doi: 10.1006/jsre.2000.6043. [DOI] [PubMed] [Google Scholar]
- 26.Krezalek MA, Hyoju S, Zaborin A, Okafor E, Chandrasekar L, et al. Can methicillin-resistant Staphylococcus aureus silently travel from the gut to the wound and cause postoperative infection? Modeling the “Trojan Horse Hypothesis.”. Ann Surg. 2018;267:749–758. doi: 10.1097/SLA.0000000000002173. [DOI] [PubMed] [Google Scholar]
- 27.Begley M, Gahan CG, Hill C. The interaction between bacteria and bile. FEMS Microbiol Rev. 2005;29:625–651. doi: 10.1016/j.femsre.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 28.Peterson KM. Expression of Vibrio cholerae virulence genes in response to environmental signals. Curr Issues Intest Microbiol. 2002;3:29–38. [PubMed] [Google Scholar]
- 29.Prouty A, Gunn J. Salmonella enterica serovar Typhimurium invasion is repressed in the presence of bile. Infect Immun. 2000;68:6763–6769. doi: 10.1128/IAI.68.12.6763-6769.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crawford RW, Keestra AM, Winter SE, Xavier MN, Tsolis RM, et al. Very long O-antigen chains enhance fitness during Salmonella-induced colitis by increasing bile resistance. PLoS Pathog. 2012;8:e1002918. doi: 10.1371/journal.ppat.1002918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nesper J, Schild S, Lauriano CM, Kraiss A, Klose KE, et al. Role of Vibrio cholerae O139 surface polysaccharides in intestinal colonization. Infect Immun. 2002;70:5990–5996. doi: 10.1128/IAI.70.11.5990-5996.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sannasiddappa TH, Hood GA, Hanson KJ, Costabile A, Gibson GR, et al. Staphylococcus aureus MnhF mediates cholate efflux and facilitates survival under human colonic conditions. Infect Immun. 2015;83:2350–2357. doi: 10.1128/IAI.00238-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prouty AM, Brodsky IE, Falkow S, Gunn JS. Bile-salt-mediated induction of antimicrobial and bile resistance in Salmonella typhimurium . Microbiol. 2004;150:775–783. doi: 10.1099/mic.0.26769-0. [DOI] [PubMed] [Google Scholar]
- 34.Trainor EA, Horton KE, Savage PB, Testerman TL, McGee DJ. Role of the HefC efflux pump in Helicobacter pylori cholesterol-dependent resistance to ceragenins and bile salts. Infect Immun. 2011;79:88–97. doi: 10.1128/IAI.00974-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin J, Sahin O, Michel LO, Zhang Q. Critical role of multidrug efflux pump CmeABC in bile resistance and in vivo colonization of Campylobacter jejuni . Infect Immun. 2003;71:4250–4259. doi: 10.1128/IAI.71.8.4250-4259.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Novick R. Properties of a cryptic high-frequency transducing phage in Staphylococcus aureus . Virology. 1967;33:155–166. doi: 10.1016/0042-6822(67)90105-5. [DOI] [PubMed] [Google Scholar]
- 37.Clarke SR, Harris LG, Richards RG, Foster SJ. Analysis of Ebh, a 1.1-megadalton cell wall-associated fibronectin-binding protein of Staphylococcus aureus . Infect Immun. 2002;70:6680–6687. doi: 10.1128/IAI.70.12.6680-6687.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen L, Shopsin B, Zhao Y, Smyth D, Wasserman GA, et al. Real-time nucleic acid sequence-based amplification assay for rapid detection and quantification of agr functionality in clinical Staphylococcus aureus isolates. J Clin Microbiol. 2012;50:657–661. doi: 10.1128/JCM.06253-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kenny JG, Ward D, Josefsson E, Jonsson I-M, Hinds J, et al. The Staphylococcus aureus response to unsaturated long chain free fatty acids: survival mechanisms and virulence implications. PLoS One. 2009;4:e4344. doi: 10.1371/journal.pone.0004344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Valle J, Toledo-Arana A, Berasain C, Ghigo J-M, Amorena B, et al. SarA and not sigmaB is essential for biofilm development by Staphylococcus aureus . Mol Microbiol. 2003;48:1075–1087. doi: 10.1046/j.1365-2958.2003.03493.x. [DOI] [PubMed] [Google Scholar]
- 41.Wolz C, Goerke C, Landmann R, Zimmerli W, Fluckiger U. Transcription of clumping factor A in attached and unattached Staphylococcus aureus in vitro and during device-related infection. Infect Immun. 2002;70:2758–2762. doi: 10.1128/IAI.70.6.2758-2762.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee N. The Operon. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1980. Molecular aspects of ara regulation; pp. 389–410. [Google Scholar]
