Abstract
Objective:
Assess outcomes of interval debulking surgery (IDS) after neoadjuvant chemotherapy via minimally invasive surgery (MIS) compared with laparotomy in patients with advanced epithelial ovarian cancer.
Methods:
Patients diagnosed with stage IIIC or IV epithelial ovarian cancer between 2013–2018 who received neoadjuvant chemotherapy and IDS were identified in the National Cancer Database. Primary outcome was overall survival. Secondary outcomes were 5-year survival, 30- and 90-day postoperative mortality, extent of surgery, residual disease, hospitalization duration, surgical conversions, and unplanned readmissions. Propensity score matching was used to compare MIS and laparotomy for IDS. Association of treatment approach with overall survival was assessed using Kaplan-Meier method and Cox regression. Sensitivity analysis was conducted for effect of unmeasured confounders.
Results:
A total of 7,897 patients met inclusion criteria; 2,021 (25.6%) underwent MIS. Percentage undergoing MIS increased from 20.3%–29.0% over the study period. After propensity score matching, mean overall survival was 35.9 months in the MIS group versus 34.5 months in the laparotomy group [hazard ratio (HR) 0.86 (95%CI 0.79–0.94)]. Five-year survival probability was higher in MIS versus laparotomy (38.3% vs 34.8%, p<0.01). There was lower 30- and 90-day mortality (0.3% vs 0.7% [p=0.04] and 1.4% vs 2.5% [p=0.01], respectively), shorter length of stay (mean 3.7 vs 5.7 days, p<0.01), lower residual disease (23.9% vs 26.7%, p<0.01), and lower additional cytoreductive procedures (59.3% vs 70.8%, p<0.01) in MIS compared to laparotomy, with similar rates of unplanned readmission (2.7% vs 3.1%, p=0.39).
Conclusions:
Patients who undergo IDS by MIS have similar overall survival and decreased morbidity compared with laparotomy.
Keywords: Laparoscopic surgery, interval debulking, minimally invasive surgery, ovarian cancer
1. Introduction
Primary debulking surgery performed via laparotomy followed by adjuvant chemotherapy has traditionally been the standard of care for advanced epithelial ovarian cancer, with a presumed survival advantage mainly based on its ability to achieve maximal cytoreduction.1 Over the past decade, four randomized controlled trials demonstrated equivalent overall and progression-free survival as well as decreased morbidity for neoadjuvant chemotherapy (NACT) followed by interval debulking surgery (IDS) compared to primary debulking surgery (PDS).2–5 Additionally, retrospective studies demonstrated higher rates of complete resection in patients who underwent NACT and IDS versus PDS followed by adjuvant chemotherapy.6 Subsequently, there has been a shift toward increased use of NACT for advanced ovarian cancer,7,8 raising the question of optimal surgical approach for IDS.
Typically, NACT for epithelial ovarian cancer is administered as three to four cycles of platinum-based chemotherapy, leaving diminished remaining abdominal tumor. This makes minimally invasive surgery (MIS) a more feasible option in order to achieve no gross residual disease on IDS.9 Prior studies of MIS for interval debulking of advanced ovarian cancer consist mainly of case reports and small series in which this approach appeared to be safe and effective.9,10 Additionally, MIS has been linked to earlier initiation of adjuvant chemotherapy, as well as decreased perioperative morbidity, postoperative pain and hernia rates when compared with laparotomy.11 We previously analyzed the National Cancer Database (NCDB) from 2010 to 2012 and identified 450 patients who underwent laparoscopic interval debulking. We observed equivalent 3-year survival with decreased morbidity compared to laparotomy.12 This initial study population likely reflected very early adopters of MIS for IDS, given early feasibility studies were published around the same time and the patients were very highly selected. In the current study, we sought to assess the survival, surgical, and clinical outcomes of interval debulking surgery performed by minimally invasive approach compared with laparotomy in a more contemporary (2013–2018) and larger population of patients with advanced epithelial ovarian cancer who had previously received neoadjuvant chemotherapy.
2. Methods
2.1. Study population and data sources
This was a retrospective cohort study using data from the NCDB, a dataset sourced from hospital registry data. The data are collected from more than 1,500 Commission on Cancer accredited facilities and represents more than 70% of all newly diagnosed cancer cases in the United States. The NCDB is a joint project of the American Cancer Society and the American College of Surgeon’s Commission on Cancer. The data used in this study were in an existing, deidentified database, therefore exemption was granted by the Institutional Review Board at MD Anderson Cancer Center.
All patients diagnosed with epithelial ovarian cancer from 2013 to 2018 in the 2019 NCDB participant user data file (the most recent file available with complete survival data) were included. Patients with American Joint Commission on Cancer (AJCC) stage IIIC and stage IV were selected. Pathologic stage was used when clinical stage was not available. The stage groups were TNM-based according to the seventh and eight editions of the AJCC Cancer Staging Manual.13,14 Serous, mucinous, clear cell, endometrioid and other histologies were selected, using the International Classification of Diseases for Oncology, Third Edition (Table 1).15 Cases were then excluded in the setting of no or unknown receipt of surgery (n=27,527) or chemotherapy (n=529), pre-existing cancer diagnosis (n=8,753), no microscopic confirmation (n=173), no treatment at reporting facility (n=3,242), surgery before chemotherapy (n=3,198), unknown sequence of treatment (n=27), and unknown surgical approach (n=1), such that the cases that remained after exclusion included only those patients with advanced epithelial ovarian cancer who underwent NACT followed by IDS with known surgical approach (Figure 1).
Table 1.
Epithelial ovarian cancer histology codes included in this analysis.
| Histologic Type | ICD-O-3 Code |
|---|---|
| Serous carcinoma | 8441, 8460–8463 |
| Mucinous carcinoma | 8470, 8471, 8480, 8481 |
| Endometrioid Carcinoma | 8380, 8381 |
| Clear cell carcinoma | 8310, 8313 |
| Other adenocarcinoma | 8050, 8140, 8144, 8255, 8260, 8263, 8290, 8320, 8323, 8340, 8440, 8450, 8490, 8560, 8574, 8940 |
Abbreviation: ICD-O-3, International Classification of Diseases for Oncology, Third Edition.
