Abstract
Theories of the relation between age at lesion onset and outcomes posit different views of the young brain: resilient and plastic (i.e., the so-called “Kennard Principle”), or vulnerable (i.e., the Early Vulnerability Hypothesis). There is support for both perspectives in previous research and questions about the “best” or “worst” times to sustain brain injury remain. Here, we present a systematic review investigating the influence of age at focal brain lesion onset on cognitive functioning. This systematic review identifies and qualitatively synthesizes empirical studies from 1985–2021 that investigated age at lesion onset as a variable of interest associated with neuropsychological outcomes. A total of 45 studies were identified from PubMed, PsycINFO, and CINAHL databases. Almost all studies indicated that brain injury earlier in the developmental period predicts worse cognitive outcomes when compared to onset either later in the developmental period or in adulthood. More specifically, the overwhelming majority of studies support an “earlier is worse” model for domains of intellect, processing speed, attention and working memory, visuospatial and perceptual skills, and learning and memory. Relatively more variability in outcomes exists for domains of language and executive functioning. Outcomes for all domains are influenced by various other age and injury variables (e.g., lesion size, lesion laterality, chronicity, a history of epilepsy). Continued interdisciplinary understanding and communication about the influence of age at lesion onset on neuropsychological outcomes will aid in promoting the best possible outcomes for patients.
Keywords: age at onset, brain damage, neuropsychological outcomes, plasticity, critical periods
It is well-known that damage to the brain can affect cognitive functioning. In fact, the study of cognitive functioning following brain damage has been a pillar of neuropsychological research for decades. The age at which damage (i.e., the brain lesion) occurs is a particularly interesting variable to consider when predicting neuropsychological outcomes. Investigation of differential outcomes depending on age at lesion onset dates back decades, with seminal studies conducted by Margaret Kennard (i.e., Kennard, 1938, 1940, 1942) and Donald Hebb (i.e., the Early Vulnerability Hypothesis; Hebb, 1942, 1949; Taylor, 1984).
1.1. Early Work Investigating Age at Lesion Onset
Results from some of Kennard’s early work indicated recovery from brain injury is aided by plasticity. She reported reorganization after brain damage can ameliorate the negative impact of the damage, with novel neural networks forming to support functions originally associated with the damaged areas of the brain, ultimately resulting in observable recovery of function (e.g., cognitive or motor; Kennard, 1938, 1940, 1942). As plasticity is maximal in the early years of life, Kennard’s work has been reframed over time to indicate that earlier age at lesion onset is more likely to be associated with greater recovery of function (i.e., the so-called “Kennard Principle”; Dennis, 2010; Teuber, 1974).
Later research expanded on Kennard’s original work and the “earlier is better” theme while examining language functions. Specifically, it was documented that young children are often less affected by brain insult, showing fewer language deficits when compared to older children or adults (e.g., Lenneberg, 1967; Tompkins, 1990). Additionally, when children exhibited language deficits after brain injury, there is evidence of a significant recovery of function over time, even back to full normality (e.g., Ballantyne et al., 2008; Basser, 1962; Teuber, 1975). These findings supported the view of the young brain as more plastic (as Kennard had proposed), with early brain-injury resulting in less detrimental outcomes than later brain-injury (e.g., Lenneberg, 1967; Tompkins, 1990). This “earlier is better” heuristic became commonly used to predict outcomes of patients in healthcare settings (Dennis, 2010; Hart and Faust, 1988; Webb et al., 1996). For instance, studies surveying healthcare providers indicated they will often predict that a younger individual will have better outcomes after brain injury than someone who sustains a brain injury at an older age (e.g., Hart and Faust, 1988; Webb et al., 1996).
Alternately, the Early Vulnerability Hypothesis states damage sustained earlier in life will lead to worse outcomes when compared to damage sustained after early developmental periods (Hebb, 1942, 1949; Taylor, 1984). Damage during early life may be especially detrimental if it occurs during a critical/sensitive period (Jacobs et al., 2007; Thomas & Johnson, 2008; for further discussion of critical periods’ relevance for neuropsychological outcomes, see Anderson et al., 2011). Ultimately, the conflicting predictions of resiliency versus vulnerability in early life led to many questions about “better” or “worse” times to sustain brain injury (e.g., Schneider, 1979).
Returning to Kennard’s early work, she was largely concerned with investigating mechanisms of recovery. She proposed that the negative effects of early brain damage may be ameliorated by reorganization of neural networks (e.g., the ipsilateral cortex or other extrapyramidal areas may aid in compensating for damaged brain areas; see Dennis, 2010). Resilient outcomes after early brain injury are supported by the fact that a young brain has a biological advantage for neural compensation after focal brain damage. For instance, a young brain is more capable of anatomical reorganization or regrowth when compared to an adult brain (e.g., Kolb & Gibb, 1993; Kolb et al., 1994).
Though, not surprisingly, plasticity is complex. Physical recovery after damage due to reorganization or regrowth can be flawed in several ways (e.g., overshooting of macrophage activity, toxic apoptosis) leading to abnormal development (Finger & Almli, 1985; Giza & Prins, 2006; Isaacson, 1975; Kolb & Gibb, 1993; Kolb et al., 2004; Mallat & Chamak, 1994; Stein & Hoffman, 2003; Vargha-Khadem et al., 1992). A flawed recovery is especially likely if damage occurs during a critical period, according to a critical periods view (e.g., Kolb, 1995; Luciana, 2003). Specifically, if damage occurs during a critical period the cognitive skills dependent on that region of the brain known to develop during or after that period may be irreversibly impacted (e.g., Kolb, 1995; Luciana, 2003). So even while it is possible a physical brain may appear to recover when examined with available tools (e.g., neuroimaging), cognitive abilities related to the damaged area of the brain may not, and therefore individuals may present with decreased functional abilities (e.g., functional plasticity; Anderson et al., 2011). Of note, this decreased functional plasticity likely indicates disruptions of neural circuits that may remain under identified due to limitations at this time in both measurements and understanding of the developing brain.
Importantly, many other factors were also identified in early work that influenced the relation between age at lesion onset and cognitive outcomes (e.g., cognitive domain, injury, age). While Kennard’s early work largely investigated motor functions, Hebb’s studies focused on intellect (Hebb, 1942, 1949; Kennard 1938, 1940, 1942). Kennard and Fulton (1942) later examined the impact of lesions made outside the motor area (e.g., occipital areas, frontal association cortex) and noted the complicated nature of early-onset lesions. For instance, the location of the damage is one important variable as some brain areas appear to be more functionally plastic than others (e.g., the motor cortex versus association cortices; gray matter versus white matter; Jacobsen et al., 1936). Other important factors include the size of the lesion, unilateral versus bilateral lesions, cortical versus subcortical lesions, the “serial lesion effect,” and the proposal of “growing into deficits” (Finger et al., 1973; Kennard, 1936, 1940; Kennard and Fulton, 1942). For reviews of Kennard’s work and other seminal studies of the influence of age at lesion onset on functional outcomes, readers are referred to Finger and Almli (1988) and Dennis (2010).
Given the conflicting findings of early work investigating “better” or “worse” times to sustain brain injury, subsequent research was conducted investigating the relation between age at onset and cognitive outcomes. Without question, age at lesion onset is a relevant variable for the prediction of cognitive functioning; however, the relation between age at lesion onset and cognitive outcomes is complicated, with many notable nuances of the research findings. While a large body of research has included age at lesion onset as a variable, the results of studies on this topic are challenging to parse and broadly summarize, for several reasons. Studies differ on methods (e.g., neuropsychological domains included, assessments used, study design, analyses), age variables (e.g., age at onset ranges, age at assessment) and injury variables (e.g., etiologies, lesion size, lesion laterality, lesion location). Thus, the literature would benefit from a review to synthesize findings due to the large number of studies investigating age at onset and the complicated nature of the relation of age at lesion onset and neuropsychological outcomes. A review with a broad scope would be especially valuable, beyond the relevant specific reviews that currently exist.
1.2. Existing Reviews
For a review of earlier literature investigating the influence of age at lesion onset on cognitive outcomes, including seminal case studies, see Schneider (1979) and Dennis (2010). Reviews written after Schneider’s (1979) review have largely opted for a refined lens on specific domains (e.g., language, Bates, 1999; Bates & Roe, 2001; Dennis, 1998; executive functioning, Rivella & Viterbori, 2021), specific injury characteristics (e.g., unilateral lesions, Vargha-Khadem et al., 1994), specific etiologies (e.g., traumatic brain injury, Babikian & Asarnow, 2009; and stroke, Fuentes et al., 2016; Gomes et al., 2014; Hogan et al., 2000; Kirton et al., 2007; Malone & Felling, 2020; Rivella & Viterbori, 2021), or specific age at lesion onset periods (e.g., birth to adolescence; Anderson et al., 2011; Babikian & Asarnow, 2009; Bates, 1999; Bates & Roe, 2001; Dennis, 1998; Ewing-Cobbs et al., 2003; Fuentes et al., 2016; Gomes et al., 2014; Hogan et al., 2000; Kirton et al., 2007; Malone & Felling, 2020; Rivella & Viterbori, 2021; Vargha-Khadem et al., 1994).
A focus on the period from birth through adolescence makes sense as the development of cognitive skills largely takes place in early life, beginning in utero and, for some higher-order skills (e.g., executive functions), extending into late adolescence and early adulthood (e.g., 25; e.g., Arain et al., 2013) with stabilization after that time (e.g., Casey et al., 2000; Tombaugh et al., 1999; Tombaugh, 2004). Interestingly, a few studies have taken a lifespan perspective and included age at lesion onset ranges into adulthood and have indicated that age at lesion onset is a potentially relevant variable across the lifespan (i.e., from birth to late adulthood; Duval et al., 2008; Montour-Proulx et al., 2004). Thus, a review incorporating work that has utilized a lifespan perspective is additive in the context of existing reviews for a broader understanding of the influence of age at lesion onset on neuropsychological outcomes.
Additionally, many studies that included age at lesion onset as a variable of interest have been conducted with samples of mixed etiologies of focal brain injury (e.g., stroke, focal TBI; Anderson et al., 2009; Anderson, Jacobs, et al., 2010; Anderson, Spencer-Smith et al., 2010; Anderson et al., 2014). The important contributions of literature including mixed samples of etiologies for focal brain injuries are not included within reviews that have a refined focus on a particular etiology of focal brain injury (e.g., stroke). As stated by Taylor and Alden (1997), “A comprehensive review of age-related influences on outcomes requires examination of studies of diverse forms of early brain disease” (p. 556). Although this statement was made over 20 years ago, no such systematic review exists at this time.
1.3. Parameters for the Current Review
To provide readers with a thorough exploration of the work completed in the field thus far, this systematic review identifies all relevant literature published between 1985 and 2021 on the influence of age at focal lesion onset on neuropsychological outcomes. Of note, the term “focal” refers to the anatomical attributes (e.g., as identified on imaging) of a restricted lesion to the brain and is not meant to indicate focal cognitive/behavioral consequences of the lesion. The method of systematic review was chosen so a reader may critically evaluate all work done in the field. This review aims to answer remaining questions about outcomes after focal injuries and will focus on 1) studies that contain samples of patients whose lesions were of heterogeneous etiology but have been designated as focal (i.e., typically defined as parenchymal tissue damage with defined borders evident on imaging), and 2) studies that focused solely on stroke, which is by definition a focal injury of the brain (Sacco et al., 2013).
It is preferable to separate focal and diffuse (e.g., traumatic brain injury causing diffuse axonal damage; anoxic injury) lesions because existing literature has documented differences between focal and diffuse brain injuries and more consistent outcomes with diffuse brain injuries, likely because diffuse lesions leave little possibility for reorganization and recovery after injury (e.g., see Anderson et al., 2004; Anderson et al., 2005; Anderson et al., 2011; Catroppa et al., 1999; Jacobs et al., 2004; Keenan et al., 2007). Importantly, due to the focus of this review on focal injuries and differences between outcomes after focal and diffuse injuries, findings are not generalizable to diffuse injuries.
The starting point of 1985 for this review was chosen due to the focus on focal lesions. Prior to the mid-1980s neuropsychological studies and examinations of brain-behavior relations were conducted without identification of the brain lesions, except by autopsy. The introduction of and subsequent increase in neuroimaging during the 1980s led to a better conceptualization of focal lesions and changed the way focal lesions were defined and incorporated into research (e.g., Bigler, 2017). Thus, studies conducted after this time utilizing neuroimaging represent another cohort of research, building on the past work done prior to the visualization of lesions on imaging (i.e., prior to the 1980s). This prior work is of great importance and has been noted in this introduction. For the later systematic review, included studies will be those conducted after the introduction of neuroimaging to prioritize a more homogeneous conceptualization of focal lesions.
1.3.1. Other Relevant Variables
Taking a broader perspective, Anderson and colleagues (2011) provided a selective review of children’s ability to recover post-brain injury and included potential contributing factors that may be associated with poorer outcomes including biological factors (e.g., injury factors, age factors; Table 4), environmental factors, and interventions/rehabilitation. The authors drew attention to the influence of these other variables and how they may interact with age at lesion onset to provide a more complete picture of resilience or vulnerability. Injury and age factors have been most consistently investigated in association with age at lesion onset. Thus, this systematic review will give special attention to injury and age variables noted by Anderson and colleagues (2011) to identify where these variables have been accounted for and where they could add to the story of risk versus resilience.
Table 4.
Age, Injury, and Methodological Variables
| Category | Variable | Definition |
|---|---|---|
| Age variables | Chronicity | Age at test minus age at lesion onset |
| Recovery over time | Within-person change in cognitive functioning after lesion onset, evaluated by a longitudinal design | |
| Age at testing | The age of the subject at the time the measure was administered | |
| Injury variables | Lesion size/volume | A measurement of the volume of tissue damaged as the result of a lesion |
| Lesion laterality | The hemispheric side on which a lesion occurs (i.e., left, right, or bilateral) | |
| Lesion location | The location of damage in the brain | |
| Seizures | A noted history of seizures, or presence of intractable seizures. Severity of seizures and classification may vary depending on the specific study | |
| Methodological variables | Study design | Specifically identifying cross-sectional versus longitudinal study designs |
| Age at lesion onset range | The range of age at lesion onset values for subjects included in each study |
1.3.2. Aim of the Current Review
This systematic review collects and synthesizes all empirical articles published from 1985–2021 that included samples with focal brain injury and examined age at brain lesion onset in association with cognitive outcomes including intellect, processing speed, attention and working memory, language, visuospatial and perceptual skills, learning and memory, and executive functioning (based on the domains described in the Lezak et al. (2012) reference text for neuropsychological assessment). This review will also note the contribution of biological (i.e., age, injury) variables for outcomes in association with age at lesion onset.
Methods
The current systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (Page et al., 2021).
2.1. Data Sources and Search Strategy
A literature search of online databases including PubMed, PsycINFO, and CINAHL was used to identify relevant studies. Search strategies and final searches were developed by the primary author and a librarian. Combinations of three key concepts, “age at lesion onset,” “focal brain damage,” and “neuropsychological outcomes” were used as the basis for search terms (e.g., subject headings and keywords) across all databases. Searches were conducted in September 2020 and then updated in October 2021. Pre-established database filters including “Human,” “English,” and “1985–2020” were used. The full search strategies for all databases are included for reference (Figure 1).
Figure 1.



Search Strategies for PubMed, PsycINFO, and CINAHL, Conducted September 2020, Updated October 2021
2.2. Inclusion and Exclusion Criteria
Articles were included in this systematic review if they met the following inclusion criteria: (i) peer-reviewed, empirical journal article published in the English language between the years of 1985–2021; (ii) included age at lesion onset as a clearly-defined variable of interest (e.g., as a continuous variable, age at lesion onset groups, comparing children and adults); (iii) identified focal brain damage (i.e., studies examining heterogenous etiologies specifically identified as focal, or solely stroke); and (iv) used standardized and validated neuropsychological assessments for the assessment of at least one of the relevant cognitive domains (see Table 3 for included cognitive domains).
Table 3.
List of Included Measures and Domains
| Intellect |
|---|
| ◦ Bayley Scales of Infant Development (BSID; Bayley, 1993, 2006) ◦ British Ability Scales (Elliot, 1983) ◦ Differential Ability Scales (DAS; Elliott, 1990, 2007) ◦ Hamburg-Wechsler-Intelligenztest für Kinder IV (HAWIK IV; Petermann & Petermann, 2010) ◦ Kaufman Brief Intelligence Test (K-ABC; Kaufman & Kaufman, 1983; Kaufman, 2004) ◦ Wechsler Preschool and Primary Scale of Intelligence (WPPSI; Wechsler, 2002, 2012) ◦ Wechsler Intelligence Scales for Children (WISC; Wechsler, 1991, 2003) ◦ Wechsler Adult Intelligence Scale (WAIS; Wechsler, 1981, 2008) ◦ Wechsler Abbreviated Scale of Intelligence (WASI; Wechsler, 1999) ◦ The short form of the WAIS (i.e., Vocabulary and Block Design subtests) ◦ Calculated mean of Verbal IQ and Performance IQ |
| Processing Speed |
| ◦ Delis-Kaplan Executive Function System (DKEFS) Trail Making Test: motor speed and Color Word Interference: color naming and word reading time (Delis, 2001) ◦ Handwriting Speed Test (Wallen et al., 1996) ◦ Processing Speed Index (PSI), or the Coding subtest on the WISC or WAIS (Wechsler, 1991, 1999, 2003, 2008) ◦ Sky Search Motor Control: motor control attention score from the Test of Everyday Attention for Children (TEA-Ch; Manly et al., 2001) |
| Attention and Working Memory |
| ◦ DKEFS Trail Making Test: visual scanning, number sequence time, and letter sequence time (Delis et al., 2001) ◦ D2 Test of Attention (Brickenkamp & Zillmer, 1998) ◦ Subtests from the K-ABC (Kaufman & Kaufman, 1983) ◦ Multilingual Aphasia Examination (MAE) Sentence Repetition subtest (Benton et al., 1994) ◦ Subtests from the NEPSY-II (Korkman et al., 2007) ◦ Test of Attention Performance (TAP; Zimmermann & Fimm, 1993) ◦ TEA-Ch: Code Transmission, Creature Counting, Sky Search (number of targets correct), Score, Walk/Don’t Walk, and a composite of subtests of the TEA-Ch (Manly et al., 2001) ◦ Working memory index (WMI), digit span subtest, and letter number sequencing from the WAIS or WISC (Wechsler, 1991, 1999, 2003, 2008) ◦ The Working Memory Test Battery for Children (WMTB-C; Pickering & Gathercole, 2001) |
| Language |
| ◦ The Clinical Evaluation of Language Fundamentals, 3rd edition (CELF-III; Semel et al., 1995) ◦ Expressive Vocabulary Test 2 (EVT-2; Williams, 2007) ◦ Kaufman’s Verbal Comprehension factor from the WISC-R (Kaufman, 1975) ◦ Subtests from the NEPSY-II (Korkman et al., 1998; adapted by Kolk & Talkvik, 2000) ◦ The Peabody Picture Vocabulary Test (PPVT; Dunn & Dunn, 2007) ◦ Rapid Automatized Naming (Semel et al., 2003) ◦ Salzburger Lese und Rechtschreibtest (SLRT-II; Moll & Landerl, 2010) ◦ Test zur Überprüfung des Grammatikverständnisses (TROG-D; Bishop, 1989; Fox, 2006) ◦ The Token Test (McNeil & Prescott, 1978; McGhee et al., 2007) ◦ Verbal comprehension index (VCI) and verbal subtests of the WISC and WAIS (Wechsler, 1991, 1999, 2003, 2008) ◦ Wingfield Object Naming Test (Oldfield & Wingfield, 1964) ◦ Wortschatz-und Wortfindungstest (Glück & Glück, 2011) |
| Visuospatial and Perceptual Skills |
| ◦ Beery-Buktenica Developmental Test of Visual Motor Integration (Beery, 2004) ◦ Bender-Gestalt Test (Bender, 1938) ◦ Subtests from the K-ABC (Kaufman & Kaufman, 1983) ◦ Kaufman’s perceptual organization factor from the WISC-R (Kaufman, 1975) ◦ Subtests from the NEPSY-II (Korkman et al., 1998; adapted by Kolk & Talkvik, 2000) ◦ Perceptual reasoning index (PRI), block design, picture completion, or object assembly from the WISC and WAIS (Wechsler, 1991, 1999, 2003, 2008), and the Hamburg-Wechsler-Intelligenztest für Kinder IV (HAWIK IV; Petermann & Petermann, 2010) ◦ Rey Complex Figure Test (RCFT): copy condition (Rey, 1941; Meyers & Meyers, 1995) |
| Learning and Memory |
| Verbal Memory: ◦ Auditory Verbal Learning Test (AVLT; Rey, 1964) ◦ California Verbal Learning Test-Children’s Version (CVLT-C; Delis et al., 1994) ◦ Children’s Memory Scale (Cohen, 1997) ◦ Verbaler Lern-und Merkfahigkeitstest (The German Version of the AVLT; Helmstaedter et al., 2001) ◦ Wechsler Memory Scale – third edition and fourth edition (WMS-III, WMS-IV; Wechsler, 1997, 2009) ◦ Wide Range Assessment of Memory and Learning-2nd ed. (WRAML-2; Sheslow & Adams, 2003) Visual Memory: ◦ Faces subtest of the Children’s Memory Scale (Cohen, 1997) ◦ Rey Complex Figure Test-recall condition (Rey, 1941) and recognition condition (Meyers & Meyers, 1995) |
| Executive Functioning |
| Questionnaires: ◦ Behavior Rating Inventory of Executive Function (BRIEF) parent and self-report (Gioia et al., 2000) Behavioral Tasks: ◦ The Contingency Naming Test, Twenty Questions Test (Anderson et al., 2000) ◦ Controlled Oral Word Association Test (COWAT; Spreen & Strauss, 1998) ◦ DKEFS: Verbal Fluency, Design Fluency, Trail Making Test: number letter switching, Color Word Inhibition: inhibition/switching, inhibition errors, and a composite of the sequencing tests (Delis et al., 2001) ◦ Porteus Maze Test (Porteus, 1950) ◦ Regensburger Wortflu ssigkeitstest (Animal Fluency; Aschenbrenner et al., 2000) ◦ Sky Search: Search Strategy score and Sky Search Dual Task subtest of the TEA-Ch (Manly et al., 2001) ◦ The Tower of London (TOL; Anderson et al., 1996; Shallice, 1982) ◦ Tower Test (Delis et al., 2001) ◦ WISC or WAIS subtests: Similarities and Matrix Reasoning (Wechsler, 1991, 1999, 2003, 2008; Petermann & Petermann, 2010) ◦ Wisconsin Card Sorting Test (Grant & Berg, 1948) |
Studies were excluded if they were reviews; case studies, or case series with no quantitative analyses; included only a motor component; or included a sample with etiologies including transient ischemic attack (TIA), only traumatic brain injury (TBI), only epilepsy, or only brain tumors.
