Abstract
Skin injuries across the body continue to disrupt everyday life for millions of patients and result in prolonged hospital stays, infection, and death. Advances in wound healing devices have improved clinical practice but have mainly focused on treating macroscale healing versus underlying microscale pathophysiology. Consensus is lacking on optimal treatment strategies using a spectrum of wound healing products, which has motivated the design of new therapies. We summarize advances in the development of novel drug, biologic products, and biomaterial therapies for wound healing for marketed therapies and those in clinical trials. We also share perspectives for successful and accelerated translation of novel integrated therapies for wound healing.
Advancements in drugs, biologic products, and biomaterial therapies accelerates wound healing.
INTRODUCTION
Overview of wounds
Wounds throughout the body are common and can be devastating injuries with long recovery times. Wounds are commonly classified as either acute or chronic and by their clinical presentation (Fig. 1). Untreated wounds converge on the same endpoint: necrosis and cellular death of integument (1) with severity dictated by depth and extent. All wounds, regardless of antecedent event [thermal (2, 3), mechanical, pressure (4, 5), etc.], demonstrate a common set of parameters that yield cumulative risk related to both initial breakdown of the skin barrier and impediment to successful healing and repair. Most of these factors are manifestations of relative ischemia (5): inadequate inflow and/or outflow [e.g., peripheral arterial disease/venous stasis (6)], microvascular damage [e.g., diabetes (7)], and vasoconstriction [e.g., effect of acute nicotine use (8)]. Other systemic factors include nutritional status, fibroblast/progenitor health [e.g., as affected by corticosteroids, radiation (9)], and infectious bioburden (1).
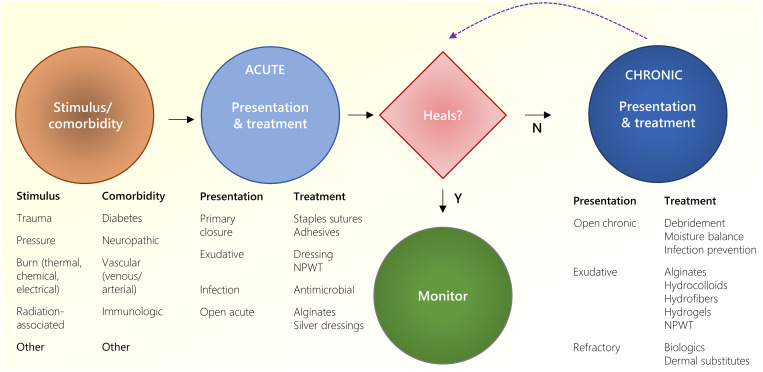
Fig. 1. Summary of wound healing types and treatments.
Following an injury stimulus, acute wounds treated either heal or persist to chronic wounds. Depending on the clinical presentation, several different treatments are provided. NPWT, negative pressure wound therapy.
Normal healing of acute wounds includes a predictable series of events: inflammation, proliferation/repair, and remodeling (1, 10). Wounds that fail to proceed through the normal phases and remain in a dysregulated inflammatory state are reclassified from acute to chronic wounds (11–13). The most common chronic wounds have several delineating nuances. Pressure ulcers typically follow a progressive, increasing depth of tissue necrosis beginning from discoloration and pain from microvascular injury alone (stage 1), ulceration and skin breakdown (stage 2), to extension to underlying fat (stage 3) or deeper structures (stage 4). Diabetic ulcers are accompanied with altered sensorium, and these paresthesias/anesthesia eliminate the protective afferent feedback (pain) that normally prevents soft tissue injury leading to initial ulceration and unnoticed progression. Venous stasis wounds are typically associated with variable levels of granulation tissue and pain with periwound skin discoloration along with significant exudate. Arterial ulcers lead to acute ischemia often accompanied with significant pain and eschar. Nevertheless, the resulting wounds still commonly converge in phenotype and chronicity that require specialty care. Through expansive research and innovation, a vast library of wound care technologies and products have been developed to facilitate progression in stalled wounds (14).
Human and economic cost of wounds
Wounds have remained a challenge throughout history (15) and continue to represent an extraordinary burden to the health care system. In the United States in 2014, wounds affected more than 8 million people costing an estimated $30 billion (16). With an increasingly aging and obese population, high risk comorbidities commensurately increase, growing wound closure product market size of 21.4 billion in 2022 and compound annual growth rate of 4.15% from 2023 to 2030 (Grand View Research) (17).
With increases in the number of surgical procedures performed and an increasing aging population, surgical wounds represent the largest wound subset. Careful surgical technique and optimal suture material remain important, as wound dehiscence can lead to a 9.6% increase in mortality, an additional 9.4 days of hospitalization, and up to $40,000 in hospital charges. For patients with diabetes, there is a 25% lifetime risk of developing a foot ulcer, of which 15% progress to amputation. In addition, pressure injuries affect 3.5 to 69% of patients in hospitals (up to 2.5 million patients per year) (18–20), and complications may result in wound-related infections and mortality (>55%) and up to 60,000 Americans deaths each year (21–24). Pressure injuries cost $9.1 to 11.6 billion per year in the United States. Last, although the number of burn injuries in the United States is decreasing (~16.8 per 100,000), burn-related inpatient stays remain approximately twice as long and costly as non-burn–related stays ($24,000 versus $10,700). Annually, burns are associated with ~$1.5 billion in medical costs and $5 billion in lost workdays.
Standard of care and emerging treatments
The current standard of care for almost all wounds relies on preparation of a viable wound bed amenable to healing (15). This may be performed via irrigation or debridement, including removal of foreign material and necrotic tissue (25, 26). Wounds may be allowed to heal by secondary intent or repaired with primary closure, while others may require a graft or flap (27). For more complex wounds, typically chronic, serial debridements awaiting secondary healing or as a bridge to closure may be required (28, 29). In the following section, we summarize several U.S. Food and Drug Association (FDA)–cleared or approved {class II [510(k)]: “K-”; class III [Premarket Approval (PMA)]: “P-”; National Drug Code “NDC-”; Biologic Product “BP-”; Biologic License Application “BLA-”; and marketed products for wound healing}.
In recent decades, surgical wound care has been supplemented with assistive technologies: Wounds amenable to primary closure may be facilitated by any combination of staples, sutures {including numerous absorbable products: poliglecaprone 25 [Monocryl (K960653) (Ethicon)] and polydiaxone 910 [Vicryl (K183183) (Ethicon)], among others}, cyanoacrylate adhesives [Dermabond (P960052; K152096) (Ethicon) (30) and Liquiband (K211878) (AMS) (31)], and adhesive strips [Steri-Strips (K813265) (3M) (32)]. All of these techniques are used to cancel dead space and minimize tension on the wound, promoting tissue repair and regeneration.
Pressure injuries are a serious problem in institutionalized patients, with incidence of approximately 12% (33). As a means of mitigating this increased capillary afterload, foam dressings [e.g., Mepilex (K123892) (Molnlycke)] and special clinical mattresses composed of foam [Ultrafoam (Amico)], water [Akva (ProActive)], and autonomously alternating air mattresses that vary pressure distributions [Protekt Aire (ProActive), Aura (Amico), Clinitron (Hillrom)], obviating nursing labor for frequent side-to-side offloading.
For open chronic wounds, the principles of management comprise debridement, moisture balance, infection prevention, and medical optimization of comorbidities such as peripheral vascular disease, nicotine use, and blood glucose control. To minimize the microbial and necrotic material impairing wound healing, serial debridements provide the environment to minimize inflammation and progression to active proliferation (29, 34). Classical debridement consists of sharp excision of necrotic or fibrinous debris typically followed by wet-to-dry woven gauze dressings for sustained microdebridements. When grossly contaminated, additional anti-infective agents may be added, such as sodium hypochlorite [Vashe Wound Solutions (K123072) (SteadMed) (35) and Dakin’s Solution (K150208) (Century) (36)], cyclic lipopeptides (37), silver impregnated materials [Mepilex Ag (K100029) (Molnlycke) (38), Contreet (K013525) (Coloplast), Allevyn Ag (K063835) (39) (Smith + Nephew)], and enzymatic debridement agents [Santyl (NDC 50484-010) (Smith + Nephew) (40)].
In highly exudative wounds, excess moisture can cause maceration of the wound bed and surrounding tissues impeding the healing process. Alginates [Kaltostat (K904488) (ConvaTec) and Tegaderm Alginate (K973036) (3M)], hydrocolloids [DuoDerm (K990368) (ConvaTec), Suprasorb H (K183208) (Lohmann and Rauscher)], and hydrofibers (Aquacel (K982116) (ConvaTec)], and hydrogels [Purilon (K971597) (Coloplast) and Hydrosorb (K041105) (Hartmann)] are capable of holding varying degrees of fluid. Negative pressure wound therapy (NPWT) [VAC (K062227) (KCI), Avelle (K180205) (ConvaTec), and Avance (K203369) (Monlycke)] can also provide moisture control in addition to enhancing several other mechanisms that can improve healing of dry or wet wounds, including increased capillary perfusion, wound contraction, evacuation of debris, and micromechanical force (41). In refractory chronic wounds over sensitive areas (for example, the pericardium, pleura, or bowel), gliding services (for example, over tendons), or surgically created wounds (for example, flap donor sites), biologics or dermal regeneration templates [Integra Dermal Regeneration Template (P900033) (Integra Lifesciences) and Novosorb (K172140) (PolyNovo), and AlloDerm (LifeCell)] with or without impregnated growth factors [Primatrix (K153690) (Integra Lifesciences) and Helisorb (Medira)] and even cultured epidermal autografts [Epicel (HDE: BH990200.34) (Vericel)] have been used.