Figure 1:
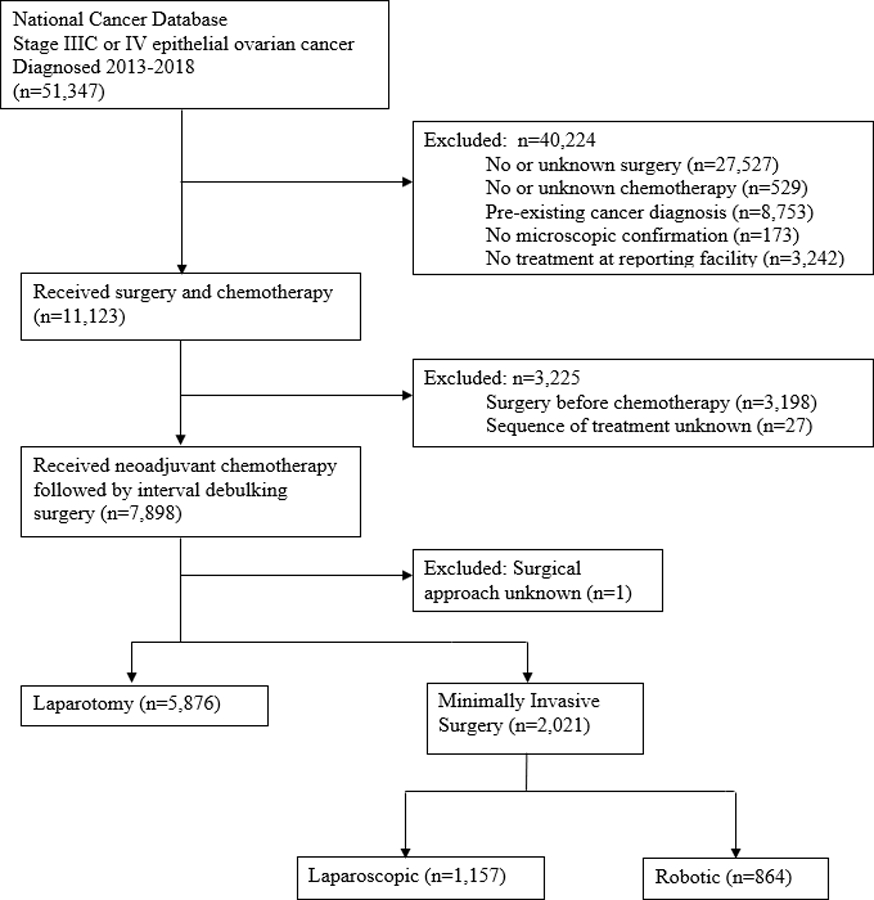
Flow diagram for the selection of patients from the National Cancer Database.
Cases were categorized based on patient and tumor characteristics. The NCDB does not provide data regarding clinical or pathologic response to chemotherapy. Age was defined as a continuous variable in years. Race and ethnicity were categorized as American Indian, Asian or Pacific Islander, Hispanic, Non-Hispanic Black, Non-Hispanic White, and none of the above. Race and ethnicity data were used because results of prior studies demonstrated different rates of minimally invasive gynecologic surgery by race and ethnicity.16,17 Geographic locations were categorized by US Census division of the reporting facility: New England, Middle Atlantic, South Atlantic, East-North Central, East-South Central, West-North Central, West-South Central, Mountain, Pacific, and unknown. Year of diagnosis was defined as the initial date of diagnosis whether clinically, pathologically, or retrospectively defined by physician. Charlson-Deyo comorbidity scores (0–3) were used to measure comorbidity. These scores are weighted and derived from the sum of scores for comorbid conditions. A value greater than or equal to 1 indicated the presence of comorbidities as defined by the International Classification of Diseases (ICD), Ninth or ICD Tenth Revision, Clinical Modification secondary diagnosis codes. Patients’ annual median household incomes were categorized as <$40,227, $40,227–50,353, $50,354–63,332 and ≥$63,333. Median income data is obtained by the NCDB based on the ZIP code of patient residence, collected via the 2016 American Community Survey. Insurance status was categorized as private insurance, Medicaid and other government insurance, Medicare, uninsured, or unknown. Rural-Urban status was categorized as metropolitan, rural, urban, or unknown, based on census data and the Rural-Urban Continuum Codes as defined by the United States Department of Agriculture Economic Research Service. The treating facility type was evaluated by program structure, services provided, and number of cases per year, as determined by the Commission on Cancer, and categorized as academic and research program, community cancer program, comprehensive community cancer program or integrated network cancer program. Medicaid expansion was classified as a non-expansion, January 2014 expansion, early expansion, late expansion, or unknown, based on the status of the state Medicaid expansion in the patient’s state at the time of diagnosis.
2.2. Statistical analysis
The primary outcome was overall survival, defined as months from cancer diagnosis to death or date of the last contact. Secondary outcomes included overall survival at 5 years after diagnosis, 30- and 90-day mortality, length of hospitalization, residual disease status, extent of surgery, percentage of surgical conversions, and unplanned readmissions. Extent of surgery was classified as “gynecologic” (hysterectomy and/or oophorectomy) with or without omentectomy, or “additional cytoreductive procedures” (including surgery to the bowel, urinary tract, other organs, or radical surgery). Analysis was performed as intention to treat based on surgical approach at initiation of surgery (laparotomy vs MIS). Patients who underwent MIS (laparoscopic or robot-assisted) that was then converted to laparotomy were included in the minimally invasive cohort.
We compared categorical variables using χ2 or Fisher’s exact tests and continuous variables using independent sample t-tests in univariate analysis of patient characteristics. We fit a multivariate logistic regression model to estimate the probability of receiving MIS; independent variables included age (categorized as <50, 50–59, 60–69, 70–79, and ≥80 years), year of diagnosis, race and ethnicity, treating facility type, Charlson-Deyo comorbidity score, state Medicaid expansion status, insurance status, annual median household income, United States region, rural-urban status, cancer stage, and histology.
Propensity score matching was performed to create a cohort in which patients who underwent MIS and patients who underwent laparotomy for IDS were balanced on the covariates used in the multivariate regression model, as those covariates may confound the effect of treatment approach on survival.18 Each patient who received MIS was matched with a patient who underwent laparotomy who had the same propensity to undergo MIS using Greedy nearest neighbor matching.19 Standardized differences of covariates in the propensity-matched cohort were assessed for balance. We then compared overall and 5-year survival between patients who underwent MIS versus laparotomy in the propensity score matched cohort using the Kaplan-Meier method and Cox proportional hazards regression model was used to estimate the hazard ratio (HR) and 95% confidence interval (CI) for the association of MIS with survival. We calculated 30- and 90-day mortality, extent of surgery, unplanned readmission rate, and residual disease between the two groups in the propensity-matched cohort using χ2 or Fisher’s exact test.
2.3. Sensitivity Analyses
We repeated all analyses for the primary outcome after excluding patients who had a conversion from MIS to laparotomy and repeated all analyses again after excluding those with clear cell, mucinous, or “other” histologies.
Although we adjusted for a large number of observed confounders using propensity score analysis, we additionally conducted a sensitivity analysis using the “E-value” to evaluate the robustness of derived estimates to potential unmeasured confounding.20 The E-value uses estimates from the study data to quantify the strength of association an unmeasured confounder must have with the exposure and outcome to fully explain an observed relationship. We used the E-value to calculate the magnitude of the association an unmeasured confounder would need to have with both the exposure (MIS versus laparotomy) and the outcome (overall survival) to fully explain the derived estimate (HR). We also calculated the E-value required to explain the confidence limit of the HR closest to the null and to explain an inverse relationship with survival.
All statistical tests were two-sided, and differences were considered statistically significant at P < 0.05. All statistical analysis was performed using SAS Enterprise Guide version 7.1.