2.3. Identification of Relevant Studies and Data Extraction
As mentioned above, a database search was first conducted in September 2020 and updated in October 2021. Descriptions of each search are included below and a combined total for the searches is depicted in Figure 2. For the first search, the three database searches produced 3,958 articles. After identification and deletion of duplicate articles via automated process on Mendeley and Rayyan, 2,959 unique articles remained. Titles and abstracts of all studies were then screened to assess if they met inclusion criteria. The titles and abstracts of 15% of the unique articles (n = 444) were also screened by another author and compared to the primary author’s determination of whether to include or exclude articles. Out of the 444 articles, agreement between the two reviewers was 92.5%; kappa was 0.41. For any articles about which the two raters did not agree, the articles were discussed, and a consensus was reached. After the initial 15% were reviewed, the first author screened all remaining articles. The methods and results sections of articles identified as relevant or potentially relevant (n = 161) were read. In all, 39 articles met inclusion and not exclusion criteria and were included in this review.
Figure 2.
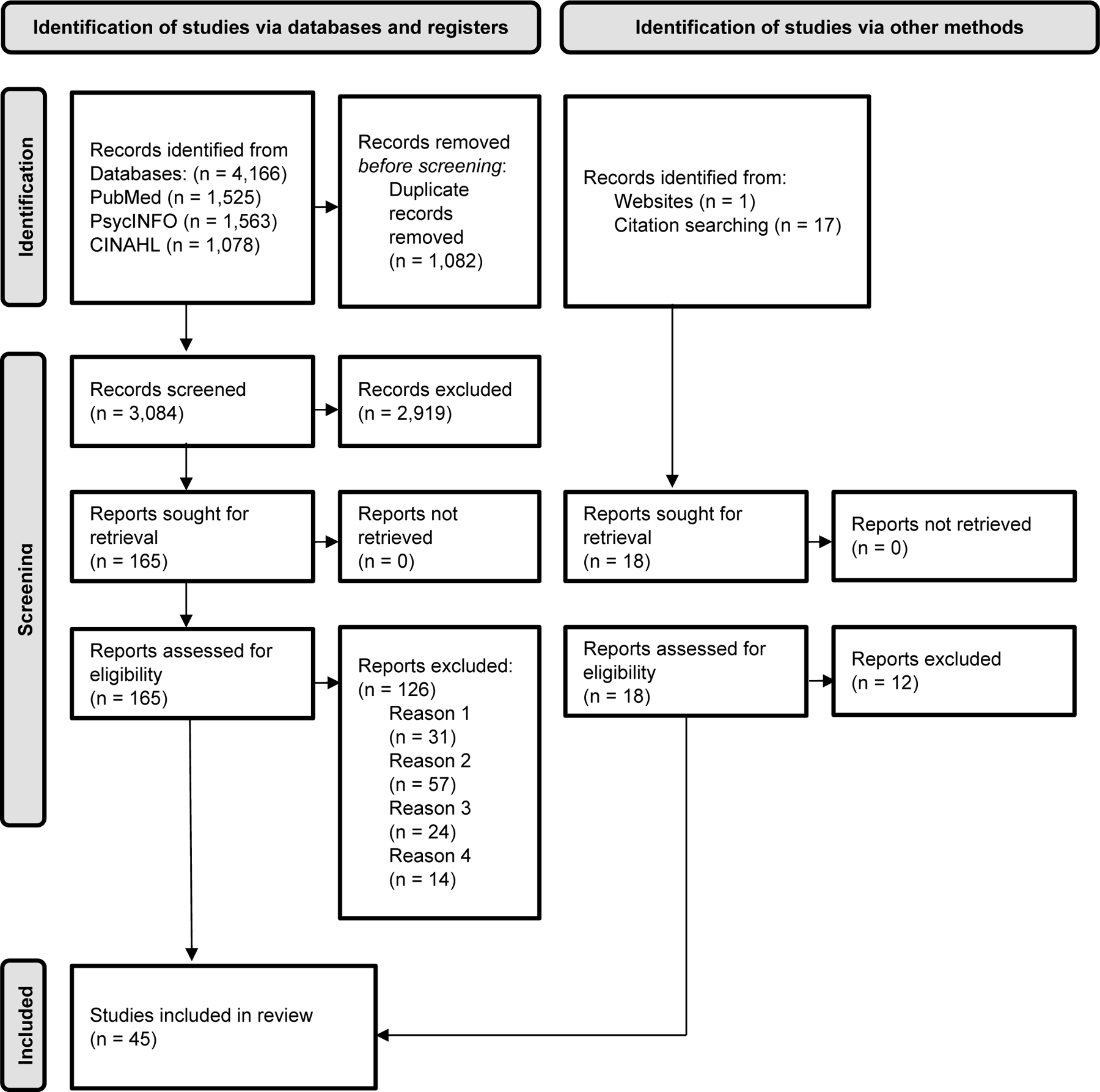
Prisma 2020 Flow Diagram
Note. Exclusion reason numbers correspond to inclusion criteria numbers in the inclusion and exclusion criteria section of the methods.
The updated search conducted in October 2021 included 208 articles, of which 125 new articles remained after the removal of duplicates. Four articles were sought for retrieval. After screening and exclusion, no new articles were retained for inclusion. Other ways of identifying relevant articles included hand searching reference lists of all included articles along with relevant reviews and websites. Hand searching identified six additional relevant articles that were included in the final review, for a total of 45 total articles.
Data were then extracted from all included articles for sample characteristics, assessment information, and age at onset findings. Extracted sample characteristics included sample size, age at onset range and classification, inclusion of a comparison group, etiology and laterality of brain lesion, and seizure history (Table 1). Extracted assessment information included age at testing, chronicity (i.e., age at testing minus age at lesion onset), domains assessed, tests administered, and study design (i.e., cross-sectional or longitudinal; see Table 2 and Table 3). Finally, results were extracted for age at onset analyses for relevant cognitive domains and other notable variables for age at onset analyses (i.e., injury and age variables controlled for or that interacted with the age at onset variable). All extracted data included in the manuscript were checked by a second individual for accuracy.
Table 1.
Articles Included in Systematic Review: Sample Characteristics
| Study | Sample size | Age at onset range (year, month) | Groups (year, month) | Comparison group included | All unilateral | Seizures included | Description of etiologies |
|---|---|---|---|---|---|---|---|
| Anderson et al. (2009) | 164 | 0y-16y | 1st-2nd tri 3rd tri-1m 2m-2y 3y-6y 7y-9y >10y |
N | N | Y | DEV, infective, ischemic, neuroplastic, TBI |
| Anderson, Jacobs, et al. (2010) | 164 | 0y-15y | 1st-2nd tri 3rd tri-1m 2m-2y 3y-6y 7y-9y >10y |
N | N | Y | DEV, infective, ischemic, neuroplasm, TBI |
| Anderson, Spencer-Smith, et al. (2010) | 164 | 0y-16y | 1st-2nd tri 3rd tri-1m 2m-2y 3y-6y 7y-9y >10y |
N | N | Y | DEV, infective, ischemic, neuroplastic, TBI |
| Anderson et al. (2014) | 138 | 0y-15y | <3y >3y |
N | N | Y | DEV, infective, neoplastic, TBI |
| Aram & Eisele (1994) | 26 | 0y-15y 11m |
n/a | N | Y | N | Vascular resulting from CVA, prenatal insult, AVM, or meningitis |
| Aram & Ekelman (1986) | 31 | 0y-15y 11m |
<1y >1y |
Y | Y | N | CVA, AVM, prenatal or perinatal insult |
| Aram & Ekelman (1987) | 28 | 0y-15y 11m |
<1y >1y |
Y | Y | Y | CVA, AVM, prenatal or perinatal, complex migraine |
| Aram & Ekelman (1988) | 32 | 0y-14y 3m |
<2y >2y |
Y | Y | N | CVA, prenatal insults, AVM, complex migraine |
| Banich et al. (1990) | 41 | 0y-9y 6m |
n/a | N | Y | -- | TBI, stroke, vasculitis, astrocytoma, meningitis, encephalitis |
| Braun et al. (2001) | 635 | 5y 2m (5y 6m) 46y 7m (18y) |
n/a | N | Y | Y | Infectious, vascular, mitotic, dysplastic, TBI, other |
| Duval et al. (2002) | 685 | 0y-71y | <7y >7y |
N | Y | Y | Static (e.g., TBI, CVA, malformations), epilepsy, progressive (e.g., tumor, cyst) |
| Duval et al. (2008) | 725 | 0y-84y | n/a | N | Y | Y | Infectious, vascular, cystic, dysplastic, TBI, tumoral, unknown |
| Gingras & Braun (2018) | 2186 | 0y-87y | n/a | N | Y | Y | Metabolic, dysplasic, infectious, CVA, TBI, porencephalic, autoimmune, AVM, mitotic, sclerotic/gliotic, unknown |
| Goodman & Yude (1996) | 124 | 0y-8y | <1m 1m-5y 5y-8y |
N | N | Y | Hemiplegia, not otherwise described |
| Isaacs et al. (1996) | 115 | 0y-13y 1m |
<0y >0y |
Y | Y | Y | Congenital, CVA, trauma, acute onset infantile hemiplegia |
| Jacobs et al. (2007) | 38 | 0y-10+y | <0y 0y-3y 4y-6y 7y-9y >10y |
N | N | Y | Tumor, abscess, DNET, stroke or cystic lesion, TBI, cerebral malformation/dysplasia, demyelinating disorder |
| Kornhuber et al. (1985) | 51 | Perinatal −12 |
<5y >5y |
Y | N | Y | Not reported |
| Levine et al. (1987) | 41 | 0y-9y 6m |
<0y >0y |
N | Y | Y | Congenital, astrocytoma, TBI, meningitis, stroke, chronic focal encephalitis |
| Montour-Proulx et al. (2004) | 635 | 0y-79y | n/a | N | Y | Y | TBI, CVA, malformations, seizure disorder, tumor, cyst |
| Riva & Cazzaniga (1986) | 48 | 0y-13y | <1y >1y |
Y | Y | N | DEV, neonatal asphyxia, encephalitis, papilloma, cyst, tumor, abscess, AVM, embolia, hematoma |
| Spencer-Smith et al. (2011) | 138 | 0y-15y | 1st-2nd tri 3rd tri-1m 2m-2y 3y-6y 7y-9y 10y-15y |
N | N | Y | DEV, ischaemic, infective, neuroplastic, TBI |
| Vargha-Khadem et al. (1985) | 53 | <0y-14y | <0y 2m–5y 5y-14y |
Y | Y | Y | CVA, seizures, surgical excision, tumor, TBI, acute onset infantile hemiplegia, subdural hematoma |
| Allman & Scott (2013) | 44 | 1m-16y | 1m-1y 1y-6y 6y-16y |
N | Y | N | Ischemic stroke |
| Anderson et al. (2020) | 61 | 0y-16y 4m |
0–28d 29d-5y 5y-16y 4m |
N | N | Y | Ischemic stroke |
| Bartha-Doering et al. (2019) | 17 | 1y 4m- 16y 7m |
n/a | Y | Y | Y | Ischemic stroke |
| Bartha-Doering et l. (2021) | 18 | 0y1m – 16y 7m |
n/a | Y | Y | N | Ischemic stroke |
| Block et al. (1999) | 11 | 6m–15y | 6m-2y 6y-15y |
Y | Y | Y | Ischemic stroke |
| de Montferrand et al. (2019) | 184 | 1m-15y 4m |
n/a | N | N | Y | Ischemic and hemorrhagic stroke |
| Everts et al. (2008) | 21 | 0y 1m- 17y 6m |
n/a | N | Y | Y | Stroke |
| Fuentes et al. (2017) | 32 | 0–14 | Perinatal 1m-5y 6y-14y |
Y | Y | N | Ischemic stroke |
| Gordon et al. (2015) | 50 | 0y-15y 6m |
0–0.36m 1.5m-15y 6m |
N | N | -- | Ischemic stroke |
| Hajek et al. (2014) | 36 | 4y 2m (4y 4m) |
<1m >1m |
Y | N | Y | Ischemic stroke |
| Ilves et al. (2014) | 12 | Perinatal −11y 3m |
<2y >2y |
Y | Y | Y | Ischemic and hemorrhagic stroke |
| Jacomb et al. (2018) | 41 | 0y-16y 8m | n/a | N | N | N | Ischemic and hemorrhagic stroke |
| Lansing et al. (2004) | 26 | 0y-13y | ≤1y >1y |
Y | Y | -- | Stroke |
| Long et al. (2011) | 28 | 0y-14y 6m |
<5y >5y |
N | N | Y | Ischemic and hemorrhagic stroke |
| Max et al. (2010) | 29 | 7y 10m (3y 2m) |
<1y ≥1y |
Y | Y | -- | Ischemic and hemorrhagic stroke |
| Mosch et al. (2005) | 38 | 3y 2m (4y 5m) 53y 2m (16y) |
children vs. adults | N | Y | Y | Stroke |
| O’Keeffe et al. (2014) | 49 | 4m-15y 8m |
n/a | N | N | Y | Ischemic stroke |
| Pavlovic et al. (2006) | 19 | 0y 11m- 16y 4m |
n/a | N | N | Y | Ischemic stroke and sinus venous thrombosis |
| Peterson et al. (2019) | 27 | 1m-18y | n/a | N | N | Y | Stroke |
| Studer et al. (2014) | 99 | 1m-16y | 1m-2y 11m 3y-5y 11m 6y-9y 11m >10y |
N | N | Y | Ischemic stroke |
| Westmacott et al. (2010) | 145 | 0y-16y | <0y-1m 1m-5y 6y-16y |
N | Y | N | Ischemic stroke |
| Westmacott et al. (2018) | 44 | 5m- 13+y |
n/a | N | N | Y | Stroke |
| Wingeier et al. (2011) | 8 | 9y-13y | n/a | N | N | -- | Hemorrhagic stroke |
Note. Studies are grouped by samples with heterogeneous etiologies first, then studies with samples that had stroke as the etiology. Descriptors for etiologies are those provided by the authors in individual papers. For age at onset ranges not explicitly provided, the mean and standard deviation for relevant groups were provided in the form mean(standard deviation). Age at onset was rounded to the nearest year and month based on information provided in each article. m = months; y = years; n/a = not applicable; tri = trimester; -- = missing information; N = not included; Y = included; AVM = arteriovenous malformation; CVA = cerebrovascular accident; DEV = developmental; TBI = traumatic brain injury.
Table 2.
