Abstract
Genetic transformation of plants by Agrobacterium tumefaciens is mediated by a virulence (vir)-specific type IV secretion apparatus assembled from 11 VirB proteins and VirD4. VirB1, targeted to the periplasm by an N-terminal signal peptide, is processed to yield VirB1*, comprising the C-terminal 73 amino acids. The N-terminal segment, which shares homology with chicken egg white lysozyme as well as lytic transglycosylases, may provide local lysis of the peptidoglycan cell wall to create channels for transporter assembly. Synthesis of VirB1* followed by its secretion to the exterior of the cell suggests that VirB1* may also have a role in virulence. In the present study, we provide evidence for the dual roles of VirB1 in tumorigenesis as well as the requirements for processing and secretion of VirB1*. Complementation of a virB1 deletion strain with constructs expressing either the N-terminal lysozyme-homologous region or VirB1* results in tumors intermediate in size between those induced by a wild-type strain and a virB1 deletion strain, suggesting that each domain has a unique role in tumorigenesis. The secretion of VirB1* translationally fused to the signal peptide indicates that processing and secretion are not coupled. When expressed independently of all other vir genes, VirB1 was processed and VirB1* was secreted. When restricted to the cytoplasm by deletion of the signal peptide, VirB1 was neither processed nor secreted and did not restore virulence to the virB1 deletion strain. Thus, factors that mediate processing of VirB1 and secretion of VirB1* are localized in the periplasm or outer membrane and are not subject to vir regulation.
Agrobacterium tumefaciens, the causative agent of crown gall disease, induces tumors on most dicotyledonous plants. During infection, A. tumefaciens transfers DNA in a DNA-protein complex (T complex) into plant cells (reviewed in references 12, 18, 58, 60, and 62). The complex comprises a single-stranded copy (T strand) of a segment (T-DNA) of the tumor-inducing plasmid (Ti), as well as the Agrobacterium proteins VirD2 and VirE2. A single molecule of VirD2 is covalently attached to the 5′ end of the T strand (32, 41). The single-strand binding protein VirE2 coats the length of the T strand (14, 29, 46), although whether binding of VirE2 occurs in the bacterium (13) or in the plant cell (8, 49) is currently unresolved. After import into the plant cell nucleus (15, 33, 52, 61), the T strand becomes integrated into a plant chromosome (43, 53). The gene products that mediate T-strand production and transfer, as well as provide structural components of the T complex, are encoded in the vir region, a nontransferred segment of the Ti plasmid. Five complementation groups, virA, virB, virD, virE, and virG, are essential for DNA transfer (reviewed in reference 58). In the plant cell, gene products of the transferred DNA promote the unregulated production of plant growth regulators that induce increased rates of cell division in the transformed cells, resulting in neoplastic growth. The T-DNA also encodes enzymes for the biosynthesis of sugar derivatives called opines. As the infecting strain also carries genes for opine metabolism on the Ti plasmid, these compounds can be specifically utilized as a carbon source (reviewed in references 20 and 31). Thus, A. tumefaciens genetically manipulates plant cells to produce a unique habitat that it is specifically equipped to exploit.
Export of the T complex is thought to be mediated by a multimeric, transmembrane apparatus assembled from 11 VirB proteins and VirD4. This apparatus belongs to a growing family of transporters called type IV secretion systems (9). Type IV systems constitute the transfer machinery of many broad-host-range (BHR) and narrow-host-range conjugal plasmids (e.g., IncF, IncW, IncP, and IncN) (12, 37, 57, 62). In addition, the ptl operon of Bordetella pertussis encodes nine proteins required for the secretion of pertussis toxin that are homologous to VirB proteins and the transfer proteins of plasmid conjugation systems (56). Homologs of VirB4, VirB7, VirB9, VirB10, VirB11, and VirD4 are implicated in transfer of a factor from Helicobacter pylori that induces secretion of interleukin-8 by epithelial cells (16), while pathogenicity of Legionella pneumophila requires homologs of VirB10 and VirB11 (54). Recently, homologs of virB4, virB8, virB9, virB10, virB11, and virD4 were identified in the genome of Rickettsia prowazekii, but the functions of their products are unknown (2). Finally, an operon in Brucella suis that is required for virulence contains homologs of all 11 virB genes (16, 40). These homologies suggest that type IV secretion systems share a common ancestor and that the secretion or transfer of substrates as diverse as single-stranded-DNA-protein complexes and proteinaceous pathogenesis factors involves common mechanisms.
Little is known about the assembly of the VirB DNA transfer apparatus or the molecular details of its operation. VirB2 to VirB11 and VirD4 are all required for virulence (7). The apparatus has a pilus (27) and may form a transmembrane channel for cell-to-cell trafficking of the T complex (12). It is unknown whether these two structures are coupled physically or functionally.
The major structural component of the pilus is VirB2 (36). VirB5 also cofractionates with VirB2 through pilus purification and may be a minor pilus component (45). VirB3 and VirB4 are homologous to TraC and TraE, respectively, which are accessory pilus proteins in the IncF system required for pilus assembly but are not structural components (34). Evidence to date for the Agrobacterium protein VirB4, however, suggests that it is a structural component of the transmembrane channel (17).
The remainder of the VirB proteins form the putative transmembrane channel (12). The core of the apparatus likely is composed of VirB7-VirB9 heterodimers that are linked by a disulfide bridge and anchored in the outer membrane by lipid modification of VirB7 (1, 4, 23, 24, 47). The VirB7-B9 heterodimer interacts, either directly or indirectly, with VirB10 (6) and is genetically required for the stability of VirB4, VirB8, VirB10, and VirB11 (24). Coordinate overexpression of VirB9, VirB10, and VirB11 relieves the dominant negative phenotype of specific VirB11 mutations, suggesting that these proteins may be required in stoichiometric amounts for the assembly of VirB transporters (59). VirB6 is firmly embedded in the inner membrane with five transmembrane regions, and its presence is required for the stability of several VirB proteins (S. Hapfelmeier, N. Domke, P. C. Zambryski, and C. Baron, unpublished data). Thus, VirB6 was suggested to form a pore in the inner membrane (12) and may anchor the VirB transfer apparatus to the inner membrane. VirB8 localizes to the inner membrane (50, 51), but a specific role in transporter assembly has not been assigned to it. Finally, VirD4, by analogy to its homologs in conjugal type IV systems, which are termed “coupling” proteins (10), may mediate delivery of the T complex to the VirB transfer apparatus.
The first product of the VirB operon, VirB1, is not absolutely required for virulence, although its deletion reduces DNA transfer 100- to 1,000-fold depending on the assay (7, 25). Sequence similarity between the N terminus of VirB1 and chicken egg white lysozyme as well as lytic transglycosylases (21, 39) suggests that it may provide local lysis of the peptidoglycan cell wall to create channels large enough for assembly of the transporter. Regions of lysozyme homology are present in proteins from many type II, III, and IV secretion systems (5, 39, 48), which suggests a broad requirement for this activity in the assembly of membrane-spanning complexes. Mutations of putative active-site residues within the region of lysozyme homology of VirB1 reduce virulence (39). Furthermore, low cellular levels of VirB4 and VirB11 (7) and the lack of T pili (26, 45) observed in virB1 deletion strains suggest that transporter assembly across an intact bacterial cell wall is inefficient or unstable. A second role for VirB1 was suggested by the observation that the C-terminal third of the protein, VirB1*, is secreted and loosely associated with the exterior of Agrobacterium cells (3). Chemical cross-linking and coimmunoprecipitation demonstrated an association between VirB1* and VirB9 (3). It has not been determined whether VirB1* has a postsecretion function.
