Supplemental Digital Content is available in the text.
Keywords: diarrhea, Saccharomyces boulardii, Lactobacillus rhamnosus, rotavirus
Abstract
Pediatric acute gastroenteritis (PAGE) is a significant cause of morbidity, mortality and healthcare costs in many countries, but differences in PAGE vary from country-to-country; thus, we limited our analysis to 1 country. Probiotics have been recommended as an adjunct to standard treatment, but the choice of probiotic is unclear. PubMed, Google Scholar, and reviews were searched from inception to May 2020 for randomized controlled trials (RCTs) in India using probiotics for a treatment for PAGE. Meta-analyses using subgroups of identical probiotic types (≥2 RCT/type) were conducted for primary outcomes (duration of diarrhea, cured by day 3, rapidity of response, and length of hospital stay). Twenty-two RCTs were included in the systematic review (N = 4059 participants) including 5 single-strained probiotics and 3 multi-strained mixtures. For the meta-analyses, 17 RCT (20 treatment arms) were included. Saccharomyces boulardii CNCM I-745 had the strongest effect on shortening the duration of diarrhea (standardized mean difference, –1.86 d; 95% confidence interval, –2.8 to –0.9), while both Lactobacillus rhamnosus GG and a mixture of 4 Bacillus clausii strains (O/C, SIN, N/R, T) significantly reduced the duration of diarrhea (–1.7 and –1.4 d, respectively). S. boulardii and L. rhamnosus GG significantly reduced hospital stays (−1.8 and −1.1 d, respectively), while B. clausii had no effect. The frequency of stools/day was significantly reduced by day 4 for S. boulardii and by day 5 for L. rhamnosus GG. In India, 2 types of probiotics (S. boulardii CNCM I-745 and L. rhamnosus GG) significantly shortened both the duration of diarrhea and hospitalization stays in pediatric patients with PAGE. While these 2 probiotic strains were safe and effective for children in India, further research is needed to confirm if other probiotic strains or mixtures may be effective.
What Is Known
Pediatric acute gastroenteritis (PAGE) is a significant cause of morbidity and mortality globally, especially in children under 5 years old and is more severe in developing countries.
Most clinical trials studying the efficacy of probiotics for PAGE have been done in developed countries.
The choice of an appropriate probiotic is strain-specific, but it is not known if efficacy differs depending upon study population’s country.
What Is New
Only 3 types of probiotics had sufficient clinical trials (total 17 randomized controlled trials) in India to be included in a meta-analysis.
Two probiotics (Saccharomyces boulardii CNCM I-745 and Lactobacillus rhamnosus GG) significantly reduced both the duration of diarrhea (1–2 d) and length of hospitalization (1–2 d) compared with controls in children living in India.
Translational Impact
Two types of probiotics added to standard treatments for PAGE were found effective and safe in clinical trials done in India, but other probiotics required further trials.
INTRODUCTION
Pediatric acute gastroenteritis (PAGE) is a leading cause of morbidity and mortality in children under 5 years old (1–3). Globally, 1.7 billion cases of PAGE occur each year, with 90% of cases occurring in developing countries (1,4). Deaths due to PAGE in children under 5 years were found to vary from country-to-country in a survey done in 2017: Africa (3%–13%), Asia (1%–9%), with the highest rates found in Syria (20%) (2). Factors influencing the incidence and severity of PAGE in different geographic areas include water sanitation, degree of malnourishment, diet, lifestyle factors, and type of diarrhea etiologies (3,5). It is difficult to account for all these factors when assessing efficacy of new treatments; thus, we focused our review on trials done in 1 country (India).
In India, improvements in water quality and use of oral rehydration therapy (ORT) has resulted in a 40% reduction of PAGE-associated mortality since 2001, but PAGE continues to be a significant cause of morbidity, mortality, increased hospital admissions, and economic burden (4–8). Etiologies of PAGE in India may also differ from European countries. In Europe, the most common etiologies were found to be Campylobacter or Salmonella (9). In 1 study of outpatient children under 5 years in Odisha, India, the most common etiologies for PAGE were E. coli (30%), rotavirus (26%), or Shigella (24%), and concurrent infection with multiple etiologies was frequent (34%) (10).
Shifts in the normally protective intestinal microbiome are found when diarrhea is present (11,12) and use of some strains of living probiotics has been found to be useful in restoring the normal microbiome (13,14). The choice of an appropriate probiotic is challenging due to the diverse types of probiotics available, inconsistent reports of efficacies and strength of available evidence (15). As the efficacy of probiotics has been demonstrated to be both disease-specific and strain-specific, it is important to assess efficacy only within the identical type of probiotic strain or strains within multi-strain mixtures for 1 type of disease (16,17). Many reviews and meta-analyses have either incorrectly pooled different strains of probiotics together (18) or limited their review to 1 strain of probiotic, but then pooled studies done in developed and developing countries (19–23). The pooled estimates of efficacy may be biased due to heterogeneity related to different probiotic strains or by different factors (microbiome profiles, socioeconomic factors, etc.) related to geographic area. The recent American Gastroenterology Association guidelines recommended against probiotics for PAGE in the United States and Canada, as most randomized controlled trials (RCTs) were done outside of these 2 countries, thus highlighting the importance of country-to-country variations (24).
This review focuses on randomized clinical trials only done in 1 country (India) in an effort to reduce these diverse sources of heterogeneity. Our aim is to determine which types of probiotics are safe and clinically effective for management of PAGE in the Indian population.
METHODS
Primary outcomes included differences in the mean duration of acute diarrhea from enrollment to resolution or the end of the study in subgroups of probiotics with the same strain or mixture of strains compared with the duration in the control groups. The other primary outcomes were the frequency of children with diarrhea resolution by day 3 or 5 (“cured”) of treatment in subgroups of probiotics with the same strain or mixture of strains compared with children in the control group and the rapidity of response (the mean number of stools/day from day 1, to day 3, 4, or 5) for the probiotic and control groups. Secondary outcomes included differences in length of hospitalization and differences in the frequency of adverse events between the study groups.
Standard search strategy, inclusion/exclusion criteria, data extraction, statistical methods, and assessment procedures were followed and described (see Text, Supplemental Digital Content 1, Methods, http://links.lww.com/PG9/A30) (25–30).
RESULTS
Search Results
The literature search yielded 235 articles on probiotic use for the treatment of PAGE (see Figure, Supplemental Digital Content 1, PRISMA Flow-Chart, http://links.lww.com/PG9/A32; Table, Supplemental Digital Content 1, PRISMA Checklist, http://links.lww.com/PG9/A40). Reasons for exclusion (n = 213 studies) are provided elsewhere (see Text, Supplemental Digital Content 2, Results, http://links.lww.com/PG9/A31). Twelve trials done in India but not meeting inclusion criteria were excluded (see Table, Supplemental Digital Content 2, Excluded Trials, http://links.lww.com/PG9/A41) (31–42).
Studies included in the systematic review (n = 22 RCTs, N = 4059 participants) included 5 single-strain probiotics and 3 multi-strained mixtures (Table 1) (43–64). Five types of probiotics were excluded from the meta-analysis (60–64), as they lacked at least 1 confirmatory trial, resulting in 17 RCTs for the meta-analysis (20 treatment arms) for 3 probiotics: Saccharomyces boulardii CNCM I-745 (9 RCTs), Lactobacillus rhamnosus GG (ATCC 53103) (6 RCT, 7 arms), and a 4-strain mixture of Bacillus clausii O/C, SIN, N/R, T (4 RCTs). Two trials compared 2 different probiotics to a control group (43,51) and 1 trial had 2 treatment arms with different doses of the probiotic (55).
