Abstract
Hidradenitis suppurativa (HS) is a rare, debilitating skin disease characterized by the presence of recurrent tender subcutaneous nodules that develop into abscesses and fistulae. Isolated perineal Crohn’s disease (CD) is unusual, diagnosis can be difficult, and distinction from HS is a challenge for the gastroenterologist. The aim of this work was to determine the criteria that distinguish perineal CD from perineal HS. Four patients with isolated perineal CD and three with perineal HS were included. Rectal or skin biopsies of all CD patients showed granulomas. No granulomas were found for HS. Fistulae were present in 4/4 CD, extended to the anal canal. All patients with HS had gluteal abscesses. They were bilateral in all cases, superficial. Perineal lesions management should involve a multidisciplinary approach in order to make an accurate diagnosis and ultimately to give the best and most effective treatment.
Keywords: Crohn’s disease, Hidradenitis suppurativa
What Is Known
Hidradenitis suppurativa (HS) is a rare, debilitating skin disease characterized by the presence of recurrent tender subcutaneous nodules that develop into abscesses and fistulae.
Isolated perineal Crohn’s disease (CD) is unusual, diagnosis can be difficult, and distinction from HS is a challenge for the gastroenterologist.
What Is New
MRI presentation of anoperineal disease may overlap between CD and HS. But, diagnosis of HS is possible with a combination of 3 features: either posterior localization of the lesions or absence of features’ predominance in perianal area, absence of rectal wall thickening, and bilaterality of features. HS lesions in MRI are superficial.
MRI with skin or rectal biopsy could be the more contributory complementary examination.
INTRODUCTION
Hidradenitis suppurativa (HS) is a rare, debilitating skin disease characterized by the presence of recurrent tender subcutaneous nodules that develop into abscesses and fistulae. Lesions may develop in the intertriginous apocrine gland-bearing areas of the body, in decreasing order of frequency axillary, inguinal, perianal, perineal, mammary, buttock, pubic, chest, scalp, retroauricular, and eyelid, usually after puberty (1, 2).
The diagnosis of HS is primarily made based on its characteristic clinical presentation and has to meet the criteria adopted by the 2nd International Conference on HS, March 5, 2009, San Francisco, CA. All three criteria must be met for establishing the diagnosis (3):
(1) Typical lesions, ie, deep-seated painful nodules: “blind boils” in early lesions; abscesses, draining sinus, bridged scars, and “tombstone” double-ended pseudocomedones in secondary lesions.
(2) Typical topography, ie, axillae, groins, perineal and perianal region, buttocks, infra, and inter mammary folds.
(3) Chronicity and recurrences.
The severity of the disease can be classified in 3 grades for each area involved according to the Hurley classification (4).
HS has an estimated prevalence of 1%–4%, with a notable female-to-male predominance of 3:1 (1, 2).
Crohn’s disease (CD) is a chronic inflammatory bowel disease that is characterized by segmental noncaseating granulomas and may affect any portion of the gastrointestinal tract from mouth to the anus.
Perineal CD presents as fistulas and fissures, associated abscesses, uncomplicated skin inflammation, and skin tags (5).
Isolated perineal CD is unusual, diagnosis can be difficult, and distinction from HS is a challenge for the gastroenterologist (6–8).
The aim of this work was to determine the criteria that distinguish perineal CD from perineal HS.
MATERIAL AND METHODS
All patients with a diagnosis of isolated perineal CD according to the European Society of Pediatric Gastroenterology and Nutrition criteria (9) or of perineal HS followed in our tertiary center were considered for inclusion in this retrospective study. Data were retrospectively obtained from medical files and recorded in an anonymous standardized form. The following baseline characteristics were recorded for each patient: gender, age at onset, weight, body mass index, anti-Saccharomyces cerevisiae antibodies (ASCA), MRI, biopsies, extraperineal locations, CD classification according to Paris classification, and HS according to Hurley classification.
Patients with luminal CD were excluded (normal colonoscopy or normal calprotectin).
RESULTS
Four patients with isolated perineal CD and 3 with perineal HS were included.
Clinical data are summarized in Table 1, and pictures shown in Figure 1.
TABLE 1.
