Abstract
Background:
Patient-reported outcomes (PROs) may improve care for patients with heart failure (HF). The Kansas City Cardiomyopathy Questionnaire-12 (KCCQ-12) is a patient survey that captures symptom frequency, symptom burden, physical limitations, social limitations, and quality of life. Despite the utility of PROs and the KCCQ-12, the implementation and routine use of these measures can be difficult. We conducted an evaluation of clinician perceptions of the KCCQ-12 to identify barriers and facilitators to implementation into clinical practice.
Methods:
We conducted interviews with cardiologists from four institutions across the United States and Canada (n=16) and observed clinic visits at one institution in Northern California (n=5). Qualitative analysis was conducted in two rounds: 1) rapid analysis constructed around major themes related to the aims of the study and 2) content analysis with codes derived from the rapid analysis and implementation science.
Results:
Most HF physicians and advanced practice clinicians reported that the KCCQ-12 was acceptable, appropriate, and useful in clinical care. Clinician engagement efforts, trialability, and the straightforward design of the KCCQ-12 facilitated its use in clinical care. Further opportunities identified to facilitate implementation include more streamlined integration into the electronic health record and comprehensive staff education on PROs. Participants highlighted that the KCCQ-12 was useful in clinic visits to improve the consistency of patient history taking, focus patient-clinician conversations, collect a more accurate account of patient quality of life, track trends in patient wellbeing over time, and refine clinical decision making.
Conclusions:
In this qualitative study, clinicians reported that the KCCQ-12 enhanced several aspects of HF patient care. Use of the KCCQ-12 was facilitated by a robust clinician engagement campaign and the design of the KCCQ-12 itself. Future implementation of PROs in HF clinic should focus on streamlining EHR integration and providing additional staff education on the value of PROs.
Keywords: heart failure, patient reported outcomes, qualitative research, cardiology, health status, quality of life
Introduction
Heart failure (HF) exacts a heavy symptom burden and significantly reduces patient quality of life.1 Optimizing patients’ health status is an essential component of HF care, but it is an outcome that is difficult to reliably measure.2 The New York Heart Association (NYHA) functional class is traditionally used to define health status;2,3 however, this classification, determined by clinicians, is often discordant with patient-reported health status. Patient-reported outcomes (PROs) allow for the systematic collection of patient-reported health status, providing additional granularity and better predicting prognosis.2,4–6 PROs thus have the potential to improve patient-centered care.4
The Kansas City Cardiomyopathy Questionnaire (KCCQ-12) is a PRO measure that captures symptom frequency, symptom burden, physical limitations, social limitations, and quality of life.2 In HF, the KCCQ-12 is among the most used and validated PRO measures.2,7 It not only summarizes health status but is also responsive to HF therapies and is a strong predictor of clinical risk.8 The short-form version, the KCCQ-12, is designed for implementation in routine care.9
Implementation and routine use of PROs can be difficult.10 Physician engagement is a prerequisite for PRO uptake.8 There are important gaps in understanding how clinician perceptions lead to successful KCCQ-12 implementation and integration in clinical practice.11 We evaluated clinician perception of KCCQ-12 implementation in the context of the Patient-Reported Outcomes in Heart Failure Clinic (PRO-HF) trial,12 which randomized patients seen in HF clinic to routine collection of the KCCQ-12 or usual care. We aimed to investigate 4 questions: (1) What are clinician perspectives on PROs and the KCCQ-12? (2) What is clinician understanding of/readiness for use of the KCCQ-12 in clinical practice? (3) What are clinicians’ on-the-ground experiences with KCCQ-12 data? (4) How are clinicians using KCCQ-12 data in clinical practice? We assessed barriers and facilitators impacting KCCQ-12 implementation to identify emergent best practices, which can guide future interventions.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
KCCQ-12 Implementation at Stanford Medicine
Setting.
This implementation evaluation is embedded in the PRO-HF trial, which sought to evaluate whether routinely collecting and displaying the KCCQ-12 to clinicians impacts health status or treatment patterns for HF patients. The PRO-HF trial was conducted at Stanford Health Care (SHC) (Palo Alto, California, US). The design of this trial is depicted in Figure 1 and has been described previously.12
Figure 1.
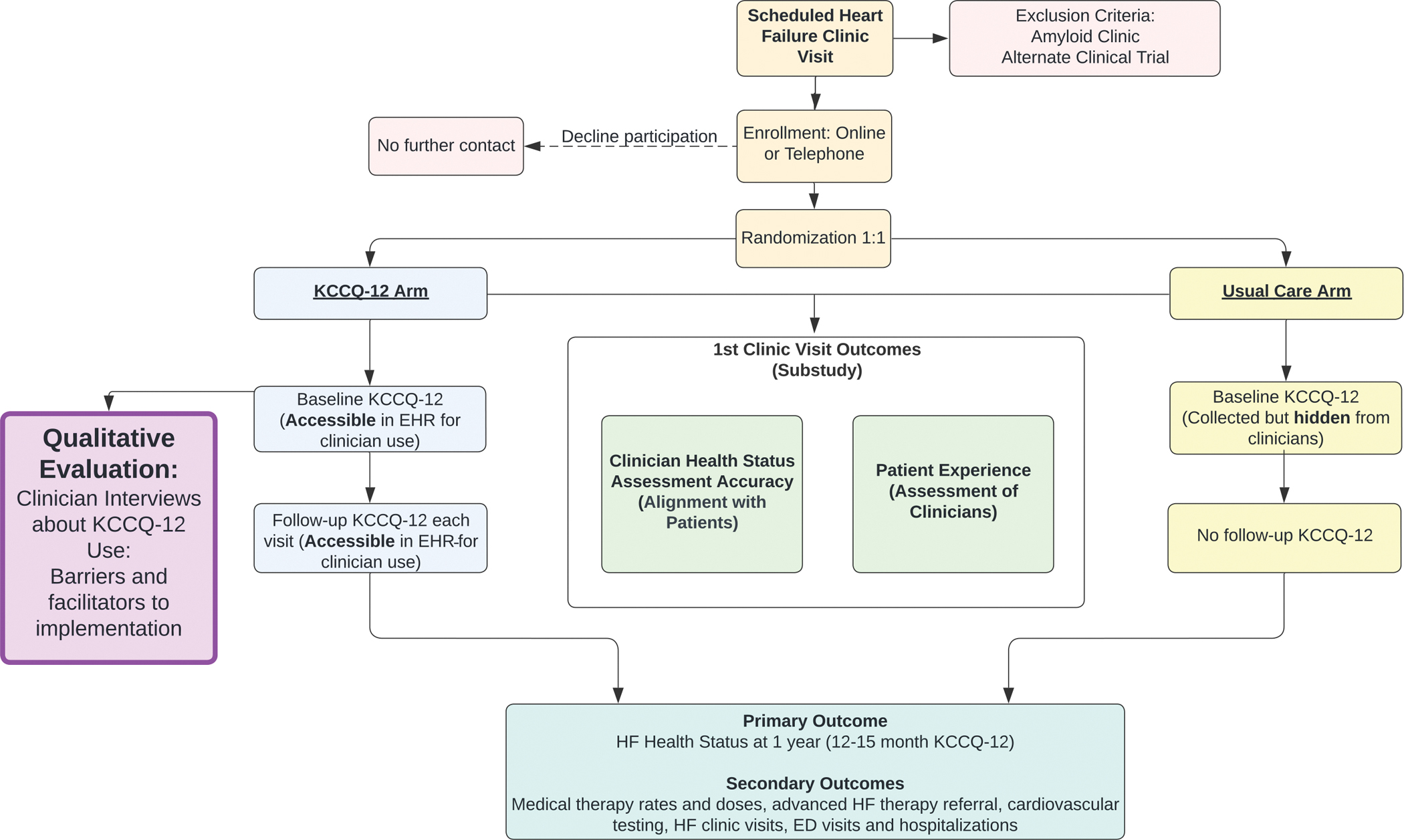
Process Map of PRO-HF trial
