Abstract
Intestinal fibrosis associated stricture is a common complication of inflammatory bowel disease usually requiring endoscopic or surgical intervention. Effective anti-fibrotic agents aiming to control or reverse intestinal fibrosis are still unavailable. Thus, clarifying the mechanism underpinning intestinal fibrosis is imperative. Fibrosis is characterized by an excessive accumulation of extracellular matrix (ECM) proteins at the injured sites. Multiple cellular types are implicated in fibrosis development. Among these cells, mesenchymal cells are major compartments that are activated and then enhance the production of ECM. Additionally, immune cells contribute to the persistent activation of mesenchymal cells and perpetuation of inflammation. Molecules are messengers of crosstalk between these cellular compartments. Although inflammation is necessary for fibrosis development, purely controlling intestinal inflammation cannot halt the development of fibrosis, suggesting that chronic inflammation is not the unique contributor to fibrogenesis. Several inflammation-independent mechanisms including gut microbiota, creeping fat, ECM interaction, and metabolic reprogramming are involved in the pathogenesis of fibrosis. In the past decades, substantial progress has been made in elucidating the cellular and molecular mechanisms of intestinal fibrosis. Here, we summarized new discoveries and advances of cellular components and major molecular mediators that are associated with intestinal fibrosis, aiming to provide a basis for exploring effective anti-fibrotic therapies in this field. (Gut Liver, Published online March 10, 2023, 2023)
Keywords: Inflammatory bowel diseases, Intestinal fibrosis, Immune system, Creeping fat, Gastrointestinal microbiota
INTRODUCTION
Fibrosis is a dysregulated outcome of wound healing, especially during chronic inflammatory disorders.1 When inflammation is persistent, the severity of the damage may exceed the ability of the affected tissue to completely heal, which then initiates fibrotic response that eventually results in fibrosis.2,3 The gastrointestinal tract is a tubular structure and therefore fibrosis is presented with the narrowing of lumen and intestinal stricture.4
Intestinal stricture is a common complication of inflammatory bowel disease (IBD) including Crohn's disease (CD) and ulcerative colitis (UC).5,6 CD is a transmural disease that can affect the entire gastrointestinal tract, while UC is a superficial inflammatory disease, restricted to the colonic mucosa and submucosa layer.7 At initial diagnosis, at least 10% of CD patients are presented with a fibrostenosis phenotype.8 However, up to 50% of CD patients ultimately progress to stricturing or penetrating complications and 70% of patients require surgery within their life time.5,9 Even though stricture formation is rather infrequent in UC, recent evidence suggested fibrosis occurs in both acute and chronic UC.10 In the past decades, despite the availability and efficacy of biological therapies in IBD, the incidence of intestinal stricture does not achieve a significant reduction.11 This implies that pure anti-inflammatory treatments do not necessarily alleviate the associated fibrosis.
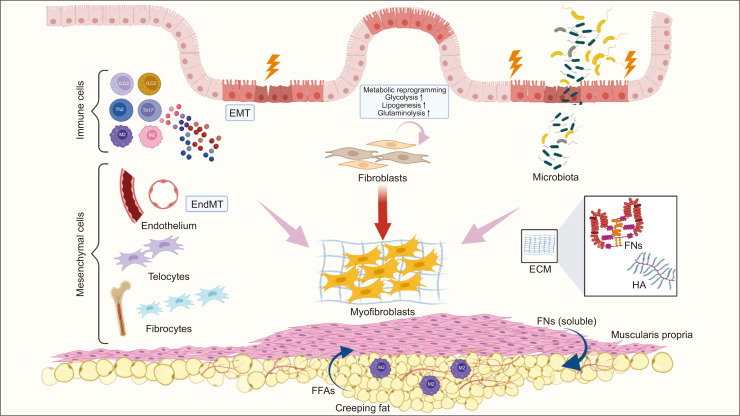
The review would provide a cellular and molecular biology of intestinal fibrosis (Fig. 1). Considering the close association between intestinal fibrosis and stricturing complications, understanding the pathogenesis of intestinal fibrosis is crucial to identify new anti-fibrotic targets for patients with intestinal strictures.
Fig. 1.
Cellular and molecular components implicated in intestinal fibrosis. Intestinal strictures are characterized by extracellular matrix (ECM) accumulation, intestinal muscularis propria thickening, and mesenteric fat wrapping. Myofibroblasts, the major source of ECM production, can originate from various types of mesenchymal cells. Immune cells contribute to persistent myofibroblasts proliferation and activation by secreting abundant cytokines. In addition, inflammatory-independent factors including gut microbiota, ECM interaction, creeping fat and metabolic reprogramming are involved in fibrosis formation.
EMT, epithelial-mesenchymal transition; EndMT, endothelial-mesenchymal transition; FFAs, free fatty acids; FNs, fibronectins; HA, hyaluronan.
CELLULAR MECHANISMS OF FIBROSIS
Intestinal fibrosis is driven by multiple cellular compartments including mesenchymal cells and immune cells.12 The histological feature of intestinal stricture is thickening of the muscularis mucosa and muscularis propria owing to the activation and proliferation of mesenchymal cells. Activated mesenchymal cells not only produce matrix components, but also secrete chemokines to recruit cells from the immune system (e.g., macrophages and T cells), thus perpetuating chronic inflammation. Reciprocal interaction between mesenchymal and immune cell populations in the intestine create a unique pro-fibrotic microenvironment, eventually resulting in fibrosis formation.13
1. Mesenchymal cells and mesenchymal progenitors
Intestinal fibrosis results from sustained activation and proliferation of myofibroblasts.14 The activated myofibroblasts, as the final effector cells, can produce extracellular matrix (ECM) proteins and secrete cytokines such as interleukin (IL)-6 and IL-11, which facilitates formation of a fibrogenic milieu.15-17 The majority of myofibroblasts derive from resident fibroblasts and smooth muscle cells (SMCs). However, they can also originate from other cell types like epithelial and endothelial cells, pericytes, bone marrow stem cells and bone marrow-derived circulating fibrocytes.18,19 The various types of cells weave together in the inflamed intestine and contribute to the development of intestinal fibrosis.20
1) Fibroblasts
Fibroblasts are characterized by an elongated or spindle-shaped morphology, which are the most abundant cell type in connective tissue. Their main function is to maintain tissue integrity.21 Fibroblasts can be activated and multiply in response to pro-inflammatory mediators, such as insulin-like growth factor (IGF)-I, fibroblast growth factor, and IL-1β.22 The growth of fibroblasts can also be induced by immune cells or inflammatory cells through a cell-to-cell contact mechanism.23,24 In addition, fibroblasts can migrate to the site of inflammation foci along the concentration gradient of pro-inflammatory cytokines via activation of NF-κB and JAK-STAT signaling pathways.16,25,26 A previous study reported that fibroblasts isolated from inflamed or fibrotic CD tissue, or inflamed UC mucosa exhibited an increased proliferation, when compared with normal tissue.27 Recently, Wohlfahrt et al.28 found that activated fibroblasts could be reprogrammed into resting fibroblasts by pharmacological and genetic inactivation of PU.1, leading to fibrosis regression. In recent years, the advent of single-cell RNA sequencing (scRNA-seq) technology may assist to reveal the heterogeneous functionality of fibroblast populations, which may shed some insights on the fibrotic pathogenesis of fibroblasts.29
2) Myofibroblasts
Myofibroblasts are characterized by the expression of α-smooth muscle actin (α-SMA), with enhanced production of collagen and increased capacity of contraction.30 Although the exact molecular mechanism of myofibroblasts in fibrosis remains incompletely understood, mediators acting on myofibroblasts are clearly demonstrated, including pro-inflammatory cytokines, paracrine and autocrine factors (e.g., IGF-1), and pathogen or damage-associated molecular patterns.31 The activated myofibroblasts initiate fibrotic process in the following ways. Firstly, myofibroblasts secrete ECM components, as well as various cytokines and chemokines, directly or indirectly contributing to the thickening of mesenchymal cell layer.32,33 Secondly, myofibroblasts participate in tissue remodeling through mechanical contractions.34,35 Thirdly, the mechanic contraction of myofibroblasts can activate latent transforming growth factor-β1 (TGF-β1) released from ECM.36 TGF-β1 and its related pathways are major drivers in the process of fibrosis.37 Recently, de Bruyn et al.38 using specimens from the same CD patients has firstly found that primary myofibroblasts isolated from stenotic ileum were phenotypically and functionally (e.g., ECM organization and collagen production) distinct from myofibroblasts isolated from normal and inflamed areas. Specifically, stenotic myofibroblasts can increase tissue stiffness, while suppress the expression of matrix metalloproteinase (MMP)-3 activity, to facilitate fibrosis development.38
3) Smooth muscle cells
SMCs are one of the three interrelated cell phenotypes (the other two being fibroblasts and myofibroblasts).33 SMCs and fibroblasts are derived from the same primitive mesenchymal cells.39 SMCs are regarded as the progenitors of myofibroblasts.39 A dynamic equilibrium exists between SMCs and myofibroblasts phenotypes.39 SMCs can change their phenotypes in response to environmental stimulation.40 A previous study found that SMCs isolated from CD ileum presented alterations in morphology and contractile activity.41 It was demonstrated that SMCs isolated from CD ileum had an overexpression of platelet-derived growth factor (PDGF)-β, which drove the myogenic phenotype switch to synthetic one. The effect of PDGF-β was paralleled to a reduced encoding of contractile genes that were responsible for quiescent smooth muscle.41 SMCs are also able to release significant amounts of IL-6, contributing to inflammatory process.42 Besides, these cells actively contribute to the development of intestinal fibrosis by inducing the production of collagens and MMPs.41 These evidences suggested that the phenomenon of smooth muscle hyperplasia/hypertrophy in CD may be a driving force, rather than simply a passive increase of stricture formation.
4) Epithelial or endothelial-mesenchymal transition
Epithelial or endothelial-mesenchymal transition (EMT or EndMT) represents a dynamic entity where epithelial or endothelial cells transform to mesenchymal cells in response to inflammatory cytokines, oxidative stress and hypoxia.43-45 EMT is implicated in CD-associated fistulas and intestinal fibrosis.46,47 During formation of CD-associated fistulas, intestinal epithelial cells start with the dissociation from the base membrane and then migrate to the lining of the fistula tracts, where they convert to mesenchymal cells.47 In CD-associated intestinal fibrosis, EMT serves as a reservoir that can generate new fibroblasts and consequently result in fibrosis formation.46,48 Results from Iwano et al. 49 study found that approximately 36% of new fibroblast specific protein-1 positive fibroblast cells originated from local EMT in mouse models of liver and renal fibrosis. Frid et al.50 previously demonstrated that endothelial cells could differentiate into SMCs in vitro. Zhang et al. 51 reported that 17% of fibroblasts/myofibroblasts in the fibrotic myocardium were EndMT-derived. Evidence of EndMT can be also detected in colonic tissues from IBD patients as well as patients with radiation-induced proctitis.52 Multiple targeted therapies aiming to inhibit EMT in cancer are already undergoing clinical evaluation.53 Targeting EMT or EndMT may hold therapeutic promise for fibrotic disorders.
