Abstract
During the growth of Clostridium cellulolyticum in chemostat cultures with ammonia as the growth-limiting nutrient, as much as 30% of the original cellobiose consumed by C. cellulolyticum was converted to cellotriose, glycogen, and polysaccharides regardless of the specific growth rates. Whereas the specific consumption rate of cellobiose and of the carbon flux through glycolysis increased, the carbon flux through the phosphoglucomutase slowed. The limitation of the path through the phosphoglucomutase had a great effect on the accumulation of glucose 1-phosphate (G1P), the precursor of cellotriose, exopolysaccharides, and glycogen. The specific rates of biosynthesis of these compounds are important since as much as 16.7, 16.0, and 21.4% of the specific rate of cellobiose consumed by the cells could be converted to cellotriose, exopolysaccharides, and glycogen, respectively. With the increase of the carbon flux through glycolysis, the glucose 6-phosphate (G6P) pool decreased, whereas the G1P pool increased. Continuous culture experiments showed that glycogen biosynthesis was associated with rapid growth. The same result was obtained in batch culture, where glycogen biosynthesis reached a maximum during the exponential growth phase. Glycogen synthesis in C. cellulolyticum was also not subject to stimulation by nutrient limitation. Flux analyses demonstrate that G1P and G6P, connected by the phosphoglucomutase reaction, constitute important branch points for the distribution of carbon fluxes inside and outside cells. From this study it appears that the properties of the G1P-G6P branch points have been selected to control excretion of carbon surplus and to dissipate excess energy, whereas the pyruvate-acetyl coenzyme A branch points chiefly regulate the redox balance of the carbon catabolism as was shown previously (E. Guedon et al., J. Bacteriol. 181:3262–3269, 1999).
Clostridium cellulolyticum, a strictly anaerobic cellulolytic bacterium, was isolated from decayed grass (18) and degrades cellulose by using a complex cellulolytic system called cellulosome (11). The cellulosome, wherein the cellulases were found to be organized into a high-molecular-weight, cellulolytic complex, has been extensively investigated (2, 27), while few studies have focused on the carbon metabolic pathway, mainly in C. cellulolyticum (5). Most studies of the carbon metabolism in cellulolytic clostridias have been performed with cellobiose, the major end product of the degradation process, which is taken up and assimilated by the cells (5, 16, 28). Our recent study (7) demonstrated that when C. cellulolyticum was grown in continuous cellobiose-limited culture, there was a shift from an acetate-ethanol fermentation at low levels of carbon flow to a lactate-ethanol fermentation at high catabolic rates; in addition, increasing levels of pyruvate in the extracellular medium were detected as the dilution rate increased. The pyruvate overflow suggested that the carbon flow through glycolysis was higher than the rate of processing by pyruvate-ferredoxin oxidoreductase (7) (Fig. 1). Consequently, under such conditions, the rates of energy production in the catabolic pathway were not correlated with the anabolic energy requirements, i.e., more ATP was produced than was needed by the biosynthetic and maintenance demands (25). Furthermore, because lignocellulosic compounds usually contain high levels of carbon and a low levels of nitrogen, growth of C. cellulolyticum on these compounds would lead to an excess of energy, and energy-spilling reactions must be utilized. In addition, it is also necessary to overcome the potentially deleterious osmotic effects of the accumulation of surplus intracellular metabolites (19). There are different types of energy-consuming reactions selected by the bacteria during the course of evolution; for instance, (i) an overflow metabolism, wherein bacteria excrete or leak partially oxidized metabolites (29); (ii) metabolic shifts, where bacteria can change their end products and alter ATP production (30); (iii) futile cycles (15); and (iv) the synthesis of intra- and extracellular polysaccharides, which are energy-consuming processes and limit the carbon flow toward glycolysis and ATP production (20–22). In addition to pyruvate, extracellular polysaccharides were previously found to be secreted by C. cellulolyticum at high rates of cellobiose consumption (7), leading to the belief that carbon flow was regulated at the branch point of the glucose phosphate pools (Fig. 1). This kind of regulation is further suggested by the fact that growth of C. cellulolyticum was inhibited in the presence of excess carbon (8). The aim of the present study was to investigate how changes in catabolic flux affected (i) the intracellular turnover of glucose 1-phosphate (G1P) and glucose 6-phosphate (G6P) and (ii) the distribution of the carbon fluxes inside and outside the cells.
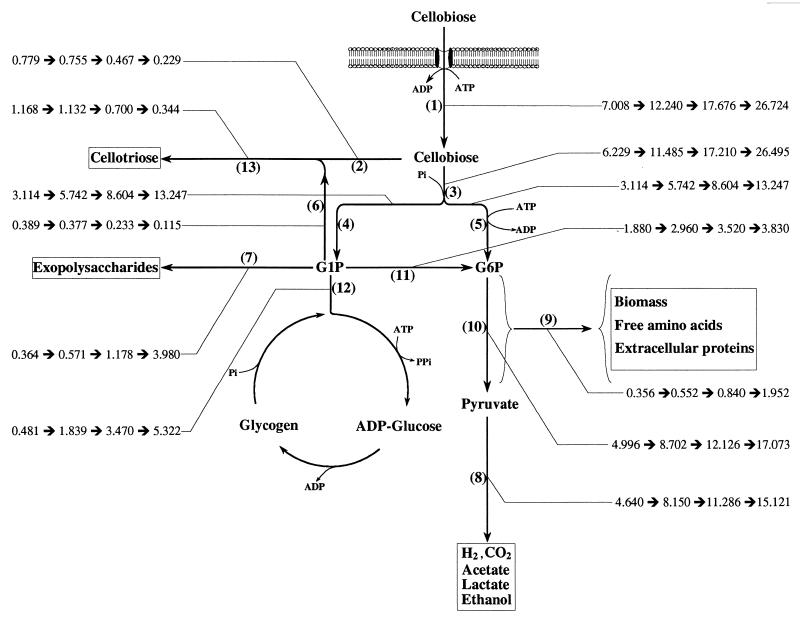
FIG. 1.