KCCQ-Kansas City Cardiomyopathy Questionnaire, HF-Heart Failure, HER- Electronic Health Record, ED-Emergency Department
As part of this trial, all SHC HF clinicians and clinic staff received periodic trainings and attended team meetings, which focused on the evidence supporting use of the KCCQ-12 and how it would be integrated into the clinic workflow. Specifically, the implementation lead (AS) presented a summary of data on the importance of the questionnaire, its interpretation, and prognostic significance. Resources and protocols on how to integrate the KCCQ-12 into clinicians’ workflows were also provided for clinic staff to utilize as a reference, including KCCQ-12 infographics posted in clinical areas. The implementation lead was available to meet with clinicians for one-on-one check-ins during the month of September 2021 before or on the day they first saw an enrolled participant.
For patients in the KCCQ-12 arm of the PRO-HF trial, KCCQ-12 responses and scores were available to clinicians in the EHR and via printed copy. The implementation lead provided instructions on how to access KCCQ-12 results and how to automatically import the results into a clinic note. Clinic staff also printed KCCQ-12 responses on paper and handed them to treating clinicians.
Data Collection
Data collected for this evaluation included semi-structured interviews. To evaluate KCCQ-12 implementation outside of SHC, we also recruited clinicians with experience using the KCCQ-12 in routine care at other sites. We conducted semi-structured interviews with HF clinicians from SHC and 3 other academic medical centers: University of Utah Health (Salt Lake City, Utah, US), Saint Luke’s Mid America Heart Institute (Kansas City, Missouri, US), and Mazankowski Alberta Heart Institute (Calgary, Alberta, Canada). The KCCQ-12 had been implemented at these institutions in routine clinical practice to varying degrees. At each site, the KCCQ-12 is collected from at least certain HF patients and incorporated into clinical care. A small number of clinic visits (n=5) at SHC were observed to triangulate data collected in the clinician interviews.
Semi-Structured Interviews
Recruitment.
We solicited attending physicians and advanced practice providers/pharmacists (APPs/PharmDs). HF clinicians at all institutions were recruited using snowball sampling. Potential participants (n=22) were contacted by email and invited to participate in a 30-minute semi-structured phone interview. No incentives were provided for clinician interviews.
Data collection and analysis.
The interview protocol was informed by focus group questions used in Wohlfahrt et al., 2020,8 the Consolidated Framework for Implementation Research 2.0 (CFIR 2.0),13 and design thinking approaches (See Appendix A). Interviews were conducted by a trained qualitative researcher (AA). During interview data collection, the interview protocol was iteratively updated in bi-weekly meetings with project partners (e.g., to include prompts for specific examples of how the KCCQ was used in clinical practice, to explore utilization of the KCCQ as a conversation prompt/guide). Project partners represented multidisciplinary views, including those of physicians (primary care, palliative care, cardiology), APPs, qualitative researchers, and nurses.
Interviews (range=15–50 minutes) were recorded and transcribed verbatim (Rev.com, Austin TX, USA). We conducted an initial rapid qualitative analysis14 after the first 9 interviews, constructed as is typical for rapid analyses around major themes of the interview guide (i.e., clinician perspectives of the KCCQ-12, on-the-ground experience with the KCCQ-12, understanding and readiness for use of the KCCQ-12). An initial report of this rapid analysis was presented to project partners and circulated among them for validation and feedback. The interview guide, rapid analysis, and subsequent expert feedback informed the codebook utilized for the next round of more traditional content analysis. Transcripts for all interviews were subsequently analyzed using these content codes as well as implementation science codes modified from CFIR 2.0.13 Codes were mapped to CFIR 2.0 codes and fell within 5 broad constructs of the framework: Innovation characteristics, Inner setting, Outer setting, Key roles and characteristics, and Implementation process. Additional project specific codes (clinical decision making, monitoring disease prevention) were incorporated to supplement CFIR 2.0. We used the Dedoose platform (Version 8.3.47, Los Angeles, CA, USA) to allow for virtual collaboration. Coauthors AA and JC were trained in Dedoose and collaboratively coded two transcripts. Analysis was discussed with the multidisciplinary group in bi-weekly meetings to validate findings with ongoing program considerations.15 Additionally, analysis techniques were reviewed in bi-weekly qualitative researcher methodology meetings. Findings and their interpretation were also validated with a broader group of implementation scientists based at Stanford.