Articles Included in Systematic Review: Assessment Information
| Study | Longitudinal | Age at testing (year, month) | Chronicity | Domain assessed | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mixed etiologies | I N |
P S |
A W |
L A |
V P |
L M |
E F |
||||
| Anderson et al. (2009) | N | 10y-16y | C | X | X | ||||||
|
| |||||||||||
| Anderson, Jacobs, et al. (2010) | N | 10y-16y | C | X | X | X | X | X | X | ||
|
| |||||||||||
| Anderson, Spencer-Smith, et al. (2010) | N | 10y-16y | C | X | X | X | |||||
|
| |||||||||||
| Anderson et al. (2014) | N | 10y-16y | C | X | X | ||||||
|
| |||||||||||
| Aram & Eisele (1994) | Y | 4y 5m-12y 5m | A/C | X | |||||||
|
| |||||||||||
| Aram & Ekelman (1986) | N | 4y 4m-16y 4m | A/C | X | X | X | |||||
|
| |||||||||||
| Aram & Ekelman (1987) | N | 6y 1m-17y 11m | C | X | |||||||
|
| |||||||||||
| Aram & Ekelman (1988) | N | 5y 1m-18y 4m | C | X | X | X | |||||
|
| |||||||||||
| Banich et al. (1990) | N | 2y 8m-18y | A/C | X | |||||||
|
| |||||||||||
| Braun et al. (2001) | N | 17y 2m (10y) 48y 11m (16y 8m) |
-- | X | |||||||
|
| |||||||||||
| Duval et al. (2002) | Y | 3y-71y | -- | X | |||||||
|
| |||||||||||
| Duval et al. (2008) | Y | -- | C | X | |||||||
|
| |||||||||||
| Everts et al. (2008) | N | 6y 9m-21y 2m | A/C | X | X | X | X | X | X | ||
|
| |||||||||||
| Gingras & Braun (2018) | N | 3y-89y | -- | X | |||||||
|
| |||||||||||
| Goodman & Yude (1996) | N | 6y-10y | -- | X | |||||||
|
| |||||||||||
| Isaacs et al. (1996) | N | 5y 3m-23y | -- | X | |||||||
|
| |||||||||||
| Jacobs et al. (2007) | N | 10y-16y | C | X | X | X | |||||
|
| |||||||||||
| Kornhuber et al. (1985) | N | 11y 5m (3y 7m) | C | X | X | ||||||
|
| |||||||||||
| Levine et al. (1987) | N | -- | -- | X | X | X | |||||
|
| |||||||||||
| Montour-Proulx et al. (2004) | N | -- | A/C | X | |||||||
|
| |||||||||||
| Riva & Cazzaniga (1986) | N | 8y 5m (average for early lesions) 11y 8m (average for late lesions) |
C | X | |||||||
|
| |||||||||||
| Spencer-Smith et al. (2011) | N | 10y-16y | C | X | X | ||||||
|
| |||||||||||
| Vargha-Khadem et al. (1985) | N | 6y-17y | C | X | X | ||||||
|
| |||||||||||
| Stroke | |||||||||||
|
| |||||||||||
| Allman & Scott (2013) | N | 7y 2m; 6y 6m; 11y 2m | C | X | X | X | X | X | X | X | |
|
| |||||||||||
| Anderson et al. (2020) | Y | -- | A/C | X | |||||||
|
| |||||||||||
| Bartha-Doering et al. (2019) | N | 7y 0m-17y 5m | C | X | |||||||
|
| |||||||||||
| Bartha-Doering et al. (2021) | N | 7y 0m-17y 5m | A/C | X | X | X | X | X | |||
|
| |||||||||||
| Block et al. (1999) | N | 8y-23y | C | X | |||||||
|
| |||||||||||
| de Montferrand et al. (2019) | N | 8m-15y 5m | A/C | X | |||||||
|
| |||||||||||
| Fuentes et al. (2017) | N | 9y 2m (5m) | C | X | |||||||
|
| |||||||||||
| Gordon et al. (2015) | Y | -- | A/C | X | X | ||||||
|
| |||||||||||
| Hajek et al. (2014) | N | 9y 2m (3y 0m) | C | X | X | X | |||||
|
| |||||||||||
| Ilves et al. (2014) | N | 6 y 4m-13y 10m | C | X | X | ||||||
|
| |||||||||||
| Jacomb et al. (2018) | Y | 4y 7m-18y 7m | A/C | X | X | ||||||
|
| |||||||||||
| Lansing et al. (2004) | N | 5y 11m-16y 8m | C | X | X | X | |||||
|
| |||||||||||
| Long et al. (2011) | N | 10y-15y | C | X | X | X | X | ||||
|
| |||||||||||
| Max et al. (2010) | N | 5y-19y | C | X | X | X | X | X | X | ||
|
| |||||||||||
| Mosch et al. (2005) | N | 12y 5m (3y 10m) 56y 6m (15y) |
C | X | X | X | X | X | X | ||
|
| |||||||||||
| O’Keeffe et al. (2014) | Y | 6y-18y 5m | C | X | X | X | |||||
|
| |||||||||||
| Pavlovic et al. (2006) | N | 2y 1m-18 y 2m | C | X | |||||||
|
| |||||||||||
| Peterson et al. (2019) | N | 9y 8m (4y 2m) | C | X | X | X | X | X | X | ||
|
| |||||||||||
| Studer et al. (2014) | N | 9y5m (4y 10m) | C | X | |||||||
|
| |||||||||||
| Westmacott et al. (2010) | N | 9y 1m (3y 10m) 8y (3y 10m) 7y 5m (2y 9m) 2y 5m (3y 9m) |
C | X | X | X | |||||
|
| |||||||||||
| Westmacott et al. (2018) | N | 6y-20y | C | X | X | X | X | X | X | X | |
|
| |||||||||||
| Wingeier et al. (2011) | N | 12y-21y | C | X | |||||||
Note. If ranges were not provided for age at testing, means and standard deviations were given in the form mean (standard deviation) for all age at onset groups. Age at testing was rounded to the nearest year and month; Y = yes; N = no; C = at least three months between injury and assessment; A = all less than three months between injury and assessment; A/C = mix of less and more than three months between injury and assessment; IN = Intellect; PS = Processing Speed; AW = Attention and Working Memory; LA = Language; VP = Visuospatial and Perceptual skills; LM = Learning and Memory; EF = Executive Function.
2.4. Transparency and Openness
This review was not preregistered. Data extracted for this review are available in the included Tables. For this manuscript, we followed the PRISMA-P checklist and the PRISMA 2020 reporting guidelines for systematic reviews (Page et al., 2021).
Results
After screening full-text articles according to the PRISMA guidelines process, 45 articles were retained for inclusion in the systematic review. For ease of interpretation, results from the relevant studies have been grouped into cognitive domains (Lezak et al., 2012; see Table 3 for included cognitive domains). Of note, the executive function domain contains numerous subskills (e.g., planning, organization, concept formation, fluency, switching and shifting, inhibition). While processing speed and working memory are also commonly thought of as executive functions (Anderson et al., 2001; Stuss & Benson, 1986) these skills are presented in separate sections from the executive function domain. The separation of processing speed and working memory into separate domains in this manuscript is to allow for easier examination of subsets of skills that make up executive functions and does not indicate the domains are not related.
Most test results were reported within the domain identified in the original research article after confirmation that the original classification aligned with the description of the measure in the Lezak and colleagues (2012) book. Occasionally the same measure was classified in different domains across studies (e.g., The Controlled Oral Word Association Test (COWAT) was classified as language in one study and executive function in others). In those cases, a decision was made to use one classification and report corresponding findings, across studies, in one domain (e.g., all COWAT findings are reported in the executive function section). The chosen domain was based on 1) the classification of the measure in the Lezak et al. (2012) text, 2) descriptive information from the specific assessment, and 3) consultation with clinical neuropsychologists blind to the current review. The original classification of the measure across studies was additionally considered with an emphasis on maintaining the majority designation if it reasonably aligned with other previously mentioned sources (i.e., the Lezak et al. (2012) text and assessment description). The names of included measures in each domain are detailed in Table 3 to aid in the interpretation of findings.
Studies that reported a significant relation between age at lesion onset and cognitive outcomes are discussed. Effect sizes are also included when reported in the studies. Individual articles may be referenced to calculate other effect sizes for those studies that did not report an effect size. Additionally, some studies reported effect sizes that did not align with the categorization of measures as described above (e.g., those papers that included a composite of many measures that fit into more than one cognitive domain) and were also not included. For a more complete overview of the literature, the studies that indicated a non-significant association between age at lesion onset and outcomes are also listed. These non-significant results are occasionally discussed in more detail (e.g., if the finding was close to significant or notable due to some other factor such as a small sample size) as there was typically limited information available in studies regarding non-significant results.
Importantly, many age and injury variables were associated with neuropsychological outcomes (Table 4). These variables are largely reported in later sections entitled Age Variables and Injury Variables for discussion across all neuropsychological domains. Especially relevant age and injury variables are discussed briefly within specific domain sections.
3.1. Intellect
Intellect is assessed by measures of general abilities or intelligence (Lezak et al., 2012; Table 3). Studies investigating a full-scale intelligence quotient (FSIQ) are included within this section. Studies that only reported subscales of Verbal IQ or Performance IQ, but not FSIQ, are reported separately. If studies included both FSIQ and subscale analyses, only the FSIQ results are included due to the overlap between the full-scale and subscales.
3.1.1. Full-Scale IQ
Full-scale IQ (FSIQ) was reported as an outcome in many studies investigating the influence of age at onset of focal brain injury (see Table 5).
Table 5.
Significant Results and Reported Effect Sizes for FSIQ
| Study | Direction | Effect size and classification | Notes |
|---|---|---|---|
| Allman & Scott (2013) | EV | Not reported | |
| Anderson et al. (2009) | EV | L R2= 0.14 |
|
| Anderson et al. (2014) | EV | Not reported | |
| Anderson, Spencer-Smith, et al. (2010) | EV | L R2= 0.14 |
|
| Anderson et al. (2020) | EV | Not reported | |
| Aram & Eisele (1994) | EV | L R2 = 0.30 |
Only significant for groups with left-sided lesions |
| Block et al. (1999) | EV | Not reported | Finding was described as tentative due to the small sample size (n = 11) |
| Braun et al. (2001) | EV | S R2 = 0.01 |
|
| Duval et al. (2008) | EV | S R2 = 0.02 |
|
| Gingras & Braun (2018) | EV | M R2 = 0.06 |
For the sample with epilepsy |
| Gingras & Braun (2018) | ER | S R2 = 0.01 |
For the sample without epilepsy |
| Goodman & Yude (1996) | U | Not reported | |
| Max et al. (2010) | EV | M R2 = 0.04 |
|
| Jacomb et al. (2018) | EV | M R2 = 0.13 |
|
| Kornhuber et al. (1985) | EV | Not reported | |
| Pavlovic et al. (2006) | U | L R2 = 0.23 |
|
| Riva & Cazzaniga (1986) | EV | Not reported | |
| Spencer-Smith et al. (2011) | EV | M R2 = 0.13 |
|
| Studer et al. (2014) | EV | Not reported | |
| Westmacott et al. (2010) | EV | M R2= 0.10 |
Group with subcortical only lesions |
| Westmacott et al. (2010) | U | M R2= 0.12 |
Group with cortical only lesions |
| Wingeier et al. (2011) | EV | L R2 = 0.61 |
Note. Effect size classifications are based on Cohen (1988)’s classification scheme. All effect sizes were converted to a common index of R2 for ease of comparison. S = small effect size; M = medium effect size, L = large effect size; ER = early resilience (earlier age of onset associated with better outcomes); EV = early vulnerability (earlier age of onset associated with worse outcomes); U = a nonlinear association.
Of the studies with statistically significant findings, the most consistent finding was that earlier age at lesion onset was associated with lower FSIQ when compared to later age at lesion onset. When provided, effect sizes of the influence of age at lesion onset on intellect outcomes ranged from small to large. Many additional studies investigated age at lesion onset and FSIQ and had non-significant findings (i.e., Aram and Ekelman, 1986; Banich et al., 1990; de Montferrand et al., 2019; Everts et al., 2008; Gordon et al., 2015; Hajek et al., 2014; Jacobs et al., 2007; Mosch et al., 2005; O’Keeffe et al., 2014; Peterson et al., 2019; Westmacott et al., 2018). Other factors that may influence the association between age at lesion onset and outcomes are discussed below.
3.1.2. Verbal IQ and Performance IQ
Some studies also reported VIQ or PIQ as an outcome measure without reporting FSIQ, as summarized below (and see Table 7).
Table 7.
Significant Results and Reported Effect Sizes for VIQ
| Study | Direction | Effect size and classification |
|---|---|---|
| Duval et al. (2002) | EV | Not reported |
| Montour-Proulx et al. (2004) | EV | M R2 = 0.04 |
Note. Effect size classifications are based on Cohen (1988)’s classification scheme. All effect sizes were converted to a common index of R2 for ease of comparison. S = small effect size; EV = early vulnerability (earlier age of onset associated with worse outcomes).
Although not reported as statistically significant, Lansing et al. (2004) also reported earlier lesions were associated with worse outcomes for both PIQ (p < .06) and VIQ (p < .08) Other studies indicated a non-significant association between VIQ or PIQ and age at lesion onset (i.e., Aram and Ekelman, 1988; Isaacs et al., 1996; Levine et al., 1987; Montour-Proulx et al., 2004).
3.1.3. Summary of Intellect Findings
Overall, in those studies that found a significant association between age at lesion onset and intellect, most indicated earlier age at lesion onset predicted worse outcomes for FSIQ, VIQ, and PIQ, with small to large effect sizes. Vulnerability is particularly high in early childhood (e.g., the first few years of life). Onset of a lesion at three years-old, or earlier, was associated with worse outcomes when compared to onset at older ages in several studies (i.e., Allman & Scott, 2013; Anderson et al., 2009; Anderson et al., 2014; Block et al., 1999; Max et al., 2010; Spencer-Smith et al., 2011; Studer et al., 2014). Though not statistically significant (p = .06), Jacobs and colleagues (2007) similarly found those with lesion onset in the prenatal period performed worse on FSIQ than those with onset between seven and nine years old. When examining VIQ and PIQ, lesion onset before the age of seven was associated with worse outcomes when compared to onset after the age of seven (Duval et al., 2002).
The relation between age at lesion onset and intellect also has the potential to be non-linear, with lesion onset during a period from one month to five years conferring more risk for poor outcomes than before or after that time. For instance, Westmacott et al. (2010) found those with lesion onset in the perinatal period (i.e., from 20 weeks gestation to one month after birth) and after the age of five perform better than those who sustain lesions during the interim period (one month-five years) when examining a sample with cortical lesions. This U-shaped relation is like that reported in Goodman and Yude (1996) where both early and later onset, predicted higher outcomes than a period of particular vulnerability from one month to five years. Importantly, Goodman and Yude (1996) reported this pattern of findings after examining several potentially confounding variables including injury severity, laterality, seizures, and head circumference.
An interaction between age at lesion onset and recovery over time may also be relevant, with those with earlier onset exhibiting worse performance across time and those with later onset exhibiting improvement across time. Anderson and colleagues (2020) and Gordon and colleagues (2015) specifically examined trajectories of intellectual functioning after stroke and found individuals with a neonatal stroke (defined as 0–28 days in Anderson et al., 2020 and 0.03–0.33 months in Gordon et al., 2015) exhibited decreasing trajectories across time (i.e., up to 12 months after stroke for Anderson et al., 2020 and six months for Gordon et al., 2015) whereas those with later age at onset (i.e., until age 16 in Anderson et al., 2020 and age five in Gordon et al., 2015) exhibited better performance over time after stroke.
Particularly relevant etiological and injury variables appear to be a history of seizures and lesion location (i.e., cortical versus subcortical) and are discussed in later sections.
3.2. Processing Speed
Processing speed is defined as the rate and efficiency with which basic information is processed and responded to (Lezak et al., 2012). Some studies included processing speed as an outcome of interest (Table 8).
Table 8.
Significant Results and Reported Effect Sizes for Processing Speed
| Study | Direction | Effect size and classification |
|---|---|---|
| Anderson, Jacobs, et al. (2010) | EV | M R2 = 0.09–0.12 |
| Anderson, Spencer-Smith, et al. (2010) | EV | M R2 = 0.10 |
| Long et al. (2011) | EV | Not reported |
Note. Effect size classifications are based on Cohen (1988)’s classification scheme. All effect sizes were converted to a common index of R2 for ease of comparison. M = medium effect size; EV = early vulnerability (earlier age of onset associated with worse outcomes).
Other studies reported a non-significant association between age at lesion onset and processing speed including Allman and Scott (2013), Aram and Ekelman (1988), Hajek et al. (2014), Peterson et al. (2019), Westmacott et al. (2010), and Westmacott et al. (2018).
3.2.1. Summary of Processing Speed Outcomes
Overall, fewer studies investigated the influence of age at lesion onset on processing speed outcomes than on intellect and many studies reported a non-significant effect of age at lesion onset on processing speed outcomes. However, like intellect, for those that did report a significant effect, earlier onset appears to be a risk factor for worse processing speed outcomes, with a medium effect. Specifically, Long and colleagues (2011) reported lesion onset before the age of five was associated with worse outcomes when compared to after the age of five. Anderson, Spencer-Smith, and colleagues (2010) reported worse outcomes with onset at or before the age of seven and Anderson, Jacobs, and colleagues (2010) reported those with onset before one-month had worse outcomes than those with onset between seven and nine years.
3.3. Attention and Working Memory
Attention and working memory are abilities that help one to concentrate, hold, and manipulate information (Lezak et al., 2012; Table 3). Extensive work has examined attention and working memory as an outcome of interest related to age at lesion onset (see Table 9).
Table 9.
Significant Results and Reported Effect Sizes for Attention and Working Memory
| Study | Direction | Effect size and classification | Notes |
|---|---|---|---|
| Anderson, Jacobs, et al. (2010) | EV | M R2 = 0.03–0.13 |
|
| Anderson, Spencer-Smith et al. (2010) | EV | M R2 = 0.12 |
|
| Lansing et al. (2004) | EV | L R2 = 0.25 |
|
| Max et al. (2010) | EV | S R2 = 0.02 |
|
| Spencer-Smith et al. (2011) | EV | M R2 = 0.11 |
|
| Westmacott et al. (2010) | EV | M R2 = 0.09–0.13 |
Results reported for the group with only subcortical lesions and separately for the group with only cortical lesions |
| Westmacott et al. (2018) | EV | L R2 = 0.21 |
Neither age at onset nor age at test significantly predicted outcomes when age at test was included in the model |
Note. Effect size classifications are based on Cohen (1988)’s classification scheme. All effect sizes were converted to a common index of R2 for ease of comparison. S = small effect size; M = medium effect size; L = large effect size; ER = early resilience (earlier age of onset associated with better outcomes); EV = early vulnerability (earlier age of onset associated with worse outcomes); U = a nonlinear association.
Other studies reported no significant association between age at lesion onset and a measure of attention and working memory (Allman & Scott, 2013; Bartha-Doering et al., 2021; Fuentes et al., 2017; Hajek et al., 2014; Long et al., 2011; Mosch et al., 2005; O’Keeffe et al., 2014; Peterson et al., 2019; Vargha-Khadem et al., 1985).
3.3.1. Summary of Attention and Working Memory Outcomes
Overall, on measures that examined working memory and attention, earlier lesion onset predicted worse outcomes when compared to later lesion onset, with small to large effect sizes. The first few years of life may be a particularly vulnerable time (e.g., in the perinatal period (Westmacott et al., 2010), before the age of one (Lansing et al., 2004; Max et al., 2010), or before the age of three (Anderson, Jacobs et al., 2010; Anderson, Spencer-Smith et al., 2010; Spencer-Smith et al., 2011)) relative to middle childhood (e.g., age seven; (Anderson, Jacobs et al., 2010; Anderson, Spencer-Smith, et al., 2010; Spencer-Smith et al., 2011), or between one month to five years and six and 16 years (Westmacott et al., 2010)), which may be a time of less vulnerability for worse outcomes in attention and working memory after brain injury. Fuentes et al. (2017) also reported those with perinatal stroke commonly performed worse on measures of working memory when compared to those with childhood-onset stroke (i.e., between one-month and 14 years), though these exploratory findings failed to reach significance, likely due to the small sample size.
3.4. Language
Language functioning includes production, comprehension, and analysis of verbal material (Lezak et al., 2012). Various studies included language functioning as an outcome of interest (Table 10).
Table 10.
Significant Results and Reported Effect Sizes for Language
| Study | Direction | Effect size and classification | Notes |
|---|---|---|---|
| Anderson, Jacobs, et al. (2010) | EV | M-L R2 = 0.03–0.14 |
|
| Aram & Ekelman (1987) | EV | Not reported | Group with left-lateralized lesions |
| Aram & Ekelman (1987) | ER | Not reported | Group with right-lateralized lesions |
| Ilves et al. (2014) | EV | Not reported | |
| Levine et al. (1987) | ER | Not reported | |
| Long et al. (2011) | EV | Not reported | |
| Max et al. (2010) | EV | M R2 = 0.08 |
|
| Vargha-Khadem et al. (1985) | ER | L R2 = 0.26 |
Note. Effect size classifications are based on Cohen (1988)’s classification scheme. All effect sizes were converted to a common index of R2 for ease of comparison. S = small effect size; M = medium effect size; L = large effect size; ER = early resilience (earlier age of onset associated with better outcomes); EV = early vulnerability (earlier age of onset associated with worse outcomes); U = a nonlinear association.