Estimation of flux distribution within the central metabolic pathways based on the steady-state kinetic data from continuous culture of C. cellulolyticum grown on cellobiose at four dilution rates (0.013, 0.036, 0.066, and 0.115 h−1). Fluxes are calculated according to the values in Table 1 and Table 2. All fluxes are expressed as meqC per gram of cells per hour and are indicated by a thin line. Specific rates: 1, cellobiose consumption; 2, cellobiose toward cellotriose formation; 3, cellobiose toward G1P and G6P formations; 4, G1P formation; 5, cellobiose toward G6P formation; 6, G1P toward cellotriose formation; 7, G1P toward exopolysaccharide formation; 8, pyruvate formation; 9, G6P toward biosyntheses; 10, G6P formation; 11, G1P through phosphoglucomutase activity; 12, G1P toward glycogen cycle; and 13, cellotriose formation.
MATERIALS AND METHODS
Chemicals.
All chemicals were of highest-purity analytical grade. Unless stated otherwise, commercial reagents, enzymes, and coenzymes were supplied by Sigma Chemical Co., St. Louis, Mo. All gases used were purchased from Air Liquide, Paris, France.
Organism and medium.
The bacterium, C. cellulolyticum ATCC 35319 used in this study was originally isolated by Petitdemange et al. (18) from decayed grass. Stock cultures of C. cellulolyticum were maintained on cellulose and were grown for one transfer in cellobiose before initiation of growth experiments. The anaerobic culture technique of Hungate (10), as modified by Bryant (3), was used.
The defined medium used in all experiments was a modification of the CM3 medium described by Weimer and Zeikus (31), in which 5 g of yeast extract per liter was replaced by oligoelement and vitamin solutions. The composition was as follows: KH2PO4 (1.40 g/liter), K2HPO4 · 3H2O (2.90 g/liter), MgCl2 · 6H2O (0.10 g/liter), CaCl2 (0.02 g/liter), and 9.15% (wt/vol) FeSO4 · 7H2O in 50 mM H2SO4 (25 μl), oligoelement solution (1.0 ml), vitamin solution (10 ml), Na2S (0.50 g/liter), and 0.2% (wt/vol) resazurin (0.5 ml). In addition, the medium contained cellobiose and (NH4)2SO4 in variable amounts as specified in the Results section.
The oligoelement solution contained the following (in grams per liter): FeSO4 · 7H2O, 5.00; ZnSO4 · 7H20, 1.44; MnSO4 · 7H2O, 1.12; CuSO4 · 5H2O, 0.25; Na2B4O7, 0.20; (Mo)7(NH4)6O24 · 4H2O, 1.00; NiCl2, 0.04; CoCl2, 0.02; HBO3, 0.03; and Na2SeO3, 0.02, as well as 50.0 ml of 10 M HCl.
The composition of the vitamin solution was (in milligrams per 100 ml of distilled water): d-biotin, 10; para-aminobenzoic acid, 25; nicotinic acid, 15; riboflavin, 25; pantothenic acid, 25; thiamin, 25; and cyanocobalamin, 10. The vitamin in solution was sterilized by filtration through a 0.2-μm (pore-size) filter.
Growth conditions.
C. cellulolyticum ATCC 35319 was batch grown and grown in chemostat culture at various dilution rates as described previously (7, 8).
Analytical procedures.
Bacterial growth, biomass, extracellular proteins, amino acid composition, ammonia, acetate, lactate, ethanol, and exopolysaccharides were determined as described previously (7).
For glycogen determinations, cells were harvested by centrifugation (8,000 × g, 2°C, 10 min) and washed twice with 9% (wt/vol) cold NaCl. The pellets were suspended in 1 ml of 0.25% sodium dodecyl sulfate. The suspension was then diluted five times in 1 ml of potassium phosphate buffer (50 mM, pH 4.5) and incubated with 2 U of amyloglucosidase from Rhizopus mold (EC 3.2.1.3) for 60 min at 55°C (14).
Samples were centrifuged (10,000 × g, 4°C, 10 min), and the glucose in the supernatant was assayed with glucose oxidase.
To determine whether the conversion to glucose was quantitative, rabbit liver glycogen was treated by using this procedure, and recovery at 100 ± 10% was obtained.
Cellobiose and cellodextrins were assayed by high-pressure liquid chromatography (Spectra Physics SP 8810) by using a refractive index detector (Spectra Physics SP 8430). Separations, were achieved on a C18 column (YMC-Pack ODS-AQ). Water or methanol (0.5% [vol/vol] in water) was used as the solvent at a flow rate of 0.8 ml min−1 and at an ambient temperature for cellobiose and cellodextrins separations, respectively.
Assay of metabolic intermediates in cell extracts.
G1P, G6P, fructose 6-phosphate (F6P), and fructose 1,6-biphosphate (FBP) were extracted from a culture broth sample by HClO4 by using the rapid system described by Thomas et al. (30) and Guedon et al. (7).
Metabolites were measured by coupling appropriate enzyme assays with fluorimetric determination of the coenzyme NAD(P)H. Emission was measured at 459 nm after excitation at 341 nm with a fluorimeter (F2000; Hitachi, Tokyo, Japan). G1P, G6P, and F6P concentrations were determined by using an assay mixture containing Tris-HCl buffer (50 mM, pH 7.5), MgCl2 (10 mM), glucose 1,6-biphosphate (3 μM), NADP+ (0.6 mM) extract, and 2 U of G6P dehydrogenase from baker yeast (EC 1.1.1.49) to initiate G6P consumption. After complete depletion of G6P in the extract, 3 U of phosphoglucomutase from rabbit muscle (EC 5.4.2.2) was added to measure the G1P concentration. Addition of 2 U of phosphoglucose isomerase from baker's yeast (EC 5.3.1.9) allowed the F6P concentration present in the extract to be measured. The FBP concentration was determined as described previously (7).
Determination of adenylate pools.
ATP, ADP, and AMP were extracted with perchloric acid as described above for metabolic intermediates. ATP levels were measured by a luminescence assay by using a luciferin-luciferase system (Microbiol Biomass Test Kit; Celsis Lumac, Landgraaf, The Netherlands). ADP was converted to ATP in a reaction mixture containing 2 ml of 14 mM phosphocreatine in glycine buffer (0.1 M, pH 9.0), 0.4 mM MgSO4, and 4 U of creatine phosphokinase from rabbit muscle (EC 2.7.3.2); after 15 min at 38°C for the conversion of AMP to ATP, 5 U of myokinase from rabbit muscle (EC 2.7.4.3) was added in the same mixture. Reactions were stopped by heating (100°C) for 3 min, and the mixtures were centrifuged (8,000 × g, 4°C, 15 min). ADP and AMP were determined by calculating the difference.
Preparation of cell extracts.
