Abstract
Molecular therapeutics are limited for Candida vaginitis because they damage normal cells and tissues of vagina, aggravating the imbalance of vaginal microbiota and increasing the recurrence. To tackle this limitation, through the combination of peroxidase-like rGO@FeS2 nanozymes [reduced graphene oxide (rGO)] with Lactobacillus-produced lactic acid and H2O2, a responsive hyaluronic acid (HA) hydrogel rGO@FeS2/Lactobacillus@HA (FeLab) is developed. FeLab has simultaneous anti–Candida albicans and vaginal microbiota–modulating activities. In particular, the hydroxyl radical produced from rGO@FeS2 nanozymes and Lactobacillus kills C. albicans isolated from clinical specimens without affecting Lactobacillus. In mice with Candida vaginitis, FeLab has obvious anti–C. albicans activity but hardly damages vaginal mucosa cells, which is beneficial to vaginal mucosa repair. Moreover, a higher proportion of Firmicutes (especially Lactobacillus) and a decrease in Proteobacteria reshape a healthy vaginal microbiota to reduce the recurrence. These results provide a combined therapeutic of nanozymes and probiotics with translational promise for Candida vaginitis therapy.
A probiotic nanozyme hydrogel regulates vaginal microenvironment and has peroxidase-like activity for anti–Candida albicans.
INTRODUCTION
Candida vaginitis, a common fungal vulvovaginal inflammatory disease usually caused by Candida albicans (1), occurs in approximately 75% of all women worldwide. Even more seriously, 5 to 8% of these women have suffered from recurrence. Because of its high incidence and nature of recurrence, Candida vaginitis affects both the physical and mental health of women (2, 3). Pathologically, vaginal microbiota plays a key role in maintaining the vaginal microenvironment and health (4). In particular, Lactobacillus is the dominant beneficial bacterium (5). It maintains the balance of vaginal microbiota, which protects the vagina from foreign microorganisms by producing lactic acid and antibacterial substances, forming a biological barrier, and protecting vaginal epithelial cells (6, 7).
Recently, the probiotic therapy has attracted great research interest partially because the current Candida vaginitis therapy is not satisfactory (8, 9). There are four most widely deployed antifungal drugs to treat Candida vaginitis, including Clotrimazole, Fluconazole, Miconazole, and Nystatin (10). However, some of them only halt the growth of fungi but do not kill them (11). Moreover, their long-term use can cause certain damage to the normal cells and tissues of vagina, which will further aggravate local vaginal microbiota imbalance, acid-base balance disorder, superinfection, and recurrence, thus greatly increasing the difficulty of clinical treatments (12). Therefore, it is urgent to explore Candida vaginitis therapies that can modulate the vaginal microenvironment.
Because of its dominance in healthy vaginal microbiota, Lactobacillus has been explored to develop probiotic Candida vaginitis therapies (9, 13). While Lactobacillus preparations have great potential in treating and preventing bacterial vaginitis, the therapeutic effect on Candida vaginitis is not satisfactory (8, 14, 15). A possible explanation is that C. albicans is a eukaryotic cell with mycelium and spores, which are more tolerant; while Lactobacillus generates some antibacterial substances, such as H2O2, lactic acid, and bacteriocin, which are too mild to kill C. albicans (16, 17). We reason that converting mild H2O2 to more toxic hydroxyl radical (•OH) would be a promising therapeutic strategy.
•OH can be generated from H2O2 through metal ion–mediated Fenton reactions (such as Fe2+) or enzyme-mediated catalysis (such as myeloperoxidase–nicotinamide adenine dinucleotide) (18, 19). Nevertheless, the metal ions for Fenton reactions can be detrimental to normal cells and tissues (20). On the other hand, enzymes are unstable and immunogenic, limiting their practical applications. Encouragingly, nanozymes (the functional nanomaterials with enzyme-like properties) have shown a promising capability to produce reactive oxygen species (ROS), including •OH, for biomedical applications (21–23). Recent studies have shown that some peroxidase (POD)–like nanozymes can produce •OH and exhibit high antimicrobial activities (24). Here, by taking advantage of the complementary merits of POD-like nanozymes and probiotic Lactobacillus, we developed a responsive hyaluronic acid (HA) hydrogel-rGO@FeS2 [reduced graphene oxide (rGO)]/Lactobacillus@HA (designated as FeLab) to effectively treat Candida vaginitis and reduce the recurrence. When applied into the vagina, the hyaluronidase (HAase) secreted by C. albicans and bacteria can degrade HA (25), thereby locally releasing Lactobacillus and rGO@FeS2 nanozymes. On the one hand, Lactobacillus ferments and generates lactic acid to normalize the vaginal microenvironment and reduce vaginal pH to 4 to 4.5 (7). On the other hand, the rGO@FeS2 nanozymes can catalyze Lactobacillus-produced H2O2 to generate a large amount of •OH, killing C. albicans (Fig. 1). Notably, our system not only inhibited the clinical isolates but also exhibited satisfactory therapeutic efficacy for C. albicans–induced vaginitis in vivo. Moreover, compared with clinical drugs, our system is much friendlier to the vagina and can reduce the recurrence. Therefore, we implemented a promising strategy to treat and reduce the recurrence of Candida vaginitis by simultaneously regulating the vaginal microenvironment and catalyzing the killing of C. albicans.
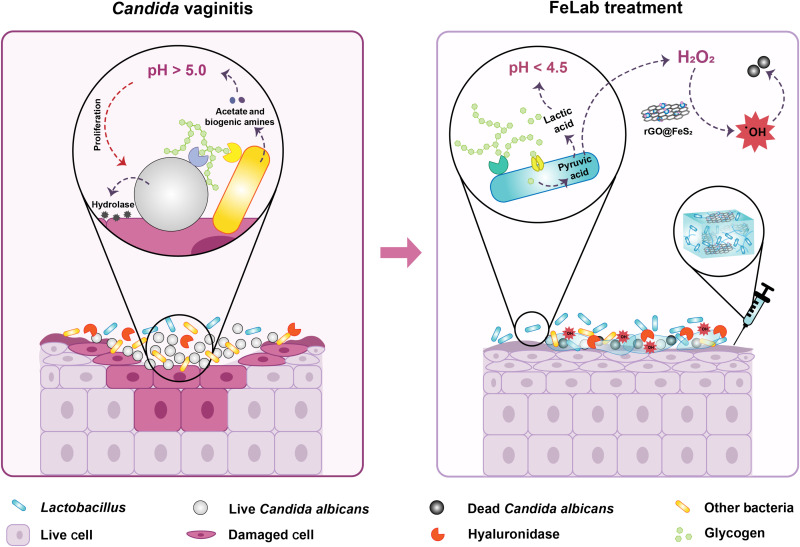
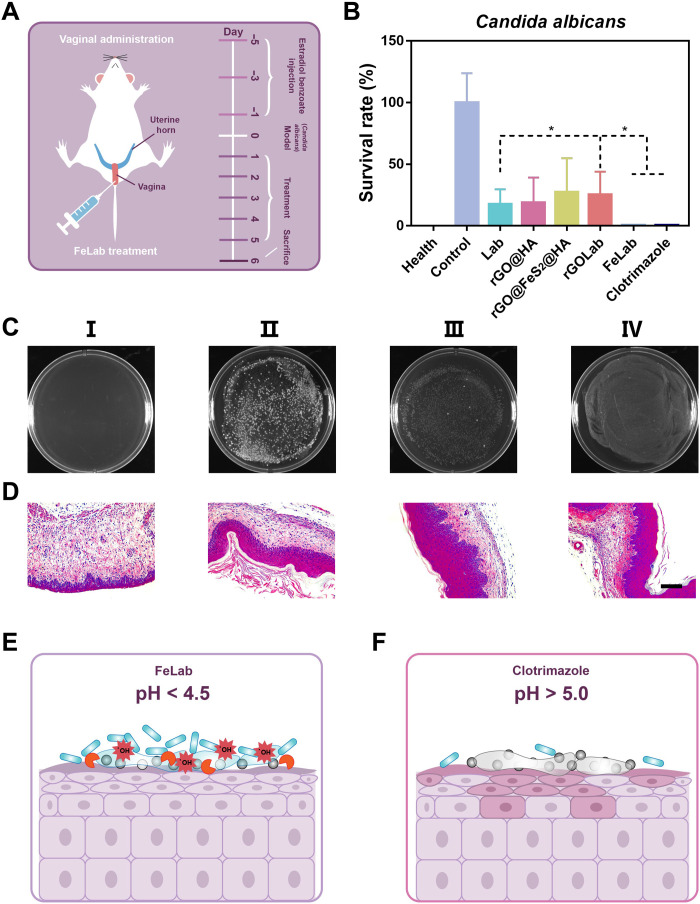
Fig. 1. Schematic illustrating Candida vaginitis microenvironment and FeLab-enabled microenvironmental regulation for Candida vaginitis therapy.
Left: Candida vaginitis microenvironment characterized with hyperproliferating C. albicans, elevated vaginal pH, imbalance of vaginal microbiota, and damaged vaginal mucosal cells. Right: FeLab-enabled microenvironmental regulation for Candida vaginitis therapy. The HAase secreted by C. albicans and bacteria can degrade HA, which releases Lactobacillus and rGO@FeS2 nanozymes. Subsequently, released Lactobacillus ferments and generates lactic acid to normalize the vaginal microenvironment and reduce vaginal pH to 4 to 4.5. Simultaneously, the released rGO@FeS2 nanozymes can catalyze Lactobacillus-produced H2O2 to generate a large amount of •OH, killing C. albicans.