Ethical considerations.
This project was deemed to be non-human subjects research (Protocol ID #65109) by the Stanford Institutional Review Board as a quality improvement project. Interview participants gave verbal consent and confidentiality was prioritized.
Results
Participants
Physicians (n=11) and APPs/PharmDs (n=5) from four academic medical centers participated in 30-minute phone interviews in 2022 (see Table 1). Most participants were male (n=9), in their 40s (n=6), and identified as white (n=9).
Table 1.
Characteristics of interview participants from 4 academic medical institutions
| Participant Characteristic | % Participants (n) |
|---|---|
|
| |
| Gender | |
| Men | 56% (9) |
| Women | 44% (7) |
| Age (Years) | |
| 20–39 | 31% (5) |
| 40–49 | 38% (6) |
| 50–79 | 31% (5) |
| Race and Ethnicity | |
| Non-White | 38% (6) |
| White | 56% (9) |
| Declined to Respond | 6% (1) |
| Role | |
| Physician | 69% (11) |
| Advanced Practice Clinician or Pharmacist | 31% (5) |
|
| |
| Total | 100.0% (16) |
Qualitative Findings
Our analysis uncovered barriers and facilitators to implementation of the KCCQ-12 by HF clinicians that fit into four categories: 1) overall perceptions of PROs and the KCCQ-12; 2) clinician understanding of and readiness for use of the KCCQ-12 in clinical practice, 3) on-the-ground experience with KCCQ-12 data, and 4) clinician use of KCCQ-12 data in clinical practice. Findings in these areas suggest emerging best practices for KCCQ-12 implementation (Table 2) and clinical use (Table 3). See Figure 2 for a summary of evaluation findings.
Table 2.
Impact of the KCCQ-12 Implementation Components on Physician Engagement
| Aspect of KCCQ-12 Implementation | Exemplar Quotes | |
|---|---|---|
| Clinician Engagement | Engaged Implementation Champion (Facilitator) | “He wanted to meet up with the APPs and the providers individually, or in a group setting to kind of break it down in the pre-rollout process. So I think that was very critical because we had some time, maybe even over a month to just prepare and understand. And then I think while it was rolling out, rolled out I think he did provide emails with refreshers, ‘By the way in case everyone forgot this is the presentation I made, if anyone needed some reference or resource.’” (Clinician 3) |
| Clinic Staff Education and Training (Proposed Solutions) | “I think, for me, it [the education] was fine. I think for the rest of my clinic team, I think they probably needed a little bit more background, like why this is useful. … where they understand the background behind … the utility of it. And then understanding how it can help them.” (Clinician 2) | |
| KCCQ-12 Trialability | Straightforward usage and interpretation of the KCCQ-12 (Facilitator) | “It’s a very straightforward questionnaire.” (Clinician 2) |
| KCCQ-12 Design | Important insight gained from the KCCQ-12 (Facilitator) | “I found that the end questions, when it does talk about quality of life, specifically that question about, ‘If you had to live with your heart failure like this for the rest of your life, how would you feel?’ I find that question particularly enlightening. And I think it’s sometimes hard to ask those questions just organically. That doesn’t really fit into conversation very well. So I think using the scale allows you to have just an opportunity to ask that question that it wouldn’t flow naturally. So we when tell people we’re doing the questionnaire, we say this is just so that we can note how you’re doing now and then how you’re doing three months later. A lot of the questions are familiar to them, but some of those questions I feel about the quality of life are particularly impactful because you get a sense of it just based on what they’re doing, their activity, their demeanor, but you don’t really get the full like, ‘How do you feel from your point of view?’ blunt question answer about the quality of life stuff. So I think it is useful.” (Clinician 13) |
| Patient Interpretation of the KCCQ-12 (Barrier) | “I think there’s such a complicated interaction between the patien’ts personality and how they interpret those forms.” (Clinician 11) | |
| KCCQ-12 Integration into Clinic Workflow | Time-consuming and new addition to workflow (Barrier) | “I did initially find it time-consuming compared to what the other tools that we have, but now, when I look at it, I don’t think i’ts time-consuming because the information we gather from it is valuable.” (Clinician 16) |
| Automation in EHR notes (Proposed solutions) | “Honestly, I would love to see it with my vital signs.” (Clinician 12) | |
KCCQ-Kansas City Cardiomyopathy Questionnaire 12, APP-Advanced Practice Providers, EHR-Eletronic Health Record
Table 3.
Clinician Use of the KCCQ-12 Data in Clinical Practice
| Major Uses of KCCQ-12 | Exemplar Quotes |
|---|---|
| Improve the consistency of patient history taking | “I think i’ts very helpful! … my history-taking can be a little bit variable from how the APP does it, from how someone else does it. But I think the nice thing about the PRO is, it standardizes that to a larger extent.” (Clinician 2) |
| Focus patient-clinician conversations | “I can tell you that it definitely facilitates the interaction process and the conversation we have as clinician and patient…for now i’ts just something I use to help guide me and speed up the process of figuring out where the patient is and what I want to do to help them.” (Clinician 14) |
| Collect a more accurate account of patient health status | “Yeah. I mean, I sometimes just ask patients about it. “It doesn’t seem like all much has changed, but yet the way you answered the questionnaire, it seems like you’re pretty limited. Is this the way you’ve always been, or is this something new?”….They answer that a little bit more honestly than to you… people just don’t want to complain, but when you ask them on a piece of paper, maybe they’re a little bit more honest.” (Clinician 7) “And the score will give me a little bit more insight into maybe where they really are as opposed to where they try to convey to me that they are. So again, that helps to minimize the time I have to spend digging with questions to come up with what I think is the right answer as far as what the patient feels.” (Clinician 14) |
| Track trends in patient wellbeing over time | “…if i’ts trended over time where they’ve done it now one or two times, I find that interesting to see for sure. I’ve used it in those cases, especially with recent hospitalizations where we’ve made medical changes to their… Changes to their medication. I’ts been helpful to see the subjective report and then try to compare it to the clinical evidence.” (Clinician 10) |
| Gather data for clinical decision making | “I don’t think it necessarily changed my care. I wouldn’t say it changed my management… But, I think it helped me acquire the data that helps me change my management a little bit differently. But ultimately, I don’t think it sort of changed, ultimately, what I ended up do[ing].” (Clinician 2) |
KCCQ- Kansas City Cardiomyopathy Questionnaire 12, APP-Advanced Practice Providers, PRO-Patient Reported Outcomes
Figure 2.