In the present study, we further characterized the dual roles of VirB1 in tumorigenesis as well as the requirements for processing and secretion of VirB1*. Complementation of virB1 deletion strains with constructs expressing either the N-terminal lysozyme-homologous region or VirB1* results in tumors intermediate in size between those induced by a wild-type strain and a virB1 deletion strain. Thus, each domain has a unique role in tumorigenesis. While processing and secretion of VirB1* occur in the absence of other vir functions, they do require signal peptide-mediated export into the periplasm. Thus, factors that mediate processing of VirB1 and secretion of VirB1* are localized between the inner and outer membranes and are not subject to vir regulation.
MATERIALS AND METHODS
Strains and growth conditions.
Two common laboratory A. tumefaciens strains, C58 and A348, and their derivatives were used. C58 carries the nopaline Ti plasmid pTiC58. A348 (28) was produced by introducing the octopine Ti plasmid pTiA6NC into A136 (C58 cured of its Ti plasmid [C58NT1] and then screened for resistance to rifampin and nalidixic acid [A136]). C58 and A348 strains with in-frame deletions of virB1 are CB1001 (45) and A348ΔB1 (7), respectively.
For induction of the vir system, single colonies were inoculated into 5 ml of YEB liquid medium (0.5% beef extract, 0.1% yeast extract, 0.5% peptone, 0.5% sucrose, 2 mM MgSO4) with appropriate antibiotics and grown at 28°C. After 48 h, cultures were diluted to an A600 of 0.1 in AB medium (10 g of glucose, 4 g of morpholine ethanesulfonic acid, 2 g of NH4Cl, 0.3 g of MgSO4 · 7H2O, 0.15 g of KCl, 0.01 g of CaCl2, and 0.0025 g of FeSO4 · 7H2O per liter and 1 mM potassium phosphate [pH 5.5]). The vir system was induced by the addition of acetosyringone (200 μM final concentration) prepared as a 1,000× stock solution in dimethyl sulfoxide. Liquid cultures were grown for 18 to 20 h at 28°C after induction.
Construction of vectors for expression of wild-type VirB1 and VirB1 mutants.
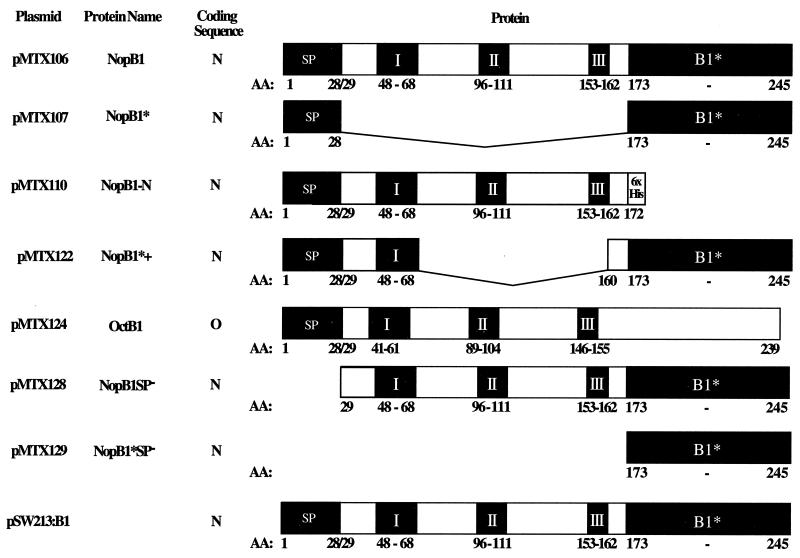
All procedures for plasmid DNA isolation and manipulations, such as digestion with restriction endonucleases or ligation, were as described by Sambrook et al. (44). Diagrams of VirB1 and derivative proteins are shown in Fig. 1.
FIG. 1.
virB1 and virB1 deletion constructs in pBP2N, a BHR vector with the nopaline virB promoter. pSW213:virB1 is also a BHR vector that contains virB1 under the control of the IPTG-inducible lactose promoter. N, coding sequences from nopaline virB1; O, coding sequences from octopine virB1; I, II, and III, the three conserved motifs in the superfamily of glycosidases that includes VirB1 and chicken egg white lysozyme (39); AA, amino acid; SP, signal peptide.
Wild-type VirB1 and its derivatives were expressed from the native Shine-Dalgarno sequence of the virB operon in plasmids pBP2N and pBP21 (Table 1). To this end, the BHR plant transformation vector pPZP200 (Hapfelmeier, Domke, Zambryski, and Baron, unpublished) was digested with ScaI and partially with BclI to remove the segment with T-DNA borders and the cloning site. A 360-bp fragment including the virB promoter with a 3′ NcoI site for direct cloning was amplified from pGV0310 (19) by PCR, using the primers BP5 and BP3-Nco (Table 1). The virB promoter fragment was digested with BclI and ScaI and ligated into pPZP200 to produce pBP2N. pBP21, also a derivative of pPZP200, differs from pB2N in that the polylinker from pBCSK+. NdeI (7) has been introduced at the 3′ end of the virB promoter. virB1 and virB1 fragments were amplified from pMTX100, a derivative of pUC119 carrying a 1.9-kbp HindIII fragment from pTiC58 that includes virB1 to virB3 and part of virB4. Constructs with PCR-amplified fragments were confirmed by sequence analysis. Additional characteristics and details of construction or use of strains, plasmids, and oligonucleotides are given in Table 1.
TABLE 1.
