Abstract
Background/Aims
We explored whether high sodium intake, assessed by urinary excretion, determines the risk of sarcopenia and nonalcoholic fatty liver disease (NAFLD).
Methods
We analyzed 10,036 adult participants with normal kidney function from the Korea National Health and Nutrition Examination Survey (2008–2011). NAFLD was identified using the fatty liver index, and the muscle mass was evaluated using dual X-ray absorptiometry. The dietary sodium intake was estimated using Tanaka’s equation.
Results
The mean 24-hour urinary sodium excretion was 144.2±36.1 mmol/day (corresponding to 3.3 g/day Na) in the total population. The 24-hour urinary sodium excretion showed moderate accuracy in predicting NAFLD (area under the receiver operating characteristic, 0.702; 95% confidence interval [CI], 0.692 to 0.712). A cutoff value of 99.96 mmol/day (corresponding to 2.30 g/day Na) for urinary sodium excretion in predicting NAFLD showed 76.1% sensitivity and 56.1% specificity. The results of multiple adjusted models indicated that the participants with the highest urinary sodium excretion had a significantly higher risk of NAFLD (odds ratio, 1.46; 95% CI, 1.27 to 1.66; p<0.001) and sarcopenia (odds ratio, 1.49; 95% CI, 1.28 to 1.73; p<0.001) than those with the lowest urinary sodium excretion. The association between a higher 24-hour urinary sodium excretion and NAFLD was independent of sarcopenia.
Conclusions
Participants with a high sodium intake, as assessed by sodium excretion, had a substantial risk of NAFLD and sarcopenia.
Keywords: Non-alcoholic fatty liver disease, Sarcopenia, Urinary sodium excretion, Metabolic syndrome
INTRODUCTION
With the rapid increase in obesity and sedentary lifestyles, nonalcoholic fatty liver disease (NAFLD) has emerged as a socioeconomic issue. NAFLD is considered the most widespread chronic liver disease worldwide, and as the clinical consequences of NAFLD increase, so too will the economic burden of NAFLD. In 2016, it was forecasted, using an estimating model, that over 100 billion dollars would be spent for NAFLD and that the expected 10-year burden of NAFLD could increase to approximately one trillion dollars in the United States.1 In Korea, the NAFLD prevalence was estimated at 21.4% to 31.4% in 2019 and is expected to increase by 6% in the next 10 years.2,3 Although simple steatosis without metabolic dysfunction has been shown to lead to favorable long-term outcomes, NAFLD per se can be a risk factor for other metabolic diseases including diabetes, hypertension, cardiovascular disease, and chronic kidney disease.3-5 Of these, a close association between NAFLD and sarcopenia has been established.6,7 Sarcopenia is regarded as an early manifestation of liver injury and is a prognostic marker for liver disease.8 Since lipid dysregulation, insulin resistance, and chronic inflammation play fundamental roles in liver and muscle tissues,8 there may be an independent association between sarcopenia and NAFLD and their interactions.
Lifestyle patterns, which are mostly accounted for by dietary intake, are associated with a higher risk of NAFLD. High fat, particularly saturated fat, and a refined carbohydrate-rich diet are well-known risk factors for NAFLD.9 Increased sodium intake has been reported to be a risk factor for insulin resistance.10 Furthermore, an association between sodium intake and obesity and body fat distribution has been proposed.11 However, the association among NAFLD, sarcopenia, and sodium intake has not been elucidated. Previous studies on the role of the high-sodium diet have focused on cardiovascular diseases, primarily hypertension.
Thus, we investigated whether a high-sodium diet, estimated using urinary sodium excretion, is related significantly to the risk of NAFLD or sarcopenia, as well as whether a high sodium intake enhances the risk of cardiovascular risk among the participants with NAFLD and sarcopenia. The aim of this paper was whether high sodium intake (exposure) has association to NAFLD or sarcopenia (as outcomes).
MATERIALS AND METHODS
1. Study population
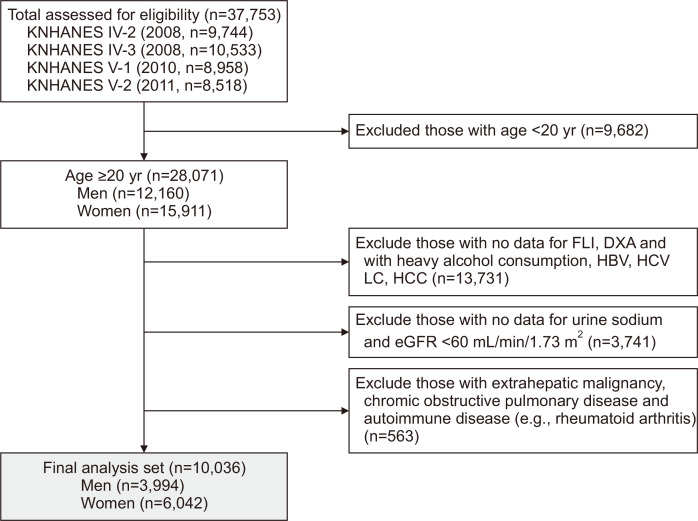
In this study, we used the Korea National Health and Nutrition Examination Survey (KNHANES) dataset from 2008 to 2011. The KNHANES is a national health survey that monitors the fitness and nutritive conditions of South Koreans. Each KNHANES was established on autonomous datasets of the civilian population, recruited randomly from 600 districts in the cities and provinces in South Korea.12 As presented Fig. 1, KNHANES 2008–2011, included 37,753 participants. Initially, we selected 28,071 adults (≥20 years old, 12,160 men and 15,911 women) and excluded 17,214 participants with the following conditions: (1) inadequate clinical and laboratory data to estimate liver steatosis or muscle mass; (2) presence of viral hepatitis (positive serologic markers of hepatitis B or C virus), history of hepatocellular carcinoma, or liver cirrhosis; (3) heavy alcohol consumption (>210 g/wk for men and 140 g/wk for women); (4) missing data for urine sodium or with chronic kidney disease. Chronic kidney disease was defined as an estimated glomerular filtration rate of <60 mL/min/1.73 m2. Additionally, as chronic disease including extrahepatic malignancy, chronic obstructive pulmonary disease and autoimmune disease such as rheumatoid arthritis can affect muscle mass, we excluded those populations. The final study analysis consisted of 10,036 participants (3,994 men and 6,042 women) with complete data.