Several other emerging technologies are now entering the market. These include products for the detection of elevated protease activity as a proxy for impaired wounds [Woundchek (DEN180014) (Systagenix) (42)], epidermal harvest and suspension systems [Cellutome (KCI) and Recell (BP170122) (Avita)], targeted pulsed electromagnetic therapy [SofPulse (K070541) (Endonovo)], topical wound oxygen therapy [TWO2 (WoundSource)], and ultrasound therapy [UltraMIST (K1407828) (WoundSource)]. In the complex milieu of healing wounds, several growth factors including epidermal growth factor, fibroblast growth factor, transforming growth factor–β, and platelet-derived growth factor (PDGF) have been described. Ongoing technology development has yielded growth factors like PDGF supplementation [Regranex (BLA103691) (Smith + Nephew)] as adjuncts for tenacious wounds, including diabetic neuropathic ulcers.
Need for new and integrated therapies
Chronic wound physiology has proven to be highly complex and intricate at the cellular level, involving multiple regulatory axes and signaling cascades. Indeed, developing technologies have begun to target these coordinated cellular processes. Despite effective and foundational interventions for the optimization of wound care, there remain challenging problems that remain incompletely understood and in need of ongoing research and innovation. While commercial products have predominantly focused on “macro” factors (e.g., moisture and pressure), there remains ample opportunity to tailor wound care based on “micro” factors (e.g., cells, proteins, and peptides).
Commercially available biomaterials for wound healing typically target the alleviation of symptoms (fluid exudation, moisture balance, scarring, pressure relief, infection, etc.). In contrast, advanced biomaterials for wound healing are being developed to provide extracellular matrix (ECM)–inspired biophysical cues and modulation of the immune response for adequate resolution of inflammation. These therapies are typically formulated as injectable or biomaterial-based delivery systems and may include integration of drug and biological product therapies. Fundamental studies have highlighted how biophysical signals (43–50) may be integrated in biomaterials to control cell behavior (51–57). Biomaterial-based delivery systems (e.g., hydrogels) can provide sustained-release (58) and stimuli-responsive release. Such principals may overcome limits and risks of systemic administration and further promote patient adherence to new therapies (59–62).
Novel biomaterials with integrated pharmacologic and tissue regenerative function are typically biodegradable and include macroporosity to allow for vascularization and cell recruitment. Fundamentally, such materials must achieve biocompatibility for successful translation. Examples of stimuli-responsive release include triggering of release by the pH of skin [ranges from pH 4 to pH 6 (63)], which is more acidic during healing (64), or harnessing differences in temperature from the core to appendicular skeleton (may approach differences up to 5°C to induce vasodilation and nutrient and oxygen supply).
In the next sections, we first highlight current preclinical research on novel integrated therapies that use combinations of biomaterials and drugs or biologic product therapies specifically designed for acute and chronic wound healing. We then discuss recently completed and ongoing clinical trials on novel drugs or biologic product therapy modalities for acute and chronic wounds.
Advanced therapies for wound healing in preclinical and clinical trials
Advanced wound therapies in preclinical trials
In acute wounds (e.g., surgical and traumatic wounds), bandages inhibit bleeding, absorb exudate, and effectively close wounds to promote healing. Therefore, recent advances in wound dressings for acute wounds focus on tight wound closure for hemostasis, absorption of wound exudate, and infection control. For example, a strongly adhesive wound dressing made of alginate and poly(N-isopropylacrylamide) actively contracted wounds based on its thermoresponsive properties and its high toughness and accelerated wound contraction in splinted mouse wounds (65). A recent effort to combine adhesive hydrogels with surgical mesh successfully demonstrated strong adhesion and flexibility, permeability, and strength by a poly(N-isopropylacrylamide)/chitosan hydrogel and a polyethylene terephthalate surgical mesh, respectively, in wounds under mechanical stress (66).
In chronic wounds, advanced bandages target the dysregulated inflammatory phase, replace skin tissue, and protect against infection. In diabetic wounds, recent attention focused on jump-starting the healing process by inducing acute inflammation. The preventive delivery of a mast cell stabilizer and the release of the neuropeptide substance P both induced strong inflammation after wounding, improved wound reepithelialization, and accelerated wound healing in diabetic mice (67, 68). Furthermore, removing tissue-damaging proinflammatory factors also improved tissue regeneration and healing in diabetic mice. Reducing reactive oxygen species and matrix metalloproteinase 9 (MMP9) activity, both continuously released by immune cells in diabetic wounds, promoted the progression into the proliferation phase, and accelerated wound healing in several mouse models of diabetes. Hydrogels with sustained release of the iron(II) scavenger deferoxamine, which inhibits the conversion of hydrogen peroxide to the highly toxic hydroxyl radical, and hydrogels releasing low molecular weight MMP9 inhibitors and MMP9-silencing RNA improved reepithelialization and accelerated wound healing in diabetic mice (69–71). A sustained release formulation of the PPCN hydrogel loaded with stromal cell derived factor-1 accelerated wound healing in diabetic mice (72). Bandages removing proinflammatory cytokines such as Monocyte Chemoattractant Protein-1 (MCP-1) and interleukin-8 via electrostatic interactions also accelerated wound closure in db/db mice, showing that reducing chronic inflammation improves wound healing in diabetic wounds (73). To replace the functionally impaired ECM in diabetic wounds, ECM-mimicking hydrogels that display laminin-derived peptides or act as a growth factor reservoir and provide cues to stromal cells were investigated in diabetic wounds. A hydrogel decorated with heparin-binding domains of laminin accelerated wound healing after topical application on db/db mouse wounds, both with and without vascular endothelial growth factor and PDGF encapsulation (74). In addition, a thermoresponsive hydrogel decorated with the tethered laminin-derived peptide A5G81 facilitated keratinocyte and dermal fibroblast migration and accelerated wound healing in db/db mice with splinted wounds (75). To reduce apoptosis of cells and inflammation in burned tissue, a peptidic derivative of heat-shock protein 90α was applied using a topical carboxymethyl cellulose hydrogel to contact burn wounds in pigs, improved reepithelialization, and accelerated wound healing in this large animal model (76).
Infection is common in acute and chronic wounds with potentially lethal consequences. A variety of anti-infective bandages have shown promising results in preclinical studies. A polymeric hydrogel made of poly(acrylic acid) and poly(acrylamide) loaded with antimicrobial silver/graphene particles showed exceptionally high swelling ratios due to the hydrophilic polyacrylamide and promoted wound healing in excised rat wounds (77). New hemostatic, absorbent, and antimicrobial wound dressings and a dressing based on a new mechanobiological strategy have therefore shown promise in animal models of surgical wound healing. Two recently reported agarose and alginate hydrogel systems showed high loading and sustained release of three antibiotics, as well as good wound enclosure and beneficial effects on burn wounds in pig models (78, 79). High barrier function was also reported for a suspension made of multilayered poly-l-lactic acid nanosheets that firmly attached to burn wounds in the absence of adhesives and prevented infection with Pseudomonas aeruginosa in mice with burn wounds over at least 3 days (80). For wound infections, several theranostic electroconductive dressings were developed that aim at sensing infection-associated wound parameters such as pH and temperature and releasing antibacterial drugs on demand (81–83). One hydrogel was based on a carbon/polyaniline working electrode capable of sensing wound pH and releasing cefazolin, which accelerated wound healing in an excisional mouse wound model. Another system used electrical stimulation to provide prohealing cues and improved wound healing in diabetic mice (84). A variety of electronics-integrated wound dressings were recently developed for electrostimulation, wound monitoring (e.g., wound pH and temperature), and on-demand drug delivery (85–87). Furthermore, several antimicrobial peptides showed promise in preclinical wound models (88–90). An antimicrobial peptide-releasing DNA hydrogel, whose retention mechanism relies on ionic interactions of the negative DNA with cationic antimicrobial peptides, decreased Staphylococcus aureus burden in ex vivo porcine skin explants and accelerated wound healing in mice (88).
A major research focus is on skin wound substitutes to replace the invasive practice of autografting, and this approach promises to provide new options for severe burn wounds where auto- and allografting are currently the standard of care. Three-dimensional (3D) bioprinting has recently received much attention in this field, with combined scanning and printing approaches generating personalized skin substitutes that allow complete wound coverage in three dimensions. A portable 3D scanning and 3D bioprinting system capable of printing autologous fibroblast (dermis) and keratinocyte cell (epidermis) layers made of collagen and thrombin-crosslinked fibrinogen showed good vascularization and reepithelialization and improved healing in excisional mouse wounds (91). Bioprinted gelatin-alginate hydrogels containing mesenchymal stem cells and an angiogenic nitric oxide source accelerated reepithelialization and wound closure in burn wounds of mice (92). As the large mesh sizes of gels used as bioinks can lead to a burst release of drugs, hydrogels have been crosslinked during the printing process to sustain drug release. 3D-printed photocrosslinked hydrogels made of chitosan methacrylate, the antibiotic levofloxacin, and the analgesic lidocaine showed sustained drug release over 3 days and accelerated wound closure in burn wounds on rats (93).