Bacterial strains, plasmids, and oligonucleotides
| Bacterial strain, plasmid, or oligonucleotide | Relevant characteristics | Reference |
|---|---|---|
| Strains | ||
| E. coli | ||
| DH5α | λ− φ80d/lacZΔM15 Δ(lacZYA-argF)U169 recA endA1 hsdR17(rK−mK+) | Laboratory stock |
| CJ236 | Camr(pCJ105) dut ung thi relA; used for site-directed mutagenesis | Laboratory stock |
| A. tumefaciens | ||
| A136 | Strain C58 heat cured of its Ti plasmid | 28 |
| A348 | A136 containing octopine pTiA6NC | 28 |
| A348ΔB1 | A348 with a precise, nonpolar deletion of virB1 from pTiA6NC | 7 |
| C58 | A136 containing the nopaline Ti plasmid pTiC58 | Laboratory stock |
| CB1001 | C58 with a precise, nonpolar deletion of virB1 from pTiC58 | 45 |
| Plasmids | ||
| pBCSK+.NdeI | Camr; source of polylinker for pBP21 | 7 |
| pBP2N | Strr Spcr; BHR vector carrying the nopaline virB promoter followed by a unique NcoI site containing the ATG start codon | This study |
| pBP21 | Strr Spcr; BHR vector carrying the nopaline virB promoter followed by the polylinker from pBCSK+.NdeI | This study |
| pGV0310 | Crbr; carries three consecutive HindIII fragments of pTiC58 that include the entire virB operon in pBR322; template for PCR amplification of the virB promoter | 19. |
| pMTX100 | Crbr; pUC119 carrying a 1.9-kbp HindIII fragment of the virB operon that includes virB1, virB2, virB3 and part of virB4 | This study |
| pMTX106 | Strr Spcr; vir-regulated expression of full-length nopaline virB1, in pBP2N | This study |
| pMTX107 | Strr Spcr, vir-regulated expression of nopaline virB1* fused to the coding sequence for the signal peptide, in pBP2N | This study |
| pMTX110 | Strr Spcr; vir-regulated expression of the signal peptide N-terminal segment of nopaline virB1 with a six-His tag, in pBP2N | This study |
| pMTX122 | Strr Spcr; similar to pMTX107, with a smaller deletion in the lysozyme-homologous region in pBP2N | This study |
| pMTX124 | Strr Spcr; vir-regulated expression of full-length octopine virB1, in pBP21 | This study |
| pMTX128 | Strr Spcr; vir-regulated expression of nopaline VirB1 without the signal peptide in pBP2N | This study |
| pMTX129 | Spcr; vir-regulated expression of nopaline virB1* without the signal peptide in pBP2N | This study |
| pPZP200 | Strr Spcr; binary vector, source of E. coli and pVS1 BHR origins of replication for pBP2 and pBP21 | 30 |
| pSW213 | Tetr; IncP BHR plasmid containing lacIq and plac with downstream polylinker sequence | 11 |
| pSW213:virB1 | Tetr; full-length VirB1 cloned behind the plac promoter using HindIII and PstI sites | This study |
| Oligonucleotides | ||
| BP5 | 5′-GGCCTGATCATCGCTGAGCTCGGACATAGG-3′; 5′ primer for PCR amplification of the virB promoter from pGV0310 | |
| BP3Nco | 5′-GGCCAGTACTCCATGGCCCATCTCCCCAAGCTCATAA-3′; 3′ primer for PCR amplification of the virB promoter from pGV0310 | |
| C-His5′ | 5′-GCATCATCATCATCATCATTAGAGATCT-3′; 5′ primer for introducing a six-His tag at the C terminus of VirB1 followed by a stop codon and a BglII site | |
| C-His3′ | 5′-AGATCTCTAATGATGATGATGATGATGC-3′; 3′ primer complementary to C-His3′ | |
| MTX4 | 5′-CCACTTTCATTTGCTGCTCAACAGCTCGTC-3′; used in site-directed mutagenesis of pMTX100 to delete coding sequence for amino acids 29 to 172 of VirB1 to produce VirB1* translationally fused to the signal peptide for pMTX107 | |
| MTX5 | 5′-GGACAACATGTTGAAGGCAACAG-3′; 5′ primer for amplification of nopaline virB1, Met codon is within an AflIII site | |
| MTX6 | 5′-GGACAAGTACTATTGCGGACCTCCT-3′; 3′ primer for amplification of nopaline virB1 with a ScaI site | |
| MTX19 | 5′-GGAAGCTTGAGCTAAGGAGATAAGG-3′; 5′ primer for PCR amplification of octopine virB1, includes HindIII site and ribosomal binding site upstream of the start codon | |
| MTX20 | 5′-GAACTGCAGCTCCTTAGTATAAGTCGA-3′; 3′ primer for PCR amplification of octopine virB1, includes PstI site after the stop codon | |
| MTX21 | 5′-CTTCCCATGGCTCAACAGCTCGTC-3′; 5′ primer for PCR amplification of virB1* lacking signal peptide coding sequence, ATG within an NcoI site is inserted in front of amino acid 173 | |
| MTX22 | 5′-CCTTCCATGGCTCCATCCGTTGCTC-3′; 5′ primer for PCR amplification of virB1 lacking the signal peptide coding sequence, ATG within an NcoI site is inserted in front of amino acid 29 | |
| VirB1-5′ | TGACAAGCTTGGGGAGATGGGGA; 5′ primer for PCR amplification of virB1 with a HindIII site upstream of the coding sequence | |
| VirB1-3′ | GCGCGAATTCATTGCGGACCTCCTTGATT; 3′ primer for PCR amplification of virB1 with an EcoRI site at the 3′ end of the coding sequence |
pMTX106 is a pBP2N derivative that encodes full-length VirB1 from pTiC58, a nopaline-type Ti plasmid. The 5′ primer (MTX5) (Table 1) introduced an AflIII site, compatible with NcoI, at the 5′ end of virB1, while the 3′ primer (MTX6) (Table 1) introduced a ScaI site after the virB1 stop codon. This PCR-amplified fragment was then digested with AflIII and ScaI and cloned into pBP2N digested with NcoI and ScaI.
pMTX107 is a pBP2N derivative that expresses VirB1* fused directly to the 28-residue signal peptide. The coding sequence for amino acids 29 to 172 of VirB1 was precisely deleted from pMTX100 by site-directed mutagenesis (35) using primer MTX4 (Table 1). This virB1 derivative was then PCR amplified with primers MTX5 and MTX6 and cloned into pBP2N as described for pMTX106. To introduce a smaller internal deletion leaving coding sequences for a few amino acids C terminal of the signal peptidase I cleavage site and N terminal of the VirB1* processing site, pMTX106 was digested with XmnI and NruI. An 8-bp HindIII linker was added to restore the frame resulting in pMTX122.
pMTX110 is a pBP2N derivative that encodes the N-terminal 172 amino acids of VirB1, followed by a six-His tag (Table 1). The six-His tag was introduced by removing overhanging nucleotides from EagI-digested pMTX100 with mung bean nuclease. Annealed oligonucleotides (C-His5′ and C-His3′) (Table 1) encoding the His tag were ligated to produce the intermediate pMTX105. This virB1 derivative was then PCR amplified with primers MTX5 and MTX6 and cloned into pBP2N as described above.
pMTX124 encodes full-length VirB1 from an octopine-type Ti plasmid. Octopine virB1 was PCR amplified with primers that introduced a HindIII site at the 5′ end of the fragment and a PstI site at the 3′ end (MTX19 and MTX20, respectively) (Table 1). This fragment also includes the ribosome binding site from the octopine virB operon upstream of the virB1 gene. HindIII and PstI sites on the vector and insert were used to introduce the PCR-amplified fragment into pBP21, resulting in pMTX124.
To produce pMTX128, the coding sequence for the signal peptide of VirB1 was deleted by PCR amplification (primers MTX22 and MTX6) so that this plasmid expresses the coding sequence for amino acids 29 to 245, VirB1SP−. For pMTX129, the coding sequence for amino acids 173 to 245, i.e., VirB1*, was PCR amplified with primers MTX21 and MTX6. In both cases, virB1 sequences were amplified from pMTX100 and an ATG within an NcoI site was introduced by the 5′ primers. Subsequently, they were cloned into pBP2N via the NcoI and ScaI sites.
To express virB1 independently of the vir system, nopaline virB1 was PCR amplified with the primers VirB1-5′ and VirB1-3′ (Table 1), which introduced a HindIII site at the 5′ end of the coding sequence and an EcoRI site at the 3′ end, respectively. These sites were used to clone virB1 into pSW213 (11) to produce pSW213::virB1. This plasmid carries E. coli plac so that gene expression can be induced with 0.5 mM isopropyl-β-d-thiogalactopyranoside (IPTG).
Tumor assays.
Virulence assays were performed on Kalanchoe diagremontiana. One-centimeter-long wound sites, created by carefully scratching the surface of a leaf with a toothpick, were inoculated with 109 CFU of the strains described above. Virulence was assayed by tumor size and time course of tumor development. The virulence of each strain carrying different constructs was assayed at least 10 times in independent experiments. Photographs were taken 6 to 7 weeks after inoculation.
