Abstract
Purpose
Today, the decision to treat patients with chemotherapy for early breast cancer (EBC) is made based on the patient’s individual risk stratification and tumor biology. In cases with chemotherapy indication, the neoadjuvant application (NACT) is the preferred option in comparison with primary surgery and adjuvant chemotherapy (ACT). Age remains a relevant factor in the decision-making process. The aim of the present study was to illustrate the impact of age on the use of systemic therapy in clinical routine.
Methods
The study separately analyzed chemotherapy use among six age cohorts of EBC patients who had been treated at 104 German breast units between January 2008 and December 2017.
Results
In total, 124,084 patients were included, 46,279 (37.3%) of whom had received chemotherapy. For 44,765 of these cases, detailed information on treatment was available. Within this cohort, chemotherapy was administered as NACT to 14,783 patients (33.0%) and as ACT to 29,982 (67.0%) patients. Due to the higher prevalence of unfavorable tumor subtypes, younger patients had a higher rate of chemotherapy (≤ 29y: 74.2%; 30–39y: 71.3%) and a higher proportion of NACT administration ( ≤ 29y: 66.9%; 30–39y: 56.0%) in comparison with elderly patients, who had lower rates for overall chemotherapy (60–69y: 37.5%; ≥ 70y: 17.6%) and NACT (60–69y: 25.5%; ≥ 70y: 22.8%). Pathologic complete response was higher in younger than in older patients (≤ 29y: 30.4% vs. ≥ 70y: 16.7%), especially for HER2− subtypes.
Conclusion
The data from the nationwide German cohort reveal relevant age-dependent discrepancies concerning the use of chemotherapy for EBC.
Keywords: Early breast cancer, Pathological complete response, Neoadjuvant chemotherapy, Age, Elderly patients
What does this study add to the clinical work
| Data from a nationwide German cohort reveal relevant discrepancies concerning the indication for and patterns of chemotherapy use for early breast cancer depending on age. Younger patients (< 40 years) more often receive chemotherapy both in general and in a neo adjuvant therapy setting. These younger patients also have higher rates of pathologic complete remission in comparison with elderly patients, especially for HER− subtypes. |
Introduction
Mortality in early breast cancer (EBC) has declined over the past decade in most developed countries — such as Germany [1] — due to new developments in screening, diagnostics, surgery, radiotherapy, and systemic therapy, due to structural improvements (e.g., multidisciplinarity, specialized breast cancer units), and due to quality improvement measures, such as evidence-based guidelines [2]. A better molecular understanding of EBC [3] suggests that systemic therapy for EBC should be tailored according to individual risk factors and intrinsic subtypes [4].
In the past decade, this process has led to a substantial decline in overall chemotherapy use in EBC due to the availability of more individualized treatment decisions. However, the expanding application of neoadjuvant chemotherapy (NACT) (in comparison with adjuvant chemotherapy; ACT) has caused more patients to have a pathological complete response (pCR), which can be regarded as a surrogate for better outcomes (in comparison with non-pCR). These developments have been demonstrated for Germany in previous single-center [5] and multicenter [6] analyses.
Although the indication for chemotherapy in EBC is mainly driven by tumor biology, age remains a relevant factor in routine decision-making. Very young and old age are particularly important factors that might impact treatment decisions: When it comes to defining which EBC patients should be considered young, the limit can be set at 40 years or younger, in keeping with recent ESMO guidelines [7]. This group of patients represents around 5% of all EBC patients [8], albeit with a rising incidence [9]. When it comes to elderly EBC patients, defining a threshold for specific therapy management is more difficult because numerical age is influenced by individual performance and frailty, with a threshold of ≥ 70 years often being used to define the group [10]. Elderly patients with comorbidities are particular often underrepresented or excluded from clinical trials [11].
No nationwide tumor registration exists in Germany, and details about the indication for chemotherapy in the cohorts of both very young and elderly EBC patients, therefore, remain unclear, as does the impact of age on treatment patterns and outcomes for EBC within the German healthcare system. The aim of the present study was, thus, to illustrate both the impact of age on systemic treatment patterns for EBC and the respective outcomes of these treatment patterns among patients by using data from a large patient cohort derived from the clinical routine. For this purpose, we present data from 124,084 patients who were treated at 104 German institutions between 2008 and 2017.
Methods
Database
The present study uses data from the West German Breast Center GmbH (WBC), Düsseldorf, Germany [12]. Participating hospitals and breast cancer units (BCUs) contribute clinical, surgical, and pathological data on patients with EBC to the database, and the collaborating institutions collect the data prospectively. Thus, the present study represents a post hoc analysis of a prospectively collected database. The dataset does not include follow-up information on oncological outcomes.
For the analysis, anonymized data from all female patients with invasive EBC who had been treated between 1 January 2008 and 31 December 2017 were extracted from the database. The final dataset comprised 124,084 patients. EBC was defined as primary (non-metastasized) breast cancer that was being treated in curative intention. All patients had undergone breast surgery. The division into adjuvant and neoadjuvant chemotherapy was determined based on the date of surgery. Patients who had received both neoadjuvant and adjuvant (i.e., post-neoadjuvant) chemotherapy were subsumed as neoadjuvant (because neoadjuvant therapy was the primary therapy in these cases).
The study was approved by the Ethics Committee of Heidelberg University and was conducted in accordance with the Declaration of Helsinki. The study was deemed to be without risk because it included only analyses of routinely collected anonymized data. Consequently, the Ethics Committee did not request approval for consent for this designated analysis. Informed consent to analyze the anonymized data was obtained from all individual participants before data acquisition as part of the benchmarking process.
Categorization of age groups
All patients were categorized into one of six different age groups, which were defined by the date of the patient’s (first) histopathologic diagnosis of EBC: Group 1: ≤ 29 years; Group 2: 30–39 years; Group 3: 40–49 years; Group 4: 50–59 years; Group 5: 60–69 years; and Group 6: ≥ 70 years.
Definitions of tumor histology, stages, and subtypes
Tumor histology was defined according to the World Health Organization criteria [14], and post-operative pathological staging was performed in line with the recent TNM classification [15]. Response to NACT was determined using the post-operative specimens along international standards, and pCR after NACT was defined as ypT0 ypN0 – that is, as the absence of invasive cancer in breast and axillary lymph nodes. The expression of the immunohistochemical (IHC) parameters of estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor 2 (HER2), and Ki-67 was assessed using formalin-fixed, paraffin-embedded tumor tissue according to international standards. For patients who were receiving NACT, IHC was based on the pre-treatment biopsy (if available); whereas for patients with ACT, IHC was based on the final post-operative pathological sample. The detailed criteria for positivity of the hormone receptors (HR) — that is, ER and PR — and of the HER2 status has been described previously [16]. HR was defined as negative if both ER/PR were negative and as positive if either ER or PR (or both) were positive. We then defined four subtypes: (1) HR+ and HER2− , (2) HR+ and HER2+ , (3) HR− and HER2+ , and (4) HR− and HER2− (i.e., “triple negative”; TN).