TABLE 1.
Probiotic and control intervention characteristics of 22 randomized controlled trials (25 treatment arms) in India for the treatment of Pediatric Acute Gastroenteritis
| Probiotic | Daily dose (CFU/d) | Formulation | Duration (d) | Type of control | ORT given | Zinc | Initiation Time (d) | Overall risk of bias | References |
|---|---|---|---|---|---|---|---|---|---|
| Saccharomyces boulardii CNCM I-745 | 1 × 1010 | NR | 6 | Open | Yes | NR | 1.2 ± 0.6 | High | Bhat et al (43) |
| S. boulardii CNCM I-745 | 1 × 1010 | Sachet | 5 | Open | Yes | Yes | <2 | Low | Burande and Burande (44) |
| S. boulardii CNCM I-745 | 1 × 1010 | Sachet | 5 | Placebo | NR | NR | 3 ± 1 | Low | Das et al (45) |
| S. boulardii CNCM I-745 | 1 × 1010 | Sachet | 5 | Open | Yes | Yes | NR | High | Dash et al (46) |
| S. boulardii CNCM I-745 | 1 × 1010 | Sachet | 3 | Open | PRN | NR | NR | High | Kumar et al (47) |
| S. boulardii CNCM I-745 | 1 × 1010 | Powder | 5 | Placebo | Yes | Yes | 0.9 ± 0.8 | Low | Riaz et al (48) |
| S. boulardii CNCM I-745 | 1 × 1010 | Powder | 5 | Open | Yes | NR | NR | High | Sirsat and Sankpal (49) |
| S. boulardii CNCM I-745 | 1 × 1010 | NR | 5 | Placebo | Yes | No | NR | Low | Vandenplas et al (50) |
| S. boulardii CNCM I-745 | 1 × 1010 | Sachet | NR | Open | Yes | Yes | NR | Low | Vidjeadevan et al (51) |
| Lactobacillus rhamnosus GG | 1 × 1010 | Capsule | 5 | Open | Yes | Yes | 2.2 ± 1.3 | Low | Aggarwal et al (52) |
| L. rhamnosus GG | 1 × 1010 | NR | 5 | Open | NR | NR | 2.5 ± 1.0 | High | Agarwal (53) |
| L. rhamnosus GG | 1.2 × 108 | Liquid | 7 | Placebo | Yes | No | NR | Low | Basu et al (54) |
| L. rhamnosus GG-low dose | 2 × 1010 | Liquid | 7 | Placebo | Yes | No | NR | Low | Basu et al (55) |
| L. rhamnosus GG-high dose | 2 × 1012 | Liquid | 7 | Placebo | Yes | No | NR | Low | Basu et al (55) |
| L. rhamnosus GG | 1 × 106–109 | Capsule | 3–10 | Placebo | NR | No | 1.9 ± NR | Low | Misra et al (56) |
| L. rhamnosus GG | 1 × 1010 | Capsule | 28 | Placebo | NR | NR | 4 ± NR | Low | Sindhu et al (57) |
| Bacillus clausii O/C, SIN, N/R, T | 4 × 109 | Spores | 6 | Open | Yes | NR | 1.2 ± 0.6 | High | Bhat et al (43) |
| B. clausii O/C, SIN, N/R, T | 4 × 109 | Liquid | 5 | Open | Yes | Yes | NR | High | Lahiri et al (58) |
| B. clausii O/C, SIN, N/R, T | 4 × 109 | Liquid | 5 | Open | Yes | Yes | NR | High | Lahiri et al (59) |
| B. clausii O/C, SIN, N/R, T | 2 × 109 | Spores | NR | Open | Yes | Yes | NR | Low | Vidjeadevan et al (51) |
| Bifilac (4 strains) | 2.5 × 108 | Sachets | 14 | Placebo | Yes | NR | <3 | Low | Narayanappa (60) |
| B. clausii UBBC-07 | 4 × 109 | Liquid | 5 | Placebo | Yes | NR | NR | Low | Sudha et al (61) |
| L. casei DN114001 | 1 × 1010 | NR | 5 | Open | NR | No | NR | Low | Agarwal and Bhasin (62) |
| L. sporogenes | 2.4 × 109 | Tablets | 5 | Placebo | Yes | No | <3 | Low | Dutta et al (63) |
| 8 strain mixture | 2.4 × 1011 | Liquid | 4 | Placebo | Yes | NR | <3 | Low | Dubey et al (64) |
L. rhamnosus GG (ATCC 53103); Bifilac: 4 strain mixture: Clostridium butyricum, Bacillus mesentericus, Streptococcus faecalis, and L. sporogenes, strains not reported, from author correspondence; 8 strain mixture: L. plantarum DSM24730, S. thermophilus DSM24731, Bifidobacterium breve DSM24732, L. delbrueckii ssp. bulgaricus DSM24733, L. paracasei DSM24734, L. acidophilus DSM24735, B. longum DSM24736, and B. infantis DSM24737. CFU/d = colony-forming units per day; Initiation Time = mean time from onset of diarrhea to initiation of probiotic; NR = not reported in article; ORT = oral rehydration therapy; PRN = given as needed.
Trial Characteristics
The study participant characteristics and safety data are described supplementary files (see Text, Supplemental Digital Content 2, Results, http://links.lww.com/PG9/A31 and in Table, Supplemental Digital Content 3, Study Population, http://links.lww.com/PG9/A42). Most trials provided complete descriptions of the probiotic intervention (Table 1), but 10 trials did not provide the manufacturer or brand name (see Table, Supplemental Digital Content 4, Probiotic Description, http://links.lww.com/PG9/A43).
Of the 17 RCTs with ≥2 RCTs/probiotic type, 59% had an overall low risk of bias, while 41% had an overall high risk of bias (Table 1). Most of the trials had a low risk of bias for randomization method (59%), attrition (88%), open/placebo controls (53%), and reporting post hoc outcomes (94%) (see Figure, Supplemental Digital Content 2, Risk of Bias, http://links.lww.com/PG9/A33). Ten (59%) of the 17 RCTs were double-blinded using placebos, while 7 (41%) used open controls (standard treatments only). There was no significant impact of blinding on the estimated treatment effects; thus, these types of controls were combined. For example, in trials with S. boulardii, the mean duration of diarrhea for unblinded (open) studies was 2.3 days for S. boulardii versus 3.6 days for open controls and comparable data were observed (2.7 d for S. boulardii versus 3.6 d for placebo controls), and results were similar for L. rhamnosus GG trials, while all B. clausii trials used open controls. Trials ranked as high risk typically did not describe the method of randomization, whether the study allocation was blinded, or did not report if the outcome was determined by blinded study staff. The risk of bias had an inconsistent impact on outcome measures by the type of probiotic: trials with S. boulardii showed the greatest reduction in duration of diarrhea in low bias risk trials (see Table Supplemental Digital Content 5, Subgroup Analysis, http://links.lww.com/PG9/A44), while the greatest reduction was seen in high-risk trials with L. rhamnosus GG and 3 of 4 trials with B. clausii mix were of high risk.