Protein analysis.
Preparation of the cell lysates and supernatant fraction by precipitation with trichloroacetic acid as well as analysis of VirB1 products by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting was performed as previously described (3).
RESULTS
Complementation with N- and C-terminal domains.
Deletion of virB1 severely attenuates but does not abolish virulence (7). In the present experiments with K. diagremontiana as a host, a strain of A. tumefaciens (A348ΔB1) carrying octopine pTiA6 with an in-frame deletion of virB1 only rarely formed a small tumor (Table 2 and Fig. 2A). In contrast, virB1 deletion from nopaline pTiC58 in CB1001 had only a slight effect on virulence regardless of whether the host was K. diagremontiana or Nicotiana tabacum (data not shown). pTiC58 may be inherently more tumorigenic than pTiA6NC, and so the deletion of virB1 has a smaller effect on virulence. Alternatively, other pTi factors, most likely non-vir, play a role in determining the requirement for VirB1 during tumorigenesis.
TABLE 2.
Effect of virB1 deletion on virulence of A. tumefaciens on K. diagremontiana and complementation with the N and C termini of VirB1
| Strain (plasmid; VirB1 fragmenta) | Tumor frequencyb | Relative tumor sizec |
|---|---|---|
| A348 | 15/15 | 1.00 |
| A136 | 0/10 | 0.00 |
| A348ΔB1 | 2/15 | 0.15 |
| A348ΔB1 (pMTX124; OctB1) | 10/10 | 1.00 |
| A348ΔB1 (pMTX106; NopB1) | 15/15 | 1.00 |
| A348ΔB1 (pMTX110; NopB1-N) | 12/15 | 0.60 |
| A348ΔB1 (pMTX107; NopB1*) | 12/15 | 0.40 |
See Fig. 1.
Number of inoculations that incited tumors per number of inoculations.
Size of tumors relative to that of tumors incited by A348 (wild type) estimated by visual inspection.
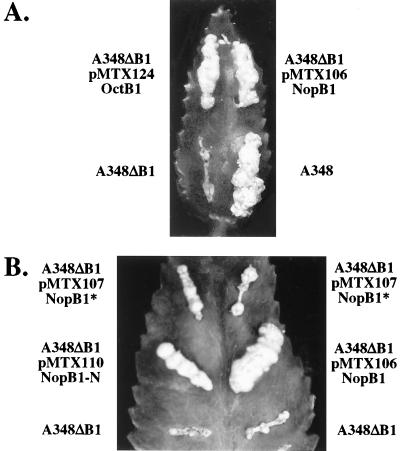
FIG. 2.
Complementation of virB1 deletion strain A348ΔB1 assessed by testing virulence on K. diagremontiana. (A) Complementation with full-length VirB1, either octopine (pMTX124) or nopaline (pMTX106), restored tumorigenicity completely. (B) Complementation of virB1 deletion strain A348ΔB1 by constructs expressing either the VirB1 region of lysozyme homology (pMTX110) or VirB1* (pMTX107) partially restored tumorigenicity. Wound sites are labeled with the strain used for inoculation. Plasmids and encoded VirB1 fragments (Fig. 1) are indicated below the strain names.
Wild-type virulence was restored to A348ΔB1 by expression of octopine VirB1 (pMTX124) (Table 2 and Fig. 2A). The resulting tumors were indistinguishable from those incited by wild-type Agrobacterium. This mutant was also restored to virulence by expression of nopaline VirB1 (pMTX106) (Table 2 and Fig. 2A). The ability of both the nopaline and octopine VirB1 proteins to complement virB1 deletion in A348ΔB1 indicates that these proteins provide identical functions during tumorigenesis. Therefore, we took advantage of this cross-complementation to characterize further the functions and processing of nopaline VirB1.
The function(s) provided by VirB1 during DNA transfer has not been demonstrated unequivocally. The homology to lysozyme suggests an early role for the N-terminal domain modifying the murein at the site of transporter assembly (21, 39). A subsequent, extracellular function is suggested by the specific processing and secretion of VirB1* and the association of VirB1* with VirB9 (3). To determine whether each domain plays a unique role during tumorigenesis, the ability of the N and C termini to independently restore A348ΔB1 to virulence was assessed.
A348ΔB1 expressing the N-terminal domain (amino acids 1 to 173, NopB1-N, pMTX110) incited tumors intermediate in size between those incited by wild-type Agrobacterium and A348ΔB1 (Table 2 and Fig. 2B). The coding sequence for VirB1* fused to the signal peptide (amino acids 1 to 28 and 173 to 245, NopB1*, pMTX107) was used to complement A348ΔB1, and this also resulted in small tumors (Table 2 and Fig. 2B). A third complementation construct, with a smaller internal deletion, retained amino acids 29 to 68 C terminal to the signal peptidase I site and 13 amino acids (amino acids 160 to 172) N terminal to the VirB1* processing site (NopB1*+, pMTX122). This clone was constructed to account for the possibility that adjacent residues were required for efficient processing of the signal peptidase I and VirB1* sites. Complementation of the virB1 deletion with pMTX122 resulted in tumors that were not significantly larger than when the deletion was complemented with pMTX107 (data not shown). That expression of the N-terminus- or C-terminus-coding sequences of virB1 partially complemented the virB1 deletion suggests that each domain performs a distinct function that is missing in A348ΔB1.
Processing and secretion of VirB1*.
As both domains of VirB1 have some function in tumorigenesis, the processing and secretion events that produce VirB1* were further studied to characterize the requirements for these reactions.
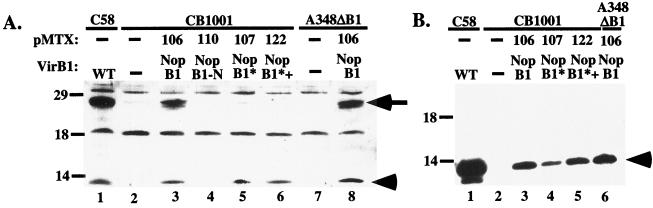
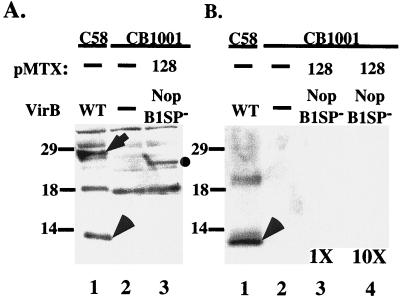
Nopaline VirB1, controlled by the virB promoter and expressed from an exogenous plasmid, was processed to yield VirB1* (Fig. 3A), which was then secreted (Fig. 3B). Furthermore, processing of nopaline VirB1 and VirB1* secretion occurred with similar efficiencies in virB1 deletions constructed in both nopaline and octopine Ti plasmids (Fig. 3A, lanes 3 and 8, and B, lanes 3 and 6). Processing of octopine VirB1 by A348 could not be assessed, however, because our anti-nopaline VirB1 polyclonal antiserum, as well as a peptide antibody raised against the C terminus of nopaline VirB1, does not cross-react efficiently with octopine VirB1. In all cases, trans-complementing plasmids expressed less VirB1 than did the wild type (Fig. 3A, lanes 1, 3, and 8, and B, lanes 1, 3, and 6). In spite of the reduction in VirB1 expression, however, A348ΔB1 carrying pMTX106, which encodes full-length nopaline VirB1, still incited tumors comparable to those incited by the wild type (Fig. 2A).