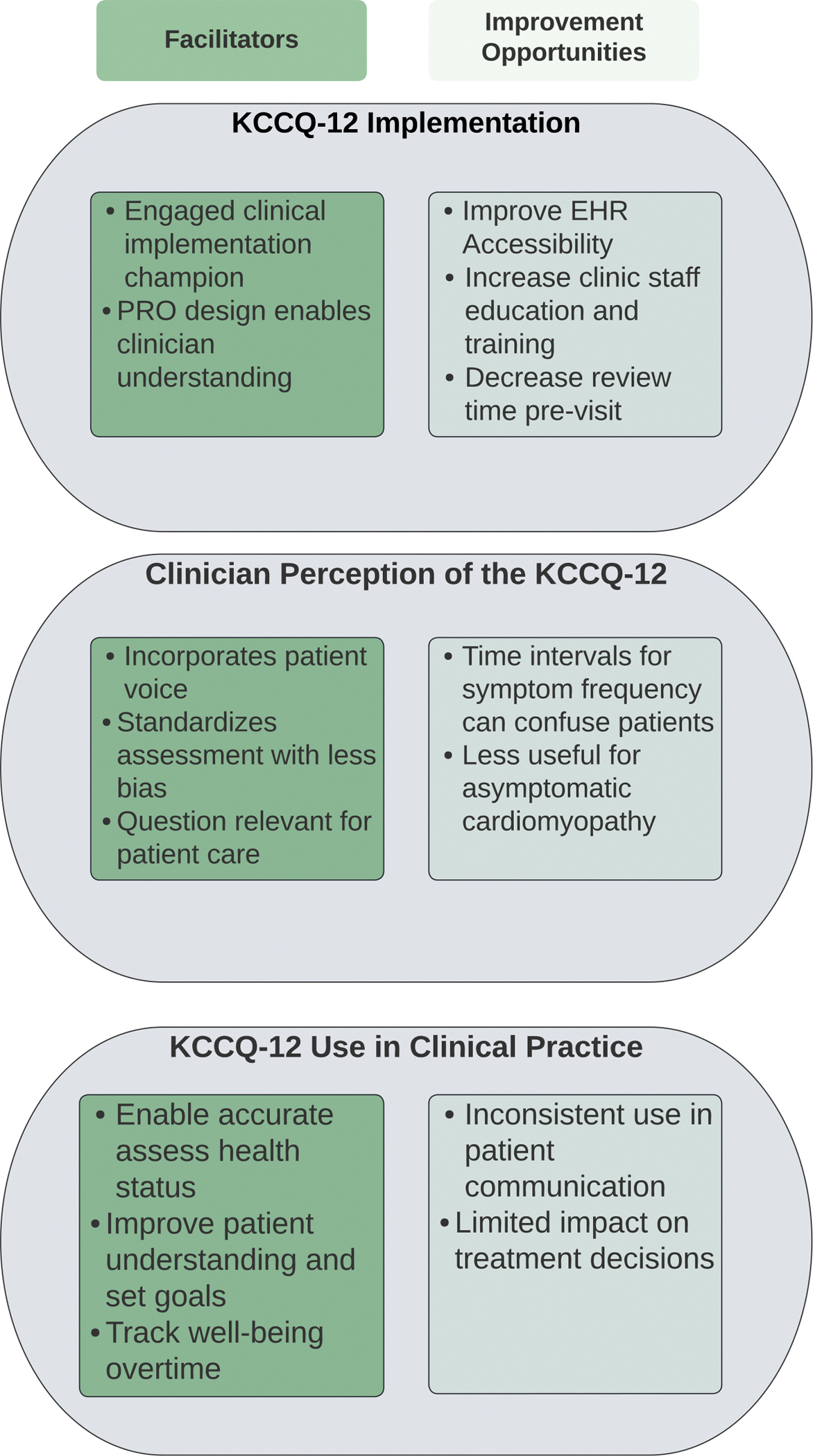
Summary of major themes of implementation of Patient Reported Outcomes
KCCQ-Kansas City Cardiomyopathy Questionnaire, PRO-Patient Reported Outcomes, EHR- Electronic Health Record
Overall Perceptions of PROs and the KCCQ
Participants had positive perceptions of both PROs and the KCCQ-12, citing that they helped bring patient perspectives into the clinic visit, were acceptable, and were appropriate. Most HF clinicians felt that PROs broadly helped to incorporate the patient’s voice into care decisions. For example, one clinician stated:
“I think patient-reported outcomes are really critical; critical for patients. Patients really want to improve how they feel and improve their overall many aspects of their quality of life. I think it’s complex. Too frequently in medicine, we’ve kind of used surrogates for patient-reported outcomes, mainly being physicians or nurses to clinicians. The concern there, is that clinicians will often kind of interject their own opinions, values, and preferences into the ascertainment of a patient-reported outcome.” (Clinician 6)
The KCCQ-12 was deemed acceptable, appropriate, and useful in clinic visits by most participants. Most participants found the KCCQ-12’s design and evidence-base to be acceptable and satisfactory. Participants noted that an area for growth is in improving access to KCCQ-12 data in routine care. They specifically cited a need to improve the integration of the KCCQ-12 with the EHR.