Cells were centrifuged (12,000 × g, 15 min, 0°C), and pellets were rapidly frozen with liquid nitrogen and stored at −80°C.
Anaerobic conditions were maintained throughout the entire procedure, and all manipulations were performed under an O2-free nitrogen atmosphere. Cells (1 g) were placed in a glass tube that contained 4 ml of Tris-HCl buffer (50 mM, pH 7.5) and 400,000 U of muramidase (EC 3.2.1.17) from chicken egg white, and the suspension was incubated at 30°C for 30 min and mixed continuously. The supernatant was collected from the cell lysate following centrifugation (12,000 × g, 20 min, 4°C). The protein content of extracts was determined by the method of Lowry (13) by using crystalline bovine serum albumin as the standard.
Enzyme assays.
All assays were performed at 34°C. The specific activities were determined in a range in which linearity with protein concentration was established.
The total cellobiose cleavage activity was determined by following the NAD(P)H-dependent oxidation of G6P into 6-phosphogluconate at 340 nm (12). The assay mixture contained 10 mM imidazole buffer (pH 6.4), 10 mM potassium phosphate buffer (pH 6.4), 9 mM MgCl2, 3 μM glucose 1,6-biphosphate, 1 mM NAD+, 1 mM ATP, 5 U of hexokinase from baker yeast (EC 2.7.1.1), 4 U of phosphoglucomutase from rabbit muscle, and 4 U of NAD(P)-dependent G6P dehydrogenase from Leuconostoc mesenteroides (EC 1.1.1.49).
Cellobiose phosphorylase activity (EC 2.4.1.20) was measured by omitting ATP and glucokinase from the mixture. β-Glucosidase (EC 3.2.1.21) content was determined by omitting potassium phosphate buffer and phosphoglucomutase (12).
ADP-glucose pyrophosphorylase (EC 2.7.7.27) was assayed in the direction of synthesis of ADP-[14C]glucose from [14C]G1P (ICN, Costa Mesa, Calif.) and ATP, as described by Shen and Preiss (26) and as modified by Robson et al. (24).
Glycogen synthase (EC 2.4.1.21) was assayed by measuring the incorporation of [14C]glucose into glycogen from ADP-[14C]glucose (NEN Life Science Products, Boston, Mass.) in the presence of primer as described by Greenberg and Preiss (6), except that washing procedures were carried out three times with 1.5 ml of cold 75% (vol/vol) methanol containing 1% (wt/vol) KCl. In addition, pellets were resuspended in 200 μl of 0.1 M NaOH for assay of their radioactivity.
Radioactivity was assayed with 5 ml of scintillation liquid (Ultima Gold XR; Packard, Groningen, The Netherlands) in a scintillation counter (LS 5000 TD; Beckman, Palo Alto, Calif.).
Phosphoglucomutase (EC 5.4.2.2) was assayed as described by Yu et al. (32) except that 3 μM glucose 1,6-diphosphate was added and 1 mM NAD+ and 4 U of G6P dehydrogenase from L. mesenteroides were used.
Glycogen phosphorylase (EC 2.4.1.1) was measured in the direction of the phosphorolysis of glycogen by coupling to phosphoglucomutase and G6P dehydrogenase by the method of Robson and Morris (23) except that 1 mg of glycogen per ml from rabbit liver, 1 mM NAD+, 4 U of NAD(P)-dependent G6P dehydrogenase from L. mesenteroides, and 4 U of phosphoglucomutase from rabbit muscle were used, and 3 μM glucose 1,6-biphosphate was added.
Glycogen was extracted and analyzed from bacterial cells by a method described previously (4). Polysaccharide samples (1 mg) were digested with 6 U of α-amylase (EC 3.2.1.1) in 1 ml of potassium phosphate buffer (20 mM, pH 7.0) with 2 U of amyloglucosidase in 1 ml of potassium phosphate buffer (50 mM, pH 4.5) or with 5,000 U of isoamylase (EC 3.2.1.68) in 1 ml of acetate buffer (50 mM, pH 3.7).
All enzymes incubation conditions were performed as recommended by the supplier. Sugar residues were separated on thin-layer chromatography plates (Silica Gel 60; Merck, Darmstadt, Germany) by developing them in butanol-acetic acid-water (3:3:2) for 12 to 24 h. Sugars were visualized by spraying with a solution of 0.2% (wt/vol) in ethanol and 20% H2SO4 in equal amounts.
Calculations and nomenclature.
The main products of cellobiose fermentation by C. cellulolyticum were acetate, ethanol, lactate, H2, and CO2 (5).
To determine how carbon flux was distributed in C. cellulolyticum and the turnover of pools, we applied the model developed by Holms (9). From the measures of cellobiose utilization, growth rate, production of biomass, and byproducts, provided that the metabolic routes are known, the flux through every enzyme can be calculated. In the steady state, the biomass generates itself at a constant rate described as the growth rate “μ” (per hour), and the fluxes are expressed as milliequivalents of carbon (meqC) gram of cells−1 hour−1. The number of millimoles of any product is multiplied by the number of carbon atoms in that molecule to obtain the meqC. Pool size is an amount expressed as micromoles or meqC of an intermediate contained as a pool within the biomass (in gram of cells). In addition to ions, cofactors, and vitamins, metabolic pools consist of anabolic pools of precursors and catabolic pools (17). In this context, glycogen which is not integrated into the macromolecular structure of C. cellulolyticum but resides in the cell as a collector or supplier of glucose, is considered a metabolic pool. The turnover of the pool is the rate of input or output divided by the pool size expressed in meqC per gram of cells per hour/meqC per gram of cells, which is a value expressed per hour and is the number of times the pools turn over every hour.
The fluxes through every step of the Fig. 1 were calculated as described in Table 1. Adenylate energy charges were calculated from the following equation: ([ATP] + 1/2 [ADP])/([ATP] + [ADP] + [AMP]).
TABLE 1.
