Abstract
The hydrogenase in Azotobacter vinelandii, like other membrane-bound [NiFe] hydrogenases, consists of a catalytic heterodimer and an integral membrane cytochrome b. The histidines ligating the hemes in this cytochrome b were identified by H2 oxidation properties of altered proteins produced by site-directed mutagenesis. Four fully conserved and four partially conserved histidines in HoxZ were substituted with alanine or tyrosine. The roles of these histidines in HoxZ heme binding and hydrogenase were characterized by O2-dependent H2 oxidation and H2-dependent methylene blue reduction in vivo. Mutants H33A/Y (H33 replaced by A or Y), H74A/Y, H194A, H208A/Y, and H194,208A lost O2-dependent H2 oxidation activity, H194Y and H136A had partial activity, and H97Y,H98A and H191A had full activity. These results suggest that the fully conserved histidines 33, 74, 194, and 208 are ligands to the hemes, tyrosine can serve as an alternate ligand in position 194, and H136 plays a role in H2 oxidation. In mutant H194A/Y, imidazole (Imd) rescued H2 oxidation activity in intact cells, which suggests that Imd acts as an exogenous ligand. The heterodimer activity, quantitatively determined as H2-dependent methylene blue reduction, indicated that the heterodimers of all mutants were catalytically active. H33A/Y had wild-type levels of methylene blue reduction, but the other HoxZ ligand mutants had significantly less than wild-type levels. Imd reconstituted full methylene blue reduction activity in mutants H194A/Y and H208A/Y and partial activity in H194,208A. These results indicate that structural and functional integrity of HoxZ is required for physiologically relevant H2 oxidation, and structural integrity of HoxZ is necessary for full heterodimer-catalyzed H2 oxidation.
Hydrogenase catalyzes the reversible reaction H2⇌2H+ + 2e− (EC 1.18.99.1 and 1.12.2.1) (12). The enzyme occurs in a wide variety of eubacteria and archaea, and the catalytic direction depends on cellular location and metabolic function. Azotobacter vinelandii has a membrane-bound hydrogenase that primarily catalyzes the oxidative reaction (38). Membrane-bound [NiFe] hydrogenases consist of a membrane-bound catalytic heterodimer and an integral membrane cytochrome b (12). While the heterodimer alone can catalyze hydrogenase reactions with artificial electron acceptors and donors, the cytochrome b is essential for physiologically relevant H2 oxidation (8–10, 20, 26, 40). Proposed functions for HoxZ, the cytochrome b in A. vinelandii, include electron transfer from the heterodimer to the respiratory chain, anchoring the heterodimer to the membrane, reduction of the catalytic site, and stabilization of the enzyme (40).
All three subunits of membrane-bound hydrogenases are metalloproteins, and the ligands in the subunits have unusual structural and functional features. The large subunit contains the site of catalysis, and the iron in the [NiFe] catalytic site has the nonprotein ligands CO and CN− (18). The small subunit of the heterodimer has three aligned FeS clusters (45). Irons in FeS clusters are usually ligated by cysteines, but the distal 4Fe4S cluster includes a histidine ligand (45), which is essential for activity (16). Substitution of one of the cysteine ligands of the central 3Fe4S cluster in A. vinelandii resulted in the loss of O2 sensitivity in hydrogenase, which is normally highly sensitive to O2 inactivation (25). The hemes and ligands of the third subunit have been the subject of recent studies (5, 7, 9, 16).
Dross et al. (9) determined that the integral membrane component of hydrogenase in Wolinella succinogenes, HydC, had four hydrophobic segments, and they suggested that this protein was a cytochrome b. From their alignment of full and partial homologous sequences to HydC, they indicated proposed α-helical transmembrane segments and two heme ligands. However, our alignment of these hydrogenase b-type cytochromes, which included subsequently released full sequences of Azotobacter chroococcum (10) and Bradyrhizobium japonicum (44), suggested that four conserved histidines may serve as ligands to two hemes, in agreement with Berks et al. (7) and Gross et al. (16). The homologous protein in Alcaligenes eutrophus, HoxZ, was shown to be a diheme cytochrome b that anchors the heterodimer to the periplasmic side of the cytoplasmic membrane and donates electrons to ubiquinone (6). When three of the conserved histidines in HydC of W. succinogenes were replaced with alanine or methionine, the mutant strains had negligible levels of H2-dependent electron transfer to the anaerobic electron acceptor, menaquinone (16). The fourth conserved histidine was suggested as the fourth ligand (7, 16) but was not identified experimentally. The requirement of three ligands for physiologically relevant electron transfer from H2 was shown (16), but further investigations of the roles of these ligands have not been carried out.
Unlike W. succinogenes, A. vinelandii is an aerobic, nitrogen-fixing bacterium that has ubiquinone as part of the respiratory chain. Along with the four conserved histidines, there are five histidines conserved to various degrees among 14 hydrogenase b-type cytochrome homologues. Four of these five residues occur in A. vinelandii HoxZ but not in W. succinogenes. We have investigated the effect of histidine substitutions in both O2-dependent H2 oxidation and H2-dependent methylene blue (MB) reduction in vivo. Substitution of histidine residues with nonligands (alanine), alternative ligands (tyrosine), and exogenous ligands (Imd) provided a means of identifying the heme ligands and probing the heme site and the role of the ligands in HoxZ. Imd rescued mutants with substitutions in one of the putative heme ligands. This is the first report of imidazole (Imd) rescue of activity in a membrane protein and the first report of in vivo rescue in a nonheterologous system.
MATERIALS AND METHODS
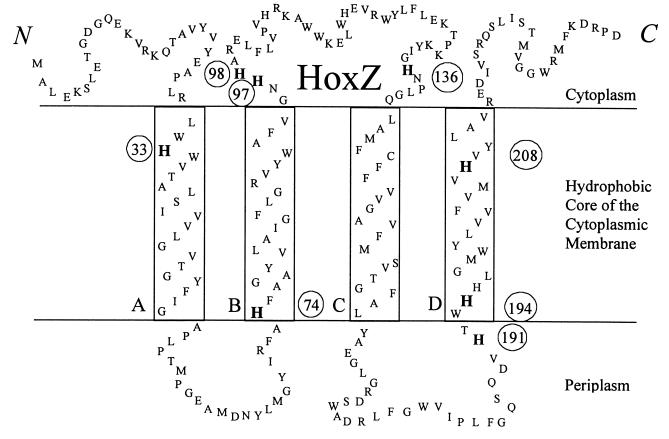
Schematic of A. vinelandii HoxZ.
The sequence of A. vinelandii HoxZ (National Center for Biotechnology Information [NCBI] GenBank accession no. L23970, submitted by Menon et al. [27]) is shown in Fig. 1. The designated transmembrane segments were most similar in selected residues and length to those predicted by NCBI Entrez protein query for accession no. P23000 (A. vinelandii HoxZ).
FIG. 1.
Schematic of A. vinelandii HoxZ.
Plasmids, bacterial strains, and mutagenesis.