Many other studies reported a non-significant association between age at lesion onset and a measure of language (i.e., Allman & Scott, 2013; Aram & Ekelman, 1986; Bartha-Doering et al., 2019; Bartha-Doering et al., 2021; Gordon et al., 2015; Mosch et al., 2005; Peterson et al., 2019; Vargha-Khadem et al., 1985; Westmacott et al., 2018).
3.4.1. Summary of Language Outcomes
Overall, findings for the language domain are more contradictory than other domains with some finding earlier onset predicts worse outcomes and others finding resilience with earlier onset, even within similar subdomains of language. In two studies examining verbal expression those with earlier age at lesion onset (before three for Anderson, Jacobs et al. (2010) and before five for Long et al. (2011)) had worse performance on a measure of naming when compared to lesion onset after that time. Alternatively, Vargha-Khadem and colleagues (1985) found later age at injury (especially after the age of five) in those with left hemisphere lesions, was associated with worse performance on a measure of object naming.
In studies examining verbal comprehension, onset before the age of one (Max et al., 2010) or before the age of three (including the perinatal period) was associated with worse outcomes than after the age of three (Anderson, Jacobs et al., 2010). Alternatively, Levine and colleagues (1987) found those with congenital onset (i.e., before birth) had significantly better performance than those with acquired (i.e., after birth) onset and Ilves and colleagues (2014) found better outcomes for those who sustained a lesion before two when compared to after. Importantly, lesion laterality plays a role in outcomes. For instance, Aram and Ekelman (1987) reported similar findings to Max and colleagues (2010) when examining only children with left lateralized lesions. However, when examining those with right lateralized lesions, performance on the Token Test was better in those with lesion onset before one year, when compared to after one year. Thus, results in those with right-lateralized lesions are opposite of those reported in the left-lateralized lesions and in the previously reported Max et al. (2010) study (Aram & Ekelman, 1987). The implications of lesion laterality are further discussed in the Injury Variables section.
3.5. Visuospatial and Perceptual Skills
Visuospatial and perceptual skills encompass visual perceptual, constructional, and spatial skills (Lezak et al., 2012). Many studies included visuospatial and perceptual skills as an outcome of interest (see Table 11).
Table 11.
Significant Results and Reported Effect Sizes for Visuospatial and Perceptual Skills
| Study | Direction | Effect size and classification | Notes |
|---|---|---|---|
| Anderson, Jacobs, et al. (2010) | EV | M R2 = 0.05–0.08 |
|
| Max et al. (2010) | EV | L R2 = 0.17 |
|
| Mosch et al. (2005) | EV | L R2 = 0.17 |
|
| Peterson et al. (2019) | EV | L R2 = 0.41 |
Perceptual reasoning index; Was described as potentially spurious |
Note. Effect size classifications are based on Cohen (1988)’s classification scheme. All effect sizes were converted to a common index of R2 for ease of comparison. S = small effect size; M = medium effect size; L = large effect size; EV = early vulnerability (earlier age of onset associated with worse outcomes).
Other studies reported no significant association between age at lesion onset and visuospatial and perceptual skills (i.e., Allman & Scott, 2013; Aram and Ekelman, 1986; Bartha-Doering et al., 2021; Ilves et al., 2014; Jacobs et al., 2007; Levine et al., 1987; Peterson et al., 2019; Westmacott et al., 2018).
3.5.1. Summary of Visuospatial and Perceptual Outcomes
Overall, earlier onset is associated with worse visuospatial and perceptual skills. For instance, childhood-onset stroke was associated with worse performance, when compared to adult-onset stroke (Mosch et al., 2005). In a more fine-grained examination of the childhood period, worse outcomes were associated with onset before the age of one (Max et al., 2010) and before the age of three (Anderson, Jacobs, et al., 2010; Jacobs et al. 2007). Effect sizes were small to large when reported for the contribution of age at lesion onset to visuospatial and perceptual skills.
3.6. Learning and Memory
Learning and memory includes abilities that allow for the acquisition, storage, and retrieval of visual and verbal information (Lezak et al., 2012). Verbal and visual memory were both investigated in association with age at lesion onset (see Table 12).
Table 12.
Significant Reported Results and Effect Sizes for Verbal and Visual Memory
| Study | Direction | Effect size and classification | Notes |
|---|---|---|---|
| Verbal Memory | |||
| Lansing et al. (2004) | EV | M-L R2 = 0.11–0.20 |
|
| Mosch et al. (2005) | EV | L R2 = 0.26 |
For right-hemisphere lesions |
| Westmacott et al. (2018) | EV | M R2 = 0.11 |
|
| Visual Memory | |||
| Max et al. (2010) | EV | L R2 = 0.27 |
|
Note. Effect size classifications are based on Cohen (1988)’s classification scheme. All effect sizes were converted to a common index of R2 for ease of comparison. M = Medium effect size; L = large effect size; EV = early vulnerability (earlier age of onset associated with worse outcomes).
Other studies reported no significant association between age at lesion onset and verbal or visual memory outcomes (Allman & Scott, 2013; Anderson, Jacobs, et al., 2010; Aram & Ekelman, 1988; Bartha-Doering et al., 2019; Bartha-Doering et al., 2021; Jacomb et al., 2018; Max et al., 2010).
3.6.1. Summary of Learning and Memory Findings
Overall, earlier onset of injury appears to predict worse outcomes for both visual and verbal memory in studies that included both childhood onset and adult-onset focal brain injuries. Within the childhood period, onset before the age of one year was found to confer increased risk for poor outcomes (Lansing et al. 2004; Max et al., 2010). Effect sizes were medium to large depending on memory subdomain, hemisphere of the lesion, and groups of comparison.
3.7. Executive Function
Executive function skills are defined as skills that guide and direct behavior in a purposive, self-directed manner (Lezak et al., 2012). Studies that assessed executive function through standardized and validated behavioral tasks or questionnaires are included in this section (see Table 13). Some of the studies utilized a questionnaire measure of executive function and others utilized behavioral tasks to measure aspects of executive function (Table 3).
Table 13.
Significant Reported Results and Effect Sizes for Executive Function
| Study | Direction | Effect size and classification |
|---|---|---|
| Anderson et al. (2009) | EV | M R2 = 0.09–0.10 |
| Anderson, Jacobs, et al. (2010) | EV | M R2 = 0.03–0.10 |
| Anderson, Spencer-Smith, et al. (2010) | EV/U | M R2 = 0.09–0.12 |
| Anderson et al. (2014) | EV | Not reported |
| Jacobs et al. (2007) | U | Not reported |
| Long et al. (2011) | EV/ER | Not reported |
| Max et al. (2010) | ER | S-L R2 = 0.01–0.14 |
| O’Keeffe et al. (2014) | ER | L R2 = 0.34–0.38 |
| Spencer-Smith et al. (2011) | EV | M R2 = 0.11 |
Note. Effect size classifications are based on Cohen (1988)’s classification scheme. All effect sizes were converted to a common index of R2 for ease of comparison. S = small effect size; M = medium effect size; L = large effect size; ER = early resilience (earlier age of onset associated with better outcomes); EV = early vulnerability (earlier age of onset associated with worse outcomes); U = a nonlinear association.
Other studies found no significant association between age at lesion onset and a measure of executive functioning (i.e., Allman & Scott, 2013; Bartha-Doering et al., 2019; Bartha-Doering et al., 2021; Mosch et al., 2005; Peterson et al., 2019; Westmacott et al., 2018).
3.7.1. Summary of Executive Function Findings
Overall, findings for executive function abilities are mixed across both questionnaire and behavioral measures of executive function. For the Behavior Rating Inventory of Executive Function (BRIEF) self or other-report earlier onset of injury was rated to have more difficulties on the global executive composite (Anderson et al., 2009; Anderson et al., 2014), behavioral regulation (Anderson et al., 2009), planning and organization (Long et al., 2011), and shifting scales (Long et al., 2011). Alternately, older age at onset was associated with more self-rated difficulties on the global executive composite, behavioral regulation, and metacognition scales in one study (O’Keeffe et al., 2014).
Other studies reported more complicated relations. For instance, Anderson, Spencer-Smith, and colleagues (2010) reported both early onset (before age three) and a period between seven and nine years old appeared to confer the most risk for deficits in the global executive composite, behavioral regulation, and metacognition. Jacobs and colleagues (2007) reported both earlier (i.e., prenatal) and later (i.e., between four and nine) lesion onset conferred less risk for deficits in behavioral regulation than a time between birth and three-years-old.
For behavioral tasks of executive functioning, earlier age at onset was associated with more difficulties on measures of planning and organization (Anderson, Jacobs, et al., 2010; Anderson, Spencer-Smith, et al., 2010), verbal fluency (Anderson, Jacobs, et al., 2010; Anderson, Spencer-Smith, et al., 2010; Jacobs et al., 2007), and inhibition and switching (Anderson, Jacobs, et al., 2010, Anderson, Spencer-Smith et al., 2010; Long et al., 2011; Spencer-Smith et al., 2011). Distinctions were made when comparing those with onset before versus after two years of age, especially when compared to onset after seven years old (Anderson, Jacobs, et al., 2010; Anderson, Spencer-Smith et al., 2010), or five years old (Long et al., 2011), with earlier lesion onset conferring increased risk for poor outcomes when compared to later lesion onset. Additional comparisons were made between the prenatal period and after 10 years of age (Jacobs et al., 2007) and between two months and two years versus seven to nine years (Spencer-Smith et al., 2011), again with earlier onset conferring increased risk for poor outcomes.
In contrast, later onset was associated with worse outcomes in some studies for planning and organization when comparing onset before five years to after five years of age (Long et al., 2011), and verbal and design fluency when comparing onset before one year to after one year (Max et al., 2010). Earlier onset of stroke was also associated with better performance on a composite of sequencing tests from the DKEFS, but this effect was no longer significant when controlling for age at test (O’Keeffe et al., 2014). Of note, Jacobs and colleagues (2007) also report a trend for a potential curvilinear relation with those who sustain lesions between four and six years old having the greatest difficulty with verbal mental flexibility, when compared to those with lesion onset before or after that period.
In summary, in contrast to many of the previous domains, several studies found evidence of older age at onset predicting worse outcomes. There is also the potential for a curvilinear relationship, with earlier and later onset both predicting worse outcomes. Inconsistency across findings spanned both questionnaire and behavioral measures of executive function as well as different aspects of executive function, though it is notable that no studies reported that earlier age at onset was significantly associated with better outcomes for behavioral measures of inhibition or switching.
3.8. Other Relevant Variables
Other biological variables (i.e., age and injury) that contributed to findings related to age at lesion onset are reported here. Contributory variables include those that influenced cognitive outcomes and especially those that interacted with age at lesion onset to influence cognitive outcomes. Related methodological factors are also discussed.
3.8.1. Age Variables
Relevant age variables include chronicity (i.e., age at test minus age at lesion), recovery over time, and age at testing (Table 4). Additionally, relevant methodological factors are discussed (i.e., study design, age at lesion onset range).
3.8.1.1. Chronicity.
The chronicity of the lesion, or the interval between time of injury and time of testing, is a notable covariate of age at lesion onset. Importantly, there is a general expected trajectory of recovery after a brain injury with the most severe deficits presenting earlier in the trajectory, recovery occurring over the following weeks and months (approximately three to six months), and stability following thereafter. However, recovery trajectories are often more complicated when brain injury occurs during the developmental period, given the brain damage occurs while skills are still developing rather than after they have stabilized. Potential trajectories for recovery after a developmental lesion include stability, increasing skill, and declining skill. Importantly, stability would not be the expectation for those who sustain lesions in the developmental period, as they are expected to continue to acquire skills. Given the increased demand placed on children to not only maintain skills, but to acquire new skills, it is also possible individuals with early onset lesions will grow into deficits (i.e., evidence a greater difference when compared to a normative population across time). This pattern may be the result of individuals attempting to acquire new skills while utilizing a compromised neural system when compared to those children who have not sustained a brain injury.
In studies that investigated chronicity, chronicity was independently associated with general intellectual outcomes (FSIQ, VIQ, PIQ) such that a longer duration since lesion onset is associated with worse outcomes, in those largely assessed in the chronic period (see Table 2 for years since stroke average; Westmacott et al., 2010). A similar trend was noted for auditory attention and inhibition/switching, with higher chronicity associated with worse outcomes (Westmacott et al., 2018). In contrast, others reported lower chronicity was associated with worse outcomes on measures of VIQ, PRI, and visual-motor integration (Montour-Proulx et al., 2004; Peterson et al., 2019) and some studies found no association between chronicity and IQ, language, attention and working memory, processing speed, visuospatial and perceptual skills, learning and memory, or executive functioning (Allman & Scott, 2013; Bartha-Doering et al., 2019, 2021; Braun et al., 2001; de Montferrand et al., 2019; Peterson et al., 2019; Wingeier et al., 2011).
Chronicity also has the potential to interact with age at onset as earlier onset and increasing duration since onset was associated with poorer outcomes (e.g., IQ, nonverbal intellectual skills, and VIQ), but later onset and longer chronicity was associated with improved outcomes for IQ (Duval et al., 2008), nonverbal intellectual skills, and VIQ (Montour-Proulx et al., 2004). Of note, in their continuous analysis, Duval and colleagues (2008) found conflicting findings when compared to the previous analysis. Specifically, lesions acquired before the age of 15 were not significantly associated with chronicity, but individuals with lesion onset after 15 exhibited a decline in IQ as a function of longer chronicity on average (Duval et al., 2008).
As chronicity is shown to be associated with outcomes, it is important to acknowledge age at lesion onset findings in the context of chronicity. While many studies did not account for chronicity in analyses, many others found significant results when accounting for chronicity. For example, Gingras and Braun (2018) reported age at lesion onset was still predictive of IQ even when accounting for chronicity of the lesion, as did Aram and Eisele (1994). Of note, age at injury, age at test, and chronicity are often intercorrelated, resulting in difficulty when assessing relative contributions of the different variables on cognitive outcomes (e.g., Westmacott et al., 2018).
3.8.1.2. Recovery Over Time.
A related concept to chronicity is the recovery over time (Table 4). Of those studies with longitudinal designs, one reported younger age at lesion onset is significantly associated with a greater change in FSIQ over time and ultimately worse intellectual outcome (Jacomb et al., 2018). Anderson and colleagues (2020) specifically identified those with lesion onset in the neonatal period exhibited declines across time in intellectual functioning whereas those with later onset strokes made gains up to 12 months after stroke. Similarly, others noted an interaction between age at lesion onset and recovery over time for receptive language, with those with onset in the neonatal period demonstrating decreasing scores on longitudinal assessments done one month and six months after diagnosis and individuals who sustained injury onset at older times exhibiting improvement across time (i.e., from one to six months after injury onset; Gordon et al., 2015). Duval and colleagues (2002) also noted differential recovery over time with those who sustained injury before the age of seven years-old showing stable profiles and those who sustained injury after seven years-old showing improvement on measures of VIQ. In a later study with a lifespan sample Duval and colleagues (2008) again demonstrated age at lesion onset interacted with recovery across time, with time elapsed between assessments associated with a decline in IQ for those with lesion onset before 18 and an increase in scores for those with lesion onset after the age of 18. When examining other domains, Jacomb and colleagues (2018) reported no significant association between age at stroke and change over time in memory (i.e., immediate memory, delayed memory, and recognition memory).
Similar to chronicity, age at lesion onset and recovery over time may interact, with studies supporting that those who sustain lesions later in life may improve across time on measures of IQ, whereas those who sustain lesions earlier in life may remain the same, or decrease in skill, depending on the domain assessed. Although chronicity may be a proxy for recovery across time in cross-sectional studies, within-person recovery can only be measured in a longitudinal design. Importantly, those studies measuring chronicity and longitudinal studies both support that earlier-onset often confers more risk for worse outcomes when compared to later-onset lesions.
3.8.1.3. Age at Testing.
The developmental period during which testing occurs is also an important consideration when examining cognitive outcomes, even though most studies use age-corrected standardized scores. Age at testing is independently, positively, associated with intellectual functioning, processing speed, attention, visuospatial and perceptual skills, language, memory, and executive functioning (e.g., Anderson, Jacobs, et al., 2010; Anderson, Spencer-Smith, et al., 2010; Braun et al., 2001). However, other studies have found no significant association with age at testing and IQ outcomes, processing speed, working memory, perceptual reasoning, visual-motor integration, language, learning and memory and executive functioning (Bartha-Doering et al., 2019; Peterson et al., 2019; Westmacott et al., 2010; Westmacott et al., 2018). Age at lesion onset can also be significantly associated with age at testing (O’Keeffe et al., 2014).
The contribution of age at testing should be accounted for when considering age at onset’s influence on cognitive outcomes, especially when not using age standardized scores. Importantly, studies have controlled for age at testing and found a significant contribution of age at onset for outcomes including attention and working memory, language, visuospatial skills, learning and memory, and executive functioning (Anderson, Jacobs, et al., 2010; Aram & Eisele, 1994; Isaacs et al., 1996; Jacobs et al., 2007; Lansing et al., 2004; O’Keeffe et al., 2014; Westmacott et al., 2018). However, studies also found an effect of age at onset on visual attention and executive function was no longer significant when age at test was accounted for (O’Keeffe et al., 2014; Westmacott et al., 2018).
3.8.1.4. Age at Onset Range.
Another age factor is the age at lesion onset range included, which may influence the ability to detect differences between periods of increased risk or resilience. A particular focus may be if perinatal lesions were included or not. Importantly past work has suggested potential differences between onset at different points in the perinatal period (i.e., between congenital and acquired lesions, e.g., Levine et al., 1987). Of those studies that included congenital lesions, some had separate groups for congenital versus acquired lesions. Studies that found significant differences reported those with congenital lesions performed significantly better on the PPVT (Levine et al., 1987) and had lower ratings on the inhibition scale of the BRIEF (Jacobs et al., 2007). There was also a nonsignificant trend for verbal IQ subtests on the WISC and WAIS, with those with congenital lesions performing better than those with acquired lesions (Levine et al., 1987). Overall, there may be a difference between lesions sustained prior to birth versus those sustained after, with those sustained prior to birth conferring less risk for outcomes. Further implications of the age at onset range are explored in the discussion.
3.8.1.5. Study Design.
As many studies have been cross-sectional in design (Table 2) it is important to understand how study design (i.e., cross-sectional versus longitudinal) may impact findings when investigating the contributions of age at lesion-onset on outcomes. Duval and colleagues (2002) examined a cross-sectional design in comparison with a longitudinal design by matching cross-sectional subjects with longitudinal subjects on several age and injury variables. The authors showed that results varied based on the use of a cross-sectional or longitudinal design, with the longitudinal design showing decreased recovery for individuals with onset early in life for IQ. Alternately, the cross-sectional design did not strongly support the findings from the longitudinal study. Duval and colleagues’ (2002) findings point to the potential importance of a longitudinal approach to account for recovery across time, which may provide additional information to the cross-sectional approach that has been used in a majority of the age at lesion onset studies. Duval and colleagues (2002) encourage the use of a longitudinal design and control of age and injury variables in analyses to further identify the contribution of age at lesion onset. Thus, moving forward it will be important to recognize the difference between cross-sectional and longitudinal studies and potentially implement further longitudinal studies to account for considerations including recovery across time. Though, it is again important to note that when examining the many studies done, there is consistency in the conclusions drawn across cross-sectional and longitudinal studies with earlier-onset conferring increased risk for worse outcomes.