Fig. 1.
Flow diagram of the inclusion and exclusion criteria used in the KNHANES IV and V. Of all the study participants (n=37,753), 10,036 were finally eligible (3,994 men and 6,042 women).
KNHANES, Korea National Health and Nutrition Examination Survey; FLI, fatty liver index; DXA, dual energy X-ray absorptiometry; HBV, hepatitis B virus; HCV, hepatitis C virus; LC, liver cirrhosis; HCC, hepatocellular carcinoma; eGFR, estimated glomerular filtration rate.
All participants provided written informed consent before the health examinations and surveys commenced, and the KNHANES complied with the requirements of the Institutional Review Board of the Korea Center for Disease Control and Prevention (2008-04EXP-01-C, 2009-01CON-03-2C, 2010-02CON-21-C, and 2011-02CON-06C).
2. Evaluation of clinical and laboratory parameters
The methods used for the current study have been described previously.6,7,11,12 Briefly, the KNHANES data consisted of three-parts: medical history, nutritional status, and laboratory tests. The medical history included disease diagnosis and/or treatment and health-related behaviors, such as smoking, alcohol consumption, and physical activity. Data were obtained using direct interviews and self-reporting. After overnight (≥8 hours) fasting, the participants underwent blood tests and spot urinalysis. The samples were refrigerated immediately and transferred to a central laboratory (NeoDin Medical Institute, Seoul, Korea). Hypertension was defined as blood pressure ≥130/85 mm Hg or taking antihypertensive medications, and diabetes as fasting plasma glucose more than 126 mg/dL or taking oral anti-hyperglycemic agents. Regular exercise was characterized as involving intense athletics, resulting in exhaustion or gasping for breath, and with engagement of over 20 minutes per session at least three times a week.6 We defined hyper low-density lipoprotein (LDL) cholesterolemia as the participant’s LDL cholesterol goal being within the recommendations of the 2004 update of the Adult Treatment Panel III guidelines or taking anti-dyslipidemia drugs.13
3. Confirmation of NAFLD and sarcopenia
Hepatic steatosis was assessed as a fatty liver index and NAFLD was defined as a fatty liver index ≥30.14 Data on the appendicular skeletal muscle mass between 2008 and 2011, was obtained using the dual-energy X-ray absorptiometry (QDR 4500A; Hologic Inc., Bedford, MA, USA) test. Sarcopenia was defined as total appendicular skeletal muscle mass (kg) divided by body weight, and we defined sarcopenia when the result was less than one standard deviation below the sex-specific mean of healthy adults aged 20 to 39 years,15 with the cutoff for men and women being 30.92% and 24.35%, respectively.
4. Assessment of salt intake by 24-hour urinary sodium excretion
The sodium intake was estimated according to the 24-hour urinary sodium that was assessed using the sodium and creatinine levels in spot urine samples with Tanaka’s equation. The estimated 24-hour urinary sodium excretion was calculated as follows (mmol/day): 21.98 × XNa0.392, where XNa=[spot urinary sodium (mmol/L)/spot urinary creatinine (mg/dL)×10]×Pr24-hourCr (mg/day).
Pr24-hourCr (mg/day)=–2.04×age (yr)+14.89×body weight (kg)+16.14×height (cm)–2,244.45.16 We classified the participants into tertile groups according to their 24-hour urinary sodium excretion levels.
5. Statistical analysis
All statistical analyses were conducted using IBM SPSS version 27.0 for Windows (IBM Corp., Armonk, NY, USA); p<0.05 was considered to be statistically significant. Results are presented as means±standard deviations for continuous variables and numbers (n) or percentages (%) for categorical variables. A one-way analysis of variance and chi-square tests were used to analyze the sex-specific tertiles of urinary sodium excretion. Both were followed by post hoc analyses using the Bonferroni method. Values that were not normally distributed (triglyceride, high-density lipoprotein cholesterol, LDL cholesterol, insulin, homeostasis model assessment of insulin resistance, aspartate aminotransferase, alanine aminotransferase, gamma glutamyl transpeptidase, and platelets) were log-transformed to the original scale to achieve approximately symmetrical distributions. Although we set high sodium intake as exposure, and NAFLD and sarcopenia as outcomes, we assessed the association of higher 24-hour urinary sodium excretion levels with the risk of sarcopenia and NAFLD, the highest 24-hour urinary sodium excretion tertile group was compared against lower 24-hour urinary sodium excretion, including both the lowest and middle tertile groups (T1 and T2). A multivariate logistic regression analysis was used to confirm the independent association among NAFLD, sarcopenia, and 24-hour urinary sodium excretion, with a multi-step adjustment. To measure the prediction accuracy of the 24-hour urinary sodium excretion for NAFLD, we analyzed the area under the receiver operating characteristic (AUROC) curve and calculated the sensitivity, specificity and cutoff value. To analyze the interaction between high sodium intake and NAFLD in sarcopenia, we applied two-way analysis of variance (NAFLD and urinary sodium excretion as fixed factors, and muscle mass adjusted by body weight as a dependent variable).