- 43.Schleif R. DNA looping. Annu Rev Biochem. 1992;61:199–223. doi: 10.1146/annurev.bi.61.070192.001215. [DOI] [PubMed] [Google Scholar]
- 44.Lynen F, Wieland O. [94] β-ketoreductase. Meth Enzymol. 1955;1:566–573. [Google Scholar]
- 45.Alarifi S, Bell A, Walton G. In vitro fermentation of gum acacia - impact on the faecal microbiota. Int J Food Sci Nutr. 2018;69:696–704. doi: 10.1080/09637486.2017.1404970. [DOI] [PubMed] [Google Scholar]
- 46.Monteagudo-Mera A, Chatzifragkou A, Kosik O, Gibson G, Lovegrove A, et al. Evaluation of the prebiotic potential of arabinoxylans extracted from wheat distillers’ dried grains with solubles (DDGS) and in-process samples. Appl Microbiol Biotechnol. 2018;102:7577–7587. doi: 10.1007/s00253-018-9171-6. [DOI] [PubMed] [Google Scholar]
- 47.Martín-Peláez S, Gibson GR, Martín-Orúe SM, Klinder A, Rastall RA, et al. In vitro fermentation of carbohydrates by porcine faecal inocula and their influence on Salmonella Typhimurium growth in batch culture systems. FEMS Microbiol Ecol. 2008;66:608–619. doi: 10.1111/j.1574-6941.2008.00610.x. [DOI] [PubMed] [Google Scholar]
- 48.Sannasiddappa TH, Costabile A, Gibson GR, Clarke SR. The influence of Staphylococcus aureus on gut microbial ecology in an in vitro continuous culture human colonic model system. PLoS One. 2011;6:e23227. doi: 10.1371/journal.pone.0023227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kempf VA, Trebesius K, Autenrieth IB. Fluorescent in situ hybridization allows rapid identification of microorganisms in blood cultures. J Clin Microbiol. 2000;38:830–838. doi: 10.1128/JCM.38.2.830-838.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Esteller A. Physiology of bile secretion. World J Gastroenterol. 2008;14:5641–5649. doi: 10.3748/wjg.14.5641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Minuk GY, Rascanin N, Sarjeant ES, Pai CH. Sepsis and cholestasis: the in vitro effects of bacterial products on 14C-taurocholate uptake by isolated rat hepatocytes. Liver. 1986;6:199–204. doi: 10.1111/j.1600-0676.1986.tb01066.x. [DOI] [PubMed] [Google Scholar]
- 52.Makino I, Nakagawa S, Mashimo K. Conjugated and unconjugated serum bile acid levels in patients with hepatobiliary diseases. Gastroenterology. 1969;56:1033–1039. [PubMed] [Google Scholar]
- 53.Rudman D, Kendall FE. Bile acid content of human serum. I. Serum bile acids in patients with hepatic disease. J Clin Invest. 1957;36:530–537. doi: 10.1172/JCI103450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xie G, Wang Y, Wang X, Zhao A, Chen T, et al. Profiling of serum bile acids in a healthy Chinese population using UPLC-MS/MS. J Proteome Res. 2015;14:850–859. doi: 10.1021/pr500920q. [DOI] [PubMed] [Google Scholar]
- 55.Kawamata Y, Fujii R, Hosoya M, Harada M, Yoshida H, et al. AG protein-coupled receptor responsive to bile acids. J Biol Chem. 2003;278:9435–9440. doi: 10.1074/jbc.M209706200. [DOI] [PubMed] [Google Scholar]
- 56.Makishima M, Okamoto AY, Repa JJ, Tu H, Learned RM, et al. Identification of a nuclear receptor for bile acids. Science. 1999;284:1362–1365. doi: 10.1126/science.284.5418.1362. [DOI] [PubMed] [Google Scholar]
- 57.Parks DJ, Blanchard SG, Bledsoe RK, Chandra G, Consler TG, et al. Bile acids: natural ligands for an orphan nuclear receptor. Science. 1999;284:1365–1368. doi: 10.1126/science.284.5418.1365. [DOI] [PubMed] [Google Scholar]
- 58.Li T, Chiang JY. Bile acids as metabolic regulators. Curr Opin Gastroenterol. 2015;31:159–165. doi: 10.1097/MOG.0000000000000156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.D’Aldebert E, Biyeyeme Bi Mve M-J, Mergey M, Wendum D, Firrincieli D, et al. Bile salts control the antimicrobial peptide cathelicidin through nuclear receptors in the human biliary epithelium. Gastroenterology. 2009;136:1435–1443. doi: 10.1053/j.gastro.2008.12.040. [DOI] [PubMed] [Google Scholar]
- 60.Nelson M, Grier M, Barbaro S, Ismail M. Polyfunctional antibiotics affecting bacterial membrane dynamics. AIAMC. 2009;8:3–16. doi: 10.2174/187152109787047779. [DOI] [Google Scholar]