The KCCQ-12 was reported to be appropriate by most participants, reflecting the perception that the KCCQ-12 was compatible with HF care and clinically useful. Many noted that the KCCQ-12 captures relevant HF data points. Notably, a few highlighted that the KCCQ-12 was only appropriate for symptomatic HF patients, but not patients with an asymptomatic cardiomyopathy, who were cared for in HF clinic:
“I think that more advanced heart failure patients would find this easier to answer or relate more to it than patients who don’t know much about heart failure, because they’ve never been in heart failure.” (Clinician 16)
Clinician Understanding of and Readiness for Use of the KCCQ-12 in Clinical Practice
Clinician engagement was viewed as critical to successful deployment of the KCCQ-12. Respondents from SHC, which was the only health system with an active rollout at the time of interviews, reported that the efforts to engage clinicians in KCCQ-12 implementation were satisfactory. A few clinicians mentioned that the accessibility and engagement of the implementation lead, the physician who championed the KCCQ-12 rollout at the clinic, was a major contributor to implementation success.
The SHC implementation included clinic staff education sessions focused on KCCQ-12 logistics, rather than on the evidence and rationale education supporting use of the KCCQ-12 received by clinicians. One participant felt that the implementation could be improved by providing non-clinician members of the clinical team (i.e., nurses and medical assistants) with additional information about the importance of the KCCQ-12 to patient care.
On-The-Ground Experience With the KCCQ-12
Several aspects of the KCCQ-12 implementation influenced clinician use of the PRO in communication with patients. These included the KCCQ-12 design and integration into the clinical workflow (see Table 2).
Design of the KCCQ-12.
Overall, participants reported that the KCCQ-12 was well-designed and usable. Some reported that the domains included were relevant to HF and well validated. A few respondents highlighted individual questions that enhanced their understanding of patient quality of life and supported clinical care discussions. Participants noted that the concrete, straightforward questions in the KCCQ-12 can help clinicians get a better sense of patients’ symptoms during their day-to-day activities:
“‘Do you get short of breath in the shower?’ It’s so concrete, it’s a personal activity that maybe is uncomfortable for someone to ask their patient, but I think really gets to the heart of, can your patient get through their day?” (Clinician 8)
In terms of the KCCQ-12’s limitations, a few felt that subset of KCCQ-12 questions could be revised to support patient understanding, which could in turn improve the accuracy of KCCQ-12 scores. Participants specifically noted concerns regarding patients misinterpreting the timeframe for the questions focused on symptom frequency with some patients reporting their average frequency of symptoms since their HF diagnosis rather than specifically over the prior 2 weeks as queried in the KCCQ-12.
Integrating the KCCQ-12 into the clinical workflow.
Participants reported that they were willing to integrate the KCCQ-12 into their clinical workflow. However, a few noted that the biggest barrier to implementation was time, highlighting that busy clinic schedules can make it a challenge to review KCCQ-12 scores before seeing each patient. To address this challenge, some noted that receiving the scores prior to clinic visits would best support the integration of the KCCQ-12 into clinic visits: “it obviously would be a better system for me to have it in real time and then use it in the clinic room.” (Clinician 5)
Clinician Use of KCCQ-12 Data in Clinical Practice
Clinicians used the KCCQ-12 during patient care to improve communication in the form of history taking and conversations, to collect a more accurate account of patient quality of life, to track trends in patient wellbeing over time, and to refine clinical decision making (Table 3).
Patient-clinician communication: History-taking and conversations.
The KCCQ-12 was used to standardize history-taking across clinicians. A few participants mentioned that history-taking can vary widely between different clinicians or even between the same clinician on different days. Clinicians reported that enlisting the KCCQ-12 ensured that patient histories would be collected in a standardized, consistent way each visit.
Overall, clinicians had mixed thoughts about the utility of the KCCQ-12 to communicate with patients about their health status and prognosis. A few participants reported that the KCCQ-12 helped focus patient-clinician conversations. Based on a patient’s survey responses, clinicians could narrow additional questions to areas of decline, ensuring that they fully investigated a patient’s functional status. Some clinicians reported that the KCCQ-12 could be used to reflect back a patient’s functional status and explain care recommendations:
“I would say, well, I can’t give a number, but I would say, more often than not, if I recommend a procedure such as a valve intervention or surgery, patients are going to be willing to move forward. But there are patients who are skeptical. I find that for patients who have skepticism and fear of moving forward with the procedure, especially a procedure that carries risk, having the [KCCQ-12] data can be very helpful. I would kind of explain where they are, and say, ‘Look, your quality of life is X. I think we can get it to Y. I think we can dramatically improve it. If I were to look at all-comers, this is kind of my concern.’ I find it very helpful in those patients” (Clinician 6)
One participant reported that directly communicating with patients about the KCCQ-12 could help patients better understand their own health status, allowing them to monitor their own disease progression and identify the benefits of HF treatments:
“So yeah, in an ideal state, it’s something that the patient not only fills out, but understands and is able to recognize changes in and appreciate, ‘Hey, I feel better and my KCCQ score’s better,’ or, ‘Hey, I don’t love this new medicine, but when I really look at the big picture, not the maybe side effect that maybe a medicine is causing me, what I can see from these scores is that my quality of life in relation to my heart failure disease burden hasn’t significantly improved. So maybe I can put up with a little bit of this side effect or that side effect if it means that from day to day, I have a lot more energy. I can do more before getting winded, can enjoy the things I enjoy with a little more capacity.’ Yeah. Ideally it would be both patient- and physician-facing.” (Clinician 14)
Some challenges in communicating directly with patients about the KCCQ-12 included misinterpretation and potential negative effects on the human connection. One participant felt that the KCCQ-12 scores could be misinterpreted by patients, causing unneeded stress: “And so I haven’t used the KCCQ-12 score because I would be concerned that it’s either like a black or white - it’s good or bad in the patient’s eyes.” (Clinician 8) Another participant worried that the KCCQ-12 may limit clinicians’ ability to connect with patients on a human level. Despite these potential concerns, the majority of participants felt there was a value in using the KCCQ-12 as part of patient conversations, especially when there are communication challenges between patients and clinician:
“Well, you might have a patient who really isn’t very communicative and really hasn’t said very much, and that’s where maybe there’s Kansas City Questionnaire might come in. You’ve got some of them, or you’ve done it, it’s there. And they say they get light-headedness or shortness of breath, or they’re not eating well or their appetite’s good.” (Clinician 11)
Accurate assessment of patient health status.