5) Telocytes
Telocytes (TCs) are a novel type of interstitial cells characterized by CD34/PDGFRα, and have been demonstrated to be involved in several disorders including CD.54-56 The function of TCs is widely linked with other cells including mast cells, macrophages, myofibroblasts, and fibroblasts.57 Milia et al.55 firstly identified that TCs were distributed in all layers of ileum, from mucosa to subserosa. Comparing normal with fibrotic resected ileal specimens from human, they found that TCs were nearly disappeared in the fibrotic ileum. A previous study showed that disappearance of TCs was accompanied by an increasement of myofibroblasts in UC, suggesting that TCs loss might be associated with the aberrant differentiation of fibroblasts into myofibroblasts.58 However, further studies are needed to elucidate the casual relationship of TC and fibrosis.
6) Fibrocytes
Fibrocytes are bone marrow-derived mesenchymal progenitors, with the features of both hematopoietic (CD34) and fibroblast markers (collagen-I). Fibrocytes play a critical role in fibrotic diseases.59 Previous studies revealed that fibrocytes could be triggered by several inflammatory cytokines.59 Specifically, within four-day following injury, activated fibrocytes typically migrate into injured sites and then participate in fibrotic reactions through a direct way by production of ECM proteins and fibrogenic cytokines, or an indirect way by differentiation into myofibroblasts.60-62 Sazuka et al.61 found that bone marrow-derived fibrocytes were associated with intestinal fibrosis, which was consistent with another study that the frequency of circulating fibrocytes was increased in fibrostenotic CD patients, compared with healthy individuals.63 A recent study has revealed that fibrocytes deposited in inflamed colon can produced tissue inhibitor of metalloproteinase to inhibit degradation of collagen.64 Therefore, circulating fibrocytes may be a therapeutic target of intestinal fibrosis.63
2. Immune cells
A variety of key innate (macrophages) and adaptive (T cell subsets) immune cell types have been well-established in orchestrating the fibrotic microenvironment in intestine. The immune cell skewing in fibrosis niche probably perpetuates inflammation and exacerbates the process of wound healing.65 Here, we will discuss several immune cell subsets, pointing toward novel immune-based therapeutic strategies in fibrosis.
1) Th2 cells
T helper 2 (Th2) cells are hallmarked by the secretion of cytokines IL-4, IL-5, and IL-13, which are responsible for type 2 immune responses.66 The type 2-associated cytokines are actively engaged in wound healing and fibrosis.65 At inflammatory sites, activated innate immune cells, such as group 2 innate lymphoid cells (ILC2) and basophils, are usually the early sources of local cytokines IL-4, IL-5, and IL-13, which trigger the activation and accumulation of Th2 cells.67,68 Th2 cells-derived IL-4 and IL-13 further promote the accumulation and proliferation of ILC2, thus creating a vicious cycle.69 Activated Th2 cells orchestrate the process of tissue fibrosis directly and indirectly by acting on immune or non-immune cells including local M2 macrophages, fibroblasts, endothelial cells and epithelial cells.70,71 Besides, as a well-known opponent of Th1 cells, Th2 cells can reverse the expression levels of Th1-associated anti-fibrotic cytokines such as interferon γ (IFN-γ).72 However, randomized controlled trials showed that blockade of IL-13 to target Th2 responses while administration of IFN-γ to stimulate Th1 responses failed to attenuate pulmonary fibrosis.73-75 Conversely, in a phase II trial, neutralization of IL-4/IL-13 effectively improved early skin fibrosis.76 The inconsistent results suggested targeting Th2 response as a therapeutic strategy for fibrosis requires further investigation.
2) Macrophages
Macrophages are highly heterogeneous and plastic cell populations, and are key regulators of tissue fibrosis in several organs.66,77 Generally, macrophages are classified into two subtypes: M1 macrophages with pro-inflammatory roles, and M2 macrophages with pro-fibrotic properties. The latter is activated by IL-4 and IL-13, and characterized by effects of inflammation resolution and tissue restoration.66 In intestine, STAT6-dependent M2 macrophages promote mucosal repair through activating Wnt signaling pathway.78 Moreover, macrophages from CD patients showed a significant enrichment in the expressions of M2-related as well as fibrotic-related genes, implying that M2 macrophages potentially exacerbated fibrosis formation.79 Results from STAT6 deficient colitis mice showed that the frequency of CD16+ macrophages was enhanced in the damaged mucosa of CD patients with stenotic or penetrating complications, and were also associated with the expression of fibrotic-related markers.80 Blockade of the interactions between inflamed macrophages and stromal cells has been proven to potentially ameliorate aberrant wound repair in zebrafish IBD model.81 Recently, the advanced scRNA-seq has revealed a novel macrophage subgroup, named with CX3CR1+SiglecF+ transitional macrophages, which are abundant in fibrotic niche and exhibit a pro-fibrotic effect in bleomycin-induced lung fibrosis.82 ScRNA-seq will be a promising technique to reveal the cellular heterogeneity of macrophages in fibrosis.
3) Th17 cells
T helper 17 (Th17) cells are characterized by RAR-related orphan receptor γt (ROR-γt) expression and signatured by producing IL-17, IL-21, and IL-22 cytokines,83,84 which have fibrogenic properties. IL-17A, the predominant Th17-assciated cytokines, exerts its fibrotic effects via acting on myofibroblasts and regulating EMT.85-87 In gut, elevated levels of tissue Th17 cells and IL-17 production are observed in patients with intestinal stenosis.87 Recently, Paul et al.88 has showed that IL-17-driven fibrosis is negatively regulated by Itch, whereas Itch deficiency leads to increased expression of collagen-I and α-SMA in response to IL-17 in myofibroblasts. In vitro, IL-17A can dose-dependently induce EMT in intestinal epithelial cells.89 In vivo, IL-17A blockade significantly ameliorates TNBS-induced intestinal fibrosis through enhancing ECM degradation and decreasing pro-fibrotic cytokines production.89,90 Of note, in clinical trials, administration with neither anti-IL-17A monoclonal antibody (secukinumab) nor its receptor monoclonal antibody (brodalumab) is effective in CD patients with stenosis.91,92 Th17 cells expressing IL-22 are also implicated in fibroblast activation, myofibroblast differentiation and ECM gene expression in skin fibrosis.93,94 The fibrotic effects of Th17 cells-derived cytokines are still largely unknown.
4) Innate lymphoid cells
ILCs are a functionally diverse but developmentally related family of innate lymphocytes, with phenotypes and functions having striking similarities to T helper (Th) cells.95 According to cytokine signatures and transcription factors expression, ILCs are divided into three groups.96 Group 1 ILCs (ILC1) subsets share common properties with Th1 cells and express the transcription factor T-bet.97 Group 2 ILCs (ILC2) express the transcription factors RORα and GATA-3, which resembles Th2 cells functionally.98 Group 3 ILCs (ILC3), expressing transcription factor RORγt, are analogous to Th17 cells.99,100 ILC2 can respond rapidly to tissue damage, followed by an increase of Th2-like cytokines.101 An increased frequency of ILC2 has been detected in intestinal tissues from CD patients.102 Lo et al.103 reported that Rorasg/sg bone marrow transplant (BMT) chimeric mice which was a model of ILC2 deficiency was resistant to salmonella-induced intestinal fibrosis, with reduced collagens deposition and fibroblasts accumulation in infected intestinal tissues. Furthermore, restoring ILC3 function in Rorasg/sg BMT mice was able to reestablish the susceptibility to intestinal fibrosis.103 Although the effect of ILCs in intestinal inflammation is explicit, their roles in fibrotic process still require more investigation.
MOLECULAR MECHANISMS OF FIBROSIS
Molecules are messengers of crosstalk between immune and non-immune cells and actively contribute to persistent inflammation.13 Although inflammation is a prerequisite of fibrosis, purely controlling intestinal inflammation cannot hold back the progression of fibrosis.8 This implies that inflammation is not the exclusive driver of fibrosis. In the following section, a detailed discussion concerning inflammation-dependent, but with a focus on inflammation-independent factors of fibrosis, will be reported.
1. Inflammation-dependent molecular mechanisms
Cytokines and chemokines secreted by immune and non-immune cells are orchestrators of sustained inflammatory microenvironment, and are also observed to possess pro-fibrotic effects, which lay foundations for uncovering novel therapeutic targets in fibrotic disorders.142 Several novel cytokines involved in fibrogenesis will be detailly described in this part, and the elaborate profile of cytokines and chemokines are shown in Table 1.
Table 1.
Cytokine and Chemokine Profiles Involved in Intestinal Fibrosis
| Cytokine | Pro- or anti-fibrosis |
Effects on intestinal fibrosis | References |
|---|---|---|---|
| IL-1 | Pro | Induce fibroblasts activation; enhance pro-fibrotic cytokines production; inhibit ECM degradation; enhance collagens expression | 104-106 |
| IL-4 | Pro | Promote myofibroblasts activation; promote type 2 immunity-induced fibrosis | 107,108 |
| IL-6 | Pro | Promote SMCs activation; promote fibroblasts activation and proliferation; enhance ECM production | 109-111 |
| IL-10 | Uncertain | Inhibit collagens deposition; no effects on fibroblasts and myofibroblasts | 112,113 |
| IL-11 | Pro | Enhance collagens expression; may promote SMCs hyperplasia | 114 |
| IL-12 | Pro | Promote inflammation | 115,116 |
| IL-13 | Pro | Promote TGF-β1 production; initiate fibrosis | 117,118 |
| IL-17 | Pro | Stimulate myofibroblasts activation; enhance collagens expression; decrease ECM degradation; induce EMT | 85,89,119 |
| IL-21 | Uncertain | Facilitate Th2 and Th17 development; enhance MMPs secretion | 120,121 |
| IL-22 | Pro | Inhibit inflammation; enhance MMPs secretion; promote myofibroblasts differentiation | 122-124 |
| IL-23 | Pro | Promote inflammation; promote fibrotic responses | 115,116,124 |
| IL-25 | Non | IL-13 production depends on IL-25; induce type 2 immunity; promote inflammation | 125,126 |
| IL-33 | Pro | Promote pro-fibrotic type 2 immunity | 127,128 |
| IL-34 | Pro | Enhance collagens expression; promote fibroblasts activation | 129 |
| IL-36 | Pro | Enhance collagens expression; promote fibroblasts activation | 130 |
| TL1A | Pro | Enhance collagens expression and pro-fibrotic molecules production; promote fibroblasts activation; induce EMT | 131,132 |
| IFN-γ | Anti | Inhibit TGF-β signaling; inhibit fibroblasts migration and myofibroblasts differentiation | 133-135 |
| TGF-β | Pro | Enhance pro-fibrotic molecules production; promote fibroblasts activation; induce EMT | 43,136-138 |
| CXCR4 | Pro | Mediate pro-fibrotic effects of PDGF-C | 139 |
| CCL11 | Pro | Induce eosinophils recruitment and in turn promote fibroblasts activation | 140 |
| CXCL8 | Pro | Promote pro-fibrotic growth factors production; enhance MMPs secretion | 141 |
IL, interleukin; ECM, extracellular matrix; SMCs, smooth muscle cells; TGF, transforming growth factor; EMT, epithelial-mesenchymal transition; Th, T helper; MMPs, matrix metalloproteinases; TL1A, tumor necrosis factor-like ligand 1A; IFN, interferon; CXCR4, C-X-C motif chemokine receptor 4; PDGF-C, platelet-derived growth factor-C; CCL11, C-C motif chemokine ligand 11; CXCL8, C-X-C motif chemokine ligand 8.