Clinical characteristics at diagnosis of isolated perineal Crohn’s disease and hidradenitis suppurativa
| Characteristics | CD1 | CD2 | CD3 | CD4 | HS1 | HS2 | HS3 |
|---|---|---|---|---|---|---|---|
| Gender | Girl | Girl | Girl | Boy | Boy | Boy | Boy |
| Age at diagnosis | 15 | 9 | 10 | 16 | 15 | 15 | 9 |
| Family history | None | None | None | None | None | Father similar lesions nape and chest without diagnosis | None |
| Symptoms duration (mo) | 36 | 1 | 4 | 3 | 14 | 4 | 36 |
| BMI | 18 | 20 | 15 | 17 | 22 | 20 | 20 |
| Perineal lesions | Fistulae | Fistulae | Fistulae | Fistulae | Abscess | Abscess gluteal | Abscess |
| Gluteal | |||||||
| Natal cleft | Bilateral | Bilateral | |||||
| Extraperineal locations | Height delay | Aphtae | Height delay | None | Chest | Nape | None |
| Paris classification | A1b | A1a | A1b | A1b | |||
| B3p | B3p | B3p | B3p | ||||
| G1 | G0 | G1 | G0 | ||||
| Hurley classification | I | III | II | ||||
| Treatment | Azathioprine and infliximab | Azathioprine and infliximab | Infliximab | Azathioprine and infliximab | Antibiotics | Infliximab | Adalimumab |
| infliximab | antibiotics | antibiotics |
BMI = body mass index; CD = Crohn’s disease; HS = Hidradenitis suppurativa.
FIGURE 1.
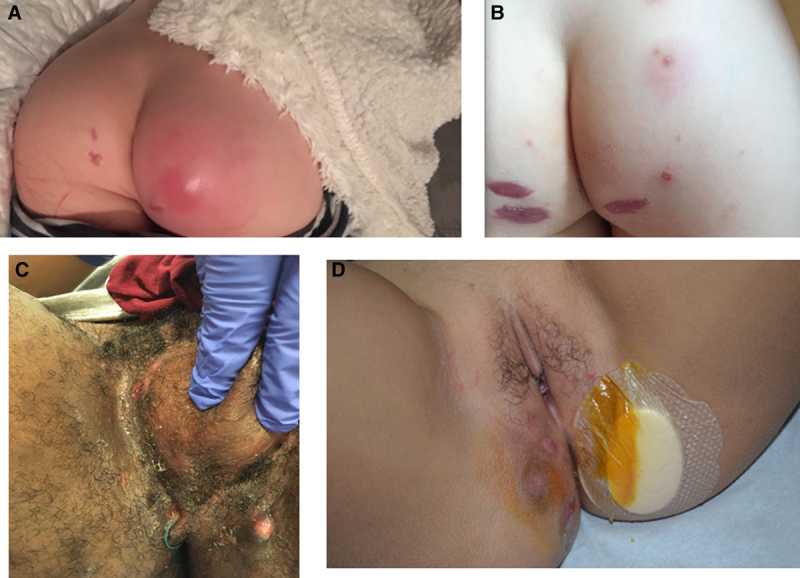
Gluteal abscess before (A) and after (B) surgery, Hurley I, in a 9-year-old boy. C) Perineal abscesses, Hurley III, in a 15-year-old boy. D) Perineal Crohn’s disease in a 10-year-old girl.
Biopsy
Among CD patients, granulomas were found on skin biopsies (n = 2) or rectal biopsies (n = 2). No granulomas were found in HS patients.
ASCA
Three (of 4) CD patients were ASCA+. This data was not available for HS patients.
MRI Features
Morphological findings in patients with HS and CD are listed in Table 2, shown in Figure 2.
TABLE 2.