FIG. 3.
Processing of VirB1 and secretion of VirB1*. Samples from vir-induced cultures of the indicated strains were taken 18 to 20 h after induction. Cell lysates (A) and supernatants (B) were subjected to SDS-PAGE and blotted onto polyvinylidene difluoride membranes (Millipore Corp.), followed by detection with VirB1-specific antiserum. pMTX, the plasmid used for complementation; VirB1, the VirB1 fragment expressed (Fig. 1); WT, wild type. The arrow indicates the position of VirB1; the arrowheads indicate the positions of VirB1*. Numbers to the left are molecular mass markers, in kilodaltons.
VirB1* fused directly to the signal peptide (NopB1*, pMTX107) was secreted into the supernatant (Fig. 3B, lane 4), suggesting that secretion is not coupled to processing. The efficiency of VirB1* synthesis and secretion depended on the construct. More VirB1* was consistently found in the medium than the amount retained by the cells when the construct encoded residues adjacent to the signal peptide and VirB1* processing sites (Fig. 3B, lanes 4 and 5). Thus, the context of the amino acid sequence at both processing sites exerts a small influence on these proteolytic reactions and possibly secretion.
The N terminus of VirB1 (pMTX110) was not immunologically detectable (Fig. 3A, lane 4). This construct, however, was able to restore virulence partially (Fig. 2B). In the wild type, only full-length protein and VirB1* are detected on Western blots. Possibly, the N terminus of nopaline VirB1 is not immunogenic or is especially susceptible to degradation.
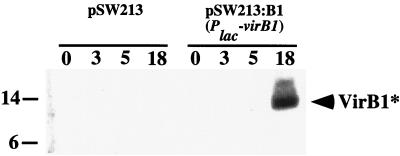
The role of vir functions in processing and secretion was assessed by using plac to express VirB1 (pSW213:virB1) in the absence of all other Vir proteins. Addition of IPTG induced expression of VirB1, and VirB1* was subsequently recovered from the medium (Fig. 4). Thus, processing and secretion are independent of other vir-encoded functions.
FIG. 4.
Synthesis and secretion of VirB1 are vir independent. Expression of VirB1 was regulated by the plac promoter in CB1001 (pSW213:virB1). Numbers above lanes indicate that samples from IPTG-induced cultures were taken 0, 3, 5, and 18 h after induction. CB1001 carrying vector alone (pSW213) was the negative control. Endogenous vir genes were not acetosyringone induced. Supernatants were subjected to SDS-PAGE and blotted onto polyvinylidene difluoride membranes, followed by detection with VirB1-specific antiserum.
Processing was studied further by expressing VirB1 without the N-terminal signal peptide (pMTX128). Deletion of this domain should confine VirB1 to the cell pellet fraction of vir-induced cultures. Indeed, VirB1SP− was found exclusively in the cell pellet (Fig. 5A, lane 3, and B, lane 3). The molecular mass of the protein was approximately 23 kDa, the approximate size predicted from the coding sequence for this segment of VirB1 (amino acids 29 to 245). VirB1* was not detected in the pellet or in the supernatant from the A348ΔB1(pMTX128) culture even when a 10-fold-concentrated supernatant was loaded (Fig. 5B, lanes 3 and 4). Thus, cleavage of the full-length protein to form VirB1* requires sec-dependent transport and must occur during or after transport across the inner membrane. VirB1* expressed without the signal peptide (pMTX129) was not immunologically detectable in the cell pellet or supernatant (data not shown), suggesting that it is rapidly degraded in the cytoplasm.
FIG. 5.
The signal peptide is required for processing and secretion of VirB1*. CB1001, a virB1 deletion strain of C58, was transformed with pMTX128, in which the coding sequence for the signal peptide was deleted. Samples from vir-induced cultures were taken 18 to 20 h after induction. Cell lysates (A) and supernatants (B) were subjected to SDS-PAGE and blotted onto polyvinylidene difluoride membranes, followed by detection with VirB1-specific antiserum. pMTX, the plasmid used for trans-complementation; VirB, the VirB1 fragment expressed by that plasmid (Fig. 1); WT, wild type. The arrow indicates the position of VirB1; the arrowhead indicates VirB1*; the circle indicates VirB1SP−. Numbers are molecular mass markers, in kilodaltons. In panel B, the supernatant fraction from CB1001(pMTX128) was applied at two concentrations: equivalent to that of other lanes (1×) and 10 times that of other lanes (10×).
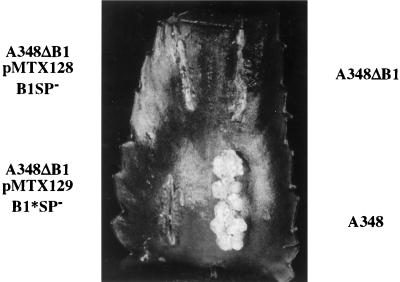
Complementation requires the signal peptide.
The clone for expression of VirB1SP− (pMTX128) was introduced into A348ΔB1 and did not restore this strain to virulence (Fig. 6). Thus, complementation of the virB1 deletion requires the sec-dependent export of VirB1 into the periplasm. The partial restoration of virulence to A348ΔB1 by expression of VirB1* (pMTX107) (Fig. 3B) was not observed when the VirB1* was expressed without a signal peptide (pMTX129) (Fig. 6). Thus, formation of smaller tumors, induced by expression of VirB1* in A348ΔB1, also requires sec-dependent export of VirB1* to the periplasm. This suggests that VirB1 and VirB1* function in the periplasm or at the exterior of the cell during DNA transfer.
FIG. 6.
The signal peptide is required for full complementation of the virB1 deletion by VirB1 or partial complementation by VirB1*. Complementation of A348ΔB1 with pMTX128 (VirB1SP−) and pMTX129 (VirB1*SP−) were assessed by testing virulence on K. diagremontiana. Wound sites are labeled with the strain used for inoculation. Plasmids and encoded VirB1 fragments (Fig. 1) are indicated below the strain names.
DISCUSSION
Here evidence is provided that nopaline VirB1 has two distinct functions that enhance tumorigenesis. The ability of either octopine or nopaline virB1 coding sequences to complement the deletion of virB1 from an octopine Ti plasmid and to restore virulence (Fig. 2A) suggests that these proteins provide identical functions. The lack of effect on tumorigenesis of deleting virB1 from the nopaline pTiC58 may reflect either the greater tumorigenicity of pTiC58 or the presence of a cryptic pTiC58 protein(s) that is functionally redundant. For example, B. suis has a virB-like operon that encodes homologs of all VirB proteins, including VirB1 (40), but also has a homolog corresponding to lytic transglycosylases, located 5′ upstream of the virB-like operon (D. O'Callaghan, personal communication).
Each VirB1 domain's performance of a unique function derives from the ability of the N- and C-terminal domains to restore partial virulence to A348ΔB1 (Fig. 2B). The formation of tumors intermediate in size between those incited by A348ΔB1 and A348ΔB1(pMTX106) expressing full-length VirB1 suggests that the functions are distinct and necessary for complete complementation. The dependence of virulence on the signal peptide of VirB1 (Fig. 6) suggests that both VirB1 and VirB1* act in the periplasm or the outer membrane or outside the cell.