- 61.Kurdi P, Kawanishi K, Mizutani K, Yokota A. Mechanism of growth inhibition by free bile acids in lactobacilli and bifidobacteria. J Bacteriol. 2006;188:1979–1986. doi: 10.1128/JB.188.5.1979-1986.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sannasiddappa TH, Lund PA, Clarke SR. In vitro antibacterial activity of unconjugated and conjugated bile salts on Staphylococcus aureus . Front Microbiol. 2017;8:1581. doi: 10.3389/fmicb.2017.01581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hofmann AF. The continuing importance of bile acids in liver and intestinal disease. Arch Intern Med. 1999;159:2647–2658. doi: 10.1001/archinte.159.22.2647. [DOI] [PubMed] [Google Scholar]
- 64.Jones BV, Begley M, Hill C, Gahan CGM, Marchesi JR. Functional and comparative metagenomic analysis of bile salt hydrolase activity in the human gut microbiome. Proc Natl Acad Sci U S A. 2008;105:13580–13585. doi: 10.1073/pnas.0804437105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Buffie CG, Bucci V, Stein RR, McKenney PT, Ling L, et al. Precision microbiome reconstitution restores bile acid mediated resistance to Clostridium difficile . Nature. 2015;517:205–208. doi: 10.1038/nature13828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ridlon JM, Kang D-J, Hylemon PB. Bile salt biotransformations by human intestinal bacteria. J Lipid Res. 2006;47:241–259. doi: 10.1194/jlr.R500013-JLR200. [DOI] [PubMed] [Google Scholar]
- 67.Baron SF, Franklund CV, Hylemon PB. Cloning, sequencing, and expression of the gene coding for bile acid 7 alpha-hydroxysteroid dehydrogenase from Eubacterium sp. strain VPI 12708. J Bacteriol. 1991;173:4558–4569. doi: 10.1128/jb.173.15.4558-4569.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Doden H, Sallam LA, Devendran S, Ly L, Doden G, et al. Metabolismof oxo-bile acids and characterization of recombinant 12α-hydroxysteroiddehydrogenases from bile acid 7α-dehydroxylating human gut bacteria. Appl Environ Microbiol. 2018;84:e00235-18. doi: 10.1128/AEM.00235-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mallonee DH, Lijewski MA, Hylemon PB. Expression in Escherichia coli and characterization of a bile acid-inducible 3 alpha-hydroxysteroid dehydrogenase from Eubacterium sp. strain VPI 12708. Curr Microbiol. 1995;30:259–263. doi: 10.1007/BF00295498. [DOI] [PubMed] [Google Scholar]
- 70.Ridlon JM, Kang D-J, Hylemon PB. Isolation and characterization of a bile acid inducible 7alpha-dehydroxylating operon in Clostridium hylemonae TN271. Anaerobe. 2010;16:137–146. doi: 10.1016/j.anaerobe.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wells JE, Hylemon PB. Identification and characterization of a bile acid 7alpha-dehydroxylation operon in Clostridium sp. strain TO-931, a highly active 7alpha-dehydroxylating strain isolated from human feces. Appl Environ Microbiol. 2000;66:1107–1113. doi: 10.1128/AEM.66.3.1107-1113.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ridlon JM, Harris SC, Bhowmik S, Kang D-J, Hylemon PB. Consequences of bile salt biotransformations by intestinal bacteria. Gut Microbes. 2016;7:22–39. doi: 10.1080/19490976.2015.1127483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kakiyama G, Muto A, Takei H, Nittono H, Murai T, et al. A simple and accurate HPLC method for fecal bile acid profile in healthy and cirrhotic subjects: validation by GC-MS and LC-MS. J Lipid Res. 2014;55:978–990. doi: 10.1194/jlr.D047506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yang SY, Li JM, He XY, Cosloy SD, Schulz H. Evidence that the fadB gene of the fadAB operon of Escherichia coli encodes 3-hydroxyacyl-coenzyme A (CoA) epimerase, delta 3-cis-delta 2-trans-enoyl-CoA isomerase, and enoyl-CoA hydratase in addition to 3-hydroxyacyl-CoA dehydrogenase. J Bacteriol. 1988;170:2543–2548. doi: 10.1128/jb.170.6.2543-2548.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yang SY, Schulz H. The large subunit of the fatty acid oxidation complex from Escherichia coli is a multifunctional polypeptide. Evidence for the existence of a fatty acid oxidation operon (fad AB) in Escherichia coli . J Biol Chem. 1983;258:9780–9785. [PubMed] [Google Scholar]
