Feng and colleagues discuss work from the labs of Spencer Freeman and Sergio Grinstein as well as the lab of Xiaochen Wang demonstrating the lysosomal H+/Cl− exchanger ClC-7 drives luminal Cl− accumulation to activate acidic hydrolases.
Abstract
Lysosomal hydrolases require an acidic lumen for their optimal activities. In this issue, two independent groups (Wu et al. 2023. J. Cell Biol. https://doi.org/10.1083/jcb.202208155; Zhang et al. 2023. J. Cell. Biol. https://doi.org/10.1083/jcb.202210063) report that hydrolase activation also requires high intralysosomal Cl−, which is established by the lysosomal Cl−/H+ exchanger ClC-7.
Lysosomes are membrane-bound acidic organelles containing >60 different types of hydrolytic enzymes: proteases, lipases, glycosidases, and nucleases, which break down cargo macro-biomolecules such as proteins, lipids, carbohydrates, and nucleotides to provide the building-block molecules for the cell (1, 2). Most lysosomal hydrolases exhibit their optimal activities in the acidic (pH 4.5–5.0) environment of the lysosomal lumen; hence, they are referred to as acidic hydrolases (2, 3). Luminal acidity is established and maintained by pumping H+ into the lysosomes through the vacuolar-type H+-ATPases (V-ATPases) at the expense of ATP, resulting in a 500–1,000-fold H+ concentration gradient across the lysosomal membrane (3). While isolated lysosomes have a “resting” membrane potential (Δψ, defined as ψCytosol – ψLumen) close to 0 mV (4), native lysosomes of the cell have a V-ATPase–mediated H+ influx that may help establish a luminal-side-positive Δψ (−20 to −30 mV, that is, ∼20–30 mV more negative in the cytosol than in the lumen; 5; Fig. 1). This modest Δψ is maintained by various “background” ionic conductances. For example, “unopposed” V-ATPase activity leading to a more negative lysosomal Δψ would cause a re-adjustment in the contributions of “background” conductances to Δψ and an inhibition of the V-ATPase activity, both in a negative feedback manner (3, 4). Lysosomal pH homeostasis is also regulated by various proton efflux mechanisms in the lysosome (5). Both lysosomal acidification and Δψ play essential roles in the regulation of lysosomal degradation, ion homeostasis, endosome and (auto)phagosome maturation, export of degradation products, and lysosomal membrane trafficking, which in turn are required for the maintenance of lysosomal membrane integrity and homeostasis (1, 5). In this issue of JCB, two studies from Wu et al. (6) and Zhang et al. (7) independently identify lysosomal Cl− as a new regulator of lysosomal degradation, and report that the lysosomal Cl−/H+ exchanger ClC-7/CLCN7 is responsible for the creation and maintenance of a two- to fourfold trans-lysosomal concentration gradient of Cl− without affecting lysosomal acidification. These studies revealed an unexpected role of ClC-7 and high intralysosomal Cl− in hydrolase activation, which is required for (phago)lysosome resolution in macrophages and protecting lysosomal membrane integrity in vivo.
Figure 1.
