Abstract
Visceral artery pseudoaneurysms are potentially lethal lesions and tend to rupture in a high proportion of cases, thereby warranting an immediate and active intervention.
We present our experience of splanchnic visceral artery pseudoaneurysms in a university hospital over a 5-year time interval with emphasis on etiology, clinical presentation, management (endovascular/surgical), and final outcome.
This was a retrospective study in which we searched our image database for pseudoaneurysms of visceral arteries over a period of 5 years. The clinical and operative details were retrieved from the medical record section of our hospital. The lesions were analyzed for the vessel of origin, size, etiology, clinical features, mode of treatment, and outcome.
Twenty-seven patients with pseudoaneurysms were encountered. Pancreatitis (8) was the most common cause, followed by previous surgery (7) and trauma (6). Fifteen were managed by the interventional radiology (IR) team, 6 by surgery, and in 6 no intervention was done. Technical and clinical success was achieved in all patients in the IR group with few minor complications. Surgery and no intervention carry a high mortality in such a setting (66 and 50%, respectively).
Visceral pseudoaneurysms are potentially fatal lesions, commonly encountered after trauma, pancreatitis, surgeries, and interventional procedures. These lesions are easily salvageable by minimally invasive interventional techniques (endovascular embolotherapy), and surgeries carry a lot of morbidity and mortality in such cases and a prolonged hospital stay.
Keywords: pseudoaneurysm, endovascular, embolization, coil
Visceral artery pseudoaneurysms are potentially lethal lesions and tend to rupture in a high proportion of cases, thereby warranting an immediate and active intervention. They lack the complete vascular wall seen in true aneurysms and result from a variety of insults (traumatic and inflammatory). 1 In a tertiary care setting, most are the result of pancreatitis, trauma, iatrogenic interventions (hepatobiliary and pancreaticoduodenal surgeries and interventional procedures like percutaneous transhepatic biliary drainage [PTBD], liver biopsies, etc.), and less commonly inflammatory and infectious vasculitis. 1 Understandably, these are usually symptomatic and may present with pain (due to the mass effect of an expanding hematoma), bleeding into the gastrointestinal tract (presenting with hematemesis and melena), bleeding into the peritoneal cavity, or through a surgical drain. 2 A pseudoaneurysm occurring in a postoperative setting is an exceedingly difficult problem to manage by a repeat surgery because of inflammation, adhesions, and distortion of anatomy by previous surgery and carries the risk of postoperative fistulization, but fortunately, many of these are amenable to endovascular treatment. 2 Surgery may still be necessary for a subset of patients who present with rupture and hemodynamic instability or other associated complications that may merit repeat surgery as such (like an anastomotic leak) but carries a high mortality. 2 Thus, an early diagnosis is mandatory for optimal management, and this is greatly facilitated by timely computed tomography (CT) angiography in patients with any index of suspicion which can identify a vast majority of these lesions. An ultrasound or Doppler study is not effective in identifying many of these lesions because of associated ileus, overlying gut gas, and postoperative dressings, etc., affording a limited acoustic window. Notwithstanding, even CT angiography may miss a few of such lesions and both CT and digital subtraction angiography (DSA) may be helpful in such a setting.
The endovascular treatment of pseudoaneurysms arising from celiac artery/superior mesenteric artery (SMA) branches is technically more challenging because of the possibility of collateral circulation, resulting in recurrences unless the pseudoaneurysms are completely excluded from the circulation by blocking both the proximal and distal “doors” of the lesion. 3 4 5 Renal artery branches are end arteries and their embolization is often technically simpler and easier and recanalization is rare. In this article, we present our experience of visceral artery pseudoaneurysm in a university hospital over a 5-year time interval (2015–2020) with emphasis on etiology, clinical presentation, management (endovascular/surgical), and final outcome. We have predominantly focused on pseudoaneurysms arising from the splanchnic vascular system and have excluded renal artery pseudoaneurysms which we have addressed in a separate paper.
Material and Methods
In this study, we have studied different types of splanchnic visceral artery pseudoaneurysm (excluding renal artery pseudoaneurysms), their clinical profile, the endovascular and surgical management, and their outcome over a period of 5 years. It was a retrospective study in which we searched our image database for pseudoaneurysms of visceral arteries over a period of 5 years as per the inclusion and exclusion criteria. The clinical and operative details were retrieved from the medical record section of our hospital. The lesions were analyzed for the vessel of origin, size, etiology, clinical features, mode of treatment, and outcome. Follow-up imaging which was usually done within a month of treatment was reviewed. Technical success was defined as exclusion of aneurysm from the circulation on table and at follow-up at 1 month in case of nonrecurrence of symptoms. Clinical success was defined by cessation of bleeding.