RESULTS
Characterization of rGO@FeS2 nanozymes
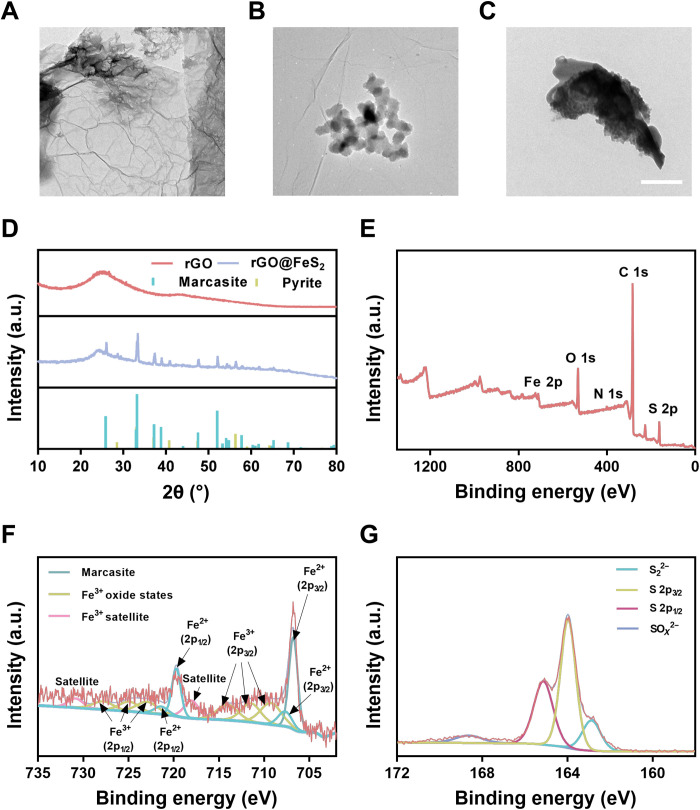
Previous studies demonstrated that Fe-N-rGO with Fe2+ exhibited excellent POD-like activity because it had an optimal adsorption energy for OH (26, 27). Therefore, in this work, we chose marcasite FeS2 nanoparticles with POD-like activity after our preliminary screening study. Compared with several other iron-containing nanozymes [i.e., Pt3Fe, Fe3O4, and pyrite FeS2 nanozymes (28–30)] (figs. S1 and S2), the rGO@FeS2 nanozymes with H2O2 generated the most amount of •OH, which was captured by 5,5-dimethyl-1-pyrroline N-oxide (DMPO) and analyzed by electron paramagnetic resonance (EPR) (fig. S3). rGO was used to stabilize marcasite FeS2 nanoparticles. Otherwise, marcasite FeS2 nanoparticles would easily aggregate, preventing the biomedical applications (31, 32). As shown via transmission electron microscopy (TEM) imaging, rGO had a two-dimensional lamellar structure (Fig. 2A), and marcasite FeS2 nanoparticles were evenly distributed on the surface of rGO (Fig. 2B). In contrast, marcasite FeS2 nanoparticles alone aggregated obviously (Fig. 2C). In addition, the high-angle annular dark-field scanning TEM (HAADF-STEM) and corresponding elemental mapping images (fig. S4) showed the even presence of C, N, Fe, and S elements without obvious large aggregates of marcasite FeS2 nanoparticles. These results showed the importance of rGO as the supports to stabilize and improve the dispersity of marcasite FeS2 nanoparticles. Furthermore, a lattice spacing of 0.24 nm corresponding to the (111) plane of marcasite FeS2 was identified by high-resolution TEM imaging (fig. S5) (33). Meanwhile, the crystalline feature of rGO@FeS2 was studied. As shown in Fig. 2D, the characteristic peaks in the x-ray diffraction (XRD) patterns were indexed to rGO and the cubic phase of marcasite FeS2 (Joint Committee on Powder Diffraction Standards (JCPDS) card number 74-1051) (30, 34). Then, we analyzed the surface chemical compositions and electronic states by x-ray spectroscopy (XPS). The XPS survey spectrum (Fig. 2E) showed typical peaks of C 1s, N 1s, O 1s, Fe 2p, and S 2p. The high-resolution C 1s XPS spectrum showed the presence of C═C/C─C, C─O/C─N, and C═O at 284.6, 285.7, and 287.1 eV, respectively (fig. S6A). The N 1s XPS spectrum could be deconvoluted into three peaks at 398.2, 400.1, and 402.8 eV (fig. S6B), which corresponded to pyridinic N, pyrrolic N, and oxidized N. For Fe 2p, the representative peaks of marcasite FeS2 nanoparticles, originating from electron deficiency of Fe2+ created by the breaking of Fe─S bonds, were around 707.4 eV (Fe 2p3/2) and 720.5 eV (Fe 2p1/2), respectively. Moreover, there were several peaks at 710 to 715 eV and 724 to 728 eV, which could be assigned to Fe3+ oxide states due to the precursor FeCl3 and slight oxidation of marcasite FeS2 in air (Fig. 2f). The S 2p peaks located at 162.8, 163.9, and 165.1 eV were attributed to S22−, S 2p3/2, and S 2p1/2, respectively. In addition, another SOx2−-related peak at 168.3 eV was correlated with the inevitable oxidation of marcasite FeS2 (Fig. 2G) (33, 35). These binding energies of both S 2p and Fe 2p were consistent with those in marcasite FeS2. The above results showed the successful synthesis of rGO@FeS2 nanozymes.
Fig. 2. Characterization of rGO@FeS2 nanozymes.
(A to C) TEM images of rGO (A), rGO@FeS2 (B), and marcasite FeS2 (C), respectively. Scale bar, 200 nm. (D) XRD patterns of rGO and rGO@FeS2. The lines at the bottom mark the reference patterns of marcasite FeS2 (JCPDS card number 74-1051) and pyrite FeS2 (JCPDS card number 42-1340). a.u., arbitrary units. (E) XPS spectrum of rGO@FeS2. (F and G) XPS spectra for Fe 2p (F) and S 2p (G) regions of rGO@FeS2.
POD-like and anti–C. albicans activities of rGO@FeS2 nanozymes
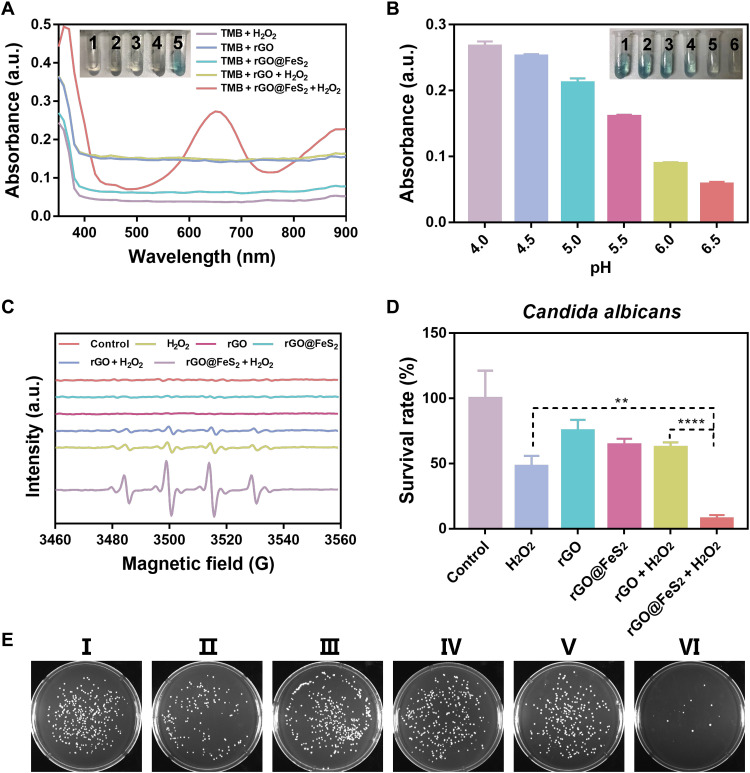
We used the oxidation of 3,3′,5,5′-tetramethylbenzidine (TMB) in the presence of H2O2 to examine the POD-like activity of rGO@FeS2 nanozymes. As shown in Fig. 3A, only the system containing rGO@FeS2 nanozymes, H2O2, and TMB turned blue [the color of oxidized TMB (oxTMB)]. The corresponding absorption spectrum of the system exhibited a strong absorption peak at 652 nm, suggesting that rGO@FeS2 nanozymes had a strong POD-like activity. Moreover, the POD-like activity of rGO@FeS2 nanozymes was pH dependent. Figure 3B shows that rGO@FeS2 nanozymes exhibited a stronger POD-like activity under acidic conditions and had an optimal activity at pH 4 to 4.5. In addition, enzymatic kinetics analysis of rGO@FeS2 nanozymes toward H2O2 and TMB substrates was performed. For comparison, other iron-containing nanozymes (i.e., Pt3Fe, Pyrite FeS2, and Fe3O4) were also investigated. Both the Michaelis-Menten constant (Km) and maximum initial velocity (vmax) were obtained (figs. S7 and S8 and table S1). We further analyzed the relationship between the kinetics parameters and anti–C. albicans activity among these four nanozymes. As shown in fig. S9, there was no direct correlation between the anti–C. albicans activities and the kinetics parameters of these four POD-like nanozymes.