Pelvic MRI findings in patients with hidradenitis suppurativa and Crohn’s disease
| Morphologic characteristics | CD | HS |
|---|---|---|
| Inflammatory features | ||
| Abscess | 0/4 | 3/3 |
| Sinus tract | 4/4 | 1/3 |
| Classification | ||
| Complex | 0/4 | 0/3 |
| Intersphincteric | 0/4 | 0/3 |
| Extrasphincteric | 4/4 | 0/3 |
| Transphincteric | 0/4 | 0/3 |
| Suprasphincteric | 0/4 | 0/3 |
| Superficial | 0/4 | 3/3 |
| Penetration into the sphincter mechanism | 0/4 | 0/3 |
| Anatomical regions | ||
| Ischiorectal fosse | 0/4 | 0/3 |
| Sacral/gluteal | 1/4 | 3/3 |
| Anterior/inguinal | 0/4 | 0/3 |
| Natal cleft | 2/4 | 0/3 |
| Perianal | 4/4 | 0/3 |
| Additional features | ||
| Lymphadenopathy | 3/4 | 0/3 |
| Rectal wall thickening | 1/4 | 0/3 |
| Myositis | 0/4 | 0/3 |
| Subcutaneous edema | 2/4 | 2/3 |
| Skin thickening | 1/4 | 1/3 |
| Global features | ||
| Predominance in perianal area | 3/4 | 0/3 |
| Bilaterality | 1/4 | 3/3 |
CD = Crohn’s disease; HS = Hidradenitis suppurativa.
FIGURE 2.
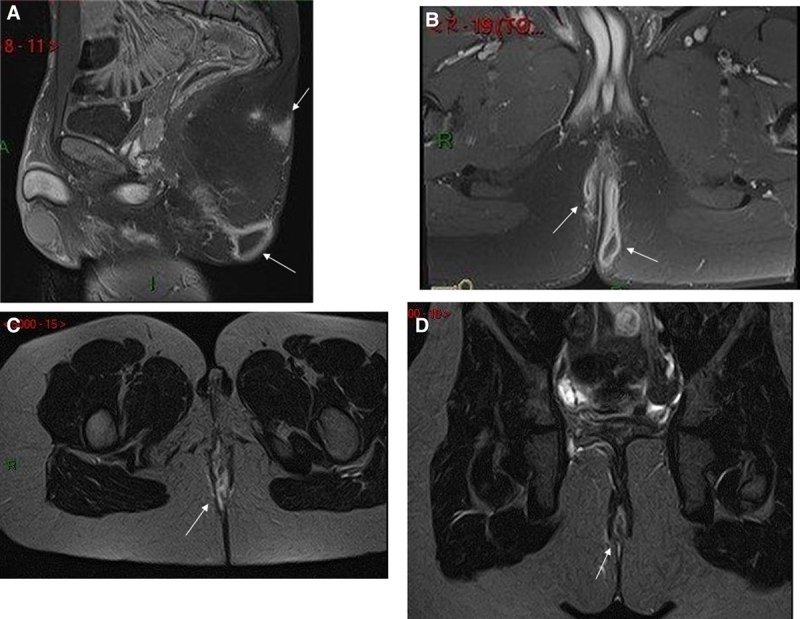
A) Fat suppressed gadolinium enhanced sagittal T1 weighted MRI in a 15-year-old boy with perineal hidradenitis shows a posterior gluteal abscess with sacral subcutaneous oedema. B) Fat suppressed gadolinium enhanced coronal T1 weighted MRI in a 15-year-old boy with perineal hidradenitis shows a posterior bilateral gluteal abscess. C) Fat suppressed axial T2 weighted MRI in a 9-year-old girl with perineal Crohn’s disease show a posterior median fistula. D) Fat suppressed coronal T2 weighted MRI in a 9-year-old girl with perineal Crohn’s disease show a posterior median fistula.
Fistulae were present in 4/4 CD. Fistulae in CD extended to the anal canal with no penetration into the sphincter mechanism. Location in CD was perianal.
All patients with HS had gluteal abscesses. They were bilateral in all cases, superficial, in one case extended to the sacral region.
Treatment
All CD patients were treated with infliximab, 3 patients received azathioprine.
All HS patients were treated with antibiotics. One HS patient improved on infliximab and another one on adalimumab. The third patient worsened on infliximab and adalimumab and required intravenous antibiotic treatment and surgery.
DISCUSSION
Only 4 patients with isolated perineal CD were identified among the 484 CD followed at our tertiary center (0.82%), whereas 15.7% (n = 76) had perianal and luminal disease. Isolated perineal CD is , and there may be confusion with other perineal lesions such as HS. Both diseases are rare; thus, we only can present a small series of patients.