3.8.2. Injury Variables
Relevant injury variables that may contribute to cognitive outcomes independently of age at lesion onset or in coordination with age at lesion onset include lesion size/volume, lesion laterality, lesion location, and a history of seizures (Table 4). To determine the potential contribution of these variables, they are reviewed in the following sections. Other factors may also contribute to outcomes, such as comorbidities. For instance, individuals with congenital lesions may have other genetic differences, individuals with acquired strokes may have comorbid heart problems, and individuals with brain tumors may have undergone treatment including chemotherapy and radiation. These potential comorbidities are often not reported in research done regarding age at lesion onset and thus are beyond the scope of this review but warrant consideration in the broader picture for expectations of performance and when thinking of implications of research for patient care.
This review included studies investigating both heterogeneous etiologies and those that included only stroke. Those studies examining heterogeneous etiologies were separated from studies examining specifically stroke to aid in identifying potential systematic differences. It is also interesting to consider the work reviewed in comparison to prior work done with animal models. Research done with animals often creates clearly defined lesions and examines the influence of the lesions on simple behaviors. Studies that include stroke samples and investigate lower-level cognitive functions are most like the original animal work. Thus, examining these studies separately also allows for considerations of similarities to the historical work in this area done with animal models.
While the focus of this paper was largely on higher-level cognitive functions and excluded basic motor functions, some simpler cognitive functions (e.g., processing speed and simple attention) were included. Importantly, studies investigating the domains of processing speed and simple attention reported similar findings for stroke and heterogeneous samples, largely indicating an early vulnerability for worse outcomes (e.g., Anderson, Jacobs, et al., 2010; Anderson, Spencer-Smith, et al., 2010; Spencer-Smith et al., 2011; Long et al., 2010; Max et al., 2010; Westmacott et al., 2018). Overall, there were no systematic differences between the studies including samples with heterogeneous etiologies and those with stroke-only, with studies including both samples reaching similar conclusions.
3.8.2.1. Lesion Size.
Of note, the methods for calculating lesion size varied across studies (e.g., Anderson et al., 2009; Vargha-Khadem et al., 1985). All studies that noted analyses related to lesion size are included in this section. See individual studies for exact methods of calculating lesion size.
Lesion size is independently predictive of many cognitive outcomes, with larger lesions predicting lower FSIQ, VIQ, PIQ, language abilities, processing speed, working memory, attention, perceptual reasoning and more difficulties with executive function (Anderson et al., 2014; Banich et al., 1990; Bartha-Doering et al., 2021; Braun et al., 2001; Duval et al., 2008; Everts et al., 2008; Hajek et al., 2014; Kornhuber et al., 1985; Levine et al., 1987; Montour-Proulx et al., 2004; Westmacott et al., 2018) and decreasing intellect across time (Anderson et al., 2020). Other studies found lesion size to not be associated with some cognitive abilities (Aram & Eisele, 1994; Bartha-Doering et al., 2021; Everts et al., 2008; Peterson et al., 2019). Importantly, lesion volume may be a contributory factor regardless of the age at which the lesion occurs. For instance, the volume of the lesion has been shown to account for more variance in predicting FSIQ, VIQ and PIQ than other age variables (i.e., chronicity, age at onset, age at testing) and to not interact with age at lesion onset in some studies, so that a larger lesion may have a more negative effect on IQ outcomes, regardless of the age at which the damage occurs (Banich et al., 1990; Montour-Proulx et al., 2004). It is important to note, however, there may be an association between age at lesion onset and lesion size such that those who sustain lesions earlier in life are more likely to have larger lesions (e.g., Banich et al., 1990). Though, this is not always true, as Max et al. (2010) found no significant difference in lesion size between early and late stroke groups.
Some studies have accounted for lesion size (e.g., by examining correlations between lesion size and age groups or controlling for lesion size in final analyses) and still saw a significant effect of age at lesion onset for IQ, processing speed, attention and working memory, language, visuospatial and perceptual skills, learning and memory, or executive function abilities (Anderson et al., 2014; Gingras & Braun, 2018; Lansing et al., 2004; Levine et al., 1987; Montour-Proulx et al., 2004; Mosch et al., 2005; Westmacott et al., 2018). Finally, the importance of the influence of lesion size versus age at onset may be domain dependent. For instance, lesion size may be most important for predicting working memory abilities (Westmacott et al., 2018).
3.8.2.2. Lesion Laterality.
The side on which a lesion occurs (i.e., left, right or bilateral) is another injury variable that affects outcomes. For instance, numerous studies report differences in outcomes depending on the laterality of the lesion such that lesion laterality is significantly associated with outcomes for VIQ, PIQ, processing speed, working memory, verbal memory, and language (Allman & Scott, 2013; Anderson et al., 2014; de Montferrand et al., 2019; Goodman & Yude, 1996; Kornhuber et al., 1985; Levine et al., 1987; Montour-Proulx et al., 2004; Pavlovic et al., 2006; Riva & Cazzaniga, 1986; Vargha-Khadem et al., 1985). Specifically, those who sustain left or bilateral strokes tend to perform worse on measures of IQ (Kornhuber et al., 1985), language (Allman & Scott, 2013; Levine et al., 1987; Vargha-Khadem et al., 1985), nonverbal abstract reasoning (O’Keeffe et al., 2014), verbal memory, working memory, and perceptual reasoning (Allman & Scott, 2013) and those who sustain right lateralized lesions tend to perform worse on visual-motor abilities (Allman & Scott, 2013), performance IQ (Anderson et al., 2014; Montour-Proulx et al., 2004), and full-scale IQ (Anderson et al., 2014). Conflicting findings were reported in the domains of verbal IQ and processing speed with some studies reporting worse outcomes for those who sustained left-sided lesions (Allman & Scott, 2013; de Montferrand et al., 2019; Montour-Proulx et al., 2004) and others reporting worse outcomes for those who sustained right or bilateral lesions (Anderson et al., 2014; Pavlovic et al., 2006). On the other hand, other studies have reported no significant effects for laterality on outcomes of IQ, language, memory, visuospatial functioning, attention and working memory, processing speed, and executive function, especially in samples with early lesion onset (Hajek et al., 2014; Isaacs et al., 1996; Lansing et al., 2004; Long et al., 2011; Pavlovic et al., 2006; Peterson et al., 2019; Studer et al., 2014; Westmacott et al., 2010). Thus, the age at which lesion onset occurs is one variable that may explain why some studies report significant findings for laterality and others do not.
In fact, variables of lesion laterality and age at lesion onset interact when predicting several cognitive functions (i.e., object naming, comprehension, verbal memory, and IQ). For verbal comprehension and PIQ, left hemisphere lesions sustained earlier in life are associated with worse outcomes and right hemisphere lesions sustained earlier in life are associated with better outcomes (Aram & Eisele, 1994; Aram & Ekelman, 1986, 1987; Montour-Proulx et al., 2004). Interestingly, for verbal memory, Mosch and colleagues (2005) found a different effect with adults who had right-sided lesions performing better than children with right-sided lesions and no age at onset effect in left sided lesions (Mosch et al., 2005). In a comparison of verbal and performance abilities Aram & Ekelman (1988) found higher PIQ than VIQ scores in subjects with lesions in the left hemisphere and onset after two years-old, and higher VIQ scores than PIQ scores in those with right hemisphere damage and onset after the age at two. Allman & Scott (2013) found the opposite effect with laterality differences for performance and processing speed only in the infancy onset group, but not other age at onset groups. However, others found no significant association between age at injury and lesion laterality for some cognitive outcomes (Max et al., 2010; O’Keeffe et al., 2014).
Overall lesion laterality is significantly and independently associated with outcomes in several cognitive domains. Early left hemisphere lesions are associated with worse outcomes for comprehension and PIQ when compared to early-onset right-hemisphere lesions. Early right-sided lesions are associated with deficits in verbal memory when compared to adult-onset right-sided lesions. Later-onset (i.e., after two years old) is associated with higher PIQ than VIQ scores in those with left hemisphere lesions and higher VIQ scores than PIQ scores in those with right hemisphere damage. There is conflicting evidence about laterality differences in earlier life, with some studies reporting no laterality differences, and others reporting laterality differences as early as the infancy period.
3.8.2.3. Lesion Location.
Lesion location is another more refined injury factor that is associated with many cognitive outcomes (e.g., Westmacott et al., 2010). Lesion location is well-known to influence outcomes depending on the domain assessed, and a review of those findings is beyond the scope of this paper as this review has a refined focus on findings particularly related to age at lesion onset. Regarding vulnerability versus plasticity, lesion location is relevant as it has the potential to influence the periods of peak vulnerability.
Westmacott et al. (2010) found those with perinatal lesions have significantly worse outcomes than those with lesion onset from after the perinatal period with groups from one month to five years and six to 16 years, in a sample including individuals with subcortical lesions. However, when analyses were refined to only include those with cortical lesions, the critical period for general intellectual outcomes shifted, with a period of vulnerability from one month to five years when compared to those whose strokes occurred both earlier and later, aligning with Goodman and Yude’s (1996) U-shaped findings (Westmacott et al., 2010). These findings were only significant for FSIQ and while Westmacott and colleagues (2010) noted a similar trend for other measures (i.e., PIQ, VIQ, WMI), they did not reach significance, likely due to small sample sizes. Westmacott and colleagues (2010) and Studer and colleagues (2014) both found those with combined cortical and subcortical lesions do worse across cognitive domains when compared to those who have only subcortical or cortical lesions. Studer and colleagues (2014) found no significant difference in outcomes when comparing cortical only to subcortical only groups. Thus, when investigating age at onset effects, it could be important to control for lesion location (e.g., subcortical versus cortical lesions), especially with larger sample sizes. Interestingly, Anderson and colleagues reported cortical/subcortical classification had a significant effect on intellect trajectory with those who had cortical-only lesions exhibiting decreasing trajectories across time. Some studies have investigated domains such as IQ, visuospatial abilities, visual memory, and verbal memory while controlling for lesion location and found age at lesion onset still significantly influences cognitive outcomes (Mosch et al., 2005; Gingras & Braun, 2018).
Of note, lesion location identification (e.g., through methods such as lesion-symptom mapping) is often more detailed and advanced for those studies examining adult-onset lesions than for those examining developmental-onset lesions. This discrepancy could be due to several factors. It is critical for future work to utilize modern methods for relating lesion location to outcomes, such as large-scale lesion-symptom mapping studies. This would be helpful in the future to consider broader networks impacted by lesions in individuals with early-onset lesions.
3.8.2.4. Seizures.
Seizure history is an independent predictor of outcomes including intellect, processing speed, attention and working memory, language, visuospatial skills, memory, and executive functions, with the presence of seizures predicting worse outcomes (Anderson, Jacobs, et al., 2010; Anderson et al., 2014; Duval et al., 2008; Goodman & Yude, 1996; O’Keeffe et al., 2014; Studer et al., 2014). Anderson and colleagues (2020) reported those who had no history of seizures after stroke had improving intellectual abilities when compared to those who had a history of seizures. While some have found that the effect of seizures exists regardless of age at onset of lesion for measures of intellect (Isaacs et al., 1996), the presence of seizures also has the potential to influence how age at lesion onset affects outcomes. For instance, Gingras and Braun (2018) reported a negative relation between age at onset and FSIQ in the subset of the sample without epilepsy and a positive relation between age at onset and FSIQ in those with a history of epilepsy, while controlling for a number of other injury variables including lesion volume, side, and location. So, for subjects without a history of epilepsy, earlier onset was associated with better outcomes, whereas in the sample with a history of epilepsy, earlier onset was associated with worse outcomes. Thus, seizure history may impact the relation between age at lesion onset and outcomes.
After accounting for the influence of seizure history on outcomes of attentional control, Anderson, Jacobs, and colleagues (2010) no longer found a significant influence of age at lesion onset. Notably, this is domain dependent as there was still an effect of age at lesion onset for processing speed, attention and working memory, visuospatial functioning, language, and executive functioning when controlling for seizure history (Anderson, Jacobs, et al., 2010). From a broader etiological perspective Montour and colleagues (2004) reported for those with lesions occurring early in life, recurrent etiologies (e.g., epilepsy) are worse for nonverbal intelligence than static etiologies and for those with lesions occurring later in life, static etiologies impaired nonverbal intellectual skills more than recurrent etiologies.
Overall, a history of seizures is known to be associated with worse cognitive outcomes. The presence of seizures has the potential to reverse the association between age at lesion onset and cognitive outcomes, such that earlier onset would predict worse outcomes in those with epilepsy, but earlier onset in those without epilepsy is associated with better outcomes. When accounting for the presence of seizures, some have found age at lesion onset is no longer associated with outcomes whereas age at lesion onset was still predictive of numerous cognitive outcomes even when controlling for seizure history.
Discussion
While questions of “better” or “worse” times to sustain brain injury and implications for cognitive functioning have existed for decades, conflicting findings and varied study characteristics make the literature difficult to synthesize. A broad review allows for an examination of general themes, which are relevant for the treatment of patients who sustain focal brain injury. Thus, this systematic review identified and synthesized literature spanning from 1985–2021 that examined age at focal lesion onset as a variable associated with neuropsychological outcomes. While several useful reviews have been published, the current systematic review of the literature adds the important contributions of studies utilizing samples with heterogeneous etiologies of focal injury and those with age at onset ranges from pre-birth to late adulthood. Additionally, it is critical to consider other factors when interpreting age at lesion onset findings and this review adds consideration of biological variables (e.g., age and injury) that influence cognitive outcomes in association with age at lesion onset.
This systematic review of the literature revealed that age at lesion onset is a relevant variable for cognitive outcomes in domains including intellect, processing speed, attention and working memory, language, visuospatial and perceptual skills, learning and memory, and executive function. Overall, earlier age at onset was associated with relatively worse outcomes across many of the domains, including intellect, processing speed, attention and working memory, visuospatial and perceptual skills, and learning and memory. More contradictory findings were shown in studies examining language and executive function (discussed in more detail below).
Many studies utilized a group comparison, basing groups on developmental time periods. This approach is sensible as it is known that brain maturation and development is stepwise rather than linear (Casey et al., 2000; Gogtay et al., 2004). However, different groups and categories were used across various studies and domains, making particular periods of vulnerability difficult to determine. Anderson and colleagues (2014) note that use of a categorical group variable may decrease the ability to detect effects and mask critical developmental periods. Of note, if utilizing a continuous variable, it is important to allow for a curvilinear relation between age at onset and outcome, which has been supported in the literature (e.g., Allman & Scott, 2013; Goodman & Yude, 1996). While interpretation is made more difficult due to different group comparisons across studies, many studies identified the first few years of life as the most vulnerable for relatively worse cognitive outcomes.
These findings are supported by what is known about neural development during the first few years of life. Specifically, the period between one month and five years is a period of peak synaptic production (e.g., synaptogenesis, dendritic arborization, myelination) and elimination (Goodman 1989, 1991; Huttenlocher, 1979). Focal damage during this time has the potential to interrupt normal migratory patterns of neurons and to create abnormal connections by preserving collaterals that otherwise would have been pruned. This disruption could then result in more widespread damage than the original injury may suggest, influencing brain regions distant from the original injury (Goodman, 1989; Hicks et al., 1984). For instance, diaschisis refers to damage to one focal area affecting distant brain regions and has been reported following focal lesions of heterogeneous etiology (e.g., Carrera & Tononi, 2014). Overall, damage to the brain during development can interrupt critical processes for development at a neural level, with severe implications for cognitive processes supported by the damaged areas of the brain and areas distant, but functionally connected, to the site of original injury (Carrera & Tononi, 2014; Goodman 1989, 1991; Huttenlocher, 1979). In other words, a brain injury determined to be anatomically “focal” (e.g., as on imaging) but sustained during development may not be comparable to focal brain injuries sustained after development. A focal brain injury early in childhood (e.g., before the age of five) may result in more severe impairments across more widespread domains due to the damage being incurred in a developing brain that still must make changes to reach its full potential. A similar focal brain injury (e.g., anatomically on imaging) in a non-developing brain (e.g., an older child or adult) may result in a more focal cognitive profile as brain development has stabilized and the acquisition of new skills has reached a plateau.
Interestingly, this period of vulnerability before the age of five has been identified as relevant across other brain-based conditions investigating the contribution of age at onset on outcomes. For instance, those with earlier age at cancer diagnosis (i.e., before the age of five) had worse academic achievement outcomes than those with later cancer diagnoses (Harshman et al., 2012). More generally, worse cognitive and affective outcomes have also been observed with earlier lesions to the cerebellum, when compared to adult-onset lesions (e.g., Wang et al., 2014). These cross-condition findings could serve as evidence of the importance and fragility of underlying developmental mechanisms that are affected by damage (broadly defined) in early life (i.e., especially before the age of five). This aligns with work supporting significant cognitive development in early childhood (Casey et al., 2000) and changes in brain structure stabilizing after the age of five (e.g., total cerebral size; Giedd et al., 1996).
Overall, age at onset is a relevant variable for focal brain injuries and is also predictive of outcomes across number of other conditions, with particular vulnerability when damage occurs before cognitive skills and neural development have stabilized, especially before the age of five. These findings emphasize the importance of recognizing potential vulnerability for worse cognitive outcomes with early-onset brain insult, when compared to later brain insult, across many brain-based conditions.
4.1. Contradictory Findings
In this review, contradictory findings emerged in the language and executive function domains, without a clear story of risk or resilience after lesion onset in early life. Returning to themes stemming from early work, claims of plasticity in the developing brain were supported in work investigating language (e.g., Ballantyne et al., 2008; Basser, 1962; Teuber, 1975). The findings of early resilience for language functions presented in some studies in this review align with this early work. Language may be unique and different from domains like intellect in several ways. For instance, the neural development of language areas is uniquely supported. Language is highly plastic with longer windows of sensitivity than other cognitive domains (Bjorklund & Ellis, 2014). When relevant areas of the left hemisphere become damaged in those with left-lateralized language, there is evidence of compensation through reorganization of language to the right-hemisphere (e.g., Lidzba et al., 2017). This reorganization of language may come at the cost of other cognitive domains, such as visual-spatial functions, as proposed by the Crowding Hypothesis, evidencing a prioritization of language functions (e.g., Danguecan & Smith, 2019). Biological advantages for compensatory reorganization after early rather than late brain injury could be one explanation for why earlier onset of brain injury would predict better language outcomes than later brain injury. In line with this theory, functional reorganization may be more successful with earlier injury, before the age of five-years-old (Lidzba et al., 2017). Given the potential for differential outcomes depending on reorganization, lesion characteristics are especially important to consider for the language domain.
Executive functioning is another domain with mixed findings for age at lesion onset. Notably, executive functioning is a heterogeneous domain, composed of several different skills. The inclusion of many different skills in one domain is relevant as the particular periods of vulnerability during early life may vary based on the skill under question. Specifically, skills undergoing development or not yet developed are more vulnerable than already established skills (Anderson et al., 2005; Dennis, 1988; Ewing-Cobbs et al., 1989; Johnson, 2005; Thomas & Johnson, 2008). Thus, the developmental stage of each skill is important to account for when considering the potential effect of focal lesions on cognitive outcomes and may explain some differences in the findings for age at lesion onset in executive functioning, as subsets of executive functions have different developmental progressions (Anderson et al., 2001; Anderson, 2002; Anderson et al., 2011).