1) Interleukin-11
IL-11, a member of IL-6 family, is recognized as a pro-fibrotic cytokine secreted by stromal cells, as well as epithelial cells during tissue injuries.143 IL-11 is upregulated in various fibro-inflammation disorders.143 Ng et al.144 reported that IL-11 was increasingly expressed in invasive lung fibroblasts isolated from patients with idiopathic pulmonary fibrosis. They demonstrated that IL-11 exerted pro-fibrotic effects by driving fibroblasts activation, while anti-IL-11 treatment reversed lung fibrosis in mice.144 Recently, Schafer et al.145 has showed that fibroblast-specific IL-11 transgene expression or administration with IL-11 in mice resulted in heart and kidney fibrosis, whereas genetic deletion of IL-11 receptor alpha chain 1 (IL-11ra1) protected against fibrosis. scRNA-seq has revealed that the expression of IL-11 is enhanced in activated fibroblasts from CD inflamed segments.146 Lim et al.114 has found that SMC-specific IL-11 transgenic expression can induce inflamed, thickened and fibrotic bowel in mice. This is in line with another animal model with fibroblast-specific expression of IL-11.114 The emerging data prioritize IL-11 as a drug target for fibrotic diseases.
2) Interleukin-33
IL-33, a member of IL-1 superfamily, is passively released upon cellular damage and necrosis and is thus considered as an alert of inflammation.147 IL-33 is also involved in the process of fibrogenesis.148 Binding of IL-33 to its receptor ST2 triggers activation of Th2 cells to produce amphiregulin, which then drove osteopontin production by eosinophils, thus forming IL-33-amphiregulin-osteopontin axis. The axis conferred to fibrotic responses in eosinophilic airway inflammation.149 With regard to intestine, the expression of IL-33 and ST2 were upregulated in mucosa from UC patients and dextran sulfate sodium (DSS) colitis model.136,137 In particular, elevated epithelial expression of IL-33 was strongly associated with fibrosis progression in pediatric Crohn’s ileitis.138 A recent study by Imai et al.127 has unveiled a novel relationship between IL-33/ST2 signaling and gut dysbiosis in intestinal fibrosis. They reported that adherent-invasive Escherichia coli (AIEC) colonization elicited ST2 expression in intestinal epithelium, which in turn augmented the sensing of IL-33/ST2 signaling and ultimately promoted intestinal fibrosis. Alternatively, targeting IL-33/ST2 signaling with a neutralizing anti-ST2 antibody attenuated fibrotic effects of AIEC.127
3) Interleukin-34
IL-34, a member of 4-helical cytokine family, is produced by a wide range of cells including fibroblasts, immune cells, epithelial cells, endothelial cells and adipocytes.150 The association of aberrant high expression of IL-34 and fibrosis has been identified in several organs, including liver, kidney, and gut.129,151,152 Production of IL-34 was enhanced in inflamed mucosa in IBD patients and in DSS-induced colitis, which was regulated by tumor necrosis factor-α (TNF-α) via NF-κB signaling.153,154 Notably, the expression of IL-34 was elevated in fibrostrictures sites of CD.129 It was observed that activated fibroblasts by TNF-α exhibited increased expression of IL-34.129,155 Besides, fibroblasts stimulated with IL-34 could enhance expression of COL1A1 and COL3A1, while this effect disappeared in fibroblast-specific IL-34 knockout mice.129 These evidences raise a possibility that fibroblast is a cellular target of IL-34.
4) Interleukin-36
IL-36, also belonging to IL-1 superfamily, consists of five isoforms: IL-36α, IL-36β, IL-36γ, IL-36Ra, and IL-38.156,157 Among them, IL-36α, IL-36β, and IL-36γ play pro-inflammatory effects through activating IL-36 receptor (IL-36R) signaling, while IL-36Ra and IL-38 have opposing effects as they are inhibitors of IL-36R signaling.156,158 It is demonstrated that IL-36α and IL-36γ are elevated in both CD and UC mucosa under inflammation stimuli.158,159 Of note, IL-36α had an increased expression in tissues of CD fibrostenosis.130 Stimulation of IL-36α and IL-36γ can induce fibroblasts activation and epithelial cells proliferation,159,160 which is associated with an enhancement of collagen-VI secretion and ultimately fibrosis development.130 Importantly, both IL-36R blockade and IL-36 genes knockout are sufficient to attenuate intestinal fibrosis, which highlights the therapeutic values of IL-36 in fibrosis.130
5) Tumor necrosis factor-like ligand 1A
Tumor necrosis factor-like ligand 1A (TL1A) is a member of TNF superfamily and interacts with death receptor-3 (DR3) to form TL1A/DR3 co-stimulatory system.161 Aberrant TL1A/DR3 signaling is involved in chronic inflammation and fibrogenesis.162-165 CD patients with higher expression of serum TL1A were prone to develop stricture.166 Another study found that CD patients presenting with specific TL1A genotype rs6478108 were susceptible to forming stricturing phenotype.167 In vivo, mice with constitutive TL1A expression exhibited exaggerated intestinal inflammation and fibrosis,166,168 while neutralizing anti-TL1A antibody attenuated and even reversed the established fibrosis.131,165,169 Additionally, pro-fibrotic effects of TL1A/DR3 may depend on the presence of microbiota, since fibrosis was resistant in transgenic TL1A mice when specific microbiota such as Mucispirillum schaedleri and Ruminococcus absented.170 In phase 2 clinical trial (NCT02840721), treatment with anti-TL1A antibody (PF-06480605) has reduced the expressions of fibrotic-related genes and alleviated ECM remodeling.171
2. Inflammation-independent molecular mechanisms
Recently, several inflammation-independent mechanisms including ECM interaction, creeping fat (CrF), gut microbiota, as well as metabolic reprogramming, have attracted much attention because of their unique roles in intestinal fibrosis, which will be discussed in the following part.14,172,173
1) ECM-cells interactions
ECM is a highly specialized and dynamic three-dimensional scaffold in tissue, which is an active player rather than a purely passive player in fibrosis.14 ECM comprises a variety of fibrous components such as collagens, hyaluronan (HA) and fibronectin.174 HA exists as a high-molecular-weight polymer in normal conditions. During excessive inflammation, the polymer is cleaved to fragments of lower molecular weight, which promotes fibroblasts proliferation and myofibroblasts differentiation, thus contributing to fibrosis process. Besides, HA in low molecular weight fragments aids in recruiting immune cells to inflammatory sites, which in turn release a variety of inflammatory mediators and growth factors to initiate fibrosis.175-177 Fibronectin can enhance the susceptibility of SMCs to proliferation through combining with αVβ3 integrin.178 Additionally, the phenotype and function of myofibroblasts are altered along with the increased ECM stiffness. Myofibroblasts isolated from stenotic intestine display enhanced contractility of ECM and decreased activity of MMP-3, resulting in a vicious circle that further leads to tissue rigidity.38 Increased ECM stiffness is also able to drive fibroblasts to produce more ECM proteins through the Hippo and yes-associated protein pathway.14
2) CrF and intestinal fibrosis
CrF indicates that mesenteric fat wrapping around more than 50% of the intestinal circumference, which is the unique hallmark of CD.179 Although CrF was first described nearly 100 years ago, whether CrF is pathogenic or protective is still a controversy.179 Previous studies found that CrF was associated with the severity of intestinal inflammation and strictures.180 Inclusion mesentery in ileocolic resection achieved a reduction of stricture recurrence and reoperation.181,182 However, Ha et al.183 has reported that translocation of Clostridium innocuum to mesenteric adipose tissue (MAT) stimulated tissue remodeling via M2 macrophages and adipose proliferation, suggesting that CrF may restrict intestinal inflammation and bacterial dissemination in CD patients.
The relationship between CrF and intestinal fibrosis is still underexplored. Our previous study has uncovered a positive feedback loop between CrF and intestinal muscularis propria.184 Firstly, CrF-derived long-chain free fatty acids significantly enhanced proliferation and activation of intestinal muscle cells, with increased production of ECM proteins and strictures formation subsequently.184,185 Vise versa, hypertrophic muscularis propria triggered migration of preadipocytes out of MAT by fibronectin production, which facilitated CrF development.186 Another study observed that adipocytes within CrF were capable to convert to fibroblasts, whereas selective ablation of CrF adipocytes attenuated collagen deposition and bowel wall thickening.187 Noteworthily, new animal models via repeated colonic biopsy or antimesenteric enterotomy have been recently established,188,189 which will make a big difference for uncovering the complex relationship between CrF and intestinal fibrosis in the future.
3) Gut microbiota
Accumulating evidence indicates that gut microbiota plays crucial roles in fibrosis.8 The direct evidence was that experimental mice when reared under specific-pathogen-free conditions displayed a minimal inflammation, whereas when injected bacteria or bacterial wall components they exhibited inflammation and fibrosis.190 The terminal ileum is the most common site of intestinal stricture, where AIEC mainly colonize, giving us a hint that AIEC may be correlated with fibrosis development.191 Indeed, research using mice with AIEC inoculation found that flagellin of AIEC via IL-33-ST2 signaling facilitated intestinal fibrosis.127 Intestinal fibroblasts isolated from CD patients are observed to have an increased expression of several Toll-like receptors (TLRs) including 2, 3, 4, 6, 7.192 It is known that TLRs can be activated by perceiving microbial components, which are called pathogen-associated molecular patterns.172 The activated TLRs then promote the differentiation of fibroblasts into myofibroblasts.192 For example, TLR-3 activation in fibroblasts can augment α-SMA expression and TGF-β1 production via NF-κB signaling.193 Additionally, lipopolysaccharide activating TLR-4 can also stimulate α-SMA expression and collagen synthesis in fibroblasts.194,195 In conclusion, when exposed to pathogen-associated molecular patterns, intestinal myofibroblasts expressed upregulated levels of α-SMA and increased production of ECM proteins, thus confirming the link between gut microbiota and intestinal fibrosis.172,174,192
4) Metabolic reprogramming
Metabolic reprogramming has been widely described in fibrotic diseases. Generally, increased glycolysis and decreased fatty acid metabolism in fibroblasts are the main manifestations of metabolic reprogramming,173 Succinate is a key regulator of glycolysis.173 A recent study has reported that the expression levels of succinate and its specific receptor SUCNR1 in both serum and intestinal tissue are significantly increased in CD patients when compared with non-CD patients. Additionally, fibroblasts isolated from damaged intestine of CD patients also displayed an enhanced expression of SUCNR1. Furtherm ore, fibroblasts treated with succinate dose-dependently increased mRNA expressions of pro-fibrotic factor (e.g., TGF-β), as well as fibrotic markers (e.g., COL1A1, α-SMA), implying that succinate may be a potential target for intestinal fibrosis.196
With regard to lipid metabolism, peroxisome proliferator-activated receptor-γ (PPAR-γ) is responsible for the uptake and oxidation of fatty acids and is recognized as an anti-fibrotic factor.173,197 Results from a mice model with intestinal fibrosis showed that the expression of PPAR-γ was significantly decreased in fibrotic colon. Additionally, the administration of PPAR-γ agonist (GED-0507-34 Levo) was able to reduce the production of collagens and the expression of pro-fibrotic molecules, as well as prevent TGF-β-induced EMT, thus attenuating fibrosis.197
FUTURE PERSPECTIVE
Despite substantial progress have been achieved over the past decades in the understanding of cellular and molecular pathogenesis of fibrosis, ideal anti-fibrotic agents that specifically target intestinal fibrosis without significant side-effects have not been identified yet. Unravelling the inflammatory-independent mechanisms concerning pathogenesis of intestinal fibrosis, such as intestinal muscularis propria thickening, microbiota colonization and mesenteric fat hypertrophy, may open a new avenue to this perplexing issue.172,198 In addition, emerging methodology such as scRNA-seq has brought about new discoveries. For example, a deeper understanding of cell populations like fibroblasts or macrophages may reveal novel therapeutic points towards fibrosis.29,82 The past few years have also witnessed a rapid evolution of multi-omics analyses, which are able to integrate data across different levels of cellular organization, including genomes, epigenomes, transcriptomes, as well as proteomes. These multifaceted approaches provide an unprecedented opportunity to decode the complex mechanisms underlying intestinal fibrosis. Promising anti-fibrotic agents targeting intestine should be available in the near future.
