Abstract
BACKGROUND:
With the development of new technologies and the changing patient profiles, cytopathology departments receive increasing numbers of adrenal gland cytology specimens. In this study, the authors analyzed archival adrenal gland cytology cases and attempted to implement a diagnostic reporting system.
DESIGN:
Retrospective electronic medical record search was performed for adrenal gland cytology specimens in seven tertiary care centers. The cytology diagnoses were grouped in 7 categories: nondiagnostic, nonneoplastic, benign adrenal cortical elements (BACE), primary neoplasm of noncortical origin (NONC), atypia of undetermined significance (AUS), suspicious for malignancy (SM), and malignant (MAL). If available, histopathology results of concurrent and/or follow-up biopsies and/or resections were documented.
RESULTS:
A total of 473 adrenal gland cytology cases were included. BACE cases comprised 21.8%, whereas MAL cases were 57.5% of all cases. For BACE and MAL categories, there were 100% and 98.9% correlation, respectively, in the cases with histopathology follow-up. Six of 10 NONC cases had histopathology diagnoses and there were 3 pheochromocytomas and 3 schwannomas. Twenty-one AUS cases had histology follow-up and 10 (47.6%) of them were malignant. Six cases of SM had histopathology follow-up, and all of them were malignant on the follow-up.
CONCLUSIONS:
The authors propose a 7-tier diagnostic scheme for adrenal gland cytology. The risk of malignancy was 98.9% in MAL cases (87/88) in the cohort. The only case with discordance was reported as “adrenal cortical adenoma with marked atypia”’ on resection. There was no difference between endoscopic ultrasound-guided and percutaneous methods. Further studies are needed to validate and make this approach universal.
Keywords: adrenal gland, categorization, cytology, fine-needle aspiration, reporting system
INTRODUCTION
The adrenal glands are 1 of the natural locations for a number of commonly seen metastatic malignancies such as carcinoma of the lung, colon, renal cell carcinoma, and melanoma.1–4 With the advances in targeted cancer therapies and changing patient profiles, the role of adrenal gland cytology has been evolving toward diagnosing metastatic tumors of extra-adrenal origin and ensuring that adequate material is collected for molecular analyses. On the other hand, adrenal gland cytology has been performed in increasing numbers to investigate adrenal masses with indeterminate radiologic features that are found incidentally in patients who undergo routine imaging studies.5
The first report from a series of adrenal fine-needle aspirations (FNAs) came from Katz et al,6 where they reported 22 cases of adrenal FNAs performed from 1976 to 1981. They found adrenal FNA to be especially helpful and showing a high accuracy in patients with metastatic neoplasms from nonadrenal sites. Since then, a lot has changed, including the ability to identify smaller lesions radiographically and the implementation of endoscopic ultrasound (EUS)-guided FNA technique. In addition, triage of the material obtained on FNA needs to meet adequacy criteria for subsequent molecular testing techniques.
Furthermore, in the recent years, we have seen standardized reporting systems like The Bethesda System for Reporting Cervical Cytology, The Bethesda System for Reporting Thyroid Cytology, The Paris System for Reporting Urinary Cytology, and The Milan System for Reporting Salivary Gland Cytopathology reach a great success in universally standardizing the criteria and terminology for reporting relevant cytology specimens.7–10 The application of these standardized reporting systems has been shown to reduce high rates of indeterminate diagnoses, increase sensitivity, and accurately stratify the risk of malignancy.11 Additionally, they improve overall effectiveness of FNA by “creation of a uniform and internationally accepted reporting systems, improved communication between pathologists and clinicians, facilitation of cytohistologic correlation, enhanced sharing of data between institutions, and promotion of research related to FNA diagnosis.”12
However, for the adrenal gland cytology, there are very few reports with a large number of cases to create a basis for a standardized reporting system. Re-defining the field of cytology, Dr. Ed Cibas13 has proposed the term “minimally invasive pathologic diagnosis” to better describe what cytology is. A number of advantages (eg, minimally invasive nature, less frequent and less severe complications, etc.) and limitations (eg, small amount of tissue and/or sample, lack of evaluation of surrounding structures, etc.) of adrenal cytology are also applicable to core biopsy specimens. Therefore, we believe a classification system for adrenal gland cytology would also be useful in the diagnosis and reporting of adrenal gland core biopsy specimens.
In this study, our aim was to add 1 of the largest case series of adrenal gland cytology to the literature and to investigate if a standardized system of reporting along with associated risk of malignancy could be formulated based on our experience from a large multi-institutional study.
MATERIALS AND METHODS
After approval by the institutional review boards, a retrospective electronic medical record search was performed for adrenal gland cytology specimens from 1995 to 2020 at 7 tertiary care academic medical centers. The patients’ demographics, clinical histories, procedure types, cytology diagnosis, presence of cell blocks, immunohistochemical and other ancillary studies done on the cell blocks, and histopathology diagnosis (concurrent or follow-up biopsy or resection) of the adrenal lesions were tabulated. Various methods of specimen preparation (including conventional smears, liquid-based cytology [ThinPrep 5000 method; Hologic Co, Marlborough, Massachusetts], cytospins, and cell blocks) and staining (including Diff-Quik staining on air-dried slides for ROSE and Papanicolaou staining on conventional slides fixed with an alcohol-based fixative) were used according to the different protocols and preferences of each institution.
The cytology specimens included adrenal gland FNA specimens from all except 1 institution. This institution’s workflow was to perform rapid on-site evaluation (ROSE) on the adrenal gland core biopsy touch prep slides. The ROSE diagnoses were considered as the cytology diagnosis for the cases included from this institution, and all the cases automatically had a concurrent core biopsy specimen.
In their recent study, Mustafa et al14 used a reporting system similar to The Milan System for Reporting Salivary Gland Cytopathology for adrenal cytology and reached a stratified risk of malignancy for their diagnostic categories. However, we believe this classification has a couple of issues: 1) overlap between pheochromocytoma and adrenal cortical nodule and/or adenoma and/or carcinoma cases in neoplasm-uncertain malignant potential category, and 2) presumption that benign adrenal cortical cells and adrenal cortical nodule and/or adenoma are cytologically distinguishable. Therefore, we formulated a diagnostic classification approach, taking these issues into consideration.