In sum, investigational bandages for acute and chronic wounds with immunomodulatory, anti-infective, skin substitutive, and sealant properties have shown promise in animal models of wound healing. These proof-of-concept studies point to a thriving preclinical pipeline and highlight the potential of addressing key properties in pathophysiology and clinical pathology of acute and chronic wounds.
Advanced wound therapies in the clinical pipeline
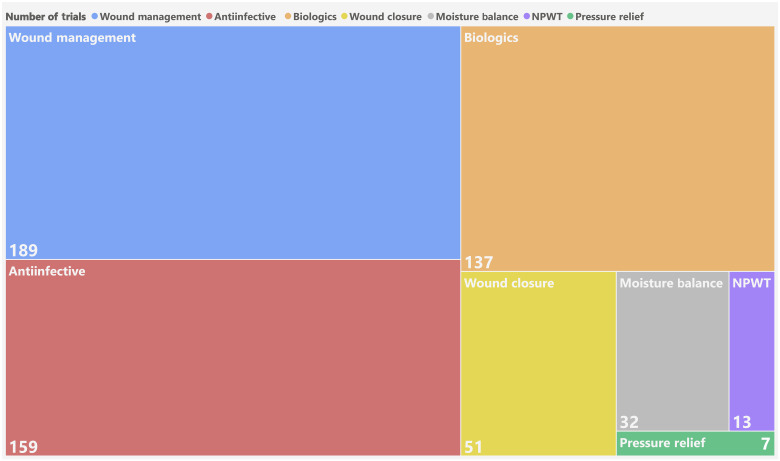
Several advanced wound therapies are in the clinical pipeline (Table 1). A search was conducted using clinicaltrials.gov to determine the most common trials ongoing, which involve wound management, anti-infectives, and biologics (Fig. 2). This section summarizes ongoing clinical trials across the many segments of wound types.
Table 1. Clinical pipeline of biologic and drug wound therapies.
RNAi, RNA interference.
| Phase | Clinical trial (NCT) | Company | Mode of action | Indication |
|---|---|---|---|---|
| 1 | NCT04803708 | Technophage | Biologic: Antibacterial bacteriophage dispersion | Diabetic foot ulcer; infections with P. aeruginosa, S. aureus, Acinetobacter |
| NCT04281992 | Aurealis Pharma | Biologic: Genetically engineered L. lactis bacteria expressing anti-inflammatory, angiogenic, and tissue-repairing proteins | Diabetic foot ulcer | |
| NCT03569267 | OLX101 | Biologic: CTGF RNAi therapeutics stem cell therapy | Cicatrix, hypertrophic | |
| NCT02590042 | ADSC-SVF-002 | Biologic: Wound healing agent | Abnormally healing wounds, scars, soft tissue defects | |
| NCT03695939 | XenoTherapeutics | Biologic: Live cell xenotransplantation skin product derived from genetically engineered (alpha-1,3-galactosyltransferase knockout) porcine donors | Deep full-thickness burn injury (disorder) | |
| NCT04890574 | RenovaCare | Biologic: Autologous stem cells obtained from donor skin using CellMist System and sprayed on wound with SkinGun device | Burns, burns second degree, burns deep second degree | |
| 2 | NCT04817228 | Mediwound | Drug: Debridement by protease-containing wound solutions | Venous leg ulcer, diabetic foot ulcer |
| NCT01898923 | Oneness Biotech | Drug: Plant extract for M2 polarization | Diabetic foot | |
| NCT02664740 | Pherecydes | Drug: Topical anti-staphylococcal bacteriophage cocktail against methicillin-resistant or susceptible S. aureus | Diabetic foot, staphylococcal infections | |
| NCT03880058 | Pharma SLI-F06 | Drug: Anti-scarring agents (FMOD peptide) | Scars | |
| NCT04331080 | Granexin | Drug: Cx43 mimetics | Mammoplasty, scarring, scar, breast reconstruction | |
| NCT01655407 | ESS | Drug: Collagen/fibroblast | Thermal injury, deep partial-thickness, burn, full-thickness burn | |
| NCT02116010 | Phagoburn | Drug: Bacteriophage | Wound infection | |
| 3 | NCT03282981 | VA Office of Research and Development | Drug: Pro-angiogenic timolol hydrogel | Chronic diabetic foot ulcers, diabetic neuropathic ulcers, nonhealing wound |
Fig. 2. The wound treatment pipeline.
Recent clinical trials for wound management, anti-infective, biologics, wound closure, moisture balance, NPWT, and pressure relief among the etiologies of surgical/trauma, ulcer, and burns. Numbers indicate the number of interventional clinical trials since 2015 recruiting, not yet recruiting, actively recruiting, completed, or enrolling by invitation.
Advanced antiscarring and healing-promoting therapies for surgical wounds are in clinical development (Table 1). OLX101 is a cell-penetrating asymmetric interfering RNA that targets connective tissue growth factor (CTGF) to combat antihypertrophic scarring (OliX Therapeutics). Instead of delivering with liposomes or nanoparticles, OLX101 has developed an small interfering RNA that can enter cells spontaneously without complex delivery systems and is currently in clinical development as an intradermal injectable for hypertrophic and keloid scars (NCT03569267). New peptide formulations are also being investigated to promote wound closure and reduce scarring. A fibromodulin (FMOD)–based amino acid peptide sequence, SLI-F06 (Scarless Laboratories), was found to stimulate fibroblast and endothelial cell migration and myofibroblast differentiation/contraction to promote timely wound closure. Following preclinical studies showing that intradermal delivery of FMOD reduced scar size, increased tensile strength, and improved dermal collagen architecture organization in pig wound models (94), an ongoing double-blind study is evaluating its effectiveness for the improvement in scar appearance and wound strength in routine surgical excisions, as well as postoperative abdominoplasty scar appearance (NCT03880058). Other peptides being developed include a connexin43 (Cx43) mimetic peptide (Granexin). Cx43 is most abundant in epidermal and dermal cutaneous layers of skin, and studies in chronic wounds find Cx43 in wound edges and in the dermis. Both knockdown of Cx43 and use of a peptide mimetic of the Cx43 carboxyl terminus improved wound closure rate and reduced scarring (95). Granexin is being evaluated in phases 1 and 2 trials in venous leg ulcers, diabetic foot ulcers, and surgical wounds (NCT04331080).
Several stem cell, exosome, and peptide therapies are in clinical trials as strategies for soft tissue defects and refractory wounds. ADSC-SVF-002 (AdiSave) is an autologous adipose-derived stem cell therapy injected subcutaneously into soft tissue defects and abnormally healing wounds with or without unprocessed autologous fat. A single-arm, open-labeled, single-center, descriptive, and exploratory safety trial is underway to demonstrate safety in a population of subjects with soft tissue defects (NCT02590042). In contrast to cell-based therapies, exosomes derived from platelets are being tested for advanced wound healing (Plexoval, ExoPharm Limited). Ongoing work is studying autologous exosomes, administered by local injection, over a 6-week time frame to examine safety, wound closure, and scarring.
Biologic-free advances for surgical wound healing are also being investigated. Several portable NPWT devices exist, including PICO (Smith & Nephew), which met noninferiority in a multicenter, phase 4–randomized comparative efficacy study and was superior compared to traditional NPWT with regard to wound progression toward healing over the 12-week treatment period (96). An ongoing randomized control trial (RCT) is examining the incidence of infection over 1 to 3 months for the prevention of surgical wound infection following cardiac surgery under extracorporeal circulation compared to single-use hydrocolloid dressings (NCT04265612). Heat therapy or noncontact normothermic, i.e., 38°C, dressings were historically of great interest, with preliminary investigations and several clinical trials (97, 98) showing promise in pressure and venous stasis ulcer healing. Application of a warming agent to a semipermeable dressing, i.e., WarmUp (99) Active Wound Therapy [510(k), Augustine Medical, Eden Prairie MN], was hypothesized to increase blood flow and subsequent immunogenicity to the area (100) and facilitate increased rates and final surface of area of successful healing. However, more contemporary studies and other investigations involving similar technologies like infrared therapy (NCT00426166) have either demonstrated muted results (98) or are still pending reports on any significant outcomes (101) and have had limited contemporary clinical translation.