Funding Statement
ACKNOWLEDGEMENTS This work was supported by the National Natural Science Foundation of China (NSFC grant numbers 81970483, and 82170537 to R.M.).
Footnotes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Wynn TA, Ramalingam TR. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med. 2012;18:1028–1040. doi: 10.1038/nm.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Henderson NC, Rieder F, Wynn TA. Fibrosis: from mechanisms to medicines. Nature. 2020;587:555–566. doi: 10.1038/s41586-020-2938-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weiskirchen R, Weiskirchen S, Tacke F. Organ and tissue fibrosis: molecular signals, cellular mechanisms and translational implications. Mol Aspects Med. 2019;65:2–15. doi: 10.1016/j.mam.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 4.Rieder F, Bettenworth D, Ma C, et al. An expert consensus to standardise definitions, diagnosis and treatment targets for anti-fibrotic stricture therapies in Crohn's disease. Aliment Pharmacol Ther. 2018;48:347–357. doi: 10.1111/apt.14853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rieder F, Zimmermann EM, Remzi FH, Sandborn WJ. Crohn's disease complicated by strictures: a systematic review. Gut. 2013;62:1072–1084. doi: 10.1136/gutjnl-2012-304353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gumaste V, Sachar DB, Greenstein AJ. Benign and malignant colorectal strictures in ulcerative colitis. Gut. 1992;33:938–941. doi: 10.1136/gut.33.7.938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang JT. Pathophysiology of inflammatory bowel diseases. N Engl J Med. 2020;383:2652–2664. doi: 10.1056/NEJMra2002697. [DOI] [PubMed] [Google Scholar]
- 8.Rieder F, Fiocchi C, Rogler G. Mechanisms, management, and treatment of fibrosis in patients with inflammatory bowel diseases. Gastroenterology. 2017;152:340–350. doi: 10.1053/j.gastro.2016.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Louis E, Collard A, Oger AF, et al. Behaviour of Crohn's disease according to the Vienna classification: changing pattern over the course of the disease. Gut. 2001;49:777–782. doi: 10.1136/gut.49.6.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gordon IO, Agrawal N, Goldblum JR, Fiocchi C, Rieder F. Fibrosis in ulcerative colitis: mechanisms, features, and consequences of a neglected problem. Inflamm Bowel Dis. 2014;20:2198–2206. doi: 10.1097/MIB.0000000000000080. [DOI] [PubMed] [Google Scholar]
- 11.Baumgart DC, Le Berre C. Newer biologic and small-molecule therapies for inflammatory bowel disease. N Engl J Med. 2021;385:1302–1315. doi: 10.1056/NEJMra1907607. [DOI] [PubMed] [Google Scholar]
- 12.D'Alessio S, Ungaro F, Noviello D, Lovisa S, Peyrin-Biroulet L, Danese S. Revisiting fibrosis in inflammatory bowel disease: the gut thickens. Nat Rev Gastroenterol Hepatol. 2022;19:169–184. doi: 10.1038/s41575-021-00543-0. [DOI] [PubMed] [Google Scholar]
- 13.Wang J, Lin S, Brown JM, van Wagoner D, Fiocchi C, Rieder F. Novel mechanisms and clinical trial endpoints in intestinal fibrosis. Immunol Rev. 2021;302:211–227. doi: 10.1111/imr.12974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herrera J, Henke CA, Bitterman PB. Extracellular matrix as a driver of progressive fibrosis. J Clin Invest. 2018;128:45–53. doi: 10.1172/JCI93557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bochaton-Piallat ML, Gabbiani G, Hinz B. The myofibroblast in wound healing and fibrosis: answered and unanswered questions. F1000Res. 2016;5(F1000 Faculty Rev):752. doi: 10.12688/f1000research.8190.1.8bcb23ed789349eb91cca33260a46d89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davidson S, Coles M, Thomas T, et al. Fibroblasts as immune regulators in infection, inflammation and cancer. Nat Rev Immunol. 2021;21:704–717. doi: 10.1038/s41577-021-00540-z. [DOI] [PubMed] [Google Scholar]
- 17.West NR, Hegazy AN, Owens B, et al. Oncostatin M drives intestinal inflammation and predicts response to tumor necrosis factor-neutralizing therapy in patients with inflammatory bowel disease. Nat Med. 2017;23:579–589. doi: 10.1038/nm.4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rock JR, Barkauskas CE, Cronce MJ, et al. Multiple stromal populations contribute to pulmonary fibrosis without evidence for epithelial to mesenchymal transition. Proc Natl Acad Sci U S A. 2011;108:E1475–E1483. doi: 10.1073/pnas.1117988108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.LeBleu VS, Taduri G, O'Connell J, et al. Origin and function of myofibroblasts in kidney fibrosis. Nat Med. 2013;19:1047–1053. doi: 10.1038/nm.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zidar N, Langner C, Jerala M, Boštjančič E, Drobne D, Tomažič A. Pathology of fibrosis in Crohn's disease-contribution to understanding its pathogenesis. Front Med (Lausanne) 2020;7:167. doi: 10.3389/fmed.2020.00167.9d571446f2f249628a2d9d98d14aed23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Driskell RR, Lichtenberger BM, Hoste E, et al. Distinct fibroblast lineages determine dermal architecture in skin development and repair. Nature. 2013;504:277–281. doi: 10.1038/nature12783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214:199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krystel-Whittemore M, Dileepan KN, Wood JG. Mast cell: a multi-functional master cell. Front Immunol. 2016;6:620. doi: 10.3389/fimmu.2015.00620.868c2a5198164785933f4b0ba3e51670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galdiero MR, Varricchi G, Seaf M, Marone G, Levi-Schaffer F, Marone G. Bidirectional mast cell-eosinophil interactions in inflammatory disorders and cancer. Front Med (Lausanne) 2017;4:103. doi: 10.3389/fmed.2017.00103.d15a005a059f472b8d1c19dad49b63f2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.D'Urso M, Kurniawan NA. Mechanical and physical regulation of fibroblast- myofibroblast transition: from cellular mechanoresponse to tissue pathology. Front Bioeng Biotechnol. 2020;8:609653. doi: 10.3389/fbioe.2020.609653.7d89b8175cd549a5839f8a8fb2288e0c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li GQ, Zhang Y, Liu D, et al. Celastrol inhibits interleukin-17A-stimulated rheumatoid fibroblast-like synoviocyte migration and invasion through suppression of NF-κB-mediated matrix metalloproteinase-9 expression. Int Immunopharmacol. 2012;14:422–431. doi: 10.1016/j.intimp.2012.08.016. [DOI] [PubMed] [Google Scholar]
- 27.Lawrance IC, Maxwell L, Doe W. Altered response of intestinal mucosal fibroblasts to profibrogenic cytokines in inflammatory bowel disease. Inflamm Bowel Dis. 2001;7:226–236. doi: 10.1097/00054725-200108000-00008. [DOI] [PubMed] [Google Scholar]
- 28.Wohlfahrt T, Rauber S, Uebe S, et al. PU.1 controls fibroblast polarization and tissue fibrosis. Nature. 2019;566:344–349. doi: 10.1038/s41586-019-0896-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muhl L, Genové G, Leptidis S, et al. Single-cell analysis uncovers fibroblast heterogeneity and criteria for fibroblast and mural cell identification and discrimination. Nat Commun. 2020;11:3953. doi: 10.1038/s41467-020-17740-1.5f2bea014f994236b4d2abd38b628f2e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baum J, Duffy HS. Fibroblasts and myofibroblasts: what are we talking about? J Cardiovasc Pharmacol. 2011;57:376–379. doi: 10.1097/FJC.0b013e3182116e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rieder F, Brenmoehl J, Leeb S, Schölmerich J, Rogler G. Wound healing and fibrosis in intestinal disease. Gut. 2007;56:130–139. doi: 10.1136/gut.2006.090456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lawrance IC, Rogler G, Bamias G, et al. Cellular and molecular mediators of intestinal fibrosis. J Crohns Colitis. 2017;11:1491–1503. doi: 10.1016/j.crohns.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rieder F, Fiocchi C. Intestinal fibrosis in IBD: a dynamic, multifactorial process. Nat Rev Gastroenterol Hepatol. 2009;6:228–235. doi: 10.1038/nrgastro.2009.31. [DOI] [PubMed] [Google Scholar]
- 34.Klingberg F, Hinz B, White ES. The myofibroblast matrix: implications for tissue repair and fibrosis. J Pathol. 2013;229:298–309. doi: 10.1002/path.4104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li C, Kuemmerle JF. The fate of myofibroblasts during the development of fibrosis in Crohn's disease. J Dig Dis. 2020;21:326–331. doi: 10.1111/1751-2980.12852. [DOI] [PubMed] [Google Scholar]
- 36.Li C, Flynn RS, Grider JR, et al. Increased activation of latent TGF-β1 by αVβ3 in human Crohn's disease and fibrosis in TNBS colitis can be prevented by cilengitide. Inflamm Bowel Dis. 2013;19:2829–2839. doi: 10.1097/MIB.0b013e3182a8452e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Frangogiannis N. Transforming growth factor-β in tissue fibrosis. J Exp Med. 2020;217:e20190103. doi: 10.1084/jem.20190103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de Bruyn JR, van den Brink GR, Steenkamer J, et al. Fibrostenotic phenotype of myofibroblasts in Crohn's disease is dependent on tissue stiffness and reversed by LOX inhibition. J Crohns Colitis. 2018;12:849–859. doi: 10.1093/ecco-jcc/jjy036. [DOI] [PubMed] [Google Scholar]