Statistical analysis
Annual percentages of chemotherapy use were calculated and presented in a longitudinal time-trend analysis for the period from 2008 to 2017 (in %) for the entire cohort. pCR rates were calculated from the subgroup of patients who had received NACT. All cases were assigned to a year (2008–2017) according to the date of the first histopathological documentation. Multivariable logistic regression modeling was used to identify factors associated with the achievement of pathological complete remission after NACT had been applied. Due to the extensive sample size of the register database, p values of < 0.05 were considered statistically significant in a descriptive sense. Missing data were not imputed. Data were analyzed descriptively using both SPSS software version 25 (IBM; Armonk, NY; USA) and R version 3.5.0.
Results
Patient and tumor characteristics
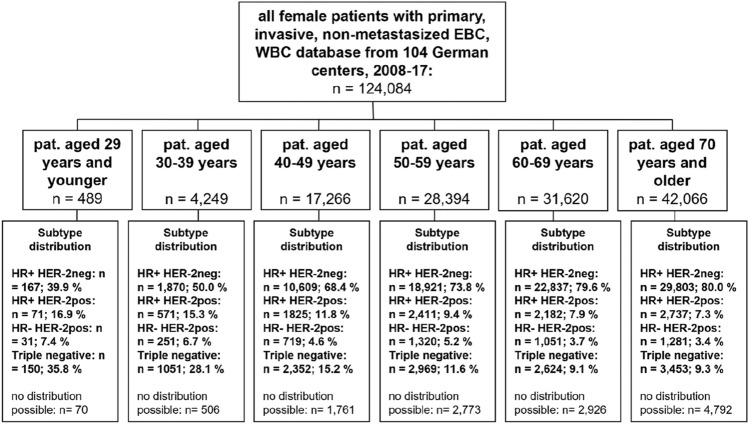
In total, 104 institutions provided a final dataset of 124,084 patients with EBC, 82.3% (n = 102,080) of whom were 50 years or older upon first diagnosis. Figure 1 presents the distribution among the six age cohorts. Menopause status relates to age, with nearly all women below age 30 being registered as pre-menopausal (95.0%) and nearly all women aged 70 and older being registered as post-menopausal (98.5%). Overall, most patients presented with tumors of stage T1/T2, with no relevant differences between the age groups. Higher tumor stages—classified as T3/T4—were most prevalent in the oldest age group and affected 6.0% and 5.4%, respectively, of the women in this group. In all other age groups, T3 and T4 tumors were less prevalent and affected between 2.6% (patients aged 60–69y) and 4.3% (patients aged 30–39y) as well as between 0.7% (patients aged 30–39y) and 1.4% (patients aged 60–69y), respectively, of the women in these groups. Overall, there was no relevant difference concerning nodal status, with most patients being nodal negative in all age groups. Regarding grading, most tumors in patients under 30 years old were graded as G3 (57.2%); whereas, most tumors in patients aged 40 and older were graded as G2 (55.8, 57.2, 61.6, and 63.1%, respectively). In relation to tumor subtype, the youngest age group displayed a rather unfavorable subtype distribution, with only 39.9% of patients presenting with the subtype HR+ HER2–, 16.9% presenting with the subtype HR+ HER2+ , 7.4% presenting with the subtype HR– HER2+ , and 35.8% presenting with the subtype HR– HER2–. In contrast, in the oldest age group, most patients presented with the subtype HR+ HER2– (80.0%), with other subtypes being relatively rare (HR+ HER2+ : 7.3%; HR– HER2+ : 3.4%; HR– HER2–: 9.3%). The younger the patient group was, the more often its members were being treated at a university hospital, with almost one-third of patients aged 29 or younger (28.6%) and only 8.5% of patients aged 70 or older being treated there. The Karnofsky Performance Status Scale indicates that functional impairment was more present in the older patient groups, with 71.8% and 20.1% of patients under 30 achieving a score of 100 or 90, respectively, while only 33.5% and 34.5%, respectively, of patients aged 70 years and older achieved the same score (Table 1).
Fig. 1.
Patient cohorts
Table 1.
Patient and tumor characteristics for all cases of early breast cancer, divided into six age groups (Group 1: ≤ 29 y; Group 2: 30–39 y; Group 3: 40–49 y; Group 4: 50–59 y; Group 5: 60–69 y; Group 6: ≥ 70 y; total n = 124,084)
| Patient characteristics (n = 124,084) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ≤ 29 y (n = 489) |
30–39 y (n = 4249) |
40–49 y (n = 17,266) |
50–59 y (n = 28,394) |
60–69 y (n = 31,620) |
≥ 70 y (n = 42,066) |
|||||||
| Number | % | Number | % | Number | % | Number | % | Number | % | Number | % | |
| Menopause status | ||||||||||||
| Pre | 453 | 95.0 | 4008 | 95.6 | 14,020 | 82.2 | 5410 | 19.4 | 201 | 0.6 | 141 | 0.3 |
| Peri | 13 | 2.7 | 87 | 2.1 | 1405 | 8.2 | 3532 | 12.6 | 348 | 1.1 | 473 | 1.1 |
| Post | 11 | 2.3 | 99 | 2.4 | 1621 | 9.5 | 18,999 | 68.0 | 30,607 | 98.2 | 40,735 | 98.5 |
| Total | 477 | 100 | 4194 | 100 | 17,046 | 100 | 27,941 | 100 | 31,156 | 100 | 41,349 | 100 |
| Missing | 12 | 55 | 220 | 453 | 464 | 717 | ||||||