Being a retrospective study ethical clearance was waived off.
Results
A total of 27 patients with pseudoaneurysms were encountered. The diagnosis was confirmed by CT angiography in the majority of these cases. Out of those 14 were treated by endovascular methods, 1 by percutaneous embolization, 6 were treated by open surgery, 3 patients showed spontaneous resolution, and 3 patients died before any surgical or interventional management could be offered.
Fourteen patients were managed by endovascular intervention and one patient was managed by percutaneous thrombin injection, out of these one patient succumbed (due to unrelated issues) and one aneurysm recurred. Coils were used in 13 patients and gel foam was used in 2 patients. Minor complications were encountered in three patients (small splenic infarcts) and a large splenic abscess was seen in one patient that required prolonged pigtail catheter drainage ( Fig. 1 ). Repeat embolization had to be performed in two patients (for the recanalization of aneurysm).
Fig. 1.
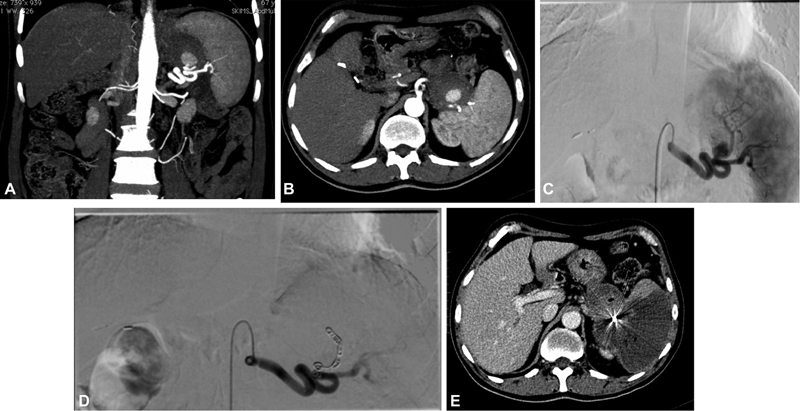
( A , B ) Computed tomography (CT) angiogram of partially thrombosed pseudoaneurysm of splenic artery in a patient with chronic pancreatitis. ( C ) Splenic artery angiogram depicting the pseudoaneurysm arising from of the branches of splenic artery. ( D ) Post-coil embolization check angiogram showing exclusion of aneurysm. ( E ) Follow-up CT showing nonopacification of pseudoaneurysm with a large upper pole splenic infarct.
Out of the 15 patients managed by interventional radiology (IR) ( Table 1 ), 7 patients had hepatic artery pseudoaneurysms ( Figs. 2 and 3 ). Six of these were posttraumatic (two related to biliary surgery, one had a gunshot injury, two patients had road traffic accidents, and one fall from height). In three of these patients who were post-laparotomy, a pseudoaneurysm was suspected because of hemorrhagic drain output and subsequently confirmed on CT angiography. The seventh patient was an unresectable case of pancreatic neuroendocrine tumor who had a percutaneous biliary drain for obstructive jaundice in place and presented with hemobilia. Five of these patients were managed successfully by superselective catheterization of bleeding vessel and deployment of microcoils. Two patients were managed by gel foam embolization, one of whom developed a large splenic infarct postprocedure which evolved into an abscess over subsequent 2 weeks. The splenic abscess was managed by pigtail catheter drainage. The other patient in whom gel foam embolization had been performed showed recanalization of the pseudoaneurysm and had to be reembolized with coils.
Table 1. Pseudoaneurysms treated by interventional radiology.