The plasmids and bacterial strains used in this study are listed in Table 1. Oligonucleotides for the mutagenesis were designed using the software Oligo (version 3; Molecular Biology Insights, Plymouth, Minn.). The oligonucleotide-directed PCR-based methods of Merino et al. (29) and Weiner et al. (46) were used for site-directed mutagenesis of the plasmid template pAVHoxZ+. The PCR product was then transformed into competent Escherichia coli DH5α (Gibco BRL, Grand Island, N.Y.), which were plated on Luria-Bertani agar medium (39) supplemented with ampicillin (60 mg/liter) (Sigma, St. Louis, Mo.) and grown at 37°C overnight. Single colonies were grown overnight in ampicillin-supplemented Luria-Bertani liquid medium, and plasmid preparations were made according to Lee and Rasheed (21). Sequencing by the Central Services Lab of the Center for Gene Research and Biotechnology, Oregon State University, was used to screen and verify the mutants.
TABLE 1.
Plasmids and bacterial strains
| Plasmid or strain | Relevant properties | Source or reference |
|---|---|---|
| Plasmids | ||
| pAVHoxZ+ | SmaI-SalI fragment (1,049 bases) of hoxGZM containing hoxZ inserted into pBluescript II SK− | This study, subclone of SalI fragment inserted into pSelect (p302L) from reference 40 |
| pAVHoxZ− | pSelect with 231-base KpnI fragment deleted from hoxZ | 40 |
| pDB303 | A. vinelandii Rifr genomic DNA fragment in pUC8; Ampr | D. R. Deana |
| pAVHoxZ H→A series | pAVHoxZ+ with H→A individually in positions 33, 74, 98, 136, 191, 194, 208, and 194 plus 208 | This study |
| pAVHoxZ H→Y series | pAVHoxZ+ with H→Y individually in positions 33, 74, 97, 194, and 208 | This study |
| Strains | ||
| A. vinelandii | ||
| DJ (Wt) | Rifs wild-type, high-transformation strain | D. R. Dean |
| DJ (WtR) | RifrA. vinelandii DJ | This study |
| HoxKG−Rifs | Rifs strain with nonfunctional hoxKG genes derived from A. vinelandii DJ | 40 |
| HoxKG−Rifr | Rifr HoxKG− derived from A. vinelandii HoxKG− Rifs | This study |
| HoxZ− Rifs | Rifs strain with nonfunctional hoxZ gene, derived from A. vinelandii DJ transformed with pAVHoxZ− | This study |
| HoxZ− Rifr | Rifr strain with nonfunctional hoxZ gene derived from A. vinelandii DJ transformed with pAVHoxZ− and pDB303 | 40 |
| HoxZ H→A series | RifrA. vinelandii H→A individually in HoxZ positions 33, 74, 98, 136, 191, 194, and 194 plus 208 | This study |
| HoxZ H→Y series | RifrA. vinelandii H→A individually in HoxZ positions 33, 74, 97, 194, and 208 | This study |
| E. coli DH5α | Amps | Gibco BRL |
Virginia Polytechnic Institute and State University.
Growth of A. vinelandii.
A. vinelandii strains were grown at 30°C in Burk medium or on Burk plates (43). For rifampin-resistant (Rifr) strains, the medium was supplemented with 20 mg of rifampin (Sigma) per liter (making Burk-Rif medium). Cultures were shaken continuously (150 cycles/min) during growth and grown to optical densities at 600 nm (OD600) of 0.8 to 1.1 with a Beckman DU-70 spectrophotometer, except for the wild-type (Wt) activity assay, in which case cultures were grown to OD600 of 0.5 to 1.2. For Imd (Sigma)-grown cultures, Imd (made to pH 7.5 with HCl) was added to a final concentration of 10 mM (4) at the time of inoculum addition. Cells were collected by centrifugation.
Transformation and screening.
A. vinelandii strains were made competent by several transfers on iron- and molybdenum-deficient, 28 mM ammonium acetate-supplemented Burk medium (36) under O2-limited conditions (33). To generate a Rifs HoxZ− A. vinelandii strain, pHoxZ− (1 μg) was transformed into competent A. vinelandii DJ (Wt) without the aid of an antibiotic marker. Except for growth in Burk medium without rifampin, other aspects of transformation and screening were the same as for other mutants. Transformations with all other HoxZ constructs were done using plasmids linearized with the restriction enzyme SmaI (U.S. Biochemical, Cleveland, Ohio; Promega, Madison, Wis.). Linearized plasmid preparations (1 μg of DNA) of pHoxZ H33, H74, H194, H208, and H194,208→A or -Y were individually cotransformed with the EcoRI (Promega) 1.7-kb fragment of pDB303 (10 ng), which confers Rifr into Rifs A. vinelandii DJ (Wt), using the method of Premakumar et al. (36). Linearized plasmids pHoxZ H97, H98, H136, and H191→A or -Y were cotransformed similarly into Rifs A. vinelandii HoxZ−. To compare only hox mutants and not differences due to the lower growth rate in medium with rifampin, A. vinelandii DJ and A. vinelandii HoxKG− were made Rifr by transformation using the 1.7-kb EcoRI fragment of pDB303. The day following transformation, bacteria were transferred to Burk-Rif agar plates to select for Rifr transformants. Single colonies were picked and transferred three times on Burk-Rif medium to allow for complete segregation (22, 36). A phenotypic assay of H2 oxidation ability similar to that of Sayavedra-Soto and Arp (40) was used to screen for coincident transformation of the HoxZ mutants. Test tubes containing 2 ml of Burk-Rif were inoculated from single colonies; cultures were grown to an OD600 of 0.4 and capped with butyl rubber stoppers, and H2 (4.46 μmol) was added (high-purity commercial grade H2 and N2 were purchased locally). Cultures were then grown on a shaker table (150 rpm) for 16 h at 30°C. For screening, culture headspace was sampled and analyzed for the presence of H2 using a Shimadzu GC-8A thermal conductivity detector gas chromatograph (Molecular Sieve 5A column; 5 ft long, 1/8-inch diameter, 200°C injection temperature, 120°C column temperature, 60-mA current). Strains with phenotypes differing from that of the background parental strain were confirmed as mutants by DNA sequence analysis of a PCR product of the genomic DNA preparation (3). Sequencing was performed at the Central Services Lab of the Center for Gene Research and Biotechnology, Oregon State University.
Whole cell O2-dependent H2 oxidation.
To determine when to harvest A. vinelandii for assays, a Wt activity assay compared OD600 (0.5 to 1.2) and protein contents (microbiuret assay [14] with bovine serum albumin as a standard) to O2-dependent H2 oxidation activity. A. vinelandii cultures for other assays were then harvested in the range of greatest activity, OD600 of 0.8 through 1.0. The specific activities in nanomoles of H2 oxidized per minute per milligram at these ODs were 0.8 (188), 0.9 (184), and 1.0 (173). Preliminary experiments showed that cells resuspended in growth medium had H2 oxidation activity, but cells resuspended in phosphate buffer did not, probably due to oxidation of the hydrogenase enzyme. Therefore 3 mM sucrose, equivalent to a starvation level of carbon for E. coli (0.1% glucose) (2), was added to the buffer to maintain respiratory protection of O2-sensitive enzymes. Suspension cultures (1.5 ml) were centrifuged for 1 min at 14,000 rpm, resuspended in 50 mM phosphate buffer (pH 7.0)–3 mM sucrose (1.5 ml), then placed in 10-ml serum vials, capped with a rubber sleeve stopper, and placed upside down in a 30°C water bath at a shaker rate of 200 cycles/min. To initiate the assay, H2 (4.46 μmol) was added to the vial. Headspace samples (200 μl) were injected into the thermal conductivity detector gas chromatograph at 0, 15, and 30 min. For assays with Imd, either cultures were grown in 10 mM Imd, or Imd and chloramphenicol (Aldrich Chemical Company, Milwaukee, Wis.) were added at the initiation of the experiment to final concentrations of 10 and 3 mM, respectively. Four individual replications of this assay were recorded for each strain. In all assays, results for the control strains grown with Imd were not significantly different (least squares difference at P = 0.05 [LSD0.05]) from the results for control strains grown without Imd.