RESULTS
1. Study population
A total of 10,036 participants (3,994 men and 6,042 women) with complete information were included in the final statistical analysis (Fig. 1). Table 1 describes the clinical characteristics of the study population, based on urinary sodium excretion. The mean 24-hour urinary sodium excretion was 144.2±36.1 mmol/day (corresponding to 3.3 g/day Na) in the total population. There was no significant difference in the 24-hour urinary sodium excretion by sex; 144.6±35.3 mmol/day for men and 143.9±36.6 mmol/day for women (p=0.364). A total of 3,784 participants (37.7%) had NAFLD, whereas the remaining participants (n=6,252, 62.3%) did not. Additionally, 1,557 participants (15.5%) were identified as having sarcopenia. The mean age, body mass index, waist circumference, blood pressure, fasting blood glucose, homeostasis model assessment of insulin resistance, total cholesterol, and triglyceride concentrations were significantly greater in participants with a higher 24-hour urinary sodium excretion than in those with a lower urinary sodium excretion (all p<0.05). The proportion of sarcopenia that divided appendicular muscle mass into body weight was significantly higher in the highest 24-hour urinary sodium excretion group than in the lowest 24-hour urinary sodium excretion group (13.4% vs 18.5%, p<0.001). The participants who excreted a higher urinary sodium had elevated liver enzymes (aspartate aminotransferase, alanine aminotransferase, and gamma glutamyl transpeptidase), triglycerides, LDL cholesterol, and homeostasis model assessment of insulin resistance than those with lower urinary sodium excretion (all p<0.05), while their kidney function (estimated glomerular filtration rate) and high-density lipoprotein cholesterol levels showed a significant attenuation (p<0.05). The prevalence of hypertension, metabolic syndrome, diabetes, NAFLD, and sarcopenia was significantly greater in the participants with the highest 24-hour urinary sodium excretion than in the other groups (all p<0.05).
Table 1.
Baseline Characteristics of the Study Population by Categories of 24-Hour Urinary Sodium Excretion
| Characteristics | Tertiles of 24-hour urinary sodium excretion (mmol/day) | p-value* | ||
|---|---|---|---|---|
| T1 (n=3,345) | T2 (n=3,345) | T3 (n=3,345) | ||
| 24-Hour urinary sodium, mmol/day | 106.1±16.4 | 142.1±8.7† | 184.3±22.3†,‡ | <0.001 |
| Daily energy intake, kcal | 1,863±819 | 1,907±785 | 1,875±763 | 0.085 |
| Age, yr | 45.6±16.3 | 49.5±15.3† | 53.1±15.0†,‡ | <0.001 |
| Male sex | 1,301 (38.9) | 1,335 (39.9) | 1,358 (40.6) | 0.157 |
| Waist circumference, cm | 78.1±10.0 | 80.4±9.3† | 83.8±9.3†,‡ | <0.001 |
| Body mass index, kg/m2 | 22.9±3.3 | 23.5±3.1† | 24.5±3.3†,‡ | <0.001 |
| Appendicular muscle mass, kg | 18.1±4.9 | 18.4±4.9† | 19.0±4.8†,‡ | <0.001 |
| Systolic blood pressure, mm Hg | 115.9±16.0 | 119.8±17.1† | 124.5±18.2†,‡ | <0.001 |
| Diastolic blood pressure, mm Hg | 75.5±10.0 | 77.2±10.3† | 79.2±10.7†,‡ | <0.001 |
| Fasting plasma glucose, mg/dL | 95.6±23.3 | 96.5±19.7 | 99.0±22.8† | <0.001 |
| Total cholesterol, mg/dL | 187.0±35.1 | 188.6±35.9 | 191.7±35.7†,‡ | <0.001 |
| HDL cholesterol, mg/dL§ | 52.8±12.5 | 51.7±12.4† | 50.8±11.8†,‡ | <0.001 |
| Triglycerides, mg/dL§ | 115.0±81.1 | 126.3±91.9† | 141.0±95.8†,‡ | <0.001 |
| LDL cholesterol, mg/dL§ | 114.9±31.5 | 115.8±31.4 | 117.3±31.2† | 0.012 |
| eGFR, CKD-EPI, mL/min/1.73 m2 | 98.2±15.6 | 96.8±15.2† | 96.1±14.4† | <0.001 |
| Platelet, 109/L§ | 258.4±55.5 | 255.0±58.0† | 256.9±58.9 | 0.113 |
| Insulin, μU/mL§ | 9.6±5.2 | 10.1±6.4† | 10.6±5.6†,‡ | <0.001 |
| HOMA-IR§ | 2.3±1.6 | 2.5±2.5† | 2.7±2.4†,‡ | <0.001 |
| AST, IU/dL | 20.9±8.7 | 21.3±10.2† | 22.4±9.1†,‡ | <0.001 |
| ALT, IU/dL | 20.1±15.1 | 20.7±15.0† | 22.2±16.2†,‡ | <0.001 |
| GGT, IU/dL | 27.4±35.8 | 27.0±30.9 | 29.3±32.3†,‡ | <0.001 |
| Vitamin D, ng/mL | 17.1±6.5 | 17.7±6.5† | 18.2±6.5†,‡ | <0.001 |
| FLI | 23.2±22.9 | 27.0±22.9† | 33.9±24.7†,‡ | <0.001 |
| FLI ≥30 | 965 (28.8) | 1,213 (36.3)† | 1,606 (48.0)†,‡ | <0.001 |
| Sarcopenia | 447 (13.4) | 492 (14.7) | 618 (18.5)†,‡ | <0.001 |
| Current smoking | 686 (20.5) | 554 (16.6)† | 473 (14.1)†,‡ | <0.001 |
| Moderate-intensity exercise | 496 (14.8) | 504 (15.1) | 497 (14.9) | 0.977 |
| Hypertension | 768 (23.0) | 878 (26.2)† | 1,144 (34.2)†,‡ | <0.001 |
| Diabetes | 273 (8.2) | 276 (8.3) | 400 (12.0)†,‡ | <0.001 |
| Metabolic syndrome | 744 (22.2) | 907 (27.1)† | 1,275 (38.1)†,‡ | <0.001 |
Data are presented as mean±SD or number (%).
T1, lowest tertile, T2, middle tertile; T3, highest tertile; HDL, high-density lipoprotein; LDL, low density lipoprotein; eGFR, estimated glomerular filtration rate; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; HOMA-IR, homeostasis model assessment of insulin resistance; AST, aspartate aminotransferase; ALT, alanine aminotransferase; GGT, gamma glutamyl transpeptidase; FLI, fatty liver index.