The role of the VirB1 N-terminal domain, suggested by its homology to lysozyme, has been discussed extensively (3, 12, 21, 39). Assembly of a membrane-spanning, multimeric complex requires penetration of the bacterial cell wall. The size of a complex composed of even a few VirB proteins would prohibit its insertion through naturally occurring channels in the peptidoglycan layer, which permit diffusion of molecules up to ca. 50 kDa (21). The N terminus of VirB1 is predicted to hydrolyze the murein layer to provide channels large enough to accommodate assembly of the VirB transporter (3, 39). The hypothesis that VirB1 provides hydrolytic activity is supported by specific mutagenesis of putative active-site residues (39). These mutants partially restore the ability to incite tumors when used to complement a virB1 deletion strain (39). The partial restoration may result from functions provided by VirB1*, which is not affected by mutations at the putative active site for polysaccharide hydrolysis.
The function provided by nopaline VirB1* is unknown. VirB1* is most likely not a component of the pilus (45). By virtue of its secretion, however, it would be in the proper location to play an early, transient role mediating pilus formation, e.g., providing chaperone activity for VirB2. Alternatively, it may modify the surface of the Agrobacterium cell during transporter assembly as a prerequisite for attachment. Association of VirB1* with VirB9 is consistent with such a role (3). Finally, the loose association of VirB1* with the exterior of Agrobacterium suggests that it may be available to interact with the site of attachment on the plant cell surface.
Processing of nopaline VirB1 to VirB1* and subsequent secretion of VirB1* to the exterior of the cell are independent events (Fig. 3) critical to the promotion of tumorigenesis by VirB1, as shown by the partial restoration of virulence (Fig. 2B). The synthesis of VirB1* from derivatives of VirB1 with deletions in the lysozyme-homologous region (Fig. 3B) suggests that full-length VirB1 is not required for VirB1* processing. The wild-type context at the signal peptidase I site and the VirB1* processing site, however, does enhance the efficiency of processing and secretion (Fig. 3B, lanes 4 and 5). Neither processing nor secretion requires any factors encoded in the vir region (Fig. 4). The specific factors involved, however, are not known. The processing and secretion that follow IPTG-induced expression of VirB1 (Fig. 4) suggest two possibilities: either VirB1 is autocatalytic for both functions or these activities are constitutively expressed.
Many extracellular virulence factors are translated as preproteins with domains that act as intramolecular chaperones (38, 42, 55). These domains assist in folding of the mature protein in the periplasm and are proteolytically removed prior to translocation across the outer membrane. Proteolysis of some intramolecular chaperones is autocatalytic. By analogy, the C terminus of VirB1 may potentiate enzymatic activity of the N terminus by ensuring proper folding after sec-dependent secretion into the periplasm. The conditions in the periplasm might then induce the autocatalytic liberation of VirB1*. If processing and secretion are not functions of VirB1 itself, they may be provided by constitutively expressed bacterial proteins. This would require a protease localized to the periplasm that has not yet been identified. In addition, a secretion system is needed. As VirB1* is delivered into the periplasm by the general secretory pathway, a type II secretion system may transport VirB1* to the exterior of the cell. This would be analogous to secretion of elastase by Pseudomonas aeruginosa (38). Elastase is exported into the periplasm by the general secretory pathway, where an intramolecular domain, which serves as a chaperone, is cleaved autoproteolytically but remains associated with the elastase. Secretion of the elastase across the outer membrane is mediated by the Xcp apparatus, a type II secretion apparatus required for pathogenicity (38).
Until recently, non-vir functions for pathogenesis were primarily associated with attachment of Agrobacterium to plant cells. This list, however, is expanding. In the final step of maturation, the T-pilin VirB2 is cyclized by the formation of an intramolecular peptide bond between the N and C termini (22). Cyclization does not require any products encoded by the Ti plasmid other than VirB2 (22). The formation of VirB7-VirB7 or VirB7-VirB9 dimers may require specific chaperones or Dsb (disulfide bond formation)-like enzymes that are not encoded within the vir region (4, 47). During infection of a plant, VirB1 is transported into the periplasm by the general secretory pathway and is processed to generate VirB1*, which is secreted to the exterior of the cell. The VirB1-VirB1* processing and secretion events, which also do not require any functions encoded by the vir region of the Ti plasmid, rely on factors encoded outside the vir region or on the bacterial chromosome.
ACKNOWLEDGMENTS
This work was supported by NSF grant IBN-9507782 to P.Z. M.L. was supported by a postdoctoral fellowship from the Spanish Ministry of Education. C.B. was supported by a fellowship from the Deutsche Forschungsgemeinschaft (DFG, Ba 1416/1-1).
We thank Peter Christie for the generous gift of A. tumefaciens strain A348ΔB1. We also thank Nicholas Kaplinsky for technical assistance in the construction of pSW213:virB1.
M.L. and J.Z. contributed equally to this work.
REFERENCES
- 1.Anderson L B, Hertzel A V, Das A. Agrobacterium tumefaciens VirB7 and VirB9 form a disulfide-linked protein complex. Proc Natl Acad Sci USA. 1996;93:8889–8894. doi: 10.1073/pnas.93.17.8889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersson S G, Zomorodipour A, Andersson J O, Sicheritz-Ponten T, Alsmark U C, Podowski R M, Naslund A K, Eriksson A S, Winkler H H, Kurland C G. The genome sequence of Rickettsia prowazekii and the origin of mitochondria. Nature. 1998;396:133–140. doi: 10.1038/24094. [DOI] [PubMed] [Google Scholar]
- 3.Baron C, Llosa M, Zhou S, Zambryski P C. VirB1, a component of the T-complex transfer machinery of Agrobacterium tumefaciens, is processed to a C-terminal secreted product, VirB1*. J Bacteriol. 1997;179:1203–1210. doi: 10.1128/jb.179.4.1203-1210.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baron C, Thorstenson Y R, Zambryski P C. The lipoprotein VirB7 interacts with VirB9 in the membranes of Agrobacterium tumefaciens. J Bacteriol. 1997;179:1211–1218. doi: 10.1128/jb.179.4.1211-1218.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bayer M, Eferl R, Zellnig G, Teferle K, Dijkstra A, Koraimann G, Hogenauer G. Gene 19 of plasmid R1 is required for both efficient conjugative DNA transfer and bacteriophage R17 infection. J Bacteriol. 1995;177:4279–4288. doi: 10.1128/jb.177.15.4279-4288.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beaupré C E, Bohne J, Dale E M, Binns A N. Interactions between VirB9 and VirB10 membrane proteins involved in movement of DNA from Agrobacterium tumefaciens into plant cells. J Bacteriol. 1997;179:78–89. doi: 10.1128/jb.179.1.78-89.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berger B R, Christie P J. Genetic complementation analysis of the Agrobacterium tumefaciens virB operon: virB2 through virB11 are essential virulence genes. J Bacteriol. 1994;176:3646–3660. doi: 10.1128/jb.176.12.3646-3660.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Binns A N, Beaupré C E, Dale E M. Inhibition of VirB-mediated transfer of diverse substrates from Agrobacterium tumefaciens by the IncQ plasmid RSF1010. J Bacteriol. 1995;177:4890–4899. doi: 10.1128/jb.177.17.4890-4899.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burns D L. Biochemistry of type IV secretion. Curr Opin Microbiol. 1999;2:25–29. doi: 10.1016/s1369-5274(99)80004-6. [DOI] [PubMed] [Google Scholar]