The cytology diagnoses were grouped using a 7-tier diagnostic classification based on the original cytology reports. The cytology reports were collected in 1 location. Two pathologists (LT and GAB) separately went over the reports and each case was placed into 1 of 7 categories. For the cases with discrepancy, the 2 pathologists had a second session where cytology reports and selected slides were reviewed together and an agreement was reached.
The diagnostic categories were as following: I) nondiagnostic (UNSAT); II) nonneoplastic (NN); III) benign adrenal cortical elements (BACE) (Figure 1; IV) primary neoplasm of noncortical origin (NONC) ( Figure 2; V) atypia of undetermined significance (AUS); VI) suspicious for malignancy (SM); and VII) malignant (MAL). The risk of malignancy for each category was calculated. See Table 1 for the diagnostic criteria of each category (Figure 3).
Figure 1.
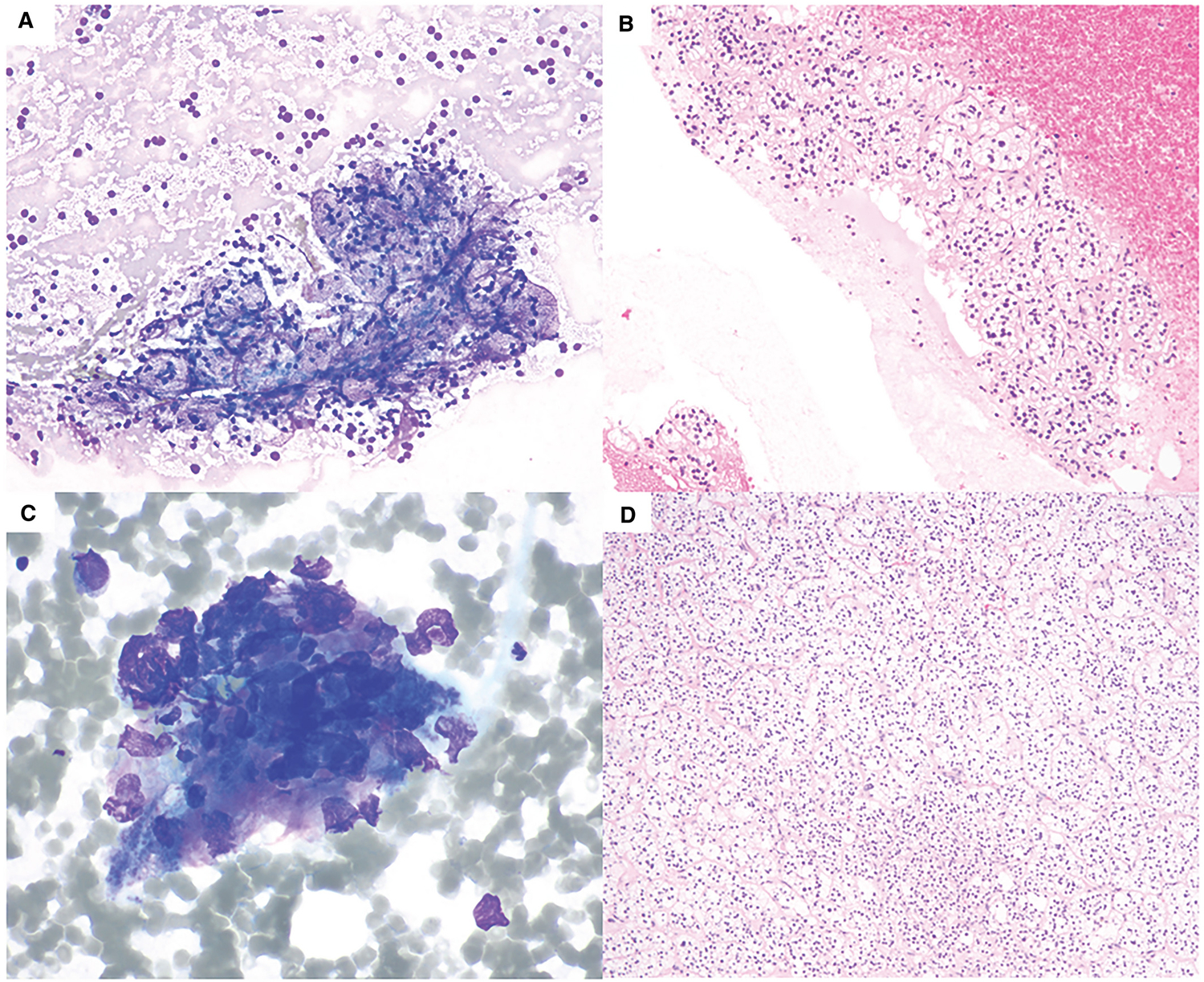
Fine-needle aspiration of adrenal cortical adenoma. (A) Benign adrenal cortical elements with “frothy” background and bland-appearing naked nuclei. Intact cells have abundant clear foamy cytoplasms (Diff-Quik, 200×). (B) Cell block of this case shows nests of bland-appearing cells with abundant clear cytoplasm (H & E, 200×). (C) A case of atypia of undetermined significance (AUS) with crushed cells with occasional hyperchromatic nuclei (Diff-Quik, 600×). (D) The subsequent adrenalectomies of both cases showed adrenal cortical adenoma. The histological section shown is from the AUS case (H & E, 100×).
Figure 2.