*Chi-square tests for qualitative variables and analysis of variance tests for quantitative variables; †p<0.05 by post hoc analyses when compared with the lowest tertile; ‡p<0.05 by post hoc analyses when compared with the middle tertile; §Log-transformed.
2. The risk of NAFLD in 24-hour urinary sodium excretion status and sarcopenia
We analyzed the risk of NAFLD according to the 24-hour urinary sodium excretion and sarcopenia, after adjusting for confounding factors (Table 2). The participants with a high urinary sodium excretion had a significantly higher risk of NAFLD than those with a low urinary sodium excretion (crude odds ratio [OR], 2.28; 95% confidence interval [CI], 2.06 to 2.52; fully adjusted model OR, 1.46; 95% CI, 1.27 to 1.66, all p<0.001). We then divided the study population according to the presence of sarcopenia and analyzed the risk of NAFLD. Similarly, a gradual increase in the risk of NAFLD from the lowest to the highest 24-hour urinary sodium excretion, was observed in both the sarcopenia present and absent groups with the risk of NAFLD being slightly higher in the sarcopenia absent group (OR 2.10, 95% CI 1.82 to 2.42, p<0.001 for sarcopenia absence group; OR 1.68, 95% CI 1.24 to 2.29, p<0.001 for sarcopenia presence group).
Table 2.
Crude and Adjusted Odds Ratios of NAFLD According to 24-Hour Urinary Sodium Excretion
| Variable | Crude | Adjusted model | |||
|---|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | ||
| Total population (n=10,036) | |||||
| T1 | Reference | Reference | |||
| T2 | 1.40 (1.27–1.56) | <0.001 | 1.25 (1.09–1.43) | 0.001 | |
| T3 | 2.28 (2.06–2.52) | <0.001 | 1.46 (1.27–1.66) | <0.001 | |
| Sarcopenia absence (n=8,464)* | |||||
| T1 | Reference | Reference | |||
| T2 | 1.49 (1.32–1.67) | <0.001 | 1.46 (1.27–1.69) | <0.001 | |
| T3 | 2.33 (2.07–2.61) | <0.001 | 2.10 (1.82–2.42) | <0.001 | |
| Sarcopenia presence (n=1,157)* | |||||
| T1 | Reference | Reference | |||
| T2 | 1.04 (0.80–1.35) | 0.775 | 1.02 (0.75–1.39) | 0.892 | |
| T3 | 1.77 (1.37–2.29) | <0.001 | 1.68 (1.24–2.29) | 0.001 | |
Adjusted model: adjusted for age, sex, exercise, smoking, body mass index, hypertension, diabetes, dyslipidemia, aspartate aminotransferase†, alanine aminotransferase†, homeostasis model assessment of insulin resistance†, vitamin D, estimated glomerular filtration rate, Chronic Kidney Disease Epidemiology Collaboration, and appendicular muscle mass.
NAFLD, nonalcoholic fatty liver disease; OR, odds ratio; CI, confidence interval; T1, lowest tertile, T2, middle tertile; T3, highest tertile.
*In subgroup analysis by sarcopenia, appendicular muscle mass was excluded in the adjusted model; †Log-transformed.
3. The risk of sarcopenia based on 24-hour urinary sodium excretion status depends on the presence of NAFLD
Since we observed an independent association between NAFLD and the 24-hour urinary sodium excretion, we further analyzed the risk of sarcopenia according to the 24-hour urinary sodium excretion and NAFLD using adjusted models (Table 3). The participants with a higher urinary sodium excretion had a significantly higher risk of sarcopenia than those with lower urinary sodium excretion (crude OR, 1.47; 95% CI, 1.29 to 1.68; fully adjusted model OR, 1.49; 95% CI, 1.28 to 1.73, all p<0.001). In the subgroup analysis according to the NAFLD status, the risk for sarcopenia was higher in participants who excreted a higher urinary sodium excretion but without NAFLD in the fully adjusted model (OR, 1.91; 95% CI, 1.49 to 2.45; p<0.001); however, this statistical significance was diminished in participants with NAFLD. This different outcome reflects that NAFLD might be an effect modifier to sarcopenia in high sodium intake, therefore, we analyzed the interaction between high sodium intake and NAFLD in the risk of sarcopenia. A two-way analysis of variance was conducted to examine the effect of NAFLD and high sodium intake on sarcopenia. There was a statistically significant interaction between the effect of NAFLD and high sodium intake on the muscle mass (F=11.70, p<0.001) (Supplementary Table 1). Bonferroni’s post hoc comparisons indicated that, as hypothesized, individuals with high sodium intake had 0.68 kg/kg (p<0.001; 95% CI of the difference, –0.94 to –0.42) lower than the lowest sodium intake group, 0.34 kg/kg (p=0.006; 95% CI of the difference, –0.60 to –0.08) lower than the middle sodium intake group.
Table 3.
Crude and Adjusted Odds Ratios of Sarcopenia According to 24-Hour Urinary Sodium Excretion
| Variable | Crude | Adjusted model | |||
|---|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | ||
| Total population (n=10,036) | |||||
| T1 | Reference | Reference | |||
| T2 | 1.12 (0.97–1.29) | 0.113 | 1.11 (0.95–1.29) | 0.178 | |
| T3 | 1.47 (1.29–1.68) | <0.001 | 1.49 (1.28–1.73) | <0.001 | |
| NAFLD absence (n=6,252)* | |||||
| T1 | Reference | Reference | |||
| T2 | 1.22 (0.99–1.51) | 0.061 | 1.41 (1.12–1.77) | 0.03 | |
| T3 | 1.34 (1.07–1.66) | 0.009 | 1.91 (1.49–2.45) | <0.001 | |
| NAFLD presence (n=3,784)* | |||||
| T1 | Reference | Reference | |||
| T2 | 0.85 (0.70–1.04) | 0.109 | 0.85 (0.68–1.05) | 0.845 | |
| T3 | 1.02 (0.85–1.22) | 0.856 | 1.09 (0.89–1.34) | 0.383 | |
Adjusted model, adjusted for age, sex, exercise, smoking, body mass index, hypertension, diabetes, dyslipidemia, aspartate aminotransferase†, alanine aminotransferase†, homeostasis model assessment of insulin resistance†, vitamin D, estimated glomerular filtration rate, Chronic Kidney Disease Epidemiology Collaboration, and appendicular muscle mass.