| Type of artery | No. of cases | Etiology | Presentation | Procedure | Clinical outcome |
|---|---|---|---|---|---|
| Hepatic artery and its branches | 7 | 1. Post-cholecystectomy, ERCP | GI bleed | Coil embolization | Technical and clinical success |
| 2. Post-choledochoduodenostomy | GI bleed/ Hemobilia on ERCP | Coil embolization | Technical and clinical success | ||
| 3. Gunshot injury | Post laparotomy hemorrhagic drain | Coil embolization | Technical and clinical success | ||
| 4. Trauma | Post laparotomy hemorrhagic drain | Gel foam embolization | Recurrence -repeat embolization | ||
| 5. Trauma | Post laparotomy hemoperitoneum | Coil embolization | Technical and clinical success | ||
| 6. Inoperable pancreatic NET | Hemorrhagic drain via PTBD catheter | Gel foam embolization | Large splenic abscess -managed by pigtail drainage | ||
| 7. Trauma | Abdominal distension and tenderness after fall from height | Coil embolization | Technical and clinical success | ||
| Gastroduodenal artery pseudoaneurysm | 3 | 1. Chronic pancreatitis | GI bleed, peripancreatic hematoma | Coil embolization | Technical and clinical success |
| 2. Post-partial gastrectomy | Recurrent hemorrhagic drainage, drop in hematocrit | Coil embolization | Technical and clinical success | ||
| 3. Acute pancreatitis | GI bleed, peripancreatic hematoma | Coil embolization | Technical and clinical success | ||
| Splenic artery pseudoaneurysm | 5 | 1. Acute pancreatitis | GI bleed, peripancreatic hematoma | Coil embolization after failed percutaneous Glue injection- | Partial (large) splenic infarct asymptomatic |
| 2. Chronic pancreatitis | GI bleed, aneurysm eroding late gastric wall | Coil embolization | Small splenic infarct | ||
| 3. Post-ERCP stenting for pancreatitis with pseudocyst | Hemorrhage via pigtail catheter | Coil embolization | Small splenic infarct | ||
| 4. Trauma (background splenomegaly) | Perisplenic hematoma | Coil embolization | Technical and clinical success | ||
| 5. Post-Whipple's | GI bleed | Percutaneous thrombin injection | Technical and clinical success |
Abbreviations: ERCP, endoscopic retrograde cholangiopancreatography; GI, gastrointestinal; NET, neuroendocrine tumor; PTBD, percutaneous transhepatic biliary drainage.
Fig. 2.
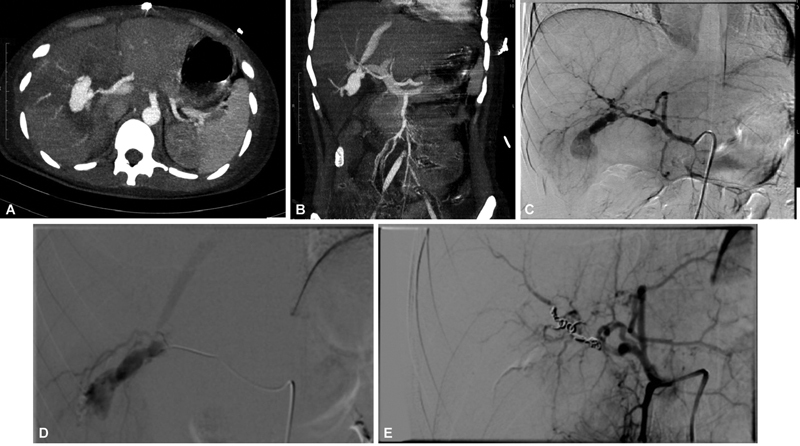
( A , B ) Computed tomography (CT) angiogram showing a large right hepatic artery pseudoaneurysm in a patient of firearm injury with shunting into hepatic vein. ( C , D ) Celiac angiogram and right hepatic artery angiogram depicting the pseudoaneurysm and the hepatic venous shunting. ( E ) Post-coil embolization angiogram showing exclusion of aneurysm.
Fig. 3.
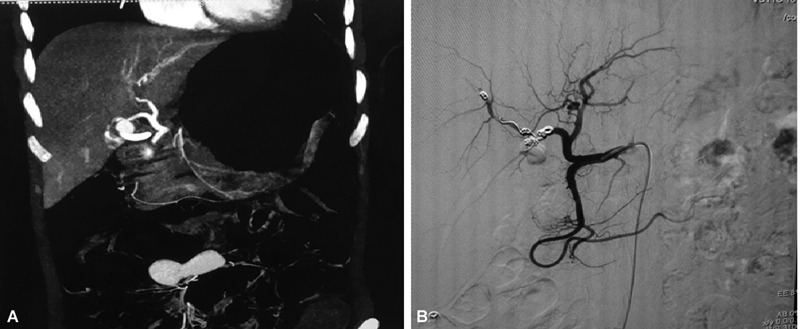
( A ) Computed tomography (CT) angiogram of a right hepatic artery pseudoaneurysm in a patient post-endoscopic retrograde cholangiopancreatography (ERCP). ( B ) Post-coil embolization common hepatic artery angiogram showing complete exclusion of aneurysm.
Out of the three gastroduodenal artery pseudoaneurysms two were related to pancreatitis (one acute and one chronic) and one followed a partial gastrectomy, who had a persistent hemorrhagic drain despite reexploration for the same. All three were managed by coil embolization with the coils being deployed distal and proximal to the pseudoaneurysm, with immediate technical and clinical success.