- 39.Kobayashi M, Khalil HA, Lei NY, et al. Bioengineering functional smooth muscle with spontaneous rhythmic contraction in vitro. Sci Rep. 2018;8:13544. doi: 10.1038/s41598-018-31992-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Allahverdian S, Chaabane C, Boukais K, Francis GA, Bochaton-Piallat ML. Smooth muscle cell fate and plasticity in atherosclerosis. Cardiovasc Res. 2018;114:540–550. doi: 10.1093/cvr/cvy022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Severi C, Sferra R, Scirocco A, et al. Contribution of intestinal smooth muscle to Crohn's disease fibrogenesis. Eur J Histochem. 2014;58:2457. doi: 10.4081/ejh.2014.2457.5aba5ef07991402681ba2dfe02857a68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ng EK, Panesar N, Longo WE, et al. Human intestinal epithelial and smooth muscle cells are potent producers of IL-6. Mediators Inflamm. 2003;12:3–8. doi: 10.1080/0962935031000096917.0ebfaacbc9524ca08e070f5c7e93a230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest. 2003;112:1776–1784. doi: 10.1172/JCI200320530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lovisa S, Genovese G, Danese S. Role of epithelial-to-mesenchymal transition in inflammatory bowel disease. J Crohns Colitis. 2019;13:659–668. doi: 10.1093/ecco-jcc/jjy201. [DOI] [PubMed] [Google Scholar]
- 45.Di Gregorio J, Robuffo I, Spalletta S, et al. The epithelial-to-mesenchymal transition as a possible therapeutic target in fibrotic disorders. Front Cell Dev Biol. 2020;8:607483. doi: 10.3389/fcell.2020.607483.2d0cfd462ae745d29e81c95336fbae44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Flier SN, Tanjore H, Kokkotou EG, Sugimoto H, Zeisberg M, Kalluri R. Identification of epithelial to mesenchymal transition as a novel source of fibroblasts in intestinal fibrosis. J Biol Chem. 2010;285:20202–20212. doi: 10.1074/jbc.M110.102012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bataille F, Rohrmeier C, Bates R, et al. Evidence for a role of epithelial mesenchymal transition during pathogenesis of fistulae in Crohn's disease. Inflamm Bowel Dis. 2008;14:1514–1527. doi: 10.1002/ibd.20590. [DOI] [PubMed] [Google Scholar]
- 48.Ortiz-Masià D, Salvador P, Macias-Ceja DC, et al. WNT2b activates epithelial-mesenchymal transition through FZD4: relevance in penetrating Crohn's disease. J Crohns Colitis. 2020;14:230–239. doi: 10.1093/ecco-jcc/jjz134. [DOI] [PubMed] [Google Scholar]
- 49.Iwano M, Plieth D, Danoff TM, Xue C, Okada H, Neilson EG. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest. 2002;110:341–350. doi: 10.1172/JCI0215518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Frid MG, Kale VA, Stenmark KR. Mature vascular endothelium can give rise to smooth muscle cells via endothelial-mesenchymal transdifferentiation: in vitro analysis. Circ Res. 2002;90:1189–1196. doi: 10.1161/01.RES.0000021432.70309.28. [DOI] [PubMed] [Google Scholar]
- 51.Zhang Y, Wu X, Li Y, et al. Endothelial to mesenchymal transition contributes to arsenic-trioxide-induced cardiac fibrosis. Sci Rep. 2016;6:33787. doi: 10.1038/srep33787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rieder F, Kessler SP, West GA, et al. Inflammation-induced endothelial-to-mesenchymal transition: a novel mechanism of intestinal fibrosis. Am J Pathol. 2011;179:2660–2673. doi: 10.1016/j.ajpath.2011.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Santamaria PG, Moreno-Bueno G, Portillo F, Cano A. EMT: present and future in clinical oncology. Mol Oncol. 2017;11:718–738. doi: 10.1002/1878-0261.12091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vannucchi MG, Traini C, Manetti M, Ibba-Manneschi L, Faussone-Pellegrini MS. Telocytes express PDGFRα in the human gastrointestinal tract. J Cell Mol Med. 2013;17:1099–1108. doi: 10.1111/jcmm.12134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Milia AF, Ruffo M, Manetti M, et al. Telocytes in Crohn's disease. J Cell Mol Med. 2013;17:1525–1536. doi: 10.1111/jcmm.12177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ibba-Manneschi L, Rosa I, Manetti M. Telocyte implications in human pathology: an overview. Semin Cell Dev Biol. 2016;55:62–69. doi: 10.1016/j.semcdb.2016.01.022. [DOI] [PubMed] [Google Scholar]
- 57.Wollheim FA. Telocytes, communicators in healthy stroma and relation to inflammation and fibrosis. Joint Bone Spine. 2016;83:615–618. doi: 10.1016/j.jbspin.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 58.Manetti M, Rosa I, Messerini L, Ibba-Manneschi L. Telocytes are reduced during fibrotic remodelling of the colonic wall in ulcerative colitis. J Cell Mol Med. 2015;19:62–73. doi: 10.1111/jcmm.12457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sahebally SM, Burke JP, Chang KH, Kiernan MG, O'Connell PR, Coffey JC. Circulating fibrocytes and Crohn's disease. Br J Surg. 2013;100:1549–1556. doi: 10.1002/bjs.9302. [DOI] [PubMed] [Google Scholar]
- 60.Mifflin RC, Pinchuk IV, Saada JI, Powell DW. Intestinal myofibroblasts: targets for stem cell therapy. Am J Physiol Gastrointest Liver Physiol. 2011;300:G684–G696. doi: 10.1152/ajpgi.00474.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sazuka S, Katsuno T, Nakagawa T, et al. Fibrocytes are involved in inflammation as well as fibrosis in the pathogenesis of Crohn's disease. Dig Dis Sci. 2014;59:760–768. doi: 10.1007/s10620-013-2813-8. [DOI] [PubMed] [Google Scholar]
- 62.Grieb G, Steffens G, Pallua N, Bernhagen J, Bucala R. Circulating fibrocytes: biology and mechanisms in wound healing and scar formation. Int Rev Cell Mol Biol. 2011;291:1–19. doi: 10.1016/B978-0-12-386035-4.00001-X. [DOI] [PubMed] [Google Scholar]
- 63.Ueno A, Jijon HB, Peng R, et al. Association of circulating fibrocytes with fibrostenotic small bowel Crohn's disease. Inflamm Bowel Dis. 2022;28:246–258. doi: 10.1093/ibd/izab157. [DOI] [PubMed] [Google Scholar]
- 64.Kuroda N, Masuya M, Tawara I, et al. Infiltrating CCR2+ monocytes and their progenies, fibrocytes, contribute to colon fibrosis by inhibiting collagen degradation through the production of TIMP-1. Sci Rep. 2019;9:8568. doi: 10.1038/s41598-019-45012-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Friedrich M, Pohin M, Powrie F. Cytokine networks in the pathophysiology of inflammatory bowel disease. Immunity. 2019;50:992–1006. doi: 10.1016/j.immuni.2019.03.017. [DOI] [PubMed] [Google Scholar]
- 66.Gieseck RL, 3rd, Wilson MS, Wynn TA. Type 2 immunity in tissue repair and fibrosis. Nat Rev Immunol. 2018;18:62–76. doi: 10.1038/nri.2017.90. [DOI] [PubMed] [Google Scholar]
- 67.Pelly VS, Kannan Y, Coomes SM, et al. IL-4-producing ILC2s are required for the differentiation of TH2 cells following Heligmosomoides polygyrus infection. Mucosal Immunol. 2016;9:1407–1417. doi: 10.1038/mi.2016.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gibbs BF, Haas H, Falcone FH, et al. Purified human peripheral blood basophils release interleukin-13 and preformed interleukin-4 following immunological activation. Eur J Immunol. 1996;26:2493–2498. doi: 10.1002/eji.1830261033. [DOI] [PubMed] [Google Scholar]
- 69.Symowski C, Voehringer D. Th2 cell-derived IL-4/IL-13 promote ILC2 accumulation in the lung by ILC2-intrinsic STAT6 signaling in mice. Eur J Immunol. 2019;49:1421–1432. doi: 10.1002/eji.201948161. [DOI] [PubMed] [Google Scholar]
- 70.Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32:593–604. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 71.Barron L, Wynn TA. Fibrosis is regulated by Th2 and Th17 responses and by dynamic interactions between fibroblasts and macrophages. Am J Physiol Gastrointest Liver Physiol. 2011;300:G723–G728. doi: 10.1152/ajpgi.00414.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wynn TA. Fibrotic disease and the T(H)1/T(H)2 paradigm. Nat Rev Immunol. 2004;4:583–594. doi: 10.1038/nri1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Maher TM, Costabel U, Glassberg MK, et al. Phase 2 trial to assess lebrikizumab in patients with idiopathic pulmonary fibrosis. Eur Respir J. 2021;57:1902442. doi: 10.1183/13993003.02442-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Raghu G, Richeldi L, Crestani B, et al. SAR156597 in idiopathic pulmonary fibrosis: a phase 2 placebo-controlled study (DRI11772) Eur Respir J. 2018;52:1801130. doi: 10.1183/13993003.01130-2018. [DOI] [PubMed] [Google Scholar]
- 75.Raghu G, Brown KK, Bradford WZ, et al. A placebo-controlled trial of interferon gamma-1b in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2004;350:125–133. doi: 10.1056/NEJMoa030511. [DOI] [PubMed] [Google Scholar]
- 76.Allanore Y, Wung P, Soubrane C, et al. A randomised, double-blind, placebo-controlled, 24-week, phase II, proof-of-concept study of romilkimab (SAR156597) in early diffuse cutaneous systemic sclerosis. Ann Rheum Dis. 2020;79:1600–1607. doi: 10.1136/annrheumdis-2020-218447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wynn TA, Vannella KM. Macrophages in tissue repair, regeneration, and fibrosis. Immunity. 2016;44:450–462. doi: 10.1016/j.immuni.2016.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cosín-Roger J, Ortiz-Masiá D, Calatayud S, Hernández C, Esplugues JV, Barrachina MD. The activation of Wnt signaling by a STAT6-dependent macrophage phenotype promotes mucosal repair in murine IBD. Mucosal Immunol. 2016;9:986–998. doi: 10.1038/mi.2015.123. [DOI] [PubMed] [Google Scholar]
- 79.Dharmasiri S, Garrido-Martin EM, Harris RJ, et al. Human intestinal macrophages are involved in the pathology of both ulcerative colitis and Crohn disease. Inflamm Bowel Dis. 2021;27:1641–1652. doi: 10.1093/ibd/izab029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Salvador P, Macías-Ceja DC, Gisbert-Ferrándiz L, et al. CD16+ macrophages mediate fibrosis in inflammatory bowel disease. J Crohns Colitis. 2018;12:589–599. doi: 10.1093/ecco-jcc/jjx185. [DOI] [PubMed] [Google Scholar]