3. Results
We identified 7,897 patients with stage IIIC and IV epithelial ovarian cancer who underwent NACT followed by IDS and met all inclusion criteria (Figure 1). A minimally invasive approach was initiated in 2,021 (25.6% overall, with increase from 20.3% of cases in 2013 to 29.0% of cases in 2018). Of the 2,021 cases, 1,157 (57.2%) underwent conventional laparoscopic approach and 864 (42.8%) underwent robotic-assisted laparoscopic approach. Conversion from MIS to laparotomy occurred in 208 cases (10.3%) and among the converted cases, residual disease was noted in 64.9%. Reasons for conversion are not provided in the NCDB dataset.
3.1. Patient demographic, clinical, and tumor characteristics
Univariate analysis of patient characteristics demonstrated several important differences among the laparotomy versus MIS groups (Table 2). Compared with patients undergoing laparotomy, those undergoing MIS were more likely to be older (65.2 years vs 63.8 years, p<0.001), have fewer comorbidities (Charlson-Deyo comorbidity score of 0; 79.6% vs 77.0%), to receive treatment at a Comprehensive Community Cancer Program (36.3% vs 26.3%), use Medicare insurance (50.8% vs 46.4%), and be diagnosed later in the cohort (p<0.001). Serous histology was the most common, comprising 6,981 cases (88.4%). There was no difference in MIS versus laparotomy according to disease stage (IIIC vs IV), race and ethnicity, or histology.
Table 2:
Patient demographic, clinical, and tumor characteristics before and after propensity score matching.
| Before propensity score matching | After propensity score matching | |||||
|---|---|---|---|---|---|---|
| MIS | Laparotomy | P value | MIS | Laparotomy | P value | |
| Variable | n=2021 | n=5876 | n=2021 | n=2021 | ||
| Age at diagnosis, years (Mean, SD) | 65.16 (10.60) | 63.83 (10.53) | <.001 | 65.16 (10.60) | 65.11 (10.25) | 0.90 |
| Race/Ethnicity (n, %) | 0.22 | 0.94 | ||||
| American Indian | 11 (0.5%) | 19 (0.3%) | 11 (0.5%) | 10 (0.5%) | ||
| Asian | 92 (4.6%) | 241 (4.1%) | 92 (4.6%) | 80 (4.0%) | ||
| Hispanic | 157 (7.8%) | 390 (6.6%) | 157 (7.8%) | 153 (7.6%) | ||
| Non-Hispanic Black | 169 (8.4%) | 510 (8.7%) | 169 (8.4%) | 178 (8.8%) | ||
| Non-Hispanic White | 1562 (77.3%) | 4644 (79.0%) | 1562 (77.3%) | 1572 (77.8%) | ||
| Unknown | 30 (1.5%) | 72 (1.2%) | 30 (1.5%) | 28 (1.4%) | ||
| Charlson-Deyo comorbidity score (n, %) | 0.03 | 0.98 | ||||
| 0 | 1608 (79.6%) | 4526 (77.0%) | 1608 (79.6%) | 1604 (79.4%) | ||
| 1 | 290 (14.3%) | 1006 (17.1%) | 290 (14.3%) | 298 (14.7%) | ||
| 2 | 84 (4.2%) | 243 (4.1%) | 84 (4.2%) | 82 (4.1%) | ||
| 3 | 39 (1.9%) | 101 (1.7%) | 39 (1.9%) | 37 (1.8%) | ||
| United States region (n, %) | <.001 | 1.0 | ||||
| New England | 111 (5.5%) | 286 (4.9%) | 111 (5.5%) | 108 (5.3%) | ||
| Middle Atlantic | 272 (13.5%) | 1009 (17.2%) | 272 (13.5%) | 267 (13.2%) | ||
| South Atlantic | 482 (23.8%) | 1053 (17.9%) | 482 (23.8%) | 500 (24.7%) | ||
| East-North Central | 276 (13.7%) | 990 (16.8%) | 276 (13.7%) | 271 (13.4%) | ||
| East-South Central | 101 (5.0%) | 440 (7.5%) | 101 (5.0%) | 98 (4.8%) | ||
| West-North Central | 142 (7.0%) | 517 (8.8%) | 142 (7.0%) | 138 (6.8%) | ||
| West-South Central | 188 (9.3%) | 617 (10.5%) | 188 (9.3%) | 190 (9.4%) | ||
| Mountain | 120 (5.9%) | 194 (3.3%) | 120 (5.9%) | 116 (5.7%) | ||
| Pacific | 307 (15.2%) | 682 (11.6%) | 307 (15.2%) | 307 (15.2%) | ||
| Unknown | 22 (1.1%) | 88 (1.5%) | 22 (1.1%) | 26 (1.3%) | ||
| Rural-Urban status (n, %) | <0.01 | 0.76 | ||||
| Metropolitan | 1691 (83.7%) | 4731 (80.5%) | 1691 (83.7%) | 1694 (83.8%) | ||
| Urban | 233 (11.5%) | 738 (12.6%) | 233 (11.5%) | 224 (11.1%) | ||
| Rural | 29 (1.4%) | 95 (1.6%) | 29 (1.4%) | 37 (1.8%) | ||
| Unknown | 68 (3.4%) | 312 (5.3%) | 68 (3.4%) | 66 (3.3%) | ||
| Annual median household income (n, %) | 0.001 | 0.73 | ||||
| < $40,227 | 260 (12.9%) | 855 (14.6%) | 260 (12.9%) | 243 (12.0%) | ||
| $40,227-$50,353 | 353 (17.5%) | 1005 (17.1%) | 353 (17.5%) | 345 (17.1%) | ||
| $50,354-$63,332 | 440 (21.8%) | 1145 (19.5%) | 440 (21.8%) | 457 (22.6%) | ||
| ≥$63333 | 704 (34.8%) | 1927 (32.8%) | 704 (34.8%) | 690 (34.1%) | ||
| Unknown | 264 (13.1%) | 944 (16.1%) | 264 (13.1%) | 286 (14.2%) | ||
| Facility type (n, %) | <.001 | 0.93 | ||||
| Community cancer program | 12 (0.6%) | 53 (0.9%) | 12 (0.6%) | 13 (0.6%) | ||
| Comprehensive community cancer program | 733 (36.3%) | 1547 (26.3%) | 733 (36.3%) | 751 (37.2%) | ||
| Academic/research program | 901 (44.6%) | 2998 (51.0%) | 901 (44.6%) | 890 (44.0%) | ||
| Integrated network | 353 (17.5%) | 1190 (20.3%) | 353 (17.5%) | 341 (16.9%) | ||
| Unknown | 22 (1.1%) | 88 (1.5%) | 22 (1.1%) | 26 (1.3%) | ||
| Insurance type (n, %) | <.001 | 0.92 | ||||
| Private | 799 (39.5%) | 2405 (40.9%) | 799 (39.5%) | 778 (38.5%) | ||
| Medicaid and other government | 130 (6.4%) | 517 (8.8%) | 130 (6.4%) | 133 (6.6%) | ||
| Medicare | 1026 (50.8%) | 2729 (46.4%) | 1026 (50.8%) | 1035 (51.2%) | ||