HS and CD share many characteristics, and the two diseases can be associated in the same patient (7). The prevalence of HS in CD patients is 12.4%–17.9%, while the prevalence of CD in HS patients is around 3%.
Family history and extraperineal skin locations can help to guide the diagnosis. Physicians should inspect the axilla, chest, inguinal creases, vulva, perineum, perianal skin, buttocks, and medial thighs for other signs guiding to HS such as comedones, acneiform papules and pustules, inflammatory nodules, sinus tracts, ulcers, erosions, drainage, granulation tissue, induration, edema, lymphedema, lichenification, dyspigmentation, scars (atrophic, hypertrophic, keloidal), strictures, and contractures (10,11).
Biopsy is warranted if CD is suspected. A biopsy of the affected skin showing granulomatous inflammation on histopathology strongly supports a diagnosis of CD. Sometimes granulomas are found only on rectal biopsies suggesting that a colonoscopy should be performed for an accurate diagnosis (12).
ASCA could help to diagnose CD. Some studies provided preliminary evidence, that inflammatory bowel disease may have a preclinical phase that might be detected by disease-specific biomarkers, such as ASCA. These biomarkers should be part of the diagnosis process (13). A recent study (14) identified serologic detection of ASCAs as a biomarker for HS, mainly in its severe (Hurley III) and inflammatory forms. The association between ASCA seropositivity and high HS tissue and/or systemic inflammatory burden provides support for a link between systemic inflammation in the absence of inflammatory bowel disease and the development of ASCAs. It has been showed in CD that positivity for ASCAs is associated with more severe disease and greater need for surgery (15).
MRI presentation of anoperineal disease may overlap between CD and HS. But, diagnosis of HS is possible with a combination of 3 features, as already shown by Monnier et al (16): either posterior localization of the lesions or absence of features’ predominance in perianal area, absence of rectal wall thickening, and bilaterality of features. HS lesions in MRI are superficial. These points should be discussed with the radiologist to guide the diagnosis (16,17), and this may be with biopsy the more contributory complementary examination.
Medical treatment of perineal disease in CD includes antibiotics, anti-tumour necrosis factor (TNF) antibodies. In HS, multimodal treatment should be discussed by gastroenterologist and dermatologists. The choice of therapy is guided by disease severity (18). Antibiotics are widely used, from oral to intravenous route. Patients with moderate to severe disease can also be treated with anti–TNF-α antibodies, especially if they have required long-term antibiotic treatments. Adalimumab was approved for the treatment of HS by the US Food and Drug Administration in 2016 (19). Infliximab may be used as an alternative anti-TNF therapy. In case of treatment failure, third-line should be discussed (anakinra, ustekinumab, dapsone, or acitretin) (20). Surgery is required to definitively treat the tunnels and scars associated with chronic HS.
In conclusion, management of perineal lesions should involve a multidisciplinary approach combining the knowledge of the gastroenterologist, the dermatologist, and the radiologist who have appropriate experience in this area in order to make an accurate diagnosis and ultimately to give the best and most effective treatment.
Footnotes
Dr. Martinez-Vinson received honoraria, speaker’s fees and/or congress fees from AbbVie, MSD, Nestle, Biogen, Adacyte. The remaining authors report no conflicts of interest.
Dr. Martinez-Vinson is the guarantor of this article.