Fig. 3. POD-like and anti–C. albicans activities of rGO@FeS2 nanozymes.
(A) Ultraviolet-visible absorption spectra and corresponding color changes of TMB in different reaction systems: 1, TMB + H2O2; 2, TMB + rGO; 3, TMB + rGO@FeS2; 4, TMB + rGO + H2O2; 5, TMB + rGO@FeS2 + H2O2 in a pH 4.5 HAc-NaAc buffer after 15 min of incubation. (B) pH-dependent POD-like activities of rGO@FeS2. The pH values of insets 1 to 6 are 4.0, 4.5, 5.0, 5.5, 6.0, and 6.5. (C) EPR monitoring the generation of •OH by rGO@FeS2 in the presence of H2O2 in a pH 4.5 HAc-NaAc buffer after 5 min of incubation. (D) Antifungal effects of rGO@FeS2 and H2O2 on C. albicans. (E) Digital images of C. albicans colonies after different treatments. I to VI correspond to the x coordinate of (D), respectively. An 80 μM amount of H2O2, rGO, and rGO@FeS2 at 25 μg/ml was used for anti–C. albicans at 37°C for 120 min. Data are presented as means ± SD (n = 3). **P < 0.01 and ****P < 0.0001.
Next, we used EPR spectroscopy to investigate the •OH generating activity of rGO@FeS2 nanozymes, which would be critical for the anti–C. albicans therapy. As shown in Fig. 3C, rGO@FeS2 nanozymes can effectively catalyze a low concentration of H2O2 (80 μM) to generate strong •OH. Then, we investigated the anti–C. albicans activity of rGO@FeS2 nanozymes in the presence of H2O2 in acetate–sodium acetate buffer solution (HAc-NaAc; pH 5.0) using the colony-forming unit (CFU) method. Compared with other groups, fungal colonies obviously decreased, indicating the lowest survival rate of C. albicans in the group treated with both rGO@FeS2 nanozymes and H2O2 (Fig. 3, D and E).
Under the same iron content, the intensity of •OH generation by rGO@FeS2, Pt3Fe, Fe3O4, and pyrite FeS2 nanozymes with H2O2 showed a positive correlation with their anti–C. albicans activities (fig. S10). It was mentioned that the rGO@FeS2 nanozymes with H2O2 had the most notable anti–C. albicans activity (figs. S11 and S12), which further highlighted the advantages of rGO@FeS2 nanozymes. Together, rGO@FeS2 nanozymes had excellent anti–C. albicans activity based on the POD-like activity to generate toxic •OH under acidic conditions, implying great potential for treating Candida vaginitis.
rGO@FeS2 nanozymes with Lactobacillus enhanced anti–C. albicans activities, including five samples of clinical isolates in vitro
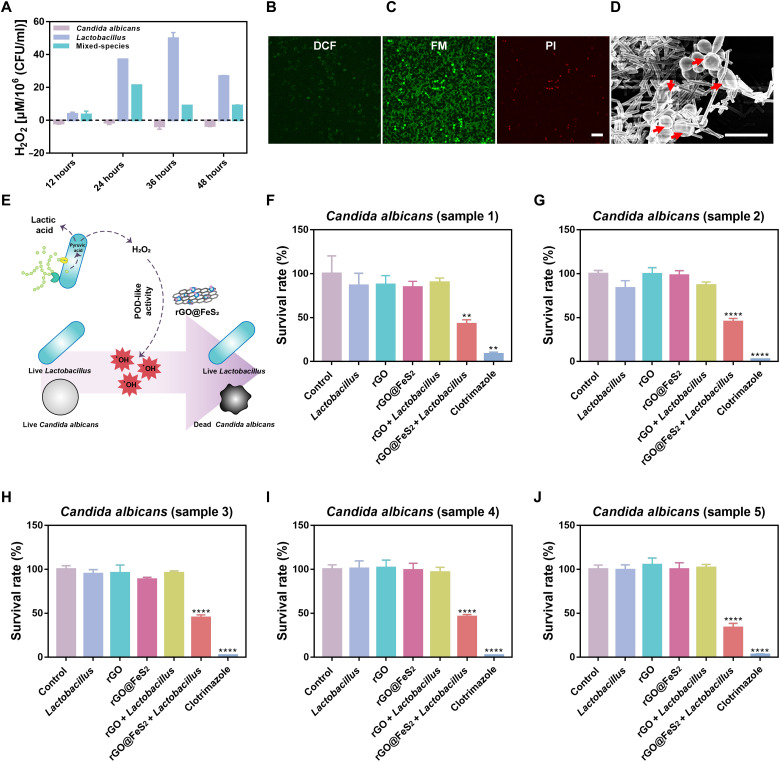
As demonstrated above, H2O2 is required and a low pH is preferred for the POD-like activity of rGO@FeS2 nanozymes. Therefore, we investigated whether the introduction of Lactobacillus would produce enough H2O2 and lower the pH substantially for rGO@FeS2 nanozymes to exert POD-like activity in treating C. albicans vaginitis. First, we detected the amounts of H2O2 generated during the Lactobacillus fermentation using a hydrogen peroxide assay kit and drew a standard curve of H2O2 accordingly (fig. S13A). As shown in Fig. 4A and fig. S13B, no matter how long it was cultured, C. albicans alone did not generate H2O2. In contrast, the Lactobacillus group generated H2O2 in a time-dependent manner, and the highest amount of H2O2 was obtained at 36 hours (about 50 μM). Moreover, the cofermentation of Lactobacillus with C. albicans (i.e., the mixed-species group) also generated H2O2. At the optimal cofermentation time (i.e., 24 hours), about 30 μM H2O2 was produced, which was lower than that of Lactobacillus alone and attributed to nutrient competition between them (16). Then, we examined whether Lactobacillus could lower the pH of de Man, Rogosa, and Sharpe (MRS) medium. Figure S13C shows that compared with C. albicans alone, the mixed-species group lowered the pH of MRS medium from 5.7 to 4.5 after 24 hours of cofermentation and maintained the pH thereafter. Further studies showed that rGO and rGO@FeS2 with Lactobacillus also lowered the pH of MRS medium (fig. S14). These results indicated that Lactobacillus can produce H2O2 and lower the pH of MRS medium, the two key requirements to achieve effective probiotic therapy of Candida vaginitis. Note that the cofermentation time of 24 hours was optimal and used in the following studies.
Fig. 4. rGO@FeS2 nanozymes with Lactobacillus enhanced anti–C. albicans activities, including five samples of clinical isolates in vitro.
(A) Amount of H2O2 in C. albicans, Lactobacillus, and mixed-species (C. albicans and Lactobacillus) groups. (B to D) ROS level (DCF) (B), cell viability staining (FM and PI) (C), and SEM images (D) of C. albicans treated with rGO@FeS2 nanozymes and Lactobacillus. Red arrows show the damaged cells. Scale bars, 20 μm (C) and 10 μm (D). (E) Schematic diagram of rGO@FeS2 catalyzing the generation of •OH from H2O2 and lactic acid produced by Lactobacillus to kill C. albicans. (F to J) Five different samples of C. albicans isolated from clinics treated with the indicated treatments. The medium was MRS medium, and the culture conditions were microaerophilic. The concentration of rGO, rGO@FeS2, and Clotrimazole was 25 μg/ml. The coincubation time was 24 hours. Data are presented as means ± SD (n = 3). **P < 0.01 and ****P < 0.0001.
Because the cofermentation of Lactobacillus with C. albicans can generate about 30 μM H2O2 (Fig. 4A), we also investigated the anti–C. albicans activity of rGO@FeS2 in the presence of H2O2 (30 μM). As shown in fig. S15, compared with other groups, fungal liabilities, and colonies decreased in the rGO@FeS2 + H2O2–treated group, indicating that rGO@FeS2 nanozymes with a lower concentration of H2O2 were still effective for anti–C. albicans activity. Then, we studied the anti–C. albicans activity of rGO@FeS2 and Lactobacillus. The colony morphology of C. albicans and Lactobacillus is different. The former grows into large and milky colonies, and the latter forms small and transparent colonies (fig. S16). These morphological characteristics enable us to distinguish them easily, facilitating the study of anti–C. albicans activity. As shown in figs. S16 and S17A, the treatment with Lactobacillus alone showed a negligible anti–C. albicans effect, whereas the treatment with rGO@FeS2 nanozymes and Lactobacillus resulted in a 70% reduction in the viability of C. albicans. In addition, rGO, rGO@FeS2, and rGO with Lactobacillus had minimal effects on the viability of C. albicans. Moreover, the treatments with rGO/Lactobacillus or rGO@FeS2/Lactobacillus hardly affected the viability of Lactobacillus (fig. S17B), which may be attributed to certain oxidative stress tolerance of Lactobacillus given its H2O2 producing property or proliferation and fermentation abilities under microaerophilic conditions (7).