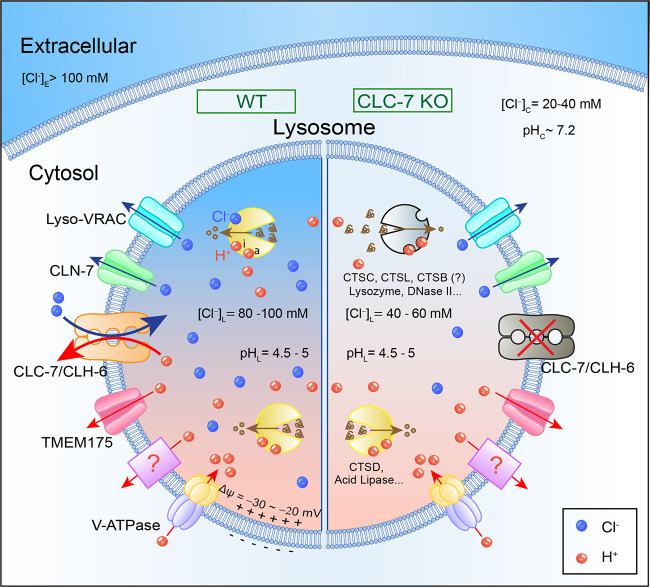
Regulation of lysosomal hydrolytic activities by intralysosomal Cl−. Lysosomes are acidic digestive organelles filled with >60 types of hydrolases. The acidic lumen (pH 4.5–5.0) is maintained by the activity of V-ATPase via pumping H+ from the cytosol into the lumen, as well as by the H+ leak pathways through TMEM175 or yet-to-be-identified H+ transporters. Lysosomal hydrolases, e.g., cathepsins, exhibit their optimal activities in an acidic and high Cl− environment to break down the endocytic and autophagic cargo materials. Luminal H+ binds to both activation (a) and inhibitory (i) sites to regulate the activities of lysosomal hydrolases, achieving maximal activation at optimal pH. Lysosomal Δψ (= ψCytosol − ψLumen) is estimated to be −20 to −30 mV. The cytosolic Cl− ranges from 20 to 40 mM, and lysosomal [Cl−] is estimated to be 80–100 mM in the WT cells (left). Hence, the ECl is approximately −25 mV, whereas EH is more than +140 mV. In the ClC-7 KO cells (right), lysosomal pH is normal, but intralysosomal [Cl−] is reduced to 40–60 mM. Hence, ClC-7, as an H+-dependent Cl− transporter, drives intralysosomal accumulation of Cl−, which is required for the activities of some hydrolases (e.g., cathepsin C [CTSC] and cathepsin L [CTSL]), likely through direct binding. In the ClC-7 KO cells, reduced intralysosomal Cl− levels lead to comprised lysosomal degradation capability.
In their efforts to identify new players in regulating lysosome function, Wu et al. and Zhang et al. converged on ClC-7/CLCN7, a lysosomal H+/Cl− transporter (6, 7). The study from Wu et al. explored the mechanisms of (phago)lysosomal resolution using macrophages, professional phagocytes with a very high demand in lysosomal cargo degradation (6). Phagosomes are formed during phagocytosis, an evolutionarily conserved process that maintains cellular homeostasis by digesting phagocytosed microbes and dead cells. Upon fusion with lysosomes, phagosomes mature to phagolysosomes. In their effort to find the phagosomal Cl− transporter/channel that is required for phagosomal degradation of IgG-opsonized sheep red blood cells (sRBCs), Wu et al. (6) identified ClC-7, a member of the ClC family Cl− channel/transporter (Fig. 1). While ClC-7 knockout (KO) macrophages exhibit normal phagosome acidification and maturation, the degradation of phagocytosed sRBC was blocked (6). Consistently, the hydrolytic activities of phagosomes, assessed by the artificial cargo dye-quenched bovine serum albumin in situ, were dramatically reduced (6). In a separate study, Zhang et al. (7) employed the Caenorhabditis elegans genetic model system to search for the molecular players that maintain lysosomal membrane integrity in vivo (7). They screened for mutant worms containing damaged lysosomal membranes by monitoring accumulation of fluorescently labeled Galectin-3, which enters ruptured lysosomes to bind to glycoconjugates in the luminal leaflet (7). In addition, fluorescently labeled lysosomal enzyme NUC-1 would also leak out of the lysosomes, but only if the lysosomal membranes are ruptured (7). CLH-6, the C. elegans ortholog of ClC-7, was identified; loss of CLH-6 caused an accumulation of endocytic and autophagic cargo, resulting in lysosomal membrane ruptures in the worm cells, particularly the hypodermal cells (7). The activities of two cysteine-type proteases, cathepsin B/CPR-2 and cathepsin L/CPL-1, were significantly reduced in multiple loss-of-function clh-6 mutants. Whereas genetic inactivation of cathepsin B or L phenocopied the clh6 mutant, overexpression of cathepsin B or L rescued the lysosomal defects associated with CLH-6 loss (7). In both studies, the effects of ClC-7 were dramatic, presumably because the cargo load and lysosomal degradation demand are high in both experimental settings.