Fluxes analysis from cellobiose to by-products and biomass
| Stepa | Nomenclature of specific flow rate (meqC g of cells−1 h−1)b | Calculationc |
|---|---|---|
| (1) | qcellobiose | (SR −  )/ )/ × D × D |
| (2) | qcellobiose toward qcellotriose | (13) × 2/3 |
| (3) | qcelliobose toward G1P and G6P | (1) − (2) |
| (4) | qcellobiose toward G1P = qG1P | (3) × 1/2 |
| (5) | qcellobiose toward G6P | (3) × 1/2 |
| (6) | qG1P toward qcellotriose | (13) × 1/3 |
| (7) | qpolysaccharides |
Cpolysaccharide/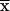 × D × D |
| (8) | qpyruvate | (Cacetate + Clactate × Cethanol + CCO2/ × D × D |
| (9) | qbiosynthesis | (Cbiomass + Camino acids + Cproteins)/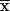 × D × D |
| (10) | qG6P | (8) + (9) |
| (11) | qphosphoglucomutase = qPGM | (10) − (5) |
| (12) | qglycogen | (4) − (6) − (7) − (11) |
| (13) | qcellotriose |
Ccellotriose/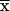 × D × D
|
Step numbers correspond to numbers in Fig. 1.
Calculated from the millimoles of substrate or products.
SR, concentration of cellobiose input; 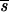 , residual concentration of cellobiose of steady-state culture;
, residual concentration of cellobiose of steady-state culture; 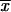 , biomass concentration of steady-state culture; D, dilution rate; C, meqC from by-products and biomass. Ratios 2/3, 1/2, and 1/3 correspond to coefficient numbers.
, biomass concentration of steady-state culture; D, dilution rate; C, meqC from by-products and biomass. Ratios 2/3, 1/2, and 1/3 correspond to coefficient numbers.
Carbon recoveries were calculated from the production of metabolites, biomass, amino acids, proteins, and exopolysaccharides present in the supernatant. CO2 production was calculated as the sum of the acetate and ethanol production.
RESULTS
Glycogen synthesis in C. cellulolyticum grown in batch culture in the presence of carbon and nitrogen excess.
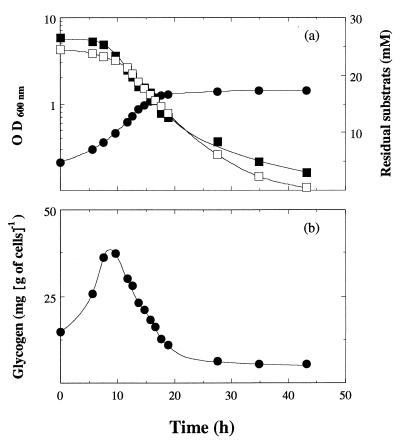
After about 20 h of incubation in batch culture, cells entered the stationary phase, although neither carbohydrate nor NH4+ was exhausted (Fig. 2a). Intracellular polysaccharide biosynthesis appeared to be associated with the vegetative growth (Fig. 2b); it reached a maximum of almost 40 mg g of cells−1 during the exponential growth phase. Then, after about 9 h, a gradual utilization of this polysaccharide took place even in the medium which contained cellobiose. This intracellular polysaccharide was obtained after KOH-ethanol extraction and was characterized by use of enzymatic digestion and comparison with several commercial preparations. Only glucose appeared on a thin-layer chromatography plate after the polysaccharide was digested with amyloglucosidase (data not shown). Partial hydrolysis occurred with α-amylase or isoamylase, indicating a polyglucan containing α-1,4 and α-1,6 glucosidic linkages. Thus, it appeared to be glycogen.
FIG. 2.
Glycogen synthesis in C. cellulolyticum grown in batch culture in the presence of carbon and nitrogen excess [cellobiose, 23.4 mM; (NH4)2SO4, 26.5 mM]. (a) Optical density at 600 nm (●), residual cellobiose (□), and ammonia (■) concentration. (b) Glycogen biosynthesis.
Glycogen synthesis in C. cellulolyticum grown in a chemostat.
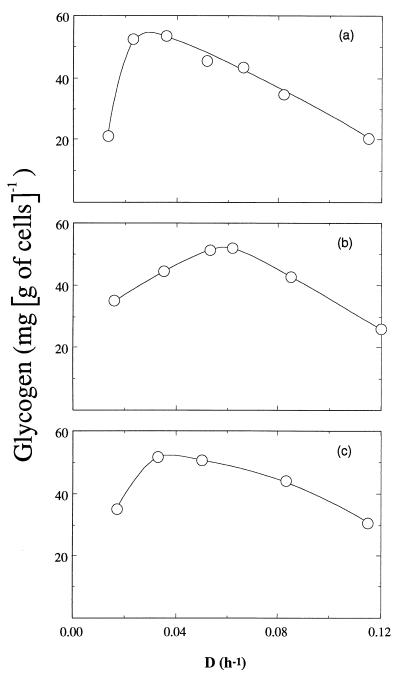
In order to determine the precise conditions where glycogen was stored in C. cellulolyticum, its production was measured during continuous cultures. Figure 3a shows the effect of dilution rate on glycogen formation in cells grown under nitrogen limitation (NH4+, 4.00 mM) and cellobiose excess (14.62 mM), which is usually the best condition for glycogen storage. Glycogen was synthesized during all growth rates, ranging from 20.6 to 53.4 mg of glucose eq g of cells−1. Similar results were obtained both under carbon limitation and nitrogen excess (Fig. 3b) or carbon and nitrogen excess (Fig. 3c). Further, the usual condition, i.e., carbon excess (14.62 mM) and nitrogen limitation (4.00 mM) was used to study the glycogen function in the distribution of the carbon flux in C. cellulolyticum.
FIG. 3.
Effect of dilution rate on glycogen formation by C. cellulolyticum. Growth conditions: a, cellobiose excess (14.6 mM) and ammonia limitation (4.0 mM); b, cellobiose limitation (5.8 mM) and ammonia excess (15.1 mM); c, cellobiose excess (14.6 mM) and ammonia excess (15.1 mM). Symbols represent the averages from three different chemostats, and the values were determined with an average accuracy of ±10%.
Effect of dilution rate on biomass and metabolite formation.
C. cellulolyticum was grown in continuous culture over a wide range of dilution rates (D = 0.013 to 0.115 h−1) with 14.62 mM cellobiose as the carbon source and 4.0 mM ammonia as the nitrogen source (Table 2). At this concentration, cellobiose was always in excess, and residual cellobiose concentrations were in the range of 6.44 to 12.11 mM. Ammonia was limiting since, over a wide range (0.013 to 0.082 h−1) of low dilution rates, the ammonia concentrations were less than 0.03 mM; at >0.082 h−1, the concentration increased and was found to be 2.20 mM at a D value of 0.115 h−1. These data are typical of a continuous culture carried out under nitrogen limitation.
TABLE 2.