NAFLD, nonalcoholic fatty liver disease; OR, odds ratio; CI, confidence interval; T1, lowest tertile, T2, middle tertile; T3, highest tertile.
*In subgroup analysis by NAFLD, aspartate aminotransferase, and alanine aminotransferase were excluded in adjusted model; †Log-transformed.
4. Association between NAFLD, sarcopenia and a higher 24-hour urinary sodium excretion
To clarify the relationship between NAFLD, sarcopenia, and urinary sodium excretion, we compared the ORs of each parameter. Both sarcopenia and NAFLD were associated independently with a higher 24-hour urinary sodium excretion (OR 1.39, 95% CI 1.24 to 1.55, p<0.001 for sarcopenia; OR 1.19, 95% CI 1.03 to 1.38, p=0.016 for NAFLD) (Table 4). After multiple and sufficient adjustments, participants with both NAFLD and sarcopenia had a significantly strong relationship with a higher 24-hour urinary sodium excretion (OR, 1.34; 95% CI, 1.10 to 1.54; p=0.004), followed by those with sarcopenia but without NAFLD (OR, 1.25; 95% CI, 1.01 to 1.54; p=0.038) and participants with NAFLD but without sarcopenia (OR, 1.16; 95% CI, 1.02 to 1.31; p=0.027).
Table 4.
Association of NAFLD, Sarcopenia and the Highest 24-Hour Urinary Sodium Excretion Tertile (T3)
| Variable | Crude | Adjusted model | |||
|---|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | ||
| Sarcopenia | |||||
| Sarcopenia (–) | Reference | Reference | |||
| Sarcopenia (+) | 1.39 (1.24–1.55) | <0.001 | 1.19 (1.03–1.38) | 0.016 | |
| NAFLD | |||||
| NAFLD (–) | Reference | Reference | |||
| NAFLD (+) | 1.91 (1.76–2.08) | <0.001 | 1.14 (1.01–1.29) | 0.031 | |
| NAFLD×sarcopenia | |||||
| NAFLD (–)×sarcopenia (–) | Reference | Reference | |||
| NAFLD (+)×sarcopenia (–) | 1.89 (1.72–2.07) | <0.001 | 1.16 (1.02–1.31) | 0.027 | |
| NAFLD (–)×sarcopenia (+) | 1.21 (1.01–1.46) | 0.048 | 1.25 (1.01–1.54) | 0.038 | |
| NAFLD (+)×sarcopenia (+) | 2.10 (1.83–2.41) | <0.001 | 1.34 (1.10–1.54) | 0.004 | |
Adjusted model, adjusted for adjusted for age, sex, exercise, smoking, body mass index, hypertension, diabetes, dyslipidemia, aspartate aminotransferase*, alanine aminotransferase*, homeostasis model assessment of insulin resistance*, vitamin D, estimated glomerular filtration rate, Chronic Kidney Disease Epidemiology Collaboration, and appendicular muscle mass.
NAFLD, nonalcoholic fatty liver disease; OR, odds ratio; CI, confidence interval.
*Log-transformed.
5. The 24-hour urinary sodium excretion AUROC, cutoff value for NAFLD and sarcopenia
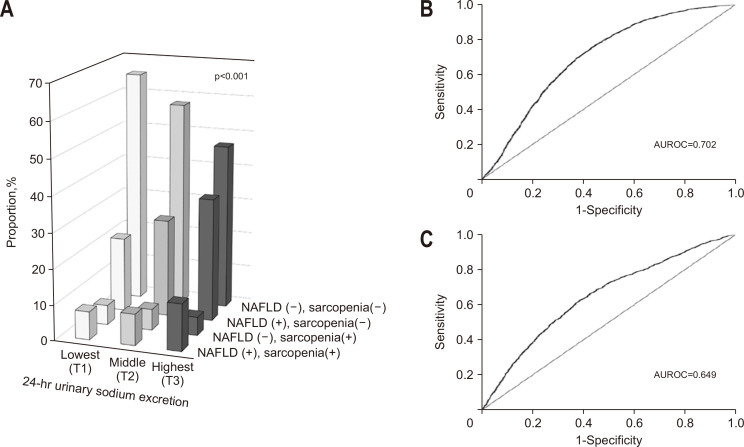
To demonstrate the association between the 24-hour urinary excretion, NAFLD, and sarcopenia, we stratified the study population according to the presence or absence of NAFLD/sarcopenia and the tertiles of 24-hour urinary sodium excretion. As shown in Fig. 2A, the 24-hour urinary sodium excretion exhibited a strong positive association with NAFLD and sarcopenia (p for trend <0.001). We then evaluated the feasibility of using the 24-hour urinary excretion levels and performed an AUROC curve analysis. The 24-hour urinary sodium excretion showed moderate accuracy in predicting NAFLD (AUROC, 0.702; 95% CI, 0.692 to 0.712) (Fig. 2B). The 24-hour urinary sodium excretion levels showed an acceptable discrimination in predicting sarcopenia (AUROC, 0.649; 95% CI, 0.634 to 0.664) (Fig. 2C). Furthermore, the diagnostic performance of the 24-hour urinary sodium excretion, with a cutoff value of 99.96 mmol/day (corresponding to 2.30 g/day Na) in predicting NAFLD, showed 76.1% sensitivity and 56.1% specificity.
Fig. 2.