Notably, the age ranges investigated for executive function were all in the childhood period, even though some executive function skills are expected to develop well into adolescence and may be especially affected by later injury (Diamond, 2002; Eslinger & Grattan, 1991). Therefore, it could be beneficial for future work to investigate age at lesion onset outcomes for executive functions with age at onset ranges expanding well into late adolescence. Of note when examining findings across language and executive functioning qualitatively, it was not the case that differences in the collected age or injury variables for this review (i.e., age at testing, chronicity, unilateral lesions, longitudinal assessment, or inclusion of those with seizures in the sample) could explain the contradictory findings for these domains.
This review indicates that when compared to others who have brain injuries, early-onset brain injury may confer increased risk for worse cognitive outcomes. However, these findings are not indicative of the risk for poor cognitive outcomes when compared to normative expectations as this study does not review the literature that examines the difference between those with early-onset injury and normative expectations or comparison groups. Of those studies reviewed, several studies indicated individuals with early-onset brain injury are below expectations when compared to normative expectations or comparison groups for intellect (Everts et al., 2008; Hajek et al., 2014; Jacomb et al., 2018; Lansing et al., 2004; Levine et al., 1987; O’Keeffe et al., 2014; Riva & Cazzaniga, 1986; Westmacott et al., 2010; Westmacott et al., 2018), processing speed (Aram & Ekelman, 1988; Everts et al., 2008; Hajek et al., 2014; Peterson et al., 2019; Westmacott et al., 2010), attention and working memory (Everts et al., 2008; Hajek et al., 2014; Jacomb et al., 2018; Lansing et al., 2004; O’Keeffe et al., 2014; Pavlovic et al., 2006; Peterson et al., 2019; Westmacott et al., 2010; Westmacott et al., 2018), language (Bartha-Doering et al., 2019; Hajek et al., 2014; Ilves et al., 2014; Vargha-Khadem et al., 1985; Westmacott et al., 2010), memory (Aram & Ekelman, 1988; Lansing et al., 2004; Westmacott et al., 2018), visuospatial and perceptual skills (Everts et al., 2008; Ilves et al., 2014; Pavlovic et al., 2006), and executive functioning (Jacomb et al., 2018; Long et al., 2011; O’Keeffe et al., 2014; Peterson et al., 2019; Westmacott et al., 2018). While statistically significant, the actual differences between normative expectations or comparison groups and those with early-onset brain injury are often small in magnitude (e.g., less than one standard deviation; Anderson, Jacobs et al., 2010; Fuentes et al., 2017; O’Keeffe et al., 2014; Studer et al., 2014). Significant but small differences potentially indicate a consistent, albeit small reduction, in cognitive skills after early-onset brain injury at a broad level.
Still, other work has indicated those with early brain injury generally perform within the average range on measures of intellect (Aram & Eisele, 1994; Aram & Ekelman, 1986, 1988; Fuentes et al., 2017; Isaacs et al., 1996; Peterson et al., 2019), processing speed (Westmacott et al., 2018), working memory (Fuentes et al., 2017), visual-spatial skills and perceptual reasoning (Peterson et al., 2019; Westmacott et al., 2018), memory (Jacomb et al., 2018), and language (Gordon et al., 2015; Peterson et al., 2019; Westmacott et al., 2018). Other recent work has indicated the majority of children with early-onset brain injury seem to recover well cognitively, with the exception of those who sustain neonatal brain injury (Anderson et al., 2020).
Overall, this review indicates those with early-onset brain injury are at increased risk for worse outcomes when compared to others who sustain later-onset brain injury. Individuals who sustain brain injury are also potentially at increased risk for worse outcomes when compared to normative expectations across a number of cognitive domains. However, at a group level, those with early-onset brain injury often recover well and can exhibit cognitive abilities within the average or low average range.
4.2. Other Relevant Variables
One etiological factor notable for further discussion is a history of seizures. Overall, it is well-known that a history of seizures has a detrimental effect on cognitive functioning and many studies did not account for this variable when investigating outcomes (on the poor consequences of early seizures see Vargha-Khadem et al., 1992). Previous work has been criticized for a lack of control of relevant variables, including a history of seizures (e.g., duration of seizures, age at seizure onset, seizure severity, history of epilepsy surgery), and findings pertaining to age at lesion onset have been called into question (e.g., Lidzba et al., 2009). The findings of Gingras and Braun (2018) draw attention to how influential a history of seizures can be for outcomes and especially age at lesion onset analyses. When considering the literature as a whole, the findings of early vulnerability are still compelling given the number of studies that reported early vulnerability across different samples and different cognitive domains. Additionally, many studies that did report significant results, with earlier age at lesion onset predicting worse outcomes, controlled for seizure history or excluded those with a history of seizures (Allman & Scott, 2013; Anderson et al., 2014; Duval et al., 2008; Jacomb et al., 2018; Westmacott et al., 2010). However, it is important to view past work with an eye towards when seizures were accounted for, or not. Moving forward it is critical to distinguish between groups of those with seizure history, or account for seizures in analyses in some way, including the additional considerations of duration and severity of seizures, and history of epilepsy surgery.
Another potential methodological contributor is sample size. Sample sizes were extracted for all included studies and can be referenced in Table 1. Unsurprisingly, due to fewer limitations on who could be included in the study, studies that included heterogeneous etiologies had larger sample sizes overall. Upon examination, sample size alone does not seem to influence the findings for FSIQ, learning and memory, language, or executive functioning as studies with similar sample sizes reported significant and non-significant results and there were no obvious or consistent patterns based on sample sizes and results. For processing speed, attention and working memory, and visuospatial and perceptual skills the studies with heterogeneous etiologies and significant results tended to have larger sample sizes than the studies with non-significant results (e.g., n = 138–164 versus n = 31–53). However, most studies in these domains included samples with stroke as the primary etiology. For those studies that included stroke as the primary etiology and investigated each of the domains (i.e., processing speed, attention and working memory, and visuospatial and perceptual skills) there was no consistent pattern with sample size and significance of the results. Studies with similar sample sizes reported significant and non-significant results. Overall, it is possible studies including heterogeneous etiologies require larger sample sizes to detect the influence of age at lesion onset on neuropsychological outcomes though the overall findings of this review do not seem to be influenced by sample sizes of included studies.
4.3. Limitations
This review had a focus on biological (i.e., age and injury) variables that influenced neuropsychological outcomes after brain injury. Importantly, there are other identified factors (e.g., sex/gender, environmental, and intervention/rehabilitation) not explored in this review that are also known to influence neuropsychological outcomes after brain injury. Anderson and colleagues (2011) discussed the importance of these factors. Specifically, variables like family function, socioeconomic status (SES), and response to disability are discussed as relevant to the recovery of cognitive functions after brain injury (e.g., Anderson et al., 2011; Breslau, 1990). Thus, while beyond the scope of this review, environmental factors, experiential factors, and gender/sex are important to account for when considering variables that contribute to outcomes post brain injury. These factors may be especially relevant for executive function and language, as socioeconomic status has been shown to have stronger associations with executive functions and language outcomes than other neuropsychological domains (Merz et al., 2019; Noble et al., 2005; Noble et al., 2007). The influence of social factors on language and executive functioning could add to an explanation of the variability seen in outcomes for specifically executive function and language.
There are several other limitations to this systematic review. While it is noted that age at lesion onset may be particularly relevant for academic functioning (i.e., reading, spelling, and math; Westmacott et al., 2018), those domains were not included in this review. A comprehensive review of academic functioning was beyond the reach of the current review and would benefit from its own review to allow for inclusion of various assessments of academic functioning. Understanding the implications of age at lesion onset on outcomes has also benefitted from cross-study comparison (e.g., comparing a study examining onset in the perinatal period and a study that examined onset in the childhood period; Stiles et al., 2012). This review only included studies that identified age at lesion onset as a variable of interest within the study and thus does not include results from studies that examined one period of age at lesion onset (e.g., perinatal onset; see Stiles et al., 2012 for an overview). However, the contributions of studies investigating age at lesion onset during a particular time should certainly not be disregarded and could supplement the findings of this review, especially in the domain of language.
It is also important to consider that many measures have changed across time, given the wide span of publication dates for studies included in this review (e.g., updated tests, new tests, different versions/editions, new normative samples; see Table 3 for the list of all measures used in included studies). Many studies used different versions of the same measures across time (e.g., the WAIS), in parallel with the updating of tests, and some of these studies were conducted decades apart. When synthesizing results, findings were collapsed across time and across different measures and versions of measures (e.g., collapsing across different versions of the WAIS). Collapsing across measures in this way could potentially add noise to the findings. The inclusion of only standardized and valid measures was one way the authors of this review tried to limit the contribution of noise due to variation in measures. Additionally, there is support for using different versions of a standardized test, especially in special patient populations (e.g., Peterson et al., 2019; Westmacott et al., 2010) and there is no indication that patterns in the results are due to differences in measures used across time.
Several studies used neuropsychological measures that were not in the English language, many of which were commonly used neuropsychological measures that had been translated into a different language (e.g., the German version of the WAIS). All included measures are noted in Table 3. As the current authors are most familiar with English language measures, studies that included non-English measures could be further evaluated by readers for measure characteristics (see Bartha-Doering et al., 2019; Bartha-Doering et al., 2021; de Montferrand et al., 2019; Everts et al., 2008; Ilves et al., 2014; Kornhuber et al., 1985; Pavlovic et al., 2006; Riva & Cazzaniga, 1986; Studer et al., 2014; Wingeier et al., 2011).
4.4. Conclusions and Future Directions
Although it is difficult to draw definitive conclusions from the literature about specific periods of vulnerability due to heterogeneous groups and differing levels of control of relevant variables (Gingras & Braun, 2018; Montour-Proulx et al., 2004), this review yields several themes that could help inform future work and clinical care. Without question, age at lesion onset is a relevant variable for outcomes in many neuropsychological domains, especially when considered with other age and injury factors. While this review is not comprehensive with regard to all relevant factors, relevant age variables include age at testing, chronicity, and recovery over time and relevant injury variables include lesion laterality, location, and size, and a history of epilepsy. Future work should account for these variables and could emphasize a longitudinal approach to better elicit recovery profiles of brain injury.
A better understanding of the influence of age and injury variables on outcomes after focal brain injury could aid in distinguishing those individuals at higher risk for poor outcomes, influencing their treatment and recovery (O’Keeffe et al., 2004). For instance, a better understanding of the implications of age at lesion onset and other age and injury variables for outcomes could lead to better screening of children who sustain focal brain injury and specific guidelines for continued care to evaluate potential acquired deficits as they age (O’Keeffe et al., 2004). Existing guides for neuropsychological evaluation of children consider various predictors of outcomes and make recommendations for who, how, when, and what to assess (e.g., Dennis et al., 2014). For these neuropsychological guidelines to be most effective it is critical that individuals are referred for neuropsychological evaluation by healthcare providers (e.g., neurologists, neurosurgeons) after experiencing a brain injury. As such, an emphasis should be placed on interdisciplinary communication of these risk factors to various healthcare professionals integral to the care of individuals after focal brain injury. Although patients may significantly benefit from neuropsychological evaluation due to evidence of its incremental value for patient care, it is not the standard of care after all focal brain injuries, as has been recommended for some other illnesses and injuries (Annett et al., 2015; Donders, 2020; Ruble et al., 2019).
Persons may have misconceptions about the long-term impacts of brain injury, especially in children, given the common perception that children often recover well from physical injuries. Some studies have identified a common belief among providers that earlier brain injury is associated with better outcomes (e.g., Hart and Faust, 1988; Webb et al., 1996). These studies draw attention to an important over-simplification of the relation between age at lesion onset and outcomes. While there is evidence of resilience after early-onset brain injury, there is also evidence of potential risk for impairments after early brain injury. Importantly, underestimation of the effects of brain injury and inadequate access to information are known to serve as barriers to neuropsychological testing (Ruble et al., 2019). As a result, children may not be referred for neuropsychological evaluations until they have begun to exhibit significant impairments, leaving less room for neuropsychological expertise to collaborate in the rehabilitation process (Hardy et al., 2017). As individuals and especially children start to exhibit deficits after brain injury, patients and their families may feel underprepared and may have difficulty advocating for resources for themselves or their loved ones (Hardy et al., 2017; Trask et al., 2009). Neuropsychological evaluations can greatly help in this regard. The purpose of a neuropsychological evaluation is to identify cognitive strengths and weaknesses and to provide recommendations regarding difficulties and compensatory strategies (e.g., Donders, 2020). Specifically, deficits identified in neuropsychological evaluations have been shown to predict outcomes in relationships, education, employment, and SES (e.g., Donders, 2020). Further, recommendations provided at the end of a neuropsychological evaluation, including referrals to other providers (e.g., counseling, neuropsychological rehabilitation, occupational therapy, physical therapy, social work, academic counselors), could be incredibly helpful for domains such as school, social life, and extracurricular activities (Margelisch et al., 2015). Given the findings in this review and in the literature about the potential for worse cognitive outcomes following brain injury in early life when compared to later in life, an emphasis should be placed on integration of knowledge from multiple relevant fields (e.g., neuropsychology, counseling, neurology, neurosurgery, physical therapy) for holistic and impactful treatment of patients, with an eye towards assessing cognition after focal brain injury in early life.
Public Significance:
This systematic review indicates early-onset brain injury confers increased risk for worse cognitive outcomes in several neuropsychological domains when compared to brain injury occurring later in life.
Acknowledgements
Thank you to Kelly Hangauer for sharing expertise and providing guidance and help with the literature search process.
This research was supported in part by a National Institutes of Health Behavioral-Biomedical Interface T32 Predoctoral Training Grant (T32GM108540) from the National Institute of General Medical Sciences. The funding source was not involved in conducting this systematic review.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest: none.
References included in this review are denoted by *
References
- *.Allman C, & Scott RB (2013). Neuropsychological sequelae following pediatric stroke: A nonlinear model of age at lesion effects. Child Neuropsychology, 19(1), 97–107. 10.1080/09297049.2011.639756 [DOI] [PubMed] [Google Scholar]
- Anderson P (2002). Assessment and development of executive function (EF) during childhood. Child Neuropsychology, 8, 71–82. 10.1076/chin.8.2.71.8724 [DOI] [PubMed] [Google Scholar]
- Anderson P, Anderson V, & Lajoie G (1996). The Tower of London Test: Validation and standardization for pediatric populations. The Clinical Neuropsychologist, 10, 54–65. 10.1080/13854049608406663 [DOI] [Google Scholar]
- Anderson PJ, Anderson V, Northam E, & Taylor HG (2000). Standardization of the Contingency Naming Test (CNT) for school-aged children: A measure of reactive flexibility. In Standardization of the Contingency Naming Test (CNT) for school-aged children: A measure of reactive flexibility (Vol. 1, pp. 247–273). [Google Scholar]
- Anderson VA, Anderson P, Northam E, Jacobs R, & Catroppa C (2001). Development of executive functions through late childhood and adolescence in an Australian sample. Developmental neuropsychology, 20(1), 385–406. 10.1207/S15326942DN2001_5 [DOI] [PubMed] [Google Scholar]
- Anderson V, Catroppa C, Morse S, Haritou F, & Rosenfeld J (2005). Functional plasticity or vulnerability after early brain injury?. Pediatrics, 116(6), 1374–1382. 10.1542/peds.2004-1728 [DOI] [PubMed] [Google Scholar]
- *.Anderson V, Darling S, Mackay M, Monagle P, Greenham M, Cooper A, … & Gordon AL (2020). Cognitive resilience following paediatric stroke: Biological and environmental predictors. European Journal of Paediatric Neurology, 25, 52–58. 10.1016/j.ejpn.2019.11.011 [DOI] [PubMed] [Google Scholar]
- *.Anderson V, Jacobs R, Spencer-Smith M, Coleman L, Anderson P, Williams J, Greenham M, & Leventer R (2010). Does early age at brain insult predict worse outcome? Neuropsychological implications. Journal of Pediatric Psychology, 35(7), 716–727. 10.1093/jpepsy/jsp100 [DOI] [PubMed] [Google Scholar]
- Anderson VA, Morse SA, Catroppa C, Haritou F, & Rosenfeld JV (2004). Thirty month outcome from early childhood head injury: a prospective analysis of neurobehavioural recovery. Brain, 127(12), 2608–2620. 10.1093/brain/awh320 [DOI] [PubMed] [Google Scholar]
- *.Anderson VA, Spencer-Smith MM, Coleman L, Anderson PJ, Greenham M, Jacobs R, Lee KJ, & Leventer RJ (2014). Predicting neurocognitive and behavioural outcome after early brain insult. Developmental Medicine and Child Neurology, 56(4), 329–336. 10.1111/dmcn.12387 [DOI] [PubMed] [Google Scholar]
- *.Anderson V, Spencer-Smith M, Coleman L, Anderson P, Williams J, Greenham M, Leventer RJ, & Jacobs R (2010). Children’s executive functions: Are they poorer after very early brain insult. Neuropsychologia, 48(7), 2041–2050. 10.1016/j.neuropsychologia.2010.03.025 [DOI] [PubMed] [Google Scholar]
- *.Anderson V, Spencer-Smith M, Leventer R, Coleman L, Anderson P, Williams J, Greenham M, & Jacobs R (2009). Childhood brain insult: Can age at insult help us predict outcome? Brain, 132(1), 45–56. 10.1093/brain/awn293 [DOI] [PubMed] [Google Scholar]
- Anderson V, Spencer-Smith M, & Wood A (2011). Do children really recover better? Neurobehavioural plasticity after early brain insult. Brain, 134(8), 2197–2221. 10.1093/brain/awr103 [DOI] [PubMed] [Google Scholar]
- Annett RD, Patel SK, &Phipps S (2015). Monitoring and assessment of neuropsychological outcomes as a standard of care in pediatric oncology. Pediatric Blood & Cancer, 62(S5), S460–S513. 10.1002/pbc.25749. [DOI] [PubMed] [Google Scholar]
- *.Aram DM, & Eisele JA (1994). Intellectual stability in children with unilateral brain lesions. Neuropsychologia, 32(1), 85–95. 10.1016/0028-3932(94)90071-X [DOI] [PubMed] [Google Scholar]
- *.Aram DM, & Ekelman BL (1986). Cognitive Profiles of Children With Early Onset of Unilateral Lesions. Developmental Neuropsychology, 2(3), 155–172. 10.1080/87565648609540339 [DOI] [Google Scholar]
- *.Aram DM, & Ekelman BL (1987). Unilateral brain lesions in childhood: Performance on the revised token test. Brain and Language, 32(1), 137–158. 10.1016/0093-934X(87)90121-0 [DOI] [PubMed] [Google Scholar]
- *.Aram DM, & Ekelman BL (1988). Scholastic aptitude and achievement among children with unilateral brain lesions. Neuropsychologia, 26(6), 903–916. 10.1016/0028-3932(88)90058-9 [DOI] [PubMed] [Google Scholar]
- Arain M, Haque M, Johal L, Mathur P, Nel W, Rais A, … & Sharma S (2013).Maturation of the adolescent brain. Neuropsychiatric disease and treatment, 9, 449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschenbrenner S, Tucha O, & Lange KW (2000). RWT: Regensburger Wortflüssigkeits-Test Hogrefe. [Google Scholar]
- Babikian T, & Asarnow R (2009). Neurocognitive outcomes and recovery after pediatric TBI: meta-analytic review of the literature. Neuropsychology, 23(3), 283. 10.1037/a0015268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballantyne A, Spilkin A, Hesselink J, & Trauner D (2008). Plasticity in the developing brain: intellectual, language and academic functions in children with ischaemic perinatal stroke. Brain, 131, 2975–2985. 10.1093/brain/awn176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Banich MT, Levine SC, Kim H, & Huttenlocher P (1990). The effects of developmental factors on IQ in hemiplegic children. Neuropsychologia, 28(1), 35–47. 10.1016/0028-3932(90)90084-2 [DOI] [PubMed] [Google Scholar]
- *.Bartha-Doering L, Novak A, Kollndorfer K, Schuler AL, Kasprian G, Langs G, … & Seidl, R. (2019). Atypical language representation is unfavorable for language abilities following childhood stroke. European Journal of Paediatric Neurology, 23(1), 102–116. 10.1016/j.ejpn.2018.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Bartha-Doering L, Gleiss A, Knaus S, Schmook MT, & Seidl R (2021). Influence of socioeconomic status on cognitive outcome after childhood arterial ischemic stroke. Developmental Medicine & Child Neurology, 63(4), 465–471. 10.1111/dmcn.14779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basser L, S. (1962). Hemiplegia of early onset and the faculty of speech with special reference to the effects of hemispherectomy. Brain, 85, 427–460. 10.1093/brain/85.3.427 [DOI] [PubMed] [Google Scholar]
- Bates E (1999). Plasticity, localization and language development. In Broman SH & Fletcher JM (Eds.), The changing nervous system: Neurobehavioral consequences of early brain disorders (pp. 214–253). Oxford University Press. [Google Scholar]
- Bates E, & Roe K (2001). Language development in children with unilateral brain injury. In Nelson CA & Luciana M (Eds.), Handbook of developmental cognitive neuroscience (pp. 281). MIT Press. [Google Scholar]
- Bayley N (1993). Bayley Scales for Infant Development (BSID-II), 2nd ed.The Psychological Corportation. [Google Scholar]
- Bayley N (2006). Bayley scales of infant and toddler development The Psychological Corporation, Pearson. [Google Scholar]
- Beery KE (2004). Beery VMI: The Beery-Buktenica developmental test of visual-motor integration (6th ed.). Pearson.