Respondents reported that a major use of the KCCQ-12 was to collect a more accurate account of patients’ health status. Many mentioned that assumptions can sometimes cloud clinical assessments, which can challenge understanding of patient prognosis and development of an appropriate care plan. Additionally, a few felt that, in traditional history-taking, patients may report rosier assessments of their symptoms to be seen as “good patients.” Because the KCCQ-12 is completed by patients independently, participants reported that the KCCQ-12 may provide a more accurate assessment of patient function:
“And I think the other reason it’s helpful, is a lot of patients I find want to be perceived as a good patient or want to sort of build rapport with a clinician by giving them a very rosy assessment of their symptoms and the medication adherence, et cetera. And so I think a standardized questionnaire can also help bring some honesty to their relationship so that even if something’s not going well, if it’s a standardized question hopefully both people will feel like this is not a personal assessment of, ‘You’re not a good patient or you’re not a good doctor.’ But just trying to get everyone on the same page.” (Clinician 8)
A few participants also mentioned that the KCCQ-12 may further support the collection of objective data by eliminating potential bias in clinician questions. Clinicians noted that they may unintentionally ask leading questions that could influence patient responses during traditional history taking: “I am not prompting, I am not unintentionally framing, et cetera. I do prefer when the patient fills out their own questionnaire.” (Clinician 16)
Some also reported that the KCCQ-12 may provide a fuller picture of a patient’s health status than other data, such as lab values or imaging results, as the KCCQ-12 captures patient perceptions of their health. This data can help to better understand how patients are feeling and inform their clinical plans.
Track trends in well-being and refine decision making.
Participants reported that an advantage of the KCCQ-12 was the ability to track trends in patient wellbeing overtime, which can enable better understanding of patient prognosis. Participants also used these trends to understand how changes in medication or major health events impacted a patient’s health status.
Finally, participants used the KCCQ-12 as a tool to refine their clinical decision making. Clinicians stated that often the KCCQ-12 would not change their care plan but might instead be used to validate the clinical decisions they had already made:
“I don’t know, maybe it’s reinforced my desire to do those sorts of things rather than sort of prompted my desire to do those things… I don’t know that it’s made huge changes in sort of what I’ve decided to do in terms of therapeutics. I’m trying to think, maybe it makes me a little bit more likely to push harder, to try harder for someone who’s worse off, whose symptoms are more pronounced by the score than I would’ve thought otherwise. But generally speaking, I think we try to be pretty aggressive, particularly in systolic heart failure with guideline directed medical therapy.” (Clinician 7)
Others used the KCCQ-12 to fine tune the care plan. For example, one clinician described looking at other clinical indicators to determine the overall trajectory of a patient’s care plan. Following this, the clinician used the KCCQ-12 to add or decrease ancillary medications that could support patient quality of life.
The level of KCCQ-12 information used varied among clinicians. Some reported relying more on individual question responses to understand patient health status because this strategy helped them to understand specific symptoms that impacted patients. Other focused more on the summary score, finding that this data point gave them a more global view of a patient’s wellbeing:
“I tend to use the individual components, the questions themselves, and I guess maybe calculate a score in my head, or at least kind of see what the theme is in terms of the symptoms that are impacting the patient. So the overall number itself, I don’t use it that directly.” (Clinician 1)
Discussion
Most HF clinicians reported that the KCCQ-12 was acceptable, appropriate, and useful in HF clinical care. Clinician engagement efforts during the KCCQ-12 rollout as well as the trialability and design of the KCCQ-12 facilitated its use. Most participants highlighted that the KCCQ-12 was helpful during clinic visits to improve the consistency of patient history taking, focus patient-clinician conversations, collect a more accurate account of patient quality of life, track trends in patient wellbeing over time, and refine clinical decision making. Engagement barriers were largely related to the changes in workflow and the accessibility of the KCCQ-12 in the EHR.
This study highlights how PROs can be used in clinical care and how they can influence clinician-patient communication, a relatively unexplored area of investigation. We found the use of PROs varied across clinicians and is potentially related to expertise, practice style, and/or patient population. Previous research has suggested that PROs may especially benefit early career physicians in connecting with patients.16 PROs may also increase the efficiency of clinic visits.17 We found that some clinicians used the KCCQ-12 to focus their conversation and line of questioning during clinical encounters.
In this study, PROs only appeared to influence clinical decision making in a limited way. This work validates prior literature, which suggests PROs have a greater impact on the detection and discussion of patient problems rather than on treatment decisions.18 This may be because physicians do not view poor or declining PRO scores as an indication for treatment.19,20 A contributing factor may be the lack of HF clinical trials using PROs to identify patients eligible for therapies. Clinicians thus face ambiguity as to the appropriate response to patient-reported health status and may instead focus on traditional therapeutic indications, such as worsening left ventricular ejection fraction. This status quo is unfortunate, as the primary goal of HF treatment is to improve quality of life, and numerous therapies and treatment strategies have been shown to improve patient-reported health status among HF patients.21–24 Though clinicians may prefer to rely on their own clinical judgement versus PRO data to determine who would benefit from therapy to improve health status,25 multiple analyses have demonstrated the substantial discordance between clinician and patient assessment of health status.26 Building the evidence base linking PROs and therapeutic interventions and increasing PRO exposure during clinical training27,28 could support clinician use of PROs in treatment decisions for HF patients.
PROs are inherently patient-focused and their use may increase patient satisfaction. Patients perceive value in sharing symptoms with their clinicians,19 and improving patient-clinician connection can positively impact health.29 Unsurprisingly, a previous qualitative assessment of patient perceptions of the KCCQ-12 demonstrated that patients only found the PRO beneficial when clinicians utilized the results during visits.30 In this evaluation, we describe how clinicians used the KCCQ-12 in care delivery to enhance communication, track patient well-being over time, and even improve clinical decision making. Using PROs in this way may have the added benefit of enhancing equity by standardizing health status assessment across populations and reducing implicit bias in clinician assessment.31
Finally, this study highlights the potential role of Implementation Science (IS) to address the challenge of how to integrate PROs in routine care. This study leveraged IS frameworks to identify best practices to replicate success and opportunities to improve future implementation of PROs. Our results further underscore the importance of an engaged implementation champion; this leadership in combination with local ownership of an initiative can further support clinical uptake.11,19
Limitations
This evaluation is limited in that it includes perspectives from 4 institutions with the majority coming from one site (SHC). Related, we did not collect details on the exposures interviewees had to the PRO-trial. Second, the SHC interviews were conducted through the PRO-HF trial; clinician experiences may have evolved during and following the trial. Finally, the interviews were conducted with physicians and APPs/PharmDs but did not capture other members of the clinical team, such as nurses and medical assistants.