- 81.Nayar S, Morrison JK, Giri M, et al. A myeloid-stromal niche and gp130 rescue in NOD2-driven Crohn's disease. Nature. 2021;593:275–281. doi: 10.1038/s41586-021-03484-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Aran D, Looney AP, Liu L, et al. Reference-based analysis of lung single-cell sequencing reveals a transitional profibrotic macrophage. Nat Immunol. 2019;20:163–172. doi: 10.1038/s41590-018-0276-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Patel DD, Kuchroo VK. Th17 cell pathway in human immunity: lessons from genetics and therapeutic interventions. Immunity. 2015;43:1040–1051. doi: 10.1016/j.immuni.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 84.Ramani K, Biswas PS. Interleukin-17: friend or foe in organ fibrosis. Cytokine. 2019;120:282–288. doi: 10.1016/j.cyto.2018.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Biancheri P, Pender SL, Ammoscato F, et al. The role of interleukin 17 in Crohn's disease-associated intestinal fibrosis. Fibrogenesis Tissue Repair. 2013;6:13. doi: 10.1186/1755-1536-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Honzawa Y, Nakase H, Shiokawa M, et al. Involvement of interleukin-17A-induced expression of heat shock protein 47 in intestinal fibrosis in Crohn's disease. Gut. 2014;63:1902–1912. doi: 10.1136/gutjnl-2013-305632. [DOI] [PubMed] [Google Scholar]
- 87.Brockmann L, Giannou AD, Gagliani N, Huber S. Regulation of TH17 cells and associated cytokines in wound healing, tissue regeneration, and carcinogenesis. Int J Mol Sci. 2017;18:1033. doi: 10.3390/ijms18051033.3f569e2046f54eefabd0167991b1fac2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Paul J, Singh AK, Kathania M, et al. IL-17-driven intestinal fibrosis is inhibited by Itch-mediated ubiquitination of HIC-5. Mucosal Immunol. 2018;11:427–436. doi: 10.1038/mi.2017.53. [DOI] [PubMed] [Google Scholar]
- 89.Zhang HJ, Zhang YN, Zhou H, Guan L, Li Y, Sun MJ. IL-17A promotes initiation and development of intestinal fibrosis through EMT. Dig Dis Sci. 2018;63:2898–2909. doi: 10.1007/s10620-018-5234-x. [DOI] [PubMed] [Google Scholar]
- 90.Li J, Liu L, Zhao Q, Chen M. Role of interleukin-17 in pathogenesis of intestinal fibrosis in mice. Dig Dis Sci. 2020;65:1971–1979. doi: 10.1007/s10620-019-05969-w. [DOI] [PubMed] [Google Scholar]
- 91.Hueber W, Sands BE, Lewitzky S, et al. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn's disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut. 2012;61:1693–1700. doi: 10.1136/gutjnl-2011-301668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Targan SR, Feagan B, Vermeire S, et al. A randomized, double-blind, placebo-controlled phase 2 study of brodalumab in patients with moderate-to-severe Crohn's disease. Am J Gastroenterol. 2016;111:1599–1607. doi: 10.1038/ajg.2016.298. [DOI] [PubMed] [Google Scholar]
- 93.McGee HM, Schmidt BA, Booth CJ, et al. IL-22 promotes fibroblast-mediated wound repair in the skin. J Invest Dermatol. 2013;133:1321–1329. doi: 10.1038/jid.2012.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liang SC, Tan XY, Luxenberg DP, et al. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med. 2006;203:2271–2279. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Klose CS, Artis D. Innate lymphoid cells as regulators of immunity, inflammation and tissue homeostasis. Nat Immunol. 2016;17:765–774. doi: 10.1038/ni.3489. [DOI] [PubMed] [Google Scholar]
- 96.Eberl G, Colonna M, Di Santo JP, McKenzie AN. Innate lymphoid cells. Innate lymphoid cells: a new paradigm in immunology. Science. 2015;348:aaa6566. doi: 10.1126/science.aaa6566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Klose C, Flach M, Möhle L, et al. Differentiation of type 1 ILCs from a common progenitor to all helper-like innate lymphoid cell lineages. Cell. 2014;157:340–356. doi: 10.1016/j.cell.2014.03.030. [DOI] [PubMed] [Google Scholar]
- 98.Kabata H, Moro K, Koyasu S. The group 2 innate lymphoid cell (ILC2) regulatory network and its underlying mechanisms. Immunol Rev. 2018;286:37–52. doi: 10.1111/imr.12706. [DOI] [PubMed] [Google Scholar]
- 99.Gladiator A, Wangler N, Trautwein-Weidner K, LeibundGut-Landmann S. Cutting edge: IL-17-secreting innate lymphoid cells are essential for host defense against fungal infection. J Immunol. 2013;190:521–525. doi: 10.4049/jimmunol.1202924. [DOI] [PubMed] [Google Scholar]
- 100.Zheng Y, Valdez PA, Danilenko DM, et al. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat Med. 2008;14:282–289. doi: 10.1038/nm1720. [DOI] [PubMed] [Google Scholar]
- 101.Messing M, Jan-Abu SC, McNagny K. Group 2 innate lymphoid cells: central players in a recurring theme of repair and regeneration. Int J Mol Sci. 2020;21:1350. doi: 10.3390/ijms21041350.659cb58ddfe047538c9c6a20bfc93ec2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Forkel M, van Tol S, Höög C, Michaëlsson J, Almer S, Mjösberg J. Distinct alterations in the composition of mucosal innate lymphoid cells in newly diagnosed and established Crohn's disease and ulcerative colitis. J Crohns Colitis. 2019;13:67–78. doi: 10.1093/ecco-jcc/jjy119. [DOI] [PubMed] [Google Scholar]
- 103.Lo BC, Gold MJ, Hughes MR, et al. The orphan nuclear receptor RORα and group 3 innate lymphoid cells drive fibrosis in a mouse model of Crohn's disease. Sci Immunol. 2016;1:eaaf8864. doi: 10.1126/sciimmunol.aaf8864. [DOI] [PubMed] [Google Scholar]
- 104.Scarpa M, Kessler S, Sadler T, et al. The epithelial danger signal IL-1α is a potent activator of fibroblasts and reactivator of intestinal inflammation. Am J Pathol. 2015;185:1624–1637. doi: 10.1016/j.ajpath.2015.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Drygiannakis I, Valatas V, Sfakianaki O, et al. Proinflammatory cytokines induce crosstalk between colonic epithelial cells and subepithelial myofibroblasts: implication in intestinal fibrosis. J Crohns Colitis. 2013;7:286–300. doi: 10.1016/j.crohns.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 106.Okuno T, Andoh A, Bamba S, et al. Interleukin-1beta and tumor necrosis factor-alpha induce chemokine and matrix metalloproteinase gene expression in human colonic subepithelial myofibroblasts. Scand J Gastroenterol. 2002;37:317–324. doi: 10.1080/003655202317284228. [DOI] [PubMed] [Google Scholar]
- 107.Andoh A, Hata K, Araki Y, Fujiyama Y, Bamba T. Interleukin (IL)-4 and IL-17 synergistically stimulate IL-6 secretion in human colonic myofibroblasts. Int J Mol Med. 2002;10:631–634. [PubMed] [Google Scholar]
- 108.Dohi T, Fujihashi K, Kiyono H, Elson CO, McGhee JR. Mice deficient in Th1- and Th2-type cytokines develop distinct forms of hapten-induced colitis. Gastroenterology. 2000;119:724–733. doi: 10.1053/gast.2000.16500. [DOI] [PubMed] [Google Scholar]
- 109.Li C, Iness A, Yoon J, et al. Noncanonical STAT3 activation regulates excess TGF-β1 and collagen I expression in muscle of stricturing Crohn's disease. J Immunol. 2015;194:3422–3431. doi: 10.4049/jimmunol.1401779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Franzè E, Monteleone I, Laudisi F, et al. Cadherin-11 is a regulator of intestinal fibrosis. J Crohns Colitis. 2020;14:406–417. doi: 10.1093/ecco-jcc/jjz147. [DOI] [PubMed] [Google Scholar]
- 111.Li C, Kuemmerle JF. Mechanisms that mediate the development of fibrosis in patients with Crohn's disease. Inflamm Bowel Dis. 2014;20:1250–1258. doi: 10.1097/MIB.0000000000000043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Theiss AL, Fuller CR, Simmons JG, Liu B, Sartor RB, Lund PK. Growth hormone reduces the severity of fibrosis associated with chronic intestinal inflammation. Gastroenterology. 2005;129:204–219. doi: 10.1053/j.gastro.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 113.Rogler G, Gelbmann CM, Vogl D, et al. Differential activation of cytokine secretion in primary human colonic fibroblast/myofibroblast cultures. Scand J Gastroenterol. 2001;36:389–398. doi: 10.1080/003655201300051216. [DOI] [PubMed] [Google Scholar]
- 114.Lim WW, Ng B, Widjaja A, et al. Transgenic interleukin 11 expression causes cross-tissue fibro-inflammation and an inflammatory bowel phenotype in mice. PLoS One. 2020;15:e0227505. doi: 10.1371/journal.pone.0227505.b9436e2a31354ff8a067085ea9933b32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Guan Q, Ma Y, Hillman CL, et al. Targeting IL-12/IL-23 by employing a p40 peptide-based vaccine ameliorates TNBS-induced acute and chronic murine colitis. Mol Med. 2011;17:646–656. doi: 10.2119/molmed.2010.00252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Guan Q, Weiss CR, Wang S, et al. Reversing ongoing chronic intestinal inflammation and fibrosis by sustained block of IL-12 and IL-23 using a vaccine in mice. Inflamm Bowel Dis. 2018;24:1941–1952. doi: 10.1093/ibd/izy142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Fichtner-Feigl S, Fuss IJ, Young CA, et al. Induction of IL-13 triggers TGF-beta1-dependent tissue fibrosis in chronic 2,4,6-trinitrobenzene sulfonic acid colitis. J Immunol. 2007;178:5859–5870. doi: 10.4049/jimmunol.178.9.5859. [DOI] [PubMed] [Google Scholar]
- 118.Fichtner-Feigl S, Young CA, Kitani A, Geissler EK, Schlitt HJ, Strober W. IL-13 signaling via IL-13R alpha2 induces major downstream fibrogenic factors mediating fibrosis in chronic TNBS colitis. Gastroenterology. 2008;135:2003–2013.E7. doi: 10.1053/j.gastro.2008.08.055. [DOI] [PubMed] [Google Scholar]
- 119.Yagi Y, Andoh A, Inatomi O, Tsujikawa T, Fujiyama Y. Inflammatory responses induced by interleukin-17 family members in human colonic subepithelial myofibroblasts. J Gastroenterol. 2007;42:746–753. doi: 10.1007/s00535-007-2091-3. [DOI] [PubMed] [Google Scholar]
- 120.Speca S, Giusti I, Rieder F, Latella G. Cellular and molecular mechanisms of intestinal fibrosis. World J Gastroenterol. 2012;18:3635–3661. doi: 10.3748/wjg.v18.i28.3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Monteleone G, Caruso R, Fina D, et al. Control of matrix metalloproteinase production in human intestinal fibroblasts by interleukin 21. Gut. 2006;55:1774–1780. doi: 10.1136/gut.2006.093187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zenewicz LA, Yancopoulos GD, Valenzuela DM, Murphy AJ, Stevens S, Flavell RA. Innate and adaptive interleukin-22 protects mice from inflammatory bowel disease. Immunity. 2008;29:947–957. doi: 10.1016/j.immuni.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Andoh A, Zhang Z, Inatomi O, et al. Interleukin-22, a member of the IL-10 subfamily, induces inflammatory responses in colonic subepithelial myofibroblasts. Gastroenterology. 2005;129:969–984. doi: 10.1053/j.gastro.2005.06.071. [DOI] [PubMed] [Google Scholar]