Meta-Analysis of Probiotic Efficacy
Duration of Diarrhea
Of the 17 RCTs (20 treatment arms), most (15, 88%) reported the mean duration of diarrhea as the primary outcome (Table 2). As shown in Figure 1, 3 types of probiotics significantly reduced the mean duration PAGE. S. boulardii CNCM I-745 had the strongest effect on the reduction of diarrhea (standardized mean difference [SMD], –1.86 d; 95% confidence interval [CI], –2.8 to –0.91 d; P < 0.001, I2 = 96.6%). L. rhamnosus GG also significantly reduced the duration of diarrhea, but to a lesser extent (SMD, –1.75 d; 95% CI, –2.73 to –0.77 d; P = 0.001; I2 = 98.8%). The 4-strain mixture of B. clausii O/C, SIN, N/R, T also significantly reduced the duration of diarrhea (SMD, –1.39; 95% CI, –2.74 to –0.04; P = 0.04; I2 = 97.3%). There was significant heterogeneity found among these studies (I2 = 97%–99%) and publication bias was noted (Egger’s t = –3.3; P = 0.005), as shown in the funnel plot (see Figure, Supplemental Digital Content 3, Publication Bias, http://links.lww.com/PG9/A34). Sensitivity analysis found no one individual trial had undue influence on the pooled outcome of the mean duration of diarrhea.
TABLE 2.
Main outcomes for probiotic and control groups from 22 randomized controlled trials (25 treatment arms) in India for the treatment of Pediatric Acute Gastroenteritis
| Probiotic | Probiotic | Control | Probiotic | Control | Probiotic | Control | References | ||
|---|---|---|---|---|---|---|---|---|---|
| N | Duration of diarrhea (mean d ± SD) | N | Duration of diarrhea (mean d ± SD) | Cured by day 3, n (%) | Cured by day 3, n (%) | AE (%) | AE (%) | ||
| Saccharomyces boulardii CNCM I-745 | 40 | 1.7 ± 0.4 | 40 | 2.4 ± 1.1 | NR | NR | NR | NR | Bhat et al (43) |
| S. boulardii CNCM I-745 | 35 | 3.4 ± 1.4 | 35 | 5.5 ± 2.1 | NR | NR | NR | NR | Burande and Burande (44) |
| S. boulardii CNCM I-745 | 30 | 2.5 ± 0.2* | 30 | 3.7 ± 0.3* | NR | NR | 0 | 0 | Das et al (45) |
| S. boulardii CNCM I-745 | 64 | 1.1 ± 0.5* | 62 | 2.0 ± 1.0* | NR | NR | 0 | 0 | Dash et al (46) |
| S. boulardii CNCM I-745 | 50 | Unclear | 50 | Unclear | 15 (30) | 8 (16) | 0* | 0* | Kumar et al (47) |
| S. boulardii CNCM I-745 | 54 | 2.2 ± 2.0 | 54 | 2.7 ± 1.3 | NR | NR | 0 | 0 | Riaz et al (48) |
| S. boulardii CNCM I-745 | 145 | NR | 145 | NR | 97 (67) | 72 (49) | NR | NR | Sirsat et al (49) |
| S. boulardii CNCM I-745 | 93 | 2.2 ± 1.6 | 95 | 2.8 ± 2.2 | 90 (97) | 85 (90) | 0 | 0 | Vandenplas et al (50) |
| S. boulardii CNCM I-745 | 34 | 3.0 ± 0.2* | 32 | 4.5 ± 0.2* | 20 (59) | 5 (16) | NR | NR | Vidjeadevan et al (51) |
| Lactobacillus rhamnosus GG | 100 | 2.5 ± 0.1 | 100 | 3.2 ± 0.1 | NR | NR | 0 | 0 | Aggarwal et al (52) |
| L. rhamnosus GG | 32 | 2.5 ± 1.9 | 33 | 3.2 ± 1.9 | NR | NR | 0 | 0 | Agarwal (53) |
| L. rhamnosus GG | 323 | 6.8 ± 2.1 | 323 | 6.6 ± 2.3 | NR | NR | 1 | 2 | Basu et al (54) |
| L. rhamnosus GG-low dose | 188 | 5.0 ± 1.3 | 185 | 7.2 ± 1.3 | NR | NR | 2 | 4 | Basu et al (55) |
| L. rhamnosus GG-high dose | 186 | 5.1 ± 1.2 | 185 | 7.2 ± 1.3 | NR | NR | 4 | 4 | Basu et al (55) |
| L. rhamnosus GG | 105 | 2.9 ± 0.5* | 105 | 3.2 ± 0.5* | NR | NR | NR | NR | Misra et al (56) |
| L. rhamnosus GG | 65 | 4.0 ± 2.2* | 59 | 4.0 ± 2.2* | NR | NR | 6 | 2 | Sindhu et al (57) |
| Bacillus clausii O/C, SIN, N/R, T | 40 | 2.2 ± 0.7 | 40 | 2.4 ± 1.1 | NR | NR | NR | NR | Bhat et al (43) |
| B. clausii O/C, SIN, N/R, T | 69 | 0.9 ± 0.4* | 62 | 1.96 ± 0.4* | NR | NR | NR | NR | Lahiri et al (58) |
| B. clausii O/C, SIN, N/R, T | 80 | 0.9 ± 1.7* | 80 | 1.4 ± 1.7* | NR | NR | NR | NR | Lahiri et al (59) |
| B. clausii O/C, SIN, N/R, T | 33 | 4.0 ± 0.2* | 32 | 4.5 ± 0.2* | 15 (45) | 5 (16) | NR | NR | Vidjeadevan et al (51) |
| Bifilac (4 strains) | 40 | 4.3 ± 1.2 | 40 | 5.4 ± 1.7 | NR | NR | 0 | 0 | Narayanappa (60) |
| B. clausii UBBC-07 | 59 | 3.1 ± 0.6 | 60 | 3.4 ± 0.6 | 41 (69.5) | 35 (58.3) | NR | NR | Sudha et al (61) |
| L. casei DN114001 | 32 | 1.5 ± 0.5 | 33 | 2.1 ± 0.7 | NR | NR | NR | NR | Agarwal and Bhasin (62) |
| L. sporogenes | 78 | 1.4 ± 0.8 | 70 | 1.5 ± 0.9 | 70 (89.7) | 58 (82.9) | 0 | 0 | Dutta et al (63) |
| 8 strain mixture | 113 | NR | 111 | NR | 101 (89.4) | 44 (39.6) | 0 | 0 | Dubey et al (64) |
*Data supplied by author; or estimated SD; Bifilac: 4 strain mixture: Clostridium butyricum, Bacillus mesentericus, Streptococcus faecalis, and L. sporogenes, strains not reported, or; 8 strain mixture: L. plantarum DSM24730, S. thermophilus DSM24731, Bifidobacterium breve DSM24732, L. delbrueckii ssp. bulgaricus DSM24733, L. paracasei DSM24734, L. acidophilus DSM24735, B. longum DSM24736, and B. infantis DSM24737. AE = adverse event; NR = not reported in article; SD = standard deviation.
FIGURE 1.
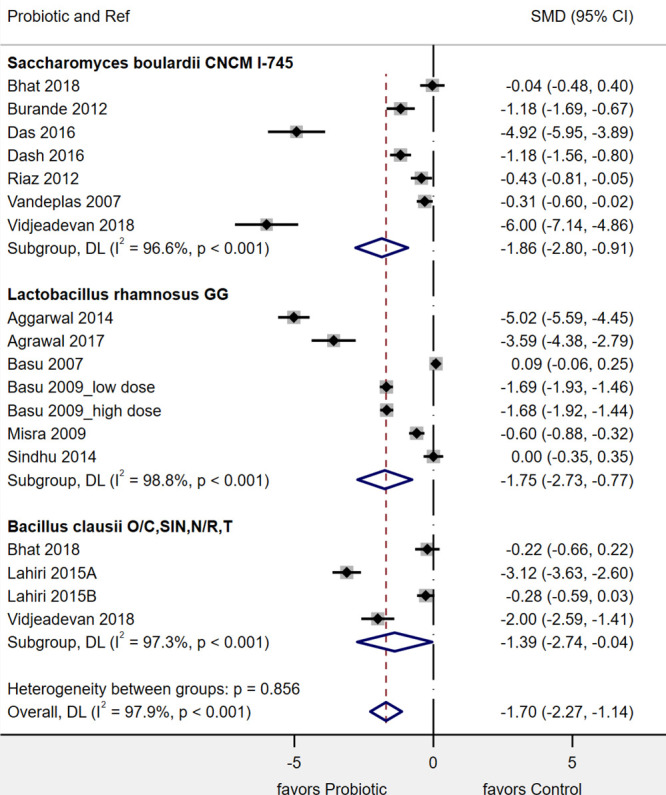
Forest plot of 17 randomized controlled trials done in India for the mean reduction in the duration of Pediatric Acute Gastroenteritis (d) with 3 different probiotics. CI = confidence interval; DL = DerSimonian-Laird estimate of between study variance; SMD = standardized mean difference.