Conclusion
In this qualitative analysis of KCCQ-12 implementation, we identified important facilitators and barriers to clinician adoption of PROs. PROs can be a valuable tool for improving patient-centered HF care, but clinicians need to successfully incorporate these measures in care delivery to realize their benefits. Future efforts to implement PROs in HF care should build on these lessons to maximize the likelihood of successful clinician adoption.
Supplementary Material
What is known.
Patient-reported outcomes (PROs) have the potential to improve care for patients with heart failure (HF).
The Kansas City Cardiomyopathy Questionnaire-12 (KCCQ-12) is among the most widely used and validated HF PRO measures.
What this study adds.
We conducted an evaluation of clinician perceptions of the KCCQ-12 to identify barriers and facilitators to its implementation into clinical practice.
KCCQ-12 use was facilitated by a robust clinician engagement campaign and the design of the KCCQ-12 itself, which allowed for enhanced patient care during visits.
Future implementation of PROs in HF clinics should focus on streamlining EHR integration and providing additional staff education on the value of PROs.
Acknowledgments.
Authors would like to thank patient and clinician participants in this study and the larger trial.
Sources of Funding.
This work was funded by the National Heart, Lung, and Blood Institute (1K23HL151672–01) and Stanford University institutional funding.
Non-standard Abbreviations and Acronyms:
- HF
Heart Failure
- NYHA
New York Heart Association
- PROs
Patient Reported Outcomes
- KCCQ-12
Kansas City Cardiomyopathy Questionnaire-12
- SHC
Stanford Health Care
- APP
advanced practice provider
- PharmD
pharmacist
- CFIR 2.0
Consolidated Framework for Implementation Research 2.0
- EHR
Electronic Health Record
Footnotes
Disclosures. The other authors have no conflicts of interest to disclose.
Registration: https://clinicaltrials.gov/ct2/show/NCT04164004
References
- 1.Juenger J, Schellberg D, Kraemer S, Haunstetter A, Zugck C, Herzog W, Haass M. Health related quality of life in patients with congestive heart failure: comparison with other chronic diseases and relation to functional variables. Heart. 2002;87(3):235–241. doi: 10.1136/heart.87.3.235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spertus JA, Jones PG, Sandhu AT, Arnold SV. Interpreting the Kansas City Cardiomyopathy Questionnaire in Clinical Trials and Clinical Care. Journal of the American College of Cardiology. 2020;76(20):2379–2390. doi: 10.1016/j.jacc.2020.09.542 [DOI] [PubMed] [Google Scholar]
- 3.Bennett JA, Riegel B, Bittner V, Nichols J. Validity and reliability of the NYHA classes for measuring research outcomes in patients with cardiac disease. Heart & Lung. 2002;31(4):262–270. doi: 10.1067/mhl.2002.124554 [DOI] [PubMed] [Google Scholar]
- 4.Hawwa N, Vest AR, Kumar R, Lahoud R, Young J, Wu Y, Gorodeski EZ, Cho L. Comparison Between the Kansas City Cardiomyopathy Questionnaire and New York Heart Association in Assessing Functional Capacity and Clinical Outcomes. Journal of Cardiac Failure. 2017;23(4):280–285. doi: 10.1016/j.cardfail.2016.12.002 [DOI] [PubMed] [Google Scholar]
- 5.Meinertz T, Münzel T. Kansas City Cardiomyopathy Quality Score Indicates Sustained Health Status Improvement in Patients After TMVr. Journal of the American College of Cardiology. 2020;75(17):2107–2109. doi: 10.1016/j.jacc.2020.03.034 [DOI] [PubMed] [Google Scholar]
- 6.Psotka MA, Teerlink JR. Patient-Reported Outcome Instruments in Heart Failure. JACC: Heart Failure. 2018;6(7):561–563. doi: 10.1016/j.jchf.2018.03.008 [DOI] [PubMed] [Google Scholar]
- 7.Chew DS, Whitelaw S, Vaduganathan M, Mark DB, Van Spall HGC. Patient-Reported Outcomes Measures in Cardiovascular Disease: An Evidence Map of the Psychometric Properties of Health Status Instruments. Ann Intern Med. Published online September 20, 2022;175(10):1431–1439. doi: 10.7326/M22-2234 [DOI] [PubMed] [Google Scholar]
- 8.Wohlfahrt P, Zickmund SL, Slager S, Allen LA, Nicolau JN, Kfoury AG, Felker GM, Conte J, Flint K, Devore AD, et al. Provider Perspectives on the Feasibility and Utility of Routine Patient-Reported Outcomes Assessment in Heart Failure: A Qualitative Analysis. JAHA. 2020;9(2): e013047. doi: 10.1161/JAHA.119.013047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spertus JA, Jones PG. Development and Validation of a Short Version of the Kansas City Cardiomyopathy Questionnaire. Circ Cardiovasc Qual Outcomes. 2015;8(5):469–476. doi: 10.1161/CIRCOUTCOMES.115.001958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spertus J Barriers to the Use of Patient-Reported Outcomes in Clinical Care. Circ: Cardiovascular Quality and Outcomes. 2014;7(1):2–4. doi: 10.1161/CIRCOUTCOMES.113.000829 [DOI] [PubMed] [Google Scholar]
- 11.Stover AM, Haverman L, van Oers HA, Greenhalgh J, Potter CM. Using an implementation science approach to implement and evaluate patient-reported outcome measures (PROM) initiatives in routine care settings. Qual Life Res. 2021;30(11):3015–3033. doi: 10.1007/s11136-020-02564-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kalwani NM, Calma J, Varghese GM, Gupta A, Zheng J, Brown-Johnson C, Amano A, Vilendrer S, Winget M, Asch S, et al. The patient-reported outcome measurement in heart failure clinic trial: Rationale and methods of the PRO-HF trial. American Heart Journal. 2023;255:137–146. doi: 10.1016/j.ahj.2022.10.081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Damschroder Laura. Introducing Version 2 CFIR Updates Updates. Presented at: Prevention Science and Methodolgy Group Virtual Grand Rounds. 2022. [Google Scholar]