How does ClC-7 deficiency reduce lysosomal hydrolase activity? ClC-7 is a lysosomal H+/Cl− exchanger, and previous studies have established an essential role of H+/Cl− transport coupling in its lysosomal functions (8). As an H+/Cl− exchanger, cells lacking ClC-7 may have an “H+ problem,” a “Cl− problem,” or both. Theoretically or mechanistically, ClC-7 can serve as an H+-dependent Cl− transporter or a Cl−-dependent H+ transporter. However, the equilibrium potential of lysosomal Cl− (ECl, approximately −25 mV) is at near “resting” lysosomal Δψ, whereas the driving force for lysosomal H+ efflux is huge (EH greater than +140 mV; Fig. 1). Therefore, from the lysosomal physiology point of view, ClC-7 may preferentially function as an H+-dependent Cl− transporter, driving H+-dependent Cl− flux. As a Cl−/H+ exchanger, ClC-7 may catalyze the uptake (influx) of two Cl− for one outward H+ (efflux) using the large pH gradient. However, even if ClC-7 KO cells have a primary Cl− problem, Cl− influx may provide the so-called counter ions for the continuous V-ATPase–mediated pumping (3, 9). In this hypothesis, Cl− influx was proposed to neutralize the accumulation of positive charges in the luminal leaflet and the lumina-side-positive Δψ due to V-ATPase–mediated H+ pumping into the lumen, and in doing so, enabling acidification of the organelles (9). In other words, ClC-7 KO cells might have a primary Cl− problem and a secondary H+ problem; the secondary acidification defects could fully explain the reduced hydrolase activities in the ClC-7 KO cells. However, both Wu et al. (6) and Zhang et al. (7) found that ClC-7 was dispensable for lysosomal acidification. Similar results have been reported previously (8–10), but in the current study by Wu et al. (6), not only the steady-state lysosomal pH, but also the rate of (phago)lysosomal acidification during phagosome maturation, were found to be normal in the ClC-7 KO macrophages.
The lack of acidification defects in ClC-7 KO raises several questions. First, is it possible that the transporter is only minimally active, based on the lack of an observed effect on the ClC-7–dependent H+ “leak” activity, which can be manifested by V-ATPase inhibition (6)? However, the dramatic lysosomal phenotypes in ClC-7 KO would suggest otherwise. Given the existence of several cationic conductances in the lysosomes of the cell, e.g., those mediated by TRPML and TPC Ca2+/Na+ channels, it is possible that in the native lysosomes of the cells no dedicated counter ion conductance is necessary. Hence, ClC-7 might only play a minor role in providing counter ions to support continuous V-ATPase H+ pumping. At the plasma membrane, Ca2+ ATPase and Na+-K+ ATPase can effectively and continuously pump Ca2+ and Na+ out of the cells at the resting membrane potential of −70 mV. As far as lysosomal Δψ is kept at approximately −30 mV, e.g., via the above-mentioned or unidentified lysosomal conductances to prevent Δψ from becoming too negative when the V-ATPase pumping is unopposed, the V-ATPases can be continuously operative in the lysosome. Second, is it true that ClC-7 plays absolutely no role in lysosomal acidification? Lysosomal acidification is a complicated process that involves the expression, lysosomal delivery, regulation, and activity of both V-ATPase and H+ leak pathways (Fig. 1; 3). In cells carrying a gain-of-function mutant allele of ClC-7, or when the tonic inhibition of ClC-7 is pharmacologically and acutely relieved, lysosomal acidification is increased (11). Hence, it is possible that in the ClC-7 KO cells there is a subtle lysosomal acidification defect, but such defect is genetically or functionally compensated through the changes in the activity of V-ATPase and/or H+ leak. For instance, it is expected that H+ leak must be elevated in the damaged lysosomes of ClC-7 KO, suggesting that the V-ATPase activity must be elevated to a certain extent as well to achieve a “normal” acidification (7). Likewise, altered lysosomal ECl due to intralysosomal Cl− accumulation is expected to cause a change in lysosomal Δψ, which is known to regulate the V-ATPase activity (3).