Subgroup analyses found several factors influenced the effect of probiotics on the mean duration of diarrhea (see Table, Supplemental Digital Content 5, Subgroups, http://links.lww.com/PG9/A44). In 4 RCTs, when S. boulardii CNCM I-745 was added to ORT and zinc, there was a significant reduction of the duration of diarrhea (SMD, –2.05 d; 95 CI, –3.4 to –0.75 d; P = 0.002). In 1 study, no zinc was given (50) and 4 RCTs did not report if zinc was given or not (43, 45, 47, 49). Trials using S. boulardii with a low risk of bias showed a greater reduction in diarrhea duration, but the difference was not significant (Cochrane’s Q = 2.1; P = 0.15). No other subgroups significantly influenced the efficacy of S. boulardii CNCM I-745.
Subgroups resulting in a significant reduction in heterogeneity for L. rhamnosus GG trials included: use of zinc (Cochrane’s Q = 38.0; P < 0.001), risk of bias (Cochrane’s Q = 5.8; P = 0.02), unblinded controls (Cochrane’s Q = 18.2; P = 0.001), and higher (≥1010 colony-forming units per day [CFU]/d) dose (Cochrane’s Q = 5.24; P = 0.02). For 2 subgroups (zinc and risk of bias), only 1 trial used zinc (52) and only 1 trial had a high risk of bias (53), so conclusions should not be made for these factors. For factors (daily dose and blinded study design), more robust conclusions can be made as there were ≥2 trials/subgroup. For L. rhamnosus GG trials, daily doses ≥1010/d resulted in a significantly greater reduction in days of diarrhea (SMD, –2.4 d; 95% CI, –3.6 to –1.1; P < 0.001) compared with lower doses (SMD, –0.2 d; 95% CI, –0.92 to 0.43; P = 0.48). For trials comparing placebo to L. rhamnosus GG, the mean reduction was not significant (SMD, –0.78; 95% CI, –1.61 to 0.05; P = 0.07), while use of open controls resulted in a significant reduction of diarrhea (SMD, –4.33; 95% CI, –5.73 to –2.93; P < 0.001).
Subgroup analysis for B. clausii trials was limited, as all trials were in inpatients and used open controls and all 4 trials gave ORT to the subjects. Zinc was also given in 3 trials but not reported in 1 trial (43). Subgroups were not significantly different: daily dose (Cochrane’s Q = 0.63; P = 0.43) and low versus high risk of bias (Cochrane’s Q = 0.63; P = 0.43).
Other subgroup analyses found factors that did not significantly impact outcome measures for PAGE for any of the 3 probiotics included: probiotic formulation, probiotic initiation times, use of ORT, rural versus urban settings, or funding sources (see Table, Supplemental Digital Content 5, Subgroups, http://links.lww.com/PG9/A44).
Duration of Diarrhea in Rotavirus-Positive Children
Only 2 types of probiotics had sufficient trials to assess rotaviral diarrhea (see Table, Supplemental Digital Content 6, Rotaviral Diarrhea, http://links.lww.com/PG9/A45). Two RCTs using S. boulardii reported outcomes for rotavirus-positive children, but different outcome measures were used. Das et al (45) found S. boulardii significantly reduced diarrhea duration by –1.2 ± 0.1 days and Sirsat and Sankpal (49) found 25% more children were cured by day 3 if given S. boulardii compared with controls. L. rhamnosus GG reported mean duration of diarrhea in 3 trials of rotavirus-positive patients (52,53,57). The reduction in diarrheal days in rotavirus-positive children was not significant for L. rhamnosus GG (SMD, –3.11 d; 95% CI, –9.29 to 3.1 d; P = 0.32; I2 = 98%). The 4 RCTs with B. clausii mixture did not report data by diarrheal etiology.
Cured by Day 3
Only S. boulardii CNCM I-745 had sufficient trials to explore this outcome, with 4 trials reporting the cure rates by day 3 (47, 49–51). There was a trend (P = 0.054) of cure by day 3 (relative risk, 1.55; 95% CI, 0.90–2.41; I2 = 90%; see Figure, Supplemental Digital Content 4, Cured by Day 3, http://links.lww.com/PG9/A35). Sensitivity analysis found no one individual trial had undue influence on the pooled outcome. The relative risk was not significantly influenced when only 2 trials of low risk of bias were included nor by the degree of blinding, or by dose, or by the addition of zinc.
Rapidity of Response (Stool Frequency/Day Over Time)
The rapidity of a response was different by the type of probiotic, but not all trials provided data for this outcome (see Table, Supplemental Digital Content 7, Rapidity of Response, http://links.lww.com/PG9/A46). In 3 RCTs with S. boulardii CNCM I-745 (43, 46, 48), the number of stools/day were equivalent at day 1 and day 3, but by day 4, the mean number of stools was significantly fewer for S. boulardii compared with controls (SMD, –0.61 stools/day; 95% CI, –1.06 to –0.17; P = 0.007), as shown in Figure (Supplemental Digital Content 5, S. boulardii Response, http://links.lww.com/PG9/A36), although only 1 trial measured this outcome on day 4. In 2 RCTs (3 study arms) with L. rhamnosus GG (54,55), a significant difference in stools/day was not observed until day 5 with SMD = –1.1 stools/day; 95% CI, –2.11 to –0.08; P = 0.03 (see Figure, Supplemental Digital Content 6, L. rhamnosus Response, http://links.lww.com/PG9/A37). In 2 RCTs with B. clausii mixture, the number of stools/day was equivalent to controls for days 1, 3, and 5 (see Figure, Supplemental Digital Content 7, B. clausii Mix Response, http://links.lww.com/PG9/A38).
Length of Hospital Stay
Of the 12 RCTs that enrolled inpatient children, 9 trials provided data on the mean length of hospitalization stay (LOS) for the probiotic compared with the control group (see Table, Supplemental Digital Content 8, LOS, http://links.lww.com/PG9/A47). As shown in Figure 2, 2 types of probiotics significantly reduced the mean duration of hospitalization. S. boulardii CNCM I-745 had the strongest effect on the reduction of mean LOS (SMD = –1.81 d; 95% CI, –3.58 to –0.04 d; P = 0.04; I2 = 98%). L. rhamnosus GG also significantly reduced the mean LOS (SMD, –1.13 d; 95% CI, –2.14 to –0.11 d; P = 0.03; I2 = 99%). The 4-strain mixture of B. clausii O/C, SIN, N/R, T did not significantly reduce the LOS (SMD, –1.14; 95% CI, –2.91 to 0.64; P = 0.21; I2 = 98%). There was significant heterogeneity found among these studies (97%–98%), but no significant publication bias was noted (Egger’s P = 0.16).
FIGURE 2.