The association between 24-hour urinary sodium excretion, nonalcoholic fatty liver disease (NAFLD), and sarcopenia. (A) Proportion of participants by NAFLD, sarcopenia and 24-hour urinary sodium creation tertiles. (B) ROC and AUROC to predict NAFLD characterized fatty liver index ≥30. (C) ROC and AUROC to predict sarcopenia.
ROC, receiver operating characteristics; AUROC, area under the receiver operating characteristics curve.
DISCUSSION
In this large and nationally representative data-based study, we demonstrated that participants with a higher sodium intake had higher risks of both NAFLD and sarcopenia. We also found that the average urinary sodium excretion was 3.3 g/day in the general Korean population. The 24-hour urinary sodium excretion levels showed a greater accuracy in predicting NAFLD (AUROC, 0.702; 95% CI, 0.692 to 0.712) than in predicting sarcopenia (AUROC, 0.649; 95% CI, 0.634 to 0.664). With regard to NAFLD risk, as reflected by the fatty liver index, the participants with the highest 24-hour urinary sodium excretion showed a 1.46-fold higher risk of NAFLD than the participants with the lowest 24-hour urinary sodium excretion. The risk was maintained independent of the presence of sarcopenia and was increased by up to 2.10 in the participants without sarcopenia. Moreover, the risk of sarcopenia increased by 1.49-fold in the participants with the highest 24-hour urinary sodium excretion; however, the risk was only maintained in the non-NAFLD group. By analyzing the interaction between sodium intake and NAFLD in sarcopenia, we found that NAFLD might be an effect modifier to sarcopenia in high sodium intake. Additionally, the association between a high sodium intake and NAFLD and sarcopenia was stronger where there was a coexistence of NAFLD and sarcopenia rather than in NAFLD or sarcopenia alone. All these findings may indicate that a reduction in sodium intake is clinically important for the general population who are at a risk of NAFLD and sarcopenia.
Our study has several clinical implications. First, high sodium intake may contribute to both NAFLD and sarcopenia, and this association was more dominant in NAFLD than in sarcopenia. Previous studies have shown the impact of high sodium intake on single metabolic dysfunction, including diabetes, hypertension, NAFLD, and sarcopenia.11,17,18 Similarly, our results demonstrated that participants in the highest urinary sodium excretion tertile group were more likely to have hypertension, diabetes, and the metabolic syndrome. Our study further demonstrated the higher risks of both NAFLD and sarcopenia with an increased sodium intake and an integration of the risks for each disease. In parallel with the well-known fact that insulin resistance is a critical cornerstone of metabolic diseases, which is exacerbated by high sodium intake,19 recent experimental models have proposed that a high sodium intake modulates pro-inflammatory cytokines and mineralocorticoid receptors, thereby activating organ fibrosis.20,21 Excessive salt intake can lead dysregulation in microvascular reactive oxygen species, resulting endothelial dysfunction.22 Decreased arteriolar responsiveness to endothelium dependent stimuli is observed in arteries and muscles.22,23 Additionally, evidences support that high sodium diet endogenous fructose production and accelerate leptin resistance which can drive sarcopenia and fatty liver disease.24,25 Thus, it appears that a high sodium intake implicates both NAFLD and sarcopenia, although the underlying mechanisms of high salt intake on liver and muscle interactions have not been fully elucidated. We also found that the risk of NAFLD was higher in the participants who excreted high urinary sodium without sarcopenia (OR: 2.10 vs 1.68), whereas the risk of sarcopenia did not increase in the participants with both high urinary sodium excretion and NAFLD, which may indicate that a high sodium intake is more profound in NAFLD than in sarcopenia. Considering the relative effect of NAFLD and sarcopenia on mortality, NAFLD alone, does not increase the death rate,26 while sarcopenia may be a more advanced metabolic derangement than NAFLD. Therefore, dietary education to enable the reduction of sodium consumption should be considered in the early stages of NAFLD before the development of sarcopenia.
Second, the current study showed that the average daily sodium consumption, assessed by urinary excretion, 3.3 g in the general Korean population. This value was similar to that of a previous study that analyzed the 24-hour urine collection in Korea.27 Although there were some efforts to reduce dietary sodium intake in Korea,28 this level was still higher than the sodium intake recommended by the World Health Organization (2 g/day).29 In Korea, kimchi and noodles are the main contributors to dietary sodium.28 Moreover, our data has suggested a potential cutoff value for sodium intake in NAFLD of 2.30 g/day. This cutoff was similar to that for systemic oxidative stress and inflammatory response as well as for cardiac remodeling after myocardial infarction.30 Similarly, it has been shown that a reduction in salt consumption by 9 g/day (e.g., from 12 to 3 g/day, 3 g/day of salt corresponding to 1.2 g/day Na) would reduce death from stroke by 36% and death from coronary heart disease by 27%.31 Considering that cardiovascular disease is the most common contributory cause of death in NAFLD,32 our results provided evidence of the usefulness of the dietary sodium intake cutoff value in NAFLD. We demonstrated previously that the coordinated impact of decreased muscle mass and NAFLD on the risk of cardiovascular disease,6 and high sodium intake may be responsible for increased occurrence of cardiovascular disease among the participants with NAFLD and sarcopenia. Furthermore, the dietary acid load derived from dietary potassium was associated with cardiovascular disease in our previous study,33 and the reduction in sodium in addition to an increase in dietary potassium may have provided the necessity for appropriate dietary intervention for participants with NAFLD and/or sarcopenia who are vulnerable to cardiovascular disease risk.
Third, our data demonstrated the clinical usefulness of urinary sodium in predicting NAFLD. Considering the burden of NAFLD on various other cardiometabolic diseases and disease progression, the characterization of the participants with NAFLD is important for identifying high-risk populations. In the current study, the AUROC value for NAFLD was 0.702, and the participants with the highest urinary sodium excretion showed a much greater risk of NAFLD after adjusting for hypertension, diabetes, obesity, and muscle mass (OR, 1.46; 95% CI, 1.27 to 1.66). Additionally, this association was strengthened when there was a coexistence with sarcopenia (OR, 1.16; 95% CI, 1.02 to 1.31 for NAFLD alone vs OR, 1.34; 95% CI, 1.10 to 1.54 for NAFLD with sarcopenia).