In contrast to the lack of an obvious acidification defect in ClC-7 KO, but in line with previous findings (9, 10), both Wu et al. (6) and Zhang et al. (7) found a dramatic decrease in the intralysosomal Cl− level. Although the intralysosomal Cl− levels are not in situ measured and calibrated in the current studies, putting all the available information together and for the sake of discussion, intralysosomal [Cl−] is likely to be as high as 80–100 mM in most lysosomes (Fig. 1). In the ClC-7 KO cells, intralysosomal [Cl−] is reduced to 40–60 mM (Fig. 1). Therefore, the primary function of ClC-7 is to function as an H+-dependent Cl− transporter, such that the reduced intralysosomal [Cl−] might be the direct cause of the reduced hydrolase activity in the ClC-7 KO cells.
Intralysosomal Cl−, like intralysosomal H+, might regulate hydrolase activity directly, or indirectly, by affecting lysosomal physiology and trafficking. To separate the primary effect from those secondarily caused by intralysosomal Cl− accumulation, it is necessary to directly assess the Cl− dependence of the hydrolase activity. Among the lysosomal hydrolases, the activity of cathepsin C is known to be regulated by Cl− through direct binding (12). Zhang et al. focused on cathepsins B and L, since cathepsin C is missing in nematodes, and the cysteine-type proteases are known to have overlapping functions (7). While Zhang et al. used the lysosomes isolated from WT and ClC-7 KO cells for their analyses, Wu et al. used purified lysosomal enzymes in their in vitro assays. In both studies, however, the authors found clear Cl− dependence of the activities of some cathepsins, as well as some other lysosomal enzymes, e.g., lysozyme and DNase II. The in vitro experiments by Zhang et al. (7) showed that Cl− could bind directly to cathepsin B/CPR-2 and cathepsin L/CPL-1 in the mM range, and their activities were found to be Cl− dependent. In macrophages, Wu et al. found that a depletion of lysosomal Cl− also decreased the hydrolytic activities of phagosomes (6). In the in vitro assays, even at the physiological pH found within phagosomes, the Cl− dependence of cathepsin C activity is still obvious (6). Hence, the simple explanation to the collective results is that high intralysosomal Cl− is required for the activities of at least some lysosomal hydrolases. The ion substitution experiments suggested that the anion-dependence (Cl− vs. ΝΟ3−) in the enzymatic assay is different from the anion dependence of ClC-7 transport activity, arguing that the effect of intralysosomal Cl− accumulation is through hydrolase activation, not caused by secondary changes due to lysosomal Cl− flux (6). However, given that Cl− dependence of hydrolase activity is a sufficient test assay, it is not clear whether intralysosomal Cl− also plays any additional role in the cells. For example, lysosomal Cl− is known to regulate lysosomal Ca2+ release (10), which in turn may regulate various other lysosomal functions, including those that can protect lysosomal integrity such as lysosomal membrane repair (5). Such hydrolase-independent but lysosomal Cl−-dependent functions may contribute to the dramatic phenotypes seen in the ClC-7 KO cells. Cl− is the most abundant anion of the cell, and recent studies have revealed signaling roles of cytosolic Cl−, e.g., by modulating the activity of WNK kinase that is known to regulate a variety of ion transporters (5). The existence of a two- to fourfold Cl− concentration gradient across the lysosomal membrane, together with the recent identification of several lysosomal Cl− channels (5), suggest that lysosomal Cl− may regulate a variety of lysosomal functions.