| pT stadium (cases without neoadjuvant chemotherapy) | ||||||||||||
| pT1 | 91 | 60.7 | 1091 | 57.8 | 6056 | 58.6 | 13,029 | 66.7 | 15,932 | 66.6 | 13,503 | 42.0 |
| pT1mic | 1 | 0.7 | 6 | 0.3 | 15 | 0.1 | 64 | 0.3 | 55 | 0.2 | 47 | 0.1 |
| pT2 | 50 | 33.3 | 683 | 36.2 | 3723 | 36.0 | 5615 | 28.8 | 6865 | 28.7 | 14,644 | 45.5 |
| pT3 | 6 | 4.0 | 92 | 4.9 | 431 | 4.2 | 618 | 3.2 | 698 | 2.9 | 2079 | 6.5 |
| pT4 | 2 | 1.3 | 16 | 0.8 | 103 | 1.0 | 204 | 1.0 | 363 | 1.5 | 1878 | 5.8 |
| Total | 150 | 100 | 1888 | 100 | 10,328 | 100 | 19,530 | 100 | 2,3913 | 100 | 32,151 | 100 |
| Missing | 102 | 692 | 2901 | 4514 | 4789 | 8343 | ||||||
| ypT stadium (cases with neoadjuvant chemotherapy) | ||||||||||||
| ypT0 | 84 | 39.1 | 539 | 36.2 | 1120 | 30.7 | 1200 | 30.8 | 770 | 29.6 | 329 | 24.4 |
| ypTis | 25 | 11.6 | 159 | 10.7 | 369 | 10.1 | 342 | 8.8 | 210 | 8.1 | 102 | 7.6 |
| ypT1 | 64 | 29.8 | 458 | 30.7 | 1237 | 33.9 | 1291 | 33.1 | 882 | 33.9 | 422 | 31.3 |
| ypT1mic | 1 | 0.5 | 14 | 0.9 | 15 | 0.4 | 33 | 0.8 | 20 | 0.8 | 14 | 1.0 |
| ypT2 | 29 | 13.5 | 241 | 16.2 | 705 | 19.3 | 800 | 20.5 | 509 | 19.6 | 338 | 25.1 |
| ypT3 | 11 | 5.1 | 68 | 4.6 | 165 | 4.5 | 135 | 3.5 | 121 | 4.6 | 72 | 5.3 |
| ypT4 | 1 | 0.5 | 11 | 0.7 | 42 | 1.1 | 98 | 2.5 | 91 | 3.5 | 72 | 5.3 |
| Total | 215 | 100 | 1490 | 100 | 3653 | 100 | 3899 | 100 | 2603 | 100 | 1349 | 100 |
| Missing | 22 | 179 | 384 | 451 | 315 | 223 | ||||||
| (y)pN stadium | ||||||||||||
| (y)pN0 | 297 | 70.9 | 2420 | 64.7 | 9967 | 64.3 | 17,874 | 69.5 | 20,802 | 73.0 | 21,731 | 64.1 |
| (y)pN1 | 80 | 19.1 | 837 | 22.4 | 3507 | 22.6 | 4854 | 18.9 | 4702 | 16.5 | 6818 | 20.1 |
| (y)pN1mi | 15 | 3.6 | 113 | 3.0 | 517 | 3.3 | 815 | 3.2 | 717 | 2.5 | 799 | 2.4 |
| (y)pN2 | 20 | 4.8 | 267 | 7.1 | 1,019 | 6.6 | 1426 | 5.5 | 1,419 | 5.0 | 2717 | 8.0 |
| (y)pN3 | 7 | 1.7 | 106 | 2.8 | 488 | 3.1 | 738 | 2.9 | 870 | 3.1 | 1,832 | 5.4 |
| Total | 419 | 100 | 3743 | 100 | 15,498 | 100 | 25,707 | 100 | 28,510 | 100 | 33,897 | 100 |
| Missing | 70 | 506 | 1768 | 2687 | 3110 | 8169 | ||||||
| Grading | ||||||||||||
| G1 | 12 | 3.3 | 209 | 6.1 | 1811 | 12.2 | 4230 | 16.8 | 4770 | 16.6 | 4129 | 11.6 |
| G2 | 146 | 39.6 | 1532 | 45.0 | 8295 | 55.8 | 14,430 | 57.2 | 17,654 | 61.6 | 22,477 | 63.1 |
| G3 | 211 | 57.2 | 1667 | 48.9 | 4764 | 32.0 | 6588 | 26.1 | 6229 | 21.7 | 8988 | 25.3 |
| Total | 369 | 100 | 3408 | 100 | 14,870 | 100 | 25,248 | 100 | 28,653 | 100 | 35,594 | 100 |
| Missing | 120 | 841 | 2396 | 3146 | 2967 | 6472 | ||||||
| Estrogen-receptor status | ||||||||||||
| Positive | 230 | 53.9 | 2385 | 62.5 | 12,379 | 78.2 | 21,370 | 81.7 | 25,158 | 86.1 | 32,975 | 86.3 |
| Negative | 197 | 46.1 | 1432 | 37.5 | 3452 | 21.8 | 4788 | 18.3 | 4059 | 13.9 | 5251 | 13.7 |
| Total | 427 | 100 | 3817 | 100 | 15,831 | 100 | 26,158 | 100 | 29,217 | 100 | 38,226 | 100 |
| Missing | 62 | 432 | 1435 | 2236 | 2403 | 3840 | ||||||
| Progesterone-receptor status | ||||||||||||
| Positive | 202 | 47.3 | 2171 | 56.9 | 11,625 | 73.4 | 18,769 | 71.8 | 21,644 | 74.1 | 28,200 | 73.8 |
| Negative | 225 | 52.7 | 1646 | 43.1 | 4204 | 26.6 | 7380 | 28.2 | 7567 | 25.9 | 10,016 | 26.2 |
| Total | 427 | 100 | 3817 | 100 | 15,829 | 100 | 26,149 | 100 | 29,211 | 100 | 38,216 | 100 |
| Missing | 62 | 432 | 1437 | 2245 | 2409 | 3850 | ||||||
| HER2-receptor status | ||||||||||||
| Positive | 104 | 24.8 | 825 | 22.0 | 2539 | 16.3 | 3687 | 14.4 | 3,205 | 11.1 | 3993 | 10.7 |
| Negative | 316 | 75.2 | 2932 | 78.0 | 12,997 | 83.7 | 21,997 | 85.6 | 25,559 | 88.9 | 33,342 | 89.3 |
| Total | 420 | 100 | 3757 | 10 | 15,536 | 100 | 25,684 | 100 | 28,764 | 10 | 37,335 | 100 |
| Missing | 69 | 492 | 1730 | 2710 | 2856 | 4731 | ||||||
| Subtype distribution | ||||||||||||
| HR+ HER2− | 167 | 39.9 | 1870 | 50.0 | 10,609 | 68.4 | 18,921 | 73.8 | 22,837 | 79.6 | 29,803 | 80.0 |
| HR+ HER2+ | 71 | 16.9 | 571 | 15.3 | 1825 | 11.8 | 2411 | 9.4 | 2182 | 7.6 | 2737 | 7.3 |
| HR− HER2+ | 31 | 7.4 | 251 | 6.7 | 719 | 4.6 | 1320 | 5.2 | 1051 | 3.7 | 1281 | 3.4 |
| HR− HER2− | 150 | 35.8 | 1051 | 28.1 | 2352 | 15.2 | 2969 | 11.6 | 2624 | 9.1 | 3453 | 9.3 |
| Total | 419 | 100 | 3743 | 100 | 15,505 | 100 | 25,621 | 100 | 28,694 | 100 | 37,274 | 100 |
| Missing | 70 | 506 | 1761 | 2773 | 2926 | 4792 | ||||||