To assess the anti–C. albicans mechanism of rGO@FeS2 and Lactobacillus, we investigated the oxidative state and structural integrity of both C. albicans and Lactobacillus. The intracellular ROS was detected by using 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA) as a fluorescence probe. DCFH-DA can be converted to 2′,7′-dichlorofluorescein (DCF) after reacting with ROS. The observation of laser scanning confocal microscopy clearly confirmed that rGO@FeS2 nanozymes and Lactobacillus treatment exhibited the strongest ability to generate intracellular ROS of C. albicans compared with other treatments (Fig. 4B and fig. S18A). Moreover, the disruption of C. albicans was further demonstrated using green fluorescent plasma membrane and vesiculation dye (FilmTracer FM 1-43) and red fluorescent propidium iodide (PI) nucleic acid dye. FilmTracer FM 1-43 is membrane-permeant, whereas PI only penetrates damaged cell membranes. As shown in Fig. 4C and fig. S18B, the confocal fluorescence images showed the most serious damage to C. albicans cell membrane in the group treated with rGO@FeS2 nanozymes and Lactobacillus. In addition, compared with other treatments, characterization of C. albicans and Lactobacillus surfaces by scanning electron microscope (SEM) imaging showed that the structural integrity of treated C. albicans was the most severely affected after the treatment with rGO@FeS2 nanozymes and Lactobacillus (Fig. 4D and fig. S18C). The oxidative state and structural integrity induced by the treatment of rGO@FeS2 nanozymes and Lactobacillus were observed only on C. albicans but not on Lactobacillus (Fig. 4, B to D). Collectively, these results demonstrated that rGO@FeS2 nanozymes can use H2O2 and lactic acid produced by Lactobacillus to exert POD-like activity and further generate ROS, thereby causing the death of C. albicans without affecting the activity of Lactobacillus. The corresponding schematic illustration of the self-cascade reaction between rGO@FeS2 nanozymes and Lactobacillus is depicted in Fig. 4E. In general, the combination of rGO@FeS2 nanozymes and Lactobacillus had great potential for treating vaginitis caused by C. albicans.
On the basis of the results above, to further demonstrate the translational potential of the self-cascade action between rGO@FeS2 nanozymes and Lactobacillus in the treatment of Candida vaginitis, we explored the effects on clinically isolated C. albicans, which were named samples 1, 2, 3, 4, and 5, respectively. Similar to the findings in figs. S14, S16, and S17, compared with Lactobacillus treatment, the cell viability and colonies of clinically isolated C. albicans treated with rGO@FeS2 nanozymes and Lactobacillus were significantly decreased (Fig. 4, F to J and fig. S19). Likewise, the pH of MRS medium could be lowered to around 4.5, and the activities of Lactobacillus were hardly affected after the treatment of rGO@FeS2 nanozymes and Lactobacillus (figs. S20 and S21). Meanwhile, we chose Clotrimazole suppository, one of the most commonly used medications in clinics (10), for a comparative study of the anti–C. albicans activity toward clinical isolates. As shown in Fig. 4 (F to J), although Clotrimazole had a strong anti–C. albicans activity toward clinical isolates, it had severe cytotoxicity at very low concentrations (fig. S22); on the other hand, it could not adjust the pH of MRS medium (fig. S20). Therefore, if Clotrimazole was used to treat C. albicans–infected vaginitis, then it would not only affect the repair of vaginal mucosa but also increase the risk of Candida vaginitis recurrence due to failure to regulate the vaginal microenvironment.
Effects of rGO@FeS2 nanozyme oxidation on their activities
It is critical to maintain the anti–C. albicans and POD-like activities of rGO@FeS2 for Candida vaginitis therapy. We investigated the sensitivity of rGO@FeS2 to oxygen by storing the material in vacuum and air for 12 days via XRD and XPS analysis. As shown in fig. S23, rGO@FeS2 nanozymes were oxidized when exposed to air, whereas their oxidation was obviously reduced in vacuum. We further monitored the anti–C. albicans activity of rGO@FeS2 after potential oxidation. It was shown that the anti–C. albicans activity of rGO@FeS2 exposed to air gradually decreased (fig. S24, A to C) and disappeared after 12 days (fig. S24D) in the presence of H2O2. In contrast, rGO@FeS2 with H2O2 retained the anti–C. albicans activity after 30 days of storage in vacuum (fig. S24E). Accordingly, the •OH generating activity of oxidized rGO@FeS2 and H2O2 was notably reduced (fig. S24F). Notably, although rGO@FeS2 nanozymes were sensitive to oxygen and the potential oxidation can affect their anti–C. albicans and POD-like activities, the proper storage would retain the activities. In practice, medications can be vacuum packed to prolong the storage time. In our study, we developed a hydrogel formulation of rGO@FeS2 and Lactobacillus, which will be easily vacuum-packed. We therefore reason that the activities of rGO@FeS2 would be effectively retained for practical use, for example, using vacuum packaging.
Effects of FeLab on C. albicans and Lactobacillus and the release profile of Lactobacillus with HAase in vitro
Since HAase secreted by Candida and bacteria can degrade HA, we used HA hydrogel to encapsulate rGO@FeS2 nanozymes and Lactobacillus to form a FeLab preparation (25, 36). It was beneficial to inject the hydrogel delivery system into the vagina and reduce leakage. Moreover, through the controllably triggered response to the C. albicans–infected microenvironment, the hydrogel preparation could achieve an enhanced ROS concentration in situ and minimize damage to vaginal mucosal cells (37). While having the above advantages, it was critical that the encapsulation within HA hydrogels neither affected the viability of Lactobacillus nor hindered anti–C. albicans effects exerted by the self-cascading reaction between rGO@FeS2 nanozymes and Lactobacillus. As shown in fig. S25A, the viability of Lactobacillus (105 CFU/ml) encapsulated in HA hydrogels was not affected. Therefore, Lactobacillus (105 CFU/ml) was encapsulated into HA hydrogels for further anti–C. albicans activities. Compared with the treatment of other HA hydrogels [i.e., Lactobacillus@HA (Lab), rGO@HA, rGO@FeS2@HA, and rGO/Lactobacillus@HA (rGOLab)], the pH of MRS medium could be lowered to around 4.5 after treatment with FeLab (fig. S25B). Moreover, the cell viability of C. albicans treated with FeLab was significantly decreased, confirming the retained antifungal activity of rGO@FeS2/Lactobacillus after HA coating (fig. S25, C and D). Then, the HAase-simulated release profile of Lactobacillus was investigated. As shown in fig. S26, stimulated by the HAase solution, Lactobacillus showed a sustained release and achieved a nearly 100% release in 24 hours. In contrast, HA hydrogels without HAase solution did not cause substantial leakage of cargoes, indicating that the HA hydrogel with HAase secreted by Candida and bacteria can controllably release contents for anti–C. albicans activities.
Antifungal effects of FeLab on C. albicans–induced vaginitis in vivo
To further assess its potential as a topical antifungal medication, we evaluated the antifungal effects of FeLab on C. albicans–induced vaginitis in vivo. Clotrimazole group (as a representative small molecule drug) was included for comparison. First, female BALB/c mice were injected with estradiol benzoate solutions every 2 days. Then, C. albicans solutions were injected into the vagina to establish a vaginitis model of C. albicans infection. After successful establishment of the model, treatments were performed intravaginally once a day for 5 days. Last, vaginal washes were obtained for colony counting and microbiota composition analysis. Subsequently, mice were euthanized to obtain vaginal tissues for histochemistry (Fig. 5A). As shown in Fig. 5 (B and C) and fig. S27, compared with Lab treatment, the cell viability and colony formation of C. albicans with both FeLab and Clotrimazole treatments were significantly decreased in vaginal washes. Histochemistry analysis showed that main organs, including the heart, liver, spleen, lung, and kidney, were not affected after 5 days of continuous vaginal administration in all treatment groups (fig. S28). However, through histological staining of vaginal tissues, vaginal keratinization and mucosal and submucosal inflammatory cell infiltration in the FeLab treatment group were notably lower than those in the group treated with Clotrimazole (Fig. 5D and fig. S29). This indicated that Clotrimazole caused serious damage to vaginal mucosa cells (consistent with the results of fig. S22), while FeLab was conducive to promoting the repair of vaginal mucosa. Together, the above results indicated that the responsive release of FeLab was triggered at the vaginal site infected with C. albicans. After release, Lactobacillus could regulate the microenvironment of the vagina by reducing the pH to about 4.5 and producing H2O2, which was conducive to the catalysis of rGO@FeS2 nanozymes to generate a large amount of •OH to kill C. albicans in situ, thereby minimizing the damage to vaginal mucosal cells (Fig. 5E) (7, 38). In contrast, although Clotrimazole had anti–C. albicans activity by affecting the permeability of the cell membrane, it caused severe damage to vaginal mucosal cells due to poor selectivity. On the other hand, Clotrimazole could not regulate the pH of the vagina (over 5.0; fig. S20), which is the optimal condition for C. albicans to grow and may lead to a greater risk of Candida vaginitis recurrence than FeLab (Fig. 5F).
Fig. 5. Antifungal effects of FeLab on C. albicans–induced vaginitis in vivo.
(A) Experimental scheme of C. albicans–infected vaginitis mice. (B) Cell viabilities of C. albicans in vaginal washes after indicated treatments. (C and D) Digital images of C. albicans colonies in the vaginal washes (C) and H&E-staining images of the vaginal tissue (D) in different groups. I, health; II, control; III, FeLab; IV, Clotrimazole. Scale bar, 100 μm. (E and F) Schematic diagram comparing FeLab (E) with Clotrimazole (F) for the treatment of Candida vaginitis in mice. Data are presented as means ± SD (n = 5). *P < 0.05.