Whole cell, H2-dependent MB reduction.
Suspension cultures (40 ml) were centrifuged 10 min at 7,500 rpm; the cells were resuspended in 1 ml of 50 mM Tris buffer–5 mM EDTA (pH 7.3) and placed in a 10-ml serum vial stoppered with a rubber sleeve cap. The vial was purged with N2 to remove O2. A sample cuvette (7.5 ml; Beckman 2097) containing a total of 1 ml of 50 mM Tris buffer–5 mM EDTA (pH 7.3) including 150 μM MB was similarly stoppered and flushed with N2, and H2 (1 ml) was added for assays with H2. All reactions were started with the addition of N2-purged culture (50 μl) to the sample cuvette. Under the conditions of these MB experiments, activation was not required for reduction of the catalytic site since the heterodimers were sufficiently reduced from the flushing of nitrogen gas and removal of O2 to reduce MB without a lag period and in the absence of dithionite. The decrease in absorbance at 690 nm was monitored with a Beckman DU-70 spectrophotometer for 1 min, and the greatest difference in absorbance in a 10-s time period was recorded. Four individual replications of this assay were recorded for each strain. Calculations were based on a molar extinction coefficient of 11.4 mM−1 cm−1 at 690 nm. MSU Stat (Montana State University, Bozeman) software was used for analysis of variance of the means for the data from both O2-dependent H2 oxidation and H2-dependent MB reduction assays.
RESULTS
Conservation of histidines in hydrogenase b-type cytochromes.
An alignment and analysis of 14 eubacterial hydrogenase b-type cytochromes (alignment not shown) show that four conserved histidines are found in the predicted transmembrane α-helical segments (Fig. 1). One histidine is near the center of helix A, one is at the beginning of helix B, and two are in helix D, in agreement with Gross et al. (16). Six histidines occur in A. vinelandii without corresponding histidines in W. succinogenes. Four of these in the loop regions were selected for mutagenesis. Three of the four are in loop BC, predicted to be on the cytoplasmic side, and the fourth is in loop CD, predicted to be on the periplasmic side of the cytoplasmic membrane (Fig. 1). A. vinelandii HoxZ is only distantly related (29% identical) to the hydrogenase cytochrome b of W. succinogenes (phylogeny not shown). However, the similarities seen in biochemical assays of these distantly related proteins imply similar structural and functional importance of all hydrogenase b-type cytochromes. To examine the roles of these histidines in HoxZ, mutants were constructed to produce altered proteins with specific histidines replaced with either alanine or tyrosine.
Effect of histidine replacements on whole cell O2-dependent H2 oxidation.
To determine whether hydrogenase is functional in physiological electron transport in A. vinelandii hoxZ mutants, H2 oxidation with O2 as the terminal electron acceptor was assayed (Table 2). A. vinelandii DJ (Wt) and Rifr A. vinelandii DJ (WtR) served as positive controls. HoxKG− Rifr (a partial deletion mutant of the catalytic heterodimer) served as a negative control. HoxZ− Rifr (a partial deletion mutant of hydrogenase cytochrome b) served as a control for the loss of a functional HoxZ. Wt and WtR were not significantly different. Physiologically relevant H2 oxidation activity was undetectable for both Hox mutants.
TABLE 2.
Comparison of strains in O2-dependent H2 oxidationa
| Strain | Mean nmol of H2 oxidized/min/ml ± SE | % | + Imidazole (10 mM) | % Wt |
|---|---|---|---|---|
| Controls | ||||
| Wt | 25.3 ± 1.0 | 100 | 26.5 ± 1.4 | 105 |
| WtR | 25.8 ± 1.1 | 102 | 24.6 ± 1.1 | 97 |
| HoxKG− Rifr, HoxZ− Rifr | 0.4 ± 0.5 | 2 | 0.7 ± 0.7 | 3 |
| No cells | 0.6 ± 0.6 | 2 | ||
| Ligands | ||||
| H33A | 0.5 ± 0.5 | 2 | 2.2 ± 1.1 | 9 |
| H33Y | 0.7 ± 0.5 | 3 | 0.2 ± 0.2 | 1 |
| H74A | 0.2 ± 0.2 | 1 | 0.5 ± 0.3 | 2 |
| H74Y | 1.5 ± 0.9 | 6 | 2.0 ± 0.9 | 8 |
| H194A | 0.7 ± 0.2 | 3 | 25.5 ± 0.8 | 101 |
| H194Y | 5.7 ± 0.8 | 23 | 23.3 ± 1.0 | 92 |
| H208A | −0.2 ± 0.5 | −1 | 1.7 ± 0.7 | 7 |
| H208Y | 0.2 ± 0.2 | 1 | 0.0 ± 0.4 | 0 |
| H194,208A | −0.2 ± 0.2 | −1 | 0.7 ± 0.8 | 3 |
| Nonligands | ||||
| H97Y,H98A,H191A | 24.8 ± 0.8 | 98 | 23.5 ± 1.0 | 93 |
| H136A | 6.4 ± 0.3 | 25 | 8.2 ± 1.5 | 32 |
Cultures (1.5 ml) at an OD600 of 0.8 to 1.1 were resuspended in 50 mM phosphate buffer (pH 7.0)–3 mM sucrose. Four individual replications of each strain were assayed; LSD0.05 = 2.2 in all cases.
No H2 oxidation was detected for the mutants with alanine substitutions in putative ligand positions 33, 74, 194, and 208 (Table 2). None of these His→Ala mutants, including the mutant, H194,208A were functional in physiological electron transport from hydrogenase to O2. These positions are the four conserved histidines in aligned sequences of hydrogenase b-type cytochromes and are therefore proposed as the ligands to the two hemes in HoxZ.
Histidines that were not fully conserved were not expected to be ligands to the hemes. Four of these histidines in loop regions near the membrane edges were replaced in A. vinelandii by alanine or tyrosine. H2 oxidation assays of putative nonligand mutants indicated that all four had H2 oxidation activity and that three of the four mutants (with replacements in positions 97, 98, and 191) had similar to Wt activity (Table 2). Residues 97 and 98 are predicted to be on the cytoplasmic side of the plasma membrane, and 191 is predicted to be on the periplasmic side. Mutant H136A had approximately one-third of Wt activity. Residue 136 is predicted to be on the cytoplasmic side of the membrane, and the decreased ability of H136A to oxidize H2 implies that the histidine in position 136 serves an important function.
Mutants with His→Tyr substitutions in positions 33, 74, and 208 showed that tyrosine in these positions gave the same result as the His→Ala mutants (Table 2). In contrast, mutant H194Y appeared to have some H2 oxidation activity (consumption in 30 min of ∼5% of the initial H2 added, which is equivalent to 23% of Wt activity) and is significantly different from both Wt and the control with no cells (Table 2).