| Hospital-type distribution | ||||||||||||
| University | 140 | 28.6 | 929 | 21.9 | 2622 | 15.2 | 3574 | 12.6 | 3557 | 11.2 | 3575 | 8.5 |
| Teaching hospital | 249 | 50.9 | 2304 | 54.2 | 10,260 | 59.4 | 17849 | 62.9 | 20,244 | 64.0 | 27,430 | 65.2 |
| Other | 100 | 20.4 | 1016 | 23.9 | 4384 | 25.4 | 6971 | 24.6 | 7819 | 24.7 | 11,061 | 26.3 |
| Total | 489 | 10 | 4249 | 100 | 17,266 | 100 | 28,394 | 100 | 31,620 | 100 | 42,066 | 100 |
| Chemotherapy | ||||||||||||
| Yes | 363 | 74.2 | 3031 | 71.3 | 10,261 | 59.4 | 13,363 | 47.1 | 11,848 | 37.5 | 7408 | 17.6 |
| No | 126 | 25.8 | 1218 | 28.7 | 7005 | 40.6 | 15,031 | 52.9 | 19,772 | 62.5 | 34,658 | 82.4 |
| Total | 489 | 100 | 4249 | 100 | 17,266 | 100 | 28,394 | 100 | 31,620 | 100 | 42,066 | 10 |
| Chemotherapy with complete information available | ||||||||||||
| NACT | 237 | 66.9 | 1669 | 56.0 | 4037 | 40.1 | 4350 | 33.4 | 2918 | 25.5 | 1572 | 22.8 |
| ACT | 117 | 33.1 | 1309 | 44.0 | 6019 | 59.9 | 8679 | 66.6 | 8540 | 74.5 | 5318 | 77.2 |
| Total | 354 | 100 | 2978 | 100 | 10,056 | 100 | 13,029 | 100 | 11,458 | 100 | 6890 | 100 |
| Karnofsky performance status scale | ||||||||||||
| 0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| 10 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| 20 | 0 | 0.0 | 0 | 0.0 | 1 | 0.0 | 3 | 0.0 | 3 | 0.0 | 15 | 0.0 |
| 30 | 0 | 0.0 | 0 | 0.0 | 3 | 0.0 | 8 | 0.0 | 13 | 0.1 | 24 | 0.1 |
| 40 | 0 | 0.0 | 1 | 0.0 | 6 | 0.0 | 25 | 0.1 | 36 | 0.1 | 167 | 0.6 |
| 50 | 2 | 0.6 | 5 | 0.2 | 27 | 0.2 | 40 | 0.2 | 88 | 0.4 | 672 | 2.2 |
| 60 | 2 | 0.6 | 11 | 0.4 | 37 | 0.3 | 77 | 0.3 | 155 | 0.6 | 1112 | 3.7 |
| 70 | 1 | 0.3 | 11 | 0.4 | 91 | 0.7 | 240 | 1.1 | 496 | 2.0 | 2136 | 7.1 |
| 80 | 22 | 6.6 | 230 | 7.4 | 850 | 6.4 | 1756 | 7.9 | 2544 | 10.2 | 5538 | 18.3 |
| 90 | 67 | 20.1 | 711 | 23.0 | 3312 | 24.9 | 6279 | 28.4 | 7943 | 32.0 | 10,433 | 34.5 |
| 100 | 239 | 71.8 | 2128 | 68.7 | 8995 | 67.5 | 13,709 | 61.9 | 13,538 | 54.5 | 10,111 | 33.5 |
| Total | 333 | 100 | 3097 | 100 | 13,324 | 100 | 22,138 | 100 | 24,820 | 100 | 30,212 | 100 |
| Missing | 156 | 1152 | 3944 | 6257 | 6804 | 11,858 | ||||||
Chemotherapy use
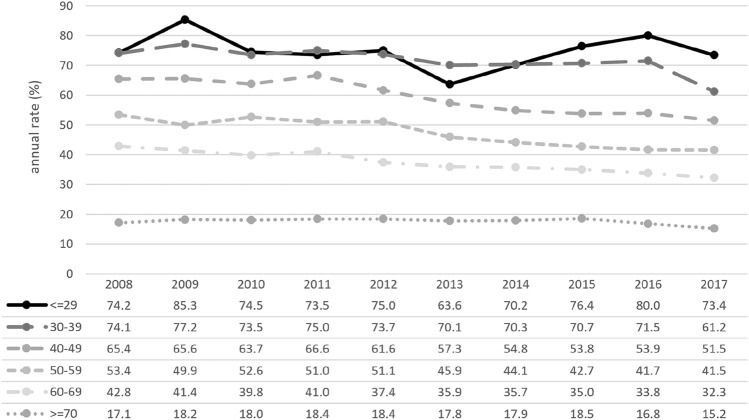
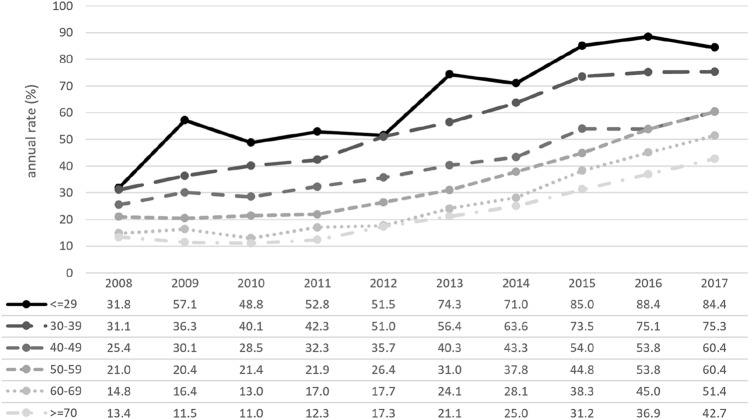
In total, 46,274 (37.3%) patients had received chemotherapy, 44,765 of whom had complete information available and 1,509 (3.3%) of whom had missing data on treatment. Of the patients with complete information, 29,982 (67.0%) had received chemotherapy as ACT, and 14,783 (33.0%) had received chemotherapy as NACT. In total, 1,367 patients had received both neoadjuvant and adjuvant chemotherapy. Younger patients had received chemotherapy more often both overall (≤ 29y: 74.2%; 30–39y: 71.3%) and as NACT ( ≤ 29y: 66.9%; 30–39y: 56.0%) in comparison with older patients regarding both overall CHT (60–69y: 37.5%; ≥ 70y: 17.6%) and NACT (60–69a: 25.5%; ≥ 70y 22.8%). Between 2008 and 2017, the proportion of patients in all age groups who had received NACT rose (Fig. 2), whereas CHT use declined overall, primarily in the age group between 40 and 70 years (Fig. 3).
Fig. 2.
Overall portion of patients receiving chemotherapy (CHT), divided into six age groups (Group 1: ≤ 29 years; Group 2: 30–39 years; Group 3: 40–49 years; Group 4: 50–59 years; Group 5: 60–69 years; Group 6: ≥ 70 years; total n = 124,084)
Fig. 3.
Relative portion of neoadjuvant chemotherapy (NACT) use (among all patients on chemotherapy), divided into six age groups (Group 1: ≤ 29 years; Group 2: 30–39 years; Group 3: 40–49 years; Group 4: 50–59 years; Group 5: 60–69 years; Group 6: ≥ 70 years; total n = 44,765; missing n = 1509)
Response to neoadjuvant chemotherapy
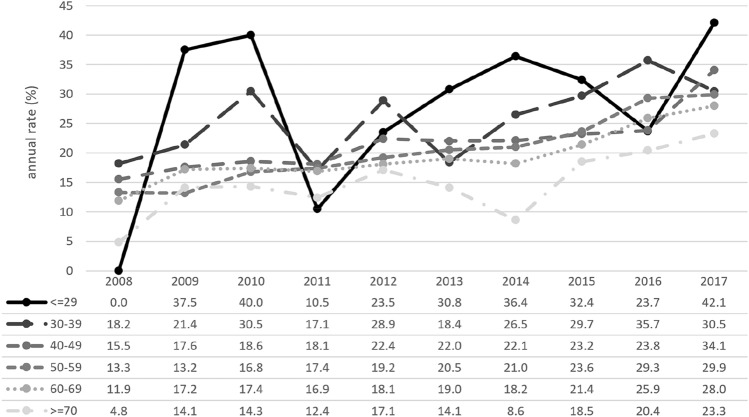
Between 2008 to 2017, the rate of pCR (ypT0 ypN0) rose for all patients after NACT (n = 14,783) in all age groups. Overall, pCR rates were higher in younger patients than in older patients (Fig. 4). Across all ages, pCR rates were highest among patients with the tumor subtype HR– HER2+ , which affected 45.1% of patients compared with 34.0% and 30.4% of patients with the tumor subtypes HR– HER2– and HR+ HER2+ , respectively. Divided by age group, pCR rates sank with rising age (≤ 29y: 28.4% vs. ≥ 70y: 16.9%) (Table 2).