To test our hypothesis, we next investigated the effects of FeLab and Clotrimazole on Candida vaginitis recurrence in mice. Candida vaginitis mice in Fig. 5A were treated with FeLab and Clotrimazole, respectively. Then, C. albicans solutions were reinjected into the vagina to establish a recurrence model. After 24 hours, vaginal washes and vaginal tissues were collected to assess recurrence effects. As shown in fig. S30, the cell viability and colonies of C. albicans treated with FeLab were lower than those treated with Clotrimazole in vaginal washes. Moreover, the hematoxylin and eosin (H&E) analysis of vaginal tissues revealed that the former had less infiltration of vaginal mucositis cells. These results suggested that although Clotrimazole had strong anti–C. albicans activity, FeLab could more notably reduce the risk of Candida vaginitis recurrence (3).
Vaginal microbiome amelioration in mice with C. albicans–induced vaginitis after FeLab treatment versus Clotrimazole
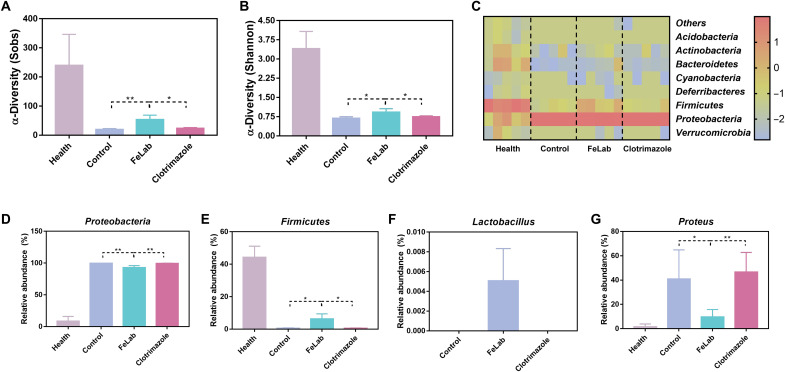
Evidence suggests that patients with Candida vaginitis are associated with dysbiosis characterized by decreased biodiversity, a lower proportion of the Firmicutes ratio, and an increase in Proteobacteria (39, 40). Thus, we investigated whether FeLab therapy could better modulate the composition of the vaginal microbiota than Clotrimazole treatment in mice through the second ribosomal internal transcribed spacer amplicon sequencing and the Sobs and Shannon entropy index of α-diversity by 16S ribosomal RNA gene sequencing. Analysis of vaginal wash samples revealed that compared with Clotrimazole, FeLab treatment markedly improved fungal and bacterial diversities in mice with Candida vaginitis, as indicated by the increase in the number of unique operational taxonomic units (Fig. 6, A and B, and fig. S31). Further analysis at the phylum level revealed that FeLab treatment markedly reduced the relative abundance of Proteobacteria and increased the relative abundance of Firmicutes, reshaping a healthier vaginal microbiome (Fig. 6, C to E). At the genus level, the relative abundance of Lactobacillus, which can promote the maintenance of vaginal homeostasis and prevent the colonization and growth of adverse microorganisms, significantly increased (Fig. 6F and fig. S32), while Proteus significantly decreased (Fig. 6G) after treatment with FeLab (41–43). Together, compared with Clotrimazole, FeLab could increase the richness and diversity of the vaginal microbiome, especially Lactobacillus, to efficiently treat Candida vaginitis and reduce the recurrence, demonstrating the promise to treat and prevent vaginal dysbiosis. We are also aware that the vaginal microenvironment and microbiota of rodent models are different from those of humans in Candida vaginitis (44, 45). Therefore, a detailed relationship between rodent models and human disease still needs further exploration.
Fig. 6. Vaginal microbiome amelioration in mice with C. albicans–induced vaginitis after FeLab treatment versus Clotrimazole.
(A and B) α-Diversity Sobs (A) and Shannon (B) indexes of the vaginal microbiome. (C) Heatmap of the relative abundance of phylum-level taxa for each mouse (columns). (D and E) Relative abundance of Proteobacteria (D) and Firmicutes (E) taxa, respectively. (F and G) Relative abundance of genus-level taxa for Lactobacillus (F) and Proteus (G), respectively. Data are presented as means ± SD (n = 4). *P < 0.05 and **P < 0.01.
DISCUSSION
In summary, a responsive hydrogel FeLab with POD-like and vaginal microenvironment regulating activities was developed to kill C. albicans and modulate vaginal microbiota balance for Candida vaginitis treatment and reduce the recurrence. The designed rGO@FeS2 structure ensured uniform dispersion of marcasite FeS2 nanoparticles on the rGO supports, and the encapsulated Lactobacillus could produce H2O2 and lower the pH, facilitating the enhancement of POD-like activity to kill C. albicans in a C. albicans–induced vaginitis model. The self-cascade catalysis hardly affected Lactobacillus, thus effectively improving vaginal microbiota and reshaping a healthier vaginal microenvironment. This work not only demonstrates the advantages of nanozyme-probiotic combinations over small molecular antifungal drugs for Candida vaginitis treatment and reducing recurrence but also shows the translational promise of nanozymes.
MATERIALS AND METHODS
Materials
Iron chloride hexahydrate (FeCl3·6H2O), ferrous sulfate (FeSO4), ferric chloride (FeCl3), thiourea [(NH2)2CS], sodium hydroxide (NaOH), hydrochloric acid (HCl), hydrogen peroxide (H2O2; 30%), benzoic acid, NaAc, ethylene glycol (EG), trichloromethane (CHCl3), and ammonia water (NH3·H2O; 25 to 28%) were purchased from Sinopharm Chemical Reagent Co. Ltd. (Shanghai, China). Polyvinylpyrrolidone (PVP; 8000) and TMB were purchased from Aladdin Chemical Co. Ltd. (Shanghai, China). Iron acetylacetonate [Fe(acac)3] and platinum acetylacetonate [Pt(acac)2] were purchased from J&K Scientific Co. Ltd. (Beijing, China). Sodium hyaluronic acid (weight-average molecular weight = 0.3 MDa), HAase, l-cysteine methyl ester hydrochloride, 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDC), and N-hydroxysuccinimide (NHS) were purchased from Yuanye Bio-Technology Co. Ltd. (Shanghai, China). DMPO was purchased from JK Chemical and Dojindo (Japan). Dimethyl sulfoxide (DMSO), DCFH-DA, PI, methylthiazolyldiphenyl-tetrazolium bromide (MTT), and Dulbecco’s modified Eagle medium (DMEM) were purchased from Beyotime Chemical Reagent Co. Ltd. (Shanghai, China). FilmTracer FM 1-43 was purchased from Thermo Fisher Scientific (USA). MRS medium was obtained from Haibo Biotechnology Co. Ltd. (Shandong, China). Estradiol benzoate injection was purchased from Quanyu Bio-Technology Animal Pharmaceutical Co. Ltd. (Shanghai, China). Clotrimazole suppository was purchased from Baiyunshan Pharmaceutical Holdings Co. Ltd. (Guangdong, China). GO was purchased from Jiangsu Xianfeng Nanomaterials Technology Co. Ltd. (Jiangsu, China). All aqueous solutions were prepared with deionized water (18.2 megohm·cm; Millipore).
Instrumentation
TEM imaging was performed on a JEM-2100 (JEOL, Japan) transmission electron microscope at an acceleration voltage of 200 kV. Powder XRD patterns were measured at 5°/min using a diffractometer (Rigaku Ultima III, Japan and Bruker D8 advance, Germany) with a Cu Kα radiation. XPS spectra were collected by using a PHI 5000 Versa Probe XPS microscope (Ulvac-Phi, Japan). Ultraviolet-visible absorption spectra were measured on a SpectraMax M2e microplate reader (Molecular Devices, USA). HAADF-STEM and the corresponding energy-dispersive spectroscopy elemental mappings were performed on a Tecnai G2 F30 (FEI, USA). EPR spectra were recorded on a Bruker A300 spectrometer (X-band). The pH value of MRS medium was measured using a pH meter (STARTER 3100, USA). Cell images of C. albicans and Lactobacillus were recorded on a laser scanning confocal microscope (Olympus, Japan). Tissue images of H&E staining were photographed by a DMi8 fluorescence microscope (Leica, Germany).
Synthesis of rGO@FeS2, Pt3Fe, Fe3O4, and pyrite FeS2 nanozymes
rGO@FeS2 nanozymes were synthesized as follows (34). Five milliliters of FeCl3 (0.135 g/ml), 5 ml of (NH2)2CS (0.038 g/ml), and 30 μl of NH3·H2O were added to 7.5 ml of GO aqueous solution (5.6 mg/ml) under mild ultrasonication. Subsequently, the mixture solution was heated at 180°C for 12 hours to form marcasite FeS2 nanoparticle–decorated rGO (rGO@FeS2). After rGO@FeS2 nanozymes were dialyzed with ultrapure water for 3 days, they were subjected to lyophilization for use. For comparison, rGO supports and pristine marcasite FeS2 nanoparticles were also prepared via similar methods without the addition of FeCl3 and (NH2)2CS or GO aqueous solutions, respectively.