Although the findings are of clinical significance, this study had several limitations. First, while we used a validated equation to assess the dietary sodium intake with spot urine, the 24-hour collection of urine provided the most accurate assessment of salt intake. Similarly, a well-established NAFLD prediction model was used in our study because it was not possible to consider imaging studies or biopsy results. Second, since the present study was based on cross-sectional data, we could not confirm the causality or interaction between status changes in urinary sodium excretion, NAFLD, and sarcopenia. Third, despite the exclusion of the participants with impaired renal function (estimated glomerular filtration rate <60 mL/min/1.73 m2), the use of spot urinary sodium excretion may have underestimated the sodium intake. Third, the identification of sarcopenia depended solely on muscle mass because of the lack of information on muscle strength or physical performance. Lastly, due to the lack of drug information in KNHANES, we could not assess the diuretics use, adrenal dysfunction or urinary tract infection which can affect urinary sodium excretion.
In conclusion, the participants with high sodium levels, as assessed by urinary sodium excretion, had a substantially increased risk of both NAFLD and sarcopenia. Sarcopenia further increased the risk of NAFLD. Further studies are necessary to clarify the role of dietary sodium intake in integrating muscle and liver interactions and whether reduction in dietary sodium consumption may decrease the metabolic burden of NAFLD and sarcopenia. It is recommended that prospective and long-term follow-up studies targeting the prognostic importance of sodium intake on NAFLD and sarcopenia be conducted in order to develop a meaningful and practical dietary guideline which may include energy and caloric restrictions, macronutrient (protein/carbohydrate/fat) compounds, and sodium consumption as a fundamental component that encourages patient education and improves understanding.
Funding Statement
ACKNOWLEDGEMENTS This research was supported by a Bisa Research Grant from Keimyung University in 2020 (20210807).
Footnotes
SUPPLEMENTARY MATERIALS
Supplementary materials can be accessed at https://doi.org/10.5009/gnl220133.
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTIONS
Study concept and design: E.H., B.K.J. Data acquisition: E.H. Data analysis and interpretation: E.H., B.K.J. Drafting of the manuscript: E.H., B.K.J. Critical revision of the manuscript for important intellectual content: M.K.K., S.S.I., H.S.K., T.K.K. Statistical analysis: E.H. Obtained funding: E.H. Administrative, technical, or material support; study supervision: E.H., B.K.J. Approval of final manuscript: all authors.
REFERENCES
- 1.Younossi Z, Anstee QM, Marietti M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15:11–20. doi: 10.1038/nrgastro.2017.109. [DOI] [PubMed] [Google Scholar]
- 2.Estes C, Chan HL, Chien RN, et al. Modelling NAFLD disease burden in four Asian regions: 2019-2030. Aliment Pharmacol Ther. 2020;51:801–811. doi: 10.1111/apt.15673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park J, Lee EY, Li J, et al. NASH/liver fibrosis prevalence and incidence of nonliver comorbidities among people with NAFLD and incidence of NAFLD by metabolic comorbidities: lessons from South Korea. Dig Dis. 2021;39:634–645. doi: 10.1159/000514953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim Y, Han E, Lee JS, et al. Cardiovascular risk is elevated in lean subjects with nonalcoholic fatty liver disease. Gut Liver. 2022;16:290–299. doi: 10.5009/gnl210084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Han E, Lee YH, Lee JS, et al. Fibrotic burden determines cardiovascular risk among subjects with metabolic dysfunction-associated fatty liver disease. Gut Liver. 2022;16:786–797. doi: 10.5009/gnl210290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Han E, Lee YH, Kim YD, et al. Nonalcoholic fatty liver disease and sarcopenia are independently associated with cardiovascular risk. Am J Gastroenterol. 2020;115:584–595. doi: 10.14309/ajg.0000000000000572. [DOI] [PubMed] [Google Scholar]
- 7.Han E, Lee YH, Kim BK, et al. Sarcopenia is associated with the risk of significant liver fibrosis in metabolically unhealthy subjects with chronic hepatitis B. Aliment Pharmacol Ther. 2018;48:300–312. doi: 10.1111/apt.14843. [DOI] [PubMed] [Google Scholar]
- 8.Bhanji RA, Narayanan P, Allen AM, Malhi H, Watt KD. Sarcopenia in hiding: the risk and consequence of underestimating muscle dysfunction in nonalcoholic steatohepatitis. Hepatology. 2017;66:2055–2065. doi: 10.1002/hep.29420. [DOI] [PubMed] [Google Scholar]
- 9.Glass O, Filozof C, Noureddin M, et al. Standardisation of diet and exercise in clinical trials of NAFLD-NASH: recommendations from the Liver Forum. J Hepatol. 2020;73:680–693. doi: 10.1016/j.jhep.2020.04.030. [DOI] [PubMed] [Google Scholar]
- 10.Vedovato M, Lepore G, Coracina A, et al. Effect of sodium intake on blood pressure and albuminuria in type 2 diabetic patients: the role of insulin resistance. Diabetologia. 2004;47:300–303. doi: 10.1007/s00125-003-1303-5. [DOI] [PubMed] [Google Scholar]