Fermentation parameters for continuous steady-state cultures of C. cellulolyticum
| Parameter | Resulta obtained at a D value (h−1) of:
|
||||||
|---|---|---|---|---|---|---|---|
| 0.013 | 0.023 | 0.036 | 0.052 | 0.066 | 0.082 | 0.115 | |
| Biomass (g liter−1) | 0.165 ± 0.007 | 0.190 ± 0.007 | 0.210 ± 0.006 | 0.232 ± 0.012 | 0.280 ± 0.010 | 0.285 ± 0.012 | 0.126 ± 0.005 |
| Residual cellobiose (mM)b | 7.20 ± 0.38 | 8.02 ± 0.43 | 8.67 ± 0.27 | 8.62 ± 0.46 | 8.37 ± 0.40 | 8.85 ± 0.36 | 12.18 ± 0.51 |
| qcellobiose (mmol g of cells−1 h−1) | 0.584 | 0.799 | 1.020 | 1.345 | 1.473 | 1.660 | 2.230 |
| qacetate (mmol g of cells−1 h−1) | 0.855 | 0.985 | 1.244 | 1.504 | 1.782 | 1.890 | 2.911 |
| qlactate (mmol g of cells−1 h−1) | 0.249 | 0.230 | 0.264 | 0.138 | 0.980 | 1.450 | 0.939 |
| qethanol (mmol g of cells−1 h−1) | 0.503 | 0.893 | 1.110 | 1.150 | 0.999 | 1.000 | 1.193 |
| Glycogen (mg g of cells−1) | 21.3 | 52.4 | 53.4 | 45.4 | 43.5 | 34.6 | 20.6 |
| Exopolysaccharides (g liter−1) | 0.139 ± 0.016 | 0.113 ± 0.011 | 0.102 ± 0.012 | 0.145 ± 0.012 | 0.150 ± 0.017 | 0.193 ± 0.021 | 0.131 ± 0.011 |
| Cellotriose (mM) | 0.829 ± 0.095 | 0.541 ± 0.083 | 0.367 ± 0.062 | 0.291 ± 0.050 | 0.165 ± 0.025 | 0.092 ± 0.020 | 0.021 ± 0.005 |
| Extracellular proteins (mg liter−1) | 44.2 ± 1.8 | 66.7 ± 2.8 | 16.5 ± 1.4 | 59.0 ± 2.7 | 34.2 ± 1.8 | 36.27 ± 1.6 | 9.3 ± 1.1 |
| Free amino acids (mg liter−1) | 71.9 ± 3.9 | 81.8 ± 4.1 | 65.7 ± 3.8 | 53.3 ± 3.2 | 58.3 ± 3.8 | 55.8 ± 3.6 | 44.6 ± 3.4 |
| Carbon recovery (%) | 100.55 | 99.58 | 96.71 | 95.82 | 93.99 | 96.81 | 97.09 |
Values represent the average determinations (± the standard deviations) from three different chemostats. All other values were determined with an average accuracy of ±10%.
The cellobiose and ammonium inputs were 14.62 and 4.00 mM, respectively.
The main products of cellobiose catabolism associated with the production of ATP were acetate, lactate, and ethanol. Glycogen was synthesized during all growth rates; exopolysaccharides, cellotriose, extracellular proteins, and free amino acids were detected in the cell-free medium regardless of the dilution rate.
High values of exopolysaccharides were found, e.g., for chemostat experiments conducted at 0.013 h−1, 0.139 g of polysaccharides liter−1 were produced by 0.165 g of cells liter−1. Cellotriose decreased from 0.83 to 0.02 mM in parrallel with an increase in growth rate; no glucose or other oligosaccharides were detected extracellularly. Extracellular protein concentrations fluctuated between 9.3 and 66.7 mg liter−1 and could be associated with the carboxymethylcellulase activity detected in the medium (data not shown).
Whatever the dilution rate, growth on ammonium led to the appearance of the usual amino acids in the medium varying from 3 to 67 μmol liter−1. Furthermore, other amino acids accumulated in the medium: phosphoserine (27 μmol liter−1), citrulline (4 μmol liter−1), aminobutyrate (2.5 μmol liter−1), ornithine (3.5 μmol liter−1), and the amino compound phosphoethanolamine (4 μmol liter−1). The appearance of all of these last compounds is a function of the growth rate: they were produced in the range of 44.6 to 81.8 mg liter−1 (Table 2). Such spilling of usual amino acids accompanied by other amino compounds could be explained by an imbalance in amino compound biosynthesis. Carbon recoveries were between 94.0 and 100.5%.
Specific consumption and production rates.
Data on the effect of D on cellobiose consumption and product formation are shown in Fig. 4. The rate of cellobiose consumption varied from 7.01 to 26.72 meqC g of cells−1 h−1 with increasing growth rate (Fig. 4a), while 0.36 to 1.95 meqC g of cells−1 h−1 were converted into biomass, proteins, and amino compounds (Fig. 4b). Cellobiose catabolism, leading to the end product formation via pyruvate, increased from 4.64 to 15.12 meqC g of cells−1 h−1 with increasing growth rate (Fig. 4c). Below a D of 0.052 h−1, the specific rate of cellotriose was stable and at levels above this value decreased sharply (Fig. 4d), concomitantly with the increase of the extracellular polysaccharide formation (Fig. 4e), indicating a switch in the carbon flux. The specific production rate of glycogen increased with dilution rate from 0.48 to 5.32 meqC g of cells−1 h−1 (Fig. 4f).
FIG. 4.
Effect of dilution rate on specific rates: a, rate of cellobiose consumption (qcellobiose); b, rate of biosynthesis (qbiosynthesis); c, rate of pyruvate formation (qpyruvate); d, rate of cellotriose formation (qcellotriose); e, rate of polysaccharide formation (qpolysaccharides); and f, rate of glycogen formation (qglycogen). Values are calculated as explained in Table 1 and Fig. 1 and are expressed as the meqC per gram of cells per hour.
Intracellular hexose-phosphate and adenylate pools of continuous steady-state cultures of C. cellulolyticum.
Previous work indicated that C. cellulolyticum was unable to regulate its cellobiose consumption (8); this explains the large amounts of G1P and G6P detected inside the cells (Table 3). G1P concentrations were found to be higher at high dilution rates, whereas the highest values of G6P were found at low dilution rates (Table 3). This could result from the decrease of the carbon flux through the phosphoglucomutase from 27 to 14.3% of the original carbon with the increase in D (Fig. 1). Moreover, no significant variation between the dilution rate and in vitro intracellular levels of F6P and FBP could be detected (data not shown).