Pt3Fe nanozymes were synthesized as follows (28). Pt(acac)2 (20.21 mg), Fe(acac)3 (21.4 mg), benzoic acid (50 mg), and PVP (80 mg) were dispersed in benzyl alcohol (5 ml) under vigorous stirring for 15 min. Subsequently, the resultant mixture was transferred into a reactor at 180°C for 12 hours. Pt3Fe nanozymes were precipitated by acetone, washed six times with an ethanol-acetone mixture, and dispersed into water for use.
Fe3O4 nanozymes were synthesized as follows (29). A mixture of FeCl3 and FeSO4 (molar ratio, 2:1) solutions was prepared under N2 protection. Then, enough NH3·H2O was dropped into it under vigorous stirring. After 30 min, the resulting Fe3O4 nanozymes were washed five times immediately with distilled water by magnetic separation.
Pyrite FeS2 nanozymes were synthesized as follows (30). Since the as-synthesized marcasite FeS2 nanoparticles were too compacted to disperse (Fig. 2C), we synthesized pyrite FeS2 nanozymes for comparison. In a typical synthesis, PVP (0.7 g), FeCl3·6H2O (0.5 g), and NaAc (3.6 g) were dispersed into 30 ml of EG solution under vigorous stirring. Next, S powder (0.4 g) was added, and the resultant mixture was ultrasonicated for 1 hour to form a homogeneous dispersion. The mixture was transferred into a reactor at 200°C for 12 hours. Pyrite FeS2 nanozymes were isolated with the addition of excess CHCl3, alcohol, and ultrapure water by centrifugation.
Synthesis of HA hydrogel
The HA hydrogel was prepared following a previously reported method (37). In detail, HA (0.4 g), NHS (0.575 g, 5 mM), and EDC (0.958 g, 5 mM) were dissolved thoroughly in deionized water (100 ml) for 1 hour at room temperature to fully activate the carboxylic groups of HA. Then, l-cysteine methyl ester hydrochloride (0.855 g, 5 mM) was added to the mixture under continuous stirring for 24 hours with light protection. The pH value of the above solution was adjusted to 4.8 by adding 1.0 M NaOH or 1.0 M HCl. The resulting solution was dialyzed (molecular weight cutoff, 1000) against dilute HCl solution (pH 3.5) to remove unnecessary agents, followed by lyophilization to obtain the target conjugates as fluffy solids (HA-SH). Then, HA-SH conjugates (16 mg), Lactobacillus (105 CFU/ml), and rGO@FeS2 nanozymes (25 μg/ml) were dissolved in phosphate-buffered saline (PBS) (400 μl; pH 8), and the mixture was vortexed for 10 s to yield a homogeneous stock solution. After that, the precursor solution was incubated at 37°C without shaking for complete FeLab formation. Similarly, a series of hydrogels with/without Lactobacillus or rGO@FeS2 nanozymes were prepared.
POD-like activity and kinetic assay
The POD-like activity of rGO@FeS2 nanozymes was determined using TMB as the substrate in the presence of H2O2. In a typical experiment, rGO@FeS2 nanozymes (25 μg/ml), TMB (0.2 mM), and H2O2 (0.08 mM) were added to 200 μl of HAc-NaAc buffer (0.1 M; pH 4.5). Then, the absorbance at 652 nm for oxTMB was recorded at a certain reaction time to evaluate the POD-like activity. The pH-dependent POD-like activity of rGO@FeS2 nanozymes was studied under different pH values, including 4.0, 4.5, 5.0, 5.5, 6.0, and 6.5, in HAc-NaAc buffer.
Steady-state kinetics assays were conducted at 25°C in 200 μl of HAc-NaAc buffer with rGO@FeS2 nanozymes (25 μg/ml) as a catalyst in the presence of H2O2 and TMB. On the one hand, TMB (0.2 mM) and different concentrations of H2O2 (0, 0.025, 0.05, 0.1, 0.2, 0.4, and 0.8 mM) were added to the reaction system with H2O2 as the substrate. On the other hand, H2O2 (0.08 mM) and different concentrations of TMB (0, 0.1, 0.2, 0.3, 0.35, 0.4, and 0.45 mM) were added to the reaction system with TMB as the substrate. The Michaelis-Menten constants were calculated according to the Michaelis-Menten saturation curve by GraphPad Prism 7.0 (GraphPad Software).
Lactobacillus and C. albicans culture
Lactobacillus capsules (S20030005) for treating bacterial vaginitis were obtained from Inner Mongolia Shuangqi Pharmaceutical Co. Ltd. The capsules were dissolved in MRS medium and inoculated on MRS medium agar plates for 12 hours (microaerophilic conditions at 37°C) via an inoculation loop to obtain a single colony. The single colony was placed in fresh MRS medium and cultured overnight for further experiments. C. albicans, including five samples of clinical isolates, was cultured in a similar method except under microaerophilic conditions.
Anti–C. albicans activities of four nanozymes
C. albicans cultures were washed three times with sterile PBS and divided into twelve groups: (i) control (C. albicans), (ii) C. albicans + H2O2, (iii) C. albicans + rGO, (iv) C. albicans + rGO@FeS2, (v) C. albicans + Pt3Fe, (vi) C. albicans + Fe3O4, (vii) C. albicans + pyrite, (viii) C. albicans + rGO + H2O2, (ix) C. albicans + rGO@FeS2 + H2O2, (x) C. albicans + Pt3Fe + H2O2, (xi) C. albicans + Fe3O4 + H2O2, and (xii) C. albicans + pyrite + H2O2. The concentrations of C. albicans, H2O2, and nanozymes were 106 CFU/ml, 80 μM, and 25 μg/ml in HAc-NaAc buffer (0.1 M; pH 5.0), respectively. These mixed suspensions were then incubated for 2 hours at 37°C, and 100 μl from them was spread evenly on MRS medium agar plates. After incubation for 48 hours at 37°C, the photos were taken, and quantitative analysis of anti–C. albicans activities was performed by ImageJ.
Electron spin resonance analysis of •OH formation
Glass capillary tubes containing four nanozymes (25 μg/ml), H2O2 (80 μM), DMPO (10 mM), and HAc-NaAc buffer (0.1 M; pH 4.5) were inserted into the EPR cavity to record •OH signals after 5 min of reaction.
Enhanced anti–C. albicans activities with Lactobacillus, including five samples of clinical isolates in vitro
Five samples of clinically isolated C. albicans were obtained from the Department of Clinical Laboratory of Nanjing Drum Tower Hospital, Medical School of Nanjing University of consent and processed with approval from the Review Board (2022-543-01). C. albicans and Lactobacillus cultured were washed three times with sterile PBS and divided into seven groups in a 24-well plate: (i) control (C. albicans), (ii) C. albicans + Lactobacillus, (iii) C. albicans + rGO, (iv) C. albicans + rGO@FeS2, (v) C. albicans + rGO + Lactobacillus, (vi) C. albicans + rGO@FeS2 + Lactobacillus, and (vii) C. albicans + Clotrimazole. The final concentrations of C. albicans, Lactobacillus, rGO@FeS2, and Clotrimazole were 106 CFU/ml, 102 CFU/ml, 25 μg/ml, and 25 μg/ml (except excipient), respectively, in 2 ml of MRS medium. These mixed suspensions were then incubated for 24 hours at 37°C under microaerophilic conditions, and 100 μl of them was spread on MRS medium agar plates. After incubation for 48 hours under the same culture conditions, the photos were taken, and the quantitative analysis of anti–C. albicans activities was performed by ImageJ. C. albicans formed large and milky colonies, whereas Lactobacillus was small and transparent. In addition, the pH values of the mixed MRS medium were measured with a pH meter.
Determination of intracellular ROS
As an oxidant-sensitive dye, DCFH-DA was used to measure intracellular ROS levels. C. albicans, Lactobacillus, or a mixture of both were stained with 10 μM DCFH-DA for 30 min in the dark at room temperature and washed twice with sterile PBS, followed by (i) to (vi) treatments. The intracellular ROS levels were measured using a confocal laser microscope with excitation and emission wavelengths at 488 and 525 nm, respectively.
Morphological observation of C. albicans and Lactobacillus
Nest circle microscope cover glass was used at the bottom of a 24-well plate. The C. albicans and Lactobacillus treated by (i) to (vii) for 24 hours were fixed with 2.5% glutaraldehyde overnight at 4°C in the dark. The fungal and bacterial cells were then dehydrated with 30, 50, 70, 80, 90, and 100% of ethanol, respectively, for 10 min. Last, the dried cells were sputter-coated with iridium (6 nm) for SEM observation.
Live/dead cell staining of C. albicans and Lactobacillus
The C. albicans and Lactobacillus treated by (i) to (vi) were stained with FilmTracer FM 1-43 and PI for 30 min and washed three times with sterile 0.9% NaCl solution for visualization via a confocal fluorescence microscope.
In vitro cytotoxicity experiments
The cell viabilities of mouse embryonic fibroblast cells (NIH/3T3) and a human normal hepatocyte line (L02) to rGO, rGO@FeS2, and Clotrimazole were determined by an MTT assay. First, the cells were incubated with DMEM in a 96-well plate (about 5000 cells per well, five wells per concentration) in a humidified incubator (37°C, 5% CO2) for 24 hours. Then, the cells were treated with different concentrations of rGO, rGO@FeS2, and Clotrimazole solutions (10, 25, 50, 80, and 100 μg/ml) for 12 hours. Subsequently, MTT (0.5 mg/ml) was added to the cells and incubated for 4 hours. Last, the medium was removed, and 100 μl of DMSO was added. The absorbance was determined at 490 nm, and the cell viabilities were expressed as a percentage of the control.