| None | 45 (2.2%) | 173 (2.9%) | 45 (2.2%) | 50 (2.5%) | ||
| Unknown | 21 (1.0%) | 52 (0.9%) | 21 (1.0%) | 25 (1.2%) | ||
| State Medicaid expansion status (n, %) | <.001 | 0.85 | ||||
| Non-expansion | 819 (40.5%) | 2242 (38.2%) | 819 (40.5%) | 832 (41.2%) | ||
| January 2014 expansion | 534 (26.4%) | 1732 (29.5%) | 534 (26.4%) | 544 (26.9%) | ||
| Early expansion | 425 (21.0%) | 1042 (17.7%) | 425 (21.0%) | 416 (20.6%) | ||
| Late expansion | 221 (10.9%) | 772 (13.1%) | 221 (10.9%) | 203 (10.0%) | ||
| Not available | 22 (1.1%) | 88 (1.5%) | 22 (1.1%) | 26 (1.3%) | ||
| Year of diagnosis (n, %) | <.001 | 0.82 | ||||
| 2013 | 200 (9.9%) | 785 (13.4%) | 200 (9.9%) | 202 (10.0%) | ||
| 2014 | 261 (12.9%) | 827 (14.1%) | 261 (12.9%) | 285 (14.1%) | ||
| 2015 | 311 (15.4%) | 982 (16.7%) | 311 (15.4%) | 324 (16.0%) | ||
| 2016 | 395 (19.5%) | 1117 (19.0%) | 395 (19.5%) | 392 (19.4%) | ||
| 2017 | 418 (20.7%) | 1097 (18.7%) | 418 (20.7%) | 394 (19.5%) | ||
| 2018 | 436 (21.6%) | 1068 (18.2%) | 436 (21.6%) | 424 (21.0%) | ||
| Histology (n, %) | 0.10 | 0.90 | ||||
| Serous carcinoma | 1786 (88.4%) | 5195 (88.4%) | 1786 (88.4%) | 1779 (88.0%) | ||
| Clear cell carcinoma | 19 (0.9%) | 96 (1.6%) | 19 (0.9%) | 24 (1.2%) | ||
| Endometrioid carcinoma | 16 (0.8%) | 53 (0.9%) | 16 (0.8%) | 15 (0.7%) | ||
| Mucinous carcinoma | 6 (0.3%) | 27 (0.5%) | 6 (0.3%) | 4 (0.2%) | ||
| Other adenocarcinoma | 194 (9.6%) | 505 (8.6%) | 194 (9.6%) | 199 (9.8%) | ||
| Cancer stage (n, %) | 0.13 | 0.23 | ||||
| IIIC | 993 (49.1%) | 3001 (51.1%) | 993 (49.1%) | 955 (47.3%) | ||
| IV | 1028 (50.9%) | 2875 (48.9%) | 1028 (50.9%) | 1066 (52.7%) | ||
Abbreviations: MIS, minimally invasive surgery; SD, standard deviation; n, number.
In a multivariate analysis, factors associated with increased use of MIS included: age ≥80 years (vs 60–69 years), Hispanic race/ethnicity (vs non-Hispanic White race/ethnicity), Charlson-Deyo comorbidity score of 0 (vs 1), New England region (vs Middle Atlantic, and all Central regions), Mountain region (vs New England), metropolitan status (vs unknown rural-urban status), treatment at a comprehensive community cancer program (vs community cancer program), income of $50,354-$63,332 (vs <$40,227), private insurance (vs Medicaid or other government insurance), and diagnosis between 2015–2018 (vs 2013) (Table 3).
Table 3:
Multivariate analysis of independent predictors of the use of minimally invasive surgery for interval debulking surgery.
| Demographic or clinical variable | OR (95%CI)* |
|---|---|
| Age, years (reference = 60–69) | |
| <50 | 0.92 (0.74–1.15) |
| 50–59 | 1.00 (0.86–1.17) |
| 70–79 | 1.14 (0.99–1.31) |
| ≥80 | 1.59 (1.28–1.96) |
| Race and ethnicity (reference = non-Hispanic White) | |
| American Indian | 1.80 (0.83–3.91) |
| Asian or Pacific Islander | 1.20 (0.92–1.55) |
| Hispanic | 1.26 (1.02–1.56) |
| Non-Hispanic Black | 1.13 (0.93–1.38) |
| None of the above | 1.28 (0.81–2.01) |
| Charlson-Deyo comorbidity score (reference = 0) | |
| 1 | 0.80 (0.69–0.92) |
| 2 | 0.92 (0.71–1.20) |
| 3 | 0.96 (0.65–1.42) |
| United States region (reference = New England) | |
| Middle Atlantic | 0.62 (0.48–0.82) |
| South Atlantic | 1.01 (0.76–1.35) |
| East-North Central | 0.73 (0.56–0.96) |
| East-South Central | 0.55 (0.40–0.78) |
| West-North Central | 0.64 (0.47–0.86) |
| West-South Central | 0.66 (0.48–0.90) |
| Mountain | 1.44 (1.03–2.01) |
| Pacific | 0.88 (0.65–1.17) |
| Rural-urban status (reference = metropolitan) | |
| Urban | 0.94 (0.79–1.12) |
| Rural | 0.95 (0.62–1.47) |
| Unknown | 0.69 (0.52–0.91) |
| Annual median household income (reference = <$40,227) | |
| $40,227-$50,353 | 1.15 (0.95–1.39) |
| $50,354-$63,332 | 1.22 (1.00–1.47) |
| ≥$63,333 | 1.14 (0.95–1.38) |
| Unknown | 0.90 (0.73–1.11) |
| Facility type (reference = community cancer program) | |
| Comprehensive community cancer program | 2.40 (1.26–4.57) |
| Academic or research program | 1.62 (0.85–3.09) |
| Integrated network | 1.48 (0.78–2.83) |
| Insurance type (reference = private) | |
| Medicaid or other government insurance | 0.71 (0.57–0.89) |
| Medicare | 1.01 (0.87–1.17) |
| Uninsured | 0.80 (0.56–1.13) |
| Unknown | 1.23 (0.72–2.09) |
| State Medicaid expansion status (reference = non-expansion) | |
| January 2014 expansion | 0.92 (0.78–1.09) |
| Early expansion | 1.08 (0.85–1.37) |
| Late expansion | 0.93 (0.75–1.16) |
| Year of diagnosis (reference = 2013) | |
| 2014 | 1.21 (0.98–1.50) |
| 2015 | 1.25 (1.02–1.54) |
| 2016 | 1.39 (1.14–1.69) |
| 2017 | 1.49 (1.22–1.81) |
| 2018 | 1.62 (1.33–1.97) |
| Histology (reference = serous carcinoma) | |
| Clear cell carcinoma | 0.62 (0.37–1.02) |
| Endometrioid carcinoma | 0.88 (0.48–1.63) |
| Mucinous carcinoma | 0.63 (0.26–1.55) |
| Other adenocarcinoma | 1.18 (0.98–1.41) |
| Stage (reference = stage IIIC) | |
| Stage IV | 1.10 (0.99–1.22) |
An odds ratio (OR) >1 indicates an increased likelihood of IDS performed via MIS. OR<1 indicates an increased likelihood to have IDS performed via laparotomy.