- 10.Cabezon E, Sastre J I, de la Cruz F. Genetic evidence of a coupling role for the TraG protein family in bacterial conjugation. Mol Gen Genet. 1997;254:400–406. doi: 10.1007/s004380050432. [DOI] [PubMed] [Google Scholar]
- 11.Chen C-Y, Winans S C. Controlled expression of the transcriptional activator gene virG in Agrobacterium tumefaciens by using the Escherichia coli lac promoter. J Bacteriol. 1991;173:1139–1144. doi: 10.1128/jb.173.3.1139-1144.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Christie P J. Agrobacterium tumefaciens T-complex transport apparatus: a paradigm for a new family of multifunctional transporters in eubacteria. J Bacteriol. 1997;179:3085–3094. doi: 10.1128/jb.179.10.3085-3094.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Christie P J, Ward J E, Winans S C, Nester E W. The Agrobacterium tumefaciens virE2 gene product is a single-stranded-DNA-binding protein that associates with T-DNA. J Bacteriol. 1988;170:2659–2667. doi: 10.1128/jb.170.6.2659-2667.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Citovsky V, Wong M L, Zambryski P. Cooperative interaction of Agrobacterium VirE2 protein with single-stranded DNA: implications for the T-DNA transfer process. Proc Natl Acad Sci USA. 1989;86:1193–1197. doi: 10.1073/pnas.86.4.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Citovsky V, Zupan J, Warnick D, Zambryski P. Nuclear localization of Agrobacterium VirE2 protein in plant cells. Science. 1992;256:1802–1805. doi: 10.1126/science.1615325. [DOI] [PubMed] [Google Scholar]
- 16.Covacci A, Telford J L, Del Giudice G, Parsonnet J, Rappuoli R. Helicobacter pylori virulence and genetic geography. Science. 1999;284:1328–1333. doi: 10.1126/science.284.5418.1328. [DOI] [PubMed] [Google Scholar]
- 17.Dang T A, Zhou X R, Graf B, Christie P J. Dimerization of the Agrobacterium tumefaciens VirB4 ATPase and the effect of ATP-binding cassette mutations on the assembly and function of the T-DNA transporter. Mol Microbiol. 1999;32:1239–1253. doi: 10.1046/j.1365-2958.1999.01436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de la Cruz F, Lanka E. Function of the Ti-plasmid Vir proteins: T-complex formation and transfer to the plant cell. In: Spaink H P, Kondorosi A, Hooykaas P J J, editors. The Rhizobiaceae: molecular biology of model plant-associated bacteria. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1998. pp. 281–301. [Google Scholar]
- 19.Depicker A, De Wilde M, De Vos G, De Vos R, Van Montagu M, Schell J. Molecular cloning of overlapping segments of the nopaline Ti-plasmid pTiC58 as a means to restriction endonuclease mapping. Plasmid. 1980;3:193–211. doi: 10.1016/0147-619x(80)90109-2. [DOI] [PubMed] [Google Scholar]
- 20.Dessaux Y, Petit A, Farrand S F, Murphy P J. Opines and opine-like molecules involved in plant-Rhizobiaceae interactions. In: Spaink H P, Kondorosi A, Hooykaas P J J, editors. The Rhizobiaceae: molecular biology of model plant-associated bacteria. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1998. pp. 173–197. [Google Scholar]
- 21.Dijkstra A J, Keck W. Peptidoglycan as a barrier to transenvelope transport. J Bacteriol. 1996;178:5555–5562. doi: 10.1128/jb.178.19.5555-5562.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eisenbrandt R, Kalkum M, Lai E M, Lurz R, Kado C I, Lanka E. Conjugative pili of IncP plasmids, and the Ti plasmid T pilus are composed of cyclic subunits. J Biol Chem. 1999;274:22548–22555. doi: 10.1074/jbc.274.32.22548. [DOI] [PubMed] [Google Scholar]
- 23.Fernandez D, Dang T A T, Spudich G M, Zhou X-R, Berger B R, Christie P J. The Agrobacterium tumefaciens virB7 gene product, a proposed component of the T-complex transport apparatus, is a membrane-associated lipoprotein exposed at the periplasmic surface. J Bacteriol. 1996;178:3156–3167. doi: 10.1128/jb.178.11.3156-3167.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fernandez D, Spudich G M, Zhou X-R, Christie P J. The Agrobacterium tumefaciens VirB7 lipoprotein is required for stabilization of VirB proteins during assembly of the T-complex transport apparatus. J Bacteriol. 1996;178:3168–3176. doi: 10.1128/jb.178.11.3168-3176.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fullner K J. Role of Agrobacterium virB genes in transfer of T complexes and RSF1010. J Bacteriol. 1998;180:430–434. doi: 10.1128/jb.180.2.430-434.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fullner K J, Lara J C, Nester E W. Pilus assembly by Agrobacterium T-DNA transfer genes. Science. 1996;273:1107–1109. doi: 10.1126/science.273.5278.1107. [DOI] [PubMed] [Google Scholar]
- 27.Fullner K J, Nester E W. Temperature affects the T-DNA transfer machinery of Agrobacterium tumefaciens. J Bacteriol. 1996;178:1498–1504. doi: 10.1128/jb.178.6.1498-1504.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garfinkel D J, Simpson R B, Ream L W, White F F, Gordon M P, Nester E W. Genetic analysis of crown gall: fine structure map of the T-DNA by site-directed mutagenesis. Cell. 1981;27:143–153. doi: 10.1016/0092-8674(81)90368-8. [DOI] [PubMed] [Google Scholar]
- 29.Gietl C, Koukolikova-Nicola Z, Hohn B. Mobilization of T-DNA from Agrobacterium to plant cells involves a protein that binds single-stranded DNA. Proc Natl Acad Sci USA. 1987;84:9006–9010. doi: 10.1073/pnas.84.24.9006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hajdukiewicz P, Svab Z, Maliga P. The small, versatile pPZP family of Agrobacterium binary vectors for plant transformation. Plant Mol Biol. 1994;25:989–994. doi: 10.1007/BF00014672. [DOI] [PubMed] [Google Scholar]
- 31.Hooykaas P J, Schilperoort R A. Agrobacterium and plant genetic engineering. Plant Mol Biol. 1992;19:15–38. doi: 10.1007/BF00015604. [DOI] [PubMed] [Google Scholar]
- 32.Howard E A, Winsor B A, De Vos G, Zambryski P. Activation of the T-DNA transfer process in Agrobacterium results in the generation of a T-strand-protein complex: tight association of VirD2 with the 5′ ends of T-strands. Proc Natl Acad Sci USA. 1989;86:4017–4021. doi: 10.1073/pnas.86.11.4017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Howard E A, Zupan J R, Citovsky V, Zambryski P C. The VirD2 protein of A. tumefaciens contains a C-terminal bipartite nuclear localization signal: implications for nuclear uptake of DNA in plant cells. Cell. 1992;68:109–118. doi: 10.1016/0092-8674(92)90210-4. [DOI] [PubMed] [Google Scholar]