Controlled-release experiment of Lactobacillus
The release behavior of Lactobacillus from HA hydrogels was monitored as follows (37). A certain amount of Lactobacillus was encapsulated in a hydrogel of 109 CFU/ml. Specifically, the prepared Lab (10 ml) was individually immersed into PBS buffer (50 ml; pH 5.0) and HAase solution (150 U/mg) with constant shaking. At predetermined time intervals, 100 μl of the surrounding medium was taken to measure the optical density at 600 nm by a microplate reader. The release rate was calculated on the basis of the percentage of released Lactobacillus within the whole encapsulated Lactobacillus in the respective release medium.
C. albicans–infected vaginitis model and therapeutic process in vivo
All animal experiments were approved by the Committee for Experimental Animals Welfare and Ethics of Nanjing University (Institutional Animal Care and Use Committee, 2202010). The anti–C. albicans effects of FeLab were evaluated by using C. albicans–infected mice. After 7 days of adaptation to the environment, female BALB/c mice (18 to 20 g) were randomly divided into eight groups with six mice per group: (i) health, (ii) control, (iii) Lab, (iv) rGO@HA, (v) rGO@FeS2@HA, (vi) rGOLab, (vii) FeLab, and (viii) Clotrimazole. Mice except the healthy group were subcutaneously injected with 0.1 ml of estradiol benzoate injection (2 mg/ml) once 2 days for 6 days. On the 7th day, 20 μl of C. albicans solution (1 × 109 CFU/ml) was injected into the vagina with a pipette gun, and the mice were fed normally for 1 day. Note that this day was designated as day 0. C. albicans–induced mice were intravaginally injected with various HA hydrogels (4%) and Clotrimazole for five consecutive days (days 1, 2, 3, 4, and 5). Among them, the concentrations of encapsulated Lactobacillus, rGO@FeS2, and Clotrimazole were 105 CFU/ml, 0.4 mg/kg, and 0.4 mg/kg (except excipient), respectively. On day 6, the vagina was washed repeatedly with sterile PBS (20 μl) by pipetting five times to obtain vaginal washes, which were used for quantitative analysis of C. albicans cell viability on MRS medium agar plates and vaginal microbiome analysis. Following anesthesia with diethyl ether, all groups of mice were euthanized, and the vagina, heart, liver, spleen, lung, and kidney were collected and fixed with paraformaldehyde (4%). These tissues were dissected, analyzed by H&E staining, and imaged by an optical microscope.
C. albicans–infected vaginitis recurrence model in vivo
On the basis of the above modeling methods, 12 C. albicans–infected mice were equally divided into two groups and then treated with FeLab and Clotrimazole, respectively. Note that this day was designated as day 0. On day 1, 20 μl of C. albicans solution (1 × 109 CFU/ml) was injected into the vagina with a pipette gun, and the mice were fed normally for 1 day. Similarly, vaginal washes were obtained for quantitative analysis of C. albicans cell viability, and vaginal tissues were collected for further H&E analysis.
Statistical analysis
Data were shown as means ± SD. Statistical analysis was performed using Student’s t test for two-group differences and one-way two-sided analysis of variance (ANOVA) for multiple comparisons. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001
Acknowledgments
Funding: This work was supported by the National Key R&D Program of China (2019YFA0709200 and 2021YFF1200700), the National Natural Science Foundation of China (21874067 and 21722503), the CAS Interdisciplinary Innovation Team (JCTD-2020-08), the PAPD Program, and Fundamental Research Funds for the Central Universities (202200325).
Author contributions: H.W. conceived the project. H.W. and G.W. designed the experiments. G.W., Q.L., W.L., Y.Z., X.Z., and W.Z. performed the experiments. G.W., Q.L., X.W., Z.Z., W.L., Y.Z., S.L., and C.Z. analyzed the results. H.W., G.W., X.W., Z.Z., W.L., and Y.Z. wrote the manuscript. All authors discussed the results and commented on the manuscript.
Competing interests: The authors declare that they have no competing interests, and the patent application is in progress.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials.
Supplementary Materials
This PDF file includes:
Figs. S1 to S32
Table S1
REFERENCES AND NOTES
- 1.M. Ilkit, A. B. Guzel, The epidemiology, pathogenesis, and diagnosis of vulvovaginal candidosis: A mycological perspective. Crit. Rev. Microbiol. 37, 250–261 (2011). [DOI] [PubMed] [Google Scholar]
- 2.L. O. Eckert, Acute vulvovaginitis. N. Engl. J. Med. 355, 1244–1252 (2006). [DOI] [PubMed] [Google Scholar]
- 3.D. W. Denning, M. Kneale, J. D. Sobel, R. Rautemaa-Richardson, Global burden of recurrent vulvovaginal candidiasis: A systematic review. Lancet Infect. Dis. 18, e339–e347 (2018). [DOI] [PubMed] [Google Scholar]
- 4.Å. Sullivan, C. Edlund, C. E. Nord, Effect of antimicrobial agents on the ecological balance of human microflora. Lancet Infect. Dis. 1, 101–114 (2001). [DOI] [PubMed] [Google Scholar]
- 5.S. L. Hillier, M. A. Krohn, L. K. Rabe, S. J. Klebanoff, D. A. Eschenbach, The normal vaginal flora, H2O2-producing Lactobacilli, and bacterial vaginosis in pregnant women. Clin. Infect. Dis. 16, 273–281 (1993). [DOI] [PubMed] [Google Scholar]
- 6.J. M. Wells, A. Mercenier, Mucosal delivery of therapeutic and prophylactic molecules using lactic acid bacteria. Nat. Rev. Microbiol. 6, 349–362 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.M. France, M. Alizadeh, S. Brown, B. Ma, J. Ravel, Towards a deeper understanding of the vaginal microbiota. Nat. Microbiol. 7, 367–378 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.S. Husain, J. Allotey, Z. Drymoussi, M. Wilks, B. M. Fernandez-Felix, A. Whiley, J. Dodds, S. Thangaratinam, C. McCourt, E. M. Prosdocimi, W. G. Wade, B. M. de Tejada, J. Zamora, K. Khan, M. Millar, Effects of oral probiotic supplements on vaginal microbiota during pregnancy: A randomised, double-blind, placebo-controlled trial with microbiome analysis. BJOG 127, 275–284 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.J. van de Wijgert, M. C. Verwijs, Lactobacilli-containing vaginal probiotics to cure or prevent bacterial or fungal vaginal dysbiosis: A systematic review and recommendations for future trial designs. BJOG 127, 287–299 (2020). [DOI] [PubMed] [Google Scholar]
- 10.Y. Lee, E. Puumala, N. Robbins, L. E. Cowen, Antifungal drug resistance: Molecular mechanisms in Candida albicans and beyond. Chem. Rev. 121, 3390–3411 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.J. Berman, P. E. Sudbery, Candida albicans: A molecular revolution built on lessons from budding yeast. Nat. Rev. Genet. 3, 918–931 (2002). [DOI] [PubMed] [Google Scholar]
- 12.M. C. Watson, J. M. Grimshaw, C. M. Bond, J. Mollison, A. Ludbrook, Oral versus intra-vaginal imidazole and triazole anti-fungal agents for the treatment of uncomplicated vulvovaginal candidiasis (thrush): A systematic review. BJOG 109, 85–95 (2002). [DOI] [PubMed] [Google Scholar]
- 13.C. M. A. Santos, M. C. V. Pires, T. L. Leão, Z. P. Hernández, M. L. Rodriguez, A. K. S. Martins, L. S. Miranda, F. S. Martins, J. R. Nicoli, Selection of Lactobacillus strains as potential probiotics for vaginitis treatment. Microbiology 162, 1195–1207 (2016). [DOI] [PubMed] [Google Scholar]
- 14.R. Palmeira-de-Oliveira, A. Palmeira-de-Oliveira, J. Martinez-de-Oliveira, New strategies for local treatment of vaginal infections. Adv. Drug. Deliver. Rev. 92, 105–122 (2015). [DOI] [PubMed] [Google Scholar]