- Bender L, Koppitz EM, & Lagos CJ (1938). Instructions for the Use of the Visual Motor Gestalt Test. The Bender Gestalt Test for Young Children: Volume II, Research and Application, 1963–1973 American Orthopsychiatric Association. [Google Scholar]
- Benton AL, Hamsher KS, Sivan AB (1994). Multilingual Aphasia Examination (3rd ed.). AJA Associates. [Google Scholar]
- Bigler ED (2017). Structural neuroimaging in neuropsychology: History and contemporary applications. Neuropsychology, 31(8), 934–953. 10.1037/neu0000418 [DOI] [PubMed] [Google Scholar]
- Bishop DVM (1989). TROG Test for Reception of Grammar Manchester: University of Manchester. [Google Scholar]
- Bjorklund DF, & Ellis BJ (2014). Children, childhood, and development in evolutionary perspective. Developmental Review, 34(3), 225–264. 10.1016/j.dr.2014.05.005 [DOI] [Google Scholar]
- *.Block GW, Nanson JL, & Lowry NJ (1999). Attention, memory, and language after pediatric ischemic stroke. Child Neuropsychology, 5(2), 81–91. 10.1076/chin.5.2.81.3169 [DOI] [Google Scholar]
- *.Braun CM, Montour-Proulx I, Daigneault S, Rouleau I, Kuehn S, Piskopos M, … & Rainville C (2000). Prevalence, and intellectual outcome of unilateral focal cortical brain damage as a function of age, sex and aetiology. Behavioural neurology, 13(3, 4), 105–116. http://www.er.uqam.ca/nobel/r31210/home.html. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslau N (1990). Does brain dysfunction increase children’s vulnerability to environmental stress?. Archives of General Psychiatry, 47(1), 15–20. doi: 10.1001/archpsyc.1990.01810130017003. [DOI] [PubMed] [Google Scholar]
- Brickenkamp R, Zillmer E (1998). The d2 Test of Attention Hogrefe & Huber Publisher. [Google Scholar]
- Carrera E, & Tononi G (2014). Diaschisis: past, present, future. Brain, 137(9), 2408–2422. 10.1093/brain/awu101 [DOI] [PubMed] [Google Scholar]
- Casey B, Giedd J, & Thomas K (2000). Structural and functional brain development and its relation to cognitive development. Biological Psychology, 54, 241–257. 10.1016/S0301-0511(00)00058-2 [DOI] [PubMed] [Google Scholar]
- Catroppa C, Anderson V, & Stargatt R (1999). A prospective analysis of the recovery of attention following pediatric head injury. Journal of the International Neuropsychological Society, 5(1), 48–57. 10.1017/S1355617799511077 [DOI] [PubMed] [Google Scholar]
- Cohen J (1988). Statistical Power Analysis for the Behavioral Sciences New York, NY: Routledge Academic. [Google Scholar]
- Cohen M (1997). Children’s Memory Scale. Administration Manual The Psychological Corporation. [Google Scholar]
- Danguecan AN, & Smith ML (2019). Re-examining the crowding hypothesis in pediatric epilepsy. Epilepsy & Behavior, 94, 281–287. 10.1016/j.yebeh.2019.01.038 [DOI] [PubMed] [Google Scholar]
- *.de Montferrand C, Vassel-Hitier J, Yvon-Chaou E, Câmara-Costa H, Dellatolas G, & Chevignard M (2019). Language and cognitive outcomes after childhood stroke: Theoretical implications for hemispheric specialization. Cortex, 120, 509–523. 10.1016/j.cortex.2019.07.020 [DOI] [PubMed] [Google Scholar]
- Delis DC, Kaplan E, & Kramer JH (2001). Delis-Kaplan executive function system Pearson. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Edith K, & Ober BA (1994). California verbal learning test children’s version (CVLT-C): Manual Pearson Inc. [Google Scholar]
- Dennis M (1988). Language and the young damaged brain. In Boll T & Bryant BK (Eds.), The Master lecture series, Vol. 7. Clinical neuropsychology and brain function: Research, measurement, and practice (pp. 89–123). American Psychological Association. 10.1037/10063-003 [DOI] [Google Scholar]
- Dennis M (1998). Discourse in children with neurodevelopmental disorder, early focal brain injury, or childhood acquired brain injury. Brain and language, 61(3), 305–307. 10.1006/brln.1997.1881 [DOI] [PubMed] [Google Scholar]
- Dennis M (2010). Margaret Kennard (1899–1975): not a ‘principle’of brain plasticity but a founding mother of developmental neuropsychology. Cortex, 46(8), 1043–1059. 10.1016/j.cortex.2009.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis M, Spiegler BJ, Simic N, Sinopoli KJ, Wilkinson A, Yeates KO, … & Fletcher JM (2014). Functional plasticity in childhood brain disorders: when, what, how, and whom to assess. Neuropsychology review, 24(4), 389–408. 10.1007/s11065-014-9261-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A (2002). Normal development of prefrontal cortex from birth to young adulthood: Cognitive functions, anatomy, and biochemistry. In Stuss DT & Knight RT (Eds.), Principles of frontal lobe function (pp. 466–503). Oxford University Press. 10.1093/acprof:oso/9780195134971.001.0001 [DOI] [Google Scholar]
- Donders J (2020). The incremental value of neuropsychological assessment: A critical review. The Clinical Neuropsychologist, 34(1), 56–87. 10.1080/13854046.2019.1575471. [DOI] [PubMed] [Google Scholar]
- Dunn DM, & Dunn LM (2007). Peabody Picture Vocabulary Test, Fourth Edition, Manual Pearson. [Google Scholar]
- *.Duval J, Braun CMJ, Montour-Proulx I, Daigneault S, Rouleau I, & Bégin J (2008). Brain lesions and IQ: Recovery versus decline depends on age of onset. Journal of Child Neurology, 23(6), 663–668. 10.1177/0883073808314161 [DOI] [PubMed] [Google Scholar]
- *.Duval J, Dumont M, Braun CM, & Montour-Proulx I (2002). Recovery of intellectual function after a brain injury: A comparison of longitudinal and cross-sectional approaches. Brain and cognition, 48(2–3), 337–342. https://pubmed.ncbi.nlm.nih.gov/12030463/ [PubMed] [Google Scholar]
- Elliott CD (1990). Differential Ability Scales Psychological Corporation. [Google Scholar]
- Elliott CD (2007). Differential Ability Scales – Second Edition (DAS-II) Psychological Corporation. [Google Scholar]
- Elliot CD (1983). The British Ability Scales NFER-Nelson. [Google Scholar]
- Eslinger PJ, & Grattan LM (1991). Perspectives on the developmental consequences of early frontal lobe damage: Introduction. Developmental Neuropsychology, 7(3), 257–260. 10.1080/87565649109540493 [DOI] [Google Scholar]
- *.Everts R, Pavlovic J, Kaufmann F, Uhlenberg B, Seidel U, Nedeltchev K, … & Steinlin M (2008). Cognitive functioning, behavior, and quality of life after stroke in childhood. Child Neuropsychology, 14(4), 323–338. 10.1080/09297040701792383 [DOI] [PubMed] [Google Scholar]
- Ewing-Cobbs L, Barnes MA, & Fletcher JM (2003). Early brain injury in children: development and reorganization of cognitive function. Developmental neuropsychology, 24(2–3), 669–704. 10.1080/87565641.2003.9651915 [DOI] [PubMed] [Google Scholar]
- Ewing-Cobbs L, Miner ME, Fletcher JM, & Levin HS (1989). Intellectual, motor, and language sequelae following closed head injury in infants and preschoolers. Journal of pediatric psychology, 14(4), 531–547. 10.1093/jpepsy/14.4.531 [DOI] [PubMed] [Google Scholar]
- Finger S, & Almli CR (1985). Brain damage and neuroplasticity: mechanisms of recovery or development?. Brain Research Reviews, 10(3), 177–186. 10.1016/0165-0173(85)90023-2 [DOI] [PubMed] [Google Scholar]
- Finger S, & Almli CR (1988). Margaret Kennard and her “principle” in historical perspective. In Brain injury and recovery (pp. 117–132). Springer, Boston, MA. [Google Scholar]
- Finger S, Walbran B, & Stein DG (1973). Brain damage and behavioral recovery: Serial lesion phenomena, Brain Research, 63, 1–18. 10.1016/0006-8993(73)90072-3 [DOI] [PubMed] [Google Scholar]
- Fox AV (2006). TROG-D: Test zur Überprüfung des Grammatikverständnisses.[Test for reception of grammar-German version] Schulz-Kirchner. [Google Scholar]
- Fuentes A, Deotto A, Desrocher M, deVeber G, & Westmacott R (2016). Determinants of cognitive outcomes of perinatal and childhood stroke: A review. Child Neuropsychology, 22(1), 1–38. 10.1080/09297049.2014.969694 [DOI] [PubMed] [Google Scholar]
- *.Fuentes A, Westmacott R, Deotto A, deVeber G, & Desrocher M (2017). Working memory outcomes following unilateral arterial ischemic stroke in childhood. Child Neuropsychology, 23(7), 803–821. 10.1080/09297049.2016.1205008 [DOI] [PubMed] [Google Scholar]
- Giedd JN, Snell JW, Lange N, Rajapakse JC, Casey BJ, Kozuch PL, … & Rapoport JL (1996). Quantitative magnetic resonance imaging of human brain development: ages 4–18. Cerebral cortex, 6(4), 551–559. 10.1093/cercor/6.4.551 [DOI] [PubMed] [Google Scholar]
- *.Gingras B, & Braun CMJ (2018). Intellectual outcome after a cortical lesion with versus without epilepsy: A life span neurodevelopmental view. Epilepsy and Behavior, 85, 129–140. 10.1016/j.yebeh.2018.05.045 [DOI] [PubMed] [Google Scholar]
- Gioia GA, Isquith PK, Guy SC, & Kenworthy L (2000). Behavior rating inventory of executive function: BRIEF Psychological Assessment Resources. [Google Scholar]
- Giza C, & Prins M (2006). Is being plastic fantastic? Mechanisms of altered plasticity after developmental traumatic brain injury. Developmental Neuroscience, 28, 364–379. 10.1159/000094163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glück CW, & Glück CW (2011). Wortschatz-und Wortfindungstest für 6-bis 10-Jährige (WWT 6–10) Urban & Fischer. [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, … & Thompson PM (2004). Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences, 101(21), 8174–8179. 10.1073/pnas.0402680101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes A, Rinehart N, Greenham M, & Anderson V (2014). A critical review of psychosocial outcomes following childhood stroke (1995–2012). Developmental neuropsychology, 39(1), 9–24. 10.1080/87565641.2013.827197 [DOI] [PubMed] [Google Scholar]
- Goodman R (1989). Neuronal misconnections and psychiatric disorder: Is there a link?. The British Journal of Psychiatry, 154(3), 292–299. 10.1192/bjp.154.3.292 [DOI] [PubMed] [Google Scholar]
- Goodman R (1991). Developmental disorders and structural brain development. In Rutter M & Casaer P (Eds.), Biological risk factors for psychosocial disorders (pp. 20–49). Cambridge University Press. [Google Scholar]
- *.Goodman R, & Yude C (1996). IQ and its predictors in childhood hemiplegia. Developmental Medicine and Child Neurology, 38(10), 881–890. 10.1111/j.1469-8749.1996.tb15045.x [DOI] [PubMed] [Google Scholar]
- *.Gordon AL, Anderson V, Ditchfield M, Coleman L, Mackay MT, Greenham M, Hunt RW, & Monagle P (2015). Factors associated with six-month outcome of pediatric stroke. International Journal of Stroke, 10(7), 1068–1073. 10.1111/ijs.12489 [DOI] [PubMed] [Google Scholar]
- Grant DA, & Berg E (1948). A behavioral analysis of degree of reinforcement and ease of shifting to new responses in a Weigl-type card-sorting problem. Journal of experimental psychology, 38(4), 404. 10.1037/h0059831 [DOI] [PubMed] [Google Scholar]
- *.Hajek CA, Yeates KO, Anderson V, Mackay M, Greenham M, Gomes A, & Lo W (2014). Cognitive outcomes following arterial ischemic stroke in infants and children. Journal of Child Neurology, 29(7), 887–894. 10.1177/0883073813491828 [DOI] [PubMed] [Google Scholar]
- Hardy KK, Olson K, Cox SM, Kennedy T, &Walsh KS (2017). Systematic review: A prevention-based model of neuropsychological assessment for children with medical illness. Journal of Pediatric Psychology, 42(8), 815–822. 10.1093/jpepsy/jsx060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harshman LA, Barron S, Button AM, Smith BJ, Link BK, Lynch CF, & Denburg NL (2012). Population-based exploration of academic achievement outcomes in pediatric acute lymphoblastic leukemia survivors. Journal of pediatric psychology, 37(4), 458–466. 10.1093/jpepsy/jsr119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart K, & Faust D (1988). Prediction of the effects of mild head injury: a message about the Kennard Principle. Journal of Clinical Psychology, 44(5), 780–782. [DOI] [PubMed] [Google Scholar]
- Hebb DO (1942). The effect of early and late brain injury upon test scores, and the nature of normal adult intelligence. Proceedings of the American Philosophical Society, 275–292. https://www.jstor.org/stable/985007
- Hebb D, O. (1949). The organization of behaviour John Wiley & Sons Inc. [Google Scholar]
- Helmstaedter C, Lendt M, & Lux S (2001). Verbaler Lern-und Merkfähigkeitstest: VLMT; Manual Beltz-test. [Google Scholar]
- Hicks SP, D’Amato CJ, & Glover RA (1984). Recovery or malformation after fetal radiation and other injuries. In Almi CR & Finger S (Eds.), Early Brain Damage (pp. 127–147). Academic Press. [Google Scholar]
- Hogan AM, Kirkham FJ, & Isaacs EB (2000). Intelligence after stroke in childhood: review of the literature and suggestions for future research. Journal of child neurology, 15(5), 325–332. 10.1177/088307380001500509 [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR (1979). Synaptic density in human frontal cortex-developmental changes and effects of aging. Brain Research, 163(2), 195–205. 10.1016/0006-8993(79)90349-4 [DOI] [PubMed] [Google Scholar]
- *.Ilves P, Tomberg T, Kepler J, Laugesaar R, Kaldoja ML, Kepler K, & Kolk A (2014). Different plasticity patterns of language function in children with perinatal and childhood stroke. Journal of child neurology, 29(6), 756–764. 10.1177/0883073813489350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Isaacs E, Christie D, Vargha-Khadem F, & Mishkin M (1996). Effects of hemispheric side of injury, age at injury, and presence of seizure disorder on functional ear and hand asymmetries in hemiplegic children. Neuropsychologia, 34(2), 127–137. 10.1016/0028-3932(95)00089–5 [DOI] [PubMed] [Google Scholar]
- Isaacson RL (1975). The myth of recovery from early brain damage. In Ellis NR (Ed.), Aberrant development in infancy: Human and animal studies, 1. Routledge. [Google Scholar]
- Jacobs RK, Anderson VA, Neale JL, Shield LK, & Kornberg AJ (2004). Neuropsychological outcome after acute disseminated encephalomyelitis: impact of age at illness onset. Pediatric neurology, 31(3), 191–197. 10.1016/j.pediatrneurol.2004.03.008 [DOI] [PubMed] [Google Scholar]
- *.Jacobs R, Harvey SA, & Anderson V (2007). Executive Function Following Focal Frontal Lobe Lesions: Impact of Timing of Lesion on Outcome. Cortex, 43, 792–805. 10.1016/S0010-9452(08)70507-0 [DOI] [PubMed] [Google Scholar]
- Jacobsen CF, Taylor FV, Haslerud GM (1936). Restitution of function after cortical injury in monkeys. Proceedings of the American Physiological Society Forty-Eighth Annual Meeting, The American Journal of Physiology, 116, 85–86 [Google Scholar]
- *.Jacomb I, Porter M, Brunsdon R, Mandalis A, & Parry L (2018). Cognitive outcomes of pediatric stroke. Child Neuropsychology, 24(3), 287–303. 10.1080/09297049.2016.1265102 [DOI] [PubMed] [Google Scholar]
- Johnson MH (2005). Sensitive periods in functional brain development: Problems and prospects. Developmental Psychobiology: The Journal of the International Society for Developmental Psychobiology, 46(3), 287–292. 10.1002/dev.20057 [DOI] [PubMed] [Google Scholar]
- Kaufman AS (1975). Factor analysis of the WISC-R at 11 age levels between 61/2 and 161/2 years. Journal of Consulting and Clinical Psychology, 43(2), 135. 10.1037/h0076502 [DOI] [PubMed] [Google Scholar]
- Kaufman AS (2004). Kaufman brief intelligence test–Second Edition American Guidance Service. [Google Scholar]
- Kaufman AS, & Kaufman NL (1983). Kaufman Assessment Battery for Children Circle Pines, MN: American Guidance Service [Google Scholar]
- Keenan HT, Hooper SR, Wetherington CE, Nocera M, & Runyan DK (2007). Neurodevelopmental consequences of early traumatic brain injury in 3-year-old children. Pediatrics, 119(3), e616–e623. 10.1542/peds.2006-2313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennard MA (1938). Reorganization of motor function in the cerebral cortex of monkeys deprived of motor and premotor areas in infancy. Journal of Neurophysiology, 1(6), 477–496. 10.1152/jn.1938.1.6.477 [DOI] [Google Scholar]
- Kennard MA (1940). Relation of age to motor impairment in man and in subhuman primates. Archives of Neurology & Psychiatry, 44(2), 377–397. 10.1001/archneurpsyc.1940.02280080137008 [DOI] [Google Scholar]
- Kennard MA (1942). Cortical reorganization of motor function: studies on series of monkeys of various ages from infancy to maturity. Archives of Neurology & Psychiatry, 48(2), 227–240. 10.1001/archneurpsyc.1942.02290080073002 [DOI] [Google Scholar]
- Kennard MA, & Fulton JF (1942). Age and reorganization of the central nervous system. Journal of Mount Sinai Hospital, 9, 594–606. [Google Scholar]
- Kirton A, Westmacott R, & deVeber G (2007). Pediatric stroke: Rehabilitation of focal injury in the developing brain. NeuroRehabilitation, 22(5), 371–382. 10.3233/NRE-2007-22504 [DOI] [PubMed] [Google Scholar]
- Kolb B (1995). Brain plasticity and behavior Psychology Press. [DOI] [PubMed] [Google Scholar]
- Kolb B, & Gibb R (1993). Possible anatomical basis of recovery of function after neonatal frontal lesions in rats. Behavioral neuroscience, 107(5), 799. 10.1037/0735-7044.107.5.799 [DOI] [PubMed] [Google Scholar]