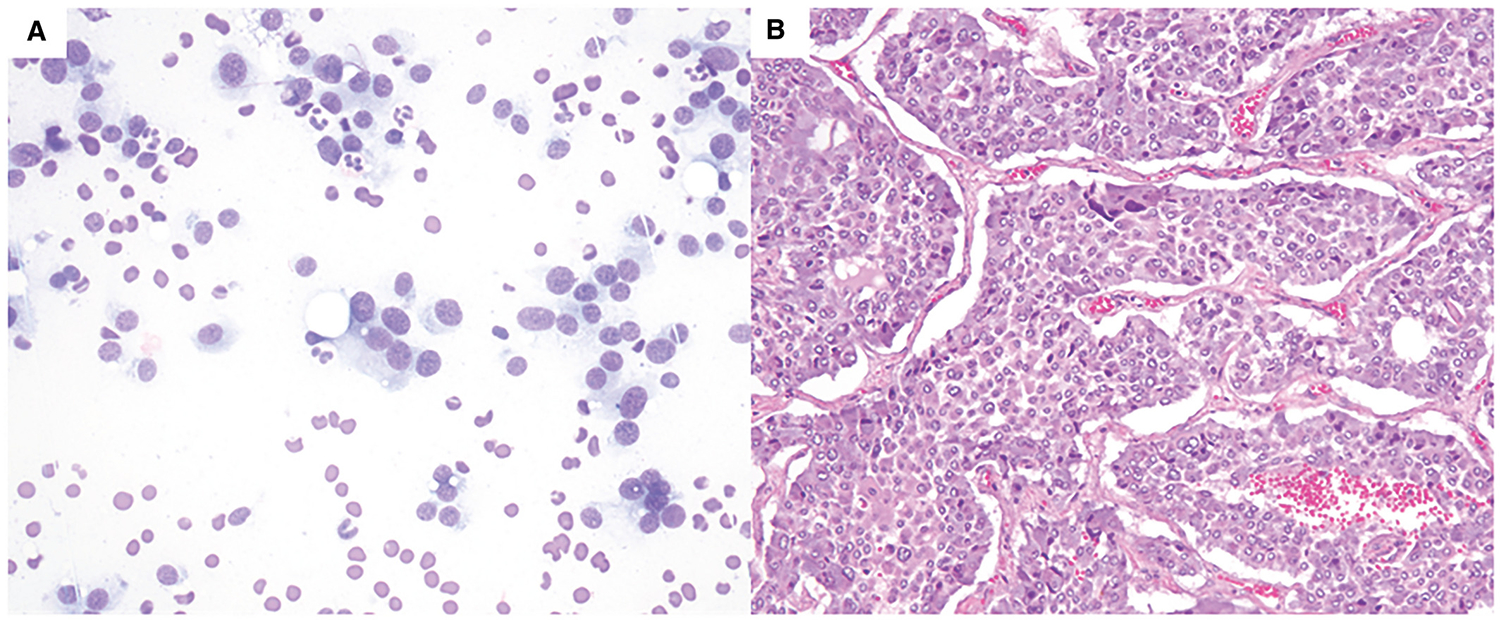
Pheochromocytoma. (A) Primary neoplasm of noncortical origin with polygonal cells with abundant cytoplasm, plasmacytoid nuclei with fine chromatin. (Diff-Quik, 400×). (B) The subsequent adrenalectomy shows pheochromocytoma (H & E, 200×).
TABLE 1.
Definitions of Diagnostic Categories
| Diagnostic Category |
Definition |
|---|---|
|
| |
| I. UNSAT | Insufficient diagnostic material (eg, lack of adrenal cortical cells or abnormal elements) |
| II. NN | Nonneoplastic elements (eg, inflammation [acute, chronic, granulomatous], infection, pseudocyst, etc) |
| III. BACE | Bland appearing adrenal cortical cells; no mitosis, no necrosis; correlates with normal adrenal cortex, adrenal cortical hyperplasia, or adrenal cortical adenoma |
| IV. NONC | Features suggestive of a primary noncortical neoplasm (eg, pheochromocytoma, ganglioneuroma, nerve sheath tumors, etc); excludes overt primary malignant tumors (eg, neuroblastoma, malignant peripheral nerve sheath tumor, etc) |
| V. AUS | Cytologic atypia that does not fulfill the criteria for the other categories; specimens have low cellularity and/or lack qualitative evidence of a malignancya |
| VI. SM | Findings highly suggestive of but not definite for malignancy due to lack of quantitative evidencea |
| VII. MAL | Specimens with high cellularity, marked atypia, frequent mitoses, and necrosisa |
Abbreviations: AUS, atypia of undetermined significance; BACE, benign adrenal cortical elements; MAL, malignant; NN, nonneoplastic; NONC, primary neoplasm of noncortical origin; SM, suspicious for malignancy; UNSAT, nondiagnostic.
Whether the cells are of adrenal cortical origin should be reported if possible by morphology and/or ancillary studies.
Figure 3.
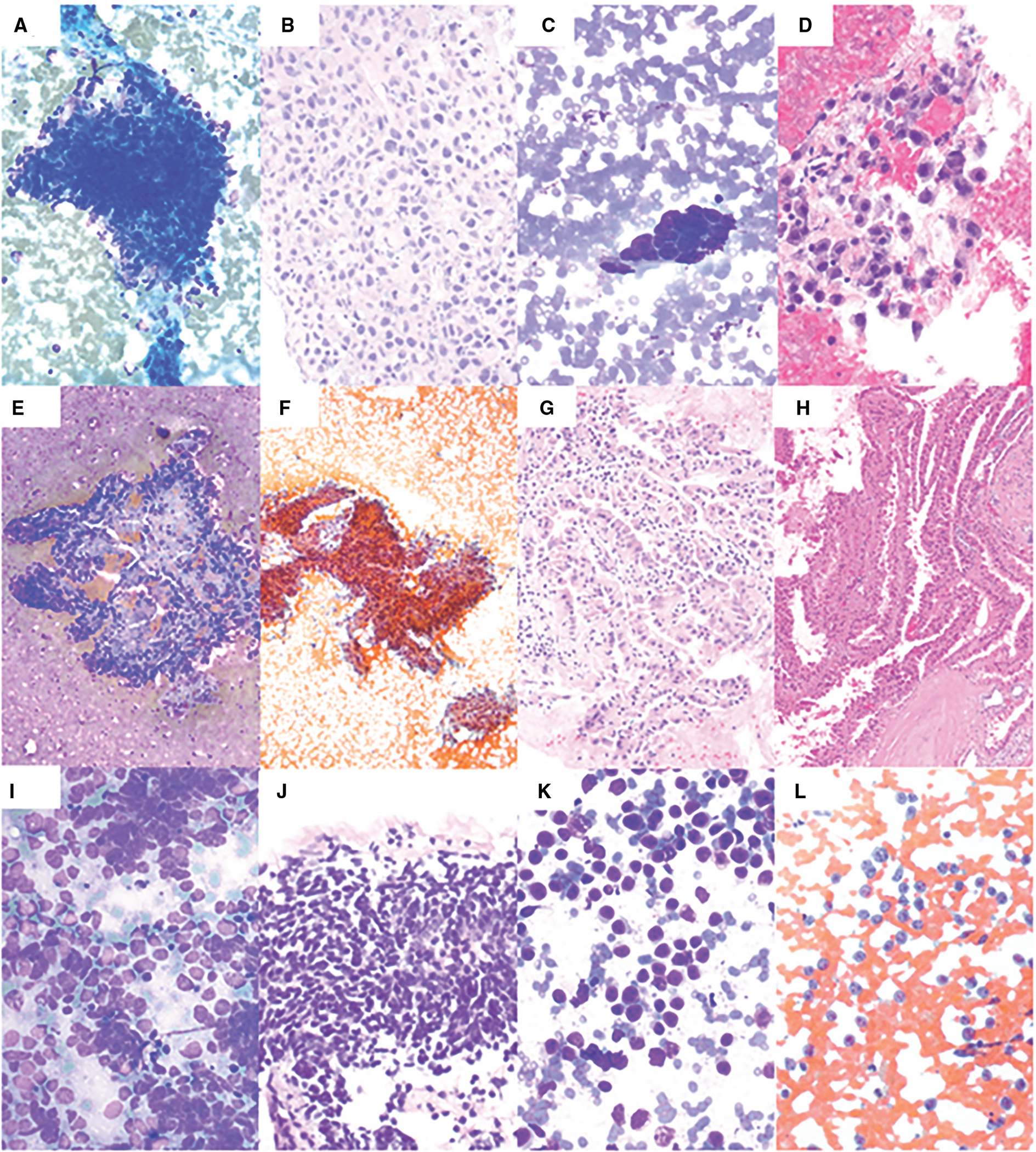
Fine-needle aspiration of malignant neoplasms. (A) Adrenal cortical carcinoma showing nuclear overlapping, hyperchromasia, and nuclear pleomorphism (Diff-Quik, 200×). (B) Cell block shows pleomorphic cells with pale eosinophilic cytoplasm (H & E, 400×). (C) Poorly differentiated adenocarcinoma of the esophagus showing cohesive epithelial cells with nuclear pleomorphism (Diff-Quik, 400×). (D) Cell block shows pleomorphic epithelial cells forming a poorly defined gland and/or cluster (H & E, 400×). (E) Papillary renal cell carcinoma showing epithelial cells forming papillary structures with fibrovascular core (Diff-Quik, 100×). (F) Papanicolaou (PAP) stain shows epithelial cells forming papillary structures (PAP, 100×). (G) Cell block shows epithelial cells with abundant eosinophilic cytoplasm forming papillary structures (H & E, 200×). (H) The subsequent radical nephrectomy shows papillary renal cell carcinoma, type-2 (H & E, 100×). (I) Small cell carcinoma showing epithelioid cells with scant cytoplasm, fine chromatin, nuclear molding, and smearing artifact (Diff-Quik, 400×). (J) Cell block shows hyperchromatic epithelioid cells with crushing artifacts (H & E, 400×). (K) Diffuse large B-cell lymphoma showing discohesive, monotonous, large cells with scant cytoplasm (Diff-Quik, 400×). (L) PAP stain shows that these cells have occasional nucleoli (PAP, 400×).