Fig. 4.
Rates for pathological complete response (pCR: ypT0 ypN0) after neoadjuvant chemotherapy, divided into six age groups (Group 1: ≤ 29 years; Group 2: 30–39 years; Group 3: 40–49 years; Group 4: 50–59 years; Group 5: 60–69 years; Group 6: ≥ 70 years; total n = 14,783)
Table 2.
Percentage of patients who achieved pathological complete remission (pCR, defined as ypT0 ypN0) after having received neoadjuvant chemotherapy (NACT), divided by age group and tumor subtype
| Percentage of patients who achieved pCR after having received NACT, divided by age group and subtype | |||||
|---|---|---|---|---|---|
| HR+ HER2− | HR+ HER2+ | HR− HER2+ | HR− HER2− | All subtypes | |
| ≤ 29 y | 14.1 | 26.2 | 36.4 | 39.1 | 28.4 |
| 30–39 y | 13.7 | 32.2 | 41.0 | 34.3 | 27.6 |
| 40–49 y | 10.9 | 27.2 | 37.2 | 31.3 | 22.5 |
| 50–59 y | 9.0 | 26.2 | 43.2 | 28.4 | 22.6 |
| 60–69 y | 8.1 | 29.4 | 37.9 | 27.7 | 21.3 |
| ≥ 70 y | 5.1 | 22.0 | 35.5 | 19.3 | 16.9 |
| All ages | 10.8 | 30.4 | 45.1 | 34.0 | 30.8 |
Multivariable model
A multivariable logistic regression of factors that influence pCR achievement after NACT was performed (Table 3). Young age was found to be positively correlated with pCR; however, this finding was not statistically significant. The odds of achieving pCR significantly increased for patients with HER2+ and TN EBC compared with for patients with the HR+ HER2– subtype. Regarding the influence of caseload, a higher caseload was associated with lower odds of achieving pCR. These findings were statistically significant.
Table 3.
Multivariable logistic regression, revealing factors that influence the achievement of pathological complete remission (vs. no pathological complete remission) (n = 8943)
| Odds ratio (95% CI) | P value | |
|---|---|---|
| Age | ||
| ≤ 29 y | Reference | Reference |
| 30–39 y | 1.4123 (0.6372–3.0996) | 0.3951 |
| 40–49 y | 1.2531 (0.5812–2.6725) | 0.5649 |
| 50–59 y | 1.0184 (0.4728–2.168) | 0.9629 |
| 60–69 y | 0.8238 (0.3776–1.775) | 0.6258 |
| ≥ 70 y | 0.776 (0.3408–1.7477) | 0.5452 |
| Grading | ||
| G1 | Reference | Reference |
| G2 | 1.2277 (0.6229–2.4658) | 0.5611 |
| G3 | 1.2791 (0.6524–2.5516) | 0.4818 |
| Subtype | ||
| HR+ HER2– | Reference | Reference |
| HR+ HER2+ | 1.8646 (1.3965–2.4914) | < 0.001 |
| HR– HER2+ | 2.4872 (1.7914–3.4574) | < 0.001 |
| HR– HER2– | 1.6639 (1.2753–2.1714) | < 0.001 |
| Hospital type | ||
| University | Reference | Reference |
| Teaching hospital | 1.1392 (0.8116–1.5995) | 0.4514 |
| Other | 1.2352 (0.868–1.758) | 0.2407 |
| Annual caseload | ||
| ≤ 100 cases | Reference | Reference |
| 101–250 cases | 0.6035 (0.4275–0.855) | 0.0043 |
| > 250 cases | 0.4867 (0.3418–0.6942) | < 0.001 |
| Karnofsky index | ||
| 50 | Reference | Reference |
| 60 | 1.3289 (0.1142–15.5768) | 0.8235 |
| 70 | 2.1119 (0.2917–16.3266) | 0.4757 |
| 80 | 1.4683 (0.2377–9.8417) | 0.6938 |
| 90 | 2.2939 (0.3789–15.1321) | 0.3906 |
| 100 | 1.6069 (0.2662–10.5641) | 0.6233 |
Discussion
This study analyzed the impact of age on systemic treatment patterns for EBC using data from a large patient cohort in clinical routine in Germany.
Since the emergence of molecular classification systems [13], it has become evident that systemic therapy for EBC must be tailored according to individual risk factors, such as tumor stage and subtype. Gene-expression profiles have been implemented in clinical routine for cases for which no other criteria enable adequate adjuvant treatment with chemotherapy. Nonetheless, age remains an important factor in the complex process of decision-making for adjuvant and neoadjuvant systemic therapy treatment in EBC [14]. While most patients who are affected with EBC are between 40 and 70 years old, patients outside of this range — that is, both very young and elderly patients — might be at risk of overtreatment or undertreatment, both of which are associated with deviations from guideline-adherent treatment. To address specific challenges for these subgroups, recommendations have been established for elderly patients [15] and for younger patients [7, 16]. Age groups differ not only in their clinico-pathological characteristics, but also in demographic factors, such as life expectancy, time of diagnosis, and differences in individual screening and treatment patterns [17], as demonstrated by our patient characteristics (Table 1). Moreover, studies have shown that molecular subtypes have different distributions and prognostic effects in elderly EBC patients compared with in younger patients, and biomarkers therefore have different implications in elderly patients compared with in their younger counterparts [18]. Comparable to these finding, our data also revealed differences in the prevalence of tumor subtypes between age cohorts, with a higher rate of unfavorable subtypes (HER2+ and TN) having been found in younger patient cohorts (Table 1).
One study from Germany demonstrated that only about 3 out of 4 patients with EBC undergo guideline-adherent therapy, which results in unfavorable outcome parameters for patients with guideline violations [19]. A major subgroup with guideline violations seems to be patients with higher age [20–23]. Several comparable results have demonstrated that higher age remains a barrier to receiving chemotherapy for EBC, as has been shown, for example, in France [24], Denmark [25], Spain [26], and the US [27]. In Germany, the most important reason for discouraging patients from undergoing chemotherapy is somatic comorbidities and age > 75 years [19]. In general, relevant comorbidity prevalence upon EBC diagnosis increases with age and likely negatively influences the chances of receiving guideline-adherent systemic treatment [28].