Effect of Imd on whole cell O2-dependent H2 oxidation of mutants.
In some proteins, Imd can serve as an exogenous ligand to heme in proteins where a natural ligand has been removed (4). To determine if Imd, a histidine analogue, could serve as a free ligand in physiologically relevant H2 oxidation, 10 mM Imd was added to the growth medium. There was no significant difference in H2 oxidation between Wt and WtR, with or without Imd (Table 2). Furthermore, Imd did not restore activity to HoxKG− Rifr or HoxZ− Rifr.
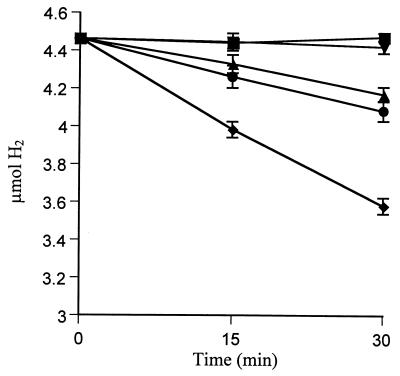
Addition of 10 mM Imd to the growth medium of His→Ala ligand mutants 33, 74, and 208 did not increase H2 oxidation activity (Table 2). Surprisingly, H194A recovered activity to a level comparable to Wt (Table 2; see Fig. 3). Hydrogenase activity in Imd-treated H194A [H194A (Imd)] indicates that Imd acts as a free ligand in this position and reconstitutes hydrogenase activity to Wt levels. Over the range of 1 to 10 mM Imd, H2 oxidation rates by H194A (Imd) and Wt (Imd) were not significantly different from Wt (data not shown). When Imd was excluded from the growth medium but added at the start of the assay, H2 oxidation activity was detected immediately and with no change throughout the course of the assay (Fig. 2). However, the rate (0.38 nmol/min/ml) was lower than when cells were grown with Imd (0.88 nmol/min/ml) (Fig. 2) (LSD0.05 = 0.11). Addition of chloramphenicol, a known inhibitor of protein synthesis in A. vinelandii (34, 37), did not prevent the restoration of activity by Imd. These results indicate that new protein synthesis was not required for Imd to bind and serve as a ligand in trans.
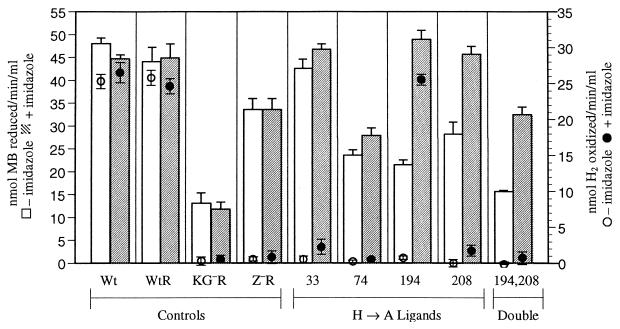
FIG. 3.
Effect of Imd on H2-dependent MB reduction and O2-dependent H2 oxidation. Bars denote standard error; only top half of column error is shown. The x axis indicates A. vinelandii controls and H→A mutants.
FIG. 2.
Recovery of O2-dependent H2 oxidation by H194A with the addition of Imd. Symbols represent H194A alone (■), H194A plus Imd added at initiation of assay (●), H194A plus Imd and chloramphenicol added at initiation of assay (▴), H194A grown in Imd (⧫), and no-cell control (▾).
The His→Tyr ligand mutants grown with Imd followed a similar pattern as the His→Ala ligand mutants in that only H194Y had activity when grown in Imd (Table 2). A comparison of both mutants grown in Imd shows that there is no significant difference among Wt, H194A (Imd), and H194Y (Imd) (LSD0.05 = 0.13) (additional data not shown).
The nonligand HoxZ mutants were also grown with Imd and assayed for O2-dependent H2 oxidation. Activities of the nonligand mutants grown with Imd were similar to activities of these mutants grown without Imd. H136A did not recover H2-oxidizing capability upon addition of Imd. We assumed that HoxZ was expressed to Wt levels in all cases since the nonligand mutants and one ligand mutant grown with Imd have high levels of activity. Because antibody to HoxZ was not available, expression of the altered proteins in HoxZ mutants was not assayed for directly.
Whole cell H2-dependent MB reduction.
MB reduction can be quantitatively measured spectroscopically and must be assayed anaerobically to prevent O2 from reoxidizing the artificial electron acceptor. MB can accept electrons directly from the heterodimer (42) and may have other nonspecific binding locations along the electron transport chain and in the cell. Since MB can also be nonspecifically reduced, experiments were conducted in the absence and presence of H2 (1) (Table 3) to account for the level of endogenous cellular reduction. In addition, assays were conducted on strains grown in the absence and presence of Imd.
TABLE 3.
Comparison of endogenous and H2-dependent MB reduction activity of strainsa
| Strain | Treatment | Mean nmol of MB reduced/min/ml ± SE (% Wt)
|
|
|---|---|---|---|
| Endogenous | H2 dependent | ||
| Controls | |||
| Wt | 15.5 ± 4.1 (100) | 48.0 ± 1.4 (100) | |
| Imd | 10.6 ± 2.2 (69) | 44.7 ± 0.8 (93) | |
| WtR | ±Imd | 13.2 ± 1.0 (86) | 44.5 ± 3.2 (92) |
| HoxKG− Rifr | ±Imd | 11.4 ± 1.4 (74) | 12.5 ± 2 (26) |
| HoxZ− Rifr | ±Imd | 15.0 ± 2.1 (97) | 33.6 ± 2.6 (70) |
| Ligands | |||
| H33A | 10.1 ± 1.3 (65) | 42.6 ± 2.2 (89) | |
| H33A | Imd | 15.9 ± 0.5 (103) | 46.8 ± 1.0 (98) |
| H74A | 10.0 ± 1.3 (65) | 23.6 ± 1.2 (49) | |
| H74A | Imd | 13.6 ± 0.8 (88) | 27.9 ± 1.6 (58) |
| H194A | 10.6 ± 0.8 | 21.5 ± 1.2 (45) | |
| H194A | Imd | 13.0 ± 1.8 (84) | 48.9 ± 2.0 (102) |
| H208A | 11.9 ± 1.7 (77) | 28.2 ± 2.7 (59) | |
| H208A | Imd | 14.6 ± 1.9 (94) | 45.7 ± 1.7 (95) |
| H194,208A | 7.9 ± 1.0 (51) | 15.7 ± 0.2 (33) | |
| H194,208A | Imd | 11.3 ± 0.9 (73) | 32.5 ± 1.6 (68) |
| Nonligands | |||
| H97Y | 7.4 ± 0.6 (48) | 32.5 ± 2.6 (68) | |
| H97Y | +Imd | 12.4 ± 0.8 (80) | 42.6 ± 1.8 (89) |
| H98A | ±Imd | 15.6 ± 1.9 (101) | 48.0 ± 1.3 (101) |
| H136A | ±Imd | 17.5 ± 1.7 (113) | 58.7 ± 2.2 (122) |
| H191A | ±Imd | 13.6 ± 2.2 (88) | 50.1 ± 1.5 (104) |
| LSD0.05 | 4.8 | 5.6 | |
Cultures (40 ml) at an OD600 of 0.8 to 1.1 were centrifuged, and the cells were resuspended in 1 ml of Tris buffer–5 mM EDTA (pH 7.3) and purged with N2. Where indicated, 10 mM Imd was added to the growth medium. For H2-dependent MB reduction, 1 ml of H2 was added. Four individual replications of each strain were assayed.