RStudio software (version 3.5.0, The R Foundation for Statistical Computing, Boston, Massachusetts) was used for analytical calculations. The χ2 test is performed to examine the difference between the observed frequencies. P values of less than .05 were considered to be statistically significant.
RESULTS
A total of 473 adrenal gland cytology cases from 197 women and 276 men (male:female ratio 1.3:1) were included in the study. The age of the patients ranged from 1 to 102 years, with a median age of 66 years for both females and males. There was a left-side predominance in our cohort (297 left, 159 right). EUS-guided approach was performed in 194 cases (42%) whereas percutaneous (computed tomography- or ultrasound-guided) approaches were performed in 269 cases (58%). Overall adequacy rate was 92.6%, with 91.4% for percutaneous FNAs (246/269), and 93.8% for EUS-guided FNAs (182/194) (P > .05).
Table 2 summarizes the case distribution and risk of malignancy for each diagnostic category.
TABLE 2.
Reporting Rates and Risk of Malignancies of Diagnostic Categories
| Diagnostic Category | No. (%) | Histology | Risk of Malignancy (%) | Malignant Tumors on Histology |
|---|---|---|---|---|
|
| ||||
| I. UNSAT | 35 (7.4) | 10 | 30 | 1 metastatic dedifferentiated liposarcoma, 1 metastatic clear cell RCC, 1 metastatic leiomyosarcoma |
| II. NN | 12 (2.5) | 1 | 0 | N/A |
| III. BACE | 103 (21.8) | 21 | 0 | N/A |
| IV. NONC | 10 (2.1) | 6 | 0 | N/A |
| V. AUS | 31 (6.6) | 21 | 47.6 | 4 adrenal cortical carcinoma, 2 metastatic clear cell RCC, 2 metastatic adenocarcinoma of the lung, 1 metastatic leiomyosarcoma, 1 posttransplant lymphoproliférative disorder |
| VI. SM | 10 (2.1) | 6 | 100 | 3 diffuse large B-cell lymphoma, 1 metastatic adenocarcinoma of the pancreas, 1 metastatic clear cell RCC, 1 adrenal cortical carcinoma |
| VII. MAL | 272 (57.5) | 88 | 98.9 | See Table 3 |
Abbreviations: AUS, atypia of undetermined significance; BACE, benign adrenal cortical elements; MAL, malignant; NN, nonneoplastic; NONC, primary neoplasm of noncortical origin; RCC, renal cell carcinoma; SM, suspicious for malignancy; UNSAT, nondiagnostic.
The cases were grouped using a 7-tier diagnostic classification, and the case distribution was as follows: UNSAT (n = 35, 7.4%), NN (n = 12, 2.5%), BACE (n = 103, 21.8%), NONC (n = 10, 2.1%), AUS (n = 31, 6.6%), SM (n = 10, 2.1%), and MAL (n = 272, 57.5%). There were 153 cases with histology diagnosis (concurrent or follow-up biopsy or resection) out of 473 cases. The risk of malignancy for each category was: UNSAT, 30%; NN, 0%; BACE, 0%; NONC, 0%; AUS, 47.6%; SM, 100%; and MAL, 98.9%.
Ten out of 35 UNSAT on cytology had histologic diagnosis, of which 3 were diagnosed as malignant on histology (1 metastatic dedifferentiated liposarcoma, 1 metastatic clear cell renal cell carcinoma, and 1 metastatic leiomyosarcoma). There was also 1 case each of pheochromocytoma, ganglioneuroma, and myelolipoma on the histology follow-up. The remaining 4 were diagnosed either nondiagnostic or benign adrenal cortical tissue on the biopsy/resection (Figure 4).
Figure 4.