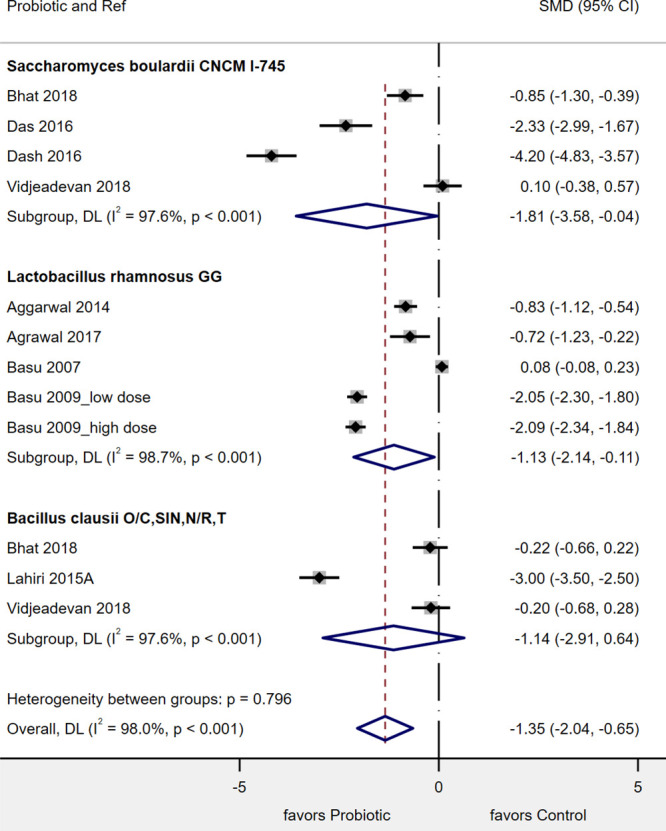
Forest plot of 11 randomized controlled trials in India for the mean reduction of hospitalization (length of stay [d]) with 3 different types of probiotics. CI = confidence interval; DL = DerSimonian-Laird estimate of between study variance; SMD = standardized mean difference.
Safety
Of the 25 study arms, 11 (44%) did not collect any adverse reaction data (Table 2), but 14 (56%) did collect and report adverse event data. Of the 14 with safety data, 10 (71%) reported no adverse events were observed during the study, while 4 (29%) reported at least 1 child with an adverse event, but the frequency was not significantly different for probiotic compared with control groups. None of the different probiotic types were associated with significant adverse events.
DISCUSSION
We conducted a systematic review and meta-analysis of 22 RCTs (with 4059 participants) to estimate the efficacy of probiotics available in India for the treatment of PAGE. Our study is a unique study focusing on clinical trials in 1 developing country (India) in an effort to limit geographic and nutritional factors that may influence rates of pediatric acute diarrhea. We included 17 RCTs in our meta-analysis of 3 different types of probiotics. We found differences in efficacy by type of probiotic: S. boulardii CNCM I-745 had the greatest reduction in mean days of diarrhea and reduction in LOS for hospitalized children and had the most rapid response by number of stools/day (reduced by day 4), whereas L. rhamnosus GG did not show a significant reduction in stool frequency until day 5. Trials using B. clausii did not show a significant difference by day 5. Most trials followed World Health Organization recommendations to use ORT and zinc in India or other countries where zinc deficiency is found (1), but only 41% used zinc as recommended. Zinc was not added to ORT in 24% of the RCTs and 35% of the RCTs failed to report if zinc was added or not.
Guidelines for which probiotics may be recommended for PAGE have been inconsistent. The 2020 American Gastroenterology Association guidelines did not recommend probiotics, citing most trials have been done in other countries besides the United States or Canada (24). In Europe, a recent update of the European Society of Paediatric Gastroenterology, Hepatology and Nutrition had weak recommendations for S. boulardii (based on 22 RCTs), L. rhamnosus GG (16 RCTs), and 2 other probiotics with L. reuteri DSM17938 (6 RCTs), but recommended against the use of B. clausii mix or a mix of L. helveticus R52 and L. rhamnosus R11 (65).
We found 7 meta-analyses previously published on probiotics and treatment of PAGE (19–23, 65, 66) and all combined results from trials done in different countries. An updated Cochrane meta-analysis included 79 RCTs in children and adults with acute gastroenteritis in different countries and found a mean of 1-day reduction in diarrhea for each of the following: S. boulardii (11 RCTs), L. rhamnosus GG (14 RCTs), and L. reuteri DSM 17938 (6 RCTs), but did not present pediatric trials separately by country (66).
Two meta-analyses of S. boulardii trials found significant efficacy for PAGE (20, 23). Feizizadeh et al (20) included 22 RCTs done in 12 different countries and found S. boulardii reduced the duration of diarrhea by –0.82 days. Szajewska et al (23) included 23 RCTs in her meta-analysis of S. boulardii trials from 11 different countries and also found a significant reduction in duration of diarrhea (SMD, –1.1 d; 95% CI, –1.3 to –0.8 d) and shorter LOS for inpatients (8 RCT; SMD, 0.85 d; 95% CI, –1.3 to –0.3 d) and in rotaviral positive patients. In general, our results of trials limited to those done in India agreed with results found in other countries, showing probiotics may be effective in varied populations. In our meta-analysis of 7 RCTs done in India using S. boulardii CNCM I-745, we found a greater reduction in the duration of diarrhea (SMD, –1.86 d; P < 0.001) and shorter hospitalization stays (4 RCTs; SMD, –1.81 d; P < 0.001) and significant efficacy in rotaviral diarrhea. Subgroup analysis determined factors not impacting the efficacy of S. boulardii included: daily probiotic dose, in/outpatient status, degree of blinding, or type of controls used. The time of probiotic initiation was not a factor in the Indian trials, as S. boulardii was started within 3 days of diarrheal onset in all reported trials. No dose-response was observed, as all trials used the same dose (1 × 1010 CFU/d).
Two previous meta-analyses were done including trials only using L. rhamnosus GG and both found a significant mean reduction in duration of diarrhea: in 15 RCTs (SMD, –0.85 d; 95% CI, –1.15 to –0.56) (22) and in 19 RCTs (SMD, –1.0 d; 95% CI, –1.5 to –0.5) (67). Szajewska et al (22) reported L. rhamnosus GG was twice as effective in European countries compared with a mix of non-European countries (South East Asia, South America, United States, and Australia). A meta-analysis by Li et al (67) found doses of L. rhamnosus GG less than 1010 CFU/d, delayed probiotic initiation (>3 d) and non-Asian/non-European countries resulted in a loss of efficacy for duration of diarrhea by L. rhamnosus GG. Li et al (67) also found L. rhamnosus GG was more effective in rotaviral diarrhea for shortening diarrheal duration (7 RCTs; SMD, –1.3 d; P < 0.001) and reduced LOS by 1.3 days. Our results agree with these 2 meta-analyses done in different countries. In our meta-analysis of 6 RCTs in India, L. rhamnosus GG appeared to be slightly more effective in reducing the duration of diarrhea (SMD, −1.75 d; P < 0.001) compared with the mixed group of different countries in the previous 2 meta-analyses. In our meta-analysis, L. rhamnosus GG also reduced the mean LOS in Indian inpatients (4 RCTs; SMD, –1.13 d; P < 0.001), but not as great as an extent as the previous 2 meta-analyses. In the Indian trials reported initiation times, L. rhamnosus GG was started within 4 days of the onset of diarrhea. Our meta-analysis also confirmed a sufficiently high dose (1 × 1010 CFU/d) was needed for L. rhamnosus GG to be significantly effective. It is also interesting that the same strain was not found to be effective in another country. A large RCT involving 971 children with PAGE admitted to 10 pediatric emergency departments across the United States failed to find efficacy for L. rhamnosus GG (68).