Analysis of the control strains of Wt and the partial deletion mutants show that without H2, all three strains have similar levels of endogenous reduction of MB. The endogenous level of Wt MB reduction was approximately 32% of total Wt MB reduction when H2 was added. Without H2, all mutant strains also had similar but low levels of endogenous activity, comparable to HoxKG− with or without H2 (LSD0.05 = 4.8) (Table 3). However, with H2, the controls showed three distinct levels of activity relative to the endogenous level of MB reduction: none, partial, and full. H2 did not stimulate reduction of MB by the KG deletion mutant, which is consistent with no hydrogenase heterodimer activity. H2 stimulated MB reduction by HoxZ−, indicating a functional hydrogenase heterodimer, but MB reduction was 70% of the Wt level (partial activity). The lack of a complete HoxZ affected the ability of the heterodimer to reduce MB. The H2-dependent MB reduction of Wt was considered to be full activity. In all strains except HoxKG−, when H2 was added, reduction of MB was also immediate, but more rapid than in the absence of H2, which indicates that the heterodimers are functional (LSD0.05 = 5.6) (Table 3).
In assays with H2, MB reduction rates by ligand mutants varied with position (Fig. 3) but were similar between alanine and tyrosine mutants at the same position (tyrosine substitution data not shown). H33A/Y had full activity, regardless of the presence of Imd. Mutants of position 74 had partial activity, whether Imd was present or not. Positions H194A/Y and H208A/Y individually had partial activity without Imd and full activity with Imd. The double ligand mutant H194,208A with Imd showed substantial increase in activity but did not regain full activity.
With or without Imd, three of the four nonligand mutants had at least full Wt activity (Table 3). With H2, but with or without Imd, H136A had significantly greater (>20% increase) MB reduction than Wt and was the only strain with significantly greater than Wt activity. H97Y was unusual in that it had less than Wt activity when assayed without Imd but was comparable to Wt in H2-dependent MB reduction when grown with Imd (Table 3).
The MB reduction results indicate that the heterodimers in the HoxZ partial deletion mutant and point mutants were catalytically active and that the lack of activity in the O2-linked H2 oxidation assay was localized to HoxZ. The rates of reduction in HoxZ− and three of the ligand mutants were not comparable to Wt, which indicates that the heme ligands in HoxZ are required for full catalytic capability of the hydrogenase heterodimer. Unlike their roles in electron transport, in which each ligand was required for hydrogenase function, the four ligands do not play equivalent structural roles, as shown by MB reduction (Fig. 3). Depending on the position, Imd differs in its ability to substitute for ligands and fulfill their functional or structural role.
DISCUSSION
Influence of residue substitutions in HoxZ on O2-dependent H2 oxidation.
In A. vinelandii HoxZ, the four histidines 33, 74, 194, and 208 are essential for physiologically relevant hydrogenase activity (Table 2) and are proposed as the ligands to two hemes in this cytochrome b. The first three residues are homologous to histidines in W. succinogenes HydC, which when substituted with other residues also resulted in cells with low or no physiologically relevant hydrogenase activity (16). In several quinone-interacting b-type cytochromes (7, 11, 16, 49), four conserved histidines occur in transmembrane segments. The invariant histidines in these proteins have been suggested to be ligands to two hemes. In succinate:ubiquinone oxidoreductase (11) and the bc1 complex (49), residue replacements of conserved histidines resulted in loss of activity and changes in absorption spectra, which supported the conclusion that these histidines were ligands to two hemes. Crystal structures of various bc1 complexes confirmed the invariant histidines as heme ligands (48). These biochemical approaches would have been valuable in demonstrating that the four conserved histidines in A. vinelandii HoxZ are indeed ligands to the hemes. Difference spectra (reduced minus oxidized) of A. vinelandii Wt and HoxZ− membranes were examined, but given the large heme background found in this bacterium (e.g., cytochrome bc1 complex and cytochrome bd oxidase), we were unable to identify the absorption associated with HoxZ. A. vinelandii hydrogenase, like most NiFe hydrogenases, was purified as a heterodimer without HoxZ (42). Our efforts to purify A. vinelandii HoxZ along with the heterodimer or HoxZ itself were not successful. However, the current site-specific mutagenesis approach shows that loss of any of these four ligands results in the complete loss of hydrogenase activity and supports the conclusion that the four fully conserved histidines in the hydrogenase b-type cytochrome are ligands to two hemes.
A tyrosine residue is capable of binding heme and is the natural ligand in catalase (31) and cytochrome d1 of nitrite reductase cytochrome cd1 (13) and serves as a ligand in natural methemoglobin mutants (35). Three of the four H→Y A. vinelandii HoxZ ligand mutants (positions 33, 74, and 208) had the same loss of hydrogenase activity as the corresponding H→A ligand mutants. However, the mutant with tyrosine in position 194 had ∼20% of Wt activity, suggesting that tyrosine substitutes as a ligand for histidine in this position but does not enable Wt levels of activity. The failure to reach Wt activity may indicate that the larger size of the tyrosine residue prevents optimal conformation and helix bundling, or perhaps the tyrosine substitution affects redox potential.
Imd has been used with several substituted heme proteins (e.g., horseradish peroxidase H170A [31], cytochrome c peroxidase H175G [24], a soluble version of heme oxygenase H25A [47], and soluble guanylate cyclase H105G [50]) to serve as an exogenous ligand and rescue heme binding. In each of these studies with purified proteins, the iron in the native protein is five-coordinate with a single histidine as the proximal ligand and Imd replaced this ligand, albeit to various degrees. Circular dichroism spectra of E. coli expressing the sperm whale myoglobin gene showed that Imd rescued myoglobin H93G (4). The present study probed multiple ligand sites and demonstrated in vivo Imd rescue of one of these sites in this integral membrane protein. Imd apparently fulfills the structure and function of a ligand in H194A/Y to the same capacity as histidine. Since hydrogenase function in H194A can be controlled simply by adding Imd to the growth medium or assay buffer, i.e., converting hydrogenase from inactive to active, this provides a system that could be used for a variety of in vivo hydrogenase studies.
The H194 site in HoxZ is unique in allowing free Imd or a tyrosine residue to restore activity, but the rationale for this is not clear. H33 and H74 are the only ligands on helices A and B, respectively, but 194 and 208 are both on helix D. With single replacements, helix D would be held in place by the unsubstituted ligand. However, the ability of Imd to function in a site cannot be due solely to being on helix D, since Imd does not restore O2-dependent H2 oxidation activity to H208A. H194 is the only site in HoxZ with a neighboring histidine (H195). In some proteins, such as A. vinelandii hydrogenase HoxK (41) and ferredoxin I FdxA (23), a neighboring or free cysteine residue substituted for a missing cysteine ligand. However, neither H195 nor its counterpart in W. succinogenes (H187) can substitute for the ligand (16). Furthermore, the W. succinogenes HydC mutant H187A had Wt activity, which implies that this neighboring histidine does not play a critical role in Wt hydrogenase activity. We do not know why Imd and tyrosine can substitute for this ligand and only this ligand. The other histidine ligands may have different local environments that do not aid in positioning or stabilizing a substitution or addition of a free ligand or are not able to provide the appropriate redox potential for the heme.