Abbreviations: OR, odds ratio; CI, confidence interval.
Propensity score matching between the MIS and laparotomy groups yielded 100% match rate for the 2,021 patients in the MIS group. After propensity score matching, there were no significant differences between the MIS and laparotomy groups with respect to clinical or demographic variables (Table 2), and an assessment between groups found all variables to be balanced (Appendix A).
3.2. Survival analysis
Figure 2 shows Kaplan-Meier survival curves for the groups in the propensity-matched cohort. The mean overall survival in this cohort was 35.9±18.3 months for the MIS group and 34.5±18.9 months in the laparotomy group (p=0.01). In addition, 5-year survival probabilities were 38.3% (95% CI, 35.5–41.2%) and 34.8% (95% CI, 32.1–37.5%) in the MIS and laparotomy groups, respectively (p<0.01). Overall, patients in the MIS group had a 14% decreased hazard of all-cause mortality compared with patients in the laparotomy group (HR=0.86; 95% CI, 0.79–0.94). Within the propensity-matched cohort, 30- and 90-day mortality probabilities were 0.3% and 1.4%, respectively, in the MIS group, and 0.7% and 2.5%, respectively, in the laparotomy group (p=0.04 for 30-day mortality, p=0.01 for 90-day mortality).
Figure 2:
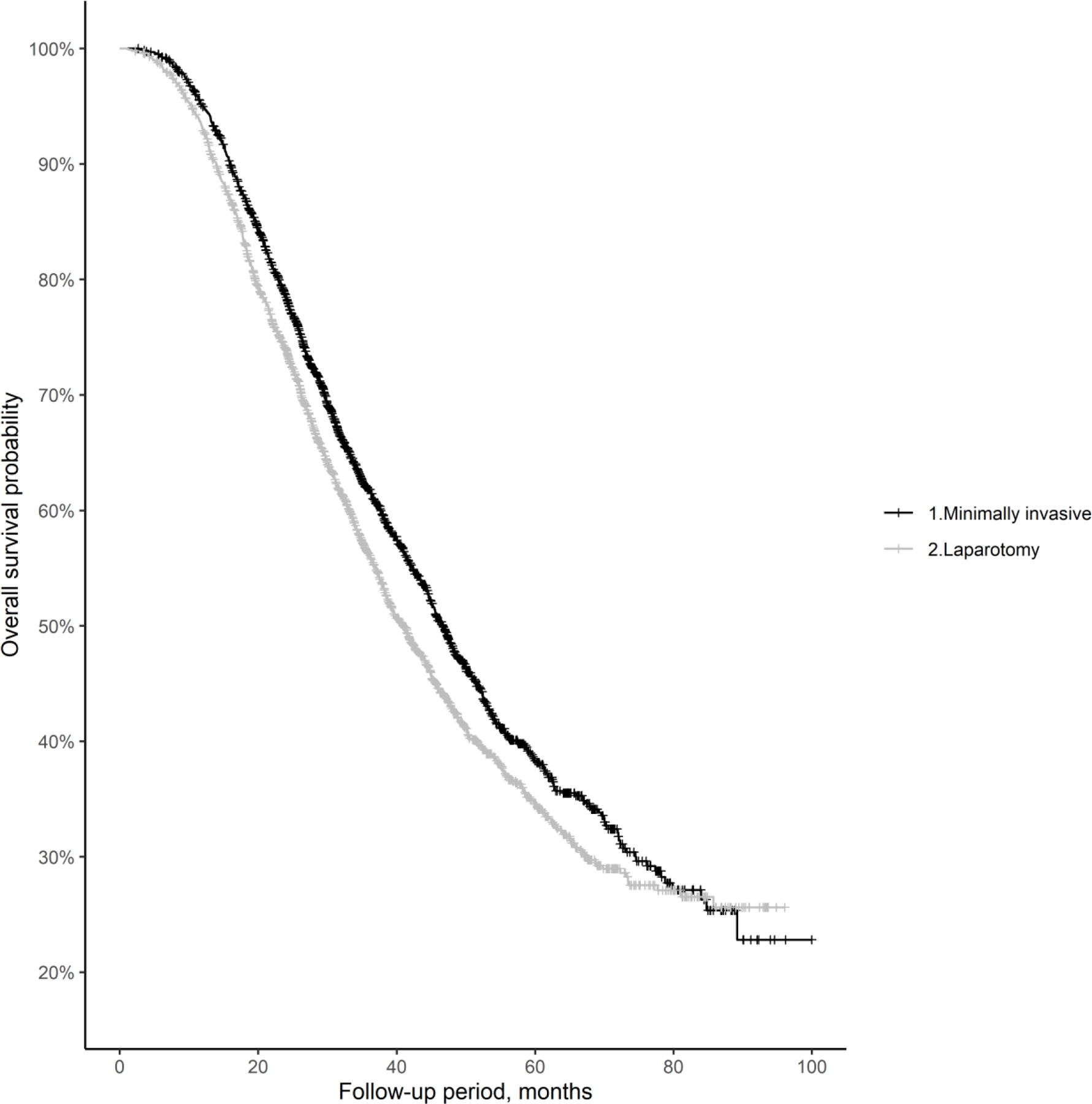
Kaplan-Meier overall survival curves for the propensity-matched cohort.
3.3. Secondary outcomes analyses
Secondary outcomes of the propensity-matched cohort are listed in Table 4. Minimally invasive surgery was associated with a significantly shorter mean hospitalization duration (3.7 days vs 5.7 days, p<0.01). There was overall a significant difference in residual disease status, with a higher percentage of no residual disease among patients undergoing IDS via MIS than laparotomy (43.2% versus 38.6%, p<0.01), although documentation of residual disease was not available for 33.8% of cases (32.9% the MIS group and 34.7% in the laparotomy group). There was a lower percentage of additional cytoreductive procedures performed as part of the surgery in the MIS group than laparotomy group (59.3% vs 70.8%, p<0.01). Readmission rates were overall low, with a non-significant difference between the groups (unplanned readmission rates of 2.7% in the MIS group and 3.1% in the laparotomy group, p=0.39).