A meta-analysis by Ianiro et al (21) included 6 RCTs using B. clausii probiotics and found a significant reduction in the duration of diarrhea (SMD, –0.4 d; 95% CI, –0.69 to –0.07; P = 0.02) and a reduction in LOS (3 RCTs; SMD, –0.85 d; 95% CI, –1.6 to –0.15; P = 0.03), but did not conduct any subgroup analyses. In a recent review of probiotics for the management of acute gastroenteritis in children, this Bacillus mixture was not recommended to treat PAGE (65). In our meta-analysis, the 4-strain mixture of B. clausii appears was effective for the reduction of diarrheal duration when trials are done in India (SMD, –1.4 d; P < 0.001), but the reduction in LOS was not significantly reduced by the B. clausii mix. Two reasons why our results may differ from the previous reviews is that we only included RCTs done in India and, second, we had rigorous inclusion criteria that excluded unpublished or duplicative trials. These excluded trials typically had nonsignificant efficacy findings. An advantage of using living probiotics is that they may possess one or more multiple mechanisms of action, which may explain why specific probiotics are effective against different etiologies of PAGE (69, 70).
Strengths of our study included an exhaustive search of all RCTs in 1 country, including gray literature and meeting abstracts. The inclusion criteria were rigorous, in that only trials with well-described probiotic treatments (strains, daily dose, and formulations) were included and only those probiotics with at least 2 RCTs were included. Most trials used a standard definition of diarrhea (≥3 loose-watery stools/day) for the inclusion criteria. However, since there is no consensus for a standard outcome measure, the trials differed in the definition of diarrheal outcome (see Table, Supplemental Digital Content 4, Probiotic Description and Definitions, http://links.lww.com/PG9/A43) and types of outcomes.
Limitations were related to trials using different outcome measures, as we could only compare efficacy when common outcomes were used. In addition, probiotics with just a single RCTs could not be assessed and the proof of efficacy for some probiotic strains must wait until confirmatory trials are published. Factors that may be sources of heterogeneity were not reported in all trials. Reasons for heterogeneity may include differences in clinical baseline characteristics (such as age, degree of malnourishment, urban/rural settings, inpatient/outpatient, breast-fed versus formula-fed infants, etc.) or may due to differences in study design (degree of blinding, attrition, study quality, study size, adjunctive therapies given, etc.) Important confounder factors and influences were often not described in papers (diet, environmental factors, malnourishment status, etiologies of diarrhea, basic health status, mortality data, frequency of breast-feeding versus formula-fed, etc.). We were able to conduct subgroup analysis on most of these factors, but most did not significantly reduce heterogeneity. Daily doses less than 1010 CFU/d were not effective for L. rhamnosus GG, while trials with S. boulardii all used 2 × 1010 CFU/d and B. clausii used a lower dose (109 CFU/d). Trials differed in quality of reporting (method of randomization not reported, allocation blinding not reported, etc.). About half (48%) used placebo, but 52% of the trials were not double-blinded. However, subgroup analysis by study design bias did not find this influenced efficacy measures. This confirms the finding by Moustgaard et al (71) who reviewed 142 meta-analyses and found no significant effect of blinding or placebo use on estimated treatment effects. When trials were grouped by low versus high risk of bias, trials with low risk had better efficacy with S. boulardii (see Figure, Supplemental Digital Content 8, Risk of Bias, http://links.lww.com/PG9/A39), while the interpretation of high risk is difficult for L. rhamnosus GG, as there is only 1 trial with high risk. This holds true for B. clausii mix trials, as only one trial was of low risk. The type of funding was also assessed (23% by academic grant, 9% funded by Pharma companies, 23% were unfunded) but 45% did not report funding sources. The type of funding did not appear to significantly influence efficacy outcomes.
Generalizability and Future Studies
As the included trials were based upon study patients who lived in India, the results may not be extrapolated to probiotic efficacy in developed countries where the level of water sanitation and hygiene differ and the frequency of breast-feeding is typically lower. In addition, microbiome profiles and etiologies of diarrhea differ geographically and how this may influence probiotic efficacy is unknown. Implications for clinical practice and policy may include a more rational choice of the types of probiotics used for PAGE. Future research should include factors not reported in previous trials (described above) and a search for other clinical probiotic strains or multi-strain mixtures that might be effective for the treatment of PAGE.
CONCLUSIONS
In India, 2 types of probiotics (S. boulardii CNCM I-745 and L. rhamnosus GG) were well tolerated and significantly shortened both the duration of diarrhea and hospitalization stays in pediatric patients with PAGE. This is of important clinical significance. Earlier recovery and reduced hospital stays translate to less morbidity and lower healthcare costs for the system and the carers of these children.
ACKNOWLEDGMENTS
We would like to thank Dr. Krishna C. Veligandla for his assistance in communication with coauthors and reviewing the article.
Planning and conducting the study (L.V.M., R.S., R.P.S., S.G., A.B., M.M., and B.R.), collecting and interpreting the data (L.V.M., R.S., R.P.S., S.G., A.B., M.M., and B.R.), statistical analysis (L.V.M.), drafting the article (L.V.M.), and writing the final article (L.V.M., R.S., R.P.S., S.G., A.B., M.M., B.R., and N.M.). L.V.M. accepts full responsibility for the conduct of the study. The author had access to the data and had control of the decision to publish. All authors approved the final draft submitted.
Supplementary Material
Footnotes
Dr McFarland is a paid lecturer and consultant for the following companies and sits on the Microbiome Advisory Board (Laboratoires Biocodex, Paris, France) and on the Scientific Advisory Board of Bio-K Plus, International (Montreal, QC, Canada), but owns no stock or equity in either company. The remaining authors report no conflicts of interest.
Funding for writing, analysis, and publication fees provided by Dr. Reddy’s Laboratories, India. The sponsor has reviewed the article, but the selection of the articles, analysis, writing, and decision to publish has been done independently by the authors.
Review registered: PROSPERO (CRD42020186739).
Supplemental digital content is available for this article.
REFERENCES
- 1.World Health Organization (WHO). Diarrhoeal Disease. 2017. Available at: https://www.who.int/en/news-room/fact-sheets/detail/diarrhoeal-disease. Accessed September 9, 2020.
- 2.UNICEF. “Diarrhoea October 2019.” 2019. Available at: https://data.unicef.org/topic/child-health/diarrhoeal-disease/. Accessed October 27, 2020.