REFERENCES
- 1.van der Zee HH, Laman JD, Boer J, et al. Hidradenitis suppurativa: viewpoint on clinical phenotyping, pathogenesis and novel treatments. Exp Dermatol. 2012; 21:735–739 [DOI] [PubMed] [Google Scholar]
- 2.Jemec GBE. Hidradenitis suppurativa. N Engl J Med. 2012; 2:158–164 [DOI] [PubMed] [Google Scholar]
- 3.Kurzen H, Kurokawa I, Jemec GB, et al. What causes hidradenitis suppurativa? Exp Dermatol. 2008; 17:455–472 [DOI] [PubMed] [Google Scholar]
- 4.Hurley HJ. Roenigk RK, Roenigk HH, Jr. Axillary hyperhidrosis, apocrine bromhidrosis, hidradenitis suppurativa and familial benign pemphigus. Surgical approach. Dermatologic Surgery. Principles and Practice. 1996; 2New York: Marcel Dekker; 623–645 [Google Scholar]
- 5.Adler J, Dong S, Eder SJ, et al. ; ImproveCareNow Pediatric IBD Learning Health System. Perianal crohn disease in a large multicenter pediatric collaborative. J Pediatr Gastroenterol Nutr. 2017; 64:e117–e124 [DOI] [PubMed] [Google Scholar]
- 6.Bassas-Vila J, González Lama Y. Hidradenitis suppurativa and perianal Crohn disease: differential diagnosis. Actas Dermosifiliogr. 2016; 107suppl 227–31 [DOI] [PubMed] [Google Scholar]
- 7.Vilarrasa Rull E, González Lama Y. Clinical features of hidradenitis suppurativa and Crohn disease: what do these two entities have in common? Actas Dermosifiliogr. 2016; 107suppl 221–26 [DOI] [PubMed] [Google Scholar]
- 8.Chen W-T, Chi C-C. Association of hidradenitis suppurativa with inflammatory bowel disease: a systematic review and meta-analysis. JAMA Dermatol. 2019; 155:1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levine A, Koletzko S, Turner D, et al. ; European Society of Pediatric Gastroenterology, Hepatology, and Nutrition. ESPGHAN revised porto criteria for the diagnosis of inflammatory bowel disease in children and adolescents. J Pediatr Gastroenterol Nutr. 2014; 58:795–806 [DOI] [PubMed] [Google Scholar]
- 10.Stewart KMA. Challenging ulcerative vulvar conditions: hidradenitis suppurativa, crohn disease, and aphthous ulcers. Obstet Gynecol Clin North Am. 2017; 44:453–473 [DOI] [PubMed] [Google Scholar]
- 11.Jørgensen AR, Thomsen SF, Karmisholt KE, et al. Clinical, microbiological, immunological and imaging characteristics of tunnels and fistulas in hidradenitis suppurativa and Crohn’s disease. Exp Dermatol. 2020; 29:118–123 [DOI] [PubMed] [Google Scholar]
- 12.Jemec GB, Hansen U. Histology of hidradenitis suppurativa. J Am Acad Dermatol. 1996; 34:994–999 [DOI] [PubMed] [Google Scholar]
- 13.Torres J, Petralia F, Sato T, et al. Serum biomarkers identify patients who will develop inflammatory bowel diseases up to 5 years before diagnosis. Gastroenterology. 2020; 159:96–104 [DOI] [PubMed] [Google Scholar]
- 14.Assan F, Gottlieb J, Tubach F, et al. Anti-Saccharomyces cerevisiae IgG and IgA antibodies are associated with systemic inflammation and advanced disease in hidradenitis suppurativa. J Allergy Clin Immunol. 2020; 146:452–455.e5 [DOI] [PubMed] [Google Scholar]
- 15.Kim BC, Park S, Han J, et al. Clinical significance of anti-Saccharomyces cerevisiae antibody (ASCA) in Korean patients with Crohn’s disease and its relationship to the disease clinical course. Dig Liver Dis. 2007; 39:610–616 [DOI] [PubMed] [Google Scholar]
- 16.Monnier L, Dohan A, Amara N, et al. Anoperineal disease in Hidradenitis Suppurativa: MR imaging distinction from perianal Crohn’s disease. Eur Radiol. 2017; 27:4100–4109 [DOI] [PubMed] [Google Scholar]
- 17.Hammer MR, Dillman JR, Smith EA, et al. Magnetic resonance imaging of perianal and perineal crohn disease in children and adolescents. Magn Reson Imaging Clin N Am. 2013; 21:813–828 [DOI] [PubMed] [Google Scholar]
- 18.Saunte DML, Jemec GBE. Hidradenitis suppurativa: advances in diagnosis and treatment. JAMA. 2017; 318:2019–2032 [DOI] [PubMed] [Google Scholar]
- 19.Kimball AB, Okun MM, Williams DA, et al. Two phase 3 trials of adalimumab for hidradenitis suppurativa. N Engl J Med. 2016; 375:422–434 [DOI] [PubMed] [Google Scholar]
- 20.Włodarek K, Ponikowska M, Matusiak Ł, et al. Biologics for hidradenitis suppurativa: an update. Immunotherapy. 2019; 11:45–59 [DOI] [PubMed] [Google Scholar]