Table 4:
Secondary surgical and clinical outcomes according to surgical approach in the propensity-matched cohort.*
| Variable | Minimally Invasive | Laparotomy | p-value |
|---|---|---|---|
| Extent of surgery, n (%) | <0.01 | ||
| Gynecologic ± omentectomy | 817 (40.7) | 588 (29.2) | |
| Additional cytoreductive procedures | 1190 (59.3) | 1428 (70.8) | |
| Residual disease, n (%) | 0.01 | ||
| No residual tumor | 873 (43.2) | 780 (38.6) | |
| Residual tumor | 483 (23.9) | 539 (26.7) | |
| Unknown | 665 (32.9) | 702 (34.7) | |
| 30-Day mortality, n (%) | 6 (0.3) | 15 (0.7) | 0.04 |
| 90-Day mortality, n (%) | 28 (1.4) | 50 (2.5) | 0.01 |
| Hospitalization duration, days (mean, SD) | 3.7 (5.0) | 5.7 (5.3) | <0.01 |
| Readmission within 30 Days, n (%) | 0.39 | ||
| No readmission | 1926 (95.3) | 1927 (95.4) | |
| Unplanned readmission | 55 (2.7) | 63 (3.1) | |
| Planned readmission | 33 (1.6) | 22 (1.1) | |
| Unknown | 7 (0.4) | 9 (0.5) | |
| Overall survival, months (mean, SD) | 35.9 (18.3) | 34.5 (18.9) | 0.01 |
Percentages in the table may not equal 100% due to rounding.
Abbreviations: n, number; SD, standard deviation.
3.4. Sensitivity analyses
We performed a sensitivity analysis using only the cases that were initiated and completed minimally invasively (n=1,813), after excluding cases that were converted to laparotomy (n=208). Propensity score matching was repeated, demonstrating 100% match rate for all 1,813 cases and balance between groups for each variable. Cox regression analysis demonstrated decreased hazard of all-cause mortality in the MIS group compared to laparotomy, consistent with results of the intention-to-treat analysis (HR=0.88 [95% CI 0.80–0.96] see Appendix B for further details). A second sensitivity analysis was performed using only those with high grade serous or endometrioid histologies (n=1,210) and found no difference in overall survival between the MIS and laparotomy groups (HR=0.94 [95% CI 0.84–1.05], see Appendix C for further details).
We calculated E-values to assess the sensitivity of our primary outcome finding to unmeasured confounding. For overall survival, the observed HR of 0.86 in favor of MIS, conditional on measured covariates, could be explained by an unmeasured confounder that was associated with both the surgical approach (MIS or laparotomy) and overall survival by a risk ratio (RR) of 1.45. To move the 95% CI to include the null, an unmeasured confounder associated with both surgical approach and overall survival by a RR of at least 1.25 was required. An unmeasured confounder would have to be associated with both surgical approach and survival by an RR of 2.96 to shift the HR to 2.0. This would reverse the finding in this study and conclude a clinically meaningful increase in HR of death for MIS compared to laparotomy.
4. Discussion
Among patients who receive NACT for advanced ovarian cancer, it may be possible to reduce morbidity by performing IDS using minimally invasive surgical techniques.9,12,21–25 As NACT has become a more common approach in the upfront management of advanced epithelial ovarian cancer,26,27 the use of minimally invasive techniques for IDS has also risen, accounting for 29% of all interval debulking surgeries in 2018 (see Appendix D for supplemental figure 1). In the present large national database study examining surgical approach after NACT, MIS was not associated with worse overall survival compared with laparotomy. This finding was robust to potential unmeasured confounders and remained significant in sensitivity analyses after excluding those requiring surgical conversions from MIS to laparotomy, and those with clear cell, mucinous, or “other” histologies.
Despite the increasing popularity of MIS for IDS, evidence reporting its oncologic safety and efficacy is limited. Among patients with ovarian cancer, resection to no residual disease is the goal of any debulking surgery. Critics of MIS in this setting state that abdominal exploration without tactile feedback may lead to higher rates of occult residual disease and that MIS may be associated with cancer spread or inability to fully debulk large volume disease.28 Furthermore, studies of minimally invasive cancer surgery for other gynecological malignancies have yielded contradictory results. Among patients with early-stage endometrial cancer, MIS improves perioperative outcomes without impairing survival.29,30 In contrast, among patients diagnosed with early-stage cervical cancer, minimally invasive radical hysterectomy, until recently a standard approach, has been found to be inferior to open radical hysterectomy.31–34
Notwithstanding these concerns, current National Comprehensive Cancer Network guidelines suggest that minimally invasive techniques may be used for IDS in “select patients.”35 It is unclear exactly who these “select” patients may be. This study found differences in likelihood of undergoing MIS by race/ethnicity, geography, comorbidity scores, and income, however, the study was not powered to address these disparities given the high percentage of non-Hispanic White patients (77%), those with comorbidity scores of 0 (79%), and relatively lower numbers of patients with annual household incomes below $50,000 or living in non-Metropolitan areas. It is more likely patient-specific factors outside of those reported here may play an important role in selection for MIS. Prior observational studies demonstrated a high rate of complete cytoreduction, good perioperative outcomes, and excellent progression-free survival among patients who underwent minimally invasive interval cytoreductive surgery after responding to NACT.9,12,21–25 In these studies, researchers may have selected the most favorable patients for MIS, such as those with better responses to chemotherapy based on pre-operative findings, those with better performance statuses, or those with fewer comorbidities, than patients who underwent laparotomy. These factors can result in differences in the distribution of prognostic factors between patients who are treated with these two surgical approaches. In the present investigation, we used propensity score matching to adjust for many variables traditionally associated with treatment approach.
Although the extent of surgery was noted to be more extensive in the laparotomy group than in the MIS group, which may explain why there was increased residual disease in the former, these differences did not result in survival gains for laparotomy. Furthermore, we tested the robustness of the overall survival HR to potential unmeasured confounders, and we found that moderate unmeasured confounding would be required to fully explain away the significance of our primary outcome, and that moderate unmeasured confounding would be required to reverse the overall survival finding in this study.
A notable aspect of the retrospective nature of this study that must be addressed is the possibility of inadvertent selection bias, as the intent of each surgeon when selecting the surgical approach was unknown, the NACT regimen(s) and the patient-specific response to NACT were not available. The sensitivity analysis performed on cases that initiated and completed debulking by a minimally invasive approach was an attempt to remove the possibility of bias due to cases that were perhaps initiated laparoscopically with intent to either pursue laparotomy or abort the debulking procedure entirely. After removal of cases that were initiated via a MIS approach and converted to laparotomy, there was no change in overall survival outcomes, suggesting that surgical conversion did not adversely affect the results of the MIS cohort.
A strength of this study was the use of information from a large national database that reflects actual practice patterns for all settings in the United States where patients with ovarian cancer receive care. Nevertheless, it is important to note several limitations. Although the NCDB does not account for surgeon intent or chemotherapy response, we used clinical factors used to assess surgical fitness (age and comorbidities). The MIS group did not appear to be definitively more healthy and thus better surgical candidates than the laparotomy group based on available information, as the surgical approach did not differ based on disease stage, and older patients were more likely to undergo MIS than younger ones. A lack of data regarding functional status and disease burden limited the ability to determine pre-surgical survival estimates for patients, and missing data regarding residual disease status may limit interpretation of this result. Additionally, mortality outcomes in the NCDB are not cancer-specific, and progression-free survival is not assessed in the NCDB, thus limiting the ability to interpret our findings. Use of propensity score matching enabled us to compare people diagnosed in similar years, thus minimizing the effect between groups of changes in therapy within the last decade owing to use of therapeutics such as bevacizumab or poly(ADP)-ribose polymerase) inhibitors. However, data on the specific chemotherapy regimens for each patient were not included in the dataset.