Regarding outcomes, adjuvant chemotherapy in elderly patients is postulated to be beneficial, as has been shown for low-risk subgroups [29] and for patients with unfavorable tumor characteristics [30]. Upon examining outcome perspectives for extremely old EBC patients, these age groups also seem to profit from adjuvant chemotherapy, as results for patients > 75 years in South Korea [31] and for patients > 80 years in Singapore [32] have demonstrated. A recent analysis from the US revealed that chemotherapy is also associated with improved overall survival in node-positive, estrogen-receptor-positive elderly patients with multiple comorbidities [33]. In this context, higher recurrence rates in elderly patients compared with in younger post-menopausal women were explained by the under-use of systemic treatment in these groups [23].
When treating elderly patients with chemotherapy, the risk of hematotoxicity must be considered, specifically when using anthracyclines [34]. However, other risks seem to increase in elderly EBC patients who undergo chemotherapy, including acute kidney injury [35] and secondary haemato-oncological diseases [36]. Cardiotoxicity might be an additional problem for the application of trastuzumab in combination with standard chemotherapy, especially in HER2+ patients. Thus, in one US study, the highest rates of non-standard chemotherapy regimens in EBC were found among elderly women and were associated with fewer toxicity-related hospitalizations but with worse survival rates [37]. In contrast, the chemotherapy regimens used in women with EBC aged 70 and above in Germany appear to be relatively standardized and correspond to the recommendations given in the respective guidelines [38]. Survey results from outside Germany reveal a relevant lack of knowledge concerning the specific management of elderly patients affected by EBC [39].
Regarding pCR rates, age has an unfavorable impact on the chances of pCR, but acceptable rates are still possible, especially in HER2+ elderly patients [40]. These results are in line with our data, which reveal a general negative likelihood of pCR among patients with higher ages (Table 3) but no relevant decrease in pCR rates for patients with HER2+ tumors— in contrast to patients with HER2− subtypes in higher age cohorts (Table 2). In the multivariable model, the trend of having lower chances of pCR among elderly patients is mainly driven by the lower prevalence of these HER2+ subtypes rather than by the elderly population itself (Table 3). The negative effect of age on pCR can, thus, be concluded to have most likely been factored out due to the increased occurrence of HR+ HER− with increasing age.
Therefore, when assessing the risks and benefits of chemotherapy for older patients, treatment must be adapted to general health and tumor biology rather than to age. In these cases, a professional geriatric assessment has been shown to benefit from therapy management [41]. It seems to be beneficial to evaluate individual risk factors in elderly EBC patients in order to avoid short-term mortality after adjuvant chemotherapy [42]. While undertreatment among elderly patients is often reported for systemic therapy, the opposite trend appears in surgical procedures, with continued overtreatment (e.g., in axillary management) causing unnecessary morbidity without any oncological benefit [43]. Moreover, for radiotherapy, this trend of reducing the therapy intensity is important: As one study demonstrated for patients aged 70 and older in low-risk EBC situations, breast irradiation after breast-conserving surgery can be avoided with a less-than-3% chance of local recurrence [44].
Younger women have poorer survival rates after breast cancer than older women: Previous research has demonstrated that young age is an independent risk factor for disease recurrence and death, although recent data suggest that this finding may not be true for all EBC subtypes [45] and that younger patients have higher proportions of HER2+ and TN subtypes than older women and are also more likely to be primarily diagnosed with advanced disease [46]. These findings are congruent with tumor characteristics in our patient cohorts (Table 1). In the literature, younger patients face higher rates of mastectomy and the use of chemotherapy, which indicates that more aggressive therapy is recommended or chosen for women in this age group in general [47]. Additionally, in these cohorts, EBC is more likely to have a hereditary background that might influence the decision to undergo treatment with a more aggressive approach [48].
Future clinical trials that focus on these specific subgroups appear to be necessary in order to find proper treatment strategies. Some prospective trials have already been established, such as the UK-based POSH study, which addresses younger patients in high-risk situations [49].
Our study has several limitations: Although the German registry is very large and covers the entire country, it is still only a sample and is not a comprehensive mandatory registry. Therefore, the results may not be entirely representative of all institutions [50]. Unfortunately, as we have a benchmarking database, information on individual patient status (e.g., comorbidities) and clinical tumor stage is not available. Thus, we were not able to adjust our data by considering these baseline patient characteristics.
Conclusion
The results of this large, nationwide cohort reveal both a relevant discrepancy concerning the use of chemotherapy based on age and the risk of undertreatment or overtreatment among the subgroups of very young patients and elderly patients with an EBC diagnosis.
Author contributions
ASH: project development, data collection and management, data analysis, manuscript writing/editing, AH: project development, data collection and management, manuscript writing/editing, MF: data analysis, MM: data collection and management, SH: manuscript writing/editing, TMD: manuscript writing/editing, RT: manuscript writing/editing, BS: manuscript writing/editing, AS: data collection and management, manuscript writing/editing, MW: manuscript writing/editing, MG: manuscript writing/editing, JH: project development, data collection and management, manuscript writing/editing, FR: project development, data collection and management, data analysis, manuscript writing/editing.
Funding
Open Access funding enabled and organized by Projekt DEAL. The authors declare that no funds, grants, or other support was received during the preparation of this manuscript.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