In A. vinelandii, when four histidines with some degree of conservation among 14 hydrogenase cytochrome b homologues were replaced by alanine or tyrosine, all four mutants were active in O2-dependent H2 oxidation activity. Three of these, H97Y, H98A, and H191A, had full Wt O2-dependent H2 oxidation activity. Substitution of three different nonligand histidines in HydC of W. succinogenes (16), including a histidine (homologous to A. vinelandii HoxZ H195), produced altered proteins that had Wt activity. One of the A. vinelandii HoxZ nonligand histidine mutants, H136A, had only one-third of Wt O2-dependent H2 oxidation activity. The histidine in this position apparently plays a role in the structure or function of HoxZ. H136 is in the BC loop region on the cytoplasmic side of the cytoplasmic membrane near the hydrophobic core of the membrane and in close proximity to the high potential heme. Of the surrounding residues, G135 is conserved in most and N137 and P138 are conserved in all HoxZ homologues examined. Besides heme ligation, other possible roles suggested for conserved histidines in b-type cytochromes are functions in quinol binding and modulating heme redox potential (7). H136 in A. vinelandii HoxZ, H217 of the bc1 complex (Rhodobacter capsulatus numbering) (15), and H13 of Bacillus subtilis succinate:ubiquinone oxidoreductase (17) are all located on the cytoplasmic side of the plasma membrane, and replacement of each of these nonligand mutants affects activity. Because redox properties of the quinone in the Qi site were affected, H217 was suggested to form part of the Qi site binding pocket (15). H13Y had half the activity of Wt. From electron paramagnetic spin studies, Hägerhäll et al. (17) concluded that there was a structural change in the local environment of the heme, bH.
Influence of alterations in HoxZ on hydrogenase activity of the HoxKG heterodimer.
To localize the loss of O2-dependent H2 oxidation activity to the substitutions in HoxZ, the hydrogenase activity in the mutants was determined with an assay (H2-dependent MB reduction) requiring only functional heterodimers. Previous studies (5, 16, 32, 40) showed that the heterodimer is active in mutants in which the cytochrome b component of hydrogenase is disrupted. As expected, the hydrogenase heterodimers were active in all the HoxZ mutants (Table 3), which indicated that heterodimer activity could still occur without a functional HoxZ. However, quantitative assays revealed that the A. vinelandii HoxZ mutants did influence the heterodimer activity and that the effects depended on the residue substituted and whether or not Imd was included (Fig. 3). H33A/Y had full Wt activity, while H74A, H194A and H208A had less than Wt activity. The mechanism by which HoxZ influences the H2-dependent MB reduction activity of the heterodimer is uncertain, particularly since MB can bind directly to the heterodimer (42). Perhaps these three HoxZ ligands, H74, 194, and 208, influence alternative and more efficient pathways of electron transfer to MB, or perhaps they fulfill a structural role in anchoring the heterodimer correctly. For H194 and H208, but not H74, Imd restored this function. Imd appears to influence a structural role in support of MB reduction by the heterodimer. The H→A substitution in position 136, which is not a heme ligand, resulted in ∼20% greater H2-dependent MB reduction and 75% lower O2-dependent H2 oxidation rate than Wt. If H136 is part of a quinone binding site, perhaps the H→A substitution decreases the efficiency of quinone binding while increasing the efficiency of MB binding. Since H136 is on the cytoplasmic side, this position probably is not involved in heterodimer binding.
Similarities between quinone-interacting proteins (26) provide insight on how cytochrome b may structurally affect the other subunits. The presence or absence of hemes in succinate-ubiquinone oxidoreductase in E. coli determined whether the heterodimer was able to bind the membrane and function (30), suggesting that incorporation of the heme resulted in a conformational change that exposed binding sites for the two subunits or that the heme may interact directly with them (19). Several lines of evidence indicate that the cytochrome b component of hydrogenase can influence the conformation of the heterodimer. First, in an E. coli deletion mutant of HyaC (a HoxZ homologue), the heterodimer occurs in three forms, unlike the single form of Wt. This heterogeneity may result from differential folding of the heterodimer (28). Second, when an Alcaligenes eutrophus HoxZ deletion mutant was analyzed for hydrogenase activity via an in-gel nitroblue tetrazolium reduction with H2 and phenazine methosulfonate, the location of activity in the HoxZ deletion mutant indicated that the heterodimer of HoxZ− was in a different conformation than the heterodimer in Wt (6). Third, ligands binding in positions 74, 194, and 208 in HoxZ of A. vinelandii are apparently structurally important for full heterodimer activity. Perhaps an Imd-induced conformational change in HoxZ point mutants in positions 194 and 208 enables the heterodimer to bind to HoxZ in a conformation that allows the heterodimer to regain full activity.
Results of the present HoxZ study of A. vinelandii indicate that specific histidine residues 33, 74, 194, and 208 in HoxZ are required for physiologically functional electron transfer. Based on this requirement, the intramolecular locations of the residues, and their conservation among HoxZ homologues, we conclude that these residues are ligands to two hemes. Residues 74, 194, and 208 are also needed structurally for full catalytic activity of the heterodimer even in the absence of electron flow through HoxZ. The four heme ligand sites in A. vinelandii HoxZ are unique with regard to their chemical tolerance to residue substitution and small molecule complementation, as measured by two hydrogenase assays (O2-dependent H2 oxidation and H2-dependent MB reduction). One nonligand histidine, H136, is also important to hydrogenase in physiological electron transport. Both ligands near the periplasmic side of the plasma membrane, those nearest the heterodimer, and one of the ligands on the cytoplasmic side are required for optimal heterodimer catalytic activity. Thus, in the hydrogenase of A. vinelandii, the ligands in the b-type cytochrome HoxZ are required structurally and functionally for optimal H2 oxidation activity. Several roles have been explored for HoxZ and its homologues (6, 8, 16, 20, 26, 32, 40), including mediating electron flow from hydrogenase to the respiratory chain. The present results identify specific histidine residues in HoxZ involved in this role.
ACKNOWLEDGMENTS
The help of Paul Bishop in A. vinelandii transformation and Doug Barrick in the use of Imd as an exogenous ligand is greatly appreciated. We are grateful to Norman Hommes for technical expertise, to Theo Dreher for help in oligonucleotide design, and to other colleagues for helpful discussions.
This work was supported by U.S. Department of Energy grant FG06-90ER20013 to D.J.A. and made possible in part by a grant from the AAUW (American Association of University Women) Educational Foundation.
Footnotes
Present address: Division of Hematology, Washington University School of Medicine, St. Louis, MO 63110.