In summary, the back-to-back publications of Wu et al. (6) and Zhang et al. (7) have convincingly demonstrated that high intralysosomal Cl− and the Cl−/H+ exchanger ClC-7 are indispensable for the activities of certain lysosomal hydrolases, as well as the physiological functions of lysosomes, including phagosome resolution and protection of lysosomal membrane integrity in vivo (6, 7). The dual regulation of lysosomal hydrolases by high intralysosomal H+ and Cl− might have evolved to ensure the lysosomal lumen as the only activation site for lysosomal hydrolases in normal physiology. The studies also raised several important questions. For instance, are the effects of lysosomal H+ and Cl− on the activities of hydrolases independent, additive, or synergistic? What is the Cl−-binding site in the Cl−-sensitive hydrolases? What are the roles of lysosomal Cl− in the regulation of lysosomal physiology, lysosomal content condensation, lysosomal Ca2+ release, and lysosomal membrane repair? Cl−-site mutation knock-in studies may help reveal the relative roles of intralysosomal Cl− in the above-mentioned lysosomal functions. How are the expression and activities of ClC-7, as well as lysosomal Cl− channels such as CLN7 and Lyso-VRACs, regulated by cellular stimuli to tune lysosomal degradation capacity. More broadly, given the cross-regulation and interdependence between different lysosomal ions, Cl−, H+, and Ca2+ in the tiny organelles, acute modulation (activation and inhibition)—as opposed to genetic manipulations—of the activities lysosomal channels/transporters might help better define their direct roles in the regulation of lysosomal degradation and membrane trafficking. Nevertheless, a firm establishment of lysosomal Cl− in hydrolase activation is an important first step toward illustrating the specific roles of lysosomal ions in lysosomal functions.
Acknowledgments
We apologize to colleagues whose works are not cited due to space limitations. We appreciate the helpful comments from Spring Gao, Richard Hume, and other members of the Xu laboratory.
The work in the authors’ laboratory is supported by the start-up funds from the Liangzhu Laboratory and the Second Affiliated Hospital of Zhejiang University School of Medicine, as well as a grant from the National Key Research and Development Program of China (No. 2022YFE0210100).
References
- 1.Ballabio, A., and Bonifacino J.S.. 2020. Nat. Rev. Mol. Cell Biol. 10.1038/s41580-019-0185-4 [DOI] [PubMed] [Google Scholar]
- 2.Kolter, T., and Sandhoff K.. 2005. Annu. Rev. Cell Dev. Biol. 10.1146/annurev.cellbio.21.122303.120013 [DOI] [PubMed] [Google Scholar]
- 3.Mindell, J.A. 2012. Annu. Rev. Physiol. 10.1146/annurev-physiol-012110-142317 [DOI] [PubMed] [Google Scholar]
- 4.Li, P., Gu M., and Xu H.. 2019. Trends Biochem. Sci. 10.1016/j.tibs.2018.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hu, M., et al. 2022. J. Cell Biol. 10.1083/jcb.202109133 [DOI] [Google Scholar]
- 6.Wu, J.Z., et al. 2023. J. Cell Biol. 10.1083/jcb.202208155 [DOI] [Google Scholar]
- 7.Zhang, Q., et al. 2023. J. Cell Biol. 10.1083/jcb.202210063 [DOI] [Google Scholar]
- 8.Weinert, S., et al. 2010. Science. 10.1126/science.1188072 [DOI] [Google Scholar]
- 9.Steinberg, B.E., et al. 2010. J. Cell Biol. 10.1083/jcb.200911083 [DOI] [Google Scholar]
- 10.Chakraborty, K., et al. 2017. Elife. 10.7554/eLife.28862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leray, X., et al. 2022. Elife. 10.7554/eLife.74136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cigic, B., and Pain R.H.. 1999. Eur. J. Biochem. 10.1046/j.1432-1327.1999.00697.x [DOI] [PubMed] [Google Scholar]