- Kolb B, Gorny G, & Gibb R (1994). Tactile stimulation enhances recovery and dendritic growth in rats with neonatal frontal lesions. Soc Neurosci Abstr, 20, 1430. [Google Scholar]
- Kolb B, Pellis S, & Robinson TE (2004). Plasticity and functions of the orbital frontal cortex. Brain and cognition, 55(1), 104–115. 10.1016/S0278-2626(03)00278-1 [DOI] [PubMed] [Google Scholar]
- Kolk A, & Talvik T (2000). Cognitive outcome of children with early-onset hemiparesis. Journal of child neurology, 15(9), 581–587. 10.1177/088307380001500903 [DOI] [PubMed] [Google Scholar]
- Korkman M, Kirk U, Kemp SL (1998). NEPSY: A Developmental Neuropsychologic Assessment. Psychological Corporation and Harcourt Brace; 1998.) adapted by Kolk, A., Talvik, T. Cognitive outcome of children with early-onset hemiparesis. J Child Neurol 2000;15:581–587 to the Estonian language. [Google Scholar]
- Korkman M, Kirk U, & Kemp SL (2007). NEPSY II. Administrative Manual Psychological Corporation. [Google Scholar]
- *.Kornhuber HH, Bechinger D, Jung H, & Sauer E (1985). A quantitative relationship between the extent of localized cerebral lesions and the intellectual and behavioural deficiency in children. European archives of psychiatry and neurological sciences, 235(3), 129–133. 10.1007/BF00380981 [DOI] [PubMed] [Google Scholar]
- Kirton A, Westmacott R, & deVeber G (2007). Pediatric stroke: Rehabilitation of focal injury in the developing brain. NeuroRehabilitation, 22(5), 371–382. 10.3233/NRE-2007-22504 [DOI] [PubMed] [Google Scholar]
- *.Lansing AE, Max JE, Delis DC, Fox PT, Lancaster J, Manes FF, & Schatz A (2004). Verbal learning and memory after childhood stroke. Journal of the InternationalNeuropsychological Society, 10(5), 742–752. 10.1017/S1355617704105122 [DOI] [PubMed] [Google Scholar]
- Lenneberg EH (1967). The biological foundations of language. Hospital Practice, 2(12), 59–67. 10.1080/21548331.1967.11707799 [DOI] [Google Scholar]
- *.Levine SC, Huttenlocher P, Banich MT, & Duda E (1987). Factors affecting cognitive functioning of hemiplegic children. Developmental Medicine & Child Neurology, 29(1), 27–35. 10.1111/j.1469-8749.1987.tb02104.x [DOI] [PubMed] [Google Scholar]
- Lezak MD, Howieson DB, Bigler ED, & Tranel D (2012). Neuropsychological Assessment (5th ed.). Oxford University Press. [Google Scholar]
- Lidzba K, Wilke M, Staudt M, & Krägeloh-Mann I (2009). Early plasticity versus early vulnerability: the problem of heterogeneous lesion types. Brain, 132(10), e128–e128. 10.1093/brain/awp197 [DOI] [PubMed] [Google Scholar]
- Lidzba K, Küpper H, Kluger G, & Staudt M (2017). The time window for successful right-hemispheric language reorganization in children. European journal of paediatric neurology, 21(5), 715–721. 10.1016/j.ejpn.2017.06.001 [DOI] [PubMed] [Google Scholar]
- *.Long B, Spencer-Smith MM, Jacobs R, Mackay M, Leventer R, Barnes C, & Anderson V (2011). Executive function following child stroke: The impact of lesion location. Journal of Child Neurology, 26(3), 279–287. 10.1177/0883073810380049 [DOI] [PubMed] [Google Scholar]
- Luciana M (2003). Cognitive development in children born preterm: Implications for theories of brain plasticity following early injury. Development and Psychopathology, 15, 1017–1047. 10.1017/S095457940300049X [DOI] [PubMed] [Google Scholar]
- Mallat M, & Chamak B (1994). Brain macrophages: neurotoxic or neurotrophic effector cells?. Journal of leukocyte biology, 56(3), 416–422. 10.1002/jlb.56.3.416 [DOI] [PubMed] [Google Scholar]
- Malone LA, & Felling RJ (2020). Pediatric stroke: unique implications of the immature brain on injury and recovery. Pediatric neurology, 102, 3–9. 10.1016/j.pediatrneurol.2019.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manly T, Anderson V, Nimmo‐Smith I, Turner A, Watson P, & Robertson IH (2001). The differential assessment of children’s attention: The Test of Everyday Attention for Children (TEA-Ch), normative sample and ADHD performance. Journal of Child Psychology and Psychiatry, 42(8), 1065–1081. 10.1111/1469-7610.00806 [DOI] [PubMed] [Google Scholar]
- Margelisch K, Studer M, Ritter BC, Steinlin M, Leibundgut K, & Heinks T (2015). Cognitive dysfunction in children with brain tumors at diagnosis. Pediatric Blood & Cancer, 62(10), 1805–1812. 10.1002/pbc.25596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Max JE, Margaret Bruce Mbbc., Eva Keatley B, & Dean Delis B (2010). Pediatric Stroke: Plasticity, Vulnerability, and Age of Lesion Onset. The Journal of Neuropsychiatry and Clinical Neurosciences, 22. http://neuro.psychiatryonline.org [DOI] [PubMed] [Google Scholar]
- McGhee RL, Ehrler DJ, & DiSimoni F (2007). Token test for children (TTFC–2) Pro-Ed. McNeil MR, & Prescott TE (1978). Revised Token Test. Pro-Ed, Inc. [Google Scholar]
- Merz EC, Wiltshire CA, & Noble KG (2019). Socioeconomic inequality and the developing brain: Spotlight on language and executive function. Child Development Perspectives, 13(1), 15–20. 10.1111/cdep.12305 [DOI] [Google Scholar]
- Meyers JE, Meyers KR (1995). Rey Complex Figure Test and Recognition Trial: Professional Manual Psychological Assessment Resources. [Google Scholar]
- Moll K, & Landerl K (2010). Lese-und Rechtschreibtest (SLRT-II). Weiterentwicklung des Salzburger Lese-und Rechtschreibtests (SLRT), 2. [Google Scholar]
- *.Montour-Proulx I, Braun CMJ, Daigneault S, Rouleau I, Kuehn S, & Bégin J (2004). Predictors of intellectual function after a unilateral cortical lesion: Study of 635 patients from infancy to adulthood. Journal of Child Neurology, 19(12), 935–943. 10.1177/08830738040190120501 [DOI] [PubMed] [Google Scholar]
- *.Mosch C,Max J, & Tranel D (2005). A matched lesion analysis of childhood versus adult-onset brain injury due to unilateral stroke: Another perspective on neural plasticity and recovery of social functioning. Cognitive and Behavioral Neurology, 18(1), 5–17. 10.1097/01.wnn.0000152207.80819.3c [DOI] [PubMed] [Google Scholar]
- Noble KG, Norman MF, & Farah MJ (2005). Neurocognitive correlates of socioeconomic status in kindergarten children. Developmental Science, 8(1), 74–87. 10.1111/j.1467-7687.2005.00394.x [DOI] [PubMed] [Google Scholar]
- Noble KG, McCandliss BD, & Farah MJ (2007). Socioeconomic gradients predict individual differences in neurocognitive abilities. Developmental Science, 10, 464–480. 10.1111/j.1467-7687.2007.00600.x. [DOI] [PubMed] [Google Scholar]
- *.O’Keeffe F, Liégeois F, Eve M, Ganesan V, King J, & Murphy T (2014). Neuropsychological and neurobehavioral outcome following childhood arterial ischemic stroke: Attention deficits, emotional dysregulation, and executive dysfunction. Child Neuropsychology, 20(5), 557–582. 10.1080/09297049.2013.832740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC, & Wingfield A (1964). The time it takes to name an object. Nature, 202(4936), 1031–1032. [DOI] [PubMed] [Google Scholar]
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, … & Moher D (2021). The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Bmj, 372. 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Pavlovic J, Kaufmann F, Boltshauser E, Mori AC, Mercati DG, Haenggeli CA, … & Steinlin M (2006). Neuropsychological problems after paediatric stroke: two year follow-up of Swiss children. Neuropediatrics, 37(01), 13–19. 10.1055/s-2006-923932 [DOI] [PubMed] [Google Scholar]
- Petermann F, Petermann U (2010). Hamburg-Wechsler-Intelligenztest für Kinder IV (HAWIK IV) Huber. [Google Scholar]
- *.Peterson RK, Williams TS, McDonald KP, Dlamini N, & Westmacott R (2019). Cognitive and academic outcomes following childhood cortical stroke. Journal of child neurology, 34(14), 897–906. 10.1177/0883073819866609 [DOI] [PubMed] [Google Scholar]
- Pickering S, & Gathercole S (2001). Working Memory Test Battery for Children (WMBT-C) Manual Pearson. [Google Scholar]
- Porteus SD (1950). The Porteus Maze Test and intelligence Pacific Books. [Google Scholar]
- Rey A (1941). L’examine psycholgique dans les cas d’encephalopathie traumatique. Archives de Psycholgique, 28, 240–286. [Google Scholar]
- Rey A (1964). L’Examen Clinique en Psychologie. Paris: Press Universitaire de France. Gavett, BE, & Horwitz, JE (2012). Immediate list recall as a measure of short-term episodic memory: Insights from the serial position effect and item response theory. Archives of Clinical Neuropsychology, 27, 125–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Riva D, & Cazzaniga L (1986). Late effects of unilateral brain lesions sustained before and after age one. Neuropsychologia, 24(3), 423–428. 10.1016/0028-3932(86)90029-1 [DOI] [PubMed] [Google Scholar]
- Rivella C, & Viterbori P (2021). Executive function following pediatric stroke. A systematic review. Child Neuropsychology, 27(2), 209–231. 10.1080/09297049.2020.1820472. [DOI] [PubMed] [Google Scholar]
- Ruble K, Paré-Blagoev J, Cooper S, Martin A, & Jacobson LA (2019). Parent perspectives on oncology team communication regarding neurocognitive impacts of cancer therapy and school reentry. Pediatric Blood & Cancer, 66(1), e27427. 10.1002/pbc.27427 [DOI] [PubMed] [Google Scholar]
- Sacco RL, Kasner SE, Broderick JP, Caplan LR, Connors JJ, Culebras A, … & Vinters HV (2013). An updated definition of stroke for the 21st century: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke, 44(7), 2064–2089. 10.1161/STR.0b013e318296aeca [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider GE (1979). Is it really better to have your brain lesion early? A revision of the “Kennard principle”. Neuropsychologia, 17(6), 557–583. 10.1016/0028-3932(79)90033-2 [DOI] [PubMed] [Google Scholar]
- Semel E, Wiig E, & Secord W (1995). Clinical evaluation of language fundamentals (3rd ed.). Psychological Corporation. [Google Scholar]
- Semel EM, Wiig EH, & Secord WA (2003). Clinical Evaluation of Language Fundamentals (4th ed). The Psychological Corporation. [Google Scholar]
- Shallice T (1982). Specific impairments of planning. Philosophical Transactions of the Royal Society of London. B, Biological Sciences, 298(1089), 199–209. 10.1098/rstb.1982.0082 [DOI] [PubMed] [Google Scholar]
- Sheslow D, & Adams W (2003). Wide range assessment of memory and learning (2nd ed.). Psychological Assessment Resources, Inc. [Google Scholar]
- *.Spencer-Smith M, Anderson P, Jacobs R, Coleman L, Long B, & Anderson V (2011). Does timing of brain lesion have an impact on children’s attention? Developmental Neuropsychology, 36(3), 353–366. 10.1080/87565641.2010.549983 [DOI] [PubMed] [Google Scholar]
- Spreen O, Strauss EA (1998). Compendium of Neuropsychological Tests: Administration, Norms and Commentary Oxford Univeristy Press. [Google Scholar]
- Stein DG, & Hoffman SW (2003). Concepts of CNS plasticity in the context of brain damage and repair. The Journal of head trauma rehabilitation, 18(4), 317–341. 10.1097/00001199-200307000-00004 [DOI] [PubMed] [Google Scholar]
- Stiles J, Reilly JS, Levine SC, Trauner DA, & Nass R (2012). Neural plasticity and cognitive development: Insights from children with perinatal brain injury Oxford University Press. [Google Scholar]
- *.Studer M, Boltshauser E, Mori AC, Datta A, Fluss J, Mercati D, … & Steinlin M (2014). Factors affecting cognitive outcome in early pediatric stroke. Neurology, 82(9), 784–792. 10.1212/WNL.0000000000000162 [DOI] [PubMed] [Google Scholar]
- Stuss DT, & Benson DF (1986). The frontal lobes Raven Press. [Google Scholar]
- Taylor H (1984). Early brain injury and cognitive development. In Almli C, & Finger S (Ed.), Early brain damage: Research orientations and clinical observations (pp. 325–345). NewYork: Academic Press [Google Scholar]
- Taylor HG, & Alden J (1997). Age-related differences in outcomes following childhood brain insults: an introduction and overview. Journal of the international Neuropsychological Society, 3(6), 555–567. 10.1017/S1355617797005559. [DOI] [PubMed] [Google Scholar]
- Teuber HL (1974). Functional recovery after lesions of the nervous system. II. Recovery of function after lesions of the central nervous system: history and prospects. Neurosciences Research Program Bulletin, 12(2), 197–211. [PubMed] [Google Scholar]
- Teuber HL (1975) Recovery of function after brain injury in man. In Porter R & Fitzsimons DW (Eds.), Outcome of Severe Damage to the Central Nervous System (pp. 159–186). Elsevier. [Google Scholar]
- Thomas MS, & Johnson MH (2008). New advances in understanding sensitive periods in brain development. Current directions in psychological science, 17(1), 1–5. 10.1111/j.1467-8721.2008.00537.x [DOI] [Google Scholar]
- Tombaugh TN, Kozak J, & Rees L (1999). Normative data stratified by age and education for two measures of verbal fluency: FAS and animal naming. Archives of clinical neuropsychology, 14(2), 167–177. [PubMed] [Google Scholar]
- Tombaugh TN (2004). Trail Making Test A and B: normative data stratified by age and education. Archives of clinical neuropsychology, 19(2), 203–214. [DOI] [PubMed] [Google Scholar]
- Tompkins CA (1990). Knowledge and strategies for processing lexical metaphor after right or left hemisphere brain damage. Journal of Speech, Language, and Hearing Research, 33(2), 307–316. 10.1044/jshr.3302.307 [DOI] [PubMed] [Google Scholar]
- Trask CL, Welch JJG, Manley P, Jelalian E, &Schwartz CL (2009). Parental needs for information related to neurocognitive late effects from pediatric cancer and its treatment. Pediatric Blood & Cancer, 52(2), 273–279. [DOI] [PubMed] [Google Scholar]
- Vargha-Khadem F, Isaacs E, & Muter V (1994). A review of cognitive outcome after unilateral lesions sustained during childhood. Journal of Child Neurology, 9(2_suppl), 2S67–2S73. 10.1177/0883073894009002101 [DOI] [PubMed] [Google Scholar]
- Vargha-Khadem F, Isaacs E, Van Der Werf SI, Robb S, & Wilson J (1992). Development of intelligence and memory in children with hemiplegic cerebral palsy: The deleterious consequences of early seizures. Brain, 115(1), 315–329. 10.1093/brain/115.1.315 [DOI] [PubMed] [Google Scholar]
- *.Vargha-Khadem F, O’gorman AM, & Watters GV (1985). Aphasia and handedness in relation to hemispheric side, age at injury and severity of cerebral lesion during childhood. Brain, 108. https://academic.oup.com/brain/article/108/3/677/312165 [DOI] [PubMed] [Google Scholar]
- Wallen M, Bonney MA, & Lennox L (1996). The handwriting speed test Adelaide: Helios Art and Book Co. [Google Scholar]
- Wang SSH, Kloth AD, & Badura A (2014). The cerebellum, sensitive periods, and autism. Neuron, 83(3), 518–532. 10.1016/j.neuron.2014.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb C, Rose FD, Johnson DA, & Attree EA (1996). Age and recovery from brain injury: clinical opinions and experimental evidence. Brain Injury, 10(4), 303–310. [DOI] [PubMed] [Google Scholar]
- Wechsler D (1981). Wechsler Adult Intelligence Scale (rev. ed.) Psychological Corporation. [Google Scholar]
- Wechsler D (1991). Wechsler Intelligence Scale for Children– Third Edition The Psychological Corporation. [Google Scholar]
- Wechsler D (1997). Wechsler Memory Scale – Third Edition (WMS-III) Psychological Corporation. [Google Scholar]
- Wechsler D (1999). Wechsler Abbreviated Scale of Intelligence (WASI) Harcourt Assessment. [Google Scholar]
- Wechsler D (2002). Wechsler preschool and primary scale of intelligence—third edition The Psychological Corporation. [Google Scholar]
- Wechsler D (2003). Wechsler Intelligence Scale for Children– Fourth Edition The Psychological Corporation. [Google Scholar]
- Wechsler D (2008). Wechsler adult intelligence scale–Fourth Edition (WAIS–IV) Pearson. [Google Scholar]
- Wechsler D (2009). Wechsler Memory Scale – Fourth Edition (WMS-IV) Psychological Corporation. [Google Scholar]
- Wechsler D (2012). Wechsler preschool and primary scale of intelligence—fourth edition The Psychological Corporation. [DOI] [PubMed] [Google Scholar]
- *.Westmacott R, Askalan R, Macgregor D, Anderson P, & Deveber G (2010). Cognitive outcome following unilateral arterial ischaemic stroke in childhood: Effects of age at stroke and lesion location. Developmental Medicine and Child Neurology, 52(4), 386–393. 10.1111/j.1469-8749.2009.03403.x [DOI] [PubMed] [Google Scholar]
- *.Westmacott R, McDonald KP, Roberts SD, deVeber G, MacGregor D, Moharir M, Dlamini N, & Williams TS (2018). Predictors of Cognitive and Academic Outcome following Childhood Subcortical Stroke. Developmental Neuropsychology, 43(8), 708–728. 10.1080/87565641.2018.1522538 [DOI] [PubMed] [Google Scholar]
- Williams KT (2007). EVT-2: Expressive vocabulary test Pearson Assessments. [Google Scholar]
- *.Wingeier K, Bigi S, El-Koussy M, Heinks-Maldonado T, Boltshauser E, & Steinlin M (2011). Long-term sequelae after acquired pediatric hemorrhagic cerebellar lesions. Child’s nervous system, 27(6), 923–931. 10.1007/s00381-010-1357-x [DOI] [PubMed] [Google Scholar]
- Zimmermann P, & Fimm B (1993). A computerized neuropsychological assessment of attention deficits (Manual) PsyTest. [Google Scholar]