- 14.Hamilton. Qualitative Methods in Rapid Turn-Around Health Services Research. Published December 11, 2013. Accessed November 18, 2022. https://www.hsrd.research.va.gov/for_researchers/cyber_seminars/archives/video_archive.cfm?SessionID=780
- 15.Miles MB, Huberman AM, Saldana J. 2019. Qualitative Data Analysis, 4th ed. Los Angeles: Sage. [Google Scholar]
- 16.Basch E, Barbera L, Kerrigan CL, Velikova G. Implementation of Patient-Reported Outcomes in Routine Medical Care. American Society of Clinical Oncology Educational Book. 2018;(38):122–134. doi: 10.1200/EDBK_200383 [DOI] [PubMed] [Google Scholar]
- 17.Weinfurt KP. Viewing assessments of patient-reported heath status as conversations: Implications for developing and evaluating patient-reported outcome measures. Qual Life Res. 2019;28(12):3395–3401. doi: 10.1007/s11136-019-02285-8 [DOI] [PubMed] [Google Scholar]
- 18.Greenhalgh J The applications of PROs in clinical practice: what are they, do they work, and why? Qual Life Res. 2009;18(1):115–123. doi: 10.1007/s11136-008-9430-6 [DOI] [PubMed] [Google Scholar]
- 19.Greenhalgh J, Long AF, Flynn R. The use of patient reported outcome measures in routine clinical practice: lack of impact or lack of theory? Social Science & Medicine. 2005;60(4):833–843. doi: 10.1016/j.socscimed.2004.06.022 [DOI] [PubMed] [Google Scholar]
- 20.Gilbody SM, House AO, Sheldon TA. Routinely administered questionnaires for depression and anxiety: systematic review. BMJ. 2001;322(7283):406–409. doi: 10.1136/bmj.322.7283.406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kitzman DW, Whellan DJ, Duncan P, Pastva AM, Mentz RJ, Reeves GR, Nelson MB, Chen H, Upadhya B, Reed SD, et al. Physical Rehabilitation for Older Patients Hospitalized for Heart Failure. New England Journal of Medicine. 2021;385(3):203–216. doi: 10.1056/NEJMoa2026141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Butler J, Filippatos G, Jamal Siddiqi T, Brueckmann M, Bohm M, Chopra VK, Pedro Ferreira J, Januzzi JL, Kual S, Piña IL, et al. Empagliflozin, Health Status, and Quality of Life in Patients With Heart Failure and Preserved Ejection Fraction: The EMPEROR-Preserved Trial. Circulation. 2022;145(3):184–193. doi: 10.1161/CIRCULATIONAHA.121.057812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Flynn KE, Piña IL, Whellan DJ, Lin L, Blumenthal JA, Ellis SJ, Fine LJ, Howlett JC, Keteyian SJ, Kitzman DW, et al. Effects of Exercise Training on Health Status in Patients With Chronic Heart Failure: Findings From the HF-ACTION Randomized Controlled Trial. JAMA. 2009;301(14):1451–1459. doi: 10.1001/jama.2009.457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Turgeon RD, Barry AR, Hawkins NM, Ellis UM. Pharmacotherapy for heart failure with reduced ejection fraction and health-related quality of life: a systematic review and meta-analysis. European Journal of Heart Failure. 2021;23(4):578–589. doi: 10.1002/ejhf.2141 [DOI] [PubMed] [Google Scholar]
- 25.Gilbody SM, House AO, Sheldon TA. Psychiatrists in the UK do not use outcomes measures. National survey. Br J Psychiatry. 2002;180:101–103. doi: 10.1192/bjp.180.2.101 [DOI] [PubMed] [Google Scholar]
- 26.Tran AT, Chan PS, Jones PG, Spertus JA. Comparison of Patient Self-reported Health Status With Clinician-Assigned New York Heart Association Classification. JAMA Network Open. 2020;3(8):e2014319. doi: 10.1001/jamanetworkopen.2020.14319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Skovlund PC, Ravn S, Seibaek L, Thaysen HV, Lomborg K, Nielsen BK. The development of PROmunication: a training-tool for clinicians using patient-reported outcomes to promote patient-centred communication in clinical cancer settings. J Patient Rep Outcomes. 2020;4(1):10. doi: 10.1186/s41687-020-0174-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Santana MJ, Haverman L, Absolom K, Takeuchi E, Feeny D, Grootenhuis M, Velikova G. Training clinicians in how to use patient-reported outcome measures in routine clinical practice. Qual Life Res. 2015;24(7):1707–1718. doi: 10.1007/s11136-014-0903-5 [DOI] [PubMed] [Google Scholar]
- 29.Street RL, Makoul G, Arora NK, Epstein RM. How does communication heal? Pathways linking clinician–patient communication to health outcomes. Patient Education and Counseling. 2009;74(3):295–301. doi: 10.1016/j.pec.2008.11.015 [DOI] [PubMed] [Google Scholar]
- 30.Mondesir FL, Zickmund SL, Yang S, Perry G, Galyean P, Nativi-Nicolau J, Kemeyou L, Spertus J, Stehlik J. Patient Perspectives on the Completion and Use of Patient-Reported Outcome Surveys in Routine Clinical Care for Heart Failure. Circ: Cardiovascular Quality and Outcomes. 2020;13(9): e007027. doi: 10.1161/CIRCOUTCOMES.120.007027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Breathett K, Yee E, Pool N, Hebdon M, Crist JD, Yee RA, Knapp SM, Solola S, Luy L, Herrera-Theut K, et al. Association of Gender and Race With Allocation of Advanced Heart Failure Therapies. JAMA Network Open. 2020;3(7):e2011044. doi: 10.1001/jamanetworkopen.2020.11044 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