- 3.Guarino A, Aguilar J, Berkley J, et al. Acute gastroenteritis in children of the world: what needs to be done? J Pediatr Gastroenterol Nutr. 2020; 70:694–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lakshminarayanan S, Jayalakshmy R. Diarrheal diseases among children in India: current scenario and future perspectives. J Nat Sci Biol Med. 2015; 6:24–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.GBD 2016 Diarrhoeal Disease Collaborators. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of diarrhoea in 195 countries: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect Dis. 2018; 18:1211–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhatnagar S, Kumar R, Dua R, et al. Outcome of children with severe acute malnutrition and diarrhea: a cohort study. Pediatr Gastroenterol Hepatol Nutr. 2019; 22:242–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Basa S. Prevalence of diarrhoea among under-five children and health seeking behavior of their mothers in an urban slum of Delhi. Asian J Biomed Pharmac Sci. 2015; 5:8– 11. [Google Scholar]
- 8.Huey SL, Finkelstein JL, Venkatramanan S, et al. Prevalence and correlates of undernutrition in young children living in Urban Slums of Mumbai, India: a cross sectional study. Front Public Health. 2019; 7:191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guarino A, Ashkenazi S, Gendrel D, et al. ; European Society for Pediatric Gastroenterology, Hepatology, and Nutrition; European Society for Pediatric Infectious Diseases. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition/European Society for Pediatric Infectious Diseases evidence-based guidelines for the management of acute gastroenteritis in children in Europe: update 2014. J Pediatr Gastroenterol Nutr. 2014; 59:132–152. [DOI] [PubMed] [Google Scholar]
- 10.Shrivastava AK, Kumar S, Mohakud NK, et al. Multiple etiologies of infectious diarrhea and concurrent infections in a pediatric outpatient-based screening study in Odisha, India. Gut Pathog. 2017; 9:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De R, Mukhopadhyay AK, Dutta S. Metagenomic analysis of gut microbiome and resistome of diarrheal fecal samples from Kolkata, India, reveals the core and variable microbiota including signatures of microbial dark matter. Gut Pathog. 2020; 12:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen SY, Tsai CN, Lee YS, et al. Intestinal microbiome in children with severe and complicated acute viral gastroenteritis. Sci Rep. 2017; 7:46130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davis EC, Dinsmoor AM, Wang M, et al. Microbiome composition in pediatric populations from birth to adolescence: impact of diet and prebiotic and probiotic interventions. Dig Dis Sci. 2020; 65:706–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dinleyici EC, Martínez-Martínez D, Kara A, et al. Time series analysis of the microbiota of children suffering from acute infectious diarrhea and their recovery after treatment. Front Microbiol. 2018; 9:1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sniffen JC, McFarland LV, Evans CT, et al. Choosing an appropriate probiotic product for your patient: an evidence-based practical guide. PLoS One. 2018; 13:e0209205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McFarland LV, Evans CT, Goldstein EJC. Strain-specificity and disease-specificity of probiotic efficacy: a systematic review and meta-analysis. Front Med. 2018:5:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Szajewska H. Pooling data on different probiotics is not appropriate to assess the efficacy of probiotics. Eur J Pediatr. 2014; 173:975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lai BY, Liang N, Cao HJ, et al. Pediatric Tui Na for acute diarrhea in children under 5 years old: a systematic review and meta-analysis of randomized clinical trials. Complement Ther Med. 2018; 41:10–22. [DOI] [PubMed] [Google Scholar]
- 19.McFarland LV, Elmer GW, McFarland M. Meta-analysis of probiotics for the prevention and treatment of acute pediatric diarrhea. Internl J Probiotics Prebiotics. 2006; 1:63–76. [Google Scholar]
- 20.Feizizadeh S, Salehi-Abargouei A, Akbari V. Efficacy and safety of Saccharomyces boulardii for acute diarrhea. Pediatrics. 2014; 134:e176–e191. [DOI] [PubMed] [Google Scholar]
- 21.Ianiro G, Rizzatti G, Plomer M, et al. Bacillus clausii for the treatment of acute diarrhea in children: a systematic review and meta-analysis of randomized controlled trials. Nutrients. 2018; 10:E1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Szajewska H, Kolodziej M, Gieruxzczak-Bialek D, et al. Systematic review with meta-analysis: Lactobacillus rhamnosus GG for treating acute gastroenteritis in children- a 2019 update. Aliment Pharmacol Ther. 2019; 49:1376–1384. [DOI] [PubMed] [Google Scholar]
- 23.Szajewska H, Kołodziej M, Zalewski BM. Systematic review with meta-analysis: Saccharomyces boulardii for treating acute gastroenteritis in children-a 2020 update. Aliment Pharmacol Ther. 2020; 51:678–688. [DOI] [PubMed] [Google Scholar]
- 24.Su GL, Ko CW, Bercik P, et al. AGA clinical practice guidelines on the role of probiotics in the management of gastrointestinal disorders. Gastroenterology. 2020; 159:697–705. [DOI] [PubMed] [Google Scholar]
- 25.Hill C, Guarner F, Reid G, et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014; 11:506–514. [DOI] [PubMed] [Google Scholar]
- 26.Moher D, Shamseer L, Clarke M, et al. ; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015; 4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. 2011. Available at: http://www.cochrane-handbook.org. Accessed July 1, 2020.
- 28.McGuinness LA. Robvis: An R Package and Web Application for Visualising Risk-of-Bias Assessments. 2019. Available at: https://github.com/mcguinlu/robvis. Accessed May 20, 2020. [DOI] [PubMed]
- 29.Palmer TM, Sterne JAC. Meta-Analysis in Stata: An Updated Collection From the Stata Journal. 2nd ed. College Station, TX: Stata Press; 2016. [Google Scholar]
- 30.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005; 5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vineeth S, Saireddy S, Keerthi T, Mantada P. Efficacy of Bacillus clausii and Saccharomyces boulardii in treatment of acute rotaviral diarrhea in pediatric patient. Indonesian J Clin Pharm. 2017; 6:91–98. [Google Scholar]
- 32.Reddy BS, Paul S, Vohra P. Comparing the role of Bacillus clausii and Saccharomyces boulardii in acute watery diarrhoea. Poster presentation at: Pedgastrocon; September 29–30, 2013; Pune, India. [Google Scholar]
- 33.Burande MA. Comparison of efficacy of Saccharomyces boulardii strain in the treatment of acute diarrhea in children: a prospective, single-blind, randomized controlled clinical trial. J Pharmacol Pharmacother. 2013; 4:205–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kiran M, Pawaskar L. Safety and efficacy of Saccharomyces boulardii for the management of diarrhoea in Indian children. Int J Sci Res Manage (IJSRM). 2018; 6:MP-2018-01-05. [Google Scholar]
- 35.Sultana R, Deka A. Role of Saccharomyces boulardii in acute watery diarrhoea. Poster presentation at: 4th Annual Conference ISPGHAN; October 28–29, 2017; Guwahati, India. [Google Scholar]
- 36.Ahmad K. Bacillus clausii as an adjuvant therapy in acute childhood diarrhoea. Indian J Appl Res. 2018; 8:182–183. [Google Scholar]
- 37.Lahiri K. NCT00457353. A Phase III, Controlled, Open-Label, Randomized, Parallel Group, Multicentric, Comparative Study to Assess the Efficacy and Safety of Oral Rehydration Therapy (ORT) in Combination With Spores of Bacillus clausii (Enterogermina™) Versus ORT Alone, Administered for 5 Days in the Treatment of Acute Diarrhea in Children. Sanofi Aventis report. 2008. Available at: http://en.sanofi.com/img/content/study/ENTER_L_01486_summary.pdf. Accessed September 9, 2020.