Five patients had splenic artery pseudoaneurysms (one complicating acute pancreatitis, one following chronic pancreatitis, one following surgery—modified Whipple's procedure, one post-endoscopic retrograde cholangiopancreatography [ERCP] pancreatic duct stenting, and one due to trauma). The patient who had a splenic pseudoaneurysm following surgery was managed by percutaneous thrombin injection, as a safe catheter position for coil deployment could not be achieved ( Fig. 4 ). One patient was initially managed by percutaneous glue injection under ultrasound guidance but the aneurysm recurred and was subsequently treated by coil embolization of splenic artery. Three patients were treated by coil embolization of splenic artery (back door-front door occlusion/exclusion of pseudoaneurysm). Two of these developed a large asymptomatic splenic infarct requiring no intervention. All patients had immediate technical and clinical success of embolotherapy.
Fig. 4.
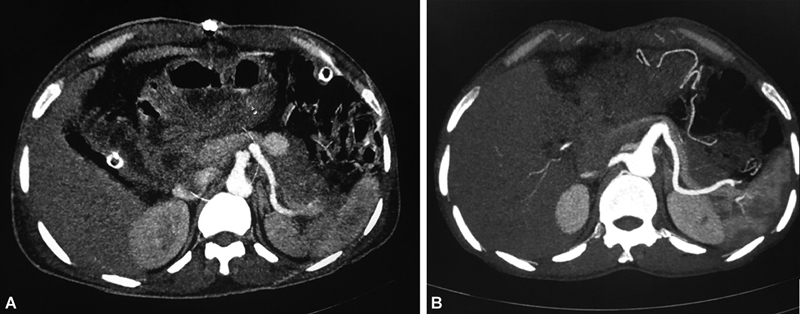
( A ) Computed tomography (CT) angiogram showing a pseudoaneurysm in relation to splenic artery in a patient post-Whipple's procedure. A safe coil deployment position could not be reached on digital subtraction angiography (DSA), so percutaneous thrombin injection performed. ( B ) Follow-up CT angiogram shows nonopacification of pseudoaneurysm.
We did not encounter recurrence of any pseudoaneurysm following coil embolization, and the technical result of aneurysm closure and clinical endpoint of cessation of bleeding was achieved in all of our patients.
Six patients were treated by surgery—two because they were to be operated due to other reasons and four because they were hemodynamically unstable and were not referred to the IR department for embolization. Overall, out of the six patients treated by surgery ( Table 2 ), two were operated electively and made a recovery while four patients who underwent emergency surgery succumbed, one of whom had initial control of bleeding but died because of the late complications of the second surgery. Thus, emergency surgery of visceral pseudoaneurysm especially in the setting of previous surgery carries considerable morbidity and mortality and an endovascular-first approach is the best treatment option. However, all of these patients were hemodynamically unstable and actively bleeding and hence taken to the operating room upon the decision of the operating surgeon.
Table 2. Pseudoaneurysms treated by primary surgical repair.
| Type of pseudoaneurysm | Presentation | Etiology | Postsurgical outcome | |
|---|---|---|---|---|
| 1. | Gastroduodenal artery pseudoaneurysm |
Detected on CT during evaluation for pancreatic mass | Chronic pancreatitis, pancreatic head mass | Whipple's done – discharged after 2 weeks |
| 2. | Splenic artery pseudoaneurysm |
Detected on CT during evaluation for pancreatitis | Chronic pancreatitis Chronic pseudocyst |
Aneurysmectomy, repair of splenic artery, pseudocyst drainage- discharged after 2 weeks |
| 3. | Hepatic artery pseudoaneurysm |
GI bleed | Post-Whipple's | Bleeding control achieved, > 2 months' hospital stay, pancreatic fistula followed by death |
| 4. | Gastroduodenal artery pseudoaneurysm |
GI bleed | Periampullary mass | Renal shutdown following surgery, multiorgan failure – died after 1 week |
| 5. | Multiple mesenteric artery pseudoaneurysms | GI bleed | Tubercular vasculitis Tubercular spondylodiscitis |
Perioperative death |
| 6. | Right hepatic artery pseudoaneurysm | GI bleed | Post-ERCP | Died in ICU in 48 hours due to multiorgan failure |
Abbreviations: CT, computed tomography; ERCP, endoscopic retrograde cholangiopancreatography; GI, gastrointestinal; ICU, intensive care unit.