Several bandages are under clinical investigation for diabetic foot ulcers. An FDA-approved ocular gel containing the beta-adrenergic antagonist timolol is being tested in a phase 3 trial (NCT03282981), as beta-adrenergic antagonists were shown to improve angiogenesis and tissue repair in vitro and in vivo (102). A bacteriophage dispersion (TP-102) for topical application is in a phase 1/2a trial (NCT04803708), which aims at reducing infections by targeting P. aeruginosa, S. aureus, and Acinetobacter baumannii. A topical dispersion of genetically engineered Lactococcus lactis bacteria (AuP1602-C) is currently in a phase 1/2a trial (NCT04281992). These bacteria express three anti-inflammatory, angiogenic, and tissue-repairing proteins (fibroblast growth factor-2, interleukin-4, and macrophage colony stimulating factor-1). To replace surgical debridement, protease-containing wound solutions for the outpatient setting are under investigation (phase 2 study, NCT04817228). In an RCT, topical application of a cream (ON101) containing two plant extracts with reported effects on macrophage polarization toward the M2 phenotype showed improved wound healing at 16 weeks compared with an absorbent wound dressing (103) (NCT01898923). Therefore, a variety of biological bandages based on auto- and allograft, or phages and bacteria, as well as drug-releasing dressings are currently under clinical investigation for diabetic foot ulcers. These promise new therapeutic options for the treatment of diabetic foot ulcer in this decade.
Next-generation debridement therapies for burns are in clinical trials. NexoBrid (KMW-1) (Kaken Pharmaceutical) is a topical agent composed of proteolytic enzymes isolated from the stem of the pineapple plant (Bromelain). Pineapple stem protein contains at least four cysteine proteinases that can hydrolyze and solubilize heat-denatured proteins that comprise the eschar (104, 105). NexoBrid provides selective and quick removal of dead or damaged tissues (debridement) within 4 hours after application. In a phase 3 clinical trial, 89% of patients had eschar completely removed with no serious adverse reactions documented.
Several cell-based therapies are under clinical investigation to enhance healing of burns. StratGraft (Mallinckrodt) is a bilayered, cellularized scaffold containing keratinocytes and dermal fibroblasts applied topically to promote endogenous skin cell recruitment. After receiving Regenerative Medicine Advanced Therapy Designation (RMAT), priority review, and orphan drug designation from the FDA, it was approved for adults with thermal burns containing intact dermal elements for which surgical intervention is clinically indicated. Expanding on cultured cell sheet technologies such as Epicel (Genzyme Corp), Engineering Skin Substitute (ESS) (Amarantus BioScience Holdings) is a tissue-engineered skin prepared from the patient’s own epithelial cells and fibroblasts with collagen. In preclinical studies, ESS generated a functional skin barrier. Completed clinical studies have investigated its use in the treatment of severe burns in pediatric patients (up to 95% total body surface area). A phase 2 trial is underway to evaluate safety and efficacy of ESS compared to meshed split thickness autografted skin for the treatment of life-threatening severe burns (NCT01655407). Last, SkinMed (BioDan) is based on autologous fibroblasts and keratinocytes obtained from a single biopsy seeded into clotted human plasma as a 3D dermal scaffold (106). Previous studies have shown that keratinocytes seeded on the plasma-based scaffold have a 1000-fold area expansion after 24 to 26 days and display expression of structural intracellular proteins and basement membrane components. Engraftment and skin regeneration have been demonstrated in patients in multiple years of follow up (106, 107). It is being developed for indications in severe burns, wound healing, oral mucosa, and urological and gynecological mucosa.
Summary and emerging opportunities to enable innovative therapy
Hundreds of wound dressings with novel mechanisms of action are in preclinical and clinical development for the treatment of acute and chronic wounds. Their mechanisms of action are highly diverse and address many phases of wound healing, potentially allowing for tight wound closure for hemostasis, immunomodulation during the inflammation phase, and ECM substitutes for the proliferative and remodeling phases. The diversity of these strategies builds confidence that clinicians will soon have novel tools to improve wound healing at their disposal. However, despite the development of a variety of new products, considerable challenges remain in the treatment of acute and chronic wounds. Here, we highlight key areas that we believe are essential to drive the field forward: (i) fundamental understanding of pathophysiological processes driving injury, (ii) improved definition and understanding of unmet clinical needs in transdisciplinary teams, and (iii) reshaping research goals to align with guidelines accepted by the FDA.
To transition from current unspecific to molecularly targeted wound dressing, we need to improve our understanding of pathophysiological processes of chronic wounds. These insights will allow us to select the best indication for products with single molecular targets (e.g., MMP9-inhibiting dressings) and will ultimately lead to personalized medicine in chronic wound therapy. Multi-omics approaches and single-cell analysis currently drive the identification of novel targets and biomarkers (108). Simulating chronic wounds with increasingly complex skin substitutes and organoids, especially with the inclusion of immune cells, also promises to yield new therapeutic targets (109, 110). Also, these targets could be diagnostically valuable and allow for molecular fingerprinting of wounds, which would complement the current macroscopic evaluations in clinical practice. Eventually, wound dressings may sense the unique wound environment of every patient and release drugs autonomously to treat the wound using a personalized medicine strategy.
To accelerate translation of new wound care products from bench to bedside, academic researchers should preferentially consider the targeted indication early on to devise an evidence-based target product profile and patenting strategy. Of highest importance is the identification of unmet clinical needs through early engagement with clinicians and targeted customer discovery. As the scientific enterprise becomes increasingly multidisciplinary, the importance of initiating collaborations and using cores or contract research organization for necessary assay expertise is ever important (111). Given the broad numbers of technologies available and in development, it is advantageous to search patent databases in addition to academic literature for prior art. Moreover, considerations on producing the product on an industrial scale are also warranted, as failure to recognize complexities in scale up and adoption results in ultimate standstill.
As wound dressings are transitioning into targeting key pathophysiologic factors in a certain wound type, identifying the clinical relevance in pathophysiology in both model systems and the targeted disease is essential. Indeed, although it is advantageous to perform certain preclinical wound healing studies in small rodents, it must be recognized that these models have limitations. At present, identifying preclinical models that illustrate human tissue and wound healing responses remains a significant translational dilemma (112). Various species have been used to model cutaneous wound healing and tissue repair responses, among which the most popular are the pig, rabbit, mouse, and rat (113). The type of injury (e.g., burn, incisional, and chronic), location of injury (e.g., plantar) (114), and patient factors influencing healing (e.g., immunocompetency, nutrition status, and diabetes) may influence the choice of animal model (115). Pig wound healing models have anatomical and physiological similarities that most closely recapitulate those of human skin. However, the pathologic responses to chronic wounds and scarring are complex, and discrepancies between the immune system and inflammatory response to injury between animal models and humans may affect translation to clinical practice (115–118). Chronic wounds are uncommon in animals and challenging to stimulate (119). FDA guidance suggests that there are no adequate animal models for chronic wounds, and multiple models should be considered to assess wound healing products (110, 120). For example, angiogenesis may be best studied in a chicken chorioallantoic membrane or rabbit cornea model, whereas reepithelialization might be studied in a rabbit ear model. Separate models might be selected for different chronic wound indications sought (e.g., diabetic ulcer, venous stasis ulcer, burn wound, pressure ulcer, etc.) (115). Moreover, incorporating human tissue validation complementary to experimental models has been proposed to avoid late-stage translation failures (121). Indeed, the FDA has identified the lack of accepted animal models, drug delivery challenges, and standardized endpoints in clinical trials as key barriers to innovation and is working to find solutions in a multistakeholder approach (8). In addition to pathophysiological relevance, in vivo study design must consider key guidelines and recommendations from the FDA related to prior work and associated standards [e.g., FDA/American Society for Testing and Materials (ASTM)/International Organization for Standardization (ISO)] to avoid unnecessary or repeating studies (122).
In many cases of hydrogel-based products, depending on the indications for use, larger animal trials are not required for FDA 510(k) clearance, and human clinical trials may be only necessary after market. This contrasts with the FDA approval process of new drugs, which often pose greater risk to the patient, thus requiring more robust safety and efficacy testing in humans before approval. While industry may be able to market these products, transforming standard of care with a new product will require strong clinical data including beneficial health economics. Only with these attributes will emerging technologies survive beyond the bench and affect the lives of patients.
Acknowledgments
Funding: The authors receive grant support through the NIH and Wyss Institute. The views and opinions expressed in this article are those of the authors and do not necessarily reflect the position of the Wyss Institute for Biologically Inspired Engineering at Harvard University or University of Montreal. This work was supported by the Wyss Institute for Biologically Inspired Engineering at Harvard and the National Institute on Aging of the NIH (K99AG065495).
Author contributions: All authors contributed to the preparation of this manuscript. All authors (B.R.F., C.H., B.H., S.T., S.M., and D.J.M.) were involved in the writing and review of this paper.
Competing interests: D.J.M. has sponsored research from Novartis, consults, and/or has stock options/stock in J&J, Medicenna, Boston Scientific, Lyell, Attivare, IVIVA, Epoulosis, Limax Biosciences, and Revela. D.J.M. is also an inventor on issued and pending patents in the wound healing field that are owned by Harvard University. B.R.F. and D.J.M. are inventors on a patent related to this work filed by Harvard University (20210338577, published 4 November 2021). D.J.M. is an inventor on a patent related to this work filed by Harvard University (20190091367, published 28 March 2019). D.J.M. is an inventor on a patent related to this work filed by Harvard University (20200000967, published 2 January 2020). B.R.F. has been a paid consultant for Amend Surgical and has the following interests: Limax Biosciences, equity; Amend Surgical, licensed IP. B.R.F. is an inventor on patents and patent applications related to certain of the publications cited in this review. S.T. is a consultant for Tissium and on a scientific advisory board for NeurAptive and Six Therapeutics. B.H. is a consultant of Limax Biosciences. The authors declare that they have no other competing interests.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper.