- 76.Pramanik A, Pawar S, Antonian E, Schulz H. Five different enzymatic activities are associated with the multienzyme complex of fatty acid oxidation from Escherichia coli . J Bacteriol. 1979;137:469–473. doi: 10.1128/jb.137.1.469-473.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bustos AY, Font de Valdez G, Fadda S, Taranto MP. New insights into bacterial bile resistance mechanisms: the role of bile salt hydrolase and its impact on human health. Food Res Int. 2018;112:250–262. doi: 10.1016/j.foodres.2018.06.035. [DOI] [PubMed] [Google Scholar]
- 78.Johnson R, Ravenhall M, Pickard D, Dougan G, Byrne A, et al. Comparison of Salmonella enterica Serovars Typhi and Typhimurium reveals typhoidal serovar-specific responses to bile. Infect Immun. 2018;86:e00490-17. doi: 10.1128/IAI.00490-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ruiz L, Margolles A, Sánchez B. Bile resistance mechanisms in Lactobacillus and Bifidobacterium . Front Microbiol. 2013;4:396. doi: 10.3389/fmicb.2013.00396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sánchez B, Champomier-Vergès M-C, Stuer-Lauridsen B, Ruas-Madiedo P, Anglade P, et al. Adaptation and response of Bifidobacterium animalis subsp. lactis to bile: a proteomic and physiological approach. Appl Environ Microbiol. 2007;73:6757–6767. doi: 10.1128/AEM.00637-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Murga MALF, de Valdez GF, Disalvo EA. Effect of lipid composition on the stability of cellular membranes during freeze-thawing of Lactobacillus acidophilus grown at different temperatures. Arch Biochem Biophys. 2001;388:179–184. doi: 10.1006/abbi.2001.2274. [DOI] [PubMed] [Google Scholar]
- 82.Taranto MP, Fernandez Murga ML, Lorca G, de Valdez GF. Bile salts and cholesterol induce changes in the lipid cell membrane of Lactobacillus reuteri . J Appl Microbiol. 2003;95:86–91. doi: 10.1046/j.1365-2672.2003.01962.x. [DOI] [PubMed] [Google Scholar]
- 83.Lowe PJ, Coleman R. Membrane fluidity and bile salt damage. Biochim Biophys Acta. 1981;640:55–65. doi: 10.1016/0005-2736(81)90531-9. [DOI] [PubMed] [Google Scholar]
- 84.Black PN, DiRusso CC. Molecular and biochemical analyses of fatty acid transport, metabolism, and gene regulation in Escherichia coli . Biochim Biophys Acta. 1994;1210:123–145. doi: 10.1016/0005-2760(94)90113-9. [DOI] [PubMed] [Google Scholar]
- 85.Campbell JW, Morgan-Kiss RM, Cronan JE. A new Escherichia coli metabolic competency: growth on fatty acids by a novel anaerobic beta-oxidation pathway. Mol Microbiol. 2003;47:793–805. doi: 10.1046/j.1365-2958.2003.03341.x. [DOI] [PubMed] [Google Scholar]
- 86.Pavoncello V, Barras F, Bouveret E. Degradation of exogenous fatty acids in Escherichia coli . Biomolecules. 2022;12:1019. doi: 10.3390/biom12081019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Horsburgh MJ, Aish JL, White IJ, Shaw L, Lithgow JK, et al. σB modulates virulence determinant expression and stress resistance: characterization of a functional rsbU strain derived from Staphylococcus aureus 8325-4. J Bacteriol. 2002;184:5457–5467. doi: 10.1128/JB.184.19.5457-5467.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kreiswirth BN, Löfdahl S, Betley MJ, O’Reilly M, Schlievert PM, et al. The toxic shock syndrome exotoxin structural gene is not detectably transmitted by a prophage. Nature. 1983;305:709–712. doi: 10.1038/305709a0. [DOI] [PubMed] [Google Scholar]
- 89.Baba T, Ara T, Hasegawa M, Takai Y, Okumura Y, et al. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol Syst Biol. 2006;2:2006.0008. doi: 10.1038/msb4100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Arnaud M, Chastanet A, Débarbouillé M. New vector for efficient allelic replacement in naturally nontransformable, low-GC-content, gram-positive bacteria. Appl Environ Microbiol. 2004;70:6887–6891. doi: 10.1128/AEM.70.11.6887-6891.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Guzman LM, Belin D, Carson MJ, Beckwith J. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J Bacteriol. 1995;177:4121–4130. doi: 10.1128/jb.177.14.4121-4130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.