In six patients no surgical or endovascular intervention was done ( Table 3 ). Out of these, two patients were planned for an elective endovascular treatment (left gastric artery pseudoaneurysm for embolization, SMA pseudoaneurysm for covered stent graft) but succumbed to a massive bleed before the procedure ( Fig. 5 ). One patient had multiple mesenteric mycotic pseudoaneurysm and severe sepsis and was not deemed fit for any intervention (because of unstable clinical condition and could not be shifted to the IR lab) and expired within a few days. Three patients were observed; one with multiple pancreaticoduodenal arcade aneurysm (technically not amenable for coil embolization due to multiplicity), one with a small pseudoaneurysm (6 mm) arising from a splenic artery (which was expected to resolve on its own due to small size as patient has no symptoms), and one with a large pseudoaneurysm arising at the celiac origin in whom a diagnostic DSA failed to clear the origin of the pseudoaneurysm and as such was not taken up for embolization because the origin could not be negotiated ( Fig. 6 ). All of these three pseudoaneurysms resolved on follow-up imaging. One of these patients with multiple tiny pancreaticoduodenal arcade aneurysms was also kept under observation and stabilized with follow-up imaging subsequently showing resolution. The patient's multiple pancreaticoduodenal arcade aneurysms were thought to be due to celiac origin stenosis and retrograde filling of the pancreaticoduodenal arcade was demonstrated on angiography.
Table 3. Pseudoaneurysms in which no intervention was done.
| Serial no. | Pathological vessel | Presentation | Etiology | Management/Outcome |
|---|---|---|---|---|
| 1. | Left gastric artery pseudo aneurysm | Intermittent GI bleed | Gastric cancer | Planned for embolization, massive GI bleed and death |
| 2. | SMA pseudoaneurysm | Post-laparotomy hemorrhagic drain | Post-Whipple's SMA repaired perop, peripancreatic collection | Planned for covered stent, death after massive hemorrhage into peritoneal cavity |
| 3. | Multiple mesenteric pseudoaneurysms | Abdominal distension | Infective endocarditis, multiple vegetations, mycotic aneurysms | Managed by intravenous antibiotics, died because of hemoperitoneum |
| 4. | Large celiac artery pseudoaneurysm | Abdominal distension and tenderness after RTA | Posttraumatic, post-laparotomy, portal vein and liver laceration repaired | Planned for balloon-assisted thrombin injection. Spontaneous thrombosis and resolution a |
| 5. | Splenic artery branch pseudoaneurysm | Detected on postoperative CT | Post-modified Whipple's | Spontaneous resolution |
| 6. | Multiple pancreaticoduodenal arcade aneurysm | Detected on CT during evaluation for pancreatitis | Acute pancreatitis | Spontaneous resolution |
Abbreviations: CT, computed tomography; GI, gastrointestinal; RTA, road traffic accident; SMA, superior mesenteric artery.
Case of vehicular accident with a large pseudoaneurysm arising from the celiac trunk. Planned for balloon-assisted percutaneous thrombin injection. However, in subsequent imaging partial thrombosis of the aneurysm was noted and the patient was kept on watchful surveillance. Complete thrombosis of the pseudoaneurysm was noted.
Fig. 5.
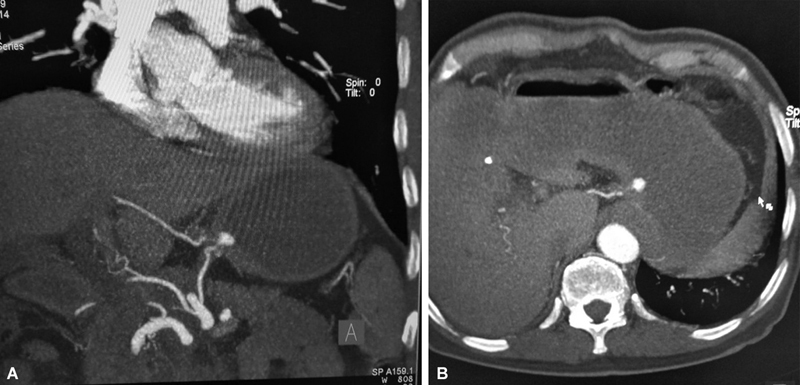
( A , B ) Computed tomography (CT) angiogram showing a left gastric artery pseudoaneurysm in a patient of gastric cancer. Patient was planned for an elective endovascular treatment, however, succumbed to a massive bout of gastrointestinal (GI) bleed prior to the procedure.
Fig. 6.
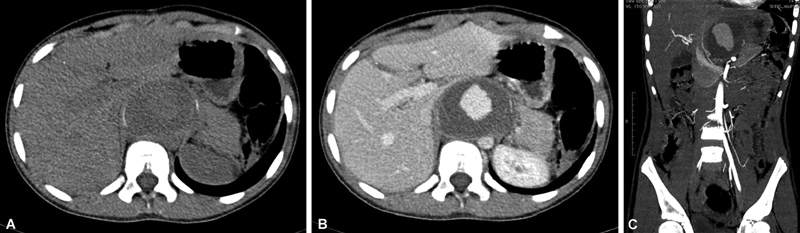
Noncontrast computed tomography (NCCT) ( A ) and CT angiogram ( B , C ) showing a large partially thrombosed pseudoaneurysm in relation to celiac artery in a patient of road traffic accident who had undergone exploratory laparotomy with portal vein repair and hepatic artery ligation. The pseudoaneurysm was thought to arise from celiac artery but could not be demonstrated on selective celiac artery angiography. Follow-up imaging showed spontaneous thrombosis of the pseudoaneurysm.