REFERENCES AND NOTES
- 1.H. N. Wilkinson, M. J. Hardman, Wound healing: Cellular mechanisms and pathological outcomes. Open Biol. 10, 200223 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.S. T. Lanier, S. A. McClain, F. Lin, A. J. Singer, R. A. Clark, Spatiotemporal progression of cell death in the zone of ischemia surrounding burns. Wound Repair Regen. 19, 622–632 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.H. O. Rennekampff, Z. Alharbi, Burn injury: Mechanisms of keratinocyte cell death. Med. Sci. (Basel) 9, 51 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.A. M. Al Aboud, B. Manna, Wound pressure injury management, in StatPearls (Treasure Island, 2022). [PubMed] [Google Scholar]
- 5.T. N. Demidova-Rice, M. R. Hamblin, I. M. Herman, Acute and impaired wound healing: Pathophysiology and current methods for drug delivery, part 1: Normal and chronic wounds: Biology, causes, and approaches to care. Adv. Skin Wound Care 25, 304–314 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.B. Z. Rayala, Skin ulcers: Prevention and diagnosis of pressure, venous leg, and arterial ulcers. FP Essent 499, 11–18 (2020). [PubMed] [Google Scholar]
- 7.D. G. Greenhalgh, Wound healing and diabetes mellitus. Clin. Plast. Surg. 30, 37–45 (2003). [DOI] [PubMed] [Google Scholar]
- 8.P. Silverstein, Smoking and wound healing. Am. J. Med. 93, S22–S24 (1992). [DOI] [PubMed] [Google Scholar]
- 9.S. S. Jadhav, C. J. Meeks, N. M. Mordwinkin, T. B. Espinoza, S. G. Louie, G. S. diZerega, K. E. Rodgers, Effect of combined radiation injury on cell death and inflammation in skin. Apoptosis 20, 892–906 (2015). [DOI] [PubMed] [Google Scholar]
- 10.T. Kondo, Y. Ishida, Molecular pathology of wound healing. Forensic Sci. Int. 203, 93–98 (2010). [DOI] [PubMed] [Google Scholar]
- 11.K. Jarbrink, G. Ni, H. Sonnergren, A. Schmidtchen, C. Pang, R. Bajpai, J. Car, The humanistic and economic burden of chronic wounds: A protocol for a systematic review. Syst. Rev. 6, 15 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.R. G. Frykberg, J. Banks, Challenges in the treatment of chronic wounds. Adv. Wound Care (New Rochelle) 4, 560–582 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.M. Spear, Acute or chronic? What's the difference? Plast. Surg. Nurs. 33, 98–100 (2013). [DOI] [PubMed] [Google Scholar]
- 14.J. Panuncialman, V. Falanga, The science of wound bed preparation. Surg. Clin. North Am. 89, 611–626 (2009). [DOI] [PubMed] [Google Scholar]
- 15.T. Brocke, J. Barr, The history of wound healing. Surg. Clin. North Am. 100, 787–806 (2020). [DOI] [PubMed] [Google Scholar]
- 16.S. R. Nussbaum, M. J. Carter, C. E. Fife, J. DaVanzo, R. Haught, M. Nusgart, D. Cartwright, An economic evaluation of the impact, cost, and medicare policy implications of chronic nonhealing wounds. Value Health 21, 27–32 (2018). [DOI] [PubMed] [Google Scholar]
- 17.G. V. Research, "Wound Care Market Size, Share & Trends Analysis Report By Product" (2023).
- 18.M. L. Shannon, P. Skorga, Pressure ulcer prevalence in two general hospitals. Decubitus 2, 38–43 (1989). [PubMed] [Google Scholar]
- 19.M. T. Manley, Incidence, contributory factors and costs of pressure sores. S. Afr. Med. J. 53, 217–222 (1978). [PubMed] [Google Scholar]
- 20.M. Fogerty, J. Guy, A. Barbul, L. B. Nanney, N. N. Abumrad, African Americans show increased risk for pressure ulcers: A retrospective analysis of acute care hospitals in America. Wound Repair Regen. 17, 678–684 (2009). [DOI] [PubMed] [Google Scholar]
- 21.R. M. Allman, Pressure ulcers among the elderly. N. Engl. J. Med. 320, 850–853 (1989). [DOI] [PubMed] [Google Scholar]
- 22.R. M. Allman, J. M. Walker, M. K. Hart, C. A. Laprade, L. B. Noel, C. R. Smith, Air-fluidized beds or conventional therapy for pressure sores. A randomized trial. Ann. Intern. Med. 107, 641–648 (1987). [DOI] [PubMed] [Google Scholar]
- 23.J. Maklebust, Pressure ulcers: Etiology and prevention. Nurs. Clin. North Am. 22, 359–377 (1987). [PubMed] [Google Scholar]
- 24.T. Velnar, T. Bailey, V. Smrkolj, The wound healing process: An overview of the cellular and molecular mechanisms. J. Int. Med. Res. 37, 1528–1542 (2009). [DOI] [PubMed] [Google Scholar]
- 25.T. J. Schaefer, K. D. Szymanski, in StatPearls (Treasure Island, 2022). [Google Scholar]
- 26.P. C. Ambe, T. Rombey, J. D. Rembe, J. Dorner, H. Zirngibl, D. Pieper, The role of saline irrigation prior to wound closure in the reduction of surgical site infection: A systematic review and meta-analysis. Patient Saf. Surg. 14, 47 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.R. Simman, Wound closure and the reconstructive ladder in plastic surgery. J. Am. Col. Certif. Wound Spec. 1, 6–11 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.R. G. Sibbald, J. A. Elliott, R. Persaud-Jaimangal, L. Goodman, D. G. Armstrong, C. Harley, S. Coelho, N. Xi, R. Evans, D. O. Mayer, X. Zhao, J. Heil, B. Kotru, B. Delmore, K. LeBlanc, E. A. Ayello, H. Smart, G. Tariq, A. Alavi, R. Somayaji, Wound bed preparation 2021. Adv. Skin Wound Care 34, 183–195 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.V. Falanga, H. Brem, W. J. Ennis, R. Wolcott, L. J. Gould, E. A. Ayello, Maintenance debridement in the treatment of difficult-to-heal chronic wounds. Recommendations of an expert panel. Ostomy Wound Manage Suppl, 2–13 (2008). [PubMed] [Google Scholar]
- 30.M. D. Nipshagen, J. J. Hage, W. H. Beekman, Use of 2-octyl-cyanoacrylate skin adhesive (Dermabond) for wound closure following reduction mammaplasty: A prospective, randomized intervention study. Plast. Reconstr. Surg. 122, 10–18 (2008). [DOI] [PubMed] [Google Scholar]
- 31.H. Jan, N. Waters, P. Haines, A. Kent, LiquiBand® surgical S topical adhesive versus sutures for the closure of laparoscopic wounds. A randomized controlled trial. Gynecol. Surg. 10, 247–252 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.P. Romero, G. Frongia, S. Wingerter, S. Holland-Cunz, Prospective, randomized, controlled trial comparing a tissue adhesive (Dermabond) with adhesive strips (Steri-Strips) for the closure of laparoscopic trocar wounds in children. Eur. J. Pediatr. Surg. 21, 159–162 (2011). [DOI] [PubMed] [Google Scholar]
- 33.L. Afzali Borojeny, A. N. Albatineh, A. Hasanpour Dehkordi, R. Ghanei Gheshlagh, The Incidence of pressure ulcers and its associations in different wards of the hospital: A systematic review and meta-analysis. Int. J. Prev. Med. 11, 171 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.G. S. Schultz, R. G. Sibbald, V. Falanga, E. A. Ayello, C. Dowsett, K. Harding, M. Romanelli, M. C. Stacey, L. Teot, W. Vanscheidt, Wound bed preparation: A systematic approach to wound management. Wound Repair Regen. 11Suppl 1, S1–S28 (2003). [DOI] [PubMed] [Google Scholar]
- 35.J. A. Niezgoda, P. J. Sordi, M. H. Hermans, Evaluation of vashe wound therapy in the clinical management of patients with chronic wounds. Adv. Skin Wound Care 23, 352–357 (2010). [DOI] [PubMed] [Google Scholar]
- 36.J. Georgiadis, V. B. Nascimento, C. Donat, I. Okereke, M. M. Shoja, Dakin's Solution: "One of the most important and far-reaching contributions to the armamentarium of the surgeons". Burns 45, 1509–1517 (2019). [DOI] [PubMed] [Google Scholar]
- 37.J. Gil, I. Pastar, R. A. Houghten, S. Padhee, A. Higa, M. Solis, J. Valdez, C. R. Head, H. Michaels, B. Lenhart, C. Simms, B. Williams, P. Cudic, S. C. Davis, Novel cyclic lipopeptides fusaricidin analogs for treating wound infections. Front. Microbiol. 12, 708904 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.S. Barrett, Mepilex Ag: An antimicrobial, absorbent foam dressing with Safetac technology. Br. J. Nurs. 18, S30–S36 (2009). [DOI] [PubMed] [Google Scholar]