- 38.Lahiri KR, Tullu MS, Taori R, Kondekar S. Abstract GE/06(0) Beneficial role of Bacillus clausii in treatment of acute diarrhea. PEDICON 2011. 48th Annual National Conference of Indian Academy of Pediatrics; January 20–23, 2011; Jaipur, India. [Google Scholar]
- 39.Kiran M, Pawaskar L. Efficacy and safety for suspension of Bacillus clausii while treating the patient of diarrhoea. Indian J Basic Appl Med Res. 2017; 7:251–257. [Google Scholar]
- 40.Khanna V, Alam S, Malik A, et al. Efficacy of tyndalized Lactobacillus acidophilus in acute diarrhea. Indian J Pediatr. 2005; 72:935–938. [DOI] [PubMed] [Google Scholar]
- 41.Agarwal KN, Bhasin SK, Faridi MM, et al. Lactobacillus casei in the control of acute diarrhea–a pilot study. Indian Pediatr. 2001; 38:905–910. [PubMed] [Google Scholar]
- 42.Basu S, Chatterjee M, Ganguly S, et al. Effect of Lactobacillus rhamnosus GG in persistent diarrhea in Indian children: a randomized controlled trial. J Clin Gastroenterol. 2007; 41:756–760. [DOI] [PubMed] [Google Scholar]
- 43.Bhat S, Shreekrishna GN, Savio CD. Efficacy of probiotics in acute diarrhoea in children. Int J Contemp Pediatr. 2018; 5:1646–1650. [Google Scholar]
- 44.Burande MA, Burande AR. Efficacy of Saccharomyces boulardii strain in acute diarrhoea in children: an Indian perspective. Interl J Recent Trends Sci Tech. 2012; 4:41–44. [Google Scholar]
- 45.Das S, Gupta PK, Das RR. Efficacy and safety of Saccharomyces boulardii in acute rotavirus diarrhea: double blind randomized controlled trial from a developing country. J Trop Pediatr. 2016; 62:464–470. [DOI] [PubMed] [Google Scholar]
- 46.Dash DK, Dash M, Mohanty MD, Acharya N. Efficacy of probiotic Saccharomyces boulardii as an adjuvant therapy in acute childhood diarrhoea. J Nepal Paediatr Soc. 2016; 36:250–255. [Google Scholar]
- 47.Kumar A, Kumar D, Rajkumar D. To study the role of Saccharomyces boulardii in treatment of acute watery diarrhea in children aged 6 months to 5 years: prospective randomized control study. J Dental Med Sci. 2018; 17:35–40. [Google Scholar]
- 48.Riaz M, Alam S, Malik A, et al. Efficacy and safety of Saccharomyces boulardii in acute childhood diarrhea: a double blind randomised controlled trial. Indian J Pediatr. 2012; 79:478–482. [DOI] [PubMed] [Google Scholar]
- 49.Sirsat GM, Sankpal DM. Role of Saccharomyces boulardii in management of acute diarrhoea of children - a randomized controlled trial. MedPulse Internl J Pediatr. 2017; 4:68–72. [Google Scholar]
- 50.Vandenplas Y, Badriul H, Thapa B, Elizabeth K, Bhave S. A multi-center dbrpc-trial in developing countries with Saccharomyces boulardii (S. boulardii) in acute gastroenteritis. J Pediatr Gastroenterl Nut. 2007; 44:e86. [Google Scholar]
- 51.Vidjeadevan D, Vinoth S, Ramesh S. Role of Saccharomyces boulardii and Bacillus clausii in reducing the duration of diarrhea: a three-armed randomised controlled trial. Int J Contemp Pediatr. 2018; 5:1811–1814. [Google Scholar]
- 52.Aggarwal S, Upadhyay A, Shah D, et al. Lactobacillus GG for treatment of acute childhood diarrhoea: an open labelled, randomized controlled trial. Indian J Med Res. 2014; 139:379–385. [PMC free article] [PubMed] [Google Scholar]
- 53.Agarwal HK. Evaluation on Lactobacillus GG for treatment of acute childhood diarrhoea: a hospital based study. Glob J Res Anal. 2017; 6:283–285. [Google Scholar]
- 54.Basu S, Chatterjee M, Ganguly S, et al. Efficacy of Lactobacillus rhamnosus GG in acute watery diarrhoea of Indian children: a randomised controlled trial. J Paediatr Child Health. 2007; 43:837–842. [DOI] [PubMed] [Google Scholar]
- 55.Basu S, Paul DK, Ganguly S, et al. Efficacy of high-dose Lactobacillus rhamnosus GG in controlling acute watery diarrhea in Indian children: a randomized controlled trial. J Clin Gastroenterol. 2009; 43:208–213. [DOI] [PubMed] [Google Scholar]
- 56.Misra S, Sabui TK, Pal NK. A randomized controlled trial to evaluate the efficacy of lactobacillus GG in infantile diarrhea. J Pediatr. 2009; 155:129–132. [DOI] [PubMed] [Google Scholar]
- 57.Sindhu KN, Sowmyanarayanan TV, Paul A, et al. Immune response and intestinal permeability in children with acute gastroenteritis treated with Lactobacillus rhamnosus GG: a randomized, double-blind, placebo-controlled trial. Clin Infect Dis. 2014; 58:1107–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lahiri K, Jadhav K, Gahlowt P, Najmuddin F. Bacillus clausii as an adjuvant therapy in acute childhood diarrhea. IOSR J Dental Med Sci. 2015; 14:74–76. [Google Scholar]
- 59.Lahiri K, D’Souza J, Gahlowt P. Beneficial role of probiotic in acute childhood diarrhea. J Harmonized Res. 2015; 2:26–30. [Google Scholar]
- 60.Narayanappa D. Randomized double blinded controlled trial to evaluate the efficacy and safety of Bifilac in patients with acute viral diarrhea. Indian J Pediatr. 2008; 75:709–713. [DOI] [PubMed] [Google Scholar]
- 61.Sudha MR, Jayanthi N, Pandey DC, Verma AK. Bacillus clausii UBBC-07 reduces severity of diarrhea in children under 5 years of age: a double-blind placebo controlled study. Bene Microb. 2019; 10:149–154. [DOI] [PubMed] [Google Scholar]
- 62.Agarwal KN, Bhasin SK. Feasibility studies to control acute diarrhea in children by feeding fermented milk preparations Actimel and Indian Dahi. Euro J Clin Nutr. 2002; 56:S56–S59. [DOI] [PubMed] [Google Scholar]
- 63.Dutta P, Mitra U, Dutta S, Rajendran K, Saha TK, Chatterjee MK. Randomised controlled clinical trial of Lactobacillus sporogenes (Bacillus coagulans), used as probiotic in clinical practice, on acute watery diarrhea in children. Trop Med Interl Health. 2011; 16:555–561. [DOI] [PubMed] [Google Scholar]
- 64.Dubey AP, Rajeshwari K, Chakravarty A, et al. Use of VSL[sharp]3 in the treatment of rotavirus diarrhea in children: preliminary results. J Clin Gastroenterol. 2008; 42(suppl 3, pt 1):S126–S129. [DOI] [PubMed] [Google Scholar]
- 65.Szajewska H, Guarino A, Hojsak I, et al. ; Working Group on Probiotics and Prebiotics of the European Society for Paediatric Gastroenterology, Hepatology and Nutrition. Use of probiotics for the management of acute gastroenteritis in children: an update. J Pediatr Gastroenterol Nutr. 2020; 71:261–269. [DOI] [PubMed] [Google Scholar]
- 66.Collinson S, Deans A, Padua-Zamora A, et al. Probiotics for treating acute infectious diarrhoea. Cochrane Database Syst Rev. 2020; 12:CD003048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li YT, Xu H, Ye JZ, et al. Efficacy of Lactobacillus rhamnosus GG in treatment of acute pediatric diarrhea: a systematic review with meta-analysis. World J Gastroenterol. 2019; 25:4999–5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schnadower D, Tarr PI, Casper TC, et al. Lactobacillus rhamnosus GG versus placebo for acute gastroenteritis in children. N Engl J Med. 2018; 379:2002–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Plaza-Diaz J, Ruiz-Ojeda FJ, Gil-Campos M, et al. Mechanisms of action of probiotics. Adv Nutr. 2019; 10(suppl_1):S49–S66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McFarland LV. Systematic review and meta-analysis of Saccharomyces boulardii in adult patients. World J Gastroenterol. 2010; 16:2202–2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Moustgaard H, Clayton GL, Jones HE, et al. Impact of blinding on estimated treatment effects in randomised clinical trials: meta-epidemiological study. BMJ. 2020; 368:l6802. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