Discussion
Visceral pseudoaneurysms occur in a wide variety of clinical settings (iatrogenic, pancreatitis, malignancy, vasculitis, etc.) and can have fatal outcome if not managed urgently. 1 Trauma, surgeries (especially Whipple's procedure and gallbladder surgeries), and pancreatitis accounts for the vast majority of pseudoaneurysms all over the world and less common causes include vasculitis, radiological interventional procedures, malignancies, and atherosclerosis. 6 In our series, pancreatitis (acute and chronic) was the etiology in eight cases, previous surgery in seven patients, and trauma (road traffic accidents, fall from height, gunshot) accounted for six cases. Three cases were seen after interventional procedures (ERCP and PTBD); thus, iatrogenic causes were implicated overall in 10 patients. 7 8
Operative management was done in six patients; out of whom four patients were operated on emergent basis. Three patients succumbed within 24 hours, demonstrating that operative management carries a high mortality and morbidity. One patient developed postoperative fistula and died after a prolonged hospital stay and an extended period of morbidity. Two patients made good recovery and were discharged within 2 weeks. Iatrogenic (postsurgical, postinterventional) pseudoaneurysms are a surgeon's nightmare and a repeat surgery to address these is an exceedingly difficult clinical scenario because of the adhesions, inflammation, and distorted postoperative anatomy. 9 Thus, operative management of pseudoaneurysms carries a high morbidity and mortality and should be employed selectively especially if associated with problems which would otherwise merit surgery. 10
Endovascular management was employed in 14 patients out of whom 7 had undergone previous surgery for different indications. Coils were used in 13 patients and 2 patients were embolized using gel foam. We adopted the usual front door-back door sandwich technique in coil embolization except two patients who had pseudoaneurysms of terminal hepatic artery branches. Three splenic infarcts and one large splenic abscess was encountered as a complication; the latter was seen in a common hepatic artery pseudoaneurysm which was embolized using gel foam and was due to unintended gel foam reflux. We had technical success and clinical cessation of bleeding in all but one pseudoaneurysm which was embolized using gel foam and recurred after a week. This was successfully managed by repeat embolization.
Endovascular embolotherapy should always be considered the treatment of choice for splanchnic pseudoaneurysms especially those arising from branches of celiac axis and is highly efficacious in this setting. Even though recanalization can occur there is always a prospect of repeating the procedure with minimal morbidity. 5 The availability of Amplatzer vascular plugs may allow more effective vascular occlusions but are costly and may not always be available in developing and resource-depleted countries. These are especially useful in occlusion of larger vessels (e.g., splenic artery) and if aneurysm recurs post-coiling. 11 Many centers have used glue (in combination with lipiodol) which is especially useful for tortuous and difficult anatomy vascular beds; it is, however, technically difficult and demands a prior experience with its use. 2 5 12 Pseudoaneurysms involving proximal segments of SMA, celiac, or proper hepatic arteries which cannot be coil embolized because of risk of ischemia downstream can be treated by covered stents. Such hardware is not immediately available at many centers in developing countries (as was unfortunately the case with a post-Whipple's procedure SMA pseudoaneurysm and posttraumatic celiac pseudoaneurysm in our series). 13 14 15 16
Pseudoaneurysms arising from splenic artery proper can be safely embolized by coils in the usual front door-back door technique and splenic perfusion in those cases is maintained by short gastric collaterals. If the distal segment of the artery is involved, splenic infarct may result and is best managed conservatively with analgesics and intravenous antibiotics to prevent development of abscess. 17 18 19 The risk of splenic infarction and abscess is higher with gel foam and liquid embolic agents. Coil embolization, wherever feasible, is the preferred technique especially if the pseudoaneurysm shows brisk flow on color Doppler; if percutaneous injection of thrombin is used for any reason (emergency control of bleeding, or when safe catheter position cannot be achieved), the patient should be carefully followed for recurrence. 17 18 19
Mycotic and vasculitis-associated pseudoaneurysms of the splanchnic arteries, especially if these are multiple, are tough treatment dilemmas and are usually managed conservatively, addressing the primary etiology plus supportive treatment. Catheter-based treatments especially placement of stent grafts is not preferred in mycotic aneurysms, due to the possibility of graft infection and in such cases, repair, if needed, is performed surgically. 20
A recurrence of pseudoaneurysms is always a possibility and is more common in gastroduodenal and hepatic pseudoaneurysms because of recruitment of complex collateral pathways. 21 22 Reintervention in such cases is challenging, if not impossible.