- 39.J. W. Beam, Topical silver for infected wounds. J. Athl. Train. 44, 531–533 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.L. Shi, D. Carson, Collagenase Santyl ointment: A selective agent for wound debridement. J. Wound Ostomy Continence Nurs. 36, S12–S16 (2009). [DOI] [PubMed] [Google Scholar]
- 41.V. Saxena, C. W. Hwang, S. Huang, Q. Eichbaum, D. Ingber, D. P. Orgill, Vacuum-assisted closure: Microdeformations of wounds and cell proliferation. Plast. Reconstr. Surg. 114, 1086–1096 (2004). [DOI] [PubMed] [Google Scholar]
- 42.A. Lockmann, T. Schill, F. Hartmann, L. L. Gronemeyer, R. Holzkamp, M. P. Schon, K. M. Thoms, Testing elevated protease activity: Prospective analysis of 160 wounds. Adv. Skin Wound Care 31, 82–88 (2018). [DOI] [PubMed] [Google Scholar]
- 43.K. S. Miller, B. K. Connizzo, E. Feeney, L. J. Soslowsky, Characterizing local collagen fiber re-alignment and crimp behavior throughout mechanical testing in a mature mouse supraspinatus tendon model. J. Biomech. 45, 2061–2065 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.S. Khetan, M. Guvendiren, W. R. Legant, D. M. Cohen, C. S. Chen, J. A. Burdick, Degradation-mediated cellular traction directs stem cell fate in covalently crosslinked three-dimensional hydrogels. Nat. Mater. 12, 458–465 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O. Chaudhuri, L. Gu, D. Klumpers, M. Darnell, S. A. Bencherif, J. C. Weaver, N. Huebsch, H. P. Lee, E. Lippens, G. N. Duda, D. J. Mooney, Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nat. Mater. 15, 326–334 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.L. Yin, D. M. Elliott, A biphasic and transversely isotropic mechanical model for tendon: Application to mouse tail fascicles in uniaxial tension. J. Biomech. 37, 907–916 (2004). [DOI] [PubMed] [Google Scholar]
- 47.N. Huebsch, E. Lippens, K. Lee, M. Mehta, S. T. Koshy, M. C. Darnell, R. M. Desai, C. M. Madl, M. Xu, X. Zhao, O. Chaudhuri, C. Verbeke, W. S. Kim, K. Alim, A. Mammoto, D. E. Ingber, G. N. Duda, D. J. Mooney, Matrix elasticity of void-forming hydrogels controls transplanted-stem-cell-mediated bone formation. Nat. Mater. 14, 1269–1277 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.D. R. Bogdanowicz, H. H. Lu, Designing the stem cell microenvironment for guided connective tissue regeneration. Ann. N. Y. Acad. Sci. 1410, 3–25 (2017). [DOI] [PubMed] [Google Scholar]
- 49.V. S. Nirmalanandhan, M. R. Dressler, J. T. Shearn, N. Juncosa-Melvin, M. Rao, C. Gooch, G. Bradica, D. L. Butler, Mechanical stimulation of tissue engineered tendon constructs: Effect of scaffold materials. J. Biomech. Eng. 129, 919–923 (2007). [DOI] [PubMed] [Google Scholar]
- 50.A. I. Goncalves, M. T. Rodrigues, M. E. Gomes, Tissue-engineered magnetic cell sheet patches for advanced strategies in tendon regeneration. Acta Biomater. 63, 110–122 (2017). [DOI] [PubMed] [Google Scholar]
- 51.S. D. Subramony, B. R. Dargis, M. Castillo, E. U. Azeloglu, M. S. Tracey, A. Su, H. H. Lu, The guidance of stem cell differentiation by substrate alignment and mechanical stimulation. Biomaterials 34, 1942–1953 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.R. Keller, D. Shook, P. Skoglund, The forces that shape embryos: Physical aspects of convergent extension by cell intercalation. Phys. Biol. 5, 015007 (2008). [DOI] [PubMed] [Google Scholar]
- 53.S. Horne-Badovinac, The Drosophila egg chamber-a new spin on how tissues elongate. Integr. Comp. Biol. 54, 667–676 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.V. H. Barocas, R. T. Tranquillo, An anisotropic biphasic theory of tissue-equivalent mechanics: The interplay among cell traction, fibrillar network deformation, fibril alignment, and cell contact guidance. J. Biomech. Eng. 119, 137–145 (1997). [DOI] [PubMed] [Google Scholar]
- 55.M. S. Peach, D. M. Ramos, R. James, N. L. Morozowich, A. D. Mazzocca, S. B. Doty, H. R. Allcock, S. G. Kumbar, C. T. Laurencin, Engineered stem cell niche matrices for rotator cuff tendon regenerative engineering. PLOS ONE 12, e0174789 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.S. B. Orr, A. Chainani, K. J. Hippensteel, A. Kishan, C. Gilchrist, N. W. Garrigues, D. S. Ruch, F. Guilak, D. Little, Aligned multilayered electrospun scaffolds for rotator cuff tendon tissue engineering. Acta Biomater. 24, 117–126 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.R. Perris, D. Perissinotto, Role of the extracellular matrix during neural crest cell migration. Mech. Dev. 95, 3–21 (2000). [DOI] [PubMed] [Google Scholar]
- 58.R. Langer, J. Folkman, Polymers for the sustained release of proteins and other macromolecules. Nature 263, 797–800 (1976). [DOI] [PubMed] [Google Scholar]
- 59.G. W. Ashley, J. Henise, R. Reid, D. V. Santi, Hydrogel drug delivery system with predictable and tunable drug release and degradation rates. Proc. Natl. Acad. Sci. U.S.A. 110, 2318–2323 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.A. T. Florence, P. U. Jani, Novel oral drug formulations. Their potential in modulating adverse effects. Drug Saf. 10, 233–266 (1994). [DOI] [PubMed] [Google Scholar]
- 61.J. Cohen, IL-12 deaths: Explanation and a puzzle. Science 270, 908 (1995). [DOI] [PubMed] [Google Scholar]
- 62.R. Langer, Drug delivery and targeting. Nature 392, 5–10 (1998). [PubMed] [Google Scholar]
- 63.H. Lambers, S. Piessens, A. Bloem, H. Pronk, P. Finkel, Natural skin surface pH is on average below 5, which is beneficial for its resident flora. Int. J. Cosmet. Sci. 28, 359–370 (2006). [DOI] [PubMed] [Google Scholar]
- 64.C. R. Kruse, M. Singh, S. Targosinski, I. Sinha, J. A. Sorensen, E. Eriksson, K. Nuutila, The effect of pH on cell viability, cell migration, cell proliferation, wound closure, and wound reepithelialization: In vitro and in vivo study. Wound Repair Regen. 25, 260–269 (2017). [DOI] [PubMed] [Google Scholar]
- 65.S. O. Blacklow, J. Li, B. R. Freedman, M. Zeidi, C. Chen, D. J. Mooney, Bioinspired mechanically active adhesive dressings to accelerate wound closure. Sci. Adv. 5, eaaw3963 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Y. Gao, X. Han, J. Chen, Y. Pan, M. Yang, L. Lu, J. Yang, Z. Suo, T. Lu, Hydrogel-mesh composite for wound closure. Proc. Natl. Acad. Sci. U.S.A. 118, e2103457118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.A. Tellechea, S. Bai, S. Dangwal, G. Theocharidis, M. Nagai, S. Koerner, J. E. Cheong, S. Bhasin, T. Y. Shih, Y. Zheng, W. Zhao, C. Zhang, X. Li, K. Kounas, S. Panagiotidou, T. Theoharides, D. Mooney, M. Bhasin, L. Sun, A. Veves, Topical application of a mast cell stabilizer improves impaired diabetic wound healing. J. Invest. Dermatol. 140, 901–911.e11 (2020). [DOI] [PubMed] [Google Scholar]
- 68.E. C. Leal, E. Carvalho, A. Tellechea, A. Kafanas, F. Tecilazich, C. Kearney, S. Kuchibhotla, M. E. Auster, E. Kokkotou, D. J. Mooney, F. W. LoGerfo, L. Pradhan-Nabzdyk, A. Veves, Substance P promotes wound healing in diabetes by modulating inflammation and macrophage phenotype. Am. J. Pathol. 185, 1638–1648 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.S. A. Castleberry, B. D. Almquist, W. Li, T. Reis, J. Chow, S. Mayner, P. T. Hammond, Self-Assembled wound dressings silence MMP-9 and improve diabetic wound healing in vivo. Adv. Mater. 28, 1809–1817 (2016). [DOI] [PubMed] [Google Scholar]