- 124.Mathur R, Alam MM, Zhao XF, et al. Induction of autophagy in Cx3cr1+ mononuclear cells limits IL-23/IL-22 axis-mediated intestinal fibrosis. Mucosal Immunol. 2019;12:612–623. doi: 10.1038/s41385-019-0146-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Borowczyk J, Shutova M, Brembilla NC, Boehncke WH. IL-25 (IL-17E) in epithelial immunology and pathophysiology. J Allergy Clin Immunol. 2021;148:40–52. doi: 10.1016/j.jaci.2020.12.628. [DOI] [PubMed] [Google Scholar]
- 126.Wang AJ, Smith A, Li Y, et al. Genetic deletion of IL-25 (IL-17E) confers resistance to dextran sulfate sodium-induced colitis in mice. Cell Biosci. 2014;4:72. doi: 10.1186/2045-3701-4-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Imai J, Kitamoto S, Sugihara K, et al. Flagellin-mediated activation of IL-33-ST2 signaling by a pathobiont promotes intestinal fibrosis. Mucosal Immunol. 2019;12:632–643. doi: 10.1038/s41385-019-0138-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kotsiou OS, Gourgoulianis KI, Zarogiannis SG. IL-33/ST2 axis in organ fibrosis. Front Immunol. 2018;9:2432. doi: 10.3389/fimmu.2018.02432.8b40a2724aec46b489db895f381f17b8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Franzè E, Dinallo V, Laudisi F, et al. Interleukin-34 stimulates gut fibroblasts to produce collagen synthesis. J Crohns Colitis. 2020;14:1436–1445. doi: 10.1093/ecco-jcc/jjaa073. [DOI] [PubMed] [Google Scholar]
- 130.Scheibe K, Kersten C, Schmied A, et al. Inhibiting interleukin 36 receptor signaling reduces fibrosis in mice with chronic intestinal inflammation. Gastroenterology. 2019;156:1082–1097. doi: 10.1053/j.gastro.2018.11.029. [DOI] [PubMed] [Google Scholar]
- 131.Shih DQ, Zheng L, Zhang X, et al. Inhibition of a novel fibrogenic factor Tl1a reverses established colonic fibrosis. Mucosal Immunol. 2014;7:1492–1503. doi: 10.1038/mi.2014.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wenxiu J, Mingyue Y, Fei H, et al. Effect and mechanism of TL1A expression on epithelial-mesenchymal transition during chronic colitis-related intestinal fibrosis. Mediators Inflamm. 2021;2021:5927064. doi: 10.1155/2021/5927064.6180d4691cdb45558e4daec70fd45862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ulloa L, Doody J, Massagué J. Inhibition of transforming growth factor-beta/SMAD signalling by the interferon-gamma/STAT pathway. Nature. 1999;397:710–713. doi: 10.1038/17826. [DOI] [PubMed] [Google Scholar]
- 134.Leeb SN, Vogl D, Gunckel M, et al. Reduced migration of fibroblasts in inflammatory bowel disease: role of inflammatory mediators and focal adhesion kinase. Gastroenterology. 2003;125:1341–1354. doi: 10.1016/j.gastro.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 135.Francoeur C, Bouatrouss Y, Seltana A, et al. Degeneration of the pericryptal myofibroblast sheath by proinflammatory cytokines in inflammatory bowel diseases. Gastroenterology. 2009;136:268–277. doi: 10.1053/j.gastro.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 136.Kobori A, Yagi Y, Imaeda H, et al. Interleukin-33 expression is specifically enhanced in inflamed mucosa of ulcerative colitis. J Gastroenterol. 2010;45:999–1007. doi: 10.1007/s00535-010-0245-1. [DOI] [PubMed] [Google Scholar]
- 137.Lopetuso LR, De Salvo C, Pastorelli L, et al. IL-33 promotes recovery from acute colitis by inducing miR-320 to stimulate epithelial restitution and repair. Proc Natl Acad Sci U S A. 2018;115:E9362–E9370. doi: 10.1073/pnas.1803613115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Masterson JC, Capocelli KE, Hosford L, et al. Eosinophils and IL-33 perpetuate chronic inflammation and fibrosis in a pediatric population with stricturing Crohn's ileitis. Inflamm Bowel Dis. 2015;21:2429–2440. doi: 10.1097/MIB.0000000000000512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lu W, Xie Y, Huang B, et al. Platelet-derived growth factor C signaling is a potential therapeutic target for radiation proctopathy. Sci Transl Med. 2021;13:eabc2344. doi: 10.1126/scitranslmed.abc2344. [DOI] [PubMed] [Google Scholar]
- 140.Takemura N, Kurashima Y, Mori Y, et al. Eosinophil depletion suppresses radiation-induced small intestinal fibrosis. Sci Transl Med. 2018;10:eaan0333. doi: 10.1126/scitranslmed.aan0333. [DOI] [PubMed] [Google Scholar]
- 141.Walana W, Ye Y, Li M, et al. IL-8 antagonist, CXCL8(3-72)K11R/G31P coupled with probiotic exhibit variably enhanced therapeutic potential in ameliorating ulcerative colitis. Biomed Pharmacother. 2018;103:253–261. doi: 10.1016/j.biopha.2018.04.008. [DOI] [PubMed] [Google Scholar]
- 142.Lenti MV, Di Sabatino A. Intestinal fibrosis. Mol Aspects Med. 2019;65:100–109. doi: 10.1016/j.mam.2018.10.003. [DOI] [PubMed] [Google Scholar]
- 143.Cook SA, Schafer S. Hiding in plain sight: interleukin-11 emerges as a master regulator of fibrosis, tissue integrity, and stromal inflammation. Annu Rev Med. 2020;71:263–276. doi: 10.1146/annurev-med-041818-011649. [DOI] [PubMed] [Google Scholar]
- 144.Ng B, Dong J, D'Agostino G, et al. Interleukin-11 is a therapeutic target in idiopathic pulmonary fibrosis. Sci Transl Med. 2019;11:eaaw1237. doi: 10.1126/scitranslmed.aaw1237. [DOI] [PubMed] [Google Scholar]
- 145.Schafer S, Viswanathan S, Widjaja AA, et al. IL-11 is a crucial determinant of cardiovascular fibrosis. Nature. 2017;552:110–115. doi: 10.1038/nature24676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Martin JC, Chang C, Boschetti G, et al. Single-cell analysis of Crohn's disease lesions identifies a pathogenic cellular module associated with resistance to anti-TNF therapy. Cell. 2019;178:1493–1508. doi: 10.1016/j.cell.2019.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Schmitz J, Owyang A, Oldham E, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–490. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 148.Lopetuso LR, Scaldaferri F, Pizarro TT. Emerging role of the interleukin (IL)-33/ST2 axis in gut mucosal wound healing and fibrosis. Fibrogenesis Tissue Repair. 2012;5:18. doi: 10.1186/1755-1536-5-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Morimoto Y, Hirahara K, Kiuchi M, et al. Amphiregulin-producing pathogenic memory T helper 2 cells instruct eosinophils to secrete osteopontin and facilitate airway fibrosis. Immunity. 2018;49:134–150. doi: 10.1016/j.immuni.2018.04.023. [DOI] [PubMed] [Google Scholar]
- 150.Baghdadi M, Umeyama Y, Hama N, et al. Interleukin-34, a comprehensive review. J Leukoc Biol. 2018;104:931–951. doi: 10.1002/JLB.MR1117-457R. [DOI] [PubMed] [Google Scholar]
- 151.Preisser L, Miot C, Le Guillou-Guillemette H, et al. IL-34 and macrophage colony- stimulating factor are overexpressed in hepatitis C virus fibrosis and induce profibrotic macrophages that promote collagen synthesis by hepatic stellate cells. Hepatology. 2014;60:1879–1890. doi: 10.1002/hep.27328. [DOI] [PubMed] [Google Scholar]
- 152.Baek JH, Zeng R, Weinmann-Menke J, et al. IL-34 mediates acute kidney injury and worsens subsequent chronic kidney disease. J Clin Invest. 2015;125:3198–3214. doi: 10.1172/JCI81166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Franzè E, Monteleone I, Cupi ML, et al. Interleukin-34 sustains inflammatory pathways in the gut. Clin Sci (Lond) 2015;129:271–280. doi: 10.1042/CS20150132. [DOI] [PubMed] [Google Scholar]
- 154.Zwicker S, Martinez GL, Bosma M, et al. Interleukin 34: a new modulator of human and experimental inflammatory bowel disease. Clin Sci (Lond) 2015;129:281–290. doi: 10.1042/CS20150176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hwang SJ, Choi B, Kang SS, et al. Interleukin-34 produced by human fibroblast-like synovial cells in rheumatoid arthritis supports osteoclastogenesis. Arthritis Res Ther. 2012;14:R14. doi: 10.1186/ar3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Mantovani A, Dinarello CA, Molgora M, Garlanda C. Interleukin-1 and related cytokines in the regulation of inflammation and immunity. Immunity. 2019;50:778–795. doi: 10.1016/j.immuni.2019.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Elias M, Zhao S, Le HT, et al. IL-36 in chronic inflammation and fibrosis: bridging the gap? J Clin Invest. 2021;131:e144336. doi: 10.1172/JCI144336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Nishida A, Hidaka K, Kanda T, et al. Increased expression of interleukin-36, a member of the interleukin-1 cytokine family, in inflammatory bowel disease. Inflamm Bowel Dis. 2016;22:303–314. doi: 10.1097/MIB.0000000000000654. [DOI] [PubMed] [Google Scholar]
- 159.Scheibe K, Backert I, Wirtz S, et al. IL-36R signalling activates intestinal epithelial cells and fibroblasts and promotes mucosal healing in vivo. Gut. 2017;66:823–838. doi: 10.1136/gutjnl-2015-310374. [DOI] [PubMed] [Google Scholar]
- 160.Takahashi K, Nishida A, Shioya M, et al. Interleukin (IL)-1β is a strong inducer of IL-36γ expression in human colonic myofibroblasts. PLoS One. 2015;10:e0138423. doi: 10.1371/journal.pone.0138423.502585d824ad4c11ba9e66730ca24a7a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Migone TS, Zhang J, Luo X, et al. TL1A is a TNF-like ligand for DR3 and TR6/DcR3 and functions as a T cell costimulator. Immunity. 2002;16:479–492. doi: 10.1016/S1074-7613(02)00283-2. [DOI] [PubMed] [Google Scholar]