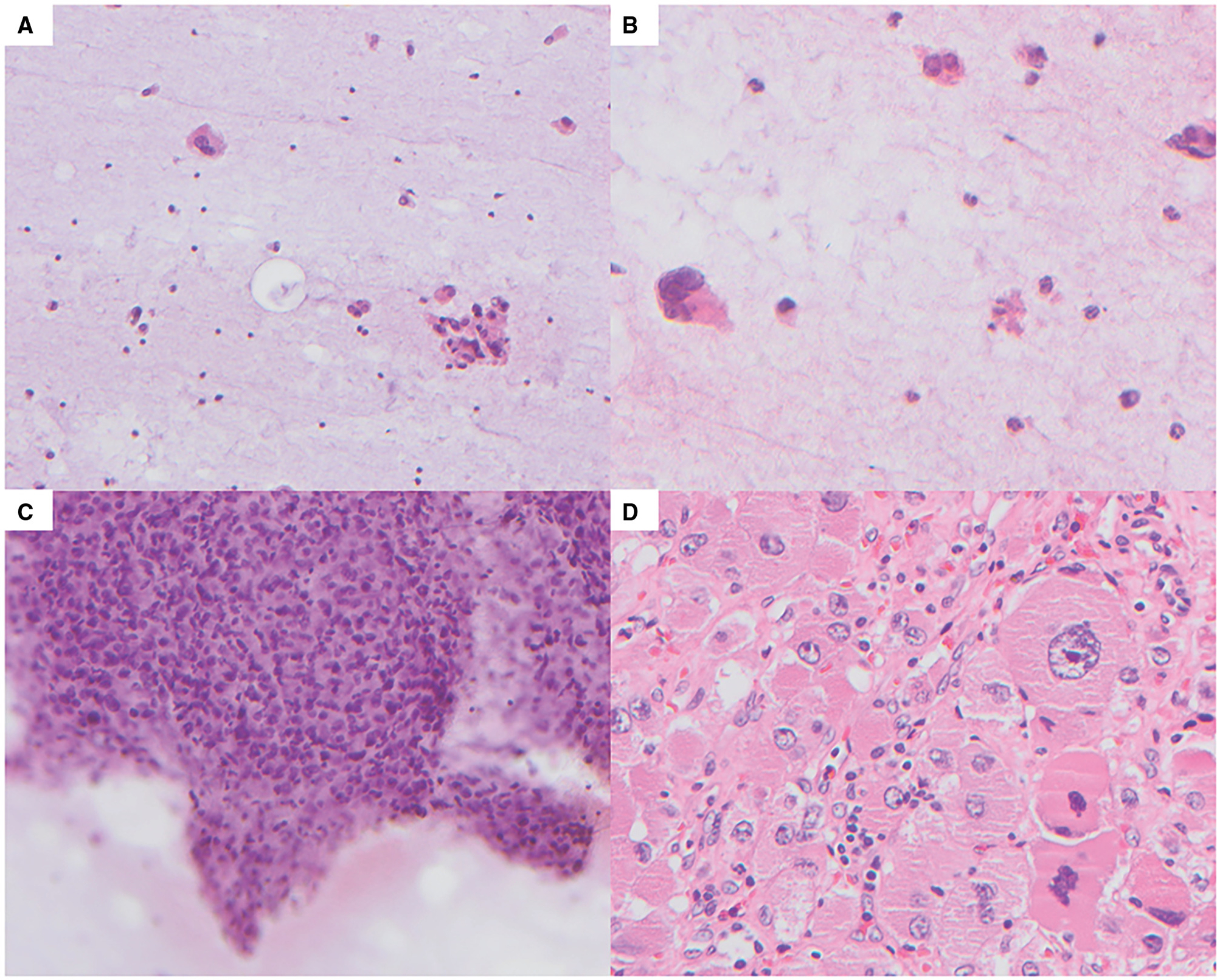
False-positive case. (A) Areas with low cellularity and a highly atypical cell with irregular nucleus (Papanicolaou [PAP], 200×). (B) Areas with low cellularity and occasional highly atypical cells with hyperchromatic irregular nuclei (PAP, 400×). (C) Hypercellular cluster with nuclear overlapping, hyperchromasia, and moderate nuclear pleomorphism (PAP, 400×). (D) The subsequent adrenalectomy shows adrenal cortical adenoma with marked endocrine atypia (H & E, 400×).
Only 1 case out of 12 NN cases on cytology had a follow-up histology diagnosis, which was reported as calcified adrenal pseudocyst.
Cytologic diagnoses of benign adrenal cortical tissue, adrenal cortical hyperplasia, and/or adenoma were classified as BACE. Twenty-one out of 103 BACE cases had histology diagnosis, of which all were benign or insufficient on histology. The BACE cases included 14 cases of adrenal cortical tissue, 3 cases of adrenal cortical adenoma, and 4 cases with insufficient material on follow-up histology.
Ten cases of NONC were identified in our cohort, of which 6 had a histology diagnosis and showed 3 cases of pheochromocytoma and 3 cases of schwannoma.
Twenty-one out of 31 AUS cases had histology diagnosis, of which 10 malignant tumors were identified (4 cases of adrenal cortical carcinoma, 2 cases of metastatic clear cell renal cell carcinoma, 2 cases of metastatic adenocarcinoma of the lung, 1 case of metastatic leiomyosarcoma, and 1 case of posttransplant lymphoproliferative disorder). There were also 7 cases of adrenal cortical adenoma and 1 case each of myelolipoma and cavernous hemangioma. The remaining 2 cases had necrosis, chronic inflammation, epithelioid histiocytes, and fibroconnective tissue on the biopsy.
There were 10 cases of SM in our cohort, of which 6 had a concurrent and/or follow-up histology and all were malignant tumors (3 cases of diffuse large B-cell lymphoma, 1 case of metastatic adenocarcinoma of the pancreas, 1 case of metastatic clear cell renal cell carcinoma, and 1 case of adrenal cortical carcinoma).
Out of 272 MAL cases on cytology, 88 had histology diagnosis, of which 87 were confirmed as malignant tumors. The only case that was discordant on histology was diagnosed as adrenal adenoma with marked endocrine atypia on resection. Of the cases with histology confirmation, 8 (9.2%) were primary malignancies and 79 (90.8%) were metastatic tumors.