- 15.M. Pirotta, J. Gunn, P. Chondros, S. Grover, P. O'Malley, S. Hurley, S. Garland, Effect of lactobacillus in preventing post-antibiotic vulvovaginal candidiasis: A randomised controlled trial. BMJ 329, 548–553 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.R. Alonso-Roman, A. Last, M. H. Mirhakkak, J. L. Sprague, L. Möller, P. Großmann, K. Graf, R. Gratz, S. Mogavero, S. Vylkova, G. Panagiotou, S. Schäuble, B. Hube, M. S. Gresnigt, Lactobacillus rhamnosus colonisation antagonizes Candida albicans by forcing metabolic adaptations that compromise pathogenicity. Nat. Commun. 13, 3192–3207 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.M. E. Falagas, G. I. Betsi, S. Athanasiou, Probiotics for the treatment of women with bacterial vaginosis. Clin. Microbiol. Infect. 13, 657–664 (2007). [DOI] [PubMed] [Google Scholar]
- 18.E. Brillas, I. Sirés, M. A. Oturan, Electro-Fenton process and related electrochemical technologies based on Fenton’s reaction chemistry. Chem. Rev. 109, 6570–6631 (2009). [DOI] [PubMed] [Google Scholar]
- 19.A. Scheeline, D. L. Olson, E. P. Williksen, G. A. Horras, M. L. Klein, R. Larter, The peroxidase-oxidase oscillator and its constituent chemistries. Chem. Rev. 97, 739–756 (1997). [DOI] [PubMed] [Google Scholar]
- 20.L. Zhang, S.-S. Wan, C.-X. Li, L. Xu, H. Cheng, X.-Z. Zhang, An adenosine triphosphate-responsive autocatalytic Fenton nanoparticle for tumor ablation with self-supplied H2O2 and acceleration of Fe(III)/Fe(II) conversion. Nano Lett. 18, 7609–7618 (2018). [DOI] [PubMed] [Google Scholar]
- 21.L. Gao, J. Zhuang, L. Nie, J. Zhang, Y. Zhang, N. Gu, T. Wang, J. Feng, D. Yang, S. Perrett, X. Yan, Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat. Nanotechnol. 2, 577–583 (2007). [DOI] [PubMed] [Google Scholar]
- 22.J. Wu, X. Wang, Q. Wang, Z. Lou, S. Li, Y. Zhu, L. Qin, H. Wei, Nanomaterials with enzyme-like characteristics (nanozymes): Next-generation artificial enzymes (II). Chem. Soc. Rev. 48, 1004–1076 (2019). [DOI] [PubMed] [Google Scholar]
- 23.B. Yang, Y. Chen, J. Shi, Reactive oxygen species (ROS)-based nanomedicine. Chem. Rev. 119, 4881–4985 (2019). [DOI] [PubMed] [Google Scholar]
- 24.M. Liu, L. Huang, X. Xu, X. Wei, X. Yang, X. Li, B. Wang, Y. Xu, L. Li, Z. Yang, Copper doped carbon dots for addressing bacterial biofilm formation, wound infection, and tooth staining. ACS Nano 16, 9479–9497 (2022). [DOI] [PubMed] [Google Scholar]
- 25.R. Stern, M. J. Jedrzejas, Hyaluronidases: Their genomics, structures, and mechanisms of action. Chem. Rev. 106, 818–839 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.M. S. Kim, S. Cho, S. H. Joo, J. Lee, S. K. Kwak, M. I. Kim, J. Lee, N- and B-codoped graphene: A strong candidate to replace natural peroxidase in sensitive and selective bioassays. ACS Nano 13, 4312–4321 (2019). [DOI] [PubMed] [Google Scholar]
- 27.M. S. Kim, J. Lee, H. S. Kim, A. Cho, K. H. Shim, T. N. Le, S. S. A. An, J. W. Han, M. I. Kim, J. Lee, Heme cofactor-resembling fe-n single site embedded graphene as nanozymes to selectively detect H2O2 with high sensitivity. Adv. Funct. Mater. 30, 5410–5420 (2019). [Google Scholar]
- 28.Z. Wang, Y. Zhang, E. Ju, Z. Liu, F. Cao, Z. Chen, J. Ren, X. Qu, Biomimetic nanoflowers by self-assembly of nanozymes to induce intracellular oxidative damage against hypoxic tumors. Nat. Commun. 9, 3334–3348 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Z. Chen, J.-J. Yin, Y.-T. Zhou, Y. Zhang, L. Song, M. Song, S. Hu, N. Gu, Dual enzyme-like activities of iron oxide nanoparticles and their implication for diminishing cytotoxicity. ACS Nano 6, 4001–4012 (2012). [DOI] [PubMed] [Google Scholar]
- 30.X. Meng, D. Li, L. Chen, H. He, Q. Wang, C. Hong, J. He, X. Gao, Y. Yang, B. Jiang, G. Nie, X. Yan, L. Gao, K. Fan, High-performance self-cascade pyrite nanozymes for apoptosis-ferroptosis synergistic tumor therapy. ACS Nano 15, 5735–5751 (2021). [DOI] [PubMed] [Google Scholar]
- 31.C.-H. Lai, M.-Y. Lu, L.-J. Chen, Metal sulfide nanostructures: Synthesis, properties and applications in energy conversion and storage. J. Mater. Chem. 22, 19–30 (2012). [Google Scholar]
- 32.X. Du, I. Skachko, A. Barker, E. Y. Andrei, Approaching ballistic transport in suspended graphene. Nat. Nanotechnol. 3, 491–495 (2008). [DOI] [PubMed] [Google Scholar]
- 33.K. Han, W. Zhao, Q. Yu, Z. Liu, P. Li, W. Wang, L. Song, F. An, P. Cao, X. Qu, Marcasite-FeS2@carbon nanodots anchored on 3D cell-like graphenic matrix for high-rate and ultrastable potassium ion storage. J. Power Sources 469, 228429–228441 (2020). [Google Scholar]
- 34.R. Wang, M. Yan, H. Li, L. Zhang, B. Peng, J. Sun, D. Liu, S. Liu, FeS2 nanoparticles decorated graphene as microbial-fuel-cell anode achieving high power density. Adv. Mater. 30, 618–625 (2018). [DOI] [PubMed] [Google Scholar]
- 35.H. Han, K. M. Kim, H. Choi, G. Ali, K. Y. Chung, Y.-R. Hong, J. Choi, J. Kwon, S. W. Lee, J. W. Lee, J. H. Ryu, T. Song, S. Mhin, Parallelized reaction pathway and stronger internal band bending by partial oxidation of metal sulfide-graphene composites: Important factors of synergistic oxygen evolution reaction enhancement. ACS Catal. 8, 4091–4102 (2018). [Google Scholar]
- 36.R. Tian, X. Qiu, P. Yuan, K. Lei, L. Wang, Y. Bai, S. Liu, X. Chen, Fabrication of self-healing hydrogels with on-demand antimicrobial activity and sustained biomolecule release for infected skin regeneration. ACS Appl. Mater. Interfaces 10, 17018–17027 (2018). [DOI] [PubMed] [Google Scholar]
- 37.Y. Xiao, C. Lu, Y. Liu, L. Kong, H. Bai, H. Mu, Z. Li, H. Geng, J. Duan, Encapsulation of Lactobacillus rhamnosus in hyaluronic acid-based hydrogel for pathogen-targeted delivery to ameliorate enteritis. ACS Appl. Mater. Interfaces 12, 36967–36977 (2020). [DOI] [PubMed] [Google Scholar]
- 38.A. Lin, Y. Liu, X. Zhu, X. Chen, J. Liu, Y. Zhou, X. Qin, J. Liu, Bacteria-responsive biomimetic selenium nanosystem for multidrug-resistant bacterial infection detection and inhibition. ACS Nano 13, 13965–13984 (2019). [DOI] [PubMed] [Google Scholar]
- 39.B. J. Callahan, D. B. DiGiulio, D. S. A. Goltsman, C. L. Sun, E. K. Costello, P. Jeganathan, J. R. Biggio, R. J. Wong, M. L. Druzin, G. M. Shaw, D. K. Stevenson, S. P. Holmes, D. A. Relman, Replication and refinement of a vaginal microbial signature of preterm birth in two racially distinct cohorts of US women. Proc. Natl. Acad. Sci. U.S.A. 114, 9966–9971 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.J. Ravel, P. Gajer, Z. Abdo, G. M. Schneider, S. S. Koenig, S. L. McCulle, S. Karlebach, R. Gorle, J. Russell, C. O. Tacket, R. M. Brotman, C. C. Davis, K. Ault, L. Peralta, L. J. Forney, Vaginal microbiome of reproductive-age women. Proc. Natl. Acad. Sci. U.S.A. 108, 4680–4687 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Human Microbiome Project Consortium , Structure, function and diversity of the healthy human microbiome. Nature 486, 207–214 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.P. Gajer, R. M. Brotman, G. Bai, J. Sakamoto, U. M. E. Schütte, X. Zhong, S. S. K. Koenig, L. Fu, Z. Ma, X. Zhou, Z. Abdo, L. J. Forney, J. Ravel, Temporal dynamics of the human vaginal microbiota. Sci. Transl. Med. 4, 132–152 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.M. N. Anahtar, D. B. Gootenberg, C. M. Mitchell, D. S. Kwon, Cervicovaginal microbiota and reproductive health: The virtue of simplicity. Cell Host Microbe 23, 159–168 (2018). [DOI] [PubMed] [Google Scholar]
- 44.A. Cassone, D. Sobel Jack, Experimental models of vaginal candidiasis and their relevance to human candidiasis. Infect. Immun. 84, 1255–1261 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.S. P. Rosshart, J. Herz, B. G. Vassallo, A. Hunter, M. K. Wall, J. H. Badger, J. A. McCulloch, D. G. Anastasakis, A. A. Sarshad, I. Leonardi, N. Collins, J. A. Blatter, S.-J. Han, S. Tamoutounour, S. Potapova, M. B. F. S. Claire, W. Yuan, S. K. Sen, M. S. Dreier, B. Hild, M. Hafner, D. Wang, I. D. Iliev, Y. Belkaid, G. Trinchieri, B. Rehermann, Laboratory mice born to wild mice have natural microbiota and model human immune responses. Science 365, 4361–4390 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figs. S1 to S32
Table S1