TABLE 3.
Metabolite levels of continuous ammonia-limited steady-state cultures of C. cellulolyticum
| Parameter | Resulta obtained at a D value (h−1) of:
|
||||||
|---|---|---|---|---|---|---|---|
| 0.013 | 0.023 | 0.036 | 0.052 | 0.066 | 0.082 | 0.115 | |
| Intracellular concn (μmol g of cells−1) | |||||||
| G1P | 16.6 ± 0.5 | 25.5 ± 0.4 | 56.6 ± 1.1 | 86.3 ± 1.7 | 109.6 ± 1.9 | 118.8 ± 2.9 | 209.6 ± 5.7 |
| G6P | 136.2 ± 2.1 | 149.7 ± 1.7 | 90.0 ± 1.6 | 83.1 ± 0.9 | 34.8 ± 0.6 | 27.4 ± 0.8 | 16.3 ± 0.6 |
| ATP | 1.66 ± 0.08 | 2.19 ± 0.15 | ND | 5.08 ± 0.21 | 6.03 ± 0.25 | 7.08 ± 0.44 | 8.81 ± 0.50 |
| ADP | 0.86 ± 0.04 | 1.43 ± 0.08 | ND | 2.63 ± 0.092 | 2.71 ± 0.14 | 2.98 ± 0.17 | 1.66 ± 0.07 |
| AMP | 0.05 ± 0.01 | 0.04 ± 0.01 | ND | 0.52 ± 0.08 | 0.64 ± 0.10 | 0.95 ± 0.15 | 0.97 ± 0.13 |
| G6P/G1P ratio | 8.20 | 5.87 | 1.59 | 0.96 | 0.32 | 0.23 | 0.08 |
| Energetic charge | 0.81 | 0.79 | ND | 0.78 | 0.79 | 0.78 | 0.84 |
Values represent the average determinations (± the standard deviation) from three different chemostats. G6P/G1P ratios and the energetic charge were determined with an average accuracy of ±10%. ND, not determined.
Whatever the D value, conversion of cellobiose into pyruvate was between 63.0 and 56.6% and into biosynthesis around 5.5%. Under these circumstances, almost 30% of the carbon flux must be partitioned into glycogen, polysaccharides, and cellotriose (Fig. 1).
From 6.8% (D = 0.013 h−1) to 19.9% (D = 0.115 h−1) of the original cellobiose uptake was used through the glycogen cycle, and from 5.2 to 14.9% was used for polysaccharide excretion; the rest was used for cellotriose biosynthesis, which decreased from 16.7 to 1.3% (Fig. 1). The conversion of G1P to cellotriose is much more efficient than to glycogen and to polysaccharides since the pool of G1P at low growth rates (high specific rates of cellotriose formation) turns over 31.2 times per hour and only 13.7 times at high growth rates (high specific rates of glycogen and polysaccharide production) (Fig. 5a). This could reflect the needs for a high rate of substrate production (G1P) to increase the carbon flux toward glycogen and polysaccharides (Fig. 1). From D = 0.013 to 0.115 h−1 (Table 3), the intracellular G6P/G1P ratios ranged from 8.2 to 0.08, meaning that the phosphoglucomutase is a limiting step at the high dilution rates but not at the low dilution rates.
FIG. 5.
Effect of dilution rate on G6P turnover (●) and G1P turnover (○) (a) and on glycogen turnover (b). The flux values are calculated as explained in Table 1 and Fig. 1; the pools of G1P and G6P are given in Table 3, and the glycogen pools are obtained from the intracellular concentrations reported in Table 2. Symbols represent the averages from three different chemostats, and the values are determined with an average accuracy of ±10%.
The pool of G6P is at a junction, and the bulk of the G6P is made by the hexokinase since the flux through the phosphoglucomutase slowed with D (Fig. 1), i.e., the carbon flux through step 11 was 27% of the cellobiose consumed at D = 0.013 h−1 and only 14.3% at D = 0.115 h−1. From D = 0.013 h−1 to D = 0.115 h−1, the G6P pool decreased (Table 3); correlatively, its turnover increased from 6.1 to 154.6 times per hour (Fig. 5a). These facts were associated with the increase of the carbon flux in glycolysis: from 3.114 to 13.247 meqC g of cells−1 h−1 (step 5, Fig. 1). Therefore, the demand for cellobiose toward step 3 increased (Fig. 1) and the cellobiose was no longer available for the cellotriose formation through the reversible phosphorylase reaction, which decreased from 0.779 to 0.229 meqC g of cells−1 h−1 (step 2, Fig. 1). This explains how large amounts of intracellular G1P accumulated with D (Table 3), since its specific rate of formation increased from 3.1 to 13.2 meqC g of cells−1 h−1 with D (step 4, Fig. 1), whereas its utilization in cellotriose production decreased from 0.389 to 0.115 meqC g of cells−1 h−1 (step 6, Fig. 1) and the G1P flux through the phosphoglucomutase slowed with D (step 11, Fig. 1). Table 3 shows that the intracellular concentrations of ATP, ADP, and AMP increased with D but that no significant variation of the adenylate charge could be detected. The energetic charge values, from 0.78 to 0.84, indicate a high level of ATP production.
Synthesis of the key enzymes of glycogen biosynthesis and mobilization.
The measurements of the total cellobiose cleavage activities (cellobiose phosphorylase and β-glucosidase) were not significantly different from the cellobiose phosphorylase activity itself. On the basis of these results, cellobiose phosphorylase and β-glucosidase activities were found at ca. 28 and 2 nmol min−1 mg of proteins−1, respectively, and it appears that C. cellulolyticum uses the phosphorylase for the cellobiose catabolism.
Glycogen synthase and ADP-glucose pyrophosphorylase, two biosynthetic enzymes, were detected with glycogen phosphorylase, the enzyme which catalyzes the first step of glycogenolysis. Glycogen synthase activity was found to vary from 125 to 375 nmol min−1 mg of proteins−1, and ADP-glucose pyrophosphorylase activity varied from 30 to 110 nmol min−1 mg of proteins−1, explaining that the synthesis of glycogen in C. cellulolyticum takes place during all growth rates (Fig. 4f) and chiefly at a high growth rate, e.g., at μ = 0.115 h−1. The value of the carbon flux through the glycogen cycle could achieve 19.9% of the specific rate of cellobiose uptake.