- 70.T. T. Nguyen, D. Ding, W. R. Wolter, R. L. Perez, M. M. Champion, K. V. Mahasenan, D. Hesek, M. Lee, V. A. Schroeder, J. I. Jones, E. Lastochkin, M. K. Rose, C. E. Peterson, M. A. Suckow, S. Mobashery, M. Chang, Validation of matrix metalloproteinase-9 (MMP-9) as a novel target for treatment of diabetic foot ulcers in humans and discovery of a potent and selective small-molecule MMP-9 inhibitor that accelerates healing. J. Med. Chem. 61, 8825–8837 (2018). [DOI] [PubMed] [Google Scholar]
- 71.D. Duscher, A. A. Trotsyuk, Z. N. Maan, S. H. Kwon, M. Rodrigues, K. Engel, Z. A. Stern-Buchbinder, C. A. Bonham, J. Barrera, A. J. Whittam, M. S. Hu, M. Inayathullah, J. Rajadas, G. C. Gurtner, Optimization of transdermal deferoxamine leads to enhanced efficacy in healing skin wounds. J. Control Release 308, 232–239 (2019). [DOI] [PubMed] [Google Scholar]
- 72.Y. Zhu, R. Hoshi, S. Chen, J. Yi, C. Duan, R. D. Galiano, H. F. Zhang, G. A. Ameer, Sustained release of stromal cell derived factor-1 from an antioxidant thermoresponsive hydrogel enhances dermal wound healing in diabetes. J. Control Release 238, 114–122 (2016). [DOI] [PubMed] [Google Scholar]
- 73.N. Lohmann, L. Schirmer, P. Atallah, E. Wandel, R. A. Ferrer, C. Werner, J. C. Simon, S. Franz, U. Freudenberg, Glycosaminoglycan-based hydrogels capture inflammatory chemokines and rescue defective wound healing in mice. Sci. Transl. Med. 9, eaai9044 (2017). [DOI] [PubMed] [Google Scholar]
- 74.J. Ishihara, A. Ishihara, K. Fukunaga, K. Sasaki, M. J. V. White, P. S. Briquez, J. A. Hubbell, Laminin heparin-binding peptides bind to several growth factors and enhance diabetic wound healing. Nat. Commun. 9, 2163 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Y. Zhu, Z. Cankova, M. Iwanaszko, S. Lichtor, M. Mrksich, G. A. Ameer, Potent laminin-inspired antioxidant regenerative dressing accelerates wound healing in diabetes. Proc. Natl. Acad. Sci. U.S.A. 115, 6816–6821 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.A. Bhatia, K. O'Brien, M. Chen, A. Wong, W. Garner, D. T. Woodley, W. Li, Dual therapeutic functions of F-5 fragment in burn wounds: Preventing wound progression and promoting wound healing in pigs. Mol. Ther. Methods Clin. Dev. 3, 16041 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Z. Fan, B. Liu, J. Wang, S. Zhang, Q. Lin, P. Gong, L. Ma, S. Yang, A novel wound dressing based on Ag/graphene polymer hydrogel: Effectively kill bacteria and accelerate wound healing. Adv. Funct. Mater. 24, 11 (2014). [Google Scholar]
- 78.K. Nuutila, J. Grolman, L. Yang, M. Broomhead, S. Lipsitz, A. Onderdonk, D. Mooney, E. Eriksson, Immediate treatment of burn wounds with high concentrations of topical antibiotics in an alginate hydrogel using a platform wound device. Adv. Wound Care (New Rochelle) 9, 48–60 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.J. M. Grolman, M. Singh, D. J. Mooney, E. Eriksson, K. Nuutila, Antibiotic-containing agarose hydrogel for wound and burn care. J. Burn Care Res. 40, 900–906 (2019). [DOI] [PubMed] [Google Scholar]
- 80.Y. Okamura, K. Kabata, M. Kinoshita, H. Miyazaki, A. Saito, T. Fujie, S. Ohtsubo, D. Saitoh, S. Takeoka, Fragmentation of poly(lactic acid) nanosheets and patchwork treatment for burn wounds. Adv. Mater. 25, 545–551 (2013). [DOI] [PubMed] [Google Scholar]
- 81.N. Tang, R. Zhang, Y. Zheng, J. Wang, M. Khatib, X. Jiang, C. Zhou, R. Omar, W. Saliba, W. Wu, M. Yuan, D. Cui, H. Haick, Highly efficient self-healing multifunctional dressing with antibacterial activity for sutureless wound closure and infected wound monitoring. Adv. Mater. 34, e2106842 (2022). [DOI] [PubMed] [Google Scholar]
- 82.G. Xu, Y. Lu, C. Cheng, X. Li, Z. Liu, J. Liu, G. Liu, Z. Shi, Z. Chen, F. Zhang, Y. Jia, D. Xu, W. Yuan, Z. Cui, S. Shin, Q. Liu, Battery-free and wireless smart wound dressing for wound infection monitoring and electrically controlled on-demand drug delivery. Adv. Funct. Mater. 31, 2100852 (2021). [Google Scholar]
- 83.T. Fu, P. Stupnitskaia, S. Matoori, Next-generation diagnostic wound dressings for diabetic wounds. ACS Meas. Sci. Au 2, 377–384 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Y. Jiang, A. A. Trotsyuk, S. Niu, D. Henn, K. Chen, C. C. Shih, M. R. Larson, A. M. Mermin-Bunnell, S. Mittal, J. C. Lai, A. Saberi, E. Beard, S. Jing, D. Zhong, S. R. Steele, K. Sun, T. Jain, E. Zhao, C. R. Neimeth, W. G. Viana, J. Tang, D. Sivaraj, J. Padmanabhan, M. Rodrigues, D. P. Perrault, A. Chattopadhyay, Z. N. Maan, M. C. Leeolou, C. A. Bonham, S. H. Kwon, H. C. Kussie, K. S. Fischer, G. Gurusankar, K. Liang, K. Zhang, R. Nag, M. P. Snyder, M. Januszyk, G. C. Gurtner, Z. Bao, Wireless, closed-loop, smart bandage with integrated sensors and stimulators for advanced wound care and accelerated healing. Nat. Biotechnol. 10.1038/s41587-022-01528-3, (2022). [DOI] [PubMed] [Google Scholar]
- 85.Q. Pang, D. Lou, S. Li, G. Wang, B. Qiao, S. Dong, L. Ma, C. Gao, Z. Wu, Smart flexible electronics-integrated wound dressing for real-time monitoring and on-demand treatment of infected wounds. Adv. Sci. (Weinh) 7, 1902673 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.P. Mostafalu, A. Tamayol, R. Rahimi, M. Ochoa, A. Khalilpour, G. Kiaee, I. K. Yazdi, S. Bagherifard, M. R. Dokmeci, B. Ziaie, S. R. Sonkusale, A. Khademhosseini, Smart bandage for monitoring and treatment of chronic wounds. Small 14, e1703509 (2018). [DOI] [PubMed] [Google Scholar]
- 87.J. W. Song, H. Ryu, W. Bai, Z. Xie, A. Vazquez-Guardado, K. Nandoliya, R. Avila, G. Lee, Z. Song, J. Kim, M. K. Lee, Y. Liu, M. Kim, H. Wang, Y. Wu, H. J. Yoon, S. S. Kwak, J. Shin, K. Kwon, W. Lu, X. Chen, Y. Huang, G. A. Ameer, J. A. Rogers, Bioresorbable, wireless, and battery-free system for electrotherapy and impedance sensing at wound sites. Sci. Adv. 9, eade4687 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.S. Obuobi, H. K. Tay, N. D. T. Tram, V. Selvarajan, J. S. Khara, Y. Wang, P. L. R. Ee, Facile and efficient encapsulation of antimicrobial peptides via crosslinked DNA nanostructures and their application in wound therapy. J. Control Release 313, 120–130 (2019). [DOI] [PubMed] [Google Scholar]
- 89.M. V. Mouritzen, M. Petkovic, K. Qvist, S. S. Poulsen, S. Alarico, E. C. Leal, L. T. Dalgaard, N. Empadinhas, E. Carvalho, H. Jenssen, Improved diabetic wound healing by LFcinB is associated with relevant changes in the skin immune response and microbiota. Mol. Ther. Methods Clin. Dev. 20, 726–739 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.S. Spiller, T. Wippold, K. Bellmann-Sickert, S. Franz, A. Saalbach, U. Anderegg, A. G. Beck-Sickinger, Protease-triggered release of stabilized CXCL12 from coated scaffolds in an ex vivo wound model. Pharmaceutics 13, 1597 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.M. Albanna, K. W. Binder, S. V. Murphy, J. Kim, S. A. Qasem, W. Zhao, J. Tan, I. B. El-Amin, D. D. Dice, J. Marco, J. Green, T. Xu, A. Skardal, J. H. Holmes, J. D. Jackson, A. Atala, J. J. Yoo, In situ bioprinting of autologous skin cells accelerates wound healing of extensive excisional full-thickness wounds. Sci. Rep. 9, 1856 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Y. Wu, T. Liang, Y. Hu, S. Jiang, Y. Luo, C. Liu, G. Wang, J. Zhang, T. Xu, L. Zhu, 3D bioprinting of integral ADSCs-NO hydrogel scaffolds to promote severe burn wound healing. Regen. Biomater. 8, rbab014 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.J. H. Teoh, S. M. Tay, J. Fuh, C. H. Wang, Fabricating scalable, personalized wound dressings with customizable drug loadings via 3D printing. J. Control Release 341, 80–94 (2021). [DOI] [PubMed] [Google Scholar]