All procedures that were performed in studies that involved human participants were undertaken in accordance both with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments, or with comparable ethical standards. The Ethics Committee of Heidelberg University Medical School did not request approval for consent for this designated analysis. Informed consent to analyze the anonymized data was obtained from all individual participants for the data acquisition of the benchmarking process.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hennigs A, Riedel F, Gondos A, Sinn P, Schirmacher P, Marme F, et al. Prognosis of breast cancer molecular subtypes in routine clinical care: a large prospective cohort study. BMC Cancer. 2016;16(1):734. doi: 10.1186/s12885-016-2766-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ditsch N, Wöcke A, Untch M, Jackisch C, Albert US, Banys-Paluchowski M, et al. AGO recommendations for the diagnosis and treatment of patients with early breast cancer: update 2022. Breast Care (Basel) 2022;17(4):403–420. doi: 10.1159/000524879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature. 2000;406(6797):747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 4.Weigelt B, Baehner FL, Reis-Filho JS. The contribution of gene expression profiling to breast cancer classification, prognostication and prediction: a retrospective of the last decade. J Pathol. 2010;220(2):263–280. doi: 10.1002/path.2648. [DOI] [PubMed] [Google Scholar]
- 5.Hennigs A, Riedel F, Marme F, Sinn P, Lindel K, Gondos A, et al. Changes in chemotherapy usage and outcome of early breast cancer patients in the last decade. Breast Cancer Res Treat. 2016;160(3):491–499. doi: 10.1007/s10549-016-4016-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Riedel F, Hoffmann AS, Moderow M, Heublein S, Deutsch TM, Golatta M, et al. Time trends of neoadjuvant chemotherapy for early breast cancer. Int J Cancer. 2020;147:3049–3058. doi: 10.1002/ijc.33122. [DOI] [PubMed] [Google Scholar]
- 7.Paluch-Shimon S, Cardoso F, Partridge AH, Abulkhair O, Azim HA, Jr, Bianchi-Micheli G, et al. ESO-ESMO 4th international consensus guidelines for breast cancer in young women (BCY4) Ann Oncol. 2020;31(6):674–696. doi: 10.1016/j.annonc.2020.03.284. [DOI] [PubMed] [Google Scholar]
- 8.DeSantis CE, Ma J, Gaudet MM, Newman LA, Miller KD, Goding Sauer A, et al. Breast cancer statistics, 2019. CA Cancer J Clin. 2019;69(6):438–451. doi: 10.3322/caac.21583. [DOI] [PubMed] [Google Scholar]
- 9.Leclère B, Molinié F, Trétarre B, Stracci F, Daubisse-Marliac L, Colonna M. Trends in incidence of breast cancer among women under 40 in seven European countries: a GRELL cooperative study. Cancer Epidemiol. 2013;37(5):544–549. doi: 10.1016/j.canep.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 10.Abdel-Razeq H, Abu Rous F, Abuhijla F, Abdel-Razeq N, Edaily S. Breast cancer in geriatric patients: current landscape and future prospects. Clin Interv Aging. 2022;17:1445–1460. doi: 10.2147/CIA.S365497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rocque GB, Caston NE, Franks JA, Williams CP, Aswani MS, Azuero A, et al. Clinical trial representativeness and treatment intensity in a real-world sample of women with early stage breast cancer. Breast Cancer Res Treat. 2021;190(3):531–540. doi: 10.1007/s10549-021-06381-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neuschwander E, Elsner G, Hettenbach A, Becker G. Überblick der medizinischen Kennzahlensysteme in Deutschland zur Qualitätssicherung der Diagnostik und therapie des mammakarzinoms: eine bewertung aus der sicht des klinikers. Geburtshilfe Frauenheilkd. 2006;66(11):1050–1058. doi: 10.1055/s-2006-924468. [DOI] [Google Scholar]
- 13.Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thurlimann B, Senn HJ. Strategies for subtypes–dealing with the diversity of breast cancer: highlights of the St. Gallen international expert consensus on the primary therapy of early breast cancer 2011. Ann Oncol. 2011;22(8):1736–47. doi: 10.1093/annonc/mdr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuijer A, King TA. Age, molecular subtypes and local therapy decision-making. Breast. 2017;34(Suppl 1):S70–S77. doi: 10.1016/j.breast.2017.06.032. [DOI] [PubMed] [Google Scholar]
- 15.Biganzoli L, Wildiers H, Oakman C, Marotti L, Loibl S, Kunkler I, et al. Management of elderly patients with breast cancer: updated recommendations of the international society of geriatric oncology (SIOG) and European society of breast cancer specialists (EUSOMA) Lancet Oncol. 2012;13(4):e148–e160. doi: 10.1016/S1470-2045(11)70383-7. [DOI] [PubMed] [Google Scholar]
- 16.Cardoso F, Loibl S, Pagani O, Graziottin A, Panizza P, Martincich L, et al. The European society of breast cancer specialists recommendations for the management of young women with breast cancer. Eur J Cancer. 2012;48(18):3355–3377. doi: 10.1016/j.ejca.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 17.Lodi M, Scheer L, Reix N, Heitz D, Carin AJ, Thiébaut N, et al. Breast cancer in elderly women and altered clinico-pathological characteristics: a systematic review. Breast Cancer Res Treat. 2017;166(3):657–668. doi: 10.1007/s10549-017-4448-5. [DOI] [PubMed] [Google Scholar]
- 18.de Kruijf EM, Bastiaannet E, Rubertá F, de Craen AJ, Kuppen PJ, Smit VT, et al. Comparison of frequencies and prognostic effect of molecular subtypes between young and elderly breast cancer patients. Mol Oncol. 2014;8(5):1014–1025. doi: 10.1016/j.molonc.2014.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schwentner L, Van Ewijk R, Kühn T, Flock F, Felberbaum R, Blettner M, et al. Exploring patient- and physician-related factors preventing breast cancer patients from guideline-adherent adjuvant chemotherapy-results from the prospective multi-center study BRENDA II. Support Care Cancer. 2016;24(6):2759–2766. doi: 10.1007/s00520-016-3088-3. [DOI] [PubMed] [Google Scholar]
- 20.Hancke K, Denkinger MD, König J, Kurzeder C, Wöckel A, Herr D, et al. Standard treatment of female patients with breast cancer decreases substantially for women aged 70 years and older: a German clinical cohort study. Ann Oncol. 2010;21(4):748–753. doi: 10.1093/annonc/mdp364. [DOI] [PubMed] [Google Scholar]
- 21.Schwentner L, Wolters R, Wischnewsky M, Kreienberg R, Wöckel A. Survival of patients with bilateral versus unilateral breast cancer and impact of guideline adherent adjuvant treatment: a multi-centre cohort study of 5292 patients. The Breast. 2012;21(2):171–177. doi: 10.1016/j.breast.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 22.Schwentner L, Wolters R, Koretz K, Wischnewsky MB, Kreienberg R, Rottscholl R, et al. Triple-negative breast cancer: the impact of guideline-adherent adjuvant treatment on survival–a retrospective multi-centre cohort study. Breast Cancer Res Treat. 2012;132(3):1073–1080. doi: 10.1007/s10549-011-1935-y. [DOI] [PubMed] [Google Scholar]
- 23.Wallwiener CW, Hartkopf AD, Grabe E, Wallwiener M, Taran FA, Fehm T, et al. Adjuvant chemotherapy in elderly patients with primary breast cancer: are women ≥65 undertreated? J Cancer Res Clin Oncol. 2016;142(8):1847–1853. doi: 10.1007/s00432-016-2194-4. [DOI] [PubMed] [Google Scholar]
- 24.Meresse M, Bouhnik AD, Bendiane MK, Retornaz F, Rousseau F, Rey D, et al. Chemotherapy in old women with breast cancer: is age still a predictor for under treatment? Breast J. 2017;23(3):256–266. doi: 10.1111/tbj.12726. [DOI] [PubMed] [Google Scholar]
- 25.Jensen JD, Cold S, Nielsen MH, Jylling AM, Søe KL, Larsen LB, et al. Trends in breast cancer in the elderly in Denmark, 1980–2012. Acta Oncol. 2016;55(Suppl 1):59–64. doi: 10.3109/0284186X.2015.1115118. [DOI] [PubMed] [Google Scholar]
- 26.Martinez-Ramos D, Simon-Monterde L, Queralt-Martin R, Suelves-Piqueres C, Menor-Duran P, Escrig-Sos J. Breast cancer in octogenarian are we doing our best? a population-registry based study. Breast. 2018;38:81–5. doi: 10.1016/j.breast.2017.12.007. [DOI] [PubMed] [Google Scholar]