- 34.Jones A L, Shirasu K, Kado C I. The product of the virB4 gene of Agrobacterium tumefaciens promotes accumulation of VirB3 protein. J Bacteriol. 1994;176:5255–5261. doi: 10.1128/jb.176.17.5255-5261.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kunkel T A, Roberts J D, Zakour R A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- 36.Lai E-M, Kado C I. Processed VirB2 is the major subunit of the promiscuous pilus of Agrobacterium tumefaciens. J Bacteriol. 1998;180:2711–2717. doi: 10.1128/jb.180.10.2711-2717.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lessl M, Lanka E. Common mechanisms in bacterial conjugation and Ti-mediated T-DNA transfer to plant cells. Cell. 1994;77:321–324. doi: 10.1016/0092-8674(94)90146-5. [DOI] [PubMed] [Google Scholar]
- 38.McIver K S, Kessler E, Olson J C, Ohman D E. The elastase propeptide functions as an intramolecular chaperone required for elastase activity and secretion in Pseudomonas aeruginosa. Mol Microbiol. 1995;18:877–889. doi: 10.1111/j.1365-2958.1995.18050877.x. [DOI] [PubMed] [Google Scholar]
- 39.Mushegian A R, Fullner K J, Koonin E V, Nester E W. A family of lysozyme-like virulence factors in bacterial pathogens of plants and animals. Proc Natl Acad Sci USA. 1996;93:7321–7326. doi: 10.1073/pnas.93.14.7321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O'Callaghan D, Cazevielle C, Allardet-Servent A, Boschiroli M L, Bourg G, Foulongne V, Frutos P, Kulakov Y, Ramuz M. A homologue of the Agrobacterium VirB and Bordetella pertussis Ptl type IV secretion systems is essential for intracellular survival of Brucella suis. Mol Microbiol. 1999;33:1210–1220. doi: 10.1046/j.1365-2958.1999.01569.x. [DOI] [PubMed] [Google Scholar]
- 41.Pansegrau W, Schoumacher F, Hohn B, Lanka E. Site-specific cleavage and joining of single-stranded DNA by VirD2 protein of Agrobacterium tumefaciens Ti plasmids: analogy to bacterial conjugation. Proc Natl Acad Sci USA. 1993;90:11538–11542. doi: 10.1073/pnas.90.24.11538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pohlner J, Halter R, Beyreuther K, Meyer T F. Gene structure and extracellular secretion of Neisseria gonorrhoeae IgA protease. Nature. 1987;325:458–462. doi: 10.1038/325458a0. [DOI] [PubMed] [Google Scholar]
- 43.Rossi L, Hohn B, Tinland B. Integration of complete transferred DNA units is dependent on the activity of virulence E2 protein of Agrobacterium tumefaciens. Proc Natl Acad Sci USA. 1996;93:126–130. doi: 10.1073/pnas.93.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 45.Schmidt-Eisenlohr H, Domke N, Angerer C, Wanner G, Zambryski P C, Baron C. Vir proteins stabilize VirB5 and mediate its association with the T pilus of Agrobacterium tumefaciens. J Bacteriol. 1999;181:7485–7492. doi: 10.1128/jb.181.24.7485-7492.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sen P, Pazour G J, Anderson D, Das A. Cooperative binding of Agrobacterium tumefaciens VirE2 protein to single-stranded DNA. J Bacteriol. 1989;171:2573–2580. doi: 10.1128/jb.171.5.2573-2580.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Spudich G M, Fernandez D, Zhou X R, Christie P J. Intermolecular disulfide bonds stabilize VirB7 homodimers and VirB7/VirB9 heterodimers during biogenesis of the Agrobacterium tumefaciens T-complex transport apparatus. Proc Natl Acad Sci USA. 1996;93:7512–7517. doi: 10.1073/pnas.93.15.7512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stone K D, Zhang H Z, Carlson L K, Donnenberg M S. A cluster of fourteen genes from enteropathogenic Escherichia coli is sufficient for the biogenesis of a type IV pilus. Mol Microbiol. 1996;20:325–337. doi: 10.1111/j.1365-2958.1996.tb02620.x. [DOI] [PubMed] [Google Scholar]
- 49.Sundberg C, Meek L, Carroll K, Das A, Ream W. VirE1 protein mediates export of the single-stranded DNA-binding protein VirE2 from Agrobacterium tumefaciens into plant cells. J Bacteriol. 1996;178:1207–1212. doi: 10.1128/jb.178.4.1207-1212.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thorstenson Y R, Kuldau G A, Zambryski P C. Subcellular localization of seven VirB proteins of Agrobacterium tumefaciens: implications for the formation of a T-DNA transport structure. J Bacteriol. 1993;175:5233–5241. doi: 10.1128/jb.175.16.5233-5241.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thorstenson Y R, Zambryski P C. The essential virulence protein VirB8 localizes to the inner membrane of Agrobacterium tumefaciens. J Bacteriol. 1994;176:1711–1717. doi: 10.1128/jb.176.6.1711-1717.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tinland B, Koukolikova-Nicola Z, Hall M N, Hohn B. The T-DNA-linked VirD2 protein contains two distinct functional nuclear localization signals. Proc Natl Acad Sci USA. 1992;89:7442–7446. doi: 10.1073/pnas.89.16.7442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tinland B, Schoumacher F, Gloeckler V, Bravo-Angel A M, Hohn B. The Agrobacterium tumefaciens virulence D2 protein is responsible for precise integration of T-DNA into the plant genome. EMBO J. 1995;14:3585–3595. doi: 10.1002/j.1460-2075.1995.tb07364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vogel J P, Andrews H L, Wong S K, Isberg R R. Conjugative transfer by the virulence system of Legionella pneumophila. Science. 1998;279:873–876. doi: 10.1126/science.279.5352.873. [DOI] [PubMed] [Google Scholar]
- 55.Wandersman C. Secretion, processing and activation of bacterial extracellular proteases. Mol Microbiol. 1989;3:1825–1831. doi: 10.1111/j.1365-2958.1989.tb00169.x. [DOI] [PubMed] [Google Scholar]
- 56.Weiss A A, Johnson F D, Burns D L. Molecular characterization of an operon required for pertussis toxin secretion. Proc Natl Acad Sci USA. 1993;90:2970–2974. doi: 10.1073/pnas.90.7.2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Winans S C, Burns D L, Christie P J. Adaptation of a conjugal transfer system for the export of pathogenic macromolecules. Trends Microbiol. 1996;4:64–68. doi: 10.1016/0966-842X(96)81513-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zambryski P C. Chronicles from the Agrobacterium-plant cell DNA transfer story. Annu Rev Plant Physiol Plant Mol Biol. 1992;43:465–490. [Google Scholar]
- 59.Zhou X-R, Christie P J. Suppression of mutant phenotypes of the Agrobacterium tumefaciens VirB11 ATPase by overproduction of VirB proteins. J Bacteriol. 1997;179:5835–5842. doi: 10.1128/jb.179.18.5835-5842.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zupan J, Zambryski P. The Agrobacterium DNA transfer complex. Crit Rev Plant Sci. 1997;16:279–295. [Google Scholar]
- 61.Zupan J R, Citovsky V, Zambryski P. Agrobacterium VirE2 protein mediates nuclear uptake of single-stranded DNA in plant cells. Proc Natl Acad Sci USA. 1996;93:2392–2397. doi: 10.1073/pnas.93.6.2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zupan J R, Ward D, Zambryski P. Assembly of the VirB transport complex for DNA transfer from Agrobacterium tumefaciens to plant cells. Curr Opin Microbiol. 1998;1:649–655. doi: 10.1016/s1369-5274(98)80110-0. [DOI] [PubMed] [Google Scholar]