Glycogen phosphorylase activity, which was found at from 12 to 37 nmol min−1 mg of protein−1, means that the stable glycogen content at each steady state could be the result of the glycogen synthesis and of glycogenolysis occurring simultaneously. A glucose equivalent turnover in the glycogen cycle from 0.7 to 7.7 times per hour takes place according to the growth rate (Fig. 5b).
DISCUSSION
This study describing the carbon fluxes in C. cellulolyticum growing on cellobiose in chemostat cultures with ammonia as the growth-limiting nutrient shows that whatever the specific growth rate, carbon flux into the cells exceeds the fluxes needed for biosynthesis of cell monomers and to sustain reactions leading to energy production. Consequently, ca. 30% of the original specific rate of cellobiose uptake by C. cellulolyticum was converted to cellotriose, glycogen, and polysaccharides rather than being used for generation of metabolic energy and biosynthetic precursors.
The data from the flux analyses are reported as a function of the growth rate in Fig. 1. It is clear that G1P and G6P connected by phosphoglucomutase constitute important branch points for distributing the carbon fluxes inside and outside the cells.
The regular increase of the fluxes through steps 1, 3, 5, and 10 (Fig. 1) contrasts with the flux through the phosphoglucomutase (step 11), which reached a limitation level at D = 0.066 h−1. The steady-state concentrations of the G6P and G1P corroborate the analysis of the carbon flux, since at low dilution rates the ratio G6P/G1P is 8.2, whereas at the high dilution rate the ratio is 0.08. This suggests that the enzyme is limiting at high dilution rates but not at low ones. The limitation of the path through the phosphoglucomutase had a great effect on the accumulation of G1P, the precursor of cellotriose, exopolysaccharides, and glycogen. The biosynthesis of these compounds are important since as much as 16.7, 19.9, and 14.9% of the specific rates of cellobiose consumed were used to supply specific rates of cellotriose, glycogen, and polysaccharide production, respectively.
One of the features of microbial phosphorylases is their ability to synthesize oligosaccharides (1); cellobiose serves as a glucosyl acceptor, and G1P serves as a glucosyl donor. In C. cellulolyticum at low growth rates, when the flux through glycolysis was low, only 88.8% of the intracellular cellobiose was used through the glycolysis and, hence, 11.2% was used to supply the specific rate of cellotriose biosynthesis. This biosynthesis was efficient and led to a high value of the G1P turnover. With the increase of the dilution rate, the flux of cellobiose through step 3 increased from 88.8 to 99.14%, whereas its flux through the step 2 decreased, thus explaining the drop in the cellotriose excretion.
In batch culture, glycogen in C. cellulolyticum reached a maximum during the exponential growth phase. This result is in contrast to many bacterial species which synthesize glycogen during a limited growth period at the outset of the stationary phase (20–22). Our chemostat studies demonstrate that glycogen was synthesized during all growth rates in carbon- and nitrogen-sufficient media, in carbon excess and nitrogen limitation, as well as in carbon limitation and ammonia excess; in these three cases, similar quantities of glycogen were detected. Similarly, the synthesis of glycogen in C. cellulolyticum was not subject to stimulation by nutrient limitation. In contrast, in many bacterial species glycogen accumulates when growth is limited, e.g., by phosphorus, sulfur, or nitrogen in the presence of an excess of a carbon source (20–22). These data, along with the fact that glycogen phosphorylase activity and glycogen content were measured throughout the growth cycle, suggest that glycogen was simultaneously synthesized and degraded during cell growth. Continuous culture experiments confirmed that high glycogen turnover in C. cellulolyticum, i.e., glycogen accumulation and degradation, was associated with rapid growth and hence with a high carbon flux. Such glycogen turnover would limit the flux of carbon through glycolysis, avoiding the potentially deleterious effects of generating high concentrations of intracellular metabolites.
Whatever the dilution rate, the values of the adenylate energy charge were high, indicating that the inefficiently regulated carbon flow also led to an excess of energy, which is consumed by the biosynthesis of cellotriose and exopolysaccharides, as well as by the turnover of the intracellular glycogen.
From this study and previous results it appears that pyruvate-acetyl coenzyme A are important branch points for regulating the redox balance of the carbon catabolism, whereas the properties of the G1P-G6P branch points have been selected to control excretion of carbon surplus and to dissipate excess energy.
Regulations at these last branch points are needed since C. cellulolyticum is not able to regulate cellobiose consumption (8); nevertheless, they are imperfect since overflow of pyruvate occurred at the second branch point. In other words, the G1P-G6P branch points carry out a coarse control of the carbon flux and the pyruvate-acetyl coenzyme A branch points to a fine control beside the regulation of the redox balance.
ACKNOWLEDGMENTS
This work was supported by the Commission of European Communities FAIR program (contract no. CT 95-0191 [DG 12 SSMA]) and by the program AGRICE (no. 97.01.041).
We thank K. Poiret for typing the manuscript, G. Raval for excellent technical assistance, and E. McRae for critical reading of the manuscript.