There were a total of 286 malignant tumors identified in our cohort, diagnosed by cytology and/or surgical pathology specimens. Of these, 15 (5.2%) were primary adrenal malignancies and 271 (94.8%) were metastatic tumors including 83 cases of lung, 44 cases of genitourinary (kidney, ureter, bladder, prostate, urethra, and testis), 25 cases of gastrointestinal and hepatobiliary tract, 16 cases of hematopoietic, 12 cases of melanoma, 7 cases of Mullerian tract (ovary, fallopian tube, endometrium, and cervix), 4 cases of breast, and 3 other origin. The remaining 77 cases were diagnosed as “metastatic carcinoma” and no further classification could be made in these cases (Table 3).
TABLE 3.
Distribution of Sites of Origin for Metastatic Malignancies and Primary Tumor Diagnoses
| Metastatic Malignancies | No. (%) | Primary Tumors | No. (%) |
|---|---|---|---|
|
| |||
| Site of origin | Benign | 22 (59.5) | |
| Lung | 83 (30.6) | Cortical adenoma | 11 (29.7) |
| GU tract (kidney, ureter, bladder, prostate, urethra, and testis) | 44 (16.2) | Pheochromocytoma | 4 (10.8) |
| GI and hepatobiliary tract | 25 (9.1) | Schwannoma | 3 (8.1) |
| Hematopoietic | 16 (5.9) | Myelolipoma | 2 (5.4) |
| Melanoma | 12 (4.4) | Ganglioneuroma | 1 (2.7) |
| Müllerian tract (ovary, fallopian tube, endometrium, and cervix) | 7 (2.6) | Cavernous hemangioma | 1 (2.7) |
| Breast | 4 (1.5) | Malignant | 15 (40.5) |
| Other | 3 (1.1) | Cortical carcinoma | 12 (3.2) |
| Metastatic carcinoma, not otherwise specified | 77 (28.4) | Neuroblastoma | 2 (5.4) |
| Primary small blue round cell tumor | 1 (2.7) | ||
Abbreviations: GI, gastrointestinal; GU, genitourinary.
A total of 277 of the cases had a cell block prepared. Immunohistochemical and other ancillary studies were performed on cell blocks in 122 cases (44%), malignant cases being the most common (n = 96). Most commonly used immunohistochemical studies were thyroid transcription factor-1 (n = 60), inhibin (n = 56), and cytokeratin 7 (n = 46).
DISCUSSION
The management of adrenal gland masses often requires a multidisciplinary approach, because biochemical workup, image analysis, and evaluation of clinical symptoms and signs are necessary components of the process. Although parallel clinical and radiologic assessments can accurately diagnose benign adrenal lesions in increasing numbers, numerous adrenal masses remain indeterminate by these assessments due to the drastically increased use of diagnostic imaging over the last decades.15 Additionally, changing patient demographics and targeted therapeutic options for metastatic diseases caused increasing sampling from the possible metastatic foci of various primary tumors.16,17 As a minimally invasive procedure, adrenal gland cytology provides valuable information to guide the management by causing minimal to no harm to the patients.18 In this study, we showed that adrenal gland cytology had a 92.6% overall adequacy rate, with no statistical difference between percutaneous and EUS-guided approaches. Our findings are in accordance with multiple prior studies that reported adequacy rates between 72% and 95% with no difference between percutaneous and EUS-guided approaches (Table 4).6,14,18–23
TABLE 4.
Previously Reported Series of Adrenal Gland Cytology
| Year | No. of Cases |
Nondiagnostic, No. (%) |
Malignant, No. (%) |
Malignant Origin (Adrenal/Other) |
False-Positive | False-Negative | |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Katz et al6 | 1984 | 22 | NA | 13 (59) | 6/7 | 0 | 2 |
| Wadih et al19 | 1992 | 50 | 6 (12) | 27 (54) | 4/23 | 0 | 0 |
| de Agustin et al18 | 1999 | 169 | 48 (28) | 56 (33) | 0/56 | 1 | 0 |
| Tirabassi et al20 | 2012 | 50 | 11 (22) | 18 (36) | 4/14 | 0 | 3 |
| Novotny et al17 | 2019 | 198 | 15 (8) | 105 (53) | 0/105 | 0 | 2 |
| Martin-Cardona et al16 | 2019 | 204 | 10 (5) | 122 (60) | NA | NA | NA |
| Point du Jour et al15 | 2020 | 139 | 12 (9) | 77 (55) | 5/72 | 0 | 1 |
| Mustafa et al21 | 2020 | 484 | 67 (14) | 199 (41) | 5/194 | 0 | 1 |
| This study | 473 | 35 (7) | 272 (58) | 11/261 | 1 | 0 | |
Abbreviation: NA, not available.
The application of standardized reporting systems provided a more uniform and risk assessment-focused approach and improved the communication between clinicians and cytopathologists in thyroid, salivary gland, and urine cytology.11,24,25 Currently, there is no standardized reporting system for adrenal gland cytology. Creating a cytodiagnostic classification requires incorporation of the common entities seen in the site, the extent of what could be determined on a cytologic specimen, and the clinical implications of the diagnostic categories. In an attempt to standardize reporting, we formulated a diagnostic classification system for adrenal gland cytologic specimens and possibly for core needle biopsies as a number of the limitations and advantages of adrenal gland cytology are also applicable to core biopsy specimens.
By far the most common clinical indication for adrenal gland cytology is to confirm a metastatic lesion in the context of an extra-adrenal malignancy, as reflected in our study, and adrenal gland cytology has been shown to be successful in distinguishing metastatic tumors from benign adrenal nodules.26 Similar to the traditional data, in our study, the most common cytology diagnosis was “malignant” mostly composed of metastatic tumors (90.8% of confirmed malignant diagnoses). In line with the prior publications, the most common metastatic tumors were of lung and genitourinary origin in our study, comprising 61.2% of all metastatic tumors.14,18 Out of 272 malignant diagnoses on cytology, 88 had a concurrent or follow-up histology and 87 of them were confirmed. The only case with discordance was reported “adrenal cortical adenoma with marked atypia” on resection.
Another challenge cytopathologists face in adrenal gland cytology is cortical elements with “gray zone” features. Bland-appearing round naked nuclei with little variation in size, absence of necrosis and mitosis, and a “frothy” background would seal the diagnosis for a benign cytology. At the opposite end of the spectrum, the presence of isolated cells with intact cytoplasm, marked nuclear atypia, frequent mitoses, and necrosis would point to a poorly differentiated adrenocortical carcinoma in a patient with an isolated adrenal tumor. However, differentiating an adrenal cortical adenoma from a well-differentiated adrenal cortical carcinoma could be very challenging when the findings are equivocal.27 This creates a need for a uniform language in reporting such lesions.