REFERENCES
- 1.Arp D J. Hydrogen-oxidizing bacteria: methods used in their investigation. In: Linskens H R, Jackson J F, editors. Gases in plant and microbial cells. New York, N.Y: Springer-Verlag; 1989. pp. 257–274. [Google Scholar]
- 2.Atlung T, Knudsen K, Heerfordt L, Brondsted L. Effects of sigmaS and the transcriptional activator AppY on induction of the Escherichia coli hya and cbdAB-appA operons in response to carbon and phosphate starvation. J Bacteriol. 1997;179:2141–2146. doi: 10.1128/jb.179.7.2141-2146.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons; 1998. [Google Scholar]
- 4.Barrick D. Replacement of the proximal ligand of sperm whale myoglobin with free imidazole in the mutant his-93→gly. Biochemistry. 1994;33:6546–6554. doi: 10.1021/bi00187a023. [DOI] [PubMed] [Google Scholar]
- 5.Bernhard M, Benelli B, Hochkoeppler A, Zannoni D, Friedrich B. Functional and structural role of the cytochrome b subunit of the membrane-bound hydrogenase complex of Alcaligenes eutrophus H16. Eur J Biochem. 1997;248:179–186. doi: 10.1111/j.1432-1033.1997.00179.x. [DOI] [PubMed] [Google Scholar]
- 6.Bernhard M, Schwartz E, Rietdorf J, Friedrich B. The Alcaligenes eutrophus membrane-bound hydrogenase gene locus encodes functions involved in maturation and electron transport coupling. J Bacteriol. 1996;178:4522–4529. doi: 10.1128/jb.178.15.4522-4529.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berks B C, Page M D, Richardson D J, Reilly A, Cavill A, Outen F, Ferguson S J. Sequence analysis of subunits of the membrane-bound nitrate reductase from a denitrifying bacterium: the integral membrane subunit provides a prototype for the dihaem electron-carrying arm of a redox loop. Mol Microbiol. 1995;15:319–331. doi: 10.1111/j.1365-2958.1995.tb02246.x. [DOI] [PubMed] [Google Scholar]
- 8.Cauvin B, Colbeau A, Vignais P M. The hydrogenase structural operon in Rhodobacter capsulatus contains a third gene, hupM, necessary for the formation of a physiologically competent hydrogenase. Mol Microbiol. 1991;5:2519–2527. doi: 10.1111/j.1365-2958.1991.tb02098.x. [DOI] [PubMed] [Google Scholar]
- 9.Dross F, Geisler V, Lenger R, Theis F, Krafft T, Fahrenhoz F, Kojro E, Duchêne A, Tripier D, Juvenal K, Kröger A. The quinone-reactive Ni/Fe-hydrogenase of Wolinella succinogenes. Eur J Biochem. 1992;206:93–102. doi: 10.1111/j.1432-1033.1992.tb16905.x. [DOI] [PubMed] [Google Scholar]
- 10.Du L, Tibelius K H, Souza W M, Garg R P, Yates M G. Sequences, organization and analysis of the hupZMNOQRTV genes from the Azotobacter chroococcum hydrogenase gene cluster. J Mol Biol. 1994;243:549–557. doi: 10.1016/0022-2836(94)90029-9. [DOI] [PubMed] [Google Scholar]
- 11.Fridén H, Hederstedt L. Role of his residues in Bacillus subtilis cytochrome b558 for haem binding and assembly of succinate:quinone oxidoreductase (complex II) Mol Microbiol. 1990;4:1045–1056. doi: 10.1111/j.1365-2958.1990.tb00677.x. [DOI] [PubMed] [Google Scholar]
- 12.Friedrich B, Schwartz E. Molecular biology of hydrogen utilization in aerobic chemolithotrophs. Annu Rev Microbiol. 1993;47:351–383. doi: 10.1146/annurev.mi.47.100193.002031. [DOI] [PubMed] [Google Scholar]
- 13.Fülöp V, Moir J W B, Ferguson S J, Hajdu J. The anatomy of a bifunctional enzyme: structural basis for reduction of oxygen to water and synthesis of nitric oxide by cytochrome cd1. Cell. 1995;81:369–377. doi: 10.1016/0092-8674(95)90390-9. [DOI] [PubMed] [Google Scholar]
- 14.Gornall A G, Bardawill C J, David M M. Determination of serum proteins by the means of the Biuret reaction. J Biol Chem. 1949;177:751–766. [PubMed] [Google Scholar]
- 15.Gray K A, Dutton P L, Daldal F. Requirement of histidine 217 for ubiquinone reductase activity (Qi site) in the cytochrome bc1 complex. Biochemistry. 1994;33:723–733. doi: 10.1021/bi00169a014. [DOI] [PubMed] [Google Scholar]
- 16.Gross R, Simon J, Lancaster C R D, Kröger A. Identification of histidine residues in Wolinella succinogenes hydrogenase that are essential for menaquinone reduction by H2. Mol Microbiol. 1998;30:639–646. doi: 10.1046/j.1365-2958.1998.01100.x. [DOI] [PubMed] [Google Scholar]
- 17.Hägerhäll C, Fridén H, Aasa R, Hederstedt L. Transmembrane topology and axial ligands to hemes in the cytochrome b subunit of Bacillus subtilis succinate:menaquinone reductase. Biochemistry. 1995;34:11080–11089. doi: 10.1021/bi00035a013. [DOI] [PubMed] [Google Scholar]
- 18.Happe R P, Roseboom W, Pierik A J, Albracht S P J, Bagley K A. Biological activation of hydrogen. Nature. 1997;385:126. doi: 10.1038/385126a0. [DOI] [PubMed] [Google Scholar]
- 19.Hederstedt L, Andersson K K. Electron-paramagnetic-resonance spectroscopy of Bacillus subtilis cytochrome b558 in Escherichia coli membranes and in succinate dehydrogenase complex from Bacillus subtilis membranes. J Bacteriol. 1986;167:735–739. doi: 10.1128/jb.167.2.735-739.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hidalgo E, Palacios J M, Murillo J, Ruiz-Arüeso T. Nucleotide sequence and characterization of four additional genes of the hydrogenase structural operon from Rhizobium leguminosarum bv. viciae. J Bacteriol. 1992;174:4130–4139. doi: 10.1128/jb.174.12.4130-4139.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee S-Y, Rasheed S. A simple procedure for maximum yield of high-quality plasmid DNA. BioTechniques. 1990;9:676–679. [PubMed] [Google Scholar]
- 22.Maldonado R, Jimenez J, Casadesus J. Changes of ploidy during the Azotobacter vinelandii growth cycle. J Bacteriol. 1994;176:3911–3919. doi: 10.1128/jb.176.13.3911-3919.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martín A E, Burgess B K, Stout C D, Cash V L, Dean D R, Jensen G M, Stephens R J. Site-directed mutagenesis of Azotobacter vinelandii ferredoxin I: [FeS] cluster-driven protein rearrangement. Proc Natl Acad Sci USA. 1990;87:598–602. doi: 10.1073/pnas.87.2.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McRee D E, Jensen G M, Fitzgerald M M, Siegel H A, Goodin D B. Construction of a bisaquo heme enzyme and binding by exogenous ligands. Proc Natl Acad Sci USA. 1994;91:12847–12851. doi: 10.1073/pnas.91.26.12847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McTavish H, Sayavedra-Soto L A, Arp D J. Substitution of Azotobacter vinelandii small-subunit cysteines by serines can create insensitivity to inhibition by O2 and preferentially damages H2 oxidation over H2 evolution. J Bacteriol. 1995;177:3960–3964. doi: 10.1128/jb.177.14.3960-3964.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Menon A L, Mortenson L E, Robson R L. Nucleotide sequences and genetic analysis of hydrogen oxidation (hox) genes in Azotobacter vinelandii. J Bacteriol. 1992;174:4549–4557. doi: 10.1128/jb.174.14.4549-4557.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Menon A L, Stults L W, Robson R L, Mortenson L E. Cloning, sequencing and characterization of the [NiFe] hydrogenase-encoding structural genes (HoxK and HoxG) from Azotobacter vinelandii. Gene. 1990;96:67–74. doi: 10.1016/0378-1119(90)90342-o. [DOI] [PubMed] [Google Scholar]