In conclusion, we found that MIS is not associated with higher mortality than laparotomy after NACT. Our findings were robust to large differences in potential unmeasured confounders. Although the data presented here is promising, it is unknown if minimally invasive interval cytoreductive surgery for advanced ovarian cancer delivers long-term oncologic outcomes similar to those achieved with laparotomy, and the role of MIS in this population remains controversial. The data presented in this study suggest MIS is increasingly utilized for IDS and clinicians and patients thus far have reasons to be optimistic about its use. Forthcoming data from a prospective, multicentric randomized trial (the LANCE Trial) may mitigate many of the limitations of this and prior retrospective studies.36
Supplementary Material
Highlights.
Minimally invasive interval debulking in advanced ovarian cancer is associated with similar survival to laparotomy.
Minimally invasive interval debulking surgery is associated with improved morbidity compared to laparotomy.
Use of minimally invasive surgery for interval debulking is gaining popularity but prospective data is missing.
Acknowledgements:
This work was supported by grants from the National Institutes of Health National Cancer Institute (NIH/NCI) under cancer center support grant number P30 CA016672 (J.A. Rauh-Hain, K. Jorgensen, R. Nitecki, P.T. Ramirez, and C-F. Wu); grant number K08 CA234333 (J.A. Rauh-Hain); grant number T32 CA101642 (K. Jorgensen and R. Nitecki). Dr. Alexander Melamed reports funding from the Department of Defense Ovarian Cancer Research Program (AM OC210024).The funding sources were not involved in the development of the research hypothesis or study design, data analysis, or manuscript writing. Editorial support was provided by Don Norwood of Editing Services, Research Medical Library at MD Anderson Cancer Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This study was accepted for presentation at the Society of Gynecologic Oncology Annual Meeting on Women’s Cancer, to be held March 25–28, 2023.
CRediT author statement:
Kirsten Jorgensen: Conceptualization, Data curation, Methodology, Writing original draft & editing. Alexander Melamed: Methodology, Writing original draft & editing. Chi-Fang Wu: Data curation, Formal analysis, Methodology, Validation, Writing original draft & editing. Roni Nitecki: Writing original draft & editing. Rene Pareja: Writing original draft & editing. Anna Fagotti: Writing original draft & editing. John O Schorge: Writing original draft & editing. Pedro T Ramirez: Writing original draft & editing. Jose Alejandro Rauh-Hain: Conceptualization, Methodology, Supervision, Writing original draft & editing.
Declaration of Competing Interest
In addition to the funding sources listed above, the following authors have additional disclosures:
Alexander Melamed: served on AstraZeneca advisory board
J. Alejandro Rauh-Hain: received consulting fees from Schlesinger Group and Guidepoint
John O. Schorge: served on Avenge, Bio, advisory boards and received royalties from McGraw-Hill and UpToDate
Pedro T. Ramirez: received support for travel to the European Society of Gynecologic Oncology (2022), FIGO Regional Meeting (2022), and IGCS Annual Meeting (2022). All other authors report no competing interests.
References
- 1.Bristow RE, Tomacruz RS, Armstrong DK, Trimble EL, Montz FJ. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. J Clin Oncol 2002;20(5):1248–1259. doi: 10.1200/JCO.2002.20.5.1248 [DOI] [PubMed] [Google Scholar]
- 2.Vergote I, Tropé CG, Amant F, et al. Neoadjuvant Chemotherapy or Primary Surgery in Stage IIIC or IV Ovarian Cancer. N Engl J Med 2010;363(10):943–953. doi: 10.1056/NEJMoa0908806 [DOI] [PubMed] [Google Scholar]
- 3.Fagotti A, Ferrandina MG, Vizzielli G, et al. Randomized trial of primary debulking surgery versus neoadjuvant chemotherapy for advanced epithelial ovarian cancer (SCORPION-NCT01461850). Int J Gynecol Cancer 2020;30(11):1657–1664. doi: 10.1136/ijgc-2020-001640 [DOI] [PubMed] [Google Scholar]
- 4.Kehoe S, Hook J, Nankivell M, et al. Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): an open-label, randomised, controlled, non-inferiority trial. Lancet 2015;386(9990):249–257. doi: 10.1016/S0140-6736(14)62223-6 [DOI] [PubMed] [Google Scholar]
- 5.Onda T, Satoh T, Saito T, et al. Comparison of treatment invasiveness between upfront debulking surgery versus interval debulking surgery following neoadjuvant chemotherapy for stage III/IV ovarian, tubal, and peritoneal cancers in a phase III randomised trial: Japan Clinical Oncology Group Study JCOG0602. Eur J Cancer 2016;64:22–31. doi: 10.1016/j.ejca.2016.05.017 [DOI] [PubMed] [Google Scholar]
- 6.Rauh-Hain JA, Rodriguez N, Growdon WB, et al. Primary debulking surgery versus neoadjuvant chemotherapy in stage IV ovarian cancer. Ann Surg Oncol 2012;19(3):959–965. doi: 10.1245/s10434-011-2100-x [DOI] [PubMed] [Google Scholar]
- 7.Wright AA, Bohlke K, Armstrong DK, et al. Neoadjuvant chemotherapy for newly diagnosed, advanced ovarian cancer: Society of Gynecologic Oncology and American Society of Clinical Oncology Clinical Practice Guideline. Gynecol Oncol 2016;143(1):3–15. doi: 10.1016/j.ygyno.2016.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patel A, Iyer P, Matsuzaki S, Matsuo K, Sood AK, Fleming ND. Emerging Trends in Neoadjuvant Chemotherapy for Ovarian Cancer. Cancers 2021;13(4):626. doi: 10.3390/cancers13040626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gueli Alletti S, Bottoni C, Fanfani F, et al. Minimally invasive interval debulking surgery in ovarian neoplasm (MISSION trial–NCT02324595): a feasibility study. Am J Obstet Gynecol 2016;214(4):503.e1–503.e6. doi: 10.1016/j.ajog.2015.10.922 [DOI] [PubMed] [Google Scholar]
- 10.Nezhat FR, Pejovic T, Finger TN, Khalil SS. Role of Minimally Invasive Surgery in Ovarian Cancer. J Minim Invasive Gynecol 2013;20(6):754–765. doi: 10.1016/j.jmig.2013.04.027 [DOI] [PubMed] [Google Scholar]
- 11.Menderes G, Black JD, Azodi M. The role of minimally invasive interval debulking surgery in advanced epithelial ovarian cancer. Expert Rev Anticancer Ther 2016;16(9):899–901. doi: 10.1080/14737140.2016.1219658 [DOI] [PubMed] [Google Scholar]