Even though all pseudoaneurysms are considered emergencies, in a few selected cases watchful surveillance may be chosen (e.g., in partially thrombosed lesion with a thick calcified wall, asymptomatic lesions that have demonstrated size reduction on serial CT scans). 23 We unfortunately encountered two cases of fatal aneurysmal rupture in patients who were scheduled for a procedure though it is a policy in our institution to treat such lesions as quickly as possible.
CT angiography prior to the procedure is a routine in our hospital; however, CT angiography missed the diagnosis of three of our patients, and if the clinical suspicion is high, we routinely perform a DSA. We also employ rotational angiography as a problem-solving tool if the DSA and CT angiography remains inconclusive or discordant and the clinical suspicion of a pseudoaneurysm remains high. 24
In conclusion, visceral pseudoaneurysms are not uncommonly encountered potentially fatal lesions, commonly encountered after trauma, pancreatitis, surgeries, and interventional procedures. These lesions are easily salvageable by minimally invasive interventional techniques (endovascular embolotherapy), and surgeries carry a lot of morbidity and mortality in such cases and a prolonged hospital stay. In a developing country and a resource-depleted environment, the instantaneous unavailability of appropriate hardware (e.g., covered stents, vascular plugs) may result in treatment failures and the attendant mortality. Procurement of essential hardware in the inventory should thus be a policy with the hospital administration so as to enable treatment without delays so as to salvage a complex surgical or interventional procedure or lethal but potentially curable condition (e.g., a posttraumatic or post-pancreatitis pseudoaneurysm).
Funding Statement
Funding None.
Conflict of Interest None declared.
Statement
The manuscript has been read and approved by all the authors, the requirements for authorship have been met, and we believe that the manuscript represents honest work.
References
- 1.Pitton M B, Dappa E, Jungmann F. Visceral artery aneurysms: Incidence, management, and outcome analysis in a tertiary care center over one decade. Eur Radiol. 2015;25(07):2004–2014. doi: 10.1007/s00330-015-3599-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tulsyan N, Kashyap V S, Greenberg R K.The endovascular management of visceral artery aneurysms and pseudoaneurysms J Vasc Surg 20074502276–283., discussion 283 [DOI] [PubMed] [Google Scholar]
- 3.Roberts K J, McCulloch N, Forde C. Emergency treatment of haemorrhaging coeliac or mesenteric artery aneurysms and pseudoaneurysms in the era of endovascular management. Eur J Vasc Endovasc Surg. 2015;49(04):382–389. doi: 10.1016/j.ejvs.2014.12.019. [DOI] [PubMed] [Google Scholar]
- 4.Duan X-H, Ren J-Z, Zhou G-F. Clinical features and endovascular treatment of visceral artery pseudoaneurysms. Ann Vasc Surg. 2015;29(03):482–490. doi: 10.1016/j.avsg.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 5.Laganà D, Carrafiello G, Mangini M. Multimodal approach to endovascular treatment of visceral artery aneurysms and pseudoaneurysms. Eur J Radiol. 2006;59(01):104–111. doi: 10.1016/j.ejrad.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 6.Regus S, Lang W. Rupture risk and etiology of visceral artery aneurysms and pseudoaneurysms: a single-center experience. Vasc Endovascular Surg. 2016;50(01):10–15. doi: 10.1177/1538574415627868. [DOI] [PubMed] [Google Scholar]
- 7.Gabrielli D, Taglialatela F, Mantini C, Giammarino A, Modestino F, Cotroneo A R. Endovascular treatment of visceral artery pseudoaneurysms in patients with chronic pancreatitis: our single-center experience. Ann Vasc Surg. 2017;45:112–116. doi: 10.1016/j.avsg.2017.05.035. [DOI] [PubMed] [Google Scholar]
- 8.Kalva S P, Yeddula K, Wicky S, Fernandez del Castillo C, Warshaw A L. Angiographic intervention in patients with a suspected visceral artery pseudoaneurysm complicating pancreatitis and pancreatic surgery. Arch Surg. 2011;146(06):647–652. doi: 10.1001/archsurg.2011.11. [DOI] [PubMed] [Google Scholar]