In this study, cytologic diagnosis of benign adrenal cortical tissue and adrenal cortical hyperplasia and/or adenoma were grouped together because our group believes there are no reliable cytomorphological features to distinguish an adrenal cortical hyperplasia and/or adenoma from a normal adrenal cortical tissue. Therefore, we propose a BACE category for the cytologically bland appearing adrenal cortical epithelial cells with no mitosis or necrosis. Mild nuclear atypia can be seen even in normal adrenal cortical tissue; therefore, we recommend a restrained approach to nuclear atypia in adrenal cortical elements before taking the case to the “indeterminate” diagnostic categories. In our study, 21 out of 103 BACE cases had a histology diagnosis and all were benign or insufficient on histology.
As much as we try to avoid it, the “indeterminate” diagnoses are virtually inevitable in cytologic diagnosis. There are essentially 2 situations, based on qualitative and quantitative factors, where an indeterminate diagnosis is rendered by a cytopathologist: 1) cytologic atypia that does not fulfill the criteria for a malignant diagnosis, and 2) cytologic abnormality that is highly suspicious for malignancy but lacks quantitative evidence. As a result, there are 2 separate indeterminate diagnostic categories in all the standardized cytology reporting systems: AUS and SM.8–10 We also used these categories to better classify the indeterminate cytologic diagnoses in our study and the risk of malignancy in AUS and SM categories were 47.6% and 100%, respectively. However, it is important to note that the data is limited due to the low number of cases. We also believe that it would be ideal to report whether the cells are of adrenal cortical origin if possible by cytomorphology or ancillary studies, because the clinical management differs in cases with primary adrenal masses and in cases with suspected adrenal metastasis of an extra-adrenal primary malignancy.
Thus, we were able to place all cases with adrenal cortical cells of varying cytomorphologic features into 1 of these 4 categories: BACE, AUS, SM, or MAL. This leaves the cases with no and/or scant cellularity and benign adrenal cortical cells along with pertinent findings that do not fit into any of the other categories such as overt acute, chronic, or granulomatous inflammation, microorganisms (fungal, bacterial, etc), and cystic changes at hand. We were again inspired by the prior standardized cytology reporting systems for these occasions and borrowed 2 more diagnostic categories: UNSAT and NN. The risk of malignancy for UNSAT cases in our cohort was 30%. The only NN case with histologic correlation was diagnosed as a pseudocyst.
Pheochromocytoma is 1 of the main clinical differential diagnoses of any adrenal mass. FNA or core needle biopsy of a suspected pheochromocytoma is avoided as a potential hypertensive crisis could be triggered by such procedure.28 Furthermore, combined biochemical and radiological screening for pheochromocytoma has a very high negative predictive value of over 0.95.15 Therefore, these lesions are rarely diagnosed on a core biopsy or cytology, as reflected in our study. There were only 5 pheochromocytoma cases diagnosed in cytology and/or follow-up histology out of 473 cases in this study (1.1% of all cases). However, it is very important to alert the clinical team if such a case is suspected on cytology because a delayed complication such as hematoma is frequent following a biopsy of a pheochromocytoma.29 This especially puts the cytopathology professional who deals with such cases on ROSE in a critical position, where ancillary studies are not in reach. Thus, we propose a NONC category for the cases with a varying composition of polygonal cells and spindle cells that can be suggestive of a pheochromocytoma, ganglioneuroma, or a nerve sheath tumor. In our study, 6 of 10 NONC cases had a histology diagnosis, 3 were reported as pheochromocytoma, and the other 3 were schwannoma. None of the 3 pheochromocytoma cases were proven to be malignant to date.
We believe our approach will improve the language confusion for the indeterminant diagnoses and the communication among cytopathologists at large and clinicians. Using this classification system, we were able to accurately stratify the risk of malignancies for the 2 indeterminant diagnostic categories (AUS, 47.6% and SM, 100%) by rendering these diagnoses only for 8.7% of the cases (AUS, 6.6% and SM, 2.1%). The NONC category allowed us to isolate pheochromocytoma and its differential diagnoses from the other indeterminant cytologic diagnoses. This approach is in concordance with the clinical approach to pheochromocytoma as it is radiologically and biochemically screened out to avoid a biopsy due to the possibly fatal complications of the procedure. However, this process is not able to identify 100% of the pheochromocytomas. When it is biopsied on very rare occasions, it is the cytopathologist’s duty to alert the clinical team about a possible pheochromocytoma by using a clear term that is not intermixed with other “indeterminant” diagnoses.
Our study has several limitations. First, the vast majority of cases were recategorized based on a retrospective review of the cytology reports. If there was a difficulty categorizing a case based on the report, only then, the slides were reviewed. As in all retrospective studies, there was a verification bias in that not all FNA cases had a follow-up core biopsy or resection. In the absence of a resection specimen, the core biopsy was taken as the gold standard, however, we know that core biopsies may also be subjected to sampling error. In addition, because we have not implemented this classification system into our routine practices, we do not have prospective data on its effectiveness.
Overall, we showed adrenal gland cytology has a high adequacy and diagnostic accuracy rate. In our study, there is only 1 case with significant diagnostic discordance where an adrenal cortical adenoma with marked atypia was diagnosed malignant on cytology. The risk of malignancy for nonneoplastic and BACE categories was 0%. With a low indeterminant (AUS and SM) diagnostic rate (8.7%), we were able to stratify the risk of malignancies for the AUS and SM categories, 47.6% and 100%, respectively. We recommend additional prospective studies on adrenal gland FNAs and core biopsies using the proposed classification system to validate the results of this study.
FUNDING SUPPORT
No specific funding was disclosed.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
The authors made no disclosures.