- 12.Melamed A, Nitecki R, Boruta DM, et al. Laparoscopy Compared With Laparotomy for Debulking Ovarian Cancer After Neoadjuvant Chemotherapy. Obstet Gynecol 2017;129(5):861–869. doi: 10.1097/AOG.0000000000001851 [DOI] [PubMed] [Google Scholar]
- 13.Amin MB, Gress DM, Vega LRM, Edge SB. AJCC Cancer Staging Manual 8th edition. (Greene FL, Byrd DR, Brookland RK, Washington MK, Compton CC, eds.). Springer; 2017. [DOI] [PubMed] [Google Scholar]
- 14.Edge SB, American Joint Committee on Cancer, eds. AJCC Cancer Staging Manual 7th ed. Springer; 2010. [DOI] [PubMed] [Google Scholar]
- 15.National Cancer Institute. SEER ICD-O-3 Guidelines. ICD-O-3 Coding Materials Published 2022. Accessed September 15, 2022. https://seer.cancer.gov/icd-o-3/index.html
- 16.Alexander AL, Strohl AE, Rieder S, Holl J, Barber EL. Examining Disparities in Route of Surgery and Postoperative Complications in Black Race and Hysterectomy. Obstet Gynecol 2019;133(1):6–12. doi: 10.1097/AOG.0000000000002990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pollack LM, Olsen MA, Gehlert SJ, Chang SH, Lowder JL. Racial/Ethnic Disparities/Differences in Hysterectomy Route in Women Likely Eligible for Minimally Invasive Surgery. J Minim Invasive Gynecol 2020;27(5):1167–1177.e2. doi: 10.1016/j.jmig.2019.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosenbaum PR, Rubin DB. The Central Role of the Propensity Score in Observational Studies for Causal Effects. Biometrika 1983;70(1):41–55. doi: 10.2307/2335942 [DOI] [Google Scholar]
- 19.Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat 2011;10(2):150–161. doi: 10.1002/pst.433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E-value. Ann Intern Med 2017;167(4):268–274. doi: 10.7326/M16-2607 [DOI] [PubMed] [Google Scholar]
- 21.Fagotti A, Alletti SG, Corrado G, et al. The INTERNATIONAL MISSION study: minimally invasive surgery in ovarian neoplasms after neoadjuvant chemotherapy. International Journal of Gynecologic Cancer 2019;29(1):5–9. doi: 10.1136/ijgc-2018-000012 [DOI] [PubMed] [Google Scholar]
- 22.Brown J, Drury L, Crane EK, et al. When Less Is More: Minimally Invasive Surgery Compared with Laparotomy for Interval Debulking After Neoadjuvant Chemotherapy in Women with Advanced Ovarian Cancer. J Minim Invasive Gynecol 2019;26(5):902–909. doi: 10.1016/j.jmig.2018.09.765 [DOI] [PubMed] [Google Scholar]
- 23.Favero G, Macerox N, Pfiffer T, et al. Oncologic Concerns regarding Laparoscopic Cytoreductive Surgery in Patients with Advanced Ovarian Cancer Submitted to Neoadjuvant Chemotherapy. Oncology 2015;89(3):159–166. doi: 10.1159/000381462 [DOI] [PubMed] [Google Scholar]
- 24.Ackroyd SA, Thomas S, Angel C, Moore R, Meacham PJ, DuBeshter B. Interval robotic cytoreduction following neoadjuvant chemotherapy in advanced ovarian cancer. J Robotic Surg 2018;12(2):245–250. doi: 10.1007/s11701-017-0720-2 [DOI] [PubMed] [Google Scholar]
- 25.Corrado G, Mancini E, Cutillo G, et al. Laparoscopic Debulking Surgery in the Management of Advanced Ovarian Cancer After Neoadjuvant Chemotherapy. Int J Gynecol Cancer 2015;25(7):1253–1257. doi: 10.1097/IGC.0000000000000491 [DOI] [PubMed] [Google Scholar]
- 26.Melamed A, Fink G, Wright AA, et al. Effect of adoption of neoadjuvant chemotherapy for advanced ovarian cancer on all cause mortality: quasi-experimental study. BMJ 2018;360:j5463. doi: 10.1136/bmj.j5463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Melamed A, Hinchcliff EM, Clemmer JT, et al. Trends in the use of neoadjuvant chemotherapy for advanced ovarian cancer in the United States. Gynecol Oncol 2016;143(2):236–240. doi: 10.1016/j.ygyno.2016.09.002 [DOI] [PubMed] [Google Scholar]
- 28.Kremer KM, Lee J, Carlson MJ, Lococo SJ, Miller DS, Lea JS. Practice patterns using minimally invasive surgery for the treatment of ovarian cancer: A survey of physician members of the Society of Gynecologic Oncologists. Gynecol Oncol Rep 2020;33:1–5. doi: 10.1016/j.gore.2020.100617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walker JL, Piedmonte MR, Spirtos NM, et al. Recurrence and survival after random assignment to laparoscopy versus laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group LAP2 Study. J Clin Oncol 2012;30(7):695–700. doi: 10.1200/JCO.2011.38.8645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Janda M, Gebski V, Davies LC, et al. Effect of Total Laparoscopic Hysterectomy vs Total Abdominal Hysterectomy on Disease-Free Survival Among Women With Stage I Endometrial Cancer: A Randomized Clinical Trial. JAMA 2017;317(12):1224–1233. doi: 10.1001/jama.2017.2068 [DOI] [PubMed] [Google Scholar]
- 31.Ramirez PT, Frumovitz M, Pareja R, et al. Minimally Invasive versus Abdominal Radical Hysterectomy for Cervical Cancer. N Engl J Med 2018;379(20):1895–1904. doi: 10.1056/NEJMoa1806395 [DOI] [PubMed] [Google Scholar]
- 32.Melamed A, Rauh-Hain JA, Ramirez PT. Minimally Invasive Radical Hysterectomy for Cervical Cancer: When Adoption of a Novel Treatment Precedes Prospective, Randomized Evidence. J Clin Oncol 2019;37(33):3069–3074. doi: 10.1200/JCO.19.01164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nitecki R, Ramirez PT, Frumovitz M, et al. Survival After Minimally Invasive vs Open Radical Hysterectomy for Early-Stage Cervical Cancer: A Systematic Review and Meta-analysis. JAMA Oncol 2020;6(7):1019–1027. doi: 10.1001/jamaoncol.2020.1694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Melamed A, Margul DJ, Chen L, et al. Survival after Minimally Invasive Radical Hysterectomy for Early-Stage Cervical Cancer. N Engl J Med 2018;379(20):1905–1914. doi: 10.1056/NEJMoa1804923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.National Comprehensive Cancer Network. Ovarian Cancer (version 1.2022) Accessed May 8, 2022. https://www.nccn.org/guidelines/guidelines-detail
- 36.Nitecki R, Rauh-Hain JA, Melamed A, et al. Laparoscopic cytoreduction After Neoadjuvant ChEmotherapy (LANCE). Int J Gynecol Cancer 2020;30(9):1450–1454. doi: 10.1136/ijgc-2020-001584 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