- 162.Valatas V, Kolios G, Bamias G. TL1A (TNFSF15) and DR3 (TNFRSF25): a co-stimulatory system of cytokines with diverse functions in gut mucosal immunity. Front Immunol. 2019;10:583. doi: 10.3389/fimmu.2019.00583.28b64bc89cd1440e99a3e97d721bf316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Takedatsu H, Michelsen KS, Wei B, et al. TL1A (TNFSF15) regulates the development of chronic colitis by modulating both T-helper 1 and T-helper 17 activation. Gastroenterology. 2008;135:552–567. doi: 10.1053/j.gastro.2008.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Jacob N, Kumagai K, Abraham JP, et al. Direct signaling of TL1A-DR3 on fibroblasts induces intestinal fibrosis in vivo. Sci Rep. 2020;10:18189. doi: 10.1038/s41598-020-75168-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Li H, Song J, Niu G, et al. TL1A blocking ameliorates intestinal fibrosis in the T cell transfer model of chronic colitis in mice. Pathol Res Pract. 2018;214:217–227. doi: 10.1016/j.prp.2017.11.017. [DOI] [PubMed] [Google Scholar]
- 166.Barrett R, Zhang X, Koon HW, et al. Constitutive TL1A expression under colitogenic conditions modulates the severity and location of gut mucosal inflammation and induces fibrostenosis. Am J Pathol. 2012;180:636–649. doi: 10.1016/j.ajpath.2011.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Yang DH, Yang SK, Song K, et al. TNFSF15 is an independent predictor for the development of Crohn's disease-related complications in Koreans. J Crohns Colitis. 2014;8:1315–1326. doi: 10.1016/j.crohns.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 168.Shih DQ, Barrett R, Zhang X, et al. Constitutive TL1A (TNFSF15) expression on lymphoid or myeloid cells leads to mild intestinal inflammation and fibrosis. PLoS One. 2011;6:e16090. doi: 10.1371/journal.pone.0016090.9d4d782a89f445189668f80a41d5488f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Clarke AW, Poulton L, Shim D, et al. An anti-TL1A antibody for the treatment of asthma and inflammatory bowel disease. MAbs. 2018;10:664–677. doi: 10.1080/19420862.2018.1440164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Jacob N, Jacobs JP, Kumagai K, et al. Inflammation-independent TL1A-mediated intestinal fibrosis is dependent on the gut microbiome. Mucosal Immunol. 2018;11:1466–1476. doi: 10.1038/s41385-018-0055-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Hassan-Zahraee M, Ye Z, Xi L, et al. Antitumor necrosis factor-like ligand 1A therapy targets tissue inflammation and fibrosis pathways and reduces gut pathobionts in ulcerative colitis. Inflamm Bowel Dis. 2022;28:434–446. doi: 10.1093/ibd/izab193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Li J, Mao R, Kurada S, et al. Pathogenesis of fibrostenosing Crohn's disease. Transl Res. 2019;209:39–54. doi: 10.1016/j.trsl.2019.03.005. [DOI] [PubMed] [Google Scholar]
- 173.Bos S, Laukens D. Metabolic modulation during intestinal fibrosis. J Dig Dis. 2020;21:319–325. doi: 10.1111/1751-2980.12882. [DOI] [PubMed] [Google Scholar]
- 174.Latella G, Rogler G, Bamias G, et al. Results of the 4th scientific workshop of the ECCO (I): pathophysiology of intestinal fibrosis in IBD. J Crohns Colitis. 2014;8:1147–1165. doi: 10.1016/j.crohns.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 175.Derkacz A, Olczyk P, Olczyk K, Komosinska-Vassev K. The role of extracellular matrix components in inflammatory bowel diseases. J Clin Med. 2021;10:1122. doi: 10.3390/jcm10051122.ed00fc1b7e0242c5ae97b5449d3aba8e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Soroosh A, Albeiroti S, West GA, Willard B, Fiocchi C, de la Motte CA. Crohn's disease fibroblasts overproduce the novel protein KIAA1199 to create proinflammatory hyaluronan fragments. Cell Mol Gastroenterol Hepatol. 2016;2:358–368. doi: 10.1016/j.jcmgh.2015.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Wight TN, Potter-Perigo S. The extracellular matrix: an active or passive player in fibrosis? Am J Physiol Gastrointest Liver Physiol. 2011;301:G950–G955. doi: 10.1152/ajpgi.00132.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Flynn RS, Murthy KS, Grider JR, Kellum JM, Kuemmerle JF. Endogenous IGF-I and alphaVbeta3 integrin ligands regulate increased smooth muscle hyperplasia in stricturing Crohn's disease. Gastroenterology. 2010;138:285–293. doi: 10.1053/j.gastro.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Sheehan AL, Warren BF, Gear MW, Shepherd NA. Fat-wrapping in Crohn's disease: pathological basis and relevance to surgical practice. Br J Surg. 1992;79:955–958. doi: 10.1002/bjs.1800790934. [DOI] [PubMed] [Google Scholar]
- 180.Borley NR, Mortensen NJ, Jewell DP, Warren BF. The relationship between inflammatory and serosal connective tissue changes in ileal Crohn's disease: evidence for a possible causative link. J Pathol. 2000;190:196–202. doi: 10.1002/(SICI)1096-9896(200002)190:2<196::AID-PATH513>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 181.Liu Q, Zhang X, Ko HM, et al. Constrictive and hypertrophic strictures in ileal Crohn's disease. Clin Gastroenterol Hepatol. 2022;20:e1292–e1304. doi: 10.1016/j.cgh.2021.08.012. [DOI] [PubMed] [Google Scholar]
- 182.Coffey CJ, Kiernan MG, Sahebally SM, et al. Inclusion of the mesentery in ileocolic resection for Crohn's disease is associated with reduced surgical recurrence. J Crohns Colitis. 2018;12:1139–1150. doi: 10.1093/ecco-jcc/jjx187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Ha C, Martin A, Sepich-Poore GD, et al. Translocation of viable gut microbiota to mesenteric adipose drives formation of creeping fat in humans. Cell. 2020;183:666–683. doi: 10.1016/j.cell.2020.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Mao R, Chandra J, Brown JM, et al. A positive feedback loop between creeping fat and intestinal stricture formation in Crohn's disease: the role of creeping fat-derived free fatty acids, extracellular matrix, and integrin. Gastroenterology. 2020;158:S-188–S-189. doi: 10.1016/S0016-5085(20)31154-9. [DOI] [Google Scholar]
- 185.Mao R, Doyon G, Kurada S, et al. Creeping-fat derived free fatty acids induce hyperplasia of intestinal muscularis propria muscle cells: a novel link between fat and intestinal stricture formation in Crohn's disease. Gastroenterology. 2018;154:S–131. doi: 10.1016/S0016-5085(18)30866-7. [DOI] [Google Scholar]
- 186.Mao R, Doyon G, Gordon IO, et al. Activated intestinal muscle cells promote preadipocyte migration: a novel mechanism for creeping fat formation in Crohn's disease. Gut. 2022;71:55–67. doi: 10.1136/gutjnl-2020-323719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Bauer-Rowe KE, Griffin M, Foster D, et al. Where there is fat there is fibrosis: elucidating the mechanisms of creeping fat-driven stricture formation. J Am Coll Surg. 2021;233:S65. doi: 10.1016/j.jamcollsurg.2021.07.114. [DOI] [Google Scholar]
- 188.Xiong S, Whitehurst CE, Li L, et al. Reverse translation approach generates a signature of penetrating fibrosis in Crohn's disease that is associated with anti-TNF response. Gut. 2022;71:1289–1301. doi: 10.1136/gutjnl-2020-323405. [DOI] [PubMed] [Google Scholar]
- 189.Bauer-Rowe KE, Foster D, Titan A, et al. A Surgical model for investigating the role of creeping fat in intestinal fibrosis. J Am Coll Surg. 2020;231:S50–S51. doi: 10.1016/j.jamcollsurg.2020.07.079. [DOI] [Google Scholar]
- 190.Rieder F. The gut microbiome in intestinal fibrosis: environmental protector or provocateur? Sci Transl Med. 2013;5:190ps10. doi: 10.1126/scitranslmed.3004731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Darfeuille-Michaud A, Boudeau J, Bulois P, et al. High prevalence of adherent-invasive Escherichia coli associated with ileal mucosa in Crohn's disease. Gastroenterology. 2004;127:412–421. doi: 10.1053/j.gastro.2004.04.061. [DOI] [PubMed] [Google Scholar]
- 192.Zorzi F, Calabrese E, Monteleone G. Pathogenic aspects and therapeutic avenues of intestinal fibrosis in Crohn's disease. Clin Sci (Lond) 2015;129:1107–1113. doi: 10.1042/CS20150472. [DOI] [PubMed] [Google Scholar]
- 193.Sugiura H, Ichikawa T, Koarai A, et al. Activation of Toll-like receptor 3 augments myofibroblast differentiation. Am J Respir Cell Mol Biol. 2009;40:654–662. doi: 10.1165/rcmb.2008-0371OC. [DOI] [PubMed] [Google Scholar]
- 194.Bhattacharyya S, Wang W, Tamaki Z, et al. Pharmacological inhibition of Toll-like receptor-4 signaling by TAK242 prevents and induces regression of experimental organ fibrosis. Front Immunol. 2018;9:2434. doi: 10.3389/fimmu.2018.02434.187e93ecbe254f39a080b50e58c9a920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Yang R, Song Z, Wu S, Wei Z, Xu Y, Shen X. Toll-like receptor 4 contributes to a myofibroblast phenotype in cardiac fibroblasts and is associated with autophagy after myocardial infarction in a mouse model. Atherosclerosis. 2018;279:23–31. doi: 10.1016/j.atherosclerosis.2018.10.018. [DOI] [PubMed] [Google Scholar]
- 196.Macias-Ceja DC, Ortiz-Masiá D, Salvador P, et al. Succinate receptor mediates intestinal inflammation and fibrosis. Mucosal Immunol. 2019;12:178–187. doi: 10.1038/s41385-018-0087-3. [DOI] [PubMed] [Google Scholar]
- 197.Di Gregorio J, Sferra R, Speca S, et al. Role of glycogen synthase kinase-3β and PPAR-γ on epithelial-to-mesenchymal transition in DSS-induced colorectal fibrosis. PLoS One. 2017;12:e0171093. doi: 10.1371/journal.pone.0171093.564d16f4f6fb409f9578eeaa512ec36d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Mao R, Kurada S, Gordon IO, et al. The mesenteric fat and intestinal muscle interface: creeping fat influencing stricture formation in Crohn's disease. Inflamm Bowel Dis. 2019;25:421–426. doi: 10.1093/ibd/izy331. [DOI] [PubMed] [Google Scholar]