- 27.Mamtani A, Gonzalez JJ, Neo DT, Friedman RS, Recht A, Hacker MR, et al. Treatment strategies in octogenarians with early-stage. High-Risk Breast Cancer Ann Surg Oncol. 2018;25(6):1495–1501. doi: 10.1245/s10434-018-6350-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Land LH, Dalton SO, Jensen MB, Ewertz M. Influence of comorbidity on the effect of adjuvant treatment and age in patients with early-stage breast cancer. Br J Cancer. 2012;107(11):1901–1907. doi: 10.1038/bjc.2012.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taubenhansl C, Ortmann O, Gerken M, Inwald EC, Klinkhammer-Schalke M. Guideline-concordant chemotherapy in patients with hormone receptor-positive and node-positive, early breast cancer leads to better overall and metastases-free survival with limited benefit in elderly patients. Arch Gynecol Obstet. 2020;301(2):573–583. doi: 10.1007/s00404-019-05387-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jueckstock J, Kasch F, Jaeger B, Schramm A, Janni W, Scholz C. Adjuvant therapeutic decisions in elderly breast cancer patients: the role of chemotherapy in a retrospective analysis. Arch Gynecol Obstet. 2015;292(5):1101–1107. doi: 10.1007/s00404-015-3728-8. [DOI] [PubMed] [Google Scholar]
- 31.Jeon YW, You SH, Lee JE, Youn HJ, Lim W, Han JH, et al. Optimal treatment of breast cancer in women older than 75 years: a Korea breast cancer registry analysis. Breast Cancer Res Treat. 2019;178(3):693–701. doi: 10.1007/s10549-019-05426-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee CM, Zheng H, Tan VK, Tan TJ, Kanesvaran R, Wong FY, et al. Surgery for early breast cancer in the extremely elderly leads to improved outcomes - an Asian population study. Breast. 2017;36:44–48. doi: 10.1016/j.breast.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 33.Tamirisa N, Lin H, Shen Y, Shaitelman SF, Sri Karuturi M, Giordano SH, et al. Association of chemotherapy with survival in elderly patients with multiple comorbidities and estrogen receptor-positive. Node-Positive Breast Cancer JAMA Oncol. 2020;6(10):1548–1554. doi: 10.1001/jamaoncol.2020.2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karavasilis V, Papadimitriou C, Gogas H, Kouvatseas G, Pentheroudakis G, Koutras A, et al. Safety and tolerability of anthracycline-containing adjuvant chemotherapy in elderly high-risk breast cancer patients. Clin Breast Cancer. 2016;16(4):291–8.e3. doi: 10.1016/j.clbc.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 35.Li S, Liu J, Virnig BA, Collins AJ. Association between adjuvant chemotherapy and risk of acute kidney injury in elderly women diagnosed with early-stage breast cancer. Breast Cancer Res Treat. 2017;161(3):515–524. doi: 10.1007/s10549-016-4074-7. [DOI] [PubMed] [Google Scholar]
- 36.Rosenstock AS, Niu J, Giordano SH, Zhao H, Wolff AC, Chavez-MacGregor M. Acute myeloid leukemia and myelodysplastic syndrome after adjuvant chemotherapy: a population-based study among older breast cancer patients. Cancer. 2018;124(5):899–906. doi: 10.1002/cncr.31144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Freedman RA, Vaz-Luis I, Barry WT, Lii H, Lin NU, Winer EP, et al. Patterns of chemotherapy, toxicity, and short-term outcomes for older women receiving adjuvant trastuzumab-based therapy. Breast Cancer Res Treat. 2014;145(2):491–501. doi: 10.1007/s10549-014-2968-9. [DOI] [PubMed] [Google Scholar]
- 38.Barinoff J, Traut A, Bauerschlag D, Bischoff J, Herr D, Lübbe K, et al. Chemotherapy for 70 Year-old Women with breast cancer in Germany: a survey by the German breast group. Geburtshilfe Frauenheilkd. 2013;73(5):433–439. doi: 10.1055/s-0032-1328612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Audisio RA, Osman N, Audisio MM, Montalto F. How do we manage breast cancer in the elderly patients? a survey among members of the british association of surgical oncologists (BASO) Crit Rev Oncol Hematol. 2004;52(2):135–141. doi: 10.1016/j.critrevonc.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 40.von Waldenfels G, Loibl S, Furlanetto J, Machleidt A, Lederer B, Denkert C, et al. Outcome after neoadjuvant chemotherapy in elderly breast cancer patients - a pooled analysis of individual patient data from eight prospectively randomized controlled trials. Oncotarget. 2018;9(20):15168–15179. doi: 10.18632/oncotarget.24586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barthélémy P, Heitz D, Mathelin C, Polesi H, Asmane I, Litique V, et al. Adjuvant chemotherapy in elderly patients with early breast cancer Impact of age and comprehensive geriatric assessment on tumor board proposals. Crit Rev Oncol Hematol. 2011;79(2):196–204. doi: 10.1016/j.critrevonc.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 42.Rosenstock AS, Lei X, Tripathy D, Hortobagyi GN, Giordano SH, Chavez-MacGregor M. Short-term mortality in older patients treated with adjuvant chemotherapy for early-stage breast cancer. Breast Cancer Res Treat. 2016;157(2):339–350. doi: 10.1007/s10549-016-3815-y. [DOI] [PubMed] [Google Scholar]
- 43.Louie RJ, Gaber CE, Strassle PD, Gallagher KK, Downs-Canner SM, Ollila DW. Trends in surgical axillary management in early stage breast cancer in elderly women: continued over-treatment. Ann Surg Oncol. 2020;27(9):3426–3433. doi: 10.1245/s10434-020-08388-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stueber TN, Diessner J, Bartmann C, Leinert E, Janni W, Herr D, et al. Effect of adjuvant radiotherapy in elderly patients with breast cancer. PLoS ONE. 2020;15(5):e0229518. doi: 10.1371/journal.pone.0229518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rosenberg SM, Partridge AH. Management of breast cancer in very young women. Breast. 2015;24(Suppl 2):S154–S158. doi: 10.1016/j.breast.2015.07.036. [DOI] [PubMed] [Google Scholar]
- 46.Keegan TH, DeRouen MC, Press DJ, Kurian AW, Clarke CA. Occurrence of breast cancer subtypes in adolescent and young adult women. Breast Cancer Res. 2012;14(2):R55. doi: 10.1186/bcr3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Plichta JK, Thomas SM, Vernon R, Fayanju OM, Rosenberger LH, Hyslop T, et al. Breast cancer tumor histopathology, stage at presentation, and treatment in the extremes of age. Breast Cancer Res Treat. 2020;180(1):227–235. doi: 10.1007/s10549-020-05542-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kast K, Rhiem K, Wappenschmidt B, Hahnen E, Hauke J, Bluemcke B, et al. Prevalence of BRCA1/2 germline mutations in 21 401 families with breast and ovarian cancer. J Med Genet. 2016;53(7):465–471. doi: 10.1136/jmedgenet-2015-103672. [DOI] [PubMed] [Google Scholar]
- 49.Copson ER, Maishman TC, Tapper WJ, Cutress RI, Greville-Heygate S, Altman DG, et al. Germline BRCA mutation and outcome in young-onset breast cancer (POSH): a prospective cohort study. Lancet Oncol. 2018;19(2):169–180. doi: 10.1016/S1470-2045(17)30891-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Riedel F, Hoffmann AS, Moderow M, Feisst M, Heublein S, Deutsch TM, et al. Do hospital type or caseload make a difference in chemotherapy treatment patterns for early breast cancer? results from 104 German institutions, 2008–2017. Breast. 2021;58:63–71. doi: 10.1016/j.breast.2021.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.