- 28.Menon N K, Robbins J, Wendt J C, Shanmugam K T, Przybyla A E. Mutational analysis and characterization of the Escherichia coli hya operon, which encodes [NiFe] hydrogenase 1. J Bacteriol. 1991;173:4851–4861. doi: 10.1128/jb.173.15.4851-4861.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Merino E, Osuna J, Bolivar F, Soberon X. A general, PCR-based method for single or combinatorial oligonucleotide-directed mutagenesis on pUC/M13 vectors. BioTechniques. 1992;12:508–510. [PubMed] [Google Scholar]
- 30.Nakamura K, Yamaki M, Sarada M, Nakayama S, Vibat C R T, Gennis R B, Nakayashiki T, Inokuchi H, Kojima S, Kita K. Two hydrophobic subunits are essential for the heme b ligation and functional assembly of complex II (succinate-ubiquinone oxidoreductase) from Escherichia coli. J Biol Chem. 1996;271:521–527. doi: 10.1074/jbc.271.1.521. [DOI] [PubMed] [Google Scholar]
- 31.Newmyer S L, Sun J, Loehr T M, Ortiz de Montellano P R. Rescue of the horseradish peroxidase his-170→ala mutant activity by imidazole: importance of proximal ligand tethering. Biochemistry. 1996;35:12788–12795. doi: 10.1021/bi9609331. [DOI] [PubMed] [Google Scholar]
- 32.Ohtsuki T, Kita Y, Fujioka T, Hashimoto D, Shimosaka M, Okazaki M. The hupC gene product is a component of the electron transport system for hydrogen oxidation in Pseudomonas hydrogenovora. FEMS Microbiol Lett. 1997;150:127–133. doi: 10.1111/j.1574-6968.1997.tb10360.x. [DOI] [PubMed] [Google Scholar]
- 33.Page W J, von Tigerstrom M. Optimal conditions for transformation of Azotobacter vinelandii. J Bacteriol. 1979;139:1058–1061. doi: 10.1128/jb.139.3.1058-1061.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peterson J B. Dependence of oxygen-tolerant nitrogenase activity on divalent cations in Azotobacter vinelandii. J Bacteriol. 1992;174:3399–3402. doi: 10.1128/jb.174.10.3399-3402.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pin S, Alpert B, Cortès R, Ascone I, Chiu M, Sligar S G. The heme iron coordination complex in his64(E7)tyr recombinant sperm whale myoglobin. Biochemistry. 1994;33:11618–11623. doi: 10.1021/bi00204a024. [DOI] [PubMed] [Google Scholar]
- 36.Premakumar R, Loveless T M, Bishop P E. Effect of amino acid substitutions in a potential metal-binding site of AnfA on expression from the anfH promoter in Azotobacter vinelandii. J Bacteriol. 1994;176:6139–6142. doi: 10.1128/jb.176.19.6139-6142.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prosser J, Graham L, Maier R J. Hydrogen-mediated enhancement of hydrogenase expression in Azotobacter vinelandii. J Bacteriol. 1988;170:1990–1993. doi: 10.1128/jb.170.4.1990-1993.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Przybyla A E, Robbins J, Menon N, Peck H D., Jr Structure-function relationships among the nickel-containing hydrogenases. FEMS Microbiol Rev. 1992;8:109–135. doi: 10.1111/j.1574-6968.1992.tb04960.x. [DOI] [PubMed] [Google Scholar]
- 39.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 40.Sayavedra-Soto L A, Arp D J. The hoxZ gene of the Azotobacter vinelandii hydrogenase operon is required for activation of hydrogenase. J Bacteriol. 1992;174:5295–5301. doi: 10.1128/jb.174.16.5295-5301.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sayavedra-Soto L A, Arp D J. In Azotobacter vinelandii hydrogenase, substitution of serine for the cysteine residues at positions 62, 65, 289, and 292 in the small (HoxK) subunit affects H2 oxidation. J Bacteriol. 1993;175:3414–3421. doi: 10.1128/jb.175.11.3414-3421.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seefeldt L C, Arp D J. Purification to homogeneity of Azotobacter vinelandii hydrogenase: a nickel and iron containing αβdimer. Biochimie. 1986;68:25–34. doi: 10.1016/s0300-9084(86)81064-1. [DOI] [PubMed] [Google Scholar]
- 43.Strandberg G W, Wilson P W. Formation of the nitrogen-fixing enzyme system in Azotobacter vinelandii. Can J Microbiol. 1968;14:25–31. doi: 10.1139/m68-005. [DOI] [PubMed] [Google Scholar]
- 44.Van Soom C, Browaeys J, Verreth C, Vanderleyden J. Nucleotide sequence analysis of four genes, hupC, hupD, hupF and hupG, downstream of the hydrogenase structural genes in Bradyrhizobium japonicum. J Mol Biol. 1993;234:508–512. doi: 10.1006/jmbi.1993.1605. [DOI] [PubMed] [Google Scholar]
- 45.Volbeda A, Charon M-H, Piras C, Hatchikian E C, Frey M, Fontecilla-Camps J C. Crystal structure of the nickel-iron hydrogenase from Desulfovibrio gigas. Nature. 1995;373:580–587. doi: 10.1038/373580a0. [DOI] [PubMed] [Google Scholar]
- 46.Weiner M P, Costa G L, Schoettlin W, Cline J, Mathur E, Bauer J C. Site-directed mutagenesis of double-stranded DNA by the polymerase chain reaction. Gene. 1994;151:119–123. doi: 10.1016/0378-1119(94)90641-6. [DOI] [PubMed] [Google Scholar]
- 47.Wilks A, Sun J, Loehr T M, Ortiz de Montellano P R. Heme oxygenase his25ala mutant: replacement of the proximal histidine iron ligand by exogenous bases restores catalytic activity. J Am Chem Soc. 1995;117:2925–2926. [Google Scholar]
- 48.Yu C A, Zhang L, Deng K P, Tian H, Xia D, Kim H, Deisenhofer J, Yu L. Structure and reaction mechanisms of multifunctional mitochondrial cytochrome bc1 complex. Biofactors. 1999;9:103–109. doi: 10.1002/biof.5520090204. [DOI] [PubMed] [Google Scholar]
- 49.Yun C-H, Crofts A R, Gennis R B. Assignment of the histidine axial ligands to the cytochrome bH and cytochrome bL components of the bc1 complex from Rhodobacter sphaeroides by site-directed mutagenesis. Biochemistry. 1991;30:6747–6754. doi: 10.1021/bi00241a017. [DOI] [PubMed] [Google Scholar]
- 50.Zhao Y, Shelvis J P M, Babcock G T, Marletta M A. Identification of histidine 105 in the B1 subunit of soluble guanylate cyclase as the heme proximal ligand. Biochemistry. 1998;37:4502–4509. doi: 10.1021/bi972686m. [DOI] [PubMed] [Google Scholar]