- 94.W. Jiang, K. Ting, S. Lee, J. N. Zara, R. Song, C. Li, E. Chen, X. Zhang, Z. Zhao, C. Soo, Z. Zheng, Fibromodulin reduces scar size and increases scar tensile strength in normal and excessive-mechanical-loading porcine cutaneous wounds. J. Cell. Mol. Med. 22, 2510–2513 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.J. Montgomery, G. S. Ghatnekar, C. L. Grek, K. E. Moyer, R. G. Gourdie, Connexin 43-based therapeutics for dermal wound healing. Int. J. Mol. Sci. 19, 1778 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.R. Kirsner, C. Dove, A. Reyzelman, D. Vayser, H. Jaimes, A prospective, randomized, controlled clinical trial on the efficacy of a single-use negative pressure wound therapy system, compared to traditional negative pressure wound therapy in the treatment of chronic ulcers of the lower extremities. Wound Repair Regen. 27, 519–529 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.L. C. Kloth, J. E. Berman, S. Dumit-Minkel, C. H. Sutton, P. E. Papanek, J. Wurzel, Effects of a normothermic dressing on pressure ulcer healing. Adv. Skin Wound Care 13, 69–74 (2000). [PubMed] [Google Scholar]
- 98.L. C. Kloth, J. E. Berman, M. Nett, P. E. Papanek, S. Dumit-Minkel, A randomized controlled clinical trial to evaluate the effects of noncontact normothermic wound therapy on chronic full-thickness pressure ulcers. Adv. Skin Wound Care 15, 270–276 (2002). [DOI] [PubMed] [Google Scholar]
- 99.C. Robinson, S. M. Santilli, Warm-up active wound therapy: A novel approach to the management of chronic venous stasis ulcers. J. Vasc. Nurs. 16, 38–42 (1998). [DOI] [PubMed] [Google Scholar]
- 100.A. A. Khan, P. E. Banwell, M. C. Bakker, P. G. Gillespie, D. A. McGrouther, A. H. Roberts, Topical radiant heating in wound healing: An experimental study in a donor site wound model. Int. Wound J. 1, 233–240 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.J. H. Yue, S. J. Zhang, Q. Sun, Z. R. Sun, X. X. Wang, B. Golianu, Y. Lu, Q. Zhang, Local warming therapy for treating chronic wounds: A systematic review. Medicine (Baltimore) 97, e9931 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.R. Kaur, C. Tchanque-Fossuo, K. West, Y. Hadian, A. Gallegos, D. Yoon, L. Ismailyan, S. Schaefer, S. E. Dahle, R. R. Isseroff, Beta-adrenergic antagonist for the healing of chronic diabetic foot ulcers: Study protocol for a prospective, randomized, double-blinded, controlled and parallel-group study. Trials 21, 496 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Y. Y. Huang, C. W. Lin, N. C. Cheng, S. M. Cazzell, H. H. Chen, K. F. Huang, K. Y. Tung, H. L. Huang, P. Y. Lin, C. K. Perng, B. Shi, C. Liu, Y. Ma, Y. Cao, Y. Li, Y. Xue, L. Yan, Q. Li, G. Ning, S. C. Chang, Effect of a novel macrophage-regulating drug on wound healing in patients with diabetic foot ulcers: A randomized clinical trial. JAMA Netw. Open 4, e2122607 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.A. D. Rowan, C. W. Christopher, S. F. Kelley, D. J. Buttle, H. P. Ehrlich, Debridement of experimental full-thickness skin burns of rats with enzyme fractions derived from pineapple stem. Burns 16, 243–246 (1990). [DOI] [PubMed] [Google Scholar]
- 105.C. Varilla, M. Marcone, L. Paiva, J. Baptista, Bromelain, a group of pineapple proteolytic complex enzymes (Ananas comosus) and their possible therapeutic and clinical effects. A summary. Foods 10, 2249 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.S. G. Llames, M. Del Rio, F. Larcher, E. Garcia, M. Garcia, M. J. Escamez, J. L. Jorcano, P. Holguin, A. Meana, Human plasma as a dermal scaffold for the generation of a completely autologous bioengineered skin. Transplantation 77, 350–355 (2004). [DOI] [PubMed] [Google Scholar]
- 107.C. Gomez, J. M. Galan, V. Torrero, I. Ferreiro, D. Perez, R. Palao, E. Martinez, S. Llames, A. Meana, P. Holguin, Use of an autologous bioengineered composite skin in extensive burns: Clinical and functional outcomes. A multicentric study. Burns 37, 580–589 (2011). [DOI] [PubMed] [Google Scholar]
- 108.G. Theocharidis, D. Baltzis, M. Roustit, A. Tellechea, S. Dangwal, R. S. Khetani, B. Shu, W. Zhao, J. Fu, S. Bhasin, A. Kafanas, D. Hui, S. H. Sui, N. A. Patsopoulos, M. Bhasin, A. Veves, Integrated skin transcriptomics and serum multiplex assays reveal novel mechanisms of wound healing in diabetic foot ulcers. Diabetes 69, 2157–2169 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.G. Rioux, M. Simard, S. Morin, I. Lorthois, S. L. Guerin, R. Pouliot, Development of a 3D psoriatic skin model optimized for infiltration of IL-17A producing T cells: Focus on the crosstalk between T cells and psoriatic keratinocytes. Acta Biomater. 136, 210–222 (2021). [DOI] [PubMed] [Google Scholar]
- 110.S. Matoori, A. Veves, D. J. Mooney, Advanced bandages for diabetic wound healing. Sci. Transl. Med. 13, eabe4839 (2021). [DOI] [PubMed] [Google Scholar]
- 111.B. R. Freedman, D. J. Mooney, E. Weber, Advances toward transformative therapies for tendon diseases. Sci. Transl. Med. 14, eabl8814 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.A. Grada, J. Mervis, V. Falanga, Research techniques made simple: Animal models of wound healing. J. Invest. Dermatol. 138, 2095–2105.e1 (2018). [DOI] [PubMed] [Google Scholar]
- 113.L. K. S. Parnell, S. W. Volk, The evolution of animal models in wound healing research: 1993-2017. Adv. Wound Care (New Rochelle) 8, 692–702 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.K. J. Stalnaker, C. Fuchs, A. Slate, J. N. Camacho, L. Pham, Y. Wang, R. R. Anderson, J. Tam, Boot camp: Training and dressing regimens for modeling plantar wounds in the swine. Lab. Anim 57, 59–68 (2022). [DOI] [PubMed] [Google Scholar]
- 115.R. Nunan, K. G. Harding, P. Martin, Clinical challenges of chronic wounds: Searching for an optimal animal model to recapitulate their complexity. Dis. Model. Mech. 7, 1205–1213 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.A. C. Drake, Of mice and men: What rodent models don't tell us. Cell. Mol. Immunol. 10, 284–285 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.J. Seok, H. S. Warren, A. G. Cuenca, M. N. Mindrinos, H. V. Baker, W. Xu, D. R. Richards, G. P. McDonald-Smith, H. Gao, L. Hennessy, C. C. Finnerty, C. M. Lopez, S. Honari, E. E. Moore, J. P. Minei, J. Cuschieri, P. E. Bankey, J. L. Johnson, J. Sperry, A. B. Nathens, T. R. Billiar, M. A. West, M. G. Jeschke, M. B. Klein, R. L. Gamelli, N. S. Gibran, B. H. Brownstein, C. Miller-Graziano, S. E. Calvano, P. H. Mason, J. P. Cobb, L. G. Rahme, S. F. Lowry, R. V. Maier, L. L. Moldawer, D. N. Herndon, R. W. Davis, W. Xiao, R. G. Tompkins; Inflammation and Host Response to Injury; Large Scale Collaborative Research Program , Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc. Natl. Acad. Sci. U.S.A. 110, 3507–3512 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.L. E. Wagar, R. M. DiFazio, M. M. Davis, Advanced model systems and tools for basic and translational human immunology. Genome Med. 10, 73 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.K. D. Verma, F. Lewis, M. Mejia, M. Chalasani, K. A. Marcus, Food and drug administration perspective: Advancing product development for non-healing chronic wounds. Wound Repair Regen. 30, 299–302 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.FDA. (2006).
- 121.G. M. Gordillo, S. F. Bernatchez, R. Diegelmann, L. A. Di Pietro, E. Eriksson, B. Hinz, H. W. Hopf, R. Kirsner, P. Liu, L. K. Parnell, G. E. Sandusky, C. K. Sen, M. Tomic-Canic, S. W. Volk, A. Baird, Preclinical models of wound healing: Is man the model? proceedings of the wound healing society symposium. Adv Wound Care (New Rochelle) 2, 1–4 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.B. R. Freedman, D. J. Mooney, Biomaterials to mimic and heal connective tissues. Adv. Mater. 31, e1806695 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]