- 9.Batagini N C, El-Arousy H, Clair D G, Kirksey L. Open versus endovascular treatment of visceral artery aneurysms and pseudoaneurysms. Ann Vasc Surg. 2016;35:1–8. doi: 10.1016/j.avsg.2016.01.035. [DOI] [PubMed] [Google Scholar]
- 10.Erben Y, De Martino R R, Bjarnason H. Operative management of hepatic artery aneurysms. J Vasc Surg. 2015;62(03):610–615. doi: 10.1016/j.jvs.2015.03.077. [DOI] [PubMed] [Google Scholar]
- 11.Laganà D, Carrafiello G, Mangini M. Indications for the use of the Amplatzer vascular plug in interventional radiology. Radiol Med (Torino) 2008;113(05):707–718. doi: 10.1007/s11547-008-0306-1. [DOI] [PubMed] [Google Scholar]
- 12.Madhusudhan K S, Gamanagatti S, Garg P. Endovascular embolization of visceral artery pseudoaneurysms using modified injection technique with N-butyl cyanoacrylate glue. J Vasc Interv Radiol. 2015;26(11):1718–1725. doi: 10.1016/j.jvir.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 13.Kim S K, Lee J, Duncan J R, Picus D D, Darcy M D, Sauk S. Endovascular treatment of superior mesenteric artery pseudoaneurysms using covered stents in six patients. AJR Am J Roentgenol. 2014;203(02):432–438. doi: 10.2214/AJR.13.11644. [DOI] [PubMed] [Google Scholar]
- 14.Rossi M, Rebonato A, Greco L, Citone M, David V. Endovascular exclusion of visceral artery aneurysms with stent-grafts: technique and long-term follow-up. Cardiovasc Intervent Radiol. 2008;31(01):36–42. doi: 10.1007/s00270-007-9167-6. [DOI] [PubMed] [Google Scholar]
- 15.Venturini M, Marra P, Colombo M. Endovascular treatment of visceral artery aneurysms and pseudoaneurysms in 100 patients: covered stenting vs transcatheter embolization. J Endovasc Ther. 2017;24(05):709–717. doi: 10.1177/1526602817717715. [DOI] [PubMed] [Google Scholar]
- 16.Stampfl U, Sommer C-M, Bellemann N. The use of balloon-expandable stent grafts for the management of acute arterial bleeding. J Vasc Interv Radiol. 2012;23(03):331–337. doi: 10.1016/j.jvir.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 17.Venkatesh S K, Kumar S, Baijal S S, Phadke R V, Kathuria M K, Gujral R B. Endovascular management of pseudoaneurysms of the splenic artery: experience with six patients. Australas Radiol. 2005;49(04):283–288. doi: 10.1111/j.1440-1673.2005.01466.x. [DOI] [PubMed] [Google Scholar]
- 18.Xin J, Xiao-Ping L, Wei G. The endovascular management of splenic artery aneurysms and pseudoaneurysms. Vascular. 2011;19(05):257–261. doi: 10.1258/vasc.2011.oa0289. [DOI] [PubMed] [Google Scholar]
- 19.Loffroy R, Guiu B, Cercueil J-P. Transcatheter arterial embolization of splenic artery aneurysms and pseudoaneurysms: short- and long-term results. Ann Vasc Surg. 2008;22(05):618–626. doi: 10.1016/j.avsg.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 20.Tsao J W, Marder S R, Goldstone J, Bloom A I. Presentation, diagnosis, and management of arterial mycotic pseudoaneurysms in injection drug users. Ann Vasc Surg. 2002;16(05):652–662. doi: 10.1007/s10016-001-0124-6. [DOI] [PubMed] [Google Scholar]
- 21.Corey M R, Ergul E A, Cambria R P. The presentation and management of aneurysms of the pancreaticoduodenal arcade. J Vasc Surg. 2016;64(06):1734–1740. doi: 10.1016/j.jvs.2016.05.067. [DOI] [PubMed] [Google Scholar]
- 22.Dave B, Sharma A, Kwolek C, Demoya M, Wicky S, Kalva S. Percutaneous transcatheter arterial embolization of inferior pancreatico-duodenal artery aneurysms associated with celiac artery stenosis or occlusion. Catheter Cardiovasc Interv. 2010;75(05):663–672. doi: 10.1002/ccd.22395. [DOI] [PubMed] [Google Scholar]
- 23.Tétreau R, Beji H, Henry L, Valette P-J, Pilleul F. Arterial splanchnic aneurysms: presentation, treatment and outcome in 112 patients. Diagn Interv Imaging. 2016;97(01):81–90. doi: 10.1016/j.diii.2015.06.014. [DOI] [PubMed] [Google Scholar]
- 24.Vittoria De Martini I, Pfammatter T, Puippe G, Clavien P A, Alkadhi H. Frequency and causes of delayed diagnosis of visceral artery pseudoaneurysms with CT: lessons learned. Eur J Radiol Open. 2020;7:100221. doi: 10.1016/j.ejro.2020.100221. [DOI] [PMC free article] [PubMed] [Google Scholar]