REFERENCES
- 1.Alexander J K. Cellobiose phosphorylase from Clostridium thermocellum. Methods Enzymol. 1972;28:944–948. [Google Scholar]
- 2.Bayer E A, Chanzy H, Lamed R, Shoham Y. Cellulose, cellulases and cellulosomes. Curr Opin Struct Biol. 1998;8:548–557. doi: 10.1016/s0959-440x(98)80143-7. [DOI] [PubMed] [Google Scholar]
- 3.Bryant M P. Commentary on the Hungate technique for culture of anaerobic bacteria. Am J Clin Nutr. 1972;25:1324–1328. doi: 10.1093/ajcn/25.12.1324. [DOI] [PubMed] [Google Scholar]
- 4.Gerhardt P R G, Murray E, Wood W A, Krieg N R. Methods for general and molecular bacteriology. Washington, D.C.: American Society for Microbiology; 1994. [Google Scholar]
- 5.Giallo J, Gaudin C, Belaich J P, Petitdemange E, Caillet-Mangin F. Metabolism of glucose and cellobiose by cellulolytic mesophilic Clostridium sp. strain H10. Appl Environ Microbiol. 1983;45:843–849. doi: 10.1128/aem.45.3.843-849.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greenberg E, Preiss J. Purification and properties of the adenosine diphosphoglucase: glycogen transglucolase of Arthrobacter species NRRL B1973. J Biol Chem. 1965;240:2341–2348. [PubMed] [Google Scholar]
- 7.Guedon E, Payot S, Desvaux M, Petitdemange H. Carbon and electron flow in Clostridium cellulolyticum grown in chemostat culture on synthetic medium. J Bacteriol. 1999;181:3262–3269. doi: 10.1128/jb.181.10.3262-3269.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guedon E, Desvaux M, Payot S, Petitdemange H. Growth inhibition of Clostridium cellulolyticum by an inefficiently regulated carbon flow. Microbiology. 1999;145:1831–1838. doi: 10.1099/13500872-145-8-1831. [DOI] [PubMed] [Google Scholar]
- 9.Holms H. Flux analysis and control of the central metabolic pathways in Escherichia coli. FEMS Microbiol Rev. 1996;19:85–116. doi: 10.1111/j.1574-6976.1996.tb00255.x. [DOI] [PubMed] [Google Scholar]
- 10.Hungate R E. A roll tube method for cultivation of strict anaerobes. Methods Microbiol. 1969;33:117–132. [Google Scholar]
- 11.Lamed R, Setter E, Kenig R, Bayer E A. The cellulosome—a discrete cell surface organelle of Clostridium thermocellum which exhibits separate antigenic, cellulose-binding and various cellulolytic activities. Biotechnol Bioeng. 1983;13:163–181. [Google Scholar]
- 12.Lou J, Dawson K A, Strobel H J. Role of phosphorolytic cleavage in cellobiose metabolism by the ruminal bacterium Prevotella ruminicola. Appl Environ Microbiol. 1996;62:1770–1773. doi: 10.1128/aem.62.5.1770-1773.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 14.Matheron C, Delort A-M, Gaudet G, Forano E. Simultaneous but differential metabolism of glucose and cellobiose in Fibrobacter succinogenes cells, studied by in vivo13C-NMR. Can J Microbiol. 1996;42:1091–1099. doi: 10.1139/m96-140. [DOI] [PubMed] [Google Scholar]
- 15.Neijssel O M, Burman E T, Teixeira de Mattos M J. The role of futile cycles in the energetics of bacterial growth. Biochim Biophys Acta. 1990;1018:252–255. doi: 10.1016/0005-2728(90)90260-b. [DOI] [PubMed] [Google Scholar]
- 16.Ng T, Zeikus J G. Differential metabolism of cellobiose and glucose by Clostridium thermocellum and Clostridium thermohydrosulfuricum. J Bacteriol. 1982;150:1391–1399. doi: 10.1128/jb.150.3.1391-1399.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pai S R, Kubitschek H E. Catabolic pools in Escherichia coli. Res Microbiol. 1992;143:173–181. doi: 10.1016/0923-2508(92)90006-a. [DOI] [PubMed] [Google Scholar]
- 18.Petitdemange E, Caillet F, Giallo J, Gaudin C. Clostridium cellulolyticum sp. nov., a cellulolytic mesophilic species from decayed grass. Int J Syst Bacteriol. 1984;34:155–159. [Google Scholar]
- 19.Prasad C, Freese E. Cell lysis of Bacillus subtilis caused by intracellular accumulation of glucose-1-phosphate. J Bacteriol. 1974;118:1111–1122. doi: 10.1128/jb.118.3.1111-1122.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Preiss J. Bacterial glycogen synthesis and its regulation. Annu Rev Microbiol. 1984;38:419–458. doi: 10.1146/annurev.mi.38.100184.002223. [DOI] [PubMed] [Google Scholar]
- 21.Preiss J, Romeo T. Physiology, biochemistry and genetics of bacterial glycogen synthesis. Adv Microb Physiol. 1989;30:183–233. doi: 10.1016/s0065-2911(08)60113-7. [DOI] [PubMed] [Google Scholar]
- 22.Preiss J. Regulation of glycogen synthesis. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B Jr, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Vol. 1. Washington, D.C.: American Society for Microbiology; 1996. pp. 1015–1024. [Google Scholar]
- 23.Robson R L, Morris J G. Mobilization of granulose in Clostridium pasteurianum. Purification and properties of granulose phosphorylase. Biochem J. 1974;144:513–517. doi: 10.1042/bj1440513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robson R L, Robson R M, Morris J G. The biosynthesis of granulose by Clostridium pasteurianum. Biochem J. 1974;144:503–511. doi: 10.1042/bj1440503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Russel J B, Cook G M. Energetics of bacterial growth: balance of anabolic and catabolic reactions. Microbiol Rev. 1995;59:48–62. doi: 10.1128/mr.59.1.48-62.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shen L, Preiss J. The activation and inhibition of bacterial adenosine-diphosphoglucose pyrophosphorylases. Biochem Biophys Res Commun. 1964;17:424–429. [Google Scholar]
- 27.Shoham Y, Lamed R, Bayer E A. The cellulosome concept as an efficient microbial strategy for the degradation of insoluble polysaccharides. Trends Microbiol. 1999;7:275–281. doi: 10.1016/s0966-842x(99)01533-4. [DOI] [PubMed] [Google Scholar]
- 28.Strobel H J, Caldwell F C, Dawson K A. Carbohydrate transport by the anaerobic thermophile Clostridium thermocellum LQRI. Appl Environ Microbiol. 1995;61:4012–4015. doi: 10.1128/aem.61.11.4012-4015.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tempest D W, Neijssel O M. Physiological and energetic aspects of bacterial metabolite overproduction. FEMS Microbiol Lett. 1992;100:169–176. doi: 10.1111/j.1574-6968.1992.tb14036.x. [DOI] [PubMed] [Google Scholar]
- 30.Thomas T D, Ellwood D C, Longyear M C. Change from homo- to heterolactic fermentation by Streptococcus lactis resulting from glucose limitation in anaerobic chemostat cultures. J Bacteriol. 1979;138:109–117. doi: 10.1128/jb.138.1.109-117.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weimer P J, Zeikus J G. Fermentation of cellulose and cellobiose by Clostridium thermocellum in the absence and presence of Methanobacterium thermoautotrophicum. Appl Environ Microbiol. 1977;33:289–297. doi: 10.1128/aem.33.2.289-297.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu J-P, Ladapo J, Whitman W B. Pathway of glycogen metabolism in Methanococcus maripaludis. J Bacteriol. 1994;176:325–332. doi: 10.1128/jb.176.2.325-332.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]