- 11.Huh JH, Lim JS, Lee MY, Chung CH, Shin JY. Gender-specific association between urinary sodium excretion and body composition: analysis of the 2008-2010 Korean National Health and Nutrition Examination Surveys. Metabolism. 2015;64:837–844. doi: 10.1016/j.metabol.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 12.Kweon S, Kim Y, Jang MJ, et al. Data resource profile: the Korea National Health and Nutrition Examination Survey (KNHANES) Int J Epidemiol. 2014;43:69–77. doi: 10.1093/ije/dyt228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stone NJ, Bilek S, Rosenbaum S. Recent National Cholesterol Education Program Adult Treatment Panel III update: adjustments and options. Am J Cardiol. 2005;96(4A):53E–59E. doi: 10.1016/j.amjcard.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 14.Bedogni G, Bellentani S, Miglioli L, et al. The Fatty Liver Index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006;6:33. doi: 10.1186/1471-230X-6-33.bfadc7e1511141379fa0188f090432a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim TN, Choi KM. Sarcopenia: definition, epidemiology, and pathophysiology. J Bone Metab. 2013;20:1–10. doi: 10.11005/jbm.2013.20.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tanaka T, Okamura T, Miura K, et al. A simple method to estimate populational 24-h urinary sodium and potassium excretion using a casual urine specimen. J Hum Hypertens. 2002;16:97–103. doi: 10.1038/sj.jhh.1001307. [DOI] [PubMed] [Google Scholar]
- 17.Kang MS, Kim CH, Jeong SJ, Park TS. Dietary sodium intake in people with diabetes in Korea: the Korean National Health and Nutrition Examination Survey for 2008 to 2010. Diabetes Metab J. 2016;40:290–296. doi: 10.4093/dmj.2016.40.4.290.3c4df60d47d948d99426d0855a0a989a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huh JH, Lee KJ, Lim JS, et al. High dietary sodium intake assessed by estimated 24-h urinary sodium excretion is associated with NAFLD and hepatic fibrosis. PLoS One. 2015;10:e0143222. doi: 10.1371/journal.pone.0143222.811e26a5d0b7451aaab322460d5edaf4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Donovan DS, Solomon CG, Seely EW, Williams GH, Simonson DC. Effect of sodium intake on insulin sensitivity. Am J Physiol. 1993;264(5 Pt 1):E730–E734. doi: 10.1152/ajpendo.1993.264.5.E730. [DOI] [PubMed] [Google Scholar]
- 20.Baudrand R, Lian CG, Lian BQ, et al. Long-term dietary sodium restriction increases adiponectin expression and ameliorates the proinflammatory adipokine profile in obesity. Nutr Metab Cardiovasc Dis. 2014;24:34–41. doi: 10.1016/j.numecd.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pizarro M, Solís N, Quintero P, et al. Beneficial effects of mineralocorticoid receptor blockade in experimental non-alcoholic steatohepatitis. Liver Int. 2015;35:2129–2138. doi: 10.1111/liv.12794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lenda DM, Boegehold MA. Effect of a high-salt diet on oxidant enzyme activity in skeletal muscle microcirculation. Am J Physiol Heart Circ Physiol. 2002;282:H395–H402. doi: 10.1152/ajpheart.0354.2001. [DOI] [PubMed] [Google Scholar]
- 23.Liu Y, Fredricks KT, Roman RJ, Lombard JH. Response of resistance arteries to reduced PO2 and vasodilators during hypertension and elevated salt intake. Am J Physiol. 1997;273(2 Pt 2):H869–H877. doi: 10.1152/ajpheart.1997.273.2.H869. [DOI] [PubMed] [Google Scholar]
- 24.Lanaspa MA, Kuwabara M, Andres-Hernando A, et al. High salt intake causes leptin resistance and obesity in mice by stimulating endogenous fructose production and metabolism. Proc Natl Acad Sci U S A. 2018;115:3138–3143. doi: 10.1073/pnas.1713837115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kohara K, Ochi M, Tabara Y, Nagai T, Igase M, Miki T. Leptin in sarcopenic visceral obesity: possible link between adipocytes and myocytes. PLoS One. 2011;6:e24633. doi: 10.1371/journal.pone.0024633.f4564d4ca54049cd9e679cdbbbc66091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim D, Wijarnpreecha K, Sandhu KK, Cholankeril G, Ahmed A. Sarcopenia in nonalcoholic fatty liver disease and all-cause and cause-specific mortality in the United States. Liver Int. 2021;41:1832–1840. doi: 10.1111/liv.14852. [DOI] [PubMed] [Google Scholar]
- 27.Nam GE, Kim SM, Choi MK, et al. Association between 24-h urinary sodium excretion and obesity in Korean adults: a multicenter study. Nutrition. 2017;41:113–119. doi: 10.1016/j.nut.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 28.Park HK, Lee Y, Kang BW, et al. Progress on sodium reduction in South Korea. BMJ Glob Health. 2020;5:e002028. doi: 10.1136/bmjgh-2019-002028.8aaa6331a010436bacab3c9cea263f46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.World Health Organization (WHO), author Guideline: sodium intake for adults and children [Internet] WHO; Geneva: c2012. [cited 2021 Dec 25]. Available from: https://www.who.int/publications/i/item/9789241504836 . [Google Scholar]
- 30.Costa AP, de Paula RC, Carvalho GF, et al. High sodium intake adversely affects oxidative-inflammatory response, cardiac remodelling and mortality after myocardial infarction. Atherosclerosis. 2012;222:284–291. doi: 10.1016/j.atherosclerosis.2012.02.037. [DOI] [PubMed] [Google Scholar]
- 31.He FJ, MacGregor GA. How far should salt intake be reduced? Hypertension. 2003;42:1093–1099. doi: 10.1161/01.HYP.0000102864.05174.E8. [DOI] [PubMed] [Google Scholar]
- 32.Paik JM, Henry L, De Avila L, Younossi E, Racila A, Younossi ZM. Mortality related to nonalcoholic fatty liver disease is increasing in the United States. Hepatol Commun. 2019;3:1459–1471. doi: 10.1002/hep4.1419.d3911e45bfb34ec2bfae99763b26004b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Han E, Kim G, Hong N, et al. Association between dietary acid load and the risk of cardiovascular disease: nationwide surveys (KNHANES 2008-2011) Cardiovasc Diabetol. 2016;15:122. doi: 10.1186/s12933-016-0436-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.