REFERENCES
- 1.Lo CY, van Heerden JA, Soreide JA, et al. Adrenalectomy for metastatic disease to the adrenal glands. Br J Surg. 1996;83:528–531. [DOI] [PubMed] [Google Scholar]
- 2.Kim SH, Brennan MF, Russo P, Burt ME, Coit DG. The role of surgery in the treatment of clinically isolated adrenal metastasis. Cancer. 1998;82:389–394. [PubMed] [Google Scholar]
- 3.Branum GD, Epstein RE, Leight GS, Seigler HF. The role of resection in the management of melanoma metastatic to the adrenal gland. Surgery. 1991;109:127–131. [PubMed] [Google Scholar]
- 4.Beitler AL, Urschel JD, Velagapudi SR, Takita H. Surgical management of adrenal metastases from lung cancer. J Surg Oncol. 1998;69:54–57. [DOI] [PubMed] [Google Scholar]
- 5.Varadarajulu S, Fockens P, Hawes RH. Best practices in endoscopic ultrasound-guided fine-needle aspiration. Clin Gastroenterol Hepatol. 2012;10:697–703. [DOI] [PubMed] [Google Scholar]
- 6.Katz RL, Patel S, Mackay B, Zornoza J. Fine-needle aspiration cytology of the adrenal gland. Acta Cytol. 1984;28:269–282. [PubMed] [Google Scholar]
- 7.Nayar R, Wilbur DC. The Pap test and Bethesda 2014. Cancer Cytopathol. 2015;123:271–281. [DOI] [PubMed] [Google Scholar]
- 8.Cibas ES, Ali SZ. The 2017 Bethesda System for Reporting Thyroid Cytopathology. Thyroid. 2017;27:1341–1346. [DOI] [PubMed] [Google Scholar]
- 9.Rossi ED, Faquin WC, Baloch Z, et al. The Milan System for Reporting Salivary Gland Cytopathology: analysis and suggestions of initial survey. Cancer Cytopathol. 2017;125:757–766. [DOI] [PubMed] [Google Scholar]
- 10.Barkan GA, Wojcik EM, Nayar R, et al. The Paris System for Reporting Urinary Cytology: the quest to develop a standardized terminology. Acta Cytol. 2016;60:185–197. [DOI] [PubMed] [Google Scholar]
- 11.Pastorello RG, Barkan GA, Saieg M. Experience on the use of The Paris System for Reporting Urinary Cytopathology: review of the published literature. J Am Soc Cytopathol. 2021;10:79–87. [DOI] [PubMed] [Google Scholar]
- 12.Rossi ED, Faquin WC. The Milan System for Reporting Salivary Gland Cytopathology (MSRSGC): an international effort toward improved patient care-when the roots might be inspired by Leonardo da Vinci. Cancer Cytopathol. 2018;126:756–766. [DOI] [PubMed] [Google Scholar]
- 13.Cibas ES. We are minimally invasive diagnosis. J Am Soc Cytopathol. 2021;10:113–114. [DOI] [PubMed] [Google Scholar]
- 14.Mustafa M, Cramer HM, Wu HH. Fine-needle aspiration cytology of adrenal lesions classified with the Bethesda-like system: a retrospective study of 484 cases. Diagn Cytopathol. 2020;48:618–622. [DOI] [PubMed] [Google Scholar]
- 15.Sherlock M, Scarsbrook A, Abbas A, et al. Adrenal incidentaloma. Endocr Rev. 2020;41:775–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amir E, Clemons M. Should a biopsy be recommended to confirm metastatic disease in women with breast cancer? Lancet Oncol. 2009;10:933–935. [DOI] [PubMed] [Google Scholar]
- 17.Woodcock DJ, Riabchenko E, Taavitsainen S, et al. Prostate cancer evolution from multilineage primary to single lineage metastases with implications for liquid biopsy. Nat Commun. 2020;11:5070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Point du Jour KS, Alwelaie Y, Coleman A, Tadros T, Aneja R, Reid MD. Adrenal gland fine-needle aspiration: a multi-institutional analysis of 139 cases. J Am Soc Cytopathol. 2021;10:168–174. [DOI] [PubMed] [Google Scholar]
- 19.Martin-Cardona A, Fernandez-Esparrach G, Subtil JC, et al. EUS-guided tissue acquisition in the study of the adrenal glands: results of a nationwide multicenter study. PLoS One. 2019;14:e0216658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Novotny AG, Reynolds JP, Shah AA, et al. Fine-needle aspiration of adrenal lesions: a 20-year single institution experience with comparison of percutaneous and endoscopic ultrasound guided approaches. Diagn Cytopathol. 2019;47:986–992. [DOI] [PubMed] [Google Scholar]
- 21.de Agustín P, López-Ríos F, Alberti N, Pérez-Barrios A. Fine-needle aspiration biopsy of the adrenal glands: a ten-year experience. Diagn Cytopathol. 1999;21:92–97. [DOI] [PubMed] [Google Scholar]
- 22.Wadih GE, Nance KV, Silverman JF. Fine-needle aspiration cytology of the adrenal gland. Fifty biopsies in 48 patients. Arch Pathol Lab Med. 1992;116:841–846. [PubMed] [Google Scholar]
- 23.Tirabassi G, Kola B, Ferretti M, et al. Fine-needle aspiration cytology of adrenal masses: a re-assessment with histological confirmation. J Endocrinol Invest. 2012;35:590–594. [DOI] [PubMed] [Google Scholar]
- 24.Sauter JL, Lehrke H, Zhang X, et al. Assessment of The Bethesda System for Reporting Thyroid Cytopathology. Am J Clin Pathol. 2019;152:502–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rivera Rolon M, Schnadig VJ, Faiz S, Nawgiri R, Clement CG. Salivary gland fine-needle aspiration cytology with the application of the Milan system for risk stratification and histological correlation: a retrospective 6-year study. Diagn Cytopathol. 2020;48:1067–1074. [DOI] [PubMed] [Google Scholar]
- 26.Kindelberger DW, Cibas ES. Evaluating metastatic carcinoma to the adrenal: utility of fine-needle aspiration cytology. Pathol Case Rev. 2005;10:257–262. [Google Scholar]
- 27.Renshaw AA, Cibas ES. Kidney and adrenal gland. In: Cibas ES, Ducatman BS, eds. Cytology: Diagnostic Principles and Clinical Correlates. Elsevier; 2021:506–507. [Google Scholar]
- 28.Baguet JP, Hammer L, Tremel F, Mangin L, Mallion JM. Metastatic phaeochromocytoma: risks of diagnostic needle puncture and treatment by arterial embolization. J Hum Hypertens. 2001;15:209–211. [DOI] [PubMed] [Google Scholar]
- 29.Vanderveen KA, Thompson SM, Callstrom MR, et al. Biopsy of pheochromocytomas and paragangliomas: potential for disaster. Surgery. 2009;146:1158–1166. [DOI] [PubMed] [Google Scholar]


